Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) causes a prolonged active infection followed by a persistent infection in lymphoid tissues lasting for several months. Pigs develop both an antibody and cell-mediated immune response following PRRSV infection, but the specific role of each type in the development of protective immunity and clearance of the virus is not yet known. The aims of this study were to characterize the dynamics of PRRSV persistence from 0 to 135 d post infection (pi), characterize the kinetics of the antibody mediated immune response following PRRSV infection, and characterize the cell mediated immune responses to PRRSV infection. Eighty, 4-month-old PRRSV-free gilts were obtained from a source known to be negative for PRRSV. On day 0, gilts were infected intranasally with 102.4 TCID/ 50 MN 30–100 PRRSV. Following infection, animals were bled between days 0 to 135 pi. Viremia was detected up to day 30. Serum antibody response (by enzyme-linked immunosorbent assay [ELISA] and virus neutralization antibody) was detected from day 14 to 120 pi. Cell-mediated immune response represented by interferon gamma (IFN-γ) was detected from day 14 to 120 pi. Persistence of PRRSV in tissues was confirmed by reverse transcriptase polymerase chain reaction (RT-PCR) between days 30 to 135. These results indicate that serum neutralizing antibodies and IFN-γ play an important role in the clearance of PRRSV. Nevertheless none of the parameters measured (virus neutralizing antibodies), either alone or in combination, are solely responsible for the clearance of the virus from the host and the development of sterilizing immunity.
Résumé
Le virus du syndrome respiratoire et reproducteur porcin (PRRSV) cause une infection active prolongée suivie d’une infection persistante dans les tissus lymphoïdes qui durent plusieurs mois. Suite à l’infection par le PRRSV les porcs développent une réponse immunitaire humorale et à médiation cellulaire, mais leur rôle spécifique dans le développement d’une immunité protectrice et l’élimination du virus n’est pas connu. Les objectifs de l’étude étaient de caractériser la dynamique de la persistance du PRRSV de 0 à 135 j post-infection (PI), caractériser la cinétique de la réponse humorale suite à l’infection par le PRRSV, et caractériser la réponse à médiation cellulaire suite à l’infection par le PRRSV. Un total de 80 cochettes âgées de 4 mois et exemptes de PRRSV ont été obtenues d’un élevage reconnu négatif pour le PRSSV. Au jour 0, les cochettes ont reçu par voie intra-nasale 102,4 TCID/50 de la souche MN 30–100 du PRRSV. Suite à l’infection, des prélèvements de sang furent obtenus des animaux entre les jours 0 et 135. Une virémie a été notée jusqu’au jour 30 pi. Une réponse humorale (détectée à l’aide d’une épreuve ELISA et d’un test de neutralisation virale) a été détectée entre les jours 14 et 120 pi. L’immunité à médiation cellulaire, représentée par l’IFN-γ a également été détectée du jour 14 jusqu’au jour 120 pi. La persistance du PRRSV dans les tissus du jour 30 au jour 135 pi a été confirmée par réaction d’amplification en chaîne par la polymérase à l’aide de la transcriptase inverse (RT-PCR). Ces résultats indiquent que les anticorps sériques neutralisants et l’INF-γ ont un rôle important dans la disparition du PRSSV. Malgré tout, aucun des paramètres mesurés, (anticorps neutralisants), soit seul on en combinaison, n’est responsable à lui seul de l’élimination du virus chez l’hôte et du développement d’une immunité stérilisante.
(Traduit par Docteur Serge Messier)
Introduction
Porcine reproductive and respiratory syndrome (PRRS) constitutes one of the most important diseases that the swine industry faces today (1). Porcine reproductive and respiratory syndrome virus (PRRSV) is an RNA virus classified in the family Arteriviridae, order Nidovirales (2). In order to properly solve PRRS problems in the field, a clear understanding of the kinetics of the virus and the immune response to PRRSV infection in the pig is necessary. Regarding the kinetics of the virus, PRRSV causes a prolonged acute infection in pigs, where the viremic period may last for 4 to 5 wk, followed by a persistent infection in lymphoid tissues lasting several months (3). Persistent infection is defined as “the continued presence of a pathogen in a host beyond the acute symptomatic phase of infection” (4). The persistence of PRRSV involves a continuous low level of viral replication but is not a true steady-state persistent infection (5). Porcine reproductive and respiratory syndrome virus persistence has been detected up to 157 d post infection (pi) in weaned pigs (3). In contrast, PRRSV persistence in adult sows appears to be of a shorter duration and has been reported only up to 42 to 86 d pi. (6). In support of this work, Batista and others (7) reported that PRRSV persistence in breeding age female swine was not detected during the period of 120 to 180 d pi. Furthermore, this study also documented that shedding of the virus from experimentally infected animals was not detected from 90 to 180 d pi.
The immune response following PRRSV infection is also very complex. In contrast to swine influenza virus that elicits inflammatory cytokines and interferon responses in the lung and is rapidly cleared from the host within 1 wk of infection (8), PRRSV infection induces a prolonged active viremia and persistent infection (9–11). Therefore, the immune response to PRRSV in pigs appears to be relatively ineffective in eliminating the virus from the circulatory system and lymphoid tissues during the acute and chronic phases of the disease.
