Abstract
Through subtraction of tumor-specific CpG methylation, we identified receptor tyrosine kinase-like orphan receptor 2 (ROR2) as a candidate tumor suppressor gene (TSG). ROR2 is a specific receptor or co-receptor for WNT5A, involved in canonical and non-canonical WNT signaling, with its role in tumorigenesis controversial. We characterized its functions and related cell signaling in common carcinomas. ROR2 was frequently silenced by promoter CpG methylation in multiple carcinomas including nasopharyngeal, esophageal, gastric, colorectal, hepatocellular, lung, and breast cancers, while no direct correlation of ROR2 and WNT5A expression was observed. Ectopic expression of ROR2 resulted in tumor suppression independent of WNT5A status, through inhibiting tumor cell growth and inducing cell cycle arrest and apoptosis. ROR2 further suppressed epithelial-mesenchymal transition and tumor cell stemness through repressing β-catenin and AKT signaling, leading to further inhibition of tumor cell migration/invasion and increased chemo-sensitivity. Thus ROR2, as an epigenetically inactivated TSG, antagonizes both β-catenin and AKT signaling in multiple tumorigenesis. Its epigenetic silencing could be a potential tumor biomarker and therapeutic target for carcinomas.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1485-z) contains supplementary material, which is available to authorized users.
Keywords: Epigenetic, Tumor suppressor, ROR2, Methylation, Carcinoma
Introduction
Receptor tyrosine kinases (RTKs) play a critical role in normal development and diseases including cancers through regulating cellular proliferation, apoptosis, differentiation, and migration [1, 2]. Receptor tyrosine kinase-like orphan receptor 2 (Ror2), a member of orphan RTKs, has been shown to be involved in multiple developmental morphogenesis [3–5] including osteoblastogenesis [6] and neurogenesis [7, 8]. Its dual role in human tumorigenesis has just been studied recently, especially for its tumor-promoting function [9]. ROR2 was reported to possess tumor-promoting activities in several malignancies including renal cancer [10], prostate cancer [11], melanoma [12], and osteosarcoma [13], and further serves as a prognostic biomarker and therapeutic target [14, 15]. On the other hand, promoter methylation-mediated silencing of ROR2 was found to promote tumor cell growth of colon cancer in vivo and in vitro [16]. Loss of Wnt5a and Ror2 protein has been identified in hepatocellular carcinoma, and associated with its poor prognosis [17]. However, the expression and tumor-suppressive function of ROR2 in the pathogenesis of other common tumor remain unclear.
As a specific receptor or co-receptor for Wnt5a [8, 18], Ror2 inhibits canonical Wnt/β-catenin signaling [19–22] and activates non-canonical c-Jun N-terminal kinase (JNK) signaling in developmental morphogenesis [23, 24]. Of note, Ror2 and Wnt5a display overlapping expression patterns, and Ror2- and Wnt5a-knockout mice share similar phenotypes [5, 24–26], suggesting a physical and functional correlation of Ror2 and Wnt5a. Although WNT5A-ROR2 signaling has been reported to be implicated in the invasiveness of several carcinomas [11–13], the underlying molecular mechanisms of ROR2 signaling in human tumorigenesis are poorly defined.
Here, we identified ROR2 as a methylated TSG through PCR subtraction of tumor-specific CpG methylation. We further investigated its epigenetic inactivation in multiple carcinomas, and demonstrated its pro-apoptotic and anti-metastatic activities in tumor cells. We found that ROR2 suppressed β-catenin and AKT signaling, as well as the epithelial-mesenchymal transition (EMT) and stemness of tumor cells, further leading to the inhibition of tumor cell migration/invasion.
Materials and methods
Cell lines and tissue samples
A series of tumor cell lines were used. Immortalized, non-transformed normal epithelial cell lines (NP69, Het-1A, NE1, NE3, CCD841-CoN, and HMEpC) were used as normal controls. Cell lines were obtained either from American Type Culture Collection or from collaborators. Cell lines were treated with 10 mmol/l 5-aza-2′-deoxycytidine (Aza) (Sigma-Aldrich, St Louis, MO, USA) for 3 days or further treated with 100 nmol/l trichostatin A (TSA) (Cayman Chemical Co., Ann Arbor, MI, USA) for an additional ~16 h as described previously [27]. Normal adult and fetal tissue RNA and protein samples were purchased commercially (Stratagene, La Jolla, CA, USA, or Millipore-Chemicon, Billerica, MA, USA). DNA samples of some normal epithelial primary carcinomas (T) and matched surgical margin normal tissues (N) have been described previously [28].
Plasmid construction and generation of stable cell pools
pcDNA3.1(+)-ROR2 construct with FLAG-tagged at the C terminus was generated as previously described [28], and sequence verified. To establish stably transfected tumor cells with ROR2 expression, full-length ROR2 expression construct was transfected into HONE1, MB231, KYSE150, and KYSE410 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). The cells were cultured in RPMI 1640 supplemented with 10 % FBS and selected in 400 or 1,200 μg/ml of G418 for 20-30 days to establish stable cell pools, with confirmed ROR2 expression.
RNA interference
Two independent pairs of unique siRNA (Origene Technologies, Rockville, MD, USA), a pool of three siRNA duplexes (Santa Cruz Biotechnologies, Santa Cruz, CA) and a pool of four unique shRNA constructs (Origene) targeting ROR2, as well as a pool of three unique siRNA duplexes (Origene) targeting WNT5A were used in this study. For siRNA or shRNA knockdown of ROR2, the cells were transfected with either targeting siRNA or shRNA, or scramble siRNA or shRNA using Lipofectamine 2000 (Invitrogen) for 48 h.
Antibodies
Antibodies used are: p27Kip1 (#3698), cleaved caspase-3 (#9661), cleaved poly (ADP-ribose) polymerase (#9541), phospho-β-Catenin (Ser552) (#9566), phospho-GSK-3β (Ser9) (#9336), GSK-3β (#9315), phospho-AKT (Ser473) (#4060), AKT (#4691) and E-cadherin (#4065) (Cell Signaling, Beverly, MA); Flag M2 (#F3165), Vimentin (#V6630) (Sigma-Aldrich) and active β-catenin (anti-ABC, #05-665, Upstate, Lake Placid, NY, USA); total β-catenin (#M3539), anti-mouse Ig G-HRP (#P0161), anti-rabbit Ig G-HRP (#P0448) (Dako, Glostrup, Denmark); N-cadherin (BD Transduction Labs, San Jose, CA, USA); ROR2 (#sc-80329), Fibronectin (#sc-9068); CCND1 (#sc-20044), c-MYC (#sc-764), WNT5A (#sc-365370) (Santa Cruz, CA); a-tubulin (Lab Vision Corporation, Fremont, CA, USA).
Semiquantitative RT-PCR, 5′-RACE (rapid amplification of cDNA ends) and quantitative real-time PCR (qRT-PCR)
Semiquantitative RT-PCR and quantitative real-time PCR were performed as described before [29]. GAPDH was amplified as a control. Real-time PCR was carried out according to the manufacturer’s protocol (HT7900 system; Applied Biosystems), with SYBR Green master mix (Applied Biosystems) used. Primers used are listed in Supplementary Table S1.
