Abstract
Obesity and metabolic disorders, such as type 2 diabetes and hypertension, have attracted considerable attention as life-threatening diseases not only in developed countries but also worldwide. Additionally, the rate of obesity in young people all over the world is rapidly increasing. Accumulated evidence suggests that the central nervous system may participate in the development of and/or protection from obesity. For example, in the brain, the hypothalamic melanocortin system senses and integrates central and peripheral metabolic signals and controls the degree of energy expenditure and feeding behavior, in concert with metabolic status, to regulate whole-body energy homeostasis. Currently, researchers are studying the mechanisms by which peripheral metabolic molecules control feeding behavior and energy balance through the central melanocortin system. Accordingly, recent studies have revealed that some inflammatory molecules and transcription factors participate in feeding behavior and energy balance by controlling the central melanocortin pathway, and have thus become new candidates as therapeutic targets to fight metabolic diseases such as obesity and diabetes.
Keywords: Hypothalamus, Pro-opiomelanocortin, Agouti-related peptide, Neuropeptide Y, Leptin, Transcription factors, Posttranslational modification, Energy balance, Melanocortin pathway
Introduction
The rate of obesity in the worldwide population is rapidly increasing; consequently, it is also gaining attention in many countries as a serious life-threatening factor. Of all adults in the worldwide population, approximately 17 % are obese and 10 % are diabetic. Obesity is a predisposing condition for several other metabolic syndromes, such as hypertension, type 2 diabetes and stroke [1–3]. The development of this obesity epidemic is highly associated with complex and various abnormalities in delicate balance between caloric excess and body weight homeostasis. In other words, the precise coordination of multi-signaling between the peripheral tissues and central nervous system (CNS) is necessary for balancing food intake and energy expenditure, and thus preserves the overall energy standard; this system is compromised in obese individuals.
Many research efforts have primarily focused on the molecular, cellular and systemic integration of the CNS in the regulation of metabolism and feeding behaviors [4–6]. These scientists found that the neuronal networks in the hypothalamus sense a variety of metabolic signals generated by several peripheral systems and transported through the circulating blood stream, and in turn produce an equivalent tone of intra- and intercellular signaling into deep brain regions responsible for coordinated energy expenditure and feeding behavior. Specifically, the neurons in the arcuate nucleus of the hypothalamus (ARC), ventromedial hypothalamus (VMH), dorsomedial hypothalamus (DMH), lateral hypothalamic area (LH) and the paraventricular nucleus of the hypothalamus (PVN) are the first cells to respond to the numerous peripheral metabolic inputs, such as leptin and ghrelin. These neurons are anatomically localized around the third ventricle (3v), and they include biochemically discrete types of cells expressing certain receptors specific to the metabolic signal molecules that originate from both the peripheral tissues and CNS [7–11].
The ARC includes two physiologically distinct types of neuronal populations, the proopiomelanocortin (POMC) neurons and the agouti-related peptide (AgRP)/neuropeptide Y (NPY) neurons [12, 13]. To date, numerous studies have focused extensively on the mechanisms relying on these neurons that are associated with energy balance. The AgRP/NPY neurons are localized more medio-centrally to the 3v, while most POMC neurons are localized more laterally. The AgRP/NPY neurons sense the hunger-related signal molecules, including ghrelin, and individually play a pivotal role in energy homeostasis [14, 15]. Particulary, recent findings revealed that stimulation of AgRP neuronal activity using channelrhodopsin-2-based photostimulation evoked animals’ feeding behavior independent of the melanocortin system [16]. In this study, activation of <1,000 AgRP neurons was sufficient to trigger an animal’s food intake, and the degree of change in feeding behavior was positively correlated with either increased duration or frequency of the photostimulation of AgRP neurons. Interestingly, this AgRP neuron-evoked feeding behavior was regulated by the γ-amino-butyric acid (GABA) input to the other brain regions, suggesting that the GABA component of AgRP neuron is crucial in the regulation of feeding behavior, independent of AgRP release from the same neuron.
Among the extra-hypothalamic brain regions, it has been reported that certain nuclei in the brainstem, including the dorsal motor vagal nucleus (DMV), the parabrachial nucleus (PBN) and nucleus of the solitary tract (NTS), also play critical roles in whole-body physiologies related to metabolism regulation, such as feeding behavior, blood pressure and gastric function [17–24]. The PBN has been specifically implicated as a participant in energy homeostasis and feeding behavior, independent of the melanocortin system [25]. The AgRP neurons in the ARC (ARC-AgRP neurons) inhibit the PBN, and, thus, ablation of ARC-AgRP neurons resulted in anorexia and starvation behaviors through the hyperactivation of PBN neurons [24]. However, anatomical and biochemical evidence revealed that a main function of the AgRP/NPY neurons in metabolism regulation is achieved through the melanocortin pathway, which provides inhibitory signals to the melanocortin system [26–28]. In reality, the AgRP/NPY system is a negative counterpart of melanocortin signaling in energy homeostasis. Thus, we next describe in detail the mechanisms how the melanocortin system of the brain affects energy homeostasis and feeding behavior.
The melanocortin system in the regulation of metabolism
The central melanocortin system detects and integrates a variety of hormonal, neuronal and nutritional metabolic signals, such as leptin, insulin, glucose, leucine and serotonin, and regulates energy homeostasis [29, 30] (Fig. 1). Melanocortins are a family of small peptides, which originate from the POMC precursor. A recent study indicated that postnatal ablation of POMC neurons in the brain leads to the development of an obese phenotype with a reduced energy expenditure, similar to that observed in leptin-deficient animal models [31]. A similar metabolic phenotype as with ablation of POMC neurons was observed in a mouse model with Pomc gene deletion [32, 33]. Several research groups reported that a mutation in POMC is highly correlated with obesity development in humans [34–36]. On the other hand, studies using animal models overexpressing POMC, either by genetic modification or by virus-associated gene delivery, have demonstrated that these animals were protected from obesity development [37–39]. These studies clearly demonstrated a role of the central POMC system in metabolic regulation and feeding behavior.
