Abstract
Current models put forward that the epidermal growth factor receptor (EGFR) is efficiently internalized via clathrin-coated pits only in response to ligand-induced activation of its intrinsic tyrosine kinase and is subsequently directed into a lysosomal-proteasomal degradation pathway by mechanisms that include receptor tyrosine phosphorylation and ubiquitylation. Herein, we report a novel mechanism of EGFR internalization that does not require ligand binding, receptor kinase activity, or ubiquitylation and does not direct the receptor into a degradative pathway. Inhibition of basal protein kinase A (PKA) activity by H89 and the cell-permeable substrate peptide Myr-PKI induced internalization of 40–60% unoccupied, inactive EGFR, and its accumulation into early endosomes without affecting endocytosis of transferrin and μ-opioid receptors. This effect was abrogated by interfering with clathrin function. Thus, the predominant distribution of inactive EGFR at the plasma membrane is not simply by default but involves a PKA-dependent restrictive condition resulting in receptor avoidance of endocytosis until it is stimulated by ligand. Furthermore, PKA inhibition may contribute to ligand-induced EGFR endocytosis because epidermal growth factor inhibited 26% of PKA basal activity. On the other hand, H89 did not alter ligand-induced internalization of EGFR but doubled its half-time of down-regulation by retarding its segregation into degradative compartments, seemingly due to a delay in the receptor tyrosine phosphorylation and ubiquitylation. Our results reveal that PKA basal activity controls EGFR function at two levels: 1) residence time of inactive EGFR at the cell surface by a process of “endocytic evasion,” modulating the accessibility of receptors to stimuli; and 2) sorting events leading to the down-regulation pathway of ligand-activated EGFR, determining the length of its intracellular signaling. They add a new dimension to the fine-tuning of EGFR function in response to cellular demands and cross talk with other signaling receptors.
INTRODUCTION
The mechanisms that regulate the endocytic behavior of epidermal growth factor receptor (EGFR) have been a long-standing subject of intense research as a model system of regulated vesicular protein traffic associated to signaling, cellular demands, and cancer (Trowbridge et al., 1993; Di Fiore and Gill, 1999; Carpenter, 2000; Ceresa and Schmid, 2000; Schlessinger, 2000; Wiley and Burke, 2001). Endocytosis provides a pathway for gradual attenuation or desensitization of receptor signaling and also allows a single receptor to transmit different signals from different locations in the cell before degradation, thus enhancing the range of modulation and response variability (Di Fiore and Gill, 1999; Ceresa and Schmid, 2000; Wiley and Burke, 2001). Defects in internalization and/or degradation pathways, as well as distinct endocytic routing displayed by different EGFR family members, have been associated with cell transformation and oncogenesis (Di Fiore and Gill, 1999; Ceresa and Schmid, 2000). The EGFR is also a downstream element of nonepidermal growth factor (EGF)-like proliferation signals (Carpenter, 1999). All this prompts to search for mechanisms and factors able to control EGFR internalization, endosomal sorting, and degradation, either in concert with ligand-induced events or independently of ligand binding. Serine-threonine kinases are interesting elements to explore because they play roles in both signaling and vesicular protein transport.
Ligand-mediated activation of the EGFR intrinsic tyrosine kinase results in phosphorylation events involved in the signaling process, receptor endocytosis, and final fate after internalization. Ligand binding induces structural changes in the EGFR that presumably expose cryptic codes in its cytosolic domain, favoring interaction with the clathrin-mediated endocytic apparatus (Boll et al., 1995; Nesterov et al., 1995a,b), mainly through its adaptor AP-2 (Cadena et al., 1994; Nesterov et al., 1995a; Sorkin et al., 1996), although AP-2–independent processes have been also invoked (Sorkin and Carpenter, 1993; Nesterov et al., 1995b). In addition, EGFR activation stimulates the endocytic apparatus through tyrosine-phosphorylation of a variety of downstream substrates needed for its efficient recruitment into coated pits (Lamaze and Schmid, 1995). The tyrosine kinase src is activated during ligand-induced EGFR endocytosis, phosphorylating and redistributing clathrin to the plasma membrane (Wilde et al., 1999). In this scenario, only ligand-activated receptors would be efficiently recognized by the clathrin-endocytic machinery. Permanency of inactive EGFR at the cell surface is implicitly believed to be by default, grounded only on properties of receptor resting structure. On the other hand, sorting of EGF/EGFR from endosomes to a lysosomal degradation pathway depends on specific targeting information residing in its intracellular domain (Kurten et al., 1996; Kil et al., 1999) and has been recently shown to involve ubiquitylation of the receptor (Levkowitz et al., 1998) in a process influenced by protein kinase C (PKC) and mediated by the proto-oncogene product c-Cbl, that is tyrosine phosphorylated by the EGFR (Bao et al., 2000).
Several studies have shown that the exocytic and endocytic pathways are tightly regulated by different combinations of serine-threonine kinases, including PKC and protein kinase A (PKA), presumably by controlling the generation of transport carriers emerging from the ER, Golgi complex, or plasma membrane (Pimplikar and Simons, 1994; Muniz et al., 1996, 1997; Goretzki and Mueller, 1997; Jamora et al., 1999; Aridor and Balch, 2000; Lee and Linstedt, 2000). PKC and PKA also have been implicated in transmodulation of ligand and/or kinase activity of the EGFR (Ciardiello and Tortora, 1998; Barbier et al., 1999; Carpenter, 1999; Schlessinger, 2000). However, only PKC has been additionally reported to modulate EGFR function by modifying its endocytic sorting (Bao et al., 2000). Instead, for several other proteins, including the low-density lipoprotein receptor-related protein (Li et al., 2001), urokinase-type plasminogen activator (Goretzki and Mueller, 1997), and Na+/H+ exchanger NHE3 (Hu et al., 2001), it has been recently described that PKA activity is required for their stimulated endocytosis. Inhibition of PKA activity or mutation of PKA phosphorylation sites inhibits the endocytosis of these proteins.
H89 is a potent, highly specific and reversible inhibitor of PKA (Chijiwa et al., 1990), which, in combination with a panel of other kinase inhibitors, provides a convenient way to analyze the function of PKA in diverse vesicular transporting pathways (Muniz et al., 1996, 1997; Goretzki and Mueller, 1997; Jamora et al., 1999; Aridor and Balch, 2000; Lee and Linstedt, 2000). Herein, we report that inhibition of PKA activity by H89 or the PKA inhibitory peptide Myr-PKI induced endocytosis and selective redistribution of empty inactive EGFR into early endocytic compartments. Thus, basal PKA activity participates in a previously unsuspected mechanism that keeps inactive EGFRs predominantly at the cell surface by abrogating their interaction with the endocytic apparatus. Additionally, we found that H89 delayed the endocytic sorting of ligand-activated EGFR to a degradative lysosomal-proteosomal pathway, very likely as a consequence of retarding the receptor autophosphorylation and ubiquitylation, suggesting that the time course of intracellular signaling before degradation is also under the influence of basal PKA activity.
MATERIALS AND METHODS
Reagents and Antibodies
Human recombinant EGF and cDNA encoding the human EGFR were provided by Drs. Pablo Valenzuela and Carlos George-Nascimento (Chiron, Emeryville, CA). cDNA encoding the N-terminal FLAG-tagged murine μ-opioid receptor was provided by Drs. Paulette Zaki and Christopher Evans (University of California, Los Angeles, CA). DMEM containing high glucose, protein A-Sepharose, butyric acid, and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum was purchased from Hyclone Laboratories (Logan, UT). H89, forskolin, H8, H7, Myr-PKI (14-22), KT5720, staurosporine, chelerythrine, calphostin C, 5,6-dicloro-1-β-d-ribofuranosylbenzimidazole (DRB), and genistein were from Calbiochem (San Diego, CA). CKI-7 was obtained from Seikagaku America (Rockville, MD) and wortmannin and [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO) were from Sigma-Aldrich.
For immunoblot detection, the enhanced chemiluminescence (ECL) system from Amersham Biosciences (Piscataway, NJ) was used. Cell surface labeling reagents EZLink sulfo-NHS-Biotin and EZLink sulfo-NHS-SS-Biotin were from Pierce Chemical (Rockford, IL). Cell culture reagents were purchased from Invitrogen (Carlsbad, CA) and Sigma-Aldrich. Tissue culture plastics were from Nalge Nunc (Naperville, IL). Polyclonal antibody EGFR984 has been previously characterized (Faúndez et al., 1992) and was obtained against the peptide described previously (Kriss et al., 1985), corresponding to residues 984–996 (DDVVDADEYLIPQ) of the EGFR cytosolic tail. Hybridomas producing monoclonal anti-EGFR antibodies HB8506 reacting with the extracellular domain of the EGFR and anti-phosphotyrosine HB8190 were purchased from American Type Culture Collection (Manassas, VA). HB8190 can detect phosphorylated tyrosines both in immunoblot and immunoprecipitation (Faúndez et al., 1992). Anti-ubiquitin antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). M2 antibody against FLAG was from Sigma-Aldrich.
Cell Culture, Transfection, and Drug Treatments
N2a cells, cultured in DMEM, 4.5 g/l glucose, supplemented with 7.5% fetal bovine serum and antibiotics (50 mU/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml fungizone) were transfected with a vector pBK-CMV into which the cDNA of the human EGFR was subcloned, or with pcDNA3 vector into which the cDNA of FLAG-μ-opioid receptor was subcloned. Transfection was done with LipofectAMINE (Invitrogen) according to the manufacturer's instructions. Permanently transfected cells were selected in 0.8 mg/ml G418 and maintained in the above-mentioned culture medium supplemented with 0.2 mg/ml G418 (Invitrogen). Expression was induced by 10 mM sodium butyrate for 12–24 h, following our protocols described previously (Marzolo et al., 1997). K721A cells expressing a point mutant kinase-negative EGFR and Her14 expressing wt EGFR (Felder et al., 1990) were a kind gift from Dr. J. Schlessinger (New York University Medical Center, New York, NY). In all the experiments, the cells were cultured to ∼80% confluence and then were serum starved by incubation during 24 h in media supplemented with only 0.3% fetal bovine serum (FBS) (Hyclone Laboratories). For H89, KT5720, CKI-7, staurosporin, and DRB, stock solutions were prepared in dimethyl sulfoxide and, therefore, control conditions included concentrations of 0.2–0.4% dimethyl sulfoxide.
