Abstract
Mitochondrial calcium uptake plays a critical role in various cellular functions. After half a century of extensive studies, the molecular components and important regulators of the mitochondrial calcium uptake complex have been identified. However, the mechanism by which these protein molecules interact with one another and coordinate to regulate calcium passage through mitochondrial membranes remains elusive. Here, we summarize recent progress in the structural and functional characterization of these important protein molecules, which are involved in mitochondrial calcium uptake. In particular, we focus on the current understanding of the molecular mechanism underlying calcium through two mitochondrial membranes. Additionally, we provide a new perspective for future directions in investigation and molecular intervention.
Keywords: Calcium signaling, Calcium channel, VDAC, MCU, MICU, EMRE, MCUR1
Introduction
Mitochondria are energy providers for various cellular activities and act as important signaling nodes to produce and transduce signals in the cell [1, 2]. Consequently, mitochondria are vital for many cellular processes, such as ion homeostasis, apoptosis, and autophagy [3]. Ca2+ is a second messenger that regulates eukaryotic cell life from proliferation to death [4]. Mitochondria were the first intracellular organelles found to closely associate with Ca2+ signaling [5]. Ca2+ homeostasis plays a crucial role in mitochondrial function [6, 7]. Cellular Ca2+ signals are finely regulated by many ion channels, pumps and exchangers, which can transport Ca2+ across the plasma membrane and various types of organelles [8]. The endoplasmic reticulum (ER) and mitochondria significantly contribute to cellular Ca2+ homeostasis [9].
Mitochondrial Ca2+ transport participates in different physiological processes, such as shaping the cytosolic Ca2+ transients [10–13] and controlling the metabolic rate for cellular energy production [14–16]. Ca2+ overload in the mitochondria leads to activation of the cell death pathway [17–21]. In the 1960s, scientists found that rat kidney mitochondria took up large quantities of Ca2+ [22, 23], after which the Ca2+ carrier was defined as the mitochondrial Ca2+ “uniporter” [24]. By patch-clamping the inner mitochondrial membrane, researchers demonstrated that the uniporter is a highly selective Ca2+ channel [25]. The mitochondrial respiratory chain proton pump components generate a large voltage across the inner mitochondrial membrane (ΔΨm), which is the main driving force for Ca2+ accumulation in the mitochondrial matrix through the uniporter [26, 27]. The uniporter rapidly transports Ca2+ across the steep electrochemical gradient and is inhibited by ruthenium red. The apparent Ca2+ affinity of the uniporter is very low (10–20 μM); therefore, the influx rate of healthy cells may become weak because of the low [Ca2+]c [28]. The discrepancy between the uniporter’s low apparent Ca2+ affinity and its role in high-flux Ca2+ uptake is explained by the existence of the local micro-domains’ concept of high Ca2+ concentrations at sites of ER–mitochondrial junctions [1, 29, 30].
A high concentration of Ca2+ is present, upon IP3-mediated ER calcium release, in close appositions between the ER and mitochondria [29]. The Ca2+ signal microheterogeneity may, therefore, determine the mitochondrial Ca2+ uptake. The Ca2+ released from the ER can be transported into mitochondria via a voltage-dependent anion channel (VDAC) on the outer membrane and a uniporter on the inner mitochondrial membrane [1].
In this review, we summarize the progress of research on mitochondrial Ca2+ uptake, focusing on molecular regulation of proteins involved in this crucial process.
VDAC in the mitochondrial outer membrane
The VDAC, the most abundant protein in the outer mitochondrial membrane, controls the flux of small molecules and ions between the cytosol and mitochondrial spaces [31]. The VDAC is a large-diameter (2.5–3 nm) channel that is permeable to solutes smaller than 5 kDa and thus to Ca2+ [32]. It also acts as a scaffold for modulator proteins and plays a key role in mitochondrial-induced apoptosis [33]. The VDAC adopts a stable, open conformation at a low or zero membrane potential, at which small ions are subject to selective conduction, and multiple conformations at potentials greater than 30–40 mV, which yield different ionic selectivities and permeabilities [31, 34]. The VDAC can finely tune cellular processes through three isoforms (VDAC1, VDAC2 and VDAC3) [35] that have common channel properties but play different roles in cell survival. The silencing of VDAC1 potentiates apoptotic challenges, whereas that of VDAC2 has the opposite effect. VDAC1 selectively transfers low-amplitude apoptotic Ca2+ signals to mitochondria. VDAC1, but not VDAC2 or VDAC3, interacts with IP3 receptors, and this interaction can be further strengthened by apoptotic stimuli [36].
VDAC transports Ca2+ across the mitochondrial outer membrane [32]. The expression levels of VDAC are directly correlated with the process in which Ca2+ is rapidly taken up into the mitochondrial matrix [37]. Overexpression of VDAC enhances the amplitude of agonist-dependent increases in the mitochondrial matrix Ca2+ concentration [36, 38] but has no effect on the ER Ca2+ concentrations. The amplitude-enhancing effect is due to the formation of new ER–mitochondria contacts and not to a general increase in the permeability of the outer membrane and/or an effect of the uptake systems. Moreover, VDAC-dependent enhancement of the mitochondrial Ca2+ responses promotes Ca2+-dependent changes in organelle morphology and in activation of mitochondrial apoptotic events.
