Abstract
This review discusses a potential role of galectins and the renin–angiotensin system (RAS) in the pathophysiology of preeclampsia (PE). Preeclampsia affects between 3 and 5 % of all pregnancies and is a heterogeneous disease, which may be caused by multiple factors. The only cure is the delivery of the placenta, which may result in a premature delivery and baby. Probably due to its heterogeneity, PE studies in human have hitherto only led to the identification of a limited number of factors involved in the pathogenesis of the disease. Animal models, particularly in mice and rats, have been used to gain further insight into the molecular pathology behind PE. In this review, we discuss the picture emerging from human and animal studies pointing to galectins and the RAS being associated with the PE syndrome and affecting a broad range of cellular signaling components. Moreover, we review the epidemiological evidence for PE increasing the risk of future cardiovascular disease later in life.
Keywords: Preeclampsia, Galectins, Acute atherosis, Renin–angiotensin system
Preeclampsia: a heterogeneous syndrome
Preeclampsia (PE) is among the leading causes of maternal death, perinatal morbidity and mortality worldwide. Pregnancy hypertension complicates 10 % of all pregnancies. Of these, PE is a syndrome affecting 3–5 % of all pregnancies, with new hypertension and de novo proteinuria developing in the second half of pregnancy [1]. PE is responsible for more than 50,000 maternal deaths annually, the majority of which occur in developing countries [2]. The only present “cure” for PE is delivery of the placenta, and thereby the baby, often necessitating a premature delivery, with subsequent augmented short-term morbidity and mortality for the newborn. Women who develop PE are at increased risk of cardiovascular disease later in life. Also, children born of preeclamptic pregnancies run increased risk for adolescent hypertension and adult cardiovascular disease.
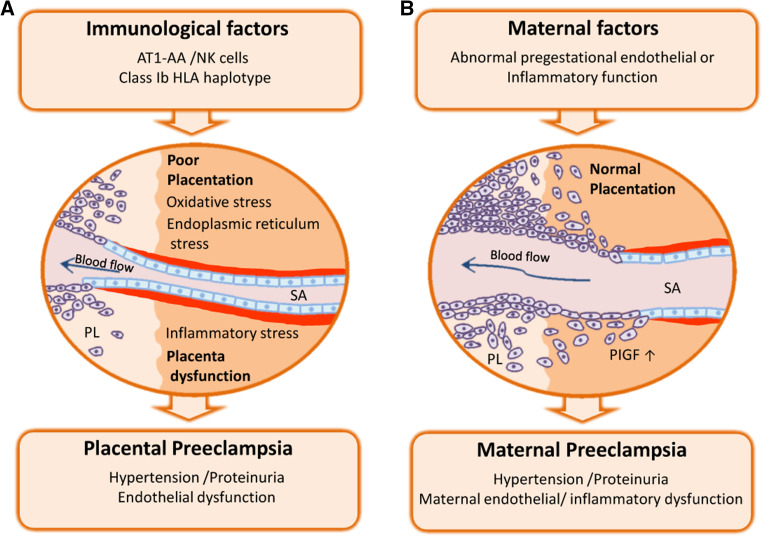
The exact etiology for PE remains unknown, but a 3-stage model is suggested for the “placental type” of PE [3, 4] illustrated in Fig. 1a. Dysregulated immunological factors (stage 1) underlying a defect placentation with reduced invasion of fetal extravillous trophoblast cells and reduced remodeling of maternal uteroplacental spiral arteries (stage 2) are initial pathophysiological events. An unfavorable uteroplacental circulation ensues, with enhanced oxidative, endoplasmic reticulum stress and release of placental factors to the maternal circulation. This causes an excessive maternal inflammatory response and endothelial dysfunction, thereby inducing the maternal clinical signs of PE with hypertension and proteinuria (stage 3) [1]. This multistep concept has recently been extended to include our present understanding of the uteroplacental vascular inflammatory lesion named “acute atherosis”, often present in preeclamptic pregnancies [5] and possibly linked to disease severity and perinatal outcome [6].
Fig. 1.
Preeclampsia: two different etiologies for a complex disease. PE is characterized by new onset hypertension and proteinuria in the mother, which result from diffuse endothelial and inflammatory dysfunction. In placental PE (a), dysregulated immune interactions with trophoblast cells impair the placentation process, with defective remodeling of the uteroplacental spiral arteries. Placental dysfunction is assocaited with enhanced oxidative, endoplasmic reticulum stress and release of inflammatory mediators to the material circulation, thereby including the clinical signs. Preeclampsia can also ensue when placental development is normal, as a result of pregestational conditions associated with maternal vascular inflammation and endothelial dysfunction (i.e., diabetes, obesity and chronic hypertension). In such cases of maternal PE (b), the syndrome arises as a consequence of an unusual susceptibility to the otherwise normal burden of placenta-derived inflammatory factors. SA spiral artery, PlGF placental growth factor, NK natural killer cells
In PE, new hypertension and proteinuria arise secondarily to diffuse maternal endothelial and inflammatory dysfunction. The placenta imposes an increasing inflammatory stress, even in normal pregnancy. In PE the inflammatory burden is excessive [3]. Placental dysfunction arises from altered perfusion of the uteroplacental circulation, due to deficient placentation occurring in the first half of pregnancy. Placentation involves a complex interaction between maternal immune cells in the uterine wall (named “decidua”, the pregnancy endometrium) and invasive trophoblasts, which are placental cells that express paternal HLA, restricted to HLA-C of the strong transplantation antigens. A normal interaction stimulates massive remodeling of the distal ends of the spiral arteries up to mid-gestation. Their media are entirely replaced by fibrinoid and their diameter increases from five to tenfold. In PE the spiral arteries are incompletely remodeled. Placental ischemia–reperfusion injury ensues [7], with oxidative and endoplasmic reticulum stress, and excess release of placental factors into the maternal circulation, causing the features of PE (reviewed in [8]).
Some women may develop PE despite normal placentation, possibly because they are unusually susceptible to the normal burden of placental-shed inflammatory factors. The new multistep model of preeclampsia depicts how women with chronic maternal vascular inflammation (such as in diabetes, obesity and chronic hypertension) could develop preeclampsia even when the placentation process appears normal, the so-called “maternal preeclampsia” [5] illustrated in Fig. 1b. However, our concepts of “placental” and maternal” causes of preeclampsia may have been incomplete, as these entities are not clinically well defined, nor is the decidual or placental tissue systematically investigated by postpartum unless for research purposes. A model of separated forms of maternal and placental preeclampsia is probably too abrupt, as maternal chronic disease is likely to impact on placentation and the early-onset form of preeclampsia. In this context, we recently suggested that there may be two placental causes of preeclampsia [9]: the first “extrinsic” placental mechanism is the dysfunctional remodeling of the uteroplacental spiral arteries feeding blood into the placenta intervillous space, the second placental mechanism could be “intrinsic” with restricted intervillous perfusion due to increasing placental size (and not due to remodeling problems of the uteroplacental spiral arteries per se).
An imbalance in maternal circulating angiogenic proteins as placenta-derived inflammatory molecules has been extensively investigated for more than a decade, with essential contributions by the Boston group of Karumanchi [10]. Elevated soluble vascular endothelial growth receptor-1 (sVEGFR-1 also known as sFlt-1) and soluble endoglin (sEng), with reduced placental growth factor (PlGF), are proposed to induce the systemic vascular inflammation and maternal signs of preeclampsia, namely new onset hypertension and proteinuria [4]. However, although in early onset preeclampsia there is typically an excess of sFlt1 and sEng and a deficiency of PlGF, the late-onset PE form is less often characterized by low circulating levels of PlGF and elevated sFlt1 [4]. An angiogenic imbalance is therefore not likely to explain all features of the heterogeneous preeclampsia syndrome.
