Abstract
Primary open-angle glaucoma is a multifactorial disease that affects the retinal ganglion cells, but currently its therapy is to lower the eye pressure. This indicates a definite involvement of the trabecular meshwork, key region in the pathogenesis of glaucoma. This is the first target of glaucoma, and its functional complexity is a real challenge to search. Its functions are those to allow the outflow of aqueous humor and not the reflux. This article describes the morphological and functional changes that happen in anterior chamber. The “primus movens” is oxidative stress that affects trabecular meshwork, particularly its endothelial cells. In these develops a real mitochondriopaty. This leads to functional impotence, the trabecular meshwork altering both motility and cytoarchitecture. Its cells die by apoptosis, losing barrier functions and altering the aqueous humor outflow. All the morphological alterations occur that can be observed under a microscope. Intraocular pressure rises and the malfunctioning trabecular meshwork endotelial cells express proteins that completely alter the aqueous humor. This is a liquid whose functional proteomics complies with the conditions of the trabecular meshwork. Indeed, in glaucoma, it is possible detect the presence of proteins which testify to what occurs in the anterior chamber. There are six classes of proteins which confirm the vascular endothelium nature of the anterior chamber and are the result of the morphofunctional trabecular meshwork decay. It is possible that, all or in part, these proteins can be used as a signal to the posterior pole.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1493-z) contains supplementary material, which is available to authorized users.
Keywords: Open angle glaucoma, Trabecular meshwork, Intraocular pressure, Endothelium, Oxidative damage, Aqueous humor, Molecular endpoints
Introduction
Glaucoma refers to several disorders having the same clinical features. They are characterized by an optic neuropathy in which retinal ganglion cell degeneration leads to a characteristic cupping of the optic nerve head (ONH) associated with typical visual field defects [1]. Glaucomas have been traditionally classified as open-angle, angle-closure (referring to the configuration of the iridocorneal angle), and congenital. Each subtype has been subdivided into two categories: primary, when no cause for the glaucoma can be identified; and secondary, when the glaucoma is caused by an underlying pathological ocular or systemic condition [1]. The majority (60–70 %) of primary glaucomas are open-angle. Primary open-angle glaucoma (POAG) is the leading cause of irreversible blindness in the world [2]. Although the precise molecular mechanisms leading to glaucoma are far from being understood, three tissues are known to be involved in POAG pathogenesis: the trabecular meshwork (TM) in the anterior chamber (AC) of the eye [3]; the ONH, specifically the retinal ganglion cells (RGCs), in the posterior segment of the eye [4]; and the lateral geniculate nuclei and the visual cortex in the central nervous system [5]. Thus, we can describe POAG as an ascending disease that begins in the anterior segment with increased intraocular pressure (IOP) [6], spreads to the posterior segment, and finally travels along the neuronal chain to the visual cortex. Growing evidence obtained from clinical and experimental studies over the past decade strongly suggests the involvement of the reactive oxygen species (ROS) in glaucoma [7] and in RGCs death [8]. Free radicals can directly induce neuronal death by a protease and phosphatase-gated mechanism [9]. In glaucoma, free radicals may damage the TM [10] while, in the posterior segment of the eye, exposure of glial cells to elevated concentrations of free radicals starts the process of apoptotic retinal ganglion cell death [11]. The final neurological damage results in progressive RGCs death, axon atrophy, and degeneration, also extending to the brain cortex (visual areas), and finally leading to the characteristic optic-cup neuropathy and irreversible visual loss [12, 13]. Cell death in the various involved tissues is due to apoptosis. Different mechanisms could trigger oxidative damage. Retinal ganglion cell death has been related to high nitric oxide (NO) levels [14], glutamate excitotoxicity [15], mitochondrial damage [16], defective axonal transport [17], and glial cell pathology [18]. Visual field defects due to RGC degeneration are directly proportional to oxidative damage in the TM [19] and are therefore linked to its endothelium cellularity [10]. TM tissue alteration has been shown to be crucial in glaucoma pathogenesis. TM malfunction, which occurs in most open-angle glaucomas, causes IOP increases [20]. The relationship between IOP and glaucomatous ONH damage is still undisclosed; however, glaucoma therapy remains focussed on decreasing IOP. Two theories were formulated to explain the glaucoma origin: the vascular one and the mechanics one. The first is based on the hypothesis that ocular blood flow is reduced [21], while according to the second hypothesis, the pressure on the axons develops a compression and loss of the neurotrophic support of the ganglion cells [22]. Neither theory explains precisely the pathogenesis of the glaucoma neuropathy, because the first does not justify the role of increased IOP, while the other one does not explain the alterations to the circulation within the ONH [23]. Indeed, the deformation of the vitreoretinal interface does not ncessarily correspond to the deformation of the anterior surface of the lamina cribrosa, and the central retinal vasculature has surprisingly little effect on ONH biomechanics [24]. Thus, scleral thickness, especially near the ONH, seems a potential factor of interest in the development of glaucomatous optic neuropathy [25]. A recent paper suggested that IOP is not only transmitted via the vitreous but also via the suprachoroidal space (SCS). Increases in IOP could be efficiently applied via the SCS to the most external axons of the ONH as they leave the eye. If there were no flow in the SCS, pressure in the AC would be transmitted unmitigated to the ONH potentially causing axonal loss. Thus, the rate of flow in the SCS determines the fraction of IOP applied to the ONH [26].
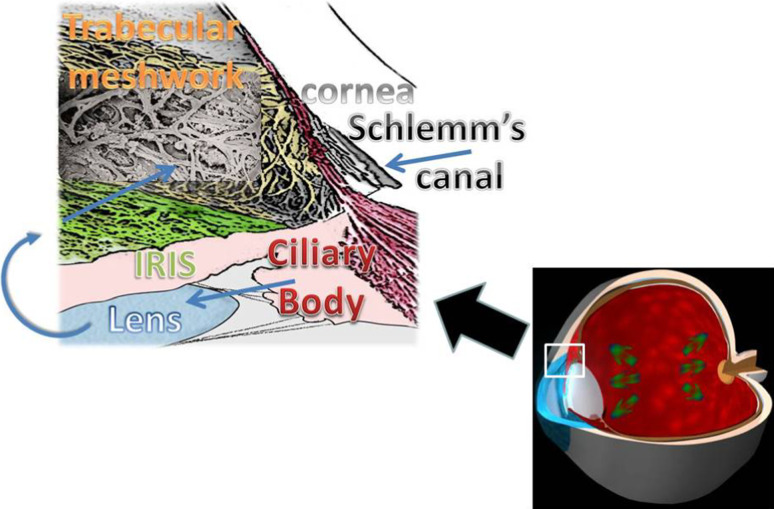
Increased intraocular pressure increases create mechanical stress transmitted in an undefined manner to the back of the eye damaging the RGCs and their axons (i.e., the optic nerves). IOP may be controlled, in part, by genes that are expressed in the eye, particularly in the ciliary body and/or the TM (the ocular sites involved in aqueous production and outflow) [27] (Fig. 1). An identified genetic risk variant for POAG is found in the caveolin gene family, members of which are expressed in the TM and RGCs [28]. This discovery suggests that the molecular pathogenesis is similar in the two regions, even if the triggering insult is different. Lütjen-Drecoll [29] and other authors [30–32] have stated that ‘‘common factors are involved in the pathogenesis of both the TM and the optic nerve changes’’. Although these two structures are distinct, they share a common embryogenic origin and are linked by the pathogenetic events that determine the beginning of the glaucoma cascade. In glaucoma pathogenesis, there are two common pathways that are involved in the cellular damage to both the TM and optic nerve: oxidative stress [33] and vascular damage [21]. The purpose of this review is to examine the molecular alterations occurring in the anterior segment which activate the entire glaucoma pathogenic cascade.
Fig. 1.
Aqueous humor (AH), the biological fluid filling both the anterior and the posterior chambers of the eye. AH is secreted in the posterior chamber by the ciliary body arrives into anterior chamber (AC) through the pupil, goes above the anterior face of the iris and leaves the AC via two routes: the conventional (anterior) route is the through the TM, and the unconventional (posterior) route is through the uveoscleral pathway along the ciliary muscle fibers
Oxidative stress: endogenous and exogenous sources in the eye
Free radicals are chemical species with a single unpaired electron. This configuration is highly reactive, as it seeks to pair with another free electron. This process results in the production of another free radical. The newly produced free radical is unstable and may trigger a chain reaction that causes damage to macromolecules including DNA, protein, and lipids.
Reactive oxygen species and nitric oxide are important reactive species in living organisms. They can both initiate events resulting in cellular damage or important physiological signal transducers. Free radicals can have endogenous or exogenous sources [34]. The most common ocular diseases are mediated by ROS [35]. Oxidative stress is the result of an impaired balance between formation and removal of ROS.
Excessive ROS levels induce apoptosis in a variety of cell types by inducing DNA damage [36]. ROS, as demonstrated for H2O2, are messengers for NF-kB activation [37]. ROS activates the transcription factor NF-κB, which induces expression of a great variety of agents, including pro-inflammatory cytokines such as IL-1/6 and TNF-α [38]. Therefore, adequate levels of the antioxidant defenses responsible for scavenging free radicals are essential for redox homeostasis. At moderate concentrations, NO and ROS play important roles as regulatory mediators of signaling processes. Normally, the amounts of ROS in the tissues are relatively low. The increase of superoxide or NO leads to a temporary imbalance constituting the basis of redox adjustment. The persistent increase in the production of large quantities of ROS or reactive nitrosative species (RNS) can lead to persistent changes in signal transduction and gene expression that can lead to pathological conditions.
Many of ROS-mediated responses actually protect cells against oxidative stress and reestablish “redox homeostasis.”, but when ROS generation overwhelms the antioxidant defences, free radicals can interact with endogenous macromolecules and alter cellular functions [39]. There is an age-dependent increase in the fraction of ROS and free radicals that may escape these cellular defense mechanisms and exert damage to cellular constituents, including DNA, RNA, lipid, and proteins. Signals triggering ROS overproduction may induce the opening of the membrane permeability transition pore in mitochondria and the release of cytochrome c and other apoptogenic factors, which ultimately lead the cell into apoptosis [40]. Exogenous ROS sources include UV light, visible light, ionizing radiation, chemotherapeutics, and environmental toxins. Endogenous sources include the activity of peroxisomes, lipoxygenases, NADH oxidase, cytochrome P450, and mitochondrial respiration [39].
Nitric oxide has physiological functions playing an important role in controlling ocular vascular tone and blood flow in the human eye [41]. Actually, NO is a potent signaling molecule in blood vessels, where its continuous formation from endothelial cells acts on the underlying smooth muscle to maintain vasodilatation and blood flow. NO is synthesized from l-arginine by a family of nitric oxide synthase (NOS) isozymes that includes neuronal (n)NOS, endothelial (e)NOS, and inducible (i)NOS. nNOS and eNOS are constitutive Ca2+ (calcium)/calmodulin-dependent enzymes and are tightly controlled by mechanisms regulating physiological intracellular Ca2+ levels, whereas iNOS is Ca2+-independent [42]. The human endothelial TM expresses mainly the eNOS isoform, with a significantly lower amount of nNOS [43]. eNOS physiologically regulates aqueous outflow in the eye by maintaining vascular endothelial cell function.
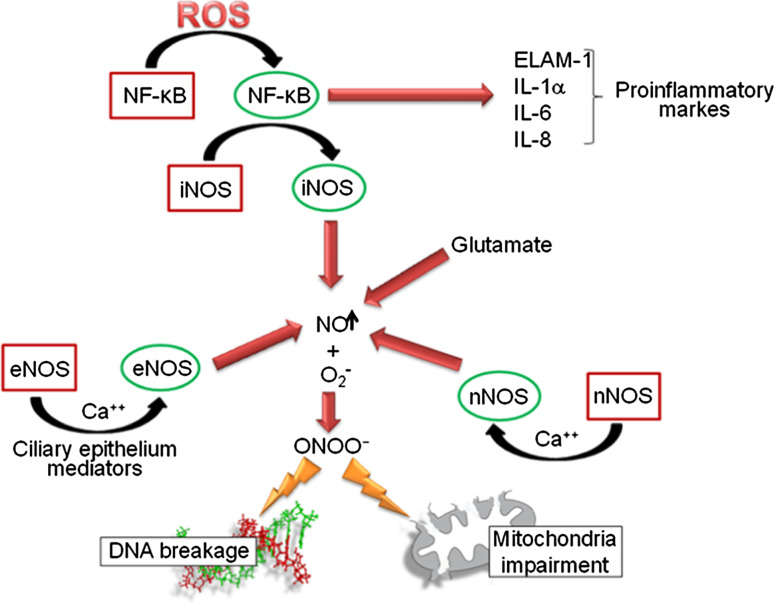
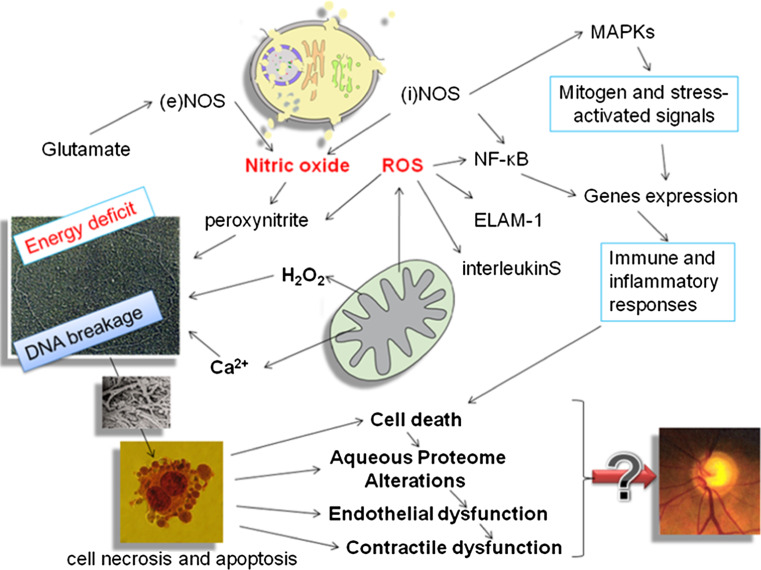
The interaction between NO and oxygen radicals that typically occurs in endothelial tissues produces peroxynitrite (ONOO−) [44], which is a potent oxidant that initiates lipid peroxidation, DNA breakage and base modification, protein tyrosine nitration and nitrosylation, alterations in cell signaling, and cell necrosis and apoptosis in cases of severe damage [45]. Peroxynitrite can affect mitochondrial respiration, causing cellular energy failure, contractile dysfunction, and cell death [46, 47]. iNOS expression is regulated by signaling pathways that involve molecules such as the redox-responsive transcription factor NF-κB and MAPK. NF-κB is the nuclear factor kappa-light-chain-enhancer of activated B cells, which is involved in cellular responses to stimuli such as cellular stress and free radical damages. MAPK are mitogen-activated protein kinases, specific protein kinases responding to extracellular stimuli and regulating a variety of cellular activities [48]. As shown in Fig. 2, an increase in ROS generation results in sustained NF-κB activation, which in turn induces the expression of proinflammatory markers, including endothelial leukocyte adhesion molecule-1 (ELAM-1), interleukin (IL)-1α, IL-6, and IL-8 [49]. Although short-term activation of these mechanisms in the TM can contribute to IOP decrease [50], their chronic activation may exert pathological effects on the TM and contribute to glaucoma progression [51].
Fig. 2.
Reactive oxygen species (ROS) and nitric oxide (NO) are signal transducers able to trigger a dramatic cascade of events resulting in cellular damage. Physiological NO levels play an important role in controlling ocular vascular tone and blood flow in the human eye. An enhanced level of ROS could induce an overproduction of NO which in turn, reacting with ROS, produces reactive peroxonitrite (ONOO −) causing DNA breakage and mitochondrial damage
Nitric oxide synthase alterations are another major source of molecular damage playing a role in glaucoma pathogenesis. A NOS deficit in the TM and Schlemm’s canal endothelia occurs in glaucomatous patients and is linked to altered contractile cell tone [43]. NO and peroxynitrite can affect mitochondrial respiration, causing cellular energy failure, contractile dysfunction, and cell death. ROS produced by mitochondria modulate cell signaling [52] inhibiting tyrosine phosphatase [53] leading to cell proliferation and translocation and activation of serine/threonine kinases such as protein kinase C [54].