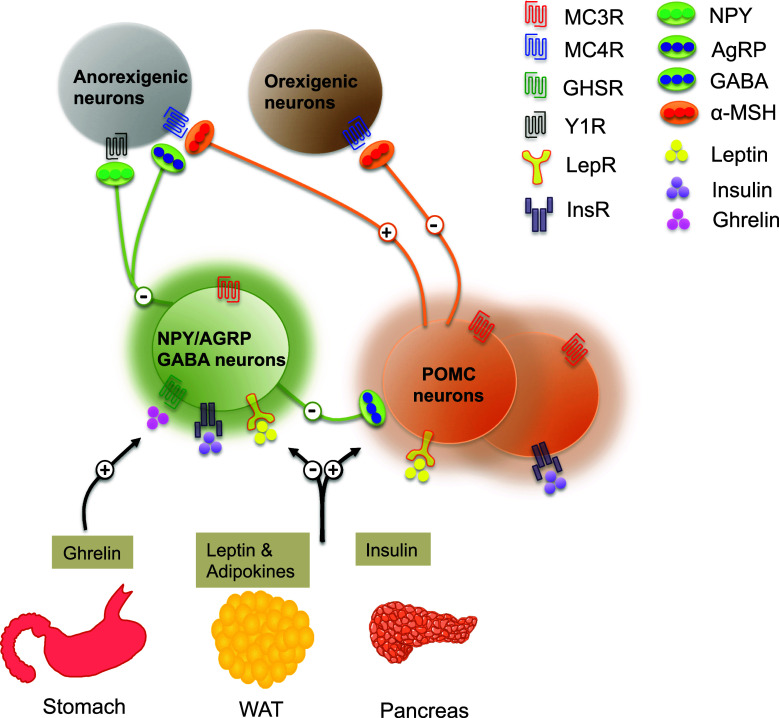
Fig. 1.
Hypothalamic melanocortin pathways regulating central energy metabolism. The hypothalamic arcuate nucleus detects a variety of metabolic signals, which originate from several peripheral organs including the gut, adipose tissue and pancreas. For example, the AgRP/NPY neurons express ghrelin receptors, and, thus, specifically detect a gut-derived and hunger-related metabolic molecule, ghrelin, to facilitate energy intake. On the other hand, distinct subtypes of POMC neurons produce either leptin or insulin receptors and respond to the anorexigenic molecules such as adipose-originated leptin or pancreas-derived insulin. Notably, POMC neurons expressing leptin receptors do not co-produce insulin receptors, and vice versa, indicating the existence of heterogeneous subtypes of POMC neurons in the hypothalamus. AgRP/NPY neurons also express leptin and insulin receptors; however, these molecules negatively regulate the AgRP/NPY neuronal activity, unlike POMC cells. POMC and AgRP/NPY neurons project their axon terminals into various brain regions and activate and/or inactivate target cells expressing the dominant melanocortin receptors MC3/4R. Indeed, POMC and AgRP/NPY neurons are in functional opposition to one another. POMC neurons stimulate a satiety response by releasing α-MSH, the most well-known POMC-derived small melanocortin associated with energy balance and feeding behavior, to the anorexigenic system. AgRP/NPY neurons release AgRP and NPY, which are naturally appearing, endogenous antagonists of α-MSH, to the anorexigenic system. Furthermore, AgRP/NPY neurons also create synaptic connections with the POMC system and release GABA to inactivate this system. Overall, a precise coordination of signaling between the POMC and AgRP/NPY systems in the hypothalamus, in concert with metabolic status, is critical for balancing feeding behavior and whole-body energy homeostasis. NPY neuropeptide Y, AgRP agouti-related peptide, GABA gamma-aminobutyric acid, α-MSH α-melanocyte-stimulating hormone, MC3/4R melanocortin-3/4 receptor, GHSR growth hormone secretagogue receptor, Y1R neuropeptide Y Y1 receptor, LepR leptin receptor, InsR insulin receptor, WAT white adipose tissue
In the brain, POMC-producing neurons are localized in several discrete regions including the pituitary, the ARC and the brainstem. In the ARC, it is believed that distinct groups of POMC neurons project into other hypothalamic areas such as the PVN and the LH, as well as the brainstem area, all of which are known to be critical for energy homeostasis [12, 13]. Recently, it has also been reported that in the ARC, POMC neurons expressing leptin receptors do not express serotonin receptors, and vice versa, suggesting the existence of heterogeneous POMC populations in the ARC [40, 41]. POMC-expressing neurons are also found in the NTS of the brainstem [20]. These cells also produce leptin receptors, but do not respond to leptin [21, 42]. It is believed that POMC neurons in the ARC are responsible for the long-term regulation of feeding behavior, which is directly correlated with adiposity, while POMC neurons in the NTS are important for the control of short-term satiety signal [19]. Using the DREAD (designer receptors exclusively activated by designer drugs) method and diphtheria toxin-induced specific ablation of POMC cells either in the ARC or NTS, Zhan et al. [19] also revealed that ablation of POMC cells, only in the ARC and not in the NTS, increased food intake and led to the development of an obese phenotype in animals. As described above, the POMC neurons monitor a variety of circulating signal molecules directly associated with energy balance. However, the detailed relationship between heterogeneous POMC populations with differential projections and their different roles associated with energy homeostasis is not yet understood.
Numerous studies have also shown that cellular signaling within POMC neurons is closely associated with the complex processes for posttranslational modification of POMC protein. POMC is a prohormone that produces several small peptides, such as alpha-melanocyte-stimulating hormone (α-MSH), through a series of protein modification. Therefore, this review will now describe in detail some recently discovered upstream signaling molecules that regulate the POMC system, as well as a special role for leptin as a representative adipokine in this system and the posttranslational modification processes of POMC in association with energy homeostasis. We will also discuss α-MSH, the most well-known POMC-derived melanocortin in the brain, and its receptor system.
Transcription factors for the melanocortin pathway
A great deal of attention has been paid recently to the identification of new signal molecules in the melanocortin system as potential candidates for the therapeutic treatment of metabolic disorders. Notably, some transcription factors are involved in appetite regulation through the transcriptional control of Agrp and Pomc genes in the brain (Fig. 2). The promoter regions of both Pomc and Agrp genes contain binding domains for forkhead protein 1 (Foxo1), which activates AgRP transcription, but inhibit POMC transcription as a downstream transcription factor of insulin signaling [43, 44]. On the contrary, signal transducer and activator of transcription 3 (STAT3) inhibits AgRP transcriptional activity and triggers gene expression of POMC by binding to the leptin-response element [45, 46]. The ablation of STAT3 in POMC neurons resulted in mild obesity development with decreased Pomc gene expression [45].
Fig. 2.
