Abstract
Type 1 diabetes is an autoimmune disease characterized by the destruction of insulin-producing pancreatic β-cells. Even though extensive scientific research has yielded important insights into the immune mechanisms involved in pancreatic β-cell destruction, little is known about the events that trigger the autoimmune process. Recent epidemiological and experimental data suggest that environmental factors are involved in this process. In this review, we discuss the role of viruses as an environmental factor on the development of type 1 diabetes, and the immune mechanisms by which they can trigger or protect against this pathology.
Keywords: Diabetes, Environment, Virus, Coxsackievirus
Introduction
Type 1 diabetes (T1D) is an organ-specific autoimmune disease characterized by the destruction of β-cells within the islets of Langerhans in the pancreas. Once 80–90 % of the β-cells have been destroyed, insulin production becomes insufficient, resulting in hyperglycemia. T1D is considered a childhood disease because most patients develop T1D by 20 years of age, and accounts for 1–5 % of all diabetes cases. Studying diabetes is often difficult because its development is very heterogeneous among patients. In some diabetic patients, it can develop rapidly without clear signs of autoimmunity, such as the presence of autoantibodies [1]. Other patients can have a subclinical phase of various durations, characterized by the presence of autoantibodies and autoreactive T cells recognizing islet antigens before the onset of overt diabetes [2]. Furthermore, some patients can harbor islet autoantibodies for many years without ever progressing to T1D [3]. The circulating autoantibodies and autoreactive T cells mostly target β-cell antigens such as proinsulin, glutamic acid decarboxylase (GAD), tyrosine phosphatase IA-2, islet-specific glucose-6-phosphatase catalytic subunit related protein (IGRP) and chromogranin A [4]. It is not yet clear what initiates the breakdown of tolerance towards β-cells, but genetic and environmental factors have been implicated, both alone and in synergy.
Relationship between genetics, environment, and autoimmune diabetes
The frequency of autoimmune diseases has increased in recent decades. This rise includes both allergic diseases such as asthma, whose incidence has more than doubled since 1980 in the United States of America, rhinitis and atopic dermatitis [5, 6], and autoimmune diseases such as T1D [7, 8], multiple sclerosis [9] and Crohn’s disease [10]. The annual increase in the frequency of T1D is estimated to be 3 % [11], although its global distribution is not homogenous. In fact, the distribution of many autoimmune diseases forms a gradient with the highest frequency in the north, which decreases towards the south of the northern hemisphere and from the south to the north in the southern hemisphere [12]. Such gradients have been observed for multiple sclerosis in the United States of America and Australia [13, 14] and for T1D, with the Canadian province of Newfoundland and Labrador and European countries having the highest rates [15]. In Europe, the highest incidence was observed in Nordic countries [16], with Finland being on top of the list (40.9/100,000/year) followed by Sweden (30/100,000/year) and Norway (20.8/1,000,000/year) while in most Asian countries the incidence was lower than 1/100,000/year. [17]. Genetic and environmental factors have been proposed to explain these differences and are discussed in this review.
Genetics
The development of T1D is under genetic control. Type 1 diabetes is particularly common in families with one or more diabetic siblings or first-degree relatives [18] and there is a high (40–60 %) concordance of diabetes in identical twins [19, 20]. More than 40 disease-susceptibility genetic loci have been identified in T1D, which include the genes coding insulin, cytotoxic T lymphocyte antigen (CTLA)-4, interleukin (IL)-2 receptor a, the tyrosine phosphatase PTPN22 and the intracellular viral RNA sensor IFIH1 [21]. However, the strongest risk for T1D is associated with HLA loci, also known as IDDM1 (insulin-dependent diabetes mellitus locus), particularly the HLA class II DR and DQ alleles, which is found in around 40 % of cases, although these loci can also confer protection against T1D. Interestingly, European countries with the highest incidence of T1D, such as Finland or Sardinia, also have the highest frequency of T1D-predisposing major histocompatibility complex (MHC) class II alleles, HLA DR3/4-DQ8 [22]. However, not all individuals carrying a susceptibility allele develop T1D, and the susceptibility alleles can have different effects in different nations. For example, while DR3/4-DQ8 alleles confer increased risk in Bahraini Arabs, they actually play a neutral role in the Lebanese population [23]. Meanwhile, Japanese individuals carry both susceptibility and protective alleles, and it is the balance between these alleles that contributes to the low incidence of T1D in Asia [24]. Thus, the distribution of different HLA alleles at least partly accounts for the differences in T1D incidence worldwide; however, other factors are also involved. For example, the frequency of susceptibility and protective HLA-DQ alleles is similar among children from Finland and Karelia, a neighboring region in Russia; however, the incidence of T1D in Finland is six times higher than that in Karelia [25]. Similarly, while the frequency of various alleles does not differ between Baltic States and Nordic countries, the incidence of T1D is higher in Nordic countries [26]. There is also a difference in the incidence of T1D between eastern and western Germany, even though both populations share the same genetic background. Another striking observation concerns immigrant families. First-generation Pakistani children born in the United Kingdom have a similar rate of T1D as the local population (11.7/100,000/year), but this is ten times higher than in the incidence in Pakistan (1/100,000/year) [27]. Another recent study showed that children with non-Swedish parents but living in Sweden have an increased risk of T1D compared to children in their native countries [28]. All of these observations support the role of non-genetic factors in the etiology of T1D. Nevertheless, the genetic background is important because the incidence of T1D in Sardinian families migrating to a country with a lower incidence of T1D remains high, similar to that in their native Sardinia [29, 30]. Clearly, genetic susceptibility is a very strong factor underlying the development of T1D, but external factors might play a decisive role in either inducing or protecting against T1D.
Environment
It is clear that genetics is a risk factor that accounts for at least some of the pattern of T1D distribution; however, it cannot explain the rapid worldwide increase estimated to be 3 % per year [31]. If this rise was solely dependent on genes, one would expect an increase in the frequency of predisposing HLA alleles among newly diabetic patients. However, this is not the case. In fact, the frequency of susceptibility alleles has actually decreased while that of protective HLA alleles has increased among newly diagnosed children [32, 33]. Therefore, the role of changing environmental factors has been proposed and examined in epidemiological and animal studies. As a result, epidemiological studies have suggested an association between the incidence of T1D and socio-economic status, which reflects the exposure to microbial agents and dietary habits. Sun exposure and vitamin D intake have also been proposed to influence T1D onset.
Socio-economic status and the role of infectious diseases
The European north–south gradient not only correlated with T1D distribution but also with the degree of development and national growth income of the countries in Europe. The richest and most developed countries have the highest incidence of T1D [34]. There are numerous differences between poor and rich countries, and one particularly interesting finding relevant for T1D is the increase in hygiene and decrease in infection because both phenomena are very recent. Developed countries have better hygiene because they invest in general cleanliness of cities, in education, and in medical care such as vaccination. These approaches have eliminated the favorable niches where pathogens used to proliferate, such as sewage, thus limiting the numbers of pathogens. In addition, if an infection occurs, its spread is often better controlled through greater accessibility to drugs and campaigns aimed at instilling people to follow basic rules of hygiene, such as frequent hand washing. Consequently, people are no longer exposed to the wide variety of pathogens that they used to be, and the age at which children encounter such pathogens has increased. Consistent with these observations, the increasing frequency of immune-mediated diseases such as T1D, allergy, and asthma in Europe has been correlated with the decreasing rate of infections with enteroviruses, tuberculosis, and hepatitis B or C viruses.
In 1989, David Strachan, a scientist studying the relationship between autoimmune hay fever, hygiene, and household factors, proposed that frequent encounters with parasites, bacteria, and viruses in early childhood favor the development of a balanced immune system [35]. Otherwise, the untrained immune system may develop inappropriate immune reactions to the self, thus provoking autoimmune diseases. His proposal was later coined “Hygiene Hypothesis”, and has since been applied to numerous autoimmune and inflammatory diseases including T1D, multiple sclerosis, Crohn’s disease, inflammatory bowel disease, allergy, and asthma. This hypothesis is supported by the observation that, in large families, the incidence of T1D is lower among the youngest children than in their oldest siblings, possibly because the youngest children are more exposed to pathogens brought home by their siblings. Most importantly this happens from a very young age. In a similar way, children who attend daycare less frequently develop autoimmune diseases compared to children who are kept at home and who do not socialize with other children as much.
Protective role of parasitic and bacterial infections
Parasites and bacteria can inhibit the development of T1D in animal models [36]. For example, infection with Schistosoma mansoni or injection of S. mansoni egg soluble antigen (SEA) prevents T1D in non-obese diabetic (NOD) mice [37]. Treatment with SEA induces a shift in the cytokine profile of dendritic cells (DCs) that produce less pro-inflammatory interleukin IL-12 and more suppressive tumor growth factor (TGF)-β. Schistosoma mansoni soluble egg antigen also promotes the differentiation of type 2 macrophages and the skewing of T lymphocytes towards the production of IL-4 and IL-10 [38]. Gastrointestinal parasites, such as Trichinella spiralis or Heligmosomoides polygyrus, can also inhibit the development of diabetes in NOD mice by diminishing insulitis, inducing the secretion of cytokines such as IL-4, IL-10, and IL-13, and skewing T lymphocyte responses towards a T helper (Th)2 profile [39, 40]. Infection with the nematode filarial activates Th2 T cells and FoxP3+ Tregs and protects NOD mice from T1D [41]. These mice are also protected from T1D by Salmonella infection, which upregulates the inhibitory programmed cell death one ligand one (PD-L1) receptor [42]. Recent studies have also highlighted the role of the gut microbiota in regulating the development of T1D in NOD mice [43]. Infection with a laboratory strain of Mycobacterium avium induced the expression of the death receptor Fas on autoreactive T lymphocytes, which enhanced their killing by other immune cells [44, 45]. Interestingly, the M. avium subspecies paratuberculosis is currently a focus of research as a possible trigger of T1D in humans [46–48]. Since many pathogens can prevent T1D, elucidation of the underlying mechanisms will provide new knowledge that could be used to develop therapeutic strategies to protect against T1D.
Cow’s milk
Early introduction of cow’s milk into the diet of children has been proposed to trigger T1D. Enhanced expression of antibodies to cow’s milk was observed in children who later develop T1D [49]. Elliott et al. [50] compared the consumption of milk proteins in 14 different countries and found that the incidence of diabetes increased with increasing consumption of milk protein β-casein A1 and the B variants, with Nordic countries having the highest intake. Iceland, where the consumption of these two proteins is lower than in Nordic countries, has a lower incidence of T1D despite similar environmental conditions [51]. Other milk proteins such as lactoferrin, bovine serum albumin, and immunoglobulin against bovine insulin are considered harmless [52]. However, other studies found no such associations and the causative role of cow’s milk remains unconfirmed. It is possible that the increased consumption of cow’s milk in young infants simply reflects the decreased tendency towards breastfeeding in wealthy Western countries.
Wheat and gluten
Type 1 diabetes and celiac disease, an immune disorder characterized by intolerance to gluten present in some cereals, share several common susceptibility HLA alleles and non-HLA alleles, such as CTLA-4 and C–C chemokine receptor type 5 [53]. Celiac disease is more frequent among T1D patients than in control subjects [54]. Therefore, dietary gluten was proposed as a link between the gut, immune activation in gut-associated lymphoid tissues, and the development of T1D. The German Babydiab Study of more than 1,600 children with T1D parents showed that the introduction of gluten before 3 months of age increased islet autoantibody risk [55]. The Babydiab investigators are currently investigating whether eliminating gluten in genetically susceptible newborns during the first year of life can delay or decrease T1D incidence.
Sun and vitamin D
The distribution of T1D along the north–south gradient is correlated with exposure to sunlight and, consequently, the amount of vitamin D produced in the presence of solar ultraviolet B rays. Vitamin D was suggested to play a protective role because of its immunosuppressive capacity, and countries with greater solar exposure and enhanced vitamin D synthesis generally show a reduced incidence of T1D [56–59]. Mohr et al. [60] reported that each time the daily recommended dose of vitamin D was lowered in Finland (from 4,500 to 2,000 IU in 1960s, to 1,000 IU in 1975, and to 400 IU in 1992), the incidence of T1D increased sharply. However, other researchers have presented contradictory results.
