Abstract
Enzyme-linked immunosorbent assay (ELISA) and culture are 2 common diagnostic tests for detecting Mycobacterium avium subsp. paratuberculosis (Map) in Johne’s disease, but they are not as sensitive as polymerase chain reaction (PCR). However, inhibitors can coextract with the target DNA and cause interference in PCR. Development of an immune capture assay followed by PCR amplification can alleviate this problem. In this study, we were able to induce an immune response in chickens using heat or formalin inactivated Map. The purified immunoglobulin (Ig)Y has a molecular weight of 160 kDa. The titers were at 1:6400 and 1:12 800 at weeks 5 to 6 and 8 to 9, respectively, as determined by the IDEXX modified ELISA kit for Johne’s disease. The IgY produced from inactivated bacterial cells had no effect on its ability to recognize live Map cells as illustrated by immunofluorescence assay and immune capture PCR results.
Résumé
L’épreuve ELISA et la culture sont deux méthodes de diagnostique usuelles pour détecter Mycobacterium avium subsp. Paratuberculosis (Map) dans les cas de maladie de Johne, mais elles ne sont pas aussi sensible que la méthode d’amplification en chaîne par la polymérase (PCR). Toutefois, des inhibiteurs peuvent être co-extraits avec l’ADN cible et causer de l’interférence dans l’épreuve PCR. Le développement d’une épreuve d’immuno-capture suivie d’une amplification par PCR permet d’éviter ce problème. Dans cette étude, nous avons été en mesure d’induire une réponse immunitaire chez des poulets en utilisant du Map inactivé par la chaleur ou la formaline. L’immunoglobuline (Ig)Y purifiée a un poids moléculaire de 160 kDa. Aux semaines 5 à 6 et 8 à 9 les titres sériques détectés à l’aide de la trousse ELISA modifié d’IDEXX pour la maladie de Johne étaient, respectivement, de 1:6400 et 1:12800. Les IgY produites suite à l’inoculation des bactéries inactivées étaient en mesure de reconnaître des cellules de Map vivantes tel que démontré par les épreuves d’immunofluorescence et de PCR avec immuno-capture.
(Traduit par Docteur Serge Messier)
Introduction
Mycobacterium avium subsp. paratuberculosis (Map) is the causative agent for Johne’s disease, an infectious, progressive chronic digestive disorder of both wild and domestic ruminants. It is a worldwide problem with great economic impact, especially in the cattle industry (1–3). Presently, culture is considered the “gold standard” for the diagnosis of Map, although the sensitivity of this test depends on the stage of the disease in the animal. The detection level is reported to be as few as 10 organisms per gram of feces using a radiometric technique with filter concentration (4) or 1000 organisms per gram of feces by conventional culture with a sedimentation technique (5). Although molecular tests such as polymerase chain reaction (PCR) are rapid and sensitive, the presence of unknown inhibitors in the bovine fecal samples can prevent amplification (6,7). An alternative means to increase test specificity is to immunocapture the organism before extracting the nucleic acid to circumvent the problem of inhibition. Immunocapture assay has been used to increase the level of detection of Map (8,9).
Antibodies have been widely used in both research and diagnostic applications and mammals, such as rabbits, have frequently been chosen for producing specific polyclonal antibodies. In recent years, focus on production of polyclonal antibodies has shifted from mammals to avian species as alternate hosts. Chickens are commonly used to produce polyclonal antibodies to some conserved mammalian proteins due to their evolutionary distance from mammals (10). Chicken eggs have been used as an excellent source of polyclonal antibodies in many studies (11–15). Each bird can produce approximately 5 to 6 eggs per week with a yolk volume of approximately 15 mL that contains an equivalent amount of immunoglobulin (Ig)G found in 90 to 100 mL of serum. This amount is 10 times higher than the normal volume of serum that can be collected from an immunized rabbit per week. Furthermore, the process of bleeding rabbits is invasive and far more stressful to the animal compared with collecting eggs from a chicken. The primary shortcoming of using egg-derived antibodies is that the extraction of immunoglobulin from the yolk is more labor intensive and time consuming than the preparation of immunoglobulin from mammalian sera.
There are 3 major immunoglobulin classes in chickens: IgG (referred to as IgY), IgA, and IgM (16). During the maturation of the egg in the oviduct, active transport of IgY from the chicken’s serum to the yolk results in significant IgY levels in the yolk (17). The IgY does not bind to protein A (18), protein G (19), mammalian Fc receptors, or mammalian complement (20); consequently, the purification of IgY is different from the purification of mammalian IgG.
The production of IgY is simple and has many applications. Therefore, the aim of this study is to immunize chickens with Map for large scale production of antibodies and to evaluate the specificity and sensitivity of IgY in capturing the bacterium for the future development of an immunomagnetic separation-PCR based diagnosis of Johne’s disease.
Materials and methods
Bacterial strains
The Map field strains FR2616, AA3814, EA4146, EQ2356, ER2945Y162, 11992, 12258, 4200, and 11520-5 were obtained from the Agri-Food Laboratories Branch, Alberta Agriculture, Food and Rural Development, Edmonton, Alberta. These field isolates were all confirmed to be Map by culture at the Mycobacterium Division of the Provincial Laboratory of Public Health (Microbiology). In addition, the following strains were included in the study to test for specificity: M. avium (ATCC 25291); M. gordonae (ATCC 14470); M. intracellulare (ATCC 13950); BCG (ATCC 27291); M. tuberculosis H37Ra (ATCC 2177); and 2 control isolates of Map, ATCC 19698 and ATCC 43544, that were purchased from the American Type Culture Collection (Manassas, Virginia, USA).
