Abstract
Bile acids (BAs) are amphipathic molecules produced from cholesterol by the liver. Expelled from the gallbladder upon meal ingestion, BAs serve as fat solubilizers in the intestine. BAs are reabsorbed in the ileum and return via the portal vein to the liver where, together with nutrients, they provide signals to coordinate metabolic responses. BAs act on energy and metabolic homeostasis through the activation of membrane and nuclear receptors, among which the nuclear receptor farnesoid X receptor (FXR) is an important regulator of several metabolic pathways. Highly expressed in the liver and the small intestine, FXR contributes to BA effects on metabolism, inflammation and cell cycle control. The pharmacological modulation of its activity has emerged as a potential therapeutic strategy for liver and metabolic diseases. This review highlights recent advances regarding the mechanisms by which the BA sensor FXR contributes to global signaling effects of BAs, and how FXR activity may be regulated by nutrient-sensitive signaling pathways.
Keywords: FXR, Nuclear receptor, Bile acids, Homeostasis, Metabolism
Introduction
Bile acid (BA) synthesis and the enterohepatic BA cycle
Bile acids are steroid molecules synthesized in hepatocytes from cholesterol. The BA biosynthetic pathway includes a number of successive enzymatic conversions involving multiple hydroxylations of cholesterol in the endoplasmic reticulum, and the mitochondrial shortening of its 17β-side chain from a C27 to a C24 molecule [1]. Primary products, resulting directly from this multi-enzymatic pathway and dependent on the key enzymes cytochrome P450 family members Cyp7A1, Cyp8B1 and Cyp27A1 [2], are the so-called primary BA cholic acid (CA) and chenodeoxycholic acid (CDCA) in humans, whereas an additional step converts CDCA into muricholic acid (α- and β-MCA) in mice. It is worth noting that ursodeoxycholic acid (UDCA), first identified in Chinese black bear bile, is also a primary BA in rodents [1, 3]. Prior to their secretion by hepatocytes into the canalicular lumen, BAs are conjugated to taurine or glycine at the C24 position by bile acid coenzyme A: amino-acid N-acyl transferase (BAAT) to decrease their hydrophobicity and allow their secretion into the bile [4–6]. In humans, BAs can also be conjugated to sulfate mostly at C3 by sulfotransferase 2A1 (SULT2A1) (Sult2A9 in mice), or glucuronidated by UDP glucuronosyltransferase family 2 (UGT2B4 and UGT2B7), a modification resulting in reduced toxic properties and increased urinary or fecal excretion [7–9]. Conjugated BAs are actively secreted into the canalicular lumen through the bile salt export pump (BSEP), stored and concentrated into the gallbladder. BA export promotes the simultaneous excretion of phospholipids and cholesterol into bile, through the ATP-binding cassette subfamily B member 4 (MDR3/ABCB4) and subfamily G member 5 and 8 (ABCG5/8) transporters, thus favoring cholesterol solubilization [10]. The human plasma bile acid pool is mainly composed of CDCA derivatives, especially glycochenodeoxycholic acid (GCDCA, 24 %), deoxycholic acid (DCA, 19 %), CA (16 %) and CDCA (15 %), whereas the mouse plasma bile acid pool is constituted of CA (24 %), ω-MCA (24 %), β-MCA (19 %) and DCA (7 %), thus revealing major differences between species. Glycine-conjugated BAs are the most abundant conjugated BAs in humans, whereas tauro-conjugated BAs are most abundant in rodents [11]. Modification of the BA pool composition may result in the accumulation of more hydrophobic BA species, causing severe hepatic alterations that may lead to liver failure and eventually extrahepatic tissue injury, as observed in cholestatic liver diseases [12].
Upon ingestion of a meal, gallbladder contraction, induced by cholecystokinin (CCK) released from the gut, discharges bile into the small intestine where it participates in lipid and lipid-soluble vitamin absorption by the intestine. BAs are also actively reabsorbed in the terminal ileum by the enterocyte transporter system composed of apical sodium-dependent bile acid transporter (ASBT/SLC10A2) and the heterodimeric organic solute transporter (OSTα/β). BAs escaping from reabsorption (~5 %) are converted into the secondary BAs DCA, lithocholic acid (LCA) and UDCA in humans and DCA, LCA, ωMCA, hyodeoxycholic acid (HDCA) and murideoxycholic acid (MDCA) in mouse. This conversion is catalyzed by the gut microbiota which may generate, according to its composition, dozens of secondary and tertiary BA metabolites [13, 14]. Secondary BAs can further be passively reabsorbed by the colonocytes or lost into feces. Reabsorbed primary and secondary BAs are then transported by the portal venous system and taken up by hepatocytes via the Na+-taurocholic acid cotransporting polypeptide (NTCP/SLC10A1), specifically involved in conjugated BA reabsorption, or by members of the organic anion transporting polypeptide family (OATPs/SLCOs [15]). In hepatocytes, BAs are then reconjugated and secreted back by the liver together with newly synthesized BAs which compensate for fecal loss. The BA pool, defined as the total amount of BAs circulating within this enterohepatic cycle, cycles between two and six times per day depending on the dietary regimen in humans.
Gut microbiota as a driver of BA pool composition
Recent reports highlighted the importance of gut microbiota as a driver of the BA pool composition. Germ-free mice display a decrease in BAs diversity, with an absence of secondary BAs. In addition, germ-free mice have an increased BA pool size, concentrated in the gallbladder and small intestine, but a decreased BA content in the cecum, colon, feces and serum [3]. This increased BA pool size results from a specific increase in βMCA conjugated and unconjugated metabolites rather than CA, and may be caused by enhanced intestinal BA reabsorption in the absence of gut microbiota [3, 16, 17]. Changes in microbiota due to probiotic, antioxidant or antibiotic treatments result in the modification of the BA pool composition [3, 18, 19]. In addition, dysbiosis has been described in diabetes and obesity [20], pathological conditions in which BA metabolism is altered [21–23]. Joyce and colleagues recently emphasized the importance of bacterial bile salt hydrolase (BSH) enzymes in determining BA pool composition. BSH enzymes are widespread enzymes in normal microbiota and are implicated in the deconjugation of glyco- and tauro-conjugated BAs. Monocolonization of germ-free mice with an Escherichia coli strain expressing a single bacterial BSH enzyme (BSH1) led to decreased plasma BAs and to a specific reduction of tauro-conjugated BAs associated with increased free CA, and α- and β-MCA content [24]. Thus, microbiotas control BA pool size and composition. Conversely, as a high concentration of DCA (0.5 mM) may inhibit bacterial overgrowth [25], BAs are also known to influence the structure and composition of gut microbiome, underlining the complex and reciprocal interaction between BA metabolism and microbiota composition [26, 27].
Roles of BAs in metabolic regulation
The role of BAs in metabolic regulation has been highlighted by the clinical use of BAs and oral non-absorbed BA sequestrants. First, BAs regulate their own synthesis and transport. UDCA, used as treatment for cholestatic manifestations such as primary biliary cirrhosis (PBC) or intrahepatic cholestasis of pregnancy (ICP) [28–33] and delays liver transplantation by increasing the rate of excretion of intracellular BA across liver cells and into the canaliculus [34], thus reducing toxic hydrophobic BA accumulation within liver cells [35]. In addition, the use of CDCA and UDCA in dissolving radiolucent cholesterol gallstone in gallstone disease patients emphasizes the role of BAs in cholesterol metabolism [36]. Lipid metabolism is impacted upon administration of BA sequestrants such as colesevelam, used to reduce cardiovascular disease (CVD) risk in prediabetic hypercholesterolemic patients [37], by increasing cholesterol efflux and lowering low-density lipoprotein cholesterol (LDL) plasma levels [38]. Finally, BA sequestration affects glucose metabolism, as pointed out by decreased blood glucose and glycated hemoglobin levels (HbA1c) in type 2 diabetic patients treated with colesevelam [39–41]. The use of BA sequestrants and BAs as treatment for various human metabolic dysfunctions emphasizes the pleiotropic role of BAs in the control of diverse metabolic pathways.
Part I: FXR, a BA receptor with pleiotropic biological functions
In 1999, the BA field witnessed a major leap forward with the discovery of a BA receptor: the nuclear receptor farnesoid X receptor (FXR) [42, 43]. Further studies revealed that BAs are also endogenous ligands for other nuclear receptors (NRs) including pregnane X receptor (PXR) [44], vitamin D receptor (VDR) [45] and the bile acid membrane receptor (TGR5/GPBAR1) [46]. The role of these NRs in metabolic regulation has been discussed previously [47, 48].
FXR structure
Farnesoid X receptor shares the common nuclear receptor structure composed of a central DNA-binding domain (DBD) close to the N-terminal ligand-independent activation function 1 domain (AF1) and a C-terminal ligand-binding domain (LBD) encompassing the ligand-dependent activation function 2 domain (AF2). The LBD and DBD are separated by a hinge region, which is considered as a flexible link between these two regions [49]. The FXR gene (also called NR1H4) is evolutionarily conserved showing high sequence similarities between several species such as Syrian hamster, mouse, rat and humans [50]. The murine and human FXR gene contains 10 introns and 11 exons [50, 51].
Using alternative promoters and splicing, four FXR isoforms have been identified (FXRα1, α2, α3 and α4 [50]) which display tissue- and species-specific expression. In humans, FXRα1 and FXRα2 are mainly expressed in the liver and in adrenals, whereas the four isoforms are present in the small intestine. The FXRα3 and α4 isoforms predominate in the colon and kidney. Contrary to humans, mouse liver and small intestine express the four isoforms, whereas mouse kidney and stomach express only the FXRα3 and α4 isoforms [50, 52]. FXR mRNA has been detected in human and mouse pancreas and the FXR protein also in reproductive tissues and human vascular smooth muscle cells [53–55]. Interestingly, FXR isoforms show distinct responsiveness to agonist-mediated activation. In transient-transfection experiments, FXRα2 is the most potent isoform to promote ligand-induced FXR target-gene expression [51, 52, 56–58]. This differential isoform efficiency is moreover gene dependent: the OSTβ gene is similarly activated by exogenous human FXR isoforms in the presence of an agonist, contrary to the small heterodimer partner (SHP) and BSEP. In addition, the isoform specificity is variable according to species, as only the hBSEP promoter is differentially transactivated by FXRα2 and FXRα1 isoforms, in contrast to rat and mouse Bsep [59].
The phenotype of FXR-deficient mice
Studies with Fxr-null (Fxr−/−) mice have convincingly demonstrated the physiological role of this nuclear receptor. Three different models of Fxr-null mice have been used in the literature. The first and most used model results from a deletion of a genomic fragment encoding a large portion of the LBD and the 3′UTR region of the Fxr mRNA [60]. The phenotype of these mice has been extensively studied and characterized by defective BA transport identified by elevated plasma BA concentrations, a decreased BA fecal loss and a high susceptibility to cholestasis upon CA feeding [60]. Fxr −/− mice have a reduced body and adipose tissue mass [61]. In addition, Fxr −/− mice fed a 1 % cholesterol-enriched diet showed an alteration of cholesterol and lipid metabolism, with increased hepatic triglycerides and cholesterol content and a pro-atherogenic serum lipoprotein profile [60, 62]. In line with this altered lipoprotein profile, Fxr −/− ApoE −/− mice develop more extensive atherosclerotic aortic plaques and display more profound lipid abnormalities than ApoE −/− mice [63]. However, a study combining Fxr and Ldlr deficiency surprisingly detected less atherosclerotic lesions in Fxr −/− Ldlr −/− compared to Ldlr −/− mice in male, but not in female mice, calling into question the beneficial effect of FXR activity on atherosclerosis [64]. In addition, high fat diet-fed Fxr −/− Ldlr −/− male mice develop hepatic lesions mimicking human NASH [65].
Fxr −/− mice display an altered glucose homeostasis. A transient hypoglycemia upon short-term fasting is associated with reduced hepatic glycogen content and decreased hepatic glucose production [66], whereas fed mice are hyperglycemic and display impaired insulin signaling in skeletal muscle and liver [67, 68]. However, a beneficial effect of Fxr deficiency on glucose parameters in leptin-deficient (ob/ob) mice has been reported [61, 69], suggesting that metabolic conditions might affect FXR activities.
Male and female Fxr −/− mice spontaneously develop liver tumors at 14 months of age [69–72] and show defective liver regeneration after partial hepatectomy [73] or upon CCl4-induced liver injury [74]. Fxr deficiency in male and female APCmin mice leads to intestinal tumor development [75], hinting at a role for FXR in cellular proliferation.
The second model of Fxr −/− mice has been generated by Deltagen Inc. and presents a deletion of a part of Fxr exon 2, thus affecting the DNA-binding domain integrity [76]. This model has been less extensively studied, but displays an increased body mass, increased liver mass, increased plasma and biliary bile salt concentration, increased BA fecal loss and increased bile acid pool size, contrary to the first model described above [76]. In agreement with the first Fxr −/− model, this model shows reduced endogenous glucose production, delayed intestinal glucose absorption and reduced hepatic and peripheral insulin sensitivity [77]. No liver function alterations as measured by ALT, AST or bilirubin plasma levels have been reported. Recently, a third model of Fxr −/− mice has been generated by deletion of Fxr exon 9, thereby removing part of the FXR LBD [78]. This mouse model has a decreased body mass associated with increased energy expenditure at the age of 21 weeks. At 30 weeks, these mice show decreased body fat mass and altered bone mineral density when compared with their wild-type littermates. These aging Fxr −/− mice spontaneously develop cholestasis and exhibit liver damage resembling NASH. In addition, and contradictory to previous models of Fxr deficiency, they display improved glucose tolerance and lower HOMA-IR index [78]. To sum up, these different models present clear and unexplained discrepancies in their phenotype which might be due to KO-induced artefacts, such as the possible occurence of a truncated protein and removal of non-coding RNAs, but also to experimental conditions of the study (age, diet, treatment, etc.). A formal comparative evaluation of the phenotype of these three different Fxr −/− strains would be of particular interest to identify true phenotypic differences. Furthermore, the use of tissue-specific knockout animal models is mandatory to dissect the actual implication of FXR in different tissues. Despite these discrepancies, FXR has undoubtedly a broad role in whole body homeostasis, emphasizing the importance of understanding its molecular and mechanistic properties.
