Abstract
The purpose of this study was to evaluate the cationic trypsinogen gene in miniature schnauzers for possible mutations. Genetic mutations have been linked with hereditary pancreatitis in humans. Four miniature schnauzers were selected on the basis of a clinical history of pancreatitis. One healthy miniature schnauzer and 1 healthy mixed breed canine were enrolled as controls. DNA was extracted from these canines using a commercial kit. Primers were designed to amplify the entire canine cationic trypsinogen cDNA sequence. A polymerase chain reaction (PCR) was performed and products were purified and sequenced. All sequences were then compared. The healthy control canine, a healthy miniature schnauzer, and the 4 miniature schnauzers with pancreatitis showed identical sequences of the cationic trypsinogen gene to the published sequence. We conclude that, in contrast to humans with hereditary pancreatitis, mutations of the cationic trypsinogen gene do not play a major role in the genesis of pancreatitis in the miniature schnauzer.
Résumé
L’objectif de cette étude était d’évaluer la présence possible de mutations dans le gène du trypsinogène cationique chez des chiens de race Schnauzer miniature étant donné que chez l’humain, des mutations génétiques ont été reliées à une pancréatite héréditaire. Quatre chiens de race Schnauzer miniature ont été sélectionnés sur la base d’une histoire de pancréatite clinique. Un chien de race Schnauzer miniature en santé et un chien de race mixte en santé ont été utilisés comme témoins. De l’ADN a été extrait de ces chiens à l’aide d’une trousse commerciale. Des amorces ont été préparées afin d’amplifier la séquence entière de l’ADNc du trypsinogène cationique canin. Une réaction d’amplification en chaîne par la polymérase (PCR) a été effectuée et les produits de la réaction purifiés et séquencés. Chez le chien témoin de race Schnauzer miniature en santé ainsi que chez les 4 chiens de race Schnauzer miniature avec pancréatite, les séquences du gène du trypsinogène cationique étaient identiques à la séquence publiée. En conclusion, contrairement aux humains souffrant de pancréatite héréditaire, des mutations dans le gène du trypsinogène cationique ne jouent pas un rôle majeur dans l’apparition de pancréatite chez le chien de race Schnauzer miniature.
(Traduit par Docteur Serge Messier)
Clinically, there appears to be a high incidence of pancreatitis in the miniature schnauzer (1,2). In humans, a similar clustering of pancreatitis in certain families has been described as hereditary pancreatitis. Hereditary pancreatitis in humans is an autosomal dominant disorder (3–5). Two major, as well as a few minor, missense mutations of the cationic trypsinogen gene (PRSS1) have been linked to hereditary pancreatitis in humans (6–13). The 1st mutation, R117H (also described as R122H), leads to the replacement of a single amino acid residue in position 117. The original arginine is replaced with histidine in this position leading to synthesis of a structurally altered trypsin. This substitution eliminates an initial hydrolysis site, thus yielding trypsinogen, trypsin, or both, that are resistant to degradation or autolysis (13). The 2nd major mutation that has been identified is a substitution of isoleucine with asparagine in position 21, N21I (4,14). In humans, this mutation results in a conformational change that brings E24 closer to R117 leading to the formation of a salt bridge. The salt bridge then leads to the formation of a phenotype that is functionally similar to the R117 mutation. The result of these so called “gain of function” mutations is a structural change at position 117 that makes the trypsinogen, trypsin, or both molecules resistant to degradation or autolysis (Figure 1) (6,13). While the delay in degradation of trypsinogen has no direct detrimental effect, the delay in autolysis of trypsin can lead to autodigestion of pancreatic tissue and pancreatitis.
Figure 1.
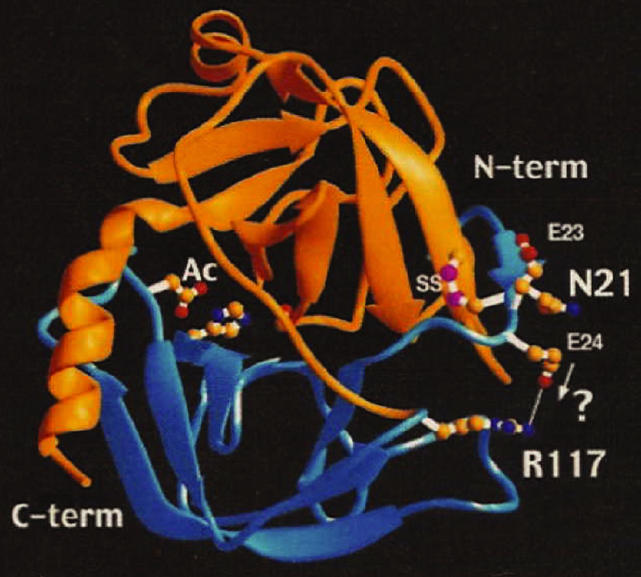
Crystallographic structure of human cationic trypsin. This figure shows the crystallographic 2 domain structure of human cationic trypsin. The structural location of N21, E24, and R117 are also shown. The N21I mutation may bring E24 closer to R117, leading to the formation of a salt bridge and a conformational change rendering the altered structure non-accessible for degradation and autolysis (13).
