Abstract
Structurally, protein kinase CK2 consists of two catalytic subunits (α and α′) and two regulatory subunits (β), which play a critical role in targeting specific CK2 substrates. Compelling evidence shows the complexity of the CK2 cellular signaling network and supports the view that this enzyme is a key component of regulatory protein kinase networks that are involved in several aspects of cancer. CK2 both activates and suppresses the expression of a number of essential oncogenes and tumor suppressors, and its expression and activity are upregulated in blood tumors and virtually all solid tumors. The prognostic significance of CK2α expression in association with various clinicopathological parameters highlighted this kinase as an adverse prognostic marker in breast cancer. In addition, several recent studies reported its implication in the regulation of the epithelial-to-mesenchymal transition (EMT), an early step in cancer invasion and metastasis. In this review, we briefly overview the contribution of CK2 to several aspects of cancer and discuss how in mammary epithelial cells, the expression of its CK2β regulatory subunit plays a critical role in maintaining an epithelial phenotype through CK2-mediated control of key EMT-related transcription factors. Importantly, decreased CK2β expression in breast tumors is correlated with inefficient phosphorylation and nuclear translocation of Snail1 and Foxc2, ultimately leading to EMT induction. This review highlights the pivotal role played by CK2β in the mammary epithelial phenotype and discusses how a modest alteration in its expression may be sufficient to induce dramatic effects facilitating the early steps in tumor cell dissemination through the coordinated regulation of two key transcription factors.
Keywords: Protein kinase CK2 catalytic subunit (CK2α), Protein kinase CK2 regulatory subunit (CK2β), Substrate specificity, Asymmetric subunit expression, Glycogen synthase kinase 3 beta (GSK3β), Epithelial-to-mesenchymal transition (EMT), Zinc finger protein Snail1, Forkhead box protein C2 (Foxc2), Homeobox protein SIX1, Hallmarks of cancer
Introduction
Protein kinase CK2 shares a quaternary structure composed of catalytic and regulatory subunits with few other protein kinases. Two catalytic subunits (α or α′) associate with a dimer of regulatory β subunits, which do not share homology with any other regulatory subunit of protein kinases [1, 2]. CK2 is a multifunctional, ubiquitously expressed, protein kinase with the unusual ability to phosphorylate serine, threonine, and tyrosine residues within clusters of acidic residues [3, 4]. It has been estimated that this kinase might be individually responsible for the generation of a substantial fraction of the eukaryotic phosphoproteome [5]. At the molecular level, CK2 participates in hierarchical phosphorylation signaling as either a priming or primed protein kinase for the phosphorylation of key protein substrates (reviewed in [6]). Although a few studies have shown that extracellular stimuli can modulate CK2 activity [7–10], most of the data suggest that this kinase is acting as a “lateral signaling player” on numerous signaling pathways that are critical for cell proliferation, differentiation, and apoptosis. This means that cellular responses such as proliferation, growth, and survival will be potentiated by CK2, while a death signal will be dampened. Following a brief overview of what is known about the contribution of CK2 to several aspects of transformation and cancer, we discuss the biological consequences of the multi-subunit structure of CK2 and consider the pivotal role of its regulatory CK2β subunit in targeting specific protein substrates. Building on this, we focus our discussions on recent evidence that shows the critical role played by CK2β and the dramatic consequences of its perturbation in the regulation of epithelial cell plasticity. Finally, we discuss how unbalanced expression of CK2 subunits in breast carcinoma could participate in the molecular circuits propagating the tumor phenotype.
Protein kinase CK2 and the “hallmarks of cancer”
Historically, a large number of growth-related proteins have been identified as CK2 substrates, suggesting their role in growth-related functions [11–16]. Accumulating observations also support the view that CK2 is a component of regulatory protein kinase networks that are involved in several aspects of transformation and cancer illustrating the extreme complexity of CK2 functions. These data have been widely reviewed in the literature [2, 17, 18]. Despite the demonstration in transgenic mouse models of a causative role of CK2α overexpression in hematopoietic and mammary oncogenesis [19–23], CK2 does not conform to the definition of oncogene since there is no evidence of point mutations in CK2 giving rise to tumors. However, a recent study revealed that mutated CK2α in association with 34 other mutated genes confers cancer resistance to immune attack [24]. Furthermore, other studies have also pointed out the frequent copy-number alteration of all CK2 genes in several malignancies [25]. Yet, there is a wealth of evidence that CK2 plays a major role in tumorigenesis, by enhancing the transforming potential of oncogenes and acting as an antiapoptotic molecule [14, 26–28]. Accordingly, elevated CK2 expression associated with high activity is a common denominator in the majority of cancers and is associated with aggressive tumor behavior [29–32]. The reliance of malignant cells on CK2 functions, which underlines a phenomenon defined as “non-oncogene addiction” [33], has been thoroughly reviewed by Ruzzene and Pinna [14]. How CK2 alterations contribute to cancer development is an important and challenging question. Although beyond the scope of the present article, it is noteworthy that a review of the literature discloses that CK2 participates to the regulation of many of the same cellular responses that characterize the “hallmarks of cancer” originally described by Hanahan and Weinberg [34, 35]. Indeed, CK2 both activates and alleviates the expression of a number of proteins essential for proliferation [9, 11, 13, 18, 28, 36–45]; evading growth suppressors [39, 41, 46–57]; avoiding immune destruction [58, 59]; enabling replicative immortality [8, 60–66], tumor-promoting inflammation [67–74], invasion, and metastasis [22, 23, 29, 31, 56, 66, 72, 75–95]; inducing angiogenesis [29, 96–106]; regulating genome instability and mutation [25, 40, 57, 107–142]; resisting cell death [26, 30, 108, 143–160]; and deregulating cellular energetics [161–166], all relevant for cancer progression (Fig. 4). Therefore, it is conceivable that modest alterations in CK2 activity and/or protein levels can influence the acquisition and maintenance of the emerging cancer hallmarks in several different ways. In light of these observations, CK2 has evidently emerged as an attractive candidate for molecular targeted cancer therapy. Numerous publications in this area have been summarized in several reviews [2, 12, 167]. Importantly, an orally available small molecule inhibitor of CK2 (CX-4945) that promotes an anti-proliferative and anti-angiogenic response in mouse cancers has recently entered Phase II clinical trials as a potential anticancer drug [168–170].
Fig. 4.
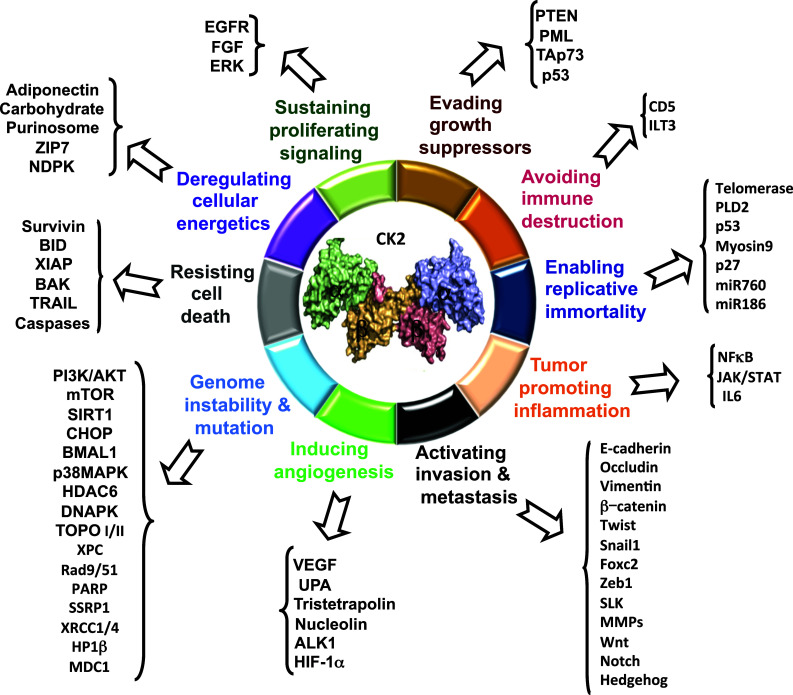
Selected targets and/or interactors of CK2 that contribute to the “hallmarks of cancer”described by Hanahan and Weinberg [34, 35]. At each phase of tumorigenesis, high CK2 activity may reinforce the progression of the disease through promotion of the hallmarks
CK2 as an atypical protein kinase
The CK2 holoenzyme can exist in α2β2, αα′β2, or α′2β2 configurations and has a low dissociation constant around 4 nM, which is a characteristic of strong heterocomplexes [171]. Unlike many signaling protein kinases, CK2 is constitutively active, independent of second messengers or phosphorylation events, and there is not yet a recognized potential model to explain how this kinase is regulated within cells. However, mounting evidence suggests that it can be regulated through mechanisms such as local recruitment into complexes or intracellular compartmentalization [142, 172, 173]. In addition, the individual CK2 subunits might also play independent functions on their own [20, 45, 174–176]. In the early 1990s, Stigare et al. [177] first reported in an epithelial Chironomus cell line that most of the catalytic α-subunit was tightly bound to nuclear structures in the absence of its β-subunit counterpart. In addition, free monomeric CK2α is relatively common in plants, and several reports have provided evidence for an unbalanced expression of CK2α and CK2β subunits in different mammalian tissues [178–180]. The dynamic properties of the molecular interaction between CK2 subunits were first revealed in living cells, by the observation of individual CK2 subunits on a short timescale using live-cell fluorescent imaging [45]. This study provided evidence of the independent movement of CK2α and CK2β in cells, showing that the majority of the two subunits are not present in a permanent holoenzyme. This apparent difference in mobility was also evident at the level of their nuclear translocation: each CK2 subunit enters the nucleus as distinct subunits rather than as a preassembled holoenzyme. Cellular and structural data configure the CK2 holoenzyme either as a strong transient or permanent complex [171]. Dissociation of the tetrameric CK2 complex in living cells has been postulated. However, the presence of noninteracting CK2 subunits within cells cannot result from the spontaneous dissociation of the complex. Rather, it can be hypothesized that at any one time, CK2 subunits can interact with each other and/or with specific partners to participate in the transient formation of distinct multimolecular complexes. In this scenario, CK2 activity may be subtly modulated by a variety of interchangeable partners. Accordingly, the CK2β subunit was characterized as a regulatory binding partner of several important protein kinases, including A-Raf, c-Mos, p90rsk [178], PKCζ, the checkpoint kinase Chk1 [181], the cell cycle Wee1 kinase [182], and the activin receptor-like kinase ALK1 [98]. Together, these data supported the view that CK2β may act as a scaffold to coordinate the regulation of kinases distinct from CK2, offering intriguing new prospects for understanding its implication in different signal transduction pathways [175].
A protein kinase on the move
As a signaling protein, CK2 appears as a moving enzyme that could be rapidly recruited to target specific nuclear proteins in response to different stress stimuli such as heat shock [183], ionizing radiation [184], hypoxia [104–106], DNA breaks [142, 172], viral infections [185–188]. Importantly, compelling evidence accumulate showing that alterations in the subcellular localization of CK2 subunits contribute to cancer development and are correlated with clinicopathological parameters. This is especially the case in prostate and colorectal cancers where enhanced nuclear localization of CK2α has been reported to correlate with poor prognostic factors [76, 189, 190]. Early immunocytochemical studies have shown that CK2 is mostly detected both in the cytoplasm and the nucleus of most cells. However, there are also evidence that CK2 can be targeted to plasma membranes to regulate ion channel activity [191–193] and the phosphorylation of various membrane-bound proteins [194–196]. JAK family tyrosine kinases bind to cytokine receptors and are activated upon cytokine binding. Activated JAKs are crucial in transmitting signals through activation of the key downstream transcriptional effector STAT3. However, activating mutations in JAK2 are frequently observed in the majority of patients with myeloproliferative disorders, with the most common mutation being V617F, resulting in constitutive activation of the JAK-STAT signaling pathway. Zheng et al., provided the first evidence that CK2 binds to JAK2 and is critical for activation of the JAK2-STAT pathway in response to cytokine stimulation and also for constitutive activation of JAK2 in cells expressing JAK2 V617F [70]. Importantly, pharmacological CK2 inhibition decreases proliferation and induce apoptosis in cells expressing JAK2 V617F raising the possibility that CK2 inhibitors might be potent inhibitors of constitutively activated JAK2 V617F and downstream pathways.