Dietary supplementation of vitamin D in pregnancy or in the first year of life in children with little sun exposure was reported to reduce the risk of T1D in some studies, but not all [61–65]. Importantly, sunlight exposure cannot explain the differences in T1D in adjacent Finland and Karelia or in eastern and western Germany, where sunlight exposure is the same. Moreover, the incidence of T1D in Sardinia remains high, despite high sunlight exposure. Therefore, the contribution of this factor to the development of T1D remains under investigation.
Role of viruses in T1D
Viruses have been documented as possible causative agents of T1D in humans, and can act via numerous mechanisms. One of these mechanisms is molecular mimicry in which a pathogen-derived peptide shows sequence homology with a self-peptide and the host’s T cells mistakenly attack self-tissue. Another mechanism is bystander activation of T cells. Viral infection can provoke significant inflammation and destruction of its target tissue with subsequent release of autoantigens that can activate autoreactive T cells. Inflammation can also induce stress in the endoplasmic reticulum, causing misfolding of proteins and the creation of new autoantigens. Even if the initial amount of autoantigen released is minimal, the small pool of autoreactive T cells, by killing target cells, could provoke the release of normally sequestered autoantigens from β-cells, a process known as antigen spreading [66].
Viruses can also protect against T1D by several mechanisms. First, when an organism is subjected to repetitive infections, its resources can be used in priority for the expansion and action of antipathogenic immune cells, which limits resources available for autoreactive cells and may therefore control their numbers and activation [67, 68, 12]. Second, natural selection has made some pathogens able to modify or dampen the host’s immune responses to promote their own survival. For example, some viruses can alter the functions of macrophages and antigen-presenting cells [69, 70] while other viruses induce the proliferation and differentiation of regulatory T cells (Tregs) [71, 72]. These mechanisms favor the maintenance of peripheral tolerance and prevent T1D onset. Moreover, not only pathogens can directly alter the immune system, but the human body can also promote immune regulatory mechanisms to avoid exacerbated responses that could damage host tissues.
Viruses and acceleration of T1D
Several murine and human viruses such as rubella, mumps, rotavirus, and cytomegalovirus (CMV) can trigger the development of diabetes (Fig. 1). It is important to note that the induction of diabetes by these viruses involves various mechanisms, and the detection of anti-islet autoimmune responses remains elusive for rubella and mumps. The association with a particular HLA allele and/or the identification of antigenic epitopes shared by viruses and islet antigens suggests that specific anti-islet responses could occur.
Fig. 1.
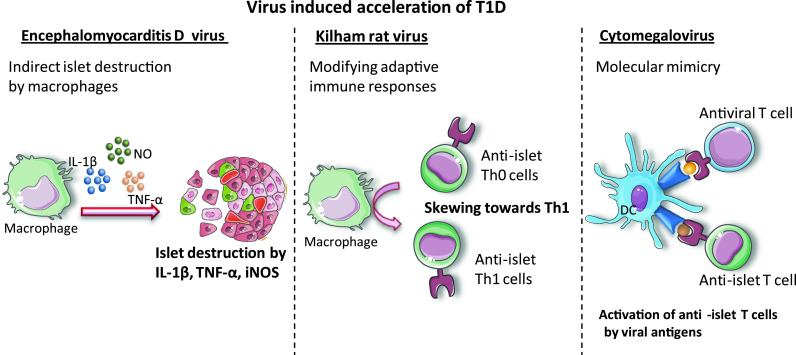
Virus-induced acceleration of T1D. Viruses can accelerate T1D by inducing immune cells such as macrophages to produce proinflammatory cytokines that can kill pancreatic β-cells or by skewing immune responses towards generation of pathogenic anti-islet Th1 cells. Molecular mimicry can be another mechanism leading to the activation of autoreactive anti-islet T cells
Infection with rubella in the first trimester of pregnancy was associated with a 20 % higher incidence of diabetes in children born with congenital rubella infection [73]. A relationship between rubella and diabetes has been shown in rabbits and hamsters [73, 74]. In New York, the incidence of diabetes increased after a rubella epidemic affecting mostly genetically predisposed children. In a cohort of over 200 infected patients, there was a decrease in the frequency of the HLA-DR2 allele and an increase in the HLA-DR3 allele among diabetic patients [75]. Molecular mimicry between human GAD65 and rubella virus protein was suggested as a triggering factor [76]. Despite the possible influence of HLA alleles and the described epitope mimicry, rubella-induced diabetes might differ from classical T1D since rubella infection affects many other organs as well [77].
Mumps virus infection was suggested to be associated with the presence of pancreatic autoantibodies [78]. Interestingly, after virtually eliminating mumps infection through a vaccination campaign in Finland, a plateau in the T1D incidence was observed [79]. However, little is known about the autoimmune process induced by mumps virus.
Several studies by Honeyman and colleagues have pointed towards a role of rotaviruses in T1D induction. For example, the appearance of antiviral IgG was linked to the appearance of autoantibodies against GAD65, insulin, and IA-2 in the Australian BabyDiab Study involving at-risk children [80]. The same authors also showed that rotavirus could infect pancreatic cells from NOD mice and macaque monkeys [81]. Interestingly, the rotaviral VP7 protein shows high sequence homology with islet autoantigens, tyrosine phosphatase IA-2, and GAD65. T cells cross-reacting with VP7 peptides and epitopes of IA2 and GAD65 were recently characterized in T1D patients. These peptides bind strongly to HLA-DRB1*04, which confers susceptibility to T1D [82]. All of these findings support the hypothesis that molecular mimicry between VP7 and islet autoantigens could facilitate the development or exacerbation of anti-islet autoimmunity and T1D onset. However, a Finnish study failed to find a link between rotavirus and T1D [83]. Concerning mouse models, high replicative rate of rhesus monkey rotavirus strain RRV infection was shown to protect NOD mice from [84].
In humans, initial reports found a correlation between CMV infection and the presence of anti-islet autoantibodies [85]. Molecular mimicry between CMV peptide and GAD65 has been proposed [86]. However, other studies failed to find a link between CMV infection and T1D in genetically predisposed children [87–89]. Interestingly, CMV can trigger T1D in susceptible LEW.1WR1 and BioBreeding (BB) rats [90, 91], and a recent study showed that CMV can infect human β-cells and induce the expression of inflammatory cytokines and chemokines [92].
Besides these viruses that can infect both humans and rodents, two other viruses whose natural host is a rodent are used in laboratory studies to decipher the mechanisms of viral pathogenesis. Even though encephalomyocarditis D (EMC-D) virus can directly infect β-cells, the mechanism by which it induced T1D is mostly indirect. After low-dose EMC-D virus infection, the infected macrophages were shown to be involved in the development of diabetes in DBA/2 mice through the production of mediators such as IL-1β, tumor necrosis factor (TNF)-α and inducible nitric oxide synthase (iNOS). The depletion of macrophages or the inhibition of these three mediators decreased the incidence of T1D [93]. Interleukin-1β and tumor necrosis factor-α can induce the expression of the death receptor Fas on β-cells, and subsequent binding of Fas to its ligand can lead to β-cell apoptosis. Notably, nitric oxide produced by iNOS can directly induce β-cell apoptosis.
Several laboratories have analyzed the pathogenic role of the Kilham rat virus (KRV) in T1D induction in diabetes-resistant BB rats [94, 95, 90]. Even though the KRV does not infect β-cells, it skews the immune responses towards a deleterious Th1 profile [94]. Subsequent studies showed that KRV induces the production of the proinflammatory cytokines, IL-12 and IL-6. Interestingly, IL-12 production was dependent on Toll-like receptor (TLR) 9, and blocking of TLR9 by chloroquine prevented the development of T1D in BB rats [95].
Protective effects of viruses against T1D
Because the protective role of viruses in T1D in humans can only be observed through the correlation between infection and reduced T1D incidence, the mechanisms by which viruses confer protection against T1D were mainly studied in rodent models [36]. Non-obese diabetic mice have been widely used because they spontaneously develop diabetes with many characteristics of human T1D. Interestingly, these mice less frequently develop T1D in animal facilities that are not pathogen-free or after treatment with pathogen-derived molecules. For example, a single Bacillus Calmette-Guérin injection is sufficient to inhibit T1D when administered to young NOD mice [96]. Similarly, treating NOD mice with complete Freund’s adjuvant reduced the number of diabetogenic T cells, while incomplete Freund’s adjuvant skewed the T cell responses towards a Th2 profile protecting against T1D [97, 98].
The protection of T1D by viral infection has been extensively analyzed using lymphocytic choriomeningitis virus (LCMV) (Fig. 2). Twenty years ago, Oldstone and colleagues [99, 100] reported that LCMV infection in diabetes-prone rats and NOD mice decreased or prevented the development of autoimmune diabetes. Subsequent studies have shown that the protection against diabetes is associated with an interferon (IFN)-γ-induced protein-10 chemokine gradient, with the highest concentration in pancreatic lymph nodes resulting in the attraction of activated T lymphocytes in this tissue, followed by their apoptosis [101]. More recently, we reported that the protective role of LCMV is dependent on a small lymphocyte population called invariant natural killer T (iNKT) cells because of their expression of an invariant Vα14Jα18 T cell receptor and typical natural killer (NK) cell receptors. These innate-like T cells exhibit various regulatory and effector functions, and inhibit the development of spontaneous T1D by dampening pathogenic autoimmune responses [102–104]. In the context of LCMV infection, iNKT cells mediate protection against spontaneous diabetes by promoting the recruitment and the activation of plasmacytoid DCs (pDCs) into the pancreas. Plasmacytoid dendritic cells produce large amounts of IFN-α and rapidly inhibit viral replication in the pancreas. This control of viral replication is a key factor in the inhibition of pancreatic tissue destruction and T1D in transgenic mice expressing the nucleoprotein of LCMV in β-cells [105]. In a second step, pDCs migrate from the pancreas to the pancreatic lymph node where they produce TGF-β and induce the conversion of naive T cells into Tregs. These induced Tregs migrate to the pancreas and locally produce TGF-β, which suppresses anti-LCMV and anti-islet T cell responses, thereby inhibiting the destruction of pancreatic islets and the development of LCMV-induced and spontaneous T1D [71].
Fig. 2.
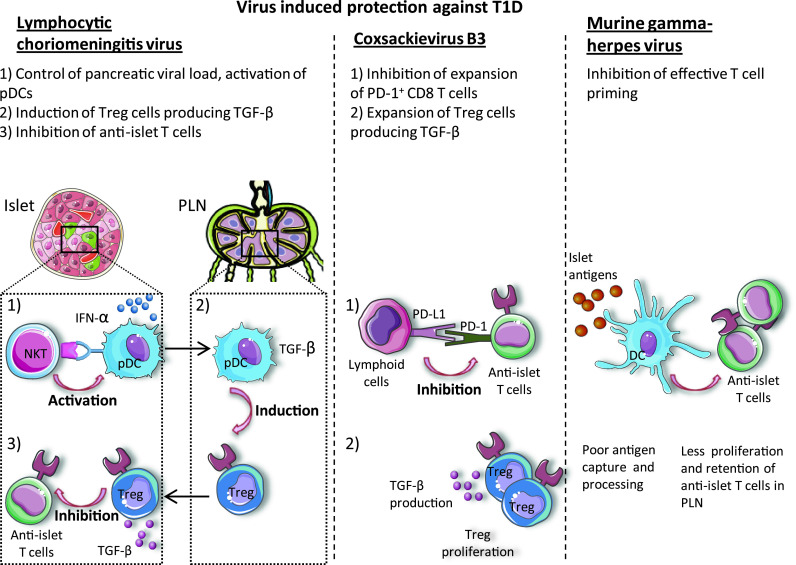
Virus-induced protection against T1D. Viruses can induce protection from T1D by eliciting immune mechanisms such as induction of Treg cells, inhibition of anti-islet T cells through suppressive receptors like PD-1 and suppressive cytokines like TGF-β or by blocking the efficient antigen capture and processing by antigen-presenting cells
Protection against T1D was also observed after infection with coxsackievirus B3 (CVB3). Two mechanisms act in synergy to delay and reduce the onset of T1D. First, CVB3 infection upregulates the expression of the inhibitory receptor PD-L1 on lymphoid cells, which hinders the expansion of PD-1-expressing autoreactive CD8+ T cells and delays T1D onset. Second, CVB3 infection enhances the proliferation of Tregs, which produce TGF-β to confer long-term protection against diabetes [72]. Studies of the CVB4 strain, which is able to both induce and protect against diabetes, depending on the context, will be discussed later in this review.
