Abstract
Hormonal regulation is essential to spermatogenesis. Sertoli cells (SCs) have functions that reach far beyond the physical support of germ cells, as they are responsible for creating the adequate ionic and metabolic environment for germ cell development. Thus, much attention has been given to the metabolic functioning of SCs. During spermatogenesis, germ cells are provided with suitable metabolic substrates, in a set of events mediated by SCs. Multiple signaling cascades regulate SC function and several of these signaling pathways are hormone-dependent and cell-specific. Within the seminiferous tubules, only SCs possess receptors for some hormones rendering them major targets for the hormonal signaling that regulates spermatogenesis. Although the mechanisms by which SCs fulfill their own and germ cells metabolic needs are mostly studied in vitro, SC metabolism is unquestionably a regulation point for germ cell development and the hormonal control of these processes is required for a normal spermatogenesis.
Keywords: Sertoli cells, Spermatogenesis, Cell metabolism, Metabolic modulation, Hormonal control
Introduction
The testes have two major functions, the synthesis of steroid hormones (steroidogenesis) and the production of mature sperm (spermatogenesis), which are achieved through coordination between the various cell types (Sertoli, Leydig, peritubular myoid, and germ cells) within the testis. The number of Sertoli cells (SCs) is an important determinant of testis size [1] and the adjacent SCs form tight junctions with each other, such that nothing larger than 1,000 Da can pass from the outside to the inside of the tubule [2], creating one of the tightest blood–tissue barriers known to exist in mammalian tissues, the blood–testis barrier (BTB) [3]. Several proteins and other products are known to be secreted by SCs, e.g., androgen-binding protein [4], transferrin [5], plasminogen activator [6], glycoproteins [7], sulpho-proteins [8], and myo-inositol [9]. They can also transport water from the interstitial space into the lumen, serving as the vehicle for moving sperm from the testis to the epididymis [10] and they can also control the pH in the seminiferous fluid [11, 12].
The SC structure is very complex, with several cup-shaped processes encompassing the various germ cell types that are distributed within the seminiferous epithelium in a very arranged way [13]. The germ cells form intimate associations with SCs and multiple germ cells are in contact with a single SC. It is this unique association between germ and SCs that is in the basis of the seminiferous epithelium cycle. Each particular association of germ cells is referred to as a stage and thus, the number of stages of spermatogenesis in a species is defined by the number of morphologically recognizable germ cell associations within the testis [14].
Often called “nurse cells”, fully differentiated SCs must ensure a physical and nutritional support for germ cells. SCs deposit extracellular matrix components (e.g., collagen and laminin), form specialized junctions and exhibit a well-organized cytoskeleton important for maintenance of the seminiferous epithelium [15]. SCs also secrete specific products that are necessary for germ cell development and survival. Amongst these products, are the anti-Müllerian hormone (AMH), the c kit ligand and the inhibin. In addition, SCs secrete peptides such as prodynorphin and nutrients or metabolic intermediates [16, 17]. This transference of metabolic products, such as amino acids, carbohydrates, lipids, vitamins, and metal ions [15] from the SCs to the developing germ cells is only possible due to the close relationship between these two cell types and, for that it is imperative that germ cells receive an adequate level of energy substrates [18–20].
Despite the abundance of published data on the response of the SCs and testis in general to hormonal treatment or hormonal deprivation, the exact roles for hormonal control over SCs metabolism and spermatogenesis remain unclear. There are several drawbacks in hormonal studies concerning SC metabolic and physiological functions. Most of these studies are performed in vitro using cultured SCs and under these circumstances there are a number of different substrates and pathways available, which makes it difficult to infer about the real impact on germ cells. Furthermore, in vivo, the relative importance of the different metabolic pathways to SCs is likely to be determined by multiple factors, including substrate concentrations, and the overlapping and synergistic actions of hormones can mask the relative contribution of each pathway to a normal spermatogenesis. In an attempt to isolate hormone-regulated effects, several genes have been knocked out selectively in SCs of rodents, mainly those encoding hormone receptors. In this work, we aim to review and discuss the literature on SCs energy metabolism (de)regulation, hormonal control of SCs metabolism, and finally on the pathways known to occur during the control of SCs metabolism.
Importance of Sertoli cell for normal spermatogenesis
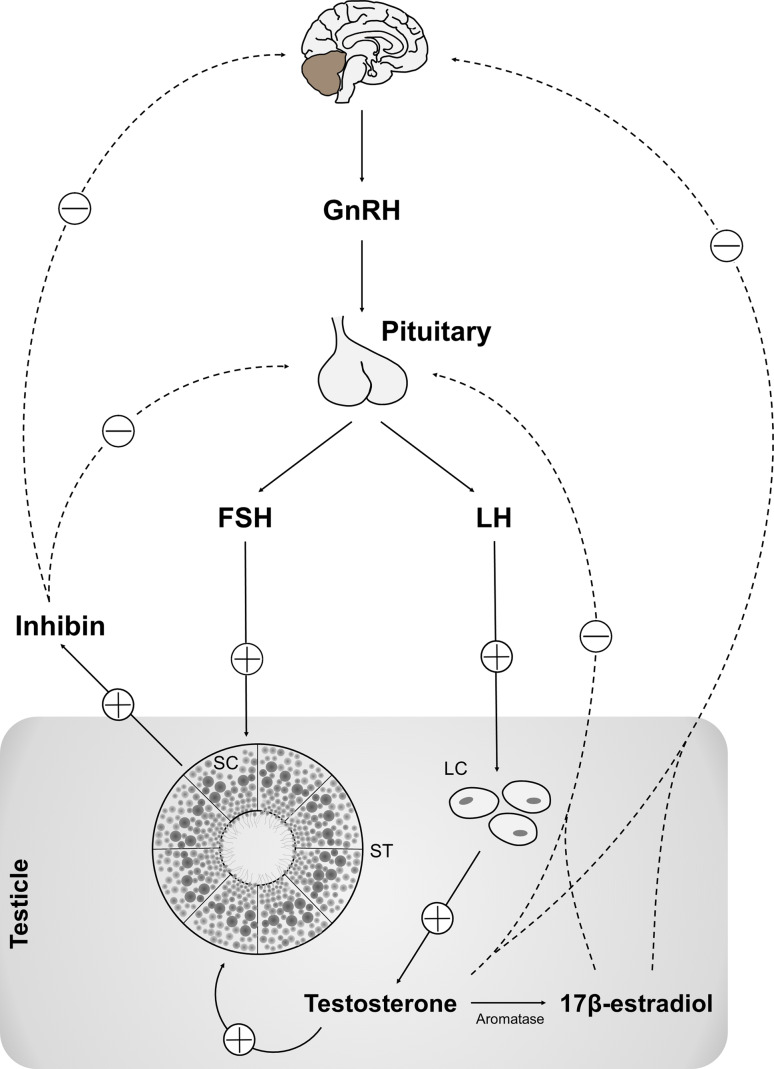
Spermatogenesis is a complex cellular event that takes place in the seminiferous tubules epithelium and requires the involvement of a complex assortment of peptides and hormones. These hormonal messengers regulate not only germ cell differentiation, but also the proliferation and function of the somatic cell types required for the proper development and function of the testis [21]. During spermatogenesis the spermatogonia (2n) undergo mitosis, followed by a cellular transformation of type B spermatogonia into spermatocytes, which enter meiosis to form spermatids (n), and finally develop to spermatozoa through spermiogenesis [14]. Spermatogenesis is regulated by the hypothalamus–pituitary–testis axis, essentially by two pituitary hormones [follicle-stimulating hormone (FSH) and luteinizing hormone (LH)] [22], which functionally connect the brain with the testis and the dysfunction of this axis leads to infertility [23]. The process is finely regulated and follows an intricate network of signals and pathways (Fig. 1). The production of inhibin by SCs, as well as testosterone (T) and 17β-estradiol (E2) by Leydig cells, provides a negative feedback loop that reduces the secretion of gonadotropin-releasing hormone (GnRH) in the hypothalamus and of LH in the pituitary, in order to maintain the homeostasis of FSH, LH, T, E2, and inhibin within the hypothalamic–pituitary–testicular axis [24, 25]. In fact, all these hormones and factors support the spermatogenesis (Fig. 1).
Fig. 1.
Simplified diagram of the hypothalamus–pituitary–testis axis control of spermatogenesis. The two pituitary hormones, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), are responsible for the connection between the brain and the testis. The production of inhibin by SCs and testosterone (T) by Leydig cells provide a negative feedback control that results in reduction of gonadotropin-releasing hormone (GnRH) production in the hypothalamus and reduced LH and FSH production on pituitary, thus maintaining the homeostasis of FSH, LH, T and inhibin. All these hormones and factors have a tight control on spermatogenesis. Circled plus positive feedback, Circled minus negative feedback
Support and nurturing by SCs is essential for the normal development of spermatogenesis and each SC has the ability to support approximately 30–50 germ cells by providing required factors for their development [26]. SC multiplication depends of several conditions that are species-specific. For instance, in rats, the SCs cease to divide by approximately 15 days postpartum [27] while in humans, they cease to maturate at birth [28] with their number remaining relatively constant in the testis throughout adulthood at about 40 million per testis in rats [27] and from 260 million at birth to approximately 3,700 million per testis in humans [29]. As such, approximately 75 % of germ cells [30] undergo spontaneous degeneration in the seminiferous epithelium via an extrinsic pathway of apoptosis involving Fas and the Fas ligand (FasL) or a mitochondrial pathway that involves the Bcl-2 family of proteins [31] so that the Sertoli:germ cell ratio can approximately be maintained between 1:30 and 1:50 [26, 27]. Thus SCs importance reaches far beyond their own biological processes since germ cells development and maintenance are intimately linked to the SCs physiological state.
Glucose, lactate, and pyruvate as fuels for germ cells
Glucose is essential for the maintenance of spermatogenesis in vivo [32, 33] however, there are low levels of this sugar in the tubular fluid, mainly due to the metabolism of glucose to different metabolic subproducts [34, 35]. These metabolic intermediates derived from glucose metabolism are crucial and point to a possible role for SCs as feeders in germ cell development because they produce lactate at a high rate [34, 36–38] and pachytene spermatocytes and round spermatids require exogenous lactate or pyruvate for energy production [36, 39, 40]. These observations, tested in vitro, reported that pachytene spermatocytes survival and activity was regulated by the supply of lactate from SCs [41]. It is known that lactate is the preferred substrate for round spermatids and the energy production is more efficient when this metabolite is present in high concentrations and pyruvate in low concentrations [39]. Nevertheless, both lactate and pyruvate are major products with a crucial role in maintaining a high rate of protein synthesis in isolated spermatocytes and spermatids during short-term incubations [42]. The importance of lactate as preferred substrate for spermatocytes and spermatids has been widely tested under in vitro conditions. For instance, in a substrate competition work, it was reported that the addition of lactate, but not glucose, results in enhanced rates of respiration and protein and RNA synthesis by isolated pachytene spermatocytes or round spermatids [36]. It has also been highlighted that lactate is a player in other metabolic pathways and crossroads in spermatids as it increases protein synthesis by producing adenosine triphosphate (ATP) [43] and thus modulates the rate of nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) oxidation and the pentose phosphate pathway [44].
Furthermore, the importance of lactate for a normal spermatogenesis was highlighted by reports showing: (1) that spermatogenesis in adult cryptorchid testis is improved by intratesticular infusion of lactate [45]; and (2) an anti-apoptotic effect of lactate on germ cells [20]. Being so, the lactate production by SCs reported both in vitro and in vivo appears as an excellent point for the metabolic control of spermatogenesis [35].
Moreover, the importance of glucose metabolism and glucose-related metabolic subproducts is far beyond the simple nutritional maintenance of developing germ cells, as in mammals the ideal spermatozoa motility and capacitation is achieved through aerobic glycolysis [46, 47]. In fact, the glycolysable substrates are essential for several functions such as protein tyrosine phosphorylation and hyperactivation. The last process is associated with capacitation [48–50], sperm motility [48, 51], and fertilization [52, 53] and thus, glucose metabolism can be ultimately responsible for fertilization ability.
Importantly, the conversion of pyruvate to lactate by lactate dehydrogenase (LDH) with the concomitant oxidation of NADH to NAD+ is essential for the continued production of ATP by glycolysis [54] (Fig. 2) and germ cells specifically express a unique isozyme of LDH [55, 56], LDH type C (LDHC), which is abundant in spermatids and spermatozoa [57]. This unique characteristic of germ cells is related to their unique dependence on lactate and as expected, the targeted disruption of the Ldhc gene results in male infertility due to a progressive decrease in sperm motility, failure in the capacity to develop the hyperactivated motility pattern essential for fertilization and a decline in ATP levels [58]. Also, the in vivo macromolecular organization of enzymes is crucial in the regulation of glycolysis in the sperm flagellum, and LDHC is an integral component of a complex containing an ATP carrier protein that re-distributes ATP from phosphoglycerate kinase 2 to hexokinase type 1, thus regulating the initial phase of glycolysis [59]. Undoubtedly, LDHC is a control point in germ cells metabolic needs and development.
