Abstract
Learning and memory depend on long-term synaptic plasticity including long-term potentiation (LTP) and depression (LTD). Activity-regulated cytoskeleton-associated protein (Arc) plays versatile roles in synaptic plasticity mainly through inducing F-actin formation, underlying consolidation of LTP, and promoting AMPA receptor (AMPAR) endocytosis, underlying LTD. Insulin can also induce LTD by facilitating the internalization of AMPARs. In neuroblastoma cells, insulin induced a dramatic increase in Arc mRNA and Arc protein levels, which may underlie the memory-enhancing action of insulin. Thus, a hypothesis was made that, in response to insulin, increased AMPAR endocytosis leads to enhanced Arc expression, and vice versa. Primary cultures of neonatal Sprague–Dawley rat cortical neurons were used. Using Western-blot analysis and immunofluorescent staining, our results reveal that inhibiting AMPAR-mediated responses with AMPAR antagonists significantly enhanced whereas blocking AMPAR endocytosis with various reagents significantly prevented insulin (200 nM, 2 h)-induced Arc expression. Furthermore, via surface biotinylation assay, we demonstrate that acute blockade of new Arc synthesis after insulin stimulation using Arc antisense oligodeoxynucleotide prevented insulin-stimulated AMPAR endocytosis. These findings suggest for the first time that an interaction exists between insulin-stimulated AMPAR endocytosis and insulin-induced Arc expression.
Keywords: Insulin, AMPA receptor endocytosis, Arc, Synaptic plasticity, Memory
Introduction
Brain insulin has been reported to exert a memory-enhancing action in experimental animals and humans [1–3]. Learning and memory depend on long-term synaptic plasticity, which requires new gene expression and protein synthesis [4, 5]. In response to synaptic activity, similar macromolecular syntheses have been reported to be essential for long-term forms of synaptic plasticity such as long-term potentiation (LTP) and depression (LTD) [6]. The induction of activity-regulated cytoskeleton-associated protein (Arc) is of particular interest. Arc mRNA is rapidly transcribed following synaptic activity and then transported to activated dendritic regions where it undergoes local translation, suggesting a role of Arc in synaptic plasticity and in learning and memory [7, 8]. In fact, accumulating evidence reveals that Arc plays versatile roles in regulating multiple forms of synaptic plasticity, which includes inducing F-actin formation underlying consolidation of LTP [8–10] and promoting AMPA receptor (AMPAR) endocytosis underlying LTD [10–12]. Disruption of Arc protein expression impairs the maintenance of LTP and the consolidation of long-term memory [13, 14]. Furthermore, in addition to synaptic activity, Arc expression is also rapidly induced by various biochemical stimuli related to synaptic plasticity, such as brain-derived neurotrophic factor (BDNF) [15, 16], group 1 metabotropic glutamate receptor (mGluR) agonist dihydroxyphenylglycine (DHPG) [17] or cholinergic agonist carbachol [18]. In SH-SY5Y human neuroblastoma cells, application of insulin (100 nM, 1 h) also induced a dramatic increase in Arc mRNA and Arc protein levels [19], which may underlie the mechanism of insulin action on learning and memory [20].
In addition to the induction of Arc expression, insulin also plays a role in synaptic plasticity by acting on AMPA-type glutamate receptor trafficking. AMPA receptors are comprised of heterotetrameric complexes made up of at least two of four subunits designated GluR1-4 (or GluA1-4) [21]. AMPARs are ligand-gated ion channels and mediate most of the fast excitatory synaptic transmission in mammalian brain [22]. The levels of AMPARs at synapses are very dynamic, which seems to be a major mechanism contributing to synaptic plasticity [23, 24]. In fact, LTP generally requires newly inserted AMPARs at synapses, and LTD results from the removal of synaptic AMPARs [25, 26]. In the brain, insulin facilitates the internalization of AMPARs in clathrin-mediated and GluR2-dependent manners and thus induces LTD of excitatory synaptic transmission [25, 27, 28]. Although insulin-induced LTD is mechanistically distinct from LTD induced by low-frequency stimulation [28], it has been proposed that insulin-induced LTD may play an important role in brain information processing [29].
Previous studies have suggested that insulin-stimulated AMPAR endocytosis leads to a rapid reduction in the number of postsynaptic AMPARs and a long-lasting depression of the receptor-mediated synaptic transmission [25, 30, 31]. Furthermore, inhibiting AMPARs by antagonists has been shown to strongly potentiate BDNF-induced Arc expression in primary rat cortical neurons, indicating that AMPARs downregulate Arc expression [32]. Therefore, we hypothesized that insulin can enhance the expression of Arc through stimulating AMPAR endocytosis. To test this hypothesis, instead of the aforementioned SH-SY5Y cells, primary cultures of neonatal rat cortical neurons that recapitulate many features of Arc regulation in vivo [32] were used, and the effects of blocking either AMPAR-mediated responses or AMPAR endocytosis on insulin-induced Arc expression were evaluated by Western-blot analysis and immunofluorescent staining. In addition, through acute blockade of new Arc synthesis in response to insulin with Arc antisense oligodeoxynucleotide, whether insulin-induced Arc expression can regulate insulin-stimulated AMPAR endocytosis was examined by surface biotinylation assay. The results can reveal whether there is a reciprocal effect between insulin-induced Arc expression and insulin-stimulated AMPAR endocytosis. Consequently, the molecular mechanisms underlying insulin’s role in learning and memory will be further clarified, which is helpful in developing a strategy for preventing or treating memory loss.
Materials and methods
Chemicals and reagents
Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), B27 supplement, Alexa Fluor 488 donkey anti-goat IgG, and Alexa Fluor 594 goat anti-rabbit IgG were purchased from Invitrogen (Carlsbad, CA, USA). Mouse anti-Arc antibody, rabbit anti-Arc antibody, goat anti-GluR2 antibody raised against an extracellular epitope of the receptor, and donkey anti-goat horseradish peroxidase-conjugated antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-actin antibody, goat-anti-mouse horseradish peroxidase-conjugated secondary antibody, and normal goat serum (NGS) were purchased from Millipore (Billerica, MA, USA). Insulin, PP2, cytosine arabinoside, bovine serum albumin (BSA), and other chemicals for buffers were purchased from Sigma (St. Louis, MO, USA). NBQX and GYKI52466 hydrochloride were purchased from Tocris Bioscience (Bristol, UK). Tat-GluR23Y and its scrambled control peptide (scr-Tat-GluR23Y) were purchased from AnaSpec (Fremont, CA, USA).
Primary cultures of neonatal rat cortical neurons
All efforts were made to minimize the number of animals used and their suffering. Animal maintenance and treatment were approved by the Institutional Animal Care and Use Committee of Kaohsiung Medical University. Adult Sprague–Dawley rats for breeding were obtained from BioLasco Taiwan Co., Ltd. Primary cortical neurons were prepared from neonatal rats of either sex as previously established [33]. Less than 5 × 105 cells/cm2 were plated on poly-d-lysine precoated plastic dishes (for Western-blot analysis) or coverslips (for immunofluorescent staining). Cultures were maintained at 37 °C in a 5 % CO2 incubator. After 24 h, the medium was replaced with DMEM containing B27 supplement. Cytosine arabinoside at 5 μM was added to inhibit the proliferation of non-neuronal cells. The culture medium was changed every 3 days. The cultured neurons were used for experiments after 14–21 days in vitro (DIV).
