Abstract
Cell signaling in response to an array of diverse stress stimuli converges on the phosphorylation of the α-subunit of eukaryotic initiation factor 2 (eIF2). Phosphorylation of eIF2α on serine 51 results in a severe decline in de novo protein synthesis and is an important strategy in the cell’s armory against stressful insults including viral infection, the accumulation of misfolded proteins, and starvation. The phosphorylation of eIF2α is carried out by a family of four kinases, PERK (PKR-like ER kinase), PKR (protein kinase double-stranded RNA-dependent), GCN2 (general control non-derepressible-2), and HRI (heme-regulated inhibitor). Each primarily responds to a distinct type of stress or stresses. Thus, while significant sequence similarity exists between the eIF2α kinases in their kinase domains, underlying their common role in phosphorylating eIF2α, additional unique features determine the regulation of these four proteins, that is, what signals activate them. This review will describe the structure of each eIF2α kinase and discuss how this is linked to their activation and function. In parallel to the general translational attenuation elicited by eIF2α kinase activation the translation of stress-induced mRNAs, most notably activating transcription factor 4 (ATF4) is enhanced and these set in motion cascades of gene expression constituting the integrated stress response (ISR), which seek to remediate stress and restore homeostasis. Depending on the cellular context and concurrent signaling pathways active, however, translational attenuation can also facilitate apoptosis. Accordingly, the role of the kinases in determining cell fate will also be discussed.
Keywords: eIF2α kinases, Cell stress, PKR-like ER kinase (PERK), Protein kinase double-stranded RNA-dependent (PKR), General control non-derepressible-2 (GCN2), Heme-regulated inhibitor (HRI), Activating transcription factor 4 (ATF4)
Introduction
The eIF2α kinases are a family of four well-characterized serine-threonine kinases, PERK (PKR-like ER kinase), PKR (protein kinase double-stranded RNA-dependent), GCN2 (general control non-derepressible-2) and HRI (heme-regulated inhibitor), which curtail general translation in response to a wide array of different cellular stresses while facilitating programs of stress-induced gene expression. They perform important and often essential functions in response to infection, proteotoxicity, and low levels of essential nutrients such as amino acids and heme, and in this way play important roles in viral pathogenicity, cancer, and during development. Although they are primarily recognized for their phosphorylation of eIF2α, additional substrates of these kinases have now been identified, which broadens the scope of their activity and likely contributes to the selectivity of signaling downstream of each of the four proteins; moreover, it is probable that additional substrates of these kinases remain to be discovered. Also, while each eIF2α kinase responds to distinct signals, which is determined by unique regulatory features, in certain cases these stimuli overlap, leading to a degree of redundancy among the kinases.
Phosphorylation of eIF2α protects cells by reducing the general rate of protein synthesis and also biases the cell’s translation initiation machinery towards translation of the mRNAs of genes with roles in stress responses [74, 76]. For these reasons, eIF2α phosphorylation and the ensuing signaling pathways have been termed an “integrated stress response” (ISR) [76]. Despite its benefit to the cell under many conditions, the question of whether phosphorylation of eIF2α is intrinsically a pro- or anti-apoptotic signal remains controversial [152, 170]. It is likely that the duration and levels of this phosphorylation event together with the other signaling pathways which are activated in parallel will determine whether or not eIF2α phosphorylation is ultimately pro-death or pro-survival.
Much of our understanding of the downstream signaling upon eIF2α phosphorylation has come from studies performed in Saccharomyces cerevisiae and we will discuss these data here where appropriate. In addition, studies in Drosophila melanogaster and Caenorhabditis elegans have also identified and characterized homologues of PERK and GCN2 (e.g., [155, 179]). However, in this review we will focus our discussions on a thorough analysis of the four mammalian eIF2α kinases with regard to their structural features, functions, regulation, and downstream signaling.
The eIF2α kinases
PERK
PERK (also known as PEK, EIF2AK3) is primarily activated by the accumulation of misfolded proteins in the endoplasmic reticulum (ER), a phenomenon termed ER stress [185]. Through its phosphorylation of eIF2α, PERK blocks the synthesis of new polypeptides, in this manner reducing the entry of nascent polypeptides into the ER lumen. This allows the ER time to refold misfolded proteins and dispose of those that are terminally misfolded, important elements of the cell’s “unfolded protein response” (UPR), which seeks to restore ER homeostasis. PERK has also been implicated in pathologies such as cancer [78], diabetes [73], and ischemia [105], and therefore its role in cell fate has attracted much attention.
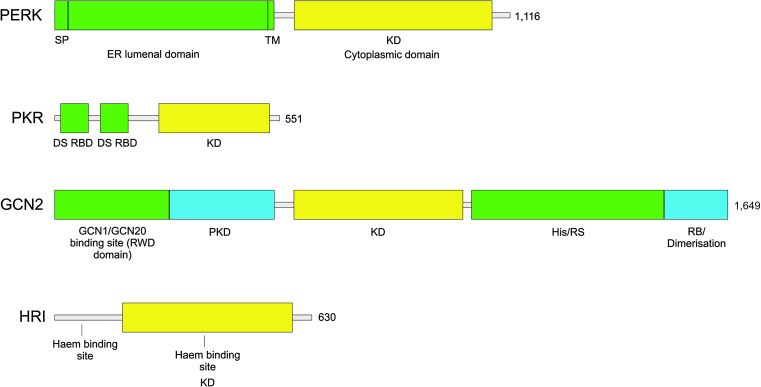
PERK is an ER transmembrane protein; its N-terminal region lies inside the ER lumen and incorporates the regions of the protein important for dimerization, regulation, and association with the ER chaperone, immunoglobulin binding protein (BiP), also known as glucose-regulated protein 78 (GRP78) and HspA5, while the C-terminal region is cytosolic and contains the kinase domain and autophosphorylation sites (Fig. 1) [75].
Fig. 1.
Domain organization of the four mammalian eIF2α kinases. Polypeptides are represented as bars running from N- to C-terminal domains from left to right. Length in amino acids is of the human proteins. SP signal peptide, TM transmembrane domain, KD kinase domain, DS RBD double-stranded RNA binding domain, PKD pseudokinase domain, His/Rs histidyl-tRNA synthetase-related domain, RB ribosome binding. Domains involved in sensing stress signals/activation are in green. Kinase domains are in yellow. Other domains are colored blue. Domains are drawn to scale
The precise trigger for PERK activation is still unclear; however, dimerization appears to be crucial. In its inactive state, PERK is bound by BiP and it has been proposed that misfolded proteins within the ER lumen compete with PERK for BiP binding. Accumulation of misfolded proteins causes BiP to dissociate from PERK allowing dimerization, autophosphorylation at several residues in the C-terminal domain, and activation of PERK to occur [15]. Intriguingly, a recent report on the effects of ischemia in the brain has described what appears to be a mechanism of PERK activation in the absence of misfolded proteins and without changes in the expression of ER chaperones, suggesting more complex and nuanced control of PERK signaling, which may be mediated through changes in ATP or Ca2+ levels within the ER, which are in turn sensed by BiP or co-chaperones [169]. In fact, some have argued that PERK functions primarily as a sensor of ER calcium levels [23, 66], and that diverse signals such as inhibition of the SERCA calcium pump, glucose deprivation, and high levels of fatty acids reduce the ER luminal calcium concentration, which in turn negatively affects the ability of BiP to maintain PERK in its inactive state.
The crystal structure of PERK’s kinase domain has recently been determined; this has facilitated the confirmation of Thr980 phosphorylation as a key event in PERK activation through stabilization of the activation loop and of the helix αG in the C-terminal lobe, which contacts eIF2α [35]. Through comparison with what is known regarding PKR activation (see below), and also with reference to the suggested mechanism of activation of a related ER-resident kinase, inositol requiring enzyme 1 (IRE1) [89, 101], a tentative model for PERK activation has been put forward [35]. In this “line-up” model, it is suggested that in the absence of misfolded proteins, PERK may be present as a population of back-to-back dimers; upon the release of BiP and/or binding of misfolded polypeptides to PERK’s C-terminal luminal domain PERK dimers line up in a row, bringing the activation loop of one dimer in proximity to the catalytic site of an adjacent dimer. Such an arrangement, it is suggested, would facilitate inter-dimer autophosphorylation of the activation loops followed by eIF2α binding and phosphorylation.
Recent data suggest that PERK is a dual specificity kinase that also possesses the ability to phosphorylate tyrosine residues [184]. This was discovered in the context of PERK activation where it was found that autophosphorylation at Tyr615 is important for full autophosphorylation of PERK and for PERK’s ability to efficiently phosphorylate eIF2α [184].
A further direct substrate of PERK is nuclear factor (erythroid-derived 2)-like two (NRF2), a bZIP transcription factor, which up-regulates the expression of a battery of antioxidant and detoxifying enzymes that aim to restore cellular redox homeostasis [138]. NRF2 is expressed constitutively but its activity is inhibited by its regulator Kelch-like ECH-associated protein 1 (KEAP1). Under normal conditions, it is constitutively degraded in a KEAP1-dependent manner (see [137] for a recent review on NRF2 regulation). In the context of the UPR, NRF2 undergoes PERK-mediated threonine phosphorylation at position 80 whereupon its binding to KEAP1 is disrupted [121] and NRF2 migrates to the nucleus where it induces the expression of its target genes. The importance of NRF2 activation during the response to ER stress is underscored by the fact that cells devoid of NRF2 are acutely sensitive to compounds that induce ER stress [36].
A link has also been made between activation of both PERK and PKR, and activation of glycogen synthase kinase-3β (GSK-3β) [11]. Specifically, signals that activate either PERK or PKR result in GSK-3β activation, which in turn promotes nuclear export and subsequent degradation of p53. The mechanism by which PERK and PKR signal to GSK-3β remains unknown but intriguingly phosphorylation of eIF2α does not seem to play a role [11].
PERK is now recognized as playing an extensive role in limiting oxidative stress and when growing cells in vitro it is recommended that cells deficient in PERK be cultured in the presence of reducing compounds [76]. Indeed, several ATF4 targets mediate responses to oxidative stress [76], and it thus appears that protection against oxidative stress is one element of the “integrated stress response”. Also, as the formation of disulphide bonds during protein maturation in the ER results in the equimolar production of reactive oxygen species (ROS) [172, 175], PERK directly ameliorates oxidative stress through its phosphorylation of eIF2α and the resulting block in protein synthesis [9].
The relationship between PERK and cancer progression is a particularly interesting aspect of PERK signaling. It is a well-established fact that solid tumors are prone to regions of hypoxia and that hypoxic tumors are particularly invasive and chemoresistant (see, among others, [173]). Hypoxia is a potent inducer of PERK-dependent eIF2α phosphorylation and in line with PERK’s important pro-survival function in cells exposed to hypoxia, PERK signaling can increase tumor size, vascularization, and rates of cell survival (reviewed in [102]). A recent study has further confirmed that PERK-deficient tumors are smaller than their wild-type counterparts and demonstrated that PERK-deficient cancer cells are compromised in their ability to progress through the cell cycle as a result of the accumulation of ROS-invoked DNA damage [16]. In contrast to this, ectopic expression of PERK in tumors has been shown to suppress growth of cancer cells in vivo [163], while inhibition of eIF2α phosphorylation can promote malignant transformation [51, 152], indicating that PERK also has tumor-suppressor characteristics. Thus, the actual role of PERK in cancer is likely complex, and contingent on cell type and oncogenic collaborators. A recent attractive model [16] based on in vivo studies in mice proposes a paradigm for PERK in cancer progression by arguing that (a) by itself, long-term loss of PERK from normal cells may increase spontaneous tumor formation (possibly through the loss of anti-oxidant tumor-suppressor effects, which protect against DNA damage and genomic instability); (b) loss of PERK triggers a significant attenuation of tumor cell proliferation in established tumors by inhibiting their ability to grow and divide in environments characterized by limited amounts of glucose and oxygen.
Although inherently cytoprotective in response to ER stress, constitutive PERK activity seems in fact to be a pro-death signal [115, 116] and it has been reported that constitutive PERK activity had detrimental effects on both proliferation and viability [116].
Consistent with an important role in regulating survival and cell death, PERK appears to be subject to additional regulation apart from simply being “turned on” by ER stress. The Hsp40 family member p58IPK, which is upregulated in response to ER stress [65], has been described to inhibit PERK through a direct interaction to ensure that eIF2α phosphorylation (and translational repression) as well as the upregulation of the downstream components of the UPR, BiP and CHOP, is transient following ER stress [192, 203]. However, this proposed role of p58IPK in inhibiting PERK is controversial, as a recent study has shown that it is localized exclusively in the ER lumen in complex with BiP where it plays a role in enhancing ER protein folding capacity [166]. Thus, it remains unclear how it can inhibit the kinase domain of PERK, which is cytoplasmic. It has been proposed that during prolonged ER stress, p58IPK, which harbors an inefficient ER targeting sequence, is retained at sufficient concentrations in the cytosol where it can inhibit PERK [166]. Recently, the Ca2+- and calmodulin-dependent phosphatase calcineurin was identified as another direct modifier of PERK signaling [18]. In contrast to p58IPK’s inhibitory function, it was described that calcineurin directly interacts with PERK’s cytosolic domain to promote PERK autophosphorylation, which in turn enhances eIF2α phosphorylation and the resulting translational attenuation. How a phosphatase, calcineurin, could promote the autophosphorylation and activation of PERK, a kinase, seems paradoxical and in fact, the mechanism behind this effect was not determined. We speculate that calcineurin may play some role in stabilizing the PERK protein. Interestingly, the interaction of calcineurin and PERK is promoted when cytosolic levels of calcium are high (and ER levels of calcium drop). This observation chimes well with the hypothesis of Cavener and coworkers that PERK functions primarily as a sensor of Ca2+ levels [23, 66].