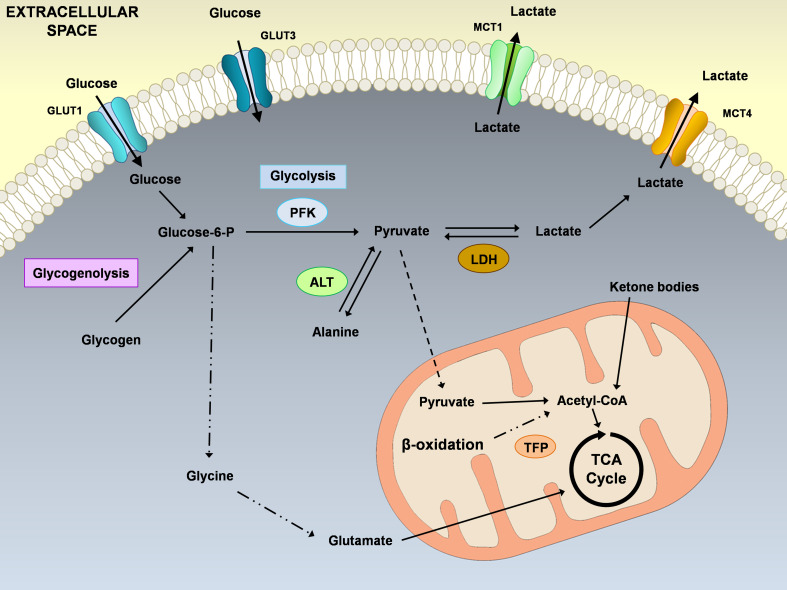
Fig. 2.
Schematic illustration of the main metabolic pathways described in Sertoli cells (SCs). The extracellular glucose enters via glucose transporters (GLUT1 and GLUT3) and is converted in pyruvate that can either be transported into the mitochondrial matrix to form Acetyl-coA, or can be converted into lactate or alanine. Sertoli cells actively consume glucose producing large quantities of lactate, for the developing germ cells, which is then exported to the extracellular medium by proton-linked plasma membrane transporters, the monocarboxylate transporters (MCT1 and MCT4). Nevertheless, although glucose is one of the main substrates for SCs, they can metabolize ketone bodies, fatty acids and glycogen. ALT alanine transaminase, GLUT glucose transporter, LDH lactate dehydrogenase, MCT monocarboxylate transporter, PFK phosphofructokinase, TFP trifunctional protein
Several biochemical steps are involved in lactate production by SCs, and glucose transport across the cell membrane is an important event for the whole process. A family of structurally related glycoproteins, designated as glucose transporters (GLUT), is responsible for mediating passive glucose transport [60] and studies with cultured mammalian cells suggest that glucose itself may regulate its own transport and metabolism [60]. Until now, the GLUT isoforms GLUT1, GLUT3, and GLUT8, have been identified in SCs [61–63]. However, GLUT8 has not been localized at the plasma membrane, thus excluding its role in glucose transport from the extracellular milieu [64, 65] while GLUT1 and GLUT3 are present at the plasma membrane, and thus, it may be assumed that they mediate glucose incorporation into SCs (Fig. 2). Also, some substances, such as hormones, cytokines or growth factors, promote an increment of glucose incorporation into SCs by differentially regulating GLUT1 and GLUT3 expression [62]. When glucose is removed from the culture medium, there is an increase in GLUT1 and a decrease in GLUT3 expression levels in SCs [66] leading to the assumption that changes in extracellular milieu glucose levels may also constitute a signal for SCs to upregulate their glucose transport system and ensure the appropriate lactate production for germ cell development. This kind of mechanism has been reported in some cell types where the absence or deprivation of glucose leads to a compensatory increase in the glucose uptake to optimize glucose utilization and maintain energy levels [60]. Of course, this assumption based on in vitro observations is correct only under these circumstances because in vivo conditions may reveal a more complex signaling cascade. Nevertheless, the expected results from in vivo punctual glucose deprivation or low glucose availability should be in line with a physiological adaptation in GLUTs levels.
Often underestimated, glycogen contribution to SC metabolism should deserve special attention. Although histochemical evidences of the presence of glycogen and glycogen phosphorylase activity in these cells have been reported for several years [67, 68], few has been done to unravel the exact role of glycogen in SCs metabolic control of spermatogenesis. It should be noted however that the presence of glycogen or glycogen metabolism machinery in SCs could be a valuable data for the explanation of the adaptive mechanisms described earlier to maintain lactate production.
The available data on SCs metabolism point to a crucial role in glucose metabolism and glucose metabolism intermediates. So, the glucose transport mediated by GLUTs, the LDH isoenzyme system that reversibly catalyzes the interconversion of pyruvate into lactate, and the lactate transport across the plasma membrane mediated by monocarboxylate transporters (MCTs) are prime targets for achieving an optimal lactate offer to germ cells (Fig. 2). Germ cells, on the other hand, have to metabolize lactate in optimal conditions and even spermatozoa maturation is dependent upon a specific metabolic need. Thus, the optimal metabolic functioning of all cells types involved in spermatogenesis, spermiogenesis, and spermatozoa maturation is crucial for the achievement of an optimal sperm quality and fertilization.
Lipids, β-oxidation, and amino acids as energy suppliers to Sertoli cells
The lipids play a crucial role in the testis since this organ has high rates of cellular proliferation, measured by phospholipid synthesis, which is related to the number of proliferating germ cells [69]. In fact, Leydig cells and SCs multiply very slowly and a testicle actively producing spermatozoa requires constant membrane synthesis and thus a large substrate pool of phospholipids. Being so, these somatic cells are not expected to contribute meaningfully to phospholipid synthesis [69]. Furthermore, in normal rats, SCs and Leydig cells have some differences regarding the amount of lipids and fatty acid composition, which is related to their specific functions in the testis [70] and thus it is difficult to isolate the role of each testicular cell in lipids metabolism. In fact, the lipid β-oxidation and glycolysis are two metabolic pathways to produce energy within cells and each cell type predominantly utilizes one of the two energy substrates for ATP production and although both lipids and glucose can be energy sources, glycolysis is an active ATP-producing pathway in the majority of cell types whereas the lipids normally function as storage of energy. The SCs produce significantly higher ATP levels than interstitial and spermatogenic cells and they can utilize lipids to produce energy via the β-oxidation pathway [71]. The importance of β-oxidation in SCs is emphasized by their function as recyclers of lipids since they can phagocytize and degrade apoptotic spermatogenic cells and residual bodies to form lipids that are then used to produce ATP during spermatogenesis [71]. Also, in mice, spermatogenesis can be compromised by inactivation of genes involved in lipid metabolism suggesting that an appropriated lipid metabolism is critical for male reproduction [72]. Interestingly, cultured rat SCs can oxidize palmitate to CO2, ketone bodies and fatty acids and the latter are known as being a major energy substrate for these cells [73]. The way SCs respond in vitro to substrate availability and media changes are often underestimate. In fact, as in other cell types, it is expected that the culture conditions as well as the media concentration exert a stoichiometric pressure toward some pathways over others. Nevertheless, several studies with different in vitro approaches regarding SCs report the presence of metabolic enzymes involved in the biosynthesis of n-6 polyunsaturated fatty acids [74–76] thus emphasizing the main role that lipid metabolism may have in SCs metabolism. However, there are differences regarding the distinct use of fatty acids by in vitro cultured testicular cells in mature and immature rats, with the rates of oleate oxidation to CO2 being higher in testicular cells from immature animals [77]. Although the in vivo situation may present a much more complex situation where fatty acids, such as oleic acids, are not often within such a high range of concentrations, it is remarkable that mature and immature testicular cells present such differences concerning the use of lipids thus pointing out for a more important role for lipids as an energy source for the prepubertal rat testis. Recently [78], it was described that in vitro cultured human SCs produce high amounts of acetate that is under strict hormonal control by insulin, E2 and 5α-dihydrotestosterone (DHT). This hormonal control of acetate metabolism was suggested to have an important role in producing sub-products essential to maintain a high rate of lipid synthesis in the developing germ cells and thus should deserve special attention as acetate metabolism in these cells remains unclear.
Besides fatty acids, the oxidation of glutamine and leucine can yield much of the required energy by SCs [44] but the relative importance of the endogenous metabolism of these substrates on the bioenergetics balance of SCs is far from being disclosed. However, in rat spermatogenic epithelium the enzyme branched-chain amino acid aminotransferase, responsible for the conversion of the branched-chain amino acids such as valine, leucine, and isoleucine to the corresponding branched-chain acids, is confined to SCs and it was reported that cultured SCs can use glutamine and other amino acids such as alanine, leucine, and valine as energy substrates, which may be very important for SCs metabolism [79]. Finally, though glycine does not seem to be an adequate energy substrate, and perhaps is not easily attained in in vivo conditions, it is effectively incorporated into proteins and partially converted to lipids as demonstrated by studies performed with glycine enriched with 14C in carbon 1 ([1-14C]glycine) showing that glycine is converted to serine in SCs, and then incorporated into phospholipids, although SCs have insignificant capacity for oxidizing glycine to CO2 [79].
The role of lipids and amino acids in SCs metabolism is not entirely understood as most of the evidences are attained under in vitro conditions. Nevertheless, the physiologic meaning of these studies is clear: SCs are capable and probably required for lipid metabolic remodeling and amino acid incorporation.
Androgens actions mediate Sertoli cell metabolism
Initiation, maintenance, and reinitiation of spermatogenesis depend on androgen action [80] and T, which is responsible for the promotion of the integrity of the BTB and the assembly of junctional complexes [81, 82], is produced by Leydig cells in response to LH stimulation. Most of the androgenic control of SCs functions is modulated by the 5α-reduced metabolites of T, such as DHT, which has a biological activity two- to threefold greater than that of T [83]. Androgen receptor (AR) activity is regulated by the steroid ligand T and its derivative DHT, the binding of which initiates nuclear translocation and the transcriptional regulatory function of AR [84]. Both androgens, T and DHT, bind to and activate the same AR protein, but DHT has a much higher affinity [85]. In hypogonadal mice genetically deficient in GnRH (hpg mice), T alone is able to restore spermatogenesis under experimental conditions where FSH is absent [86], which demonstrates the importance of T and ARs to spermatogenesis. Using the same hpg mouse model, which lacks germ cell development, it was reported that T and DHT have similar effects on the development of spermatogenesis [86]. However, the differential induction of FSH levels by T, but not by DHT, makes it very likely that in hpg models the aromatization of T leads to stimulation of pituitary FSH release. Importantly, in hpg mice with SCs lacking ARs, the non-aromatizable androgen does not stimulate spermatogenesis, suggesting that the androgen stimulation of spermatogenesis requires direct androgen action on SCs [87]. The ARs also play an important role in the regulation of T levels that occurs through autocrine feedback on the Leydig cells, via hypothalamic GnRH production and through inhibition of LH secretion by the pituitary [88]. They are localized in the testicular somatic cells, including Leydig cells, peritubular myoid, and blood vessel smooth muscle cells and also in SCs. Conversely, germ cells of the mature testis do not seem to require functional ARs. Thus, androgens must affect spermatogenesis indirectly via somatic SCs as these cells interact directly with developing germ cells and express functional ARs [89–91].
The ARs present in the SC play a critical role in maintaining the SC ability to support normal spermatogenesis, as when ARs are ablated in these cells the germ cell development stop at the spermatocyte stages [91, 92] or early spermatid [93], highlighting the fact that SC is the primary site that mediates androgen support to a regular spermatogenesis. In contrast, deletion of AR has no effect on germ cell development [94]. Additionally, although it was originally suggested that androgen action in peritubular myoid cells (PTM) was not required for male fertility [95], recent data revealed an essential role in vivo for ARs signaling via the PTM cells in normal spermatogenesis and male fertility, using PTM-specific androgen receptor knockout (PTM-ARKO) mouse model [96]. These animals presented impaired SC function and reduced expression of some androgen-dependent SC genes, though the exact mode of action by which androgens play their role in SCs via PTM is not yet fully disclosed.
In mice lacking ARs through either a natural mutation (Tfm) or genomic manipulation (ARKO), there is severe interruption in spermatogenesis with a failure of germ cells to progress from the early stages of meiosis [91]. In contrast, mice lacking FSH or the FSH receptor are fertile, although they present a reduced germ cell number [97–99]. Thus, FSH seems to primarily act in optimization of spermatogenesis and germ cell number, while androgens are critical for the completion of meiosis and, consequently, to fertility. In fact, rats lacking both FSH receptors and ARs in SCs (FSHRKO/SCARKO) show a marked reduction in the total number of germ cells [100] although the total number of SCs was not significantly different between FSHRKO and FSHRKO/SCARKO. Thus, FSH and androgen interact through the SC to regulate testicular function by an additive and synergistic way.
