Abstract
Gap junction channels link cytoplasms of adjacent cells. Connexins, their constitutive proteins, are essential in cell homeostasis and are implicated in numerous physiological processes. Spermatogenesis is a sophisticated model of germ cell proliferation, differentiation, survival, and apoptosis, in which a connexin isotype, connexin 43, plays a crucial role as evidenced by genomic approaches based on gene deletion. The balance between cell proliferation/differentiation/apoptosis is a prerequisite for maintaining levels of spermatozoa essential for fertility and for limiting anarchic cell proliferation, a major risk of testis tumor. The present review highlights the emerging role of connexins in testis pathogenesis, focusing specifically on two intimately interconnected human testicular diseases (azoospermia with impaired spermatogenesis and testicular germ cell tumors), whose incidence increased during the last decades. This work proposes connexin 43 as a potential cancer diagnostic and prognostic marker, as well as a promising therapeutic target for testicular diseases.
Keywords: Azoospermia, Connexin 43, Gap junction, Pathogenesis, Testicular germ cell tumors
Introduction
Disruption of testis development and of spermatogenesis, consequently to neonatal exposure to endocrine disrupters, is now widely recognized [1]. Identification of the mechanisms that drive these features is important because fetal exposure is held responsible for the increasing incidence of male infertility and testicular cancer (reviewed in [2, 3]). Spermatogenesis is a highly regulated process of germ cell proliferation, differentiation, survival, and apoptosis, starting from gonocytes to spermatocytes and giving rise to spermatids, the future spermatozoa, which occurs in the testis within the seminiferous epithelium (Fig. 1). During spermatogenesis, the spermatogonia (2n) undergo mitosis, followed by a cellular transformation from type B spermatogonia into spermatocytes, which enter meiosis to form spermatids (1n) and finally develop into spermatozoa via spermiogenesis. Spermatogenesis and spermiogenesis occur within the seminiferous epithelium and their normal progression depends on cell–cell interactions between the germ cells and Sertoli cells, which have been described as the “nurse cells”, capable of creating a proper environment for the development of germ cells and to provide essential nutriments and growth factors. This local control may be partly mediated through direct intercellular communication channels made up by gap junctions and a family of constituted proteins, the connexins (Cx) [4].
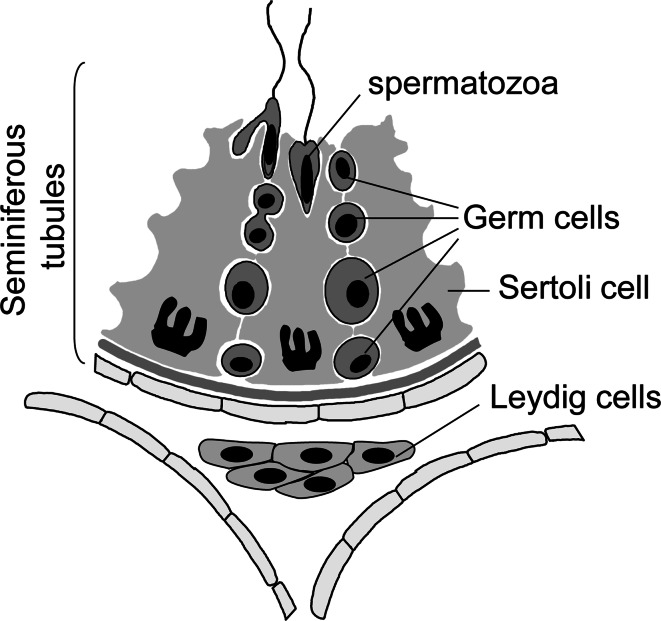
Fig. 1.
Schematization of testis section illustrating the seminiferous tubules composed by Sertoli cells and germ cells at different stages of development and the interstitium with Leydig cells
Based on our recent findings, we hypothesized that testicular Cx dysregulation could partly participate in the etiopathology of human infertility and testicular cancer [5, 6]. A large number of experimental studies suggest that targeting Cx may be a novel approach either to inhibit tumor cell growth directly or to restore normal cell growth, indicating that the therapeutic potential of connexins is undeniable [7]. This review focuses on the regulation and the function of Cx43, the predominant testicular gap junction protein, in human male infertility and testicular germ cell cancer with emphasis on their therapeutic potentials.
Human male infertility
Unexplained male infertility occurs in 30–40 % of men with abnormal semen parameters [8]. Obstructive azoospermia with normal spermatogenesis and nonobstructive azoospermia with distinct degrees of abnormal spermatogenesis represent the most cases of male infertility. The origins of spermatogenic defects in infertile patients are multifactorial. Endocrine disruption of testis development during neonatal period, due to environmental pollution, genetic, and epigenetic factors, is the most potential explanation evoked for the unexplained male infertility [2, 8, 9]. This defect can lead to testicular dysgenesis, male infertility, and to an increased risk of testicular malignancy, as recently suggested [2]. However, the underlying molecular mechanisms that drive these testicular pathologies are mostly unknown, and remain to be investigated [10].
Within the seminiferous epithelium, cell–cell interaction between the somatic cells, Sertoli cells, and the different germ cells are essential in the local control of spermatogenesis and are mediated through paracrine pathways and direct intercellular communication channels made up by Cx [4]. By using transgenic animals, it has been well established that one of these Cx, Cx43, the most predominant Cx in the testis, is a prerequisite for normal spermatogenesis [11–16] and it has been hypothesized that testicular Cx dysregulations could partly participate in the etiopathology of human male infertility [5, 6].
Gap junction channels, connexins, and associated human diseases
Gap junctions are intercellular plasma membrane channels that create electric and metabolic coupling from cell to cell in a wide variety of tissues. Small molecules (<1 kDa), metabolic precursors, nutrients, and signaling factors such as inositol 1,4,5-tri-phosphate, cAMP, cGMP, glutathione, and ions can be transferred directly from one cell to adjacent cells through gap junction channels whereas larger molecules as proteins, polysaccharides and complex lipids are retained within the cytoplasm (reviewed in [17]). Gap junctions are formed by the docking of two hemi-channels, termed connexons, present at opposing plasma membranes of adjacent cells, resulting from the oligomerization of six molecules of Cx (Fig. 2). To date, at least 21 members of this multigene family have been cloned in mammals [18]. Current nomenclature is based on their molecular weights. This process of molecular signal exchange through gap junctions, called gap junction intercellular communication (GJIC), has been involved in several cellular processes including control of cell growth, intercellular synchronization, and co-operation and hormone responsiveness. Cx can be regulated at different levels including transcription, mRNA processing, protein synthesis, post-translational modification (phosphorylation), assembly in the Golgi apparatus, trafficking to the plasma membrane, connexon docking, and gating of the channel (reviewed in [19]).
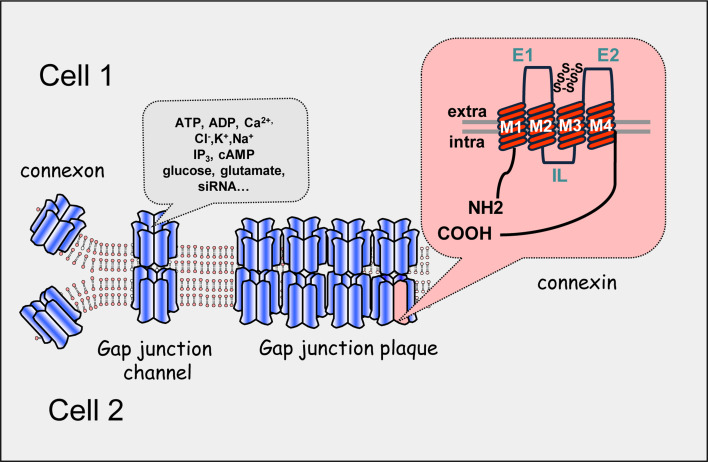
Fig. 2.
Schematic architecture of gap junction plaque, gap junction channel, gap junction hemi-channel or connexon and connexin. Each intercellular channel or gap junction provides an axial channel that interconnects the cytoplasms of two adjacent cells (cell 1 and cell 2) and allow the direct exchange of molecules with a relative molecular mass up to 1 kDa. Hemi-channels or connexons are formed of six connexin subunits that exhibit four transmembranous domains (M1–M4), two extracellular loops (E1 and E2), and three cytoplasmic domains including one intracellular loop (IL) and cytoplasmic NH2- and COOH-termini
Disruption of GJIC could be due to different processes such as mutations of Cx, altered expression of the Cx genes, or impaired trafficking of the protein to the plasma membrane [20]. Genetic approaches have uncovered a still growing number of mutations in Cx genes related to human diseases, including peripheral and central neuropathies, deafness, skin disease, cataracts, and cardiovascular dysfunctions, suggesting that Cx are crucial for a large number of physiological processes [21, 22] (see Table 1). In addition, it has been reported that a loss of gap junctions also appears as one of the primary events of uncontrolled cell proliferation, giving rise to tumor development [23]. Indeed, impaired GJIC and Cx expression have been reported in many human tumors, in nearly all malignant cell lines and after exposure to tumor promoters and oncogene expression (Table 1) [24, 25]. Cx have been considered as tumor-suppressor genes since Cx transfection into transformed cells restores in most cases normal cell growth, leading to the proposal that pharmacological Cx up-regulation may have therapeutic implications in cancer [26].
