Abstract
During the development of the central nervous system (CNS), oligodendrocyte precursors (OPCs) are generated in specific sites within the neural tube and then migrate to colonize the entire CNS, where they differentiate into myelin-forming oligodendrocytes. Demyelinating diseases such as multiple sclerosis (MS) are characterized by the death of these cells. The CNS reacts to demyelination and by promoting spontaneous remyelination, an effect mediated by endogenous OPCs, cells that represent approximately 5–7 % of the cells in the adult brain. Numerous factors influence oligodendrogliogenesis and oligodendrocyte differentiation, including morphogens, growth factors, chemotropic molecules, extracellular matrix proteins, and intracellular cAMP levels. Here, we show that during development and in early adulthood, OPCs in the murine cerebral cortex contain phosphodiesterase-7 (PDE7) that metabolizes cAMP. We investigated the effects of different PDE7 inhibitors (the well-known BRL-50481 and two new ones, TC3.6 and VP1.15) on OPC proliferation, survival, and differentiation. While none of the PDE7 inhibitors analyzed altered OPC proliferation, TC3.6 and VP1.15 enhanced OPC survival and differentiation, processes in which ERK intracellular signaling played a key role. PDE7 expression was also observed in OPCs isolated from adult human brains and the differentiation of these OPCs into more mature oligodendroglial phenotypes was accelerated by treatment with both new PDE7 inhibitors. These findings reveal new roles for PDE7 in regulating OPC survival and differentiation during brain development and in adulthood, and they may further our understanding of myelination and facilitate the development of therapeutic remyelination strategies for the treatment of MS.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1340-2) contains supplementary material, which is available to authorized users.
Keywords: PDE7 inhibitors, Oligodendrocyte differentiation, Multiple sclerosis
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disorder of the central nervous system (CNS) that is characterized by inflammation. As MS progresses, it produces axonal demyelination due to oligodendrocyte loss, which is followed by astrogliosis and eventual axonal degeneration [1, 2]. MS is a major cause of disability, affecting over 2.5 million people worldwide and it is the most common neurological disease among young and middle-aged adults. The etiology of MS is poorly understood and as yet there is no effective, although several therapies can ameliorate or delay the characteristic outbreaks/relapses. Currently available MS treatments rely on an immunomodulatory mechanism of action but they do not promote remyelination [3, 4], highlighting the urgent need for the development of novel neuroreparative treatments [5].
During embryonic development, oligodendrocyte precursor cells (OPCs) migrate to their final destination where they differentiate into myelin-forming oligodendrocytes [6, 7]. OPCs represent 5–7 % of the total cells in the mature CNS of both healthy and sick individuals, making this cell type an interesting target for therapies that aim to produce tissue repair in demyelinating diseases such as MS [8, 9].
Several studies suggest that 3’-5’-cyclic adenosine monophosphate (cAMP) plays an important role in neuroprotection and in the neuroinflammatory response. Accordingly, controlling the levels of this nucleotide could regulate the pathological neuroinflammatory process and decelerate the progression of MS [10–12]. Intracellular cAMP levels depend on its synthesis by adenylyl cyclases and its degradation by cyclic nucleotide 3’-5’-phosphodiesterases (PDEs). PDE7, one of the 11 PDEs isoenzymes identified to date, is a cAMP-specific enzyme that is insensitive to rolipram (a PDE4 inhibitor), and that is expressed in T cells and in multiple brain structures [13–15]. PDE7 has emerged as a novel therapeutic target to alleviate chronic inflammation in a variety of immunological conditions and conditions of immunodeficiency, as well as in neurodegenerative disorders like MS in which both the immune system and CNS are implicated [12, 16, 17].
There is little information regarding the physiological activities regulated by PDE7, although it has been implicated in pro-inflammatory processes and it is necessary to induce T-cell proliferation [18]. Moreover, specific inhibitors of PDE7 have recently been proposed as potential treatments for neurological disorders due to their modulatory effects on inflammation, a well-established neuroprotective strategy [12, 19, 20]. These data suggest that this class of PDE7 inhibitors is a potential source candidate drugs to treat MS. The effectiveness of current pharmacological treatments for MS is limited by several severe drawbacks, including poor efficacy and pharmacokinetic properties, and the search for new treatments can be considered as a rapidly growing field of research.
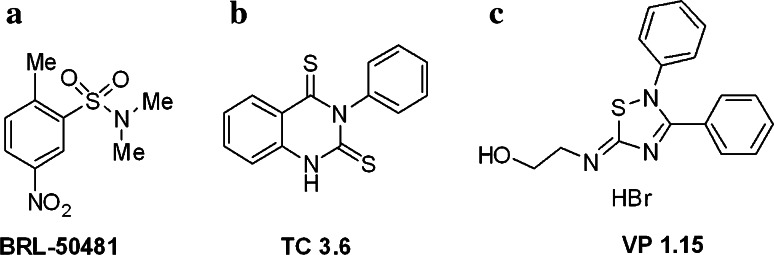
In the present study, we investigated the effects of PDE7 inhibitors on oligodendrocyte precursor cells (OPCs). Inhibition of PDE7 activity accelerated the differentiation of newborn cortical OPCs and increased their survival in culture via a pathway dependent on PKA-cAMP. These effects were reproduced in OPCs from P15 mouse brain and in OPCs isolated from biopsies of adult individuals that did not suffer MS. Indeed, this is one of the first studies to perform a parallel analysis of OPCs from mice (P0 and P15) and human. To modulate PDE7 activity in OPCs, we used small molecules to characterize the biological mechanism [21, 22]. The well-known PDE7 inhibitor BRL-50481 (BRL; Fig. 1a) and two newly discovered PDE7 inhibitors, the TC3.6 quinazoline (Fig. 1b) and the 5-imino-1,2,4-thiadiazole derivative, VP1.15 (Fig. 1c). As a result, we reveal novel roles for PDE7 in regulating OPC survival and differentiation in the brain, which may further our understanding of myelination and improve the possibility of defining adequate remyelination strategies to treat diseases such as MS.
Fig. 1.
PDE7 inhibitors used as chemical probes. a Structure of commercial PDE7 inhibitor BRL-50481. b Structure of the quinazoline TC3.6 c Structure of the 5-imino-1,2,4-thiadiazole VP1.15
Materials and methods
Animals
Postnatal P0 and P15 mice were obtained from Charles River Laboratories (Wilmington, MA, USA) and maintained in the animal facilities at the Hospital Nacional de Parapléjicos (Toledo, Spain). All animal experiments were carried out in accordance with Spanish (RD223/88) and European (2010/63/EU) regulations, and they were approved by the Animal Review Board at the Hospital Nacional de Parapléjicos (SAPA001).
Human samples
Human biopsies were obtained from major surgery of non-responders to drug treatment adult temporal epilepsy patients (29–59 years old) performed by the Neurosurgery Service at the Hospital Universitario de La Princesa (Madrid, Spain). All experiments involving human samples were carried out in accordance with the guidelines of the Research Ethics Committee of Toledo (Spain) and all subjects provided informed consent.
PDE7 inhibitors
BRL-50481 (BRL; Fig. 1a) was supplied by Calbiochem, while Quinazoline TC3.6 (Fig. 1b [23]) and 5-imino-1,2,4-thiadiazole, VP1.15 (Fig. 1c [24]), were synthesized at the Instituto de Química Médica-CSIC. Both compounds were discovered by our research group by employing various medicinal chemistry strategies in the search of new PDE7 inhibitors. The quinazolines were identified via ligand-based virtual screening using CODES descriptors [25] while 5-imino-1,2,4-thiadizoles were discovered using a specific artificial neuronal network [26]. Hit-to-lead programs led to the identification of TC3.6 and VP1.15 based on the optimization of biological activity and drug-like properties, such as blood–brain barrier penetration. These compounds have IC50 values for PDE7 inhibition of 1.04 ± 0.08 and 1.1 ± 0.17 μM, respectively [26, 27]. Both compounds have demonstrated significant therapeutic potential for the treatment of neurological disorders [28, 29].
Cell culture
To isolate OPCs, primary mixed glial cultures were prepared as described previously for the rat forebrain [30, 31] using the modified technique of [32]. Briefly, the cerebral cortex of P0 and P15 mice was dissected out and dissociated enzymatically for 15 min at 37 °C in HBSS (Invitrogen) containing papain (0.9 mg/ml; Worthington Biochemical), l-cysteine (0.2 mg/ml; Sigma), and EDTA (0.2 mg/ml; Sigma). The digested tissue was then filtered through a 100-μm nylon mesh strainer (BD Biosciences) and seeded on poly-l-ornithine-coated 75-cm2 flasks in DMEM medium containing 10 % fetal bovine serum (FBS; BioWhittaker) and a 1 % antibiotic anti-mycotic solution (Sigma). The cultures were maintained at 37 °C and 5 % CO2, and the medium was changed every 4 days, adding 10 ng/ml human PDGF-AA (Millipore) fromP15 (for 2 weeks). When the cultures reached confluence, they were shaken overnight at 230 rpm and at 37 °C to detach the remaining oligodendrocyte progenitors located on top of the confluent astrocyte monolayer. The medium was then filtered through a 40-μm nylon mesh strainer (BD Biosciences) and centrifuged at 900 rpm. The cells were seeded twice (45 min each) in bacterial grade Petri dishes (Sterilin) to remove the loosely adherent microglia. After another round of centrifugation, the resulting enriched oligodendrocyte progenitor cell suspension was counted and seeded. After 2 days in culture the PDGFRα-positive oligodendrocyte precursors represented 87 ± 0.2 % of the total cells (mean ± SEM; n = 50 independent cultures) at P0 and 82 ± 0.7 % (n = 30 independent cultures) at P15 (Suppl. Fig. 1j).
