Abstract
Human endogenous retroviruses (HERVs) and related genetic elements form 504 distinct families and occupy ~8 % of human genome. Recent success of high-throughput experimental technologies facilitated understanding functional impact of HERVs for molecular machinery of human cells. HERVs encode active retroviral proteins, which may exert important physiological functions in the body, but also may be involved in the progression of cancer and numerous human autoimmune, neurological and infectious diseases. The spectrum of related malignancies includes, but not limits to, multiple sclerosis, psoriasis, lupus, schizophrenia, multiple cancer types and HIV. In addition, HERVs regulate expression of the neighboring host genes and modify genomic regulatory landscape, e.g., by providing regulatory modules like transcription factor binding sites (TFBS). Indeed, recent bioinformatic profiling identified ~110,000 regulatory active HERV elements, which formed at least ~320,000 human TFBS. These and other peculiarities of HERVs might have played an important role in human evolution and speciation. In this paper, we focus on the current progress in understanding of normal and pathological molecular niches of HERVs, on their implications in human evolution, normal physiology and disease. We also review the available databases dealing with various aspects of HERV genetics.
Keywords: Human endogenous retrovirus, Long terminal repeat, Genome evolution, Cancer, Autoimmune disorders, Infectious diseases
Introduction
Human endogenous retroviruses (HERVs) and related genetic elements occupy ~8 % of human genome. Genomic copies of HERVs are of particular interest because in addition to functional viral genes, they have multitude of regulatory DNA regions serving as promoters [1–3], enhancers [4, 5], polyadenylation signals [6, 7], insulators [8, 9] and binding sites for various nuclear proteins [2, 10–13]. Many families of HERVs exhibit high transcriptional activity in human tissues [1, 14–17]. HERVs are believed to be remnants of numerous retroviral infections [18–20] that occurred repeatedly during primate evolution [18, 21]. This was supported by the artificial reconstruction of an active HERV element [20]. This element successfully amplified via an extracellular pathway involving retroviral particle exit from the cell and reinfection, thus recapitulating ex vivo the molecular events responsible for its dissemination in the host genomes. HERVs fixed in the genome and became inheritable because their insertions occurred in the germ cell lineage [22–25].
HERVs are composed of sequences related to retroviral genes and are flanked from both ends by ~1 kb long so-called long terminal repeats (LTRs). A structure of an LTR comprises functional enhancers [26], promoters and polyadenylation signals [18] normally used for retroviral gene expression. However, the LTRs may drive the transcription of adjacent host genomic sequences [27, 28]. Most of HERVs reside in the human genome as solitary LTRs arisen due to homologous recombinations between the two 5′- and 3′-flanking LTRs of the same full-length HERV elements [29–31]. However, some full-length HERVs express viral genes in a variety of human tissues [32] and even form virus-like particles [33, 34]. Expression of HERV-encoded proteins is directly or indirectly associated with progression of many human diseases [35].
In this review, we tried to elucidate the current progress in the identification of the HERV-linked genomic features dealing with the human gene expression. We consider structure of HERVs, their protein-coding potential, their influence on the transcription of host genes, their implication in human diseases and evolutional aspects of HERV accumulation, mutation and selection. A special attention is paid to the databases featuring functional peculiarities of HERVs.
Structure of HERVs
Genomic structure
Human endogenous retroviruses are represented in the human genome by 504 families including ~520,000 individual members, which make up ~8 % of human DNA. Genomic structure of a full-length HERV includes retroviral genes, typically Gag, Prot, Env and Pol, and flanking ~1 kb long sequences termed long terminal repeats (LTRs). Most of the HERVs exist in the form of solitary LTRs, arisen most probably due to homologous recombinations between LTRs of full-length elements [29–31]. In turn, recombinations between the different HERV elements may cause further genomic rearrangements including copy number variation (CNV) of known human genes. For example, this mechanism may be responsible for at least 78 CNV cases encompassing known human genes [36].
Evolutionary recent HERVs have more intact open reading frames (ORFs) and less mutated regulatory sequences, as compared to the ancient elements [37]. The most recent endogenous retroviral group of human genome, HERV-K/HML-2 (ERVK), comprises at least fifty-five full-length members (termed proviruses) [21] and ~2000 solitary LTRs [38].
Having a variety of potential regulatory sequences such as promoters, enhancers, transcriptional factor binding sites, splice sites and polyadenylation signals, LTRs are believed to possess the major transcriptional regulatory potential of endogenous retroviruses. Solitary LTRs are differentially methylated in different human tissues [39–41], they may specifically bind host cell nuclear proteins [42, 43], serve as tissue-specific transcriptional promoters and enhancers [4, 26, 39, 44], and, finally, are transcribed in vivo in many tissues [1, 45–48]. In addition, LTRs may contribute to the host gene regulation network by acting in cis (by providing regulatory elements) or in trans (by driving expression of antisense transcripts) [30, 48–51]. Overall, the LTR sequences have a higher substitution rate than the rest of the non-coding part of the genome [21]. This higher mutation rate underlines LTR regulatory potential, since it may lead to its inactivation, thus counteracting its deleterious effects for the host cell [21].
HERV life cycle
The life cycle of a HERV comprises reverse transcription of viral RNA, followed by the integration of a nascent DNA copy into genomic DNA of the host cell [52, 53]. Importantly, retroviral genomic RNA differs from genomic copy by the absence of LTRs, which are built during the reverse transcription, a multistep complex process including several template switching events [54–56].
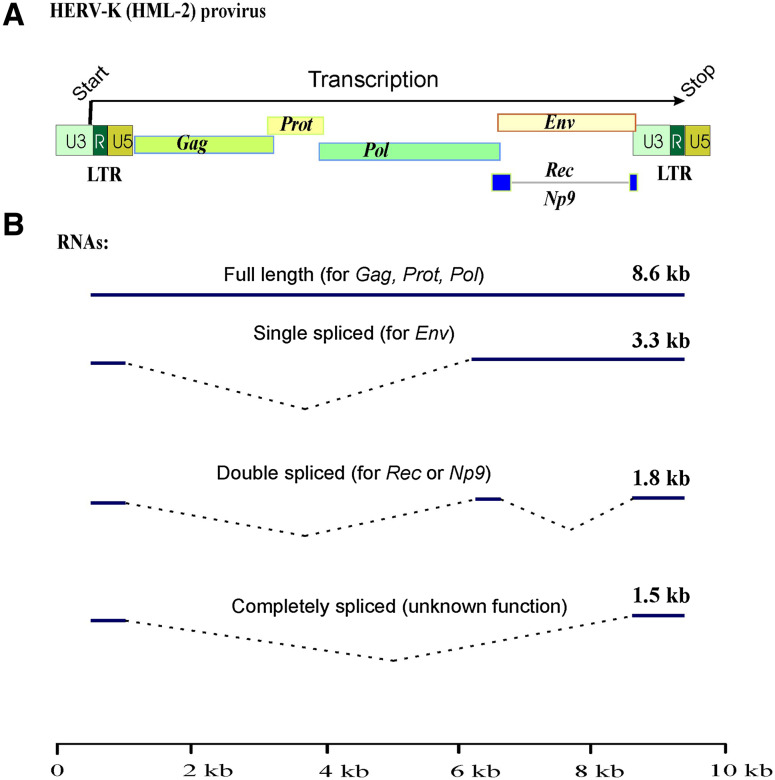
Transcription of an endogenous retrovirus can be shown on the example of HERV-K/HML-2 (ERVK) family members, which have the most complex organization among all HERV elements [19]. The inserted full-length proviral copy is normally transcribed using its functional promoter on the 5′ LTR (Fig. 1a). Transcription stops at the polyadenylation signal of the 3′-terminal LTR. The polyadenylated full-length transcript can be further spliced, thus generating at least three different spliceforms (Fig. 1b). Fully unspliced transcript encodes for 160-kDa viral polyprotein Gag–Prot–Pol. Its translation requires two (−1) ribosome frameshifts [37]. The Polyprotein is then processed by the Prot (retroviral protease) intramolecular activity, and the mature proteins are released. The Gag protein is further cleaved to release matrix, core and nucleocapsid proteins [37, 57]. Pol is the retroviral reverse transcriptase (RT), possessing also RNase H activity. The single-spliced transcript encodes for the envelope protein (Env) that is needed to infect the cells via binding to a cellular receptor [37]. Double-spliced RNA encodes a short regulatory protein. Type 1 HERV-K/HML-2 (ERVK) elements share a 292 nt deletion in the Env region. Apart from fusion of Pol and Env genes, this deletion also gives rise to a difference between the two isoforms of regulatory proteins encoded by the double-spliced transcripts. Type 2 HERV-K/HML-2 (ERVK) 1.8 kb-long proviral transcript codes for the 15-kDa accessory protein Rec (also called cORF [58]), which is the only known auxillary factor encoded by HERVs [37]. Type 1-specific double-spliced RNA product called Np9, is a 9-kDa protein that shares only the N-terminal 15 aa residues with Rec [37, 59, 60]. Rec is specifically accumulated in the nucleoli. It has a striking functional homology to lentiviral RNA-binding nuclear export proteins like the HIV and HTLV proteins Rev and Rex, respectively [37]. Similarly to those proteins, Rec binds to unspliced or partially spliced viral transcripts and mediates their transfer to the cytoplasm where they escape the cellular splicing machinery and can be translated into retroviral polyprotein [61]. Rec may act via interaction with the host-encoded protein Staufen-1 that facilitates transfer of unspliced transcripts to the cytoplasm [62]. The Rec binding site, termed Rec Responsive Element (RRE), is a highly structured RNA motif within the U3R region of the 3′ LTR. Interestingly, this functional motif can be recognized by the HTLV Rex protein that can at least partly substitute for the Rec function [37, 61, 63]. Similarly to Rec, Np9 accumulates in the nucleus. Although Np9 expression was found in many tissues and cell lines, the exact molecular function for this protein remains unclear.
Fig. 1.
Functional genes encoded by HERVs, on the example of HERV-K (HML-2) elements. a Genomic organization of the reconstituted full-size provirus. Apart from “classical” retroviral genes Gag, Prot, Pol and Env, an additional gene Rec or Np9, depending on the provirus type, is encoded. b Different types of proviral transcripts. Full-length subgenomic transcript encodes for Gag–Prot–Pol polyprotein, single-spliced product codes Envelope protein, double-spliced RNA is for Rec/Np9, whereas ~1.5 kb long completely spliced transcript of unknown function appears to lack any functional open reading frames
Finally, ~1.5 kb-long completely spliced proviral transcripts (Fig. 1b) appear to lack any protein coding regions and may have only some regulatory functions, if any [64, 65].
Expression of HERV proteins
Human endogenous retrovirus proteins are actively expressed in a variety of human tissues [37]. For example, autologous antibodies against multiple HERV-K/HML-2 (ERVK) proviral Env epitopes were found in ~30 % of healthy individuals [66]. Increased HERV protein production was also detected in placentas and in embryonic tissues, in line with the identification of putative responsive elements for several pregnancy hormones within the HERV LTRs [67, 68]. Gag protein expression may induce massive T cell stimulation or apoptosis [69]. Endogenous Prot genes may help to exogenous retroviruses, such as lentiviruses, to infect the host cells [70–72]. Rec and Np9 activities may interfere with normal nuclear cytoplasmic transport mechanisms [73] or even serve as inducers of organ-specific tumorogenesis [74]. Finally, Env protein has an immunosuppressive domain that inhibits T and B cell activation and proliferation and induces modulation of the expression of many cytokines [75, 76]. This may be functionally linked to an increased HERV expression in some tumors [37, 77]. Of note, human primary lymphocytes express up to 32 different HERV-K/HML-2 (ERVK) envelopes, and at least two of the most highly expressed Env genes retain the protein-coding capacity [78].
HERV proliferation in the genome
Although traces of several hundreds retroviral groups can be identified in human DNA, there are no evidence that any of them remains active in terms of generating new copies in human genome. However, up to recent times in human evolution, few retroviral families were prolific [24]. Some HERV inserts are specific to human DNA, which means that they happened already after the radiation of human and chimpanzee ancestor lineages, that occurred ~6 million years ago [38, 79, 80]. HERV-K/HML-2 (ERVK) is the sole group of HERVs known to contain human specific members, totally ~140 such elements found in previous works [1, 21, 24, 38, 47, 81–86], that contributed ~330 kb of the human DNA [87]. Similarly to other HERVs, this group comprises mostly proliferation deficient and transcriptionally silent elements [88]. However, many family members are transcriptionally active [45] and theoretically may possess an infectious potential [79, 86]. According to the number of literature citations, this group may be considered the most biologically active human endogenous retroviral family [31, 89, 90]. The detailed analysis of the human-specific LTR structures provided evidence that at least three HERV-K/HML-2 (ERVK) master genes were active in the hominid lineage soon after the human-chimpanzee ancestral radiation [24]. Moreover, few dozens of HERVs are also insertionally polymorphic in human population [25, 79, 86, 91–94], thus suggesting that this family remained prolific up to recent times in the human evolutionary history [74, 86, 89, 95]. The bioinformatic screening of human specific HERVs revealed that they may encode a total of 11 functional genes for Gag, 12 genes for Prot, 9 genes for Pol, 8 for Env, and 9—for Rec [19].
In contrast to humans, in other mammals, endogenous retroviruses may be actively proliferating and highly invasive [96, 97]. For example, murine ERVs are very active and form a significant source of mutation of the murine germ-line. Approximately, one in ten spontaneous phenotypes that have been described in mice are caused by insertional mutagenesis by an ERV [98].
In addition to standard mechanism for HERV proliferation involving reverse transcription and genomic integration of retroviral RNA using a complex of HERV-encoded proteins, at least two alternative possibilities can be mentioned. First, new HERV copies may be generated due to unrelated DNA duplication mechanisms, as in the case of widespread expansion of centromeric human endogenous retroviruses [99]. Multiple copies of a single recently inserted HERV-K/HML-2 (ERVK) provirus, named K111, present in at least 100 copies spread across the centromeres of fifteen human chromosomes. In the chimpanzee genome, K111 is present as a single copy, and it is most likely absent from the DNA of other primates [99]. Second, new HERV copies may appear in the form of processed pseudogenes generated using the reverse transcriptase machinery of a LINE-1 retrotransposon rather than using HERV self-encoded enzymes [10]. Such HERV copies lack LTRs and most frequently are transcriptionally silent [100]. This phenomenon is not unique to HERVs and represents a general mechanism, e.g., there are on an average 1–10 processed pseudogenes per each human gene [101].
Regulatory potential of endogenous retroviruses
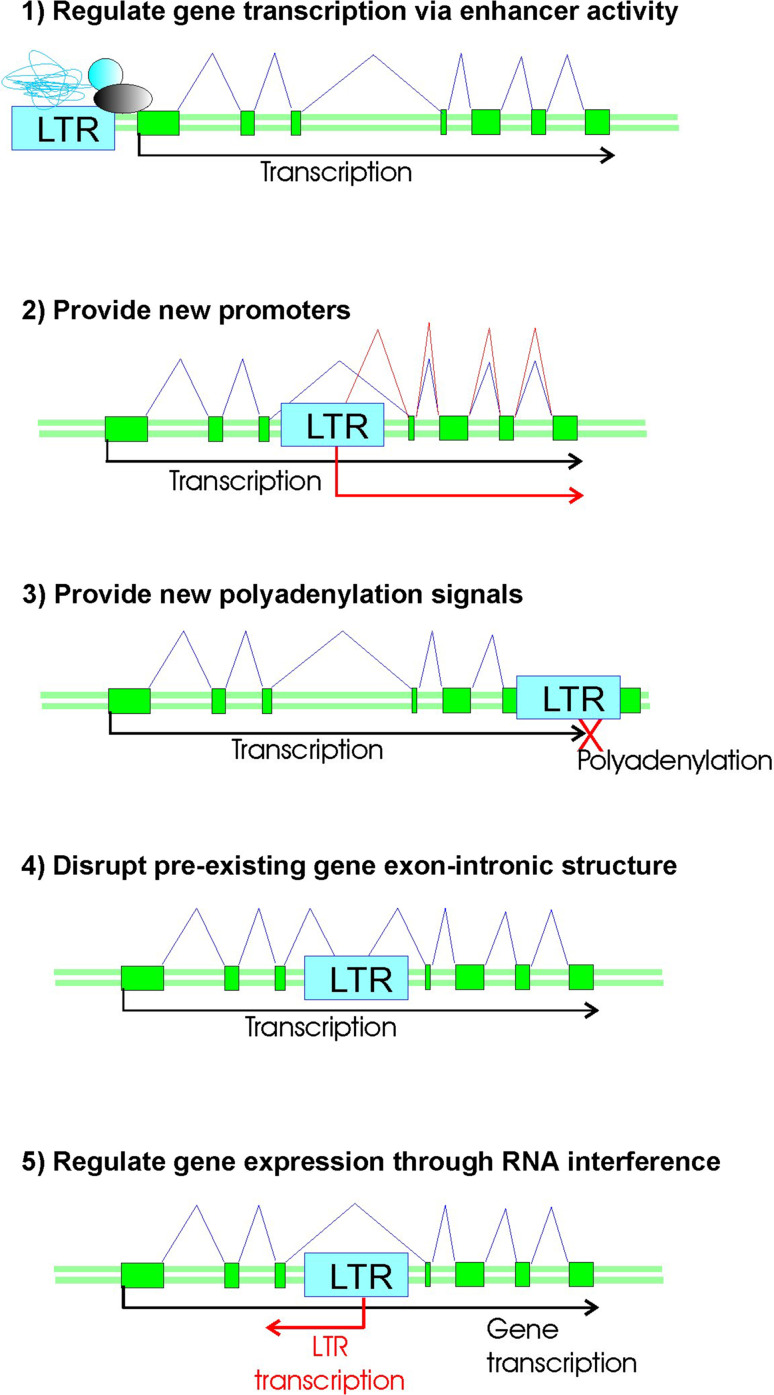
Not only proteins, but also non-coding sequences including viral regulatory elements, considerably shaped human genome and transcriptome [32, 102–104]. HERV LTRs have functional enhancers, promoters, polyadenylation signals and splice sites. They can regulate transcription in human cells by using the following five major mechanisms: (1) LTR enhancer/transcriptional repressor activity may alter expression of the neighboring genes; (2) LTRs may drive transcription of downstream genomic sequences, thus creating new genes and non-coding RNAs; (3) LTR polyadenylation sites may cause premature termination of the read-through transcripts; (4) LTR splice sites may change exon–intron structure of genes; (5) LTRs may regulate host genes via RNA interference mechanisms (Fig. 2). Below, we discuss examples of such functional roles published in the literature.