Western-blot analysis
Cell lysate was centrifuged and the supernatant was quantified for total protein concentration by the BCA protein assay kit (Thermo Scientific, Pierce, CA, USA). Protein samples (30 μg per sample) were resolved by 10 % Bis–Tris gel (Invitrogen) using MOPS running buffer for 50 min and then were transferred to polyvinylidene difluoride (PVDF) membrane using NuPage transfer buffer for 60 min. Membranes were incubated at room temperature in TBS-T (0.1 % Tween 20, 0.05 M Tris base in 0.9 % NaCl, pH 7.4) containing 5 % nonfat milk for 30 min to block the nonspecific binding. Following incubation with the primary antibody that recognizes Arc (1:400) or actin (1:5,000) for 1 h at room temperature or at 4 °C overnight, the blots were washed in TBS-T and then incubated with the horseradish peroxidase-conjugated secondary antibody (1:4,000) for 1 h. After washing the blots with TBS-T, immunolabeling was detected using enhanced chemiluminescence reagents (Perkin Elmer, Waltham, MA, USA) and the protein levels were quantified using densitometric analysis. Three or four independent experiments were performed.
Surface biotinylation assay
AMPA receptor endocytosis was measured by surface biotinylation assay as described in previous studies [27, 34] with some modifications. Live cultured neurons were washed with ice-cold PBS (with calcium and magnesium, pH 7.4) and then were incubated in Sulfo-NHS-SS-Biotin (0.3 mg/ml in ice-cold PBS; Thermo Scientific) for 30 min to biotinylate surface proteins. Surface biotinylation was subsequently quenched with 50 mM glycine for 20 min to remove all unreacted biotin. After washes, neurons were incubated in medium alone or in medium containing various reagents in the presence or absence of insulin at 37 °C for durations indicated in the results. Following this incubation, during which the internalization occurred, medium was removed from dishes and neurons were incubated with 50 mM glutathione (dissolved in 75 mM NaCl, 10 mM EDTA, 1 % BSA, and 0.075 N NaOH) for 20 min at 4 °C to remove remaining surface-bound biotin. Then, neurons were washed with cold PBS and lysed with radio-immunoprecipitation assay (RIPA) buffer. Cell lysates were centrifuged and cleared supernatant containing 90 μg of protein was incubated with NeutrAvidin agarose (Thermo Scientific) overnight, rotating at 4 °C to capture internalized biotinylated proteins. After that, biotinylated proteins were eluted from NeutrAvidin agarose by boiling in sample buffer, separated by SDS-PAGE, transferred to a PVDF membrane, and immunoblotted to identify internalized biotinylated GluR2 using a goat anti-GluR2 antibody (1:500) followed by a donkey anti-goat horseradish peroxidase-conjugated antibody (1:2,000). In addition, total GluR2 level was determined by immunoblotting 30 μg of protein from each cleared supernatant not incubated with NeutrAvidin agarose. All immunoreactive band intensities for internalized biotinylated GluR2 were normalized to the band intensity for their respective total GluR2 within the same sample. Three independent experiments were performed.
Immunofluorescent staining
All procedures were performed at room temperature unless otherwise indicated. Primary cortical neurons on coverslips were washed briefly with PBS and then fixed for 15 min with 4 % paraformaldehyde in PBS under nonpermeabilizing conditions for detecting the expression of cell-surface GluR2 subunits. To minimize surface receptor internalization, 4 % sucrose was added to the fixation solution [35]. After fixation, neurons were washed followed by incubation in PBS containing 2 % BSA for l h to block nonspecific binding. Then, neurons were incubated for 1 h at room temperature followed by overnight at 4 °C with goat anti-GluR2 antibody that recognizes the extracellular domain of GluR2 (1:50) in PBS containing 0.1 % BSA. After washes, neurons were then incubated in PBS containing 0.1 % BSA and Alexa Fluor 488-conjugated anti-goat IgG (1:1,000) for 1 h. Subsequently, for detecting the expression of Arc protein, neurons were washed and then permeabilized with 0.1 % Triton X-100 in PBS (PBST) for 5 min. Blocking was performed in PBST containing 10 % NGS for l h. After this, neurons were incubated overnight at 4 °C in PBS containing 0.1 % NGS and rabbit anti-Arc antibody (1:50). Neurons were then washed and incubated in PBS containing 0.1 % NGS and Alexa Fluor 594-conjugated anti-rabbit IgG (1:1,000) for 1 h. Finally, neurons were washed extensively and mounted with Antifade Mounting Media containing 4,6-diamino-2-phenylindole dihydrochloride (DAPI) (Vector Laboratories, Burlingame, CA, USA) to reveal the nuclei. All images were taken under an Axioscope fluorescent microscope (Zeiss, Oberkochen, Germany) at the same exposure time for each fluorescent dye respectively.
Oligodeoxynucleotide design and treatments
As described in previous studies [13, 36], an antisense oligodeoxynucleotide (ODN) against Arc mRNA was designed to acutely block new synthesis of Arc and a scrambled ODN was also designed to serve as a control. The following sequences were used (“~” denotes a phosphorothioate linkage): 5′-G~T~C~CAGCTCCATCTGCT~C~G~C-3′ (antisense) and 5′-C~G~T~GCACCTCTCGCAGC~T~T~C-3′ (scrambled). Either the Arc antisense ODN or the control scrambled ODN was introduced into cultured neurons using jetPRIME transfection reagent (Polyplus Transfection, Illkirch, France).
Statistical analysis
Data shown in the results and figures are expressed as mean ± SEM. Statistical significance was analyzed by one-way ANOVA followed by a post hoc test (LSD multiple comparison test) except the analysis for Fig. 6b in which Student’s t test was used. The significance level was set at p < 0.05.
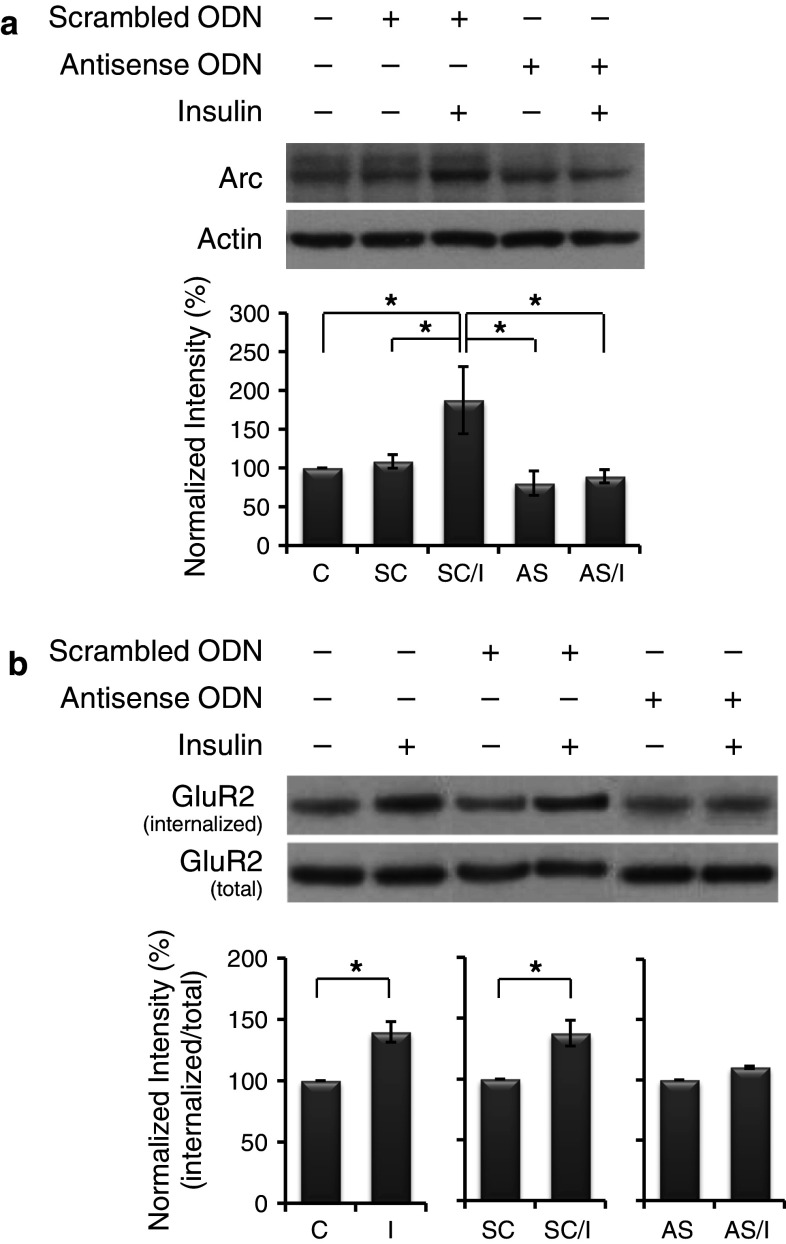
Fig. 6.
