Abstract
Bone morphogenetic proteins (BMPs) are one of the main classes of multi-faceted secreted factors that drive vertebrate development. A growing body of evidence indicates that BMPs contribute to the formation of the central nervous system throughout its development, from the initial shaping of the neural primordium to the generation and maturation of the different cell types that form the functional adult nervous tissue. In this review, we focus on the multiple activities of BMPs during spinal cord development, paying particular attention to recent results that highlight the complexity of BMP signaling during this process. These findings emphasize the unique capacity of these signals to mediate various functions in the same tissue throughout development, recruiting diverse effectors and strategies to instruct their target cells.
Keywords: Bone morphogenetic proteins (BMPs), Central nervous system development, Neural tube closure, Neural patterning, Neurogenesis, Gliogenesis
Introduction
The formation of a functional central nervous system (CNS) involves a series of intricate and overlapping developmental processes. In vertebrates, this occurs over a long period, beginning during early embryogenesis and terminating in adulthood. Nervous tissue is first induced at the end of gastrulation by neuralization of the medial part of the ectoderm [1]. From this point onwards, the neural tissue will evolve from a pseudo-stratified monolayer of neuroepithelial cells, or neural stem cells, to generate a mature organ composed of multiple cell types and subtypes that form complex networks of intercellular connections.
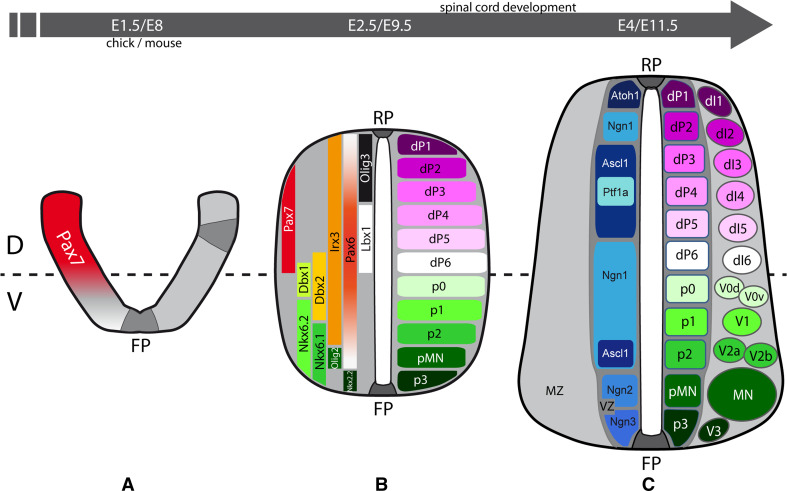
Studies of the development of the spinal cord, the caudal, and most anatomically straightforward part of the vertebrate CNS, have been instrumental in defining the various cellular processes involved in CNS formation. First, cytoskeletal rearrangements within neuroepithelial cells allow the neural plate to be progressively shaped into a neural tube that closes at the dorsal midline (Fig. 1a, b, reviewed in [2]). Meanwhile, neuroepithelial cells proliferate to ensure the growth of this tissue. In addition, they enter a patterning process, which allows each neuroepithelial cell to acquire a specific progenitor identity in function of its initial spatial coordinates along the anterior–posterior (AP) and dorsal–ventral (DV) axes (Fig. 1a, b). In amniotes, patterning of the caudal neural tube along the DV axis leads to the generation of 11 distinct domains of neural progenitors with a restricted lineage potential (Fig. 1b, reviewed in [3, 4]).
Fig. 1.
Early steps of spinal cord development in higher vertebrates. Schematic representation of transverse sections from three stages of higher vertebrate spinal cord development. a At the neural plate stage (chick embryonic day E1.5, mouse embryonic day E8), cytoskeletal rearrangements (dark grey) in neuroepithelial cells situated in the floor plate (FP) and at dorsal–lateral positions, drive the bending and progressive closure of the neural plate. Concurrently, neuroepithelial cells enter the patterning process and lead to the establishment of the ventral (V) and the Pax7+ (red) dorsal (D) territories of the developing neural tube. b After closure at the dorsal midline, the roof plate (RP) is generated, and once dorsal–ventral (DV) patterning is established (chick E2.5, mouse E9.5), the neural tube can be subdivided into 11 domains of neural progenitors: six dorsal domains (dP1-6) and five ventral domains (p3, pMN, p2-0). Each progenitor domain expresses a particular combination of cell fate determinants from the homeodomain (HD) and basic helix-loop-helix (bHLH) transcription factor families (left side), which defines their lineage potential. c During the first wave of neurogenesis (chick E4, mouse E11.5), each of the progenitor domains that contributes to the apically located ventricular zone (VZ) and that expresses particular pro-neural bHLH factors (blue), will produce one population or various sub-populations of post-mitotic neurons. These neurons migrate basally and they form the mantle zone (MZ), progressively differentiating and acquiring their particular morphological and molecular features
Once the boundaries of these domains are established, neural progenitors enter the phase of neurogenesis in which each progenitor domain gives rise to one population or various sub-populations of neurons (Fig. 1c, reviewed in [3, 4]). This process must be finely regulated to ensure the on-going expansion of the progenitor pools located in the ventricular zone and the generation of post-mitotic neurons that migrate basally to form the mantle zone (Fig. 1c). The progressive acquisition of the morphological and molecular features of these differentiating neurons is accompanied by their migration along the DV axis to reach their final destination [4]. Moreover, these cells enter into processes of axonogenesis, dendritogenesis, and synaptogenesis. The size of these neuronal populations can be further adjusted during development by selective phases of programmed cell death.
As neurogenesis terminates, the remaining neural progenitors, whose lineage potential has been somehow further restricted, enter a phase of gliogenesis. During this step, diverse types of glial cells are generated, mainly oligodendrocytes and astrocytes [5]. Eventually, these diverse specialized neuronal and glial cells mature to establish the functional network of intercellular connections required for the correct CNS activity to occur.
Remarkably, there is increasing evidence that the same class of extracellular signals can be re-used throughout development to fulfill various functions. One example of such a signal is that provided by the family of bone morphogenetic proteins (BMPs), which participate in various aspects of spinal cord development, from neural tube closure to the generation and maturation of selected neuronal and glial cell types. In this review, we shall first describe the basis of BMP signaling (see [6–8] for detailed reviews). Subsequently, we will attempt to define the precise contribution of the BMP pathway to various neuro-developmental processes, focusing mainly on studies related to spinal cord development.
