Abstract
Parkinson’s disease (PD), the second most common neurodegenerative disorder, affects 1–2 % of humans aged 60 years and older. The diagnosis of PD is based on motor symptoms such as bradykinesia, rigidity, tremor, and postural instability associated with the striatal dopaminergic deficit that is linked to neurodegenerative processes in the substantia nigra (SN). In the past, cellular replacement strategies have been evaluated for their potential to alleviate these symptoms. Adult neurogenesis, the generation of new neurons within two proliferative niches in the adult brain, is being intensively studied as one potential mode for cell-based therapies. The subventricular zone provides new neurons for the olfactory bulb functionally contributing to olfaction. The subgranular zone of the hippocampus produces new granule neurons for the dentate gyrus, required for memory formation and proper processing of anxiety provoking stimuli. Recent years have revealed that PD is associated with non-motor symptoms such as hyposmia, anhedonia, lack of novelty seeking behavior, depression, and anxiety that are not directly associated with neurodegenerative processes in the SN. This broad spectrum of non-motor symptoms may partly rely on proper olfactorial processing and hippocampal function. Therefore, it is conceivable that some non-motor deficits in PD are related to defective adult neurogenesis. Accordingly, in animal models and postmortem studies of PD, adult neurogenesis is severely affected, although the exact mechanisms and effects of these changes are not yet fully understood or are under debate due to conflicting results. Here, we review the current concepts related to the dynamic interplay between endogenous cellular plasticity and PD-associated pathology.
Keywords: Adult neurogenesis, Parkinson’s disease, Alpha-synuclein, LRKK2, 6-Hydroxydopmaine, MPTP, Olfactory bulb, Hippocampus
Introduction
Parkinson’s disease
Parkinson’s disease (PD) is a progressive, chronic neurodegenerative disease with a complex, multifactorial etiology. Initially described by James Parkinson in 1817, it is at present the most common movement disorder. Its prevalence is age-associated, with approximately 1 % of the population being affected at 65 years, increasing to 4–5 % in 85 year olds [1]. The past 20 years have shed important insights into the genetic and molecular pathogenesis of the disease, although a causal explanation for the underlying pathophysiology is still lacking. It is currently accepted that PD is a multisystem disorder, diffusely affecting the central nervous system rather than single circuits within the basal ganglia [2].
Initial pathological studies have associated PD almost solely with the degeneration of dopaminergic neurons of the substantia nigra (SN) pars compacta projecting into the striatum, which are tightly associated with the prototypical motor features of the disease such as hypokinesia, rigidity, tremor, and postural instability. However, Braak and collegues [3] proposed a model for the progressive nature of the disease starting within brainstem nuclei and the olfactory bulb (OB) that continues with a stepwise affection of midbrain, mesocortical, and finally cortical regions. Interestingly, neuropathological alterations in affected brain regions correlate with the onset of clinical features other than the cardinal motor symptoms. These non-motor features are present at early premotor stages of the disease and include olfactory dysfunction, cardiac sympathetic denervation and constipation, REM sleep disorder, depression, and anxiety. More importantly, other non-motor symptoms such as mild cognitive impairment often appear in the early motor phase of the disease [4, 5]. Although current dopaminergic treatment controls motor symptoms, non-motor symptoms, first of all neuropsychiatric symptoms play a key role in leading to severe disability and loss of quality of life.
The pathological hallmarks of PD are eosinophilic intracellular inclusion bodies termed Lewy-bodies (LB), and argyrophilic processes (Lewy neurites, LN). The identification of families with autosomal dominant forms of PD led to the identification of the presynaptic protein alpha-synuclein as the major component of LB and LN [1]. In sporadic PD, the natively unfolded or tetrameric protein alpha-synuclein forms oligomers and consecutively fibrillary isoforms aggregate as LB and LN [6–10]. Furthermore, release of alpha-synuclein into the extracellular space and subsequent uptake of neighboring neurons [11], astrocytes [12], as well as microglia points towards mechanisms of cell-to-cell propagation. These findings may offer a putative explanation for the neuropathological studies by Braak [3] and the alpha-synuclein pathology in fetal midbrain derived grafts observed more than 10 years after transplantation in PD patients [13, 14]. Moreover, neuron to glia transmission is accompanied by microglia activation and astrocytosis in postmortem studies of PD brains [15, 16]. These inflammatory responses may reflect important disease-modifying mechanisms most likely influencing the course of disease progression [17]. Other autosomal dominant (leucin-rich repeat kinase 2; LRRK2) or recessive forms (i.e., PINK1 or DJ1) of PD (for review, see [1]) have helped tremendously to conceive PD as a proteinopathy, influenced by oxidative stress and defects in distinct protein degradation systems. Currently, 15 gene loci have been linked to PD, causing up to 3 % of the late onset forms and up to 50 % of early onset PD. Nevertheless, the majority of PD cases remain sporadic.
Adult neurogenesis
Adult neurogenesis is defined as the generation of new neurons in the adult forebrain. It was first discovered 40 years ago in rats [18], is restricted to the OB and hippocampus, and has also been confirmed in the human brain [19, 20]. Upon aging, rodent adult neurogenesis declines dramatically. During the past decade, large efforts were undertaken to characterize these regions and their physiological role. Numerous factors modulating adult neurogenesis have been identified and the therapeutic potential of adult neurogenesis has been studied extensively (for a detailed review, see [21, 22]).
Adult neural stem cells (aNSCs) are multipotent cells defined by stem cell characteristics such as lifetime maintenance, proliferation, self-renewal, and differentiation. Adult NSCs, specialized glial fibrillary acidic protein (GFAP)-positive astrocyte-like cells [23], express stem cell markers like nestin, sry-box2, (Sox2; [24]), and Hes5. During proliferation, aNSCs express the proliferating cell nuclear antigen (PCNA) and reside within the subventricular zone (SVZ) of the lateral ventricles, as well as in the subgranular zone (SGZ) of the dentate gyrus (DG) [25]. At their respective origin, they generate new neurons for their final target regions, the OB and the DG.
Adult OB neurogenesis is initiated within in the SVZ just beneath the ependymal layer of the lateral ventricular walls. From there, newly generated cells migrate within the rostral migratory stream (RMS) into the OB where they terminally differentiate and integrate into the local network [18, 19, 26] (Fig. 1a). In humans, the number of migrating neuroblasts is high during infancy, but decreases dramatically upon ageing [27]. In rodents, slowly cycling putative stem cells are GFAP-, Hes5-, and nestin-positive [23, 28–31]. The derived transiently amplifying progenitor cells are GFAP-negative and downregulate nestin, but express the epidermal growth factor receptor (EGFR) [32]. With their initiation of migration and their fate determination to a neuronal lineage, precursor cells upregulate doublecortin (DCX; [33]) and proteins like poly-sialated neural cell adhesion molecule PSA-NCAM [34, 35]. These neuroblasts migrate tangentially in chains of elongated cells [36, 37], ensheathed by astrocytes [38], into the OB turning radially towards the granular and the periglomerular cell layer [39]. After reaching their final target region after 3–4 weeks, the cells integrate either as GABAergic interneurons in the granule cell layer or as coexpressing GABAergic and dopaminergic interneurons (about 20 %) into the periglomerular cell layer [40].
Fig. 1.
Adult olfactory bulb neurogenesis in rodent models of Parkinson’s disease (PD). a In the neurogenic niche of the subventicular zone (SVZ) adjacent to the lateral ventricle (LV), stem cells proliferate (depicted in green) and give rise to migrating neuroblasts (blue). These cells migrate along the rostral migratory stream (RMS) into the olfactory bulb (OB) where they differentiate into granular neurons (GCL; red) or glomerular neurons (Glom; orange). b–g Each triptych demonstrates the changes in adult neurogenesis in the respective rodent model of PD with reference to the naïve situation in (a). Changes in the SVZ are depicted in the right picture, changes in migrating cells in the RMS are depicted in the center and results for OB neurogenesis are shown on the left. b–d Summary of the results from acute dopaminergic lesion models of PD. b Dopaminergic deafferentation results in decreased proliferation and an increase of newly generated dopaminergic glomerular neurons. c Upon stimulation with epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF-2), newly generated cells migrate into the adjacent striatum, causing a reduction in granular neurogenesis in the OB, whereas dopaminergic neurogenesis in the OB remains elevated. d Dopamine agonist treatment increases proliferation in the SVZ, resulting in an increased OB neurogenesis. e–g Delineates adult neurogenesis in transgenic mouse models of PD. e Wild-type (wt) alpha-synuclein expression in migrating neuroblasts results in a decrease both in granular and glomerular neurogenesis. f Mutant (mt) alpha-synuclein expression in the OB as well as in maturing newly generated neurons decreases granular neurogenesis as well as dopaminergic glomerular neurogenesis. Conditional suppression of alpha-synuclein expression ameliorates this effect. g In transgenic mice expresing mt LRRK2, OB neurogenesis is affected at each stage of adult neurogenesis. Deficits in proliferation and reduced numbers of migrating neuroblasts result in a deficit in olfactory bulb neurogenesis in the granular as well as the glomerular cell layer
The DG, a substructure of the hippocampal formation, receives its glutamatergic input from the entorhinal cortex as well as from frontal cortical areas. Dentate gyrus granular neurons project almost exclusively towards the CA3 region of the hippocampus, forming glutamatergic synapses on CA3 neuronal dendrites [41]. Within the SGZ of rodents, about 9,000 cells daily are migrating a short distance into the adjacent granular cell layer [42, 43]. During their migration along radial glia cells, the immature hippocampal neurons transiently express PSA-NCAM and DCX. These new neurons form synapses at the CA3 target region 2 weeks after their birth. The complexity of efferent projections and the dendritic arborization continues to be shaped upon further maturation [44] (Fig. 2). During this integration process (third and fourth weeks after cell cycle exit), mature granule neurons start expressing the neuronal marker neuronal nuclei (NeuN; [40]). During the critical period of integration, more than 50 % of the newly generated cells undergo apoptosis [45].