Infection of NOD mice with the murine gammaherpesvirus-68 delays the onset of T1D by reducing the capacity of CD11c+ DCs to capture and process autoantigens. This results in the retention of autoreactive T cells in the spleen and pancreatic lymph node, limiting their homing to the pancreas and the killing of islet β-cells. The numbers of Tregs do not change during the course of infection [69].
Even though the reovirus strain type 3 Abney (T3A) can infect β-cells and induce their apoptosis, the infection of newborn NOD mice with this virus reduces or delays the onset of T1D without preventing insulitis [106]. While the exact mechanism by which the T3A strain inhibits T1D has not been determined, the authors proposed two non-exclusive scenarios. The first one is based on the observation that the T3A strain infects various organs, including the thymus. Infection of the thymus of young animals could somehow eliminate autoreactive T cells in this tissue. In the second scenario, the infection and destruction of pancreatic islets could lead to the release of β-cell antigens and the establishment of an active tolerance through Treg cells.
Chronic mouse hepatitis virus (MHV) infection can prevent the development of T1D in NOD mice [107]. Studies outside of the context of T1D have shown that MHV can, in some cases, suppress the activation of splenic T lymphocytes and reduce the functions of antigen-presenting cells, such as macrophages, DCs, and B lymphocytes [108, 109].
Finally, lactate dehydrogenase virus infection also suppresses T1D onset in NOD mice by reducing the numbers of inflammatory macrophages in the peritoneum [70].
Dual role of enteroviruses in T1D
The role of enteroviruses in T1D is of great interest. They seem to represent a perfect illustration of the hygiene hypothesis. In times when enterovirus infections were frequent, the incidence of diabetes was low [110, 111]. Currently, however, the rarity of enterovirus infection is thought to make them more aggressive in susceptible individuals and can favor the onset of T1D.
The enterovirus genus belongs to the Picornaviridae family. Their genome is composed of a single positive RNA molecule encapsulated in capsid without an envelope. Human enteroviruses include polioviruses, echoviruses, rhinoviruses, enterovirus 71, and coxsackieviruses, which is the most prevalent group after the introduction of poliovirus vaccination. Coxsackieviruses are divided into two groups, A and B, with 24 and six serotypes, respectively. Coxsackievirus group B (CVB), particularly CVB4, is widely implicated in T1D. Infections with enteroviruses are mostly asymptomatic and only rarely cause complications such as hand, foot, and mouth disease, acute hemorrhagic conjunctivitis, aseptic meningitis, myocarditis, and severe neonatal sepsis-like disease. They are predominantly transmitted via the oral–fecal route (i.e., consumption of food or water containing contaminated feces).
Epidemiological perspectives of enteroviruses
Studies examining the relationship between enteroviruses and T1D probably started with two articles published by Gamble and Taylor in 1969. In their first article, they reported that patients with a recent onset of T1D (<3 months) had higher antibody titers against CVB4 compared to control individuals and patients whose T1D was not recent [112]. In their second article, they reported a seasonal onset of T1D with peaks in late fall/early winter, which followed the seasonal outbreak of CVB infection in late summer/early fall [113]. Since then, many studies have analyzed the role of CVB in T1D. The major difficulty in providing direct proof for the pathogenic role of enteroviruses is the interval between enteroviral infection and onset of T1D, meaning the enteroviral infection may be undetectable. Enteroviral infection is mostly identified by the detection of viral RNA in the serum. However, in healthy individuals, enteroviral RNA is detectable for only a few days after infection and most studies analyzed serum samples at intervals of several months. Nevertheless, with the exception of a few studies [114, 115], more than 24 retrospective and prospective studies have detected a link between enteroviruses and T1D [116].
Prospective studies
In 1995, the Finnish (DiMe) study group showed that enterovirus infections, identified by the presence of antibodies against enteroviral antigens, were almost two times more frequent in siblings who developed clinical T1D compared to siblings who remained diabetes-free [117]. Similarly, in the Finnish Diabetes Prediction and Prevention (DIPP) Study, anti-enteroviral antibodies were detected in 26 % of children who developed diabetes compared to 18 % of children in the control group. They reported a temporal relationship between enteroviral infection and the onset of T1D since 57 % of cases had autoantibodies present within 6 months after infection [118]. Similar results were observed in the Finnish trial to reduce IDDM in genetically at-risk (TRIGR) project, which revealed that enteroviral RNA was more frequently detected in children developing autoantibodies than in children who did not have autoantibodies [119]. The diabetes and autoimmunity study in the young (DAISY) conducted in Denver, CO, USA, followed 2,365 at-risk children, of which 140 subsequently developed autoantibodies. Among them, 61 % of children with enteroviral RNA developed T1D as compared to 28 % of children who did not have enteroviral RNA [120]. Even in Cuba where the incidence of T1D is low, the presence of enteroviral RNA was reported to be associated with T1D [121]. Thus, enterovirus infection is often considered a pivotal event that shifts the balance from chronic subclinical autoimmunity towards destructive autoimmunity.
Retrospective studies
In the United Kingdom, the study by Nairn et al. [122] found that 27 % of newly diagnosed patients harbored enteroviral RNA in serum samples taken within 1 week after confirmation of diagnosis, as compared to only 4.9 % of healthy controls. A recent study by Schulte et al. [123] also detected enteroviral RNA at the onset of T1D whereas no enteroviral RNA was detected in healthy controls. This study also underlined the importance of assessing peripheral blood mononuclear cells because 4/10 patients with T1D were positive for enteroviral RNA in peripheral blood mononuclear cells as compared to 2/10 in serum [123]. In a Swedish study, anti-CVB4 neutralizing antibodies were more frequently detected in patients with newly diagnosed T1D as compared to healthy controls [124].
Because detecting enteroviral presence in the pancreas requires biopsies or pancreatic samples from postmortem donors, such analyses have been limited. However in 1979, Yoon et al. [125] isolated a virus from the pancreas of a deceased patient diagnosed with diabetic ketoacidosis. This virus was later identified as being related to the diabetogenic CVB4 strain. When injected into mice, this virus had infected pancreatic islets and caused β-cell loss. Similarly, Dotta et al. [126], using immunohistochemistry, detected enteroviral VP1 capsid protein in islets from patients with T1D, whereas islets from healthy donors were virus-free. They sequenced the viral genome present in the pancreatic islets of one out of three VP1-positive diabetic donors and identified this virus as CVB4. The isolated CVB4 could infect human islets from healthy donors and reduce insulin secretion [126]. Richardson et al. [127] detected enteroviral capsid VP1 protein in the pancreatic islets of 44/72 (66 %) patients with recently diagnosed T1D, while this protein was virtually non-existent in healthy controls. In this study, VP1 was also detected in 42 % of T2D patients. Interestingly, the authors propose that enteroviral infection could also play a role in T2D, since enteroviral infection of β-cells in vitro decreases glucose-induced insulin secretion. Thus, in individuals with increased insulin resistance and higher insulin requirements, enteroviral infection could contribute to the development of T2D. Enteroviral RNA was also detected by in situ hybridization of pancreatic islets from two deceased donors with fulminant CVB3 infection and in some CVB3-infected patients with T1D [128]. Indirectly, the presence of a virus has also been suggested by the detection of the antiviral cytokine IFN-α in the pancreas and β-cells of patients with T1D [129, 130].
Finally, echoviruses 4, 6, 9, 14, and 30, other members of the enterovirus family, have been implicated in the etiology of T1D and were shown to impair or kill human β-cells in vitro [131–137]. However, studies focusing on echoviruses remain very scarce.
Understanding the relationship between enteroviral infections and T1D: studies in NOD mice
Because the sequence of P2-C protein of CVB4 is very similar to that of the highly immunogenic GAD65 expressed in β-cells, molecular mimicry was proposed to explain the pathogenic effects of CVB4 in T1D in humans [138]. This hypothesis implies that anti-CVB4 T lymphocytes cross-react with GAD65, destroy β-cells, and induce T1D. However, molecular mimicry was ruled out in later studies [139].
Coxsackievirus group B 4 infection in NOD mice seems to yield contradictory results. The discrepancy might reflect the complexity of the interactions among CVB4, the immune system and pancreatic β-cells, which could lead to the induction or prevention of T1D. Indeed, several parameters, including timing of infection, type of mice, viral dose, and viral strain with its particular virulence, play important roles in the outcome of CVB infection in animal models (Fig. 3). All of these factors, in combination, might explain why CVB4 could accelerate T1D in some individuals while remaining asymptomatic in others.
Fig. 3.
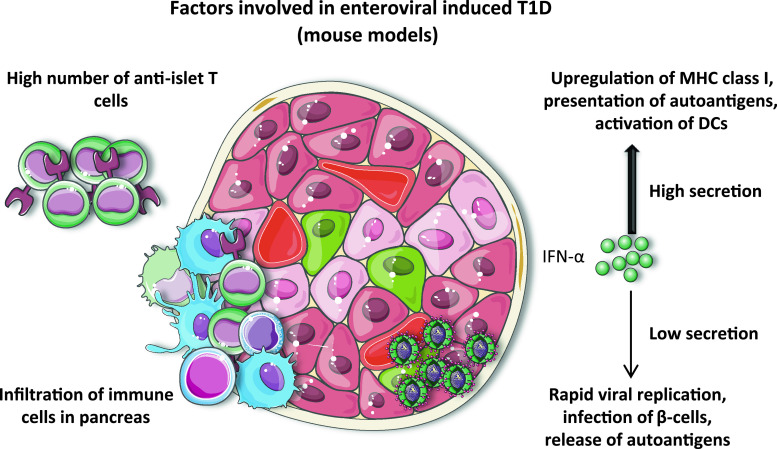
Factors involved in enteroviral-induced T1D. Studies in mouse models have revealed that in order for enteroviruses to accelerate T1D, a high number of autoreactive anti-islet T cells are necessary. In addition, pancreas must be infiltrated by cells of the immune system. As for the IFN-α, its high secretion can result in a strong activation of the immune system favoring the presentation of islet autoantigens. On the other hand, if IFN-α secretion is too low, viruses will replicate freely, infect islet β-cells, and provoke the release of autoantigens leading to the activation of anti-islet T cells and provoking diabetes
Age and associated numbers of autoreactive T cells
Using BDC2.5 transgenic NOD mice, Horwitz et al. [140] showed that CVB4 infection of prediabetic mice, harboring a greater number of anti-islet BDC2.5 CD4+ T cells compared to younger mice, induces the acceleration of T1D through bystander activation of T cells. This study suggested that the number of anti-islet T cells at the time of infection is a critical factor. Two subsequent studies strengthened this hypothesis by infecting non-transgenic NOD mice at different ages. Coxsackievirus group B 4 infection of young NOD mice, aged 4–8 weeks, did not accelerate or induce T1D [141, 142]. However, CVB4 infection of older mice accelerated T1D onset in 61 % of mice [142]. Interestingly, it seems that both the number and location of autoreactive T cells are important factors. F1 mice, obtained by crossing BDC2.5 NOD mice with BALB/c or C57BL/6 mice, had the same numbers of peripheral autoreactive T cells. However, these T cells only infiltrated the pancreas in BDC2.5NOD × BALB/c mice. Upon CVB4 infection, BDC2.5NOD × BALB/c mice, but not BDC2.5NOD × C57BL/6 mice, developed diabetes, indicating that infiltration of pancreas by diabetogenic T cells before infection is an important factor that promotes the onset of diabetes [143].
Based on these experimental results, the pre-existence of a pool of anti-islet T cells and the infiltration of the pancreas by immune cells in children could determine whether T1D will develop following enteroviral infection.
Viral titer
A comparison of non-diabetogenic CVB3/GA and diabetogenic CVB3/28 strains prompted Tracy and coworkers [144] to propose that viral dose and replication rate play an important role in the initiation of T1D. Infection of 12-week-old NOD mice with 5 × 105 TCID50 (50 % tissue culture infective dose) per mouse of the non-diabetogenic CVB3/GA strain did not induce diabetes, whereas a 100-fold higher dose induced diabetes in 30 % of mice. On the other hand, the diabetogenic CVB3/28 strain induced T1D in up to 70 % of NOD mice, although this rate decreased when a lower dose of CVB3/28 was administered. These results indicate that the pathogenicity of the strain could be dependent on its dose. Thus, children infected with a high viral dose or whose immune system would allow viruses to quickly reach high titers, could be at increased risk of developing T1D.