We determined the ROR2 transcription start site using the 5′-RACE system for rapid amplification of cDNA ends (Version 2.0, Invitrogen). Briefly, first-strand cDNA was synthesized from trachea RNA using primer ROR2R2 (Supplementary Table S1). Homopolymeric tails were then added to the 3′-ends with terminal deoxynucleotidyl transferase. PCR was performed using Abridged Anchor Primer and a second gene-specific primer ROR2R (Supplementary Table S1). The RACE product was enriched by semi-nest amplifying with the Abridged Universal Amplification Primer and ROR2R2. PCR products were then cloned and sequenced.
Bisulfite treatment and promoter methylation analysis
Bisulfite modification of genomic DNA was carried out as described previously [30, 31]. Bisulfited DNA was amplified with either methylation-specific primer sets or unmethylation-specific primer sets. MSP primers are shown in Table 2. MSP primers have been tested for not amplifying any not bisulfited DNA (Supplementary Fig. S1A). For BGS, bisulfite-treated DNA was amplified using a BGS primer set (Supplementary Table S1). PCR products were then cloned into pCR4-Topo vector (Invitrogen, Carlsbad, CA), with 8–10 clones randomly picked and sequenced.
Table 2.
Reduced expression of ROR2 in tumors
| Tissue type | Sample number | Median of expression intensity (log 2) | p value |
|---|---|---|---|
| Bladder | 48 | 0.453 | |
| Bladder cancer | 28 | −2.635 | 1.73E−13 |
| Prostate | 8 | 2.277 | |
| Prostate cancer | 13 | 1.264 | 1.90E−05 |
| Cervix | 5 | 0.468 | |
| Cervical cancer | 40 | 0.016 | 1.26E−14 |
| Colon | 19 | 0.252 | |
| Colon adenocarcinoma | 101 | −0.623 | 3.56E−06 |
| Pancreas | 39 | 0.477 | |
| Pancreatic cancer | 39 | 0.093 | 7.69E−07 |
| Brain | 23 | −1.997 | |
| Glioblastoma | 81 | −2.858 | 6.78E−04 |
| Lung | 2 | 1.642 | |
| Squamous cell lung carcinoma | 10 | 0.627 | 2.88E−04 |
| Ovary | 4 | 2.539 | |
| Ovarian cancer | 28 | 1.815 | 3.73E−05 |
| Kidney | 3 | −0.991 | |
| Renal cancer | 19 | −3.497 | 2.00E−03 |
| Breast | 15 | 1.279 | |
| Breast cancer | 7 | 0.939 | 3.00E−03 |
| Liver | 220 | −1.221 | |
| HCC | 225 | −1.258 | 4.00E−03 |
Data extracted from the cancer microarray database Oncomine: www.oncomine.org/
Colony formation assay
Colony formation assay was carried out as previously described [32, 33]. Briefly, cells were cultured overnight in a 12-well plate (1.0 × 105 per well) and transfected with empty vector (pcDNA3.1) or ROR2-expressing plasmid (pcDNA3.1(+)ROR2-Flag) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Forty-eight hours later, the transfectants were replated in triplicate and cultured for 10–15 days in complete medium containing G418 (400 μg/ml). Surviving colonies were stained with gentian violet after methanol fixation, with visible colonies (≥50 cells) counted. ZR-75-1 cells were treated with independent two scramble siRNA, or ROR2 siRNA. KYSE140 cells were treated with scramble shRNA, or pooled ROR2 shRNA. The cells were stained with gentian violet after methanol fixation 4 days after siRNA or shRNA treatment.
Soft agar assay
Anchorage-independent growth of tumor cells was determined by soft agar assay as described. Briefly, cells were cultured and transfected with empty vector (pcDNA3.1), ROR2-expressing plasmid (pcDNA3.1(+)ROR2-Flag) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Forty-eight hours after transfection, cells were resuspended in complete medium containing G418 (400 μg/ml). With 1/10 volume of heated 3.3 % soft agar added, 5 × 103 cells were seeded into each well of a 24-well plate. Colonies were counted after 17 days.
Doxorubicin treatment
Doxorubicin was purchased from EBEWE Pharma (Ebewe Pharma Ges.m.b.H.N fg., Unterach am Attersee, Austria). Stable expressing-ROR2 cell pools were treated with doxorubicin at the concentration of 10 μg/ml for 2 h, and then collected for the further analysis.
TUNEL assay
Cells cultured on coverslips were fixed with 4 % paraformaldehyde in phosphate-buffered saline for 15 min at room temperature, and permeabilized with 0.1 % Triton X-100 in phosphate-buffered saline for 2 min on ice. TUNEL staining was done using the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany).
Flow cytometry analysis of cell cycle
Flow cytometry analysis of cell cycle was described previously [34, 35]. Stable cell pools were used for detecting the effect of ectopic expression of ROR2. Cells transiently transfected with scramble and ROR2 siRNA were used for testing its effect of ROR depletion. Cells transfected with sramble#1 and #2 siRNA were mixed as one group. Cells were fixed in ice-cold 70 % ethanol and stained with propidium iodide (PI). Cell-cycle profiles were obtained using the Elite ESP flow cytometry at 488 nm, with data analyzed using the CELL quest software (BD Biosciences, San Jose, CA, USA).
Western blot
Cell lysates were prepared by incubating cell pellets in lysis buffer (50 mmol/l Tris–HCl, pH 8.0; 150 mmol/l NaCl, 0.5 % NP-40) for 30 min on ice, followed by centrifugation at 14,000 × g for 15 min at 4 °C. For Western-blot analysis, membranes were incubated with primary antibodies for 1 h at room temperature or overnight at 4 °C, followed by incubation with secondary antibodies. Immunoreactive bands were visualized using Western blot Luminol reagent (GE Healthcare Bio-Sciences, Piscataway, NJ, USA).
Dual-luciferase reporter assay
TcF transcriptional activity and its target gene-promoter activities were determined by luciferase reporter assays. The TcF-responsive luciferase construct pTOPFlash (kindly provided by Prof. Christof Niehrs, German Cancer Research Center, DKFZ), FOPFlash reporter containing mutant TCF/LEF binding sites (gift from Dr. Jin Dong-Yan, University of Hong Kong) were cotransfected with either pcDNA3.1-ROR2 or empty vector, together with an internal control Renilla luciferase reporter phRL-TK (Promega, Madison, WI, USA). Forty-eight hours after transfection, luciferase activities were determined using a dual-luciferase reporter assay kit (Promega). For testing the effect of ROR2 depletion on luciferase activity, after scramble siRNA, and ROR2 siRNA transfection for 24 h, the cells were then transiently transfected with reporter construct TOPflash or FOPflash for another 48 h. Relative luciferase activities were determined and normalized using Renilla reniformis luciferase activity as an internal control.
Indirect immunofluorescence
Cells grown on coverslips were stained by indirect immuno-fluorescence as described previously [28, 34]. Briefly, cells were incubated with primary antibody against ROR2, E-cadherin, or Vimentin and then incubated with Alexa Fluor 594- (Invitrogen Molecular Probes, Carlsbad, CA, USA) or FITC-conjugated (F313, Dako) secondary antibody against mouse or rabbit IgG. To analyze the effects of ROR2 on actin stress fiber formation, cells were serum starved for 24 h before incubation in normal 10 % fetal bovine serum medium. After 1 h, cells were fixed and stained by Rhodamine-labeled phalloidin (Invitrogen Molecular Probes). Cells were then counterstained with DAPI and imaged with an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan).