In isolated rat SCs, it was reported [101] that both T and DHT can act through an alternative pathway characterized by a rapid increase in intracellular Ca2+ concentration triggering a transcriptional response that leads to essential signals to the developing germ cells regulating by this way the microenvironment within the seminiferous epithelium and creating the perfect conditions for germ cell differentiation. However, this alternative pathway of androgen signaling is not easily supported by in vivo evidences. Moreover, not all actions occurring through this alternative pathway are mediated via a transcriptional response, which makes these mechanisms very unlikely to occur in vivo. In fact, the fatty acid profile of in vitro cultured SCs is modified by T through modulation in fatty acid desaturases activity [102] and as discussed earlier, the hormonal control of lipid metabolism in SC, is important not only for maintaining the energy of the cells themselves but also for the control of the spermatogenesis process [103] (Fig. 3).
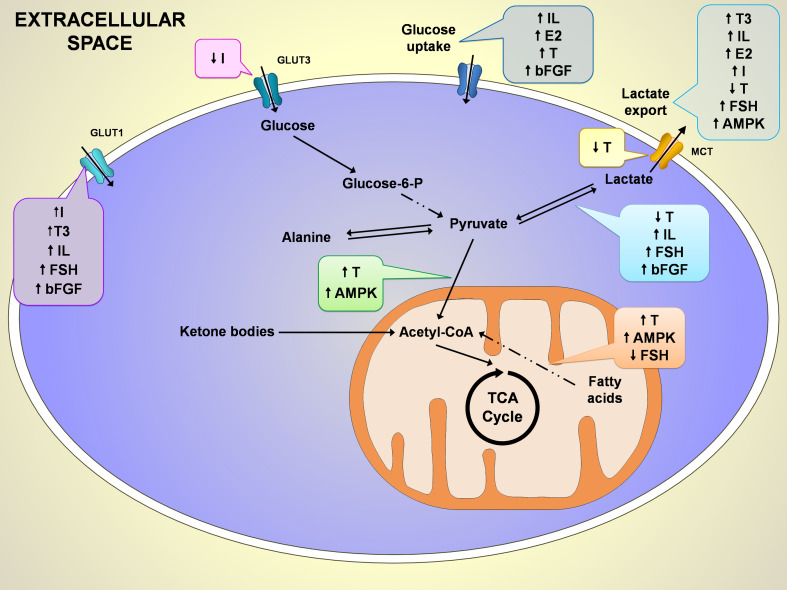
Fig. 3.
Schematic representation of the hormonal control on some of the most important metabolic pathways of Sertoli cells (SCs) metabolism. So far, the hormonal control described is mainly related to glucose and fatty acids metabolism. While in glucose metabolism the described effects result in stimulation of glucose uptake, fatty acids metabolism is stimulated by testosterone (T) but inhibited by follicle-stimulating hormone (FSH). Importantly, the conversion of pyruvate to lactate by lactate dehydrogenase and the lactate export by monocarboxylate transporters seem to be a hot spot for hormonal direct or indirect action. The known effects of hormonal and signaling control on SCs metabolism is signed as ↑up arrow for stimulation and ↓down arrow for inhibition. AMPK 5′ adenosine monophosphate-activated protein kinase, bFGF basic fibroblast growth factor, E 2 17β-estradiol, FSH follicle-stimulating hormone, GLUT glucose transporter, I insulin, IL interleukin, MCT monocarboxylate transporter, T testosterone, T3 triiodothyronine
In rat [38] and human [37] cultured SCs, the glucose consumption is stimulated after DHT treatment. Interestingly, that increase in glucose consumption is not followed by an increase in lactate production thus, it is probably related with a reduced transport of lactate to the extracellular medium, via MCTs, or a decrease of the conversion of pyruvate into lactate catalyzed by lactate dehydrogenase isozyme (LDHA) [38]. In fact, the mRNA levels of MCT4 decrease after 50-h treatment with DHT, which is obviously concomitant with less lactate production and export. The decrease of lactate production by DHT-treated cells can also be a consequence of a lower cellular conversion of pyruvate to lactate catalyzed by LDH A, as the mRNA levels of LDH A were found to be decreased. Interestingly, non-treated cells consumed more glucose in the first 6 h of incubation, but this consumption was highly decreased after 50 h, suggesting that SCs can change their metabolism according to the available substrates and that androgens, known to have a major role on SCs physiological functioning and male fertility [93, 104], are also crucial for the regular metabolic functioning of these testicular cells. These in vitro observations are very difficult to follow under in vivo conditions but present clear evidences that SCs possess a certain degree of metabolic plasticity, which can be very useful for infertility treatments targeting metabolic therapies. It is not entirely expected that in vitro hormonal control of DHT may be overlapping with the in vivo situation, but knowing that DHT is capable of inhibiting the lactate production/export in both rat [38] and human SCs [37] give us clear evidences that androgens are key regulators of lactate production and thus of spermatogenesis (Fig. 3).
SCs from rats exposed to flutamide, an antagonist of the ARs, also presented a decrease in lactate production [105]. However, the use of the non-hydrolysable ARs agonist R1881 to stimulate isolated SCs from 15-day-old rats showed an increase of LDH A gene expression within 6 h of stimulation [106]. Nevertheless, R1881 does not exactly duplicates the effects of T or DHT [107] and it may exhibit some features that can be called “side-effects” [108] thus explaining why the stimulation of R1881 is not able to reproduce the same kind of results of DHT in LDH expression levels. Also, in DHT-treated rat [38] and human [37] cultured SCs, a decrease in alanine and lactate production was reported, suggesting that DHT is responsible for signals that allow SCs to switch their normal glucose metabolism to the Krebs cycle and in this condition cells become metabolically more efficient although lactate production can be compromised (Fig. 3). In accordance with this hypothesis tested in vitro, is an in vivo study in the rhesus monkey epididymis reporting succinate dehydrogenase and malate dehydrogenase activity stimulation by DHT [109].
Androgen action in SCs in culture is under constant debate and it remains unclear whether these cells lose their responsiveness to this hormone. When looking for androgen effects in SCs, it should be noted that some androgen responsive genes, such as Pem, a homeobox gene that responds with an up to 50-fold increase in expression in response to androgens [110], is lost 24 h after SCs isolation [111]. These phenomena can be either associated with the fact that most of these studies use immature SCs, which may be less androgen-responsive than adult cells [112], or with a low level of ARs expression resulting in poor androgen responsiveness. Nevertheless, differences in experimental conditions may improve androgen responsiveness and some endogenous genes may maintain responsive to androgens [113]. Also, there are no indications that the ARs in immature SCs would be physically or functionally defective and microarray experiments reported that cultured SCs respond to androgens with a moderate but consistent modulation of a few endogenous target genes [113].
The mechanisms by which androgens modulate SCs metabolism are not fully understood but it is clear that they are responsible for signals that can change the preferred substrate pathways and modulate one of the main functions of SCs: lactate production. Further works will be needed to clarify the role of androgens on SCs metabolism and thus, in regulating spermatogenesis.
Estrogens are key mediators of Sertoli cell metabolism
The involvement of estrogen in the initiation and maintenance of testicular function and spermatogenesis is a very complex subject due to estrogen involvement in numerous functions of male reproductive physiology including in the hypothalamus–pituitary–testis axis, Leydig cells, SCs, germ cells, and epididymis. There is a high concentration of estrogen in rete testis fluid [114] and, the concentration of estrogen in rat epididymis is about 25 times the level measured in the plasma [115, 116], suggesting that estrogen has a central role in the control of spermatogenesis. Furthermore, some studies led to the assertion that SC is the major source of estrogens in the immature individuals although Leydig cells synthesize these hormones in the adults [117]. The estrogens are produced within the testes by the aromatization of T and they can disrupt the development of fetal Leydig cells, enhance spermatogenesis by inhibiting apoptosis of the postmeiotic spermatogenic cells, affect proliferation and differentiation of gonocytes and spermatogonia and inhibit T production by Leydig cells [118–120].
Two subtypes of estrogen receptors (ERs), ERα and ERβ, have been described so far in the human male reproductive tract [121–123] but the expression sites of ERα in the testis are controversial. Some studies refer that ERα mainly locates in the efferent ductule epithelium, SCs and Leydig cells in human testis while ERβ locates mainly in somatic cells and/or primary spermatocytes [124–126]. Other studies show no ERα expression in human testis [126], although in human immature germ cells and rat testis two ERα isoforms (66 and 46 kDa) have been found [127, 128]. More recently, ERα mRNA and protein expression were described in human testicular biopsies [123]. Nevertheless, this controversy is not extended to the importance of ERs to spermatogenesis. For instance, ERα is known to control the reabsorption of the fluid of the seminiferous tubules just before the spermatozoa enter into the epididymis [129]. Also, the inhibitory effects of estrogens on testicular steroidogenesis are mediated by ERα [130] and the induction of spermatogenesis and the increased serum FSH levels in hpg mice by E2 are dependent on an ERα mechanism [131]. Given that it is accepted that ERα is expressed throughout the hypothalamic–pituitary–testicular axis, the increase in FSH by E2 suggests both a direct and indirect pathway for estrogenic stimulation of spermatogenesis [132].
Importantly, estrogens are involved in the function of mature spermatozoa and the incubation of human spermatozoa with estrogens is known to stimulate sperm functions such as motility [133], lactate production [134], and the metabolization of several substrates [135]. Estrogens are also able to directly induce the spermatogenic cell apoptosis by the FasL pathway and the cytochrome c release from mitochondria [136]. Recently, the role of E2 in human [37] and rat SCs [38] metabolism was investigated and E2-treated cells produced high amounts of alanine. This fact is remarkable as the appearance of high alanine content can be associated with a reduced redox cytosolic state [37, 38] (Fig. 3). It is true that these in vitro observations may not represent exactly an in vivo situation but the isolated effect of E2 on SCs metabolism presents clear evidences that under non-physiologic conditions, such as those occurring in pathologies associated with E2 dysfunction, the estrogen concentration that targets SCs is crucial to the development of a normal spermatogenesis. Also, these results go a step further to identify key mechanisms by which E2 regulates metabolite production/export in SCs. Although little is known about these control mechanisms on SC metabolism in vivo, there are growing evidences that point to a close relation between estrogens and SC metabolism affecting germ cell development.
FSH exerts metabolic control in Sertoli cell
FSH is secreted by the pituitary and acts via specific G-coupled receptors that are exclusively located on SCs [137]. FSH importance to the reproductive potential is very well known as FSH controls SCs proliferation during the perinatal and/or pubertal period and thus determines the adult spermatogenic capacity. The FSH molecular mechanism of action involves the adenylyl cyclase/cAMP pathway, via activation of a G protein and FSH increases phosphorylated protein kinase B (p-PKB) levels in a phosphatidylinositol 3-kinase (PI3K)-dependent manner, a phenomenon closely related to rat SCs function [138]. This interaction between FSH and PI3K is very important as PI3K is a key enzyme involved in the regulation of several biological responses including mitogenesis, oxidative burst and glucose uptake [139] and there have also been reported close associations between cAMP, PI3K and PKB signaling pathways in different cell types, such as thyroid and granulosa cells [140].
In fact, although FSH exerts a metabolic control over SCs, little is known about these mechanisms. In SCs from immature rat testes, amino acid accumulation is stimulated by FSH [141] and FSH stimulation of lactate production and LDH activity are partially blocked when the cells are pre-incubated with Wortmannin, a PI3K inhibitor, which suggests that PI3K/PKB signaling pathway is a key step in the FSH regulation of those processes [138]. The Wortmannin inhibition of glucose uptake mediated by FSH stimulation points toward an intervention of a PI3K-dependent pathway in the glucose transport mechanism through the plasma membrane in SCs [138]. Interestingly, the stimulation of glucose uptake by FSH was not altered in the presence of the PKA inhibitor H89 suggesting that glucose transport in SCs is not dependent of the cAMP/PKA pathway [138] (Fig. 3). Also, as described earlier, in vivo studies in FSHRKO/SCARKO rats reported that FSH and androgens have additive effects in the regulation of spermatogenesis and SCs activity, thus explaining why is so difficult to isolate FSH effects on SCs metabolism.
Insulin controls Sertoli cell metabolism
It has been reported that insulin increases the rate of in vitro lactate production by SCs. This effect was observed with low insulin concentrations [142] and described as mediated by insulin-specific receptors existing in SCs [143]. Others have also reported that micromolar concentrations of insulin are responsible for a small stimulatory effect not only on DNA and protein synthesis, but also in lactate production by SCs from 2-week-old rats [144]. These were the first clear evidences that insulin could control SC metabolism. Recently, it was reported that in in vitro cultured human SCs the first hours of insulin-deprivation are critical [145]. Insulin-deprived SCs presented altered glucose consumption and lactate secretion, as well as altered expression of metabolism-associated genes involved in lactate production and export [145]. Noteworthy was the adaptation exhibited by these cells on the glucose uptake, by differentially modulating the expression of GLUT1 and GLUT3 [145]. This hormonal regulation of GLUT1 and GLUT3 has been observed in several cell types, as in Ishikawa endometrial cancer cells, where GLUT1 is up-regulated by estrogen and progesterone, while the protein expression levels of GLUT3 are not affected [146]. A similar effect was also observed in ovarian cells stimulated with interleukin 1 (IL-1) where both GLUT1 and GLUT3 protein levels increase in response to insulin [147].