Pancreatitis in canines is common, but the exact etiology remains unknown in most cases (1). Potential causes and risk factors are thought to include dietary indiscretion, hypercalcemia, blunt trauma, surgical trauma, hypotension, hyperlipidemia, iatrogenic by the use of L-asparaginase, thiazides, potassium bromide, and other pharmaceutical agents (1). Some have also proposed glucocorticoid administration as a risk factor for pancreatitis in canines, but there is little evidence to suggest a cause and effect relationship. Also, glucocorticoids have recently been removed from the list of drugs and drug classes that are implicated in causing pancreatitis in humans. Infectious agents, such as canine parvovirus, may also cause pancreatitis but evidence for a cause and effect relationship is rather weak. Finally, other causes may include type III hypersensitivity reactions and genetic predisposition.
Vomiting and abdominal pain are the key clinical signs observed in dogs with pancreatitis (1). Other clinical signs include fever, tachycardia, dehydration, jaundice, hypovolemic shock, and abdominal effusion. Animals may demonstrate high serum amylase and lipase activities, however, neither one of these tests are either sensitive or specific for canine pancreatitis. Some canines have also been found to have high serum trypsin-like immunoreactivity concentrations (cTLI) (1). Recently, a new diagnostic test, canine pancreatic lipase immunoreactivity (cPLI), has been developed and validated, and initial studies would suggest that this new assay is the most sensitive diagnostic test for canine pancreatitis. With chronic pancreatitis, the pancreas undergoes progressive fibrosis, ultimately leading to a reduction of functional acinar mass leading to, in some cases, exocrine pancreatic insufficiency (EPI) (1,2).
Even though the specific cause of pancreatitis in canines is often unknown, the pathophysiologic cascade is believed to be similar in most cases. Different insults to the exocrine pancreas lead to a decrease in exocrine pancreatic secretion, followed by the formation of giant vacuoles that are the fusion product of lysosomes and zymogen granules (15). Trypsinogen is prematurely activated in those giant vacuoles and in turn leads to the activation of more trypsinogen but also to activation of other pancreatic enzymes such as elastase and phospholipase (16). While the premature activation, release, or both, are responsible for initiating pancreatitis, the progression of the disease is now believed to be mainly due to the release of inflammatory cytokines leading to systemic effects, such as distant organ failure.
The exocrine pancreas has several protective mechanisms against autodigestion and resulting pancreatitis. Most pancreatic enzymes, especially proteolytic and phospholipolytic enzymes are synthesized as inactive proenzymes, also known as zymogens. Trypsinogen activation does not take place until the trypsinogen reaches the duodenum. In addition, even though acinar cells produce lysosomal enzymes, which are capable of activating zymogens, these are strictly segregated from zymogens early during posttranslational modification, thereby preventing premature activation of the zymogens (17). Also, a pancreatic secretory trypsin inhibitor, which is synthesized together with trypsinogen, rapidly inhibits any trace amount of trypsin that is generated within the pancreatic acinar cells. In addition, prematurely activated trypsin is able to autodigest itself containing the premature activation when only few molecules have been activated. When all of these protective mechanisms are overwhelmed, pancreatitis can then develop. Minute quantities of trypsin, which reach the circulatory system during the development of pancreatitis, are reversibly inhibited by α1 -proteinase inhibitor and are then transferred to α2-macroglobulin. Alternatively, trypsin molecules may be bound by α2-macroglobulin directly. These large trypsin α2-macroglobulin complexes are rapidly removed by the reticuloendothelial system, rendering them harmless (1).
Since pancreatitis in miniature schnauzers appears to be inherited, and one of the most important causes of hereditary pancreatitis in humans has been shown to be mutations of the cationic trypsinogen gene, the goal of this project was to investigate whether the same or similar mutations of the cationic trypsinogen gene also occur in miniature schnauzers with pancreatitis.