Wound-healing and Matrigel invasion assays
Cell motility was assessed using a scratch wound-healing assay [28, 33]. Stably transfected cells were cultured in six-well plates until confluent. Cell layers were carefully wounded using sterile tips and washed twice with fresh medium. After incubation for 12, 24, and 40 h, cells were photographed under a phase-contrast microscope. Experiments were performed in triplicate. In-vitro invasion assays were carried out in BD BioCoat Matrigel chambers (Transwell, BD Biosciences, Heidelberg, Germany) as described previously [28].
Statistical analysis
Results are shown as values of mean ± SD. Statistical analyses were performed using Student’s t test to determine p values.
Results
Epigenetic identification of ROR2 as a methylated candidate TSG
We performed methylation-sensitive representational difference analysis (MS-RDA) [27] of an NPC cell line and its demethylated counterpart with Aza treatment and identified ROR2 as a methylated candidate TSG (Fig. 1a). We then examined the expression profile and methylation status of ROR2 in multiple normal and tumor cell lines. ROR2 is widely expressed in all normal human adult and fetal tissues as well as immortalized normal cell lines (Fig. 1b, c), but silenced or downregulated in multiple carcinoma cell lines including nasopharyngeal (NPC), esophageal (ESCC), lung, gastric, hepatocellular (HCC), colorectal (CRC), and breast carcinomas (Fig. 1c). To study the epigenetic regulation of ROR2 silencing, we first applied 5′-RACE to define its transcriptional start site, which is found to be 65-bp ahead of the first base of the ROR2 cDNA sequence (NM_004560) in genome database (Fig. 1a). The ROR2 promoter contains a typical CpG island (CGI) and we thus investigated its methylation status. By methylation-specific PCR (MSP), promoter methylation of ROR2 was detected in tumor cell lines with silenced ROR2 but not in any normal cell line (Fig. 1c; Table 1), which was further confirmed by detailed methylation analysis with bisulfite genomic sequencing (BGS) (Supplementary Fig. S1A).
Fig. 1.
ROR2 is frequently silenced by promoter CpG methylation in multiple carcinomas. a Schematic structure of the ROR2 CpG island. The hypermethylated fragment identified by MS-RDA (indicated with a thick bar), 5′-RACE fragment, exon 1, CpG sites (short vertical lines), MSP sites and BGS region analyzed are shown. Transcription start site is indicated by a curved arrow. b ROR2 is broadly expressed in human normal adult tissues, PBMCs, and fetal tissues, with GAPDH as a control. Sk.M skeleton muscle, BM bone marrow, L.N. lymph node. c ROR2 is frequently silenced and methylated in multiple carcinoma cell lines, but expressed and unmethylated in immortalized but non-transformed epithelial cell lines (names underlined). d Pharmacologic demethylation with Aza alone or combined with TSA (A + T) restored ROR2 expression in methylated/silenced tumor cell lines. Ca carcinoma, NPC nasopharyngeal carcinoma, ESCC esophageal carcinoma, HCC hepatocellular carcinoma, CRC colorectal cancer, M methylated, U unmethylated
Table 1.
Summary of ROR2 methylation in tumors and normal tissues
| Cell lines (% methylated) | Tumors (% methylated) | |
|---|---|---|
| Carcinoma | ||
| Nasopharyngeal | 100 (5/5) | 72 (21/29) |
| Esophageal | 56 (5 + 5 w/18) | 76 (13/17) |
| Lung | 50 (4/8) | |
| Gastric | 56 (9/16) | 77 (40/52) |
| Hepatocellular | 54 (5 + 2 w/13) | 54 (20/37) |
| Colorectal | 63 (4 + 1 w/8) | 64 (7/11) |
| Breast | 78 (7/9) | 47 (9/19) |
| Immortalized normal epithelial cell lines | ||
| NP69, NE1, NE3 | 0 (0/3) | |
| Normal epithelial cell lines | ||
| Het-1A, HMEC, HMEpC, CCD841-CoN | 0 (0/4) | |
| Surgical margin tissues of tumors | ||
| Esophageal tissues | 18 (3/17) | |
| Normal tissues | ||
| Normal nasopharyngeal tissues | 11 (1 w/9) | |
| Normal breast tissues | 14 (1/7) | |
W weak methylation
ROR2 expression in methylated and silenced cell lines could be restored by treatment with the DNA methyltransferase inhibitor Aza, alone or combined with histone deacetylase inhibitor trichostatin A (Fig. 1d). BGS analysis further confirmed the pharmacological demethylation of ROR2, concomitantly with decreased methylated and increased unmethylated promoter alleles (Supplementary Fig. S1C). These results suggest that promoter methylation directly mediates ROR2 silencing in tumor cells.
Frequent promoter methylation and downregulation of ROR2 in primary carcinomas
We further evaluated ROR2 promoter methylation in primary carcinomas. Methylation was detected in multiple common carcinoma tissues, including 72 % (21/29) of NPC, 76 % (13/17) of esophageal, 77 % (40/52) of gastric, 54 % (20/37) of HCC, 64 % (7/11) of colon and 47 % (9/19) of breast carcinomas, but rarely in normal epithelial tissues (Fig. 2a; Table 1). RT-PCR analysis also showed that reduced ROR2 expression in tumor samples, consistent with its methylation status (Fig. 2c). Further high-resolution methylation analysis by BGS showed densely methylated alleles in tumors, while only scattered CpG sites were methylated in normal tissues (Fig. 2b). Moreover, ROR2 was significantly downregulated in multiple common carcinoma tissues, through analyzing the online microarray database (Oncomine, Compendia Bioscience, Ann Arbor, MI) (Table 2; Fig 2c) [36]. These results demonstrate that ROR2 silencing by promoter methylation is a frequent event in multiple tumorigenesis.
Fig. 2.
a Representative MSP analysis of ROR2 methylation in primary carcinomas and normal tissues. Ca carcinoma, NPC nasopharyngeal carcinoma, ESCC esophageal carcinoma, HCC hepatocellular carcinoma, CRC colorectal cancer, N paired tumor-adjacent normal tissues, T tumor, M methylated, U unmethylated. b Representative BGS analyses of ROR2 promoter methylation in normal nasopharyngeal tissue and primary tumors. c Representative analysis of ROR2 expression in primary tumors and paired tumor-normal tissues by semi-quantitative RT-PCR. T tumor, N tumor margin normal tissue
ROR2 expression exhibits anti-tumorigenic effects independent of WNT5A
Due to the relationship of Ror2 and Wnt5a in a mouse model, we thus investigated their relationship in normal tissue and tumor cell lines. WNT5A was readily expressed in normal adult tissues with varying expression levels as measured by semi-quantitative RT-PCR and quantitative RT-PCR (qRT-PCR), except for normal peripheral blood mononuclear cells (PBMC) (Supplementary Fig. S2A and C), while frequently downregulated in multiple carcinoma cell lines (Supplementary Fig. S2B and C). No direct correlation was found between the expression of ROR2 and WNT5A in human cancer cell lines, indicating that ROR2 may exert its tumor suppressive function independent of WNT5A. Thus, we selected representative tumor cell lines with methylated/silenced ROR2 and/or WNT5A (WNT5A+/ROR2-: HONE1, MB231 and KYSE410; WNT5A-/ROR2-: KYSE150), together with the ZR-75-1 and KYSE140 cell lines naturally expressing ROR2 and WNT5A, as tumor models for further functional and mechanical studies.