FXR function in bile acid homeostasis
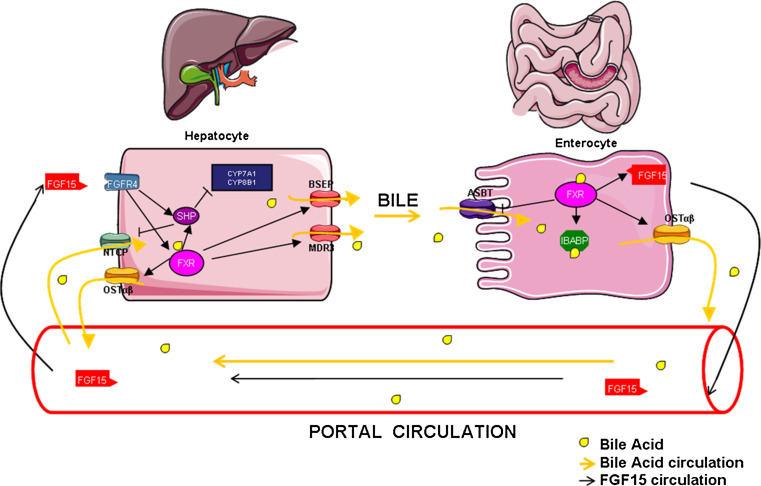
Bile acid synthesis and transport need to be tightly regulated because of their high cellular toxicity and FXR plays a critical role in this process. In the liver, FXR activation increases Shp gene expression [79], a known repressor of Cyp7A1 expression. SHP represses liver receptor homolog 1 (LRH-1) transcriptional activity through direct interaction with LRH-1 and the recruitment of corepressors [80–82]. CYP8B1 gene regulation by FXR stems from two opposite mechanisms in humans, but only one in rats. The common mechanism in both species involves an SHP-dependent inhibition of LRH1 activity and the subsequent decrease of CYP8B1 expression. In human hepatocytes, a second FXR-dependent mechanism coexists, leading to the FXR-mediated transactivation of the CYP8B1 promoter through increased hepatocyte nuclear factor 4α (HNF4α) transcriptional activity. These two opposite FXR-dependent regulations of CYP8B1 counteract each other, explaining probably why CDCA treatment in humans strongly represses CYP7A1 expression but has little effect on CYP8B1 expression [83–85], whereas in the mouse CA treatment represses Cyp8B1 and Cyp7A1 expression to a similar extent [60]. The differential activation of the human CYP7A1 and CYP8B1 genes may modulate BA pool hydrophobicity, as CYP8B1 is required for CA synthesis [86]. FXR also represses BA uptake by the liver by repressing Ntcp through an SHP-dependent mechanism [87, 88]. FXR increases the efflux of BAs from the liver to the canalicular lumen through upregulation of ABCB11/BSEP and ABCB4/MDR3 gene expression and BA efflux from the liver to the portal vein by increasing OSTα and OSTβ expression [85, 89]. Finally, FXR also regulates key enzymes involved in BA conjugation and detoxification, such as UGTs, SULTs and glutathione S-transferase [90].
In the intestine, FXR induces the expression of a fibroblast growth factor family member, FGF15 (in mouse) and FGF19 (in humans) in vitro and in vivo [91]. FGF15/19 expression and secretion by enterocytes contributes to BA synthesis regulation, through the activation of the hepatic fibroblast growth factor receptor 4 (FGFR4) membrane receptor. FGF19 is induced upon FXR activation in primary human hepatocytes revealing, in addition to endocrine signaling from the intestine, a possible hepatic autocrine or paracrine FGF19 signaling pathway [92]. Activation of FGFR4 and of its immediate target, fibroblast growth factor receptor substrate 2α (FRS2α), leads to the activation of the JNK and ERK pathways, resulting in decreased Cyp7A1 gene expression [93]. Intestinal deletion of the Fxr gene prevents Cyp7A1 repression caused by an FXR agonist in normal physiological conditions [94–96]. These observations therefore highlight the major regulatory role of intestinal FXR (iFXR) in regulating hepatic BA synthesis. Interestingly, numerous links have been made between the iFXR/FGF15 pathway and hepatic FXR activity (lFXR) on BA synthesis. Both in Fgf15 and Fgfr4 KO mice, Cyp7a1 expression is not inhibited after oral administration of an FXR agonist [94, 97]. Furthermore, a component of hepatic FGFR4 signaling, cytoplasmic tyrosine phosphatase SHP2, is necessary for the induction of Shp expression after FXR agonist treatment [98] and hepatic SHP is required for FGF15-mediated repression of Cyp7a1 [97, 99]. This suggests that FGFR4 signaling is necessary for lFXR-mediated repression of BA synthesis, making iFXR a strong driver of liver BA synthesis. In addition to BA synthesis, FXR also regulates BA transepithelial transport in the intestine, by regulating BA absorption and export to the portal system. Indeed, FXR inhibits intestinal Asbt expression responsible for BA reabsorption in mice, but not in rats [100], and increases fatty acid binding protein 6 (Fabp6/Ibabp) transporter expression, which shuttles BAs within the enterocytes [101]. Expression of the heterodimeric transporter OSTα/β, the major player of BA transport from enterocyte to the portal vein, is also regulated by FXR [102]. Thus, by reducing BA synthesis, favoring BA reabsorption in the intestine and promoting export and biotransformation in hepatocytes, FXR reduces metabolic requirements for BA synthesis and protects against BA toxicity (Fig. 1). In line with this protective effect, FXR activation leads to improvement of cholestasis in models of extra- and intrahepatic cholestasis by decreasing liver injury [103–106]. Paradoxically, Fxr −/− mice have less liver injury after bile duct ligation, an aggressive surgical model of cholestasis, probably due to the downregulation of the canalicular transporter Bsep expression, thereby reducing biliary pressure. By generating a more hydrophilic bile acid pool and enhancing phase I detoxification of BAs through polyhydroxylation by cytochrome P450 member 3A11 (CYP3A11), Fxr−/− mice display increased urinary BA excretion [107–109]. A decreased FXR activity can also be generated upon intestinal Sirtuin 1 (Sirt1) knockout which, as a result, inhibits HNF1α binding to the Fxr promoter [110]. This suggests that aging, which is associated with decreased Sirt1 expression, may also affect the FXR signaling pathway [111]. Cholestatic conditions such as progressive familial intrahepatic cholestasis type 1 (PFIC1) and intrahepatic cholestasis of pregnancy (ICP) have been also associated with decreased FXR activity and/or expression [112–114], suggesting that FXR activation may be a promising therapeutic option in the treatment of intrahepatic cholestasis.
Fig. 1.
FXR controls the enterohepatic cycle of bile acids. FXR activation by BAs protects the liver against BAs’ accumulation through a multistep mechanism involving a communication between the liver and intestine. In the intestine, FXR activation by BAs reduces bile acid uptake from the intestinal lumen and increases BAs’ cellular trafficking and export into the portal circulation. In addition, FXR activation in the intestine (distal ileum) increases FGF15 synthesis and export into the portal system. FGF15 through its membrane receptor FGFR4 decreases hepatic bile acid biosynthesis through SHP-dependent and SHP-independent mechanisms, mostly affecting Cyp7a1 activity, and hence CDCA synthesis. Hepatic FXR activation increases bile acid efflux from hepatocytes through the regulation of transporters’ expression (OSTα/β, BSEP and MDR3). FXR represses bile acid uptake into the hepatocyte through the repression of the NTCP transporter. This figure has been made using Servier Medical Art according to the license Creative Commons Attribution 3.0 France
Role of FXR in lipoprotein metabolism
In addition to its role in BA metabolism, FXR also regulates lipoprotein metabolism. As mentioned above with respect to the Fxr-deficient mice phenotype, the implication of FXR in HDL/LDL metabolism is controversial depending on the genotype background. In vitro, treatment of immortalized human hepatocytes with CDCA or the synthetic FXR agonist GW4064 reduces proprotein convertase subtilisin/kexin type 9 (PCSK9) expression, a natural inhibitor of LDL-R activity [115]. Although suggestive of a plasma LDL-C(holesterol) lowering capacity of activated FXR, in vivo treatment with CDCA did not alter or even increase LDL-C plasma concentrations upon short or prolonged treatment, respectively [116, 117]. A recent clinical trial in NAFLD diabetic patients reported increased LDL-C plasma levels after administration of the semi-synthetic FXR agonist INT747 during 6 weeks [118]. FXR also acts on reverse-cholesterol transport and has a profound effect on HDL metabolism, by repressing apolipoprotein A1 (ApoAI) expression and increasing cholesteryl ester transfer protein (CETP) expression [119]. Moreover, FXR regulates hepatic HDL-C uptake and HDL remodeling, by modulating phospholipid transfer protein (PLTP) and hepatic lipase (HL) [120, 121]. In a clinical study, CDCA decreased HDL-cholesterol levels [122]. Administration of the FXR agonist PX20606 decreases plasma cholesterol and reduces aortic plaque formation in CETP-transgenic LDLR−/− mice and in cynomolgus monkey, probably through increased HDL-C subclass 2 uptake by the liver resulting from increased hepatic SR-BI expression [123]. This counterintuitive observation in light of the plasma lipoprotein profile, but concordant with the pro-atherogenic profile of Fxr-deficient mice, makes FXR a potential anti-atherogenic target. More studies are needed to determine whether prolonged FXR activation limits atherosclerotic risk in controlled clinical studies.
FXR function in lipid metabolism and inflammation
The farnesoid X receptor regulates lipogenesis and triglyceride synthesis by repressing sterol regulatory binding protein 1c (Srebp1c) gene transcription, a key transcription factor stimulating the transcription of lipogenic genes, fatty acid desaturases and elongases, through an SHP-mediated inhibition of LRH-1/LXR transactivation [124]. A similar mechanism operates in the repression of microsomal transfer protein (Mtp) expression, which controls triglyceride assembly with apolipoprotein B in very low-density lipoproteins (VLDL) [125]. In agreement with its role in modulating hepatic triglyceride content, FXR is implicated in the progression of non-alcoholic fatty liver disease (NAFLD), a spectrum of liver disorders ranging from benign hepatic steatosis to steatohepatitis. FXR activation indeed protects against steatosis in rat and mice [126, 127]. FXR activation by the synthetic agonist WAY-362450 attenuates liver inflammation and fibrosis in mice fed a methionine and choline-deficient diet [128]. Moreover, FXR also regulates orosomucoid-1 (AGP) acute-phase protein expression, which is secreted in plasma upon liver inflammation, and hepatic FXR may modulate adipose tissue inflammation in mice [129].
FXR function in glucose homeostasis
Fxr-deficient mice display mild systemic glucose intolerance with hepatic and peripheral insulin resistance [66–68]. Moreover, Fxr expression is reduced in the liver of diabetic animal models [130] and its expression can be increased by insulin [130]. FXR activation by intraperitoneal injection of GW4064 promotes insulin sensitivity in the liver and skeletal muscle in ob/ob mice [66, 67, 130]. By contrast, long-term high fat diet feeding and concomitant treatment with GW4064 reduced insulin sensitivity, decreased energy expenditure and promoted weight gain [131]. These contradictory results may result from differences in experimental design such as the use of two different administration modes for GW4064. Given the poor bioavailability of this compound, the activation of peripheral FXR by orally administrated GW4064 is questionable. Indeed, Prawitt et al. [61] demonstrated that hepatic FXR deficiency does not protect against diet-induced obesity and insulin resistance, suggesting the involvement of peripheral FXR in metabolic homeostasis. Another point of divergence is the use of different mouse models of obesity. The importance of the genetic background in assessing FXR functions is emphasized by two different studies. Fxr deficiency in the obesogen ob/ob background improves glucose homeostasis and prevents weight gain [61, 69], whereas in the pro-atherogenic Ldlr −/− background, Fxr deficiency alters neither insulin sensitivity nor glucose tolerance [69]. Further studies with tissue-specific FXR knockouts are needed to decipher FXR-dependent mechanisms involved in peripheral metabolic effects.
FXR regulates different pathways of hepatic glucose metabolism by increasing glycogen synthesis [68, 132] and decreasing glycolysis by downregulation of L-type pyruvate kinase gene expression (LPK) [133]. Interestingly, the effect of FXR activation on gluconeogenesis changes according to the physiological context. In fed mice, FXR activation represses the expression of phosphoenolpyruvate carboxykinase 1 (Pepck) and glucose-6-phosphatase (G6pase) genes, whereas in fasted mice, FXR activation increased their expression via a mechanism involving GR [134]. The evaluation of FXR differential activity in fasted versus fed mice needs to be mechanistically studied by transcriptomic and genomic analysis. In addition to its hepatotropic effects, FXR has a general protective role in β-cell function and notably glucose-induced insulin secretion, thereby affecting glucose homeostasis [53, 135–137].
FXR functions in cellular proliferation
In addition to its metabolic phenotype, aging Fxr −/− mice spontaneously develop hepatocellular carcinoma (HCC), characterized by the upregulation of genes involved in inflammation and cell cycle control [70, 138]. The mechanism underlying the oncosuppressive property of FXR in the liver remains ill defined, but could be partially explained by an SHP-mediated increase of apoptosis, repression of BA synthesis and signal transducer and activator of transcription 3 (STAT3) inactivation through increased suppressor of cytokine signaling 3 (SOCS3) expression [139–141]. In addition, Deuschle et al. [142] identified the tumor suppressor N-myc downstream-regulated gene 2 (NDRG2) as a novel FXR target gene implicated in cell cycle control. In the liver, the tumorigenic effect of Fxr deficiency seems to stem from increased inflammation and fibrosis, and the Fxr −/− phenotype recapitulates the progression of human HCC [72]. Furthermore, treatment of human HCC cell lines by a combination of an acyclic retinoid [targeting retinoid X receptor α (RXRα)] and GW4064 inhibits growth and causes apoptosis [143]. Moreover, lentiviral-mediated FXR overexpression represses liver cancer cell proliferation and tumor growth in nude mice [144]. In agreement with these in vivo results, FXR expression is decreased and inversely correlated to disease progression in human HCC [72]. Interestingly, Vaquero et al. [145, 146] reported that FXR activation stimulates the chemoprotection of hepatocytes against genotoxic compounds, thus reducing the response of tumor cells to pharmacologically induced cellular toxicity in vitro. This mechanism possibly involves increased expression of genes implicated in drug efflux, DNA repair and cell survival. Recently, Degirolamo et al. [147] showed that intestinal Fxr expression protects Fxr −/− mice from spontaneous HCC development by decreasing BA overload, hepatic inflammation and lesions, emphasizing the importance of the FGF15 signaling pathway in HCC progression. FGF19-based therapy has been proposed as a potential therapeutic option in hepatocellular carcinoma treatment. However, FGF19 also appears to play a role in tumorigenesis by favoring hepatocyte proliferation, raising a potential safety concern. Recently, a specific variant of FGF19 displaying a selective activity on the regulation of bile acid metabolism without exhibiting adverse effects on tumorigenesis has been described, pointing to the therapeutic potential of FGF19 as a hepatic oncosuppressor [148, 149].