Very recently, PERK was shown to be a direct target of AKT [131]. Intriguingly, it was shown that AKT can regulate PERK signaling through inhibitory phosphorylation [131]. A key aspect of this newly defined relationship is that AKT dampens PERK signaling, specifically eIF2α phosphorylation and ATF4 and CHOP expression, to promote survival under conditions of ER stress. Inhibition of AKT signaling was previously shown to sensitize cells to ER stress-induced apoptosis [84]; Mounir and colleagues show that this is at least partly due to unbridled PERK activity as overexpression of mutant PERK K618A cDNA in AKT 1/2 double-knockout cells afforded protection against ER stress-induced apoptosis [131]. These experiments suggest that, while essentially pro-survival in response to ER stress, PERK activation must also be regulated, i.e., there exists an optimal level of PERK signaling beyond which, due to higher levels of CHOP etc., PERK signaling becomes toxic. Indeed, as mentioned above, constitutively active PERK has been shown to promote apoptotic cell death [116], and PERK activation and signaling (in the absence of ATF6 and IRE1 activation) has been suggested to represent the terminal, pro-apoptotic phase of the UPR in response to prolonged ER stress [115].
The function of PERK at a physiological level is unclear. What has been established unequivocally is that loss of PERK is the cause of Wolcott–Rallison syndrome (WRS) in humans, a disease characterized by neonatal onset of diabetes that persists for life as well as defects in the pancreas and in the skeletal system [41]. PERK −/− mice recapitulate these main symptoms and display profound dysfunction in the absence of any stress stimuli, suffering from permanent neonatal diabetes [73], as well as skeletal dysplasias and postnatal growth retardation [211]. What are less clear are the underlying reasons for how loss of PERK leads to these abnormalities. Although it was postulated that the cause of diabetes in PERK −/− mice stems from high levels of ER stress in pancreatic beta cells [54], this notion has now been firmly challenged; using pancreas-specific PERK −/− mice it was shown that beta cells do not in fact die at a faster rate in the absence of PERK and do not exhibit dysregulation of genes associated with ER stress and apoptosis [213]. Rather, it was shown that the diminished beta cell mass in PERK −/− mice is as a result of reduced proliferation and that these cells are defective in their trafficking of proinsulin [213]. This is due to a reduced capacity of the ER to dispose of terminally misfolded proteins through ER-associated degradation (ERAD); [66], although more studies are certainly needed to definitively elucidate the molecular determinants connecting loss of PERK with diabetes. An additional physiological function of PERK appears to be to promote maturation of the mammary gland as mice deleted for PERK are compromised in their development of the mammary epithelium and fail to adequately induce lipogenic enzymes which results in the secretion of milk with an altered lipid profile and an impairment in the growth of pups [17].
PKR
PKR (or EIF2AK2) was initially discovered as a kinase that phosphorylates eIF2α in response to viral infection, thereby blocking the translation of viral mRNAs and promoting apoptosis in response to viral infection [56]. However, PKR also plays a more general role in cellular physiology; it can be activated in response to signals as diverse as oxidative and ER stress [87, 132, 142, 167], as well as cytokine signaling and growth factors [29, 61]. In addition, it has been implicated in the pathology of obesity [132], as well as cancer [100, 147].
PKR is the best characterized eIF2α kinase with regard to its structural features and mechanism of activation. Unlike PERK, the PKR protein is localized in the cytosol and the nucleus, and at its regulatory N-terminal region contains a double-stranded RNA binding domain (dsRBD) composed of two ~70-amino-acid dsRNA binding motifs that are separated by an unstructured linker region. The C-terminal kinase domain constitutes the dimerization interface crucial for PKR activation and carries out the catalytic functions of the protein. Transcription of PKR is induced by interferons, which are secreted early in the cellular response to viral infection [129]; before its activation by dsRNA or other stimuli PKR is resident in the cytoplasm and nucleus in a latent state. Earlier work stressed an important role for the dsRBDs in inhibiting activation of the kinase domains, in large part due to the observations that dsRBM2 contacts the catalytic domain while deletion of the entire dsRBD results in constitutively active PKR [200, 133]. In such a model, in the absence of dsRNA binding the interaction of dsRBM2 with the kinase domain gives a closed structure, which blocks the activation of PKR, while binding of dsRNA evicts dsRBM2 from the kinase domain, allowing activation to occur. However, based on several lines of evidence, including, small angle scattering [193], atomic force microscopy (AFM) [111], and equilibrium chemical denaturation experiments [5], it appears that PKR monomers, in fact, exist in an equilibrium between closed and open states, an interpretation supported by the observation that even in its latent state PKR’s kinase domain can be accessed by nucleotides [111]. Moreover, there also exists a large body of evidence that attributes a key role to dimerization of PKR in its activation. PKR undergoes phosphorylation when overexpressed [12], and promotion of PKR dimerization induces PKR autophosphorylation and activation [191, 194]. Thus, a further model for PKR activation has been proposed [31]. According to this model, prior to activation PKR molecules are present in the cell in conformations that flip back and forth between monomers and dimers. In response to binding of dsRNA, dimerization is induced, which is mediated by the C-terminal kinase domains. This in turn leads to autophosphorylation, stabilizing the dimer and resulting in a PKR–PKR complex capable of phosphorylating eIF2α [31]. In support of this model, a recent study showed that dimerization of human immunodeficiency virus type 1 transactivation-responsive region (TAR) RNA monomers induces recruitment of multiple PKR molecules allowing PKR dimerization and activation to proceed [79]. Many sites on PKR have been reported as sites of activating autophosphorylation [186]; however, there is strong evidence to suggest that phosphorylation on Thr 446, which is in the activation loop of the kinase domain of PKR (analogous to residue Thr 980 in the activation loop of PERK), is of particular significance. It is required for the full kinase activity of PKR [164], and mutation of this residue impairs phosphorylation of eIF2α [45]. It has also been reported that PKR is also phosphorylated at three tyrosine residues in vitro and in vivo and that these phosphorylation events play important roles in the binding of dsRNA and also in the dimerization, autophosphorylation, and activation of the kinase as well as subsequent phosphorylation of eIF2α [183].
As mentioned above, PKR is activated by several distinct stress signals [199], in a manner that appears to be independent of the presence of dsRNA. An important regulator of PKR in response to cellular stresses is PACT (Protein Activator); [151], which becomes phosphorylated in response to various insults [150], leading to its dissociation from the inhibitory protein TRBP (human immunodeficiency virus (HIV)-1 transactivation response (TAR) RNA-binding protein) [176]. It is unclear at present if this interaction is mediated by another macromolecule, as both proteins also bind RNA. PACT goes on to associate with and activate PKR by dint of the dsRBDs, which it shares with PKR [154]. PACT has been shown to be the link between stress signals such as ER stress [107, 177], serum starvation, and arsenite exposure [150].
An important feature that distinguishes PKR from the other eIF2α kinases is the number of its targets. As well as eIF2α, PKR phosphorylates p53 [33, 34], facilitates activation of the STAT transcription factors [40, 109, 161], in addition to promoting MAPK activity [61]. PKR also mediates NF-κB activation, seemingly through activation of the IKK complex [30].
Activation of PKR leads to apoptosis [58], a process that is dependent on phosphorylation of eIF2α [180], and has also been linked with NF-κB signaling [60]. Importantly, PKR induces expression of the pro-apoptotic transcription factor CHOP in response to stimuli such as hyperoxia [122], and ER stress [113, 177]. In general, the role of PKR in response to ER stress seems to be in direct opposition to that of PERK (the primary eIF2α kinase activated in response to ER stress). While PERK −/− cells are sensitive to cell death induced by ER stress (e.g., [74]), PKR −/− cells have been described to display a marked resistance to ER stress-induced apoptosis [107, 142, 177]. How two kinases that act on the same substrate can promote opposing outcomes in response to the same stimulus is a puzzling observation and the reasons behind these phenomena remain enigmatic. It is possible that the additional substrates of the two kinases may determine these effects and that PKR’s apoptotic role in response to ER stress may be determined in part by its activation of p53 [33] and or other factors. As discussed below, PERK signaling in fact limits p53 activity [11].
PKR signaling is not always pro-apoptotic in nature; in response to certain stress stimuli and in certain cell types PKR may also function to promote cellular survival, e.g., through its activation of NF-κB signaling [37]. In several cancer cell lines, its expression levels have been described to be elevated [99, 147], while knockdown resulted in cancer cell death [147].
Moreover, PKR signaling is regulated by several cellular factors. These can both serve to inhibit or activate PKR signaling and in this manner drive cells towards proliferation or death, respectively. For example, along with PACT, the tumor suppressor Mda7 has been shown to be an additional direct activator of PKR activity [149]. Overexpression of Mda7 induces apoptosis in a range of cancer cell lines in a PKR-dependent manner [148]. An additional inhibitor of PKR is the chaperone Hsp90 [50], which, consistent with its other anti-apoptotic functions, interacts with PKR to block its phosphorylation and to suppress apoptosis. The mechanisms underlying these effects are not completely clear. It was demonstrated that Hsp90-binding sites are present in the N-terminus regulatory domain and in the kinase domain of PKR and it was proposed that Hsp90 binding may enhance the inhibitory effects of PKR’s N-terminus on its activation. This may involve either enhancing the interaction between the N-terminal inhibitory region and the kinase domain or the covering of the kinase domain [50]. In addition, the nucleolar phosphoprotein nucleophosmin has been reported to physically interact with PKR and prevent its activity [146].
An additional cellular regulator of PKR is the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome 10) [130], which limits cell growth by inhibiting activity of the phosphoinositide 3-kinase (PI3 K) pathway through its reversal of the reaction catalyzed by PI3 K [59]. In fact, it was shown that PKR activation and eIF2α phosphorylation are additional key elements in the growth suppressive functions of PTEN and establish PKR as an element of a key signaling pathway, which is mutated in many cancers [212]. The mechanism by which PTEN activates PKR phosphorylation of eIF2α remains to be fully elucidated but requires the C-terminal PDZ-binding motif of PTEN [130].
Attenuation of mRNA translation through eIF2α phosphorylation functions to inhibit viral replication by blocking synthesis of viral proteins and by executing apoptosis; therefore, it is no surprise that viruses have evolved multiple mechanisms to try to prevent PKR activation. These include the synthesis of viral proteins that sequester dsRNA molecules, RNA inhibitors that bind PKR but prevent its activation, and prevention of the dimerization (and thus activation) of PKR (reviewed in [106]).
PKR −/− mice display no physical or behavioral phenotype under normal conditions and produce litters of normal size [1, 205]. There have been conflicting reports regarding the requirement of PKR for the mounting of adequate anti-viral responses [1, 10, 181, 205], indicating possible redundancy in the cell’s antiviral defense mechanisms and differential requirements for PKR activity depending on the infectious agent. Interestingly, despite its putative tumor suppressor role, mice with no functional PKR were not observed to develop tumors at an increased rate compared to wild-type counterparts [1], and PKR −/− fibroblasts did not give rise to tumors when injected into mice [205]. Thus, although a large number of signaling pathways have been linked with PKR, the in vivo significance of these interactions remains an open question.
GCN2
GCN2 (or EIF2AK4) is primarily a sensor of amino acid availability and a regulator of changes in gene expression in response to amino acid deprivation [43]. It is also activated by glucose deprivation in both yeast [204], and mammalian cells [206], although the exact mechanism remains to be defined. GCN2 was recently shown to be also activated in response to viral infection [14, 103] and UV irradiation [63].
