Abstract
Hepatic fibrosis is a dynamic chronic liver disease occurring as a consequence of wound-healing responses to various hepatic injuries. This disorder is one of primary predictors for liver-associated morbidity and mortality worldwide. To date, no pharmacological agent has been approved for hepatic fibrosis or could be recommended for routine use in clinical context. Cellular and molecular understanding of hepatic fibrosis has revealed that peroxisome proliferator-activated receptor-γ (PPARγ), the functioning receptor for antidiabetic thiazolidinediones, plays a pivotal role in the pathobiology of hepatic stellate cells (HSCs), whose activation is the central event in the pathogenesis of hepatic fibrosis. Activation of PPARγ inhibits HSC collagen production and modulates HSC adipogenic phenotype at transcriptional and epigenetic levels. These molecular insights indicate PPARγ as a promising drug target for antifibrotic chemotherapy. Intensive animal studies have demonstrated that stimulation of PPARγ regulatory system through gene therapy approaches and PPARγ ligands has therapeutic promise for hepatic fibrosis induced by a variety of etiologies. At the same time, thiazolidinedione agents have been investigated for their clinical benefits primarily in patients with nonalcoholic steatohepatitis, a common metabolic liver disorder with high potential to progress to fibrosis and liver-related death. Although some studies have shown initial promise, none has established long-term efficacy in well-controlled randomized clinical trials. This comprehensive review covers the 10-year discoveries of the molecular basis for PPARγ regulation of HSC pathophysiology and then focuses on the animal investigations and clinical trials of various therapeutic modalities targeting PPARγ for hepatic fibrosis.
Keywords: Hepatic fibrosis, Peroxisome proliferator-activated receptor-γ, Hepatic stellate cell, Thiazolidinedione, Nonalcoholic steatohepatitis, Metabolic syndrome
Introduction
Hepatic fibrosis is an integral clinicopathological condition of chronic liver disease predisposing to cirrhosis and hepatocellular carcinoma (HCC). It is characterized by overproduction of extracellular matrix (ECM), mainly type I and III collagens. The net deposition of ECM distorts hepatic architecture and leads to portal hypertension [1, 2]. The evolution of liver fibrosis is influenced by a broad spectrum of environmental and genetic factors that perform in concert. Globally, infection of hepatitis B virus (HBV) or C virus (HCV) and non-alcoholic fatty liver disease (NAFLD) are the most common etiologies for liver fibrosis. Although conventional therapies such as antiviral strategy can attenuate or prevent liver fibrosis, a large number of subjects are either resistant to causal treatment or diagnosed with end-stage fibrosis [3]. Currently, understanding of hepatic fibrosis has moved into some new frontiers. The pathogenesis of liver fibrosis due to NAFLD has been thought to be associated with the metabolic syndrome and insulin resistance (IR), suggesting that mediators of the metabolic syndrome are also effectors of liver fibrogenesis and correlate with a more severe fibrogenic progression [4]. Clinical studies have demonstrated that IR not only determines the responsiveness to interferon therapy for liver fibrosis in chronic HCV patients [5] but also is an independent predictor of advanced fibrosis in HCV infection and NAFLD [6, 7]. Furthermore, the overwhelming prevalence of obesity worldwide has also led to increasing incidence of nonalcoholic steatohepatitis (NASH), which implies a risk of HCC once fibrosis develops [8]. Therefore, hepatic fibrosis is a broader category subdivided by progressive status and complication with the metabolic syndrome under some circumstances.
The limitations in preventive measures and lack of established therapies for chronic liver diseases highlight the urgent need to develop novel antifibrotic agents. It has been growingly established that there is a vast complexity in the transcriptional control pathways in the diseased liver [9]. These molecular discoveries provide potential drug targets for intervention of hepatic fibrosis. Transcription factor peroxisome proliferator-activated receptor-γ (PPARγ) is one of the attractive targets. The dysfunction of PPARγ transcription plays a pivotal role in liver fibrosis progression [10]. Here, we will provide a brief but up-to-date summary on PPARγ and its regulatory system in the pathophysiology of liver fibrosis. Subsequently, we will comprehensively review preclinical and clinical studies of PPARγ agonists. The goal of this work is to highlight these findings that have potential to be translated into novel therapeutics for hepatic fibrosis.
An update on PPARγ molecular biology and physiology
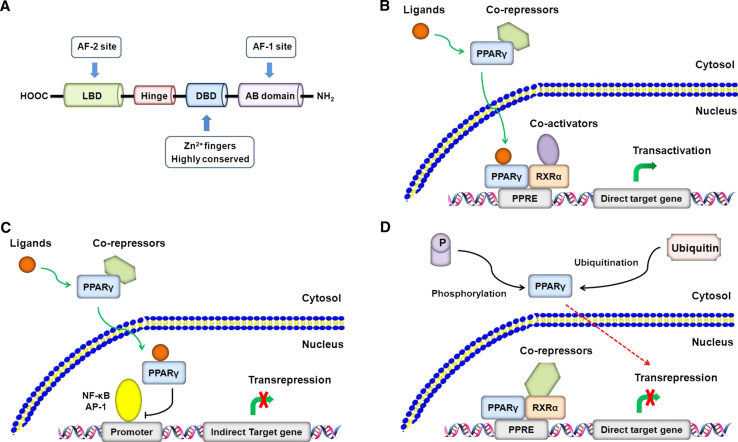
PPARγ is predominantly present in liver and adipose tissue and belongs to the superfamily of nuclear receptors controlling the transcription of a subset of genes [11]. It has four major isoforms, namely γ1, γ2, γ3, and γ4, which differ only in their N-terminal sequence and have similar transcriptional activities [12]. Upon ligand binding, PPARγ activates gene expression by directly binding to specific DNA sequences called peroxisome proliferator response elements (PPRE) in target gene promoters as heterodimers with the retinoid X receptor (RXR). This process is termed ligand-dependent transactivation, which is linked to the recruitment of co-activators that promote assembly of general transcriptional machinery at the promoter [13]. In addition, PPARγ can exert biological functions via ligand-dependent transrepression and ligand-independent transrepression [14]. Ligand-activated PPARγ can interfere with other transcription factors such as nuclear factor-κB (NF-κB) and activator protein-1 (AP-1), leading to transrepression of gene expression [15, 16]. In contrast, ligand-independent transrepression of PPARγ is associated with the recruitment of co-repressors that function to antagonize the actions of co-activator complexes [17]. Moreover, ubiquitination [18] and phosphorylation [19] also contribute to the ligand-independent transrepression of PPARγ (Fig. 1).
Fig. 1.
Mechanisms of PPARγ regulation of gene transcription. a The primary structure of PPARγ mainly comprises a C-terminal ligand binding domain (LBD) that harbors the ligand-dependent activation function (AF2), a zinc-finger DNA binding domain (DBD), and an N-terminal AB domain that harbors a ligand-independent function (AF1). A hinge domain links the LBD to DBD. b Ligand-dependent transactivation. In the absence of ligands, PPARγ is bound to co-repressors and prevents gene transcription. Upon binding to ligands, PPARγ undergoes conformational changes. It dissociates the co-repressors and translocates to nucleus where it recruits co-activators that have histone acetylase activity, and forms a heterodimer with RXRα. The active transcription complex then binds to the DNA response elements termed PPRE located in the promoter regions of target genes, allowing transcription activation. A number of co-activator proteins have been identified and their combinatorial usage may provide the basis for cell type- or gene-specific transcriptional responses. c Ligand-dependent transrepression. Following activated by ligands, PPARγ can interfere with the activity and function of other transcription factors such as NF-κB and AP-1, leading to disruption of their binding to target gene promoters and thereby to transcription repression. In this mechanism, PPARγ does not bind to specific DNA response elements. This transcriptional model may underlie the ability of PPARγ to negatively regulate the inflammatory responses and collagen production in hepatic fibrosis. d Ligand-independent transrepression. In the absence of ligands, PPARγ can recruit co-repressors that antagonize the effects of co-activators, leading to transcription repression of direct target genes. The most defined co-repressors are NCoR (nuclear-receptor co-repressor) and SMRT (silencing mediator of retinoic-acid and thyroid hormone receptors). In addition, protein modifications such as ubiquitination and phosphorylation have also been shown to suppress the transcription activity of PPARγ and thus play a role in the ligand-independent transrepression mechanism
Ligands for PPARγ include endogenous fatty acids and synthetic antidiabetic thiazolidinediones, as well as nonthiazolidinediones farglitazar and KR62776 [20]. Ligand activation of PPARγ commonly initiates transcription of adipogenesis-associated genes regulating adipocyte differentiation and lipid metabolism [21]. PPARγ is also genetically associated with type 2 diabetes and IR. PPARγ activation can restore insulin sensitivity via enhancing expression of glucose transporter type 4 and adiponectin that promotes fatty acid oxidation and insulin sensitivity [22]. Advances in PPARγ biology show that the pathophysiological functions are far beyond fatty acids and glucose. These new discoveries are briefly highlighted: (1) Activation of PPARγ attenuates matrix remolding [23] and oxidative stress [24], showing protection for tissue repair. (2) PPARγ regulates communication between metabolic and inflammatory reactions, exhibiting anti-inflammatory properties [25–27]. (3) PPARγ modulates cell division cycle, cell survival, and death [28, 29]. (4) PPARγ shows circadian expression in liver and adipose tissue [30]. (5) Activation of PPARγ is associated with bone loss and bone marrow adipogenesis [31]. Taken together, current knowledge suggests pleiotropic roles of PPARγ in a wide range of fundamental pathways with broad pathophysiological implications (Fig. 2). Given the importance of PPARγ in metabolic disorders and liver pathology [4], PPARγ has been suggested as a novel target for liver fibrosis therapy. Since persistent inflammatory infiltration contributes to the pathogenesis of liver fibrosis [32], the beneficial role of PPARγ in tissue repair and the anti-inflammatory property may further support the notion that targeting PPARγ is a promising strategy for treatment and prevention of liver fibrosis.
Fig. 2.
Representative natural and synthetic PPARγ ligands and ligand-specific PPARγ biological functions. PPARγ ligands include a wide range of chemical structures from endogenous and synthetic sources. The naturally occurring PPARγ agonist 15d-PGJ2 is most commonly used in laboratory investigations. Thiazolidinediones including troglitazone, rosiglitazone, and pioglitazone represent an important class of therapeutic agents for type 2 diabetes. Meanwhile, nonthiazolidinedione PPARγ agonists have been increasingly developed with attractive pharmacological properties. Activation of PPARγ by ligands has been shown to produce varied transcriptional profiles and biological functions. PPARγ is originally thought to regulate adipogenesis and insulin sensitivity and thus serves as a master integrator of energy consumption and glucose homeostasis. Accumulating evidence shows that the biological outcomes of PPARγ activation are far beyond regulation of fat and glucose. PPARγ exhibits a pleiotropic role affecting a variety of pathophysiological pathways as illustrated in this schema, indicating that PPARγ may have broad implications in pharmacotherapy of diseases. The beneficial role in tissue repair and the anti-inflammatory property implicate PPARγ regulatory system in the management of hepatic fibrosis. Progress toward understanding PPARγ biology still continues
PPARγ regulation of stellate cell pathophysiology in hepatic fibrosis
Hepatic stellate cells (HSCs) are a type of mesenchymal cells anatomically located in the space of Disse between hepatocytes and liver sinusoidal endothelial cells. They contain a specialized organelle called lipid droplet responsible for hepatic retinoid and vitamin A storage [33]. Under physiological conditions, HSCs are quiescent and maintain liver lipid homoeostasis. Upon fibrogenic injury, HSCs proliferate and undergo myofibroblastic transdifferentiation, a dramatic phenotypic change from quiescent cells to myofibroblasts responsible for the overproduction of ECM. This process is termed HSC activation and has been defined as the central event in hepatic fibrogenesis. Activated HSCs characteristically express α-smooth muscle actin (α-SMA), and meanwhile lose their lipid droplets, making them no longer retain the adipogenic phenotype [34]. The molecular insights into PPARγ regulation of HSC pathophysiology in hepatic fibrosis are critically summarized and discussed below.