After 48 h of insulin deprivation, human SCs presented a significant decrease in MCT4 and LDH A mRNA levels, indicating that lactate interconversion from pyruvate and the export of lactate are modulated by insulin (Fig. 3). This was concomitant with a lower lactate concentration in extracellular media of insulin-deprived cells [145] and opens new, important insights of the mechanisms by which insulin can regulate the glucose metabolism in SCs. Although insulin regulatory signaling mechanisms in SC metabolism are still unknown, there are clear evidences that insulin may be crucial for a normal metabolism of these cells and thus, for spermatogenesis. These in vitro studies with insulin-deprivation or insulin-stimulation are crucial to elucidate the effects of pathological conditions, such as diabetes, that are intimately related with insulin dysfunction.
Thyroid hormones effects on Sertoli cell metabolism
Thyroid hormones (TH) are essential to postnatal growth and development and are known to play a crucial role in the regulation of energy metabolism in several tissues and organs. In the testis, TH control the early development although their role during adulthood remains largely unknown [148]. Early clinical observations showed that TH are crucial for a normal spermatogenesis. Hyperthyroid men are reported to have oligospermia and loss of spermatozoa [149] and males with thyroid deregulation usually develop erectile dysfunction and are associated with a loss of libido or impotence [150, 151]. The effect of TH in SCs proliferation is well studied and the presence of TH receptors in SCs is well characterized [152]. In this regard, it was reported that the injection of triiodothyronine (T3) to neonatal rats completely stopped SCs proliferation [153] and this control of T3 over SCs proliferation was suggested to be mediated through aromatase activity and estradiol [154] and/or through specific cyclin-dependent kinase inhibitors [155]. The interaction between TH and estradiol is under debate but it is known that TH modulate ER content in SCs [156] thus suggesting that both hormones may have a synergistic role.
The TH effects in SCs go far beyond the control of these cells proliferation, as they are also known to increase insulin-like growth factor-I (IGF-I) [157], inhibin [153], and to inhibit androgen-binding protein (ABP) production [158] and testosterone metabolism [159]. Concerning the effect of TH in SCs metabolism, it was described that the addition of physiological concentrations (1 nM) of T3 to the culture medium of rat SCs stimulated protein synthesis and lactate production [159]. Besides, T3 is also known to stimulate GLUT1 mRNA synthesis [61] and both the MCTs and the anion-transporting polypeptides demonstrate a high specificity towards TH [160, 161], suggesting that TH are also able to modulate SCs metabolism in its most relevant function: lactate production. Although the mechanisms are not yet known, T3 has been reported to play a role in amino acids metabolism, as it stimulates amino acids accumulation in immature rat testes [162].
The clinical effects of disturbance in the normal euthyroid state and the later identification of functional thyroid receptors in SCs, clearly indicated that TH affect the morphological and functional development of the testis. Several studies have reported that TH control is essential to spermatogenesis, sperm motility and ultimately fertility. Although most of the mechanisms remain obscure, it is undeniable that TH contribute to the hormonal control of SCs metabolism.
bFGF and interleukins as possible points for hormonal control of metabolism in Sertoli cell
There are a number of growth factors, paracrine and autocrine mediators affecting SC and germ cell function but there are few studies versed on the control of such factors in SC metabolism. In fact, their role in the overall signaling for lactate production and germ cells development is far from being disclosed. For instance, basic fibroblast growth factor (bFGF), an intratesticular regulator of numerous crucial cellular processes implicated in the normal functioning and maintenance of spermatogenesis [163], exerts its effects by binding to the tyrosine kinase family receptors and is known to have an important role as intermediary in SC metabolism. bFGF is expressed in germ cells although other cells in the testis, such as SCs, also express it [164, 165]. Cultured SCs express bFGF receptors [166] and bFGF modulates several processes such as transferrin, E2 and lactate secretion [163, 165], glucose uptake, LDH activity, GLUT1 and LDH A mRNA levels [18], and the number of FSH receptors [167] thus assuming an important role on SC metabolic control. The mode of action of bFGF on SC metabolism is not fully understood, but the short-term bFGF stimulation of glucose involves an increase in glucose transport rate, LDH activity and GLUT1 and LDH A mRNA levels (Fig. 3), in order to increase lactate production [18]. The mechanism by which bFGF regulates transferrin production, LDH activity and glucose transport into the cell is very distinct. The first two involve the MAPK pathway while glucose transport is mediated by a PI3K/PKB pathway. Although these results were attained in vitro, they elucidate that, at least in SCs, there are different signals elicited by bFGF that regulate distinct biological responses [168].
Other important point for hormonal control in SC metabolism is IL-1, which is a multifunctional cytokine that exists in two isoforms, IL-1α and IL-1β [169]. The functions of IL-1 are very important and within the seminiferous tubules it is able to modulate SCs functions [170–172], differentiation of spermatogonia and preleptotene spermatocytes and DNA synthesis [173, 174], although the last steps of spermiogenesis do not require direct action of IL-1 and the only germ cell types that do not express IL-1 receptor type I (IL-IRI) mRNA are the elongating and elongated spermatids [175].
The cytokines belong to a set of local testicular regulators that may be involved in the signaling of the metabolic cooperation existing between SCs and germ cells. It has been suggested that spermatogenesis uses the redistribution of LDH isoforms, mainly through an increase in LDH A expression and the activity of LDH A4, under IL-1α control, as a key mechanism to enhance lactate production by SCs [176]. In fact, IL-1α produced in the seminiferous tubules by SCs [177, 178] and germ cells [179] has a direct inhibitory action on spermatogonia development and Leydig cell steroidogenesis in vitro [180–182]. Leydig cells and interstitial macrophages produce IL-1β [183, 184] and IL-1 receptor type I and II are constitutively expressed by SCs [175]. IL-1β stimulates LDH activity, glucose uptake and, consequently, lactate production in SCs [19, 176] (Fig. 3) and also regulates several processes in SC such as GTP activity [185] and proliferation [186], IL-6 expression [187], transferrin and gelatinase A secretion [188, 189] and E2 production [170], among other functions.
Finally, bFGF, IL-1β and FSH regulate GLUT1 expression on SC (Fig. 3). These cells express GLUT1 and GLUT3 throughout pubertal development but only GLUT1 is regulated by bFGF, IL1β and FSH [62], which points towards a crucial role for hormonal control on GLUT1 function and ultimately on spermatogenesis. Although most of these studies were performed in vitro and the in vivo situation is expected to be different, the control exerted by these growth factors and these paracrine and autocrine mediators of SC metabolism, is a first step to understand the signals that hormones can exert in SCs.
Activation of AMPK in Sertoli cells is related to energy supply to germ cells
AMPK is broadly distributed in testicular cells and is present in the SC [66]. The action of AMPK in eukaryotic cells is well known and when cells are subjected to metabolic stresses, such as glucose and/or nutrient deprivation, specific metabolic adaptations are triggered and the intracellular signaling involved in the responses are usually AMPK-dependent [66] thus AMPK is sensitive to metabolic stress and, interestingly, becomes activated when the ratio AMP/ATP increases. Once activated, AMPK suppresses the biosynthesis of fatty acids and cholesterol and activates ATP-generating catabolic pathways, such as glycolysis and fatty acid oxidation, regulating the cellular ATP levels [190] (Fig. 3). Nevertheless, other intracellular signaling systems, such as the stress-activated p38 MAPK [191] and the survival pathway PI3K/PKB [192], may be involved in the metabolic response to nutrient deprivation.
AMPK pathway is activated under glucose deprivation and since the pharmacological activation of AMPK is followed by a decrease in GLUT3 expression, it has been suggested that the downregulation of GLUT3 is the result of AMPK activation [193]. In SCs, the activation of AMPK by 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) promotes a dose- and time-dependent increase in lactate secretion, increases glucose uptake, GLUT1 and MCT4 levels and decreases MCT1 expression [193] (Fig. 3). Interestingly, with the increase on lactate secretion, there is an increase in the AMP/ATP ratio directly linked with the activation of AMPK in SCs and thus, the maintenance of the energy supply to germ cells depends upon these signals [193] and as expected, the knockout male mice for an upstream activator of the AMPK, the LKB1 mice, do not possess these signals and are infertile [194]. It is interesting that MCT1 mRNA levels are downregulated by AICAR treatment, while MCT4 mRNA levels are upregulated leading to the postulation that MCT1 has a role in lactate import from the extracellular milieu. On the other hand, MCT4, which has a much lower affinity for lactate than MCT1, has been proposed to serve as lactate exporter so, the manner in which both transporters are regulated in SCs by long-term AMPK activation may reflect a situation of increased lactate export and decreased lactate recapture from the extracellular milieu leading to adequate lactate levels made available to germ cells.
Conclusions
Successful spermatogenesis is in the basis of male fertility and due to SCs role as supporters and feeders of developing germ cells, these cells certainly merits a special attention in this process. The understanding of their energy metabolism, the preferred substrates, the metabolic sub-products produced and the mechanism of adaptation to different conditions are primary steps to identify and promote new therapeutic approaches against several disorders that result in infertility. The characterization of hormonal control of SCs metabolism is crucial to understand how the endocrine system, particularly the hypothalamo-pituitary–testis axis, can exert a tied control on male physiology and reproductive potential.
Much of what is known about the molecular regulation and function of SCs has been inferred from in vitro studies using immature SCs and this should be taken in consideration as adult and immature cells differ in significant ways. They have structural differences [195] and alterations in the expression of some factors such as cathepsin and transferrin [171]. Also, immature SCs are more responsive to FSH and less responsive to androgens [196]. Interestingly, SCs isolated from aged rat testis, also respond differently to germ cells in co-culture studies [197]. SCs in culture may also lose their differentiated function thus making it difficult to interpret the results. All these considerations should be taken in account when looking to a major finding in SC function and hormonal response.
Most of the studies focused on SCs metabolic pathways are done in in vitro conditions and present some important limitations, as already discussed. Nevertheless, there is a growing number of studies, especially concerning the hormonal control of spermatogenesis and the role of hormone receptors, which take advantage of selective knockout technologies in SCs. These knockout models may be able to provide a more detailed and mechanistic assessment of SCs metabolic pathways. Nevertheless, much remains unexplored concerning the use of these selective knockout rodent models, especially regarding the role of some enzymes and transporters in in vivo conditions. In the next years new challenges and answers are expected as the use of these models is becoming more widespread.
Further studies will be needed to extend the knowledge over the activated pathways and signaling events behind the hormonal response of SCs and the metabolic adaptations that occur. It is unquestionable that SC metabolism is a key step for germ cell development and the hormonal control of these processes is crucial for a normal spermatogenesis.
Acknowledgments
We would like to acknowledge the authors that positively answered our call for a paper copy or some punctual doubts that we had about technical or theoretical data interpretation. This work was supported by the Portuguese “Fundação para a Ciência e a Tecnologia”—FCT (PTDC/QUI-BIQ/121446/2010) co-funded by FEDER via Programa Operacional Factores de Competitividade—COMPETE/QREN. MG Alves (SFRH/BPD/80451/2011 L Rato (SFRH/BD/72733/2010) were financed by FCT. P.F. Oliveira was financed by FCT through FSE and POPH funds (Programa Ciência 2008).
