Abstract
A proper balance between androgen and oestrogen is fundamental for normal male reproductive development and function in both animals and humans. This balance is governed by the cytochrome P450 aromatase, which is expressed also under spatio-temporal control. Oestrogen receptors ERα and/or ERβ, together with the membrane-associated G-protein-coupled functional ER (GPER), mediate the effects of oestrogen in the testis. Oestrogen action in male reproduction is more complex than previously predicted. The androgen/oestrogen balance and its regulation in the masculinisation programming window (MPW) during foetal life is the most critical period for the development of the male reproductive system. If this balance is impaired during the MPW, the male reproductive system may be negatively affected. Recent data from genetically modified mice and human infertile patients have shown that oestrogens may promote the engulfment of live Leydig cells by macrophages leading to male infertility. We also discuss recent data on environmental oestrogen exposure in men and rodents, where a rodent–human distinction is crucial and analyse some aspects of male fertility potentially related to impaired oestrogen/androgen balance.
Keywords: Oestrogen, Oestrogen receptor, Male fertility
Introduction
Androgen plays an important role in masculinization and the determination of male fertility. The notion “androgen maketh a man” has long been established. Without this hormonal action, genetic males would become phenotypically infertile females. Unlike androgens, oestrogen action in males has long been accepted as having a minor role in gonadotropin secretion from the pituitary. The generation of the oestrogen receptor α knockout mouse (αERKO) [1], the aromatase knockout mouse (ArKO) [2] as well as the discovery of mutations in human aromatase [3], and the identification of ERα-deficient male patients [4] have broadened our understanding of oestrogen action, particularly in male reproduction [5].
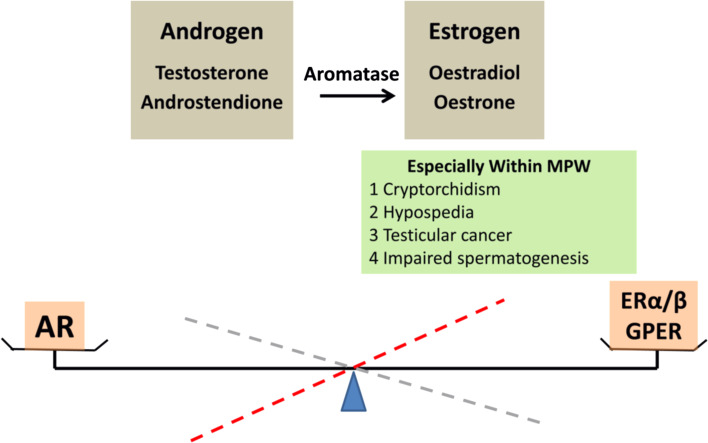
Typically, androgens can be converted into oestrogens, thus the proposed concept that androgen and oestrogen represent opposite sides of the same coin [6] is rational and valid. The balance between androgen and oestrogen that is required for normal male reproductive development is regulated by cytochrome P450 aromatase (P450arom, the product of the CYP19A1 gene). Any change in this balance may disturb the normal development and function of the male genital tract and may translate into a wide range of urogenital disorders, which can affect normal fertility. In this review, we will not discuss the fundamental functions and molecular actions of androgens and androgen receptor (AR) on male reproduction, as these topics have been extensively reviewed elsewhere [7]. We will concentrate on the molecular effects of oestrogen, the nuclear ERα and ERβ, the different ERα and ERβ splice variants, membrane bound G-protein-coupled functional ER (GPER) [8], and the multiple cellular signalling pathways that regulate the oestrogen/androgen balance (Fig. 1).
Fig. 1.
The balance of androgen and oestrogen
In recent years, exposure to environmental oestrogen-like chemicals (also termed endocrine disruptive compounds, EDCs) has been proposed to be associated with poor semen quality and male reproductive tract disorders, including cryptorchidism, hypospadias and testicular cancer [9–11]. EDCs seem to mimic the oestrogen effects (through oestrogen receptor α/β) in males. Due to variable results from individual investigations, pharmacological doses of EDCs used in experiments, perhaps varying quality of antibodies in immunohistochemical studies, and above all the major differences in endocrinology between humans and rodents, etc. make the exact effects of EDCs on the parameters of male reproduction still debatable [12]. Based on these studies, many questions have arisen regarding the specific contribution of oestrogen action on testicular development and disease. A growing body of evidence indicates that masculinization is a pivotal event in the development of a phenotypic male and that masculinization depends on the adequate production of testosterone (T) by the foetal testis within the specific “masculinisation programming window” (MPW) [13].
Last year, we reported a novel finding that excess oestrogens may promote the engulfment of live testicular Leydig cells by adjacent macrophages in infertile men as well as in genetically modified transgenic mice overexpressing aromatase [14]. In this review, we will focus on the recent research from our laboratory and other groups’ on oestrogen activity and the effects of an impaired oestrogen/androgen ratio on the male reproductive system, both in experimental animal models and human clinical studies.
Aromatase and its expression in males
Aromatase and its promoters
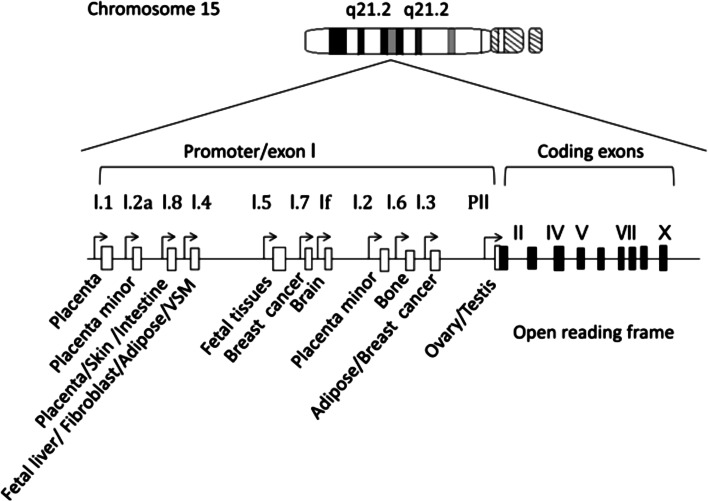
The balance between androgens and oestrogens is governed by cytochrome P450 aromatase within the endoplasmic reticulum of cells. P450 aromatase is responsible for catalysing the series of reactions that lead to the irreversible conversion of C19 androgenic substrates (T and androstenedione) into C18 oestrogens (oestradiol and oestrone) (reviewed in [5]). Therefore, aromatase enzyme activity affects both androgen metabolism and oestrogen production. The human CYP19A1 gene contains a coding region that spans 9 exons, starting at exon II, and is expressed in various tissue types within the body [5]. The expression of CYP19A1 in different locations in humans is regulated by at least 11 different tissue-specific promoters that are situated upstream of a series of 11 alternative 5′ untranslated first exons [5, 15] (Fig. 2). Of the 11 noncoding exons, promoter II is the main one utilized in the testis and ovary for the expression of the CYP19A1 gene in humans [5]. The expression of promoter II starts as early as week 13 and ceases at week 35 during embryonic development [16]. In particular, exon 1.4 seems to play a critical role in the regulation of oestrogen biosynthesis in males because this exon contains the major promoter for extra-gonadal tissues [5]. Regardless of the expression localization and the different 5′UTR CYP19A1 variants, each transcript generates a highly conserved 55 kDa aromatase protein [17]. Thus, the spatio-temporal expression of CYP19A1 is critical for the maintenance of the oestrogen/androgen balance during foetal and postnatal development.
Fig. 2.
Genomic organization of human CYP19A gene. Open boxes aromatase exon Is. Closed box aromatase exons II–X encoding the open reading frame (a modified graph from [110])
Aromatase expression in testis
As Simpson et al. suggested, it is difficult to find a tissue without CYP19A1 expression [18]; its expression has been detected along the entire male urogenital track. To ensure the subsequent normal differentiation and growth of all male reproductive organs, sufficient androgen exposure in the male foetus must occur within the MPW in rodents [13, 19]. This proposed foetal MPW in rodents is similar to that in human males [20]. Deficient androgen exposure during this critical foetal MPW can result in decreased masculinisation of the urogenital tract, an increased risk of hypospadias and cryptorchidism, low sperm production, and lower T levels [13, 19].
Human and rodent studies have demonstrated that foetal Leydig cells are responsible for foetal and neonatal masculinisation [21]. The foetal testis becomes steroidogenically active around week 6 of gestation [22]. High expression levels of StAR and CYP11A1 have been observed in foetal testis at 15–19 weeks of gestation, whereas the expression of CYP19A1 is much lower than that of the two rate-limiting enzymes for de novo steroidogenesis in human foetal testis during gestational weeks 15–19 [23]. Moreover, CYP19A1 is localized in the cytoplasm of both interstitial and intratubular cells of the foetal testis at weeks 13–14 of life [16]. CYP19A1 expression increases gradually, peaks by weeks 17–18, and starts to decrease thereafter. CYP19A1 expression nearly completely ceases at 35 weeks [16].