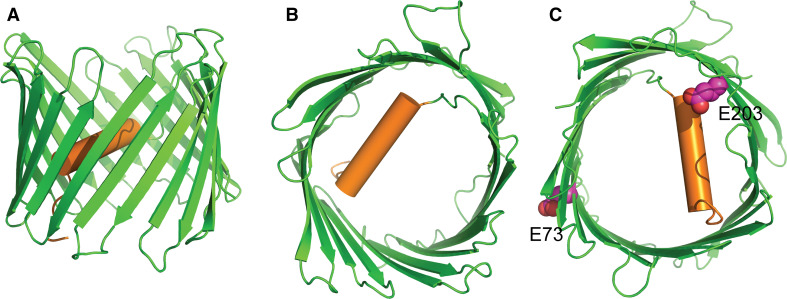
The structure of VDAC1 was previously determined using different approaches (Fig. 1a, b). VDAC1 adopts a β-barrel architecture composed of 19 β-strands with an N-terminal α-helix located horizontally midway within the pore [39–41]. The N-terminal segment (1–26 amino acids) that forms a hydrogen-bond pattern with β-strands 8–19 facilitates its orientation against the interior wall of the pore [40]. The general consensus in the field is that the N-terminal segment of VDAC is involved in voltage gating and, therefore, may adopt different conformations. The N-terminal α-helix of VDAC1 can translocate from the internal pore to the channel face and then interact with anti-apoptotic proteins (hexokinase, Bcl-2 and Bcl-xL), which demonstrates its important role in channel gating and cell survival [42–44]. Ca2+-binding sites have been identified in VDAC1 (Fig. 1c) [45], and studies show that the inhibitor ruthenium red interacts with Ca2+-binding sites [46] and reduces VDAC conductance [31].
Fig. 1.
VDAC structure. a, b Cartoon representation of mouse VDAC (PDB code: 3EMN) with different perspectives. c Proposed Ca2+-binding sites in mouse VDAC. Residues E73 and E203 are shown as magenta spheres
The uniporter complex in the mitochondrial inner membrane
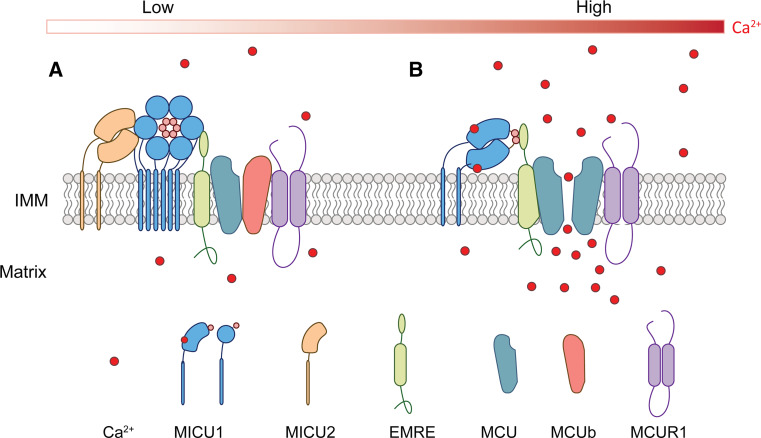
Although uniporter physiology has been studied extensively for approximately half a century, the molecular identity of the uniporter has remained elusive until recently. In 2010, based on clues from comparative physiology, evolutionary genomics, organelle proteomics and genome-wide RNAi screening, the mitochondrial Ca2+ uptake 1 (MICU1) protein was identified as an essential element in mitochondrial Ca2+ uptake [47, 48]. The identification of MICU1 has greatly facilitated research on the remaining uniporter complex components. The uniporter is an approximately 480-kDa multiple protein complex, referred to as the uniporter complex, rather than a single protein [49]. The molecular components of the uniporter include the channel subunit MCU, the endogenous MCU dominant-negative protein MCUb, the two Ca2+-sensing regulatory proteins MICU1 and MICU2, and the essential MCU regulator EMRE (Fig. 2).
Fig. 2.
Schematic representation of the mitochondrial calcium uptake complex in the low Ca2+ state (a) and high Ca2+ state (b). These models are based on our current understanding of the published data. IMM inner mitochondrial membrane
MICU1
MICU1, the first protein in the uniporter complex to be characterized, is located in the inner mitochondrial membrane and expressed in the most mammalian tissues. MICU1 is an approximately 54-kDa protein with an amino-terminal mitochondrial targeting sequence, a transmembrane helix and a cytosolic C-terminus (amino acids 53–476) containing two classic EF-hands, which acts as a Ca2+ sensor to regulate the MCU channel activity [50].
MICU1 and MCU have a close relationship; they are homologs of both MICU1 and MCU in metazoan, plants, and protozoa but not fungi [51]. Co-immunoprecipitation experiments demonstrated that MICU1 and MCU physically interact with each other [52–55]. In all vertebrates, the MICU1 and MCU genes are adjacent and share a potential bidirectional promoter, which may be the mechanistic basis for their coordinated expression [51].
MICU1 is an essential component of the uniporter complex. shRNA knockdown of MICU1 in HeLa cells strongly disrupts the mitochondrial Ca2+ uptake phenotype after histamine stimulation. Moreover, expression of the MICU1 cDNA in the knockdown cells was sufficient to fully rescue mitochondrial Ca2+ uptake. By contrast, cDNA expression of the MICU1 EF-hands mutant did not rescue the mitochondrial Ca2+ uptake phenotype [47]. Silencing MICU1 did not disrupt mitochondrial respiration or membrane potential, but it attenuated metabolic coupling between cytosolic Ca2+ transients and activation of matrix dehydrogenases. These results demonstrate the cooperative role of MICU1 in regulating mitochondrial Ca2+ uptake and that the EF-hands of MICU1 play a key role in this process.
