Abstract
Invasiveness is a common feature of trophoblast and tumors; however, while tumor invasion is uncontrolled, trophoblast invasion is strictly regulated. Both trophoblast and tumor cells express high levels of the immunomodulatory progesterone-induced blocking factor (PIBF), therefore, we aimed to test the possibility that PIBF might be involved in invasion. To this aim, we used PIBF-silenced or PIBF-treated trophoblast (HTR8/Svneo, and primary trophoblast) and tumor (HT-1080, A549, HCT116, PC3) cell lines. Silencing of PIBF increased invasiveness as well as MMP-2,-9 secretion of HTR8/SVneo, and decreased those of HT-1080 cells. PIBF induced immediate STAT6 activation in both cell lines. Silencing of IL-4Rα abrogated all the above effects of PIBF, suggesting that invasion-related signaling by PIBF is initiated through the IL-4Rα/PIBF-receptor complex. In HTR-8/SVneo, PIBF induced fast, but transient Akt and ERK phosphorylation, whereas in tumor cells, PIBF triggered sustained Akt, ERK, and late STAT3 activation. The late signaling events might be due to indirect action of PIBF. PIBF induced the expression of EGF and HB-EGF in HT-1080 cells. The STAT3-activating effect of PIBF was reduced in HB-EGF-deficient HT-1080 cells, suggesting that PIBF-induced HB-EGF contributes to late STAT3 activation. PIBF binds to the promoters of IL-6, EGF, and HB-EGF; however, the protein profile of the protein/DNA complex is different in the two cell lines. We conclude that in tumor cells, PIBF induces proteins, which activate invasion signaling, while—based on our previous data—PIBF might control trophoblast invasion by suppressing proinvasive genes.
Keywords: PIBF, Invasion, Tumor, Trophoblast, Zebrafish
Introduction
The capacity to invade basal membranes and the extracellular matrix (ECM) is a common feature of first-trimester trophoblast and malignant tumors. However, while tumor invasion is a pathological process characterized by imbalanced regulation of motility and proteolysis together with unlimited metastatic capacity, trophoblast invasion is a strictly controlled physiological event, restricted in time to the implantation window, and localized in space to the endometrium and the proximal third of the myometrium. The smallest disturbances in the precise control of trophoblast invasion may lead to pathological pregnancies [1, 2].
Invasion is a multistep process, involving detachment of primary cells, degradation of basal membranes and extracellular matrix (ECM) components, and migration through the eroded stromal tissue. Degradation of ECM is initiated by proteolytic enzymes, e.g., matrix metalloproteinases (MMPs). A variety of extracellular stimuli, including cytokines, growth factors, and cell–ECM interactions regulates the transcription of MMP genes via diverse pathways, e.g., STAT3, Akt, and MAPK cascades [3].
Previously, we reported that progesterone-induced blocking factor (PIBF) is secreted by maternal lymphocytes and mediates the immunological effects of progesterone during pregnancy [4, 5]. PIBF inhibits natural killer (NK) cell activity and enhances Th2-type cytokine production via the JAK1/STAT6 and PKC/Ca++ pathways [6, 7], thus supporting the maintenance of pregnancy.
Progesterone plays a crucial role during implantation via governing trophoblast invasion [8, 9]. Within the first-trimester decidua, the expression pattern of PIBF coincides with sites of trophoblast invasion and the most intense PIBF staining appears at the extravillous trophoblast [10]. Early detection of PIBF may be related to premature trophoblast invasion possibly into an endometrium not yet prepared for the trophoblast and might lead to early immune rejection of the fetus [11].
It was recently shown that PIBF is up-regulated in undifferentiated proliferating cells (e.g., trophoblast cells) as well as in a set of malignancies [10–14]. Several breast cancers over-express PIBF compared to normal breast tissues [12]; moreover, MCF-7 breast carcinoma cells produce PIBF independently of progesterone [12]. The PIBF1 gene is located on the chromosomal region 13q21-q22, which has been implicated as a common site for somatic deletions in a variety of malignant tumors [15]. Moreover, the full-length form of PIBF is associated with the centrosome [12] as a component of the pericentriolar satellites [16], which are involved in cell cycle regulation. Furthermore, PIBF might facilitate tumor growth by suppressing local antitumor immune responses [17].
Based on these data, we aimed to investigate the possible involvement of PIBF in trophoblast and tumor invasion in different cell lines. Neither of the available trophoblast cell lines [18] represent the physiological conditions since these are either choriocarcinoma-derived (e.g., JEG-3, BeWo, JAR) or immortalized by transformation of human extravillous trophoblast cells with the SV40 large T antigen (e.g., HTR-8/SVneo, SGHPL-4, HIPEC65). For studying differences between trophoblast and tumor invasion, we selected the transformed HTR-8/SVneo cell line. HTR-8/SVneo possesses progenitor-like characteristics [19]. Recent data have demonstrated differences in gene expression profiles of integrins, proteases, hormones, and growth factors between trophoblast cell lines, primary cytotrophoblast and extravillous trophoblast cells; furthermore, neither of the cell lines showed a close similarity to the primary trophoblast [20]. Therefore, we used freshly isolated primary trophoblast cells to confirm our findings in HTR-8/SVneo.
Here, we report a novel role of PIBF in invasion-regulation. We demonstrate for the first time that PIBF is able to directly regulate gene expression; we show that PIBF can bind the promoter region of IL-6, EGF, and HB-EGF and induce or suppress transcription of these genes in certain tumor and trophoblast cells, respectively. The newly induced proteins in tumor cells activate signaling pathways that might be connected to altered MMP-9 and MMP-2 activity. We show that PIBF inhibits trophoblast invasiveness by down-regulating MMP-2 and MMP-9 activity while facilitating tumor invasion by inducing MMP-2 and MMP-9 genes, in an indirect manner.
Materials and methods
Cells and treatments
The immortalized human first-trimester extravillous trophoblast cell line HTR-8/SVneo was cultured in RPMI (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum (FBS). The HT-1080 fibrosarcoma, HCT-116 colorectal carcinoma, A549 lung carcinoma, and PC-3 prostate cancer cell lines were maintained in DMEM (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10 % FBS.
Primary trophoblast cells were isolated from first-trimester human elective abortion material according to a method described earlier [21]. Informed consent was obtained from all participants. Experiments were performed in accordance with the ethical guidelines of the Declaration of Helsinki. Approval from the Regional Ethical Committee at University of Pécs, Medical School was obtained.
After starving overnight, 6 × 105 cells were incubated at 37 °C for 5, 20, 60 min, 6 or 24 h with medium as a control or with recombinant human PIBF at a concentration of 100 ng/ml. The recombinant human PIBF was produced in our laboratory as described earlier [22].
RNA interference
Oligonucleotides were pre-designed by Ambion (Austin, TX, USA) to interfere with PIBF, IL-4Rα, or HB-EGF mRNA. Diluted oligonucleotides were pre-incubated with Oligofectamine (Invitrogen Life Technologies, Carlsbad, CA, USA) for 20 min at room temperature then the mixtures were added dropwise to 3 × 105 cells in OptiMEM (Invitrogen Life Technologies, Carlsbad, CA, USA). After 4 h at 37 °C, DMEM with 30 % FCS was added to the cultures. Cells were lysed after 24, 48, or 72 h for Western-blot analyses.
Invasion assay
Oris Cell Invasion and Detection Assay (Platypus Technologies, Madison, WI, USA) was performed according to the manufacturer’s instructions. Briefly; basement membrane extract (BME, 3 mg/ml) was applied on 96-well plates and the latter populated with Cell Seeding Stoppers to restrict cell seeding to the outer annular regions of the wells. Then wells were seeded with 75,000 cells. After 24 h, stoppers were removed, resulting in a 2-mm-diameter un-seeded region in the center of each well (i.e., the detection zone), and cells were overlaid with 12 mg/ml BME containing 15 % FBS. After 72 h, cells were labeled with calcein AM and the detection zone was analyzed using multiarea scan by an Olympus FluoView FV-1000 confocal microscope. Cell invasion was assessed by measuring the area of the detection zone in the scrambled and knock-down images using ImageJ analysis software. Invasion was determined by area closure, which was calculated as follows: invaded area of detection zone/full area of detection zone ×100.
Xenotransplantation
The Tg(fli1:EGFP) zebrafish (Danio rerio) line was maintained in compliance with the local animal care regulations and standard protocols of the University of Pécs. Approval from the Regional Ethical Committee at the University of Pécs, Medical School was obtained.
Fish were kept at 28 °C in aquaria with day/night light cycles. Embryos were obtained using standard mating conditions. The developing embryos were kept in an incubator at constant temperatures.
