Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurological disease characterized by degeneration of upper and lower motor neurons. There are currently no clinically impactful treatments for this disorder. Death occurs 3–5 years after diagnosis, usually due to respiratory failure. ALS pathogenesis seems to involve several pathological mechanisms (i.e., oxidative stress, inflammation, and loss of the glial neurotrophic support, glutamate toxicity) with different contributions from environmental and genetic factors. This multifaceted combination highlights the concept that an effective therapeutic approach should counteract simultaneously different aspects: stem cell therapies are able to maintain or rescue motor neuron function and modulate toxicity in the central nervous system (CNS) at the same time, eventually representing the most comprehensive therapeutic approach for ALS. To achieve an effective cell-mediated therapy suitable for clinical applications, several issues must be addressed, including the identification of the most performing cell source, a feasible administration protocol, and the definition of therapeutic mechanisms. The method of cell delivery represents a major issue in developing cell-mediated approaches since the cells, to be effective, need to be spread across the CNS, targeting both lower and upper motor neurons. On the other hand, there is the need to define a strategy that could provide a whole distribution without being too invasive or burdened by side effects. Here, we review the recent advances regarding the therapeutic potential of stem cells for ALS with a focus on the minimally invasive strategies that could facilitate an extensive translation to their clinical application.
Keywords: Amyotrophic lateral sclerosis, Stem cells, Transplantation, Clinical translation
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurological disease characterized by the degeneration of upper and lower motor neurons. Currently, there are no clinically impactful treatments. Death occurs approximately after 3–5 years after the diagnosis, usually due to motor neuron loss and weakness of skeletal muscles responsible for airway and respiratory control [1]. ALS represents an extremely high burden on the life of the affected patients, their families, and society. Patients usually require 24-h assistance with the use of special equipment and aids (walkers, wheelchairs, ventilators).
Stem cell therapies represent new hope for patients affected by conditions such as traumatic injuries and neurological diseases [2]. The possibility to exploit stem cells as therapeutic strategy is supported by their plasticity and ability to direct their differentiation in response to extracellular signals. Current cell-therapy strategies take advantage of multiple types of stem cells to modify disease pathophysiology, support neurons and surrounding cells through the release of neurotrophic factors, or directly replace cells. Cell-mediated therapy is a field of intensive research in many neurodegenerative disorders.
Many different pathological mechanisms appear to contribute to ALS onset. Regarding familial forms of ALS, several genetic mutations have been identified as connected with the disease, including mutations in Cu2+/Zn2+ superoxide dismutase (SOD1) and TAR DNA binding protein-43 (TDP-43). More recently, hexanucleotide repeat expansions in the 5′ noncoding region of the C9orf72 gene have been discovered as the most common mutation underlying familial forms of ALS. Neurodegeneration in the more common form of sporadic ALS might result from a complex interaction of multiple cell types and different mechanisms, including cytoplasmic protein aggregates, glutamate excitotoxicity, and the generation of free radicals eventually combined with mitochondrial dysfunction. All of these factors can ultimately lead to the disruption of axonal transport processes through accumulation of neurofilament intracellular aggregates [1, 3].
Furthermore, impaired peripheral immunological responses and neuroinflammation, due to the contribution of toxic glia, have been identified as important effectors of the ALS disease process. Altering the diseased environment of ALS spinal cord by modulating astrogliosis and providing neurotrophic support for motor neurons will probably be crucial to achieve a therapeutic result [4, 5]. Indeed, therapeutic molecular or cellular strategies directed against only one of these pathological mechanisms have provided insufficient results, with only a minimal impact on disease phenotype [6].
In summary, stem cells seem to be an interesting therapeutic tool to approach diseases still without an effective treatment. However, some important issues need to be addressed before clinical translation, such as the identification of the ideal stem cell source and the best route of cell administration that should provide the greatest efficacy with the least invasiveness. Other relevant topics that must be clarified are the optimal therapeutic window for cell administration, the ideal balance between differentiation and persistence of stem cells into the targeted tissue, and the precise mechanism of tissue repair to promote (i.e., cell replacement and/or environmental enrichment).
Non-cell-mediated therapeutic approaches to ALS
In the last years, research aimed at the discovery of an effective treatment for ALS has been increased. The discovery of new causative genes and the development of new technologies (i.e., advanced RNA sequencing techniques, the finding and characterization of regulatory RNAs, the investigation and therapeutic use of antisense oligonucleotides, the discovery and optimization of cell reprogramming techniques) have certainly provided new inputs to this field. However, to date, riluzole is the only therapeutic drug approved for ALS with regard to modestly prolonging survival and delaying the use of supportive approaches, such as tracheotomy and mechanical ventilation [7]. Numerous potential reasons underlie the unsuccessful research of an effective therapy: the availability of representative preclinical models and the design of human trials account for some of the current problems. International conferences have been organized to standardize the methodology in animal models of ALS to search for reasons behind these difficulties and to suggest possible solutions [2].
The research for an effective treatment has been focused on different therapeutic targets and varied approaches. Among these, interventions influencing proteins near the neuromuscular junction and therapies that directly increase muscular strength are being tested through clinical trials [2]: as a matter of fact, skeletal muscle is a source of anabolic signals that influences neuron survival, axonal growth, and maintenance of synaptic connections. Nogo-A is a neuromuscular junction protein that was shown to inhibit axonal regrowth; Nogo A levels are increased in ALS patients’ muscles and in ALS murine models. GSK1223249, a humanized IgG1-type antibody (mABb) that binds to Nogo-A, antagonizes its biological function. In experimental models, anti-Nogo-A antibodies increase neurite outgrowth in vitro and functional outcome in vivo [2]. Other strategies derive from the observations that, in transgenic SOD1 animals, motor neuron death is correlated with metabolic status. In this model, mutant strains have lower BMI than wild-type mice, probably due to altered lipid storage in white adipose tissue [8]. Two clinical trials are assessing alternative therapies involving nutritional support: the first one (trial NCT00983983) is evaluating the efficacy of high-fat diet administered to patients with ALS. The second one (trial NCT00876772), tested the safety and efficacy of olanzapine, a drug that promotes weight gain, in ALS [8].