Fig. 2.
Functional roles played by HERV elements (defined as LTRs) in the regulation of gene expression. 1 HERVs may serve as transcriptional enhancers or silencers by regulating activities of downstream promoters. 2 HERVs may act as transcriptional promoters for host non-repetitive DNA, thus creating new genes. 3 HERV polyadenylation sites may cause premature termination of transcription of the host genes. 4 HERV sequences may disrupt exon–intron structure of genes by donating new splice sites. 5 HERVs may initiate antisense transcripts overlapping with RNAs of the host genes
Functional DNA regions
DNaseI hypersensitivity sites (DHS) are probably the most important genomic landmarks for regions of open (functionally active) chromatin, whereas transcription factor binding sites (TFBS) denote regions of DNA with nuclear protein binding properties [105, 106]. Garazha et al. combined investigation of both DHS and TFBS content of HERVs on a genomic scale. To this end, we devised a bioinformatic algorithm mapping relevant TFBS identified by annotating all the HERVs in the human DNA (504 families and ~720,000 copies) [107]. For the whole set of HERVs, ~140,000 inserts (~19 %) had at least one mapped DHS and ~110,000 inserts (~15 %) had at least one mapped TFBS. The total numbers of all DHS and TFBS in all HERV elements amounted to ~155,000 and ~320,000, respectively [107]. All the 504 HERV families were characterized with regard to their TFBS content (available at http://herv.pparser.net/TotalStatistic.php). The individual families differ dramatically in copy number, ranging from just few copies as for the HERV-F (ERVFH21-1, ERVH48-1) family, to more than 22,000 members as for the THE1B family. The total number of TFBS was also strikingly different varying from 0 (families LTR5, LTR7A) to ~13,000 (MLT1K family). The maximum absolute number of the TFBS-positive members was observed for the MLT1K family (~4000). The families with the greatest densities of TFBS may be regarded as the most functionally active ones among the HERVs. However, it is also important to consider the absolute numbers of TFBS contributed by each family. For example, the family LTR12 has the highest proportion of TFBS-positive members and contributed a total of ~1300 TFBS to the human genome, whereas the family MLT1K donated the greatest number of TFBS (~13,000), but has a rather small density of TFBS-positive members. A definite trend was seen when HERV-related DNase hypersensitivity sites (DHS) and TFBS distributions were compared. The probability that an individual HERV element has DHS increases proportionate to the increase in mapped TFBS. Further experiments confirmed that TFBS density may be an overall measure of the functional activity of HERVs [107]. These results provide clues for identification and functional validation of tens of thousands of previously unknown regulatory sequences of the human genome. Moreover, these results are likely an underestimation of the HERV-related TFBS pool. Due to the repetitive nature of HERVs, it is impossible in many cases to directly map TFBS on any particular element. Those TFBS that were successfully mapped, corresponded mostly to the 5′- or 3′-terminal regions of HERVs, no further than ~200 bp from the border with the unique flanking genomic sequence [107].
Enhancer activity
Human endogenous retroviruses and their LTRs include numerous transcription factor binding sites and may be involved in regulation of the neighboring host genes. One of the first striking reports of the involvement of HERVs in tissue-specific gene transcriptional regulation was for the human amylase locus [108]. In humans, amylase is produced in pancreas and in salivary glands. Human amylase locus includes two genes of pancreatic amylase (AMY2A and AMY2B) and three genes of salivary amylase (AMY1A, AMY1B, AMY1C). The latter three genes are likely products of a recent triplication, because in the chimpanzee genome there is only one gene for AMY1. All genes for salivary amylase contain a full-length insert of HERV-E (ERVE) upstream their transcription start site. The insertion of a full length endogenous retrovirus activated a cryptic promoter that drives the transcription of amylase in salivary glands.
Promoter activity
Promoter strengths of HERVs were investigated in many experimental assays. The application of novel high-throughput techniques such as cap analysis of gene expression (CAGE) and paired-end ditag (PET) sequencing revealed 51197 HERV-derived promoter sequences. 114 HERV-derived transcription start sites appeared to drive transcription of at least 97 human genes, thus producing chimeric transcripts initiated within LTR and read-through into known gene sequences [109]. In transient transfection experiments, a human-specific HERV-K/HML-2 (ERVK) LTR from contig L47334 displayed very low promoter activity in three out of ten cell lines tested, moderate activity (10–20 % of the SV40 promoter) in six cell lines and, finally, the maximal value of ~100 % of the SV40 activity—in embryonal teratocarcinoma cells Tera-1 [44]. Similarly, in another laboratory, five other individual HERV-K/HML-2 (ERVK) LTRs showed high promoter strengths in the same cell line [39].
The comprehensive study of the expression of human-specific LTRs in human germ-line tissue (testicular parenchyma) and in the corresponding tumor (seminoma) [1] showed that different individual LTRs were expressed at markedly different levels differing ~3000 times in magnitude [47]. The LTR status (solitary, 5′ or 3′ proviral) was an important factor affecting LTR activity: promoter strengths of solitary and 3′ proviral LTRs were almost identical, whereas 5′ proviral LTRs showed higher promoter activity (~2-fold and ~5-fold greater in testicular parenchyma and seminoma, respectively). Another important factor influencing promoter activity was the LTR distance from genes: the relative content of promoter-active LTRs in gene-rich regions was significantly higher than in gene-poor loci.
The data obtained suggest also a selective suppression of transcription for the LTRs located in gene introns. Such a transcriptional suppression might be aimed at silencing of the proviral gene expression in gene-rich regions and may serve to minimize possible destructive effects of undesirable transcription. Transcriptional peculiarities of the LTRs are tightly associated with their capability of binding the host transcription factors, e.g., DUX-4 by MaLR, HERV-L (ERVL) and HERV-K/HML-2 (ERVK) promoters [12].
Polyadenylation
Polyadenylation is an essential step for the maturation of almost all eukaryotic mRNAs. A polyadenylation signal (AAUAAA) nearby the 3′ end of pre-mRNA is required for poly(A) synthesis. HERVs encode proteins and utilize functional poly(A) signals at the 3′-termini of their genes. Therefore, insertions of HERVs in the sense orientation can influence the expression of neighboring genes by providing new poly(A) signals. This consideration may explain the clearly seen strong negative selection pressure on such elements inserted in gene introns and oriented in the same transcriptional direction as the enclosing gene [110, 111].
Consistently, HERV polyadenylation signal may be used for the non-retroviral human transcripts. For example, eight human mRNAs are polyadenylated at the sequence of HERV-K/HML-2 (ERVK) LTR [19, 112]. One of these transcripts encodes a 8-kDa protein of unknown function, highly similar to human protein GON4L, a transcription factor that functions in cell cycle control [113]. 5′ LTR of the retrovirus HERV-F (ERVFH21-1, ERVH48-1) may function as an alternative polyadenylation site for known gene ZNF195 [114]. Human genes HHLA2 and HHLA3 utilize HERV-H (ERVH) LTRs as the major polyadenylation signals. In the baboon genome, orthologous loci lack retroviral inserts and these genes recruit other polyadenylation motifs [115].
Antisense regulation of gene expression
This regulatory mechanism is based on formation of the double-stranded RNA between mRNA and the antisense transcript, followed by catalytic degradation of RNAs containing the sites homologous to the double stranded fragment [50]. Among twenty-eight antisense-oriented human-specific HERV-K/HML-2 (ERVK) LTRs located in gene introns, fifteen elements were shown to be promoter active in human germ cells [1]. High expression levels of certain intronic LTRs might suggest the possibility of their involvement in antisense regulation of the enclosing genes [30]. Recently, we found the first evidence for the human specific antisense regulation of gene expression occurred due to promoter activity of HERV-K/HML-2 (ERVK) endogenous retroviral inserts [48]. The human-specific LTRs located in the introns of genes SLC4A8 (for sodium bicarbonate cotransporter) and IFT172 (for intraflagellar transport protein 172) in vivo generate transcripts that are complementary to exons within the corresponding mRNAs in a variety of human tissues. Overexpression of antisense transcripts resulted in ~4- and 3-fold decrease in mRNA levels for these genes, respectively [48].
Splicing
Human gene Hpr sequence for haptoglobin related protein is 92 % identical to haptoglobin gene HP [116]. Both genes are transcribed at the highest level in liver. Hpr promoter is stronger than HP promoter, but the concentration of Hpr liver transcripts is ~17-fold lower than for the HP mRNA [117]. The major distinction between these genes is the endogenous retroviral sequence RTVL-Ia in the intron of Hpr [118]. RTVL-Ia fragment demonstrated significant silencer activity in a series of luciferase transient transfection experiments [117]. The mechanism of the negative Hpr regulation by the RTVL-Ia endogenous retrovirus is most probably linked with aberrant splicing of the Hpr transcript at the retroviral sequences.
Host regulation of HERVs
Suppression of HERVs by the host-encoded mechanisms
Expression of HERVs is tightly controlled by the host cell because it may be deleterious. Even the physical presence of such a number of repetitive sequences in the genome can generate considerable problems dealing with homologous recombination between the different HERV elements, which may disrupt functional genes located in their neighborhood [10]. HERV-derived transcription and gene regulation can bias normal gene expression regulatory networks [4, 10, 48]. Expression of HERV proteins in various human tissues may result in dangerous inflammatory or immunosuppressive effects [35].
In the mouse, endogenous retroviruses are transcriptionally repressed using the tetrapod-specific KRAB-containing zinc finger proteins (KRAB-ZFPs) and their cofactor TRIM28. Recent study demonstrated that KRAB/TRIM28-mediated regulation is responsible for controlling a broad range of HERVs in human embryonic stem cells, too. The authors revealed reciprocal dependence between TRIM28 recruitment at specific families of HERVs and their DNA methylation, which suggests that KRAB/TRIM28 complex recruits methylation machinery to HERV copies [119]. These data are in line with the striking correlation observed across vertebrate genomes between the number of LTR retroelements and the number of host tandem KRAB domain zinc finger genes [120]. Similarly, zinc finger protein Yin Yang 1 may be one of the crucial components restricting HERV transcription in embryonic cells by suppressing promoter activities of the LTRs [121]. Other known mechanisms suppressing endogenous and exogenous retroviruses deal with the functions of APOBEC3, BST2, TREX, Tetherin, TRIM5α and Toll-like receptor proteins [122–124].
They are able to limit viral replication by targeting specific steps of the viral life cycle. Tetherin is interferon-induced transmembrane protein that blocks the release of particles of many enveloped viruses, including HIV [125]. It is associated with lipid rafts at the plasma membrane, and at the trans-Golgi network [122]. Tetherin sequence is highly variable among the mammalian organisms. It appears to inhibit virus release, by connecting both viral and host cell membranes [122]. However, the Envelope proteins (Env) of HERV-K/HML-2 (ERVK) proviruses are able to inhibit Tetherin using a yet unknown mechanism mediated by the recognition of Tetherin by the surface subunit of Env. In experimental tests, two out of six natural complete alleles of HERV-K/HML-2 (ERVK) Env were able to inhibit Tetherin and block Tetherin-mediated viral restriction [126]. Notably, since many HERV-K/HML-2 (ERVK) elements are polymorphic in the human population, it is likely that all individuals will not all possess the same anti-Tetherin potential, which may have functional consequences for individual responses to infection [126].
APOBEC3 protein family consists of seven members in humans. Human APOBEC3G (hA3G) inhibits the infectivity of HIV-1 variants lacking a Vif gene. Vif (virion infectivity factor) prevents hA3G activity by binding and inducing its degradation through ubiquitination [127]. If not inactivated by Vif, hA3G enters HIV-1 particles, and then induces hypermutation of HIV-1 proviruses by editing the proviral genome during reverse transcription, leading to G to A substitutions [122, 128]. Similarly, hA3G inhibits the replication of many other exogenous retroviruses and HERVs. In addition to hA3G, hA3F contributes to proviral hypermutation by deaminating minus-strand of viral cDNA during reverse transcription [122]. In mice, the activity of endogenous retroviruses is also suppressed by the nucleic acid-recognizing Toll-like receptors 3, 7, and 9 (TLR 3, TLR7, and TLR9). Loss of TLR7 function caused spontaneous retroviral viremia that coincided with the absence of ERV-specific antibodies. Additional TLR3 and TLR9 deficiency led to acute T cell lymphoblastic leukemia. Experimental ERV infection induced a TLR3-, TLR7-, and TLR9-dependent group of “acute-phase” genes previously described in HIV and SIV infections [124].
Helpful HERVs
Co-evolution with the human genome resulted in a recruitment of certain HERVs to the execution of important molecular functions. HERV-H (ERVH) is a family of endogenous retroviruses expressed preferentially in human embryonic stem cells (hESCs) [129]. Recently, it was published simultaneously by two research teams that transcriptional regulation of the HERV-H (ERVH) LTRs may be one of the primary mediators of cell fate reprogramming, e.g., induced pluripotency stem cells generation using “Yamanaka cocktail” (by overexpressing OCT3/4, SOX2, and KLF4 proteins) [130, 131]. This effect appeared to be most probably mediated by the HERV-H (ERVH)-driven intergenic long noncoding regulatory RNAs [130, 131]. HERV-R (ERV3-1)-encoded Env protein is also suggested as possible developmental mediator, as it is overexpressed in the developing tissues, like kidney, tongue, heart, liver and brain [132].
The envelope proteins of HERV-W (ERVW-1) family members (corresponding to human protein Syncytin) may serve human physiology through their fusogenic or immunosuppressive properties. For example, Syncytin is essential for placentation, by mediating cell fusion of syncytial cell layers, and for maternal tolerance of the fetus, by immunosuppression [98, 133]. Syncytin, the product of individual HERV-W (ERVW-1) proviral locus ERVW-1, binds to its extramembrane receptor SLC1A5/ASCT-2/RDR/ATB(0) and initiates formation of trophoblast cell fusion, most likely via Cx43-mediated gap junctional intercellular communication [134]. The deficiencies in Syncytin expression, e.g., caused by hypermethylation of ERVW-1, were reported to be associated with various placental abnormalities [135]. As shown in cell culture experiments, Syncytin activity may be negatively regulated by an RNA-binding protein LIN28A, whose target downregulation, in turn, appeared to release Syncytin functionality and promoted fusion of cultured human trophoblast cells [136]. This fusogenic effect was specific to human, but not mouse trophoblast cells, which suggests long-term species-specific molecular evolution of mechanisms controlling spatio-temporal activation of Syncytin in placenta [136]. Other retroviral Env proteins encoded by the endogenous elements ERV-3 and HERV-FRD (ERVFRD), may be also implicated in placentation, as they promote intercellular fusion in cell culture model [137] and are normally expressed in placenta [138]. Interestingly, in other mammalian species, other retroviral Env proteins may behave similarly to Syncytin in placentation, like EnvV protein encoded by ERV-V-1 and ERVV-2 proviruses in the Old World monkeys, but not in the great apes [139].
HERVs may serve as major transcriptional regulators of human genes by direct enhancer or promoter activities, or using other mechanisms, sometimes involving long non-coding transcripts [140]. For example, human specific transcription of human gene PRODH in hippocampus is regulated by an enhancer element created by the insert of a HERV-K/HML-2 (ERVK) LTR [4]. This might have an important impact on human evolution since PRODH metabolizes neuromediator molecules and has a strong implication in higher nervous activity [4]. The ERV9 LTR element upstream of the DNase I hypersensitive site 5 (HS5) of the locus control region in the human β-globin cluster, 40–70 kb upstream of the human fetal gamma- and adult beta-globin genes, is responsible for controlling expression of this cluster in erythroid cells [141]. The enhancer effect is caused by LTR-initiated transcription driven in the direction of associated gene promoter [9, 142]. The LTR contains multiple CCAAT and GATA motifs and competitively recruits a high concentration of NF-Y and GATA-2 transcription factors present in low abundance in adult erythroid cells to assemble an LTR/RNA polymerase II complex. The LTR complex transcribes intergenic RNAs unidirectionally through the intervening DNA to loop with and modulate transcription factor occupancies at the far downstream globin promoters, thereby regulating globin gene switching by a competitive way [140].
Solitary ERVL LTR was shown to promote transcription of a known human gene β3GAL-T5 in various tissues, being especially active in colon, where it is responsible for the majority of gene transcripts [143]. β3GAL-T5 is involved in the synthesis of type 1 carbohydrate chains in gastrointestinal and pancreatic tissues. Interestingly, murine β3GAL-T5 gene is also expressed primarily in colon, despite the absence of an orthologous LTR in the mouse genome. It is likely that in humans, the LTR adopted the function of an ancestral mammalian promoter active in colon [143]. Another interesting example of gene transcriptional regulation by LTR was shown for NAIP (BIRC1) gene coding for neuronal apoptosis inhibitory protein [144]. Although human and rodent NAIP promoter regions share no similarity, in both cases LTR serve as an alternative promoter. Thus, two evolutionary distinct LTR elements were recruited independently in primate and rodent genomes for transcriptional regulation of this gene.