Inhibition of HSC activation and collagen production
Pathological proliferation and activation of HSCs correlate directly with depleted PPARγ expression. A pioneering study by Miyahara and colleagues demonstrated that the expression of PPARγ1 was reduced and its ability to bind to PPRE was dramatically impaired in activated HSCs, but treatment with PPARγ agonists was able to prevent HSC activation [35]. Treatment with PPARγ agonists also inhibited proliferation and induced apoptosis in HSCs, and markedly inhibited transforming growth factor (TGF)-β1-induced expression of connective tissue growth factor (CTGF), a key regulator of ECM production in hepatic fibrosis [36]. Because the effects of exogenous PPARγ ligands can be mediated by receptor-independent mechanisms [37], studies with ectopic PPARγ expression provide additional evidence supporting PPARγ as a key regulatory molecule during HSC activation. Infection with a recombinant adenoviral vector encoding PPARγ in activated HSCs resulted in restoration of morphologic characteristics of quiescent HSCs, such as reappearance of cytoplasmic lipid droplets and capacity to concentrate retinyl palmitate [38]. On the other hand, PPARγ inhibition of HSC activation leads to decreased collagen production due to the transrepression function of PPARγ. Ligand-induced PPARγ activation reduced collagen and fibronectin synthesis induced by TGF-β1 in human HSCs and directly inhibited procollagen type I promoter activity by interfering with AP-1 transactivation [39]. PPARγ was also able to directly interact with the transcription factor JunD but did not affect its expression, disrupting JunD binding to AP-1 promoter that promotes collagen I transcription in activated HSCs [38]. A site-directed mutagenesis study has identified a region proximal to -133 bp at α1(I) procollagen promoter as the action site, showing that PPARγ abrogated p300-facilitated NF-I transactivity for collagen expression in HSCs [40]. Furthermore, synergism between PPARγ and farnesoid X receptor (FXR) contributes to reducing profibrogenic activities of HSCs. For instance, ligands targeting FXR reversed the effect of depleted PPARγ expression and inhibited α1(I) procollagen production in rat HSCs [41]. PPARγ promoter could be activated by a FXR-SHP (small heterodimer partner) regulatory cascade that counter-regulated the pro-inflammatory phenotype in HSCs [42]. Studies have suggested that FXR was expressed in rat HSCs and modulated their functions [43, 44]. However, a recent report demonstrated the functional absence of FXR in mouse and human HSCs [45], which is in stark contrast to the above findings [43, 44]. An explanation involving an interspecies difference may be too simplistic and not reassuring. Therefore, further investigation is needed to address this discrepancy. The new findings would fundamentally influence novel therapies to be designed, given the role of FXR or FXR-PPARγ axis in hepatic fibrosis and the synergistic activity of FXR-PPARγ that could limit the side-effects due to single use of PPARγ agonists.
Transcriptional regulation of HSC adipogenic phenotype
HSCs indeed have an adipocyte phenotype, despite the absence of adipocyte-specific PPARγ2 isoform in HSCs [38]. Activation of HSCs shares similar cellular and molecular mechanisms with differentiation of preadipocytes [46]. However, the adipogenic phenotype of HSCs is lost concomitantly with profound changes in expression profile of adipocyte-specific genes during activation [47, 48]. There has been growing evidence that PPARγ is a pivotal molecule for adipogenic transcriptional regulation and for maintenance of quiescence in HSCs. Ectopic expression of PPARγ restored the coordinated expression of a panel of transcription factors associated with adipogenesis in cultured rat HSCs, including CCAAT/enhancer binding protein α (C/EBPα), C/EBPβ, C/EBPδ, liver X receptor α, and sterol regulatory element-binding protein 1c, whose expression was rapidly depleted during HSC transdifferentiation to myofibroblastic cells. These genetic events induced adipogenic differentiation in HSCs and led to HSC phenotypic reversal to be quiescent [49]. Moreover, a cascade of transcription factors involving PEX16 and catalase, two downstream genes of PPARγ, and adipsin, a typical adipogenesis marker, controlled the HSC switch between the lipid storing and the myofibroblast phenotypes in liver fibrotic processes [50]. PPARγ was also able to induce HSC adipogenic phenotype and quiescence via albumin that plays a direct role in the formation of cytoplasmic lipid droplets, suggesting that albumin may be a downstream effector of PPARγ in HSCs [51].
HSC transdifferentiation is manifested by a shift of the phenotype from adipogenic nature to myogenic lineages due to repressed adipogenic transcription. Core to this alteration is the depleted expression of PPARγ, the master adipogenic regulator. Restoration of PPARγ expression can achieve the fat-storing phenotype that favors suppression of HSC activation marker genes. However, there are some paradoxes. Hepatic PPARγ overexpression has been linked to exacerbated steatosis by mechanisms involving activation of lipogenic genes and de novo lipogenesis as well as increased hepatic triglyceride concentrations [52]. PPARγ could induce adipogenic conversion of hepatocytes in PPARα−/− mice via upregulating adipocyte-specific genes and lipid accumulation [53]. These suggest that PPARγ may play a role in stimulating hepatic lipogenesis. Therefore, hepatic PPARγ overexpression, if without cellular selectivity, is likely to promote a lipogenic microenvironment in the liver, which renders the liver more sensitive to various profibrogenic stimuli. Moreover, enhanced adipogenic transcriptional regulation participates in the pathogenesis of NAFLD. Interestingly, under these adipogenic conditions, HSCs are still activated continuously and lose their adipogenic phenotype. Thus, activated HSCs seem to be resistant to the adipogenic signals that make hepatocytes steatotic [54]. More recently, a study has demonstrated that loss of retinoid-containing lipid droplets during HSC transdifferentiation did not affect liver fibrogenesis but reduced hepatocarcinogenesis [55], indicating that reversal of HSC adipogenic phenotype may have no potential for antifibrogenesis. All these findings constitute a lipid myth concerning HSC transdifferentiation. It is postulated that there should be some regulatory commonalities between HSC adipogenesis and hepatocyte lipogenesis under pathological conditions, which are poorly understood. A detailed understanding of these lipid paradoxes in hepatic fibrogenesis and the critical involvement of PPARγ will help provide new insights into HSC pathophysiology.
Epigenetic regulation of HSC transdifferentiation
PPARγ-mediated molecular events that orchestrate the coordinated expression of adipogenic genes in HSCs can also be modulated at the epigenetic level. It was found that diminished PPARγ expression was associated with DNA methylation mediated by methyl-CpG binding protein 2 (MeCP2) in activated HSCs, suggesting PPARγ modulation of chromatin packaging. DNA methylation inhibitor enhanced PPARγ expression in cultured HSCs and blocked HSC myofibroblastic transdifferentiation [56]. Some details of how PPARγ expression was suppressed by MeCP2 were subsequently elucidated, pointing to the role of microRNAs (miRNAs). Downregulation of miRNA-132 released a translational block on MeCP2, which bound to the 5′ end of PPARγ gene, where it facilitated H3K9-mediated methylation and recruited the transcription repressor HP1α. MeCP2 also promoted EZH2 (an epigenetic regulatory protein) expression and H3K27 methylation to form a repressive chromatin structure in the 3′ exons of PPARγ [57]. These molecular recognitions indicate that MeCP2 and DNA methylation exert epigenetic control over HSC adipogenic phenotype and that the repressed PPARγ expression is induced at least partially by a combination of miRNA-132, MeCP2, and EZH2 in a relay pathway. Moreover, the epigenetic PPARγ repression has been shown to be involved in necdin-Wnt pathway that mediated the anti-adipogenic HSC transdifferentiation. It was found that necdin, a melanoma antigen family protein that inhibits adipogenesis, was selectively expressed in HSCs and stimulated HSC myofibroblastic transdifferentiation via transactivation of canonical Wnt and Wnt-mediated suppression of PPARγ. Silencing of necdin abrogated the recruitment of MeCP2 and HP1α to PPARγ promoter and decreased H3K27 dimethylation at the exon 5 locus, leading to restoration of PPARγ expression and reversal of culture-activated HSCs to be quiescent cells [58]. More recently, delta-like 1 homolog (DLK1), which was selectively expressed in HSCs in the adult rodent liver, was shown to be critically involved in epigenetic repression of PPARγ. DLK1 knockdown reversed activated HSCs to be fat-storing quiescent cells via restoring PPARγ expression dependent on suppression of canonical Wnts [59]. These findings reinforce the concept that loss of adipogenic regulation underlies the shift of HSC cell fate to myofibroblastic cells, given the established role for DLK1 as an anti-adipogenic mediator [60]. Collectively, these discoveries preliminarily address the epigenetic mechanisms for PPARγ-mediated HSC transdifferentiation. Other epigenetic events such as mRNA stabilization [61] and miRNA interactions [62] have also been characterized in HSC pathophysiology in hepatic fibrosis, increasingly touching on the fundamental question that how HSC transdifferentiation is achieved by global mechanisms. Given the notion that the increased collagen expression in liver fibrosis is primarily post-transcriptional [63], it would be attractive to further explore the regulatory role of PPARγ in these epigenetic mechanisms, because modulators that selectively interfere with these pathways could be developed to pharmacologically reduce hepatic fibrosis. For example, rosmarinic acid and baicalin, two active phytochemicals in the herbal prescription Yang-Gan-Wan, have been recently shown to epigenetically derepress PPARγ expression via suppressing canonical Wnt signaling in HSCs, leading to attenuated HSC activation and reduced fibrogenesis [64].
Targeting PPARγ for hepatic fibrosis: data from animal studies
Gene therapy
The aforementioned molecular discoveries strongly highlight the critical role for PPARγ as a potential therapeutic target for hepatic fibrosis. Accumulating in vivo evidence supports this notion. PPARγ-deficient rodents subjected to bile duct ligation (BDL) [65] or fed the methionine- and choline-deficient (MCD) diet [66] developed more severe fibrosis or steatohepatitis than the wild-type ones. These observations raise a concept that gene therapy for PPARγ activation can be a therapeutic approach for hepatic fibrosis. More recently, this hypothesis has been tested in several studies with animal models. Adenovirus-mediated PPARγ overexpression downregulated a set of fibrogenic genes leading to inhibited HSC activation and reduced fibrogenesis in C57BL6 mice with MCD diet-induced hepatic fibrosis [67]. These effects were recaptured in activated human HSCs, where ectopic PPARγ overexpression arrested cell cycle at G0/G1 checkpoint and downregulated mRNA expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2, two proteins preventing matrix metalloproteinase (MMP)-directed degradation of collagen, and resultantly decreased collage production [67]. These findings support the notion that normalized PPARγ function favors matrix degradation conditions in fibrogenic HSCs. In addition, adenovirus-mediated PPARγ overexpression ameliorated hepatic inflammation and fibrosis in nutritional steatohepatitis in mice fed the MCD diet via upregulating adiponectin and hemeoxygenase-1, and downregulating tumor necrosis factor-α (TNF-α), interleukin (IL)-6, MMP-2, and MMP-9 [68]. Similar treatments also decreased HSC accumulation and collagen production in BDL-induced fibrosis in rats [65]. Furthermore, lentiviral-mediated PPARγ overexpression decreased HSC proliferation and type I collagen expression and effectively ameliorated the sustained fibrosis in CCl4-intoxiciated rats. However, this manipulation showed no significant benefits in the early phase of the fibrosis [69]. Although current data on gene therapy targeting PPARγ have indeed shown therapeutic benefits for liver fibrosis, how to achieve HSC-selective delivery needs to be further addressed, especially given the aforementioned lipid paradoxes. Encouragingly, several kinds of delivery system targeting activated HSCs have been explored with enhanced targeted potential in vivo [70]. It is tempting to develop HSC-targeted PPARγ delivery to improve the antifibrotic effects but with less off-target adverse effects.