CVB4, β-cell infection and the antiviral response
Some CVB strains have been reported to infect, proliferate, affect the metabolism, and destroy human pancreatic islet cells in vitro. In human pancreas, CVB4 was detected in islets but not in the exocrine tissue, whereas it was mostly detected in pancreatic exocrine tissue in mice [145–147]. The effects of infection of mouse β-cells with CVB4 are contradictory. It has been described that CVB4 primarily uses the coxsackie and adenovirus receptor (CAR) to enter cells [128, 148]. Although CAR and CVB4 were not detected in some studies [149, 150], others have detected CVB4 in murine islets. Importantly, the permissiveness of islet β-cells was suggested to determine the outcomes of infections with different CVB strains. Using immunohistochemistry, Horwitz et al. [143, 151] demonstrated the presence of CVB4 in pancreatic islets at 7 days post-infection in BDC2.5 mice, and the pathogenic effect of the CVB4 strain was attributed to its capacity to infect β-cells whereas CVB3 could not. Meanwhile in old prediabetic NOD mice, even the non-diabetogenic CVB3 could infect islets and accelerate diabetes [152]. If infection of islet β-cells is an important factor, the efficacy of an individual’s response to the virus could determine the progression to T1D.
Interferon-α, interferon-β and interferon-γ are the major cytokines that are rapidly secreted after viral infection. The expression of IFN-γ in pancreatic islet β-cells of IFN-γ—deficient mice allows them to control viral replication and survive after CVB4 infection [153]. Similarly in transgenic NOD mice in which IFN signaling is inhibited in pancreatic β-cells, CVB4 induces diabetes in 95 % of mice while non-transgenic mice remain diabetes-free. This inability to respond to IFNs rendered β-cells permissive to CVB4 infection resulting in their killing by activated NK cells [154]. It has been shown that during coxsackievirus infection, IFN-α increases the expression of intracellular double-stranded RNA sensor 2–5AS, which activates the enzyme RNase L that cleaves viral RNA and therefore protects β-cells. Interferon-γ activates the enzyme PKR (dsRNA-dependent protein kinase), which can disturb protein synthesis and block CVB replication [155]. Thus, the rapid islet response to viral infection through IFN secretion has a strong influence on diabetes outcome because it determines whether enteroviruses can infect islet β-cells. As described above, uncontrolled viral replication, which can yield high viral titers very quickly, can make an otherwise harmless viral strain highly pathogenic.
Islet neogenesis
Islet neogenesis has been proposed to be a factor determining the outcome of CVB infection in mice. Yap et al. [150] used two CVB4 strains, the diabetogenic CVB4/E2 strain and the non-diabetogenic CVB4/JVB strain, and found that the severity of pancreatic acinar tissue damage caused by viral infection influences T1D development. While the E2 strain caused massive destruction of acinar tissue without islet destruction, the JVB strain caused only minor damage. This allowed regeneration of the acinar tissue and new pancreatic islets, which were not observed in the severely destroyed pancreas of CVB4/E2-infected NOD mice [150]. Although it is unknown whether islet neogenesis occurs in the human exocrine tissue, a marker for cell proliferation, Ki-67, was expressed in human pancreatic islets positive for enteroviral VP1 protein [156]. Therefore, the capacity of islet cells to proliferate after the infection might protect against T1D.
Ambivalent roles of enteroviruses in T1D
Hygiene hypothesis
According to the hygiene hypothesis, the rarity of enteroviral infections could lead to decreased immunity against these viruses, thus increasing their invasiveness and pathogenicity. To estimate the frequency of enteroviral infections, Viskari et al. [157] studied the prevalence of enteroviral meningitis in Finnish children and concluded that the frequency of enteroviral infection in children aged ≥6 months had decreased. However, they found that the infection rate was actually increasing in younger children aged 0–6 months. One explanation for this rise in young infants could be the lack of protective anti-enteroviral antibodies of maternal origin that would pass to the child through the placenta and breastfeeding. Indeed, Sadeharju et al. [158] found that children who were breastfed for >2 weeks after the birth had a lower incidence of enteroviral infections than children breastfed for <2 weeks. Moreover, the percentage of women lacking antibodies against the CVB4 strain increased from 6 to 17 % between 1983 and 1995 while the percentage of women lacking antibodies against enteroviral peptides increased from 13 to 42 % during the same period [110, 111].
With fewer infections, pregnant women nowadays might lack anti-enteroviral antibodies or may have a repertoire that covers fewer serotypes compared to women at the beginning of the 20th century. Thus, young children would receive fewer or no protective antibodies from their mothers. In children with a weaker immune defense against enteroviruses, a single strong infection could then allow increased virus replication, promoting the development of T1D. Interestingly, this scenario proposing the increased pathogenic role of CVB in T1D is supported by observations of polioviral infections, another member of the enteroviral family. At the end of the 19th century, poliovirus infections became rarer, and the number of children developing a severe complication of polioviral infection, paralytic poliomyelitis, increased drastically. It is suggested that with frequent polioviral infections children encountered the virus at an early age when they still had protective antibodies transmitted by their mothers. These antibodies protected against paralysis by forming an immediate barrier and blocking the invasion of the central nervous system by poliovirus. Thus the infected individual had time to make his own protective antibodies to further eliminate the virus. However, because of improved sanitary conditions, the virus became rare and children were infected at an older age when the level of maternal antibodies had strongly decreased. Without any immediate protection, poliovirus attacked the central nervous system easier, thus increasing the chances to develop paralytic poliomyelitis [159].
The reduced frequency of enteroviral infection could favor delayed enteroviral encounters in genetically predisposed older children. The older the child is at the time of infection, the more likely the child is to have accumulated anti-islet T cells. It is important to remember that studies of NOD mice have shown that the number of anti-islet T cells and the degree of islet inflammation are critical factors and could explain why some children develop T1D while others do not.
RNA sensors and T1D
To further support the pathogenic role of enteroviruses, the genomic region coding the viral RNA sensor IFIH1 (IFN induced with helicase C domain 1), otherwise known as MDA5 (melanoma differentiation-associated protein 5), has been identified as a T1D susceptibility locus. This is particularly important because MDA5 is critical for the recognition of picornaviruses to which the enterovirus genus belongs [160]. This intracellular receptor recognizes double-stranded viral RNA that forms during viral replication, induces the expression of type I IFNs (IFN-α and β), and activates the immune system. Four rare mutations that reduce the expression of IFIH1 and type I IFNs were reported to have a protective role in T1D [161–163]. These findings suggest that wild-type IFIH1 and effective recognition of viral infection actually predispose individuals to T1D. This role of MDA5 is supported by several previous studies. For example, IFN-α was detected in plasma samples of 70 % of newly diagnosed T1D patients, of whom 50 % carried enteroviral RNA in their blood samples; none of the IFN-α–negative patients had enteroviral RNA [164]. The scenario proposes that after viral RNA binding to IFIH1, large amounts of type I IFNs are secreted, leading to the upregulation of MHC class I molecule expression on β-cells, increased presentation of autoantigens, and activation of DCs [165]. Type I IFNs could also regulate cells of the innate and adaptive immune system, which could facilitate the killing of β-cells and destruction of pancreatic islets [166, 167].
Even though much less documented than MDA5, it has been proposed that another RNA sensor, TLR7, could be implicated in the etiology of T1D [168]. Toll-like receptor 7, mainly expressed by pDCs, can recognize coxsackieviruses and initiate an antiviral immune response by inducing the production of type I IFNs [169]. Treatment with a TLR7 agonist, particularly in combination with other immune stimulatory molecules such as CD40, induced T1D in transgenic NOD mice bearing an increased number of autoreactive T cells [170]. Interestingly, NOD mice deficient for Myd88, an adaptor molecule required for the signaling of most TLRs, including TLR7, do not develop diabetes in a specific pathogen-free mouse facility [43].
While an efficient MDA5-mediated response is associated with T1D susceptibility in humans, several studies have highlighted the critical role of IFN-α production in diabetes prevention in infected mice [105, 154, 171, 172]. The amount and the environment in which IFN-α is secreted could perhaps account for this phenomenon. IFN-α is required to block viral replication, but excessive secretion in the context of an ongoing autoimmune response could render IFN-α pathogenic.
Could pollutants promote virus-induced diabetes?
Since the frequency of autoimmune diseases, including T1D, is higher in more industrialized countries, it is tempting to speculate that pollutants present in the environment might be involved. Heavy metals such as mercury or cadmium worsen the course of autoimmune diseases in mouse models [173–175]. Concerning T1D, arsenic present in drinking water in western Bangladesh and Taipei city, Taiwan, was associated with an increased incidence of T1D in these populations [176, 177]. Pollutants have also been found at higher levels in diabetic mothers [178]. In a mouse model, exposure to mercury caused dysfunction and apoptosis of pancreatic β-cells [179, 180]. However, other studies in Bangladesh and the United States found no link between arsenic and T1D [181, 182], and mercury was found to delay T1D onset in NOD mice [183]. The discrepancy between these results may be due to the fact that the pollutants by themselves do not have a noticeable effect on autoimmune diseases in humans. Instead, they may create an environment that is aggravated by an infection. For example, exposing mice to mercury and bacterial lipopolysaccharide exacerbated the onset of autoimmune disease in genetically susceptible mice or rendered resistant mice susceptible to autoimmunity [184]. Funseth et al. [185] showed that CVB3 infection can cause an accumulation of an environmental pollutant in the viral target organ, such as the pancreas, and aggravate the inflammation by its toxicity. However, additional epidemiological and experimental studies are required to determine the precise role of pollutants in virus-induced T1D.
Conclusions
Many recent studies in humans and animal models have implicated viruses in the development of T1D. The interactions between viruses, the immune system, and β-cells are probably key in determining their pathogenic or beneficial roles. Most importantly, the conditions in which the disease develops might have changed in recent years because the role of HLA is becoming less prominent. Other genes are clearly involved and can interfere with environmental factors such as infection, diet, and pollutants. Interdisciplinary studies in the fields of genetics, immunology, microbiology, nutrition, and epidemiology are essential to elucidate the etiology of T1D and develop efficient preventive strategies.
Acknowledgments
We apologize to all the authors whose work we could not cite owing to space constrictions. This work was supported by funds from the Institut National de la Santé et de la Recherche Médicale and the Centre National pour la Recherche Scientifique, grant from ANR-09-GENO-023 and LABEX INFLAMEX to AL. Liana Ghazarian and Yannick Simoni were supported by doctoral fellowships from the Ministère de l'Education Nationale et de la Recherche et Technique and from Région Île-de-France.
Competing interests statement
The authors declare no competing financial interests.
Contributor Information
Liana Ghazarian, Phone: +33-1-43217384, FAX: +33-1-40488352, Email: liana.ghazarian@inserm.fr.
Agnès Lehuen, Email: agnes.lehuen@inserm.fr.
