Abstract
Two-pore channel proteins (TPC) encode intracellular ion channels in both animals and plants. In mammalian cells, the two isoforms (TPC1 and TPC2) localize to the endo-lysosomal compartment, whereas the plant TPC1 protein is targeted to the membrane surrounding the large lytic vacuole. Although it is well established that plant TPC1 channels activate in a voltage- and calcium-dependent manner in vitro, there is still debate on their activation under physiological conditions. Likewise, the mode of animal TPC activation is heavily disputed between two camps favoring as activator either nicotinic acid adenine dinucleotide phosphate (NAADP) or the phosphoinositide PI(3,5)P2. Here, we investigated TPC current responses to either of these second messengers by whole-vacuole patch-clamp experiments on isolated vacuoles of Arabidopsis thaliana. After expression in mesophyll protoplasts from Arabidopsis tpc1 knock-out plants, we detected the Arabidopsis TPC1-EGFP and human TPC2-EGFP fusion proteins at the membrane of the large central vacuole. Bath (cytosolic) application of either NAADP or PI(3,5)P2 did not affect the voltage- and calcium-dependent characteristics of AtTPC1-EGFP. By contrast, PI(3,5)P2 elicited large sodium currents in hTPC2-EGFP-containing vacuoles, while NAADP had no such effect. Analogous results were obtained when PI(3,5)P2 was applied to hTPC2 expressed in baker’s yeast giant vacuoles. Our results underscore the fundamental differences in the mode of current activation and ion selectivity between animal and plant TPC proteins and corroborate the PI(3,5)P2-mediated activation and Na+ selectivity of mammalian TPC2.
Keywords: TPC channels, Plant vacuole, Yeast vacuole, Patch clamp, Lysosome
Introduction
Two-pore channel (TPC) proteins are ubiquitous intracellular cation channels in animals and plants. They are formed by 12 transmembrane spanning segments (TM) that can be grouped into two Shaker-like repeats. A Shaker-like unit, the fundamental building block of members of the superfamily of voltage-gated ion channels [1], is formed by six TM, of which segment S4 acts as a voltage sensor, due to the presence of several positively charged residues, and the re-entrant loop between segment S5 and S6, the so-called P-loop, participates in pore formation. In agreement with the fact that four P-loops are necessary to form a functional pore, TPC proteins possess a dimeric structure [2].
Plant TPCs are targeted to the tonoplast [3–5], the membrane surrounding the central lytic vacuole of plant cells, and mediate large poorly selective cation currents. This vacuolar conductance was originally named slow vacuolar (SV) channel, because of its slow activation time course [6, 7], and later related to the TPC1 protein [3]. Although calcium permeability has been demonstrated [8–10], the available evidence raises doubts on the involvement of plant TPCs in the generation of cytosolic calcium signals in response to a variety of environmental stimuli [4], but rather favor a role in cellular cation homeostasis [11, 12]. The activation of SV channels depends both on the membrane potential and on the cytosolic calcium concentration. These concepts are well established, yet on closer inspection contain open aspects. The SV voltage dependence shows pronounced outward rectification under most experimental conditions, i.e., the channel is active at positive voltages, but closed at physiological tonoplast potentials, which are assumed to be slightly negative. The calcium dependence of SV channels is conferred by two EF-hand motifs present in the central cytosolic loop connecting the two hydrophobic domains of plant TPC1 [13] (but not in mammalian homologues). Slow vacuolar channel activation appears to require unphysiologically high cytosolic calcium concentrations, although there is considerable variability of the calcium sensitivity between TPC channels from different plant species and cell types [13, 14]. Consequently, efforts were made in search for additional factors favoring SV channel activation and identified cytosolic magnesium [15, 16], reducing agents [17–19], the aminoglycoside neomycin [20] and polyunsaturated fatty acids [21].
Mammalian TPC channels have received increasing attention as important intracellular Ca2+ permeable channels, since Calcraft et al. [22] proposed TPC2 channels as receptors of nicotinic acid adenine dinucleotide phosphate (NAADP), one of the most powerful intracellular calcium-mobilizing second messengers [23]. Recently, however, NAADP photoaffinity labeling experiments strongly suggested that the NAADP binding site is not located on the TPC2 protein itself but rather on an accessory component [24, 25]. Several subsequent studies from different labs confirmed that TPC2 was a NAADP-gated calcium-permeable channel (reviewed in [26]). Its potassium permeability was, however, controversially discussed: Pitt et al. [27] determined a permeability ratio P Ca/P K of 2.6, while in the study of Schieder et al. [28] this ratio was >1,000. A possible sodium permeability of TPC channels was not considered in these studies.
By contrast, Wang et al. [29] presented substantial experimental evidence in support of TPC2 activation by the second messenger lipid phosphatidylinositol-(3,5)-bisphosphate (PI(3,5)P2) but not by NAADP. In addition, they showed that the channel is essentially sodium-selective with very low permeability for both potassium and calcium. The discovery of PI(3,5)P2 as an activator of mammalian TPC2 prompted us to test here if this molecule, which represents a low-abundance phosphoinositide also in plant cells [30, 31], modulates the activation of the plant TPC homologue.
