Abstract
Coxsackievirus B3 (CVB3) is one of the most prevalent causes of viral myocarditis and is associated with many other pathological conditions. CVB3 replication relies on host cellular machineries and causes direct damage to host cells. MicroRNAs have been found to regulate viral infections but their roles in CVB3 infection are still poorly understood. Here we describe a novel mechanism by which miR-126 regulates two signal pathways essential for CVB3 replication. We found that CVB3-induced ERK1/2 activation triggered the phosphorylation of ETS-1 and ETS-2 transcription factors, which induced miR-126 upregulation. By using both microRNA mimics and inhibitors, we proved that the upregulated miR-126 suppressed sprouty-related, EVH1 domain containing 1 (SPRED1) and in turn enhanced ERK1/2 activation. This positive feedback loop of ERK1/2–miR-126–ERK1/2 promoted CVB3 replication. Meanwhile, miR-126 expression stimulated GSK-3β activity and induced degradation of β-catenin through suppressing LRP6 and WRCH1, two newly identified targets in the Wnt/β-catenin pathway, which sensitized the cells to virus-induced cell death and increased viral progeny release to initiate new infections. Our results demonstrate that upregulated miR-126 upon CVB3 infection targets SPRED1, LRP6, and WRCH1 genes, mediating cross-talk between ERK1/2 and Wnt/β-catenin pathways, and thus promoting viral replication and contributes to the viral cytopathogenicity.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1411-4) contains supplementary material, which is available to authorized users.
Keywords: CVB3, Myocarditis, MiR-126, ERK1/2, Wnt
Introduction
Viruses are obligate parasites and interact with their hosts by manipulating cellular signal transduction to either evade the host immune responses to maximize their replication or initiate viral progeny release to further infect surrounding healthy tissue [1]. Coxsackievirus B3 (CVB3) belongs to the enterovirus genus in the Picornaviridae family. It is one of the major pathogens causing viral myocarditis, an inflammatory heart disease accounting for ~20 % of cases of sudden unexpected death in children and young adults [2, 3]. Unlike some other viruses which inhibit cell apoptosis to increase viral particle production, CVB3 has been found to induce host cell death at the late stage of infection to facilitate the viral progeny release, enabling the infection of surrounding cells [4–6]. To date, the incomplete understanding of virus–host interactions still impedes the development of effective treatments. CVB3 has adopted many strategies to hijack host cellular machineries to benefit its own replication. The viral infection can lead to systematical changes of host gene transcription and translation, resulting in dramatic reprogramming of the signal transduction networks [7–10]. The extracellular signal regulated protein kinases (ERK1/2) signaling pathway has been found to be one of the most critical pillars of these networks during CVB3 infection. We and others have observed that CVB3 infection induces ERK1/2 phosphorylation and that the inhibition of ERK1/2 activation attenuated viral replication and subsequent cell death [11, 12]. Recent reports also showed that ERK1/2 regulates the entry of CVB3 into the host cells [13]. It is speculated that ERK1/2 phosphorylation is triggered by viral protease-mediated cleavage of RasGAP during CVB3 infection [14]. However, the regulation of downstream RAF, especially the involvement of its negative regulator, sprouty-related, EVH1 domain containing 1 (SPRED1) [15], has not been studied in CVB3 infection. β-Catenin is another essential signal involved in CVB3 infection. Our group previously showed that CVB3 infection activates glycogen synthase kinase (GSK)-3β through tyrosine kinase during early infection, leading to the inhibition of β-catenin. This signal pathway induces cytopathic effect (CPE) and subsequent cell death that benefits viral progeny release [16]. However, it is not clear why the activity of GSK-3β is only transiently increased at approximately 1 h post-infection (hpi) while the degradation of β-catenin starts much later (5–7 hpi). It is also unknown whether other upstream regulators of GSK-3β/β-catenin cascade, such as mediators in the Wnt signaling pathways [17], regulate this process.
MicroRNAs (miRNAs) are recently identified small endogenous non-coding RNAs that regulate gene expression at post-transcriptional levels [18]. One or more miRNAs can regulate multiple genes simultaneously by recognizing the target sites on the 3′ untranslated regions (3′ UTRs) of target genes [19]. The vast regulation by miRNAs enables them to modulate various physiological and pathological conditions including viral infections [20]. Viruses have been found to alter the host miRNA profiles to benefit their own replication; on the other hand, the host may respond to viral infection by adjusting its miRNA levels to inhibit viral replication. Hepatitis C virus utilizes host miR-122 to facilitate its gene translation and viral replication [21] while shrimp miR-7 is found to be upregulated in response to white spot syndrome virus (WSSV) infection and inhibits viral replication [22]. Nevertheless, the understanding of miRNA function in viral infection is still very limited. Particularly, most studies until now have focused on the regulation of a specific pathway by miRNAs while the modulation of cross-talk among multiple pathways by a single miRNA in viral infection has not been well studied. MiR-126 is a heart-enriched miRNA [23] involved in cardiovascular diseases [24, 25] but its function in CVB3-induced infectious heart disease is still entirely unknown. It is not clear whether miR-126 is a positive or negative regulator in CVB3 infection. To address this issue, we first explored the expression of miR-126 during CVB3 infection and found that it was upregulated during viral infection. We showed that the induction of miR-126 was associated with the activation of transcription factor E-twenty-six (ETS) by ERK1/2 signaling. Further studies have demonstrated that miR-126 promoted CVB3 replication and virus-induced cell death through mediating cross-talk between ERK1/2 and Wnt/β-catenin pathways by targeting SPRED1, low-density lipoprotein receptor-related protein 6 (LRP6), and Wnt-responsive Cdc42 homolog 1, also named as RhoU (WRCH1).
Materials and methods
Cell culture and viral infection
HeLa cells were cultured in DMEM (Lonza) with 10 % FBS (Sigma). Cardiomyocyte HL-1 cells were cultured in Claycomb medium (Sigma) with 10 % FBS. Human umbilical vein endothelial (HUVEC) cells were cultured using the EGM BulletKit (Lonza). Cell cultures were infected with CVB3 for 1 h, washed with phosphate buffered saline (PBS) (Lonza), and then replenished with fresh medium. Cell morphologies were photographed under a phase-contrast microscope connected to a camera (Nikon) with 100× magnification. Details are given in the supplementary material.