Cells were stained with CM-Dil (Vybrant; Invitrogen Life Technologies, Carlsbad, CA, USA) as described elsewhere [23] and resuspended in PBS at a concentration of 106 cells per 50 μl and kept on ice before microinjection. Forty-eight hours post-fertilization, zebrafish embryos were dechorionated and anesthetized with tricaine (Sigma-Aldrich, Steinheim, Germany). Approximately 100 cells were injected into the yolk sac of the embryos using an Eppendorf FemtoJet microinjector equipped with a glass capillary (1 μm inner diameter). The approximate injection parameters were injection pressure = 140 kPa, holding pressure = 25 kPa, injection time = 0.8 s. After injection, embryos were incubated for 1 h at 31 °C and checked for cell presence at 2-h post-transplantation. Fish with fluorescent cells outside the implantation area were excluded from further analysis. All other fish were incubated at 33 °C for 5 days. Disseminations of cells were monitored by an Olympus FluoView FV-1000 confocal microscope. Cell invasion within the zebrafish embryos was analyzed by using ImageJ software. The area of disseminated cells and area of cells remaining in the yolk sac was measured and invasion index was calculated as follows: area of disseminated cells/(area of disseminated cells + area of non-disseminated cells) × 100.
Substrate zymography
Cell-conditioned media from the invasion assay were subjected to electrophoresis under non-reducing conditions in a 7.5 % SDS/polyacrylamide gel containing 1 mg/ml gelatin. After electrophoresis, gels were re-natured and stained as described earlier [24]. Semi-quantification of bands was performed by densitometry using ImageJ software.
Protein arrays
Cell lysates of PIBF-treated and PIBF-knock down HTR-8/SVneo and HT-1080 cells were analyzed by proteome profiler antibody array (Human Angiogenesis Array Kit, R and D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Array data on developed X-ray film were quantitated with ImageJ software.
Western blotting
Cell lysates were separated on 10 % SDS-PAGE and transferred to nitrocellulose membrane at 54 mA overnight. Membranes were blocked with TBS-Tween (pH 7.4) containing 5 % non-fat dried milk for an hour and incubated with rabbit polyclonal anti-human PIBF [19], rabbit polyclonal phospho-specific (Tyr-641) anti-human STAT6, or rabbit polyclonal phospho-specific (Ser473) anti-human Akt antibodies (all from Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) in TBS-Tween with 3 % non-fat dried milk for an hour at room temperature, rabbit anti-human phospho-STAT3 (Tyr705), rabbit anti-human phospho-STAT3 (Ser727) or rabbit anti-human phospho-p44/42 MAPK (Thr202/Tyr204) (all from Cell Signaling Technology Inc., Danvers, MA, USA) antibodies in TBS-Tween with 5 % BSA overnight at 4 °C or as control with rabbit anti-human β-actin (Sigma-Aldrich, Steinheim, Germany) in TBS-Tween with 3 % non-fat dried milk for an hour. After washing, bound antibodies were detected with 1:2,000 diluted horseradish peroxidase-conjugated anti-rabbit IgG (Dako, Glostrup, Denmark), followed by development with ECL reagents (Perkin Elmer Life Sciences, Waltham, MA, USA). Semi-quantification of the bands was performed by densitometry using ImageJ software.
Cytometric bead arrays
IL-6 concentrations were determined from the supernatants of PIBF knock-down HTR-8/SVneo and HT-1080 cells by cytometric bead array (CBA; BD Biosciences, San Diego, CA, USA) according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qRT-PCR)
HTR-8/SVneo and HT-1080 cells were seeded on six-well plates. After silencing of PIBF, total RNA was isolated using RNeasy Mini kit (Qiagen). One microgram of total RNA was reverse-transcribed to cDNA using SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Quantitative gene expression was performed for MMP-9 (Hs00234579_m1), TIMP-1 (Hs00171558_m1) and beta-actin (Hs99999903_m1) in a total volume of 20 μl using TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer’s instructions. All PCRs were carried out in triplicate. Relative expression levels were calculated using the ΔΔCT method [25] after normalization to β-actin as endogenous control.
Confocal microscopy
Cells were plated on poly-l-lysine-coated slides, then fixed and permeabilized with acetone. After blocking, cells were incubated with anti-PIBF (1:100) for 1 h at room temperature, following incubation with Cy3-conjugated anti-rabbit-Ig (1:200) (Invitrogen Life Technologies, Carlsbad, CA, USA). Nuclei were stained by Hoechst 33258 (Sigma-Aldrich, Steinheim, Germany) then cells were mounted with DABCO (Sigma-Aldrich, Steinheim, Germany) and slides were closed by nail polish. Microscopic analysis was performed with an Olympus FluoView FV-1000 confocal microscope with 60× oil immersion objective. Images were analyzed using the Olympus FluoView 1.7 software.
Chromatin immunoprecipitation (ChIP)
Chromatin immunoprecipitation was performed according to a method described elsewhere [26]. Protein/DNA complexes were immunoprecipitated using rabbit polyclonal anti-PIBF antibodies. DNA was recovered using Qiagen PCR Purification Kit (Qiagen). PCR was performed with primers to the promoter regions of EGF (5′-AGCGAGTTATCTCCTCTTTGGCAGT-3′ and 5′-ACAGAGCAAGGCAAAGGCTTAGAGA-3′), IL-6 (5′-CATAATCCCAGGCTTGGGGG-3′ and 5′-GGTCGTCATTGAGGCTAGCG-3′), PlGF (5′-GTCTGGACCTGCCGAGAG-3′ and 5′-AGGTTCCCGAGCCGAGTT-3′) and HB-EGF (5′-TCCAGAAGACCCCTGCAAAA-3′ and 5′-TCCCAGGCTTCTGAGATTGG-3′). PCR products were separated on 1.5 % agarose gels and visualized with ethidium bromide staining.
Statistical analysis
Statistical analysis was performed using Student’s t test. Data were presented as mean ± SEM and p values of ≤0.05 were considered as statistically significant.
Results
PIBF regulates invasiveness of trophoblast and tumor cells
The invasive potential of PIBF-silenced trophoblast (HTR-8/SVneo and primary first-trimester human trophoblast) and tumor cells (HT-1080, HCT-116, A549, PC-3) was investigated. Quantitative analyses indicated that silencing of PIBF markedly increased the invasiveness of both HTR8/SVneo and primary first-trimester trophoblast cells (Fig. 1a, c), while the same treatment significantly decreased the invasive behavior of the tumor cell lines (Fig. 1b, c).
Fig. 1.
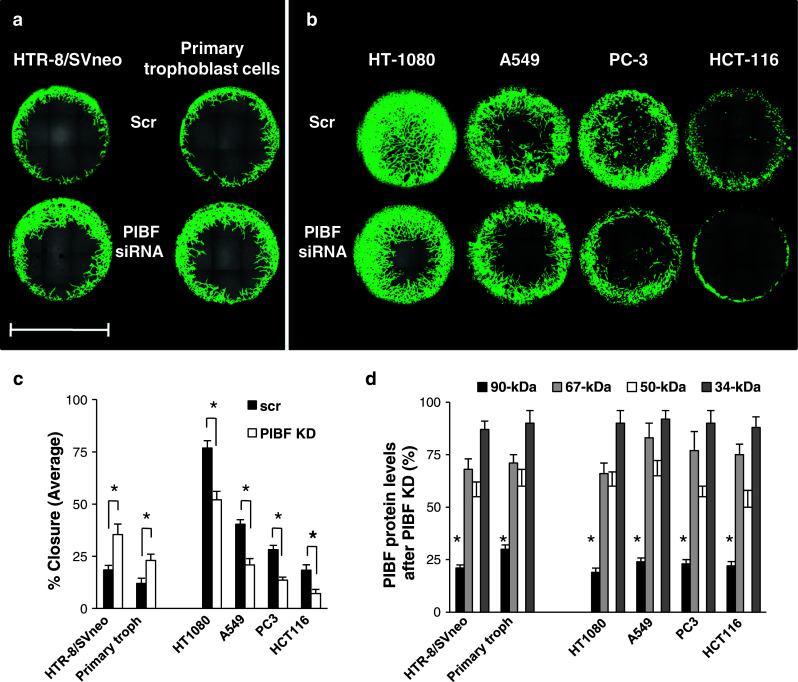
The effect of PIBF knock-down on invasiveness of trophoblast and tumor cells. The HTR-8/SVneo trophoblast cell line and primary trophoblast cells isolated from first-trimester abortion material (a) as well as HT-1080 (fibrosarcoma), A549 (lung carcinoma), HCT-116 (colorectal cancer), PC-3 (prostate cancer) tumor cell lines (b) were transfected with PIBF siRNA, or scrambled oligonucleotides (scr). Wells were seeded with control (scr) or PIBF siRNA-treated cells. Invasion of cells into the detection zones after 72 h is shown. Cells were stained with calcein AM. Images were captured using multiarea scan by a confocal microscope (bar 2 mm). c Quantification of area closure (%) calculated from measured areas of cell invasion at 72 h. Data are presented as mean ± SEM from 12 wells (HTR-8/SVneo, HT-1080) or six wells (other cell lines and primary trophoblast cells) per condition (asterisk indicates significant difference from scrambled at p < 0.05). d The efficiency of PIBF knock down (KD) was controlled by measuring PIBF levels (90-, 67-, 50-, and 34-kDa isoforms) using Western blotting at each individual experiment. Statistical analysis of Western blots are shown as mean ± SEM; asterisk indicates significant difference from scrambled at p < 0.05
We selected the HT-1080 fibrosarcoma cell line to model tumor invasion. Since PIBF affected the invasive behavior of the immortalized HTR-8/SVneo cells and primary trophoblast cells in the same manner, we used the HTR-8/SVneo trophoblast cells to further study the underlying molecular mechanisms.