However, Mallilankaraman et al. [53] observed a different phenomenon; they found that MICU1 is required to preserve normal [Ca2+]m under basal conditions. MICU1 knockdown resulted in basal [Ca2+]m accumulation, which triggered generation of excessive reactive oxygen species (ROS) and sensitivity to apoptotic stress. [Ca2+]m accumulated in MICU1 knockdown cells through MCU-mediated Ca2+ uptake. Thus, MICU1 is a gatekeeper that sets a [Ca2+] threshold of MCU-mediated mitochondrial Ca2+ uptake. Below 3–4 μM [Ca2+]c, MICU1 strongly inhibited MCU-mediated Ca2+ uptake, but this inhibition did not affect the overall kinetics of MCU-mediated Ca2+ uptake at higher [Ca2+]c. Moreover, the EF-hands mutant of MICU1 lost the gatekeeper function, which suggests that EF-hands provide a high-affinity [Ca2+]m-sensing mechanism that enables MICU1 regulation.
Csordas et al. [56] found that MICU1 regulation of MCU-mediated Ca2+ uptake was dose-dependent based on [Ca2+]c. These authors confirmed the role of MICU1 in setting a [Ca2+]c threshold that prevents mitochondria from taking up low [Ca2+]c. In addition, they found that MICU1 facilitates cooperative activation of MCU as the [Ca2+]c increased. Although the EF-hands-mutated MICU1 could block the Ca2+ uniporter at low [Ca2+]c, which is similar to wild-type MICU1 (in contrast to Mallilankaraman’s results), it could not conduct cooperative activation as the [Ca2+]c increased. The function transition of MICU1 must be mediated by Ca2+ binding to two MICU1 EF-hands. MICU1 is a molecular switch that binds Ca2+, which sharply enhances MCU-mediated mitochondrial Ca2+ uptake. Therefore, a brief MICU1 regulation model includes low [Ca2+]c conditions, as MICU1 does not bind Ca2+; Ca2+-free MICU1 inhibits Ca2+ uptake into mitochondria and thus sets a threshold for MCU. With increasing [Ca2+]c, MICU1 binds Ca2+ and then activates mitochondrial Ca2+ uptake. Csordas et al. also found that MICU1 depletion produced an adaptive increase in mitochondrial matrix Ca2+ chelation.
Discrepancies in results from different research groups may be due to different experimental conditions. In our experience, fluorescent dyes used for Ca2+ measurements have resulted in different interpretations. Therefore, the fluorescent dye for Ca2+ must be consistent with the calcium concentration range measured in the experiment. We reported that the affinity of MICU1 for Ca2+ is approximately 15–20 μM [55]; this result indicates that MICU1 is less likely to bind Ca2+ in a resting cell. We support the model proposed by Csordas et al. [56].
MICU1 acts as a calcium sensor. Studying its topology can aid in understanding the mechanism underlying its operation. However, the results are contradictory. It was predicted that MICU1 includes a single transmembrane domain at its N-terminus. Alkaline carbonate extraction of mitochondria from HEK293T cells showed that MICU1 is strongly associated with the inner mitochondrial membrane. A proteinase K (PK) digestion experiment showed that the large, soluble C-terminal portion of MICU1 was located in the inter-membrane space of the mitochondria, which is consistent with the function by which it senses changes in the [Ca2+]c and regulates MCU-mediated Ca2+ uptake [55–57]. By contrast, Hoffman et al. [54] concluded that MICU1 was compartmentalized primarily in the matrix side of the inner mitochondrial membrane by imaging tagged mitochondrial proteins in plasma membrane-permeabilized cells in response to outer and inner membrane permeabilization. Recently, Hung et al. [58] combined APEX technology with stable isotope labeling using amino acids in a cell culture (SILAC)-based ratiometric tagging strategy and detected MICU1 in the inner membrane space proteome. Hoffman et al. demonstrated that the polybasic motif (amino acids 99–110) of human MICU1 is essential for MCU binding and that the EF-hands of MICU1 do not participate in this process. Kamer et al. and our group found that the MICU1 C-terminal helix (amino acids 445–476) plays a key role in the interaction with MCU [55, 59]. In our opinion, the argument that the C-terminal portion of MICU1 is located in the periplasmic space of mitochondria is more consistent with most published experimental data [56, 57].
Our group recently described the atomic structures of both Ca2+-free and Ca2+-bound human MICU1 (Fig. 3a, b) [55]. The structures revealed that the C-terminal portion of MICU1 includes four regions: the N-domain (residues 103–177), the N-lobe (residues 183–318), the C-lobe (residues 319–445) and the C-helix (residues 446–476). Each MICU1 molecule can maximally bind two calcium ions. Our studies showed that Ca2+-free MICU1 forms a hexamer that binds and inhibits MCU. Upon Ca2+ binding, MICU1 undergoes large conformational changes, which yields a mixture of oligomers that activate MCU (Fig. 3c). In the Ca2+-free MICU1 hexamer, the C-helix is located in the center of the six molecules and is packed as a helix bundle. Removal of the C-helix abolishes the interaction between MICU1 and MCU under both Ca2+-free and Ca2+-bound conditions, resulting in a loss of MICU1-activated mitochondrial Ca2+ uptake.
Fig. 3.