RNA extraction and real-time RT-PCR
Cellular RNAs were extracted using miRCURY RNA Isolation Kits (Exiqon) according to the manufacturer’s instructions. RNAs were then transcribed using the TaqMan MicroRNA Reverse Transcription Kit (Life Technologies) and miRNA levels were detected by TaqMan MicroRNA Assay (Life Technologies) using relatively quantitative methods as described previously (Ho et al. [34]). U6, a small non-coding RNA, was detected as the endogenous control. All real-time quantitative RT-PCR (q-RT-PCR) experiments were performed in triplicate with no-template as a control. Details are given in the supplementary material.
Western blot
Cells were lysed with RIPA lysis buffer. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), transferred to nitrocellulose membranes, and detected by antibodies accordingly. β-Actin was detected as a loading control. Signal intensities were quantified with the ImageJ program and normalized to one of the samples (usually the control samples, set as 1.00). All the experiments were conducted at least three times. Details are given in the supplementary material.
Transfection of miRNA mimics, siRNAs, and miRNA inhibitors
miRNA mimics (Ambion Pre-miR miRNA Precursors, Life Technologies), siRNAs (On-Target Plus SmartPool, Dharmacon) and miRNA inhibitors (Ambion Anti-miR miRNA Inhibitors, Life Technologies) were transfected into cells using Oligofectamine (Life Technologies) according to the manufacturer’s instructions. Further analysis of the samples (infection or RNA isolation) was performed at 48 h post-transfection. Details are given in the supplementary material.
Treatment of ERK1/2 or caspase-3 inhibitor
Cells were serum starved overnight and treated with ERK1/2 inhibitor U0126 (Cell Signaling Technology) (20 μM), caspase-3 inhibitor Z-VAD (Cedarlane Labs) (50 μM) or equal volume of DMSO (Sigma) starting from 30 min prior to infection [11]. Cells were then incubated with CVB3 at multiplicity of infection (MOI) of 10 for 1 h. After infection, cells were washed with PBS and replenished with serum-free medium with DMSO, U0126, or Z-VAD.
Viral plaque assay
CVB3 plaque assays were performed as previously described [26]. Briefly, sample supernatants were serially diluted and added onto HeLa cells in six-well plates (8 × 105 cells/well). After incubation for 1 h, cells were washed with PBS twice again, overlaid with 0.75 % soft agar medium, and incubated for 3 days. Cells were fixed with Carnoy’s fixative for 30 min and stained with 1 % crystal violet. The viral plaques were counted manually. The virus titers were calculated as the plaque-forming units per ml (pfu/ml). All the assays were conducted at least three times.
MTS cell viability assay
Cell viability was analyzed by CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS) (Promega) following the manufacturer’s instructions as described previously [26]. Briefly, MTS reagents were added to the wells and incubated for 2 h. The absorbance at wavelength 490 nm was measured by using an enzyme-linked immunosorbent assay (ELISA) reader (SLT Lab Instruments). All the assays were performed at least three times and the data were normalized to that of sham-infected samples (set as 100 %).
Constructs and Dual-Luciferase assay
The wild-type (wt) or mutant (mut) LRP6 or WRCH1 binding sites were synthesized, annealed, and inserted into the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega) according to the manufacturer’s instructions. The oligonucleotides used for annealing are listed in supplementary information, Fig. S6b. Dual-luciferase assay was conducted using Dual-Luciferase Reporter Assay System (Promega). The SPRED1 (Human cDNA clone) overexpression plasmid (pCMV6–SPRED1) and the corresponding empty vector (pCMV6) were purchased from Origene. Details are given in the supplementary material.
Statistical analysis
All experiments were repeated at least three times. Student’s t test was used for the paired comparison among the samples. Error bars represent mean ± SD. A p value <0.05 (labeled with “*”) in two-tailed tests was considered as statistically significant; “**” was used for labeling differences with p value <0.01.
Results
CVB3 infection induces miR-126 through ERK1/2–ETS cascade
To investigate the roles of miR-126 in CVB3 infection, particularly the association between viral infection phases and miR-126 expression levels, we infected HeLa cells with CVB3 and detected the miR-126 levels. At the early stages of infection (0–1 h), no significant change of miR-126 expression was detected between CVB3-infected and sham-infected (sh) control cells (Fig. 1a). We observed a temporal decrease of miR-126 expression at 3 hpi but a robust increase (1.5–2 folds) afterwards (Fig. 1b). Prolonged infection (16 h) of CVB3 further induced miR-126 (3–5 folds) with a positive correlation between viral titers and levels of miR-126 induction (Fig. 1c).
Fig. 1.
CVB3 infection upregulates miR-126 through ERK-ETS cascade. a–c Time- and viral titer-dependent regulation of miR-126 by CVB3 in HeLa cells. Cells were infected with CVB3 at indicated titer and time points. Cellular RNAs were isolated for detection of miR-126 using real-time RT-PCR. All data were normalized to the sham-infected samples [“*” (p < 0.05) or “**” (p < 0.01), n = 6]. d ERK1/2 and ETS-1/2 phosphorylation in CVB3-infected HeLa cells. Cells were infected by as indicated. Cellular proteins were isolated for WB detection of indicated signal proteins. β-Actin was detected as a loading control. e Inhibition of ERK1/2 blocks ETS phosphorylation during CVB3 infection. HeLa cells were treated with U0126 or DMSO (control) and then infected with CVB3. WB was conducted to detect the indicated signal proteins. f Inhibition of ERK1/2 suppresses miR-126 upregulation during CVB3 infection. HeLa cells were treated with U0126 or DMSO and then infected as indicated. MiR-126 levels were detected by q-RT-PCR
To elucidate the mechanism by which CVB3 upregulates host miR-126, we detected the expression of ETS-1 and ETS-2, two transcription factors involved in miR-126 induction [27]. Surprisingly, both ETS-1 and ETS-2 declined gradually throughout the course of CVB3 infection (Fig. 1d). As the activities of ETS-1/2 are enhanced by ERK1/2-mediated phosphorylation at threonine 38 (T38) for ETS-1 and (T72) for ETS-2 [28], we thus further examined the phosphorylation levels of ERK1/2 and ETS-1/2. The data showed that ERK1/2 had a temporal phosphorylation at 30 min followed by a continuous activation starting from 5 hpi. The later ERK1/2 activation correlated well with the elevated phosphorylation levels of ETS-1/2 (Fig. 1d). In prolonged infection with a low virus titer, ETS-1/2 phosphorylation was also enhanced (Fig. 1d). Similar correlations between miR-126 induction and ERK1/2–ETS phosphorylation were also found in cardiomyocytes HL-1 and HUVEC cells (supplemental Fig. S1a–d). In addition, we used UV-inactivated CVB3, which can only enter the cells by endocytosis but not conduct successful replication to infect HeLa cells. The results showed that UV-treated CVB3 failed to initiate the expression of VP-1 or induce miR-126 upregulation (supplemental Fig. S1e, f).
