Abstract
Pancreatitis is recognized as an important cause for morbidity and mortality in cats, but diagnosis remains difficult in many cases. As a first step in trying to identify a better diagnostic tool for feline pancreatitis the objective of this project was to develop and analytically validate a radioimmunoassay for the measurement of feline pancreatic lipase immunoreactivity (fPLI). Feline pancreatic lipase (fPL) was purified from pancreatic tissue and antiserum against fPL was raised in rabbits. Tracer was produced by iodination of fPL using the chloramine T method. A radioimmunoassay was established and analytically validated by determination of sensitivity, dilutional parallelism, spiking recovery, intra-assay variability, and interassay variability. A control range for fPLI in cat serum was established from 30 healthy cats using the central 95th percentile. The sensitivity of the assay was 1.2 μg/L. Observed to expected ratios for serial dilutions ranged from 98.8% to 164.3% for 3 different serum samples. Observed to expected ratios for spiking recovery ranged from 76.9% to 147.6% for 3 different serum samples. Coefficients of variation for intra- and interassay variability for 4 different serum samples were 10.1%, 4.5%, 2.2%, and 3.9% and 24.4%, 15.8%, 16.6%, and 21.3%, respectively. A reference range for fPLI was established as 1.2 to 3.8 μg/L. We conclude that the assay described is sensitive, accurate, and precise with limited linearity in the lower and limited reproducibility in the lower and higher end of the working range. Further studies to evaluate the clinical usefulness of this assay are needed and in progress.
Résumé
La pancréatite est reconnue comme une cause importante de morbidité et de mortalité chez les chats mais son diagnostic demeure une difficulté dans plusieurs cas. Cette étude avait comme objectif de développer et valider de manière analytique un radio-immuno-essai pour mesurer l’immuno-réactivité de la lipase pancréatique féline (fPLI) afin d’identifier un meilleur outil diagnostique pour la pancréatite féline. De la lipase pancréatique féline (fPL) a été purifiée à partir de tissu pancréatique et un antisérum dirigé contre fPL fut produit chez le lapin. Un marqueur a été produit par iodination de fPL à l’aide de la méthode par la chloramine T. Un radio-immuno-essai a été développé et validé de manière analytique en déterminant sa sensibilité, son parallélisme de dilution, son efficacité de détection d’échantillons ensemencés, la variabilité intra-essai et la variabilité inter-essai.Un écart témoin pour la fPLI dans le sérum de chat a été établi en utilisant le 95e percentile central des resultants de 30 chats. La sensibilité de l’épreuve était de 1,2 μg/L. Pour les valeurs de dilutions sériées, le ratio des données obtenues:données attendues variait de 98,8 % à 164,3 % pour 3 échantillons de sérum différents. Pour les valeurs d’échantillons ensemencés le ratio des données obtenues: données attendues variait de 76,9 % à 147,6 % pour 3 échantillons de sérum différents. Les coefficients de variation pour la variabilité intra- et inter-essai pour 4 échantillons de sérum différents étaient, respectivement, 10,1 %, 4,5 %, 2,2 % et 3,9 %, et 24,4 %, 15,8 %, 16,6 % et 21,3 %. Un écart de valeurs de référence pour fPLI a été établi comme étant 1,2 à 3,8 μg/L. L’épreuve décrite est sensible, exacte et précise avec une linéarité limitée dans ses valeurs limites inférieures et une reproductibilité limitée dans les extrémités inférieure et supérieure de son étendue de travail. Des travaux supplémentaires sont nécessaires et sont en cours pour évaluer l’utilité clinique de cette épreuve.
(Traduit par Docteur Serge Messier)
Introduction
Until recently, pancreatitis has been considered uncommon in cats. However, recent data would suggest that feline pancreatitis leads to significant morbidity and mortality, which has led to an increased index of suspicion for this disease (1,2). Unfortunately, definitive diagnosis of feline pancreatitis remains elusive in many cases (3). This is, in part, due to the fact that clinical signs are non-specific and key clinical signs commonly seen in humans and dogs with pancreatitis, such as vomiting and abdominal pain, are reported infrequently in cats (4). Also, abnormalities observed during routine clinical testing are non-specific. Serum amylase activity has been shown to be decreased and serum lipase activity to be increased in cats with experimentally induced pancreatitis, but serum amylase and lipase activities have not been shown to be of clinical value in cats with spontaneous pancreatitis (5,6). Abdominal radiography can show features that are compatible with pancreatitis, but do not allow for a definitive diagnosis. Finally, when stringent criteria are applied, abdominal ultrasound is highly specific for feline pancreatitis but its sensitivity only reaches 11% to 35% (7,8). Measurement of serum feline trypsin-like immunoreactivity (fTLI) is also highly specific for pancreatitis but its sensitivity of 40% to 60% is less than optimal (5,7,9,10). Thus, a minimally-invasive diagnostic test that is both highly sensitive and specific for feline pancreatitis is needed.
