Abstract
Extracorporeal shock wave therapy is becoming increasingly used in equine practice to treat musculoskeletal disorders. Although the exact effects of extracorporeal shock waves on tissues still need to be elucidated, transient cutaneous analgesia has been observed within treated areas in humans and horses. The purpose of this study was to determine the onset, magnitude, and duration of cutaneous analgesia after non-focused extracorporeal shock wave (ESW) application by comparing the limb withdrawal reflex latency (LWRL) in treated and untreated areas on the metacarpus of horses, using a focused light source. A single treatment with non-focused ESW was applied to 3 areas over the dorsal aspect of one metacarpus in 12 horses. The LWRL was measured to assess cutaneous sensation in treated and untreated control areas of the treated metacarpus and in an untreated area on the opposite metacarpus, before and at various time points after ESW application. Most treated and control areas revealed a significant decrease in LWRL over time compared with baseline values. Although the results of our study do not suggest cutaneous analgesia after ESW application to the equine metacarpus, we advise cautious use of this treatment modality for horses in training or before competition until further research is done.
Résumé
L’utilisation de la thérapie à l’aide d’ondes de choc extracorporelle (ESW) prend de l`ampleur en médecine équine pour traiter les problèmes d’ordre musculo-squelettique. Bien que les effets exacts de cette thérapie sur les tissus sont encore à être élucidés, une analgésie cutanée transitoire a été observée dans les régions traitées chez les humains et les chevaux. Dans cette étude nous avons voulu déterminer le début, l’ampleur et la durée de l’analgésie cutanée après un traitement ESW non-ciblé en comparant le temps de latence du réflexe de retrait du membre (LWRL) de régions traitées et non-traitées du métacarpe de chevaux, à l’aide d’une source lumineuse ciblée. Un traitement unique d’ESW non-ciblé a été appliqué à 3 régions sur l’aspect dorsal d’un des métacarpes chez 12 chevaux. Le LWRL a été mesuré afin d’évaluer la sensation cutanée dans les régions traitées d’un métacarpe et les régions non-traitées du métacarpe opposé, avant et après différents temps de traitement par ESW. La plupart des régions traitées et témoin ont montré une diminution marquée du LWRL dans le temps lorsque comparée aux valeurs de base. Bien que les résultats de notre étude ne suggèrent pas qu’il y ait analgésie cutanée après application d’ESW au métacarpe équin, nous recommandons une utilisation judicieuse de ce mode de traitement chez les chevaux à l’entraînement ou avant une compétition jusqu’à ce que des études supplémentaires soient effectuées.
(Traduit par Docteur Serge Messier)
Introduction
Periostitis and stress fractures of the dorsal cortex of the 3rd metacarpal bone represent conditions that are often observed in young fast-gaited performance horses at 2 to 3 y of age (1). This disease complex is referred to as dorsal metacarpal disease (DMD) and is believed to occur because of a failure of the adaptive response to altered biomechanical stresses that arise from high-speed work. Although racing Thoroughbreds have the greatest prevalence of DMD, young quarter horses and occasionally racing standardbreds can also be affected (1). Conventional treatment of DMD includes combinations of stall rest, altered training regimens, percutaneous periosteal scraping, osteostixis, and systemically administered non-steroidal antiinflammatory drugs (NSAIDs) (1). Alternative treatment modalities, such as cryotherapy (2) and, more recently, extracorporeal shock wave (ESW) therapy (3,4) have gained increasing interest in the treatment of DMD. In a clinical report, 29 Thoroughbred racehorses with DMD that were unresponsive to conventional treatment were treated with pneumatically generated, non-focused ESW. After 3 treatments at 3-week intervals, 24 horses (83%) resumed speed-work without recurrence of lameness or other clinical signs associated with DMD (3). In another case series, 10 horses with dorsal or palmar metacarpal cortical stress fractures were treated with 1 to 3 applications of focused shock waves administered under ultrasonographic control (4). Ninety days after treatment, 8 of these 10 horses (80%) had returned to racing or training and 6 (60%) were pain-free, revealed radiographic signs of fracture healing, or both (4). Despite the lack of reliable controls, these reports suggest that ESW therapy may have a place in the treatment of DMD.