In summary, preeclampsia is a heterogeneous syndrome, presenting clinically in a variety of severity forms, affecting multiple maternal organs, placental function and fetal growth, with large impact on maternal and offspring health, both on short and long terms. The pathophysiology is also likely multifactorial and may differ between the clinical subgroups, which calls for improved molecular understanding of the syndrome, to progress with prophylactic, intervention and treatment innovations for the diverse target groups. In this review, we focus our discussion on less investigated aspects of the pathogenesis of PE, in particular on the contribution of galectins as well as the involvement of the renin–angiotensin system. In addition, we review how PE compromises the risk of future cardiovascular disease.
Galectins: a lectin family with potential role in the pathogenesis of PE
Galectins are characterized by β-galactoside binding affinity and the presence of an evolutionary conserved sequence, the carbohydrate recognition domain (CRD), which mediates their specific interaction with N-acetyllactosamine [Galβ(1-4)-GlcNAc]-enriched glycoconjugates [11]. Twelve from the 15 mammalian galectins identified to date are found in humans. Classification based on their molecular structure divides galectins into three main types: while some of these galectins contain one CRD and are biologically active as monomers (i.e. galectin-1, galectin-13) or as oligomers that aggregate though their non-lectin domain (galectin-3), others contain two CRDs connected by a short linker peptide (e.g. galectin-9). Being present in the cell cytoplasm, galectins exert intracellular functions modulating various processes including cell growth, differentiation, survival and migration [12]. In addition, some of these endogenous lectins translocate to the nucleus and participate in transcriptional regulation and mRNA splicing [12, 13]. However, galectins can be also found on the cell surface or secreted through a non-classical endoplasmic reticulum (ER)/Golgi-independent pathway to the extracellular compartment [14]. There galectins bind their carbohydrate ligands on cell surface or ECM molecules and regulate a diverse combination of biological functions such as cell adhesion, apoptosis, regulation of the innate and adaptive immune responses [13, 15]. We will now discuss each of the three galectins with potential function in the pathogenesis of PE.
Galectin-1
Galectin-1 (gal-1), the protein product encoded by the LGALS1 locus, was identified more than three decades ago as the first member of this conserved family of lectins [16]. As a prototype galectin, gal-1 consists of one CRD that can function as a monomer or a non-covalent homodimer, which exerts a wide variety of intra- and extracellular biological activities by engaging in protein–protein and protein–carbohydrate interactions, respectively [17]. The best described function of gal-1 is probably the modulation of immune responses, acting as a tolerogenic signal to control the proliferation, survival and activation of effector T cells and promote the secretion of Th2 cytokines [18–20]. Immune regulation by gal-1 plays a pivotal role in fetomaternal tolerance during pregnancy, where it has been shown to promote the generation of tolerogenic dendritic cells (DC) and modulate maternal T cell function in vivo [21]; as well as to favor immune escape mechanisms of human placental trophoblasts in vitro [22].
Gal-1 is probably the most abundant galectin expressed in female reproductive tissues, particularly in the ovary and the uterine endometrium [23, 24]. Endometrial gal-1 expression fluctuates with hormonal variations during the reproductive cycle and is further increased in decidual tissue, concomitant with a possible role in supporting decidual development and maintaining maternal immune homeostasis during pregnancy [22]. It has become increasingly evident, however, that the important pregnancy-protective role played by this lectin results not only from maternal sources of expression but also from regulatory functions exerted by fetal/placental derived gal-1 [25]. For instance, expression of gal-1 is detected in human pre-implantation embryos [22], localizing to both the inner cell mass and the trophectoderm that will eventually originate the different lineages of placental trophoblasts. Later on, placental gal-1 expression is differentially distributed in the villous and extravillous trophoblast cell lineages, suggesting a role in the modulation of trophoblast differentiation during placentation. In the first trimester, differentiation of villous cytotrophoblasts (CTB) along the syncytial pathway occurs together with an apparent loss of gal-1 expression [22, 26], and the lectin has been shown to stimulate cell fusion and syncytin expression by villous CTB in vitro [27]. On the other hand, gal-1 is able to stimulate adhesion and invasion of HTR-8/SVneo cells and primary extravillous trophoblasts (EVT) cultured in Matrigel [28], and increased gal-1 expression correlates with trophoblast differentiation along the invasive pathway [22, 29]. The important role of gal-1 as an endogenous modulator of EVT differentiation is further evidenced by its ability to modulate the expression of human leukocyte antigen (HLA)-G isoforms [22], which are considered a molecular signature of invasive trophoblasts and a chief feto-maternal tolerance strategy due to its suppressive effects on maternal CD8+ T lymphocytes and NK cells [30].
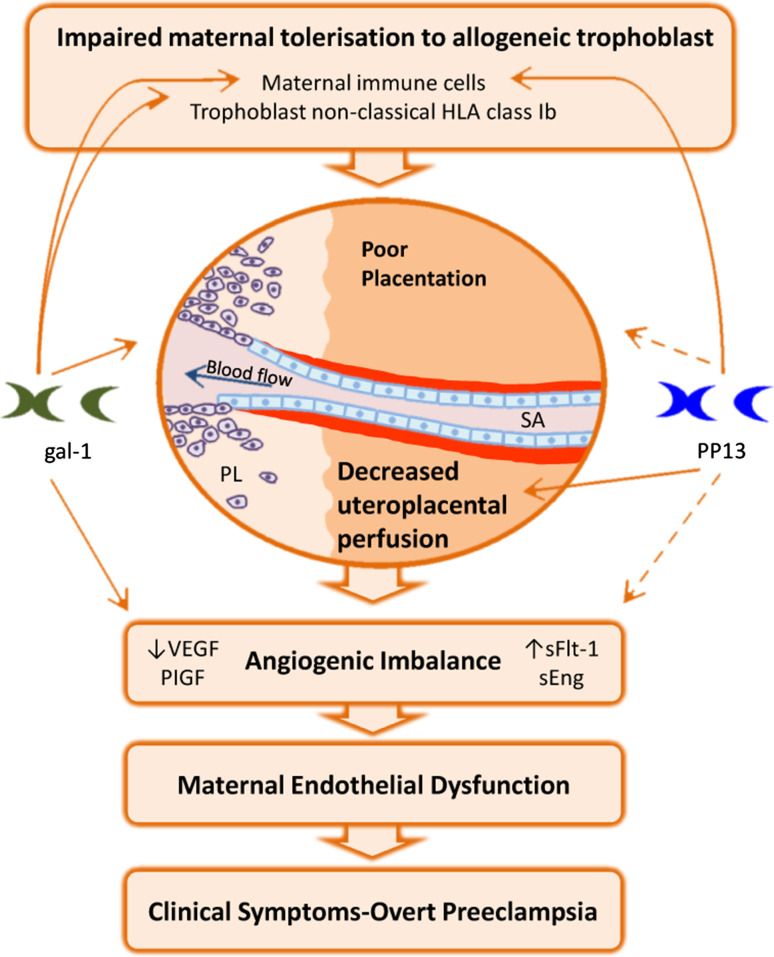
Another well-described function of gal-1 is its ability to promote angiogenic responses, which results from its effects on endothelial cell activation via H-Ras signaling [31] as well as from interaction with the neuropilin (NRP)-1/VEGFR2 signaling pathway enhancing several steps of the angiogenesis process including endothelial cell adhesion, migration and proliferation [32]. Signaling through VEGFR2 has been found critical for the physiological adaptation of the maternal vasculature to embryo implantation [33], which together with the high local expression of NRP-1 during peri-implantation stages [34] points to an important role of gal-1 mediated pathways in the control of pregnancy-associated angiogenesis. This was recently demonstrated in vivo in a mouse model of early pregnancy loss caused by attenuated decidual vascular expansion [35], by showing that administration of gal-1 promotes embryo survival associated with enhanced VEGFR2 activation [36]. Furthermore, treatment with anginex (an artificial β-peptide with potent anti-angiogenic effects targeting gal-1) resulted in decreased adhesion and capillary tube formation in SGHPL-4 EVT-like cells in vitro and impaired spiral artery remodeling and placental function in vivo, causing fetal growth restriction. Interestingly, these studies demonstrated that both anginex-treated and Lgals1-deficient pregnant mice developed similar preeclampsia-like symptoms during late gestation, suggesting that pro-angiogenic functions of the lectin are critical for healthy pregnancies (Fig. 2) [36].