Serum lipase activity has been used for several decades to diagnose pancreatitis in humans and dogs, but it is commonly recognized that these assays are neither sensitive nor specific for pancreatitis in the dog and clinically useless in the cat (5,11–15). This is most likely due to the fact that many different lipases are synthesized and secreted by cells of various organs. Many different lipases share the same function, the hydrolysis of nonpolar lipids into more polar lipolysis products. For example, triglyceridases all hydrolyze triglycerides and thus may all be detected by kinetic lipase assays. In contrast, different lipases often share little amino acid homology and would not cross-react in an immunoassay. To test this hypothesis immunoassays for the measurement of canine pancreatic lipase immunoreactivity have recently been developed and analytically validated (16,17). Serum canine pancreatic lipase immunoreactivity (cPLI) concentration has been shown to be highly specific for exocrine pancreatic function in the dog and also highly sensitive for canine pancreatitis (18,19). Therefore, based on these findings in the dog, it would be interesting to evaluate the clinical usefulness of feline pancreatic lipase immunoreactivity (fPLI) for the diagnosis of feline pancreatitis.
As a first step, the goal of the study described here was to develop and analytically validate a radioimmunoassay (RIA) for the measurement of fPLI in serum.
Materials and methods
Purification of feline pancreatic lipase
Feline pancreatic lipase (fPL) was purified from feline pancreatic tissue by delipidation, anion-exchange chromatography, size-exclusion chromatography, and cation-exchange chromatography, as described previously (20).
Antibody production
Polyclonal antibody production was conducted (Express-Line PLUS protocol; Lampire Biological Laboratories [LBL], Pipersville, Pennsylvania, USA) using New Zealand white rabbits. Feline pancreatic lipase was prepared in phosphate buffered saline solution (PBSS), pH 7.2, and sent to LBL for antibody production. Two rabbits were used and both were inoculated with 250 μg fPL in 500 μL PBSS emulsified with 500 μL of adjuvant (Complete Freund’s adjuvant; Sigma Chemical Company, St. Louis, Missouri, USA). Three weeks after the 1st injection, each rabbit received a booster injection of 250 μg fPL in 500 μL PBSS emulsified with an equal volume of adjuvant (Incomplete Freund’s adjuvant; Sigma Chemical Company). After another 3 wk period, each rabbit was again injected with 250 μg fPL in 500 μL PBSS emulsified with 500 μL adjuvant (Incomplete Freund’s adjuvant). A production bleed was completed 1 wk after the last booster injection and an exsanguination bleed was performed 2 wk after the last booster. The polyclonal antiserum was purified by affinity chromatography. Briefly, an affinity chromatography column (HiTrap; Amersham Pharmacia Biotech, Piscataway, New Jersey, USA) for fPL was prepared following manufacturer’s instructions. Antiserum was applied to the column after lipoprotein precipitation and buffer change to 75 mM Tris-HCl, 150 mM NaCl, pH 8.0. After the absorbance (280 nm) of the eluent had returned to baseline values the column was washed with 100 mM glycine (Sigma Chemical Company), 500 mM NaCl, pH 3.0, in order to elute the bound antibodies. The buffer of the purified polyclonal antibody was changed to PBSS, pH 7.2 (100 mM sodium phosphate, 150 mM NaCl, pH 7.2, BupHTM dry blend buffers; Pierce Chemical Company, Rockford, Illinois, USA), the concentration adjusted to approximately 1 mg/mL and stored at –80°C.