Extracorporeal shock waves are acoustic pressure gradient waves that are used to treat a variety of orthopedic disorders in human and animal patients. Although first introduced for non-invasive treatment of urolithiasis in man (5), ESW were subsequently investigated for their effect on wound healing (6) and their osteogenic potential (7,8). Humans are currently treated with ESW for a variety of conditions including fracture non-unions (9,10) and painful soft tissue syndromes, such as plantar calcaneal spurs (11) and tennis elbow (12). Several clinical investigations have revealed promising results in the treatment of a variety of orthopedic disorders in horses, such as bone spavin (13), stress fractures (4), high suspensory desmitis (14), and navicular disease (15). Horses are often treated using non-focused or radial shock waves that are characterized by their distinct physical characteristics including lower wave energies, a slower rise time, and their inferior penetration into the patient’s body when compared with focused shock waves (16). However, beneficial effects in treatment of orthopedic disorders with non-focused shock waves have been reported in man (17–19) and horses (3,14).
Although the precise mechanism by which ESW exert their effects on tissues are currently not known, transient cutaneous analgesia over treated areas has been observed in man (20) and horses (16). This analgesic effect is likely to be independent of any other beneficial effect on healing of deeper lying injured structures and could place performance horses with predisposing bone and soft tissue lesions at risk of sustaining a life- and career-threatening injury when exercised too soon after application of shock wave therapy. The recognition of these safety concerns resulted in the development of regulations concerning the use of ESW therapy prior to competition and training by racing jurisdictions and the Federation Equine International (16).
The purpose of this investigation was to evaluate the local cutaneous analgesic effect of ESW in the distal limb in horses. We hypothesized that a single treatment with non-focused ESW applied dorsally over the 3rd metacarpal bone would induce transient cutaneous analgesia and that altered peripheral pain perception could be assessed by evaluating the response to a standardized thermal pain stimulus. Our specific objective was to document the latency of onset, magnitude, and duration of cutaneous analgesia in shock wave-treated metacarpal areas in comparison to non-treated areas.
Materials and methods
Animals
This study was approved by the Institutional Animal Care and Use Committee of the Louisiana State University. Twelve adult horses from the institutional research herd were used in the study: 5 quarter horses (3 geldings, 2 mares), 6 Thoroughbreds (4 geldings, 2 mares), and 1 Arabian, with a mean age of 14 y (range 5 to 19 y). Prior to inclusion in the study, all animals were clinically evaluated and determined to be free of lameness and apparent abnormalities in their metacarpal bones. Individual housing was provided at a nearby research facility and all horses were fed a routine pelleted diet and had free access to water.
Extracorporeal shock wave therapy
Prior to ESW administration, each horse was sedated with detomidine hydrochloride (0.02 mg/kg of body weight, IV) and butorphanol tartrate (0.02 mg/kg of body weight, IV) and the hair on the medial, lateral, and dorsal aspect of both metacarpi was clipped. One forelimb of each animal was randomly assigned to be treated. The skin of 5 circular areas (15 mm diameter, same diameter as the applicator head of the shock wave generator) on the treated metacarpus (lateral-mid-diaphysis = point 1; dorsal-mid-diaphysis = point 2; medial-mid-diaphysis = point 3; dorsal-proximal diaphysis = point 4; dorsal-distal diaphysis = point 5) and on one area on the control limb (dorsal-metacarpal mid-diaphysis = point 6) of each animal were darkened with a black ink felt pen to improve light absorption for assessment of the limb withdrawal reflex latency (LWRL). After application of a coupling gel, provided by the manufacturer, 2000 pulses with a non-focused ESW generator (Swiss DolorClast Vet; EMS Electro Medical Systems, Nyon, Switzerland) were applied over each of the 3 mid-diaphyseal circular areas of the treatment limb (points 1, 2, 3). The regimen was administered at a frequency of 12 Hz, with a machine pressure of 0.25 MPa, using a 15-mm diameter applicator head. The proximal and distal diaphyseal areas on the treated limb (points 4 and 5) and the mid-diaphyseal area on the opposite limb (point 6) served as controls (Figure 1).