Fig. 2.
Regulatory functions of galectins involved in the pathogenesis of PE. Both gal-1 and PP13 may paly an important immunomodulatory function during early stages of pregnancy by regulating maternal T cell survival and activation. Additionally, gal-1 appears to be important for the control of trophoblast lineage differentiation along the invasive pathway, thereby directly influencing the process of placentation and immunomodulation by inducing HLA-G expression on EVTcells. The precise function of PP13 during placentation is still elusive, but this lectin showed the ability to increase blood flow to the implantation site by influencing uteroplacental arterial remodeling. Pro-angiogenic properties of galectins and their interactions with the VEGF signaling pathway may, when deregulated, contribute to the angiogenic imbalance typical of the syndrome. This has been clearly demonstrated for gal-1 in vitro and in vivo, but direct effects of PP13 in the regulation of angiogenesis await further investigation
The important functions ascribed to gal-1 in placental development, maternal immune homeostasis and angiogenesis make this lectin a suitable candidate to play a significant role in the pathogenesis of preeclampsia. Indeed, pioneer studies have demonstrated an up-regulation of gal-1 expression in decidual tissue, villous trophoblast and EVT cells in preeclampsia and HELLP syndrome-derived biopsies [37, 38]. More recently, profiling studies of galectin expression in uteroplacental samples we have shown that gal-1 is the only member dysregulated in preeclampsia [36], implying that aberrant gal-1 biosynthesis is associated with the pathogenesis of this complex syndrome. These studies further showed that variations in placental gal-1 expression are opposite between early-onset and late-onset diseases, supporting the hypothesis that the two clinical entities have different etiologies. Thus, decreased expression of gal-1 in early-onset PE could be related to defective placentation, whereas its overexpression in the context of late-onset disease could represent a compensation mechanism to cope with the excessive inflammatory response that characterizes the syndrome. This is consistent with the switch toward decreased gal-1 expression observed in circulating T and NK cells from preeclampsia patients [39], which might contribute to increased immune cell activation further amplifying the maternal inflammatory response. Adding up to these evidences, the potential of gal-1 as a preclinical biomarker for the development of preeclampsia has recently emerged in a prospective study showing that circulating levels of the lectin during the second trimester are significantly decreased prior to the onset of the syndrome [36]. However, once the disease is diagnosed, serum levels of gal-1 are comparable between early-onset cases and controls and significantly lower than those observed in late-onset patients [36, 39], suggesting that determination of circulating gal-1 may not provide further diagnostic information in certain cases of preeclampsia. Since systemic gal-1 levels reflect both maternal and placental contributions, further studies in sufficiently large and homogeneous patient cohorts would greatly improve our understanding of its diagnostic value in preeclampsia.
Galectin-3
Galectin-3 (gal-3) is the only chimera-type member identified so far, consisting of a C-terminal CRD and an N-terminal domain of proline- and glycine-rich short tandem repeats responsible for multimerization and proteolytical regulation [40]. Like other galectins, gal-3 is abundantly expressed at the fetal maternal interface, showing a localization pattern that partially overlaps that of gal-1 [24, 41]. The interplay between these lectins may be important for fine-tuning the maternal immune response during early pregnancy, as gal-3 is mainly considered a pro-inflammatory signal promoting the activation, degranulation and cytokine secretion of diverse innate immune cell subsets [42, 43]. Gal-3 can also promote T cell proliferation and activation [42, 44], whereas its effect on T cell survival can be either stimulatory or inhibitory depending on its association with intracellular or extracellular targets [18, 45].
Despite considerable research profiling gal-3 expression at the fetal maternal interface of different species, the physiological relevance of this lectin during pregnancy is still ill-defined. During mouse pregnancy, gal-3 is selectively up-regulated on the uterine luminal epithelium and primary decidual zone at early stages, being later on predominantly expressed in placental tissue [46]. This pattern of distribution points to an important role in the embryo–maternal dialog driving implantation, which is further highlighted by the decreased implantation rates recently reported upon uterine tissue-specific knock-down of Lgals3 expression [47]. The spatiotemporal expression of this lectin in humans shows a quite similar pattern, being up-regulated in the late secretory phase endometrium and the decidua of early pregnancy and switching to placental villous and extravillous trophoblast as gestation progresses [24, 48]. Given the functional effects of gal-3 on cells of the innate immune system, one possibility is that it may play a regulatory function controlling the activation and cytotoxicity of uterine NK cells. Indeed, biosynthesis of gal-3 has been reported by mouse uterine NK cells [49] and it has been speculated that interaction with cubilin, an utero-placental-specific counterreceptor for this lectin, may reflect some mechanism for altering immune function by modifying the cytolytic potential of perforin-rich uterine NK cell granules [50]. Additionally, a role for gal-3 in the modulation of angiogenic responses has been demonstrated in a variety of physiopathological settings [51–54], which is anticipated to be significant for neovascularization responses associated with early pregnancy. Like gal-1, gal-3 has been shown to stimulate VEGF-mediated angiogenesis by retaining VEGFR2 on the plasma membrane of endothelial cells and promoting its phosphorylation [55], thereby enhancing exposure to its ligands and intracellular signal transduction. More recently, in vitro studies have suggested that angiogenic responses may be delicately modulated by an interplay between gal-1 and gal-3, as combined administration of both galectins not only induced mitogenic effects mediated by VEGFR2 activation but also promoted VEGFR1 phosphorylation, which is mainly involved in endothelial tube formation [56].
Besides pioneer work by Jeschke’s group showing a significant up-regulation of gal-1 and gal-3 expression in extravillous trophoblasts of preeclamptic and HELLP placentas [37], the implication of this lectin in the pathogenesis of preeclampsia remains largely unexplored. The immune regulatory and pro-angiogenic properties ascribed to gal-3 as well as its functional interactions with gal-1 mediated pathways merit further investigation in the context of pregnancy, making this lectin an attractive candidate for studies assessing the molecular basis of preeclampsia.
Galectin-13
Expression of this prototype galectin is largely restricted to the human placenta, where it was originally identified as 16-kDa protein with lysophospholipase activity named placental protein 13 (PP13) [57]. Synthesized mainly by the placental syncytiotrophoblast, PP13 is externalized to the cell surface and secreted in microvesicles associated with actin and annexin II [58] and like other placenta-specific galectins has been suggested to play an important role in feto-maternal tolerance by promoting apoptosis of activated T cells and macrophages [59]. Thus, PP13 is selectively associated with T cell-, neutrophil- and macrophage-rich decidual foci of necrosis [60], suggesting that it might act to attract, activate and kill maternal immune cells facilitating trophoblast invasion and conversion of the maternal spiral arterioles. While evidence supporting a role for PP13 per se in the regulation of angiogenic responses is still lacking, recent studies suggest that this lectin is important for the regulation of maternal vascular adaptations to pregnancy [61]. In this study, a single i.v. injection or continuous infusion of PP13 over 5 days showed hypotensive effects in pregnant rats, increasing heart rate and decreasing peripheral resistance due to general vasodilation. At day 20 of pregnancy PP13-infused animals displayed an increased utero-placental vascularity evidenced as larger arterial and venous diameters, suggesting that PP13 may be involved in generating a systemic endothelial effect (i.e., vascular remodeling and vasodilation) in the mother.