Pigs develop both antibody (AMIR) and cell-mediated (CMIR) immune responses following PRRSV infection, but the specific role of each type in the development of protective immunity and clearance of the virus is not yet known. The immunoglobulin (Ig) M antibodies are detected approximately 5 to 7 d pi and then decline rapidly to undetectable levels after 2 to 3 wk (12). The IgG antibodies are first detected by enzyme-linked immunosorbent assay (ELISA) 7 to 10 d pi, peak at 2 to 4 wk pi, remain constant for months, and then decline to low levels by 300 d pi (13). Antibody responses detected by ELISA and indirect fluorescent antibody test (IFA) do not appear to be protective and, thus, may be directed against the viral nucleocapsid. The IgG antibodies that neutralize viral infectivity and are directed against glycosylated protein (GP)5, GP4, and matrix (M) can be detected as early as 3 wk pi and as long as 604 d pi (14). Recently, Osorio and others (15) demonstrated, using passive transfer, that PRRSV neutralizing antibodies may play a role in protective immunity against the reproductive form of the disease. Following PRRSV clearance, the pig apparently has lifelong immunity against the homologous strain (15).
A comprehensive understanding of the CMIR is not available at this time (16). An antigen-specific CMIR has been described following PRRSV infection with field isolates. T-cell responses are detectable in blood approximately 4 wk pi and are present for extended periods (> 1 y) (17). A measurement of T-cell response to PRRSV infection that has been used extensively is the production of the interferon gamma (IFN-γ). The IFN-γ is a cytokine produced by T-cells and has been associated with protection against viral infections (18–20). The major function of IFN-γ is to regulate expression of molecules involved in antigen processing and presentation, such as proteasomes and the major histocompatibility complex (MHC), which are required for T-cell stimulation and recognition of cytotoxic T-cells (21).
Recently it has been hypothesized that both virus-specific IFN-γ-secreting T-cells and virus neutralizing antibodies are correlates of protective immunity following PRRSV infection. Meier and others (17) reported that virus-specific IFN-γ-producing lymphocytes in the blood of pigs were not detected until 13 wk pi with virulent PRRSV or 8 wk after a live, attenuated booster vaccination. In another study using an Aujeszky’s Disease live, attenuated vaccine, high numbers of IFN-γ-producing lymphocytes were detected within 2 wk after a secondary immunization. In contrast, IFN-γ response was not achieved until 23 wk after a similar immunization protocol using a PRRSV vaccine (22–23).
A key population in the swine breeding herd is the replacement gilt pool. In commercial farms, it is important to promote natural exposure of replacement gilts to PRRSV to induce protective immune response and to mitigate persistent infection. Therefore, groups of young replacement females are deliberately infected to allow sufficient time for viral clearance and to develop a protective immune response. To better understand the dynamics of PRRSV in a large population, we assessed the virological and immunological parameters of PRRSV infection in a large group of breeding age female swine under commercial conditions. The aims of this study were to characterize the dynamics of PRRSV persistence from 0 to 135 d pi, the kinetics of AMIR following PRRSV infection, and the CMIR to PRRSV infection. The ultimate objective was to establish improved control protocols leading to the eradication of PRRSV.
Materials and methods
Source of animals and housing
Eighty, 4-month-old PRRSV-free gilts were obtained from a source known to be negative for PRRSV based on 5 y of diagnostic data and the absence of clinical signs of PRRSV in all phases of production (1,24,25). The gilts were housed at the University of Minnesota Swine Disease Eradication Center research farm in a mechanically ventilated finishing building consisting of 10 pens, each being 10 m by 2.5 m in size with partially slatted floors. Animals were placed 12 per pen and provided 2 m2 space. During the entire study, animals were cared for according to approved guidelines of the University of Minnesota Institutional Animal Care and Use Committee (IACUC).
Experimental infection
Upon arrival, all gilts were individually identified using numbered ear tags. On day 0, gilts were infected intranasally with 5 mL of cell culture fluid containing 102.4 TCID/ 50 of the PRRSV field isolate of MN 30–100 (passage 3) (26). To assess the PRRSV status of the population over the course of the study, a monitor group of 15 index gilts was organized by randomly selecting 1 to 2 animals from each pen. This sample size was sufficient to estimate prevalence when the true expected prevalence was ≤ 10% or ≥ 90% at a 95% confidence with ± 10% accuracy (27). On day 0, 3 8-week-old PRRSV naïve gilts originating from the same source were housed in a separate facility, 30 m from the experimental facility and served as negative controls.
Assessment of clinical signs
Clinical signs (depression, anorexia, fever, respiratory distress) and mortality were observed once a day for the first 7 d pi (26).
Assessment of PRRSV viremia
Following experimental infection, animals were bled on days 0, 3, 7, 21, 30, 50, 70, 90, 100, 110, 120, and 135 pi. Sera were tested for PRRSV nucleic acid by reverse transcriptase polymerase chain reaction (RT-PCR) and for active PRRSV by virus isolation (VI). Specifically, the Taqman PCR (Perkin-Elmer Applied Biosystems, Foster City, California, USA) (28) was used and samples were assessed by VI for active virus using MARC-145 continuous cell lines and porcine alveolar macrophages (29).