Concomitantly, the oestrogen wave parallels the intratesticular T wave (starts at week 9 and continues until week 20), with a slight delay during the MPW. The androgen-dependent phase of testis descent into the scrotum occurs during weeks 27–35 of human gestation [24, 25]. This fine-tuned regulation of androgen/oestrogen action suggests that direct autocrine and paracrine effects of locally produced oestrogens on the human foetal testis regulate the oestrogen-mediated activation of testis development during weeks 13–35 of foetal life [24]. The concurrent high expression of aromatase suggests significant local oestrogen production via the aromatization of the elevated quantity of available androgenic precursors. T enhances CYP19A1 gene expression in Leydig and germ cells [26]. This window of aromatase expression suggests direct or indirect regulation of this enzyme by T in the CYP19A1 [18] promoter region.
CYP19A1 is expressed in adult testes, and the majority of oestrogen biosynthesis takes place mainly in the Leydig cells, whereas CYP19A1 is not present in the peritubular myoid cells [24, 27, 28] or spermatogonia [29]. CYP19A1 expression has been observed in different stages of germ cell development (pachytene spermatocytes; round, elongating and elongated spermatids; and spermatozoa), in all somatic cells within the adult testis, and in spermatozoa in the mammalian epididymis [30, 31]. Based on these reports, one could speculate that stem-like cell types, such as peritubular and spermatogonia, may programme their own protective mechanism against the deleterious effects of oestrogen action and thereby support the self-renewal of interstitial and germ cells.
Although some seasonal breeding species exhibit a switch in CYP19A1 localization during the breeding period, CYP19A1 can be detected by immunostaining in many testicular cell types [32]. Moreover, Aquila et al. reported that human spermatozoa contain active aromatase after ejaculation [33]. In addition, Lambard et al. demonstrated that CYP19A1 mRNA levels and aromatase activity were 30–50 % higher in the motile fraction compared with the immotile fraction of spermatozoa [34, 35]. Although the physiological relevance of oestrogen synthesis in spermatozoa remains to be elucidated, the data encourage an investigation of the function of aromatase in male fertility.
ER molecular signalling pathways
ER molecular action
Oestrogen elicits its molecular actions via several signalling pathways [36–39]. Classically, oestrogens diffuse into a cell and bind to nuclear ERs, thus forming an oestrogen–ER complex. After crossing the nuclear membrane, this oestrogen–ER complex binds to consensus oestrogen response elements (EREs) or non-consensus EREs directly or indirectly via protein–protein interactions in the promoter region of oestrogen-responsive genes, which results in the recruitment of co-regulatory proteins (such as coactivators or co-repressors) to the promoter that regulate gene expression and associated protein production, leading to a physiological response. This classical, or “genomic”, mechanism usually occurs over the course of hours.
Oestrogens can act more rapidly via “non-genomic” mechanisms through ERs located in or adjacent to the plasma membrane or by other non-ER plasma membrane-associated oestrogen-binding proteins that induce cellular responses, such as increased levels of Ca2+ or NO and kinase cascade activation. It has been accepted that activation of GPERs via cross-talk with peptide growth factors, such as epidermal growth factor (EGF) and/or insulin-like growth factor-1 (IGF-1), may increase the expression of oestrogen-responsive genes [37]. In addition, membrane-associated ERs, or GPERs, mediate the rapid biological effects of oestrogens via a non-genomic pathway; this may regulate gene expression via the activation of kinase signal transduction pathways that regulate target transcription factors [40–42]. Moreover, ERs can be targets of the mitogen-activated protein kinase (MAPK) signalling pathway [43], which may indicate that non-genomic pathways activated by oestrogens can modulate the functions of ERs themselves [40, 41]. This rapid “non-genomic” signalling occurs within seconds.
Structure and function of ERs
ERα and ERβ and their isoforms
There are three known ERs that mediate all oestrogenic effects. The first identified and well-described ERα was cloned in 1986 [44]. The second ER, ERβ, is less well characterized; ERβ was cloned in the rat prostate in 1996 [45] GPER was first proposed to be involved in oestrogen signalling in 2000 [46]. ERα has at least three different isoforms, and ERβ has at least five different isoforms. Unlike full-length ERα, the two shorter ERα isoforms, ERα36 [47] and ERα46 [48], both lack the N-terminal DNA-binding domain. Because they have the ability to heterodimerise with full-length ERα, they can repress ERα activity. Recent publications have reported that ERα36 also functions as a membrane-localized ER that can interact with GPER [49].
Ten different variant mRNA isoforms of the human ERβ gene with deletions in various combinations of exons have been identified in extracts from human cell lines and tissues [50]. Both long (59.2 kDa) and short (53.3 and 54.2 kDa) isoforms [50] of ERβ protein have been identified in humans, and the functional equivalency of these isoforms was demonstrated in transfection studies [51]. In 1998, human ERβcx, which is shorter than wild-type ERβ and contains a unique C-terminal stretch of 26 amino acids, was identified [52]. The four shorter ERβ isoforms differ from full-length ERβ predominantly in the ligand-binding domain, resulting in compromised ligand-binding ability. Different ERα and ERβ isoforms have a very diverse influence on oestrogen signalling and target gene regulation [53, 54]; for example, some ERβ isoforms lack the ability to bind ligands or coactivators, and certain isoforms affect ERα/β heterodimerization, hence silencing ERα signalling. Furthermore, homodimers of ERα and ERβ have been shown to regulate distinct sets of genes from those regulated by ERα/β heterodimers. These data are supported by the observation of completely distinct cellular responses to ER subtype-specific and non-specific agonists. It has also been suggested that ERβ has an inhibitory effect on ERα-mediated signalling. In some instances, opposite roles of ERα and ERβ have been revealed by differences in their expression in various tissues and organs [5].
GPER
GPER, a plasma membrane receptor previously known as orphan G-protein-coupled receptor 30 (GPR30), is structurally and genetically unrelated to ERα and ERβ. It is located on the cell surface and responsible for rapid oestrogen signalling, although certain ERα and ERβ variants have also been associated with the plasma membrane and non-genomic signalling [53, 54]. GPER expression and function occur independently of the two nuclear ERs. It has a high affinity for oestrogens but only a limited binding capacity, with single oestrogen-binding sites. Compared with nuclear ERs, its binding affinity for 17β-oestradiol is considerably lower, and the association and dissociation rates are very rapid, on the order of minutes. The specificity of GPER for oestrogens is emphasized by the fact that other steroid hormones possess very low binding affinities for this receptor [30, 55, 56].
Localization of ERs and GPER in the male urogenital track
ERα and ERβ expression is localized along the male urogenital track [57, 58]. Within the testis, ERα and ERβ expression is highly variable, with differences between species as well as between individuals within a species [56]. There are conflicting reports on the immunohistochemical localization and mRNA expression patterns of these receptors in testicular tissues and cells [59–61]. This discrepancy could be explained by the use of different antibodies for immunohistochemical analysis or different tissue preservation techniques [62]. GPR30 protein expression is localized in murine tissues along the male reproductive tract, including in the testes, epididymis, vas deferens, and seminal vesicles as well as in the prostate [63]. As there are no reports on GPR30 localization within the testis, it is of great importance to obtain these data in the near future.
Testicular phenotype of oestrogen-related genetically modified male murine models
The development of genetically modified murine models, such as knockout (KO) or overexpressing models, in the last decade has significantly increased our knowledge of ER and oestrogen action. Targeted disruption of ER in mice revealed the crucial and specific roles of oestrogen and ER action in male reproduction. The models include mice lacking functional ERα (αERKO) [64], ERβ (βERKO) [64, 65], both oestrogen receptors (αβERKO) [65], or aromatase (ArKO) [2] as well as knockout models of AF2ERK1, GPER, EST, or VAMP7 and mice that overexpress aromatase (AROM+ and int-5/aromatase) (please see below).
Murine models with oestrogen deficiency
αERKO (Esr1−/−) mice
Studies with KO mice have shown that spermatogenesis, steroidogenesis and fertility are impaired in Esr1−/− and Esr1−/−/Esr2−/− (αERKO and αERKO/βERKO) mice [64, 65]. αERKO male mice are infertile, and they have a very low sperm count and significantly decreased sperm motility with dilated seminiferous tubules compared with WT mice [66]. A possible explanation for the likely explanation of the dilated seminiferous tubules could be the significant decrease in ductal fluid reabsorption-related proteins, such as sodium-hydrogen exchanger 3 (NHE3/slc9a3) and/or carbonic anhydrase (car2) [67, 68]. αERKO males have altered steroidogenic hormone levels, namely, elevated T or LH levels [66]. It has also been postulated that testicular spermatogenic cells may not require ERα for development or function, whereas ERα in somatic cells is pivotal for spermatogenesis and male fertility [69, 70]. The ERαKO (Esr1tm1KSK) mouse expresses trace levels of N-terminal truncated mutant ERα (E1-ERα) [1], which is generated by non-controlled alternative splicing [71]. Esr1−/− mice with additional exclusion of exon 3 by the cre-loxP system (Esr1tm4.2KSK) have identical major phenotypes as the ERα null strains [66, 72]. It would be important and very interesting to see more testis-specific, such as Leydig cell-specific (see below also) and/or peritubular cell-specific, ERα KO phenotypes in the future. In the mouse, ERα is expressed in Leydig cells and peritubular cells [64, 65]. Male infertility could possibly stem from spermatogenic defects in ER knockout mice. In vitro exposure of WT and αERKO Leydig cells to tamoxifen has no effect on androgen biosynthesis, although treatment with ICI 182,780 decreases T production in WT, but not in αERKO Leydig cells. These observations may suggest that ERα plays regulatory role in Leydig cell steroidogenic function [73].