GCN2′s structure is more complex than that of PERK and PKR. As well as a typical eukaryotic kinase domain, it also harbors a pseudo-kinase domain and a histidyl-tRNA synthetase (HisRS)-related domain, which binds uncharged tRNAs with higher affinity than it does charged tRNAs (Fig. 1). Activation of GCN2 is thought to occur through the binding of uncharged transfer RNAs (tRNAs) to the HisRS domain of the protein. These accumulate in response to starvation for essential amino acids or when the synthesis of a normally non-essential amino acid is inhibited [98]. As with other eIF2α kinases, dimerization appears to be key for activation [134], while autophosphorylation in the kinase activation loop at Thr882 and Thr887 is also required for optimal kinase activity [164]. Yeast Gcn2p has also been shown to be subject to inhibitory phosphorylation at residue Ser577 [57]. While the identity of the kinase responsible for this phosphorylation event is still unknown, it appears that it is under the negative control of TOR signaling [28]. Interestingly, multiple sequence alignment of the S. cerevisiae kinase with the S. pombe, murine and human GCN2 proteins demonstrate that this serine site is specific to S. cerevisiae. Sequences at both the N- and C-termini of GCN2 have been shown to be important for efficient sensing of the starvation signal. At the extreme N-terminus of the protein lies a binding site for the GCN1/GCN20 protein complex (also known as the RWD domain, so named as it is present in RING finger and WD repeat containing proteins and DEXDc-like helicases). These proteins are stimulators of GCN2 activation and together ensure that amino acid starvation is efficiently sensed by a mechanism that may involve the coordination of tRNA binding to the HisRS domain [49, 82]. Lysine residues in the C-terminus, meanwhile, have also been shown to be required for tRNA binding and kinase activity [49], and residues at the tip of the C-terminal region confer ribosome binding capabilities on GCN2, which are important for translational control [215].
GCN2 is also known to be activated by UV-irradiation, resulting in a translational arrest, although how UV irradiation results in the accumulation of uncharged tRNAs remains a mystery. In fact, there is evidence that the mechanism of action of GCN2 in the response to UV irradiation may not be via its effect on eIFα as a phosphorylatable form of eIF2α is not necessary for this to occur [63]. This strongly suggests that additional GCN2 substrates remain to be discovered. GCN2 also halts DNA replication after UV light in a manner which may or may not be dependent on eIF2α phosphorylation [63].
GCN2 can also be activated by viral infection and proteasome inhibition. The mechanism of GCN2 activation in response to virus was shown to proceed via direct binding of the genomic RNA of Sindbis Virus to the HisRs domain [14]. In addition, GCN2 has also been implicated in inhibiting the replication of vesicular stomatitis virus in a manner, which interestingly is not dependent on phosphorylation of eIF2α [103]. The activation of GCN2 in response to proteasome inhibition has now been noted by several groups [3, 92, 136]. The mechanism for this remains to be described; however, it seems plausible that interfering with the cell’s ability to recycle the building blocks of proteins through the proteasome might result in the accumulation of uncharged tRNAs.
At present, the only characterized substrate of GCN2 is eIF2α. A significant subset of the genes upregulated by ATF4, meanwhile, are involved in amino acid import and metabolism and a hallmark of ATF4 −/− cells is their impaired metabolism of amino acids [76] (discussed in detail below). Recent studies have also demonstrated the involvement of GCN2 signaling in cancer progression and in cellular responses to classical therapeutic agents. Interestingly, it has been shown that GCN2 can be activated by hypoxia [120], at least in murine cells in vitro; more significantly, GCN2 activation has shown to be a consequence of not only amino acid deprivation but also glucose deprivation, two stresses commonly encountered by solid tumors, in human cancer cells. Importantly, both human and mouse tumors were described to exhibit pronounced GCN2 activation while compromising GCN2 signaling severely hampered tumor growth in vivo [206]. As glucose deprivation and hypoxia can also activate PERK, it is likely that GCN2 and PERK cooperate in regulating stress signaling in tumors in vivo.
With regard to proteins that regulate GCN2 activity, in yeast it has been reported that the protein Snf1, which is the ortholog of the catalytic subunit of adenosine monophosphate activated kinase (AMPK), stimulates GCN2 activation through a direct interaction [27]. It appears that this stimulatory activity requires an intact Snf1 activation loop but actually proceeds through autophosphorylation of GCN2 [27].
It was initially described that GCN2 −/− mice develop normally in the absence of amino acid starvation [211]. However, a subsequent study reported that the survival rate of litters of GCN2 −/− mice is diminished versus GCN2 wild-type counterparts [6]. GCN2 is emerging as a key regulator of metabolism in response to amino acid starvation. Recently, it has been demonstrated that GCN2 is also involved in the utilization of lipid stores in response to amino acid deprivation [64]. When fed a diet deficient in leucine, GCN2 +/+ mice exhibited drastic decreases in the mass of both their liver and adipose tissue; these changes were nearly completely absent in GCN2 −/− mice. These mutant mice also experienced pronounced steatosis of the liver, which was followed by accumulation of triglycerides in the same organ. This was found to be due to a failure to downregulate genes involved in fatty acid transport, oxidation, and lipogenesis in response to leucine deprivation. Interestingly, this was not dependent on ATF4 as ATF4 −/− mice did not develop fatty liver and were able to repress expression of the fatty acid synthase mRNA [64].
GCN2 is expressed prominently in the brain and GCN2 has been implicated in behavioral adaptation to diets deficient in amino acids (discussed briefly in [44]). In mice, uncharged tRNA seems to act as a signal that directs the animal to reject diets deficient in amino acids. Firstly, food intake lacking essential amino acids was shown to result in GCN2-dependent eIF2α phosphorylation; secondly, and more intriguingly, mutant mice with no functional GCN2 were shown to readily ingest amino acid-deficient feed in contrast to their wild-type counterparts who reject diets lacking essential amino acids [71, 126]. It remains to be determined whether phosphorylation of eIF2α is required for this aversion behavior and if so how this could lead to mice rejecting amino acid-deficient feed; i.e., what are the molecular signals downstream of eIF2α and possibly ATF4 that govern feeding behavior?
HRI
The HRI kinase (or EIF2AK1) has two key roles during development; it serves to couple the synthesis of globin genes to the amount of heme present in the cell and it promotes the survival of erythroid precursors when iron levels are low [24]. It has also been implicated in responses to stresses as diverse as proteasome inhibition [207], and arsenite exposure [123], through mechanisms which are uncharacterized at present.
The HRI protein has five domains (Fig. 1). The N-terminus and C-terminus are unique while the protein also harbors two kinase regions flanking a central kinase insert (KI) domain. Binding sites for heme are present in both the N-terminus and KI regions. The N-terminus contains the stable heme binding site, required for heme’s regulation of HRI while the KI harbors the reversible heme-binding site [157]. The heme molecule is coordinated at both sites by flanking histidine residues (Fig. 1).
When levels of heme are low, HRI is activated by autophosphorylation. The current model proposes that once synthesized, HRI is bound by heme at its N-terminus. This binding of heme to HRI triggers intermolecular autophosphorylation, which stabilizes HRI against aggregation and generates a HRI–HRI dimer competent for sensing intracellular heme concentrations. When levels of heme are high, the HRI dimer is bound by heme and further phosphorylation leading to activation is inhibited; in conditions of heme deficiency, HRI is activated by further autophosphorylation and proceeds to phosphorylate eIF2α [13, 24, 157, 158].
It was initially thought that HRI expression is limited to erythrocytes [32], but recent studies have demonstrated that it is also present in the liver and in macrophages. Its role in red blood cells is best characterized and consists in ensuring that the production of α- and β-globin chains is commensurate with the amount of heme available to the cell. An imbalance between these three components will be toxic to the cell [171]. Attenuating general protein translation in red blood cells is a suitable mechanism for achieving this as α- and β-globin chains are expressed at very high levels in red blood cells. A recent direct comparative study between HRI +/+ and HRI −/− erythroid precursors from mouse embryos revealed that HRI signaling is important for erythroid differentiation, and identified genes for Gata1 and Fog1 transcription factors regulated by HRI, which may be necessary to maintain the differentiated state in developing erythroid cells [117, 118]. However, the link between HRI signaling and these transcription factors is not yet clear.
As mentioned above, it has been demonstrated that HRI is also present and physiologically active in the liver where it negatively regulates a key enzyme involved in the utilization of l-tryptophan [114], and in murine macrophages where it is important for macrophage maturation and for a robust inflammatory response [119]. A recent report indicates that HRI signaling may also be involved in ER homeostasis in the liver [2].
Very recently, activation of HRI was shown to hold promise as a therapeutic strategy in treating cancers. It was found that treatment of cancer cells with N,N′-diarylureas, simple aromatic urea molecules, led to phosphorylation of eIF2α, which was directly dependent on HRI. Treatment with these molecules inhibited tumor growth without any toxic side effects, indicating that selective induction of HRI-mediated eIF2α phosphorylation may represent a viable anticancer strategy in hepatocellular cancers, for example, as HRI is expressed in the liver [25].
HRI −/− mice display no gross abnormalities in the absence of stress, except for a slight increase in red blood cell volume and hemoglobin content. However, when these animals were fed a low-iron diet, this led to a failure to attenuate protein synthesis in red blood cells, the formation of globin inclusion bodies, and an increased rate of apoptosis in erythroid precursors [70]. Additionally, HRI deficiency has been demonstrated to exacerbate the phenotypes of hemochromatosis [118], through its regulation of hepcidin, a key player in iron homeostasis, and the red blood cell disorders erythropoietic protoporphyria and β-thalassemia [69], underscoring the crucial role of control of translation in red blood cell physiology.
Signaling downstream of the eIF2α kinases
eIF2 and the regulation of translation
Regulation of mRNA translation has evolved as an efficient way for cells to cope with fluctuations in the environment and with various cellular insults. Compared with control at the level of transcription, control of protein synthesis holds the key advantage of allowing rapid changes in the proteome, which is of particular importance when cells are faced with stressful conditions [83].
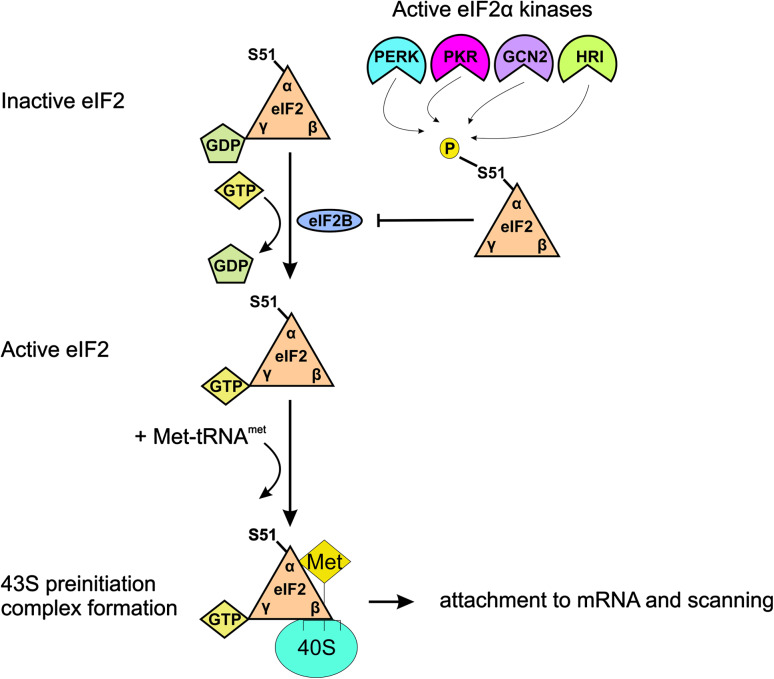
Regulation of translation occurs mostly at the level of initiation, generally the rate-limiting step in protein synthesis, and initiation itself depends on the formation of the preinitiation complex, which in turn is determined largely by eIF2 status. eIF2 participates in translation initiation by forming a 43S preinitiation complex together with a 40S ribosomal subunit and the translation initiation factors, eIF1, eIF1A, and eIF3. In this manner, eIF2, bound by GTP and Met-tRNAmet, facilitates recognition of the initiation codon by the scanning 43S complex allowing translation to proceed. After this recognition has occurred, the GTP bound to eIF2 is hydrolyzed and eIF2 (now complexed with GDP) is released by the translation machinery. Importantly, the hydrolysis of GTP now means that eIF2′s affinity for Met-tRNAmet is reduced. In order to return to its activated state, ready for subsequent rounds of translation, exchange of GDP for GTP on eIF2 is carried out by eIF2B. When eIF2-GDP is phosphorylated on its α-subunit at Ser51 by one of the eIF2α kinases, it switches from being a substrate to an inhibitor of eIF2B. Thus, levels of active GTP-bound eIF2 fall dramatically and general translation is curtailed (Figs. 2, 3) [90]. An important implication of eIF2-GDP’s inhibitory effects on eIF2B is that the overall ratio of eIF2:eIF2B levels will determine the sensitivity of the system to eIF2α phosphorylation, i.e., the inhibitory effects of eIF2α phosphorylation on translation will depend on the levels of eIF2α relative to eIF2B.
