Abstract
Mesenchymal stem/stromal cells (MSCs) can regenerate tissues by direct differentiation or indirectly by stimulating angiogenesis, limiting inflammation, and recruiting tissue-specific progenitor cells. MSCs emerge and multiply in long-term cultures of total cells from the bone marrow or multiple other organs. Such a derivation in vitro is simple and convenient, hence popular, but has long precluded understanding of the native identity, tissue distribution, frequency, and natural role of MSCs, which have been defined and validated exclusively in terms of surface marker expression and developmental potential in culture into bone, cartilage, and fat. Such simple, widely accepted criteria uniformly typify MSCs, even though some differences in potential exist, depending on tissue sources. Combined immunohistochemistry, flow cytometry, and cell culture have allowed tracking the artifactual cultured mesenchymal stem/stromal cells back to perivascular anatomical regions. Presently, both pericytes enveloping microvessels and adventitial cells surrounding larger arteries and veins have been described as possible MSC forerunners. While such a vascular association would explain why MSCs have been isolated from virtually all tissues tested, the origin of the MSCs grown from umbilical cord blood remains unknown. In fact, most aspects of the biology of perivascular MSCs are still obscure, from the emergence of these cells in the embryo to the molecular control of their activity in adult tissues. Such dark areas have not compromised intents to use these cells in clinical settings though, in which purified perivascular cells already exhibit decisive advantages over conventional MSCs, including purity, thorough characterization and, principally, total independence from in vitro culture. A growing body of experimental data is currently paving the way to the medical usage of autologous sorted perivascular cells for indications in which MSCs have been previously contemplated or actually used, such as bone regeneration and cardiovascular tissue repair.
Keywords: Blood vessels, Stem cells, Pericytes, Cell therapy, Tissue repair, Mesenchymal stem cells
Introduction
It is intuitive that any adult tissue with the capacity to repair or regenerate harbors specific stem cells, defined by their ability to self-renew and retain sufficient proliferative and differentiation potential. Bone has an impressive ability to repair and it is therefore not surprising that stem cells with bone-regenerative characteristics have been identified from cultures of bone-derived cells. The presence of non-hematopoietic stem cells in the bone marrow (BM) was first described by Conheim, who proposed that bone marrow was also a source of fibroblasts contributing to bone healing [1]. In the 1960s, the Russian scientist Friedenstein [2–4] identified a population of cells within rodent bone marrow that were rapidly adherent to plastic, had the appearance of fibroblasts, and formed clonal colonies in vitro (colony-forming unit (CFU)—fibroblast). These cells were also capable of osteogenic differentiation in culture and could generate bone when implanted in ectopic locations in vivo. In addition, their demonstrated ability to regenerate heterotopic bone tissue in serial implants suggested their self-renewal [5]. Since Friedenstein’s early descriptions, numerous laboratories have confirmed and expanded these findings, showing that cells with similar abilities to be sub-passaged and differentiated in vitro into a variety of mesodermal cell types such as osteoblasts, chondrocytes, adipocytes, and myoblasts could be isolated from human bone marrow [6–9]. Some investigators suggested that perivascular cells in the bone marrow are the precursors of connective tissue lineages in vivo [10, 11]. Friedenstein had isolated from the bone marrow of rodents what would later be coined “mesenchymal stem cells (MSCs)” by Caplan [12].
MSCs have now been isolated from multiple different human tissue types including fat [13, 14], dental pulp [15], periodontal ligament [16], tendon [17, 18], umbilical cord [19], skin [20], placenta [21], amniotic fluid [22], synovial membrane [23], muscle [24], indeed almost all post-natal [25, 26], and fetal tissues [27, 28] (Table 1). Considerable work has been done to characterize and expand these cells in vitro, and to explore strategies to maintain these cells in their stem-like state [29–34]. This work was driven by the promise of therapeutic translation using these progenitors to replace or repair damaged musculoskeletal tissues. Therefore, current knowledge of MSCs is almost entirely based on characterization and observations of behavior in culture—the setting in which they are defined—and until recently the in vivo counterpart of culture-expanded MSCs remained a mystery.
Table 1.
Human MSCs derived from different sources
| Human tissue | Study |
|---|---|
| Aorta | [25] |
| Adipose | [13, 27, 228–233, 257] |
| Amniotic fluid | [22, 41] |
| Bone marrow | [2–4, 7, 15, 25, 27, 234] |
| Blood | [235] |
| Brain | [25, 27, 113] |
| Cartilage | [236, 237] |
| Cord blood | [234, 238–241] |
| Dental pulp | [15, 44, 242–246] |
| Endometrium | [247, 248] |
| Eye | [27] |
| Gut | [27, 249] |
| Heart | [27] |
| Kidney | [25] |
| Liver | [25, 234] |
| Lung | [25, 27] |
| Muscle | [24, 25, 27] |
| Pancreas | [25, 27] |
| Perichondrium | [250, 251] |
| Periodontal ligament | [16] |
| Placenta | [21, 27, 252] |
| Salivary gland | [253] |
| Skin | [20, 27] |
| Spleen | [25] |
| Synovial membrane | [23] |
| Tendon | [17, 18] |
| Thymus | [25] |
| Umbilical cord | [19, 27, 254] |
| Vein | [25, 255] |
With interest so far focused on multipotency and tissue engineering, little is currently understood regarding the ontogeny of these cells, their anatomical localization or their natural role in tissue homeostasis, physiology, or pathology. Characterization of native MSCs could allow for either pharmacological or genetic manipulations of this cellular pool in vivo, or facilitate the purification of populations for tissue engineering applications. In this review, we summarize recent developments in our understanding of the anatomical and developmental origins of MSCs and discuss related ongoing controversies. We discuss how advances in our understanding of the in vivo location of MSCs will facilitate the expanding medical applications of these cells.
Definitions and in vitro behaviors of MSCs
Attempts have been made to standardize the nomenclature used in MSC research, however the variation in methods of isolation, culture, and assays used to examine them has made this issue both difficult and at times misleading. In 2006, the International Society for Cellular Therapy (ISCT) produced a position statement in which it suggested the minimum criteria required to define MSCs [35]. They stated that cells must:
Be plastic adherent
Express the cell surface antigens CD105, CD73, and CD90
Not express the cell surface antigens CD45, CD34, CD14, CD11b, CD79α, CD19, or HLA-DR
Differentiate into osteoblasts, adipocytes, and chondroblasts in vitro
These criteria were established to standardize human MSC isolation but may not apply uniformly to other species. For example, murine MSCs differ in marker expression and behavior compared with human MSCs [36].
Although not included within defining criteria, MSCs are recognized to perform a number of roles beyond multipotency including immune modulation, hematopoiesis support, and the release of trophic factors in response to injury.
Multilineage potential
The ability of MSCs to differentiate into mesodermal cell lineages (cartilage, bone, tendon, ligament, adipose tissue, marrow stroma, connective tissue) in appropriate conditions is well established [12]. This is routinely achieved in vitro by supplementation of cultures with lineage-specific growth factor combinations. For example, dexamethasone, 3-isobutyl-1-methylxanthine (IBMX), and insulin are used to induce adipogenic differentiation while dexamethasone, β-glycerophosphate (BGP), and ascorbic acid are used to promote osteogenic differentiation.
Support of hematopoiesis
The crucial role of BM stromal progenitors in supporting hematopoiesis was first described by Friedenstein et al. [3] who observed the formation of heterotopic ossicles containing bone and hematopoietic tissue upon ectopic CFU-fibroblast-derived colony transplantation in semi-syngeneic animals. The hematopoietic cells were of recipient origin whereas bone-forming cells originated from the donor suggesting that transplanted colonies provided a microenvironment favorable for hematopoietic stem cell (HSC) homing and subsequent establishment of hematopoiesis. Subsequently, Dexter et al. [37] established a system of murine long-term cultures to demonstrate that BM stromal cells can maintain hematopoiesis for several months. It was confirmed that a subset of human BM stromal cells expressing the STRO-1 antigen possesses hematopoiesis supporting ability, along with the potential to differentiate into multiple mesenchymal cell lineages [38, 39]. There is accumulating evidence to suggest that BM MSCs also have promoting effects on HSC engraftment and repopulation. Several studies have demonstrated that co-transplantation of human HSCs and MSCs results in increased chimerism and/or hematopoietic recovery, in both animal models and humans [40–46].
Immune regulation
The immunomodulatory properties of bone marrow-derived MSCs, including their immunosuppressive effects during allogeneic stem cell transplantation, have been well documented [47–51]. The immunoactivity of the cells is mediated by direct cell-to-cell contact and through secreted bioactive molecules involving dendritic cells, B and T cells including T regulatory cells, and T helper cells and killer cells [51, 52]. The immunomodulatory effect of MSCs has been suggested to involve the release of bio-molecules (IL-10, interferon-γ, indoleamine 2,3-dioxygenase [53]), cell-to-cell contacts [54], T-cell regulation [55], and alloantigen-pulsed dendritic cells [56]. Furthermore, umbilical cord perivascular mesenchymal progenitors [57] as well as pericytes purified from pancreas, skeletal muscle and placenta [Corselli et al., unpublished] can reduce lymphocyte proliferation as bone marrow MSCs do. More studies will be required to elucidate thoroughly the role of MSCs and related perivascular mesenchymal progenitors during the inflammatory and immunosuppressive responses.
Secretion of trophic factors
Experiments using transplantation of cultured MSCs into animals led to the realization that MSCs’ therapeutic effects could not be explained by differentiation into tissue-specific cells alone [58, 59]. As such, transplanted MSCs may exert beneficial effects through their vast secretome beyond immune regulation [60, 61]. Bioactive factors secreted by MSCs have angiogenic and antiapoptotic properties that serve to limit the extent of tissue damage at the injured sites, re-establish blood supply, and possibly recruit local progenitors. These MSC paracrine effects have been referred to as trophic effects [54].
Due to the lack of a unique MSC function and anatomic identity, these cells were termed MSCs or more or less synonymously “marrow stromal cells”, “BM stromal cells” and “mesenchymal stromal cells” [1, 62, 63]. Populations of cells that fulfilled the ISCT MSC criteria yet exhibit broader differentiation capacity have also been described [64]. Investigators described such cells as multipotent adult progenitor cells (MAPC) [65], marrow isolated multilineage inducible cells (MIAMI) [66], or multipotent adult stem cells (MASC) [67]. The relationship of these cells to MSCs is currently not clear.
For example, cord blood (CB) contains different non-hematopoietic CD45−, CD34− adherent cell populations: cord blood mesenchymal stromal cells (CB MSCs) that behave almost like MSCs from bone marrow (BM MSCs), very small embryonic-like stem cells (VSEL), and unrestricted somatic stem cells (USSCs) that differentiate into cells of all three germ layers [68–70]. Distinguishing between these populations is difficult due to overlapping features such as the immunophenotype or the osteogenic and chondrogenic differentiation pathways. Functional differences in the differentiation potentials suggest different developmental stages or different cell populations.
The immunophenotype of MSCs
Assuming that MSCs represent a distinct cell population, it is intuitive that they would have a specific repertoire of cell surface antigens that would enable identification, isolation, and purification based on phenotype. Flow cytometry is a powerful and relatively easy-to-handle approach for phenotyping of cells using fluorescence labeled monoclonal antibodies (mAbs) against cell surface antigens. The cell surface antigen profile of MSCs has been well explored, and in recent years various combinations of cell surface markers were published for characterizing MSCs (Tables 2, 3) [71, 72]. A particular challenge for the field has been the absence of any specific marker to define MSCs, although a large number of different determinants have been associated, albeit not exclusively, with them (reviewed by Lindner et al. [73] 2010; for human MSCs).
Table 2.