Restriction enzyme analysis and southern hybridization
Bacterial cultures of ATCC 19698, ATCC 43544, EQ2356, FR2616, EA4146, 4200, and AA3814 were grown in Middlebrook 7H9 liquid medium and washed twice in phosphate buffered saline solution (PBSS), pH 7.4. Two hundred microliters of siliconized GLC 40 mesh glass beads (BDH, Toronto, Ontario), were added to the pellet and vortexed vigorously to resuspend the pellet. After adding 500 μL of Tris-EDTA (TE) buffer (pH 8.4), an equal volume of phenol-chloroform was added. The mixture was vortexed for 1 min, followed by centrifugation at 13 500 × g for 10 min. The aqueous layer was removed to another microfuge tube and the phenol-chloroform extraction step was repeated. The aqueous layer was removed and was extracted with chloroform. The DNA was precipitated in the presence of 3.0 M sodium acetate, pH 5.2, and isopropanol. The DNA was quantified using a DyNA Quant 200 fluorometer (Hoefer Pharmacia Biotech, San Francisco, California, USA). One-hundred and fifty nanograms of DNA were digested using 1 unit of BstEII (Invitrogen, Burlington, Ontario) or PvUII (Invitrogen) at 37°C for 3 h. The digested DNA was electrophoresed in a 0.8% agarose gel at 35V for 22 h. The DNA was denatured in 0.5 M NaOH per 1.5 M NaCl for 15 min, and neutralized in 1 M Tris per 1.5 M NaCl buffer (pH 8.0) for 1 h. The DNA fragments were passively transferred from the gel to a Hybond N nylon membrane (Amersham, Arlington Heights, Illinois, USA) by a standard blotting protocol (21) using 6 × SSC buffer (0.9 M NaCl per 0.09 M Na3-Citrate-2H2O). The DNA fragments were cross-linked to the membrane using the cross-linking program preset for southern damp membrane (GS Gene Linker UV chamber; BioRad, Mississauga, Ontario). The membrane was stored at –20°C in the freezer until required for southern hybridization.
The probe for southern hybridization was prepared using PCR and established primers designated J5A (forward 5′-ATGTGGTTGCTGTGTTGGATGG-3′), and J5B (reverse 5′-CCGCCGCAATCAACTCCAG-3) (22) targeting the Map IS900 insertion element. The size of the amplified product was 298 base pairs (bp). Ten microliters of DNA was added to a cocktail containing 1 × PCR buffer, 1.5 mM magnesium chloride, 0.1 mM deoxynucleotide triphosphates (dNTPs) (DIG DNA Labelling Mix; Roche-Boehringer Mannheim, Mississauga, Ontario) and 0.75 μM of each primer. Touchdown cycling parameters were as follows: 94°C for 5 min; 94°C for 30 s, 66°C for 30 s, and 72°C for 1 min for 4 cycles; 94°C for 30 s, 64°C for 30 s, and 72°C for 1 min for 4 cycles; 94°C for 30 s, 62°C for 30 s, and 72°C for 1 min for 4 cycles; 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min for 24 cycles; and a final extension at 72°C for 7 min. The amplicons were detected by electrophoresis in a 1% agarose gel containing 0.5% ethidium bromide and visualized by ultra violet (UV) ) illumination. A 1 kb DNA size marker (Invitrogen) was included in each gel. The amplicons were purified using a kit (QIAquick PCR Purification Kit; Qiagen, Valencia, California, USA), as per manufacturer’s instruction. The amount of DIG labelling in the purified amplicon was determined by visual comparison of color to a set of standard DNA dotted on a control strip (Roche-Boehringer Mannheim). The probe was stored at –20°C in the freezer until use.
Southern hybridization was performed as follows. The membrane was prehybridized for 2 h at 68°C with gentle shaking in 20 mL of pre-hybridization solution consisting of 6 × SSC (0.5 M sodium chloride, 0.015 M sodium citrate, pH 7.0), 5 × Denhardt solution (0.1% polyvinylpyrrolodone, 0.1% Ficoll, 0.1% bovine serum albumin, 1.0% sodium dodecyl sulfate [SDS]), and 100 μg/mL denatured salmon sperm DNA. After prehybridization, the denatured DIG probe was added to a final concentration of 15 pg/20 mL of hybridizing buffer. Hybridization was carried out overnight at 68°C in a circulating water bath. Membranes were rinsed twice in 6 × SSC (pH 7.0) and once in 6 × SSC with 0.1% SDS for 1.5 h at 68°C. The membranes were rinsed briefly with 1 × maleate buffer (0.1 M maleaic acid per 0.15 M sodium chloride) followed by 1 × maleate buffer with 1.5% blocking solution (Roche Diagnostics Corporation, Indianapolis, Indiana, USA) for 2 h at room temperature. The buffer was replaced with 1 × maleate buffer containing 3 units of anti-digoxigenin, 1% blocking reagent and incubated for 1 h at room temperature. After 4 washes of 15 min each with 1 × maleate buffer with 0.3% Tween 20, the membrane was equilibrated with buffer C (100 mM Tris per 100 mM NaCl) for 5 min before incubating the membrane in 0.25 mM of CSPD (Disodium3-[4-methoxyspiro (1,2-dioxetane-3,2′ -[5′ -chloro] tricyclo [3.3.1.13,7]decan)-4-yl] phenyl phosphate) in buffer C containing 0.1% magnesium chloride at 37°C for 5 min. The membrane was placed in cassettes with X-Omat AR X-ray film (Eastman, Rochester, New York, USA) and developed. The bands were examined visually and analysis was performed (Gel Doc System; BioRad and BioNumerics Software; Applied Maths, Austin, Texas, USA). A dendrogram was generated using Dice coefficient with 1% tolerance.