Assessment of PRRSV persistence
Ten randomly selected animals were marketed on days 30, 50, 70, 90, 100, 110, 120, and 135 pi. In order to avoid cross contamination, animals were transported in a truck that had been washed, disinfected thoroughly, and had a 24 h period of no contact with other animals prior to transport. Animals were transported directly to a slaughterhouse 200 miles away from the research farm and were housed for 12 h in isolated pens. The experimental animals were always the first to be slaughtered and the ear tag was not removed until the researchers could individually identify both the head and the carcass before processing. Then selected tissues were collected at slaughter and tested for the presence of virus. The samples were always taken by the same investigators, who wore latex gloves and changed them between animals. The sampling equipment was also disinfected between animals. The sample size of 10 gilts per slaughter group could detect at least 1 PRRSV-infected gilt, assuming an estimated prevalence of 20% and a 95% confidence (26). Tonsils, superficial inguinal, and sternal lymph nodes (LN) were collected from each gilt, based on ease of accessing these sites at slaughter. Data from a previous study demonstrated frequent detection of PRRSV nucleic acid by Taqman PCR in these samples (26). Additional lymphoid tissue was also collected (tracheobronchial LN, medial iliac LN, and/or lateral retropharyngeal LN) when they could be clearly identified. Samples were collected during the evisceration process on the kill floor or as carcasses were kept at 4°C. Samples were pooled by individual animal, transported on ice to the University of Minnesota Veterinary Diagnostic Laboratory, and tested for PRRSV by RT-PCR and VI. Prior to pooling, all tissues were confirmed to be of lymphoid origin by microscopic examination and evaluated for the presence of lesions suggestive of PRRSV infection (11,30). The open reading frame 5 (ORF 5) region of representative PRRSV isolates was sequenced in order to determine the homology with the isolate used for the infection (31). Sera from negative controls were tested for the presence of PRRSV antibodies (sample-to-positive ratio < 0.4) by IDEXX ELISA (IDEXX Laboratories Westbrook, Maine, USA) (32).
Antibody and cell-mediated immune response
Animals were bled on days 0, 3, 7, 21, 30, 50, 70, 90, 100, 110, 120, and 135 pi. Sera were tested for the presence of PRRSV antibodies by IDEXX ELISA and serum neutralization test (SN), currently a titer of > 1:4 is considered positive (33).
Blood mononuclear cells (BMC) were analyzed for the presence of IFN-γ-producing cells by enzyme-linked immunospot assay (ELISPOT), as previously described by Zuckermann and others (22), flow cytometry (FC) using a FACS flow cytometer (Becton Dickson FACS Caliber), and using computer software (Cell Quest; BD Bioscience, San Jose, California, USA) (34–36).
Results
Clinical signs
Clinically, 90% of the animals were depressed, anorexic, and pyrexic (40 to 41.5°C) for approximately 48 to 72 h pi. There was no mortality.
Detection of PRRSV and virus-specific antibody
All 15 gilts from the monitor group were PRRSV-negative on arrival, as verified by PCR, VI, and ELISA. Serial testing of the monitor gilts indicated successful experimental infection. On day 3 pi, 15 out of 15 gilts were PCR positive and 0 out of 15 were VI positive (Figure 1). The ORF 5 region of a randomly selected PRRSV isolate from an index pig was 100% homologous with the isolate used for the experimental infection. On day 7 pi, 15 out of 15 gilts in the monitoring group were PCR positive and 8 out of 15 were VI positive; however, all were still ELISA negative. On day 14 pi, 15 out of 15 gilts in the monitoring group were ELISA positive, while 15 out of 15 and 9 out of 15 were PCR and VI positive, respectively. The number of ELISA positive monitoring gilts detected from days 30 to 135 pi were as follows: 15 out of 15 (days 30, 50, 70, 90, 100, and 110 pi) and 12/15 (day 135 pi) (Figure 2). The number of serum neutralizing antibodies positive gilts were 0 out of 15 for days 0 and 7 pi, 8 out of 15 for day 14 pi, 10 out of 15 for day 21, 15 out of 15 for days 30 to 120, and 6 out of 6 for day 135 (Figure 2) with serum neutralizing antibody titers ranging from 1:2 to 1:128 (Figure 2). All sera samples collected on days 30 to 135 were PCR and VI negative except for 1 out of 15 for PCR on day 30.
Figure 1.
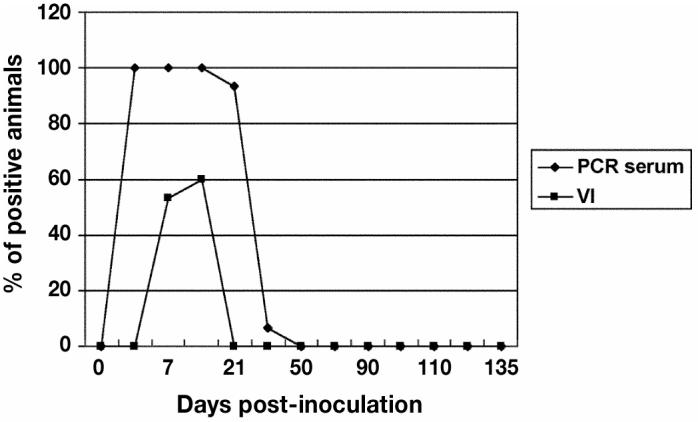
Detection of porcine reproductive and respiratory syndrome virus (PRRSV) in pig sera between days 0 to 135 postinoculation (pi). The polymerase chain reaction (PCR) detected PRRSV in the blood from days 3 to 30 pi and by virus isolation only between days 7 and 15 pi.