Radioiodination
For the RIA, tracer was produced by iodination of fPL with 125I, using the chloramine T method (21). A mini stir bar (8 mm × 1.5 mm; VWR Scientific, West Chester, Pennsylvania, USA) was placed in a polypropylene test tube (75 mm × 12 mm; VWR Scientific) that was situated over a stir plate. Then 10 μL of free 125I (NaI, 0.1 mCi/μL at time of production; NEN Life Sciences Products, Boston, Massachusetts, USA) were added to the test tube using a Hamilton syringe (VWR Scientific) and mixed with 10 μL 250 mM sodium phosphate buffer (Sigma Chemical Company), pH 7.5. This was followed by the addition of approximately 5 μg of pure fPL in 10 μL PBSS, pH 7.2, 10 μL of 2 mg/mL chloramine T (Sigma Chemical Company) in 50 mM sodium phosphate buffer, pH 7.5, 100 μL of 400 mg/mL sodium metabisulfite (Sigma Chemical Company) in 50 mM sodium phosphate buffer, pH 7.5, and 860 μL of 2 mg/mL potassium iodide (Sigma Chemical Company) in 50 mM sodium phosphate, pH 7.5 in rapid succession. The iodinated protein fraction was separated from the free iodide by size-exclusion chromatography on a disposable column (PD-10; Amersham Pharmacia Biotech, Piscataway, New Jersey, USA) following the manufacturer’s directions. Briefly, RIA buffer (RIAB, 50 mM sodium phosphate buffer, pH 7.5, with 5 g/L bovine serum albumin [Sigma Chemical Company] and 0.2 g sodium azide [Sigma Chemical Company]) was used as the mobile phase. Fractions of 1 mL each were collected and the fraction containing the peak protein concentration was collected. The tracer was adjusted to approximately 40 000 counts/minute/100 μL tracer and kept in a lockable refrigerator at 4°C until further use.
Feline pancreatic lipase immunoreactivity-radioimmunoassay procedure
Polypropylene tubes (VWR Scientific) were set up in duplicate fashion. The first 2 tubes, labeled total count (TC), received 100 μL tracer only. The next 2 tubes, labeled nonspecific binding (NB), received 100 μL tracer and 200 μL RIAB; followed by 2 tubes, labeled reference (B0), that received 100 μL tracer, 100 μL antibody solution against fPL (approximately 1 mg/mL affinity-purified antibody against fPL diluted at 1 in 16 000 with RIAB), and 100 μL RIAB. The following 14 tubes were used as standards and received 100 μL tracer, 100 μL antibody solution, and 100 μL standard solution of 128, 64, 32, 16, 8, 4, or 2 μg/L fPL in RIAB. All of the following tubes were used for unknown samples and received 100 μL tracer, 100 μL antibody solution, and 100 μL of an unknown sample. Tubes were vortexed and incubated for 2 h at room temperature. After the incubation, all tubes, except the tubes labeled TC, received 100 μL rabbit carrier serum (1 mL normal rabbit serum mixed with 99 mL RIAB) and 1 mL of a commercially available precipitation solution (N6; Diagnostic Products Corporation, Los Angeles, California, USA). Again, all tubes were vortexed and then centrifuged at 3000 × g and 4°C for 20 min. The supernatant of all tubes, except the tubes labeled TC, was carefully decanted and all tubes were counted for 1 min in a gamma counter (Riastar; Packard Instrument Company, Meriden, Connecticut, USA). A standard curve was calculated using a log/logit curve fit. The fPL concentrations were plotted along the x-axis in a logarithmic fashion. Values on the y-axis were calculated using the formula y = loge([Bstandard/B0]/(1 – [Bsample/B0]) with Bstandard being the counts per minute (CPM) for each standard and B0 being the CPM for the reference.
Feline pancreatic lipase immunoreactivity-radioimmunoassay validation
The assay was analytically validated by determining the assay sensitivity, control range, linearity, accuracy, precision, and reproducibility by testing assay sensitivity, dilutional parallelism, spiking recovery, intra-assay variability, and interassay variability. Assay sensitivity was determined by setting up 10 duplicates of B0 and calculating the standard deviation of the raw counts of these 10 duplicates. Three standard deviations were subtracted from the mean count and the resulting value estimated on the standard curve. The sensitivity also served as the lower limit of the working range. The highest standard was taken as the upper limit of the working range. Serum samples were selected from random feline serum samples to fall into different areas of the working range of the assay. Because a volume of 1 mL or greater was not available for most serum samples, samples used for validation were generated from a pool of several serum samples. Linearity was assessed by determination of dilutional parallelism by evaluating 3 serum samples at full strength and at dilutions of 1 in 2, 1 in 4, and 1 in 8. Accuracy was assessed by determination of spiking recovery by adding 0, 2, 4, 6, 8, 16, and 32 μg/L fPL in RIAB to each 1 of 3 serum samples. Precision was evaluated by calculating intra-assay variability for 4 different serum samples that were measured 10 times within the same assay run. Finally, reproducibility was evaluated by calculating interassay variability for 4 different serum samples that were measured in 10 consecutive assay runs. The control range for the RIA for fPLI was determined from the central 95th percentile (2.5th to the 97.5th percentile) of serum fPLI concentrations measured in 30 healthy random source cats. None of the cats had clinical signs of disease but no further testing was performed in order to exclude occult pancreatitis.
