Abstract
Excitatory amino acid transporters (EAATs) are high-affinity Na+-dependent carriers of major importance in maintaining glutamate homeostasis in the central nervous system. EAAT3, the human counterpart of the rodent excitatory amino acid carrier 1 (EAAC1), is encoded by the SLC1A1 gene. EAAT3/EAAC1 is ubiquitously expressed in the brain, mostly in neurons but also in other cell types, such as oligodendrocyte precursors. While most of the glutamate released in the synapses is taken up by the “glial-type” EAATs, EAAT2 (GLT-1 in rodents) and EAAT1 (GLAST), the functional role of EAAT3/EAAC1 is related to the subtle regulation of glutamatergic transmission. Moreover, because it can also transport cysteine, EAAT3/EAAC1 is believed to be important for the synthesis of intracellular glutathione and subsequent protection from oxidative stress. In contrast to other EAATs, EAAT3/EAAC1 is mostly intracellular, and several mechanisms have been described for the rapid regulation of the membrane trafficking of the transporter. Moreover, the carrier interacts with several proteins, and this interaction modulates transport activity. Much less is known about the slow regulatory mechanisms acting on the expression of the transporter, although several recent reports have identified changes in EAAT3/EAAC1 protein level and activity related to modulation of its expression at the gene level. Moreover, EAAT3/EAAC1 expression is altered in pathological conditions, such as hypoxia/ischemia, multiple sclerosis, schizophrenia, and epilepsy. This review summarizes these results and provides an overall picture of changes in EAAT3/EAAC1 expression in health and disease.
Keywords: EAAC1, EAAT3, Glutamate, Oxidative stress, Glutathione, Retinoids
Introduction
EAAT3, the human counterpart of the EAAC1 rodent transporter, is a member of the EAAT family of high-affinity, sodium-dependent glutamate carriers encoded by genes of the SLC1 family [1–3]. EAAT3/EAAC1 is coded by SLC1A1/Slc1a1 and was the first sodium-dependent mammalian amino acid transporter to be cloned [4]. When studying the activity of EAAT3/EAAC1 in cultured human fibroblasts before its cloning, early researchers demonstrated that the transporter can accept the D isomer of aspartate but not the D isomer of glutamate [5]. This stereoselective anomaly has been consistently confirmed for all the transporters for anionic amino acids of the SLC1 family and is one of the functional hallmarks of EAAT carriers.
Like other EAAT carriers, EAAT3/EAAC1 uses the transmembrane gradients of Na+, K+, and H+ electrochemical potentials as the driving force to accumulate glutamate from the extracellular space [6, 7]. Glutamate is co-transported with 3 Na+ and 1 H+, while 1 K+ is extruded in the same cycle, ensuring the electrogenicity of the transport cycle and the formation of a high transmembrane gradient of the transported substrates.
The expected molecular weight of the transporter is 64 kDa, but electrophoretic migration yields higher values, ascribed to a high degree of glycosylation. Moreover, Western-blot experiments have demonstrated high-molecular-weight bands, referred to as trimers of the transporter. In fact, trimers are thought to constitute the functional unit of EAAT3/EAAC1 in situ [8, 9]. Although experimental data on the carrier structure are incomplete, EAATs have 8–10 transmembrane domains, with glycosylation sites in the extracellular loop between TM3 and TM4 and several putative intracellular consensus phosphorylation sites [10].
EAAT3/EAAC1 is expressed not only in the central nervous system (CNS) but also in Schwann cells [11]. Moreover, the carrier is also expressed in the intestine and kidney, where it accounts for the absorption/reabsorption of dicarboxylic amino acids, and in many other tissues. Defective expression of functional EAAT3/EAAC1 results in dicarboxylic aminoaciduria (OMIM222730) [12]. In the CNS, of the five EAATs characterized so far, EAAT4 [13] and EAAT5 [14] are highly expressed in cerebellar Purkinje cells and in retinal photoreceptors and bipolar cells, respectively. In contrast, EAAT1 and EAAT2 predominately have a glial distribution, while EAAT3/EAAC1 is highly expressed in glutamatergic and GABAergic neurons [15], especially in somata and dendrites (with particular abundance in the neck of dendritic spines [16]) but not in synaptic terminals of axons or spine heads [16–18]. Other neuronal populations express EAAT3/EAAC1, such as dopaminergic neurons; these neurons are very sensitive to carrier dysfunction [19, 20] or inhibition [21]. EAAT3/EAAC1 is also expressed in cells of glial origin, such as in subsets of oligodendrocytes [22], in immature oligodendrocytes [23], and in oligodendrocyte progenitor cells [24]. An isolated report describes EAAT3/EAAC1 expression also in microglial cells after traumatic brain injury [25]. Mature astrocytes are generally believed to be EAAT3/EAAC1-negative, but it should be noted that astroglial tumor cells, such as rat C6 glioma cells and several human glioma cell models, express the transporter [26]. At variance with other EAAT transporters [27, 28], the expression of EAAT3/EAAC1 is mainly intracellular [22, 29, 30]; only a minor portion of the carriers are targeted to the plasma membrane under control conditions, while most are associated with cytoplasmic vesicles [15, 16].
This peculiar distribution has relevant consequences for the regulation of transporter activity. In cells of both nervous and non-nervous origin, EAAT3/EAAC1 activity is modulated by post-translational mechanisms that rapidly modify its trafficking and abundance on the plasma membrane. EAAT3/EAAC1 abundance on the plasma membrane is readily enhanced upon PKC activation [31] or stimulation with PDGF [32]. Several PKC enzymes may be involved in these regulatory effects at different levels [31–34]. In addition, other kinases have been implicated in the regulation of EAAT3/EAAC1 trafficking and activity in Xenopus oocytes injected with the transporter cDNA, including phosphatidylinositol-3 (PI3)-kinase, the phosphoinositide-dependent kinase PDK1, the serum- and glucocorticoid-inducible kinase SGK1, and the phosphatidylinositol-3-phosphate-5-kinase PIKfyve [35]. Interestingly, the gene encoding PIKfyve is associated with schizophrenia and the mutant form expressed in patients seems to exert a dominant-negative effect on EAAT3 trafficking in Xenopus oocytes [36]. In the same experimental model, mTOR increases EAAT3/EAAC1 activity [37], which, instead, is decreased by the mTOR inhibitor AMPK [38].
These effects may be due to a direct interaction between EAAT3/EAAC1 and the kinase or, more often, to changes in the interaction between the transporter and several intracellular proteins, such as the endoplasmic reticulum proteins addicsin/glutamate transporter-associated protein 3-18 (GTRAP3-18, [39]) and reticulon (RTN2B, [40]), which are believed to play opposite regulatory roles in the membrane-targeting of the transporter [41, 42]. Interestingly, the gene for GTRAP3-18, which is a negative modulator of EAAT3/EAAC1 activity, maps to human chromosome 3p14, a susceptibility locus for cancer and epilepsy [43]. GTRAP3-18 is negatively regulated by ADP-ribosylation factor-like 6-interacting protein 1 (Ar16ip1), which causes a decreased interaction between GTRAP3-18 and EAAT3/EAAC1 in neurons [44]. Moreover, EAAT3/EAAC1 trafficking involves several steps dependent on Rab11 [45], SNAP-23 (a SNARE protein) [46], and caveolin-1 [47], although evidence for a direct interaction between the transporter and these proteins is lacking. The δ-opioid receptor is a G-protein that also modulates EAAT3/EAAC1 activity [48]. EAAT3/EAAC1 also interacts with the cytoskeletal protein α-adducin [49], which is particularly abundant in dendritic spines [50], although, in this case, the functional implications of the interaction have not been defined.
Compared to these multiple sites of regulation at the protein level, the characterization of mechanisms modulating EAAT3/EAAC1 synthesis at the transcriptional level has been much slower. This review aims to recount the information available on this subject as well as on pathological situations in which the expression of EAAT3/EAAC1 is altered. Before examining the regulatory mechanisms, a brief summary on the physiological role of EAAT3 is discussed.
EAAT3/EAAC1 functions in physiology and pathology
In general, the activity of EAAT transporters is considered the main mechanism responsible for the termination of glutamatergic transmission. As such, EAAT transporters are believed to be important factors for the prevention of excitotoxic damage, i.e., neuronal loss due to excessive influx of calcium mediated by the overstimulation of glutamate receptors of the NMDA and AMPA/kainate classes.
However, the roles of the three most important EAATs, EAAT1, EAAT2, and EAAT3/EAAC1, are thought to be diverse [51]. This is well exemplified by experiments with knock-out animals. Whereas the knock-outs for GLAST (the rodent counterpart of EAAT1) and GLT-1 (the rodent EAAT2) exhibit early signs of severe excitotoxic damage [52], the knock-out for EAAT3/EAAC1 has an apparently normal development [53]. However, these animals present symptoms of precocious neurodegeneration and subtle neurologic damage, associated with cell loss in the cerebral cortex, increased aggressiveness and impaired self-grooming [54]. In particular, EAAT3/EAAC1-deficient mice exhibit spatial learning/memory dysfunction, loss of dopaminergic neurons in the substantia nigra, and movement disorders; these disturbances typically appear at senescence [20, 54] but have also been reported in young animals [55]. Moreover, patients affected by dicarboxylic aminoaciduria also display hints of neurologic damage [12, 56].
The prevalent opinion is that the basic mechanism for termination of glutamatergic transmission resides in EAAT1 and EAAT2 function. Consistent with this hypothesis, these transporters are endowed with higher affinity for substrates than EAAT3/EAAC1 and are localized in glial cell processes close to synaptic clefts, while EAAT3/EAAC1 is mostly extra-synaptic [16]. Therefore, EAAT3/EAAC1 may be important in situations where glutamate spills out from the synaptic zone or reaches abnormally high extracellular concentrations, for example under ischemic conditions (see below). However, EAAT3/EAAC1 may play an important role in glutamatergic termination in areas such as the hippocampus, where most synapses are not closely surrounded by astrocytic processes [57]. However, EAAT3/EAAC1 is far less abundant than EAAT2 also in these regions [18].