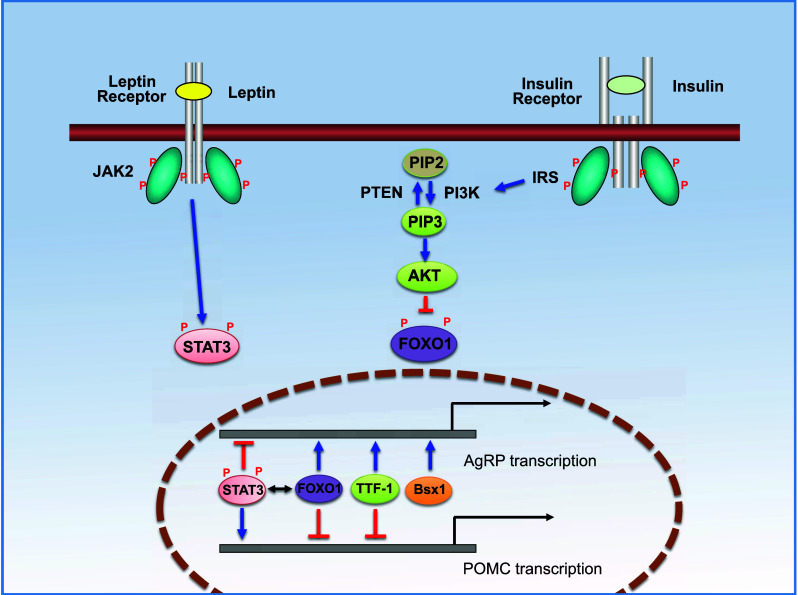
Intracellular signaling pathways of leptin and insulin receptors in the central melanocortin system. Hypothalamic POMC and AgRP/NPY neurons express both leptin and insulin receptors. Binding of leptin and insulin to their receptors triggers sequential intracellular signaling cascades, finally affecting the transcriptional activity of the melanocortin system. Specifically, the binding of leptin to its receptor induces auto-phosphorylation of the receptor as well as the phosphorylation of JAK (pJAK2) that subsequently activates STAT3 signaling by phosphorylation of STAT3 (pSTAT3). pSTAT3 is then translocated into the nucleus and regulates the expression of genes encoding AgRP and POMC. Independent of leptin signaling, insulin binding to its receptor activates IRS, and further stimulates the PI3K pathway. PI3K phosphorylates PIP2 to produce PIP3 that becomes PIP2 reversely by PTEN-mediated dephosphorylation. PIP3 induces phosphorylation of AKT (pAKT) and pAKT inhibits phosphorylation of FOXO1. The dephosphorylated-FOXO1 directly binds to the promoter region of Agrp and Pomc genes and regulates their transcriptional activities. Interestingly, STAT3 and FOXO1 compete with each other for the binding domain. Recently, the involvement of some transcription factors in the regulation of central energy balance through the melanocortin system has been determined. Homeobox-containing transcription factors such as TTF-1 and Bsx are expressed in the hypothalamus. TTF-1 activates Agrp gene expression, but inhibits Pomc transcriptional activity via binding to its specific binding motifs in the Agrp and Pomc gene promoters. Unlike other transcription factors described above, Bsx1 triggers Agrp but not Pomc gene expression. JAK2 janus kinase 2, STAT3 signal transducer and activator of transcription 3, AKT serine/threonine-specific protein kinase, FOXO1 forkhead box protein O1, IRS insulin receptor substrate, PI3K, phosphoinositide 3 kinase, PIP2 phosphatidylinositol (4,5)-bisphosphate, PIP3 phosphatidylinositol (3,4,5)-triphosphate, PTEN phosphatase and tensin homolog, TTF-1 thyroid transcription factor-1, Bsx brain-specific homeobox transcription factor
Several lines of evidence have also demonstrated that homeobox-containing transcription factors such as thyroid transcription factor-1 (TTF-1) and brain-specific homeobox transcription factor (Bsx) are expressed in the hypothalamus and play a pivotal role in the regulation of Pomc and Agrp gene expression, thus influencing feeding behavior [47–49]. TTF-1 is also a member of the NKx family of homeodomain genes important for the development of the diencephalic brain region. Knockdown of TTF-1 biosynthesis in the rodent brain inhibited gene expression of Agrp, but activated Pomc transcription, and further resulted in a decrease in appetite and daily body weight gain of animals [47, 48]. Unlike TTF-1, ablation of Bsx in mice resulted in decreased Npy and Agrp gene expression as well as reduced locomotion and food-seeking behavior. Furthermore, the deletion of Bsx alleviated hyperphagic behavior of animals, which is generally observed in the leptin-deficient mouse model, and thereby prevented the animals from obesity development. Unlike other transcription factors mentioned above, Bsx is required only for Agrp gene transcription [49].
Role of inflammatory cytokines in POMC neuron function
In addition to the transcription factors, researchers have recently paid a great deal of attention to the function of inflammatory cytokines in feeding behavior. In rodents, some proinflammatory cytokines such as interleukin 6 (IL6) and tumor necrosis factor alpha (TNFα) were up-regulated, with an increased astrocytic density in the hypothalamus, during early phases of high-fat diet (HFD) treatment [50]. Up-regulation of inflammatory signals in the hypothalamus was also observed in obese humans [50]. An independent study demonstrated in detail the participation of inflammatory signaling in the transcriptional activity of the melanocortin system: rodents receiving lipopolysaccharide (LPS) treatment exhibited an increased level of Pomc gene expression in the ARC [51]. In addition, both POMC and AgRP/NPY neurons expressed type 1 interleukin-1 receptor 1 (IL-1R1), and the exogenous administration of IL-1-beta resulted in an increase in the firing rate of POMC neurons [52] and a decrease in the spontaneous release of AgRP [53]. Furthermore, inflammatory signaling in the hypothalamic melanocortin system is strongly associated with disease-related negative energy balance such as anorexia and cachexia [54–56]. These results clearly demonstrate the participation of inflammatory signaling in central regulation of metabolism, primarily through the melanocortin signaling pathway.
Leptin in the control of energy balance
Over the past decade, great scientific progress has been achieved to understand participation of the adipose tissue-derived cytokines (adipokines) in the control of energy homeostasis, including feeding behavior, thermogenesis and other neuroendocrine functions. These adipokines, including adiponectin, visfatin, vaspin, apellin and leptin [57–62], play critical roles for the control of hypothalamic melanocortin pathway. Among them, leptin participates in a variety of physiological and behavioral controls, as a representative adipocyte-derived signal modulator in association with energy homeostasis [13, 63–65]. For example, a mouse model with depletion of leptin (Lepob/ob) displayed a phenotype that was both obese and diabetic, with cold-intolerance, infertility and hyperphagic behavior [13, 64, 66]. Similarly, when leptin receptors in the brain were deleted, the animals displayed a hyperphagic phenotype concomitant with reduced whole-body energy expenditure [67–69]. Indeed, leptin-associated physiological effects are dominantly mediated by the brain [70]. The physiologically active form of leptin receptors is expressed in several regions of the brain including the hypothalamus, midbrain and brainstem of both neonatal and adult animals [71–75]. Among these brain regions, dense leptin receptor expression was detected in the discrete hypothalamic nuclei including the ARC, VMH and PVN. Consequentially, leptin directly modulates neural activity in these hypothalamic regions in a multi-directional manner [7] and influences whole-body energy metabolism in areas such as energy expenditure, feeding behavior and, as a consequence, body weight change [68, 76].