FXR expression is reduced in human colorectal cancers [150, 151], and accumulating evidence reveals a link between intestinal inflammation and tumorigenesis. The role of FXR in intestinal inflammation is underlined by the ability of activated FXR to decrease the production of proinflammatory cytokines such as IL1β, IL2, IL6, TNFα and IFNγ [152]. In addition, iFXR activation induces the transcription of genes involved in enteroprotection and prevention of bacterial translocation in the intestinal tract [153]. In addition, FXR deficiency increased the number and size of tumors in experimental models of intestinal cancer [75]. In line with the oncosuppressive activity of FXR, a chimeric, constitutively active FXR (VP16 AD-FXR) led to a markedly increased apoptosis and reduction of tumor growth in a xenograft mouse model [154]. FXR depletion in 8-week-old APC+/− mice increases adenoma-like lesions and aberrant crypt foci displaying extensive intestinal inflammation, suggesting the implication of FXR in all cancer progression stages [154]. In contrast, FXR upregulation is correlated to the induction of carcinogenesis and metastasis in the stomach and esophagus, via a mechanism involving probably the activation of the caudal-related homeobox 2 (Cdx2) gene, a transcription factor implicated in cancer development [155]. Consequently, increasing FXR signaling may be an interesting strategy in the management of inflammation and possibly carcinogenesis in the liver or the intestine, whereas such beneficial effects in other cancers remain to be firmly established.
FXR function in liver regeneration
Farnesoid X receptor deficiency strongly inhibits liver growth and increases mortality after a 70 % partial hepatectomy (PH) [73]. Fxr-deficient mice exhibit increased hepatocyte apoptosis after CCl4 treatment, a chemical compound promoting hepatic fibrosis, by delaying activation of the STAT3 signaling pathway. Mirroring these effects, FXR expression promotes liver regeneration [73] and stimulates liver repair after CCl4-induced liver injury [74]. These data suggest a dual role of FXR in promoting liver regeneration and protecting from hepatocyte cell death. The liver regenerative action of FXR stems from its ability to activate the forkhead box M1 transcription factor (FOXM1b), a key cell cycle regulator controlling the G1/S and G2/M transitions [73, 156]. Interestingly, overexpression of SIRT1 in mice and the subsequent reduction in FXR activity resulted in impaired hepatocyte proliferation after PH [157]. Strikingly, intestinal-specific FXR deficiency results in an impaired liver regeneration process, indicating that iFXR participates in hepatic regeneration. Exogenous delivery of FGF15 in iFXR-deficient mice restores liver repair capacity, showing that the iFXR effect is mediated through the endocrine FGF15 pathway independently of FoxM1b activation and may involve decreased BA synthesis and FGF15 mitogenic activity [158].
Part II: mechanism of transcriptional regulation by FXR
To date, the vast majority of FXR biological activities has been ascribed to its ability to interact with cis-acting DNA sequences. The molecular basis for these activities are described below.
DNA-binding properties of FXR
Farnesoid X receptor regulates gene expression by binding directly to DNA in vitro as a heterodimer with RXR or as a monomer [159]. The FXR DNA-binding domain confers specific recognition of different DNA motifs called FXR response element (FXRE), for which genome-wide studies have revealed that the most prominently recognized DNA sequence is an inverted repeat of the consensus sequence AGGTCA separated by one nucleotide (IR1) in mouse liver and intestine and in the human hepatoma HepG2 cell line [160–162]. In addition, FXR has been reported to bind other response elements with various geometries [161, 163–166] such as IR0, everted repeats separated by two or eight nucleotides (ER2–8) or direct repeats separated by one, four or five nucleotides (DR1–4–5). Interestingly, only 11 % of FXR binding sites are shared between mouse liver and intestine, revealing a clear tissue specificity of the FXR cistrome, which emphasizes its tissue-specific functions [161]. Indeed, gene ontology analysis of genes associated with FXR binding sites in the liver and intestine revealed tissue-specific pathway/function enrichment, according to which iFXR binding sites are strikingly associated with genes involved in catalytic reactions, oxidoreductase activity, monooxygenase activity, cofactor binding and the complement and coagulation cascade. In contrast, lFXR binding sites are in close vicinity of genes involved in cholesterol, lipid and glucose homeostasis which coincides with FXR biological activities deduced from studies in Fxr −/− mice [161]. In addition, motif analysis reveals that FXR preferentially binds in intestinal cells to ER2 motifs in addition to the IR1, underlining possible tissue-specific motif recognition by FXR. How this specificity is achieved is not yet known [161]. FXR thus demonstrates a broad diversity in bound DNA motifs, which could emphasize the high variety of mechanisms by which it regulates the expression of its target genes.
Ligand-binding properties of FXR
As other NR LBDs, the FXR LBD is constituted of 12 α-helices, forming a hydrophobic pocket allowing binding of ligands such as endogenous BAs and synthetic BA analogs. The orientation of the BA steroid scaffold in the ligand-binding pocket (LBP) is opposite to that found observed in other steroid NRs, with the carboxylic moiety being buried inside the LBP [167]. As mentioned above, BAs exhibit distinct potencies to activate FXR, with CDCA being the most active natural human FXR activator (EC50 = 20 µM in a mammalian 2-hybrid system) followed by DCA and LCA [42, 43, 52]. BAs have an “α” side on which hydroxyls are exposed at the 3α, 7α and 12α positions. The hydrophobic “β” side is similar between primary and secondary BAs. Relative affinities of BAs for FXR are dictated by their specific pattern of axial hydroxyl groups at the 7 and 12 positions [167]. Intriguingly, the potency of BAs to activate FXR is species specific and may involve cell-specific expression of membrane transporters and/or of transcriptional modulators. In HepG2 cells, CA treatment fails to activate FXR [52]. A possible explanation is the absence of the NTCP transporter in those cell lines [168]. In addition, human intestinal cell lines are sensitive to CDCA, but not to its conjugated derivatives GCDCA and TCDCA at similar intracellular concentrations, whereas FXR-target genes are activated by these CDCA conjugates in human hepatocyte cell lines, suggesting cell type-specific FXR activation by conjugated BAs not related to BA uptake properties [52].
Due to the poor selectivity of endogenous BAs hampering the dissection of FXR-dependent from FXR-independent pathways, synthetic and semi-synthetic ligands have been developed with improved selectivity for FXR. The semi-synthetic ligand INT747 (6-ECDCA) is derived from the structure of CDCA and is the most advanced in clinical trials (phase III trial, Primary Biliary Cirrhosis, Intercept Pharmaceuticals, NCT01473524). The non-steroidal synthetic agonist GW4064 is extensively used for in vitro and in vivo evaluation of FXR activity; it activates intestinal FXR, but displays poor bioavailability. As mentioned above, GW4064 has opposite metabolic effects in short- versus long-term treatment, which might stem from the different administration routes and preferential activation of liver (intraperitoneal) or intestinal FXR (oral), thereby inducing bias and discrepancies between studies [66, 68, 131]. Novel, less characterized agonists such as Px-102, WAY-362450 and fexaramine have been reported [169]. Surprisingly, fexaramine and GW4064 induce different gene expression profiles in primary mouse hepatocytes, suggesting that, in line with other NRs, FXR has distinct transcriptional properties when bound to structurally different ligands [170]. However, these differences can also be partly explained by the relaxed specificity of GW4064 which activates the estrogen-related receptor (ERR) isoforms, calling for a careful pharmacological evaluation of newly synthesized ligands [171].
In addition to natural or synthetic FXR agonists, FXR antagonists have been discovered. The secondary murine BA tauro-β muricholic acid (T-βMCA) has antagonistic properties [3]. Interestingly, the gut microbiotas modulate the FXR signaling pathway through modulation of T-βMCA conversion [18, 19]. Germ-free mice show increased T-βMCA concentrations, leading to the inhibition of iFXR. This in turn leads to increased hepatic BA synthesis and a larger total BA pool size as a result of decreased FGF15 signaling. Despite the increase in T-βMCA concentrations in the liver of germ-free mice, lFXR activity is not regulated as a function of gut microbiotas, suggesting a lack of sensitivity of lFXR to T-βMCA [3]. T-βMCA is however not produced in humans and further studies are needed to evaluate the presence of a “T-βMCA-like” antagonist activity in the human BA pool. Guggulsterone, a natural extract from the guggul tree, has FXR antagonistic activity on several genes, but surprisingly enhances FXR-induced Bsep gene expression [172]. This selective antagonism may be explained by the poor selectivity of guggulsterone which interacts also with other NRs [173]. Other antagonists such as 15d-PGJ2, suvanine or theonellasterol have been described in structural and screening studies [174–177], but remain poorly characterized with respect to their in vivo and in vitro activities.
FXR transactivation mechanisms
Nuclear receptors activate gene transcription through three currently described mechanisms: simple transactivation, composite transactivation and tethering transactivation, in analogy to other NR such as glucocorticoid receptor (GR) [178]. Simple transactivation is the “classical” way by which an NR activates transcription. Monomeric, homodimeric or RXR heterodimeric NRs directly bind to nuclear receptor response elements (NRE), while ligand (agonist) binding triggers a multistep process initiated by a conformational change of the LBD. This allows the dismissal of a corepressor complex and the subsequent recruitment of coactivating complexes. These are essentially, but not exclusively, made of histone writers which alter chromatin structure through post-translational histone modifications, promoting access of the general transcription machinery to proximal promoters or enhancers. Composite transactivation is defined as the direct binding of the NR to an NRE and its synergy with other DNA-bound NRs or other transcription factors (TF) present in close proximity. The tethering transactivation mechanism requires indirect binding of the NR to DNA through protein–protein interaction and has been described for several NRs, including GR which synergizes with DNA-bound signal transducer and activator of transcription 5 (STAT5) [179] or COUP-transcription factor 2 (COUP-TFII) [180]. RXR/FXR heterodimers have often been shown to act through a simple transactivation mechanism by the use of reporter-gene assays [89]. However, monomeric transactivation has been suggested for UGT2B4 and the glucose transporter (GLUT4) [181, 182]. Composite transactivation has equally been proposed for FXR. Genome-wide analyses indeed showed co-binding of FXR and of the orphan nuclear receptor LRH-1, both cooperating in activating hepatic lipid metabolism [160, 183]. Moreover, the existence of a synergy between FXR and HNF4α has been suggested, as FXR activation by the synthetic agonist GW4064 in vivo increases HNF4α binding in the vicinity of an FXRE [184]. This synergy is restricted to a particular set of regions and associated genes implicated in the complement and coagulation cascade. These two transcription factors were suggested to have cooperative, compensatory or independent effects on transcriptional regulations depending of the target, revealing a complex relationship between these two factors [184].
FXR regulates transcription through the recruitment of coregulators able to modulate chromatin properties. This occurs through the recognition of a hydrophobic groove at the surface of the NR generated upon agonist binding and ensuing LBD structural transitions. This hydrophobic groove accommodates LXXLL motifs found in single or multiple copies in coactivating proteins. Unexpectedly, the FXR LBD harbors two distinct docking sites for LXXLL motifs, the first one being responsible for coactivator recruitment and the second generating a binding interface for coactivators with more than one LXXLL motif, thereby increasing their relative affinity for FXR [167]. Steroid receptor coactivator 1 (SRC1) [185, 186], peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α) [187], thyroid hormone receptor-associated protein (TRAP) [188], SET domain-containing lysine transferase (SET7/9) [189] as well as histone methyltransferases such as PRMT1 [190], CARM1 [191], ASCOM (MLL3/MLL4) [192, 193], histone acetylases such as P300/CBP [186, 194] and chromatin remodelers of the Swi/Snf ATPases family (BRG1, [195]) have been identified as FXR coactivators (Table 1). Corepressors have also been identified, including the silencing mediator for retinoid and thyroid hormone receptors (SMRT) and the nuclear receptor corepressor 1 (NCoR1) complexes [196], Ku autoantigen proteins [197], the nuclear receptor 0B1 (DAX-1/NR0B1) [198] and the histone deacetylase SIRT1 [199] (Table 2). The composition of the FXR-tethered bound cofactor complex appears gene specific. SHP activation by FXR is highly dependent on PGC1α and SIRT1, whereas BSEP and FGF19 are activated independent of PGC1α and SIRT1 in HepG2 cells [200]. FXR can also regulate genes from more distal regulatory regions or enhancers. A genome-wide study revealed that FXR binds often to intergenic and intronic regions, as 41 % of FXR binding sites in mouse liver are located at more than 10 kb upstream of a Refseq gene [161]) and can possibly induce chromatin looping to connect distant binding sites and the promoter-bound transcriptional machinery. A head-to-tail chromatin looping between an FXRE located in the Shp/Nr0b2 promoter and another found in a downstream enhancer has been demonstrated [79], and similar looping processes have been proposed for the Cyp8b1 and Gr gene regulation by FXR [82, 201]. Emerging Hi-C technologies (three-dimensional analysis of genome architecture) might elucidate the functions of FXR in distal chromatin looping and whether gene activation by enhancer-bound FXR is also mediated through a cohesin-dependent mechanism as described for the estrogen receptor [202]. Undoubtedly, these various mechanisms implicating different FXR partners establish another layer of complexity in FXR transcriptional regulation.