Fig. 2.
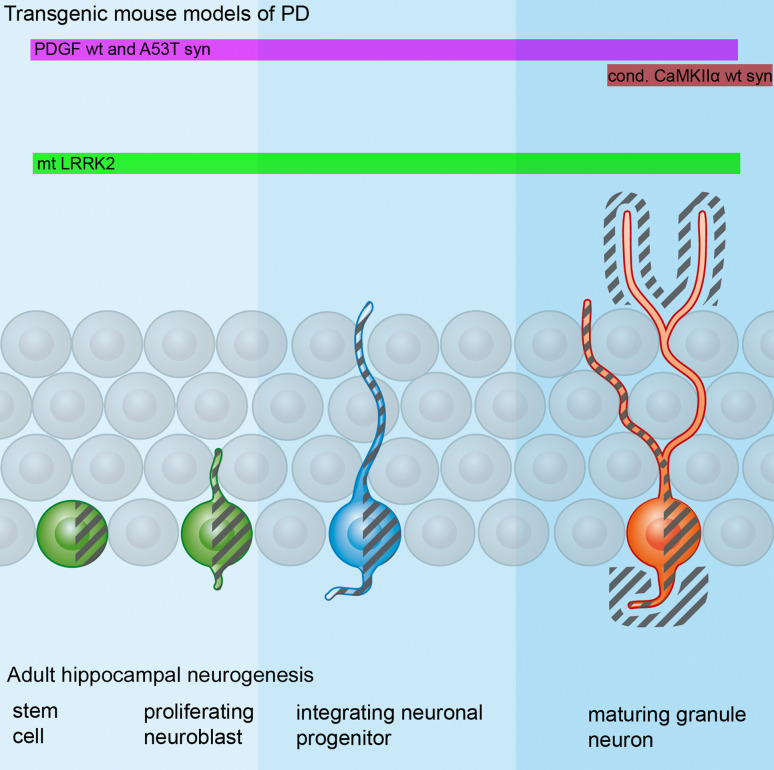
Adult hippocampal neurogenesis and changes in rodent models of Parkinson’s disease (PD). Stem cells and proliferating neuroblasts (depicted in green) give rise to neuronal progenitors (blue) which migrate a short distance into the granule cell layer (not shown). After 2–3 weeks the dendritic arborization and axonal connectivity starts to be shapened (blue). The dendritic arborization of these cells is further being refined and the cells start to express markers of mature neurons after 3–4 weeks (red). Different influences affect adult hippocampal neurogenesis in PD. In transgenic mouse models of PD, hippocampal neurogenesis is decreased either by affecting proliferation within the neurogenic niche (light blue), by hindering proper integration of neuroblasts (blue) or by influencing maturing newly generated neurons (dark blue). The influence of alpha-synuclein or mutant (mt) LRRK2 is visualized by gray shadings, revealing either intracellular expression or expression in the surrounding microenvironment. The PDGF promoter (pink bar) drives expression of A53T and wild-type alpha-synuclein in stem cells, and affects proliferation and integration of neuronal precursors as well as maturing new neurons. In addition, human alpha-synulein is expressed in the surrounding tissue. Taken together, this results in decreased proliferation, a loss of integrating neuronal progenitors, and a defective maturation of newly generated granule neurons. Conditional expression of wild-type alpha-synuclein driven by the Calcium/calmodulin dependant kinase II alpha (CaMKIIα) promoter (red bar) results in transgene expression outside newly generated neurons or in mature granule neurons. This influences proper integration of maturing granule neurons. In mutant LRRK2 transgenic animals, measures of all three steps (proliferation, integration, and maturation) are significantly reduced, with a prominent dendritic phenotype featuring shorter dendrite lengths and a reduced survival of mature newborn neurons
Function of adult neurogenesis
The functional role of adult neurogenesis has been intensively studied for both neurogenic regions. Even though the exact function of periglomerular and granular neurons in the OB is not fully understood, several studies point towards an important role of adult OB neurogenesis in olfaction (for a detailed review, see [46]). Olfactory bulb neurogenesis is not altered by passive odor enrichment [47–50]. Furthermore, defective OB neurogenesis does not result in overt changes in odor discrimination [51, 52]. However, odor-learning paradigms, i.e., operant odor conditioning increases OB neurogenesis [50]. Moreover, perceptual and associative learning of odors specifically increases the survival of integrating new neurons within a time window of 30 days after cell birth. After integration, adult-born OB neurons unlike preexisting neurons are characterized by features such as long-term potentiation (LTP; [53, 54]). Thus, a distinct and precisely timed role of OB neurogenesis in odor learning is very likely [46]. In addition to its influence on distinct aspects of olfactory functioning, OB neurogenesis may also contribute to more global behaviors like social recognition [55] and mood [56, 57].
The traditional role of the hippocampus as a “pinhole structure” relaying information, and thus contributing to the acquisition of new memory, has been studied extensively [58, 59]. A contribution of adult hippocampal neurogenesis to memory formation and pattern separation appears very likely. Initial and recent studies specifically ablating adult hippocampal neurogenesis have identified a role of newly generated granule cells in spatial memory [60–62]. Interestingly, the generation of neural progenitors in the adult hippocampus is an experience-dependent mechanism and social isolation reduces hippocampal neurogenesis [63].
Besides its role in memory formation, the hippocampus, a central part of the mesolimbic system, participates in the processing of emotions [64]. Consistently, transgenic ablation of adult neurogenesis increases anxiety-related behavior, whereas increasing hippocampal neurogenesis enhances contextual fear discrimination [62, 65]. Consecutively, a role of hippocampal neurogenesis in depression has been frequently proposed, but its distinct role in emotion and mood regulation is still far from fully understood. Nevertheless, a substantial increase of adult hippocampal neurogenesis upon antidepressant treatment is evident and has been linked to functional improvements [66–71].
Why study adult neurogenesis in Parkinson’s disease?
In an age-related neurodegenerative disease, it may appear “far-fetched” to analyze endogenous cellular plasticity that physiologically declines with age. However, it has become apparent that the disease process in PD starts several years if not decades prior to the onset of motor symptoms. More than half of the cells within the SN are already lost 5–10 years prior to the onset of motor symptoms, finally leading to the clinical diagnosis of PD [72]. During the premotor phase in PD, one of the earliest premotor symptoms is hyposmia as well as changes in mood and cognition [2, 4]. Depression and anxiety are frequently observed, sometimes predating motor symptoms for up to 20 years [73]. While LB and LN are present in the OB in early PD, LB are usually absent in archi- and paleocortical regions [3, 74]. Intriguingly, deficits in adult neurogenesis are considered to contribute to depression and anxiety as well as hyposmia typical non-motor symptoms in PD (see “Function of adult neurogenesis”). Since adult neurogenesis at present is the only endogenous neuronal replacement mechanism that offers a putative restoration of neuronal function, the elucidation of neurogenic processes in PD is crucial. Additionally, the process of proliferation, migration, and integration of new neurons is an ideal model system to study early cellular and neuritic alterations, probably leading to a better understanding of initial pathogenic events in PD.
Adult neurogenesis in Parkinson’s disease
PD patients
At present, postmortem analyses of adult neurogenesis in PD patients are limited. In addition, recent studies have led to partially conflicting results. Studies in mice and humans showed a dense dopaminergic innervation of the SVZ which is most likely derived from projections of the SN [32, 75]. In addition a decreased number of PCNA-positive cells were observed in the SVZ of PD patients [32]. Moreover, the number of EGF receptor positive cells is decreased in the adult SVZ in human PD as an indirect sign of reduced OB neurogenesis [76]. Decreased EGF levels have been described in the striatum and the prefrontal cortex of PD patients, possibly related to dopaminergic nigral deafferentation [77].