Markers used for the POSITIVE identification of MSCs and MSC precursors
| Marker | Also known as | “Conventional” MSCs | Pericytes | Adventitial cells |
|---|---|---|---|---|
| CD3 | [256] | |||
| CD9 | Tetraspanin-29 | [256–259] | ||
| CD10 | Neural endopeptidase | [27, 257] | [27] | |
| CD13 | Alanine aminopeptidase N | [27, 257] | [27] | |
| CD18 | [261] | |||
| CD29 | Integrin beta 1 | [148, 256–259, 261, 262] | [260] | |
| CD34* | Mucosialin | [106, 135, 257, 258, 262, 263, 265] | [135, 263–265] | |
| CD44 | Receptor for hyaluronic acid | [148, 256–259, 261, 262] | [27, 260, 263, 295] | [135] |
| CD49A | Half of α1β1 integrin duplex | [256, 257, 259, 261] | ||
| CD49B | Half of α2β1 integrin duplex | [256, 257, 259, 261] | ||
| CD49C | Integrin α 3 | [256, 257, 259, 261] | ||
| CD49E | Integrin α 5 | [256, 257, 259, 261] | ||
| CD49F | Integrin α 6 | [256, 257, 259, 261] | ||
| CD51 | Integrin α V | [261] | ||
| CD54 | Intercellular adhesion molecule 1 | [257–259, 261] | ||
| CD55 | [257–259] | |||
| CD56 | Neural cell adhesion molecule (NCAM) | [261, 263] | ||
| CD58 | Lymphocyte function-associated antigen 3 | [256] | ||
| CD59 | Protectin | [257, 266] | ||
| CD61 | Integrin β 3 | [258] | ||
| CD63 | Lysosomal-associated membrane protein 3 (LAMP-3) | [258] | ||
| CD71 | Transferrin receptor | [256, 258, 259] | ||
| CD73 | 5′-nucleotidase, ecto | [27, 35, 148, 256, 259, 262] | [27, 106, 260, 264] | [106, 135] |
| CD90 | Thy-1 | [27, 35, 148, 256, 258, 259, 262, 263] | [27, 106, 260, 263–265, 295] | [13, 15–17] |
| CD97 | Leucocyte antigen | [258] | ||
| CD98 | [258] | |||
| CD99 | E2 antigen | [258] | ||
| CD104 | Integrin β4 | [261] | ||
| CD105 | Endoglin | [27, 35, 148, 257–259] | [27, 260, 264, 295] | [135] |
| CD106 | Vascular cell adhesion molecule 1 (VCAM1) | [257, 261] | ||
| CD120A | Tumor necrosis factor receptor | [7] | ||
| CD124 | Interleukin-4 receptor | [7] | ||
| CD140 | Platelet-derived growth factor receptor beta (PDGFRβ) | [27, 263, 267, 268] | [27, 260, 263, 264] | |
| CD146 | Melanoma cell adhesion molecule (MCAM) | [27, 106, 257, 263, 265] | [27, 106, 260, 263–265, 295] | |
| CD166 | Activated leukocyte cell adhesion molecule (ALCAM) | [257–259, 261, 262] | [295] | |
| CD271 | Low-affinity nerve growth factor receptor (LNGFR) | [263, 267, 269] | ||
| CD276 | [258] | |||
| CD304 | [258] | |||
| CD324 | [261] | |||
| CD340 | [267] | |||
| CD349 | [267] | |||
| αSMA | [27, 263] | [27, 263, 264] | ||
| NG2 | [258, 263] | [27, 260, 263, 264] | [263] | |
| STRO-1 | [264, 295] |
* CD34 expression is rapidly lost in culture
Table 3.
Markers used for the NEGATIVE identification of MSCs and MSC precursors
| Marker | Also known as | “Conventional” MSCs | Pericytes | Adventitial cells |
|---|---|---|---|---|
| CD11a | Integrin αL chain | [7, 35] | ||
| CD11b | Integrin αM chain | [35] | ||
| CD14 | LPS receptor | [7, 35, 148, 258] | [260, 295] | |
| CD16 | [256] | |||
| CD19 | [7, 35] | |||
| CD27 | [256] | |||
| CD28 | [256] | |||
| CD31 | Platelet/endothelial cell adhesion molecule 1 (PECAM-1) | [106, 256, 259, 265] | [27, 106, 260, 263, 264, 270, 295] | [263, 264, 270] |
| CD33 | [256] | |||
| CD34 | Mucosialin | [35, 148, 256, 263] | [27, 106, 263, 264, 270] | |
| CD36 | [256] | |||
| CD45 | Leucocyte common antigen (LCA) | [35, 148, 256, 258, 259] | [27, 106, 260, 264, 270, 295] | [135, 270] |
| CD50 | [261] | |||
| CD56 | [27, 264] | |||
| CD79a | Ig-α | [35] | ||
| CD102 | Intercellular adhesion molecule 2 | [261] | ||
| CD106 | Vascular cell adhesion molecule (VCAM1) | [295] | ||
| CD117 | c-kit | [270] | [270] | [270] |
| CD133 | Prominin-1 | [148, 258] | [27] | |
| CD144 | Vascular endothelial (VE)-cadherin | [27, 148] | [27] | |
| CD146 | Melanoma cell adhesion molecule (MCAM) | [106, 135, 263, 270] | ||
| CD243 | [256] | |||
| αSMA | [135, 263] | |||
| NG2 | [135] |
Simmons et al. reported that a population of human bone marrow-derived cells expressing STRO-1 was considerably enriched in clonogenic cells that were capable of differentiation into multiple mesenchymal cell lineages and included CFUs. It was subsequently demonstrated that the homogeneity of the STRO-1-positive population could be further improved by co-selecting for VCAM-1 [74]. Similarly, CD146 (MCAM)+ populations isolated from bone marrow were shown to adhere to plastic, demonstrate clonogenicity, and self renew in vitro [75, 76]. When transplanted subcutaneously into mice, this CD146+ population can generate bone and support hematopoiesis. Any relationship between the STRO-1+VCAM-1+ population and the CD146+ population remains to be determined. While these markers have been used to enrich MSC-like populations, they do not appear to participate in the molecular processes regulating self-renewal versus differentiation [77].
Defining MSCs in vitro adds complexity to their study because the culture conditions may introduce experimental artifacts. It has been proposed that certain natively expressed surface markers are modified following explantation, while new markers may be acquired. For example, an MSC line was isolated that uniformly expressed human leukocyte antigen-DR (HLA-DR) (a marker that should not be expressed on MSCs by the above definition) while also expressing CD90 and CD105, adhering to plastic in culture, and being capable of differentiating into osteoblasts, adipocytes, and chondroblasts [78].
Similarly, the expression of CD105, CD73, and CD90 is not uniform and can be modulated by in vitro conditioning. The expression or absence of these factors does not appear to be inclusive or exclusive of multipotency and discrete subpopulations of MSC-like cells have been isolated with varying levels of expression [79]. Numerous other markers have been suggested, including platelet-derived growth factor receptor β (PDGFRβ), CD271 [80, 81], and recently decorin, a marker specific to MSCs in adipose tissue has been identified [82]. A nomenclature that focuses on anatomically defined in vivo populations is preferential to one that is based on inherently variable and imprecise in vitro populations.
MSCs isolated from different organs exhibit unique features
The equivalency of MSC populations of distinct anatomic origins has not been robustly demonstrated. Despite fulfilling the ISCT criteria, differences have been observed with respect to the immunophenotype, secreted cytokine profile, and results obtained by proteome analysis depending on the source and the native or cultivated state of the MSC population characterized [83–85]. Cloned human MSCs isolated from fat and bone marrow default to an adipogenic or osteogenic potential respectively, suggesting that the tissue environment of origin imprints such character. Clonal analysis varies between donors and tissues yielding values between 30 and 50 % for tripotent cells that can differentiate into adipocytes, chondrocytes and osteoblasts [86, 87]. The ability of MSCs to differentiate in vitro into adipocytes, chondrocytes, osteoblasts, myoblasts, and of late into hematopoiesis- or osteogenesis-supporting stromal cells has been used to stratify the multipotency of these cells as well as to search for markers indicative of lineage commitment. While surface antigens like CD105, CD73, and CD29 are conserved by most MSCs [35], others such as Sca-1 (rodents), CD24 [88–90], CD140-a, -b, CD146 [91], CD271 [92, 93], CD338 [94], and many others [88] betray the underlying heterogeneity in these cells. Some markers like PDGFRα correlated with the adipogenic potential of these cells, both in humans and in rodents, while others like CD146 may be associated with greater multipotency, and a higher colony-forming efficiency and proliferation rate [87].
Anatomical location of MSCs
With interest focused on multipotency and tissue engineering and repair, the native origin and physiological roles in vivo of MSCs have been considerably overlooked. As such, cells that could be identified only retrospectively in long-term culture were being proposed for therapeutic purposes, without a true understanding of their native origin or function. The real, in vivo counterpart of culture expanded MSCs was unknown, and it could be argued that based on the ISCT definition, MSCs represented a mere artifact of culture with no exact equivalent in the living organism. Somewhat surprisingly, the lack of understanding of the in vivo origin of these cells did not constrain their clinical uses. However, such a retrospective characterization in vitro meant that any clinical exploitation of MSCs would make use of a heterogeneous population of cells exposed to the hazards of extended culture. In search of ways to fully exploit the therapeutic characteristics of MSCs, researchers sought an improved understanding of the native identity and biology of these cells.
The massive stem cell recruitment, expansion, migration, and differentiation that can be visualized at early embryonic stages wanes with maturity. As development proceeds, stem cells become less prevalent and tissue regeneration and repair become quantitatively marginal. This makes the documentation of stem cell presence and activity in anatomic terms increasingly challenging. It is established that adult tissue-specific stem cells are located in specialized “niches” in their corresponding tissues of origin [95]. For example, HSCs can be found in the bone marrow [96] [93], epidermal stem cells in mammalian hair follicles [97], and neural SCs in the subventricular zone [98]. MSCs have perhaps proved to be the most elusive of all adult stem cells.
The main cell types suggested to descend from MSCs including bone, cartilage, fat, and muscle are not limited to one anatomical region. Wherever MSCs originate, they must be capable of reaching these tissues throughout the body or be locally available. With this in mind, a number of potential explanations have been suggested [99]. Firstly, MSCs may originate from a single organ, from which they migrate towards areas of need in response to systemic signals. In support of this, experiments using rats exposed to low-oxygen conditions suggest that MSCs are specifically mobilized into peripheral blood as a consequence of hypoxia [100], while elevated numbers of MSCs were noted in the peripheral blood of patients immediately following traumatic hip injury [101]. However, the origin(s) of the mobilized cells remains unclear and it has proved extremely difficult to establish MSC cultures from conventional blood either in physiological conditions or following stimulation with cytokines [25, 102, 103].
Conversely, the ability to derive apparently identical MSCs from multiple tissues led to the hypothesis that these cells share a common in vivo location. A growing body of published reports has described perivascular cells that appear indistinguishable from vascular pericytes as a possible source of MSCs [15, 27, 43, 75], a situation that would explain why MSCs can be isolated from all vascularized organs. Association of these mesenchymal progenitor cells with the vasculature would allow them to function as a source of new cells for physiological turnover and for the repair or regeneration of local lesions. The establishment of MSC-like cultures from blood vessels alone supports this hypothesis [9]. Lineage tracing studies have confirmed that vascular pericytes do contribute to regeneration of bone following tooth injury although other populations of cells are also involved [44]. More recently, another subset of vascular cells, namely adventitial cells, have been identified that may behave in a similar manner to pericytes [135].
Pericytes at the origin of MSCs
Recent results have acknowledged the regenerative potential, under certain conditions, of a subset population residing in the wall of blood vessels [45, 46]. Pericytes have been recognized as a distinct cellular entity that share a common immunophenotype and differentiation potential to mesenchymal stem/progenitor cells [26]. In a variety of human organs, perivascular mesenchymal progenitor cells can be identified by a combination of perivascular (CD146, NG2, PDGFRβ) and MSC (CD29, CD44, CD73, CD90, CD105, alkaline phosphatase) markers, as well as lack of hemato-endothelial cell markers [CD31, CD34, CD45, CD144, von Willebrand factor (vWF)] expression. Pericytes have been shown to differentiate into multiple mesodermal lineages including bone [104, 105], fat [106], cartilage [27], and skeletal muscle [107, 108]. The T-lymphocyte surveillance shut-down effects observed with culture-expanded bone marrow-derived MSCs have also been reported in studies evaluating pericytes [109, 110].
Tottey et al. [111] demonstrated that perivascular cells isolated from human fetal muscle proliferate at a higher rate under hypoxic conditions (6 %) than normoxia (21 %) and that they migrate more rapidly when exposed to degraded ECM products. This indicates some degree of activation in the presence of injury. Perivascular cells can release various cytokines, including basic-fibroblast growth factor (b-FGF), a well-known chemotactic and mitogenic agent and vascular endothelial growth factor (VEGF), a regulator of angiogenesis, which can also participate in tumor progression [112].
Pericytes participate in vivo in the development, renewal, and repair of several distinct tissues
Most interestingly, there is now increasing evidence that pericytes—aka mural cells—can play a natural role as progenitor cells in development and in various injured tissues. Perivascular cells represent a ubiquitous cell population, distinct from tissue-specific stem cells such as brain neural stem cells [113], hepatic stellate cells [114], supra adventitial-adipose stromal cells [106], and myogenic satellite cells [115]. However, Leydig cells in the rat testis were suggested to be regenerated by pericytes following chemical injury [116] and mural cells were proposed as normal progenitors of white adipocytes in murine fat tissue [117]. It has also been demonstrated that pericytes resident in postnatal skeletal muscle differentiate into muscle fibers and generate satellite cells following chemical damage to the muscle [118]. Most recently, direct differentiation of pericytes into follicular dendritic cells was documented in the mouse [119]. More generally, pericytes appear to give rise in situ to multiple mesodermal derivatives [120], in response notably to PDGFRβ signaling [121]. Therefore it appears that the ability of pericytes to yield MSCs in culture mirrors an intrinsic broad developmental potential of these cells, or at least of subsets thereof.
Pericytes and the bone marrow hematopoietic niche
Although hematopoietic stem cells were originally localized in the endosteal regions of bone marrow, recent findings suggested the existence of a distinct perivascular niche in which pericytes support HSC stemness. Perivascular reticular cells expressing CXCL12 were found to play a role in murine HSC maintenance [122]. Mendez-Ferrer et al. [123] demonstrated the existence in mouse bone marrow of perivascular nestin+ MSCs associated with HSCs. Ablation of these nestin+ MSCs led to a significant reduction in the number and homing ability of HSCs. Furthermore, a direct role for perivascular cells in hematopoiesis regulation was recently confirmed by Ding et al. [124] in a stem cell factor (SCF) knock-in mouse model. Here, selective shut-off of c-kit ligand expression in leptin receptor (Lep-R)-positive cells surrounding bone marrow blood vessels significantly reduced the frequency of long-term reconstituting hematopoietic stem cells. More recently, Corselli et al. [125] demonstrated that human bone marrow and adipose tissue-resident pericytes express in vivo nestin, CXCL12, and Lep-R, and support the ex vivo maintenance of human HSCs. Furthermore, it was found that pericytes support HSCs through direct contact and Notch/Jagged1 signaling. Conversely, conventional unfractionated MSCs did not maintain HSC stemness, favoring differentiation. Pericytes can therefore be considered as the bona fide human equivalents of the hematopoietic perivascular niche components recently described in the mouse.