Table 1.
| Human diseases | Connexins |
|---|---|
| X-linked Charcot–Marie–Tooth disease (CMTX) | Cx32 |
| Atrial fibrillation | Cx40 |
| Viscero-atrial heterotaxia | Cx43 |
| Cataract | Cx46, Cx50 |
| Oculodentodigital dysplasia (ODDD) | Cx43 |
| Clouston syndrome | Cx30 |
| Hearing loss (non-syndromic or associated with skin | Cx26, Cx30, Cx31 |
| Keratitis ichthyosis deafness syndrome | Cx26, Cx30 |
| Erythrokeratodermia variabilis | Cx30.3, Cx31 |
| Pelizaeus–Merzbacher-like | Cx46.6, Cx47 |
| Vohwinkel syndrome | Cx26 |
| Human cancers | Connexins |
|---|---|
| Head and neck carcinoma | Cx31.1 |
| Nasopharyngeal carcinoma | Cx43, Cx45 |
| Meningioma and hemangiopericytoma | Cx26, Cx43 |
| Human larynx carcinoma | Cx26, Cx30, Cx32, Cx40 |
| Liver tumor | Cx32, Cx43 |
| Colon cancer | Cx26, Cx43 |
| Esophageal cancer | Cx26, Cx32, Cx43 |
| Breast cancer | Cx26, Cx43 |
| Mesothelioma tumor | Cx37, Cx43, Cx45 |
| Glioblastoma | Cx43 |
| Lung cancer | Cx43 |
| Adrenocortical tumors | Cx43 |
| Renal cell cancer | Cx32, Cx43 |
| Cervical carcinoma | Cx43 |
| Ovarian carcinoma | Cx43 |
| Endometrial carcinoma | Cx43 |
| Prostate tumor | Cx26, Cx32, Cx43 |
| Bladder cancer | Cx26 |
| Thyroid carcinoma | Cx26, Cx32, Cx43 |
| Testis cancer | Cx26, Cx40, Cx43 |
| Hepatocarcinogenesis | Cx26, Cx32 |
| Skin tumor | Cx26, Cx30 |
Gap junction, connexin 43, and testicular diseases
Connexin and testis
Transcripts for at least 11 different Cx (Cx26, Cx30.2, Cx31, Cx31.1, Cx32, Cx33, Cx37, Cx40, Cx43, Cx46, and Cx50) have been detected in the rodent testis [27] and many of them are also present in the male genital tract within prostate, seminal vesicles, epididymis, and corpus cavernosum (reviewed in [28]). In the testis, Cx43, also named GJA1 (gap junction protein alpha 1), is present in Leydig cells and in seminiferous epithelium between Sertoli cells and between Sertoli cell and germ cells [29].
In the human testis, Cx40 transcripts were identified [30], Cx26 protein has been detected within seminiferous tubules [31] and Cx31.9 was found expressed in testicular vascular smooth muscle [32, 33]. The Cx43, which appears to be the most abundant Cx within the human testis, is ubiquitously expressed within most cell types [29]. Cx43 mRNA and protein are present in Leydig cells and within the seminiferous tubules between Sertoli cells and spermatogonia or primary spermatocytes [34–37].
To characterize the role of these Cx isoforms into the reproductive function, genomic deletion of these proteins was achieved. If knock-out (KO) animals for Cx31, Cx32, Cx40, Cx46, and Cx50 show normal fertility [38], Cx43-deficient mice die at birth [39] but exhibit a 50 % depletion in primordial germ cells [11]. To overcome this perinatal lethality, testes from Cx43-null mutant fetuses were grafted under the kidney capsules of adult males [13], or Cx43 gene was substituted by the coding sequences of Cx26, Cx32, or Cx40 [12, 16]. In both cases, the reproductive function was strongly impaired with abnormal proliferation and differentiation of germ cells or spermatogenesis arrest at the level of spermatogonia, leading to a Sertoli-cell-only (SCO) phenotype. Even if these studies supported the unique role of Cx43 in spermatogenesis, the contribution of Cx43 deletion in other tissues was nevertheless not verified. Thus, in order to avoid both perinatal lethality and pleiotropic effects on other developmental tissues, authors generated a conditional Cx43 KO mouse using the Cre/loxP recombination system, which lacks the Cx43 gene solely in Sertoli cells by crossing two transgenic mouse lines, AMH-Cre mice and Cx43-floxed LacZ mice [14, 15]. Adult male transgenic animals showed normal testis descent and development of the genital tract. However, the size of the testes was reduced and most seminiferous tubules were devoid of germ cells, with abnormal proliferation and intermediate phenotype expression of Sertoli cells [40]. If these conditional KO experiments confirmed the key function of Cx43 in spermatogenesis, they did not, however, give more information on the role of this Cx within each testis cells.
Since fetuses from Cx43 KO mice exhibit a marked depletion in primordial germ cells (PGCs), it is likely that Cx43 participates in the control of PGC survival and/or migration [11]. Previous studies demonstrated that Cx43 is essential for PGC motility in 8.5- and 11.5-day-old embryos and cell survival in older embryos, with abnormal p53 activation playing a crucial role in the apoptotic loss of PGCs at the latter stages in the Cx43 KO mouse embryos [41]. Within Sertoli cells, the detection of Cx43 expression has been found to correlate with cell differentiation [35, 42]. Then, it has been hypothesized that Cx43 could actively control Sertoli cell proliferation and differentiation during neonatal period, as suggested by the observations in Sertoli cell conditional Cx43 KO mice [14, 15]. Recently, we reinforced this possibility by developing a Cx inhibitory mimetic peptide strategy, which reveals that Cx43 between Sertoli cells control Sertoli cell proliferation whereas Cx43 between Sertoli cells and spermatogonia regulate germ cell survival rather than germ cell proliferation [43].
During the time course of testis development, it has been postulated that Cx43 gap junctions, present in the perinatal testis, control Sertoli cell differentiation and through this way maintain the number of germ cells (gonocytes or primitive spermatogonia). Afterwards, Cx43 could control germ cell differentiation and appears essential for meiotic progression of spermatocytes [44, 45]. Sertoli cells would ensure metabolic and signaling coupling to germ cells through Cx43 gap junction channels and allow synchronization of male germ cell proliferation, differentiation, and survival [43]. Consistently, microarray analysis of pre-pubertal mice in which Cx43 was specifically depleted in Sertoli cells revealed that an important amount of germ cell-specific genes, essential for mitotic and meiotic progression of spermatogenesis, were down-regulated. Moreover, other genes controlling transcription, metabolism, cell migration, and cytoskeleton organization were also altered [46]. Recent studies suggested that Cx43 could also indirectly drive the spermatogenic process by controlling tight junction proteins, which form the blood–testis barrier (BTB) [47, 48]. The involvement of such a regulation is of interest since it is well established that the BTB plays a crucial role in spermatogenesis by forming an intricate proteinaceous network, which segregates and protects the post-meiotic germ cell from unwanted biomolecules or environmental toxicants.
Connexin expression in testis with impaired spermatogenesis
Although endocrine disruption of testis development, during the neonatal period, is the most potential explanation evoked for both the increased incidence of male hypofertility and testicular malignancy, the molecular mechanisms at the origin of these testicular pathologies are unknown. To date, there is strong evidence suggesting that most cases of this testicular dysgenesis syndrome are due to environmental factors (reviewed in [2]). We recently hypothesized that testicular Cx43 dysregulations could participate in the etiopathology of human male infertility and testis cancer since Cx43 is (1) essential for spermatogenesis, (2) altered during tumoral process, (3) a specific target for testicular Cx (reviewed in [5, 6, 25]).
There are few data reported in the literature that inform on the status of testicular gap junctions in the pathological testis of patients with abnormal spermatogenesis. Previous freeze-fracture studies have not reported any variation in the number of gap junctional particles in the seminiferous tubules of azoospermic and oligozoospermic patients [49]. However, no information was given on the origin and degree of the spermatogenesis defect. Inversely, gap junction-like cell membrane specializations are very rare in hypo- or azoospermic patients [50] and are affected in SCO seminiferous tubules [51]. This alteration is accompanied by a decrease in immunoreactive Cx43 [34]. Moreover, recent studies from our laboratory demonstrated that the Cx43 mRNA amount decrease in the testis of rat, with impaired spermatogenesis following experimental cryptorchidism, is concomitant with a disappearance of Cx43 protein in the seminal epithelium, whereas Cx43 is still present in the interstitial tissue [35]. Similar observations were reported in other species [52]. In addition to cryptorchidism, inflammation of the male reproductive tract, resulting from interstitial infiltration of T lymphocytes, is an important etiological factor of infertility [53]. A recent study reported that the germ-cell loss observed in rats with experimental autoimmune orchitis is associated with early alterations of Cx43 cell–cell gap junction communication [54]. In azoospermic patients with severe spermatogenesis failure, we reported that the disturbed expression of Cx43 mRNA might be related to a defect in the maturation of Sertoli cells [35]. Thus, the possibility that Sertoli cell Cx43 impairment could be a sign of undifferentiated Sertoli cell functionality has been hypothesized. More precisely, other authors found a significant positive correlation between the histological score and intensity of the testicular Cx43 expression, when the spermatogenesis defect was evaluated in oligozoospermic men by Johnsen score [36]. While Cx43 staining is quite similar in human testes with hypospermatogenesis and spermatogenic arrest at the level of round spermatids or spermatocytes compared to healthy normal adult testis, seminiferous tubules with spermatogenic arrest at the level of spermatogonia and Sertoli-cell-only syndrome were completely immunonegative [34]. From these data, we suggested that the communication between Sertoli cells and germ cells through Cx43 may be important for the progression of spermatogenesis specifically at some precise stages of germ cell development. For unknown reasons, sterility induced by epididymal and vasal ligation was able to affect Cx43 expression in the testis of the experimental rat model, suggesting that Cx43 is a key molecule in the reproductive process [55].