References
- 1.Hanafusa T, Imagawa A. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab. 2007;3(1):36–45. doi: 10.1038/ncpendmet0351. [DOI] [PubMed] [Google Scholar]
- 2.Knip M. Natural course of preclinical type 1 diabetes. Horm Res. 2002;57(Suppl 1):6–11. doi: 10.1159/000053305. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity. 2010;32(4):468–478. doi: 10.1016/j.immuni.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallone R, Brezar V, Boitard C. T cell recognition of autoantigens in human type 1 diabetes: clinical perspectives. Clin Dev Immunol. 2011;2011:513210. doi: 10.1155/2011/513210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wadonda-Kabondo N, Sterne JA, Golding J, Kennedy CT, Archer CB, Dunnill MG. A prospective study of the prevalence and incidence of atopic dermatitis in children aged 0–42 months. Br J Dermatol. 2003;149(5):1023–1028. doi: 10.1111/j.1365-2133.2003.05605.x. [DOI] [PubMed] [Google Scholar]
- 6.Williams HC. Is the prevalence of atopic dermatitis increasing? Clin Exp Dermatol. 1992;17(6):385–391. doi: 10.1111/j.1365-2230.1992.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 7.Ma RC, Chan JC. Diabetes: incidence of childhood type 1 diabetes: a worrying trend. Nat Rev Endocrinol. 2009;5(10):529–530. doi: 10.1038/nrendo.2009.180. [DOI] [PubMed] [Google Scholar]
- 8.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 9.Sellner J, Kraus J, Awad A, Milo R, Hemmer B, Stuve O. The increasing incidence and prevalence of female multiple sclerosis—a critical analysis of potential environmental factors. Autoimmun Rev. 2011;10(8):495–502. doi: 10.1016/j.autrev.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Gismera CS, Aladren BS. Inflammatory bowel diseases: a disease(s) of modern times? Is incidence still increasing? World J Gastroenterol. 2008;14(36):5491–5498. doi: 10.3748/wjg.14.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23(10):1516–1526. doi: 10.2337/diacare.23.10.1516. [DOI] [PubMed] [Google Scholar]
- 12.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 13.Hernan MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology. 1999;53(8):1711–1718. doi: 10.1212/WNL.53.8.1711. [DOI] [PubMed] [Google Scholar]
- 14.McLeod JG, Hammond SR, Hallpike JF. Epidemiology of multiple sclerosis in Australia. With NSW and SA survey results. Med J Aust. 1994;160(3):117–122. [PubMed] [Google Scholar]
- 15.Newhook LA, Grant M, Sloka S, Hoque M, Paterson AD, Hagerty D, Curtis J. Very high and increasing incidence of type 1 diabetes mellitus in Newfoundland and Labrador Canada. Pediatr Diabetes. 2008;9(3 Pt 2):62–68. doi: 10.1111/j.1399-5448.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- 16.Green A, Patterson CC. Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia. 2001;44(Suppl 3):B3–B8. doi: 10.1007/PL00002950. [DOI] [PubMed] [Google Scholar]
- 17.Daimond Project Group Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23(8):857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 18.The Eurodiab Ace Study Group. The Eurodiab Ace Substudy 2 Study Group Familial risk of type I diabetes in European children. Diabetologia. 1998;41(10):1151–1156. doi: 10.1007/s001250051044. [DOI] [PubMed] [Google Scholar]
- 19.Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med. 2008;359(26):2849–2850. doi: 10.1056/NEJMc0805398. [DOI] [PubMed] [Google Scholar]
- 20.Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes. 2003;52(4):1052–1055. doi: 10.2337/diabetes.52.4.1052. [DOI] [PubMed] [Google Scholar]
- 21.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41(6):703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronningen KS, Keiding N, Green A. Correlations between the incidence of childhood-onset type I diabetes in Europe and HLA genotypes. Diabetologia. 2001;44(Suppl 3):B51–B59. doi: 10.1007/PL00002955. [DOI] [PubMed] [Google Scholar]
- 23.Almawi WY, Busson M, Tamim H, Al-Harbi EM, Finan RR, Wakim-Ghorayeb SF, Motala AA. HLA class II profile and distribution of HLA-DRB1 and HLA-DQB1 alleles and haplotypes among Lebanese and Bahraini Arabs. Clin Diagn Lab Immunol. 2004;11(4):770–774. doi: 10.1128/CDLI.11.4.770-774.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park Y, Eisenbarth GS. Genetic susceptibility factors of type 1 diabetes in Asians. Diabetes Metab Res Rev. 2001;17(1):2–11. doi: 10.1002/1520-7560(2000)9999:9999<::AID-DMRR164>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Kondrashova A, Reunanen A, Romanov A, Karvonen A, Viskari H, Vesikari T, Ilonen J, Knip M, Hyoty H. A sixfold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann Med. 2005;37(1):67–72. doi: 10.1080/07853890410018952. [DOI] [PubMed] [Google Scholar]
- 26.Skrodeniene E, Marciulionyte D, Padaiga Z, Jasinskiene E, Sadauskaite-Kuehne V, Sanjeevi CB, Ludvigsson J. HLA class II alleles and haplotypes in Lithuanian children with type 1 diabetes and healthy children (HLA and type 1 diabetes) Medicina (Kaunas) 2010;46(8):505–510. [PubMed] [Google Scholar]
- 27.Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwright R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ. 1992;304(6833):1020–1022. doi: 10.1136/bmj.304.6833.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delli AJ, Lindblad B, Carlsson A, Forsander G, Ivarsson SA, Ludvigsson J, Marcus C, Lernmark A. Type 1 diabetes patients born to immigrants to Sweden increase their native diabetes risk and differ from Swedish patients in HLA types and islet autoantibodies. Pediatr Diabetes. 2010;11(8):513–520. doi: 10.1111/j.1399-5448.2010.00637.x. [DOI] [PubMed] [Google Scholar]
- 29.Bruno G, Pagano G, Faggiano F, De Salvia A, Merletti F. Effect of Sardinian heritage on risk and age at onset of type 1 diabetes: a demographic case-control study of Sardinian migrants. Int J Epidemiol. 2000;29(3):532–535. doi: 10.1093/ije/29.3.532. [DOI] [PubMed] [Google Scholar]
- 30.Muntoni S, Fonte MT, Stoduto S, Marietti G, Bizzarri C, Crino A, Ciampalini P, Multari G, Suppa MA, Matteoli MC, Lucentini L, Sebastiani LM, Visalli N, Pozzilli P, Boscherini B. Incidence of insulin-dependent diabetes mellitus among Sardinian-heritage children born in Lazio region Italy. Lancet. 1997;349(9046):160–162. doi: 10.1016/S0140-6736(96)04241-9. [DOI] [PubMed] [Google Scholar]
- 31.Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmun Rev. 2010;9(5):A355–A365. doi: 10.1016/j.autrev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, Gale EA. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364(9446):1699–1700. doi: 10.1016/S0140-6736(04)17357-1. [DOI] [PubMed] [Google Scholar]
- 33.Hermann R, Knip M, Veijola R, Simell O, Laine AP, Akerblom HK, Groop PH, Forsblom C, Pettersson-Fernholm K, Ilonen J. Temporal changes in the frequencies of HLA genotypes in patients with type 1 diabetes—indication of an increased environmental pressure? Diabetologia. 2003;46(3):420–425. doi: 10.1007/s00125-003-1045-4. [DOI] [PubMed] [Google Scholar]
- 34.Patterson CC, Dahlquist G, Soltesz G, Green A. Is childhood-onset type I diabetes a wealth-related disease? An ecological analysis of European incidence rates. Diabetologia. 2001;44(Suppl 3):B9–B16. doi: 10.1007/PL00002961. [DOI] [PubMed] [Google Scholar]
- 35.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10(7):501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 37.Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJ, Dunne DW. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21(4):169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 38.Zaccone P, Burton OT, Gibbs S, Miller N, Jones FM, Dunne DW, Cooke A. Immune modulation by Schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. J Biomed Biotechnol. 2010;2010:795210. doi: 10.1155/2010/795210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75(1):397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Q, Sundar K, Mishra PK, Mousavi G, Liu Z, Gaydo A, Alem F, Lagunoff D, Bleich D, Gause WC. Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect Immun. 2009;77(12):5347–5358. doi: 10.1128/IAI.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hubner MP, Stocker JT, Mitre E. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cells. Immunology. 2009;127(4):512–522. doi: 10.1111/j.1365-2567.2008.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newland SA, Phillips JM, Mastroeni P, Azuma M, Zaccone P, Cooke A. PD-L1 blockade overrides Salmonella typhimurium-mediated diabetes prevention in NOD mice: no role for Tregs. Eur J Immunol. 2011;41(10):2966–2976. doi: 10.1002/eji.201141544. [DOI] [PubMed] [Google Scholar]
- 43.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455(7216):1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bras A, Aguas AP. Diabetes-prone NOD mice are resistant to Mycobacterium avium and the infection prevents autoimmune disease. Immunology. 1996;89(1):20–25. doi: 10.1046/j.1365-2567.1996.d01-717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martins TC, Aguas AP. Mechanisms of Mycobacterium avium-induced resistance against insulin-dependent diabetes mellitus (IDDM) in non-obese diabetic (NOD) mice: role of Fas and Th1 cells. Clin Exp Immunol. 1999;115(2):248–254. doi: 10.1046/j.1365-2249.1999.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosu V, Ahmed N, Paccagnini D, Gerlach G, Fadda G, Hasnain SE, Zanetti S, Sechi LA. Specific immunoassays confirm association of Mycobacterium avium Subsp. paratuberculosis with type-1 but not type-2 diabetes mellitus. PLoS ONE. 2009;4(2):e4386. doi: 10.1371/journal.pone.0004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sechi LA, Paccagnini D, Salza S, Pacifico A, Ahmed N, Zanetti S. Mycobacterium avium subspecies paratuberculosis bacteremia in type 1 diabetes mellitus: an infectious trigger? Clin Infect Dis. 2008;46(1):148–149. doi: 10.1086/524084. [DOI] [PubMed] [Google Scholar]
- 48.Rani PS, Sechi LA, Ahmed N. Mycobacterium avium subsp. paratuberculosis as a trigger of type-1 diabetes: destination Sardinia, or beyond? Gut Pathog. 2010;2(1):1. doi: 10.1186/1757-4749-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luopajarvi K, Savilahti E, Virtanen SM, Ilonen J, Knip M, Akerblom HK, Vaarala O. Enhanced levels of cow’s milk antibodies in infancy in children who develop type 1 diabetes later in childhood. Pediatr Diabetes. 2008;9(5):434–441. doi: 10.1111/j.1399-5448.2008.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elliott RB, Harris DP, Hill JP, Bibby NJ, Wasmuth HE. Type I (insulin-dependent) diabetes mellitus and cow milk: casein variant consumption. Diabetologia. 1999;42(3):292–296. doi: 10.1007/s001250051153. [DOI] [PubMed] [Google Scholar]
- 51.Birgisdottir BE, Hill JP, Harris DP, Thorsdottir I. Variation in consumption of cow milk proteins and lower incidence of type 1 diabetes in Iceland vs the other 4 Nordic countries. Diabetes Nutr Metab. 2002;15(4):240–245. [PubMed] [Google Scholar]
- 52.Birgisdottir BE, Hill JP, Thorsson AV, Thorsdottir I. Lower consumption of cow milk protein A1 beta-casein at 2 years of age, rather than consumption among 11- to 14-year-old adolescents, may explain the lower incidence of type 1 diabetes in Iceland than in Scandinavia. Ann Nutr Metab. 2006;50(3):177–183. doi: 10.1159/000090738. [DOI] [PubMed] [Google Scholar]