Immunization and IgY preparation
For immunization of the chickens, single comb white leghorn (SCWL) chickens at age 23 wk of age were obtained, housed, and cared for in accordance with the Canadian Council on Animal Care guidelines for animal welfare. They were immunized according to the procedure described by Sunwoo et al (23). Briefly, 2 pools containing 4 different strains of Map (FR2616, AA3814, EA4146, EQ2356) were inactivated either in 10% formalin overnight or by heating at 90°C for 1 h. The organisms in the pools were washed in sterile PBSS, pH 7.4, and cell density was adjusted to a McFarland #1 standard. Cells were emulsified with an equal volume of Freund’s incomplete adjuvant (Fisher Scientific, Nepean, Ontario) and injected intramuscularly into the breast muscles using 0.5 mL per site. Each prepared pool was inoculated into 2 chickens. Booster immunizations were given 3 and 7 wk after the initial immunization. Eggs were collected daily and stored at 4°C until processed. For the control chickens, PBSS without Map was injected in an identical manner.
The IgY antibody was purified using a kit (EggSTRACT kit; Promega, Madison, Wisconsin, USA), as per the manufacturer’s instruction. Briefly, each egg yolk was separated from the white using an egg separator. The yolk membrane was ruptured and the volume was measured. Three times the yolk volume of precipitation solution A was added to the yolk while stirring with a magnetic stir bar to precipitate the lipids. Centrifugation was performed at 10 000 × g for 10 min at 4°C. The supernatant was filtered through sterile gauze and the volume was measured. One part precipitation solution B was slowly added to 3 parts supernatant while stirring on a magnetic stirrer at room temperature to precipitate the IgY. After centrifugation at 10 000 × g for 10 min at 4°C, the supernatant was discarded and the pellet was dissolved in a volume of PBSS equal to the volume of the yolk as measured previously and stored at –20°C. The protein concentration of the purified IgY was determined (Protein Assay; BioRad). A random sample of 10 IgY preparations was examined for purity by comparison to an IgY standard (Promega) using polyacrylamide gel electrophoresis (PAGE). A precast 12% polyacrylamide mini gel (NuPAGE Bis-Tris SDS-PAGE; Invitrogen) using an Xcell SureLock Mini-Cell (Invitrogen) was run at 200V for 45 min. The bands were visualized by staining the gel (SimplyBlue SafeStain; Invitrogen), as per the manufacturer’s instruction.
Enzyme-linked immunosorbent assay (ELISA) and immunofluorescent (IF) assay
The immune response titer of the IgY to inactivated Map by heat or formalin was monitored by ELISA using a Johne’s test kit (IDEXX Laboratories, Westbrook, Maine, USA). The secondary antibody (anti-bovine IgG) was replaced with anti-chicken IgY (Southern Biotechnology Associates, Birmingham Alabama, USA). The testing procedure was performed according to the IDEXX version of Map ELISA protocols (Herdchek Map Antibody Test Kit; IDEXX Laboratories). Purified IgY samples diluted from 1:500 to 1:16,000 were added to the Map antigen-coated 96 well microplate and incubated for 30 min at 37°C. After incubation, the wells were washed 4 times with 350 μL PBSS in an automatic microplate washer (Spectramax 340 PC; Molecular Devices, Sunnyvale, California, USA). A 100 μL volume of diluted rabbit anti-chicken IgY:HRPO (horseradish peroxidase) conjugate (Promega Corporation) was added to each well and the plate was incubated for 30 min at room temperature. The washing step was repeated as above followed by the addition of 100 μL of TMB (3,3′ 5,5′ Tetramethybenzidine; IDEXX Laboratories) substrate solution to develop the enzymatic color reaction. The reaction was stopped by adding 100 μL of 0.125% hydrofluoric acid (IDEXX Laboratories). Optical density values were read at 650 nm using a Vmax microplate reader (Molecular Devices). Initially, IgY from 3 eggs was randomly chosen to determine the immune response titer for each week. When an immune response was detected, all IgY preparations from subsequent weeks were tested.
Specificity of the IgY to Map and other closely related Mycobacteria sp. was determined using IF microscopy. Map strains (FR2616, ATCC 19698), M. avium, M. gordonae, M. intracellulare, BCG, and M. tuberculosis H37Ra were standardized to a concentration of 104 cells/mL. One hundred microliters of standardized cells were combined with IgY dilutions of 1:10, 1:50, 1:100, 1:250, 1:500, 1:750, 1:1000, 1:1500, and 1:2000. After 1 h of incubation at 37°C, the cells were washed 5 times with PBSS, pH 7.4. The cells were resuspended in 100 μL of PBSs and goat anti-chicken IgG (H and L) FITC (Southern Biotechnology Associates) was added to a final concentration of 1:20, as specified by the manufacturer. After incubation at 37°C for 30 min, the cells were washed as described above and resuspended in 100 μL of PBSS. Ten microliters of cells were placed on glass slides and examined using an epi fluoresecent microscope (Zeiss Axioskop 20; Carl Zeiss, Oberkochen, Germany). The IgY preparations that showed cross reactivity were subjected to a pre-adsorption protocol. Five millilitres of M. avium cells adjusted to a concentration of 108 cells/mL were centrifuged at 13 000 × g for 5 min and the pellet was suspended in 5 mL of pooled IgY (titer > 1/2000). The cell suspension was incubated, with gentle rotation, at 4°C overnight. After incubation, the cells were centrifuged at 13 000 × g and the IgY was removed and filtered through a 0.22 micron filter and retested with Map and M. avium cells.