Indirect Immunofluorescence and Colocalization with Transferrin-Texas Red (TxR)
Cells were grown on glass coverslips, subjected to the different experimental conditions, and then washed with phosphate-buffered saline (PBS) and fixed for 30 min at room temperature with 4% paraformaldehyde in PBS supplemented with 0.1 mM CaCl2 and 1 mM MgCl2 (PBS-CM). After washing three times with PBS plus 0.2% gelatin (300 Bloom; Sigma-Aldrich) for 5 min each, the cells were permeabilized with 0.2% Triton X-100 for 10 min at room temperature and incubated for 1 h at 25°C with anti-EGFR monoclonal antibody (mAb) HB8506 (1/100 in PBS). Incubation with the secondary antibody anti-mouse IgG fluorescein isothiocyanate (FITC) (1/100 in PBS, 1 h at 25°C) was made after six washes in PBS-gelatin. Trafficking of the internalized EGFR through the early endocytic compartments was analyzed together with transferrin-TxR. The cells were washed three times with cold dMEM and then incubated with 50 μg/ml transferrin-TxR (Molecular Probes, Eugene, OR) for different time periods at 37°C, washed, and processed for indirect immunofluorescence of the EGFR. Fluorescence images were collected on a Zeiss Axioplan microscope and photographed using a 63× immersion objective and the Axiocam camera. Images were transferred to a computer workstation running Axiovision imaging software.
Receptor Binding and Internalization Assays
Human 125I-EGF was prepared by the chloramine T method as described previously (Faúndez et al., 1992). The specific activities of labeled ligand were typically 50,000–70,000 cpm/ng. Binding assays were done in Hanks' solution with 20 mM HEPES and 0.1% bovine serum albumin (BSA) during 2 h at 4°C. Free ligand was removed by washing four times with ice-cold PBS-0.1% BSA. Cell-bound ligand was released in 1 M NaOH for 1 h at room temperature. Nonspecific binding was assessed in the presence of 1 μg/ml unlabeled human EGF and subtracted from the total. Samples were quantified using a gamma counter (COBRA II; Packard, Canberra, Australia). Scatchard analysis was performed with the LIGAND program (Munson and Rodbard, 1980). To monitor 125I-EGF internalization, cells in 12-well dishes were incubated with 50 ng/ml 125I-EGF in binding medium for 2 h at 4°C. After washing the unbound radioligand, the cells were transferred to 37°C for different time periods. Assays were ended by rapidly cooling the monolayers in ice water followed by four washes with ice-cold PBS-0.1% BSA to remove unbound ligand and then surface and internalization assessment. The established acid-wash method (Haigler et al., 1980) was made incubating the cells for 6 min with 0.2 M acetic acid, pH 2.5, containing 0.5 M NaCl at 4°C. The acid wash was combined with another short rinse with the same acidic solution to determine the amount of surface-bound (SUR) 125I-EGF. The cells were then lysed in 1 N NaOH to quantitate internalized (IN) radioactivity. 125I-EGF degradation was assessed by trichloroacetic acid precipitation of incubation media. All data represent specific 125I-EGF binding assessed by adding 100-fold molar excess of cold EGF with each point in duplicate.
Diferric transferrin (diTf) was prepared from apoTf as described previously (Bali and Harris, 1990) and iodinated with the chloramine T method in similar conditions as for EGF, achieving a specific activity of 13,500 cpm/ng.
The IN/SUR method (Wiley and Cunningham, 1982) was used to estimated the endocytic rate constant ke, as the slope calculated by linear regression of the biphasic curve resulting from the plot of the ratio of IN to SUR ligand internalized (IN) to surface (SUR) versus time.
Cell Surface Biotinylation and Immunodetection of EGFR
The EGFR present at the cell surface was assessed by biotinylation assays and by surface immunoprecipitation with the antibody HB8506 followed by protein A-Sepharose (Bravo-Zehnder et al., 2000). Briefly, for biotinylation, cells were washed three times in PBS-CM at 4°C and then sulfo-NHS-Biotin (Pierce Chemical) was added to a final concentration of 0.5 mg/ml for 30 min. The monolayers were washed six times in ice-cold PBS and incubated with 50 mM NH4Cl /PBS-CM for 10 min to block free biotin. Biotin assay for endocytosis experiments with reducible sulfo-NHS-SS-Biotin was made as described previously (Le Bivic et al., 1989), reducing surface sulfo-NHS-SS-Biotin with 50 mM glutathione for 30 min, in 90 mM NaCl, 1 mM MgCl2, 0.1 mM CaCl2, 60 mM NaOH, and 10% FBS. The cells were then lysed and immunoprecipitated with anti-EGFR antibody HB8506 previously linked to protein A-Sepharose beads for 2 h. The beads were washed six times in ice-cold buffer for 5 min each and incubated in sample buffer for 5 min at 65°C. The eluates were run in a 7.5% acrylamide gel. Protein detection was made by blot analysis with streptavidin-horseradish peroxidase (HRP) conjugate and the ECL detection kit (Amersham Biosciences) in nitrocellulose filters, after electrotransferring the biotinylated surface proteins.
Immunoprecipitation
Immunoprecipitations were performed following established procedures with minimal modifications (Faúndez et al., 1992; Marzolo et al., 1997). Cells were lysed with 1% Triton X-100 in 50 mM HEPES, 150 mM NaCl, 1 mM EGTA, 2 mM MgCl2, and glycerol 10% supplemented with an antiprotease mixture (4 mM phenylmethylsulfonyl fluoride; 0.28 trypsin inhibitory units/ml aprotinin; and 4 μg/ml pepstatin, leupeptin, and antipain). Total cell protein was determined by Bio-Rad protein assay (Bio-Rad, Hercules, CA) by using bovine serum albumin as standard. Immunoprecipitations of equivalent total protein amounts were performed at 4°C for 2 h by using the primary antibody previously bound to 30 μl of protein A-Sepharose CL-4B beads per sample. The beads were washed six times with ice-cold buffer (20 mM HEPES, 150 mM NaCl, 10% glycerol, and 0.1% Triton X-100). The beads in each tube were heated to 65°C for 5 min in 20 μl of sample buffer (62.5 mM Tris, pH 6.8, 2% SDS, 100 mM dithiothreitol, 10% glycerol, 0.005% bromphenol blue), and then clarified by centrifugation. Proteins were separated by SDS-PAGE (Laemmli, 1970) on 7.5% acrylamide gels and transferred to nitrocellulose membranes (Towbin et al., 1979). Membranes were blotted for proteins as indicated and visualized using horseradish peroxidase-conjugated secondary antibody and ECL detection system. The tyrosine phosphorylation experiments were made in the lysis buffer supplemented with 1.5 mM MnCl2, 1 mM MgCl2, and 1 mM sodium orthovanadate in which case intact cells were incubated at 37°C either in the absence or presence of 100 ng/ml EGF. For immuneprecipitation of EGFR present on the cell surface, the cells were first metabolically labeled and then incubated with HB8506 antibody at 4°C for 2 h. Cells were starved in methionine/cysteine-free dMEM (Invitrogen) with 0.3% FBS for 30 min, and then incubated with 0.1 mCi (EasyTaq, Express Labeling Mix) of 35S-labeled methionine and cysteine (ICN Pharmaceuticals Biochemicals Division, Aurora, OH) in 1 ml of dMEM for 12 h at 37°C. Fluorograms were developed on preflashed KodaK AR X-Omat films (Eastman Kodak, Rochester, NY). Fluorograms or immunoblots were digitalized in a VISTA-T630 UMax scanner driven by Adobe Photoshop 3.1 (Adobe Systems, Mountain View, CA) and quantitative analysis was done with the NIH Image 1.55 (fpu) software.
Inhibition of Clathrin-mediated Endocytosis by Cytosol Acidification, and Potassium or Cholesterol Depletion
Cells were incubated in DMEM 0.3% FBS overnight before the experiments. Cytosol acidification (Sandvig et al., 1987) was carried out by washing the cells twice in DMEM, pH 7.4, and then incubating them at 37°C for 10 min in DMEM plus 10 mM acetic acid, pH 5.0. For the intracellular potassium depletion (Larkin et al., 1983), the cells were rinsed twice with buffer A (20 mM HEPES, 140 mM NaCl, 1 mM CaCl2, and 1 mM MgCl2, pH 7.4), incubated in hypotonic buffer A (buffer A diluted 1:1 with water) for 5 min at 37°C, and then rinsed and incubated again in buffer A for additional 30 min at 37°C. Cholesterol depletion was done with methyl-β-cyclodextrin (MβCD) (Rodal et al., 1999; Subtil et al., 1999) in Her14 cells. The cells were washed twice in PBS, incubated in DMEM 0.3% FBS with 10 mM MβCD for 30 min at 37°C, and then incubated with or without H89 in the same medium for additional 10 min. After these procedures, the cells were rinsed twice with PBS before adding either EGF (100 ng/ml) for 10 min or 20 μM H89 for 1 h at 37°C.
RESULTS
H89 Provokes a Delay in EGF/EGFR Complex Degradation
To analyze the effects of H89 on EGF/EGFR endocytosis and degradation we first used transfected neuroblastoma N2a cells as a model system that provided cells expressing different levels of the receptor. We selected colonies of transfected cells expressing ∼200,000 receptors/cell, hereafter called N2a-15 cells. These transfected cells displayed high- (Kd = 0.08 nM) and low-affinity (Kd = 0.56 nM) forms of the EGFR (our unpublished data), as described for most cellular systems. Instead, in nontransfected N2a cells, we could not detect endogenous EGF-R by 125I-EGF binding, immunoblot, and immunofluorescence.
Cells treated for 1 h with 20 μM H89 and exposed to 125I-EGF at 4°C for 2 h were incubated at 37°C for various time periods to allow internalization of the ligand–receptor complex. As previously shown in human melanoma cells (Goretzki and Mueller, 1997), H89 did not affect 125I-EGF endocytosis (Figure 1A). In fact, the internalization rate showed no significant differences between control (ke = 0.149 min−1) and H89-treated cells (ke = 0.143 min−1) (Figure 2C). However, H89 diminished the degradation of the internalized 125I-EGF (Figure 1, B and C) and provoked a delay in the degradation of ligand-activated EGFR, increasing its half-time of down-regulation (Figure 2A). N2a-15 cells incubated with saturating concentrations of EGF (100 ng/ml) showed a progressive disappearance of the EGFR with a half-time of ∼34 min, which increased to 77 min in cells pretreated for 1 h with H89 (Figure 2B). The level of receptor degradation after the delay was finally similar (80%) to that achieved by ligand alone.