Table 1.
List of FXR coactivators, their functions and the context (cell type, gene) of their association with FXR
| Coactivator | Function | Substrate | Model | Genes | Reference |
|---|---|---|---|---|---|
| P300 | Histone acetyl transferase (HAT) | H3K9/K14 | HepG2; IHH; mouse liver | SHP TXNIP | [133, 194, 227] |
| CARM1 | Arginine methyltransferase | H3R17 | HepG2 | BSEP | [191] |
| PRMT1 | Arginine methyltransferase | H4R3 | HepG2 | SHP BSEP | [190] |
| MLL3 | Lysine methyltransferase | H3K4 | HepG2; mouse liver | SHP BSEP Mrp2 | [193] |
| MLL4 | Lysine methyltransferase | H3K4 | HepG2 | SHP BSEP | [193] |
| ASC2 (NCOA6) | Recruitment of MML3/4 | HepG2; mouse liver | SHP BSEP KNG1 Mrp2 | [192, 193, 200] | |
| Brg1 | ATPase chromatin remodeler | HepG2; mouse liver | SHP | [195] | |
| TRRAP | Recruitment of HAT | HepG2 | Reporter assay with FXRE | [188] | |
| Set7/9 | Non-histone methyltransferase | FXR | Huh7 | SHP BSEP | [189] |
| SRC1 | HepG2 | Two-hybrid assay SHP BSEP OSTα | [198, 228, 229] | ||
| SRC2 | HepG2 | KNG1 | [200] | ||
| PGC1α | Recruitment of other cofactors | HepG2 | IBABP BSEP | [187] | |
| SIRT1 | Deacetylase | PGC1α, FXR | Mouse liver and intestine | Shp Ostβ | [220] |
Table 2.
List of FXR corepressors, their functions and the context (cell type, gene) of their association with FXR
| Corepressor | Function | Substrate | Model | Genes | Reference |
|---|---|---|---|---|---|
| SIRT1 | Deacetylase | H3K9/14, FXR | HepG2; mouse liver | SHP | [199] |
| DAX1 | NCOR recruitment | HepG2 | SHP BSEP | [198] | |
| KU80 and 70 | DNA-dependent protein kinases | unknown | HepG2 | BSEP | [197] |
| SMRT | HEK; IHH | Two-hybrid assay TXNIP | [133, 177] | ||
| NCOR | HEK | Two-hybrid assay | [177] |
FXR transrepression mechanisms
Similarly to transactivation processes, several mechanisms have been described for transrepression of target genes by NRs, including simple transrepression, competitive transrepression, tethering transrepression and coactivator squelching. Like simple transactivation, simple transrepression involves direct binding of NR as a monomer, homo- or heterodimer to a negative nuclear receptor response element (nNRE). Tethering transrepression requires indirect binding of the NR to DNA via interaction with another TF, thereby preventing coactivator recruitment or stabilizing corepressor binding to this TF. This scenario has been abundantly documented to explain the inhibitory effect of several NRs such as GR, peroxisome proliferator-activated receptor γ (PPARγ or retinoid receptors on the activator protein 1 (AP-1) and nuclear factor of kappa light polypeptide gene enhancer in B-cells (NF-kappaB) signaling pathways. Coactivator squelching implies the competition for cofactors between the ligand-activated NRs and other TF.
Transrepression by FXR has been reported for the regulation of human APOAI in human ApoA1 transgenic mice treated with taurocholic acid (TCA), which occurs independently of RXR upon binding to a negative FXRE [203]. These results, raising questions about the potential atherogenic properties of FXR agonists as they lower HDL-C, have however been recently challenged by Gardès et al. [204], who suggested that the observed decrease is unlikely to be an FXR-dependent process, as synthetic FXR agonists do not mimic the effect of TCA in vivo. Competitive transrepression has been described at the APOA/Lp(A) promoter, as ligand-activated FXR competes for binding to a HNF4α-bound DR1 site in its promoter. In addition, an increased hepatic BA concentration caused by bile duct ligation in transgenic hAPOA mice decreased APOA expression, suggesting that BAs repress hAPOA expression in vivo in an FXR-dependent manner [205]. Transrepression has also been described for the ApoCIII gene which encodes for an inhibitor of lipoprotein lipase, providing a molecular basis for the triglyceride-lowering effect of FXR agonists. BA treatment induces the binding of FXR and RXR heterodimers and displaces HNF4α from a DR-1 site. Since this repressive effect is also observed in hepatic HNF4a-deficient mice, a direct repressive role of FXR has been proposed [166]. In the colonic Caco2 cell line, LCA treatment decreased UGT2B7 gene expression through a mechanism dependent on RXR and involving a negative FXRE composed of the sequence “GATCCTTGATATTA” [206]. A similar mechanism has been identified for the negative regulation of SULT2A1 by FXR in HepG2 cells and in vivo [207]. FXR represses liver pyruvate kinase (LPK) gene expression through tethering transrepression. At high glucose concentrations, HNF4α and carbohydrate response element-binding protein (ChREBP) bind to the LPK promoter and activate LPK expression. Agonist-activated FXR interacts with CHREBP and HNF4α, leading to the release of the transcriptionally active CHREBP and stabilizing the SMRT corepressor onto the FXR/HNF4α-containing complex, hence decreasing LPK transcription [133]. In addition, FXR repressed autophagic genes in the liver through the transrepression of cAMP response element-binding protein (CREB). Activated FXR interacts with CREB bound at autophagic gene promoters and disrupts the transcriptionally active CREB/CRTC2 complex [208]. FXR-mediated repression may also operate through competition with PPARα at autophagic gene promoters [209]. Furthermore, FXR cross talks with the NF-kappaB pathway. FXR activation inhibits the NF-kappaB-mediated hepatic inflammatory response without, surprisingly, affecting NF-kappaB-activated antiapoptotic genes [210]. This observation has been replicated in murine macrophages and data were suggestive of a model by which FXR activation led to the stabilization of the NcoR complex on the NF-kappaB-responsive element of the Il-1β promoter [211]. Conversely, NF-kappaB represses FXR activity in liver and intestinal cells [210, 212]. More studies are needed to dissect the mechanism of transrepression of NF-kappaB by FXR, and in particular whether it occurs independently of direct FXR DNA binding as shown for PPARα [213]. Genome-wide studies, together with comprehensive transcriptomic analyses, are required to address the gene selectivity of such a mechanism, its functional significance in vivo and the relative importance of the transrepression mechanism in the FXR activity spectrum.
Part III: mechanism of modulation of FXR activity
As emphasized above, questions remain about the role of endogenous BAs in activating FXR in different organs, as normal plasma BA concentrations are in the low micrometer range and EC50s in the high micrometer range. In vivo imaging of FXR activity in mice underlines the poor sensibility of lFXR to a CDCA-enriched diet, which only leads to increased iFXR activity. The FXR agonist GW4064 dramatically increases FXR activity in ileum, kidney, adrenals and liver [214], albeit that this efficiency was not replicated in another study [200]. The mechanism by which lFXR is activated in physiological conditions thus remains enigmatic, as no significant changes in hepatic BA concentrations were reported during a 24-h cycle in mice fed a chow diet [215]. Fxr gene expression oscillates weakly during a 24-h cycle in the mouse [216]. In contrast, the expression of FXR-target genes such as Shp, Cyp7A1, Ntcp and Bsep in the liver and Fgf15 and Ostα in the intestine clearly show circadian rhythmicity, suggesting a possible regulation of FXR activity through ligand-independent mechanisms [215, 217]. These observations suggest that in physiological conditions, lFXR activity may not be modulated by varying BA concentrations, in contrast to ileal FXR. In addition, Renga et al. [134] demonstrated that FXR activity and expression are dependent on the nutritional status, as activation of FXR exerts opposite effects on gluconeogenic gene expression in fasting versus fed conditions. This study raised the possibility of a differential FXR transcriptional activity as a function of the pathophysiological conditions, which was further strengthened by a genome-wide comparison of FXR binding sites in chow versus high fat diet-fed mice, which evidenced distinct FXR cistromes in both conditions [218].
These different results suggest that FXR activity is modulated by nutrient fluxes through a mechanism which may be, at least in part, independent of BA composition and concentration in the liver. A recent set of studies established that FXR-dependent transcriptional responses can also be modulated by nutrients essentially through post-translational modifications (PTMs). Glucose favors FXR expression and transcriptional activity [132] through the hexosamine biosynthetic pathway (HBP) and O-GlcNac transferase (OGT), which catalyzes FXR O-GlcNacylation [162]. Another signaling pathway regulated by the cellular energetic status is the AMP-activated protein kinase (AMPK) pathway. In low-energy conditions, AMPK is activated and targets a range of physiological processes to block anabolic reactions and to favor ATP synthesis [219]. Phosphorylation of FXR on serine 250 by AMPK decreases its transcriptional activity via the inhibition of coactivator recruitment [200]. In addition to AMPK, SIRT1 is activated in low-energy conditions by increased NAD+ concentrations. By counteracting the effect of p300-mediated FXR acetylation, which decreases its interaction with RXR thereby preventing its interaction with DNA, SIRT1 favors FXR recycling to regulated promoters. In line, deletion of hepatic SIRT1 negatively affects the FXR signaling pathway and triggers negative metabolic outcomes [111, 194, 199, 220]. However, SIRT1 is also a regulator of HNF1α, a known inducer of FXR expression in both liver and intestine, whose hepatic or intestinal deletion decreases hepatic and intestinal Fxr expression [110, 220]. SIRT1 transgenic mice display FXR persistent deacetylation correlated with decreased FXR protein content, leading to decreased FXR activity [157]. These paradoxical results highlighted a more complex regulation of FXR signaling by SIRT1, which acts at different levels, including direct deacetylation of FXR and also of its transcriptional partners and histones. Other FXR PTMs have been described, including phosphorylation by PKCα [221] and PKCζ [222], ubiquitination and sumoylation [223]. Further studies are warranted to understand the contribution of each PTMs to FXR activation in distinct physiological contexts.
Concluding remarks
Given the multiple roles of FXR in whole body metabolic regulation, FXR activation may be generally beneficial to normalize metabolic parameters in metabolically disturbed individuals. Recently, FXR signaling has been shown to be essential for the positive effect of vertical sleeve gastrectomy on weight loss and metabolic improvement [224], although the phenotype of Fxr −/− mice, which display a decreased body mass, calls for a careful evaluation of this study [225]. Further studies using tissue-specific Fxr deletion are needed to unravel FXR functions. Nevertheless, the potential benefit of FXR activation in many diseases including NASH and cholestasis has been highlighted in several clinical studies using 6α-ethyl CDCA (INT747, obeticholic acid). A phase II clinical trial in type 2 diabetes and NAFLD patients demonstrated that INT747 treatment increased insulin sensitivity, as evidenced by an increased glucose infusion rate in a hyperinsulinemic–euglycemic clamp study. There was also a drop in plasma γ-glutamyltransferase (GGT) and alanine aminotransferase (ALT), suggesting reduced liver injury. Liver fibrosis, which occurred in 81 % of the patients, was also improved significantly after INT747 treatment by reducing hyaluronic acid, procollagen III amino terminal peptide and tissue inhibitor of metalloproteinase 1 [118, 226]. A phase IIb clinical study with NASH patients has been stopped prematurely in January 2014 because of early achievement of the pre-defined efficacy criterion (Intercept Pharmaceuticals, FLINT trial NCT01999101). In addition, INT747 has also been tested in primary biliary cirrhotic (PBC) patients, an autoimmune disease which causes progressive cholestasis, fibrosis and ultimately cirrhosis. Clinical trials have shown beneficial effects of INT747 treatment in PBC patients. Short- and long-term treatment of INT747 together with UDCA reduced alanine aminotransferase (ALT) and alkaline phosphatase (ALP) levels in PBC patients which did not respond to UDCA alone (Intercept Pharmaceutical NCT00550862). A phase III clinical trial is currently ongoing (Intercept Pharmaceuticals NCT01473524). The more commonly observed adverse effect observed with INT747 treatment is pruritus, but HDL-C and LDL-C levels need to be monitored in long-term treatments. Other phase II clinical trials are planned on different hepatic pathologies such as primary sclerosing cholangitis (PSC) (NCT02177136) and alcoholic steatohepatitis (ASH) (NCT02039219). Another non-steroidal FXR agonist Px104 is being evaluated in a clinical phase II trial in NAFLD patients (Phenex Pharmaceuticals AG NCT01999101).
Furthermore, the comparative evaluation of several FXR agonists in rodent models of atherosclerosis and obesity and normolipemic cynomolgus monkeys evidenced distinct effects on lipoprotein and cholesterol plasma levels, while equally reducing aortic atherogenic plaque size [123]. These observations demonstrate that development of selective bile acid receptor modulators (SBARM) is warranted, allowing the partial and selective activation of the FXR transcriptome in a tissue-specific manner.
Acknowledgments
CM was supported by a fellowship from Institut National de la Santé et de la Recherche Médicale (INSERM) and from Région Nord-Pas-de-Calais. This work was supported by grants from INSERM, Agence Nationale de la Recherche (ANR) (FXRen), EGID (ANR-10-LABX-46), Région Nord-Pas de Calais, Fond Européen de Développement Régional (FEDER) and Cost Action BM0602. B Staels is a member of the Institut Universitaire de France.
Footnotes
This review has been written on the basis of a Pubmed database search using the following keywords: “bile acids” or “FXR” to provide a detailed overview of the contribution of FXR in bile acid signaling and the regulation of FXR activity by environmental stimulus.