Human gene CYP19 codes for aromatase P450, the key enzyme in estrogen biosynthesis. LTR insertion upstream CYP19 led to the formation of alternative promoter located 100 kb upstream of the coding region [28]. This event resulted in the primate-specific transcription of CYP19 in the syncytiotrophoblast layer of placenta. Placental-specific expression plays an important role in controlling estrogen levels during pregnancy. Cases of placental-specific transcription driven from endogenous retroviral promoters were also shown for Mid1 gene linked with inheritable Opitz syndrome [145], endothelin B receptor [146] and insulin-like growth factor INSL4 [147]. HERVs may also serve as unique promoters for human genes. For example, the only apparent promoter of the liver-specific gene BAAT implicated in familial hypercholanemia is an ancient LTR in human but not in mouse [148].
In addition, a polymorphic HERV-K/HML-2 (ERVK) insert in the ninth intron of the complement component C4 gene was reported as a novel marker of type 1 diabetes that accounts for the disease association previously attributed to some key HLA-DQB1 alleles raising the possibility that this retroviral insertion element contributes to functional protection against type 1 diabetes using any of the above mechanisms [149].
HERVs and human diseases
Recent studies evidence that different activities of HERVs may be involved in various human diseases including autoimmune disorders, neurological, infectious diseases and cancer [150].
Autoimmune diseases
The biased expression of proviral proteins in human tissues may trigger autoimmune diseases [151, 152]. This was indicated first by increased proviral transcript levels [153] and finding anti-HERV protein antibodies in sera from several groups of patients suffering from these systemic disorders [37]. Immune reactivity to HERV products can often occur spontaneously in infection or cancer and is considered the driving force of several autoimmune disorders also in mice. Immune reactivity against ERV proteins can be experimentally induced in mice and non-human primates, suggesting that immunological tolerance to ERV-derived products is not complete [98].
The apparent overexpression of HERV genes may be associated with global hypomethylation as observed for the DNA of T cells from systemic lupus erythematosus (SLE) patients. However, different HERV families behave differently in SLE. For example, HERV-E (ERVE) mRNA expression was higher in lupus CD4+ T cells than in healthy controls, whereas the expression of HERV-K/HML-2 (ERVK) and HERV-W (ERVW-1) family members were similar in SLE patients and healthy controls. Additionally, the HERV-E (ERVE) mRNA expression level was positively correlated with SLE disease activity. Consistently, the HERV-E (ERVE) LTR methylation level was decreased and negatively correlated to the HERV-E (ERVE) mRNA expression in lupus CD4+ T cells [154]. Overexpression of the following HERVs is considered as possibly implicated in SLE: HRES-1, ERV-3, HERV-E 4-1 (ERVE 1–4), HERV-K10 (ERVK-10) and HERV-K18 (ERVK-18) [155]. In addition to HERV-E (ERVE) LTRs, the HERV-K/HML-2 (ERVK) LTRs were also found significantly undermethylated in various types of T lymphocytes in SLE patients [156]. Note that another study showed decreased expression of most HERV genes in purified monocytes from SLE [157].
In the patients with rheumatoid arthritis, statistically significant increase in IgG antibody response to HERV-K10 (ERVK-10) Gag protein was detected, as compared to normal controls [158]. HERV-W (ERVW-1) transcripts and protein products (isoforms of Syncytin) were overrepresented in cartilage of osteoarthritis patients [159]. In osteoarthritis, the patients health status and the disease severity index were also correlated with the expression of HERV-K18 (ERVK-18) provirus, thus suggesting its possible involvement in the aetiopathogenesis of this disease [160]. The implication of HERV-K/HML-2 (ERVK) elements in autoimmune disorders may be connected with the presense of multiple binding sites for various inflammation-linked transcription factors in their LTRs [161].
However, inflammatory diseases may be equally associated with decreased expression of HERV genes [162]. For example, Lichen planus (LP) is a common inflammatory skin disease of unknown etiology. In LP subjects, a significant decrease in the HERV-K/HML-2 (ERVK) Gag and Env, as well as HERV-K18 (ERVK-18) and HERV-W (ERVW-1) Env mRNA expression was detected, compared to healthy controls. Overall, HERV-K/HML-2 (ERVK) Gag expression strongly correlated with other HERV sequences. The decrease of HERV expression in this case may be at least partly explained by observed significant up-regulation of known retroviral restriction factors like cytidine deaminase APOBEC 3G gene, and the GTPase MxA (Myxovirus resistance A) gene [162]. Other inflammation-related transcripts, such as the master regulator of interferon-dependent immune responses, STING, IRF-7 (interferon regulatory factor 7), IFN-β and the inflammasome NALP3, also had increased levels in LP, when compared to healthy controls [162]. This study evidences that interferon-inducible factors may contribute to the negative transcriptional control of HERVs [162]. For psoriasis, which is a multifactorial chronic disease of skin, a significant decrease in antibody response against HERV-K/HML-2 (ERVK) protein products Gag and Env was detected in plasma of the affected patients [163]. Congruently, the expression of HERV-K/HML-2 (ERVK) and ERV-9 gene transcripts was significantly lower in lesional psoriatic skin as compared to healthy skin [163].
Interestingly, although there are currently no indications that HERV reverse transcriptase (RT) is involved in autoimmune disorders, some of the drugs targeting RT enzymatic activity manifested good clinical effects for inflammation-linked diseases [164].
Neurological diseases
Enhanced expression of the HERV-encoded proteins is a promising biomarker for several neurological diseases [165, 166]. Pro-inflammatory cytokine IFNγ plays a key role in the pathology of several HERV-associated neurological diseases. In model experiments, IFNγ signalling markedly enhanced the levels of HERV-K/HML-2 (ERVK) protein expression in both human astrocytes and neurons. These findings again indicate that HERV expression may be inducible under inflammatory conditions [165]. For example, expression of HERV-K/HML-2 (ERVK) genes was significantly increased in postmortal brains of Amyotrophic lateral sclerosis (ALS) patients [167].
For multiple sclerosis (MS), a hypothesis was proposed that HERV-encoded envelope proteins (Env) can act as strong immune stimulators [168]. Thus, slow disease progression following neurodegeneration might be induced by re-activation of HERV expression directly, while relapses in parallel to inflammation might be secondary to the expression of HERV-encoded superantigens [169]. In Northern and Southern European cohorts, an association with susceptibility to bout-onset MS was established and confirmed for the HERV-Fc1 (ERVFC1) sequence in chromosome X and the enclosing polymorphism rs391745 [170]. In addition, two polymorphisms mapped within the HERV-K18 (ERVK-18) locus on the chromosome 1 appeared to be significantly associated with susceptibility to MS in the Spanish cohort of patients [171]. MS occurs more frequently in women than in men, and a possible link between the HERV-W (ERVW-1) copy on chromosome Xq22.3, and the gender differential prevalence in MS has been suggested. This copy contains an almost intact open reading frame for Env, but it is interrupted by a premature stop codon, so the resulting protein, if any, is heavily truncated [172]. Several MS-linked genetic polymorphisms were recently reported that were located within the same genomic region [172]. HERV-W (ERVW-1) Env protein was detected in MS brain lesions within microglia and perivascular macrophages and was shown to induce proinflammatory response in human macrophage cells through TLR4 activation pathway [173]. The corresponding HERV-W (ERVW-1) mRNA levels were enriched in blood, spinal fluid, and brain samples of the MS patients [174]. Furthermore, HERV-W (ERVW-1) Env was even utilized as a superantigen to develop a MS model in mice [173]. While increasing during MS manifestation, HERV-W (ERVW-1) expression decreases with the decline of this disease. Natalizumab is a humanized monoclonal antibody against the cell adhesion molecule α4-integrin. Since 2004, it is widely used as the target drug against MS. In a cohort of MS patients efficiently treated with Natalizumab, both mRNA and protein levels of HERV-W (ERVW-1) Env were significantly reduced [175].
There is frequently a cross-talk between MS and previous infections of the human CNS cells [168]. It was recently demonstrated that infectious mononucleosis may lead to enhanced levels of HERV-W (ERVW-1) Env protein and mRNA in blood mononuclear cells [174]. Flow cytometry data showed increased percentages of cells exposing surface HERV-W (ERVW-1) Env protein, that occur differently in specific cell subsets, and in acute disease and past infection [174]. Expression of the same retroviral protein was considerably increased in various human cell types following influenza A virus infection [2]. Importantly, treatment with neuroleptics and antidepressants (e.g., valproic acid, haloperidol, risperidone, and clozapine) may greatly upregulate the expression of HERV-W (ERVW-1) and ERV9 elements in CNS cells [176]. Moreover, expression of HERV-W (ERVW-1) may be significantly upregulated by some ubiquitous nutrients and medicines like caffeine and aspirin [177]. Besides HERV-W (ERVW-1), MS is genetically associated in Scandinavians with one human endogenous retroviral locus related to the HERV-F (ERVFH21-1, ERVH48-1) element [123]. HERV-F (ERVFH21-1, ERVH48-1) Gag RNA in plasma was increased fourfold in patients with recent history of attacks, relative to patients in a stable state and to healthy controls [178]. It can be extrapolated that infections sometimes can upregulate HERV expression in the CNS cells, thus provoking deleterious autoimmune response [179]. Indeed, genetic variant in some genes restricting retroviral infections were statistically linked with the risk of getting MS, as shown for TRIM5, TRIM22 and BST2, but not for APOBEC3s and TREXs genes [123]. Interestingly, HIV infection is associated with a significantly decreased risk of developing MS. Mechanisms of this observed protective association may include immunosuppression induced by chronic HIV infection and antiretroviral medications [180].
In schizophrenia and bipolar disorder, abnormally high levels of HERV-W (ERVW-1) Env gene product in the plasma were also detected [176, 181]. The seroprevalence for Toxoplasma gondii yielded low but significant association with HERV-W (ERVW-1) transcriptional level in a subgroup of bipolar disorder and schizophrenia, suggesting a potential role in particular patients [182]. However, for the HERV-K18 (ERVK-18) provirus, there were no significant associations with the susceptibility to schizophrenia [183].
Finally, HERVs may also cause neurological disorders using quite distinct mechanism comprising HERV-linked genomic rearrangements. For example, HERV-H (ERVH)-mediated 3q13.2-q13.31 deletions cause a syndrome of hypotonia and motor, language, and cognitive delays [184].
Infectious diseases
The long-term spontaneous evolution of humans and the human viruses might generate various mechanisms involving cooperation or interference of endogenous and exogenous retroviruses. For example, the primate lentiviruses HIV and simian immunodeficiency virus (SIV) do not express their own dUTPase, and it is believed that a host cell endogenous retroviral enzyme (Prot) provides this activity during reverse transcription [70–72], in line with the recent observations that HIV-1 infection may increase the expression of HERV-K/HML-2 (ERVK) proviruses in vitro [185] and in vivo [185, 186]. The envelope glycoprotein of one of HERV-K/HML-2 (ERVK) members, HERV-K18 (ERVK-18), is incorporated into HIV-1 in an HIV matrix-specific fashion [78]. In HIV patients, HERV-K/HML-2 (ERVK) proviruses are expressed at significantly higher levels in peripheral blood mononuclear cells than in those from uninfected individuals [187]. Proviruses were expressed in multiple blood cell types, and the magnitude of HML-2 expression was not related to HIV-1 disease markers [187]. In addition, a controversial data were reported on whether HERV-K/HML-2 (ERVK) viral particles are present or not in the plasma of HIV-infected patients [187]. Increased levels of HERV-K/HML-2 (ERVK) RNA were detected in plasma of HIV patients from Uganda, but not from the USA [188]. In contrast, Esqueda et al. argue that there was no correlation between HERV-K/HML-2 (ERVK) RNA levels and HIV viral loads in plasma specimens they profiled [189]. However, presence of antibodies against HERV-K/HML-2 (ERVK) Env protein in blood was proposed as the new biomarker of HIV-1 infection, because HIV-1 can modify HERV-K/HML-2 (ERVK) Env mRNA expression, resulting in the expression of a fully N-glycosylated HERV-K/HML-2 (ERVK) envelope protein on the cell surface [190]. Moreover, HERV-K/HML-2 (ERVK)-specific CD8+ T cells obtained from HIV-1-infected human subjects, exhibited complete elimination of human cells infected with a panel of globally diverse HIV-1, HIV-2, and SIV isolates in vitro. This supports the consideration of HERV-K/HML-2 (ERVK)-specific and cross-reactive T cell responses for exploring HERV-K/HML-2 (ERVK)-targeted HIV vaccines and immunotherapeutics [191]. The mechanism of HIV-1 induced transactivation of HERV-K/HML-2 (ERVK) and other transposable elements possibly involves the activity of an HIV-1 Tat protein [192]. Recent studies showed that in model experiments with peripheral blood lymphocytes out of 91 annotated HERV-K/HML-2 (ERVK) proviruses, Tat significantly activates expression of 26 unique HERV-K/HML-2 (ERVK) proviruses, silences 12, and does not significantly alter the expression of the rest proviruses [193]. In addition, HIV infection may cause transactivation of HERV-W (ERVW-1) family with their Env genes and Syncytin [194].
On the other hand, endogenous retroviral Env production theoretically can provide to the host cell a partial resistance to infection of pathogenic exogenous counterparts or related retroviruses by receptor interference [77, 195, 196], as this is the case for endogenous Jaagsiekte sheep retrovirus (JSRV) that blocks the entry of the corresponding exogenous virus. Both forms use the same protein receptor for entry, implying interference between endogenous and exogenous viruses [195]. Endogenous Gag protein may be also involved in antiviral host cell protection. For instance, the expression of murine endogenous Gag-sequence Fv1 blocks certain strains of mouse leukemia virus (MLV) soon after entry [197], most probably, due to a direct encounter with the incoming viral capsid [37]. Similar cases were reported also for the chicken, ground squirrel and cat endogenous retroviral elements [198]. No direct evidence exists so far for the human elements, but theoretically this may be possible since human DNA harbors hundreds of intact or largely intact retroviral env genes [199]. The recent association study of ~23,000 participants indicates that susceptibility to herpes zoster caused by the varicella zoster virus (VZV) is linked with the non-coding gene HCP5 (HLA Complex P5) in the major histocompatibility complex. This gene is an endogenous retrovirus that likely suppresses viral activity through regulatory functions. Variants in this genetic region are also known to be associated with delay in development of AIDS in people infected by HIV [200].
Cancer
The role of HERVs in cancer is most likely limited to retrovirus-driven gene expression and does not involve their insertional activity [22]. In this regard, data from cancer genome sequencing identified over 180 somatic integrations in human cancer cells caused by LINE-1 retrotransposon activity [98, 201, 202]. In contrast, there was only a single insertion of a small HERV fragment, which was most likely the result of a microhomology-mediated DNA repair mechanism [98, 201].
Abnormal expression of HERVs in cancer is well known. For example, HERV-K/HML-2 (ERVK) elements are overexpressed in germ cell tumors and in melanoma [22, 203–205]. Upregulation of HERVs may be mediated by the cancer-specific combinations of transcription factors, as shown for the HERV-K/HML-2 (ERVK) activation by the melanoma-specific transcription factor MITF-M [206]. A significant increase in frequency and titer of antibodies against proviral proteins in patients suffering from testicular cancers has been documented (60 % against 4 % in healthy control group) [57]. Importantly, shortly after the elimination of the tumor, the antibody titers dropped and became undetectable by 5 years after surgery [57]. Both HERV-K/HML-2 (ERVK) mRNA and antibodies against proviral proteins were significantly overrepresented in plasma of the patients with primary breast cancer [207]. Serum proviral mRNA levels tended to be higher in the patients who later on developed the metastatic disease [207]. Aberrant overexpression of HERV-K/HML-2 (ERVK) proviruses was found also in prostate cancer [208] and HERV-K/HML-2 (ERVK) encoded transcripts or proteins are considered as possible biomarkers of malignization, being overexpressed for the patients with poor prognosis [209]. Increased HERV-K/HML-2 (ERVK) Env gene expression was detected in the prostate tumors in 40 % of European-American and 61 % of African-American patients [208]. Expression of some individual HERV-K/HML-2 (ERVK) elements can be of an outstanding importance to follow prostate cancer development, as recently shown for the HERV-K/HML-2 (ERVK) (ch22q11.23) Gag gene [209]. In a cohort of patients with renal cell carcinoma, bone marrow transplantation was reported to result in tumor regression likely due to a graft-versus-tumor effect. In such patients, anti-tumor cytotoxic lymphocytes were targeting a HERV-E (ERVE)-encoded epitope [210], thus demonstrating the importance of anti-HERV immune responses in the progression or cure of human diseases [98].
Chronic lymphocytic leukemia (CLL) cells demonstrate increased transcription of auxillary HERV-K/HML-2 (ERVK) gene Np9, which was previously published as possible oncogene [211]. Indeed, silencing of Np9 inhibits the growth of myeloid and lymphoblastic leukemic cells, whereas its overexpression promotes the growth in vitro and in vivo. In human leukemia cells, Np9 protein level is substantially higher than that in normal cells, e.g., in normal hematopoietic stem cells. Np9 may act by activating ERK, AKT and Notch1 signaling pathways and through the upregulation of β-catenin, essential for survival of leukemia stem cells [212]. Another group of researchers found that Np9 may directly interact with the RING-type E3 ubiquitin ligase LNX protein [59]. This interaction affects the subcellular localization of LNX, whereas LNX can target the cell fate determinant and Notch antagonist Numb for proteasome degradation, thereby promoting Notch signaling. The LNX-interacting Np9 is also unstable and degraded via the proteasome pathway. Combined, these findings point to the possibility that Np9 affects tumorigenesis by influencing the LNX/Numb/Notch pathway [59]. In addition, Np9-positive leukemia samples highly expressed HERV-K/HML-2 (ERVK) Pol-Env polyprotein, Env and transmembrane proteins as well as entire viral particles [212].