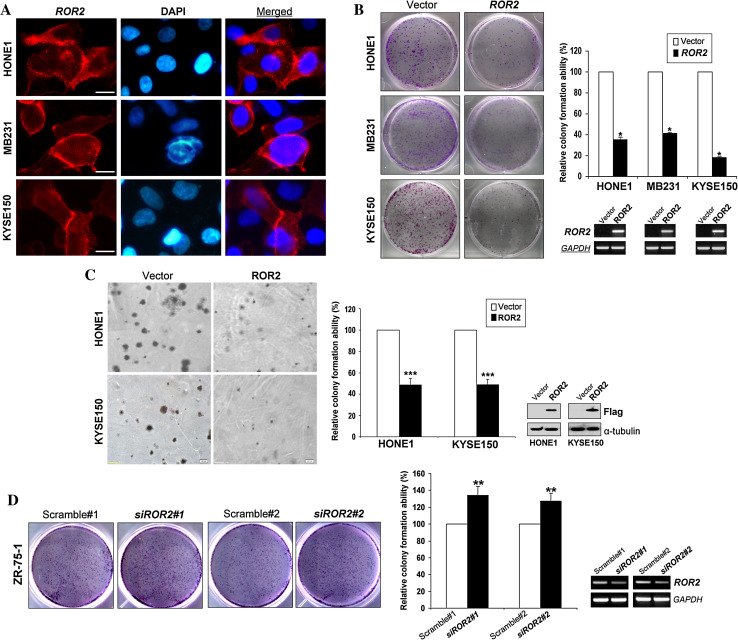
To assess the role of ROR2 in tumorigenesis, we investigated its effects on cell growth, cell cycle, and apoptosis. Immunostaining showed that ROR2 was located at the cell membrane and less often in cytoplasm in ROR2-transiently transfected cells (Fig. 3a). Colony formation assay showed that ROR2 significantly suppressed tumor cell colony formation (colony numbers down to ~20–40 % of controls) (* p < 0.05) (Fig. 3b), with the WNT5A-ROR2 double-negative cell line KYSE150 had the greatest effect. Soft agar assay also showed that colony numbers were significantly decreased to ~50 % in ROR2-expressing HONE1 and KYSE150 cells, along with reduced colony size (*** p < 0.001) (Fig. 3c). On the contrary, RNAi-mediated knockdown of endogenous ROR2 significantly enhanced the growth of ZR-75-1 and KYSE140 cells by ~30 % (** p < 0.01, *** p < 0.001) (Fig. 3d; Supplementary Fig. S3A). These data suggest that ROR2 does act as a tumor suppressor through inhibiting cell growth of tumor cells.
Fig. 3.
ROR2 inhibits tumor cell growth and induces tumor cell apoptosis. a Immunostaining showed that ROR2 (red) was localized at the cell membrane in ROR2-transfected cells using mouse anti-ROR2 monoclonal antibody. DAPI counterstaining (blue) was used to visualize DNA. Original magnification, ×400. Scale bar 20 μm. b, d Representative colony formation assays. Quantitative analyses of colony numbers are shown as values of mean ± SD (left panel), ROR2 expression was measured by RT-PCR (right panel), *p < 0.05, **p < 0.01. c The effect of ectopic expression of ROR2 on tumor cell growth as measured by soft agar assay. Quantitative analyses of colony numbers are shown as values of mean ± SD. ROR2 expression was measured by Western-blot analysis, ***p < 0.001
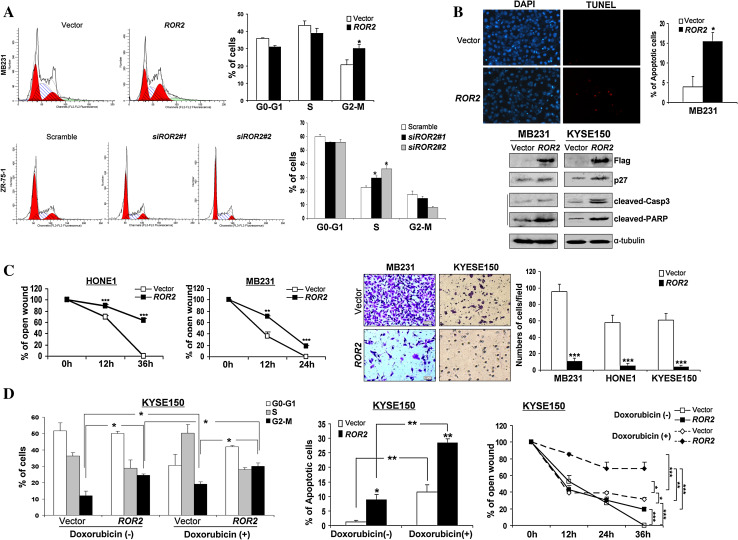
Ectopic expression of ROR2 also significantly increased the proportion of G2/M phase cells (* p < 0.05) (Fig. 4a, d), together with upregulated p27 protein level (Fig. 4b), while knockdown of ROR2 by siRNA significantly increased the progression of cell cycle S phase (* p < 0.05) (Fig. 4a). TUNEL staining revealed a significant increase of apoptotic cells in ROR2-expressing MB231 and KYSE150 cells (* p < 0.05) (Fig. 4b, d), as also confirmed by elevated-cleaved caspase 3 and poly (ADP-ribose) polymerase (Fig. 4b). Thus, ROR2 functions as a TSG in tumor cells, irrespective of their WNT5A status.
Fig. 4.
a Effect of ROR2 on cell cycle distribution of tumor cells. Representative cell cycle analysis and summarized flow cytometry data are shown. Results are represented as mean ± SD and based on three independent experiments. *p < 0.05. b TUNEL assay of ROR2 and vector-expressing MB231 tumor cells. *p < 0.05. Western blot showing upregulation of p27, cleaved caspase 3 and cleaved-PARP in ROR2-expressing MB231 and KYSE150 cells. c Migration of ROR2- or empty vector-transfected tumor cells by scratch wound healing assay. Width of remaining open wound measured in relation to time 0 h separation. **p < 0.01, ***p < 0.001. Transwell migration assay of ROR2-expressing tumor cells. Migrated cells at the lower surface of the transwell filter were stained (left panel) and counted (right panel). Original magnification, ×400. ***p < 0.0001. d Effects of cell cycle, apoptosis, and migration on KYSE150 cells treated with or without doxorubicin. *p < 0.05, **p < 0.01, ***p < 0.001
ROR2 inhibits tumor cell migration/invasion and promotes chemosensitivity
We next employed wound-healing and Matrigel assays to assess the effects of ROR2 expression on cell migration and invasion in WNT5A+/ROR2-, WNT5A-/ROR2-, and WNT5A+/ROR2+ cells. Scratch wound-healing assay showed that ROR2 expression significantly inhibited the wound closure of tumor cells (** p < 0.01, *** p < 0.001) (Fig. 4c; Supplementary Fig. S4A). In contrast, ROR2 depletion by pooled shRNA promoted the wound closure of tumor cells (Supplementary Fig. S3B). Matrigel assay revealed a significant suppression of invasion across the Matrigel (by ~90 %) for ROR2-expressing tumor cells (*** p < 0.001) (Fig. 4c), suggesting its anti-metastatic feature.