References
- 1.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 2.Russell DW, Setchell KD. Bile acid biosynthesis. Biochemistry. 1992;31:4737–4749. doi: 10.1021/bi00135a001. [DOI] [PubMed] [Google Scholar]
- 3.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall H, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Kwakye JB, Barnes S, Diasio RB. Identification of bile acid coenzyme a synthetase in rat kidney. J Lipid Res. 1993;34:95–99. [PubMed] [Google Scholar]
- 5.Falany CN, Johnson MR, Barnes S, Diasio RB. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J Biol Chem. 1994;269:19375–19379. [PubMed] [Google Scholar]
- 6.Solaas K, Ulvestad A, Söreide O, Kase BF. Subcellular organization of bile acid amidation in human liver: a key issue in regulating the biosynthesis of bile salts. J Lipid Res. 2000;41:1154–1162. [PubMed] [Google Scholar]
- 7.Jansen PL, Mulder GJ, Burchell B, Bock KW. New developments in glucuronidation research: report of a workshop on “glucuronidation, its role in health and disease”. Hepatology. 1992;15:532–544. doi: 10.1002/hep.1840150328. [DOI] [PubMed] [Google Scholar]
- 8.Stiehl A. Disturbances of bile acid metabolism in cholestasis. Clin Gastroenterol. 1977;6:45–67. [PubMed] [Google Scholar]
- 9.Yousef I, Mignault D, Tuchweber B. Effect of complete sulfation of bile acids on bile formation: role of conjugation and number of sulfate groups. Hepatology. 1992;15:438–445. doi: 10.1002/hep.1840150314. [DOI] [PubMed] [Google Scholar]
- 10.Elferink RPJO, Ottenhoff R, Fricker G, Seward DJ, Ballatori N, Boyer J. Lack of biliary lipid excretion in the little skate, Raja erinacea, indicates the absence of functional Mdr2, Abcg5, and Abcg8 transporters. Am J Physiol Gastrointest Liver Physiol. 2004;286:G762–G768. doi: 10.1152/ajpgi.00424.2003. [DOI] [PubMed] [Google Scholar]
- 11.García-Cañaveras JC, Donato MT, Castell JV, Lahoz A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J Lipid Res. 2012;53:2231–2241. doi: 10.1194/jlr.D028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attili AF, Angelico M, Cantafora A, Alvaro D, Capocaccia L. Bile acid-induced liver toxicity: relation to the hydrophobic-hydrophilic balance of bile acids. Med Hypotheses. 1986;19:57–69. doi: 10.1016/0306-9877(86)90137-4. [DOI] [PubMed] [Google Scholar]
- 13.Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4523–4530. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridlon JM, Kang D, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- 16.Riottot M, Sacquet E. Increase in the ileal absorption rate of sodium taurocholate in germ-free or conventional rats given an amylomaize-starch diet. Br J Nutr. 1985;53:307–310. doi: 10.1079/bjn19850038. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson BE, Bergstrom S, Lindstedt S, Norman A. Turnover and nature of fecal bile acids in germfree and infected rats fed cholic acid-24-14C; bile acids and steroids 41. Proc Soc Exp Biol Med. 1957;94:467–471. doi: 10.3181/00379727-94-22981. [DOI] [PubMed] [Google Scholar]
- 18.Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014;7:12–18. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014;63:1513–1521. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 21.Vincent RP, Omar S, Ghozlan S, Taylor DR, Cross G, Sherwood RA, Fandriks L, Olbers T, Werling M, Alaghband-Zadeh J, le Roux CW. Higher circulating bile acid concentrations in obese patients with type 2 diabetes. Ann Clin Biochem. 2013;50:360–364. doi: 10.1177/0004563212473450. [DOI] [PubMed] [Google Scholar]
- 22.Wewalka M, Patti M, Barbato C, Houten SM, Goldfine AB. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab. 2014;99:1442–1451. doi: 10.1210/jc.2013-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner C, Othman A, Saely CH, Rein P, Drexel H, von Eckardstein A, Rentsch KM. Bile acid metabolites in serum: intraindividual variation and associations with coronary heart disease, metabolic syndrome and diabetes mellitus. PLoS ONE. 2011;6:e25006. doi: 10.1371/journal.pone.0025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, Hill C, Gahan CGM. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci USA. 2014;111:7421–7426. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Trauner M, Fickert P, Tilg H. Bile acids as modulators of gut microbiota linking dietary habits and inflammatory bowel disease: a potentially dangerous liaison. Gastroenterology. 2013;144:844–846. doi: 10.1053/j.gastro.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 27.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heathcote EJ, Cauch-Dudek K, Walker V, Bailey RJ, Blendis LM, Ghent CN, Michieletti P, Minuk GY, Pappas SC, Scully LJ, et al. The Canadian multicenter double-blind randomized controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1994;19:1149–1156. [PubMed] [Google Scholar]
- 29.Poupon RE, Poupon R, Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group. N Engl J Med. 1994;330:1342–1347. doi: 10.1056/NEJM199405123301903. [DOI] [PubMed] [Google Scholar]
- 30.Combes B, Carithers RLJ, Maddrey WC, Lin D, McDonald MF, Wheeler DE, Eigenbrodt EH, Muñoz SJ, Rubin R, Garcia-Tsao G, et al. A randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1995;22:759–766. [PubMed] [Google Scholar]
- 31.Lindor KD, Dickson ER, Jorgensen RA, Anderson ML, Wiesner RH, Gores GJ, Lange SM, Rossi SS, Hofmann AF, Baldus WP. The combination of ursodeoxycholic acid and methotrexate for patients with primary biliary cirrhosis: the results of a pilot study. Hepatology. 1995;22:1158–1162. doi: 10.1016/0270-9139(95)90624-x. [DOI] [PubMed] [Google Scholar]
- 32.Kuiper EMM, Hansen BE, Lesterhuis W, Robijn RJ, Thijs JC, Engels LGJB, Koek GH, Aparicio MN, Kerbert-Dreteler MJ, van Buuren HR. The long-term effect of ursodeoxycholic acid on laboratory liver parameters in biochemically non-advanced primary biliary cirrhosis. Clin Res Hepatol Gastroenterol. 2011;35:29–33. doi: 10.1016/j.gcb.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Glantz A, Reilly S, Benthin L, Lammert F, Mattsson L, Marschall H. Intrahepatic cholestasis of pregnancy: amelioration of pruritus by UDCA is associated with decreased progesterone disulphates in urine. Hepatology. 2008;47:544–551. doi: 10.1002/hep.21987. [DOI] [PubMed] [Google Scholar]
- 34.Jazrawi RP, de Caestecker JS, Goggin PM, Britten AJ, Joseph AE, Maxwell JD, Northfield TC. Kinetics of hepatic bile acid handling in cholestatic liver disease: effect of ursodeoxycholic acid. Gastroenterology. 1994;106:134–142. doi: 10.1016/s0016-5085(94)94899-2. [DOI] [PubMed] [Google Scholar]
- 35.Combes B, Markin RS, Wheeler DE, Rubin R, West AB, Mills AS, Eigenbrodt EH, Maddrey WC, Munoz SJ, Garcia-Tsao G, Bonner GF, Boyer JL, Luketic VA, Shiffman ML, Peters MG, White HM, Zetterman RK, Carithers RLJ. The effect of ursodeoxycholic acid on the florid duct lesion of primary biliary cirrhosis. Hepatology. 1999;30:602–605. doi: 10.1002/hep.510300315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 2014;55:1553–1595. doi: 10.1194/jlr.R049437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Out C, Groen AK, Brufau G. Bile acid sequestrants: more than simple resins. Curr Opin Lipidol. 2012;23:43–55. doi: 10.1097/MOL.0b013e32834f0ef3. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg RB, Rosenson RS, Hernandez-Triana E, Misir S, Jones MR. Colesevelam improved lipoprotein particle subclasses in patients with prediabetes and primary hyperlipidaemia. Diab Vasc Dis Res. 2013;10:256–262. doi: 10.1177/1479164112461657. [DOI] [PubMed] [Google Scholar]
- 39.Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31:1479–1484. doi: 10.2337/dc08-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zieve FJ, Kalin MF, Schwartz SL, Jones MR, Bailey WL. Results of the glucose-lowering effect of WelChol Study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther. 2007;29:74–83. doi: 10.1016/j.clinthera.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168:1531–1540. doi: 10.1001/archinte.168.14.1531. [DOI] [PubMed] [Google Scholar]
- 42.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 43.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 44.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 46.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 47.Stepanov V, Stankov K, Mikov M. The bile acid membrane receptor TGR5: a novel pharmacological target in metabolic, inflammatory and neoplastic disorders. J Recept Signal Transduct Res. 2013;33:213–223. doi: 10.3109/10799893.2013.802805. [DOI] [PubMed] [Google Scholar]
- 48.Keitel V, Häussinger D. Perspective: TGR5 (Gpbar-1) in liver physiology and disease. Clin Res Hepatol Gastroenterol. 2012;36:412–419. doi: 10.1016/j.clinre.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Zwart W, de Leeuw R, Rondaij M, Neefjes J, Mancini MA, Michalides R. The hinge region of the human estrogen receptor determines functional synergy between AF-1 and AF-2 in the quantitative response to estradiol and tamoxifen. J Cell Sci. 2010;123:1253–1261. doi: 10.1242/jcs.061135. [DOI] [PubMed] [Google Scholar]
- 50.Huber RM, Murphy K, Miao B, Link JR, Cunningham MR, Rupar MJ, Gunyuzlu PL, Haws TF, Kassam A, Powell F, Hollis GF, Young PR, Mukherjee R, Burn TC. Generation of multiple farnesoid-X-receptor isoforms through the use of alternative promoters. Gene. 2002;290:35–43. doi: 10.1016/s0378-1119(02)00557-7. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Kast-Woelbern HR, Edwards PA. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J Biol Chem. 2003;278:104–110. doi: 10.1074/jbc.M209505200. [DOI] [PubMed] [Google Scholar]
- 52.Vaquero J, Monte MJ, Dominguez M, Muntané J, Marin JJG. Differential activation of the human farnesoid X receptor depends on the pattern of expressed isoforms and the bile acid pool composition. Biochem Pharmacol. 2013;86:926–939. doi: 10.1016/j.bcp.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 53.Popescu IR, Helleboid-Chapman A, Lucas A, Vandewalle B, Dumont J, Bouchaert E, Derudas B, Kerr-Conte J, Caron S, Pattou F, Staels B. The nuclear receptor FXR is expressed in pancreatic beta-cells and protects human islets from lipotoxicity. FEBS Lett. 2010;584:2845–2851. doi: 10.1016/j.febslet.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 54.Anaya-Hernández A, Méndez-Tepepa M, Laura GH, Pacheco P, Martínez-Gómez M, Castelán F, Cuevas E. Farnesoid X receptor immunolocalization in reproductive tissues of adult female rabbits. Acta Histochem. 2014;116:1068–1074. doi: 10.1016/j.acthis.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci USA. 2004;101:3668–3673. doi: 10.1073/pnas.0400046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee FY, Kast-Woelbern HR, Chang J, Luo G, Jones SA, Fishbein MC, Edwards PA. Alpha-crystallin is a target gene of the farnesoid X-activated receptor in human livers. J Biol Chem. 2005;280:31792–31800. doi: 10.1074/jbc.M503182200. [DOI] [PubMed] [Google Scholar]
- 57.Anisfeld AM, Kast-Woelbern HR, Meyer ME, Jones SA, Zhang Y, Williams KJ, Willson T, Edwards PA. Syndecan-1 expression is regulated in an isoform-specific manner by the farnesoid-X receptor. J Biol Chem. 2003;278:20420–20428. doi: 10.1074/jbc.M302505200. [DOI] [PubMed] [Google Scholar]
- 58.Anisfeld AM, Kast-Woelbern HR, Lee H, Zhang Y, Lee FY, Edwards PA. Activation of the nuclear receptor FXR induces fibrinogen expression: a new role for bile acid signaling. J Lipid Res. 2005;46:458–468. doi: 10.1194/jlr.M400292-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Song X, Chen Y, Valanejad L, Kaimal R, Yan B, Stoner M, Deng R. Mechanistic insights into isoform-dependent and species-specific regulation of bile salt export pump by farnesoid X receptor. J Lipid Res. 2013;54:3030–3044. doi: 10.1194/jlr.M038323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 61.Prawitt J, Abdelkarim M, Stroeve JHM, Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, van Dijk TH, Lucas A, Dorchies E, Daoudi M, Lestavel S, Gonzalez FJ, Oresic M, Cariou B, Kuipers F, Caron S, Staels B. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 2011;60:1861–1871. doi: 10.2337/db11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lambert G, Amar MJA, Guo G, Brewer HBJ, Gonzalez FJ, Sinal CJ. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 63.Hanniman EA, Lambert G, McCarthy TC, Sinal CJ. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein e-deficient mice. J Lipid Res. 2005;46:2595–2604. doi: 10.1194/jlr.M500390-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Wang X, Vales C, Lee FY, Lee H, Lusis AJ, Edwards PA. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2006;26:2316–2321. doi: 10.1161/01.ATV.0000235697.35431.05. [DOI] [PubMed] [Google Scholar]