Expression of Syncytin, or Syncytin-1, which is Env protein of HERV-W (ERVW-1) family, is normally restricted to the placenta. However, it was also found in many pathologies including cancers and it is hypothesized that Syncytin-mediated cell fusion participates in cancer cell transformation or metastasis [98]. Endogenous retroviral Env proteins possess immunosuppressive and fusogenic activities [98]. As in the placenta, the expression of immunosuppressive domain of HERV-W (ERVW-1) and HERV-K/HML-2 (ERVK) Env in tumors may suppress immune responses and thus prevent rejection of the tumour and the embryo [76]. Immunosuppression is most likely mediated by the transmembrane subunit of the envelope protein of several retroviruses [213]. Indeed, overexpression of Moloney MLV transmembrane subunit in murine tumor cell lines led to tumor growth in recipient mice that would otherwise immunologically reject them [98, 214]. Consistently, knockdown of env transcripts in melanoma and neuroblastoma cell lines, both of which produce infectious MLVs derived from endogenous retroviral precursors, rendered them susceptible to immune rejection in vivo [215, 216]. In human DNA, in addition to Syncytins, the Env genes of HERV-E (ERVE) [217] and HERV-H (ERVH) [218] were shown to possess immunosuppressive potential [98]. MRNA contents of HERV-R (ERV3-1), HERV-H (ERVH), HERV-K/HML-2 (ERVK), and HERV-P Env genes were significantly increased in breast cancer patients and dropped to normal levels following chemotherapy [219]. Syncytins and other Env genes like Erv-3, envT and envFc2 were also upregulated in endometrial carcinomas [220].
In addition, HERVs may promote cellular transformation by regulating downstream human gene expression through their LTRs [98]. Transcriptional derepression of the CSF1R gene, encoding colony stimulating factor-1 receptor, by a demethylated MaLR LTR acting as an alternative promoter has been linked with survival of cancer B cells in Hodgkin’s lymphoma [221]. Finally, the discovery of HTLV-1 (human T-lymphotropic virus 1) unequivocally proved the existence of a tumor-inducing exogenous human retrovirus [98].
Impact on human evolution
Various HERV families were active at the different timepoints during human evolution [8, 10, 18, 222]. For primate-specific regions, ~63 % of mapped DNase I hypersensitivity sites representing open chromatin regions corresponded to HERV sequences [105]. The apparent emergence of ~320,000 transcription factor binding sites donated by HERVs to human genome, must have a deep impact on the regulation of intracellular molecular networks [107]. Expression of viral genes, HERV-driven transcription of neighboring DNA, attenuation of splicing, polyadenylation and RNA degradation might all considerably influence the human cell and organism [10]. In a comprehensive genome-wide study, Subramanian and coauthors recently found that both solitary LTRs and full-size proviruses of human HERV-K/HML-2 (ERVK) family are preferably located on gene-rich chromosomes and close to gene regions [223]. A small group of HERVs belonging to ERVK elements was actively proliferating in the genome after the divergence of human and chimpanzee ancestor lineages [19]. Few group members were even specific to non-human hominid genomes of Neanderthals and Denisovans [224]. Human specific HERVs, in turn, are presented by ~130–140 members that shaped ~330 kb of the human DNA. This group modified the human genome activity by endogenizing ~50 functional copies of viral genes that may have important implications in the immune response, cancer progression and anti-retroviral host defence. 134 potential promoters and enhancers have been added to the human DNA, about 50 % of them—in the close gene vicinity, and 22 %—in gene introns [1]. For sixty such human specific promoters their activity was confirmed by in vivo assays, with the transcriptional level varying ~1000-fold from hardly detectable to the level comparable with the expression of major housekeeping genes. New polyadenylation signals have been provided to four human RNAs, and a number of potential antisense regulators of known human genes appeared due to human-specific retroviral insertional activity [47, 48].
When looking at all, not only human-specific, HERV elements, a number of remarkable trends can be observed dealing with transcription factor binding site (TFBS) densities. Garazha et al. analyzed the distribution of the TFBS-containing HERVs relatively to their divergence from the consensus sequence (this divergence positively reflects evolutionary age of each HERV element) [225, 226]. Overall, the TFBS-activity of HERVs was decreasing with evolutional age [107]. However, a significant heterogeneity between the proportions of TFBS-positive elements was found for the evolutionary “young” HERVs (divergence less than 10 %). However, the proportion of TFBS-positive elements drops as the divergence increases (>15 %) and further tends to lay within a sharp interval of 0.12–0.18, which is ~6-times less wide than for the young elements. This clearly demonstrates that the low-diverged (evolutionally recent) HERVs have a significantly lower likelihood of functional activity in human DNA compared to the “older” elements. This observation may suggest that genomic “domestication” of the newly integrated HERV sequences involves reshaping of their active TFBS profiles and their further “standardization”, e.g., upon accumulation of mutations [107]. Another aspect of the same concept was uncovered when looking at the distributions of TFBS for the different transcription factors. For example, the protein NF-YA has highly abundant TFBS in the evolutionary young HERVs (divergence 5–8 %), whereas for the older elements, the TFBS ratio is significantly lower. In contrast, for the protein Rad21 there is a relatively low ratio of TFBS for the “young” elements followed by a subsequent increase for the older elements, reaching a maximum value at ~22 % divergence [107]. This example shows, that for some transcription factors (e.g., NF-YA), the recently inserted HERVs are enriched in TFBS, whereas further genomic domestication and mutation pressure progressively decrease the TFBS proportion. In contrast, for another group of transcription factors like Rad21, the older elements accumulate increased proportions of TFBS [107]. Overall, most of the transcription factors showed one common feature in their TFBS evolutionary dynamics: a decrease in the proportion of TFBS in the divergence interval around 5–15 %. This indicates that functional adaptation and modification of a HERV insert includes strong initial silencing of the original TFBS that came from this element, and further accumulation of new functional TFBS in tight co-evolution with the host genome [107]. Support for this hypothesis comes from the studies showing that the newly integrated inserts are initially under strong DNA methylation repression [119, 227]. This preserves the cell genome from viral gene expression and from the deleterious influence of these elements on the host gene regulatory ensembles. Upon de-methylation, a number of HERVs release their regulatory potential and provide functional TFBS to the human genome. However, de-methylation is followed by progressive mutation of the HERV sequence, which is reflected by a further decline of the “original” TFBS. However, mutations do not only cause removal of these sites, but also create new TFBS in the HERVs that, in turn, fall under selection pressure according to their implication in the overall genomic context [228]. Finally, the highly diverged HERVs become roughly equilibrated with the enclosing genomic regions and show little difference compared to the average genomic sequence [107].
In a much closer evolutional scale, this theory is exemplified by the investigations of the promoter activity of an evolutionally recent family HERV-K/HML-2 (ERVK) [27]. Mapping of the transcriptional start sites for several actively transcribed HERV-K/HML-2 (ERVK) LTRs evidenced for the presence of two functional promoter regions within their sequence [27]. The first promoter was the canonical element located in the LTR U3 region, whereas the second one was mapped in the very 3′ terminus of the LTR R region. Both promoters appeared to be active in solitary LTRs and in full-length proviruses. Surprisingly, this second non-canonical element was even more active than the classical U3-based retroviral promoter. Therefore, the R region is excluded from most transcripts initiated on LTRs, whereas a classical retroviral life cycle model implies that the transcription is driven from between the LTR U3 and R elements (first promoter), and the R transcript is a 5′-terminal component of the newly synthesized proviral RNA [27]. Such a mode of proviral DNA transcription is a basis of the HERV life cycle that provides the possibility of template jumps during proviral RNA reverse transcription. Further studies confirmed the prevailing activity of a non-canonical HERV-K/HML-2 (ERVK) LTR promoter and showed it is regulated by Sp1 and Sp3 transcription factors via a TATA box-independent transcription initiation mechanism [229]. A shift of the transcriptional start site can be explained by the initial adaptation to the human genomic context, which can be an early step in the complex process of the HERV sequence domestication and reshaping by the host genome. Another important mechanism guiding coevolution of HERVs with the human genome deals with inactivation of full-size proviruses. This mechanism may include recombinations, most frequently eliminating viral genes and producing solitary LTRs instead of complete proviral sequences. An outstanding case of full-size provirus disruption by recombination was published by Hughes and Coffin in 2004 [29]. The authors found a genomic locus, which may exist in three alternative states: it may lack proviral insert, may have a complete provirus, or may have a solitary LTR instead. Overall, apparent polymorphic solitary LTR formation from full-size proviruses was documented in 5/13 of the studied cases and was found to be a rather frequent event in the human population [29]. Moreover, the same authors previously found that ~16 % of all recently inserted ERVK elements are linked with traceable genomic rearrangement events [230].
Databases linked with HERV structure and functions
The ubiquitous nature of HERVs and the plurality of their molecular functions stress the importance of organization and maintaining related databases. Some of the HERV sequences and features were annotated and collected in various databases. For example, in 2002 the first database was published termed HERVd that was collecting structural data on most on the HERV elements known at that time point [231]. However, this database was not updated since 2003 and nowadays misses important information available elsewhere. Villesen et al. published a database putting together the sequences of HERV-encoded open reading frames of the human genome available to the date of publication (2005). Fifty-nine intact or almost intact endogenous retroviral genes were identified, of them 29 encoding envelope proteins [232]. The database of human retrotransposon insertion polymorphisms [233] presents the data on the HERV elements that appeared polymorphic in human population, and the database of human specific transposable elements [234] contains information on the genomic location of human specific HERVs and other retrotransposons. However, many human specific HERVs were unique to further database [19] which encompassed 134 human-specific endogenous retroviral inserts. It has genomic coordinates of HERVs, their polymorphic state in human populations, distances from known genes or mapped RNAs, structural status of HERVs (solitary LTR/provirus), information about open reading frames putatively encoded by the corresponding HERVs, and the corresponding deduced aminoacid sequences. It also accumulates data on methylation of individual elements, and also on individual HERV transcription. This database was probably the first attempt to characterize in detail a particular group of HERVs [19]. However, it was focused solely on human-specific HERV-K/HML-2 (ERVK) elements and was not updated since 2007. More recently, Subramanian et al. cataloged all available HERV-K/HML-2 (ERVK) sequences of the human genome, including both proviruses and solitary LTRs [223]. However, other human endogenous retroviral groups left outside the framework of this study.
RNA of HERVs and other transposable elements may form an intrinsic hairpin structures and/or serve as microRNA precursor when inserted into transcriptionally active genomic regions [235, 236]. To catalog the data on transposable elements that may have an impact on gene regulation and functioning via RNA interference, a database termed “TranspoGene database” has been constructed that covers not only human, but also mouse, chicken, zebrafish, fruit fly, nematode and sea squirt genomes [237]. A variant of this database termed “microTranspoGene” collects data on human, mouse, zebrafish and nematode TE-derived microRNAs [237]. HESAS (HERVs Expression and Structure Analysis System) database was developed to link HERV sequences with the neighboring genes to identify the elements having impact on the expression of human functional genes. Inserts of HERV elements were found into 17,317 of human genes and linked (to the opinion of the authors) to expression of 898 genes [238]. However, the database was not updated since 2004 and lacks information on functional signatures of regulatory sequences such as DNase I hypersensitivity sites and transcription factor binding sites.
A bioinformatic algorithm termed RetroTector and related database published in 2007 utilize automated recognition of retroviral sequences in genomic data. Retroviral sequences were detected in various vertebrate genomes, including human. Most RetroTector-detected chains were coincident with Repeatmasker output and the HERVd database. However, RetroTector did not report many evolutionary old HERV sequences, and was useless for the detection of solitary LTRs [239].
Most recently, Garazha and coworkers published an interactive comprehensive HERV database that groups the individual inserts according to their familial nomenclature, number of mapped TFBS and divergence from their consensus sequence [107]. Database encompasses data from 717,612 individual elements represented by 504 different HERV families, which covers ~8 % of the human DNA. Detailed information on any particular HERV element can be easily extracted by the user. To facilitate data navigation, we created a genome browser tool enabling quick mapping and finding of any HERV insert according to genomic coordinates, known human genes and densities of TFBS. This browser is cross-linked with the UCSC Genome Browser to enable easy mapping of other genetic features of the interest. This database may be widely used for quickly locating functionally relevant individual HERVs, and for analyzing their impact on the regulation of human genes. In addition, option of browsing TFBS distribution for any particular transcription factor among the HERVs is enabled [107]. These resources are freely available at http://herv.pparser.net.
Concluding remarks
In the last decade, human endogenous retroviruses attracted attention of the research society because of multiplicity of ways they can influence human physiology in health and disease. Since 2005, the grant funding for HERV-related projects by governmental agencies increased ~4-times ([240]; International Aging Research Portfolio, http://www.agingportfolio.org/) and the number of PubMed-indexed publications featuring HERVs increased by ~1.4-fold reaching a number of approximately 200 publications per year. This growing interest of biomedical community is linked with the immense role that these elements played, play and may play in shaping of human genome and transcriptome, in molecular evolution and in the progression of many autoimmune, neurological, infectious and oncological diseases. Contemporary experimental and bioinformatic methods enable investigation of HERVs at the unprecedental levels of whole transcriptomes and even proteomes. This promises further acceleration of the progress in decoding molecular functions of HERVs, hopefully at the level of each individual genomic element.
Acknowledgments
This work was supported by the Pathway Pharmaceuticals Research Initiative (Hong-Kong) and by the Program of the Presidium of the Russian Academy of Sciences “Dynamics and Conservation of Genomes”. The authors thank “UMA Foundation” for their help in preparation of the manuscript.
