Abstract
Nucleoli perform a crucial cell function, ribosome biogenesis, and of critical relevance to the subject of this review, they are also extremely sensitive to cellular stresses, which can cause loss of function and/or associated structural disruption. In recent years, we have learned that cells take advantage of this stress sensitivity of nucleoli, using them as stress sensors. One major protein regulated by this role of nucleoli is the tumor suppressor p53, which is activated in response to diverse cellular injuries in order to exert its onco-protective effects. Here we discuss a model of nucleolar regulation of p53, which proposes that key steps in the promotion of p53 degradation by the ubiquitin ligase MDM2 occur in nucleoli, thus providing an explanation for the observed link between nucleolar disruption and p53 stability. We review current evidence for this compartmentalization in p53 homeostasis and highlight current limitations of the model. Interestingly, a number of current chemotherapeutic agents capable of inducing a p53 response are likely to do so by targeting nucleolar functions and these compounds may serve to inform further improved therapeutic targeting of nucleoli.
Keywords: Nucleolus, p53, MDM2, Stress, Ribosome, Cancer therapy
Introduction
What are called structures are slow processes of long duration, functions are quick processes of short duration Ludwig von Bertalanffy, 1952 [1].
As biology textbooks have taught us for years, the main function of the nucleolus is the synthesis and assembly of ribosomal particles. This function carries such a heavy metabolic burden that it makes the nucleolus and its function exquisitely sensitive to stress. This stress sensitivity of nucleolar function is often manifested in a loss of nucleolar structure, in perhaps the most striking example of interdependence between cellular structure and function. In the last few years, interest in nucleolar biology has been extended since other cellular pathways distinct from ribosomal biogenesis have been shown to also operate in association with nucleoli, perhaps because they can take advantage of existing processes in ribosome production such as nuclear export [2–4]. These non-classical roles of the nucleolus are likely to be subject to the same stress sensitivity that ribosome biosynthesis displays and it should therefore not be surprising to find that pathways of response to oncogenic stresses may also utilize nucleoli in order to induce an onco-protective response. In this review, we shall discuss the role of the nucleolus in regulating the stability of the tumor suppressor p53 and will review evidence that sheds light on the mechanisms relating to this process. The nucleolus-associated biology of p53 and its main regulator MDM2 may represent a new paradigm in our understanding of non-classical nucleolar functions and, importantly, may also provide us with new targets for the therapeutic regulation of p53. We will also briefly argue that some common chemotherapeutic approaches currently in use may act, at least partially, through a nucleolar mechanism and may therefore already provide valuable information on the therapeutic targeting of nucleoli. We suggest that not only is therapeutic targeting of p53 through the nucleolar machinery a hope for future therapies, but indeed, it is already being used by many current ones.
TP53 is probably the single most important mammalian tumor suppressor gene, and is mutated or lost in more than half of all human cancers (for more information on the role of p53 as a tumor suppressor and its therapeutic exploitation see for example [5]). It exerts its tumor suppressor function through the capacity of its product, the p53 protein, to induce a wide range of phenotypes including apoptosis, cell cycle arrest (G1 and G2 phases), senescence, and enhanced DNA repair [6, 7], in addition to modulating metabolic processes [8], although the relative contribution of each of these to tumor suppression remains unclear. It has been observed routinely that an increase in the steady-state levels of p53 (stabilization) can promote apoptosis and cell cycle arrest [5]. This is frequently accompanied by a number of post-translational modifications such as phosphorylation and acetylation, the precise patterns of which appear to be dependent on the nature of the stress that initiated the p53 response [7, 9]. Since the range of p53-inducing stresses can be so varied (see for example an extensive compilation of stresses in [10]), their very diversity presents a problem: how can they all converge upon activation of p53? Moreover, since iatrogenic activation of p53 continues to be a major strategy of cancer therapy [11, 12], understanding how a p53 response is initiated is important for a number of reasons if we are to use therapies more effectively, and/or develop new approaches to therapeutic interventions that target p53. Firstly, it may tell us if therapeutically effective levels of p53, as currently obtained with DNA damaging agents, can be achieved in the absence of DNA damage and thus identify new ways to target cancer cells that lack the longer-term risks of genotoxicity associated with many p53-activating agents in current use. Secondly, it might shed light on the bases of the wide range of therapeutic indices of current genotoxic agents in different tissues [13]. Thirdly, and perhaps most importantly, an understanding of the mechanistic details will be increasingly important for identifying rational combinatorial therapeutic strategies envisaged as part of personalizing medical treatments for cancer [14].
The stability of p53 is primarily determined by the action of the MDM2 protein, which is an E3 ubiquitin ligase for p53 as well as a transcriptional target of p53, properties that result in the formation of an auto-regulatory feedback loop [15, 16]. In addition to ubiquitylation, MDM2 also regulates NEDDylation and SUMOylation of p53, and thus the interplay between p53 and MDM2 is a complex one [17–19]. MDM2-promotes ubiquitylation of p53 and poly-ubiquitylated p53 is subjected to proteasomal degradation. This degradation is an intrinsic characteristic of the p53-MDM2 feedback loop and it maintains p53 at low levels in the absence of stress. p53 degradation can be inhibited either by exogenous cell stresses or by alterations in oncogene expression that cause an increase in the levels of ARF (alternate reading frame product of the CDKN2A gene, which encodes the p14ARF protein [p19ARF in mice], hereafter referred to collectively simply as ARF [20]). The interaction between ARF and MDM2 can promote sequestration of MDM2 in nucleoli, an observation which originally drew attention to the possibility of an involvement of nucleoli in some step(s) of the p53/MDM2 regulatory loop. The realization that stresses that disrupted nucleoli would in general also induce p53 ([21], see also [22–24] and, for a recent review, see [25]), suggested that a link between nucleolar function and p53 stability might lead to a unifying model for the induction of p53 by a diverse range of cellular stresses. Here we review some of the landmark findings that implicated nucleoli in the regulation of p53 and discuss the mechanisms by which this regulation might be exerted.
Nucleolar disruption and p53 induction
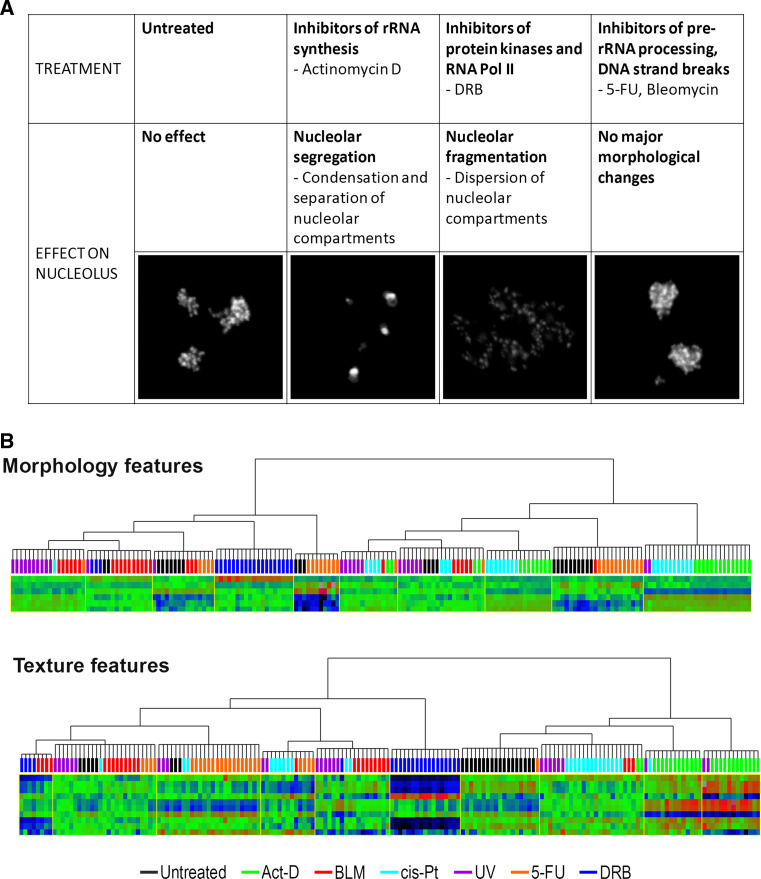
The concept of nucleolar regulation of p53 homeostasis depends on the notion that cells take advantage of the exquisite sensitivity of nucleoli to stresses, many of which potentially oncogenic, to initiate a rapid p53 response (increase in p53 steady-state levels). A priori, it is certainly a biologically appealing notion [22–24] because, as Rudra and Warner referring to cellular stress put it, “what better measure than ribosome synthesis” [24]. However, the general applicability of the model of nucleolar disruption to all p53-inducing stresses is not certain. This is further complicated by the definition of what constitutes nucleolar disruption. Alterations of nucleolar function, whether they affect synthesis or processing of rRNA (and perhaps other functions) often, but not always, cause visible changes in nucleolar morphology [26]. These morphological changes can be accompanied by significant re-localization of nucleolar proteins to the nucleoplasm and this re-localization is easily quantifiable by various methods [21, 25]. Indeed, it has long been known that re-localization of B23/Nucleophosmin (NPM1, which we shall call B23/NPM) can be an indicator of drug cytotoxicity [27, 28]. However, some inhibitors of nucleolar function, for example 5-fluorouracil (5-FU), which inhibits rRNA processing, disrupt nucleolar morphology only minimally, if at all [25, 26]. Moreover, it is not entirely clear how inhibition or interference with a particular nucleolar function determines the type of morphological change that it causes. It is nevertheless possible to make the following broad classification: agents that inhibit rRNA synthesis cause nucleolar segregation, defined by condensation and separation of nucleolar fibrillar centers and granular components [25, 29–31]; protein kinase inhibitors induce a massive dispersion of nucleolar components called nucleolar fragmentation [25, 29] probably due to the role of kinases in maintaining nucleolar organization [32, 33]; and inhibition of pre-rRNA processing fails to introduce major nucleolar morphological alterations [25, 29]. This classification is summarized in Fig. 1a. We have also observed that different nucleolar stresses tend to induce characteristic changes in nucleoli that appear to be identifiable. Figure 1b shows two approaches to classification of images of variously stressed nucleoli (image shape and image texture descriptors applied to confocal sections of fibrillarin immunofluorescence), which are able to cluster a significant proportion of images into treatment groups (CPR unpublished). These preliminary studies suggest that there is potential for statistical analysis of nucleolar disruption of single cells by agents that produce limited or no nucleolar/ribosomal protein re-localization. Burger et al. [26] have extensively cataloged the effects of a large number of cytostatic drugs on both rRNA synthesis and nucleolar integrity, providing a very useful reference to compare mechanisms of action with nucleolar or ribosomal outcomes. Certain CDK inhibitors such as DRB (5,6-Dichlorobenzimidazole-1-β-D-ribofuranoside) and roscovitine are peculiar since they can cause extensive nucleolar reorganization, yet their inhibitory effect appears to be exerted mainly on processing of rRNA rather than on rDNA transcription [26, 32]. In addition, work by Sirri et al. [34] using CDK inhibitors suggests that in fact these two processes may be uncoupled. However, we do not yet know whether the reverse is true, in other words, whether the classification of a nucleolar disruption event into one of the categories defined above can inform us about the mechanism involved. Regardless, one thing which is clear is that all of these classes of nucleolar/ribosomal stress result in p53 stabilization. Thus, to encompass all three types of changes in nucleoli and rRNA processing that lead to p53 stabilization we hereafter use the term nucleolar disruption, bearing in mind that such disrupted phenotypes may not be easy to visualize by microscopy (either by nucleolar morphology or by protein re-localization) and may require biochemical analyses (see for example [26]).
Fig. 1.
Nucleolar disruption. a Modes of nucleolar disruption and example treatments. b Characterization of nucleolar disruption: examples of clustering of images of treated nucleoli using morphology (top) and texture (Haralick) descriptors (bottom). Images shown in a and used for analysis in b are single confocal sections of immunofluorescence for fibrillarin, each image containing all nucleoli within a single nucleus
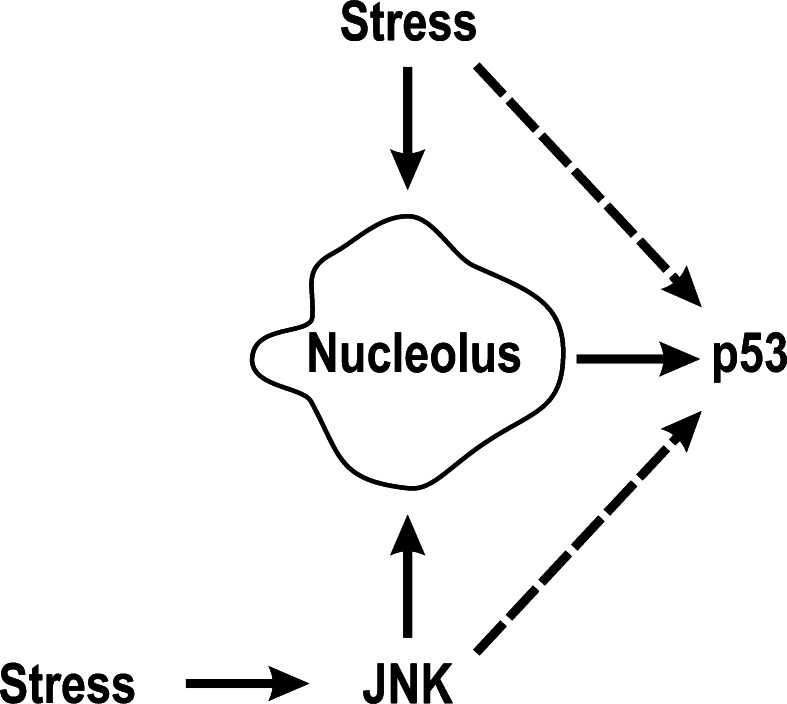
A major obstacle to understanding whether the correlation between nucleolar disruption and p53 stabilization denotes causality is that many agents used to study this have pleiotropic cellular effects and some of them may directly impact on p53 [7, 9, 10]. These modes of relaying a stress signal to p53 are depicted in Fig. 2. A further complication is that some stresses may not impinge on nucleoli directly, but may link to pathways that lead to inhibition of a nucleolar function. For example, oxidative stress can inhibit rRNA synthesis by activating the JNK2 kinase, and this in turn phosphorylates and inhibits the RNA Pol I factor RRN3 (commonly and hereafter referred to as TIF-IA) [35, 36]. From this observation, it is tempting to speculate that cells might make use of the robust stress response pathways that involve nucleolar disruption to deal with stresses that would otherwise not affect nucleolar function directly, rather than evolve new pathways. This possibility is depicted in Fig. 2 as the JNK pathway. Regardless, probably the clearest indication of a direct connection between disruption of nucleolar function and p53 stabilization in the absence of potentially confounding pleiotropic effects induced by exogenous physical/chemical cell stresses, particularly DNA damage, comes from studies of the Bop 1, Pes1, and hUTP18 proteins [37–39]. Bop and Pes1 are involved in pre-rRNA processing and, together with WDR12, form the PeBoW complex, which is critical for 28S rRNA maturation [40]; while the hUTP18 processing factor is required for 18S rRNA maturation [39]. As shown by the pioneering work of Pestov and coworkers for Bop1, and later by others for Pes1 and hUTP18, mutation or depletion of any one of these three proteins both inhibits pre-rRNA processing and stabilizes p53 [37–39]. In these conditions, it is unlikely that any other exogenous cellular stress is present. Likewise, Cre-mediated depletion of TIF-IA in mice has both been shown to cause nucleolar disruption and to be a powerful p53 inducer (leading to embryonic lethality by day 9.5 [41]). While it is likely in such experiments employing plasmid vector-driven expression, RNAi-mediated knock-down, etc., that unpredictable transcriptome changes will occur, which may impact other pathways in the cell, the fact that alterations at different points of rRNA processing all cause p53 stabilization surely argues for a direct link between nucleolar function and p53 stability.