BMP signaling in the neural tissue
The basics of BMP signaling
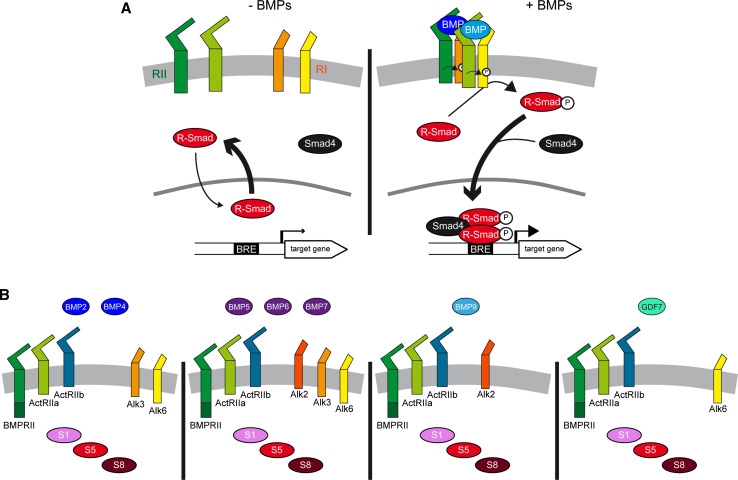
Bone morphogenetic proteins (BMPs) are a subgroup of secreted molecules that belong to the transforming growth factor β (TGF-β) superfamily [8]. The basic canonical BMP signaling machinery is remarkably simple (Fig. 2a). Dimers of ligands induce the formation of a stable tetrameric receptor complex consisting of two type 1 and two type 2 transmembrane serine/threonine kinases [8]. Within this complex, the type 2 receptors phosphorylate and thereby activate type 1 receptors. In turn, the type 1 receptors propagate the signal intracellularly by phosphorylating serine residues in the carboxy-terminal tail of the R-Smads, a class of transcription factors homologous to Drosophila mother’s against decapentaplegic (MAD) [8]. These phosphorylated R-Smads interact with their co-partner Smad4 to form a heterotrimeric complex with enhanced nuclear stability [9] that modulates the transcription of target genes in conjunction with other co-factors [10, 11] (Fig. 2a).
Fig. 2.
Basic elements and promiscuity in the BMP signaling pathway. a In the absence of BMP ligands, the transcription factors R-Smad (Smad1/5/8) undergo continuous nuclear–cytoplasmic shuttling. Since R-Smad are exported faster from the nucleus than they are imported, this favors their cytoplasmic accumulation [8]. Homo- or hetero-dimers of BMP ligands promote the formation of a complex of transmembrane serine/threonine kinases, in which type 2 receptors (RII) phosphorylate and activate type 1 receptors (RI), which in turn phosphorylate R-Smads on serine residues in their carboxy-terminal tail. This facilitates the interaction of two R-Smads with their partner Smad4, forming heterotrimeric complexes with enhanced nuclear stability that bind to BMP-responsive elements (BRE) in target gene promoters. These complexes cooperate with transcriptional co-factors (not shown) to modulate the expression of target genes. b Various members of the four BMP subgroups are expressed during spinal cord development. The type 2 and type 1 receptors for which they exhibit preferential affinities, and through which they probably act, are also represented, as well as the three R-Smads, all of which are expressed during spinal cord development
The diversity of BMPs that act in the developing spinal cord
Of the nearly 40 members of the TGF-β superfamily, nearly 20 have been assigned to the BMP subfamily, although only a dozen represent canonical BMP ligands [6]. Based on homology, BMPs can be further subdivided into four subgroups: the BMP2/4 group, the BMP5/6/7/8 group, the BMP9/10 group, and the growth and differentiation factor (GDF) group comprising GDF5/6/7 [6]. BMP2/4 are functional orthologues of the Drosophila decapentaplegic (Dpp), while members of the BMP5/6/7/8 subgroup are more closely related to Drosophila glass bottom boat (Gbb/60A) and Screw (Scw) [8].
During spinal cord development, members of all four BMP subgroups are expressed within the neural tissue and surrounding areas, including BMP2,-4,-5,-6,-7,-9 and GDF7 (Fig. 2b). Before neural tube closure, various members are expressed in the notochord (BMP7) and in the epidermal ectoderm surrounding the neural plate (BMP2/4/5/7) [12–15], as well as in the neural plate itself (BMP4/5) [13, 14]. Following closure of the neural tube, both these ligands and additional ones (BMP6/9, GDF7) are produced mainly by the roof plate [12–18], a specialized group of cells located in the dorsal midline of the neural tube (Fig. 1b). During neurogenesis, the expression of members such as BMP4 remains restricted to the roof plate [19], whereas new domains of BMP7 expression emerge ventrally within the developing spinal cord [19]. Thus, the diversity of BMP ligands and their dynamic expression during development complicate our understanding of the roles BMP signaling fulfils during spinal cord development.
The promiscuity of the ligand–receptor interactions
BMP ligands can bind to diverse type 2 receptors (Fig. 2b), including the BMP-specific BMPR2 that harbors an atypical carboxy-terminal tail [6]. Alternatively, they can bind to ActR2a/2b (Fig. 2b), which also interact with ligands from the Activin/TGF-β subfamily [6, 7]. These type 2 receptors can themselves recruit and activate various type 1 receptors (Fig. 2b), including Alk1, Alk2/Acvr1, Alk3/BMPR1a, and Alk6/BMPR1b [6, 7]. Biochemical studies have demonstrated that distinct BMP ligands have different affinities for the various BMP receptors [6]. BMP2/4 bind to Alk3 and Alk6 (Fig. 2b) and while members of the BMP5/6/7/8 subgroup also bind to Alk3 and Alk6, they can additionally interact with Alk2 for which they show a higher affinity (Fig. 2b). BMP9/10 can only interact with Alk1 and Alk2 (Fig. 2b), and like GDF5 [20], GDFs appear to preferentially bind to Alk6 (Fig. 2b). To further complicate matters, recent reports indicate that ligands from the Activin/TGF-β subgroup can also recruit receptors that were assumed to be BMP-specific [7]. Thus, there is considerable promiscuity within the cascade, given that the same ligand can interact with different type 2 and type 1 receptors, and the same receptor can interact with various ligands.
With the exception of Alk1, whose expression appears to be restricted to the vascular system [21], all these receptors are expressed in neural tissue [6, 22]. The precise contribution of type 2 receptors to the generation of the nervous system has yet to be determined. Indeed, BMPR2 mutant mice exhibit early embryonic lethality [23], while ActR2a and ActR2b knockouts produce no obvious neural defects [24–26]. Notably, the CNS-restricted double knockout of Alk3/6 receptors provokes spinal cord defects that are not seen in either of the single mutants [27], suggesting that receptors exert redundant functions. In an elegant study it was recently suggested that only BMP heterodimers appear to possess sufficient receptor affinity to elicit the signaling response required for DV patterning during zebrafish development [28]. This argues that the activities of BMPs are potentiated when they act as heterodimers, as such recruiting hetero-tetrameric rather than homo-tetrameric receptors complexes.
Canonical vs. non-canonical intracellular signaling
Smad1/5/8 are the canonical R-Smad effectors assumed to specifically transduce BMP signals into a transcriptional response [10, 11] (Fig. 2a). These three Smads are expressed in neural tissue during development and they might therefore mediate BMP activity [19]. Analysis of mutant mice carrying a hypomorphic Smad8 only revealed minor defects in the developing CNS [29], suggesting a weak contribution of Smad8 or the possible compensatory activity of Smad1/5. The early embryonic lethality of both Smad1 and Smad5 mutant mice precluded the determination of their functions during neural development [30, 31]. However, emerging strategies for conditional ablation have started to shed light on their contribution to several neuro-developmental processes [32].
Until recently, it was assumed that transduction of BMP activity inevitably converges on Smad1/5/8 activation to trigger a transcriptional response. However, there is a growing body of evidence suggesting that several BMP activities are independent of transcription and that non-canonical signaling cascades exist (reviewed in [6, 33]). During neural development, the effects of BMPs on axonal orientation and neuritogenesis are transduced through BMP receptors by the recruitment of the PI3K–Akt cascade [34] and the LIM kinases (LIMK) [35], respectively.