References
- 1.Buzdin A, Kovalskaya-Alexandrova E, Gogvadze E, Sverdlov E. At least 50 % of human-specific HERV-K (HML-2) long terminal repeats serve in vivo as active promoters for host nonrepetitive DNA transcription. J Virol. 2006;80(21):10752–10762. doi: 10.1128/JVI.00871-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li F, Nellåker C, Sabunciyan S, Yolken RH, Jones-Brando L, Johansson A-S, Br Owe-Larsson, Karlsson H. Transcriptional derepression of the ERVWE1 locus following influenza A virus infection. J Virol. 2014;88(8):4328–4337. doi: 10.1128/JVI.03628-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs NV, Loewer S, Daley GQ, Izsvak Z, Lower J, Lower R. Human endogenous retrovirus K (HML-2) RNA and protein expression is a marker for human embryonic and induced pluripotent stem cells. Retrovirology. 2013;10:115. doi: 10.1186/1742-4690-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suntsova M, Gogvadze EV, Salozhin S, Gaifullin N, Eroshkin F, Dmitriev SE, Martynova N, Kulikov K, Malakhova G, Tukhbatova G, Bolshakov AP, Ghilarov D, Garazha A, Aliper A, Cantor CR, Solokhin Y, Roumiantsev S, Balaban P, Zhavoronkov A, Buzdin A. Human-specific endogenous retroviral insert serves as an enhancer for the schizophrenia-linked gene PRODH. Proc Natl Acad Sci USA. 2013;110(48):19472–19477. doi: 10.1073/pnas.1318172110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuong EB, Rumi MA, Soares MJ, Baker JC. Endogenous retroviruses function as species-specific enhancer elements in the placenta. Nat Genet. 2013;45(3):325–329. doi: 10.1038/ng.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HS. Genomic impact, chromosomal distribution and transcriptional regulation of HERV elements. Mol Cells. 2012;33(6):539–544. doi: 10.1007/s10059-012-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu HL, Zhao ZK, Zhu F. The role of human endogenous retroviral long terminal repeat sequences in human cancer (review) Int J Mol Med. 2013;32(4):755–762. doi: 10.3892/ijmm.2013.1460. [DOI] [PubMed] [Google Scholar]
- 8.Schumann GG, Gogvadze EV, Osanai-Futahashi M, Kuroki A, Munk C, Fujiwara H, Ivics Z, Buzdin AA. Unique functions of repetitive transcriptomes. Int Rev Cell Mol Biol. 2010;285:115–188. doi: 10.1016/B978-0-12-381047-2.00003-7. [DOI] [PubMed] [Google Scholar]
- 9.Ling J, Pi W, Yu X, Bengra C, Long Q, Jin H, Seyfang A, Tuan D. The ERV-9 LTR enhancer is not blocked by the HS5 insulator and synthesizes through the HS5 site non-coding, long RNAs that regulate LTR enhancer function. Nucleic Acids Res. 2003;31(15):4582–4596. doi: 10.1093/nar/gkg646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gogvadze E, Buzdin A. Retroelements and their impact on genome evolution and functioning. Cell Mol Life Sci CMLS. 2009;66(23):3727–3742. doi: 10.1007/s00018-009-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarumi C, Matsunuma N, Miyakoshi N, Yamashita K, Masuda H. Long term oral administration study of pravastatin sodium to beagles for 104 weeks. J Toxicol Sci. 1989;14(Suppl 1):85–101. doi: 10.2131/jts.14.SupplementI_85. [DOI] [PubMed] [Google Scholar]
- 12.Young JM, Whiddon JL, Yao Z, Kasinathan B, Snider L, Geng LN, Balog J, Tawil R, van der Maarel SM, Tapscott SJ. DUX4 binding to retroelements creates promoters that are active in FSHD muscle and testis. PLoS Genet. 2013;9(11):e1003947. doi: 10.1371/journal.pgen.1003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arjan-Odedra S, Swanson CM, Sherer NM, Wolinsky SM, Malim MH. Endogenous MOV10 inhibits the retrotransposition of endogenous retroelements but not the replication of exogenous retroviruses. Retrovirology. 2012;9:53. doi: 10.1186/1742-4690-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maliniemi P, Vincendeau M, Mayer J, Frank O, Hahtola S, Karenko L, Carlsson E, Mallet F, Seifarth W, Leib-Mosch C, Ranki A. Expression of human endogenous retrovirus-w including syncytin-1 in cutaneous T-cell lymphoma. PLoS ONE. 2013;8(10):e76281. doi: 10.1371/journal.pone.0076281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Babaian A, Gagnier L, Mager DL. Visualized computational predictions of transcriptional effects by intronic endogenous retroviruses. PLoS ONE. 2013;8(8):e71971. doi: 10.1371/journal.pone.0071971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosenca D, Gabriel U, Steidler A, Mayer J, Diem O, Erben P, Fabarius A, Leib-Mosch C, Hofmann WK, Seifarth W. HERV-E-mediated modulation of PLA2G4A transcription in urothelial carcinoma. PLoS ONE. 2012;7(11):e49341. doi: 10.1371/journal.pone.0049341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Triviai I, Ziegler M, Bergholz U, Oler AJ, Stubig T, Prassolov V, Fehse B, Kozak CA, Kroger N, Stocking C. Endogenous retrovirus induces leukemia in a xenograft mouse model for primary myelofibrosis. Proc Natl Acad Sci USA. 2014;111(23):8595–8600. doi: 10.1073/pnas.1401215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sverdlov ED. Retroviruses and primate evolution. BioEssays: news and reviews in molecular, cellular and developmental biology. 2000;22(2):161–171. doi: 10.1002/(SICI)1521-1878(200002)22:2<161::AID-BIES7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Buzdin A. Human-specific endogenous retroviruses. Sci World J. 2007;7:1848–1868. doi: 10.1100/tsw.2007.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belshaw R, Pereira V, Katzourakis A, Talbot G, Paces J, Burt A, Tristem M. Long-term reinfection of the human genome by endogenous retroviruses. Proc Natl Acad Sci USA. 2004;101(14):4894–4899. doi: 10.1073/pnas.0307800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romano CM, Ramalho RF, Zanotto PM. Tempo and mode of ERV-K evolution in human and chimpanzee genomes. Arch Virol. 2006;151(11):2215–2228. doi: 10.1007/s00705-006-0792-1. [DOI] [PubMed] [Google Scholar]
- 22.Hohn O, Hanke K, Bannert N. HERV-K(HML-2), the best preserved family of HERVs: endogenization, expression, and implications in health and disease. Front Oncol. 2013;3:246. doi: 10.3389/fonc.2013.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewannieux M, Heidmann T. Endogenous retroviruses: acquisition, amplification and taming of genome invaders. Curr Opin Virol. 2013;3(6):646–656. doi: 10.1016/j.coviro.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Buzdin A, Ustyugova S, Khodosevich K, Mamedov I, Lebedev Y, Hunsmann G, Sverdlov E. Human-specific subfamilies of HERV-K (HML-2) long terminal repeats: three master genes were active simultaneously during branching of hominoid lineages. Genomics. 2003;81(2):149–156. doi: 10.1016/S0888-7543(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 25.Wildschutte JH, Ram D, Subramanian R, Stevens VL, Coffin JM. The distribution of insertionally polymorphic endogenous retroviruses in breast cancer patients and cancer-free controls. Retrovirology. 2014;11:62. doi: 10.1186/s12977-014-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruda VM, Akopov SB, Trubetskoy DO, Manuylov NL, Vetchinova AS, Zavalova LL, Nikolaev LG, Sverdlov ED. Tissue specificity of enhancer and promoter activities of a HERV-K(HML-2) LTR. Virus Res. 2004;104(1):11–16. doi: 10.1016/j.virusres.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 27.Kovalskaya E, Buzdin A, Gogvadze E, Vinogradova T, Sverdlov E. Functional human endogenous retroviral LTR transcription start sites are located between the R and U5 regions. Virology. 2006;346(2):373–378. doi: 10.1016/j.virol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 28.van de Lagemaat LN, Landry JR, Mager DL, Medstrand P. Transposable elements in mammals promote regulatory variation and diversification of genes with specialized functions. Trends Genet. 2003;19(10):530–536. doi: 10.1016/j.tig.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Hughes JF, Coffin JM. Human endogenous retrovirus K solo-LTR formation and insertional polymorphisms: implications for human and viral evolution. Proc Natl Acad Sci USA. 2004;101(6):1668–1672. doi: 10.1073/pnas.0307885100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buzdin AA, Lebedev IuB, Sverdlov ED. Human genome-specific HERV-K intron LTR genes have a random orientation relative to the direction of transcription, and possibly, participated in antisense gene expression regulation. Bioorg Khim. 2003;29(1):103–106. doi: 10.1023/a:1022294906202. [DOI] [PubMed] [Google Scholar]
- 31.Mayer J, Stuhr T, Reus K, Maldener E, Kitova M, Asmus F, Meese E. Haplotype analysis of the human endogenous retrovirus locus HERV-K(HML-2.HOM) and its evolutionary implications. J Mol Evol. 2005;61(5):706–715. doi: 10.1007/s00239-005-0066-7. [DOI] [PubMed] [Google Scholar]
- 32.Seifarth W, Frank O, Zeilfelder U, Spiess B, Greenwood AD, Hehlmann R, Leib-Mosch C. Comprehensive analysis of human endogenous retrovirus transcriptional activity in human tissues with a retrovirus-specific microarray. J Virol. 2005;79(1):341–352. doi: 10.1128/JVI.79.1.341-352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyden TW, Johnson PM, Mwenda JM, Rote NS. Ultrastructural characterization of endogenous retroviral particles isolated from normal human placentas. Biol Reprod. 1994;51(1):152–157. doi: 10.1095/biolreprod51.1.152. [DOI] [PubMed] [Google Scholar]
- 34.Medstrand P, Blomberg J. Characterization of novel reverse transcriptase encoding human endogenous retroviral sequences similar to type A and type B retroviruses: differential transcription in normal human tissues. J Virol. 1993;67(11):6778–6787. doi: 10.1128/jvi.67.11.6778-6787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho K, Lee YK, Greenhalgh DG. Endogenous retroviruses in systemic response to stress signals. Shock. 2008;30(2):105–116. doi: 10.1097/SHK.0b013e31816a363f. [DOI] [PubMed] [Google Scholar]
- 36.Campbell IM, Gambin T, Dittwald P, Beck CR, Shuvarikov A, Hixson P, Patel A, Gambin A, Shaw CA, Rosenfeld JA, Stankiewicz P. Human endogenous retroviral elements promote genome instability via non-allelic homologous recombination. BMC Biol. 2014;12:74. doi: 10.1186/s12915-014-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bannert N, Kurth R. Retroelements and the human genome: new perspectives on an old relation. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14572–14579. doi: 10.1073/pnas.0404838101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medstrand P, Mager DL. Human-specific integrations of the HERV-K endogenous retrovirus family. J Virol. 1998;72(12):9782–9787. doi: 10.1128/jvi.72.12.9782-9787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavie L, Kitova M, Maldener E, Meese E, Mayer J. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K(HML-2) J Virol. 2005;79(2):876–883. doi: 10.1128/JVI.79.2.876-883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khodosevich KV, Lebedev IuB, Sverdlov ED. The tissue-specific methylation of human-specific endogenous retroviral long terminal repeats. Bioorg Khim. 2004;30(5):493–498. doi: 10.1023/b:rubi.0000043787.07628.2a. [DOI] [PubMed] [Google Scholar]
- 41.Khodosevich K, Lebedev Y, Sverdlov ED. Large-scale determination of the methylation status of retrotransposons in different tissues using a methylation tags approach. Nucleic Acids Res. 2004;32(3):e31. doi: 10.1093/nar/gnh035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akopov SB, Nikolaev LG, Khil PP, Lebedev YB, Sverdlov ED. Long terminal repeats of human endogenous retrovirus K family (HERV-K) specifically bind host cell nuclear proteins. FEBS Lett. 1998;421(3):229–233. doi: 10.1016/S0014-5793(97)01569-X. [DOI] [PubMed] [Google Scholar]
- 43.Trubetskoy DO, Zavalova LL, Akopov SB, Nikolaev LG. Purification of proteins specifically binding human endogenous retrovirus K long terminal repeat by affinity elution chromatography. J Chromatogr A. 2002;976(1–2):95–101. doi: 10.1016/S0021-9673(02)01236-0. [DOI] [PubMed] [Google Scholar]
- 44.Domansky AN, Kopantzev EP, Snezhkov EV, Lebedev YB, Leib-Mosch C, Sverdlov ED. Solitary HERV-K LTRs possess bi-directional promoter activity and contain a negative regulatory element in the U5 region. FEBS Lett. 2000;472(2–3):191–195. doi: 10.1016/S0014-5793(00)01460-5. [DOI] [PubMed] [Google Scholar]
- 45.Vinogradova TV, Leppik LP, Nikolaev LG, Akopov SB, Kleiman AM, Senyuta NB, Sverdlov ED. Solitary human endogenous retroviruses-K LTRs retain transcriptional activity in vivo, the mode of which is different in different cell types. Virology. 2001;290(1):83–90. doi: 10.1006/viro.2001.1134. [DOI] [PubMed] [Google Scholar]
- 46.Vinogradova T, Leppik L, Kalinina E, Zhulidov P, Grzeschik KH, Sverdlov E. Selective Differential Display of RNAs containing interspersed repeats: analysis of changes in the transcription of HERV-K LTRs in germ cell tumors. Mol Genet Genomics. 2002;266(5):796–805. doi: 10.1007/s00438-001-0596-7. [DOI] [PubMed] [Google Scholar]
- 47.Buzdin A, Kovalskaya-Alexandrova E, Gogvadze E, Sverdlov E. GREM, a technique for genome-wide isolation and quantitative analysis of promoter active repeats. Nucleic Acids Res. 2006;34(9):e67. doi: 10.1093/nar/gkl335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gogvadze E, Stukacheva E, Buzdin A, Sverdlov E. Human-specific modulation of transcriptional activity provided by endogenous retroviral insertions. J Virol. 2009;83(12):6098–6105. doi: 10.1128/JVI.00123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buzdin AA. Retroelements and formation of chimeric retrogenes. Cell Mol Life Sci CMLS. 2004;61(16):2046–2059. doi: 10.1007/s00018-004-4041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mack M, Bender K, Schneider PM. Detection of retroviral antisense transcripts and promoter activity of the HERV-K(C4) insertion in the MHC class III region. Immunogenetics. 2004;56(5):321–332. doi: 10.1007/s00251-004-0705-y. [DOI] [PubMed] [Google Scholar]
- 51.Sverdlov ED. Retroviral regulators of gene expression in the human genome as possible factors for its evolution. Bioorg Khim. 1999;25(11):821–827. [PubMed] [Google Scholar]
- 52.Dewannieux M, Harper F, Richaud A, Letzelter C, Ribet D, Pierron G, Heidmann T. Identification of an infectious progenitor for the multiple-copy HERV-K human endogenous retroelements. Genome Res. 2006;16(12):1548–1556. doi: 10.1101/gr.5565706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee YN, Bieniasz PD. Reconstitution of an infectious human endogenous retrovirus. PLoS Pathog. 2007;3(1):e10. doi: 10.1371/journal.ppat.0030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kandel ES, Nudler E. Template switching by RNA polymerase II in vivo. Evidence and implications from a retroviral system. Mol Cell. 2002;10(6):1495–1502. doi: 10.1016/S1097-2765(02)00777-3. [DOI] [PubMed] [Google Scholar]
- 55.Coffin JM. Molecular mechanisms of nucleic acid integration. J Med Virol. 1990;31(1):43–49. doi: 10.1002/jmv.1890310109. [DOI] [PubMed] [Google Scholar]
- 56.Coffin JM. Retroviridae: the viruses and their replication. Fields virology. Philadelphia: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 57.Boller K, Konig H, Sauter M, Mueller-Lantzsch N, Lower R, Lower J, Kurth R. Evidence that HERV-K is the endogenous retrovirus sequence that codes for the human teratocarcinoma-derived retrovirus HTDV. Virology. 1993;196(1):349–353. doi: 10.1006/viro.1993.1487. [DOI] [PubMed] [Google Scholar]
- 58.Lower R, Tonjes RR, Korbmacher C, Kurth R, Lower J. Identification of a Rev-related protein by analysis of spliced transcripts of the human endogenous retroviruses HTDV/HERV-K. J Virol. 1995;69(1):141–149. doi: 10.1128/jvi.69.1.141-149.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Armbruester V, Sauter M, Roemer K, Best B, Hahn S, Nty A, Schmid A, Philipp S, Mueller A, Mueller-Lantzsch N. Np9 protein of human endogenous retrovirus K interacts with ligand of numb protein X. J Virol. 2004;78(19):10310–10319. doi: 10.1128/JVI.78.19.10310-10319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Armbruester V, Sauter M, Krautkraemer E, Meese E, Kleiman A, Best B, Roemer K, Mueller-Lantzsch N. A novel gene from the human endogenous retrovirus K expressed in transformed cells. Clin Cancer Res. 2002;8(6):1800–1807. [PubMed] [Google Scholar]
- 61.Magin C, Lower R, Lower J. cORF and RcRE, the Rev/Rex and RRE/RxRE homologues of the human endogenous retrovirus family HTDV/HERV-K. J Virol. 1999;73(11):9496–9507. doi: 10.1128/jvi.73.11.9496-9507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanke K, Hohn O, Liedgens L, Fiddeke K, Wamara J, Kurth R, Bannert N. Staufen-1 interacts with the human endogenous retrovirus family HERV-K(HML-2) rec and gag proteins and increases virion production. J Virol. 2013;87(20):11019–11030. doi: 10.1128/JVI.03031-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magin-Lachmann C, Hahn S, Strobel H, Held U, Lower J, Lower R. Rec (formerly Corf) function requires interaction with a complex, folded RNA structure within its responsive element rather than binding to a discrete specific binding site. J Virol. 2001;75(21):10359–10371. doi: 10.1128/JVI.75.21.10359-10371.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kleiman A, Senyuta N, Tryakin A, Sauter M, Karseladze A, Tjulandin S, Gurtsevitch V, Mueller-Lantzsch N. HERV-K(HML-2) GAG/ENV antibodies as indicator for therapy effect in patients with germ cell tumors. Int J Cancer. 2004;110(3):459–461. doi: 10.1002/ijc.11649. [DOI] [PubMed] [Google Scholar]