Fig. 2.
Possible pathways of delivery of cell stress to p53. For a long time it has been considered that cell stresses directly activate p53 (dashed arrow), mainly through stress-induced kinases. The nucleolus model proposes that many of these stresses may stabilize p53 indirectly, by disrupting nucleoli (solid arrow). For most stresses, it is difficult to resolve which pathway predominates or whether the two pathways co-exist. Some stresses such as oxidative stress (see text) do not appear to affect nucleolar structure/function directly, but activate JNK, which in turn inhibits rDNA transcription (solid arrows). This pathway suggests stresses that normally would not affect nucleoli might be turned into nucleolar stresses by JNK, possibly to elicit the more general stress response that nucleoli support. JNK also phosphorylates p53 in response to stress (see text for further details and references)
In addition to the regulation by JNK2 described above, RNA Pol I (and also Pol III) activity is also regulated by other cancer-related proteins. The mitogen-activated protein kinases (MAPK) ERK (extracellular signal-regulated kinase) and RSK (ribosomal S6 kinase) cause direct activation of the RNA Pol I transcription factor TIF-IA [42], and also Upstream Binding Factor (UBF; reviewed in [43, 44]). The Myc oncoprotein also directly regulates RNA Pol I and III transcription [43–47]. Importantly, mTOR (mammalian target of rapamycin) signaling regulates rDNA transcription through influencing both the activity and localization of TIF-IA [48] and UBF [49], thus providing a link between nutrient availability and nucleolar rRNA synthesis. p53 has also been reported to inhibit rDNA transcription although reports have differed as to whether this action is direct [50] or indirect [51]. More recently, studies using a nucleolar disruption model based on Cre/loxP deletion of TIF-IA have suggested that p53 induction feeds back to inhibit rDNA transcription via inhibiting mTOR [52]. The ARF tumor suppressor, in addition to its direct role on the p53/MDM2 axis described below, also inhibits rRNA processing (but not transcription) in a p53- and MDM2-independent manner [53], possibly through its interaction with B23/NPM [54]. Finally, the retinoblastoma protein (Rb) has been shown to regulate both RNA Pol I and III activity (reviewed in [43, 44]). Collectively, these observations demonstrate that nucleolar activity is regulated by a number of signaling pathways that are not only known to be altered in tumor cells, but moreover, promote tumorigenesis.
As argued below, nucleolar disruption probably provides the most general explanation for the mechanisms of action of a number of chemotherapeutic agents with respect to the induction of p53. However, testing this hypothesis has presented two problems: firstly, that many p53-inducing agents cause other primary cellular injuries in addition to their effects on nucleoli (the pleiotropic effects referred to above); secondly, that it is necessary to de-couple DNA damage from nucleolar disruption in order to demonstrate that cells can tolerate DNA damage without p53 induction (providing that nucleoli remain unaffected under such conditions). Of the many other DNA-damaging agents that can induce p53, UV irradiation has the unique practical advantage that it can easily be delivered in a localized sub-cellular manner. Using UV in this way, it became possible to demonstrate that cells in which DNA damage was induced by local UV-C irradiation (with doses larger than those necessary to induce p53) failed to display both nucleolar disruption (as indicated by B23/NPM translocation) and p53 stabilization. This indicates that p53 stabilization is associated with nucleolar disruption, rather than with induction of DNA damage per se [21]. It is noteworthy that the reason that nucleoli are disrupted by UV irradiation may not necessarily (or not exclusively) be due to damage to rDNA. Cockayne syndrome (CS) fibroblasts cannot repair damage to RNA Pol II transcribed strands and therefore cannot efficiently recover from RNA synthesis inhibition following UV irradiation. As a consequence, they undergo p53 stabilization at UV irradiation doses (≤2 J/m2) that are too low to induce p53 in normal cells [21, 55, 56]. Since these low UV doses also cause nucleolar disruption in CS but not in normal fibroblasts, it is likely that disruption of nucleoli by UV irradiation is caused by diminished mRNA synthesis, and therefore that the effect of localizing the irradiation is to preserve the vast majority of nuclear Pol II-mediated RNA synthesis, rather than to protect nucleoli from UV damage to ribosomal genes. Regardless, using locally delivered UV has demonstrated that DNA damage induced by UV irradiation can be de-coupled from p53 induction when nucleoli are not disrupted. The complementary experiment, disrupting nucleoli without introducing DNA damage, has been performed by microinjecting an antibody against the RNA Pol I transcription factor UBF. These studies showed that p53 could be stabilized by microinjection of anti-UBF antibody and thus it appears that inhibition of rRNA synthesis leads to p53 stabilization [21]. In the last few years, low-dose actinomycin D has become so widely accepted as a highly specific inhibitor of rRNA synthesis that the DNA damaging capacity of this agent appears neglected. Actinomycin D is a DNA intercalator that stimulates topoisomerase I cleavage and stabilizes the resulting single-strand breaks, an effect manifested at all doses tested (Trask and Muller [57] tested doses as low as 64 nM). However, some reports indicate the absence of detectable p53 phosphorylation at serines 15 and 20 in the presence of 5 nM actinomycin D [58], while others have shown induction of serine 15 phosphorylation at 10 nM actinomycin D, in the absence of detectable histone H2AX phosphorylation [59]. This suggests that these low doses of actinomycin D do not induce detectable DNA damage and therefore do not signal to p53 through the DNA damage signaling response, but rather activate p53 through other paths. More recently, studies have been published using the novel rRNA synthesis inhibitory compound CX-5461. This acts as a selective transcriptional inhibitor of rRNA synthesis, displaying in the order of 200-fold more activity towards rRNA synthesis than towards mRNA synthesis ([60], similar in effect to actinomycin D [61]). Exposing cells to CX-5461 induces p53 as might be expected, albeit perhaps not as strongly as low-dose actinomycin D [60]. While further studies are needed to investigate details of the mechanism of action of CX-5461, it is interesting to note that in solid tumors it has been shown to induce apoptosis independently of p53 status [60], which suggests that targeting nucleolar function may also be applicable to tumors where p53 is mutated/deleted. It should be noted however, that this may be context-dependent, as studies in lymphomas found that the pro-apoptotic action of CX-5461 appeared to depend on wild-type p53 [62].
Regardless, the de-coupling of DNA damage from nucleolar disruption described above, suggesting that it is nucleolar disruption that correlates with p53 induction rather than DNA damage, obviously does not permit us to conclude that all stresses utilize this pathway to stabilize p53, let alone to conclude that nucleolar disruption is the critical mechanism. Therefore the question of the generality of the nucleolar disruption model remains. A strong argument in favor of this generality is the fact that most, if not all, stresses studied that cause p53 stabilization also cause nucleolar disruption and vice versa (see table in [25]). The fact that for such an extensively studied stress response protein as p53 (with inducing agents as diverse as DNA lesions, DNA strand breaks, kinase activation and inhibition, metabolic alterations, etc.; see the extensive review of Ljungman [10]), no agents have yet been found that induce p53 without affecting nucleoli or vice versa, makes this correlation compelling. The exceptions are inhibitors of proteasome function (e.g., MG132) where poly-ubiquitylated p53 cannot be degraded, or competitors of the MDM2-p53 interaction (e.g., Nutlins [63]), where again, p53 cannot be poly-ubiquitylated, in both cases leading to stabilization irrespective of the integrity or activity of nucleoli. While the fact remains that absence of evidence is not evidence of absence, the notable absence of agents that could differentially induce nucleolar disruption and p53 stabilization suggests that nucleolar control of p53 might indeed be general. Furthermore, the apparent variety of targets for inhibition of nucleolar function may offer the potential for combinatorial therapies based on p53 induction through targeting the same regulatory pathway at different points. It will also be of great value to determine whether and to what extent existing p53-inducing agents utilize a nucleolar route, since studies of these agents may already provide a wealth of information to elucidate nucleolar mechanisms and to understand how to use these therapeutically. In the next section, we will discuss the possible molecular mechanisms that might determine how nucleolar disruption, in any of its various forms, causes p53 stabilization.
Riding the ribosome
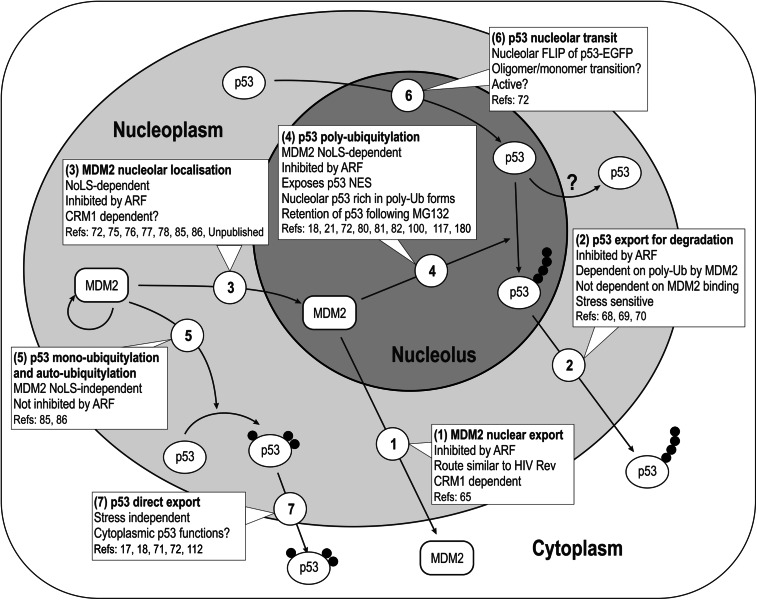
The most significant indication of nucleolar involvement in p53 regulation has undoubtedly come from studies of p53 stabilization by ARF. The early developments in this field were thoroughly reviewed by Sherr and Weber [64], who originally proposed the idea of nucleolar transit of p53 and MDM2, suggesting that these proteins might “ride the ribosome” as a complex. Support for this view came from several observations of both p53 and MDM2. MDM2 shuttles between the nucleus and cytoplasm and it has been shown that this shuttling of MDM2 is important for p53 degradation [65]. MDM2 shuttling can be inhibited by a peptide from HTLV1 rex that is a competitive inhibitor of the nuclear export of HIV Rev protein [65], suggesting that both proteins use a common export mechanism. Rev localizes to the nucleolus and this localization is important for its export function [66]. The export of both MDM2 and Rev is mediated by CRM1/Exportin-1, a RanGTP-dependent export protein, and in accord with the need for MDM2 export for p53 degradation, the specific CRM1 inhibitor leptomycin B (LMB) [67] efficiently blocks p53 degradation [68, 69]. Figure 3 summarizes the experimental results that underpin a model of the nucleolar regulation of p53, with the results discussed above corresponding to pathway 1. However, p53 export is also inhibited by LMB [68, 69] and since p53 and MDM2 co-compartmentalization appears to be required for p53 degradation [70], it has been concluded that p53 had to be exported to the cytoplasm in a CRM1-dependent manner, for proteasomal degradation (pathway 2 in Fig. 3). However, evidence also exists for a significant role of nuclear proteasomes. Several reports argue that p53 is (or can be) degraded in the nucleus. Li et al. [71] studied conditions of relatively low and high MDM2 levels and proposed that in the former case, p53 is mainly mono-ubiquitylated and therefore is exported from nuclei, while in the latter circumstance it is poly-ubiquitylated and consequently is degraded. Together with these observations they proposed that p53 degradation is nuclear on the basis that conditions promoting p53 poly-ubiquitylation lead to nuclear accumulation of p53 when proteasomes are also inhibited. However, as we have shown [72] p53 continues to shuttle between the nucleus and cytoplasm under conditions of proteasome inhibition, such that the observed steady-state level in the nuclear compartment is likely to reflect the rates of import and export, and thus may not simply indicate retention in inhibited nuclear proteasomes. Shirangi et al. [73] reported that p53 can be degraded in the nucleus in the presence of LMB when MDM2 is overexpressed and that this nuclear degradation takes place during the recovery period after p53 stabilization has been induced by stress once the inducing stress has been removed. These observations were later extended in follow-up studies of endogenous levels of p53 [74]. While the data are compelling, the observations appear to contradict abundant literature demonstrating nuclear stabilization of p53 by LMB, for example the detailed series of studies of the degradation behavior of p53 and MDM2 compartmentalization mutants mentioned above, which included LMB-induced p53 stabilization [70]. The latter work, however, also reported that a p53 nuclear export signal (NES) mutant can efficiently undergo MDM2-mediated degradation, supporting the notion of nuclear degradation of p53. Therefore, there is evidence for nuclear proteasomal degradation of p53, but whether this occurs in normal circumstances or only in conditions of overexpression, mutation or prolonged inhibition is not yet clear.
Fig. 3.