- 65.Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116–122. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, Caron S, Torpier G, Fruchart J, Gonzalez FJ, Kuipers F, Staels B. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 67.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Ge X, Heemstra LA, Chen W, Xu J, Smith JL, Ma H, Kasim N, Edwards PA, Novak CM. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in Ob/Ob mice. Mol Endocrinol. 2012;26:272–280. doi: 10.1210/me.2011-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 71.Wolfe A, Thomas A, Edwards G, Jaseja R, Guo GL, Apte U. Increased activation of the Wnt/Β-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J Pharmacol Exp Ther. 2011;338:12–21. doi: 10.1124/jpet.111.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu N, Meng Z, Lou G, Zhou W, Wang X, Zhang Y, Zhang L, Liu X, Yen Y, Lai L, Forman BM, Xu Z, Xu R, Huang W. Hepatocarcinogenesis in FXR−/− mice mimics human HCC progression that operates through HNF1α regulation of FXR expression. Mol Endocrinol. 2012;26:775–785. doi: 10.1210/me.2011-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 74.Meng Z, Wang Y, Wang L, Jin W, Liu N, Pan H, Liu L, Wagman L, Forman BM, Huang W. FXR regulates liver repair after CCl4-induced toxic injury. Mol Endocrinol. 2010;24:886–897. doi: 10.1210/me.2009-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maran RRM, Thomas A, Roth M, Sheng Z, Esterly N, Pinson D, Gao X, Zhang Y, Ganapathy V, Gonzalez FJ, Guo GL. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther. 2009;328:469–477. doi: 10.1124/jpet.108.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kok T, Hulzebos CV, Wolters H, Havinga R, Agellon LB, Stellaard F, Shan B, Schwarz M, Kuipers F. Enterohepatic circulation of bile salts in farnesoid X receptor-deficient mice: efficient intestinal bile salt absorption in the absence of ileal bile acid-binding protein. J Biol Chem. 2003;278:41930–41937. doi: 10.1074/jbc.M306309200. [DOI] [PubMed] [Google Scholar]
- 77.van Dijk TH, Grefhorst A, Oosterveer MH, Bloks VW, Staels B, Reijngoud D, Kuipers F. An increased flux through the glucose 6-phosphate pool in enterocytes delays glucose absorption in Fxr−/− mice. J Biol Chem. 2009;284:10315–10323. doi: 10.1074/jbc.M807317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bjursell M, Wedin M, Admyre T, Hermansson M, Böttcher G, Göransson M, Lindén D, Bamberg K, Oscarsson J, Bohlooly-Y M. Ageing Fxr deficient mice develop increased energy expenditure, improved glucose control and liver damage resembling NASH. PLoS ONE. 2013;8:e64721. doi: 10.1371/journal.pone.0064721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li G, Thomas AM, Hart SN, Zhong X, Wu D, Guo GL. Farnesoid X receptor activation mediates head-to-tail chromatin looping in the Nr0b2 gene encoding small heterodimer partner. Mol Endocrinol. 2010;24:1404–1412. doi: 10.1210/me.2010-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 81.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 82.Sanyal S, Båvner A, Haroniti A, Nilsson L, Lundåsen T, Rehnmark S, Witt MR, Einarsson C, Talianidis I, Gustafsson J, Treuter E. Involvement of corepressor complex subunit GPS2 in transcriptional pathways governing human bile acid biosynthesis. Proc Natl Acad Sci USA. 2007;104:15665–15670. doi: 10.1073/pnas.0706736104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abrahamsson A, Gustafsson U, Ellis E, Nilsson L, Sahlin S, Björkhem I, Einarsson C. Feedback regulation of bile acid synthesis in human liver: importance of HNF-4alpha for regulation of CYP7A1. Biochem Biophys Res Commun. 2005;330:395–399. doi: 10.1016/j.bbrc.2005.02.170. [DOI] [PubMed] [Google Scholar]
- 84.Ellis E, Axelson M, Abrahamsson A, Eggertsen G, Thörne A, Nowak G, Ericzon B, Björkhem I, Einarsson C. Feedback regulation of bile acid synthesis in primary human hepatocytes: evidence that CDCA is the strongest inhibitor. Hepatology. 2003;38:930–938. doi: 10.1053/jhep.2003.50394. [DOI] [PubMed] [Google Scholar]
- 85.Liu J, Lu H, Lu Y, Lei X, Cui JY, Ellis E, Strom SC, Klaassen CD (2014) Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol Sci [DOI] [PMC free article] [PubMed]
- 86.Pandak WM, Bohdan P, Franklund C, Mallonee DH, Eggertsen G, Björkhem I, Gil G, Vlahcevic ZR, Hylemon PB. Expression of sterol 12alpha-hydroxylase alters bile acid pool composition in primary rat hepatocytes and in vivo. Gastroenterology. 2001;120:1801–1809. doi: 10.1053/gast.2001.24833. [DOI] [PubMed] [Google Scholar]
- 87.Zollner G, Wagner M, Fickert P, Geier A, Fuchsbichler A, Silbert D, Gumhold J, Zatloukal K, Kaser A, Tilg H, Denk H, Trauner M. Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver. Am J Physiol Gastrointest Liver Physiol. 2005;289:G798–G805. doi: 10.1152/ajpgi.00319.2004. [DOI] [PubMed] [Google Scholar]
- 88.Maeda T, Miyata M, Yotsumoto T, Kobayashi D, Nozawa T, Toyama K, Gonzalez FJ, Yamazoe Y, Tamai I. Regulation of drug transporters by the farnesoid X receptor in mice. Mol Pharm. 2004;1:281–289. doi: 10.1021/mp0499656. [DOI] [PubMed] [Google Scholar]
- 89.Plass JRM, Mol O, Heegsma J, Geuken M, Faber KN, Jansen PLM, Müller M. Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology. 2002;35:589–596. doi: 10.1053/jhep.2002.31724. [DOI] [PubMed] [Google Scholar]
- 90.Lee FY, de Aguiar Vallim TQ, Chong HK, Zhang Y, Liu Y, Jones SA, Osborne TF, Edwards PA. Activation of the farnesoid X receptor provides protection against acetaminophen-induced hepatic toxicity. Mol Endocrinol. 2010;24:1626–1636. doi: 10.1210/me.2010-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song K, Li T, Owsley E, Strom S, Chiang JYL. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhan L, Liu H, Fang Y, Kong B, He Y, Zhong X, Fang J, Wan YY, Guo GL. Genome-wide binding and transcriptome analysis of human farnesoid X receptor in primary human hepatocytes. PLoS ONE. 2014;9:e105930. doi: 10.1371/journal.pone.0105930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou W, Feng X, Wu Y, Benge J, Zhang Z, Chen Z. FGF-receptor substrate 2 functions as a molecular sensor integrating external regulatory signals into the FGF pathway. Cell Res. 2009;19:1165–1177. doi: 10.1038/cr.2009.95. [DOI] [PubMed] [Google Scholar]
- 94.Kong B, Wang L, Chiang JYL, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Modica S, Petruzzelli M, Bellafante E, Murzilli S, Salvatore L, Celli N, Di Tullio G, Palasciano G, Moustafa T, Halilbasic E, Trauner M, Moschetta A. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology. 2012;142:355–365. doi: 10.1053/j.gastro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 96.Stroeve JHM, Brufau G, Stellaard F, Gonzalez FJ, Staels B, Kuipers F. Intestinal FXR-mediated FGF15 production contributes to diurnal control of hepatic bile acid synthesis in mice. Lab Invest. 2010;90:1457–1467. doi: 10.1038/labinvest.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 98.Li S, Hsu DDF, Li B, Luo X, Alderson N, Qiao L, Ma L, Zhu HH, He Z, Suino-Powell K, Ji K, Li J, Shao J, Xu HE, Li T, Feng G. Cytoplasmic tyrosine phosphatase Shp2 coordinates hepatic regulation of bile acid and FGF15/19 signaling to repress bile acid synthesis. Cell Metab. 2014;20:320–332. doi: 10.1016/j.cmet.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kir S, Zhang Y, Gerard RD, Kliewer SA, Mangelsdorf DJ. Nuclear receptors HNF4α and LRH-1 cooperate in regulating Cyp7a1 in vivo. J Biol Chem. 2012;287:41334–41341. doi: 10.1074/jbc.M112.421834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ, Breslow J, Ananthanarayanan M, Shneider BL. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem. 2003;278:19909–19916. doi: 10.1074/jbc.M207903200. [DOI] [PubMed] [Google Scholar]
- 101.Hwang ST, Urizar NL, Moore DD, Henning SJ. Bile acids regulate the ontogenic expression of ileal bile acid binding protein in the rat via the farnesoid X receptor. Gastroenterology. 2002;122:1483–1492. doi: 10.1053/gast.2002.32982. [DOI] [PubMed] [Google Scholar]
- 102.Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai S, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1124–G1130. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- 103.Cui YJ, Aleksunes LM, Tanaka Y, Goedken MJ, Klaassen CD. Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol Sci. 2009;110:47–60. doi: 10.1093/toxsci/kfp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fiorucci S, Clerici C, Antonelli E, Orlandi S, Goodwin B, Sadeghpour BM, Sabatino G, Russo G, Castellani D, Willson TM, Pruzanski M, Pellicciari R, Morelli A. Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J Pharmacol Exp Ther. 2005;313:604–612. doi: 10.1124/jpet.104.079665. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez JG, Evans RM, Downes M. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci USA. 2006;103:11323–11328. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marschall H, Wagner M, Bodin K, Zollner G, Fickert P, Gumhold J, Silbert D, Fuchsbichler A, Sjövall J, Trauner M. Fxr(−/−) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J Lipid Res. 2006;47:582–592. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- 109.Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, Marschall H, Denk H, Trauner M. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- 110.Kazgan N, Metukuri MR, Purushotham A, Lu J, Rao A, Lee S, Pratt-Hyatt M, Lickteig A, Csanaky IL, Zhao Y, Dawson PA, Li X. Intestine-specific deletion of SIRT1 in mice impairs DCoH2-HNF-1α-FXR signaling and alters systemic bile acid homeostasis. Gastroenterology. 2014;146:1006–1016. doi: 10.1053/j.gastro.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lefebvre P, Staels B. DCo(H2)Ding the Metabolic Functions of SIRT1 in the Intestine. Gastroenterology. 2014;146:893–896. doi: 10.1053/j.gastro.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 112.Alvarez L, Jara P, Sánchez-Sabaté E, Hierro L, Larrauri J, Díaz MC, Camarena C, De la Vega A, Frauca E, López-Collazo E, Lapunzina P. Reduced hepatic expression of farnesoid X receptor in hereditary cholestasis associated to mutation in ATP8B1. Hum Mol Genet. 2004;13:2451–2460. doi: 10.1093/hmg/ddh261. [DOI] [PubMed] [Google Scholar]
- 113.Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, Strautnieks SS, Thompson RJ, Magid MS, Gordon R, Balasubramanian N, Suchy FJ, Shneider BL. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology. 2004;126:756–764. doi: 10.1053/j.gastro.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 114.Zimmer V, Müllenbach R, Simon E, Bartz C, Matern S, Lammert F. Combined functional variants of hepatobiliary transporters and FXR aggravate intrahepatic cholestasis of pregnancy. Liver Int. 2009;29:1286–1288. doi: 10.1111/j.1478-3231.2009.02026.x. [DOI] [PubMed] [Google Scholar]
- 115.Langhi C, Le May C, Kourimate S, Caron S, Staels B, Krempf M, Costet P, Cariou B. Activation of the farnesoid X receptor represses PCSK9 expression in human hepatocytes. FEBS Lett. 2008;582:949–955. doi: 10.1016/j.febslet.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 116.Wang Y, Jones PJH, Woollett LA, Buckley DD, Yao L, Granholm NA, Tolley EA, Heubi JE. Effects of chenodeoxycholic acid and deoxycholic acid on cholesterol absorption and metabolism in humans. Transl Res. 2006;148:37–45. doi: 10.1016/j.lab.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 117.Pérez-Aguilar F, Bretó M, Alegre B, Berenguer J. Increase in serum total cholesterol and low-density lipoprotein cholesterol by high-dose chenodeoxycholic acid in patients with radiolucent gallstones significantly reversed during preventive low dose after gallstone dissolution. Digestion. 1985;31:225–233. doi: 10.1159/000199204. [DOI] [PubMed] [Google Scholar]
- 118.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall H, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 119.Gautier T, de Haan W, Grober J, Ye D, Bahr MJ, Claudel T, Nijstad N, Van Berkel TJC, Havekes LM, Manns MP, Willems SM, Hogendoorn PCW, Lagrost L, Kuipers F, Van Eck M, Rensen PCN, Tietge UJF. Farnesoid X receptor activation increases cholesteryl ester transfer protein expression in humans and transgenic mice. J Lipid Res. 2013;54:2195–2205. doi: 10.1194/jlr.M038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Urizar NL, Dowhan DH, Moore DD. The farnesoid X-activated receptor mediates bile acid activation of phospholipid transfer protein gene expression. J Biol Chem. 2000;275:39313–39317. doi: 10.1074/jbc.M007998200. [DOI] [PubMed] [Google Scholar]
- 121.Sirvent A, Verhoeven AJM, Jansen H, Kosykh V, Darteil RJ, Hum DW, Fruchart J, Staels B. Farnesoid X receptor represses hepatic lipase gene expression. J Lipid Res. 2004;45:2110–2115. doi: 10.1194/jlr.M400221-JLR200. [DOI] [PubMed] [Google Scholar]
- 122.Leiss O, von Bergmann K. Different effects of chenodeoxycholic acid and ursodeoxycholic acid on serum lipoprotein concentrations in patients with radiolucent gallstones. Scand J Gastroenterol. 1982;17:587–592. doi: 10.3109/00365528209181063. [DOI] [PubMed] [Google Scholar]
- 123.Hambruch E, Miyazaki-Anzai S, Hahn U, Matysik S, Boettcher A, Perović-Ottstadt S, Schlüter T, Kinzel O, Krol HD, Deuschle U, Burnet M, Levi M, Schmitz G, Miyazaki M, Kremoser C. Synthetic farnesoid X receptor agonists induce high-density lipoprotein-mediated transhepatic cholesterol efflux in mice and monkeys and prevent atherosclerosis in cholesteryl ester transfer protein transgenic low-density lipoprotein receptor (−/−) mice. J Pharmacol Exp Ther. 2012;343:556–567. doi: 10.1124/jpet.112.196519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hirokane H, Nakahara M, Tachibana S, Shimizu M, Sato R. Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor-4. J Biol Chem. 2004;279:45685–45692. doi: 10.1074/jbc.M404255200. [DOI] [PubMed] [Google Scholar]
- 126.Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in zucker (Fa/Fa) obese rats. J Lipid Res. 2010;51:771–784. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schmitt J, Kong B, Stieger B, Tschopp O, Schultze SM, Rau M, Weber A, Müllhaupt B, Guo GL,Geier A (2014) Protective effects of farnesoid X receptor (FXR) on hepatic lipid accumulation are mediated by hepatic FXR and independent of intestinal FGF15 signal. Liver Int [DOI] [PMC free article] [PubMed]