Based on our previous results showing the targeting of murine TPC2 to the central vacuole of Arabidopsis protoplasts [32] and the usefulness of the plant vacuole as a heterologous system for the functional characterization of the lysosomal chloride transporter CLC-7 [33], we used mesophyll cells of Arabidopsis plants lacking endogenous TPC1 (tpc1–2) and giant yeast vacuoles, to test the ligand-sensitivity and ion selectivity of human TPC2. Our results provide evidence for a direct activation by the phosphoinositide PI(3,5)P2 and a high Na+:K+ permeability of hTPC2, while Arabidopsis TPC1 was not activated by this ligand. Our study demonstrates the suitability of yeast and plant vacuoles for the functional characterization of membrane transport proteins from the endo-lysosomal system of animal cells.
Materials and methods
Plant material and protoplast transformation
Plants of Arabidopsis thaliana Columbia-0 wild type and tpc1–2 [4] were grown on soil in a growth chamber at 22 °C and 8 h light/16 h dark regime. For protoplast expression, the hTPC2 coding sequence was subcloned into the plant expression vector pSAT6-EGFP-N1 [34]. Arabidopsis TPC1 in the Gateway vector p2GWF7 [13] was used to express TPC1-EGFP. Mesophyll protoplasts were isolated from tpc1–2 plants and transiently transformed using the polyethylene glycol method [35, 36]. Protoplasts were maintained at 23 °C in the dark. The transformation efficiency judged by EGFP fluorescence in protoplasts was routinely >80 %.
Yeast expression
For localization experiments, hTPC2-GFP(S65T) was subcloned into the yeast expression vector pKT10 [37]. For patch-clamp experiments, hTPC2 was subcloned into the yeast expression vector pDR197 [38].
Saccharomyces cerevisiae SH1007 cells (BJ5458 background [39]) were transformed with empty vector or with hTPC2-containing plasmids, and enlarged using the spheroplast incubation method, as described previously [39–41].
Patch-clamp recordings
Patch-clamp experiments on Arabidopsis vacuoles were performed ≥40 h after protoplast transformation, as described elsewhere [4, 10]. Two-pore channel-containing vacuoles were identified by EGFP fluorescence.
For experiments on hTPC2, the pipette (vacuolar side) solution contained (in mM): 200 NaCl, 2 MgCl2, 10 MES-Tris, pH 5.5. The standard bath (cytoplasmic side) solution contained (in mM): 100 NaCl, 2 MgCl2, 10 HEPES–Tris, pH 7.5. In the K+-based bath solution, NaCl was replaced with equimolar KCl. The osmolarity of the vacuolar and cytoplasmic solutions was adjusted to 550 and 600 mOsm, respectively, by the addition of d-sorbitol. In calcium permeability experiments, the pipette solution was (in mM): 50 CaCl2, 2 MgCl2, 10 HEPES–Tris, pH 7.5. The bath solution was identical to the pipette solution except that CaCl2 was substituted with 200 mM NaCl. The osmolarity of both solutions was adjusted to 600 mOsm by adding d-sorbitol.
For experiments on AtTPC1, the pipette solution contained (in mM): 100 K-gluconate, 2 MgCl2, 10 EGTA, 10 MES-Tris, pH 5.5. The bath solution contained 50 K-gluconate, 1 MgCl2, 1 or 0.01 CaCl2, 10 HEPES–Tris, pH 7.5. The osmolarity of the solutions was adjusted to 430 mOsm by the addition of d-sorbitol. Dithiothreitol (DTT; 2 mM) was added to the bath solution prior to the measurements. DTT was prepared as 1 M stock solution the day of the experiment and stored in ice. PI(3,5)P2 was purchased as dioctanyl ester (diC8) from AG Scientific or Echelon Biosciences Inc (USA). Other chemicals were purchased from Sigma-Aldrich (Italy, Germany) and Carl Roth (Germany). PI(3,5)P2 and NAADP were prepared as 1 mM stock solutions and stored at −20 °C.
For patch-clamp experiments on yeast vacuoles, giant yeast cells were treated with a hypotonic solution (100 mM KCl, 150 mM sorbitol, 10 mM Tris–MES, pH 7.5) to disrupt the plasma membrane and release the intact vacuole into the bath. The whole-vacuole configuration was achieved with a voltage pulse of 1.2 V for 20 ms. Recordings were done using a patch-clamp amplifier (CEZ2400, Nihon-Koden) and a digital data recorder (EX-RP10, Sony).
Data analysis
Positive currents correspond to anions flowing from the lumen of the vacuole to the cytoplasmic side or cations moving in the opposite direction. Steady-state current amplitudes were normalized to the vacuolar membrane capacitance. The reversal voltage of sodium was corrected for a calculated liquid-junction potential of 3.6 mV.
Unless otherwise indicated, data are reported as mean ± SEM. Data analysis and figure preparation were done with IgorPro software (Wavemetrics, Lake Oswego, OR, USA) or Photoshop (Adobe Inc., USA).