During the past decades, the discovery of mutations in the SOD1 gene and, more recently, other genes causative of motor neuron degeneration, directed attention to the promising new therapeutic approach of antisense therapy. Antisense therapy is based on the administration of short synthetic and chemically modified nucleic acids that bind to a specific target on the mRNA, a process which ultimately leads to the silencing of the target mRNA [9]. Antisense oligonucleotides (AONs) are currently employed in different clinical trials for several applications.
Several growth factors, such as insulin-like growth factor 1 (IGF-1), glial cell line-derived growth factor, brain-derived growth factor, vascular endothelial growth factor and ciliary neurotrophic factor, have been evaluated in experimental models of ALS, and have been shown to have positive effects in the majority of cases [9]. However, human trials showed modest or no effect of neurotrophic factor on ALS.
During the last years, increasing scientific interest has been paid to the possibility of exploiting stem cells as a therapeutic tool. Stem cell transplantation could represent a possible therapeutic strategy for ALS via multiple mechanisms, including the reduction of inflammation, the protection of endogenous motor neurons and neuronal circuitry and, ultimately, the replacement of degenerated cells [10].
Stem cells
Stem cells are characterized by their potential to continuously renew themselves by symmetric division and to generate more mature progenitors of multiple lineages through asymmetric division [10]. Many classifications of stem cells exist, reflecting the different cell types they can give rise to and the ways in which the stem cells are derived (Fig. 1). Embryonic stem (ES) cells are originated from the inner cell mass of a developing blastocyst and possess the ability to produce all the three germ layers [10]. Mesenchymal stem cells (MS) can be derived from multiple sources, including umbilical cord and adult bone marrow; they naturally give rise to osteoblasts, chondrocytes, and adipocytes.
Fig. 1.
Cell-mediated therapies. Multiple cell types can be employed as a source for cell-mediated therapies
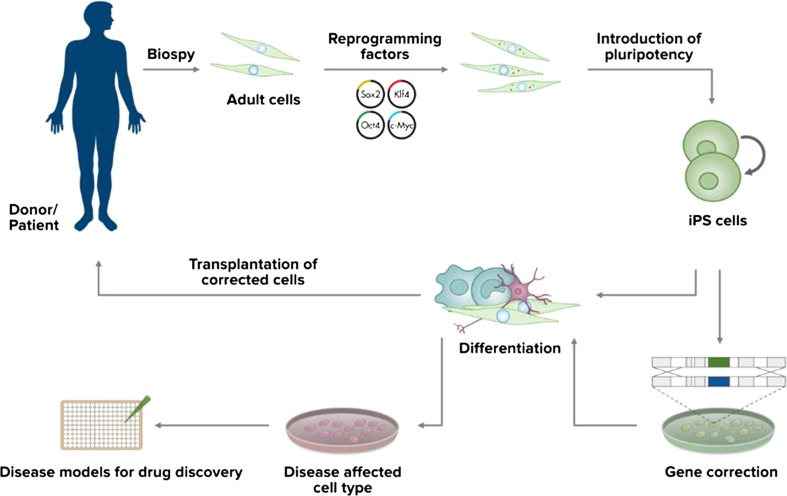
Progenitor cells are derived from more developed fetal or adult tissues. These cells are multipotent with the capacity to produce more restricted lineages than ES cells, usually determined by the germ layer of origin. Among them, neural stem cells (NSCs) are able to differentiate into different cell types within a neural lineage. NSCs can be obtained directly from fetal or adult neural tissue, or by directed differentiation of ES cells through cell culture manipulation [10]. Induced pluripotent stem cells (iPSCs) represent a rather novel source of autologous stem cells (Fig. 2). They are obtained from somatic tissue such as fibroblasts and reprogrammed to an embryonic stem cell-like pluripotent state by the induction of specific factors expressed in embryonic stem cells. iPSC were first generated in 2006 from mouse fibroblasts [11], followed by the generation of human iPSC in 2007 [12]. iPSCs have features similar to ES with respect to morphology, surface antigens, gene-expression profiles and differentiation potential with the great advantage of bypassing rejection-related issues and ethical controversies.
Fig. 2.
Derivation and application of iPSCs. Induced pluripotent stem cells can be derived through specific factor reprogramming of patients’ somatic cells. Pluripotent stem cells are then grown in culture and differentiated into cell types, such as neuronal cells, which are useful for the treatment of a particular disease. Derived neurons and other neural cell types can be investigated in vitro and used for disease modeling. Neural cells can also be genetically corrected in vitro and employed for therapeutic transplantation
Among the different cell sources, the selection can be further deepened aiming to identify a specific population provided with the most appropriate biological features to survive, migrate to the disease site, engraft, and properly differentiate. It was previously described that the selection of a specific subset of NSCs can provide a major advantage in terms of therapeutic efficacy compared with mixed NSC/neuronal precursor populations [13–15]. Corti et al. [13, 16] reported the positive therapeutic effect of a subset of self-renewing, multipotent rodent NSCs selected for their aldehyde dehydrogenase (ALDH) activity. When intrathecally transplanted into mice models of motor neuron disease, these cells could efficiently migrate into the spinal cord and modulate the disease phenotype. The transplantation of a NSC subpopulation selected for the double positivity for Lewis X and the chemokine receptor CXCR4 (LeX+/CXCR4+) modified disease progression in the SOD1G93A transgenic mice [14]. Indeed, CXCR4 and its ligand SDF-1 are known critical mediators for the ischemia-specific recruitment of circulating progenitor cells [17] and SDF is expressed also in ischemic brain [18]. As a result of injury, surviving or invading glial cells (including activated astrocytes and microglia) produce chemoattractants such as SDF-1 that may direct cell migration. The chemotactic signal provided by the presence of SDF can promote the recruitment of CXCR4 cells into the CNS, facilitating their transendothelial migration.