Intracellular signaling in POMC neurons
The mechanisms of intracellular signaling in the POMC system are complex. However, it is understood that both Pomc gene expression and the posttranslational modification of precursor protein, POMC, are important for tissue-specific POMC functions. In the ARC, for example, a certain population of POMC neurons produces leptin receptors, and leptin triggers POMC neuronal activity, which can be measured either by an electrophysiological recording or by biochemical determination of the degree of STAT3 phosphorylation and c-fos expression [11, 77–79]. Leptin also activates POMC gene expression in these cells [80]. In addition to leptin, other hormonal and nutritional signaling molecules such as insulin and glucose affect POMC neuronal activity and gene expression. A distinct population of POMC neurons expresses insulin receptors, through which insulin activates the phosphatidylinositol-3 kinase (PI3K) pathway (see Fig. 2) in POMC neurons [81]. Leptin and insulin activate different intracellular signaling pathways in distinct subtypes of POMC neurons in the ARC, and may act together synergistically, and/or antagonistically, to modulate POMC-associated physiology [82, 83]. POMC neurons also sense circulating glucose, which stimulates ATP production in POMC neurons. Notably, impairment of glucose sensing by POMC neurons is linked to development of type 2 diabetes, partially mediated by mitochondrial uncoupling protein 2 (UCP2) signaling within POMC cells [29].
Alpha-melanocyte-stimulating hormone production by enzymatic processing of POMC
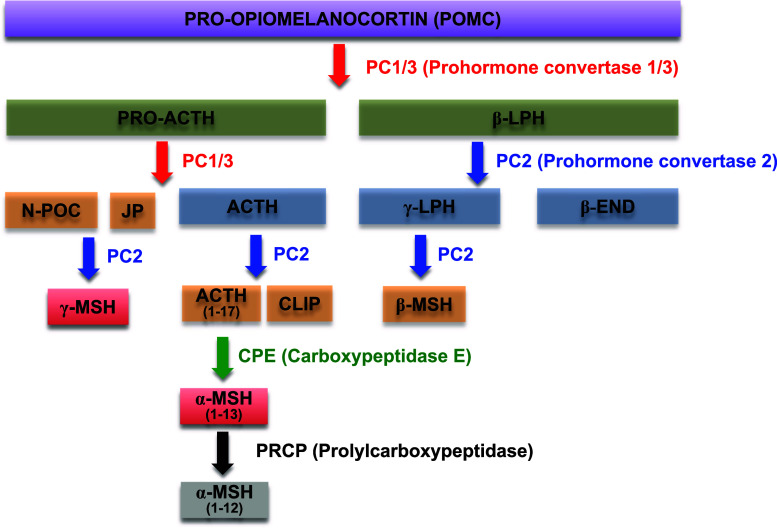
POMC is a prohormone for several small peptides such as adrenocorticotropic hormone (ACTH), α-MSH, γ-MSH and β-endorphin [84–86]. During the posttranslational process, the POMC precursor undergoes several modifications by a variety of proteases and enzymes, such as prohormone convertases (PCs), carboxypeptidase E (CPE) and peptidyl α-amidating monooxygenase (PAM) (Fig. 3). The differential expression of these proteases in distinct tissues and cells leads to the production of a variety of POMC-derived small peptides in the target regions [86]. In the hypothalamus, the POMC precursor is first processed to ACTH, β-lipoprotein (β-LPH) and the N-terminal POMC fragment (N-POC) by the action of PC1/3. Then, ACTH is further processed to α-MSH through sequential modification processes, including participation of several proteases and enzymes such as CPE, PAM and N-acetyltransferase, while β-LPH is processed to β-endorphin by PC2. A majority of POMC synthesized is converted to the physiologically active form of α-MSH in hypothalamic POMC neurons [86].
Fig. 3.
Posttranslational processing of the POMC precursor protein. POMC is a precursor for several small melanocortin peptides such as α-MSH, γ-MSH and β-endorphins. Various enzymes and proteases responsible for the cleavage of POMC, such as prohormone convertases and carboxypeptidase, are expressed in several tissues. Different combinations of these enzymes are responsible for production of the different tissue-specific melanocortins. In the hypothalamus, POMC predominantly produces a physiologically active form of α-MSH (α-MSH1–13), the most well-known melanocortin in the CNS related to metabolic regulation and feeding behavior, through sequential steps of protein cleavages and modifications. Recently, the molecular mechanism responsible for the degradation of α-MSH has also been determined. In this regard, PRCP cleaves one amino acid at the C-terminal end of α-MSH, producing a biologically inactive form of α-MSH (α-MSH1–12). ACTH adrenocorticotropic hormone, β- and γ-LPH β- and γ-lipotropin, JP joining peptide, β-END β-endorphin, CLIP corticotropin-like intermediate lobe peptide, α-, β- and γ-MSH α-, β- and γ-melanocyte-stimulating hormone, PC1/2/3 prohormone convertase 1/2/3, CPE carboxypeptidase E, N-POC N-terminal pro-opiomelanocortin, PRCP prolylcarboxypeptidase
Of all the POMC products, α-MSH is the best-understood and most abundant signaling molecule in the hypothalamus associated with energy homeostasis. Several groups of scientists have demonstrated a physiological function of α-MSH in the regulation of metabolism [33, 39, 87]. The production of α-MSH in the brain closely coordinates with metabolic status. Studies using animal models, either with acute administration of an α-MSH agonist or with long-term overexpression of α-MSH in the brain, revealed that α-MSH inhibits feeding behaviors concomitant with an increase in energy expenditure, and therefore counteracts obesity.
Recently, a cellular mechanism responsible for the inactivation of α-MSH has also been reported [88–92]. A small serine peptidase, prolylcarboxypeptidase (PRCP), is expressed in several hypothalamic regions and is released into distinct brain areas where α-MSH is also secreted by POMC-terminals [88]. PRCP effectively cleaves one amino acid from the C-terminal end of α-MSH (Fig. 3), thereby producing a physiologically inactive form of α-MSH [88]. Hypothalamic PRCP gene expression was shown to be affected by metabolic status, i.e., an increased level of PRCP gene expression was observed in the hypothalamus of fasted animals [92]. Once PRCP expression was abolished (PRCPgt/gt), α-MSH levels in the brain were continuously increased. Consequently, PRCPgt/gt animals showed higher energy expenditure, reduced food intake and, as a consequence, lower body weight gain. Therefore, they were protected from diet-induced obesity [89, 90]. These evidences suggest that small population of POMC cells governs multiple physiologies by producing a variety of neuropeptides processed by posttranslational modification.