Results
The ligand-sensitivity of TPC proteins was tested following transient expression of TPC-EGFP fusion proteins in A. thaliana mesophyll protoplasts and yeast cells. In Arabidopsis, the TPC1 gene is the only representative of the TPC family. Expression of AtTPC1-EGFP in the TPC1 null background (tpc1–2) is able to fully restore the Ca2+- and voltage-dependent cation currents of the vacuolar membrane [13]. The AtTPC1-EGFP fusion was localized in the vacuolar membrane, indicated by the EGFP fluorescence excluding the chloroplasts (arrow in Fig. 1a). In the presence of saturating Ca2+-concentrations (1 mM) at the cytosolic side of the membrane, AtTPC1-EGFP mediated depolarization-activated whole-vacuolar K+ currents of up to 10 nA (Fig. 1b). Addition of 10 µM NAADP to the bath solution did not alter the current amplitudes within 3 min. The experiments were repeated in the presence of only 10 µM Ca2+, a concentration well below the half-maximal activation concentration [13], in order to test any stimulatory effect of NAADP. Under these low-Ca2+ conditions, in which TPC1 currents were largely reduced or macroscopic currents could not be resolved, NAADP did not induce a current increase (Fig. 1b), indicating that the voltage- and Ca2+-dependent channel characteristics were not modified by the second messenger. We further concluded that NAADP, under the conditions tested, did not activate other cation channel types in the vacuolar membrane of Arabidopsis mesophyll cells either.
Fig. 1.
PI(3,5)P2 and NAADP do not activate Arabidopsis TPC1 channels. a Vacuolar membrane localization of AtTPC1-EGFP in Arabidopsis mesophyll protoplasts. Left confocal fluorescence overlay image of the EGFP (green) and chlorophyll (red) signals; right bright-field image of the same cell. The arrow indicates the vacuolar membrane. Scale bar 5 μm. b AtTPC1 currents are not stimulated by NAADP. Whole-vacuolar currents in control conditions (black traces, left) and after addition of 10 µM NAADP to the cytosolic side of the membrane (red traces, middle). Currents were elicited by 1-s voltage pulses from −70 to +150 mV in 20-mV increments. Time course (right graph) of normalized current amplitudes at +150 mV (n = 5 for each condition) in response to NAADP application (t = 0 s), as determined in voltage ramps. AtTPC1 was either preactivated by 1 mM (upper row) or not fully activated in the presence of 10 µM (lower row). c AtTPC1 currents are not stimulated by PI(3,5)P2. Current traces, time course (n = 6 for each condition) and responses to voltage ramps as described in b. PI(3,5)P2 (10 µM) was applied at t = 0 s
The effect of the signaling lipid PI(3,5)P2 was investigated using a water-soluble dioctanyl ester (di-C8). Bath application of 1 µM (data not shown) or 10 µM PI(3,5)P2 did not change TPC1 current amplitudes within 3 min, neither in the presence of high (1 mM) or low (10 µM) Ca2+-concentrations in the bath solution (Fig. 1b). In some measurements, a time-dependent current reduction was observed, however independently of the lipid. In summary, our results showed that PI(3,5)P2 is not able to directly activate TPC1 in vacuoles of Arabidopsis mesophyll cells.
Similarly to AtTPC1, also human TPC2 fused to EGFP was targeted to the membrane of the large central vacuole, following transient expression in Arabidopsis tpc1–2 mesophyll protoplasts (Fig. 2a). In patch-clamp experiments on EGFP-decorated whole vacuoles, membrane currents increased strongly upon bath application of 100 nM PI(3,5)P2 (Fig. 2b, c). This current increase was larger at negative than at positive membrane potentials (Fig. 2c), pointing to the activation of a cationic conductance in our experimental conditions (NaClcyt 100 mM/NaClvac 200 mM). PI(3,5)P2-evoked currents (I P) were only observed in hTPC2-EGFP-containing vacuoles (n = 10), but not in untransformed control vacuoles (n = 9; Fig. 2d). The variability of the I P density likely reflects differences between individual protoplasts in the amount of hTPC2-EGFP targeted to the central vacuole. By contrast, bath application of 1 µM NAADP did not elicit an increase of the vacuolar membrane currents (n = 4; Fig. 2d).
Fig. 2.
PI(3,5)P2 but not NAADP activates hTPC2 in Arabidopsis vacuoles. a Vacuolar membrane localization of hTPC2-EGFP in Arabidopsis mesophyll protoplasts. Confocal fluorescence images of an isolated vacuole released from a hTPC2-EGFP-expressing protoplast. Left EGFP signal (green); middle chlorophyll signal (red); right merge. Scale bar 7 μm. b, c Time course of current amplitudes (b) recorded in a hTPC2-EGFP-containing vacuole in response to bath application of 100 nM PI(3,5)P2 (arrow). Time points indicated by numbers correspond to the current traces (c) in control conditions (black) and in the presence of PI(3,5)P2 (red). d Summary plot of current responses upon bath application of PI(3,5)P2 (330 nM) or NAADP (1 µM) in untransformed vacuoles (control) and hTPC2-EGFP-containing vacuoles (hTPC2). Current densities were calculated by normalizing the evoked current at −40 mV by the vacuolar membrane capacitance. The number of vacuoles is given in brackets. The average capacitance of plant vacuoles was 25 ± 4 pF
As an independent approach, we examined hTPC2 channels after expression in baker’s yeast. The hTPC2-GFP fusion was targeted to the vacuolar membrane of giant yeast cells (Fig. 3a). In patch-clamp experiments on whole giant vacuoles, addition of PI(3,5)P2 to the cytoplasmic side of the vacuole caused an increase of vacuolar membrane currents (Fig. 3b). No such PI(3,5)P2-evoked current activation was observed in vacuoles from giant yeast cells transformed with the empty vector (Fig. 3c). These results obtained in two independent expression systems concurrently showed that hTPC2 acts as a PI(3,5)P2-gated ion channel.