For cells obtained from human biopsies, the same protocol was used except that the medium was supplemented with 10 ng/ml human PDGF-AA (Millipore) for 2 weeks, and then incubated for 2 weeks without growth factors before shaking. After 2 days in culture the PDGFRα-positive oligodendrocyte precursors represented 70 ± 0.04 % (n = 20 independent cultures; Suppl. Fig. 1).
Differentiation assay
Purified OPCs were placed on coverslips coated with poly-l-lysine and laminin in 24-well tissue culture dishes at a density of 2 × 104 cells/well. To promote differentiation, the cells were maintained in serum-free differentiation medium, as described previously [33], consisting of BME:F12 (1:1) supplemented with 100 μg/ml transferrin, 20 μg/ml putrescine, 12.8 ng/ml progesterone, 10.4 ng/ml selenium, 25 μg/ml insulin, 0.8 μg/ml thyroxine, 0.6 % glucose, and 6.6 mM glutamine. The PDE7 inhibitors (TC3.6, VP1.15 or BRL; 1 μM) were added to the culture medium to study their effects: and after 5 (for P0 and human cultures) or 7 DIV (for P15 cultures), the cells were fixed with 4 % PFA for further immunocytochemical analyses.
For the kinetics assays, OPCs were maintained for 2, 3, 5, or 7 DIV, to compare their rate of differentiation. Oligodendroglial cultures were examined by phase-contrast microscopy and differentiation was confirmed by immunostaining with antibodies against oligodendroglial cell-specific markers (CNPase and MBP), as described below.
To determine whether the cAMP/PKA pathway was involved in differentiation, cultures were treated with the cAMP antagonist Rp-cAMP (100 μM, BIOMOL Research Laboratories) to block PKA activation.
Proliferation assay
Purified OPCs were placed on coverslips coated with poly-l-lysine and laminin (2 × 104 cells/well) and they were incubated with the PDE7 inhibitors TC3.6, VP1.15 or BRL (1 μM) in differentiation medium. After 42 h in culture, BrdU (50 μM; Sigma) was added for 6 h and after a total of 72 h in culture, the cells were fixed and BrdU incorporation was detected by immunocytochemistry (1:20, G3G4 Hybridoma Bank) combined with the detection of the oligodendroglial markers described above.
Cell viability
OPCs were cultured with PDE7 inhibitors (TC3.6, VP1.15 or BRL; 1 μM) in differentiation medium and after 2 DIV, they were fixed with 4 % PFA and subjected to immunocytochemistry for active caspase 3 (1:200, Abcam) and the oligodendroglial markers described above.
Immunocytochemistry
OPCs were characterized as described previously [25, 26]. The different stages of oligodendroglial differentiation were detected using the following markers: PDGFRα (anti-PDGFRα, 1:200; Santa Cruz Biotechnology), NG2 (anti-NG2, 1:200; Millipore), or A2B5 (anti-A2B5, 1:10; Hybridoma Bank) for OPCs; CNPase (anti-CNPase, 1:200; Covance) for pre-oligodendrocytes; MBP (anti-MBP, 1:100; Abcam) or PLP (anti-PLP, 1:200; Abcam) for mature oligodendrocytes; and Olig2 (anti-Olig2, 1:250; Millipore) as a marker for all oligodendroglial stages. Cultures were co-stained with anti-PDE7A (1:50; Santa Cruz Biotechnology) or anti-PDE7B (1:50; Santa Cruz Biotechnology). The secondary antibodies used were donkey anti-goat Alexa 594, donkey anti-mouse Alexa 488, donkey anti-rat Alexa 594 and donkey anti-rabbit Alexa 488 (all diluted 1:1,000).
Western blotting
ERK signaling was analyzed in 96-well tissue culture plates seeded with 5 × 104 cells per well. Cells plated in OPC medium were maintained at 37 °C in a 5 % CO2 atmosphere at 95 % relative humidity. After 1 DIV, the cells were deprived of serum for 24 h and subsequently stimulated with PDE7 inhibitors (30 μM). After 10 min the cells were fixed for a further 10 min at RT with 4 % PFA. An In-Cell Western blot was carried out using the Odyssey Infrared Imaging System and the OPCs were immunostained with anti-pERK (1:250; Santa Cruz) and anti-ERK (1:300; Santa Cruz) following the manufacturer’s instructions. Antibody binding was assessed using secondary antibodies conjugated to 680 or 800 IRDye and the 96-well tissue culture plates were scanned using the Odyssey Infrared Imaging System (LICOR). ERK and pERK expression were analyzed according to the Odyssey LICOR instruction manual and the amount of pERK relative to that of total ERK was normalized to the pERK/ERK value in control samples. Values are represented as the mean ± SEM.
To quantify pCREB and CREB, we performed a quantitative Western blot using the Odyssey Infrared Imaging System (LICOR) for simultaneous analysis of two different proteins in separate fluorescent channels. The membranes were incubated with secondary antibodies conjugated to 680 or 800 IRDye and scanned using the Odyssey infrared scanner (LICOR). The bands corresponding to pCREB and CREB were analyzed according to the Odyssey LICOR instruction manual. The amount of pCREB relative to total CREB was normalized against pCREB/CREB values in control samples and presented as the mean ± SEM.
To quantify the expression of PDE7A/B, and PDE4B/D, at different stages we also carried out an In-Cell Western blot using the Odyssey Infrared Imaged System, as described above. In this case, OPCs were immunostained with anti-PDE7A (1:50), anti-PDE7B (1:50), anti-PDE4B (1:50), anti-PDE4D (1:50; all from Santa Cruz Biotechnology) and with anti-tubulin (1:80,000; Sigma), following the manufacturer’s instructions. OPC cultures were analyzed by immunocytochemistry after 1, 3, and 5 DIV in differentiation medium to compare PDEs expression at different stages of oligodendrocyte differentiation. The expression of PDE7 and PDE4 isoforms was analyzed as indicated in the Odyssey LICOR instruction manual, and the relative phosphodiesterase levels were normalized against the corresponding tubulin levels and expressed as the mean ± SEM.
Imaging analysis
Digital fluorescence images were obtained with a DFC480 FX digital camera (Leica) coupled to a Leica DM5000B microscope or using a SP5 resonant scanner (Leica Microsystems).
Statistical analysis
The data is shown as the ratio to the oligodendroglial lineage cells, and were analyzed using SigmaPlot software (Jandel Scientific). A comparative analysis was performed using a Student’s t test (or Mann–Whitney rank-sum test) or one-way ANOVA. Statistical significance was set at p < 0.05, *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Phosphodiesterase 7 is expressed by oligodendrocyte precursor cells
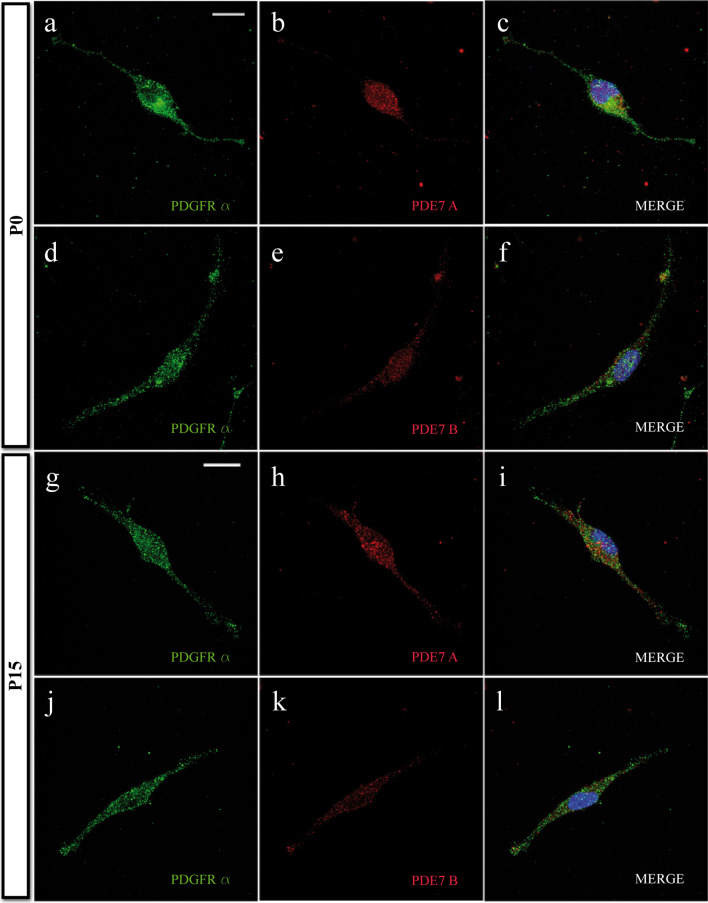
To analyze the expression of both PDE7 isoforms (PDE7A and PDE7B) in rodent OPCs, we cultured isolated cortical OPCs from P0 postnatal mouse brains in vitro and performed dual immunocytochemistry for PDE7A and PDE7B together with the well-characterized OPC markers: NG2 (not shown) and PDGFRα (Fig. 2a–f). Double immunocytochemistry studies clearly revealed that all OPCs expressed both PDE7A and PDE7B, isoforms which were also expressed by all OPCs isolated from the cortex at P15 (Fig. 2g-l and Suppl. Fig. 2).