Abbreviations
- [1-14C]glycine
Glycine enriched with 14C in carbon 1
- ABP
Androgen-binding protein
- AICAR
5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside
- AMH
Anti-Müllerian hormone
- cAMP
Cyclic adenosine monophosphate
- AMPK
5′ Adenosine monophosphate-activated protein kinase
- AR
Androgen receptor
- ARKO
Androgen receptor knockout
- ATP
Adenosine triphosphate
- bFGF
Basic fibroblast growth factor
- BTB
Blood–testis barrier
- DHT
5α-Dihydrotestosterone
- E2
17β-estradiol
- ER
Estrogen receptor
- FSH
Follicle-stimulating hormone
- gGTP
Guanosine triphosphate
- GLUTs
Glucose transporters
- GnRH
Gonadotropin-releasing hormone
- IGF-I
Insulin-like growth factor-I
- IL-1
Interleukin-1
- LDH
Lactate dehydrogenase
- LH
Luteinizing hormone
- MCTs
Monocarboxylate transporters
- NADPH
Nicotinamide adenine dinucleotide phosphate-oxidase
- PI3K
Phosphatidylinositol 3-kinase
- PKB
Protein kinase b
- PTM
Peritubular myoid cells
- SCs
Sertoli cells
- T
Testosterone
- T3
Triiodothyronine (T3)
- TH
Thyroid hormones
- TNF
Tumor necrosis factor
Contributor Information
Marco G. Alves, Email: alvesmarc@gmail.com, Email: alvesmar@gmail.com
Pedro F. Oliveira, Email: pfobox@gmail.com
References
- 1.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125(6):769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 2.Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130(1):15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- 3.Rato L, Socorro S, Cavaco JE, Oliveira PF. Tubular fluid secretion in the seminiferous epithelium: ion transporters and aquaporins in Sertoli cells. J Membr Biol. 2010;236(2):215–224. doi: 10.1007/s00232-010-9294-x. [DOI] [PubMed] [Google Scholar]
- 4.Fritz IB, Rommerts FG, Louis BG, Dorrington JH. Regulation by FSH and dibutyryl cyclic AMP of the formation of androgen-binding protein in Sertoli cell-enriched cultures. J Reprod Fertil. 1976;46(1):17–24. doi: 10.1530/jrf.0.0460017. [DOI] [PubMed] [Google Scholar]
- 5.Skinner MK, Griswold MD. Sertoli cells synthesize and secrete transferrin-like protein. J Biol Chem. 1980;255(20):9523–9525. [PubMed] [Google Scholar]
- 6.Marzowski J, Sylvester SR, Gilmont RR, Griswold MD. Isolation and characterization of Sertoli cell plasma membranes and associated plasminogen activator activity. Biol Reprod. 1985;32(5):1237–1245. doi: 10.1095/biolreprod32.5.1237. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien DA, Gabel CA, Eddy EM. Mouse Sertoli cells secrete mannose 6-phosphate containing glycoproteins that are endocytosed by spermatogenic cells. Biol Reprod. 1993;49(5):1055–1065. doi: 10.1095/biolreprod49.5.1055. [DOI] [PubMed] [Google Scholar]
- 8.Elkington JS, Fritz IB. Regulation of sulfoprotein synthesis by rat Sertoli cells in culture. Endocrinology. 1980;107(4):970–976. doi: 10.1210/endo-107-4-970. [DOI] [PubMed] [Google Scholar]
- 9.Robinson R, Fritz IB. Myoinositol biosynthesis by Sertoli cells, and levels of myoinositol biosynthetic enzymes in testis and epididymis. Can J Biochem. 1979;57(6):962–967. doi: 10.1139/o79-117. [DOI] [PubMed] [Google Scholar]
- 10.Setchell BP, Scott TW, Voglmayr JK, Waites GM. Characteristics of testicular spermatozoa and the fluid which transports them into the epididymis. Biol Reprod. 1969;1(Suppl 1):40–66. doi: 10.1095/biolreprod1.Supplement_1.40. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira PF, Sousa M, Barros A, Moura T, Rebelo da Costa A. Intracellular pH regulation in human Sertoli cells: role of membrane transporters. Reproduction. 2009;137(2):353–359. doi: 10.1530/REP-08-0363. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira PF, Sousa M, Barros A, Moura T, Rebelo da Costa A. Membrane transporters and cytoplasmatic pH regulation on bovine Sertoli cells. J Membr Biol. 2009;227(1):49–55. doi: 10.1007/s00232-008-9139-z. [DOI] [PubMed] [Google Scholar]
- 13.Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am J Anat. 1952;90(2):167–215. doi: 10.1002/aja.1000900202. [DOI] [PubMed] [Google Scholar]
- 14.Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 15.Mruk DD, Cheng CY. Sertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25(5):747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 16.Griswold M. The central role of Sertoli cells in spermatogenesis. London: Academic Press; 1998. pp. 411–416. [DOI] [PubMed] [Google Scholar]
- 17.Griswold M, McLean D. The Sertoli cell. In: Neill J, editor. Knobil and Neill’s physiology of reproduction. San Diego: Elsevier; 2006. pp. 949–975. [Google Scholar]
- 18.Riera MF, Meroni SB, Schteingart HF, Pellizzari EH, Cigorraga SB. Regulation of lactate production and glucose transport as well as of glucose transporter 1 and lactate dehydrogenase A mRNA levels by basic fibroblast growth factor in rat Sertoli cells. J Endocrinol. 2002;173(2):335–343. doi: 10.1677/joe.0.1730335. [DOI] [PubMed] [Google Scholar]
- 19.Riera MF, Meroni SB, Gomez GE, Schteingart HF, Pellizzari EH, Cigorraga SB. Regulation of lactate production by FSH, iL1beta, and TNFalpha in rat Sertoli cells. Gen Comp Endocrinol. 2001;122(1):88–97. doi: 10.1006/gcen.2001.7619. [DOI] [PubMed] [Google Scholar]
- 20.Erkkila K, Aito H, Aalto K, Pentikainen V, Dunkel L. Lactate inhibits germ cell apoptosis in the human testis. Mol Hum Reprod. 2002;8(2):109. doi: 10.1093/molehr/8.2.109. [DOI] [PubMed] [Google Scholar]
- 21.Sofikitis N, Giotitsas N, Tsounapi P, Baltogiannis D, Giannakis D, Pardalidis N. Hormonal regulation of spermatogenesis and spermiogenesis. J Steroid Biochem Mol Biol. 2008;109(3–5):323–330. doi: 10.1016/j.jsbmb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6(7):380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sokol RZ. Endocrinology of male infertility: evaluation and treatment. Semin Reprod Med. 2009;27(2):149–158. doi: 10.1055/s-0029-1202303. [DOI] [PubMed] [Google Scholar]
- 24.Flier JS, Underhill LH, Marshall JC, Kelch RP. Gonadotropin-releasing hormone: role of pulsatile secretion in the regulation of reproduction. N Engl J Med. 1986;315(23):1459–1468. doi: 10.1056/NEJM198612043152306. [DOI] [PubMed] [Google Scholar]
- 25.Tilbrook A, Clarke I. Negative feedback regulation of the secretion and actions of gonadotropin-releasing hormone in males. Biol Reprod. 2001;64(3):735. doi: 10.1095/biolreprod64.3.735. [DOI] [PubMed] [Google Scholar]
- 26.Weber JE, Russell LD, Wong V, Peterson RN. Three-dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli–Sertoli and Sertoli–germ-cell relationships. Am J Anat. 1983;167(2):163–179. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- 27.Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982;203(4):485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- 28.Nistal M, Abaurrea MA, Paniagua R. Morphological and histometric study on the human Sertoli cell from birth to the onset of puberty. J Anat. 1982;134(Pt 2):351–363. [PMC free article] [PubMed] [Google Scholar]
- 29.Cortes D, Muller J, Skakkebaek NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int J Androl. 1987;10(4):589–596. doi: 10.1111/j.1365-2605.1987.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 30.Sinha Hikim AP, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod. 1999;4(1):38–47. doi: 10.1530/ror.0.0040038. [DOI] [PubMed] [Google Scholar]
- 31.Tripathi R, Mishra DP, Shaha C. Male germ cell development: turning on the apoptotic pathways. J Reprod Immunol. 2009;83(1–2):31–35. doi: 10.1016/j.jri.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Mancine RE, Penhos JC, Izquierdo IA, Heinrich JJ. Effects of acute hypoglycemia on rat testis. Proc Soc Exp Biol Med. 1960;104:699–702. doi: 10.3181/00379727-104-25957. [DOI] [PubMed] [Google Scholar]
- 33.Zysk JR, Bushway AA, Whistler RL, Carlton WW. Temporary sterility produced in male mice by 5-thio-d-glucose. J Reprod Fertil. 1975;45(1):69–72. doi: 10.1530/jrf.0.0450069. [DOI] [PubMed] [Google Scholar]
- 34.Robinson R, Fritz IB. Metabolism of glucose by Sertoli cells in culture. Biol Reprod. 1981;24(5):1032–1041. doi: 10.1095/biolreprod24.5.1032. [DOI] [PubMed] [Google Scholar]
- 35.Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, Oliveira PF. Metabolic regulation is important for spermatogenesis. Nat Rev Urol. 2012;9:330–338. doi: 10.1038/nrurol.2012.77. [DOI] [PubMed] [Google Scholar]
- 36.Jutte NH, Grootegoed JA, Rommerts FF, van der Molen HJ. Exogenous lactate is essential for metabolic activities in isolated rat spermatocytes and spermatids. J Reprod Fertil. 1981;62(2):399–405. doi: 10.1530/jrf.0.0620399. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira PF, Alves MG, Rato L, Silva J, Sa R, Barros A, Sousa M, Carvalho RA, Cavaco JE, Socorro S. Influence of 5α-dihydrotestosterone and 17β-estradiol on human Sertoli cells metabolism. Int J Androl. 2011;34(6pt2):e612–e620. doi: 10.1111/j.1365-2605.2011.01205.x. [DOI] [PubMed] [Google Scholar]
- 38.Rato L, Alves MG, Socorro S, Carvalho RA, Cavaco JE, Oliveira PF. Metabolic modulation induced by oestradiol and DHT in immature rat Sertoli cells cultured in vitro. Biosci Rep. 2012;32(1):61–69. doi: 10.1042/BSR20110030. [DOI] [PubMed] [Google Scholar]
- 39.Mita M, Hall PF. Metabolism of round spermatids from rats: lactate as the preferred substrate. Biol Reprod. 1982;26(3):445–455. doi: 10.1095/biolreprod26.3.445. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura M, Okinaga S, Arai K. Metabolism of round spermatids: evidence that lactate is preferred substrate. Am J Physiol. 1984;247(2 Pt 1):E234–E242. doi: 10.1152/ajpendo.1984.247.2.E234. [DOI] [PubMed] [Google Scholar]
- 41.Jutte NH, Jansen R, Grootegoed JA, Rommerts FF, Clausen OP, van der Molen HJ. Regulation of survival of rat pachytene spermatocytes by lactate supply from Sertoli cells. J Reprod Fertil. 1982;65(2):431–438. doi: 10.1530/jrf.0.0650431. [DOI] [PubMed] [Google Scholar]
- 42.Jutte NH, Jansen R, Grootegoed JA, Rommerts FF, van der Molen HJ. FSH stimulation of the production of pyruvate and lactate by rat Sertoli cells may be involved in hormonal regulation of spermatogenesis. J Reprod Fertil. 1983;68(1):219–226. doi: 10.1530/jrf.0.0680219. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura M, Hino A, Yasumasu I, Kato J. Stimulation of protein synthesis in round spermatids from rat testes by lactate. J Biochem. 1981;89(4):1309–1315. [PubMed] [Google Scholar]
- 44.Grootegoed JA, Oonk RB, Jansen R, van der Molen HJ. Metabolism of radiolabelled energy-yielding substrates by rat Sertoli cells. J Reprod Fertil. 1986;77(1):109–118. doi: 10.1530/jrf.0.0770109. [DOI] [PubMed] [Google Scholar]
- 45.Courtens JL, Ploen L. Improvement of spermatogenesis in adult cryptorchid rat testis by intratesticular infusion of lactate. Biol Reprod. 1999;61(1):154–161. doi: 10.1095/biolreprod61.1.154. [DOI] [PubMed] [Google Scholar]
- 46.Miki K. Energy metabolism and sperm function. Soc Reprod Fertil Suppl. 2007;65:309–325. [PubMed] [Google Scholar]
- 47.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O’Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA. 2004;101(47):16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams AC, Ford WC. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl. 2001;22(4):680–695. [PubMed] [Google Scholar]
- 49.Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, Diaz-Cueto L, Storey BT, Kopf GS, Moss SB. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem. 2001;276(10):7630–7636. doi: 10.1074/jbc.M006217200. [DOI] [PubMed] [Google Scholar]
- 50.Urner F, Leppens-Luisier G, Sakkas D. Protein tyrosine phosphorylation in sperm during gamete interaction in the mouse: the influence of glucose. Biol Reprod. 2001;64(5):1350–1357. doi: 10.1095/biolreprod64.5.1350. [DOI] [PubMed] [Google Scholar]
- 51.Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71(2):540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 52.Urner F, Sakkas D. Glucose participates in sperm–oocyte fusion in the mouse. Biol Reprod. 1996;55(4):917–922. doi: 10.1095/biolreprod55.4.917. [DOI] [PubMed] [Google Scholar]