Polymerase chain reaction
In order to confirm the binding specificity of the different Map and other Mycobacterium strains, 200 μL aliquots of each of the above bacterial cell suspensions were centrifuged at 13 000 × g for 5 min and the pellets were resuspended in 200 μL of pre-adsorbed antibody at a final concentration of 1/500 for 1 h followed by incubation with 2 μL of rabbit anti-chicken IgG (MagaBeads; Cortex BioChem, San Leandro, California, USA) for 1 h at room temperature. After 5 washes with PBSS, 1% SDS in 12 mM Tris-HCl (pH 7.4) was added and the bacteria was eluted from the bacteria complex (MagaBeads) at room temperature for 15 min. A magnet was applied to the beads and DNA was extracted (MagaZorb DNA extraction kit; Cortex BioChem) from the supernatant. Briefly, 200 μL of eluted bacterial cells were added to 20 μL of proteinase K (pK) solution, followed by the addition of 200 μL of lysis buffer. After vortexing for 15 s, the tubes were incubated for 10 min at 56°C. Five hundred microliters of binding buffer and 20 μL of magnetic particles were added, followed by incubation with mixing at room temperature for 10 min. A magnetic rack was used to sediment the particle-bound DNA. After 3 washings with 1 mL wash buffer, the particles (MagaZorb) were sedimented by magnetic rack and the final wash was discarded. The DNA was eluted from the beads by adding 200 μL of 12 mM Tris-HCl, pH 7.4. After mixing for 10 min at 56°C, the particles were separated from the DNA solution by using the magnetic rack. In order to check the specificity of the immunocapture, PCR assays were run using Map specific primers, J5A and J5B, and universal primers directed to the 23S rRNA sequence (24). The primer sequences for the latter target were as follows: forward-5′ GCGATTTCYGAAYGGGGRAACCC and reverse- TTCGCCTTTCCCTCACGGTACT (where Y is C or T and R is A or G). The PCR master mix consisted of 1 × PCR buffer, 2.0 mM magnesium chloride, 150 μM of dNTPs, 1.0 μM of each primer, and 2 μL of template DNA in a total volume of 50 μL. The PCR conditions consisted of: 95°C for 5 min; 95°C for 15 s, 55°C for 15 s, and 72°C for 15s for 25 cycles; 95°C for 15 s, 65°C for 30 s, and 72°C for 15s for 25 cycles; and a final extension at 72°C for 7 min. The PCR product was subjected to agarose gel electrophoresis and the bands were visualized after staining with ethidium bromide, as described above.
Results
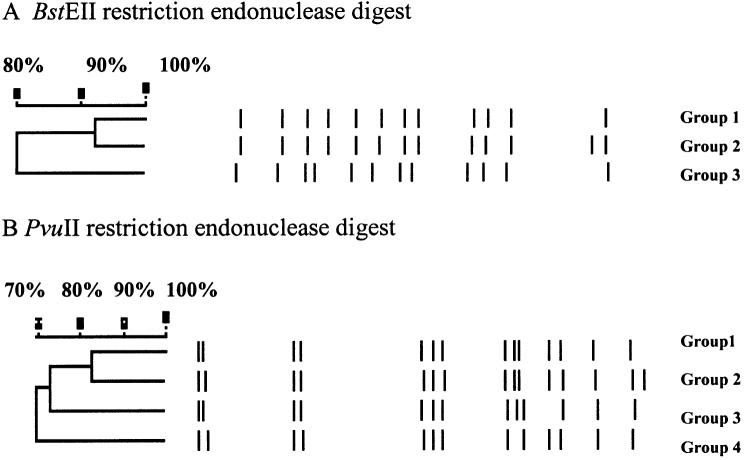
Restricted fragment length polymorphism (RFLP) generated by Southern hybridization is shown in Figure 1. Five isolates of Map (FR2616, AA3814, EA4146, EQ2356, and 4200) collected from 5 geographical regions showed 3 different fingerprinting patterns using BstEII or PvuII restriction digest. Group 1 of the BstEII digests consisted of EQ2356, FR2616, and AA3814; groups 2 and 3 have only 1 isolate in each group and they are EA4146 and 4200, respectively. The 2 reference strains (ATCC 43544 and ATCC 19698) also shared the BstEII group 1 pattern. However, the PvuII restriction digest pattern differentiates the reference strain ATCC 43544 into a separate group designated as group 4. No change in the groupings were observed with respect to the other isolates. A recent study by Motiwala (25) clearly shows that there is a high degree of genetic similarity among bovine Map isolates regardless of geographic origin.
Figure 1.
Panels A and B. Mycobacterium avium subsp. paratuberculosis (Map) DNA isolated from FR2616, AA3814, EA4146, EQ2356, 4200, ATCC 43544, and ATCC 19698 was cleaved using restriction endonucleases BstEII and PvuII and followed by Southern hybridization with probe targeting IS900.
The average volume and concentration of IgY purified (EggSTRACT kit), based on 100 eggs was 5.55 mL and 5.75 mg/mL per egg, respectively. Polyacrylamide gel analysis of 25 different purified IgY preparations showed 2 bands (Figure 2). The molecular weight of the major band corresponds to the IgY standard fragment (MW = 160 kDa) indicating the success of the extraction. There was also a minor contaminating band with a molecular weight of 65 kDa.
Figure 2.