Although these findings demonstrate that testicular Cx43 expression is reduced in mutant rodents, with testicular defects, and in hypofertile men, the existence of a direct relationship between disrupted Cx and spermatogenesis arrest has not been clearly established. Consequently, it is still questionable if the altered Cx expression is a consequence of impaired testicular function rather than the cause of the testicular pathology (reviewed in [28]). Since experimental studies have demonstrated that Cx43 is essential in the initiation and maintenance of spermatogenesis, as revealed by the analysis of mice lacking Cx43 through germ line knock-out of Gja1 [11–16], the possibility that Cx43 drives and controls spermatogenesis has been suggested. To test this hypothesis, phenotype analysis of Cx43 gene mutation and consequence on testicular function have to be analyzed. Over the last decades, the role of Cx signaling in human physiology has been highlighted by the discovery of numerous Cx gene mutations and Cx protein alteration, in a wide range of tissues. These Cx defects cause a large variety of severe human pathologies, such as myelin-related neuropathy diseases, skin diseases, hearing loss, congenital cataract, or more complex syndromes, such as cardiovascular dysfunctions and the oculodentodigital (ODDD) dysplasia (reviewed in [21, 22]) (Table 1). However, no effective and consequent testis dysfunction was examined and reported in men for most Cx gene mutations (reviewed in [56]). Since Cx43 is the predominant Cx in the human testis, the consequence of Cx43 mutation needs to be analyzed. To date, there is only one report in which human genital tract abnormalities (hypospadias, undescended testis) have been reported in male patients and infertility in a female [57]. Experimentally, a Cx43 mutant from a mouse model for ODDD, with a Cx43 dominant loss of function, exhibits impaired oogenesis due to granulosa cells dysfunction [58]. This interesting result is in agreement with the existence of a direct relationship between altered Cx43 expression and reproductive defects, since granulosa cells and Sertoli cells play similar supporting functions for germ cells during oogenesis and spermatogenesis, respectively. However, it must remain cautious because in the last data cited phenotypic descriptions of ODDD male patients have not concerned reproductive function. Thus, since this feature has not been investigated in the clinical protocols, these patients have not been reported to be infertile. Recently, the exploration of Gja1Jrt/+ mice, which carry a dominant mutation that causes an amino acid substitution (G60S) and mimic the phenotype of ODDD, revealed a loss of germ cells in some seminiferous tubules and reduced sperm count and sperm velocity parameters, supporting, for the first time, the possibility of subfertility in ODDD human males [59]. Thus, it is possible that, as in mutant mice, many ODDD patients could present signs of hypofertility, but this defect is not sufficiently severe to lead to infertility. This hypothesis is in agreement with our previous data demonstrating a relationship between the decrease in testicular Cx43 and the severity of spermatogenic defect in men [35]. Further studies must be developed in male subjects harboring Cx43 mutations with ODDD syndrome and accurate analysis of reproductive function and sperm parameters (number, viability, velocity) must be performed.
Testis cancer and connexin 43
Competent differentiating Sertoli cells are required for spermatogenesis, spermiogenesis, and the production of normal spermatozoa. Therefore, dysfunction of Sertoli cells leads to hypospermatogenesis, which may lead to hypofertility or complete infertility [60]. During neonatal development, Sertoli cell dysfunction is associated with an arrest of gonocyte production at an early stage of development. These undifferentiated primitive germ cells are thought to be the forerunners to carcinoma-in situ (CIS) testis syndrome that, in turn, can develop into testicular germ cell cancer (reviewed in [61]). Interestingly, it has been proposed that testis tumor progression may be correlated with a disrupted Sertoli cell function resulting from an abnormal and undifferentiated status of the somatic cells (reviewed in [62]). According to this hypothesis, epidemiologic studies demonstrated an increased incidence of testicular cancer in infertile men who exhibit abnormal semen analysis [63–67]. In addition to genetic abnormalities due to SRY mutations, male infertility and testicular cancer have been associated with defects in DNA repair genes, tumor suppressor gene mutations, and epimutations [68]. Several genes that could be involved in the pathogenesis of testicular germ cell tumors have been identified [69, 70]. In addition to these genes, Cx43 could be a good candidate in the etiopathogeny of testis diseases. In agreement with this hypothesis, Cx gene expression is abnormally down-regulated in most tumoral tissues (listed in Table 1) and cell lines [23–25]. Moreover, restoration of normal phenotype in transformed cells, by transfection of exogenous Cxs, gave rise to the concept that the gap junction proteins can act as tumor suppressor genes (reviewed in [26, 71]). In the human testis, the two gap junction proteins Cx43 and Cx26 were found to be associated with neoplasic progression of CIS. Indeed, down-regulation of Cx43 expression was found in the pathological testes of patients with CIS, Sertoli cell tumor, seminoma, and non-seminomatous germ cell tumors, as compared to healthy testis [30, 31, 72, 73]. It is noteworthy that the down-regulation of Cx43 gene expression during tumoral progression from CIS to seminoma is concomitantly associated with an up-regulation of another Cx, Cx26 [31]. Cx26 overexpression and cytoplasmic accumulation has also been reported in numerous carcinomas (pancreas, head and neck, breast, colon, prostate), in keratinocyte-derived skin tumors and in human papillary thyroid and follicular thyroid cancers, suggesting a non-tissue-specific role of this Cx in the tumoral process (reviewed in [23]). Thus, it is possible that the compensatory mechanism that occurs between Cx43 and Cx26 was unable to overtake the function of Cx43, since Cx43 exerts a unique role in the testis as mentioned above.
In addition to reduced mRNA and protein expression, delocalization of the membrane protein within the cytoplasm has been reported in many human cancers such as prostate, colorectal, gastric adenocarcinomas, breast, and hepatocellular carcinoma [74–78]. In neoplasic cells originating from a seminoma cell line, we reported that Cx43 protein was aberrantly trafficked with accumulation of the protein within the cell cytoplasms [72]. Overexpression of wild-type Cx43 by transfection of a Cx43 vector not only restored gap junctional intercellular communication but also blocked abnormal proliferation of these cells. In agreement with this previous study, aberrant cytoplasmic Cx43 accumulation has also been demonstrated in pure human testicular seminoma tissues but the precise intracytoplasmic compartment was not clearly evidenced [79]. In Leydig cell tumors, the presence of Cx43 was detected within early endosomes, suggesting an excessive internalization of the gap junction protein [80]. Although the role of this intracytoplasmic presence of the gap junction protein is questionable, this aberrant localization in tumoral tissue has been suggested to stimulate tumor processing [74, 81] and has been observed in many cell types exposed to carcinogens (reviewed in [25]). This result is in agreement with previous findings demonstrating that Cx itself could control cell growth independently of cell–cell communication [82–84].
Consequently from these data, several causes for the invasive potential of CIS cells could be advanced. First, it may be speculated that the independence of these pre-invasive tumor cells to proliferate anarchically could result from impaired control of germ cells proliferation by mature Sertoli cells through functional Cx43 gap junctions (Fig. 3). This could happen if developing Sertoli cells failed to differentiate due to Cxs disruptor [5] and/or mature Sertoli cells dedifferentiate [31, 85]. Indeed, disruption of Sertoli cell control on germ cell proliferation is in agreement with our previous experimental observations demonstrating that (1) the transfer of signaling molecules through gap junctions occurs unidirectionally from Sertoli to germ cells [86], (2) such an independence may also occur in physiological conditions during specific stages of spermatogenesis between Sertoli cells and some germ cells, such as spermatogonia [87]. Second, it is also possible that the undifferentiated and preinvasive tumor germ cells, which escape Sertoli cell control, could accumulate Cx43 protein within their cytoplasms. This aberrant intracytoplasmic accumulation could further favor human seminoma development through GJIC-independent mechanisms. Such dual effect of Cx has been previously suggested in the breast where Cx first acts as a tumor suppressor in the primary tumors and then as an enhancer of tumor progression (reviewed in [88]). This possibility is also supported by recent findings demonstrating that a loss of intercellular gap junctions and a gain of intracytoplasmic Cx26 and Cx32 can play an important role in the formation of gastric and colorectal adenocarcinomas [77, 78]. Interestingly, cytoplasmic Cx32 exerts stimulatory effects on some steps of hepatocellular carcinoma progression, such as invasion and metastasis, when the cells have acquired a malignant phenotype [81]. The molecular basis of such a GJIC-independent role of Cxs is still unknown. Nevertheless, a recent study evidenced that cytosolic Cx43 regulates the dynamic of microtubule network required for cell migration as well as cellular polarity [89]. Thus, it could be postulated that such drastic effects on cytoskeleton and polarity could participate in the epithelial–mesenchymal transition initiating cancer development and metastasis.
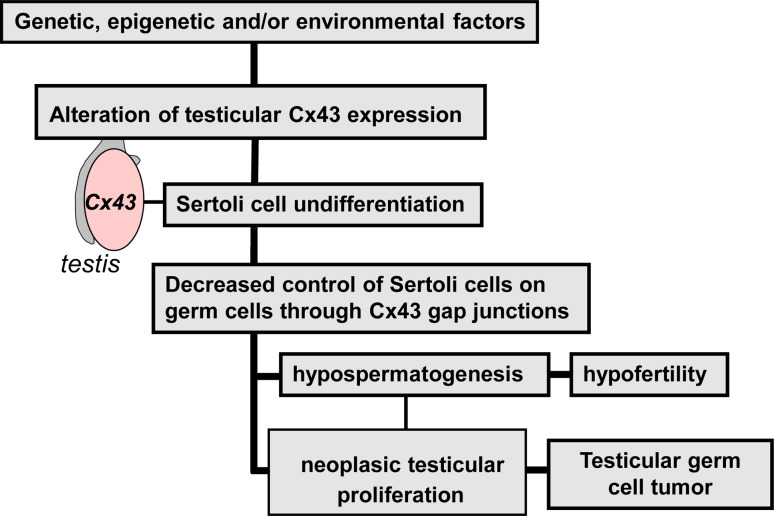
Fig. 3.
Schematic illustration of human testicular diseases associated with impairment of Cx43. In physiological situations, Sertoli cell Cx43 independently or dependently of gap junction channels participates in the control of germ cell proliferation, differentiation, survival, and apoptosis required for spermatogenesis. In physiopathological conditions, for example under the influence of genetic, epigenetic and/or environmental factors, a decreased or complete rupture of the control of germ cells by Sertoli cells through Cx43 can conduce either to hypospermatogenesis, which first leads to man hypofertility, or to abnormal tumor cell proliferation, which finally leads to testicular germ cell tumors
Altogether, these data suggest that the down-regulation of Cx43 and the shift in its localization, from the cell membrane to the cytoplasm, could be early events of tumor progression associated with uncontrolled cell proliferation. These two events could serve as indicators of carcinogenesis and, as such, as additional neoplasic biomarkers during tumor germ cell progression. In the testis, the Cx43 down-regulation and delocalization [31, 80], concomitant with Cx26 up-regulation, have been postulated to be early signs of carcinogenesis [73]. The recent demonstration of a similar shift of expression and delocalization in some areas of the contralateral testis biopsies of patients with non-seminomatous testicular germ cells, although no CIS was evidenced, strongly supports this assumption and could be indicative of malignant dormant germ cell tumors [90].
