Abstract
Identifiable causes of fetal growth restriction (FGR) account for 30 % of cases, but the remainders are idiopathic and are frequently associated with placental dysfunction. We have shown that the angiogenic factor endocrine gland-derived VEGF (EG-VEGF) and its receptors, prokineticin receptor 1 (PROKR1) and 2, (1) are abundantly expressed in human placenta, (2) are up-regulated by hypoxia, (3) control trophoblast invasion, and that EG-VEGF circulating levels are the highest during the first trimester of pregnancy, the period of important placental growth. These findings suggest that EG-VEGF/PROKR1 and 2 might be involved in normal and FGR placental development. To test this hypothesis, we used placental explants, primary trophoblast cultures, and placental and serum samples collected from FGR and age-matched control women. Our results show that (1) EG-VEGF increases trophoblast proliferation ([3H]-thymidine incorporation and Ki67-staining) via the homeobox-gene, HLX (2) the proliferative effect involves PROKR1 but not PROKR2, (3) EG-VEGF does not affect syncytium formation (measurement of syncytin 1 and 2 and β hCG production) (4) EG-VEGF increases the vascularization of the placental villi and insures their survival, (5) EG-VEGF, PROKR1, and PROKR2 mRNA and protein levels are significantly elevated in FGR placentas, and (6) EG-VEGF circulating levels are significantly higher in FGR patients. Altogether, our results identify EG-VEGF as a new placental growth factor acting during the first trimester of pregnancy, established its mechanism of action, and provide evidence for its deregulation in FGR. We propose that EG-VEGF/PROKR1 and 2 increases occur in FGR as a compensatory mechanism to insure proper pregnancy progress.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-012-1141-z) contains supplementary material, which is available to authorized users.
Keywords: EG-VEGF, Prokineticin, FGR, Placenta, Angiogenesis
Introduction
It is well established that placental development depends on the controlled growth, invasion, and differentiation of the trophoblast cells and on an adequate vascular development [1]. A failure in these processes leads to the development of placental pathologies such as preeclampsia (PE) and fetal growth restriction (FGR). FGR is a leading cause of perinatal mortality and morbidity and is an important antecedent to childhood and adult diseases [2–4]. This disease affects approximately 8 % of all pregnancies [5–7] and its occurrence is highly related to abnormal placental development [8–10]. The placenta has a key role in this process via its actions on the transport of nutrients and gases through the placental barrier, composed of syncytiotrophoblast (ST) and cytotrophoblast layers (CT), connective tissue, and fetal vascular endothelium [11, 12].
Placentas from FGR pregnancies are characterized by a number of pathological defects, such as increased placental apoptosis [13–17], higher thickness of the placental barrier [18], poor invasion of maternal vessels [19, 20], reduced ST [18], and aberrant angiogenesis [21, 22].
Recent literature suggests that a failure in the establishment of the feto-maternal interface, a key component of early placental development, is tightly associated to the development of FGR. During the first trimester of pregnancy, proliferative CT form plugs within the maternal arteries to restrict blood flow into the intervillous space (IVS), resulting in a low-oxygen environment (hypoxia), which is essential for both placental and embryonic development [23, 24]. At around 10–12 weeks of gestation (wg), CT that are present in anchoring villi generate multilayered columns of extravillous trophoblast (EVT) that colonize the maternal decidua and the uterine blood vessels. Poor invasion of maternal vessels causes abnormal maintenance of IVS hypoxia, or rapid variation of partial oxygen pressure, associated with the development of PE and FGR [25].
To date, neither the causes of FGR development nor the compensatory mechanisms that allow the pregnancy to progress are fully understood. Several indications converge to incriminate deregulations of growth and angiogenic factors during placental development [20, 26–28]. Various growth factors and cytokines, such as epidermal growth factor (EGF) and transforming growth factor-β (TGF-β), have been reported to be involved in the coordination of these processes and to be deregulated in FGR [29–31]. Also, alterations in the placental growth control genes, called homeobox gene (HLX, ESX1L, DLX3, and DLX4) have been reported in FGR [32–35]. More specifically, HLX has been shown to be predominantly expressed in proliferating but not invading CT, and to be a downstream effector gene for the hepatocyte growth factor (HGF) [36] and for the colony-stimulating factor-1 (CSF-1) [37], two key regulators of CT proliferation.
In the last decade, we and others have been interested in the study of the new angiogenic factor EG-VEGF (endocrine gland-derived vascular endothelial growth factor) that was characterized and sequenced by the group of Ferrara [38] and was shown to be expressed in testis, adrenal gland, ovary, and placenta [38]. Human EG-VEGF exerts its effects via G protein-coupled receptors prokineticins (PROKRs), termed PROKR1 and PROKR2 [39]. EG-VEGF expression in the placenta was briefly described in the initial report by LeCouter et al. [38]. In recent reports from our group, we have shown that EG-VEGF and its receptors are abundant in human placenta during the first trimester of pregnancy, with the highest expression of EG-VEGF found in the ST; their expression is up-regulated by hypoxia; EG-VEGF controls trophoblast invasion and placental angiogenesis; and EG-VEGF circulating levels are significantly higher during the first trimester of pregnancy and in PE [40–43].
The specificity and magnitude of EG-VEGF expression in the placenta, its control of placental angiogenesis, its up-regulation by hypoxia and deregulation in PE [40–44], suggests that EG-VEGF plays an important role in the placental development and that its expression might be deregulated in FGR. Here we determined the direct role of EG-VEGF on placental development during the first trimester of pregnancy by demonstrating its effects on trophoblast proliferation, survival, differentiation, and vascular development. Furthermore, we identified the effect of EG-VEGF-mediated control of the homeobox gene HLX on trophoblast proliferation. More importantly, we determined the EG-VEGF circulating levels in FGR and gestation age-matched controls (AMC) and compared the expression of EG-VEGF, PROKR1, and PROKR2 in control and FGR placentas.
Materials and methods
Tissue collection
Collection and processing of human placentas were approved by the local hospital ethical committees. Informed patient consent was obtained in all cases. First-trimester human placentas from 7–10 wg were obtained from elective terminations of pregnancies from Grenoble Hospital. FGR and gestational age-matched controls placentas were collected between 30 and 39 wg at the Poissy-Saint Germain Hospital and at the Grenoble University Hospital. Table 1 describes the clinical characteristics of FGR-affected pregnancies and the AMC controls that were included in this study. Inclusion criteria for this study were a birth weight less than the 10th percentile for gestation age using growth charts of the French population. Exclusion criteria for both control and FGR-affected pregnancies were pre-eclampsia, placental abruption, prolonged rupture of the membranes, fetal congenital abnormalities, or suspicion of intrauterine viral infection. Pre-eclampsia is often associated with FGR; however, in this study, we aimed to investigate a population of idiopathic FGR, and therefore all patients were normotensive. The gestation age for both FGR and control patients included in this study was calculated based on the last menstrual period dates and confirmed by second-trimester ultrasound in 90 % of the patients or solely based on the first-trimester scan in the remaining 10 % of the patients. Control pregnancies were selected to match FGR cases according to gestation age. Control women either presented in spontaneous idiopathic preterm labor or underwent elective delivery. All control placentas obtained were grossly normal. Placentas were obtained within 20 min of delivery. Placental tissue samples were excised from random areas of the placental cotyledons, excluding the peripheral margin and infarcted areas. Samples from each placenta were either snap-frozen and stored at −80 °C for RNA and protein extraction or fixed in 4 % paraformaldehyde for immunohistochemical analyses.
Table 1.
Clinical characteristics of samples included in the study
| Characteristics | Control (n = 44) | FGR (n = 25) | Significance |
|---|---|---|---|
| Gestation age (mean ± SD) | 35.4 ± 2.02 (30–39) | 35.2 ± 2.2 (30–38) | ns |
| Maternal age (mean ± SD) | 30.4 ± 5.3 | 32 ± 5.5 | ns |
| Placental weight (mean ± SD) | 515.4 ± 142 | 333.5 ± 115 | p < 0.01 |
| Parity | |||
| Primiparous | 25 % | 24 % | ns |
| Multiparous | 75 % | 76 % | ns |
| Newborn characteristics | |||
| Male | 45.45 % | 44 % | ns |
| Female | 54.55 % | 56 % | ns |
| Birth weight (mean ± SD) | 2,940.7 ± 607 (2,010–3,722) | 2,172.5 ± 570 (670–2,960) | p < 0.001 |
Blood sample collection
Analysis of circulating EG-VEGF levels in FGR and AMC was performed using a bank of sera that have been collected at Hospital Poissy-Saint Germain. To increase the sampling of the third trimester AMC sera, we also used some samples from a previously published bank of sera [43]. All patients gave informed consent and the study was approved by the local ethics committee.