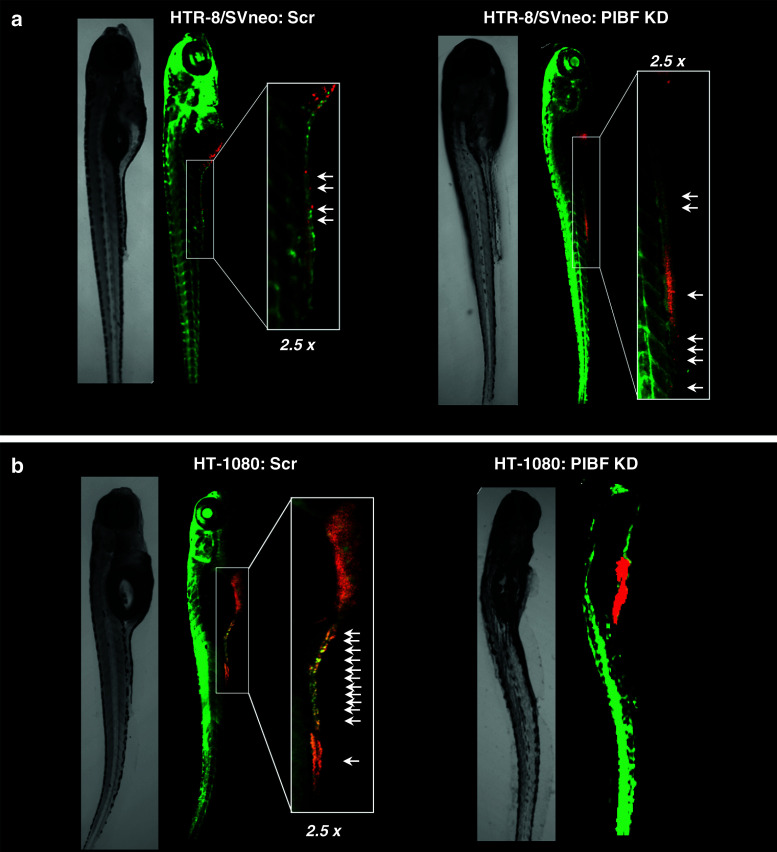
Invasion of PIBF-silenced trophoblast and tumor-cell xenografts in zebrafish embryos
Zebrafish is a favored in vivo platform for studying tumor development and progression because in the immunologically immature, transparent embryos human xenografts are not rejected and dissemination of cancer cells can be easily monitored in real time [27].
To confirm the differential role of PIBF in trophoblast and tumor invasion, fluorescent labeled, PIBF-silenced trophoblast or tumor cells were microinjected into the yolk sac of 2-day-old Tg(fli1:EGFP) transgenic zebrafish embryos, exhibiting a green fluorescent vasculature; and their invasiveness was monitored by confocal microscopy. The survival rates of the embryos were between 75 and 95 % (Table 1). Both cell types invaded embryonic tissue, entered circulation, and disseminated via the vessels within the embryos, from the yolk sac towards the tail (Fig. 2). When PIBF-silenced HTR-8/SVneo cells were transplanted, more cells were disseminated within the zebrafish embryos (invasion index 46.29 %) compared to the embryos injected with the control HTR-8/SVneo cells (invasion index 20.81 %) (Fig. 2a; Table 1), verifying that PIBF inhibits trophoblast invasion. PIBF knock-down tumor cells showed decreased invasive potential within the zebrafish embryos (invasion index 2.03 %) in contrast to the scrambled-injected control group (invasion index 55.58 %), confirming that PIBF facilitates tumor invasion (Fig. 2b; Table 1).
Table 1.
In vivo invasion assay: xenotransplantations of HTR-8/SVneo and HT-1080 cells into 2-day-old zebrafish embryos
| Microinjections | Number of injected embryos (analyzed) †: dead | Area of non-disseminated cells (mean, mm2) | Area of disseminated cells (mean, mm2) | Invasion index (%) | p value |
|---|---|---|---|---|---|
| HTR-8/Svneo | |||||
| scr | 20 (18) †1 | 0.01227 | 0.003225 | 20.81 | 0.0034 |
| PIBF KD | 20 (17) †2 | 0.008497 | 0.007323 | 46.29 | |
| HT-1080 | |||||
| scr | 20 (14) †5 | 0.01815 | 0.03071 | 55.58 | 0.0024 |
| PIBF KD | 20 (17) †2 | 0.0476 | 0.000984 | 2.03 | |
Number of embryos micro-injected and analyzed is shown in the table. If 2-h post-injection fluorescent cells were detected, the embryo was excluded from further analysis. After micro-injection with scrambled HT-1080 cells, one embryo developed yolk sac edema and was thus not analyzed. When HTR-8/SVneo cells were injected, survival rates of embryos were 95 % (scrambled, scr) and 90 % (PIBF knock down, KD); when HT-1080 cells were transplanted, survival rates of embryos were 75 % (scr) and 90 % (PIBF KD). Invasion index was calculated in live embryos at 5 days post-injection as follows: area of disseminated cells/area of total cells ×100
Fig. 2.
Xenotransplantation of PIBF-silenced trophoblast (a) and tumor (b) cells into the yolk sac of Tg(fli1:EGFP) zebrafish embryos. HTR-8/SVneo and HT-1080 cells were transfected with PIBF siRNA (PIBF KD) or scrambled (scr) oligonucleotides (control). The cells were then labeled with DiI (shown as red) and microinjected into the yolk sac of 48-h post-fertilization zebrafish embryos expressing EGFP under an endothelial promoter (green vasculature). Pictures were taken 5 days post-injection. Cells invaded the caudal part of zebrafish embryos. Arrows indicate distant micrometastases
PIBF affects invasion of trophoblast and tumor cells by modulating MMP-2 and MMP-9 activity
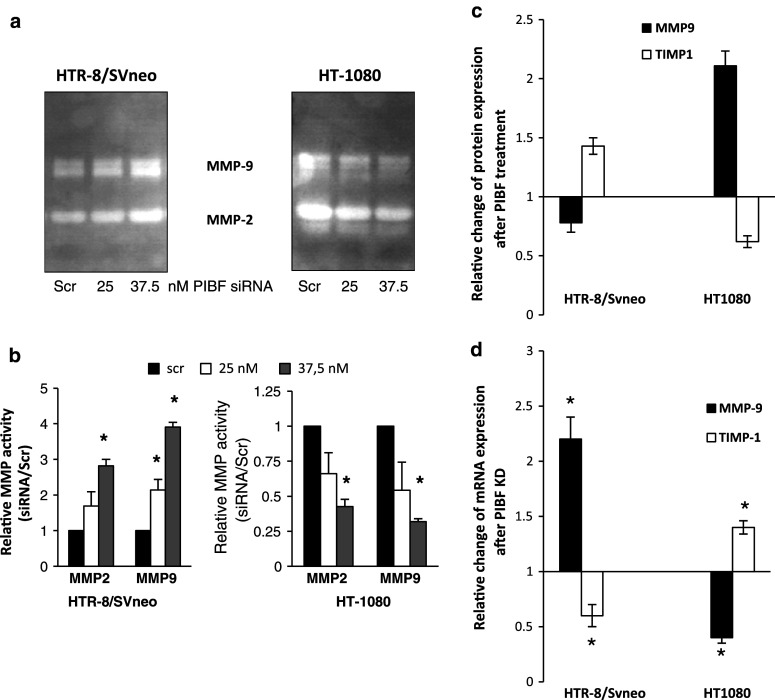
Matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) are the key mediators of both trophoblast and tumor invasion. MMP-9 and MMP-2, named gelatinases, cleave type IV collagen, the main component of basal lamina [28, 29]. Therefore, we measured MMP-2 and MMP-9 activity in cell-conditioned media from the invasion assay by substrate zymography. Silencing of PIBF resulted in increased gelatinase activity in HTR-8/SVneo cells, while PIBF knockdown HT-1080 cells showed reduced MMP-2 and MMP-9 activity (Fig. 3a, b).