Fig. 2.
OPCs express PDE7. a–f High-magnification immunofluorescence images of PDE7A/PDGFRα a–c or PDE7B/PDGFRα d-f double-labeled P0-derived OPCs. Both PDE7 isoforms were expressed by almost every OPC (see text) identified with the OPC molecular marker PDGFRα, after 1 DIV in differentiation medium. g–l High-magnification immunofluorescence images of PDE7A/PDGFRα g–i or PDE7B/PDGFRα j–l double-labeled P15-derived OPCs in similar conditions as P0 OPC cultures, with similar results. Scale bars are 10 μm in a–f and 7.5 μm in g–l
PDE7 inhibitors promote the differentiation of P0 cortical-derived OPCs
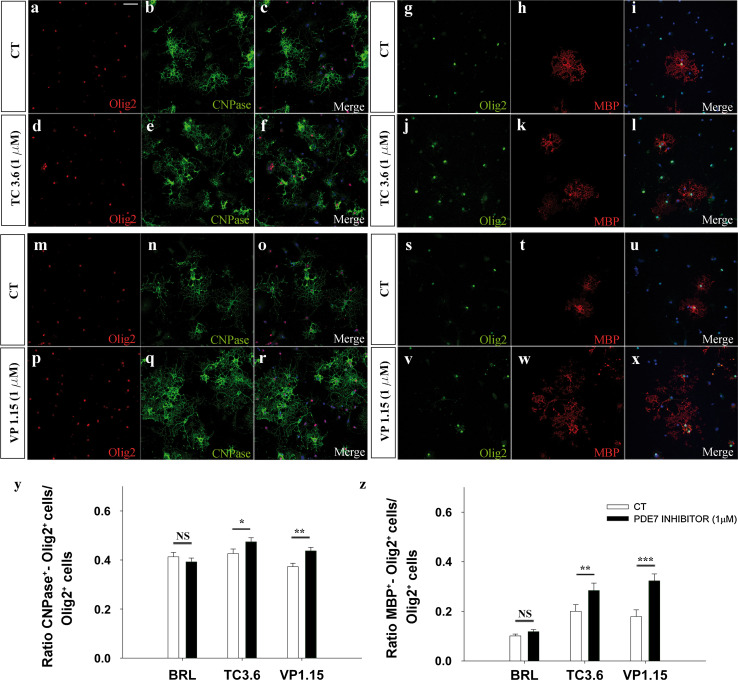
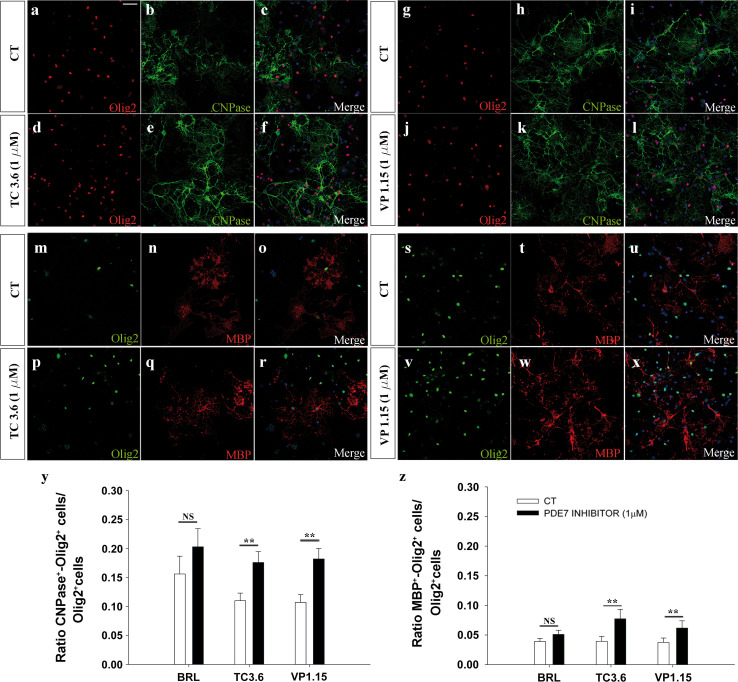
We investigated the effects of the PDE7 inhibitors, TC3.6 and VP1.15, on cultures of P0 cortical OPCs (see Materials and methods), using the commercial PDE7 inhibitor BRL to set a threshold for further comparison with the new inhibitors. We first carried out experiments using OPCs cultured for 5 DIV in differentiation medium (see Materials and methods) in the presence of both inhibitors and the commercial one: TC3.6, VP1.15, BRL, and their corresponding control. While BRL had no effect on OPC differentiation, exposure to 1 μM of TC3.6 produced a significant increase in the number of OPCs that became pre-oligodendrocyte (CNPase+/Olig2+ cells) relative to the total number of Olig2 cells in the cultures, compared with the controls (Fig. 3a–f, y) and the increase produced by VP1.15 was stronger (Fig. 3m–r, y). When the number of OPCs that formed mature oligodendrocytes (MBP+/Olig2+ cells) was assessed after 5DIV in differentiation medium, and relative to the total number of oligodendrocytes (Olig2+) similar increases were observed to those detected for CNPase+/Olig2+ cells (Fig. 3g–l, s–x, z): again, TC3.6 and VP1.15 exerted more potent and significant effects than BRL.
Fig. 3.
Both TC3.6 and VP 1.15 PDE7 inhibitors promote the differentiation of OPCs from P0 mice brain cortices. a–l Immunofluorescence images of Olig2 and CNPase (a–f) or Olig2 and MBP (g–l) expression in OPCs isolated from P0 brains grown for 5 DIV in control differentiation medium (a–c, g–i) and in the presence of 1 μM of TC3.6 (d–f, j–l). Cultures were counterstained with DAPI. m–x Images shown CNPase (m–r) or Olig2 and MBP (s–x) expression when differentiation was studied in control conditions (m–o, s–t) versus. In the presence of 1 μM of VP 1.15 (p–r, v–x) (y) the plot represents the number of pre-oligodendrocytes (CNPase+/Olig2+) in respect to the total number of oligodendrocyte (Olig2+). In the presence of both PDE7 inhibitors, the percentage of differentiated cell was significantly higher than in control conditions, except for BRL. (z) Quantification of mature oligodendrocytes (MBP/Olig2+) differentiated in respect to the total number of oligodendrocyte (Olig2+). Both new PDE7inhibitors (TC3.6 and VP1.15) were more efficient than commercial BRL. Scale bar represents 50 μm for a–x. Values are given as mean ± SEM and the results of Student’s t test are represented as *p < 0.05, **p < 0.01, and ***p < 0.001
A more detailed analysis of the differentiation rate in the presence of PDE7 inhibitors at different time points (2, 3, 5, and 7 DIV in differentiation medium) revealed that PDE7 inhibition induced OPCs differentiation earlier than in control conditions, producing more CNPase-positive cells from 2 DIV until 5 DIV (Suppl Fig. 3a). This difference only disappeared at the seventh day (Suppl Fig. 3a). To rule out the possibility that the differences in differentiation were due to changes in PDE7 expression along the oligodendrocyte lineage, we analyzed the expression of both isoforms (PDE7A and PDE7B) in our cultures at 1, 3, and 5 DIV. In the In-Cell Western blot (see Methods), there were no changes in PDE7A and PDE7B protein at different stages of oligodendrocyte differentiation (Suppl. Fig. 3b). However, when we analyzed the expression kinetics of different isoforms of another well-known PDE, as PDE4 (PDE4B and PDE4D), the OPCs increasingly expressed more PDE4B as they were cultured for more days, but differences were not significant for PDE4D (Suppl. Fig. 3b).
PDE7 inhibition exerts a neuroprotective effect on cultured OPCs
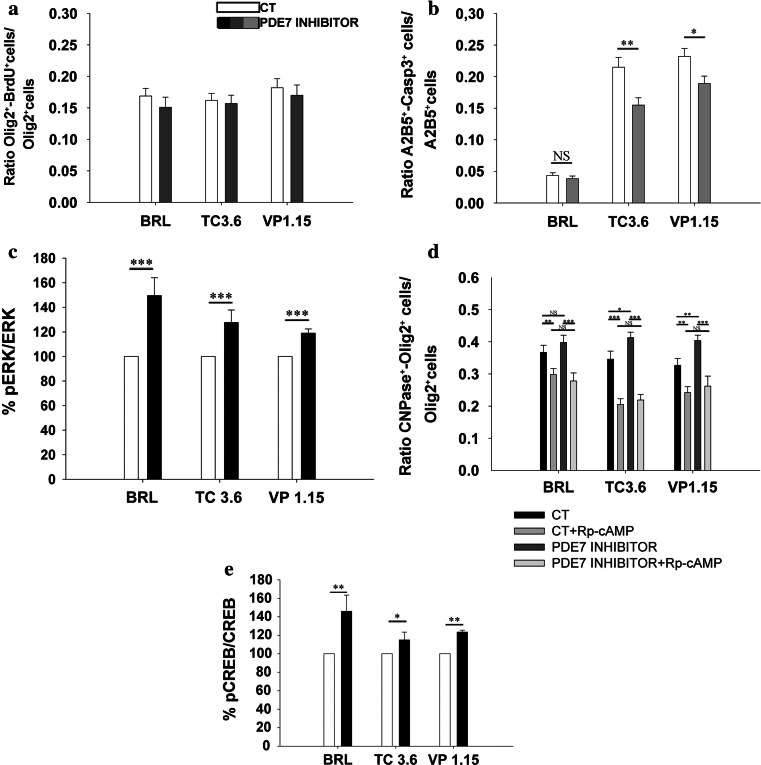
Analysis of OPC cultures incubated in the presence of PDE7 inhibitors revealed that the number of oligodendroglial cells, identified as Olig2+ cells, increased compared with the corresponding controls. This effect was observed for all three PDE7 inhibitors used in this study (% Olig2+ cells versus CT: BRL: 125 ± 6,p = 0.002; TC3.6: 130 ± 8, p = 0.023 and VP1.15: 179 ± 12, p < 0.001), being this increase higher when the inhibitor was VP1.15.