Although the physiological role played by PP13 during pregnancy is only emerging, several lines of evidence have highlighted its potential role in the pathogenesis of preeclampsia. For instance, decreased placental expression of LGALS13 mRNA and protein has been reported in both early- and late-onset disease patients respect to normal pregnant women [62]. This decreased expression contrasts with the high PP13 immunoreactivity observed in syncytiotrophoblast-derived cytoplasm protrusions, membrane blebs and microparticles of these patients, suggesting that preeclampsia may be associated with increased membrane shedding of PP13 to the maternal circulation. Indeed, circulating PP13 levels are increased during the third trimester in pregnancies complicated with preeclampsia and HELLP syndrome [62], which were assumed to result from increased shedding of syncytiotrophoblast-derived PP13 as observed for other brush border-localized placenta-specific proteins (i.e., PP5/TFPI-2) that are up-regulated during the course of preeclampsia [63]. Decreased placental LGALS13 expression has also been described during the first trimester in normal pregnancies that subsequently derived in preeclampsia [64], suggesting that PP13 may serve as an early diagnosis biomarker for this condition. The results of a recent meta-analysis covering studies published between 2006 and 2012 have strengthened this concept [65], showing that systemic PP13 levels are lower in the first trimester among women who will subsequently develop preeclampsia and that combined determination with other markers (i.e., PlGF, Doppler pulsatility index of uterine arteries) enhances their predictive value by increasing preeclampsia detection rates (Fig. 2) [66, 67]. In view of these findings the authors have speculated on the preventive and therapeutic potential of restoring PP13 levels during early pregnancy, an attractive possibility that requires further investigation given the still ill-defined biological effects of PP13 on gestation in vivo. In particular, while the proven vasodilatory and hypotensive effects of PP13 in pregnant rodents are undoubtedly promising, the same pilot study revealed unexpected psychopharmacological relaxation and behavioral changes in response to PP13 that encourage further research into possible neuromuscular or central nervous system effects exerted by this lectin [61].
Role of the renin–angiotensin system in the pathogenesis of preeclampsia
The renin–angiotensin system (RAS) is the major blood pressure regulating system in humans and although it participates in the development of preeclampsia, the mechanisms are poorly understood. Plasma renin activity (PRA) is increased in normal pregnancy [68] because of increased renin substrate [69], but PRA is actually decreased in preeclampsia [70]. We reported threefold higher PRA values in nonpreeclamptic women compared to preeclamptic women [71]. Angiotensin II (Ang II) levels are increased during normal pregnancy, but these women remain normotensive despite a twofold increase in Ang II. A key feature of normal pregnancy is decreased responsiveness to Ang II [72]. However, circulating Ang II, the effector molecule of the renin–angiotensin system, is decreased in PE [73]. Pregnant women who subsequently develop preeclampsia are highly sensitive to infused Ang II. Gant et al. infused Ang II into pregnant patients from first trimester onward and found that women who later developed preeclampsia required diminishing amounts of Ang II to obtain a similar pressor response, whereas pregnant women without preeclampsia are resistant [74].
We have previously described circulating agonistic autoantibodies directed at the angiotensin Ang II receptor AT1 in women with preeclampsia (AT1-AA) [75]. We showed that AT1-AA cross the ureteroplacental barrier, but were present only in fetuses of preeclamptic mothers [71]. Passive transfer of AT1-AA induces a preeclamptic phenotype in mice and rats, fulfilling Koch’s postulates for infectious agents [76, 77]. Infusion of purified rat AT1-AA into healthy pregnant rats induced hypertension and activated the endothelin system [76]. In similar experiments in mice Zhou et al. showed an induction of sFlt-1 leading to proteinuria and hypertension [77]. Recent data by the group of LaMarca indicate that AT1-AA are involved in an immunomodulatory function during pregnancy. Suppressing B-cells by rutiximab reduced AT1-AA and ameliorated the hypertension in an established rat model for preeclampsia induced by placental ischemia (RUPP model) [78]. RUPP induced CD4+ T cells, which upon adoptive transfer increased inflammation and AT1AA in normal pregnant rats [79]. Karumanchi summarized that AT1-AA may be an early event leading to the activation of a cascade, ending in the production of sFlt-1 [80]. However, prospective clinical studies are needed to evaluate whether AT1-AA are present before the onset of the clinical syndrome.
Recently we have learned that AT1-AA are not specific for preeclampsia. AT1-AA are found in patients with C4d-negative humoral rejection after kidney transplantation [81] in most patients with systemic sclerosis [82]. So far, AT1-AA in preeclamptic patients can only be detected functionally by a cardiac contraction assay [75], but for the other indications a commercially available ELISA (Thermofisher) can be used and has already a clinical utility [83].
The increased sensitivity to Ang II in preeclamptic patients persists postpartum [84, 85]. Saxena et al. showed that the increased Ang II sensitivity persisted in women with preeclampsia even after 21 months of pregnancy. Whereas women with a normotensive pregnancy modulated their response to Ang II on the basis of sodium intake, former preeclamptic women had high blood pressure responses to Ang II after both high- and low-salt diets. Hladunewich et al. showed that women with a history of severe early-onset preeclampsia had a blunted rise in circulating RAS mediators in response to simulated orthostatic stress using incremental lower body negative pressure as compared to controls 6–18 months postpartum [85]. These data underscore a dysregulation of the renin–angiotensin system in this population, which could be based on a genetic background. Interestingly, we have recently shown that a risk allele for hypertension also was a risk for PE in two Norwegian cohorts (RGS2 1114G allele), possibly contributing to the altered angiotensin II signaling of PE. Important for this study, the allele was more frequent in the group with acute atherosis [86]. Likewise, the polymorphism was also associated with increased risk of future hypertension in a Norwegian population-based cohort. Other mechanisms that have been proposed to result in increased Ang II responsiveness in preeclampsia include AT1-AA [87] heterodimerisation of AT1- and Bradykinin-receptors [88] and a redox state of Angiotensinogen [89].
Although there is agreement on the renin–angiotensin system (RAS) as a participant in the development of preeclampsia; the relative contributions from the circulating RAS and the tissue-based, uteroplacental RAS are unknown [90, 91]. During pregnancy, there is an additional tissue-based RAS in the uteroplacental unit, consisting of the placenta (fetal origin, with fetal cells) and the decidua (maternal origin, where extravillous trophoblasts invade the maternal endometrium of pregnancy, named decidua). Thus, there are actually two RAS in the uteroplacental unit: a maternal and a fetal one. In our previous study [71], we found that all components of the RAS, especially Renin, Angiotensinogen and ACE-1, are higher in the decidua compared to the placenta in normal pregnancy. In a subsequent study, we could show that local tissue Ang II stimulates trophoblast invasion in vivo in the rat and in vitro in human cells, a hitherto unrecognized function of the local RAS [92]. However, tissue RAS is also dysregulated in preeclampsia. Shah et al. introduced the hypothesis to look at preeclampsia as a pregnancy variation of the 2 Kidney–1 Clip Goldblatt model [93]. Due to the reduced perfusion of the uteroplacental unit in preeclampsia, it behaves like the “clipped kidney” and the two maternal kidneys being the “nonclipped kidney”. Anton et al. showed that RAS is dysregulated in chorionic villi in preeclamptic patients [94]. They found a threefold Ang II increase compared to chorionic villi from healthy pregnancies. Ang II was the predominant peptide in the chorionic villi, with a 30-fold higher concentration than Ang I. These data are in line with our earlier study, where we could show that the AT1 receptor is up to tenfold increased in the preeclamptic placenta [71].