Nonpericyte perivascular cells as MSC ancestors
Multipotent progenitors displaying MSC phenotypic and developmental properties have also been described in the bovine artery wall [126] and have recently been isolated from the tunica adventitia of the human pulmonary artery [127]. The tunica adventitia was long considered an inactive component of blood vessels mainly functioning as structural support for the tunica media. Only recently has it been demonstrated that the adventitia plays a crucial role in vascular remodeling and the development of vascular diseases including arteriosclerosis and restenosis [128]. Activation of adventitial cells has been described in response to physical stressors including injury [129], vein grafting [130], hypoxia [131], and hypertension [132]. In these settings, adventitial cells may differentiate into myofibroblasts that migrate into the inner layers of the vascular wall, alter extracellular matrix deposition, and release paracrine factors regulating vascular remodeling [133]. In apoE−/− mice, Hu et al. [130] identified and isolated Sca1+ adventitial progenitor cells that are able to differentiate in vitro and in vivo into smooth muscle cells. Following transplantation of Sca1+ βgal- cells carrying the LacZ gene under the control of the smooth muscle-specific promoter SM22 into the adventitia of murine vein grafts, the authors observed the presence of β-gal+ smooth muscle cells in the neointima up to 4 weeks after grafting. This indicates the contribution of adventitial progenitors in the progression of vascular diseases. It has subsequently been demonstrated that the differentiation potential of adventitial cells is not restricted to myofibroblasts. These observations suggest, indirectly, that pericytes exclusively present around capillaries and microvessels are not the only ancestors of MSCs, as hypothesized previously [134].
Along a systematic search by flow cytometry purification for alternative non-pericyte cells at the origin of MSCs, Corselli et al. identified a subset of CD34+ CD45− CD56− CD146− NG2− cells in the tunica adventitia of human arteries and veins [135]. These adventitial cells grew like MSCs in culture and exhibited typical MSC differentiation properties. Interestingly, adventitial progenitor cells express natively the MSC markers CD44, CD73, CD90, and CD105. No potential to give rise to MSCs in culture was detected outside the perivascular subsets including pericytes and adventitial cells.
Although pericytes and adventitial perivascular cells have been described for more than a century, it is only recently that the blood vessel wall was demonstrated as a reservoir of progenitor cells. We showed that perivascular cells, i.e., pericytes and adventitial cells, are in vivo counterparts of MSCs obtained in culture from various organs [27, 135]. These perivascular cells can be prospectively purified by flow cytometry using a well-defined surface marker combination, common in all human organs tested. Importantly, pericytes and adventitial cells dissociated from vessel walls contain multipotent precursors with robust regeneration properties similar to those of classic heterogeneous MSCs.
Mesenchymal stem cells from umbilical cord blood: do pericytes enter the blood circulation?
Over the last years, the clinical use of allogeneic umbilical CB for hematopoietic cell transplantation has increased dramatically. In comparison with other stem cell sources, well-characterized CB grafts are immediately available in numerous CB banks worldwide [136, 137]. Despite publication of many relevant reports over the past 10 years, controversy still exists as to whether MSCs or their forerunners are present in human CB. Clearly though, non-hematopoietic cells contributing to tissue repair circulate in fetal blood at term, as recently reported by some of us [138–140] and confirmed by others [141].
Even though cells present in CB possess overlapping features with MSCs derived from usual sources, some particularities have fed the debate on their very nature. One of the differences between regular MSCs and CB-derived cells is the isolation rate, which varies considerably between investigators [138, 142, 143]. In addition, the growth of the latter can be markedly delayed, up to 20 days from the seeding.
Moreover, at least two different cell population kinetics can be described within CB MSC like cells, endowed with short-term (a few passages) or long-term (more than ten passages) expansion ability, characterized by different growth curves with lower or higher cumulative population doublings (CPD). There is no consensus either regarding the nomenclature of these multipotent cells, diversely named USSCs [68, 69, 144], multilineage progenitor cells (MLPCs) [145], embryonic-like stem cells [146], very small embryonic-like stem cells (VSELs) [70], or more simply CB MSCs.
The morphology of CB-derived stromal cells is quite similar to that of bone marrow MSCs, even though CB stromal cells are smaller and less spindle-shaped, with a higher nucleus/cytoplasm ratio. Over the long term, these cells remain healthy, homogenous, and non-senescent till numerous passages, and reach higher CPD than BM MSCs.
Flow cytometry analysis showed that these cells are negative for lineage markers such as CD45 and CD34, but positive for human MSC markers such as CD90, CD105, and CD73, as well as other integrins and matrix receptors, defining an immunophenotype consistent with that of BM-derived MSCs [147]. The ability of CB stromal cells to differentiate into osteoblasts that produce mineralized matrices and chondrocytes that produce type-II collagen has been confirmed by several authors [148]. Regarding adipogenic differentiation, some authors report very poor potential [141] and others a complete absence [149]. Some authors claimed to distinguish short- and long-term CB stromal stem cells by their adipogenic differentiation potential and delta-like 1 (DLK-1) expression profile [69], but others did not confirm these differences [141]. Worthy of consideration is the fact that the proliferation and differentiation abilities of customary MSCs may decrease with donor age, while the MSC-like cells contained in CB can be considered as, by essence, very young. Therefore, a considerable volume of work has already been carried out investigating the use of CB stromal stem cells in animal tissue regeneration, including kidney and lung repair [138, 140]. Clinical trials are already going on (see http://www.clinicaltrials.gov) to evaluate the safety and efficacy of CB-derived MSCs to promote hematopoietic stem cell engraftment and prevent graft-versus-host disease. Clinical applications are also contemplated in patients affected by focal glomerulosclerosis and preterm newborns with bronchopulmonary dysplasia.
Nothing is known regarding the origin of CB MSCs and the possible affiliation thereof with perivascular cells. Some authors have speculated that short- and long-term CB MSCs are released from fetal bone marrow or liver into the blood circulation [69]. Considering the physical trauma inflicted to the placenta and umbilical cord at birth and during blood collection, it remains possible that perivascular cells have simply been released mechanically from severed blood vessels, their presence in blood being of no physiological relevance.
Developmental origins of MSCs
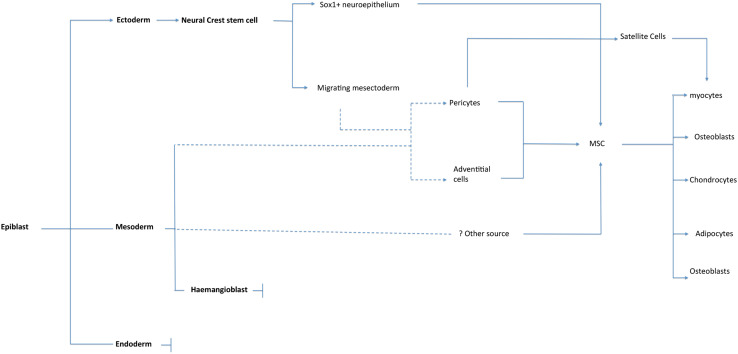
There have been few basic studies on the emergence of MSCs and their developmental origins remain an area of considerable ongoing mystery. However, the limited available data do suggest that MSCs derive from multiple developmental origins [150–152] (Fig. 1). The mesoderm is the primary source of mesenchymal cells giving rise to skeletal and connective tissues [153]. Pericytes that develop around the developing trunk vessels in the axial and lateral plate areas are thought to derive from mesoderm [154]. Coronary vessel mural cells may derive from epicardial cells, which are themselves derived from the splanchnic mesoderm [155]. An early Flk1+ mesodermal precursor, with the potential to differentiate into endothelial cells, blood, muscle, and mesenchymal lineage cells (bone and cartilage), was identified in the E9.5 mouse dorsal aorta [156]. Evaluation of osteogenic, chondrogenic, and adipogenic potentials of cells isolated from different anatomical sites in the E11.5 mouse embryo revealed intraembryonic hematopoietic tissues [aorta-gonad-mesonephros (AGM)] as a site of origin for cells with mesenchymal differentiation potential [157]. However, immediate mesodermal precursors that give rise to expandable multipotential MSC lines are not identified and characterized.
Fig. 1.
Schematic illustrating the known developmental and anatomical origins of MSCs. Dashed lines indicate where associations are inferred
Studies in the mouse embryo demonstrated the origin of some MSCs from neural crest [158, 159]. A recent study showed that the earliest lineage providing MSC-like cells during embryonic trunk development is generated from Sox1(+) neuroepithelium, at least in part through a neural crest intermediate stage [150]. These early MSCs are then replaced, later in development, by MSCs from other origins. As embryogenesis progresses, the neural crest cells can be classified in different series, with respect to the territory they colonize: (1) the cranial neural crest, (2) the cardiac neural crest, (3) the trunk neural crest, and (4) the vagal and sacral neural crest. Terminal differentiation of these cells gives rise to neural as well as mesenchymal tissues. In the peripheral nervous system, these cells contribute to neurons and glia of sensory, sympathetic and parasympathetic systems, and the neural plexuses within specific tissues and organs. Neural crest cells migrating to the pharyngeal arches form a mixed tissue type known as “mesectoderm” or “ectomesenchyme” to distinguish them from mesenchymal cells derived from the mesoderm [152].
Similarly, using interspecies transplantation of quail and chick embryonic tissues, the lineage of cephalic pericytes was traced to neuroectodermal tissue [160]. The vasculature of the avian embryo exhibits a mosaic pattern where neural crest- and mesoderm-derived pericytes and smooth muscle cells occupy sharply delineated and mutually exclusive regions in the face and brain [161]. Evidence that these neural crest-derived cells persist in some adult tissues has come from several studies [162]. The neural lineage potential of neural crest-derived MSCs is preserved in adult rats and can be reactivated when they form mesenspheres, express various neural crest cell markers, and undergo differentiation, in vitro, into neuron- and glia-like cells [163–166]. While the gene expression profiles of neural crest cells and MSCs are unique [167], there is evidence that cultivation of neural crest cells in the presence of serum evokes an MSC-like phenotype. For example, human embryonic stem cells differentiated in vitro toward a neural crest cell phenotype can differentiate into neurons and glia when cultured under serum-free conditions, while cultivation in medium containing serum evokes an MSC-like phenotype capable of differentiation to mesodermal cells [168].
Perhaps relevant to these observations, it has been recently demonstrated that neural crest-derived cells migrate to the bone marrow through the bloodstream [169]. These cells are still present in the adult bone marrow, and can differentiate in vitro into neurons, glial cells, and myofibroblasts. The potential link, if any, between these cells, the cells identified by Takashima et al. [150] and conventional MSCs isolated according to Friedenstein’s protocol [4] remains unclear.
It has also been reported that endothelial progenitors and mural cells may derive from a common vascular progenitor [170]. Lineage-tracing experiments have shown that cells originated from the primary vascular plexus also give rise to mural cells with the capability to differentiate into osteoblasts and adipocytes [171]. These findings may suggest that MSCs, HSCs, and endothelium progenitor cells (EPCs) arise from a common progenitor.
The mixed developmental origins of pericytes, and thus MSCs, may reflect the finding that a core set of expressed genes governs the general hallmarks of MSCs [172]. Regulation of this battery of genes may allow cells originating from different germ layers to undergo mesenchymal “activation”. As such, the ontogeny of MSCs is different from that of other specialized cell types, for which the traditionally held view is that they undergo differentiation along a linear path, requiring successive specifications of progenitor cells paralleled by progressive restrictions in potency.
There is a need to define developmentally distinct MSC subsets and the hierarchy of their progenitors to advance our understanding of heterogeneity within MSCs and its implications for the developmental and therapeutic potential of these cells.
Therapeutic significance of understanding the native anatomical origins of MSCs
The multipotency as well as the trophic and immunoregulatory effects of MSCs have vast potential clinical applications, with many treatments already in the stages of clinical trials (305 registered on clinicaltrials.gov at the time of writing) (Table 4). However, conventional unpurified MSC preparations have significant drawbacks including contamination from non-MSC populations and the requirement of in vitro culture to enrich the MSC population. Approaches to delivering cell-based therapies are increasingly being guided by regulatory frameworks. Within these frameworks, cells that require in vitro manipulation or culture must undergo stringent safety trials prior to approval for clinical use while cells that can be directly implanted bypass much of this legislation. Many of these drawbacks can be addressed with the ability to identify and isolate pure populations of MSC precursors as perivascular cells using FACS. The practical and therapeutic consequences of understanding the identity and anatomical origin of native MSCs have therefore been considerable.
Table 4.