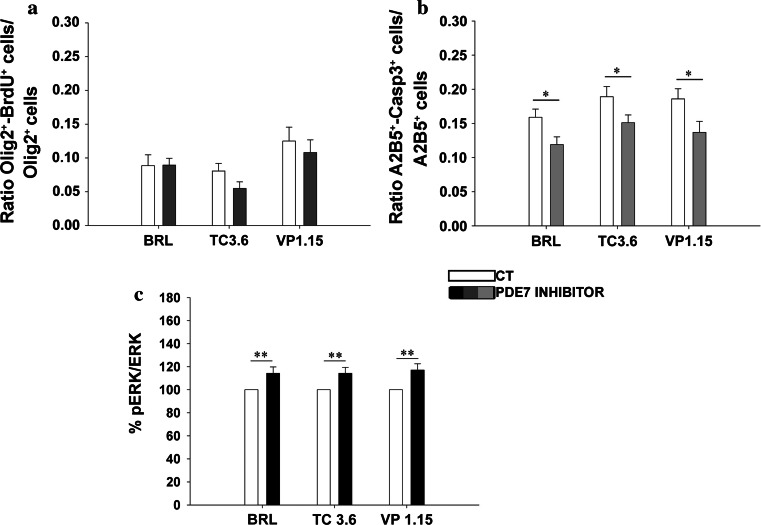
To determine whether this increase was due to an increase in OPC proliferation or survival, we analyzed BrdU incorporation and the number of caspase3+ cells in the cultures. The analysis of BrdU incorporation in P0 OPC cultures revealed that none the PDE7 inhibitors had any effect on oligodendrocyte proliferation (Fig. 4a). However, in survival assays, we found that there were fewer dying OPCs (expressing caspase3) in the presence of the PDE7 inhibitors than in control cultures (Fig. 4b). Remarkably, fewer dying OPCs were observed in cultures treated with the new PDE7 inhibitors than with the commercial inhibitor (BRL; Fig. 4b). To define the signaling pathways underlying the effects of PDE7 inhibitors on oligodendrocyte maturation and survival, we first investigated the contribution of ERK activation since this kinase has been implicated in OPC biology and it plays a key role in triggering the switch between OPC proliferation and differentiation [34]. OPC cultures from P0 mice were serum deprived for 24 h and treated for 10 min with PDE7 inhibitors, after which pERK/ERK was analyzed. All three inhibitors significantly increased the pERK/ERK levels (Fig. 4c), suggesting that PDE7 inhibition involves the activation of this signaling pathway.
Fig. 4.
TC3.6 and VP 1.15 do not affect OPC proliferation but increase their survival at P0, by activating ERK, PKA, and CREB intracellular pathways. a Quantification of BrdU incorporation by P0 OPCs characterized with the oligodendroglial marker Olig2. The presence of BRL, TC3.6, or VP1.15 (1 μM) did not modify the number of P0 BrdU+/Olig2+ OPCs after 3 DIV in comparison to their respective controls. b Determination of dead OPCs (active caspase3+/A2B5+) versus the total number of OPCs (A2B5+cells) after 2 DIV in the presence of PDE7 inhibitors. With both new PDE7 inhibitors survival of OPCs was increased, while the commercial BRL, did not have any effect. c Estimation of p-ERK/ERK quotient by measurement of fluorescence intensity in purified OPCs cultures by immunostaining with anti-pERK and anti-ERK. ERK was significantly more activated when OPCs were exposed to BRL, TC3.6, and VP1.15 (30 μM). d Detection and quantification of CNPase+/Olig2+ pre-oligodendrocytes in purified cultures treated with PDE7 inhibitors plus the cAMP antagonist, Rp-cAMP (100 μM), which inhibits PKA. The effects observed after PDE7 inhibition on OPCs differentiation disappeared when PKA pathway was inhibited, which strongly suggest the implication of cAMP/PKA pathway in OPC differentiation. e Determination of pCREB/CREB quotient by measurement of fluorescence intensity. An increase of pCREB/CREB was also observed in the isolated OPCs under the presence of the three PDE7 inhibitors studied but, in this case, commercial BRL and VP1.15 showed stronger effects than the other new inhibitor, TC3.6. Values are given as mean ± SEM and the results of Student’s t test are represented as *p < 0.05, **p < 0.01, and ***p < 0.001
PDE7 is a cAMP-specific enzyme and the most common intracellular target for cAMP is protein kinase A (PKA), whose activation is responsible for many cAMP-related effects [35]. Thus, we assessed whether PKA activation was required for the pro-differentiation effects of TC3.6 and VP1.15 in OPCs. Treatment of OPC cultures with the cAMP antagonist Rp-cAMP (which blocks cAMP/PKA activation) prevented the increase in oligodendroglial differentiation induced by the PDE7 inhibitors (Fig. 4d), suggesting that the cAMP/PKA pathway mediates the effects of these inhibitors. We next analyzed the phosphorylation state of the cAMP response element-binding protein (CREB), a known target of the cAMP/PKA signaling pathway. At P0, the pCREB/CREB ratio increased in the presence of PDE7 inhibitors compared with the corresponding controls (Fig. 4e), suggesting that the new PDE7 inhibitors acted through the PKA/CREB pathway. Notably, the effect of TC3.6 was significantly weaker than that of the other two inhibitors studied.
PDE7 inhibitors promote the differentiation and survival of cortical-derived P15 OPCs
Based on the studies on OPCs isolated from P0 mouse brains, we investigated the effects of our PDE7 inhibitors on OPCs isolated from cortices of older mice, purifying OPCs from the cortex of P15 mice when a peak of PLP mRNA expression occurs [36].
Both new PDE7 inhibitors accelerated the differentiation of P15 OPCs to pre-oligodendrocyte while the commercial one did not show a significant effect (Fig. 5a–l, y) and, moreover, we observed an increase in the number of mature oligodendrocytes after 7 DIV in differentiation medium (Fig. 5m–x, z). As seen in OPCs isolated at P0, exposure to PDE7 inhibitors had no effect on the number of P15 oligodendrocytes that incorporated BrdU (Fig. 6a) while they enhanced OPC survival (Fig. 6b). As P0 and P15 OPCs responded similarly to these PDE7 inhibitors, we investigated the role of the pERK/ERK signaling pathway in the former. As seen in younger OPCs, all three inhibitors induced an increase in pERK/ERK activity (Fig. 6c), suggesting that the PDE7 inhibitors employ a similar mechanism of action in P0 and P15 OPCs, although the increase in pERK/ERK activity observed in P0 OPCs was greater than that observed in P15 OPCs (Fig. 4c).
Fig. 5.
TC3.6 and VP 1.15 PDE7 inhibitors increased the differentiation of OPCs at P15. a–l Immunofluorescence images of Olig2 and CNPase expression in P15-derived OPCs grown for 7 DIV in control differentiation medium (a–c, g–i) or in the presence of 1 μM of TC3.6 (d–f) or 1 μM of VP1.15 (j–l). Cultures were counterstained with DAPI. m–x Images show mature oligodendrocytes differentiated after 7 DIV in differentiation medium of P15-derived OPCs cultures identified as MBP/Olig2+ cells in control medium (m–o, s–u) and in the presence of 1 μM of TC3.6 (p–r) or 1 μM of VP1.15 (v–x). Cultures were counterstained with DAPI. y Total number of differentiated pre-oligodendrocytes (CNPase+/Olig2+) in respect to the total number of oligodendrocyte cells (Olig2+). In the presence of both new PDE7 inhibitors, the number of differentiated cell was significantly higher than in control conditions. z Quantification of mature oligodendrocytes (MBP/Olig2+) differentiated. In the presence of TC3.6 and VP1.15 PDE7 inhibitors, the number of mature oligodendrocytes was significantly higher than in control conditions. Both TC3.6 and VP1.15 were more efficient than commercial BRL. Scale bar represents 50 μm for a–x. Values are given as mean ± SEM and the results of Student’s t test are represented as *p < 0.05, **p < 0.01, and ***p < 0.001
Fig. 6.