The nucleolar model of p53 regulation. Map of the proposed mechanisms for p53 homeostasis. The text boxes summarize the experimental observations that determine the sub-cellular localization and roles of the corresponding steps (numbers in circles)
Crucial additional supporting evidence for a nucleolar role in the process of p53 regulation comes from studies showing that MDM2 export can be inhibited by ARF-mediated nucleolar sequestration of MDM2 resulting in p53 stabilization. This effect depends upon the nucleolar localization of ARF since nucleolar-localization defective ARF mutants fail to stabilize p53 [75–77]. Significantly, some of these ARF mutants have been found in tumors [76]. Nucleolar transit of MDM2, and its inhibition by actinomycin D, was later directly demonstrated by Mekhail et al. [78] using photobleaching. These results introduced the notion that MDM2 export to the cytoplasm follows a nucleolar route (pathway 3 in Fig. 3). Interestingly, MDM2 shuttling mutants (deficient in either the nuclear localization signal [NLS] or NES) have been shown to be unable to promote p53 degradation [79]. Since exclusively cytoplasmic (NLS-deficient) MDM2 fails to degrade p53, the work of Tao and Levine [79] suggested that to achieve p53 degradation it is not enough for both MDM2 and p53 to co-localize in the cytoplasm, but rather MDM2 had to be exported from the nucleus together with p53. These observations lent additional support to the concept of p53 and MDM2 using the ribosomal export machinery (“riding the ribosome”) as part of a complex in order to reach the cytoplasm where poly-ubiquitylated p53 could then be degraded [64]. In Fig. 3, this would imply that pathways 1 and 2 occur as a single export route with p53 and MDM2 forming a complex. However, subsequent studies have cast doubt on this putative mechanism. For example, studies of the function of the p53 NES showed that it is masked in p53 oligomers, but can be exposed following MDM2-mediated p53 ubiquitylation [18, 80–83]. This suggests that MDM2 might be required to expose the p53 NES, rather than to export p53 directly (pathway 4 in Fig. 3, and see below for an explanation of the nucleolar localization of this pathway). It should be noted, however, that over-expression of ARF inhibits MDM2-promoted p53 degradation without the apparent need for MDM2 re-localization to nucleoli (and arguably, nucleolar retention of it) [84]. These observations led to questioning of the hypothesis that p53 and MDM2 would “ride the ribosome” as a complex. Nevertheless, at the same time as these discoveries were made, further evidence was also accumulating that supported a nucleolar involvement in the process. Expression of ARF was shown to have a differential effect on MDM2-mediated p53 ubiquitylation, inhibiting poly-ubiquitylation, but having little or no effect on mono-ubiquitylation [85]. Interestingly, ARF expression does not affect MDM2 ubiquitylation, presumably this refers to auto-ubiquitylation since it is does not occur when an MDM2 RING finger mutant C462A (murine) that lacks ubiquitin ligase activity is used [85]. This differential effect of ARF expression on p53 and MDM2 ubiquitylation is similar to the effect of an MDM2 nucleolar localization signal mutant (NoLS, residues 466-473, as described by Lohrum et al. [86]), which also does not affect either MDM2 auto-ubiquitylation or p53 mono-ubiquitylation, but which inhibits p53 poly-ubiquitylation (indicated as pathway 5 in Fig. 3). As the authors of these studies pointed out, since the NoLS mutations overlap/juxtapose the RING finger domain of MDM2, it may be that the differential effects of these mutations on ubiquitylation are not related to the nucleolar localization deficiency, but rather to an alteration of the ubiquitin-ligase activity of MDM2 (though this would have to selectively affect auto- and trans-ubiquitylation) [85, 86]. Another possibility is that interactions with other factors required for efficient MDM2-dependent p53 poly-ubiquitylation (for example p300; see [15]) are impaired by the NoLS mutation. Regardless, the striking phenocopying between the effects of ARF over-expression and of MDM2 NoLS mutation [85] demonstrates that loss of MDM2 nucleolar localization has an effect similar to ARF over-expression and strongly suggests that the ability of MDM2 to poly-ubiquitylate p53 may depend on its ability to reach nucleoli, whereas MDM2 auto-ubiquitylation or p53 mono–ubiquitylation may be independent of the nucleolar localization of MDM2. Taken together, the ARF and MDM2 NoLS observations suggest that p53 poly-ubiquitylation occurs in nucleoli. This idea has been further supported by our observation that nucleolar p53 is enriched in poly-ubiquitylated forms [72] and prompts us to propose that pathway 4 in Fig. 3 is in fact nucleolar.
In addition to the proposed role of the nucleolus/nucleolar function as a co-ordinator of stress responses signaling to p53 and as a site that provides for the efficient poly-ubiquitylation of p53 in unstressed cells, it is interesting to speculate that this compartment might also function to protect poly-ubiquitylated p53 from ubiquitin specific proteases (USPs). Such a mechanism would explain both our observation that poly-ubiquitylated p53 appears enriched in nucleoli with respect to the nucleoplasm and that p53 continues exchanging between nucleolus and nucleoplasm even during proteasomal inhibition [72]. Of the USPs identified as nucleolar in v3.0 of the nucleolar proteome database ([87]; http://www.lamondlab.com/NOPdb3.0/), only HAUSP (USP7) has been reported to de-ubiquitylate p53, though since it also de-ubiquitylates MDM2, it is not clear whether nucleolar HAUSP activity would result in p53 stabilization or de-stabilization [88–91]. Regardless, the potential role of nucleoli as USP-free environments with respect to p53 is certainly worthy of further consideration.
Interestingly, the nucleolar compartmentalization of p53 regulation has the potential to explain the somewhat paradoxical effects of inhibition of nucleocytoplasmic export on p53 stability. While inhibition of CRM1 with LMB inhibits MDM2-mediated p53 degradation, p53 degradation promoted by the HPV E6 protein is, by contrast, not efficiently inhibited by LMB [68, 92]. This implies either that MDM2-mediated p53 degradation depends on nuclear export while E6/E6-AP-mediated degradation does not, or that LMB selectively prevents MDM2-mediated p53 poly-ubiquitylation. In this regard, it may be noteworthy that different p53 poly-ubiquitylation patterns are induced by LMB and MG132 treatment and this is perhaps more compatible with a mechanism in which LMB impairs MDM2-mediated p53 ubiquitylation [72]. In addition, Laín et al. [69] have reported that LMB induces accumulation of MDM2 in nuclear bodies, and we have obtained preliminary data suggesting that the nucleolar accumulation of MDM2 induced by actinomycin D (see for example [86, 93]) is efficiently inhibited by LMB (CPR unpublished). It is appropriate here to quote a conclusion of Xirodimas et al. [70] to their studies of p53 and MDM2 compartmentalization mutants: “If nuclear export and cytoplasmic degradation was the only pathway for Mdm2-mediated degradation of p53, inhibition of nuclear export by LMB should provide a similar profile to use of proteasome inhibitors. However, the observation that LMB caused a clear stabilization of p53 without accumulation of ubiquitinated protein (as MG132 does) suggests that LMB inhibits a step in the p53 degradation pathway at or before, but not after, ubiquitination”. Thus it is possible that CRM1 is required to transport MDM2 to nucleoli (pathway 3 in Fig. 3). This is perhaps not surprising, since there is now abundant evidence that CRM1 plays a role in intranuclear transport [94–97], and more particularly in the intranucleolar trafficking of several proteins [98].
At the time that the concept of MDM2–p53 complexes riding the ribosome was first proposed, it was unclear whether p53 localized to nucleoli [64], since p53 immunostaining shows nucleoli as poorly or unstained areas compared with the surrounding nucleoplasm. To address this, we showed that nucleolar p53 can be clearly revealed by permeabilizing cells before fixation, which removes the majority of soluble p53 [99]. Subsequently, Klibanov et al. [100] reported that proteasome inhibition visibly increases the retention of nucleolar p53 under permeabilized conditions and we have now directly demonstrated the presence of p53 in nucleoli using sub-cellular fractionation [72]. Nevertheless, the difficulty in detecting the relatively low steady-state levels of nucleolar p53 by immunofluorescence of whole cells, led Sherr and Weber [64] to suggest that “as p53 has not been visualized in nucleoli, trans-nucleolar export of Mdm2–p53 complexes, if it occurs, would have to be an extremely efficient and rapid process”. This prediction has proven insightful since this is indeed the case. Fluorescence loss in photobleaching (FLIP) studies of cells stably expressing p53-EGFP have demonstrated that essentially all of the nucleoplasmic p53 transits rapidly through nucleoli [72] and this p53 nucleolar transit is indicated as pathway 6 in Fig. 3.
Since p53 stabilizing cell stresses also disrupt nucleoli (see previous section), the idea emerged that nucleoli might not only be involved in ARF-induced p53 stabilization but also in the response to a wide variety of stresses. We hypothesized that the correlation between nucleolar disruption and p53 stabilization might result from a causal relationship, which was supported by experiments de-coupling DNA damage and nucleolar disruption as described above. However, nucleolar disruption induced by cellular stresses introduces pleiotropic effects, not simply leading to the inhibition of specific nucleolar functions. Unlike other sub-cellular organelles, the nucleolus is membraneless and therefore its structure and function are intimately connected, such that alterations at almost any stage of ribosome biogenesis induce nucleolar reorganization [25, 31]. Indeed, the nucleolus has been called “an organelle formed by the act of building a ribosome” [101]. A consequence of this is that stresses that impinge on any step of ribosome biogenesis cause the translocation of many ribosomal and nucleolar proteins from the nucleolus to the nucleoplasm, often accompanied by changes in nucleolar morphology such as segregation and fragmentation as already described, which are dependent on the inducing stress (reviewed in [25, 29, 31]). Within stressed nucleoli, trafficking rates of nucleolar/ribosomal proteins may change in a protein-specific manner, with some trafficking faster and others more slowly or not at all [31, 93]. Therefore, when any of the biochemical processes described above involving nucleolar MDM2 and p53 are studied in the presence of putative inhibitors or in stress conditions, a number of nucleolar events are likely to occur that confound interpretation of the results.
There is another important mechanism by which nucleolar stresses could be translated into p53 stabilization. MDM2 and p53 both interact with a number of ribosomal and nucleolar proteins [25, 102]. Indeed, binding of ribosomal proteins to MDM2 is commonly observed when screening for MDM2 protein–protein interactions [103]. Due to the fact that many ribosomal/nucleolar proteins can both translocate to the nucleoplasm under conditions of nucleolar stress and bind to MDM2 and/or p53, a large body of literature supports the notion that it is the re-localization of these nucleolar components that is responsible for the p53 stabilization observed under conditions of nucleolar stress (for a review, see [104]). We call these “factor re-localization models”, as opposed to the model described in this section and in Fig. 3, which is a direct nucleolar control model. For stresses that cause nucleolar/ribosomal protein re-localization, both models can account for the ensuing p53 stabilization and it is quite possible that they both operate simultaneously. However, a direct nucleolar control model implies that even stresses that do not cause detectable factor re-localization can still induce p53 stabilization which, importantly, implies that there may be many nucleolar functions that can be targeted for therapeutic activation of p53. Consequently, it is important to understand the relative contributions of factor re-localization versus direct nucleolar models in inducing p53 stabilization in response to stress. In the following sections, we will discuss recent work on cellular aspects of p53 homeostasis that allow for discrimination between the two models, in particular the phenomenon that we have termed “local control” of p53 [72]. We will also identify current limitations in both models to our understanding of the nucleolar regulation of p53 homeostasis.
Local control of p53
All of the proteins that have thus far been implicated in linking nucleolar stress with p53 regulation have been amply demonstrated to shuttle between nucleus and cytoplasm [77, 105–109]. In addition, we have confirmed this shuttling for MDM2, B23/NPM, and RPL11 in stressed cells, as well as for p53 itself (see supplementary Fig. 1 in [72]). Therefore, one should expect that any signaling for p53 stabilization mediated by these proteins should also be able to reach all the cellular compartments that they shuttle to or through. On the other hand, a requirement for a complete and functional nucleolus to promote p53 degradation might determine that control of p53 steady-state levels is a property intrinsic to a cell nucleus. This question can be addressed by using the technique of cell fusion (heterokaryon) that has long been used to study shuttling between cellular compartments, having windows of observation of around 6–8 h post-fusion before nuclear envelopes breakdown during mitosis (for a review on techniques and historical developments see [110]). In the case of p53, human U2Os cells and murine NIH 3T3 fibroblasts differ significantly in their p53 degradation rates (approximate half-lives of 30 vs. 15 min, respectively [72]), thus providing a system to study whether the p53 homeostatic system of one cell nucleus might be able to influence that of another nucleus. The first important information that the p53 human/murine heterokaryon system produced was that each nucleus regulates p53 steady-state levels independently, and that the expression level in each nucleus inversely correlates with the p53 half-life of the parental cells, independently of the origin of the p53 being studied (human or murine). We have termed this behavior “local control” of p53 steady-state levels. This observation puts a constraint on models of p53 regulation, which must be able to accommodate local control. It must be stressed that local control refers to steady-state p53 levels, that therefore have to be physiologically controlled in order for heterokaryon data to be meaningful. For instance, conditions in which levels of p53 or MDM2 are artificially raised (e.g., transient over-expression of either wild-type proteins or of mutants that alter compartmentalization) cannot be guaranteed to reflect the normal cellular mechanism of p53 regulation. A reasonable criterion for a valid test of local vs. global regulation might be the stable expression of any gene tested, to ensure that the protein product is physiologically tolerated by the cell. For example, in a heterokaryon experiment intended to study normal (non-stressed) regulation of p53 steady-state levels, if transfecting a construct commits cells to apoptosis, then this is hardly likely to reflect physiologically relevant modulation of p53 levels.
The second important observation from heterokaryons is that p53 stabilization introduced by DNA damage to one co-cytoplasmic nuclei remains local to that nucleus. This implies that whatever factors determine the loss of capacity to degrade p53, they are not able to transfer the activity to another nucleus. As mentioned above, ribosomal and nucleolar proteins are difficult to envisage as locally acting due to their ability to rapidly shuttle between compartments. Interestingly, analysis of p53 phosphorylation has demonstrated that this is not a determinant of local control, since the prominently modified residue Ser15 [9] was observed to be phosphorylated in all co-cytoplasmic nuclei of a heterokaryon [72]. We do not know whether the global distribution of Ser15-phosphorylated p53 is due to global distribution of the kinase involved or to shuttling of Ser15-phosphorylated p53 after phosphorylation, but in any event, the fact remains that Ser15 phosphorylation does not appear to provide an explanation for local control. It is interesting to note that local p53 stabilization in heterokaryons following ionizing radiation (IR) has also been reported, however, the authors focused on the association with cell cycle rather than comparing irradiated and non-irradiated nuclei [111]. Nevertheless, these results probably reflect the same local p53 regulation phenomenon that we are discussing here.