We also investigated the effect of ROR2 on the chemosensitivity of tumor cells to doxorubicin. In ROR2-expressing KYSE150 cells treated with doxorubicin, significant increase of G2/M arrest (* p < 0.05), cell apoptosis (** p < 0.01), and cell motility repression (* p < 0.05, ** p < 0.01, ***p < 0.001) (Fig. 4d) was observed, suggesting that ROR2 increases the chemosensitivity of doxorubicin to tumor cells.
ROR2 suppresses epithelial-mesenchymal transition (EMT)
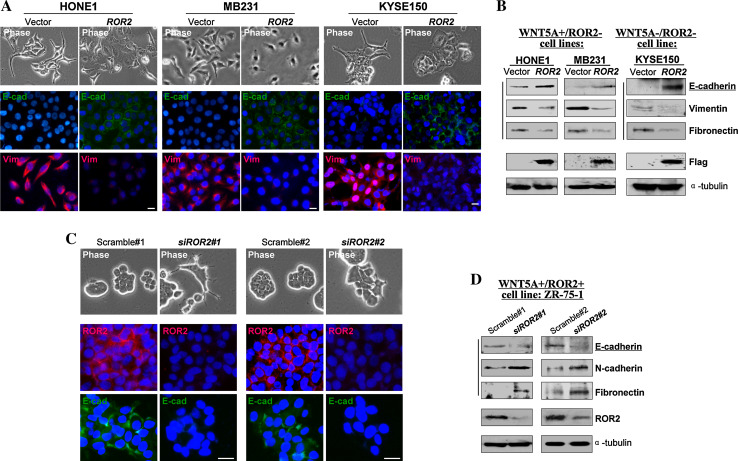
As EMT plays a critical role in tumor cell metastasis, the effect of ROR2 on tumor cell EMT was further assessed. By ectopically expressing ROR2 in WNT5A+/ROR2- (HONE1 and MB231) and WNT5A-/ROR2- (KYSE150) tumor cells, we found that ROR2-expressing tumor cells endured morphological changes from spindle-like shape to cobblestone-like appearance (Fig. 5a). Consistent with morphology change, dramatic abrogation in actin cytoskeleton formation was observed (Supplementary Fig. S4B). Meanwhile, we detected increased E-cadherin expression and decreased expression of vimentin and fibronectin, by both immunofluorescence staining and Western blot (Fig. 5a, b), while the cell WNT5A status did not affect this effect. On the other hand, in WNT5A+/ROR2+ ZR-75-1 cells, siRNA-mediated silencing of ROR2 induced EMT through downregulating E-cadherin and upregulating N-cadherin and fibronectin, as evidenced by more spindle-like cells (Fig. 5c, d). These data demonstrate that ROR2 inhibits the EMT of tumor cells, which is also WNT5A-independent.
Fig. 5.
ROR2 inhibited tumor cells EMT. a Morphology changes of HONE1, MB231, and KYSE150 cells after ROR2 transfection by phase contrast microscopy (upper panel). Indirect immunofluorescence detecting the expression of E-cadherin and vimentin (lower panel). Original magnification, ×400. Scale bar 20 μm. b Western blot showing the expression of E-cadherin, vimentin and fibronectin in ROR2- or vector-transfected cells. α-tubulin was used as a loading control. c Immunofluorescent analysis of E-cadherin in scramble- and ROR2-siRNA treated ZR-75-1 cells. Original magnification, ×400. Scale bar 20 μm. d Western blot showing the knock down of ROR2 which resulted in a decreased expression of E-cadherin and an increased expression of N-cadherin and fibronectin in ZR-75-1 cells, compared with control siRNA scramble-treated cells
ROR2 antagonizes β-catenin and AKT signaling and represses cell stemness
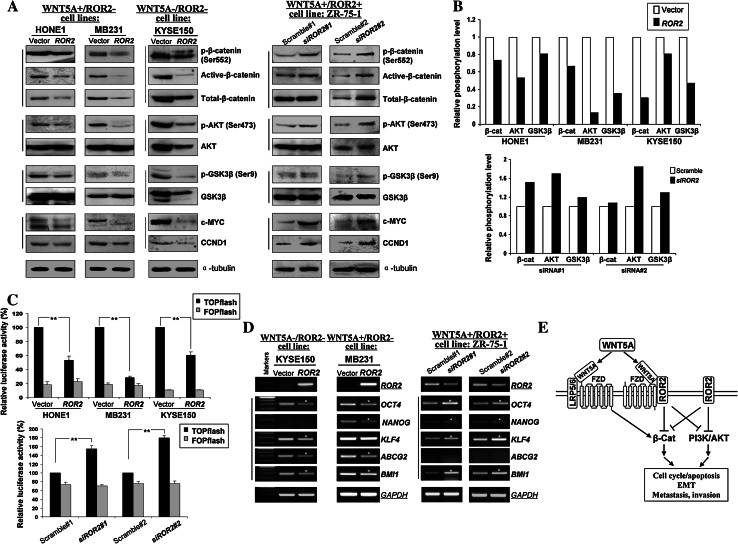
As β-catenin and AKT pathways are critical molecular events regulating cell EMT, we tested whether ROR2 could counteract β-catenin and AKT signaling in tumor cells. Ectopic expression of ROR2 in WNT5A+ and WNT5A-negative tumor cells led to significantly decreased active- and phosphor (Ser552)-β-catenin, as well as its downstream targets CCND1 and c-MYC (Fig. 6a). Moreover, inhibition of phosphorylated AKT (Ser473) was detected in ROR2-expressing cells, accompanied by decreased phosphorylation (Ser9) of its downstream target GSK3β (Fig. 6a; left panel). We further assessed the effects of ROR2 on β-catenin and AKT signaling by knocking down WNT5A and overexpressing ROR2 in KYSE410 cells (WNT5A+/ROR2-). We found that ROR2 downregulated phosphorylation of β-catenin and AKT in WNT5A-depleted cells (Supplementary Fig. S5), consistent with the finding observed in cells with physiological-depleted WNT5A and ROR2. Moreover, knock-down of ROR2 by siRNA or shRNA induced the activation of β-catenin and AKT signaling in WNT5A+/ROR2+ ZR-75-1 and KYSE140 cells (Fig. 6a, right panel, Supplementary Fig. S3C).
Fig. 6.