Although glutamate uptake represents the best-characterized activity of the carrier, EAAT3/EAAC1 also transports cysteine [58, 59] and may provide the majority of the influx of the amino acid in neurons [60]. Through cysteine uptake, EAAT3/EAAC1 contributes to the intracellular synthesis of glutathione [59, 61], one of the most important intracellular antioxidants. EAAT3/EAAC1-mediated uptake may be the major contributor of cysteine to glutathione synthesis, because the knock-out mouse for xCT, the catalytic subunit of the sodium-independent transport system for cystine, is apparently normal and shows no changes in brain glutathione content [62]. Indeed, the modulation of EAAT3/EAAC1 activity correlates with neuronal glutathione content [41]. Further, the impaired Rab11-dependent trafficking of EAAT3/EAAC1 is associated with enhanced sensitivity to oxidative stress in a murine model of Huntington disease [63]. The mouse knocked-out for EAAT3/EAAC1, which presents a decreased glutathione content in neurons and early onset brain aging, exhibits significant biochemical and functional improvements if treated with the antioxidant N-acetyl-cysteine [64].
The high expression of EAAT3/EAAC1 in GABAergic neurons supports its role in the metabolic fueling of GABA synthesis. This role is also supported by the increase in paroxysmal activity in animals treated with antisense oligonucleotides that hinder transporter expression (see below and [65]).
In addition, EAAT3/EAAC1 also has an important function in the regulation of synaptic transmission. EAAT3/EAAC1 is involved in long-term potentiation and, hence, in memory and higher cerebral functions [66–69]. Acute regulation of EAAT3/EAAC1 membrane expression and activity by volatile anesthetics [70, 71] and carbamazepine [72] may represent examples of pharmacological modulation of these mechanisms.
Regulatory mechanisms at the gene and protein levels
The expression of EAAT3/EAAC1 varies during brain development [73, 74], and the transporter is expressed before EAAT1 and EAAT2 both in vitro [75] and in vivo [73]. Early expression suggests a role for EAAT3/EAAC1 in neuroprotection of CNS cells during brain development. Moreover, because GABAergic neurons reach maturation before excitatory glutamatergic cells in the hippocampus, the early expression of EAAT3/EAAC1 may play an important role in developing the glutamatergic neuronal network [76]. These data are consistent with recent evidence showing that the EAATs are differentially regulated during the differentiation of human astroglial progenitors [77]. In particular, EAAT3/EAAC1 is more expressed than EAAT1 and EAAT2 in A2B5-positive cells and decreases upon differentiation to the astrocytic phenotype. Interestingly, early precursors of the oligodendroglial cell lineage are A2B5 positive [78].
Notwithstanding these data, studies focused on the modulation of the SLC1A1 gene under differentiating stimuli are lacking. In Hs683 human oligodendroglioma cells valproic acid (VPA) and trichostatin A, two inhibitors of histone deacetylases (HDACs), strongly increase SLC1A1 mRNA as well as EAAT3/EAAC1 protein expression and transport activity [79]. In the same model, VPA significantly also enhances the expression of PDGFRA, an early marker of oligodendrocyte precursors cells (OPCs), while it does not affect the expression of either TUBBIII or GFAP, markers of neuronal and glial cells, respectively. VPA is a well-known differentiating agent, and its effects on the differentiation of CNS cells have been repeatedly reported. In particular, VPA promotes neuronal differentiation of multipotent adult neural progenitor cells and influences the timing of oligodendrocyte differentiation in the developing rat brain [80, 81].
In another model of immature CNS cells, the rat glioma cell line C6, the differentiating agent all–trans retinoic acid (ATRA) markedly increases EAAT3/EAAC1 expression at both the mRNA and protein levels. ATRA effect is specific for the Slc1a1 gene because it does not affect either GLT-1 (Slc1a2) or GLAST (Slc1a3) protein levels [82]. RAR-specific but not RXR-specific inhibitors prevent EAAT3/EAAC1 induction after ATRA treatment, while the RAR-specific agonist Am80 (tamibarotene, retinobenzoic acid) mimics the ATRA effect. Conversely, EAAT3/EAAC1 induction is prevented by Rarbeta silencing, demonstrating that the ATRA effect on EAAT3/EAAC1 strictly depends upon the RARβ receptor [83]. A consensus site for RARβ has been identified in the rat Slc1a1 promoter but not in the human SLC1A1 promoter region [83]. Along with Slc1a1 induction, the expression of the oligodendrocytic marker Plp1 is also enhanced by ATRA in C6 cells [82, 84], suggesting that retinoids may be involved in the activation of the oligodendrocytic differentiation pathway.
Soon after the cloning of the transporter, increased expression of EAAT3/EAAC1 was reported in bovine NBL-1 renal epithelial cells incubated under hypertonic conditions. Incubation of these cells in medium supplemented with 200 mM sucrose leads to a marked increase in EAAT3/EAAC1 mRNA and protein, as well as to the stimulation of anionic amino acid transport [85]. Although it has not yet been demonstrated, it is likely that, as with other amino acid transporters [86], EAAT3/EAAC1 induction also involves the activation of the osmosensitive transcription factor NFAT5/TonEBP [87]. Incubation in the presence of tunicamycin, an inhibitor of protein glycosylation that triggers endoplasmic reticulum (ER) stress, increases EAAT3/EAAC1 mRNA and protein [88]. In contrast, incubation of NBL-1 cells in the absence of amino acids, a condition that promotes the expression of other sodium-dependent transporters, such as SNAT2 [89], stimulates aspartate transport but not EAAT3/EAAC1 expression [90]. Thus, it seems that in NBL-1 renal epithelial cells Slc1a1 is induced by several (but not all) forms of stress associated with increased protein unfolding and ER stress. Unfortunately, these experiments have not been repeated in CNS-derived cell models.
Ma et al. [91] have found that a binding sequence for the regulatory factor X1 (RFX1) exists in the promoter region of SLC1A1, but not in the promoters of SLC1A2 (encoding EAAT2) and SLC1A3 (encoding EAAT1). RFX proteins are transcription factors that bind to X-boxes of DNA sequences. In the mouse brain, RFX1 is expressed from the embryonic state (E15) to adulthood, and its knock-out is lethal in the early embryonic state, suggesting an indispensable role in brain development [92]. In rats and mice, RFX1 is highly expressed in several regions of the brain, such as the hippocampus, the cerebellum (Purkinje cells), and the olfactory bulb, with localization in the nuclei of neurons and microglial cells but not in the nuclei of astrocytes [93]. In the study by Feng and Zuo [93], 24 h after transfection of C6 glioma cells with human RFX1, EAAT3/EAAC1 expression increased by 60 % and transporter activity increased by 40 %. Conversely, knock-down of RFX1 expression by antisense oligonucleotides decreased EAAT3/EAAC1 expression in rat cortical neurons in culture. Moreover, transfection with RFX1 stimulated the transcriptional activity of the SLC1A1 promoter in both rat glioma C6 cells and human neuroblastoma SHSY-5Y cells. Thus, although SLC1A1 mRNA was not directly measured in that study, these data suggest that the increase in EAAT3/EAAC1 expression was due to the interaction of RFX1 with the promoter [93]. However, the natural activators of RFX1 are not known, and it is difficult to assess its physiological role.
Although most of the previous studies on EAAT3/EAAC1 function have focused on its role in the regulation of glutamate homeostasis, accumulating evidence suggests that the carrier plays an essential function in controlling cell redox potential (see above). Astrocytes support neuronal antioxidant capacity by releasing glutathione, the most important anti-oxidant agent in the CNS, which is cleaved to cysteine in brain extracellular space [94]. Free cysteine supply is the limiting step for glutathione synthesis, and neuronal cysteine influx depends upon EAAT3/EAAC1 activity [95]. The activation of the nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant responsive element (ARE) pathway by oxidative stress promotes not only glutathione release from astrocytes but also the transcriptional regulation of neuronal EAAT3/EAAC1 both in vitro and in vivo [95]. Moreover, Nrf2-dependent overexpression of EAAT3/EAAC1 protein is associated with an increase in neuronal glutathione content, and these effects are abrogated in mice genetically deficient in either Nrf2 or EAAT3/EAAC1. Selective overexpression of Nrf2 in brain neurons by lentiviral gene transfer is sufficient to up-regulate both neuronal EAAT3/EAAC1 protein and glutathione content. These findings support a mechanism whereby Nrf2 activation can coordinate astrocyte glutathione release with neuronal glutathione synthesis through transcriptional up-regulation of neuronal EAAT3/EAAC1 expression. EAAT3/EAAC1-mediated cysteine uptake has also recently been implicated in the increased neuronal glutathione content of mice treated with caffeine or uric acid [96], although the underlying mechanism has not yet been elucidated. Interestingly, recent contributions demonstrate that fumarates can activate Nrf2-dependent responses and thereby ameliorate the disease course of experimental autoimmune encephalomyelitis (EAE), a rodent model of multiple sclerosis (MS) [97, 98]. The protective effect of fumarates is associated with improved preservation of myelin and neurons. Unfortunately, no investigation was performed on possible changes in EAAT3/EAAC1 induced by fumarates in these models.
In a report by Molteni et al. [99], EAAT3/EAAC1 was up-regulated in parallel with glutamate NMDA receptor subunits after chronic periods of exercise, providing further support for a functional role of the transporter in the modulation of glutamatergic transmission.
Overall, these data suggest that the transcriptional regulation of EAAT3/EAAC1 expression has peculiar features because most of the reported mechanisms specifically affect the transporter, which, conversely, appears insensitive to well-characterized inducers of EAAT1 and EAAT2 [100, 101]. Similarly, studies on knock-out models for glutamate receptors demonstrate that EAAT1 and EAAT2 expression is lower in mGluR3−/− mice, while EAAT3/EAAC1 is decreased in mGluR2−/− animals [102]. However, examples of a parallel regulation of EAAT1, EAAT2, and EAAT3/EAAC1 proteins have been also reported [103].