Moreover, it could be significant for the therapeutic effect on motor neuron disease animal models that injected cells differentiate in a good percentage into the targeted motor neuron phenotype. Indeed, transplanted motor neuron progenitors were shown to be effective in promoting neuronal sparing and functional recovery in animal models of neurodegenerative diseases and spinal cord lesions [19, 20]. Overall, this evidence supports the idea that the identification and selection of a specific stem cell subpopulation could be crucial for clinical translation, conferring major advantages to cell-mediated therapy, including the possibility to use a minimally invasive administration protocol.
Potential therapeutic effects of stem cells
Cellular therapies exploit cells or tissue grafts as a tool for treating different pathologies. Treatment goals of stem cell therapies are commonly focused on two main objectives that can occur simultaneously.
First, the process of cellular replacement implies the differentiation of stem cells into the specific neuronal subtypes affected by the disease, and their proper engraftment within the injury sites. In this setting, transplanted neuron integration and cross-talk with endogenous cells may lead to the restoration of a neural network mimicking the one lost in the disease.
Cell-mediated strategies have been extensively investigated in murine models of disease, but some practical issues might significantly limit the clinical translation of the direct motor neuron replacement approach: transplanted cells should be able to form synapses, direct axons properly to the target through the inhibitory white matter and, in the case of ALS, reach the muscular target far from the cell body in order to recreate the neuromuscular junction. For all of these reasons, the direct replacement of the lost motor neurons is unlikely the principal therapeutic aim to foster.
During the last years, increasing attention has been paid to the toxic function acquired by astrocytes and microglia during the development of the disease process through different mechanisms including loss of metabolic support, altered neuron-glia interactions and release of toxic metabolites instead of neurotrophic factors [4, 21]. As a consequence, cell therapies could be efficacious through a combined effect consisting of modulation of glial dysfunction, support of the endogenous population, and enrichment of the diseased spinal cord. Preclinical studies have shown that astrocytes expressing wild-type SOD1 are able to positively influence the degenerative process of mutant SOD1 motor neurons [22]. Glial-restricted precursors transplanted into the spinal cords of SOD1 rodents have demonstrated to differentiate into astrocytes, restore the levels of astrocyte glutamate transporter, and prolong survival of treated animals [23].
The second important and complementary aspect is the environmental enrichment/neurotrophic support stem cells may provide by releasing trophic factors, scavenging toxic ones, or creating secondary neural networks around diseased areas. Many strategies for environmental enrichment exploit stem cells in order to ensure de novo synthesis and further delivery of neuroprotective growth factors at the site of disease. Growth factors such as glial-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), insulin-like growth factor-I (IGF-I), and vascular endothelial growth factor (VEGF) have been shown to be protective in neurodegenerative disease models and to provide local support within the affected CNS [24, 25]. Taken together, these data reinforce the idea that cell-mediated therapies might support motor neurons in ALS, especially by creating a more trophic and enriched microenvironment in the spinal cord.
Stem cells preclinical application for amyotrophic lateral sclerosis
Therapeutic approaches based on stem cell transplantation were investigated with different results in many neurological diseases [26]. Preclinical studies have reported the vitality of endogenous neurons and rescue of function through the transplantation of multiple types of human stem cells (i.e., human mesenchymal stem cells derived from umbilical cord or bone marrow, human neural precursor, human-induced pluripotent stem cells) expressing functional genes, especially growth factors, in animal models of different neurological disorders, such as stroke, spinal cord injury, and ALS [26–28]. Many studies will be needed to assess the safety and efficacy and also to establish a protocol of administration feasible for clinical application. Over the last years, a great number of preclinical studies in ALS models have been performed with the aim of testing the therapeutic effects of varying types of stem cells selected for different characteristics in order to increase their possibilities to reach the injury site, survive, and properly engraft [14, 15, 29–33]. Taken together, results provided by the preclinical studies are promising and could pave the way for clinical trials in human patients (Table 1). However, more studies will be needed to obtain data allowing to precisely define safety and efficacy of this strategy, the therapeutic effects of stem cell therapy, and to establish an effective, minimally invasive, and standardized protocol of administration.
Table 1.
