Abstract
The midbrain-hindbrain boundary (MHB) is a highly conserved vertebrate signalling centre, acting to pattern and establish neural identities within the brain. While the core signalling pathways regulating MHB formation have been well defined, novel genetic and mechanistic processes that interact with these core components are being uncovered, helping to further elucidate the complicated networks governing MHB specification, patterning and shaping. Although formation of the MHB organiser is traditionally thought of as comprising three stages, namely positioning, induction and maintenance, we propose that a fourth stage, morphogenesis, should be considered as an additional stage in MHB formation. This review will examine evidence for novel factors regulating the first three stages of MHB development and will explore the evidence for regulation of MHB morphogenesis by non-classical MHB-patterning genes.
Keywords: Midbrain–hindbrain boundary, Isthmic organiser, Neural tube, Morphogenesis
Introduction
The vertebrate brain is the single most complex and dynamic organ to arise in the animal kingdom throughout evolutionary development. The brain originates from the neural ectoderm, formed during gastrulation, and begins its development as a flat sheet of neuroepithelial cells, termed the neural plate. Although some temporal, spatial and mechanistic differences exist, the formation and folding of the vertebrate brain is a remarkably well-conserved evolutionary process [1, 2]. The early neural plate undergoes a convergence and extension process (neurulation) to form the neural tube (embryonic brain and spinal cord). Patterning of this tube along the dorso-ventral axis is well understood and depends on the relative gradients of BMP4 (dorsalising) and Shh (ventralising) factors [3, 4]. Similarly, patterning along the anterior-posterior axis is also well defined, particularly with respect to the positioning, induction and maintenance of the most crucial neural developmental signalling centre, the midbrain-hindbrain boundary (MHB). Originally identified in chick [5–8], the isthmic organiser located at the MHB consists of cells that influence the fate of neighbouring cells to adopt either a mesencephalic (midbrain) or metancephalic (hindbrain) fate through expression of transcription factors and soluble signalling molecules [9]. This organiser is powerful enough to induce surrounding cells to re-fate to become ectopic midbrain and hindbrain if it is transplanted to other areas of the brain [10], indicating that correct positioning and patterning of organiser formation must be tightly and precisely regulated at an early stage in brain formation.
The subsequent use of a variety of developmental vertebrate models has helped to identify conserved patterning signals, which position and influence the formation of the MHB. Although the precise spatio-temporal expression of genes within the MHB cascade varies between vertebrate species [9], and different gene orthologues perform the same functions between species, broadly speaking, the conserved pathway operates as follows. The position of the prospective MHB is specified at the interface of the expression domains of the transcription factors Otx2 and Gbx1/2 within the neural plate [10–12]. A cascade of signalling (FGF8/Wnt1) and transcription (Pax2/5/8, Eng1/2) factors within the Otx2/Gbx1/2 boundary induces formation of the MHB [9, 13–15], and subsequent interplay between these factors is critical for maintenance of the MHB. These factors (Otx, Gbx, FGF8, Wnt, Pax, and Eng) classically comprise the core MHB cascade, and disturbance of any of these factors leads to severe functional disruption in the formation of the isthmic organiser.
This core MHB cascade has been the subject of numerous excellent reviews [9, 10, 16–18] and will therefore not be the topic of this work. Rather, our focus will centre on novel factors and mechanisms that regulate the positioning, induction and maintenance of the MHB. This review will also examine the evidence that a fourth stage of MHB development, namely morphogenesis, is actively governed by a specific set of signalling cues and is not merely a passive consequence of correct patterning of the neural territory by genes of the core MHB cascade.
Novel factors regulating the stages of MHB development
Stage 1: Positioning and establishment
As the Otx and Gbx factors are critical for positioning the presumptive MHB within the neural territory, the spatio-temporal regulation of their expression is pivotal. Planar signals operating within the neural plate are central to multiple aspects of neurulation, from initial regionalisation of the neural plate to the ultimate morphogenesis of the neural tube [19]. Studies in zebrafish have shown that “posteriorisation” (conversion of part of the neuroectoderm to a more posterior fate) during early neurulation, mediated by Wnt8, is a critical requirement for the onset of gbx1 and otx2 expression [20]. The importance of the notochord in signalling to the neural plate to set up the Otx2/Gbx1/2 boundary has been debated, although as the establishment of this interface in mice is unaffected in embryos lacking a (node-derived) notochord [21], the contribution of this signalling to the establishment of the MHB territory is now thought to be minor, and rather the non-axial mesendoderm underlying the neural plate appears to be a more critical source of ventral signalling to the neural plate [20].
A large body of work suggests that FGF8 is the critical organiser molecule (Fig. 1). FGF8 can regulate the expression of both Otx2 and Gbx2 by activating Gbx2 and repressing Otx2 within the isthmic region [22] and by suppressing Otx2 expression in the hindbrain [23]. The expression domain of FGF8 itself is in turn regulated by the boundaries of Otx2/Gbx2 expression [24], indicating the presence of a feedback loop. Most interestingly, FGF8 is still observed within the neuroectoderm in the absence of both these factors [24], indicating that Otx2 and Gbx2 may serve to refine rather than induce the expression of FGF8. Experiments in the chick model suggest that de-repression of Otx2 transcriptional activity may be critical in the initial positioning of the midbrain-hindbrain domains. Overexpression of the midbrain-patterning gene Meis2 [25] greatly increased the transactivation potential of Otx2, suggesting that in addition to specifying the midbrain domain, Otx2 may also play an active process in gene induction of the core MHB cascade. Although Meis2 clearly regulates the function of Otx2, it does not impact on the initiation of the MHB cascade, or possess MHB-organiser function itself [25], suggesting that despite regulating a critical early MHB-patterning gene, Meis2 in itself is not an MHB-patterning factor.
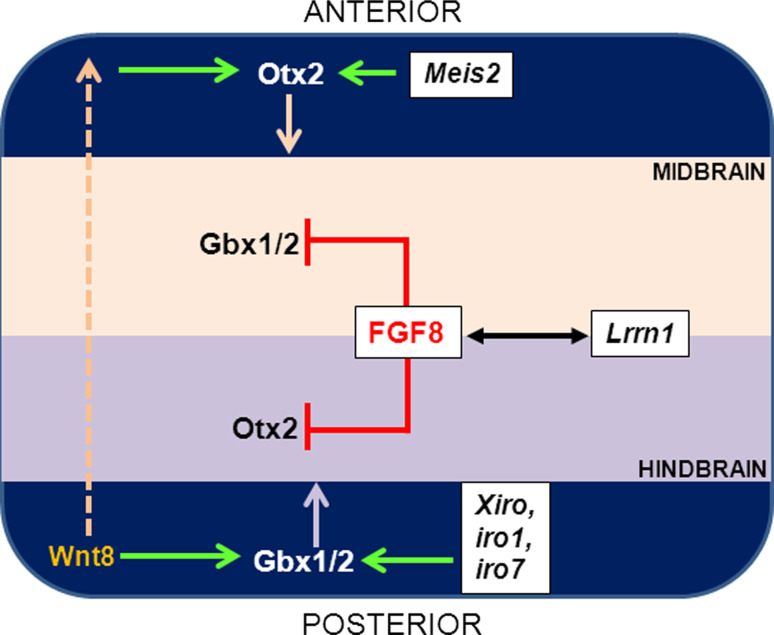
Fig. 1.