Arc antisense oligodeoxynucleotides (ODNs) diminished insulin-stimulated GluR2 internalization. a Arc antisense ODNs block new synthesis of Arc in response to insulin. Primary neurons were divided into five groups: control (C), scrambled ODNs alone (SC), antisense ODNs alone (AS), and pretreatment of scrambled or antisense ODNs followed by insulin treatment (SC/I, AS/I). Data are shown as mean ± SEM (n = 3). One-way ANOVA and a post hoc test were used. *p < 0.05 compared with the group of SC/I. b Insulin-stimulated GluR2 internalization was prevented by blocking the new synthesis of Arc protein. Data are shown as mean ± SEM (n = 3). Student’s t test was used. *p < 0.05 compared with the group of C or SC
Results
Insulin enhanced Arc protein expression in primary cultured neonatal rat cortical neurons
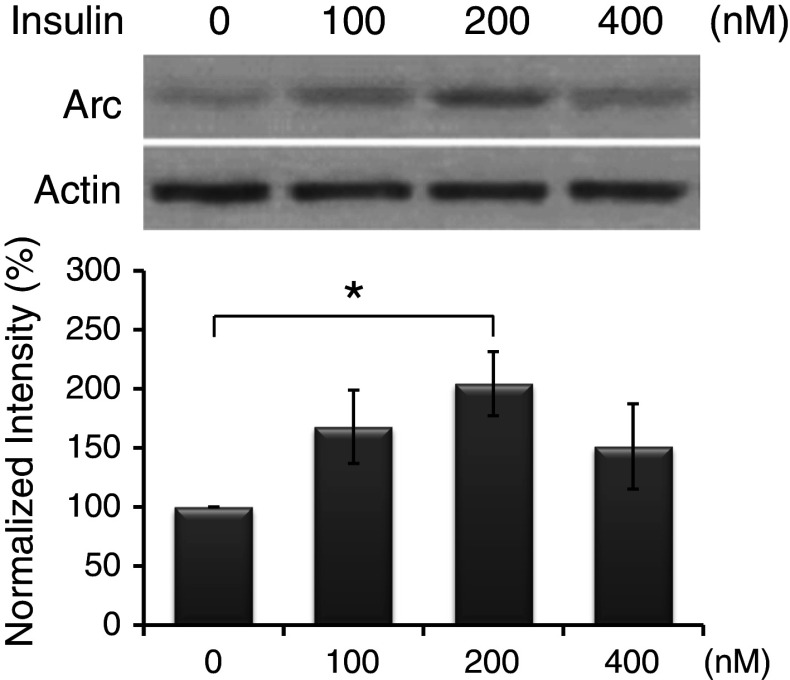
Primary cultured neurons were incubated with insulin (dissolved in 1 % glacial acetic acid) at 100, 200, and 400 nM for 1 or 2 h. Under both non- and vehicle (1 % glacial acetic acid)-treated conditions, the levels of Arc protein detected by the Western-blot analysis were very low. Thus, to reduce the number of groups, vehicle-treated neurons were used as the controls for comparisons in the following experiments. As shown in Fig. 1, without insulin treatment, the basal level of Arc protein was low (normalized to 100 %). After treating cultures with insulin for 1 h, no significant change was found (data not shown). Following 2-h insulin stimulation, a significant increase in Arc expression was reached at 200 nM (200 ± 27 %). Therefore, in the following experiments, cultures were treated with insulin at 200 nM for 2 h to induce significant Arc expression.
Fig. 1.
Insulin induced Arc expression in primary neurons. Cultures were treated with insulin at indicated concentrations for 2 h. Arc levels were normalized to actin and expressed in percent relative to the control group (normalized to 100 %). Data are shown as mean ± SEM (n = 4). One-way ANOVA and a post hoc test were used. *p < 0.05 compared with the control group
Blocking AMPAR-mediated responses enhanced insulin-induced Arc expression
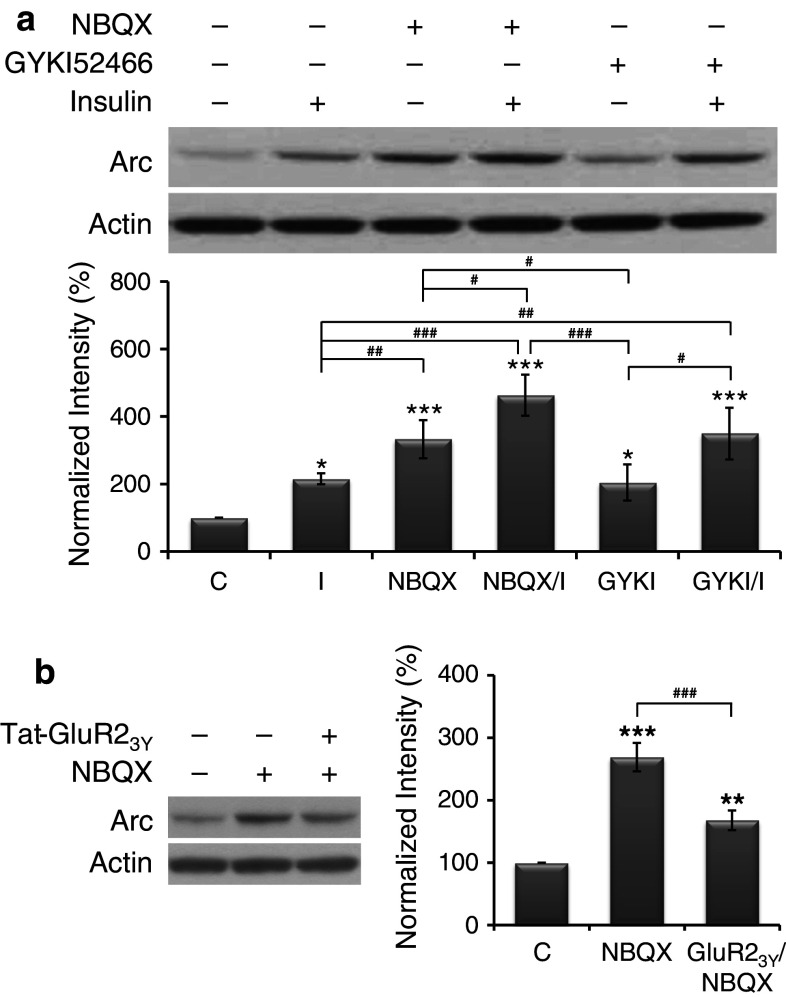
To evaluate the effect of blocking AMPAR-mediated transmission on insulin-induced Arc expression, AMPAR-mediated responses were blocked by competitive (NBQX) or non-competitive (GYKI52466) AMPAR antagonists. Cultures were grouped into control, insulin alone, NBQX (10 μM, 2.5 h) alone, GYKI52466 (15 μM, 2.5 h) alone, and pretreatments of NBQX or GYKI52466 (30 min) followed by insulin (2 h) in the presence of NBQX or GYKI52466. The results shown in Fig. 2a reveal that, when compared with the basal level of Arc protein (normalized to 100 %), all treatments significantly increased Arc expression. Interestingly, the level of Arc expression induced by NBQX (333 ± 57 %) but not GYKI52466 (204 ± 53 %) was significantly higher than that induced by insulin (216 ± 16 %), and, most importantly, pretreatments of both NBQX (463 ± 61 %) and GYKI52466 (349 ± 77 %) significantly enhanced insulin-induced Arc expression.