Ongoing and completed cell-based clinical trials on ALS patients
| Study | Status | Location | Procedure | Number |
|---|---|---|---|---|
| Phase II/III: effect of intrathecal administration of hematopoietic stem cells in patients with amyotrophic lateral sclerosis | Recruiting | Mexico | Intrathecal injections | NCT01933321 |
| Phase I/II clinical trial on the use of umbilical cord mesenchymal stem cells in amyotrophic lateral sclerosis | Enrolling by invitation | China | Intrathecal injections | NCT01494480 |
| Phase I/II clinical trial on the use of autologous bone marrow stem cells in amyotrophic lateral sclerosis | Active, not recruiting | Spain | Arm 1: laminectomy and bone marrow stem cell transplantation; Arm 2: intrathecal infusion of autologous bone marrow stem cells; Arm 3: intrathecal infusion of placebo (saline solution) | NCT01254539 |
| Phase I, single-center, prospective, non-randomized, open-label, safety/efficacy study of the infusion of autologous bone marrow-derived stem cells, in patients with amyotrophic lateral sclerosis | Active, not recruiting | US | Intrathecal infusion of the cell product suspended in infusion medium | NCT01082653 |
| Phase I/II clinical trial on the use of autologous bone marrow stem cells in amyotrophic lateral sclerosis | Completed | Spain | C1-C2 laminectomy and mononuclear autologous cells intraspinal transplantation | NCT00855400 |
| Open-label, phase I trial for safety study of HLA-haplo-matched allogenic bone marrow-derived stem cells (“HYNR-CS-Allo Inj”) treatment in amyotrophic lateral sclerosis (ALS) | Recruiting | Korea | Intrathecal injections | NCT01758510 |
| Phase I: a dose-escalation safety trial for intrathecal autologous mesenchymal stem cell therapy in amyotrophic lateral sclerosis | Recruiting | US | Single vs. double doses of cells administered by intrathecal injections | NCT01609283 |
| Phase I: safety of intravenous transplantation of bone marrow-derived mesenchymal stem cell in patients with ALS | Completed | Iran | Intravenous injections | NCT01759797 |
| Phase I: safety of intraventricular injection of Bone marrow-derived mesenchymal stem cell in patients with ALS | Recruiting | Iran | Intraventricular injections by stereotaxis | NCT01759784 |
| Phase I: intrathecal transplantation of autologous bone marrow-derived mesenchymal stem cell in patients with ALS | Recruiting | Iran | Intrathecal injections | NCT01771640 |
| A Phase IIa, open-label, dose-escalating clinical study to evaluate the safety, tolerability and therapeutic effects of transplantation of autologous cultured mesenchymal bone marrow stromal cells secreting neurotrophic factors (MSC-NTF), in patients with amyotrophic lateral sclerosis (ALS) | Ongoing, not recruiting | Israel | Multiple intramuscular injections at 24 separate sites, in addition to a single intrathecal injections | NCT01777646 |
| Autologous mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a Phase I clinical trial | Completed | Italy | Cell-direct transplantation into the spinal cord at a high thoracic level with a surgical procedure | 16454-pre21-823 |
| Safety and immunological effects of autologous mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis | Completed | Israel | Intrathecal and intravenous administration | NCT00781872 |
| A phase 2, randomized, double blind, placebo controlled multicenter study to evaluate safety and efficacy of transplantation of autologous mesenchymal stem cells secreting neurotrophic factors (MSC-NTF) in patients with ALS | Not yet open for recruitment | US | Combined intramuscular and intrathecal administration | NCT02017912 |
| Phase l, open-label, first in human, feasibility and safety study of human spinal cord-derived neural stem cell transplantation for the treatment of amyotrophic lateral sclerosis | Active, not recruiting | US | Intraspinal transplantation (surgical implantation) | NCT01348451 |
| Phase II, open-label, dose-escalation and safety study of human spinal cord-derived neural stem cell transplantation for the treatment of amyotrophic lateral sclerosis | Enrolling by invitation | US | Human spinal cord stem cell implantation | NCT01730716 |
| Intra-spinal cord delivery of human neural stem cells in ALS patients: proposal for a Phase I study | Recruiting | Italy | Surgical microinjections of human neural stem cells into spinal cord | NCT01640067 |
Another fascinating possibility of application of human stem cells (especially focusing on induced pluripotent stem cells) in addition to the appealing promise of the therapeutic transplantation, is the one of disease modeling. Indeed, with standardized methods for stem cell differentiation becoming available, it is now possible to obtain a great number of human neuronal cells to investigate and modulate disease processes [34]. The discovery and assessment of reprogramming strategies that exploit specific factors to derive patient-specific human-induced pluripotent stem cells (hiPSC) provided a further progress in this direction (Fig. 2). By using hiPSCs, it has become possible to obtain human neuronal cells affected by the combination of genetic variants that caused neurodegeneration in a single patient. These neurons allow the investigation of pathological mechanisms not easily identifiable in postmortem tissues, including some pathogenetic events maybe occurring before overt disease. Relentless optimization of these modeling methods holds the premises to provide new insights into diseases of the nervous system [35].
Minimally invasive strategies of administrations to ease the clinical translation
Neurodegenerative disorders share some common mechanisms leading to neuronal dysfunction and, eventually, cell death. Current therapies alleviate the symptoms, but they are not able to rescue or restore the cell function, or even modulate the disease process leading to neuronal death [36].
Several issues need to be addressed in order to achieve an effective clinically translatable therapy: among them there are the identification of a suitable cell source and the selection of the most performing cell population allowing to asses a feasible administration protocol.
Regarding the cell source, iPSCs represent a rather novel source of autologous stem cells. They can be obtained from somatic tissue and reprogrammed to an embryonic stem cell-like pluripotent state with the use of non-viral methods that, even if less efficacious than the viral ones, possess the great advantage to constitute a translational approach, avoiding the major issues of using viral-treated cells for patients’ treatment [15].
Additional strategies based on the use of bone marrow-derived MSCs are being implemented for clinical translation. MSCs substantial amount and relative accessibility favor their therapeutic potential for autologous cell therapy in comparison with other stem cell types.
When considering the route of delivery and the administration protocol, both crucial aspects for clinical translation, the issue of cell migration must be addressed. During the last years, many preclinical studies have provided results on the possibility of minimally invasive transplanted neural stem cells (intravenous cell injections or intrathecal ones) to effectively improve the phenotype of CNS disease model [37–39]. The ideal route of administration is the one that allows obtaining the best therapeutic effect with the minimal invasiveness in order to establish a protocol suitable for human patients (Fig. 3). Indeed, the method of cell delivery represents a major issue in supporting the clinical translation of cell-mediated approaches: to be efficacious, cells must be distributed along the CNS being able to reach both upper and lower motor neurons. Non-invasive methods are burdened by minor side effects but they should ensure a sufficient engraftment. Intrathecal and systemic intravenous transplantations could represent effective approaches for ALS treatment: both of these strategies are non-invasive and allow repeated administrations of cells. These features are essential for achieving a widespread distribution in the CNS of a significant amount of cells, which may be reasonably proportional to the therapeutic efficacy.