Local p53 stabilization in stressed (DNA-damaged) heterokaryons has a further implication, which is that nucleocytoplasmic p53 export is linked to stress and is also locally regulated. Comparison of heterokaryons with and without protein synthesis inhibition (cycloheximide) shows that non-stressed nuclei maintain their ability to promote p53 degradation, which implies that the fraction of p53 that is stabilized in a UV-irradiated nucleus does not reach the non-stressed nucleus. This interpretation, namely that stress induces a reduction in p53 nuclear export has been directly confirmed by cytoplasmic photobleaching of stably expressed p53-EGFP and quantitation of loss of nuclear signal (FLIP [72], see pathway 7 in Fig. 3). Interestingly, both heterokaryon and FLIP studies indicate that p53 nuclear export following UV irradiation is “leaky” and some p53 still reaches the cytoplasm, leading us to propose the existence of two pathways for p53 export; stress sensitive export and constitutive stress insensitive export (SSE and SIE, respectively; see [72]). SIE might be important, for example, for cytoplasmic transactivation-independent functions of p53 (pathway 7 in Fig. 3; see [17, 71, 112]), which may also be regulated by NEDDylation and SUMOylation [17, 18].
Limitations of the nucleolar model of p53 regulation
There are two important aspects of p53 regulation that remain unaccounted for by the nucleolar model: the accumulation of poly-ubiquitylated p53 in nucleoli and the maintenance of low levels of p53 in conditions of physiologically low nucleolar activity.
When proteasome-mediated degradation is inhibited, for example using MG132, nucleolar p53 is enriched in poly-ubiquitylated forms of p53 compared to nucleoplasmic and cytoplasmic p53 [72]. A probably related observation is that MG132 treatment causes the nucleolar retention of p53 as observed by immunofluorescence of cells permeabilized before fixation [100] (more recently, Krüger and Scheer [113] have described studies in which the nature of these nuclear sites has been investigated, as discussed below). In addition, we have also detected a lower (albeit not statistically significant) rate of p53 nucleolar transit following MG132 treatment, which is more marked after UV irradiation. Thus, the fate of poly-ubiquitylated p53 still raises further questions. While all models of p53 stabilization have concentrated mainly on inhibition of the E3 ligase activity of MDM2 towards p53, it is apparent that p53 degradation can be impaired even when it is poly-ubiquitylated. For example, it has been reported that IR and UV can induce different p53 ubiquitylation patterns, with IR inducing strong poly-ubiquitylation, while UV induces no poly-ubiquitylation, or barely detectable levels at low doses [114]. In both cases, however, similar steady-state levels and half-lives of p53 are achieved. It should be stressed that, as mentioned above, nucleolar stress is likely to play a role in p53 stabilization by IR since stabilization is local [111], with DNA strand break-induced p53 phosphorylation being global [72]. Stabilization of p53 without reduction in poly-ubiquitylation has also been reported for other agents such as DRB [115] and nitric oxide [116]. Clearly, models based on nucleolar/ribosomal factor re-localization and inhibition of MDM2-mediated p53 poly-ubiquitylation would not be applicable to p53 stabilization in these conditions, but a direct nucleolar model, at present, also fails to explain this. Nevertheless, as stated earlier, proteasomal inhibition (MG132) causes retention of p53 in nucleoli [100, 117, 118], and this nucleolar p53 is enriched in poly-ubiquitylated forms [72], which suggests that a mechanism must exist to support the transit of p53 through nucleoli and the delivery of poly-ubiquitylated p53 to proteasomes (that is, to support pathway 2 in Fig. 3). It is possible that p53 that becomes poly-ubiquitylated in nucleoli is retained there unless it can be mobilized to proteasomes, whereas non-ubiquitylated p53 might return to the nucleoplasm (indicated in Fig. 3), causing both the nucleolar retention and relative enrichment in poly-ubiquitylated forms as discussed. It is worth noting that we did not find a statistically significant difference in the nucleolar transit rates of p53-EGFP by nucleolar FLIP (discussed below) in cells treated with MG132, but did see this when cells had been UV irradiated. Within the quantitative limitations of the FLIP technique, this would agree with total p53 not transiting through massively disrupted nucleoli (UV) but largely transiting through nucleoli of MG132-treated cells, with retention of a less abundant, poly-ubiquitylated fraction. Interestingly, Krüger and Scheer [113] have reported that under conditions of proteasome inhibition with MG132 p53 accumulates in nucleolar cavities of “low-density” (characterized by electron microscopy) and not with RNA Pol I-containing fibrillar centers. This p53 accumulation becomes detectable after 8 h of MG132 treatment and is most marked at 16 h, hence it is maximally observable after long-term proteasome inhibition. While its origin and function are not yet entirely clear, based on these electron microscopy observations, the authors propose that these cavities “might correspond to nucleoplasmic spaces enclosed within the nucleolus” and also observe that proteasomal components accumulate in them, suggesting that they might constitute a site of proteasomal activity, in agreement with an earlier report on proteasome-containing, nucleoplasmic invaginations into nucleoli [119]. Considering the extensive use of proteasomal inhibition in nucleolar studies, this compartment deserves further attention. This co-localization of inhibited proteasomes and p53 appears to agree with (and indeed might be the basis of) earlier propositions that p53 is degraded in the nucleus [70, 71, 73, 74].
There is experimental evidence to support the idea that nucleolar transit of p53 might not be merely due to diffusion but involve post-translational modifications of p53 or active transport. Firstly, p53-EGFP in the absence of unmodified endogenous p53 displays a reduced rate of transit through nucleoli [72]. It is not clear whether this is due to a reduction or a complete impairment of transit because the photobleaching technique used (FLIP) is not precise enough to resolve this. Unfortunately, fluorescence recovery after photobleaching (FRAP [120]) is also impractical for quantitative studies of nucleolar p53 diffusion rates due to the lower nucleolar steady-state level compared with the surrounding nucleoplasm. Secondly, considering the lower steady-state levels of nucleolar p53, the rate of p53-nucleolar transit must be higher than p53-nucleoplasmic diffusion, since clearance half-lives of p53 (FLIP) are similar for both nucleoplasmic and nucleolar photobleaching. There are a number of different ways through which this could occur. The p53 nuclear export signal is masked in oligomeric p53 and is exposed by dissociation of the oligomer [80]. Since our work links nucleoli to p53 poly-ubiquitylation and also to export, it is also possible that an oligomer/monomer transition occurs, which might explain the more rapid nucleolar transit. To complicate matters further, it has been reported that nucleolar localization of p53 in vitro is energy-dependent and that it can be reduced by DNA damage [121]. If this process occurs in vivo, it would add another level of complexity to the interpretation of mechanistic studies of p53 nucleolar transit [121]. Interestingly, recent work by Brangwynne et al. [122] suggests that nucleolar viscosity is also energy-dependent, being increased by ATP depletion. Recently, non-coding RNAs from the ribosomal intragenic spacer regions have been shown to bind and retain proteins in the nucleolus, among them MDM2 [123, 124]. This is an exciting new development since such a mechanism may explain the variety of behaviors observed in the transit of proteins through the nucleoli under stress. From these data, one may speculate that the nucleolar transit of p53 might be a stress-sensitive process which, though dependent on poly-ubiquitylation, might nevertheless be separable from the process of poly-ubiquitylation. For example, a recent comprehensive study comparing nucleolar changes elicited by UV and IR demonstrated that while UV induces nucleolar fragmentation and relocation of components, IR by contrast, only causes subtle nucleolar changes with maintenance of overall morphology and rRNA synthetic activity [125]. It is conceivable that nucleolar fragmentation or segregation induced by UV irradiation prevents MDM2 from poly-ubiquitylating p53, while nucleolar changes induced by agents such as IR do not prevent MDM2-mediated p53 poly-ubiquitylation, but nevertheless prevent the poly-ubiquitylated p53 from reaching proteasomes. If correct, this would provide a further level at which nucleolar stress could impede p53 degradation. A corollary of these studies is that nucleolar compartmentalization is a complex and novel issue that deserves attention from the systems biology point of view: it is clear that molecular transactions involving nucleoli cannot be understood exclusively in terms of possible protein–protein interactions, and compartmentalization-related processes such as transit and formation/stability and regulation of macromolecular complexes must also be considered.
An alternative explanation for the nucleolar retention of p53 following proteasome inhibition could partly be provided by the existence of nucleolar proteasomes. While plausible and in accordance with the apparently extensive nucleolar ubiquitylation observed under conditions of proteasome inhibition, evidence seems to favor the conclusion that there is no proteasomal activity, nor indeed proteasomes, in nucleoli. This conclusion is based upon both functional studies using cleavable fluorogenic precursors [126] and cell fractionation experiments with samples analyzed by western blot or mass spectrometry [126, 127]. Nevertheless, a number of reports showing detection of nucleolar proteasomal components by immunofluorescence, albeit with no demonstration of proteasomal function, argue for the presence of nucleolar proteasomes [117]. Regardless, in the event that nucleoli possess proteasomes, this remains insufficient of itself to explain the retention of poly-ubiquitylated p53 in nucleoli under conditions of proteasomal inhibition, since the ubiquitylated p53 would otherwise be exported from nucleoli (see above).
With respect to the second issue with the nucleolar model, there are cellular states such as quiescence or senescence, in which nucleolar activity is very much lower than in highly proliferating cells. Cells with low nucleolar activity have nucleoli that display a distinctive morphology [29, 128–130]. Under these conditions, the maintenance of low p53 levels may be more difficult to reconcile with a model that depends on nucleolar function to promote p53 degradation, than with a model where constitutive, nucleolus-independent p53 degradation is inhibited by stress-induced re-localization of nucleolar/ribosomal components. However, it is interesting to recall that early work on p53 regulation showed that p53 mRNA levels were undetectable in quiescent 3T3 fibroblasts [131] and in resting peripheral blood lymphocytes [132], whereas p53 mRNA was expressed at much higher levels in cycling 3T3 fibroblasts and in lectin-stimulated or transformed peripheral blood lymphocytes, respectively. This suggests that in resting cells, p53 protein levels may be controlled transcriptionally, rather than post-translationally. In this event, low nucleolar activity would no longer constitute a problem for the nucleolar model of p53 regulation, since it would be by-passed in such conditions and this is currently under investigation. It is worth considering that the maintenance of low levels of p53 at a transcriptional level in such non-proliferating cells might also contribute to the therapeutic index of nucleolus-stressing chemotherapeutic agents.
Limitations of factor re-localization models
As described above, re-localization of nucleolar/ribosomal proteins accompanies many nucleolar stresses. Due to the capacity of some of these proteins to interact with MDM2 and/or p53 and to inhibit MDM2-mediated p53 ubiquitylation, this re-localization can be expected to contribute to p53 stabilization. It is, however, not clear which stresses use this mechanism and, for those that do, whether such re-localization is a major (or exclusive) mechanism of action or merely an adjunct to a direct nucleolar regulatory pathway. The most extensively studied factor that is re-localized by stress and interacts with MDM2 is probably the large subunit ribosomal protein L11. Lohrum et al. [133] originally reported that following low-dose actinomycin D treatment, levels of nucleoplasmic L11 increased with a concomitant increase in MDM2 that co-immunoprecipitated with L11 and also with p53 stabilization (importantly, these experiments examined endogenous L11 and MDM2). The authors proposed that nucleolar/ribosomal stress causes an increase in nucleoplasmic L11 concentration through translocation, with L11 then interacting with MDM2, leading to inhibition of MDM2-mediated degradation of p53 [133]. One major problem with L11-dependent transduction of nucleolar stress into a p53 response is that the increased L11-MDM2 co-immunoprecipitation observed when cells are treated with actinomycin D is absent following UV irradiation. As discussed above, UV irradiation is the p53-stabilizing stress for which a nucleolar involvement is most clearly demonstrable. This suggests that an L11-dependent mechanism might not be applicable to other p53-inducing stresses. Others, however, have proposed that L11, MDM2, p53, and ARF form a quaternary complex in the absence of nucleolar stress, as has been demonstrated in transient transfection experiments [134]. It was further suggested that L11 does not prevent p53–MDM2 interaction, but rather that it inhibits the ubiquitylation of p53 that would otherwise result from this [134], thus potentially confirming and extending the observations of Lohrum et al. [133]. However, the opposite effect was described for MDMX poly-ubiquitylation, since binding of L11 (but not of L5 or L23) to MDM2 promotes MDM2-mediated poly-ubiquitylation of MDMX [135], which is also promoted by ARF binding to MDM2 [136]. MDMX is another protein that can contribute to the regulation of p53 steady-state levels by augmenting MDM2-mediated ubiquitylation of p53 and interestingly it can also be sequestered in nucleoli by binding to ARF [137, 138]. Whether the nucleolar localization of MDMX has any relevance to the p53 regulation model discussed here is not yet known and clearly this merits further investigation. Other studies have suggested that the mechanism by which L11 modulates p53 protein levels may be somewhat more complicated than simply inhibiting p53 poly-ubiquitylation. Using transient transfection of p53, MDM2, and L11 (in p53-null H1299 cells) Dai et al. [139] showed that transfected p53 was poly-ubiquitylated and stabilized in the presence of co-transfected L11 or ARF, but not with co-transfected L5 or L23 (albeit L23 does stabilize p53 to a lesser extent). This study also demonstrated that transfected MDM2 was poly-ubiquitylated in the presence of L11 and this was likely the result of auto-ubiquitylation, since it depended upon an intact MDM2 RING finger in MDM2-/- murine fibroblasts. Using an in vitro degradation system, the authors showed that L11 could inhibit 26S proteasome-mediated degradation of ubiquitylated MDM2. These studies suggest that MDM2 in L11-MDM2-p53 ternary complexes can still promote p53 poly-ubiquitylation and since such complexes also appeared to protect poly-ubiquitylated MDM2 from 26S proteasomal degradation, the authors hypothesized that p53 might similarly be protected from degradation, though this was not explicitly tested. Further studies are clearly needed to determine how L11, MDM2, and p53 interact in cells at physiological levels and what the consequences of such interactions are. Given the role of sub-cellular compartmentalization in p53 stabilization, including spatial and dynamic information in such future studies might prove particularly informative. As discussed earlier in the context of heterokaryon studies, the use of transient transfections to increase the expression of p53, MDM2, and any interacting proteins presents potential problems for studies of p53 regulation. For example, differences in the transfection ratios of MDM2 and L11 can have dramatic effects on the nuclear localization of both proteins, with L11:MDM2 ratios of 2:1 showing a predominantly nucleoplasmic localization of both proteins and the higher ration of 4:1 showing a more nucleolar localization [133]. In addition, transient expression systems make it difficult to determine whether any protein–protein interactions or changes in ubiquitylation result in changes in steady-state levels.