Critical role of CK2β in targeting specific CK2 substrates
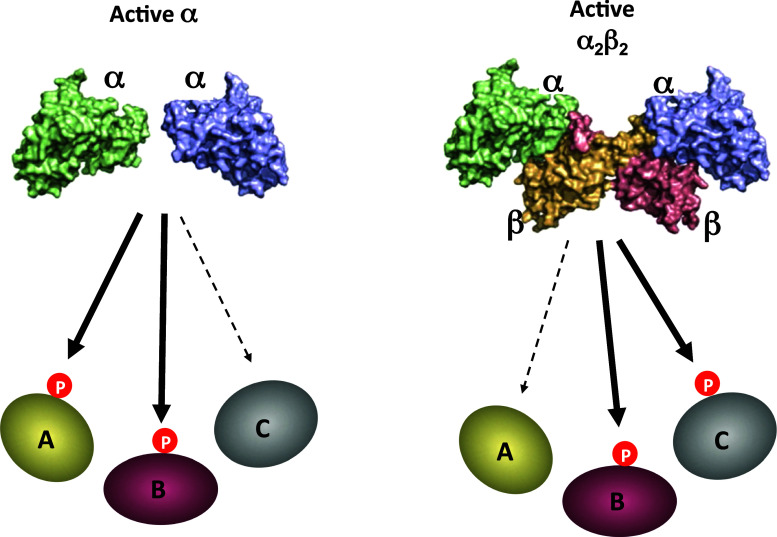
Unlike the regulatory subunit of other hetero-oligomeric kinases, the CK2β subunit is not required for the activity of the catalytic subunits. Both the isolated CK2α subunits and the holoenzyme are endowed with constitutive activity. However, binding of the regulatory subunit to CK2α results in the phosphorylation of a range of substrates that are not or are only weakly phosphorylated in its absence. In contrast, a limited number of protein substrates are phosphorylated by the noncomplexed catalytic subunit but not by the holoenzyme [197, 198] (Fig. 1). Interestingly, it has been reported that the tight association between CK2 and some of its substrates is often bridged by the CK2β dimer [2, 39, 78, 80, 122, 199–201]. This means that any change in CK2β expression might lead to a shift in the balance of phosphorylated CK2α- and holoenzyme-specific substrates. Furthermore, since CK2 substrates localize in many different subcellular compartments, a dynamic rather than a static interaction of the CK2 subunits may help adjust the kinase specificity to ensure that the relevant form of the catalytic subunit is present at each of these locations. To fine-tune these activities, it is likely that cells have developed specific mechanisms to actively segregate the CK2 subunits or to differentially downregulate their expression. Very little is known about the kinetics of the assembly of CK2 subunits into intact cells, a process that could be controlled by interactions with other cellular proteins. This raises the possibility that CK2α could be locally and transiently recruited into multimolecular complexes in which the CK2β dimer serves as a scaffold or a docking subunit through high-affinity interactions with substrate or non-substrate protein partners [174, 198, 200]. Alternatively, it has been suggested that upon activation of Src family kinases, a pool of CK2α not associated with the β-subunits becomes tyrosine phosphorylated with a resultant increase in its activity toward a subset of specific substrates [202]. Altogether, it can be predicted that such mechanisms might be crucial in the control of the many cellular processes that are governed by this pleiotropic kinase.
Fig. 1.
Role of CK2β in targeting CK2 substrates. CK2 substrates can be phosphorylated by the noncomplexed CK2α subunits (e.g., substrate A). Binding of the regulatory CK2β subunit to CK2α results in the phosphorylation of a range of substrates that are not, or are only weakly, phosphorylated in its absence (e.g., substrate C), whereas other proteins can be equally phosphorylated by the isolated CK2α subunits or by the holoenzyme (e.g., substrate B). This model suggests that any change in CK2β expression may lead to a shift in the balance of phosphorylated CK2α- and holoenzyme-specific substrates
Dysregulation of CK2 in mammary tumorigenesis
Early studies postulated that CK2 could contribute to breast cancer carcinoma development because enforced overexpression of CK2α in the mammary gland of transgenic mice promotes hyperplasia and neoplasia in this organ [82, 203]. Moreover, upregulation of CK2 protein and activity was observed during the development of DMBA-induced mammary tumors [204]. In mouse models, CK2 cooperatively promotes oncogenesis and tumor progression with overexpression of oncogenes such as c-myc [205] or with loss of tumor suppressors such as p53 [19, 21, 206]. Later on, studies have shown that human breast cancer tissues contain high CK2 catalytic activity usually correlated with CK2 overexpression, suggesting a pathologic relationship between CK2 expression and mammary tumorigenesis [82]. Because of the importance of subgroup classification based on a tumor’s biology and clinical behavior, Giusiano et al. evaluated the prognostic significance of CK2α expression in association with various clinicopathological parameters in a large cohort of breast tumor patients. This study demonstrated a strong association between CK2α overexpression and breast tumor aggressiveness, highlighting this kinase as an adverse prognostic marker [29]. Furthermore, at the mRNA level, both CK2α and CK2β are elevated and associated with a poor survival prognosis [25, 30]. This is consistent with two studies that have identified CK2 as a component of an “invasiveness gene signature” predictive of metastasis and poor survival in breast cancer [32, 88]. Clinically, breast tumors have been categorized into three basic therapeutic groups: oestrogen receptor (ER+), ERBB2 expressing (HER2+) and triple-negative breast cancers (TNBCs) lacking expression of ER, progesterone receptor (PR) and HER2. A recent analysis of transcript expression for CK2 subunits revealed significant levels of both CK2α and CK2β overexpression but strong CK2α′ underexpression in all breast cancer subtypes, features that were correlated with lower survival rates [30]. Although a correlation between transcript and protein deregulation remains to be addressed, these data suggest that deregulated CK2 gene transcript expression may be a mechanism underlying the increased CK2 activity and protein levels detected in specific breast tumor subtypes.
CK2 contribution to the epithelial phenotype
Epithelial cells usually exist as sheets of immotile, tightly packed, polarized cells with distinct apical, basal, and lateral surfaces. Remarkably, these cells can dramatically alter their morphology, losing their epithelial apicobasal polarity to become motile, fibroblast-like mesenchymal cells in a process of epithelial–mesenchymal transition (EMT). In the early steps, aberrant reactivation of EMT in cancer epithelial cells may facilitate the dissemination of tumor cells and the generation of cancer stem cells, fueling both initiation and metastatic spread [207–210].
There is compelling evidence for the multiple functional contributions that CK2 makes to maintain the epithelial phenotype and polarity [211–213]. In particular, studies have suggested its implication in the regulation of the actin cytoskeleton via phosphorylation of the Wiskott-Aldrich syndrome protein (WASP) [214]. CK2 is also required for proper microtubule organization to facilitate neurite outgrowth in neuroblastoma cells [215], documenting the potential role of CK2 in the regulation of cell polarity and morphology. Although CK2α activity and expression were found to be upregulated in breast tumors, the contribution of CK2β to this dysregulation has not been explored [29, 82]. A differential expression of CK2 subunit transcripts was observed in Basal and Luminal A molecular subtypes. Interestingly, Luminal A were depleted for CK2β transcripts while higher expression of all three subunits was observed in Basal subtype [25]. Analysis of CK2 subunits at the protein level in breast tumor samples showed that whereas most samples expressed equivalent amounts of catalytic and regulatory subunits, their ratio was unexpectedly deviant in a subset of samples. Importantly, these clinical breast tumor samples displayed high concordance between CK2β underexpression and EMT markers, emphasizing the coupling between an asymmetric expression of CK2 subunits and EMT in vivo [80].
Mechanistically, EMT appears to be a dynamic process, resulting from the execution of several interconnected cellular programs that controls epithelial plasticity [216–218]. A plethora of pathways including the RTK, TGFβ, Notch, Wnt, and BMP pathways are able to induce EMT [219–223]. These pathways signal through intracellular kinase cascades to activate EMT transcription factors (Snail1) ([224, 225], Snail2 [226], Twist [227], Zeb1 [228], Zeb2 [229], Foxc2 [230], and others), which together with the loss of E-cadherin transcription are considered important hallmarks of the process [217]. In breast tumors, Snail expression precedes the expression of other factors [231–233]. Snail1 expression seems to be required for EMT initiation, whereas other EMT inducers are required to maintain late EMT [232, 234]. Thus, a temporal hierarchy in the activation of the EMT transcription factors may lead to the sequential repression of the epithelial phenotype, the acquisition of mesenchymal traits, extracellular matrix (ECM) remodeling and the appearance of invasive properties associated with acquired multidrug resistance [235]. Late recurrence in breast cancer associated with tumor dormancy is common and is associated with very poor outcomes. Interestingly, it has been recently reported that a high degree of epithelial–mesenchymal plasticity in primary breast tumors is strongly associated with late recurrence, as opposed to the conventional classification based on expression status of HER2, ER and PR. These findings suggest that in primary tumor, an EMT-related gene signature that is independent of disease subtype could predict the transition of tumor cells to a dormant phenotype with potential outgrowth as recurrent disease [236]. Since low CK2β expression is associated with an EMT-related gene signature and ECM remodeling, this molecular alteration is likely a common phenomenon applicable to all breast cancer subtypes.
Linking CK2β to SNAIL1
Analysis of clinical breast tumor samples exhibiting a wide range of CK2α expression levels showed that Snail1 expression was significantly increased in low CK2β-expressing tumor samples. This suggests that CK2β underexpression may be associated with EMT, which is a common feature of aggressive human breast tumors. Furthermore, downregulation of CK2β in MCF10A mammary epithelial cells clearly promoted EMT [80]. This is in accordance with previous observations showing that Snail1 expression is elevated in highly aggressive breast tumors of the basal-like phenotype [237].
Snail1 is a labile zinc finger protein and its turnover is tightly controlled by the E3 ligase-mediated proteasome degradation process [238]. Snail1-mediated EMT confers cellular plasticity by regulating genes involved in cell death and stem cell maintenance [239]. As a transcriptional repressor, Snail1 interacts with several corepressors and epigenetic remodeling complexes to directly repress specific target genes such as the E-cadherin gene [240].
A wide range of signaling pathways have been found to activate Snail1 expression including TGFβ [241, 242], Notch [243, 244], and Wnt pathways [245]; reactive oxygen species [246]; and hypoxic stress [247–249]. The central role of Snail1 in the regulation of EMT has been linked to its subcellular location and functions through different phosphorylation events. There are at least five kinases that can phosphorylate Snail1 on five distinct regions for regulating Snail1 protein transcriptional activity, nuclear location, and protein stability. For example, the p21-activated kinase (PAK1) phosphorylates Snail1, promotes the accumulation of Snail1 in the nucleus and subsequent Snail1-mediated transcriptional repression of target genes [250]. In contrast, GSK3β phosphorylates two Ser residues on Snail1, one of which targets Snail1 for ubiquitination and degradation [251, 252]. Snail1 is also phosphorylated by CK2 on Ser92 [81]. Importantly, CK2β is required for CK2-mediated Snail1 phosphorylation, and pull-down assays using MCF10A cell lysates revealed that Snail1 binds the CK2 holoenzyme through its CK2β subunit [80]. CK2β-dependent phosphorylation had a cumulative positive effect on GSK3β-mediated Snail1 phosphorylation, showing that both kinases can negatively regulate Snail1 stability through its hierarchal phosphorylation. Strikingly, Snail1 silencing was sufficient to prevent the EMT phenotype induced in response to CK2β attenuation, highlighting the key role of CK2 in Snail1-mediated EMT [80].
A comparative genome-wide characterization of CK2β-regulated mRNA expression could identify an EMT core signature consisting upregulation of mesenchymal genes (CDH2, FN1, MYL9, VIM, SNAIL1, TWIST1, ZEB1/ZEB2, SIX1), genes involved in the regulation of the extracellular matrix (FN1, FBN1, COL6A1, COL17A1, LAD1), the cytoskeleton (MAP1B, MYL9, MYLK), the metalloproteases (ADAM19, ADAM23, ADAMTS4), and downregulation of epithelial genes (CDH1, CDH3, CLDN1, CLDN7, OCLN, KRT5, KRT6B, COL2A1, MUC1) (Fig. 2). This is in accordance with the observations that CK2β downregulation in epithelial cells induces dramatic changes in cell adhesion and migration [213].
Fig. 2.