Glutamate has also been shown to modulate NO production [55] and mediators released by the ciliary epithelium may influence eNOS activity in the cells of the inflow and outflow pathways [56].
Oxidative/nitrosative stress is recognized to be a prominent feature of many acute and chronic diseases and of the normal aging process [57]. There is much evidence of the relationship between aging, increased oxidative damage, and mitochondrial dysfunction. Mitochondrial integrity declines with age, aged organelles being morphologically altered and producing more oxidants and less ATP than younger organelles [58]. Generation of ROS is a side effect of oxidative phosphorylation which take place in mitochondria. ROS are converted to H2O2 by Mn-dependent superoxide dismutase (SOD) in the mitochondrial matrix [59]. Thus, mitochondria are the main endogenous source of ROS [60]. 8-hydroxy-2′-deoxyguanosine (8-OH-dG) is an established biomarker of oxidative DNA damage. The amount of 8-OH-dG in nuclear and mitochondrial DNA (mtDNA) progressively increases with normal aging. However, the rate of increase is greater in mtDNA [61–63] because of mtDNA proximity to the oxidant source, the lack of any protective histone covering, and limited mtDNA repair mechanism [64]. Such a high level of mtDNA oxidative damage results in guanosine loss and apurinic site formation, which are very fagile and whose dyruption results in the formation of mtDNA deletions. The most typical and frequent mtDNA deletion related to oxidative damage is the mtDNA 4977 common deletion, causing the loss of one-third of the whole mtDNA and of mitochondrial genes encoding for enzymes involved in the oxidative phosphorylation. Mitochondria bearing this deletion are less efficient in ATP production and release more ROS than intact mitochondria [64]. Mitochondria bearing the mtDNA 4977 deletion have a shorter genome than normal mitochondria, thus replicating more quickly and prevailing in the cytoplasm throughout time. These mechanisms result in the long-term accumulation of high levels of mitochondrial damage in aged tissues targeted by endogenous oxidative stress, such as the TM [65], and causes an energy deficit and tissue atrophy [66].
The relative amount of mtDNA deletions correlates with the levels of 8-OH-dG [67]. The level of mtDNA damage detected in glaucomatous TMs is remarkably high [16]. The bioenergetics consequences of mtDNA and nuclear DNA derangements, with the associated mitochondrial dysfunction, contribute to the development of POAG [68]. MtDNA lesions tend to accumulate with age, possibly influencing mitochondrial function [69, 70] and providing a link between ageing and glaucoma.
Izzotti et al. [65] demonstrated that mitochondrial deletions specifically accumulate more in POAG and pseudoexfoliative glaucoma (PEXG) but not in other glaucoma types compared with unaffected control TM samples. Similar findings have also been obtained for oxidative damage to nuclear DNA, which was significantly increased in POAG and PEXG but not in other glaucoma types. By contrast, the total number of cells in the TM decreased in all glaucoma types. These findings imply that the mechanism causing glaucomatous damage in the TM is not identical in all types of glaucoma, even if the target tissues are the same. Furthermore, analysing molecular proteomic endpoints in the AH, oxidative and mitochondrial damage as occurring in glaucomatous TM has also been demonstrated [71].
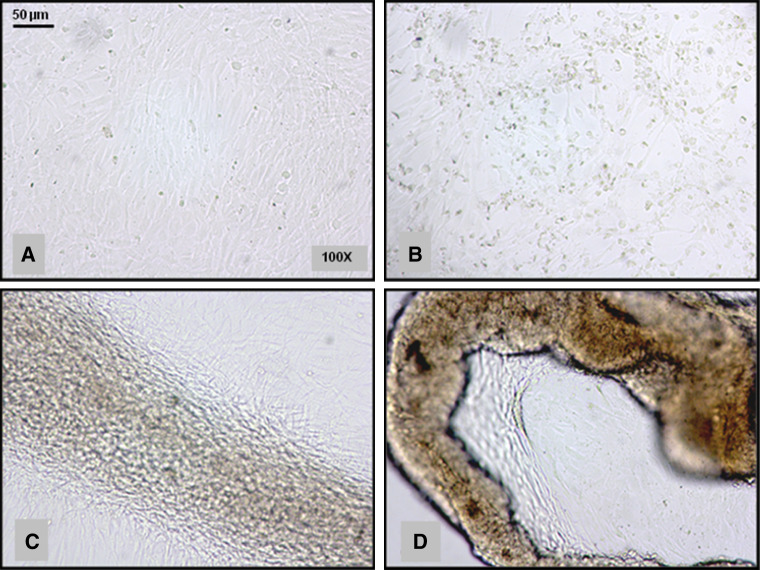
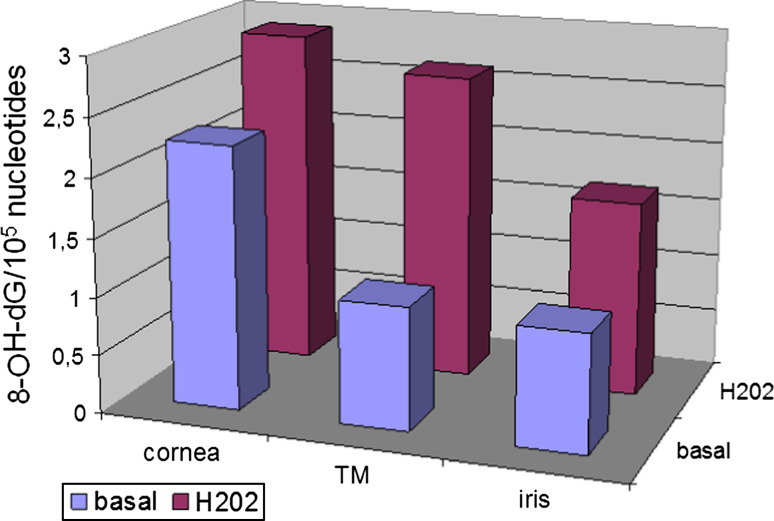
The high sensitivity of mtDNA to oxidative damage led to the concept of a “vicious cycle” in which an initial ROS-induced mitochondrial impairment leads to increased oxidant production that, in turn, leads to further mitochondrial damage [60]. Oxidative damage to mitochondria influences different structural and functional components of mtDNA, proteins and membrane lipids. TM cells of POAG patients have a decreased activity in the mitochondrial respiratory chain complex I [72]. Human trabecular meshwork (HTM) cells exposed to 1 mmol of hydrogen peroxide show reduced adhesiveness to the extracellular matrix (ECM) proteins fibronectin, laminin, and collagen types I and IV, with the consequence that the effect of H2O2 on the adhesion of HTM cells to ECM proteins results in a rearrangement of cytoskeletal structures that may lead to HTM disruption [73]. Figure 3 reports the oxidant effect of H2O2 on the adhesion of TM cells to extra-cellular matrix proteins resulting in a rearrangement of cytoskeletal structures leading to TM disruption. Under physiological conditions, this effect is reversed by reduced GSH, catalase and ascorbic acid [20]. The GSH redox system is thought to protect ocular tissues from the damage induced at low H2O2 concentrations, whereas catalase protects tissues at higher H2O2 concentrations [74]. Patients with glaucoma have decreased levels of circulating GSH as compared to controls [75]. The TM is the most sensitive tissue of the anterior chamber to oxidative damage. Indeed, after H2O2 in vitro exposure of fresh human biopsies, oxidative damage dramatically increased in the TM but not in the cornea and iris [76] (Fig. 4). TM sensitivity to oxidative stress has been explained by the fact that ocular tissues directly exposed to light, i.e. cornea and iris, possess effective antioxidant defence mechanisms that are not activated in the TM, which being in the sclero-cornal angle is not directly exposed to light [76]. This bears relevance for glaucoma therapy, because a direct exposure of TM to light has been demonstrated to activate antioxidant defences in in vitro cultured HTM cells [77]. Nerve cells are also quite sensitive to oxidative damage, and the oxidative protein modifications occurring during glaucomatous neurodegeneration increase neuronal susceptibility to damage and lead to glial dysfunction [30]. In the aging eye and in several neurodegenerative diseases, there is a decline in the normal antioxidant defence mechanisms, which increase the vulnerability of the eye to the deleterious effects of oxidative damage [78].
Fig. 3.
Oxidant effect of H2O2 (hydrogen peroxide) on the adhesion of HTM cells to ECM proteins results in a rearrangement of cytoskeletal structures that may lead to TM disruption. a Untreated HTM cells, b HTM cells 0 min after treatment with H2O2 (400 μM for 15 min), c HTM cells after 1 h since H2O2 treatment, d HTM cells after 3 h since H2O2 treatment. Magnification ×100
Fig. 4.
Different sensibility to oxidative damage of the anterior chamber tissues. Basal damage (blue columns) is greater in cornea than in trabecular meshwork and iris. Cornea is the most exposed tissue to exogenous ROS sources. After treatment with H2O2 (red columns) the most relevant increase in oxidative DNA damage is detected in trabecular meshwork (TM), whose natural defences against reactive oxygen species are minimal due to its position repaired from the direct action of ROS exogenous sources (data from Izzotti et al. [76])
Figure 5 resumes the complex network of mechanisms interlacing oxidative stress, mitochondria impairment and pathogenic events leading to glaucoma. These mechanisms could be at the base of the decrease in the TM cellularity and its malfunction in anging and glaucoma but also in ONH.
Fig. 5.
The complex network of mechanisms interlacing oxidative stress, mitochondria impairment and pathogenic events leading to glaucoma
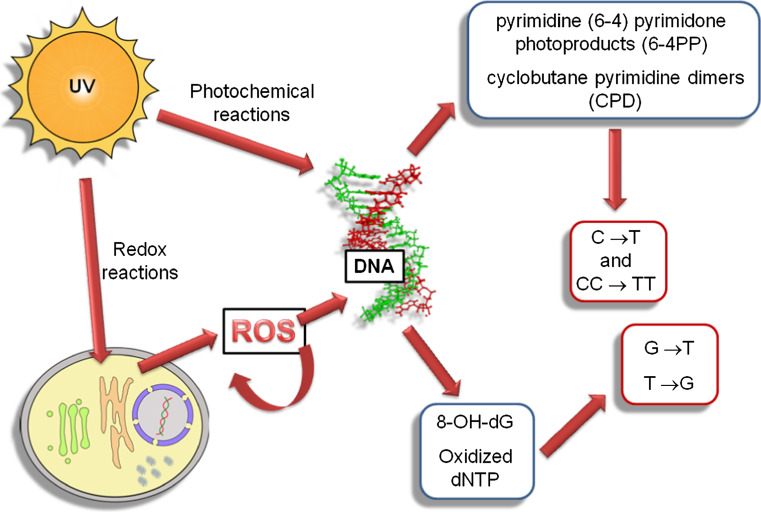
Both oxidative stress and AH antioxidant defences plays an important role in glaucoma pathogenesis. Antioxidant enzymes including SOD, catalase, glutathione peroxidase, and glutathione reductase display reduced activities in the eyes of patients with glaucoma [20, 79]. Microarray gene-expression analysis identified antioxidant downregulation and endothelial stress-response upregulation as hallmarks of glaucomatous TM [51]. 8-OH-dG levels were significantly higher in the TM of patients affected by glaucoma than in the control group [19]. The decline in the human TM endothelial cells cellularity is linearly related to age and plays a major role in glaucoma pathogenesis; [80–82], glaucomatous subjects having a lower TM cellularity than non-glaucomatous subjects [82], UVB light comparable to solar irradiation levels readily causes the formation of intracellular ROS in human corneal epithelial cells [83]. In particular, UV induces oxidative stress in irradiated cells through the production of ROS by activating riboflavin, tryptophan and porphyrin, that in their turn activate cellular oxygen [84]. Iris surface and corneal endothelium are clearly visible and exposed to direct light; the TM is hidden between them in the iris corneal corner. As a consequence of that, the TME have fewer antioxidant defences [85]. Light can cause cell dysfunction through the action of ROS on DNA, [86]. Light has a direct oxidising effect that is exerted locally and at a systemic level in exposed organisms [87, 88]. At the eye level, the apoptotic death of photoreceptor cells by light-induced stress in retinal degenerative disorders is well documented [85, 89, 90]. In conjunctiva, UV radiation induces a typical caspase-dependent apoptotic cell death [91]. In the AC, light induces the formation of oxidative radicals that can indirectly target the TM by altering the oxidant–antioxidant balance in the AH, thus contributing to glaucoma pathogenesis. This mechanism generates both a decrease in the total antioxidant potential of the AC [79] and TM damage in terms of 8-oxo-dG formation and DNA fragmentation [19], contributing to endothelial dysfunction. Thus, UV represents an exogenous ROS source targeting the ocular structures. As reported in Fig. 6, UV can cause DNA lesions directly through the formation of photochemical products, such as pyrimidine-(6-4) pyrimidone and cyclobutane T–T dymers, or indirectly generating ROS, which in turn produce DNA lesions, such as 8-OH-dG and oxidized nucleotides. If repair mechanisms are ineffective, these damages result in stable DNA damage and mutations, which, after a long-term accumulation, are in perennial cells, such as those composing TM, a stimulus towards apoptosis and cell loss [92].
Fig. 6.
UV can cause DNA lesions directly through photochemical reaction (6-4 PP and CPD) or indirectly through redox reaction-generating ROS, which in turn produce DNA lesions (8-OH-dG and oxidized dNTPs). If repair mechanisms are ineffective, such damage results in stable DNA damage and mutations
Anterior Chamber: Functional anatomy and antioxidant defences
AC is a space filled by aqueous humor (AH) between the inner endothelial face of cornea and the anterior limiting layer of the iris, and surrounded peripherally for 360° by TM (Fig. 1). AH has an ionic composition very similar to the blood plasma and has two main functions: to provide nutrients to the tissues surrounding AC and to maintain IOP.
The cornea
The cornea protects inner ocular tissues, such as the lens and retina, against external environmental insults as a physical and biochemical barrier. The human cornea absorbs 92 % of ultra violet (UV)-B at 300 nm, [93]. UVB light comparable to solar irradiation levels readily causes the formation of intracellular ROS in human corneal epithelial cells [83]. The cornea contains both low molecular weight antioxidants such as ferritin [94], ascorbic acid [95], reduced glutathione (GSH) [96], α-tocopherol [97], and high molecular weight antioxidants such as catalase, SOD, glutathione peroxidase, and reductase [98]. l-Ascorbate is present in aqueous compartments (e.g. cytosol, plasma, and other body fluids) and can reduce the tocopheroxyl radical; it also has a number of metabolically important cofactor functions in enzyme reactions, notably hydroxylations. The water-soluble antioxidant vitamin C can reduce tocopheroxyl radicals directly or indirectly thus supporting the antioxidant activity of vitamin E; such functions can be performed also by other appropriate reducing compounds such as GSH [99]. GSH is ubiquitously synthesized in all cell types [100] and directly protects cells from ROS through the direct scavenging of radicals and acting as a cofactor for glutathione peroxidases during the metabolism of H2O2 and lipid peroxides [101]. GSH plays a fundamental role in cellular metabolism, including differentiation, proliferation, cellular senescence, and apoptosis [102, 103]. In the cornea GSH is differentially distributed and the highest levels of GSH are found in the epithelium fivefold higher than that in the stroma [104, 105]. Its presence is related with its pivotal role in maintaining optimum hydration of the cornea [106]. The α-tocopherol is the major peroxyl radical scavenger in biological lipid phases such as membranes or low-density lipoproteins. It can also regenerate other antioxidants, including GSH and ascorbate [99]. The synergistic effect between vitamin E and ascorbate significantly reduce oxidative stress by preventing lipid peroxidation and apoptosis in corneal endothelial cells [107]. Another antioxidant enzyme relatively abundant in the human cornea is SOD. SOD isoenzymes exert their actions in their respective compartments: the CuZn–SOD in cytosol and in extracellular compartments, while Mn–SOD is located in the mitochondrial matrix [108]. The total level of SOD activity is much higher in the normal corneal epithelium than the activity of catalase and this is higher than the activity of glutathione peroxidase [109]. SOD neutralizes H2O2 producing superoxide anion, which is still a potent ROS and need to be neutralized by catalase. Accordingly, SOD exerts antioxidant effect only when coupled with catalase. Even if catalase is a major enzyme involved in the detoxification of H2O2 from cells, its expression under oxidative stress is not predictable and its activity may be decreased, increased, or unchanged [110]. Anyway, H2O2 induces a great amount of catalase expression, and smaller increases in glutathione peroxidase (GPx) and Mn–SOD expression [111]. The regulation of SOD isoenzymes in mammalian tissues primarily occurs in a manner coordinated by inflammatory cytokines, rather than as a response of individual cells to oxidants [112].