Figure 2.
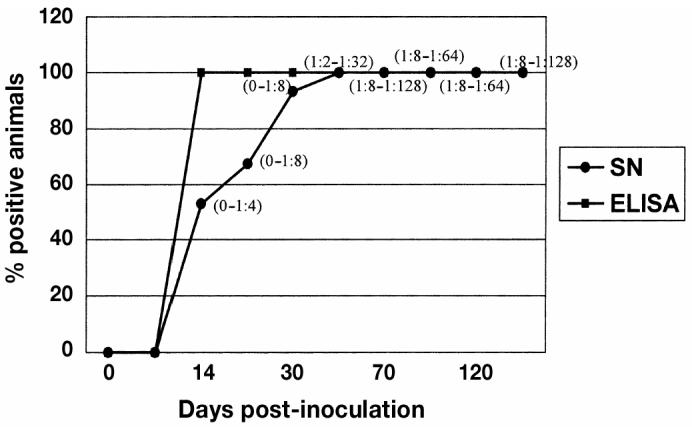
A comparison of the mean of enzyme-linked immunosorbent assay (ELISA) and virus neutralizing antibodies (ranges in parenthesis) response between days 0 and 135 of the study. Antibodies were detected in 100% of the experimental animals from day 14 to 135 postinoculation (pi). In comparison, virus neutralizing antibodies were detected in the experimental animals on day 14 but were not present in 100% of the experimental animals until day 50 pi.
Porcine reproductive and respiratory syndrome virus persistence
On days 30 to 100 pi, all tissue pools were positive for PRRSV with 1 exception at day 90 (Figure 3). On day 110, 120, and 135 pi, 80%, 30%, and 20% of the tissue pools, respectively, were positive for PRRSV. Histological lesions typical of PRRSV infection characterized by germinal center hypertrophy and hyperplasia, germinal center necrosis, and cystic spaces with polykaryocytes were observed in lymphoid tissue in samples collected at all sampling times (30). All samples were negative for VI with the exception of the samples from day 30. The negative control gilts remained ELISA and VI negative throughout the study.
Figure 3.
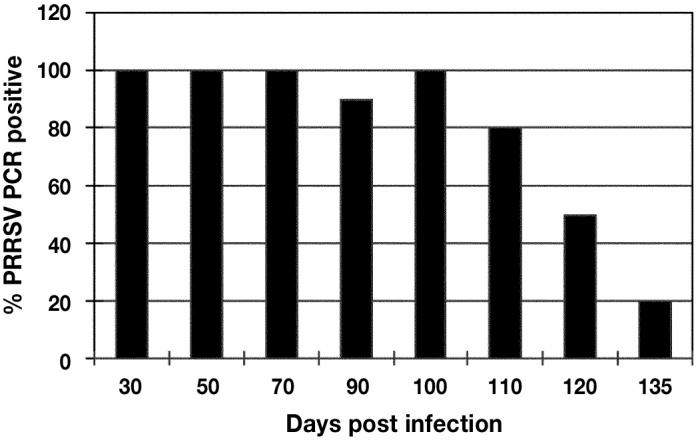
The percentage of gilts with porcine reproductive and respiratory syndrome virus (PRRSV) in tissues between days 0 and 135 post infection (pi) of the study. Tissue homogenates were positive between day 30 to 135 pi but decreased from 100% on day 100 to 20% positives on day 135 pi.
Porcine reproductive and respiratory syndrome virus cell-mediated immune response
Both FC and ELISPOT assays were employed to assess antigen-specific IFN-γ production from day 0 to 135 of the experiment. The ELISPOT assay resulted in a similar response pattern. There was an early response detected at 14 d, which peaked between 50 to 70 d and started decreasing thereafter (Figures 4 and 5). T-cell proliferation encompassed CD4, CD8, and γδ T-cell population. T-cell proliferation followed a pattern similar to IFN-γ production with the initial response detected at 14 d pi with a peak between 50 to 70 d (Figure 6).
Figure 4.
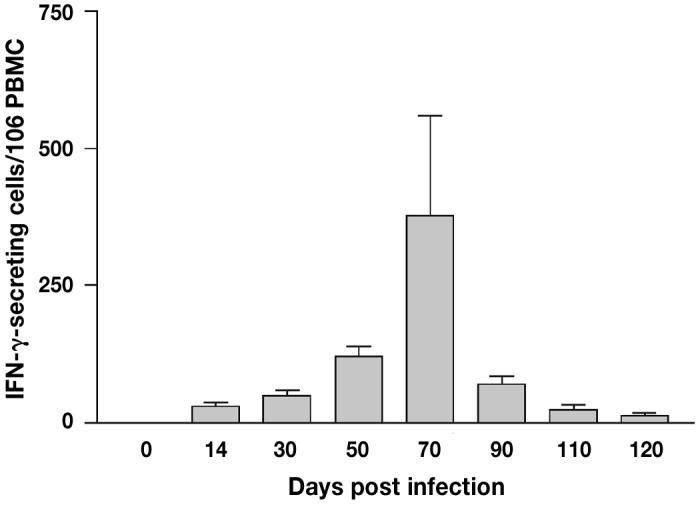
Interferon-gamma (IFN-γ) secreting cells from day 14 to 120 post infection (pi) as reported using ELISPOT. The IFN-γ production was first detected on day 14 pi, peaked on day 70, and decreased thereafter.