Fig. 3.
PIBF modulates MMP-2 and MMP-9 activity. a Supernatants from the invasion assay were subjected to gelatin zymography to detect MMP-2 and MMP-9 activity. b Densitometric evaluation of three separate zymograms. Data are represented as mean ± SEM (asterisk indicates significant difference from the control at p < 0.05). c Changes in relative expression of MMP-9 and TIMP-1 in PIBF-treated (24 h) HTR-8/SVneo trophoblast and HT-1080 fibrosarcoma cells (protein array, n = 2). d The expression of MMP-9 and TIMP-1 was also analyzed at mRNA level in PIBF knock down (KD) cells by real-time PCR. Cells were analyzed at 48 h post-transfection with siRNA. Changes in relative mRNA levels are shown. Data are represented as mean ± SEM
In line with this, after 24 h, PIBF treatment MMP-9 expression increased in HT-1080 cells and decreased in HTR-8/SVneo cells, whereas TIMP-1—an inhibitor of MMP-9—was down-regulated in tumor and up-regulated in trophoblast cells (protein array, Fig. 3c). We also analyzed the mRNA expression of MMP-9 and TIMP-1 in HTR-8/SVneo and HT-1080 cells after silencing of PIBF for 48 h by real-time qPCR. MMP-9 mRNA was up-regulated in PIBF-silenced HTR-8/SVneo cells and down-regulated in HT-1080 cells, whereas TIMP-1 mRNA was down-regulated in HTR-8/SVneo and up-regulated in HT-1080 cells (Fig. 3d).
PIBF-induced signaling pathways in trophoblast and tumor cells
In order to reveal the underlying mechanisms, we investigated the PIBF-induced signaling pathways in HTR-8/SVneo trophoblast and HT-1080 tumor cells.
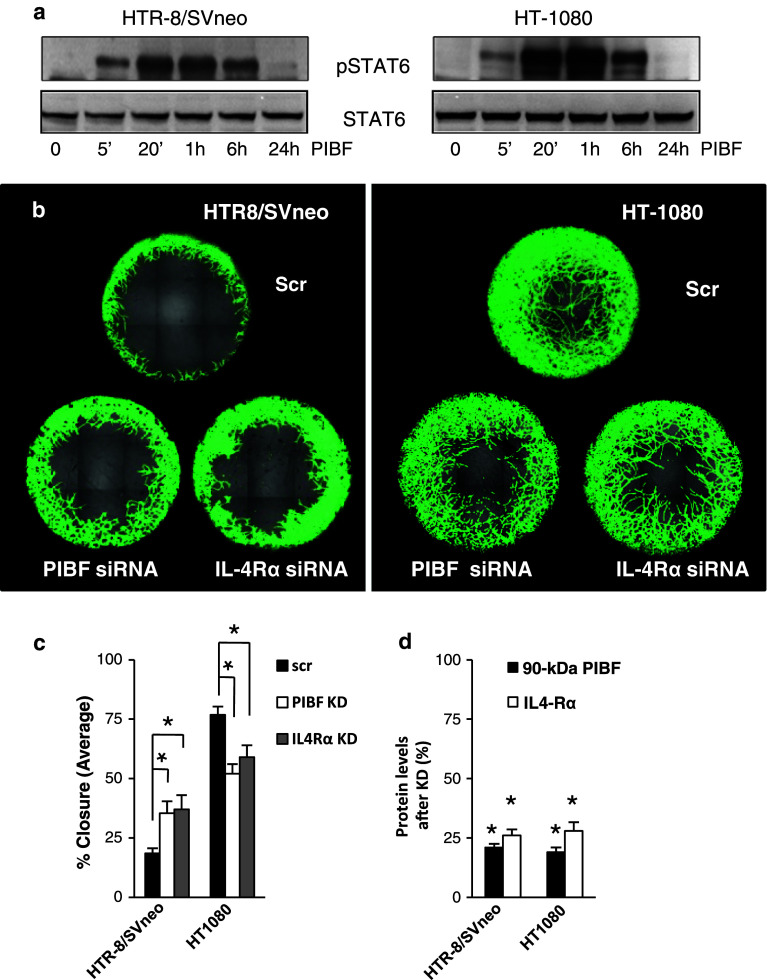
Earlier we showed that the PIBF receptor is a GPI-anchored protein which upon ligation forms a temporary association with the alpha chain of IL-4 receptor [7] and activates STAT6 in peripheral lymphocytes. Therefore, we investigated the involvement of IL-4Rα in PIBF-regulated invasion. PIBF-induced STAT6 phosphorylation (Fig. 4a) and silencing of IL-4Rα by siRNA abrogated the effect of PIBF on invasion in both cell lines (Fig. 4b, c), suggesting that IL-4Rα is involved in the PIBF-induced invasion-related mechanism. Therefore, we focused on IL-4Rα-associated signaling pathways.
Fig. 4.
PIBF signals via the IL-4Rα/PIBFR complex in trophoblast and tumor cells. a Serum-starved HTR-8/SVneo and HT-1080 cells were treated with PIBF (100 ng/ml) for the indicated time points. Total STAT6 and phosphorylated STAT6 levels were detected by Western blotting. b Invasion of control (scrambled, scr), IL-4Rα, or PIBF siRNA (KD)-treated HTR8/SVneo and HT1080 cells. Invasion of cells into the detection zones (2 mm) after 72 h is shown. Cells were stained with calcein AM. Images were captured using multiarea scan by a confocal microscope. c Quantification of area closure (%) calculated from measured areas of cell invasion at 72 h. Data are presented as mean ± SEM from 12 wells per condition (asterisk indicates significant difference from scrambled at p < 0.05). d Efficiency of PIBF or IL-4Rα knock down (KD) was determined by Western blotting. Statistical analysis of Western blots are shown as mean ± SEM; asterisk indicates significant difference from scrambled at p < 0.05
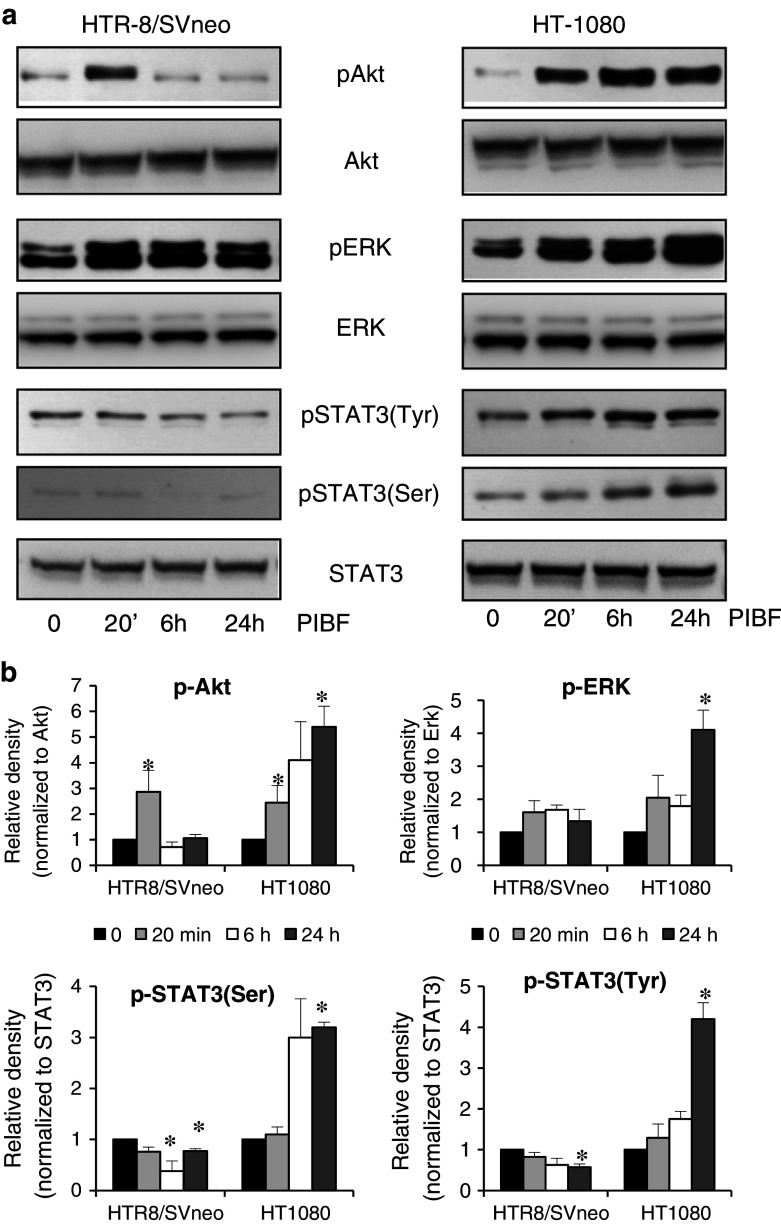
Insulin receptor substrate (IRS), associated with IL-4Rα, can activate the PI-3 K/Akt pathway and the MAPK cascade [30, 31], that have been implicated in invasion and tumorigenesis. In trophoblast cells, PIBF treatment resulted in fast, transient phosphorylation of Akt and ERK, whereas in the tumor cell line, PIBF treatment induced sustained activation of these molecules (Fig. 5a, b).