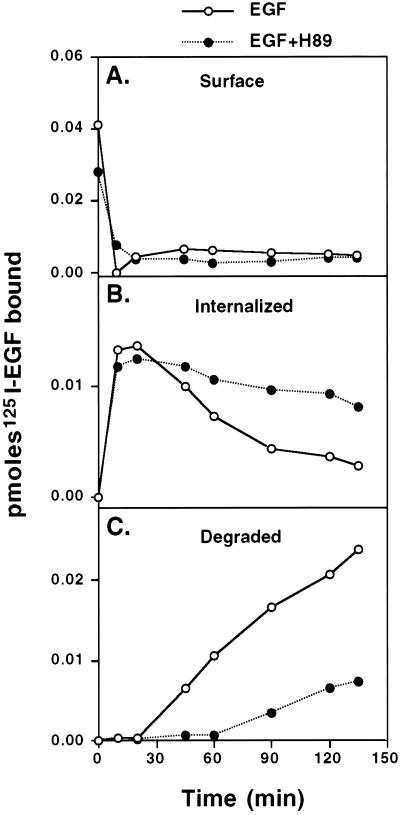
Figure 1.
Effect of H89 on the internalization and degradation kinetics of previously bound 125I-EGF. Transfected N2a-15 cells were incubated in the absence or presence of 20 μM H89 for 1 h at 37°C and then with 125I-EGF (50 ng/ml) for 2 h at 4°C to saturate the EGFR at the cell surface. After washing off unbound ligand, the cells were warmed to 37°C and incubated for different periods of time. Cells previously treated with H89 were kept in the presence of the drug. 125I-EGF disappearance from the surface (A) and internalization (B) were assessed by acid wash. Degradation (C) was estimated from trichloroacetic acid-soluble radioactivity in the media. H89 did not affected ligand-induced internalization but clearly inhibited ligand degradation.
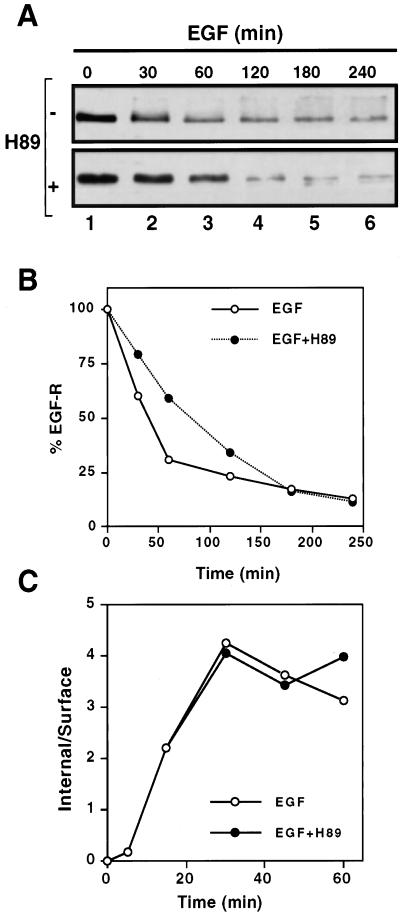
Figure 2.
H89 provokes a delay in EGFR down-regulation without affecting internalization kinetics. Permanently transfected N2a-15 cells expressing EGFRs were treated with EGF (100 ng/ml) at 37°C in the absence or presence of 20 μM H89 for the indicated time periods. (A) Immunoblot of EGFR detected with polyclonal antibody (EGFR984) and revealed by ECL. (B) Relative amount of EGFR assessed in the immunoblot is expressed as percentage of control. EGF induced almost 70% receptor degradation during the first hour versus 40% in the presence of H89. After 2 h, receptor degradation became similar in cells treated with or without H89. (C) Cells were incubated during different time periods at 37°C with 125I-EGF (50 ng/ml) alone or together with 20 μM H89. The amount of internalized (Internal) and surface-associated (Surface) radiolabel was determined as described under MATERIALS AND METHODS. Data were plotted using the IN/SUR method showing a ke of 0.149 and 0.143 min−1 for the control and H89-treated cells, respectively.
H89 Inhibits Ligand-induced Tyrosine Phosphorylation and Ubiquitylation of EGFR
Ligand binding induces EGFR tyrosine phosphorylation and subsequently ubiquitylation, which has been functionally related to sorting into a degradation pathway (Levkowitz et al., 1998; Bao et al., 2000). Under the effect of H89, the levels of EGFR tyrosine phosphorylation and ubiquitylation remained undetectable within the first 5 min of EGF stimulation (Figure 3A), appearing in the next time period of 10 min (Figure 3A, lane 7), when almost 90% of the ligand-activated receptors have been already internalized, as shown by cell surface biotinylation assays (Figure 3B, lane 4). The slower kinetics of these ligand-induced modifications could account for the delayed down-regulation of the receptor seen under H89 treatment.
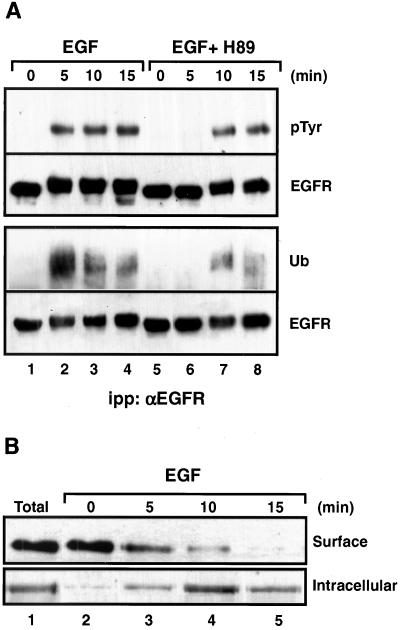
Figure 3.
H89 delayed both tyrosine phosphorylation and ubiquitylation of the EGFR. (A) N2a-15 cells were treated with EGF (200 ng/ml) alone or together with 20 μM H89 for 5, 10, and 15 min at 37°C. The cells were lysed in the presence of 200 μM sodium orthovanadate and the EGFR was immunoprecipitated with HB8506 antibody and analyzed by Western blot with either anti-phosphotyrosine (pTyr) or anti-ubiquitin (Ub) antibodies. The blots were then stripped and incubated with the polyclonal anti-EGFR antibody (EGFR984) to assess total EGFR mass. (B) Cells treated with EGF for the indicated periods of time were placed at 4°C and biotinylated with sulfo-NHS-Biotin to determine the percentage of EGFR remaining on the cell surface (top). In another experiment (bottom), the cells were first incubated with reducible sulfo-NHS-SS-Biotin at 4°C and then placed at 37°C in the presence or absence of EGF. At the indicated time points, the sulfo-NHS-SS-Biotin in the cell surface was reduced with 50 mM glutathione at 4°C. Only internalized EGFRs remain biotinylated. In both experiments the EGFRs were immunoprecipitated and analyzed by Western blot with streptavidin-HRP and ECL. Note that ∼90% of the receptor mass was already internalized after 10 min of EGF incubation.
H89 Induces Ligand-independent Internalization of EGFR
During the acid wash experiments we noticed that pretreating cells with 20 μM H89 decreased the levels of 125I-EGF binding. Scatchard analysis showed that H89 induces complete loss of the high-affinity forms of the EGFR and a dramatic decrease in the low-affinity binding sites (Figure 4A). Overall, >50% of the binding sites disappeared in 1 h of H89 treatment. To study whether this was due to endocytic removal of empty inactive EGFR from the cell surface, we performed cell surface biotinylation assays (Figure 4B, lanes 1 and 2) and cell-surface immunodetection with an antibody against the external domain of the EGFR (Figure 4B, lanes 3 and 4). Both experiments showed decreased levels of EGFR at the cell surface after 1 h of 20 μM H89 treatment, but the total cellular content of the receptor remaining unchanged (Figure 4B, lanes 5 and 6). In contrast with its fate after ligand-induced endocytosis, the H89-internalized receptor was not degraded for at least 4 h (Figure 4C). Immunofluorescence made in N2a-15 and in Her14 cells (Honegger et al., 1987), which are more flattened, showed the receptor accumulated in a juxtanuclear compartment (Figure 4D), probably corresponding to recycling endosomes that in many cells appear as a bright perinuclear spot (Trowbridge et al., 1993; Mukherjee et al., 1997).
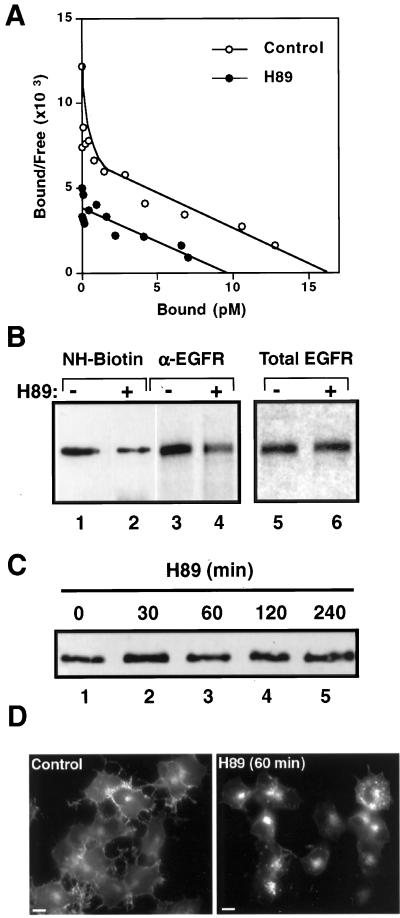
Figure 4.