Figure 5.
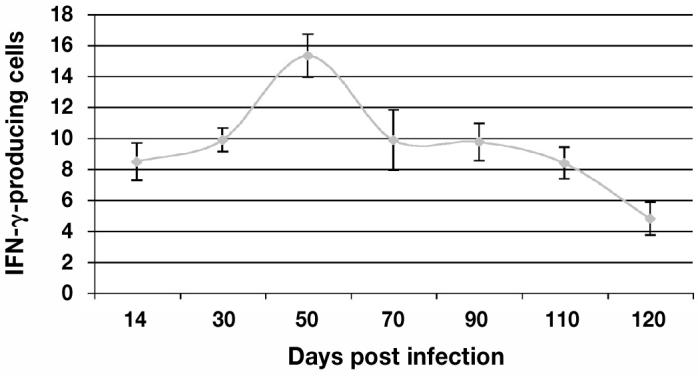
The percentage of interferon-gamma (IFN-γ) producing cells from day 14 to 120 post infection (pi) as reported using flow cytometry. The IFN-γ production was first detected on day 14 pi, peaked on day 50, and decreased thereafter.
Figure 6.
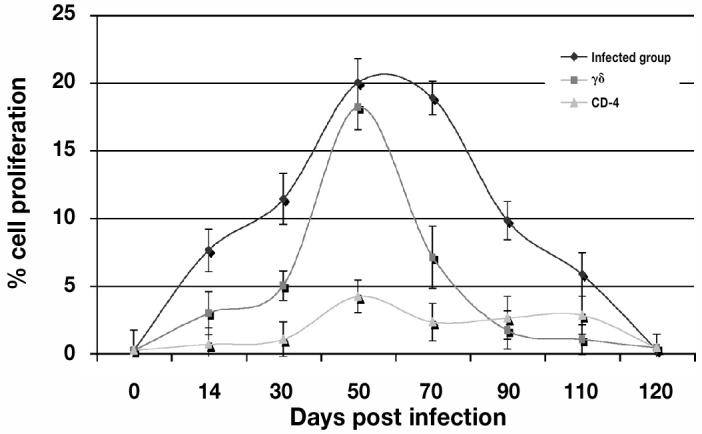
The percentage of total cell proliferation (CD-4 and γ-δ) against porcine reproductive and respiratory syndrome virus (PRRSV) from day 0 to day 135 post infection (pi) of the experiment using flow cytometry. T-cell proliferation was detected on day 14, peaked around day 50 to 70 pi, and decreased thereafter.
Discussion
This study was undertaken to describe PRRSV persistence and the immune response to infection in a large population of experimentally challenged gilts. The study was unique in that it involved 80 4-month-old gilts housed under commercial conditions for an extended period of time (6 mo). It was designed to improve understanding of the kinetic of the virus and the immunological response in gilt acclimatization practices that are currently used in the field. The first parameter assessed was the kinetic of the virus following experimental infection. Data from the monitor gilt group indicated successful experimental infection with detectable viremia for up to 30 d pi. Sera samples from the index gilts were positive to RT-PCR on days 3, 7, 14, 21, and 30. In contrast, a lower number of sera collected on the same sampling days were VI positive demonstrating the superior sensitivity of the PCR assay.
Persistence of PRRSV in tissues was confirmed between days 30 and 135 by PCR. Lesions related to PRRSV infection were detected histologically following microscopic evaluation of lymphoid tissue collected between days 30 and 135, as described by Rossow (30). Sera samples were also positive by VI on days 7 and 14. As shown, lower numbers of samples were VI positive, indicating that the PCR test possesses a higher degree of sensitivity than VI. Sensitivity of the TaqMan PCR used in this study has been reported to be 0.1 TCID50/mL. This lower sensitivity of VI demands the use of different diagnostic tests to confirm results during an experimental study. The PRRSV RNA was detected in breeding age female swine up to 135 d, although a reduction in the number of persistently infected animals was observed between day 100 and day 135 pi. These data differ from our previous study that reported the inability to detect PRRSV persistence on days 120, 150, and 180 pi using animals of the same age and the same viral strain (7). One explanation for this difference might be that some gilts in the present study may have harbored PRRSV in lymphoid sites that were not sampled in the former study. Another explanation might be the individual variation in the immune response in a population of pigs, resulting in the animals taking longer to clear the virus. However, both studies used the same strain of the virus and the same genetics of animals from the same farm. This suggests that PRRSV persistence may not necessarily be associated with genetic line or farm source.