Fig. 5.
PIBF-induced signaling in trophoblast and tumor cells. a Serum-starved HTR-8/SVneo and HT-1080 cells were treated with PIBF (100 ng/ml) for the indicated time points. Phospho(Ser473)-Akt, total Akt, phospho(Thr202/Tyr204)-ERK, total ERK, phospho(Ser727)-STAT3, phospho(Tyr705)-STAT3, and total STAT3 levels were detected by Western blotting. b Densitometric evaluation of the Western blots. The bars indicate mean ± SEM of three separate experiments (*p < 0.05)
Since the STAT3 pathway is important in invasion signaling [32–35], we also tested the effect of PIBF treatment on STAT3 activation in the trophoblast and tumor cells. In HTR-8/SVneo cells, 6- and 24-h PIBF treatment inhibited activation of STAT3; while in HT-1080 cells, PIBF treatment resulted in late STAT3 Ser727- and Tyr705-phosphorylation, suggesting an indirect role of PIBF in STAT3 activation (Fig. 5a, b).
PIBF-induced proteins are responsible for late signaling events
The late signaling events may be attributed to indirect effects of PIBF.
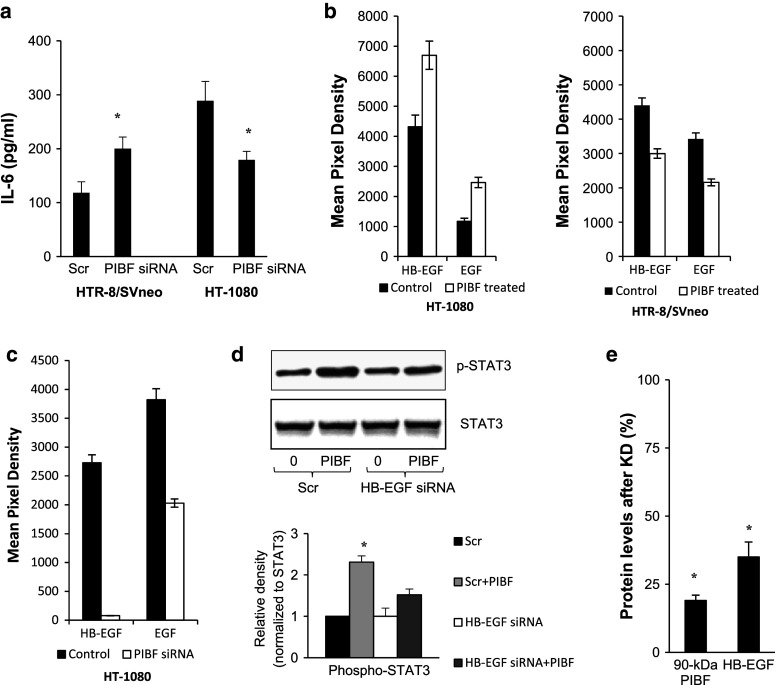
IL-6 activates STAT3 [36–38], therefore, we measured secreted IL-6 levels in the supernatants of control and PIBF knock down HTR-8/SVneo and HT-1080 cells by cytometric bead array. IL-6 production of PIBF-silenced trophoblast cells increased, whereas that of PIBF knock down tumor cells was reduced after 48 h (Fig. 6a). Levels of secreted IL-6 in HTR-8/SVneo cells increased from 118.54 ± 20.06 to 200.03 ± 21.56 ng/ml after silencing of PIBF. Levels of secreted IL-6 in HT-1080 cells decreased from 288.99 ± 35.7 to 179.22 ± 15.87 ng/ml after PIBF knock-down.
Fig. 6.
PIBF-regulated proteins. a IL-6 secretion of PIBF knock-down HTR-8/SVneo and HT-1080 cells (cytometric bead array). Supernatants were analyzed at 48 h post-transfection with siRNA. Data are represented as mean ± SEM of three separate experiments (*p < 0.05). b EGF and HB-EGF expression in control and 24-h PIBF-treated HTR-8/SVneo and HT-1080 cells in a protein array (n = 2). c EGF and HB-EGF expression in control (scrambled) and PIBF knock-down HT-1080 cells in a protein array (n = 2). d The effect of 24 h PIBF treatment on STAT3 activation in control (Scr) and HB-EGF knock down HT-1080 cells. Upper panel Western blot for phospho-STAT3 (Tyr). Lower panel mean ± SEM of densitograms from three separate experiments (asterisk p < 0.05). e Efficiency of HB-EGF knock down was analyzed by Western blotting. Statistical analysis of Western blots is shown as mean ± SEM; asterisk indicates significant difference from scrambled at p < 0.05
To further identify PIBF-regulated molecules, protein array-detecting invasion and angiogenesis-related proteins was performed on lysates of PIBF knock down and on those of 24-h PIBF-treated HT-1080 cells (Fig. 6b, c). PIBF treatment resulted in increased EGF and HB-EGF expression of the tumor cell line (Fig. 6b). Silencing of PIBF reduced the expression of EGF and HB-EGF in HT-1080 cells (Fig. 6c). Either of these might account for the late PIBF-induced signaling events.
To confirm that late STAT3 activation observed in the tumor cells was indeed due to gene induction, we tested the effect of PIBF treatment on STAT3 phosphorylation in HB-EGF knock-down HT-1080 cells. In HB-EGF-silenced tumor cells, the STAT3-activating effect of PIBF was decreased in contrast to the PIBF-treated control (scrambled), suggesting that PIBF-induced HB-EGF might contribute to late STAT3 activation in the tumor cells (Fig. 6d, e).
PIBF binds to the promoter of IL-6, EGF, and HB-EGF
The full-length PIBF contains leucine zippers, basic leucine zipper (bZIP) domain, and nuclear localization signals (NLS), which enable the molecule to bind DNA and modulate the transcription of genes [19]. To confirm that PIBF enters the nucleus, we visualized the subcellular localization of PIBF by confocal microscopy (Fig. 7a). PIBF is present in the cytoplasm and nuclei of both HTR-8/SVneo and HT-1080 cells.
Fig. 7.
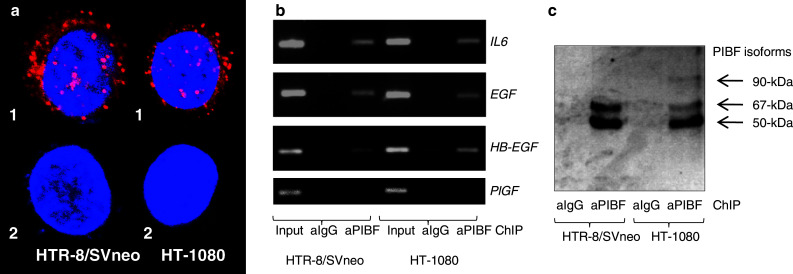
PIBF binds to the promoter of IL-6, HB-EGF, and EGF. a Subcellular localization of PIBF in HTR-8/SVneo and HT-1080 cells. (1) Cells were labeled with anti-PIBF antibody, and reacted with anti-rabbit Ig-Cy3 (shown as red). Nuclei were counterstained with Hoechst (shown as blue). Merged images taken by confocal microscope. (2) Control, without anti-PIBF antibody. b PCR of non-immunoprecipitated (input) and PIBF or control IgG immunoprecipitated DNA from ChIP assay of HTR-8/SVneo and HT-1080 cells, using primers to the promoter of IL-6, HB-EGF, EGF, and PlGF. PlGF ChIP was used as a negative control. c Detection of PIBF isoforms by Western blotting on the eluted protein/DNA complex immunoprecipitated by anti-PIBF
To confirm that PIBF is capable of regulating the transcription of IL-6, HB-EGF, and EGF, chromatin immunoprecipitation (ChIP) was performed with anti-PIBF antibody. This revealed that PIBF has the capacity to bind the promoter of IL-6, HB-EGF, and EGF in trophoblast and tumor cells (Fig. 7b). However, the protein content of the protein/DNA complex immunoprecipitated by anti-PIBF antibody differed in HTR-8/SVneo and HT-1080 cells (Fig. 7c); in trophoblast cells, the complex contained the 50- and 67-kDa PIBF isoforms while in tumor cells the full-length PIBF was also included in the DNA-binding complex.