H89 diminishes both high- and low-affinity binding sites and the amount of empty EGFR at the surface of transfected N2a cells. (A) Scatchard analysis of 125I-EGF (327,100 cpm/pmol) shows that 20 μM H89 provoked 50% reduction of total number of binding sites and disappearance of the high-affinity component. (B) Amount of EGFR at the plasma membrane was assessed by cell-surface biotinylation, followed by immunoprecipitation and streptavidin-peroxidase detection (lanes 1 and 2), and by immunodetection of the receptor at the cell surface of intact [35S]methionine-cysteine–labeled cells, by using the anti-EGFR mAb HB8506, followed by precipitation of immune complexes with protein A-Sepharose (lanes 3 and 4). In each case, the cells were incubated for 1 h in the absence (lanes 1 and 3) or presence of 20 μM H89 (lanes 2 and 4). Both methods show a decreased mass of empty EGFR at the cell surface of ∼50% when treated with H89. Total EGFR inmunoprecipitated from N2a-15 cells did not change under the effects of H89 (lanes 5 and 6). (C) Immunoblot of 300,000 cells treated for up to 4 h with 20 μM H89 shows no degradation of the EGFR. (D) N2a-15 cells were incubated with or without 20 μM H89 for 1 h at 37°C and then analyzed by immunofluorescence with HB8506 anti-EGFR mAb and FITC–anti-mouse. An intense intracellular EGFR staining appears upon H89 treatment. Bars, 10 μm.
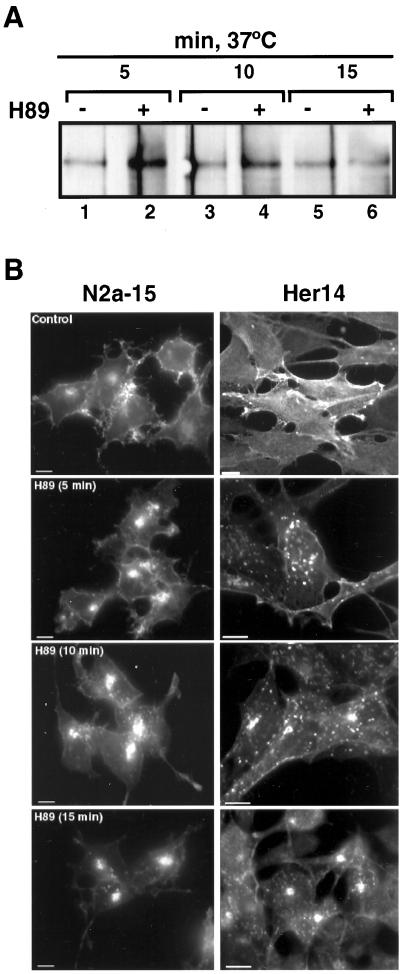
Experiments of cell surface biotinylation with the cleavable reagent sulfo-NHS-SS-Biotin (Le Bivic et al., 1989) directly demonstrated H89-induced endocytosis of unoccupied EGFR. Glutathione added to the medium eliminates the biotin only from cell surface proteins because internalized proteins become inaccessible to it. N2a-15 cells were first incubated with sulfo-NHS-SS-Biotin at 4°C and then warmed to 37°C in the presence or absence of 20 μM H89 for 5, 10, and 15 min. Internalization was readily detectable within the first 5 min of H89 treatment (Figure 5A, lane 2), whereas it was not observed under control conditions (Figure 5A, lanes 1, 3, and 5). The EGFR lost biotin label during the next time periods of 10 and 15 min (Figure 5A, lane 6), indicating that a proportion of the internalized receptors recycled back to the cell surface.
Figure 5.
H89 induces the internalization and accumulation of EGFR in intracellular compartments. (A) Internalization of the EGFR was monitored by incubation with EZ-Link sulfo-NH-SS-Biotin at 4°C and incubation at 37°C in the absence or presence of H89 for 5, 10, and 15 min. After reduction with glutathione, the EGFR was immunoprecipited with the HB8506 antibody and protein A-Sepharose, and then analyzed by Western blot with streptavidin-HRP and ECL. Endocytosis occurred already after the first 5 min of H89, as detected by resistance of biotinylated-EGFR to treatment with glutathione at 4°C. (B) N2a-15 and Her14 cells incubated for the indicated times at 37°C with 20 μM H89 were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. EGFR was detected by immunofluorescence with HB8506 anti-EGFR mAb and FITC–anti-mouse. Bars, 10 μm.
The endocytic effect of H89 was also seen by immunofluorescence (Figure 5B). After 1 h of incubating N2a-15 or NIH-3T3 Her14 cells with H89, the EGFR ends up accumulated in a juxtanuclear endocytic compartment reminiscent of recycling endosomes.
EGFR Endocytosis Induced by H89 Does Not Involve Intrinsic Protein Tyrosine Kinase Activity, Tyrosine Phosphorylation, and Ubiquitylation of Receptor
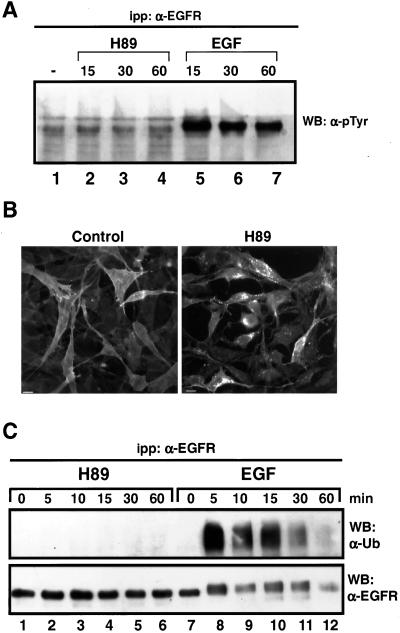
In contrast with the effects induced by EGF, we could not detect any significant change in the phosphotyrosine content of the EGFR when cells were treated with 20 μM H89 (Figure 6A). Furthermore, H89 was able to induce internalization of the mutant receptor K721A, which lacks tyrosine kinase activity (Honegger et al., 1987), as seen by immunofluorescence (Figure 6B), ligand-binding, and cell surface biotinylation experiments (our unpublished data). In addition, cells incubated with 1 mCi/ml 32P for 4 h showed no changes in the phosphorylation status of the EGFR upon H89 treatment (our unpublished data), making unlikely modifications of serine-threonine phosphorylation of the receptor.
Figure 6.
EGFR internalization induced by H89 does not involve tyrosine phosphorylation, tyrosine kinase activity, or ubiquitylation of the receptor. (A) Transfected N2a-15 cells expressing EGFR were incubated for the indicated times with either 20 μM H89 (lanes 2–4) or 100 ng/ml EGF (lanes 5–7) at 37°C. The EGFR was immunoprecipitated with the mAb-HB8506 and its phosphotyrosine content was assessed by immunoblot with an anti-phosphotyrosine mAb (HB8190). In contrast with EFG treatment, H89 did not provoke a detectable increase in tyrosine phosphorylation of the EGFR. (B) K721A cells expressing a kinase-minus EGFR were incubated with or without 20 μM H89 and then fixed, permeabilized, and the cellular distribution of the EGFR analyzed by indirect immunofluorescence. H89 induced internalization and intracellular accumulation of empty EGFR. (C) Her14 cells were incubated with 20 μM H89 (lanes 2–6) or 200 ng/ml EGF (lanes 8–12) for different times at 37°C. The EGFR was then immunoprecipitated with anti-EGFR antibodies (HB8506) and analyzed by immunoblot with anti-ubiquitin antibody. H89 did not induce ubiquitylation of the receptor. The membranes were stripped and incubated with polyclonal anti-EGFR antibody (EGFR984).
It has been recently described that EGFR ubiquitylation can occur at the cell surface and therefore might provide additional endocytic information (Stang et al., 2000). However, in contrast with the effects of ligand stimulation (Figure 6C, lanes 8–12), we could not find evidence of EGFR ubiquitylation during H89-induced endocytosis (Figure 6C, lanes 2–6), congruent with the fact that EGFR ubiquitylation requires a previously tyrosine phosphorylated receptor (Levkowitz et al., 1998).
H89-induced Internalization of EGFR Occurs via Clathrin-mediated Endocytosis
The endocytic pathway induced by H89 seems to be saturable. The average levels of EGFR endocytosis induced by 20 μM H89 for 1 h and measured as remnant ligand binding were 57 ± 5% (n = 10) for N2a-15 cells, which express 200,000 receptors/cell and 44 ± 4% (n = 6) for Her14 cells, expressing 270,000 receptors/cell. Instead, it was only 4 ± 1% (n = 6) in A431 cells expressing ∼4000,000 receptors/cell and 3 ± 2% (n = 4) in another colony (N2a-3) of transfected N2a cells, which express about 1,000,000 receptors/cell. Similar saturability has been demonstrated for ligand-induced internalization of EGFR via clathrin-coated pits (Wiley, 1988; Wiley et al., 1991).
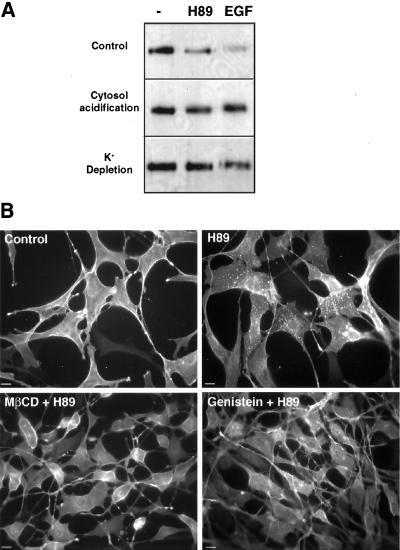
Cytosol acidification (Sandvig et al., 1987) and potassium depletion (Larkin et al., 1983), which block endocytosis by clathrin-coated pits, inhibited H89-induced as well as EGF-induced disappearance of EGFR from the cell surface (Figure 7A). Furthermore, treatment with 10 mM MβCD, which causes acute cholesterol depletion from the plasma membrane and has been recently shown to decrease clathrin-coated pit function (Rodal et al., 1999; Subtil et al., 1999), was also able to inhibit receptor endocytosis induced by H89 (Figure 7B). Clathrin-mediated EGFR endocytosis induced by ligand is also inhibited by the tyrosine kinase inhibitor genistein that would interfere with phosphorylation events mediated not only by the intrinsic receptor kinase but also by src (Wilde et al., 1999). Genistein also abolished EGFR endocytosis induced by H89 (Figure 7B). All these observations indicate that H89 promotes an interaction of EGFR with the clathrin-mediated endocytic machinery.
Figure 7.