EG-VEGF ELISA
EG-VEGF was measured by ELISA (PeproTech, France) in the collected sera. Two separated standard curves were constructed to allow accurate readings of samples at upper and lower ranges of the assay. All samples were in the linear range of the standard curves. The detection limit of the assay was 16 pg/ml.
Cells and explants culture (PEX)
Isolation of cytotrophoblasts
Cytotrophoblasts were isolated from first-trimester human placentas (7–10 wg, n = 12) and cultured as previously described [41]. Cytotrophoblasts were seeded at a density of 106 cells/ml in DMEM supplemented with 2 mM glutamine, 10 % fetal bovine serum, 25 mM Hepes, and antibiotics.
BeWo culture
The human choriocarcinoma-derived trophoblast cell line, BeWo, was a kind gift from Dr. J. Badet (Faculty of Pharmacy, Paris V). Cells were grown in F12 K medium supplemented with 10 % fetal calf serum (FCS) (w/v) and antibiotics.
Human PEX culture and EG-VEGF treatment
PEX were established from first trimester human placentas (7–10 wg, n = 8) and cultured as previously described [43]. After 24 h of culture, the medium was changed and explants were incubated in the absence or presence of 10–100 ng/ml recombinant human EG-VEGF (Tebu, France).
Assessment of EG-VEGF effect on trophoblast proliferation
The effect of EG-VEGF was assessed on primary CT, BeWo cell line, and on PEX using [3H]-thymidine incorporation and Ki67 staining, respectively. Recombinant EG-VEGF concentrations ranged from 10 to 100 ng/ml of EG-VEGF (concentrations that correspond to 0.1–10 nM). This range covers the EC50 of EG-VEGF for its receptors (1.1–7.7 nM) [45]. An amount of 50 ng/ml of EG-VEGF was chosen as an optimal concentration in several assays as it gave the maximal response in our system. Cells were cultured overnight (7 × 104 cell/well, 37 °C, 5 % CO2). The cells were serum-starved for 6 h and then incubated for 24 h in serum-free media containing 10–100 ng/ml EG-VEGF, labeled with 0.5 μCi/ml [3H]-thymidine (Amersham, France) and were subsequently washed in HBSS and incubated in 2 ml ice–cold 5 % trichloroacetic acid for 20 min at RT. After washing, 0.4 ml of 0.1 M NaOH and 0.1 % SDS were added; the lysates were transferred into a vial containing scintillation liquid and the radioactivity was counted in β counter (Beckman, Germany). For Ki67 staining, the PEX were incubated for 24 h in serum-free medium containing 10–100 ng/ml EG-VEGF, fixed in paraformaldehyde for 20 min, and then immunostained for Ki67 (Dako).
Inactivation of PROKR1, PROKR2, and HLX in BeWo cells
BeWo were transfected with siRNAs targeting human PROKR1, PROKR2, or HLX (Qiagen) as previously described [36, 37, 40].
Assessment of EG-VEGF effect on trophoblast survival
Apoptosis was detected on paraffin sections by ApopTag peroxidase labeling of fragmented DNA (KIT QIA33, Calbiochem, France) according to the manufacturer’s instructions. Briefly, paraffin-embedded PEX were deparaffinized, pretreated with proteinase K, rinsed, and quenched in 1 % H2O2. Samples were then incubated with terminal deoxynucleotidyl transferase (TdT) in the presence of nucleotides. The slides were then incubated with stop-wash buffer, incubated with anti-dioxigenin peroxidase, rinsed, and then stained with AEC-chromogen. Sections were counterstained with Mayer’s hematoxylin. A dark-brown DAB signal indicates positive staining while shades of blue-green to greenish tan signify a nonreactive cell.
Immunohistochemistry
Placental tissues of FGR and AMC were collected and fixed for 24 h at 4 °C in 4 % (v/v) paraformaldehyde, embedded in paraffin, and processed as previously described [41]. Immunoreactive PROKR1 and PROKR2 were detected using in-house rabbit polyclonal antibodies, CD31 and Ki67 staining were detected using commercial antibodies (Dako, France), anti-carbonic anydrase-IX (CA-IX) was purchased from Novus Biologicals, Littleton, CO, USA.
Western-blot analysis
Frozen placental samples from FGR, from AMC, and from CT were homogenized in RIPA lysis buffer as previously described [41]. Membranes from cells were blotted with anti-MAP kinases, anti-phospho-MAP kinases, anti-AKT, and anti-P-AKT antibodies (Cell Signaling, Danvers, MA, USA) and membranes from tissue samples were blotted with anti-CD31; anti- PROKR1, anti-PROKR2, and with anti-EG-VEGF. As described previously [41] a specific Western-blot protocol was set up to detect EG-VEGF protein (10-12 kD). Briefly, we used 100 μg of placental protein that was separated on 0.1 % SDS-17 % polyacrylamide gels in Tris-Tricine-SDS Buffer (Sigma-Aldrich, St. Louis, MO, USA), and electrically transferred onto 0.2-μm polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The transfer of the proteins was reduced to 30 min at 90 V. The blots were washed with PBS-Tween 0.1 % and incubated overnight in blocking solution (2.5 % skimmed milk in PBS-T). Subsequently, the membranes were immunoblotted with a rabbit antibody against EG-VEGF (0.48 μg/ml) (Covalab Lyon, France) for 2 h. The blots were then rinsed with PBS-T and incubated with biotinylated goat anti-rabbit IgG (450 ng/ml, 1:2000, dilution in blocking solution; DakoCytomation) for 30 min. After three PBS-T washes, the membrane was incubated with a peroxidase-conjugated extravidin (1:2000 dilution in blocking solution; Sigma-Aldrich) for 30 min. Blots were washed six times with PBS-T and the antibody-antigen complex was detected using the ECL plus detection system (Amersham Pharmacia Biotech). β-actin was used to standardize the loading.
RNA isolation and real-time PCR analysis
Total RNA was extracted from FGR, and AMC placental tissue using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) as described [40]. Reverse transcription was performed on 1 μg total RNA (Invitrogen). Primers used are listed in Table 2. EG-VEGF, PROKR1, PROKR2, βhCG, ST1, ST2, HLX and GAPDH mRNA expressions were quantified by real-time RT-PCR using a Bio-Rad CFX96 apparatus and GoTaq® qPCR Master Mix (Promega, Madison, WI, USA) or ABI 7500 and TaqMan probe and Universal mastermix (Applied Biosystems, Carlsbad, CA, USA). PCR conditions were: step 1, 94 °C for 10 min; step 2, 45 cycles consisting of 95 °C for 15 s, temperature indicated in Table 2 for 5 s, and 72 °C for 10 s. The results were normalized to GAPDH.
Table 2.
Primers used for real-time RT-PCR
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) | Temperature (°C) |
|---|---|---|---|
| EG-VEGF | AGGTCCCCTTCTTCAGGAAACG | GGCTTGTCACATCTGCAAGTA | 60 |
| PROKR1 | GTCCTCGTCATTGTCAAGAGCC | AAACACGGTGGGGAAGAAGTCG | 60 |
| PROKR2 | CATCCCATCGCCTTACTTTGC | CTTTTCCTTCACGAACACAGTGG | 60 |
| βhCG | TACTGCCCCACCATGACC | GGACTCGAAGCGCACATC | 60 |
| ST1 | CGGACATCCAAAGTGATACATCT | TGATGTATCCAAGACTCCACTCCA | 60 |
| ST2 | GCCTGCAAATAGTCTTCTTT | ATAGGGGCTATTCCCATTAG | 60 |
| HLX | GACACGTTTCCAGGTCCCTA | CTGGAACCACACCTTCACCT | 60 |
| GAPDH | ACCCAGAAGACTGTGGATGG | TTCTAGACGGCAGGTCAGGT | 60 |
Desmoplakin immunofluorescence
Desmoplakin staining was performed as previously described [46]. BeWo were washed, fixed, and permeabilized in methanol at −20 °C for 25 min. A monoclonal anti-desmoplakin (Abcam, France) was then applied, followed by (FITC)-conjugated immunoglobulin (Jackson ImmunoResearch, West Grove, PA, USA). After washing, samples were mounted in DAPI (Vector Laboratories, Burlingame, CA, USA).