These results indicating a reduced proliferative capacity in the PD brain have recently been challenged [78]. Studying ten postmortem PD brains, several confounding variables could be excluded, and this study provides first evidence that the proliferation of aNSCs measured by PCNA and phospho histone-H3 is not altered in the SVZ of PD patients compared to controls, irrespective of dopaminergic treatment. Moreover, neurospheres were generated from the SVZ of an 80-year-old PD patient, strongly indicating the presence of aNSCs even in the SVZ of older PD patients. Interestingly, although OB volumes may be reduced in PD [79], the number of dopaminergic neurons may be increased in the OB of male patients [80–82]. Furthermore, a reduction of nestin- and β3-tubulin-positive cells in the DG has been described in the hippocampus of PD patients [32].
In summary, a detailed and comprehensive study of proliferation and survival of aNSCS in PD is still lacking, in particular with short postmortem time delay to obtain unequivocal results. Moreover, dopaminergic denervation of the SVZ in PD [32] has not yet been demonstrated in humans. Ideally, acquired data should be correlated to age and sex differences, disease stage and duration, clinical symptoms, and drug treatment. The difficulty of preserving human tissue postmortem, variable postmortem delay with different levels of tissue deterioration, the antigen-masking effect of fixation, and the limited specificity of numerous antibodies for human tissue are significant caveats to obtaining reliable results for adult neurogenesis in PD. Therefore, further and long-term clinico-neuropathological studies are urgently required to translate findings derived from preclinical models to the human brain.
Lesion models
Adult neurogenesis has been extensively explored in different acute lesion models (Fig. 1b–d). These models predominantly replicate the dopaminergic nigrostriatal deficit in PD and result in a distinct motor phenotype. After injection into the SN or the medial forebrain bundle, 6-hydroxydopamine (6-OHDA) is selectively taken up by dopaminergic neurons, and leads to dopaminergic denervation of the striatum and the SVZ [83, 84]. The precise anatomical origin of the dopaminergic input into the SVZ is still under debate. A subset of dopaminergic neurons located between the SN and the ventral tegmental area (VTA) appear to specifically project to the SVZ [85]. Dopamine receptors (DRs) expressed in the SVZ may be remnants of the embryonic neurogenic region where D2-like dopamine receptors (D2LRs) are abundantly expressed along the proliferative lining of the neural tube [86]. In the adult SVZ, dopaminergic loss decreases SVZ proliferation by affecting the pool of rapidly dividing, transiently amplifying, EGFR positive aNSCs via D2LR, most likely the D3R [32, 75, 76, 87, 88] (Fig. 1b). The lesion-induced proliferation deficit may be restored by dopaminergic treatment, resulting in an increased EGF release from aNSCs [76]. Accordingly, systemic treatment with DA agonists such as pramipexole and ropinirole increases SVZ proliferation in 6-OHDA-lesioned rats [32, 88] (Fig. 1d). These data are in contrast to mice lacking this response to dopaminergic treatment [89]. It is even noteworthy that dopamine receptor blockade by antipsychotic treatment with haloperidol leads to increased neuroblast proliferation [90, 91]. Moreover, the effects of 6-OHDA on progenitor cell proliferation seem to depend on the site and severity of the lesion: the application of lower amounts of 6-OHDA into the medial forebrain bundle or the striatum caused a transient increase of SVZ proliferation during the first 14 days post-lesioning, accompanied by swelling of the SVZ and the generation of astroglial cells [92, 93].
Intracerebroventricular administration of EGF and FGF-2 results in massive proliferation and leads to migration of neuroblasts into the adjacent striatum, indicating that the endogenous potential for proliferation may be exogenously modified in the SVZ [77, 94, 95] (Fig. 1b). In acute lesion models, dopaminergic deafferentation decreases olfactory bulb granule cell neurogenesis, paralleled by an increase of newborn dopaminergic neurons in the glomerular layer of the OB [87] (Fig. 1b).
Chronic or acute intraperitoneal administration of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is widely used to model the dopaminergic deficit of PD. MPTP crosses the blood–brain barrier, oxidizes to 1-methyl-4-phenylpyridinium (MPP+), is taken up by dopaminergic neurons, and impairs mitochondrial function by inhibiting complex I. Primates (and humans) injected with MPTP develop a severe parkinsonism [96]. Subacute administration in mice results in cell death of dopaminergic neurons associated with bradykinesia and gait abnormalities. In contrast, chronically administrated MPTP at lower doses results in a milder phenotype with spontaneous recovery [97–99]. Similar to 6-OHDA lesions, MPTP-injected mice show reduced proliferation in the SVZ, fewer newborn granule cells in the OB, and an increased dopaminergic neurogenesis in the glomerular cell layer [32] [100] (Fig. 1b), even in non-human primates [83]. In contrast to 6-OHDA leading to a permanent deafferentation [101], acute MPTP lesions (delivering i.e., 40 mg/kg of MPTP) have been found to show dopaminergic reinnervation. Interestingly, this reinnervation process is paralleled by a restoration of proliferation in the SVZ, indicating a direct correlation of dopaminergic innervation and proliferation of murine aNSCs [32]. In contrast, chronic MPTP administration of a total of 250 mg/kg over 5 weeks does not alter proliferation in the SVZ [78].
In general, studies on the influence of dopaminergic denervation show conflicting results for the proliferation in the SVZ. However, a decreased OB neurogenesis upon dopaminergic deafferentation is a consistent observation. In contrast, the impact of dopaminergic deafferentation on hippocampal neurogenesis is ambiguous. The DG receives dopaminergic innervation not only from the VTA but also from the SN [32, 102, 103]. While mature granular neurons respond with long-term depression upon dopaminergic stimulation, immature neurons lower their excitatory postsynaptic current, resulting in a subsequent decrease in the potential to induce LTP [104]. Dopamine depletion after acute MPTP application (of a total dose of 40 mg/kg) results in a transient decrease of PCNA-positive cells in the DG [32]. In contrast, a transient increase of PCNA-positive as well as in BrdU-positive profiles was observed in a study using a total dose of 80 mg/kg MPTP [105]. The different MPTP dosages may account for these differences. Nigral 6-OHDA injections, however, decrease both the proliferation of aNSCs in the DG and the survival of newly generated granule neurons [103].
Besides the SN, the raphe nuclei are among the brainstem regions to be affected early in PD [3]. Serotonergic input from the dorsal nuclei of raphe negatively affects proliferation both in the SGZ and the SVZ [106]. In the SVZ, the serotonin receptors (5-hydroxytryptamine receptors; 5-HT1a and 5-HT2c) mediate this effect, whereas 5-HT1a and 5-HT2a receptors are detected in the hippocampus (reviewed in [107]).
In summary, lesion models ablating two important modulatory systems for the forebrain, namely the dopaminergic and serotonergic system, have provided insight into the regulation of the neurogenic niche, predominantly the SVZ. Dopaminergic deafferentation results in either impaired or unchanged precursor cell proliferation in the SVZ and an increased number of dopaminergic neurons in the glomerular cell layer of the OB. These effects are most likely mediated via D2LRs and might be reversed by either EGF, a combination of EGF and FGF-2, or dopamine agonists. In addition, serotonergic deafferentation reduces proliferation of aNSCs in the SVZ and the hippocampus.
Acute lesions, however, induce a sudden and severe neurotransmitter deficit, thus not mirroring the slowly progressing nature of neurodegenerative processes in PD. Moreover, these models are not recapitulating processes associated with alpha-synuclein aggregation.
Transgenic animal models
Transgenic PD mouse models expressing alpha-synuclein under the control of different promoters have been studied and reviewed (see [108, 109]). The majority of these models capture alpha-synuclein-associated pathology, and feature specific functional non-motor deficits observed in PD, but at present are not able to produce a severe dopaminergic deficit within the nigrostriatal system. In contrast to acute lesion models, current transgenic animals do not result in a severe motor phenotype. Nevertheless, these models offer important insights into cellular and molecular mechanisms associated with alpha-synuclein, significantly contributing to neuronal dysfunction in PD.
Adult neurogenesis has been studied in transgenic mice expressing human wild-type or A53T mutant alpha-synuclein under the control of the platelet-derived growth factor β (PDGF)-promoter [110, 111] and A53T mutant alpha-synuclein under the control of the calcium/calmodulin-dependent kinase II alpha (CaMKIIα)-promoter [112]. Moreover, conditional mouse models expressing wild-type human alpha-synuclein or A30P mutant alpha-synuclein under the control of the CaMKIIα promoter allow a transient suppression of transgene expression, prior to the analysis of adult neurogenesis [113]. In addition to alpha-synuclein transgenic mice, mice overexpressing mutant LRRK2 have been evaluated for changes in adult neurogenesis.