Pathological conditions involving changes in EAAT3/EAAC1 expression
Alterations in EAAT3/EAAC1 have been associated with several pathological conditions. In particular, there is a strong association between single-nucleotide polymorphisms (SNPs) in SLC1A1 and obsessive–compulsive disorder [104–107], but these polymorphisms seem more correlated with changes in transporter function than with its expression. Dicarboxylic aminoaciduria is a Mendelian disorder (OMIM 222730) where EAAT3/EAAC1 function is defective due to the expression of a mutant, functionally impaired transporter that is also scarcely sorted to the cell membrane [12]. Clear-cut, although contrasting, changes in EAAT3/EAAC1 expression have been described in Alzheimer’s disease (AD). Results in mouse models of AD are conflicting. Schallier et al. [108] reported an increase in EAAT3/EAAC1 expression (although overwhelmed by a concomitant decrease in GLT-1 and GLAST levels) in AβPP23 mice, while a decrease in the expression of all three glutamate transporters has been reported by Cassano et al. [109] in triple transgenic (3×Tg) AD mice. In humans, aberrant intracellular accumulation of a detergent insoluble EAAT3/EAAC1 was described in hippocampal neurons of AD patients, although overall cortical expression of the transporter was comparable to controls, suggesting a defect in transporter processing rather than synthesis [110].
Four conditions in which alterations in EAAT3/EAAC1 expression have been identified will be discussed: hypoxia/ischemia, MS, epilepsy, and schizophrenia.
Ischemia/hypoxia
Due to perturbations of ionic gradients, glutamate uptake through EAAT transporters is impaired during ischemia and the rise of extracellular glutamate is believed to contribute significantly to tissue damage. The possibility that changes in the expression of EAAT transporters also occur under ischemic conditions has been investigated for many years.
The potential protective role of EAAT3/EAAC1 was proposed following the cloning of the transporter [4] and discussed in one of the first reviews concerning glutamate transporters [111]. The first experimental evidence of ischemia-related expression changes was provided by Martin et al. [112]; immunoblotting showed that EAAT3/EAAC1 was reduced by 55 % in the putamen of newborn piglets 24 h after an ischemic stress caused by 30 min of hypoxia (arterial O2 saturation, 30 %) followed by 7 min of airway occlusion (O2 saturation, 5 %). This decrease was possibly secondary to neuronal loss, consistent with the Slc1a1 mRNA decrease demonstrated in gerbil CA1 hippocampal neurons 24 h after a transient ischemia [113] and the marked decrease (42–68 %) in EAAT3/EAAC1 protein detected in the same model [114]. The latter study compared the decrease of GLT-1, which precedes neuronal loss, with that of EAAT3/EAAC1, which parallels cell death. Their results suggest that decreased EAAT3/EAAC1 may have a role in GABAergic dysfunction. Decrease of EAAT3/EAAC1 expression as a consequence of neuronal loss has been more recently shown by Han et al. [115] following microsphere embolism in rats. However, the possibility that adaptive changes in ischemic cells may directly affect transporter expression should not be excluded a priori [38].
A predominant role for GLT-1 in the protection from ischemic damage was proposed by Rao et al. [116], who showed that knock-down of this transporter, but not of EAAT3/EAAC1, exacerbated the neuronal damage induced by transient focal cerebral ischemia in rat brain. Douen et al. [117] also stressed the importance of GLT-1, using as a model rats that were preconditioned 3 days before ischemia with cortical spreading depression (CSD) and showed relative protection from ischemic damage. These authors noted that extracellular brain glutamate after ischemia was indeed lower in the preconditioned animals and that the change was associated with a decrease of EAAT1 and EAAT2, but not EAAT3/EAAC1, expression in the plasma membrane. The authors suggested that a lower expression of EAAT transporters contributes to less glutamate efflux through the reverse operation, originally described as an alternative functional mode of EAAT transporters under hypoxic conditions [118]. A decrease in Slc1a1 expression under comparable conditions was described by Montori et al. [119].
The first experimental contribution pointing to an adaptive and/or protective role of increased EAAT3/EAAC1 expression in ischemia came in 2000 from the Matute laboratory [120]. These researchers compared the expression of the main EAAT transporters in the rat hippocampus and cerebral cortex in both early (8 h and 1 day) and late (28 days) phases of recovery from transient forebrain ischemia. They found a moderate increase in EAAT3/EAAC1 protein early after the insult, followed by a late reduction in both CA1 pyramidal neurons and in layer V pyramidal neurons. Moreover, the authors observed a sustained increase in EAAT3/EAAC1 expression in oligodendroglial progenitor cells of subcortical white matter [120], although, in a later study, the same group reported that EAAT1 and EAAT2 were also up-regulated in this area [121]. Transient changes in EAAT3/EAAC1 expression during ischemia and the recovery phase were also reported by Hwang [122], who described a fast decrease of transporter immunoreactivity in spinal cord neurons just after ischemia/reperfusion, followed by an increase of expression (after 3 h of reperfusion) and a further subsequent decrease. EAAT3/EAAC1 expression was also slowly increased, at both the mRNA and protein levels, under “hypoxia-pure” conditions [123] in rat PC12 cells. In this case, the maximum increase was reached after 18 h of incubation at 1 % O2 and corresponded to an eightfold increase at protein level and a four-fold increase in transport activity. However, GLT-1 was also up-regulated under the same conditions.
These results are, at least in part, consistent with the evidence obtained by Romera et al. [124] in rat cortical cultures exposed to sublethal oxygen-glucose deprivation (an in vitro model for ischemic preconditioning), and by Pradillo et al. [125] in preconditioned rat brain in vivo. In both studies, EAAT3/EAAC1 was up-regulated by increased TNF-α signaling. However, in the same studies, TNF-α-independent EAAT2 up-regulation was also reported. A generalized up-regulation of EAAT transporters was described in rat brain upon ischemic pre-conditioning by Bigdeli et al. [126, 127].
Results from EAAC1 knock-out mice support the protective role of the transporter under ischemic conditions [128, 129]. In particular, Won et al. demonstrated that EAAC1−/− mice subjected to transient cerebral ischemia exhibit much more widespread hippocampal neuronal death than wild-type mice. These authors suggested that impaired cysteine uptake would severely hinder intracellular glutathione synthesis, cell capability to sustain oxidative stress (expected under ischemic conditions), and maintenance of zinc homeostasis. N-acetyl-cysteine restored neuronal glutathione, normalized basal zinc levels, and attenuated ischemia-induced zinc translocation, superoxide production, and neuron death.
Given the putative role of EAAT3/EAAC1 as a protective factor under ischemic conditions, attempts to up-regulate its expression have been performed as a means to limit ischemic damage. Chen et al. [130] used intrathecal granulocyte colony-stimulating factor (G–CSF) to improve neurological defects in the ischemic spinal cord of Wistar rats. Using microdialysis, they found that G–CSF significantly hampers the increase in spinal concentrations of excitatory amino acids induced by ischemia or by exogenous intrathecal administration of glutamate. These effects are associated with the increased expression of GLAST, GLT-1 and EAAT3/EAAC1.
Multiple sclerosis
Excitotoxicity and oxidative stress have been implicated in MS-related damage that involves not only white matter but also gray matter. However, evidence for a role of the EAAT3/EAAC1 transporter in MS is thus far limited, although, as detailed in the Introduction, the transporter is also expressed in cells of oligodendrocytic lineage and, in particular, in oligodendrocyte precursor cells [23, 24, 131].
Ohgoh et al. [132], prompted by the evidence of the involvement of AMPA/kainate receptor in experimental autoimmune encephalomyelitis (EAE, the animal model of MS) [133], examined the expression of GLT-1, GLAST, and EAAT3/EAAC1 in the spinal cord of the EAE Lewis rat. While EAAT3/EAAC1 was dramatically increased at both the protein and mRNA levels, GLT-1 and GLAST were down-regulated. The AMPA/kainate receptor inhibitor NBQX ameliorated EAE symptoms and suppressed changes in transporter expression. No attempt was made to identify the cell type(s) in which changes in carrier expression occur.
This issue was instead addressed by Vallejo-Illarramendi et al. [134] who found an increase of SLC1A3 and SLC1A2 mRNA in optic nerves from MS patients, while SLC1A1 mRNA did not change. The expression increase was confirmed at the protein level for both EAAT1 (in oligodendrocytes) and EAAT2 (in astrocytes), while EAAT3/EAAC1 expression was not investigated. Increased expression of the rodent counterparts of EAAT1 and EAAT2 (at the mRNA, protein and function levels) were also observed in rat optic nerves incubated with excitotoxic glutamate concentrations.
More recently, Newcombe et al. [135] studied glutamate receptors, transporters, and enzymes in human MS lesions. They found that the AMPA GluR-1 receptor subunit was overexpressed in oligodendrocytes at the borders of MS active lesions, while reactive astrocytes, macrophages, and neuronal structures expressed a different receptor repertoire. Unexpectedly, EAAT3/EAAC1 was expressed in macrophages and reactive astrocytes, leading the authors to propose that this ectopic expression may have a protective, although not completely effective, function.
The positive effects of retinoids on EAAT3/EAAC1 expression (see above) may be important in light of the evidence for a positive effect of retinoids in EAE mice [136, 137]. Klemann et al. attributed the protective effect of the synthetic retinoid Am80 to immunomodulation. On the other hand, Huang et al. [137] attributed the protective effect of the natural compound 9-cis-retinoic acid to a RXR-γ-dependent stimulation of oligodendrocytic differentiation. Neither study, however, investigated the expression of EAAT3/EAAC1 or other glutamate transporters.
In conclusion, while some evidence suggests a role for EAAT3/EAAC1 in the pathophysiology of MS, a definite demonstration is still lacking.
Epilepsy
The role of EAAT3/EAAC1 dysregulation in epilepsy was proposed in the pivotal paper in which antisense EAAT-knock-out models were investigated for the first time [52]. While the EAAT1 and EAAT2 knock-outs produced severe neurotoxicity and progressive paralysis, the phenotype of the EAAT3/EAAC1 knock-out was milder but associated with epilepsy. Using the same experimental approach, it was then demonstrated that the repression of EAAT3/EAAC1 expression was associated with decreased tonic inhibition and to 50 % loss of hippocampal GABA levels, attributable to the impairment of GABA synthesis from extracellular glutamate [65].
These early findings spurred many investigations with experimental models of epilepsy. Compared to controls, Slc1a1 mRNA was markedly increased in dentate granule cells from rats with pilocarpine-induced epilepsy and in human dentate granule cells from patients with temporal lobe epilepsy (TLE) [138]. In cortical dysplasia, the transporter mRNA was elevated in human dysplastic neurons compared with non-dysplastic neurons. These changes were confirmed at the protein level and were interpreted as an attempt to compensate for the higher-than-normal extracellular glutamate levels caused by recurrent seizures [138]. Pilocarpine-induced status epilepticus has been associated with an increase in EAAT3/EAAC1 protein in rat hippocampus and with a ~15-fold increase in Slc1a1 mRNA in rat synaptoneurosomes [139], although the authors attributed the change more to the targeting of the messenger to dendrites than to an effect on transcription [140].