Fig. 3.
PI(3,5)P2 activates hTPC2 in giant yeast vacuoles. a Vacuolar membrane localization of hTPC2-GFP in baker’s yeast. Fluorescence microscopy images of a hTPC2-GFP(S65T)-expressing giant yeast cell. Left GFP signal (green); middle bright-field; right merge. VM vacuolar membrane. Scale bar 10 μm. b, c Patch-clamp recordings on giant vacuoles isolated from yeast cells transformed with empty pDR197 vector (b) or pDR197-hTPC2 (c). Current densities versus voltage relationships of vacuolar membrane currents (n = 3 for each condition) in control conditions and in the presence of 1 μM PI(3,5)P2 were determined by application of 7-s voltage ramps from −80 to +80 mV. Error bars represent standard deviation. The average capacitance of yeast vacuoles was 13 ± 1 pF
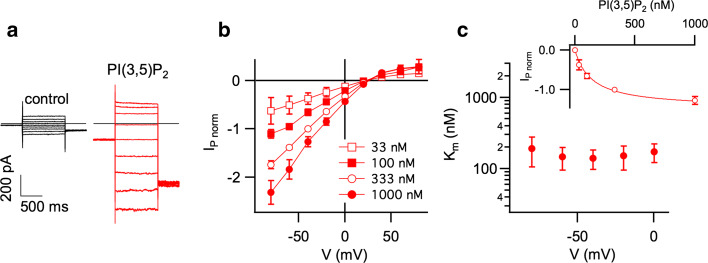
We next investigated the voltage- and dose-dependence of IP in hTPC2-EGFP-containing Arabidopsis vacuoles. In the presence of 100 nM PI(3,5)P2, application of 1-s voltage steps in the range from +80 to −80 mV elicited instantaneously activating current responses lacking any time-dependent inactivation (Fig. 4a). These currents increased in a [PI(3,5)P2]-dependent manner (Fig. 4b). Fitting the dose-dependent IP activation at a given membrane potential with a Michaelis–Menten function (inset of Fig. 4c), we determined a constant of half-maximal I P activation (K m) of about 160 nM (Fig. 4c). K m values did not vary in the voltage range between 0 and –80 mV, indicating that the PI(3,5)P2 binding site on the TPC2 protein is located outside the electrical field.
Fig. 4.
PI(3,5)P2 interaction with hTPC2 in Arabidopsis vacuoles is voltage-independent. a Whole-vacuolar current recordings in control conditions (black traces; left) and in the presence of 100 nM PI(3,5)P2 in the bath solution (red traces; right), elicited by 1-s voltage pulses from +80 to −80 mV in 20-mV decrements. b Current–voltage relationships of PI(3,5)P2-evoked hTPC2 currents (I P) as shown in a. For each vacuole, current amplitudes determined at different [PI(3,5)P2] were normalized to the value at −40 mV in the presence of 330 nM PI(3,5)P2. The number (n) of vacuoles is given in brackets. c Dose–response analysis of PI(3,5)P2-evoked inward currents. I P amplitudes recorded at a given membrane potential were fitted with a Michaelis–Menten function (inset: V = −40 mV). The constant of half-maximal activation (K m) is plotted against the membrane potential in the range from −80 to 0 mV
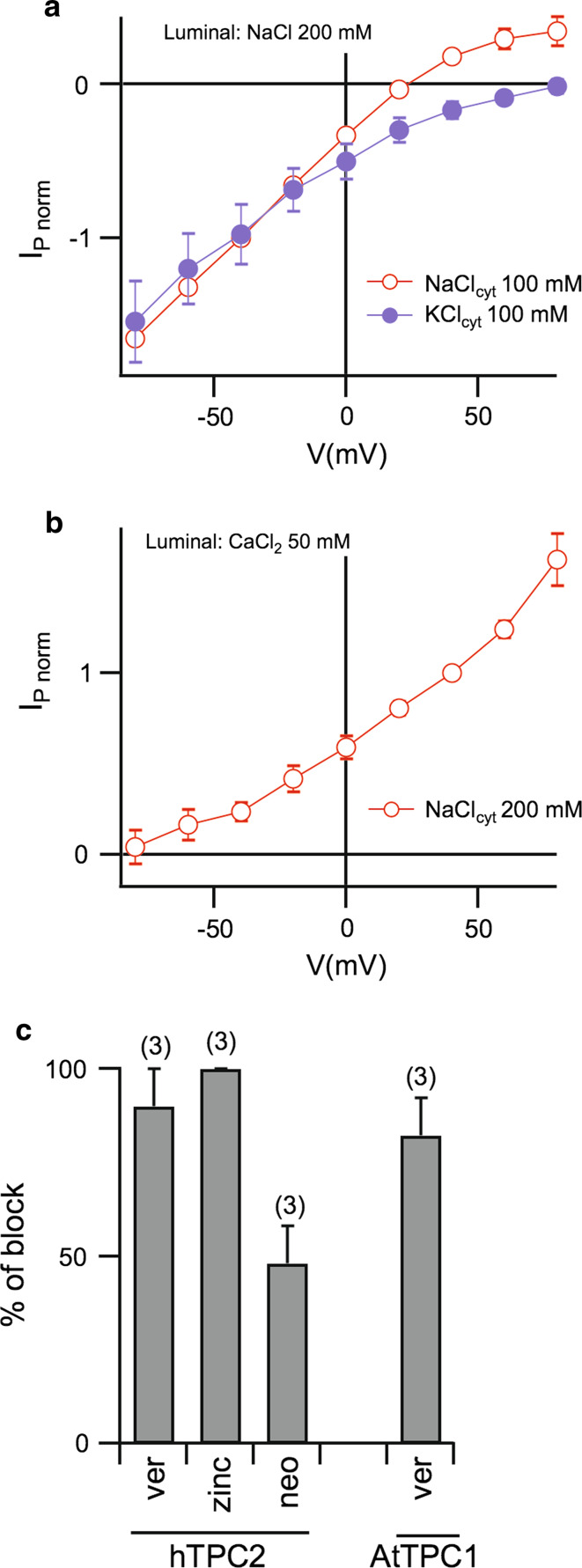