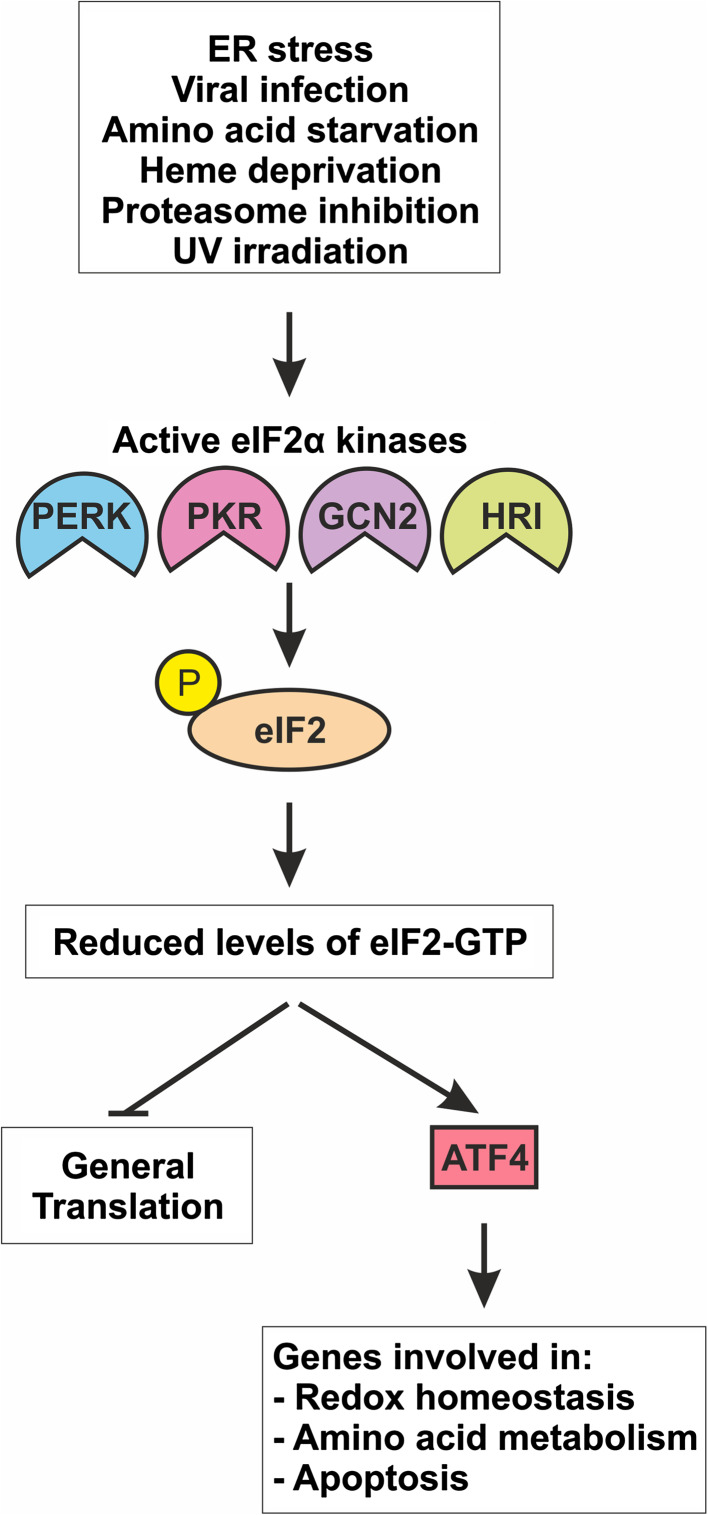
Fig. 2.
The activation of the eIF2α kinases and consequences of eIF2α phosphorylation. A large number of signals result in activation of the four eIF2α kinases, which then proceed to phosphorylate the α subunit of eIF2 blocking the recycling of GDP to GTP on the γ subunit of eIF2 by eIF2B. This has the effect of reducing levels of active eIF2-GTP, which leads to a block in general translation while concomitantly promoting enhanced translation of elements of the integrated stress response (ISR) such as ATF4. ATF4 upregulates numerous genes involved in amino acid homeostasis, redox metabolism, and apoptosis. For more details, see text. Not all activating signals for the eIF2α kinases are shown. Arrows denote activation or induction, while blunt lines indicate inhibition
Fig. 3.
A simplified schematic depicting eIF2 status in unstressed cells and in response to eIF2 kinase activation. In the absence of eIF2α kinase activation levels of eIF2-GTP are replenished by the nucleotide exchange factor, eIF2B allowing frequent assembly of the 43S preinitiation complex and efficient recognition of translational start codons (not shown). In response to diverse stress stimuli eIF2α kinases phosphorylate eIF2; this prevents eIF2B from exchanging eIF2′s GDP for GTP and results in a fall in the levels of eIF2-GTP-Met-tRNA. This ensures that formation of the preinitiation complex is hampered and that start codons are recognized with a lower frequency. See text for more details. For the sake of clarity and simplicity, not all the subunits of the 43S preinitiation complex are depicted. The subunits are not drawn to scale
eIF2α regulation and of the phosphorylation of eIF2α by the eIF2α kinases
In a screen conducted in an effort to identify mutants that suppress the slow-growth phenotype of a constitutively active GCN2 allele in yeast key amino acid residues in addition to Ser 51 involved in regulating eIF2α activity were identified. Specifically, four loss-of-function mutations (I58 M, L84F, R88C, and V89I), which alter the structural gene for eIF2α in yeast, known as SUI2, were characterized [195]. These mutants overcome the toxicity of constitutively activated forms of GCN2 by diminishing the inhibitory effects of phosphorylated eIF2α on translation initiation, rather than decreasing the proportion of eIF2α that is phosphorylated [195]. These residues are conserved in eIF2α of human, rat, and mouse. Most likely, the phosphorylated forms of these mutant eIF2α proteins lack the ability to inhibit eIF2B catalytic function and these mutations define a region in eIF2α that participates directly in a physical interaction with eIF2B [178]. In contrast, substitution of Asp by Ala at position 83 eliminated phosphorylation by GCN2 and PKR both in vivo and in vitro, establishing the critical contributions of the Ala 83 residue to kinase-substrate recognition [46]. Thus, two distinct regulators of eIF2α function, eIF2α kinases and eIF2B, have evolved to recognize the same surface and overlapping determinants on eIF2α.
Analysis of the interaction of PKR and a viral eIF2α analog, Vaccinia virus K3L, which acts as a competitive inhibitor of eIF2α and a pseudosubstrate for PKR has enhanced our understanding of how the eIF2α kinases phosphorylate eIF2α. The K3L protein has significant similarity to eIF2α at its N-terminal region [174], and mutation analysis of specific residues in K3L conserved with eIF2α (residues 79–83 in eIF2α) could abolish its inhibitory activity towards PKR [96].
The Myxoma virus protein M156R is also an efficient substrate for phosphorylation by PKR and can compete with eIF2α [162]. The similarity between the NMR structure of M156R and the beta-barrel structure in the N-terminus of eIF2α suggests that the viral homologs mimic eIF2α structure in order to compete for binding to PKR [162]. Comparison of the structures of the M156R and human eIF2α indicated that residues important for binding to PKR are located at conserved positions on the surface of the beta-barrel and in the mobile loop.
The X-ray crystal structures of the catalytic domain of PKR in complex with eIF2α have further confirmed the importance of PKR dimerization mediated by the kinase domains in PKR activation and have also revealed how the eIF2α substrate docks to the PKR kinase [39, 45]. The PKR:eIF2α structure demonstrates the importance of two distinct regions within the C lobe of the catalytic domain of PKR and in particular the αG-helix of PKR in PKR:eIF2α binding [39, 45]. The structure of eIF2α bound to PKR shows that substrate docking is a highly dynamic process where PKR docking to a site distal from Ser51 contributes to the accessibility of the Ser51 phospho site. A key feature of the PKR:eIF2α interaction and subsequent phosphorylation appears to be a disordering, which takes place in eIF2α upon PKR binding, in which the helix insert of eIF2α unfolds to allow PKR access to the Ser51 site. A subsequent study has further elucidated the mechanism of the PKR-induced disordering in eIF2α and has demonstrated that PKR binding elicits a conformational change in the Ser51 loop rendering it accessible to phosphorylation by PKR [47]. This may be due to the disruption of a hydrophobic network of protein interactions clustered around the Ser51 phosphorylation site. These experiments provide an obvious explanation for why a short peptide containing the Ser51 phosphorylation site is a poor substrate for PKR, unlike full-length eIF2α [128].
Selective translation as a result of eIF2α phosphorylation
Along with general inhibition of translation, a second key output and result of eIF2α phosphorylation is enhanced translation of bZIP transcription factors such as ATF4, [72] and ATF5 [214]. The mechanism underlying the increased translation of these transcription factors after eIF2α phosphorylation involves short upstream open reading frames (uORFs). These short uORFs are small (~30 codons) translated regions that lie upstream of about half of all mammalian genes. The mechanisms underlying the promotion of stress-induced gene expression by eIF2α phosphorylation were first elucidated for the S. cerevisiae homologue of ATF4, GCN4 (reviewed in [81]). There are four open reading frames in the 5′ untranslated region (UTR) of GCN4 mRNA that regulate its translation. When levels of amino acids, and thus eIF2-GTP, are high, the first uORF along with one of the subsequent ones (proximal to the GCN4 start site) are translated, which impairs the ability of scanning ribosomes to then reinitiate in time for the bona fide GCN4 AUG start codon. However, when amino acids are limiting many ribosomes translate the first uORF but then, because of decreased assembly of eIF2-GTP Met-tRNAmet and thus decreased preinitiation complex formation, fail to reinitiate in time for one of the three downstream ones. This has the effect of increasing the number of scanning ribosomes that will begin translation from the GCN4 start codon [80, 187]. In mice and humans, the configuration and size of these uORFs in the 5′ UTR differs; humans harbor a total of three, one of which actually overlaps the ATF4 ORF, while in mice there are two, one of which runs into the ATF4 ORF. However, the reinitiation model also holds true and the uORFs are configured in such a way as to favor translation of the bona fide ATF4 protein-coding ORF when levels of eIF2-GTP are low [72, 214]. Thus, when eIF2α-GTP levels are high, there is a greater possibility that all two or three uORFs are translated, which prevents the 40S subunit from rescanning or backtracking to the ATF4 ORF, thus resulting in no ATF4 expression. However, under stressed conditions, levels of eIF2-GTP will drop dramatically, and a higher number of 40S ribosomes will acquire eIF2-GTP Met-tRNAmet only after scanning past the last uORF and in time to recognize the ATF4 start codon, resulting in higher levels of ATF4 protein [90].
ATF4 and the ISR
ATF4 is the best characterized transcription factor preferentially translated upon eIF2α phosphorylation and is required for optimal induction of ATF3 [93] and ATF5 [214], additional transcription factors that participate in the ISR. In addition to translational control, ATF4 expression is subject to regulation at the level of transcription [7, 48], while its levels are further influenced by post-translational modifications such as phosphorylation [55]. Upon induction, ATF4 has been described to activate and repress a large number of genes involved in amino acid transport and metabolism, redox homoeostasis, and signaling (for a comprehensive list see [4, 76]) and it is this diversity of outputs coupled with the variety of signals which can activate the eIF2 kinases which has led to the coining of the term “integrated stress response” [76]. ATF4 is active as a homodimer [67], but can also regulate the expression of several of its target genes as a heterodimer with other bZIP transcription factors such as NRF2 [77] and ATF3 [93, 196]. Reflecting this diversity of function, cells lacking ATF4 require supplementation with amino acids and are also hypersensitive to oxidative stress [76, 206]. In addition, recent reports implicate both ATF4 and CHOP in inducing autophagy under hypoxic conditions [165, 168]. Another target of ATF4 is GADD34, which promotes the dephosphorylation of eIF2α, allowing the resumption of general translation and the expression of other ATF4 targets to take place (see below) [140]; importantly, both the human and murine mRNAs for GADD34 have also been shown to harbor uORFs, which facilitate translation under conditions of eIF2α phosphorylation. The mechanisms leading to this are incompletely characterized but appear to be distinct from the reinitiation model thought to regulate ATF4 translation [110]. ATF4 also upregulates the pro-apoptotic transcription factor CHOP, also known as GADD153, [143, 216], and Tribbles 3 (TRB3) [141], a pseudokinase that contributes to cell death under certain conditions in part by inhibiting the AKT pathway [20]. Thus, while ATF4 is a key regulator of genes involved in maintaining amino acid and redox homeostasis, it has also been implicated in contributing to cell death as part of the ISR.
While the enhanced translation of ATF4 is the best characterized element of the ISR induced upon eIF2α phosphorylation, it is known that up to one half of human mRNA transcripts harbor uORFs that might facilitate their translation upon depletion of active eIF2 [198]. Indeed, in addition to ATF4 and ATF5, CHOP mRNA also contains a single uORF, which allows efficient translation under conditions of eIF2α phosphorylation [145].
There is much evidence to indicate that phosphorylation of eIF2α and the ISR are closely linked with progression through the cell cycle. Several studies have now shown that activation of PERK and GCN2 promotes G1 arrest and exit from the cell cycle in response to ER stress and hypoxia, with both proteins playing overlapping roles in regulating cell cycle progression in response to these stress stimuli [19, 68, 120]. The molecular mechanisms connecting eIF2α phosphorylation with regulation of the cell cycle involve suppression of cyclin D1 expression [19, 68], while it has recently been reported that activation of PERK results in checkpoint kinase 1 (CHK1) phosphorylation and cell cycle delay in G2 [125].
The formation of stress granules, sites of the accumulation of untranslated mRNAs, is dependent on eIF2α phosphorylation under certain conditions [97], and as stress granules appear to play important anti-apoptotic functions [188], it would seem logical to consider the formation of these bodies as additional elements of the ISR.