Conformational changes in human MICU1 upon Ca2+ binding. Cartoon representation of the overall MICU1 structure in the Ca2+-free (a) and Ca2+-bound states (b). PDB codes: 4NSC for the Ca2+-free MICU1 and 4NSD for the Ca2+-bound MICU1. Calcium is shown in red. c Potential MICU1 oligomer conversion upon Ca2+ binding. The Ca2+-free MICU1 hexamer is shown in red, blue, green, cyan, yellow and orange, respectively. The Ca2+-bound MICU1 dimer is shown in green and blue, respectively
MICU2
MICU2 is a vertebrate paralog of MICU1 and shares approximately 42 % protein sequence similarity with MICU1. MICU2 also contains two conserved EF-hands [60]. Like MICU1, MICU2 is located on the inner membrane of mitochondria with its N-terminal mitochondrial targeting sequence. MICU2 is broadly expressed in various mouse tissues, particularly in visceral organs. The protein expression levels of MICU2 depend largely on MICU1, and MICU1 silencing dramatically reduced MICU2 expression [57, 59, 60]. However, MICU2 knockdown reduced MICU1 expression in HeLa cells but not HEK293T cells. MICU1 and MICU2 physically interact with each other in the presence of Ca2+ or EGTA. The 480-kDa uniporter complex includes MICU2, and MICU2 knockdown alters the complex size [60].
Previous studies of MICU1 function did not consider contributions from MICU2. Recently, functional studies of MICU2 have made significant progress, but large discrepancies remain. Kamer et al. [59] showed that the knockdown of MICU1 or MICU2 alters the threshold for mitochondrial Ca2+ uptake. Transfection of the EF-hand mutants for MICU1 or MICU2 constitutively inhibits mitochondrial Ca2+ uptake. Moreover, the EF-hand mutation for MICU1 or MICU2 also abolished the cooperative function of mitochondrial Ca2+ uptake, which suggests that MICU1 and MICU2 play a non-redundant role in regulating MCU-mediated Ca2+ influx. Kamer et al. also found that in the absence of MICU1, MICU2 did not associate with the MCU complex but the converse was not true. MICU1 without functional EF-hands inhibited Ca2+ uptake in the absence of MICU2, but, again, the converse was not true [59, 61].
Patron et al. [57] performed an FRET-based protein–protein interaction assay and confirmed a MICU1–MICU2 interaction in living cells; these authors found that MICU1 and MICU2 formed a heterodimer through a disulfide bond. Mutation of these cysteine residues abolished the MICU1–MICU2 interaction. The short loop between the two transmembrane helices of MCU participated in the interaction with the MICU1–MICU2 dimer. Mutation of the acidic residues of this loop disturbed their interaction. The activity of MCU expressed in vitro in planar lipid bilayers was measured. Adding MICU1 to the system did not alter the channel activity under low [Ca2+]. By contrast, MICU1 increased the open probability of MCU when the [Ca2+] was high, which suggests a clear activation effect of MICU1 on MCU activity but not an inhibitory effect at low [Ca2+]. Using the same method, Patron et al. verified that MICU2 is an MCU inhibitor. Thus, MICU1 and MICU2 form a regulatory dimer and modulate MCU with opposing effects.
The reasons for the contradictory results are unclear, and further investigation is necessary. In our opinion, MICU1–MICU2 heterodimer formation through a disulfide bond seems difficult because of a reducing environment in the periplasmic space of mitochondria. The dimer detected using SDS-PAGE gels in the absence of reducing agent may be interpreted as a MICU1–MICU2 heterodimer or hetero-oligomer in vivo. We prefer the model in which MICU1 and MICU2 cooperate rather than operate independently to regulate MCU function.
MCU
The channel component in mitochondrial Ca2+ uptake, MCU, was independently identified by two research groups in 2011 [52, 62]. MCU is ubiquitously expressed in mammalian tissues and most eukaryotes but is absent in Saccharomyces cerevisiae. MCU is a 40-kDa protein located at the inner mitochondrial membrane. It has two transmembrane helices and an acidic-residue-rich loop, which are highly conserved among MCU orthologs. A co-immunoprecipitation experiment showed that MCU forms oligomers. A recent study demonstrated that MCU assembles as a tetramer in vitro and in vivo, similar to other cation channels [63]. Topology studies have indicated that the N- and C-terminal portions of MCU are located in the mitochondrial matrix and that the linker between two transmembrane helices faces the inter-membrane space [52, 56, 64]. The negatively charged residues in the linker are critical for Ca2+ transport. Mutating these residues (E257A, D261A, and E264A) severely damaged mitochondrial calcium uptake. Baughman et al. showed that the mutation of Ser259 had little influence on mitochondrial Ca2+ uptake; however, the sensitivity toward ruthenium red was lost.
In intact cells, permeabilized cells and purified mouse liver mitochondria, MCU silencing severely impaired mitochondrial calcium uptake in response to histamine stimulation or an extra-mitochondrial Ca2+ pulse, but cytosolic Ca2+ mobilization remained intact. The mitochondrial Ca2+ uptake phenotype was rescued through the expression of MCU cDNA. Downregulating MCU did not affect other mitochondrial properties, such as O2 consumption, ATP synthesis, ΔΨm or organelle shape.
The most important finding in the study by Baughman et al. was that purified MCU protein that was reconstituted in a planar lipid bilayer showed channel activity when Ca2+ was the only cation contained in the medium. The channel activity measured was similar to that reported by Kirichok et al. and was inhibited by ruthenium red. The mutant MCU protein in which two acidic residues (D260 and E263) in the loop connecting two transmembrane helices were mutated to glutamine failed to show Ca2+-permeable channel activity in bilayer experiments [62].