Clinical and therapeutic applications of connexin 43 in testicular diseases
Cx43 as diagnostic biomarker of testicular diseases
The incidence of two diseases related to germ cell proliferation, hypospermatogenesis and testicular cancer, has been reported to continuously increase over the last 3–5 decades in men of different industrialized countries [91, 92]. In addition, epidemiological studies argued for an increased risk of testicular germ cell cancer among infertile men [66, 93–95]. Consequently, the early recognition of the precursor lesion before CIS stage in the non-obstructive azoospermic testes and the determination of imaging and/or serum tumors markers in the failing human testes are challenging issues. Indeed, tumor marker identification could be useful in the prognostic diagnosis and staging of the disease, for monitoring the therapeutic response and for analyzing tumor recurrence. Several markers of testis cancers have been evaluated in serum, semen, and tissue samples [96]. Regarding testicular dysgenesis syndrome, recent studies proposed the Hiwi protein and chromosome 12 aneuploidy, DNA mismatch repair, and Y-chromosome instability as possible connections between male infertility and testicular germ cell tumor [95]. The present review suggests that alterations of Cx expression, particularly its aberrant localization within testicular cells, could be a prognostic indicator for uncontrolled cell proliferation and consequently of tumor progression in the infertile testis. Analysis of Cx43 disruption may be particularly useful for screening young men at high risk of testicular cancer (hypofertility, bilateral cryptorchidism, contralateral testicular cancer).
Characterization of noninvasive prognostic biomarkers is another strategy that could be developed to facilitate the management of infertile patients and to evaluate the potential of cancer progression. However, previous studies have reported that analysis of the levels of AFP, hCG, PLAP, and LDH, the most routinely used markers, do not appear as good predictors of testicular cancer in this population of men at high risk [97, 98] and few of them exhibited high diagnostic specificity and sensitivity [99]. Recent studies have demonstrated that the production patterns in body fluids of microRNAs (miRNAs) is highly correlated with various pathologies and could be used as a novel class of noninvasive biomarkers to diagnose and monitor human cancers [100, 101]. These RNA-based elements are small non-coding RNAs with a broad range of functions essentially in gene translation regulation. Recently, altered profiles of miRNAs have been reported in the seminal plasma of infertile men [102] and in human testis samples with CIS [103]. Interestingly, circulating specific miRNAs and miRNAs coding for Cx have been postulated to be biomarkers or mediators of cardiovascular diseases [104, 105]. Thus, further research is needed to determine if alterations of circulating levels of miRNAs targeting human Cx43 mRNA, in the serum and also in the seminal plasma, could be associated with male hypofertility and the increased risk of testicular germ cell tumor development.
Cx43 as a potential therapeutic target
Based on the experimental data that support a tumor suppressor role for Cx, (reviewed in [26, 71]), the gap junction proteins have been viewed as potential therapeutic targets for inhibiting tumor development. Thus, it is likely that the restoration of Cx expression could be an attractive strategy to restore male fertility and inhibit tumor germ cell development. Various strategies, including the use of therapeutic agents, Cx gene therapy or both approaches, have been developed [106].
Combination of Cx-dependent tumor-suppressive effect and chemotherapeutic agents, which has been used for clinical tumor treatment, could be effective in enhancing the sensitivity of the tumor to cytotoxic treatment. Previous studies demonstrated that overexpression of Cx43 increases cell susceptibility to several common chemotherapeutic agents, including etoposide, paclitaxel (Taxol), and doxorubicin in a gap junction communication-dependent or -independent manner [24, 25, 106, 107]. The enhanced cell toxicity can result from intercellular diffusion of toxic/apoptotic signals mediated through gap junction channels and the degree of this toxic “bystander” effect generally correlates with the level of GJIC (reviewed in [108]). The possibility that Cx43 sensitizes cells to drug-initiated cytotoxicity, possibly through hemichannel-mediated effects on intracellular oxidative status, has also been hypothesized [109]. In addition, recent findings demonstrated that, in response to cisplatin, a widely used chemotherapeutic agent to treat testis cancer, gap junctions propagate opposite in vitro effects: protective in normal cells and toxic among cancer testicular cells [110]. However, little data are related to pathological testis in in vivo situations. This is worrying since epidemiological studies argued, during the last decades, for both an increased incidence of testis cancer, which is the most common malignant disease occurring in young adult men, and for an increased risk of testicular germ cell tumor development among the infertile male population [66, 93–95].
From our point of view, in the research of therapeutic physiological or chemical effectors that can target human testis diseases, a particular interest has been given to the granulocyte colony-stimulating factor (G-CSF). G-CSF is a member of the hematopoietic growth factor family, which controls the proliferation, differentiation, and survival of hematopoietic progenitor cells [111]. This cytokine, which has recently been described as a protector of spermatogenesis [112], has successfully been used in combination with chemotherapeutic agents in the treatment of testis cancer. Testicular germ cell tumor is the most frequent cancer occurring in young men and originates from transformed gonocytes or undifferentiated spermatogonia, which respectively derived from fetal germ cells and adult germ stem cells. Seminoma is the most frequent (50–70 %) testicular germ cell tumor. Interestingly, G-CSF, which increases Cx43 expression and redistribution of Cx43 at the plasma membrane, has recently been used as a therapeutic tool for heart disease [113, 114]. Whether such a treatment could be useful for restoring Cx43 expression in the pathological testes, stimulating germ cell progression in the testis of man with hypospermatogenesis or blocking abnormal germ cell proliferation in the tumoral testis, deserves to be investigated.
In addition, since in most cases of testicular tumors, Cx43 have been detected mainly present within the cytoplasm and absent at the plasma membrane, the hypothesis to stimulate the trafficking of Cx43 towards the membrane or to inhibit the endocytosis of membranous Cx43, has been postulated to correct this defect. However, to date, a better understanding of the molecular interactions that occur between Cxs and their protein partners, during the full trafficking of the gap junction proteins, is of primary importance (reviewed in [115]). Thereafter, a future clinical challenge will be to identify new pharmaceutical agents, capable of restoring normal Cx43 trafficking, localization, and functionality of gap junction channels, and consequently to prevent or reverse the tumoral processing in the pathological testis.
Another strategy to increase Cx expression in the pathological testis is Cx gene transfer. In the testis, germ cells are appropriate target cells for generating transgenic animals whereas the somatic cells, Sertoli and Leydig cells are more appropriate target cells for clinical applications. This molecular approach has been successfully developed in laboratory animals for correcting infertility resulting from Sertoli cell defect [116]. Thus, Cx gene transfer has been proposed as a revolutionary advance for reproductive treatment in patients with male infertility and for testicular cancers [117]. There is growing evidence that germ cell tumors, which represent 95 % of all testicular cancers, derive from germ cells that exhibit at a precise time of development abnormal gene programming, and that testicular tumoral progression is strongly reinforced by the absence or altered communication between Sertoli cells and germ cells. However, due to the complexity of germ cell tumor pathogenesis, the multitude of potential candidate male fertility-associated genes [118, 119] and the potential side-effects caused by the integration of the transgene in the germ line, there is to date rare data on gene therapy for germ cell tumors. Such genetic approach has been, however, successfully developed for REIC/Dkk-3 gene, which is down-regulated in seminoma and which can be used as a gene-therapeutic agent against testicular cancer [120]. In the absence of a general strategy that focuses on restoration of specific germ cell-deficient proteins, which may be missing or mutated in the diseased individual, therapeutically Cx43 increase through Cx transfer gene therapy could be another useful alternative for controlling tumor progression. Indeed, overexpression of Cx43, in pathological Sertoli cells unable to control their own proliferation and differentiation and those of germ cells, could reverse hypospermatogenesis in the azoospermic testis and prevent tumor progression. In keeping with this hypothesis, Cx43 gene transfer has been successfully validated in animal models [121, 122] and attempted in preclinical human studies for diseases, such as loss of hearing function [123] and atrial arrhythmias [124].
Another clinical approach for enhancing the reduced Cx43 levels found in the pathologic testis is to control miRNAs. As discussed above, their involvement in the neoplasic development of germ cell tumors has been well documented (reviewed in [100, 101, 125]). In addition, alteration of miRNAs associated with male infertility and testis tumor has also been reported [102, 103]. Thus, viewed from this angle, the discovery of potential regulatory miRNAs targeting human Cx43 is of interest for developing a new form of gene therapy. Cx43 has been shown to be targeted by two miRNAs miR-1 and miR-206 that are able to down-regulate Cx43 expression [126, 127]. In agreement with these findings, a recent clinical study reported that the misregulation of Cx43, resulting from altered miR-1 processing, could be responsible of the human cardiac dysfunctions in patients with myotonic dystrophy [128]. Although miR-206 was previously viewed as a muscle-specific miRNA, another study demonstrated its presence in osteoblasts and its capacity to inhibit their differentiation [129]. In the testis, it is unknown if Cx43, a target of miR-1 and of miR-206, could be controlled by these two miRNAs and if their disturbing expression could be associated with testicular diseases. In this context, it is worth noting that the expression of miR-1 is altered in testicular tissues of patients with non-obstructive azoospermia [130]. Thus, modulation of miRNA either by using anti-sense oligonucleotides or by transfection or infection could provide a new potential therapeutic window to control Cx43 expression and subsequently testis infertility and neoplasia. Interestingly, it has been suggested that down-regulation of another microRNA-383 is associated with male infertility and is able to promote testicular germ cell tumors [131].