To further confirm the association between ERK1/2–ETS phosphorylation and miR-126 induction during CVB3 infection, we treated the cells with ERK1/2 inhibitor U0126. We found that U0126 dramatically inhibited ERK1/2 and ETS phosphorylation compared with DMSO (control) during CVB3 infection (Fig. 1e). Correspondingly, miR-126 level was downregulated significantly when treated with U0126 during CVB3 infection (Fig. 1f). These data imply that CVB3 infection induces miR-126 through ERK1/2–ETS signal cascade.
MiR-126 promotes CVB3 replication
To study the effect of CVB3-induced miR-126 expression on host–virus interactions, we first transfected cells with miRNA mimics to test their effect on CVB3 replication. Two different concentrations (0.1, 1 nM) were used to evaluate potential dose effect. q-RT-PCR showed that miR-126 mimics increased cellular miR-126 levels at 48 h post-transfection compared to the scrambled control miRNA mimics (miR-CL) (supplemental Fig. S2a). The cells were then infected with CVB3 for 7 h. Western blot (WB) results showed that 0.1 and 1 nM of miR-126 mimics led to increased viral protein (VP-1) expression by 1.8- and 2.2-fold, respectively (Fig. 2a). Viral plaque assay using the collected supernatants post-infection demonstrated that miR-126 significantly enhanced viral progeny release and this effect was positively correlated with the miR-126 concentration as well (Fig. 2c).
Fig. 2.
miR-126 promotes CVB3 replication. HeLa cells were transfected with miRNA mimics (a, c) or miRNA inhibitors (b, d) as indicated for 48 h. Cells were then infected by CVB3 at 10 MOI for 7 h. Total proteins were isolated for detection of VP-1 by WB as an indicator for viral replication (a, b). The intensities of the bands were measured with ImageJ software and the signal ratios are listed below. Viral titer was determined by plaque assay using the supernatants collected at 7 hpi. p < 0.05, n = 3 (c, d)
To confirm that the above observation was not due to the non-specific effect of miRNA overexpression, we applied miRNA inhibitors to knock down the endogenous miR-126 level. Q-RT-PCR showed the successful inhibition of cellular miR-126 by miR-126 inhibitor (126-in) as compared to control (CL-in) (supplemental Fig. S2b). In contrast to miR-126 mimics, 126-in significantly suppressed VP-1 expression by 50 % and viral progeny release by 80 % as compared to the control inhibitor (Fig. 2b, d). Together, our results show that miR-126 benefits CVB3 replication.
MiR-126 promotes CVB3 replication by targeting SPRED1
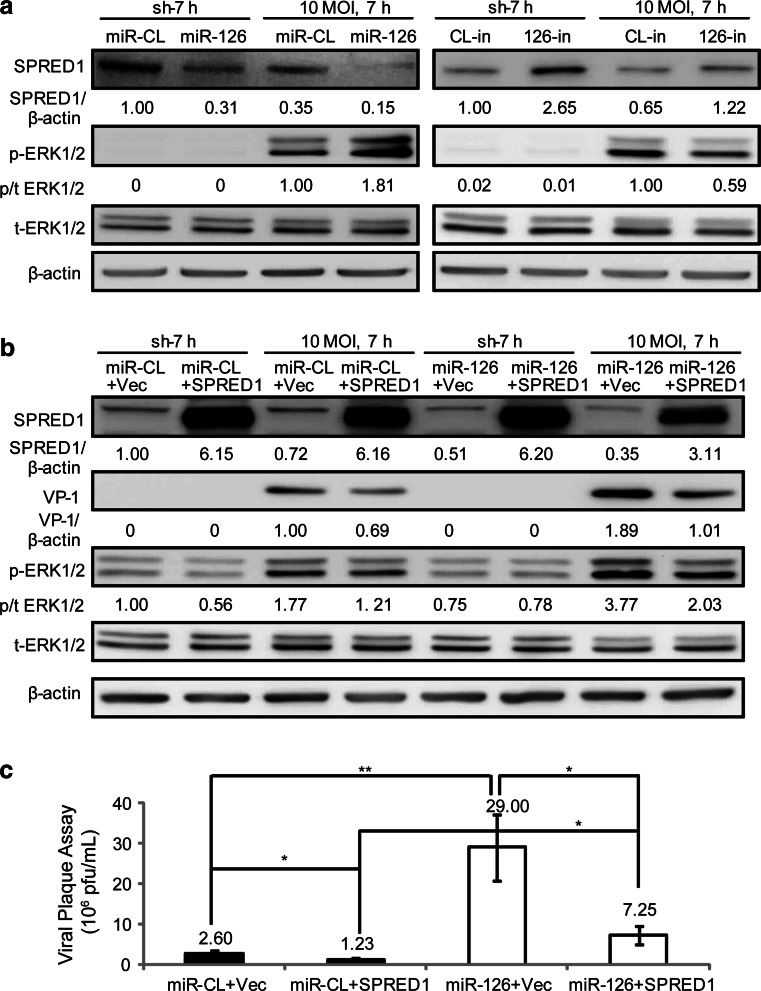
SPRED1 has been reported as a miR-126 target gene that negatively regulates the ERK1/2 signaling pathway [24]. Further, the ERK1/2 pathway is known to be essential for CVB3 replication [11, 29]. However, the role of SPRED1 in making the connection among these factors has not been studied. Thus, investigation of this question may elucidate the underlying mechanism by which miR-126 regulates CVB3 replication. To this end, we transfected the cells with miR-126 mimics (0.1 nM) or 126-in (50 nM) to explore their influences on SPRED1 and ERK1/2 activation and subsequently on CVB3 replication. In all four groups (miR-CL, miR-126, CL-in, and 126-in), a significant reduction in SPRED1 was found in CVB3-infected cells (10 MOI, 7 h) as compared to control (no virus, sh-7 h). In miR-126 mimic-transfected samples, the SPRED1 level was 0.31 and 0.15 in sham- and CVB3-infected cells, respectively (Fig. 3a). This is approximately 70 % lower than that of the miR-CL group with 1.00 in sham and 0.55 in CVB3-infected samples. Corresponding to the inhibition in SPRED1 expression, miR-126 mimic enhanced the ERK1/2 activation by 1.8-fold after CVB3 infection (Fig. 3a). On the contrary, when treated with 126-in to knock down cellular miR-126, SPRED1 levels were increased by ~2.5-fold while ERK1/2 activation was decreased by 40 % during viral infection (Fig. 3a).