Positioning of the presumptive MHB is regulated by the expression of Otx and Gbx genes. Following overall posteriorisation of the neuroectoderm by Wnt8, Otx2 is expressed within the presumptive midbrain region, and Gbx1/2 is expressed within the presumptive hindbrain territory. Otx2 expression in the midbrain is regulated partially by Meis2, whereas Gbx1/2 expression in the hindbrain is regulated by members of the Iroquois (iro) gene family. The tightly demarcated interface of Otx/Gbx gene expression is regulated by FGF8, which inhibits Gbx1/2 expression in the midbrain and Otx2 expression in the hindbrain, respectively, resulting in defined lineage restriction. Lrrn1-mediated suppression of FGF8 results in defective boundary demarcation, lineage-mixing, and defective organiser formation, indicating that the establishment of a sharp boundary is critical for further MHB development
Once established, the boundary demarcates the future midbrain (Otx2 +) and hindbrain (Gbx1 +) territories, generating an apparent lineage restriction (Fig. 1), whereby once fated, cells from the respective territories do not intermix [26, 27]. The expression of the bHLH gene Her5 has been postulated to act as the earliest fate-determinant during gastrulation, acting to establish identities of future midbrain and hindbrain cells [28]. Although lineage restriction boundaries exist throughout the neural tube demarcating distinct regions (neuromeres), the evidence for a lineage restriction at the MHB is not definitive. In vitro cell mixing and in vivo cell labelling studies in the chick MHB suggested that cells from both mesencephalic and metancephalic territories intermingled freely [26]. A subsequent study in the zebrafish MHB, tracking the fate of live cells in vivo, indicated that a tight lineage restriction was apparent [27]. While these observations may suggest that different lineage restriction mechanisms may operate within disparate species, this is unlikely given the overall conservation of MHB development within vertebrates. In support of this were further experiments in chick examining the role of a boundary-demarcating protein, Lrrn1, in MHB development. Lrrn1 is a vertebrate orthologue of Drosophila tartan/capricious, transmembrane proteins that regulate cellular adhesion and are typically expressed in the midbrain, and not in the anterior hindbrain. Precisely how Lrrn1 maintains lineage restriction is not known, but both loss of function in the midbrain, and overexpression of Lrrn1 ectopically within the hindbrain resulted in the mixing of lineage-restricted cells [29]. Loss of Lrrn1 led to the loss of isthmic organiser potential, whereas Lrrn1 overexpression resulted in a loss of restriction of midbrain (Otx2) and hindbrain (Gbx2) markers, and subsequently of MHB organiser genes Wnt1 and Fgf8. Whether the loss of isthmic organiser potential is specifically due to the loss of lineage restriction, or whether this observation is merely correlative, remains to be determined. However, it is known that Lrrn1 interacts with FGF8 at the MHB [29], suggesting that irrespective of the importance of a lineage restriction boundary, this feedback loop may be an important element of MHB formation.
However, the establishment of a lineage-restricted boundary as well as expression of Otx and Gbx factors is not sufficient in itself to initiate the MHB programme or confer organiser activity onto the isthmic territory. For example, the primitive invertebrate lancelet, Amphioxus, shows expression of both Otx and Gbx factors in the presumptive MHB region, yet this region does not have organiser activity, and the MHB cascade is not activated over this boundary [30]. The converse experiments, examining the effects of the absence of Otx or Gbx gene expression on MHB formation, showed that mice lacking Otx2 and/or Gbx2 could initiate the MHB cascade, but the positioning of the MHB was located either anteriorly or posteriorly to its usual location [24]. These data clearly indicate that in addition to global positioning indicators within the neural plate, active instructional cues are also required to initiate the MHB cascade at the junction of the tightly patterned Otx2 + and Gbx1 + territories.
Stage 2: Induction
How then does the positional information conferred by Otx2 and Gbx1/2 expression translate to activation of the downstream MHB cascade? As both Otx2 and Gbx2 are thought to be transcriptional repressors [31], alleviation of repression may be a logical first step of MHB cascade induction. Induction of the MHB is characterised by the expression of members of the Wnt, Pax, Engrailed and FGF families [9, 10, 16, 17]. Seminal experiments in the chick indicated that FGF8 possesses inductive potential (Fig. 2), as overexpression in the forebrain could ectopically induce the MHB cascade, leading to formation of both tectal and cerebellar tissues [32]. The formation of various isthmic proximal and distal neural structures is directly linked to the duration and extent of FGF activity [23], and vertebrate studies have shown that animals lacking FGF8 do not form a cerebellum [33]. The critical importance of the FGF8 pathway was again highlighted by novel work uncovering the role of the first known micro-RNA (miR) to regulate MHB patterning, miR-9 [34]. This study demonstrated that miR-9 in zebrafish was able to negatively regulate several members of the FGF pathway at the MHB, namely fgf8, fgfr1 and canopy1, thereby suggesting a regulatory network operating at the level of the MHB. Supporting this theory was the finding that miR-9 could also directly inhibit a separate pathway regulating neurogenesis in and around the MHB via direct inhibition of another MHB marker gene, her5, a gene known to be critical in its own right for restricting neurogenesis at the MHB [35]. These data show that the gradients of gene expression, particularly FGF8, at the MHB must be critically regulated to ensure correct MHB development.