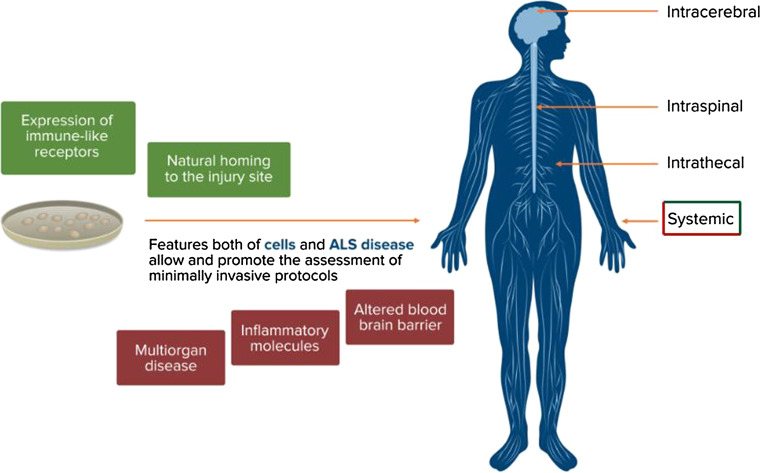
Fig. 3.
Rationale for minimally invasive protocols of administration. Features of both neural stem cells (green boxes) and ALS disease (red boxes) make the assessment of minimally invasive protocols of administration feasible
Neural stem cells have been shown to be able to target the injury sites within the CNS and to have the natural habit to migrate into the CNS [40]. It appears that systemically injected murine and human neural stem cells share the expression of a variety of functional immune-like receptors, such as functional cell adhesion molecules (e.g., CD44 and VLA-4) and inflammatory chemokine receptors (e.g., CCR2, CCR5, and CXCR4). This feature provides neural stem cells with a leukocyte-like molecular signature that allows them to interact with activated endothelial and ependymal cells: this cross-talk is an essential requirement for systemic delivery [40].
In the spinal cord and brain of both ALS patients and animal models, infiltration of inflammatory cells and IgG deposits have been demonstrated together with increased levels of albumin and IgG in the cerebrospinal fluid of ALS patients. These findings suggest altered barrier permeability in ALS, which probably occurs at both early and late stage of the disease [41, 42].
In ALS disease, neuronal death leads to the production of a huge amount of inflammatory molecules, which represents an attractive target for NSCs homing. This substrate, taken together with the alterations in the blood–brain barrier reported above, supports the feasibility of a systemic protocol of administration, which could combine the therapeutic effect with minimal side effects on the patients.
Moreover, increasing evidence suggests that ALS is probably a multi-organ disorder where motor neurons are selectively affected in the wider context of a systemic disease. Indeed, a number of documented alterations in ALS occur in the immune system, skeletal muscle tissue, skin, and lipid metabolism [43]. These considerations also promote an approach that is as much systemic as possible: systemically injected stem cells can reach peripheral organs such as draining lymph nodes and spleen, where they gather at the boundaries of blood vessels. Here, they can interact closely with lymphocytes and antigen-presenting cells (APCs), modifying their maturation and functional activation [40]. Stem cells can also exert a therapeutic effect peripherally through the release of neuroprotective factors at skeletal muscular level, contributing to the maintenance of neuromuscular junction integrity. Finally, if cells are injected systemically, they can be delivered repeatedly achieving a widespread distribution of significant amounts of cells in the CNS. It can be speculated that the therapeutic effect achieved in clinical translation might be directly proportional to the amount of cells that reach the CNS, administered with repeated injections. Even if many aspects need to be further investigated (see paragraph below) before translating most of these fascinating premises to clinical applications, minimally invasive methods of administration (i.e., intrathecal and systemic ones) may hold the key to effectively make cell-mediated therapies clinically feasible.
Indeed, most of these considerations were tested in our recent study where we isolated a specific neural stem cell (NSC) population from human iPSCs based on high aldehyde dehydrogenase activity, low side scatter, and integrin VLA4 positivity. VLA4 is a surface protein that mediates transendothelial migration; it is expressed on some hematopoietic cell fractions, in particular on T lymphocytes, and mediates their ability to cross the blood–brain barrier (BBB) [44]. NSCs expressing VLA4 are able to cross the BBB, particularly in the presence of inflammation or with a weakened BBB, as in ALS animal models and human patients. We assessed the therapeutic effects of these NSCs on the phenotype of ALS mice after minimally invasive methods of injection (intrathecal and intravenous injections). Transplanted NSCs migrated and engrafted into the central nervous system with both routes of administrations. Treated ALS mice exhibited improved neuromuscular function and motor unit pathology and significantly increased survival, compared with controls. Intrathecal and systemic transplantation of VLA4 + cells into SOD1G93A mice significantly prolonged survival, by 10 days for the former method and 23 days for the latter, compared with vehicle-treated animals. The rotarod test was exploited to assess motor function in transplanted and untreated animals: at 4 weeks after starting transplantation, treated animals showed a significant improvement in rotarod performance relative to untreated (p < 0.001) and fibroblast-treated animals (p < 0.001) [15].
Ongoing clinical trials with stem cells for ALS patients
A great number of cell-based clinical trials for ALS are based on the use of mesenchymal stem cells (MSCs). Several trials based on autologous MSC treatment have demonstrated the safety and feasibility of intraspinal, intrathecal, and intracerebral MSC transplants worldwide. A Phase 1 clinical study was conducted in Italy to assess the feasibility and toxicity of mesenchymal stem cell transplantation in ALS patients. Autologous MSCs were isolated from bone marrow, expanded in vitro, and suspended in the autologous cerebrospinal fluid (CSF). Cells were then directly transplanted into the spinal cord at a high thoracic level with a surgical procedure. Ten ALS patients were enrolled and evaluated before and after transplantation by clinical, psychological, neuroradiological, and neurophysiological assessments. No immediate or delayed transplant-related toxicity was reported [45]. Moreover, a controlled pilot study assessing the safety of intracranial MSC administration in 20 ALS patients reported a significantly longer survival in treated patients [46]. A trial on 34 ALS patients conducted in Israel evaluated the feasibility, safety, and immunological effects of intrathecal and intravenous administration of autologous MSCs: no major adverse effects were reported during follow-up and immunological analysis revealed immediate immunomodulatory effects [47]. As alternative strategy, reported clinical trials have exploited the granulocyte colony-stimulating factor (GCSF) to mobilize ALS patients’ MSCs. Trials conducted in Italy and Canada have assessed safety of this strategy, demonstrated the mobilization of MSCs, and reported the occurrence of anti-inflammatory reactions into the spinal cord [48, 49]. Overall, these trials provide important data about the safety and feasibility of MSC-based therapies in ALS patients.