Fig. 2.
AMPAR antagonists enhanced insulin-induced Arc expression. a AMPAR antagonist alone induced Arc expression and further enhanced insulin-induced Arc expression. Primary neurons were divided into six groups: control (C), insulin alone (I), NBQX alone (NBQX), GYKI52466 alone (GYKI), and pretreatments of NBQX or GYKI52466 followed by insulin (NBQX/I or GYKI/I). Data are shown as mean ± SEM (n = 4). One-way ANOVA and a post hoc test were used. *p < 0.05 and ***p < 0.001 compared with the control group; # p < 0.05, ## p < 0.01, and ### p < 0.001 compared with the group of I, NBQX, or GYKI. b Blocking AMPAR endocytosis reduced the enhanced effect of AMPAR antagonist on Arc expression. Primary neurons were divided into three groups: control (C), NBQX alone (NBQX), and pretreatment of Tat-GluR23Y followed by NBQX (GluR23Y/NBQX). Data are shown as mean ± SEM (n = 3). One-way ANOVA and a post hoc test were used. **p < 0.01 and ***p < 0.001 compared with the control group; ### p < 0.001 compared with the NBQX group
Clathrin-mediated endocytosis blocker reduced both basal and insulin-induced Arc expression
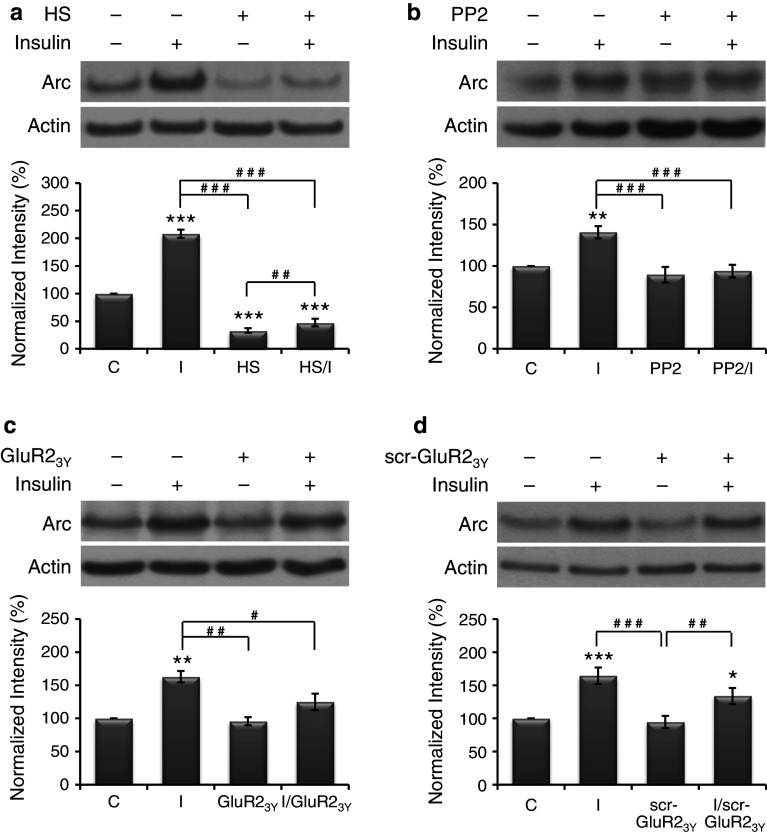
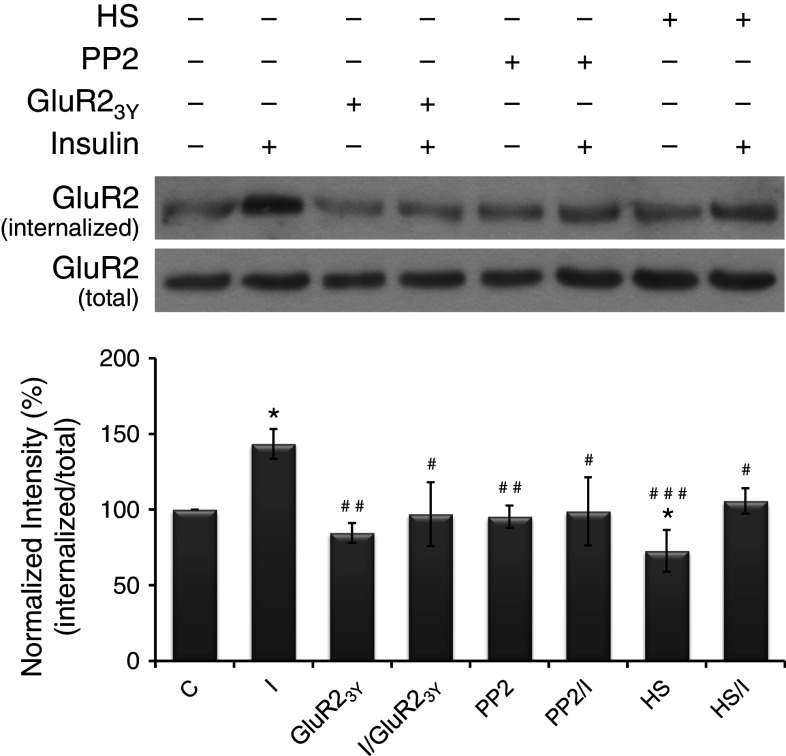
Hypertonic sucrose (0.45 M), which perturbs the formation of clathrin-coated vesicles, is a blocker of clathrin-mediated endocytosis [37]. Pretreatment of cells with hypertonic sucrose for 10–30 min prevented both constitutive and insulin-enhanced internalization of GluR2 receptors [25]. Therefore, in this study, cultures were grouped into control, insulin alone, hypertonic sucrose (2.5 h) alone, and pretreatment of hypertonic sucrose (30 min) followed by insulin (2 h) in the presence of hypertonic sucrose. The results shown in Fig. 3a reveal that, when compared with the basal level of Arc protein (normalized to 100 %), insulin significantly increased (208 ± 7 %) whereas hypertonic sucrose significantly decreased (33 ± 4 %) the expression of Arc protein. Pretreatment of hypertonic sucrose significantly reduced insulin-enhanced Arc expression to a value (48 ± 7 %) significantly lower than the basal level. These results indicate that clathrin-mediated endocytosis may play a role in both basal and insulin-induced Arc expression. However, hypertonic sucrose is a very crude blocker of endocytosis, whether AMPAR endocytosis plays a specific role needs further investigations.
Fig. 3.