Partial list of potential clinical applications of MSCs
| Bone regeneration |
| Skeletal defect healinga [271] |
| Osteoporosis [201, 202] |
| Osteogenesis imperfecta [272] |
| Cartilage regeneration |
| Cartilage defect healinga [271] |
| Meniscus injury [273] |
| Osteoarthritisa [276] |
| Muscle regeneration |
| Skeletal muscle regenerationa [279] |
| Cardiac muscle regenerationa [214] |
| Smooth muscle regeneration [277] |
| Tendon regeneration |
| Repair of tendon defectsa [278] |
| Neural regeneration and injury prevention |
| Traumatic brain injury [296] |
| Spinal cord injurya [297] |
| Multiple sclerosisa [298] |
| Parkinson’s diseasea [299] |
| Multiple system atrophya [300] |
| Ischemic strokea [280] |
| Prevention of injury in acute ischemia |
| Limb ischemiaa [281, 282] |
| Acute lung injurya [283] |
| Myocardial infarctiona [284] |
| Acute kidney injurya [285, 286] |
| Other immunomodulatory applications |
| Diabetes, type Ia [287] |
| Sepsisa [301] |
| Acute lung injurya [302] |
| Rheumatoid arthritisa [274, 275] |
| Hepatic cirrhosis [288–290] |
| GVHDa [291, 292] |
| Other |
| Renal failurea [303] |
| Skin graftinga [293] |
| Urinary incontinencea [294] |
aPhase 1 trials started for this application
Perivascular stem cells (PSCs) can be sorted to purity
Whole bone marrow cell suspensions and the stromal vascular fraction (SVF) of adipose tissue have been used directly with the aim of harnessing the potential of the contained stem cells. However, both represent highly heterogeneous cell populations, which include non-mesenchymal stem cell types, such as inflammatory cells, hematopoietic cells, endothelial cells, and non-viable cells, among others [173]. Available studies using SVF show poor and unreliable tissue formation [174], or lower tissue regeneration efficacy relative to cultured MSCs [175]. In fact, recent studies have suggested that the presence of endothelial cells has inhibiting effects on bone differentiation, among other lineages [176, 177]. Despite the process of enrichment through plastic adherence, it is inevitable that preparations will be contaminated by non-MSC populations, and the contribution of each contained population to the repair process cannot be definitively established. However, it is likely that subsets of functionally distinct cells exist even within purified populations of PSCs and MSCs. Identification of MSC subsets with the most desirable characteristics for clinical applications is likely to be a major focus of future research. Finally, variability in cell composition presents clear disadvantages for regulatory body [for example the Food and Drug Administration (FDA)] approval of a future stem cell-based therapy, potentially including reduced safety, purity, identity, potency, and efficacy. With these regulatory hurdles in mind the use of purified MSCs, i.e., pericytes and adventitial cells, collectively designated as PSCs, has clear practical advantages.
PSCs do not require in vitro selection
The selection and preparation of MSCs through adherence to culture plastic is time consuming, and introduces additional risks such as immunogenicity and infection through exposure to animal-derived culture products. Investigators have documented the influence of MSC culture on genetic instability [178], and tumorigenicity [179, 180], although these results have been challenged [181]. Multipotency, and hence therapeutic potency, has been shown to diminish with serial passaging, with human BM MSCs progressively losing their adipogenic and chondrogenic differentiation potentials as the number of cell divisions increases [86]. In addition, expression of adhesion molecules and chemokines, and the ability to respond to chemokines decline with time in culture [86].
PSCs can be isolated in sufficient numbers to negate ex vivo expansion
In addition to the advantages of negating the need for in vitro selection of MSCs, the ability to isolate PSCs from adipose tissue in clinically relevant numbers has significant therapeutic implications. Low stem cell numbers and high donor site morbidity limit the use of fresh autologous bone marrow [179, 182], periosteum [183], and the majority of other MSC sources. Adipose tissue represents a largely dispensable source of MSCs that are readily accessible through lipoaspiration, even in patients of healthy weight [184]. It has attracted much attention as a potentially plentiful source of MSCs, particularly using uncultured cells (SVF or PSCs) but also with cells following in vitro expansion. Relative to the lower yield, limited donor sites, and high morbidity associated with bone marrow or periosteal harvest, adipose tissue is now a well-documented, easily accessible, abundant source of such cells. James et al. [105] reported the yields from lipoaspirates isolated from 60 consecutive donors in cosmetic procedures. From 100 ml of whole lipoaspirate, the mean yield of total nucleated cells (SVF) was 39.4 × 106 (range, 10 × 106–70 × 106). On FACS sorting pericytes most frequently represented 30 % or less of total SVF (mean 19.5 %) with adventitial cells representing 40 % or less (mean, 23.8 %) of total SVF. When added in combination, the total PSC content most commonly fell between 30 and 60 % of total viable SVF (mean, 43.2 %, median, 41.7 %). Given this prevalence of PSCs, it has been estimated that <200 ml of lipoaspirate would be sufficient starting material for the clinical application of PSCs in localized bone repair. For example, 200 ml of lipoaspirate would theoretically yield 31 million cells, which would be sufficient for healing of a 2-cm mid-diaphyseal femoral defect (cell seeding density of 1 million per 0.4 ml) [105, 185]. In cases where there is a requirement for an extremely large number of cells (for example GVHD where 1–2 million cells/kg body weight may be required for infusion) or where the availability of fat for lipoaspirate is limited, some expansion in culture would inevitably be required.
In addition to the requirement for robust trials to demonstrate safety and efficacy of PSCs for tissue regeneration, a number of practical challenges must also be overcome before widespread clinical application of this technology. There are currently few flow sorting facilities with formal accreditation from the relevant regulatory bodies to produce clinical-grade cells. The financial costs of clinical-grade sorting, taking into account the price of the antibodies (also certified for clinical purposes) is high. However, this may be offset by savings made by the lack of requirement for expansion in culture. The use of an automated clinical-grade immunodepletion system has been proposed as a more affordable alternative for bulk sorting to FACS. However, the complex phenotype of PSCs, and the requirement for both positive and negative selections render this impractical.
In summary, the therapeutic consequences of understanding the native identity of MSCs are considerable. MSCs can now be prospectively purified to homogeneity based on expressed cell surface markers from adipose tissue in quantities large enough to avoid ex vivo expansion with its associated risks and disadvantages. Sorting of MSC precursors in this way addresses many of the issues that currently limit the translation of MSC-related therapies including the availability, purity, and potency of progenitors isolated through conventional culture methods, and the poor regenerative efficiency of SVF. The high numbers of MSC precursors that can be sorted from adipose tissue reduce the delays associated with expansion and may prevent exposure of patients to multiple anesthetic procedures while widening possible applications to include trauma where a time delay between extraction and implantation excludes their use. Furthermore, the high degree of MSC purity and potency will greatly facilitate demonstration of the product safety and efficacy required for regulatory body approval.
Conventionally derived MSCs and purified PSCs–emerging pre-clinical and clinical data
There is a rapidly expanding body of pre-clinical data evaluating the potential therapeutic benefits of exogenous MSCs. Both autologous and allogeneic MSCs isolated from multiple sources have been injected into tissue such as the heart or infused within the bloodstream and have been observed to localize to sites of injury involving damaged or inflamed blood vessels. The list of MSC-related applications includes a broad and diverse range of clinical targets and indications including graft-versus-host disease, stroke, acute myocardial infarction (MI), spinal cord lesions, acute kidney failure, liver fibrosis, multiple sclerosis (MS), amyotrophic lateral sclerosis, tendinitis, burns and wound healing, bone regeneration, juvenile diabetes, lupus, autism, inflammatory bowel disease, urinary incontinence, and sepsis [51]. Almost all of these trials and preclinical models utilize conventionally derived MSCs for their immunomodulatory or trophic effects rather than their ability to differentiate in different cell lineages. The limited emerging data from animal studies confirm that PSCs are at least as effective as conventionally derived MSCs in terms of clinical effect [28, 185]. It is expected that the added benefits of prospective isolation and the avoidance of culture will enable these treatments to become more accessible to patients from a wider range of conditions. Here we summarize the results of pre-clinical studies evaluating MSCs in bone healing and cardiac regeneration and where available compare results of studies using PSCs.
Conventionally derived MSCs and PSCs in bone healing
Osseous defects and other diseases of bone have been treated with success in preclinical studies using conventionally derived MSCs. Multiple investigators have shown the efficacy and feasibility of either allogeneic or autologous MSC-based implants to heal large osseous defects [186–188], including critical-sized defects of the appendicular and calvarial skeleton in mouse [189–191], rat [192–194], dog [195, 196], and sheep [197–199] models among others [200]. Notably, the majority of successful MSC-based healings of skeletal defects have been reported using pre-differentiated cells, although studies have also determined that pre-differentiation is not an absolute requirement. The success in MSC-mediated regeneration of osseous defects led some investigators to look into autologous MSC-based treatments of osteoporosis [201, 202]. Several in vivo studies demonstrated that transplantation of pre-differentiated autologous MSCs strengthened osteoporotic bone in ovariectomized (OVX) animal models, including rabbit [203] and rat [204]. Furthermore, direct intra-osseous injection of differentiated allogeneic MSCs showed potential in the improvement of osteoporotic trabecular bone in terms of increased osteogenic differentiation as well as collagen synthesis [204]. Finally, genetic manipulation of autologous MSCs has been investigated, utilizing MSCs as a vehicle for gene therapeutics [75]. Most prominently, the bone morphogenetic protein family, including bone morphogenetic protein (BMP)-2, BMP-4, BMP-6, and BMP-7, have been investigated for their bone-forming effects and ability to direct MSCs towards an osteogenic lineage [205]. BMPs are involved in various developmental processes including embryogenesis, skeletal formation, hematopoiesis, and neurogenesis, and belong to the transforming growth factor-β superfamily [206, 207]. For instance, MSCs transduced with BMP-2 and seeded onto a coral-hydroxyapatite scaffold led to successful healing of large mandibular defects in OVX rats [208]. In osteoporotic mice and sheep, MSCs transduced with BMP-2 were shown to increase bone regeneration and improve fracture healing [205, 209, 210]. BMP-4, BMP-7, and BMP-9 gene transduction of MSCs have yielded similar results to BMP-2 ex vivo [211, 212].
Non-cultured adipose-derived PSCs show an enhanced ability to repair bone defects when compared to unsorted SVF. In addition, cells lose their osteogenic potential with increasing passage [185]. In a murine gluteo-femoral muscle pocket model, James et al. [185, 213] reported that PSCs undergo osteogenic differentiation in vitro and form bone after intramuscular implantation without the need for pre-differentiation. Patient-matched, purified PSCs formed significantly more bone in comparison with traditionally derived SVF by all parameters tested. Recombinant BMP-2 increased in vivo bone formation but with a massive adipogenic response. In contrast, recombinant Nel-like molecule 1 (NELL-1: a novel osteoinductive growth factor) selectively enhanced bone formation.
Conventionally derived MSCs and PSCs in cardiac regeneration
In the case of cardiac muscle, various studies have shown improved cardiac function following the delivery of MSCs in animals, either via direct injection or intravenous administration [214, 215]. Although not as effective as cardiac stem cells (CSCs) [216], MSCs serve to reduce fibrosis, contractile strain alterations, and cardiomyocyte apoptosis while upregulating angiogenesis through secretion of multiple paracrine factors [214, 217, 218]. In the case of cardiac therapy, MSCs function primarily through the promotion of trophic responses in myocytes, and the elaboration of HGF, IGF-II, and VEGF, which contribute to the cardiac repair mechanism through enhancement of cell survival and angiogenesis [219–221]. Interestingly, allogeneic MSCs retain neither their immunoprivilege nor functional efficacy late after myocardial implantation [222]. Various studies have explored factors to optimize MSC efficacy, which include selection for MSC overexpressing regulators of cardiogenesis [223], use of MSCs induced via co-culture with cardiomyocytes [224], and exogenous expression of VEGF to recruit CSCs [225]. In summary, preclinical studies have demonstrated efficacy in the use of MSCs for cardiac diseases, primarily through a trophic effect on surrounding cells, and to a lesser extent through direct myogenic differentiation.
A recent study has reported long-term improvement in cardiac function following direct implantation of pericytes using a mouse myocardial infarction model [226]. In this study, the transplantation of saphenous vein pericytes (SVPs) into the peri-infarct zone of immunodeficient CD1/Foxn-1(nu/nu) or immunocompetent CD1 mice attenuated left ventricular dilatation and improved ejection fraction compared to control. Moreover, pericytes reduced myocardial scar, cardiomyocyte apoptosis, and interstitial fibrosis, improved myocardial blood flow and neovascularization, and attenuated vascular permeability. The authors demonstrated that pericytes secrete VEGF-A, angiopoietin-1, and chemokines and induce an endogenous angiocrine response by the host, through recruitment of VEGF-B expressing monocytes. The association of donor- and recipient-derived stimuli activates the proangiogenic and prosurvival Akt/eNOS/Bcl-2 signaling pathway. Moreover, microRNA-132 (miR-132) was constitutively expressed and secreted by SVPs and remarkably upregulated, together with its transcriptional activator cyclic AMP response element-binding protein, on stimulation by hypoxia/starvation or VEGF-B. In vitro, SVP conditioned medium stimulates endothelial tube formation and reduces myofibroblast differentiation through inhibition of Ras-GTPase activating protein and methyl-CpG-binding protein 2, which are validated miR-132 targets. Furthermore, miR-132 inhibition by antimiR-132 decreased SVP capacity to improve contractility, reparative angiogenesis, and interstitial fibrosis in infarcted hearts.