- 53.Bone W, Jones NG, Kamp G, Yeung CH, Cooper TG. Effect of ornidazole on fertility of male rats: inhibition of a glycolysis-related motility pattern and zona binding required for fertilization in vitro. J Reprod Fertil. 2000;118(1):127–135. doi: 10.1530/reprod/118.1.127. [DOI] [PubMed] [Google Scholar]
- 54.Kreisberg RA. Lactate homeostasis and lactic acidosis. Ann Intern Med. 1980;92(2 Part 1):227. doi: 10.7326/0003-4819-92-2-227. [DOI] [PubMed] [Google Scholar]
- 55.Coonrod S, Vitale A, Duan C, Bristol-Gould S, Herr J, Goldberg E. Testis-specific lactate dehydrogenase (LDH-C4; Ldh3) in murine oocytes and preimplantation embryos. J Androl. 2006;27(4):502–509. doi: 10.2164/jandrol.05185. [DOI] [PubMed] [Google Scholar]
- 56.Goldberg E. Reproductive implications of LDH-C4 and other testis-specific isozymes. Exp Clin Immunogenet. 1985;2(2):120–124. [PubMed] [Google Scholar]
- 57.Li SS, O’Brien DA, Hou EW, Versola J, Rockett DL, Eddy EM. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart), and C (testis) in mouse spermatogenic cells. Biol Reprod. 1989;40(1):173–180. doi: 10.1095/biolreprod40.1.173. [DOI] [PubMed] [Google Scholar]
- 58.Odet F, Duan C, Willis WD, Goulding EH, Kung A, Eddy EM, Goldberg E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol Reprod. 2008;79(1):26–34. doi: 10.1095/biolreprod.108.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Odet F, Gabel SA, Williams J, London RE, Goldberg E, Eddy EM. Lactate dehydrogenase C and energy metabolism in mouse sperm. Biol Reprod. 2011;85(3):556–564. doi: 10.1095/biolreprod.111.091546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klip A, Tsakiridis T, Marette A, Ortiz PA. Regulation of expression of glucose transporters by glucose: a review of studies in vivo and in cell cultures. FASEB J. 1994;8(1):43–53. doi: 10.1096/fasebj.8.1.8299889. [DOI] [PubMed] [Google Scholar]
- 61.Carosa E, Radico C, Giansante N, Rossi S, D’Adamo F, Di Stasi SM, Lenzi A, Jannini EA. Ontogenetic profile and thyroid hormone regulation of type-1 and type-8 glucose transporters in rat Sertoli cells. Int J Androl. 2005;28(2):99–106. doi: 10.1111/j.1365-2605.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 62.Galardo MN, Riera MF, Pellizzari EH, Chemes HE, Venara MC, Cigorraga SB, Meroni SB. Regulation of expression of Sertoli cell glucose transporters 1 and 3 by FSH, IL1 beta, and bFGF at two different time-points in pubertal development. Cell Tissue Res. 2008;334(2):295–304. doi: 10.1007/s00441-008-0656-y. [DOI] [PubMed] [Google Scholar]
- 63.Ulisse S, Jannini EA, Pepe M, De Matteis S, D’Armiento M. Thyroid hormone stimulates glucose transport and GLUT1 mRNA in rat Sertoli cells. Mol Cell Endocrinol. 1992;87(1–3):131–137. doi: 10.1016/0303-7207(92)90241-W. [DOI] [PubMed] [Google Scholar]
- 64.Piroli GG, Grillo CA, Hoskin EK, Znamensky V, Katz EB, Milner TA, McEwen BS, Charron MJ, Reagan LP. Peripheral glucose administration stimulates the translocation of GLUT8 glucose transporter to the endoplasmic reticulum in the rat hippocampus. J Comp Neurol. 2002;452(2):103–114. doi: 10.1002/cne.10368. [DOI] [PubMed] [Google Scholar]
- 65.Reagan LP, Gorovits N, Hoskin EK, Alves SE, Katz EB, Grillo CA, Piroli GG, McEwen BS, Charron MJ. Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proc Natl Acad Sci USA. 2001;98(5):2820–2825. doi: 10.1073/pnas.051629798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riera MF, Galardo MN, Pellizzari EH, Meroni SB, Cigorraga SB. Molecular mechanisms involved in Sertoli cell adaptation to glucose deprivation. Am J Physiol Endocrinol Metab. 2009;297(4):E907–E914. doi: 10.1152/ajpendo.00235.2009. [DOI] [PubMed] [Google Scholar]
- 67.Leiderman B, Mancini RE. Glycogen content in the rat testis from postnatal to adult ages. Endocrinology. 1969;85(3):607–609. doi: 10.1210/endo-85-3-607. [DOI] [PubMed] [Google Scholar]
- 68.Slaughter GR, Means AR. Follicle-stimulating hormone activation of glycogen phosphorylase in the Sertoli cell-enriched rat testis. Endocrinology. 1983;113(4):1476–1485. doi: 10.1210/endo-113-4-1476. [DOI] [PubMed] [Google Scholar]
- 69.Aaronson DS, Iman R, Walsh TJ, Kurhanewicz J, Turek PJ. A novel application of 1H magnetic resonance spectroscopy: non-invasive identification of spermatogenesis in men with non-obstructive azoospermia. Hum Reprod. 2010;25(4):847–852. doi: 10.1093/humrep/dep475. [DOI] [PubMed] [Google Scholar]
- 70.Hurtado de Catalfo GE, De Gomez Dumm INT. Lipid dismetabolism in Leydig and Sertoli cells isolated from streptozotocin-diabetic rats. Int J Biochem Cell Biol. 1998;30(9):1001–1010. doi: 10.1016/S1357-2725(98)00055-7. [DOI] [PubMed] [Google Scholar]
- 71.Xiong W, Wang H, Wu H, Chen Y, Han D. Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction. 2009;137(3):469–479. doi: 10.1530/REP-08-0343. [DOI] [PubMed] [Google Scholar]
- 72.Chung S, Wang SP, Pan L, Mitchell G, Trasler J, Hermo L. Infertility and testicular defects in hormone-sensitive lipase-deficient mice. Endocrinology. 2001;142(10):4272–4281. doi: 10.1210/en.142.10.4272. [DOI] [PubMed] [Google Scholar]
- 73.Jutte NH, Eikvar L, Levy FO, Hansson V. Metabolism of palmitate in cultured rat Sertoli cells. J Reprod Fertil. 1985;73(2):497–503. doi: 10.1530/jrf.0.0730497. [DOI] [PubMed] [Google Scholar]
- 74.Coniglio JG, Sharp J. Biosynthesis of [14C]arachidonic acid from [14C]linoleate in primary cultures of rat Sertoli cells. Lipids. 1989;24(1):84–85. doi: 10.1007/BF02535270. [DOI] [PubMed] [Google Scholar]
- 75.Huynh S, Oulhaj H, Bocquet J, Nouvelot A. Metabolic utilization of linoleate and alpha-linolenate in cultured Sertoli cells. Comp Biochem Physiol B. 1991;99(2):265–270. doi: 10.1016/0305-0491(91)90039-g. [DOI] [PubMed] [Google Scholar]
- 76.Oulhaj H, Huynh S, Nouvelot A. The biosynthesis of polyunsaturated fatty acids by rat Sertoli cells. Comp Biochem Physiol B. 1992;102(4):897–904. doi: 10.1016/0305-0491(92)90099-d. [DOI] [PubMed] [Google Scholar]
- 77.Yount EA, Harris RA. Ketone body and acetate formation from oleate by isolated rat testicular cells. Arch Biochem Biophys. 1982;217(2):503–511. doi: 10.1016/0003-9861(82)90531-8. [DOI] [PubMed] [Google Scholar]
- 78.Alves MG, Socorro S, Silva J, Barros A, Sousa M, Cavaco JE, Oliveira PF. In vitro cultured human Sertoli cells secrete high amounts of acetate that is stimulated by 17β-estradiol and suppressed by insulin deprivation. Biochim Biophys Acta. 2012;1823(8):1389–1394. doi: 10.1016/j.bbamcr.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Kaiser GR, Monteiro SC, Gelain DP, Souza LF, Perry ML, Bernard EA. Metabolism of amino acids by cultured rat Sertoli cells. Metabolism. 2005;54(4):515–521. doi: 10.1016/j.metabol.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 80.Roberts KP, Zirkin BR. Androgen regulation of spermatogenesis in the rat. Ann N Y Acad Sci. 1991;637:90–106. doi: 10.1111/j.1749-6632.1991.tb27303.x. [DOI] [PubMed] [Google Scholar]
- 81.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood–testis barrier. Proc Natl Acad Sci USA. 2005;102(46):16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, Wu CC, di Sant’Agnese PA, deMesy-Bentley KL, Tzeng CR, Chang C. Androgen receptor in Sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147(12):5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- 83.Robaire B, Viger RS. Regulation of epididymal epithelial cell functions. Biol Reprod. 1995;52(2):226–236. doi: 10.1095/biolreprod52.2.226. [DOI] [PubMed] [Google Scholar]
- 84.Lindzey J, Kumar MV, Grossman M, Young C, Tindall DJ. Molecular mechanisms of androgen action. Vitam Horm. 1994;49:383–432. doi: 10.1016/S0083-6729(08)61151-6. [DOI] [PubMed] [Google Scholar]
- 85.Deslypere JP, Young M, Wilson JD, McPhaul MJ. Testosterone and 5 alpha-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol. 1992;88(1–3):15–22. doi: 10.1016/0303-7207(92)90004-P. [DOI] [PubMed] [Google Scholar]
- 86.Singh J, O’Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136(12):5311–5321. doi: 10.1210/en.136.12.5311. [DOI] [PubMed] [Google Scholar]
- 87.O’Shaughnessy PJ, Verhoeven G, De Gendt K, Monteiro A, Abel MH. Direct action through the Sertoli cells is essential for androgen stimulation of spermatogenesis. Endocrinology. 2010;151(5):2343–2348. doi: 10.1210/en.2009-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amory JK, Bremner W. Endocrine regulation of testicular function in men: implications for contraceptive development. Mol Cell Endocrinol. 2001;182(2):175–179. doi: 10.1016/S0303-7207(01)00562-7. [DOI] [PubMed] [Google Scholar]
- 89.Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23(6):870–881. [PubMed] [Google Scholar]
- 90.Lyon MF, Glenister PH, Lamoreux ML. Normal spermatozoa from androgen-resistant germ cells of chimaeric mice and the role of androgen in spermatogenesis. Nature. 1975;258(5536):620–622. doi: 10.1038/258620a0. [DOI] [PubMed] [Google Scholar]
- 91.De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101(5):1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101(18):6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131(2):459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 94.Tsai MY, Yeh SD, Wang RS, Yeh S, Zhang C, Lin HY, Tzeng CR, Chang C. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc Natl Acad Sci USA. 2006;103(50):18975–18980. doi: 10.1073/pnas.0608565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang C, Yeh S, Chen YT, Wu CC, Chuang KH, Lin HY, Wang RS, Chang YJ, Mendis-Handagama C, Hu L, Lardy H, Chang C. Oligozoospermia with normal fertility in male mice lacking the androgen receptor in testis peritubular myoid cells. Proc Natl Acad Sci USA. 2006;103(47):17718–17723. doi: 10.1073/pnas.0608556103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 2009;23(12):4218–4230. doi: 10.1096/fj.09-138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141(5):1795–1803. doi: 10.1210/en.141.5.1795. [DOI] [PubMed] [Google Scholar]
- 98.Krishnamurthy H, Danilovich N, Morales CR, Sairam MR. Qualitative and quantitative decline in spermatogenesis of the follicle-stimulating hormone receptor knockout (FORKO) mouse. Biol Reprod. 2000;62(5):1146–1159. doi: 10.1095/biolreprod62.5.1146. [DOI] [PubMed] [Google Scholar]
- 99.O’Shaughnessy PJ, Monteiro A, Verhoeven G, De Gendt K, Abel MH. Effect of FSH on testicular morphology and spermatogenesis in gonadotropin-deficient hypogonadal mice lacking androgen receptors. Reproduction. 2010;139(1):177–184. doi: 10.1530/REP-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abel MH, Baker PJ, Charlton HM, Monteiro A, Verhoeven G, De Gendt K, Guillou F, O’Shaughnessy PJ. Spermatogenesis and Sertoli cell activity in mice lacking Sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology. 2008;149(7):3279–3285. doi: 10.1210/en.2008-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gorczynska E, Handelsman DJ. Androgens rapidly increase the cytosolic calcium concentration in Sertoli cells. Endocrinology. 1995;136(5):2052–2059. doi: 10.1210/en.136.5.2052. [DOI] [PubMed] [Google Scholar]
- 102.Hurtado de Catalfo GE, de Gomez Dumm IN. Influence of testosterone on polyunsaturated fatty acid biosynthesis in Sertoli cells in culture. Cell Biochem Funct. 2005;23(3):175–180. doi: 10.1002/cbf.1135. [DOI] [PubMed] [Google Scholar]
- 103.Guma FC, Wagner M, Martini LH, Bernard EA. Effect of FSH and insulin on lipogenesis in cultures of Sertoli cells from immature rats. Braz J Med Biol Res. 1997;30(5):591–597. doi: 10.1590/S0100-879X1997000500004. [DOI] [PubMed] [Google Scholar]
- 104.Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol. 2003;1:52. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goddard I, Florin A, Mauduit C, Tabone E, Contard P, Bars R, Chuzel F, Benahmed M. Alteration of lactate production and transport in the adult rat testis exposed in utero to flutamide. Mol Cell Endocrinol. 2003;206(1–2):137–146. doi: 10.1016/S0303-7207(02)00433-1. [DOI] [PubMed] [Google Scholar]