βERKO (Esr2−/−) mice
No impaired male phenotypes were found in βERKO (Esr2 −/− ) males [64, 65, 74]. βERKO male mice are fertile and exhibit a similar phenotype as WT littermates [64, 65]. An alternative-splicing transcript that functionally compensates for the lack of full-length ERβ could explain the Esr2 −/− phenotype [64, 65]. In another Esr2 −/− murine KO model, generated via Cre/LoxP-mediated excision of Esr2 exon 3 [75], the mice are infertile despite seemingly normal spermatogenesis and normal morpho-functional gonadal characteristics. The specific reason for the infertility in this Esr2 −/− mice lacking Esr2 exon 3 is still unknown [75]. In our opinion, a functional phenotypic comparison study between these two existing Esr2 −/− male mouse models is eagerly awaited and could be of great importance.
Double knockout ERα and ERβ (αβERKO) (Esr1−/−/Esr2−/−) mice
αβERKO [65] mice display a very similar phenotype to αERKO mice [64]. A plausible explanation for the similar phenotype between the αERKO and αβERKO mice could be that ERβ is most likely unable to compensate for ERα in the αERKO model.
Tissue-specific ER KO and knock-in mice
Multiple ERα mouse models have been developed using Cre-loxP technology to produce mice lacking functional ER in specific cell types or tissues (mainly non-relevant to the male reproductive system) [76, 77], but very few of these models have a male phenotype [76]. Mice lacking ERα in uterine epithelial cells, the UtEpiαERKO mouse model, did not show a testicular phenotype [76].
AF2ERKI mice
Murine models have been made with deletions of AF-1 or AF-2 (AF-10 and AF-20) or point mutations in the core AF-2 (AF2ERKI) [78, 79] to elucidate the physiological functions of AF-1 and AF-2 of ERα. The Korach group developed a knock-in mouse model (AF2ERKI, Esr1tm3.1KSK) that contains two-point mutations in the AF-2 region, thereby disrupting AF-2-mediated transactivation [80]. Although the AF2ER mutant ERα protein is expressed in AF2ERKI mice, the phenotype of the AF2ERKI males is similar to that of αERKO male mice, suggesting that AF-2 is necessary for ERα-mediated physiological responses. It has also been shown that the selective oestrogen receptor modulator tamoxifen partially rescues the male phenotype in AF2ERKI males [81]. We believe that further studies on this AF2ERKI murine model are needed to determine the different roles of AF-1 and AF-2 of ERα in oestrogen-regulated tissues and in hormone responsiveness.
ERαf/f CYP17iCre mice
A transgenic mouse line that expresses iCre under regulation of the cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17) promoter was developed to obtain a conditional deletion of genes specific for ovarian theca/interstitial cells and testicular Leydig cells [77]. In ERαf/f CYP17iCre males, spermatogenesis is normal, but fertility is decreased at 12 mo. The ERαf/f CYP17iCre mouse testis has normal seminiferous histology at 3–16 mo of age, and these mice present with normal T levels until 12 mo of age; T levels increase significantly at 16 mo of age [82]. In the ERαf/f CYP17iCre mouse testis, StAR mRNA levels are significantly higher compared with WT mice at 3–16 mo of age, 17β-HSD3 mRNA levels are significantly higher at 12 mo of age, and LHR mRNA levels are significantly higher at 16 mo of age [82]. It would be of great interest to have a better characterization of and more experimental functional data on the testicular phenotype of this ERαf/f CYP17iCre model. Additionally, data on the response of ERαf/f CYP17iCre males to ICI, tamoxifen, or aromatase inhibitors or on the in vitro stimulation of Leydig cells with ERα and ERβ agonists and comparisons of these results with those from αERKO mice would be very interesting.
GPER knockout mouse
Although it has been reported that GPER may regulate the proliferative and apoptotic pathways involved in spermatogenesis [83], there are no clear developmental or functional defects in the reproductive organs of GPER-KO males [84, 85]. By contrast, ERα-KO mice exhibit multiple reproductive defects, as stated above. Overall, the roles of GPER in the reproductive system are complex and require further in-depth investigation, particularly with regard to human physiology.
ArKO knockout mouse
Two individual groups generated ArKO mice through the targeted disruption of CYP19A1 [2, 86]. ArKO males are initially fertile [2], although they show impaired sexual behaviour, reduced capacity to mount and reduced mounting frequency [86]. Thereafter, ArKO males develop progressive infertility until their ability to sire pups is severely impaired [87, 88]. Spermatogenesis in ArKO males arrests at 4.5 mo of age. In addition, ArKO males exhibit significant reductions in the number of round and elongated spermatids, decreased sperm motility and an inability of the spermatozoa to fertilize the ova during in vitro fertilization [89].
Male murine models of excess oestrogen activity
AROM+ transgenic mouse
AROM+ mice were generated by fusing the human ubiquitin C promoter to the human P450 aromatase gene in the FVB/N and C57BL6 genetic backgrounds. AROM+ male mice are hypo-androgenic and hyper-oestrogenic [90, 91]. Cryptorchidism occurs in 100 % of FVB/N AROM+ male mice, which are infertile with a significantly reduced testicular weight [90]. Cryptorchidism is the main reproductive disorder in C57BL6 AROM+ mice; some lines initially present with normally descended testis, but progressive inguinal hernia begins at 4 mo [91]. The AROM+ testis exhibits Leydig cell hypertrophy and hyperplasia; in some instances, Leydig cell adenomas are present, but no malignant or metastatic tumours were observed in a follow-up at 15 mo of age [90, 91]. Interestingly, spermatogenesis is disrupted with ageing. At 9 mo, there is a notable reduction in the number of all germ cell types, and by 15 mo, the seminiferous tubules are atrophic with only Sertoli cells remaining inside the tubules [92]. Giant multinucleated macrophages accumulate in the interstitium of all mice regardless of age, and increase in number along with ageing [14, 92]. Intratesticular oestradiol concentrations also rise with age, and the increased aromatization parallels the severity of the testicular phenotype [14, 92]. Treating rats with oestrogens or xenoestrogens results in a similar testicular phenotype as that observed in the AROM+ mice [93]. The impaired testicular phenotype in the AROM+ males might not be exclusively related to cryptorchidism, as males from one line of AROM+ founders in the C57BL6 background are not cryptorchid; they exhibit normal spermatogenesis and are infertile after 4 mo of age, and they have identical testicular pathology as the other founder lines [14]. More data on AROM+ mice regarding male infertility are presented in “Novel aspects of oestrogen action in males” below (7. Novel aspects of oestrogen action in males), along with data on human infertile men with excess oestrogen.
Int-5/aromatase
Another aromatase overexpressing murine model utilizes the mouse mammary tumour virus promoter (MMTV) [94]. The MMTV promoter is typically activated during puberty. Int-5/aromatase mice present with normally descended testes. The serum E2 levels are twofold higher in int-5/aromatase mice, and the expression of CYP19A1 and ER is highly upregulated in testicular tissue. Moreover, 50 % of int-5/aromatase male mice are infertile with larger than normal testicles compared with age-matched WT mice. Histological analysis of the testes from these mice revealed unilateral or bilateral Leydig cell tumours [95]. The authors suggested that the increased E2 production most likely induced testicular Leydig cell tumours in the int-5/aromatase testes [95, 96].
EST knockout mouse
There is an oestrogen sulfotransferase (EST) KO mouse model that also reflects the effects of excessive oestrogen in males [97]. EST KO males are fertile and phenotypically present with normal testis at 2 mo of age; afterwards, they develop age-dependent Leydig cell hypertrophy/hyperplasia and seminiferous tubule damage with sperm motility reduced by 80 %, and they produce smaller litters, presumably due to local oestrogen action [97]. The presence of numerous hypertrophic or hyperplastic Leydig cells is accompanied by numerous multinuclear giant yellow cells within the testicular interstitium. In males aged 12 mo or older, seminiferous tubule damage is observed in testicular sections from most EST KO mice [97]. The late onset testicular abnormalities observed in EST KO males are identical to those observed in the AROM+ testis and may suggest a role for EST in the male reproductive system. These similarities further suggest that a ligand transformation enzyme may modulate the intracrine and paracrine oestrogen activity even under physiological conditions.
VAMP7 knockout mouse
Based on clinical data from 116 children presenting with idiopathic cases of 46XY disorders of sexual development, including cryptorchidism and penile malformation, scientists utilized genomic microarray analysis to identify the duplication of the SXXq28 region localized in the VAMP gene that correlates with the above-mentioned disorders [98]. The authors further generated a bacterial artificial chromosome (BAC clone) encoding human VAMP7 to create transgenic mice. This TG VAMP7 mouse model reproduces the defective urogenital traits found in boys with masculinization disorders (such as cryptorchidism, urethral defects and hypospadias), focal spermatogenic anomalies, diminished sperm motility and subfertility [99]. Mechanistically, upregulated VAMP7 expression enhanced oestrogen/ER binding and activity by increasing ERα stability and decreasing protein turnover by perturbing the ubiquitin–proteasome pathway [99]. Such aberrant enhanced ER activity in male genitourinary tissues affects the virilisation of the reproductive tract and may result in genitourinary birth defects in males.