The multiple activities of BMPs during spinal cord development
Neural tube formation
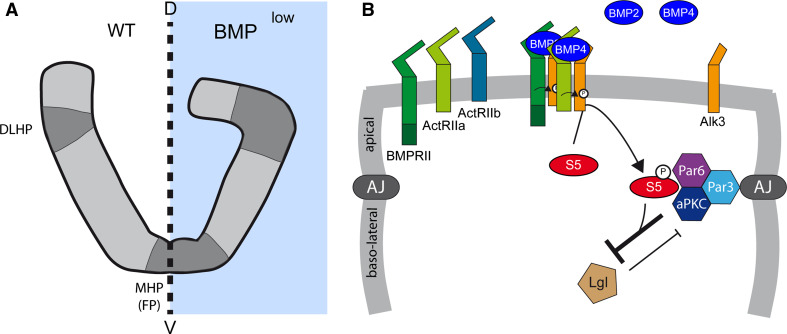
The formation of the neural tube involves the progressive ventral invagination and lateral bending of the neural plate, which closes at the dorsal midline. This morphological transformation relies on a median hinge point (MHP) located in the floor plate (FP), a particular group of cells forming the ventral midline of the neural plate (Figs. 1a, 3a). Depending on the rostro-caudal position, additional hinge points can be found dorsally (DLHPs, Fig. 3a and [2]). These hinge points are formed by groups of cells that undergo cytoskeletal rearrangements, provoking apical cell constriction, basal cell thickening, and basal nuclear migration. These hinge points serve as anchors to support the mechanical forces applied to the tissue during neural tube closure.
Fig. 3.
BMP activity during neural tube closure. a Representation of the developing spinal cord at the neural plate stage under normal conditions (WT) and when BMP signaling is dampened (BMPlow). Blocking BMP signaling can result in the formation of exaggerated median and dorsal lateral hinge points (MHP, DLHP), which exacerbate apical cell constriction and the bending of the neural plate, thereby perturbing neural tube closure. b Hypothetical molecular mechanism by which BMPs regulate neural tube closure. BMP ligands, probably BMP2 and BMP4, activate a receptor complex that is likely to contain BMPR2 and Alk3, and which promotes Smad5 activation. During this process, Smad5 acts in a non-classical (transcription-independent) manner by physically interacting with and stabilizing the apical polarity complex formed by Par3, Par6, and aPKC at the level of adherens junctions (AJ). This prevents components such as lethal giant larvae (Lgl) from destabilizing the polarity complex and disrupting the adherens junctions, a pre-requisite for apical cellular constriction
The importance of neural tube closure is reflected by the severe defects that arise when this process is impaired, such as exencephaly (rostrally) and spina bifida (caudally) [2]. Such defects may be produced by inhibiting or overactivating BMP signaling. BMP2-null embryos exhibit premature, exaggerated DLHPs (Fig. 2a, [36]). Moreover, local overexpression of Noggin, an extracellular BMP inhibitor with preferential affinity for the BMP2/4 subgroup, or overexpression of a dominant-negative form of Alk3, also provoke ectopic hinge point formation and exacerbate bending in both chick and mouse (Fig. 3a, [36, 37]). While mutant mice lacking BMP5, BMP6 or BMP7 [12, 13, 18, 38, 39] do not suffer defects in neurulation, early cranial exencephaly is provoked in the BMP5/BMP7 double knockout [39]. Conversely, the overactivation of BMP signaling triggered by the genetic invalidation of Noggin produces defective neural tube closure in mouse [40], and Noggin mutant mice also develop defects in FP maturation at caudal levels [40]. In addition, local release of BMP2 close to the neural plate inhibits neural fold bending in mice [36], and in ovo electroporation of BMP4 in the chick also leads to defective neural tube closure at caudal positions [41]. Thus, it appears that the dosage of BMP signaling is critical for neural tube closure to proceed correctly.
Interestingly, a recent study proposed that the contribution of BMPs to neural tube closure might depend on Smad activity, yet through a non-classical transcription-independent mechanism ([37], Fig. 3b). This study first revealed that the activity of Smad1/5/8 follows a bi-dimensional gradient, with higher levels of activity observed in the dorsal and apical regions of the neural plate. Intriguingly, physical interactions were reported between the activated forms of Smad1/5/8 (phospho-S1/5/8) and the components of the apical polarity complex (Par3, Par6 and aPKC). Having demonstrated that these factors co-localize with activated Smads at apical junctions, this interaction was proposed to favor the maintenance of the apical domain (Fig. 3b). Thus, inhibition of Smad activity would be required to destabilize this apical PAR complex, a pre-requisite for the apical constriction of the hinge point-forming cells [37].
Neural patterning
Neural patterning represents a crucial step in the generation of the diverse cell types and subtypes that form the CNS. In the developing spinal cord of amniotes, DV patterning leads to the generation of 11 distinct domains of neural progenitors (Fig. 1b, reviewed in [3, 4]). The neural tube can thus be subdivided into a dorsal part composed of 6 domains of progenitors (dP1-6, from dorsal to ventral), and a ventral part consisting of 5 domains (p3, pMN, p2-0, from ventral to dorsal, Fig. 1b). Each progenitor domain can be identified by the expression of a particular code of transcription factors from the homeodomain (HD) and basic helix-loop-helix (bHLH) families, a combination that determines the lineage potential of neural progenitors (Fig. 1b). Expression of these fate determinants is driven by extracellular cues secreted from various sources and that influence cell identity by acting in a graded manner along the DV axis [3, 4].