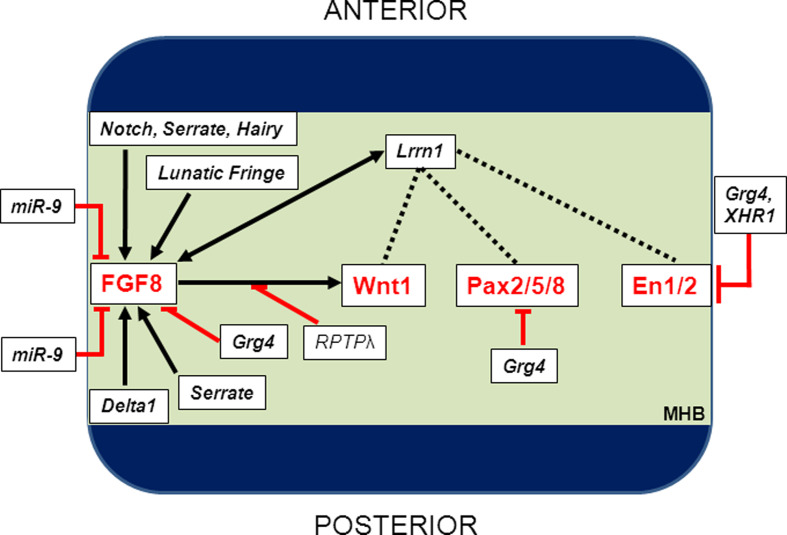
Fig. 2.
Induction of the core-MHB cascade is regulated by several factors. Once the presumptive MHB territory has been established, genes of the FGF, Wnt, Pax and Engrailed gene families (the core-MHB cascade) are rapidly induced within this region. FGF8 activity is critical, and expression and function of this molecule are regulated by miR-9 and members of the Notch pathway (Serrate, Hairy, Lunatic Fringe and Delta1). The interplay between members of the core-MHB cascade begins, and this is regulated by Lrrn1, Grg4 and XHR1 at multiple levels within the cascade. Specific interactions between members have also been described, notably RPTPλ-mediated activation of Wnt1 following FGF8 stimulation and the transcription of Engrailed 2a by FGF8-mediated activation of grhl2b
Although FGF8 may be thought of as the “master” MHB initiator, several lines of evidence indicate that it does not operate alone. The most convincing datum is that the MHB cascade is still initiated in animals lacking FGF8 [33, 36], although the expression of these genes is not subsequently maintained. Similarly, although the isthmus and cerebellum do not form, the tectum of zebrafish lacking FGF8 is largely unaffected [33], indicating that FGF8 is dispensable for at least some of the patterning functions attributed to the isthmic organiser. While the involvement of the Pax, Eng and Wnt families has been well described in the MHB cascade, it is clear that the transcriptional and signalling network that operates at the MHB comprises additional factors. For example, the receptor protein tyrosine phosphatase λ (RPTPλ) gene has been shown in the chick model to mediate the FGF8-induced activation of Wnt1, as overexpression of RPTPλ showed a decrease in Wnt1 expression at the MHB, whereas RNAi-mediated knockdown of RPTPλ resulted in an expanded Wnt1 domain [37]. Depending on the strength of FGF8 dosage, RPTPλ is either downregulated (at high doses) or upregulated (at low doses) by FGF8 [37], suggesting that this is a novel mechanism for the regulation of Wnt1 expression in MHB development. Further work is necessary to determine the overall importance of this interaction to the MHB cascade, as FGF8-RPTPλ signalling appeared to be specific to the regulation of Wnt1; the expression of other MHB-markers including En1, Pax2 or Pax5 was not differentially regulated.
Studies in Xenopus have shown that Xiro, a member of the Iroquois family that is co-expressed with both Otx2 and Gbx2 before MHB induction, activates Gbx2 expression in the hindbrain and appears to be critical for subsequent induction of FGF8 at the isthmic organiser [31], suggesting this gene family may be responsible for mediating the inductive organiser signal. Like Xenopus Iro, two members of the Iroquois family in zebrafish, iro1 and iro7, are temporally expressed before either gbx1 or otx2, and loss of function of these genes shows a marked reduction in downstream genes of the core-MHB cascade [38]. Other studies suggest that the Notch signalling pathway may be a crucial signalling cascade that regulates induction of the MHB programme [39]. The Notch-pathway genes Serrate 1 (midbrain and hindbrain), Serrate 2 (MHB), Delta 1 (hindbrain), Lunatic Fringe (midbrain) and Hairy1/2 (MHB) are expressed in and around the MHB following formation of the Otx2/Gbx2 border, and critically, downregulation of Notch signalling at the MHB leads to a loss of FGF8 and subsequent disrupted MHB formation [39], suggesting that Notch functions upstream of both FGF8 and the MHB cascade. Other pathways have also been discovered to operate upstream of induction of the MHB cascade. Additionally, transcriptional repression by Grg4, a vertebrate orthologue of Drosophila groucho, can negatively impact on expression of MHB genes [40], as does expression of dominant-negative forms of the HES-related gene Xhr1 in Xenopus [41].
Experiments from the chick indicate that FGF8 can also regulate Lrrn1. Although Lrrn1 is expressed within the neural tube during the onset of neurulation, its expression is strongly downregulated at the MHB as neurulation proceeds, although it continues to be expressed both anteriorly and posteriorly [29]. Downregulation of Lrrn1 led to failure of MHB constriction formation, with concomitant loss of FGF8, suggesting that a feedback loop exists between these two molecules as MHB development proceeds. These data indicate that Lrrn1 appears to be a novel gene required for both induction and maintenance of the MHB organiser. It will be interesting to see if in addition to its interaction with FGF8, Lrrn1 can also directly interact with Eng, Pax or Wnt proteins in maintenance of the MHB (Fig. 2), and also whether its role is conserved in other vertebrates.
These data suggest that many of the genes outside the core MHB cascade that regulate induction of the MHB programme seem to do so via interaction with FGF8, consistent with the organiser function attributed to this molecule.
Stage 3: Maintenance
FGF8 is critical and indispensable for induction of the MHB genetic programme following establishment of the Otx2/Gbx2 boundary, and this gene family continues to play a critical role as MHB development proceeds. The third phase of MHB development, that of maintenance, relies largely on continued expression and interdependence of FGF with Wnt, Eng and Pax factors [42, 43]. Loss of any of these factors during the maintenance stage leads to a breakdown of the expression of the other factors, loss of isthmic stability and subsequent altered positional patterning (Fig. 3). A defining feature of genes operating as maintenance factors is the observation that loss of function does not compromise the initial induction of the MHB cascade, but the expression of the core MHB cascade genes is rapidly decreased as MHB formation proceeds. An example of failed maintenance comes from our laboratory, in experiments showing that zebrafish grhl2b, a vertebrate orthologue of Drosophila grainy head, acts downstream of FGF8 to regulate the transcription of eng2a [44]. Although eng2a expression is reduced during the induction phase of MHB development, the expression of other core MHB patterning components, pax2a, wnt1, her5 and fgf8, is unaffected during this stage. However, the expression of these genes was lost in grhl2b morphants during the maintenance phase, indicating that initial eng2a deficiency in this model impacts on MHB maintenance, putatively through loss of transcriptional interdependence. The activation of grhl2b by FGF8 in contributing to the MHB cascade is putatively mediated by Erk signalling, as FGF8 is responsible for phosphorylating Erk [45, 46], and studies in Drosophila have shown that erk activation phosphorylates grainy head, thereby influencing its expression and transcriptional activity [47].