Studies of the role of ribosomal protein L11 in regulating p53 stabilization have also explored other modes of modulation of levels of free L11, which may represent a distinct situation to stress-induced re-localization from the nucleolus to the nucleoplasm. For example, treatment with 5-FU, concomitant with p53 induction, increases the level of ribosome-free ribosomal proteins L5, L11, and L23 [140]. Fumagalli et al. [141] studied the effect of siRNA-mediated reduction of ribosomal protein S6, which was also associated with p53 stabilization, and reported an increase in ribosome-free L11 (although down-regulation of L7 had the opposite effect on L11). Given that both studies found increased L11 binding to MDM2, both groups proposed that these ribosome-free proteins, found in the cytoplasmic fractions loaded onto gradients, would inhibit MDM2-mediated p53 poly-ubiquitylation in a manner similar to that proposed for actinomycin D-induced nucleoplasmic accumulation of L11 (above). However, it seems unlikely that this re-distribution of L11 could provide a general mechanism for the regulation of p53 homeostasis by cellular stresses since, as discussed earlier, it does not explain p53 stabilization local to a nucleus [72, 111], nor the local regulation of p53 levels by micronuclei [72, 142]. Nevertheless, a key feature of the report by Fumagalli et al. [141] is that it confirms the ribosomal biogenesis-linked regulation of p53 stabilization in circumstances that will not induce significant nucleolar disruption as measured by nucleolar/nucleoplasmic re-localization of NHP2, nucleolin, or ribosomal protein L7a. In related studies, Hölzel et al. [39] showed that depletion of the 18S rRNA maturation factor hUTP18 results in p53 stabilization and that this is dependent on the presence of L11. It is interesting to note that the main evidence for involvement of L11 in p53 stabilization in these reports derived from siRNA-mediated depletion of L11 which, in all cases, resulted in the abrogation of p53 stabilization. However, depletion of ribosomal proteins causes selective changes in the protein and mRNA composition of polysomes [140, 141], particularly for the mRNAs of ribosomal proteins and of β-actin [141]. It would therefore be important to determine whether the ribosomal and polysomal changes induced might also cause alterations in the polysomal loading of p53 mRNA or affect p53 translation. Since the main support for the involvement of L11 in such mechanisms comes from abrogation of p53 stabilization by siRNA-mediated depletion of L11, potential changes in p53 synthesis have to be addressed in order to be able to interpret these experiments. This is an important question in view of reports describing variously: interaction of p53 with 5.8S rRNA [143]; association of cytoplasmic p53 with ribosomes [144]; and translational control of p53 being dependent both on ribosomal proteins [145–147] and binding of p53 protein to the p53 5′UTR [148, 149]. It seems likely that the issue of p53 protein stabilization under conditions of altered polysomal profiles, such as those induced by down-regulation of some ribosomal proteins, is more complex than simply the effect of varying the levels of free L11 on the interaction between p53 and MDM2.
In summary, it is clear that binding of endogenous ribosomal protein L11 to MDM2 is increased following actinomycin D treatment at doses expected to have a selective effect on nucleoli [133], and it seems reasonable to assume that this binding must have an effect on p53 homeostasis. However, as stated above, the generality of this mechanism may be limited. For example, this process does not appear to be involved in the UV-induced stress response [133]. Furthermore, p53 regulation by L11 may not be simply a matter of competitive or allosteric inhibition of the ubiquitin ligase activity of MDM2 towards p53, since there is evidence of stabilization of p53 in poly-ubiquitylated forms by transient transfection of L11 [139].
Spoiling the ride
As discussed above, the present model of direct nucleolar control of p53 homeostasis can explain the broad range of conditions that modulate p53 steady-state levels and also incorporates the observation of “local” regulation of p53 levels. Importantly, it provides a framework that might explain the many interactions between MDM2 and nucleolar/ribosomal proteins so conspicuously reported, as well as offering a possible physiological role for them. As discussed elsewhere in this review and represented in Fig. 2, p53 stabilizing agents have pleiotropic effects, causing cell stresses that signal to p53 and MDM2 by a variety of post-translational modifications, some of which impair the interaction between p53 and MDM2 [7, 9, 10]. Key modifications implicated in this process are phosphorylation of evolutionarily conserved residues Ser-15 and Ser-20 by the ATM and (depending upon the stress) CHK1/CHK2/DAPK kinases, respectively [150]. Since these modifications have been proposed to stabilize p53 [7, 9], they provide plausible mechanisms directly linking stresses to p53 induction. As discussed above, a key issue in nucleolar control of p53 is to determine which stresses activate p53 directly and which (if any) do so via nucleolar disruption. However, there are two circumstances in which p53 stabilization has so far eluded explanation through direct action of stresses over p53. These are delayed exit from mitosis and micronucleation, in neither of which does the p53-dependent cell cycle arrest that ensues appear to depend on sensing DNA damage, since it has been observed both when checkpoint kinases are inhibited [151], and when DNA damage could not be detected [72, 151–153]. The nucleolar model of p53 regulation not only offers a potential explanation for these conditions leading to p53 stabilization, and in fact it could be argued that it predicts that they must occur, since both are situations akin to “cells without” nucleoli and therefore these are cells that lack the ability to promote p53 degradation. Colcemid micronucleated cells harbor micronuclei with and without nucleoli since some micronuclei will not contain nucleolar organizer regions (NORs [154]). In such cells, the nucleolar model not only explains p53 stabilization but it also explains the fact that not all micronuclei show high p53 levels [153]. Indeed, high p53 levels anti-correlate with the presence of nucleoli in this system [72] (see also [142]). During mitosis, nucleolar function ceases and nucleoli are largely disassembled [32, 155] and therefore the period without nucleolar function is expected to be prolonged when mitotic exit is delayed which, according to the direct nucleolar model, would result in raised p53 levels. In accordance with this, a p53-dependent post-mitotic arrest is in fact observed [151, 152] and it would be interesting to confirm whether the delay in nucleolar reformation is responsible, since inhibition of mitotic exit has been reported to be a better chemotherapeutic target than the mitotic spindle assembly [156].
An important consequence of nucleolar control of the regulation of p53 stability is that the dependence on fully functional nucleoli demands that cells actively overcome a number of metabolic and biochemical hurdles to promote p53 degradation. For example, there must be a sufficient pool of NTPs in order to synthesize massive amounts of rRNA [157]; RNA Pol I transcription must be able to proceed unimpeded, for which rDNA must suffer no DNA damage, chemical intercalation, or topological constraint; RNA Pol II must be able to support mRNA production (presumably critical for nucleolar/ribosomal protein turnover); mitosis must finish correctly and not be delayed in order to allow for timely nucleolar reformation; and finally, it is likely that there must be no environmental stresses activating JNK kinases, which would otherwise suppress rRNA synthesis. Only if these conditions are simultaneously met will proliferating cells be able to keep steady-state levels of p53 low enough to avoid induction of cell cycle arrest or apoptosis. However, we do not yet fully understand how failures in these requirements are translated into disruption of nucleolar function, and this information would be crucial for the development of therapeutically useful ways of activating p53. Cancer therapy has long used agents that impair one or more of these cellular requirements, and therefore we may already have a wealth of information on mechanisms of nucleolar disruption and their therapeutic consequences. However, since these agents also cause other cellular stresses, notably DNA damage, to make use of this information we must first determine whether nucleolar disruption is in fact a significant component of the mechanisms of action of current anti-cancer agents. Furthermore, the effectiveness of many of these compounds in cancers with mutant p53 suggests that if they really involve nucleolar disruption as a mechanism of action, the direct targeting of nucleolar function might provide therapeutic benefit to a wider range of patients. Therefore, we will next discuss some of the evidence implicating a direct nucleolar mode of action for some common chemotherapeutic agents.
5-fluorouracyl (5-FU)
It is generally accepted that 5-FU acts both by competitive inhibition of thymidylate synthase (TS) and by incorporation of metabolites into DNA and RNA (for a recent review see [158]). Since TS participates in the de novo synthesis of thymidine (converting dUMP into dTMP), inhibition with 5-FU impairs DNA replication and repair (“thymineless death”). In addition, dUTP accumulates and is misincorporated into DNA causing futile cycles of DNA repair that can lead to strand breaks, and the further accumulation and misincorporation of FdUTP (a metabolite of 5-FU) [158]. Thus, DNA replication defects constitute the best understood mechanism of 5-FU induced cell death. However, it is notable that the addition of uridine, rather than thymidine can inhibit p53 induction by 5-FU [158, 159]. Since TS inhibition alone cannot fully explain the failure to suppress the effects of 5-FU with thymidine (the induction of apoptosis by the TS inhibitor Tomudex, for example, can be effectively abolished by thymidine [159]), this suggests that effects on RNA may play an important role in the induction of p53 by 5-FU. While an RNA-mediated mechanism of action for p53 induction by 5-FU remains unclear, it has been demonstrated that 5-FU inhibits pre-rRNA processing, suggesting that this may be an important mechanism of action [160, 161]. Interestingly, 5-FU inhibits ribosome maturation and causes accumulation of pre-rRNA [29], but no major alterations of nucleolar morphology are observed, and indeed there is little or no re-localization of B23/NPM [21, 29]. It is therefore possible that impairment of ribosomal biogenesis is a major mechanism for p53 induction by 5-FU.
Cisplatin
Cisplatin (cis-diammineplatinum(II) dichloride–cis-Pt) is one of the most commonly used anti-cancer drugs. Since it severely bends DNA (from 39° up to 78° towards the major groove, depending on the DNA and state in which the structure is studied [162]), it has been assumed that it induces p53 stabilization through DNA damage, as well as by inhibition of transcription [163, 164]. As a consequence of this DNA bending, cisplatin–DNA adducts also bind high-mobility-group domain (HMG) proteins [165]. In contrast, the trans isomer transplatin, while also a DNA damaging agent does not bind HMG proteins and is a poor inducer of cell death [163]. Both cisplatin and transplatin inhibit replication, but only cisplatin causes significant nucleolar alterations [166]. The RNA Pol I factor UBF, contains HMG boxes [44] and efficiently binds cisplatin–DNA adducts. When cisplatin–DNA adducts are introduced, UBF is redistributed out of nucleoli [166], leading to significant nucleolar segregation. Zhai et al. [167] have shown that cisplatin–DNA adducts (and adducts of other therapeutically effective Pt compounds, but not of transplatin) efficiently bind UBF and that this “UBF hijacking” inhibits rRNA transcription in vitro. In addition, over-expression of HMG proteins, particularly UBF, has been linked with cisplatin sensitivity [168, 169]. Thus, the differential effect of cis- and trans-platin on HMG box-containing proteins, and in particular on UBF and nucleoli, may provide an explanation for the previously unexplained difference between the cellular effects of cis- and trans-platin. It is also interesting to note that cisplatin is toxic for post-mitotic neurons, which provides further support for a mechanism of action that does not depend on replication [170].
Camptothecin
It is widely accepted that the topoisomerase I (Topo I) inhibitor and anti-cancer agent camptothecin induces apoptosis by stabilizing DNA breaks introduced by Topo I, which subsequently stall replication and cause DNA damage [171]. However, since Topo I is required to relieve topological constraints in transcription it is, not surprisingly, abundant in nucleoli where it participates in rDNA transcription and translocates away from nucleoli upon inhibition of rRNA synthesis [172]. Likewise, camptothecin treatment leads to de-localization of both Topo I and B23/NPM from nucleoli [173]. This suggests that in addition to a role in S-phase (stalling replication forks) camptothecin may also have a nucleolar-targeting mechanism of action. The possibility of an S-phase-independent mechanism of cell death induction by camptothecin is supported by studies showing that camptothecin promotes apoptosis in post-mitotic, differentiated neurons where the effect is expected to be independent of DNA replication [174, 175]. Interestingly, inhibitors of topoisomerase II at the cleavable complex stage seem to have the same effect on post-mitotic neurons [176].
Concluding remarks
It is now well accepted that disruption of nucleolar function can have an impact on p53 stability, a concept that is now guiding the development of new drugs for the therapeutic activation of p53 such as CX-5461 [44, 62, 177]. In this review, we have argued that the link between nucleolar function and p53 homeostasis is due to the fact that key steps in the regulation of p53 by MDM2, such as p53 poly-ubiquitylation and nuclear export, occur within nucleoli and probably make use of parts of the ribosomal biosynthesis machinery. The molecular processes involved in the nucleolar stage of p53 regulation are still to be elucidated. So far we know that both p53 and MDM2 transit through nucleoli; that mutation of a nucleolar localization region of MDM2 selectively reduces poly-ubiquitylation, but not mono-ubiquitylation of p53 or auto-ubiquitylation (the same effect on ubiquitylation that is caused by ARF over-expression); and that nucleolar p53 is selectively enriched in poly-ubiquitylated forms. At the cellular level, we have observed that the steady-state level of p53 displayed by a cell nucleus is not affected by the presence of another nucleus co-cytoplasmic with it, whatever the history of stress of the latter. The next step will be to understand how all these events link together into a mechanism that explains the nucleolar stages of p53 and MDM2 biology and it is possible that these mechanistic insights may inform work on the nucleolar regulation of other important cancer-related proteins such as myc and VHL. We also need to determine whether there is a nucleolar involvement for other p53 regulators such as MDMX, which has been proposed to involve ribosomal proteins in its regulation of p53 [135, 178] and other proteins involved in p53 regulation such as p300, COP1, ARF-BP1, Pirh2, etc. [15].
The role of nucleoli and nucleolar function in regulating p53 stability means that nucleoli may offer many opportunities to impair MDM2-mediated p53 degradation and, as mentioned above, has already resulted in new compounds with therapeutic potential. So far, the specificity for nucleolar function of newly developed agents appears to derive mainly from rDNA binding that results in inhibition of rRNA synthesis [62, 177]. These initial developments should be extended to non-DNA targets of rRNA synthesis, in order to avoid potential off-target effects due to similar DNA motifs that may lie outside rDNA [179], and also to protein targets downstream of rRNA synthesis. As discussed, it is likely that the mechanisms of action of many existing compounds involve a significant nucleolar component. This suggests that there are likely to be several other points in nucleolar function, as well as extra-nucleolar steps in ribosome biosynthesis, not only those affecting rRNA synthesis, with potential for cancer therapy. One of the most significant outcomes of our growing understanding of the role of nucleoli in regulating stress responses is that we should increasingly be able to target different, non-overlapping functions with the potential to enable novel combinatorial approaches that will enhance both the effectiveness and the therapeutic index of existing and novel cancer therapies.