Ectopic expression of ROR2 disrupts β-catenin and AKT signaling. a Western blot was performed in ROR2-expressing and ROR2 siRNA-transfected tumor cells, using antibodies against phospho-β-catenin (Ser552), active β-catenin, total β-catenin, phospho-AKT (Ser473), AKT, phospho-GSK3β (Ser9), GSK3β, c-MYC and CCND1. α-tubulin was used as a control. b Relative phosphorylation levels were calculated as ratios of phosphorylated towards total protein levels using densitometry. c TOP/FOP luciferase reporter activity assay in vector- and ROR2-expressing tumor cells, and scramble siRNA and ROR2 siRNA transfected cells. **p < 0.01. d Expression of representative stem cell markers in ROR2- and siRNA ROR2-transfected tumor cells. *Indicates significantly downregulated/upregulated bands. e Proposed model of epigenetic inactivation of ROR2 disrupts WNT/β-catenin and AKT signaling pathways in tumorigenesis, resulting in the inhibition of EMT, metastasis, and invasion
As changed levels of active versus total beta-catenin, phosphor- versus total Akt and phosphor- versus total GSK3β were observed, we further evaluated quantitatively the phosphorylation level changes of β-catenin, AKT and GSK-3β by calculating their phospho/total ratios in assayed tumor cells (Fig. 6b). These quantitative analyses clearly showed that ROR2 could deregulate β-catenin and AKT signaling to varying levels in tumor cells with ectopic expression of ROR2, while knockdown of ROR2 led to activated β-catenin and AKT signaling in tumor cells, indicating ROR2 does suppress β-catenin and AKT signaling in tumorigenesis.
Furthermore, TOPflash/FOPflash reporter assay showed that ROR2 expression significantly repressed β-catenin/TCF-dependent transcription (decreased to 10–20 %) in both WNT5A + and WNT5A- tumor cells (** p < 0.01) (Fig. 6c, upper), while ROR2 knockdown increased the TOPflash reporter activity by more than 50 % (** p < 0.01) (Fig. 6c, lower), together with no significant difference in FOPflash transfected groups.
As EMT endows tumor cells with stem-cell like features for more growth advantage, representative stem cell markers were analyzed by RT-PCR in tumor cells after re-expressing ROR2. ROR2-expressing KYSE150 and MB231 cells showed reduced expression of OCT4, ABCG2, BMI1 and/or NANOG, while ZR-75-1 cells transfected with ROR2-siRNA showed elevated OCT4, NANOG, KLF4, and BMI1 (Fig. 6d), suggesting that ROR2 is involved in regulating the stemness of cancer cells.
Discussion
In this study, through tumor-specific methylation subtraction, we identified receptor tyrosine kinase-like orphan receptor 2 (ROR2), a component of the WNT signaling pathway, as a methylated TSG. We characterized its expression and function in common cancers and found that ROR2 is broadly expressed in normal adult and fetal tissues, but frequently methylated and silenced in multiple carcinoma cell lines and primary tumors but seldom in normal tissues. We also found that ROR2 exhibited tumor-suppressive activities in tumor cells, but different from previous report on mouse Ror2, this activity is independent of WNT5A. ROR2 antagonizes β-catenin and AKT signaling, and further inhibits the growth, migration/invasion, and EMT feature of tumor cells, resulting in increased chemosensitivity (Fig. 6e). Our study thus demonstrated that ROR2 is a broad functional tumor suppressor for multiple carcinomas.
Epigenetic inactivation of TSGs through promoter CpG methylation and histone modifications is a key cause of tumor initiation and progression [37, 38]. Using MS-RDA, a power technique to isolate differentially methylated DNA fragments [39], we successfully identified a series of methylated/silenced target genes, including PCDH10 [27], GADD45G [40], DLEC1 [41], and PAX5 [42]. We have characterized these genes to be functional TSGs and potential tumor markers for multiple malignancies, such as NPC, ESCC, gastric, colorectal, and cervical carcinomas [43]. ROR2 is another candidate TSG identified, frequently methylated and silenced in common carcinomas. We also found that in few carcinoma cell lines, ROR2 was downregulated without obvious methylation detected, suggesting that histone modifications could also be alternative mechanism contributing to ROR2 silencing in some tumors.
Although overexpression and oncogenic property of ROR2 have been reported in melanoma and sarcomas [11–15], emerging evidences [16], together with ours, support that ROR2 does function as a tumor suppressor in common carcinoma cells, indicating a complex role of ROR2 in tumorigenesis. Like other RTKs, Ror2 receptor function requires its tyrosine kinase activity. Recent reports showed that ROR2 was identified as a specific receptor or co-receptor for Wnt5a in vivo and in vitro [8, 18]. Moreover, the diverse interactions of Ror2 with other Wnt ligands and non-Wnt ligands have also been reported, most of which mediate ROR2 activation of tyrosine kinase activity [44]. The mouse Ror2, a receptor protein tyrosine kinase, inhibits Wnt/β-catenin signaling which requires its cysteine-rich domain (CRD) and Ig-like domain (Ig). In return, Wnt5a directly modulates Ror2 tyrosine kinase activity [20, 45], confirming that Ror2 is a bona fide inhibitor of Wnt/β-catenin signaling [3, 20]. Ror2 forms homodimers that result in tyrosine phosphorylation [46]. Casein kinase iepsilon (CKIε), a regulator of Wnt signaling, could regulate the tyrosine kinase activity of Ror2 as its binding partner [47].
However in human cancers, we failed to detect an obvious direct correlation between ROR2 and WNT5A expression, although the WNT-dependent role of ROR2 has been suggested in colon cancer cells [16]. Our previous studies also showed that WNT5A is frequently silenced in colorectal and esophageal squamous carcinomas [33, 48], and functions as a tumor suppressor through antagonizing Wnt/β-catenin signaling, independent of the expression status of ROR2. In this study, we further found that ROR2 exerts its tumor suppressive functions through inducing cell cycle arrest and apoptosis and inhibiting cell growth, independent of cell WNT5A status. However so far, the detailed molecular mechanisms of Ror signaling still remain mostly a mystery. With more binding partners found, the biological functions and underlying mechanisms of ROR2 in tumorigenesis will be unveiled more.
β-catenin and AKT signaling pathways have been identified involved in receptor-mediated tumor signaling. For example, activation of β-catenin and AKT pathways are critical for the maintenance of EMT [49] which is associated with cancer stem cell-like characters and chemosensitivity. It has been suggested that tumor cells with cancer stem cell features have the surviving advantage of being protected from chemotherapeutic agents through activating PI3K/AKT and WNT signaling [50]. Moreover, EMT indirectly contributes to chemoresistance by inducing a stem-like phenotype of cancer cells.
Another receptor-tyrosine-kinase-like orphan receptor member, ROR1, as another receptor for Wnt5a, shares high protein sequence similarity with ROR2. ROR1 has been reported possessing oncogenic properties in multiple cancers including breast cancer and lung adenocarcinoma [51, 52]. ROR1 activates AKT signaling through interacting with CKIε [52] or EGFR [51], leading to tumor-cell growth and sustained EGFR survival signaling. Recently, ROR1 has been reported being expressed on hematogones (non-neoplastic human B-lymphocyte precursors) and a minority of precursor-B acute lymphoblastic leukemia [53], indicating its association with cancer cell stemness. In this context, we found that ROR2 inhibits β-catenin and AKT signaling independent of WNT5A, and further suppresses the EMT and stem cell markers of cancer cells thus contributing to the inhibition of tumor cell migration and invasion. Furthermore, ROR2 depletion increased the proportion of S phase cells and the proliferation of tumor cells, together with downregulated E-cadherin, upregulated N-cadherin and fibronectin, indicating that the inhibition of ROR2 on cell migration and invasion is related to the suppression of cell proliferation and/or apoptosis. We further found that ROR2 renders drug-resistant cancer cells vulnerable to chemotherapy. These results suggest that ROR2 connects EMT, stemness and cell signaling. Further investigation is needed to test this theory through the study of tumor cell sphere formation in vitro or tumor formation in vivo from single tumor cells.