Statistical analysis
Statistical comparisons were made using Student’s t test and one-way ANOVA (both parametric and nonparametric) followed by Dunn’s, Dunnett’s, or Bonferroni tests. All data were checked for normality and equal variance. Mann–Whitney rank-sum test was used when normality or equal variance failed. When multiple comparisons were performed, all pairwise and comparison versus control were used (SigmaPlot and SigmaStat, Systat Software, San Jose, CA) (** p < 0.01; *p < 0.05).
Results
EG-VEGF effect on trophoblast proliferation
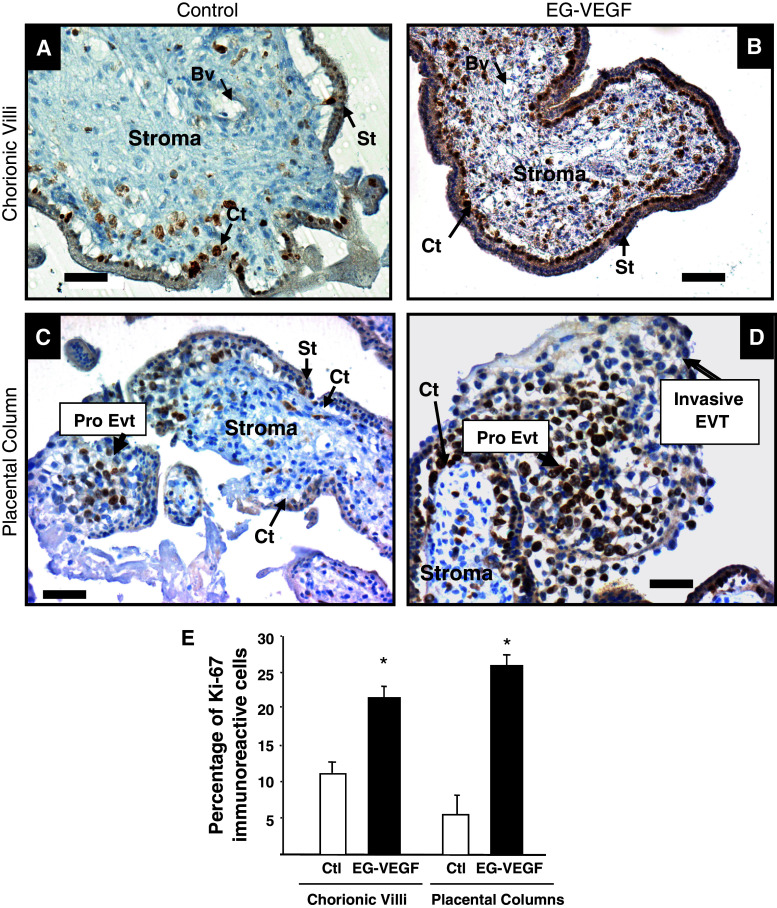
In a previous report from our group [40], we have shown that EG-VEGF increased the proliferation of human placental microvascular endothelial cells. Here, we investigated its effect on trophoblast proliferation in an ex vivo model, the PEX. It was particularly relevant to study EG-VEGF effects on trophoblast proliferation in a system in which the villous tissue architecture is maintained. PEX in culture preserve the topology of intact villi and closely mimic the formation of anchoring villi occurring in vivo [47, 48]. Explants collected at 10 wg were incubated for 24 h in the absence or presence of EG-VEGF (50 ng/ml) and proliferation was assessed using Ki67 staining. Figure 1 shows representative sections of placental villi (A, B) or placental columns (C, D) that have been incubated in the absence (A, C) or presence of EG-VEGF (B, D) and stained for Ki67. Following EG-VEGF treatment, we observed a significant increase in the number of Ki67-positive cytotrophoblasts in chorionic villi but also a significant increase in the number of proliferative CT in placental column. The quantification of three independent experiments indicated a two and threefold increase in the proliferation index of the chorionic villi and placental columns, respectively (Fig. 1e). An increase within the stroma of the villi was also observed. These data demonstrate that EG-VEGF exerts a trophic effect on the placental villi.
Fig. 1.
EG-VEGF increases the proliferation of villi and anchoring cytotrophoblast cells in PEX: the figure shows Ki-67 staining in PEX treated or not with 50 ng/ml EG-VEGF. a, c Ki67 staining in control placental villi and placental column, respectively. b, d The same staining under EG-VEGF treatment. e Percentage of Ki67-positive cytotrophoblast cells quantified in three independent experiments. Data represent the mean ± SEM of triplicates *p < 0.05 versus control. Scale bar: 50 μm. Ct cytotrophoblast, St syncytiotrophoblast, Bv blood vessel, Pro-Evt proliferative extravillous trophoblasts
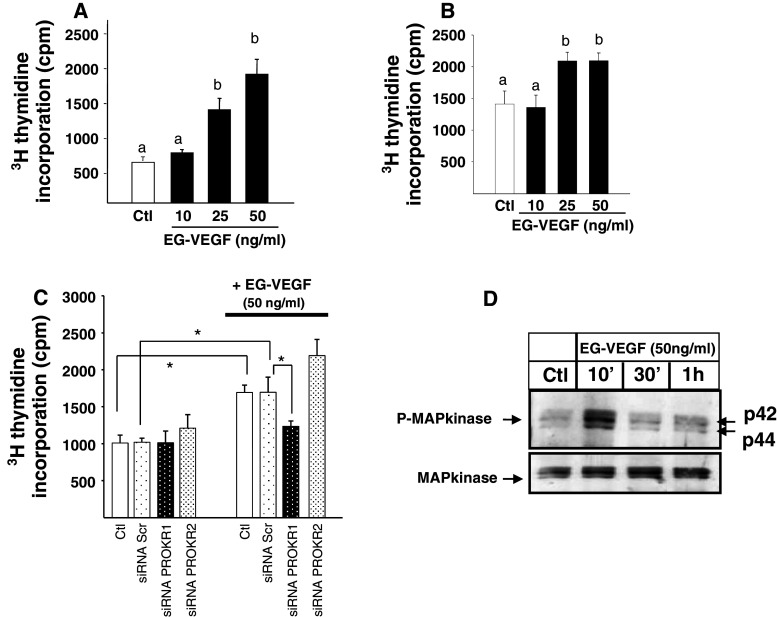
To further confirm the proliferative effect of EG-VEGF on CT, we measured [3H]-thymidine incorporation into primary cultures of cytotrophoblasts, and in the BeWo cell line, upon EG-VEGF treatment for 24 h. Figure 2a, b show that EG-VEGF increased both CT and Bewo proliferation. In previous reports, we have shown that both PROKR1 and PROKR2 receptors are present within the placental villi and that CT preferentially express PROKR1. Silencing either PROKR1 or PROKR2 using specific siRNAs shows that PROKR1 but not PROKR2 siRNA reversed the EG-VEGF proliferative effect, suggesting the direct involvement of PROKR1 in this process (Fig. 2c). In other systems it has been shown that EG-VEGF mediates its mitogenic effects via the induction of p42/44MAPK phosphorylation [49]. Figure 2d shows a representative Western-blot analysis of phospho-MAPKs upon CT treatment by EG-VEGF. A strong phosphorylation of p42/44 MAPKs protein was observed in response to a short-term treatment (10′) with EG-VEGF (Fig. 2d).
Fig. 2.