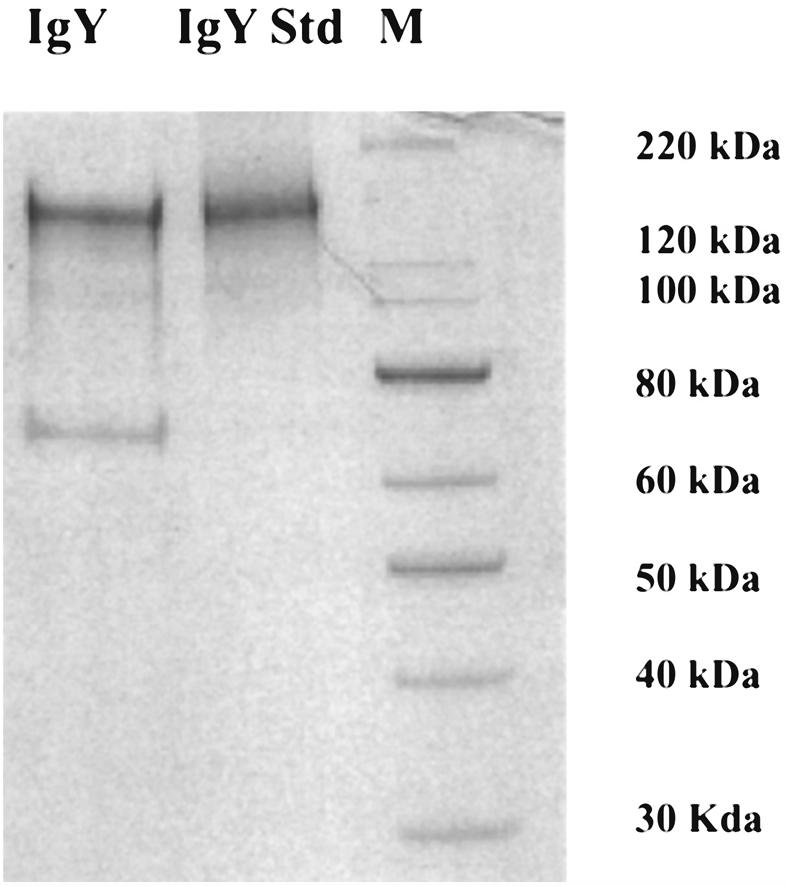
Polyacrylamide gel electrophoresis analysis of immunoglobulin (Ig)Y prepared from chicken egg yolk. Lanes 1 to 3: IgY purified from chicken immunized with heat inactivated Mycobacterium avium subsp. paratuberculosis (Map), standard IgY, and protein standards (MagicMark XP), respectively.
The OD values of the IgY from the negative control chickens were less than 0.10. Samples were classified as positive for Map antibodies if the OD was at least 2 × greater than the OD of the negative control (≥ 0.20). The titer was defined as the highest dilution of the sample yielding a positive ELISA result. The IgY responses to formalin and heat inactivated Map by the immunized chickens are shown in Figure 3. After a booster immunization at week 3 with formalin inactivated antigen, there was an immediate antibody titer rise the following week, reaching a peak at weeks 5 and 6. By week 7, the antibody level dropped, but with the administration of a 2nd booster at this time, the immune response was immediate and reached a higher peak than previously recorded. Once again, the antibody titer slowly decreased over a period of 6 wk. A similar observation was seen with the antibody response curve when the heat inactivated antigen was injected into the chickens. Although the OD readings were consistently higher with the IgY produced using heat inactivated Map than the formalin inactivated Map, there was no difference detected in the titers. The titers at 5 to 6 wk and 8 to 9 wk were 1/6400 and 1/12 800, respectively, for both types of immunogens. The IgY produced from heat inactivated Map was chosen for the subsequent immunocapture experiments.
Figure 3.
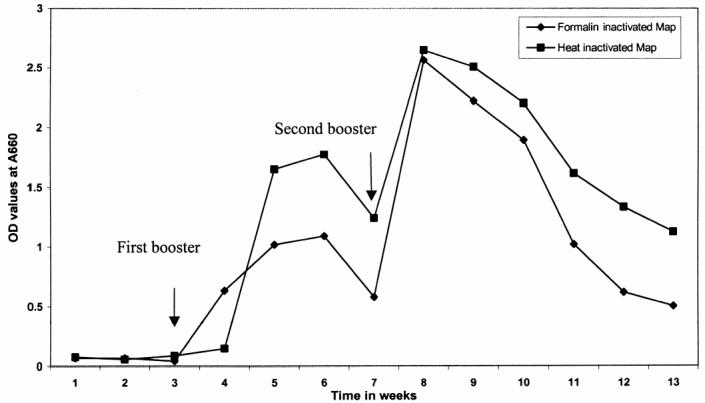
Immunoglobulin (Ig)Y response to the immunization of chickens with heat and formalin inactivated Mycobacterium avium subsp. paratuberculosis (Map) over a period of 13 wk.
Initial IF studies showed cross reactivity of the IgY with the M. avium strain. Using 1:10, 1:50, and 1:100 dilutions of the IgY with M. avium cells, all of the fields examined were positive for fluorescence. No fluorescence was seen with similar dilutions for the remaining Mycobacterium species. After the pre-adsorption with M. avium cells, none of the dilutions of IgY used for the IF assay cross reacted with M. avium cells, but there was a slight decrease in fluorescence with Map when incubated with 1:1000 dilution of IgY.
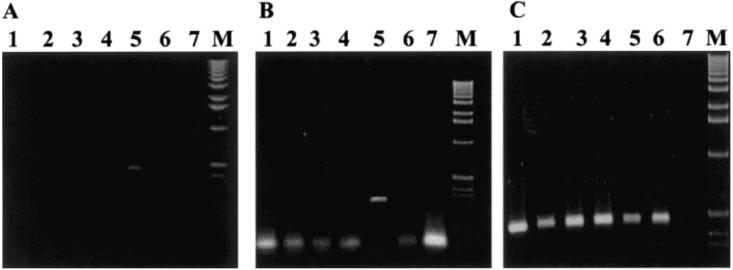
Mycobacterium tuberculosis, M. intracellulare, BCG, M. gordonae, Map, and M. avium were included in the capture study. Only Map was captured by the IgY as shown in Figure 4A using primers directed to the 23S rRNA and further confirmed by PCR using primers J5A and J5B directed to the insertion element IS900 (Figure 4B). As a control, DNA extracted from all strains was tested by PCR with the 23S rRNA primers (Figure 4C). The 23S rRNA primers amplified DNA from all the strains tested. In addition, 6 strains of Map that were not included in the immunization of the chicken (ATCC 19698, 11520-5, 12258, 11992, ER2945Y162, ATCC 43544) and the 4 strains used to inject the chickens (FR2616, AA3814, EA4146, and EQ2356) were all positive by PCR using primers J5A and J5B following the IgY immunocapture assay (Figure 5).