According to information on the website ClinicalTrials.gov, a Phase l, open-label, First in Human, Feasibility and Safety Study of Human Spinal Cord-Derived Neural Stem Cell Transplantation for the Treatment of Amyotrophic Lateral Sclerosis is currently ongoing in the United States, at Emory University, in Atlanta, Georgia. The first aim of the trial is to assess the safety and feasibility of direct intraspinal transplantation of human spinal stem cells (HSSCs) in ALS patients. The Phase I trial consists of 18 patients divided into six cohorts. The cohorts comprise three patients each and progress from non-ambulatory to ambulatory patients, from unilateral to bilateral injections, and from lumbar to cervical targets. This “risk escalation” paradigm reflects the gradual increase in risk between cohorts regarding disease severity and number and placement of HSSC injections. Patients are evaluated at defined time intervals for functional and quality of life assessments; life-long follow-up is carried on.
Interim results for safety and functional outcomes were published for thoracolumbar-only microinjection patients supporting the safety of serial bilateral thoracolumbar microinjection in ambulatory patients. No acceleration of disease related to the injection procedure was shown; an early finding of clinical improvement in one patient was demonstrated. Unilateral cervical and cervical plus thoracolumbar microinjections to the ventral horn have been recently completed in ambulatory patients: one patient developed a post-operative kyphotic deformity prompting completion of a laminoplasty, and another required re-operation for wound complications. The single patient with bulbar ALS required perioperative reintubation. The FDA recently approved the progress into a Phase II trial aimed to define a dose-limiting toxicity threshold and a maximum tolerated dose for the ALS spinal cord. It will also assess the range of modifiable infusion parameters that may be tolerated (injection number, sites injected, total dose delivered) [50].
A similar clinical trial on ALS patients with stem cells started in 2010 in Terni, Italy. The trial was named “Intramedullary transplantation of human neural stem cells as a putative therapy for ALS: Proposal for a Phase I clinical trial”. In the trial design, 18 patients have been recruited and divided into groups of six patients of different functional severity starting from patients with greater impairment. The treatment, in this first phase of the study, was performed in six patients; neural stem cells have been transplanted in the lumbar spinal cord. Regarding the safety of the technique and the surgical procedure, intraoperative complications also did not occur due to the anesthetic. From the point of view of clinical follow-up, patients did not reveal any symptoms or signs of complications of the experimental procedure. No patients experienced adverse reactions to immunosuppressive therapy that has been practiced in the 6 months following transplantation.
According to information on the website ClinicalTrials.gov, there are currently more than 15 ongoing trials of various types of stem cell transplantation alone; five that have completed enrolment, and up to ten trials actively enrolling patients in Spain, China, Italy, the USA, South Korea, Iran, and Israel. The number of studies is illustrative of the rapid progress taking place in the field with the perspective to finally find an effective treatment to this still orphan disease (Table 1).
From benchside to clinical application: issues to be addressed
Even if stem cell-mediated therapy can represent a new promising strategy to cure ALS and other neurodegenerative diseases, some crucial issues still need to be addressed before supporting the extensive clinical translation.
First, the appropriate delivery of cells to the central nervous system should be established including not only the way of administration but also standardized protocols considering the proper “dose” of cells and the timing and number of treatments. Furthermore, it is not fully assessed if transplanted cells need to be delivered with a permissive matrix or growth factors to promote proper migration and integration of cells into the host nervous system. Second, confirmation of graft survival is crucial to state the efficacy. In many preclinical studies, the identification of grafted human cells in host animal models is achieved through immunohistochemical identification with human-specific markers [51]. Upon clinical translation, however, more sophisticated techniques to detect and state the fate of grafted cells will be required.
Once stated the best way of delivery, another important issue is preventing the oncogenic transformation of transplanted cells. The use of more differentiated cells such as neural precursors or fully differentiated neurons could address this issue: transplanted cells would have a limited capacity of further proliferation. Another strategy to avoid tumor complications involves genetic engineering of stem cells in order to insert specific genes, which can induce cell death upon specific stimulation. In this way, transplanted cells could be selectively killed, if necessary, by administration of a specific drug.
An additional concern is avoiding the immunorejection of transplanted cells. This hurdle could be addressed by exploiting patient-derived stem cells; it can be hypothesized that these autologous cells will be considered as self by the immune system and thus not rejected. Even if this is probable, it has not been rigorously proved yet and there are reasons to question this assumption, including recent studies that have tested iPSC immunogenicity in various ways and have reported that reprogrammed cells may provoke an immune reaction after transplantation, even causing rejection, in animal models [52]. There are several potential causes of iPSC-derived cell immunogenicity, and results may depend on the cell preparation protocol: stem cells obtained with retroviral transduction triggered most strongly the host immune response [53]. Moreover, the random viral integration might activate or inactivate crucial endogenous genes. As an alternative to viruses, plasmid-based transient transfection [14] as well as the introduction of proteins, RNA, or small molecules able to reprogram target cells have been tested with varying reprogramming efficacy [53]. It has also to be considered that iPSCs-derived cells may be useful to treat some genetic diseases, but only if the underlying mutation is corrected in these cells. However, the expression of proteins that the patient’s immune system has never been exposed to, or only in a truncated form, may lead to an immune response.