As mentioned earlier, a remarkable property of nucleoli is their vastly increased activity in cancer cells, and more generally in rapidly proliferating cells. The difference between these cells and quiescent cells, where nucleoli have low activity, is likely to contribute to the therapeutic index of agents that cause nucleolar disruption and may be the basis for the clinical effectiveness of current and future nucleolar function inhibitors. A key challenge will be to determine whether we can improve on this discrimination between replicating and quiescent cells by selectively activating a nucleolar stress response in cancer cells as opposed to normal replicating cells. The fact that nucleolar function is modulated by signaling through kinase pathways that are altered in cancer cells, as well as by oncogenes and tumor suppressor genes, suggests that this might be the case. To answer this, the nucleolar response to stress must be examined in the context of cancer-associated mutations and also under the effect of therapeutically useful protein kinase inhibitors. Differential sensitization of nucleoli by exploiting mutations in pathways that regulate nucleolar function might ultimately lead to powerful new combinatorial therapeutic approaches.
Acknowledgments
Work in our laboratory was supported by Mersey Kidney Research, Cancer Research UK, The North West Cancer Research Fund, The Isle of Man Anti Cancer Association, and by The Hampson Trust of the Royal Liverpool University Hospital.
References
- 1.von Bertalanffy L. Problems of life: an evaluation of modern biological and scientific thought. New York: Harper; 1960. [Google Scholar]
- 2.Warner JR, McIntosh KB. How common are extraribosomal functions of ribosomal proteins? Mol Cell. 2009;34(1):3–11. doi: 10.1016/j.molcel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson MO, Hingorani K, Szebeni A. Conventional and nonconventional roles of the nucleolus. Int Rev Cytol. 2002;219:199–266. doi: 10.1016/S0074-7696(02)19014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pederson T, Tsai RYL. In search of nonribosomal nucleolar protein function and regulation. J Cell Biol. 2009;184(6):771–776. doi: 10.1083/jcb.200812014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7(12):979–987. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]
- 6.Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol. 2009;1(5):a001883. doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9(10):714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 8.Bensaad K, Tsuruta A, Selak MA, Vidal MNC, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 9.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 10.Ljungman M. Dial 9-1-1 for p53: mechanisms of p53 activation by cellular stress. Neoplasia. 2000;2:208–225. doi: 10.1038/sj.neo.7900073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane D, Lain S. Therapeutic exploitation of the p53 pathway. Trends Mol Med. 2002;8:S38–S42. doi: 10.1016/s1471-4914(02)02309-2. [DOI] [PubMed] [Google Scholar]
- 12.Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9(12):862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 13.Vlatkovic N, Crawford K, Rubbi CP, Boyd MT. Tissue-specific therapeutic targeting of p53 in cancer: one size does not fit all. Curr Pharm Des. 2011;17(6):618–630. doi: 10.2174/138161211795222568. [DOI] [PubMed] [Google Scholar]
- 14.Senderowicz AM. Targeting cell cycle and apoptosis for the treatment of human malignancies. Curr Opin Cell Biol. 2004;16(6):670–678. doi: 10.1016/j.ceb.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Marine JC, Lozano G. Mdm2-mediated ubiquitylation: p53 and beyond. Cell Death Differ. 2010;17(1):93–102. doi: 10.1038/cdd.2009.68. [DOI] [PubMed] [Google Scholar]
- 16.Freedman DA, Wu L, Levine AJ. Functions of the MDM2 oncoprotein. Cell Mol Life Sci. 1999;55(1):96–107. doi: 10.1007/s000180050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter S, Vousden KH. p53-Ubl fusions as models of ubiquitination, sumoylation and neddylation of p53. Cell Cycle. 2008;7(16):2519–2528. doi: 10.4161/cc.7.16.6422. [DOI] [PubMed] [Google Scholar]
- 18.Carter S, Bischof O, Dejean A, Vousden KH. C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat Cell Biol. 2007;9:428–435. doi: 10.1038/ncb1562. [DOI] [PubMed] [Google Scholar]
- 19.Xirodimas DP. Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochem Soc Trans. 2008;036(5):802–806. doi: 10.1042/BST0360802. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Xiong Y. Control of p53 ubiquitination and nuclear export by MDM2 and ARF. Cell Growth Differ. 2001;12(4):175–186. [PubMed] [Google Scholar]
- 21.Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 2003;22:6068–6077. doi: 10.1093/emboj/cdg579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horn HF, Vousden KH. Cancer: guarding the guardian? Nature. 2004;427:110–111. doi: 10.1038/427110a. [DOI] [PubMed] [Google Scholar]
- 23.Olson MO. Sensing cellular stress: another new function for the nucleolus? Sci STKE. 2004;2004(224):pe10. doi: 10.1126/stke.2242004pe10. [DOI] [PubMed] [Google Scholar]
- 24.Rudra D, Warner JR. What better measure than ribosome synthesis? Genes Dev. 2004;18:2431–2436. doi: 10.1101/gad.1256704. [DOI] [PubMed] [Google Scholar]
- 25.Boulon S, Westman BJ, Hutten S, Boisvert F-M, Lamond AI. The nucleolus under stress. Mol Cell. 2010;40(2):216–227. doi: 10.1016/j.molcel.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burger K, Mühl B, Harasim T, Rohrmoser M, Malamoussi A, Orban M, Kellner M, Gruber-Eber A, Kremmer E, Hölzel M, Eick D. Chemotherapeutic drugs inhibit ribosome biogenesis at various levels. J Biol Chem. 2010;285(16):12416–12425. doi: 10.1074/jbc.M109.074211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan PK. Characterization and cellular localization of nucleophosmin/B23 in HeLa cells treated with selected cytotoxic agents (studies of B23-translocation mechanism) Exp Cell Res. 1992;203:174–181. doi: 10.1016/0014-4827(92)90053-b. [DOI] [PubMed] [Google Scholar]
- 28.Chan PK, Qi Y, Amley J, Koller CA. Quantitation of the nucleophosmin/B23-translocation using imaging analysis. Cancer Lett. 1996;100:191–197. doi: 10.1016/0304-3835(95)04100-1. [DOI] [PubMed] [Google Scholar]
- 29.Hadjiolov AA. The nucleolus and ribosome biogenesis. Wien: Springer; 1985. [Google Scholar]
- 30.Hernandez-Verdun D. Nucleolus: from structure to dynamics. Histochem Cell Biol. 2006;125:127–137. doi: 10.1007/s00418-005-0046-4. [DOI] [PubMed] [Google Scholar]
- 31.Sirri V, Urcuqui-Inchima S, Roussel P, Hernandez-Verdun D. Nucleolus: the fascinating nuclear body. Histochem Cell Biol. 2008;129(1):13–31. doi: 10.1007/s00418-007-0359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sirri V, Hernandez-Verdun D, Roussel P. Cyclin-dependent kinases govern formation and maintenance of the nucleolus. J Cell Biol. 2002;156:969–981. doi: 10.1083/jcb.200201024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louvet E, Junera HR, Berthuy I, Hernandez-Verdun D. Compartmentation of the nucleolar processing proteins in the granular component is a CK2-driven process. Mol Biol Cell. 2006;17:2537–2546. doi: 10.1091/mbc.E05-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sirri V, Roussel P, Hernandez-Verdun D. In vivo release of mitotic silencing of ribosomal gene transcription does not give rise to precursor ribosomal RNA processing. J Cell Biol. 2000;148:259–270. doi: 10.1083/jcb.148.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Digman MA, Brown CM, Sengupta P, Wiseman PW, Horwitz AR, Gratton E. Measuring fast dynamics in solutions and cells with a laser scanning microscope. Biophys J. 2005;89(2):1317–1327. doi: 10.1529/biophysj.105.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer C, Grummt I. Cellular stress and nucleolar function. Cell Cycle. 2005;4:1036–1038. doi: 10.4161/cc.4.8.1925. [DOI] [PubMed] [Google Scholar]
- 37.Grimm T, Hölzel M, Rohrmoser M, Harasim T, Malamoussi A, Gruber-Eber A, Kremmer E, Eick D. Dominant-negative Pes1 mutants inhibit ribosomal RNA processing and cell proliferation via incorporation into the PeBoW-complex. Nucleic Acids Res. 2006;34(10):3030–3043. doi: 10.1093/nar/gkl378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pestov DG, Strezoska Z, Lau LF. Evidence of p53-dependent cross-talk between ribosome biogenesis and the cell cycle: effects of nucleolar protein Bop1 on G(1)/S transition. Mol Cell Biol. 2001;21:4246–4255. doi: 10.1128/MCB.21.13.4246-4255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hölzel M, Orban M, Hochstatter J, Rohrmoser M, Harasim T, Malamoussi A, Kremmer E, Längst G, Eick D. Defects in 18 S or 28 S rRNA processing activate the p53 pathway. J Biol Chem. 2010;285(9):6364–6370. doi: 10.1074/jbc.M109.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohrmoser M, Hölzel M, Grimm T, Malamoussi A, Harasim T, Orban M, Pfisterer I, Gruber-Eber A, Kremmer E, Eick D. Interdependence of Pes1, Bop1, and WDR12 controls nucleolar localization and assembly of the PeBoW complex required for maturation of the 60S ribosomal subunit. Mol Cell Biol. 2007;27(10):3682–3694. doi: 10.1128/MCB.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan X, Zhou Y, Casanova E, Chai M, Kiss E, Gröne H-J, Schütz G, Grummt I. Genetic inactivation of the transcription factor TIF-IA leads to nucleolar disruption, cell cycle arrest, and p53-mediated apoptosis. Mol Cell. 2005;19(1):77–87. doi: 10.1016/j.molcel.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 42.Zhao J, Yuan X, Frödin M, Grummt I. ERK-dependent phosphorylation of the transcription initiation factor TIF-IA is required for RNA Polymerase I transcription and cell growth. Mol Cell. 2003;11(2):405–413. doi: 10.1016/s1097-2765(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 43.White RJ. RNA polymerases I and III, growth control and cancer. Nat Rev Mol Cell Biol. 2005;6(1):69–78. doi: 10.1038/nrm1551. [DOI] [PubMed] [Google Scholar]
- 44.Drygin D, Rice WG, Grummt I. The RNA Polymerase I transcription machinery: an emerging target for the treatment of cancer. Annu Rev Pharmacol Toxicol. 2010;50(1):131–156. doi: 10.1146/annurev.pharmtox.010909.105844. [DOI] [PubMed] [Google Scholar]
- 45.Grewal SS, Li L, Orian A, Eisenman RN, Edgar BA. Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nat Cell Biol. 2005;7:295–302. doi: 10.1038/ncb1223. [DOI] [PubMed] [Google Scholar]
- 46.Grandori C, Gomez-Roman N, Felton-Edkins ZA, Ngouenet C, Galloway DA, Eisenman RN, White RJ. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nat Cell Biol. 2005;7:311–318. doi: 10.1038/ncb1224. [DOI] [PubMed] [Google Scholar]
- 47.Arabi A, Wu S, Ridderstrale K, Bierhoff H, Shiue C, Fatyol K, Fahlen S, Hydbring P, Soderberg O, Grummt I, Larsson LG, Wright AP. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat Cell Biol. 2005;7:303–310. doi: 10.1038/ncb1225. [DOI] [PubMed] [Google Scholar]
- 48.Mayer C, Zhao J, Yuan X, Grummt I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 2004;18(4):423–434. doi: 10.1101/gad.285504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannan KM, Brandenburger Y, Jenkins A, Sharkey K, Cavanaugh A, Rothblum L, Moss T, Poortinga G, McArthur GA, Pearson RB, Hannan RD. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol. 2003;23(23):8862–8877. doi: 10.1128/MCB.23.23.8862-8877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhai W, Comai L. Repression of RNA polymerase I transcription by the tumor suppressor p53. Mol Cell Biol. 2000;20:5930–5938. doi: 10.1128/mcb.20.16.5930-5938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Budde A, Grummt I. p53 represses ribosomal gene expression. Oncogene. 1999;19:1119–1124. doi: 10.1038/sj.onc.1202402. [DOI] [PubMed] [Google Scholar]
- 52.Rieker C, Engblom D, Kreiner G, Domanskyi A, Schober A, Stotz S, Neumann M, Yuan X, Grummt I, Schütz G, Parlato R. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J Neurosci. 2011;31(2):453–460. doi: 10.1523/JNEUROSCI.0590-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugimoto M, Kuo ML, Roussel MF, Sherr CJ. Nucleolar Arf tumor suppressor inhibits ribosomal RNA processing. Mol Cell. 2003;11:415–424. doi: 10.1016/s1097-2765(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 54.Bertwistle D, Sugimoto M, Sherr CJ. Physical and functional interactions of the Arf tumor suppressor protein with Nucleophosmin/B23. Mol Cell Biol. 2004;24(3):985–996. doi: 10.1128/MCB.24.3.985-996.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaizumi M, Sugano T. U.V.-induced nuclear accumulation of p53 is evoked through DNA damage of actively transcribed genes independent of the cell cycle. Oncogene. 1994;9:2775–2784. [PubMed] [Google Scholar]
- 56.Ljungman M, Zhang F. Blockage of RNA polymerase as a possible trigger for u.v. light-induced apoptosis. Oncogene. 1996;13:823–831. [PubMed] [Google Scholar]
- 57.Trask DK, Muller MT. Stabilization of type I topoisomerase-DNA covalent complexes by actinomycin D. Proc Natl Acad Sci USA. 1988;85(5):1417–1421. doi: 10.1073/pnas.85.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashcroft M, Taya Y, Vousden KH. Stress signals utilize multiple pathways to stabilize p53. Mol Cell Biol. 2000;20:3224–3233. doi: 10.1128/mcb.20.9.3224-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choong ML, Yang H, Lee MA, Lane DP. Specific activation of the p53 pathway by low dose actinomycin D: a new route to p53 based cyclotherapy. Cell Cycle. 2009;8(17):2810–2818. doi: 10.4161/cc.8.17.9503. [DOI] [PubMed] [Google Scholar]