Thiazolidinedione agonists
Antidiabetic thiazolidinediones, which were identified to be potent PPARγ-selective ligands for the first time in 1995 [71], have shown therapeutic promise for hepatic fibrosis in intensive animal investigations. Troglitazone is the first marketed thiazolidinedione agent, but it has been withdrawn due to its severe hepatotoxicity [72]. Despite this, troglitazone is still useful in the proof-of-concept studies with cell culture systems or animal models. Marra and coworkers [73] first reported that troglitazone inhibited HSC proliferation and chemotaxis in cultured human HSCs. Subsequently, they investigated the in vivo effects of troglitazone in rats with BDL-induced fibrosis [74]. Troglitazone treatment retarded liver fibrosis as indicated by significant decrease in type I collagen expression and hydroxyproline level, and also attenuated HSC activation as indicated by decreased α-SMA expression. Histochemical evaluation and reduced activity of γ-glutamyltransferase (γ-GT) further indicated troglitazone inhibition of ductular reaction. Additionally, troglitazone was found to affect epithelial-mesenchymal interaction, a significant ongoing controversy concerning the origin of fibrogenic cells in hepatic fibrosis [75–79], in this chronic cholestasis system.
Several studies have described the antifibrotic properties of rosiglitazone. Tahan and coworkers [80] first assessed the effects of rosiglitazone on a MCD diet model of NASH in rats, showing that rosiglitazone ameliorated inflammation and improved alanine transaminase (ALT), alkaline phosphatase (ALP), and IL-6 levels in the induction study and IL-1β, IL-6 and TNF-α levels in the treatment study. The anti-inflammatory property of rosiglitazone implicated in antifibrotic therapy was recaptured in mice with liver fibrosis caused by Schistosoma japonicum infection, where rosiglitazone substantially inhibited the expression of TGF-β1, α-SMA, and type I collagen concomitant with reduction in inflammatory mediators including IL-6, TNF-α, and NF-κB [81]. In nutrition-related fibrosis in mice, rosiglitazone significantly attenuated MCD diet-induced fibrosis by repressing profibrogenic factors TGF-β1 and CTGF, and also ameliorated nutritional fibrosing steatohepatitis evidenced clearly by morphological and biochemical observations [82]. Recently, a study aiming at characterizing a mice model that may faithfully recapitulate the pathophysiology of human NASH showed that rosiglitazone substantially attenuated liver steatosis, inflammation, and fibrosis via stimulation of antioxidant gene expression and mitochondrial β-oxidation, and suppression of oxidative stress [83]. Furthermore, rosiglitazone could be synergistic with agonists targeting retinoic acid receptor (RAR) in reducing thioacetamide-induced hepatic fibrosis in rats via inhibiting HSC proliferation and reducing TGF-β1 and TNF-α [84], and also protected against alcoholic fatty liver correlated closely with adiponectin signaling and the stimulated sirtuin 1-AMP-activated kinase system in mice [85]. However, Garcia-Ruiz and coworkers revealed that rosiglitazone was ineffective in ob/ob mice with NASH. Rosiglitazone treatment for 12 weeks improved neither the NAFLD inflammatory activity nor the mitochondrial injury in the model. On the contrary, rosiglitazone increased liver steatosis, particularly microvesicular steatosis and oxidative stress. The anti-inflammatory property of rosiglitazone was absent in this controlled study [86]. These conflicting results may be due to the different pathogenetic pathways of the NAFLD models. Young ob/ob mice are known to have fatty livers without overt histological evidence of steatohepatitis, whereas MCD diet-fed rats, a model used in previous studies [80], exhibit characteristics of NASH with histological evidence of both significant hepatic steatosis and inflammation.
Much work has focused on pioglitazone in experimental liver fibrosis treatment. Kon and coworkers [87] first studied the therapeutic effects of pioglitazone on early phase liver fibrosis caused by CCl4 intoxication in rats, showing that pioglitazone inhibited the promoter activity of α1(I) procollagen gene and HSC proliferation and prevented hepatic inflammation and necrosis. Similar results were later obtained by others using the same rodent model [88]. Moreover, pioglitazone prevented the activation of Kupffer cells that may produce toxic mediators resulting in hepatocyte impairment and profibrogenic responses in the liver [87]. Consistent with this, pioglitazone suppressed the sensitization of Kupffer cell to lipopolysaccharide (LPS) and attenuated ethanol-induced liver injury in rats [89]. It was elucidated that pioglitazone prevention of LPS-induced liver injury was mediated by suppression of TNF-α secretion from Kupffer cells [90]. Another report also confirmed that the therapeutic effects of pioglitazone on hepatic inflammation and necrosis induced by ethanol and LPS were dependent on suppression of TNF-α [91]. Furthermore, Tomita and coworkers investigated the mechanisms underlying pioglitazone protection against alcoholic liver injury. They demonstrated that pioglitazone significantly attenuated steatosis and lipid peroxidation in rats through activating hepatocyte growth factor (HGF)/c-Met signaling, enhancing very low-density lipoprotein-dependent lipid retrieval, and suppressing triglyceride synthesis [92]. In choline-deficient l-amino acid-defined (CDAA) diet-induced NASH model, pioglitazone not only improved hepatic steatosis and prevented fibrogenesis in the rat liver, but also reduced the mRNA expression of TIMP-1 and TIMP-2, which were upregulated during fibrogenesis [93]. In the same model, pioglitazone at a clinical dose for type 2 diabetes ameliorated hepatic fibrosis via downregulation of procollagen, α-SMA and TGF-β1, as well as serum hyaluronic acid [94]. A recent mechanistic investigation showed that pioglitazone inhibited the suppressor of cytokine signaling-3 (SOCS-3), a key intermediator between impaired inflammatory and insulin pathways, in rats fed a high-cholesterol and fructose diet [95]. This may at least partially explain pioglitazone’s benefits in dietary hepatic injury.
Although the aforementioned data strongly favor the therapeutic potential of pioglitazone for hepatic fibrosis, not all studies have reached consistent conclusions. Leclercq and coworkers assessed pioglitazone efficacy for established liver fibrosis in three different rat models caused by CCl4 injection, CDAA diet, and BDL. Early administration of pioglitazone reduced hepatic fibrosis, type I collagen content, and profibrotic genes, as well as the number of activated HSCs, but did not significantly affect necroinflammation. When pioglitazone treatment was initiated in the later course of pathogenesis, no antifibrotic effects were observed. Similarly, pioglitazone reduced the severity of fibrosis induced by CDAA diet when introduced early, while delayed treatment remained ineffective. In contrast, pioglitazone failed to interrupt progression of fibrosis due to BDL, irrespective of the timing of its administration [96]. These results suggest the limited efficacy of pioglitazone for established fibrosis and imply that the therapeutic outcomes can be affected by the nature of hepatic injury, disease duration, and the time when medication is introduced. It is also presumed that PPARγ ligands cannot be sufficient to reverse the pathogenesis at the advanced stage when a larger number of HSCs have been activated. Later, this group further used BALB/c and C57BL6/J mice injected with CCl4 to perform in vivo and in vitro evaluation of pioglitazone’s antifibrotic potential. The data showed that pioglitazone neither prevented hepatic fibrogenesis in two different strains of mice, nor impacted collagen I gene expression and HSC activation, although serum adiponectin concentration was elevated [97]. Furthermore, pioglitazone did not inhibit the myofibroblastic transdifferentiation in cultured HSCs isolated form the liver tissues [97]. These are in sharp contrast to other reports on the antifibrotic properties of pioglitazone in various models of fibrosis in rats [87–95]. The interspecies variations and polymorphisms in the expression and tissue distribution of PPARγ may offer explanations for the therapeutic failure of pioglitazone in mice. Noteworthy, these paradoxical results somewhat complicate the interpretation of the antifibrotic profiles of thiazolidinediones.
Nonthiazolidinedione agonists
Several nonthiazolidinedione PPARγ agonists such as KR62776 and GW570 also exhibited therapeutic benefits in experimental fibrosis. Two independent studies with CCl4 toxication rat model demonstrated that these two agents prevented hepatic fibrogenic responses to injury via inhibiting HSC activation and abrogating collagen production [98, 99]. Of interest is that KR62776 selectively stimulated rat HSC apoptosis with little effects on hepatoma cells, suggesting that KR62776 is likely to possess specific inhibitory actions on HSCs without affecting the hepatic origin cells [98]. This selectivity could be explained by the finding that KR62776 adopted a mode distinct from that of thiazolidinediones for binding to the receptor domain [100]. Furthermore, PPARγ ligands from natural products also deserve attention. We have reported that the nontoxic natural compound curcumin was a potent PPARγ activator and was able to disrupt TGF-β and NF-κB signalings, leading to inhibited expression of CTGF and ECM in activated HSCs, and that curcumin also protected against liver injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation in rats [101–103]. These discoveries expand the structural type of PPARγ agonists with antifibrotic potential. This is truly illuminating given the urgent need to develop novel antifibrotics in the present-day where chronic liver diseases are overwhelmingly widespread. The preclinical animal studies of targeting PPARγ regulatory system for hepatic fibrosis are summarized in Table 1.
Table 1.
Preclinical animal studies of PPARγ-targeted therapy for hepatic fibrosis
| Agent | Animal model | Mode of action | References |
|---|---|---|---|
| Ectopic PPARγ | BDL (rat) | Reduce activated HSCs, inhibit collagen production | [65] |
| MCD diet (mouse) | Inhibit HSC activation, anti-inflammation, upregulate adiponectin, reduce ECM | [67, 68] | |
| CCl4 (rat) | Inhibit HSC activation, reduce collagen production | [69] | |
| Troglitazone | BDL (rat) | Attenuate HSC activation, reduce γ-GT level | [74] |
| Rosiglitazone | MCD diet (rat, mouse) | Improve transaminases, anti-inflammation, inhibit TGF-β and CTGF | [80, 82] |
| Schistosoma japonicum (mouse) | Reduce TGF-β and collagen production, anti-inflammation | [81] | |
| High-fat diet (mouse) | Anti-inflammation, enhance mitochondrial β-oxidation, inhibit oxidative stress | [83] | |
| Thioacetamide (rat) | Synergistic with RAR ligands, inhibit HSC proliferation, reduce TGF-β1, anti-inflammation | [84] | |
| Ethanol (mice) | Stimulate adiponectin signaling, activate sirtuin 1-AMP-activated kinase system | [85] | |
| ob/ob mice with NASH | Ineffective, increase oxidative stress and steatosis | [86] | |
| Pioglitazone | CCl4 (rat) | Reduce HSC proliferation, inhibit α1(I) procollagen gene promoter, inhibit Kupffer cell activation, anti-inflammation | [87, 88] |
| CCl4 (mouse) | Ineffective | [97] | |
| Ethanol (rat) | Inhibit Kupffer cell activation, suppress TNF-α, activate HGF/c-met signaling, anti-inflammation | [89–92] | |
| CDAA diet (rat) | Reduce matrix, inhibit TGF-β and collagen production, downregulate SOCS-3 | [93–95] | |
| BDL (rat) | Ineffective | [96] | |
| KR62776 | CCl4 (rat) | Inhibit HSC activation, stimulate HSC apoptosis | [98] |
| GW570 | BDL (rat), CCl4 (rat) | Inhibit collagen production | [99] |
| Curcumin | CCl4 (rat) | Inhibit oxidative stress, anti-inflammation, reduce HSC activation | [103] |
Targeting PPARγ for NASH and hepatic fibrosis: data from clinical trials
NASH is characterized by excessive fat accumulation in the liver coexisting with cell injury and inflammation. It is suggested that between 10 and 29 % of individuals with NASH develop fibrosis and even cirrhosis within 10 years [104] and 4 to 27 % of individuals with NASH-induced cirrhosis develop HCC [105]. The pathophysiology of NASH is generally defined by a “two-hit” theory. The first hit is considered as the increase of free fatty acids in hepatocytes, which inhibits β-oxidation and further amplifies the accumulation of fatty acids. The second hit involves all of the mechanisms that contribute to the development of inflammation and fibrosis [106]. Thus, NASH is closely linked to the metabolic syndrome such as abnormal lipid metabolism and IR. In general, drugs that reverse IR may be beneficial in NASH. Indeed, the past decade has witnessed intensive clinical evaluations of PPARγ agonists, mainly thiazolidinediones, for treating NASH and hepatic fibrosis.
