Abstract
Kir3 channels control excitability in the nervous system and the heart. Their surface expression is strictly regulated, but mechanisms responsible for channel removal from the membrane remain incompletely understood. Using transfected cells, we show that Kir3.1/3.2 channels and delta opioid receptors (DORs) associate in a complex which persists during receptor activation, behaving as a scaffold that allows beta-arrestin (βarr) to interact with both signaling partners. This organization favored co-internalization of DORs and Kir3 channels in a βarr-dependent manner via a clathrin/dynamin-mediated endocytic path. Taken together, these findings identify a new way of modulating Kir3 channel availability at the membrane and assign a putatively novel role for βarrs in regulating canonical effectors for G protein-coupled receptors.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-015-1899-x) contains supplementary material, which is available to authorized users.
Keywords: Kir channels, Trafficking, Signaling complexes, Arrestin, Endocytosis, Opioid
Introduction
Kir3 channels control neuronal excitability in response to G-protein coupled receptor (GPCR) activation and their surface expression is strictly modulated both, constitutively and dynamically. Primary sequence determines the ability of Kir3 subunits to interact with sorting [1–3] or scaffolding proteins [4] that control channel export to the membrane, their presence at the synapse and their capacity to respond to G proteins. Kir3 subunits may also be dynamically regulated either via their own phosphorylation or that of the activating GPCR [5, 6]. Thus, phosphorylation of Kir3 subunits was shown to influence activation kinetics [7] and channel export to the membrane [8] while GABAB receptor phosphorylation was associated with poor membrane recycling of associated channel subunits [5, 6, 9]. GABAB receptors do not internalize upon phosphorylation but most Kir3-activating receptors do [10, 11], raising the question of whether Kir3 subunits could follow. To investigate this possibility, we monitored Kir3 channel trafficking upon delta opioid receptor (DOR) activation. Working with transfected cell lines and neurons we found that DORs and Kir3.1/3.2 subunits formed signaling complexes that maintained their integrity over prolonged receptor stimulation. Over this same period, βarr2 associated with receptors and channels driving their co-internalization in a clathrin/dynamin-dependent manner.
Materials and methods
DNA constructs
Plasmids encoding murine DORs tagged with YFP at the C-terminus [11], human Kir3.1 subunits bearing Rluc or GFP at the C-terminus, DOR-Rluc constructs and GαoA subunits tagged with the donor (Rluc II) at position 99 of the helical domain have been previously described [12, 13] as have constructs consisting of YFP fused at the N-terminus of human Gγ2 and the C-terminus of CD8 or βarr2 tagged with Rluc at its C-terminal end [14]. Native human Kir3.1 and Kir3.2 subunits, Kir3.1 subunits bearing Flag at position 114 as well as HA-F prostanoid receptor were kindly provided by Dr Terence Hébert (McGill University). Murine DORs tagged with the Myc-epitope at the N-terminus was a generous gift of Dr. Louis Gendron (University of Sherbrooke) and HA-CXCR4 was a generous gift of Dr. Nikolaus Heveker (University of Montreal).
Cell culture and transfections
Immortalized cell lines
Human embryonic kidney 293 (HEK293) cells were cultured in DMEM supplemented with 10 % fetal bovine serum, 2 mM l-glutamine, 100 unit/ml penicillin–streptomycin, at 37 °C in a humidified atmosphere at 95 % air and 5 % CO2. For transient expression of recombinant proteins, HEK293 cells were seeded at a density of 4.2 × 106 cells in 100 mm Petri dishes, cultured for 24 h, and transfected with vectors encoding BRET constructs for Kir3, DORs, G-protein subunits, or βarr2 in combination with complementary signaling partners, as detailed below. Transfections were done with polyethylenimine (Polysciences) according to a previously published protocol [15].
Primary neuronal cultures
Cultures were prepared from rat postnatal prefrontal cortex (P0–P2) using a previously described method [11] and transfected with indicated constructs by means of a modified calcium phosphate transfection protocol [14]. In experiments assessing DOR colocalization with Kir3 channels, cells were transfected with Flag-Kir3.1/Kir3.2 and myc-DOR constructs. In experiments in which siRNA was used, rat βarr2 or scrambled siRNA (Dharmacon) were transfected with lipofectamine 24 hs after the constructs at a concentration of 100 pmol/35 mm dish. For visualization of channel or receptor colocalization with βarr2, cells were transfected either with Flag-Kir3.1/Kir3.2, untagged DORs and βarr2-Rluc (βarr2/Kir3 colocalization) or Flag-DOR, untagged Kir3.1/Kir3.2 and βarr2-Rluc (βarr2/DOR colocalization).
Immunocytochemistry in neurons
Experiments were performed 2 days after transfection using a protocol to specifically label proteins present at the membrane [11, 14]. Flag-Kir3.1 subunits and Myc-DORs were simultaneously labeled using rabbit anti-Flag M1 (1:100; Sigma) and mouse anti-Myc antibodies (1:200; Covance) added to the incubation medium of live neurons at 37 °C [11]. 30 min after their introduction, cells were exposed to the DMSO 0.01 % (this condition was labeled “control”), SNC-80 (1 µM) or morphine (1 µM) as indicated in figures. Following treatment, neurons were washed three times with ice-cold phosphate-buffered saline (PBS) and fixed with 4 % PFA before being permeabilized (surface + intracellular labeling) or not (surface labeling) with in PBS/0.1 % Triton. Nonspecific sites were blocked with PBS/BSA 1 % (10 min at RT), and neurons were then exposed to second antibodies (Goat anti-Rabbit ALEXA 488; 1:1000; Invitrogen) and (Goat anti-Mouse ALEXA 594; 1:1000; Invitrogen), to respectively visualize channels and receptors. Images were acquired using Zeiss LSM-510 META confocal microscope. When clathrin (Pitstop 2, Abcam; 15 µM) or dynamin (Dynasore, Sigma; 80 µM) inhibitors were used, they were, respectively, introduced 10 or 20 min before agonists, and were washed away before the latter were added. In experiments assessing Flag-Kir3.1/3.2 channel colocalization with HA-CXCR4 or HA-PF receptors were labeled with mouse anti-HA antibody (1:300; Sigma) and second antibodies as above. Since permeabilization reduced membrane labeling as compared to non-permeabilized cells, receptor and channel removal from the cell surface was inferred by monitoring increases in their intracellular immunoreactivities. Mean cytosolic labeling intensity was quantified using ImageJ according to a previously described method [14]. Briefly, for each neuron soma in the slide, we established a perimeter delimited by the inner surface of membrane immunoreactivity and measured integrated fluorescence intensity present within this perimeter but outside the nucleus. Values resulting from this computation were divided by the area comprised between nuclear and inner membrane perimeters of the cell to yield mean cytosolic intensity. Nuclear fluorescence was considered as the background, and its mean intensity subtracted from the quotient described above.
In experiments assessing channel and receptor colocalization with βarr2-Rluc, cells expressing Flag-Kir3.1/Kir3.2 + DOR + βarr2-Rluc or Flag-DOR + Kir3.1/Kir3.2 + βarr2-Rluc were incubated with rabbit anti-Flag antibody (1:100; 37 °C; 30 min) to label receptors or channels and then treated with morphine or SNC-80 as described above. After treatment cells were fixed, permeabilized and blocked before introducing mouse anti-Rluc antibody (1:100; Millipore). Cells were then washed three times with PBS and incubated with PBS/BSA 1 % (10 min at RT) before exposure to same second antibodies as above. In order to maintain a similar color code throughout the manuscript, ImageJ was used to assign red color to the receptor, green to Kir3 subunits and blue for βarr2-Rluc. Finally, line scans were carried out using the ImageJ plot profile function.