TC3.6 and VP 1.15 do not affect OPC proliferation but favor OPC survival at P15. a Quantification of BrdU incorporation by double immunocytochemistry in P15 OPCs characterized with the oligodendroglial marker Olig2. Like at P0, the presence of TC3.6, VP1.15, or BRL (1 μM) did not modify the number of BrdU+/Olig2+ OPCs after 3DIV versus the total number of oligodendrocytes (Olig2+). b Determination of dead OPCs percentage (active caspase 3+/A2B5+) versus the total number of OPCs (A2B5+) after 2 DIV. OPC survival increased in the presence of PDE7 inhibitors (1 μM). c Estimation of p-ERK/ERK quotient by measurement of fluorescence intensity in purified P15 OPCs cultures by immunostaining with anti-pERK and anti-ERK. p-ERK/ERK was equally activated in these cells when exposed to BRL, TC3.6, and VP 1.15 (30 μM). Values are given as mean ± SEM and the results of Student’s t test are represented as *p < 0.05, **p < 0.01, and ***p < 0.001
Effects in isolated human OPCs
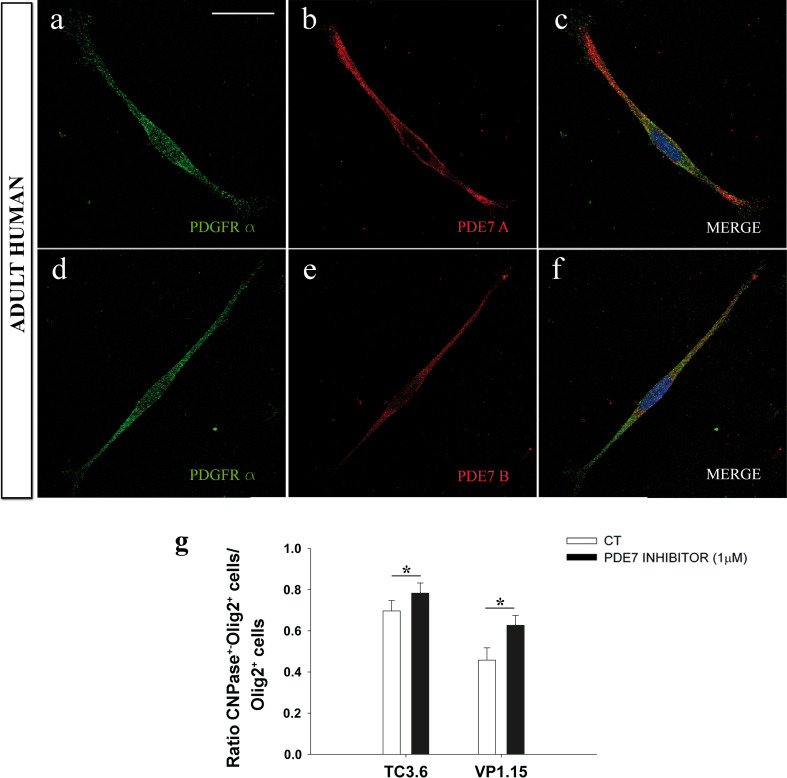
We next confirmed that PDE7A and PDE7B were expressed by all OPCs in cultures of OPCs isolated from adult human biopsies (Fig. 7a–f). In the presence of VP1.15 and TC3.6, there were more pre-oligodendrocytes in human OPC cultures exposed to the inhibitors than in their corresponding controls (Fig. 7g). These findings suggest that PDE7 activity in adult human OPCs is likely to be similar to that observed in mouse OPCs.
Fig. 7.
PDE7 inhibition on adult human OPCs potentiates their differentiation process to form myelin. a–f High-magnification immunofluorescence images of PDE7A/PDGFRα (a–c) or PDE7B/PDGFRα (d–f) double-labeled OPCs derived from adult human brain biopsies. Both isoforms was expressed on adult human OPCs. (g) The number of pre-oligodendrocytes (CNPase+/Olig2+) respect total number of Olig2 cells in comparison to control conditions in the presence of VP1.15 (1 μM) for 5 DIV was significantly higher than in control conditions. With TC3.6, there is also a significant increase in the number of pre-oligodendrocytes. Scale bar represents 25 μm in a–f. Values are given as mean ± SEM and the results of Student’s t test are represented as *p < 0.05, **p < 0.01, and ***p < 0.001
Discussion
In the present study, we demonstrate that both PDE7 isoforms (PDE7A and PDE7B) are expressed by murine and human OPCs in culture. We also show that two new PDE7-inhibitors (TC3.6 and VP1.15) promote OPCs survival and differentiation towards myelin-forming phenotypes in both murine and human OPCs.
Although PDE expression has been analyzed in different tissues, the expression of the different PDE isoforms remains to be fully characterized [37, 38]. Multiple PDE isoforms have been identified in neural populations, including PDE3, 4, 7, and 9a [15, 38–40] and PDE9a expression has also been described in a subpopulation of astrocytes [41]. PDE expression was first reported in myelin-forming cells over two decades ago [42], and since then, only one further study has examined PDE4 expression in Schwann cells [43], although alterations in PDE expression following CNS injury have also been reported [17, 44, 45], Thus, the present study is the first to demonstrate PDE7 expression and activity in oligodendroglial cells, and common enzymatic activity in OPCs from different neuronal types [15].
Increase in intracellular levels of cAMP appears to favor the differentiation of oligodendroglial and Schwann cells towards myelin-forming phenotypes [46, 47]. This suggests that inhibition of PDE7 activity would be a more effective remyelination strategy than the use of olesoxime [48] or FTY720 (Fingolimod), for example, which have proven effects on adult human oligodendrocytes [49]. LINGO-1 antagonists [50, 51], one of the best studied as a potential treatment, also displays significant therapeutic potential.
Myelination appears to be mediated by a limited number of molecules that vary widely in their function, including chemokines [52, 53], kinases like Cdk2 (only after demyelination) [54] and MAPK [55, 56], semaphorins [57, 58], transferrin [59], and the Na+/Ca2+ exchanger-3 [60]. Other molecules are implicated in myelination, such as retinoic acid [61], although to date this has only been demonstrated in Schwann cells. To the best of our knowledge, of all the factors that promote oligodendrocyte differentiation, only Fingolimod [49] and PDE7 inhibitors (present work) have been shown to exhibit such effects in human oligodendroglia.
While the thyroid hormone T3 is classically considered fundamental for OPC survival, proliferation, and maturation/myelin gene expression [62], a panoply of other molecules subsequently promote oligodendroglial survival and protection in animal models of demyelination, including: neurotrophins (CNTF, PDGF, IGF-1), neuregulin-1, and interleukins [63]. Many of these effects are combined with an influence on proliferation and/or differentiation. Indeed, the neurotrophin BDNF was recently shown to exert potent and selective neuroprotective effects on oligodendrocytes in murine EAE, without affecting maturation [64, 65]. Both BDNF and FGF are components of the neurotrophic soup produced by the endothelium to give rise to the so called “oligovascular” niche in the CNS, which protects OPCs during development [66]. The tetracycline antibiotic minocycline was recently proposed as an effective neuroprotector of oligodendrocytes in conditions of oxidative stress [68]. In the present study, the effect of PDE7 inhibitors on OPC survival (~20–40 %) was more marked than that previously reported for minocycline (~10 %; [67]), and similar to that effect described for the endothelium-produced neurotrophic soup (~40 %; [66]).
Unlike cell survival and differentiation, oligodendroglial proliferation was not affected when PDE7 activity was blocked in OPCs. OPC proliferation is strongly promoted by sonic hedgehog (both during development and in adulthood), HGF and, to a lesser extent, other growth factors like PDGF-AA, IGF neurotrophin-3, and some chemokines. By contrast, the role of mitogen FGF-2 in this process remains to be fully clarified [6, 68–72].
PDE7 inhibitors produce therapeutic affects in in vitro and in vivo models of MS, Parkinson disease, spinal cord injury, and stroke [12, 17, 19, 28]. For example, the commercial PDE4-inhibitor rolipram protects oligodendrocytes from cell death secondary to spinal cord injury, promoting their survival and eventually increasing their size and number in rodent models, establishing axonal conduction across the lesion [73, 74]. Rolipram also promotes remyelination possibly via the MEK-ERK pathway, in the cuprizone-induced mouse demyelination model [75], and its combination with lovastatin promotes neurorepair in an animal model of experimental autoimmune encephalomyelitis (EAE; [76]). The prevention of demyelination by rolipram has also been described in primates [77]. Moreover, the PDE1-inhibitor vinpocetine also exerts an effect on oligodendroglial differentiation and myelin expression [78].