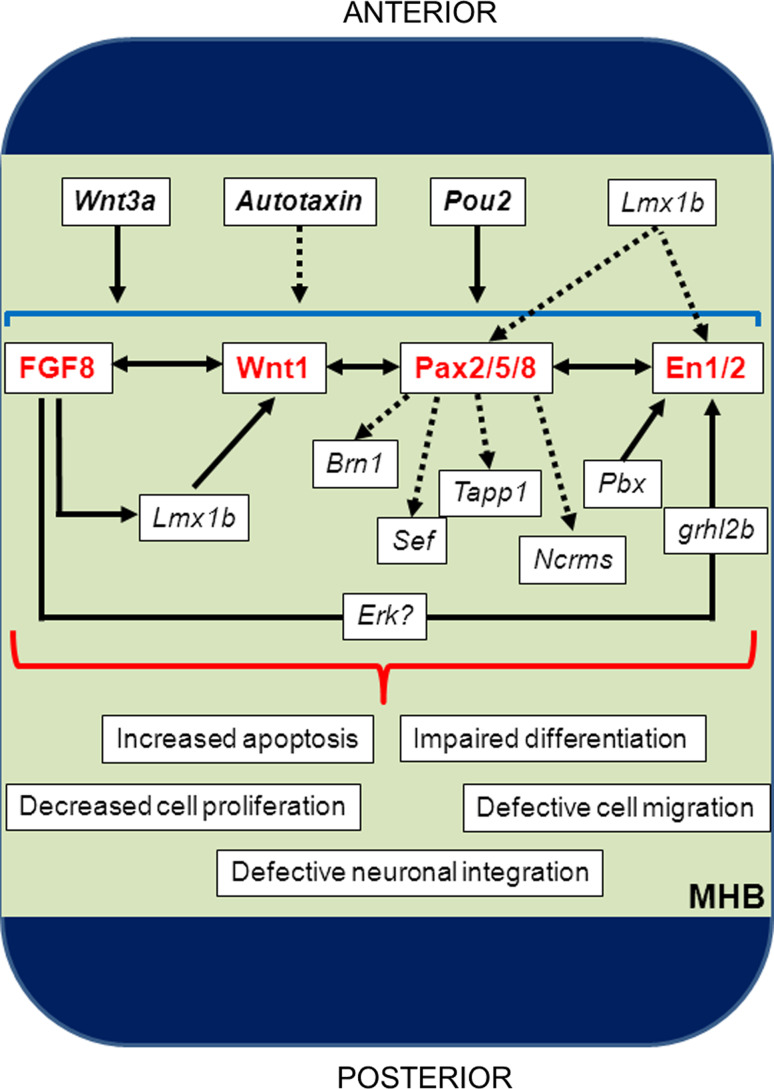
Fig. 3.
Maintenance of MHB development relies on transcriptional interdependence between members of the core MHB cascade. During this stage of MHB development, functional interplay among FGF8, Wnt1, Pax2/5/8 and En1/2 is critical, as loss of any of these factors here leads to complete loss of isthmic stability. Several other factors are also postulated to regulate this cascade, namely Wnt3a, Autotaxin and Pou2 which seem to globally regulate the cascade, as well as Lmx1b, Pbx and grhl2b, which exert their effects on specific genes within the cascade. In addition to regulation of Wnt1, Lmx1b may also regulate Eng1/2 and Pax2/5/8 in this context. Which pathways are activated by the interdependence of the core MHB cascade remain largely unknown, although some candidates (such as Brn1, Sef, Tapp1 and Ncrms) are known to lie downstream of certain cascade members, in this case Pax2. Failure of MHB interdependence during this stage leads to a variety of pleiotropic defects with profound implications for organiser integrity, namely increased cell death, defective precursor cell proliferation, and impaired differentiation, migration and functional integration of more mature neurons within the midbrain and hindbrain territories
Another example of disrupted maintenance is seen following the downregulation of Wnt3a, a factor not traditionally thought to be part of the core MHB cascade. Wnt3a loss does not impact significantly on the initial expression of the MHB factors, but these factors rapidly disappear because of failed maintenance [48]. Another critical factor for MHB maintenance appears to be Lmx1b, which is required for the continued maintenance of Wnt1 during MHB ontogeny following induction by FGF8 [49]; En2 and Pax2a are also induced but rapidly lost following Lmx1b loss [50, 51]. Similarly, loss of the Hox and Eng co-factor gene Pbx leads to normal initiation of the MHB cascade, but complete failure to maintain signalling, leading to non-formation of the MHB constriction. [52]. A recent study has also suggested that Autotaxin (ATX) may be important in the maintenance of the MHB, as ATX-deficient mouse embryos exhibit aberrant cranial neurulation and MHB marker expression and increased apoptosis [53]. One caveat here is that ATX deficiency results in pleiotropic brain defects, and further conditional deletions may be required to independently assess the role that ATX plays in MHB maintenance specifically.
Another transcription factor that appears to be critical for maintenance of the MHB cascade is pou2. Zebrafish spiel ohne grenzen mutants, which lack this gene, exhibit loss of the isthmus in a manner consistent with defective maintenance. The positioning of the otx2/gbx1 boundary is not affected, and the initial expression of fgf8 also does not differ from that of WT embryos [54]. These mutants also successfully initiate the expression of the other MHB cascade genes, albeit at somewhat reduced levels. However, the expression of all these markers, including fgf8, is not maintained, and therefore the MHB is not formed, suggesting a maintenance defect [54]. Pou2 does not appear to be an instructive signal for MHB formation, as fish mutants lacking either fgf8 or pax2 express pou2 normally, yet still fail to form the MHB [54]. This suggests that the function of pou2 may be to create a permissive environment for transcriptional MHB gene maintenance, either through direct regulation of MHB cascade genes or through maintenance of transcriptional interdependence via as yet unidentified factors.
While some of these novel genes required for MHB maintenance operate concurrently with the core MHB cascade to maintain the MHB, others undoubtedly lie downstream of this transcriptional network, as both Pax2 and Engrailed are transcription factors. For example, microarray analysis of differentially regulated genes in the isthmus of Pax2a −/− mice revealed four novel factors—the transcription factors Brn1 (Pou3f3), the intracellular signalling modifiers Sef and Tapp1, and the non-coding RNA Ncrms (as well as En1)—whose expression in the MHB was dependent on Pax2a [55]. However, the specific functions of these genes within the isthmic organiser (with the exception of En1) in maintaining the MHB remain unknown.