EG-VEGF increases cytotrophoblast proliferation via PROKR1 but not PROKR2. a [3H]-Thymidine incorporation into cytotrophoblasts, in the absence (white bars) or presence of EG-VEGF at indicated concentrations (black bars) (*p < 0.05, **p < 0.01 vs. control). Data represent the mean ± SEM of triplicate determinations in three independent experiments. b Dose–response effect of EG-VEGF on BeWo cells proliferation. c [3H]-Thymidine incorporation into BeWo cells that have been transfected with scramble or siRNA for PROKR1 or PROKR2 and treated with EG-VEGF (*p < 0.05). Data represent the mean ± SEM. d Representative Western blot of MAP kinase phosphorylations after treatment with 50 ng/ml of EG-VEGF of cytotrophoblasts. Standardization of the protein signals was done with antibodies against total-MAP kinases. Data are expressed as mean + SE. Values overwritten with different letters are significantly different from each other (p < 0.05)
EG-VEGF increases the expression of HLX in CT and in PEX
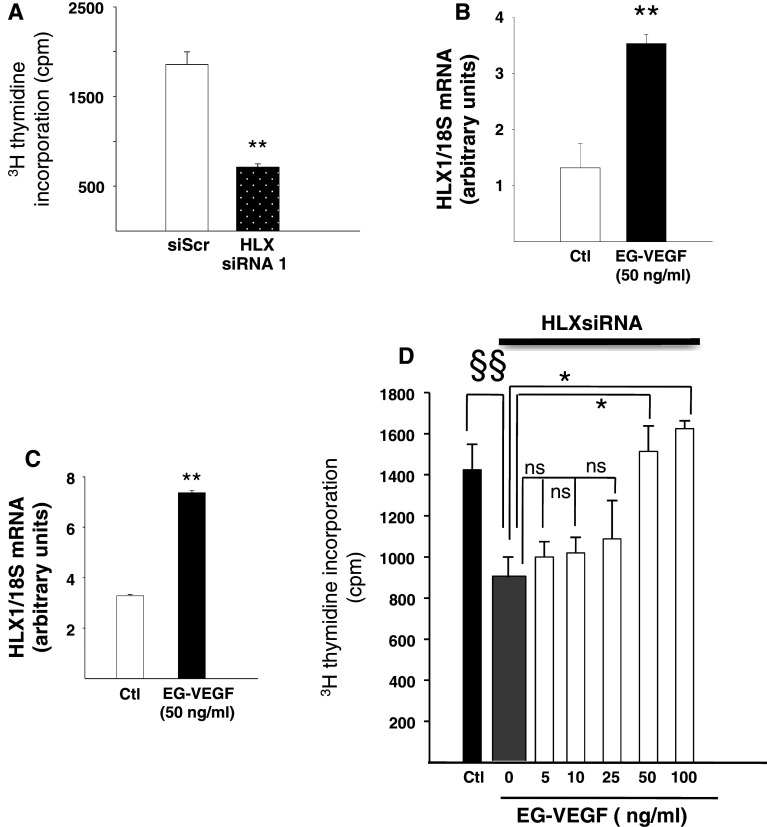
As the homeobox gene HLX has been shown to control trophoblast proliferation and to mediate the proliferative effect of two key placental growth factors, CSF-1 and HGF [36, 37], we first determined its involvement in BeWo proliferation. Figure 3a shows that silencing of the HLX gene expression using two independent siRNAs (siRNA1 and siRNA2) significantly decreased [3H]-thymidine incorporation (65 and 77 %, respectively), suggesting a direct involvement of HLX in the control of proliferation in BeWo. Interestingly, we also found that EG-VEGF (50 ng/ml) significantly increased HLX expression in PEX and in BeWo by 2.7- and 2.6-fold, respectively, (Fig. 3b, c). These data suggest a potential role for EG-VEGF induced HLX on placental growth.
Fig. 3.
EG-VEGF effect on HLX expression in PEX and in BeWos. a Effect of HLX inactivation on BeWo proliferation. b, c Effect of EG-VEGF on HLX mRNA expression in PEX cells and in BeWo, respectively. d Depiction of EG-VEGF concentration-dependent (5–100 ng/ml) rescue of proliferation of BeWo cells in the presence of HLX inactivation using siRNA. Data are expressed as mean ± SEM. §§Corresponds to the comparison between the control and the HLXsiRNA in the absence of EG-VEGF. *Represents the comparison between the HLXsiRNA in the absence and in the presence of EG-VEGF (50 and 100 ng/ml). ns Corresponds to the comparison between the HLXsiRNA in the absence and in the presence of EG-VEGF (5–25 ng/ml) (*p < 0.05, **p < 0.01 vs. control)
Proliferation assay was performed on the BeWo cells following HLX siRNA transfection in the presence of EG-VEGF stimulation at 5, 10, 25, 50, and 100 ng/ml. As depicted in Fig. 3d, EG-VEGF at 5–25 ng/ml did not affect BeWo proliferation following HLX inactivation, while at 50 and 100 ng/ml EG-VEGF significantly counteracted the inhibitory effect of HLX siRNA on BeWo proliferation. These results suggest that EG-VEGF rescued trophoblast proliferation and controls their survival.
EG-VEGF insures the survival of the placental villi
To get more insight into the role of EG-VEGF on the growth of the placental villi, we investigated its potential effect on survival upon stressful conditions such as serum starvation. Serum-starved PEX were incubated in the absence or presence of EG-VEGF (25 and 50 ng/ml) for 24 h. TUNEL staining was used to detect apoptotic cells. Under control conditions, serum starvation increased the staining (brown color), suggesting an endurance of the placental villi under these conditions. The treatment of the explants with EG-VEGF reversed this phenomenon (greenish staining) and decreased TUNEL staining (Fig. 4a–c). These results suggest that EG-VEGF is a survival factor for the placental villi during the first trimester of pregnancy. Pro-survival effects often involve the induction of the PI3 K/Akt pathway [49]. Hence, we examined EG-VEGF effect on AKT phosphorylation in PEX. Figure 4b and c show phospho-Akt levels upon EG-VEGF treatment. Strong phosphorylation of Akt in response to EG-VEGF was observed after only 2 min.
Fig. 4.
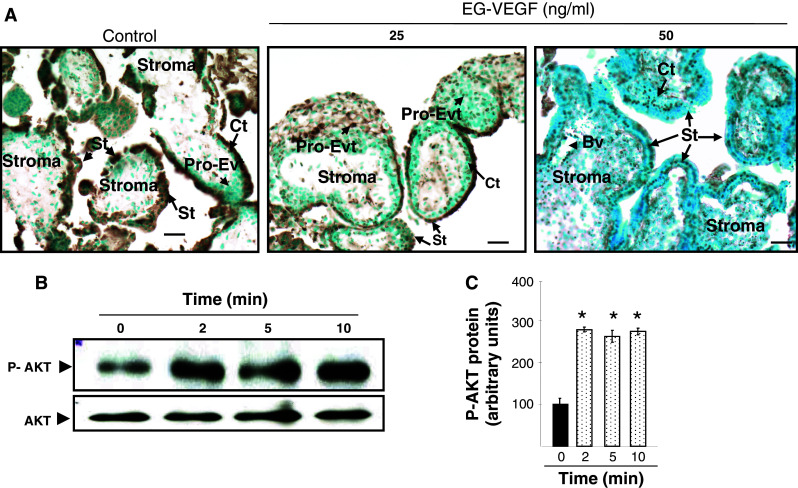
EG-VEGF effect on placental villi survival. a Representative photographs of TUNEL staining (in brown) in PEX that have been treated or not with EG-VEGF. PEX (10 wg) were serum starved for 72 h and treated for 24 h with EG-VEGF (25 and 50 ng/ml). EG-VEGF condition shows much less TUNEL-positive cells compared to the control condition. b Representative Western blot of AKT phosphorylation after treatment with 50 ng/ml EG-VEGF. Standardization of the protein signals was done with antibodies against total-AKT. Quantification of the intensity of the bands is illustrated in c. Data represent the mean ± SEM of triplicates *p < 0.05 vs. control. Scale bar: 20 μm. Ct cytotrophoblast, St syncytiotrophoblast, Bv blood vessel, Pro-Evt proliferative extravillous trophoblasts
EG-VEGF increases placental vascularization
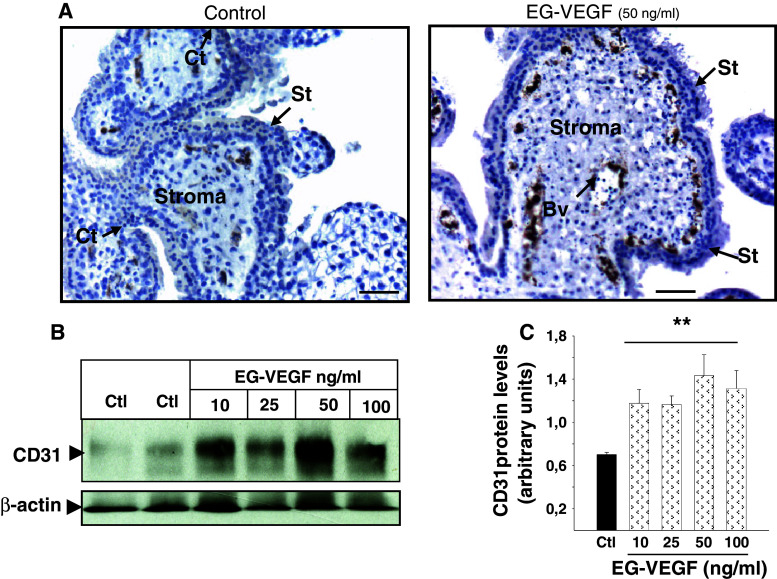
Because the microvascular system within the placental villi drives the growth and the development of this unit during the first trimester of pregnancy, we wondered whether EG-VEGF also affected this aspect of placental growth. Figure 5a shows PEX that have been incubated in the absence or presence of EG-VEGF at 50 ng/ml and stained for an endothelial cell marker, CD31/PECAM. EG-VEGF treatment markedly increased CD31 staining, suggesting an increase in the vascularization within the placental villi. Figure 5b and c show a Western-blot analysis that confirmed this increase under EG-VEGF treatment at concentrations ranging from 10 to 100 ng/ml.