Transgenic mice overexpressing wild-type alpha-synuclein under the control of the PDGF-promoter develop intracytoplasmic alpha-synuclein inclusions 2 months after birth, and show unchanged proliferation in both neurogenic regions at an age of 4 months. However, an impaired survival of newborn neurons, with a concomitant increase in cell death was observed in both neurogenic regions [110, 111] (Figs. 1e, 2). In the hippocampus, alpha-synuclein is expressed in DCX-positive adult neural precursors throughout maturation but also in target regions of adult neurogenesis with transgene expression in the hippocampal CA3 region and the OB [114] (Fig. 2). Thus, impaired adult neurogenesis in these mice may be due to cell-autonomous or cell-non-autonomous effects of the transgene. Specific cell-autonomous overexpression of alpha-synuclein in SVZ-derived progenitor cells by retroviral injections resulted in reduced proliferation and reduced the speed of migration, but did not affect the survival of newborn neurons [115].
In mice overexpressing human A53T mutant alpha-synuclein, adult neurogenesis was even more impaired than in wild-type alpha-synuclein-expressing animals [116]. Furthermore, an age-dependent reduction in proliferation and a consecutively reduced survival of new neurons in the SVZ of these animals point towards additional toxicity of A53T alpha-synuclein during ageing [116]. In the SGZ, these animals already show defective proliferation at 4 months of age which is completely reversed by treatment with the selective serotonin reuptake inhibitor fluoxetin [117]. Mice expressing A53T mutant alpha-synuclein under the control of the CaMKII promoter express the transgene within dentate granule cells and already show decreased numbers of granule neurons 3 weeks after birth, accompanied by a loss of presynaptic markers indicating synaptic disintegration [112].
Conditional mouse models of alpha-synucleinopathy, expressing tet-regulatable human wild-type alpha-synuclein under control of the murine CaMKIIα promoter, showed nigral and hippocampal neurodegeneration, along with neurochemical changes and impaired motor performance upon ageing [113]. The survival of adult newborn neurons in the OB was decreased in human A30P-mutant alpha-synuclein overexpressing mice [113, 118]). The deficit in OB neurogenesis of A30P alpha-synuclein mice was prevented by suppression of the transgene expression starting 1 month before BrdU application (Fig. 1f). This regulation directly correlates the vulnerability of newborn neurons to the presence of human alpha-synuclein. Furthermore, the late induction of alpha-synuclein expression by the CaMKIIα promoter in these models indicates a defect at the integration site of newborn neurons [118]. In conditional wild-type alpha-synuclein overexpressing mice, impaired hippocampal neurogenesis was also restored [113] (Fig. 2).
Currently, the exact mechanisms and factors interfering with proliferation or integration are under investigation. Defective Notch signaling induced by overexpression of human alpha-synuclein might be one factor that influences integration of the newly generated neurons’ arborization [119]. The role of Notch signaling in dendritic arborization substantiates this hypothesis [120].
In summary, these studies show a detrimental effect of human alpha-synuclein on the generation and the survival of newly generated neurons, depending either on alpha-synuclein accumulation at the expression site or the endogenous expression during proliferation, maturation, and integration.
When interpreting these data, one has to keep in mind that the physiological function of alpha-synuclein is still not fully understood. No phenotype was detected in inbred mice with a spontaneous deletion of the alpha-synuclein gene [121, 122], and mice with a single knockout of alpha-synuclein or a double knockout of alpha- and beta-synuclein merely showed minor differences in the dopaminergic system [123, 124]. Compensatory mechanisms during development may explain the absence of a severe phenotype. First, upregulation of other members of the synuclein protein family might counterbalance the lack of alpha-synuclein because triple-knockout of alpha-, beta- and gamma-synuclein resulted in a clear, age-dependent phenotype with shortened life-span, reduced synaptic size, and retinal dysfunction [125]. Second, RNAi-mediated knock-down of alpha-synuclein in naïve mice leads to synaptic changes in vitro and to nigral neurodegeneration in vivo [126]. Still, alpha-synuclein knockout mice (and alpha-synuclein-RNAi treated mice) are less sensitive to dopaminergic toxins like MPTP and 6-OHDA [127–134]. These data suggest a modulatory function of alpha-synuclein in the presynaptic compartment of dopaminergic nerve terminals and a protective role in respect to mitochondrial toxins. Therefore, it will be of interest to study adult neurogenesis in synuclein-knockout and -transgenic mice in conjunction with mitochondrial or inflammatory damage.
Leucin-rich repeat kinase 2 mutations are the most frequent cause of autosomal dominant PD. Animal models overexpressing human G2019S mutant LRRK2 show altered dopamine levels and abnormal exploratory behaviors with increased path length, and altered thigmotaxis indicating increased anxiety [135]. High expression of LRRK2 was present within all neurogenic regions. Accordingly, proliferation and survival were markedly reduced [136] (Figs. 1g, 2). The reduced survival of hippocampal neuroblasts observed in LRRK2 transgenic mice was only partly reversed by physical activity known to be one of the most potent stimulus-enhancing adult hippocampal neurogenesis [136]. Consistent with previous reports that wild-type LRRK2 plays an important role in neurite outgrowth, dendritogenesis, and axon guidance [137], dendritic development of newborn neuronal progenitors was severely altered in transgenic mice [136] (Fig. 2).
In summary, transgenic models have provided insights into the dysregulation of precursor cells and show consistent effects. Important caveats of these studies include the usage of numerous mice strains with both different levels of transgene expression and different promoters, resulting in a diverse transgenic expression pattern. Cell-autonomous effects (i.e., caused by transgene expression within the newborn cell) and cell-non-autonomous effects (i.e., caused by transgene expression in the environment: extracellular, glial or pre-/postsynaptic) as well as underlying molecular mechanisms have to be further delineated in future studies. This will also facilitate the development of potentially therapeutic strategies aimed at reversing an impaired adult neurogenesis.
Orchestration of adult neurogenesis in disease
Considerable efforts have been undertaken to interfere with the proliferative capacity within the neurogenic niche, to direct migration of neuronal precursors towards diseased regions, and to promote the integration of new neurons. In a physiological context, proliferation in the SGZ is increased by physical activity like running [138, 139]. The survival of new neurons is promoted by an enriched environment [140] and spatial learning tasks [141]. Among the pathologic conditions, seizures have been identified as robustly promoting proliferation [142]. Pharmacologically, infusion of platelet-derived growth factor β and brain-derived growth factor increases cell proliferation and enhances integration [143]. Furthermore, epidermal growth factor (EGF) and fibroblast growth factor-2 (FGF-2) infusion into the ventricular system is able to replace lost pyramidal neurons in the CA1 region, contributing to functional recovery [144]. Although directed migration has not been achieved so far, some progenitor cells migrate from the SVZ into the damaged striatum after cerebral ischemia [145–147]. Although adult neurogenesis is precisely regulated, these studies indicate that it may be stimulated and directed to lesioned areas. Thus, adult neurogenesis may replace neurons outside their a priori target region. However, only limited success for the integration of newly generated cells has been observed for the lesioned striatum [146, 148].
An alternative putative mechanism of cell replacement is the stimulation of locally residing quiescent neural precursors. From numerous brain regions, neural precursor cells have been generated in vitro and differentiated into glial and neuronal cells [149]. In this regard, an early report about adult neurogenesis in the substantia nigra raised the possibility of local endogenous cellular replacement [150]. However, several follow-up studies were unable to confirm these initial results [151–155]. Thus, the existence of adult neurogenesis in the SN appears less likely. However, SN-derived progenitor cells propagated by growth factors in vitro differentiated into new neurons after retransplantation into the hippocampus, but not into the SN [152]. A characterization of the anti-neurogenic microenvironment in the SN is still elusive [156]. After dopaminergic lesion, D3 receptor activation by continuous intracerebroventricular administration of 7-OH-DPAT led to the observation of newborn nigral neurons after 8 weeks [157, 158]. This correlated with functional and structural recovery, but more studies are needed to define the phenotype of these newborn neurons.