Conclusive remarks
Increased incidence of male infertility and testicular cancer is a general feature of the last decades. In the testis, the gap junction protein Cx43, which is present between the somatic Sertoli cells and between Sertoli and germ cells, is essential for normal spermatogenesis and appears to be generally impaired in testicular diseases. In seminiferous tubules, Sertoli cells exert crucial functions: they initiate, promote, and maintain spermatogenesis and Cx43 plays an essential role in these process as recently evidenced by the use of transgenic Sertoli cell conditional Cx43 knock-out mice [14, 15]. As proposed for other human pathologies such as heart diseases [132, 133] and erectile dysfunction [134], the present review raises hope that the restoration of Cx expression and/or gap junction intercellular communication between Sertoli cells and germ cells might be a significant tool to correct impaired spermatogenesis and to prevent possible associated testicular germ cell tumors in the hypofertile man population. Transcriptional and post-transcriptional processings of mRNA could successfully reinitialize Cx43 expression in most cancer cells [106], either by overexpression of the lacking or altered Cx or by modulating the miRNAs that control Cx43 mRNA. If miR-1 and miR-206 are the two best-explored miRNA regulators of Cx expression, the potential role of other identified miRNAs, such as miR-145 and miR-218 [135, 136], or still unidentified miRNAs, which also target Cx43, the crucial predominant Cx for spermatogenesis, remains to be explored. If this strategy for enhancing Cx43 levels by gene-transfer technique is clinically challenging in many organs, such an approach raises, however, practical difficulties that still seem to be far from being resolved for testicular diseases. Moreover, the other testicular Cxs and corresponding miRNAs remain to be examined in these pathologies to determine their therapeutic potentials. Indeed, controlling the expression of key Cxs, which are able to regulate other Cx isoforms and/or proteins of the BTB, could be a powerful therapeutic approach. Gene transfer into the testis also requires careful consideration, particularly for biosafety and ethical concerns, since the transgene does not pass to the germ line if used for gene therapy in the testis [137]. This strategy must have clinical potential for treatment of specific types of infertility and testicular cancer associated with a deficiency of Cx43 in the somatic Sertoli cells and must use technologies that specifically target the impaired somatic cells. To date, adenoviral vectors appear to be the most appropriate biotechnology tools for future clinical applications, since there is consequent information from clinical trials concerning other diseases [138]. In conclusion, therapeutic opportunities offered by gap junctional intercellular communication mediated by Cx43 in the testis are still widely open. New findings regarding the regulation of both Cx43 and Cx43 partners provide original tools to support this approach in hypofertile patients with impaired spermatogenesis and/or testicular cancer.
Acknowledgments
The preparation of this review was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM). The authors would like to thank Laure Gilleron for critically reading the manuscript and Jeannine Colombani for secretarial assistance. JG is a doctoral and postdoctoral research fellow of the French Ministry of Research and Technology and of EMBO.
Abbreviations
- AFP
Alpha-fetoprotein
- AMH
Anti-Müllerian hormone
- BTB
Blood–testis barrier
- cAMP
Cyclic adenosine monophosphate
- cGMP
Cyclic guanosine monophosphate
- CIS
Carcinoma in situ
- Cx
Connexin
- G-CSF
Granulocyte colony-stimulating factor
- GJA1
Gap junction protein alpha 1
- GJIC
Gap junction intercellular communication
- hCG
Human chorionic gonadotropin
- KO
Knock-out
- LDH
Lactate dehydrogenase
- miRNA
MicroRNA
- ODDD
Oculodentodigital
- PGC
Primordial germ cell
- PLAP
Placental alkaline phosphatase
- SCO
Sertoli-cell-only
References
- 1.Sharpe RM. Pathways of endocrine disruption during male sexual differentiation and masculinisation. Best Pract Res Clin Endocrinol Metab. 2006;20:91–110. doi: 10.1016/j.beem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Skakkebæk NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 3.Hardell L, Bavel B, Lindström G, Eriksson M, Carlberg M. In utero exposure to persistent organic pollutants in relation to testicular cancer risk. Int J Androl. 2006;29:228–234. doi: 10.1111/j.1365-2605.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- 4.Pointis G, Segretain D. Role of connexin-based gap junction channels in testis. Trends Endocrinol Metab. 2005;16:300–306. doi: 10.1016/j.tem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Gilleron J, Malassiné A, Carette D, Segretain D, Pointis G. Chemical connexin impairment in the developing gonad associated with offspring infertility. Curr Med Chem. 2011;18:5145–5158. doi: 10.2174/092986711797636117. [DOI] [PubMed] [Google Scholar]
- 6.Pointis G, Gilleron J, Carette D, Segretain D. Testicular connexin 43, a precocious molecular target for the effect of environmental toxicants on male fertility. Spermatogenesis. 2011;14:1–15. doi: 10.4161/spmg.1.4.18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leithe E, Sirnes S, Omori Y, Rivedal E. Downregulation of gap junctions in cancer cells. Crit Rev Oncog. 2006;12:225–256. doi: 10.1615/critrevoncog.v12.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 8.Nieschlag E, Behre HM. Andrology, male reproductive health and dysfunction. Berlin: Springer; 2000. pp. 83–87. [Google Scholar]
- 9.Rajender S, Avery K, Agarwal A. Epigenetics, spermatogenesis and male infertility. Mutat Res. 2011;727:62–71. doi: 10.1016/j.mrrev.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Singh K, Jaiswal D. Human male infertility: a complex multifactorial phenotype. Reprod Sci. 2011;18:418–425. doi: 10.1177/1933719111398148. [DOI] [PubMed] [Google Scholar]
- 11.Juneja SC, Barr KJ, Enders GC, Kidder GM. Defects in the germ line and gonads of mice lacking connexin43. Biol Reprod. 1999;60:1263–1270. doi: 10.1095/biolreprod60.5.1263. [DOI] [PubMed] [Google Scholar]
- 12.Plum A, Hallas G, Magin T, Dombrowski F, Hagendorff A, Schumacher B, Wolpert C, Kim J, Lamers WH, Evert M, Meda P, Traub O, Willecke K. Unique and shared functions of different connexins in mice. Curr Biol. 2000;10:1083–1091. doi: 10.1016/s0960-9822(00)00690-4. [DOI] [PubMed] [Google Scholar]
- 13.Roscoe WA, Barr KJ, Mhawi AA, Pomerantz DK, Kidder GM. Failure of spermatogenesis in mice lacking connexin43. Biol Reprod. 2001;65:829–838. doi: 10.1095/biolreprod65.3.829. [DOI] [PubMed] [Google Scholar]
- 14.Brehm R, Zeiler M, Rüttinger C, Herde K, Kibschull M, Winterhager E, Willecke K, Guillou F, Lécureuil C, Steger K, Konrad L, Biermann K, Failing K, Bergmann MA. Sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am J Pathol. 2007;171:19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sridarhan S, Simon L, Meling DD, Cyr DG, Gutstein DE, Fishman GI, Guillou F, Cooke PS. Proliferation of adult Sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin43) in mice. Biol Reprod. 2007;76:804–812. doi: 10.1095/biolreprod.106.059212. [DOI] [PubMed] [Google Scholar]
- 16.Winterhager E, Pielensticker N, Freyer J, Ghanem A, Schrickel JW, Kim JS, Behr R, Grümmer R, Maass K, Urschel S, Lewalter T, Tiemann K, Simoni M, Willecke K. Replacement of connexin43 by connexin26 in transgenic mice leads to dysfunctional reproductive organs and slowed ventricular conduction in the heart. BMC Dev Biol. 2007;7:26. doi: 10.1186/1471-213X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruzzone R, White TW, Goodenough DA. The cellular Internet: on-line with connexins. Bioessays. 1996;18:709–718. doi: 10.1002/bies.950180906. [DOI] [PubMed] [Google Scholar]
- 18.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, Deutsch U, Söhl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 19.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 20.Chipman JK, Mally A, Edwards GO. Disruption of gap junctions in toxicity and carcinogenicity. Toxicol Sci. 2003;71:146–153. doi: 10.1093/toxsci/71.2.146. [DOI] [PubMed] [Google Scholar]
- 21.Zoidl G, Dermietzel R. Gap junctions in inherited human disease. Pflugers Arch. 2010;460:451–466. doi: 10.1007/s00424-010-0789-1. [DOI] [PubMed] [Google Scholar]
- 22.Pfenniger A, Wohlwend A, Kwak BR. Mutations in connexin genes and disease. Eur J Clin Invest. 2011;41:103–116. doi: 10.1111/j.1365-2362.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 23.Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M. Gap junctions and cancer: new functions for an old story. Antioxid Redox Signal. 2009;11:323–338. doi: 10.1089/ars.2008.2153. [DOI] [PubMed] [Google Scholar]
- 24.Trosko JE, Ruch RJ. Gap junctions as targets for cancer chemoprevention and chemotherapy. Curr Drug Targets. 2002;3:465–482. doi: 10.2174/1389450023347371. [DOI] [PubMed] [Google Scholar]