The non-selective PDE-inhibitor Ibudilast exerts protective effects against hypoxic white-matter lesions [79] and exhibits some neuroprotective effects in MS patients, possibly by regulating the Th1/Th2 cytokine balance [80]. The immunomodulatory effects of both PDE3 and PDE4-inhibitors have potential applications in the treatment of Th1 autoimmune disorders, like MS [81, 82]. Indeed, PDE4B2 activity is dramatically increased around vessels in the EAE model of demyelination [83]. The administration of the PDE7-inhibitors studied in the present work significantly attenuates clinical symptoms in a mouse model of EAE in vivo, demonstrating the potential of these compounds in the treatment of MS [12, 20]. Together with the data presented here, these findings highlight the need to advance the study of TC3.6 and VP1.15 towards pre-clinical trials in order for these new therapies to eventually benefit MS patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1: Cultures are enriched in OPCs. Low-magnification immunofluorescence images of PDGFRα cells after 1 DIV isolated from P0 brains (a-c), P15 brains (d-f) and human biopsies (g-i). (j) Plot show the quantification of the percentage of OPCs, identified as PDGFRα+ cells after 1DIV, respect to the total number of cells. Scale bars are 50 µm in a-i. (JPEG 1741 kb)
Supplementary Figure 2: Every OPC expresses PDE7. (a-h) Low-magnification immunofluorescence images of PDE7A/PDGFRα (a-c) or PDE7B/PDGFRα (e-g) double-labeled P0 derived OPCs to show how both PDE7 isoforms were expressed by almost every OPC (see text), after 1 DIV in differentiation medium. In these pictures, several OPCs per field are observed, while in Figure 1 we show individual cells for illustration purposes. (d,h) shown immunocitochemistry without primary antibodies. (i-p) Low-magnification immunofluorescence images of PDE7A/PDGFRα (i-k) or PDE7B/PDGFRα (m-o) double-labeled P15 derived OPCs in similar conditions as P0 OPC cultures, with similar results. (l,p) shown immunocitochemistry with our primary antibodies. Scale bars are 25 µm in a-p. (JPEG 1421 kb)
Supplementary Figure 3: Kinetics of PDE7-promoted oligodendroglial differentiation and PDE expression in oligodendroglial lineage. (a) Quantification of pre-oligodendrocytes after 2, 3, 5 and 7DIV. The number of CNPase/Olig2+ cells was higher since the second day of culture until the fifth in the presence of both new PDE7 inhibitors. (b) Plot shown the measurement of fluorescence intensity in purified OPCs cultures immunostained for anti-PDE7A, PDE7B, PDE4B and PDE4D antibodies. It is remarkable that no differences in the expression of PDE7 were found along the days in culture for the oligodendrocyte lineage, although there is a significant difference in the PDE4B expression between the first and the other days in culture. Values are given as mean ± SEM and the results of ANOVA on Ranks are represented as *; P<0.05, **; P<0.01 and ***; P<0.001. (JPEG 487 kb)
Acknowledgments
We thank Dr. Jose Angel Rodríguez Alfaro, Dr. Javier Mazarío, I. Machín, R. Lebrón, I. Sánchez and J. Sarmentero for technical support. This work was supported by grants from the Spanish Ministerio de Economía y Competitividad––MINECO (SAF2009–07842, SAF2012–40023 ADE10–0010, RD07–0060–2007, RD12–0032–12, partially supported by F.E.D.E.R.-European Union “Una manera de hacer Europa”) and the Fundación Eugenio Rodríguez Pascual (Spain) to F.dC,, MINECO (SAF2009–13015 and RD07/0060/0015, partially supported by F.E.D.E.R.-European Union “Una manera de hacer Europa”-) to A.M. and the Spanish Institute of Health-ISCIII (PS09/02116) to J.P. EM.M-R. is a recipient of a predoctoral fellowship from the MINECO FPI program (associated to SAF2009–07842). A.B. holds a postdoctoral contract funded by the “Sara Borrell” program of the FIS-ISCIII/Spanish Ministry of Health. M.R and V.P. were recipients of a pre-doctoral fellowship from the CSIC (JAE program). F.dC. is on contract to SESCAM.
Conflict of interest
The authors declare no conflicts of interest.
Abbreviations
- CNS
Central nervous system
- OPCs
Oligodendrocyte precursor cells
- MS
Multiple sclerosis
- PDE
Phosphodiesterase
- cAMP
3’-5’-cyclic adenosine monophosphate
- PKA
Protein kinase A
- CREB
cAMP response element-binding protein
- DIV
Days in vitro
Contributor Information
A. Bribián, Phone: ++ 34 925 247782, FAX: ++34925247745, Email: ajbribian@sescam.jccm.es
F. de Castro, Phone: +34-92-5247782, FAX: +34-92-5247745, Email: fdec@sescam.jccm.es
References
- 1.Henderson AP, Barnett MH, Parratt JD, Prineas JW. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–753. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH, Lucchinetti C, Rodríguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 3.Steinman L. Blocking adhesion molecules as therapy for multiple sclerosis: natalizumab. Nat Rev Drug Discov. 2005;4:510–518. doi: 10.1038/nrd1752. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Forero I, Peláez A, Villoslada P. Pharmacogenomics of multiple sclerosis: in search for a personalized therapy. Expert Opin Pharmacother. 2008;9:3053–3067. doi: 10.1517/14656560802515553. [DOI] [PubMed] [Google Scholar]
- 5.Martino G, Franklin RJ, Van Baron EA, Kerr DA. Stem cell transplantation in multiple sclerosis: current status and future prospects. Nat Rev Neurol. 2010;6:247–255. doi: 10.1038/nrneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- 6.de Castro F, Bribián A. The molecular orchestra of the migration of oligodendrocyte precursors during development. Brain Res Brain Res Rev. 2005;49:227–241. doi: 10.1016/j.brainresrev.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González-Pérez O, Álvarez-Buylla A. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res Rev. 2011;67:147–156. doi: 10.1016/j.brainresrev.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 10.Giembycz MA, Smith SJ. Phosphodiesterase 7A: a new therapeutic target for alleviating chronic inflammation? Curr Pharm Des. 2006;12:3207–3220. doi: 10.2174/138161206778194123. [DOI] [PubMed] [Google Scholar]
- 11.Page CP, Spina D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb Exp Pharmacol. 2011;204:391–414. doi: 10.1007/978-3-642-17969-3_17. [DOI] [PubMed] [Google Scholar]
- 12.Redondo M, Brea J, Pérez DI, Soteras I, Val C, Pérez C, Morales-García JA, Alonso-Gil S, Paul-Fernández N, Martín-Álvarez R, Cadavid MI, Loza MI, Pérez-Castillo A, Mengod G, Campillo NE, Martínez A, Gil C. Effect of phosphodiesterase 7 (PDE7) inhibitors in experimental autoimmune encephalomyelitis mice. Discovery of a new chemically diverse family of compounds. J Med Chem. 2012;55:3274–3284. doi: 10.1021/jm201720d. [DOI] [PubMed] [Google Scholar]
- 13.Bloom TJ, Beavo JA. Identification and tissue-specific expression of PDE7 phosphodiesterase splice variants. Proc Natl Acad Sci U S A. 1996;93:14188–14192. doi: 10.1073/pnas.93.24.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miró X, Pérez-Torres S, Palacios JM, Puigdomenech P, Mengod G. Differential distribution of cAMP-specific phosphodiesterase 7A mRNA in rat brain and peripheral organs. Synapse. 2001;40:201–214. doi: 10.1002/syn.1043. [DOI] [PubMed] [Google Scholar]
- 15.Reyes-Irisarri E, Pérez-Torres S, Mengod G. Neuronal expression of cAMP-specific phosphodiesterase 7B mRNA in the rat brain. Neuroscience. 2005;32:1173–1185. doi: 10.1016/j.neuroscience.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Torres S, Cortes R, Tolnay M, Probst A, Palacios JM, Mengod G. Alterations on phosphodiesterase type 7 and 8 isozyme mRNA expression in Alzheimer’s disease brains examined by in situ hybridization. Exp Neurol. 2003;182:322–334. doi: 10.1016/S0014-4886(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 17.Morales-Garcia JA, Redondo M, Alonso-Gil S, Gil C, Pérez C, Martínez A, Santos A, Pérez-Castillo A. Phosphodiesterase 7 inhibition preserves dopaminergic neurons in cellular and rodent models of Parkinson disease. PLoS ONE. 2011;6:e17240. doi: 10.1371/journal.pone.0017240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J, Watson A, Kempson J, Carlsen M, Barbosa J, Stebbins K, Lee D, Dodd J, Nadler SG, McKinnon M, Barrish J, Pitts WJ. Identification of potent pyrimidine inhibitors of phosphodiesterase 7 (PDE7) and their ability to inhibit T cell proliferation. Bioorg Med Chem Lett. 2009;19:1935–1938. doi: 10.1016/j.bmcl.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 19.Paterniti I, Mazzon E, Gil C, Impellizzeri D, Palomo V, Redondo M, Pérez DI, Espósito E, Martínez A, Cuzzocrea S. PDE 7 inhibitors: new potential drugs for the therapy of spinal cord injury. PLoS ONE. 2011;6:e15937. doi: 10.1371/journal.pone.0015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redondo M, Palomo V, Brea J, Pérez DI, Martín-Álvarez R, Pérez C, Paúl-Fernández N, Conde S, Cadavid MI, Loza MI, Mengod G, Martínez A, Gil C, Campillo NE. Identification in silico and experimental validation of novel phosphodiesterase 7 inhibitors with efficacy in experimental autoimmune encephalomyelitis mice. ACS Chem Neurosci. 2012;3:793–803. doi: 10.1021/cn300105c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen LC, Færgeman NJ. Chemical genomics and emerging DNA technologies in the identification of drug mechanisms and drug targets. Curr Top Med Chem. 2012;12:1331–1345. doi: 10.2174/156802612801319025. [DOI] [PubMed] [Google Scholar]