BRET assays
All BRET assays were performed in HEK293 cells. To monitor interactions between DORs, Kir3 channel subunits and heterotrimeric G proteins (GαoAβ1γ2), we first completed titration assays which allowed to determine the specificity of association among different interaction partners [16–20]. To do so, a fixed amount of the donor-tagged (Rluc) construct was co-transfected with increasing amounts of the corresponding interaction partner bearing the acceptor (YFP or GFP). Untagged complementary signaling partners were also included in amounts to ensure that donor availability was the limiting factor for complex formation. Donor–acceptor levels were monitored by direct measurement of total fluorescence and luminescence on transfected cells as detailed elsewhere [21]. DNA ratios corresponding to the beginning of the saturation plateau were subsequently used for single point assays [12]. Amounts of DNA constructs transfected in the different experiments are shown in Supplementary Table 1. Two days after transfection, HEK293 cells expressing different BRET pairs and accessory subunits (as indicated in figures) were exposed to vehicle or SNC-80 (1 µM) for 30 min at 37 °C. Treatment was stopped by washing cells with ice-cold phosphate-buffered solution (PBS) following which they were transferred to 96-well plates at a protein concentration of 1.5 mg/ml (Optiplate; PerkinElmer Life Sciences). BRET1 measures were obtained in a Victor3 plate reader (PerkinElmer Life Sciences) 5 min after manual addition of coelenterazine h (Nanolight) and BRET ratios were determined by dividing the intensity of light emitted by YFP (520–550 nm) over the light emitted by Rluc (440–480 nm). BRET values were then corrected by subtracting the background signal (detected when Rluc-tagged constructs were expressed without acceptor) from the BRET signal detected in cells coexpressing both donor and acceptor constructs (Net BRET).
For assessing βarr2-Rluc recruitment to DOR-YFP or Kir3.1-GFP, BRET assays were conducted using a protocol that we had previously optimized for this purpose [14]. Briefly, HEK293 cells were transfected as indicated in Supplementary Table 1 and two days after transfection, cells were exposed to vehicle or SNC-80 (1 μM) for 30 min at 37 °C. Treatment was stopped and BRET readings taken as above. BRET2 measures (βarr2-Rluc/Kir3.1-GFP) were obtained in a Envision plate reader (Perkin Elmer) 5 min after addition of coelenterazine DeepBlueC (Biotium) and BRET ratios were determined by dividing light emitted by GFP (500–530 nm) over the light emitted by Rluc (370–450 nm).
Co-immunopurification and western blot analysis
This procedure was adapted from a previously described method [21]. Cells were transfected with Kir3.1-Rluc, YFP-Gγ2, GαoA together with Flag-DOR or cMyc-DOR as indicated. Two days after transfection cells were exposed to SNC-80 (1 µM; 30 min), or vehicle at 37 °C and at the end of treatment they were washed with ice-cold PBS and immediately used for membrane preparation. Resulting gels were transferred onto nitrocellulose (GE Healthcare) and Gαo, YFP-Gγ2 and Kir3.1-Rluc recovered with Flag-DORs were revealed using, respectively, rabbit anti-Gαo (1:1000; Millipore), rabbit anti-GFP (1:10,000; Abcam), mouse anti-Rluc (1:1000; Millipore) antibodies, followed by corresponding secondary HRP-conjugated antibodies (1:40,000; GE Healthcare). Flag-DORs in each sample were detected by probing membranes with rabbit anti-Flag antibody (1:5000; Sigma) and the corresponding secondary antibody (1:40,000; GE Healthcare). Chemiluminescence detection reagents (GE Healthcare) were used to reveal the blotted proteins, and relative intensities of the labeled bands were analyzed by densitometric scanning using MCID (Imaging Research). Densitometric values were used to calculate Flag-DOR/Gαo, Flag-DOR/YFP-Gγ2 and Flag-DOR/Kir3.1-Rluc ratios for each condition.
Western blots were also completed to assess βarr knock-down in siRNA experiments. Neurons transfected with βarr2 or scrambled siRNA were lysed in RIPA buffer (50 mM Tris–HCL pH 7.4, 1 % triton X100, 0.25 % deoxycholic acid, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 μg/ml aprotinin, 5 µg/ml leupeptine, 5 µg/ml soybean trypsin inhibitor, and 10 µg/ml benzamidine, 1 mM Na3VO4 and 1 mM NaF) for 90 min. Following centrifugation at 20,000×g for 30 min, the supernatant containing membranes and cytosol was recovered. βarrs were revealed using an antibody (1:1000) kindly provided by Dr Stéphane Laporte (McGill University). Secondary anti-rabbit horseradish-conjugated antibody was used at 1:20.000 dilution (Amersham Biosciences, Piscataway, NJ, USA). The first antibody recognizes βarr1/2 but a single band was visible which disappeared in cells transfected with siRNA for βarr2. Nonetheless, we have interpreted knock-down data in terms of general βarr function, avoiding speculation with respect to βarr1/2 subtype specificity.
ELISA-based assays
Measurement of surface-expressed Flag-tagged channels or receptors in HEK293 cells and quantification of their internalization was assessed using an ELISA-based method adapted from a previously published protocol [22].
Colocalization analyses of confocal images
Analysis of βarr2-Rluc colocalization with DORs or with Kir3.1 subunits was performed using NIH ImageJ software (intensity correlation analysis plug-in) as previously described [23]. The product of differences from the mean PDM = (red intensity − mean red intensity) × (green intensity − mean green intensity) was calculated for each pixel after background removal. PDMs were then represented as pseudo-colored images with a scale bar in which yellow and blue, respectively, indicate high and low co-localization. To aid visualization, surface plot profiles were created from PDM images, with higher peaks indicating stronger co-localization. The plug-in also provided Pearson’s correlation coefficients ranging between 0 and 1, where 1 indicates perfect correlation and 0 represents perfect exclusion. These coefficients were used for statistical comparisons of the degree of colocalization induced by different DOR agonists.
Results
DOR activation promotes internalization of Kir3 channel subunits
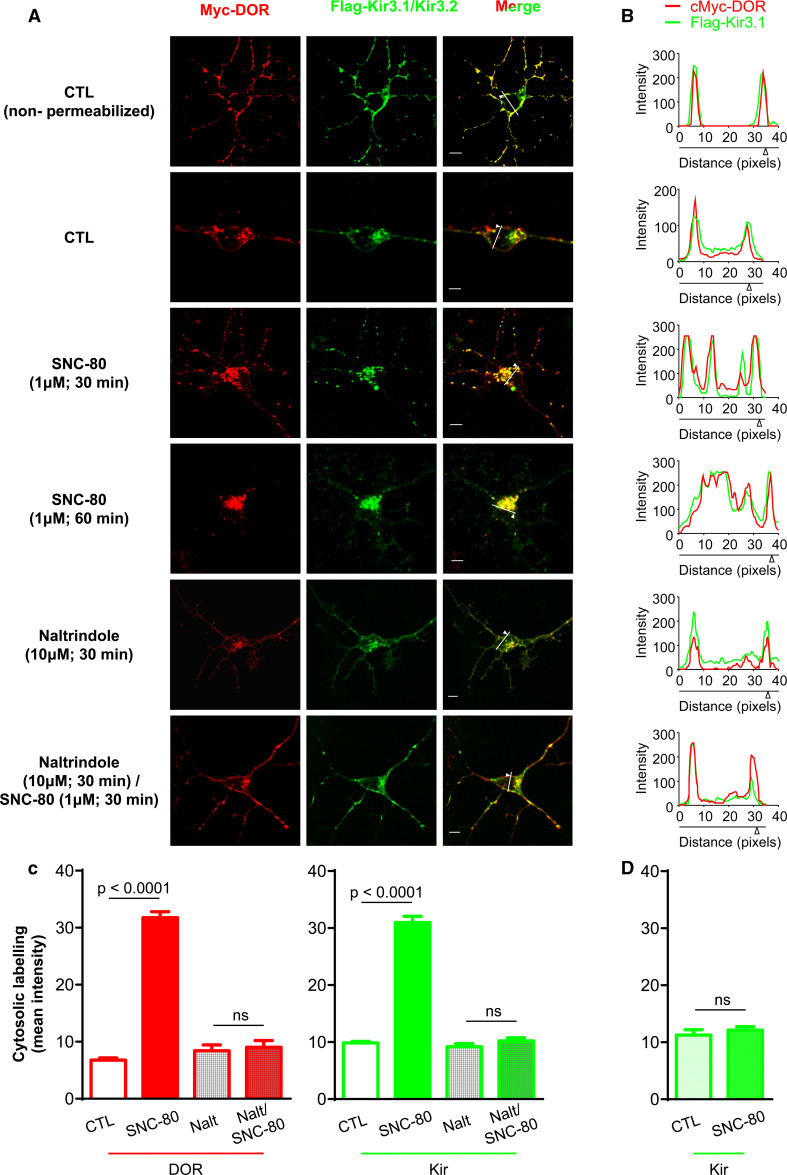
We first investigated whether DOR activation could cause Kir3 subunits to translocate to the intracellular compartment. For this purpose, primary cortical neuron cultures were co-transfected with extracellularly tagged myc-DORs and Flag-Kir3.1 channel subunits (+Kir3.2) and both species were specifically labeled at the membrane. In the absence of receptor stimulation, some intracellular immunofluorescence was evident (Fig. 1a permeabilized neurons) indicating that the two membrane-labeled species could translocate to the cytosol in the absence of ligand. Following exposure to SNC-80, intracellular labeling intensity was increased in a time-dependent manner both for receptors and Kir3.1 subunits (Fig. 1a–c; quantification at 60 min: DORbasal: 6.79 ± 0.37, DORSNC-80: 31.73 ± 1.06, Kir3.1basal: 9.86 ± 0.24, Kir3.1SNC-80: 30.99 ± 1.09). Interestingly, despite translocation, receptors and channels remained co-localized indicating that they both trafficked to the same post-endocytic compartment(s) (Fig. 1a, b). Moreover, in cells in which DORs were not expressed, SNC-80 induced no channel translocation showing that channel trafficking was a DOR-mediated event (Fig. 1d, Supplementary Fig. 1).