Endocytosis blockers reduced insulin-induced Arc expression. Primary neurons were given various treatments, which include control (C), insulin alone (I), hypertonic sucrose alone (HS), PP2 alone (PP2), pretreatments of hypertonic sucrose or PP2 followed by insulin (HS/I or PP2/I), Tat-GluR23Y or its scrambled control peptide scr-Tat-GluR23Y alone (GluR23Y or scr-GluR23Y), and pretreatment of insulin followed by Tat-GluR23Y or scr-Tat-GluR23Y (I/GluR23Y or I/scr-GluR23Y). Data are shown as mean ± SEM (n = 3–4). One-way ANOVA and a post hoc test were used. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with the control group; # p < 0.05, ## p < 0.01 and ### p < 0.001 compared with the group of I, HS or scr-GluR23Y
Src family tyrosine kinase inhibitor reduced insulin-induced Arc expression
Tyrosine phosphorylation of GluR2 intracellular carboxyl-terminal region by Src family tyrosine kinases is required for insulin-stimulated AMPAR endocytosis [38, 39]. A specific inhibitor of Src family tyrosine kinases, PP2, has been shown to prevent the insulin-stimulated AMPAR endocytosis [38]. Therefore, in this study, cultures were grouped into control, insulin alone, PP2 (10 μM, 2.5 h) alone, and pretreatment of PP2 (30 min) followed by insulin (2 h) in the presence of PP2. The results shown in Fig. 3b reveal that, when compared with the basal level of Arc protein (normalized to 100 %), insulin significantly increased the expression of Arc protein (141 ± 7 %), whereas PP2 alone had no significant effect (89 ± 9 %). Pretreatment of PP2 significantly reduced insulin-induced Arc expression (94 ± 8 %). These results support the possibility that blocking insulin-stimulated AMPAR endocytosis by inhibiting tyrosine phosphorylation of GluR2 can reduce insulin-induced but not basal Arc expression. Similar to hypertonic sucrose, PP2 has multiple effects, thus, a synthetic peptide specifically block AMPAR endocytosis was used in the following experiment to examine the role of AMPAR endocytosis in Arc expression.
AMPAR endocytosis blocker reduced insulin-induced Arc expression
Tat-GluR23Y, a synthetic membrane permeable GluR2-derived interference peptide that can disrupt the interactions between the GluR2 subunit and endocytic machineries, has been demonstrated to specifically block the GluR2-dependent, regulated (stimulated) AMPAR endocytosis [40, 41]. In cultured hippocampal neurons, intracellular application of GluR23Y prevented insulin-stimulated AMPAR endocytosis [38]. Therefore, in this study, cultures were grouped into control, insulin alone, Tat-GluR23Y or its scrambled control peptide (scr-Tat-GluR23Y) (1 μM, 2 h) alone, and pretreatment of insulin (5 min) followed by Tat-GluR23Y or scr-Tat-GluR23Y (1 h 55 min) in the presence of insulin. The results shown in Fig. 3c and d reveal that, when compared with the basal level of Arc protein (normalized to 100 %), insulin significantly increased the expression of Arc protein (163 ± 9 and 164 ± 12 %, respectively), whereas Tat-GluR23Y (96 ± 6 %) or its scrambled control peptide (scr-Tat-GluR23Y) (95 ± 9 %) alone had no significant effect. Tat-GluR23Y (125 ± 12 %) but not scr-Tat-GluR23Y (134 ± 12 %) significantly reduced insulin-induced Arc expression. These findings indicate that insulin-stimulated AMPAR endocytosis is involved in insulin-induced but not basal Arc expression.
Hypertonic sucrose, PP2, and Tat-GluR23Y prevented insulin-stimulated AMPAR endocytosis
To confirm whether hypertonic sucrose, PP2, and Tat-GluR23Y prevented insulin-stimulated AMPAR endocytosis in our primary neurons, surface biotinylation assay was performed. Cultures were treated with various reagents as mentioned previously. The results (Fig. 4) show that, when compared with the basal level of internalized GluR2 (normalized to 100 %), insulin treatment significantly increased (143 ± 9 %) whereas hypertonic sucrose significantly decreased (73 ± 13 %) the level of internalized GluR2. Treating cultures with PP2 or Tat-GluR23Y alone (95 ± 7 and 85 ± 6 %, respectively) did not significantly change the basal level of internalized GluR2. Combined treatments with hypertonic sucrose, PP2, or Tat-GluR23Y, insulin failed to induce a significant increase in the internalization of GluR2 (106 ± 8, 99 ± 22, and 97 ± 21 %, respectively), indicating that these reagents effectively prevented insulin-stimulated AMPAR endocytosis in our cultured neurons.
Fig. 4.
Endocytosis blockers prevented insulin-stimulated GluR2 internalization. Primary neurons were given various treatments as described in Fig. 3. Data are shown as mean ± SEM (n = 3). One-way ANOVA and a post hoc test were used. *p < 0.05 compared with the control group; # p < 0.05, ## p < 0.01, and ### p < 0.001 compared with the group of insulin alone
Insulin-induced reduction in surface GluR2 expression and enhancement in Arc expression were reversed by combined treatment with Tat-GluR23Y
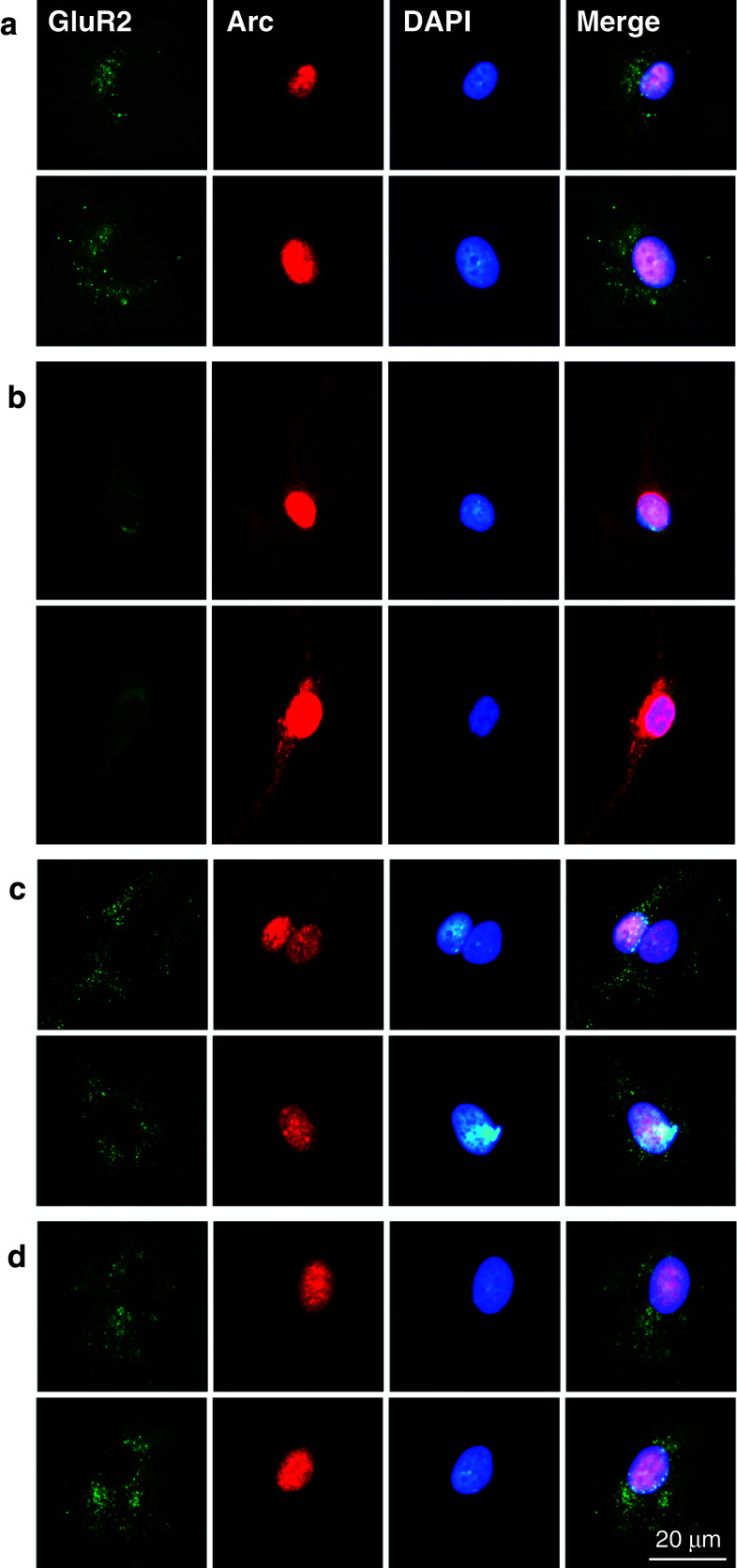
Double-immunofluorescent staining was carried out to reveal the distribution of cell-surface GluR2 and the expression of Arc protein in cultured neurons. In the control group, neurons exhibited numerous GluR2 subunit clusters shown as light green dots on the surface of cell body and proximal dendrites, and Arc immunostaining was also detected mainly in the nucleus (Fig. 5a). After the treatment with insulin, the distribution of cell-surface GluR2 was reduced obviously (Fig. 5b), indicating the occurrence of insulin-stimulated AMPAR endocytosis. In contrast, the expression of Arc protein was detected in the nucleus with a very strong fluorescence and along the dendrites with a moderate fluorescence (Fig. 5b). This finding confirms that insulin induces a de novo synthesis of Arc protein, and these newly formed Arc proteins not only accumulate in the nucleus but also in the dendrites where they play an important role in synaptic plasticity. As for the neurons treated with Tat-GluR23Y (1 μM, 2 h) in the absence or presence of insulin, results similar to those shown in control neurons were found (Fig. 5c, d), indicating that Tat-GluR23Y has no detectable effects on constitutive AMPAR endocytosis and Arc expression but, most importantly, can effectively reduce the effects of insulin treatment.