Doi et al. [141] examined the induction of epilepsy (kindling) by pentylenetetrazol (PTZ) in rats. They found that the expression of hippocampal glutamate transporters (not only EAAT3/EAAC1 but also GLAST and GLT-1) increased within 24 h by kindling but returned to basal levels 30 days after the last seizure. These findings led the authors to suggest that glutamate transporters may contribute to the occurrence of seizures. Interestingly, easily-kindled rats had levels of EAAT3/EAAC1 and GAT-1 (the GABA transporter) that were 30 % lower compared to epilepsy-resistant animals, again suggesting that EAAT3/EAAC1 level is negatively associated with the convulsive threshold [141].
Voutsinos-Porche et al. reported the temporal dependence of epilepsy-associated changes in transporter expression, demonstrating that EAAT3/EAAC1 increased in several portions of the hippocampus during the acute period of status epilepticus, but returned to control levels in the CA1, 2, and 3 layers during the latent period. The authors concluded that “it is not clear to what extent the overexpression of EAAT3/EAAC1 contributes to epileptogenesis and in which area it may represent a preventive or compensatory or response to injury” [142].
Using the model of kainate-induced convulsions, Simantov et al. [143] reached apparently divergent conclusions. Kainate acutely lowered EAAT3/EAAC1 in the stratum lacunosum moleculare, and the decrease in protein level was associated with a decrease in mRNA. Opposite effects were reported for GLT-1, leading the authors to conclude that differential regulation of neuronal and glial EAAT may contribute to kainate-induced seizures. In another model of experimental epilepsy due to prenatal exposure to the teratogen methylazoxymethanol, heterotopic hippocampal neurons exhibited striking reductions in EAAT3/EAAC1, as well as in GluR1 NMDA receptor subunit expression [144]. In contrast, a significant increase in the carrier expression was detected in hippocampal and motor cortex area neurons of rats with chest compression-induced audiogenic epilepsy [145].
Data from human tissues are contradictory. Proper et al. [146] reported an increase in EAAT3/EAAC1 in individual neurons of the hippocampus of TLE patients, although the functional consequences of the reported changes are unclear. Increased EAAT3/EAAC1 expression was also reported by Mathern et al. [147], but only in epileptic patients with hippocampal sclerosis. On the other hand, Rakhade and Loweb [148] measured the differential expression of EAAT1–4 at the mRNA and protein levels in electrically mapped human neocortical tissues and found significant reductions of EAAT3/EAAC1 and EAAT4) at both the mRNA and protein level, not associated with changes in the relative neuronal density. The authors concluded that “regional reductions in EAAT expression at human neocortical epileptic foci could produce increased local glutamate levels that in turn may contribute to both hyperexcitability and the spontaneous generation of epileptic discharges that characterize human epileptic foci” [148].
As far as genetic forms of epilepsy are concerned, Dutuit et al. [149] described a 32 % decrease in EAAT3/EAAC1 expression in the thalamus of young genetic absence epilepsy rats from Strasbourg (GAERS) compared to controls. However, the expression of GLT-1 and GLAST also decreased, and the changes were not observed in adult animals.
Interestingly, in human gangliogliomas, which are neuronal-glial tumors strongly associated with epilepsy, SLC1A1 is among the genes that are down-regulated compared to normal tissue [150]. This alteration has been attributed to a true transcriptional change rather than to a compensatory response to high extracellular glutamate due to repeated seizures. In contrast, the high expression of SLC1A1 mRNA detected in the lesions of patients affected by tuberous sclerosis, another condition exhibiting high seizure incidence, has been considered compensatory [151].
Schizophrenia
The possibility that glutamatergic transmission is altered in schizophrenic patients has been debated for several years. Changes in the expression of EAAT1 and EAAT2, but not EAAT3/EAAC1, have been detected in the thalamus [152] or the superior temporal gyrus and the hippocampus [153]. While these studies do not support a role for EAAT3/EAAC1 in schizophrenia, the findings suggest that, in addition to glutamate receptors, alterations in glutamate transport may also contribute to the glutamatergic dysfunction implicated in schizophrenia.
Other reports present evidence for a role of EAAT3/EAAC1 in schizophrenia. Increased carrier transcripts and proteins were reported in schizophrenic subjects (dorsolateral prefrontal and anterior cingulated cortex) by Bauer et al. [154]; these findings were, more recently, confirmed by Rao et al. [155] in the frontal cortex at both the protein and mRNA levels. Conversely, Lauriat et al. [156] did not observe differences in EAAT3/EAAC1 expression in either the dorsolateral prefrontal or the primary visual cortex. However, the authors also highlighted the possibility that the results were influenced by antipsychotic treatment that is often prolonged for many years in these patients [156].
On the contrary, decreased SLC1A1 transcript expression in the striatum was observed in schizophrenia (and in bipolar disorder) by other authors [157] and confirmed in schizophrenic patients in a later study [158].
Conclusions and perspectives
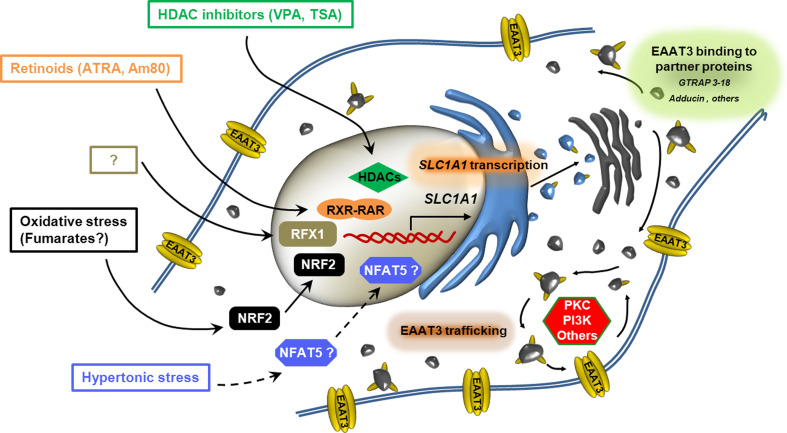
The regulation of EAAT3/EAAC1 expression at the gene level is far less characterized than mechanisms acting on transporter trafficking. Nevertheless, accumulating data yield an increasingly complex picture (Fig. 1), indicating that at least three levels of control modulate the expression of the transporter on the plasma membrane: transcriptional changes in SLC1A1 expression (Table 1), changes in protein interaction, and trafficking modulation. Overall, this information suggests that EAAT3/EAAC1 can be finely regulated at the gene level; however, detailed information is not yet available on regulations acting at post-transcriptional or translational sites. With respect to the well-known examples of fast trafficking modulation, transcriptional regulations are clearly slower but may be more relevant to explain the somewhat contradictory changes in EAAT3/EAAC1 expression described in situations of pathophysiological interest and associated with chronic alterations in nervous functions. Improved knowledge of these mechanisms is necessary to clarify the role of EAAT3/EAAC1 in these conditions and, more importantly, to envisage novel therapeutic approaches.
Fig. 1.
Sites of regulation of EAAT3/EAAC1 expression on the plasma membrane. The sketch depicts the three documented sites of regulation of EAAT3/EAAC1 expression: changes in SLC1A1 transcription, modulation of carrier interaction with binding proteins and modification of transporter trafficking to and from the plasma membrane. For changes in SLC1A1 transcription, transcription factors, chromatin-modifying enzymes, and extracellular stimuli known to be involved in expression changes are detailed
Table 1.
Selected conditions and stimuli associated with changes in the expression of EAAT3/EAAC1
| Condition/stimuli |
SLC1A1/Slc1a1
mRNA |
EAAT3/EAAC1 | Models | References |
|---|---|---|---|---|
| Alzheimer dementia | ND | ↑ | Mouse (Abeta PP23) hippocampus | [108] |
| ND | ↓ | Mouse (3×Tg-Alzheimer’s disease) hippocampus | [109] | |
| Dicarboxylic aminoaciduria | ND | ↓ | Canine kidney cell line | [12] |
| Epilepsy (kainate-induced convulsions) | ↓ | ↓ | Rat hippocampus | [143] |
| Epilepsy (methylazoxymethanol) | ↓ | ↓ | Rat hippocampal neurons | [144] |
| Epilepsy (pilocarpine-induced, temporal lobe epilepsy, TLE) | ↑ | ↑ | Human hippocampus | [138] |
| ↑ | ↑ | Rat hippocampal neurons | [139] | |
| ↑ | ↑ | Rat hippocampal granule cells | [140] | |
| ↓ | ↓ | Human neocortical tissues | [148] | |
| Exercise | ↑ | ND | Rat hippocampus in vivo | [99] |
| Experimental autoimmune encephalomyelitis (EAE) | ↑ | ↑ | Rat spinal cord | [132] |
| G–CSF | ND | ↑ | Rat spinal cord | [130] |
|
HDAC inhibitors (valproic acid, trichostatin A) |
↑ | ↑ | Human oligodendroglioma Hs683 cells | [79] |
| Hypertonic stress | ↑ | ↑ | Bovine renal epithelial NBL-1 cells | [85, 88] |
| Hypoxia (pure) | ↑ | ↑ | Rat pheochromocytoma PC-12 cells | [123] |
| Ischemia/hypoxia | ND | ↓ | Piglet striatum | [112] |
| ↓ | ↓ | Gerbil hippocampus | [113] | |
| ↓ | ↓ | Gerbil hippocampus | [114] | |
| ↓ | ND | Rat hippocampus and cerebral cortex | [119] | |
| ND | ↓ | Rat neurons and oligodendrocytes | [120] | |
| ND | ↑ | Rabbit spinal cord | [122] | |
|
Ischemic preconditioning (oxygen-glucose deprivation) |
↑ | ↑ | Rat cortical neurons in culture | [124] |
| ↑ | ↑ | Rat brain in vivo | [125] | |
| NrF2 | ↑ | ↑ | Rat brain neurons in situ and in vitro | [95] |
| Retinoids (all-trans retinoic acid and Am80, a synthetic retinoid) | ↑ | ↑ | Rat glioma C6 cells | [82, 83] |
| RFX1 | ND | ↑ | Rat glioma C6 cells; rat brain neurons in situ | [91] |
| Schizophrenia | ↑ | ↑ | Human cortex | [154] |
| ↑ | ↑ | Human frontal cortex | [155] | |
| ↓ | ND | Human striatum | [157] | |
| Tunicamycin (ER stress?) | ↑ | ↑ | Bovine renal epithelial NBL-1 cells | [88] |
ND not determined
Acknowledgments
Research in the authors’ laboratory has been funded by FISM (Fondazione Italiana Sclerosi Multipla onlus), grant no. 2010/R/9 to OB.