The reversal potential of I P was close to the theoretical Nernst potential for Na+ in NaClcyt 100 mM/NaClvac 200 mM (ENa = +17.5 mV) at all PI(3,5)P2 concentrations (Fig. 4b), but far from the Nernst potential for Cl− (ECl = −17.5 mV), indicating that hTPC2 is selective for cations. Since the cation permeability of mammalian TPC2, in particular for potassium and calcium, is a matter of debate [26], we conducted experiments in which cytosolic NaCl was substituted by equimolar KCl (Fig. 5a): outwardly directed I P (i.e., cations moving from the cytosol to the vacuolar lumen) disappeared in the presence of cytosolic KCl, leading to a shift of the reversal potential from 20 ± 2 mV (n = 7) to values larger than +80 mV (n = 4). We then investigated the calcium permeability in hTPC2 channels using a calcium-based (50 mM) vacuolar solution in order to favor the movement of Ca2+ from the luminal to the cytosolic side. As shown in Fig. 5b, no inwardly directed currents could be recorded in the presence of 200 mM cytosolic sodium, indicating that in this experimental condition PNa ≫ PCa. These data strongly suggest that TPC2 channels are mainly permeable to Na+, in agreement with the results obtained by Wang et al. [29].
Fig. 5.
PI(3,5)P2-evoked hTPC2 currents in Arabidopsis vacuoles are sodium-selective and sensitive to blockers of plant TPC channels. a Current–voltage relationships of PI(3,5)P2-evoked hTPC2 currents (I P) in the presence of 100 mM NaCl (open circles, n = 7) or 100 mM KCl (closed circles, n = 4) in the bath solution. For each vacuole, current amplitudes evoked by 330 nM PI(3,5)P2 were normalized to the value at −40 mV in the NaCl-condition. b Current–voltage relationships of PI(3,5)P2-evoked hTPC2 currents (I P; n = 3) in the presence of 200 mM NaCl in the bath and 50 mM CaCl2 in the pipette solution. For each vacuole, current amplitudes evoked by 330 nM PI(3,5)P2 were normalized to the value at +40 mV. c Sensitivity of hTPC2 to verapamil (200 µM; ver), Zn2+ (500 µM; zinc) and neomycin (200 µM; neo) and of AtTPC1 to verapamil (200 µM; ver). hTPC2 currents evoked by 330 nM PI(3,5)P2 at −40 mV; AtTPC1 currents activated by a voltage step to +60 mV in bath and pipette solutions containing 100 and 200 mM KCl, respectively. Error bars represent SD. The number of vacuoles is given in brackets
Zinc ions (Zn2+) and the aminoglycoside antibiotic neomycin are well-known blockers of plant TPC/SV channels [7, 11]. We found that I P was inhibited completely by 500 μM Zn2+ and less efficiently by 200 μM neomycin in the bath solution (Fig. 5c). Moreover, human TPC2 in the plant vacuole was also sensitive to verapamil (200 µM; Fig. 5c), confirming results obtained in patch clamp recordings from mammalian endolysosomes [29]. Similarly, AtTPC1-mediated outward currents were abolished upon addition of 200 μM verapamil (Fig. 5c), revealing a similar pharmacology of two-pore channels from the two kingdoms.
Discussion
A major question arising from previous studies on mammalian TPC channels in endolysosomes or in artificial bilayers was whether mammalian TPC channels are directly activated by the signaling lipid PI(3,5)P2 or by the cytosolic second messenger NAADP, or both. Another question relates to the degree of conservation in the activation mechanism, ion selectivity, and pharmacology between animal and plant two-pore channels.