- 53.Smyth DJ, Plagnol V, Walker NM, Cooper JD, Downes K, Yang JH, Howson JM, Stevens H, McManus R, Wijmenga C, Heap GA, Dubois PC, Clayton DG, Hunt KA, van Heel DA, Todd JA. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359(26):2767–2777. doi: 10.1056/NEJMoa0807917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, Ilonen J, Laurila K, Dahlbom I, Hansson T, Hopfl P, Knip M. Prevalence of Celiac disease among children in Finland. N Engl J Med. 2003;348(25):2517–2524. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 55.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290(13):1721–1728. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 56.Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51(8):1391–1398. doi: 10.1007/s00125-008-1061-5. [DOI] [PubMed] [Google Scholar]
- 57.Sloka S, Grant M, Newhook LA. The geospatial relation between UV solar radiation and type 1 diabetes in Newfoundland. Acta Diabetol. 2010;47(1):73–78. doi: 10.1007/s00592-009-0100-0. [DOI] [PubMed] [Google Scholar]
- 58.Griffin MD, Lutz WH, Phan VA, Bachman LA, McKean DJ, Kumar R. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun. 2000;270(3):701–708. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]
- 59.van Halteren AG, van Etten E, de Jong EC, Bouillon R, Roep BO, Mathieu C. Redirection of human autoreactive T cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D(3) Diabetes. 2002;51(7):2119–2125. doi: 10.2337/diabetes.51.7.2119. [DOI] [PubMed] [Google Scholar]
- 60.Mohr SB, Garland FC, Garland CF, Gorham ED, Ricordi C. Is there a role of vitamin D deficiency in type 1 diabetes of children? Am J Prev Med. 2010;39(2):189–190. doi: 10.1016/j.amepre.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 61.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 62.Fronczak CM, Baron AE, Chase HP, Ross C, Brady HL, Hoffman M, Eisenbarth GS, Rewers M, Norris JM. In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care. 2003;26(12):3237–3242. doi: 10.2337/diacare.26.12.3237. [DOI] [PubMed] [Google Scholar]
- 63.The Eurodiab Substudy 2 Study Group Vitamin D supplement in early childhood and risk for type I (insulin-dependent) diabetes mellitus. Diabetologia. 1999;42(1):51–54. doi: 10.1007/s001250051112. [DOI] [PubMed] [Google Scholar]
- 64.Simpson M, Brady H, Yin X, Seifert J, Barriga K, Hoffman M, Bugawan T, Baron AE, Sokol RJ, Eisenbarth G, Erlich H, Rewers M, Norris JM. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the diabetes autoimmunity study in the young (DAISY) Diabetologia. 2011;54(11):2779–2788. doi: 10.1007/s00125-011-2278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marjamaki L, Niinisto S, Kenward MG, Uusitalo L, Uusitalo U, Ovaskainen ML, Kronberg-Kippila C, Simell O, Veijola R, Ilonen J, Knip M, Virtanen SM. Maternal intake of vitamin D during pregnancy and risk of advanced beta cell autoimmunity and type 1 diabetes in offspring. Diabetologia. 2010;53(8):1599–1607. doi: 10.1007/s00125-010-1734-8. [DOI] [PubMed] [Google Scholar]
- 66.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358(6382):155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 67.Liacopoulos P, Ben-Efraim S. Antigenic competition. Prog Allergy. 1975;18:97–204. doi: 10.1159/000395257. [DOI] [PubMed] [Google Scholar]
- 68.Pross HF, Eidinger D. Antigenic competition: a review of nonspecific antigen-induced suppression. Adv Immunol. 1974;18:133–168. doi: 10.1016/S0065-2776(08)60309-0. [DOI] [PubMed] [Google Scholar]
- 69.Smith KA, Efstathiou S, Cooke A. Murine gammaherpesvirus-68 infection alters self-antigen presentation and type 1 diabetes onset in NOD mice. J Immunol. 2007;179(11):7325–7333. doi: 10.4049/jimmunol.179.11.7325. [DOI] [PubMed] [Google Scholar]
- 70.Takei I, Asaba Y, Kasatani T, Maruyama T, Watanabe K, Yanagawa T, Saruta T, Ishii T. Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J Autoimmun. 1992;5(6):665–673. doi: 10.1016/0896-8411(92)90184-R. [DOI] [PubMed] [Google Scholar]
- 71.Diana J, Brezar V, Beaudoin L, Dalod M, Mellor A, Tafuri A, von Herrath M, Boitard C, Mallone R, Lehuen A. Viral infection prevents diabetes by inducing regulatory T cells through NKT cell-plasmacytoid dendritic cell interplay. J Exp Med. 2011;208(4):729–745. doi: 10.1084/jem.20101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Filippi CM, Estes EA, Oldham JE, von Herrath MG. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J Clin Invest. 2009;119(6):1515–1523. doi: 10.1172/JCI38503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menser MA, Forrest JM, Bransby RD. Rubella infection and diabetes mellitus. Lancet. 1978;1(8055):57–60. doi: 10.1016/S0140-6736(78)90001-6. [DOI] [PubMed] [Google Scholar]
- 74.Rayfield EJ, Kelly KJ, Yoon JW. Rubella virus-induced diabetes in the hamster. Diabetes. 1986;35(11):1278–1281. doi: 10.2337/diabetes.35.11.1278. [DOI] [PubMed] [Google Scholar]
- 75.McEvoy RC, Fedun B, Cooper LZ, Thomas NM, Rodriguez de Cordoba S, Rubinstein P, Ginsberg-Fellner F. Children at high risk of diabetes mellitus: New York studies of families with diabetes and of children with congenital rubella syndrome. Adv Exp Med Biol. 1988;246:221–227. doi: 10.1007/978-1-4684-5616-5_27. [DOI] [PubMed] [Google Scholar]
- 76.Ou D, Mitchell LA, Metzger DL, Gillam S, Tingle AJ. Cross-reactive rubella virus and glutamic acid decarboxylase (65 and 67) protein determinants recognised by T cells of patients with type I diabetes mellitus. Diabetologia. 2000;43(6):750–762. doi: 10.1007/s001250051373. [DOI] [PubMed] [Google Scholar]
- 77.Gale EA. Congenital rubella: citation virus or viral cause of type 1 diabetes? Diabetologia. 2008;51(9):1559–1566. doi: 10.1007/s00125-008-1099-4. [DOI] [PubMed] [Google Scholar]
- 78.Lindberg B, Ahlfors K, Carlsson A, Ericsson UB, Landin-Olsson M, Lernmark A, Ludvigsson J, Sundkvist G, Ivarsson SA. Previous exposure to measles, mumps, and rubella—but not vaccination during adolescence—correlates to the prevalence of pancreatic and thyroid autoantibodies. Pediatrics. 1999;104(1):e12. doi: 10.1542/peds.104.1.e12. [DOI] [PubMed] [Google Scholar]
- 79.Hyoty H, Hiltunen M, Reunanen A, Leinikki P, Vesikari T, Lounamaa R, Tuomilehto J, Akerblom HK. Decline of mumps antibodies in type 1 (insulin-dependent) diabetic children and a plateau in the rising incidence of type 1 diabetes after introduction of the mumps–measles–rubella vaccine in Finland. Childhood Diabetes in Finland Study Group. Diabetologia. 1993;36(12):1303–1308. doi: 10.1007/BF00400810. [DOI] [PubMed] [Google Scholar]
- 80.Honeyman MC, Coulson BS, Stone NL, Gellert SA, Goldwater PN, Steele CE, Couper JJ, Tait BD, Colman PG, Harrison LC. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes. 2000;49(8):1319–1324. doi: 10.2337/diabetes.49.8.1319. [DOI] [PubMed] [Google Scholar]
- 81.Coulson BS, Witterick PD, Tan Y, Hewish MJ, Mountford JN, Harrison LC, Honeyman MC. Growth of rotaviruses in primary pancreatic cells. J Virol. 2002;76(18):9537–9544. doi: 10.1128/JVI.76.18.9537-9544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Honeyman MC, Stone NL, Falk BA, Nepom G, Harrison LC. Evidence for molecular mimicry between human T cell epitopes in rotavirus and pancreatic islet autoantigens. J Immunol. 2010;184(4):2204–2210. doi: 10.4049/jimmunol.0900709. [DOI] [PubMed] [Google Scholar]
- 83.Blomqvist M, Juhela S, Erkkila S, Korhonen S, Simell T, Kupila A, Vaarala O, Simell O, Knip M, Ilonen J. Rotavirus infections and development of diabetes-associated autoantibodies during the first 2 years of life. Clin Exp Immunol. 2002;128(3):511–515. doi: 10.1046/j.1365-2249.2002.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Graham KL, O’Donnell JA, Tan Y, Sanders N, Carrington EM, Allison J, Coulson BS. Rotavirus infection of infant and young adult nonobese diabetic mice involves extraintestinal spread and delays diabetes onset. J Virol. 2007;81(12):6446–6458. doi: 10.1128/JVI.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pak CY, Eun HM, McArthur RG, Yoon JW. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet. 1988;2(8601):1–4. doi: 10.1016/S0140-6736(88)92941-8. [DOI] [PubMed] [Google Scholar]
- 86.Roep BO, Hiemstra HS, Schloot NC, De Vries RR, Chaudhuri A, Behan PO, Drijfhout JW. Molecular mimicry in type 1 diabetes: immune cross-reactivity between islet autoantigen and human cytomegalovirus but not coxsackie virus. Ann NY Acad Sci. 2002;958:163–165. doi: 10.1111/j.1749-6632.2002.tb02961.x. [DOI] [PubMed] [Google Scholar]
- 87.Ivarsson SA, Lindberg B, Nilsson KO, Ahlfors K, Svanberg L. The prevalence of type 1 diabetes mellitus at follow-up of Swedish infants congenitally infected with cytomegalovirus. Diabet Med. 1993;10(6):521–523. doi: 10.1111/j.1464-5491.1993.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 88.Hiltunen M, Hyoty H, Karjalainen J, Leinikki P, Knip M, Lounamaa R, Akerblom HK. Serological evaluation of the role of cytomegalovirus in the pathogenesis of IDDM: a prospective study. The Childhood Diabetes in Finland Study Group. Diabetologia. 1995;38(6):705–710. doi: 10.1007/BF00401843. [DOI] [PubMed] [Google Scholar]
- 89.Aarnisalo J, Veijola R, Vainionpaa R, Simell O, Knip M, Ilonen J. Cytomegalovirus infection in early infancy: risk of induction and progression of autoimmunity associated with type 1 diabetes. Diabetologia. 2008;51(5):769–772. doi: 10.1007/s00125-008-0945-8. [DOI] [PubMed] [Google Scholar]
- 90.Tirabassi RS, Guberski DL, Blankenhorn EP, Leif JH, Woda BA, Liu Z, Winans D, Greiner DL, Mordes JP. Infection with viruses from several families triggers autoimmune diabetes in LEW*1WR1 rats: prevention of diabetes by maternal immunization. Diabetes. 2010;59(1):110–118. doi: 10.2337/db09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hillebrands JL, van der Werf N, Klatter FA, Bruggeman CA, Rozing J. Role of peritoneal macrophages in cytomegalovirus-induced acceleration of autoimmune diabetes in BB-rats. Clin Dev Immunol. 2003;10(2–4):133–139. doi: 10.1080/10446670310001626517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smelt MJ, Faas MM, de Haan BJ, Draijer C, Hugenholtz GC, de Haan A, Engelse MA, de Koning EJ, de Vos P. Susceptibility of human pancreatic beta cells for cytomegalovirus infection and the effects on cellular immunogenicity. Pancreas. 2012;41(1):39–49. doi: 10.1097/MPA.0b013e31821fc90c. [DOI] [PubMed] [Google Scholar]
- 93.Hirasawa K, Jun HS, Maeda K, Kawaguchi Y, Itagaki S, Mikami T, Baek HS, Doi K, Yoon JW. Possible role of macrophage-derived soluble mediators in the pathogenesis of encephalomyocarditis virus-induced diabetes in mice. J Virol. 1997;71(5):4024–4031. doi: 10.1128/jvi.71.5.4024-4031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chung YH, Jun HS, Son M, Bao M, Bae HY, Kang Y, Yoon JW. Cellular and molecular mechanism for Kilham rat virus-induced autoimmune diabetes in DR-BB rats. J Immunol. 2000;165(5):2866–2876. doi: 10.4049/jimmunol.165.5.2866. [DOI] [PubMed] [Google Scholar]
- 95.Zipris D, Lien E, Nair A, Xie JX, Greiner DL, Mordes JP, Rossini AA. TLR9-signaling pathways are involved in Kilham rat virus-induced autoimmune diabetes in the biobreeding diabetes-resistant rat. J Immunol. 2007;178(2):693–701. doi: 10.4049/jimmunol.178.2.693. [DOI] [PubMed] [Google Scholar]
- 96.Harada M, Kishimoto Y, Makino S. Prevention of overt diabetes and insulitis in NOD mice by a single BCG vaccination. Diabetes Res Clin Pract. 1990;8(2):85–89. doi: 10.1016/0168-8227(90)90017-N. [DOI] [PubMed] [Google Scholar]