- 106.Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101(30):10919–10924. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baum MJ, Kingsbury PA, Erskine MS. Failure of the synthetic androgen 17 beta-hydroxy-17 alpha-methyl-estra-4,9,11-triene-3-one (methyltrienolone, R1881) to duplicate the activational effect of testosterone on mating in castrated male rats. J Endocrinol. 1987;113(1):15–20. doi: 10.1677/joe.0.1130015. [DOI] [PubMed] [Google Scholar]
- 108.Takeda AN, Pinon GM, Bens M, Fagart J, Rafestin-Oblin ME, Vandewalle A. The synthetic androgen methyltrienolone (r1881) acts as a potent antagonist of the mineralocorticoid receptor. Mol Pharmacol. 2007;71(2):473–482. doi: 10.1124/mol.106.031112. [DOI] [PubMed] [Google Scholar]
- 109.Gupta G, Srivastava A, Setty BS. Androgen–estrogen synergy in the regulation of energy metabolism in epididymis and vas deferens of rhesus monkey. Endocr Res. 1991;17(3–4):383–394. doi: 10.1080/07435809109106815. [DOI] [PubMed] [Google Scholar]
- 110.Barbulescu K, Geserick C, Schuttke I, Schleuning WD, Haendler B. New androgen response elements in the murine pem promoter mediate selective transactivation. Mol Endocrinol. 2001;15(10):1803–1816. doi: 10.1210/me.15.10.1803. [DOI] [PubMed] [Google Scholar]
- 111.Sutton KA, Maiti S, Tribley WA, Lindsey JS, Meistrich ML, Bucana CD, Sanborn BM, Joseph DR, Griswold MD, Cornwall GA, Wilkinson MF. Androgen regulation of the Pem homeodomain gene in mice and rat Sertoli and epididymal cells. J Androl. 1998;19(1):21–30. [PubMed] [Google Scholar]
- 112.Bremner WJ, Millar MR, Sharpe RM, Saunders PT. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology. 1994;135(3):1227–1234. doi: 10.1210/en.135.3.1227. [DOI] [PubMed] [Google Scholar]
- 113.Denolet E, Gendt KD, Swinnen JV, Verrijdt G, Deboel L, Roskams T, Verhoeven G. Transfection with steroid-responsive reporter constructs shows glucocorticoid rather than androgen responsiveness in cultured Sertoli cells. J Steroid Biochem Mol Biol. 2006;98(2–3):164–173. doi: 10.1016/j.jsbmb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 114.Free MJ, Jaffe RA. Collection of rete testis fluid from rats without previous efferent duct ligation. Biol Reprod. 1979;20(2):269–278. doi: 10.1095/biolreprod20.2.269. [DOI] [PubMed] [Google Scholar]
- 115.Kumari GL, Allag IS, Das RP, Datta JK. Regional differences in steroidogenesis and hormone levels in the epididymis and vas deferens of adult rats. Int J Androl. 1980;3(3):267–281. doi: 10.1111/j.1365-2605.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 116.Hess RA. Oestrogen in fluid transport in efferent ducts of the male reproductive tract. Rev Reprod. 2000;5(2):84–92. doi: 10.1530/ror.0.0050084. [DOI] [PubMed] [Google Scholar]
- 117.Carreau S, Delalande C, Galeraud-Denis I. Mammalian sperm quality and aromatase expression. Microsc Res Tech. 2009;72(8):552–557. doi: 10.1002/jemt.20703. [DOI] [PubMed] [Google Scholar]
- 118.O’Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev. 2001;22(3):289–318. doi: 10.1210/er.22.3.289. [DOI] [PubMed] [Google Scholar]
- 119.Li H, Papadopoulos V, Vidic B, Dym M, Culty M. Regulation of rat testis gonocyte proliferation by platelet-derived growth factor and estradiol: identification of signaling mechanisms involved. Endocrinology. 1997;138(3):1289–1298. doi: 10.1210/en.138.3.1289. [DOI] [PubMed] [Google Scholar]
- 120.Miura T, Higuchi M, Ozaki Y, Ohta T, Miura C. Progestin is an essential factor for the initiation of the meiosis in spermatogenetic cells of the eel. Proc Natl Acad Sci USA. 2006;103(19):7333–7338. doi: 10.1073/pnas.0508419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aschim EL, Giwercman A, Stahl O, Eberhard J, Cwikiel M, Nordenskjold A, Haugen TB, Grotmol T, Giwercman YL. The RsaI polymorphism in the estrogen receptor-beta gene is associated with male infertility. J Clin Endocrinol Metab. 2005;90(9):5343–5348. doi: 10.1210/jc.2005-0263. [DOI] [PubMed] [Google Scholar]
- 122.Aschim EL, Saether T, Wiger R, Grotmol T, Haugen TB. Differential distribution of splice variants of estrogen receptor beta in human testicular cells suggests specific functions in spermatogenesis. J Steroid Biochem Mol Biol. 2004;92(1–2):97–106. doi: 10.1016/j.jsbmb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 123.Cavaco JE, Laurentino SS, Barros A, Sousa M, Socorro S. Estrogen receptors alpha and beta in human testis: both isoforms are expressed. Syst Biol Reprod Med. 2009;55(4):137–144. doi: 10.3109/19396360902855733. [DOI] [PubMed] [Google Scholar]
- 124.Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J Clin Endocrinol Metab. 2000;85(12):4835–4840. doi: 10.1210/jc.85.12.4835. [DOI] [PubMed] [Google Scholar]
- 125.Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24(1):145–155. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- 126.Makinen S, Makela S, Weihua Z, Warner M, Rosenlund B, Salmi S, Hovatta O, Gustafsson JA. Localization of oestrogen receptors alpha and beta in human testis. Mol Hum Reprod. 2001;7(6):497–503. doi: 10.1093/molehr/7.6.497. [DOI] [PubMed] [Google Scholar]
- 127.Lambard S, Galeraud-Denis I, Saunders PT, Carreau S. Human immature germ cells and ejaculated spermatozoa contain aromatase and oestrogen receptors. J Mol Endocrinol. 2004;32(1):279–289. doi: 10.1677/jme.0.0320279. [DOI] [PubMed] [Google Scholar]
- 128.Staub C, Rauch M, Ferriere F, Trepos M, Dorval-Coiffec I, Saunders PT, Cobellis G, Flouriot G, Saligaut C, Jegou B. Expression of estrogen receptor ESR1 and its 46-kDa variant in the gubernaculum testis. Biol Reprod. 2005;73(4):703–712. doi: 10.1095/biolreprod.105.042796. [DOI] [PubMed] [Google Scholar]
- 129.Picciarelli-Lima P, Oliveira AG, Reis AM, Kalapothakis E, Mahecha GA, Hess RA, Oliveira CA. Effects of 3-beta-diol, an androgen metabolite with intrinsic estrogen-like effects, in modulating the aquaporin-9 expression in the rat efferent ductules. Reprod Biol Endocrinol. 2006;4:51. doi: 10.1186/1477-7827-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cederroth CR, Schaad O, Descombes P, Chambon P, Vassalli JD, Nef S. Estrogen receptor alpha is a major contributor to estrogen-mediated fetal testis dysgenesis and cryptorchidism. Endocrinology. 2007;148(11):5507–5519. doi: 10.1210/en.2007-0689. [DOI] [PubMed] [Google Scholar]
- 131.Allan CM, Couse JF, Simanainen U, Spaliviero J, Jimenez M, Rodriguez K, Korach KS, Handelsman DJ. Estradiol induction of spermatogenesis is mediated via an estrogen receptor-{alpha} mechanism involving neuroendocrine activation of follicle-stimulating hormone secretion. Endocrinology. 2010;151(6):2800–2810. doi: 10.1210/en.2009-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schleicher G, Khan S, Nieschlag E. Differentiation between androgen and estrogen receptor mediated effects of testosterone on FSH using androgen receptor deficient (Tfm) and normal mice. J Steroid Biochem. 1989;33(1):49–51. doi: 10.1016/0022-4731(89)90356-7. [DOI] [PubMed] [Google Scholar]
- 133.Cheng CY, Boettcher B. The effect of steroids on the in vitro migration of washed human spermatozoa in modified Tyrode’s solution or in fasting human blood serum. Fertil Steril. 1979;32(5):566–570. doi: 10.1016/s0015-0282(16)44361-x. [DOI] [PubMed] [Google Scholar]
- 134.Revelli A, Massobrio M, Tesarik J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr Rev. 1998;19(1):3–17. doi: 10.1210/er.19.1.3. [DOI] [PubMed] [Google Scholar]
- 135.Hicks JJ, Pedron N, Rosado A. Modifications of human spermatozoa glycolysis by cyclic adenosine monophosphate (cAMP), estrogens, and follicular fluid. Fertil Steril. 1972;23(12):886–893. [PubMed] [Google Scholar]
- 136.Mishra DP, Shaha C. Estrogen-induced spermatogenic cell apoptosis occurs via the mitochondrial pathway: role of superoxide and nitric oxide. J Biol Chem. 2005;280(7):6181–6196. doi: 10.1074/jbc.M405970200. [DOI] [PubMed] [Google Scholar]
- 137.McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, Robertson DM. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–179. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- 138.Meroni SB, Riera MF, Pellizzari EH, Cigorraga SB. Regulation of rat Sertoli cell function by FSH: possible role of phosphatidylinositol 3-kinase/protein kinase B pathway. J Endocrinol. 2002;174(2):195–204. doi: 10.1677/joe.0.1740195. [DOI] [PubMed] [Google Scholar]
- 139.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274(13):8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 140.Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS. Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-induced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol. 2000;14(8):1283–1300. doi: 10.1210/me.14.8.1283. [DOI] [PubMed] [Google Scholar]
- 141.Wassermann GF, Bloch LM, Grillo ML, Silva FR, Loss ES, McConnell LL. Biochemical factors involved in the FSH action on amino acid transport in immature rat testes. Horm Metab Res. 1992;24(6):276–279. doi: 10.1055/s-2007-1003312. [DOI] [PubMed] [Google Scholar]
- 142.Oonk RB, Grootegoed JA, van der Molen HJ. Comparison of the effects of insulin and follitropin on glucose metabolism by Sertoli cells from immature rats. Mol Cell Endocrinol. 1985;42(1):39–48. doi: 10.1016/0303-7207(85)90005-X. [DOI] [PubMed] [Google Scholar]
- 143.Oonk RB, Grootegoed JA. Identification of insulin receptors on rat Sertoli cells. Mol Cell Endocrinol. 1987;49(1):51–62. doi: 10.1016/0303-7207(87)90063-3. [DOI] [PubMed] [Google Scholar]
- 144.Borland K, Mita M, Oppenheimer CL, Blinderman LA, Massague J, Hall PF, Czech MP. The actions of insulin-like growth factors I and II on cultured Sertoli cells. Endocrinology. 1984;114(1):240–246. doi: 10.1210/endo-114-1-240. [DOI] [PubMed] [Google Scholar]
- 145.Oliveira PF, Alves MG, Rato L, Laurentino S, Silva J, Sa R, Barros A, Sousa M, Carvalho RA, Cavaco JE, Socorro S. Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochim Biophys Acta. 2012;1820(2):84–89. doi: 10.1016/j.bbagen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 146.Medina RA, Meneses AM, Vera JC, Guzman C, Nualart F, Rodriguez F, de los Angeles Garcia M, Kato S, Espinoza N, Monso C, Carvajal A, Pinto M, Owen GI. Differential regulation of glucose transporter expression by estrogen and progesterone in Ishikawa endometrial cancer cells. J Endocrinol. 2004;182(3):467–478. doi: 10.1677/joe.0.1820467. [DOI] [PubMed] [Google Scholar]
- 147.Kol S, Ben-Shlomo I, Ruutiainen K, Ando M, Davies-Hill TM, Rohan RM, Simpson IA, Adashi EY. The midcycle increase in ovarian glucose uptake is associated with enhanced expression of glucose transporter 3. Possible role for interleukin-1, a putative intermediary in the ovulatory process. J Clin Invest. 1997;99(9):2274–2283. doi: 10.1172/JCI119403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sakai Y, Yamashina S, Furudate S. Developmental delay and unstable state of the testes in the rdw rat with congenital hypothyroidism. Dev Growth Differ. 2004;46(4):327–334. doi: 10.1111/j.1440-169x.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- 149.Clyde HR, Walsh PC, English RW. Elevated plasma testosterone and gonadotropin levels in infertile males with hyperthyroidism. Fertil Steril. 1976;27(6):662–666. [PubMed] [Google Scholar]
- 150.Wagner MS, Wajner SM, Maia AL. The role of thyroid hormone in testicular development and function. J Endocrinol. 2008;199(3):351–365. doi: 10.1677/JOE-08-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Krassas GE, Tziomalos K, Papadopoulou F, Pontikides N, Perros P. Erectile dysfunction in patients with hyper- and hypothyroidism: how common and should we treat? J Clin Endocrinol Metab. 2008;93(5):1815–1819. doi: 10.1210/jc.2007-2259. [DOI] [PubMed] [Google Scholar]