In a separate study, human skeletal muscle-derived pericytes were significantly better than myogenic progenitors at treating ischemic heart disease and mediating associated repair mechanisms in a murine model of myocardial infarction [227]. Echocardiography revealed that pericyte transplantation attenuated left ventricular dilatation and significantly improved cardiac contractility, being superior to CD56+ myogenic progenitor transplantation in acutely infarcted mouse hearts. Pericyte treatment substantially reduced myocardial fibrosis and significantly diminished infiltration of host inflammatory cells at the infarct site. Hypoxic pericyte-conditioned medium suppressed murine fibroblast proliferation and inhibited macrophage proliferation in vitro. High expression by pericytes of immunoregulatory molecules, including interleukin-6, leukemia inhibitory factor, cyclooxygenase-2, and heme oxygenase-1, was sustained under hypoxia, except for monocyte chemotactic protein-1. Host angiogenesis was significantly increased. Pericytes supported microvascular structures in vivo and formed capillary-like networks with or without endothelial cells in three-dimensional co-cultures. Under hypoxia, pericytes dramatically increased expression of VEGF-A, platelet-derived growth factor-β, transforming growth factor-β1 and corresponding receptors while expression of basic fibroblast growth factor, hepatocyte growth factor, epidermal growth factor, and angiopoietin-1 was repressed. The capacity of pericytes to differentiate into and/or fuse with cardiac cells was revealed by green fluorescence protein labeling, although to a minor extent.
Conclusions
The recent discovery that MSCs derive from a perivascular location where they reside as pericytes or adventitial cells has generated some momentum in the field of adult stem cell research and provided some insight into the developmental origins of these much exploited but little understood cells. It is now evident that the perivasculature represents an MSC niche in vivo, where local cues coordinate the transition to progenitor and mature cell phenotypes. Here, MSCs can stabilize blood vessels and contribute to tissue and immune system homeostasis under physiological conditions and assume a more active role in tissue repair in response to injury. The establishment of a perivascular compartment as the MSC niche provides a basis for the rational design of additional in vivo therapeutic approaches.
Disclosure
B.P., and C.S. are inventors of perivascular stem cell-related patents filed from UCLA. Dr C.S. is a founder of Scarless Laboratories Inc. which sublicenses perivascular stem cell-related patents from the UC Regents, and who also hold equity in the company. Dr C.S. is also an officer of Scarless Laboratories, Inc. This work was supported by the CIRM Early Translational II Research Award TR2-01821.
Abbreviations
- AGM
Aorta-gonad-mesonephros
- BGP
β-glycerophosphate
- BM
Bone marrow
- BMP
Bone morphogenetic protein
- CB
Cord blood
- CD
Cluster of differentiation
- CFU
Colony-forming unit
- CPD
Cumulative population doubling
- CSC
Cardiac stem cell
- DLK-1
Delta-like 1
- ECM
Extracellular matrix
- EPC
Endothelial progenitor cell
- FACS
Fluorescence-activated cell sorting
- FDA
Food and Drug Administration
- HGF
Hepatocyte growth factor
- HLADR
Human leukocyte antigen-DR
- HSC
Hematopoietic stem cell
- IBMX
3-isobutyl-1-methylxanthine
- IGF
Insulin-like growth factor
- ISCT
International Society for Cellular Therapy
- Lep-R
Leptin receptor
- mAbs
Monoclonal antibodies
- MAPC
Multipotent adult progenitor cell
- MASC
Multipotent adult stem cell
- MCAM
Melanoma cell adhesion molecule
- MI
Myocardial infarction
- MIAMI
Marrow-isolated adult multilineage inducible cell
- MLPC
Multilineage progenitor cell
- MSC
Mesenchymal stem cell
- NELL1
Nel-like molecule 1
- OVX
Ovariectomized
- PDGFRβ
Platelet-derived growth factor receptor β
- PSC
Perivascular stem cell
- SCF
Stem cell factor
- SVF
Stromal vascular fraction
- SVP
Saphenous vein pericyte
- USSC
Unrestricted somatic stem cell
- VCAM
Vascular cell adhesion molecule
- VEGF
Vascular endothelial growth factor
- VESL
Very small embryonic-like stem cell
- vWF
von Willebrand factor
Footnotes
C. C. West, W. R. Hardy, A. W. James, and T. S. Park contributed equally to this work.
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–390. [PubMed] [Google Scholar]
- 5.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 6.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9(1):204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Flores L, Gutierrez R, Gonzalez P, et al. Inducible perivascular cells contribute to the neochondrogenesis in grafted perichondrium. Anat Rec. 1991;229(1):1–8. doi: 10.1002/ar.1092290102. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Flores L, Gutierrez R, Lopez-Alonso A, et al. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res. 1992;275:280–286. [PubMed] [Google Scholar]
- 12.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 13.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Malladi P, Wagner DR, et al. Adipose-derived mesenchymal cells as a potential cell source for skeletal regeneration. Curr Opin Mol Ther. 2005;7(4):300–305. [PubMed] [Google Scholar]
- 15.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 16.Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 17.Salingcarnboriboon R, Yoshitake H, Tsuji K, et al. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res. 2003;287(2):289–300. doi: 10.1016/s0014-4827(03)00107-1. [DOI] [PubMed] [Google Scholar]
- 18.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 19.Rogers I, Casper RF. Umbilical cord blood stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18(6):893–908. doi: 10.1016/j.bpobgyn.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Toma JG, Akhavan M, Fernandes KJ, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3(9):778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 21.Igura K, Zhang X, Takahashi K, et al. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004;6(6):543–553. doi: 10.1080/14653240410005366-1. [DOI] [PubMed] [Google Scholar]
- 22.Tsai MS, Lee JL, Chang YJ, et al. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19(6):1450–1456. doi: 10.1093/humrep/deh279. [DOI] [PubMed] [Google Scholar]
- 23.De Bari C, Dell’Accio F, Tylzanowski P, et al. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68(4–5):245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 25.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 26.Covas DT, Panepucci RA, Fontes AM, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36(5):642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Crisan M. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Chen CW, Montelatici E, Crisan M, et al. Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev. 2009;20(5–6):429–434. doi: 10.1016/j.cytogfr.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Tsutsumi S, Shimazu A, Miyazaki K, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288(2):413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- 30.Kulterer B, Friedl G, Jandrositz A, et al. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomics. 2007;8:70. doi: 10.1186/1471-2164-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pochampally RR, Smith JR, Ylostalo J, et al. Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood. 2004;103(5):1647–1652. doi: 10.1182/blood-2003-06-1967. [DOI] [PubMed] [Google Scholar]
- 32.Hishikawa K, Miura S, Marumo T, et al. Gene expression profile of human mesenchymal stem cells during osteogenesis in three-dimensional thermoreversible gelation polymer. Biochem Biophys Res Commun. 2004;317(4):1103–1107. doi: 10.1016/j.bbrc.2004.03.165. [DOI] [PubMed] [Google Scholar]
- 33.Kratchmarova I, Blagoev B, Haack-Sorensen M, et al. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308(5727):1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 34.Song L, Webb NE, Song Y, et al. Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells. 2006;24(7):1707–1718. doi: 10.1634/stemcells.2005-0604. [DOI] [PubMed] [Google Scholar]
- 35.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 36.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103(5):1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 37.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 38.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78(1):55–62. [PubMed] [Google Scholar]
- 39.Dennis JE, Carbillet JP, Caplan AI, et al. The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs. 2002;170(2–3):73–82. doi: 10.1159/000046182. [DOI] [PubMed] [Google Scholar]
- 40.Devine SM, Bartholomew AM, Mahmud N, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29(2):244–255. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 41.In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102(4):1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 42.Bensidhoum M, Chapel A, Francois S, et al. Homing of in vitro expanded Stro-1− or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood. 2004;103(9):3313–3319. doi: 10.1182/blood-2003-04-1121. [DOI] [PubMed] [Google Scholar]
- 43.Farrington-Rock C, Crofts NJ, Doherty MJ, et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110(15):2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 44.Feng J, Mantesso A, De Bari C, et al. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci USA. 2011;108(16):6503–6508. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sims DE. The pericyte: a review. Tissue Cell. 1986;18(2):153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 46.Diaz-Flores L, Martin Herrera AI, Garcia Montelongo R, et al. Role of pericytes and endothelial cells in tissue repair and related pathological processes. J Cutan Pathol. 1990;17(3):191–192. doi: 10.1111/j.1600-0560.1990.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 47.Savvatis K, van Linthout S, Miteva K, et al. Mesenchymal stromal cells but not cardiac fibroblasts exert beneficial systemic immunomodulatory effects in experimental myocarditis. Plos One. 2012;7(7):e41047. doi: 10.1371/journal.pone.0041047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jia Z, Jiao C, Zhao S, et al. Immunomodulatory effects of mesenchymal stem cells in a rat corneal allograft rejection model. Exp Eye Res. 2012;102:44–49. doi: 10.1016/j.exer.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 50.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 51.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 53.Jui HY, Lin CH, Hsu WT, et al. Autologous mesenchymal stem cells prevent transplant arteriosclerosis by enhancing local expression of interleukin-10, interferon-gamma, and indoleamine 2,3-dioxygenase. Cell Transplant. 2012;21(5):971–984. doi: 10.3727/096368911X627525. [DOI] [PubMed] [Google Scholar]
- 54.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 55.Kuo YR, Chen CC, Shih HS, et al. Prolongation of composite tissue allotransplant survival by treatment with bone marrow mesenchymal stem cells is correlated with T-cell regulation in a swine hind-limb model. Plast Reconstr Surg. 2011;127(2):569–579. doi: 10.1097/PRS.0b013e318200a92c. [DOI] [PubMed] [Google Scholar]
- 56.Ikeguchi R, Sacks JM, Unadkat JV, et al. Long-term survival of limb allografts induced by pharmacologically conditioned, donor alloantigen-pulsed dendritic cells without maintenance immunosuppression. Transplantation. 2008;85(2):237–246. doi: 10.1097/TP.0b013e31815e870e. [DOI] [PubMed] [Google Scholar]
- 57.Sarugaser R, Ennis J, Stanford WL, et al. Isolation, propagation, and characterization of human umbilical cord perivascular cells (HUCPVCs) Methods Mol Biol. 2009;482:269–279. doi: 10.1007/978-1-59745-060-7_17. [DOI] [PubMed] [Google Scholar]
- 58.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112(2):214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 59.Noiseux N, Gnecchi M, Lopez-Ilasaca M, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14(6):840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 61.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 62.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8(3):301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schafer R, Dominici M, Muller I, et al. Progress in characterization, preparation and clinical applications of non-hematopoietic stem cells, 29–30 September 2006, Tubingen, Germany. Cytotherapy. 2007;9(4):397–405. doi: 10.1080/14653240701392949. [DOI] [PubMed] [Google Scholar]
- 64.Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, et al. Hunt for pluripotent stem cell—regenerative medicine search for almighty cell. J Autoimmun. 2008;30(3):151–162. doi: 10.1016/j.jaut.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 66.D’Ippolito G, Diabira S, Howard GA, et al. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117(Pt 14):2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 67.Beltrami AP, Cesselli D, Bergamin N, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110(9):3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 68.Bosch J, Houben AP, Radke TF, et al. Distinct differentiation potential of “MSC” derived from cord blood and umbilical cord: are cord-derived cells true mesenchymal stromal cells? Stem Cells Dev. 2012;21(11):1977–1988. doi: 10.1089/scd.2011.0414. [DOI] [PubMed] [Google Scholar]
- 69.Kluth SM, Buchheiser A, Houben AP, et al. DLK-1 as a marker to distinguish unrestricted somatic stem cells and mesenchymal stromal cells in cord blood. Stem Cells Dev. 2010;19(10):1471–1483. doi: 10.1089/scd.2010.0070. [DOI] [PubMed] [Google Scholar]
- 70.Kucia M, Halasa M, Wysoczynski M, et al. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2007;21(2):297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- 71.Ratajczak MZ, Zuba-Surma EK, Machalinski B, et al. Bone-marrow-derived stem cells—our key to longevity? J Appl Genet. 2007;48(4):307–319. doi: 10.1007/BF03195227. [DOI] [PubMed] [Google Scholar]
- 72.Rojewski MT, Weber BM, Schrezenmeier H. Phenotypic characterization of mesenchymal stem cells from various tissues. Transfus Med Hemother. 2008;35(3):168–184. doi: 10.1159/000129013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindner U, Kramer J, Behrends J, et al. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy. 2010;12(8):992–1005. doi: 10.3109/14653249.2010.510503. [DOI] [PubMed] [Google Scholar]