The eIF2α kinases and NF-κB
The NF-кB family of transcription factors promote the expression of over 200 genes in response to numerous cellular stress stimuli [144]. The eIF2α kinases play a key role in the activation of NF-кB in response to many stimuli, through eIF2α phosphorylation [42, 94], and as discussed above, in the case of PKR activation of NF-кB may even be direct [104]. The mechanism by which eIF2α activates NF-κB appears to be largely due to decreased synthesis of the inhibitory proteins of NF-κB, the IκBs [42]. The eIF2α kinases have been demonstrated to be the link between stress and NF-кB activation in response to UV irradiation [92], amino acid starvation (in both cases through GCN2), and ER stress [94]. Considering the link between PERK signaling and cancer progression discussed above and the acknowledged role of NF-кB in promoting the growth of malignant cells [135], PERK’s cytoprotective effects in tumorigenesis may at least partly be mediated by NF-кB activity.
The eIF2α kinases and p53
As well as promoting cell survival through stimulating the ISR and NF-κB activity, the eIF2 kinases, specifically PERK, PKR, and GCN2, have also been implicated in controlling the levels and activity of p53. Both PERK and GCN2 have been shown to protect cells against hypoxia by limiting p53 levels and transcriptional activity, at least in murine cells in vitro [120].
PKR’s relationship with p53 appears particularly complex and interesting; while certain studies have documented an activating influence of PKR on p53-dependent transcription and death signaling [33, 208], a recent report showed that activation of PKR and PERK results in nucleocytoplasmic transport and proteasomal degradation of p53 [11]. To further add to the complexity, PKR was recently shown to be a transcriptional target of p53 in response to certain [209], but not all genotoxic stresses [159]. This raises the possibility that under certain circumstances PKR regulates p53 activity in a negative feedback loop. Further studies are certainly needed to unravel the relationship between p53 and PKR. Intriguingly, it has also been reported that p53 binds to the promoter of the PERK gene in vivo [197], although a functional consequence of this binding awaits elucidation.
As mentioned above, PERK signaling has been implicated in the degradation of p53 in response to ER stress [11] but this has been reported to be independent of eIF2α phosphorylation. Further, acute ablation of PERK was reported to result in increased expression of p53 [210]. Therefore, we envision that the control of p53 levels by PERK is likely to be also exerted indirectly through PERK’s role in reducing oxidative stress.
The eIF2α kinases and CHOP
As previously described, CHOP is a key downstream target of ATF4 and is also efficiently translated when eIF2α is phosphorylated [95]. Much of our understanding of CHOP signaling comes from studies on its role during ER stress (reviewed in [143]) and as part of the amino acid response pathway [21]. CHOP is rapidly upregulated by PERK/ATF4 during ER stress and, through its transcriptional activator and repressor functions, it promotes apoptosis via three main mechanisms. The first is regulation of genes involved in apoptotic pathways, such as the Bcl-2 family members BCL-2 and BIM, and the death receptor DR5 [127, 156, 202]. The second mechanism involves upregulation of GADD34, which promotes eIF2α dephosphorylation by interacting with protein phosphatase 1 (PP1) [22]. This relief of translational repression is required for the transcriptional program of the UPR [140], but also functions to allow polypeptides to enter the already-stressed ER, thus exacerbating the ER stress [139]. Finally, CHOP has also been shown to directly ERO1-α, which promotes hyperoxidation of the ER environment and an enhanced burden of oxidized protein complexes, and which also goes on to activate the inositol 1,4,5-triphosphate (IP3) receptor (IP3R), which mediates calcium release from the ER and further promotes apoptotic cell death [112]. Thus, CHOP regulation is a key factor in cell fate with regard to death or survival. Interestingly, there are now a number of reports in the literature that activation of GCN2 and PERK do not regulate CHOP expression in the same way. A number of studies have indicated that eIF2α phosphorylation resulting from GCN2 activation is a less potent inducer of both ATF4 and CHOP [38, 91, 182]. These results may be resolved by invoking a model where eIF2α phosphorylation comprises the overlapping region of a Venn diagram of cellular stress responses, i.e., the response of a cell to a given stress will be determined by the crosstalk between the pathways that are activated downstream of the insult. Certainly, it is known that in the case of PERK, other elements of the UPR can influence CHOP expression [124]; conversely, a recent study has argued that mTOR signaling (which is expected to be inhibited in response to amino acid deprivation) has a positive influence on CHOP expression [26]. However, a recent study on murine liver cells that utilized a system where mTOR signaling was not affected revealed differences in the transcriptional programs activated by PERK and GCN2 [38]. We envisage that the different subcellular localizations of the four kinases as well as the high probability that additional targets of the eIF2α kinases remain to be discovered likely play some role in these phenomena. An additional intriguing possibility [38] is that the four kinases (or stimuli that activate them) may differentially regulate the activity of eIF2B, and in this way alter the sensitivity of the system to eIF2α phosphorylation.
Nitric oxide as a regulator of the eIF2α kinases
Nitric oxide (NO) can activate all four of the eIF2α kinases and downstream signaling pathways. NO-mediated activation of the eIF2α kinases occurs through distinct mechanisms. In the case of HRI, NO facilitates the reduction of the inhibitory heme-Fe(III) bound to HRI to heme-Fe(II) [53]. NO goes on to bind this heme-Fe(II), leading to HRI autophosphorylation and activation [86].
NO can induce ER stress [62], and PERK activation by NO proceeds indirectly through NO-mediated ER stress. PERK signaling has been described to be very sensitive to fluctuations in the calcium content of the ER [52], and one mechanism through which NO might activate PERK might be through its disruption of ER calcium homeostasis, which it does on a number of levels [85, 201]. NO has also been described to compromise ER protein folding capacity through S-nitrosylation of a key ER chaperone, protein disulphide isomerase [189].
The activation of GCN2 by NO stems not from NO itself, but is rather an indirect effect of the production of NO by NOS. NOS requires l-Arg for the production of NO [88], and so NOS activation leads to a depletion of cellular l-Arg, which then results in activation of GCN2 [108].
NO has also been described to activate PKR [153]. NO appears to displace PKR from ribosomes where it is inhibited [160], possibly through S-nitrosylation, to facilitate PKR dimerization and activation. Interestingly, in the case of PKR, NO production has been described as an outcome of eIF2α kinase activation [190]. This PKR-mediated stimulation of NO production is an NF-κB-dependent phenomenon [8, 190].
Conclusions
Recent research into the eIF2α kinases has significantly enhanced our knowledge of the breadth of different stimuli, which can regulate this family of proteins and also of the effects that phosphorylation of eIF2α has on cellular physiology. A key feature of eIF2α kinase biology is the role of both conserved and unique structural features in the determination of the function of the kinases. Much is now known about how each kinase is regulated by distinct stress stimuli and also regarding the structural features of eIF2α phosphorylation by the four kinases. However, there are important gaps in our understanding, which future research should address. These are some of the key questions which remain to be elucidated: Are there additional substrates of the eIF2α kinases, which remain to be identified? How is specificity of signaling downstream of each kinase achieved, or how is the signaling downstream of each kinase tailored to address the stimulus that activates it? What other uORF- or IRES-containing mRNAs are preferentially translated in response to eIF2α phosphorylation? How does eIF2α kinase signaling intersect with key pro- and anti-apoptotic signaling pathways? It remains to be determined how certain stress stimuli activate the different eIF2α kinases. Salient examples in this regard are the activation of GCN2 by UV light and of PKR by ER stress. Are there additional regulators of eIF2α kinase function that have not yet been identified? Does the sub-cellular localization of the different eIF2α kinases lead to compartmentalization of eIF2α phosphorylation and does this have implications for the ISR? For example, PERK is at the ER membrane whereas the other three members of the family are all located in the cytoplasm.
Future research will certainly tease out additional elements of eIF2α kinase function and regulation, and will address some of these outstanding questions.
Acknowledgments
Our research is supported by Science Foundation Ireland (09/RFP/BIC2371; 09/RFP/BMT2153), the Health Research Board (HRA/2009/59) and Breast Cancer Campaign (2008NovPhD21; 2010NovPR13).
References
- 1.Abraham N, Stojdl DF, Duncan PI, Methot N, Ishii T, Dube M, Vanderhyden BC, Atkins HL, Gray DA, McBurney MW, Koromilas AE, Brown EG, Sonenberg N, Bell JC. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274:5953–5962. doi: 10.1074/jbc.274.9.5953. [DOI] [PubMed] [Google Scholar]
- 2.Acharya P, Chen JJ, Correia MA. Hepatic heme-regulated inhibitor (HRI) eukaryotic initiation factor 2alpha kinase: a protagonist of heme-mediated translational control of CYP2B enzymes and a modulator of basal endoplasmic reticulum stress tone. Mol Pharmacol. 2010;77:575–592. doi: 10.1124/mol.109.061259. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Acharya P, Engel JC, Correia MA. Hepatic CYP3A suppression by high concentrations of proteasomal inhibitors: a consequence of endoplasmic reticulum (ER) stress induction, activation of RNA-dependent protein kinase-like ER-bound eukaryotic initiation factor 2alpha (eIF2alpha)-kinase (PERK) and general control nonderepressible-2 eIF2alpha kinase (GCN2), and global translational shutoff. Mol Pharmacol. 2009;76:503–515. doi: 10.1124/mol.109.056002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol. 2008;40:14–21. doi: 10.1016/j.biocel.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Anderson E, Cole JL. Domain stabilities in protein kinase R (PKR): evidence for weak interdomain interactions. Biochemistry. 2008;47:4887–4897. doi: 10.1021/bi702211j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem. 2004;279:36553–36561. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong JL, Flockhart R, Veal GJ, Lovat PE, Redfern CP. Regulation of endoplasmic reticulum stress-induced cell death by ATF4 in neuroectodermal tumor cells. J Biol Chem. 2010;285:6091–6100. doi: 10.1074/jbc.M109.014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auch CJ, Saha RN, Sheikh FG, Liu X, Jacobs BL, Pahan K. Role of protein kinase R in double-stranded RNA-induced expression of nitric oxide synthase in human astroglia. FEBS Lett. 2004;563:223–228. doi: 10.1016/S0014-5793(04)00302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balachandran S, Roberts PC, Brown LE, Truong H, Pattnaik AK, Archer DR, Barber GN. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13:129–141. doi: 10.1016/S1074-7613(00)00014-5. [DOI] [PubMed] [Google Scholar]
- 11.Baltzis D, Pluquet O, Papadakis AI, Kazemi S, Qu LK, Koromilas AE. The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem. 2007;282:31675–31687. doi: 10.1074/jbc.M704491200. [DOI] [PubMed] [Google Scholar]
- 12.Barber GN, Tomita J, Hovanessian AG, Meurs E, Katze MG. Functional expression and characterization of the interferon-induced double-stranded RNA activated P68 protein kinase from Escherichia coli . Biochemistry. 1991;30:10356–10361. doi: 10.1021/bi00106a038. [DOI] [PubMed] [Google Scholar]
- 13.Bauer BN, Rafie-Kolpin M, Lu L, Han A, Chen JJ. Multiple autophosphorylation is essential for the formation of the active and stable homodimer of heme-regulated eIF2alpha kinase. Biochemistry. 2001;40:11543–11551. doi: 10.1021/bi010983s. [DOI] [PubMed] [Google Scholar]
- 14.Berlanga JJ, Ventoso I, Harding HP, Deng J, Ron D, Sonenberg N, Carrasco L, de Haro C. Antiviral effect of the mammalian translation initiation factor 2alpha kinase GCN2 against RNA viruses. EMBO J. 2006;25:1730–1740. doi: 10.1038/sj.emboj.7601073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 16.Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C, Cavener D, Diehl JA. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29:3881–3895. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bobrovnikova-Marjon E, Hatzivassiliou G, Grigoriadou C, Romero M, Cavener DR, Thompson CB, Diehl JA. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc Natl Acad Sci USA. 2008;105:16314–16319. doi: 10.1073/pnas.0808517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bollo M, Paredes RM, Holstein D, Zheleznova N, Camacho P, Lechleiter JD. Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs. PLoS ONE. 2010;5:e11925. doi: 10.1371/journal.pone.0011925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci USA. 2000;97:12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromati CR, Lellis-Santos C, Yamanaka TS, Nogueira TC, Leonelli M, Caperuto LC, Gorjao R, Leite AR, Anhe GF, Bordin S. UPR induces transient burst of apoptosis in islets of early lactating rats through reduced AKT phosphorylation via ATF4/CHOP stimulation of TRB3 expression. Am J Physiol Regul Integr Comp Physiol. 2011;300:R92–R100. doi: 10.1152/ajpregu.00169.2010. [DOI] [PubMed] [Google Scholar]