Maintenance of the MHB is also dependent on tightly regulated cell proliferation and migration, as well as differentiation into the functional neurons of both the midbrain and hindbrain [28]. Several genes within the MHB cascade, such as FGF8 (as well as related family members FGF17 and FGF18) [56, 57], Wnt1 [58] and En1/2 [59], regulate cellular proliferation and/or survival independently of their patterning roles in the MHB cascade within the isthmic region; similarly, overexpression of RPTPλ also led to a reduction in progenitor proliferation [37]. Apoptosis undoubtedly also plays a role in failed maintenance of the MHB, as mouse deletion mutants lacking Wnt1, FGF8 or En1 display substantial apoptosis in and around the MHB, coinciding with failed or impaired development of either mid- or hindbrain structures [36]. In addition to disrupted MHB patterning, fish lacking grhl2b also displayed severe apoptosis in the MHB [44], consistent with studies that have shown that the grhl2b target, eng2a, is critical for the maintenance of neuronal survival [59, 60]. Taken together, data from these studies suggest that in addition to the critical role of the MHB genes in promoting correct fate acquisition, they may also be critical for ensuring cellular survival.
Stage 4: Morphogenesis?
Despite our knowledge of the specification and patterning of the neural tube, it is clear that the genes that control MHB positioning, induction and maintenance play only minimal roles in MHB morphogenesis. Although the genes of the core MHB cascade are critical for correct patterning and fating of the MHB, thereby allowing morphogenesis to proceed, we propose that morphogenesis should be thought of as a separate stage from that of MHB maintenance. Disruption of MHB morphogenesis in vertebrates with deficiencies in genes of the core MHB cascade is likely to be a secondary defect of impaired patterning. Rather than merely being a passive consequence of correct specification, however, recent data indicate that MHB morphogenesis is in fact actively regulated by genes that control cell shaping, cellular migration, cytoskeletal plasticity and cellular polarity (Fig. 4). In the zebrafish brain, the first morphological demarcation of the neural tube into a prospective folded structure appears at the 16 somite stage, where a small invagination between the midbrain and the hindbrain becomes apparent [27]. Defective polarity across the neuroepithelium (apico-basal polarity) still allows the correct neural boundaries to be specified (as seen by marker expression), but MHB folding and/or ventricle inflation is severely disrupted [2], indicating that morphogenesis in this context occurs independently from induction of the MHB cascade. Preliminary data from our laboratory suggest that grhl2b may interact with several members of the Wnt/PCP pathway in regulating MHB morphogenesis at this stage (Dworkin, unpublished), although the precise molecular mechanisms underpinning this failed folding are currently unknown.
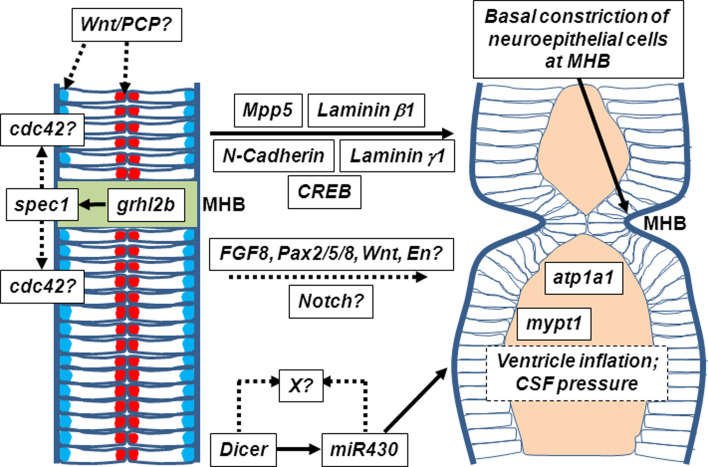
Fig. 4.
MHB morphogenesis is genetically separable from the actions of genes of the core MHB cascade. The Wnt/PCP pathway plays critical roles in the establishment of planar polarity of the neural plate during neurulation and may also regulate correct apico-basal polarity of neuroepithelial cells during morphogenesis of the MHB. Formation of the MHB constriction is likely to involve regulation of cytoskeleton plasticity, regulated by cdc42, following grhl2b-mediated activation of spec1. Several classes of adhesion molecules, junctional complex and extracellular matrix proteins, such as laminin γ1, laminin β1, N-cadherin and Mpp5, as well as the transcription factor CREB, also play a role in shaping the MHB independently of the core-MHB cascade; precisely what active or instructive role the core-MHB cascade plays during this stage is not clear. Loss of Dicer leads to aberrant morphology of the neural tube, a defect that can be rescued by miR430. However, the identity of other mature miRNAs that are lost in Dicer mutants and direct genetic targets of miR430 (both indicated by an “X?” on the figure) remain to be elucidated. Many factors, both genetic (e.g. Atp1a1, mypt1) and cell-intrinsic (cytoskeleton fidelity, cell polarity), are likely to play critical roles during basal constriction of MHB cells during morphogenesis, and extrinsic forces, such as ventricle inflation and cerebrospinal fluid (CSF) pressure, may play a role in allowing full ventricular expansion and MHB morphogenesis
Further data indicating that neural tube morphogenesis is actively regulated by non-neural patterning genes comes from early experiments examining deletion of the transmembrane adhesion molecule N-cadherin in mouse [61] and zebrafish [62]. The morphology of the entire neural tube is dismorphic and MHB formation in the fish is severely disrupted. Importantly, however, the expression of MHB genes such as wnt1 or pax2 is not affected [62], highlighting the presence of morphological defects in the context of correct MHB specification and patterning. A similar phenomenon is seen in zebrafish mis-expressing the transcription factor CREB [63], whereby overexpression of both a constitutively active (CREB-FY), or inactive (CREB-M1) form of CREB led to defects in MHB morphology, but no loss of the classical MHB markers, further supporting a separation of these processes.
Supporting data showing that the processes of MHB patterning and morphogenesis are dissociable came from investigation of deletion mutants of the miRNA processing molecule, Dicer. Maternal-zygotic (mz) Dicer-deleted zebrafish (which lack mature processed miRNAs) showed severe defects in neural morphology, particularly at the level of the MHB, without a disruption in expression of the MHB markers pax2a or eng2 [64]. This group further analysed which specific miRNAs contributed to the morphology loss and found that the MHB defects in the mz-Dicer mutants could be rescued through injection of the miR430 miRNAs. These data show that miRNAs can specifically regulate MHB morphology, and as miRNAs generally act to repress transcription or translation of targets, it suggests that an over-abundance of some factors, or a disruption in the tightly controlled homeostatic regulatory network operating at the MHB, is responsible for the observable defects. Future work will undoubtedly focus on both the specific targets of miR430 and other miRNAs regulated by Dicer in this context.