UVB modulates corneal epithelial cell expression of antioxidants and proinflammatory mediators by distinct mechanisms [113]. The suppression of proinflammatory cytokines strongly reduces matrix metalloproteinase and xanthine oxidase expression in the UVB-irradiated corneal epithelium [114]. Modulation of the expression of these mediators is important in regulating inflammation and protecting the cornea from UVB-induced oxidative stress [85].
The glutathione peroxidase catalyzes the reduction of lipid hydroperoxides to water or alcohol and H2O2 to water and molecular oxygen with concomitant oxidation of GSH [115] and is localized predominantly in the epithelium and endothelium of the cornea [116]. This reaction thereby minimizes the destructive effects of ROS in these corneal tissue layers, preventing the propagation of peroxide-dependent chain reactions that result in cell membrane degeneration [115]. Antioxidant enzyme activity decreases in aged corneas [117]; these enzymes are able to handle basal or low levels of ROS, but can be overwhelmed during acute stress [116].
The iris
The iris epithelium consists of two monocellular layers. The anterior epithelium is highly specialized; it is a myoepithelium that has an epithelial portion and a muscular portion that functions as the radially oriented dilator muscle [118]. The posterior epithelium is deeply pigmented to prohibit scattering of light.
Also, the iris tissue contributes to the antioxidant defenses of AC, the protective antioxidant system of the iris including low-molecular-weight antioxidants that are present in this pigmented tissue at high concentrations [119]. This tissue contains melanocytes bearing the pigment melanin, whose function is to prevent light scattering. This is a potent antioxidant likely to be a major contributor to the poor sensitivity of the iris to hydrogen peroxide [76]. Further melanin absorbs UV radiation and blue light more efficiently than visible light of longer wavelengths [120]. Not much is known about the role of the pigment in the glaucoma pathogenesis. Melanin granules, released from the iris, are present in the circulating AH of the human AC in low but quantifiable numbers [121] many of the retained granules being phagocytized by TM cells [122]. There is little evidence suggesting that melanin is digested in situ in TM; it remains within cells and gradually leads to pigmentation in the ageing meshwork, as can be observed by gonioscopy [123]. Patients with POAG have a higher incidence of iris transillumination defects than controls [124]. Together with melanin, the spectral properties of the ECM contribute to the antioxidant activity defending the iris [125]. The opening in the iris, the pupil, expands and contracts to control the amount of incoming light. Therefore, the iris modulates the amount of the damage that the light may cause to the posterior segment.
The aqueous humor
The AH is a crystal-clear fluid filling the AC. The concept of the AH as a stagnant liquid was refuted in the early twentieth century by Leber, Lauber and Troncoso. In 1923, Seidel, working with stains, concluded that there is continuous AH flow from the AC. In 1965, it was discovered that AH passes through the TM and uveoscleral pathways [126, 127]. AH is continuously formed from plasma by the epithelial cells of the ciliary processes. It is secreted into the posterior chamber, passes from the posterior chamber through the pupil into the anterior chamber, and is drained at the AC angle. There is a small loss of liquid through the limbar sclera [128]. AH drainage is also influenced by the selective uptake of certain substances by the iris [129]. The volume of the AC AH is approximately 0.25 ml, and the posterior chamber AH volume is 0.06 ml. It is estimated that the daytime flow is approximately 2.5 μl/min in the normal eye, and the tonography value of “C” (i.e., the coefficient of aqueous outflow facility) is 0.3 μl × min−1 × mmHg−1 [130]. Approximately 1 % of the AC and 3 % of the posterior chamber volume of AH is replaced each minute [131]. IOP in ocular-hypertensive patients is due to a reduced TM and uveoscleral outflow, while aqueous production remains normal [132]. Conversely, in the healthy aging eye, there are both reduced production of AH and reduced drainage through the uveoscleral outflow pathway [133]. AH production shows an age-related decline that approximately amounts to 15–35 % over the age range of 20–80 years [133–135]. Also, outflow facility decreases with age. The age-related decrease is about 30 % from 40- to 60-year-old subjects [134].
Increased intraocular pressure is associated with increased fluorophotometric outflow facility as has been demonstrated by administering apraclonidine to patients with ocular hypertension [136]. The AH provides nutrients for the avascular lens and cornea and an egress for the waste products of these structures. It contains approximately 1/100 of the serum protein concentration [137] that directly enters the AC by diffusion through the root of the iris. The aqueous fluid has 0.1–0.2 % the concentration of plasma proteins and a higher amino acid concentration than plasma. Ascorbate [138], lactate [139], and bicarbonate concentrations are also elevated in the AH [140]. Hydrogen peroxide, the main oxidant in AH, is normally present [141] as a result of reactions of ascorbic acid and trace metals [142]. Additional hydrogen peroxide and ROS are generated by light-catalyzed reactions, metabolic pathways, and phagocytic or inflammatory processes [141, 142]. Antioxidant defenses of the aqueous humor include vitamins, enzymes, and proteins such as albumins that have a protective role toward the TM [20, 143]. A high level of ascorbic acid in the AH is necessary for maintaining a filter-like function against UV radiation in the central corneal epithelium and the AH [138, 144].
The trabecular meshwork and the conventional outflow pathways
Anterior chamber inner cornea and trabecular meshwork walls are lined with endothelium, while the anterior surface of the iris does not have it. During embryologic development, the anterior surface of the iris is covered with a continuous layer of endothelium, which disappears at or soon after birth [145]. Anyway, the AC endothelium is constantly immersed in AH having both plastic and trophic functions [146]. AH is secreted by the ciliary body into the posterior chamber of the eye. Aqueous humor cannot traverse the intact iris and thus it passes through the pupil to reach the AC of the eye. At the iris–corneal angle, the main part of this flow enters a pathway composed of the TM, the juxta canalicular connective tissue (JCT), the endothelial lining of the inner wall of Schlemm’s canal, Schlemm’s canal itself, and the collecting channels that lead to the episcleral veins and episcleral vessels. This outflow pathway is called the “conventional way” to distinguish it from the non-conventional outflow called the uveoscleral way. The posterior way or uveoscleral outflow passes through the iris root and the anterior face of ciliary muscle in the connective tissue interposed between the bundles of ciliary muscle to SCS. This pathway carries less than 10 % of the total flow in the older adult human eye [147]. The TM resides in the ocular limbus between the cornea and the sclera and comprizes perforated, interlacing collagenous lamellae, called the TM beams. These have a core of collagenous and elastic fibers, and are covered by flat cells which rest on a basal lamina. Although some authors still believe that spaces between the beams are occupied only by AH, other authors propose that the space between the beams is filled in with ECM where the AH filters through [148]. The beams are encapsulated by a single layer of endothelial-like cells [149]. The outermost JCT or cribriform region has no beams, but rather several cell layers which some authors claim to be immersed in loose extracellular material/matrix [150]. Between the beams of the corneoscleral meshwork and the basal lamina of the inner wall of Schlemm’s canal there is the JCT. ECM components have a major role in contributing to outflow resistance in human eyes. Several ECM proteins may contribute to homeostatic modifications of AH outflow resistance, being up- or downregulated [151]. Low concentrations of oxidized low-density lipoproteins stimulate ECM remodeling [152]. An increased fibronectin synthesis in ECM could result in concomitant increase of IOP [153]. Thus, ECM turnover is important in the regulation of aqueous humor outflow facility [154]. Transforming growth factors (TGFs) controls the expression of a wide variety of ECM genes, including elastin, collagens, fibrillin, laminin, and fibulin. Levels of one of its isoform, the TGF-b2, are elevated in glaucomatous human AH [155] altering ECM metabolism [156]. TGF-b2 is also responsible for anterior chamber-associated immune deviation, a mechanism that protects the eye from inflammation and immune-related tissue damage [157]. TGF-β2 is associated with glaucomatous neuropathy, primarily via the increased synthesis and secretion of ECM proteins and remodeling of the ONH [158]. In TM, ECM production may be modulated by vitamin C [159, 160] that stimulate hyaluronic acid synthesis in glaucomatous TM cells [161] and increase outflow through the TM reducing the viscosity of hyaluronic acid [162]. Thus, it has long been known that high doses of vitamin C decreases IOP [163].
Other molecules that seem to play a very important role in collagen remodeling are the metalloproteinases (MMPs) matrix. These calcium- and zinc-dependent extracellular endoproteinases degrade ECM proteins [164] and interact with cells and their surrounding structures [165], decreasing collagen deposition and increasing AH outflow facility [3]. Nevertheless, it remains unclear what fraction of total resistance is attributable to the JCT and how ECM or specific ECM molecules might be involved in the generation of this resistance [166]. AH mainly crosses the inner endothelium wall of Schlemm’s canal by two different mechanisms: a paracellular route through the junctions formed between the endothelial cells [167] and a transcellular pathway through intracellular pores of the same cells [168]. Nevertheless, TM pores contribute only 10 % of the aqueous outflow resistance [169] and the density of inner wall pores increases with the volume of fixative perfused through the outflow pathway on experimental conditions [170]. Anyway, the inner wall and underlying JCT work together to regulate outflow resistance [171]. In the conventional aqueous outflow pathway, there are two endothelial cell barriers separating the venous circulation from AH, which are specialized and positioned in series: the TM endothelial cells (TME) and subsequently the endothelial cells that line the lumen of Schlemm’s canal (SCE). Between these two barriers, there is the JCT, which contains a loose ECM through which the AH flows [172]. The TME cells release factors into the AH that flow downstream from TMEs to bind and actively regulate the permeability properties of the SCEs. These factors, upon binding to SCE cells, increase the permeability of the SCE barrier [173], inducing a 400 % enhance in SCE conductivity by means of the activation of specific TME genes [174]. In particular IL-1α and 1β and tumor necrosis factor-α released by TME cells induce cell division and migration [175] in the cells near Schwalbe’s line, while inducing the release of matrix metalloproteinases [176] and an increase of fluid flow across ECM tissues near JCT [173]. Therefore, the cytokines released by TME cells regulate the permeability of the SCE barrier in an active way [173].
Trabecular meshwork cells express aquaporin-1, a multiple water channel protein transporting water through membranes that can modulate cell volume [177] and tissue permeability. Indeed, outflow facility is increased by hyperosmotic solutions and decreased by hyposmotic solutions [178, 179]. Aquaporins also facilitate cell migration, [180], hydration, neuroexcitation, cell proliferation, fat metabolism, and other cell functions [181]. In experimental animal models, elevated IOP reduces aquaporin expression, suggesting a role for this molecule in the pathogenesis of glaucomatous optic neuropathy [182]. During glaucoma course, accumulated oxidative stress arising from the environment, vascular dysregulation, aging and/or the pathogenic processes could induce a sublethal damage to the outflow pathways [183]. These molecular changes in the surviving cells determine the expression of new genes [184] dependent on the nature of the damaging stimulus and on the other tissue type [185, 186]. In the AC, oxidized lipoproteins and free radicals are considered to be major causes of tissue stress and serve as local triggers for tissue inflammation [187]. Primary glaucoma is associated with an aqueous inflammatory response and is associated with changes in the aqueous cytokine profile [188]. A large number of inflammatory genes, including genes involved in complement activation and inflammatory cytokine/chemokine production, are upregulated, this in turn causing abnormal leukocyte–endothelial interactions and ultimately vascular damage [187]. Furthermore, the innate immune system in general and monocytes in particular play an important role in aqueous outflow homeostasis. Indeed, under the influence of chemotactic signals, the monocytes circulate through the TM in the normal state and cytokines regulate the permeability of Schlemm’s canal endothelial cells [189] and monocytes increase aqueous outflow [190].
Glaucomatous eyes exhibit a higher level of TM cell loss, than age-matched controls [82]. The decline of human TM cellularity is linearly related to age [82]. Noxious insults, such as free radical attack, trigger and enhance this mechanism [191, 192].
Contractile elements composing TM help to regulate the outflow facility [193]. Human TM cells contain smooth muscle-specific α-actin and have been identified as functional contractile cells, and in intact eye, the contractility balance between ciliary muscle and TM determines the total AH outflow [194]. Therefore, opening or fastening its slots, TM can change the quantity of cells involved in the passage of AH from AC to SC. As collector channels become altered with age or disease, other collector channels are available to assume the functional burden [195].
Similarity between anterior chamber and blood vessels
From a structural point of view, the AC may be seen as a specialized circulatory vessel in which the walls are lined with endothelial cells (i.e., corneal and trabecular) and the AH acts as the “blood”. Malfunction in this endothelium may lead to outflow deterioration and result in increased IOP. The three-dimensional architecture of the human TM considerably increases the filtration surface between the TME and AH. The adjacent Schlemm’s canal is a continuous endothelium-lined channel that drains AH to the general venous circulation [150]. These cells display an endothelial cell-like morphology, are avid phagocytes [196], possess a contractile and migratory apparatus [197, 198], have the capacity to produce ECM elements [199], and can transduce signals after stress-induced protein kinase C (PKC) attaches to the ECM [200]. The AC endothelium (ACE) not only acts as a barrier between AH and the surrounding tissues but, like in vessels, is a real organ with the function of modulating the tone and the flow rate in response to humoral, nervous, and mechanics stimuli. Under physiological conditions, the ACE plays an active role in cellular interchange, being able to adapt functionally and structurally to changes in the environment [201]. The normal endothelial function depends on both the anatomical continuity of cellular monolayer and its functional integrity [202].
The endothelium plays an important role in AC homeostasis. In particular, the TM endothelium plays a fundamental role in AH transit from the AC to Schlemm’s canal (SC). TM endothelium releases into the media cytokines, which increase the SC endothelium barrier permeability upon SC endothelium binding [174] and flow downstream with the AH to influence SCE barrier function and regulate the egress of AH [172]. When the IOP is greater than the venous pressure, the increased tension makes the trabecular beams and cords taut, which then triggers the stretch receptors to activate the TM endothelium to release vasoactive factors that will increase the flow across SC endothelium [174]. The presence of stretch receptor in TM is supported by recent literature indicating that epithelial sodium channel proteins may function as sensors of pressure-induced vascular stretch and laminar flow [203]. Indeed, IOP is regulated by the transport of AH across epithelial eye structures, which in turn is associated with ion flux. The specific upregulation of epithelial sodium channel proteins acts as a protection mechanism against IOP increase [204]. Therefore, the endothelial cells of the TM and Schlemm’s canal constitute a system that governs the outflow through complex interactions. These interactions proceed in both directions and involve TM–SC mutual relationships [174].
A further element supporting the similarity between AC and blood vessel is the presence of protein marker typically detected in atherosclerotic vascular lesions. ELAM-1 has been detected in TM and its increase observed in POAG [205]. Recently, our group discovered in AH the presence of several proteins usually linked to the atherosclerotic plaque. Levels of these vascular proteins (Table 1) were significantly increased in AH of glaucomatous patients compared with expression levels in healthy controls [206].
Table 1.