References
- 1.Amara SG, Fontana AC. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int. 2002;41(5):313–318. doi: 10.1016/s0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 2.Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch. 2004;447(5):469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- 3.Kanai Y, Hediger MA. The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur J Pharmacol. 2003;479(1–3):237–247. doi: 10.1016/j.ejphar.2003.08.073. [DOI] [PubMed] [Google Scholar]
- 4.Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360(6403):467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 5.Gazzola GC, Dall’Asta V, Bussolati O, Makowske M, Christensen HN. A stereoselective anomaly in dicarboxylic amino acid transport. J Biol Chem. 1981;256(12):6054–6059. [PubMed] [Google Scholar]
- 6.Billups B, Rossi D, Oshima T, Warr O, Takahashi M, Sarantis M, Szatkowski M, Attwell D. Physiological and pathological operation of glutamate transporters. Prog Brain Res. 1998;116:45–57. doi: 10.1016/s0079-6123(08)60429-x. [DOI] [PubMed] [Google Scholar]
- 7.Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383(6601):634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- 8.Nothmann D, Leinenweber A, Torres-Salazar D, Kovermann P, Hotzy J, Gameiro A, Grewer C, Fahlke C. Hetero-oligomerization of neuronal glutamate transporters. J Biol Chem. 2010;286(5):3935–3943. doi: 10.1074/jbc.M110.187492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leary GP, Holley DC, Stone EF, Lyda BR, Kalachev LV, Kavanaugh MP. The central cavity in trimeric glutamate transporters restricts ligand diffusion. Proc Natl Acad Sci USA. 2011;108(36):14980–14985. doi: 10.1073/pnas.1108785108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- 11.Perego C, Di Cairano ES, Ballabio M, Magnaghi V. Neurosteroid allopregnanolone regulates EAAC1-mediated glutamate uptake and triggers actin changes in Schwann cells. J Cell Physiol. 2011;227(4):1740–1751. doi: 10.1002/jcp.22898. [DOI] [PubMed] [Google Scholar]
- 12.Bailey CG, Ryan RM, Thoeng AD, Ng C, King K, Vanslambrouck JM, Auray-Blais C, Vandenberg RJ, Broer S, Rasko JE. Loss-of-function mutations in the glutamate transporter SLC1A1 cause human dicarboxylic aminoaciduria. J Clin Invest. 2011;121(1):446–453. doi: 10.1172/JCI44474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375(6532):599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 14.Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA. 1997;94(8):4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13(3):713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 16.Coco S, Verderio C, Trotti D, Rothstein JD, Volterra A, Matteoli M. Non-synaptic localization of the glutamate transporter EAAC1 in cultured hippocampal neurons. Eur J Neurosci. 1997;9(9):1902–1910. doi: 10.1111/j.1460-9568.1997.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 17.Shashidharan P, Huntley GW, Murray JM, Buku A, Moran T, Walsh MJ, Morrison JH, Plaitakis A. Immunohistochemical localization of the neuron-specific glutamate transporter EAAC1 (EAAT3) in rat brain and spinal cord revealed by a novel monoclonal antibody. Brain Res. 1997;773(1–2):139–148. doi: 10.1016/s0006-8993(97)00921-9. [DOI] [PubMed] [Google Scholar]
- 18.Holmseth S, Dehnes Y, Huang YH, Follin-Arbelet VV, Grutle NJ, Mylonakou MN, Plachez C, Zhou Y, Furness DN, Bergles DE, Lehre KP, Danbolt NC. The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. J Neurosci. 2012;32(17):6000–6013. doi: 10.1523/JNEUROSCI.5347-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plaitakis A, Shashidharan P. Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: implications for the pathogenesis of Parkinson’s disease. J Neurol. 2000;247(Suppl 2):II25–II35. doi: 10.1007/pl00007757. [DOI] [PubMed] [Google Scholar]
- 20.Berman AE, Chan WY, Brennan AM, Reyes RC, Adler BL, Suh SW, Kauppinen TM, Edling Y, Swanson RA. N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1−/− mouse. Ann Neurol. 2011;69(3):509–520. doi: 10.1002/ana.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nafia I, Re DB, Masmejean F, Melon C, Kachidian P, Kerkerian-Le Goff L, Nieoullon A, Had-Aissouni L. Preferential vulnerability of mesencephalic dopamine neurons to glutamate transporter dysfunction. J Neurochem. 2008;105(2):484–496. doi: 10.1111/j.1471-4159.2007.05146.x. [DOI] [PubMed] [Google Scholar]
- 22.Kugler P, Schmitt A. Glutamate transporter EAAC1 is expressed in neurons and glial cells in the rat nervous system. Glia. 1999;27(2):129–142. doi: 10.1002/(sici)1098-1136(199908)27:2<129::aid-glia3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 23.Domercq M, Sanchez-Gomez MV, Areso P, Matute C. Expression of glutamate transporters in rat optic nerve oligodendrocytes. Eur J Neurosci. 1999;11(7):2226–2236. doi: 10.1046/j.1460-9568.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 24.Domercq M, Matute C. Expression of glutamate transporters in the adult bovine corpus callosum. Brain Res Mol Brain Res. 1999;67(2):296–302. doi: 10.1016/s0169-328x(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 25.van Landeghem FK, Stover JF, Bechmann I, Bruck W, Unterberg A, Buhrer C, von Deimling A. Early expression of glutamate transporter proteins in ramified microglia after controlled cortical impact injury in the rat. Glia. 2001;35(3):167–179. doi: 10.1002/glia.1082. [DOI] [PubMed] [Google Scholar]
- 26.Palos TP, Ramachandran B, Boado R, Howard BD. Rat C6 and human astrocytic tumor cells express a neuronal type of glutamate transporter. Brain Res Mol Brain Res. 1996;37(1–2):297–303. doi: 10.1016/0169-328x(95)00331-l. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15(3):711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 28.Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci. 1998;18(10):3606–3619. doi: 10.1523/JNEUROSCI.18-10-03606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conti F, DeBiasi S, Minelli A, Rothstein JD, Melone M. EAAC1, a high-affinity glutamate transporter, is localized to astrocytes and GABAergic neurons besides pyramidal cells in the rat cerebral cortex. Cereb Cortex. 1998;8(2):108–116. doi: 10.1093/cercor/8.2.108. [DOI] [PubMed] [Google Scholar]
- 30.Yang W, Kilberg MS. Biosynthesis, intracellular targeting, and degradation of the EAAC1 glutamate/aspartate transporter in C6 glioma cells. J Biol Chem. 2002;277(41):38350–38357. doi: 10.1074/jbc.M202052200. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez MI, Kazanietz MG, Robinson MB. Regulation of the neuronal glutamate transporter excitatory amino acid carrier-1 (EAAC1) by different protein kinase C subtypes. Mol Pharmacol. 2002;62(4):901–910. doi: 10.1124/mol.62.4.901. [DOI] [PubMed] [Google Scholar]
- 32.Fournier KM, Gonzalez MI, Robinson MB. Rapid trafficking of the neuronal glutamate transporter, EAAC1: evidence for distinct trafficking pathways differentially regulated by protein kinase C and platelet-derived growth factor. J Biol Chem. 2004;279(33):34505–34513. doi: 10.1074/jbc.M404032200. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez MI, Bannerman PG, Robinson MB. Phorbol myristate acetate-dependent interaction of protein kinase Calpha and the neuronal glutamate transporter EAAC1. J Neurosci. 2003;23(13):5589–5593. doi: 10.1523/JNEUROSCI.23-13-05589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Feng X, Sando JJ, Zuo Z. Critical role of serine 465 in isoflurane-induced increase of cell-surface redistribution and activity of glutamate transporter type 3. J Biol Chem. 2006;281(50):38133–38138. doi: 10.1074/jbc.M603885200. [DOI] [PubMed] [Google Scholar]
- 35.Klaus F, Gehring EM, Zurn A, Laufer J, Lindner R, Strutz-Seebohm N, Tavare JM, Rothstein JD, Boehmer C, Palmada M, Gruner I, Lang UE, Seebohm G, Lang F. Regulation of the Na(+)-coupled glutamate transporter EAAT3 by PIKfyve. Neurochem Int. 2009;54(5–6):372–377. doi: 10.1016/j.neuint.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Fedorenko O, Tang C, Sopjani M, Foller M, Gehring EM, Strutz-Seebohm N, Ureche ON, Ivanova S, Semke A, Lang F, Seebohm G, Lang UE. PIP5K2A-dependent regulation of excitatory amino acid transporter EAAT3. Psychopharmacology. 2009;206(3):429–435. doi: 10.1007/s00213-009-1621-5. [DOI] [PubMed] [Google Scholar]
- 37.Almilaji A, Pakladok T, Guo A, Munoz C, Foller M, Lang F. Regulation of the glutamate transporter EAAT3 by mammalian target of rapamycin mTOR. Biochem Biophys Res Commun. 2012;421(2):159–163. doi: 10.1016/j.bbrc.2012.03.109. [DOI] [PubMed] [Google Scholar]
- 38.Sopjani M, Alesutan I, Dermaku-Sopjani M, Fraser S, Kemp BE, Foller M, Lang F. Down-regulation of Na+-coupled glutamate transporter EAAT3 and EAAT4 by AMP-activated protein kinase. J Neurochem. 2010;113(6):1426–1435. doi: 10.1111/j.1471-4159.2010.06678.x. [DOI] [PubMed] [Google Scholar]
- 39.Lin CI, Orlov I, Ruggiero AM, Dykes-Hoberg M, Lee A, Jackson M, Rothstein JD. Modulation of the neuronal glutamate transporter EAAC1 by the interacting protein GTRAP3-18. Nature. 2001;410(6824):84–88. doi: 10.1038/35065084. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Vidensky S, Ruggiero AM, Maier S, Sitte HH, Rothstein JD. Reticulon RTN2B regulates trafficking and function of neuronal glutamate transporter EAAC1. J Biol Chem. 2008;283(10):6561–6571. doi: 10.1074/jbc.M708096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aoyama K, Watabe M, Nakaki T. Modulation of neuronal glutathione synthesis by EAAC1 and its interacting protein GTRAP3-18. Amino Acids. 2011;42(1):163–169. doi: 10.1007/s00726-011-0861-y. [DOI] [PubMed] [Google Scholar]