Fig. 1.
DORs and Kir3.1/3.2 channels co-internalize upon receptor activation by an agonist. a Cortical neuron cultures transfected with myc-DOR and Flag-Kir3.1/3.2 subunits were treated as indicated. Neurons were all processed for intracellular and surface labeling (permeabilized) except as indicated in the figure for exclusive visualization of surface immunoreactivities (non-permeabilized). Scale bars 5 μm. b Line scans correspond to traces shown in merged images (Kir3.1 = green, DOR = red). c Histograms correspond to mean intracellular fluorescence intensity and represent mean ± SEM of 3–9 experiments with 39–59 neurons analyzed per condition. Changes in DOR and Kir3.1 immunoreactivities were analyzed separately using one-way ANOVA to compare the different treatments (including those in next figure). Post hoc comparisons were done using Bonferroni correction and are indicated in the figure. d Histograms correspond to mean intracellular fluorescence intensity of Flag-Kir3 subunits in cells that were transfected only with the channel and treated or not with SNC-80 (1 μM; 60 min). Data represent mean ± SEM of 3 experiments with 36–40 neurons analyzed per condition. Statistical comparisons were done using Student’s t test; (n = 3), p = 0.4762
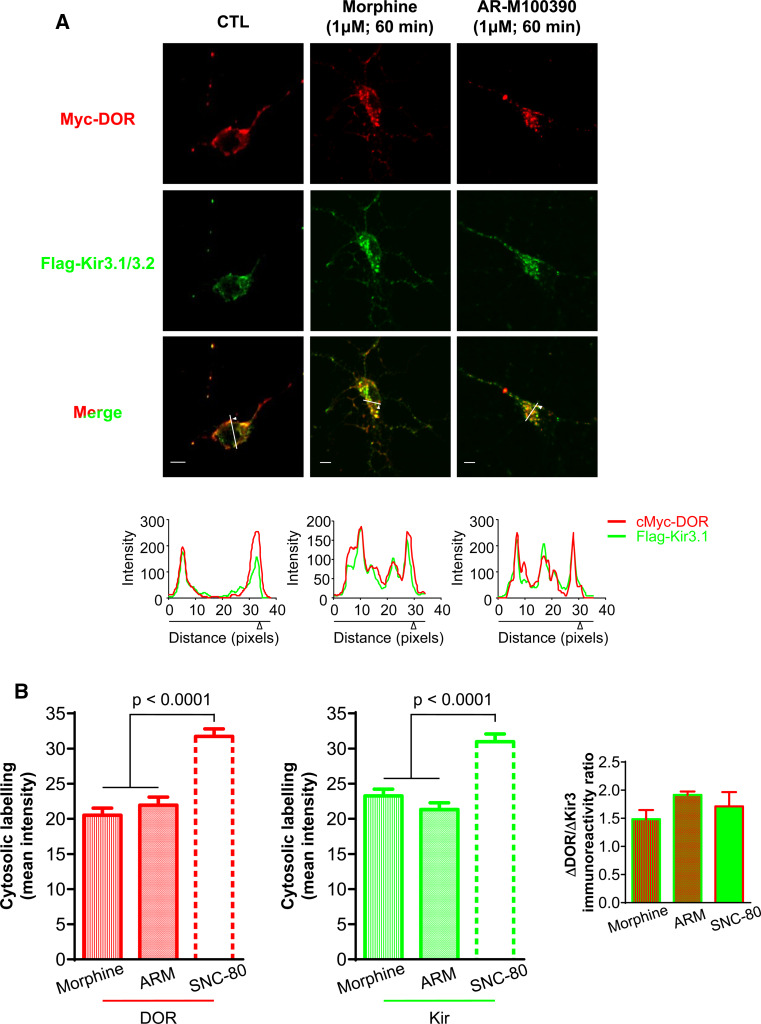
To further establish whether channel internalization was triggered by receptor activation, we determined if Kir3 subunit sequestration displayed similar pharmacological properties as other DOR-mediated responses. SNC-80 was previously shown to produce greater DOR internalization than a similar dose of morphine or ARM100390 [11, 24], and this internalization could be blocked by an antagonist [25]. Pre-incubation with the antagonist naltrindole (30 min, 10 µM) abolished SNC-80-induced increases in DORs and Kir3 intracellular immunoreactivities (Fig. 1a–c), further consistent with the fact that DOR activation drives Kir3 channel sequestration. DOR and Kir3 channel internalization by morphine (DORMOR: 20.53 ± 0.99, Kir3.1MOR: 23.27 ± 0.94) and ARM100390 (DORARM: 21.95 ± 1.15, Kir3.1ARM: 21.33 ± 0.96) were also assessed (Fig. 2), and in both cases sequestration was less pronounced than for SNC-80 (Fig. 2b). Moreover, the proportion of receptors and channels that accumulated intracellularly was similar for all agonists (DOR/Kir3.1 ratioSNC-80: 1.7 ± 0.3; ratioMOR: 1.5 ± 0.2; ratioARM: 1.9 ± 0.1; inset Fig. 2b), indicating that both membrane species internalized as a unit.
Fig. 2.
DOR and Kir3 internalization display similar pharmacological profiles. a Cortical neuron cultures were exposed to morphine or ARM100390 (1 µM, 60 min) and permeabilized for visualization of membrane and intracellular labeling. Graphs below line scans corresponding to traces shown in merged images (Kir3.1 = green, DOR = red). Scale bars 5 μm. b Histograms correspond to mean ± SEM of 3–9 experiments with 42–90 neurons analyzed per condition; SNC-80 was introduced for comparison. Data were analyzed as in Fig. 1; ARM100390 vs CTL: p < 0.0001; morphine vs CTL: p < 0.0001. Additional comparisons are shown in the figure. Inset ratios of ligand-induced changes in intracellular immunoreactivities for DOR and Kir3 (p = 0.5162; one way ANOVA; n = 3–8)
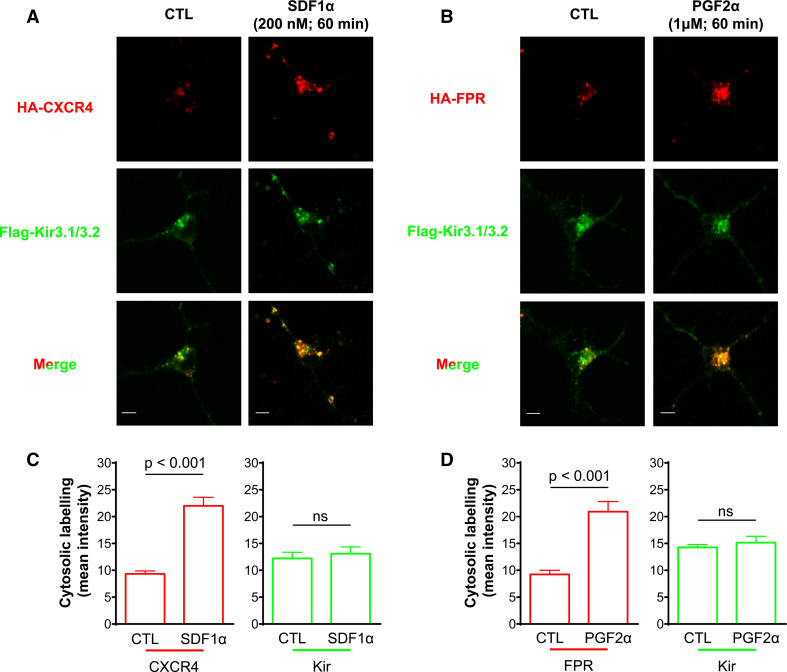
We then sought to establish the specificity of these observations by assessing if other GPCRs could induce Kir3 channel internalization. Like DORs, CXCR4 receptors couple to Gαi/o [26], they internalize upon activation [10] and activate Kir3 currents in neurons [27]. We therefore assessed if CXCR4 and Kir3.1/3.2 channels co-internalized upon exposure to SDF-1α (200 nM; 60 min). CXCR4 receptors and Kir3 subunits both displayed ligand-independent intracellular labeling (Fig. 3a). However, stimulation with SDF-1α at a concentration (200 nM; 60 min) that produces maximal CXCR4 sequestration [10] failed to induce channel sequestration (Fig. 3a, c). Kir3.1/3.2 subunits were also co-expressed with F prostanoid receptors (FPR) which couple to Gαq [28]. FPR stimulation by PGF2α (1 µM; 60 min) increased intracellular labeling of the receptor but did not modify channel labeling (Fig. 3b, d). Taken together, these data indicate that overexpression of receptors and channels cannot simply account for Kir3 channel internalization associated with DOR stimulation.