- 65.Wang-Johanning F, Liu J, Rycaj K, Huang M, Tsai K, Rosen DG, Chen DT, Lu DW, Barnhart KF, Johanning GL. Expression of multiple human endogenous retrovirus surface envelope proteins in ovarian cancer. Int J Cancer. 2007;120(1):81–90. doi: 10.1002/ijc.22256. [DOI] [PubMed] [Google Scholar]
- 66.Herve CA, Lugli EB, Brand A, Griffiths DJ, Venables PJ. Autoantibodies to human endogenous retrovirus-K are frequently detected in health and disease and react with multiple epitopes. Clin Exp Immunol. 2002;128(1):75–82. doi: 10.1046/j.1365-2249.2002.01735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ono M, Kawakami M, Ushikubo H. Stimulation of expression of the human endogenous retrovirus genome by female steroid hormones in human breast cancer cell line T47D. J Virol. 1987;61(6):2059–2062. doi: 10.1128/jvi.61.6.2059-2062.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andersson AC, Svensson AC, Rolny C, Andersson G, Larsson E. Expression of human endogenous retrovirus ERV3 (HERV-R) mRNA in normal and neoplastic tissues. Int J Oncol. 1998;12(2):309–313. doi: 10.3892/ijo.12.2.309. [DOI] [PubMed] [Google Scholar]
- 69.Hugin AW, Vacchio MS, Morse HC., 3rd A virus-encoded “superantigen” in a retrovirus-induced immunodeficiency syndrome of mice. Science. 1991;252(5004):424–427. doi: 10.1126/science.1850169. [DOI] [PubMed] [Google Scholar]
- 70.Mayer J, Meese EU. Presence of dUTPase in the various human endogenous retrovirus K (HERV-K) families. J Mol Evol. 2003;57(6):642–649. doi: 10.1007/s00239-003-2514-6. [DOI] [PubMed] [Google Scholar]
- 71.Harris JM, McIntosh EM, Muscat GE. Expression and cytoplasmic localisation of deoxyuridine triphosphate pyrophosphatase encoded by a human endogenous retrovirus. Arch Virol. 2000;145(2):353–363. doi: 10.1007/s007050050027. [DOI] [PubMed] [Google Scholar]
- 72.Harris JM, McIntosh EM, Muscat GE. Structure/function analysis of a dUTPase: catalytic mechanism of a potential chemotherapeutic target. J Mol Biol. 1999;288(2):275–287. doi: 10.1006/jmbi.1999.2680. [DOI] [PubMed] [Google Scholar]
- 73.Boese A, Galli U, Geyer M, Sauter M, Mueller-Lantzsch N. The Rev/Rex homolog HERV-K cORF multimerizes via a C-terminal domain. FEBS Lett. 2001;493(2–3):117–121. doi: 10.1016/S0014-5793(01)02280-3. [DOI] [PubMed] [Google Scholar]
- 74.Galli UM, Sauter M, Lecher B, Maurer S, Herbst H, Roemer K, Mueller-Lantzsch N. Human endogenous retrovirus rec interferes with germ cell development in mice and may cause carcinoma in situ, the predecessor lesion of germ cell tumors. Oncogene. 2005;24(19):3223–3228. doi: 10.1038/sj.onc.1208543. [DOI] [PubMed] [Google Scholar]
- 75.Denner J, Persin C, Vogel T, Haustein D, Norley S, Kurth R. The immunosuppressive peptide of HIV-1 inhibits T and B lymphocyte stimulation. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12(5):442–450. doi: 10.1097/00042560-199608150-00002. [DOI] [PubMed] [Google Scholar]
- 76.Morozov VA, Dao Thi VL, Denner J. The transmembrane protein of the human endogenous retrovirus-K (HERV-K) modulates cytokine release and gene expression. PLoS ONE. 2013;8(8):e70399. doi: 10.1371/journal.pone.0070399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melder DC, Pankratz VS, Federspiel MJ. Evolutionary pressure of a receptor competitor selects different subgroup a avian leukosis virus escape variants with altered receptor interactions. J Virol. 2003;77(19):10504–10514. doi: 10.1128/JVI.77.19.10504-10514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brinzevich D, Young GR, Sebra R, Ayllon J, Maio SM, Deikus G, Chen BK, Fernandez-Sesma A, Simon V, Mulder LC. HIV-1 interacts with human endogenous retrovirus K (HML-2) envelopes derived from human primary lymphocytes. J Virol. 2014;88(11):6213–6223. doi: 10.1128/JVI.00669-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belshaw R, Dawson AL, Woolven-Allen J, Redding J, Burt A, Tristem M. Genomewide screening reveals high levels of insertional polymorphism in the human endogenous retrovirus family HERV-K(HML2): implications for present-day activity. J Virol. 2005;79(19):12507–12514. doi: 10.1128/JVI.79.19.12507-12514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buzdin A, Khodosevich K, Mamedov I, Vinogradova T, Lebedev Y, Hunsmann G, Sverdlov E. A technique for genome-wide identification of differences in the interspersed repeats integrations between closely related genomes and its application to detection of human-specific integrations of HERV-K LTRs. Genomics. 2002;79(3):413–422. doi: 10.1006/geno.2002.6705. [DOI] [PubMed] [Google Scholar]
- 81.Lebedev YB, Belonovitch OS, Zybrova NV, Khil PP, Kurdyukov SG, Vinogradova TV, Hunsmann G, Sverdlov ED. Differences in HERV-K LTR insertions in orthologous loci of humans and great apes. Gene. 2000;247(1–2):265–277. doi: 10.1016/S0378-1119(00)00062-7. [DOI] [PubMed] [Google Scholar]
- 82.Lavrentieva I, Khil P, Vinogradova T, Akhmedov A, Lapuk A, Shakhova O, Lebedev Y, Monastyrskaya G, Sverdlov ED. Subfamilies and nearest-neighbour dendrogram for the LTRs of human endogenous retroviruses HERV-K mapped on human chromosome 19: physical neighbourhood does not correlate with identity level. Hum Genet. 1998;102(1):107–116. doi: 10.1007/s004390050662. [DOI] [PubMed] [Google Scholar]
- 83.Mamedov I, Batrak A, Buzdin A, Arzumanyan E, Lebedev Y, Sverdlov ED. Genome-wide comparison of differences in the integration sites of interspersed repeats between closely related genomes. Nucleic Acids Res. 2002;30(14):e71. doi: 10.1093/nar/gnf071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buzdin A, Vinogradova T, Lebedev Y, Sverdlov E. Genome-wide experimental identification and functional analysis of human specific retroelements. Cytogenet Genome Res. 2005;110(1–4):468–474. doi: 10.1159/000084980. [DOI] [PubMed] [Google Scholar]
- 85.Barbulescu M, Turner G, Seaman MI, Deinard AS, Kidd KK, Lenz J. Many human endogenous retrovirus K (HERV-K) proviruses are unique to humans. Curr Biol. 1999;9(16):861–868. doi: 10.1016/S0960-9822(99)80390-X. [DOI] [PubMed] [Google Scholar]
- 86.Turner G, Barbulescu M, Su M, Jensen-Seaman MI, Kidd KK, Lenz J. Insertional polymorphisms of full-length endogenous retroviruses in humans. Curr Biol. 2001;11(19):1531–1535. doi: 10.1016/S0960-9822(01)00455-9. [DOI] [PubMed] [Google Scholar]
- 87.Tristem M. Identification and characterization of novel human endogenous retrovirus families by phylogenetic screening of the human genome mapping project database. J Virol. 2000;74(8):3715–3730. doi: 10.1128/JVI.74.8.3715-3730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lower R, Lower J, Kurth R. The viruses in all of us: characteristics and biological significance of human endogenous retrovirus sequences. Proc Natl Acad Sci USA. 1996;93(11):5177–5184. doi: 10.1073/pnas.93.11.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dewannieux M, Blaise S, Heidmann T. Identification of a functional envelope protein from the HERV-K family of human endogenous retroviruses. J Virol. 2005;79(24):15573–15577. doi: 10.1128/JVI.79.24.15573-15577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frank O, Giehl M, Zheng C, Hehlmann R, Leib-Mosch C, Seifarth W. Human endogenous retrovirus expression profiles in samples from brains of patients with schizophrenia and bipolar disorders. J Virol. 2005;79(17):10890–10901. doi: 10.1128/JVI.79.17.10890-10901.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Macfarlane C, Simmonds P. Allelic variation of HERV-K(HML-2) endogenous retroviral elements in human populations. J Mol Evol. 2004;59(5):642–656. doi: 10.1007/s00239-004-2656-1. [DOI] [PubMed] [Google Scholar]
- 92.Mamedov I, Lebedev Y, Hunsmann G, Khusnutdinova E, Sverdlov E. A rare event of insertion polymorphism of a HERV-K LTR in the human genome. Genomics. 2004;84(3):596–599. doi: 10.1016/j.ygeno.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 93.Marchi E, Kanapin A, Magiorkinis G, Belshaw R. Unfixed endogenous retroviral insertions in the human population. J Virol. 2014;88(17):9529–9537. doi: 10.1128/JVI.00919-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kahyo T, Tao H, Shinmura K, Yamada H, Mori H, Funai K, Kurabe N, Suzuki M, Tanahashi M, Niwa H, Ogawa H, Tanioka F, Yin G, Morita M, Matsuo K, Kono S, Sugimura H. Identification and association study with lung cancer for novel insertion polymorphisms of human endogenous retrovirus. Carcinogenesis. 2013;34(11):2531–2538. doi: 10.1093/carcin/bgt253. [DOI] [PubMed] [Google Scholar]
- 95.Rakoff-Nahoum S, Kuebler PJ, Heymann JJ, E Sheehy M, Ortiz GM, S Ogg G, Barbour JD, Lenz J, Steinfeld AD, Nixon DF. Detection of T lymphocytes specific for human endogenous retrovirus K (HERV-K) in patients with seminoma. AIDS Res Hum Retroviruses. 2006;22(1):52–56. doi: 10.1089/aid.2006.22.52. [DOI] [PubMed] [Google Scholar]
- 96.Denner J. Xenotransplantation and hepatitis E virus. Xenotransplantation. 2015 doi: 10.1111/xen.12156. [DOI] [PubMed] [Google Scholar]
- 97.Fiebig U, Keller M, Moller A, Timms P, Denner J. Lack of antiviral antibody response in koalas infected with koala retroviruses (KoRV) Virus Res. 2015;198:30–34. doi: 10.1016/j.virusres.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Kassiotis G. Endogenous retroviruses and the development of cancer. J Immunol. 2014;192(4):1343–1349. doi: 10.4049/jimmunol.1302972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Contreras-Galindo R, Kaplan MH, He S, Contreras-Galindo AC, Gonzalez-Hernandez MJ, Kappes F, Dube D, Chan SM, Robinson D, Meng F, Dai M, Gitlin SD, Chinnaiyan AM, Omenn GS, Markovitz DM. HIV infection reveals widespread expansion of novel centromeric human endogenous retroviruses. Genome Res. 2013;23(9):1505–1513. doi: 10.1101/gr.144303.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Parseval N, Diop G, Blaise S, Helle F, Vasilescu A, Matsuda F, Heidmann T. Comprehensive search for intra- and inter-specific sequence polymorphisms among coding envelope genes of retroviral origin found in the human genome: genes and pseudogenes. BMC Genomics. 2005;6:117. doi: 10.1186/1471-2164-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brosius J. RNAs from all categories generate retrosequences that may be exapted as novel genes or regulatory elements. Gene. 1999;238(1):115–134. doi: 10.1016/S0378-1119(99)00227-9. [DOI] [PubMed] [Google Scholar]
- 102.Leib-Mosch C, Haltmeier M, Werner T, Geigl EM, Brack-Werner R, Francke U, Erfle V, Hehlmann R. Genomic distribution and transcription of solitary HERV-K LTRs. Genomics. 1993;18(2):261–269. doi: 10.1006/geno.1993.1464. [DOI] [PubMed] [Google Scholar]
- 103.Hohenadl C, Leib-Mosch C, Hehlmann R, Erfle V. Biological significance of human endogenous retroviral sequences. J Acqui Immune Defic Syndr Hum Retrovirol. 1996;13(Suppl 1):S268–S273. doi: 10.1097/00042560-199600001-00040. [DOI] [PubMed] [Google Scholar]
- 104.Perot P, Cheynet V, Decaussin-Petrucci M, Oriol G, Mugnier N, Rodriguez-Lafrasse C, Ruffion A, Mallet F. Microarray-based identification of individual HERV loci expression: application to biomarker discovery in prostate cancer. J Vis Exp. 2013;81:e50713. doi: 10.3791/50713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jacques PE, Jeyakani J, Bourque G. The majority of primate-specific regulatory sequences are derived from transposable elements. PLoS Genet. 2013;9(5):e1003504. doi: 10.1371/journal.pgen.1003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ho B, Baker PM, Singh S, Shih SJ, Vaughan AT. Localized DNA cleavage secondary to genotoxic exposure adjacent to an Alu inverted repeat. Genes Chromosom Cancer. 2012;51(5):501–509. doi: 10.1002/gcc.21938. [DOI] [PubMed] [Google Scholar]
- 107.Garazha A, Ivanova A, Suntsova M, Malakhova G, Roumiantsev S, Zhavoronkov A, Buzdin A. New bioinformatic tool for quick identification of functionally relevant endogenous retroviral inserts in human genome. Cell Cycle. 2015;14:1476–1484. doi: 10.1080/15384101.2015.1022696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meisler MH, Ting CN. The remarkable evolutionary history of the human amylase genes. Crit Rev Oral Biol Med. 1993;4(3–4):503–509. doi: 10.1177/10454411930040033501. [DOI] [PubMed] [Google Scholar]
- 109.Conley AB, Piriyapongsa J, Jordan IK. Retroviral promoters in the human genome. Bioinformatics. 2008;24(14):1563–1567. doi: 10.1093/bioinformatics/btn243. [DOI] [PubMed] [Google Scholar]
- 110.van de Lagemaat LN, Medstrand P, Mager DL. Multiple effects govern endogenous retrovirus survival patterns in human gene introns. Genome Biol. 2006;7(9):R86. doi: 10.1186/gb-2006-7-9-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cutter AD, Good JM, Pappas CT, Saunders MA, Starrett DM, Wheeler TJ. Transposable element orientation bias in the Drosophila melanogaster genome. J Mol Evol. 2005;61(6):733–741. doi: 10.1007/s00239-004-0243-0. [DOI] [PubMed] [Google Scholar]
- 112.Baust C, Seifarth W, Germaier H, Hehlmann R, Leib-Mosch C. HERV-K-T47D-Related long terminal repeats mediate polyadenylation of cellular transcripts. Genomics. 2000;66(1):98–103. doi: 10.1006/geno.2000.6175. [DOI] [PubMed] [Google Scholar]
- 113.Kuryshev VY, Vorobyov E, Zink D, Schmitz J, Rozhdestvensky TS, Munstermann E, Ernst U, Wellenreuther R, Moosmayer P, Bechtel S, Schupp I, Horst J, Korn B, Poustka A, Wiemann S. An anthropoid-specific segmental duplication on human chromosome 1q22. Genomics. 2006;88(2):143–151. doi: 10.1016/j.ygeno.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 114.Kjellman C, Sjogren HO, Salford LG, Widegren B. HERV-F (XA34) is a full-length human endogenous retrovirus expressed in placental and fetal tissues. Gene. 1999;239(1):99–107. doi: 10.1016/S0378-1119(99)00372-8. [DOI] [PubMed] [Google Scholar]
- 115.Mager DL, Hunter DG, Schertzer M, Freeman JD. Endogenous retroviruses provide the primary polyadenylation signal for two new human genes (HHLA2 and HHLA3) Genomics. 1999;59(3):255–263. doi: 10.1006/geno.1999.5877. [DOI] [PubMed] [Google Scholar]
- 116.Maeda N. Nucleotide sequence of the haptoglobin and haptoglobin-related gene pair. The haptoglobin-related gene contains a retrovirus-like element. J Biol Chem. 1985;260(11):6698–6709. [PubMed] [Google Scholar]
- 117.Hatada S, Grant DJ, Maeda N. An intronic endogenous retrovirus-like sequence attenuates human haptoglobin-related gene expression in an orientation-dependent manner. Gene. 2003;319:55–63. doi: 10.1016/S0378-1119(03)00791-1. [DOI] [PubMed] [Google Scholar]
- 118.Maeda N, Kim HS. Three independent insertions of retrovirus-like sequences in the haptoglobin gene cluster of primates. Genomics. 1990;8(4):671–683. doi: 10.1016/0888-7543(90)90254-R. [DOI] [PubMed] [Google Scholar]
- 119.Turelli P, Castro-Diaz N, Marzetta F, Kapopoulou A, Raclot C, Duc J, Tieng V, Quenneville S, Trono D. Interplay of TRIM28 and DNA methylation in controlling human endogenous retroelements. Genome Res. 2014;24(8):1260–1270. doi: 10.1101/gr.172833.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thomas JH, Schneider S. Coevolution of retroelements and tandem zinc finger genes. Genome Res. 2011;21(11):1800–1812. doi: 10.1101/gr.121749.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schlesinger S, Lee AH, Wang GZ, Green L, Goff SP. Proviral silencing in embryonic cells is regulated by Yin Yang 1. Cell Rep. 2013;4(1):50–58. doi: 10.1016/j.celrep.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bae EH, Jung YT. Comparison of the effects of retroviral restriction factors involved in resistance to porcine endogenous retrovirus. J Microbiol Biotechnol. 2014;24(4):577–583. doi: 10.4014/jmb.1312.12079. [DOI] [PubMed] [Google Scholar]