Fig. 5.
EG-VEGF effect on the vascularization of first-trimester placental villi. a Representative photographs of CD31 staining (in brown) in PEX that have been treated or not with EG-VEGF. b Representative Western blot of CD31 expression after treatment with 10–50 and 100 ng/ml of EG-VEGF. Standardization of the protein signals was done with antibodies against β actin. Quantification of the intensity of the bands is illustrated in c. Data represent the mean ± SEM of triplicates *p < 0.05 vs. control. Scale bar: 50 μm. Ct cytotrophoblast, St syncytiotrophoblast, Bv blood vessel
EG-VEGF effect on trophoblast syncytialization
Placental trophoblast differentiation is an important phenomenon for normal placental development. Under physiological conditions, cytotrophoblasts differentiate and fuse to form the multinucleated epithelium known as syncytiotrophoblast. The fusogenic membrane glycoproteins of retroviral origin, syncytin-1 and syncytin-2, are critical to this process [50–52]. Trophoblast differentiation also enhances secretion of two hormones, the human chorionic gonadotropin (hCG) and placental lactogen [53–55]. Here we assessed the effect of EG-VEGF on syncytialization by measuring syncytin-1, syncytin-2, and β-hCG mRNA expression in trophoblastic cells after a 24-h exposure to 50 ng/ml of EG-VEGF (Fig. S1A). No significant effect of EG-VEGF on syncytin-1, syncytin-2, or β-hCG mRNA expression was observed. Larger ranges of EG-VEGF concentration (10–100 ng/ml) and time exposure (24, 48, and 72 h and 5 days) were tested, but no effect of EG-VEGF was observed (data not shown). However, the same cells responded positively to forskolin (50 μM), used as a positive control after 24 h (Fig. S1B), 48 and 72 h (data not shown). Syncytialization can also be followed morphologically using intercellular protein markers such as desmoplakin to identify mononuclear and multinuclear cell boundaries and morphology [56]. Quantification of nuclei within desmoplakin boundaries and syncytia following 24-h treatment with EG-VEGF did not show any effect on syncytialization (Fig. S1C-D).
As all aforementioned experiments conducted in trophoblasts and in PEX demonstrated the involvement of EG-VEGF in placental growth and survival during the first trimester of pregnancy, we hypothesized that EG-VEGF might be deregulated in placental pathologies associated with growth defects, such as FGR. Hence, we investigated EG-VEGF expression level and that of its receptors PROKR1 and PROKR2 in a bank of sera and placental tissues collected from AMC and FGR patients during the third trimester of pregnancy.
Levels of carbonic anhydrase-IX (CA-IX), an endogenous hypoxia marker, in AMC and FGR pregnancies
Hypoxia, a key parameter of placental development that has been shown to up-regulate the expression of EG-VEGF and its receptors [38, 41], is also known to be associated to the occurrence of FGR [57, 58]. In the bank of tissues collected for this study, we confirmed that FGR placentas were hypoxic. Figure S2A shows two representative controls and two representative FGR sections collected at 30 and 36 wg and stained for CA-IX. As expected, the level of CA-IX protein was up-regulated (threefold increase) in FGR samples compared to AMC (Fig. S2B-C).
EG-VEGF circulating levels and placental expression are increased in FGR pregnancies
We compared EG-VEGF circulating levels in FGR mothers’ sera and AMC during the third trimester of pregnancy. Figure 6a shows that EG-VEGF levels were significantly higher in the FGR group as compared to AMC (p < 0.011). To determine whether the increase in circulating EG-VEGF results from increased placental synthesis, we compared EG-VEGF mRNA and protein levels in AMC and FGR placental tissues. Both at the mRNA (Fig. 6b) and protein levels (Fig. 6c–e), we found that EG-VEGF levels are significantly increased in FGR placentas as compared to AMC.
Fig. 6.
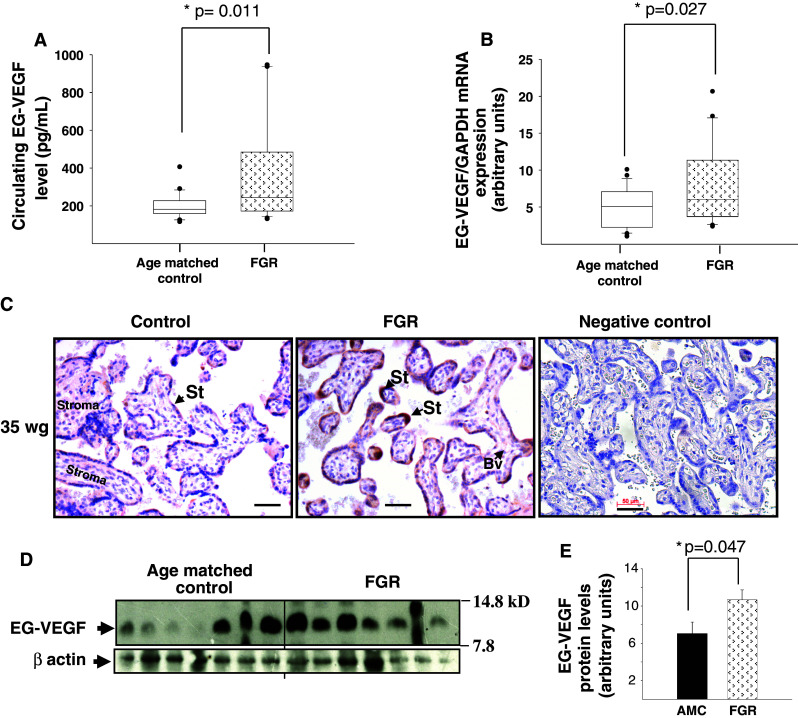
EG-VEGF expression is up-regulated in FGR pregnancies. a EG-VEGF serum levels in FGR and AMC pregnant women. EG-VEGF levels were measured by ELISA (*p < 0.05). b EG-VEGF mRNA levels in FGR and AMC placentas (25 AMC and 21 FGR) (*p < 0.05). c Chorionic villi sections immunostained with anti-EG-VEGF antibody. d Representative Western-blot analysis that compares AMC versus FGR placentas (14 AMC and 14 FGR). Quantification of the intensity of the bands is illustrated in e (*p < 0.05). Villous cytotrophoblasts and syncytiotrophoblasts were positively stained. Scale bar: 50 μm. Ct cytotrophoblast, St syncytiotrophoblast, Bv blood vessel
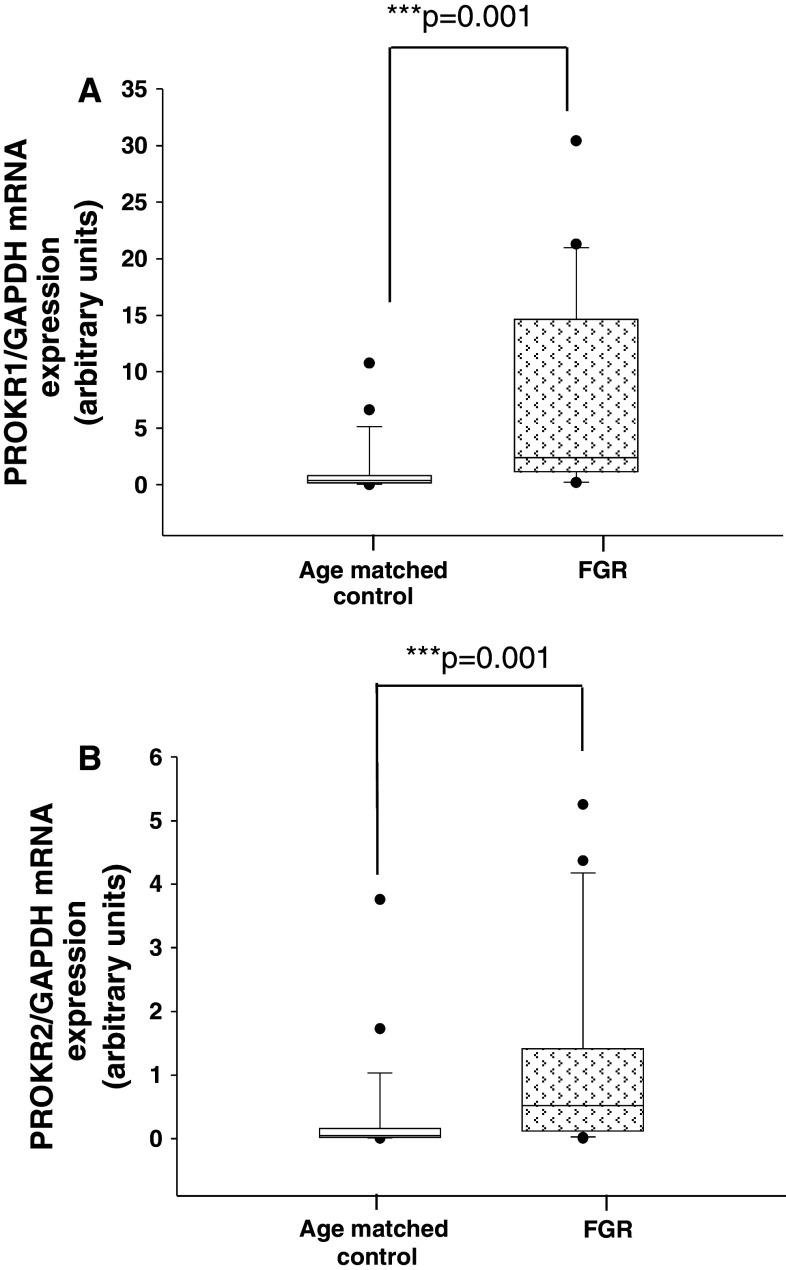
PROKR1 and PROKR2 in AMC and FGR pregnancies
As EG-VEGF acts via PROKR1 and PROKR2, we wondered whether these receptors are also deregulated in FGR. Both mRNA and protein expression levels were determined using placental tissues from AMC and FGR. Figure 7a and b show that both PROKR1 and PROKR2 mRNA levels were significantly increased in the FGR placentas. The effect on mRNA levels was substantiated at the protein levels. Figure 8a shows representative photographs of PROKR1 and PROKR2 protein expression in AMC and FGR placentas. For both receptors, an increase in the intensity of the staining was observed. The levels of PROKR1 and PROKR2 proteins were up-regulated (twofold increase) in FGR samples compared to AMC (Fig. 8b, c).