Here, we answered these questions by functional expression of the human TPC2 channel in plant and yeast vacuoles. As a prerequisite, the localization of hTPC2 in the vacuolar membrane was shown. In contrast to AtTPC1-GFP, a major fraction of hTPC2-EGFP fusion proteins was detected in other membranes of the secretory pathway in Arabidopsis protoplasts, pointing to a less efficient trafficking process for the latter. This may be specific to the human homologue, since TPC2 from mouse efficiently targeted to the vacuole [32]. However, hTPC2 proteins inserted into the vacuolar membrane were functional.
Human TPC2 activation was observed upon application of PI(3,5)P2 from 30 nM up to 1 μM in Arabidopsis vacuoles lacking endogenous TPC proteins. In contrast, no change in currents was recorded when vacuoles were challenged by 1 μM cytosolic NAADP. Similar results were obtained when hTPC2 was expressed in baker’s yeast, which does not possess members of the TPC family [11, 42, 43]. Our results are in full agreement with those obtained by Wang et al. [29] and, considering the immediate response upon PI(3,5)P2 application, strongly suggest a direct activation mechanism. While we ruled out the possibility that NAADP serves as an activating ligand of mammalian TPC2, it may act via an additional modulator not present in plant and yeast cells; such a NAADP-binding regulatory factor has yet to be identified (see [26] and references therein).
The activation mechanism does not seem to be conserved between mammalian and plant TPC proteins. AtTPC1 is activated upon Ca2+ binding to the second of two EF-hand motifs in the central cytosolic loop [13] and has low open probability at resting membrane potentials, while channel opening is favored by membrane depolarization. Here, we showed that NAADP and PI(3,5)P2 do not act as additional activators of AtTPC1. Since PI(3,5)P2 binding to hTPC2 possibly occurs outside the electrical field, it is tempting to speculate that the ligand binding function of the central cytosolic linker domain has diverged in the different phyla.
In addition to the striking differences in the activation mechanism between plant and animal TPC channels, their permeation pathways also differ strongly. Plant TPC1 channels can be classified as non-selective cation channels, in which potassium and sodium pass the channel pore equally well [9, 44, 45]. Here, as previously suggested by Wang et al. [29], we verified that human TPC2 channels are permeable to sodium but not to potassium. Different experimental approaches have demonstrated that plant TPC1 channels are permeable to calcium (see [10] and references therein). However, attempts to directly measure TPC1-mediated vacuolar calcium release failed, therefore raising doubts on the physiological relevance of its calcium permeability [11]. Human TPC2 channels have been proposed as a route for NAADP-gated calcium release from the lysosome to the cytosol. Our measurements, performed in conditions largely favoring calcium mobilization from the luminal to the cytosolic side, did not reveal any Ca2+ currents mediated by hTPC2 channels, in agreement with previous results obtained by Wang et al. [29]. In the light of these data, the role of hTPC2 in cytosolic calcium signaling needs to be re-evaluated.
Interestingly, however, our data indicate that plant and animal TPC channels share similarities in their pharmacology. Verapamil, zinc, and the antibiotic neomycin inhibit both plant TPC1 and human TPC2 channels. We can thus speculate that some structural features, responsible for the common blocking effects, are still conserved between plant and animal TPC channels.
In this study, we demonstrated that the vacuole of plant and yeast cells provide useful systems for the characterization of intracellular transport proteins, which will help elucidating the structural and functional diversification of endolysosomal TPC proteins.
Acknowledgments
We thank Anna Moroni (University of Milan) for critical comments on the manuscript. The technical assistance of Francesca Quartino, Alessandro Barbin, Damiano Magliozzi (IBF-CNR, Italy) was highly appreciated. ACa was supported by the Italian “Progetti di Ricerca di Interesse Nazionale” (PRIN2010CSJX4F) and by Compagnia di San Paolo Research Foundation (ROL 291) and PD by the “Deutsche Forschungsgemeinschaft” (FOR964).