- 123.Nexo BA, Hansen B, Nissen KK, Gundestrup L, Terkelsen T, Villesen P, Bahrami S, Petersen T, Pedersen FS, Laska MJ. Restriction genes for retroviruses influence the risk of multiple sclerosis. PLoS ONE. 2013;8(9):e74063. doi: 10.1371/journal.pone.0074063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu P, Lubben W, Slomka H, Gebler J, Konert M, Cai C, Neubrandt L, Prazeres da Costa O, Paul S, Dehnert S, Dohne K, Thanisch M, Storsberg S, Wiegand L, Kaufmann A, Nain M, Quintanilla-Martinez L, Bettio S, Schnierle B, Kolesnikova L, Becker S, Schnare M, Bauer S. Nucleic acid-sensing Toll-like receptors are essential for the control of endogenous retrovirus viremia and ERV-induced tumors. Immunity. 2012;37(5):867–879. doi: 10.1016/j.immuni.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 125.Liberatore RA, Bieniasz PD. Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc Natl Acad Sci USA. 2011;108(44):18097–18101. doi: 10.1073/pnas.1113694108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lemaitre C, Harper F, Pierron G, Heidmann T, Dewannieux M. The HERV-K human endogenous retrovirus envelope protein antagonizes Tetherin antiviral activity. J Virol. 2014;88(23):13626–13637. doi: 10.1128/JVI.02234-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goila-Gaur R, Strebel K. HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology. 2008;5:51. doi: 10.1186/1742-4690-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Holmes RK, Malim MH, Bishop KN. APOBEC-mediated viral restriction: not simply editing? Trends Biochem Sci. 2007;32(3):118–128. doi: 10.1016/j.tibs.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 129.Santoni FA, Guerra J, Luban J. HERV-H RNA is abundant in human embryonic stem cells and a precise marker for pluripotency. Retrovirology. 2012;9:111. doi: 10.1186/1742-4690-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ohnuki M, Tanabe K, Sutou K, Teramoto I, Sawamura Y, Narita M, Nakamura M, Tokunaga Y, Nakamura M, Watanabe A, Yamanaka S, Takahashi K. Dynamic regulation of human endogenous retroviruses mediates factor-induced reprogramming and differentiation potential. Proc Natl Acad Sci USA. 2014;111(34):12426–12431. doi: 10.1073/pnas.1413299111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu X, Sachs F, Ramsay L, Jacques PE, Goke J, Bourque G, Ng HH. The retrovirus HERVH is a long noncoding RNA required for human embryonic stem cell identity. Nat Struct Mol Biol. 2014;21(4):423–425. doi: 10.1038/nsmb.2799. [DOI] [PubMed] [Google Scholar]
- 132.Andersson AC, Venables PJ, Tonjes RR, Scherer J, Eriksson L, Larsson E. Developmental expression of HERV-R (ERV3) and HERV-K in human tissue. Virology. 2002;297(2):220–225. doi: 10.1006/viro.2002.1428. [DOI] [PubMed] [Google Scholar]
- 133.Frendo JL, Olivier D, Cheynet V, Blond JL, Bouton O, Vidaud M, Rabreau M, Evain-Brion D, Mallet F. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol Cell Biol. 2003;23(10):3566–3574. doi: 10.1128/MCB.23.10.3566-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dunk CE, Gellhaus A, Drewlo S, Baczyk D, Potgens AJ, Winterhager E, Kingdom JC, Lye SJ. The molecular role of connexin 43 in human trophoblast cell fusion. Biol Reprod. 2012;86(4):115. doi: 10.1095/biolreprod.111.096925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ruebner M, Strissel PL, Ekici AB, Stiegler E, Dammer U, Goecke TW, Faschingbauer F, Fahlbusch FB, Beckmann MW, Strick R. Reduced syncytin-1 expression levels in placental syndromes correlates with epigenetic hypermethylation of the ERVW-1 promoter region. PLoS ONE. 2013;8(2):e56145. doi: 10.1371/journal.pone.0056145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seabrook JL, Cantlon JD, Cooney AJ, McWhorter EE, Fromme BA, Bouma GJ, Anthony RV, Winger QA. Role of LIN28A in mouse and human trophoblast cell differentiation. Biol Reprod. 2013;89(4):95. doi: 10.1095/biolreprod.113.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rote NS, Chakrabarti S, Stetzer BP. The role of human endogenous retroviruses in trophoblast differentiation and placental development. Placenta. 2004;25(8–9):673–683. doi: 10.1016/j.placenta.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 138.Lokossou AG, Toudic C, Barbeau B. Implication of human endogenous retrovirus envelope proteins in placental functions. Viruses. 2014;6(11):4609–4627. doi: 10.3390/v6114609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Esnault C, Cornelis G, Heidmann O, Heidmann T. Differential evolutionary fate of an ancestral primate endogenous retrovirus envelope gene, the EnvV syncytin, captured for a function in placentation. PLoS Genet. 2013;9(3):e1003400. doi: 10.1371/journal.pgen.1003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pi W, Zhu X, Wu M, Wang Y, Fulzele S, Eroglu A, Ling J, Tuan D. Long-range function of an intergenic retrotransposon. Proc Natl Acad Sci USA. 2010;107(29):12992–12997. doi: 10.1073/pnas.1004139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Long Q, Bengra C, Li C, Kutlar F, Tuan D. A long terminal repeat of the human endogenous retrovirus ERV-9 is located in the 5′ boundary area of the human beta-globin locus control region. Genomics. 1998;54(3):542–555. doi: 10.1006/geno.1998.5608. [DOI] [PubMed] [Google Scholar]
- 142.Ling J, Pi W, Bollag R, Zeng S, Keskintepe M, Saliman H, Krantz S, Whitney B, Tuan D. The solitary long terminal repeats of ERV-9 endogenous retrovirus are conserved during primate evolution and possess enhancer activities in embryonic and hematopoietic cells. J Virol. 2002;76(5):2410–2423. doi: 10.1128/jvi.76.5.2410-2423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dunn CA, van de Lagemaat LN, Baillie GJ, Mager DL. Endogenous retrovirus long terminal repeats as ready-to-use mobile promoters: the case of primate beta3GAL-T5. Gene. 2005;364:2–12. doi: 10.1016/j.gene.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 144.Romanish MT, Lock WM, van de Lagemaat LN, Dunn CA, Mager DL. Repeated recruitment of LTR retrotransposons as promoters by the anti-apoptotic locus NAIP during mammalian evolution. PLoS Genet. 2007;3(1):e10. doi: 10.1371/journal.pgen.0030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Landry JR, Rouhi A, Medstrand P, Mager DL. The Opitz syndrome gene Mid1 is transcribed from a human endogenous retroviral promoter. Mol Biol Evol. 2002;19(11):1934–1942. doi: 10.1093/oxfordjournals.molbev.a004017. [DOI] [PubMed] [Google Scholar]
- 146.Medstrand P, Landry JR, Mager DL. Long terminal repeats are used as alternative promoters for the endothelin B receptor and apolipoprotein C-I genes in humans. J Biol Chem. 2001;276(3):1896–1903. doi: 10.1074/jbc.M006557200. [DOI] [PubMed] [Google Scholar]
- 147.Bieche I, Laurent A, Laurendeau I, Duret L, Giovangrandi Y, Frendo JL, Olivi M, Fausser JL, Evain-Brion D, Vidaud M. Placenta-specific INSL4 expression is mediated by a human endogenous retrovirus element. Biol Reprod. 2003;68(4):1422–1429. doi: 10.1095/biolreprod.102.010322. [DOI] [PubMed] [Google Scholar]
- 148.Carlton VE, Harris BZ, Puffenberger EG, Batta AK, Knisely AS, Robinson DL, Strauss KA, Shneider BL, Lim WA, Salen G, Morton DH, Bull LN. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet. 2003;34(1):91–96. doi: 10.1038/ng1147. [DOI] [PubMed] [Google Scholar]
- 149.Mason MJ, Speake C, Gersuk VH, Nguyen QA, O’Brien KK, Odegard JM, Buckner JH, Greenbaum CJ, Chaussabel D, Nepom GT. Low HERV-K(C4) copy number is associated with type 1 diabetes. Diabetes. 2014;63(5):1789–1795. doi: 10.2337/db13-1382. [DOI] [PubMed] [Google Scholar]
- 150.Young GR, Stoye JP, Kassiotis G. Are human endogenous retroviruses pathogenic? An approach to testing the hypothesis. BioEssays. 2013;35(9):794–803. doi: 10.1002/bies.201300049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tugnet N, Rylance P, Roden D, Trela M, Nelson P. Human endogenous retroviruses (HERVs) and autoimmune rheumatic disease: is there a link? Open Rheumatol J. 2013;7:13–21. doi: 10.2174/1874312901307010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cusick MF, Libbey JE, Fujinami RS. Multiple sclerosis: autoimmunity and viruses. Curr Opin Rheumatol. 2013;25(4):496–501. doi: 10.1097/BOR.0b013e328362004d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ehlhardt S, Seifert M, Schneider J, Ojak A, Zang KD, Mehraein Y. Human endogenous retrovirus HERV-K(HML-2) Rec expression and transcriptional activities in normal and rheumatoid arthritis synovia. J Rheumatol. 2006;33(1):16–23. [PubMed] [Google Scholar]
- 154.Wu Z, Mei X, Zhao D, Sun Y, Song J, Pan W, Shi W. DNA methylation modulates HERV-E expression in CD4+ T cells from systemic lupus erythematosus patients. J Dermatol Sci. 2015;77(2):110–116. doi: 10.1016/j.jdermsci.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 155.Nelson P, Rylance P, Roden D, Trela M, Tugnet N. Viruses as potential pathogenic agents in systemic lupus erythematosus. Lupus. 2014;23(6):596–605. doi: 10.1177/0961203314531637. [DOI] [PubMed] [Google Scholar]
- 156.Nakkuntod J, Sukkapan P, Avihingsanon Y, Mutirangura A, Hirankarn N. DNA methylation of human endogenous retrovirus in systemic lupus erythematosus. J Hum Genet. 2013;58(5):241–249. doi: 10.1038/jhg.2013.6. [DOI] [PubMed] [Google Scholar]
- 157.Shi L, Zhang Z, Yu AM, Wang W, Wei Z, Akhter E, Maurer K, Costa Reis P, Song L, Petri M, Sullivan KE. The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLoS ONE. 2014;9(5):e93846. doi: 10.1371/journal.pone.0093846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nelson PN, Roden D, Nevill A, Freimanis GL, Trela M, Ejtehadi HD, Bowman S, Axford J, Veitch AM, Tugnet N, Rylance PB. Rheumatoid arthritis is associated with IgG antibodies to human endogenous retrovirus gag matrix: a potential pathogenic mechanism of disease? J Rheumatol. 2014;41(10):1952–1960. doi: 10.3899/jrheum.130502. [DOI] [PubMed] [Google Scholar]
- 159.Bendiksen S, Martinez-Zubiavrra I, Tummler C, Knutsen G, Elvenes J, Olsen E, Olsen R, Moens U. Human endogenous retrovirus W activity in cartilage of osteoarthritis patients. Biomed Res Int. 2014;2014:698609. doi: 10.1155/2014/698609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Garcia-Montojo M, Varade J, Villafuertes E, de La Hera B, Hoyas-Fernandez J, Dominguez-Mozo MI, Rodriguez-Rodriguez L, Tornero-Esteban P, Arias-Leal A, Leon L, Lamas JR, Alvarez-Lafuente R, Urcelay E, Fernandez-Gutierrez B. Expression of human endogenous retrovirus HERV-K18 is associated with clinical severity in osteoarthritis patients. Scand J Rheumatol. 2013;42(6):498–504. doi: 10.3109/03009742.2013.779021. [DOI] [PubMed] [Google Scholar]
- 161.Manghera M, Douville RN. Endogenous retrovirus-K promoter: a landing strip for inflammatory transcription factors? Retrovirology. 2013;10:16. doi: 10.1186/1742-4690-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.de Sousa Nogueira MA, Biancardi Gavioli CF, Pereira NZ, de Carvalho GC, Domingues R, Aoki V, Sato MN. Human endogenous retrovirus expression is inversely related with the up-regulation of interferon-inducible genes in the skin of patients with lichen planus. Arch Dermatol Res. 2015;307(3):259–264. doi: 10.1007/s00403-014-1524-0. [DOI] [PubMed] [Google Scholar]
- 163.Gupta R, Michaud HA, Zeng X, Debbaneh M, Arron ST, Jones RB, Ormsby CE, Nixon DF, Liao W. Diminished humoral responses against and reduced gene expression levels of human endogenous retrovirus-K (HERV-K) in psoriasis. J Transl Med. 2014;12:256. doi: 10.1186/s12967-014-0256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fowler BJ, Gelfand BD, Kim Y, Kerur N, Tarallo V, Hirano Y, Amarnath S, Fowler DH, Radwan M, Young MT, Pittman K, Kubes P, Agarwal HK, Parang K, Hinton DR, Bastos-Carvalho A, Li S, Yasuma T, Mizutani T, Yasuma R, Wright C, Ambati J. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science. 2014;346(6212):1000–1003. doi: 10.1126/science.1261754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Manghera M, Ferguson J, Douville R. ERVK polyprotein processing and reverse transcriptase expression in human cell line models of neurological disease. Viruses. 2015;7(1):320–332. doi: 10.3390/v7010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Christensen T. HERVs in neuropathogenesis. J Neuroimmune Pharmacol. 2010;5(3):326–335. doi: 10.1007/s11481-010-9214-y. [DOI] [PubMed] [Google Scholar]
- 167.Alfahad T, Nath A. Retroviruses and amyotrophic lateral sclerosis. Antiviral Res. 2013;99(2):180–187. doi: 10.1016/j.antiviral.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Libbey JE, Cusick MF, Fujinami RS. Role of pathogens in multiple sclerosis. Int Rev Immunol. 2014;33(4):266–283. doi: 10.3109/08830185.2013.823422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Emmer A, Staege MS, Kornhuber ME. The retrovirus/superantigen hypothesis of multiple sclerosis. Cell Mol Neurobiol. 2014;34(8):1087–1096. doi: 10.1007/s10571-014-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.de la Hera B, Varade J, Garcia-Montojo M, Alcina A, Fedetz M, Alloza I, Astobiza I, Leyva L, Fernandez O, Izquierdo G, Antiguedad A, Arroyo R, Alvarez-Lafuente R, Vandenbroeck K, Matesanz F, Urcelay E. Human endogenous retrovirus HERV-Fc1 association with multiple sclerosis susceptibility: a meta-analysis. PLoS ONE. 2014;9(3):e90182. doi: 10.1371/journal.pone.0090182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.de la Hera B, Varade J, Garcia-Montojo M, Lamas JR, de la Encarnacion A, Arroyo R, Fernandez-Gutierrez B, Alvarez-Lafuente R, Urcelay E. Role of the human endogenous retrovirus HERV-K18 in autoimmune disease susceptibility: study in the Spanish population and meta-analysis. PLoS ONE. 2013;8(4):e62090. doi: 10.1371/journal.pone.0062090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Garcia-Montojo M, de la Hera B, Varade J, de la Encarnacion A, Camacho I, Dominguez-Mozo M, Arias-Leal A, Garcia-Martinez A, Casanova I, Izquierdo G, Lucas M, Fedetz M, Alcina A, Arroyo R, Matesanz F, Urcelay E, Alvarez-Lafuente R. HERV-W polymorphism in chromosome X is associated with multiple sclerosis risk and with differential expression of MSRV. Retrovirology. 2014;11:2. doi: 10.1186/1742-4690-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Perron H, Dougier-Reynaud HL, Lomparski C, Popa I, Firouzi R, Bertrand JB, Marusic S, Portoukalian J, Jouvin-Marche E, Villiers CL, Touraine JL, Marche PN. Human endogenous retrovirus protein activates innate immunity and promotes experimental allergic encephalomyelitis in mice. PLoS ONE. 2013;8(12):e80128. doi: 10.1371/journal.pone.0080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mameli G, Madeddu G, Mei A, Uleri E, Poddighe L, Delogu LG, Maida I, Babudieri S, Serra C, Manetti R, Mura MS, Dolei A. Activation of MSRV-type endogenous retroviruses during infectious mononucleosis and Epstein-Barr virus latency: the missing link with multiple sclerosis? PLoS ONE. 2013;8(11):e78474. doi: 10.1371/journal.pone.0078474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Arru G, Leoni S, Pugliatti M, Mei A, Serra C, Delogu LG, Manetti R, Dolei A, Sotgiu S, Mameli G. Natalizumab inhibits the expression of human endogenous retroviruses of the W family in multiple sclerosis patients: a longitudinal cohort study. Mult Scler. 2014;20(2):174–182. doi: 10.1177/1352458513494957. [DOI] [PubMed] [Google Scholar]
- 176.Diem O, Schaffner M, Seifarth W, Leib-Mosch C. Influence of antipsychotic drugs on human endogenous retrovirus (HERV) transcription in brain cells. PLoS ONE. 2012;7(1):e30054. doi: 10.1371/journal.pone.0030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu C, Chen Y, Li S, Yu H, Zeng J, Wang X, Zhu F. Activation of elements in HERV-W family by caffeine and aspirin. Virus Genes. 2013;47(2):219–227. doi: 10.1007/s11262-013-0939-6. [DOI] [PubMed] [Google Scholar]
- 178.Nissen KK, Laska MJ, Hansen B, Terkelsen T, Villesen P, Bahrami S, Petersen T, Pedersen FS, Nexo BA. Endogenous retroviruses and multiple sclerosis-new pieces to the puzzle. BMC Neurol. 2013;13:111. doi: 10.1186/1471-2377-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hon GM, Erasmus RT, Matsha T. Multiple sclerosis-associated retrovirus and related human endogenous retrovirus-W in patients with multiple sclerosis: a literature review. J Neuroimmunol. 2013;263(1–2):8–12. doi: 10.1016/j.jneuroim.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 180.Gold J, Goldacre R, Maruszak H, Giovannoni G, Yeates D, Goldacre M. HIV and lower risk of multiple sclerosis: beginning to unravel a mystery using a record-linked database study. J Neurol Neurosurg Psychiatry. 2015;86(1):9–12. doi: 10.1136/jnnp-2014-307932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li S, Liu ZC, Yin SJ, Chen YT, Yu HL, Zeng J, Zhang Q, Zhu F. Human endogenous retrovirus W family envelope gene activates the small conductance Ca2+-activated K+ channel in human neuroblastoma cells through CREB. Neuroscience. 2013;247:164–174. doi: 10.1016/j.neuroscience.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 182.Perron H, Hamdani N, Faucard R, Lajnef M, Jamain S, Daban-Huard C, Sarrazin S, LeGuen E, Houenou J, Delavest M, Moins-Teisserenc H, Bengoufa D, Yolken R, Madeira A, Garcia-Montojo M, Gehin N, Burgelin I, Ollagnier G, Bernard C, Dumaine A, Henrion A, Gombert A, Le Dudal K, Charron D, Krishnamoorthy R, Tamouza R, Leboyer M. Molecular characteristics of human endogenous retrovirus type-W in schizophrenia and bipolar disorder. Transl Psychiatry. 2012;2:e201. doi: 10.1038/tp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nyegaard M, Demontis D, Thestrup BB, Hedemand A, Sorensen KM, Hansen T, Werge T, Hougaard DM, Yolken RH, Mortensen PB, Mors O, Borglum AD. No association of polymorphisms in human endogenous retrovirus K18 and CD48 with schizophrenia. Psychiatr Genet. 2012;22(3):146–148. doi: 10.1097/YPG.0b013e328353953c. [DOI] [PubMed] [Google Scholar]
- 184.Shuvarikov A, Campbell IM, Dittwald P, Neill NJ, Bialer MG, Moore C, Wheeler PG, Wallace SE, Hannibal MC, Murray MF, Giovanni MA, Terespolsky D, Sodhi S, Cassina M, Viskochil D, Moghaddam B, Herman K, Brown CW, Beck CR, Gambin A, Cheung SW, Patel A, Lamb AN, Shaffer LG, Ellison JW, Ravnan JB, Stankiewicz P, Rosenfeld JA. Recurrent HERV-H-mediated 3q13.2-q13.31 deletions cause a syndrome of hypotonia and motor, language, and cognitive delays. Hum Mutat. 2013;34(10):1415–1423. doi: 10.1002/humu.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Contreras-Galindo R, Lopez P, Velez R, Yamamura Y. HIV-1 infection increases the expression of human endogenous retroviruses type K (HERV-K) in vitro. AIDS Res Hum Retroviruses. 2007;23(1):116–122. doi: 10.1089/aid.2006.0117. [DOI] [PubMed] [Google Scholar]