Within recent years, important roles of glial cells have been emerging for adult neurogenesis and PD. The two adult neurogenic niches which exclusively allow neuronal production in an adult environment are tightly regulated by a complex interplay of many different factors that are far from being fully understood. In this regard, glial cells play a key role. First, astrocytes represent an important cellular component of the niche [28] in conjunction with endothelial cells and regulate the composition of extracellular soluble factors with direct influence on aNSCs [159, 160]. In PD models, on the other hand, astrocytes are activated by alpha-synuclein [15, 16, 161], and alpha-synuclein propagates from neurons to astroglia [12]. The interplay of astrocytes and microglia is highly relevant in dopaminergic neuronal survival [162, 163]. Inflammatory responses and the activation of microglia in an MPTP-model of PD are partly mediated by the chemokine receptor CX3CR1 [163], which is also a known regulator of adult neurogenesis [164]. Indeed, microglial cells have been described to regulate adult neurogenesis in the non-inflammatory state as well as in inflammation, and may exert distinct effects on aNSCs [165]. This highlights the necessity of a detailed analysis of glial cells in the adult neurogenic niche of PD patients and PD models.
In addition, astrocytes are abundantly present in most brain regions and may constitute a source for cell replacement strategies: in vitro, they could be trans-differentiated into electrophysiologically functional neurons upon overexpression of neurogenic transcription factors [166]. Considering that in vitro trans-differentiation of fibroblasts into dopaminergic neurons has been established [167], corresponding in vivo studies will need to address feasibility and security of this kind of trans-differentiation approach.
A putative model for the regulation of adult neurogenesis during progression of PD
The vast majority of in vivo studies of adult neurogenesis in animal models of PD provide insight into the affection of neurogenic regions using distinct experimental settings (i.e., lesion or specific transgene expression). The absence of an “ideal” animal model for PD, that features a progressive, spreading phenotype with the affection of multiple neurotransmitter systems, currently hinders the precise analysis of the dynamics of adult neurogenesis upon ageing and disease progression. Nevertheless, combining the results of preclinical rodent studies in conjunction with postmortem human pathology allows the proposing of a putative model for the role of adult neurogenesis in PD (Fig. 3).
Fig. 3.
Adult neurogenesis in Parkinson’s disease (PD). According to Braak stages of Lewy pathology in PD, alpha-synuclein pathology leads to neurodegeneration in distinct brain regions and progresses along the neuraxis upon disease progression. These stages and respective brain regions are depicted in columns 1 and 2. Respective stages are color-coded and shown in a schematic drawing of the human brain. Stage I is shown in red, stages II and III are in light red, stages IV and V are in yellow. Each stage leads to distinct neuropathological events, summarized in column 3. Adult neurogenesis in the subventricular zone/olfactory bulb system (SVZ/OB) features the proliferation of stem cells in the SVZ of the lateral ventricular wall (small green dots), the migration of neuroblasts along the rostral migratory stream (blue arrow) and their integration into the OB (blue dots). Hippocampal neurogenesis takes place in the dentate gyrus of the hippocampus in the medial temporal lobe (simplified by the large green stem cell and the orange mature granule neuron). Neuropathological changes in PD likely influence adult neurogenesis in the SVZ/OB and in the dentate gyrus of the hippocampus (column 4). Studies describing the proposed alterations of adult neurogenesis in PD are summarized in column 5
In PD, pathological alpha-synuclein accumulation in the central nervous system may first occur in the OB and lower brainstem regions (Braak stage I; Fig. 3). Upon the transition of premotor into motor PD, Lewy pathology emerges in midbrain regions like the SN (Braak stages II and III; Fig. 3). Underlying neurodegenerative processes influence the proper modulatory function of neurotransmitter systems. As sequelae, proliferation in both neurogenic regions may be affected (Fig. 3).
One frequently observed premotor symptom in PD is hyposmia with a severe affection of olfactory discrimination [72]. The increased numbers of dopaminergic neurons in the OB after dopaminergic deafferentation of the SVZ parallels postmortem studies in the OB of PD patients [80, 81, 95]. This may explain the proportional increase of dopaminergic neurons that is only present in the OB of PD patients. In this context, it is noteworthy that glomerular dopaminergic neurons, in contrast to nigral dopaminergic neurons, are specialized interneurons with short neurites, dendritodendritic synapses, and the coexpression of dopaminergic as well as GABAergic markers. This increase in dopaminergic neurons may interfere with proper odor discrimination. However, local overexpression of alpha-synuclein in the glomerular cell layer predominantly in dopaminergic neurons results in decreased dopaminergic glomerular neurogenesis [118]. Interestingly, comparable expression pattern in the OB leads to astrogliosis and neuroinflammation resulting in reduced dopamine neurotransmitter levels in the OB without a loss in TH-positive neurons [168] (Braak stage I; Fig. 3). Therefore, local alpha-synuclein expression in the OB and effects of dopaminergic deafferentation of the SVZ may counterbalance each other during the course of PD. Additional studies are necessary to dissociate the interplay of overexpression of alpha-synuclein in the OB and dopaminergic deafferentation in the SVZ.
Besides hyposmia, anxiety and depression are common neuropsychological features in the premotor phase of PD. In general, these neuropsychiatric symptoms have been correlated with defective adult neurogenesis in non-PD animal models. Lesion models characterized by the loss of dopaminergic and serotonergic projections from the SN and the raphe nuclei to the hippocampus show reduced proliferation of aNSCs. Interestingly, specific ablation of hippocampal neurogenesis induces anxiety-related behavior in mice [65]. However, defective dopaminergic innervation of the dorsal striatum might also play an important role in establishing anxiety-related behaviors [169]. Thus, it seems likely that impaired adult neurogenesis, in addition to compromised striatal neuronal circuities, contributes to the development of these neuropsychiatric symptoms during the premotor phase of PD (Braak stages II and III; Fig. 3).
Upon disease progression, alpha-synuclein pathology progresses to meso- and allocortical regions reaching the hippocampus (Braak stages IV and V; Fig. 3). In addition to motor symptoms resulting from progressive nigrostriatal degeneration, the initial mild cognitive impairment of premotor PD often progresses and cognitive impairment correlates with allocortical alpha-synuclein pathology [170]. At this later stage, excessive alpha-synuclein expression in target regions of adult neurogenesis like the CA3 region might hinder proper axonal integration of newly generated neurons (Braak stages IV and V; Fig. 3).
Of course, the question arises how anxiety, depression, and cognitive impairment as early PD symptoms may be related to or influenced by impaired hippocampal neurogenesis. First, distinct connections of the ventral and the dorsal hippocampus may contribute separately to these symptoms, since the ventral hippocampus is involved instead in affective behavioral changes, whereas the dorsal hippocampus might preferentially regulate learning and memory [171]. Moreover, it is noteworthy that adult neurogenesis-dependent memory performance is highly variable depending crucially on the different maturation stages of the newly generated cells [21]. Thus, it is crucial to investigate the modulation of adult neurogenesis more precisely. Perturbations of post- and presynaptic integration might cause functional impairment of newborn neurons besides quantitative reduction by decreased proliferation resulting from deafferentation in both the SVZ and the SGZ [172]. We hypothesize that deafferentation accounts for alterations in emotional behavior, whereas perturbed integration of adult-born neurons plays a more important role in cognitive processing. In this regard, behavioral studies assessing anxiety-related behavior and memory function in toxic as well as transgenic animal models are crucial to dissociate the precise contribution of defective proliferation and/or integration to certain behavioral aspects of the disease. Currently, the description of the precise role of adult hippocampal neurogenesis in humans is still lacking. Thus, research on the contribution of adult neurogenesis to anxiety, depression, and cognitive impairment in PD patients still requires a strong translational effort.
Endogenous cellular replacement using adult neurogenesis: a potential target for future therapies?
In PD, the survival of newly generated neurons is decreased both in the OB and the DG. Adult neurogenesis is vulnerable to deafferentation of serotonergic and dopaminergic modulatory inputs as well as to alpha-synuclein expression. It is currently unclear if this results in a robust decrease in proliferation or does not affect proliferation at all, but a lack of site-specific integration of newly generated cells appears very likely. If proliferation was unchanged upon dopaminergic or serotonergic deafferentation, the capacity of the neurogenic niche may be more easily accessible, whereas if proliferation was affected, additional pharmacological efforts with currently undetermined growth factor/neurotransmitter combinations would be needed to enable endogenous cell replacement.
The mechanisms underlying the effect of alpha-synuclein at the integration sites remains still unclear, but this knowledge is crucial for the implementation of endogenous cell replacement in PD [173]. The LB-like pathology in grafted tissue in PD patients [13, 14] emphasizes the relevance to further study the initial steps that influence the integration of new cells into a hostile pathogenic environment.