In summary, our study identifies ROR2 as a functional tumor suppressor with frequent epigenetic inactivation in common carcinomas. We also demonstrate that ROR2 inhibits β-catenin and AKT signaling, further contributing to the suppression of tumor cell EMT and migration/invasion. The methylation-mediated silencing of ROR2 may serve as a potential tumor biomarker and therapeutic target.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Drs. George Tsao, Sun Young Rha, Bert Vogelstein, and Michael Obster for some cell lines, DSMZ (German Collection of Microorganisms and Cell Cultures) for the KYSE cell lines (Shimada et al., Cancer 69: 277-284, 1992). This study was supported by grants from National Natural Science Foundation (No. 81372898 and 81172582), Hong Kong RGC (GRF # 474710), and Group Research Schemes of The Chinese University of Hong Kong.
Conflict of interest
The authors declare no conflicts of interest.
Contributor Information
Yajun Guo, Phone: +86-21-81870801, FAX: +86-21-81870810, Email: yjguo@smmu.edu.cn.
Qian Tao, Phone: +852-2632-1340, FAX: +852-2648-8842, Email: qtao@cuhk.edu.hk.
References
- 1.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 2.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 3.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20(2):163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oldridge M, Fortuna AM, Maringa M, Propping P, Mansour S, Pollitt C, DeChiara TM, Kimble RB, Valenzuela DM, Yancopoulos GD, Wilkie AO. Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat Genet. 2000;24(3):275–278. doi: 10.1038/73495. [DOI] [PubMed] [Google Scholar]
- 5.DeChiara TM, Kimble RB, Poueymirou WT, Rojas J, Masiakowski P, Valenzuela DM, Yancopoulos GD. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet. 2000;24(3):271–274. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- 6.Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, Nishita M, Marumo K, Martin TJ, Minami Y, Takahashi N. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012;18(3):405–412. doi: 10.1038/nm.2653. [DOI] [PubMed] [Google Scholar]
- 7.Takai A, Inomata H, Arakawa A, Yakura R, Matsuo-Takasaki M, Sasai Y. Anterior neural development requires Del1, a matrix-associated protein that attenuates canonical Wnt signaling via the Ror2 pathway. Development. 2010;137(19):3293–3302. doi: 10.1242/dev.051136. [DOI] [PubMed] [Google Scholar]
- 8.Hikasa H, Shibata M, Hiratani I, Taira M. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development. 2002;129(22):5227–5239. doi: 10.1242/dev.129.22.5227. [DOI] [PubMed] [Google Scholar]
- 9.Ford CE, Qian Ma SS, Quadir A, Ward RL. The dual role of the novel Wnt receptor tyrosine kinase, ROR2, in human carcinogenesis. Int J Cancer. 2012 doi: 10.1002/ijc.27984. [DOI] [PubMed] [Google Scholar]
- 10.Wright TM, Brannon AR, Gordan JD, Mikels AJ, Mitchell C, Chen S, Espinosa I, van de Rijn M, Pruthi R, Wallen E, Edwards L, Nusse R, Rathmell WK. Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene. 2009;28(27):2513–2523. doi: 10.1038/onc.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto H, Oue N, Sato A, Hasegawa Y, Matsubara A, Yasui W, Kikuchi A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29(14):2036–2046. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- 12.O’Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, French AD, Dissanayake SK, Indig FE, Bernier M, Taub DD, Hewitt SM, Weeraratna AT. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene. 2009;29(1):34–44. doi: 10.1038/onc.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren D, Minami Y, Nishita M. Critical role of Wnt5a-Ror2 signaling in motility and invasiveness of carcinoma cells following Snail-mediated epithelial-mesenchymal transition. Genes Cells. 2011;16(3):304–315. doi: 10.1111/j.1365-2443.2011.01487.x. [DOI] [PubMed] [Google Scholar]
- 14.Morioka K, Tanikawa C, Ochi K, Daigo Y, Katagiri T, Kawano H, Kawaguchi H, Myoui A, Yoshikawa H, Naka N, Araki N, Kudawara I, Ieguchi M, Nakamura K, Nakamura Y, Matsuda K. Orphan receptor tyrosine kinase ROR2 as a potential therapeutic target for osteosarcoma. Cancer Sci. 2009;100(7):1227–1233. doi: 10.1111/j.1349-7006.2009.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edris B, Espinosa I, Muhlenberg T, Mikels A, Lee CH, Steigen SE, Zhu S, Montgomery KD, Lazar AJ, Lev D, Fletcher JA, Beck AH, West RB, Nusse R, van de Rijn M. ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J Pathol. 2012 doi: 10.1002/path.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lara E, Calvanese V, Huidobro C, Fernandez AF, Moncada-Pazos A, Obaya AJ, Aguilera O, Gonzalez-Sancho JM, Sanchez L, Astudillo A, Munoz A, Lopez-Otin C, Esteller M, Fraga MF. Epigenetic repression of ROR2 has a Wnt-mediated, pro-tumourigenic role in colon cancer. Mol Cancer. 2010;9:170. doi: 10.1186/1476-4598-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ, Liu XH. Loss of Wnt5a and Ror2 protein in hepatocellular carcinoma associated with poor prognosis. World J Gastroenterol. 2012;18(12):1328–1338. doi: 10.3748/wjg.v18.i12.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24(22):2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem. 2009;284(44):30167–30176. doi: 10.1074/jbc.M109.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billiard J, Way DS, Seestaller-Wehr LM, Moran RA, Mangine A, Bodine PV. The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol Endocrinol. 2005;19(1):90–101. doi: 10.1210/me.2004-0153. [DOI] [PubMed] [Google Scholar]
- 22.Ho HY, Susman MW, Bikoff JB, Ryu YK, Jonas AM, Hu L, Kuruvilla R, Greenberg ME. Wnt5a-Ror-dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci USA. 2012;109(11):4044–4051. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12(5):779–792. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8(7):645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, Tamura S, Ueda T, Hatta T, Otani H, Terashima T, Takada S, Yamamura H, Akira S, Minami Y. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5(1):71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, Tarui H, Sasaki H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15(1):23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Ying J, Li H, Seng TJ, Langford C, Srivastava G, Tsao SW, Putti T, Murray P, Chan AT, Tao Q. Functional epigenetics identifies a protocadherin PCDH10 as a candidate tumor suppressor for nasopharyngeal, esophageal and multiple other carcinomas with frequent methylation. Oncogene. 2006;25(7):1070–1080. doi: 10.1038/sj.onc.1209154. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Ying J, Li H, Zhang Y, Shu X, Fan Y, Tan J, Cao Y, Tsao SW, Srivastava G, Chan AT, Tao Q. The human cadherin 11 is a pro-apoptotic tumor suppressor modulating cell stemness through Wnt/beta-catenin signaling and silenced in common carcinomas. Oncogene. 2011;31(34):3901–3912. doi: 10.1038/onc.2011.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seng TJ, Low JS, Li H, Cui Y, Goh HK, Wong ML, Srivastava G, Sidransky D, Califano J, Steenbergen RD, Rha SY, Tan J, Hsieh WS, Ambinder RF, Lin X, Chan AT, Tao Q. The major 8p22 tumor suppressor DLC1 is frequently silenced by methylation in both endemic and sporadic nasopharyngeal, esophageal, and cervical carcinomas, and inhibits tumor cell colony formation. Oncogene. 2007;26(6):934–944. doi: 10.1038/sj.onc.1209839. [DOI] [PubMed] [Google Scholar]