Discussion
Both trophoblast and malignant tumors are invasive, and share the same underlying signaling pathways (i.e., PI-3 K/Akt, MAPK, FAK, ROCK, STAT3) and mediators (i.e., MMPs and TIMPs) [39]. However, in contrast to the unlimited invasive capacity of tumors, trophoblast invasion is under strict spatiotemporal control. We aimed to identify differences between regulatory mechanisms of invasion in tumor cells and trophoblast cells. Our data indicate that PIBF activates or inhibits the transcription of invasion-related genes, in a tissue-specific manner.
PIBF-mediated regulation of invasiveness was initiated through the IL-4Rα/PIBF-receptor complex. Silencing of IL-4Rα affected invasion of trophoblast and tumor cells in a manner similar to PIBF deficiency. Earlier data from our laboratory suggest that PIBF inhibits trophoblast invasion by down-regulating invasion-promoting genes, e.g., leptin [40]. Furthermore, Akt and ERK, which were rapidly but transiently phosphorylated in PIBF-treated trophoblast, are both involved in promoting (rather than inhibiting) invasion. Therefore, though the involvement of IL-4Rα is crucial, IL-4-dependent signaling is unlikely to account for PIBF-induced down-regulation of invasive genes. We hypothesize that the role of the IL-4Rα/PIBF complex would be rather to internalize [41] and allow PIBF to enter the nucleus and initiate transcription. The PIBF-induced proteins would be responsible for late signaling events, i.e., inhibition of STAT3 in trophoblast cells and induction of late Akt, ERK, and STAT3 phosphorylation in tumor cells.
Various cytokines and growth factors, e.g., EGF, IL-6 signal through the PI-3 K/Akt, JAK/STAT, and MAPK, etc., pathways. These pathways are frequently altered in different cancers and promote tumor cell invasion by inducing cell motility and altering MMP expression [34, 35, 42, 43]. Moreover, these pathways are continuously active in many transformed cells since they continuously produce both growth factors and their receptors, thereby providing an auto-stimulatory growth impulse [44, 45].
Among other proteins, IL-6, EGF, and HB-EGF were differentially regulated by PIBF in trophoblast and tumor cells.
EGF is expressed by both decidual and trophoblast cells, and induces differentiation, invasion, and proliferation of the latter. Qiu et al. [46] reported that EGF increases MMP-9 through the PI-3 K/Akt and MAPK pathways in HTR-8/SVneo cells. In JEG-3, EGF induces the expression of MMP-9 through the activation of p38MAPK and ERK signaling pathways [47]. Haslinger et al. [48] demonstrated that in SGHPL-5 trophoblast cells, PI-3 K/Akt signaling is required for trophoblast migration involving the downstream effector mTORC1. The JAK/STAT system is associated with mTOR signaling; mTORC1 mediates human trophoblast proliferation and invasion through regulation of matrix remodeling enzymes (e.g., MMP-2,-9, uPA, PAI-1) and requires STAT3 Ser-phosphorylation [49]. Based on the above-mentioned data, it is plausible that PIBF, via down-regulating EGF, negatively regulates trophoblast invasion. Inhibited STAT3 phosphorylation after PIBF treatment (6, 24 h) might be the result of suppressed EGF thus AUTHOR: Sentence not clear in meaning; please revise accordingly.-->inhibited PI-3 K/Akt/mTOR pathway that might lead to decreased expression of MMP-2 and MMP-9.
In HT-1080 fibrosarcoma cells, EGF induces the expression of MMP-9 through a Src-dependent mechanism [50]. In line with this, PIBF induced EGF in HT-1080 cells might contribute to increased expression of MMP-9 and increased invasiveness (Fig. 8).
Fig. 8.
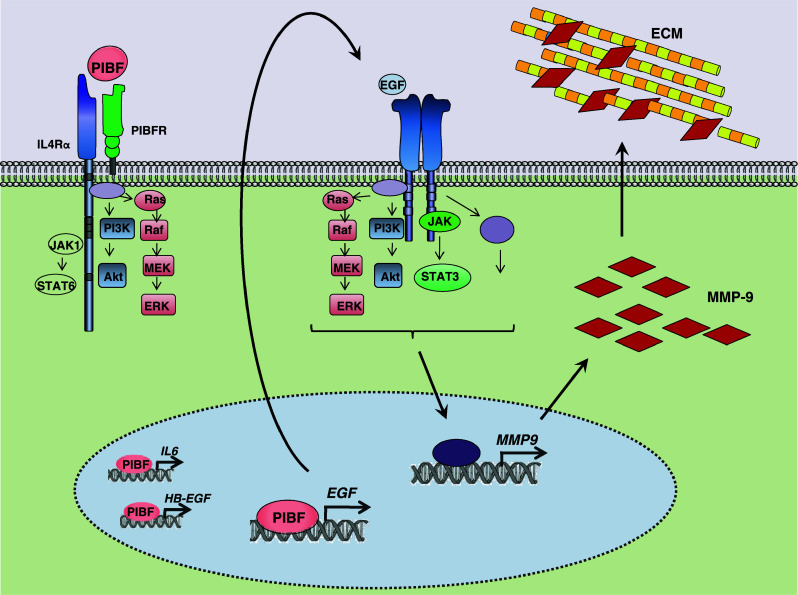
Transcriptional regulation of tumor invasion by PIBF. PIBF activates not only the Jak1/STAT6 pathway but also the PI-3 K/Akt and ERK cascades through the IL-4Rα/PIBFR complex. Moreover, PIBF is able to enter the nucleus where it binds the promoter regions of EGF, IL-6, and HB-EGF and induces their transcription. The expressed and secreted EGF then binds its own receptor and further triggers the PI-3 K/Akt, MAPK, and Jak/STAT3 pathways, resulting in proliferation, survival, and increased MMP-9 expression, thus increased invasive behavior. (ECM extracellular matrix)
HB-EGF is another member of the EGF family [51] that, based on our data, is regulated by PIBF. HB-EGF has a prominent role during the peri-implantation period [52]. HB-EGF promotes differentiation of trophoblast cells to an invasive phenotype by inducing integrin switching [53], thus required for successful invasion. HB-EGF is downregulated in pre-eclampsia, a disorder associated with shallow trophoblast invasion [53]. The membrane-anchored pro-HB-EGF is cleaved by metalloproteinases in order to release the soluble form of HB-EGF [54]. HB-EGF provokes differentiation of trophoblast cells to an invasive phenotype through coordinate activation of PI-3 K/Akt, ERK, MAPK14, and JNK pathways [55]. By downregulating HB-EGF in the trophoblast, PIBF might control physiologic trophoblast invasion.
HB-EGF expression is also altered in different cancer types (e.g., breast, colon, prostate, ovarian etc.,) [56]. HB-EGF binding to EGFR/ErbB1 activates downstream signaling that converges on PI-3 K/Akt and ERK cascades to promote survival and proliferation [56]. Thus, we hypothesize that PIBF-induced HB-EGF might contribute to elevated Akt and ERK levels found after 6- and 24-h PIBF treatments in HT-1080 cells.
In our hands, the STAT3-activating effect of PIBF was reduced in HB-EGF knock-down HT-1080 cells, suggesting that, at least in part, HB-EGF was responsible for late STAT3 activation. STAT3 promotes proliferation, cell growth, invasion, and metastasis formation and inhibits apoptosis in tumor cells [34, 35, 38].
The secretion of IL-6: another activator of STAT3 signaling was induced by PIBF in the tumor and inhibited in trophoblast cells. In primary extravillous trophoblast cells, IL-6 phosphorylates STAT3 [57], which contributes to invasiveness in this cell type [58]. Therefore, PIBF induced IL-6 might be responsible for late Akt, ERK, and STAT3 phosphorylation in PIBF-treated tumor cells, while the lack of STAT3 activation in the trophoblast might be attributed to the inhibitory effect of PIBF on IL-6 production.