H89 induces internalization of empty EGFR through a clathrin-mediated pathway involving a tyrosine kinase activity distinct from the receptor. (A) Endocytosis of the EGFR was stimulated with either 20 μM H89 for 1 h or 100 ng/ml EGF for 10 min in control conditions or after cytosol acidification or potassium depletion in Her14 cells. Biotinylation of cell surface proteins was then done at 4°C and the EGFR was immunoprecipitated with HB8506 and its biotinylation analyzed by Western blot with streptavidin-HRP and ECL. Disappearance of the EGFR from the cell surface was lowered by these two procedures that inhibit clathrin-mediated endocytosis. The result is representative of three independent experiments. (B) Her14 cells were treated with or without 10 mM MβCD for 30 min or incubated for 60 min with or without 50 μM genistein, a tyrosine kinase inhibitor, before the addition of 20 μM H89 for an additional 10 min at 37°C. The cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and analyzed by indirect immunofluorescence with HB8506 antibody for EGFR distribution. Both MβCD and genistein abrogate H89-induced endocytosis of EGFR. Because we previously showed that under H89 the intrinsic tyrosine kinase of the receptor is not activated, other tyrosine kinase activities should be involved.
H89 Effects on EGFR Endocytosis Correlated with Inhibition of PKA Activity
H89 has been currently used as a potent, highly selective, and reversible inhibitor of PKA (Chijiwa et al., 1990; Muniz et al., 1996, 1997. It is approximately a 1000-fold more efficient inhibitor for PKA than for PKC or other known kinases (Chijiwa et al., 1990). At 30 μM, H89 maintains selectivity for PKA inhibition in intact cells without affecting other kinases measured in extracts of treated cells (Chijiwa et al., 1990), whereas at 90 μM, H89 inhibits Golgi vesiculation induced by the drug illimaquinone involving PKD instead of PKA (Jamora et al., 1999).
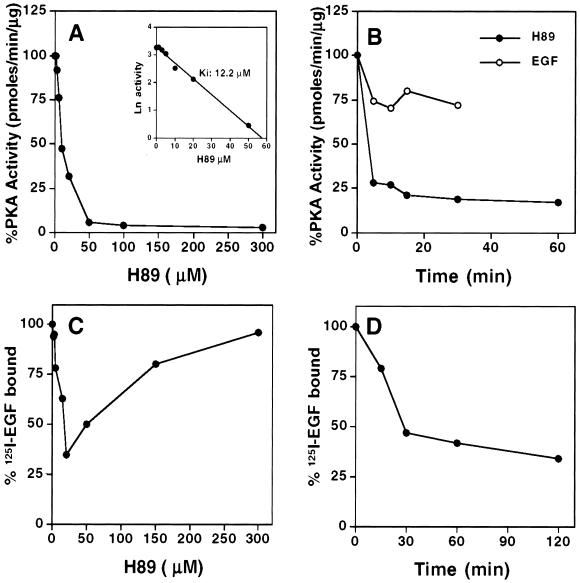
In our conditions, assessment of PKA activity in extracts of intact cells treated with H89 for 1 h showed an IC50 of ∼12 μM (Figure 8A). This correlated with the IC50 of 13 μM estimated for the effect of H89 on 125I-EGF binding (Figure 8C). Ligand-binding activity decreased within the range of 5–20 μM H89, whereas at higher H89 concentrations this effect was gradually lost until it became completely abolished at 300 μM. Thus, other serine-threonine kinases inhibited at these high H89 concentrations (Chijiwa et al., 1990; Cotlin et al., 1999) are probably required for the endocytosis of EGFR acting downstream of PKA. The time course of PKA inhibition was also congruent with an involvement of PKA in the endocytic effect of H89. Substantial inhibition of PKA activity was achieved as fast as 5 min of H89 coincident with the time course of H89-induced endocytosis of EGFR (Figures 5 and 8, B and D). Strikingly, EGF by itself causes a 26% decrease in the levels of PKA activity (Figure 8B), suggesting that this effect might play a role in EGFR ligand-induced endocytosis.
Figure 8.
Correlation between H89 effects on PKA inhibition and decrease of 125I-EGF binding in transfected N2a cells. Cells were treated with different concentrations of H89 for 1 h at 37°C (A and C), or for different times with 20 μM H89 (B and D), or 100 ng/ml EGF alone (B) and then the PKA activity was assessed in cell extracts with the Signa TECT kit (Promega) (A and B), and 125I-EGF binding was determined using saturation conditions (C and D). Concentration dependency of PKA inhibition in vivo by H89 showed an IC50 of 12.2 μM (inset), in correlation with the IC50 of 13.3 μM observed for the decrease in ligand binding within a range of 5–20 μM H89, although at higher concentrations the effect became gradually lost. Almost maximal effect of 20 μM H89 was achieved between 5 and 15 min for PKA inhibition and at ∼30 min for the decrease in 125I-EGF binding. Strikingly, EGF provoked 26% inhibition of PKA in N2a-15 cells.
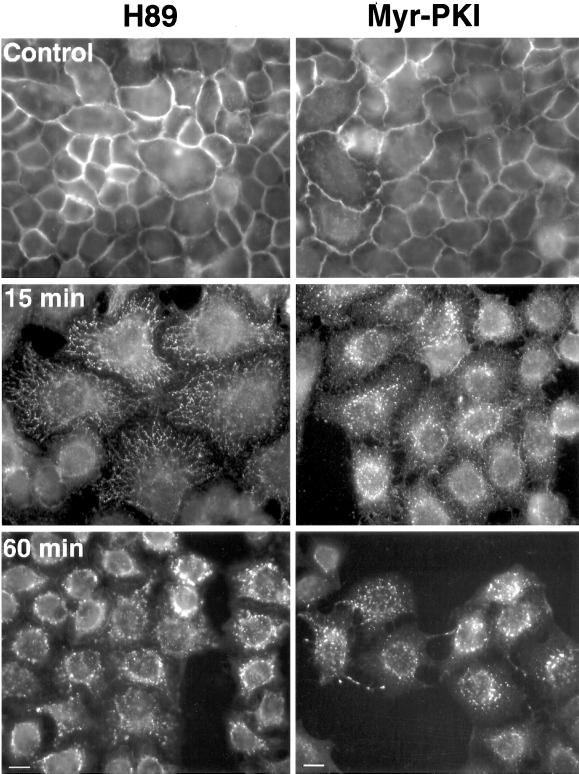
Myr-PKI, a specific peptide substrate for PKA considered to be one of its most highly selective inhibitors (Harris et al., 1997), was also able to induce EGFR endocytosis. This was most clearly seen in HeLa cells (Figure 9). Either 20 μM H89 or 100 μM Myr-PKI led to redistribution of the receptor from the cell surface to intracellular compartments, progressively concentrated in perinuclear regions. Within the first 15 min of H89 treatment, linear arrays of vesicles resembling tubulo-vesicular structures were frequently seen converging toward these perinuclear regions.
Figure 9.
H89 and the cell-permeable peptide Myr-PKI induce internalization of EGFR in HeLa cells. HeLa cells were treated with or without 20 μM H89 or 100 μM Myr-PKI (14-22) peptide for the indicated time periods at 37°C. Cells were fixed with methanol for 2 min at room temperature and then incubated with HB8506 (1/25) antibody and FITC–anti-mouse. Both PKA inhibitors showed a similar endocytic effect upon EGFR. Linear arrays of vesicles or tubulo-vesicular structures were seen, more frequently under H89 treatment. Bars, 10 μM
Instead, inhibitors of PKC (H-7, calphostin C, and chelerythrine), casein kinase I and II (CKI-7 and DRB), and tyrosine kinases (genistein) were all ineffective as inductors of EGFR endocytosis (our unpublished data). Wortmannin, an inhibitor of phosphatidylinositol 3-kinase that participates in the endocytosis of several proteins (Chen and Wang, 2001), has no effect either (our unpublished results).
Changes in Intracellular Pathway of EGFR and Redistribution of Transferrin (Tf) Endocytic Compartments upon H89 Treatment
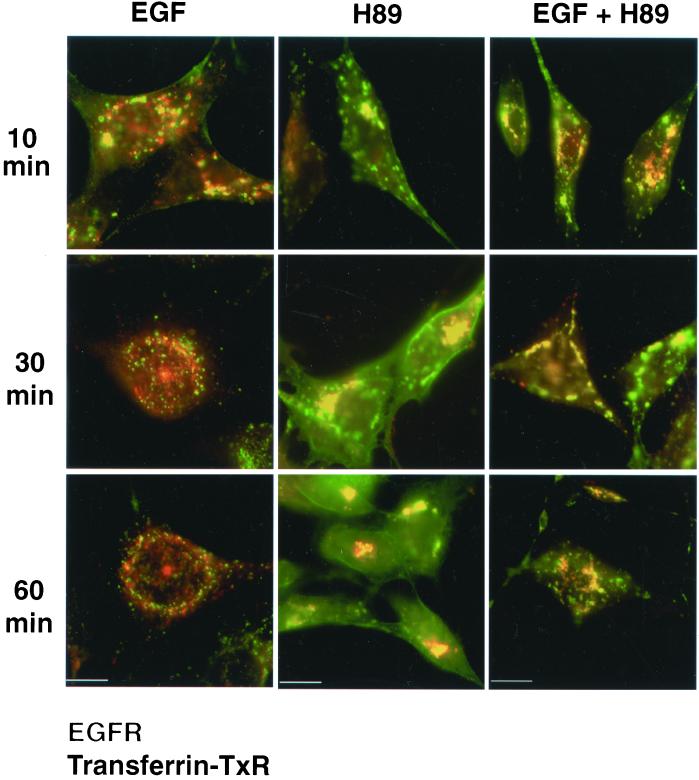
The endosomal compartment is a system of interconnected tubulo-vesicular elements currently and broadly divided by functional and structural criteria into early (sorting and recycling) and late (prelysosomal) endosomes (Trowbridge et al., 1993; Mukherjee et al., 1997). The endocytic pathway followed by EGFR under the effect of H89 was analyzed by double fluorescence with Tf-TxR as marker for early endosomal compartments (Trowbridge et al., 1993; Mukherjee et al., 1997). The EGFR internalized by EGF stimulation extensively colocalized with Tf-TxR during the first 10–15 min and then was segregated from Tf-TxR into distinct vesicular structures, as expected for proteins following either recycling or degradation pathways (Figure 10, 30 min). After 1 h, the staining of the EGFR diminished in most cells. Instead, the EGFR internalized during H89 treatment in the absence of ligand showed a maintained colocalization with Tf-TxR and both were gradually concentrated in a juxtanuclear compartment very likely corresponding to recycling endosomes (Mukherjee et al., 1997). Such distribution was not achieved by the EGFR in cells treated with EGF alone. Finally, when Her14 cells were incubated with EGF and H89, the pattern of EGFR endocytosis frequently showed tubulo-vesicular endocytic compartments and the colocalization of EGFR and Tf-TxR lasted longer than in cells treated with EGF alone, because it was still observed in many cells after 60 min of H89 treatment (Figure 10). This means that EGF/EGFR complexes were more slowly segregated from early endosomes to lysosomes under the effects of H89, as suggested also by the delayed receptor down-regulation and ligand degradation observed previously.