Preeclampsia increases the risk of future cardiovascular disease (CVD)
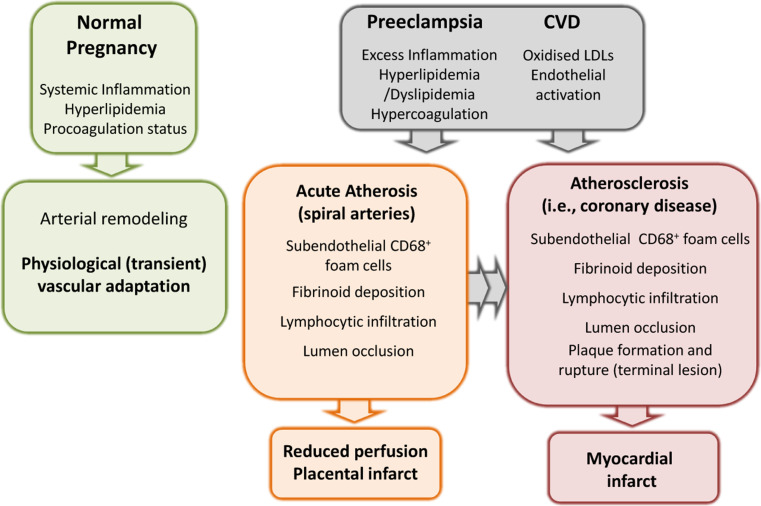
Cardiovascular disease (CVD) includes coronary heart disease, stroke, and other atherosclerotic conditions and is the leading cause of death both for men and women in developed countries and most emerging economies [95]. Women with PE, growth restricted fetus or diabetes in pregnancy run increased risk for later CVD [96–98]. The association strengthens with more severe PE [99], including early-onset PE (reviewed in [4]). Two theories are discussed that link PE and CVD. The first is that PE and atherosclerosis share risk factors for systemic inflammation and endothelial dysfunction, which are unmasked by the “stress” of pregnancy. Such factors include obesity, dyslipidemia, diabetes mellitus, other forms of insulin resistance, hypertension, endothelial dysfunction and a family history [8]. An important paradox is smoking, which is associated with an augmented risk of atherosclerosis and CVD, but a reduced risk of PE. The second possibility, which does not exclude the first, is that pregnancy and especially PE may induce permanent arterial changes, mediating risk for future CVD (Fig. 3).
Fig. 3.
Preeclampsia and the risk for future CVD. Normal pregnancy poses a maternal stress in terms of systemic inflammation, lipid mobilization and pro-coagulant activity, but vascular modifications are transient and contribute to physiological adaptation of the maternal organism to promote fetal growth. These features are exacerbated in PE, which together with increased oxidative stress and endothelial dysfunction are shared common predisposing factors for CVD. Acute atherosis lesions often present in the uteroplacental arteries of PE patients closely resemble the early phases of atherosclerotic lesion development, and may result in permanent vascular changes increasing the risk for CVD
Acute atherosis (AA) is an arterial lesion uniquely affecting the maternal uteroplacental spiral arteries. AA is seen in 20–40 % of cases of PE [100], not affecting the systemic maternal arteries. It also develops with other pregnancy complications and in 1 of 6 women with apparently normal pregnancies, including normal spiral artery remodeling [100]. It resembles early stages of atherosclerosis, with some striking similarity to coronary artery atherosclerotic lesions. Acute atherosis comprises subendothelial lipid-filled, CD68-positive foam cells [101], derived from macrophages and possibly smooth muscle cells (reviewed in [102]), associated with an inflammatory cell infiltrate. AA usually develops in the decidua (the endometrium of pregnancy), where the spiral arteries feed into the placenta. AA can sometimes also occur in cases of fetal growth restriction or certain autoimmune diseases [8]. Its foam cell features can also be seen in some uncomplicated pregnancies [100].
It is possible that the pro-atherogenic stress of PE could activate arterial wall inflammation that fails to resolve after delivery. Only subsets of women with preeclampsia have however the highest risk for severe or premature CVD (Fig. 3), and we lack the appropriate tools to identify them. We have recently proposed that postpartum detection of AA could be such a tool to identify a subset of women that are more susceptible for developing atherosclerotic disease and cardiovascular CVD later in life [102, 103]. If this was the case, more intensified prevention strategies could be offered to the women with highest CVD risk, thereby benefiting from pregnancy information to improve future maternal health. Women that have undergone pregnancy complications such as preeclampsia are today not routinely followed up after pregnancy for future cardiovascular risk despite general recommendations [95], and the effects of preventive strategies have not been evaluated. Also offspring of preeclamptic pregnancies run an increased risk of hypertension as adolescent [104–106], but the mechanisms are not fully known [107].
Future perspectives
We propose that further molecular dissection of the heterogeneity of preeclampsia is necessary to progress in the identification of novel prophylactic and therapeutic strategies. In this regard, we propose that further understanding of the role of galectins and RAS may improve pathophysiological understanding and hopefully aid to improve the short- and long-term outcome of preeclampsia both for mothers and offsprings worldwide.
Acknowledgments
We apologize to the many authors whose excellent papers could not be cited in this review for space limitations. The work discussed in this review was supported by Deutsche Forschungsgemeinschaft (DFG) Grant BL1115/2-1 and Fritz Thyssen Stiftung (Az. 10.10.2.125) to S.M.B. The Research Council of Norway and Regional Health Authorities (South-Eastern Norway) to A.C.S; and G.B. is supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina).
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63(6):534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 4.Staff AC, Benton SJ, von Dadelszen P, Roberts JM, Taylor RN, Powers RW, Charnock-Jones DS, Redman CW. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013;61(5):932–942. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 5.Staff AC, Johnsen GM, Dechend R, Redman CW. Preeclampsia and uteroplacental acute atherosis: immune and inflammatory factors. J Reprod Immunol. 2014;101–102:120–126. doi: 10.1016/j.jri.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Stevens DU, Al-Nasiry S, Bulten J, Spaanderman ME. Decidual vasculopathy in preeclampsia: lesion characteristics relate to disease severity and perinatal outcome. Placenta. 2013;34(9):805–809. doi: 10.1016/j.placenta.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia—novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010;56(6):1026–1034. doi: 10.1161/HYPERTENSIONAHA.110.157743. [DOI] [PubMed] [Google Scholar]
- 9.Redman CW, Sargent IL, Staff AC. IFPA Senior Award Lecture: making sense of pre-eclampsia—two placental causes of preeclampsia? Placenta. 2014;35(Suppl):S20–25. doi: 10.1016/j.placenta.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76(4):597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 12.Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572(2–3):263–273. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez JD, Baum LG. Ah, sweet mystery of death! Galectins and control of cell fate. Glycobiology. 2002;12(10):127R–136R. doi: 10.1093/glycob/cwf081. [DOI] [PubMed] [Google Scholar]
- 14.Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6(8):607–614. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 15.Rabinovich GA, Liu FT, Hirashima M, Anderson A. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand J Immunol. 2007;66(2–3):143–158. doi: 10.1111/j.1365-3083.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- 16.Hirabayashi J, Ayaki H, Soma G, Kasai K. Cloning and nucleotide sequence of a full-length cDNA for human 14 kDa beta-galactoside-binding lectin. Biochim Biophys Acta. 1989;1008(1):85–91. doi: 10.1016/0167-4781(89)90173-5. [DOI] [PubMed] [Google Scholar]