- 186.Contreras-Galindo R, Gonzalez M, Almodovar-Camacho S, Gonzalez-Ramirez S, Lorenzo E, Yamamura Y. A new real-time-RT-PCR for quantitation of human endogenous retroviruses type K (HERV-K) RNA load in plasma samples: increased HERV-K RNA titers in HIV-1 patients with HAART non-suppressive regimens. J Virol Methods. 2006;136(1–2):51–57. doi: 10.1016/j.jviromet.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 187.Bhardwaj N, Maldarelli F, Mellors J, Coffin JM. HIV-1 infection leads to increased transcription of human endogenous retrovirus HERV-K (HML-2) proviruses in vivo but not to increased virion production. J Virol. 2014;88(19):11108–11120. doi: 10.1128/JVI.01623-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li L, Deng X, Linsuwanon P, Bangsberg D, Bwana MB, Hunt P, Martin JN, Deeks SG, Delwart E. AIDS alters the commensal plasma virome. J Virol. 2013;87(19):10912–10915. doi: 10.1128/JVI.01839-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Esqueda D, Xu F, Moore Y, Yang Z, Huang G, Lennon PA, Hu PC, Dong J. Lack of correlation between HERV-K expression and HIV-1 viral load in plasma specimens. Ann Clin Lab Sci. 2013;43(2):122–125. [PubMed] [Google Scholar]
- 190.Michaud HA, de Mulder M, SenGupta D, Deeks SG, Martin JN, Pilcher CD, Hecht FM, Sacha JB, Nixon DF. Trans-activation, post-transcriptional maturation, and induction of antibodies to HERV-K (HML-2) envelope transmembrane protein in HIV-1 infection. Retrovirology. 2014;11:10. doi: 10.1186/1742-4690-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jones RB, Garrison KE, Mujib S, Mihajlovic V, Aidarus N, Hunter DV, Martin E, John VM, Zhan W, Faruk NF, Gyenes G, Sheppard NC, Priumboom-Brees IM, Goodwin DA, Chen L, Rieger M, Muscat-King S, Loudon PT, Stanley C, Holditch SJ, Wong JC, Clayton K, Duan E, Song H, Xu Y, SenGupta D, Tandon R, Sacha JB, Brockman MA, Benko E, Kovacs C, Nixon DF, Ostrowski MA. HERV-K-specific T cells eliminate diverse HIV-1/2 and SIV primary isolates. J Clin Investig. 2012;122(12):4473–4489. doi: 10.1172/JCI64560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jones RB, Song H, Xu Y, Garrison KE, Buzdin AA, Anwar N, Hunter DV, Mujib S, Mihajlovic V, Martin E, Lee E, Kuciak M, Raposo RA, Bozorgzad A, Meiklejohn DA, Ndhlovu LC, Nixon DF, Ostrowski MA. LINE-1 retrotransposable element DNA accumulates in HIV-1-infected cells. J Virol. 2013;87(24):13307–13320. doi: 10.1128/JVI.02257-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gonzalez-Hernandez MJ, Cavalcoli JD, Sartor MA, Contreras-Galindo R, Meng F, Dai M, Dube D, Saha AK, Gitlin SD, Omenn GS, Kaplan MH, Markovitz DM. Regulation of the human endogenous retrovirus K (HML-2) transcriptome by the HIV-1 Tat protein. J Virol. 2014;88(16):8924–8935. doi: 10.1128/JVI.00556-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Uleri E, Mei A, Mameli G, Poddighe L, Serra C, Dolei A. HIV Tat acts on endogenous retroviruses of the W family and this occurs via Toll-like receptor 4: inference for neuroAIDS. AIDS. 2014;28(18):2659–2670. doi: 10.1097/QAD.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 195.Spencer TE, Mura M, Gray CA, Griebel PJ, Palmarini M. Receptor usage and fetal expression of ovine endogenous betaretroviruses: implications for coevolution of endogenous and exogenous retroviruses. J Virol. 2003;77(1):749–753. doi: 10.1128/JVI.77.1.749-753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ponferrada VG, Mauck BS, Wooley DP. The envelope glycoprotein of human endogenous retrovirus HERV-W induces cellular resistance to spleen necrosis virus. Arch Virol. 2003;148(4):659–675. doi: 10.1007/s00705-002-0960-x. [DOI] [PubMed] [Google Scholar]
- 197.Best S, Le Tissier PR, Stoye JP. Endogenous retroviruses and the evolution of resistance to retroviral infection. Trends Microbiol. 1997;5(8):313–318. doi: 10.1016/S0966-842X(97)01086-X. [DOI] [PubMed] [Google Scholar]
- 198.Fujino K, Horie M, Honda T, Merriman DK, Tomonaga K. Inhibition of Borna disease virus replication by an endogenous bornavirus-like element in the ground squirrel genome. Proc Natl Acad Sci USA. 2014;111(36):13175–13180. doi: 10.1073/pnas.1407046111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Malfavon-Borja R, Feschotte C. Fighting fire with fire: endogenous retrovirus envelopes as restriction factors. J Virol. 2015 doi: 10.1128/JVI.03653-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Crosslin DR, Carrell DS, Burt A, Kim DS, Underwood JG, Hanna DS, Comstock BA, Baldwin E, de Andrade M, Kullo IJ, Tromp G, Kuivaniemi H, Borthwick KM, McCarty CA, Peissig PL, Doheny KF, Pugh E, Kho A, Pacheco J, Hayes MG, Ritchie MD, Verma SS, Armstrong G, Stallings S, Denny JC, Carroll RJ, Crawford DC, Crane PK, Mukherjee S, Bottinger E, Li R, Keating B, Mirel DB, Carlson CS, Harley JB, Larson EB, Jarvik GP. Genetic variation in the HLA region is associated with susceptibility to herpes zoster. Genes Immun. 2015;16(1):1–7. doi: 10.1038/gene.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee E, Iskow R, Yang L, Gokcumen O, Haseley P, Luquette LJ, 3rd, Lohr JG, Harris CC, Ding L, Wilson RK, Wheeler DA, Gibbs RA, Kucherlapati R, Lee C, Kharchenko PV, Park PJ, Cancer Genome Atlas Research Network Landscape of somatic retrotransposition in human cancers. Science. 2012;337(6097):967–971. doi: 10.1126/science.1222077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gualtieri A, Andreola F, Sciamanna I, Sinibaldi-Vallebona P, Serafino A, Spadafora C. Increased expression and copy number amplification of LINE-1 and SINE B1 retrotransposable elements in murine mammary carcinoma progression. Oncotarget. 2013;4(11):1882–1893. doi: 10.18632/oncotarget.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Oricchio E, Sciamanna I, Beraldi R, Tolstonog GV, Schumann GG, Spadafora C. Distinct roles for LINE-1 and HERV-K retroelements in cell proliferation, differentiation and tumor progression. Oncogene. 2007;26(29):4226–4233. doi: 10.1038/sj.onc.1210214. [DOI] [PubMed] [Google Scholar]
- 204.Muster T, Waltenberger A, Grassauer A, Hirschl S, Caucig P, Romirer I, Fodinger D, Seppele H, Schanab O, Magin-Lachmann C, Lower R, Jansen B, Pehamberger H, Wolff K. An endogenous retrovirus derived from human melanoma cells. Cancer Res. 2003;63(24):8735–8741. [PubMed] [Google Scholar]
- 205.Schmitt K, Reichrath J, Roesch A, Meese E, Mayer J. Transcriptional profiling of human endogenous retrovirus group HERV-K(HML-2) loci in melanoma. Genome Biol Evol. 2013;5(2):307–328. doi: 10.1093/gbe/evt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Katoh I, Mirova A, Kurata S, Murakami Y, Horikawa K, Nakakuki N, Sakai T, Hashimoto K, Maruyama A, Yonaga T, Fukunishi N, Moriishi K, Hirai H. Activation of the long terminal repeat of human endogenous retrovirus K by melanoma-specific transcription factor MITF-M. Neoplasia. 2011;13(11):1081–1092. doi: 10.1593/neo.11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang-Johanning F, Li M, Esteva FJ, Hess KR, Yin B, Rycaj K, Plummer JB, Garza JG, Ambs S, Johanning GL. Human endogenous retrovirus type K antibodies and mRNA as serum biomarkers of early-stage breast cancer. Int J Cancer. 2014;134(3):587–595. doi: 10.1002/ijc.28389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wallace TA, Downey RF, Seufert CJ, Schetter A, Dorsey TH, Johnson CA, Goldman R, Loffredo CA, Yan P, Sullivan FJ, Giles FJ, Wang-Johanning F, Ambs S, Glynn SA. Elevated HERV-K mRNA expression in PBMC is associated with a prostate cancer diagnosis particularly in older men and smokers. Carcinogenesis. 2014;35(9):2074–2083. doi: 10.1093/carcin/bgu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Reis BS, Jungbluth AA, Frosina D, Holz M, Ritter E, Nakayama E, Ishida T, Obata Y, Carver B, Scher H, Scardino PT, Slovin S, Subudhi SK, Reuter VE, Savage C, Allison JP, Melamed J, Jager E, Ritter G, Old LJ, Gnjatic S. Prostate cancer progression correlates with increased humoral immune response to a human endogenous retrovirus GAG protein. Clin Cancer Res. 2013;19(22):6112–6125. doi: 10.1158/1078-0432.CCR-12-3580. [DOI] [PubMed] [Google Scholar]
- 210.Takahashi Y, Harashima N, Kajigaya S, Yokoyama H, Cherkasova E, McCoy JP, Hanada K, Mena O, Kurlander R, Tawab A, Srinivasan R, Lundqvist A, Malinzak E, Geller N, Lerman MI, Childs RW. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J Clin Investig. 2008;118(3):1099–1109. doi: 10.1172/JCI34409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fischer S, Echeverria N, Moratorio G, Landoni AI, Dighiero G, Cristina J, Oppezzo P, Moreno P. Human endogenous retrovirus np9 gene is over expressed in chronic lymphocytic leukemia patients. Leuk Res Rep. 2014;3(2):70–72. doi: 10.1016/j.lrr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen T, Meng Z, Gan Y, Wang X, Xu F, Gu Y, Xu X, Tang J, Zhou H, Zhang X, Gan X, Van Ness C, Xu G, Huang L, Zhang X, Fang Y, Wu J, Zheng S, Jin J, Huang W, Xu R. The viral oncogene Np9 acts as a critical molecular switch for co-activating beta-catenin, ERK, Akt and Notch1 and promoting the growth of human leukemia stem/progenitor cells. Leukemia. 2013;27(7):1469–1478. doi: 10.1038/leu.2013.8. [DOI] [PubMed] [Google Scholar]
- 213.Lavialle C, Cornelis G, Dupressoir A, Esnault C, Heidmann O, Vernochet C, Heidmann T. Paleovirology of ‘syncytins’, retroviral env genes exapted for a role in placentation. Philos Trans R Soc Lond B Biol Sci. 2013;368(1626):20120507. doi: 10.1098/rstb.2012.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mangeney M, Heidmann T. Tumor cells expressing a retroviral envelope escape immune rejection in vivo. Proc Natl Acad Sci USA. 1998;95(25):14920–14925. doi: 10.1073/pnas.95.25.14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pothlichet J, Heidmann T, Mangeney M. A recombinant endogenous retrovirus amplified in a mouse neuroblastoma is involved in tumor growth in vivo. Int J Cancer. 2006;119(4):815–822. doi: 10.1002/ijc.21935. [DOI] [PubMed] [Google Scholar]
- 216.Mangeney M, Pothlichet J, Renard M, Ducos B, Heidmann T. Endogenous retrovirus expression is required for murine melanoma tumor growth in vivo. Cancer Res. 2005;65(7):2588–2591. doi: 10.1158/0008-5472.CAN-04-4231. [DOI] [PubMed] [Google Scholar]
- 217.Naito T, Ogasawara H, Kaneko H, Hishikawa T, Sekigawa I, Hashimoto H, Maruyama N. Immune abnormalities induced by human endogenous retroviral peptides: with reference to the pathogenesis of systemic lupus erythematosus. J Clin Immunol. 2003;23(5):371–376. doi: 10.1023/A:1025369500466. [DOI] [PubMed] [Google Scholar]
- 218.Mangeney M, de Parseval N, Thomas G, Heidmann T. The full-length envelope of an HERV-H human endogenous retrovirus has immunosuppressive properties. J Gen Virol. 2001;82(Pt 10):2515–2518. doi: 10.1099/0022-1317-82-10-2515. [DOI] [PubMed] [Google Scholar]
- 219.Rhyu DW, Kang YJ, Ock MS, Eo JW, Choi YH, Kim WJ, Leem SH, Yi JM, Kim HS, Cha HJ. Expression of human endogenous retrovirus env genes in the blood of breast cancer patients. Int J Mol Sci. 2014;15(6):9173–9183. doi: 10.3390/ijms15069173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Strissel PL, Ruebner M, Thiel F, Wachter D, Ekici AB, Wolf F, Thieme F, Ruprecht K, Beckmann MW, Strick R. Reactivation of codogenic endogenous retroviral (ERV) envelope genes in human endometrial carcinoma and prestages: emergence of new molecular targets. Oncotarget. 2012;3(10):1204–1219. doi: 10.18632/oncotarget.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lamprecht B, Walter K, Kreher S, Kumar R, Hummel M, Lenze D, Kochert K, Bouhlel MA, Richter J, Soler E, Stadhouders R, Johrens K, Wurster KD, Callen DF, Harte MF, Giefing M, Barlow R, Stein H, Anagnostopoulos I, Janz M, Cockerill PN, Siebert R, Dorken B, Bonifer C, Mathas S. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat Med. 2010;16(5):571–579. doi: 10.1038/nm.2129. [DOI] [PubMed] [Google Scholar]
- 222.Hayes M, Whitesell M, Brown MA. Pathological and evolutionary implications of retroviruses as mobile genetic elements. Genes. 2013;4(4):573–582. doi: 10.3390/genes4040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Subramanian RP, Wildschutte JH, Russo C, Coffin JM. Identification, characterization, and comparative genomic distribution of the HERV-K (HML-2) group of human endogenous retroviruses. Retrovirology. 2011;8:90. doi: 10.1186/1742-4690-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lee A, Huntley D, Aiewsakun P, Kanda RK, Lynn C, Tristem M. Novel Denisovan and Neanderthal retroviruses. J Virol. 2014;88(21):12907–12909. doi: 10.1128/JVI.01825-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Beaune J, Nony P, Chassignolle J, Loire R, Gros P, Delaye J. Aortic insufficiency caused by dystrophic aneurysm of the ascending aorta: study of development in 95 cases. Value of cutaneous biopsy in the etiologic diagnosis. Arch Mal Coeur Vaiss. 1989;82(8):1389–1396. [PubMed] [Google Scholar]
- 226.Anisimova M, Liberles DA. The quest for natural selection in the age of comparative genomics. Heredity. 2007;99(6):567–579. doi: 10.1038/sj.hdy.6801052. [DOI] [PubMed] [Google Scholar]
- 227.Kao TH, Liao HF, Wolf D, Tai KY, Chuang CY, Lee HS, Kuo HC, Hata K, Zhang X, Cheng X, Goff SP, Ooi SK, Bestor TH, Lin SP. Ectopic DNMT3L triggers assembly of a repressive complex for retroviral silencing in somatic cells. J Virol. 2014;88(18):10680–10695. doi: 10.1128/JVI.01176-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kuzmin D, Gogvadze E, Kholodenko R, Grzela DP, Mityaev M, Vinogradova T, Kopantzev E, Malakhova G, Suntsova M, Sokov D, Ivics Z, Buzdin A. Novel strong tissue specific promoter for gene expression in human germ cells. BMC Biotechnol. 2010;10:58. doi: 10.1186/1472-6750-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fuchs NV, Kraft M, Tondera C, Hanschmann KM, Lower J, Lower R. Expression of the human endogenous retrovirus (HERV) group HML-2/HERV-K does not depend on canonical promoter elements but is regulated by transcription factors Sp1 and Sp3. J Virol. 2011;85(7):3436–3448. doi: 10.1128/JVI.02539-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hughes JF, Coffin JM. Evidence for genomic rearrangements mediated by human endogenous retroviruses during primate evolution. Nat Genet. 2001;29(4):487–489. doi: 10.1038/ng775. [DOI] [PubMed] [Google Scholar]
- 231.Paces J, Pavlicek A, Zika R, Kapitonov VV, Jurka J, Paces V. HERVd: the human endogenous retroviruses database: update. Nucleic Acids Res. 2004;32(Database issue):D50. doi: 10.1093/nar/gkh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Villesen P, Aagaard L, Wiuf C, Pedersen FS. Identification of endogenous retroviral reading frames in the human genome. Retrovirology. 2004;1:32. doi: 10.1186/1742-4690-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang J, Song L, Grover D, Azrak S, Batzer MA, Liang P. dbRIP: a highly integrated database of retrotransposon insertion polymorphisms in humans. Hum Mutat. 2006;27(4):323–329. doi: 10.1002/humu.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mills RE, Bennett EA, Iskow RC, Luttig CT, Tsui C, Pittard WS, Devine SE. Recently mobilized transposons in the human and chimpanzee genomes. Am J Hum Genet. 2006;78(4):671–679. doi: 10.1086/501028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hernandez-Pinzon I, de Jesus E, Santiago N, Casacuberta JM. The frequent transcriptional readthrough of the tobacco Tnt1 retrotransposon and its possible implications for the control of resistance genes. J Mol Evol. 2009;68(3):269–278. doi: 10.1007/s00239-009-9204-y. [DOI] [PubMed] [Google Scholar]
- 236.Piriyapongsa J, Jordan IK. A family of human microRNA genes from miniature inverted-repeat transposable elements. PLoS ONE. 2007;2(2):e203. doi: 10.1371/journal.pone.0000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Levy A, Sela N, Ast G. TranspoGene and microTranspoGene: transposed elements influence on the transcriptome of seven vertebrates and invertebrates. Nucleic acids research. 2008;36(Database issue):D47–D52. doi: 10.1093/nar/gkm949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kim TH, Jeon YJ, Kim WY, Kim HS. HESAS: HERVs expression and structure analysis system. Bioinformatics. 2005;21(8):1699–1700. doi: 10.1093/bioinformatics/bti194. [DOI] [PubMed] [Google Scholar]
- 239.Sperber GO, Airola T, Jern P, Blomberg J. Automated recognition of retroviral sequences in genomic data—RetroTector. Nucleic Acids Res. 2007;35(15):4964–4976. doi: 10.1093/nar/gkm515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhavoronkov A, Cantor CR. Methods for structuring scientific knowledge from many areas related to aging research. PLoS ONE. 2011;6(7):e22597. doi: 10.1371/journal.pone.0022597. [DOI] [PMC free article] [PubMed] [Google Scholar]