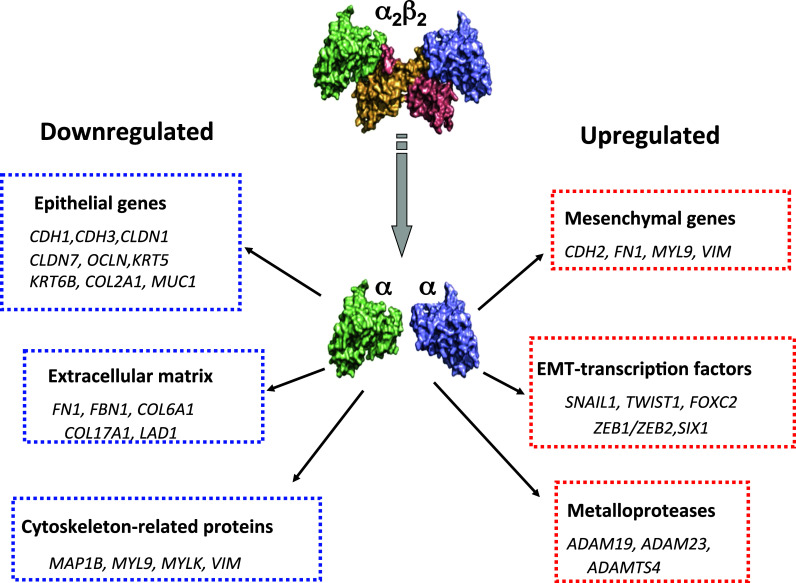
Selected transcripts modulated in CK2β-depleted MCF-10A mammary epithelial cells. Downregulated and upregulated genes are in blue and red, respectively. Microarray data were deposited in the Gene Expression Omnibus (GEO) public database at NCBI, under Accession number GSE28569
Linking CK2β to FOXC2
The forkhead box proteins, Foxc1 and Foxc2, belong to the forkhead family of transcription factors, which are important in regulating the expression of genes involved in cell growth, proliferation, and differentiation [253–255]. It has been reported that Foxc2 is maintained in the cytoplasm of injured renal cells where it promotes an epithelial phenotype, suggesting that this protein may have regulatory functions independent of its nuclear transcriptional activity [256]. In contrast, nuclear Foxc2 localization is implicated in EMT induction and plays causal role in metastatic dissemination [230]. Recently, Golden and Cantley provided strong evidence that a CK2-mediated phosphorylation of Foxc2 at serine 124 promotes cytoplasmic retention of this transcription factor in normal epithelial cells [78]. In agreement with the mechanism already observed for CK2-dependent phosphorylation of Snail1 [80], the authors found that CK2β is also required for CK2-mediated phosphorylation of Foxc2. Consistent with these findings, nuclear localization of Foxc2 was correlated with decreased expression of CK2β and upregulation of EMT markers in breast metastatic tumor cell lines. Phosphorylation of Snail1 and Foxc2 by the CK2 holoenzyme alters their stability and subcellular localization, respectively. Therefore, the CK2β threshold level is critical in governing Snail1 and Foxc2 fate. This is consistent with a model in which increased CK2β expression may impinge upon Snail1 and Foxc2 functions to reinforce epithelial integrity [78, 80]. These findings also illuminate CK2β as a key regulatory protein that acts as a substrate-dependent modulator of CK2α activity.
SIX1 is overexpressed in CK2β-depleted cells
The SIX1 homeoprotein has been implicated in both tumor initiation and tumor progression in many human cancers [257–262]. Overexpression of SIX1 mRNA was observed in 44 % of primary breast cancers and 90 % of metastatic lesions. Recently, several studies provided evidence that SIX1 both participates in TGFβ signaling in mammary cells [262, 263] and induces EMT to promote tumor development [264].
It has been reported that CK2 phosphorylates SIX1 and this phosphorylation negatively affects its DNA binding activity [115]. Interestingly, we found that in vitro, SIX1 was not phosphorylated by CK2α alone, whereas optimal phosphorylation was observed in the presence of the CK2 holoenzyme. Furthermore, a potential negative impact of this phosphorylation on SIX1 expression is suggested by the observation that in MCF10A cells, SIX1 is upregulated both at the mRNA and protein levels in CK2β-depleted epithelial cells. In contrast, forced expression of exogenous CK2β in these cells downregulated SIX1 mRNA expression [213].
Conclusions
As the complexity of the cellular CK2 signaling network unfolds, it becomes increasingly important to put individual CK2 protein substrates and partners into context and understand their dynamics in normal versus cancerous cells. The recent research discussed in this review describes the contribution of the deregulated expression of CK2 to cancer development and highlights CK2β as a gatekeeper of epithelial differentiation. CK2β appears to be a key factor that tips the balance in favor of epithelial cell differentiation through the coordinated negative regulation of key EMT-inducing transcription factors. Therefore, the CK2 holoenzyme, through stoichiometric expression of its two subunits, is critical to maintain a normal epithelial morphology. In this situation, CK2β may target CK2α to specific proteins (Snail1, Foxc2, SIX1, and others) whose phosphorylation is highly dependent on the presence of CK2β. However, this cellular mechanism can be overcome in specific conditions such as a low ratio of CK2β to CK2α expression. In this setting, the inefficient CK2-mediated phosphorylation of these key proteins may lead to their stabilization/activation and to consequent disruption of the epithelial phenotype (Fig. 3). Therefore, reduced CK2β may be a novel molecular alteration during malignant tumor progression. How the relative abundance of CK2 subunits is regulated in response to the activation of specific signaling pathways remains a challenging question. Recent data suggest that the kinase activity of the CK2 catalytic subunits is implicated in the regulation of their own gene expression [265]. Intriguingly, miR-125b, whose expression is downregulated in breast tumors, was found to perform its tumor suppressor function via the direct targeting of the 3′-UTRs of CK2α [266]. Conversely, no effector modulating CK2β expression is known, although a link between hypoxia and CK2 was aptly revealed by the demonstration that under hypoxic conditions, the CK2 holoenzyme is dissociated, allowing free CK2α to induce stabilization of the HIF-1α protein [106]. Moreover, it has been observed that tumor samples expressing low levels of CK2β had upregulated HIF-1α expression, suggesting that CK2β underexpression may be associated with the hypoxic conditions found in human breast tumors [80]. Although traditionally considered to be the regulatory subunit of CK2, it appears that this highly conserved protein also has cellular functions that are independent of CK2, reinforcing the importance of delineating its regulation. Therefore, we can anticipate that the identification of new partners for CK2β will certainly tease out additional mechanistic insights on the multiple functions of CK2 during tumorigenesis (Fig. 4). While there are many unanswered questions with regard to how differential levels of CK2β regulate distinct proteins involved in normal versus cancerous cells, the potential for CK2 to be an efficacious target in treating cancer patients remains high.
Fig. 3.
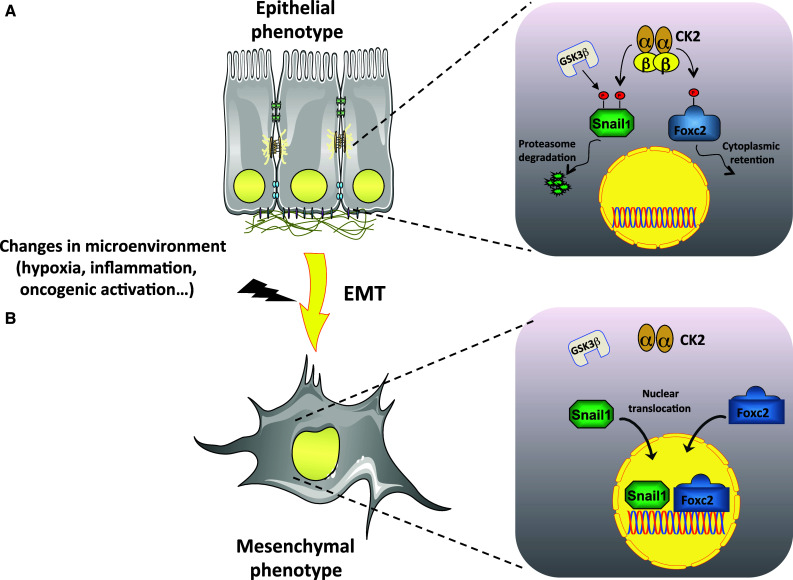
A hypothetical model in which CK2β is at center stage in the regulation of epithelial plasticity. a Snail1 and Foxc2 are master transcription regulators that trigger the formation of a signaling network responsible for establishing and maintaining mesenchymal cell phenotypes. In epithelial cells, both GSK3β and CK2 can negatively regulate Snail1 stability through its hierarchal phosphorylation. CK2-mediated phosphorylation of Foxc2 promotes its cytoplasmic retention. Importantly, CK2-dependent phosphorylation of both transcription factors is mediated by the holoenzyme and thus requires the presence of CK2β. b Silencing of CK2β or unbalanced expression of CK2 subunits in response to changes in the microenvironment leads to inefficient phosphorylation of Snail1 and Foxc2, thereby leading to their nuclear translocation and to EMT induction associated with enhanced migratory potential and acquisition of apoptosis resistance
Acknowledgments
This research was supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Ligue Nationale et Regionale contre le Cancer, the Commissariat à l’Energie Atomique and the University of Grenoble Alpes and the Espoir foundation.
Contributor Information
Odile Filhol, Email: odile.filhol-cochet@cea.fr.
Claude Cochet, Phone: 33 4 38 78 42 04, Email: claude.cochet@cea.fr.
References
- 1.Allende JE, Allende CC. Protein kinases. 4. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995;9(5):313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 2.Guerra B, Issinger OG. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis. 1999;20(2):391–408. doi: 10.1002/(SICI)1522-2683(19990201)20:2<391::AID-ELPS391>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 3.Pinna LA. Casein kinase 2: an ‘eminence grise’ in cellular regulation? Biochim Biophys Acta. 1990;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- 4.Basnet H, Su XB, Tan Y, Meisenhelder J, Merkurjev D, Ohgi KA, Hunter T, Pillus L, Rosenfeld MG. Tyrosine phosphorylation of histone H2A by CK2 regulates transcriptional elongation. Nature. 2014;516(7530):267–271. doi: 10.1038/nature13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvi M, Sarno S, Cesaro L, Nakamura H, Pinna LA. Extraordinary pleiotropy of protein kinase CK2 revealed by weblogo phosphoproteome analysis. Biochim Biophys Acta. 2009;1793(5):847–859. doi: 10.1016/j.bbamcr.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Gabriel M, Litchfield DW. Protein kinase CK2: at the crossroads of pathways controlling cell proliferation and survival, vol Protein kinase CK2. New York: John Wiley & Sons, Inc; 2013. [Google Scholar]
- 7.Arevalo MA, Rodriguez-Tebar A. Activation of casein kinase II and inhibition of phosphatase and tensin homologue deleted on chromosome 10 phosphatase by nerve growth factor/p75(NTR) inhibit glycogen synthase kinase-3 beta and stimulate axonal growth. Mol Biol Cell. 2006;17(8):3369–3377. doi: 10.1091/mbc.E05-12-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauck L, Harms C, An JF, Rohne J, Gertz K, Dietz R, Endres M, von Harsdorf R. Protein kinase CK2 links extracellular growth factor signaling with the control of p27(Kip1) stability in the heart (vol 14, pg 315) Nat Med. 2008;14(5):585. doi: 10.1038/nm1729. [DOI] [PubMed] [Google Scholar]
- 9.Ji H, Wang J, Nika H, Hawke D, Keezer S, Ge Q, Fang B, Fang X, Fang D, Litchfield DW, Aldape K, Lu Z. EGF-induced ERK activation promotes CK2-mediated disassociation of alpha-Catenin from beta-Catenin and transactivation of beta-Catenin. Mol Cell. 2009;36(4):547–559. doi: 10.1016/j.molcel.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mead JR, Hughes TR, Irvine SA, Singh NN, Ramji DP. Interferon-gamma stimulates the expression of the inducible cAMP early repressor in macrophages through the activation of casein kinase 2: a potentially novel pathway for interferon-gamma-mediated inhibition of gene transcription. J Biol Chem. 2003;278(20):17741–17751. doi: 10.1074/jbc.M301602200. [DOI] [PubMed] [Google Scholar]
- 11.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17(3):349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 12.Duncan JS, Litchfield DW. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim Biophys Acta. 2008;1784(1):33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Guerra B, Issinger OG. Protein kinase CK2 in human diseases. Curr Med Chem. 2008;15(19):1870–1886. doi: 10.2174/092986708785132933. [DOI] [PubMed] [Google Scholar]
- 14.Ruzzene M. Pinna LA (2010) Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim Biophys Acta. 1804;3:499–504. doi: 10.1016/j.bbapap.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Trembley JH, Unger GM, Tobolt DK, Korman VL, Wang G, Ahmad KA, Slaton JW, Kren BT, Ahmed K. Systemic administration of antisense oligonucleotides simultaneously targeting CK2alpha and alpha’ subunits reduces orthotopic xenograft prostate tumors in mice. Mol Cell Biochem. 2011;356(1–2):21–35. doi: 10.1007/s11010-011-0943-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tawfic S, Yu S, Wang H, Faust R, Davis A, Ahmed K. Protein kinase CK2 signal in neoplasia. Histol Histopathol. 2001;16(2):573–582. doi: 10.14670/HH-16.573. [DOI] [PubMed] [Google Scholar]
- 17.Pinna LA, Allende JE. Protein kinase CK2 in health and disease: protein kinase CK2: an ugly duckling in the kinome pond. Cell Mol Life Sci. 2009;66(11–12):1795–1799. doi: 10.1007/s00018-009-9148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St-Denis NA, Litchfield DW. Protein kinase CK2 in health and disease: from birth to death: the role of protein kinase CK2 in the regulation of cell proliferation and survival. Cell Mol Life Sci. 2009;66(11–12):1817–1829. doi: 10.1007/s00018-009-9150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seldin DC, Leder P. Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science. 1995;267(5199):894–897. doi: 10.1126/science.7846532. [DOI] [PubMed] [Google Scholar]