Fig. 5.
AMPAR endocytosis blocker disturbed the effects of insulin on the distribution of cell-surface GluR2 and the expression of Arc protein. Primary neurons were treated with insulin and Tat-GluR23Y as described in Fig. 3. Two representative images for each group, respectively, are shown. a In the control neurons, numerous light green dots representing GluR2 subunit clusters were shown on the surface of cell body and proximal dendrites, and Arc immunostaining (red) was detected mainly in the nucleus. b Insulin treatment reduced the distribution of cell-surface GluR2 but enhanced the expression of Arc in both the nucleus and the dendrites. c, d Results similar to those of control neurons were observed after the treatment of Tat-GluR23Y in the absence or presence of insulin
Blocking new synthesis of Arc in response to insulin prevented insulin-stimulated AMPAR endocytosis
The specificity and efficacy of the Arc antisense ODNs were evaluated by Western-blot analysis. Thirty minutes after ODNs delivery, cultured neurons were treated with insulin (2 h) in the presence of ODNs. As shown in Fig. 6a, neither scrambled (SC) nor antisense (AS) ODNs (108 ± 8 and 80 ± 15 %, respectively) significantly changed the basal Arc level (normalized to 100 %). In the presence of scrambled ODNs, insulin treatment induced a significant increase in Arc expression (187 ± 43 %), whereas Arc antisense ODNs effectively blocked insulin-induced increase in Arc expression (89 ± 8 %) (Fig. 6a). These results confirm that Arc antisense ODNs blocked new synthesis of Arc in response to insulin.
Subsequently, whether insulin-induced Arc synthesis regulates insulin-stimulated AMPAR endocytosis was evaluated. In the presence of ODNs, basal or insulin-stimulated AMPAR endocytosis was examined by surface biotinylation assay. As shown in Fig. 6b, when compared with the basal level of internalized GluR2 (normalized to 100 %), insulin treatment significantly increased the level of internalized GluR2 (140 ± 8 %). In the presence of scrambled ODNs, insulin also significantly increased the level of internalized GluR2 (138 ± 10 %), whereas in the presence of Arc antisense ODNs, the enhanced effect of insulin was prevented (110 ± 1 %). Scrambled or antisense ODNs alone did not significantly change the basal level of internalized GluR2. These results indicate that new synthesis of Arc in response to insulin is required for the occurrence of insulin-stimulated AMPAR endocytosis.
Discussion
In contrast to the previous finding that Arc accelerates AMPAR endocytosis, we hypothesized that insulin-stimulated AMPAR endocytosis can lead to an increase in Arc expression. Previous evidence has shown that, in cultured hippocampal neurons, basal AMPAR endocytosis reached a plateau after 10–15 min. Insulin (10 μM) enhanced the rate and degree of AMPAR endocytosis over 30 min without reaching a plateau [27]. In transfected HEK293 cells, brief insulin exposure (0.5 μM, 10–15 min) resulted in a rapid, long-lasting decrease in cell-surface GluR2 receptors without affecting the total number of GluR2 receptors, and, in cultured hippocampal neurons, insulin treatment for both 15 and 60 min increased the loss of the surface AMPARs when compared with respective control values [25]. These findings indicate that insulin can induce significantly enhanced AMPAR endocytosis within 15 min. In contrast, although in SH-SY5Y cells, both Arc mRNA and Arc protein are detectable as soon as 30 min treatments of insulin (100 nM) and a maximal level of Arc mRNA or Arc protein can be observed after 1–2 h treatments [19], in our cultured cortical neurons, the expression of Arc protein did not significantly increased after insulin treatment at 100, 200, and 400 nM for 1 h. These time courses of AMPAR endocytosis and Arc expression in response to insulin provide support for our hypothesis.
In Fig. 2a, treating cultures with competitive (NBQX) or non-competitive (GYKI52466) AMPAR antagonist alone significantly increased Arc protein levels. In particular, NBQX induced a significantly higher level than GYKI52466. In cultured hippocampal neurons, another competitive AMPAR antagonist CNQX (30 μM) alone induced AMPAR endocytosis, whereas GYKI52466 (30 μM) did not, suggesting that a ligand-induced conformational change in the receptor was enough to stimulate AMPAR endocytosis, independent of receptor activation [27]. According to this finding, it is possible that NBQX alone induces higher Arc expression than GYKI52466 alone through blocking AMPAR-mediated responses and stimulating AMPAR endocytosis. To examine whether AMPAR endocytosis plays a role in NBQX-induced Arc expression, cultures were grouped into control, NBQX (10 μM, 2 h) alone, and pretreatment of Tat-GluR23Y (1 μM, 5 min) followed by NBQX (1 h 55 min) in the presence of Tat-GluR23Y. The results shown in Fig. 2b reveal that NBQX-enhanced Arc expression (269 ± 23 %) was significantly reduced by blocking AMPAR endocytosis with Tat-GluR23Y (168 ± 16 %), confirming that AMPAR endocytosis, at least partly, contributes to Arc expression induced by NBQX.
It has been demonstrated that Arc accelerates AMPAR endocytosis through interacting with components of the endocytic machinery [11, 17, 42, 43], therefore, increased Arc expression is expected under a condition of reduced AMPAR endocytosis. However, in the present study, blocking insulin-stimulated AMPAR endocytosis decreased insulin-induced Arc expression (Fig. 3c). In cultured neurons, hypertonic sucrose [25], PP2 [38], and GluR23Y [38] have been used to block insulin-stimulated AMPAR endocytosis. In Fig. 3a–c, treatments with these reagents prevented insulin-induced Arc expression. Only the effects of PP2 on activity-dependent Arc expression have been examined previously. In primary neonatal rat cortical neurons (12 DIV), PP2 (1 μM) exerted no effect on BDNF-induced Arc expression [32]. However, in SH-SY5Y cells [19] and in our primary neonatal rat cortical neurons (14–21 DIV) (Fig. 3b), PP2 (10 μM) abolished insulin-induced Arc expression effectively. This discrepancy may be due to the differences in the concentration of PP2 and cultured days of primary neurons. However, it cannot be excluded that PP2 may disturb BDNF- and insulin-mediated signaling pathways with different effects. In addition, hypertonic sucrose but not PP2 or Tat-GluR23Y significantly reduced the basal level of Arc protein (Fig. 3a–c). The results shown in Fig. 4 may account for this finding, that is, although all reagents prevented insulin-enhanced GluR2 internalization, only hypertonic sucrose significantly reduced the basal level of internalized GluR2, which is suggested to result in the decrease in the basal level of Arc protein. In fact, previous studies have shown that hypertonic sucrose effectively blocks both insulin-stimulated and constitutive AMPAR endocytosis [25]. However, tyrosine phosphorylation of GluR2 by Src family tyrosine kinases is required for insulin-stimulated but not constitutive AMPAR endocytosis [38, 39], and GluR23Y specifically blocks the regulated AMPAR endocytosis but has little effect on constitutive AMPAR endocytosis [38].