Results
The sensitivity of the assay was calculated to be 1.2 μg/L, leading to a lower limit of the working range of 1.2 μg/L. Observed to expected ratios for dilutional parallelism for 3 serum samples ranged from 98.8% to 164.3% (mean ± standard deviation [s], 120.5% ± 23.4%; Table I). Observed to expected ratios for spiking recovery for 3 serum samples ranged from 76.9% to 147.6% (mean ± s, 106.2% ± 22.9%; Table II). Coefficients of variation for intra-assay variability of the 4 serum samples were 10.1%, 4.5%, 2.2%, and 3.9% (Table III). Also, coefficients of variation for interassay variability of 4 serum samples were 24.4%, 15.8%, 16.6%, and 21.3% (Table III). The control range established from the central 95th percentile of serum fPLI concentrations in 30 healthy random-source cats was 1.2 to 3.8 μg/L (Figure 1).
Table I.
Dilutional parallelism for the radioimmunoassay (RIA) for feline pancreatic lipase immunoreactivity (fPLI) shown for 3 serum samples at dilutions of 1 in 1, 1 in 2, 1 in 4, and 1 in 8
| Sample 1 | |||
| Dilution | Observed (μg/L) | Expected (μg/L) | O/E ratio (%) |
| 1 in 1 | 4.48 | ||
| 1 in 2 | 3.50 | 2.24 | 156.3 |
| 1 in 4 | 1.28 | 1.12 | 114.3 |
| 1 in 8 | 0.92 | 0.56 | 164.3 |
| Sample 2 | |||
| Dilution | Observed (μg/L) | Expected (μg/L) | O/E ratio (%) |
| 1 in 1 | 7.53 | ||
| 1 in 2 | 3.95 | 3.77 | 104.9 |
| 1 in 4 | 2.20 | 1.88 | 116.9 |
| 1 in 8 | 0.93 | 0.94 | 98.8 |
| Sample 3 | |||
| Dilution | Observed (μg/L) | Expected (μg/L) | O/E ratio (%) |
| 1 in 1 | 45.06 | ||
| 1 in 2 | 24.45 | 22.53 | 108.5 |
| 1 in 4 | 11.90 | 11.27 | 105.6 |
| 1 in 8 | 6.47 | 5.63 | 114.9 |
O/E — observed/expected
Table II.
Spiking recovery for the radioimmunoassay (RIA) for feline pancreatic lipase immunoreactivity (fPLI) shown for 3 serum samples and 6 spiking concentrations
| Sample 1 | |||
| Added (μg/L) | Observed (μg/L) | Expected (μg/L) | O/E ratio (%) |
| 0.0 | 3.0 | ||
| 2.0 | 4.1 | 5.0 | 81.3 |
| 4.0 | 5.8 | 7.0 | 82.2 |
| 8.0 | 9.2 | 11.0 | 83.1 |
| 16.0 | 14.6 | 19.0 | 76.9 |
| 32.0 | 27.9 | 35.0 | 79.6 |
| Sample 2 | |||
| Added (μg/L) | Observed (μg/L) | Expected (μg/L) | O/E ratio (%) |
| 0.0 | 16.9 | ||
| 2.0 | 22.3 | 18.9 | 118.0 |
| 4.0 | 26.2 | 20.9 | 125.4 |
| 8.0 | 29.8 | 24.9 | 119.8 |
| 16.0 | 43.7 | 32.9 | 132.8 |
| 32.0 | 72.2 | 48.9 | 147.6 |
| Sample 3 | |||
| Added (μg/L) | Observed (μg/L) | Expected (μg/L) | O/E ratio (%) |
| 0.0 | 35.9 | ||
| 2.0 | 51.1 | 37.9 | 134.8 |
| 4.0 | 53.4 | 55.1 | 96.9 |
| 8.0 | 62.8 | 61.4 | 102.3 |
| 16.0 | 85.1 | 78.8 | 108.0 |
| 32.0 | 121.9 | 117.1 | 104.1 |
O/E — observed/expected
Table III.