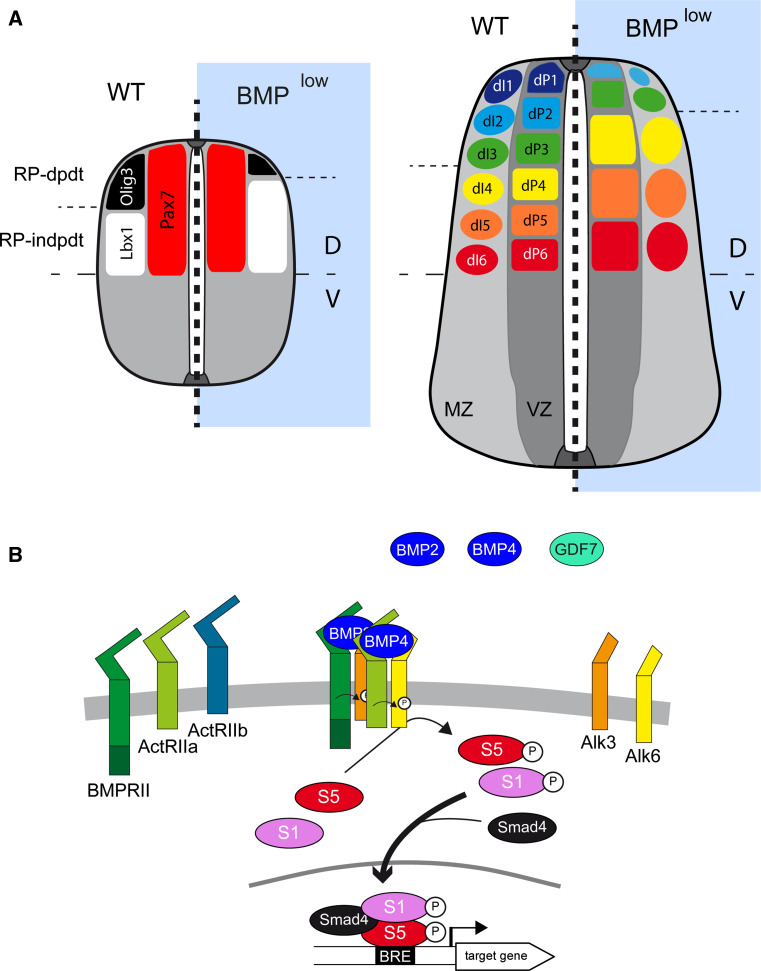
The idea that BMPs act as morphogens to pattern the neural tube was first postulated 15 years ago following the demonstration that BMPs can promote the generation of cells of the dP1 and dP3 lineages from explants in a concentration-dependent manner [42, 43]. In vivo, BMP activity also appears to be crucial for the generation of the most-dorsal progenitors dP1-3 (Fig. 4a). Overexpression of Noggin in chick embryos leads to the loss of the dP1–2 domain [44], while fewer dP1–3 cells are generated when Follistatin is overexpressed, another extracellular BMP antagonist [45]. Similarly, silencing both Alk3 and Alk6 in the developing murine CNS results in complete loss of the dP1 domain, in conjunction with a strongly diminished dP2 domain [27] (Fig. 4a). This reduction of the dorsal-most domains is accompanied by a reciprocal dorsal expansion of the more ventral dP4–6 domains (Fig. 4a), while no obvious changes in the ventral domains are reported [27, 44].
Fig. 4.
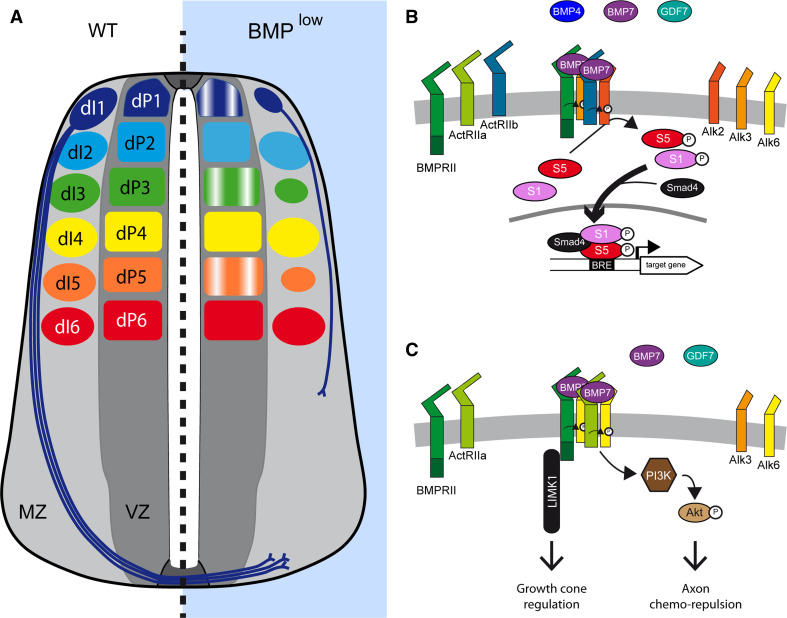
BMP activity during neural patterning. a Representation of DV patterning at two stages of spinal cord development in higher vertebrates, under normal conditions (WT) and in conditions of reduced BMP signaling (BMPlow). Only the dorsal progenitor domains are represented. The dorsal region (Pax7+) of the neural tube can be subdivided in two territories: the three dorsal-most progenitor populations (dP1–3) expressing Olig3 (black) that are generated through extracellular cues secreted from the roof plate (RP-dpdt); and the three more ventral dorsal progenitor domains (dP4–6) expressing Lbx1 (white) that can be formed independently of the RP-derived signals (RP-indpdt). Early blockade of BMP signaling alters this patterning, strongly diminishing the Olig3+ territory while expanding the Lbx1+ domain reciprocally. Consequently, in such circumstances dP1 is absent, dP2 is reduced, dP3 is unchanged or only slightly smaller, while dP4/5/6 expand reciprocally. The numbers of the corresponding dorsal interneurons (dI1–6) are modified accordingly. b Hypothetical molecular mechanism by which BMPs regulate neural patterning. BMP ligands, probably BMP2 and BMP4, with a restricted contribution of GDF7 involved in the induction of dP1, activate a receptor complex likely to contain BMPR2, Alk3, and Alk6, and which promotes Smad1/5 activation. The concentration and duration of exposure to the BMP ligands is transduced into distinct levels of Smad1/5 transcriptional activity, with the highest levels of Smad1/5 activity favoring more dorsal progenitor identities
It is interesting to notice that BMPs harbor several basic amino-acid motifs in their amino-terminal tail [46]. These motifs allow BMPs to interact with components of the extracellular matrix such as heparan sulphate proteoglycans (HSPGs), which can thereby modulate their range of action [47, 48]. Restricted overexpression in the dorsal part of the developing CNS (under the control of the Wnt1 promoter) of a BMP4 with a mutated basic motif produced stronger dorsalization of the intermediate neural tube territories than that produced by wild-type BMP4 [47]. Thus, BMPs appear to have a limited range of action within the developing neural tube, which might explain the restriction of their influences to the most-dorsal progenitor domains (Fig. 4a).
The initial experiments of Jessell and colleagues suggested that many BMPs participate in neural patterning, as all BMP4, BMP5, BMP7, and BMP9/Dsl1 showed the ability to stimulate the generation of cells from the dP1 and dP3 lineages ex vivo [42, 43]. However, the BMP ligands that effectively mediate patterning activity in vivo still remain elusive. The mutation of zebrafish BMP2 and BMP7 suggested they were involved in neural patterning [49], yet no such defects were observed after BMP7 loss-of-function in mice or chicks [12, 19]. Similarly, the analysis of mutant mice for BMP5/6/9 did not clarify their role in neural patterning [13, 16, 18]. As for BMP2, the early embryonic lethality provoked by its genetic invalidation in mice has so far hindered any analysis of its contribution to neural patterning in mammals [15], as occurs with BMP4 [14]. Currently, GDF7 is the only BMP ligand whose physiological contribution to neural patterning has been precisely established [17]. In GDF7-null mutant embryos there is a premature reduction in the pool of dP1 progenitors, which compromises the production of a specific subpopulation of dI1 interneurons (dI1A) [17]. Thus, GDF7 plays a discrete role in dorsal spinal cord patterning and its activity cannot account for the full contribution of BMP signaling to this process.