Moreover, patient-derived stem cells should not keep any epigenetic footprint of the original cell (i.e., fibroblast). This chromatin-based mechanism might limit the developmental potential and, eventually, interfere with the derivation of completely differentiated and functional neurons. An epigenetic footprint of the original cell could also result in aberrant surface antigen expression when iPSCs are differentiated into other cell types. Accordingly, modifications in cell surface proteins due to genetic mutations could lead to a response by the host immune system [54]. Further advances in our understanding of reprogramming need to address these concerns.
Finally, although different methods have been reported for directed differentiation of stem cells into specific neural types and their morphological and functional characterization, it is fundamental to increase our understanding of how these neurons change in culture. Moreover, many variables exist between different reported experimental protocols that need to be assessed and standardized [55].
To summarize, the future use of human stem cells for patients’ therapy should be promoted taking into account the many issues described above. Their application must also be guided by rigorous ethical principles with a precise selection of patients suitable for cell-mediated therapies.
Conclusions
Overall, results from different preclinical studies provide evidence that stem cells may represent an effective approach for the treatment of ALS. The therapeutic effects derived from stem cell transplantation are probably due to multiple events that counteract different aspects of the disease; this feature is crucial for multifactorial disease such as ALS. The possibility to exploit patients’ specific cells, such as iPSCs, and to administer them with minimally invasive strategies pave the way to future extensive clinical applications. More specifically, focusing the investigations on the selection of stem cell populations provided with the best biological features in terms of migration to the CNS and ability to engraft will allow optimizing minimally invasive administration protocols, highly suitable for clinical applications.
It can also be speculated that a combination of complementary therapeutic strategies (e.g., pharmacology, molecular therapy, and cell transplantation) that target different pathogenic mechanisms of the disease will increase the possibilities of achieving a clinically impactful therapy for ALS.
Acknowledgments
This work was supported by the ALS foundation to S.C. We also thank the ‘Associazione Amici del Centro Dino Ferrari’ for support.
References
- 1.Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- 2.Gordon P, Corcia P, Meininger V. New therapy options for amyotrophic lateral sclerosis. Expert Opin Pharmacother. 2013;14:1907–1917. doi: 10.1517/14656566.2013.819344. [DOI] [PubMed] [Google Scholar]
- 3.Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 4.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(Suppl 1):S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 6.Yang W, Strong MJ. Widespread neuronal and glial hyperphosphorylated tau deposition in ALS with cognitive impairment. Amyotroph Lateral Scler. 2012;13:178–193. doi: 10.3109/17482968.2011.622405. [DOI] [PubMed] [Google Scholar]
- 7.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2012;3:CD001447. doi: 10.1002/14651858.CD001447.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupuis L, Gonzalez de Aguilar JL, Echaniz-Laguna A, Eschbach J, Rene F. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PLoS One. 2009;4:e5390. doi: 10.1371/journal.pone.0005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nizzardo M, Simone C, Falcone M, Riboldi G, Rizzo F, et al. Research advances in gene therapy approaches for the treatment of amyotrophic lateral sclerosis. Cell Mol Life Sci. 2012;69:1641–1650. doi: 10.1007/s00018-011-0881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulis NM, Federici T, Glass JD, Lunn JS, Sakowski SA, et al. Translational stem cell therapy for amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;8:172–176. doi: 10.1038/nrneurol.2011.191. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 13.Corti S, Locatelli F, Papadimitriou D, Donadoni C, Del Bo R, et al. Transplanted ALDHhiSSClo neural stem cells generate motor neurons and delay disease progression of nmd mice, an animal model of SMARD1. Hum Mol Genet. 2006;15:167–187. doi: 10.1093/hmg/ddi446. [DOI] [PubMed] [Google Scholar]
- 14.Corti S, Locatelli F, Papadimitriou D, Del Bo R, Nizzardo M, et al. Neural stem cells LewisX+ CXCR4+ modify disease progression in an amyotrophic lateral sclerosis model. Brain. 2007;130:1289–1305. doi: 10.1093/brain/awm043. [DOI] [PubMed] [Google Scholar]
- 15.Nizzardo M, Simone C, Rizzo F, Ruggieri M, Salani S, et al. Minimally invasive transplantation of iPSC-derived ALDHhiSSCloVLA4+ neural stem cells effectively improves the phenotype of an amyotrophic lateral sclerosis model. Hum Mol Genet. 2014;23:342–354. doi: 10.1093/hmg/ddt425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corti S, Nizzardo M, Nardini M, Donadoni C, Salani S, et al. Neural stem cell transplantation can ameliorate the phenotype of a mouse model of spinal muscular atrophy. J Clin Invest. 2008;118:3316–3330. doi: 10.1172/JCI35432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 18.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyatt TJ, Rossi SL, Siegenthaler MM, Frame J, Robles R, et al. Human motor neuron progenitor transplantation leads to endogenous neuronal sparing in three models of motor neuron loss. Stem Cells Int. 2011;2011:207230. doi: 10.4061/2011/207230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erceg S, Ronaghi M, Oria M, Rosello MG, Arago MA, et al. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells. 2010;28:1541–1549. doi: 10.1002/stem.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valori CF, Brambilla L, Martorana F, Rossi D. The multifaceted role of glial cells in amyotrophic lateral sclerosis. Cell Mol Life Sci. 2014;71:287–297. doi: 10.1007/s00018-013-1429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucherie C, Schafer S, Lavand’homme P, Maloteaux JM, Hermans E. Chimerization of astroglial population in the lumbar spinal cord after mesenchymal stem cell transplantation prolongs survival in a rat model of amyotrophic lateral sclerosis. J Neurosci Res. 2009;87:2034–2046. doi: 10.1002/jnr.22038. [DOI] [PubMed] [Google Scholar]