- 17.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16(11):137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 18.Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176(2):778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 19.Blaser C, Kaufmann M, Muller C, Zimmermann C, Wells V, Mallucci L, Pircher H. Beta-galactoside-binding protein secreted by activated T cells inhibits antigen-induced proliferation of T cells. Eur J Immunol. 1998;28(8):2311–2319. doi: 10.1002/(SICI)1521-4141(199808)28:08<2311::AID-IMMU2311>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Motran CC, Molinder KM, Liu SD, Poirier F, Miceli MC. Galectin-1 functions as a Th2 cytokine that selectively induces Th1 apoptosis and promotes Th2 function. Eur J Immunol. 2008;38(11):3015–3027. doi: 10.1002/eji.200838295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13(12):1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 22.Tirado-Gonzalez I, Freitag N, Barrientos G, Shaikly V, Nagaeva O, Strand M, Kjellberg L, Klapp BF, Mincheva-Nilsson L, Cohen M, Blois SM. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol Hum Reprod. 2013;19(1):43–53. doi: 10.1093/molehr/gas043. [DOI] [PubMed] [Google Scholar]
- 23.Choe YS, Shim C, Choi D, Lee CS, Lee KK, Kim K. Expression of galectin-1 mRNA in the mouse uterus is under the control of ovarian steroids during blastocyst implantation. Mol Reprod Dev. 1997;48(2):261–266. doi: 10.1002/(SICI)1098-2795(199710)48:2<261::AID-MRD14>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 24.von Wolff M, Wang X, Gabius HJ, Strowitzki T. Galectin fingerprinting in human endometrium and decidua during the menstrual cycle and in early gestation. Mol Hum Reprod. 2005;11(3):189–194. doi: 10.1093/molehr/gah144. [DOI] [PubMed] [Google Scholar]
- 25.Barrientos G, Freitag N, Tirado-Gonzalez I, Unverdorben L, Jeschke U, Thijssen VL, Blois SM. Involvement of galectin-1 in reproduction: past, present and future. Human Repro Update. 2014;20(2):175–193. doi: 10.1093/humupd/dmt040. [DOI] [PubMed] [Google Scholar]
- 26.Bevan BH, Kilpatrick DC, Liston WA, Hirabayashi J, Kasai K. Immunohistochemical localization of a beta-D-galactoside-binding lectin at the human maternofetal interface. Histochem J. 1994;26(7):582–586. doi: 10.1007/BF00158592. [DOI] [PubMed] [Google Scholar]
- 27.Fischer I, Redel S, Hofmann S, Kuhn C, Friese K, Walzel H, Jeschke U. Stimulation of syncytium formation in vitro in human trophoblast cells by galectin-1. Placenta. 2010;31(9):825–832. doi: 10.1016/j.placenta.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Kolundzic N, Bojić-Trbojević T, Kovaćević T, Stefanoska I, Kadoya T, Vićovac L. Galectin-1 is part of human trophoblast invasion machinery: a functional study in vitro. PloS One. 2011;6(12):e28514. doi: 10.1371/journal.pone.0028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aplin JD. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci. 1991;99(Pt 4):681–692. doi: 10.1242/jcs.99.4.681. [DOI] [PubMed] [Google Scholar]
- 30.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. Faseb J. 2005;19(7):681–693. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 31.Thijssen VL, Barkan B, Shoji H, Aries IM, Mathieu V, Deltour L, Hackeng TM, Kiss R, Kloog Y, Poirier F, Griffioen AW. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010;70(15):6216–6224. doi: 10.1158/0008-5472.CAN-09-4150. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh SH, Ying NW, Wu MH, Chiang WF, Hsu CL, Wong TY, Jin YT, Hong TM, Chen YL. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene. 2008;27(26):3746–3753. doi: 10.1038/sj.onc.1211029. [DOI] [PubMed] [Google Scholar]
- 33.Douglas NC, Tang H, Gomez R, Pytowski B, Hicklin DJ, Sauer CM, Kitajewski J, Sauer MV, Zimmermann RC. Vascular endothelial growth factor receptor 2 (VEGFR-2) functions to promote uterine decidual angiogenesis during early pregnancy in the mouse. Endocrinology. 2009;150(8):3845–3854. doi: 10.1210/en.2008-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halder JB, Zhao X, Soker S, Paria BC, Klagsbrun M, Das SK, Dey SK. Differential expression of VEGF isoforms and VEGF(164)-specific receptor neuropilin-1 in the mouse uterus suggests a role for VEGF(164) in vascular permeability and angiogenesis during implantation. Genesis. 2000;26(3):213–224. [PubMed] [Google Scholar]
- 35.Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Investig. 2008;118(12):3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freitag N, Tirado-Gonzalez I, Barrientos G, Herse F, Thijssen VL, Weedon-Fekjaer SM, Schulz H, Wallukat G, Klapp BF, Nevers T, Sharma S, Staff AC, Dechend R, Blois SM. Interfering with Gal-1-mediated angiogenesis contributes to the pathogenesis of preeclampsia. Proc Natl Acad Sci USA. 2013;110(28):11451–11456. doi: 10.1073/pnas.1303707110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeschke U, Mayr D, Schiessl B, Mylonas I, Schulze S, Kuhn C, Friese K, Walzel H. Expression of galectin-1, -3 (gal-1, gal-3) and the Thomsen–Friedenreich (TF) antigen in normal, IUGR, preeclamptic and HELLP placentas. Placenta. 2007;28(11–12):1165–1173. doi: 10.1016/j.placenta.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Than NG, Erez O, Wildman DE, Tarca AL, Edwin SS, Abbas A, Hotra J, Kusanovic JP, Gotsch F, Hassan SS, Espinoza J, Papp Z, Romero R. Severe preeclampsia is characterized by increased placental expression of galectin-1. J Matern Fetal Neonatal Med. 2008;21(7):429–442. doi: 10.1080/14767050802041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molvarec A, Blois SM, Stenczer B, Toldi G, Tirado-Gonzalez I, Ito M, Shima T, Yoneda S, Vasarhelyi B, Rigo J, Jr, Saito S. Peripheral blood galectin-1-expressing T and natural killer cells in normal pregnancy and preeclampsia. Clinical Immunol. 2011;139(1):48–56. doi: 10.1016/j.clim.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin–saccharide-mediated cellular interactions. Curr Opin Struct Biol. 2002;12(5):616–623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 41.Vicovac L, Jankovic M, Cuperlovic M. Galectin-1 and -3 in cells of the first trimester placental bed. Human Repro (Oxford, England) 1998;13(3):730–735. doi: 10.1093/humrep/13.3.730. [DOI] [PubMed] [Google Scholar]
- 42.Chen HY, Liu FT, Yang RY. Roles of galectin-3 in immune responses. Archivum Immunologiae et Therapiae Experimentalis. 2005;53(6):497–504. [PubMed] [Google Scholar]
- 43.Alves CM, Silva DA, Azzolini AE, Marzocchi-Machado CM, Carvalho JV, Pajuaba AC, Lucisano-Valim YM, Chammas R, Liu FT, Roque-Barreira MC, Mineo JR. Galectin-3 plays a modulatory role in the life span and activation of murine neutrophils during early Toxoplasma gondii infection. Immunobiology. 2010;215(6):475–485. doi: 10.1016/j.imbio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Iglesias MM, Rabinovich GA, Ambrosio AL, Castagna LF, Sotomayor CE, Wolfenstein-Todel C. Purification of galectin-3 from ovine placenta: developmentally regulated expression and immunological relevance. Glycobiology. 1998;8(1):59–65. doi: 10.1093/glycob/8.1.59. [DOI] [PubMed] [Google Scholar]
- 45.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93(13):6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips B, Knisley K, Weitlauf KD, Dorsett J, Lee V, Weitlauf H. Differential expression of two beta-galactoside-binding lectins in the reproductive tracts of pregnant mice. Biol Reprod. 1996;55(3):548–558. doi: 10.1095/biolreprod55.3.548. [DOI] [PubMed] [Google Scholar]