Fig. 3.
MiR-126 promotes CVB3 replication by targeting SPRED1 and enhancing ERK1/2 activation. a MiR-126 suppressed SPRED1 and enhanced ERK1/2 phosphorylation. HeLa cells were transfected with miRNA mimics (0.1 nM) or miRNA inhibitors (50 nM) and then infected with CVB3 as indicated. Cellular proteins were applied to WB detection of indicated signals. The intensities of the bands were measured by ImageJ software and the signal ratios are listed. b Overexpression of SPRED1 partially reversed the effect of miR-126 on VP-1 production and ERK1/2 activation. HeLa cells were co-transfected with miRNA mimics (0.1 nM) and SPRED1 plasmid or empty vector (Vec) and infected with CVB3 as indicated. The signals were detected by WB. The intensities of the bands were measured by ImageJ and the signal ratios are listed. c Overexpression of SPRED1 inhibited viral progeny release. HeLa cells were co-transfected with miRNA mimics and SPRED1 plasmid and then infected with CVB3. Viral titers were determined by plaque assay using the supernatants. p < 0.05 (“*”), p < 0.01 (“**”), n = 3
To further confirm that SPRED1 downregulation by miR-126 benefits CVB3 replication, we overexpressed SPRED1 in the presence of miR-126 by co-transfection. WB results showed that compared with empty vector (miR-CL + Vec), overexpression of SPRED1 (miR-CL + SPRED1) inhibited both VP-1 production and ERK1/2 phosphorylation by approximately 30 % (Fig. 3b). When the SPRED1 overexpression vector was co-transfected with miR-126 (miR-126 + SPRED1), the VP-1 expression and ERK1/2 activation was almost restored to the levels in the miR-CL + Vec group. In addition, SPRED1 overexpression reduced the viral progeny release by ~50 % in the absence of miR-126, but it failed to completely diminish the effect of miR-126 on viral progeny release as evidenced by the production of more viral plaque in the miR-126 + SPRED1 group than in the miR-CL + Vec group (p < 0.05) (Fig. 3c). We also used small interfering RNA (siRNA) to knock down the expression of SPRED1. We found that SPRED1 siRNA induced a ~70 % inhibition of the SPRED1 expression, which was accompanied with a 1.5-fold increase in both ERK1/2 phosphorylation and VP-1 synthesis during CVB3 infection (supplemental Fig. S3a). Viral plaque assay also showed that SPRED1 siRNA enhanced viral release by approximately twofold (supplemental Fig. S3b). To further confirm the role of miR-126 in SPRED1 inhibition and ERK activation, we also tested the miR-126 effect on CVB3 replication using HL-1 cells. The results showed that the miR-126 inhibitor suppressed cellular miR-126 expression, ERK1/2 activation, VP-1 synthesis, and viral progeny release in HL-1 cells (supplemental Fig. S3c–e).
To validate that VP-1 upregulation by the miR-126-SPRED1 cascade is indeed through the ERK1/2 pathway, we treated cells with ERK1/2 inhibitor U0126 in the presence of miR-126 mimics or SPRED1 siRNA. Though miR-126 and SPRED1 siRNA still successfully inhibited SPRED1, their effect on upregulation of VP-1 was no longer observed in the presence of U0126, which inhibited ERK activation (supplemental Fig. S4a). This indicates that ERK1/2 activation is necessary downstream effecters for miR-126-SPRED1 pathway to promote CVB3 replication. Surprisingly, in the presence of U0126, a ~7-fold increase in viral progeny release was still observed in miR-126-transfected cells (8.25 × 106 pfu/ml) as compared to miR-CL (1.23 × 106 pfu/ml) (supplemental Fig. S4b). In contrast, U0126 efficiently diminished the effect of SPRED1 siRNA on viral progeny release (supplemental Fig. S4c). This indicates that other miR-126 targets may also contribute to CVB3 progeny release in addition to SPRED1. The above data showed that suppression of SPRED1 by miR-126 enhanced ERK1/2 activation and viral replication during CVB3 infection.
MiR-126 sensitizes cells to CVB3-induced cell death and enhances viral progeny release
As mentioned above, neither SPRED1 overexpression nor ERK1/2 inhibition can completely block the effect of miR-126 on enhancing viral progeny release, which indicates that other mechanisms of miR-126 action also play an important role in this regard. Previous studies showed that CVB3-induced CPE and cell death is essential to viral progeny release [4, 5, 16]. We therefore investigated whether miR-126 would affect virus-induced CPE and cell death. Morphological data showed that transfection of miR-126 induced more degenerative changes (cell rounding and detaching) and CPE in CVB3-infected cells than in miR-CL cells (Fig. 4a). The cell morphological degeneration was more severe in the cells transfected with a higher concentration of miR-126, suggesting that miR-126 enhances CVB3-induced cell death. In support of this observation, we conducted WB to detect pro-caspase-3 cleavage after CVB3 infection. As expected, we found a substantial elevation of pro-caspase-3 cleavage in the miR-126 groups compared to miR-CL (Fig. 4b). This elevation of cleavage is particularly apparent in cells transfected with higher concentrations of miR-126. In contrast to miR-126 mimics, 126-in significantly suppressed the virus-induced CPE and caspase-3 activation post-CVB3 infection (Fig. 4c, d). To quantify the effect of miR-126 on cell death during CVB3 infection, we conducted MTS assay. The results showed that miR-126 mimic transfected at 0.1 nM led to ~25 and ~50 % reduction in cell survival compared with miR-CL at 7 and 16 hpi, respectively (Fig. 4e). When the concentration of miR-126 increased, the reduction in cell survival was also enhanced. We also confirmed this effect by using 126-in, which increase cell survival by 1.2- and 2-fold at 7 and 16 hpi, respectively (Fig. 4f). Together, our results showed that miR-126 sensitized the cells to CVB3-induced cell death.
Fig. 4.