We recently showed that PIBF expression in the trophoblast is inversely related to invasiveness, as well as to leptin and leptin receptor expression [40], suggesting that by suppressing invasion-related genes, PIBF might act as an intrinsic negative regulator of trophoblast invasion. In choriocarcinoma, the loss of PIBF results in increased invasive behavior [40] since the pro-invasive genes that are normally suppressed by PIBF will be transcribed.
Our data allow the conclusion that PIBF facilitates invasion in tumor cells by activating genes of molecules, e.g., EGF that initiate invasion signaling (Fig. 8). The secreted proteins combine with their receptors and activate signaling pathways, which in turn, might further trigger the expression of invasion-promoting molecules, e.g., MMP-9 and MMP2.
We provided evidence that PIBF binds the promoter of IL-6, EGF, and HB-EGF genes; however, in tumor cells, the protein/DNA complex includes the full-length PIBF, in addition to the 50- and 67-kDa PIBF isoforms found in the trophoblast. Thus, we hypothesize that the different composition of the DNA-binding PIBF complex might explain the differential regulation of trophoblast and tumor invasion by PIBF.
Based on these data, PIBF might serve as a potential therapeutic target to control tumor invasion.
Acknowledgments
We thank Dr. Charles Graham (Department of Anatomy and Cell Biology, Queen’s University, Kingston, Ontario, Canada L7L 3N6) for the gift of HTR8/SVneo cell line. We also thank Dr. Istvan Magyary (Faculty of Animal Science, Kaposvar University, Kaposvar, Hungary) for the zebrafish strain. This work was supported by the Hungarian National Research Fund (OTKA 77717), the University of Pécs (34039/KA-PostDoc12-03), the Hungarian Ministry of Health (ETT 286/2009), and the Research Team on Innovation (SROP-4.2.2/08/1/2008-0011), TÁMOP-4.2.1/B-10/1-2010-0002, and by TÁMOP-4.2.2.A-11/1/KONV-2012-0053.
Abbreviations
- BME
Basement membrane extract
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- EGFP
Enhanced green fluorescent protein
- Erk
Extracellular signal-regulated protein kinase
- Fli1
Friend leukemia integration 1
- GPI
Glycophosphatidylinositol
- HB-EGF
Heparin-binding EGF-like growth factor
- JAK
Janus kinase
- IL
Interleukin
- IL-4Rα
Interleukin-4 receptor alpha
- MAPK
Mitogen-activated protein kinase
- MMP
Matrix metalloproteinase
- NK
Natural killer
- PI-3K
Phosphoinositide-3-kinase
- PIBF
Progesterone-induced blocking factor
- PIBFR
PIBF-receptor
- PKC
Protein kinase C
- PlGF
Placental growth factor
- sHB-EGF
soluble HB-EGF
- STAT
Signal transducer and activator of transcription
- SV40
Simian vacuolating virus 40
- Tg
Transgene
- Th
T helper
- TIMP
Tissue inhibitor of metalloproteinase
References
- 1.Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376:717–729. doi: 10.1016/S0140-6736(10)60280-2. [DOI] [PubMed] [Google Scholar]
- 2.Cudihy D, Lee RV. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynaecol. 2009;29:576–582. doi: 10.1080/01443610903061751. [DOI] [PubMed] [Google Scholar]
- 3.Knöfler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54:269–280. doi: 10.1387/ijdb.082769mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szekeres-Bartho J, Kilar F, Falkay G, Csernus V, Torok A, Pacsa AS. The mechanism of the inhibitory effect of progesterone on lymphocyte cytotoxicity: I. Progesterone-treated lymphocytes release a substance inhibiting cytotoxicity and prostaglandin synthesis. Am J Reprod Immunol Microbiol. 1985;9:15–18. doi: 10.1111/j.1600-0897.1985.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 5.Szekeres-Bartho J, Autran B, Debre P, Andreu G, Denver L, Chaouat G. Immunoregulatory effects of a suppressor factor from healthy pregnant women’s lymphocytes after progesterone induction. Cell Immunol. 1989;122:281–294. doi: 10.1016/0008-8749(89)90077-4. [DOI] [PubMed] [Google Scholar]
- 6.Kozma N, Halasz M, Palkovics T, Szekeres-Bartho J. The progesterone-induced blocking factor modulates the balance of PKC and intracellular Ca. Am J Reprod Immunol. 2006;55:122–129. doi: 10.1111/j.1600-0897.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 7.Kozma N, Halasz M, Polgar B, Poehlmann TG, Markert UR, Palkovics T, Keszei M, Par G, Kiss K, Szeberenyi J, Grama L, Szekeres-Bartho J. Progesterone-induced blocking factor activates STAT6 via binding to a novel IL-4 receptor. J Immunol. 2006;176:819–826. doi: 10.4049/jimmunol.176.2.819. [DOI] [PubMed] [Google Scholar]
- 8.Shimonovitz S, Hurwitz A, Hochner-Celnikier D, Dushnik M, Anteby E, Yagel S. Expression of gelatinase B by trophoblast cells: down-regulation by progesterone. Am J Obstet Gynecol. 1998;178:457–461. doi: 10.1016/S0002-9378(98)70420-X. [DOI] [PubMed] [Google Scholar]
- 9.Chen JZ, Wong MH, Brennecke SP, Keogh RJ. The effects of human chorionic gonadotropin, progesterone and oestradiol on trophoblast function. Mol Cell Endocrinol. 2011;342:73–80. doi: 10.1016/j.mce.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Anderle C, Hammer A, Polgar B, Hartmann M, Wintersteiger R, Blaschitz A, Dohr G, Desoye G, Szekeres-Bartho J, Sedlmayr P. Human trophoblast cells express the immunomodulator progesterone-induced blocking factor. J Reprod Immunol. 2008;79:26–36. doi: 10.1016/j.jri.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Check JH, Nazari P, Check ML, Szekeres-Bartho J, Yuan W. Evidence that the adverse effect of controlled ovarian hyperstimulation on successful pregnancy outcome following embryo transfer may be related to premature trophoblast invasion. Clin Exp Obstet Gynecol. 2002;29:83–86. [PubMed] [Google Scholar]
- 12.Lachmann M, Gelbmann D, Kalman E, Polgar B, Buschle M, Von Gabain A, Szekeres-Bartho J, Nagy E. PIBF (progesterone induced blocking factor) is overexpressed in highly proliferating cells and associated with the centrosome. Int J Cancer. 2004;112:51–60. doi: 10.1002/ijc.20326. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava MD, Thomas A, Srivastava BI, Check JH. Expression and modulation of progesterone induced blocking factor (PIBF) and innate immune factors in human leukemia cell lines by progesterone and mifepristone. Leuk Lymphoma. 2007;48:1610–1617. doi: 10.1080/10428190701471999. [DOI] [PubMed] [Google Scholar]
- 14.Check JH, Dix E, Sansoucie L. Support for the hypothesis that successful immunotherapy of various cancers can be achieved by inhibiting a progesterone-associated immunomodulatory protein. Med Hypotheses. 2009;72:87–90. doi: 10.1016/j.mehy.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 15.Rozenblum E, Vahteristo P, Sandberg T, Bergthorsson JT, Syrjakoski K, Weaver D, Haraldsson K, Johannsdottir HK, Vehmanen P, Nigam S, Golberger N, Robbins C, Pak E, Dutra A, Gillander E, Stephan DA, Bailey-Wilson J, Juo SH, Kainu T, Arason A, et al. A genomic map of a 6-Mb region at 13q21-q22 implicated in cancer development: identification and characterization of candidate genes. Hum Genet. 2002;110:111–121. doi: 10.1007/s00439-001-0646-6. [DOI] [PubMed] [Google Scholar]
- 16.Kim K, Rhee K. The pericentriolar satellite protein CEP90 is crucial for integrity of the mitotic spindle pole. J Cell Sci. 2011;124:338–347. doi: 10.1242/jcs.078329. [DOI] [PubMed] [Google Scholar]
- 17.Szekeres-Bartho J, Polgar B. PIBF: the double edged sword. Pregnancy and tumor. Am J Reprod Immunol. 2010;64:77–86. doi: 10.1111/j.1600-0897.2010.00833.x. [DOI] [PubMed] [Google Scholar]
- 18.Hannan NJ, Paiva P, Dimitriadis E, Salamonsen LA. Models for study of human embryo implantation: choice of cell lines? Biol Reprod. 2009;82:235–245. doi: 10.1095/biolreprod.109.077800. [DOI] [PubMed] [Google Scholar]