The best-characterized form of LTD depends on the activation of either N-methyl-d-aspartate receptors (NMDARs) and/or mGluRs [44, 45]. The subsequent induction of GluR2-dependent AMPAR endocytosis has been shown to play important roles in these forms of LTD, which occurs in a number of brain regions [46] and is involved in memory [47]. In particular, mGluR-dependent LTD in the hippocampus has been demonstrated to require rapid de novo synthesis of Arc protein leading to AMPAR endocytosis [11, 17, 42, 43, 48]. Similarly, insulin stimulation can induce LTD (insulin-LTD) in the hippocampus and cerebellum, which is also reported to be closely related to insulin-stimulated GluR2-dependent AMPAR endocytosis [25, 27, 28, 30, 31, 49]. In addition, insulin-LTD is also dependent on a novel protein synthesis because protein synthesis inhibitors effectively prevented the induction of insulin-LTD in the dendritic sites [28]. However, the exact role of insulin-induced Arc expression in insulin-LTD is still uncertain.
Previous studies have found that Arc protein localizes to activated dendritic regions [7, 8] and the nucleus [50]. In Fig. 5a, Arc nuclear expression was observed in the control group. After insulin treatment, an enhanced expression of nuclear Arc combined with a moderate expression of dendritic Arc was detected (Fig. 5b). In contrast to the synaptic function of Arc, the role of nuclear Arc is uncertain until a recent study shows that Arc nuclear localization regulates homeostatic plasticity through decreasing GluA1 transcription [51]. They found that nuclear Arc decreases surface GluA1 (GluR1) expression through suppressing GluA1 transcription leading to a decrease in total amounts of GluA1, and thus suggested that Arc expression decreases AMPAR surface expression not only through promoting AMPAR endocytosis [11, 42] but also through translocating Arc to the nucleus. However, in Fig. 4, no significant change was found in the amounts of total GluR2 after insulin treatment that enhanced nuclear Arc expression. Further investigation is needed to examine the role of nuclear Arc in GluR2 internalization.
Consistent with the Western-blot results, immunofluorescent staining shown in Fig. 5 reveals that insulin reduced the distribution of cell-surface GluR2 but enhanced the expression of Arc protein, and Tat-GluR23Y prevented these insulin actions. In Fig. 6, blocking insulin-induced Arc expression by Arc antisense ODNs significantly inhibited insulin-stimulated AMPAR endocytosis. These findings demonstrate a continuing interaction, in which, at least partly, insulin can enhance Arc expression through stimulating AMPAR endocytosis and, conversely, insulin can facilitate AMPAR endocytosis through inducing Arc expression. Under this condition, AMPAR endocytosis gradually prevails over AMPAR insertion, and Arc mRNA and protein are degraded rapidly by translation-dependent mRNA decay and proteasomal degradation, respectively [12, 32, 52, 53]. Finally, the stop of this interaction may be due to a progressive decrease in the number of surface AMPARs and/or a reduction in Arc protein level.
Clarifying the mechanisms underlying the involvement of AMPAR endocytosis in modulating Arc expression is beyond the scope of this study. However, previous evidence may provide possible links between AMPAR endocytosis and the subsequent Arc expression. As mentioned previously, insulin-stimulated AMPAR endocytosis is GluR2-dependent [25, 27, 28]. GluR2-containing AMPARs are calcium-impermeable whereas GluR2-lacking AMPARs are calcium-permeable [54]. Although calcium signaling is essential for the induction of Arc transcription and/or translation following various stimuli [12, 53, 55], a previous study reported an important role of GluR2-containing calcium-impermeable AMPAR in Arc expression. They found that, in primary rat cortical neurons, AMPAR antagonists strongly potentiated BDNF-induced increases in Arc mRNA and protein, however, a specific antagonist of GluR2-lacking AMPAR did not potentiate BDNF-induced Arc protein expression, indicating that GluR2-containing AMPAR is the possible candidate responsible for regulating BDNF-induced Arc expression [32]. They concluded that AMPARs negatively regulate Arc transcription but not translation through activating a pertussis toxin-sensitive G protein. AMPAR endocytosis may prevent the action of AMPAR on G protein leading to an enhanced Arc expression. In addition, their results revealed that the relative extent of NMDAR and AMPAR activation may be a critical determinant of Arc expression [32]. Interestingly, insulin not only stimulates AMPAR endocytosis but also promotes the delivery of NMDAR to the cell surface by exocytosis [56], and Arc can be induced by synaptic activity in an NMDAR-dependent manner [7, 8, 57]. Therefore, it is speculated that insulin may induce an increase in the NMDAR/AMPAR ratio leading to an enhancement of Arc expression.
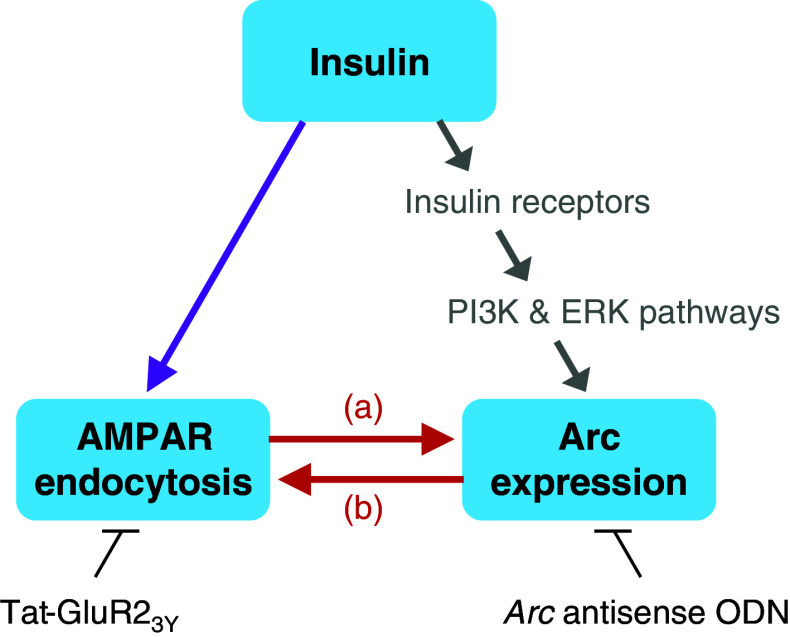
In summary, as shown in Fig. 7, our findings demonstrate for the first time that insulin-stimulated AMPAR endocytosis can lead to enhanced Arc expression (a) and insulin-induced Arc expression can facilitate AMPAR endocytosis (b). Because the insulin receptor signal transduction cascades mainly include the phosphatidylinositol-3 kinase (PI3K) and the extracellular signal-regulated kinase (ERK) pathways [3], insulin may enhance Arc expression through not only stimulating AMPAR endocytosis but also activating these two pathways. In fact, we have demonstrated that both PI3K and ERK pathways participate in BDNF-induced Arc expression [33], and our unpublished data has shown that blocking these pathways with inhibitors significantly reduced insulin-induced Arc expression. Therefore, an interaction between insulin-stimulated AMPAR endocytosis and insulin-induced Arc expression is demonstrated in the present study, which further clarifies the molecular mechanism underlying the memory-enhancing effect of brain insulin.