- 47.Yang H, Lei C, Zhang W. Expression of galectin-3 in mouse endometrium and its effect during embryo implantation. Repro Biomed Online. 2012;24(1):116–122. doi: 10.1016/j.rbmo.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Maquoi E, van den Brule FA, Castronovo V, Foidart JM. Changes in the distribution pattern of galectin-1 and galectin-3 in human placenta correlates with the differentiation pathways of trophoblasts. Placenta. 1997;18(5–6):433–439. doi: 10.1016/s0143-4004(97)80044-6. [DOI] [PubMed] [Google Scholar]
- 49.Lee VH, Lee AB, Phillips EB, Roberts JK, Weitlauf HM. Spatio-temporal pattern for expression of galectin-3 in the murine utero-placental complex: evidence for differential regulation. Biol Reprod. 1998;58(5):1277–1282. doi: 10.1095/biolreprod58.5.1277. [DOI] [PubMed] [Google Scholar]
- 50.Crider-Pirkle S, Billingsley P, Faust C, Hardy DM, Lee V, Weitlauf H. Cubilin, a binding partner for galectin-3 in the murine utero-placental complex. J Biol Chem. 2002;277(18):15904–15912. doi: 10.1074/jbc.M200331200. [DOI] [PubMed] [Google Scholar]
- 51.Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156(3):899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell. 2004;15(8):3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan SY, Zhang TF, Ding Y. Galectin-3 enhances proliferation and angiogenesis of endothelial cells differentiated from bone marrow mesenchymal stem cells. Transpl Proc. 2011;43(10):3933–3938. doi: 10.1016/j.transproceed.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 54.Machado CM, Andrade LN, Teixeira VR, Costa FF, Melo CM, dos Santos SN, Nonogaki S, Liu FT, Bernardes ES, Camargo AA, Chammas R. Galectin-3 disruption impaired tumoral angiogenesis by reducing VEGF secretion from TGFbeta1-induced macrophages. Cancer Med. 2014;3(2):201–214. doi: 10.1002/cam4.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markowska AI, Jefferies KC, Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem. 2011;286(34):29913–29921. doi: 10.1074/jbc.M111.226423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Haene N, Sauvage S, Maris C, Adanja I, Le Mercier M, Decaestecker C, Baum L, Salmon I. VEGFR1 and VEGFR2 involvement in extracellular galectin-1- and galectin-3-induced angiogenesis. PLoS One. 2013;8(6):e67029. doi: 10.1371/journal.pone.0067029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Than NG, Sumegi B, Than GN, Berente Z, Bohn H. Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13), a new lysophospholipase, homologue of human eosinophil Charcot–Leyden Crystal protein. Placenta. 1999;20(8):703–710. doi: 10.1053/plac.1999.0436. [DOI] [PubMed] [Google Scholar]
- 58.Than NG, Pick E, Bellyei S, Szigeti A, Burger O, Berente Z, Janaky T, Boronkai A, Kliman H, Meiri H, Bohn H, Than GN, Sumegi B. Functional analyses of placental protein 13/galectin-13. Eur J Biochem/FEBS. 2004;271(6):1065–1078. doi: 10.1111/j.1432-1033.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 59.Than NG, Romero R, Goodman M, Weckle A, Xing J, Dong Z, Xu Y, Tarquini F, Szilagyi A, Gal P, Hou Z, Tarca AL, Kim CJ, Kim JS, Haidarian S, Uddin M, Bohn H, Benirschke K, Santolaya-Forgas J, Grossman LI, Erez O, Hassan SS, Zavodszky P, Papp Z, Wildman DE. A primate subfamily of galectins expressed at the maternal–fetal interface that promote immune cell death. Proc Natl Acad Sci USA. 2009;106(24):9731–9736. doi: 10.1073/pnas.0903568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kliman HJ, Sammar M, Grimpel YI, Lynch SK, Milano KM, Pick E, Bejar J, Arad A, Lee JJ, Meiri H, Gonen R. Placental protein 13 and decidual zones of necrosis: an immunologic diversion that may be linked to preeclampsia. Repro Sci. 2012;19(1):16–30. doi: 10.1177/1933719111424445. [DOI] [PubMed] [Google Scholar]
- 61.Gizurarson S, Huppertz B, Osol G, Skarphedinsson JO, Mandala M, Meiri H. Effects of placental protein 13 on the cardiovascular system in gravid and non-gravid rodents. Fetal Diagn Ther. 2013;33(4):257–264. doi: 10.1159/000345964. [DOI] [PubMed] [Google Scholar]
- 62.Than NG, Abdul Rahman O, Magenheim R, Nagy B, Fule T, Hargitai B, Sammar M, Hupuczi P, Tarca AL, Szabo G, Kovalszky I, Meiri H, Sziller I, Rigo J, Jr, Romero R, Papp Z. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch. 2008;453(4):387–400. doi: 10.1007/s00428-008-0658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogawa M, Yanoma S, Nagashima Y, Okamoto N, Ishikawa H, Haruki A, Miyagi E, Takahashi T, Hirahara F, Miyagi Y. Paradoxical discrepancy between the serum level and the placental intensity of PP5/TFPI-2 in preeclampsia and/or intrauterine growth restriction: possible interaction and correlation with glypican-3 hold the key. Placenta. 2007;28(2–3):224–232. doi: 10.1016/j.placenta.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 64.Sekizawa A, Purwosunu Y, Yoshimura S, Nakamura M, Shimizu H, Okai T, Rizzo N, Farina A. PP13 mRNA expression in trophoblasts from preeclamptic placentas. Repro Sci Calif. 2009;16(4):408–413. doi: 10.1177/1933719108328615. [DOI] [PubMed] [Google Scholar]
- 65.Huppertz B, Meiri H, Gizurarson S, Osol G, Sammar M. Placental protein 13 (PP13): a new biological target shifting individualized risk assessment to personalized drug design combating pre-eclampsia. Human Repro Update. 2013;19(4):391–405. doi: 10.1093/humupd/dmt003. [DOI] [PubMed] [Google Scholar]
- 66.Nicolaides KH, Bindra R, Turan OM, Chefetz I, Sammar M, Meiri H, Tal J, Cuckle HS. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet Gynecol. 2006;27(1):13–17. doi: 10.1002/uog.2686. [DOI] [PubMed] [Google Scholar]
- 67.Wortelboer EJ, Koster MP, Cuckle HS, Stoutenbeek PH, Schielen PC, Visser GH. First-trimester placental protein 13 and placental growth factor: markers for identification of women destined to develop early-onset pre-eclampsia. BJOG. 2010;117(11):1384–1389. doi: 10.1111/j.1471-0528.2010.02690.x. [DOI] [PubMed] [Google Scholar]
- 68.Ganten D, Minnich JL, Granger P, Hayduk K, Brecht HM, Barbeau A, Boucher R, Genest J. Angiotensin-forming enzyme in brain tissue. Science. 1971;173(3991):64–65. doi: 10.1126/science.173.3991.64. [DOI] [PubMed] [Google Scholar]
- 69.Weinberger MH, Kramer NJ, Grim CE, Petersen LP. The effect of posture and saline loading on plasma renin activity and aldosterone concentration in pregnant, non-pregnant and estrogen-treated women. J Clin Endocrinol Metab. 1977;44(1):69–77. doi: 10.1210/jcem-44-1-69. [DOI] [PubMed] [Google Scholar]
- 70.Gordon RD, Parsons S, Symonds EM. A prospective study of plasma-renin activity in normal and toxaemic pregnancy. Lancet. 1969;1(7590):347–349. doi: 10.1016/s0140-6736(69)91302-6. [DOI] [PubMed] [Google Scholar]
- 71.Herse F, Dechend R, Harsem NK, Wallukat G, Janke J, Qadri F, Hering L, Muller DN, Luft FC, Staff AC. Dysregulation of the circulating and tissue-based renin–angiotensin system in preeclampsia. Hypertension. 2007;49(3):604–611. doi: 10.1161/01.HYP.0000257797.49289.71. [DOI] [PubMed] [Google Scholar]
- 72.Li C, Ansari R, Yu Z, Shah D. Definitive molecular evidence of renin–angiotensin system in human uterine decidual cells. Hypertension. 2000;36(2):159–164. doi: 10.1161/01.hyp.36.2.159. [DOI] [PubMed] [Google Scholar]