Figure 1.
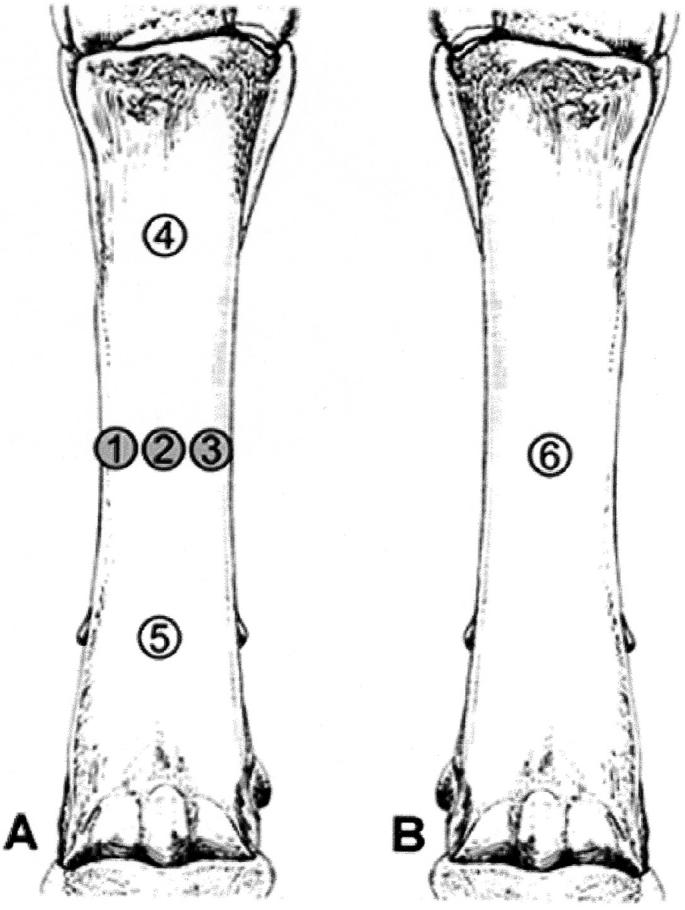
Evaluation of the limb withdrawal reflex latency (LWRL) on the 3rd metacarpal bones of horses treated with non-focused extracorporeal shock waves. A = shock wave-treated limb. B = untreated control limb. Points 1, 2, 3 = treated areas. Points 4, 5, 6 = untreated control areas.
Assessment of the LWRL
The degree of cutaneous analgesia produced by non-focused ESW was quantified by assessment of alterations in a nociceptive threshold, using a focused light beam as a stimulus. Briefly, horses were led into the testing area and a focused beam of light, of a standardized intensity, was emitted from a distance of 20 cm onto the target areas on the 3rd metacarpal bones. The time from stimulus when the light beam was emitted until withdrawal of the limb was observed, and this response was designated as the LWRL. Baseline values were obtained in both treated and control areas prior to sedation and administration of the ESW regimen. Following treatment of the designated areas, LWRL was assessed in random order in all areas of both limbs every 2 h for 12 h, followed by measurements every 8 h for 48 h after treatment. Each LWRL measurement was repeated 3 times in each area in random order at all time points.
Data analysis
Time to withdrawal from the light source was considered continuous and determined to follow a normal distribution using the Shapiro-Wilk test with rejection of the null hypothesis of normality at P ≤ 0.05. Results were summarized as mean ± standard error (sχ̄) values. Using each horse as its own control (blocking by horse), the effects of treatment and time were evaluated using a mixed effect linear model accounting for the random variance of horses and repeated measurements on each horse. Where there were significant main and interaction effects, selected multiple comparisons were made to determine the between treatment effects at various time points and the effect of time within treatments. A software program (PROC UNIVARIATE and PROC MIXED, SAS version 8.0; SAS Institute, Cary, North Carolina, USA) was used for analysis.
Results
The ESW application was well tolerated by all of the animals. Eight of the 12 horses developed mild to moderate swelling and skin abrasions over the treated areas. These changes were observed immediately after the end of ESW administration.