These above-mentioned murine models have added highly significant value to the understanding of oestrogen action in male reproduction (Table 1). It would be of great interest to determine whether the above-mentioned cellular effects are the result of direct effects on developing germ cells or indirect effects through Sertoli cells.
Table 1.
Reproductive phenotype of oestrogen-related genetically modified male mice
| Genotype | Reproductive tract development | Fertility | Testicular morphology | Sperm motility | REF |
|---|---|---|---|---|---|
| αERKO | Normal | Infertile | Dysmorphogenic | Reduced | 66 |
| βERKO | Normal | Fertile | Normal | Normal | 64, 65, 75 |
| αβERKO | Normal | Infertile | Dysmorphogenic | Reduced | 65 |
| AF2ERK1 | Normal | Infertile | Dysmorphogenic | Reduced | 78, 79, 81 |
| UtEpiαERKO | Normal, no male phenotype | Fertile | Normal | Fertile | 76 |
| GPER | Normal | Fertile | Normal, not well characterized | Not well characterized | 83 |
| ArKO | Normal, impaired sexual behaviour, reduced capacity to mount | Initially fertile, later infertile | Initially normal, arrested spermatogenesis at 4.5-mo-age | Decreased motility, inability to fertilize ova during IVF | 2, 86, 87, 88, 89 |
| AROM+ | Initially normal, cryptorchidism, BOO | Infertile, reduced fertility | Initially normal, later Leydig cell hyperplasia | Not well characterized | 14, 90, 91, 92, 93 |
| Int5/aromatase | Normal | 50 % fertile | Uni/bilateral Leydig cell tumours | Not well characterized | 95,96 |
| EST | Normal, smaller litters | Fertile until 2-mo-age | Age-dependent Leydig cell hyper-troph/plasia | 80 % reduced motility | 97 |
| VAMP7 | Defective urogenital tract, cryptorchidism, hypospadias | Infertile | Dysmorphogenic | Diminished motility | 99 |
| ERαf/fCYP17iCre | Normal (?), yet to be characterized | Decreased fertility | Normal (?),yet to be characterized | Yet to be characterized | 77, 82 |
αERKO, Esr1 −/− mice; βERKO, Esr2 −/−; αβERKO, double knock out for Esr1 −/− /Esr2 −/− mice; AF2ERK1, KO of AF1 and AF2 deletions of Esr1 −/− and knock-in containing 2 point mutations in core AF-2; UtEpiαERKO, tissue-specific Esr1 −/− mice; ArKO, mice with disruption of CYP19A1; AROM+, aromatase/CYP19A overexpressing mice under human ubiquitin promoter; BOO, bladder outlet obstruction, Int5-aromatase, aromatase overexpressing mice under mouse mammary tumour promoter/MMTV; EST, oestrogen sulfotransferase KO mice; VAMP7, mice encoding human bacterial artificial/BAC clone VAMP7; ERαf/fCYP17iCre, iCRE under the regulation of CYP17 promoter for conditional Leydig cell-specific KO
Clinical investigations of oestrogen deficiency and aromatase excess syndrome in males
Aromatase deficiency
CYP19A1 mutations in patients are rare; there have been approximately 9 males out of 25 cases reported [3, 100–107]. In these patients, oestrogen levels are undetectable, but normal to high levels of T and gonadotropins are observed. Although the extra-gonadal clinical features of this condition are similar among all the cases [62], there are no consistent findings on the testicular phenotype [108]. Testicular size is variable, and cryptorchidism is evident in some cases. Due to ethical reasons, it was difficult to acquire semen samples and testicular biopsies from these patients with CYP19A1 mutations. Therefore, no solid conclusions on the effects of aromatase deficiency on spermatogenesis could be stated. Available data may indicate that aromatase deficiency is associated with Sertoli cell only syndrome, hypospermatogenesis, oligospermia, reduced motility or complete sperm immobility (reviewed in ref [108]). Qualitative testicular histopathological analyses have revealed that these patients have seminiferous tubules with a normal appearance [102] or hypotrophic seminiferous tubules [103]. Whether aromatase deficiency directly affects male fertility has yet to be determined, because data on only one aromatase-deficient man have been published up to date [3]. This reported aromatase-deficient man does not have any children. Based on the reported phenotypic observations of ArKO males, it is highly possible that the reproductive fertility of all aromatase-deficient males is severely impaired. Nevertheless, it would be of considerable importance to follow-up these clinical patients with CYP19A1 mutation-induced aromatase deficiency in the future. An alternative explanation for the variable testicular/spermatogenic phenotype of these male patients with inactivating mutations in CYP19 individuals is that they may harbour additional mutations explaining the phenotype, especially if they are the result of a consanguineous relationship. This could apply also to ERα or other human mutants.
ER mutations
Human ER mutations are extremely rare, with only one male out of two cases reported to date [4, 109]. The patient had normal male genitalia with bilateral descended testes and a normal sperm count, but the sperm motility was quite low (18 % compared with a normal motility of >50 %), which compromises the sperm quality and suggests impaired male fertility/subfertility [4].
Aromatase excess syndrome (AEXS)
Aromatase excess syndrome (AEXS), a very rare genetic disease formerly known as familial gynaecomastia, is characterized by the pre- or peri-pubertal onset of mammary development in males [110]. Familial gynaecomastia is an autosomal genetic disease caused by a gain-of-function mutation in CYP19A1. A sub-chromosomal inversion of CYP19A1 as a causative mutation of AEXS was first reported in 2003 [111]. To date, 12 mutant alleles have been identified in 15 families composed of 30 affected males [110]. The clinical features of AEXS have been well documented, and the phenotype is due to increased aromatization in adipose tissue and other tissues [111]. AEXS patients exhibit mild to severe pre- or peri-pubertal onset of gynaecomastia and small testes but have relatively preserved masculinization. Notably, 9 out of these patients were over 20 years of age, and 8 out of these 9 adults presented with normal spermatogenesis [110]. Circulating oestrone levels were elevated in 17 out of the 18 measured patients. Furthermore, oestradiol levels were elevated in only 13 out of 27 patients and were normal in the remaining 14 patients. This is consistent with the finding that oestrone, instead of oestradiol, is the major oestrogen produced in male patients with AEXS. Circulating androstenedione and T levels were low or subnormal for chronological age in more than one-half of the patients. The oestradiol (pg/ml)/T (ng/ml) ratio, a reflection of aromatization, was high in many, but not all, patients: the ratio was >10 in 75 % of the AEXS patients. Patients with AEXS may show mild hypogonadotropic hypogonadism. Decreased T levels are consistent among patients of all ages with AEXS. Testicular volume is subnormal in teens but is normal in adults and gender identity is not compromised. Mild oligozoospermia was noted in one patient, but the fecundity in these patients was normal. Interestingly, hypospadia or cryptorchidism was not reported in any of these 30 patients [110].
The MPW is a critical period in both humans and rodents [112, 113]. Any impairment of androgen activity during this MPW results in hypospadias or cryptorchidism and/or abnormal function as well as decreased anogenital distance (AGD) [114]. Therefore, it is likely that the gain-of-function of CYP19A1 via promoter regulation is expressed later than the MPW. Genomic analysis demonstrated that a 79,156 bp tandem duplication encompasses 7 of the 11 noncoding exon 1 s of CYP19A1 in some families. Notably, exon I.4 and I.8 are the major promoters of CYP19A1 overexpression in these families. In one family, a 211,631 bp deletion in exons 2–43 of DMXL2 and exons 5–10 of GLDN leads to the cryptic usage of DMXL2 exon 1 as an extra-transcriptional start site for CYP19A1 [115]. The most proximal promoter (PII) is almost exclusively expressed in gonadal tissues, whereas exon I.4, which is located upstream of PII, is almost exclusively expressed in adipose tissue. The most upstream promoter (I.8) is expressed in the placenta (Fig. 2). Considering these above-mentioned reports, AEXS patients may not serve as a suitable model to study the role of oestrogen activity on male fertility.