Fig. 3.
CXCR4 and F prostanoid (FP) receptor stimulation does not induce Kir3 internalization. Cortical neuron cultures transfected with a HA-CXCR4/Flag-Kir3.1/3.2 or b HA-FPR/Flag-Kir3.1/3.2 were, respectively, exposed to SDF-1α or PGF2α and then permeabilized for visualization of membrane and intracellular labeling. Scale bars 5 μm. c Histograms correspond to intracellular labeling intensity for CXCR4 receptors and Kir3 subunits and correspond to mean ± SEM of 3 experiments with 24–32 neurons analyzed per condition. Changes in DOR and Kir3.1 immunoreactivities were analyzed separately using Student’s t test, and significance is given in the figure, comparison Kir p = 0.6215. d Histograms correspond to intracellular labeling intensity for FP receptors and Kir3 subunits, (n = 3). Data represented and analyzed as in (c). Comparison Kir p = 0.5233
DORs and Kir3.1/3.2 channels remain associated over the time course of receptor internalization
The previous section showed that DORs and Kir3 channels internalize as a unit. To evaluate if DORs and Kir3 subunits remained associated during this time lapse, we carried out bioluminescence resonance energy transfer (BRET) assays, but first verified if Kir3.1/3.2 channels also underwent DOR-promoted internalization in the cellular background where BRET readings were obtained. We observed that in HEK293 cells, SNC-80 (1 µM) produced time-dependent decrease in membrane labeling of both species, each attaining maximal internalization with similar half-lives (Supplementary Fig. 2).
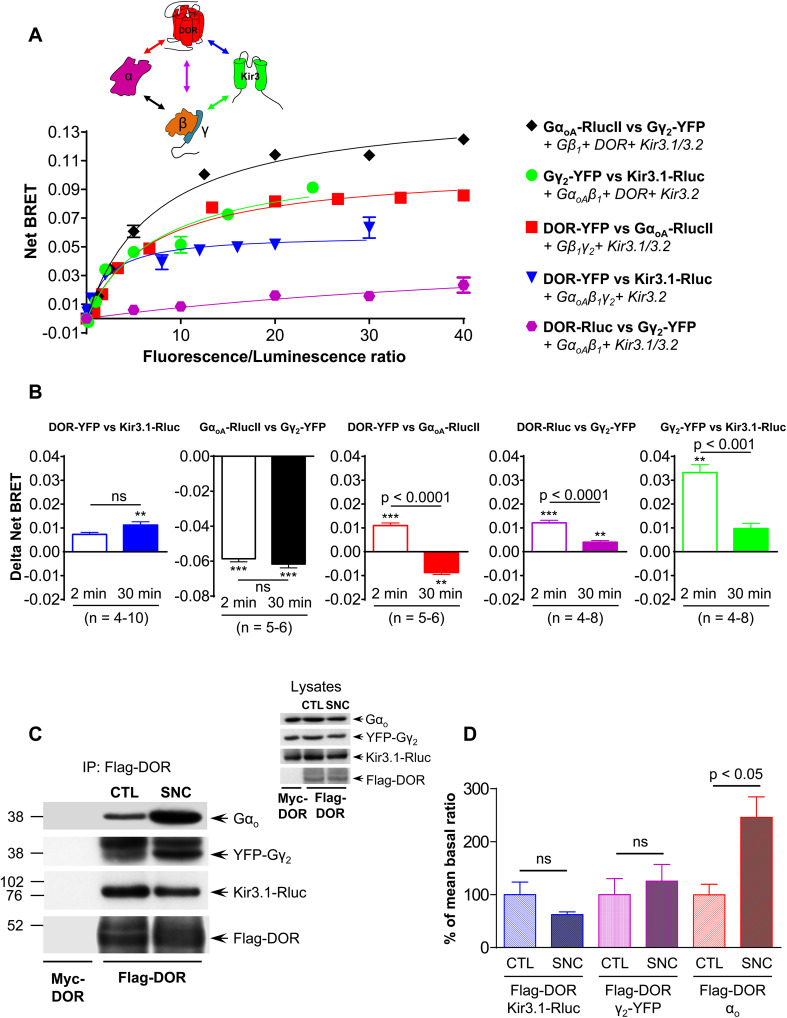
Then, to determine if channels and receptors remained associated within the time frame of sequestration we used BRET-based biosensors. The interactions that were monitored and donor–acceptor BRET pairs that were used for this purpose are shown in Fig. 4a. In a first approach, we used “titrations assays” in which a fixed amount of one of the protein of interest was tagged with the donor Rluc and was co-expressed with increasing amounts of the corresponding interaction partner labeled with the acceptor YFP (Fig. 4a). For the different pairs tested, increasing concentrations of the acceptor induced a progressive increase in spontaneous energy transfer until increments became minimal, corresponding to the theoretical situation in which all donor constructs are in close proximity of an acceptor [29]. This saturation behavior is typical of specific interactions as opposed to minimal and non-saturated bystander BRET curves [29] generated by proteins that have similar distribution as the signaling partners of interest but do not associate into specific complexes (Supplementary Fig. 3). Thus, titration curves carried out in non-stimulated cells confirmed that: (a) DORs constitutively and specifically associate with GαoA subunits, with the Gβ1γ2 dimer and with Kir3.1 subunits (Fig. 4a, Supplementary Fig. 3A–C); (b) the Gβ1γ2 dimer associated with Kir3.1 channel subunits (Fig. 4a, Supplementary Fig. 3D) and (c) GαoAβ1γ2 formed the expected heterotrimer (Fig. 4a, Supplementary Fig. 3E).
Fig. 4.