Melanocortin receptors
Anatomical and functional profiles of the melanocortin receptor subtypes in the mammalian brain have been understood since the 1990s. It was revealed that the melanocortin receptor types 3 and 4 (MC3R and MC4R, respectively) are widely expressed in the brain and play a dominant role as brain-specific melanocortin receptors, although other subtypes of melanocortin receptors are also expressed in the brain of various species [4, 93–97]. Interestingly, genetically modified animals with a deletion of either MC3R or MC4R (Mc3R or Mc4R knockout mice) matured into an obese phenotype, and thus provided strong evidence for the involvement of MC3R and MC4R in the regulation of metabolism and feeding behavior in concert with melanocortins [1, 4, 98, 99].
Anatomical studies demonstrated that MC3R mRNA expression is abundant and widely present in mammalian brain, including the hypothalamic and limbic regions [100, 101]. Within the hypothalamus, MC3R expression is observed in certain nuclei, such as the ARC and the VMH, to be highly associated with feeding behavior and the regulation of metabolism. Particularly in the ARC, MC3R is produced both by POMC- and AgRP/NPY-ergic neurons [102, 103]. These anatomical evidences indicate the involvement of MC3R in energy homeostasis via transferring the auto-feedback signal of α-MSH on POMC neurons. However, a pathophysiological function of MC3R, in association with metabolic regulation, is ambiguous and still under debate. For example, variants of the Mc3R gene were not directly correlated with an obese phenotype in humans [104, 105]. However, a mutation of Mc3R in mice leads to the development of a moderately obese phenotype under a standard rodent diet, with an increase in adiposity, a decrease in locomotion and abnormal feeding behavior, without an observed hyperphagic syndrome [106]. In addition, obesity became pronounced in Mc3R KO animals when fed a high-fat diet [98, 99]. Recent data also proposed a role of MC3R in adaptation to food restriction [106]. Nevertheless, the pathophysiological significance and the detailed mechanisms by which MC3R plays a role in the control of energy homeostasis have yet to be elucidated.
When compared to other melanocortin receptor subtypes, MC4R has been established as a critical node for body weight homeostasis for a long time [107–109]. MC4R is expressed mostly in the CNS, although its expression is not restricted to the brain [93, 101, 110–114]. Studies profiling a pattern of MC4R expression revealed that gene expression of Mc4R is widespread in the brain, although it is predominantly expressed in the hypothalamus, the brainstem and the amygdala [114]. More specifically, the PVN, DMH, VMH and LH in the hypothalamus and the superior colliculus, NTS and DMV in the brainstem showed the highest levels of expression of MC4R, comparatively. This anatomical evidence supports a unique and complex function of MC4R in reproduction, stress, emotional behavior, and cardiovascular function as well as in energy homeostasis. Indeed, rare mutations in the Mc4R gene are associated with an obesity syndrome in humans [115–117].
Recently, much effort has also been expended to uncover MC4R-associated intracellular signaling and trafficking mechanisms for a better understanding of MC4R-mediated disease processes, and, accordingly, the participation of melanocortin receptor accessory proteins (MRAPs) was revealed in MC4R signaling [118–121]. MRAP was first identified as an MC2R accessory protein, and MRAP2 is a brain-expressed homologue of MRAP. In the brain, MRAP2 is most abundantly expressed in the hypothalamus and directly interacts with MC4R, influencing the sensitivity of MC4R to α-MSH as well as MC4R-mediated intracellular signaling, both in development and adulthood [120, 121]. In addition, mice with a deletion of MRAP2 developed a severely obese phenotype, clearly demonstrating the participation of MRAP2 in the MC4R-associated energy homeostasis [121]. Overall, the participation of MC4R signaling in energy homeostasis is clear. However, the brain region-specific differential roles of MC4R in metabolic regulation, in association with intracellular signal molecules interacting with MC4R, will require further study in order to provide better understanding of the pathophysiological effect of MC4R dysfunction.
Conclusions and perspectives
As obesity and other diseases associated with metabolic dysfunction, such as hypertension and type 2 diabetes, gain more attention worldwide as potentially life-threatening health factors, scientists have invested significant effort in developing preventative and curative therapies against these metabolic syndromes. In this regard, the participation of the CNS has been a focus in the study of metabolic regulation and whole-body energy homeostasis. In the brain, hypothalamic neural networks including the melanocortin system play an important role in sensing and integrating a variety of metabolic signals, and further triggering signaling cascades into deep brain regions to produce an adequate level of feeding behavior and energy expenditure, thereby preserving whole-body energy balance. Diverse studies have recently been performed to uncover unknown mechanisms underlying the hypothalamic melanocortin system. These studies have found that certain brain-specific transcription factors and inflammatory molecules participate in metabolic regulation through the melanocortin pathway.
Unfortunately, the mechanisms underlying the melanocortin pathway are complex and are still under debate. However, there is no doubt that the central melanocortin pathway plays a fundamental role in controlling whole-body energy homeostasis. Therefore, further investigation is necessary to clearly understand this system, which will in turn aid the fight against obesity and metabolic disorders.
Acknowledgments
This study was supported by the Research Fund of University of Ulsan.
Footnotes
Jin Kwon Jeong and Jae Geun Kim have contributed equally to preparation of the manuscript.