Figure 4.
Polymerase chain reaction (PCR) results with immunocapture using immunoglobulin (Ig)Y isolated from chickens immunized with heat inactivated Mycobacterium avium subsp. paratuberculosis (Map). Lanes 1 to 7: M. tuberculosis H37Ra, M. intracellulare, BCG, M. gordonae, Map, M. avium, negative control. M: 1 kb marker. The bacterial cells were subjected to immunocapture in panels A and B. The PCR primers were directed against the 23S rRNA sequence in panel A (amplicon size is 495 base pairs [bp]) and IS900 (amplicon size is 298 bp) in panel B. Cells were not captured in panel C and the primers for the PCR assay were targeting the 23S RNA region.
Figure 5.
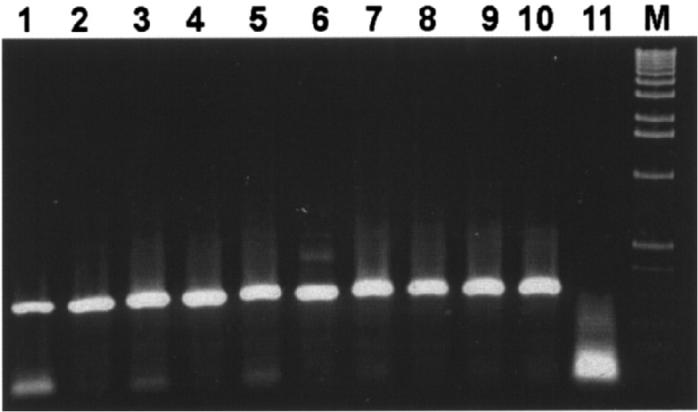
Different strains of Mycobacterium avium subsp. paratuberculosis (Map) were captured by immunoglobulin (Ig)Y and polymerase chain reaction (PCR) performed using primers J5A and J5B. The size of the amplicon is 298 base pairs (bp). Lanes 1 to 11: AA3814, ATCC 19698, FR2616, 11520-5, 12258, 11992, ER2945Y162, EA4146, ATCC 43544, EQ2356, negative control. M: 1 kb molecular size marker.
Discussion
Our previous study (26) showed that screening for Map using PCR on feces from cattle encounters major problems with inhibition. In order to circumvent the problem, a 1:100 dilution of the extracted DNA was required. Consequently, specimens with low bacterial loads could produce false negative results. To increase the sensitivity of PCR by eliminating the need for diluting the template DNA, production and characterization of IgY for an immunocapture assay was investigated. Applications using IgY for detecting pathogens such as Salmonella (14,15,27,28), E. coli (11,29–33), Streptococcus (12,34,35), Staphylococcus (28), rotavirus (36,37), and coronavirus (38) have been widely published in the literature. In this investigation, Map, the infectious agent for Johne’s disease in cattle, was successful in inducing an immune response in chickens. A pool consisting of 4 different Map strains were included in the immunization program. Since Map is a potential pathogen for both the handlers and the chickens, inactivation of the bacteria was required. Inactivation by heat or formalin had no effect on the ability of the purified IgY to recognize live Map cells as illustrated by the IF assay and immunocapture PCR. Secondly, the ELISA titers were identical when both types of antigens were used for immunization.
Although both bacterial suspensions were standardized to the same concentration as measured by the OD before inactivation, the immune response measured in OD values was higher with the cells that were inactivated by heat than those inactivated by formalin. Standardization of the bacterial suspensions for immunization was based on 1 OD550 being equivalent to 2.8 × 106 to 2.8 × 107 mycobacterial cells/mL (39). This “range” in cell count could explain the slight difference in immune response between the 2 types of inactivated antigen used. The secondary response after booster was much higher than the primary response independent of the type of antigen being used in the immunization. The IgY was specific and there was no cross reactivity observed with other closely related Mycobacterium species except with M. avium. Adsorption of the IgY with M. avium, eliminated the non-specific binding without affecting the binding of the IgY to the 10 different Map strains tested, independent of whether they had been included as immunogen for the immunization program. However, there was a slight decrease in binding efficiency of the antibody to Map after the pre-adsorption as indicated by the result of the fluorescent assay using 1:1000 dilution of the antibody. This can be explained by loss of IgY bounded to M. avium during the pre-adsorption step. The IgY preparation (EggSTRACT kit) can yield high quality and quantity antibodies. A contaminating band, with a molecular weight at 65 kDa, was detected by PAGE, but did not seem to have an effect on the binding capacity of IgY to Map.
At present, the diagnosis of Johne’s is mainly based on ELISA or culture. The sensitivity of ELISA is low and reported to be in a range from 15% to 87% depending on the stage of the disease (40). The turn-around time for culture can vary from between 8 and 20 wk (4 to 6 wk for liquid based systems) depending on the bacterial load of the infected animal. In recent years, with the development of molecular methods, PCR is one of the amplification tests that can provide increased sensitivity and rapid detection of microorganisms. However, due to the complexity of bovine fecal material, both known and unknown inhibitors can be co-extracted with target DNA resulting in PCR failure. Dilution of the target DNA can, at times, eliminate the effect of inhibitory factors but it also dilutes out the target DNA resulting in a loss of sensitivity. It is, therefore, important to search for other means to improve the detection of Map by PCR.
In this study, we have demonstrated success in eliciting immune responses by injecting SCWL chickens with heat or formalin inactivated Map. The IgY antibody isolated from the eggs is specific, able to capture live organisms and has potential in the development of an immunocapture assay for Map in bovine fecal samples. The use of immunocapture will enable rapid PCR detection of Map by eliminating the inhibitory problems associated with complex clinical specimens, such as bovine feces.