- 97.Niu X, Zhou Z, Jiang T, Su H. The effects of complete Freund’s adjuvant on prevention of pancrentitis and diabetes mellitus in non-obese diabetic mice. Zhonghua Nei Ke Za Zhi. 2002;41(4):229–232. [PubMed] [Google Scholar]
- 98.Liddi R, Beales PE, Rosignoli G, Pozzilli P. Incomplete Freund’s adjuvant reduces diabetes in the non-obese diabetic mouse. Horm Metab Res. 2000;32(6):201–206. doi: 10.1055/s-2007-978622. [DOI] [PubMed] [Google Scholar]
- 99.Dyrberg T, Schwimmbeck P, Oldstone M. The incidence of diabetes in BB rats is decreased following acute LCMV infection. Adv Exp Med Biol. 1988;246:397–402. doi: 10.1007/978-1-4684-5616-5_48. [DOI] [PubMed] [Google Scholar]
- 100.Oldstone MB. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science. 1988;239(4839):500–502. doi: 10.1126/science.3277269. [DOI] [PubMed] [Google Scholar]
- 101.Christen U, Benke D, Wolfe T, Rodrigo E, Rhode A, Hughes AC, Oldstone MB, von Herrath MG. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J Clin Invest. 2004;113(1):74–84. doi: 10.1172/JCI200417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Novak J, Griseri T, Beaudoin L, Lehuen A. Regulation of type 1 diabetes by NKT cells. Int Rev Immunol. 2007;26(1–2):49–72. doi: 10.1080/08830180601070229. [DOI] [PubMed] [Google Scholar]
- 103.Beaudoin L, Laloux V, Novak J, Lucas B, Lehuen A. NKT cells inhibit the onset of diabetes by impairing the development of pathogenic T cells specific for pancreatic beta cells. Immunity. 2002;17(6):725–736. doi: 10.1016/S1074-7613(02)00473-9. [DOI] [PubMed] [Google Scholar]
- 104.Laloux V, Beaudoin L, Jeske D, Carnaud C, Lehuen A. NK T cell-induced protection against diabetes in V alpha 14-J alpha 281 transgenic nonobese diabetic mice is associated with a Th2 shift circumscribed regionally to the islets and functionally to islet autoantigen. J Immunol. 2001;166(6):3749–3756. doi: 10.4049/jimmunol.166.6.3749. [DOI] [PubMed] [Google Scholar]
- 105.Diana J, Griseri T, Lagaye S, Beaudoin L, Autrusseau E, Gautron AS, Tomkiewicz C, Herbelin A, Barouki R, von Herrath M, Dalod M, Lehuen A. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 2009;30(2):289–299. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 106.Wetzel JD, Barton ES, Chappell JD, Baer GS, Mochow-Grundy M, Rodgers SE, Shyr Y, Powers AC, Thomas JW, Dermody TS. Reovirus delays diabetes onset but does not prevent insulitis in nonobese diabetic mice. J Virol. 2006;80(6):3078–3082. doi: 10.1128/JVI.80.6.3078-3082.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilberz S, Partke HJ, Dagnaes-Hansen F, Herberg L. Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia. 1991;34(1):2–5. doi: 10.1007/BF00404016. [DOI] [PubMed] [Google Scholar]
- 108.Cook-Mills JM, Munshi HG, Perlman RL, Chambers DA. Mouse hepatitis virus infection suppresses modulation of mouse spleen T cell activation. Immunology. 1992;75(3):542–545. [PMC free article] [PubMed] [Google Scholar]
- 109.de Souza MS, Smith AL. Characterization of accessory cell function during acute infection of BALB/cByJ mice with mouse hepatitis virus (MHV), strain JHM. Lab Anim Sci. 1991;41(2):112–118. [PubMed] [Google Scholar]
- 110.Viskari HR, Koskela P, Lonnrot M, Luonuansuu S, Reunanen A, Baer M, Hyoty H. Can enterovirus infections explain the increasing incidence of type 1 diabetes? Diabetes Care. 2000;23(3):414–416. doi: 10.2337/diacare.23.3.414. [DOI] [PubMed] [Google Scholar]
- 111.Viskari H, Ludvigsson J, Uibo R, Salur L, Marciulionyte D, Hermann R, Soltesz G, Fuchtenbusch M, Ziegler AG, Kondrashova A, Romanov A, Kaplan B, Laron Z, Koskela P, Vesikari T, Huhtala H, Knip M, Hyoty H. Relationship between the incidence of type 1 diabetes and maternal enterovirus antibodies: time trends and geographical variation. Diabetologia. 2005;48(7):1280–1287. doi: 10.1007/s00125-005-1780-9. [DOI] [PubMed] [Google Scholar]
- 112.Gamble DR, Kinsley ML, FitzGerald MG, Bolton R, Taylor KW. Viral antibodies in diabetes mellitus. Br Med J. 1969;3(5671):627–630. doi: 10.1136/bmj.3.5671.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gamble DR, Taylor KW. Seasonal incidence of diabetes mellitus. Br Med J. 1969;3(5671):631–633. doi: 10.1136/bmj.3.5671.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hierholzer JC, Farris WA. Follow-up of children infected in a coxsackievirus B-3 and B-4 outbreak: no evidence of diabetes mellitus. J Infect Dis. 1974;129(6):741–746. doi: 10.1093/infdis/129.6.741. [DOI] [PubMed] [Google Scholar]
- 115.Fuchtenbusch M, Irnstetter A, Jager G, Ziegler AG. No evidence for an association of coxsackie virus infections during pregnancy and early childhood with development of islet autoantibodies in offspring of mothers or fathers with type 1 diabetes. J Autoimmun. 2001;17(4):333–340. doi: 10.1006/jaut.2001.0550. [DOI] [PubMed] [Google Scholar]
- 116.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hyoty H, Hiltunen M, Knip M, Laakkonen M, Vahasalo P, Karjalainen J, Koskela P, Roivainen M, Leinikki P, Hovi T, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995;44(6):652–657. doi: 10.2337/diabetes.44.6.652. [DOI] [PubMed] [Google Scholar]
- 118.Lonnrot M, Korpela K, Knip M, Ilonen J, Simell O, Korhonen S, Savola K, Muona P, Simell T, Koskela P, Hyoty H. Enterovirus infection as a risk factor for beta-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes. 2000;49(8):1314–1318. doi: 10.2337/diabetes.49.8.1314. [DOI] [PubMed] [Google Scholar]
- 119.Sadeharju K, Hamalainen AM, Knip M, Lonnrot M, Koskela P, Virtanen SM, Ilonen J, Akerblom HK, Hyoty H. Enterovirus infections as a risk factor for type I diabetes: virus analyses in a dietary intervention trial. Clin Exp Immunol. 2003;132(2):271–277. doi: 10.1046/j.1365-2249.2003.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stene LC, Oikarinen S, Hyoty H, Barriga KJ, Norris JM, Klingensmith G, Hutton JC, Erlich HA, Eisenbarth GS, Rewers M. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: the diabetes and autoimmunity study in the young (DAISY) Diabetes. 2010;59(12):3174–3180. doi: 10.2337/db10-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sarmiento L, Cabrera-Rode E, Lekuleni L, Cuba I, Molina G, Fonseca M, Heng-Hung L, Borroto AD, Gonzalez P, Mas-Lago P, Diaz-Horta O. Occurrence of enterovirus RNA in serum of children with newly diagnosed type 1 diabetes and islet cell autoantibody-positive subjects in a population with a low incidence of type 1 diabetes. Autoimmunity. 2007;40(7):540–545. doi: 10.1080/08916930701523429. [DOI] [PubMed] [Google Scholar]
- 122.Nairn C, Galbraith DN, Taylor KW, Clements GB. Enterovirus variants in the serum of children at the onset of type 1 diabetes mellitus. Diabet Med. 1999;16(6):509–513. doi: 10.1046/j.1464-5491.1999.00098.x. [DOI] [PubMed] [Google Scholar]
- 123.Schulte BM, Bakkers J, Lanke KH, Melchers WJ, Westerlaken C, Allebes W, Aanstoot HJ, Bruining GJ, Adema GJ, Van Kuppeveld FJ, Galama JM. Detection of enterovirus RNA in peripheral blood mononuclear cells of type 1 diabetic patients beyond the stage of acute infection. Viral Immunol. 2010;23(1):99–104. doi: 10.1089/vim.2009.0072. [DOI] [PubMed] [Google Scholar]
- 124.Frisk G, Tuvemo T. Enterovirus infections with beta-cell tropic strains are frequent in siblings of children diagnosed with type 1 diabetes children and in association with elevated levels of GAD65 antibodies. J Med Virol. 2004;73(3):450–459. doi: 10.1002/jmv.20111. [DOI] [PubMed] [Google Scholar]
- 125.Yoon JW, Austin M, Onodera T, Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300(21):1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 126.Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci USA. 2007;104(12):5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52(6):1143–1151. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- 128.Ylipaasto P, Klingel K, Lindberg AM, Otonkoski T, Kandolf R, Hovi T, Roivainen M. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia. 2004;47(2):225–239. doi: 10.1007/s00125-003-1297-z. [DOI] [PubMed] [Google Scholar]
- 129.Foulis AK, Farquharson MA, Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet. 1987;2(8573):1423–1427. doi: 10.1016/S0140-6736(87)91128-7. [DOI] [PubMed] [Google Scholar]
- 130.Huang X, Yuang J, Goddard A, Foulis A, James RF, Lernmark A, Pujol-Borrell R, Rabinovitch A, Somoza N, Stewart TA. Interferon expression in the pancreases of patients with type I diabetes. Diabetes. 1995;44(6):658–664. doi: 10.2337/diabetes.44.6.658. [DOI] [PubMed] [Google Scholar]
- 131.Otonkoski T, Roivainen M, Vaarala O, Dinesen B, Leipala JA, Hovi T, Knip M. Neonatal type I diabetes associated with maternal echovirus six infection: a case report. Diabetologia. 2000;43(10):1235–1238. doi: 10.1007/s001250051518. [DOI] [PubMed] [Google Scholar]
- 132.Vreugdenhil GR, Schloot NC, Hoorens A, Rongen C, Pipeleers DG, Melchers WJ, Roep BO, Galama JM. Acute onset of type I diabetes mellitus after severe echovirus nine infection: putative pathogenic pathways. Clin Infect Dis. 2000;31(4):1025–1031. doi: 10.1086/318159. [DOI] [PubMed] [Google Scholar]
- 133.Diaz-Horta O, Bello M, Cabrera-Rode E, Suarez J, Mas P, Garcia I, Abalos I, Jofra R, Molina G, Diaz–Diaz O, Dimario U. Echovirus four and type 1 diabetes mellitus. Autoimmunity. 2001;34(4):275–281. doi: 10.3109/08916930109014696. [DOI] [PubMed] [Google Scholar]
- 134.Cabrera-Rode E, Sarmiento L, Molina G, Perez C, Arranz C, Galvan JA, Prieto M, Barrios J, Palomera R, Fonseca M, Mas P, Diaz–Diaz O, Diaz-Horta O. Islet cell related antibodies and type 1 diabetes associated with echovirus 30 epidemic: a case report. J Med Virol. 2005;76(3):373–377. doi: 10.1002/jmv.20368. [DOI] [PubMed] [Google Scholar]
- 135.Paananen A, Savolainen-Kopra C, Kaijalainen S, Vaarala O, Hovi T, Roivainen M. Genetic and phenotypic diversity of echovirus 30 strains and pathogenesis of type 1 diabetes. J Med Virol. 2007;79(7):945–955. doi: 10.1002/jmv.20922. [DOI] [PubMed] [Google Scholar]
- 136.Cabrera-Rode E, Sarmiento L, Tiberti C, Molina G, Barrios J, Hernandez D, Diaz-Horta O, Di Mario U. Type 1 diabetes islet associated antibodies in subjects infected by echovirus 16. Diabetologia. 2003;46(10):1348–1353. doi: 10.1007/s00125-003-1179-4. [DOI] [PubMed] [Google Scholar]
- 137.Roivainen M, Ylipaasto P, Savolainen C, Galama J, Hovi T, Otonkoski T. Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains. Diabetologia. 2002;45(5):693–702. doi: 10.1007/s00125-002-0805-x. [DOI] [PubMed] [Google Scholar]
- 138.Atkinson MA, Bowman MA, Campbell L, Darrow BL, Kaufman DL, Maclaren NK. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest. 1994;94(5):2125–2129. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schloot NC, Willemen SJ, Duinkerken G, Drijfhout JW, de Vries RR, Roep BO. Molecular mimicry in type 1 diabetes mellitus revisited: T cell clones to GAD65 peptides with sequence homology to Coxsackie or proinsulin peptides do not crossreact with homologous counterpart. Hum Immunol. 2001;62(4):299–309. doi: 10.1016/S0198-8859(01)00223-3. [DOI] [PubMed] [Google Scholar]
- 140.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by coxsackievirus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4(7):781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 141.Tracy S, Drescher KM, Chapman NM, Kim KS, Carson SD, Pirruccello S, Lane PH, Romero JR, Leser JS. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J Virol. 2002;76(23):12097–12111. doi: 10.1128/JVI.76.23.12097-12111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Serreze DV, Ottendorfer EW, Ellis TM, Gauntt CJ, Atkinson MA. Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T cells in pancreatic islets. Diabetes. 2000;49(5):708–711. doi: 10.2337/diabetes.49.5.708. [DOI] [PubMed] [Google Scholar]