Fig. 7.
PROKR1 and PROKR2 mRNA expression in placental tissue from AMC and FGR pregnancies. a Quantification of PROKR1 mRNA levels expression in placental tissues from AMC and FGR pregnancies. b Quantification of PROKR2 mRNA levels expression in placental tissues from AMC and FGR pregnancies in the third trimester. We analyzed 25 AMC and 21 FGR placentas
Fig. 8.
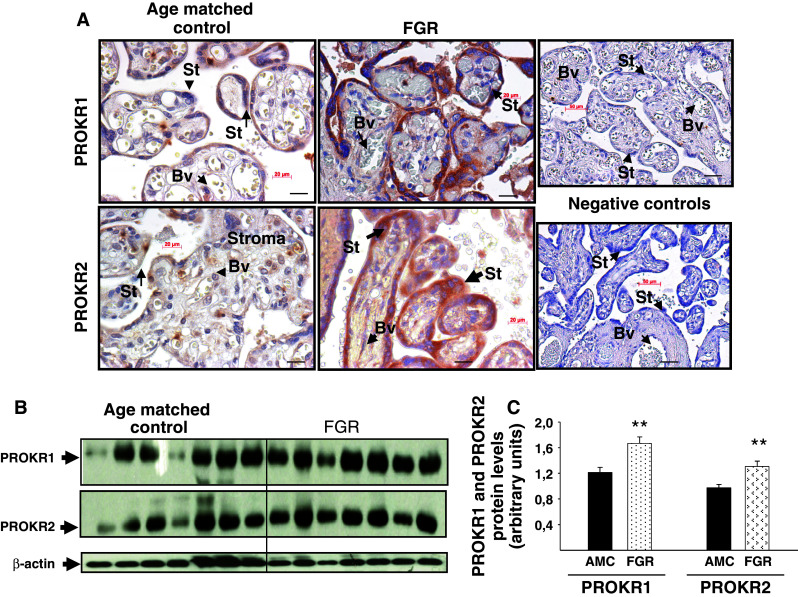
PROKR1 and PROKR2 protein expression in placental tissue from AMC and FGR pregnancies. a Representative photographs of PROKR1 and PROKR2 staining in AMC and FGR placentas. b Representative Western blots of PROKR1 and PROKR2 expression in AMC and FGR (14 AMC and 14 FGR). Standardization of the protein signals was done using beta actin. Quantification of the intensity of the bands is illustrated in c. Data represent the mean ± SEM of triplicate *p < 0.05 versus control. Ct cytotrophoblast, St syncytiotrophoblast, Bv blood vessel
Discussion
The present work demonstrates the direct involvement of EG-VEGF in the growth of the placental villi during the first trimester of pregnancy and brings evidences of its deregulation and that of its receptors in a major placental pathology, the FGR. Hence, we propose that EG-VEGF is a physiological placental growth factor that should be ranked among the important factors in FGR. These statements are based on three observations. First, we showed that EG-VEGF increased the proliferation of the villi and anchoring CT, without affecting their differentiation toward the formation of syncytium. These results are of great interest in terms of physiological perspective, as the specific effect on CT is mandatory for the continuous growth of the villi in a hypoxic environment. By increasing the proliferation of anchoring cytotrophoblasts, EG-VEGF participates to the formation of trophoblast plugs that are known to protect the growing villi, and therefore the embryo from harmful high oxygen levels against which the placenta is not equipped at this gestational age. The absence of EG-VEGF effect on syncytium formation further demonstrates that this factor drives the cytotrophoblast towards a proliferative pathway in preference to a differentiated one. Second, we showed that EG-VEGF is a survival factor for the placental villi under stress conditions. This result was observed upon serum starvation of PEX ex vivo, but clearly reflects what could happen when the placenta is subjected to diverse stressors such as a hypoxic environment, a condition that is often associated with FGR. The increase in EG-VEGF and its receptor in FGR is a confirmation of this speculation and it suggests that the EG-VEGF/PROKR1 and PROKR2 signaling complex might well be a compensatory system for placental and fetal development. Third, we showed that EG-VEGF increases the vascularization of the placental villi as reflected by an increase in CD31 expression. This result further supports an involvement of EG-VEGF in the development of the villi, a process that is critical for the establishment and the increase of the feto-maternal exchanges. In the context of the FGR, the increase in EG-VEGF could be a compensatory mechanism in response to vascularization defects observed in FGR placentas [22]. One can therefore speculate that EG-VEGF is increased in response to a failure in the FGR vascular system.
Our study also demonstrated that one of the compensatory mechanisms of EG-VEGF might involve EG-VEGF-induced control over the expression of the homeobox gene HLX, a key gene of trophoblast proliferation shown to be significantly decreased in this pathology [33].
Speculations about EG-VEGF as a compensatory actor in FGR are to be taken with caution, since another explanation for the elevation of EG-VEGF could be drawn in respect to the control of EVT invasion by EG-VEGF. In fact, we have previously demonstrated that EG-VEGF is a negative regulator of EVT invasion, a crucial process for spiral arteries remodeling [43]. Hence, a sustained expression of EG-VEGF over the first trimester of pregnancy in response to external stimuli can compromise the remodeling of the spiral arteries, a defect that is known to be a major cause of utero-placental hypoxia and a central event in most FGR [19, 59].
To date, we do not have a direct demonstration of this hypothesis, as only a prospective study examining the expression of EG-VEGF and/or its receptors during the first trimester of pregnancy in women who go on to develop FGR will allow to determine whether elevated expression of this factor in FGR pregnancies is a cause or a consequence of the pathology.
Hence, two hypotheses could be considered to explain the association between EG-VEGF/EG-VEGF receptors increase and the FGR condition. The first one proposes that the observed increase in EG-VEGF levels in FGR pregnancies could be due to abnormal prolonged expression of EG-VEGF over the end of the first trimester of pregnancy (still to be demonstrated). The second one proposes that FGR development is caused by other insults and that EG-VEGF and its receptors levels increase in response to the stressful condition in FGR placentas. Importantly, we know that EG-VEGF and its receptors are activated by hypoxia and hCG [41, 44], and that FGR placentas are known to be hypoxic and to have higher hCG levels [19, 59–61]. Hence, these parameters might trigger the increase of EG-VEGF and its receptors expression.