Novel aspects of oestrogen action in males
We demonstrated the possible molecular mechanisms underlying oestrogen-mediated male infertility [14]. E2 or an increased E2/T ratio not only promotes testicular macrophage activation but also regulates production of growth arrest-specific 6 (GAS6) and induces phosphatidylserine (PS) exposure on the surface of LCs in an ERα-dependent manner. The exposure of PS on the surface of dead, dying, or aged cells usually is the universal recognition cue for engulfment by phagocytes [116]. Phagocytes interact with PS on target cells with high affinity, either directly through the PS receptor or indirectly through bridging molecules like GAS6 [117]. This ERα-dependent macrophage activation leads to E2/ERα-mediated phagocytosis independent of apoptosis in the AROM + testis. In addition to their pivotal role in reproduction, E2/ERα influences inflammatory responses [118, 119]. Moreover, E2 induces macrophage activation in the modulation of the immune response [120–122]. As LCs express abundant ERα and GAS6 [123, 124], we demonstrated that E2/ERα promotes LC hyperplasia, GAS6 production, transactivation of the GAS6 promoter and Ca2+-dependent PS exposure on the surface of LCs. Simultaneously, we showed that E2/ERα activates macrophages. The subsequent interactions between GAS6, LCs, and testicular macrophages resulted in the formation of an AXL-GAS6-PS complex that leads to LC engulfment. This again confirmed the finely tuned balance between testicular macrophages and LCs in normal spermatogenesis [125–127]. Consequently, the depletion of T-producing LCs results in disrupted spermatogenesis, thereby leading to male infertility. To provide clinical relevance to our transgenic mice findings, we performed a microarray analysis on testicular biopsy specimens from patients with nonobstructive azoospermia (NOA) (n = 47, aged 24–57) or obstructive azoospermia (OA) (the OA patient samples served as controls for the NOA patients), an immunohistochemical analysis of the NOA (n = 20) and OA (n = 10) specimens, and a semen analysis of NOA and OA patients and healthy donors. The analysis of the molecular markers identified in the mouse study revealed significantly enhanced expression of CYP19A1, GAS6 and AXL in human testes from patients with NOA. These human data further confirmed that the obtained results from the AROM + mouse model reflected human infertility and provided the novel finding that GAS6 may potentially be a clinical biomarker and therapeutic target for male infertility [14].
EDCs, oestrogens, phytoestrogens, anti-androgens and male fertility
EDCs are synthetic or natural compounds that may have oestrogenic, anti-oestrogenic or anti-androgenic activity. Therefore, they could interfere with endogenous endocrine actions. EDCs have been proposed to be associated with poor male reproductive parameters [9–11]. However, it is still a subject of intense debate, how EDCs affect male reproduction. Androgen could pre-programme masculinization; in terms of the MPW, this pre-programming could occur before morphological changes in the male reproductive system are actually observed in rats [112]. A series of rat studies have established that sufficient androgen exposure within the MPW is a prerequisite for later normal differentiation and growth of all the male reproductive organs [19, 112, 128]. Deficient androgen action during the MPW results in smaller reproductive organs and an increased incidence of hypospadias, cryptorchidism, low sperm production, lower T levels and reduced anogenital distance (AGD) in the postnatal life of rats [19, 112, 128]. In agreement with the data on deficient androgen action during the MPW in males, mice treated during the MPW with a potent oestrogen, diethylstilboestrol (DES), present with similar urogenital disorders [129]. This could be a partial explanation for the high incidence of urogenital disorders in boys exposed to DES in utero. In this regard, one could propose a positive relationship between EDCs and poor male reproductive parameters [9], as the milder male reproductive disorders cryptorchidism and hypospadias occur in 2.4–9 and 1–4.6 %, respectively, of boys at birth [130, 131]. The concept therefore has the potential to be clinically useful in assessing androgen deficiency during human gestation. However, there are no valid non-invasive parameters for assessing androgen action during the MPW to predict the incidence of reproductive disorders, such as hypospadias and cryptorchidism. Although AGD has the highest potential as a clinical biomarker for predicting changes in the foetal androgen/oestrogen balance, a recent animal study demonstrated the variability in AGD [132].
Despite genetic polymorphisms that introduce variability into the susceptibility to EDCs, human disorders are presumably the result of chronic exposure to low levels of EDCs. It is challenging to establish a relationship between exposure to EDCs and the occurrence of clinical disorders. Studies on EDCs have shown that even common pesticides, such as methoxychlor (MXC), may influence sex differentiation [133] and impair semen quality and steroid production [134, 135]. Most of the previous studies were carried out using toxicological doses of MXC (over 100 mg/kg/day) [136, 137], but humans and wildlife would not be exposed to such conditions/doses. We have recently shown that maternal (from gestational embryonic day E0.5 till lactational period postnatal day P21.5) exposure to low-dose MXC (1 mg/kg/day) disturbs testicular development in mice and significantly downregulates certain spermatogenic genes (Dazl, Boll, Rarg, Stra8 and Cyclin-A1) [138]. In a similar recent study, we reported that maternal low-dose exposure to another widely used synthetic pyrethroid and potential EDC, cypermethrin (CYP) (1 mg/kg/day), also affected murine testicular development and adult function and downregulated the expression of T production-related, mitosis-related, and meiosis-related genes [139]. When analysing the EDCs potential effects on male reproduction data, one should consider the major striking differences in endocrinology between humans and rodents.
Natural chemicals found in human and animal food products (e.g. phytoestrogens such as genistein and coumestrol) may also act as EDCs. Phytoestrogens are natural oestrogenic compounds that bind to both ERα and ERβ due to their conformational similarity to E2 [140]. Phytoestrogens are abundant in soy and tofu products as well as in many other food products. Urinary concentrations of the phytoestrogens genistein and daidzein are approximately 500-fold higher in infants fed with soy formula than in those fed with cow milk formula [141]. Therefore, it is important to analyse the putative potential for endocrine disruption by phytoestrogens. Phytoestrogens may negatively affect male fertility, as supported by the epidemiological data on deteriorating male reproductive function (fertility and/or semen quality) due to exposure to EDCs/phytoestrogens [142, 143]. Although there are reports indicating a negative association between exposure to certain EDCs and sperm quality, no solid evidence has been published to support this claim [143].
An evolving body of evidence suggests that decreased semen quality could be related to environmental exposure to oestrogen-like compounds, although some studies have demonstrated conflicting results [144]. Data from randomized human studies show a clear and consistent decline in serum T levels in healthy American men over the years [145], but the trend in the decline in serum T concentration in EDC-exposed men has been more uniform [144]. Reports have shown lower general sperm counts in Japanese men compared with Caucasian men, which could be associated with the massive consumption of phytoestrogens in Asia, although correlations with other factors, such as genetic diversity or lifestyle issues, cannot be ruled out [146, 147]. Until recently, limited research was available on the effects of EDCs on human couple fecundity, but a correlation between higher serum concentrations of EDCs and decreased fecundity has been reported [148], especially in men [149, 150].
The discrepancy, or rather the lack of consistency between human and animal study results on male reproductive parameters after EDCs could be due to the pharmacological doses used in animal studies. Humans are not exposed to such high EDC doses. A second major obstacle to study the in vitro effects of EDCs has been a lack of good model for screening potential reproductive and developmental toxicants [151, 152]. Current protocols and models are inadequate for the screening of numerous chemicals, metabolites, and mixtures that may impair testicular steroidogenesis [151–153]. A recently characterized murine Leydig cell line, BLTK1, that produces androgens and steroids in response to hCG/LH stimulation and a novel complementary recombinant human chorionic gonadotropin (rhCG)-inducible Leydig-based model could fill this missing gap and be used to assess the effects on steroidogenic gene expression, intracellular cAMP levels, and the concentrations of P, T, and E2 in media [154, 155].
Conclusion and insight into the future
A detailed analysis of the oestrogen-related mutant patients and the genetically modified murine models suggests a complex contribution of oestrogen actions. Additionally, the oestrogen/androgen ratio balance, specifically within the MPW is a prerequisite for normal male reproduction (Fig. 1). αERKO and αβERKO, but not βERKO, males are infertile due to a defect in efferent duct fluid reabsorption [156, 157]. ArKO males, although initially fertile, develop progressive infertility with arrested spermatogenesis [2]. Although the phenotypes of the αERKO and ArKO males are indicative of the critical roles of oestrogens mediated via ERα activation, on adult rodent fertility, the role of oestrogens on human masculine fertility has not yet been completely elucidated. The limited observations of testicular function in adult humans with a disruption of the ERα gene [4] or incapable oestrogen synthesis secondary to congenital aromatase deficiency [3, 100] have failed to provide clear conclusions. The available literature may suggest that oestrogens are required for normal fertility in men. The non-genomic effect of oestrogens provides a new basis for understanding the roles of oestrogen and GPER in the regulation of spermatogenesis and in reproductive disorders. Thus, the generation of a triple KO murine model (ERs and GPER) could be an important contribution to further understand mechanisms of oestrogen signalling.
Ample data suggest that EDCs, including environmental chemicals, directly or indirectly may affect the function of the male reproductive system. However, convincing human evidence that EDC exposure to the general population impairs male fertility is still lacking. There is perhaps no direct evidence that oestrogen actions per se in the MPW period are important, although in rodents over-exposure to oestrogens in foetal life can perturb this MPW via inhibiting androgen production. It is possible that these rodent effects could have played an important role in generating the “oestrogen hypothesis” with putative knock on concerns for oestrogenic EDCs. Numerous in vitro studies on human foetal testes by Rene Habert group [158–160] or a recent study in a xenograft model [161] showed no effect of oestrogens, arguably due to the lack of ERα in human foetal Leydig cells. Whilst this phenomenon does not invalidate the idea that oestrogens can impact the human male at other life stages, it would be crucial to highlight the rigorous rodent–human distinction, while assessing EDC risk issues.