- 25.Pointis G, Fiorini C, Gilleron J, Carette D, Segretain D. Connexins as precocious markers and molecular targets for chemical and pharmacological agents in carcinogenesis. Curr Med Chem. 2007;14:2288–2303. doi: 10.2174/092986707781696564. [DOI] [PubMed] [Google Scholar]
- 26.Yamasaki H, Omori Y, Krutovskikh V, Zhu W, Mironov N, Yamakage K, Mesnil M. Connexins in tumour suppression and cancer therapy. Novartis Found Symp. 1999;219:241–254. doi: 10.1002/9780470515587.ch15. [DOI] [PubMed] [Google Scholar]
- 27.Risley MS. Connexin gene expression in seminiferous tubules of the Sprague–Dawley rat. Biol Reprod. 2000;62:748–754. doi: 10.1095/biolreprod62.3.748. [DOI] [PubMed] [Google Scholar]
- 28.Pointis G, Fiorini C, Gilleron J, Carette D, Segretain D. Connexins in the male reproductive system. In: Harris A, Locke D, editors. Connexins, a guide. Totowa: Humana Press; 2008. pp. 495–510. [Google Scholar]
- 29.Pointis G, Gilleron J, Carette D, Segretain D. Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Phil Trans R Soc B. 2010;365:1607–1620. doi: 10.1098/rstb.2009.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada K, Katagiri T, Tsunoda T, Mizutani Y, Suzuki Y, Kamada M, Fujioka T, Shuin T, Miki T, Nakamura Y. Analysis of gene-expression profiles in testicular seminomas using a genome-wide cDNA microarray. Int J Oncol. 2003;23:1615–1635. [PubMed] [Google Scholar]
- 31.Brehm R, Marks A, Rey R, Kliesch S, Bergmann M, Steger K. Altered expression of connexins 26 and 43 in Sertoli cells in seminiferous tubules infiltrated with carcinoma-in-situ or seminoma. J Pathol. 2002;197:647–653. doi: 10.1002/path.1140. [DOI] [PubMed] [Google Scholar]
- 32.White TW, Srinivas M, Ripps H, Trovato-Salinaro A, Condorelli DF, Bruzzone R. Virtual cloning, functional expression, and gating analysis of human connexin31.9. Am J Physiol Cell Physiol. 2002;283:C960–C970. doi: 10.1152/ajpcell.00163.2002. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen PA, Kumar NM. Differences in expression patterns between mouse connexin-30.2 (Cx30.2) and its putative human orthologue, connexin-31.9. FEBS Lett. 2003;540:151–156. doi: 10.1016/s0014-5793(03)00252-7. [DOI] [PubMed] [Google Scholar]
- 34.Steger K, Tetens F, Bergmann M. Expression of connexin 43 in human testis. Histochem Cell Biol. 1999;112:215–220. doi: 10.1007/s004180050409. [DOI] [PubMed] [Google Scholar]
- 35.Defamie N, Berthaut I, Mograbi B, Chevallier D, Dadoune JP, Fenichel P, Segretain D, Pointis G. Impaired gap junction connexin43 in Sertoli cells of patients with secretory azoospermia: a marker of undifferentiated Sertoli cells. Lab Invest. 2003;83:449–456. doi: 10.1097/01.lab.0000059928.82702.6d. [DOI] [PubMed] [Google Scholar]
- 36.Matsuo Y, Nomata K, Eguchi J, Aoki D, Hayashi T, Hishikawa Y, Kanetake H, Shibata Y, Koji T. Immunohistochemical analysis of connexin43 expression in infertile human testes. Acta Histochem Cytochem. 2007;40:69–75. doi: 10.1267/ahc.07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotula-Balak M, Hejmej A, Sadowska J, Bilinska B. Connexin 43 expression in human and mouse testes with impaired spermatogenesis. Eur J Histochem. 2007;51:261–268. [PubMed] [Google Scholar]
- 38.Willecke K, Kirchhoff S, Plum A, Temme A, Thonnissen E, Ott T. Biological functions of connexin genes revealed by human genetic defects, dominant negative approaches and targeted deletions in the mouse. Novartis Found Symp. 1999;219(76–88):88–96. doi: 10.1002/9780470515587.ch6. [DOI] [PubMed] [Google Scholar]
- 39.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 40.Weider K, Bergmann M, Giese S, Guillou F, Failing K, Brehm R. Altered differentiation and clustering of Sertoli cells in transgenic mice showing a Sertoli cell specific knockout of the connexin 43 gene. Differentiation. 2011;82:38–49. doi: 10.1016/j.diff.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Francis RJ, Lo CW. Primordial germ cell deficiency in the connexin 43 knockout mouse arises from apoptosis associated with abnormal p53 activation. Development. 2006;133:3451–3460. doi: 10.1242/dev.02506. [DOI] [PubMed] [Google Scholar]
- 42.Anniballo R, Brehm R, Steger K. Recognising the Sertoli-cell-only (SCO) syndrome: a case study. Andrologia. 2011;43:78–83. doi: 10.1111/j.1439-0272.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 43.Gilleron J, Carette D, Durand P, Pointis G, Segretain D. Connexin 43 a potential regulator of cell proliferation and apoptosis within the seminiferous epithelium. Int J Biochem Cell Biol. 2009;41:1381–1390. doi: 10.1016/j.biocel.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Lee NP, Leung KW, Wo JY, Tam PC, Yeung WS, Luk JM. Blockage of testicular connexins induced apoptosis in rat seminiferous epithelium. Apoptosis. 2006;11:1215–1229. doi: 10.1007/s10495-006-6981-2. [DOI] [PubMed] [Google Scholar]
- 45.Godet M, Sabido O, Gilleron J, Durand P. Meiotic progression of rat spermatocytes requires mitogen-activated protein kinases of Sertoli cells and close contacts between the germ cells and the Sertoli cells. Dev Biol. 2008;315:173–188. doi: 10.1016/j.ydbio.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 46.Giese S, Hossain H, Markmann M, Chakraborty T, Tchatalbachev S, Guillou F, Bergmann M, Failing K, Weider K, Brehm R (2012) Sertoli-cell-specific knockout of connexin 43 leads to multiple alterations in testicular gene expression in prepubertal mice. Dis Model Mech. doi:10.1242/dmm.008649 [DOI] [PMC free article] [PubMed]
- 47.Li MW, Mruk DD, Lee WM, Cheng CY. Connexin 43 is critical to maintain the homeostasis of the blood–testis barrier via its effects on tight junction reassembly. Proc Natl Acad Sci USA. 2010;107:17998–18003. doi: 10.1073/pnas.1007047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carette D, Weider K, Gilleron J, Giese S, Dompierre J, Bergmann M, Brehm R, Denizot JP, Segretain D, Pointis G. Major involvement of connexin 43 in seminiferous epithelial junction dynamics and male fertility. Dev Biol. 2010;346:54–67. doi: 10.1016/j.ydbio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Bigliardi E, Vegni-Talluri M. Gap junctions between Sertoli cells in the infertile human testis. Fertil Steril. 1977;28:755–758. doi: 10.1016/s0015-0282(16)42679-8. [DOI] [PubMed] [Google Scholar]
- 50.Schleiermacher E. Ultrastructural changes of the intercellular relationship in impaired human spermatogenesis. Hum Genet. 1980;54:391–404. doi: 10.1007/BF00291587. [DOI] [PubMed] [Google Scholar]
- 51.Cavicchia JC, Sacerdote FL, Ortiz L. The human blood–testis barrier in impaired spermatogenesis. Ultrastruct Pathol. 1996;20:211–218. doi: 10.3109/01913129609016317. [DOI] [PubMed] [Google Scholar]
- 52.Hejmej A, Bilińska B. The effects of cryptorchidism on the regulation of steroidogenesis and gap junctional communication in equine testes. Endokrynol Pol. 2008;59:112–118. [PubMed] [Google Scholar]
- 53.Schuppe HC, Meinhardt A. Immune privilege and inflammation of the testis. Chem Immunol Allergy. 2005;88:1–14. doi: 10.1159/000087816. [DOI] [PubMed] [Google Scholar]
- 54.Pérez C, Sobarzo C, Jacobo P, Jarazo Dietrich S, Theas M, Denduchis B, Lustig L. Impaired expression and distribution of adherens and gap junction proteins in the seminiferous tubules of rats undergoing autoimmune orchitis. Int J Androl. 2011;34:566–577. doi: 10.1111/j.1365-2605.2011.01165.x. [DOI] [PubMed] [Google Scholar]
- 55.Altay B, Turna B, Oktem G, Aktuğ H, Semerci B, Bilir A. Immunohistochemical expression of connexin 43 and occludin in the rat testis after epididymal and vasal ligation. Fertil Steril. 2008;90:141–147. doi: 10.1016/j.fertnstert.2007.05.065. [DOI] [PubMed] [Google Scholar]
- 56.Lai-Cheong JE, Arita K, McGrath JA. Genetic diseases of junctions. J Invest Dermatol. 2007;127:2713–2725. doi: 10.1038/sj.jid.5700727. [DOI] [PubMed] [Google Scholar]
- 57.Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Van Maldergem L, Boyadjiev SA, Bodurtha JN, Jabs EW. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat. 2009;30:724–733. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- 58.Tong D, Colley D, Thoo R, Li TY, Plante I, Laird DW, Bai D, Kidder GM. Oogenesis defects in a mutant mouse model of oculodentodigital dysplasia. Dis Model Mech. 2009;2:157–167. doi: 10.1242/dmm.000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gregory M, Kahiri CN, Barr KJ, Smith CE, Hermo L, Cyr DG, Kidder GM. Male reproductive system defects and subfertility in a mutant mouse model of oculodentodigital dysplasia. Int J Androl. 2011;34:630–641. doi: 10.1111/j.1365-2605.2011.01224.x. [DOI] [PubMed] [Google Scholar]
- 60.Wohlfahrt-Veje C, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome: foetal origin of adult reproductive problems. Clin Endocrinol (Oxf) 2009;71:459–465. doi: 10.1111/j.1365-2265.2009.03545.x. [DOI] [PubMed] [Google Scholar]