- 20.Landesman-Bollag E, Belkina A, Hovey B, Connors E, Cox C, Seldin DC. Developmental and growth defects in mice with combined deficiency of CK2 catalytic genes. Mol Cell Biochem. 2011;356(1–2):227–231. doi: 10.1007/s11010-011-0967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landesman-Bollag E, Channavajhala PL, Cardiff RD, Seldin DC. p53 deficiency and misexpression of protein kinase CK2alpha collaborate in the development of thymic lymphomas in mice. Oncogene. 1998;16(23):2965–2974. doi: 10.1038/sj.onc.1201854. [DOI] [PubMed] [Google Scholar]
- 22.Eddy SF, Guo S, Demicco EG, Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Inducible IkappaB kinase/IkappaB kinase epsilon expression is induced by CK2 and promotes aberrant nuclear factor-kappaB activation in breast cancer cells. Cancer Res. 2005;65(24):11375–11383. doi: 10.1158/0008-5472.CAN-05-1602. [DOI] [PubMed] [Google Scholar]
- 23.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Protein kinase CK2 promotes aberrant activation of nuclear factor-kappaB, transformed phenotype, and survival of breast cancer cells. Cancer Res. 2002;62(22):6770–6778. [PubMed] [Google Scholar]
- 24.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray GK, McFarland BC, Rowse AL, Gibson SA, Benveniste EN. Therapeutic CK2 inhibition attenuates diverse prosurvival signaling cascades and decreases cell viability in human breast cancer cells. Oncotarget. 2014;5(15):6484–6496. doi: 10.18632/oncotarget.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed K, Gerber DA, Cochet C. Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol. 2002;12(5):226–230. doi: 10.1016/s0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad KA, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2––a key suppressor of apoptosis. Adv Enzyme Regul. 2008;48:179–187. doi: 10.1016/j.advenzreg.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trembley JH, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2 in health and disease: CK2: a key player in cancer biology. Cell Mol Life Sci. 2009;66(11–12):1858–1867. doi: 10.1007/s00018-009-9154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giusiano S, Cochet C, Filhol O, Duchemin-Pelletier E, Secq V, Bonnier P, Carcopino X, Boubli L, Birnbaum D, Garcia S, Iovanna J, Charpin C. Protein kinase CK2alpha subunit over-expression correlates with metastatic risk in breast carcinomas: quantitative immunohistochemistry in tissue microarrays. Eur J Cancer. 2011;47(5):792–801. doi: 10.1016/j.ejca.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 30.Ortega CE, Seidner Y, Dominguez I. Mining CK2 in cancer. PLoS ONE. 2014;9(12):e115609. doi: 10.1371/journal.pone.0115609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae JS, Park SH, Kim KM, Kwon KS, Kim CY, Lee HK, Park BH, Park HS, Lee H, Moon WS, Chung MJ, Sylvester KG, Jang KY. CK2alpha phosphorylates DBC1 and is involved in the progression of gastric carcinoma and predicts poor survival of gastric carcinoma patients. Int J Cancer. 2015;136(4):797–809. doi: 10.1002/ijc.29043. [DOI] [PubMed] [Google Scholar]
- 32.Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, Sherlock G, Lewicki J, Shedden K, Clarke MF. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356(3):217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 33.Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Cell. 2007;130(6):986–988. doi: 10.1016/j.cell.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Lebrin F, Chambaz EM, Bianchini L. A role for protein kinase CK2 in cell proliferation: evidence using a kinase-inactive mutant of CK2 catalytic subunit alpha. Oncogene. 2001;20(16):2010–2022. doi: 10.1038/sj.onc.1204307. [DOI] [PubMed] [Google Scholar]
- 37.Bailly K, Soulet F, Leroy D, Amalric F, Bouche G. Uncoupling of cell proliferation and differentiation activities of basic fibroblast growth factor. FASEB J. 2000;14(2):333–344. [PubMed] [Google Scholar]
- 38.Bonnet H, Filhol O, Truchet I, Brethenou P, Cochet C, Amalric F, Bouche G. Fibroblast growth factor-2 binds to the regulatory beta subunit of CK2 and directly stimulates CK2 activity toward nucleolin. J Biol Chem. 1996;271(40):24781–24787. doi: 10.1074/jbc.271.40.24781. [DOI] [PubMed] [Google Scholar]
- 39.Filhol O, Baudier J, Delphin C, Loue-Mackenbach P, Chambaz EM, Cochet C. Casein kinase II and the tumor suppressor protein P53 associate in a molecular complex that is negatively regulated upon P53 phosphorylation. J Biol Chem. 1992;267(29):20577–20583. [PubMed] [Google Scholar]
- 40.Bliesath J, Huser N, Omori M, Bunag D, Proffitt C, Streiner N, Ho C, Siddiqui-Jain A, O’Brien SE, Lim JK, Ryckman DM, Anderes K, Rice WG, Drygin D. Combined inhibition of EGFR and CK2 augments the attenuation of PI3 K-Akt-mTOR signaling and the killing of cancer cells. Cancer Lett. 2012;322(1):113–118. doi: 10.1016/j.canlet.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 41.Cox ML, Meek DW. Phosphorylation of serine 392 in p53 is a common and integral event during p53 induction by diverse stimuli. Cell Signal. 2010;22(3):564–571. doi: 10.1016/j.cellsig.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Skjerpen CS, Nilsen T, Wesche J, Olsnes S. Binding of FGF-1 variants to protein kinase CK2 correlates with mitogenicity. EMBO J. 2002;21(15):4058–4069. doi: 10.1093/emboj/cdf402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.So KS, Kim CH, Rho JK, Kim SY, Choi YJ, Song JS, Kim WS, Choi CM, Chun YJ, Lee JC. Autophagosome-mediated EGFR down-regulation induced by the CK2 inhibitor enhances the efficacy of EGFR-TKI on EGFR-mutant lung cancer cells with resistance by T790M. PLoS ONE. 2014;9(12):e114000. doi: 10.1371/journal.pone.0114000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ritt DA, Zhou M, Conrads TP, Veenstra TD, Copeland TD, Morrison DK. CK2 Is a component of the KSR1 scaffold complex that contributes to Raf kinase activation. Curr Biol. 2007;17(2):179–184. doi: 10.1016/j.cub.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 45.Filhol O, Nueda A, Martel V, Gerber-Scokaert D, Benitez MJ, Souchier C, Saoudi Y, Cochet C. Live-cell fluorescence imaging reveals the dynamics of protein kinase CK2 individual subunits. Mol Cell Biol. 2003;23(3):975–987. doi: 10.1128/MCB.23.3.975-987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fragoso R, Barata JT. Kinases, tails and more: regulation of PTEN function by phosphorylation. Methods. 2014 doi: 10.1016/j.ymeth.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Lallemand-Breitenbach V, de The H. CK2 and PML: regulating the regulator. Cell. 2006;126(2):244–245. doi: 10.1016/j.cell.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Keller DM, Lu H. p53 serine 392 phosphorylation increases after UV through induction of the assembly of the CK2.hSPT16.SSRP1 complex. J Biol Chem. 2002;277(51):50206–50213. doi: 10.1074/jbc.M209820200. [DOI] [PubMed] [Google Scholar]
- 49.Scaglioni PP, Yung TM, Cai LF, Erdjument-Bromage H, Kaufman AJ, Singh B, Teruya-Feldstein J, Tempst P, Pandolfi PP. A CK2-dependent mechanism for degradation of the PML tumor suppressor. Cell. 2006;126(2):269–283. doi: 10.1016/j.cell.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 50.Sivachandran N, Cao JY, Frappier L. Epstein–Barr virus nuclear antigen 1 Hijacks the host kinase CK2 to disrupt PML nuclear bodies. J Virol. 2010;84(21):11113–11123. doi: 10.1128/JVI.01183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martel V, Filhol O, Colas P, Cochet C. p53-dependent inhibition of mammalian cell survival by a genetically selected peptide aptamer that targets the regulatory subunit of protein kinase CK2. Oncogene. 2006;25(56):7343–7353. doi: 10.1038/sj.onc.1209722. [DOI] [PubMed] [Google Scholar]
- 52.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276(2):993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 53.Torres J, Rodriguez J, Myers MP, Valiente M, Graves JD, Tonks NK, Pulido R. Phosphorylation-regulated cleavage of the tumor suppressor PTEN by caspase-3: implications for the control of protein stability and pten–protein interactions. J Biol Chem. 2003;278(33):30652–30660. doi: 10.1074/jbc.M212610200. [DOI] [PubMed] [Google Scholar]
- 54.Kang NI, Yoon HY, Kim HA, Kim KJ, Han MK, Lee YR, Hwang PH, Soh BY, Shin SJ, Im SY, Lee HK. Protein kinase CK2/PTEN pathway plays a key role in platelet-activating factor-mediated murine anaphylactic shock. J Immunol. 2011;186(11):6625–6632. doi: 10.4049/jimmunol.1100007. [DOI] [PubMed] [Google Scholar]
- 55.Barata JT. The impact of PTEN regulation by CK2 on PI3 K-dependent signaling and leukemia cell survival. Adv Enzyme Regul. 2011;51(1):37–49. doi: 10.1016/j.advenzreg.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Lu H, Yan C, Quan XX, Yang X, Zhang J, Bian Y, Chen Z, Van Waes C. CK2 phosphorylates and inhibits TAp73 tumor suppressor function to promote expression of cancer stem cell genes and phenotype in head and neck cancer. Neoplasia. 2014;16(10):789–800. doi: 10.1016/j.neo.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keller DM, Zeng X, Wang Y, Zhang QH, Kapoor M, Shu H, Goodman R, Lozano G, Zhao Y, Lu H. A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16, and SSRP1. Mol Cell. 2001;7(2):283–292. doi: 10.1016/s1097-2765(01)00176-9. [DOI] [PubMed] [Google Scholar]
- 58.Sestero CM, McGuire DJ, De Sarno P, Brantley EC, Soldevila G, Axtell RC, Raman C. CD5-dependent CK2 activation pathway regulates threshold for T cell anergy. J Immunol. 2012;189(6):2918–2930. doi: 10.4049/jimmunol.1200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ulges A, Klein M, Reuter S, Gerlitzki B, Hoffmann M, Grebe N, Staudt V, Stergiou N, Bohn T, Bruhl TJ, Muth S, Yurugi H, Rajalingam K, Bellinghausen I, Tuettenberg A, Hahn S, Reissig S, Haben I, Zipp F, Waisman A, Probst HC, Beilhack A, Buchou T, Filhol-Cochet O, Boldyreff B, Breloer M, Jonuleit H, Schild H, Schmitt E, Bopp T. Protein kinase CK2 enables regulatory T cells to suppress excessive T2 responses in vivo. Nat Immunol. 2015 doi: 10.1038/ni.3083. [DOI] [PubMed] [Google Scholar]
- 60.Dixit D, Sharma V, Ghosh S, Mehta VS, Sen E. Inhibition of Casein kinase-2 induces p53-dependent cell cycle arrest and sensitizes glioblastoma cells to tumor necrosis factor (TNFalpha)-induced apoptosis through SIRT1 inhibition. Cell Death Dis. 2012;3:e271. doi: 10.1038/cddis.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HR, Kim K, Lee KH, Kim SJ, Kim J. Inhibition of casein kinase 2 enhances the death ligand- and natural killer cell-induced hepatocellular carcinoma cell death. Clin Exp Immunol. 2008;152(2):336–344. doi: 10.1111/j.1365-2249.2008.03622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee YH, Bae YS. Phospholipase D2 downregulation induces cellular senescence through a reactive oxygen species-p53-p21Cip1/WAF1 pathway. FEBS Lett. 2014;588(17):3251–3258. doi: 10.1016/j.febslet.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Lee YH, Kang BS, Bae YS. Premature senescence in human breast cancer and colon cancer cells by tamoxifen-mediated reactive oxygen species generation. Life Sci. 2014;97(2):116–122. doi: 10.1016/j.lfs.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 64.Lee YH, Kim SY, Bae YS. Upregulation of miR-760 and miR-186 is associated with replicative senescence in human lung fibroblast cells. Mol Cells. 2014;37(8):620–627. doi: 10.14348/molcells.2014.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caino MC, Meshki J, Kazanietz MG. Hallmarks for senescence in carcinogenesis: novel signaling players. Apoptosis Int J Program Cell Death. 2009;14(4):392–408. doi: 10.1007/s10495-009-0316-z. [DOI] [PubMed] [Google Scholar]