The LWRL responses were similar in all treated and control areas over time with a significant decrease noted at most sites and time points compared with the baseline values. Between point comparisons at different times after treatment revealed that there was no significant difference between points during baseline LWRL measurements, and at 2, 4, 6, and 28 h after ESW application (Table I). There was a significant difference in LWRL decrease at most sites on the treated limb, compared with the control area on the opposite limb (point 6), at 8, 10, 12, 20, and 48 h after treatment.
Table I.
Mean ± standard error (sχ̄) LWRL values of horses after application of non-focused shock waves to the metacarpus
| Hours after treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Point | Baseline | 2 | 4 | 6 | 8 | 10 | 12 | 20 | 28 | 48 |
| 1 | 13.41 (1.71) | 10.76 (0.89)a | 8.96 (0.54)a | 7.76 (0.55)a | 7.32 (0.35)a,b | 6.93 (0.35)a,b | 6.32 (0.45)a,b | 9.36 (1.18) | 6.68 (0.41)a | 8.11 (1.00)a,b |
| 2 | 15.18 (1.65) | 11.73 (1.36) | 10.46 (0.82)a | 8.27 (0.84)a | 7.64 (0.59)a,b | 7.36 (0.62)a | 6.96 (0.44)a,b | 9.43 (1.14)a | 7.06 (0.37)a | 8.06 (1.15)a,b |
| 3 | 17.66 (2.54) | 12.11 (0.10)a | 9.66 (0.92)a | 8.52 (0.74)a | 8.05 (0.44)a,b | 7.32 (0.46)a | 7.82 (0.59)a,b | 11.80 (2.70)a | 7.08 (0.60)a | 8.95 (1.02)a |
| 4 | 13.47 (1.28) | 10.89 (0.87) | 9.17 (0.75)a | 8.30 (0.88)a | 7.28 (0.59)a,b | 7.18 (0.55)a,b | 8.13 (0.46)a | 9.13 (1.20)a | 6.89 (0.48)a | 9.44 (1.16) |
| 5 | 14.79 (1.59) | 11.70 (0.42) | 10.04 (0.80)a | 8.26 (1.13)a | 8.92 (0.80)a | 8.05 (0.67)a | 7.48 (0.62)a,b | 8.53 (1.14)a,b | 9.44 (2.01)a | 9.99 (1.27)a |
| 6 | 17.26 (2.45) | 12.43 (0.72)a | 10.84 (1.20)a | 9.36 (0.75)a | 11.06 (1.38)a | 9.53 (0.83)a | 10.06 (0.82)a | 14.55 (1.72) | 9.44 (0.64)a | 13.21 (1.79) |
Points 1,2,3 = treated areas on mid-diaphysis of the treated limb; points 4,5 = untreated control areas on the treated limb; point 6 = mid-diaphyseal area on the opposite limb (untreated control)
Denotes means that are significantly different from the baseline value at P ≤ 0.05, within each treatment point
Indicates means that are significantly different from point 6 (untreated control) at P ≤ 0.05, within each time point
Discussion
Non-focused ESW applied to the 3rd metacarpal bone of horses did not result in noticeable cutaneous analgesia in our study. A significant decrease in LWRL was observed over time in both treated and untreated metacarpi, but there was no difference between treated (points 1, 2, 3) and control areas (point 4, 5) on ESW-treated metacarpi alone. A significant difference in decrease of LWRL in some areas of the treated limb was noted, when compared to the control area on the opposite limb (point 6) at 8, 10, 12, 20, and 48 h after treatment. Because swelling and skin abrasions were observed in several horses, a local inflammatory response secondary to treatment-induced trauma could potentially have overwhelmed any local cutaneous analgesic effect. However, considering the similarity of our findings in treated and untreated areas over time, it appears more likely that no local analgesia resulted from the ESW treatment. Tissue trauma and inflammation after ESW therapy may have also induced hypersensitivity in all testing sites (treated and untreated areas) in treated limbs.