- 19.Takao T, Asanoma K, Kato K, Fukushima K, Tsunematsu R, Hirakawa T, Matsumura S, Seki H, Takeda S, Wake N. Isolation and characterization of human trophoblast side-population (SP) cells in primary villous cytotrophoblasts and HTR-8/SVneo cell line. PLoS One. 2011;6:e21990. doi: 10.1371/journal.pone.0021990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilban M, Tauber S, Haslinger P, Pollheimer J, Saleh L, Pehamberger H, Wagner O, Knöfler M. Trophoblast invasion: assessment of cellular models using gene expression signatures. Placenta. 2010;31:989–996. doi: 10.1016/j.placenta.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS. Isolation and culture of term human trophoblast cells. In: Soares MJ, Hunt JS, editors. Placenta and trophoblast, methods in molecular medicine. Totowa: Humana Press; 2006. pp. 203–217. [DOI] [PubMed] [Google Scholar]
- 22.Polgar B, Kispal G, Lachmann M, Paar C, Nagy E, Csere P, Miko E, Szereday L, Varga P, Szekeres-Bartho J. Molecular cloning and immunologic characterization of a novel cDNA coding for progesterone-induced blocking factor. J Immunol. 2003;171:5956–5963. doi: 10.4049/jimmunol.171.11.5956. [DOI] [PubMed] [Google Scholar]
- 23.Marques IJ, Weiss FU, Vlecken DH, Nitsche C, Bakkers J, Lagendijk AK, Partecke LI, Heidecke CD, Lerch MM, Bagowski CP. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer. 2009;9:128. doi: 10.1186/1471-2407-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Rocca G, Pucci-Minafra I, Marrazzo A, Taormina P, Minafra S. Zymographic detection and clinical correlations of MMP-2 and MMP-9 in breast cancer sera. Br J Cancer. 2004;90:1414–1421. doi: 10.1038/sj.bjc.6601725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Carey MF, Peterson CL, Smale ST (2009) Chromatin immunoprecipitation (ChIP). In: Transcriptional regulation in eukaryotes: concepts, strategies, and techniques AUTHOR: Please double-check this reference-->CSHL Press, Cold Spring Harbour, New York
- 27.Mione MC, Trede SN. The zebrafish as a model for cancer. Dis Model Mech. 2010;3:517–523. doi: 10.1242/dmm.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol. 2011;21:228–237. doi: 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2:59. doi: 10.1186/1477-7827-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carey GB, Semenova E, Qi X, Keegan AD. IL-4 protects the B-cell lymphoma cell line CH31 from anti-IgM-induced growth arrest and apoptosis: contribution of the PI-3 kinase/AKT pathway. Cell Res. 2007;17:942–955. doi: 10.1038/sj.cr.2007.90. [DOI] [PubMed] [Google Scholar]
- 31.So EY, Oh J, Jang JY, Kim JH, Lee CE. Ras/Erk pathway positively regulates Jak1/STAT6 activity and IL-4 gene expression in Jurkat T cells. Mol Immunol. 2007;44:3416–3426. doi: 10.1016/j.molimm.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann NY Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann NY Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devarajan E, Huang S. STAT3 as a central regulator of tumor metastases. Curr Mol Med. 2009;9:626–633. doi: 10.2174/156652409788488720. [DOI] [PubMed] [Google Scholar]
- 35.Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, Huang S. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- 36.Waldner MJ, Foersch S, Neurath MF. Interleukin-6: a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8:1248–1253. doi: 10.7150/ijbs.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu CT, Hsieh CC, Lin CC, Chen WC, Hong JH, Chen MF. Significance of IL-6 in the transition of hormone-resistant prostate cancer and the induction of myeloid-derived suppressor cells. J Mol Med. 2012;90:1343–1355. doi: 10.1007/s00109-012-0916-x. [DOI] [PubMed] [Google Scholar]
- 38.Tu B, Du L, Fan QM, Tang Z, Tang TT. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012;325:80–88. doi: 10.1016/j.canlet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 2007;13:121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 40.Miko E, Halasz M, Jericevic-Mulac B, Wicherek L, Arck P, Arato G, Skret Magierlo J, Rukavina D, Szekeres-Bartho J. Progesterone-induced blocking factor (PIBF) and trophoblast invasiveness. J Reprod Immunol. 2011;90:50–57. doi: 10.1016/j.jri.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Halasz M, Polgar B, Kozma N, Berta G, Toth G, Szekeres-Bartho J. Identifying the receptor-binding part of PIBF. Am J Reprod Immunol. 2008;60:88. doi: 10.1111/j.1600-0897.2008.00626_8.x. [DOI] [Google Scholar]
- 42.Kim D, Kim S, Koh H, Yoon S, Chung A, Cho KS, Chung J. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–1962. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- 43.Reddy KB, Nabha SM, Atanaskova N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003;22:395–403. doi: 10.1023/A:1023781114568. [DOI] [PubMed] [Google Scholar]
- 44.Soundararajan R, Jagannadha R. Trophoblast “pseudo-tumorigenesis”: significance and contributory factors. Reprod Biol Endocrinol. 2004;2:15. doi: 10.1186/1477-7827-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang SY, Miah A, Pabari A, Winslet M. Growth factors and their receptors in cancer metastases. Front Biosci. 2011;16:531–538. doi: 10.2741/3703. [DOI] [PubMed] [Google Scholar]
- 46.Qiu Q, Yang M, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction. 2004;128:355–363. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- 47.Busch S, Renaud SJ, Schleussner E, Graham CH, Markert UR. mTOR mediates human trophoblast invasion through regulation of matrix-remodeling enzymes and is associated with serine phosphorylation of STAt3. Exp Cell Res. 2009;135:1724–1733. doi: 10.1016/j.yexcr.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 48.Haslinger P, Haider S, Sonderegger S, Otten JV, Pollheimer J, Whitley G, Knofler M. AKT isoforms 1 and 3 regulate basal and epidermal growth factor-stimulated SGHPL-5 trophoblast cell migration in humans. Biol Reprod. 2013;88(54):1–11. doi: 10.1095/biolreprod.112.104778. [DOI] [PubMed] [Google Scholar]
- 49.Ren HB, Jiang ZY, Sun LZ, Fan MS, Zou YF. Effect of epidermal growth factor on the expression of matrix metalloproteinase-9 and the signalling pathways involved in the trophoblast cell line JEG-3. Zhonghua Fu Chan Ke Za Zhi. 2011;46:521–526. [PubMed] [Google Scholar]
- 50.Fiorelli A, Ricciardi C, Pannone G, Santoro A, Bufo P, Santini M, Serpico R, Rullo R, Pierantoni GM, Domenico M. Interplay between steroid receptors and neoplastic progression in sarcoma tumors. J Cell Physiol. 2011;226:2997–3003. doi: 10.1002/jcp.22645. [DOI] [PubMed] [Google Scholar]
- 51.Higashiyama S, Iwabuki H, Morimoto C, Hieda M, Inoue H, Matsushita N. Membrane-anchored growth factors, the epidermal growth factor family: beyond receptor ligands. Cancer Sci. 2008;99:214–220. doi: 10.1111/j.1349-7006.2007.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jessmon P, Leach RE, Armant DR. Diverse functions of HBEGF during pregnancy. Mol Reprod Dev. 2009;76:1116–1127. doi: 10.1002/mrd.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leach RE, Kilburn B, Wang J, Liu Z, Romero R, Armant DR. Heparin binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol. 2004;15:223–237. doi: 10.1016/j.ydbio.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 54.Miyazono K. Ectodomain shedding of HB-EGF: a potential target for cancer therapy. J Biochem. 2012;151:1–3. doi: 10.1093/jb/mvr120. [DOI] [PubMed] [Google Scholar]
- 55.Jessmon P, Kilburn BA, Romero R, Leach RE, Armant DR. Function-specific intracellular signalling pathways downstream of heparin-binding EGF-like growth factor utilized by human trophoblasts. Biol Reprod. 2010;82:921–929. doi: 10.1095/biolreprod.109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 57.Champion H, Innes BA, Robson SC, Lash GE, Bulmer JN. Effects of interleukin-6 on extravillous trophoblast invasion in early human pregnancy. Mol Hum Reprod. 2012;18:391–400. doi: 10.1093/molehr/gas010. [DOI] [PubMed] [Google Scholar]
- 58.Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR. Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3) Hum Reprod Update. 2008;14:335–344. doi: 10.1093/humupd/dmn010. [DOI] [PubMed] [Google Scholar]