Troglitazone therapy
In 2001, Caldwell and coworkers published the first open-label pilot study of troglitazone in NASH patients, which was completed before this drug was withdrawn. In this trial, 10 women with biopsy-proven NASH, some of whom complicated with obesity, type 2 diabetes or cirrhosis, were received troglitazone therapy for up to 6 months. Although 70 % of subjects had normalized ALT levels, only mild histological improvements were observed in follow-up biopsies [107]. This short-term trial indicates an absent correlation between liver enzyme levels and histology, and the limited efficacy of troglitazone for persistent steatohepatitis at the histological level. Later, this group surveyed the extended follow-up data of NASH patients, who were previously treated with troglitazone, to assess how lifestyle modifications affect clinical and histological parameters related to NASH recurrence. They reported that short-term troglitazone therapy led to histological improvements and discontinued progression of fibrosis in two subjects, who had sustained exercise programs and weight reduction [108]. These benefits cannot reflect long-term effects of troglitazone, but suggest that thiazolidinedione therapy combined with sustained lifestyle changes could be preventive for NASH relapse that is not inevitable after medication discontinuation.
Rosiglitazone therapy
Neuschwander-Tetri and coworkers performed the first open-label uncontrolled trial of rosiglitazone in 30 overweight patients with well-characterized NASH. Their interim results showed that rosiglitazone therapy for 24 weeks restored insulin sensitivity, reduced hepatic fat content, and improved biochemical evidence of hepatocyte injury, indicating that IR is indeed critical to NASH pathogenesis [109]. Their final results revealed that treatment for 48 weeks significantly improved insulin sensitivity and histological markers of NASH [110]. Importantly, although there was no significant improvement in overall fibrosis, some patients had improved zone 3 fibrosis, suggesting that hepatic fibrosis may occur in the absence of characteristic features of NASH. Whether longer treatment durations are associated with resolution of the zone 3 fibrosis or improvement in the overall fibrosis remains uncertain; and the nature of zone 3 fibrosis may be worthy of detailed examination in future trials. Of equal importance regarding this trial is the 6-month post-treatment follow-up evaluation, showing that liver enzyme levels had increased to near pretreatment levels [110]. This implies that permanent use of thiazolidinediones for insulin sensitization is probably essential in preventing progressive liver injury in patients with response to the initial treatment. Moreover, in a prospective, longitudinal controlled study enrolling 74 individuals with NASH, 48 weeks of rosiglitazone treatment reduced histological features of steatohepatitis including steatosis and ballooning in contrast to the diet and exercise group, but fibrosis was not significantly improved [111]. Examination of liver biopsies from NASH patients demonstrated that rosiglitazone effects were associated with increased crystalline inclusions in hepatocyte mitochondria [112]. However, whether these are adaptive or pathological remains unknown and further studies are warranted to assess hepatic mitochondrial function during thiazolidinedione therapy for NASH. With regard to drug safety, an open-label, inadequately controlled trial enrolling 68 type 2 diabetes patients with NASH showed that rosiglitazone treatment for 24 weeks led to normalized ALT levels in one-third of patients without serious adverse events [113]. More recently, a small-scale uncontrolled study recaptured the similar results in 27 NASH subjects for 6-month treatment, showing that rosiglitazone was well tolerated and significantly improved liver function without adversely affecting the lipid profiles [114].
Ratziu and coworkers [115] conducted a randomized placebo-controlled trial of rosiglitazone in 63 patients with NASH. Rosiglitazone improved steatosis and transaminase levels, but did not affect any other liver histological lesions including fibrosis and necroinflammation. Rosiglitazone was also proven safe in general, despite weight gain and an asymptomatic low-grade reduction in hemoglobin level occurred in some patients. This study had a limitation of low baseline NAS (NAFLD activity score, a histological feature scoring system with reasonable inter-rater reproducibility addressing the full spectrum of lesions of NAFLD [116]), leaving little room for improvements. Thus, this group enlarged the investigation, in which 53 patients who had been subjected to a 1-year randomized trial were enrolled in an open-label extension trial for 2 additional years [117]. Rosiglitazone showed substantial antisteatogenic effects only in the first year of treatment without additional benefits in longer therapy, despite the maintained effects on insulin sensitivity and transaminase levels. In addition, no significant improvements in fibrosis during 2 or 3 years of treatment were seen, which was consistent with their data in 2008 [115], rendering it improbable that the inefficacy observed after only 1 year of treatment could be due to the short treatment duration. An explanation could be that ameliorated steatosis and decreased aminotransferases are directly associated with improved insulin sensitivity, whereas fibrosis may not be reduced significantly by simply correcting IR.
Given the modest effects of rosiglitazone on NASH resolution and fibrosis regression, studies aimed at combination therapies that synergistically improve IR seem to be laudable. Recently, two open-label, randomized trials have assessed the efficacy of rosiglitazone and metformin in combination versus rosiglitazone or metformin alone. Rosiglitazone therapy seemed to be more effective in metabolic control and histological improvement in 64 NAFLD patients with impaired glucose metabolism and NAFLD activity score of at least 5 in liver biopsy [118]. Although combination therapy effectively decreased the NAFLD score in follow-up biopsy, fibrosis did not change significantly after the treatment [118]. Another trial enrolling 137 subjects (108 completed the trial) with biopsy-proven NASH showed that 48 weeks of combination of rosiglitazone and metformin conferred no greater benefit than rosiglitazone alone in all histologic parameters including steatosis, hepatocellular inflammation, and fibrosis, leading to the conclusion that adjuvant therapies were ineffective [119].
Pioglitazone therapy
Shadid and colleagues [120] first examined pioglitazone’s effects on liver fatty acid metabolism in a trial enrolling 20 upper-body obese subjects, five of whom with NASH, showing that pioglitazone improved liver function in NASH. Subsequently, a pilot study involving 18 non-diabetic patients with NASH demonstrated that pioglitazone therapy for 48 weeks led to improvement in biochemical and histological features of NASH, as well as resolution of fibrosis [121]. However, this study was not well controlled and had a narrow margin of statistical significance for fibrosis improvement. Belfort and coworkers performed the first placebo-controlled trial using diet plus pioglitazone in 55 patients with NASH for 6 months. Pioglitazone led to clear metabolic and histological improvements, but no significant reduction in fibrosis was observed [122]. A similar placebo-controlled trial enrolling 74 non-diabetic patients with histological NASH for 1 year demonstrated that pioglitazone resulted in improvements in metabolic and histological parameters, most notably in liver injury and fibrosis indicated by biopsy [123]. This trial offered clear evidence of pioglitazone benefits for fibrosis, which was contrary to the Belfort’s data [122]. Furthermore, a pilot study, in which 21 NASH patients received 48-week pioglitazone therapy with significant improvements and 13 of them were followed for repeated liver biopsy for an additional 48 weeks showed that pioglitazone discontinuation resulted in elevated serum ALT levels and marked worsening of parenchymal inflammation and steatosis, but no change in fibrosis, suggesting that long-term therapy with pioglitazone may be necessary to maintain improvements in NASH patients [124]. Furthermore, several lines of clinical evidence indicated that improvements in liver histology during pioglitazone treatment for NASH might correlate with increased secretion of adiponectin by peripheral adipocytes [125], and with restoration of adipose tissue insulin sensitivity, as well as increased oxidation of free fatty acids in the liver [126].
There are also several clinical reports about pioglitazone versus vitamin E for NASH therapy. It is proposed that the antioxidant vitamin E may have benefits for NASH, because oxidative stress contributes critically to the pathogenesis of NASH and hepatic fibrosis [127]. A small-sized pilot study showed that pioglitazone combined with vitamin E led to greater improvements in NASH histology, but vitamin E alone did not significantly reduce histological parameters other than steatosis [128]. This group later planned a randomized, double-blind, placebo-controlled trial for 96 weeks to evaluate the therapeutic efficacy of pioglitazone and vitamin E in 247 non-diabetic patients with NASH [129]. The results demonstrated that pioglitazone had no benefits over placebo for the primary outcome, but led to significant improvements in some histological features of NASH, such as steatosis and lobular inflammation. However, neither pioglitazone nor vitamin E ameliorated fibrosis and portal inflammation [130]. A conclusion still cannot be drawn about the relative efficacy of pioglitazone and vitamin E in NASH treatment. Larger extended trials are warranted to assess the long-term and sustained effects of pioglitazone on the biochemical and histological features of NASH and hepatic fibrosis.
Farglitazar therapy
More recently, McHutchison and coworkers [131] evaluated the antifibrotic potential of farglitazar, a nonthiazolidinedione insulin-sensitizing agent approximately 100-fold more potent than rosiglitazone in its ability to both bind to and activate PPARγ in a large-scale randomized placebo-controlled trial in 265 patients with HCV infection and biopsy-proven fibrosis of Ishak stages 2–4. Treatment with farglitazar for 52 weeks failed to improve immunohistochemical markers of fibrogenesis, including well-accepted biological markers of HSC activation and collagen synthesis. There was also no significant improvement in other more standard histological assessments such as ranked assessment of the paired biopsy specimens, and Ishak fibrosis and necroinflammation scores, although minor decreases in steatosis and serum ALT values of uncertain clinical significance were observed. Of note, farglitazar failure in clinically reducing fibrosis is in contrast to the preclinical evidence that farglitazar inhibited activation of isolated HSCs and decreased collagen content in a rodent model of fibrosis [131]. It is possible that animal models of hepatic fibrosis that mimic injury over weeks to months are unable to reflect the duration of injury and fibrogenesis in HCV infection that results in maturation of ECM and scar tissue over many years [132]. The cross-linked ECM components in established fibrosis may be resistant to resorption with the antifibrotic agents, which have no effects on the underlying cause of injury (e.g., HCV infection) and are given over a relatively short period of time.