Another explanation for the progressive decrease of LWRL in all tested areas over time could be that the animals became conditioned to testing during the study and that a painful thermal stimulus was anticipated. However, models assessing perception of thermal stimuli have been previously used in horses to quantify the analgesic effects of intravenously (21) and locally (22,23) administered drugs, and to demonstrate local analgesia after acupuncture and electroacupuncture treatment (24). To our knowledge, conditioning of animals to the testing procedure has not been reported in these studies, although preventive measures, such as blindfolding the animals prior to testing (23) or intermittent use of an unfocused (sham) light source (22) have been mentioned in some of the experimental protocols. No such measures were performed in our study.
In our study, the effect of ESW application to the 3rd metacarpal bone on LWRL was not assessed beyond 48 h. This follow-up period was chosen based on clinical observations in humans (20) where the analgesic response appears to be biphasic. An immediate initial improvement in signs is normally followed by return of the pain 3 to 4 d after treatment and then a second gradual improvement over the ensuing 3 to 4 wk. The initial response is believed to result from the direct effects of shock waves on nociceptors and impaired substance P synthesis, whereas the second phase of pain relief is believed to result from tissue healing associated with angiogenesis and tissue matrix remodeling (20). A study in sheep, however, failed to identify depletion of the neuropeptides substance P and calcitonin gene-related peptide (CGRP) in the soft tissues overlying metacarpi after treatment with focused or non-focused ESW (25).
Our results appear to be somewhat contradictory to the observations of another investigator who detected cutaneous analgesia of the overlying skin during the first 72 h after application of focused or non-focused ESW to the 3rd metacarpal bone in horses (25). In that study, however, cutaneous sensation was assessed only once every 24 h and an electric stimulus was used to test for local cutaneous analgesia (25).
In human medicine, shock wave therapy is often used to provide relief in painful conditions at the soft tissue and bone junction, such as tennis elbow, calcific shoulder tendonitis, or plantar calcaneal spurs (9,12,26). The rapid improvement of clinical signs after shock wave therapy is not usually accompanied by concurrent radiographic changes and has been attributed to an analgesic effect that appears to be independent of the healing response in tissues (9). Similar observations were made in a study in horses with osteoarthritis of the distal intertarsal and tarsometatarsal joints. Eighty percent of the animals improved at least 1 lameness grade and 18% were completely sound at 90 d after a single treatment with focused ESW. No signs of facilitated ankylosis or other changes in radiographic appearance were evident at that time (13).
Haist and von Keitz-Steiger (27) proposed 3 hypotheses for shock wave-induced analgesia in man: 1st, shock waves induce cell damage and the peripheral nociceptors, therefore, can not build up a membrane potential to transmit pain signals; 2nd, the nociceptors are over-stimulated by shock waves and emit high frequency impulses to peripheral nerve fibers, which are suppressed by a gate control mechanism; and 3rd, shock wave-induced pericellular free radicals induce the local release of unknown pain-suppressing substances.
Direct effects of shock waves on peripheral nerves could indeed contribute substantially to regional analgesia after ESW therapy. A study in isolated frog sciatic nerves revealed repetitive generation of action potentials during application of ESW (28). Although this in vitro mechanism was believed to not be applicable to the distal equine limb in vivo (25), it may be possible that ESW could result in over-stimulation of peripheral sensory nerve fibers and that signal transmission may be impaired by modulation via a gate control-like mechanism (27). A study in horses in our laboratory revealed a significant decrease in sensory nerve conduction velocity in palmar digital nerves after direct treatment with non-focused ESW, when compared to untreated controls (29). Transmission electron microscopy of treated nerves showed disruption and separation within the myelin sheaths of large- to medium-sized myelinated axons (29). The lack of a cutaneous analgesic effect in our study, therefore, could possibly be explained by the absence of peripheral nerves with large myelinated axons in our treatment areas.
It has been recommended to avoid peripheral nerves, large blood vessels, and growth plates when treating horses with ESW (16). However, peripheral nerves appear often to be directly targeted when shock waves are used to treat conditions in the distal limb, such as navicular disease (15). Although clinical improvement of navicular disease after ESW therapy has been observed (15), an experimental study in horses failed to identify local cutaneous analgesia in the heel area following ESW application to the palmar digital nerves in the pastern (25).