Vascular protein as detected in AH of POAG pateints by antibody microarray (data from Saccà et al. [206])
| Protein | Role | Target |
|---|---|---|
| ELAM1 | Inflammatory responses | Endothelium and cytoskeleton |
| Apo B | Inflammatory responses | Endothelium |
| Apo E | Oxidative stress response | Endothelium |
| VASP | Cell adhesion and motility | Endothelium |
| Hsp60 | Mitochondrial chaperonin | Endothelium |
| Hsp70 | Immunoreactivity | Cytoskeleton |
| Myogenin | Muscle growth and regeneration | Cytoskeleton |
| Myogenic factor 3 | Regulator of skeletal myogenesis | Cytoskeleton |
| Myotrophin | Myofibrillar growth pattern | Cytoskeleton |
| Ankyrin | Anchors cytoskeletal components | Cytoskeleton |
| Phospholipase C | PKC activator | Cytoskeleton |
| Ubiquitin | Regulation of endothelial NOS activity | Endothelium |
The presence of these proteins in AH indicates that POAG course damages the trabecular meshwork and in particular its endothelial and cytoskeletal components thus altering both TM functionality and motility
Endothelial dysfunction
Vascular dysfunction is commonly observed in many diseases and is closely related to oxidative stress [207, 208]. Higher levels of reactive oxygen and nitrogen species occurring in vasculature have been found in hypertension, hyperlipidemia, atherosclerosis, and diabetes mellitus being established that these reactive species induce oxidative damage in vascular tissue [209].
Chronic eye diseases are characterized by decreased biosynthesis and/or bioavailability of NO [210], excess of superoxide [211], and endothelin production [212]. TNF-α (tumor necrosis factor-alpha) regulates NOS expression and activity, which exert direct effects on NO production. TNF-α increases iNOS expression by activating NF-κB. Increased TNF-α expression induces ROS production. TNF-α also activates NF-κB transcription, which regulates the expression of genes involved in inflammation and oxidative stress [213–215]. Glaucoma syndromes are characterized by these bio-humoral changes. A balance between vasoconstrictors and vasodilators is necessary for maintaining the physiological structure and function of endothelia [194], modulating the permeability of the endothelial barrier, and releasing of ET and NO. This balance is impaired in glaucoma, with major consequences. Cyclic GMP and NO2 concentrations were lower in the AH of POAG patients than in normal eyes [216]. NO donors decrease IOP by increasing the aqueous outflow facility in the TM and/or Schlemm’s canal through cellular mechanisms known to regulate outflow facility, including changes in cell volume and cell contractility. In fact, the NO promotes the conversion of GTP to cGMP. This process activates protein kinase G (PKG), which then determines the phosphorylation and therefore the activation of the BKCa ionic channel [217]. These potassium (K+) ion channels are activated by calcium (Ca++), are located at the cell membrane, and are activated by electric potential changes across the membrane or by an increase of intracellular Ca++ concentration. Potassium on channel activation determines the exit of K+ from cells and the consequent reduction of cell volume and osmotic pressure [218]. TM cell volume reduction allows for enlarged intertrabecular spaces and consequently greater exposure of the cell surface and enhanced AH outflow. Furthermore, the NO-dependent soluble guanylate cyclase/cyclic guanosine monophosphate system plays an important role in regulating the AH dynamics that control AH production in the ciliary processes [219, 220], with subsequent decreases in IOP [221].
Endothelins also participate in IOP regulation; despite TM mobility, ET-1 (endothelin 1) induces TM contraction and increased outflow resistance, whereas TM relaxation increases outflow [194]. ET-1 is the most powerful constricting substance produced by endothelium, and it acts on specific ETA and ETB receptors. ETA receptors are present only in smooth muscle cells and cause vasoconstriction and cell growth. ETB receptors (which also lead to vasoconstriction) are present on smooth muscle cells, the expansion of which stimulates NO production and provides negative feedback by inhibiting the further production of ET-1. This negative feedback mechanism is compromized when the bioavailability of NO is reduced, and vasoconstriction effect is consequently increased [222]. The effects of endothelin-induced vasoconstriction in the anterior part of the eye cause a decrease in ocular blood flow, followed by pathological changes in the retina and the ONH; these processes are assumed to contribute to the degeneration of the RGCs. Nevertheless, trabecular outflow is modulated by TM contractility, which is affected by endothelin [223]. High ET-1 levels have been reported in the AH of patients with glaucoma [224, 225], and abnormal vascular responses to endothelin or its receptor antagonists have been shown in patients with normal tension glaucoma (NTG) [226]. Although direct evidence for local ocular endothelial dysfunction (EDf) is difficult to obtain, Henry et al. [227] demonstrated general EDf in a group of patients with NTG. This association could be due to attenuated ETA receptor-mediated tone, increased ETB receptor-mediated contraction, or impaired ETB receptor-mediated endothelial NO release [228]. There may be both a dysregulated vascular response to increased endothelin levels and a direct endothelin effect on target tissues, depending on the expression and distribution of their endothelin receptors [229]. NO can modulate the expression, sensitivity, and signal termination of endothelin receptors [230].
Primary open-angle glaucoma is also associated with peripheral vascular EDf [231]. This dysfunction can include the TM endothelium, which exhibits mitochondrial dysfunction in POAG patients [16] and a higher susceptibility to ROS than other tissues that constitute the AC [76]. Siasos et al. [232] have reported that POAG patients have significantly impaired endothelial function, which has been linked to an increased inflammatory status that is a causative mechanism of EDf. Furthermore, ET-1 has been linked to various other glaucoma-associated effects on the optic nerve and RGC including astrogliosis, ECM remodeling, and NO-induced damage [233]. Additional evidence of EDf arises from data showing increased levels of ELAM-1 in the AH. ELAM-1 is the earliest marker of atherosclerotic plaques in the vasculature. It is activated in human TM cells collected from patients with glaucoma [205], and it represents the sustained activation of a stress response that results in the expression of pro-inflammatory markers [234] and an index of endothelial damage.
Aqueous humor proteome during glaucoma course
An intriguing component of the glaucomatous AH is its protein fraction. Some stimuli, including elevated IOP, exert physical forces on HTM cells, causing mechanical stretch. This stretching can have a profound effect on their gene expression profile and may dramatically change the AH proteomics during glaucoma. To date, several molecules that appear to be involved in POAG pathogenesis have been identified. Many of these molecules are involved in the control of ECM turnover in TM [235]. Evidence that molecular alterations in the AH reflect glaucoma pathogenesis has been published by Izzotti et al. [71, 236]. The expression of 1,264 proteins was analyzed by detecting remarkable changes in the AH proteins of glaucomatous patients relative to matched controls. These findings shed light on the biomolecular mechanisms involved in human glaucoma pathogenesis. AH proteome profile undergoes dramatic changes in POAG patients compared to matched controls, even if total protein amount was only slightly, and not to a statistically significant extent, increased.
Proteins having a significant (more than two-fold) variation in expression between POAG patients and controls can be classified into 6 groups reflecting glaucoma pathogenesis. The first group includes mitochondrial proteins that are involved in the electron transport chain, trans-membrane transport, protein repair, and mitochondrial integrity maintenance. Under physiological conditions, these proteins are segregated inside cells in functional mitochondria. The presence of these proteins in AH provides evidence of both mitochondrial dysfunction and distinct injury occurring during glaucoma. This molecular event is linked to myocilin and its mutations impairing mitochondrial functions in HTM cells [237], affecting the actin cytoskeletal structure [238], and conferring differential sensitivity to oxidative stress [239]. Myocilin mutations are typically associated with high IOP, while optineurin (OPTN) mutations are associated with normal tension glaucoma. These associations imply that myocilin alteration has more impact on the cells of the aqueous outflow pathway, i.e. the TM, than those in the ONH and in the retina, i.e. RGCs, and vice versa in the case of OPTN [240]. OPTN expression in TM is increased by IOP increases [241, 242]. OPTN expression is regulated by various cytokines, particularly NF-κB [243], which can be activated by increased IOP, ageing, vascular diseases, and oxidative stress. Furthermore, OPTN also plays an important role in regulating several genes, including myocilin [244].
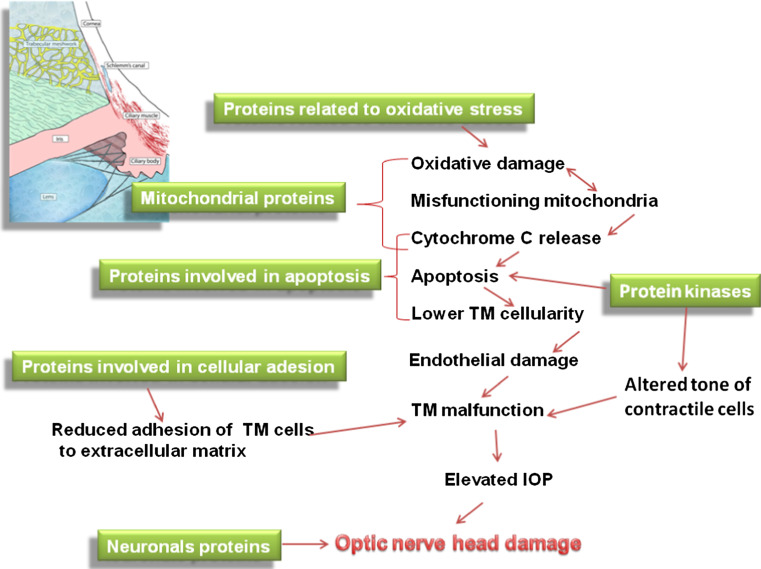
The second group consists of proteins involved in apoptosis induction through the intrinsic (i.e., mitochondrial-dependent) pathway. The relationship between TM cell loss and the apoptosis observed in POAG patients can be triggered by many events, including oxidative stress [10, 245], intense phagocytosis, and inflammation, either alone or in combination with [246] mechanical stress [247]. Nevertheless, these pathways induce endothelial cell death, which first leads to EDf and then to complete TM malfunction with the consequent increase in IOP (Fig. 7). Cytochrome c is prominent in this group of proteins. It is released from the mitochondrial cristae following elevated hydrostatic pressure or oxidative stress and leads to apoptotic cell death. Cytochrome c release on day 3 of pressure treatment is correlated with caspase-3 activation and apoptotic cell death in differentiated RGC-5 cells [248]. The presence of cytochrome c in the AC is due to an apoptotic mechanism led towards to TM cells. It is conceivable that AH proteome alteration reflect from anterior to the posterior ocular segment thus establishing a communication mediated by proteins between AC and ONH. Many proteins enter the AH of the AC by diffusing through the root of the iris [249]. From there, they reach the supraciliary space and move as far as the suprachoroid layer of the peripapillary retina in the posterior segment [250]. This process demonstrates how different molecules, particularly proteins, spread freely in the anterior and posterior segments. Therefore, these proteins may acquire the task of transmitting signals to both the AC and posterior segment cells. This situation bears pathogenic relevance in particular for AH proteins highly expressed in glaucoma and having neurological relevance.
Fig. 7.
Histopathological and ultrastructural functional alterations occurring in glaucomatous TM as highlighted by AH proteome analysis
Indeed, the third group is composed by neuronal proteins. It includes optineurin and growth and differentiation factors involved in neurogenesis and neuronal survival. Their presence in the AC reflects the neural embryologic origin of the TM cells, which have a neuro-ectodermal origin and, in part, a neural-like phenotype [32]. Indeed, TM cells are derived from the mesenchymal cells of the ectodermal neural crest [251]. This is unique situation in human body because all vascular endothelial are of mesodermal origin. Conversely, TM is an endothelium of ectodermal origin, thus bearing cross-reacting antigens with ONH lamina cribrosa. Indeed, TM and lamina cribrosa display remarkable similarity in protein expression [252]. Neural protein may be detected by proteome analysis in AH under physiological conditions [253] and are enhanced in vitreous humor in case of proliferative retinopathy [254]. Accordingly, it has been proposed [71] that the high amount of neural proteins detectable in POAG AH derives from ONH damage, reflecting on both vitreous and AH composition due to the protein exchange occurring through the uveo-scleral pathway [250].
The fourth group consists of proteins involved in intercellular adhesion. These proteins include catenins, junctional plaque protein, dynein, and cadherins. The presence of these proteins confirms that EDf plays an important role in the pathogenesis of TM damage by permeabilizing the barrier between the AC and Schlemm’s canal. Thus, outflow decrease in glaucoma is related to loss of mechanical integrity in the TM as a consequence of reduced cell–cell and cell–matrix adhesion. Catenins are WNT proteins, and the WNT signaling pathway controls the activity of the genes needed at specific times during development and regulates the interactions between cells as organs and tissues are forming. Interestingly, normal myocilin is a modulator of WNT signaling and provides a useful tool for reorganizing the cytoskeleton of TM cells and regulating IOP [255]. E-cadherin is one of the most important cell surface glycoproteins involved in cell morphogenesis. It is closely related to metalloproteinase and has an important but not well-understood role in the onset and progression of POAG [256]. N-cadherin, a Ca2+-dependent cell–cell adhesion molecule, is an effector of the optimedin gene, which is a neural protein playing an important role in differentiating the brain and retina by modulating cytoskeleton organisation, cell–cell adhesion, and migration [257]. Proteins interconnecting TM cells exert a role in glaucoma pathogenesis because their alteration hampers TM function and integrity. Indeed, it has been recently reported that phosphorylation of Tyr14 in caveolin-1 (CAV-1) and transcriptional regulation of CAV-1 expression have a role in glaucomatous alterations in TM cells [258]. Furthermore, polymorphisms of genes encoding for caveolins have been established to act as risk factor for POAG by genome-wide association studies [28].
The fifth group includes protein kinases, which are key regulators of cell function constituting one of the largest and most functionally diverse gene families. PKC pathway modulation is relevant in glaucoma. PKC inhibitors relax TM and affect expression of matrix metalloproteinase and PGF2 alpha thus increasing AH outflow by regulating myosin light chain phosphorylation and the morphological and cytoskeletal characteristics of TM and SC cells [259]. PKC has been implicated in activating iROS production by NAD(P)H oxidase in endothelial and smooth muscle cells and contribute to induce iROS production [260]. PKC gamma is a unique PKC isoform that is found in neuronal cells and eye tissues. This isoform is activated by ROS, such as H2O2, which activates protein kinase Cγ through the C1 domain, this activation resulting in the inhibition of gap junctions [261]. This confirms the key role of EDf in the course of glaucoma. PKCs are involved in apoptosis activation and signal transduction [262, 263].
Finally, the sixth group of AH proteins altered in glaucoma is related to oxidative stress and includes nitric oxide synthase, SOD, and microsomal glutathione S-transferase 1. In a study by our group [264], the levels of the antioxidant enzymes SOD and glutathione transferase in the AH were significantly lower in POAG patients than in controls, whereas the levels of the pro-oxidant enzyme NOS and glutamine synthase in the AH were significantly higher in patients with POAG than in controls. This finding confirms that the AH antioxidant defenses in glaucoma patients are insufficient, and failure of these antioxidant defenses results in damage to the TM due to the remarkable susceptibility of this tissue to oxidative injury [76].
The origin of these AH proteins may leave some doubt. However, it is unlikely that their origin is in either iris or cornea. In fact, cornea endothelia do not suffer specific abnormalities in the course of glaucoma, and iris loses the endothelium after the birth. Plasma-derived proteins enter AH from the ciliary body at the iris root [265], but a reflux in the course of POAG from Schlemm’s canal or from the iris root has never been disclosed. TM damages both functional and morphological are in contrast well known, validating the hypothesis of a TM origin for AH protein changes.
Furthermore, vascular endothelial growth factor (VEGF) is increased in AH of glaucomatous eyes [266]. VEGF induces endothelial cell proliferation, promotes cell migration, and inhibits apoptosis. Its presence in glaucomatous AH might be justified with the mechanism of activation of endothelial cells and then with an increase in endothelial permeability. Indeed, the permeability-inducing factor and the endothelial cell growth factor are encoded by a single VEGF gene [267]. In addition, vascular permeability factor had been characterized as a protein that promotes extravasation of proteins from tumor-associated blood vessels [268]. Therefore, a trabecular origin of AH’s proteins is strongly plausible.