- 74.Gronthos S, Zannettino AC, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116(Pt 9):1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 75.Bianco P, Riminucci M, Gronthos S, et al. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 76.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 77.Schipani E, Kronenberg HM. Adult mesenchymal stem cells. Cambridge: Harvard Stem Cell Institute; 2008. [PubMed] [Google Scholar]
- 78.Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46(12):3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 79.Levi B, Wan DC, Glotzbach JP, et al. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor beta1 (TGF-beta1) signaling. J Biol Chem. 2011;286(45):39497–39509. doi: 10.1074/jbc.M111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quirici N, Soligo D, Bossolasco P, et al. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30(7):783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 81.Meyerrose TE, De Ugarte DA, Hofling AA, et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25(1):220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Daquinag AC, Zhang Y, Amaya-Manzanares F, et al. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell. 2011;9(1):74–86. doi: 10.1016/j.stem.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 83.Katz AJ, Tholpady A, Tholpady SS, et al. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23(3):412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 84.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 85.Kilroy GE, Foster SJ, Wu X, et al. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212(3):702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 86.Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(Pt 7):1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- 87.Russell KC, Phinney DG, Lacey MR, et al. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28(4):788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 88.De Ugarte DA, Alfonso Z, Zuk PA, et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89(2–3):267–270. doi: 10.1016/s0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 89.Vogel W, Grunebach F, Messam CA, et al. Heterogeneity among human bone marrow-derived mesenchymal stem cells and neural progenitor cells. Haematologica. 2003;88(2):126–133. [PubMed] [Google Scholar]
- 90.Mihu CM, Mihu D, Costin N, et al. Isolation and characterization of stem cells from the placenta and the umbilical cord. Rom J Morphol Embryol. 2008;49(4):441–446. [PubMed] [Google Scholar]
- 91.Bottai D, Cigognini D, Nicora E, et al. Third-trimester amniotic fluid cells with the capacity to develop neural phenotypes and with heterogeneity among sub-populations. Restor Neurol Neurosci. 2012;30(1):55–68. doi: 10.3233/RNN-2011-0620. [DOI] [PubMed] [Google Scholar]
- 92.Kuci S, Kuci Z, Kreyenberg H, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95(4):651–659. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Battula VL, Treml S, Bareiss PM, et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica. 2009;94(2):173–184. doi: 10.3324/haematol.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nichols JE, Niles JA, Dewitt D, et al. Neurogenic and neuro-protective potential of a novel subpopulation of peripheral blood-derived CD133+ ABCG2+ CXCR4+ mesenchymal stem cells: development of autologous cell based therapeutics for traumatic brain injury. Stem Cell Res Ther. 2013;4(1):3. doi: 10.1186/scrt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287(5457):1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 96.Kunisaki Y, Frenette PS. The secrets of the bone marrow niche: enigmatic niche brings challenge for HSC expansion. Nat Med. 2012;18(6):864–865. doi: 10.1038/nm.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Braun KM, Niemann C, Jensen UB, et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130(21):5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 98.Gould E, Reeves AJ, Graziano MS, et al. Neurogenesis in the neocortex of adult primates. Science. 1999;286(5439):548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 99.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 100.Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24(10):2202–2208. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- 101.Alm JJ, Koivu HM, Heino TJ, et al. Circulating plastic adherent mesenchymal stem cells in aged hip fracture patients. J Orthop Res. 2010;28(12):1634–1642. doi: 10.1002/jor.21167. [DOI] [PubMed] [Google Scholar]
- 102.Lazarus HM, Haynesworth SE, Gerson SL, et al. Human bone marrow-derived mesenchymal (stromal) progenitor cells (MPCs) cannot be recovered from peripheral blood progenitor cell collections. J Hematother. 1997;6(5):447–455. doi: 10.1089/scd.1.1997.6.447. [DOI] [PubMed] [Google Scholar]
- 103.Wexler SA, Donaldson C, Denning-Kendall P, et al. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121(2):368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 104.James AW, Zara JN, Corselli M, et al. Use of human perivascular stem cells for bone regeneration. J Vis Exp: JoVE. 2012;63:e2952. doi: 10.3791/2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.James AW, Zara JN, Corselli M, et al. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med. 2012;1(9):673–684. doi: 10.5966/sctm.2012-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zimmerlin L, Donnenberg VS, Rubin JP, et al. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry Part A: J Int Soc Anal Cytol. 2013;83:134–140. doi: 10.1002/cyto.a.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crisan M, Chen CW, Corselli M, et al. Perivascular multipotent progenitor cells in human organs. Ann NY Acad Sci. 2009;1176:118–123. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 108.Park TS, Gavina M, Chen CW, et al. Placental perivascular cells for human muscle regeneration. Stem Cells Dev. 2011;20(3):451–463. doi: 10.1089/scd.2010.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tu Z, Li Y, Smith DS, et al. Retinal pericytes inhibit activated T cell proliferation. Invest Ophthalmol Vis Sci. 2011;52(12):9005–9010. doi: 10.1167/iovs.11-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maier CL, Pober JS. Human placental pericytes poorly stimulate and actively regulate allogeneic CD4 T cell responses. Arterioscler Thromb Vasc Biol. 2011;31(1):183–189. doi: 10.1161/ATVBAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tottey S, Corselli M, Jeffries EM, et al. Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng Part A. 2011;17(1–2):37–44. doi: 10.1089/ten.tea.2010.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beck B, Driessens G, Goossens S, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478(7369):399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- 113.Paul G, Ozen I, Christophersen NS, et al. The adult human brain harbors multipotent perivascular mesenchymal stem cells. Plos One. 2012;7(4):e35577. doi: 10.1371/journal.pone.0035577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gerlach JC, Over P, Turner ME, et al. Perivascular mesenchymal progenitors in human fetal and adult liver. Stem Cells Dev. 2012;21(18):3258–3269. doi: 10.1089/scd.2012.0296. [DOI] [PubMed] [Google Scholar]
- 115.Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 116.Davidoff MS, Middendorff R, Enikolopov G, et al. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167(5):935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang W, Zeve D, Suh JM, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dellavalle A, Maroli G, Covarello D, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 119.Krautler NJ, Kana V, Kranich J, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150(1):194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bouacida A, Rosset P, Trichet V, et al. Pericyte-like progenitors show high immaturity and engraftment potential as compared with mesenchymal stem cells. Plos One. 2012;7(11):e48648. doi: 10.1371/journal.pone.0048648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Olson LE, Soriano P. PDGFRbeta signaling regulates mural cell plasticity and inhibits fat development. Dev Cell. 2011;20(6):815–826. doi: 10.1016/j.devcel.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 123.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Corselli M, Chin CJ, Parekh C, et al. Perivascular support of human hematopoietic cells. Blood. 2013;21:2891–2901. doi: 10.1182/blood-2012-08-451864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tintut Y, Alfonso Z, Saini T, et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108(20):2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 127.Hoshino A, Chiba H, Nagai K, et al. Human vascular adventitial fibroblasts contain mesenchymal stem/progenitor cells. Biochem Biophys Res Commun. 2008;368(2):305–310. doi: 10.1016/j.bbrc.2008.01.090. [DOI] [PubMed] [Google Scholar]
- 128.Sartore S, Chiavegato A, Faggin E, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89(12):1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 129.Siow RC, Mallawaarachchi CM, Weissberg PL. Migration of adventitial myofibroblasts following vascular balloon injury: insights from in vivo gene transfer to rat carotid arteries. Cardiovasc Res. 2003;59(1):212–221. doi: 10.1016/s0008-6363(03)00292-x. [DOI] [PubMed] [Google Scholar]
- 130.Hu Y, Zhang Z, Torsney E, et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113(9):1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75(4):679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 132.Herrmann J, Samee S, Chade A, et al. Differential effect of experimental hypertension and hypercholesterolemia on adventitial remodeling. Arterioscler Thromb Vasc Biol. 2005;25(2):447–453. doi: 10.1161/01.ATV.0000152606.34120.97. [DOI] [PubMed] [Google Scholar]
- 133.Stenmark KR, Davie N, Frid M, et al. Role of the adventitia in pulmonary vascular remodeling. Physiology (Bethesda) 2006;21:134–145. doi: 10.1152/physiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 134.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3(3):229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 135.Corselli M, Chen CW, Sun B, et al. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21(8):1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rao M, Ahrlund-Richter L, Kaufman DS. Concise review: cord blood banking, transplantation and induced pluripotent stem cell: success and opportunities. Stem Cells. 2012;30(1):55–60. doi: 10.1002/stem.770. [DOI] [PubMed] [Google Scholar]
- 137.Broxmeyer HE (2008) Cord blood hematopoietic stem cell transplantation. In: StemBook [Internet]. Harvard Stem Cell Institute, Cambridge. Available from: http://www.ncbi.nlm.nih.gov/books/NBK44751/ [PubMed]
- 138.Morigi M, Rota C, Montemurro T, et al. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010;28(3):513–522. doi: 10.1002/stem.293. [DOI] [PubMed] [Google Scholar]
- 139.Zanier ER, Montinaro M, Vigano M, et al. Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit Care Med. 2011;39(11):2501–2510. doi: 10.1097/CCM.0b013e31822629ba. [DOI] [PubMed] [Google Scholar]
- 140.Pierro M, Ionescu L, Montemurro T, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013;68(5):475–484. doi: 10.1136/thoraxjnl-2012-202323. [DOI] [PubMed] [Google Scholar]
- 141.Zhang X, Hirai M, Cantero S, et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: re-evaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 2011;112(4):1206–1218. doi: 10.1002/jcb.23042. [DOI] [PubMed] [Google Scholar]
- 142.Avanzini MA, Bernardo ME, Cometa AM, et al. Generation of mesenchymal stromal cells in the presence of platelet lysate: a phenotypic and functional comparison of umbilical cord blood- and bone marrow-derived progenitors. Haematologica. 2009;94(12):1649–1660. doi: 10.3324/haematol.2009.006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zeddou M, Briquet A, Relic B, et al. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int. 2010;34(7):693–701. doi: 10.1042/CBI20090414. [DOI] [PubMed] [Google Scholar]
- 144.Kogler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200(2):123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van de Ven C, Collins D, Bradley MB, et al. The potential of umbilical cord blood multipotent stem cells for non-hematopoietic tissue and cell regeneration. Exp Hematol. 2007;35(12):1753–1765. doi: 10.1016/j.exphem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 146.McGuckin C, Jurga M, Ali H, et al. Culture of embryonic-like stem cells from human umbilical cord blood and onward differentiation to neural cells in vitro. Nat Protoc. 2008;3(6):1046–1055. doi: 10.1038/nprot.2008.69. [DOI] [PubMed] [Google Scholar]
- 147.Bieback K, Kern S, Kluter H, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22(4):625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 148.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 149.Montesinos JJ, Flores-Figueroa E, Castillo-Medina S, et al. Human mesenchymal stromal cells from adult and neonatal sources: comparative analysis of their morphology, immunophenotype, differentiation patterns and neural protein expression. Cytotherapy. 2009;11(2):163–176. doi: 10.1080/14653240802582075. [DOI] [PubMed] [Google Scholar]
- 150.Takashima Y, Era T, Nakao K, et al. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129(7):1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 151.LaBonne C, Bronner-Fraser M. Molecular mechanisms of neural crest formation. Annu Rev Cell Dev Biol. 1999;15:81–112. doi: 10.1146/annurev.cellbio.15.1.81. [DOI] [PubMed] [Google Scholar]
- 152.Le Douarin NM, Creuzet S, Couly G, et al. Neural crest cell plasticity and its limits. Development. 2004;131(19):4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- 153.Dennis JE, Charbord P. Origin and differentiation of human and murine stroma. Stem Cells. 2002;20(3):205–214. doi: 10.1634/stemcells.20-3-205. [DOI] [PubMed] [Google Scholar]
- 154.Hungerford JE, Little CD. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J Vasc Res. 1999;36(1):2–27. doi: 10.1159/000025622. [DOI] [PubMed] [Google Scholar]
- 155.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, Poelmann RE. Smooth muscle cells and fibroblasts of the coronary arteries derive from epithelial–mesenchymal transformation of the epicardium. Anat Embryol (Berl) 1999;199(4):367–378. doi: 10.1007/s004290050235. [DOI] [PubMed] [Google Scholar]
- 156.Minasi MG, Riminucci M, De Angelis L, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129(11):2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 157.Mendes SC, Robin C, Dzierzak E. Mesenchymal progenitor cells localize within hematopoietic sites throughout ontogeny. Development. 2005;132(5):1127–1136. doi: 10.1242/dev.01615. [DOI] [PubMed] [Google Scholar]