- 61.Skakkebaek NE, Berthelsen JG, Giwercman A, Müller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987;10:19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 62.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 63.Petersen PM, Skakkebaek NE, Giwercman A. Gonadal function in men with testicular cancer: biological and clinical aspects. APMIS. 1998;106:24–34. doi: 10.1111/j.1699-0463.1998.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 64.Møller H, Skakkebaek NE. Risk of testicular cancer in subfertile men: case-control study. BMJ. 1999;318:559–562. doi: 10.1136/bmj.318.7183.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, Skakkebaek NE, Moller H. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000;321:789–792. doi: 10.1136/bmj.321.7264.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raman JD, Nobert CF, Goldstein M. Increased incidence of testicular cancer in men presenting with infertility and abnormal semen analysis. J Urol. 2005;174:1819–1822. doi: 10.1097/01.ju.0000177491.98461.aa. [DOI] [PubMed] [Google Scholar]
- 67.Olesen IA, Hoei-Hansen CE, Skakkebaek NE, Petersen JH, Rajpert-De Meyts E, Jørgensen N. Testicular carcinoma in situ in subfertile Danish men. Int J Androl. 2007;30:406–411. doi: 10.1111/j.1365-2605.2007.00798.x. [DOI] [PubMed] [Google Scholar]
- 68.Paul C, Povey JE, Lawrence NJ, Selfridge J, Melton DW, Saunders PT. Deletion of genes implicated in protecting the integrity of male germ cells has differential effects on the incidence of DNA breaks and germ cell loss. PLoS ONE. 2007;2:989. doi: 10.1371/journal.pone.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12:303–323. doi: 10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- 70.Looijenga LH, Gillis AJ, Stoop HJ, Hersmus R, Oosterhuis JW. Chromosomes and expression in human testicular germ-cell tumors: insight into their cell of origin and pathogenesis. Ann NY Acad Sci. 2007;1120:187–214. doi: 10.1196/annals.1411.000. [DOI] [PubMed] [Google Scholar]
- 71.Yamasaki H, Naus CC. Role of connexin genes in growth control. Carcinogenesis. 1996;17:1199–1213. doi: 10.1093/carcin/17.6.1199. [DOI] [PubMed] [Google Scholar]
- 72.Roger C, Mograbi B, Chevallier D, Michiels JF, Tanaka H, Segretain D, Pointis G, Fenichel P. Disrupted traffic of connexin 43 in human testicular seminoma cells: overexpression of Cx43 induces membrane location and cell proliferation decrease. J Pathol. 2004;202:241–246. doi: 10.1002/path.1509. [DOI] [PubMed] [Google Scholar]
- 73.Brehm R, Rüttinger C, Fischer P, Gashaw I, Winterhager E, Kliesch S, Bohle RM, Steger K, Bergmann M. Transition from preinvasive carcinoma in situ to seminoma is accompanied by a reduction of connexin 43 expression in Sertoli cells and germ cells. Neoplasia. 2006;8:499–509. doi: 10.1593/neo.05847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Omori Y, Li Q, Nishikawa Y, Yoshioka T, Yoshida M, Nishimura T, Enomoto K. Pathological significance of intracytoplasmic connexin proteins: implication in tumor progression. J Membr Biol. 2007;218:73–77. doi: 10.1007/s00232-007-9048-6. [DOI] [PubMed] [Google Scholar]
- 75.Nakashima Y, Ono T, Yamanoi A, El-Assal ON, Kohno H, Nagasue N. Expression of gap junction protein connexin32 in chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J Gastroenterol. 2004;39:763–768. doi: 10.1007/s00535-003-1386-2. [DOI] [PubMed] [Google Scholar]
- 76.Kanczuga-Koda L, Sulkowski S, Koda M, Sulkowska M. Alterations in connexin26 expression during colorectal carcinogenesis. Oncology. 2005;68:217–222. doi: 10.1159/000086777. [DOI] [PubMed] [Google Scholar]
- 77.Hong R, Lim SC. Pathological significance of connexin 26 expression in colorectal adenocarcinoma. Oncol Rep. 2008;19:913–919. [PubMed] [Google Scholar]
- 78.Jee H, Nam KT, Kwon HJ, Han SU, Kim DY. Altered expression and localization of connexin32 in human and murine gastric carcinogenesis. Dig Dis Sci. 2011;56:1323–1332. doi: 10.1007/s10620-010-1467-z. [DOI] [PubMed] [Google Scholar]
- 79.Mauro V, Chevallier D, Gilleron J, Carette D, Defamie N, Gasc JM, Senegas-Balas F, Segretain D, Pointis G. Aberrant cytoplasmic accumulation of connexin 43 in human testicular seminoma. Open Biomarkers J. 2008;1:20–27. [Google Scholar]
- 80.Segretain D, Decrouy X, Dompierre J, Escalier D, Rahman N, Fiorini C, Mograbi B, Siffroi JP, Huhtaniemi I, Fenichel P, Pointis G. Sequestration of connexin43 in the early endosomes: an early event of Leydig cell tumor progression. Mol Carcinog. 2003;38:179–187. doi: 10.1002/mc.10160. [DOI] [PubMed] [Google Scholar]
- 81.Li Q, Omori Y, Nishikawa Y, Yoshioka T, Yamamoto Y, Enomoto K. Cytoplasmic accumulation of connexin32 protein enhances motility and metastatic ability of human hepatoma cells in vitro and in vivo. Int J Cancer. 2007;121:536–546. doi: 10.1002/ijc.22696. [DOI] [PubMed] [Google Scholar]
- 82.Huang RP, Fan Y, Hossain MZ, Peng A, Zeng ZL, Boynton AL. Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (cx43) Cancer Res. 1998;58:5089–5096. [PubMed] [Google Scholar]
- 83.Quin H, Shao Q, Thomas T, Kalra J, Alaoui-Jamali MA, Laird DW. Connexin26 regulates the expression of angiogenesis-related genes in human breast tumor cells by both GJIC-dependent and -independent mechanisms. Cell Commun Adhes. 2003;10:387–393. doi: 10.1080/cac.10.4-6.387.393. [DOI] [PubMed] [Google Scholar]
- 84.Kalra J, Shao Q, Qin H, Thomas T, Alaoui-Jamali MA, Laird DW. Cx26 inhibits breast MDA-MB-435 cell tumorigenic properties by a gap junctional intercellular communication-independent mechanism. Carcinogenesis. 2006;27:2528–2537. doi: 10.1093/carcin/bgl110. [DOI] [PubMed] [Google Scholar]
- 85.Kliesch S, Behre HM, Hertle L, Bergmann M. Alteration of Sertoli cell differentiation in the presence of carcinoma in situ in human testes. J Urol. 1998;160:1894–1898. [PubMed] [Google Scholar]
- 86.Decrouy X, Gasc JM, Pointis G, Segretain D. Functional characterization of Cx43 based gap junctions during spermatogenesis. J Cell Physiol. 2004;200:146–154. doi: 10.1002/jcp.10473. [DOI] [PubMed] [Google Scholar]
- 87.Fiorini C, Decrouy X, Defamie N, Segretain D, Pointis G. Opposite regulation of connexin33 and connexin43 by LPS and IL-1alpha in spermatogenesis. Am J Physiol Cell Physiol. 2006;290:C733–C740. doi: 10.1152/ajpcell.00106.2005. [DOI] [PubMed] [Google Scholar]
- 88.McLachlan E, Shao Q, Laird DW. Connexins and gap junctions in mammary gland development and breast cancer progression. J Membr Biol. 2007;218:107–121. doi: 10.1007/s00232-007-9052-x. [DOI] [PubMed] [Google Scholar]
- 89.Francis R, Xu X, Park H, Wei CJ, Chang S, Chatterjee B, Lo C. Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PLoS ONE. 2011;6:e26379. doi: 10.1371/journal.pone.0026379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Steiner M, Weipoltshammer K, Viehberger G, Meixner EM, Lunglmayr G, Schöfer C. Immunohistochemical expression analysis of Cx43, Cx26, c-KIT and PlAP in contralateral testis biopsies of patients with non-seminomatous testicular germ cell tumor. Histochem Cell Biol. 2011;135:73–81. doi: 10.1007/s00418-010-0769-8. [DOI] [PubMed] [Google Scholar]
- 91.Richiardi L, Bellocco R, Adami HO, Torrång A, Barlow L, Hakulinen T, Rahu M, Stengrevics A, Storm H, Tretli S, Kurtinaitis J, Tyczynski JE, Akre O. Testicular cancer incidence in eight northern European countries: secular and recent trends. Cancer Epidemiol Biomarkers Prev. 2004;12:2157–2166. [PubMed] [Google Scholar]
- 92.Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES, Jr, Agarwal A. Critical appraisal of World Health Organization’s new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79:16–22. doi: 10.1016/j.urology.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 93.Walsh T, Croughan M, Schembri M, Chan J, Turek P. Increased risk of testicular germ cell cancer among infertile men. MD Arch Intern Med. 2009;169:351–356. doi: 10.1001/archinternmed.2008.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng X, Zeng X, Peng S, Deng D, Zhang J. The association risk of male subfertility and testicular cancer: a systematic review. PLoS ONE. 2009;4:e5591. doi: 10.1371/journal.pone.0005591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hotaling JM, Walsh TJ. Male infertility: a risk factor for testicular cancer. Nat Rev Urol. 2009;10:550–556. doi: 10.1038/nrurol.2009.179. [DOI] [PubMed] [Google Scholar]
- 96.Favilla V, Cimino S, Madonia M, Morgia G. New advances in clinical biomarkers in testis cancer. Front Biosci. 2010;1:456–477. doi: 10.2741/e105. [DOI] [PubMed] [Google Scholar]
- 97.Trigo JM, Tabernero JM, Paz-Ares L, García-Llano JL, Mora J, Lianes P, Esteban E, Salazar R, López-López JJ, Cortés-Funes H. Tumor markers at the time of recurrence in patients with germ cell tumors. Cancer. 2000;1:162–168. doi: 10.1002/(sici)1097-0142(20000101)88:1<162::aid-cncr22>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 98.Chieffi P. New prognostic markers and potential therapeutic targets in human testicular germ cell tumors. Curr Med Chem. 2011;18:5033–5040. doi: 10.2174/092986711797636054. [DOI] [PubMed] [Google Scholar]
- 99.Huang S, Li H, Ding X, Xiong C. Presence and characterization of cell-free seminal RNA in healthy individuals: implications for noninvasive disease diagnosis and gene expression studies of the male reproductive system. Clin Chem. 2009;55:1967–1976. doi: 10.1373/clinchem.2009.131128. [DOI] [PubMed] [Google Scholar]