Significantly, it has only recently been possible to demonstrate graded BMP activity during neural patterning in vivo [50]. Analyzing the distribution and course of Smad1/5/8 activity during chick neural development (using a BRE:GFP reporter) revealed that the levels of canonical BMP activity are similar along the DV axis before NT closure, while BMP activity becomes progressively restricted to the dorsal-most dP1-3 domains shortly after closure. Interestingly, explants experiments showed that it is the duration of cell exposure to the BMP ligand rather than its concentration that defines progenitor identities. For instance, the generation of dP1 progenitors requires a longer exposure to BMP4 than that of dP2. Variations in the duration of the stimuli trigger distinct levels of intracellular signaling, such that a longer duration leads to higher and more stable levels of Smad1/5/8 activity [50]. These results are in accordance with previous data, whereby overexpression of high doses of constitutively active Alk3 or Alk6 isoforms in chick neural tubes favored a dP1 identity over dP2-3, whereas lower doses promoted dP2-3 fates over dP1 [51]. The physiological importance of the length of exposure to BMPs was highlighted in chick embryos by overexpressing Smad6, an intracellular inhibitor of BMP signaling [50]. Blocking the activity of the pathway at successively later stages demonstrated that dP3 progenitors were recovered earlier than dP1 progenitors. From these studies a model was proposed in which neural progenitors from the dP3, dP2, and dP1 domains are instructed sequentially in the dorsal spinal cord by increasing levels of a canonical BMP activity that is distributed in a dorsal to ventral gradient [50].
On the basis of these results, a model can be postulated regarding the role of BMP signaling in neural patterning (Fig. 4a, b). Before neural tube closure, the short range-diffusing BMPs expressed throughout the entire neural plate can signal over the entire DV axis and maintain the primary dorsal identity of the neural progenitors. As the neural tube closes and the source of most BMPs becomes restricted to the roof plate, progressively fewer neural progenitors are exposed to BMP signals for the time required to promote or maintain their dorsal identity. This induction of dorsal progenitor identity depends on the level and duration of Smad1/5 transcriptional activity, which is likely to be mainly elicited by BMP2/4 acting through Alk3/6 (Fig. 4b).
Neurogenesis
During the first wave of neurogenesis in birds and mammals, motor neurons (MN) emerge in the ventral part of the neural tube, as well as four populations of ventral interneurons (vIN, v0-3) that can be further divided into distinct subpopulations (see Fig. 1c, reviewed in [3, 4]). In the dorsal part, six distinct populations of dorsal interneurons (dIN, dI1-6, from dorsal to ventral) are generated from their corresponding progenitor domains (Figs. 1c, 5a, reviewed in [3, 4]). Later in development, a second wave of neurogenesis produces two populations of interneurons (dILate) that invade the upper dorsal horn of the developing spinal cord [4].
Fig. 5.
BMP activity during neurogenesis. a Representation of a transverse section of the developing spinal cord of higher vertebrates during neurogenesis, under normal conditions (WT), and in conditions of reduced BMP signaling (BMPlow). Only the dorsal progenitor domains (dP1–6) and the corresponding populations of interneurons (dI1–6) are considered. dI1 represent the commissural neurons whose axons project contra-laterally after crossing the ventral midline under the FP. Blocking BMP signaling at this stage alters dorsal neurogenesis in various ways. First, the rate of production is disturbed, resulting in the generation of fewer dI1/3/5 neurons while dI2/4/6 numbers are barely affected. Second, the orientation and outgrowth rate of the commissural axons are altered, whereby axons are misrouted and/or their ventral progression is slowed. b Suggested molecular mechanism by which BMPs regulate dIN generation during neurogenesis. BMP ligands, specifically BMP7 with a restricted contribution of BMP4 and GDF7 for dI1 generation, activate a receptor complex that probably contains ActR2b and Alk2, leading to Smad1/5 activation and probably producing a transcriptional response. The events directly controlled by BMP signaling during the neurogenic step remain elusive. c Suggested molecular mechanism by which BMPs regulate axon formation during neurogenesis. BMP ligands, specifically BMP7 with the contribution of GDF7, activate a receptor complex that probably contains BMPR2, ActR2a, and Alk6, leading to the activation of non-canonical elements of the pathway. The chemo-repulsion of dI1 axons from the RP relies on the recruitment of the PI3K–Akt cascade. The regulation of axonal outgrowth proximal to the RP depends on the activation of LIMK1 by BMPR2 through its atypical carboxy-terminal tail
Neurogenesis involves distinct cellular and molecular events that lead to the generation of functional neurons. These events can be considered to be of two types: those leading to the generation of post-mitotic neurons; and those ensuring the functional maturation of these differentiating neurons.
Generation of post-mitotic neurons
Until recently, it was assumed that all the changes in neuronal number that are observed after modulating BMP activity were the consequence of alterations in neural patterning. However, this point of view has been challenged recently by several studies. The blockade of BMP signaling by overexpressing inhibitory Smad6 and Smad7 in the chick neural tube at late developmental stages reduced the number of dI1 and dI3 neurons generated, without obviously altering the expression of progenitor markers (Fig. 5a, [52]), arguing in favor of a discrete role for BMP signaling during neurogenesis. Furthermore, we recently identified several of the elements effectively involved in this BMP activity [19]. We found that while reducing BMP4 activity only affected the generation of dI1 neurons, the loss of BMP7 function in both mouse and chick embryos resulted in fewer dI1, dI3, and dI5 neurons, whereas the number of dI2/4/6 was barely affected (Fig. 5a, [19]). Importantly, this phenotype was obtained without altering neural patterning. Interestingly, in vivo analysis of Smad1/5/8 activity along the DV axis (using a BRE:GFP reporter) revealed that the canonical BMP activity extends more ventrally at the onset of neurogenesis that at patterning stages [19, 50]. Moreover, while interfering with chick Smad8 function produced a specific reduction in dI1 neurons, dampening Smad1 or Smad5 activity mimicked the BMP7 loss-of-function phenotype [19], strongly suggesting that BMP7 acts via the canonical BMP pathway (Fig. 5a, b). A similar role was recently proposed for Smad5 in mice [32], while the CNS-restricted loss of Smad1 did not provoke significant alterations in dIN number [32].
Intriguingly, early overexpression of constitutively active forms of Alk3 and Alk6 in the chick neural tube triggers an increase in dI1/3 generation, while overexpression at later stages has no effect on dIN number [53]. Since the CNS-restricted double Alk3/6 knockout leads to an expected phenotype for early blockade of BMP signaling during patterning [27], it is tempting to speculate that other BMP receptors transduce BMP7 activity to Smad1/5 signaling during neurogenesis. Notably, BMP7 has a high affinity for Alk2, the other type-1 BMP receptor expressed in the developing CNS [54]. However, the contribution of Alk2 to neural development remains to be established due to the embryonic lethality of Alk2 mutant mice [55].
The cellular processes that are directly modulated by BMPs at this stage also remain unclear. The generation of post-mitotic neurons depends on several cellular responses, including the proliferation of neural progenitors, the balance between proliferative and neurogenic divisions, and the differentiation process per se. The ability of BMP signaling to modulate proliferation or differentiation in the neural tube has been tested in several studies. Gain-of-function experiments have demonstrated that grafts of BMP7-producing COS cells increased the mitotic index of neural progenitors [56]. Moreover, transgenic mice overexpressing a constitutively active form of Alk3 in the developing CNS showed overgrowth, whereas overexpression of the constitutively active form of Alk6 triggered neuronal differentiation [57]. Blocking BMP signaling in the chick neural tube by overexpressing Noggin slows the proliferation rate of spinal cord neural progenitors [44], whereas there is no change in proliferation or apoptosis in the CNS-restricted double Alk3/6 knockout [27]. It has also been proposed that BMP signaling can counteract Wnt-induced proliferation of spinal neural progenitors, and that Wnt signaling antagonizes BMP-induced neuronal differentiation [58, 59]. Thus, further studies will be required to define the direct cellular response to BMP signaling during neurogenesis.