Conclusions
The molecular alterations described as occurring in TM are the first events triggering POAG. Shedding light on this early TM molecular damage can be useful to develop preventive strategies aimed at protecting TM before the occurrence of irreversible neural damage. Ageing, ROS, and other pathogenic events can decrease TM cellularity until levels of inadequacy for the passage of AH from AC to Schlemm’s canal. This situation results in IOP increase and unleashes a vicious circle of molecular events leading to POAG development, including mitochondrial dysfunction [66], endothelial cell dysfunction [269], and finally functional TM impairment [3]. When severe damage occurs in TM endothelial cells in tight contact with AH, intracellular proteins leak from damaged cells, thereby changing the AH composition [71]. The presence of neurological proteins in glaucomatous AH [71] reflects the occurrence of a similar pathological condition affecting both TM and ONH [252].
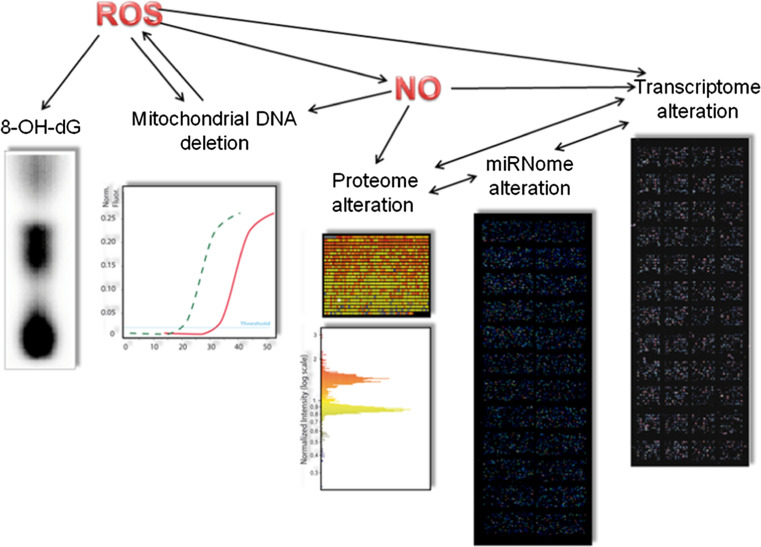
Trabecular meshwork actively modulates AH outflow from AC to Schlemm’s Canal and not a mere passive way of egress. Little is known about the mechanism transmitting glaucomatous molecular signals from the AC to the retina and then to the central nervous system. It is conceivable that protein produced by damaged TM behave as a communicating signal for the posterior segment. Early molecular events happening in AC and AH can be studied by mean of newly developed biomolecular techniques thus disclosing new horizons for understanding glaucoma patho-physiology. The understanding of this molecular language of communication between AC and ONH is a new frontier for glaucoma. Thanks to the use of new omics methodologies molecular bases of POAG are beginning to be understood (Fig. 8). In the near future, this knowledge ought to be applied in the diagnostic and therapeutic field.
Fig. 8.
Omics techniques so far applied to the study of glaucoma. Genomic methods include analysis of nuclear DNA oxidative damage (8-OH-dG) by 32P postlabeling and analysis of mitochondiral DNA deletion by qPCR. Micorarray-based postgenomic methods include expression analysis of gene (transcriptome), microRNA (miRNome), and proteins (proteome)
Microarray-based omics techniques able to detect early molecular damage in glaucoma include gene expression profiling as applied in vitro in endothelial cells [270] and in vivo in rat filtering belbs [271] and in mouse retina [272]. In human, microarray gene expression analysis in blood leukocytes has been recently reported to discriminate between glaucomatous patients and controls [273]. MicroRNA expression profiling has been applied in vitro in cultured TM cells [274, 275].
The antibody microarray method seems to be very promising [71], and, at variance with other methods, is able to quantify glaucoma protein hallmarks by a moderately invasive sample such as corneal puncture. It is conceivable that the invasiveness of this approach might be improved by the application of micropuncture techniques [236]. This method relies on the identification of different proteins differentially expressed in AH in healthy or sick subjects by means of specific antibody- and fluorescence-labeled probes. Fluorescent signals are acquired by means of a laser scanner and analyzed by means of software able to evaluate expression patterns of sets of proteins, thus being able to distinguish a normal from a POAG expression pattern.
This protein signature distinguishes different glaucoma types and could be useful for making a diagnosis at early stage. These techniques will furnish a very useful instrument of diagnosis and prevention, leading to a reduction of costs for public health. Indeed, the performances of the diagnostic test are increased by increasing the number of tested AH proteins. Performances include sensitivity (number of false negative results), specificity (number of false positive results), and accuracy (the transferability of the results from a low to a great number of subjects). AH antibody microarray analysis is able to test up to thousands of proteins in a single analysis using hallmark proteins to identify glaucoma-affected patients [71, 236].
The knowledge of molecular mechanisms leading to POAG onset will further help to develop new therapeutic approaches, counteracting TM oxidative stress and its molecular consequences, improving the energetic metabolism, correcting mitochondrial impairment, and decreasing the apoptosis rate. This molecular approach will further provide us with the tools to prevent the propagation of damage leading to apoptosis from the anterior chamber and ONH.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgment
This study was supported by the US Glaucoma Research Foundation (TGF) (New York, USA).
Abbreviations
- 8-OHdg
8-Hydroxydeoxyguanosine
- AC
Anterior chamber
- AF
Actin microfilaments
- AH
Aqueous humor
- ECM
Extracellular matrix
- ELAM-1
Endothelial leukocyte adhesion molecule-1
- GSH
Reduced glutathione
- IF
Intermediate filaments
- IL
Interleukin
- JCT
Juxtacanalicular connective tissue
- MT
Microtubules
- mtDNA
Mitochondrial DNA
- NO
Nitric oxide
- ONH
Optic nerve head
- POAG
Primary open-angle glaucoma
- RGCs
Retinal ganglion cells
- RNFL
Retinal nerve fibre layer
- ROS
Reactive oxygen species
- SCS
Suprachoroidal space
- SOD
Superoxide dismutase
- TM
Trabecular meshwork
- UV
Ultra violet radiation
References
- 1.Shields M, Ritch R, Krupin T. Classifications of the glaucomas. In: Ritch R, Shields M, Krupin T, editors. The glaucomas. St Louis: Mosby; 1996. pp. 717–725. [Google Scholar]
- 2.Leske MC. Open-angle glaucoma: an epidemiologic overview. Ophthalmic Epidemiol. 2007;14:166–172. doi: 10.1080/09286580701501931. [DOI] [PubMed] [Google Scholar]
- 3.Saccà SC, Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Prog Brain Res. 2008;173:385–407. doi: 10.1016/S0079-6123(08)01127-8. [DOI] [PubMed] [Google Scholar]
- 4.Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta N, Ang LC, Noël de Tilly L, Bidaisee L, Yücel YH. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006;90:674–678. doi: 10.1136/bjo.2005.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 7.Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;12:105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sée V, Loeffler JP. Oxidative stress induces neuronal death by recruiting a protease and phosphatase-gated mechanism. J Biol Chem. 2001;276:35049–35059. doi: 10.1074/jbc.M104988200. [DOI] [PubMed] [Google Scholar]
- 10.Saccà SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 11.Nakazawa T, Nakazawa C, Matsubara A, Noda K2, Hisatomi T (2006) Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci 26:12633–12641 [DOI] [PMC free article] [PubMed]
- 12.Weber AJ, Harman CD. Structure–function relations of parasol cells in the normal and glaucomatous primate retina. Invest Ophthalmol Vis Sci. 2005;46:3197–3207. doi: 10.1167/iovs.04-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yucel YH, Zhang Q, Gupta N, Kaufman PL, Weinreb RN. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol. 2000;118:378–384. doi: 10.1001/archopht.118.3.378. [DOI] [PubMed] [Google Scholar]
- 14.Brown GC. Nitric oxide and neuronal death. Nitric Oxide. 2010;23:153–165. doi: 10.1016/j.niox.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Brandt SK, Weatherly ME, Ware L, Linn DM, Linn CL. Calcium preconditioning triggers neuroprotection in retinal ganglion cells. Neuroscience. 2011;172:387–397. doi: 10.1016/j.neuroscience.2010.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izzotti A, Saccà SC, Longobardi M, Cartiglia C. Mitochondrial damage in the trabecular meshwork of patients with glaucoma. Arch Ophthalmol. 2010;128:724–730. doi: 10.1001/archophthalmol.2010.87. [DOI] [PubMed] [Google Scholar]
- 17.Band LR, Hall CL, Richardson G, Jensen OE, Siggers JH, Foss AJ. Intracellular flow in optic nerve axons: a mechanism for cell death in glaucoma. Invest Ophthalmol Vis Sci. 2009;50:3750–3758. doi: 10.1167/iovs.08-2396. [DOI] [PubMed] [Google Scholar]
- 18.Johnson EC, Morrison JC. Friend or foe? Resolving the impact of glial responses in glaucoma. J Glaucoma. 2009;18:341–353. doi: 10.1097/IJG.0b013e31818c6ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izzotti A, Saccà SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003;114:638–646. doi: 10.1016/s0002-9343(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 20.Saccà SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84:389–399. doi: 10.1016/j.exer.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Flammer J. The vascular concept of glaucoma. Surv Ophthalmol. 1994;38:3–6. doi: 10.1016/0039-6257(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 22.Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 23.Flammer J, Orgul S. Optic nerve blood flow abnormalities in glaucoma. Prog Retin Eye Res. 1998;17:267–289. doi: 10.1016/s1350-9462(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 24.Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Finite element modeling of optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2004;45:4378–4387. doi: 10.1167/iovs.04-0133. [DOI] [PubMed] [Google Scholar]
- 25.Norman RE, Flanagan JG, Sigal IA, Rausch SM, Tertinegg I, Ethier CR. Finite element modeling of the human sclera: influence on optic nerve head biomechanics and connections with glaucoma. Exp Eye Res. 2011;93:4–12. doi: 10.1016/j.exer.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Gerometta R, Escobar D, Candia OA. An hypothesis on pressure transmission from anterior chamber to optic nerve. Med Hypotheses. 2011;77:827–831. doi: 10.1016/j.mehy.2011.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dan J, Belyea D, Gertner G, Leshem I, Lusky M, Miskin R. Plasminogen activator inhibitor-1 in the aqueous humor of patients with and without glaucoma. Arch Ophthalmol. 2005;123:220–224. doi: 10.1001/archopht.123.2.220. [DOI] [PubMed] [Google Scholar]
- 28.Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010;42:906–909. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lütjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005;81:1–4. doi: 10.1016/j.exer.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Zhong Y, Wang J, Luo X. Integrins in trabecular meshwork and optic nerve head: possible association with the pathogenesis of glaucoma. Biomed Res Int. 2013;2013:202905. doi: 10.1155/2013/202905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kernt M, Neubauer AS, Eibl KH, Wolf A, Ulbig MW, et al. Minocycline is cytoprotective in human trabecular meshwork cells and optic nerve head astrocytes by increasing expression of XIAP, survivin, and Bcl-2. Clin Ophthalmol. 2010;4:591–604. doi: 10.2147/opth.s11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steely HT, Jr, English-Wright SL, Clark AF. The similarity of protein expression in trabecular meshwork and lamina cribrosa: implications for glaucoma. Exp Eye Res. 2000;70:17–30. doi: 10.1006/exer.1999.0764. [DOI] [PubMed] [Google Scholar]
- 33.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagchi K, Puri S. Free radicals and antioxidants in health and disease. East Mediterr Health. 1998;4:350–360. [Google Scholar]
- 35.Saccà SC, Bolognesi C, Battistella A, Bagnis A, Izzotti A. Gene-environment interactions in ocular diseases. Mutat Res. 2009;667:98–117. doi: 10.1016/j.mrfmmm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 37.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 38.Genestra M. Oxyl radicals, redox-sensitive signaling cascades and antioxidants. Cell Signal. 2007;19:1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 40.Tatton WG, Olanow CW. Apoptosis in neurodegenerative diseases: the role of mitochondria. Biochim Biophys Acta. 1999;1410:195–213. doi: 10.1016/s0005-2728(98)00167-4. [DOI] [PubMed] [Google Scholar]
- 41.Mann RM, Riva CE, Stone RA, Barnes GE, Cranstoun SD. Nitric oxide and choroidal blood flow regulation. Invest Ophthalmol Vis Sci. 1995;36:925–930. [PubMed] [Google Scholar]
- 42.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 43.Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995;36:1774–1784. [PubMed] [Google Scholar]
- 44.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virag L, Szabo E, Gergely P, Szabo C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett. 2003;140–141:113–124. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]
- 46.Ghafourifar P, Schenk U, Klein SD, Richter C. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J Biol Chem. 1999;274:31185–31188. doi: 10.1074/jbc.274.44.31185. [DOI] [PubMed] [Google Scholar]
- 47.Estevez AG, Crow JP, Sampson JB, Reiter C, Zhuang Y, et al. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 48.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 49.Li G, Luna C, Liton PB, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis. 2007;13:2282–2288. [PMC free article] [PubMed] [Google Scholar]
- 50.Liton PB, Luna C, Bodman M, Hong A, Epstein DL, Gonzalez P. Induction of IL-6 expression by mechanical stress in the trabecular meshwork. Biochem Biophys Res Commun. 2005;337:1229–1236. doi: 10.1016/j.bbrc.2005.09.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liton PB, Luna C, Challa P, Epstein DL, Gonzalez P. Genomewide: expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol Vis. 2006;12:774–790. [PMC free article] [PubMed] [Google Scholar]
- 52.Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med. 1999;31:53–59. doi: 10.1038/emm.1999.9. [DOI] [PubMed] [Google Scholar]
- 53.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 54.Novalija E, Kevin LG, Camara AK, Bosnjak ZJ, Kampine JP, et al. Reactive oxygen species precede the epsilon isoform of protein kinase C in the anesthetic preconditioning signaling cascade. Anesthesiology. 2003;99:421–428. doi: 10.1097/00000542-200308000-00024. [DOI] [PubMed] [Google Scholar]
- 55.Kosenko E, Llansola M, Montoliu C, Monfort P, Rodrigo R, et al. Glutamine synthase activity and glutamine content in brain: modulation by NMDA receptors and nitric oxide. Neurochem Int. 2003;43:493–499. doi: 10.1016/s0197-0186(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 56.Coca-Prados M, Ghosh S (2008) Functional modulators linking inflow with outflow of aqueous humor. In: Mortimer C (ed) Current topics in membranes, Edition 2. Elsevier, Amsterdam, pp 123–160