An important observation was the detection of PRRSV in tissues despite the presence of virus neutralizing antibodies and antibodies detected by ELISA and IFN-γ. Neutralizing antibody response is presumed to play an important role in resistance to reinfection and in prevention or reduction of viral spread from animal to animal, since virus neutralization antibodies have the potential to clear virus from the circulation (37). Nevertheless there are conflicting reports about the significance of antibodies in PRRSV protection, especially with regard to the PRRSV-specific neutralization response (5,10,37). Concurrent detection of neutralizing antibodies and infectious PRRSV in the blood of infected animals has led to the hypothesis that neutralizing antibodies do not play a role in protection against PRRSV infection (5,10,36). In the present study, PRRSV in the blood (days 3 to 30 pi), as well as PRRSV in the tissue (days 30 to 120 pi), was observed concurrently with SN antibodies (days 21 to 135 pi), although it could be argued that viremia decreased over time following the appearance of SN antibodies. The SN test was performed using PRRSV MN 30–100, the same strain that was used for the experimental infection. This finding might be due to the fact that the early, low amounts of neutralizing antibodies may not be sufficient to control replication of the virus. Osorio and others (17) reported that passive maternal antibodies transferred to piglets in colostrums resulted in the protection of piglets against the development of clinical symptoms and reduction of viremia, however, in that study the SN titers were higher (1:16) than those early SN titers (0 to 1:8) detected in our study. This finding might be due to the fact that the early level of neutralizing antibodies may not be sufficient to control replication of the virus. A full understanding of the role in protection of AMIR and neutralizing antibodies is further complicated by the lack of information about protective epitopes and when effective levels of relevant antibodies are attained.
The present results also suggest that IFN-γ is probably involved in the clearance of virus from blood since IFN-γ was detected as early as 14 days pi, peaking between 50 and 70 d pi. Nevertheless, it appears that the low levels of SN titers present during viremia were not sufficient to clear the virus before it spread to the lymphoid tissue. Also, the fact that the virus persisted in the lymphoid tissue after IFN-γ levels dropped, suggests that some other immune factor is associated with viral clearance and sterilizing immunity. The results from both the ELISPOT and FC assays demonstrated that PRRSV-specific IFN-γ secreting-cells were detected early after infection and persisted at low levels until day 135 pi. The number of IFN-γ secreting cells increased after the acute viremia had resolved, but decreased before resolution of the presence of virus in tissues. This finding contrasts with previous results indicating that virus-specific IFN-γ-producing lymphocytes in the blood of pigs were not detected until 13 wk after infection with virulent PRRSV or 8 wk after a live, attenuated virus booster vaccination (22,23). It is not clear why, in the present study, results differ in the chronology of the IFN-γ response. Differences may be due to the viral strain used, such as that this was a field strain and not an attenuated strain or to differences in the sensitivity of the assays. The differences found between the ELISPOT and FC could be explained by differences in the 2 tests. The ELISPOT incubates PBMC for only 20 h, avoiding cell proliferation. In contrast, flow cytometry has a longer incubation period of 72 h. Polyclonal activation of B cells, simultaneous with development of antiviral immune response, has already been shown in other arterivirus infections, such as murine LDV infection (38). Asai et al (39) have demonstrated that interleukin (IL)-6 increased in sera from PRRSV-infected pigs during the 1st wk of infection. Production of this cytokine by activated macrophages may partially explain the polyclonal activation of B and T cells. Interleukin-6 is known to be a multifunctional cytokine, acting both in the differentiation of B cells into plasma cells and in T cell activation (40). The longer period of incubation might allow for non-specific T-cell proliferation, which could explain the increase in IFN-γ secreting-cells detected in flow cytometry but not in the ELISPOT assay. Alternatively, these differences may only be a reflection of the test sensitivity.
Finally, it must be stated that our study evaluated CD-4, CD-8, and γδ lymphocyte subset populations following exposure to PRRSV. It is clear that γδ lymphocytes had a higher response in both antigen specific proliferation and IFN-γ production. Therefore, we could hypothesize that this population might be responsible for the clearance of the virus in conjunction with SN antibodies. Nevertheless, this hypothesis remains to be proven.
To the author’s knowledge, this was the largest immunological and virological PRRSV infection study ever performed in gilts. The information generated from this study is important for the swine industry since it brought forth a new perspective on the dynamics of PRRSV persistence and on the kinetics of the AMIR and CMIR of PRRSV in a large population of gilts. This information can hopefully be used to better define the time of gilt quarantine after exposure, thus enhancing the efficacy of gilt acclimatization protocols, resulting in improved disease control. Also, knowing the kinetics of the AMIR and CMIR will better define the necessary amount of time for farm closure to avoid persistent infection during a farm closure protocol, which is used in the control and eradication of PRRSV in the field.
In conclusion, this study enhanced the understanding of the virological and immunological response following PRRSV infection in a large population of breeding age female swine. The kinetics of both SN antibodies and IFN-γ in the early and late phases of the infection are now better understood. It is clear that none of the parameters measured (SN antibodies, IFN-γ), either alone or in combination, are solely responsible for clearance of the virus from the host and the development of sterilizing immunity. Future studies should focus on repeating this study using larger sample sizes resembling actual commercial conditions, as well as assessing other key immunological responses that bring about the elimination of virus from persistently infected gilts. Answers to these issues could prove to be very helpful in refining protocols for the control of PRRS throughout the global swine industry.
Acknowledgments
The authors recognize and thank the National Pork Board and Genetiporc for their financial support and animal resources during this study.