References
- 1.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 2.Boden-Albala B, Sacco RL. Lifestyle factors and stroke risk: exercise, alcohol, diet, obesity, smoking, drug use, and stress. Curr Atheroscler Rep. 2000;2:160–166. doi: 10.1007/s11883-000-0111-3. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen T, Lau DC. The obesity epidemic and its impact on hypertension. Can J Cardiol. 2012;28:326–333. doi: 10.1016/j.cjca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Cone RD. The central melanocortin system and energy homeostasis. Trends Endocrinol Metab. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 5.Zimanyi IA, Pelleymounter MA. The role of melanocortin peptides and receptors in regulation of energy balance. Curr Pharm Des. 2003;9:627–641. doi: 10.2174/1381612033391234. [DOI] [PubMed] [Google Scholar]
- 6.Seeley RJ, Drazen DL, Clegg DJ. The critical role of the melanocortin system in the control of energy balance. Annu Rev Nutr. 2004;24:133–149. doi: 10.1146/annurev.nutr.24.012003.132428. [DOI] [PubMed] [Google Scholar]
- 7.Ghamari-Langroudi M, Srisai D, Cone RD. Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. Proc Natl Acad Sci USA. 2011;108:355–360. doi: 10.1073/pnas.1016785108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fekete C, Legradi G, Mihaly E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM. Alpha-melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci. 2000;20:1550–1558. doi: 10.1523/JNEUROSCI.20-04-01550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell ME, Bhatnagar S, Akana SF, Choi S, Dallman MF. Disruption of arcuate/paraventricular nucleus connections changes body energy balance and response to acute stress. J Neurosci. 2000;20:6707–6713. doi: 10.1523/JNEUROSCI.20-17-06707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker RA, Herkenham M. Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. J Comp Neurol. 1995;358:518–530. doi: 10.1002/cne.903580405. [DOI] [PubMed] [Google Scholar]
- 11.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 12.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 13.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 14.Palou M, Sanchez J, Rodriguez AM, Priego T, Pico C, Palou A. Induction of NPY/AgRP orexigenic peptide expression in rat hypothalamus is an early event in fasting: relationship with circulating leptin, insulin and glucose. Cell Physiol Biochem. 2009;23:115–124. doi: 10.1159/000204100. [DOI] [PubMed] [Google Scholar]
- 15.Goto K, Inui A, Takimoto Y, Yuzuriha H, Asakawa A, Kawamura Y, Tsuji H, Takahara Y, Takeyama C, Katsuura G, Kasuga M. Acute intracerebroventricular administration of either carboxyl-terminal or amino-terminal fragments of agouti-related peptide produces a long-term decrease in energy expenditure in rats. Int J Mol Med. 2003;12:379–383. [PubMed] [Google Scholar]
- 16.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141:1332–1337. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- 18.Richardson J, Cruz MT, Majumdar U, Lewin A, Kingsbury KA, Dezfuli G, Vicini S, Verbalis JG, Dretchen KL, Gillis RA, Sahibzada N. Melanocortin signaling in the brainstem influences vagal outflow to the stomach. J Neurosci. 2013;33:13286–13299. doi: 10.1523/JNEUROSCI.0780-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology. 2012;153:1219–1231. doi: 10.1210/en.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garfield AS, Patterson C, Skora S, Gribble FM, Reimann F, Evans ML, Myers MG, Jr, Heisler LK. Neurochemical characterization of body weight-regulating leptin receptor neurons in the nucleus of the solitary tract. Endocrinology. 2012;153:4600–4607. doi: 10.1210/en.2012-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q, Howell MP, Palmiter RD. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. J Neurosci. 2008;28:9218–9226. doi: 10.1523/JNEUROSCI.2449-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Q, Zheng R, Srisai D, McKnight GS, Palmiter RD. NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus. Proc Natl Acad Sci USA. 2013;110:14765–14770. doi: 10.1073/pnas.1314137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur J Pharmacol. 2011;660:21–27. doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korner J, Wissig S, Kim A, Conwell IM, Wardlaw SL. Effects of agouti-related protein on metabolism and hypothalamic neuropeptide gene expression. J Neuroendocrinol. 2003;15:1116–1121. doi: 10.1111/j.1365-2826.2003.01113.x. [DOI] [PubMed] [Google Scholar]
- 27.Small CJ, Kim MS, Stanley SA, Mitchell JR, Murphy K, Morgan DG, Ghatei MA, Bloom SR. Effects of chronic central nervous system administration of agouti-related protein in pair-fed animals. Diabetes. 2001;50:248–254. doi: 10.2337/diabetes.50.2.248. [DOI] [PubMed] [Google Scholar]
- 28.Tolle V, Low MJ. In vivo evidence for inverse agonism of agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes. 2008;57:86–94. doi: 10.2337/db07-0733. [DOI] [PubMed] [Google Scholar]
- 29.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Elmquist JK, Fukuda M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann NY Acad Sci. 2011;1243:1–14. doi: 10.1111/j.1749-6632.2011.06248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenman Y, Kuperman Y, Drori Y, Asa SL, Navon I, Forkosh O, Gil S, Stern N, Chen A. Postnatal ablation of POMC neurons induces an obese phenotype characterized by decreased food intake and enhanced anxiety-like behavior. Mol Endocrinol. 2013;27:1091–1102. doi: 10.1210/me.2012-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, Oliver RL, Millington G, Aparicio SA, Colledge WH, Russ AP, Carlton MB, O’Rahilly S. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY (3–36) Proc Natl Acad Sci USA. 2004;101:4695–4700. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 34.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 35.Clement K, Dubern B, Mencarelli M, Czernichow P, Ito S, Wakamatsu K, Barsh GS, Vaisse C, Leger J. Unexpected endocrine features and normal pigmentation in a young adult patient carrying a novel homozygous mutation in the POMC gene. J Clin Endocrinol Metab. 2008;93:4955–4962. doi: 10.1210/jc.2008-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubern B, Lubrano-Berthelier C, Mencarelli M, Ersoy B, Frelut ML, Bougle D, Costes B, Simon C, Tounian P, Vaisse C, Clement K. Mutational analysis of the pro-opiomelanocortin gene in French obese children led to the identification of a novel deleterious heterozygous mutation located in the alpha-melanocyte stimulating hormone domain. Pediatr Res. 2008;63:211–216. doi: 10.1203/PDR.0b013e31815ed62b. [DOI] [PubMed] [Google Scholar]
- 37.Mizuno TM, Kelley KA, Pasinetti GM, Roberts JL, Mobbs CV. Transgenic neuronal expression of proopiomelanocortin attenuates hyperphagic response to fasting and reverses metabolic impairments in leptin-deficient obese mice. Diabetes. 2003;52:2675–2683. doi: 10.2337/diabetes.52.11.2675. [DOI] [PubMed] [Google Scholar]
- 38.Li G, Mobbs CV, Scarpace PJ. Central pro-opiomelanocortin gene delivery results in hypophagia, reduced visceral adiposity, and improved insulin sensitivity in genetically obese Zucker rats. Diabetes. 2003;52:1951–1957. doi: 10.2337/diabetes.52.8.1951. [DOI] [PubMed] [Google Scholar]