Tissue-specific ER modulators and selective aromatase inhibitors have been tested for effects on male infertility, although more mechanistic data on their therapeutic or pharmacological potential as well as adverse effects on male fertility and/or contraception are yet to come. Our recent report [14] presented data on a novel GAS6-mediated molecular mechanism that underlies oestrogen-mediated Leydig cell hyperplasia and the subsequent macrophage engulfment process in a murine model and in infertile men. Hence, this study highlighted the importance of the oestrogen/androgen ratio in human fertility/infertility. Further studies on the novel potential application of GAS6 are necessary, since it may represent a clinical biomarker for male infertility and/or a potential therapeutic target for a subset of infertile men with an impaired oestrogen/androgen ratio.
Acknowledgments
This work was supported by grants from the National Science and Technology Major Project (2013ZX10004608), Natural Science Foundation of China (NSFC31071316 and NSFC81261130024), National Science and Technology Major Project (2012AA020601), Ministry of Science/Technology (2009CB941701), the CAU Scientific Fund (No. 2012YJ034) and Academy of Finland (No. 256433). The authors thank Dr. Andreina Kero for correcting the English language of the revised manuscript.
Conflict of interest
None.
References
- 1.Lubahn DB, et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90(23):11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher CR, et al. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA. 1998;95(12):6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carani C, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337(2):91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 4.Smith EP, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 5.Simpson ER, et al. Aromatase–a brief overview. Annu Rev Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe RM. Do males rely on female hormones? Nature. 1997;390(6659):447–448. doi: 10.1038/37236. [DOI] [PubMed] [Google Scholar]
- 7.Wang RS, et al. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 2009;30(2):119–132. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirianni R, et al. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology. 2008;149(10):5043–5051. doi: 10.1210/en.2007-1593. [DOI] [PubMed] [Google Scholar]
- 9.Toppari J, et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104(Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toppari J, et al. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res A Clin Mol Teratol. 2010;88(10):910–919. doi: 10.1002/bdra.20707. [DOI] [PubMed] [Google Scholar]
- 11.Carlsen E, et al. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jørgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, Juul A, Andersson A-M, Carlsen E, Petersen JH, Toppari J, Skakkebæk NE. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2(4):e000990. doi: 10.1136/bmjopen-2012-000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welsh M, et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118(4):1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu W, et al. Estrogen promotes Leydig cell engulfment by macrophages in male infertility. J Clin Invest. 2014;124(6):2709–2721. doi: 10.1172/JCI59901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulun SE, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 16.Boukari K, et al. Human fetal testis: source of estrogen and target of estrogen action. Hum Reprod. 2007;22(7):1885–1892. doi: 10.1093/humrep/dem091. [DOI] [PubMed] [Google Scholar]
- 17.Conley A, Hinshelwood M. Mammalian aromatases. Reproduction. 2001;121(5):685–695. doi: 10.1530/rep.0.1210685. [DOI] [PubMed] [Google Scholar]
- 18.Simpson ER, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15(3):342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 19.van den Driesche S, et al. Relative importance of prenatal and postnatal androgen action in determining growth of the penis and anogenital distance in the rat before, during and after puberty. Int J Androl. 2011;34(6 Pt 2):e578–e586. doi: 10.1111/j.1365-2605.2011.01175.x. [DOI] [PubMed] [Google Scholar]
- 20.Dean A, Sharpe RM. Anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J Clin Endocrinol Metab. 2013;98(6):2230–2238. doi: 10.1210/jc.2012-4057. [DOI] [PubMed] [Google Scholar]
- 21.Saez JM. Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev. 1994;15(5):574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- 22.Tapanainen J, et al. Age-related changes in endogenous steroids of human fetal testis during early and midpregnancy. J Clin Endocrinol Metab. 1981;52(1):98–102. doi: 10.1210/jcem-52-1-98. [DOI] [PubMed] [Google Scholar]
- 23.Pezzi V, et al. Profiling transcript levels for steroidogenic enzymes in fetal tissues. J Steroid Biochem Mol Biol. 2003;87(2–3):181–189. doi: 10.1016/j.jsbmb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm D, Koopman P. The makings of maleness: towards an integrated view of male sexual development. Nat Rev Genet. 2006;7(8):620–631. doi: 10.1038/nrg1903. [DOI] [PubMed] [Google Scholar]
- 25.Wyndham NR (1943) A morphological study of testicular descent. J Anat 77(Pt 2):179–188 [PMC free article] [PubMed]
- 26.Bourguiba S, et al. Regulation of aromatase gene expression in Leydig cells and germ cells. J Steroid Biochem Mol Biol. 2003;86(3–5):335–343. doi: 10.1016/S0960-0760(03)00343-1. [DOI] [PubMed] [Google Scholar]
- 27.Silandre D, et al. Three promoters PII, PI.f, and PI.tr direct the expression of aromatase (cyp19) gene in male rat germ cells. J Mol Endocrinol. 2007;39(2):169–181. doi: 10.1677/JME-07-0046. [DOI] [PubMed] [Google Scholar]
- 28.Carreau S, Wolczynski S, Galeraud-Denis I. Aromatase, oestrogens and human male reproduction. Philos Trans R Soc Lond B Biol Sci. 2010;365(1546):1571–1579. doi: 10.1098/rstb.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inkster S, Yue W, Brodie A. Human testicular aromatase: immunocytochemical and biochemical studies. J Clin Endocrinol Metab. 1995;80(6):1941–1947. doi: 10.1210/jcem.80.6.7539819. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell L, et al. Estrogen and spermatogenesis. Endocr Rev. 2001;22(3):289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- 31.Carreau S, Bouraima-Lelong H, Delalande C. Role of estrogens in spermatogenesis. Front Biosci (Elite Ed) 2012;4:1–11. doi: 10.2741/E356. [DOI] [PubMed] [Google Scholar]
- 32.Bilinska B, et al. Photoperiod-dependent capability of androgen aromatization and the role of estrogens in the bank vole testis visualized by means of immunohistochemistry. Mol Cell Endocrinol. 2001;178(1–2):189–198. doi: 10.1016/S0303-7207(01)00427-0. [DOI] [PubMed] [Google Scholar]
- 33.Aquila S, et al. Human ejaculated spermatozoa contain active P450 aromatase. J Clin Endocrinol Metab. 2002;87(7):3385–3390. doi: 10.1210/jcem.87.7.8633. [DOI] [PubMed] [Google Scholar]
- 34.Carreau S, et al. Estrogens and male reproduction: a new concept. Braz J Med Biol Res. 2007;40(6):761–768. doi: 10.1590/S0100-879X2007000600003. [DOI] [PubMed] [Google Scholar]
- 35.Lambard S, Carreau S. Aromatase and oestrogens in human male germ cells. Int J Androl. 2005;28(5):254–259. doi: 10.1111/j.1365-2605.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson S, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81(4):1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 37.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276(40):36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 38.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3(5):281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 39.Gibson DA, Saunders PT. Estrogen dependent signaling in reproductive tissues—a role for estrogen receptors and estrogen related receptors. Mol Cell Endocrinol. 2012;348(2):361–372. doi: 10.1016/j.mce.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 40.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 41.Chimento A, et al. GPER signaling in spermatogenesis and testicular tumors. Front Endocrinol (Lausanne) 2014;5:30. doi: 10.3389/fendo.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 43.Sharpe RM (2010) Bisphenol A exposure and sexual dysfunction in men: editorial commentary on the article ‘Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction’ Li et al., 2009. Hum Reprod 25(2):292–294 [DOI] [PubMed]
- 44.Green S, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 45.Kuiper GG, et al. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filardo EJ, et al. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14(10):1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Hsu CL, Chang C. Androgen receptor corepressors: an overview. Prostate. 2005;63(2):117–130. doi: 10.1002/pros.20170. [DOI] [PubMed] [Google Scholar]
- 48.Flouriot G, et al. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J. 2000;19(17):4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakraborty P, Roy SK. Expression of estrogen receptor alpha 36 (ESR36) in the hamster ovary throughout the estrous cycle: effects of gonadotropins. PLoS One. 2013;8(3):e58291. doi: 10.1371/journal.pone.0058291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scobie GA, et al. Human oestrogen receptors: differential expression of ER alpha and beta and the identification of ER beta variants. Steroids. 2002;67(12):985–992. doi: 10.1016/S0039-128X(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 51.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140(12):5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa S, et al. Modifications of testosterone-dependent behaviors by estrogen receptor-alpha gene disruption in male mice. Endocrinology. 1998;139(12):5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 53.Rochira V, et al. Estrogens in males: what have we learned in the last 10 years? Asian J Androl. 2005;7(1):3–20. doi: 10.1111/j.1745-7262.2005.00018.x. [DOI] [PubMed] [Google Scholar]
- 54.Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- 55.Lazari MF, et al. Estrogen receptors and function in the male reproductive system. Arq Bras Endocrinol Metabol. 2009;53(8):923–933. doi: 10.1590/S0004-27302009000800005. [DOI] [PubMed] [Google Scholar]