DORs, Gαβγ subunits and Kir3.1/3.2 channels remain associated but undergo conformational rearrangements during sustained receptor stimulation. a Interactions among different complex components (inset) were studied in BRET titration assays. For this purpose, HEK293 cells were transfected with indicated BRET pairs and accessory signaling partners as described in text. Titration curves corresponding to a single experiment carried out in triplicate are shown. b HEK293 cells were transfected with a stable proportion of indicated BRET pairs, and exposed or not to SNC-80 (1 µM) for 2 or 30 min. Histograms represent agonist-induced changes in net BRET and correspond to 3–10 experiments carried out in triplicates. Significance of agonist-induced changes in energy transfer was established by one-way ANOVA comparing net BRET signals generated in controls to those generated in cells that were treated for 2 or 30 min **p < 0.01; ***p < 0.0001 (actual net BRET values are shown in Supplementary information, Supplementary Table 1). Comparison of ligand-induced BRET changes at the two stimulation time points was done by Student’s t test and appears in the figure. c HEK293 cells were transfected to express Flag-DOR (or Myc-DOR as indicated), GαoAβ1YFP-γ2 and Kir3.1-Rluc/3.2 channel subunits and were exposed or not to SNC-80 (1 µM; 30 min). A representative example of signaling partners recovered with Flag-DORs is shown. Inset proteins present in lysate. d Immunoreactivity of different complex components recovered with the receptor was expressed as a ratio of FLAG-DORs in the corresponding sample. Results were expressed as percentage of basal ratios and represent mean ± SEM of 5 independent experiments. Statistical comparisons done using Student’s t test; p values appear in the figure
HEK293 cells were then transfected with donor/acceptor pairs in amounts which produced maximal energy transfer in non-stimulated cells and were subsequently exposed to SNC-80 (1 µM) for 2 or 30 min. Comparison of BRET values obtained in control cells and cells exposed to the agonist for 2 min indicated that during this short period, the transfer of energy between DOR and channel subunits was not significantly changed, it was reduced at the GαoA–Gβγ interface but increased at biosensors assessing DOR/GαoA, DOR/Gβγ and Gβγ/Kir3 channel interactions (Fig. 4b). From these changes and from the position of donor–acceptor tags in each construct, it is possible to infer that after 2 min receptor stimulation DORs, the GαoGβ1γ2 heterotrimer and Kir3 channel subunits reorganized among themselves, but remained within the complex. Conformational changes underlying these initial BRET changes are represented in Supplementary Fig. 4A, B and further described in supplementary information section. Beyond this initial activation point, we saw that following 30 min exposure to SNC-80 (1 µM), the initial BRET increase between receptors and channels was not reduced but rather moderately enhanced (Fig. 4b), implying that both signaling partners remained associated over sustained DOR activation. In contrast, BRET increases that were initially observed at Gβγ/Kir3 and Gβγ/DOR interfaces decreased, though the reduction did not go below values observed in non-stimulated cells (Fig. 4b), implying that Gβγ remained as integral part of the DOR-Kir3 complex. Observations for GαoA were quite different since the initial BRET increase at the GαoA/DOR interface was replaced by a significant reduction (Fig. 4b), arguing that the energy transfer between the C-terminal end of the receptor and the helical domain of GαoA was reduced beyond its initial resting position. Energy transfer at the interface of GαoA and Gβγ subunits remained as low as it was after 2 min stimulation and no BRET signal was detected at the GαoA/channel interface, making spectroscopic data insufficient to conclude whether GαoA actually dissociated from the complex or not. Co-immunopurification assays carried out in control cells and in cells pre-exposed to SNC-80 (1 µM; 30 min) helped clarify this issue (Fig. 4c, d), indicating that the amount of GαoA recovered with the receptor was higher following exposure to SNC-80 than in controls. Thus, taken together, biochemical and spectroscopic data indicate that complex components not only remained associated during sustained receptor activation, but that the stability of GαoA association to other complex components was enhanced by conformational changes that took place over this time frame. Supplementary Fig. 4C and supplementary information describe putative conformational reorganizations that support BRET changes induced by sustained activation of the receptor with SNC-80.
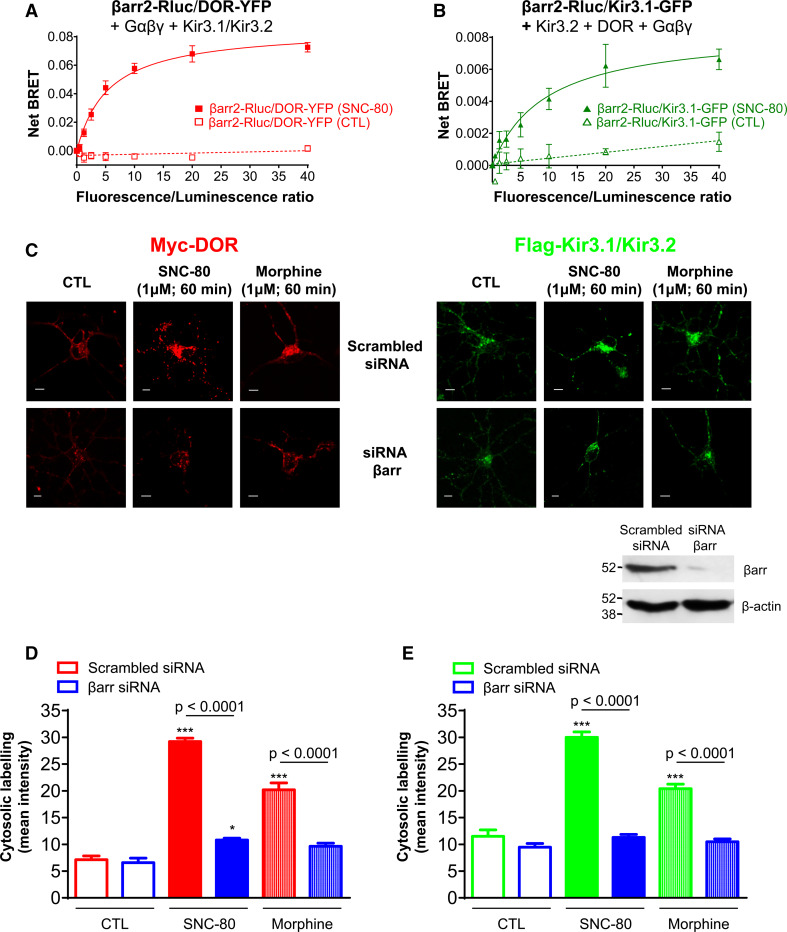
βarr is recruited to Kir3.1/3.2 channel subunits and promotes their co-internalization with DORs
Within the time frame of internalization DORs, G proteins and Kir3.1/3.2 subunits remained associated but reorganized within the complex. We therefore hypothesized that this rearrangement could represent the interaction of complex components with regulatory proteins. We recently showed that DOR activation may induce βarr interaction with Gβγ subunits as well as DORs [14] and given that here the dimer remained associated to the channel, we assessed whether Kir3 channel subunits could also interact with this regulatory protein. To do so, HEK293 cells were transfected with a fixed amount of donor-tagged βarr2 (βarr2-Rluc) and increasing amounts of either DOR-YFP or Kir3.1-GFP acceptors together with complementary Kir3.2 and GαoAβ1γ2 subunits (Fig. 5a, b). Cells were then treated for 30 min with SNC-80 (1 µM) and BRET measures were subsequently taken. No BRET signal was detected in untreated cells but, in those exposed to SNC-80, there was a saturable increase in energy transfer both at DOR/βarr2 and Kir3/βarr2 interfaces, indicating that the regulatory protein was recruited to Kir3 channel subunits as well as receptors. Given this interaction, we then sought to verify if channel sequestration was also βarr-dependent. For this purpose cortical cultures were transfected with Myc-DOR and Flag-Kir3.1/3.2 subunits together with βarr siRNA or scrambled siRNA (Fig. 5c). We detected no difference in basal cytosolic immunoreactivities for receptors and channels in cells transfected with scrambled or active siRNA (Fig. 5d, e). On the other hand, internalization of the two signaling partners was significantly reduced in βarr-silenced cells exposed to SNC-80 (1 µM; 60 min). When tested in heterologous systems, opioid receptor agonists differ considerably in their ability to recruit βarr and induce internalization of the receptor. For example, SNC-80 produces marked and stable DOR-βarr interaction while recruitment by morphine is minimal. These patterns of βarr recruitment are considered to, respectively, produce high or marginal receptor internalization [11, 14]. Thus, we determined whether βarrs were distinctively involved in the internalization of DOR-Kir3 complexes by the two agonists (Fig. 5c–e). As for SNC-80, knock-down of the regulatory protein reduced morphine-induced accumulation of intracellular DOR and Kir3.1 immunoreactivities indicating that internalization of the complex by morphine was also dependent on βarr.
Fig. 5.