Further work has come from the laboratory of Hazel Sive, who showed that during the morphogenesis phase of MHB development, cells at the MHB shorten to approximately 75 % of the length of their neighbours and concomitantly undergo a process termed “basal constriction”, whereby these cells narrow at their basal side, closest to the developing ventricular lumen [65]. This appears to be an active process, rather than passive “pushing” from the cerebrospinal fluid (CSF) following ventricle formation, as ventricle-inflation-deficient mutants snakehead (atp1a1) and nagie oko (Mpp5) form a defined basement membrane constriction. However, presumptive folds are visible within the neural tube of snakehead, but no such morphological distinction appears in nagie oko [66], suggesting that mechanisms of tube morphology are distinct from those of ventricle development. In contrast to these two ventricle-inflation mutants, the laminin-deficient mutants sleepy (laminin γ1) and grumpy (laminin β1) do not form a tight constriction, thereby defining a novel role for the basement membrane, and particularly laminin proteins, in MHB morphogenesis. Furthermore, this process again appears to be independent of the first three stages of MHB patterning, as MHB-induction genes such as eng2a and pax2a are expressed normally in laminin mutants [65]. A requirement for other patterning genes such as wnt1, fgf8 or her5 has not been reported in these mutants; however, thereby not entirely excluding the MHB cascade from impacting on this process.
Work from our laboratory has shown that in addition to its transcriptional roles in the induction and maintenance of eng2a, loss of function of grhl2b, leads to a disruption in the characteristic shaping of the MHB folds [44]. Critically, it appears as though the folding defects occur independently of eng2a downregulation, as both our studies and previous data [52] did not provide evidence that eng2a regulates MHB morphogenesis. Rather, we identified a second grhl2b target gene, spec1 (small protein effector of cdc42), which when downregulated phenocopied the non-folded MHB morphology seen in grhl2b morphants. These data were particularly interesting in light of the fact that expression of the core MHB cascade in these morphants was not disrupted, and the spec1-morphants did not exhibit significantly increased apoptosis at the MHB region [44]. The folding defects following loss of spec1 may be due to a defect in cell polarity caused by disruption of cdc42, a small molecule that influences actin accumulation in polarised T cells [67–69]. SPECs may generally be involved in cdc42-mediated polarity establishment in other cell types and may also regulate cell shape [67]. Interestingly, blocking of the Notch signalling cascade through electroporation of an inhibitor ligand into the MHB also resulted in a mis-folded neuroepithelium together with loss of MHB-gene expression, suggesting that like grhl2b, Notch signalling may also affect both MHB patterning and morphogenesis programmes via independent mechanisms [39]. Taken together, these studies demonstrate that specific defects in MHB morphogenesis can be caused through dysregulation of non-MHB cascade genes. Importantly, these defects are seen even in the context of correct specification and patterning by the MHB cascade, supporting the idea that MHB morphogenesis is in itself an active process, albeit one that requires that the neural territory be correctly patterned.
Conclusions
Despite these recent advances in the field, deciphering the genetic complexity of the regulatory network controlling MHB development remains a work in progress. In addition to a candidate gene approach, identification of factors that regulate any, some or all of the four stages of MHB development described in this review will most likely come from both forward genetic and mapping analyses of mutagenesis screens and reverse genetic studies of genes that are expressed at the MHB. The zebrafish forms a crucial model here, owing to the number of phenotypic mutants identified with MHB defects, where the responsible gene(s) remain unknown [70, 71], and conversely, also because of the large number of genes that have been shown to be expressed at the MHB (detected by high-throughput in situ hybridisation screening [72]), whose function in isthmic patterning remains unknown. These studies will further serve to shape our knowledge of how the MHB is formed and could provide valuable therapeutic insights for the multitude of human neural patterning defects.
References
- 1.Hirth F. On the origin and evolution of the tripartite brain. Brain Behav Evol. 2010;76(1):3–10. doi: 10.1159/000320218. [DOI] [PubMed] [Google Scholar]
- 2.Lowery LA, Sive H. Totally tubular: the mystery behind function and origin of the brain ventricular system. BioEssays: news and reviews in molecular, cellular and developmental biology. 2009;31(4):446–458. doi: 10.1002/bies.200800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Echelard Y, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75(7):1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Ann Rev Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- 5.Marin F, Puelles L. Patterning of the embryonic avian midbrain after experimental inversions: a polarizing activity from the isthmus. Dev Biol. 1994;163(1):19–37. doi: 10.1006/dbio.1994.1120. [DOI] [PubMed] [Google Scholar]
- 6.Martinez S, et al. Induction of ectopic engrailed expression and fate change in avian rhombomeres: intersegmental boundaries as barriers. Mech Dev. 1995;51(2–3):289–303. doi: 10.1016/0925-4773(95)00376-2. [DOI] [PubMed] [Google Scholar]
- 7.Martinez S, Wassef M, Alvarado-Mallart RM. Induction of a mesencephalic phenotype in the 2-day-old chick prosencephalon is preceded by the early expression of the homeobox gene en. Neuron. 1991;6(6):971–981. doi: 10.1016/0896-6273(91)90237-T. [DOI] [PubMed] [Google Scholar]
- 8.Alvarado-Mallart RM. The chick/quail transplantation model: discovery of the isthmic organizer center, brain research. Brain Res Rev. 2005;49(2):109–113. doi: 10.1016/j.brainresrev.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Rhinn M, Brand M. The midbrain–hindbrain boundary organizer. Curr Opinion Neurobiol. 2001;11(1):34–42. doi: 10.1016/S0959-4388(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 10.Joyner AL, Liu A, Millet S. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr Opin Cell Biol. 2000;12(6):736–741. doi: 10.1016/S0955-0674(00)00161-7. [DOI] [PubMed] [Google Scholar]