MiR-126 enhances CVB3-induced CPE and cell death. HeLa cells were transfected with miRNA mimics (a, b, e) or miRNA inhibitors (c, d, f) as indicated for 48 h. Cells were then infected by CVB3 at 10 MOI for 7 h or 0.05 MOI for 16 h. The morphologies of cells were observed under a phase contrast microscope. Magnification ×100; Scale bar 30 μm (a, c). Caspase-3 cleavage was evaluated by WB as an indicator of cell apoptosis (b, d). The intensities of the bands were measured by ImageJ and the signal ratios of cleaved to procaspase-3 are listed. Cell survival rates were measured by MTS assay (e, f). p < 0.01, n = 5
To test whether the enhanced cell death mediated by miR-126 expression benefited viral progeny release, we used caspase-3 inhibitor Z-VAD to suppress virus-induced cell apoptosis. Cells were transfected with miR-CL or miR-126, treated with Z-VAD and then infected with CVB3. The results showed that Z-VAD inhibited the effect of miR-126 on cell death (supplemental Fig. S5a). Though miR-126 still effectively suppressed SPRED1 and increased VP-1 in the presence of Z-VAD (supplemental Fig. S5b), its influence on viral progeny release was reduced from tenfold (in DMSO group) to fourfold (in the Z-VAD group) (supplemental Fig. S5c). Particularly, miR-126 + Z-VAD showed similar viral progeny release level as miR-CL + DMSO. These results indicated that miR-126 enhanced the CVB3-induced cell death that benefits viral progeny release.
MiR-126 sensitizes cells to CVB3-induced cell death by targeting WRCH1 and LRP6 and promoting β-catenin degradation
To explore the signal transduction pathways regulated by miR-126 during the process of CVB3-induced CPE and cell death, we searched for its potential targets in a miRNA target database, TargetScan [30]. A total of 154 targets were identified, with 25 conserved and 129 non-conserved ones. Conservation was determined by the sequence homology among different species. Among the top ten conserved targets across different species, we selected LRP6, a receptor of the Wnt/β-catenin signaling pathway [31], for further investigation. This is due to our previous finding that β-catenin is a major signal molecule contributing to CVB3-induced CPE and cell death [16] (Fig. 5a; supplemental Table S1). More interestingly, another Wnt signaling-related gene, WRCH1, is one of the non-conserved potential targets of miR-126 (Fig. 5a). We thus further studied these two genes during CVB3 infection and their regulation by miR-126.
Fig. 5.
LRP6 and WRCH1 are specific targets of miR-126. a Prediction of miR-126 target on LRP6 and WRCH1. Bioinformatic prediction was conducted using TargetScan. The base pairing between miR-126 and its potential target sites are listed. The “G–U” paring is labeled with “•”. The perfect matching between the seed region and seed-match region is boxed. b MiR-126 suppressed the expression of LRP6 and WRCH1. HeLa cells were transfected with miR-126 (0.1 nM) and infected with CVB3 as indicated. Cellular proteins were collected for WB analysis of the expression of LRP6 and WRCH1. The intensities of the bands were measured by ImageJ and the signal ratios are listed. c Validation of miR-126 targeting on LRP6 and WRCH1 by luciferase assay. HeLa cells were co-transfected with miR-126 or miR-CL and luciferase reporter vectors harboring the wt or mut targeting sites. Firefly and Renilla luciferase activities were detected by dual-luciferase assay and the firefly/Renilla luciferase ratios were calculated. All data were normalized to that of cells co-transfected with miR-CL and wt reporter vector (set as 1.0). p < 0.01, n = 4
To study how miR-126 regulates LRP6 and WRCH1, we transfected HeLa cells with miR-126 or miR-CL mimics at the final concentration of 0.1 nM and detected the expression levels of these genes. WB results showed that miR-126 suppressed the expression of LRP6 and WRCH1 as compared to the control (miR-CL set as 1.00) (Fig. 5b). In addition, correlated to the upregulation of miR-126 after CVB3 infection (Fig. 1b), the expression levels of LRP6 and WRCH1 were downregulated in CVB3-infected samples compared to the sham-infected ones. These results suggested that miR-126 can suppress the expression of LRP6 and WRCH1. To verify that LRP6 and WRCH1 are true targets of miR-126, we constructed luciferase reporter vectors harboring either wild-type or mutant miR-126 targeting sites in the WRCH1 or LRP6 3′ UTR. The mutations were introduced by changing four base-pairs in the seed match regions (supplemental Fig. S6). Luciferase assay showed that miR-126 significantly decreased the luciferase activities in both LRP6 and WRCH1 reporter constructs containing the wt 3′UTR but not the mut ones (Fig. 5c), indicating that WRCH1 and LRP6 are two novel targets of miR-126.
To understand the roles of LRP6 and WRCH1 in miR-126-mediated Wnt/β-catenin signaling pathway, we examined the downstream effecter gene expression after CVB3 infection. We first confirmed that miR-126 mimic suppressed the expression of LRP6 and WRCH1 while miR-126 inhibitor enhanced their expression (Fig. 6a). Previous studies showed that GSK-3β induces β-catenin degradation and this process plays important roles in CVB3-induced CPE and viral progeny release [16]. However, GSK-3β phosphorylation inhibits its activity in inducing β-catenin degradation [16] while LRP6 and the homolog of WRCH1, cdc42, are associated with GSK-3β/β-catenin cascade [17, 32]. We therefore tested the GSK-3β phosphorylation and β-catenin degradation after miR-126 transfection and CVB3 infection. Significant inhibition of GSK-3β phosphorylation and increases in β-catenin degradation were observed in miR-126 transfected group (Fig. 6a). This finding was confirmed by using miR-126 inhibitor, which promoted GSK-3β phosphorylation and preserved β-catenin from degradation (Fig. 6a). To further investigate the roles of LRP6 and WRCH1 in GSK-3β/β-catenin cascade during CVB3 infection, we used siRNAs to knock down their expression levels. As expected, inhibition of these two genes by siRNAs promoted β-catenin degradation (Fig. 6b, c). WRCH1 siRNA suppressed GSK-3β phosphorylation, similar to the function of miR-126. LRP6 siRNA showed almost no effect on GSK-3β phosphorylation. In addition, LRP6 and WRCH1 siRNA had little effect on VP-1 levels (Fig. 6b, c). These data indicate that miR-126 promotes β-catenin degradation by targeting LRP6 and WRCH1.
Fig. 6.