- 30.Tao Q, Huang H, Geiman TM, Lim CY, Fu L, Qiu GH, Robertson KD. Defective de novo methylation of viral and cellular DNA sequences in ICF syndrome cells. Hum Mol Genet. 2002;11(18):2091–2102. doi: 10.1093/hmg/11.18.2091. [DOI] [PubMed] [Google Scholar]
- 31.Tao Q, Swinnen LJ, Yang J, Srivastava G, Robertson KD, Ambinder RF. Methylation status of the Epstein–Barr virus major latent promoter C in iatrogenic B cell lymphoproliferative disease. Application of PCR-based analysis. Am J Pathol. 1999;155(2):619–625. doi: 10.1016/S0002-9440(10)65157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin H, Wang X, Ying J, Wong AH, Cui Y, Srivastava G, Shen ZY, Li EM, Zhang Q, Jin J, Kupzig S, Chan AT, Cullen PJ, Tao Q. Epigenetic silencing of a Ca(2+)-regulated Ras GTPase-activating protein RASAL defines a new mechanism of Ras activation in human cancers. Proc Natl Acad Sci USA. 2007;104(30):12353–12358. doi: 10.1073/pnas.0700153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying J, Li H, Yu J, Ng KM, Poon FF, Wong SC, Chan AT, Sung JJ, Tao Q. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/beta-catenin signaling, and is frequently methylated in colorectal cancer. Clin Cancer Res. 2008;14(1):55–61. doi: 10.1158/1078-0432.CCR-07-1644. [DOI] [PubMed] [Google Scholar]
- 34.Hu XT, Zhang FB, Fan YC, Shu XS, Wong AH, Zhou W, Shi QL, Tang HM, Fu L, Guan XY, Rha SY, Tao Q, He C. Phospholipase C delta 1 is a novel 3p22.3 tumor suppressor involved in cytoskeleton organization, with its epigenetic silencing correlated with high-stage gastric cancer. Oncogene. 2009;28(26):2466–2475. doi: 10.1038/onc.2009.92. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Li J, Cui Y, Li T, Ng KM, Geng H, Li H, Shu XS, Liu W, Luo B, Zhang Q, Mok TS, Zheng W, Qiu X, Srivastava G, Yu J, Sung JJ, Chan AT, Ma D, Tao Q, Han W. CMTM3, located at the critical tumor suppressor locus 16q22.1, is silenced by CpG methylation in carcinomas and inhibits tumor cell growth through inducing apoptosis. Cancer Res. 2009;69(12):5194–5201. doi: 10.1158/0008-5472.CAN-08-3694. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. doi: 10.1016/S1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 38.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6(2):107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 39.Ushijima T. Detection and interpretation of altered methylation patterns in cancer cells. Nat Rev Cancer. 2005;5(3):223–231. doi: 10.1038/nrc1571. [DOI] [PubMed] [Google Scholar]
- 40.Ying J, Srivastava G, Hsieh WS, Gao Z, Murray P, Liao SK, Ambinder R, Tao Q. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin Cancer Res. 2005;11(18):6442–6449. doi: 10.1158/1078-0432.CCR-05-0267. [DOI] [PubMed] [Google Scholar]
- 41.Qiu GH, Salto-Tellez M, Ross JA, Yeo W, Cui Y, Wheelhouse N, Chen GG, Harrison D, Lai P, Tao Q, Hooi SC. The tumor suppressor gene DLEC1 is frequently silenced by DNA methylation in hepatocellular carcinoma and induces G1 arrest in cell cycle. J Hepatol. 2008;48(3):433–441. doi: 10.1016/j.jhep.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Cheung KF, Ma X, Tian L, Zhao J, Go MY, Shen B, Cheng AS, Ying J, Tao Q, Sung JJ, Kung HF, Yu J. Epigenetic inactivation of paired box gene 5, a novel tumor suppressor gene, through direct upregulation of p53 is associated with prognosis in gastric cancer patients. Oncogene. 2011;31(29):3419–3430. doi: 10.1038/onc.2011.511. [DOI] [PubMed] [Google Scholar]
- 43.Yu J, Cheng YY, Tao Q, Cheung KF, Lam CN, Geng H, Tian LW, Wong YP, Tong JH, Ying JM, Jin H, To KF, Chan FK, Sung JJ. Methylation of protocadherin 10, a novel tumor suppressor, is associated with poor prognosis in patients with gastric cancer. Gastroenterology. 2009;136(2):640 e641–651 e641. doi: 10.1053/j.gastro.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 44.Green JL, Kuntz SG, Sternberg PW. Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol. 2008;18(11):536–544. doi: 10.1016/j.tcb.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Bellamy WP, Seabra MC, Field MC, Ali BR. ER-associated protein degradation is a common mechanism underpinning numerous monogenic diseases including Robinow syndrome. Hum Mol Genet. 2005;14(17):2559–2569. doi: 10.1093/hmg/ddi259. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Ross JF, Bodine PV, Billiard J. Homodimerization of Ror2 tyrosine kinase receptor induces 14-3-3(beta) phosphorylation and promotes osteoblast differentiation and bone formation. Mol Endocrinol. 2007;21(12):3050–3061. doi: 10.1210/me.2007-0323. [DOI] [PubMed] [Google Scholar]
- 47.Kani S, Oishi I, Yamamoto H, Yoda A, Suzuki H, Nomachi A, Iozumi K, Nishita M, Kikuchi A, Takumi T, Minami Y. The receptor tyrosine kinase Ror2 associates with and is activated by casein kinase iepsilon. J Biol Chem. 2004;279(48):50102–50109. doi: 10.1074/jbc.M409039200. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Ying J, Fan Y, Wu L, Ying Y, Chan AT, Srivastava G, Tao Q. WNT5A antagonizes WNT/beta-catenin signaling and is frequently silenced by promoter CpG methylation in esophageal squamous cell carcinoma. Cancer Biol Ther. 2010;10(6):617–624. doi: 10.4161/cbt.10.6.12609. [DOI] [PubMed] [Google Scholar]
- 49.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maugeri-Sacca M, Vigneri P, De Maria R. Cancer stem cells and chemosensitivity. Clin Cancer Res. 2011;17(15):4942–4947. doi: 10.1158/1078-0432.CCR-10-2538. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi T, Yanagisawa K, Sugiyama R, Hosono Y, Shimada Y, Arima C, Kato S, Tomida S, Suzuki M, Osada H, Takahashi T. NKX2-1/TITF1/TTF-1-Induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell. 2012;21(3):348–361. doi: 10.1016/j.ccr.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Zhang S, Chen L, Cui B, Chuang HY, Yu J, Wang-Rodriguez J, Tang L, Chen G, Basak GW, Kipps TJ. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS One. 2012;7(3):e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Broome HE, Rassenti LZ, Wang HY, Meyer LM, Kipps TJ. ROR1 is expressed on hematogones (non-neoplastic human B-lymphocyte precursors) and a minority of precursor-B acute lymphoblastic leukemia. Leuk Res. 2011;35(10):1390–1394. doi: 10.1016/j.leukres.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.