- 57.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 58.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kadenbach B, Ramzan R, Vogt S. Degenerative diseases, oxidative stress and cytochrome c oxidase function. Trends Mol Med. 2009;15:139–147. doi: 10.1016/j.molmed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Mecocci P, MacGarvey U, Kaufman AE, Koontz D, Shoffner JM, et al. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol. 1993;34:609–616. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- 62.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of ageing. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yakes FM, van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izzotti A. Gene environment interactions in noncancer degenerative diseases. Mutat Res Fundam Mol Mech Mutagen. 2009;667:1–3. doi: 10.1016/j.mrfmmm.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Izzotti A, Longobardi M, Cartiglia C, Saccà SC. Mitochondrial damage in the trabecular meshwork occurs only in primary open-angle glaucoma and in pseudoexfoliative glaucoma. PLoS ONE. 2011;6:e14567. doi: 10.1371/journal.pone.0014567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morris MA. Mitochondrial mutations in neuro-ophthalmological diseases. A review. J Clin Neuroophthalmol. 1990;10:159–166. [PubMed] [Google Scholar]
- 67.Hayakawa M, Hattori K, Sugiyama S, Ozawa T. Age-associated oxygen damage and mutations in mitochondrial DNA in human hearts. Biochem Biophys Res Commun. 1992;189:979–985. doi: 10.1016/0006-291x(92)92300-m. [DOI] [PubMed] [Google Scholar]
- 68.Lascaratos G, Garway-Heath DF, Willoughby CE, Chau KY, Schapira AH. Mitochondrial dysfunction in glaucoma: understanding genetic influences. Mitochondrion. 2012;12:202–212. doi: 10.1016/j.mito.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Kujoth GC, Hiona A, Pugh TD. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 70.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:670–686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- 71.Izzotti A, Longobardi M, Cartiglia C, Saccà SC. Proteome alterations in primary open angle glaucoma aqueous humor. J Proteome Res. 2010;9:4831–4838. doi: 10.1021/pr1005372. [DOI] [PubMed] [Google Scholar]
- 72.He Y, Leung KW, Zhang YH, Duan S, Zhong XF, Jiang RZ, Peng Z, Tombran-Tink J, Ge J. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: protection by antioxidants. Invest Ophthalmol Vis Sci. 2008;49:1447–1458. doi: 10.1167/iovs.07-1361. [DOI] [PubMed] [Google Scholar]
- 73.Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: the trabecular meshwork. J Cell Physiol. 1999;180:182–189. doi: 10.1002/(SICI)1097-4652(199908)180:2<182::AID-JCP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 74.Costarides AP, Riley MV, Green K. Roles of catalase and the glutathione redox cycle in the regulation of the anterior-chamber hydrogen peroxide. Ophthalmic Res. 1991;23:284–294. doi: 10.1159/000267124. [DOI] [PubMed] [Google Scholar]
- 75.Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:877–883. doi: 10.1167/iovs.04-0777. [DOI] [PubMed] [Google Scholar]
- 76.Izzotti A, Saccà SC, Longobardi M, Cartiglia C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Invest Ophthalmol Vis Sci. 2009;50:5251–5258. doi: 10.1167/iovs.09-3871. [DOI] [PubMed] [Google Scholar]
- 77.Izzotti A, Longobardi M, Ratschuller F, Cartiglia C, Saccà SC. Trabecular meshwork gene expression after selective laser trabeculoplasty (TGF) Plos ONE. 2011;6:E2010. doi: 10.1371/journal.pone.0020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 79.Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137:62–69. doi: 10.1016/s0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- 80.Alvarado JA, Murphy CG, Polansky JR, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21:714–727. [PubMed] [Google Scholar]
- 81.Alvarado J, Murphy C, Polansky J, Juster R. Studies on pathogenesis of primary open angle glaucoma: regional analyses of trabecular meshwork cellularity and dense collagen. In: Ticho U, David R, editors. Recent advances in glaucoma. Amsterdam: Elsevier; 1984. pp. 3–8. [Google Scholar]
- 82.Alvarado JA, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- 83.Shimmura S, Suematsu M, Shimoyama M, Tsubota K, Oguchi Y, et al. Subthreshold UV radiation-induced peroxide formation in cultured corneal epithelial cells: the protective effects of lactoferrin. Exp Eye Res. 1996;63:519–526. doi: 10.1006/exer.1996.0142. [DOI] [PubMed] [Google Scholar]
- 84.Ikehata H, Ono T. The mechanisms of UV mutagenesis. J Radiat Res. 2011;52:115–125. doi: 10.1269/jrr.10175. [DOI] [PubMed] [Google Scholar]
- 85.Saccà SC, Roszkowska AM, Izzotti A. Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases. Mutat Res. 2013;752:153–171. doi: 10.1016/j.mrrev.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Godley BF, Shamsi FA, Liang FQ, Jarrett SG, Davies S, Boulton M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J Biol Chem. 2005;280:21061–21066. doi: 10.1074/jbc.M502194200. [DOI] [PubMed] [Google Scholar]
- 87.Wei H, Ca Q, Rahn R, Zhang X, Wang Y, Lebwohl M. DNA structural integrity and base composition affect ultraviolet light-induced oxidative DNA damage. Biochemistry. 1998;37:6485–6490. doi: 10.1021/bi972702f. [DOI] [PubMed] [Google Scholar]
- 88.Balansky RM, Izzotti A, D’Agostini F, Camoirano A, Bagnasco M, et al. Systemic genotoxic effects produced by light, and synergism with cigarette smoke in the respiratory tract of hairless mice. Carcinogenesis. 2003;24:1525–1532. doi: 10.1093/carcin/bgg108. [DOI] [PubMed] [Google Scholar]
- 89.Osborne NN, Lascaratos G, Bron AJ, Chidlow G, Wood JP. A hypothesis to suggest that light is a risk factor in glaucoma and the mitochondrial optic neuropathies. Br J Ophthalmol. 2006;90:237–241. doi: 10.1136/bjo.2005.082230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanan Y, Moiseyev G, Agarwal N, Ma JX, Al-Ubaidi MR. Light induces programmed cell death by activating multiple independent proteases in a cone photoreceptor cell line. Invest Ophthalmol Vis Sci. 2007;48:40–51. doi: 10.1167/iovs.06-0592. [DOI] [PubMed] [Google Scholar]
- 91.Buron N, Micheau O, Cathelin S, Lafontaine PO, Creuzot-Garcher C, et al. Differential mechanisms of conjunctival cell death induction by ultraviolet irradiation and benzalkonium chloride. Invest Ophthalmol Vis Sci. 2006;47:4221–4230. doi: 10.1167/iovs.05-1460. [DOI] [PubMed] [Google Scholar]
- 92.De Flora S, Izzotti A, Randerath K, Randerath E, Bartsch H, et al. DNA adducts and chronic degenerative diseases. Pathogenetic relevance and implications in preventive medicine. Mutat Res. 1996;366:197–238. [PubMed] [Google Scholar]
- 93.Chandler HL, Reuter KS, Sinnott LT, Nichols JJ. Prevention of UV-induced damage to the anterior segment using class I UV-absorbing hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010;51:172–178. doi: 10.1167/iovs.09-3996. [DOI] [PubMed] [Google Scholar]
- 94.Linsenmayer TF, Cai CX, Millholland JM, Beazley KE, Fitch JM. Nuclear ferritin in corneal epithelial cells: tissue-specific nuclear transport and protection from UV-damage. Prog Retin Eye Res. 2005;24:139–159. doi: 10.1016/j.preteyeres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 95.Suh MH, Kwon JW, Wee WR, Han YK, Kim JH, et al. Protective effect of ascorbic Acid against corneal damage by ultraviolet B irradiation: a pilot study. Cornea. 2008;27:916–922. doi: 10.1097/ICO.0b013e31816f7068. [DOI] [PubMed] [Google Scholar]
- 96.Araie M, Shirasawa E, Hikita M. Effect of oxidized glutathione on the barrier function of the corneal endothelium. Invest Ophthalmol Vis Sci. 1988;29:1884–1887. [PubMed] [Google Scholar]
- 97.Brancato R, Fiore T, Papucci L, Schiavone N, Formigli L, et al. Concomitant effect of topical ubiquinone Q10 and vitamin E to prevent keratocyte apoptosis after excimer laser photoablation in rabbits. J Refract Surg. 2002;18:135–139. doi: 10.3928/1081-597X-20020301-06. [DOI] [PubMed] [Google Scholar]
- 98.Marchitti SA, Chen Y, Thompson DC, Vasiliou V. Ultraviolet radiation: cellular antioxidant response and the role of ocular aldehyde dehydrogenase enzymes. Eye Contact Lens. 2011;37:206–213. doi: 10.1097/ICL.0b013e3182212642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann NY Acad Sci. 1992;669:7–20. doi: 10.1111/j.1749-6632.1992.tb17085.x. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J Biol Chem. 2005;280:33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- 101.Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57:1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Watson WH, Chen Y, Jones DP. Redox state of glutathione and thioredoxin in differentiation and apoptosis. BioFactors. 2003;17:307–314. doi: 10.1002/biof.5520170130. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y, Johansson E, Fan Y, Shertzer HG, Vasiliou V, et al. Early onset senescence occurs when fibroblasts lack the glutamate–cysteine ligase modifier subunit. Free Radic Biol Med. 2009;47:410–418. doi: 10.1016/j.freeradbiomed.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kannan R, Tang D, Mackic JB, Zlokovic BV, Fernandez-Checa JC. A simple technique to determine glutathione (GSH) levels and synthesis in ocular tissues as GSH–bimane adduct: application to normal and galactosemic guinea-pigs. Exp Eye Res. 1993;56:45–50. doi: 10.1006/exer.1993.1007. [DOI] [PubMed] [Google Scholar]
- 105.Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 106.Riley MV. A role for glutathione and glutathione reductase in control of cornal hydration. Exp Eye Res. 1984;39:751–758. doi: 10.1016/0014-4835(84)90074-5. [DOI] [PubMed] [Google Scholar]
- 107.Serbecic N, Beutelspacher SC. Anti-oxidative vitamins prevent lipidperoxidation and apoptosis in corneal endothelial cells. Cell Tissue Res. 2005;320:465–475. doi: 10.1007/s00441-004-1030-3. [DOI] [PubMed] [Google Scholar]
- 108.Crouch R, Ling Z, Hayden BJ. Corneal oxygen scavenging systems: lysis of corneal epithelial cells by superoxide anions. Basic Life Sci. 1988;49:1043–1046. doi: 10.1007/978-1-4684-5568-7_172. [DOI] [PubMed] [Google Scholar]
- 109.Behndig A, Svensson B, Marklund SL, Karlsson K. Superoxide dismutase isoenzymes in the human eye. Invest Ophthalmol Vis Sci. 1998;39:471–475. [PubMed] [Google Scholar]
- 110.Choi SI, Kim TI, Kim KS, Kim BY, Ahn SY, et al. Decreased catalase expression and increased susceptibility to oxidative stress in primary cultured corneal fibroblasts from patients with granular corneal dystrophy type II. Am J Pathol. 2009;175:248–261. doi: 10.2353/ajpath.2009.081001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shull S, Heintz NH, Periasamy M, Manohar M, Janssen YM, et al. Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem. 1991;266:24398–24403. [PubMed] [Google Scholar]
- 112.Strålin P, Marklund SL. Effects of oxidative stress on expression of extracellular superoxide dismutase, CuZn-superoxide dismutase and Mn-superoxide dismutase in human dermal fibroblasts. Biochem J. 1994;298:347–352. doi: 10.1042/bj2980347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Black AT, Gordon MK, Heck DE, Gallo MA, Laskin DL, et al. UVB light regulates expression of antioxidants and inflammatory mediators in human corneal epithelial cells. Biochem Pharmacol. 2011;81:873–880. doi: 10.1016/j.bcp.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cejková J, Ardan T, Cejka C, Luyckx J. Favorable effects of trehalose on the development of UVB-mediated antioxidant/pro-oxidant imbalance in the corneal epithelium, proinflammatory cytokine and matrix metalloproteinase induction, and heat shock protein 70 expression. Graefes Arch Clin Exp Ophthalmol. 2011;249:1185–1194. doi: 10.1007/s00417-011-1676-y. [DOI] [PubMed] [Google Scholar]
- 115.Atalla LR, Sevanian A, Rao NA. Immunohistochemical localization of glutathione peroxidase in ocular tissue. Curr Eye Res. 1988;7:1023–1027. doi: 10.3109/02713688809015149. [DOI] [PubMed] [Google Scholar]
- 116.Lassen N, Black WJ, Estey T, Vasiliou V. The role of corneal crystallins in the cellular defense mechanisms against oxidative stress. Semin Cell Dev Biol. 2008;19:100–112. doi: 10.1016/j.semcdb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 117.Cejková J, Vejrazka M, Pláteník J, Stípek S. Age-related changes in superoxide dismutase, glutathione peroxidase, catalase and xanthine oxidoreductase/xanthine oxidase activities in the rabbit cornea. Exp Gerontol. 2004;39:1537–1543. doi: 10.1016/j.exger.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 118.Kivelä T, Uusitalo M. Structure, development and function of cytoskeletal elements in non-neuronal cells of the human eye. Prog Retin Eye Res. 1998;17:385–428. doi: 10.1016/s1350-9462(98)00001-9. [DOI] [PubMed] [Google Scholar]
- 119.Roberts JE. Screening for ocular phototoxicity. Int J Toxicol. 2002;21:491–500. doi: 10.1080/10915810290169918. [DOI] [PubMed] [Google Scholar]
- 120.Liu Y, Simon JD. Isolation and biophysical studies of natural eumelanins: applications of imaging technologies and ultrafast spectroscopy. Pigment Cell Res. 2003;16:606–618. doi: 10.1046/j.1600-0749.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 121.Kuchle M, Mardin CY, Nguyen NX, Wartus P, Naumann GO. Quantification of aqueous melanin granules in primary pigment dispersion syndrome. Am J Ophthalmol. 1998;126:442–445. doi: 10.1016/s0002-9394(98)00098-1. [DOI] [PubMed] [Google Scholar]
- 122.Buller C, Johnson DH, Tschumper RC. Human trabecular meshwork phagocytosis. Observations in an organ culture system. Invest Ophthalmol Vis Sci. 1990;31:2156–2163. [PubMed] [Google Scholar]
- 123.Cracknell KP, Grierson I, Hogg P, Majekodunmi AA, Watson P, et al. Melanin in the trabecular meshwork is associated with age, POAG but not Latanoprost treatment. A masked morphometric study. Exp Eye Res. 2006;82:986–993. doi: 10.1016/j.exer.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 124.Christen R, Pache M, Teuchner B, Meyer P, Prünte C, et al. Iris transillumination defects in patients with primary open angle glaucoma. Eur J Ophthalmol. 2003;13:365–369. doi: 10.1177/112067210301300406. [DOI] [PubMed] [Google Scholar]
- 125.Eagle RC. Congenital, developmental and degenerative disorders of the iris and ciliary body. In: Albert DM, Jakobiec FA, editors. Principles and practice of ophthalmology. Philadelphia: Saunders; 1994. pp. 367–388. [Google Scholar]
- 126.Bill A. The aqueous drainage mechanism in the cynomolgus monkey (Macaca irus) with evidence for unconventional routes. Invest Ophthalmol. 1965;4:911–919. [PubMed] [Google Scholar]
- 127.Bill A, Hellsing K. Production and drainage of aqueous humor in the cynomolgus monkey (Macaca irus) Invest Ophthalmol. 1965;4:920–926. [PubMed] [Google Scholar]
- 128.Bill A. Aqueous humor dynamics in monkeys (Macaca irus and Cercopithecus ethiops) Exp Eye Res. 1971;11:195–206. doi: 10.1016/s0014-4835(71)80023-4. [DOI] [PubMed] [Google Scholar]
- 129.Raviola G, Butler JM. Asymmetric distribution of charged domains on the two fronts of the endothelium of iris blood vessels. Invest Ophthalmol Vis Sci. 1985;26:597–608. [PubMed] [Google Scholar]
- 130.Bill A, MaepeaO (1999) Meccanismi e vie di drenaggio dell’umor acqueo. In: Albert DM, Jackobiec FA (eds) Principi e pratica di Oftalmologia, Verducci, Roma, pp 221–241
- 131.Stamper R. Aqueous humor: secretion and dynamics. In: Tasman W, Jaeger E, editors. Duane’s foundations of clinical ophthalmology. Philadelphia: Lippincott; 1992. pp. 1–30. [Google Scholar]