Regulation of GSK-3β/β-catenin cascade by miR-126 through targeting WRCH1 and LRP6. a miR-126 regulates GSK-3β phosphorylation and β-catenin degradation. HeLa cells were transfected with miRNA mimics (0.1 nM) or miRNA inhibitors (50 nM) and infected with CVB3. Indicated signals were detected by WB and analyzed using ImageJ software. b, c Knocking down of LRP6 or WRCH1 inhibited GSK-3β phosphorylation and enhanced β-catenin degradation. HeLa cells were transfected with LRP6 siRNA (b) or WRCH1 siRNA (c) and infected with CVB3. Indicated signals were detected by WB and analyzed using ImageJ software
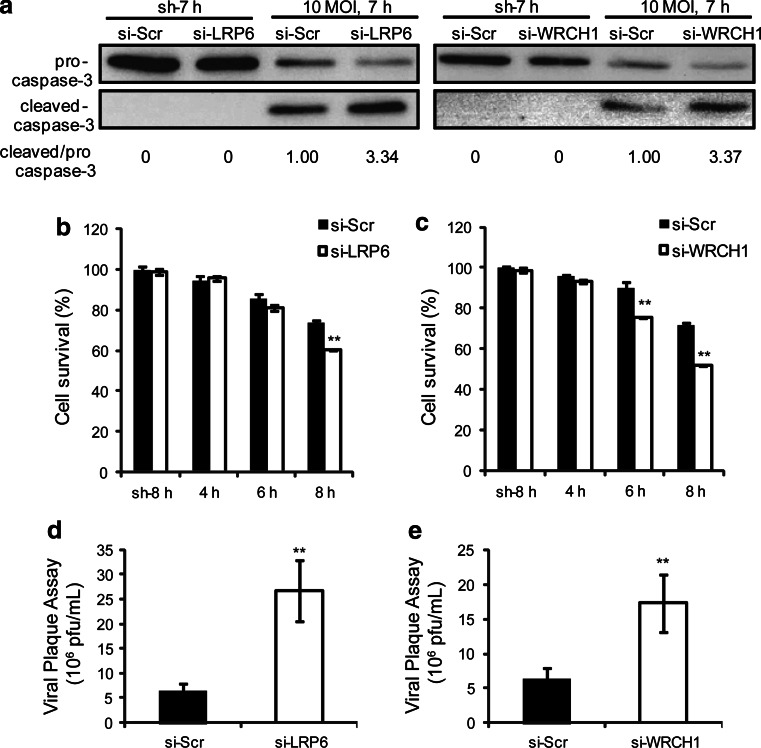
We then further confirmed the roles of LRP6 and WRCH1 on CVB3-induced cell death and viral progeny release. Consistent with the β-catenin degradation data, both siRNAs significantly enhanced the virus-induced cell death as evidenced by caspase-3 activation and MTS assay data (Fig. 7a–c). As mentioned above, CVB3 induces cell death to benefit its progeny release [4–6]. As expected, LRP6 and WRCH1 siRNAs both enhanced viral progeny release (Fig. 7d, e). To further confirm the targeting effect of miR-126 on LRP6 and WRCH1, we repeated the test using another human cell line, HUVEC. We found that miR-126 inhibitor successfully repressed cellular miR-126 levels, preserved LRP6 and WRCH1 expression, increased GSK-3β phosphorylation, delayed β-catenin degradation and inhibited CVB3-induced caspase-3 activation (supplemental Fig. S7). The above findings reveal that miR-126 targets LRP6 and WRCH1 and promotes β-catenin degradation through regulating GSK-β activity, contributing to the virus-induced cell death and viral progeny release.
Fig. 7.
Knockdown of LRP6 or WRCH1 sensitizes the cells to CVB3-induced cell death and enhances viral progeny release. HeLa cells were transfected with siRNAs targeting LRP6 or WRCH1 and then infected with CVB3. Caspase-3 activation was detected by WB (a). Cell survival rates were measured by MTS assay (b, c) and viral progeny release was quantified by plaque assay (d, e). p < 0.01, n = 4
Discussion
Virus–host interactions determine viral pathogenesis and are modulated by a complicated signaling network. miRNA, as a novel regulator in gene expression, plays an essential role in this interplay [20]. To study the functional role of miRNAs in CVB3 replication and cytopathogenicity, we selected a heart-abundant miRNA, miR-126, to study its regulatory role in the signaling network governing the replication of this cardiotropic virus. We found that miR-126 expression was regulated in a dynamic manner coinciding with the activity of transcription factor ETS. During CVB3 infection, miR-126 was upregulated following ETS activation. By targeting SPRED1, miR-126 enhanced ERK1/2 activation that supported CVB3 replication. In addition, we identified two novel targets of miR-126, LRP6 and WRCH1, in the Wnt/β-catenin signal pathway. Suppression of these two target genes by miR-126 sensitized the cells to virus-induced cell death and promoted viral progeny release in the late stages of infection.
Upon further analysis, we found that CVB3 upregulated miR-126 in a time- and viral titer-dependent manner. During the early phase of infection (3 hpi), miR-126 was transiently decreased, which could be explained by the overall inhibition of host RNA synthesis by CVB3 infection [33]. In later stages of infection, miR-126 was highly induced. We speculated that this was regulated by transcription factors ETS-1/2. A recent study showed that silencing of ETS-1/2 suppressed the expression of miR-126 and identified the binding sites of ETS-1/2 on miR-126 promoter region [27]. It is, however, not clear whether this regulation is mainly dependent on the total level of ETS proteins or their activity, which is regulated by their phosphorylation mediated by ERK1/2 [28]. Our results showed that CVB3 infection induced gradual downregulation of total ETS-1 and ETS-2 proteins, which may be due to the shutdown of host protein translation [34]. However, the phosphorylation of ETS-1/2 increased at the late stages of infection, coinciding with the activation of the ERK1/2 signaling and the induction of miR-126 at the same phase. This observation was confirmed in three different cell types and further solidified by experiment using UV-inactivated CVB3. It has been reported that UV-inactivated CVB3 cannot induce ERK1/2 signaling [11]. Our experiment showed that when infected with UV-irradiated CVB3, miR-126 was not induced. Importantly, ERK1/2 inhibitor U0126 suppressed the phosphorylation of ETS and upregulation of miR-126 during CVB3 infection, further supporting the previous data. These findings reveal a novel mechanism of miR-126 regulation mediated by ERK1/2–ETS cascade. On the other hand, the upregulated miR-126 targets SPRED1 and in turn enhances ERK1/2 activation and CVB3 replication. ERK1/2 activation is essential to CVB3 replication [11], but it is not clear whether its upstream negative regulator, SPRED1 [15], is involved in regulating viral replication. Here, we showed that CVB3 infection downregulates SPRED1, correlating well with the induction of miR-126. The suppression of SPRED1 by miR-126 or siRNA is beneficial to ERK1/2 activation and CVB3 replication. This notion was further supported by using the ERK1/2 inhibitor U0126, which blocked the effect of miR-126 or SPRED1 siRNA on VP-1 upregulation. The above findings suggest a positive feedback loop of ERK1/2–ETS–miR-126–SPRED1–ERK1/2 that is hijacked by CVB3 for the sake of its own replication. Previous report showed that Marek’s disease virus downregulated miR-126 in T-lymphoma cells [35], suggesting that the alteration of miR-126 during viral infection depends on the virus species. Our studies provide new evidence to support the notion that virus hijacks host miRNA expression.