Acknowledgments
The authors express their appreciation to Catherine Taylor for her technical support and to the Canada Alberta Beef Industry Development Fund for financial support.
References
- 1.Chiodini RJ, Van Kruinigen HJ, Merkat RS. Ruminant paratuberculosis (Johne’s disease): the current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- 2.Merkal RS, Whipple DL, Sacks JM, Snyder GR. Prevalence of Mycobacterium paratuberculosis in ileocecal lymph nodes of cattle culled in the United States. J Am Vet Med Assoc. 1987;190:676–680. [PubMed] [Google Scholar]
- 3.Pandey GS, Musonda TL, Chizyuka HGB, Scheebeli M. Paratuberculosis (Johne’s disease) in a herd of Friesian cattle in Zambia. Vet Rec. 1987;120:369. doi: 10.1136/vr.120.15.369. [DOI] [PubMed] [Google Scholar]
- 4.Collins MT, Kenefick BK, Sockett DC, Lambrecht RS, McDonald J, Jorgensen JB. Enhanced radiometric detection of Mycobacterium paratuberculosis by using filter-concentrated bovine fecal specimens. J Clin Microbiol. 1990;28:2514–2519. doi: 10.1128/jcm.28.11.2514-2519.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whipple DL, Merkal RS. Procedures for the field and laboratory processing of fecal specimens for the isolation of Mycobacterium paratuberculosis. Proc Annu Meet US Anim Health Assoc. 1985;89:475–479. [Google Scholar]
- 6.Al-Soud WA, Rådström R. Effects of amplification facilitators on diagnostic PCR in the presence of blood, feces and meat. J Clin Microbiol. 2000;28:4463–4470. doi: 10.1128/jcm.38.12.4463-4470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilde J, Eiden J, Yolken R. Removal of inhibitory substances from human specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J Clin Microbiol. 1990;28:1300–1307. doi: 10.1128/jcm.28.6.1300-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant IR, Pope CM, O’Riordan LM, Ball HJ, Rowe MT. Improved detection of Mycobacterium avium subsp. paratuberculosis in milk by immunomagnetic PCR. Vet Microbiol. 2000;77:369–378. doi: 10.1016/s0378-1135(00)00322-9. [DOI] [PubMed] [Google Scholar]
- 9.Djonne B, Jensen MR, Grant IR, Holstad G. Detection by immunomagnetic PCR of Mycobacterium avium subsp. paratuberculosis in milk from dairy goats in Norway. Vet Microbiol. 2003;92:135–143. doi: 10.1016/s0378-1135(02)00355-3. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt P, Wanke R, Linckh E, Wiedemann V, Kuhlmann R, Losch U. Chicken egg antibodies for prophylaxis and therapy of infectious intestinal diseases. II. In vitro studies on gastric and enteric digestion of egg yolk antibodies specific against pathogenic Escherichia coli strains. J Vet Med. 1989;36:619–628. doi: 10.1111/j.1439-0450.1989.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 11.Sunwoo HH, Lee E, Manninen K, Suresh MR, Sim JS. Growth inhibitory effect of chicken egg yolk antibody (IgY) on Escherichia coli 0157:H7. J Food Sci. 2002;67:227–252. [Google Scholar]
- 12.Chang HM, Ou-Yang R, Chen YT, Chen CC. Productivity and some properties of immunoglobulin specific against Streptococcus mutans serotype c in chicken egg yolk (IgY) J Agric Food Chem. 1999;47:61–66. doi: 10.1021/jf980153u. [DOI] [PubMed] [Google Scholar]
- 13.Hatta H, Tsuda K, Akachi S, Kim M, Yamamoto T, Ebina T. Oral passive immunization effect of anti-human rotavirus IgY and its behavior against proteolytic enzymes. Biosci Biotech Biochem. 1993;57:1077–1081. doi: 10.1271/bbb.57.1077. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama H, Umeda K, Peralta RC, et al. Oral passive immunization against experimental salmonellosis in mice using chicken egg yolk antibodies specific for Salmonella enteritidis and Salmonella typhimurium. Vaccine. 1998;16:388–393. doi: 10.1016/s0264-410x(97)80916-4. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama H, Peralta RC, Umeda K, et al. Prevention of fatal salmonellosis in neonatal calves, using orally administered chicken egg yolk Salmonella-specific antibodies. Am J Vet Res 1998;59:4,416–420. [PubMed]
- 16.Schranner I, Losch U. Immunological identification of avian monomeric and polymeric immunoglobulin M and immunoglobulin A after fractionation on sodium dodecylsulfate pore gradient polyacrylamide gels. Poult Sci. 1986;65:360. doi: 10.3382/ps.0650360. [DOI] [PubMed] [Google Scholar]
- 17.Rose ME, Orlans E, Buttress N. Immunoglobulin classes in the hen’s egg: their segregation in yolk and white. Eur J Immunol. 1974;4:521–523. doi: 10.1002/eji.1830040715. [DOI] [PubMed] [Google Scholar]
- 18.Kronvall G, Seal US, Finstad J. Phylogenetic insight into evolution of mammalian Fc fragment of gamma G globulin using staphylococcal protein A. J Immunol. 1970;104:140–147. [PubMed] [Google Scholar]
- 19.Larsson A, Balow RM, Lindahl TL, Forsberg PO. Chicken antibodies: Taking advantage of Evolution — A Review. Poult Sci. 1993;2:1807–1812. doi: 10.3382/ps.0721807. [DOI] [PubMed] [Google Scholar]
- 20.Benson HN, Brumfield HP, Pomeroy BS. Requirement of avian C1 for fixation of guinea pig complement by avian antibodyantigen complexes. J Immunol. 1961;87:616–622. [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual, Vol. 2, 2nd ed. Cold Spring Harbour Lab Press 1989:931–937.