- 158.Morikawa S, Mabuchi Y, Niibe K, et al. Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Commun. 2009;379(4):1114–1119. doi: 10.1016/j.bbrc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 159.Trentin A, Glavieux-Pardanaud C, Le Douarin NM, et al. Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proc Natl Acad Sci USA. 2004;101(13):4495–4500. doi: 10.1073/pnas.0400629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Korn J, Christ B, Kurz H. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J Comp Neurol. 2002;442(1):78–88. doi: 10.1002/cne.1423. [DOI] [PubMed] [Google Scholar]
- 161.Etchevers HC, Vincent C, Le Douarin NM, et al. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128(7):1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- 162.Dupin E, Coelho-Aguiar JM. Isolation and differentiation properties of neural crest stem cells. Cytometry A. 2013;83(1):38–47. doi: 10.1002/cyto.a.22098. [DOI] [PubMed] [Google Scholar]
- 163.Shi H, Zhang T, Qiang L, et al. Mesenspheres of neural crest-derived cells enriched from bone marrow stromal cell subpopulation. Neurosci Lett. 2013;532:70–75. doi: 10.1016/j.neulet.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 164.Kruger GM, Mosher JT, Bixby S, et al. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35(4):657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Morikawa S, Mabuchi Y, Kubota Y, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Morrison SJ, White PM, Zock C, et al. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96(5):737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 167.Wislet-Gendebien S, Laudet E, Neirinckx V, et al. Mesenchymal stem cells and neural crest stem cells from adult bone marrow: characterization of their surprising similarities and differences. Cell Mol Life Sci. 2012;69(15):2593–2608. doi: 10.1007/s00018-012-0937-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 168.Lee G, Kim H, Elkabetz Y, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25(12):1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 169.Nagoshi N, Shibata S, Nakamura M, et al. Neural crest-derived stem cells display a wide variety of characteristics. J Cell Biochem. 2009;107(6):1046–1052. doi: 10.1002/jcb.22213. [DOI] [PubMed] [Google Scholar]
- 170.Yamashita J, Itoh H, Hirashima M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408(6808):92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 171.Brachvogel B, Moch H, Pausch F, et al. Perivascular cells expressing annexin A5 define a novel mesenchymal stem cell-like population with the capacity to differentiate into multiple mesenchymal lineages. Development. 2005;132(11):2657–2668. doi: 10.1242/dev.01846. [DOI] [PubMed] [Google Scholar]
- 172.Nelander S, Mostad P, Lindahl P. Prediction of cell type-specific gene modules: identification and initial characterization of a core set of smooth muscle-specific genes. Genome Res. 2003;13(8):1838–1854. doi: 10.1101/gr.1197303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Paredes B, Santana A, Arribas MI, et al. Phenotypic differences during the osteogenic differentiation of single cell-derived clones isolated from human lipoaspirates. J Tissue Eng Regen Med. 2010;5:589–599. doi: 10.1002/term.351. [DOI] [PubMed] [Google Scholar]
- 174.Muller AM, Mehrkens A, Schafer DJ, et al. Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cell Mater. 2010;19:127–135. doi: 10.22203/ecm.v019a13. [DOI] [PubMed] [Google Scholar]
- 175.Cheung WK, Working DM, Galuppo LD, et al. Osteogenic comparison of expanded and uncultured adipose stromal cells. Cytotherapy. 2010;12(4):554–562. doi: 10.3109/14653241003709694. [DOI] [PubMed] [Google Scholar]
- 176.Rajashekhar G, Traktuev DO, Roell WC, et al. IFATS collection: adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: role of canonical Wnt signaling. Stem Cells. 2008;26(10):2674–2681. doi: 10.1634/stemcells.2008-0277. [DOI] [PubMed] [Google Scholar]
- 177.Meury T, Verrier S, Alini M. Human endothelial cells inhibit BMSC differentiation into mature osteoblasts in vitro by interfering with osterix expression. J Cell Biochem. 2006;98(4):992–1006. doi: 10.1002/jcb.20818. [DOI] [PubMed] [Google Scholar]
- 178.Dahl JA, Duggal S, Coulston N, et al. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int J Dev Biol. 2008;52(8):1033–1042. doi: 10.1387/ijdb.082663jd. [DOI] [PubMed] [Google Scholar]
- 179.Rosland GV, Svendsen A, Torsvik A, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69(13):5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 180.Ren Z, Wang J, Zhu W, et al. Spontaneous transformation of adult mesenchymal stem cells from cynomolgus macaques in vitro. Exp Cell Res. 2011;317(20):2950–2957. doi: 10.1016/j.yexcr.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 181.Torsvik A, Rosland GV, Svendsen A, et al. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track—letter. Cancer Res. 2010;70(15):6393–6396. doi: 10.1158/0008-5472.CAN-10-1305. [DOI] [PubMed] [Google Scholar]
- 182.Meliga E, Strem BM, Duckers HJ, et al. Adipose-derived cells. Cell Transplant. 2007;16(9):963–970. doi: 10.3727/096368907783338190. [DOI] [PubMed] [Google Scholar]
- 183.Tarnok A, Ulrich H, Bocsi J. Phenotypes of stem cells from diverse origin. Cytometry A. 2010;77(1):6–10. doi: 10.1002/cyto.a.20844. [DOI] [PubMed] [Google Scholar]
- 184.Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25(4):818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 185.James AW, Zara JN, Zhang X, et al. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med. 2012;1(6):510–519. doi: 10.5966/sctm.2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bruder SP, Jaiswal N, Ricalton NS, et al. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res. 1998;355 Suppl:S247–S256. doi: 10.1097/00003086-199810001-00025. [DOI] [PubMed] [Google Scholar]
- 187.Kang SW, Bae JH, Park SA, et al. Combination therapy with BMP-2 and BMSCs enhances bone healing efficacy of PCL scaffold fabricated using the 3D plotting system in a large segmental defect model. Biotechnol Lett. 2012;34(7):1375–1384. doi: 10.1007/s10529-012-0900-0. [DOI] [PubMed] [Google Scholar]
- 188.Zhu S, Zhang B, Man C, et al. NEL-like molecule-1-modified bone marrow mesenchymal stem cells/poly lactic-co-glycolic acid composite improves repair of large osteochondral defects in mandibular condyle. Osteoarthritis Cartilage. 2011;19(6):743–750. doi: 10.1016/j.joca.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 189.Monteiro BS, Del Carlo RJ, Argolo-Neto NM, et al. Association of mesenchymal stem cells with platelet rich plasma on the repair of critical calvarial defects in mice. Acta Cir Bras. 2012;27(3):201–209. doi: 10.1590/s0102-86502012000300001. [DOI] [PubMed] [Google Scholar]
- 190.Monteiro BS, Argolo-Neto NM, Nardi NB, et al. Treatment of critical defects produced in calvaria of mice with mesenchymal stem cells. An Acad Bras Cienc. 2012;84(3):841–851. doi: 10.1590/s0001-37652012000300026. [DOI] [PubMed] [Google Scholar]
- 191.Koob S, Torio-Padron N, Stark GB, et al. Bone formation and neovascularization mediated by mesenchymal stem cells and endothelial cells in critical-sized calvarial defects. Tissue Eng Part A. 2011;17(3–4):311–321. doi: 10.1089/ten.TEA.2010.0338. [DOI] [PubMed] [Google Scholar]
- 192.Agacayak S, Gulsun B, Ucan MC, et al. Effects of mesenchymal stem cells in critical size bone defect. Eur Rev Med Pharmacol Sci. 2012;16(5):679–686. [PubMed] [Google Scholar]
- 193.Osugi M, Katagiri W, Yoshimi R, et al. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A. 2012;18(13–14):1479–1489. doi: 10.1089/ten.tea.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stephan SJ, Tholpady SS, Gross B, et al. Injectable tissue-engineered bone repair of a rat calvarial defect. Laryngoscope. 2010;120(5):895–901. doi: 10.1002/lary.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang Q, Peng J, Lu SB, et al. Evaluation of an extracellular matrix-derived acellular biphasic scaffold/cell construct in the repair of a large articular high-load-bearing osteochondral defect in a canine model. Chin Med J (Engl) 2011;124(23):3930–3938. [PubMed] [Google Scholar]
- 196.Mokbel A, El-Tookhy O, Shamaa AA, et al. Homing and efficacy of intra-articular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. Clin Exp Rheumatol. 2011;29(2):275–284. [PubMed] [Google Scholar]
- 197.Field JR, McGee M, Stanley R, et al. The efficacy of allogeneic mesenchymal precursor cells for the repair of an ovine tibial segmental defect. Vet Comp Orthop Traumatol. 2011;24(2):113–121. doi: 10.3415/VCOT-10-03-0046. [DOI] [PubMed] [Google Scholar]
- 198.Reichert JC, Cipitria A, Epari DR, et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci Transl Med. 2012;4(141):141ra93. doi: 10.1126/scitranslmed.3003720. [DOI] [PubMed] [Google Scholar]
- 199.Marquass B, Schulz R, Hepp P, et al. Matrix-associated implantation of predifferentiated mesenchymal stem cells versus articular chondrocytes: in vivo results of cartilage repair after 1 year. Am J Sports Med. 2011;39(7):1401–1412. doi: 10.1177/0363546511398646. [DOI] [PubMed] [Google Scholar]
- 200.Khojasteh A, Behnia H, Dashti SG, et al. Current trends in mesenchymal stem cell application in bone augmentation: a review of the literature. J Oral Maxillofac Surg. 2012;70(4):972–982. doi: 10.1016/j.joms.2011.02.133. [DOI] [PubMed] [Google Scholar]
- 201.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56(3):283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cao L, Liu G, Gan Y, et al. The use of autologous enriched bone marrow MSCs to enhance osteoporotic bone defect repair in long-term estrogen deficient goats. Biomaterials. 2012;33(20):5076–5084. doi: 10.1016/j.biomaterials.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 203.Wang Z, Goh J, De Das S, et al. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006;12(7):1753–1761. doi: 10.1089/ten.2006.12.1753. [DOI] [PubMed] [Google Scholar]
- 204.Nde Ocarino M, Boeloni JN, Jorgetti V, et al. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats. Connect Tissue Res. 2010;51(6):426–433. doi: 10.3109/03008201003597049. [DOI] [PubMed] [Google Scholar]
- 205.Turgeman G, Zilberman Y, Zhou S, et al. Systemically administered rhBMP-2 promotes MSC activity and reverses bone and cartilage loss in osteopenic mice. J Cell Biochem. 2002;86(3):461–474. doi: 10.1002/jcb.10231. [DOI] [PubMed] [Google Scholar]
- 206.Bragdon B, Moseychuk O, Saldanha S, et al. Bone morphogenetic proteins: a critical review. Cell Signal. 2011;23(4):609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 207.Voumvourakis KI, Antonelou R, Kitsos DK, et al. TGF-beta/BMPs: crucial crossroad in neural autoimmune disorders. Neurochem Int. 2011;59(5):542–550. doi: 10.1016/j.neuint.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 208.Tang YC, Tang W, Tian WD, et al. A study on repairing mandibular defect by means of tissue-engineering and human bone morphogenetic protein-2 gene transfection in osteoporotic rats. Zhonghua Kou Qiang Yi Xue Za Zhi. 2006;41(7):430–431. [PubMed] [Google Scholar]
- 209.Turgeman G, Pittman DD, Muller R, et al. Engineered human mesenchymal stem cells: a novel platform for skeletal cell mediated gene therapy. J Gene Med. 2001;3(3):240–251. doi: 10.1002/1521-2254(200105/06)3:3<240::AID-JGM181>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 210.Egermann M, Baltzer AW, Adamaszek S, et al. Direct adenoviral transfer of bone morphogenetic protein-2 cDNA enhances fracture healing in osteoporotic sheep. Hum Gene Ther. 2006;17(5):507–517. doi: 10.1089/hum.2006.17.507. [DOI] [PubMed] [Google Scholar]
- 211.Zhang XS, Linkhart TA, Chen ST, et al. Local ex vivo gene therapy with bone marrow stromal cells expressing human BMP4 promotes endosteal bone formation in mice. J Gene Med. 2004;6(1):4–15. doi: 10.1002/jgm.477. [DOI] [PubMed] [Google Scholar]
- 212.Hu J, Qi MC, Zou SJ, et al. Callus formation enhanced by BMP-7 ex vivo gene therapy during distraction osteogenesis in rats. J Orthop Res. 2007;25(2):241–251. doi: 10.1002/jor.20288. [DOI] [PubMed] [Google Scholar]
- 213.Askarinam A, James AW, Zara JN, et al. Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nell-1 protein. Tissue Eng Part A. 2013;19:1386–1397. doi: 10.1089/ten.tea.2012.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhao Y, Li T, Wei X, et al. Mesenchymal stem cell transplantation improves regional cardiac remodeling following ovine infarction. Stem Cells Transl Med. 2012;1(9):685–695. doi: 10.5966/sctm.2012-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Roura S, Bago JR, Soler-Botija C, et al. Human umbilical cord blood-derived mesenchymal stem cells promote vascular growth in vivo. Plos One. 2012;7(11):e49447. doi: 10.1371/journal.pone.0049447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zheng SX, Weng YL, Zhou CQ, et al. Comparison of cardiac stem cells and mesenchymal stem cells transplantation on the cardiac electrophysiology in rats with myocardial infarction. Stem Cell Rev. 2012;9:339–349. doi: 10.1007/s12015-012-9367-6. [DOI] [PubMed] [Google Scholar]
- 217.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS ONE. 2012;7(4):e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.van den Akker F, Deddens JC, Doevendans PA, et al. Cardiac stem cell therapy to modulate inflammation upon myocardial infarction. Biochim Biophys Acta. 2012;830:2449–2458. doi: 10.1016/j.bbagen.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 219.Hu X, Yu SP, Fraser JL, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135(4):799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 220.Shabbir A, Zisa D, Suzuki G, et al. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296(6):H1888–H1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhu K, Lai H, Guo C, et al. Novel vascular endothelial growth factor gene delivery system-manipulated mesenchymal stem cells repair infarcted myocardium. Exp Biol Med (Maywood) 2012;237(6):678–687. doi: 10.1258/ebm.2012.011430. [DOI] [PubMed] [Google Scholar]
- 222.Huang XP, Sun Z, Miyagi Y, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122(23):2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 223.Gao XR, Tan YZ, Wang HJ. Overexpression of Csx/Nkx2.5 and GATA-4 enhances the efficacy of mesenchymal stem cell transplantation after myocardial infarction. Circ J. 2011;75(11):2683–2691. doi: 10.1253/circj.cj-11-0238. [DOI] [PubMed] [Google Scholar]