- 152.Palmero S, Maggiani S, Fugassa E. Nuclear triiodothyronine receptors in rat Sertoli cells. Mol Cell Endocrinol. 1988;58(2–3):253–256. doi: 10.1016/0303-7207(88)90161-X. [DOI] [PubMed] [Google Scholar]
- 153.van Haaster LH, de Jong FH, Docter R, de Rooij DG. High neonatal triiodothyronine levels reduce the period of Sertoli cell proliferation and accelerate tubular lumen formation in the rat testis, and increase serum inhibin levels. Endocrinology. 1993;133(2):755–760. doi: 10.1210/en.133.2.755. [DOI] [PubMed] [Google Scholar]
- 154.Ando S, Sirianni R, Forastieri P, Casaburi I, Lanzino M, Rago V, Giordano F, Giordano C, Carpino A, Pezzi V. Aromatase expression in prepuberal Sertoli cells: effect of thyroid hormone. Mol Cell Endocrinol. 2001;178(1–2):11–21. doi: 10.1016/S0303-7207(01)00443-9. [DOI] [PubMed] [Google Scholar]
- 155.Holsberger DR, Cooke PS. Understanding the role of thyroid hormone in Sertoli cell development: a mechanistic hypothesis. Cell Tissue Res. 2005;322(1):133–140. doi: 10.1007/s00441-005-1082-z. [DOI] [PubMed] [Google Scholar]
- 156.Panno ML, Sisci D, Salerno M, Lanzino M, Mauro L, Morrone EG, Pezzi V, Palmero S, Fugassa E, Ando S. Effect of triiodothyronine administration on estrogen receptor contents in peripuberal Sertoli cells. Eur J Endocrinol. 1996;134(5):633–638. doi: 10.1530/eje.0.1340633. [DOI] [PubMed] [Google Scholar]
- 157.Palmero S, Prati M, Barreca A, Minuto F, Giordano G, Fugassa E. Thyroid hormone stimulates the production of insulin-like growth factor I (IGF-I) by immature rat Sertoli cells. Mol Cell Endocrinol. 1990;68(1):61–65. doi: 10.1016/0303-7207(90)90170-D. [DOI] [PubMed] [Google Scholar]
- 158.Fugassa E, Palmero S, Gallo G. Triiodothyronine decreases the production of androgen-binding protein by rat Sertoli cells. Biochem Biophys Res Commun. 1987;143(1):241–247. doi: 10.1016/0006-291X(87)90656-5. [DOI] [PubMed] [Google Scholar]
- 159.Palmero S, Prati M, Bolla F, Fugassa E. Tri-iodothyronine directly affects rat Sertoli cell proliferation and differentiation. J Endocrinol. 1995;145(2):355–362. doi: 10.1677/joe.0.1450355. [DOI] [PubMed] [Google Scholar]
- 160.Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev. 2001;22(4):451–476. doi: 10.1210/er.22.4.451. [DOI] [PubMed] [Google Scholar]
- 161.Visser WE, Friesema EC, Jansen J, Visser TJ. Thyroid hormone transport in and out of cells. Trends Endocrinol Metab. 2008;19(2):50–56. doi: 10.1016/j.tem.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 162.Silva FR, Leite LD, Barreto KP, D’Agostini C, Zamoner A. Effect of 3,5,3′-triiodo-l-thyronine on amino acid accumulation and membrane potential in Sertoli cells of the rat testis. Life Sci. 2001;69(8):977–986. doi: 10.1016/S0024-3205(01)01186-9. [DOI] [PubMed] [Google Scholar]
- 163.Schteingart HF, Meroni SB, Canepa DF, Pellizzari EH, Cigorraga SB. Effects of basic fibroblast growth factor and nerve growth factor on lactate production, gamma-glutamyl transpeptidase and aromatase activities in cultured Sertoli cells. Eur J Endocrinol Oslo. 1999;141(5):539–545. doi: 10.1530/eje.0.1410539. [DOI] [PubMed] [Google Scholar]
- 164.Mullaney BP, Skinner MK. Basic fibroblast growth factor (bFGF) gene expression and protein production during pubertal development of the seminiferous tubule: follicle-stimulating hormone-induced Sertoli cell bFGF expression. Endocrinology. 1992;131(6):2928–2934. doi: 10.1210/en.131.6.2928. [DOI] [PubMed] [Google Scholar]
- 165.Han IS, Sylvester SR, Kim KH, Schelling ME, Venkateswaran S, Blanckaert VD, McGuinness MP, Griswold MD. Basic fibroblast growth factor is a testicular germ cell product which may regulate Sertoli cell function. Mol Endocrinol. 1993;7(7):889–897. doi: 10.1210/me.7.7.889. [DOI] [PubMed] [Google Scholar]
- 166.Le Magueresse-Battistoni B, Wolff J, Morera AM, Benahmed M. Fibroblast growth factor receptor type 1 expression during rat testicular development and its regulation in cultured Sertoli cells. Endocrinology. 1994;135(6):2404–2411. doi: 10.1210/en.135.6.2404. [DOI] [PubMed] [Google Scholar]
- 167.Jaillard C, Chatelain PG, Saez JM. In vitro regulation of pig Sertoli cell growth and function: effects of fibroblast growth factor and somatomedin-C. Biol Reprod. 1987;37(3):665–674. doi: 10.1095/biolreprod37.3.665. [DOI] [PubMed] [Google Scholar]
- 168.Riera MF, Meroni SB, Pellizzari EH, Cigorraga SB. Assessment of the roles of mitogen-activated protein kinase and phosphatidyl inositol 3-kinase/protein kinase B pathways in the basic fibroblast growth factor regulation of Sertoli cell function. J Mol Endocrinol. 2003;31(2):279–289. doi: 10.1677/jme.0.0310279. [DOI] [PubMed] [Google Scholar]
- 169.Eisenberg SP, Brewer MT, Verderber E, Heimdal P, Brandhuber BJ, Thompson RC. Interleukin 1 receptor antagonist is a member of the interleukin 1 gene family: evolution of a cytokine control mechanism. Proc Natl Acad Sci USA. 1991;88(12):5232–5236. doi: 10.1073/pnas.88.12.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Khan SA, Nieschlag E. Interleukin-1 inhibits follitropin-induced aromatase activity in immature rat Sertoli cells in vitro. Mol Cell Endocrinol. 1991;75(1):1–7. doi: 10.1016/0303-7207(91)90238-N. [DOI] [PubMed] [Google Scholar]
- 171.Karzai AW, Wright WW. Regulation of the synthesis and secretion of transferrin and cyclic protein-2/cathepsin L by mature rat Sertoli cells in culture. Biol Reprod. 1992;47(5):823–831. doi: 10.1095/biolreprod47.5.823. [DOI] [PubMed] [Google Scholar]
- 172.Syed V, Stephan JP, Gerard N, Legrand A, Parvinen M, Bardin CW, Jegou B. Residual bodies activate Sertoli cell interleukin-1 alpha (IL-1 alpha) release, which triggers IL-6 production by an autocrine mechanism, through the lipoxygenase pathway. Endocrinology. 1995;136(7):3070–3078. doi: 10.1210/en.136.7.3070. [DOI] [PubMed] [Google Scholar]
- 173.Pollanen P, Soder O, Parvinen M. Interleukin-1 alpha stimulation of spermatogonial proliferation in vivo. Reprod Fertil Dev. 1989;1(1):85–87. doi: 10.1071/RD9890085. [DOI] [PubMed] [Google Scholar]
- 174.Parvinen M, Soder O, Mali P, Froysa B, Ritzen EM. In vitro stimulation of stage-specific deoxyribonucleic acid synthesis in rat seminiferous tubule segments by interleukin-1 alpha. Endocrinology. 1991;129(3):1614–1620. doi: 10.1210/endo-129-3-1614. [DOI] [PubMed] [Google Scholar]
- 175.Gomez E, Morel G, Cavalier A, Lienard MO, Haour F, Courtens JL, Jegou B. Type I and type II interleukin-1 receptor expression in rat, mouse, and human testes. Biol Reprod. 1997;56(6):1513–1526. doi: 10.1095/biolreprod56.6.1513. [DOI] [PubMed] [Google Scholar]
- 176.Nehar D, Mauduit C, Boussouar F, Benahmed M. Interleukin 1alpha stimulates lactate dehydrogenase A expression and lactate production in cultured porcine Sertoli cells. Biol Reprod. 1998;59(6):1425–1432. doi: 10.1095/biolreprod59.6.1425. [DOI] [PubMed] [Google Scholar]
- 177.Gerard N, Syed V, Bardin W, Genetet N, Jegou B. Sertoli cells are the site of interleukin-1 alpha synthesis in rat testis. Mol Cell Endocrinol. 1991;82(1):R13–R16. doi: 10.1016/0303-7207(91)90019-O. [DOI] [PubMed] [Google Scholar]
- 178.Syed V, Söder O, Arver S, Lindh M, Khan S, Ritzen E. Ontogeny and cellular origin of an interleukin 1 like factor in the reproductive tract of the male rat. Int J Androl. 1988;11(5):437–447. doi: 10.1111/j.1365-2605.1988.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 179.Haugen TB, Landmark BF, Josefsen GM, Hansson V, Hogset A. The mature form of interleukin-1 [alpha] is constitutively expressed in immature male germ cells from rat. Mol Cell Endocrinol. 1994;105(2):R19–R23. doi: 10.1016/0303-7207(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 180.Calkins JH, Guo H, Sigel MM, Lin T. Differential effects of recombinant interleukin-1 alpha and beta on Leydig cell function. Biochem Biophys Res Commun. 1990;167(2):548–553. doi: 10.1016/0006-291X(90)92059-9. [DOI] [PubMed] [Google Scholar]
- 181.Soder O, Syed V, Callard GV, Toppari J, Pollanen P, Parvinen M, Froysa B, Ritzen EM. Production and secretion of an interleukin-1-like factor is stage-dependent and correlates with spermatogonial DNA synthesis in the rat seminiferous epithelium. Int J Androl. 1991;14(3):223–231. doi: 10.1111/j.1365-2605.1991.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 182.Hales DB. Interleukin-1 inhibits Leydig cell steroidogenesis primarily by decreasing 17 alpha-hydroxylase/C17-20 lyase cytochrome P450 expression. Endocrinology. 1992;131(5):2165–2172. doi: 10.1210/en.131.5.2165. [DOI] [PubMed] [Google Scholar]
- 183.Lin T, Wang D, Nagpal ML. Human chorionic gonadotropin induces interleukin-1 gene expression in rat Leydig cells in vivo. Mol Cell Endocrinol. 1993;95(1–2):139–145. doi: 10.1016/0303-7207(93)90039-M. [DOI] [PubMed] [Google Scholar]
- 184.Hayes R, Chalmers SA, Nikolic-Paterson DJ, Atkins RC, Hedger MP. Secretion of bioactive interleukin 1 by rat testicular macrophages in vitro. J Androl. 1996;17(1):41–49. [PubMed] [Google Scholar]
- 185.Meroni SB, Suburo AM, Cigorraga SB. Interleukin-1beta regulates nitric oxide production and gamma-glutamyl transpeptidase activity in Sertoli cells. J Androl. 2000;21(6):855–861. [PubMed] [Google Scholar]
- 186.Petersen C, Boitani C, Froysa B, Soder O. Interleukin-1 is a potent growth factor for immature rat Sertoli cells. Mol Cell Endocrinol. 2002;186(1):37–47. doi: 10.1016/S0303-7207(01)00680-3. [DOI] [PubMed] [Google Scholar]
- 187.Okuda Y, Bardin CW, Hodgskin LR, Morris PL. Interleukins-1 alpha and -1 beta regulate interleukin-6 expression in Leydig and Sertoli cells. Recent Prog Horm Res. 1995;50:367–372. doi: 10.1016/b978-0-12-571150-0.50022-6. [DOI] [PubMed] [Google Scholar]
- 188.Hoeben E, Van Damme J, Put W, Swinnen JV, Verhoeven G. Cytokines derived from activated human mononuclear cells markedly stimulate transferrin secretion by cultured Sertoli cells. Endocrinology. 1996;137(2):514–521. doi: 10.1210/en.137.2.514. [DOI] [PubMed] [Google Scholar]
- 189.Hoeben E, Van Aelst I, Swinnen JV, Opdenakker G, Verhoeven G. Gelatinase A secretion and its control in peritubular and Sertoli cell cultures: effects of hormones, second messengers and inducers of cytokine production. Mol Cell Endocrinol. 1996;118(1–2):37–46. doi: 10.1016/0303-7207(96)03764-1. [DOI] [PubMed] [Google Scholar]
- 190.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100(3):328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 191.Cheung WD, Hart GW. AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J Biol Chem. 2008;283(19):13009–13020. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346(Pt 3):561–576. doi: 10.1042/0264-6021:3460561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Galardo MN, Riera MF, Pellizzari EH, Cigorraga SB, Meroni SB. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-d-ribonucleoside, regulates lactate production in rat Sertoli cells. J Mol Endocrinol. 2007;39(4):279–288. doi: 10.1677/JME-07-0054. [DOI] [PubMed] [Google Scholar]
- 194.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24(10):1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Russell LD, Steinberger A. Sertoli cells in culture: views from the perspectives of an in vivoist and an in vitroist. Biol Reprod. 1989;41(4):571–577. doi: 10.1095/biolreprod41.4.571. [DOI] [PubMed] [Google Scholar]
- 196.Anway MD, Folmer J, Wright WW, Zirkin BR. Isolation of Sertoli cells from adult rat testes: an approach to ex vivo studies of Sertoli cell function. Biol Reprod. 2003;68(3):996–1002. doi: 10.1095/biolreprod.102.008045. [DOI] [PubMed] [Google Scholar]
- 197.Syed V, Hecht NB. Selective loss of Sertoli cell and germ cell function leads to a disruption in Sertoli cell-germ cell communication during aging in the Brown Norway rat. Biol Reprod. 2001;64(1):107–112. doi: 10.1095/biolreprod64.1.107. [DOI] [PubMed] [Google Scholar]