- 22.Hermon-Taylor J, Bull TJ, Sheridan JM, Cheng JM, Stellakis L, Sumar N. Causation of Crohn’s disease by Mycobacterium avium subspecies paratuberculosis. Can J Gastroenterol. 2000;14:521–539. doi: 10.1155/2000/798305. [DOI] [PubMed] [Google Scholar]
- 23.Sunwoo HH, Nakano T, Dixon WT, Sim JS. Immune responses in chickens against lipopolysaccharide of Escherica coli and Salmonella typhimurium. Poult Sci. 1996;75:342–345. doi: 10.3382/ps.0750342. [DOI] [PubMed] [Google Scholar]
- 24.Anthony RM, Brown TJ, French GL. Rapid diagnosis of bacteria by universal amplification of 23S ribosomal DNA followed by hybridization to an oligonucleotide array. J Clin Microbiol. 2000;38:781–788. doi: 10.1128/jcm.38.2.781-788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motiwala AS, Strother M, Amonsin A, et al. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis: evidence for limited strain diversity, strain sharing, and identification of unique targets for diagnosis. J Clin Microbiol. 2003;41:2015–2026. doi: 10.1128/JCM.41.5.2015-2026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chui LW, King R, Lu P, Manninen K, Sim J. Evaluation of four DNA extraction methods for the detection of Mycobacterium avium subsp. paratuberculosis by polymerase chain reaction. Diagn Microbiol Infect Dis. 2004;48:39–45. doi: 10.1016/j.diagmicrobio.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Opitz HM, El-Begearmi M, Flegg P, Beane D. Effectiveness of five feed additives in chicks infected with Salmonella enteritidis Phage Type 13A. J Appl Poult Res. 1993;2:147–153. [Google Scholar]
- 28.Yoshiko SK, Shibata K, Yun SS, Yukiko HK, Yamaguchi K, Kumagai S. Immune functions of immunoglobulin Y isolated from egg yolk of hens immunized with various infectious bacteria. Biosci Biotech Biochem. 1996;60:886–888. doi: 10.1271/bbb.60.886. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama H, Peralta RC, Diaz R, Sendo S, Ikemori Y, Kodama Y. Passive protective effect of chicken egg yolk immunoglobulins against experimental enterotoxigenic Escherichia coli infection in neonatal piglets. Infect Immun. 1992;60:998–1007. doi: 10.1128/iai.60.3.998-1007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikemori Y, Kuroki M, Peralta RC, Yokoyama H, Kodama Y. Protection of neonatal calves against fatal enteric colibacillosis by administration of egg. Am J Vet Res. 1992;53:2005–2008. [PubMed] [Google Scholar]
- 31.O’Farrelly C, Branton D, Wanke CA. Oral ingestion of egg yolk immunoglobulin from hens immunized with an enterotoxigenic Escherichia coli strain prevents diarrhea in rabbits challenged with the same strain. Infect Immun. 1992;60:2593–2597. doi: 10.1128/iai.60.7.2593-2597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imberechts H, Deprez P, Van Driessche E, Pohl P. Chicken egg yolk antibodies against F18ab fimbriae of Escherichia coli inhibit shedding of F18 positive E. coli by experimentally infected pigs. Vet Microbiol. 1997;54:329–341. doi: 10.1016/s0378-1135(96)01293-x. [DOI] [PubMed] [Google Scholar]
- 33.Wiedemann V, Linckh E, Kuhlmann R, Schmidt P, Losch U. Chicken egg antibodies for prophylaxis and therapy of infectious intestinal diseases. J Vet Med. 1991;38:283–291. doi: 10.1111/j.1439-0450.1991.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 34.Hamada S, Horikoshi T, Minami T, et al. Oral passive immunization against dental caries in rats by use of hen egg yolk antibodies specific for cell-associated glucosyltransferase of Streptococcus mutans. Infect Immun. 1991;59:4161–4167. doi: 10.1128/iai.59.11.4161-4167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatta H, Tsuda K, Ozeki M, et al. Passive immunization against dental plaque formation in humans: Effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptoccoccus mutans. Caries Res. 1997;31:268–274. doi: 10.1159/000262410. [DOI] [PubMed] [Google Scholar]
- 36.Kuroki M, Ikemori Y, Yokoyama H, Peralta RC, Icatlo FC, Jr, Kodama Y. Passive protection against bovine rotavirus-induced diarrhea in murine model by specific immunoglobulins from chicken egg yolk. Vet Microbiol. 1993;37:135–146. doi: 10.1016/0378-1135(93)90188-d. [DOI] [PubMed] [Google Scholar]
- 37.Kuroki M, Ohta M, Ikemori Y, et al. Field evaluation of chicken egg yolk immunoglobulins specific for bovine rotavirus in neonatal calves. Arch Virol. 1997;142:843–851. doi: 10.1007/s007050050123. [DOI] [PubMed] [Google Scholar]
- 38.Ikemori Y, Ohta M, Umeda K, et al. Passive protection of neonatal calves against bovine coronavirus induced diarrhea by administration of egg yolk or colostrum antibody powder. Vet Microbiol. 1997;58:105–111. doi: 10.1016/S0378-1135(97)00144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang J, Hein F, Schaffrath N, Welters S. Rapid isolation of mycobacterial DNA using QIAamp. News Qiagen Product application Issue No. 1 1996.
- 40.Sweeney RW, Whitlock RH, Buckley CL, Spencer PA. Evaluation of a commercial enzyme-linked immunosorbent assay for the diagnosis of paratuberculosis in dairy cattle. J Vet Diagn Invest. 1995;7:488–493. doi: 10.1177/104063879500700411. [DOI] [PubMed] [Google Scholar]