Intra-assay and interassay variability for the radioimmunoassay (RIA) for feline pancreatic lipase immunoreactivity (fPLI) shown for 4 serum samples
| Intra-assay variability | ||||
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| Number of repeats | 10 | 10 | 10 | 10 |
| Mean (μg/L) | 3.35 | 7.51 | 19.82 | 39.78 |
| Standard deviation (μg/L) | 0.34 | 0.33 | 0.44 | 1.54 |
| CV (%) | 10.1 | 4.5 | 2.2 | 3.9 |
| Interassay variability | ||||
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | |
| Number of repeats | 10 | 10 | 10 | 10 |
| Mean (μg/L) | 3.80 | 6.98 | 27.75 | 57.40 |
| Standard deviation (μg/L) | 0.93 | 1.10 | 4.62 | 12.23 |
| CV (%) | 24.4 | 15.8 | 16.6 | 21.3 |
Figure 1.
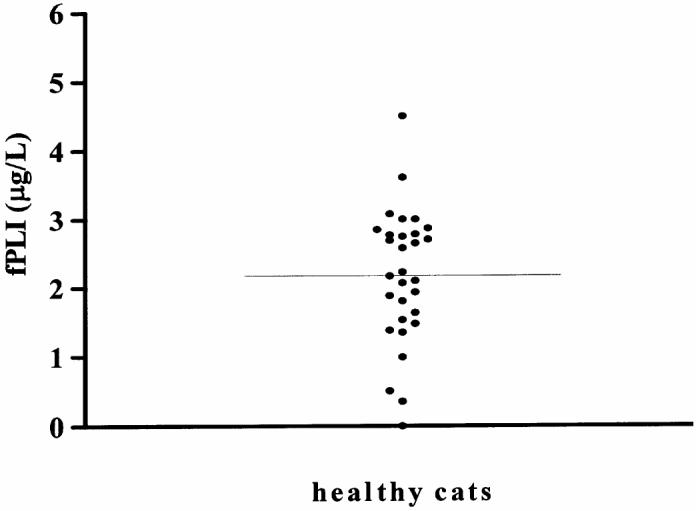
Serum feline pancreatic lipase immunoreactivity (fPLI) concentrations in 30 healthy cats.
Discussion
A RIA for the measurement of fPLI in serum from cats was successfully established. The reference range for serum fPLI concentration was determined to be 1.2 to 3.8 μg/L. Since the sensitivity of the assay was determined to be 1.2 μg/L, the reference range can also be expressed as < 3.8 μg/L.
Although many RIAs have been developed for use in both human and veterinary medicine, very little is known about the minimal performance characteristics for a clinically useful assay (22,23). Most investigators simply state the performance characteristics of the assay they develop and validate (24–26). We have previously targeted observed to expected ratios for dilutional parallelism and spiking recovery between 80% to 120%, but are not aware of any reference to support these targeted values (17,27). Also, we have previously targeted coefficients of variation for intra-assay and interassay variability to reach a maximum of 10% to 15%, but again we are not aware of any published rationale for these target values (17,27). Observed to expected ratios for serial dilution ranged between 98.8% and 164.3%. Some of these values are outside the range of 80% to 120%. The 2 highest values, 164.3% and 156.3% were observed for the sample with the lowest fPLI concentration of 4.48 μg/L. This suggests that this assay has a limited linearity in the lower limit of the working range. This finding may be due to an increased impact of nonspecific binding or matrix effects when the serum fPLI concentration is low. However, for the diagnosis of feline pancreatitis one would expect serum fPLI concentrations that are in the upper rather than in the lower area of the working range. Thus the limited degree of linearity in the lower range of the working range should not affect the clinical usefulness of this assay for the diagnosis of feline pancreatitis.
Observed to expected values for spiking recovery were between 76.9% and 147.6%. Again, some of these values are outside the range of 80% to 120%. There is no apparent explanation for these findings. However, the relatively high interassay variability (see below) may be in part responsible for these findings.