Many factors are regulating neurogenesis in animal models of PD. This is reflected by the enormous complexity of the neurogenic niche. In addition, alterations within the neurogenic niche might change upon disease progression. As an example, the lack of olfactory signals from the OB, detected early in the course of PD, might also influence hippocampal neurogenesis causing an opposite effect than the compromised dopaminergic and serotonergic input into the hippocampus [174]. Alpha-synuclein accumulation in neurogenic regions is an important pathological event occurring later in the course of the disease. This further hinders neurogenic processes most likely via a negative influence on the integration of the newly generated neurons. Furthermore, propagation of alpha-synuclein is another putative mechanism that influences adult neurogenesis by inducing alpha-synuclein spreading to aNSCs or young progenitor cells. The mechanisms by which alpha-synuclein influences adult neurogenesis either directly or indirectly via defective signaling require future analysis. In contrast to this complex interplay of multiple factors, the rescue of adult neurogenesis after blocking alpha-synclein expression in both neurogenic regions [113, 118] defines alpha-synuclein as an important target to restore impaired adult neurogenesis in PD.
Acknowledgments
This study was supported by the Bavarian State Ministry of Sciences, Research and the Arts, ForNeuroCell (J.W.; Erlangen, Germany), the Elite Network Bavaria (F.M., M.R., J.W.), and the Adalbert-Raps-Foundation (J.W.). F.M. is supported by the Interdisciplinary Center for Clinical Research (IZKF) Erlangen. The authors thank Julius Ecke for the graphic artwork.
Conflict of interest
None of the author has to declare a conflict of interest.
References
- 1.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7(4):306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 2.Tolosa E, Poewe W. Premotor Parkinson disease. Neurology. 2009;72(7 Suppl):S1. doi: 10.1212/wnl.0b013e318198dace. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 4.O’Sullivan SS, Williams DR, Gallagher DA, Massey LA, Silveira-Moriyama L, Lees AJ. Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov Disord. 2008;23(1):101–106. doi: 10.1002/mds.21813. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA. 2000;97(2):571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477(7362):107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desplats P, Lee H-J, Bae E-J, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee S-J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27(34):9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winner B, Jappelli R, Maji SK, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108(10):4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danzer KM, Krebs SK, Wolff M, Birk G, Hengerer B. Seeding induced by α-synuclein oligomers provides evidence for spreading of α-synuclein pathology. J Neurochem. 2009;111(1):192–203. doi: 10.1111/j.1471-4159.2009.06324.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee H-J, Suk J-E, Patrick C, Bae E-J, Cho J-H, Rho S, Hwang D, Masliah E, Lee S-J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285(12):9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J-Y, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 14.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14(5):504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 15.McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23(4):474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 16.Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience. 1993;52(1):1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8(4):382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 18.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137(4):433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 19.Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 21.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 22.Mu Y, Lee SW, Gage FH. Signaling in adult neurogenesis. Curr Opin Neurobiol. 2010;20(4):416–423. doi: 10.1016/j.conb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia ADR, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7(11):1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 24.Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2 + neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1(5):515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13(5):543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Corotto FS, Henegar JR, Maruniak JA. Odor deprivation leads to reduced neurogenesis and reduced neuronal survival in the olfactory bulb of the adult mouse. Neuroscience. 1994;61(4):739–744. doi: 10.1016/0306-4522(94)90397-2. [DOI] [PubMed] [Google Scholar]
- 27.Sanai N, Nguyen T, Ihrie RA, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478(7369):382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doetsch F, Caillé I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 29.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 31.Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Götz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6(5):445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Höglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7(7):726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 33.Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of double cortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 34.Tomasiewicz H, Ono K, Yee D, Thompson C, Goridis C, Rutishauser U, Magnuson T. Genetic deletion of a neural cell adhesion molecule variant (N-CAM-180) produces distinct defects in the central nervous system. Neuron. 1993;11(6):1163–1174. doi: 10.1016/0896-6273(93)90228-j. [DOI] [PubMed] [Google Scholar]
- 35.Seki T, Arai Y. Temporal and spacial relationships between PSA-NCAM-expressing, newly generated granule cells, and radial glia-like cells in the adult dentate gyrus. J Comp Neurol. 1999;410(3):503–513. doi: 10.1002/(sici)1096-9861(19990802)410:3<503::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 36.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11(1):173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 37.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 38.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271(5251):978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez-Buylla A, Garcia-Verdugo J-M. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winner B, Cooper-Kuhn CM, Aigner R, Winkler J, Kuhn HG. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur J Neurosci. 2002;16(9):1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- 41.Sandler R, Smith AD. Coexistence of GABA and glutamate in mossy fiber terminals of the primate hippocampus: an ultrastructural study. J Comp Neurol. 1991;303(2):177–192. doi: 10.1002/cne.903030202. [DOI] [PubMed] [Google Scholar]
- 42.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15(6):4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge S, Yang C-H, Hsu K-S, Ming G-L, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biebl M, Winner B, Winkler J. Caspase inhibition decreases cell death in regions of adult neurogenesis. NeuroReport. 2005;16(11):1147–1150. doi: 10.1097/00001756-200508010-00003. [DOI] [PubMed] [Google Scholar]
- 46.Lazarini F, Lledo P-M. Is adult neurogenesis essential for olfaction? Trends Neurosci. 2011;34(1):20–30. doi: 10.1016/j.tins.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Mouret A, Gheusi G, Gabellec M–M, de Chaumont F, Olivo-Marin J-C, Lledo P-M. Learning and survival of newly generated neurons: when time matters. J Neurosci. 2008;28(45):11511–11516. doi: 10.1523/JNEUROSCI.2954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alonso M, Viollet C, Gabellec M–M, Meas-Yedid V, Olivo-Marin J-C, Lledo P-M. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci. 2006;26(41):10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandairon N, Linster C. Odor perception and olfactory bulb plasticity in adult mammals. J Neurophysiol. 2009;101(5):2204–2209. doi: 10.1152/jn.00076.2009. [DOI] [PubMed] [Google Scholar]
- 50.Sultan S, Mandairon N, Kermen F, Garcia S, Sacquet J, Didier A. Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory memory. FASEB J. 2010;24(7):2355–2363. doi: 10.1096/fj.09-151456. [DOI] [PubMed] [Google Scholar]
- 51.Valley MT, Mullen TR, Schultz LC, Sagdullaev BT, Firestein S. Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning. Front Neurosci. 2009;3:51. doi: 10.3389/neuro.22.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazarini F, Mouthon M-A, Gheusi G, de Chaumont F, Olivo-Marin J-C, Lamarque S, Abrous DN, Boussin FD, Lledo P-M. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One. 2009;4(9):e7017. doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nissant A, Bardy C, Katagiri H, Murray K, Lledo P-M. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat Neurosci. 2009;12(6):728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- 54.Kelsch W, Lin C-W, Mosley CP, Lois C. A critical period for activity-dependent synaptic development during olfactory bulb adult neurogenesis. J Neurosci. 2009;29(38):11852–11858. doi: 10.1523/JNEUROSCI.2406-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feierstein CE, Lazarini F, Wagner S, Gabellec M–M, de Chaumont F, Olivo-Marin J-C, Boussin FD, Lledo P-M, Gheusi G. Disruption of adult neurogenesis in the olfactory bulb affects social interaction but not maternal behavior. Front Behav Neurosci. 2010;4:176. doi: 10.3389/fnbeh.2010.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, Hummel T. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience. 2010;169(1):415–421. doi: 10.1016/j.neuroscience.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Yang D, Li Q, Fang L, Cheng K, Zhang R, Zheng P, Zhan Q, Qi Z, Zhong S, Xie P. Reduced neurogenesis and pre-synaptic dysfunction in the olfactory bulb of a rat model of depression. Neuroscience. 2011;192:609–618. doi: 10.1016/j.neuroscience.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 58.Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75(2):143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274(5293):1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 60.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 61.Clelland CD, Choi M, Romberg C, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dranovsky A, Picchini AM, Moadel T, Sisti AC, Yamada A, Kimura S, Leonardo ED, Hen R. Experience dictates stem cell fate in the adult hippocampus. Neuron. 2011;70(5):908–923. doi: 10.1016/j.neuron.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papez JW. Connections of the pulvinar. Arch Neurol Psychiatry. 1939;41(2):277–289. [Google Scholar]