Questionnaires were sent to ACVIM diplomats requesting case information for miniature schnauzers with a history of pancreatitis. Four miniature schnauzers were identified and enrolled into this study on the basis of their clinical record reporting either: 1) a pancreatic biopsy showing pancreatic inflammation; 2) the presence of clinical signs compatible with pancreatitis (such as vomiting, abdominal pain, or both) in conjunction with a 5-fold increase in serum lipase activity; or 3) the presence of clinical signs compatible with pancreatitis and ultrasonographic evidence of pancreatitis (pancreatic edema, ascites in the area of the pancreas, and changes in the echogenicity of the pancreas). In addition, a healthy miniature schnauzer and a healthy mixed breed canine were selected for comparison.
All owners were asked to sign an informed consent form and whole blood samples were obtained from all of the canines. The blood was placed into 10 mL ethylenediaminetetraacetic acid (EDTA) vacutainer tubes (BD Vacutainer; Preanalytical Solutions, Franklin Lakes, New Jersey, USA) and shipped at room temperature for next day delivery. Upon arrival at the laboratory, the DNA was immediately extracted from the samples using a commercially available kit (Purgene DNA Purification Kit; Gentra Systems, Minneapolis, Minnesota, USA). Briefly, erythrocytes were lysed and the remaining white cells were washed. Then the white blood cells were also lysed and proteins precipitated from the remaining solution. The DNA was then washed with 70% ethanol (Sigma Chemical; St Louis, Missouri, USA), centrifuged, and allowed to dry overnight. The DNA was resuspended the next day using a hydration solution (Purgene DNA Purification Kit; Gentra Systems). The DNA was evaluated for purity and quantity by spectrophotometry. Oligonucleotide primers (Figure 2) were designed using a commercially available primer design software (Oligo, version 5.1; Molecular Biology Insights, Cascade, Colorado, USA) and were synthesized using the PerSeptive Biosystems Expedite Moss Synthesis System (Expedite Moss Synthesis System; PerSeptive Biosystems, Framingham, Massachusetts, USA) on a 50 nm scale. Five sets of primers were designed to amplify each exon of the cationic trypsinogen gene so that the entire cDNA sequence could be evaluated.
Figure 2.

Primers for canine cationic trypsinogen. Design Software: Oligo, version 5.1, Synthesis Instrument: McFly (II Column Id) Protocol: Moss 0.5 μmole HV-Memsyn.
T = thymine, A = adenosine; G = guanine, C = cytosine
A polymerase chain reaction (PCR) was performed and optimized on all of the samples and each product was purified from contaminants using a commercially available kit (Promega Wizard PCR Preps DNA Purification System; Promega Corporation, Madison, Wisconsin, USA). The samples were then sequenced on an automated sequencer (Perkin Elmer Applied Biosystems model 337 XL; Perkin Elmer, Wellesley, Massachusetts, USA).
The sequence of the cationic trypsinogen gene from the healthy and diseased miniature schnauzers was compared with that of the healthy mixed breed canine and the published sequence for canine cationic trypsinogen (NCBI Accession M11590).
Examination of the potential mutation site at residue 117 revealed no mutations in either the healthy miniature schnauzer or in any of the 4 miniature schnauzers with pancreatitis (Figure 3). In the canine, amino acid residue 21 of cationic trypsinogen is a threonine (T). This is in contrast to humans where residue 21 is an isoleucine and in the mutation it is replaced with an asparagine (N) residue (18). However, this difference was observed in all miniature schnauzers, as well as in the healthy mixed breed canine (Figure 4). The entire sequence of cationic trypsinogen in the healthy mixed breed canine was identical to the one published previously (18). Additionally, the entire sequence for cationic trypsinogen determined in the healthy miniature schnauzer was identical to the one determined in the healthy mixed breed canine. Finally, the entire sequence for the cationic trypsinogen in all 4 miniature schnauzers with pancreatitis was identical to the sequence identified in the healthy miniature schnauzer and the healthy mixed breed canine.
Figure 3.
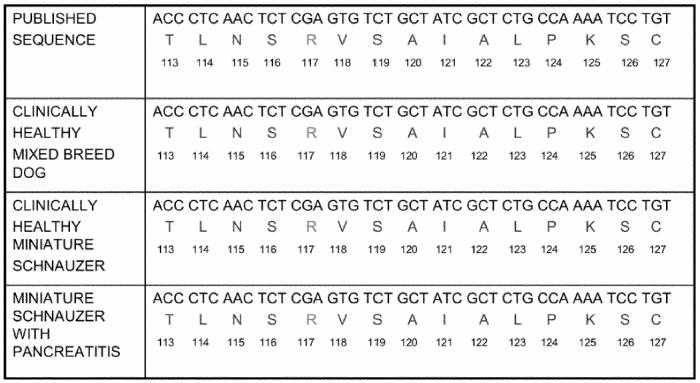
cDNA sequence encoding an area of canine cationic trypsinogen around amino acid in position 117. This figure shows the cDNA sequence encoding an area of canine cationic trypsinogen around the amino acid residue in position 117. The corresponding amino acid sequence is shown as well. Both cDNA and amino acid sequence are shown for the previously published sequence, a healthy mixed breed canine, a healthy miniature schnauzer, and 4 miniature schnauzers with recurrent pancreatitis.