Figure 10.
Different endocytic behavior of EGFR induced by H89 versus EGF. Her14 cells were incubated in the presence of 100 ng/ml EGF or 20 μM H89, or both, together with 50 μg/ml Tf-TxR for the indicated time periods at 37°C. The cells were then washed in PBS, fixed with 4% paraformaldehyde, and permeabilized with 0.2% Triton X-100 for immunofluorescence with HB8506 anti-EGFR mAb and FITC–anti-mouse. Merged pictures of the EGFR (green) and Tf (red) are presented. The EGFR stimulated by EGF colocalizes (yellow) with Tf in peripheral vesicles mostly during the first 10 min, whereas segregation to different compartments is clearly apparent at 30–60 min. In contrast, during H89 treatment the EGFR colocalizes all the time with Tf and ends up accumulated in a juxtanuclear compartment seen as a bright spot. Incubation with EGF together with H89 shows frequent tubular endocytic compartments and colocalization of EGFR with Tf-TxR as a predominant pattern, even after 30–60 min of incubation, suggesting a delay in receptor segregation out of early endosomes. Bars, 10 μm.
H89 Does Not Induce Endocytosis of Transferrin and μ-Opioid Receptors, but Provoked Changes in Early Endocytic Compartments
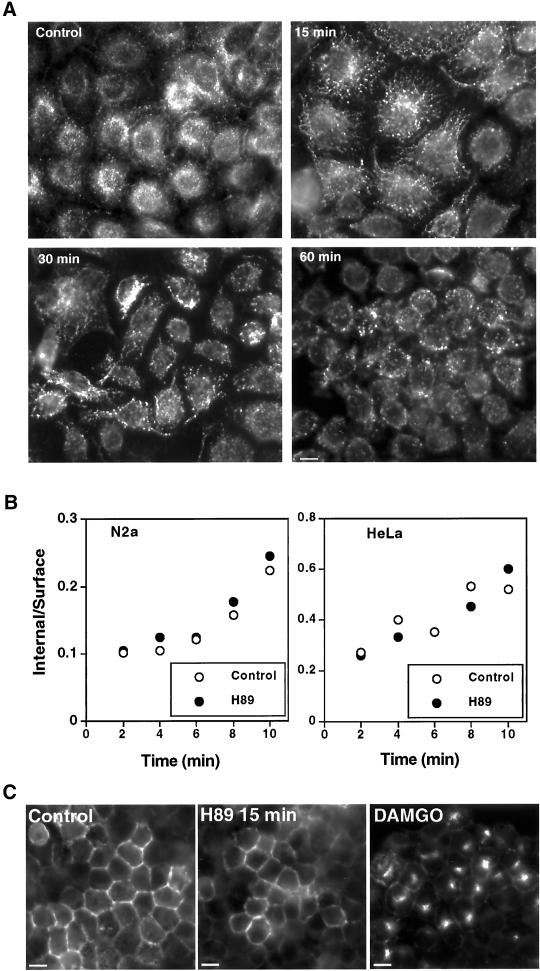
Indirect immunofluorescence of the human transferrin receptor (TfR) made in HeLa cells showed that H89 causes changes in the distribution of early endocytic compartments. During the first 5–10 min of H89 incubation, the TfR was seen in linear arrays of vesicles, possibly corresponding to tubulo-vesicular structures, converging toward the perinuclear region (Figure 11A), a pattern similar to that showed by the EGFR under the effects of H89 alone (Figure 9). After 60 min of H89 treatment, these linear arrays were rarely observed, whereas perinuclear vesicles became predominant. In spite of these changes, the internalization rate of Tf was not affected by 20 μM H89, neither in N2a (control, ke = 0.013 min−1; H89, ke = 0.017 min−1, n = 6) nor in HeLa cells (control, ke = 0.107 min−1; H89, ke = 0.109 min−1, n = 6) (Figure 11B). This is consistent with previous reports in HeLa cells and A431 cells in which the internalization rate of Tf was not affected by low concentrations of H89, being only decreased at higher concentrations (300 μM) due to casein kinase II inhibition (Cotlin et al., 1999). Treatment with either H89 (20 μM) or Myr-PKI peptide (100 μM) for 60 min did not modify the cell surface 125I-diTf binding activity (our unpublished data), indicating that recycling of continuously internalized TfR was also not altered.
Figure 11.
H89 provoked changes in the intracellular distribution of transferrin receptors but did not affect its internalization rate and did not cause internalization of the G protein-coupled μ-opioid receptor. (A) HeLa cells were incubated during the indicated time periods with 20 μM H89 and the distribution of the TfR was analyzed by indirect immunofluorescence. The pattern of the its intracellular distribution changed first to linear arrays of vesicles converging toward perinuclear vesicles which then became predominant after 60 min of treatment. (B) For the internalization assays of 125I-diTf, the cells were incubated in serum-free media during 1 h at 37°C before the experiments and then incubated with 250 ng/ml 125I-diTf for 2 h at 4°C. After extensive washes with PBS-BSA 0.1% to eliminate the unbound 125I-diTf, internalization was allowed by incubating the cells for different time periods at 37°C, in the presence or absence of 20 μM H89. H89 did not affect the internalization rate determined by the IN/SUR method. (C) N2a cells permanently transfected with FLAG-tagged μ-opioid receptors were incubated with either 20 μM H89 or their ligand DAMGO (10 μM) for 15 min. No evidence of internalization was observed with H89, in contrast to the endocytic pattern induced by the ligand. Bars, 10 μM.
In addition, to test other kinds of signaling receptors whose clathrin-mediated endocytosis is induced by ligand we assessed the effects of H89 on μ-opioid receptors, pertaining to the class of G protein-coupled receptors (Keith et al., 1996). H89 did not induce endocytosis of a FLAG-tagged μ-opioid receptor expressed in permanently transfected N2a cells, contrasting with the clear endocytic effect exerted by its ligand DAMGO (Figure 11C). Therefore, the mechanism involving basal PKA activity by which the EGFR avoids the endocytic apparatus seems to be rather selective.
DISCUSSION
Our results revealed new regulatory systems controlling the permanency of inactive EGFR at the cell surface and the efficiency of intracellular segregation of ligand-activated EGFR to a degradation pathway, both at the expense of basal PKA activity.
The most striking finding was that inhibition of PKA basal activity, by either low concentrations of H89 (IC50 = 12 μM) or the PKA substrate peptide Myr-PKI, in the absence of ligand, induced rapid internalization of inactive EGFR through the clathrin-mediated pathway. Such an effect was not generalized. We could not detect H89 induction of endocytosis either in transferrin receptors that are constitutively internalized (Trowbridge et al., 1993; Mukherjee et al., 1997) or in μ-opioid receptors that undergo endocytosis upon ligand binding (Keith et al., 1996). The magnitude of the endocytosis depended on the PKA inhibitor used, the cell line, and the levels of receptor expressed. Close to 60% of EGFR was removed from the cell surface in N2a-15 cells and accumulated in early endosomes without degradation for at least 4 h. All this reveals the existence of mechanisms controlling the relative distribution of EGFR between the cell surface and endocytic compartments, indicating also that the predominant distribution of inactive EGFR at the plasma membrane is not simply by default but through a regulated process of “endocytic evasion,” amenable to modulation according to cellular demands. Basal PKA activity exerts some kind of restrictive condition resulting in EGFR avoidance of endocytosis until it is stimulated by ligand, either maintaining the receptor endocytic codes cryptic or an EGFR internalization-promoting element inactive.
Accepted models of EGFR endocytosis state that activation of its intrinsic tyrosine kinase domain is required for efficient receptor recruitment into clathrin-coated pits by exposing cryptic endocytic codes that interact with AP-2 adaptors (Cadena et al., 1994; Nesterov et al., 1995a; Sorkin et al., 1996). EGFR could also become ubiquitylated at the cell surface and this modification might eventually serve as endocytic information (Stang et al., 2000). However, we could detect neither an increased tyrosine phosphorylation nor ubiquitylation of the EGFR receptor during H89 treatment. Furthermore, H89 provoked internalization of the mutant receptor K721A, which lacks tyrosine kinase activity (Felder et al., 1990) and is not a substrate of c-Cbl ubiquitylation (Levkowitz et al., 1998). In spite of lacking these modifications several observations involved a clathrin-mediated pathway in the EGFR endocytic effects of H89. Analysis of cells expressing high levels of EGFR showed that the endocytic process activated by H89 was saturable, as described previously for receptors internalized via clathrin-coated pits (Wiley, 1988; Wiley et al., 1991). Three procedures that inhibit clathrin-coated pit function, cytosol acidification (Sandvig et al., 1987), potassium depletion (Larkin et al., 1983), and acute cholesterol depletion (Rodal et al., 1999), effectively blocked internalization of EGFR induced by either EGF or H89. Finally, internalization through caveolae, where EGFR has been localized in fibroblasts (Smart et al., 1995; Mineo et al., 1996), could be discarded because N2a cells do not express caveolin-1 and therefore do not form caveolae (Gorodinsky and Harris, 1995; Shyng et al., 1994). Whether H89 induces interaction of EGFR with AP-2 is an interesting possibility to explore in future experiments. In certain conditions, this interaction can occur independently of receptor tyrosine phosphorylation levels and ligand binding (Sorkin and Carpenter, 1993; Boll et al., 1995), or might not even be necessary for EGFR endocytosis, as suggested by studies made with mutant receptors lacking AP-2 binding sites (Nesterov et al., 1995b) and with an AP-2 dominant negative μ2 subunit responsible for recognizing endocytic codes (Nesterov et al., 1999).