- 143.Horwitz MS, Fine C, Ilic A, Sarvetnick N. Requirements for viral-mediated autoimmune diabetes: beta-cell damage and immune infiltration. J Autoimmun. 2001;16(3):211–217. doi: 10.1006/jaut.2000.0486. [DOI] [PubMed] [Google Scholar]
- 144.Kanno T, Kim K, Kono K, Drescher KM, Chapman NM, Tracy S. Group B coxsackievirus diabetogenic phenotype correlates with replication efficiency. J Virol. 2006;80(11):5637–5643. doi: 10.1128/JVI.02361-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Szopa TM, Gamble DR, Taylor KW. Coxsackie B4 virus induces short-term changes in the metabolic functions of mouse pancreatic islets in vitro. Cell Biochem Funct. 1986;4(3):181–187. doi: 10.1002/cbf.290040304. [DOI] [PubMed] [Google Scholar]
- 146.Roivainen M, Rasilainen S, Ylipaasto P, Nissinen R, Ustinov J, Bouwens L, Eizirik DL, Hovi T, Otonkoski T. Mechanisms of coxsackievirus-induced damage to human pancreatic beta-cells. J Clin Endocrinol Metab. 2000;85(1):432–440. doi: 10.1210/jc.85.1.432. [DOI] [PubMed] [Google Scholar]
- 147.Flodstrom M, Horwitz MS, Maday A, Balakrishna D, Rodriguez E, Sarvetnick N. A critical role for inducible nitric oxide synthase in host survival following coxsackievirus B4 infection. Virology. 2001;281(2):205–215. doi: 10.1006/viro.2000.0801. [DOI] [PubMed] [Google Scholar]
- 148.Bergelson JM, Krithivas A, Celi L, Droguett G, Horwitz MS, Wickham T, Crowell RL, Finberg RW. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72(1):415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mena I, Fischer C, Gebhard JR, Perry CM, Harkins S, Whitton JL. Coxsackievirus infection of the pancreas: evaluation of receptor expression, pathogenesis, and immunopathology. Virology. 2000;271(2):276–288. doi: 10.1006/viro.2000.0332. [DOI] [PubMed] [Google Scholar]
- 150.Yap IS, Giddings G, Pocock E, Chantler JK. Lack of islet neogenesis plays a key role in beta-cell depletion in mice infected with a diabetogenic variant of coxsackievirus B4. J Gen Virol. 2003;84(Pt 11):3051–3068. doi: 10.1099/vir.0.19150-0. [DOI] [PubMed] [Google Scholar]
- 151.Horwitz MS, Ilic A, Fine C, Balasa B, Sarvetnick N. Coxsackieviral-mediated diabetes: induction requires antigen-presenting cells and is accompanied by phagocytosis of beta cells. Clin Immunol. 2004;110(2):134–144. doi: 10.1016/j.clim.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 152.Drescher KM, Kono K, Bopegamage S, Carson SD, Tracy S. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology. 2004;329(2):381–394. doi: 10.1016/j.virol.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 153.Horwitz MS, Krahl T, Fine C, Lee J, Sarvetnick N. Protection from lethal coxsackievirus-induced pancreatitis by expression of gamma interferon. J Virol. 1999;73(3):1756–1766. doi: 10.1128/jvi.73.3.1756-1766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Flodstrom M, Maday A, Balakrishna D, Cleary MM, Yoshimura A, Sarvetnick N. Target cell defense prevents the development of diabetes after viral infection. Nat Immunol. 2002;3(4):373–382. doi: 10.1038/ni771. [DOI] [PubMed] [Google Scholar]
- 155.Flodstrom-Tullberg M, Hultcrantz M, Stotland A, Maday A, Tsai D, Fine C, Williams B, Silverman R, Sarvetnick N. RNase L and double-stranded RNA-dependent protein kinase exert complementary roles in islet cell defense during coxsackievirus infection. J Immunol. 2005;174(3):1171–1177. doi: 10.4049/jimmunol.174.3.1171. [DOI] [PubMed] [Google Scholar]
- 156.In’t Veld P. Insulitis in the human endocrine pancreas: does a viral infection lead to inflammation and beta cell replication? Diabetologia. 2011 doi: 10.1007/s00125-011-2224-3. [DOI] [PubMed] [Google Scholar]
- 157.Viskari H, Ludvigsson J, Uibo R, Salur L, Marciulionyte D, Hermann R, Soltesz G, Fuchtenbusch M, Ziegler AG, Kondrashova A, Romanov A, Knip M, Hyoty H. Relationship between the incidence of type 1 diabetes and enterovirus infections in different European populations: results from the EPIVIR project. J Med Virol. 2004;72(4):610–617. doi: 10.1002/jmv.20033. [DOI] [PubMed] [Google Scholar]
- 158.Sadeharju K, Knip M, Virtanen SM, Savilahti E, Tauriainen S, Koskela P, Akerblom HK, Hyoty H. Maternal antibodies in breast milk protect the child from enterovirus infections. Pediatrics. 2007;119(5):941–946. doi: 10.1542/peds.2006-0780. [DOI] [PubMed] [Google Scholar]
- 159.Nathanson N, Kew OM. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol. 2010;172(11):1213–1229. doi: 10.1093/aje/kwq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 161.Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324(5925):387–389. doi: 10.1126/science.1167728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shigemoto T, Kageyama M, Hirai R, Zheng J, Yoneyama M, Fujita T. Identification of loss of function mutations in human genes encoding RIG-I and MDA5: implications for resistance to type I diabetes. J Biol Chem. 2009;284(20):13348–13354. doi: 10.1074/jbc.M809449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, Guja C, Ionescu-Tirgoviste C, Widmer B, Dunger DB, Savage DA, Walker NM, Clayton DG, Todd JA. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38(6):617–619. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 164.Chehadeh W, Weill J, Vantyghem MC, Alm G, Lefebvre J, Wattre P, Hober D. Increased level of interferon-alpha in blood of patients with insulin-dependent diabetes mellitus: relationship with coxsackievirus B infection. J Infect Dis. 2000;181(6):1929–1939. doi: 10.1086/315516. [DOI] [PubMed] [Google Scholar]
- 165.Alba A, Puertas MC, Carrillo J, Planas R, Ampudia R, Pastor X, Bosch F, Pujol-Borrell R, Verdaguer J, Vives-Pi M. IFN beta accelerates autoimmune type 1 diabetes in nonobese diabetic mice and breaks the tolerance to beta-cells in nondiabetes-prone mice. J Immunol. 2004;173(11):6667–6675. doi: 10.4049/jimmunol.173.11.6667. [DOI] [PubMed] [Google Scholar]
- 166.Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA. 2008;105(34):12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li Q, McDevitt HO. The role of interferon alpha in initiation of type I diabetes in the NOD mouse. Clin Immunol. 2011;140(1):3–7. doi: 10.1016/j.clim.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 168.Cooper JD, Walker NM, Smyth DJ, Downes K, Healy BC, Todd JA. Follow-up of 1715 SNPs from the Wellcome Trust Case Control Consortium genome-wide association study in type I diabetes families. Genes Immun. 2009;10(Suppl 1):S85–S94. doi: 10.1038/gene.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang JP, Asher DR, Chan M, Kurt-Jones EA, Finberg RW. Cutting edge: antibody-mediated TLR7-dependent recognition of viral RNA. J Immunol. 2007;178(6):3363–3367. doi: 10.4049/jimmunol.178.6.3363. [DOI] [PubMed] [Google Scholar]
- 170.Lee AS, Ghoreishi M, Cheng WK, Chang TY, Zhang YQ, Dutz JP. Toll-like receptor 7 stimulation promotes autoimmune diabetes in the NOD mouse. Diabetologia. 2011;54(6):1407–1416. doi: 10.1007/s00125-011-2083-y. [DOI] [PubMed] [Google Scholar]
- 171.Colonna M. Toll-like receptors and IFN-alpha: partners in autoimmunity. J Clin Invest. 2006;116(9):2319–2322. doi: 10.1172/JCI29879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McCartney SA, Vermi W, Lonardi S, Rossini C, Otero K, Calderon B, Gilfillan S, Diamond MS, Unanue ER, Colonna M. RNA sensor-induced type I IFN prevents diabetes caused by a beta cell-tropic virus in mice. J Clin Invest. 2011;121(4):1497–1507. doi: 10.1172/JCI44005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Via CS, Nguyen P, Niculescu F, Papadimitriou J, Hoover D, Silbergeld EK. Low-dose exposure to inorganic mercury accelerates disease and mortality in acquired murine lupus. Environ Health Perspect. 2003;111(10):1273–1277. doi: 10.1289/ehp.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Havarinasab S, Hultman P. Alteration of the spontaneous systemic autoimmune disease in (NZB × NZW)F1 mice by treatment with thimerosal (ethyl mercury) Toxicol Appl Pharmacol. 2006;214(1):43–54. doi: 10.1016/j.taap.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 175.Leffel EK, Wolf C, Poklis A, White KL., Jr Drinking water exposure to cadmium, an environmental contaminant, results in the exacerbation of autoimmune disease in the murine model. Toxicology. 2003;188(2–3):233–250. doi: 10.1016/S0300-483X(03)00092-1. [DOI] [PubMed] [Google Scholar]
- 176.Rahman M, Tondel M, Ahmad SA, Axelson O. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am J Epidemiol. 1998;148(2):198–203. doi: 10.1093/oxfordjournals.aje.a009624. [DOI] [PubMed] [Google Scholar]
- 177.Lai MS, Hsueh YM, Chen CJ, Shyu MP, Chen SY, Kuo TL, Wu MM, Tai TY. Ingested inorganic arsenic and prevalence of diabetes mellitus. Am J Epidemiol. 1994;139(5):484–492. doi: 10.1093/oxfordjournals.aje.a117031. [DOI] [PubMed] [Google Scholar]
- 178.Kolachi NF, Kazi TG, Afridi HI, Kazi N, Khan S, Kandhro GA, Shah AQ, Baig JA, Wadhwa SK, Shah F, Jamali MK, Arain MB. Status of toxic metals in biological samples of diabetic mothers and their neonates. Biol Trace Elem Res. 2011;143(1):196–212. doi: 10.1007/s12011-010-8879-7. [DOI] [PubMed] [Google Scholar]
- 179.Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, Lin-Shiau SY, Liu SH. Methylmercury induces pancreatic beta-cell apoptosis and dysfunction. Chem Res Toxicol. 2006;19(8):1080–1085. doi: 10.1021/tx0600705. [DOI] [PubMed] [Google Scholar]
- 180.Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, Lin-Shiau SY, Liu SH. The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic beta-cell dysfunction in vitro and in vivo. Diabetes. 2006;55(6):1614–1624. doi: 10.2337/db06-0029. [DOI] [PubMed] [Google Scholar]
- 181.Chen Y, Ahsan H, Slavkovich V, Peltier GL, Gluskin RT, Parvez F, Liu X, Graziano JH. No association between arsenic exposure from drinking water and diabetes mellitus: a cross-sectional study in Bangladesh. Environ Health Perspect. 2010;118(9):1299–1305. doi: 10.1289/ehp.0901559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Steinmaus C, Yuan Y, Liaw J, Smith AH. Low-level population exposure to inorganic arsenic in the United States and diabetes mellitus: a reanalysis. Epidemiology. 2009;20(6):807–815. doi: 10.1097/EDE.0b013e3181b0fd29. [DOI] [PubMed] [Google Scholar]
- 183.Brenden N, Rabbani H, Abedi-Valugerdi M. Analysis of mercury-induced immune activation in nonobese diabetic (NOD) mice. Clin Exp Immunol. 2001;125(2):202–210. doi: 10.1046/j.1365-2249.2001.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Abedi-Valugerdi M, Nilsson C, Zargari A, Gharibdoost F, DePierre JW, Hassan M. Bacterial lipopolysaccharide both renders resistant mice susceptible to mercury-induced autoimmunity and exacerbates such autoimmunity in susceptible mice. Clin Exp Immunol. 2005;141(2):238–247. doi: 10.1111/j.1365-2249.2005.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Funseth E, Wicklund-Glynn A, Friman G, Ilback N. Redistribution of accumulated 2,3,7,8-tetrachlorodibenzo-p-dioxin during coxsackievirus B3 infection in the mouse. Toxicol Lett. 2000;116(1–2):131–141. doi: 10.1016/S0378-4274(00)00217-4. [DOI] [PubMed] [Google Scholar]