- 21.Bruhat A, Jousse C, Wang XZ, Ron D, Ferrara M, Fafournoux P. Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both transcriptional and post-transcriptional levels. J Biol Chem. 1997;272:17588–17593. doi: 10.1074/jbc.272.28.17588. [DOI] [PubMed] [Google Scholar]
- 22.Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23:1292–1303. doi: 10.1128/MCB.23.4.1292-1303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavener DR, Gupta S, McGrath BC. PERK in beta cell biology and insulin biogenesis. Trends Endocrinol Metab. 2010;21:714–721. doi: 10.1016/j.tem.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias. Blood. 2007;109:2693–2699. doi: 10.1182/blood-2006-08-041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T, Ozel D, Qiao Y, Harbinski F, Chen L, Denoyelle S, He X, Zvereva N, Supko JG, Chorev M, Halperin JA, Aktas BH. Chemical genetics identify eIF2alpha kinase heme-regulated inhibitor as an anticancer target. Nat Chem Biol. 2011;7:610–616. doi: 10.1038/nchembio.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YJ, Tan BC, Cheng YY, Chen JS, Lee SC. Differential regulation of CHOP translation by phosphorylated eIF4E under stress conditions. Nucleic Acids Res. 2010;38:764–777. doi: 10.1093/nar/gkp1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cherkasova V, Qiu H, Hinnebusch AG. Snf1 promotes phosphorylation of the alpha subunit of eukaryotic translation initiation factor 2 by activating Gcn2 and inhibiting phosphatases Glc7 and Sit4. Mol Cell Biol. 2010;30:2862–2873. doi: 10.1128/MCB.00183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherkasova VA, Hinnebusch AG. Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes Dev. 2003;17:859–872. doi: 10.1101/gad.1069003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheshire JL, Williams BR, Baldwin AS., Jr Involvement of double-stranded RNA-activated protein kinase in the synergistic activation of nuclear factor-kappaB by tumor necrosis factor-alpha and gamma-interferon in preneuronal cells. J Biol Chem. 1999;274:4801–4806. doi: 10.1074/jbc.274.8.4801. [DOI] [PubMed] [Google Scholar]
- 30.Chu WM, Ostertag D, Li ZW, Chang L, Chen Y, Hu Y, Williams B, Perrault J, Karin M. JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity. 1999;11:721–731. doi: 10.1016/S1074-7613(00)80146-6. [DOI] [PubMed] [Google Scholar]
- 31.Cole JL. Activation of PKR: an open and shut case? Trends Biochem Sci. 2007;32:57–62. doi: 10.1016/j.tibs.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crosby JS, Lee K, London IM, Chen JJ. Erythroid expression of the heme-regulated eIF-2 alpha kinase. Mol Cell Biol. 1994;14:3906–3914. doi: 10.1128/mcb.14.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuddihy AR, Li S, Tam NW, Wong AH, Taya Y, Abraham N, Bell JC, Koromilas AE. Double-stranded-RNA-activated protein kinase PKR enhances transcriptional activation by tumor suppressor p53. Mol Cell Biol. 1999;19:2475–2484. doi: 10.1128/mcb.19.4.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuddihy AR, Wong AH, Tam NW, Li S, Koromilas AE. The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro. Oncogene. 1999;18:2690–2702. doi: 10.1038/sj.onc.1202620. [DOI] [PubMed] [Google Scholar]
- 35.Cui W, Li J, Ron D, Sha B. The structure of the PERK kinase domain suggests the mechanism for its activation. Acta Crystallogr D Biol Crystallogr. 2011;67:423–428. doi: 10.1107/S0907444911006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 37.D’Acquisto F, Ghosh S (2001) PACT and PKR: turning on NF-kappa B in the absence of virus. Sci STKE 2001: re1 [DOI] [PubMed]
- 38.Dang Do AN, Kimball SR, Cavener DR, Jefferson LS. eIF2alpha kinases GCN2 and PERK modulate transcription and translation of distinct sets of mRNAs in mouse liver. Physiological genomics. 2009;38:328–341. doi: 10.1152/physiolgenomics.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dar AC, Dever TE, Sicheri F. Higher-order substrate recognition of eIF2alpha by the RNA-dependent protein kinase PKR. Cell. 2005;122:887–900. doi: 10.1016/j.cell.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 40.Deb A, Zamanian-Daryoush M, Xu Z, Kadereit S, Williams BR. Protein kinase PKR is required for platelet-derived growth factor signaling of c-fos gene expression via Erks and Stat3. EMBO J. 2001;20:2487–2496. doi: 10.1093/emboj/20.10.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott–Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 42.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deval C, Chaveroux C, Maurin AC, Cherasse Y, Parry L, Carraro V, Milenkovic D, Ferrara M, Bruhat A, Jousse C, Fafournoux P. Amino acid limitation regulates the expression of genes involved in several specific biological processes through GCN2-dependent and GCN2-independent pathways. FEBS J. 2009;276:707–718. doi: 10.1111/j.1742-4658.2008.06818.x. [DOI] [PubMed] [Google Scholar]
- 44.Dever TE, Hinnebusch AG. GCN2 whets the appetite for amino acids. Mol Cell. 2005;18:141–142. doi: 10.1016/j.molcel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 45.Dey M, Cao C, Dar AC, Tamura T, Ozato K, Sicheri F, Dever TE. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2alpha substrate recognition. Cell. 2005;122:901–913. doi: 10.1016/j.cell.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 46.Dey M, Trieselmann B, Locke EG, Lu J, Cao C, Dar AC, Krishnamoorthy T, Dong J, Sicheri F, Dever TE. PKR and GCN2 kinases and guanine nucleotide exchange factor eukaryotic translation initiation factor 2B (eIF2B) recognize overlapping surfaces on eIF2alpha. Mol Cell Biol. 2005;25:3063–3075. doi: 10.1128/MCB.25.8.3063-3075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dey M, Velyvis A, Li JJ, Chiu E, Chiovitti D, Kay LE, Sicheri F, Dever TE. Requirement for kinase-induced conformational change in eukaryotic initiation factor 2alpha (eIF2alpha) restricts phosphorylation of Ser51. Proc Natl Acad Sci USA. 2011;108:4316–4321. doi: 10.1073/pnas.1014872108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dey S, Baird TD, Zhou D, Palam LR, Spandau DF, Wek RC. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J Biol Chem. 2010;285:33165–33174. doi: 10.1074/jbc.M110.167213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong J, Qiu H, Garcia-Barrio M, Anderson J, Hinnebusch AG. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol Cell. 2000;6:269–279. doi: 10.1016/S1097-2765(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 50.Donze O, Abbas-Terki T, Picard D. The Hsp90 chaperone complex is both a facilitator and a repressor of the dsRNA-dependent kinase PKR. EMBO J. 2001;20:3771–3780. doi: 10.1093/emboj/20.14.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donze O, Jagus R, Koromilas AE, Hershey JW, Sonenberg N. Abrogation of translation initiation factor eIF-2 phosphorylation causes malignant transformation of NIH 3T3 cells. EMBO J. 1995;14:3828–3834. doi: 10.1002/j.1460-2075.1995.tb00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DuRose JB, Tam AB, Niwa M. Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol Biol Cell. 2006;17:3095–3107. doi: 10.1091/mbc.E06-01-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandez BO, Lorkovic IM, Ford PC. Mechanisms of ferriheme reduction by nitric oxide: nitrite and general base catalysis. Inorg Chem. 2004;43:5393–5402. doi: 10.1021/ic049532x. [DOI] [PubMed] [Google Scholar]
- 54.Fonseca SG, Urano F, Burcin M, Gromada J. Stress hypERactivation in the beta-cell. Islets. 2010;2:1–9. doi: 10.4161/isl.2.1.10456. [DOI] [PubMed] [Google Scholar]
- 55.Frank CL, Ge X, Xie Z, Zhou Y, Tsai LH. Control of activating transcription factor 4 (ATF4) persistence by multisite phosphorylation impacts cell cycle progression and neurogenesis. J Biol Chem. 2010;285:33324–33337. doi: 10.1074/jbc.M110.140699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008;4:e1000018. doi: 10.1371/journal.ppat.1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Barrio M, Dong J, Cherkasova VA, Zhang X, Zhang F, Ufano S, Lai R, Qin J, Hinnebusch AG. Serine 577 is phosphorylated and negatively affects the tRNA binding and eIF2alpha kinase activities of GCN2. J Biol Chem. 2002;277:30675–30683. doi: 10.1074/jbc.M203187200. [DOI] [PubMed] [Google Scholar]
- 58.Garcia MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Georgescu MM. PTEN tumor suppressor network in PI3 K-Akt pathway control. Genes Cancer. 2010;1:1170–1177. doi: 10.1177/1947601911407325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gil J, Alcami J, Esteban M. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-kappaB. Mol Cell Biol. 1999;19:4653–4663. doi: 10.1128/mcb.19.7.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goh KC, de Veer MJ, Williams BR. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000;19:4292–4297. doi: 10.1093/emboj/19.16.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gotoh T, Mori M. Nitric oxide and endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol. 2006;26:1439–1446. doi: 10.1161/01.ATV.0000223900.67024.15. [DOI] [PubMed] [Google Scholar]
- 63.Grallert B, Boye E. The Gcn2 kinase as a cell cycle regulator. Cell Cycle Georgetown Tex. 2007;6:2768–2772. doi: 10.4161/cc.6.22.4933. [DOI] [PubMed] [Google Scholar]
- 64.Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–114. doi: 10.1016/j.cmet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Gupta S, Cuffe L, Szegezdi E, Logue SE, Neary C, Healy S, Samali A. Mechanisms of ER stress-mediated mitochondrial membrane permeabilization. Int J Cell Biol. 2010;2010:170215. doi: 10.1155/2010/170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gupta S, McGrath B, Cavener DR. PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes. 2010;59:1937–1947. doi: 10.2337/db09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamanaka RB, Bennett BS, Cullinan SB, Diehl JA. PERK and GCN2 contribute to eIF2alpha phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Mol Biol Cell. 2005;16:5493–5501. doi: 10.1091/mbc.E05-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han AP, Fleming MD, Chen JJ. Heme-regulated eIF2alpha kinase modifies the phenotypic severity of murine models of erythropoietic protoporphyria and beta-thalassemia. J Clin Investig. 2005;115:1562–1570. doi: 10.1172/JCI24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han AP, Yu C, Lu L, Fujiwara Y, Browne C, Chin G, Fleming M, Leboulch P, Orkin SH, Chen JJ. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 2001;20:6909–6918. doi: 10.1093/emboj/20.23.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, Gietzen DW. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–1778. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- 72.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/S1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 73.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/S1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 74.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 75.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 76.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 77.He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AM, Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 78.Healy SJ, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol. 2009;625:234–246. doi: 10.1016/j.ejphar.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 79.Heinicke LA, Wong CJ, Lary J, Nallagatla SR, Diegelman-Parente A, Zheng X, Cole JL, Bevilacqua PC. RNA dimerization promotes PKR dimerization and activation. J Mol Biol. 2009;390:319–338. doi: 10.1016/j.jmb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hinnebusch AG. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc Natl Acad Sci USA. 1984;81:6442–6446. doi: 10.1073/pnas.81.20.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 82.Hinnebusch AG, Natarajan K. Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot Cell. 2002;1:22–32. doi: 10.1128/EC.01.1.22-32.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 84.Hu P, Han Z, Couvillon AD, Exton JH. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem. 2004;279:49420–49429. doi: 10.1074/jbc.M407700200. [DOI] [PubMed] [Google Scholar]
- 85.Huang G, Yao J, Zeng W, Mizuno Y, Kamm KE, Stull JT, Harding HP, Ron D, Muallem S. ER stress disrupts Ca2+-signaling complexes and Ca2+ regulation in secretory and muscle cells from PERK-knockout mice. J Cell Sci. 2006;119:153–161. doi: 10.1242/jcs.02731. [DOI] [PubMed] [Google Scholar]