- 11.Broccoli V, Boncinelli E, Wurst W. The caudal limit of Otx2 expression positions the isthmic organizer. Nature. 1999;401(6749):164–168. doi: 10.1038/43670. [DOI] [PubMed] [Google Scholar]
- 12.Simeone A. Positioning the isthmic organizer where Otx2 and Gbx2meet. Trends Genetics : TIG. 2000;16(6):237–240. doi: 10.1016/S0168-9525(00)02000-X. [DOI] [PubMed] [Google Scholar]
- 13.Brand M, et al. Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development. 1996;123:179–190. doi: 10.1242/dev.123.1.179. [DOI] [PubMed] [Google Scholar]
- 14.Picker A, et al. A novel positive transcriptional feedback loop in midbrain–hindbrain boundary development is revealed through analysis of the zebrafish pax2.1 promoter in transgenic lines. Development. 2002;129(13):3227–3239. doi: 10.1242/dev.129.13.3227. [DOI] [PubMed] [Google Scholar]
- 15.Borello U, Pierani A. Patterning the cerebral cortex: traveling with morphogens. Curr Opin Genet Dev. 2010;20(4):408–415. doi: 10.1016/j.gde.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci. 2001;2(2):99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- 17.Hidalgo-Sanchez M, et al. Specification of the meso-isthmo-cerebellar region: the Otx2/Gbx2 boundary. Brain Res Brain Res Rev. 2005;49(2):134–149. doi: 10.1016/j.brainresrev.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Barkovich AJ, Millen KJ, Dobyns WB. A developmental and genetic classification for midbrain–hindbrain malformations. Brain J Neurol. 2009;132(Pt 12):3199–3230. doi: 10.1093/brain/awp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallingford JB. Planar cell polarity, ciliogenesis and neural tube defects. Hum Mol Genet. 2006;15(Spec No 2):R227–R234. doi: 10.1093/hmg/ddl216. [DOI] [PubMed] [Google Scholar]
- 20.Rhinn M, et al. Positioning of the midbrain–hindbrain boundary organizer through global posteriorization of the neuroectoderm mediated by Wnt8 signaling. Development. 2005;132(6):1261–1272. doi: 10.1242/dev.01685. [DOI] [PubMed] [Google Scholar]
- 21.Klingensmith J, et al. Neural induction and patterning in the mouse in the absence of the node and its derivatives. Dev Biol. 1999;216(2):535–549. doi: 10.1006/dbio.1999.9525. [DOI] [PubMed] [Google Scholar]
- 22.Liu A, Joyner AL. EN and GBX2 play essential roles downstream of FGF8 in patterning the mouse mid/hindbrain region. Development. 2001;128(2):181–191. doi: 10.1242/dev.128.2.181. [DOI] [PubMed] [Google Scholar]
- 23.Sato T, Joyner AL. The duration of Fgf8 isthmic organizer expression is key to patterning different tectal-isthmo-cerebellum structures. Development. 2009;136(21):3617–3626. doi: 10.1242/dev.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JY, Joyner AL. Otx2 and Gbx2 are required for refinement and not induction of mid-hindbrain gene expression. Development. 2001;128(24):4979–4991. doi: 10.1242/dev.128.24.4979. [DOI] [PubMed] [Google Scholar]
- 25.Agoston Z, Schulte D. Meis2 competes with the Groucho co-repressor Tle4 for binding to Otx2 and specifies tectal fate without induction of a secondary midbrain–hindbrain boundary organizer. Development. 2009;136(19):3311–3322. doi: 10.1242/dev.037770. [DOI] [PubMed] [Google Scholar]
- 26.Jungbluth S, et al. Cell mixing between the embryonic midbrain and hindbrain. Curr Biol. 2001;11(3):204–207. doi: 10.1016/S0960-9822(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 27.Langenberg T, Brand M. Lineage restriction maintains a stable organizer cell population at the zebrafish midbrain–hindbrain boundary. Development. 2005;132(14):3209–3216. doi: 10.1242/dev.01862. [DOI] [PubMed] [Google Scholar]
- 28.Tallafuss A, Bally-Cuif L. Tracing of her5 progeny in zebrafish transgenics reveals the dynamics of midbrain–hindbrain neurogenesis and maintenance. Development. 2003;130(18):4307–4323. doi: 10.1242/dev.00662. [DOI] [PubMed] [Google Scholar]
- 29.Tossell K, et al. Lrrn1 is required for formation of the midbrain–hindbrain boundary and organiser through regulation of affinity differences between midbrain and hindbrain cells in chick. Dev Biol. 2011;352(2):341–352. doi: 10.1016/j.ydbio.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland LZ, Short S. Gene duplication, co-option and recruitment during the origin of the vertebrate brain from the invertebrate chordate brain. Brain Behav Evol. 2008;72(2):91–105. doi: 10.1159/000151470. [DOI] [PubMed] [Google Scholar]
- 31.Glavic A, Gomez-Skarmeta JL, Mayor R. The homeoprotein Xiro1 is required for midbrain–hindbrain boundary formation. Development. 2002;129(7):1609–1621. doi: 10.1242/dev.129.7.1609. [DOI] [PubMed] [Google Scholar]
- 32.Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380(6569):66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- 33.Reifers F, et al. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain–hindbrain boundary development and somitogenesis. Development. 1998;125(13):2381–2395. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- 34.Leucht C, et al. MicroRNA-9 directs late organizer activity of the midbrain–hindbrain boundary. Nat Neurosci. 2008;11(6):641–648. doi: 10.1038/nn.2115. [DOI] [PubMed] [Google Scholar]
- 35.Geling A, et al. bHLH transcription factor Her5 links patterning to regional inhibition of neurogenesis at the midbrain–hindbrain boundary. Development. 2003;130(8):1591–1604. doi: 10.1242/dev.00375. [DOI] [PubMed] [Google Scholar]
- 36.Chi CL, et al. The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development. 2003;130(12):2633–2644. doi: 10.1242/dev.00487. [DOI] [PubMed] [Google Scholar]
- 37.Badde A, Schulte D. A role for receptor protein tyrosine phosphatase lambda in midbrain development. J Neurosci Off J Soc Neurosci. 2008;28(24):6152–6164. doi: 10.1523/JNEUROSCI.5593-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itoh M, et al. A role for iro1 and iro7 in the establishment of an anteroposterior compartment of the ectoderm adjacent to the midbrain–hindbrain boundary. Development. 2002;129(10):2317–2327. doi: 10.1242/dev.129.10.2317. [DOI] [PubMed] [Google Scholar]
- 39.Tossell K, et al. Notch signalling stabilises boundary formation at the midbrain–hindbrain organiser. Development. 2011;138(17):3745–3757. doi: 10.1242/dev.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugiyama S, Funahashi J, Nakamura H. Antagonizing activity of chick Grg4 against tectum-organizing activity. Dev Biol. 2000;221(1):168–180. doi: 10.1006/dbio.2000.9643. [DOI] [PubMed] [Google Scholar]
- 41.Shinga J, et al. Early patterning of the prospective midbrain–hindbrain boundary by the HES-related gene XHR1 in Xenopus embryos. Mech Dev. 2001;109(2):225–239. doi: 10.1016/S0925-4773(01)00528-7. [DOI] [PubMed] [Google Scholar]