- 23.Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M, McHugh J, Tork C, Shelley B, Hayes A, et al. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol Ther. 2008;16:2002–2010. doi: 10.1038/mt.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lunn JS, Sakowski SA, Federici T, Glass JD, Boulis NM, et al. Stem cell technology for the study and treatment of motor neuron diseases. Regen Med. 2011;6:201–213. doi: 10.2217/rme.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borlongan CV. Recent preclinical evidence advancing cell therapy for Alzheimer’s disease. Exp Neurol. 2012;237:142–146. doi: 10.1016/j.expneurol.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 28.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 29.Corti S, Nizzardo M, Nardini M, Donadoni C, Salani S, et al. Systemic transplantation of c-kit+ cells exerts a therapeutic effect in a model of amyotrophic lateral sclerosis. Hum Mol Genet. 2010;19:3782–3796. doi: 10.1093/hmg/ddq293. [DOI] [PubMed] [Google Scholar]
- 30.Kerr DA, Llado J, Shamblott MJ, Maragakis NJ, Irani DN, et al. Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J Neurosci. 2003;23:5131–5140. doi: 10.1523/JNEUROSCI.23-12-05131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- 32.Mitrecic D, Nicaise C, Gajovic S, Pochet R. Distribution, differentiation, and survival of intravenously administered neural stem cells in a rat model of amyotrophic lateral sclerosis. Cell Transpl. 2010;19:537–548. doi: 10.3727/096368910X498269. [DOI] [PubMed] [Google Scholar]
- 33.Teng YD, Benn SC, Kalkanis SN, Shefner JM, Onario RC, et al. Multimodal actions of neural stem cells in a mouse model of ALS: a meta-analysis. Sci Transl Med. 2012;4:165ra164. doi: 10.1126/scitranslmed.3004579. [DOI] [PubMed] [Google Scholar]
- 34.Sandoe J, Eggan K. Opportunities and challenges of pluripotent stem cell neurodegenerative disease models. Nat Neurosci. 2013;16:780–789. doi: 10.1038/nn.3425. [DOI] [PubMed] [Google Scholar]
- 35.Han SS, Williams LA, Eggan KC. Constructing and deconstructing stem cell models of neurological disease. Neuron. 2011;70:626–644. doi: 10.1016/j.neuron.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Madhavan L, Collier TJ. A synergistic approach for neural repair: cell transplantation and induction of endogenous precursor cell activity. Neuropharmacology. 2010;58:835–844. doi: 10.1016/j.neuropharm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Chu K, Kim M, Jeong SW, Kim SU, Yoon BW. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci Lett. 2003;343:129–133. doi: 10.1016/s0304-3940(03)00174-5. [DOI] [PubMed] [Google Scholar]
- 38.Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87:2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 39.Jeong SW, Chu K, Jung KH, Kim SU, Kim M, et al. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34:2258–2263. doi: 10.1161/01.STR.0000083698.20199.1F. [DOI] [PubMed] [Google Scholar]
- 40.Pluchino S, Cossetti C. How stem cells speak with host immune cells in inflammatory brain diseases. Glia. 2013;61:1379–1401. doi: 10.1002/glia.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pluchino S, Zanotti L, Deleidi M, Martino G. Neural stem cells and their use as therapeutic tool in neurological disorders. Brain Res Brain Res Rev. 2005;48:211–219. doi: 10.1016/j.brainresrev.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, et al. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Amico E, Factor-Litvak P, Santella RM, Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;65C:509–527. doi: 10.1016/j.freeradbiomed.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinman L. A molecular trio in relapse and remission in multiple sclerosis. Nat Rev Immunol. 2009;9:440–447. doi: 10.1038/nri2548. [DOI] [PubMed] [Google Scholar]
- 45.Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a phase I clinical trial. Exp Neurol. 2010;223:229–237. doi: 10.1016/j.expneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Martinez HR, Gonzalez-Garza MT, Moreno-Cuevas JE, Caro E, Gutierrez-Jimenez E, et al. Stem-cell transplantation into the frontal motor cortex in amyotrophic lateral sclerosis patients. Cytotherapy. 2009;11:26–34. doi: 10.1080/14653240802644651. [DOI] [PubMed] [Google Scholar]
- 47.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cashman N, Tan LY, Krieger C, Madler B, Mackay A, et al. Pilot study of granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood stem cells in amyotrophic lateral sclerosis (ALS) Muscle Nerve. 2008;37:620–625. doi: 10.1002/mus.20951. [DOI] [PubMed] [Google Scholar]
- 49.Chio A, Mora G, La Bella V, Caponnetto C, Mancardi G, et al. Repeated courses of granulocyte colony-stimulating factor in amyotrophic lateral sclerosis: clinical and biological results from a prospective multicenter study. Muscle Nerve. 2011;43:189–195. doi: 10.1002/mus.21851. [DOI] [PubMed] [Google Scholar]
- 50.Riley J, Glass J, Feldman EL, Polak M, Bordeau J, et al. Intraspinal stem cell transplantation in ALS: a phase I trial, cervical microinjection and final surgical safety outcomes. Neurosurgery. 2014;74:77–87. doi: 10.1227/NEU.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 51.Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol. 2014;5:7. doi: 10.3389/fimmu.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheiner ZS, Talib S, Feigal EG. The potential for immunogenicity of autologous induced pluripotent stem cell-derived therapies. J Biol Chem. 2014;289:4571–4577. doi: 10.1074/jbc.R113.509588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyazaki S, Yamamoto H, Miyoshi N, Takahashi H, Suzuki Y, et al. Emerging methods for preparing iPS cells. Jpn J Clin Oncol. 2012;42:773–779. doi: 10.1093/jjco/hys108. [DOI] [PubMed] [Google Scholar]
- 54.Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nizzardo M, Simone C, Falcone M, Locatelli F, Riboldi G, et al. Human motor neuron generation from embryonic stem cells and induced pluripotent stem cells. Cell Mol Life Sci. 2010;67:3837–3847. doi: 10.1007/s00018-010-0463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]