- 65.Revest J-M, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza P-V, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14(10):959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- 66.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manev H, Uz T, Smalheiser NR, Manev R. Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur J Pharmacol. 2001;411(1–2):67–70. doi: 10.1016/s0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- 68.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 69.Perera TD, Coplan JD, Lisanby SH, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27(18):4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64(4):293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 71.Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34(11):2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winkler J, Ehret R, Büttner T, Dillmann U, Fogel W, Sabolek M, Winkelmann J, Kassubek J. Parkinson’s disease risk score: moving to a premotor diagnosis. J Neurol. 2011;258(Suppl 2):S311–S315. doi: 10.1007/s00415-011-5952-x. [DOI] [PubMed] [Google Scholar]
- 73.Shiba M, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Anxiety disorders and depressive disorders preceding Parkinson’s disease: a case-control study. Mov Disord. 2000;15(4):669–677. doi: 10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 74.Jellinger KA. Neuropathology of sporadic Parkinson’s disease: evaluation and changes of concepts. Mov Disord. 2012;27(1):8–30. doi: 10.1002/mds.23795. [DOI] [PubMed] [Google Scholar]
- 75.Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20(2):575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- 76.O’Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci USA. 2009;106(21):8754–8759. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iwakura Y, Piao Y-S, Mizuno M, Takei N, Kakita A, Takahashi H, Nawa H. Influences of dopaminergic lesion on epidermal growth factor-ErbB signals in Parkinson’s disease and its model: neurotrophic implication in nigrostriatal neurons. J Neurochem. 2005;93(4):974–983. doi: 10.1111/j.1471-4159.2005.03073.x. [DOI] [PubMed] [Google Scholar]
- 78.van den Berge SA, van Strien ME, Korecka JA, et al. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain. 2011;134(Pt 11):3249–3263. doi: 10.1093/brain/awr256. [DOI] [PubMed] [Google Scholar]
- 79.Brodoehl S, Klingner C, Volk GF, Bitter T, Witte OW, Redecker C (2012) Decreased olfactory bulb volume in idiopathic parkinson's disease detected by 3.0-tesla magnetic resonance imaging. Mov Disord [Epub ahead of print] [DOI] [PubMed]
- 80.Mundiñano I-C, Caballero M-C, Ordóñez C, Hernandez M, DiCaudo C, Marcilla I, Erro M-E, Tuñon M-T, Luquin M-R. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. 2011;122(1):61–74. doi: 10.1007/s00401-011-0830-2. [DOI] [PubMed] [Google Scholar]
- 81.Huisman E, Uylings HBM, Hoogland PV. A 100 % increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov Disord. 2004;19(6):687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- 82.Huisman E, Uylings HBM, Hoogland PV. Gender-related changes in increase of dopaminergic neurons in the olfactory bulb of Parkinson’s disease patients. Mov Disord. 2008;23(10):1407–1413. doi: 10.1002/mds.22009. [DOI] [PubMed] [Google Scholar]
- 83.Freundlieb N, François C, Tandé D, Oertel WH, Hirsch EC, Höglinger GU. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci. 2006;26(8):2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318(1):215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- 85.Lennington JB, Pope S, Goodheart AE, Drozdowicz L, Daniels SB, Salamone JD, Conover JC. Midbrain dopamine neurons associated with reward processing innervate the neurogenic subventricular zone. J Neurosci. 2011;31(37):13078–13087. doi: 10.1523/JNEUROSCI.1197-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coronas V, Bantubungi K, Fombonne J, Krantic S, Schiffmann SN, Roger M. Dopamine D3 receptor stimulation promotes the proliferation of cells derived from the post-natal subventricular zone. J Neurochem. 2004;91(6):1292–1301. doi: 10.1111/j.1471-4159.2004.02823.x. [DOI] [PubMed] [Google Scholar]
- 87.Winner B, Geyer M, Couillard-Despres S, Aigner R, Bogdahn U, Aigner L, Kuhn G, Winkler J. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp Neurol. 2006;197(1):113–121. doi: 10.1016/j.expneurol.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 88.Winner B, Desplats P, Hagl C, et al. Dopamine receptor activation promotes adult neurogenesis in an acute Parkinson model. Exp Neurol. 2009;219(2):543–552. doi: 10.1016/j.expneurol.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baker SA, Baker KA, Hagg T. D3 dopamine receptors do not regulate neurogenesis in the subventricular zone of adult mice. Neurobiol Dis. 2005;18(3):523–527. doi: 10.1016/j.nbd.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 90.Dawirs RR, Hildebrandt K, Teuchert-Noodt G. Adult treatment with haloperidol increases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm. 1998;105(2–3):317–327. doi: 10.1007/s007020050061. [DOI] [PubMed] [Google Scholar]
- 91.Kippin TE, Kapur S, van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci. 2005;25(24):5815–5823. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aponso PM, Faull RLM, Connor B. Increased progenitor cell proliferation and astrogenesis in the partial progressive 6-hydroxydopamine model of Parkinson’s disease. Neuroscience. 2008;151(4):1142–1153. doi: 10.1016/j.neuroscience.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 93.Liu BF, Gao EJ, Zeng XZ, Ji M, Cai Q, Lu Q, Yang H, Xu QY. Proliferation of neural precursors in the subventricular zone after chemical lesions of the nigrostriatal pathway in rat brain. Brain Res. 2006;1106(1):30–39. doi: 10.1016/j.brainres.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 94.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17(15):5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Winner B, Couillard-Despres S, Geyer M, Aigner R, Bogdahn U, Aigner L, Kuhn HG, Winkler J. Dopaminergic lesion enhances growth factor-induced striatal neuroblast migration. J Neuropathol Exp Neurol. 2008;67(2):105–116. doi: 10.1097/nen.0b013e3181630cff. [DOI] [PubMed] [Google Scholar]
- 96.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 97.Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4(3):257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 98.Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav Brain Res. 2001;125(1–2):109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 99.Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77(4):1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- 100.Yamada M, Onodera M, Mizuno Y, Mochizuki H. Neurogenesis in olfactory bulb identified by retroviral labeling in normal and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated adult mice. Neuroscience. 2004;124(1):173–181. doi: 10.1016/j.neuroscience.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 101.Iancu R, Mohapel P, Brundin P, Paul G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res. 2005;162(1):1–10. doi: 10.1016/j.bbr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 102.Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(1):1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki K, Okada K, Wakuda T, et al. Destruction of dopaminergic neurons in the midbrain by 6-hydroxydopamine decreases hippocampal cell proliferation in rats: reversal by fluoxetine. PLoS One. 2010;5(2):e9260. doi: 10.1371/journal.pone.0009260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mu Y, Zhao C, Gage FH. Dopaminergic modulation of cortical inputs during maturation of adult-born dentate granule cells. J Neurosci. 2011;31(11):4113–4123. doi: 10.1523/JNEUROSCI.4913-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park J-H, Enikolopov G. Transient elevation of adult hippocampal neurogenesis after dopamine depletion. Exp Neurol. 2010;222(2):267–276. doi: 10.1016/j.expneurol.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89(4):999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- 107.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25(1):253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 108.Melrose HL, Lincoln SJ, Tyndall GM, Farrer MJ. Parkinson’s disease: a rethink of rodent models. Exp Brain Res. 2006;173(2):196–204. doi: 10.1007/s00221-006-0461-3. [DOI] [PubMed] [Google Scholar]
- 109.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron. 2010;66(5):646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287(5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 111.Winner B, Lie DC, Rockenstein E, Aigner R, Aigner L, Masliah E, Kuhn HG, Winkler J. Human wild-type alpha-synuclein impairs neurogenesis. J Neuropathol Exp Neurol. 2004;63(11):1155–1166. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- 112.Lim Y, Kehm VM, Lee EB, Soper JH, Li C, Trojanowski JQ, Lee VM-Y. α-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies. J Neurosci. 2011;31(27):10076–10087. doi: 10.1523/JNEUROSCI.0618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nuber S, Petrasch-Parwez E, Winner B, et al. Neurodegeneration and motor dysfunction in a conditional model of Parkinson’s disease. J Neurosci. 2008;28(10):2471–2484. doi: 10.1523/JNEUROSCI.3040-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68(5):568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- 115.Tani M, Hayakawa H, Yasuda T, Nihira T, Hattori N, Mizuno Y, Mochizuki H. Ectopic expression of α-synuclein affects the migration of neural stem cells in mouse subventricular zone. J Neurochem. 2010;115(4):854–863. doi: 10.1111/j.1471-4159.2010.06727.x. [DOI] [PubMed] [Google Scholar]