Outcome interpretation and the safety issues
To date, the only treatment for NASH is moderate weight loss and lifestyle modification. However, the majority of patients fail to execute these lifestyle measures persistently, and thus pharmacological treatment should be considered [106]. Current clinical trials testing PPARγ agonists in NASH are mainly based on the logical assumption that improving IR will lead to an improvement in liver disease. The outcomes generally demonstrate that reduction in aminotransferases and loss of steatosis are the two most reproducible hepatic effects of PPARγ agonists, but there are still a large proportion of subjects who are non-responders or only partial responders. Noteworthy, no studies have convincingly shown significant histological improvement for NASH fibrosis, although two uncontrolled trials [110, 121] and one controlled trial [123] reported improved fibrosis. Several meta-analyses also suggest the modest efficacy of thiazolidinediones for fibrosis [133–135], except one indicating that pioglitazone significantly improves fibrosis in NASH [136]. There is awareness that small sample size, short treatment duration, and heterogeneity of trials may influence the evaluation of drug efficacy for fibrosis. In addition to these, some other issues should also be taken into consideration. First, thiazolidinediones may act directly on adipose tissue, with secondary effects on the liver, because they improved glucose homeostasis and triglyceride levels in the absence of liver PPARγ [137], suggesting that hepatic PPARγ expression contributes relatively little to the beneficial effects of thiazolidinediones. It can thus be postulated that thiazolidinediones improve NASH as a result of its primary insulin-sensitizing effects on adipose tissue, which reduces the flux of free fatty acids from adipose tissue to the liver; whereas PPARγ activation may induce steatogenic responses in the liver [52, 53, 86] and compromise thiazolidinediones’ benefits for liver histology. Therefore, correcting IR is not enough to reverse liver injury in NASH, and different pathways, such as inflammatory and fibrotic cascades, should be targeted in order to achieve higher antifibrotic potency. Second, although PPARγ activation inhibits HSC activation and ECM production, and has therapeutic potential for liver fibrosis, it should be noted that PPARγ expression is dramatically depleted during excessive HSC activation [34–36, 38]. Under this condition, ligands probably cannot fully activate PPARγ signaling pathways, due to too less amount of receptor (PPARγ) available for binding, which is supported by the finding that rosiglitazone did not affect NASH in PPARγ-deficient mice fed the MCD diet [66]. This may also account for some cases where thiazolidinediones are ineffective in rodents with established fibrosis [96, 97]. It will be tempting to test whether PPARγ ligands combined with PPARγ-targeted gene therapy can coordinately yield more potent antifibrotic efficacy in animals and humans. The third challenging issue is that the lack of solid evidence-based justification of therapeutic endpoints may produce negative histological results, because the interplay between IR and hepatic effects remains to be defined. Some limitations in current fibrosis biomarkers, such as lack of sensitivity during the initial stages of fibrosis and lack of etiology-specificity, and the sampling variability of liver biopsy may affect examination of the outcomes. More sensitive biomarkers for fibrosis assessment are critically needed in evaluation of drug efficacy, particularly in situations where control of the liver injury is of principal importance, and the optimal biomarkers should be able to predict both liver histology and clinically significant endpoints [138]. It is anticipated that proteomic and metabonomic approaches may afford the possibility to characterize sensitive and specific biomarkers or biomarker panels for assessment of hepatic fibrosis [139, 140].
Weight loss can upregulate PPARγ expression and improve insulin sensitivity [141, 142], which may reinforce the idea that thiazolidinediones combined with lifestyle modification have synergistic benefits in NASH and fibrosis [108, 111]. However, these agents are approved for use only in diabetes patients; any extension of their indications should carefully assess the adverse effects and safety issues in non-diabetics, especially given that many studies [108–110, 124] argue for prolonged therapy in NASH. Apart from weight gain and fluid retention, cardiovascular effects, such as myocardial infarction, are emerging in thiazolidinedione therapy [143]. Recently, rosiglitazone has been withdrawn from the European market [144] and limited by the U.S. Food and Drug Administration [145], because of cardiovascular risk. It is still unclear whether thiazolidinedione-associated weight gain and cardiovascular events would ultimately limit the long-term benefits in patients with NASH and related liver diseases [133, 134]. Furthermore, a large proportion of patients with progressing liver diseases have high liver enzyme levels, whereas thiazolidinedione treatments are usually recommended to be avoided in these patients due to potential hepatotoxicity, especially given that there have been clinical reports on the rosiglitazone- and pioglitazone-induced hepatotoxicity [146]. Recently, thiazolidinediones have also been linked to cancer, since pioglitazone long-term use was associated with an increased risk of bladder cancer [147, 148]. Moreover, ligand-activated PPARγ mutant was also likely to enhance Angptl4-mediated angiogenesis and promote ErbB2 oncogene-induced tumorigenesis, indicating the necessity of epidemiologic analysis in patients treated with PPARγ agonists for possible effects on tumor development [149]. These findings imply a potential risk for patients with HCC or complicated with other cancers when receiving thiazolidinedione therapy. In summary, it is clear that available data represent clues for subsequent trials and highlight the methodological challenges of trial design in this disease. Whether thiazolidinedione efficacy and safety can be successfully demonstrated by better selection of NASH or fibrosis patients in non-diabetic population needs to be further determined before clinical recommendations.
Prospects for novel PPARγ modulators for hepatic fibrosis
The 10-year discoveries on PPARγ in hepatic fibrosis from bench to bedside not only portray a novel paradigm of hepatic fibrosis but also illuminate a framework for developing new antifibrotic PPARγ modulators. Some specific issues that remain unanswered should be prioritized in future studies.
Novel agents targeting PPARγ for antifibrogenesis should have selectivity and remove some of the side-effects associated with thiazolidinediones. It was found that activation of PPARγ2 not only induced lipogenesis in hepatocytes [150], which further led to HSC proliferation and apoptosis resistance [151], but also inhibited hepatocyte growth by disrupting cyclin D1 transcription [152]. These findings indicate that future PPARγ ligands for hepatic fibrosis should be HSC-specific with high target selectivity toward PPARγ1 isoform so as to maximize the therapeutic benefits of PPARγ activation. More intriguingly, several non-agonist PPARγ ligands have recently been developed exhibiting a separate biochemical activity, i.e., blocking cyclin-dependent kinase 5-mediated phosphorylation of PPARγ. Their unique mode of binding to PPARγ made them have no serious side-effects of thiazolidinediones such as fluid retention and weight gain [153, 154]. These new compounds clearly suggest that the development of safer PPARγ ligands for hepatic fibrosis is feasible.
Regulatory mechanisms of HSC by PPARγ are becoming clear, but their roles in wound response of the liver as a whole are still elusive and complex. For example, it is unclear whether reversal of HSC activation by PPARγ impairs liver regenerative responses. It has been increasingly understood that HSC activation also has pro-regenerative purposes, because the soluble factors (e.g., HGF) secreted by activated HSCs may be essential for liver regeneration [155]. Hence the best therapeutic strategy for hepatic fibrosis should focus on suppressing the former while promoting the latter. It is conceivable that inhibition of HSC activation combined with protection of hepatocytes may represent an optimal approach. Pharmacological potential for crosstalk of ligands between PPARα and PPARγ may exactly afford this possibility. Activation of PPARα, which is mostly expressed in hepatocytes in the liver, stimulates fatty acid transport and β-oxidation to prevent triglyceride accumulation, and controls inflammatory responses [156]. A number of data showed that PPARα agonists prevented liver steatosis and inflammation and reversed fibrosis in animals [157–160] and had histological benefits in NASH patients [161, 162]. Based on these promising effects of PPAR ligands, it can be proposed that dual agonists for PPARα/γ could be more perspective for therapeutic development against NASH and fibrosis owing to their optimal effects in the liver. Currently, such dual agonists have been successfully developed for pharmacological evaluation [163–165]. However, in vivo investigations testing their efficacy for the treatment of hepatic fibrosis have not been reported so far. Such studies may be highly illuminating and should be planned.
Acknowledgments
We thank the financial support from the Open Project Program of National First-Class Key Discipline for Traditional Chinese Medicine of Nanjing University of Chinese Medicine (2011ZYX4-008), Doctoral Discipline Foundation of Ministry of Education of China (20103237110010), National Natural Science Foundation of China (30873424), Jiangsu Natural Science Foundation (BK2008456), Project for Supporting Jiangsu Provincial Talents in Six Fields (2009112), Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (ysxk-2010), and “Eleven-Five” National Science and Technology Supporting Program (2008BAI51B02). We are grateful to Dr. Shile Huang, Louisiana State University Health Sciences Center, USA, for his critical reading and reversion of the manuscript.
References
- 1.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 2.Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53(5):976–980. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12(4):733–746. doi: 10.1016/j.cld.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Anty R, Lemoine M. Liver fibrogenesis and metabolic factors. Clin Res Hepatol Gastroenterol. 2011;35(Suppl 1):S10–S20. doi: 10.1016/S2210-7401(11)70003-1. [DOI] [PubMed] [Google Scholar]
- 5.Romero-Gomez M, Del Mar Viloria M, Andrade RJ, Salmeron J, Diago M, Fernandez-Rodriguez CM, Corpas R, Cruz M, Grande L, Vazquez L, Munoz-De-Rueda P, Lopez-Serrano P, Gila A, Gutierrez ML, Perez C, Ruiz-Extremera A, Suarez E, Castillo J. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128(3):636–641. doi: 10.1053/j.gastro.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 6.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression. Gastroenterology. 2003;125(6):1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 7.Bugianesi E, Marchesini G, Gentilcore E, Cua IH, Vanni E, Rizzetto M, George J. Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: role of insulin resistance and hepatic steatosis. Hepatology. 2006;44(6):1648–1655. doi: 10.1002/hep.21429. [DOI] [PubMed] [Google Scholar]
- 8.De Minicis S, Svegliati-Baroni G. Fibrogenesis in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5(2):179–187. doi: 10.1586/egh.11.28. [DOI] [PubMed] [Google Scholar]
- 9.Mann J, Mann DA. Transcriptional regulation of hepatic stellate cells. Adv Drug Deliv Rev. 2009;61(7–8):497–512. doi: 10.1016/j.addr.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Wagner M, Zollner G, Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53(3):1023–1034. doi: 10.1002/hep.24148. [DOI] [PubMed] [Google Scholar]