We observed that overexpression of SPRED1 did not fully block the effect of miR-126 on viral progeny release, indicating that other signals may be involved in this regulation. In search for other signals, we found that miR-126 enhanced CVB3-induced cell death through GSK-3β/β-catenin cascade by targeting LRP6 and WRCH1. We previously found that virus-induced CPE, cell death, and subsequent viral progeny release are partially mediated by β-catenin degradation via GSK-3β [16]. However, some major questions were not clear. First, GSK-3β activation peaked at 1 hpi but declined to normal level afterwards; while the degradation of β-catenin starts long after 1 hpi (from 5 hpi) [16]. Why is there such a delay and how does this transient activation of GSK-3β continue to support the degradation of β-catenin until the end stage of infection? Second, CVB3 infection induces dramatic phosphorylation of GSK-3β at later stage of infection [36] and phosphorylation of GSK-3β inhibits its activity. Then why does the GSK-3β activity remain quite stable at the late stage of infection as evidenced by the activity assay [16]? What are the factors involved in maintaining GSK-3β activity? Here, we showed that miR-126 mimic enhanced β-catenin degradation while miR-126 inhibitor suppressed this process. All these actions are through regulation of LRP6 and WRCH1 expression. The effect of WRCH1 on β-catenin activity has not been studied previously, but the homolog of WRCH1, cdc42, was shown to stabilize β-catenin through inhibiting GSK-3β activity via phosphorylation [17]. This implies that WRCH1 may have comparable functions as its homolog in β-catenin signaling. We did not find a significant effect of LRP6 on GSK-3β phosphorylation, which is consistent with previous findings showing that LRP6 directly inhibits β-catenin degradation by preventing phosphorylation of β-catenin by GSK-3β [37]. Our findings suggest that increases in miR-126 during the late stage of viral infection contribute to the downregulation of LRP6 and WRCH1. The decline of WRCH1 may counteract the phosphorylation of GSK-3β to extend its activity, while the suppression of LRP6 makes β-catenin more sensitive to GSK-3β mediated degradation. Thus, both target gene suppressions by miR-126 play an important role in the continuation of GSK-3β-mediated β-catenin degradation and the further facilitation of CVB3-induced cell death and progeny release.
In summary, we identified miR-126 as a novel regulator of CVB3 replication. This miRNA has three specific targets, SPRED1, LRP6, and WRCH1, distributed in the ERK1/2 and Wnt/β-catenin signal pathways. Through suppression of these three genes during viral infection, miR-126 can coordinate the collaboration of these two signal pathways, which maintains a dynamic balance of viral particle formation and release to initiate new infections. This regulatory process of host-virus interactions is beneficial to the CVB3 life cycle. To our knowledge, this is the first report on the miRNA-mediated cross talk of different signal pathways during picornaviral infection. These findings enrich our understanding of the functional roles of miRNAs in viral replication (summarized in Fig. 8) and provide novel insights into the development of therapeutic strategies.
Fig. 8.
Proposed model of regulatory roles of miR-126 in CVB3 infection. CVB3 infection induces ERK1/2 phosphorylation, which triggers the activation of ETS transcription factors, leading to miR-126 upregulation. On one hand, miR-126 targets SPRED1 and initiates a positive feedback loop of ERK1/2 signaling pathway to accelerate the viral replication (orange arrows). On the other hand, miR-126 suppresses LRP6 and WRCH1 to inhibit Wnt/β-catenin cascade and promote virus-induced cell death, contributing to viral progeny release in the late stage of infection. Blue arrows indicate down-regulation and red arrows indicate up-regulation
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by a grant from Canadian Institute of Health Research (MOP231119).
Footnotes
X. Ye and M. G. Hemida contributed equally to this work.
References
- 1.Granville DJ, Carthy CM, Yang D, Hunt DW, McManus BM. Interaction of viral proteins with host cell death machinery. Cell Death Differ. 1998;5(8):653–659. doi: 10.1038/sj.cdd.4400388. [DOI] [PubMed] [Google Scholar]
- 2.Huber SA, Gauntt CJ, Sakkinen P. Enteroviruses and myocarditis: viral pathogenesis through replication, cytokine induction, and immunopathogenicity. Adv Virus Res. 1998;51:35–80. doi: 10.1016/S0065-3527(08)60783-6. [DOI] [PubMed] [Google Scholar]
- 3.Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, Pearse LA, Virmani R. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141(11):829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 4.Carthy CM, Granville DJ, Watson KA, Anderson DR, Wilson JE, Yang D, Hunt DW, McManus BM. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J Virol. 1998;72(9):7669–7675. doi: 10.1128/jvi.72.9.7669-7675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carthy CM, Yanagawa B, Luo H, Granville DJ, Yang D, Cheung P, Cheung C, Esfandiarei M, Rudin CM, Thompson CB, Hunt DW, McManus BM. Bcl-2 and Bcl-xL overexpression inhibits cytochrome c release, activation of multiple caspases, and virus release following coxsackievirus B3 infection. Virology. 2003;313(1):147–157. doi: 10.1016/S0042-6822(03)00242-3. [DOI] [PubMed] [Google Scholar]
- 6.Yuan JP, Zhao W, Wang HT, Wu KY, Li T, Guo XK, Tong SQ. Coxsackievirus B3-induced apoptosis and caspase-3. Cell Res. 2003;13(3):203–209. doi: 10.1038/sj.cr.7290165. [DOI] [PubMed] [Google Scholar]
- 7.Taylor LA, Carthy CM, Yang D, Saad K, Wong D, Schreiner G, Stanton LW, McManus BM. Host gene regulation during coxsackievirus B3 infection in mice: assessment by microarrays. Circ Res. 2000;87(4):328–334. doi: 10.1161/01.RES.87.4.328. [DOI] [PubMed] [Google Scholar]
- 8.Zhang ZC, Li SJ, Yang YZ, Chen RZ, Ge JB, Chen HZ. Microarray analysis of extracellular matrix genes expression in myocardium of mouse with Coxsackie virus B3 myocarditis. Chin Med J (Engl) 2004;117(8):1228–1231. [PubMed] [Google Scholar]