- 116.Winner B, Rockenstein E, Lie DC, Aigner R, Mante M, Bogdahn U, Couillard-Despres S, Masliah E, Winkler J. Mutant alpha-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol Aging. 2008;29(6):913–925. doi: 10.1016/j.neurobiolaging.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kohl Z, Winner B, Ubhi K, Rockenstein E, Mante M, Münch M, Barlow C, Carter T, Masliah E, Winkler J. Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur J Neurosci. 2012;35(1):10–19. doi: 10.1111/j.1460-9568.2011.07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marxreiter F, Nuber S, Kandasamy M, Klucken J, Aigner R, Burgmayer R, Couillard-Despres S, Riess O, Winkler J, Winner B. Changes in adult olfactory bulb neurogenesis in mice expressing the A30P mutant form of alpha-synuclein. Eur J Neurosci. 2009;29(5):879–890. doi: 10.1111/j.1460-9568.2009.06641.x. [DOI] [PubMed] [Google Scholar]
- 119.Crews L, Mizuno H, Desplats P, Rockenstein E, Adame A, Patrick C, Winner B, Winkler J, Masliah E. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci. 2008;28(16):4250–4260. doi: 10.1523/JNEUROSCI.0066-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci USA. 2007;104(51):20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Specht CG, Schoepfer R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6 J inbred mice. BMC Neurosci. 2001;2:11. doi: 10.1186/1471-2202-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen PE, Specht CG, Morris RGM, Schoepfer R. Spatial learning is unimpaired in mice containing a deletion of the alpha-synuclein locus. Eur J Neurosci. 2002;16(1):154–158. doi: 10.1046/j.1460-9568.2002.02062.x. [DOI] [PubMed] [Google Scholar]
- 123.Abeliovich A, Schmitz Y, Fariñas I, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 124.Chandra S, Fornai F, Kwon H-B, et al. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci USA. 2004;101(41):14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Greten-Harrison B, Polydoro M, Morimoto-Tomita M, et al. αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc Natl Acad Sci USA. 2010;107(45):19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gorbatyuk OS, Li S, Nash K, et al. In vivo RNAi-mediated alpha-synuclein silencing induces nigrostriatal degeneration. Mol Ther. 2010;18(8):1450–1457. doi: 10.1038/mt.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dauer W, Kholodilov N, Vila M, et al. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci USA. 2002;99(22):14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schlüter OM, Fornai F, Alessandrí MG, Takamori S, Geppert M, Jahn R, Südhof TC. Role of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neuroscience. 2003;118(4):985–1002. doi: 10.1016/s0306-4522(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 129.Drolet RE, Behrouz B, Lookingland KJ, Goudreau JL. Mice lacking alpha-synuclein have an attenuated loss of striatal dopamine following prolonged chronic MPTP administration. Neurotoxicology. 2004;25(5):761–769. doi: 10.1016/j.neuro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 130.Robertson DC, Schmidt O, Ninkina N, Jones PA, Sharkey J, Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89(5):1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- 131.Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW, Abeliovich A, Beal MF. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis. 2006;21(3):541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 132.Alvarez-Fischer D, Henze C, Strenzke C, Westrich J, Ferger B, Höglinger GU, Oertel WH, Hartmann A. Characterization of the striatal 6-OHDA model of Parkinson’s disease in wild type and alpha-synuclein-deleted mice. Exp Neurol. 2008;210(1):182–193. doi: 10.1016/j.expneurol.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 133.Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. α-Synuclein cooperates with CSPα in preventing neurodegeneration. Cell. 2005;123(3):383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 134.Hayashita-Kinoh H, Yamada M, Yokota T, Mizuno Y, Mochizuki H. Down-regulation of alpha-synuclein expression can rescue dopaminergic cells from cell death in the substantia nigra of Parkinson’s disease rat model. Biochem Biophys Res Commun. 2006;341(4):1088–1095. doi: 10.1016/j.bbrc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 135.Melrose HL, Dächsel JC, Behrouz B, et al. Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol Dis. 2010;40(3):503–517. doi: 10.1016/j.nbd.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Winner B, Melrose HL, Zhao C, et al. Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice. Neurobiol Dis. 2011;41(3):706–716. doi: 10.1016/j.nbd.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sakaguchi-Nakashima A, Meir JY, Jin Y, Matsumoto K, Hisamoto N. LRK-1, a C. elegans PARK8-related kinase, regulates axonal-dendritic polarity of SV proteins. Curr Biol. 2007;17(7):592–598. doi: 10.1016/j.cub.2007.01.074. [DOI] [PubMed] [Google Scholar]
- 138.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 139.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10(3):355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 142.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mohapel P, Frielingsdorf H, Häggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132(3):767–776. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 144.Nakatomi H, Kuriu T, Okabe S, Yamamoto S-I, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 145.Osman AM, Porritt MJ, Nilsson M, Kuhn HG. Long-term stimulation of neural progenitor cell migration after cortical ischemia in mice. Stroke. 2011;42(12):3559–3565. doi: 10.1161/STROKEAHA.111.627802. [DOI] [PubMed] [Google Scholar]
- 146.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 147.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52(6):802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 148.Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24(3):739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 149.Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6(5):474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 150.Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci USA. 2003;100(13):7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kay JN, Blum M. Differential response of ventral midbrain and striatal progenitor cells to lesions of the nigrostriatal dopaminergic projection. Dev Neurosci. 2000;22(1–2):56–67. doi: 10.1159/000017427. [DOI] [PubMed] [Google Scholar]
- 152.Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22(15):6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Frielingsdorf H, Schwarz K, Brundin P, Mohapel P. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc Natl Acad Sci USA. 2004;101(27):10177–10182. doi: 10.1073/pnas.0401229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yoshimi K, Ren Y-R, Seki T, et al. Possibility for neurogenesis in substantia nigra of parkinsonian brain. Ann Neurol. 2005;58(1):31–40. doi: 10.1002/ana.20506. [DOI] [PubMed] [Google Scholar]
- 155.Steiner B, Winter C, Hosman K, Siebert E, Kempermann G, Petrus DS, Kupsch A. Enriched environment induces cellular plasticity in the adult substantia nigra and improves motor behavior function in the 6-OHDA rat model of Parkinson’s disease. Exp Neurol. 2006;199(2):291–300. doi: 10.1016/j.expneurol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 156.Arias-Carrión O, Yamada E, Freundlieb N, et al. Neurogenesis in substantia nigra of parkinsonian brains? J Neural Transm Suppl. 2009;73:279–285. doi: 10.1007/978-3-211-92660-4_23. [DOI] [PubMed] [Google Scholar]
- 157.Van Kampen JM, Robertson HA. A possible role for dopamine D3 receptor stimulation in the induction of neurogenesis in the adult rat substantia nigra. Neuroscience. 2005;136(2):381–386. doi: 10.1016/j.neuroscience.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 158.Van Kampen JM, Eckman CB. Dopamine D3 receptor agonist delivery to a model of Parkinson’s disease restores the nigrostriatal pathway and improves locomotor behavior. J Neurosci. 2006;26(27):7272–7280. doi: 10.1523/JNEUROSCI.0837-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 160.Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15(3):407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Klegeris A, Giasson BI, Zhang H, Maguire J, Pelech S, McGeer PL. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006;20(12):2000–2008. doi: 10.1096/fj.06-6183com. [DOI] [PubMed] [Google Scholar]
- 162.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137(1):47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 164.Bachstetter AD, Morganti JM, Jernberg J, et al. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32(11):2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158(3):1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 166.Heinrich C, Blum R, Gascón S, Masserdotti G, Tripathi P, Sánchez R, Tiedt S, Schroeder T, Götz M, Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8(5):e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim J, Su SC, Wang H, et al. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011;9(5):413–419. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nuber S, Petrasch-Parwez E, Arias-Carrión O, et al. Olfactory neuron-specific expression of A30P alpha-synuclein exacerbates dopamine deficiency and hyperactivity in a novel conditional model of early Parkinson’s disease stages. Neurobiol Dis. 2011;44(2):192–204. doi: 10.1016/j.nbd.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 169.Groman SM, Lee B, London ED, et al. Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. J Neurosci. 2011;31(20):7291–7299. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Braak H, Rüb U, Del Tredici K. Cognitive decline correlates with neuropathological stage in Parkinson’s disease. J Neurol Sci. 2006;248(1–2):255–258. doi: 10.1016/j.jns.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 171.Bannerman DM, Matthews P, Deacon RMJ, Rawlins JNP. Medial septal lesions mimic effects of both selective dorsal and ventral hippocampal lesions. Behav Neurosci. 2004;118(5):1033–1041. doi: 10.1037/0735-7044.118.5.1033. [DOI] [PubMed] [Google Scholar]
- 172.Toni N, Sultan S. Synapse formation on adult-born hippocampal neurons. Eur J Neurosci. 2011;33(6):1062–1068. doi: 10.1111/j.1460-9568.2011.07604.x. [DOI] [PubMed] [Google Scholar]
- 173.Hagell P, Brundin P. Cell survival and clinical outcome following intrastriatal transplantation in Parkinson disease. J Neuropathol Exp Neurol. 2001;60(8):741–752. doi: 10.1093/jnen/60.8.741. [DOI] [PubMed] [Google Scholar]
- 174.Jaako-Movits K, Zharkovsky T, Pedersen M, Zharkovsky A. Decreased hippocampal neurogenesis following olfactory bulbectomy is reversed by repeated citalopram administration. Cell Mol Neurobiol. 2006;26(7–8):1559–1570. doi: 10.1007/s10571-006-9090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]