- 73.Massani ZM, Sanguinetti R, Gallegos R, Raimondi D. Angiotensin blood levels in normal and toxemic pregnancies. Am J Obstet Gynecol. 1967;99(3):313–317. doi: 10.1016/s0002-9378(16)34536-7. [DOI] [PubMed] [Google Scholar]
- 74.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52(11):2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54(4):905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14(8):855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN, Jr, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension. 2011;57(4):865–871. doi: 10.1161/HYPERTENSIONAHA.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN, Jr, Dechend R, Lamarca B. Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. Am J Physiol Regul Integr Comp Physiol. 2012;302(10):R1197–1201. doi: 10.1152/ajpregu.00623.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parikh SM, Karumanchi SA. Putting pressure on pre-eclampsia. Nat Med. 2008;14(8):810–812. doi: 10.1038/nm0808-810. [DOI] [PubMed] [Google Scholar]
- 81.Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schonemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352(6):558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 82.Riemekasten G, Philippe A, Nather M, Slowinski T, Muller DN, Heidecke H, Matucci-Cerinic M, Czirjak L, Lukitsch I, Becker M, Kill A, van Laar JM, Catar R, Luft FC, Burmester GR, Hegner B, Dragun D. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011;70(3):530–536. doi: 10.1136/ard.2010.135772. [DOI] [PubMed] [Google Scholar]
- 83.Giral M, Foucher Y, Dufay A, Van Huyen JP, Renaudin K, Moreau A, Philippe A, Hegner B, Dechend R, Heidecke H, Brouard S, Cesbron A, Castagnet S, Devys A, Soulillou JP, Dragun D. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. 2013;13(10):2567–2576. doi: 10.1111/ajt.12397. [DOI] [PubMed] [Google Scholar]
- 84.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension. 2010;55(5):1239–1245. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hladunewich MA, Kingdom J, Odutayo A, Burns K, Lai V, O’Brien T, Gandhi S, Zimpelmann J, Kiss A, Miller J, Cherney D. Postpartum assessment of the renin angiotensin system in women with previous severe, early-onset preeclampsia. J Clin Endocrinol Metab. 2011;96(11):3517–3524. doi: 10.1210/jc.2011-1125. [DOI] [PubMed] [Google Scholar]
- 86.Kvehaugen AS, Melien O, Holmen OL, Laivuori H, Oian P, Andersgaard AB, Dechend R, Staff AC. Single nucleotide polymorphisms in G protein signaling pathway genes in preeclampsia. Hypertension. 2013;61(3):655–661. doi: 10.1161/HYPERTENSIONAHA.111.00331. [DOI] [PubMed] [Google Scholar]
- 87.Wenzel K, Rajakumar A, Haase H, Geusens N, Hubner N, Schulz H, Brewer J, Roberts L, Hubel CA, Herse F, Hering L, Qadri F, Lindschau C, Wallukat G, Pijnenborg R, Heidecke H, Riemekasten G, Luft FC, Muller DN, Lamarca B, Dechend R. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension. 2011;58(1):77–84. doi: 10.1161/HYPERTENSIONAHA.111.171348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med. 2001;7(9):1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- 89.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, Stein PE, Broughton Pipkin F, Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468(7320):108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anton L, Brosnihan KB. Systemic and uteroplacental renin–angiotensin system in normal and pre-eclamptic pregnancies. Ther Adv Cardiovasc Dis. 2008;2(5):349–362. doi: 10.1177/1753944708094529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herse F, Staff AC, Hering L, Muller DN, Luft FC, Dechend R. AT1-receptor autoantibodies and uteroplacental RAS in pregnancy and pre-eclampsia. J Mol Med (Berl) 2008;86(6):697–703. doi: 10.1007/s00109-008-0332-4. [DOI] [PubMed] [Google Scholar]
- 92.Hering L, Herse F, Geusens N, Verlohren S, Wenzel K, Staff AC, Brosnihan KB, Huppertz B, Luft FC, Muller DN, Pijnenborg R, Cartwright JE, Dechend R. Effects of circulating and local uteroplacental angiotensin II in rat pregnancy. Hypertension. 2010;56(2):311–318. doi: 10.1161/HYPERTENSIONAHA.110.150961. [DOI] [PubMed] [Google Scholar]
- 93.Shah DM, Banu JM, Chirgwin JM, Tekmal RR. Reproductive tissue renin gene expression in preeclampsia. Hypertens Pregnancy. 2000;19(3):341–351. doi: 10.1081/prg-100101996. [DOI] [PubMed] [Google Scholar]
- 94.Anton L, Merrill DC, Neves LA, Gruver C, Moorefield C, Brosnihan KB. Angiotensin II and angiotensin-(1-7) decrease sFlt1 release in normal but not preeclamptic chorionic villi: an in vitro study. Reprod Biol Endocrinol. 2010;8:135. doi: 10.1186/1477-7827-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the american heart association. Circulation. 2011;123(11):1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323(7323):1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 99.Wikstrom AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005;112(11):1486–1491. doi: 10.1111/j.1471-0528.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 100.Harsem NK, Roald B, Braekke K, Staff AC. Acute atherosis in decidual tissue: not associated with systemic oxidative stress in preeclampsia. Placenta. 2007;28(8–9):958–964. doi: 10.1016/j.placenta.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 101.Hanssens M, Pijnenborg R, Keirse MJ, Vercruysse L, Verbist L, Van Assche FA. Renin-like immunoreactivity in uterus and placenta from normotensive and hypertensive pregnancies. Eur J Obstet Gynecol Reprod Biol. 1998;81(2):177–184. doi: 10.1016/s0301-2115(98)00187-0. [DOI] [PubMed] [Google Scholar]
- 102.Staff AC, Dechend R, Redman CW. Review: preeclampsia, acute atherosis of the spiral arteries and future cardiovascular disease: two new hypotheses. Placenta. 2013;34(Suppl):S73–78. doi: 10.1016/j.placenta.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 103.Staff AC, Redman CW. IFPA Award in Placentology Lecture: preeclampsia, the decidual battleground and future maternal cardiovascular disease. Placenta. 2014;35(Suppl):S26–31. doi: 10.1016/j.placenta.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 104.Tenhola S, Rahiala E, Martikainen A, Halonen P, Voutilainen R. Blood pressure, serum lipids, fasting insulin, and adrenal hormones in 12-year-old children born with maternal preeclampsia. J Clin Endocrinol Metab. 2003;88(3):1217–1222. doi: 10.1210/jc.2002-020903. [DOI] [PubMed] [Google Scholar]
- 105.Vatten LJ, Romundstad PR, Holmen TL, Hsieh CC, Trichopoulos D, Stuver SO. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet Gynecol. 2003;101(3):529–533. doi: 10.1016/s0029-7844(02)02718-7. [DOI] [PubMed] [Google Scholar]
- 106.Oglaend B, Forman MR, Romundstad PR, Nilsen ST, Vatten LJ. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J Hypertens. 2009;27(10):2051–2054. doi: 10.1097/HJH.0b013e328330052a. [DOI] [PubMed] [Google Scholar]
- 107.Ros HS, Lichtenstein P, Ekbom A, Cnattingius S. Tall or short? Twenty years after preeclampsia exposure in utero: comparisons of final height, body mass index, waist-to-hip ratio, and age at menarche among women, exposed and unexposed to preeclampsia during fetal life. Pediatr Res. 2001;49(6):763–769. doi: 10.1203/00006450-200106000-00008. [DOI] [PubMed] [Google Scholar]