Fig. 7.
An interaction exists between insulin-stimulated AMPAR endocytosis and insulin-induced Arc expression. Based on the results shown here, a model is suggested as follows. In response to insulin, both AMPAR endocytosis and Arc expression are enhanced. AMPAR endocytosis blocker Tat-GluR23Y reduced insulin-induced Arc expression, indicating that insulin-stimulated AMPAR endocytosis can lead to enhanced Arc expression (a). In contrast, Arc antisense ODN prevented insulin-stimulated AMPAR endocytosis, indicating that insulin-induced Arc expression can facilitate AMPAR endocytosis (b). Arrows in gray indicate an alternative possible way for insulin to induce Arc expression through activating the PI3K and ERK pathways, which is not examined in this study
Acknowledgments
This work was supported by grants from the National Science Council of Taiwan (NSC97-2320-B-037-017-MY3) and the Kaohsiung Medical University Research Foundation (KMU-M103010). The authors declare that they have no conflicts of interest.
References
- 1.Kern W, Born J, Schreiber H, Fehm HL. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes. 1999;48:557–563. doi: 10.2337/diabetes.48.3.557. [DOI] [PubMed] [Google Scholar]
- 2.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68:509–514. doi: 10.1016/S0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 3.Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A. Insulin in the brain: sources, localization and functions. Mol Neurobiol. 2013;47:145–171. doi: 10.1007/s12035-012-8339-9. [DOI] [PubMed] [Google Scholar]
- 4.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 9.Messaoudi E, Kanhema T, Soulé J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soulé J, Tiron A, Wibrand K. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bösl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci USA. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TVP, Bramham CR. Brain-derived neurotrophic factor induces longterm potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teber I, Köhling R, Speckmann EJ, Barnekow A, Kremerskothen J. Muscarinic acetylcholine receptor stimulation induces expression of the activity-regulated cytoskeleton-associated gene (ARC) Brain Res Mol Brain Res. 2004;121:131–136. doi: 10.1016/j.molbrainres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Kremerskothen J, Wendholt D, Teber I, Barnekow A. Insulin-induced expression of the activity-regulated cytoskeleton-associated gene (ARC) in human neuroblastoma cells requires p21(ras), mitogen-activated protein kinase/extracellular regulated kinase and src tyrosine kinases but is protein kinase C-independent. Neurosci Lett. 2002;321:153–156. doi: 10.1016/S0304-3940(01)02532-0. [DOI] [PubMed] [Google Scholar]
- 20.Solas M, Aisa B, Mugueta MC, Del Río J, Tordera RM, Ramírez MJ. Interactions between age, stress and insulin on cognition: implications for Alzheimer’s disease. Neuropsychopharmacology. 2010;35:1664–1673. doi: 10.1038/npp.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seeburg PH. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- 22.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 23.Lüscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 24.Turrigiano GG. AMPA receptors unbound: membrane cycling and synaptic plasticity. Neuron. 2000;26:5–8. doi: 10.1016/S0896-6273(00)81131-9. [DOI] [PubMed] [Google Scholar]
- 25.Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/S0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 26.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 27.Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- 28.Huang CC, Lee CC, Hsu KS. An investigation into signal transduction mechanisms involved in insulin-induced long-term depression in the CA1 region of the hippocampus. J Neurochem. 2004;89:217–231. doi: 10.1111/j.1471-4159.2003.02307.x. [DOI] [PubMed] [Google Scholar]
- 29.Huang CC, Lee CC, Hsu KS. The role of insulin receptor signaling in synaptic plasticity and cognitive function. Chang Gung Med J. 2010;33:115–125. [PubMed] [Google Scholar]
- 30.Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 31.Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/S0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 32.Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci. 2006;9:887–895. doi: 10.1038/nn1708. [DOI] [PubMed] [Google Scholar]
- 33.Chen TJ, Wang DC, Chen SS. Amyloid-beta interrupts the PI3K-Akt-mTOR signaling pathway that could be involved in brain-derived neurotrophic factor-induced Arc expression in rat cortical neurons. J Neurosci Res. 2009;87:2297–2307. doi: 10.1002/jnr.22057. [DOI] [PubMed] [Google Scholar]
- 34.Arancibia-Cárcamo IL, Fairfax BP, Moss SJ, Kittler JT (2006) Studying the localization, surface stability and endocytosis of neurotransmitter receptors by antibody labeling and biotinylation approaches. In: Kittler JT, Moss SJ (eds) The dynamic synapse: molecular methods in ionotropic receptor biology, chap. 6. CRC Press, Boca Raton [PubMed]
- 35.Zhao WQ, Santini F, Breese R, Ross D, Zhang XD, Stone DJ, Ferrer M, Townsend M, Wolfe AL, Seager MA, Kinney GG, Shughrue PJ, Ray WJ. Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J Biol Chem. 2010;285:7619–7632. doi: 10.1074/jbc.M109.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen SH, Sandvig K, van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmadian G, Ju W, Liu L, Wyszynski M, Lee SH, Dunah AW, Taghibiglou C, Wang Y, Lu J, Wong TP, Sheng M, Wang YT. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO J. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci. 2004;24:6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox CJ, Russell K, Titterness AK, Wang YT, Christie BR. Tyrosine phosphorylation of the GluR2 subunit is required for long-term depression of synaptic efficacy in young animals in vivo. Hippocampus. 2007;17:600–605. doi: 10.1002/hipo.20302. [DOI] [PubMed] [Google Scholar]
- 41.Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, Titterness AK, Brebner K, Liu L, Weinberg J, Christie BR, Phillips AG, Wang YT. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc Natl Acad Sci USA. 2007;104:11471–11476. doi: 10.1073/pnas.0702308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 45.Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- 46.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 47.Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- 48.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 49.Huang CC, You JL, Lee CC, Hsu KS. Insulin induces a novel form of postsynaptic mossy fiber long-term depression in the hippocampus. Mol Cell Neurosci. 2003;24:831–841. doi: 10.1016/S1044-7431(03)00238-0. [DOI] [PubMed] [Google Scholar]
- 50.Bloomer WA, VanDongen HM, VanDongen AM. Activity-regulated cytoskeleton-associated protein Arc/Arg3.1 binds to spectrin and associates with nuclear promyelocytic leukemia (PML) bodies. Brain Res. 2007;1153:20–33. doi: 10.1016/j.brainres.2007.03.079. [DOI] [PubMed] [Google Scholar]
- 51.Korb E, Wilkinson CL, Delgado RN, Lovero KL, Finkbeiner S. Arc in the nucleus regulates PML-dependent GluA1 transcription and homeostatic plasticity. Nat Neurosci. 2013;16:874–883. doi: 10.1038/nn.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bateup HS, Denefrio CL, Johnson CA, Saulnier JL, Sabatini BL. Temporal dynamics of a homeostatic pathway controlling neural network activity. Front Mol Neurosci. 2013;6:28. doi: 10.3389/fnmol.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soulé J, Alme M, Myrum C, Schubert M, Kanhema T, Bramham CR. Balancing Arc synthesis, mRNA decay, and proteasomal degradation: maximal protein expression triggered by rapid eye movement sleep-like bursts of muscarinic cholinergic receptor stimulation. J Biol Chem. 2012;287:22354–22366. doi: 10.1074/jbc.M112.376491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-d-aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci USA. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/S0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]