- 132.Toris CB, Koepsell SA, Yablonski ME, Camras CB. Aqueous humor dynamics in ocular hypertensive patients. J Glaucoma. 2002;11:253–258. doi: 10.1097/00061198-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 133.Toris CB, Yablonski ME, Wang YL, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol. 1999;127:407–412. doi: 10.1016/s0002-9394(98)00436-x. [DOI] [PubMed] [Google Scholar]
- 134.Gaasterland D, Kupfer C, Milton R, Ross K, McCain L, et al. Studies of aqueous humor dynamics in man. VI. Effect of age upon parameters of intraocular pressure in normal human eyes. Exp Eye Res. 1978;26:651–656. doi: 10.1016/0014-4835(78)90099-4. [DOI] [PubMed] [Google Scholar]
- 135.Brubaker RF, Nagataki S, Townsend DJ, Burns RR, Higgins RG, et al. The effect of age on aqueous humor formation in man. Ophthalmology. 1981;88:283–288. doi: 10.1016/s0161-6420(81)35037-4. [DOI] [PubMed] [Google Scholar]
- 136.Toris CB, Tafoya ME, Camras CB, Yablonski ME. Effects of apraclonidine on aqueous humor dynamics in human eyes. Ophthalmology. 1995;102:456–461. doi: 10.1016/s0161-6420(95)31000-7. [DOI] [PubMed] [Google Scholar]
- 137.Pavao AF, Lee DA, Ethier CR, Johnson M, Anderson PJ, et al. Two-dimensional gel electrophoresis of calf aqueous humor, serum and filter-bound proteins. Invest Ophthalmol Vis Sci. 1989;30:731–738. [PubMed] [Google Scholar]
- 138.Ringvold A, Anderssen E, Kjonniksen I. Distribution of ascorbate in the anterior bovine eye. Invest Ophthalmol Vis Sci. 2000;41:20–23. [PubMed] [Google Scholar]
- 139.Tokuda K, Zorumski CF, Izumi Y. Effects of ascorbic acid on UV light-mediated photoreceptor damage in isolated rat retina. Exp Eye Res. 2007;84:537–543. doi: 10.1016/j.exer.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gerometta RM, Malgor LA, Vilalta E, Leiva J, Candia OA. Cl- concentrations of bovine, porcine and ovine aqueous humor are higher than in plasma. Exp Eye Res. 2005;80:307–312. doi: 10.1016/j.exer.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 141.Rose RC, Richer SP, Bode AM. Ocular oxidants and antioxidant protection. Proc Soc Exp Biol Med. 1998;217:397–407. doi: 10.3181/00379727-217-44250. [DOI] [PubMed] [Google Scholar]
- 142.Spector A, Garner WH. Hydrogen peroxide and human cataract. Exp Eye Res. 1981;33:673–681. doi: 10.1016/s0014-4835(81)80107-8. [DOI] [PubMed] [Google Scholar]
- 143.Russell P, Johnson DH. Enzymes protective of oxidative damage present in all decades of life in the trabecular meshwork, as detected bytwo-dimensional gel electrophoresis protein maps. J Glaucoma. 1996;5:317–324. [PubMed] [Google Scholar]
- 144.Giblin FJ, McCready JP, Kodama T, Reddy VN. A direct correlation between the levels of ascorbic acid and H2O2 in aqueous humor. Exp Eye Res. 1984;38:87–93. doi: 10.1016/0014-4835(84)90142-8. [DOI] [PubMed] [Google Scholar]
- 145.Vrabec F. The anterior superficial endothelium of the human iris. Ophthalmologica. 1952;123:20–30. doi: 10.1159/000301125. [DOI] [PubMed] [Google Scholar]
- 146.Izzotti A, Saccà SC, Di Marco B, Penco S, Bassi AM. Antioxidant activity of timolol on endothelial cells and its relevance for glaucoma course. Eye (Lond) 2008;22:445–453. doi: 10.1038/sj.eye.6702737. [DOI] [PubMed] [Google Scholar]
- 147.Gabelt BT, Kaufman PL. Prostaglandin F2 alpha increases uveoscleral outflow in the cynomolgus monkey. Exp Eye Res. 1989;49:389–402. doi: 10.1016/0014-4835(89)90049-3. [DOI] [PubMed] [Google Scholar]
- 148.Chen JZ, Kadlubar FF. A new clue to glaucoma pathogenesis. Am J Med. 2003;114:697–698. doi: 10.1016/s0002-9343(03)00199-2. [DOI] [PubMed] [Google Scholar]
- 149.Polansky J, Alvarado J. Cellular mechanisms influencing the aqueous humor outflow pathway. In: Albert DM, Jakobiec FA, editors. Principles and practice of ophthalmology: basic science. Philadelphia: Saunders; 1994. pp. 226–251. [Google Scholar]
- 150.Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–623. [PubMed] [Google Scholar]
- 151.Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005;46:2857–2868. doi: 10.1167/iovs.05-0075. [DOI] [PubMed] [Google Scholar]
- 152.Bachem MG, Wendelin D, Schneiderhan W, Haug C, Zorn U, et al. Depending on their concentration oxidized low density lipoproteins stimulate extracellular matrix synthesis or induce apoptosis in human coronary artery smooth muscle cells. Clin Chem Lab Med. 1999;37:319–326. doi: 10.1515/CCLM.1999.054. [DOI] [PubMed] [Google Scholar]
- 153.Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, et al. TGF-b2 induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–234. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- 154.Bradley JM, Vranka J, Colvis CM, Conger DM, Alexander JP, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- 155.Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 156.Wordinger RJ, Fleenor DL, Hellberg PE, Pang IH, Tovar TO, et al. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- 157.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). III. Induction of ACAID upon intraocular transforming growth factor-beta. Eur J Immunol. 1992;22:165–173. doi: 10.1002/eji.1830220125. [DOI] [PubMed] [Google Scholar]
- 158.Zode GS, Sethi A, Brun-Zinkernagel AM, Chang IF, Clark AF, et al. Transforming growth factor-β2 increases extracellular matrix proteins in optic nerve head cells via activation of the Smad signaling pathway. Mol Vis. 2011;17:1745–1758. [PMC free article] [PubMed] [Google Scholar]
- 159.Epstein DL, De Kater AW, Lou M, Patel J. Influences of glutathione and sulfhydryl containing compounds on aqueous humor outflow function. Exp Eye Res. 1990;50:785–793. doi: 10.1016/0014-4835(90)90129-i. [DOI] [PubMed] [Google Scholar]
- 160.Sawaguchi S, Yue BY, Chang IL, Wong F, Higginbotham EJ. Ascorbic acid modulates collagen type I gene expression by cells from an eye tissue-trabecular meshwork. Cell Mol Biol. 1992;38:587–604. [PubMed] [Google Scholar]
- 161.Schachtschabel DO, Binninger E. Stimulatory effects of ascorbic acid on hyaluronic acid synthesis of in vitro cultured normal and glaucomatous trabecular meshwork cells of the human eye. Gerontology. 1993;26:243–246. [PubMed] [Google Scholar]
- 162.McCarty MF. Primary open-angle glaucoma may be a hyaluronic acid deficiency disease: potential for glucosamine in prevention and therapy. Med Hypotheses. 1998;51:483–484. doi: 10.1016/s0306-9877(98)90068-8. [DOI] [PubMed] [Google Scholar]
- 163.Virno M, Bucci MG, Pecori-Giraldi J, Cantore G. Intravenous glycerol-vitamin C (sodium salt) as osmotic agents to reduce intraocular pressure. Am J Ophthalmol. 1966;62:824–833. doi: 10.1016/0002-9394(66)91905-2. [DOI] [PubMed] [Google Scholar]
- 164.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 165.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res. 2009;88:656–670. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Epstein DL, Rohen JW. Morphology of the trabecular meshwork and inner-wall endothelium after cationized ferritin perfusion in the monkey eye. Invest Ophthalmol Vis Sci. 1991;32:160–171. [PubMed] [Google Scholar]
- 168.Johnson M, Erickson K. Aqueous humor and the dynamics of its flow. In: Albert DM, Jakobiec FA, editors. Principles and practice of ophthalmology. Philadelphia: Saunders; 2000. pp. 2577–2595. [Google Scholar]
- 169.Sit AJ, Coloma FM, Ethier CR, Johnson M. Factors affecting the pores of the inner wall endothelium of Schlemm’s canal. Invest Ophthalmol Vis Sci. 1997;38:1517–1525. [PubMed] [Google Scholar]
- 170.Johnson M, Chan D, Read AT, Christensen C, Sit A, et al. The pore density in the inner wall endothelium of Schlemm’s canal of glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002;43:2950–2955. [PubMed] [Google Scholar]
- 171.Scott PA, Lu Z, Liu Y, Gong H. Relationships between increased aqueous outflow facility during washout with the changes in hydrodynamic pattern and morphology in bovine aqueous outflow pathways. Exp Eye Res. 2009;89:942–949. doi: 10.1016/j.exer.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 172.Alvarado JA, Betanzos A, Franse-Carman L, Chen J, González-Mariscal L. Endothelia of Schlemm’s canal and trabecular meshwork: distinct molecular, functional, and anatomic features. Am J Physiol Cell Physiol. 2004;286:C621–C634. doi: 10.1152/ajpcell.00108.2003. [DOI] [PubMed] [Google Scholar]
- 173.Alvarado JA, Alvarado RG, Yeh RF, Franse-Carman L, Marcellino GR, et al. A new insight into the cellular regulation of aqueous outflow: how trabecular meshwork endothelial cells drive a mechanism that regulates the permeability of Schlemm’s canal endothelial cells. Br J Ophthalmol. 2005;89:1500–1505. doi: 10.1136/bjo.2005.081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Alvarado JA, Yeh RF, Franse-Carman L, Marcellino G, Brownstein MJ. Interactions between endothelia of the trabecular meshwork and of Schlemm’s canal: a new insight into the regulation of aqueous outflow in the eye. Trans Am Ophthalmol Soc. 2005;103:148–162. [PMC free article] [PubMed] [Google Scholar]
- 175.Bradley JM, Anderssohn AM, Colvis CM, Parshley DE, Zhu XH, et al. Mediation of laser trabeculoplasty-induced matrix metalloproteinase expression by IL-1beta and TNFalpha. Invest Ophthalmol Vis Sci. 2000;41:422–430. [PubMed] [Google Scholar]
- 176.Kelley MJ, Rose AY, Song K, Chen Y, Bradley JM, et al. Synergism of TNF and IL-1 in the induction of matrix metalloproteinase-3 in trabecular meshwork. Invest Ophthalmol Vis Sci. 2007;48:2634–2643. doi: 10.1167/iovs.06-1445. [DOI] [PubMed] [Google Scholar]
- 177.Stamer WD, Peppel K, O’Donnell ME, Roberts BC, Wu F, et al. Expression of aquaporin-1 in human trabecular meshwork cells: role in resting cell volume. Invest Ophthalmol Vis Sci. 2001;42:1803–1811. [PubMed] [Google Scholar]
- 178.Al-Aswad LA, Gong H, Lee D, O’Donnell ME, Brandt JD, et al. Effects of Na-K-2CI cotransport regulators on outflow facility in calf and human eyes in vitro. Invest Ophthalmol Vis Sci. 1999;40:1695–1701. [PubMed] [Google Scholar]
- 179.Gual A, Llobet A, Gilabert R, Borras M. Effects of time of storage, albumin, and osmolality changes on outflow facility (C) of bovine anterior segment in vitro. Invest Ophthalmol Vis Sci. 1997;38:2165–2171. [PubMed] [Google Scholar]
- 180.Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 181.Tradtrantip L, Tajima M, Li L, Verkman AS. Aquaporin water channels in transepithelial fluid transport. J Med Invest. 2009;56:179–184. doi: 10.2152/jmi.56.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Naka M, Kanamori A, Negi A, Nakamura M. Reduced expression of aquaporin-9 in rat optic nerve head and retina following elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2010;51:4618–4626. doi: 10.1167/iovs.09-4712. [DOI] [PubMed] [Google Scholar]
- 183.Flammer J, Haefliger IO, Orgul S, Resnick T. Vascular deregulation: a principal risk factor for glaucomatous damage? J Glaucoma. 1999;8:212–219. [PubMed] [Google Scholar]
- 184.Dunn CJ (1995) Cytokines as mediators of chronic inflammatory disease. In: Kimball ES (ed) Cytokines and inflammation. CRC, London, pp 1–34
- 185.Mercurio F, Manning AM. NF-κB as a primary regulator of the stress response. Oncogene. 1999;18:6163–6171. doi: 10.1038/sj.onc.1203174. [DOI] [PubMed] [Google Scholar]
- 186.Itoh H, Nakao K. Vascular stress response and endothelial vasoactive factors for vascular remodeling. Diabetes Res Clin Pract. 1999;45:83–88. doi: 10.1016/s0168-8227(99)00035-2. [DOI] [PubMed] [Google Scholar]
- 187.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 188.Chua J, Vania M, Cheung CM, Ang M, Chee SP, et al. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Mol Vis. 2012;18:431–438. [PMC free article] [PubMed] [Google Scholar]
- 189.Shifera AS, Trivedi S, Chau P, Bonnemaison LH, Iguchi R, et al. Constitutive secretion of chemokines by cultured human trabecular meshwork cells. Exp Eye Res. 2010;91:42–47. doi: 10.1016/j.exer.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 190.Alvarado JA, Katz LJ, Trivedi S, Shifera AS. Monocyte modulation of aqueous outflow and recruitment to the trabecular meshwork following selective laser trabeculoplasty. Arch Ophthalmol. 2010;128:731–737. doi: 10.1001/archophthalmol.2010.85. [DOI] [PubMed] [Google Scholar]
- 191.Yan DB, Trope GE, Ethier CR, Menon A, Wakeham A. Effects of hydrogen peroxide-induced oxidative damage on outflow facility and washout in pig eyes. Invest Ophthal Vis Sci. 1991;32:2515–2520. [PubMed] [Google Scholar]
- 192.Padgaonkaret V, Giblin FJ, Leverenz V, Lin LR, Reddy VN. Studies of H2O2-induced effects on cultured bovine trabecular meshwork cells. J Glaucoma. 1994;3:123–131. [PubMed] [Google Scholar]
- 193.Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res. 2000;19(271):295. doi: 10.1016/s1350-9462(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 194.Wiederholt M. New aspects in acueous humor dynamics. In: Orgul S, Flammer J, editors. Pharmacotherapy in glaucoma. Bern: Hans Huber; 2000. pp. 65–72. [Google Scholar]
- 195.Hann CR, Fautsch MP. Preferential fluid flow in the human trabecular meshwork near collector channels. Invest Ophthalmol Vis Sci. 2009;50:1692–1697. doi: 10.1167/iovs.08-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhou L, Fukuchi T, Kawa JE, Higginbotham EJ, Yue BY. Loss of cell-matrix cohesiveness after phagocytosis by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1995;36:787–795. [PubMed] [Google Scholar]
- 197.Calthorpe CM, Grierson I. Fibronectin induces migration of bovine trabecular meshwork cells in vitro. Exp Eye Res. 1990;51:39–48. doi: 10.1016/0014-4835(90)90168-t. [DOI] [PubMed] [Google Scholar]
- 198.Stumpff F, Wiederholt M. Regulation of trabecular meshwork contractility. Ophthalmologica. 2000;214:33–53. doi: 10.1159/000027471. [DOI] [PubMed] [Google Scholar]
- 199.Yue BY. The extracellular matrix and its modulation in the trabecular meshwork. Surv Ophthalmol. 1996;40:379–390. doi: 10.1016/s0039-6257(96)80066-x. [DOI] [PubMed] [Google Scholar]
- 200.Zhou L, Cheng EL, Rege P, Yue BY. Signal transduction mediated by adhesion of human trabecular meshwork cells to extracellular matrix. Exp Eye Res. 2000;70:457–465. doi: 10.1006/exer.1999.0806. [DOI] [PubMed] [Google Scholar]
- 201.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 202.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 203.Drummond HA. The (F)low down on the endothelial epithelial sodium channel: epithelial sodium channel as a brake on flow-mediated vasodilation. Hypertension. 2009;53:903–904. doi: 10.1161/HYPERTENSIONAHA.109.128868. [DOI] [PubMed] [Google Scholar]
- 204.Dyka FM, May CA, Enz R. Subunits of the epithelial sodium channel family are differentially expressed in the retina of mice with ocular hypertension. J Neurochem. 2005;94:120–128. doi: 10.1111/j.1471-4159.2005.03177.x. [DOI] [PubMed] [Google Scholar]
- 205.Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001;7:304–309. doi: 10.1038/85446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Saccà SC, Centofanti M, Izzotti A. New proteins as vascular biomarkers in primary open angle glaucomatous aqueous humor. Invest Ophthalmol Vis Sci. 2012;53:4242–4253. doi: 10.1167/iovs.11-8902. [DOI] [PubMed] [Google Scholar]