Marchi et al. [65] found that miR-25 targeted the 3′UTR of MCU and then drastically reduced MCU levels as well as mitochondrial Ca2+ uptake, which is a key aspect of human colon cancer progression. Kovacs-Bogdan et al. [66] found that mitochondrial Ca2+ uniporter activity could be reconstituted in yeast through expressing Dictyostelium discoideum (Dd) MCU. By contrast, reconstitution of the human uniporter required the co-expression of MCU and EMRE.
Unexpectedly, mice lacking MCU did not exhibit an apparent phenotype of mitochondrial Ca2+ uptake [67, 68]. In MCU-deficient skeletal muscle, significant levels of Ca2+ were detected in the mitochondrial matrix and the muscle exhibited alterations in the phosphorylation and activity of pyruvate dehydrogenase, which suggests the existence of a compensatory mechanism in vivo. The basis for this result is unclear, and further investigation is necessary. One likely explanation is that alternative pathways exist for transporting Ca2+ into mitochondria. Through patch-clamping mitoplasts, researchers have recorded different types of Ca2+ currents [69]; these uncharacterized Ca2+ transporters may functionally compensate for the loss of MCU in mice. An alternative explanation is that mouse mitochondria may contain MCU splice variants (see review [70]), which may also be functional for Ca2+ uptake.
MCUb and EMRE
MCUb is a paralog of MCU [63]. The MCUb gene is present in vertebrates but absent in other organisms, such as Plants, Kinetoplastids, Nematoda and Arthropoda. MCUb is located on the inner mitochondrial membrane and contains approximately 330 amino acids [70]. MCUb is conserved among all species and shares a 50 % similarity with MCU. Predictions suggest that MCUb, like MCU, has two transmembrane helices. RT-PCR results showed that MCUb was expressed at low levels and exhibited a different expression profile in HeLa cells and mouse tissues. MCU and MCUb physically interact with each other and form a hetero-oligomer (tetramer). Ca2+ measurements in intact cells showed that, under histamine stimulation, MCUb silencing increased the [Ca2+]m, whereas MCUb overexpression markedly reduced the [Ca2+]m. Electrophysiological recordings demonstrated that MCUb is a dominant-negative form of MCU, which alters Ca2+ permeation across the heteromeric channel [63]. Sancak et al. [49] also found that MCUb is a component of the mitochondrial Ca2+ uniporter complex.
In an effort to establish a full molecular characterization of the uniporter complex, Sancak et al. discovered an additional component: essential MCU regulator (EMRE) [71]. EMRE is a 10-kDa, metazoan-specific inner mitochondrial membrane protein with a single predicted transmembrane helix. EMRE RNA is broadly expressed in all mouse tissues. EMRE silencing leads to a loss of mitochondrial Ca2+ uptake in permeabilized HEK-293T and HeLa cells after histamine stimulation. EMRE interacts with both MICU1/2 and MCU. EMRE knockout abolished the interaction between MICU1/2 and MCU. Loss of EMRE reduced the uniporter complex size to approximately 300 kDa on a native gel, similar to cells lacking MICU1. As a result, these authors proposed that EMRE interacts with MICU1/2 in the intermembrane and with MCU oligomers in the inner membrane [49].
MCUR1 and SLC25A23
Mallilankaraman et al. [72] reported that MCUR1 (mitochondrial Ca2+ uniporter regulator 1) plays a crucial role in mitochondrial Ca2+ uptake. MCUR1 is an inner mitochondrial membrane protein containing two transmembrane regions, with most of the protein located in the matrix. MCUR1 binds MCU (but not MICU1) and regulates MCU-dependent Ca2+ uptake. Silencing MCUR1 abrogated mitochondrial Ca2+ uptake in intact and permeabilized cells. MCUR1 knockdown disrupted oxidative phosphorylation, activated AMPK and induced macroautophagy. As a result, these authors proposed that MCUR1 is a critical component of the mitochondrial uniporter complex [70, 72].
Another regulator of mitochondrial Ca2+ uptake was recently identified: solute carrier 25A23 (SLC25A23) [73]. SLC25A23 contains two EF-hands and lies on the mitochondrial inner membrane. SLC25A23 knockdown decreased mitochondrial calcium uptake after histamine stimulation. Ectopic expression of SLC25A23 EF-hand mutants reduced mitochondrial Ca2+ uptake, which indicates that SLC25A23 EF-hands play an important role in regulating mitochondrial Ca2+ uptake. In addition, SLC25A23 interacts with MCU as well as MICU1 and increases I MCU. SLC25A23 increases basal reactive oxygen species (ROS) and induces oxidative stress-mediated cell death [73], providing a mechanism that targets MCU-dependent Ca2+ overload.
Other possible calcium transporters in the inner mitochondrial membrane
In addition to the uniporter complex, several other types of mitochondrial Ca2+ uptake transporters have been proposed [74], including mitochondrial ryanodine receptor (mRyR1), uncoupling proteins (UCP), leucine zipper-EF-hand-containing transmembrane protein 1 (LETM1), mitochondrial Ca2+ current type 2 (mCa2), rapid mode of Ca uptake (RaM), coenzyme Q10, and canonical transient receptor potential channel 3 (TRPC3) [75].
MRyR1 is a ryanodine receptor in the inner mitochondrial membrane [76] that participates in mitochondrial Ca2+ influx in both cardiomyocytes and neurons [77, 78]. mRyR may be a skeletal-muscle isoform of RyR type 1, which is present not only in native cardiomyocytes but also in cultured cardiac myoblasts and even in knockout mouse hearts [79]. mRyR can accumulate Ca2+ in mitochondria in response to cytosolic Ca2+ elevation and can be blocked by high concentrations of ryanodine and ruthenium red [77, 79–81]. More importantly, lipid bilayer experiments produced a large Ca2+-sensitive conductance (500–800 pS) [80]. Therefore, mRyR may be another route to Ca2+ uptake in mitochondria.