The coefficients of variation (CVs) for the intra-assay variability for 3 of the 4 serum samples were well below 10% and the 4th value was just above it at 10.1%. These values suggest that the assay is precise. The CVs for interassay variability of the 4 serum samples were 24.4%, 15.8%, 16.6%, and 21.3%. These CVs are slightly higher than optimal, especially for samples with low or high serum fPLI concentrations, this would suggest that this assay shows a limited degree of reproducibility in the lower and the higher ends of the working range. Considering the control range of 1.2 to 3.8 μg/L it is hypothesized that cats with significantly increased serum fPLI concentrations would fall into a range with better reproducibility or have such high serum fPLI concentrations that a limited degree of reproducibility would not change the clinical interpretation of the assay result.
In conclusion, the RIA described here is sensitive, accurate, and precise with a limited linearity in the lower end of the working range and a limited reproducibility in the lower and upper ends of the working range. Clinical studies will be necessary in order to determine whether this degree of assay linearity and reproducibility will be sufficient to definitively distinguish cats with pancreatitis from healthy cats. These studies are currently ongoing. Should the results of these clinical studies suggest that the performance characteristics described here are insufficient to reliably differentiate between cats with and those without pancreatitis measures would need to be taken to improve the performance characteristics of the assay. Such measures may include an increase of sample incubation, alterations of the RIAB, production of monoclonal antibodies, or development of an enzyme-linked immunosorbent assay (ELISA).
Footnotes
This material has been presented in part as a research abstract at the 2002 ACVIM Forum in Dallas, Texas, USA.
References
- 1.Ferreri JA, Hardam E, Kimmel SE, et al. Clinical differentiation of acute necrotizing from chronic nonsuppurative pancreatitis in cats: 63 cases (1996–2001) J Am Vet Med Assoc. 2003;223:469–474. doi: 10.2460/javma.2003.223.469. [DOI] [PubMed] [Google Scholar]
- 2.Hänichen T, Minkus G. Retrospektive Studie zur Pathologie der Erkrankungen des exokrinen Pankreas bei Hund und Katze. Tierärztliche Umschau. 1990;45:363–368. [Google Scholar]
- 3.Steiner JM. Diagnosis of pancreatitis. Vet Clin North Am Small Anim Pract. 2003;33:1181–1195. doi: 10.1016/s0195-5616(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 4.Hill RC, Van Winkle TJ. Acute necrotizing pancreatitis and acute suppurative pancreatitis in the cat. A retrospective study of 40 cases (1976–1989) J Vet Int Med. 1993;7:25–33. doi: 10.1111/j.1939-1676.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- 5.Parent C, Washabau RJ, Williams DA, et al. Serum trypsin-like immunoreactivity, amylase and lipase in the diagnosis of feline acute pancreatitis [Abstract] J Vet Int Med. 1995;9:194. [Google Scholar]
- 6.Kitchell BE, Strombeck DR, Cullen J, et al. Clinical and pathologic changes in experimentally induced acute pancreatitis in cats. Am J Vet Res. 1986;47:1170–1173. [PubMed] [Google Scholar]
- 7.Gerhardt A, Steiner JM, Williams DA, et al. Comparison of the sensitivity of different diagnostic tests for pancreatitis in cats. J Vet Intern Med. 2001;15:329–333. [PubMed] [Google Scholar]
- 8.Saunders HM, VanWinkle TJ, Drobatz K, et al. Ultrasonographic findings in cats with clinical, gross pathologic, and histologic evidence of acute pancreatic necrosis: 20 cases (1994–2001) J Am Vet Med Assoc. 2002;221:1724–1730. doi: 10.2460/javma.2002.221.1724. [DOI] [PubMed] [Google Scholar]
- 9.Swift NC, Marks SL, MacLachlan NJ, et al. Evaluation of serum feline trypsin-like immunoreactivity for the diagnosis of pancreatitis in cats. J Am Vet Med Assoc. 2000;217:37–42. doi: 10.2460/javma.2000.217.37. [DOI] [PubMed] [Google Scholar]