Code for nucleosides: A = adenosine, C = cytidine, T = thymidine, G = guanosine
1-letter code for amino acids: T = threonine, L = leucine, N = asparagine, S = serine, R = arginine, V = valine, A = alanine, I = isoleucine, P = proline, K = lysine, C = cysteine
Figure 4.
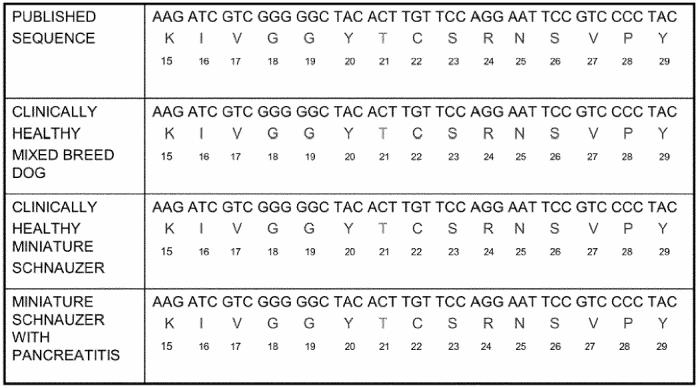
cDNA sequence encoding an area of canine cationic trypsinogen around amino acid in position 21. This figure shows the cDNA sequence encoding an area of canine cationic trypsinogen around the amino acid residue in position 21. The corresponding amino acid sequence is shown as well. Both cDNA and amino acid sequence are shown for the previously published sequence, a healthy mixed breed canine, a healthy miniature schnauzer, and 4 miniature schnauzers with recurrent pancreatitis.
Code for nucleosides: A = adenosine, C = cytidine, T = thymidine, G = guanosine
1-letter code for amino acids: K = lysine, I = isoleucine, V = valine, G = glycine, Y = tyrosine, T = threonine, C = cysteine, S = serine, R = arginine, N = asparagine, P = proline
The purpose of this project was to discover any possible mutations in the cationic trypsinogen gene in order to determine if such a putative mutation may be the cause for pancreatitis in the miniature schnauzer. No mutation was identified in the area most commonly affected in human beings with hereditary pancreatitis; the area around amino acid residue 117 of human trypsinogen gene. When compared to human cationic trypsinogen, canine cationic trypsinogen showed a different sequence at residue 21, the second most common site for a mutation of the cationic trypsinogen gene in human patients with hereditary pancreatitis. The isoleucine present at this position in human cationic trypsinogen is a threonine residue in the canine. This difference may account for not finding a mutation in the cationic trypsinogen gene in canines or it could be that a mutation exists in another gene, such as canine anionic trypsinogen, which appears to also be highly conserved between humans and canines (18). However, the cDNA sequence identified in the healthy control canine was identical to the sequence previously reported and was also identical to the sequence identified in the healthy miniature schnauzer and the 4 miniature schnauzers with pancreatitis.
No differences in the entire cDNA sequence of canine cationic trypsinogen were identified between the sequence previously published, the sequence found the healthy mixed breed canine, the healthy miniature schnauzer, and the 4 miniature schnauzers with a history of pancreatitis (18). Canine and human cationic trypsinogen, while having a few sequence differences, appear to be highly conserved (18). Thus, there is no evidence that pancreatitis in the miniature schnauzer may be caused by mutations of the cationic trypsinogen gene.
In conclusion, in contrast to humans with hereditary pancreatitis, the high incidence of pancreatitis in the miniature schnauzer does not appear to be due to mutations of the cationic trypsinogen gene. Further studies are in progress in order to investigate other potential mutations that may be responsible for pancreatitis in the miniature schnauzer. Candidate genes for such possible mutations could include the gene for anionic trypsinogen, pancreatic secretory trypsin inhibitor (SPINK1), the cystic fibrosis transmembrane conductance regulator gene (CFTR), or the lipoprotein lipase gene, all of which have been shown to play a role in hereditary pancreatitis in humans (19,20).
Footnotes
This material has been presented as a research abstract at the 2002 ACVIM Forum in Dallas, Texas.