Abbreviations
- EGFP
Enhanced green fluorescent protein
- EGTA
Ethylene glycol tetraacetic acid
- NAADP
Nicotinic acid adenine dinucleotide phosphate
- PI(3,5)P2
Phosphatidylinositol-(3,5)-bisphosphate
- TPC
Two-pore channel
- VM
Vacuolar membrane
Footnotes
A. Boccaccio, J. Scholz-Starke, and S. Hamamoto contributed equally to this work.
References
- 1.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57(4):387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 2.Rietdorf K, Funnell TM, Ruas M, Heinemann J, Parrington J, Galione A. Two-pore channels form homo- and heterodimers. J Biol Chem. 2011;286(43):37058–37062. doi: 10.1074/jbc.C111.289835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434(7031):404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 4.Ranf S, Wunnenberg P, Lee J, Becker D, Dunkel M, Hedrich R, Scheel D, Dietrich P. Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J. 2008;53(2):287–299. doi: 10.1111/j.1365-313X.2007.03342.x. [DOI] [PubMed] [Google Scholar]
- 5.Dadacz-Narloch B, Beyhl D, Larisch C, Lopez-Sanjurjo EJ, Reski R, Kuchitsu K, Muller TD, Becker D, Schonknecht G, Hedrich R. A novel calcium binding site in the slow vacuolar cation channel TPC1 senses luminal calcium levels. Plant Cell. 2011;23(7):2696–2707. doi: 10.1105/tpc.111.086751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedrich R, Neher E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature. 1987;329:833–835. doi: 10.1038/329833a0. [DOI] [Google Scholar]
- 7.Gambale F, Cantu AM, Carpaneto A, Keller BU. Fast and slow activation of voltage-dependent ion channels in radish vacuoles. Biophys J. 1993;65(5):1837–1843. doi: 10.1016/S0006-3495(93)81241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen GJ, Sanders D. Control of ionic currents in guard cell vacuoles by cytosolic and luminal calcium. Plant J. 1996;10:1055–1069. doi: 10.1046/j.1365-313X.1996.10061055.x. [DOI] [PubMed] [Google Scholar]
- 9.Pottosin II, Dobrovinskaya OR, Muniz J. Conduction of monovalent and divalent cations in the slow vacuolar channel. J Membrane Biol. 2001;181:55–65. doi: 10.1007/s0023200100073. [DOI] [PubMed] [Google Scholar]
- 10.Gradogna A, Scholz-Starke J, Gutla PV, Carpaneto A. Fluorescence combined with excised patch: measuring calcium currents in plant cation channels. Plant J. 2009;58:175–182. doi: 10.1111/j.1365-313X.2008.03762.x. [DOI] [PubMed] [Google Scholar]
- 11.Hedrich R, Marten I. TPC1-SV channels gain shape. Mol Plant. 2011;4(3):428–441. doi: 10.1093/mp/ssr017. [DOI] [PubMed] [Google Scholar]
- 12.Hedrich R. Ion channels in plants. Physiol Rev. 2012;92(4):1777–1811. doi: 10.1152/physrev.00038.2011. [DOI] [PubMed] [Google Scholar]
- 13.Schulze C, Sticht H, Meyerhoff P, Dietrich P. Differential contribution of EF-hands to the Ca2+-dependent activation in the plant two-pore channel TPC1. Plant J. 2011;68(3):424–432. doi: 10.1111/j.1365-313X.2011.04697.x. [DOI] [PubMed] [Google Scholar]
- 14.Rienmuller F, Beyhl D, Lautner S, Fromm J, Al-Rasheid KA, Ache P, Farmer EE, Marten I, Hedrich R. Guard cell-specific calcium sensitivity of high density and activity SV/TPC1 channels. Plant Cell Physiol. 2010;51(9):1548–1554. doi: 10.1093/pcp/pcq102. [DOI] [PubMed] [Google Scholar]
- 15.Pei ZM, Ward JM, Schroeder JI. Magnesium sensitizes slow vacuolar channels to physiological cytosolic calcium and inhibits fast vacuolar channels in fava bean guard cell vacuoles. Plant Physiol. 1999;121(3):977–986. doi: 10.1104/pp.121.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpaneto A, Cantù AM, Gambale F. Effects of cytoplasmic Mg2+ on slowly activating channels in isolated vacuoles of Beta vulgaris . Planta. 2001;213:457–468. doi: 10.1007/s004250100519. [DOI] [PubMed] [Google Scholar]
- 17.Carpaneto A, Cantu AM, Gambale F. Redox agents regulate ion channel activity in vacuoles from higher plant cells. FEBS Lett. 1999;442(2–3):129–132. doi: 10.1016/S0014-5793(98)01642-1. [DOI] [PubMed] [Google Scholar]
- 18.Scholz-Starke J, De Angeli A, Ferraretto C, Paluzzi S, Gambale F, Carpaneto A. Redox-dependent modulation of the carrot SV channel by cytosolic pH. FEBS Lett. 2004;576(3):449–454. doi: 10.1016/j.febslet.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 19.Scholz-Starke J, Gambale F, Carpaneto A. Modulation of plant ion channels by oxidizing and reducing agents. Arch Biochem Biophys. 2005;434(1):43–50. doi: 10.1016/j.abb.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Scholz-Starke J, Carpaneto A, Gambale F. On the interaction of neomycin with the slow vacuolar channel of Arabidopsis thaliana . J Gen Physiol. 2006;127(3):329–340. doi: 10.1085/jgp.200509402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutla PVK, Boccaccio A, De Angeli A, Gambale F, Carpaneto A. Modulation of plant TPC channels by polyunsaturated fatty acids. J Exp Bot. 2012;63(17):6187–6197. doi: 10.1093/jxb/ers272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calcraft PJ, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459(7246):596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guse AH, Lee HC. NAADP: a universal Ca2+ trigger. Sci Signal. 2008;1(44):re10. doi: 10.1126/scisignal.144re10. [DOI] [PubMed] [Google Scholar]