- 11.Sugii S, Evans RM. Epigenetic codes of PPARgamma in metabolic disease. FEBS Lett. 2011;585(13):2121–2128. doi: 10.1016/j.febslet.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10(4):355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 13.Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, Balint BL, Nagy L, Yamamoto K, Schwabe JW. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol. 2008;15(9):924–931. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual G, Glass CK. Nuclear receptors versus inflammation: mechanisms of transrepression. Trends Endocrinol Metab. 2006;17(8):321–327. doi: 10.1016/j.tem.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Bonfield TL, Thomassen MJ, Farver CF, Abraham S, Koloze MT, Zhang X, Mosser DM, Culver DA. Peroxisome proliferator-activated receptor-gamma regulates the expression of alveolar macrophage macrophage colony-stimulating factor. J Immunol. 2008;181(1):235–242. doi: 10.4049/jimmunol.181.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassaganya-Riera J, Song R, Roberts PC, Hontecillas R. PPAR-gamma activation as an anti-inflammatory therapy for respiratory virus infections. Viral Immunol. 2010;23(4):343–352. doi: 10.1089/vim.2010.0016. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki S, Sasaki S, Morita H, Oki Y, Turiya D, Ito T, Misawa H, Ishizuka K, Nakamura H. The role of the amino-terminal domain in the interaction of unliganded peroxisome proliferator-activated receptor gamma-2 with nuclear receptor co-repressor. J Mol Endocrinol. 2010;45(3):133–145. doi: 10.1677/JME-10-0007. [DOI] [PubMed] [Google Scholar]
- 18.Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277(6):4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 19.Hosooka T, Noguchi T, Kotani K, Nakamura T, Sakaue H, Inoue H, Ogawa W, Tobimatsu K, Takazawa K, Sakai M, Matsuki Y, Hiramatsu R, Yasuda T, Lazar MA, Yamanashi Y, Kasuga M. Dok1 mediates high-fat diet-induced adipocyte hypertrophy and obesity through modulation of PPAR-gamma phosphorylation. Nat Med. 2008;14(2):188–193. doi: 10.1038/nm1706. [DOI] [PubMed] [Google Scholar]
- 20.Doshi LS, Brahma MK, Bahirat UA, Dixit AV, Nemmani KV. Discovery and development of selective PPAR gamma modulators as safe and effective antidiabetic agents. Expert Opin Investig Drugs. 2010;19(4):489–512. doi: 10.1517/13543781003640169. [DOI] [PubMed] [Google Scholar]
- 21.Abbott BD. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reprod Toxicol. 2009;27(3–4):246–257. doi: 10.1016/j.reprotox.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Semple RK, Chatterjee VK, O’Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116(3):581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao GH, Niu XL, Gao DF, Wei J, Wang NP. Agonists at PPAR-gamma suppress angiotensin II-induced production of plasminogen activator inhibitor-1 and extracellular matrix in rat cardiac fibroblasts. Br J Pharmacol. 2008;153(7):1409–1419. doi: 10.1038/bjp.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Y, Sun C, Sun Y, Tan H, Wu Y, Cui B, Wu Z. PPAR gamma protects cardiomyocytes against oxidative stress and apoptosis via Bcl-2 upregulation. Vascul Pharmacol. 2009;51(2–3):169–174. doi: 10.1016/j.vph.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Alleva DG, Johnson EB, Lio FM, Boehme SA, Conlon PJ, Crowe PD. Regulation of murine macrophage proinflammatory and anti-inflammatory cytokines by ligands for peroxisome proliferator-activated receptor-gamma: counter-regulatory activity by IFN-gamma. J Leukoc Biol. 2002;71(4):677–685. [PubMed] [Google Scholar]
- 26.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6(2):137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Bouhlel MA, Staels B, Chinetti-Gbaguidi G. Peroxisome proliferator-activated receptors—from active regulators of macrophage biology to pharmacological targets in the treatment of cardiovascular disease. J Intern Med. 2008;263(1):28–42. doi: 10.1111/j.1365-2796.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- 28.Milam JE, Keshamouni VG, Phan SH, Hu B, Gangireddy SR, Hogaboam CM, Standiford TJ, Thannickal VJ, Reddy RC. PPAR-gamma agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L891–L901. doi: 10.1152/ajplung.00333.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dionne S, Levy E, Levesque D, Seidman EG. PPARgamma ligand 15-deoxy-delta 12,14-prostaglandin J2 sensitizes human colon carcinoma cells to TWEAK-induced apoptosis. Anticancer Res. 2010;30(1):157–166. [PubMed] [Google Scholar]
- 30.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126(4):801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 31.Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol. 2006;209(3):967–976. doi: 10.1002/jcp.20804. [DOI] [PubMed] [Google Scholar]
- 32.Wasmuth HE, Tacke F, Trautwein C. Chemokines in liver inflammation and fibrosis. Semin Liver Dis. 2010;30(3):215–225. doi: 10.1055/s-0030-1255351. [DOI] [PubMed] [Google Scholar]
- 33.Higashi N, Sato M, Kojima N, Irie T, Kawamura K, Mabuchi A, Senoo H. Vitamin A storage in hepatic stellate cells in the regenerating rat liver: with special reference to zonal heterogeneity. Anat Rec A Discov Mol Cell Evol Biol. 2005;286(2):899–907. doi: 10.1002/ar.a.20230. [DOI] [PubMed] [Google Scholar]
- 34.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyahara T, Schrum L, Rippe R, Xiong S, Yee HF, Jr, Motomura K, Anania FA, Willson TM, Tsukamoto H. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275(46):35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 36.Sun K, Wang Q, Huang XH. PPAR gamma inhibits growth of rat hepatic stellate cells and TGF beta-induced connective tissue growth factor expression. Acta Pharmacol Sin. 2006;27(6):715–723. doi: 10.1111/j.1745-7254.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 37.Higashi Y, Holder K, Delafontaine P. Thiazolidinediones up-regulate insulin-like growth factor-1 receptor via a peroxisome proliferator-activated receptor gamma-independent pathway. J Biol Chem. 2010;285(47):36361–36368. doi: 10.1074/jbc.M110.137661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hazra S, Xiong S, Wang J, Rippe RA, Krishna V, Chatterjee K, Tsukamoto H. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J Biol Chem. 2004;279(12):11392–11401. doi: 10.1074/jbc.M310284200. [DOI] [PubMed] [Google Scholar]
- 39.Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, Ridolfi F, Trozzi L, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122(7):1924–1940. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- 40.Yavrom S, Chen L, Xiong S, Wang J, Rippe RA, Tsukamoto H. Peroxisome proliferator-activated receptor gamma suppresses proximal alpha1(I) collagen promoter via inhibition of p300-facilitated NF-I binding to DNA in hepatic stellate cells. J Biol Chem. 2005;280(49):40650–40659. doi: 10.1074/jbc.M510094200. [DOI] [PubMed] [Google Scholar]
- 41.Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, Morelli A, Pruzanski M, Pellicciari R. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor gamma contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J Pharmacol Exp Ther. 2005;315(1):58–68. doi: 10.1124/jpet.105.085597. [DOI] [PubMed] [Google Scholar]
- 42.Renga B, Mencarelli A, Migliorati M, Cipriani S, D’Amore C, Distrutti E, Fiorucci S. SHP-dependent and -independent induction of peroxisome proliferator-activated receptor-gamma by the bile acid sensor farnesoid X receptor counter-regulates the pro-inflammatory phenotype of liver myofibroblasts. Inflamm Res. 2011;60(6):577–587. doi: 10.1007/s00011-010-0306-1. [DOI] [PubMed] [Google Scholar]
- 43.Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127(5):1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pruzanski M, Morelli A, Pellicciari R. A farnesoid x receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther. 2005;314(2):584–595. doi: 10.1124/jpet.105.084905. [DOI] [PubMed] [Google Scholar]
- 45.Fickert P, Fuchsbichler A, Moustafa T, Wagner M, Zollner G, Halilbasic E, Stoger U, Arrese M, Pizarro M, Solis N, Carrasco G, Caligiuri A, Sombetzki M, Reisinger E, Tsybrovskyy O, Zatloukal K, Denk H, Jaeschke H, Pinzani M, Trauner M. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am J Pathol. 2009;175(6):2392–2405. doi: 10.2353/ajpath.2009.090114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauvant P, Cansell M, Atgie C. Vitamin A and lipid metabolism: relationship between hepatic stellate cells (HSCs) and adipocytes. J Physiol Biochem. 2011;67(3):487–496. doi: 10.1007/s13105-011-0101-7. [DOI] [PubMed] [Google Scholar]
- 47.Tsukamoto H, She H, Hazra S, Cheng J, Miyahara T. Anti-adipogenic regulation underlies hepatic stellate cell transdifferentiation. J Gastroenterol Hepatol. 2006;21(Suppl 3):S102–S105. doi: 10.1111/j.1440-1746.2006.04573.x. [DOI] [PubMed] [Google Scholar]
- 48.De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132(5):1937–1946. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 49.She H, Xiong S, Hazra S, Tsukamoto H. Adipogenic transcriptional regulation of hepatic stellate cells. J Biol Chem. 2005;280(6):4959–4967. doi: 10.1074/jbc.M410078200. [DOI] [PubMed] [Google Scholar]
- 50.Guimaraes EL, Franceschi MF, Andrade CM, Guaragna RM, Borojevic R, Margis R, Bernard EA, Guma FC. Hepatic stellate cell line modulates lipogenic transcription factors. Liver Int. 2007;27(9):1255–1264. doi: 10.1111/j.1478-3231.2007.01578.x. [DOI] [PubMed] [Google Scholar]
- 51.Kim N, Choi S, Lim C, Lee H, Oh J. Albumin mediates PPAR-gamma or C/EBP-alpha-induced phenotypic changes in pancreatic stellate cells. Biochem Biophys Res Commun. 2010;391(1):640–644. doi: 10.1016/j.bbrc.2009.11.112. [DOI] [PubMed] [Google Scholar]
- 52.Bedoucha M, Atzpodien E, Boelsterli UA. Diabetic KKAy mice exhibit increased hepatic PPARgamma1 gene expression and develop hepatic steatosis upon chronic treatment with antidiabetic thiazolidinediones. J Hepatol. 2001;35(1):17–23. doi: 10.1016/s0168-8278(01)00066-6. [DOI] [PubMed] [Google Scholar]
- 53.Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem. 2003;278(1):498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 54.Tsukamoto H. Fat paradox in liver disease. Keio J Med. 2005;54(4):190–192. doi: 10.2302/kjm.54.190. [DOI] [PubMed] [Google Scholar]
- 55.Kluwe J, Wongsiriroj N, Troeger JS, Gwak GY, Dapito DH, Pradere JP, Jiang H, Siddiqi M, Piantedosi R, O’Byrne SM, Blaner WS, Schwabe RF. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut. 2011;60(9):1260–1268. doi: 10.1136/gut.2010.209551. [DOI] [PubMed] [Google Scholar]
- 56.Mann J, Oakley F, Akiboye F, Elsharkawy A, Thorne AW, Mann DA. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. 2007;14(2):275–285. doi: 10.1038/sj.cdd.4401979. [DOI] [PubMed] [Google Scholar]
- 57.Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, Mann DA. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010;138(2):705–714. doi: 10.1053/j.gastro.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu NL, Wang J, Tsukamoto H. The Necdin-Wnt pathway causes epigenetic peroxisome proliferator-activated receptor gamma repression in hepatic stellate cells. J Biol Chem. 2010;285(40):30463–30471. doi: 10.1074/jbc.M110.156703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu NL, Asahina K, Wang J, Ueno A, Lazaro R, Miyaoka Y, Miyajima A, Tsukamoto H. Hepatic stellate cell-derived delta-like homolog 1 (DLK1) protein in liver regeneration. J Biol Chem. 2012;287(13):10355–10367. doi: 10.1074/jbc.M111.312751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smas CM, Kachinskas D, Liu CM, Xie X, Dircks LK, Sul HS. Transcriptional control of the pref-1 gene in 3T3-L1 adipocyte differentiation. Sequence requirement for differentiation-dependent suppression. J Biol Chem. 1998;273(48):31751–31758. doi: 10.1074/jbc.273.48.31751. [DOI] [PubMed] [Google Scholar]