Uncoupling proteins (UCP) are transporters that are located on the inner mitochondrial membrane and that create proton leaks, thereby uncoupling oxidative phosphorylation from ATP synthesis [82]. Trenker et al. reported that UCP2 and UCP3 are fundamental for the mitochondrial Ca2+ uniporter in response to cell stimulation through overexpression, siRNA and mutagenesis experiments. These authors also confirmed a lack of ruthenium red-sensitive Ca2+ uptake in liver mitochondria isolated from UCP2 −/− mice [83]. The UCP2 structure was determined using NMR molecular fragment replacement in 2011 [84]. UCP2 consists mainly of six transmembrane helices that form a channel-like structure. The UCP2 structure can be divided into three pseudo repeats with similar folds. Each of the two transmembrane helices, along with the loop and amphipathic helix between them, forms a repeat. The structure of UCP2 closely resembles that of the bovine ADP/ATP carrier (ANT1), but the difference in the third repeat yields a more open structure on the matrix side of the carrier in UCP2 compared with ANT1. These results suggest that it is unlikely that UCP proteins transport Ca2+ directly into mitochondria.
LETM1 is a highly conserved eukaryotic protein located in the inner mitochondrial membrane [85, 86]. The function of LETM1 is controversial. LETM1 was originally identified as a K+/H+ exchanger [85, 87, 88]. However, genome-wide siRNA screening in Drosophila suggested that LETM1 is a Ca2+/H+ exchanger and is inhibited by ruthenium red [89]. Recent studies in liposomes containing purified LETM1 protein indicate that LETM1 is a Ca2+/H+ antiporter but is insensitive to ruthenium red [38]. Therefore, LETM1 most likely mediates mitochondrial Ca2+ efflux, not Ca2+ uptake [74].
The rapid mode (RaM) of Ca2+ uptake is a mechanism that functions at the beginning of each of the cytosolic Ca2+ pulses and allows mitochondria to rapidly sequester Ca2+ from short pulses. Furthermore, this uptake rapidly recovers between pulses, which facilitates a mitochondrial response to repetitive Ca2+ transients [90]. mCa2 is also a voltage-gated mitochondrial Ca2+ selective channel similar to MCU, but it is ruthenium red-insensitive and has low sensitivity. mCa2 was less active under failing heart conditions than in mitoplasts from non-failing hearts [91]. Coenzyme Q10 is a central electron carrier in the mitochondrial electron-transport chain (ETC). The hydroxylated coenzyme 10 binds and transports Ca2+ across artificial biomimetic membranes [92]. TRPC3 is a member of the large superfamily of transient receptor potential channels. It is permeable to the cations: Ca2+, Na+ and K+ [93]. A portion of TRPC3 is localized to the inner mitochondrial membrane. HeLa cells with stably overexpressed TRPC3 and siRNA-down-regulated MCU showed significant mitochondrial Ca2+ uptake when extramitochondrial Ca2+ concentrations were relatively high, indicating that the TRPC3 channel may be another mitochondrial Ca2+ uptake pathway [75].
Concluding remarks
Studies of mitochondrial calcium uptake have resulted in significant progress recently, and the components of the complicated uniporter complex and several regulators have been identified and characterized. In the future, we anticipate that more intensive studies will address the delicate regulation of this uniporter complex. In addition, the uniporter complex acts predominantly in the mitochondrial Ca2+ uptake mode. Alternative mitochondrial Ca2+ uptake mechanisms may exist; research on this topic may broaden our understanding of mitochondrial Ca2+ uptake.
Acknowledgments
This work was supported by the 973 Program (Grants 2012CB917200 and 2013CB910400 to YS; Grant 2014CB910201 to XY), the Natural Science Foundation of China (Grants 31370826 to YS and 31300628 to XY), Tianjin Basic Research Program (Grant 14JCQNJ09300 to XY) and the Fundamental Research Funds for the Central Universities (Grants 65142007 to YS and 65121016 to XY).