- 66.Wang D, Jang DJ. Protein kinase CK2 regulates cytoskeletal reorganization during ionizing radiation-induced senescence of human mesenchymal stem cells. Cancer Res. 2009;69(20):8200–8207. doi: 10.1158/0008-5472.CAN-09-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim KJ, Cho KD, Jang KY, Kim HA, Kim HK, Lee HK, Im SY. Platelet-activating factor enhances tumour metastasis via the reactive oxygen species-dependent protein kinase casein kinase 2-mediated nuclear factor-kappaB activation. Immunology. 2014;143(1):21–32. doi: 10.1111/imm.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aparicio-Siegmund S, Sommer J, Monhasery N, Schwanbeck R, Keil E, Finkenstadt D, Pfeffer K, Rose-John S, Scheller J, Garbers C. Inhibition of protein kinase II (CK2) prevents induced signal transducer and activator of transcription (STAT) 1/3 and constitutive STAT3 activation. Oncotarget. 2014;5(8):2131–2148. doi: 10.18632/oncotarget.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang H, Minamishima YA, Yan Q, Schlisio S, Ebert BL, Zhang X, Zhang L, Kim WY, Olumi AF, Kaelin WG., Jr pVHL acts as an adaptor to promote the inhibitory phosphorylation of the NF-kappaB agonist Card9 by CK2. Mol Cell. 2007;28(1):15–27. doi: 10.1016/j.molcel.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng Y, Qin H, Frank SJ, Deng L, Litchfield DW, Tefferi A, Pardanani A, Lin FT, Li J, Sha B, Benveniste EN. A CK2-dependent mechanism for activation of the JAK-STAT signaling pathway. Blood. 2011;118(1):156–166. doi: 10.1182/blood-2010-01-266320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh NN, Ramji DP. Protein kinase CK2, an important regulator of the inflammatory response? J Mol Med. 2008;86(8):887–897. doi: 10.1007/s00109-008-0352-0. [DOI] [PubMed] [Google Scholar]
- 72.Su YW, Xie TX, Sano D, Myers JN. IL-6 stabilizes Twist and enhances tumor cell motility in head and neck cancer cells through activation of casein kinase 2. PLoS ONE. 2011;6(4):e19412. doi: 10.1371/journal.pone.0019412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koch S, Capaldo CT, Hilgarth RS, Fournier B, Parkos CA, Nusrat A. Protein kinase CK2 is a critical regulator of epithelial homeostasis in chronic intestinal inflammation. Mucosal Immunol. 2013;6(1):136–145. doi: 10.1038/mi.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drygin D, Ho CB, Omori M, Bliesath J, Proffitt C, Rice R, Siddiqui-Jain A, O’Brien S, Padgett C, Lim JK, Anderes K, Rice WG, Ryckman D. Protein kinase CK2 modulates IL-6 expression in inflammatory breast cancer. Biochemical and biophysical research communications. 2011;415(1):163–167. doi: 10.1016/j.bbrc.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 75.Serres M, Filhol O, Lickert H, Grangeasse C, Chambaz EM, Stappert J, Vincent C, Schmitt D. The disruption of adherens junctions is associated with a decrease of E-cadherin phosphorylation by protein kinase CK2. Exp Cell Res. 2000;257(2):255–264. doi: 10.1006/excr.2000.4895. [DOI] [PubMed] [Google Scholar]
- 76.Zou J, Luo H, Zeng Q, Dong Z, Wu D, Liu L. Protein kinase CK2alpha is overexpressed in colorectal cancer and modulates cell proliferation and invasion via regulating EMT-related genes. J Trans Med. 2011;9:97. doi: 10.1186/1479-5876-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dorfel MJ, Westphal JK, Bellmann C, Krug SM, Cording J, Mittag S, Tauber R, Fromm M, Blasig IE, Huber O. CK2-dependent phosphorylation of occludin regulates the interaction with ZO-proteins and tight junction integrity. CCS. 2013;11(1):40. doi: 10.1186/1478-811X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Golden D, Cantley LG. Casein kinase 2 prevents mesenchymal transformation by maintaining Foxc2 in the cytoplasm. Oncogene. 2014 doi: 10.1038/onc.2014.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu H, Symes K, Seldin DC, Dominguez I. Threonine 393 of beta-catenin regulates interaction with Axin. J Cell Biochem. 2009;108(1):52–63. doi: 10.1002/jcb.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deshiere A, Duchemin-Pelletier E, Spreux E, Ciais D, Combes F, Vandenbrouck Y, Coute Y, Mikaelian I, Giusiano S, Charpin C, Cochet C, Filhol O. Unbalanced expression of CK2 kinase subunits is sufficient to drive epithelial-to-mesenchymal transition by Snail1 induction. Oncogene. 2013;32(11):1373–1383. doi: 10.1038/onc.2012.165. [DOI] [PubMed] [Google Scholar]
- 81.MacPherson MR, Molina P, Souchelnytskyi S, Wernstedt C, Martin-Perez J, Portillo F, Cano A. Phosphorylation of serine 11 and serine 92 as new positive regulators of human Snail1 function: potential involvement of casein kinase-2 and the cAMP-activated kinase protein kinase A. Mol Biol Cell. 2010;21(2):244–253. doi: 10.1091/mbc.E09-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001;20(25):3247–3257. doi: 10.1038/sj.onc.1204411. [DOI] [PubMed] [Google Scholar]
- 83.Kim H, Choi K, Kang H, Lee SY, Chi SW, Lee MS, Song J, Im D, Choi Y, Cho S. Identification of a novel function of CX-4945 as a splicing regulator. PLoS ONE. 2014;9(4):e94978. doi: 10.1371/journal.pone.0094978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu M, Yeh J, Van Waes C. Protein kinase casein kinase 2 mediates inhibitor-kappaB kinase and aberrant nuclear factor-kappaB activation by serum factor(s) in head and neck squamous carcinoma cells. Cancer Res. 2006;66(13):6722–6731. doi: 10.1158/0008-5472.CAN-05-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brenneisen P, Wlaschek M, Schwamborn E, Schneider LA, Ma W, Sies H, Scharffetter-Kochanek K. Activation of protein kinase CK2 is an early step in the ultraviolet B-mediated increase in interstitial collagenase (matrix metalloproteinase-1; MMP-1) and stromelysin-1 (MMP-3) protein levels in human dermal fibroblasts. Biochem J. 2002;365(Pt 1):31–40. doi: 10.1042/BJ20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaar Z, O’Reilly P, Gelman I, Sabourin LA. v-Src-dependent down-regulation of the Ste20-like kinase SLK by casein kinase II. J Biol Chem. 2006;281(38):28193–28199. doi: 10.1074/jbc.M605665200. [DOI] [PubMed] [Google Scholar]
- 87.Ranganathan P, Vasquez-Del Carpio R, Kaplan FM, Wang H, Gupta A, VanWye JD, Capobianco AJ. Hierarchical phosphorylation within the ankyrin repeat domain defines a phosphoregulatory loop that regulates Notch transcriptional activity. J Biol Chem. 2011;286(33):28844–28857. doi: 10.1074/jbc.M111.243600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rozanov DV, Savinov AY, Williams R, Liu K, Golubkov VS, Krajewski S, Strongin AY. Molecular signature of MT1-MMP: transactivation of the downstream universal gene network in cancer. Cancer Res. 2008;68(11):4086–4096. doi: 10.1158/0008-5472.CAN-07-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Groot RE, Rappel SB, Lorenowicz MJ, Korswagen HC. Protein kinase CK2 is required for Wntless internalization and Wnt secretion. Cell Signal. 2014;26(12):2601–2605. doi: 10.1016/j.cellsig.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 90.Ku MJ, Park JW, Ryu BJ, Son YJ, Kim SH, Lee SY. CK2 inhibitor CX4945 induces sequential inactivation of proteins in the signaling pathways related with cell migration and suppresses metastasis of A549 human lung cancer cells. Bioorg Med Chem Lett. 2013;23(20):5609–5613. doi: 10.1016/j.bmcl.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 91.Cheng P, Kumar V, Liu H, Youn JI, Fishman M, Sherman S, Gabrilovich D. Effects of notch signaling on regulation of myeloid cell differentiation in cancer. Cancer Res. 2014;74(1):141–152. doi: 10.1158/0008-5472.CAN-13-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jia H, Liu Y, Xia R, Tong C, Yue T, Jiang J, Jia J. Casein kinase 2 promotes Hedgehog signaling by regulating both smoothened and Cubitus interruptus. J Biol Chem. 2010;285(48):37218–37226. doi: 10.1074/jbc.M110.174565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang S, Wang Y, Mao JH, Hsieh D, Kim IJ, Hu LM, Xu Z, Long H, Jablons DM, You L. Inhibition of CK2alpha down-regulates Hedgehog/Gli signaling leading to a reduction of a stem-like side population in human lung cancer cells. PLoS ONE. 2012;7(6):e38996. doi: 10.1371/journal.pone.0038996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang S, Yang YL, Wang Y, You B, Dai Y, Chan G, Hsieh D, Kim IJ, Fang L, Au A, Stoppler HJ, Xu Z, Jablons DM, You L. CK2 inverted question mark, over-expressed in human malignant pleural mesothelioma, regulates the Hedgehog signaling pathway in mesothelioma cells. J Exp Clin Cancer Res. 2014;33(1):93. doi: 10.1186/s13046-014-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu D, Sui C, Meng F, Tian X, Fu L, Li Y, Qi X, Cui H, Liu Y, Jiang Y. Stable knockdown of protein kinase CK2-alpha (CK2alpha) inhibits migration and invasion and induces inactivation of hedgehog signaling pathway in hepatocellular carcinoma Hep G2 cells. Acta Histochem. 2014;116(8):1501–1508. doi: 10.1016/j.acthis.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 96.Farina HG, Benavent Acero F, Perera Y, Rodriguez A, Perea SE, Castro BA, Gomez R, Alonso DF, Gomez DE. CIGB-300, a proapoptotic peptide, inhibits angiogenesis in vitro and in vivo. Exp Cell Res. 2011;317(12):1677–1688. doi: 10.1016/j.yexcr.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Noy P, Sawasdichai A, Jayaraman PS, Gaston K. Protein kinase CK2 inactivates PRH/Hhex using multiple mechanisms to de-repress VEGF-signalling genes and promote cell survival. Nucleic Acids Res. 2012;40(18):9008–9020. doi: 10.1093/nar/gks687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee NY, Haney JC, Sogani J, Blobe GC. Casein kinase 2beta as a novel enhancer of activin-like receptor-1 signaling. FASEB J. 2009;23(11):3712–3721. doi: 10.1096/fj.09-131607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stepanova V, Jerke U, Sagach V, Lindschau C, Dietz R, Haller H, Dumler I. Urokinase-dependent human vascular smooth muscle cell adhesion requires selective vitronectin phosphorylation by ectoprotein kinase CK2. J Biol Chem. 2002;277(12):10265–10272. doi: 10.1074/jbc.M109057200. [DOI] [PubMed] [Google Scholar]
- 100.Xiao S, Caglar E, Maldonado P, Das D, Nadeem Z, Chi A, Trinite B, Li X, Saxena A. Induced expression of nucleolin phosphorylation-deficient mutant confers dominant-negative effect on cell proliferation. PLoS ONE. 2014;9(10):e109858. doi: 10.1371/journal.pone.0109858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Montenarh M. Protein kinase CK2 and angiogenesis. Adv Clin Exp Med. 2014;23(2):153–158. doi: 10.17219/acem/37040. [DOI] [PubMed] [Google Scholar]
- 102.Lee WH, Lee HH, Vo MT, Kim HJ, Ko MS, Im YC, Min YJ, Lee BJ, Cho WJ, Park JW. Casein kinase 2 regulates the mRNA-destabilizing activity of tristetraprolin. J Biol Chem. 2011;286(24):21577–21587. doi: 10.1074/jbc.M110.201137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guerra B, Rasmussen TD, Schnitzler A, Jensen HH, Boldyreff BS, Miyata Y, Marcussen N, Niefind K, Issinger OG. Protein kinase CK2 inhibition is associated with the destabilization of HIF-1alpha in human cancer cells. Cancer Lett. 2015;356(2):751–761. doi: 10.1016/j.canlet.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 104.Hubert A, Paris S, Piret JP, Ninane N, Raes M, Michiels C. Casein kinase 2 inhibition decreases hypoxia-inducible factor-1 activity under hypoxia through elevated p53 protein level. J Cell Sci. 2006;119(Pt 16):3351–3362. doi: 10.1242/jcs.03069. [DOI] [PubMed] [Google Scholar]
- 105.Mottet D, Ruys SP, Demazy C, Raes M, Michiels C. Role for casein kinase 2 in the regulation of HIF-1 activity. Int J Cancer. 2005;117(5):764–774. doi: 10.1002/ijc.21268. [DOI] [PubMed] [Google Scholar]
- 106.Pluemsampant S, Safronova OS, Nakahama K, Morita I. Protein kinase CK2 is a key activator of histone deacetylase in hypoxia-associated tumors. Int J Cancer. 2008;122(2):333–341. doi: 10.1002/ijc.23094. [DOI] [PubMed] [Google Scholar]
- 107.Guerra B. Protein kinase CK2 subunits are positive regulators of AKT kinase. Int J Oncol. 2006;28(3):685–693. [PubMed] [Google Scholar]
- 108.Zheng Y, McFarland BC, Drygin D, Yu H, Bellis SL, Kim H, Bredel M, Benveniste EN. Targeting protein kinase CK2 suppresses prosurvival signaling pathways and growth of glioblastoma. Clin Cancer Res. 2013;19(23):6484–6494. doi: 10.1158/1078-0432.CCR-13-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parker R, Clifton-Bligh R, Molloy MP. Phosphoproteomics of MAPK inhibition in BRAF-mutated cells and a role for the lethal synergism of dual BRAF and CK2 inhibition. Mol Cancer Ther. 2014;13(7):1894–1906. doi: 10.1158/1535-7163.MCT-13-0938. [DOI] [PubMed] [Google Scholar]
- 110.Olsen BB, Fritz G, Issinger OG. Characterization of ATM and DNA-PK wild-type and mutant cell lines upon DSB induction in the presence and absence of CK2 inhibitors. Int J Oncol. 2012;40(2):592–598. doi: 10.3892/ijo.2011.1227. [DOI] [PubMed] [Google Scholar]