- 61.Fritz D, Stefanovic B. RNA-binding protein RBMS3 is expressed in activated hepatic stellate cells and liver fibrosis and increases expression of transcription factor Prx1. J Mol Biol. 2007;371(3):585–595. doi: 10.1016/j.jmb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venugopal SK, Jiang J, Kim TH, Li Y, Wang SS, Torok NJ, Wu J, Zern MA. Liver fibrosis causes downregulation of miRNA-150 and miRNA-194 in hepatic stellate cells, and their overexpression causes decreased stellate cell activation. Am J Physiol Gastrointest Liver Physiol. 2010;298(1):G101–G106. doi: 10.1152/ajpgi.00220.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindquist JN, Parsons CJ, Stefanovic B, Brenner DA. Regulation of alpha1(I) collagen messenger RNA decay by interactions with alphaCP at the 3′-untranslated region. J Biol Chem. 2004;279(22):23822–23829. doi: 10.1074/jbc.M314060200. [DOI] [PubMed] [Google Scholar]
- 64.Yang MD, Chiang YM, Higashiyama R, Asahina K, Mann DA, Mann J, Wang CC, Tsukamoto H. Rosmarinic acid and baicalin epigenetically derepress peroxisomal proliferator-activated receptor gamma in hepatic stellate cells for their antifibrotic effect. Hepatology. 2012;55(4):1271–1281. doi: 10.1002/hep.24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L, Chan CC, Kwon OS, Liu S, McGhee J, Stimpson SA, Chen LZ, Harrington WW, Symonds WT, Rockey DC. Regulation of peroxisome proliferator-activated receptor-gamma in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G902–G911. doi: 10.1152/ajpgi.00124.2006. [DOI] [PubMed] [Google Scholar]
- 66.Wu CW, Chu ES, Lam CN, Cheng AS, Lee CW, Wong VW, Sung JJ, Yu J. PPARgamma is essential for protection against nonalcoholic steatohepatitis. Gene Ther. 2010;17(6):790–798. doi: 10.1038/gt.2010.41. [DOI] [PubMed] [Google Scholar]
- 67.Yu J, Zhang S, Chu ES, Go MY, Lau RH, Zhao J, Wu CW, Tong L, Poon TC, Sung JJ. Peroxisome proliferator-activated receptors gamma reverses hepatic nutritional fibrosis in mice and suppresses activation of hepatic stellate cells in vitro. Int J Biochem Cell Biol. 2010;42(6):948–957. doi: 10.1016/j.biocel.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Nan YM, Han F, Kong LB, Zhao SX, Wang RQ, Wu WJ, Yu J. Adenovirus-mediated peroxisome proliferator activated receptor gamma overexpression prevents nutritional fibrotic steatohepatitis in mice. Scand J Gastroenterol. 2011;46(3):358–369. doi: 10.3109/00365521.2010.525717. [DOI] [PubMed] [Google Scholar]
- 69.Wang Z, Xu JP, Zheng YC, Chen W, Sun YW, Wu ZY, Luo M. Peroxisome proliferator-activated receptor gamma inhibits hepatic fibrosis in rats. Hepatobiliary Pancreat Dis Int. 2011;10(1):64–71. doi: 10.1016/s1499-3872(11)60009-x. [DOI] [PubMed] [Google Scholar]
- 70.Li F, Wang JY. Targeted delivery of drugs for liver fibrosis. Expert Opin Drug Deliv. 2009;6(5):531–541. doi: 10.1517/17425240902936834. [DOI] [PubMed] [Google Scholar]
- 71.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 72.Faich GA, Moseley RH. Troglitazone (Rezulin) and hepatic injury. Pharmacoepidemiol Drug Saf. 2001;10(6):537–547. doi: 10.1002/pds.652. [DOI] [PubMed] [Google Scholar]
- 73.Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M, Gentilini P. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119(2):466–478. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- 74.Marra F, DeFranco R, Robino G, Novo E, Efsen E, Pastacaldi S, Zamara E, Vercelli A, Lottini B, Spirli C, Strazzabosco M, Pinzani M, Parola M. Thiazolidinedione treatment inhibits bile duct proliferation and fibrosis in a rat model of chronic cholestasis. World J Gastroenterol. 2005;11(32):4931–4938. doi: 10.3748/wjg.v11.i32.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282(32):23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 76.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, Syn WK, Baroni GS, Benedetti A, Schuppan D, Diehl AM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118(10):3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, Brenner DA. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51(3):1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139(3):987–998. doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53(5):1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tahan V, Eren F, Avsar E, Yavuz D, Yuksel M, Emekli E, Imeryuz N, Celikel C, Uzun H, Haklar G, Tozun N. Rosiglitazone attenuates liver inflammation in a rat model of nonalcoholic steatohepatitis. Dig Dis Sci. 2007;52(12):3465–3472. doi: 10.1007/s10620-007-9756-x. [DOI] [PubMed] [Google Scholar]
- 81.Chen H, He YW, Liu WQ, Zhang JH. Rosiglitazone prevents murine hepatic fibrosis induced by Schistosoma japonicum . World J Gastroenterol. 2008;14(18):2905–2911. doi: 10.3748/wjg.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nan YM, Fu N, Wu WJ, Liang BL, Wang RQ, Zhao SX, Zhao JM, Yu J. Rosiglitazone prevents nutritional fibrosis and steatohepatitis in mice. Scand J Gastroenterol. 2009;44(3):358–365. doi: 10.1080/00365520802530861. [DOI] [PubMed] [Google Scholar]
- 83.Gupte AA, Liu JZ, Ren Y, Minze LJ, Wiles JR, Collins AR, Lyon CJ, Pratico D, Finegold MJ, Wong ST, Webb P, Baxter JD, Moore DD, Hsueh WA. Rosiglitazone attenuates age- and diet-associated nonalcoholic steatohepatitis in male low-density lipoprotein receptor knockout mice. Hepatology. 2010;52(6):2001–2011. doi: 10.1002/hep.23941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bruck R, Weiss S, Aeed H, Pines M, Halpern Z, Zvibel I. Additive inhibitory effect of experimentally induced hepatic cirrhosis by agonists of peroxisome proliferator activator receptor gamma and retinoic acid receptor. Dig Dis Sci. 2009;54(2):292–299. doi: 10.1007/s10620-008-0336-5. [DOI] [PubMed] [Google Scholar]
- 85.Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G364–G374. doi: 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia-Ruiz I, Rodriguez-Juan C, Diaz-Sanjuan T, Martinez MA, Munoz-Yague T, Solis-Herruzo JA. Effects of rosiglitazone on the liver histology and mitochondrial function in ob/ob mice. Hepatology. 2007;46(2):414–423. doi: 10.1002/hep.21687. [DOI] [PubMed] [Google Scholar]
- 87.Kon K, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Kitamura T, Takei Y, Sato N. Pioglitazone prevents early-phase hepatic fibrogenesis caused by carbon tetrachloride. Biochem Biophys Res Commun. 2002;291(1):55–61. doi: 10.1006/bbrc.2002.6385. [DOI] [PubMed] [Google Scholar]
- 88.Yuan GJ, Zhang ML, Gong ZJ. Effects of PPARg agonist pioglitazone on rat hepatic fibrosis. World J Gastroenterol. 2004;10(7):1047–1051. doi: 10.3748/wjg.v10.i7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Enomoto N, Takei Y, Hirose M, Konno A, Shibuya T, Matsuyama S, Suzuki S, Kitamura KI, Sato N. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-gamma, pioglitazone. J Pharmacol Exp Ther. 2003;306(3):846–854. doi: 10.1124/jpet.102.047217. [DOI] [PubMed] [Google Scholar]
- 90.Enomoto N, Takei Y, Yamashima S, Ikejima K, Kitamura T, Sato N. Protective effect of pioglitazone against endotoxin-induced liver injury through prevention of Kupffer cell sensitization. Alcohol Clin Exp Res. 2005;29(12 Suppl):216S–219S. doi: 10.1097/01.alc.0000192394.26573.10. [DOI] [PubMed] [Google Scholar]
- 91.Ohata M, Suzuki H, Sakamoto K, Hashimoto K, Nakajima H, Yamauchi M, Hokkyo K, Yamada H, Toda G. Pioglitazone prevents acute liver injury induced by ethanol and lipopolysaccharide through the suppression of tumor necrosis factor-alpha. Alcohol Clin Exp Res. 2004;28(8 Suppl Proceedings):139S–144S. doi: 10.1097/01.alc.0000134412.38510.f7. [DOI] [PubMed] [Google Scholar]
- 92.Tomita K, Azuma T, Kitamura N, Nishida J, Tamiya G, Oka A, Inokuchi S, Nishimura T, Suematsu M, Ishii H. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology. 2004;126(3):873–885. doi: 10.1053/j.gastro.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 93.Kawaguchi K, Sakaida I, Tsuchiya M, Omori K, Takami T, Okita K. Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient l-amino acid-defined diet. Biochem Biophys Res Commun. 2004;315(1):187–195. doi: 10.1016/j.bbrc.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 94.Uto H, Nakanishi C, Ido A, Hasuike S, Kusumoto K, Abe H, Numata M, Nagata K, Hayashi K, Tsubouchi H. The peroxisome proliferator-activated receptor-gamma agonist, pioglitazone, inhibits fat accumulation and fibrosis in the livers of rats fed a choline-deficient, l-amino acid-defined diet. Hepatol Res. 2005;32(4):235–242. doi: 10.1016/j.hepres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 95.Collino M, Aragno M, Castiglia S, Miglio G, Tomasinelli C, Boccuzzi G, Thiemermann C, Fantozzi R. Pioglitazone improves lipid and insulin levels in overweight rats on a high cholesterol and fructose diet by decreasing hepatic inflammation. Br J Pharmacol. 2010;160(8):1892–1902. doi: 10.1111/j.1476-5381.2010.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Leclercq IA, Sempoux C, Starkel P, Horsmans Y. Limited therapeutic efficacy of pioglitazone on progression of hepatic fibrosis in rats. Gut. 2006;55(7):1020–1029. doi: 10.1136/gut.2005.079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Da Silva Morais A, Abarca-Quinones J, Horsmans Y, Starkel P, Leclercq IA. Peroxisome proliferated-activated receptor gamma ligand, Pioglitazone, does not prevent hepatic fibrosis in mice. Int J Mol Med. 2007;19(1):105–112. [PubMed] [Google Scholar]
- 98.Bae MA, Rhee SD, Jung WH, Ahn JH, Song BJ, Cheon HG. Selective inhibition of activated stellate cells and protection from carbon tetrachloride-induced liver injury in rats by a new PPARgamma agonist KR62776. Arch Pharm Res. 2010;33(3):433–442. doi: 10.1007/s12272-010-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang L, Stimpson SA, Chen L, Wallace Harrington W, Rockey DC. Effectiveness of the PPARgamma agonist, GW570, in liver fibrosis. Inflamm Res. 2010;59(12):1061–1071. doi: 10.1007/s00011-010-0226-0. [DOI] [PubMed] [Google Scholar]
- 100.Ahn JH, Shin MS, Jung SH, Kang SK, Kim KR, Rhee SD, Jung WH, Yang SD, Kim SJ, Woo JR, Lee JH, Cheon HG, Kim SS. Indenone derivatives: a novel template for peroxisome proliferator-activated receptor gamma (PPARgamma) agonists. J Med Chem. 2006;49(15):4781–4784. doi: 10.1021/jm060389m. [DOI] [PubMed] [Google Scholar]
- 101.Zheng S, Chen A. Activation of PPARgamma is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem J. 2004;384(Pt 1):149–157. doi: 10.1042/BJ20040928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng S, Chen A. Disruption of transforming growth factor-beta signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-gamma in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G113–G123. doi: 10.1152/ajpgi.00200.2006. [DOI] [PubMed] [Google Scholar]
- 103.Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73(2):399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 104.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):511–531. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 105.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51(5):1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 106.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Caldwell SH, Hespenheide EE, Redick JA, Iezzoni JC, Battle EH, Sheppard BL. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96(2):519–525. doi: 10.1111/j.1572-0241.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 108.Argo CK, Iezzoni JC, Al-Osaimi AM, Caldwell SH. Thiazolidinediones for the treatment in NASH: sustained benefit after drug discontinuation? J Clin Gastroenterol. 2009;43(6):565–568. doi: 10.1097/MCG.0b013e31818f4fc2. [DOI] [PubMed] [Google Scholar]
- 109.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Sponseller CA, Hampton K, Bacon BR. Interim results of a pilot study demonstrating the early effects of the PPAR-gamma ligand rosiglitazone on insulin sensitivity, aminotransferases, hepatic steatosis and body weight in patients with non-alcoholic steatohepatitis. J Hepatol. 2003;38(4):434–440. doi: 10.1016/s0168-8278(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 110.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38(4):1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 111.Idilman R, Mizrak D, Corapcioglu D, Bektas M, Doganay B, Sayki M, Coban S, Erden E, Soykan I, Emral R, Uysal AR, Ozden A. Clinical trial: insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;28(2):200–208. doi: 10.1111/j.1365-2036.2008.03723.x. [DOI] [PubMed] [Google Scholar]