- 56.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365(1546):1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nie R, et al. Differential expression of estrogen receptors alpha and beta in the reproductive tracts of adult male dogs and cats. Biol Reprod. 2002;66(4):1161–1168. doi: 10.1095/biolreprod66.4.1161. [DOI] [PubMed] [Google Scholar]
- 58.Zhou Q, et al. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23(6):870–881. [PubMed] [Google Scholar]
- 59.Saunders PT, et al. Differential expression of oestrogen receptor alpha and beta proteins in the testes and male reproductive system of human and non-human primates. Mol Hum Reprod. 2001;7(3):227–236. doi: 10.1093/molehr/7.3.227. [DOI] [PubMed] [Google Scholar]
- 60.Ra HS, Rubin L, Crang RF. Structural impacts on thallus and algal cell components of two lichen species in response to low-level air pollution in pacific northwest forests. Microsc Microanal. 2004;10(2):270–279. doi: 10.1017/S1431927604040048. [DOI] [PubMed] [Google Scholar]
- 61.Carreau S, et al. Estrogens: a new player in spermatogenesis. Folia Histochem Cytobiol. 2007;45(Suppl 1):S5–S10. [PubMed] [Google Scholar]
- 62.Jones ME, et al. Recognizing rare disorders: aromatase deficiency. Nat Clin Pract Endocrinol Metab. 2007;3(5):414–421. doi: 10.1038/ncpendmet0477. [DOI] [PubMed] [Google Scholar]
- 63.Plackett TP, et al. Lack of aromatase improves cell-mediated immune response after burn. Burns. 2006;32(5):577–582. doi: 10.1016/j.burns.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 64.Couse JE, et al. Molecular mechanism of estrogen action in the male: insights from the estrogen receptor null mice. Reprod Fertil Dev. 2001;13(4):211–219. doi: 10.1071/RD00128. [DOI] [PubMed] [Google Scholar]
- 65.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 66.Goulding EH, et al. Ex3alphaERKO male infertility phenotype recapitulates the alphaERKO male phenotype. J Endocrinol. 2010;207(3):281–288. doi: 10.1677/JOE-10-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Q, et al. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci USA. 2001;98(24):14132–14137. doi: 10.1073/pnas.241245898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee KH, et al. Estrogen regulation of ion transporter messenger RNA levels in mouse efferent ductules are mediated differentially through estrogen receptor (ER) alpha and ER beta. Biol Reprod. 2001;65(5):1534–1541. doi: 10.1095/biolreprod65.5.1534. [DOI] [PubMed] [Google Scholar]
- 69.Mahato D, et al. Spermatogenic cells do not require estrogen receptor-alpha for development or function. Endocrinology. 2000;141(3):1273–1276. doi: 10.1210/endo.141.3.7439. [DOI] [PubMed] [Google Scholar]
- 70.Mahato D, et al. Estrogen receptor-alpha is required by the supporting somatic cells for spermatogenesis. Mol Cell Endocrinol. 2001;178(1–2):57–63. doi: 10.1016/S0303-7207(01)00410-5. [DOI] [PubMed] [Google Scholar]
- 71.Couse JF, et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9(11):1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 72.Hewitt SC, et al. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem. 2010;285(4):2676–2685. doi: 10.1074/jbc.M109.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akingbemi BT, et al. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology. 2003;144(1):84–93. doi: 10.1210/en.2002-220292. [DOI] [PubMed] [Google Scholar]
- 74.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 75.Antal MC, et al. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci USA. 2008;105(7):2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winuthayanon W, et al. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA. 2010;107(45):19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bridges PJ, et al. Generation of Cyp17iCre transgenic mice and their application to conditionally delete estrogen receptor alpha (Esr1) from the ovary and testis. Genesis. 2008;46(9):499–505. doi: 10.1002/dvg.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Billon-Gales A, et al. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120(25):2567–2576. doi: 10.1161/CIRCULATIONAHA.109.898445. [DOI] [PubMed] [Google Scholar]
- 79.Billon-Gales A, et al. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci USA. 2011;108(32):13311–13316. doi: 10.1073/pnas.1105632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arao Y, et al. Estrogen receptor alpha AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proc Natl Acad Sci USA. 2011;108(36):14986–14991. doi: 10.1073/pnas.1109180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arao Y, et al. Transactivating function (AF) 2-mediated AF-1 activity of estrogen receptor alpha is crucial to maintain male reproductive tract function. Proc Natl Acad Sci USA. 2012;109(51):21140–21145. doi: 10.1073/pnas.1216189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee SY, et al. ERalpha/E2 signaling suppresses the expression of steroidogenic enzyme genes via cross-talk with orphan nuclear receptor Nur77 in the testes. Mol Cell Endocrinol. 2012;362(1–2):91–103. doi: 10.1016/j.mce.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 83.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martensson UE, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150(2):687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 85.Otto C, et al. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80(1):34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 86.Honda S, et al. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun. 1998;252(2):445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- 87.Robertson KM, et al. The phenotype of the aromatase knockout mouse reveals dietary phytoestrogens impact significantly on testis function. Endocrinology. 2002;143(8):2913–2921. doi: 10.1210/endo.143.8.8957. [DOI] [PubMed] [Google Scholar]
- 88.Robertson KM, et al. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci USA. 1999;96(14):7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robertson KM, et al. Characterization of the fertility of male aromatase knockout mice. J Androl. 2001;22(5):825–830. [PubMed] [Google Scholar]
- 90.Li X, et al. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 2001;142(6):2435–2442. doi: 10.1210/endo.142.6.8211. [DOI] [PubMed] [Google Scholar]
- 91.Lin W, et al. Molecular mechanisms of bladder outlet obstruction in transgenic male mice overexpressing aromatase (Cyp19a1) Am J Pathol. 2011;178(3):1233–1244. doi: 10.1016/j.ajpath.2010.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li X, et al. Transgenic mice expressing p450 aromatase as a model for male infertility associated with chronic inflammation in the testis. Endocrinology. 2006;147(3):1271–1277. doi: 10.1210/en.2005-0654. [DOI] [PubMed] [Google Scholar]
- 93.Balasinor NH, et al. Effect of high intratesticular estrogen on global gene expression and testicular cell number in rats. Reprod Biol Endocrinol. 2010;8:72. doi: 10.1186/1477-7827-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tekmal RR, et al. Overexpression of int-5/aromatase in mammary glands of transgenic mice results in the induction of hyperplasia and nuclear abnormalities. Cancer Res. 1996;56(14):3180–3185. [PubMed] [Google Scholar]
- 95.Fowler KA, et al. Overexpression of aromatase leads to development of testicular leydig cell tumors : an in vivo model for hormone-mediated TesticularCancer. Am J Pathol. 2000;156(1):347–353. doi: 10.1016/S0002-9440(10)64736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mandava U, Kirma N, Tekmal RR. Aromatase overexpression transgenic mice model: cell type specific expression and use of letrozole to abrogate mammary hyperplasia without affecting normal physiology. J Steroid Biochem Mol Biol. 2001;79(1–5):27–34. doi: 10.1016/S0960-0760(01)00133-9. [DOI] [PubMed] [Google Scholar]
- 97.Qian YM, et al. Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology. 2001;142(12):5342–5350. doi: 10.1210/endo.142.12.8540. [DOI] [PubMed] [Google Scholar]
- 98.Tannour-Louet M, et al. Identification of de novo copy number variants associated with human disorders of sexual development. PLoS ONE. 2010;5(10):e15392. doi: 10.1371/journal.pone.0015392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tannour-Louet M, et al. Increased gene copy number of VAMP7 disrupts human male urogenital development through altered estrogen action. Nat Med. 2014;20(7):715–724. doi: 10.1038/nm.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morishima A, et al. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80(12):3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 101.Herrmann BL, et al. Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. J Clin Endocrinol Metab. 2002;87(12):5476–5484. doi: 10.1210/jc.2002-020498. [DOI] [PubMed] [Google Scholar]
- 102.Maffei L, et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab. 2004;89(1):61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- 103.Maffei L, et al. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf) 2007;67(2):218–224. doi: 10.1111/j.1365-2265.2007.02864.x. [DOI] [PubMed] [Google Scholar]
- 104.Bouillon R, et al. Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab. 2004;89(12):6025–6029. doi: 10.1210/jc.2004-0602. [DOI] [PubMed] [Google Scholar]
- 105.Lanfranco F, et al. A novel mutation in the human aromatase gene: insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone. 2008;43(3):628–635. doi: 10.1016/j.bone.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 106.Mittre Herve MH, Kottler ML, Pura M. Human gene mutations. Gene symbol: cYP19. Disease: aromatase deficiency. Hum Genet. 2004;114(2):224. [PubMed] [Google Scholar]
- 107.Deladoey J, et al. Aromatase deficiency caused by a novel P450arom gene mutation: impact of absent estrogen production on serum gonadotropin concentration in a boy. J Clin Endocrinol Metab. 1999;84(11):4050–4054. doi: 10.1210/jcem.84.11.6135. [DOI] [PubMed] [Google Scholar]