- 111.Olsen BB, Issinger OG, Guerra B. Regulation of DNA-dependent protein kinase by protein kinase CK2 in human glioblastoma cells. Oncogene. 2010;29(45):6016–6026. doi: 10.1038/onc.2010.337. [DOI] [PubMed] [Google Scholar]
- 112.Finlan LE, Nenutil R, Ibbotson SH, Vojtesek B, Hupp TR. CK2-site phosphorylation of p53 is induced in DeltaNp63 expressing basal stem cells in UVB irradiated human skin. Cell Cycle. 2006;5(21):2489–2494. doi: 10.4161/cc.5.21.3393. [DOI] [PubMed] [Google Scholar]
- 113.Kang H, Jung JW, Kim MK, Chung JH. CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. PLoS ONE. 2009;4(8):e6611. doi: 10.1371/journal.pone.0006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ubeda M, Habener JF. CHOP transcription factor phosphorylation by casein kinase 2 inhibits transcriptional activation. J Biol Chem. 2003;278(42):40514–40520. doi: 10.1074/jbc.M306404200. [DOI] [PubMed] [Google Scholar]
- 115.Ford HL, Landesman-Bollag E, Dacwag CS, Stukenberg PT, Pardee AB, Seldin DC. Cell cycle-regulated phosphorylation of the human SIX1 homeodomain protein. J Biol Chem. 2000;275(29):22245–22254. doi: 10.1074/jbc.M002446200. [DOI] [PubMed] [Google Scholar]
- 116.Tamaru T, Hattori M, Ninomiya Y, Kawamura G, Vares G, Honda K, Mishra DP, Wang B, Benjamin I, Sassone-Corsi P, Ozawa T, Takamatsu K. ROS stress resets circadian clocks to coordinate pro-survival signals. PLoS ONE. 2013;8(12):e82006. doi: 10.1371/journal.pone.0082006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miyata Y, Nishida E. CK2 binds, phosphorylates, and regulates its pivotal substrate Cdc37, an Hsp90-cochaperone. Mol Cell Biochem. 2005;274(1–2):171–179. doi: 10.1007/s11010-005-2949-8. [DOI] [PubMed] [Google Scholar]
- 118.Sayed M, Kim SO, Salh BS, Issinger OG, Pelech SL. Stress-induced activation of protein kinase CK2 by direct interaction with p38 mitogen-activated protein kinase. J Biol Chem. 2000;275(22):16569–16573. doi: 10.1074/jbc.M000312200. [DOI] [PubMed] [Google Scholar]
- 119.Watabe M, Nakaki T. Protein kinase CK2 regulates the formation and clearance of aggresomes in response to stress. J Cell Sci. 2011;124(Pt 9):1519–1532. doi: 10.1242/jcs.081778. [DOI] [PubMed] [Google Scholar]
- 120.Olsen BB, Svenstrup TH, Guerra B. Downregulation of protein kinase CK2 induces autophagic cell death through modulation of the mTOR and MAPK signaling pathways in human glioblastoma cells. Int J Oncol. 2012;41(6):1967–1976. doi: 10.3892/ijo.2012.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bandyopadhyay K, Li P, Gjerset RA. CK2-mediated hyperphosphorylation of topoisomerase I targets serine 506, enhances topoisomerase I-DNA binding, and increases cellular camptothecin sensitivity. PLoS ONE. 2012;7(11):e50427. doi: 10.1371/journal.pone.0050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Escargueil AE, Plisov SY, Filhol O, Cochet C, Larsen AK. Mitotic phosphorylation of DNA topoisomerase II alpha by protein kinase CK2 creates the MPM-2 phosphoepitope on Ser-1469. J Biol Chem. 2000;275(44):34710–34718. doi: 10.1074/jbc.M005179200. [DOI] [PubMed] [Google Scholar]
- 123.Grecu D, Assairi L. CK2 phosphorylation of human centrins 1 and 2 regulates their binding to the DNA repair protein XPC, the centrosomal protein Sfi1 and the phototransduction protein transducin beta. FEBS Open Bio. 2014;4:407–419. doi: 10.1016/j.fob.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guerra B, Iwabuchi K, Issinger OG. Protein kinase CK2 is required for the recruitment of 53BP1 to sites of DNA double-strand break induced by radiomimetic drugs. Cancer Lett. 2014;345(1):115–123. doi: 10.1016/j.canlet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 125.Yata K, Lloyd J, Maslen S, Bleuyard JY, Skehel M, Smerdon SJ, Esashi F. Plk1 and CK2 act in concert to regulate Rad51 during DNA double strand break repair. Mol Cell. 2012;45(3):371–383. doi: 10.1016/j.molcel.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ohashi E, Takeishi Y, Ueda S, Tsurimoto T. Interaction between Rad9-Hus1-Rad1 and TopBP1 activates ATR-ATRIP and promotes TopBP1 recruitment to sites of UV-damage. DNA Repair. 2014;21:1–11. doi: 10.1016/j.dnarep.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 127.Takeishi Y, Ohashi E, Ogawa K, Masai H, Obuse C, Tsurimoto T. Casein kinase 2-dependent phosphorylation of human Rad9 mediates the interaction between human Rad9-Hus1-Rad1 complex and TopBP1. Genes Cells. 2010;15(7):761–771. doi: 10.1111/j.1365-2443.2010.01418.x. [DOI] [PubMed] [Google Scholar]
- 128.Zhao T, Jia H, Li L, Zhang G, Zhao M, Cheng Q, Zheng J, Li D. Inhibition of CK2 enhances UV-triggered apoptotic cell death in lung cancer cell lines. Oncol Rep. 2013;30(1):377–384. doi: 10.3892/or.2013.2407. [DOI] [PubMed] [Google Scholar]
- 129.Krohn NM, Stemmer C, Fojan P, Grimm R, Grasser KD. Protein kinase CK2 phosphorylates the high mobility group domain protein SSRP1, inducing the recognition of UV-damaged DNA. J Biol Chem. 2003;278(15):12710–12715. doi: 10.1074/jbc.M300250200. [DOI] [PubMed] [Google Scholar]
- 130.Loizou JI, El-Khamisy SF, Zlatanou A, Moore DJ, Chan DW, Qin J, Sarno S, Meggio F, Pinna LA, Caldecott KW. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell. 2004;117(1):17–28. doi: 10.1016/s0092-8674(04)00206-5. [DOI] [PubMed] [Google Scholar]
- 131.Luo H, Chan DW, Yang T, Rodriguez M, Chen BP, Leng M, Mu JJ, Chen D, Songyang Z, Wang Y, Qin J. A new XRCC1-containing complex and its role in cellular survival of methyl methanesulfonate treatment. Mol Cell Biol. 2004;24(19):8356–8365. doi: 10.1128/MCB.24.19.8356-8365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Strom CE, Mortusewicz O, Finch D, Parsons JL, Lagerqvist A, Johansson F, Schultz N, Erixon K, Dianov GL, Helleday T. CK2 phosphorylation of XRCC1 facilitates dissociation from DNA and single-strand break formation during base excision repair. DNA Repair. 2011;10(9):961–969. doi: 10.1016/j.dnarep.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 133.Bekker-Jensen S, Fugger K, Danielsen JR, Gromova I, Sehested M, Celis J, Bartek J, Lukas J, Mailand N. Human Xip1 (C2orf13) is a novel regulator of cellular responses to DNA strand breaks. J Biol Chem. 2007;282(27):19638–19643. doi: 10.1074/jbc.C700060200. [DOI] [PubMed] [Google Scholar]
- 134.Clements PM, Breslin C, Deeks ED, Byrd PJ, Ju L, Bieganowski P, Brenner C, Moreira MC, Taylor AM, Caldecott KW. The ataxia-oculomotor apraxia 1 gene product has a role distinct from ATM and interacts with the DNA strand break repair proteins XRCC1 and XRCC4. DNA Repair. 2004;3(11):1493–1502. doi: 10.1016/j.dnarep.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 135.Ayoub N, Jeyasekharan AD, Bernal JA, Venkitaraman AR. HP1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature. 2008;453(7195):682–686. doi: 10.1038/nature06875. [DOI] [PubMed] [Google Scholar]
- 136.Ayoub N, Jeyasekharan AD, Bernal JA, Venkitaraman AR. Paving the way for H2AX phosphorylation: chromatin changes in the DNA damage response. Cell Cycle. 2009;8(10):1494–1500. doi: 10.4161/cc.8.10.8501. [DOI] [PubMed] [Google Scholar]
- 137.Ayoub N, Jeyasekharan AD, Venkitaraman AR. Mobilization and recruitment of HP1: a bimodal response to DNA breakage. Cell Cycle. 2009;8(18):2945–2950. [PubMed] [Google Scholar]
- 138.Chapman JR, Jackson SP. Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. EMBO Rep. 2008;9(8):795–801. doi: 10.1038/embor.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu L, Luo K, Lou Z, Chen J. MDC1 regulates intra-S-phase checkpoint by targeting NBS1 to DNA double-strand breaks. Proc Natl Acad Sci USA. 2008;105(32):11200–11205. doi: 10.1073/pnas.0802885105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Spycher C, Miller ES, Townsend K, Pavic L, Morrice NA, Janscak P, Stewart GS, Stucki M. Constitutive phosphorylation of MDC1 physically links the MRE11-RAD50-NBS1 complex to damaged chromatin. J Cell Biol. 2008;181(2):227–240. doi: 10.1083/jcb.200709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Melander F, Bekker-Jensen S, Falck J, Bartek J, Mailand N, Lukas J. Phosphorylation of SDT repeats in the MDC1 N terminus triggers retention of NBS1 at the DNA damage-modified chromatin. J Cell Biol. 2008;181(2):213–226. doi: 10.1083/jcb.200708210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Filhol O, Cochet C. Protein kinase CK2 in health and disease: cellular functions of protein kinase CK2: a dynamic affair. Cell Mol Life Sci. 2009;66(11–12):1830–1839. doi: 10.1007/s00018-009-9151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tapia JC, Torres VA, Rodriguez DA, Leyton L, Quest AF. Casein kinase 2 (CK2) increases survivin expression via enhanced beta-catenin-T cell factor/lymphoid enhancer binding factor-dependent transcription. Proc Natl Acad Sci USA. 2006;103(41):15079–15084. doi: 10.1073/pnas.0606845103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barrett RM, Colnaghi R, Wheatley SP. Threonine 48 in the BIR domain of survivin is critical to its mitotic and anti-apoptotic activities and can be phosphorylated by CK2 in vitro. Cell Cycle. 2011;10(3):538–548. doi: 10.4161/cc.10.3.14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ponce DP, Maturana JL, Cabello P, Yefi R, Niechi I, Silva E, Armisen R, Galindo M, Antonelli M, Tapia JC. Phosphorylation of AKT/PKB by CK2 is necessary for the AKT-dependent up-regulation of beta-catenin transcriptional activity. J Cell Physiol. 2011;226(7):1953–1959. doi: 10.1002/jcp.22527. [DOI] [PubMed] [Google Scholar]
- 146.Ponce DP, Yefi R, Cabello P, Maturana JL, Niechi I, Silva E, Galindo M, Antonelli M, Marcelain K, Armisen R, Tapia JC. CK2 functionally interacts with AKT/PKB to promote the beta-catenin-dependent expression of survivin and enhance cell survival. Mol Cell Biochem. 2011;356(1–2):127–132. doi: 10.1007/s11010-011-0965-4. [DOI] [PubMed] [Google Scholar]
- 147.Olsen BB, Boldyreff B, Niefind K, Issinger OG. Purification and characterization of the CK2alpha’-based holoenzyme, an isozyme of CK2alpha: a comparative analysis. Protein Expr Purif. 2006;47(2):651–661. doi: 10.1016/j.pep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 148.Hellwig CT, Ludwig-Galezowska AH, Concannon CG, Litchfield DW, Prehn JH, Rehm M. Activity of protein kinase CK2 uncouples Bid cleavage from caspase-8 activation. J Cell Sci. 2010;123(Pt 9):1401–1406. doi: 10.1242/jcs.061143. [DOI] [PubMed] [Google Scholar]
- 149.Izeradjene K, Douglas L, Delaney A, Houghton JA. Influence of casein kinase II in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human rhabdomyosarcoma cells. Clin Cancer Res. 2004;10(19):6650–6660. doi: 10.1158/1078-0432.CCR-04-0576. [DOI] [PubMed] [Google Scholar]
- 150.Izeradjene K, Douglas L, Delaney A, Houghton JA. Casein kinase II (CK2) enhances death-inducing signaling complex (DISC) activity in TRAIL-induced apoptosis in human colon carcinoma cell lines. Oncogene. 2005;24(12):2050–2058. doi: 10.1038/sj.onc.1208397. [DOI] [PubMed] [Google Scholar]
- 151.Ravi R, Bedi A. Sensitization of tumor cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of casein kinase II. Cancer Res. 2002;62(15):4180–4185. [PubMed] [Google Scholar]
- 152.Wang G, Ahmad KA, Ahmed K. Role of protein kinase CK2 in the regulation of tumor necrosis factor-related apoptosis inducing ligand-induced apoptosis in prostate cancer cells. Cancer Res. 2006;66(4):2242–2249. doi: 10.1158/0008-5472.CAN-05-2772. [DOI] [PubMed] [Google Scholar]
- 153.Li PF, Li J, Muller EC, Otto A, Dietz R, von Harsdorf R. Phosphorylation by protein kinase CK2: a signaling switch for the caspase-inhibiting protein ARC. Mol Cell. 2002;10(2):247–258. doi: 10.1016/s1097-2765(02)00600-7. [DOI] [PubMed] [Google Scholar]
- 154.Duncan JS, Turowec JP, Duncan KE, Vilk G, Wu C, Luscher B, Li SS, Gloor GB, Litchfield DW. A peptide-based target screen implicates the protein kinase CK2 in the global regulation of caspase signaling. Sci Signal. 2011;4(172):ra30. doi: 10.1126/scisignal.2001682. [DOI] [PubMed] [Google Scholar]