References
- 1.Williams DA. The Pancreas. In: Strombeck DR, Guilford WG, Center SA, Williams DA, Meyer DJ, eds. Small Animal Gastroenterology. Philadelphia: W.B. Saunders, 1996:381–410.
- 2.Drazner FH. Diseases of the pancreas. In: Jones BD, Liska WD, eds. Canine and Feline Gastroenterology. W.B. Saunders Company, 1986:295–344.
- 3.Perrault J. Hereditary pancreatitis. Gastroenterol Clin North Am. 1994;23:743–752. [PubMed] [Google Scholar]
- 4.Creighton J, Lyall R, Wilson DI, et al. Mutations of the cationic trypsinogen gene in patients with chronic pancreatitis. Lancet. 1999;354:42–43. doi: 10.1016/S0140-6736(99)01814-0. [DOI] [PubMed] [Google Scholar]
- 5.Ellis I, Lerch MM, Whitcomb DC. Genetic testing for hereditary pancreatitis: guidelines for indications, counselling, consent and privacy issues. Pancreatology. 2001;1:405–415. doi: 10.1159/000055840. [DOI] [PubMed] [Google Scholar]
- 6.Tautermann G, Ruebsamen H, Beck M, et al. R116C mutation in the cationic trypsinogen gene in a Turkish family illustrates genetic microheterogeniety of hereditary pancreatitis. Gastroenterology. 2001;64:226–232. doi: 10.1159/000048866. [DOI] [PubMed] [Google Scholar]
- 7.Gorry MC, Gabbaizedeh D, Furey W, et al. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology. 1997;113:1063–1068. doi: 10.1053/gast.1997.v113.pm9322498. [DOI] [PubMed] [Google Scholar]
- 8.Nishimori I, Kamakura M, Fujikawa-Adachi K, et al. Mutations in exons 2 and 3 of the cationic trypsinogen gene in Japanese families with hereditary pancreatitis. Gut. 1999;44:259–263. doi: 10.1136/gut.44.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witt H, Luck W, Becker M. A signal peptide cleavage site mutation in the cationic trypsinogen gene is strongly associated with chronic pancreatitis. Gastroenterology. 1999;117:7–10. doi: 10.1016/s0016-5085(99)70543-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen JM, Piepoli Bis A, LeBodic L, et al. Mutational screening of the cationic trypsinogen gene in a large cohort of subjects with idiopathic chronic pancreatitis. Clinical Genetics. 2001;59:189–193. doi: 10.1034/j.1399-0004.2001.590308.x. [DOI] [PubMed] [Google Scholar]
- 11.Truninger K, Kock J, Wirth HP, et al. Trypsinogen gene mutations in patients with chronic or recurrent acute pancreatitis. Pancreas. 2001;22:18–23. doi: 10.1097/00006676-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Chen JM, Montier T, Férec C. Molecular pathology and evolutionary and physiological implications of pancreatitis-associated cationic trypsinogen mutations. Hum Genet. 2001;109:245–252. doi: 10.1007/s004390100580. [DOI] [PubMed] [Google Scholar]
- 13.Whitcomb DC. Hereditary pancreatitis: new insights into acute and chronic pancreatitis. Gut. 1999;45:317–322. doi: 10.1136/gut.45.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JM, Férec C. Origin and implication of the hereditary pancreatitis-associated N21I mutation in the cationic trypsinogen gene. Hum Genet. 2000;106:125–126. doi: 10.1007/s004390051019. [DOI] [PubMed] [Google Scholar]
- 15.Steer ML, Saluja AK. Experimental acute pancreatitis: studies of the early events that lead to cell injury. In: Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, eds. The pancreas: biology, pathobiology and disease. New York: Raven Press, 1993:489–500.
- 16.Steer ML. Etiology and pathophysiology of acute pancreatitis. In: Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, eds. The pancreas: biology, pathobiology and disease. New York: Raven Press, 1993:581–591.
- 17.Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest. 1986;77:1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinsky SD, LaForge KS, Scheele G. Differential regulation of trypsinogen mRNA translation: full-length mRNA sequences encoding two oppositely charged trypsinogen isoenzymes in the dog pancreas. Mol Cell Biol. 1985;5:2669–2676. doi: 10.1128/mcb.5.10.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jap TS, Jenq SF, Wu YC, et al. Mutations in the lipoprotein lipase gene as a cause of hypertriglyceridemia and pancreatitis in taiwan. Pancreas. 2003;27:122–126. doi: 10.1097/00006676-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Arduino C, Gaia E. Genetics of chronic pancreatitis. Biomed Pharmacother. 2000;54:394–399. doi: 10.1016/S0753-3322(01)80007-X. [DOI] [PubMed] [Google Scholar]