- 60.Drygin D, Lin A, Bliesath J, Ho CB, O’Brien SE, Proffitt C, Omori M, Haddach M, Schwaebe MK, Siddiqui-Jain A, Streiner N, Quin JE, Sanij E, Bywater MJ, Hannan RD, Ryckman D, Anderes K, Rice WG. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res. 2011;71(4):1418–1430. doi: 10.1158/0008-5472.CAN-10-1728. [DOI] [PubMed] [Google Scholar]
- 61.Perry RP, Kelley DE. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- 62.Bywater Megan J, Poortinga G, Sanij E, Hein N, Peck A, Cullinane C, Wall M, Cluse L, Drygin D, Anderes K, Huser N, Proffitt C, Bliesath J, Haddach M, Schwaebe Michael K, Ryckman David M, Rice William G, Schmitt C, Lowe Scott W, Johnstone Ricky W, Pearson Richard B, McArthur Grant A, Hannan Ross D. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell. 2012;22(1):51–65. doi: 10.1016/j.ccr.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vassilev LT. MDM2 inhibitors for cancer therapy. Trends Mol Med. 2007;13(1):23–31. doi: 10.1016/j.molmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Sherr CJ, Weber JD. The ARF/p53 pathway. Curr Opin Genet Dev. 2000;10:94–99. doi: 10.1016/s0959-437x(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 65.Roth J, Dobbelstein M, Freedman DA, Shenk T, Levine AJ. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 1998;17:554–564. doi: 10.1093/emboj/17.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hiscox JA. RNA viruses: hijacking the dynamic nucleolus. Nat Rev Microbiol. 2007;5:119–127. doi: 10.1038/nrmicro1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 68.Freedman DA, Levine AJ. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol. 1998;18:7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laín S, Midgley C, Sparks A, Lane EB, Lane DP. An inhibitor of nuclear export activates the p53 response and induces the localization of HDM2 and p53 to U1A-positive nuclear bodies associated with the PODs. Exp Cell Res. 1999;248:457–472. doi: 10.1006/excr.1999.4433. [DOI] [PubMed] [Google Scholar]
- 70.Xirodimas DP, Stephen CW, Lane DP. Cocompartmentalization of p53 and Mdm2 is a major determinant for Mdm2-mediated degradation of p53. Exp Cell Res. 2001;270:66–77. doi: 10.1006/excr.2001.5314. [DOI] [PubMed] [Google Scholar]
- 71.Li M, Brooks CL, Wu-Baer F, Chen D, Baer R, Gu W. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science. 2003;302:1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- 72.Boyd MT, Vlatković N, Rubbi CP. The nucleolus directly regulates p53 export and degradation. J Cell Biol. 2011;194(5):689–703. doi: 10.1083/jcb.201105143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shirangi TR, Zaika A, Moll UM. Nuclear degradation of p53 occurs during down-regulation of the p53 response after DNA damage. FASEB J. 2002;16:420–422. doi: 10.1096/fj.01-0617fje. [DOI] [PubMed] [Google Scholar]
- 74.Joseph TW, Zaika A, Moll UM. Nuclear and cytoplasmic degradation of endogenous p53 and HDM2 occurs during down-regulation of the p53 response after multiple types of DNA damage. FASEB J. 2003;17:1622–1630. doi: 10.1096/fj.02-0931com. [DOI] [PubMed] [Google Scholar]
- 75.Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Xiong Y. Mutations in the human ARF exon 2 disrupt its nucleolar localization and impair its ability to block nuclear export of MDM2 and p53. Mol Cell. 1999;3:579–591. doi: 10.1016/s1097-2765(00)80351-2. [DOI] [PubMed] [Google Scholar]
- 77.Tao W, Levine AJ. p19ARF stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc Natl Acad Sci USA. 1999;96:6937–6941. doi: 10.1073/pnas.96.12.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mekhail K, Gunaratnam L, Bonicalzi ME, Lee S. HIF activation by pH-dependent nucleolar sequestration of VHL. Nat Cell Biol. 2004;6:642–647. doi: 10.1038/ncb1144. [DOI] [PubMed] [Google Scholar]
- 79.Tao W, Levine AJ. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc Natl Acad Sci USA. 1999;96:3077–3080. doi: 10.1073/pnas.96.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 1999;18:1660–1672. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyd SD, Tsai KY, Jacks T. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nat Cell Biol. 2000;2:563–568. doi: 10.1038/35023500. [DOI] [PubMed] [Google Scholar]
- 82.Geyer RK, Yu ZK, Maki CG. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nat Cell Biol. 2000;2:569–573. doi: 10.1038/35023507. [DOI] [PubMed] [Google Scholar]
- 83.Gu J, Nie L, Wiederschain D, Yuan ZM. Identification of p53 sequence elements that are required for MDM2-mediated nuclear export. Mol Cell Biol. 2001;21:8533–8546. doi: 10.1128/MCB.21.24.8533-8546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Llanos S, Clark PA, Rowe J, Peters G. Stabilization of p53 by p14ARF without relocation of MDM2 to the nucleolus. Nat Cell Biol. 2001;3:445–452. doi: 10.1038/35074506. [DOI] [PubMed] [Google Scholar]
- 85.Xirodimas D, Saville MK, Edling C, Lane DP, Lain S. Different effects of p14ARF on the levels of ubiquitinated p53 and Mdm2 in vivo. Oncogene. 2001;20:4972–4983. doi: 10.1038/sj.onc.1204656. [DOI] [PubMed] [Google Scholar]
- 86.Lohrum MA, Ashcroft M, Kubbutat MH, Vousden KH. Identification of a cryptic nucleolar-localization signal in MDM2. Nat Cell Biol. 2000;2:179–181. doi: 10.1038/35004057. [DOI] [PubMed] [Google Scholar]
- 87.Ahmad Y, Boisvert F-M, Gregor P, Cobley A, Lamond AI. NOPdb: nucleolar Proteome Database—2008 update. Nucleic Acids Res. 2009;37(suppl 1):D181–D184. doi: 10.1093/nar/gkn804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- 89.Cummins JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B. Disruption of HAUSP gene stabilizes p53. Nature. 2004;428:1. doi: 10.1038/nature02501. [DOI] [PubMed] [Google Scholar]
- 90.Cummins JM, Vogelstein B. HAUSP is required for p53 destabilization. Cell Cycle. 2004;3:689–692. [PubMed] [Google Scholar]
- 91.Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol Cell. 2004;13:879–886. doi: 10.1016/s1097-2765(04)00157-1. [DOI] [PubMed] [Google Scholar]
- 92.Gray LJ, Bjelogrlic P, Appleyard VCL, Thompson AM, Jolly CE, Lain S, Herrington CS. Selective induction of apoptosis by leptomycin B in keratinocytes expressing HPV oncogenes. Int J Cancer. 2007;120(11):2317–2324. doi: 10.1002/ijc.22591. [DOI] [PubMed] [Google Scholar]
- 93.Mekhail K, Khacho M, Carrigan A, Hache RR, Gunaratnam L, Lee S. Regulation of ubiquitin ligase dynamics by the nucleolus. J Cell Biol. 2005;170:733–744. doi: 10.1083/jcb.200506030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boulon S, Verheggen C, Jady BE, Girard C, Pescia C, Paul C, Ospina JK, Kiss T, Matera AG, Bordonne R, Bertrand E. PHAX and CRM1 are required sequentially to transport U3 snoRNA to nucleoli. Mol Cell. 2004;16:777–787. doi: 10.1016/j.molcel.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 95.Sleeman J. A regulatory role for CRM1 in the multi-directional trafficking of splicing snRNPs in the mammalian nucleus. J Cell Sci. 2007;120(9):1540–1550. doi: 10.1242/jcs.001529. [DOI] [PubMed] [Google Scholar]
- 96.Sleeman JE, Ajuh P, Lamond AI. snRNP protein expression enhances the formation of Cajal bodies containing p80-coilin and SMN. J Cell Sci. 2001;114(24):4407–4419. doi: 10.1242/jcs.114.24.4407. [DOI] [PubMed] [Google Scholar]
- 97.Pradet-Balade B, Girard C, Boulon S, Paul C, Azzag K, Bordonne R, Bertrand E, Verheggen C. CRM1 controls the composition of nucleoplasmic pre-snoRNA complexes to licence them for nucleolar transport. EMBO J. 2011;30(11):2205–2218. doi: 10.1038/emboj.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Muro E, Hoang TQ, Jobart-malfait A, Dl Hernandez-verdun. In nucleoli, the steady state of nucleolar proteins is leptomycin B-sensitive. Biol Cell. 2008;100(5):303–313. doi: 10.1042/BC20070117. [DOI] [PubMed] [Google Scholar]
- 99.Rubbi CP, Milner J. Non-activated p53 co-localizes with sites of transcription within both the nucleoplasm and the nucleolus. Oncogene. 2000;19:85–96. doi: 10.1038/sj.onc.1203378. [DOI] [PubMed] [Google Scholar]
- 100.Klibanov SA, O’Hagan HM, Ljungman M. Accumulation of soluble and nucleolar-associated p53 proteins following cellular stress. J Cell Sci. 2001;114:1867–1873. doi: 10.1242/jcs.114.10.1867. [DOI] [PubMed] [Google Scholar]
- 101.Mélèse T, Xue Z. The nucleolus: an organelle formed by the act of building a ribosome. Curr Opin Cell Biol. 1995;7(3):319–324. doi: 10.1016/0955-0674(95)80085-9. [DOI] [PubMed] [Google Scholar]
- 102.Macias E, Jin AW, Deisenroth C, Bhat K, Mao H, Lindstrom MS, Zhang YP. An ARF-Independent c-MYC-activated tumor suppression pathway mediated by ribosomal protein-Mdm2 interaction. Cancer Cell. 2010;18(3):231–243. doi: 10.1016/j.ccr.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Polanski R (2009) Identification of MDM2-interacting proteins in renal cell carcinoma and characterization of phenotypic properties acquired by renal cell carcinoma cells which over-express MDM2 and p53. PhD Thesis, Liverpool, Liverpool
- 104.Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 2009;16(5):369–377. doi: 10.1016/j.ccr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56:379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- 106.Chen D, Huang S. Nucleolar components involved in ribosome biogenesis cycle between the nucleolus and nucleoplasm in interphase cells. J Cell Biol. 2001;153:169–176. doi: 10.1083/jcb.153.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Finch RA, Chan PK. ATP depletion affects NPM translocation and exportation of rRNA from nuclei. Biochem Biophys Res Commun. 1996;222:553–558. doi: 10.1006/bbrc.1996.0782. [DOI] [PubMed] [Google Scholar]
- 108.Tsai RY, McKay RD. A multistep, GTP-driven mechanism controlling the dynamic cycling of nucleostemin. J Cell Biol. 2005;168:179–184. doi: 10.1083/jcb.200409053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lam YW, Lamond AI, Mann M, Andersen JS. Analysis of nucleolar protein dynamics reveals the nuclear degradation of ribosomal proteins. Curr Biol. 2007;17(9):749–760. doi: 10.1016/j.cub.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cassio D (2006) Viable hybrids between adherent cells: generation, yield improvement, and analysis. In: Julio EC (ed) Cell biology. 3rd edn. Academic Press, Burlington, pp 231–235. doi:10.1016/b978-012164730-8/50030-7
- 111.Komarova EA, Zelnick CR, Chin D, Zeremski M, Gleiberman AS, Bacus SS, Gudkov AV. Intracellular localization of p53 tumor suppressor protein in gamma-irradiated cells is cell cycle regulated and determined by the nucleus. Cancer Res. 1997;57:5217–5220. [PubMed] [Google Scholar]
- 112.Marchenko ND, Wolff S, Erster S, Becker K, Moll UM. Monoubiquitylation promotes mitochondrial p53 translocation. EMBO J. 2007;26:923–934. doi: 10.1038/sj.emboj.7601560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Krüger T, Scheer U. p53 localizes to intranucleolar regions distinct from the ribosome production compartments. J Cell Sci. 2010;123(8):1203–1208. doi: 10.1242/jcs.062398. [DOI] [PubMed] [Google Scholar]
- 114.Maki CG, Howley PM. Ubiquitination of p53 and p21 is differentially affected by ionizing and UV radiation. Mol Cell Biol. 1997;17(1):355–363. doi: 10.1128/mcb.17.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Hagan HM, Ljungman M. Nuclear accumulation of p53 following inhibition of transcription is not due to diminished levels of MDM2. Oncogene. 2004;23:5505–5512. doi: 10.1038/sj.onc.1207709. [DOI] [PubMed] [Google Scholar]
- 116.Solovei I, Schermelleh L, Albiez H, Cremer T. Detection of cell cycle stages in situ in growing cell populations. In: Celis JE, editor. Cell biology. 3. Burlington: Academic Press; 2006. pp. 291–299. [Google Scholar]
- 117.Latonen L, Moore HM, Bai B, Jaamaa S, Laiho M. Proteasome inhibitors induce nucleolar aggregation of proteasome target proteins and polyadenylated RNA by altering ubiquitin availability. Oncogene. 2011;30:790–805. doi: 10.1038/onc.2010.469. [DOI] [PubMed] [Google Scholar]
- 118.Wild T, Horvath P, Wyler E, Widmann B, Badertscher L, Zemp I, Kozak K, Csucs G, Lund E, Kutay U. A protein inventory of human ribosome biogenesis reveals an essential function of Exportin 5 in 60S subunit export. PLoS Biol. 2010;8(10):e1000522. doi: 10.1371/journal.pbio.1000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scharf A, Rockel T, von Mikecz A. Localization of proteasomes and proteasomal proteolysis in the mammalian interphase cell nucleus by systematic application of immunocytochemistry. Histochem Cell Biol. 2007;127(6):591–601. doi: 10.1007/s00418-006-0266-2. [DOI] [PubMed] [Google Scholar]
- 120.Lippincott-Schwartz J, Altan-Bonnet N, Patterson GH (2003) Photobleaching and photoactivation: following protein dynamics in living cells. Nat Cell Biol Suppl:S7–14. doi:10.1038/ncb1032 [PubMed]
- 121.Karni-Schmidt O, Friedler A, Zupnick A, McKinney K, Mattia M, Beckerman R, Bouvet P, Sheetz M, Fersht A, Prives C. Energy-dependent nucleolar localization of p53 in vitro requires two discrete regions within the p53 carboxyl terminus. Oncogene. 2007 doi: 10.1038/sj.onc.1210162. [DOI] [PubMed] [Google Scholar]