- 42.Aoyama K, Wang F, Matsumura N, Kiyonari H, Shioi G, Tanaka K, Kinoshita C, Kikuchi-Utsumi K, Watabe M, Nakaki T. Increased neuronal glutathione and neuroprotection in GTRAP3-18-deficient mice. Neurobiol Dis. 2012;45(3):973–982. doi: 10.1016/j.nbd.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Butchbach ME, Lai L, Lin CL. Molecular cloning, gene structure, expression profile and functional characterization of the mouse glutamate transporter (EAAT3) interacting protein GTRAP3-18. Gene. 2002;292(1–2):81–90. doi: 10.1016/s0378-1119(02)00669-8. [DOI] [PubMed] [Google Scholar]
- 44.Akiduki S, Ikemoto MJ. Modulation of the neural glutamate transporter EAAC1 by the addicsin-interacting protein ARL6IP1. J Biol Chem. 2008;283(46):31323–31332. doi: 10.1074/jbc.M801570200. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez MI, Susarla BT, Fournier KM, Sheldon AL, Robinson MB. Constitutive endocytosis and recycling of the neuronal glutamate transporter, excitatory amino acid carrier 1. J Neurochem. 2007;103(5):1917–1931. doi: 10.1111/j.1471-4159.2007.04881.x. [DOI] [PubMed] [Google Scholar]
- 46.Fournier KM, Robinson MB. A dominant-negative variant of SNAP-23 decreases the cell surface expression of the neuronal glutamate transporter EAAC1 by slowing constitutive delivery. Neurochem Int. 2006;48(6–7):596–603. doi: 10.1016/j.neuint.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez MI, Krizman-Genda E, Robinson MB. Caveolin-1 regulates the delivery and endocytosis of the glutamate transporter, excitatory amino acid carrier 1. J Biol Chem. 2007;282(41):29855–29865. doi: 10.1074/jbc.M704738200. [DOI] [PubMed] [Google Scholar]
- 48.Xia P, Pei G, Schwarz W. Regulation of the glutamate transporter EAAC1 by expression and activation of delta-opioid receptor. Eur J Neurosci. 2006;24(1):87–93. doi: 10.1111/j.1460-9568.2006.04897.x. [DOI] [PubMed] [Google Scholar]
- 49.Bianchi MG, Gatti R, Torielli L, Padoani G, Gazzola GC, Bussolati O. The glutamate transporter excitatory amino acid carrier 1 associates with the actin-binding protein alpha-adducin. Neuroscience. 2010;169(2):584–595. doi: 10.1016/j.neuroscience.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 50.Seidel B, Zuschratter W, Wex H, Garner CC, Gundelfinger ED. Spatial and sub-cellular localization of the membrane cytoskeleton-associated protein alpha-adducin in the rat brain. Brain Res. 1995;700(1–2):13–24. doi: 10.1016/0006-8993(95)00962-p. [DOI] [PubMed] [Google Scholar]
- 51.Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, Lortet S. The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse? J Neurochem. 2006;98(4):1007–1018. doi: 10.1111/j.1471-4159.2006.03978.x. [DOI] [PubMed] [Google Scholar]
- 52.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 53.Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997;16(13):3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9(1):119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 55.Lee S, Park SH, Zuo Z. Effects of isoflurane on learning and memory functions of wild-type and glutamate transporter type 3 knockout mice. J Pharm Pharmacol. 2012;64(2):302–307. doi: 10.1111/j.2042-7158.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- 56.Smith CP, Weremowicz S, Kanai Y, Stelzner M, Morton CC, Hediger MA. Assignment of the gene coding for the human high-affinity glutamate transporter EAAC1 to 9p24: potential role in dicarboxylic aminoaciduria and neurodegenerative disorders. Genomics. 1994;20(2):335–336. doi: 10.1006/geno.1994.1183. [DOI] [PubMed] [Google Scholar]
- 57.Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol. 1999;9(3):293–298. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- 58.Zerangue N, Kavanaugh MP. Interaction of L-cysteine with a human excitatory amino acid transporter. J Physiol. 1996;493(Pt 2):419–423. doi: 10.1113/jphysiol.1996.sp021393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem. 2003;84(6):1332–1339. doi: 10.1046/j.1471-4159.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- 60.Shanker G, Allen JW, Mutkus LA, Aschner M. The uptake of cysteine in cultured primary astrocytes and neurons. Brain Res. 2001;902(2):156–163. doi: 10.1016/s0006-8993(01)02342-3. [DOI] [PubMed] [Google Scholar]
- 61.Himi T, Ikeda M, Yasuhara T, Nishida M, Morita I. Role of neuronal glutamate transporter in the cysteine uptake and intracellular glutathione levels in cultured cortical neurons. J Neural Transm. 2003;110(12):1337–1348. doi: 10.1007/s00702-003-0049-z. [DOI] [PubMed] [Google Scholar]
- 62.De Bundel D, Schallier A, Loyens E, Fernando R, Miyashita H, Van Liefferinge J, Vermoesen K, Bannai S, Sato H, Michotte Y, Smolders I, Massie A. Loss of system x(c)- does not induce oxidative stress but decreases extracellular glutamate in hippocampus and influences spatial working memory and limbic seizure susceptibility. J Neurosci. 2011;31(15):5792–5803. doi: 10.1523/JNEUROSCI.5465-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X, Valencia A, Sapp E, Masso N, Alexander J, Reeves P, Kegel KB, Aronin N, Difiglia M. Aberrant Rab11-dependent trafficking of the neuronal glutamate transporter EAAC1 causes oxidative stress and cell death in Huntington’s disease. J Neurosci. 2010;30(13):4552–4561. doi: 10.1523/JNEUROSCI.5865-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao L, Li L, Zuo Z. N-Acetylcysteine reverses existing cognitive impairment and increased oxidative stress in glutamate transporter type 3 deficient mice. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, Coulter DA, Rothstein JD. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci. 2002;22(15):6372–6379. doi: 10.1523/JNEUROSCI.22-15-06372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5(2):155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- 67.Pita-Almenar JD, Collado MS, Colbert CM, Eskin A. Different mechanisms exist for the plasticity of glutamate reuptake during early long-term potentiation (LTP) and late LTP. J Neurosci. 2006;26(41):10461–10471. doi: 10.1523/JNEUROSCI.2579-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andin J, Hallbeck M, Mohammed AH, Marcusson J. Influence of environmental enrichment on steady-state mRNA levels for EAAC1, AMPA1 and NMDA2A receptor subunits in rat hippocampus. Brain Res. 2007;1174:18–27. doi: 10.1016/j.brainres.2007.06.101. [DOI] [PubMed] [Google Scholar]
- 69.Scimemi A, Tian H, Diamond JS. Neuronal transporters regulate glutamate clearance, NMDA receptor activation, and synaptic plasticity in the hippocampus. J Neurosci. 2009;29(46):14581–14595. doi: 10.1523/JNEUROSCI.4845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Do SH, Fang HY, Ham BM, Zuo Z. The effects of lidocaine on the activity of glutamate transporter EAAT3: the role of protein kinase C and phosphatidylinositol 3-kinase. Anesth Analg. 2002;95(5):1263–1268. doi: 10.1097/00000539-200211000-00030. [DOI] [PubMed] [Google Scholar]
- 71.Huang Y, Zuo Z. Isoflurane enhances the expression and activity of glutamate transporter type 3 in C6 glioma cells. Anesthesiology. 2003;99(6):1346–1353. doi: 10.1097/00000542-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 72.Lee G, Huang Y, Washington JM, Briggs NW, Zuo Z. Carbamazepine enhances the activity of glutamate transporter type 3 via phosphatidylinositol 3-kinase. Epilepsy Res. 2005;66(1–3):145–153. doi: 10.1016/j.eplepsyres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci. 1997;17(21):8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Furuta A, Takashima S, Yokoo H, Rothstein JD, Wada K, Iwaki T. Expression of glutamate transporter subtypes during normal human corticogenesis and type II lissencephaly. Brain Res Dev Brain Res. 2005;155(2):155–164. doi: 10.1016/j.devbrainres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Guillet B, Lortet S, Masmejean F, Samuel D, Nieoullon A, Pisano P. Developmental expression and activity of high affinity glutamate transporters in rat cortical primary cultures. Neurochem Int. 2002;40(7):661–671. doi: 10.1016/s0197-0186(01)00110-3. [DOI] [PubMed] [Google Scholar]
- 76.Lortet S, Canolle B, Masmejean F, Nieoullon A. Plasma membrane expression of the neuronal glutamate transporter EAAC1 is regulated by glial factors: evidence for different regulatory pathways associated with neuronal maturation. Neurochem Int. 2008;52(7):1373–1382. doi: 10.1016/j.neuint.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 77.Maragakis NJ, Dietrich J, Wong V, Xue H, Mayer-Proschel M, Rao MS, Rothstein JD. Glutamate transporter expression and function in human glial progenitors. Glia. 2004;45(2):133–143. doi: 10.1002/glia.10310. [DOI] [PubMed] [Google Scholar]
- 78.Abney ER, Williams BP, Raff MC. Tracing the development of oligodendrocytes from precursor cells using monoclonal antibodies, fluorescence-activated cell sorting, and cell culture. Dev Biol. 1983;100(1):166–171. doi: 10.1016/0012-1606(83)90207-5. [DOI] [PubMed] [Google Scholar]
- 79.Bianchi MG, Franchi-Gazzola R, Reia L, Allegri M, Uggeri J, Chiu M, Sala R, Bussolati O. Valproic acid induces the glutamate transporter excitatory amino acid transporter-3 in human oligodendroglioma cells. Neuroscience. 2012;227:260–270. doi: 10.1016/j.neuroscience.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 80.Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA. 2004;101(47):16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shen S, Li J, Casaccia-Bonnefil P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J Cell Biol. 2005;169(4):577–589. doi: 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bianchi MG, Gazzola GC, Tognazzi L, Bussolati O. C6 glioma cells differentiated by retinoic acid overexpress the glutamate transporter excitatory amino acid carrier 1 (EAAC1) Neuroscience. 2008;151(4):1042–1052. doi: 10.1016/j.neuroscience.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 83.Bianchi MG, Gazzola GC, Cagnin S, Kagechika H, Bussolati O. The ATRA-dependent overexpression of the glutamate transporter EAAC1 requires RARbeta induction. Biochim Biophys Acta. 2009;1788(9):1861–1868. doi: 10.1016/j.bbamem.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 84.Zhu W, Kanoh M, Ye P, Laszkiewicz I, Royland JE, Wiggins RC, Konat G. Retinoic acid-regulated expression of proteolipid protein and myelin-associated glycoprotein genes in C6 glioma cells. J Neurosci Res. 1992;31(4):745–750. doi: 10.1002/jnr.490310418. [DOI] [PubMed] [Google Scholar]