References
- 1.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 2.Chandel NS. Mitochondria as signaling organelles. BMC Biol. 2014;12:34. doi: 10.1186/1741-7007-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagliarini DJ, Rutter J. Hallmarks of a new era in mitochondrial biochemistry. Genes Dev. 2013;27:2615–2627. doi: 10.1101/gad.229724.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Carafoli E. The fateful encounter of mitochondria with calcium: how did it happen? Biochim Biophys Acta. 2010;1797:595–606. doi: 10.1016/j.bbabio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Graier WF, Frieden M, Malli R. Mitochondria and Ca(2+) signaling: old guests, new functions. Pflugers Arch. 2007;455:375–396. doi: 10.1007/s00424-007-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529(Pt 1):57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feske S, Skolnik EY, Prakriya M. Ion channels and transporters in lymphocyte function and immunity. Nat Rev Immunol. 2012;12:532–547. doi: 10.1038/nri3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/S0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- 11.Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol. 1997;136:833–844. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- 13.Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denton RM, McCormack JG. The role of calcium in the regulation of mitochondrial metabolism. Biochem Soc Trans. 1980;8:266–268. doi: 10.1042/bst0080266. [DOI] [PubMed] [Google Scholar]
- 15.McCormack JG, Denton RM. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979;180:533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 18.Hajnoczky G, Csordas G, Madesh M, Pacher P. Control of apoptosis by IP(3) and ryanodine receptor driven calcium signals. Cell Calcium. 2000;28:349–363. doi: 10.1054/ceca.2000.0169. [DOI] [PubMed] [Google Scholar]
- 19.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 22.Deluca HF, Engstrom GW. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci USA. 1961;47:1744–1750. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasington FD, Murphy JV. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem. 1962;237:2670–2677. [PubMed] [Google Scholar]
- 24.Brand MD, Chen CH, Lehninger AL. Stoichiometry of H+ ejection during respiration-dependent accumulation of Ca2+ by rat liver mitochondria. J Biol Chem. 1976;251:968–974. [PubMed] [Google Scholar]
- 25.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 26.Rottenberg H, Scarpa A. Calcium uptake and membrane potential in mitochondria. Biochemistry. 1974;13:4811–4817. doi: 10.1021/bi00720a020. [DOI] [PubMed] [Google Scholar]
- 27.Drago I, Pizzo P, Pozzan T. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO J. 2011;30:4119–4125. doi: 10.1038/emboj.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 29.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 30.Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shoshan-Barmatz V, Ben-Hail D. VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion. 2012;12:24–34. doi: 10.1016/j.mito.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC) J Biol Chem. 2006;281:17347–17358. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- 33.Shoshan-Barmatz V, Israelson A, Brdiczka D, Sheu SS. The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr Pharm Des. 2006;12:2249–2270. doi: 10.2174/138161206777585111. [DOI] [PubMed] [Google Scholar]
- 34.Hodge T, Colombini M. Regulation of metabolite flux through voltage-gating of VDAC channels. J Membr Biol. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- 35.Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med. 2010;31:227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 36.De Stefani D, Bononi A, Romagnoli A, Messina A, De Pinto V, Pinton P, Rizzuto R. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ. 2012;19:267–273. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem J. 2001;358:147–155. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai MF, Jiang D, Zhao L, Clapham D, Miller C. Functional reconstitution of the mitochondrial Ca2+/H+ antiporter Letm1. J Gen Physiol. 2014;143:67–73. doi: 10.1085/jgp.201311096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K. Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci USA. 2008;105:15370–15375. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, Ping P, Abramson J. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, Ben-Romano R, Friedman O, Shoshan-Barmatz V. The VDAC1N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J Cell Sci. 2009;122:1906–1916. doi: 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]
- 43.Zheng L, Stathopulos PB, Li GY, Ikura M. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem Biophys Res Commun. 2008;369:240–246. doi: 10.1016/j.bbrc.2007.12.129. [DOI] [PubMed] [Google Scholar]
- 44.Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, et al. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Israelson A, Abu-Hamad S, Zaid H, Nahon E, Shoshan-Barmatz V. Localization of the voltage-dependent anion channel-1 Ca2+-binding sites. Cell Calcium. 2007;41:235–244. doi: 10.1016/j.ceca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Israelson A, Zaid H, Abu-Hamad S, Nahon E, Shoshan-Barmatz V. Mapping the ruthenium red-binding site of the voltage-dependent anion channel-1. Cell Calcium. 2008;43:196–204. doi: 10.1016/j.ceca.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins S, Meyer T. Cell biology: a sensor for calcium uptake. Nature. 2010;467:283. doi: 10.1038/467283a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hajnoczky G, Csordas G. Calcium signalling: fishing out molecules of mitochondrial calcium transport. Curr Biol. 2010;20:R888–R891. doi: 10.1016/j.cub.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bick AG, Calvo SE, Mootha VK. Evolutionary diversity of the mitochondrial calcium uniporter. Science. 2012;336:886. doi: 10.1126/science.1214977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 2012;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoffman NE, Chandramoorthy HC, Shamugapriya S, Zhang X, Rajan S, Mallilankaraman K, Gandhirajan RK, Vagnozzi RJ, Ferrer LM, Sreekrishnanilayam K, et al. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell Rep. 2013;5:1576–1588. doi: 10.1016/j.celrep.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L, Yang X, Li S, Wang Z, Liu Y, Feng J, Zhu Y, Shen Y. Structural and mechanistic insights into MICU1 regulation of mitochondrial calcium uptake. EMBO J. 2014;33:594–604. doi: 10.1002/embj.201386523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, Perez SDLF, Bogorad R, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 2013;17:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabo I, De Stefani D, Rizzuto R. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell. 2014;53:726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hung V, Zou P, Rhee HW, Udeshi ND, Cracan V, Svinkina T, Carr SA, Mootha VK, Ting AY. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol Cell. 2014;55:332–341. doi: 10.1016/j.molcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamer KJ, Mootha VK. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep. 2014;15:299–307. doi: 10.1002/embr.201337946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS ONE. 2013;8:e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahuja M, Muallem S. The gatekeepers of mitochondrial calcium influx: mICU1 and MICU2. EMBO Rep. 2014;15:205–206. doi: 10.1002/embr.201438446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabo I, Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martell JD, Deerinck TJ, Sancak Y, Poulos TL, Mootha VK, Sosinsky GE, Ellisman MH, Ting AY. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat Biotechnol. 2012;30:1143–1148. doi: 10.1038/nbt.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marchi S, Lupini L, Patergnani S, Rimessi A, Missiroli S, Bonora M, Bononi A, Corra F, Giorgi C, De Marchi E, et al. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol. 2013;23:58–63. doi: 10.1016/j.cub.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kovacs-Bogdan E, Sancak Y, Kamer KJ, Plovanich M, Jambhekar A, Huber RJ, Myre MA, Blower MD, Mootha VK. Reconstitution of the mitochondrial calcium uniporter in yeast. Proc Natl Acad Sci USA. 2014;111:8985–8990. doi: 10.1073/pnas.1400514111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herzig S, Maundrell K, Martinou JC. Life without the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1398–1400. doi: 10.1038/ncb2891. [DOI] [PubMed] [Google Scholar]
- 68.Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bondarenko AI, Jean-Quartier C, Malli R, Graier WF. Characterization of distinct single-channel properties of Ca(2+) inward currents in mitochondria. Pflugers Arch. 2013;465:997–1010. doi: 10.1007/s00424-013-1224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pendin D, Greotti E, Pozzan T. The elusive importance of being a mitochondrial Ca(2+) uniporter. Cell Calcium. 2014;55:139–145. doi: 10.1016/j.ceca.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 71.Marchi S, Pinton P. The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J Physiol. 2014;592:829–839. doi: 10.1113/jphysiol.2013.268235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffman NE, Chandramoorthy HC, Shanmughapriya S, Zhang XQ, Vallem S, Doonan PJ, Malliankaraman K, Guo S, Rajan S, Elrod JW, et al. SLC25A23 augments mitochondrial Ca(2)(+) uptake, interacts with MCU, and induces oxidative stress-mediated cell death. Mol Biol Cell. 2014;25:936–947. doi: 10.1091/mbc.E13-08-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dedkova EN, Blatter LA. Calcium signaling in cardiac mitochondria. J Mol Cell Cardiol. 2013;58:125–133. doi: 10.1016/j.yjmcc.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng S, Li H, Tai Y, Huang J, Su Y, Abramowitz J, Zhu MX, Birnbaumer L, Wang Y. Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc Natl Acad Sci USA. 2013;110:11011–11016. doi: 10.1073/pnas.1309531110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ryu SY, Beutner G, Dirksen RT, Kinnally KW, Sheu SS. Mitochondrial ryanodine receptors and other mitochondrial Ca2+ permeable channels. FEBS Lett. 2010;584:1948–1955. doi: 10.1016/j.febslet.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276:21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 78.Jakob R, Beutner G, Sharma VK, Duan Y, Gross RA, Hurst S, Jhun BS, O-Uchi J, Sheu SS. Molecular and functional identification of a mitochondrial ryanodine receptor in neurons. Neurosci Lett. 2014;575:7–12. doi: 10.1016/j.neulet.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim Biophys Acta. 2005;1717:1–10. doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 80.Altschafl BA, Beutner G, Sharma VK, Sheu SS, Valdivia HH. The mitochondrial ryanodine receptor in rat heart: a pharmaco-kinetic profile. Biochim Biophys Acta. 2007;1768:1784–1795. doi: 10.1016/j.bbamem.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 81.Ryu SY, Beutner G, Kinnally KW, Dirksen RT, Sheu SS. Single channel characterization of the mitochondrial ryanodine receptor in heart mitoplasts. J Biol Chem. 2011;286:21324–21329. doi: 10.1074/jbc.C111.245597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stuart JA, Cadenas S, Jekabsons MB, Roussel D, Brand MD. Mitochondrial proton leak and the uncoupling protein 1 homologues. Biochim Biophys Acta. 2001;1504:144–158. doi: 10.1016/S0005-2728(00)00243-7. [DOI] [PubMed] [Google Scholar]
- 83.Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berardi MJ, Shih WM, Harrison SC, Chou JJ. Mitochondrial uncoupling protein 2 structure determined by NMR molecular fragment searching. Nature. 2011;476:109–113. doi: 10.1038/nature10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashimi H, McDonald L, Stribrna E, Lukes J. Trypanosome Letm1 protein is essential for mitochondrial potassium homeostasis. J Biol Chem. 2013;288:26914–26925. doi: 10.1074/jbc.M113.495119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nowikovsky K, Bernardi P. LETM1 in mitochondrial cation transport. Front Physiol. 2014;5:83. doi: 10.3389/fphys.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nowikovsky K, Froschauer EM, Zsurka G, Samaj J, Reipert S, Kolisek M, Wiesenberger G, Schweyen RJ. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf–Hirschhorn syndrome. J Biol Chem. 2004;279:30307–30315. doi: 10.1074/jbc.M403607200. [DOI] [PubMed] [Google Scholar]
- 88.Dimmer KS, Navoni F, Casarin A, Trevisson E, Endele S, Winterpacht A, Salviati L, Scorrano L. LETM1, deleted in Wolf–Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum Mol Genet. 2008;17:201–214. doi: 10.1093/hmg/ddm297. [DOI] [PubMed] [Google Scholar]
- 89.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem. 1995;270:27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- 91.Michels G, Khan IF, Endres-Becker J, Rottlaender D, Herzig S, Ruhparwar A, Wahlers T, Hoppe UC. Regulation of the human cardiac mitochondrial Ca2+ uptake by 2 different voltage-gated Ca2+ channels. Circulation. 2009;119:2435–2443. doi: 10.1161/CIRCULATIONAHA.108.835389. [DOI] [PubMed] [Google Scholar]
- 92.Bogeski I, Gulaboski R, Kappl R, Mirceski V, Stefova M, Petreska J, Hoth M. Calcium binding and transport by coenzyme Q. J Am Chem Soc. 2011;133:9293–9303. doi: 10.1021/ja110190t. [DOI] [PubMed] [Google Scholar]
- 93.Beech DJ, Bahnasi YM, Dedman AM, Al-Shawaf E. TRPC channel lipid specificity and mechanisms of lipid regulation. Cell Calcium. 2009;45:583–588. doi: 10.1016/j.ceca.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]