In conclusion, our findings suggest that a single application of non-focused ESW had no noticeable cutaneous analgesic effect in the equine metacarpus. However, clinical observations of post-treatment analgesia in humans and horses, results of other experimental studies (25), and distinct changes in directly treated peripheral nerves following ESW application (29), indicate that this treatment modality should be used carefully when treating equine athletes in training or before competition.
Acknowledgments
This study was funded by a grant of the Louisiana State University Equine Health Studies Program. The non-focused shock wave generator was kindly provided by EMS Electro Medical Systems, Nyon, Switzerland. The authors thank Catherine Koch, Jessica Carey, and Misty Gray for technical assistance.
Footnotes
Dr. Bolt’s current address is the Veterinary Teaching Hospital, University of California, 1 Shields Avenue, Davis, California 95616, USA.
Submitted to the Graduate School of the Louisiana State University by the primary author, as partial fulfillment of a MS degree.
Funding provided by the Louisiana State University Equine Health Studies Progam
References
- 1.Bertone AL. The metacarpus and metatarsus. In: Stashak TS, ed. Adam’s Lameness in Horses. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2002:800–830.
- 2.Montgomery TC, Johnson JH, McClure JM, et al. Cryotherapy of dorsal metacarpal disease. Mod Vet Pract. 1981;61:219–220. [PubMed] [Google Scholar]
- 3.Palmer SE. Treatment of dorsal metacarpal disease in the Thoroughbred racehorse with radial extracorporeal shock wave therapy. Proc 48th Annu Conv Am Assoc Equine Pract 2002:318–321.
- 4.Scheuch B, Whitcomb MB, Galuppo L, et al. Clinical evaluation of high-energy extracorporeal shock waves on equine orthopedic injuries. Proc 19th Annu Meet Assoc Eq Sports Med 2000:18–20.
- 5.Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet. 1980;2:1265–1268. doi: 10.1016/s0140-6736(80)92335-1. [DOI] [PubMed] [Google Scholar]
- 6.Haupt G, Chvapil P. Effects of shock waves on the healing of partial-thickness wounds in piglets. J Surg Res. 1990;49:45. doi: 10.1016/0022-4804(90)90109-f. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda K, Tomita K, Takayama K. Application of extracorporeal shock wave on bone: preliminary report. J Trauma. 1999;47:947–950. doi: 10.1097/00005373-199911000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Johannes EJ, Kaulesar Sukul DM, Matura E. High-energy shock waves for the treatment of nonunions: An experiment on dogs. J Surg Res. 1994;57:246–252. doi: 10.1006/jsre.1994.1139. [DOI] [PubMed] [Google Scholar]
- 9.Haupt G. Use of extracorporeal shock waves in the treatment of pseudarthrosis, tendinopathy and other orthopedic diseases. J Urol. 1997;158:4–11. doi: 10.1097/00005392-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Valchanov VD, Michailov P. High energy shock waves in the treatment of delayed and non-union fractures. Int Orthopaed. 1991;15:181–184. doi: 10.1007/BF00192289. [DOI] [PubMed] [Google Scholar]
- 11.Rompe JD, Decking J, Schoellner C, et al. Shock wave application for chronic plantar fasciitis in running athletes. A prospective, randomized, placebo-controlled trial. Am J Sports Med. 2003;31:268–275. doi: 10.1177/03635465030310021901. [DOI] [PubMed] [Google Scholar]
- 12.Rompe JD, Riedel C, Betz U, et al. Chronic lateral epicondylitis of the elbow. A prospective study of low-energy shockwave therapy and low-energy shockwave therapy plus manual therapy of the cervical spine. Arch Phys Med Rehabil. 2001;82:578–582. doi: 10.1053/apmr.2001.22337. [DOI] [PubMed] [Google Scholar]
- 13.McCarrol D, McClure SR. Extracorporeal shock wave therapy for treatment of osteoarthritis of the tarsometatarsal and distal intertarsal joint of the horse. Proc 46th Annu Conv Am Assoc Equine Pract 2000:200–202.