In conclusion, we appended to EG-VEGF functions, mostly reported by our group in the placenta, a new role directly related to placental growth and development. Recent findings from our team further support the proposal that EG-VEGF is not an independent actor in the villi and that its profile of secretion is under a fine regulation by strong placental stimuli such as hypoxia and hCG. Importantly, these parameters are deregulated in FGR.
Altogether, our results show that EG-VEGF can be considered as a novel growth factor in the placenta via its stimulatory effects on the proliferation and survival of cytotrophoblasts, its inhibition of EVT invasion [43], and via its angiogenic effect on placental endothelial cells [40]. Further studies are however required to determine whether its deregulation is a cause or consequence of FGR. These considerations are summarized in Fig. 9.
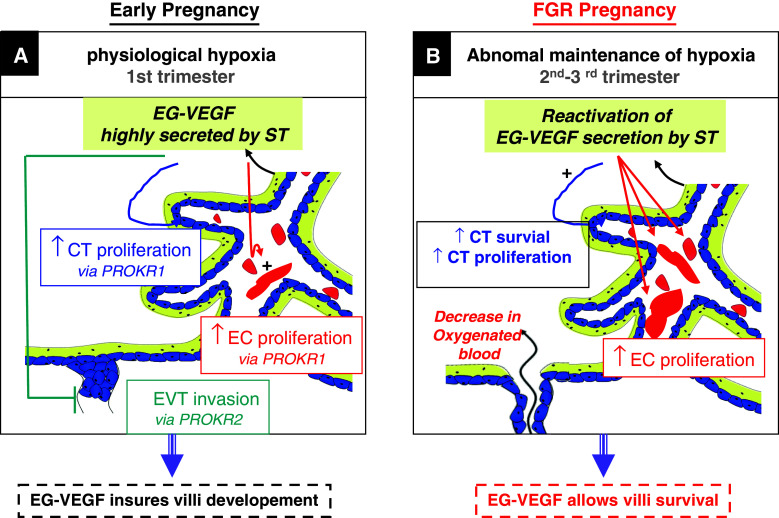
Fig. 9.
A proposed model for EG-VEGF-mediated effects in human placenta during the first trimester of pregnancy and in FGR. During the first trimester of human pregnancy (a), EG-VEGF is highly secreted from the ST layer and stimulates cytotrophoblast and endothelial cell proliferation via PROKR1, respectively. EG-VEGF also inhibits EVT invasion. These local effects contribute to the growth of the villi and to the formation of trophoblast plugs within the maternal spiral arteries; this protects the villi from the potentially harmful effects of oxygen species against which the placenta is not yet protected. In FGR placentas (b), we showed that EG-VEGF and its receptors are up-regulated, we propose that these increases occur as responses to hypoxia associated with this pathology, and that EG-VEGF and its receptors play a compensatory role to allow villi growth through effects on CT and endothelial cells proliferation, a process strongly affected in FGR. The EG-VEGF/PROKRs system acts then as survival machinery, central to the pregnancy progress
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank the staff of the Department of Gynaecology/Obstetrics (Pr. Fabrice Sergent) at the University Hospital of Grenoble for giving us access to human placentas. We acknowledge the following sources of funding: INSERM (U1036), University Joseph Fourier, Commissariat à l’Energie Atomique (DSV/iRTSV/BCI), the Région Rhône-Alpes (CIBLE-2008), the GEFLUC (Groupement des Entreprises Françaises pour la Luttte contre le Cancer) Comité Dauphiné-Savoie. Sophie Brouillet was supported by doctoral scholarships from the French Ministry of Education and Research and from the Fondation pour la Recherche Médicale.
References
- 1.Cross JC, Baczyk D, Dobric N, Hemberger M, Hughes M, Simmons DG, Yamamoto H, Kingdom JC. Genes, development and evolution of the placenta. Placenta. 2003;24(2–3):123–130. doi: 10.1053/plac.2002.0887. [DOI] [PubMed] [Google Scholar]
- 2.Henriksen T. Foetal nutrition, foetal growth restriction and health later in life. Acta Paediatr Suppl. 1999;88(429):4–8. doi: 10.1111/j.1651-2227.1999.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ. Fetal growth and adult disease. Br J Obstet Gynaecol. 1992;99(4):275–276. doi: 10.1111/j.1471-0528.1992.tb13719.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol. 2000;182(1 Pt 1):198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- 5.Baschat AA. Fetal responses to placental insufficiency: an update. BJOG. 2004;111(10):1031–1041. doi: 10.1111/j.1471-0528.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 6.Bullock CM, Li JD, Zhou QY. Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol Pharmacol. 2004;65(3):582–588. doi: 10.1124/mol.65.3.582. [DOI] [PubMed] [Google Scholar]
- 7.Pollack RN, Divon MY. Intrauterine growth retardation: definition, classification, and etiology. Clin Obstet Gynecol. 1992;35(1):99–107. doi: 10.1097/00003081-199203000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Regnault TR, Galan HL, Parker TA, Anthony RV (2002) Placental development in normal and compromised pregnancies—a review. Placenta 23(Suppl A):S119–S129. doi:10.1053/plac.2002.0792 [DOI] [PubMed]
- 9.Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CA, D’Souza SW, Glazier JD, Greenwood SL, Jansson T, Powell T. Placental phenotypes of intrauterine growth. Pediatr Res. 2005;58(5):827–832. doi: 10.1203/01.PDR.0000181381.82856.23. [DOI] [PubMed] [Google Scholar]
- 10.Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol. 1996;175(6):1534–1542. doi: 10.1016/s0002-9378(96)70103-5. [DOI] [PubMed] [Google Scholar]
- 11.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92(1):35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 12.Kingdom JC, Kaufmann P. Oxygen and placental vascular development. Adv Exp Med Biol. 1999;474:259–275. doi: 10.1007/978-1-4615-4711-2_20. [DOI] [PubMed] [Google Scholar]
- 13.Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. Am J Obstet Gynecol. 1997;177(6):1395–1401. doi: 10.1016/s0002-9378(97)70081-4. [DOI] [PubMed] [Google Scholar]
- 14.Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96(2):271–276. doi: 10.1016/s0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- 15.DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155(1):293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186(1):158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 17.Levy R, Nelson DM (2000) To be, or not to be, that is the question. Apoptosis in human trophoblast. Placenta 21(1):1–13. doi:10.1053/plac.1999.0450 [DOI] [PubMed]
- 18.Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C, Ong SS. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta. 2003;24(2–3):219–226. doi: 10.1053/plac.2002.0900. [DOI] [PubMed] [Google Scholar]
- 19.Khong TY, Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 20.Arroyo JA, Winn VD. Vasculogenesis and angiogenesis in the IUGR placenta. Semin Perinatol. 2008;32(3):172–177. doi: 10.1053/j.semperi.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Torry DS, Hinrichs M, Torry RJ. Determinants of placental vascularity. Am J Reprod Immunol. 2004;51(4):257–268. doi: 10.1111/j.1600-0897.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen CP, Bajoria R, Aplin JD. Decreased vascularization and cell proliferation in placentas of intrauterine growth-restricted fetuses with abnormal umbilical artery flow velocity waveforms. Am J Obstet Gynecol. 2002;187(3):764–769. doi: 10.1067/mob.2002.125243. [DOI] [PubMed] [Google Scholar]
- 23.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277(5332):1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 24.Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol. 1999;181(3):718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- 25.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 26.Jarvenpaa J, Vuoristo JT, Savolainen ER, Ukkola O, Vaskivuo T, Ryynanen M. Altered expression of angiogenesis-related placental genes in pre-eclampsia associated with intrauterine growth restriction. Gynecol Endocrinol. 2007;23(6):351–355. doi: 10.1080/09513590701350291. [DOI] [PubMed] [Google Scholar]
- 27.Roh CR, Budhraja V, Kim HS, Nelson DM, Sadovsky Y. Microarray-based identification of differentially expressed genes in hypoxic term human trophoblasts and in placental villi of pregnancies with growth restricted fetuses. Placenta. 2005;26(4):319–328. doi: 10.1016/j.placenta.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy C, Cotter FE, McElwaine S, Twomey A, Mooney EE, Ryan F, Vaughan J. Altered gene expression patterns in intrauterine growth restriction: potential role of hypoxia. Am J Obstet Gynecol. 2007;196(1):70–76. doi: 10.1016/j.ajog.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Cartwright JE, Holden DP, Whitley GS. Hepatocyte growth factor regulates human trophoblast motility and invasion: a role for nitric oxide. Br J Pharmacol. 1999;128(1):181–189. doi: 10.1038/sj.bjp.0702757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irving JA, Lala PK. Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGF-beta, IGF-II, and IGFBP-1. Exp Cell Res. 1995;217(2):419–427. doi: 10.1006/excr.1995.1105. [DOI] [PubMed] [Google Scholar]