The fate of internalized EGFR under the effect of H89 was different from that seen in cells stimulated with EGF. It was not substantially delivered to a proteasomal-lysosomal pathway as indicated by a lack of degradation during 4 h of H89 treatment and an extensive colocalization with Tf-TxR seen first in peripheral endocytic compartments and then in a punctate juxtanuclear region with the characteristic pattern of recycling endosomes. This supports the notion that receptor tyrosine phosphorylation and ubiquitylation, which are not induced by H89, are indeed required for its sorting to a degradation pathway (Levkowitz et al., 1998; Bao et al., 2000). The effects of H89 are also different from those described for PKC activation, which results in transient internalization of unoccupied EGFR from the cell surface but the receptors recycled to the cell surface in just 1 h (Beguinot et al., 1985; Lin et al., 1986). PKA inhibition also changed the intracellular distribution of TfR but seemingly without functional consequences. Although after 1 h of PKA inhibition almost 50% of the EGF binding activity disappeared from the cell surface due to internalization, no changes were detected in Tf binding, indicating that its recycling remained unaffected, as occurred also with its internalization rate.
EGFR endocytosis induced by PKA inhibition is the first example of protein segregation into a vesicular pathway induced by kinase inhibition and highlights the importance of basal kinase activity in selective protein trafficking. All previous reports showed that kinase activation or inhibition resulted in the corresponding induction or abrogation of protein vesicular transport, both in the exocytic and endocytic routes (Pimplikar and Simons, 1994; Jilling and Kirk, 1996; Muniz et al., 1996, 1997; Goretzki and Mueller, 1997; Jamora et al., 1999; Aridor and Balch, 2000; Lee and Linstedt, 2000). For instance, H89 inhibits trans-Golgi network-to-cell surface transport by decreasing PKA-dependent formation of transport vesicles (Muniz et al., 1996, 1997). Inhibition of PKA and abrogation of PKA phosphorylation sites also decreased the stimulated endocytosis of several proteins (Goretzki and Mueller, 1997; Hu et al., 2001; Li et al., 2001).
Ligand-induced EGFR endocytosis involves several downstream substrates of the receptor and activation of the protein tyrosine kinase src (Lamaze and Schmid, 1995; Benmerah et al., 1998; Wilde et al., 1999; Confalonieri et al., 2000). The EGFR endocytic pathway primarily triggered by PKA inhibition seems also to require several kinds of kinases, including other serine-threonine kinases besides PKA as well as tyrosine kinases. In fact, the effect of H89 on EGFR endocytosis diminished gradually at concentrations higher than 50 μM H89 and disappeared at 300 μM, most likely due to inhibition of additional serine-threonine kinases. Because the endocytic effect of H89 was abolished by genistein and we showed that neither the tyrosine kinase activity of the receptor nor its tyrosine phosphorylation was required, a tyrosine kinase distinct from that of the EGFR, which might correspond to src, is also involved.
There is increasing interest in disclosing control systems able to modulate intracellular trafficking of ligand-activated EGFR because it continues signaling from endocytic compartments before degradation (Trowbridge et al., 1993; Di Fiore and Gill, 1999; Carpenter, 2000; Ceresa and Schmid, 2000; Schlessinger, 2000; Wiley and Burke, 2001). Recent studies have shown that PKC activation prevents EGFR ubiquitylation by c-Cbl ligase and causes the receptor to recycle to the cell surface instead of being sorted to lysosomes (Bao et al., 2000). Our results indicate that PKA could be an additional regulatory element acting in an opposite way to PKC, that is, fostering segregation of EGF/EGFR complexes from early endosomes to degradation. In fact, H89 caused a delay in the trafficking of ligand-activated EGFR out of early endosomes, as indicated by an expanded time of colocalization with Tf-TxR, determining a twofold increased halftime of the receptor down-regulation, whereas its internalization rate remained unchanged. Both ligand-induced autophosphorylation as well as ubiquitylation of the EGFR were retarded and reduced by H89, and this probably interfered with the ubiquitylation-dependent process that drives the EGFR into its degradation pathway (Levkowitz et al., 1998; Bao et al., 2000). Interestingly, both modifications occurred at a time when most receptors were already internalized under EGF stimuli, suggesting that when PKA is inhibited the EGF/EGFR complexes start to signal from intracellular compartments. This might provide a model system to compare cell surface versus intracellular signaling, an issue of great interest (Trowbridge et al., 1993; Di Fiore and Gill, 1999; Carpenter, 2000; Ceresa and Schmid, 2000; Schlessinger, 2000; Wiley and Burke, 2001).
There are two isoforms of PKA, defined as PKA-I and PKA-II, that share identical catalytic subunits but differ in their regulatory subunits, RI and RII, respectively (Taylor et al., 1990). It is tempting to speculate that PKA-II is the one involved in controlling the EGFR endocytic behavior. PKA-II has been localized in membranes and organelles, including Golgi complex, plasma membrane, and early endosomes, whereas PKA-I is cytosolic (Griffiths et al., 1990). In principle, changes in the EGFR endocytic behavior induced by decreasing PKA activity could result from variations in the expression of these enzyme isoforms, known to occur during differentiation, cell growth, and neoplastic transformation (Ciardiello and Tortora, 1998).
Our present results together with recent observations on the regulation of phosphodiesterase (Grange et al., 2000) allow to propose an attractive mechanism of transmodulation of EGFR endocytic behavior by other growth factors and hormones. PKA activity decreased when cAMP levels diminished as the result of stimulation of c-AMP-phosphodiesterase PDE4D3 by endogenously produced phosphatidic acid (Grange et al., 2000). The ubiquitous enzyme phospholipase D (PLD), which produces phosphatidic acid and plays an important role in vesicular trafficking (Roth, 1999), is transiently activated by multiple external stimuli, including hormones and growth factors, acting through G protein-coupled or tyrosine kinase cell surface receptors (Jones et al., 1999). Therefore, it is expected that stimuli leading to activation of PLD should result in decreased PKA activity conducive to EGFR endocytosis. Furthermore, this mechanism also could be operative in the ligand-induced EGFR endocytosis. EGF is able to activate PLD (Yeo and Exton, 1995) and PLD inhibition decreases ligand-induced EGFR endocytosis (Shen et al., 2001). Interestingly, we showed a 26% inhibition of PKA activity in N2a-15 cells stimulated by EGF.
The identification of PKA-regulated elements responsible for maintaining the EGFR at the cell surface would be necessary to further understand the receptor endocytic mechanisms and to disclose potential new targets for the interference with its function in cancer. It would be also of interest to assess whether other members of the EGFR family respond to H89 in a similar manner.
ACKNOWLEDGMENTS
We thank Dr. Joseph Schlessinger for providing the Her14 and K721 cells and Drs. Paulette Zaki and Christopher Evans for the cDNA of FLAG-μ-opioid receptor. We also thank Drs. Jorge Garrido and Enrique Rodriguez-Boulan for useful comments and critical reading of the manuscript, and Dr. Tulio Núñez for advice with the transferrin binding experiments. This work received financial support from Fondo Nacional de Cienciay Tecnolgia grants 2970069 (to G.S.), 198-0974 (to A.G.); Fondo Nacional de Áreas Prioritarias grant 13980001; and Cátedra Presidencial en Ciencias (to A.G.). The Millennium Institute for Fundamental and Applied Biology is financed in part by the Ministerio de Planificación y Cooperación de Chile.
Abbreviations used:
- DAMGO
[d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- 125I-diTf
125I-diferric transferring
- MβCD
methyl-β-cyclodextrin
- PKA
protein kinase A
- PKC
protein kinase C
- Tf
transferrin
- TfR
transferrin receptor
- TxR
Texas Red
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–08–0403. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–08–0403.