βarr is necessary for DOR and Kir3 channel internalization. HEK293 cells transfected with a fixed amount of βarr2-Rluc and increasing amounts of a DOR-YFP or b Kir3.1-GFP plus indicated complex components were incubated or not with SNC-80 (1 µM, 30 min). Results correspond to the mean of 4–5 independent experiments and are expressed as mean ± SEM of netBRET values. Curves were analyzed by two-way ANOVA revealing an: effect of drug (p < 0.0001), effect of donor/acceptor ratios (p < 0.0001) and interaction (p < 0.0001). c Cortical neuron cultures were transfected with myc-DOR and Flag-Kir3.1/3.2 channels together with scrambled or βarr2 siRNA, and then treated or not with SNC-80 or morphine (1 µM; 30 min). Scale bars 5 μm. Inset illustrates effect of βarr2 siRNA on expression of endogenous βarr using an antibody that recognizes βarr1/2. Histograms represent mean intracellular fluorescence intensity of d myc-DORs and e Flag-Kir3.1 subunits and correspond to mean ± SEM of 3 experiments with 25–29 neurons analyzed per condition. DOR and Kir3 internalization were analyzed independently using two-way ANOVA. In both cases there was an effect of drug (p < 0.0001), of siRNA (p < 0.0001) and interaction (p < 0.0001). Post hoc comparisons indicated that both agonists significantly enhanced DOR and Kir3 immunoreactivities in cells transfected with scrambled controls (***p < 0.0001) while only SNC-80 specifically increased DOR immunoreactivity in βarr-silenced cells (*p < 0.05). Additional comparisons appear in the figure
To further characterize βarr interaction with DORs and Kir3 channels and to ascertain possible ligand-specific differences in the way this regulatory protein associates with complex components, we monitored ligand-induced subcellular distribution of the two signaling partners. For this purpose, βarr2-Rluc was alternatively co-transfected with Flag-DORs and untagged channel subunits or with Flag-Kir3.1/3.2 subunits and untagged receptors. In non-stimulated cells, βarr2-Rluc was distributed throughout the cytosol while receptors and channels were predominantly found at the membrane, where their interaction with βarr2 was minimal. In contrast, constitutively internalized complex components displayed some degree of colocalization with the regulatory protein (βarr2/DORs, Pearson’s coefficient, 0.54 ± 0.04; βarr2/Kir3 channels (Pearson’s coefficient: 0.55 ± 0.03; n = 3) (Fig. 6). Sustained stimulation with SNC-80 (1 µM; 60 min) induced redistribution and concentration of cytosolic βarr2 to the sites where receptor (arrow head, Fig. 6a) and channels (arrow head, Fig. 6b) had accumulated, significantly increasing its colocalization with either signaling partner (Fig. 6c–f). After 60-min stimulation with morphine, the situation was quite different, with no evidence of βarr2 redistribution and no significant increase in Pearson’s coefficient (Supplementary Fig. 5). Thus, despite similarly relying on βarr function for DOR/Kir3 channel internalization, the stability of βarr2 interaction with the complex differed considerably for SNC-80 and morphine since only the former induced active accumulation of DORs, Kir3 subunits and βarr2.
Fig. 6.
SNC-80 promoted βarr2 colocalization with receptors and channels. Cortical neuron cultures expressing either βarr2-Rluc, Flag-DORs and untagged channel subunits (a) or βarr2-Rluc, Flag-Kir3.1/3.2 subunits and untagged DORs (b) were exposed to SNC-80 as indicated. Arrow heads show redistribution of βarr2-Rluc to the site where receptors or channels also accumulate. Scale bars 5 μm. Cells shown in a, c and in b, d were processed using ImageJ to display the product of the differences from mean labeling intensity (PDM) as pseudocolored panels. Yellow on the scale bar indicates a large PDM value and high degree of colocalization, while blue indicates no overlap. Note difference in scales in (c). Histograms correspond to Pearson’s correlation coefficient for colocalization of Flag-DOR and βarr2-Rluc (e) or Flag-Kir3.1 and βarr2-Rluc (f) in unstimulated cells and in cells exposed to SNC-80 (1 µM; 60 min). They represent mean ± SEM of 3–4 independent experiments with 18–26 neurons analyzed per condition. Statistical comparisons of correlation coefficients was done by one ANOVA to compare SNC-80 and morphine (shown in Supplementary information and Supplementary Fig. 5) to non-treated controls
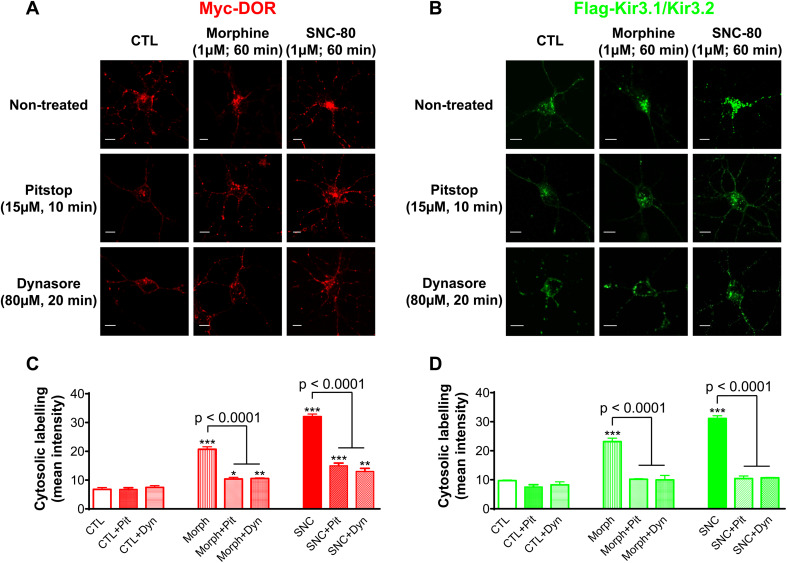
DOR-Kir3.1/3.2 signaling complexes internalize via a clathrin/dynamin mediated mechanism
βarrs cooperate with adaptor protein 2 (AP2) to promote the accumulation of membrane cargo in clathrin-coated vesicles, and these are then pinched off to the cytosol by the action of dynamin [30]. To determine whether internalization of DOR-Kir3.1/3.2 channel complexes followed this path, we used pharmacological inhibitors that either stall clathrin pit dynamics [31] or block dynamin’s GTPase activity [32]. We did not observe any significant effect of clathrin (Pitstop 2, 15 µM) nor dynamin inhibitor (Dynasore, 10 µM) on constitutive internalization of either membrane species, but both blockers reduced DOR (Fig. 7a, c) and Kir3 (Fig. 7b, d) internalization by morphine and SNC-80, indicating that the complex followed one common endocytic route independent of the agonist that activates the receptor. Interestingly, neither Pitstop 2 nor Dynasore eliminated DOR internalization by morphine or SNC-80, but they both abolished Kir3 channel internalization (Fig. 7c, d). A similar observation was obtained with βarr silencing, though residual internalization of the receptor only attained significance following SNC-80 treatment (Fig. 5d). A plausible explanation for these observations is that a small subpopulation of receptors had internalized independent of the channel via a mechanism that relies neither on βarr, clathrin nor dynamin.
Fig. 7.
Inhibition of clathrin and dynamin function interferes with DOR and Kir3 channel internalization. Cortical neuron cultures were pre-treated with clathrin inhibitor, Pitstop 2 or dynamin inhibitor, Dynasore and exposed to DOR agonists to then visualize internalization of a receptors and b channels. Scale bars 5 μm. Histograms correspond to quantification of intracellular fluorescence for DORs c or channels d and represent mean ± SEM of 3-5 experiments with 26–42 neurons analyzed per condition. Statistical significance for the effects of Pitsop 2 and Dynasore were established independently, using two-way ANOVA to determine the effect of each blocker on DORs and on Kir3 channel internalization. Both comparisons revealed an effect of agonist (p < 0.0001), of the internalization inhibitor (p < 0.0001), and interaction (p < 0.0001). Intracellular labeling of agonist-treated cells versus corresponding controls: *p < 0.05; **p < 0.01 and ***p < 0.0001. Additional post hoc comparisons are indicated in figure
Discussion
Dynamic regulation of Kir3 channel availability at the membrane affords powerful modulation of neuronal excitability and ultimately of behavior [5, 6]. The number of channels present at the surface at any given time results from a fine equilibrium between their incorporation to and their removal from the plasma membrane. Considerable evidence has been gathered with respect to the way incorporation is driven and modulated establishing that primary sequence [1] and tyrosine phosphorylation [8] may regulate Kir3 subunit export to the membrane. Similarly, dephosphorylation of either the channel [33] or of associated GABAB receptors [5, 6] were shown to interfere with membrane re-insertion of channel subunits via the recycling path, but much less was known about mechanisms participating in surface removal of channel subunits. The observation that M2 muscarinic receptors (M2R) and Kir3 subunits accumulate in the cytosol of acetylcholine-releasing PC12 cells had led to the proposal that these receptors would internalize and drag the channel intracellularly [34]. Here, we have addressed this hypothesis and demonstrated that DOR activation by different agonists caused receptors and Kir3 channels to actually translocate from the membrane to the cytosol. The extent of translocation was distinct for SNC-80, morphine and ARM100390 but the proportion of receptors and channels internalized by each ligand did not differ, indicating that both signaling partners trafficked as a unit.