- 10.Steiner JM, Williams DA. Disagrees with criteria for diagnosing pancreatitis in cats. J Am Vet Med Assoc. 2000;217:816–817. doi: 10.2460/javma.2000.217.816. [DOI] [PubMed] [Google Scholar]
- 11.Strombeck DR, Farver T, Kaneko JJ. Serum amylase and lipase activities in the diagnosis of pancreatitis in dogs. Am J Vet Res. 1981;42:1966–1970. [PubMed] [Google Scholar]
- 12.Brobst D, Ferguson AB, Carter JM. Evaluation of serum amylase and lipase activity in experimentally induced pancreatitis in the dog. J Am Vet Med Assoc. 1970;157:1697–1702. [PubMed] [Google Scholar]
- 13.Ventrucci M, Gullo L, Daniele C, et al. Comparative study of serum pancreatic isoamylase, lipase, and trypsin-like immunoreactivity in pancreatic disease. Digestion. 1983;28:114–121. doi: 10.1159/000198973. [DOI] [PubMed] [Google Scholar]
- 14.Tetrault GA. Lipase activity in serum measured with ektachem is often increased in nonpancreatic disorders. Clin Chem. 1991;37:447–451. [PubMed] [Google Scholar]
- 15.Simpson KW, Simpson JW, Lake S, et al. Effect of pancreatectomy on plasma activities of amylase, isoamylase, lipase and trypsin-like immunoreactivity in dogs. Res Vet Sci. 1991;51:78–82. doi: 10.1016/0034-5288(91)90035-m. [DOI] [PubMed] [Google Scholar]
- 16.Steiner JM, Teague SR, Williams DA. Development and analytic validation of an enzyme-linked immunosorbent assay for the measurement of canine pancreatic lipase immunoreactivity in serum. Can J Vet Res. 2003;67:175–182. [PMC free article] [PubMed] [Google Scholar]
- 17.Steiner JM, Williams DA. Development and validation of a radioimmunoassay for the measurement of canine pancreatic lipase immunoreactivity in serum of dogs. Am J Vet Res. 2003;64:1237–1241. doi: 10.2460/ajvr.2003.64.1237. [DOI] [PubMed] [Google Scholar]
- 18.Steiner JM, Finco DR, Gumminger SR, Williams DA. Serum canine pancreatic lipase immunoreactivity (cPLI) in dogs with experimentally induced chronic renal failure [Abstract] J Vet Int Med. 2001;15:311. [Google Scholar]
- 19.Steiner JM, Gumminger SR, Rutz GM, Williams DA. Serum canine pancreatic lipase immunoreactivity (cPLI) concentrations in dogs with exocrine pancreatic insufficiency [Abstract] J Vet Int Med. 2001;15:274. [Google Scholar]
- 20.Steiner JM, Wilson BG, Williams DA. Purification and partial characterization of feline classical pancreatic lipase. Comp Biochem Physiol B Biochem Mol Biol. 2003;134:151–159. doi: 10.1016/s1096-4959(02)00222-1. [DOI] [PubMed] [Google Scholar]
- 21.Hunter WM, Greenwood FC. Preparation of 131iodine-labeled human growth hormone of high specific activity. Nature. 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- 22.Berson SA, Yalow RS. Radioimmunoassay in gastroenterology. Gastroenterology. 1972;62:1061–1084. [PubMed] [Google Scholar]
- 23.Allen WE, Porter DJ. Comparison of radioimmunoassay and enzyme-linked immunoassay for the measurement of progestogen in equine plasma and milk. Vet Rec. 1987;120:429–431. doi: 10.1136/vr.120.18.429. [DOI] [PubMed] [Google Scholar]
- 24.Yalow RS, Berson SA. Radioimmunoassay of gastrin. Gastroenterology. 1970;58:1–14. [PubMed] [Google Scholar]
- 25.Camarillo IG, Thordarson G, Ilkbahar YN, et al. Development of a homologous radioimmunoassay for mouse growth hormone receptor. Endocrinology. 1998;139:3585–3589. doi: 10.1210/endo.139.8.6160. [DOI] [PubMed] [Google Scholar]
- 26.John H, Cammann K, Schlegel W. Development and review of radioimmunoassay of 12-S-hydroxyheptadecatrienoic acid. Prostaglandins Other Lipid Mediat. 1998;56:53–76. doi: 10.1016/s0090-6980(98)00043-4. [DOI] [PubMed] [Google Scholar]
- 27.Steiner JM, Medinger TL, Williams DA. Development and validation of a radioimmunoassay for feline trypsin-like immunoreactivity. Am J Vet Res. 1996;57:1417–1420. [PubMed] [Google Scholar]