REFERENCES
- Aridor M, Balch WE. Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J Biol Chem. 2000;275:35673–35676. doi: 10.1074/jbc.C000449200. [DOI] [PubMed] [Google Scholar]
- Bali PK, Harris WR. Site-specific rate constants for iron removal from diferric transferrin by nitrilotris(methylenephosphonic acid) and pyrophosphate. Arch Biochem Biophys. 1990;281:251–256. doi: 10.1016/0003-9861(90)90440-a. [DOI] [PubMed] [Google Scholar]
- Bao J, Alroy I, Waterman H, Schejter ED, Brodie C, Gruenberg J, Yarden Y. Threonine phosphorylation diverts internalized epidermal growth factor receptors from a degradative pathway to the recycling endosome. J Biol Chem. 2000;275:26178–26186. doi: 10.1074/jbc.M002367200. [DOI] [PubMed] [Google Scholar]
- Barbier AJ, Poppleton HM, Yigzaw Y, Mullenix JB, Wiepz GJ, Bertics PJ, Patel TB. Transmodulation of epidermal growth factor receptor function by cyclic AMP-dependent protein kinase. J Biol Chem. 1999;274:14067–14073. doi: 10.1074/jbc.274.20.14067. [DOI] [PubMed] [Google Scholar]
- Beguinot L, Hanover JA, Ito S, Richert ND, Willingham MC, Pastan I. Phorbol esters induce transient internalization without degradation of unoccupied epidermal growth factor receptors. Proc Natl Acad Sci USA. 1985;82:2774–2778. doi: 10.1073/pnas.82.9.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmerah A, Lamaze C, Begue B, Schmid SL, Dautry-Varsat A, Cerf-Bensussan N. AP-2/eps15 interaction is required for receptor-mediated endocytosis. J Cell Biol. 1998;140:1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W, Gallusser A, Kirchhausen T. Role of the regulatory domain of the EGF-receptor cytoplasmic tail in selective binding of the clathrin-associated complex AP-2. Curr Biol. 1995;5:1168–1178. doi: 10.1016/s0960-9822(95)00233-8. [DOI] [PubMed] [Google Scholar]
- Bravo-Zehnder M, Orio P, Norambuena A, Wallner M, Meera P, Toro L, Latorre R, Gonzalez A. Apical sorting of a voltage- and Ca2+-activated K+ channel alpha-subunit in Madin-Darby canine kidney cells is independent of N-glycosylation. Proc Natl Acad Sci USA. 2000;97:13114–13119. doi: 10.1073/pnas.240455697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena DL, Chan CL, Gill GN. The intracellular tyrosine kinase domain of the epidermal growth factor receptor undergoes a conformational change upon autophosphorylation. J Biol Chem. 1994;269:260–265. [PubMed] [Google Scholar]
- Carpenter G. Employment of the epidermal growth factor receptor in growth factor-independent signaling pathways. J Cell Biol. 1999;146:697–702. doi: 10.1083/jcb.146.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. The EGF receptor: a nexus for trafficking and signaling. Bioessays. 2000;22:697–707. doi: 10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ceresa BP, Schmid SL. Regulation of signal transduction by endocytosis. Curr Opin Cell Biol. 2000;12:204–210. doi: 10.1016/s0955-0674(99)00077-0. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang Z. Regulation of intracellular trafficking of the EGF receptor by Rab5 in the absence of phosphatidylinositol 3-kinase activity. EMBO rep. 2001;2:68–74. doi: 10.1093/embo-reports/kve005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Ciardiello F, Tortora G. Interactions between the epidermal growth factor receptor and type I protein kinase A: biological significance and therapeutic implications. Clin Cancer Res. 1998;4:821–828. [PubMed] [Google Scholar]
- Confalonieri S, Salcini AE, Puri C, Tacchetti C, Di Fiore PP. Tyrosine phosphorylation of Eps15 is required for ligand-regulated, but not constitutive, endocytosis. J Cell Biol. 2000;150:905–912. doi: 10.1083/jcb.150.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotlin LF, Siddiqui MA, Simpson F, Collawn JF. Casein kinase II activity is required for transferrin receptor endocytosis. J Biol Chem. 1999;274:30550–30556. doi: 10.1074/jbc.274.43.30550. [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, Gill GN. Endocytosis and mitogenic signaling. Curr Opin Cell Biol. 1999;11:483–488. doi: 10.1016/s0955-0674(99)80069-6. [DOI] [PubMed] [Google Scholar]
- Faúndez V, Krauss R, Holuigue L, Garrido J, Gonzalez A. Epidermal growth factor receptor in synaptic fractions of the rat central nervous system. J Biol Chem. 1992;267:20363–20370. [PubMed] [Google Scholar]
- Felder S, Miller K, Moehren G, Ullrich A, Schlessinger J, Hopkins CR. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Goretzki L, Mueller BM. Receptor-mediated endocytosis of urokinase-type plasminogen activator is regulated by cAMP-dependent protein kinase. J Cell Sci. 1997;110:1395–1402. doi: 10.1242/jcs.110.12.1395. [DOI] [PubMed] [Google Scholar]
- Gorodinsky A, Harris DA. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol. 1995;129:619–627. doi: 10.1083/jcb.129.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange M, Sette C, Cuomo M, Conti M, Lagarde M, Prigent AF, Nemoz G. The cAMP-specific phosphodiesterase PDE4D3 is regulated by phosphatidic acid binding. Consequences for cAMP signaling pathway and characterization of a phosphatidic acid binding site. J Biol Chem. 2000;275:33379–33387. doi: 10.1074/jbc.M006329200. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Hollinshead R, Hemmings BA, Nigg EA. Ultrastructural localization of the regulatory (RII) subunit of cyclic AMP-dependent protein kinase to subcellular compartments active in endocytosis and recycling of membrane receptors. J Cell Sci. 1990;96:691–703. doi: 10.1242/jcs.96.4.691. [DOI] [PubMed] [Google Scholar]
- Haigler H, Maxfield F, Willingham M, Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980;255:1239–1241. [PubMed] [Google Scholar]
- Harris TE, Persaud SJ, Jones PM. Pseudosubstrate inhibition of cyclic AMP-dependent protein kinase in intact pancreatic islets: effects on cyclic AMP-dependent and glucose-dependent insulin secretion. Biochem Biophys Res Commun. 1997;232:648–651. doi: 10.1006/bbrc.1997.6344. [DOI] [PubMed] [Google Scholar]
- Honegger AM, Dull TJ, Felder S, Van Obberghen E, Bellot F, Szapary D, Schmidt A, Ullrich A, Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987;51:199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. Dopamine Acutely Stimulates Na+/H+ Exchanger (NHE3) Endocytosis via clathrin-coated vesicles. dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem. 2001;276:26906–26915. doi: 10.1074/jbc.M011338200. [DOI] [PubMed] [Google Scholar]
- Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V. G βγ-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Jilling T, Kirk KL. Cyclic AMP and chloride-dependent regulation of the apical constitutive secretory pathway in colonic epithelial cells. J Biol Chem. 1996;271:4381–4387. doi: 10.1074/jbc.271.8.4381. [DOI] [PubMed] [Google Scholar]
- Jones D, Morgan C, Cockcroft S. Phospholipase D and membrane traffic. Potential roles in regulated exocytosis, membrane delivery and vesicle budding. Biochim Biophys Acta. 1999;1439:229–244. doi: 10.1016/s1388-1981(99)00097-9. [DOI] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Kil SJ, Hobert M, Carlin C. A leucine-based determinant in the epidermal growth factor receptor juxtamembrane domain is required for the efficient transport of ligand-receptor complexes to lysosomes. J Biol Chem. 1999;274:3141–3150. doi: 10.1074/jbc.274.5.3141. [DOI] [PubMed] [Google Scholar]
- Kriss RM, Lax I, Gullick W, Waterfield MD, Ullrich A, Fridkin M, Schlessinger J. Antibodies against a synthetic peptide as a probe for the kinase activity of the avian EGF receptor and v-erbB protein. Cell. 1985;40:619–625. doi: 10.1016/0092-8674(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Kurten RC, Cadena DL, Gill GN. Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science. 1996;272:1008–1010. doi: 10.1126/science.272.5264.1008. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Schmid SL. Recruitment of epidermal growth factor receptors into coated pits requires their activated tyrosine kinase. J Cell Biol. 1995;129:47–54. doi: 10.1083/jcb.129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JM, Brown MS, Goldstein JL, Anderson RG. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Le Bivic A, Real FX, Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci USA. 1989;86:9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Linstedt AD. Potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-Golgi transport revealed by protein kinase inhibitor H89. Mol Biol Cell. 2000;11:2577–2590. doi: 10.1091/mbc.11.8.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, van Kerkhof P, Marzolo MP, Strous GJ, Bu G. Identification of a major cyclic AMP-dependent protein kinase A phosphorylation site within the cytoplasmic tail of the low-density lipoprotein receptor-related protein: implication for receptor-mediated endocytosis. Mol Cell Biol. 2001;21:1185–1195. doi: 10.1128/MCB.21.4.1185-1195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CR, Chen WS, Lazar CS, Carpenter CD, Gill GN, Evans RM, Rosenfeld MG. Protein kinase C phosphorylation at Thr 654 of the unoccupied EGF receptor and EGF binding regulate functional receptor loss by independent mechanisms. Cell. 1986;44:839–848. doi: 10.1016/0092-8674(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Marzolo MP, Bull P, González A. Apical sorting of hepatitis B surface antigen (HBsAg) is independent of N-glycosylation and glycosylphosphatidylinositol-anchored protein segregation. Proc Natl Acad Sci USA. 1997;94:1834–1839. doi: 10.1073/pnas.94.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo C, James GL, Smart EJ, Anderson RG. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271:11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Muniz M, Alonso M, Hidalgo J, Velasco A. A regulatory role for cAMP-dependent protein kinase in protein traffic along the exocytic route. J Biol Chem. 1996;271:30935–30941. doi: 10.1074/jbc.271.48.30935. [DOI] [PubMed] [Google Scholar]
- Muniz M, Martin ME, Hidalgo J, Velasco A. Protein kinase A activity is required for the budding of constitutive transport vesicles from the trans-Golgi network. Proc Natl Acad Sci USA. 1997;94:14461–14466. doi: 10.1073/pnas.94.26.14461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson PJ, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Carter RE, Sorkina T, Gill GN, Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO J. 1999;18:2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterov A, Kurten RC, Gill GN. Association of epidermal growth factor receptors with coated pit adaptins via a tyrosine phosphorylation-regulated mechanism. J Biol Chem. 1995a;270:6320–6327. doi: 10.1074/jbc.270.11.6320. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Wiley HS, Gill GN. Ligand-induced endocytosis of epidermal growth factor receptors that are defective in binding adaptor proteins. Proc Natl Acad Sci USA. 1995b;92:8719–8723. doi: 10.1073/pnas.92.19.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimplikar SW, Simons K. Activators of protein kinase A stimulate apical but not basolateral transport in epithelial Madin-Darby canine kidney cells. J Biol Chem. 1994;269:19054–19059. [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MG. Lipid regulators of membrane traffic through the Golgi complex. Trends Cell Biol. 1999;9:174–179. doi: 10.1016/s0962-8924(99)01535-4. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Olsnes S, Petersen OW, van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987;105:679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Shen Y, Xu L, Foster DA. Role for phospholipase D in receptor-mediated endocytosis. Mol Cell Biol. 2001;21:595–602. doi: 10.1128/MCB.21.2.595-602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Heuser JE, Harris DA. A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J Cell Biol. 1994;125:1239–1250. doi: 10.1083/jcb.125.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science. 1993;261:612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Mazzotti M, Sorkina T, Scotto L, Beguinot L. Epidermal growth factor receptor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif. J Biol Chem. 1996;271:13377–13384. doi: 10.1074/jbc.271.23.13377. [DOI] [PubMed] [Google Scholar]
- Stang E, Johannessen LE, Knardal SL, Madshus IH. Polyubiquitination of the epidermal growth factor receptor occurs at the plasma membrane upon ligand-induced activation. J Biol Chem. 2000;275:13940–13947. doi: 10.1074/jbc.275.18.13940. [DOI] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Wilde A, Beattie EC, Lem L, Riethof DA, Liu SH, Mobley WC, Soriano P, Brodsky FM. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- Wiley HS. Anomalous binding of epidermal growth factor to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J Cell Biol. 1988;107:801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley HS, Burke PM. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2001;2:12–18. doi: 10.1034/j.1600-0854.2001.020103.x. [DOI] [PubMed] [Google Scholar]
- Wiley HS, Cunningham DD. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982;257:4222–4229. [PubMed] [Google Scholar]
- Wiley HS, Herbst JJ, Walsh BJ, Lauffenburger DA, Rosenfeld MG, Gill GN. The role of tyrosine kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. J Biol Chem. 1991;266:11083–11094. [PubMed] [Google Scholar]
- Yeo EJ, Exton JH. Stimulation of phospholipase D by epidermal growth factor requires protein kinase C activation in Swiss 3T3 cells. J Biol Chem. 1995;270:3980–3988. doi: 10.1074/jbc.270.8.3980. [DOI] [PubMed] [Google Scholar]