- 85.Ferrer-Martinez A, Felipe A, Nicholson B, Casado J, Pastor-Anglada M, McGivan J. Induction of the high-affinity Na(+)-dependent glutamate transport system XAG- by hypertonic stress in the renal epithelial cell line NBL-1. Biochem J. 1995;310(Pt 2):689–692. doi: 10.1042/bj3100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Franchi-Gazzola R, Visigalli R, Dall’Asta V, Sala R, Woo SK, Kwon HM, Gazzola GC, Bussolati O. Amino acid depletion activates TonEBP and sodium-coupled inositol transport. Am J Physiol Cell Physiol. 2001;280(6):C1465–C1474. doi: 10.1152/ajpcell.2001.280.6.C1465. [DOI] [PubMed] [Google Scholar]
- 87.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci USA. 1999;96(5):2538–2542. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McGivan JD, Nicholson B. Regulation of high-affinity glutamate transport by amino acid deprivation and hyperosmotic stress. Am J Physiol. 1999;277(4 Pt 2):F498–F500. doi: 10.1152/ajprenal.1999.277.4.F498. [DOI] [PubMed] [Google Scholar]
- 89.Gazzola RF, Sala R, Bussolati O, Visigalli R, Dall’Asta V, Ganapathy V, Gazzola GC. The adaptive regulation of amino acid transport system A is associated to changes in ATA2 expression. FEBS Lett. 2001;490(1–2):11–14. doi: 10.1016/s0014-5793(01)02126-3. [DOI] [PubMed] [Google Scholar]
- 90.Nicholson B, McGivan JD. Induction of high affinity glutamate transport activity by amino acid deprivation in renal epithelial cells does not involve an increase in the amount of transporter protein. J Biol Chem. 1996;271(21):12159–12164. doi: 10.1074/jbc.271.21.12159. [DOI] [PubMed] [Google Scholar]
- 91.Ma K, Zheng S, Zuo Z. The transcription factor regulatory factor X1 increases the expression of neuronal glutamate transporter type 3. J Biol Chem. 2006;281(30):21250–21255. doi: 10.1074/jbc.M600521200. [DOI] [PubMed] [Google Scholar]
- 92.Feng C, Xu W, Zuo Z. Knockout of the regulatory factor X1 gene leads to early embryonic lethality. Biochem Biophys Res Commun. 2009;386(4):715–717. doi: 10.1016/j.bbrc.2009.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feng C, Zuo Z. Regulatory factor X1-induced down-regulation of transforming growth factor beta2 transcription in human neuroblastoma cells. J Biol Chem. 2012;287(27):22730–22739. doi: 10.1074/jbc.M111.338590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Escartin C, Won SJ, Malgorn C, Auregan G, Berman AE, Chen PC, Deglon N, Johnson JA, Suh SW, Swanson RA. Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. J Neurosci. 2011;31(20):7392–7401. doi: 10.1523/JNEUROSCI.6577-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aoyama K, Matsumura N, Watabe M, Wang F, Kikuchi-Utsumi K, Nakaki T. Caffeine and uric acid mediate glutathione synthesis for neuroprotection. Neuroscience. 2011;181:206–215. doi: 10.1016/j.neuroscience.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 97.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Bruck W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134(Pt 3):678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 98.Scannevin RH, Chollate S, Jung MY, Shackett M, Patel H, Bista P, Zeng W, Ryan S, Yamamoto M, Lukashev M, Rhodes KJ. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther. 2012;341(1):274–284. doi: 10.1124/jpet.111.190132. [DOI] [PubMed] [Google Scholar]
- 99.Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16(6):1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 100.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 101.Ganel R, Ho T, Maragakis NJ, Jackson M, Steiner JP, Rothstein JD. Selective up-regulation of the glial Na+ -dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiol Dis. 2006;21(3):556–567. doi: 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 102.Lyon L, Kew JN, Corti C, Harrison PJ, Burnet PW. Altered hippocampal expression of glutamate receptors and transporters in GRM2 and GRM3 knockout mice. Synapse. 2008;62(11):842–850. doi: 10.1002/syn.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin JA, Tsai RY, Lin YT, Lee MS, Cherng CH, Wong CS, Tzeng JI. Amitriptyline pretreatment preserves the antinociceptive effect of morphine in pertussis toxin-treated rats by lowering CSF excitatory amino acid concentrations and reversing the downregulation of glutamate transporters. Brain Res. 2008;1232:61–69. doi: 10.1016/j.brainres.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 104.Veenstra-VanderWeele J, Kim SJ, Gonen D, Hanna GL, Leventhal BL, Cook EH., Jr Genomic organization of the SLC1A1/EAAC1 gene and mutation screening in early-onset obsessive-compulsive disorder. Mol Psychiatry. 2001;6(2):160–167. doi: 10.1038/sj.mp.4000806. [DOI] [PubMed] [Google Scholar]
- 105.Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH, Jr, Hanna GL. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63(7):778–785. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- 106.Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, Jenike E, Chabane N, Leboyer M, Delorme R, Jenike MA, Pauls DL. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):1027–1033. doi: 10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- 107.Veenstra-VanderWeele J, Xu T, Ruggiero AM, Anderson LR, Jones ST, Himle JA, Kennedy JL, Richter MA, Hanna GL, Arnold PD. Functional studies and rare variant screening of SLC1A1/EAAC1 in males with obsessive-compulsive disorder. Psychiatr Genet. 2012;22(5):256–260. doi: 10.1097/YPG.0b013e328353fb63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schallier A, Smolders I, Van Dam D, Loyens E, De Deyn PP, Michotte A, Michotte Y, Massie A. Region- and age-specific changes in glutamate transport in the AbetaPP23 mouse model for Alzheimer’s disease. J Alzheimers Dis. 2011;24(2):287–300. doi: 10.3233/JAD-2011-101005. [DOI] [PubMed] [Google Scholar]
- 109.Cassano T, Serviddio G, Gaetani S, Romano A, Dipasquale P, Cianci S, Bellanti F, Laconca L, Romano AD, Padalino I, LaFerla FM, Nicoletti F, Cuomo V, Vendemiale G. Glutamatergic alterations and mitochondrial impairment in a murine model of Alzheimer disease. Neurobiol Aging. 2012;33(6):1121. doi: 10.1016/j.neurobiolaging.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 110.Duerson K, Woltjer RL, Mookherjee P, Leverenz JB, Montine TJ, Bird TD, Pow DV, Rauen T, Cook DG. Detergent-insoluble EAAC1/EAAT3 aberrantly accumulates in hippocampal neurons of Alzheimer’s disease patients. Brain Pathol. 2009;19(2):267–278. doi: 10.1111/j.1750-3639.2008.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci. 1998;19(8):328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 112.Martin LJ, Brambrink AM, Lehmann C, Portera-Cailliau C, Koehler R, Rothstein J, Traystman RJ. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997;42(3):335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- 113.Fujita H, Sato K, Wen TC, Peng Y, Sakanaka M. Differential expressions of glycine transporter 1 and three glutamate transporter mRNA in the hippocampus of gerbils with transient forebrain ischemia. J Cereb Blood Flow Metab. 1999;19(6):604–615. doi: 10.1097/00004647-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 114.Raghavendra Rao VL, Rao AM, Dogan A, Bowen KK, Hatcher J, Rothstein JD, Dempsey RJ. Glial glutamate transporter GLT-1 down-regulation precedes delayed neuronal death in gerbil hippocampus following transient global cerebral ischemia. Neurochem Int. 2000;36(6):531–537. doi: 10.1016/s0197-0186(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 115.Han F, Shioda N, Moriguchi S, Qin ZH, Fukunaga K. Downregulation of glutamate transporters is associated with elevation in extracellular glutamate concentration following rat microsphere embolism. Neurosci Lett. 2008;430(3):275–280. doi: 10.1016/j.neulet.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 116.Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci. 2001;21(6):1876–1883. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Douen AG, Akiyama K, Hogan MJ, Wang F, Dong L, Chow AK, Hakim A. Preconditioning with cortical spreading depression decreases intraischemic cerebral glutamate levels and down-regulates excitatory amino acid transporters EAAT1 and EAAT2 from rat cerebral cortex plasma membranes. J Neurochem. 2000;75(2):812–818. doi: 10.1046/j.1471-4159.2000.0750812.x. [DOI] [PubMed] [Google Scholar]
- 118.Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348(6300):443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- 119.Montori S, Martinez-Villayandre B, Dos-Anjos S, Llorente IL, Burgin TC, Fernandez-Lopez A. Age-dependent modifications in the mRNA levels of the rat excitatory amino acid transporters (EAATs) at 48hour reperfusion following global ischemia. Brain Res. 2010;1358:11–19. doi: 10.1016/j.brainres.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 120.Gottlieb M, Domercq M, Matute C. Altered expression of the glutamate transporter EAAC1 in neurons and immature oligodendrocytes after transient forebrain ischemia. J Cereb Blood Flow Metab. 2000;20(4):678–687. doi: 10.1097/00004647-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 121.Arranz AM, Gottlieb M, Perez-Cerda F, Matute C. Increased expression of glutamate transporters in subcortical white matter after transient focal cerebral ischemia. Neurobiol Dis. 2009;37(1):156–165. doi: 10.1016/j.nbd.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 122.Hwang I, Lee JC, Yoo KY, Cho JH, Jung JY, Kang TC, Oh YS, Kim WK, Won M. Transient ischemia-induced changes of excitatory amino acid carrier 1 in the ventral horn of the lumbar spinal cord in rabbits. Neurol Res. 2007;29(3):310–316. doi: 10.1179/016164107X159153. [DOI] [PubMed] [Google Scholar]