- 31.Lysiak JJ, Han VK, Lala PK. Localization of transforming growth factor alpha in the human placenta and decidua: role in trophoblast growth. Biol Reprod. 1993;49(5):885–894. doi: 10.1095/biolreprod49.5.885. [DOI] [PubMed] [Google Scholar]
- 32.Chui A, Tay C, Cocquebert M, Sheehan P, Pathirage NA, Donath S, Fournier T, Badet J, Evain-Brion D, Brennecke SP, Kalionis B, Murthi P. Homeobox gene Distal-less 3 is a regulator of villous cytotrophoblast differentiation and its expression is increased in human idiopathic foetal growth restriction. J Mol Med (Berlin, Germany) 2012;90(3):273–284. doi: 10.1007/s00109-011-0836-1. [DOI] [PubMed] [Google Scholar]
- 33.Murthi P, Doherty V, Said J, Donath S, Brennecke SP, Kalionis B. Homeobox gene HLX1 expression is decreased in idiopathic human fetal growth restriction. Am J Pathol. 2006;168(2):511–518. doi: 10.2353/ajpath.2006.050637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murthi P, Said JM, Doherty VL, Donath S, Nowell CJ, Brennecke SP, Kalionis B. Homeobox gene DLX4 expression is increased in idiopathic human fetal growth restriction. Mol Hum Reprod. 2006;12(12):763–769. doi: 10.1093/molehr/gal087. [DOI] [PubMed] [Google Scholar]
- 35.Murthi P, So M, Gude NM, Doherty VL, Brennecke SP, Kalionis B. Homeobox genes are differentially expressed in macrovascular human umbilical vein endothelial cells and microvascular placental endothelial cells. Placenta. 2007;28(2–3):219–223. doi: 10.1016/j.placenta.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Rajaraman G, Murthi P, Brennecke SP, Kalionis B Homeobox gene HLX is a regulator of HGF/c-met-mediated migration of human trophoblast-derived cell lines. Biol Reprod 83(4):676–683 [DOI] [PubMed]
- 37.Rajaraman G, Murthi P, Leo B, Brennecke SP, Kalionis B. Homeobox gene HLX1 is a regulator of colony stimulating factor-1 dependent trophoblast cell proliferation. Placenta. 2007;28(10):991–998. doi: 10.1016/j.placenta.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 38.LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, Peale F, Gurney A, Hillan KJ, Ferrara N. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412(6850):877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 39.Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem. 2002;277(22):19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- 40.Brouillet S, Hoffmann P, Benharouga M, Salomon A, Schaal JP, Feige JJ, Alfaidy N. Molecular characterization of EG-VEGF-mediated angiogenesis: differential effects on microvascular and macrovascular endothelial cells. Mol Biol Cell. 2010;21(16):2832–2843. doi: 10.1091/mbc.E10-01-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann P, Feige JJ, Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology. 2006;147(4):1675–1684. doi: 10.1210/en.2005-0912. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann P, Feige JJ, Alfaidy N. Placental expression of EG-VEGF and its receptors PKR1 (prokineticin receptor-1) and PKR2 throughout mouse gestation. Placenta. 2007;28(10):1049–1058. doi: 10.1016/j.placenta.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann P, Saoudi Y, Benharouga M, Graham CH, Schaal JP, Mazouni C, Feige JJ, Alfaidy N. Role of EG-VEGF in human placentation: physiological and pathological implications. J Cell Mol Med. 2009;13(8B):2224–2235. doi: 10.1111/j.1582-4934.2008.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brouillet S, Hoffmann P, Chauvet S, Salomon A, Chamboredon S, Sergent F, Benharouga M, Feige JJ, Alfaidy N. Revisiting the role of hCG: new regulation of the angiogenic factor EG-VEGF and its receptors. Cell Mol Life Sci. 2012;69(9):1537–1550. doi: 10.1007/s00018-011-0889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Kuei C, Sutton S, Wilson S, Yu J, Kamme F, Mazur C, Lovenberg T, Liu C. Identification and pharmacological characterization of prokineticin 2 beta as a selective ligand for prokineticin receptor 1. Mol Pharmacol. 2005;67(6):2070–2076. doi: 10.1124/mol.105.011619. [DOI] [PubMed] [Google Scholar]
- 46.Frendo JL, Vidaud M, Guibourdenche J, Luton D, Muller F, Bellet D, Giovagrandi Y, Tarrade A, Porquet D, Blot P, Evain-Brion D. Defect of villous cytotrophoblast differentiation into syncytiotrophoblast in Down’s syndrome. J Clin Endocrinol Metab. 2000;85(10):3700–3707. doi: 10.1210/jcem.85.10.6915. [DOI] [PubMed] [Google Scholar]
- 47.Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, Post M, Lye SJ. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest. 1999;103(12):1641–1650. doi: 10.1172/JCI6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Genbacev O, Jensen KD, Powlin SS, Miller RK. In vitro differentiation and ultrastructure of human extravillous trophoblast (EVT) cells. Placenta. 1993;14(4):463–475. doi: 10.1016/s0143-4004(05)80466-7. [DOI] [PubMed] [Google Scholar]
- 49.Kisliouk T, Levy N, Hurwitz A, Meidan R. Presence and regulation of endocrine gland vascular endothelial growth factor/prokineticin-1 and its receptors in ovarian cells. J Clin Endocrinol Metab. 2003;88(8):3700–3707. doi: 10.1210/jc.2003-030492. [DOI] [PubMed] [Google Scholar]
- 50.Potgens AJ, Schmitz U, Bose P, Versmold A, Kaufmann P, Frank HG (2002) Mechanisms of syncytial fusion: a review. Placenta 23(Suppl A):S107–S113. doi:10.1053/plac.2002.0772 [DOI] [PubMed]
- 51.Frendo JL, Olivier D, Cheynet V, Blond JL, Bouton O, Vidaud M, Rabreau M, Evain-Brion D, Mallet F. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol Cell Biol. 2003;23(10):3566–3574. doi: 10.1128/MCB.23.10.3566-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blaise S, Parseval N, Benit L, Heidmann T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc Natl Acad Sci USA. 2003;100(22):13013–13018. doi: 10.1073/pnas.2132646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118(4):1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 54.Morrish DW, Dakour J, Li H. Functional regulation of human trophoblast differentiation. J Reprod Immunol. 1998;39(1–2):179–195. doi: 10.1016/s0165-0378(98)00021-7. [DOI] [PubMed] [Google Scholar]
- 55.Shi QJ, Lei ZM, Rao CV, Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology. 1993;132(3):1387–1395. doi: 10.1210/endo.132.3.7679981. [DOI] [PubMed] [Google Scholar]
- 56.Douglas GC, King BF. Differentiation of human trophoblast cells in vitro as revealed by immunocytochemical staining of desmoplakin and nuclei. J Cell Sci. 1990;96(Pt 1):131–141. doi: 10.1242/jcs.96.1.131. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka M, Natori M, Ishimoto H, Miyazaki T, Kobayashi T, Nozawa S. Experimental growth retardation produced by transient period of uteroplacental ischemia in pregnant Sprague-Dawley rats. Am J Obstet Gynecol. 1994;171(5):1231–1234. doi: 10.1016/0002-9378(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 58.Tapanainen PJ, Bang P, Wilson K, Unterman TG, Vreman HJ, Rosenfeld RG. Maternal hypoxia as a model for intrauterine growth retardation: effects on insulin-like growth factors and their binding proteins. Pediatr Res. 1994;36(2):152–158. doi: 10.1203/00006450-199408000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99(9):2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fitzgerald B, Levytska K, Kingdom J, Walker M, Baczyk D, Keating S. Villous trophoblast abnormalities in extremely preterm deliveries with elevated second trimester maternal serum hCG or inhibin-A. Placenta. 2011;32(4):339–345. doi: 10.1016/j.placenta.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 61.Krantz D, Goetzl L, Simpson JL, Thom E, Zachary J, Hallahan TW, Silver R, Pergament E, Platt LD, Filkins K, Johnson A, Mahoney M, Hogge WA, Wilson RD, Mohide P, Hershey D, Wapner R. Association of extreme first-trimester free human chorionic gonadotropin-beta, pregnancy-associated plasma protein A, and nuchal translucency with intrauterine growth restriction and other adverse pregnancy outcomes. Am J Obstet Gynecol. 2004;191(4):1452–1458. doi: 10.1016/j.ajog.2004.05.068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.