- 128.Zhang S, Wang J, Liu Q, Harnish DC. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol. 2009;51:380–388. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 129.Porez G, Gross B, Prawitt J, Gheeraert C, Berrabah W, Alexandre J, Staels B, Lefebvre P. The hepatic orosomucoid/Α1-acid glycoprotein gene cluster is regulated by the nuclear bile acid receptor FXR. Endocrinology. 2013;154:3690–3701. doi: 10.1210/en.2013-1263. [DOI] [PubMed] [Google Scholar]
- 130.Duran-Sandoval D, Mautino G, Martin G, Percevault F, Barbier O, Fruchart J, Kuipers F, Staels B. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 2004;53:890–898. doi: 10.2337/diabetes.53.4.890. [DOI] [PubMed] [Google Scholar]
- 131.Watanabe M, Horai Y, Houten SM, Morimoto K, Sugizaki T, Arita E, Mataki C, Sato H, Tanigawara Y, Schoonjans K, Itoh H, Auwerx J. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J Biol Chem. 2011;286:26913–26920. doi: 10.1074/jbc.M111.248203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Duran-Sandoval D, Cariou B, Percevault F, Hennuyer N, Grefhorst A, van Dijk TH, Gonzalez FJ, Fruchart J, Kuipers F, Staels B. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J Biol Chem. 2005;280:29971–29979. doi: 10.1074/jbc.M501931200. [DOI] [PubMed] [Google Scholar]
- 133.Caron S, Huaman Samanez C, Dehondt H, Ploton M, Briand O, Lien F, Dorchies E, Dumont J, Postic C, Cariou B, Lefebvre P, Staels B. Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes. Mol Cell Biol. 2013;33:2202–2211. doi: 10.1128/MCB.01004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Renga B, Mencarelli A, D’Amore C, Cipriani S, Baldelli F, Zampella A, Distrutti E, Fiorucci S. Glucocorticoid receptor mediates the gluconeogenic activity of the farnesoid X receptor in the fasting condition. FASEB J. 2012;26:3021–3031. doi: 10.1096/fj.11-195701. [DOI] [PubMed] [Google Scholar]
- 135.Seyer P, Vallois D, Poitry-Yamate C, Schütz F, Metref S, Tarussio D, Maechler P, Staels B, Lanz B, Grueter R, Decaris J, Turner S, da Costa A, Preitner F, Minehira K, Foretz M, Thorens B. Hepatic glucose sensing is required to preserve β cell glucose competence. J Clin Invest. 2013;123:1662–1676. doi: 10.1172/JCI65538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Düfer M, Hörth K, Wagner R, Schittenhelm B, Prowald S, Wagner TFJ, Oberwinkler J, Lukowski R, Gonzalez FJ, Krippeit-Drews P, Drews G. Bile acids acutely stimulate insulin secretion of mouse Β-cells via farnesoid X receptor activation and K(ATP) channel inhibition. Diabetes. 2012;61:1479–1489. doi: 10.2337/db11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Renga B, Mencarelli A, Vavassori P, Brancaleone V, Fiorucci S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. 2010;1802:363–372. doi: 10.1016/j.bbadis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 138.Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li G, Kong B, Zhu Y, Zhan L, Williams JA, Tawfik O, Kassel KM, Luyendyk JP, Wang L, Guo GL. Small heterodimer partner overexpression partially protects against liver tumor development in farnesoid X receptor knockout mice. Toxicol Appl Pharmacol. 2013;272:299–305. doi: 10.1016/j.taap.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li G, Zhu Y, Tawfik O, Kong B, Williams JA, Zhan L, Kassel KM, Luyendyk JP, Wang L, Guo GL. Mechanisms of STAT3 activation in the liver of FXR knockout mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G829–G837. doi: 10.1152/ajpgi.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu Z, Huang G, Gong W, Zhou P, Zhao Y, Zhang Y, Zeng Y, Gao M, Pan Z, He F. FXR ligands protect against hepatocellular inflammation via SOCS3 induction. Cell Signal. 2012;24:1658–1664. doi: 10.1016/j.cellsig.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 142.Deuschle U, Schüler J, Schulz A, Schlüter T, Kinzel O, Abel U, Kremoser C. FXR controls the tumor suppressor NDRG2 and FXR agonists reduce liver tumor growth and metastasis in an orthotopic mouse xenograft model. PLoS ONE. 2012;7:e43044. doi: 10.1371/journal.pone.0043044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ohno T, Shirakami Y, Shimizu M, Kubota M, Sakai H, Yasuda Y, Kochi T, Tsurumi H, Moriwaki H. Synergistic growth inhibition of human hepatocellular carcinoma cells by acyclic retinoid and GW4064, a farnesoid X receptor ligand. Cancer Lett. 2012;323:215–222. doi: 10.1016/j.canlet.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 144.Su H, Ma C, Liu J, Li N, Gao M, Huang A, Wang X, Huang W, Huang X. Downregulation of nuclear receptor FXR is associated with multiple malignant clinicopathological characteristics in human hepatocellular carcinoma. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1245–G1253. doi: 10.1152/ajpgi.00439.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vaquero J, Briz O, Herraez E, Muntané J, Marin JJG. Activation of the nuclear receptor FXR enhances hepatocyte chemoprotection and liver tumor chemoresistance against genotoxic compounds. Biochim Biophys Acta. 2013;1833:2212–2219. doi: 10.1016/j.bbamcr.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 146.Herraez E, Gonzalez-Sanchez E, Vaquero J, Romero MR, Serrano MA, Marin JJG, Briz O. Cisplatin-induced chemoresistance in colon cancer cells involves FXR-dependent and FXR-independent up-regulation of ABC proteins. Mol Pharm. 2012;9:2565–2576. doi: 10.1021/mp300178a. [DOI] [PubMed] [Google Scholar]
- 147.Degirolamo C, Modica S, Vacca M, Di Tullio G, Morgano A, D’Orazio A, Kannisto K, Parini P,Moschetta A (2014) Prevention of spontaneous hepatocarcinogenesis in FXR null mice by intestinal specific FXR re-activation. Hepatology [DOI] [PubMed]
- 148.Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu J, Yang H, Humphrey M, Ding X, Arora T, Learned RM, DePaoli AM, Tian H, Ling L. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res. 2014;74:3306–3316. doi: 10.1158/0008-5472.CAN-14-0208. [DOI] [PubMed] [Google Scholar]
- 149.Luo J, Ko B, Elliott M, Zhou M, Lindhout DA, Phung V, To C, Learned RM, Tian H, DePaoli AM, Ling L. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med. 2014;6:247ra100. doi: 10.1126/scitranslmed.3009098. [DOI] [PubMed] [Google Scholar]
- 150.De Gottardi A, Touri F, Maurer CA, Perez A, Maurhofer O, Ventre G, Bentzen CL, Niesor EJ, Dufour J. The bile acid nuclear receptor FXR and the bile acid binding protein IBABP are differently expressed in colon cancer. Dig Dis Sci. 2004;49:982–989. doi: 10.1023/b:ddas.0000034558.78747.98. [DOI] [PubMed] [Google Scholar]
- 151.Lax S, Schauer G, Prein K, Kapitan M, Silbert D, Berghold A, Berger A, Trauner M. Expression of the nuclear bile acid receptor/farnesoid X receptor is reduced in human colon carcinoma compared to nonneoplastic mucosa independent from site and may be associated with adverse prognosis. Int J Cancer. 2012;130:2232–2239. doi: 10.1002/ijc.26293. [DOI] [PubMed] [Google Scholar]
- 152.Stojancevic M, Stankov K, Mikov M. The impact of farnesoid X receptor activation on intestinal permeability in inflammatory bowel disease. Can J Gastroenterol. 2012;26:631–637. doi: 10.1155/2012/538452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Inagaki T, Moschetta A, Lee Y, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589–9594. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]
- 155.Xu Y, Watanabe T, Tanigawa T, Machida H, Okazaki H, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y, Oshitani N, Arakawa T. Bile acids induce Cdx2 expression through the farnesoid X receptor in gastric epithelial cells. J Clin Biochem Nutr. 2010;46:81–86. doi: 10.3164/jcbn.09-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen W, Wang Y, Zhang L, Shiah S, Wang M, Yang F, Yu D, Forman BM, Huang W. Farnesoid X receptor alleviates age-related proliferation defects in regenerating mouse livers by activating Forkhead box M1b transcription. Hepatology. 2010;51:953–962. doi: 10.1002/hep.23390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.García-Rodríguez JL, Barbier-Torres L, Fernández-Álvarez S, Gutiérrez-de Juan V, Monte MJ, Halilbasic E, Herranz D, Álvarez L, Aspichueta P, Marín JJG, Trauner M, Mato JM, Serrano M, Beraza N, Martínez-Chantar ML. SIRT1 controls liver regeneration by regulating bile acid metabolism through farnesoid X receptor and mammalian target of rapamycin signaling. Hepatology. 2014;59:1972–1983. doi: 10.1002/hep.26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang L, Wang Y, Chen W, Wang X, Lou G, Liu N, Lin M, Forman BM, Huang W. Promotion of liver regeneration/repair by farnesoid X receptor in both liver and intestine in mice. Hepatology. 2012;56:2336–2343. doi: 10.1002/hep.25905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Seol W, Choi HS, Moore DD. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 160.Chong HK, Infante AM, Seo Y, Jeon T, Zhang Y, Edwards PA, Xie X, Osborne TF. Genome-wide interrogation of hepatic FXR reveals an asymmetric IR-1 motif and synergy with LRH-1. Nucleic Acids Res. 2010;38:6007–6017. doi: 10.1093/nar/gkq397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Thomas AM, Hart SN, Kong B, Fang J, Zhong X, Guo GL. Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology. 2010;51:1410–1419. doi: 10.1002/hep.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Berrabah W, Aumercier P, Gheeraert C, Dehondt H, Bouchaert E, Alexandre J, Ploton M, Mazuy C, Caron S, Tailleux A, Eeckhoute J, Lefebvre T, Staels B, Lefebvre P. Glucose sensing O-GlcNAcylation pathway regulates the nuclear bile acid receptor farnesoid X receptor (FXR) Hepatology. 2014;59:2022–2033. doi: 10.1002/hep.26710. [DOI] [PubMed] [Google Scholar]
- 163.Song CS, Echchgadda I, Baek BS, Ahn SC, Oh T, Roy AK, Chatterjee B. Dehydroepiandrosterone sulfotransferase gene induction by bile acid activated farnesoid X receptor. J Biol Chem. 2001;276:42549–42556. doi: 10.1074/jbc.M107557200. [DOI] [PubMed] [Google Scholar]
- 164.Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 165.Laffitte BA, Kast HR, Nguyen CM, Zavacki AM, Moore DD, Edwards PA. Identification of the DNA binding specificity and potential target genes for the farnesoid X-activated receptor. J Biol Chem. 2000;275:10638–10647. doi: 10.1074/jbc.275.14.10638. [DOI] [PubMed] [Google Scholar]
- 166.Claudel T, Inoue Y, Barbier O, Duran-Sandoval D, Kosykh V, Fruchart J, Fruchart J, Gonzalez FJ, Staels B. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125:544–555. doi: 10.1016/s0016-5085(03)00896-5. [DOI] [PubMed] [Google Scholar]
- 167.Mi L, Devarakonda S, Harp JM, Han Q, Pellicciari R, Willson TM, Khorasanizadeh S, Rastinejad F. Structural basis for bile acid binding and activation of the nuclear receptor FXR. Mol Cell. 2003;11:1093–1100. doi: 10.1016/s1097-2765(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 168.Kullak-Ublick GA, Beuers U, Paumgartner G. Molecular and functional characterization of bile acid transport in human hepatoblastoma HepG2 cells. Hepatology. 1996;23:1053–1060. doi: 10.1002/hep.510230518. [DOI] [PubMed] [Google Scholar]
- 169.Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. 2012;53:1723–1737. doi: 10.1194/jlr.R024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Downes M, Verdecia MA, Roecker AJ, Hughes R, Hogenesch JB, Kast-Woelbern HR, Bowman ME, Ferrer J, Anisfeld AM, Edwards PA, Rosenfeld JM, Alvarez JGA, Noel JP, Nicolaou KC, Evans RM. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol Cell. 2003;11:1079–1092. doi: 10.1016/s1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dwivedi SKD, Singh N, Kumari R, Mishra JS, Tripathi S, Banerjee P, Shah P, Kukshal V, Tyagi AM, Gaikwad AN, Chaturvedi RK, Mishra DP, Trivedi AK, Sanyal S, Chattopadhyay N, Ramachandran R, Siddiqi MI, Bandyopadhyay A, Arora A, Lundåsen T, Anakk SP, Moore DD, Sanyal S. Bile acid receptor agonist GW4064 regulates PPARγ coactivator-1α expression through estrogen receptor-related receptor Α. Mol Endocrinol. 2011;25:922–932. doi: 10.1210/me.2010-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cui J, Huang L, Zhao A, Lew J, Yu J, Sahoo S, Meinke PT, Royo I, Pelaez F, Wright SD. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J Biol Chem. 2003;278:10214–10220. doi: 10.1074/jbc.M209323200. [DOI] [PubMed] [Google Scholar]
- 173.Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recept Signal. 2010;8:e005. doi: 10.1621/nrs.08005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu P, Xu X, Chen L, Ma L, Shen X, Hu L. Discovery and SAR study of hydroxyacetophenone derivatives as potent, non-steroidal farnesoid X receptor (FXR) antagonists. Bioorg Med Chem. 2014;22:1596–1607. doi: 10.1016/j.bmc.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 175.Xu X, Lu Y, Chen L, Chen J, Luo X, Shen X. Identification of 15d-PGJ2 as an antagonist of farnesoid X receptor: molecular modeling with biological evaluation. Steroids. 2013;78:813–822. doi: 10.1016/j.steroids.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 176.Yu DD, Lin W, Chen T, Forman BM. Development of time resolved fluorescence resonance energy transfer-based assay for FXR antagonist discovery. Bioorg Med Chem. 2013;21:4266–4278. doi: 10.1016/j.bmc.2013.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Di Leva FS, Festa C, D’Amore C, De Marino S, Renga B, D’Auria MV, Novellino E, Limongelli V, Zampella A, Fiorucci S. Binding mechanism of the farnesoid X receptor marine antagonist suvanine reveals a strategy to forestall drug modulation on nuclear receptors. Design, synthesis, and biological evaluation of novel ligands. J Med Chem. 2013;56:4701–4717. doi: 10.1021/jm400419e. [DOI] [PubMed] [Google Scholar]