- 86.Igarashi J, Sato A, Kitagawa T, Yoshimura T, Yamauchi S, Sagami I, Shimizu T. Activation of heme-regulated eukaryotic initiation factor 2alpha kinase by nitric oxide is induced by the formation of a five-coordinate NO-heme complex: optical absorption, electron spin resonance, and resonance Raman spectral studies. J Biol Chem. 2004;279:15752–15762. doi: 10.1074/jbc.M310273200. [DOI] [PubMed] [Google Scholar]
- 87.Ito T, Yang M, May WS. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J Biol Chem. 1999;274:15427–15432. doi: 10.1074/jbc.274.22.15427. [DOI] [PubMed] [Google Scholar]
- 88.Iwanaga T, Yamazaki T, Kominami S. Kinetic studies on the successive reaction of neuronal nitric oxide synthase from l-arginine to nitric oxide and l-citrulline. Biochemistry. 1999;38:16629–16635. doi: 10.1021/bi991277i. [DOI] [PubMed] [Google Scholar]
- 89.Iwawaki T, Akai R, Kohno K. IRE1alpha disruption causes histological abnormality of exocrine tissues, increase of blood glucose level, and decrease of serum immunoglobulin level. PLoS ONE. 2010;5:e13052. doi: 10.1371/journal.pone.0013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang HY, Wek RC. GCN2 phosphorylation of eIF2alpha activates NF-kappaB in response to UV irradiation. Biochem J. 2005;385:371–380. doi: 10.1042/BJ20041348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang HY, Wek RC. Phosphorylation of the alpha-subunit of the eukaryotic initiation factor-2 (eIF2alpha) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J Biol Chem. 2005;280:14189–14202. doi: 10.1074/jbc.M413660200. [DOI] [PubMed] [Google Scholar]
- 93.Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol. 2004;24:1365–1377. doi: 10.1128/MCB.24.3.1365-1377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003;23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jousse C, Bruhat A, Carraro V, Urano F, Ferrara M, Ron D, Fafournoux P. Inhibition of CHOP translation by a peptide encoded by an open reading frame localized in the chop 5′UTR. Nucleic Acids Res. 2001;29:4341–4351. doi: 10.1093/nar/29.21.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawagishi-Kobayashi M, Silverman JB, Ung TL, Dever TE. Regulation of the protein kinase PKR by the vaccinia virus pseudosubstrate inhibitor K3L is dependent on residues conserved between the K3L protein and the PKR substrate eIF2alpha. Mol Cell Biol. 1997;17:4146–4158. doi: 10.1128/mcb.17.7.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kilberg MS, Pan YX, Chen H, Leung-Pineda V. Nutritional control of gene expression: how mammalian cells respond to amino acid limitation. Annu Rev Nutr. 2005;25:59–85. doi: 10.1146/annurev.nutr.24.012003.132145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim SH, Forman AP, Mathews MB, Gunnery S. Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. Oncogene. 2000;19:3086–3094. doi: 10.1038/sj.onc.1203632. [DOI] [PubMed] [Google Scholar]
- 100.Kim SH, Gunnery S, Choe JK, Mathews MB. Neoplastic progression in melanoma and colon cancer is associated with increased expression and activity of the interferon-inducible protein kinase, PKR. Oncogene. 2002;21:8741–8748. doi: 10.1038/sj.onc.1205987. [DOI] [PubMed] [Google Scholar]
- 101.Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457:687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koumenis C. ER stress, hypoxia tolerance and tumor progression. Curr Mol Med. 2006;6:55–69. doi: 10.2174/156652406775574604. [DOI] [PubMed] [Google Scholar]
- 103.Krishnamoorthy J, Mounir Z, Raven JF, Koromilas AE. The eIF2alpha kinases inhibit vesicular stomatitis virus replication independently of eIF2alpha phosphorylation. Cell cycle Georgetown Tex. 2008;7:2346–2351. doi: 10.4161/cc.6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar A, Haque J, Lacoste J, Hiscott J, Williams BR. Double-stranded RNA-dependent protein kinase activates transcription factor NF-kappa B by phosphorylating I kappa B. Proc Natl Acad Sci USA. 1994;91:6288–6292. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar R, Azam S, Sullivan JM, Owen C, Cavener DR, Zhang P, Ron D, Harding HP, Chen JJ, Han A, White BC, Krause GS, DeGracia DJ. Brain ischemia and reperfusion activates the eukaryotic initiation factor 2alpha kinase, PERK. J Neurochem. 2001;77:1418–1421. doi: 10.1046/j.1471-4159.2001.00387.x. [DOI] [PubMed] [Google Scholar]
- 106.Langland JO, Cameron JM, Heck MC, Jancovich JK, Jacobs BL. Inhibition of PKR by RNA and DNA viruses. Virus Res. 2006;119:100–110. doi: 10.1016/j.virusres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 107.Lee ES, Yoon CH, Kim YS, Bae YS. The double-strand RNA-dependent protein kinase PKR plays a significant role in a sustained ER stress-induced apoptosis. FEBS Lett. 2007;581:4325–4332. doi: 10.1016/j.febslet.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 108.Lee J, Ryu H, Ferrante RJ, Morris SM, Jr, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci USA. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee JH, Park EJ, Kim OS, Kim HY, Joe EH, Jou I. Double-stranded RNA-activated protein kinase is required for the LPS-induced activation of STAT1 inflammatory signaling in rat brain glial cells. Glia. 2005;50:66–79. doi: 10.1002/glia.20156. [DOI] [PubMed] [Google Scholar]
- 110.Lee YY, Cevallos RC, Jan E. An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2alpha phosphorylation. J Biol Chem. 2009;284:6661–6673. doi: 10.1074/jbc.M806735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lemaire PA, Tessmer I, Craig R, Erie DA, Cole JL. Unactivated PKR exists in an open conformation capable of binding nucleotides. Biochemistry. 2006;45:9074–9084. doi: 10.1021/bi060567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191:1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liao M, Pabarcus MK, Wang Y, Hefner C, Maltby DA, Medzihradszky KF, Salas-Castillo SP, Yan J, Maher JJ, Correia MA. Impaired dexamethasone-mediated induction of tryptophan 2,3-dioxygenase in heme-deficient rat hepatocytes: translational control by a hepatic eIF2alpha kinase, the heme-regulated inhibitor. J Pharma Exp Ther. 2007;323:979–989. doi: 10.1124/jpet.107.124602. [DOI] [PubMed] [Google Scholar]
- 115.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin JH, Li H, Zhang Y, Ron D, Walter P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS ONE. 2009;4:e4170. doi: 10.1371/journal.pone.0004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu S, Bhattacharya S, Han A, Suragani RN, Zhao W, Fry RC, Chen JJ. Heme-regulated eIF2alpha kinase is necessary for adaptive gene expression in erythroid precursors under the stress of iron deficiency. Br J Haematol. 2008;143:129–137. doi: 10.1111/j.1365-2141.2008.07293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu S, Suragani RN, Han A, Zhao W, Andrews NC, Chen JJ. Deficiency of heme-regulated eIF2alpha kinase decreases hepcidin expression and splenic iron in HFE−/− mice. Haematologica. 2008;93:753–756. doi: 10.3324/haematol.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu S, Suragani RN, Wang F, Han A, Zhao W, Andrews NC, Chen JJ. The function of heme-regulated eIF2alpha kinase in murine iron homeostasis and macrophage maturation. J Clin Investig. 2007;117:3296–3305. doi: 10.1172/JCI32084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu Y, Laszlo C, Liu W, Chen X, Evans SC, Wu S. Regulation of G(1) arrest and apoptosis in hypoxia by PERK and GCN2-mediated eIF2alpha phosphorylation. Neoplasia. 2010;12:61–68. doi: 10.1593/neo.91354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lo SC, Li X, Henzl MT, Beamer LJ, Hannink M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006;25:3605–3617. doi: 10.1038/sj.emboj.7601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lozon TI, Eastman AJ, Matute-Bello G, Chen P, Hallstrand TS, Altemeier WA. PKR-dependent CHOP induction limits hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2011;300:L422–L429. doi: 10.1152/ajplung.00166.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol. 2001;21:7971–7980. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/S0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 125.Malzer E, Daly ML, Moloney A, Sendall TJ, Thomas SE, Ryder E, Ryoo HD, Crowther DC, Lomas DA, Marciniak SJ. Impaired tissue growth is mediated by checkpoint kinase 1 (CHK1) in the integrated stress response. J Cell Sci. 2010;123:2892–2900. doi: 10.1242/jcs.070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maurin AC, Jousse C, Averous J, Parry L, Bruhat A, Cherasse Y, Zeng H, Zhang Y, Harding HP, Ron D, Fafournoux P. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 2005;1:273–277. doi: 10.1016/j.cmet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 127.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mellor H, Proud CG. A synthetic peptide substrate for initiation factor-2 kinases. Biochem Biophys Res Commun. 1991;178:430–437. doi: 10.1016/0006-291X(91)90125-Q. [DOI] [PubMed] [Google Scholar]
- 129.Meurs E, Chong K, Galabru J, Thomas NS, Kerr IM, Williams BR, Hovanessian AG. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-N. [DOI] [PubMed] [Google Scholar]
- 130.Mounir Z, Krishnamoorthy JL, Robertson GP, Scheuner D, Kaufman RJ, Georgescu MM, Koromilas AE. Tumor suppression by PTEN requires the activation of the PKR-eIF2alpha phosphorylation pathway. Sci Signal. 2009;2:ra85. doi: 10.1126/scisignal.2000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mounir Z, Krishnamoorthy JL, Wang S, Papadopoulou B, Campbell S, Muller WJ, Hatzoglou M, Koromilas AE (2011) Akt determines cell fate through inhibition of the PERK-eIF2alpha phosphorylation pathway. Sci Signal 4: ra62 [DOI] [PMC free article] [PubMed]
- 132.Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nanduri S, Rahman F, Williams BR, Qin J. A dynamically tuned double-stranded RNA binding mechanism for the activation of antiviral kinase PKR. EMBO J. 2000;19:5567–5574. doi: 10.1093/emboj/19.20.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Narasimhan J, Staschke KA, Wek RC. Dimerization is required for activation of eIF2 kinase Gcn2 in response to diverse environmental stress conditions. J Biol Chem. 2004;279:22820–22832. doi: 10.1074/jbc.M402228200. [DOI] [PubMed] [Google Scholar]
- 135.Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Neznanov N, Dragunsky EM, Chumakov KM, Neznanova L, Wek RC, Gudkov AV, Banerjee AK. Different effect of proteasome inhibition on vesicular stomatitis virus and poliovirus replication. PLoS ONE. 2008;3:e1887. doi: 10.1371/journal.pone.0001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Niture SK, Kaspar JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxicol Appl Pharmacol. 2010;244:37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 2003;22:1180–1187. doi: 10.1093/emboj/cdg112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Onuki R, Bando Y, Suyama E, Katayama T, Kawasaki H, Baba T, Tohyama M, Taira K. An RNA-dependent protein kinase is involved in tunicamycin-induced apoptosis and Alzheimer’s disease. EMBO J. 2004;23:959–968. doi: 10.1038/sj.emboj.7600049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 144.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 145.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286:10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pang Q, Christianson TA, Koretsky T, Carlson H, David L, Keeble W, Faulkner GR, Speckhart A, Bagby GC. Nucleophosmin interacts with and inhibits the catalytic function of eukaryotic initiation factor 2 kinase PKR. J Biol Chem. 2003;278:41709–41717. doi: 10.1074/jbc.M301392200. [DOI] [PubMed] [Google Scholar]
- 147.Pataer A, Swisher SG, Roth JA, Logothetis CJ, Corn PG. Inhibition of RNA-dependent protein kinase (PKR) leads to cancer cell death and increases chemosensitivity. Cancer Biol Ther. 2009;8:245–252. doi: 10.4161/cbt.8.3.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pataer A, Vorburger SA, Barber GN, Chada S, Mhashilkar AM, Zou-Yang H, Stewart AL, Balachandran S, Roth JA, Hunt KK, Swisher SG. Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via up-regulation of the double-stranded RNA-dependent protein kinase (PKR) Cancer Res. 2002;62:2239–2243. [PubMed] [Google Scholar]
- 149.Pataer A, Vorburger SA, Chada S, Balachandran S, Barber GN, Roth JA, Hunt KK, Swisher SG. Melanoma differentiation-associated gene-7 protein physically associates with the double-stranded RNA-activated protein kinase PKR. Mol Ther. 2005;11:717–723. doi: 10.1016/j.ymthe.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 150.Patel CV, Handy I, Goldsmith T, Patel RC. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J Biol Chem. 2000;275:37993–37998. doi: 10.1074/jbc.M004762200. [DOI] [PubMed] [Google Scholar]
- 151.Patel RC, Sen GC. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 1998;17:4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Perkins DJ, Barber GN. Defects in translational regulation mediated by the alpha subunit of eukaryotic initiation factor 2 inhibit antiviral activity and facilitate the malignant transformation of human fibroblasts. Mol Cell Biol. 2004;24:2025–2040. doi: 10.1128/MCB.24.5.2025-2040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pervin S, Tran AH, Zekavati S, Fukuto JM, Singh R, Chaudhuri G. Increased susceptibility of breast cancer cells to stress-mediated inhibition of protein synthesis. Cancer Res. 2008;68:4862–4874. doi: 10.1158/0008-5472.CAN-08-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Peters GA, Hartmann R, Qin J, Sen GC. Modular structure of PACT: distinct domains for binding and activating PKR. Mol Cell Biol. 2001;21:1908–1920. doi: 10.1128/MCB.21.6.1908-1920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pomar N, Berlanga JJ, Campuzano S, Hernández G, Elías M, de Haro C. Functional characterization of Drosophila melanogaster PERK eukaryotic initiation factor 2alpha (eIF2alpha) kinase. Eur J Biochem. 2003;270:293–306. doi: 10.1046/j.1432-1033.2003.03383.x. [DOI] [PubMed] [Google Scholar]