Axon guidance, neurite outgrowth, and synapse formation
There is data now accumulating to indicate that BMP signaling plays additional roles in other aspects of spinal cord neurogenesis, including the orientation of the initial trajectory of commissural axons, axonal growth, and during synaptogenesis. There is compelling evidence that BMP7 reproduces the chemo-repulsant influence that the roof plate exerts on dI1 commissural axons [60, 61]. In fact, BMP7/GDF7 heterodimers exhibit even stronger chemo-repulsant activity [61], suggesting that this activity probably originates from the cooperation between various BMP ligands in vivo (Fig. 5a, c). However, not all BMPs secreted by the developing spinal cord at this stage possess such chemo-repulsive ability [60, 61]. Subtle differences in the structure and sequence of the diverse BMPs appear to explain these distinct effects, since a single amino-acid mutation (Q48R) in the sequence of the normally inefficient BMP6 conferred chemo-repulsive potential to this protein similar to that of its close paralogue BMP7 [62].
BMPs appear to regulate axonal guidance by recruiting classical BMP receptors, which distinguishes them from other extracellular cues, such as Shh and Wnt (reviewed in [63]). Both ActR2A and BMPR2 can mediate BMP7-induced chemotaxis in vitro [64] and Alk6 has been shown to exert a physiological influence on dI1 axonal guidance [53]. Abrogating Alk3 in the dP1 domain does not produce defects in axonal growth [53], although its invalidation on an Alk6 null background accentuates the defects in orientation observed in the single Alk6 mutant, implicating Alk3 in this process, albeit to a lesser extent [53] (Fig. 5a, c).
Several findings recently indicated that the effects of BMPs on axon orientation, axon outgrowth, and dendritogenesis are elicited by non-canonical intracellular signaling cascades. Axon orientation is influenced by concentrations of BMP7 lower than those that efficiently activate Smad1/5/8, and inhibition of the PI3K–Akt cascade blocks this activity but not the formation of dI1 neurons [34] (Fig. 5c). The carboxy-terminal tail of BMPR2 binds to the LIM Kinase 1 (LIMK1) [65], the recruitment of which appears to be essential for BMP7 to promote cone growth/dendritogenesis in cultured neurons [35] (Fig. 5c). Active LIMK triggers a molecular cascade that favors the cytoskeletal rearrangements and actin polymerization necessary to advance the growth cone (reviewed in [66]). BMPR2 and LIMK are also involved in mediating the effects of BMPs on axon orientation and extension in vivo. Overexpression in the dP1 domain of an active form of LIMK reduces the number of commissural axons [67], while LIMK deletion and BMPR2 inhibition both accelerate axon outgrowth and stimulate sprouting, effects that are associated with the misrouting of axonal tracts [67] (Fig. 4c). Notably, Smad- and transcription-independent effects of BMPs are also observed during axon guidance and dendritogenesis in invertebrates [66]. Intriguingly, it was recently reported that Smad1 activity is also required for correct axon guidance as its loss slows axon outgrowth [32]. Based on these opposing activities of Smad1 and LIMK1, it was proposed that LIMK might act proximal to the RP to restrain axonal outgrowth, thereby preventing early misrouting, whereas Smad1 may promote outgrowth more distally [32].
Finally, several recent studies have described the retrograde synaptic transport of several components of the BMP signaling machinery, which appears to be related to distinct activities of the pathway [68–70]. On the one hand, this finely regulated phenomenon has been correlated with the readjustment of neuronal subtype specification by BMPs [69]. On the other hand, several studies reported a role for BMPs in synaptic scaling [68, 71, 72], a process by which the numbers and properties of synapses are continually re-adapted to their targets [73]. As such, studies in Drosophila have proposed that Gbb forms part of a feedback loop from the muscles to the motor neuron presynaptic compartments that co-ordinately regulates muscle growth and innervation [68, 71, 72].
Gliogenesis
In vertebrates, neurogenesis is followed by a phase of gliogenesis, during which macroglial cells are generated, and there is evidence that the BMP pathway regulates the generation of the two main types of macroglial cells: oligodendrocytes and astrocytes.
Oligodendrogenesis
In the vertebrate CNS, oligodendrocytes control the production of the myelin sheaths that envelop the axons and that are required for optimal conduction of nerve impulses. Upon completion of motor neuron generation in the developing spinal cord, most oligodendrocytes emerge from a restricted ventral area that corresponds to the Olig2-expressing domain [5]. Since BMP signaling influences neural patterning during early embryogenesis, its modulation can alter oligodendrocyte specification. Indeed, overactivation and blockade of BMP signaling respectively inhibits and expands the oligodendrocyte lineage in the chick embryo, both in vitro and in vivo [74].
BMPs also appear to regulate later steps of oligodendrogenesis by opposing oligodendrocyte differentiation and maturation, as suggested by a recent study that elegantly demonstrated the requirement of the Smad-interacting protein 1 (Sip1) for proper CNS myelination [75]. Sip1 promotes oligodendrocyte differentiation by inhibiting BMP signaling in two ways: by physically interacting with and repressing the activity of the Smad1/5/8–Smad4-p300 complex; and by binding to and activating the promoter of Smad7, which in turn inhibits both the canonical BMP signaling cascade and the canonical (β-catenin-dependent) Wnt pathway [75]. This dual inhibitory action of Sip1 represses the expression of several Smad target genes known to inhibit oligodendrocyte differentiation (Id2, Id4, and Hes1), relieving the blockade on oligodendrocyte differentiation and maturation. Thus, inhibition of canonical BMP signaling appears to be a pre-requisite for correct oligodendrogenesis and myelination. Accordingly, repressing BMP signaling might represent an interesting strategy to promote regeneration following disease- and injury-related demyelination [76].
Astrocytogenesis
Astrocytes are the most abundant cell type in the human brain and they fulfill a wide variety of roles that help maintain CNS homeostasis [77]. In vertebrates, astrocytes are generated in the later phases of embryogenesis and in the postnatal period [77]. Studies on brain-derived cell cultures suggest that BMPs promote astrocyte generation (reviewed in [78]). Mechanistically, this function appears to rely on the physical cooperation between the BMP-activated Smads and the JAK–STAT signaling cascade activated by members of the CNTF–LIF family of cytokines [79].
A recent study revealed that BMP signaling controls the spatiotemporal generation of astrocytes in the developing chick spinal cord [80]. By combining cultured explants and in vivo experiments, BMP activity was shown to repress astrocyte generation in the intermediate spinal cord at early embryonic stages (until E5), while it promoted this process at later developmental stages (from E6 onwards). This switch in activity was correlated with the expression of Alk3 and the appearance of Smad1/5/8 activity in the intermediate spinal cord at E6 [80], suggesting that astrocytogenesis is regulated by a canonical BMP signaling pathway.