Footnotes
Dr. Batista’s current address is the University of Montreal, Department of Clinical Sciences, CP 5000, Saint-Hyacinthe, Quebec J2S 7C6.
References
- 1.Dee SA, Joo HS, Polson DD, Marsh WE. Evaluation of the effects of nursery depopulation on the profitability of 34 pig farms. Vet Rec. 1997;140:498–500. doi: 10.1136/vr.140.19.498. [DOI] [PubMed] [Google Scholar]
- 2.Cavanaugh D. Nidovirales: A new order comprising Coronoviridae and Artiriviridae. Arch Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- 3.Wills RW, Zimmerman JJ, Yoon KJ, et al. Porcine reproductive and respiratory syndrome virus: a persistent infection. Vet Microbiol. 1997;55:231–240. doi: 10.1016/s0378-1135(96)01337-5. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed R, Morrison LA, Knipe DM. Persistence of viruses. In: Fields BN, Knipe DM, Howley PM, eds. Field’s Virology. Philadelphia: Lippencott-Raven, 1996:219–249.
- 5.Allende R, Laegreid WW, Kutish GF, Galeota JA, Wills RW, Osorio FA. Porcine reproductive and respiratory syndrome virus: description of persistence in individual pigs upon experimental infection. J Virol. 2000;74:10834–10837. doi: 10.1128/jvi.74.22.10834-10837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bierk MD, Dee SA, Rossow KD, Collins JE, Otake S, Molitor TW. Transmission of porcine reproductive and respiratory syndrome virus from persistently infected sows to contact controls. Can J Vet Res. 2001;65:261–266. [PMC free article] [PubMed] [Google Scholar]
- 7.Batista L, Dee SA, Rossow K, Deen J, Pijoan C. Assessing the duration of porcine reproductive and respiratory syndrome virus persistence and shedding in a large population of breeding age female swine. Can J Vet Res. 2002;66:196–200. [PMC free article] [PubMed] [Google Scholar]
- 8.Brown IH, Done SH, Spencer YI, et al. Pathogenicity of a swine influenza H1N1 virus antigenically distinguishable from classical and European strains. Vet Rec. 1993;132:598–602. doi: 10.1136/vr.132.24.598. [DOI] [PubMed] [Google Scholar]
- 9.Albina E, Madec F, Cariolet R, Torrison J. Immune response and persistence of the porcine reproductive and respiratory syndrome virus in infected pigs and farm units. Vet Rec. 1994;133:567–573. doi: 10.1136/vr.134.22.567. [DOI] [PubMed] [Google Scholar]
- 10.Christianson WT, Collins JE, Benfield DA, et al. Experimental reproduction of swine infertility and respiratory syndrome in pregnant sows. Am J Vet Res. 1992;53:485–488. [PubMed] [Google Scholar]
- 11.Rossow KD, Collins JE, Goyal SM, et al. Pathogenesis of porcine reproductive and respiratory syndrome virus infection in gnotobiotic pigs. Vet Pathol. 1995;32:361–373. doi: 10.1177/030098589503200404. [DOI] [PubMed] [Google Scholar]
- 12.Joo HS, Park BK, Dee SA, Pijoan C. Indirect fluorescent IgM antibody response of pigs infected with porcine reproductive and respiratory syndrome virus. Vet Microbiol. 1997;55:303–307. doi: 10.1016/s0378-1135(96)01332-6. [DOI] [PubMed] [Google Scholar]
- 13.Nelson EA, Christopher-Hennings J, Benfield DA. Serum immune response to the proteins of porcine reproductive and respiratory syndrome (PRRS) virus. J Vet Diagn Invest. 1994;6:410–415. doi: 10.1177/104063879400600402. [DOI] [PubMed] [Google Scholar]
- 14.Lager KM, Mengeling WL, Brockmeier SL. Duration of homologous porcine reproductive and respiratory syndrome virus immunity in pregnant swine. Vet Microbiol. 1997;58:127–133. doi: 10.1016/s0378-1135(97)00159-4. [DOI] [PubMed] [Google Scholar]
- 15.Osorio FA, Galeota JA, Nelson E, et al. Passive transfer of virus-specific antibodies confers protection against reproductive failure induced by a virulent strain of porcine reproductive and respiratory syndrome virus and establishes sterilizing immunity. Virology. 2002;302:9–20. doi: 10.1006/viro.2002.1612. [DOI] [PubMed] [Google Scholar]
- 16.Murtaugh MP, Xiao Z, Zuckermann F. Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral Immunol. 2002;15:533–547. doi: 10.1089/088282402320914485. [DOI] [PubMed] [Google Scholar]
- 17.Meier W, Wheeler J, Husmann RJ, et al. Characteristics of the immune response of pigs to PRRS virus. Vet Rec. 2000;31:41. [Google Scholar]
- 18.Finke D, Brinckmann UG, ter Meulen V, Liebertg UG. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J Virol. 1995;69:5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bautista EM, Molitor TW. IFN-γ inhibits porcine reproductive and respiratory syndrome virus replication in macrophages. Arch Virol. 1999;144:1191–1200. doi: 10.1007/s007050050578. [DOI] [PubMed] [Google Scholar]