- 155.Duncan JS, Turowec JP, Vilk G, Li SS, Gloor GB. Litchfield DW (2010) Regulation of cell proliferation and survival: convergence of protein kinases and caspases. Biochim Biophys Acta. 1804;3:505–510. doi: 10.1016/j.bbapap.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 156.Filhol O, Cochet C. Protein kinases curb cell death. Sci Signal. 2011;4(172):pe26. doi: 10.1126/scisignal.2001921. [DOI] [PubMed] [Google Scholar]
- 157.Turowec JP, Vilk G, Gabriel M, Litchfield DW. Characterizing the convergence of protein kinase CK2 and caspase-3 reveals isoform-specific phosphorylation of caspase-3 by CK2alpha’: implications for pathological roles of CK2 in promoting cancer cell survival. Oncotarget. 2013;4(4):560–571. doi: 10.18632/oncotarget.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Di Maira G, Brustolon F, Bertacchini J, Tosoni K, Marmiroli S, Pinna LA, Ruzzene M. Pharmacological inhibition of protein kinase CK2 reverts the multidrug resistance phenotype of a CEM cell line characterized by high CK2 level. Oncogene. 2007;26(48):6915–6926. doi: 10.1038/sj.onc.1210495. [DOI] [PubMed] [Google Scholar]
- 159.Kreutzer JN, Ruzzene M, Guerra B. Enhancing chemosensitivity to gemcitabine via RNA interference targeting the catalytic subunits of protein kinase CK2 in human pancreatic cancer cells. BMC Cancer. 2010;10:440. doi: 10.1186/1471-2407-10-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zanin S, Borgo C, Girardi C, O’Brien SE, Miyata Y, Pinna LA, Donella-Deana A, Ruzzene M. Effects of the CK2 inhibitors CX-4945 and CX-5011 on drug-resistant cells. PLoS ONE. 2012;7(11):e49193. doi: 10.1371/journal.pone.0049193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Heiker JT, Wottawah CM, Juhl C, Kosel D, Morl K, Beck-Sickinger AG. Protein kinase CK2 interacts with adiponectin receptor 1 and participates in adiponectin signaling. Cell Signal. 2009;21(6):936–942. doi: 10.1016/j.cellsig.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 162.An S, Kyoung M, Allen JJ, Shokat KM, Benkovic SJ. Dynamic regulation of a metabolic multi-enzyme complex by protein kinase CK2. J Biol Chem. 2010;285(15):11093–11099. doi: 10.1074/jbc.M110.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yanagawa T, Funasaka T, Tsutsumi S, Raz T, Tanaka N, Raz A. Differential regulation of phosphoglucose isomerase/autocrine motility factor activities by protein kinase CK2 phosphorylation. J Biol Chem. 2005;280(11):10419–10426. doi: 10.1074/jbc.M409457200. [DOI] [PubMed] [Google Scholar]
- 164.AlQuobaili F, Montenarh M. CK2 and the regulation of the carbohydrate metabolism. Metabolism. 2012;61(11):1512–1517. doi: 10.1016/j.metabol.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 165.Taylor KM, Hiscox S, Nicholson RI, Hogstrand C, Kille P. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci Signal. 2012;5(210):11. doi: 10.1126/scisignal.2002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taylor KM, Kille P, Hogstrand C. Protein kinase CK2 opens the gate for zinc signaling. Cell Cycle. 2012;11(10):1863–1864. doi: 10.4161/cc.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim J, Kim SH. Druggability of the CK2 inhibitor CX-4945 as an anticancer drug and beyond. Arch Pharmacal Res. 2012;35(8):1293–1296. doi: 10.1007/s12272-012-0800-9. [DOI] [PubMed] [Google Scholar]
- 168.Siddiqui-Jain A, Drygin D, Streiner N, Chua P, Pierre F, O’Brien SE, Bliesath J, Omori M, Huser N, Ho C, Proffitt C, Schwaebe MK, Ryckman DM, Rice WG, Anderes K. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010;70(24):10288–10298. doi: 10.1158/0008-5472.CAN-10-1893. [DOI] [PubMed] [Google Scholar]
- 169.Pierre F, Chua PC, O’Brien SE, Siddiqui-Jain A, Bourbon P, Haddach M, Michaux J, Nagasawa J, Schwaebe MK, Stefan E, Vialettes A, Whitten JP, Chen TK, Darjania L, Stansfield R, Bliesath J, Drygin D, Ho C, Omori M, Proffitt C, Streiner N, Rice WG, Ryckman DM, Anderes K. Pre-clinical characterization of CX-4945, a potent and selective small molecule inhibitor of CK2 for the treatment of cancer. Mol Cell Biochem. 2011;356(1–2):37–43. doi: 10.1007/s11010-011-0956-5. [DOI] [PubMed] [Google Scholar]
- 170.Siddiqui-Jain A, Bliesath J, Macalino D, Omori M, Huser N, Streiner N, Ho CB, Anderes K, Proffitt C, O’Brien SE, Lim JK, Von Hoff DD, Ryckman DM, Rice WG, Drygin D. CK2 inhibitor CX-4945 suppresses DNA repair response triggered by DNA-targeted anticancer drugs and augments efficacy: mechanistic rationale for drug combination therapy. Mol Cancer Ther. 2012;11(4):994–1005. doi: 10.1158/1535-7163.MCT-11-0613. [DOI] [PubMed] [Google Scholar]
- 171.Lolli G, Ranchio A, Battistutta R. Active form of the protein kinase CK2 alpha2beta2 holoenzyme is a strong complex with symmetric architecture. ACS Chem Biol. 2014;9(2):366–371. doi: 10.1021/cb400771y. [DOI] [PubMed] [Google Scholar]
- 172.Olsen BB, Wang SY, Svenstrup TH, Chen BP, Guerra B. Protein kinase CK2 localizes to sites of DNA double-strand break regulating the cellular response to DNA damage. BMC Mol Biol. 2012;13:7. doi: 10.1186/1471-2199-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.St-Denis NA, Bailey ML, Parker EL, Vilk G, Litchfield DW. Localization of phosphorylated CK2alpha to the mitotic spindle requires the peptidyl-prolyl isomerase Pin1. J Cell Sci. 2011;124(Pt 14):2341–2348. doi: 10.1242/jcs.077446. [DOI] [PubMed] [Google Scholar]
- 174.Filhol O, Martiel JL, Cochet C. Protein kinase CK2: a new view of an old molecular complex. EMBO Rep. 2004;5(4):351–355. doi: 10.1038/sj.embor.7400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bibby AC, Litchfield DW. The multiple personalities of the regulatory subunit of protein kinase CK2: CK2 dependent and CK2 independent roles reveal a secret identity for CK2beta. Int J Biol Sci. 2005;1(2):67–79. doi: 10.7150/ijbs.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bolanos-Garcia VM, Fernandez-Recio J, Allende JE, Blundell TL. Identifying interaction motifs in CK2beta–a ubiquitous kinase regulatory subunit. Trends Biochem Sci. 2006;31(12):654–661. doi: 10.1016/j.tibs.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 177.Stigare J, Buddelmeijer N, Pigon A, Egyhazi E. A majority of casein kinase II alpha subunit is tightly bound to intranuclear components but not to the beta subunit. Mol Cell Biochem. 1993;129(1):77–85. doi: 10.1007/BF00926578. [DOI] [PubMed] [Google Scholar]
- 178.Guerra B, Siemer S, Boldyreff B, Issinger OG. Protein kinase CK2: evidence for a protein kinase CK2beta subunit fraction, devoid of the catalytic CK2alpha subunit, in mouse brain and testicles. FEBS Lett. 1999;462(3):353–357. doi: 10.1016/s0014-5793(99)01553-7. [DOI] [PubMed] [Google Scholar]
- 179.Stalter G, Siemer S, Becht E, Ziegler M, Remberger K, Issinger OG. Asymmetric expression of protein kinase CK2 subunits in human kidney tumors. Biochem Biophys Res Commun. 1994;202(1):141–147. doi: 10.1006/bbrc.1994.1904. [DOI] [PubMed] [Google Scholar]
- 180.Pinna LA, Meggio F. Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog Cell Cycle Res. 1997;3:77–97. doi: 10.1007/978-1-4615-5371-7_7. [DOI] [PubMed] [Google Scholar]
- 181.Guerra B, Issinger OG, Wang JY. Modulation of human checkpoint kinase Chk1 by the regulatory beta-subunit of protein kinase CK2. Oncogene. 2003;22(32):4933–4942. doi: 10.1038/sj.onc.1206721. [DOI] [PubMed] [Google Scholar]
- 182.Olsen BB, Kreutzer JN, Watanabe N, Holm T, Guerra B. Mapping of the interaction sites between Wee1 kinase and the regulatory beta-subunit of protein kinase CK2. Int J Oncol. 2010;36(5):1175–1182. doi: 10.3892/ijo_00000600. [DOI] [PubMed] [Google Scholar]
- 183.Gerber DA, Souquere-Besse S, Puvion F, Dubois MF, Bensaude O, Cochet C. Heat-induced relocalization of protein kinase CK2. Implication of CK2 in the context of cellular stress. J Biol Chem. 2000;275(31):23919–23926. doi: 10.1074/jbc.M002697200. [DOI] [PubMed] [Google Scholar]
- 184.Yamane K, Kinsella TJ. CK2 inhibits apoptosis and changes its cellular localization following ionizing radiation. Cancer Res. 2005;65(10):4362–4367. doi: 10.1158/0008-5472.CAN-04-3941. [DOI] [PubMed] [Google Scholar]
- 185.Souquere-Besse S, Pichard E, Filhol O, Legrand V, Rosa-Calatrava M, Hovanessian AG, Cochet C, Puvion-Dutilleul F. Adenovirus infection targets the cellular protein kinase CK2 and RNA-activated protein kinase (PKR) into viral inclusions of the cell nucleus. Microsc Res Tech. 2002;56(6):465–478. doi: 10.1002/jemt.10060. [DOI] [PubMed] [Google Scholar]
- 186.Wadd S, Bryant H, Filhol O, Scott JE, Hsieh TY, Everett RD, Clements JB. The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. J Biol Chem. 1999;274(41):28991–28998. doi: 10.1074/jbc.274.41.28991. [DOI] [PubMed] [Google Scholar]
- 187.Koffa MD, Kean J, Zachos G, Rice SA, Clements JB. CK2 protein kinase is stimulated and redistributed by functional herpes simplex virus ICP27 protein. J Virol. 2003;77(7):4315–4325. doi: 10.1128/JVI.77.7.4315-4325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Medina-Palazon C, Gruffat H, Mure F, Filhol O, Vingtdeux-Didier V, Drobecq H, Cochet C, Sergeant N, Sergeant A, Manet E. Protein kinase CK2 phosphorylation of EB2 regulates its function in the production of Epstein-Barr virus infectious viral particles. J Virol. 2007;81(21):11850–11860. doi: 10.1128/JVI.01421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Laramas M, Pasquier D, Filhol O, Ringeisen F, Descotes JL, Cochet C. Nuclear localization of protein kinase CK2 catalytic subunit (CK2alpha) is associated with poor prognostic factors in human prostate cancer. Eur J Cancer. 2007;43(5):928–934. doi: 10.1016/j.ejca.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 190.Lin KY, Tai C, Hsu JC, Li CF, Fang CL, Lai HC, Hseu YC, Lin YF, Uen YH. Overexpression of nuclear protein kinase CK2 alpha catalytic subunit (CK2alpha) as a poor prognosticator in human colorectal cancer. PLoS ONE. 2011;6(2):e17193. doi: 10.1371/journal.pone.0017193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bachhuber T, Almaca J, Aldehni F, Mehta A, Amaral MD, Schreiber R, Kunzelmann K. Regulation of the epithelial Na+ channel by the protein kinase CK2. J Biol Chem. 2008;283(19):13225–13232. doi: 10.1074/jbc.M704532200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brechet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol. 2008;183(6):1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pagano MA, Arrigoni G, Marin O, Sarno S, Meggio F, Treharne KJ, Mehta A, Pinna LA. Modulation of protein kinase CK2 activity by fragments of CFTR encompassing F508 may reflect functional links with cystic fibrosis pathogenesis. Biochemistry. 2008;47(30):7925–7936. doi: 10.1021/bi800316z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang Y, Guo W, Liang L, Esselman WJ. Phosphorylation of CD45 by casein kinase 2. Modulation of activity and mutational analysis. J Biol Chem. 1999;274(11):7454–7461. doi: 10.1074/jbc.274.11.7454. [DOI] [PubMed] [Google Scholar]
- 195.Greer SF, Wang Y, Raman C, Justement LB. CD45 function is regulated by an acidic 19-amino acid insert in domain II that serves as a binding and phosphoacceptor site for casein kinase 2. J Immunol. 2001;166(12):7208–7218. doi: 10.4049/jimmunol.166.12.7208. [DOI] [PubMed] [Google Scholar]
- 196.Axtell RC, Xu L, Barnum SR, Raman C. CD5-CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J Immunol. 2006;177(12):8542–8549. doi: 10.4049/jimmunol.177.12.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Meggio F, Boldyreff B, Marin O, Pinna LA, Issinger OG. Role of the beta subunit of casein kinase-2 on the stability and specificity of the recombinant reconstituted holoenzyme. Eur J Biochem. 1992;204(1):293–297. doi: 10.1111/j.1432-1033.1992.tb16636.x. [DOI] [PubMed] [Google Scholar]
- 198.Pinna LA. Protein kinase CK2: a challenge to canons. J Cell Sci. 2002;115(Pt 20):3873–3878. doi: 10.1242/jcs.00074. [DOI] [PubMed] [Google Scholar]
- 199.Maizel A, Tassetto M, Filhol O, Cochet C, Prochiantz A, Joliot A. Engrailed homeoprotein secretion is a regulated process. Development. 2002;129(15):3545–3553. doi: 10.1242/dev.129.15.3545. [DOI] [PubMed] [Google Scholar]