- 24.Walseth TF, Lin-Moshier Y, Jain P, Ruas M, Parrington J, Galione A, Marchant JS, Slama JT. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J Biol Chem. 2012;287(4):2308–2315. doi: 10.1074/jbc.M111.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J Biol Chem. 2012;287(4):2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan AJ, Galione A. Two-pore channels (TPCs): current controversies. BioEssays. 2013;36(2):173–183. doi: 10.1002/bies.201300118. [DOI] [PubMed] [Google Scholar]
- 27.Pitt SJ, Funnell TM, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, Parrington J, Galione A, Sitsapesan R. TPC2 is a novel NAADP-sensitive Ca2+release channel, operating as a dual sensor of luminal pH and Ca2+ . J Biol Chem. 2010;285(45):35039–35046. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schieder M, Rotzer K, Bruggemann A, Biel M, Wahl-Schott CA. Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+currents in isolated lysosomes. J Biol Chem. 2010;285(28):21219–21222. doi: 10.1074/jbc.C110.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151(2):372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijer HJ, Munnik T. Phospholipid-based signaling in plants. Annu Rev Plant Biol. 2003;54:265–306. doi: 10.1146/annurev.arplant.54.031902.134748. [DOI] [PubMed] [Google Scholar]
- 31.Heilmann I. Using genetic tools to understand plant phosphoinositide signalling. Trends Plant Sci. 2009;14(3):171–179. doi: 10.1016/j.tplants.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Larisch N, Schulze C, Galione A, Dietrich P. An N-terminal dileucine motif directs two-pore channels to the tonoplast of plant cells. Traffic. 2012;13(7):1012–1022. doi: 10.1111/j.1600-0854.2012.01366.x. [DOI] [PubMed] [Google Scholar]
- 33.Costa A, Gutla PV, Boccaccio A, Scholz-Starke J, Festa M, Basso B, Zanardi I, Pusch M, Schiavo FL, Gambale F, Carpaneto A. The Arabidopsis central vacuole as an expression system for intracellular transporters: functional characterization of the Cl−/H+exchanger CLC-7. J Physiol. 2012;590(Pt 15):3421–3430. doi: 10.1113/jphysiol.2012.230227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzfira T, Tian GW, Lacroix B, Vyas S, Li J, Leitner-Dagan Y, Krichevsky A, Taylor T, Vainstein A, Citovsky V. pSAT vectors: a modular series of plasmids for autofluorescent protein tagging and expression of multiple genes in plants. Plant Mol Biol. 2005;57(4):503–516. doi: 10.1007/s11103-005-0340-5. [DOI] [PubMed] [Google Scholar]
- 35.Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2(7):1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- 36.Abel S, Theologis A. Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. Plant J. 1994;5(3):421–427. doi: 10.1111/j.1365-313X.1994.00421.x. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka K, Nakafuku M, Tamanoi F, Kaziro Y, Matsumoto K, Toh-e A. IRA2, a second gene of Saccharomyces cerevisiae that encodes a protein with a domain homologous to mammalian ras GTPase-activating protein. Mol Cell Biol. 1990;10(8):4303–4313. doi: 10.1128/mcb.10.8.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rentsch D, Laloi M, Rouhara I, Schmelzer E, Delrot S, Frommer WB. NTR1 encodes a high affinity oligopeptide transporter in Arabidopsis. FEBS Lett. 1995;370(3):264–268. doi: 10.1016/0014-5793(95)00853-2. [DOI] [PubMed] [Google Scholar]
- 39.Hamamoto S, Marui J, Matsuoka K, Higashi K, Igarashi K, Nakagawa T, Kuroda T, Mori Y, Murata Y, Nakanishi Y, Maeshima M, Yabe I, Uozumi N. Characterization of a tobacco TPK-type K+ channel as a novel tonoplast K+ channel using yeast tonoplasts. J Biol Chem. 2008;283(4):1911–1920. doi: 10.1074/jbc.M708213200. [DOI] [PubMed] [Google Scholar]
- 40.Yabe I, Horiuchi K, Nakahara K, Hiyama T, Yamanaka T, Wang PC, Toda K, Hirata A, Ohsumi Y, Hirata R, Anraku Y, Kusaka I. Patch clamp studies on V-type ATPase of vacuolar membrane of haploid Saccharomyces cerevisiae: preparation and utilization of a giant cell containing a giant vacuole. J Biol Chem. 1999;274(49):34903–34910. doi: 10.1074/jbc.274.49.34903. [DOI] [PubMed] [Google Scholar]
- 41.Nakanishi Y, Yabe I, Maeshima M. Patch clamp analysis of a H+ pump heterologously expressed in giant yeast vacuoles. J Biochem. 2003;134(4):615–623. doi: 10.1093/jb/mvg184. [DOI] [PubMed] [Google Scholar]
- 42.Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186(2):201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu MX, Ma J, Parrington J, Galione A, Evans AM. TPCs: endolysosomal channels for Ca2+ mobilization from acidic organelles triggered by NAADP. FEBS Lett. 2010;584(10):1966–1974. doi: 10.1016/j.febslet.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carpaneto A, Cantu AM, Busch H, Gambale F. Ion channels in the vacuoles of the seagrass Posidonia oceanica . FEBS Lett. 1997;412(1):236–240. doi: 10.1016/S0014-5793(97)00786-2. [DOI] [PubMed] [Google Scholar]
- 45.Ivashikina N, Hedrich R. K+ currents through SV-type vacuolar channels are sensitive to elevated luminal sodium levels. Plant J. 2005;41(4):606–614. doi: 10.1111/j.1365-313X.2004.02324.x. [DOI] [PubMed] [Google Scholar]