- 20.Pace JL, Russell SW, Le Blanc PA, Murasko DM. Comparative effects of various classes of mouse interferons on macrophage activation for tumor cell killing. J Immunol. 1985;134:977–981. [PubMed] [Google Scholar]
- 21.Boehm U, Klam T, Groot M, Howard JC. Cellular response to interferon-γ. Ann Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 22.Zuckermann FA, Husmann RJ, Schwartz R, et al. Interleukin-12 enhances the virus-specific interferon gamma response of pigs to an inactivated pseudorabies virus vaccine. Vet Immunol Immunopathol. 1998;63:57–67. doi: 10.1016/s0165-2427(98)00082-8. [DOI] [PubMed] [Google Scholar]
- 23.Zuckermann FA, Martin S, Husmann RJ, et al. Use of interleukin 12 to enhance the cellular immune response of swine to an inactivated herpesvirus vaccine. Adv Vet Med. 1999;41:447–461. doi: 10.1016/s0065-3519(99)80034-2. [DOI] [PubMed] [Google Scholar]
- 24.Dee SA, Joo HS. Clinical investigation of recurrent reproductive failure with PRRS virus in a swineherd. J Am Vet Med Assoc. 1994;204:1017–1018. [PubMed] [Google Scholar]
- 25.Dee SA, Philips RE. Use of polymerase chain reaction to detect vertical transmission of PRRS virus in piglets from gilt litters. Swine Health and Production. 1999;7:237–239. [Google Scholar]
- 26.Bierk MD, Dee SA, Rossow KD, et al. A diagnostic investigation of chronic PRRS virus infection in a swine breeding herd. Vet Rec. 2001;148:687–690. doi: 10.1136/vr.148.22.687. [DOI] [PubMed] [Google Scholar]
- 27.Cannon RM, Roe RT. Livestock Disease Surveys: A Field Manual for Veterinarians. Australian Bureau of Animal Health. Canberra. 1982.
- 28.Molitor TW, Tune KA, Shin J, Collins J, Kapur V. Applications of TaqMan PCR in the detection of PRRS virus. Proc Allen D. Leman Swine Conf 1997:173–175.
- 29.Bautista EM, Goyal S, Yoon IJ, Joo HS, Collins J. Comparison of porcine alveolar macrophages and CL 2621 for the detection of porcine reproductive and respiratory syndrome virus and anti-PRRS antibody. J Vet Diagn Invest. 1993;5:163–165. doi: 10.1177/104063879300500204. [DOI] [PubMed] [Google Scholar]
- 30.Rossow KD. Porcine reproductive and respiratory syndrome. Vet Pathol. 1998;35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- 31.Murtaugh MP, Elam MR, Kokach LT. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the porcine reproductive and respiratory syndrome virus. Arch Virol. 1998;140:1451–1460. doi: 10.1007/BF01322671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder ML, Mermer B, Anderson PR, Wensvoort G, Hill HT. Evaluative data for an immunodiagnostic ELISA for PRRS. Proc 2nd Int Symp on PRRS 1995:15.
- 33.Yoon IJ, Joo HS, Goyal SM, Molitor TW. A modified serum neutralization test for the detection of antibody to porcine reproductive and respiratory syndrome virus in swine sera. J Vet Diagn Invest. 1994;6:289–292. doi: 10.1177/104063879400600326. [DOI] [PubMed] [Google Scholar]
- 34.Fuertes LL, Domenech N, Alvarez B, Ezquerra A, et al. Analysis of cellular immune response in pigs recovered from porcine respiratory and reproductive syndrome infection. Virus Res. 1999;64:33–42. doi: 10.1016/s0168-1702(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 35.Iyo M, Kojiro N, Kokubu T. Increased Proportions of Peripheral Blood gd T Cells in Patients with Pulmonary Tuberculosis. CHEST. 1992;102:195–197. doi: 10.1378/chest.102.1.195. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu M, Osorio F, Brandt J. Changes in lymphocyte sub-populations in pigs infected with porcine reproductive and respiratory syndrome (PRRS) virus. Vet Immunol Immunopath. 1992;50:19–27. doi: 10.1016/0165-2427(95)05494-4. [DOI] [PubMed] [Google Scholar]
- 37.Molitor TW, Bautista EM, Choi CS. Immunity to PRRSV: double-edged sword. Vet Microbiol. 1997;55:265–276. doi: 10.1016/S0378-1135(96)01327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cafruny WA, Chan SP, Harty JT, et al. Antibody response of mice to lactate dehydrogenase-elevating virus during infection and immunization with inactivated virus. Virus Res. 1986;5:357–375. doi: 10.1016/0168-1702(86)90029-8. [DOI] [PubMed] [Google Scholar]
- 39.Asai T, Mori M, Okada M, Uruno K, Yazawa S, Shibata I. Elevated serum haptoglobulin in pigs infected with porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 1999;70:143–148. doi: 10.1016/s0165-2427(99)00069-0. [DOI] [PubMed] [Google Scholar]
- 40.Lamontagne L, Page C, Larochelle R, Longtin D, Magar R. Polyclonal activation of B cells occurs in lymphoid organs from porcine reproductive and respiratory syndrome virus (PRRSV)-infected pigs. Vet Immunol and Immunopathol. 2001;82:165–181. doi: 10.1016/s0165-2427(01)00335-x. [DOI] [PubMed] [Google Scholar]