- 178.Newton R, Holden NS. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol Pharmacol. 2007;72:799–809. doi: 10.1124/mol.107.038794. [DOI] [PubMed] [Google Scholar]
- 179.Stoecklin E, Wissler M, Schaetzle D, Pfitzner E, Groner B. Interactions in the transcriptional regulation exerted by Stat5 and by members of the steroid hormone receptor family. J Steroid Biochem Mol Biol. 1999;69:195–204. doi: 10.1016/s0960-0760(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 180.De Martino MU, Alesci S, Chrousos GP, Kino T. Interaction of the glucocorticoid receptor and the chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII): implications for the actions of glucocorticoids on glucose, lipoprotein, and xenobiotic metabolism. Ann N Y Acad Sci. 2004;1024:72–85. doi: 10.1196/annals.1321.006. [DOI] [PubMed] [Google Scholar]
- 181.Barbier O, Torra IP, Sirvent A, Claudel T, Blanquart C, Duran-Sandoval D, Kuipers F, Kosykh V, Fruchart J, Staels B. FXR induces the UGT2B4 enzyme in hepatocytes: a potential mechanism of negative feedback control of FXR activity. Gastroenterology. 2003;124:1926–1940. doi: 10.1016/s0016-5085(03)00388-3. [DOI] [PubMed] [Google Scholar]
- 182.Shen H, Zhang Y, Ding H, Wang X, Chen L, Jiang H, Shen X. Farnesoid X receptor induces GLUT4 expression through FXR response element in the GLUT4 promoter. Cell Physiol Biochem. 2008;22:1–14. doi: 10.1159/000149779. [DOI] [PubMed] [Google Scholar]
- 183.Chong HK, Biesinger J, Seo Y, Xie X, Osborne TF. Genome-wide analysis of hepatic LRH-1 reveals a promoter binding preference and suggests a role in regulating genes of lipid metabolism in concert with FXR. BMC Genom. 2012;13:51. doi: 10.1186/1471-2164-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thomas AM, Hart SN, Li G, Lu H, Fang Y, Fang J, Zhong X, Guo GL. Hepatocyte nuclear factor 4 alpha and farnesoid X receptor co-regulates gene transcription in mouse livers on a genome-wide scale. Pharm Res. 2013;30:2188–2198. doi: 10.1007/s11095-013-1006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fujino T, Sato Y, Une M, Kanayasu-Toyoda T, Yamaguchi T, Shudo K, Inoue K, Nishimaki-Mogami T. In vitro farnesoid X receptor ligand sensor assay using surface plasmon resonance and based on ligand-induced coactivator association. J Steroid Biochem Mol Biol. 2003;87:247–252. doi: 10.1016/j.jsbmb.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 186.Kassam A, Miao B, Young PR, Mukherjee R. Retinoid X receptor (RXR) agonist-induced antagonism of farnesoid X receptor (FXR) activity due to absence of coactivator recruitment and decreased DNA binding. J Biol Chem. 2003;278:10028–10032. doi: 10.1074/jbc.M208312200. [DOI] [PubMed] [Google Scholar]
- 187.Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. Peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004;18:157–169. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Unno A, Takada I, Takezawa S, Oishi H, Baba A, Shimizu T, Tokita A, Yanagisawa J, Kato S. TRRAP as a hepatic coactivator of LXR and FXR function. Biochem Biophys Res Commun. 2005;327:933–938. doi: 10.1016/j.bbrc.2004.12.095. [DOI] [PubMed] [Google Scholar]
- 189.Balasubramaniyan N, Ananthanarayanan M, Suchy FJ. Direct methylation of FXR by Set7/9, a lysine methyltransferase, regulates the expression of FXR target genes. Am J Physiol Gastrointest Liver Physiol. 2012;302:G937–G947. doi: 10.1152/ajpgi.00441.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rizzo G, Renga B, Antonelli E, Passeri D, Pellicciari R, Fiorucci S. The methyl transferase PRMT1 functions as co-activator of farnesoid X receptor (FXR)/9-cis retinoid X receptor and regulates transcription of FXR responsive genes. Mol Pharmacol. 2005;68:551–558. doi: 10.1124/mol.105.012104. [DOI] [PubMed] [Google Scholar]
- 191.Ananthanarayanan M, Li S, Balasubramaniyan N, Suchy FJ, Walsh MJ. Ligand-dependent activation of the farnesoid X-receptor directs arginine methylation of histone H3 by CARM1. J Biol Chem. 2004;279:54348–54357. doi: 10.1074/jbc.M410021200. [DOI] [PubMed] [Google Scholar]
- 192.Ananthanarayanan M, Li Y, Surapureddi S, Balasubramaniyan N, Ahn J, Goldstein JA, Suchy FJ. Histone H3K4 trimethylation by MLL3 as part of ASCOM complex is critical for NR activation of bile acid transporter genes and is downregulated in cholestasis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G771–G781. doi: 10.1152/ajpgi.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim D, Lee J, Lee B, Lee JW. ASCOM controls farnesoid X receptor transactivation through its associated histone H3 lysine 4 methyltransferase activity. Mol Endocrinol. 2009;23:1556–1562. doi: 10.1210/me.2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fang S, Tsang S, Jones R, Ponugoti B, Yoon H, Wu S, Chiang C, Willson TM, Kemper JK. The P300 acetylase is critical for ligand-activated farnesoid X receptor (FXR) induction of SHP. J Biol Chem. 2008;283:35086–35095. doi: 10.1074/jbc.M803531200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Miao J, Fang S, Lee J, Comstock C, Knudsen KE, Kemper JK. Functional specificities of Brm and Brg-1 Swi/Snf ATPases in the feedback regulation of hepatic bile acid biosynthesis. Mol Cell Biol. 2009;29:6170–6181. doi: 10.1128/MCB.00825-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kainuma M, Makishima M, Hashimoto Y, Miyachi H. Design, synthesis, and evaluation of non-steroidal farnesoid X receptor (FXR) antagonist. Bioorg Med Chem. 2007;15:2587–2600. doi: 10.1016/j.bmc.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 197.Ohno M, Kunimoto M, Nishizuka M, Osada S, Imagawa M. Ku proteins function as corepressors to regulate farnesoid X receptor-mediated gene expression. Biochem Biophys Res Commun. 2009;390:738–742. doi: 10.1016/j.bbrc.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 198.Li J, Lu Y, Liu R, Xiong X, Zhang Z, Zhang X, Ning G, Li X. DAX1 suppresses FXR transactivity as a novel co-repressor. Biochem Biophys Res Commun. 2011;412:660–666. doi: 10.1016/j.bbrc.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 199.Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu S, Chiang C, Veenstra TD. FXR acetylation is normally dynamically regulated by P300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lien F, Berthier A, Bouchaert E, Gheeraert C, Alexandre J, Porez G, Prawitt J, Dehondt H, Ploton M, Colin S, Lucas A, Patrice A, Pattou F, Diemer H, Van Dorsselaer A, Rachez C, Kamilic J, Groen AK, Staels B, Lefebvre P. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J Clin Invest. 2014;124:1037–1051. doi: 10.1172/JCI68815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Renga B, D’Amore C, Cipriani S, Mencarelli A, Carino A, Sepe V, Zampella A, Distrutti E, Fiorucci S. FXR mediates a chromatin looping in the GR promoter thus promoting the resolution of colitis in rodents. Pharmacol Res. 2013;77:1–10. doi: 10.1016/j.phrs.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 202.Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X, Oh S, Kim H, Glass CK, Rosenfeld MG. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498:516–520. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, Kosykh V, Fruchart J, Dallongeville J, Hum DW, Kuipers F, Staels B. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein a-i transcription via a negative FXR response element. J Clin Invest. 2002;109:961–971. doi: 10.1172/JCI14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gardès C, Blum D, Bleicher K, Chaput E, Ebeling M, Hartman P, Handschin C, Richter H, Benson GM. Studies in mice, hamsters, and rats demonstrate that repression of hepatic apoA-i expression by taurocholic acid in mice is not mediated by the farnesoid-X-receptor. J Lipid Res. 2011;52:1188–1199. doi: 10.1194/jlr.M012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chennamsetty I, Claudel T, Kostner KM, Baghdasaryan A, Kratky D, Levak-Frank S, Frank S, Gonzalez FJ, Trauner M, Kostner GM. Farnesoid X receptor represses hepatic human APOA gene expression. J Clin Invest. 2011;121:3724–3734. doi: 10.1172/JCI45277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lu Y, Heydel J, Li X, Bratton S, Lindblom T, Radominska-Pandya A. Lithocholic acid decreases expression of UGT2B7 in Caco-2 Cells: a potential role for a negative farnesoid X receptor response element. Drug Metab Dispos. 2005;33:937–946. doi: 10.1124/dmd.104.003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Miyata M, Matsuda Y, Tsuchiya H, Kitada H, Akase T, Shimada M, Nagata K, Gonzalez FJ, Yamazoe Y. Chenodeoxycholic acid-mediated activation of the farnesoid X receptor negatively regulates hydroxysteroid sulfotransferase. Drug Metab Pharmacokinet. 2006;21:315–323. doi: 10.2133/dmpk.21.315. [DOI] [PubMed] [Google Scholar]
- 208.Seok S, Fu T, Choi S, Li Y, Zhu R, Kumar S, Sun X, Yoon G, Kang Y, Zhong W, Ma J, Kemper B,Kemper JK (2014) Transcriptional regulation of autophagy by an FXR-CREB axis. Nature [DOI] [PMC free article] [PubMed]
- 209.Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA, Moore DD (2014) Nutrient-sensing nuclear receptors coordinate autophagy. Nature [DOI] [PMC free article] [PubMed]
- 210.Wang Y, Chen W, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48:1632–1643. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- 212.Gadaleta RM, Oldenburg B, Willemsen ECL, Spit M, Murzilli S, Salvatore L, Klomp LWJ, Siersema PD, van Erpecum KJ, van Mil SWC. Activation of bile salt nuclear receptor FXR is repressed by pro-inflammatory cytokines activating NF-κb signaling in the intestine. Biochim Biophys Acta. 2011;1812:851–858. doi: 10.1016/j.bbadis.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 213.Pawlak M, Baugé E, Bourguet W, De Bosscher K, Lalloyer F, Tailleux A, Lebherz C, Lefebvre P, Staels B. The transrepressive activity of peroxisome proliferator-activated receptor alpha is necessary and sufficient to prevent liver fibrosis in mice. Hepatology. 2014;60:1593–1606. doi: 10.1002/hep.27297. [DOI] [PubMed] [Google Scholar]
- 214.Houten SM, Volle DH, Cummins CL, Mangelsdorf DJ, Auwerx J. In vivo imaging of farnesoid X receptor activity reveals the ileum as the primary bile acid signaling tissue. Mol Endocrinol. 2007;21:1312–1323. doi: 10.1210/me.2007-0113. [DOI] [PubMed] [Google Scholar]
- 215.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JAJ, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang YJ, Guo GL, Klaassen CD. Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. PLoS ONE. 2011;6:e16683. doi: 10.1371/journal.pone.0016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pan X, Zhang Y, Wang L, Hussain MM. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 2010;12:174–186. doi: 10.1016/j.cmet.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lee J, Seok S, Yu P, Kim K, Smith Z, Rivas-Astroza M, Zhong S, Kemper JK. Genomic analysis of hepatic farnesoid X receptor binding sites reveals altered binding in obesity and direct gene repression by farnesoid X receptor in mice. Hepatology. 2012;56:108–117. doi: 10.1002/hep.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Purushotham A, Xu Q, Lu J, Foley JF, Yan X, Kim D, Kemper JK, Li X. Hepatic deletion of SIRT1 decreases hepatocyte nuclear factor 1α/farnesoid X receptor signaling and induces formation of cholesterol gallstones in mice. Mol Cell Biol. 2012;32:1226–1236. doi: 10.1128/MCB.05988-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gineste R, Sirvent A, Paumelle R, Helleboid S, Aquilina A, Darteil R, Hum DW, Fruchart J, Staels B. Phosphorylation of farnesoid X receptor by protein kinase C promotes its transcriptional activity. Mol Endocrinol. 2008;22:2433–2447. doi: 10.1210/me.2008-0092. [DOI] [PubMed] [Google Scholar]
- 222.Frankenberg T, Miloh T, Chen FY, Ananthanarayanan M, Sun A, Balasubramaniyan N, Arias I, Setchell KDR, Suchy FJ, Shneider BL. The membrane protein ATPase class I type 8B member 1 signals through protein kinase C zeta to activate the farnesoid X receptor. Hepatology. 2008;48:1896–1905. doi: 10.1002/hep.22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Balasubramaniyan N, Luo Y, Sun A, Suchy FJ. SUMOylation of the farnesoid X receptor (FXR) regulates the expression of FXR target genes. J Biol Chem. 2013;288:13850–13862. doi: 10.1074/jbc.M112.443937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kuipers F, Groen AK. FXR: the key to benefits in bariatric surgery? Nat Med. 2014;20:337–338. doi: 10.1038/nm.3525. [DOI] [PubMed] [Google Scholar]
- 226.Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today. 2012;17:988–997. doi: 10.1016/j.drudis.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 227.Kemper JK. Regulation of FXR transcriptional activity in health and disease: emerging roles of FXR cofactors and post-translational modifications. Biochim Biophys Acta. 2011;1812:842–850. doi: 10.1016/j.bbadis.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lew J, Zhao A, Yu J, Huang L, De Pedro N, Peláez F, Wright SD, Cui J. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J Biol Chem. 2004;279:8856–8861. doi: 10.1074/jbc.M306422200. [DOI] [PubMed] [Google Scholar]
- 229.Bramlett KS, Yao S, Burris TP. Correlation of farnesoid X receptor coactivator recruitment and cholesterol 7alpha-hydroxylase gene repression by bile acids. Mol Genet Metab. 2000;71:609–615. doi: 10.1006/mgme.2000.3106. [DOI] [PubMed] [Google Scholar]