- 39.Savontaus E, Breen TL, Kim A, Yang LM, Chua SC, Jr, Wardlaw SL. Metabolic effects of transgenic melanocyte-stimulating hormone overexpression in lean and obese mice. Endocrinology. 2004;145:3881–3891. doi: 10.1210/en.2004-0263. [DOI] [PubMed] [Google Scholar]
- 40.Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71:488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sohn JW, Williams KW. Functional heterogeneity of arcuate nucleus pro-opiomelanocortin neurons: implications for diverging melanocortin pathways. Mol Neurobiol. 2012;45:225–233. doi: 10.1007/s12035-012-8240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55:567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- 43.Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- 44.Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 45.Xu AW, Ste-Marie L, Kaelin CB, Barsh GS. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology. 2007;148:72–80. doi: 10.1210/en.2006-1119. [DOI] [PubMed] [Google Scholar]
- 46.Ernst MB, Wunderlich CM, Hess S, Paehler M, Mesaros A, Koralov SB, Kleinridders A, Husch A, Munzberg H, Hampel B, Alber J, Kloppenburg P, Bruning JC, Wunderlich FT. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci. 2009;29:11582–11593. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JG, Nam-Goong IS, Yun CH, Jeong JK, Kim ES, Park JJ, Lee YC, Kim YI, Lee BJ. TTF-1, a homeodomain-containing transcription factor, regulates feeding behavior in the rat hypothalamus. Biochem Biophys Res Commun. 2006;349:969–975. doi: 10.1016/j.bbrc.2006.08.147. [DOI] [PubMed] [Google Scholar]
- 48.Kim JG, Park BS, Yun CH, Kim HJ, Kang SS, D’Elia AV, Damante G, Lee KU, Park JW, Kim ES, Namgoong IS, Kim YI, Lee BJ. Thyroid transcription factor-1 regulates feeding behavior via melanocortin pathway in the hypothalamus. Diabetes. 2011;60:710–719. doi: 10.2337/db10-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakkou M, Wiedmer P, Anlag K, Hamm A, Seuntjens E, Ettwiller L, Tschop MH, Treier M. A role for brain-specific homeobox factor Bsx in the control of hyperphagia and locomotory behavior. Cell Metab. 2007;5:450–463. doi: 10.1016/j.cmet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sergeyev V, Broberger C, Hokfelt T. Effect of LPS administration on the expression of POMC, NPY, galanin, CART and MCH mRNAs in the rat hypothalamus. Brain Res Mol Brain Res. 2001;90:93–100. doi: 10.1016/s0169-328x(01)00088-2. [DOI] [PubMed] [Google Scholar]
- 52.Scarlett JM, Jobst EE, Enriori PJ, Bowe DD, Batra AK, Grant WF, Cowley MA, Marks DL. Regulation of central melanocortin signaling by interleukin-1 beta. Endocrinology. 2007;148:4217–4225. doi: 10.1210/en.2007-0017. [DOI] [PubMed] [Google Scholar]
- 53.DeBoer MD, Scarlett JM, Levasseur PR, Grant WF, Marks DL. Administration of IL-1beta to the 4th ventricle causes anorexia that is blocked by agouti-related peptide and that coincides with activation of tyrosine-hydroxylase neurons in the nucleus of the solitary tract. Peptides. 2009;30:210–218. doi: 10.1016/j.peptides.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marks DL, Ling N, Cone RD. Role of the central melanocortin system in cachexia. Cancer Res. 2001;61:1432–1438. [PubMed] [Google Scholar]
- 55.Huang QH, Hruby VJ, Tatro JB. Role of central melanocortins in endotoxin-induced anorexia. Am J Physiol. 1999;276:R864–R871. doi: 10.1152/ajpregu.1999.276.3.R864. [DOI] [PubMed] [Google Scholar]
- 56.Wisse BE, Frayo RS, Schwartz MW, Cummings DE. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology. 2001;142:3292–3301. doi: 10.1210/endo.142.8.8324. [DOI] [PubMed] [Google Scholar]
- 57.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 58.Guillod-Maximin E, Roy AF, Vacher CM, Aubourg A, Bailleux V, Lorsignol A, Penicaud L, Parquet M, Taouis M. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J Endocrinol. 2009;200:93–105. doi: 10.1677/JOE-08-0348. [DOI] [PubMed] [Google Scholar]
- 59.Park BS, Jin SH, Park JJ, Park JW, Namgoong IS, Kim YI, Lee BJ, Kim JG. Visfatin induces sickness responses in the brain. PLoS One. 2011;6:e15981. doi: 10.1371/journal.pone.0015981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brunetti L, Di Nisio C, Recinella L, Chiavaroli A, Leone S, Ferrante C, Orlando G, Vacca M. Effects of vaspin, chemerin and omentin-1 on feeding behavior and hypothalamic peptide gene expression in the rat. Peptides. 2011;32:1866–1871. doi: 10.1016/j.peptides.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Kloting N, Kovacs P, Kern M, Heiker JT, Fasshauer M, Schon MR, Stumvoll M, Beck-Sickinger AG, Bluher M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia. 2011;54:1819–1823. doi: 10.1007/s00125-011-2137-1. [DOI] [PubMed] [Google Scholar]
- 62.Reaux-Le Goazigo A, Bodineau L, De Mota N, Jeandel L, Chartrel N, Knauf C, Raad C, Valet P, Llorens-Cortes C. Apelin and the proopiomelanocortin system: a new regulatory pathway of hypothalamic alpha-MSH release. Am J Physiol Endocrinol Metab. 2011;301:E955–E966. doi: 10.1152/ajpendo.00090.2011. [DOI] [PubMed] [Google Scholar]
- 63.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 64.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 65.Ahima RS, Kelly J, Elmquist JK, Flier JS. Distinct physiologic and neuronal responses to decreased leptin and mild hyperleptinemia. Endocrinology. 1999;140:4923–4931. doi: 10.1210/endo.140.11.7105. [DOI] [PubMed] [Google Scholar]
- 66.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 67.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 69.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149:2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG., Jr LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes. 2004;53:3067–3073. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- 72.Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- 73.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patterson CM, Leshan RL, Jones JC, Myers MG., Jr Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res. 2011;1378:18–28. doi: 10.1016/j.brainres.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol. 2010;518:459–476. doi: 10.1002/cne.22219. [DOI] [PubMed] [Google Scholar]
- 76.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG, Jr, Schwartz GJ, Chua SC., Jr Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korner J, Chua SC, Jr, Williams JA, Leibel RL, Wardlaw SL. Regulation of hypothalamic proopiomelanocortin by leptin in lean and obese rats. Neuroendocrinology. 1999;70:377–383. doi: 10.1159/000054499. [DOI] [PubMed] [Google Scholar]
- 78.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 79.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 80.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 81.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith AI, Funder JW. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev. 1988;9:159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 85.Castro MG, Morrison E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit Rev Neurobiol. 1997;11:35–57. doi: 10.1615/critrevneurobiol.v11.i1.30. [DOI] [PubMed] [Google Scholar]