- 42.Canning CA, et al. Sustained interactive Wnt and FGF signaling is required to maintain isthmic identity. Dev Biol. 2007;305(1):276–286. doi: 10.1016/j.ydbio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Wittmann DM, et al. Spatial analysis of expression patterns predicts genetic interactions at the mid-hindbrain boundary. PLoS Comput Biol. 2009;5(11):e1000569. doi: 10.1371/journal.pcbi.1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dworkin S, et al. Midbrain–hindbrain boundary patterning and morphogenesis are regulated by diverse grainy head-like 2-dependent pathways. Development. 2012;139(3):525–536. doi: 10.1242/dev.066522. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura H, Sato T, Suzuki-Hirano A. Isthmus organizer for mesencephalon and metencephalon. Dev Growth Differ. 2008;50(Suppl 1):S113–S118. doi: 10.1111/j.1440-169X.2008.00995.x. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki-Hirano A, Sato T, Nakamura H. Regulation of isthmic Fgf8 signal by sprouty2. Development. 2005;132(2):257–265. doi: 10.1242/dev.01581. [DOI] [PubMed] [Google Scholar]
- 47.Kim M, McGinnis W. Phosphorylation of Grainy head by ERK is essential for wound-dependent regeneration but not for development of an epidermal barrier. Proc Nat Acad Sci USA. 2011;108(2):650–655. doi: 10.1073/pnas.1016386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buckles GR, et al. Combinatorial Wnt control of zebrafish midbrain–hindbrain boundary formation. Mech Dev. 2004;121(5):437–447. doi: 10.1016/j.mod.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 49.Adams KA, et al. The transcription factor Lmx1b maintains Wnt1 expression within the isthmic organizer. Development. 2000;127(9):1857–1867. doi: 10.1242/dev.127.9.1857. [DOI] [PubMed] [Google Scholar]
- 50.O’Hara FP, et al. Zebrafish Lmx1b.1 and Lmx1b.2 are required for maintenance of the isthmic organizer. Development. 2005;132(14):3163–3173. doi: 10.1242/dev.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo C, et al. Lmx1b is essential for Fgf8 and Wnt1 expression in the isthmic organizer during tectum and cerebellum development in mice. Development. 2007;134(2):317–325. doi: 10.1242/dev.02745. [DOI] [PubMed] [Google Scholar]
- 52.Erickson T, et al. Pbx proteins cooperate with Engrailed to pattern the midbrain–hindbrain and diencephalic-mesencephalic boundaries. Developmental biology. 2007;301(2):504–517. doi: 10.1016/j.ydbio.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koike S, et al. Autotaxin is required for the cranial neural tube closure and establishment of the midbrain–hindbrain boundary during mouse development. Dev Dyn Off Publ Am Assoc Anat. 2011;240(2):413–421. doi: 10.1002/dvdy.22543. [DOI] [PubMed] [Google Scholar]
- 54.Belting HG, et al. spiel ohne grenzen/pou2 is required during establishment of the zebrafish midbrain–hindbrain boundary organizer. Development. 2001;128(21):4165–4176. doi: 10.1242/dev.128.21.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bouchard M, et al. Identification of Pax2-regulated genes by expression profiling of the mid-hindbrain organizer region. Development. 2005;132(11):2633–2643. doi: 10.1242/dev.01833. [DOI] [PubMed] [Google Scholar]
- 56.Xu J, Liu Z, Ornitz DM. Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development. 2000;127(9):1833–1843. doi: 10.1242/dev.127.9.1833. [DOI] [PubMed] [Google Scholar]
- 57.Xu FX, Chye ML. Expression of cysteine proteinase during developmental events associated with programmed cell death in brinjal. Plant J Cell Mol Biol. 1999;17(3):321–327. doi: 10.1046/j.1365-313X.1999.00370.x. [DOI] [PubMed] [Google Scholar]
- 58.Panhuysen M, et al. Effects of Wnt1 signaling on proliferation in the developing mid-/hindbrain region. Mol Cell Neurosci. 2004;26(1):101–111. doi: 10.1016/j.mcn.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Sgado P, et al. Slow progressive degeneration of nigral dopaminergic neurons in postnatal Engrailed mutant mice. Proc Nat Acad Sci USA. 2006;103(41):15242–15247. doi: 10.1073/pnas.0602116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alavian KN, et al. Elevated P75NTR expression causes death of engrailed-deficient midbrain dopaminergic neurons by Erk1/2 suppression. Neural Dev. 2009;4:11. doi: 10.1186/1749-8104-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Radice GL, et al. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181(1):64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 62.Lele Z, et al. parachute/n-cadherin is required for morphogenesis and maintained integrity of the zebrafish neural tube. Development. 2002;129(14):3281–3294. doi: 10.1242/dev.129.14.3281. [DOI] [PubMed] [Google Scholar]
- 63.Dworkin S, et al. CREB activity modulates neural cell proliferation, midbrain–hindbrain organization and patterning in zebrafish. Dev Biol. 2007;307(1):127–141. doi: 10.1016/j.ydbio.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 64.Giraldez AJ, et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308(5723):833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 65.Gutzman JH, et al. Formation of the zebrafish midbrain–hindbrain boundary constriction requires laminin-dependent basal constriction. Mech Dev. 2008;125(11–12):974–983. doi: 10.1016/j.mod.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lowery LA, Sive H. Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development. 2005;132(9):2057–2067. doi: 10.1242/dev.01791. [DOI] [PubMed] [Google Scholar]
- 67.Pirone DM, Fukuhara S, Gutkind JS, Burbelo PD. SPECs, small binding proteins for Cdc42. J Biol Chem. 2000;275(30):22650–22656. doi: 10.1074/jbc.M002832200. [DOI] [PubMed] [Google Scholar]
- 68.Ching KH, Kisailus AE, Burbelo PD. The role of SPECs, small Cdc42-binding proteins, in F-actin accumulation at the immunological synapse. J Biol Chem. 2005;280(25):23660–23667. doi: 10.1074/jbc.M500128200. [DOI] [PubMed] [Google Scholar]
- 69.Ching KH, Kisailus AE, Burbelo PD. Biochemical characterization of distinct regions of SPEC molecules and their role in phagocytosis. Exp Cell Res. 2007;313(1):10–21. doi: 10.1016/j.yexcr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 70.Mullins MC, et al. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol. 1994;4(3):189–202. doi: 10.1016/S0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 71.Kudoh T, et al. A gene expression screen in zebrafish embryogenesis. Genome Res. 2001;11(12):1979–1987. doi: 10.1101/gr.209601. [DOI] [PubMed] [Google Scholar]
- 72.Thisse B, Thisse C (2004) Fast Release Clones: A High Throughput Expression Analysis. ZFIN Direct Data Submission (http://zfin.org)