- 100.Corsini LR, Bronte G, Terrasi M, Amodeo V, Fanale D, Fiorentino E, Cicero G, Bazan V, Russo A. The role of microRNAs in cancer: diagnostic and prognostic biomarkers and targets of therapies. Expert Opin Ther Targets. 2012;16:S103–S109. doi: 10.1517/14728222.2011.650632. [DOI] [PubMed] [Google Scholar]
- 101.Zen K, Zhang CY. Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev. 2012;32:326–348. doi: 10.1002/med.20215. [DOI] [PubMed] [Google Scholar]
- 102.Wang C, Yang C, Chen X, Yao B, Yang C, Zhu C, Li L, Wang J, Li X, Shao Y, Liu Y, Ji J, Zhang J, Zen K, Zhang CY, Zhang C. Altered profile of seminal plasma microRNAs in the molecular diagnosis of male infertility. Clin Chem. 2011;57:1722–1731. doi: 10.1373/clinchem.2011.169714. [DOI] [PubMed] [Google Scholar]
- 103.Novotny GW, Belling K, Bramsen JB, Nielsen JE, Bork-Jensen J, Almstrup K, Sonne SB, Kjems J, Rajpert-De Meyts E, Leffers H. MicroRNA expression profiling of carcinoma in situ (CIS) cells of the testis. Endocr Relat Cancer. 2012;19:365–379. doi: 10.1530/ERC-11-0271. [DOI] [PubMed] [Google Scholar]
- 104.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 105.Fichtlscherer S, Zeiher AM, Dimmeler S. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol. 2011;31:2383–2390. doi: 10.1161/ATVBAHA.111.226696. [DOI] [PubMed] [Google Scholar]
- 106.Kandouz M, Batist G. Gap junctions and connexins as therapeutic targets in cancer. Expert Opin Ther Targets. 2010;14:681–692. doi: 10.1517/14728222.2010.487866. [DOI] [PubMed] [Google Scholar]
- 107.King TJ, Bertram JS. Connexins as targets for cancer chemoprevention and chemotherapy. Biochim Biophys Acta. 2005;1719:146–160. doi: 10.1016/j.bbamem.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 108.Nicholas TW, Read SB, Burrows FJ, Kruse CA. Suicide gene therapy with Herpes simplex virus thymidine kinase and ganciclovir is enhanced with connexins to improve gap junctions and bystander effects. Histol Histopathol. 2003;18:495–507. doi: 10.14670/HH-18.495. [DOI] [PubMed] [Google Scholar]
- 109.Fang X, Huang T, Zhu Y, Yan Q, Chi Y, Jiang JX, Wang P, Matsue H, Kitamura M, Yao J. Connexin43 hemichannels contribute to cadmium-induced oxidative stress and cell injury. Antioxid Redox Signal. 2011;14:2427–2439. doi: 10.1089/ars.2010.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hong X, Wang Q, Yang Y, Zheng S, Tong X, Zhang S, Tao L, Harris AL. Gap junctions propagate opposite effects in normal and tumor testicular cells in response to cisplatin. Cancer Lett. 2012;317:165–171. doi: 10.1016/j.canlet.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 111.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. [PubMed] [Google Scholar]
- 112.Kim J, Lee S, Jeon B, Jang W, Moon C, Kim S. Protection of spermatogenesis against gamma ray-induced damage by granulocyte colony-stimulating factor in mice. Andrologia. 2011;43:87–93. doi: 10.1111/j.1439-0272.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 113.Kuwabara M, Kakinuma Y, Katare RG, Ando M, Yamasaki F, Doi Y, Sato T. Granulocyte colony-stimulating factor activates Wnt signal to sustain gap junction function through recruitment of beta-catenin and cadherin. FEBS Lett. 2007;581:4821–4830. doi: 10.1016/j.febslet.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 114.Milberg P, Klocke R, Frommeyer G, Quang TH, Dieks K, Stypmann J, Osada N, Kuhlmann M, Fehr M, Milting H, Nikol S, Waltenberger J, Breithardt G, Eckardt L. G-CSF therapy reduces myocardial repolarization reserve in the presence of increased arteriogenesis, angiogenesis and connexin 43 expression in an experimental model of pacing-induced heart failure. Basic Res Cardiol. 2011;106:995–1008. doi: 10.1007/s00395-011-0230-8. [DOI] [PubMed] [Google Scholar]
- 115.Gilleron J, Carette D, Chevallier D, Segretain D, Pointis G (2012) Molecular connexin partner remodeling orchestrates connexin traffic: from physiology to pathophysiology. Crit Rev Biochem Mol Biol. doi:10.3109/10409238.2012.683482 [DOI] [PubMed]
- 116.Yomogida K, Yagura Y, Nishimune Y. Electroporated transgene-rescued spermatogenesis in infertile mutant mice with a Sertoli cell defect. Biol Reprod. 2002;67:712–717. doi: 10.1095/biolreprod.101.001743. [DOI] [PubMed] [Google Scholar]
- 117.Kojima Y, Kurokawa S, Mizuno K, Umemoto Y, Sasaki S, Hayashi Y, Kohri K. Gene transfer to sperm and testis: future prospects of gene therapy for male infertility. Curr Gene Ther. 2008;8:121–134. doi: 10.2174/156652308784049390. [DOI] [PubMed] [Google Scholar]
- 118.O’Bryan MK, de Kretser D. Mouse models for genes involved in impaired spermatogenesis. Int J Androl. 2006;29:76–89. doi: 10.1111/j.1365-2605.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- 119.Miyamoto T, Tsujimura A, Miyagawa Y, Koh E, Namiki M, Sengoku K. Male infertility and its causes in human. Adv Urol. 2012;2012:384520. doi: 10.1155/2012/384520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanimoto R, Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kumon H, Huh NH. REIC/Dkk-3 as a potential gene therapeutic agent against human testicular cancer. Int J Mol Med. 2007;19:363–368. [PubMed] [Google Scholar]
- 121.Bikou O, Thomas D, Trappe K, Lugenbiel P, Kelemen K, Koch M, Soucek R, Voss F, Becker R, Katus HA, Bauer A. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc Res. 2011;92:218–225. doi: 10.1093/cvr/cvr209. [DOI] [PubMed] [Google Scholar]
- 122.Igarashi T, Finet JE, Takeuchi A, Fujino Y, Strom M, Greener ID, Rosenbaum DS, Donahue JK. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation. 2012;125:216–225. doi: 10.1161/CIRCULATIONAHA.111.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Staecker H, Brough DE, Praetorius M, Baker K. Drug delivery to the inner ear using gene therapy. Otolaryngol Clin North Am. 2004;37:1091–1108. doi: 10.1016/j.otc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 124.Abraham MR, Henrikson CA, Tung L, Chang MG, Aon M, Xue T, Li RA, O’ Rourke B, Marban E. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97:159–167. doi: 10.1161/01.RES.0000174794.22491.a0. [DOI] [PubMed] [Google Scholar]
- 125.McIver SC, Roman SD, Nixon B, McLaughlin EA. miRNA and mammalian male germ cells. Hum Reprod Update. 2012;18:44–59. doi: 10.1093/humupd/dmr041. [DOI] [PubMed] [Google Scholar]
- 126.Anderson C, Catoe H, Werner R. MIR-206 regulates connexin43 expression during skeletal muscle development. Nucleic Acids Res. 2006;34:5863–5871. doi: 10.1093/nar/gkl743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 128.Rau F, Freyermuth F, Fugier C, Villemin JP, Fischer MC, Jost B, Dembele D, Gourdon G, Nicole A, Duboc D, Wahbi K, Day JW, Fujimura H, Takahashi MP, Auboeuf D, Dreumont N, Furling D, Charlet-Berguerand N. Misregulation of miR-1 processing is associated with heart defects in myotonic dystrophy. Nat Struct Mol Biol. 2011;18:840–845. doi: 10.1038/nsmb.2067. [DOI] [PubMed] [Google Scholar]
- 129.Inose H, Ochi H, Kimura A, Fujita K, Xu R, Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, Saito K, Nakamura T, Siomi H, Ito H, Arai Y, Shinomiya K, Takeda S. A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci USA. 2009;106:20794–20799. doi: 10.1073/pnas.0909311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lian J, Zhang X, Tian H, Liang N, Wang Y, Liang C, Li X, Sun F. Altered microRNA expression in patients with non-obstructive azoospermia. Reprod Biol Endocrinol. 2009;7:13. doi: 10.1186/1477-7827-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lian J, Tian H, Liu L, Zhang XS, Li WQ, Deng YM, Yao GD, Yin MM, Sun F. Downregulation of microRNA-383 is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation by targeting IRF1. Cell Death Dis. 2010;1:e94. doi: 10.1038/cddis.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song YN, Zhang H, Zhao JY, Guo XL. Connexin 43, a new therapeutic target for cardiovascular diseases. Pharmazie. 2009;64:291–295. [PubMed] [Google Scholar]
- 134.Pointis G. Connexin43: emerging role in erectile function. Int J Biochem Cell Biol. 2006;38:1642–1646. doi: 10.1016/j.biocel.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 135.Lee SK, Teng Y, Wong HK, Ng TK, Huang L, Lei P, Choy KW, Liu Y, Zhang M, Lam DS, Yam GH, Pang CP. MicroRNA-145 regulates human corneal epithelial differentiation. PLoS ONE. 2011;6:e21249. doi: 10.1371/journal.pone.0021249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alajez NM, Lenarduzzi M, Ito E, Hui AB, Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J, O’Sullivan B, Liu FF. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 2011;71:2381–2391. doi: 10.1158/0008-5472.CAN-10-2754. [DOI] [PubMed] [Google Scholar]
- 137.Parrington J, Coward K, Gadea J. Sperm and testis mediated DNA transfer as a means of gene therapy. Syst Biol Reprod Med. 2011;57:35–42. doi: 10.3109/19396368.2010.514022. [DOI] [PubMed] [Google Scholar]
- 138.Kojima Y, Mizuno K, Umemoto Y, Sasaki S, Hayashi Y, Kohri K (2011) Therapeutic potential of gene transfer to testis; myth or reality? In: Kang C (ed) Gene therapy applications. InTech. ISBN: 978-953-307-541-9. Available from: http://www.intechopen.com/books/gene-therapy-applications/therapeutic-potential-of-gene-transfer-to-testis-myth-or-reality-