- 207.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 208.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 209.Minuz P, Fava C, Lechi A. Lipid peroxidation, isoprostanes and vascular damage. Pharmacol Rep. 2006;58:57–68. [PubMed] [Google Scholar]
- 210.Napoli C, Ignarro LJ. Nitric oxide and atherosclerosis. Nitric Oxide. 2001;5:88–97. doi: 10.1006/niox.2001.0337. [DOI] [PubMed] [Google Scholar]
- 211.Daiber A, Oelze M, Wenzel P, Wickramanayake JM, Schuhmacher S, et al. Nitrate tolerance as a model of vascular dysfunction: roles for mitochondrial aldehyde dehydrogenase and mitochondrial oxidative stress. Pharmacol Rep. 2009;61:33–48. doi: 10.1016/s1734-1140(09)70005-2. [DOI] [PubMed] [Google Scholar]
- 212.Ruschitzka F, Quaschning T, Noll G, deGottardi A, Rossier MF, et al. Endothelin 1 type a receptor antagonism prevents vascular dysfunction and hypertension induced by 11beta-hydroxysteroid dehydrogenase inhibition: role of nitric oxide. Circulation. 2001;103:3129–3135. doi: 10.1161/01.cir.103.25.3129. [DOI] [PubMed] [Google Scholar]
- 213.Rimbach G, Valacchi G, Canali R, Virgili F. Macrophages stimulated with IFN-γ activate NF-κB and induce MCP-1 gene expression in primary human endothelial cells. Mol Cell Biol Res Commun. 2000;3:238–242. doi: 10.1006/mcbr.2000.0219. [DOI] [PubMed] [Google Scholar]
- 214.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-κB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 215.Dela Paz NG, Simeonidis S, Leo C, Rose DW, Collins T. Regulation of NF-κB-dependent gene expression by the POU domain transcription factor. J Biol Chem. 2007;282:8424–8434. doi: 10.1074/jbc.M606923200. [DOI] [PubMed] [Google Scholar]
- 216.Doganay S, Evereklioglu C, Turkoz Y, Er H. Decreased nitric oxide production in primary open-angle glaucoma. Eur J Ophthalmol. 2002;12:44–48. doi: 10.1177/112067210201200109. [DOI] [PubMed] [Google Scholar]
- 217.Dismuke WM, Mbadugha CC, Ellis DZ. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol. 2008;294:C1378–C1386. doi: 10.1152/ajpcell.00363.2007. [DOI] [PubMed] [Google Scholar]
- 218.Dismuke WM, Ellis DZ. Activation of the BKCa channel increases outflow facility and decreases trabecular meshwork cell. J Ocul Pharmacol Ther. 2009;25:309–314. doi: 10.1089/jop.2008.0133. [DOI] [PubMed] [Google Scholar]
- 219.Ellis DZ, Nathanson JA, Rabe J, Sweadner KJ. Carbachol and nitric oxide inhibition of Na, K-ATPase activity in bovine ciliary processes. Invest Ophthalmol Vis Sci. 2001;42:2625–2631. [PubMed] [Google Scholar]
- 220.Shahidullah M, Delamere NA. NO donors inhibit Na, K-ATPase activity by a protein kinase G-dependent mechanism in the nonpigmented ciliary epithelium of the porcine eye. Br J Pharmacol. 2006;148:871–880. doi: 10.1038/sj.bjp.0706795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kotikoski H, Alajuuma P, Moilanen E, Salmenperä P, et al. Comparison of nitric oxide donors in lowering intraocular pressure in rabbits: role of cyclic GMP. J Ocul Pharmacol Ther. 2002;18:11–23. doi: 10.1089/108076802317233171. [DOI] [PubMed] [Google Scholar]
- 222.Haynes WG, Webb DJ. Endothelin as a regulator of cardiovascular function in health and disease. J Hypertens. 1998;16:1081–1098. doi: 10.1097/00004872-199816080-00001. [DOI] [PubMed] [Google Scholar]
- 223.Rosenthal R, Fromm M. Endothelin antagonism as an active principle for glaucoma therapy. Br J Pharmacol. 2011;162:806–816. doi: 10.1111/j.1476-5381.2010.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Noske W, Hensen J, Wiederholt M. Endothelin-like immunoreactivity in aqueous humor of patients with primary open angle glaucoma and cataract. Graefes Arch Clin Exp Ophthalmol. 1997;235:551–552. doi: 10.1007/BF00947082. [DOI] [PubMed] [Google Scholar]
- 225.Tezel G, Kass MA, Kolker AE, Becker B, Wax MB. Plasma and aqueous humor endothelin levels in primary open angle glaucoma. J Glaucoma. 1997;6:83–89. [PubMed] [Google Scholar]
- 226.Buckley C, Hadoke PW, Henry E, O’Brien C. Systemic vascular endothelial cell dysfunction in normal pressure glaucoma. Br J Ophthalmol. 2002;86:227–232. doi: 10.1136/bjo.86.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Henry E, Newby DE, Webb DJ, O’Brien C. Peripheral endothelial dysfunction in normal pressure glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1710–1714. [PubMed] [Google Scholar]
- 228.Henry E, Newby DE, Webb DJ, Hadoke PW, O’Brien CJ. Altered endothelin-1 vasoreactivity in patients with untreated normal-pressure glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2528–2532. doi: 10.1167/iovs.05-0240. [DOI] [PubMed] [Google Scholar]
- 229.Yorio T, Krishnamoorthy R, Prasanna G. Endothelin: is it a contributor to glaucoma pathophysiology? J Glaucoma. 2002;11:259–270. doi: 10.1097/00061198-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 230.Redmond EM, Cahill PA, Hodges R, Zhang S, Sitzmann JV. Regulation of endothelin receptors by nitric oxide in cultured rat vascular smooth muscle cells. J Cell Physiol. 1996;166:469–479. doi: 10.1002/(SICI)1097-4652(199603)166:3<469::AID-JCP1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 231.Su WW, Cheng ST, Ho WJ, Tsay PK, Wu SC, Chang SH. Glaucoma is associated with peripheral vascular endothelial dysfunction. Ophthalmology. 2008;115:1173–1178. doi: 10.1016/j.ophtha.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 232.Siasos G, Tousoulis D, Siasou G, Moschos MM, Oikonomou E, et al. The association between glaucoma, vascular function and inflammatory process. Int J Cardiol. 2011;146:113–115. doi: 10.1016/j.ijcard.2010.09.083. [DOI] [PubMed] [Google Scholar]
- 233.Good TJ, Kahook MY. The role of endothelin in the pathophysiology of glaucoma. Expert Opin Ther Targets. 2010;14:647–654. doi: 10.1517/14728222.2010.487065. [DOI] [PubMed] [Google Scholar]
- 234.Luna C, Li G, Liton PB, Qiu J, Epstein DL, et al. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem Toxicol. 2009;47:198–204. doi: 10.1016/j.fct.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tamm ER (2008) Molecular approaches to glaucoma: intriguing clues for pathology. In: Mortimer C (ed) Current topics in membranes. Elsevier, Amsterdam, pp 379–425
- 236.Izzotti A, Centofanti M, Saccà SC. Molecular diagnostic of ocular diseases: the application of antibody microarray. Exp Rev Mol Diagn. 2012;12:629–643. doi: 10.1586/erm.12.57. [DOI] [PubMed] [Google Scholar]
- 237.He Y, Leung KW, Zhuo Y-H, Ge J. Pro370Leu mutant myocilin impairs mitochondrial functions in human trabecular meshwork cells. Mol Vis. 2009;15:815–825. [PMC free article] [PubMed] [Google Scholar]
- 238.Shen X, Koga T, Park BC, SundarRaj N, Yue BY. Rho GTPase and cAMP/protein kinase A signaling mediates myocilin-induced alterations in cultured human trabecular meshworkcells. J Biol Chem. 2008;283:603–612. doi: 10.1074/jbc.M708250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Joe MK, Tomarev SI. Expression of myocilin mutants sensitizes cells to oxidative stress-induced apoptosis: implication for glaucoma pathogenesis. Am J Pathol. 2010;176:2880–2890. doi: 10.2353/ajpath.2010.090853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Koga T, Shen X, Park JS, Qiu Y, Park BC, Shyam R, Yue BY. Differential effects of myocilin and optineurin, two glaucoma genes, on neurite outgrowth. Am J Pathol. 2010;176:343–352. doi: 10.2353/ajpath.2010.090194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kroeber M, Ohlmann A, Russell P, Tamm ER. Transgenic studies on the role of optineurin in the mouse eye. Exp Eye Res. 2006;82:1075–1085. doi: 10.1016/j.exer.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 242.Vittow J, Borras T. Expression of optineurin, a glaucoma-linked gene, is influenced by elevated intraocular pressure. Biochem Biophys Res Commun. 2002;298:67–74. doi: 10.1016/s0006-291x(02)02395-1. [DOI] [PubMed] [Google Scholar]
- 243.Sudhakar C, Nagabhushana A, Jain N, Swarup G. NF-kappaB mediates tumor necrosis factor alpha-induced expression of optineurin, a negative regulator of NF-kappaB. PLoS ONE. 2009;4:e5114. doi: 10.1371/journal.pone.0005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Park BC, Tibudan M, Samaraweera M, Shen X, Yue BY. Interaction between two glaucoma genes, optineurin and myocilin. Genes Cells. 2007;12:969–979. doi: 10.1111/j.1365-2443.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 245.Fernández-Durango R, Fernández-Martínez A, García-Feijoo J, Castillo A, de la Casa JM, et al. Expression of nitrotyrosine and oxidative consequences in the trabecular meshwork of patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2008;49:2506–2511. doi: 10.1167/iovs.07-1363. [DOI] [PubMed] [Google Scholar]
- 246.Johnson DH, Richardson TM, Epstein DL. Trabecular meshwork recovery after phagocytic challenge. Curr Eye Res. 1989;8:1121–1130. doi: 10.3109/02713688909000037. [DOI] [PubMed] [Google Scholar]
- 247.Grierson I. What is open angle glaucoma? Eye. 1987;1:15–28. doi: 10.1038/eye.1987.3. [DOI] [PubMed] [Google Scholar]
- 248.Ju WK, Kim KY, Lindsey JD, Angert M, Patel A, et al. Elevated hydrostatic pressure triggers release of OPA1 and cytochrome C, and induces apoptotic cell death in differentiated RGC-5 cells. Mol Vis. 2009;15:120–134. [PMC free article] [PubMed] [Google Scholar]
- 249.Johnson M, Gong H, Freddo TF, Ritter N, Kamm R. Serum proteins and aqueous outflow resistance in bovine eyes. Invest Ophthalmol Vis Sci. 1993;34:3549–3557. [PubMed] [Google Scholar]
- 250.Smith PJ, Samuelson DA, Brooks DE, Whitley RD. Unconventional aqueous humor outflow of microspheres perfused into the equine eye. Am J Vet Res. 1986;47:2445–2453. [PubMed] [Google Scholar]
- 251.Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioassays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Steely HT, Jr, English-Wright SL, Clark AF. The similarity of protein expression in trabecular meshwork and lamina cribrosa: implications for glaucoma. Exp Eye Res. 2000;70:17–30. doi: 10.1006/exer.1999.0764. [DOI] [PubMed] [Google Scholar]
- 253.Chowdhury U, Madden BJ, Charlesworth MC, Fautsch MP. Proteome analysis of aqueous humor. Invest Ophthamol Vis Sci. 2010;51:4921–4931. doi: 10.1167/iovs.10-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yu J, Liu F, Cui SJ, Liu Y, Song ZY, et al. Vitreous proteomic analysis of proliferative vitreoretinopathy. Proteomics. 2008;8:3667–3678. doi: 10.1002/pmic.200700824. [DOI] [PubMed] [Google Scholar]
- 255.Wang WH, McNatt LG, Pang IH, Millar JC, Hellberg PE, et al. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J Clin Invest. 2008;118:1056–1064. doi: 10.1172/JCI33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lin HJ, Tsai FJ, Hung P, Chen WC, Chen HY, et al. Association of E-cadherin gene 3′-UTR C/T polymorphism with primary open angle glaucoma. Ophthalmic Res. 2006;38:44–48. doi: 10.1159/000089523. [DOI] [PubMed] [Google Scholar]
- 257.Lee HS, Tomarev SI. Optimedin induces expression of N-cadherin and stimulates aggregation of NGF-stimulated PC12 cells. Exp Cell Res. 2007;13:98–108. doi: 10.1016/j.yexcr.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Surgucheva I, Surguchov A. Expression of caveolin in trabecular meshwork cells and its possible implication in pathogenesis of primary open angle glaucoma. Mol Vis. 2011;17:2878–2888. [PMC free article] [PubMed] [Google Scholar]
- 259.Khurana RN, Deng PF, Epstein DL, Vasantha Rao P. The role of protein kinase C in modulation of aqueous humor outflow facility. Exp Eye Res. 2003;76:39–47. doi: 10.1016/s0014-4835(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 260.Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, et al. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003;14:S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- 261.Lin D, Takemoto DJ. Oxidative activation of protein kinase Cgamma through the C1 domain. Effects on gap junctions. J Biol Chem. 2005;280:13682–13693. doi: 10.1074/jbc.M407762200. [DOI] [PubMed] [Google Scholar]
- 262.Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, et al. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKC alpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79:393–403. doi: 10.1016/j.exer.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 263.Kim YH, Kim YS, Park CH, Chung IY, Yoo JM, et al. Protein kinase C-delta mediates neuronal apoptosis in the retinas of diabetic rats via the Akt signaling pathway. Diabetes. 2008;57:2181–2190. doi: 10.2337/db07-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bagnis A, Izzotti A, Centofanti M, Saccà SC. Aqueous humor oxidative stress proteomic levels in primary open angle glaucoma. Exp Eye Res. 2012;103:55–62. doi: 10.1016/j.exer.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 265.Sit AJ, Gong H, Ritter N, Freddo TF, Kamm R, et al. The role of soluble proteins in generating aqueous outflow resistance in the bovine and human eye. Exp Eye Res. 1997;64:813–821. doi: 10.1006/exer.1997.0276. [DOI] [PubMed] [Google Scholar]
- 266.Hu DN, Ritch R, Liebmann J, Liu Y, Cheng B, et al. Vascular endothelial growth factor is increased in aqueous humor of glaucomatous eyes. J Glaucoma. 2002;11:406–410. doi: 10.1097/00061198-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 267.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 268.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 269.Resch H, Garhofer G, Fuchsjäger-Mayrl G, Hommer A, Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87:4–12. doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 270.Lo WR, Rowlette LL, Caballero M, Yang P, Hernandez MR, et al. Tissue differential microarray analysis of dexamethasone induction reveals potential mechanisms of steroid glaucoma. Invest Ophthalmol Vis Sci. 2003;44:473–485. doi: 10.1167/iovs.02-0444. [DOI] [PubMed] [Google Scholar]
- 271.Esson DW, Popp MP, Liu L, Schultz GS, Sherwood MB. Microarray analysis of the failure of filtering blebs in a rat model of glaucoma filtering surgery. Invest Ophthalmol Vis Sci. 2004;45:4450–4462. doi: 10.1167/iovs.04-0375. [DOI] [PubMed] [Google Scholar]
- 272.Steele MR, Inman DM, Calkins DJ, Horner PJ, Vetter ML. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:977–985. doi: 10.1167/iovs.05-0865. [DOI] [PubMed] [Google Scholar]
- 273.Colak D, Morales J, Bosley TM, Al-Bakheet A, Alyounes B, et al. Genome-wide expression profiling of patients with primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2012;18:431–438. doi: 10.1167/iovs.12-9634. [DOI] [PubMed] [Google Scholar]
- 274.Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol Vis. 2009;15:2488–2497. [PMC free article] [PubMed] [Google Scholar]
- 275.Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. MicroRNA-24 regulates the processing of latent TGFβ1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J Cell Physiol. 2011;226:1407–1414. doi: 10.1002/jcp.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.