- 123.Kobayashi S, Millhorn DE. Hypoxia regulates glutamate metabolism and membrane transport in rat PC12 cells. J Neurochem. 2001;76(6):1935–1948. doi: 10.1046/j.1471-4159.2001.00214.x. [DOI] [PubMed] [Google Scholar]
- 124.Romera C, Hurtado O, Botella SH, Lizasoain I, Cardenas A, Fernandez-Tome P, Leza JC, Lorenzo P, Moro MA. In vitro ischemic tolerance involves upregulation of glutamate transport partly mediated by the TACE/ADAM17-tumor necrosis factor-alpha pathway. J Neurosci. 2004;24(6):1350–1357. doi: 10.1523/JNEUROSCI.1596-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pradillo JM, Hurtado O, Romera C, Cardenas A, Fernandez-Tome P, Alonso-Escolano D, Lorenzo P, Moro MA, Lizasoain I. TNFR1 mediates increased neuronal membrane EAAT3 expression after in vivo cerebral ischemic preconditioning. Neuroscience. 2006;138(4):1171–1178. doi: 10.1016/j.neuroscience.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 126.Bigdeli MR, Hajizadeh S, Froozandeh M, Heidarianpour A, Rasoulian B, Asgari AR, Pourkhalili K, Khoshbaten A. Normobaric hyperoxia induces ischemic tolerance and upregulation of glutamate transporters in the rat brain and serum TNF-alpha level. Exp Neurol. 2008;212(2):298–306. doi: 10.1016/j.expneurol.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 127.Bigdeli MR, Rahnema M, Khoshbaten A. Preconditioning with sublethal ischemia or intermittent normobaric hyperoxia up-regulates glutamate transporters and tumor necrosis factor-alpha converting enzyme in the rat brain. J Stroke Cerebrovasc Dis. 2009;18(5):336–342. doi: 10.1016/j.jstrokecerebrovasdis.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 128.Won SJ, Yoo BH, Brennan AM, Shin BS, Kauppinen TM, Berman AE, Swanson RA, Suh SW. EAAC1 gene deletion alters zinc homeostasis and exacerbates neuronal injury after transient cerebral ischemia. J Neurosci. 2010;30(46):15409–15418. doi: 10.1523/JNEUROSCI.2084-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011;31(5):1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen WF, Sung CS, Jean YH, Su TM, Wang HC, Ho JT, Huang SY, Lin CS, Wen ZH. Suppressive effects of intrathecal granulocyte colony-stimulating factor on excessive release of excitatory amino acids in the spinal cerebrospinal fluid of rats with cord ischemia: role of glutamate transporters. Neuroscience. 2010;165(4):1217–1232. doi: 10.1016/j.neuroscience.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 131.DeSilva TM, Kabakov AY, Goldhoff PE, Volpe JJ, Rosenberg PA. Regulation of glutamate transport in developing rat oligodendrocytes. J Neurosci. 2009;29(24):7898–7908. doi: 10.1523/JNEUROSCI.6129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ohgoh M, Hanada T, Smith T, Hashimoto T, Ueno M, Yamanishi Y, Watanabe M, Nishizawa Y. Altered expression of glutamate transporters in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;125(1–2):170–178. doi: 10.1016/s0165-5728(02)00029-2. [DOI] [PubMed] [Google Scholar]
- 133.Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6(1):67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- 134.Vallejo-Illarramendi A, Domercq M, Perez-Cerda F, Ravid R, Matute C. Increased expression and function of glutamate transporters in multiple sclerosis. Neurobiol Dis. 2006;21(1):154–164. doi: 10.1016/j.nbd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 135.Newcombe J, Uddin A, Dove R, Patel B, Turski L, Nishizawa Y, Smith T. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol. 2008;18(1):52–61. doi: 10.1111/j.1750-3639.2007.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klemann C, Raveney BJ, Klemann AK, Ozawa T, von Horsten S, Shudo K, Oki S, Yamamura T. Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol. 2009;174(6):2234–2245. doi: 10.2353/ajpath.2009.081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Baron-Van Evercooren A, Chambon P, Ffrench-Constant C, Franklin RJ. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011;14(1):45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crino PB, Jin H, Shumate MD, Robinson MB, Coulter DA, Brooks-Kayal AR. Increased expression of the neuronal glutamate transporter (EAAT3/EAAC1) in hippocampal and neocortical epilepsy. Epilepsia. 2002;43(3):211–218. doi: 10.1046/j.1528-1157.2002.35001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ross JR, Porter BE, Buckley PT, Eberwine JH, Robinson MB. mRNA for the EAAC1 subtype of glutamate transporter is present in neuronal dendrites in vitro and dramatically increases in vivo after a seizure. Neurochem Int. 2011;58(3):366–375. doi: 10.1016/j.neuint.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang G, Raol YS, Hsu FC, Brooks-Kayal AR. Long-term alterations in glutamate receptor and transporter expression following early-life seizures are associated with increased seizure susceptibility. J Neurochem. 2004;88(1):91–101. doi: 10.1046/j.1471-4159.2003.02124.x. [DOI] [PubMed] [Google Scholar]
- 141.Doi T, Ueda Y, Nagatomo K, Willmore LJ. Role of glutamate and GABA transporters in development of pentylenetetrazol-kindling. Neurochem Res. 2009;34(7):1324–1331. doi: 10.1007/s11064-009-9912-0. [DOI] [PubMed] [Google Scholar]
- 142.Voutsinos-Porche B, Koning E, Clement Y, Kaplan H, Ferrandon A, Motte J, Nehlig A. EAAC1 glutamate transporter expression in the rat lithium-pilocarpine model of temporal lobe epilepsy. J Cereb Blood Flow Metab. 2006;26(11):1419–1430. doi: 10.1038/sj.jcbfm.9600295. [DOI] [PubMed] [Google Scholar]
- 143.Simantov R, Crispino M, Hoe W, Broutman G, Tocco G, Rothstein JD, Baudry M. Changes in expression of neuronal and glial glutamate transporters in rat hippocampus following kainate-induced seizure activity. Brain Res Mol Brain Res. 1999;65(1):112–123. doi: 10.1016/s0169-328x(98)00349-0. [DOI] [PubMed] [Google Scholar]
- 144.Harrington EP, Moddel G, Najm IM, Baraban SC. Altered glutamate receptor—transporter expression and spontaneous seizures in rats exposed to methylazoxymethanol in utero. Epilepsia. 2007;48(1):158–168. doi: 10.1111/j.1528-1167.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- 145.Lu Z, Zhang W, Zhang N, Jiang J, Luo Q, Qiu Y. The expression of glutamate transporters in chest compression-induced audiogenic epilepsy: a comparative study. Neurol Res. 2008;30(9):915–919. doi: 10.1179/174313208X327964. [DOI] [PubMed] [Google Scholar]
- 146.Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, Schrama LH, van Veelen CW, van Rijen PC, van Nieuwenhuizen O, Gispen WH, de Graan PN. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2002;125(Pt 1):32–43. doi: 10.1093/brain/awf001. [DOI] [PubMed] [Google Scholar]
- 147.Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, Danbolt NC, Nelson N, Leite JP, Chimelli L, Born DE, Sakamoto AC, Assirati JA, Fried I, Peacock WJ, Ojemann GA, Adelson PD. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52(3):453–472. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- 148.Rakhade SN, Loeb JA. Focal reduction of neuronal glutamate transporters in human neocortical epilepsy. Epilepsia. 2008;49(2):226–236. doi: 10.1111/j.1528-1167.2007.01310.x. [DOI] [PubMed] [Google Scholar]
- 149.Dutuit M, Touret M, Szymocha R, Nehlig A, Belin MF, Didier-Bazes M. Decreased expression of glutamate transporters in genetic absence epilepsy rats before seizure occurrence. J Neurochem. 2002;80(6):1029–1038. doi: 10.1046/j.0022-3042.2002.00768.x. [DOI] [PubMed] [Google Scholar]
- 150.Samadani U, Judkins AR, Akpalu A, Aronica E, Crino PB. Differential cellular gene expression in ganglioglioma. Epilepsia. 2007;48(4):646–653. doi: 10.1111/j.1528-1167.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 151.White R, Hua Y, Scheithauer B, Lynch DR, Henske EP, Crino PB. Selective alterations in glutamate and GABA receptor subunit mRNA expression in dysplastic neurons and giant cells of cortical tubers. Ann Neurol. 2001;49(1):67–78. doi: 10.1002/1531-8249(200101)49:1<67::aid-ana10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 152.Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158(9):1393–1399. doi: 10.1176/appi.ajp.158.9.1393. [DOI] [PubMed] [Google Scholar]
- 153.Shan D, Lucas EK, Drummond JB, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporters in temporal lobe areas in elderly patients with schizophrenia. Schizophr Res. 2013;144(1–3):1–8. doi: 10.1016/j.schres.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res. 2008;104(1–3):108–120. doi: 10.1016/j.schres.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rao JS, Kellom M, Reese EA, Rapoport SI, Kim HW. Dysregulated glutamate and dopamine transporters in postmortem frontal cortex from bipolar and schizophrenic patients. J Affect Disord. 2012;136(1–2):63–71. doi: 10.1016/j.jad.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 156.Lauriat TL, Dracheva S, Chin B, Schmeidler J, McInnes LA, Haroutunian V. Quantitative analysis of glutamate transporter mRNA expression in prefrontal and primary visual cortex in normal and schizophrenic brain. Neuroscience. 2006;137(3):843–851. doi: 10.1016/j.neuroscience.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 157.McCullumsmith RE, Meador-Woodruff JH. Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology. 2002;26(3):368–375. doi: 10.1016/S0893-133X(01)00370-0. [DOI] [PubMed] [Google Scholar]
- 158.Nudmamud-Thanoi S, Piyabhan P, Harte MK, Cahir M, Reynolds GP. Deficits of neuronal glutamatergic markers in the caudate nucleus in schizophrenia. J Neural Transm Suppl. 2007;72:281–285. doi: 10.1007/978-3-211-73574-9_34. [DOI] [PubMed] [Google Scholar]