- 14.Boening KJ, Loeffeld S, Weitkamp S, et al. Radial extracorporeal shock wave therapy for chronic insertion desmopathy of the proximal suspensory ligament. Proc 46th Annu Conv Am Assoc Equine Pract 2000:203–207.
- 15.Baer K, Weiler M, Bodamer J, et al. Extracorporeal shock wave therapy — a remedial procedure for navicular disease. Tieraerztl Praxis Grosstiere. 2001;29:163–167. [Google Scholar]
- 16.McClure SR, Merritt DK. Extracorporeal shock-wave therapy for equine musculoskeletal disorders. Comp Cont Educ Pract Vet. 2003;25:68–75. [Google Scholar]
- 17.Lohrer H, Schoell J, Arentz S. Radial shock wave therapy (RSWT) for the treatment of “Jumper’s Knee” and Achilles tendon. Proc Ann Symp Can Acad Sports Med 2001:87.
- 18.Lohrer H, Schoell J, Arentz S, et al. Effectiveness of radial shock wave therapy (RSWT) on tennis elbow and plantar fasciitis. Proc Ann Symp Can Acad Sports Med 2001:88.
- 19.Gremion G, Gobelet C, Siegrist O, et al. Efficacy of radial shock wave therapy in the chronic patellar tendonitis. Proc 3rd Bienn Congr of the ISAKOS 2001:76.
- 20.Ogden JA, Ogden DA. Electrohydraulic SWT: Bimodal response. Proc 5th Congr ISMST 2002:18.
- 21.Kamerling SG, Weckman TJ, DeQuick DJ, et al. A method for studying cutaneous pain perception and analgesia in horses. J Pharmacol Meth. 1985;13:267–274. doi: 10.1016/0160-5402(85)90027-0. [DOI] [PubMed] [Google Scholar]
- 22.Harkins JD, Mundy GD, Stanley S, et al. Determination of highest no effect dose (HNED) for local anaesthetic responses to procaine, cocaine, bupivacaine and benzocaine. Equine Vet J. 1996;28:30–37. doi: 10.1111/j.2042-3306.1996.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Sanroman FJ, Cruz JM, Santos M, et al. Evaluation of the local analgesic effect of ketamine in the palmar digital nerve block at the base of the proximal sesamoid (abaxial sesamoid block) in horses. Am J Vet Res. 2002;63:475–478. doi: 10.2460/ajvr.2003.64.475. [DOI] [PubMed] [Google Scholar]
- 24.Skarda RT, Tejwani GA, Muir WW. Cutaneous analgesia, hemodynamic and respiratory effects, and β-endorphin concentration in spinal fluid and plasma of horses after acupuncture and electroacupuncture. Am J Vet Res. 2002;63:1435–1442. doi: 10.2460/ajvr.2002.63.1435. [DOI] [PubMed] [Google Scholar]
- 25.McClure S, Sonea IM, Yeager M, et al. Safety of shock wave therapy in performance horses. Proc 49th Annu Conv Am Assoc Equine Pract 2003:62–65.
- 26.Rompe JD, Hopf C, Nafe B, et al. Low-energy extracorporeal shock wave therapy for painful heel: a prospective controlled single-blind study. Arch Orthop Trauma Surg. 1996;115:75–79. doi: 10.1007/BF00573445. [DOI] [PubMed] [Google Scholar]
- 27.Haist J, von Keitz-Steeger D. Stosswellentherapie knochennaher Weichteilschmerzen — Ein neues Behandlungskonzept. In: Chaussy C, Eisenberger F, Jocham D, Wilbert DM, eds. Die Stosswelle — Forschung und Klinik. Tuebingen: Attempto Verlag 1995:162–165.
- 28.Schelling G, Delius M, Gschwender M, et al. Extracorporeal shock waves stimulate frog sciatic nerves indirectly via a cavitation-mediated mechanism. Biophys J. 1994;66:133–140. doi: 10.1016/S0006-3495(94)80758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolt DM, Burba DJ, Hubert JD, et al. Functional and morphological changes in palmar digital nerves following extracorporeal shock wave application in horses. Proc 30th Annu Conf Vet Orthopedic Soc 2003:67.