- 86.Wardlaw SL. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur J Pharmacol. 2011;660:213–219. doi: 10.1016/j.ejphar.2010.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee M, Kim A, Chua SC, Jr, Obici S, Wardlaw SL. Transgenic MSH overexpression attenuates the metabolic effects of a high-fat diet. Am J Physiol Endocrinol Metab. 2007;293:E121–E131. doi: 10.1152/ajpendo.00555.2006. [DOI] [PubMed] [Google Scholar]
- 88.Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. Prolylcarboxypeptidase regulates food intake by inactivating alpha-MSH in rodents. J Clin Invest. 2009;119:2291–2303. doi: 10.1172/JCI37209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jeong JK, Szabo G, Raso GM, Meli R, Diano S. Deletion of prolyl carboxypeptidase attenuates the metabolic effects of diet-induced obesity. Am J Physiol Endocrinol Metab. 2012;302:E1502–E1510. doi: 10.1152/ajpendo.00544.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeong JK, Szabo G, Kelly K, Diano S. Prolyl carboxypeptidase regulates energy expenditure and the thyroid axis. Endocrinology. 2012;153:683–689. doi: 10.1210/en.2011-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jeong JK, Diano S. Prolyl carboxypeptidase and its inhibitors in metabolism. Trends Endocrinol Metab. 2013;24:61–67. doi: 10.1016/j.tem.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jeong JK, Kim JD, Diano S. Ghrelin regulates hypothalamic prolyl carboxypeptidase expression in mice. Mol Metab. 2013;2:23–30. doi: 10.1016/j.molmet.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mountjoy KG, Wild JM. Melanocortin-4 receptor mRNA expression in the developing autonomic and central nervous systems. Brain Res Dev Brain Res. 1998;107:309–314. doi: 10.1016/s0165-3806(98)00015-7. [DOI] [PubMed] [Google Scholar]
- 94.Tatro JB. Melanotropin receptors in the brain are differentially distributed and recognize both corticotropin and alpha-melanocyte stimulating hormone. Brain Res. 1990;536:124–132. doi: 10.1016/0006-8993(90)90016-5. [DOI] [PubMed] [Google Scholar]
- 95.Xia Y, Wikberg JE, Chhajlani V. Expression of melanocortin 1 receptor in periaqueductal gray matter. NeuroReport. 1995;6:2193–2196. doi: 10.1097/00001756-199511000-00022. [DOI] [PubMed] [Google Scholar]
- 96.Mountjoy KG. Distribution and function of melanocortin receptors within the brain. Adv Exp Med Biol. 2010;681:29–48. doi: 10.1007/978-1-4419-6354-3_3. [DOI] [PubMed] [Google Scholar]
- 97.Cone RD, Mountjoy KG. Molecular genetics of the ACTH and melanocyte-stimulating hormone receptors. Trends Endocrinol Metab. 1993;4:242–247. doi: 10.1016/1043-2760(93)90129-3. [DOI] [PubMed] [Google Scholar]
- 98.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 99.Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, Marsh DJ, Forrest MJ, Gopal-Truter S, Fisher J, Camacho RE, Strack AM, Mellin TN, Maclntyre DE, Chen HY, Van der Ploeg LH. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 100.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- 102.Jegou S, Boutelet I, Vaudry H. Melanocortin-3 receptor mRNA expression in pro-opiomelanocortin neurones of the rat arcuate nucleus. J Neuroendocrinol. 2000;12:501–505. doi: 10.1046/j.1365-2826.2000.00477.x. [DOI] [PubMed] [Google Scholar]
- 103.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li WD, Joo EJ, Furlong EB, Galvin M, Abel K, Bell CJ, Price RA. Melanocortin 3 receptor (MC3R) gene variants in extremely obese women. Int J Obes Relat Metab Disord. 2000;24:206–210. doi: 10.1038/sj.ijo.0801114. [DOI] [PubMed] [Google Scholar]
- 105.Calton MA, Ersoy BA, Zhang S, Kane JP, Malloy MJ, Pullinger CR, Bromberg Y, Pennacchio LA, Dent R, McPherson R, Ahituv N, Vaisse C. Association of functionally significant melanocortin-4 but not melanocortin-3 receptor mutations with severe adult obesity in a large North American case-control study. Hum Mol Genet. 2009;18:1140–1147. doi: 10.1093/hmg/ddn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Begriche K, Marston OJ, Rossi J, Burke LK, McDonald P, Heisler LK, Butler AA. Melanocortin-3 receptors are involved in adaptation to restricted feeding. Genes Brain Behav. 2012;11:291–302. doi: 10.1111/j.1601-183X.2012.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 108.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 109.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 110.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 111.Mountjoy KG, Jenny Wu CS, Dumont LM, Wild JM. Melanocortin-4 receptor messenger ribonucleic acid expression in rat cardiorespiratory, musculoskeletal, and integumentary systems. Endocrinology. 2003;144:5488–5496. doi: 10.1210/en.2003-0570. [DOI] [PubMed] [Google Scholar]
- 112.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 113.Daniel PB, Fernando C, Wu CS, Marnane R, Broadhurst R, Mountjoy KG. 1 kb of 5′ flanking sequence from mouse MC4R gene is sufficient for tissue specific expression in a transgenic mouse. Mol Cell Endocrinol. 2005;239:63–71. doi: 10.1016/j.mce.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 114.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Geller F, Reichwald K, Dempfle A, Illig T, Vollmert C, Herpertz S, Siffert W, Platzer M, Hess C, Gudermann T, Biebermann H, Wichmann HE, Schafer H, Hinney A, Hebebrand J. Melanocortin-4 receptor gene variant I103 is negatively associated with obesity. Am J Hum Genet. 2004;74:572–581. doi: 10.1086/382490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stutzmann F, Vatin V, Cauchi S, Morandi A, Jouret B, Landt O, Tounian P, Levy-Marchal C, Buzzetti R, Pinelli L, Balkau B, Horber F, Bougneres P, Froguel P, Meyre D. Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Hum Mol Genet. 2007;16:1837–1844. doi: 10.1093/hmg/ddm132. [DOI] [PubMed] [Google Scholar]
- 117.Heid IM, Vollmert C, Hinney A, Doring A, Geller F, Lowel H, Wichmann HE, Illig T, Hebebrand J, Kronenberg F, Group K. Association of the 103I MC4R allele with decreased body mass in 7937 participants of two population based surveys. J Med Genet. 2005;42:e21. doi: 10.1136/jmg.2004.027011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chan LF, Webb TR, Chung TT, Meimaridou E, Cooray SN, Guasti L, Chapple JP, Egertova M, Elphick MR, Cheetham ME, Metherell LA, Clark AJ. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci USA. 2009;106:6146–6151. doi: 10.1073/pnas.0809918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Noon LA, Franklin JM, King PJ, Goulding NJ, Hunyady L, Clark AJ. Failed export of the adrenocorticotrophin receptor from the endoplasmic reticulum in non-adrenal cells: evidence in support of a requirement for a specific adrenal accessory factor. J Endocrinol. 2002;174:17–25. doi: 10.1677/joe.0.1740017. [DOI] [PubMed] [Google Scholar]
- 120.Sebag JA, Zhang C, Hinkle PM, Bradshaw AM, Cone RD. Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science. 2013;341:278–281. doi: 10.1126/science.1232995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, Ramanathan V, Strochlic DE, Ferket P, Linhart K, Ho C, Novoselova TV, Garg S, Ridderstrale M, Marcus C, Hirschhorn JN, Keogh JM, O’Rahilly S, Chan LF, Clark AJ, Faroogi IS, Majzoub JA. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341:275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]