- 224.Li XH, Fu YH, Lin QX, et al. Induced bone marrow mesenchymal stem cells improve cardiac performance of infarcted rat hearts. Mol Biol Rep. 2012;39(2):1333–1342. doi: 10.1007/s11033-011-0867-2. [DOI] [PubMed] [Google Scholar]
- 225.Tang JM, Wang JN, Zhang L, et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91(3):402–411. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Katare R, Riu F, Mitchell K, et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. 2011;109(8):894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chen CW, Okada M, Proto JD, et al. Human pericytes for ischemic heart repair. Stem Cells. 2013;31(2):305–316. doi: 10.1002/stem.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.De Ugarte DA, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 229.Rodriguez AM, et al. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87(1):125–128. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 230.Lindroos B, et al. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009;11(7):958–972. doi: 10.3109/14653240903233081. [DOI] [PubMed] [Google Scholar]
- 231.Schreml S, et al. Harvesting human adipose tissue-derived adult stem cells: resection versus liposuction. Cytotherapy. 2009;11(7):947–957. doi: 10.3109/14653240903204322. [DOI] [PubMed] [Google Scholar]
- 232.Sensebe L, Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87(9 Suppl):S49–S53. doi: 10.1097/TP.0b013e3181a28635. [DOI] [PubMed] [Google Scholar]
- 233.Franco Lambert AP, et al. Differentiation of human adipose-derived adult stem cells into neuronal tissue: does it work? Differentiation. 2009;77(3):221–228. doi: 10.1016/j.diff.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 234.Campagnoli C, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 235.Villaron EM, et al. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89(12):1421–1427. [PubMed] [Google Scholar]
- 236.Alsalameh S, et al. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50(5):1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 237.Hiraoka K, et al. Mesenchymal progenitor cells in adult human articular cartilage. Biorheology. 2006;43(3–4):447–454. [PubMed] [Google Scholar]
- 238.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 239.Secco M, et al. Gene expression profile of mesenchymal stem cells from paired umbilical cord units: cord is different from blood. Stem Cell Rev. 2009;5(4):387–401. doi: 10.1007/s12015-009-9098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jager M, et al. Cord blood—an alternative source for bone regeneration. Stem Cell Rev. 2009;5(3):266–277. doi: 10.1007/s12015-009-9083-z. [DOI] [PubMed] [Google Scholar]
- 241.Bieback K, Kluter H. Mesenchymal stromal cells from umbilical cord blood. Curr Stem Cell Res Ther. 2007;2(4):310–323. doi: 10.2174/157488807782793763. [DOI] [PubMed] [Google Scholar]
- 242.Gronthos S, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nakamura S, et al. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod. 2009;35(11):1536–1542. doi: 10.1016/j.joen.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 244.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Valtieri M, Sorrentino A. The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol. 2008;217(2):296–300. doi: 10.1002/jcp.21521. [DOI] [PubMed] [Google Scholar]
- 246.Shi S, et al. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8(3):191–199. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 247.Schuring AN, et al. Characterization of endometrial mesenchymal stem-like cells obtained by endometrial biopsy during routine diagnostics. Fertil Steril. 2011;95(1):423–426. doi: 10.1016/j.fertnstert.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 248.Spitzer TL, et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod. 2012;86(2):58. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lanzoni G, et al. Isolation of stem cell populations with trophic and immunoregulatory functions from human intestinal tissues: potential for cell therapy in inflammatory bowel disease. Cytotherapy. 2009;11(8):1020–1031. doi: 10.3109/14653240903253840. [DOI] [PubMed] [Google Scholar]
- 250.Arai F, et al. Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J Exp Med. 2002;195(12):1549–1563. doi: 10.1084/jem.20011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.O’Driscoll SW, Fitzsimmons JS. The role of periosteum in cartilage repair. Clin Orthop Relat Res. 2001;391:S190–S207. doi: 10.1097/00003086-200110001-00019. [DOI] [PubMed] [Google Scholar]
- 252.In ‘t Anker PS. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 253.Rotter N, et al. Isolation and characterization of adult stem cells from human salivary glands. Stem Cells Dev. 2008;17(3):509–518. doi: 10.1089/scd.2007.0180. [DOI] [PubMed] [Google Scholar]
- 254.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21(1):105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 255.Campagnolo P, et al. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121(15):1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mariotti E, Mirabelli P, Abate G, Schiattarella M, Martinelli P, Fortunato G, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow and placenta: CD10, CD49d, and CD56 make a difference. Stem Cells Dev. 2008;17(6):1039–1041. doi: 10.1089/scd.2008.0212. [DOI] [PubMed] [Google Scholar]
- 257.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189(1):54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 258.Niehage C, Steenblock C, Pursche T, Bornhäuser M, Corbeil D, Hoflack B. The cell surface proteome of human mesenchymal stromal cells. Plos One. 2011;6(5):e20399. doi: 10.1371/journal.pone.0020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, et al. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125(1):87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- 261.Brooke G, Tong H, Levesque J-P, Atkinson K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008;17(5):929–940. doi: 10.1089/scd.2007.0156. [DOI] [PubMed] [Google Scholar]
- 262.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 263.Tallone T, Realini C, Böhmler A, Kornfeld C, Vassalli G, Moccetti T, et al. Adult human adipose tissue contains several types of multipotent cells. J Cardiovasc Transl Res. 2011;4(2):200–210. doi: 10.1007/s12265-011-9257-3. [DOI] [PubMed] [Google Scholar]
- 264.Psaltis PJ, Harbuzariu A, Delacroix S, Holroyd EW, Simari RD. Resident vascular progenitor cells—diverse origins, phenotype, and function. J Cardiovasc Transl Res. 2011;4(2):161–176. doi: 10.1007/s12265-010-9248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2009;77(1):22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Campioni DD, Moretti SS, Ferrari LL, Punturieri MM, Castoldi GLG, Lanza FF. Immunophenotypic heterogeneity of bone marrow-derived mesenchymal stromal cells from patients with hematologic disorders: correlation with bone marrow microenvironment. Haematologica. 2006;91(3):364–368. [PubMed] [Google Scholar]
- 267.Bűhring H-J, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann NY Acad Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 268.Masuda H, Anwar SS, Bűhring H-J, Rao JR, Gargett CE. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transpl. 2012;21(10):2201–2214. doi: 10.3727/096368911X637362. [DOI] [PubMed] [Google Scholar]
- 269.Flores-Torales E, Orozco-Barocio A, Gonzalez-Ramella OR, Carrasco-Yalan A, Gazarian K, Cuneo-Pareto S. The CD271 expression could be alone for establisher phenotypic marker in bone marrow-derived mesenchymal stem cells. Folia Histochem Cytobiol. 2010;48(4):682–686. doi: 10.2478/v10042-010-0063-6. [DOI] [PubMed] [Google Scholar]
- 270.Zimmerlin L, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77(1):22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Djouad F, Fritz V, Apparailly F, et al. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52(5):1595–1603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- 272.Guillot PV, De Bari C, Dell’Accio F, et al. Comparative osteogenic transcription profiling of various fetal and adult mesenchymal stem cell sources. Differentiation. 2008;76(9):946–957. doi: 10.1111/j.1432-0436.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 273.Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 274.Hillel AT, Taube JM, Cornish TC, et al. Characterization of human mesenchymal stem cell-engineered cartilage: analysis of its ultrastructure, cell density and chondrocyte phenotype compared to native adult and fetal cartilage. Cells Tissues Organs. 2010;191(1):12–20. doi: 10.1159/000225985. [DOI] [PubMed] [Google Scholar]
- 275.Erickson IE, Huang AH, Chung C, et al. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15(5):1041–1052. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Noth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4(7):371–380. doi: 10.1038/ncprheum0816. [DOI] [PubMed] [Google Scholar]
- 277.Wang N, Ren GD, Zhou Z, et al. Cooperation of myocardin and Smad2 in inducing differentiation of mesenchymal stem cells into smooth muscle cells. IUBMB Life. 2012;64(4):331–339. doi: 10.1002/iub.1003. [DOI] [PubMed] [Google Scholar]
- 278.Uysal AC, Mizuno H. Tendon regeneration and repair with adipose derived stem cells. Curr Stem Cell Res Ther. 2010;5(2):161–167. doi: 10.2174/157488810791268609. [DOI] [PubMed] [Google Scholar]
- 279.Leroux L, Descamps B, Tojais NF, et al. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther. 2010;18(8):1545–1552. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73(6):778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 281.Rosova I, Dao M, Capoccia B, et al. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26(8):2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gupta PK, Chullikana A, Parakh R, et al. A double-blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11:143. doi: 10.1186/1479-5876-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Serbeniuk TsV, Sychev VS, Lelekova TV. Bilevel organization of the spinal center of the frog lymph heart. Nauchnye Doki Vyss Shkoly Biol Nauki. 1976;7:82–86. [PubMed] [Google Scholar]
- 284.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23(7):845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 285.Burst V, Putsch F, Kubacki T, et al. Survival and distribution of injected haematopoietic stem cells in acute kidney injury. Nephrol Dial Transpl. 2013;28:1131–1139. doi: 10.1093/ndt/gfs513. [DOI] [PubMed] [Google Scholar]
- 286.Wise AF, Ricardo SD. Mesenchymal stem cells in kidney inflammation and repair. Nephrology (Carlton) 2012;17(1):1–10. doi: 10.1111/j.1440-1797.2011.01501.x. [DOI] [PubMed] [Google Scholar]
- 287.Domínguez-Bendala J, Lanzoni G, et al. Concise review: mesenchymal stem cells for diabetes. Stem Cells Transl Med. 2012;1(1):59–63. doi: 10.5966/sctm.2011-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Dai LJ, Li HY, Guan LX, et al. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2009;2(1):16–25. doi: 10.1016/j.scr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 289.Ishikawa T, Banas A, Hagiwara K, et al. Stem cells for hepatic regeneration: the role of adipose tissue derived mesenchymal stem cells. Curr Stem Cell Res Ther. 2010;5(2):182–189. doi: 10.2174/157488810791268636. [DOI] [PubMed] [Google Scholar]
- 290.Aquino JB, Bolontrade MF, Garcia MG, et al. Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma. Gene Ther. 2010;17(6):692–708. doi: 10.1038/gt.2010.10. [DOI] [PubMed] [Google Scholar]
- 291.Kim N, Im KI, Lim JY, et al. Mesenchymal stem cells for the treatment and prevention of graft-versus-host disease: experiments and practice. Ann Hematol. 2013;92(10):1295–1308. doi: 10.1007/s00277-013-1796-z. [DOI] [PubMed] [Google Scholar]
- 292.Silla L, Valim V, Amorin B et al (2013) A safety and feasibility study with platelet lysate expanded bone marrow mesenchymal stromal cells for the treatment of acute GVHD in Brazil. Leuk Lymphoma. doi:10.3109/10428194.2013.823495 [DOI] [PubMed]
- 293.Xia Z, Zhang C, Zeng Y et al (2012) Transplantation of BMSCs expressing hVEGF(165)/hBD3 promotes wound healing in rats with combined radiation-wound injury. Int Wound J. doi:10.1111/j.1742-481x.2012.01090.x [DOI] [PMC free article] [PubMed]
- 294.Kim SO, Na HS, Kwon D, et al. Bone-marrow-derived mesenchymal stem cell transplantation enhances closing pressure and leak point pressure in a female urinary incontinence rat model. Urol Int. 2011;86(1):110–116. doi: 10.1159/000317322. [DOI] [PubMed] [Google Scholar]
- 295.Zannettino ACW, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214(2):413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 296.Zhang R, Liu Y, Yan K, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10(1):106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kumagai G, Tsoulfas P, Toh S, et al. Genetically modified mesenchymal stem cells (MSCs) promote axonal regeneration and prevent hypersensitivity after spinal cord injury. Exp Neurol. 2013;248:369–380. doi: 10.1016/j.expneurol.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 298.Jadasz JJ, Kremer D, Göttle P, et al. Mesenchymal stem cell conditioning promotes rat oligodendroglial cell maturation. PLoS One. 2013;8(8):e71814. doi: 10.1371/journal.pone.0071814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Danielyan L, Schäfer R, Ameln-Mayerhofer A, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011;14(1):3–16. doi: 10.1089/rej.2010.1130. [DOI] [PubMed] [Google Scholar]
- 300.Stemberger S, Jamnig A, Stefanova N, et al. Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection. PLoS One. 2011;6(5):e19808. doi: 10.1371/journal.pone.0019808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hall SR, Tsoyi K, Ith B, Padera RF, et al. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophils. Stem Cells. 2013;31(2):397–407. doi: 10.1002/stem.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Curley GF, Ansari B, Hayes M, et al. Effects of intratracheal mesenchymal stromal cell therapy during recovery and resolution after ventilator-induced lung injury. Anesthesiology. 2013;118(4):924–932. doi: 10.1097/ALN.0b013e318287ba08. [DOI] [PubMed] [Google Scholar]
- 303.Cheng K, Rai P, Plagov A, et al. Transplantation of bone marrow-derived MSCs improves cisplatinum-induced renal injury through paracrine mechanisms. Exp Mol Pathol. 2013;94(3):466–473. doi: 10.1016/j.yexmp.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]