- 108.Haverfield JT, et al. Teasing out the role of aromatase in the healthy and diseased testis. Spermatogenesis. 2011;1(3):240–249. doi: 10.4161/spmg.1.3.18037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quaynor SD, et al. Delayed puberty and estrogen resistance in a woman with estrogen receptor alpha variant. N Engl J Med. 2013;369(2):164–171. doi: 10.1056/NEJMoa1303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shozu M, Fukami M, Ogata T. Understanding the pathological manifestations of aromatase excess syndrome: lessons for clinical diagnosis. Expert Rev Endocrinol Metab. 2014;9(4):397–409. doi: 10.1586/17446651.2014.926810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shozu M, et al. Estrogen excess associated with novel gain-of-function mutations affecting the aromatase gene. N Engl J Med. 2003;348(19):1855–1865. doi: 10.1056/NEJMoa021559. [DOI] [PubMed] [Google Scholar]
- 112.Simpson ER, Brown KA. Obesity and breast cancer: role of inflammation and aromatase. J Mol Endocrinol. 2013;51(3):T51–T59. doi: 10.1530/JME-13-0217. [DOI] [PubMed] [Google Scholar]
- 113.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30(7):883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- 114.Ra HJ, Ha JK, Kim JG. One-stage revision anterior cruciate ligament reconstruction with impacted bone graft after failed primary reconstruction. Orthopedics. 2013;36(11):860–863. doi: 10.3928/01477447-20131021-07. [DOI] [PubMed] [Google Scholar]
- 115.Fukami M, Shozu M, Ogata T. Molecular bases and phenotypic determinants of aromatase excess syndrome. Int J Endocrinol. 2012;2012:584807. doi: 10.1155/2012/584807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207(9):1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scott RS, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411(6834):207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 118.Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283(5406):1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 119.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 120.Serbina NV, et al. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Calippe B, et al. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185(2):1169–1176. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- 122.Taylor PR, et al. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 123.Mo R, et al. GAS6 is an estrogen-inducible gene in mammary epithelial cells. Biochem Biophys Res Commun. 2007;353(1):189–194. doi: 10.1016/j.bbrc.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang H, et al. Immunoexpression of Tyro 3 family receptors–Tyro 3, Axl, and Mer–and their ligand Gas6 in postnatal developing mouse testis. J Histochem Cytochem. 2005;53(11):1355–1364. doi: 10.1369/jhc.5A6637.2005. [DOI] [PubMed] [Google Scholar]
- 125.Hedger MP. Macrophages and the immune responsiveness of the testis. J Reprod Immunol. 2002;57(1–2):19–34. doi: 10.1016/S0165-0378(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 126.Hutson JC. Interactions between testicular macrophages and Leydig cells. J Androl. 1998;19(4):394–398. [PubMed] [Google Scholar]
- 127.Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol. 2002;57(1–2):3–18. doi: 10.1016/S0165-0378(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 128.Giovannone D, et al. Slits affect the timely migration of neural crest cells via Robo receptor. Dev Dyn. 2012;241(8):1274–1288. doi: 10.1002/dvdy.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McLachlan JA, Newbold RR, Bullock B. Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science. 1975;190(4218):991–992. doi: 10.1126/science.242076. [DOI] [PubMed] [Google Scholar]
- 130.Boisen KA, et al. Hypospadias in a cohort of 1072 Danish newborn boys: prevalence and relationship to placental weight, anthropometrical measurements at birth, and reproductive hormone levels at three months of age. J Clin Endocrinol Metab. 2005;90(7):4041–4046. doi: 10.1210/jc.2005-0302. [DOI] [PubMed] [Google Scholar]
- 131.Boisen KA, et al. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet. 2004;363(9417):1264–1269. doi: 10.1016/S0140-6736(04)15998-9. [DOI] [PubMed] [Google Scholar]
- 132.Kim MK, et al. Comparison of second-look arthroscopic findings and clinical results according to the amount of preserved remnant in anterior cruciate ligament reconstruction. Knee. 2014;21(3):774–778. doi: 10.1016/j.knee.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 133.Gioiosa L, et al. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav. 2007;52(3):307–316. doi: 10.1016/j.yhbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 134.Cupp AS, et al. Effect of transient embryonic in vivo exposure to the endocrine disruptor methoxychlor on embryonic and postnatal testis development. J Androl. 2003;24(5):736–745. doi: 10.1002/j.1939-4640.2003.tb02736.x. [DOI] [PubMed] [Google Scholar]
- 135.Aly HA, Azhar AS. Methoxychlor induced biochemical alterations and disruption of spermatogenesis in adult rats. Reprod Toxicol. 2013;40:8–15. doi: 10.1016/j.reprotox.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 136.Gray LE, Jr, et al. The estrogenic and antiandrogenic pesticide methoxychlor alters the reproductive tract and behavior without affecting pituitary size or LH and prolactin secretion in male rats. Toxicol Ind Health. 1999;15(1–2):37–47. doi: 10.1191/074823399678846655. [DOI] [PubMed] [Google Scholar]
- 137.Chapin RE, et al. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam Appl Toxicol. 1997;40(1):138–157. doi: 10.1006/faat.1997.2381. [DOI] [PubMed] [Google Scholar]
- 138.Du X, et al. Perinatal exposure to low-dose methoxychlor impairs testicular development in C57BL/6 mice. PLoS ONE. 2014;9(7):e103016. doi: 10.1371/journal.pone.0103016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang C, Li X. Maternal cypermethrin exposure during the perinatal period impairs testicular development in C57BL male offspring. PLoS One. 2014;9(5):e96781. doi: 10.1371/journal.pone.0096781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuiper GG, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 141.Cao Y, et al. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol. 2009;19(2):223–234. doi: 10.1038/jes.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Juul A, et al. Possible fetal determinants of male infertility. Nat Rev Endocrinol. 2014;10(9):553–562. doi: 10.1038/nrendo.2014.97. [DOI] [PubMed] [Google Scholar]
- 143.Giwercman A. Estrogens and phytoestrogens in male infertility. Curr Opin Urol. 2011;21(6):519–526. doi: 10.1097/MOU.0b013e32834b7e7c. [DOI] [PubMed] [Google Scholar]
- 144.Fisch H, Braun SR. Trends in global semen parameter values. Asian J Androl. 2013;15(2):169–173. doi: 10.1038/aja.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Travison TG, et al. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92(1):196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- 146.Iwamoto T, et al. Semen quality of 324 fertile Japanese men. Hum Reprod. 2006;21(3):760–765. doi: 10.1093/humrep/dei362. [DOI] [PubMed] [Google Scholar]
- 147.Nozawa S, Iwamoto T. Effects of endocrine disrupting chemicals on male reproductive function–epidemiological views. Nihon Eiseigaku Zasshi. 2006;61(1):32–37. doi: 10.1265/jjh.61.32. [DOI] [PubMed] [Google Scholar]
- 148.Buck Louis GM. Persistent environmental pollutants and couple fecundity: an overview. Reproduction. 2014;147(4):R97–R104. doi: 10.1530/REP-13-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Buck Louis GM, et al. Persistent environmental pollutants and couple fecundity: the LIFE study. Environ Health Perspect. 2013;121(2):231–236. doi: 10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cole DC, et al. Environmental contaminant levels and fecundability among non-smoking couples. Reprod Toxicol. 2006;22(1):13–19. doi: 10.1016/j.reprotox.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 151.Sanderson JT. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci. 2006;94(1):3–21. doi: 10.1093/toxsci/kfl051. [DOI] [PubMed] [Google Scholar]
- 152.Whitehead SA, Rice S. Endocrine-disrupting chemicals as modulators of sex steroid synthesis. Best Pract Res Clin Endocrinol Metab. 2006;20(1):45–61. doi: 10.1016/j.beem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 153.Wilson VS, et al. Diverse mechanisms of anti-androgen action: impact on male rat reproductive tract development. Int J Androl. 2008;31(2):178–187. doi: 10.1111/j.1365-2605.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- 154.Forgacs AL, et al. Triazine herbicides and their chlorometabolites alter steroidogenesis in BLTK1 murine Leydig cells. Toxicol Sci. 2013;134(1):155–167. doi: 10.1093/toxsci/kft096. [DOI] [PubMed] [Google Scholar]
- 155.Forgacs AL, et al. BLTK1 murine Leydig cells: a novel steroidogenic model for evaluating the effects of reproductive and developmental toxicants. Toxicol Sci. 2012;127(2):391–402. doi: 10.1093/toxsci/kfs121. [DOI] [PubMed] [Google Scholar]
- 156.Korach KS. Insights from the study of animals lacking functional estrogen receptor. Science. 1994;266(5190):1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- 157.Hess RA, et al. Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl. 1997;18(6):602–611. [PubMed] [Google Scholar]
- 158.Habert R, et al. Concerns about the widespread use of rodent models for human risk assessments of endocrine disruptors. Reproduction. 2014;147(4):R119–R129. doi: 10.1530/REP-13-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.N’Tumba-Byn T, et al. Differential effects of bisphenol A and diethylstilbestrol on human, rat and mouse fetal leydig cell function. PLoS ONE. 2012;7(12):e51579. doi: 10.1371/journal.pone.0051579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Muczynski V, et al. Effect of mono-(2-ethylhexyl) phthalate on human and mouse fetal testis: in vitro and in vivo approaches. Toxicol Appl Pharmacol. 2012;261(1):97–104. doi: 10.1016/j.taap.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 161.Mitchell RT, et al. Diethylstilboestrol exposure does not reduce testosterone production in human fetal testis xenografts. PLoS One. 2013;8(4):e61726. doi: 10.1371/journal.pone.0061726. [DOI] [PMC free article] [PubMed] [Google Scholar]