- 156.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 157.Rafie-Kolpin M, Chefalo PJ, Hussain Z, Hahn J, Uma S, Matts RL, Chen JJ. Two heme-binding domains of heme-regulated eukaryotic initiation factor-2alpha kinase. N terminus and kinase insertion. J Biol Chem. 2000;275:5171–5178. doi: 10.1074/jbc.275.7.5171. [DOI] [PubMed] [Google Scholar]
- 158.Rafie-Kolpin M, Han AP, Chen JJ. Autophosphorylation of threonine 485 in the activation loop is essential for attaining eIF2alpha kinase activity of HRI. Biochemistry. 2003;42:6536–6544. doi: 10.1021/bi034005v. [DOI] [PubMed] [Google Scholar]
- 159.Rahman M, Lem C, Muaddi H, Koromilas AE. PKR is not a universal target of tumor suppressor p53 in response to genotoxic stress. Cell cycle Georgetown Tex. 2009;8:3598–3599. doi: 10.4161/cc.8.21.9848. [DOI] [PubMed] [Google Scholar]
- 160.Raine DA, Jeffrey IW, Clemens MJ. Inhibition of the double-stranded RNA-dependent protein kinase PKR by mammalian ribosomes. FEBS Lett. 1998;436:343–348. doi: 10.1016/S0014-5793(98)01163-6. [DOI] [PubMed] [Google Scholar]
- 161.Ramana CV, Grammatikakis N, Chernov M, Nguyen H, Goh KC, Williams BR, Stark GR. Regulation of c-myc expression by IFN-gamma through Stat1-dependent and -independent pathways. EMBO J. 2000;19:263–272. doi: 10.1093/emboj/19.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ramelot TA, Cort JR, Yee AA, Liu F, Goshe MB, Edwards AM, Smith RD, Arrowsmith CH, Dever TE, Kennedy MA. Myxoma virus immunomodulatory protein M156R is a structural mimic of eukaryotic translation initiation factor eIF2alpha. J Mol Biol. 2002;322:943–954. doi: 10.1016/S0022-2836(02)00858-6. [DOI] [PubMed] [Google Scholar]
- 163.Ranganathan AC, Ojha S, Kourtidis A, Conklin DS, Aguirre-Ghiso JA. Dual function of pancreatic endoplasmic reticulum kinase in tumor cell growth arrest and survival. Cancer Res. 2008;68:3260–3268. doi: 10.1158/0008-5472.CAN-07-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Romano PR, Garcia-Barrio MT, Zhang X, Wang Q, Taylor DR, Zhang F, Herring C, Mathews MB, Qin J, Hinnebusch AG. Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2alpha kinases PKR and GCN2. Mol Cell Biol. 1998;18:2282–2297. doi: 10.1128/mcb.18.4.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, Lambin P, van der Kogel AJ, Koritzinsky M, Wouters BG. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rutkowski DT, Kang SW, Goodman AG, Garrison JL, Taunton J, Katze MG, Kaufman RJ, Hegde RS. The role of p58IPK in protecting the stressed endoplasmic reticulum. Mol Biol Cell. 2007;18:3681–3691. doi: 10.1091/mbc.E07-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ruvolo PP, Gao F, Blalock WL, Deng X, May WS. Ceramide regulates protein synthesis by a novel mechanism involving the cellular PKR activator RAX. J Biol Chem. 2001;276:11754–11758. doi: 10.1074/jbc.M011400200. [DOI] [PubMed] [Google Scholar]
- 168.Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, Winchester L, Pires I, Hammond E, Ragoussis I, Harris AL. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene. 2010;29:4424–4435. doi: 10.1038/onc.2010.191. [DOI] [PubMed] [Google Scholar]
- 169.Sanderson TH, Deogracias MP, Nangia KK, Wang J, Krause GS, Kumar R. PKR-like endoplasmic reticulum kinase (PERK) activation following brain ischemia is independent of unfolded nascent proteins. Neuroscience. 2010;169:1307–1314. doi: 10.1016/j.neuroscience.2010.05.076. [DOI] [PubMed] [Google Scholar]
- 170.Scheuner D, Patel R, Wang F, Lee K, Kumar K, Wu J, Nilsson A, Karin M, Kaufman RJ. Double-stranded RNA-dependent protein kinase phosphorylation of the alpha-subunit of eukaryotic translation initiation factor 2 mediates apoptosis. J Biol Chem. 2006;281:21458–21468. doi: 10.1074/jbc.M603784200. [DOI] [PubMed] [Google Scholar]
- 171.Schrier SL. Thalassemia: pathophysiology of red cell changes. Annu Rev Med. 1994;45:211–218. doi: 10.1146/annurev.med.45.1.211. [DOI] [PubMed] [Google Scholar]
- 172.Sevier CS, Kaiser CA. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim Biophys Acta. 2008;1783:549–556. doi: 10.1016/j.bbamcr.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 173.Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297–307. doi: 10.1016/S0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 174.Sharp TV, Witzel JE, Jagus R. Homologous regions of the alpha subunit of eukaryotic translational initiation factor 2 (eIF2alpha) and the vaccinia virus K3L gene product interact with the same domain within the dsRNA-activated protein kinase (PKR) Eur J Biochem. 1997;250:85–91. doi: 10.1111/j.1432-1033.1997.00085.x. [DOI] [PubMed] [Google Scholar]
- 175.Shimizu Y, Hendershot LM. Oxidative folding: cellular strategies for dealing with the resultant equimolar production of reactive oxygen species. Antioxid Redox Signal. 2009;11:2317–2331. doi: 10.1089/ars.2009.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Singh M, Castillo D, Patel CV, Patel RC. Stress-induced phosphorylation of PACT reduces its interaction with TRBP and leads to PKR activation. Biochemistry. 2011;50:4550–4560. doi: 10.1021/bi200104h. [DOI] [PubMed] [Google Scholar]
- 177.Singh M, Fowlkes V, Handy I, Patel CV, Patel RC. Essential role of PACT-mediated PKR activation in tunicamycin-induced apoptosis. J Mol Biol. 2009;385:457–468. doi: 10.1016/j.jmb.2008.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sonenberg N, Dever TE. Eukaryotic translation initiation factors and regulators. Curr Opin Struct Biol. 2003;13:56–63. doi: 10.1016/S0959-440X(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 179.Sood R, Porter AC, Ma K, Quilliam LA, Wek RC. Pancreatic eukaryotic initiation factor-2alpha kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem J. 2000;346(Pt 2):281–293. doi: 10.1042/0264-6021:3460281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Srivastava SP, Kumar KU, Kaufman RJ. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 181.Stojdl DF, Abraham N, Knowles S, Marius R, Brasey A, Lichty BD, Brown EG, Sonenberg N, Bell JC. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J Virol. 2000;74:9580–9585. doi: 10.1128/JVI.74.20.9580-9585.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Su N, Kilberg MS. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem. 2008;283:35106–35117. doi: 10.1074/jbc.M806874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Su Q, Wang S, Baltzis D, Qu LK, Wong AH, Koromilas AE. Tyrosine phosphorylation acts as a molecular switch to full-scale activation of the eIF2alpha RNA-dependent protein kinase. Proc Natl Acad Sci USA. 2006;103:63–68. doi: 10.1073/pnas.0508207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Su Q, Wang S, Gao HQ, Kazemi S, Harding HP, Ron D, Koromilas AE. Modulation of the eukaryotic initiation factor 2 alpha-subunit kinase PERK by tyrosine phosphorylation. J Biol Chem. 2008;283:469–475. doi: 10.1074/jbc.M704612200. [DOI] [PubMed] [Google Scholar]
- 185.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Taylor DR, Lee SB, Romano PR, Marshak DR, Hinnebusch AG, Esteban M, Mathews MB. Autophosphorylation sites participate in the activation of the double-stranded-RNA-activated protein kinase PKR. Mol Cell Biol. 1996;16:6295–6302. doi: 10.1128/mcb.16.11.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Thireos G, Penn MD, Greer H. 5′ untranslated sequences are required for the translational control of a yeast regulatory gene. Proc Natl Acad Sci USA. 1984;81:5096–5100. doi: 10.1073/pnas.81.16.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thomas MG, Loschi M, Desbats MA, Boccaccio GL. RNA granules: the good, the bad and the ugly. Cell Signal. 2011;23:324–334. doi: 10.1016/j.cellsig.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 190.Uetani K, Der SD, Zamanian-Daryoush M, de La Motte C, Lieberman BY, Williams BR, Erzurum SC. Central role of double-stranded RNA-activated protein kinase in microbial induction of nitric oxide synthase. J Immunol. 2000;165:988–996. doi: 10.4049/jimmunol.165.2.988. [DOI] [PubMed] [Google Scholar]
- 191.Ung TL, Cao C, Lu J, Ozato K, Dever TE. Heterologous dimerization domains functionally substitute for the double-stranded RNA binding domains of the kinase PKR. EMBO J. 2001;20:3728–3737. doi: 10.1093/emboj/20.14.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.van Huizen R, Martindale JL, Gorospe M, Holbrook NJ. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2alpha signaling. J Biol Chem. 2003;278:15558–15564. doi: 10.1074/jbc.M212074200. [DOI] [PubMed] [Google Scholar]
- 193.VanOudenhove J, Anderson E, Krueger S, Cole JL. Analysis of PKR structure by small-angle scattering. J Mol Biol. 2009;387:910–920. doi: 10.1016/j.jmb.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vattem KM, Staschke KA, Wek RC. Mechanism of activation of the double-stranded-RNA-dependent protein kinase, PKR: role of dimerization and cellular localization in the stimulation of PKR phosphorylation of eukaryotic initiation factor-2 (eIF2) Eur J Biochem. 2001;268:3674–3684. doi: 10.1046/j.1432-1327.2001.02273.x. [DOI] [PubMed] [Google Scholar]
- 195.Vazquez de Aldana CR, Dever TE, Hinnebusch AG. Mutations in the alpha subunit of eukaryotic translation initiation factor 2 (eIF-2 alpha) that overcome the inhibitory effect of eIF-2 alpha phosphorylation on translation initiation. Proc Natl Acad Sci USA. 1993;90:7215–7219. doi: 10.1073/pnas.90.15.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T, Ron D, Chen W, Trenkle W, Wiestner A, Ye Y. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci USA. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, Liu J, Zhao XD, Chew JL, Lee YL, Kuznetsov VA, Sung WK, Miller LD, Lim B, Liu ET, Yu Q, Ng HH, Ruan Y. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 198.Wethmar K, Smink JJ, Leutz A. Upstream open reading frames: molecular switches in (patho)physiology. BioEssays. 2010;32:885–893. doi: 10.1002/bies.201000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 200.Wu S, Kaufman RJ. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J Biol Chem. 1997;272:1291–1296. doi: 10.1074/jbc.272.2.1291. [DOI] [PubMed] [Google Scholar]
- 201.Xu WM, Liu LZ. Nitric oxide: from a mysterious labile factor to the molecule of the Nobel Prize. Recent progress in nitric oxide research. Cell Res. 1998;8:251–258. doi: 10.1038/cr.1998.25. [DOI] [PubMed] [Google Scholar]
- 202.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 203.Yan W, Gale MJ, Jr, Tan SL, Katze MG. Inactivation of the PKR protein kinase and stimulation of mRNA translation by the cellular co-chaperone P58(IPK) does not require J domain function. Biochemistry. 2002;41:4938–4945. doi: 10.1021/bi0121499. [DOI] [PubMed] [Google Scholar]
- 204.Yang R, Wek SA, Wek RC. Glucose limitation induces GCN4 translation by activation of Gcn2 protein kinase. Mol Cell Biol. 2000;20:2706–2717. doi: 10.1128/MCB.20.8.2706-2717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang YL, Reis LF, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams BR, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yerlikaya A, Kimball SR, Stanley BA. Phosphorylation of eIF2alpha in response to 26S proteasome inhibition is mediated by the heme-regulated inhibitor (HRI) kinase. Biochem J. 2008;412:579–588. doi: 10.1042/BJ20080324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yeung MC, Liu J, Lau AS. An essential role for the interferon-inducible, double-stranded RNA-activated protein kinase PKR in the tumor necrosis factor-induced apoptosis in U937 cells. Proc Natl Acad Sci USA. 1996;93:12451–12455. doi: 10.1073/pnas.93.22.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yoon CH, Lee ES, Lim DS, Bae YS. PKR, a p53 target gene, plays a crucial role in the tumor-suppressor function of p53. Proc Natl Acad Sci USA. 2009;106:7852–7857. doi: 10.1073/pnas.0812148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang F, Hamanaka RB, Bobrovnikova-Marjon E, Gordan JD, Dai MS, Lu H, Simon MC, Diehl JA. Ribosomal stress couples the unfolded protein response to p53-dependent cell cycle arrest. J Biol Chem. 2006;281:30036–30045. doi: 10.1074/jbc.M604674200. [DOI] [PubMed] [Google Scholar]
- 211.Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang S, Yu D. PI(3)king apart PTEN’s role in cancer. Clin Cancer Res. 2010;16:4325–4330. doi: 10.1158/1078-0432.CCR-09-2990. [DOI] [PubMed] [Google Scholar]
- 213.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 214.Zhou D, Palam LR, Jiang L, Narasimhan J, Staschke KA, Wek RC. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem. 2008;283:7064–7073. doi: 10.1074/jbc.M708530200. [DOI] [PubMed] [Google Scholar]
- 215.Zhu S, Wek RC. Ribosome-binding domain of eukaryotic initiation factor-2 kinase GCN2 facilitates translation control. J Biol Chem. 1998;273:1808–1814. doi: 10.1074/jbc.273.3.1808. [DOI] [PubMed] [Google Scholar]
- 216.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]