Results also showed that Kir3 channel sequestration is not universally triggered by all receptors that regulate channel activity. Thus, although Gαq proteins modulate Kir3 channel activity via PIP2 depletion [35], activation of the Gαq-coupled FPR did not modify channel translocation to the cytosol. Similarly, despite their ability to generate Kir3 channel currents [10] and to undergo SDF-1α-dependent sequestration [28], exposure to the agonist caused CXCR4 receptors but not Kir3 subunits to internalize. Receptor-dependent differences have also been reported in the endocytic trafficking of N-type Ca2+ (Cav2.2) channels whose sequestration is triggered by opioid-like receptors (ORL1) but not MORs [36]. The distinct ability of ORL1 receptors to promote channel sequestration was conferred by direct physical association between the C-terminal end of the receptor and that of Cav2.2 alpha subunits [36]. Although this type of interaction has not been reported for Kir3 channels subunits, there is considerable evidence that Kir3 channels and GPCRs may organize into multimeric arrays. Studies carried out on native membranes indicate that Kir3 channel subunits may be recovered by immunoprecipitating striatal D2 receptors [37], cerebellar GABAB receptors [38] or β2-adrenergic receptors present in heart and whole brain lysates [37]. Spectroscopic studies have confirmed these observations [37, 38] but have also shown that not all receptors display the same tendency to associate into multimeric arrays [39]. The tendency to form constitutive complexes may be influenced by the type of Gαi/o subunit with which the receptor interacts [40], the level of constitutive activity of the receptors [39] as well as by scaffolding proteins that confine and concentrate Kir3 channels to specialized compartments together with receptors [4, 41]. As would be expected from high levels of constitutive activity displayed by DORs [42], these receptors, GαoAβ1γ2 heterotrimers and Kir3.1/3.2 subunits spontaneously associate into a complex [12].
Here, we show that despite considerable rearrangements among interaction partners, the complex maintained its integrity from initial moments of receptor stimulation until maximal internalization was attained, providing a platform for persistent βarr2 interaction. We had recently shown that SNC-80 induces rapid βarr2 recruitment, not only to receptors but also to the Gβγ dimer and supplied evidence that these interactions were maintained over prolonged exposure to the agonist [14]. Here, we show that canonical Gβγ effectors are also subject to direct and prolonged interaction with βarr2, and establish proof that endogenous βarr in cortical neuron cultures drives joint sequestration of DOR and Kir3 channels via the clathrin/dynamin endocytic route. This type of channel regulation is in contrast with the one described for G protein-coupled receptor kinase 2 (GRK2), which takes place directly at the cell surface and rather than removing Kir3 subunits from the membrane involves conformational rearrangements that prevent Gβγ from effectively activating the channel [43]. Results obtained in neurons further suggest that βarrs, DORs and Kir3 channels remain persistently associated after exposure to SNC-80 but not morphine, since only the former caused βarr to colocalize with the two signaling partners that accumulated in the cytosol.
Distinct modes of opioid receptor association with βarrs have been proposed as possible contributors to ligand-specific potential for generating tolerance [44, 45]. SNC-80 and morphine both induce analgesic tolerance that involves βarrs [14, 24, 46], but in keeping with differences observed in the present study, their tolerance profiles are quite distinct. While a single injection of SNC-80 may abolish analgesic responsiveness for as long as 4 h [24] it takes at least 3 days of continuous morphine administration for its analgesic actions to disappear [46]. Differential sequestration of receptors and channels within βarr-containing complexes may contribute to these two distinct tolerance profiles, particularly the rapidity and persistence of SNC-80’s tolerance profile. In this sense, we have previously shown that the stable DOR-βarr2 association promoted by SNC-80 interfered with receptor recycling to membrane leading to receptor desensitization and marked development of acute analgesic tolerance. These are much less severe or absent with DPDPE, another DOR agonist which, similar to morphine, fails to promote sustained βarr2 association with the activated receptor [14].
In conclusion, this study shows that βarr recognizes DORs and Kir3 channels as a complex producing their joint internalization via a clathrin/dynamin-dependent mechanism.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grant [311997] from the Natural Sciences and Engineering Research Council of Canada (NSERC) to GP. KN holds a studentship from Ste-Justine Hospital Research Center and the Faculty of Graduate and Postdoctoral Studies, University of Montreal. IC holds an FRQS fellowship.
Conflict of interest
None.
References
- 1.Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron. 2002;33(5):715–729. doi: 10.1016/S0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 2.Lunn ML, Nassirpour R, Arrabit C, Tan J, McLeod I, Arias CM, Sawchenko PE, Yates JR, 3rd, Slesinger PA. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat Neurosci. 2007;10(10):1249–1259. doi: 10.1038/nn1953. [DOI] [PubMed] [Google Scholar]
- 3.Munoz MB, Slesinger PA. Sorting nexin 27 regulation of G protein-gated inwardly rectifying K(+) channels attenuates in vivo cocaine response. Neuron. 2014;82(3):659–669. doi: 10.1016/j.neuron.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hibino H, Inanobe A, Tanemoto M, Fujita A, Doi K, Kubo T, Hata Y, Takai Y, Kurachi Y. Anchoring proteins confer G protein sensitivity to an inward-rectifier K(+) channel through the GK domain. EMBO J. 2000;19(1):78–83. doi: 10.1093/emboj/19.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hearing M, Kotecki L, Fernandez Marron, de Velasco E, Fajardo-Serrano A, Chung HJ, Lujan R, Wickman K. Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron. 2013;80(1):159–170. doi: 10.1016/j.neuron.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, Martinez-Hernandez J, Watanabe M, Moss SJ, Lujan R, Luscher C, Slesinger PA. Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA. Neuron. 2012;73(5):978–989. doi: 10.1016/j.neuron.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ippolito DL, Temkin PA, Rogalski SL, Chavkin C. N-terminal tyrosine residues within the potassium channel Kir3 modulate GTPase activity of Galphai. J Biol Chem. 2002;277(36):32692–32696. doi: 10.1074/jbc.M204407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mora SI, Escobar LI. Phosphorylation of a tyrosine at the N terminus regulates the surface expression of GIRK5 homomultimers. FEBS Lett. 2005;579(14):3019–3023. doi: 10.1016/j.febslet.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 9.Terunuma M, Pangalos MN, Moss SJ. Functional modulation of GABAB receptors by protein kinases and receptor trafficking. Adv Pharmacol. 2010;58:113–122. doi: 10.1016/S1054-3589(10)58005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng ZJ, Zhao J, Sun Y, Hu W, Wu YL, Cen B, Wu GX, Pei G. beta-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4. J Biol Chem. 2000;275(4):2479–2485. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- 11.Charfi I, Nagi K, Mnie-Filali O, Thibault D, Balboni G, Schiller PW, Trudeau LE, Pineyro G. Ligand- and cell-dependent determinants of internalization and cAMP modulation by delta opioid receptor (DOR) agonists. Cell Mol Life Scie. 2014;71(8):1529–1546. doi: 10.1007/s00018-013-1461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard-Lalonde M, Nagi K, Audet N, Sleno R, Amraei M, Hogue M, Balboni G, Schiller PW, Bouvier M, Hebert TE, Pineyro G. Conformational dynamics of Kir3.1/Kir3.2 channel activation via delta-opioid receptors. Mol Pharmacol. 2013;83(2):416–428. doi: 10.1124/mol.112.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dacres H, Michie M, Trowell SC. Comparison of enhanced bioluminescence energy transfer donors for protease biosensors. Anal Biochem. 2012;424(2):206–210. doi: 10.1016/j.ab.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 14.Audet N, Charfi I, Mnie-Filali O, Amraei M, Chabot-Dore AJ, Millecamps M, Stone LS, Pineyro G. Differential association of receptor-Gbetagamma complexes with beta-arrestin2 determines recycling bias and potential for tolerance of delta opioid receptor agonists. J Neurosci. 2012;32(14):4827–4840. doi: 10.1523/JNEUROSCI.3734-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gales C, Rebois RV, Hogue M, Trieu P, Breit A, Hebert TE, Bouvier M. Real-time monitoring of receptor and G-protein interactions in living cells. Nat Methods. 2005;2(3):177–184. doi: 10.1038/nmeth743. [DOI] [PubMed] [Google Scholar]