- 200.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369(Pt 1):1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huillard E, Ziercher L, Blond O, Wong M, Deloulme JC, Souchelnytskyi S, Baudier J, Cochet C, Buchou T. Disruption of CK2beta in embryonic neural stem cells compromises proliferation and oligodendrogenesis in the mouse telencephalon. Mol Cell Biol. 2010;30(11):2737–2749. doi: 10.1128/MCB.01566-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Donella-Deana A, Cesaro L, Sarno S, Ruzzene M, Brunati AM, Marin O, Vilk G, Doherty-Kirby A, Lajoie G, Litchfield DW, Pinna LA. Tyrosine phosphorylation of protein kinase CK2 by Src-related tyrosine kinases correlates with increased catalytic activity. Biochem J. 2003;372(Pt 3):841–849. doi: 10.1042/BJ20021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Landesman-Bollag E, Song DH, Romieu-Mourez R, Sussman DJ, Cardiff RD, Sonenshein GE, Seldin DC. Protein kinase CK2: signaling and tumorigenesis in the mammary gland Protein kinase CK2 in mammary gland tumorigenesis. Mol Cell Biochem. 2001;227(1–2):153–165. [PubMed] [Google Scholar]
- 204.Belguise K, Guo S, Yang S, Rogers AE, Seldin DC, Sherr DH, Sonenshein GE. Green tea polyphenols reverse cooperation between c-Rel and CK2 that induces the aryl hydrocarbon receptor, slug, and an invasive phenotype. Cancer Res. 2007;67(24):11742–11750. doi: 10.1158/0008-5472.CAN-07-2730. [DOI] [PubMed] [Google Scholar]
- 205.Channavajhala P, Seldin DC. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene. 2002;21(34):5280–5288. doi: 10.1038/sj.onc.1205640. [DOI] [PubMed] [Google Scholar]
- 206.Kelliher MA, Seldin DC, Leder P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIalpha. EMBO J. 1996;15(19):5160–5166. [PMC free article] [PubMed] [Google Scholar]
- 207.Scheel C, Weinberg RA. Cancer stem cells and epithelial–mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22(5–6):396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ansieau S. EMT in breast cancer stem cell generation. Cancer Lett. 2013;338(1):63–68. doi: 10.1016/j.canlet.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 209.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16(6):488–494. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- 211.Canton DA, Litchfield DW. The shape of things to come: an emerging role for protein kinase CK2 in the regulation of cell morphology and the cytoskeleton. Cell Signal. 2006;18(3):267–275. doi: 10.1016/j.cellsig.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 212.Deshiere A, Theis-Febvre N, Martel V, Cochet C, Filhol O. Protein kinase CK2 and cell polarity. Mol Cell Biochem. 2008;316(1–2):107–113. doi: 10.1007/s11010-008-9845-y. [DOI] [PubMed] [Google Scholar]
- 213.Filhol O, Deshiere A, Cochet C. Role of CK2 in the control of cell plasticity in breast carcinoma progression, vol Protein kinase CK2 Protein kinase CK2. New York: John Wiley & sons; 2013. [Google Scholar]
- 214.Galovic M, Xu D, Areces LB, van der Kammen R, Innocenti M. Interplay between N-WASP and CK2 optimizes clathrin-mediated endocytosis of EGFR. J Cell Sci. 2011;124(Pt 12):2001–2012. doi: 10.1242/jcs.081182. [DOI] [PubMed] [Google Scholar]
- 215.Ulloa L, Diaz-Nido J, Avila J. Depletion of casein kinase II by antisense oligonucleotide prevents neuritogenesis in neuroblastoma cells. EMBO J. 1993;12(4):1633–1640. doi: 10.1002/j.1460-2075.1993.tb05808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 217.Nieto MA, Cano A. The epithelial–mesenchymal transition under control: global programs to regulate epithelial plasticity. Semin Cancer Biol. 2012;22(5–6):361–368. doi: 10.1016/j.semcancer.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 218.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial–mesenchymal transition. Sci Signal. 2014;7(344):re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 220.Moustakas A, Heldin CH. Signaling networks guiding epithelial–mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98(10):1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yang J, Weinberg RA. Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 222.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 223.Foubert E, De Craene B, Berx G. Key signalling nodes in mammary gland development and cancer. The Snail1-Twist1 conspiracy in malignant breast cancer progression. Breast Cancer Res. 2010;12(3):206. doi: 10.1186/bcr2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2(2):84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 225.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 226.Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62(6):1613–1618. [PubMed] [Google Scholar]
- 227.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 228.Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24(14):2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 229.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7(6):1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 230.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA. 2007;104(24):10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA. Metastatic colonization requires the repression of the epithelial–mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22(6):709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 232.Olmeda D, Montes A, Moreno-Bueno G, Flores JM, Portillo F, Cano A. Snai1 and Snai2 collaborate on tumor growth and metastasis properties of mouse skin carcinoma cell lines. Oncogene. 2008;27(34):4690–4701. doi: 10.1038/onc.2008.118. [DOI] [PubMed] [Google Scholar]
- 233.Dave N, Guaita-Esteruelas S, Gutarra S, Frias A, Beltran M, Peiro S, de Herreros AG. Functional cooperation between Snail1 and twist in the regulation of ZEB1 expression during epithelial to mesenchymal transition. J Biol Chem. 2011;286(14):12024–12032. doi: 10.1074/jbc.M110.168625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tran DD, Corsa CA, Biswas H, Aft RL, Longmore GD. Temporal and spatial cooperation of Snail1 and Twist1 during epithelial–mesenchymal transition predicts for human breast cancer recurrence. Mol Cancer Res. 2011;9(12):1644–1657. doi: 10.1158/1541-7786.MCR-11-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342(6159):1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 236.Cheng Q, Chang JT, Gwin WR, Zhu J, Ambs S, Geradts J, Lyerly HK. A signature of epithelial–mesenchymal plasticity and stromal activation in primary tumor modulates late recurrence in breast cancer independent of disease subtype. Breast Cancer Res. 2014;16(4):407. doi: 10.1186/s13058-014-0407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial–mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68(4):989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 238.Vinas-Castells R, Beltran M, Valls G, Gomez I, Garcia JM, Montserrat-Sentis B, Baulida J, Bonilla F, de Herreros AG, Diaz VM. The hypoxia-controlled FBXL14 ubiquitin ligase targets SNAIL1 for proteasome degradation. J Biol Chem. 2010;285(6):3794–3805. doi: 10.1074/jbc.M109.065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lim S, Becker A, Zimmer A, Lu J, Buettner R, Kirfel J. SNAI1-mediated epithelial–mesenchymal transition confers chemoresistance and cellular plasticity by regulating genes involved in cell death and stem cell maintenance. PLoS ONE. 2013;8(6):e66558. doi: 10.1371/journal.pone.0066558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tam WL, Weinberg RA. The epigenetics of epithelial–mesenchymal plasticity in cancer. Nat Med. 2013;19(11):1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278(23):21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 242.Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem. 2008;283(48):33437–33446. doi: 10.1074/jbc.M802016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial–mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18(1):99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105(17):6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhou BP, Hung MC. Wnt, hedgehog and snail: sister pathways that control by GSK-3beta and beta-Trcp in the regulation of metastasis. Cell Cycle. 2005;4(6):772–776. doi: 10.4161/cc.4.6.1744. [DOI] [PubMed] [Google Scholar]
- 246.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436(7047):123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, Konishi I. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;163(4):1437–1447. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial–mesenchymal transition in hypoxic breast cancer cells. J Cell Biol. 2007;178(3):425–436. doi: 10.1083/jcb.200701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1(6–7):303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail’s subcellular localization and functions. Cancer Res. 2005;65(8):3179–3184. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- 251.Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial–mesenchymal transition. J cell Biol. 2005;168(1):29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat Cell Biol. 2004;6(10):931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 253.Birkenkamp KU, Coffer PJ. Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem Soc Trans. 2003;31(Pt 1):292–297. doi: 10.1042/bst0310292. [DOI] [PubMed] [Google Scholar]
- 254.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27(16):2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 255.Obsil T, Obsilova V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene. 2008;27(16):2263–2275. doi: 10.1038/onc.2008.20. [DOI] [PubMed] [Google Scholar]
- 256.Hader C, Marlier A, Cantley L. Mesenchymal–epithelial transition in epithelial response to injury: the role of Foxc2. Oncogene. 2010;29(7):1031–1040. doi: 10.1038/onc.2009.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ford HL, Kabingu EN, Bump EA, Mutter GL, Pardee AB. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc Natl Acad Sci USA. 1998;95(21):12608–12613. doi: 10.1073/pnas.95.21.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10(2):175–181. doi: 10.1038/nm966. [DOI] [PubMed] [Google Scholar]
- 259.Behbakht K, Qamar L, Aldridge CS, Coletta RD, Davidson SA, Thorburn A, Ford HL. Six1 overexpression in ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and is associated with poor survival. Cancer Res. 2007;67(7):3036–3042. doi: 10.1158/0008-5472.CAN-06-3755. [DOI] [PubMed] [Google Scholar]
- 260.Coletta RD, Christensen KL, Micalizzi DS, Jedlicka P, Varella-Garcia M, Ford HL. Six1 overexpression in mammary cells induces genomic instability and is sufficient for malignant transformation. Cancer Res. 2008;68(7):2204–2213. doi: 10.1158/0008-5472.CAN-07-3141. [DOI] [PubMed] [Google Scholar]
- 261.McCoy EL, Iwanaga R, Jedlicka P, Abbey NS, Chodosh LA, Heichman KA, Welm AL, Ford HL. Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial–mesenchymal transition. J Clin Invest. 2009;119(9):2663–2677. doi: 10.1172/JCI37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Micalizzi DS, Christensen KL, Jedlicka P, Coletta RD, Baron AE, Harrell JC, Horwitz KB, Billheimer D, Heichman KA, Welm AL, Schiemann WP, Ford HL. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial–mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. J Clin Invest. 2009;119(9):2678–2690. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial–mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15(2):117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.McCoy EL, Kawakami K, Ford HL, Coletta RD. Expression of Six1 homeobox gene during development of the mouse submandibular salivary gland. Oral Dis. 2009;15(6):407–413. doi: 10.1111/j.1601-0825.2009.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lupp S, Gumhold C, Ampofo E, Montenarh M, Rother K. CK2 kinase activity but not its binding to CK2 promoter regions is implicated in the regulation of CK2alpha and CK2beta gene expressions. Mol Cell Biochem. 2013;384(1–2):71–82. doi: 10.1007/s11010-013-1782-8. [DOI] [PubMed] [Google Scholar]
- 266.Feliciano A, Castellvi J, Artero-Castro A, Leal JA, Romagosa C, Hernandez-Losa J, Peg V, Fabra A, Vidal F, Kondoh H, Ramon YCS, Lleonart ME. miR-125b acts as a tumor suppressor in breast tumorigenesis via its novel direct targets ENPEP, CK2-alpha, CCNJ, and MEGF9. PLoS ONE. 2013;8(10):e76247. doi: 10.1371/journal.pone.0076247. [DOI] [PMC free article] [PubMed] [Google Scholar]