- 122.Brangwynne CP, Mitchison TJ, Hyman AA. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci USA. 2011;108(11):4334–4339. doi: 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Audas Timothy E, Jacob Mathieu D, Lee S. Immobilization of proteins in the nucleolus by ribosomal intergenic spacer noncoding RNA. Mol Cell. 2012;45(2):147–157. doi: 10.1016/j.molcel.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 124.Audas TE, Jacob MD, Lee S. The nucleolar detention pathway: a cellular strategy for regulating molecular networks. Cell Cycle. 2012;11(11):2059–2062. doi: 10.4161/cc.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moore HM, Bai B, Boisvert F-M, Latonen L, Rantanen V, Simpson JC, Pepperkok R, Lamond AI, Laiho M (2011) Quantitative proteomics and dynamic imaging of the nucleolus reveal distinct responses to UV and ionizing radiation. Mol Cell Proteomics 10(10). doi: 10.1074/mcp.M111.009241 [DOI] [PMC free article] [PubMed]
- 126.Rockel TD, Stuhlmann D, von Mikecz A. Proteasomes degrade proteins in focal subdomains of the human cell nucleus. J Cell Sci. 2005;118(22):5231–5242. doi: 10.1242/jcs.02642. [DOI] [PubMed] [Google Scholar]
- 127.Andersen JS, Lyon CE, Fox AH, Leung AK, Lam YW, Steen H, Mann M, Lamond AI. Directed proteomic analysis of the human nucleolus. Curr Biol. 2002;12:1–11. doi: 10.1016/s0960-9822(01)00650-9. [DOI] [PubMed] [Google Scholar]
- 128.Baserga R, Huang C-H, Rossini M, Chang H, Ming P-ML. The role of nuclei and nucleoli in the control of cell proliferation. Cancer Res. 1976;36((11 Part 2)):4297–4300. [PubMed] [Google Scholar]
- 129.Puvion-Dutilleul F, Macieira-Coelho A. Aging-dependent nucleolar and chromatin changes in cultivated fibroblasts. Cell Biol Int Rep. 1983;7(1):61–71. doi: 10.1016/0309-1651(83)90105-4. [DOI] [PubMed] [Google Scholar]
- 130.Bowman PD, Meek RL, Daniel CW. Decreased synthesis of nucleolar RNA in aging human cells in vitro. Exp Cell Res. 1976;101(2):434–437. doi: 10.1016/0014-4827(76)90399-2. [DOI] [PubMed] [Google Scholar]
- 131.Reich NC, Levine AJ. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- 132.Lubbert M, Miller CW, Kahan J, Koeffler HP. Expression, methylation and chromatin structure of the p53 gene in untransformed and human T-cell leukemia virus type I-transformed human T-lymphocytes. Oncogene. 1989;4(5):643–651. [PubMed] [Google Scholar]
- 133.Lohrum MA, Ludwig RL, Kubbutat MH, Hanlon M, Vousden KH. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell. 2003;3:577–587. doi: 10.1016/s1535-6108(03)00134-x. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Y, Wolf GW, Bhat K, Jin A, Allio T, Burkhart WA, Xiong Y. Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol Cell Biol. 2003;23:8902–8912. doi: 10.1128/MCB.23.23.8902-8912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gilkes DM, Chen L, Chen J. MDMX regulation of p53 response to ribosomal stress. EMBO J. 2006;25:5614–5625. doi: 10.1038/sj.emboj.7601424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pan Y, Chen J. MDM2 promotes ubiquitination and degradation of MDMX. Mol Cell Biol. 2003;23(15):5113–5121. doi: 10.1128/MCB.23.15.5113-5121.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Welf ES, Ahmed S, Johnson HE, Melvin AT, Haugh JM. Migrating fibroblasts reorient directionality by a metastable, PI3 K-dependent mechanism. J Cell Biol. 2012;197(1):105–114. doi: 10.1083/jcb.201108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Okamoto K, Taya Y, Nakagama H. Mdmx enhances p53 ubiquitination by altering the substrate preference of the Mdm2 ubiquitin ligase. FEBS Lett. 2009;583(17):2710–2714. doi: 10.1016/j.febslet.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 139.Dai M-S, Shi D, Jin Y, Sun X–X, Zhang Y, Grossman SR, Lu H. Regulation of the MDM2-p53 pathway by ribosomal protein L11 involves a post-ubiquitination mechanism. J Biol Chem. 2006;281(34):24304–24313. doi: 10.1074/jbc.M602596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun X–X, Dai M-S, Lu H. 5-Fluorouracil activation of p53 involves an MDM2-ribosomal protein interaction. J Biol Chem. 2007;282(11):8052–8059. doi: 10.1074/jbc.M610621200. [DOI] [PubMed] [Google Scholar]
- 141.Fumagalli S, Di Cara A, Neb-Gulati A, Natt F, Schwemberger S, Hall J, Babcock GF, Bernardi R, Pandolfi PP, Thomas G. Absence of nucleolar disruption after impairment of 40S ribosome biogenesis reveals an rpL11-translation-dependent mechanism of p53 induction. Nat Cell Biol. 2009;11(4):501–508. doi: 10.1038/ncb1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Granetto C, Ottaggio L, Abbondandolo A, Bonatti S. p53 accumulates in micronuclei after treatment with a DNA breaking chemical, methylnitrosourea, and with the spindle poison, vinblastine. Mutat Res. 1996;352:61–64. doi: 10.1016/0027-5107(95)00235-9. [DOI] [PubMed] [Google Scholar]
- 143.Fontoura BM, Sorokina EA, David E, Carroll RB. p53 is covalently linked to 5.8S rRNA. Mol Cell Biol. 1992;12:5145–5151. doi: 10.1128/mcb.12.11.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fontoura BM, Atienza CA, Sorokina EA, Morimoto T, Carroll RB. Cytoplasmic p53 polypeptide is associated with ribosomes. Mol Cell Biol. 1997;17:3146–3154. doi: 10.1128/mcb.17.6.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen J, Guo K, Kastan MB. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J Biol Chem. 2012;287(20):16467–16476. doi: 10.1074/jbc.M112.349274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takagi M, Absalon MJ, McLure KG, Kastan MB. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 147.Ofir-Rosenfeld Y, Boggs K, Michael D, Kastan MB, Oren M. Mdm2 regulates p53 mRNA translation through inhibitory interactions with ribosomal protein L26. Mol Cell. 2008;32(2):180–189. doi: 10.1016/j.molcel.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fu L, Benchimol S. Participation of the human p53 3′UTR in translational repression and activation following gamma-irradiation. EMBO J. 1997;16:4117–4125. doi: 10.1093/emboj/16.13.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fu L, Minden MD, Benchimol S. Translational regulation of human p53 gene expression. EMBO J. 1996;15(16):4392–4401. [PMC free article] [PubMed] [Google Scholar]
- 150.Maclaine NJ, Hupp TR. How phosphorylation controls p53. Cell Cycle. 2011;10(6):916–921. doi: 10.4161/cc.10.6.15076. [DOI] [PubMed] [Google Scholar]
- 151.Vogel C, Kienitz A, Hofmann I, Muller R, Bastians H. Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene. 2004;23(41):6845–6853. doi: 10.1038/sj.onc.1207860. [DOI] [PubMed] [Google Scholar]
- 152.Uetake Y, Sluder G. Prolonged prometaphase blocks daughter cell proliferation despite normal completion of mitosis. Curr Biol. 2010;20(18):1666–1671. doi: 10.1016/j.cub.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sablina AA, Ilyinskaya GV, Rubtsova SN, Agapova LS, Chumakov PM, Kopnin BP. Activation of p53-mediated cell cycle checkpoint in response to micronuclei formation. J Cell Sci. 1998;111(Pt 7):977–984. doi: 10.1242/jcs.111.7.977. [DOI] [PubMed] [Google Scholar]
- 154.Hernandez-Verdun D, Robert-Nicoud M, Geraud G, Masson C. Behaviour of nucleolar proteins in nuclei lacking ribosomal genes. A study by confocal laser scanning microscopy. J Cell Sci. 1991;98((Pt 1)):99–105. doi: 10.1242/jcs.98.1.99. [DOI] [PubMed] [Google Scholar]
- 155.Leung AKL, Gerlich D, Miller G, Lyon C, Lam YW, Lleres D, Daigle N, Zomerdijk J, Ellenberg J, Lamond AI. Quantitative kinetic analysis of nucleolar breakdown and reassembly during mitosis in live human cells. J Cell Biol. 2004;166(6):787–800. doi: 10.1083/jcb.200405013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huang H-C, Shi J, Orth JD, Mitchison TJ. Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell. 2009;16(4):347–358. doi: 10.1016/j.ccr.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Raška I, Koberna K, Malínský J, Fidlerová H, Mašata M. The nucleolus and transcription of ribosomal genes. Biol Cell. 2004;96(8):579–594. doi: 10.1016/j.biolcel.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 158.Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 159.Pritchard DM, Watson AJM, Potten CS, Jackman AL, Hickman JA. Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil: evidence for the involvement of RNA perturbation. Proc Natl Acad Sci USA. 1997;94:1795–1799. doi: 10.1073/pnas.94.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ghoshal K, Jacob ST. Specific inhibition of pre-ribosomal RNA processing in extracts from the lymphosarcoma cells treated with 5-fluorouracil. Cancer Res. 1994;54:632–636. [PubMed] [Google Scholar]
- 161.Ghoshal K, Jacob ST. An alternative molecular mechanism of action of 5-fluorouracil, a potent anticancer drug. Biochem Pharmacol. 1997;53:1569–1575. doi: 10.1016/s0006-2952(97)00040-3. [DOI] [PubMed] [Google Scholar]
- 162.Gelasco A, Lippard SJ. NMR solution structure of a DNA dodecamer suplex containing a cis-Diammineplatinum(II) d(GpG) intrastrand cross-link, the major adduct of the anticancer drug cisplatin. Biochemistry (Mosc) 1998;37(26):9230–9239. doi: 10.1021/bi973176v. [DOI] [PubMed] [Google Scholar]
- 163.Jordan P, Carmo-Fonseca M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci. 2000;57(8–9):1229–1235. doi: 10.1007/PL00000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ljungman M, Lane DP. Transcription—guarding the genome by sensing DNA damage. Nat Rev Cancer. 2004;4:727–737. doi: 10.1038/nrc1435. [DOI] [PubMed] [Google Scholar]
- 165.Ohndorf U-M, Rould MA, He Q, Pabo CO, Lippard SJ. Basis for recognition of cisplatin-modified DNA by high-mobility-group proteins. Nature. 1999;399:708–712. doi: 10.1038/21460. [DOI] [PubMed] [Google Scholar]
- 166.Jordan P, Carmo-Fonseca M. Cisplatin inhibits synthesis of ribosomal RNA in vivo. Nucleic Acids Res. 1998;26:2831–2836. doi: 10.1093/nar/26.12.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhai X, Beckmann H, Jantzen HM, Essigmann JM. Cisplatin-DNA adducts inhibit ribosomal RNA synthesis by hijacking the transcription factor human upstream binding factor. Biochemistry (Mosc) 1998;37:16307–16315. doi: 10.1021/bi981708h. [DOI] [PubMed] [Google Scholar]
- 168.Huang R, Wu T, Xu L, Liu A, Ji Y, Hu G. Upstream binding factor up-regulated in hepatocellular carcinoma is related to the survival and cisplatin-sensitivity of cancer cells. FASEB J. 2002;16:293–301. doi: 10.1096/fj.01-0687com. [DOI] [PubMed] [Google Scholar]
- 169.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33(1):9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McKeage MJ, Hsu T, Screnci D, Haddad G, Baguley BC. Nucleolar damage correlates with neurotoxicity induced by different platinum drugs. Br J Cancer. 2001;85:1219–1225. doi: 10.1054/bjoc.2001.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pizzolato JF, Saltz LB. The camptothecins. The Lancet. 2003;361(9376):2235–2242. doi: 10.1016/S0140-6736(03)13780-4. [DOI] [PubMed] [Google Scholar]
- 172.Mao Y, Mehl IR, Muller MT. Subnuclear distribution of topoisomerase I is linked to ongoing transcription and p53 status. Proc Natl Acad Sci USA. 2002;99:1235–1240. doi: 10.1073/pnas.022631899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Buckwalter CA, Lin AH, Tanizawa A, Pommier YG, Cheng Y-C, Kaufmann SH. RNA synthesis inhibitors alter the subnuclear distribution of DNA topoisomerase I. Cancer Res. 1996;56:1674–1681. [PubMed] [Google Scholar]
- 174.Morris EJ, Geller HM. Induction of neuronal apoptosis by camptothecin, an inhibitor of DNA topoisomerase-I: evidence for cell cycle-independent toxicity. J Cell Biol. 1996;134:757–770. doi: 10.1083/jcb.134.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kalita K, Makonchuk D, Gomes C, Zheng J–J, Hetman M. Inhibition of nucleolar transcription as a trigger for neuronal apoptosis. J Neurochem. 2008;105(6):2286–2299. doi: 10.1111/j.1471-4159.2008.05316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tomkins CE, Edwards SN, Tolkovsky AM. Apoptosis is induced in post-mitotic rat sympathetic neurons by arabinosides and topoisomerase II inhibitors in the presence of NGF. J Cell Sci. 1994;107:1499–1507. doi: 10.1242/jcs.107.6.1499. [DOI] [PubMed] [Google Scholar]
- 177.Drygin D, Siddiqui-Jain A, O’Brien S, Schwaebe M, Lin A, Bliesath J, Ho CB, Proffitt C, Trent K, Whitten JP, Lim JKC, Von Hoff D, Anderes K, Rice WG. Anticancer activity of CX-3543: a direct inhibitor of rRNA biogenesis. Cancer Res. 2009;69(19):7653–7661. doi: 10.1158/0008-5472.CAN-09-1304. [DOI] [PubMed] [Google Scholar]
- 178.Zhu Y, Poyurovsky MV, Li YC, Biderman L, Stahl J, Jacq X, Prives C. Ribosomal protein S7 is both a regulator and a substrate of MDM2. Mol Cell. 2009;35(3):316–326. doi: 10.1016/j.molcel.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Balasubramanian S, Hurley LH, Neidle S. Targeting G-quadruplexes in gene promoters: a novel anticancer strategy? Nat Rev Drug Discov. 2011;10(4):261–275. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Latonen L, Kurki S, Pitkänen K, Laiho M. p53 and MDM2 are regulated by PI-3-kinases on multiple levels under stress induced by UV radiation and proteasome dysfunction. Cell Signal. 2003;15(1):95–102. doi: 10.1016/s0898-6568(02)00044-x. [DOI] [PubMed] [Google Scholar]