Interestingly, the temporal competence of the BMP pathway to promote astrocyte fate might depend on the presence of specific Smad co-factors, such as the pro-neural bHLH. Indeed, an elegant study demonstrated that the ability of Smad1 to stimulate the expression of one target gene or another is driven by Ngn1 [81]. During neurogenesis, Ngn1 sequesters the activated Smad complex and prevents it from acting on target genes of the astrocyte lineage, such as GFAP [81]. Once Ngn1 expression is extinguished, when neurogenesis terminates, active Smad1 and Stat3 can interact and cooperatively enhance GFAP expression [81]. This mechanism provides a valid explanation for the temporal competence of canonical BMP signaling to promote one cell fate or another, in this case promoting the switch from neurogenesis to gliogenesis.
Conclusions
In vertebrates, BMPs are key signaling molecules that are employed throughout neural development to control the intricate processes involved in generating a functional CNS. As highlighted in this review, our understanding of the implications of BMP signaling in spinal cord development has advanced greatly in the past 15 years, enabling us to draw several key conclusions.
First, BMP signaling appears to be very dynamic during spinal cord development, at least along the DV axis. Before the closing of the neural tube and during the early stages of patterning, the canonical pathway is active throughout most of the neural plate [37, 50]. Following closure, when the neural tube is being patterned into precisely defined progenitor domains, BMP signaling is progressively restricted to the dorsal-most (dP1-3) neural tube [50]. This pathway is then re-deployed throughout most of the DV axis to control the rate of differentiation when neurogenesis commences [19], and to regulate axon orientation and growth [32, 34]. Later BMP activity appears to again be spatially modulated during gliogenesis [80].
The diverse activities of BMPs are transduced by distinct cellular components in function of the process they affect. The changes in cell shape and cytoskeletal organization controlled by BMPs appear to rely mainly on non-classical intracellular signaling. This may be Smad-dependent but independent of any transcriptional regulation, as proposed during neural tube closure [37]. Alternatively, BMP signaling may involve the recruitment of non-canonical effectors by BMP receptors, such as the PI3K–Akt cascade or LIMK in the case of repulsive axon orientation and outgrowth, respectively [34, 67]. By contrast, the control of neural progenitor identity and lineage commitment by BMPs appears to depend on Smad transcriptional activity [50, 75, 81].
Most of the functions ascribed to BMPs during neural development are not those of a classical morphogen that triggers different cellular responses in function of the strength and duration of the stimulus provided through the extracellular gradient emanating from a restricted source [82]. For instance, the activity of BMP7 during neurogenesis does not fulfill the classical definition of a morphogen [19]. Thus, some care should be taken when considering BMPs as classical morphogens in the context of neural development.
Finally, it should be noted that BMP signaling must be inhibited in many neuro-developmental processes, including: the closure of the neural tube [37]; the establishment of most progenitor domains during patterning [57]; the commitment to neuronal differentiation [19]; the specification and maturation of oligodendrocytes [75]; and the temporal restriction of astrocytogenesis [80]. Therefore, it would appear that BMPs favor the maintenance of a primary/stem cell state rather than actively instructing lineage progression or the acquisition of novel features in neural cells.
Acknowledgments
Work in EM’s laboratory was supported by Grants BFU2010-18959 and CSD2007-00008.
Contributor Information
Gwenvael Le Dréau, Phone: +34-93-4034972, FAX: +34-93-4034979, Email: gldbmc@ibmb.csic.es.
Elisa Martí, Email: elisa.marti@ibmb.csic.es.
References
- 1.Mizutani CM, Bier E. EvoD/Vo: the origins of BMP signalling in the neuroectoderm. Nat Rev Genet. 2008;9(9):663–677. doi: 10.1038/nrg2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4(10):784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- 3.Le Dréau G, Martí E. Dorsal–ventral patterning of the neural tube: a tale of three signals. Dev Neurobiol. 2012;72(12):1471–1481. doi: 10.1002/dneu.22015. [DOI] [PubMed] [Google Scholar]
- 4.Lewis KE. How do genes regulate simple behaviours? Understanding how different neurons in the vertebrate spinal cord are genetically specified. Philos Trans R Soc Lond B Biol Sci. 2006;361(1465):45–66. doi: 10.1098/rstb.2005.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468(7321):214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- 6.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147(1):35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 7.Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136(22):3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 8.Schmierer B, Hill CS. TGFbeta–SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8(12):970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 9.Schmierer B, Hill CS. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor beta-dependent nuclear accumulation of Smads. Mol Cell Biol. 2005;25(22):9845–9858. doi: 10.1128/MCB.25.22.9845-9858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 12.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9(22):2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 13.Kingsley DM, et al. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell. 1992;71(3):399–410. doi: 10.1016/0092-8674(92)90510-J. [DOI] [PubMed] [Google Scholar]
- 14.Winnier G, et al. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9(17):2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122(10):2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 16.Basler K, et al. Control of cell pattern in the neural tube: regulation of cell differentiation by dorsalin-1, a novel TGF beta family member. Cell. 1993;73(4):687–702. doi: 10.1016/0092-8674(93)90249-P. [DOI] [PubMed] [Google Scholar]
- 17.Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998;12(21):3394–3407. doi: 10.1101/gad.12.21.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solloway MJ, et al. Mice lacking Bmp6 function. Dev Genet. 1998;22(4):321–339. doi: 10.1002/(SICI)1520-6408(1998)22:4<321::AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Le Dreau G, et al. Canonical BMP7 activity is required for the generation of discrete neuronal populations in the dorsal spinal cord. Development. 2012;139(2):259–268. doi: 10.1242/dev.074948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishitoh H, et al. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem. 1996;271(35):21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- 21.Oh SP, et al. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA. 2000;97(6):2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bragdon B, et al. Bone morphogenetic proteins: a critical review. Cell Signal. 2011;23(4):609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Beppu H, et al. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221(1):249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- 24.Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374(6520):356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- 25.Oh SP, Li E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev. 1997;11(14):1812–1826. doi: 10.1101/gad.11.14.1812. [DOI] [PubMed] [Google Scholar]
- 26.Song J, et al. The type II activin receptors are essential for egg cylinder growth, gastrulation, and rostral head development in mice. Dev Biol. 1999;213(1):157–169. doi: 10.1006/dbio.1999.9370. [DOI] [PubMed] [Google Scholar]
- 27.Wine-Lee L, et al. Signalling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord. Development. 2004;131(21):5393–5403. doi: 10.1242/dev.01379. [DOI] [PubMed] [Google Scholar]
- 28.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. 2009;11(5):637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hester M, et al. Smad1 and Smad8 function similarly in mammalian central nervous system development. Mol Cell Biol. 2005;25(11):4683–4692. doi: 10.1128/MCB.25.11.4683-4692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tremblay KD, Dunn NR, Robertson EJ. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development. 2001;128(18):3609–3621. doi: 10.1242/dev.128.18.3609. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, et al. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development. 1999;126(8):1571–1580. doi: 10.1242/dev.126.8.1571. [DOI] [PubMed] [Google Scholar]