- 22.Walsh DP, Chang YT. Chemical genetics. Chem Rev. 2006;106:2476–2530. doi: 10.1021/cr0404141. [DOI] [PubMed] [Google Scholar]
- 23.Gil C, Castaño T, Campillo N, Ballester S, González C. Hernández J. Compound that is a dual inhibitor of enzymes PDE7 and/or PDE4, pharmaceutical compositions and uses thereof. WO2008113881
- 24.Martínez A, Gil C, Palomo V, Pérez DI, Pérez C, Pérez-Castillo A, Loza MI, Cadavid MI, Brea J. 5-imino substituted 1,2,4-thiadiazoles useful for the treatment of neurodegenerative diseases. WO2011039403
- 25.Castro A, Jerez MJ, Gil C, Calderón F, Doménech T, Nueda A, Martínez A. CODES, a novel procedure for ligand-based virtual screening: PDE7 inhibitors as an application example. Eur J Med Chem. 2008;43:1349–1359. doi: 10.1016/j.ejmech.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Redondo M, Palomo V, Brea J, Pérez DI, Martín-Álvarez R, Pérez C, Paúl-Fernández N, Conde S, Cadavid MI, Loza MI, Mengod G, Martínez A, Gil C, Campillo NE. Identification in silico and experimental validation of novel phosphodiesterase 7 inhibitors with efficacy in experimental autoimmune encephalomyelitis mice. ACS Chem Neurosci. 2012;3:793–803. doi: 10.1021/cn300105c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castaño T, Wang H, Campillo NE, Ballester S, González-García C, Hernández J, Pérez C, Cuenca J, Pérez-Castillo A, Martínez A, Huertas O, Gelpí JL, Luque FJ, Ke H, Gil C. Synthesis, structural analysis, and biological evaluation of thioxoquinazoline derivatives as phosphodiesterase 7 inhibitors. Chem Med Chem. 2009;4:866–876. doi: 10.1002/cmdc.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redondo M, Zarruk JG, Ceballos P, Pérez DI, Pérez C, Perez-Castillo A, Moro MA, Brea J, Val C, Cadavid MI, Loza MI, Campillo NE, Martínez A, Gil C. Neuroprotective efficacy of quinazoline type phosphodiesterase 7 inhibitors in cellular cultures and experimental stroke model. Eur J Med Chem. 2012;47:175–185. doi: 10.1016/j.ejmech.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Paterniti I, Mazzon E, Gil C, Impellizzeri D, Palomo V, Redondo M, Perez DI, Esposito E, Martinez A, Cuzzocrea S. PDE 7 inhibitors: new potential drugs for the therapy of spinal cord injury. PLoS ONE. 2011;6(1):e15937. doi: 10.1371/journal.pone.0015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almazan G, Afar DE, Bell JC. Phosphorylation and disruption of intermediate filament proteins in oligodendrocyte precursor cultures treated with calyculin A. J Neurosci Res. 1993;36:163–172. doi: 10.1002/jnr.490360206. [DOI] [PubMed] [Google Scholar]
- 31.Molina-Holgado E, Vela JM, Arévalo-Martín A, Guaza C. LPS/IFN-gamma cytotoxicity in oligodendroglial cells: role of nitric oxide and protection by the anti-inflammatory cytokine IL-10. Eur J Neurosci. 2001;13:493–502. doi: 10.1046/j.0953-816x.2000.01412.x. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casaccia-Bonnefil P, Tikoo R, Kiyokawa H, Friedrich V, Jr, Chao MV, Koff A. Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1. Genes Dev. 1997;11:2335–2346. doi: 10.1101/gad.11.18.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joubert L, Foucault I, Sagot Y, Bernasconi L, Duval F, Alliod C, Frossard MJ, Pescini Gobert R, Curchod ML, Salvat C, Nichols A, Pouly S, Rommel C, Roach A, Hooft van Huijsduijnen R. Chemical inducers and transcriptional markers of oligodendrocyte differentiation. J Neurosci Res. 2010;88:2546–2557. doi: 10.1002/jnr.22434. [DOI] [PubMed] [Google Scholar]
- 35.Houslay MD, Kolch W. Cell-type specific integration of cross-talk between extracellular signal-regulated kinase and cAMP signaling. Mol Pharmacol. 2000;58:659–668. [PubMed] [Google Scholar]
- 36.Furusho M, Dupree JL, Nave KA, Bansal R. Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J Neurosci. 2012;32:6631–6641. doi: 10.1523/JNEUROSCI.6005-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansson EM, Reyes-Irisarri E, Mengod G. Comparison of cAMP-specific phosphodiesterase mRNAs distribution in mouse and rat brain. Neurosci Lett. 2012;525:1–6. doi: 10.1016/j.neulet.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 38.Nunes AR, Sample V, Xiang YK, Monteiro EC, Gauda E, Zhang J. Effect of oxygen on phosphodiesterases (PDE) three and four isoforms and PKA activity in the superior cervical ganglia. Adv Exp Med Biol. 2012;758:287–294. doi: 10.1007/978-94-007-4584-1_39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Staveren WC, Glick J, Markerink-van IM, Shimizu M, Beavo JA, Steinbusch HW, de Vente J. Cloning and localization of the cGMP-specific phosphodiesterase type 9 in the rat brain. J Neurocytol. 2002;31:729–741. doi: 10.1023/A:1025704031210. [DOI] [PubMed] [Google Scholar]
- 40.Castro LR, Gervasi N, Guiot E, Cavellini L, Nikolaev VO, Paupardin-Tritsch D, Vincent P. Type 4 phosphodiesterase plays different integrating roles in different cellular domains in pyramidal cortical neurons. J Neurosci. 2010;30:6143–6151. doi: 10.1523/JNEUROSCI.5851-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Susin C, Morales-García JA, Aguilar-Morante D, Palomo V, Sanz-Sancristóbal M, Alonso-Gil S, Gil C, Santos A, Martínez A, Pérez-Castillo A. The new iminothiadiazole derivative VP1.14 ameliorates hippocampal damage after an excitotoxic injury. J Neurochem. 2012;122:1193–1202. doi: 10.1111/j.1471-4159.2012.07866.x. [DOI] [PubMed] [Google Scholar]
- 42.Monge M, Yuan J, Cabon F, Zalc B, Kanfer JN. Glycerophosphorylcholine phosphocholine phosphodiesterase activity during the differentiation of glial progenitor cells. J Neurosci Res. 1993;36:441–445. doi: 10.1002/jnr.490360410. [DOI] [PubMed] [Google Scholar]
- 43.Walikonis RS, Poduslo JF. Activity of cyclic AMP phosphodiesterases and adenylyl cyclase in peripheral nerve after crush and permanent transection injuries. J Biol Chem. 1998;273:9070–9077. doi: 10.1074/jbc.273.15.9070. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh M, Garcia-Castillo D, Aguirre V, Golshani R, Atkins CM, Bramlett HM, Dietrich WD, Pearse DD. Proinflammatory cytokine regulation of cyclic AMP-phosphodiesterase four signaling in microglia in vitro and following CNS injury. Glia. 2012;60:1839–1859. doi: 10.1002/glia.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliva AA, Jr, Kang Y, Furones C, Alonso OF, Bruno O, Dietrich WD, Atkins CM. Phosphodiesterase isoform-specific expression induced by traumatic brain injury. J Neurochem. 2012;123:1019–1029. doi: 10.1111/jnc.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghandour MS, Feutz AC, Jalabi W, Taleb O, Bessert D, Cypher M, Carlock L, Skoff RP. Trafficking of PLP/DM20 and cAMP signaling in immortalized jimpy oligodendrocytes. Glia. 2002;40:300–311. doi: 10.1002/glia.10122. [DOI] [PubMed] [Google Scholar]
- 47.Azim K, Butt AM. GSK3β negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia. 2011;59:540–553. doi: 10.1002/glia.21122. [DOI] [PubMed] [Google Scholar]
- 48.Magalon K, Zimmer C, Cayre M, Khaldi J, Bourbon C, Robles I, Tardif G, Viola A, Pruss RM, Bordet T, Durbec P. Olesoxime accelerates myelination and promotes repair in models of demyelination. Ann Neurol. 2012;71:213–226. doi: 10.1002/ana.22593. [DOI] [PubMed] [Google Scholar]
- 49.Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol. 2008;63:61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- 50.Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, Hession C, Sah D, Trapp B, He Z, Jung V, McCoy JM, Pepinsky RB. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- 51.Mi S, Miller RH, Tang W, Lee X, Hu B, Wu W, Zhang Y, Shields CB, Zhang Y, Miklasz S, Shea D, Mason J, Franklin RJ, Ji B, Shao Z, Chédotal A, Bernard F, Roulois A, Xu J, Jung V, Pepinsky B. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009;65:304–315. doi: 10.1002/ana.21581. [DOI] [PubMed] [Google Scholar]
- 52.Maysami S, Nguyen D, Zobel F, Pitz C, Heine S, Höpfner M, Stangel M. Modulation of rat oligodendrocyte precursor cells by the chemokine CXCL12. Neuro Report. 2006;17:1187–1190. doi: 10.1097/01.wnr.0000227985.92551.9a. [DOI] [PubMed] [Google Scholar]
- 53.Patel JR, McCandless EE, Dorsey D, Klein RS. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci U S A. 2010;107:11062–11067. doi: 10.1073/pnas.1006301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caillava C, Vandenbosch R, Jablonska B, Deboux C, Spigoni G, Gallo V, Malgrange B, Baron-Van Evercooren A. Cdk2 loss accelerates precursor differentiation and remyelination in the adult central nervous system. J Cell Biol. 2011;193:397–407. doi: 10.1083/jcb.201004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santra M, Chopp M, Zhang ZG, Lu M, Santra S, Nalani A, Santra S, Morris DC. Thymosin beta four mediates oligodendrocyte differentiation by upregulating p38 MAPK. Glia. 2012;60:1826–1838. doi: 10.1002/glia.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishii A, Furusho M, Bansal R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and Schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J Neurosci. 2013;33:175–186. doi: 10.1523/JNEUROSCI.4403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piaton G, Aigrot MS, Williams A, Moyon S, Tepavcevic V, Moutkine I, Gras J, Matho KS, Schmitt A, Soellner H, Huber AB, Ravassard P, Lubetzki C. Class three semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain. 2011;134(Pt 4):1156–1167. doi: 10.1093/brain/awr022. [DOI] [PubMed] [Google Scholar]