- 112.Caldwell SH, Patrie JT, Brunt EM, Redick JA, Davis CA, Park SH, Neuschwander-Tetri BA. The effects of 48 weeks of rosiglitazone on hepatocyte mitochondria in human nonalcoholic steatohepatitis. Hepatology. 2007;46(4):1101–1107. doi: 10.1002/hep.21813. [DOI] [PubMed] [Google Scholar]
- 113.Wang CH, Leung CH, Liu SC, Chung CH. Safety and effectiveness of rosiglitazone in type 2 diabetes patients with nonalcoholic Fatty liver disease. J Formos Med Assoc. 2006;105(9):743–752. doi: 10.1016/S0929-6646(09)60202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saryusz-Wolska M, Szymanska-Garbacz E, Jablkowski M, Bialkowska J, Pawlowski M, Kwiecinska E, Omulecka A, Borkowska A, Ignaczak A, Loba J, Czupryniak L. Rosiglitazone treatment in nondiabetic subjects with nonalcoholic fatty liver disease. Pol Arch Med Wewn. 2011;121(3):61–66. [PubMed] [Google Scholar]
- 115.Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E, Grimaldi A, Poynard T. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled fatty liver improvement with rosiglitazone therapy (FLIRT) Trial. Gastroenterology. 2008;135(1):100–110. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 116.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 117.Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51(2):445–453. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 118.Omer Z, Cetinkalp S, Akyildiz M, Yilmaz F, Batur Y, Yilmaz C, Akarca U. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22(1):18–23. doi: 10.1097/MEG.0b013e32832e2baf. [DOI] [PubMed] [Google Scholar]
- 119.Torres DM, Jones FJ, Shaw JC, Williams CD, Ward JA, Harrison SA. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open- label trial. Hepatology. 2011;54(5):1631–1639. doi: 10.1002/hep.24558. [DOI] [PubMed] [Google Scholar]
- 120.Shadid S, Jensen MD. Effect of pioglitazone on biochemical indices of non-alcoholic fatty liver disease in upper body obesity. Clin Gastroenterol Hepatol. 2003;1(5):384–387. doi: 10.1053/s1542-3565(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 121.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, Liang TJ, Yanovski JA, Kleiner DE, Hoofnagle JH. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39(1):188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 122.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 123.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 124.Lutchman G, Modi A, Kleiner DE, Promrat K, Heller T, Ghany M, Borg B, Loomba R, Liang TJ, Premkumar A, Hoofnagle JH. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46(2):424–429. doi: 10.1002/hep.21661. [DOI] [PubMed] [Google Scholar]
- 125.Lutchman G, Promrat K, Kleiner DE, Heller T, Ghany MG, Yanovski JA, Liang TJ, Hoofnagle JH. Changes in serum adipokine levels during pioglitazone treatment for nonalcoholic steatohepatitis: relationship to histological improvement. Clin Gastroenterol Hepatol. 2006;4(8):1048–1052. doi: 10.1016/j.cgh.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 126.Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50(4):1087–1093. doi: 10.1002/hep.23116. [DOI] [PubMed] [Google Scholar]
- 127.Urtasun R, Conde de la Rosa L, Nieto N. Oxidative and nitrosative stress and fibrogenic response. Clin Liver Dis. 2008;12(4):769–790. doi: 10.1016/j.cld.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2(12):1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 129.Chalasani NP, Sanyal AJ, Kowdley KV, Robuck PR, Hoofnagle J, Kleiner DE, Unalp A, Tonascia J. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials. 2009;30(1):88–96. doi: 10.1016/j.cct.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McHutchison J, Goodman Z, Patel K, Makhlouf H, Rodriguez-Torres M, Shiffman M, Rockey D, Husa P, Chuang WL, Levine R, Jonas M, Theodore D, Brigandi R, Webster A, Schultz M, Watson H, Stancil B, Gardner S. Farglitazar lacks antifibrotic activity in patients with chronic hepatitis C infection. Gastroenterology. 2010;138(4):1365–1373. doi: 10.1053/j.gastro.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 132.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117(3):539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 134.Mahady SE, Webster AC, Walker S, Sanyal A, George J. The role of thiazolidinediones in non-alcoholic steatohepatitis—a systematic review and meta analysis. J Hepatol. 2011;55(6):1383–1390. doi: 10.1016/j.jhep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 135.Rakoski MO, Singal AG, Rogers MA, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2010;32(10):1211–1221. doi: 10.1111/j.1365-2036.2010.04467.x. [DOI] [PubMed] [Google Scholar]
- 136.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278(36):34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 138.Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. J Hepatol. 2007;46(4):734–742. doi: 10.1016/j.jhep.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 139.Hannivoort RA, Hernandez-Gea V, Friedman SL. Genomics and proteomics in liver fibrosis and cirrhosis. Fibrogenesis Tissue Repair. 2012;5(1):1. doi: 10.1186/1755-1536-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Constantinou MA, Theocharis SE, Mikros E. Application of metabonomics on an experimental model of fibrosis and cirrhosis induced by thioacetamide in rats. Toxicol Appl Pharmacol. 2007;218(1):11–19. doi: 10.1016/j.taap.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 141.Verreth W, De Keyzer D, Pelat M, Verhamme P, Ganame J, Bielicki JK, Mertens A, Quarck R, Benhabiles N, Marguerie G, Mackness B, Mackness M, Ninio E, Herregods MC, Balligand JL, Holvoet P. Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation. 2004;110(20):3259–3269. doi: 10.1161/01.CIR.0000147614.85888.7A. [DOI] [PubMed] [Google Scholar]
- 142.Gastaldi G, Russell A, Golay A, Giacobino JP, Habicht F, Barthassat V, Muzzin P, Bobbioni-Harsch E. Upregulation of peroxisome proliferator-activated receptor gamma coactivator gene (PGC1A) during weight loss is related to insulin sensitivity but not to energy expenditure. Diabetologia. 2007;50(11):2348–2355. doi: 10.1007/s00125-007-0782-1. [DOI] [PubMed] [Google Scholar]
- 143.Tolman KG. The safety of thiazolidinediones. Expert Opin Drug Saf. 2011;10(3):419–428. doi: 10.1517/14740338.2011.534982. [DOI] [PubMed] [Google Scholar]
- 144.Blind E, Dunder K, de Graeff PA, Abadie E. Rosiglitazone: a European regulatory perspective. Diabetologia. 2011;54(2):213–218. doi: 10.1007/s00125-010-1992-5. [DOI] [PubMed] [Google Scholar]
- 145.Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N Engl J Med. 2010;363(16):1489–1491. doi: 10.1056/NEJMp1010788. [DOI] [PubMed] [Google Scholar]
- 146.Marcy TR, Britton ML, Blevins SM. Second-generation thiazolidinediones and hepatotoxicity. Ann Pharmacother. 2004;38(9):1419–1423. doi: 10.1345/aph.1E072. [DOI] [PubMed] [Google Scholar]
- 147.Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Jr, Vaughn DJ, Nessel L, Selby J, Strom BL. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34(4):916–922. doi: 10.2337/dc10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34(6):1369–1371. doi: 10.2337/dc10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tian L, Zhou J, Casimiro MC, Liang B, Ojeifo JO, Wang M, Hyslop T, Wang C, Pestell RG. Activating peroxisome proliferator-activated receptor gamma mutant promotes tumor growth in vivo by enhancing angiogenesis. Cancer Res. 2009;69(24):9236–9244. doi: 10.1158/0008-5472.CAN-09-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab. 2005;288(6):E1195–E1205. doi: 10.1152/ajpendo.00513.2004. [DOI] [PubMed] [Google Scholar]
- 151.Wobser H, Dorn C, Weiss TS, Amann T, Bollheimer C, Buttner R, Scholmerich J, Hellerbrand C. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009;19(8):996–1005. doi: 10.1038/cr.2009.73. [DOI] [PubMed] [Google Scholar]
- 152.Sharma C, Pradeep A, Pestell RG, Rana B. Peroxisome proliferator-activated receptor gamma activation modulates cyclin D1 transcription via beta-catenin-independent and cAMP-response element-binding protein-dependent pathways in mouse hepatocytes. J Biol Chem. 2004;279(17):16927–16938. doi: 10.1074/jbc.M309045200. [DOI] [PubMed] [Google Scholar]
- 153.Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466(7305):451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, Kuruvilla DS, Shin Y, He Y, Bruning JB, Marciano DP, Cameron MD, Laznik D, Jurczak MJ, Schurer SC, Vidovic D, Shulman GI, Spiegelman BM, Griffin PR. Antidiabetic actions of a non-agonist PPARgamma ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477(7365):477–481. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Steiling H, Muhlbauer M, Bataille F, Scholmerich J, Werner S, Hellerbrand C. Activated hepatic stellate cells express keratinocyte growth factor in chronic liver disease. Am J Pathol. 2004;165(4):1233–1241. doi: 10.1016/S0002-9440(10)63383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tailleux A, Wouters K, Staels B. Roles of PPARs in NAFLD: Potential therapeutic targets. Biochim Biophys Acta. 2012;1821(5):809–818. doi: 10.1016/j.bbalip.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 157.Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39(5):1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 158.Stienstra R, Mandard S, Patsouris D, Maass C, Kersten S, Muller M. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology. 2007;148(6):2753–2763. doi: 10.1210/en.2007-0014. [DOI] [PubMed] [Google Scholar]
- 159.Kondo K, Sugioka T, Tsukada K, Aizawa M, Takizawa M, Shimizu K, Morimoto M, Suematsu M, Goda N. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, improves hepatic microcirculatory patency and oxygen availability in a high-fat-diet-induced fatty liver in mice. Adv Exp Med Biol. 2010;662:77–82. doi: 10.1007/978-1-4419-1241-1_10. [DOI] [PubMed] [Google Scholar]
- 160.Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr. 2011;141(4):603–610. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fernandez-Miranda C, Perez-Carreras M, Colina F, Lopez-Alonso G, Vargas C, Solis-Herruzo JA. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2008;40(3):200–205. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 162.Thulin P, Rafter I, Stockling K, Tomkiewicz C, Norjavaara E, Aggerbeck M, Hellmold H, Ehrenborg E, Andersson U, Cotgreave I, Glinghammar B. PPARalpha regulates the hepatotoxic biomarker alanine aminotransferase (ALT1) gene expression in human hepatocytes. Toxicol Appl Pharmacol. 2008;231(1):1–9. doi: 10.1016/j.taap.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 163.Sanwald-Ducray P, Liogier D’ardhuy X, Jamois C, Banken L. Pharmacokinetics, pharmacodynamics, and tolerability of aleglitazar in patients with type 2 diabetes: results from a randomized, placebo-controlled clinical study. Clin Pharmacol Ther. 2010;88(2):197–203. doi: 10.1038/clpt.2009.259. [DOI] [PubMed] [Google Scholar]
- 164.Hansen BC, Tigno XT, Benardeau A, Meyer M, Sebokova E, Mizrahi J. Effects of aleglitazar, a balanced dual peroxisome proliferator-activated receptor alpha/gamma agonist on glycemic and lipid parameters in a primate model of the metabolic syndrome. Cardiovasc Diabetol. 2011;10:7. doi: 10.1186/1475-2840-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lindblom P, Berg AL, Zhang H, Westerberg R, Tugwood J, Lundgren H, Marcusson-Stahl M, Sjogren N, Blomgren B, Ohman P, Skanberg I, Evans J, Hellmold H. Tesaglitazar, a dual PPAR-alpha/gamma agonist, hamster carcinogenicity, investigative animal and clinical studies. Toxicol Pathol. 2012;40(1):18–32. doi: 10.1177/0192623311429972. [DOI] [PubMed] [Google Scholar]