- 9.Rassmann A, Henke A, Zobawa M, Carlsohn M, Saluz HP, Grabley S, Lottspeich F, Munder T. Proteome alterations in human host cells infected with coxsackievirus B3. J Gen Virol. 2006;87(Pt 9):2631–2638. doi: 10.1099/vir.0.81819-0. [DOI] [PubMed] [Google Scholar]
- 10.Hammer E, Phong TQ, Steil L, Klingel K, Salazar MG, Bernhardt J, Kandolf R, Kroemer HK, Felix SB, Volker U. Viral myocarditis induced by Coxsackievirus B3 in A.BY/SnJ mice: analysis of changes in the myocardial proteome. Proteomics. 2010;10(9):1802–1818. doi: 10.1002/pmic.200900734. [DOI] [PubMed] [Google Scholar]
- 11.Luo H, Yanagawa B, Zhang J, Luo Z, Zhang M, Esfandiarei M, Carthy C, Wilson JE, Yang D, McManus BM. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J Virol. 2002;76(7):3365–3373. doi: 10.1128/JVI.76.7.3365-3373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham KA, Chapman NM, Carson SD. Caspase-3 activation and ERK phosphorylation during CVB3 infection of cells: influence of the coxsackievirus and adenovirus receptor and engineered variants. Virus Res. 2003;92(2):179–186. doi: 10.1016/S0168-1702(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 13.Marchant D, Sall A, Si X, Abraham T, Wu W, Luo Z, Petersen T, Hegele RG, McManus BM. ERK MAP kinase-activated Arf6 trafficking directs coxsackievirus type B3 into an unproductive compartment during virus host-cell entry. J Gen Virol. 2009;90(Pt 4):854–862. doi: 10.1099/vir.0.005868-0. [DOI] [PubMed] [Google Scholar]
- 14.Huber M, Watson KA, Selinka HC, Carthy CM, Klingel K, McManus BM, Kandolf R. Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J Virol. 1999;73(5):3587–3594. doi: 10.1128/jvi.73.5.3587-3594.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakioka T, Sasaki A, Kato R, Shouda T, Matsumoto A, Miyoshi K, Tsuneoka M, Komiya S, Baron R, Yoshimura A. Spred is a Sprouty-related suppressor of Ras signalling. Nature. 2001;412(6847):647–651. doi: 10.1038/35088082. [DOI] [PubMed] [Google Scholar]
- 16.Yuan J, Zhang J, Wong BW, Si X, Wong J, Yang D, Luo H. Inhibition of glycogen synthase kinase 3beta suppresses coxsackievirus-induced cytopathic effect and apoptosis via stabilization of beta-catenin. Cell Death Differ. 2005;12(8):1097–1106. doi: 10.1038/sj.cdd.4401652. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Quondamatteo F, Lefever T, Czuchra A, Meyer H, Chrostek A, Paus R, Langbein L, Brakebusch C. Cdc42 controls progenitor cell differentiation and beta-catenin turnover in skin. Genes Dev. 2006;20(5):571–585. doi: 10.1101/gad.361406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 19.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13(4):271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan CS, Ganem D. MicroRNAs and viral infection. Mol Cell. 2005;20(1):3–7. doi: 10.1016/j.molcel.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Jangra RK, Yi M, Lemon SM. Regulation of hepatitis C virus translation and infectious virus production by the microRNA miR-122. J Virol. 2010;84(13):6615–6625. doi: 10.1128/JVI.00417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang T, Zhang X. Functional analysis of a crustacean microRNA in host-virus interactions. J Virol. 2012;86(23):12997–13004. doi: 10.1128/JVI.01702-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu B, Peng XC, Zheng XL, Wang J, Qin YW. MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer. 2009;66(2):169–175. doi: 10.1016/j.lungcan.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. MiR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Small EM, Frost RJ, Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation. 2010;121(8):1022–1032. doi: 10.1161/CIRCULATIONAHA.109.889048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye X, Liu Z, Hemida MG, Yang D. Targeted delivery of mutant tolerant anti-coxsackievirus artificial microRNAs using folate conjugated bacteriophage Phi29 pRNA. PLoS One. 2011;6(6):e21215. doi: 10.1371/journal.pone.0021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris TA, Yamakuchi M, Kondo M, Oettgen P, Lowenstein CJ. Ets-1 and Ets-2 regulate the expression of microRNA-126 in endothelial cells. Arter Thromb Vasc Biol. 2010;30(10):1990–1997. doi: 10.1161/ATVBAHA.110.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foulds CE, Nelson ML, Blaszczak AG, Graves BJ. Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Mol Cell Biol. 2004;24(24):10954–10964. doi: 10.1128/MCB.24.24.10954-10964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim BK, Nam JH, Gil CO, Yun SH, Choi JH, Kim DK, Jeon ES. Coxsackievirus B3 replication is related to activation of the late extracellular signal-regulated kinase (ERK) signal. Virus Res. 2005;113(2):153–157. doi: 10.1016/j.virusres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol. 2011;18(10):1139–1146. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh JC, He X. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135(2):367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu G, Huang H, Garcia Abreu J, He X. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One. 2009;4(3):e4926. doi: 10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–155. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 34.Ho BC, Yu SL, Chen JJ, Chang SY, Yan BS, Hong QS, Singh S, Kao CL, Chen HY, Su KY, Li KC, Cheng CL, Cheng HW, Lee JY, Lee CN, Yang PC. Enterovirus-induced miR-141 contributes to shutoff of host protein translation by targeting the translation initiation factor eIF4E. Cell Host Microbe. 2011;9(1):58–69. doi: 10.1016/j.chom.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Morgan R, Anderson A, Bernberg E, Kamboj S, Huang E, Lagasse G, Isaacs G, Parcells M, Meyers BC, Green PJ, Burnside J. Sequence conservation and differential expression of Marek’s disease virus microRNAs. J Virol. 2008;82(24):12213–12220. doi: 10.1128/JVI.01722-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esfandiarei M, Luo H, Yanagawa B, Suarez A, Dabiri D, Zhang J, McManus BM. Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. J Virol. 2004;78(8):4289–4298. doi: 10.1128/JVI.78.8.4289-4298.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3’s phosphorylation of beta-catenin. Proc Natl Acad Sci USA. 2008;105(23):8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.