- 58.Bernard F, Moreau-Fauvarque C, Heitz-Marchaland C, Zagar Y, Dumas L, Fouquet S, Lee X, Shao Z, Mi S, Chédotal A. Role of transmembrane semaphorin Sema6A in oligodendrocyte differentiation and myelination. Glia. 2012;60:1590–1604. doi: 10.1002/glia.22378. [DOI] [PubMed] [Google Scholar]
- 59.Perez MJ, Ortiz EH, Roffé M, Soto EF, Pasquini JM. Fyn kinase is involved in oligodendroglial cell differentiation induced by apotransferrin. J Neurosci Res. 2009;87:3378–3389. doi: 10.1002/jnr.21962. [DOI] [PubMed] [Google Scholar]
- 60.Boscia F, D’Avanzo C, Pannaccione A, Secondo A, Casamassa A, Formisano L, Guida N, Annunziato L. Silencing or knocking out the Na(+)/Ca(2+) exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ. 2012;19:562–572. doi: 10.1038/cdd.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Latasa MJ, Ituero M, Moran-Gonzalez A, Aranda A, Cosgaya JM. Retinoic acid regulates myelin formation in the peripheral nervous system. Glia. 2010;58:1451–1464. doi: 10.1002/glia.21020. [DOI] [PubMed] [Google Scholar]
- 62.Jones SA, Jolson DM, Cuta KK, Mariash CN, Anderson GW. Triiodothyronine is a survival factor for developing oligodendrocytes. Mol Cell Endocrinol. 2003;199:49–60. doi: 10.1016/S0303-7207(02)00296-4. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Kramer EG, Mahase S, Dutta DJ, Bonnamain V, Argaw AT, John GR. Targeting oligodendrocyte protection and remyelination in multiple sclerosis. Mt Sinai J Med. 2011;78:244–257. doi: 10.1002/msj.20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Linker RA, Lee DH, Demir S, Wiese S, Kruse N, Siglienti I, Gerhardt E, Neumann H, Sendtner M, Lühder F, Gold R. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain. 2010;133:2248–2263. doi: 10.1093/brain/awq179. [DOI] [PubMed] [Google Scholar]
- 65.Lee DH, Geyer E, Flach AC, Jung K, Gold R, Flugel A, Linker RA, Lühder F. Central nervous system rather than immune cell-derived BDNF mediates axonal protective effects early in autoimmune demyelination. Acta Neuropathol. 2012;123:247–258. doi: 10.1007/s00401-011-0890-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmitz T, Endesfelder S, Chew LJ, Zaak I, Buhrer C. Minocycline protects oligodendroglial precursor cells against injury caused by oxygen-glucose deprivation. J Neurosci Res. 2012;90:933–944. doi: 10.1002/jnr.22824. [DOI] [PubMed] [Google Scholar]
- 68.Loulier K, Ruat M, Traiffort E. Increase of proliferating oligodendroglial progenitors in the adult mouse brain upon sonic hedgehog delivery in the lateral ventricle. J Neurochem. 2006;98:530–542. doi: 10.1111/j.1471-4159.2006.03896.x. [DOI] [PubMed] [Google Scholar]
- 69.Merchán P, Bribián A, Sánchez-Camacho C, Lezameta M, Bovolenta P, de Castro F. Sonic hedgehog promotes the migration and proliferation of optic nerve oligodendrocyte precursors. Mol Cell Neurosci. 2007;36:355–368. doi: 10.1016/j.mcn.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 70.McMorris FA, Dubois-Dalcq M. Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J Neurosci Res. 1998;21:199–209. doi: 10.1002/jnr.490210212. [DOI] [PubMed] [Google Scholar]
- 71.McKinnon RD, Matsui T, Dubois-Dalcq M, Aaronson SA. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- 72.Furusho M, Kaga Y, Ishii A, Hebert JM, Bansal R. Fibroblast growth factor signaling is required for the generation of oligodendrocyte progenitors from the embryonic forebrain. J Neurosci. 2011;31:5055–5066. doi: 10.1523/JNEUROSCI.4800-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whitaker CM, Beaumont E, Wells MJ, Magnuson DS, Hetman M, Onifer SM. Rolipram attenuates acute oligodendrocyte death in the adult rat ventrolateral funiculus following contusive cervical spinal cord injury. Neurosci Lett. 2008;438:200–204. doi: 10.1016/j.neulet.2008.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beaumont E, Whitaker CM, Burke DA, Hetman M, Onifer SM. Effects of rolipram on adult rat oligodendrocytes and functional recovery after contusive cervical spinal cord injury. Neuroscience. 2009;163:985–990. doi: 10.1016/j.neuroscience.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun X, Liu Y, Liu B, Xiao Z, Zhang L. Rolipram promotes remyelination possibly via MEK-ERK signal pathway in cuprizone-induced demyelination mouse. Exp Neurol. 2012;237:304–311. doi: 10.1016/j.expneurol.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 76.Paintlia AS, Paintlia MK, Singh I, Skoff RB, Singh AK. Combination therapy of lovastatin and rolipram provides neuroprotection and promotes neurorepair in inflammatory demyelination model of multiple sclerosis. Glia. 2009;57:182–193. doi: 10.1002/glia.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Genain CP, Roberts T, Davis RL, Nguyen MH, Uccelli A, Faulds D, Li Y, Hedgpeth J, Hauser SL. Prevention of autoimmune demyelination in non-human primates by a cAMP-specific phosphodiesterase inhibitor. Proc Natl Acad Sci U S A. 1995;92:3601–3605. doi: 10.1073/pnas.92.8.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Torres KJ, Göttle P, Kremer D, Rivera JF, Aguirre-Cruz L, Corona T, Hartung HP, Küry P. Vinpocetine inhibits oligodendroglial precursor cell differentiation. Cell Physiol Biochem. 2012;30:711–722. doi: 10.1159/000341451. [DOI] [PubMed] [Google Scholar]
- 79.Wakita H, Tomimoto H, Akiguchi I, Lin JX, Ihara M, Ohtani R, Shibata M. Ibudilast, a phosphodiesterase inhibitor, protects against white matter damage under chronic cerebral hypoperfusion in the rat. Brain Res. 2003;992:53–59. doi: 10.1016/j.brainres.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 80.Barkhof F, Hulst HE, Drulovic J, Uitdehaag BM, Matsuda K, Landin R. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology. 2010;74:1033–1040. doi: 10.1212/WNL.0b013e3181d7d651. [DOI] [PubMed] [Google Scholar]
- 81.Jiang H, Bielekova B, Okazaki H, Clarence-Smith K, Johnson KP, Bergey G, Martin R, Dhib-Jalbut S. The effect of vesnarinone on TNF alpha production in human peripheral blood mononuclear cells and microglia: a preclinical study for the treatment of multiple sclerosis. J Neuroimmunol. 1999;97:134–145. doi: 10.1016/S0165-5728(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 82.Bielekova B, Lincoln A, McFarland H, Martin R. Therapeutic potential of phosphodiesterase-4 and 3 inhibitors in Th1-mediated autoimmune diseases. J Immunol. 2000;164:1117–1124. doi: 10.4049/jimmunol.164.2.1117. [DOI] [PubMed] [Google Scholar]
- 83.Reyes-Irisarri E, Sánchez AJ, García-Merino JA, Mengod G. Selective induction of cAMP phosphodiesterase PDE4B2 expression in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2007;66:923–931. doi: 10.1097/nen.0b013e3181567c31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Cultures are enriched in OPCs. Low-magnification immunofluorescence images of PDGFRα cells after 1 DIV isolated from P0 brains (a-c), P15 brains (d-f) and human biopsies (g-i). (j) Plot show the quantification of the percentage of OPCs, identified as PDGFRα+ cells after 1DIV, respect to the total number of cells. Scale bars are 50 µm in a-i. (JPEG 1741 kb)
Supplementary Figure 2: Every OPC expresses PDE7. (a-h) Low-magnification immunofluorescence images of PDE7A/PDGFRα (a-c) or PDE7B/PDGFRα (e-g) double-labeled P0 derived OPCs to show how both PDE7 isoforms were expressed by almost every OPC (see text), after 1 DIV in differentiation medium. In these pictures, several OPCs per field are observed, while in Figure 1 we show individual cells for illustration purposes. (d,h) shown immunocitochemistry without primary antibodies. (i-p) Low-magnification immunofluorescence images of PDE7A/PDGFRα (i-k) or PDE7B/PDGFRα (m-o) double-labeled P15 derived OPCs in similar conditions as P0 OPC cultures, with similar results. (l,p) shown immunocitochemistry with our primary antibodies. Scale bars are 25 µm in a-p. (JPEG 1421 kb)
Supplementary Figure 3: Kinetics of PDE7-promoted oligodendroglial differentiation and PDE expression in oligodendroglial lineage. (a) Quantification of pre-oligodendrocytes after 2, 3, 5 and 7DIV. The number of CNPase/Olig2+ cells was higher since the second day of culture until the fifth in the presence of both new PDE7 inhibitors. (b) Plot shown the measurement of fluorescence intensity in purified OPCs cultures immunostained for anti-PDE7A, PDE7B, PDE4B and PDE4D antibodies. It is remarkable that no differences in the expression of PDE7 were found along the days in culture for the oligodendrocyte lineage, although there is a significant difference in the PDE4B expression between the first and the other days in culture. Values are given as mean ± SEM and the results of ANOVA on Ranks are represented as *; P<0.05, **; P<0.01 and ***; P<0.001. (JPEG 487 kb)