- 32.Hazen VM, et al. BMP receptor-activated Smads confer diverse functions during the development of the dorsal spinal cord. Dev Biol. 2012;367(2):216–227. doi: 10.1016/j.ydbio.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19(1):128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perron JC, Dodd J. Inductive specification and axonal orientation of spinal neurons mediated by divergent bone morphogenetic protein signalling pathways. Neural Dev. 2011;6:36. doi: 10.1186/1749-8104-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee-Hoeflich ST, et al. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. EMBO J. 2004;23(24):4792–4801. doi: 10.1038/sj.emboj.7600418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ybot-Gonzalez P, et al. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007;134(17):3203–3211. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- 37.Eom DS, et al. Bone morphogenetic proteins regulate neural tube closure by interacting with the apicobasal polarity pathway. Development. 2011;138(15):3179–3188. doi: 10.1242/dev.058602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo G, et al. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9(22):2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 39.Solloway MJ, Robertson EJ. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126(8):1753–1768. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- 40.McMahon JA, et al. Noggin-mediated antagonism of BMP signalling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12(10):1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misra K, Matise MP. A critical role for sFRP proteins in maintaining caudal neural tube closure in mice via inhibition of BMP signaling. Dev Biol. 2010;337(1):74–83. doi: 10.1016/j.ydbio.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Liem KF, Jr, Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91(1):127–138. doi: 10.1016/S0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- 43.Liem KF, Jr, et al. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82(6):969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- 44.Chesnutt C, et al. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274(2):334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Liem KF, Jr, Jessell TM, Briscoe J. Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development. 2000;127(22):4855–4866. doi: 10.1242/dev.127.22.4855. [DOI] [PubMed] [Google Scholar]
- 46.Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237(1):295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- 47.Hu Q, Ueno N, Behringer RR. Restriction of BMP4 activity domains in the developing neural tube of the mouse embryo. EMBO Rep. 2004;5(7):734–739. doi: 10.1038/sj.embor.7400184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohkawara B, et al. Action range of BMP is defined by its N-terminal basic amino acid core. Curr Biol. 2002;12(3):205–209. doi: 10.1016/S0960-9822(01)00684-4. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen VH, et al. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127(6):1209–1220. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- 50.Tozer S, et al. Temporal control of BMP signalling determines neuronal subtype identity in the dorsal neural tube. Development. 2013;140(7):1467–1474. doi: 10.1242/dev.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Timmer JR, Wang C, Niswander L. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development. 2002;129(10):2459–2472. doi: 10.1242/dev.129.10.2459. [DOI] [PubMed] [Google Scholar]
- 52.Hazen VM, et al. Inhibitory Smads differentially regulate cell fate specification and axon dynamics in the dorsal spinal cord. Dev Biol. 2011;356(2):566–575. doi: 10.1016/j.ydbio.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamauchi K, Phan KD, Butler SJ. BMP type I receptor complexes have distinct activities mediating cell fate and axon guidance decisions. Development. 2008;135(6):1119–1128. doi: 10.1242/dev.012989. [DOI] [PubMed] [Google Scholar]
- 54.Desgrosellier JS, et al. Activin receptor-like kinase 2 and Smad6 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2005;280(1):201–210. doi: 10.1016/j.ydbio.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 55.Mishina Y, et al. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol. 1999;213(2):314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- 56.Arkell R, Beddington RS. BMP-7 influences pattern and growth of the developing hindbrain of mouse embryos. Development. 1997;124(1):1–12. doi: 10.1242/dev.124.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Panchision DM, et al. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 2001;15(16):2094–2110. doi: 10.1101/gad.894701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ille F, et al. Wnt/BMP signal integration regulates the balance between proliferation and differentiation of neuroepithelial cells in the dorsal spinal cord. Dev Biol. 2007;304(1):394–408. doi: 10.1016/j.ydbio.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 59.Xie Z, et al. Smad6 promotes neuronal differentiation in the intermediate zone of the dorsal neural tube by inhibition of the Wnt/beta-catenin pathway. Proc Natl Acad Sci USA. 2011;108(29):12119–12124. doi: 10.1073/pnas.1100160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Augsburger A, et al. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 1999;24(1):127–141. doi: 10.1016/S0896-6273(00)80827-2. [DOI] [PubMed] [Google Scholar]
- 61.Butler SJ, Dodd J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 2003;38(3):389–401. doi: 10.1016/S0896-6273(03)00254-X. [DOI] [PubMed] [Google Scholar]
- 62.Perron JC, Dodd J. Structural distinctions in BMPs underlie divergent signaling in spinal neurons. Neural Dev. 2012;7(1):16. doi: 10.1186/1749-8104-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez-Camacho C, Bovolenta P. Emerging mechanisms in morphogen-mediated axon guidance. BioEssays. 2009;31(10):1013–1025. doi: 10.1002/bies.200900063. [DOI] [PubMed] [Google Scholar]
- 64.Perron JC, Dodd J. ActRIIA and BMPRII Type II BMP receptor subunits selectively required for Smad4-independent BMP7-evoked chemotaxis. PLoS ONE. 2009;4(12):e8198. doi: 10.1371/journal.pone.0008198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foletta VC, et al. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol. 2003;162(6):1089–1098. doi: 10.1083/jcb.200212060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez-Camacho C, et al. Morphogens as growth cone signalling molecules. Brain Res Brain Res Rev. 2005;49(2):242–252. doi: 10.1016/j.brainresrev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Phan KD, et al. The bone morphogenetic protein roof plate chemorepellent regulates the rate of commissural axonal growth. J Neurosci. 2010;30(46):15430–15440. doi: 10.1523/JNEUROSCI.4117-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ball RW, et al. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron. 2010;66(4):536–549. doi: 10.1016/j.neuron.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 69.Ji SJ, Jaffrey SR. Intra-axonal translation of SMAD1/5/8 mediates retrograde regulation of trigeminal ganglia subtype specification. Neuron. 2012;74(1):95–107. doi: 10.1016/j.neuron.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith RB, et al. Relay of retrograde synaptogenic signals through axonal transport of BMP receptors. J Cell Sci. 2012;125:3752–3764. doi: 10.1242/jcs.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.James RE, Broihier HT. Crimpy inhibits the BMP homolog Gbb in motoneurons to enable proper growth control at the Drosophila neuromuscular junction. Development. 2011;138(15):3273–3286. doi: 10.1242/dev.066142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCabe BD, et al. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39(2):241–254. doi: 10.1016/S0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 73.Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010;2(4):a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mekki-Dauriac S, et al. Bone morphogenetic proteins negatively control oligodendrocyte precursor specification in the chick spinal cord. Development. 2002;129(22):5117–5130. doi: 10.1242/dev.129.22.5117. [DOI] [PubMed] [Google Scholar]
- 75.Weng Q, et al. Dual-mode modulation of Smad signaling by Smad-interacting protein Sip1 is required for myelination in the central nervous system. Neuron. 2012;73(4):713–728. doi: 10.1016/j.neuron.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 77.Molofsky AV, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26(9):891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bond AM, Bhalala OG, Kessler JA. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev Neurobiol. 2012;72(7):1068–1084. doi: 10.1002/dneu.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakashima K, et al. Synergistic signaling in fetal brain by STAT3–Smad1 complex bridged by p300. Science. 1999;284(5413):479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 80.Agius E, et al. Role of BMPs in controlling the spatial and temporal origin of GFAP astrocytes in the embryonic spinal cord. Dev Biol. 2010;344(2):611–620. doi: 10.1016/j.ydbio.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 81.Sun Y, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104(3):365–376. doi: 10.1016/S0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- 82.Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135(15):2489–2503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]