- 17.Rebois RV, Robitaille M, Gales C, Dupre DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hebert TE. Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci. 2006;119(Pt 13):2807–2818. doi: 10.1242/jcs.03021. [DOI] [PubMed] [Google Scholar]
- 18.Ayoub MA, Maurel D, Binet V, Fink M, Prezeau L, Ansanay H, Pin JP. Real-time analysis of agonist-induced activation of protease-activated receptor 1/Galphai1 protein complex measured by bioluminescence resonance energy transfer in living cells. Mol Pharmacol. 2007;71(5):1329–1340. doi: 10.1124/mol.106.030304. [DOI] [PubMed] [Google Scholar]
- 19.Audet N, Pineyro G. Using BRET to detect ligand-specific conformational changes in preformed signalling complexes. Methods Mol Biol. 2011;756:149–163. doi: 10.1007/978-1-61779-160-4_7. [DOI] [PubMed] [Google Scholar]
- 20.Gales C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol. 2006;13(9):778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- 21.Audet N, Gales C, Archer-Lahlou E, Vallieres M, Schiller PW, Bouvier M, Pineyro G. Bioluminescence resonance energy transfer assays reveal ligand-specific conformational changes within preformed signaling complexes containing delta-opioid receptors and heterotrimeric G proteins. J Biol Chem. 2008;283(22):15078–15088. doi: 10.1074/jbc.M707941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Archer-Lahlou E, Audet N, Amraei MG, Huard K, Paquin-Gobeil M, Pineyro G. Src promotes delta opioid receptor (DOR) desensitization by interfering with receptor recycling. J Cell Mol Med. 2009;13(1):147–163. doi: 10.1111/j.1582-4934.2008.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Felice V, Cappello F, Montalbano A, Ardizzone NM, De Luca A, Macaluso F, Amelio D, Cerra MC, Zummo G. HSP90 and eNOS partially co-localize and change cellular localization in relation to different ECM components in 2D and 3D cultures of adult rat cardiomyocytes. Biol Cell. 2007;99(12):689–699. doi: 10.1042/BC20070043. [DOI] [PubMed] [Google Scholar]
- 24.Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PloS One. 2009;4(5):e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong EW, Xue L, Olmstead MC, Cahill CM. Prolonged morphine treatment alters delta opioid receptor post-internalization trafficking. Brit J Pharmacol. 2014 doi: 10.1111/bph.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajetto A, Bonavia R, Barbero S, Piccioli P, Costa A, Florio T, Schettini G. Glial and neuronal cells express functional chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor 1. J Neurochem. 1999;73(6):2348–2357. doi: 10.1046/j.1471-4159.1999.0732348.x. [DOI] [PubMed] [Google Scholar]
- 27.Guyon A, Nahon JL. Multiple actions of the chemokine stromal cell-derived factor-1alpha on neuronal activity. J Mol Endocrinol. 2007;38(3):365–376. doi: 10.1677/JME-06-0013. [DOI] [PubMed] [Google Scholar]
- 28.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46(2):205–229. [PubMed] [Google Scholar]
- 29.Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M. Quantitative assessment of beta 1- and beta 2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J Biolchem. 2002;277(47):44925–44931. doi: 10.1074/jbc.M205767200. [DOI] [PubMed] [Google Scholar]
- 30.Raimondi A, Ferguson SM, Lou X, Armbruster M, Paradise S, Giovedi S, Messa M, Kono N, Takasaki J, Cappello V, O’Toole E, Ryan TA, De Camilli P. Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron. 2011;70(6):1100–1114. doi: 10.1016/j.neuron.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Krausslich HG, Shupliakov O, Robinson PJ, McCluskey A, Haucke V. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell. 2011;146(3):471–484. doi: 10.1016/j.cell.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Barrias ES, Reignault LC, De Souza W, Carvalho TM. Dynasore, a dynamin inhibitor, inhibits Trypanosoma cruzi entry into peritoneal macrophages. PloS One. 2010;5(1):e7764. doi: 10.1371/journal.pone.0007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung HJ, Qian X, Ehlers M, Jan YN, Jan LY. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci USA. 2009;106(2):629–634. doi: 10.1073/pnas.0811615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clancy SM, Boyer SB, Slesinger PA. Coregulation of natively expressed pertussis toxin-sensitive muscarinic receptors with G-protein-activated potassium channels. J Neurosci. 2007;27(24):6388–6399. doi: 10.1523/JNEUROSCI.1190-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobrinsky E, Mirshahi T, Zhang H, Jin T, Logothetis DE. Receptor-mediated hydrolysis of plasma membrane messenger PIP2 leads to K+-current desensitization. Nat Cell Biol. 2000;2(8):507–514. doi: 10.1038/35019544. [DOI] [PubMed] [Google Scholar]
- 36.Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Ferguson SS, Mezghrani A, Dubel SJ, Bourinet E, McRory JE, Zamponi GW. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9(1):31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- 37.Lavine N, Ethier N, Oak JN, Pei L, Liu F, Trieu P, Rebois RV, Bouvier M, Hebert TE, Van Tol HH. G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J Biol Chem. 2002;277(48):46010–46019. doi: 10.1074/jbc.M205035200. [DOI] [PubMed] [Google Scholar]
- 38.Ciruela F, Fernandez-Duenas V, Sahlholm K, Fernandez-Alacid L, Nicolau JC, Watanabe M, Lujan R. Evidence for oligomerization between GABAB receptors and GIRK channels containing the GIRK1 and GIRK3 subunits. Eur J Neurosci. 2010;32(8):1265–1277. doi: 10.1111/j.1460-9568.2010.07356.x. [DOI] [PubMed] [Google Scholar]
- 39.Hein P, Frank M, Hoffmann C, Lohse MJ, Bunemann M. Dynamics of receptor/G protein coupling in living cells. EMBO J. 2005;24(23):4106–4114. doi: 10.1038/sj.emboj.7600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank M, Thumer L, Lohse MJ, Bunemann M. G Protein activation without subunit dissociation depends on a G{alpha}(i)-specific region. J Biol Chem. 2005;280(26):24584–24590. doi: 10.1074/jbc.M414630200. [DOI] [PubMed] [Google Scholar]
- 41.Nassirpour R, Bahima L, Lalive AL, Luscher C, Lujan R, Slesinger PA. Morphine- and CaMKII-dependent enhancement of GIRK channel signaling in hippocampal neurons. J Neurosci. 2010;30(40):13419–13430. doi: 10.1523/JNEUROSCI.2966-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fenalti G, Giguere PM, Katritch V, Huang XP, Thompson AA, Cherezov V, Roth BL, Stevens RC. Molecular control of delta-opioid receptor signalling. Nature. 2014;506(7487):191–196. doi: 10.1038/nature12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raveh A, Cooper A, Guy-David L, Reuveny E. Nonenzymatic rapid control of GIRK channel function by a G protein-coupled receptor kinase. Cell. 2010;143(5):750–760. doi: 10.1016/j.cell.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Raehal KM, Schmid CL, Groer CE, Bohn LM. Functional selectivity at the mu-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol Rev. 2011;63(4):1001–1019. doi: 10.1124/pr.111.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagi K, Pineyro G. Regulation of opioid receptor signalling: implications for the development of analgesic tolerance. Mol Brain. 2011;4:25. doi: 10.1186/1756-6606-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408(6813):720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.