Abstract
An analysis of the scientific literature published in the last 10 years reveals a constant growth of laccase applicative research in several industrial fields followed by the publication of a great number of patents. The Green Chemistry journal devoted the cover of its September 2014 issue to a laccase as greener alternative for chemical oxidation. This indicates that laccase “never-ending story” has found a new promising trend within the constant search for efficient (bio)catalysts able to meet the 12 green chemistry principles. A survey of ancient and cutting-edge uses of laccase in different industrial sectors is offered in this review with the aim both to underline their potential and to provide inspiration for new ones. Applications in textile and food fields have been deeply described, as well as examples concerning polymer synthesis and laccase-catalysed grafting. Recent applications in pharmaceutical and cosmetic industry have also been reviewed.
Keywords: Green chemistry, Industrial applications, Textile, Food, Polymer, Pharmaceutical, Cosmetics
Introduction
First uses of laccases in industry date back to 1990s [1, 2]. Since then, a careful study of reactions catalysed in vivo by fungal laccases has led to the discovery of an ever-increasing number of biotechnological applications reaching a peak in the last 10 years.
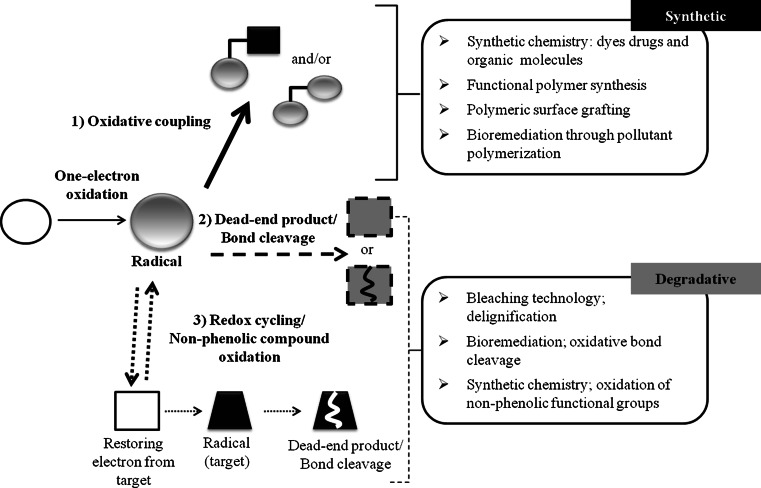
Laccase-catalyzed oxidation gives rise to radical species that can evolve towards both synthetic and degradative processes. Radical fate can follow three different pathways depending on many factors (Fig. 1). Oxidative coupling (1) is the main mechanism leading to homo- or cross-coupling of molecules resulting into dimers and/or also polymeric species. Such synthetic route translates in vitro into biotechnological applications in dye and polymer synthesis and grafting for surface functionalization. On the other hand, depending on their stability and redox reversibility, radicals may undergo rearrangement per se, yielding dead end products (2), or, in the third option (3), act as mediators for further oxidation of non-phenolic compound causing bond cleavage. The latter two kinds of laccase actions can be reproduced in vitro into biotechnological applications such as bleaching technology, and bioremediation.
Fig. 1.
Laccase-catalyzed oxidation of small organics gives rise to the corresponding radicals that can evolve towards either synthetic or degradative processes, depending on functions performed in vivo by laccases. Adapted from Jeon and coworkers [3, 4] with author permission
This review focuses on some emerging trends of laccase uses covering textile, food, pharmaceutical and cosmetic fields. Examples concerning polymer synthesis and laccase-catalyzed grafting have also been reviewed.
Textile industry
Among the explored industrial fields, laccases have found wide applications in several sectors of textile industry, where these enzymes have been integrated in already existing processes such as fiber bleaching and dyeing and are also emerging as important “green tools” to modify and/or improve textile properties. Bioremediation of textile wastewaters also occupies a wide slice of laccase applications, although it has not been reviewed in this paragraph.
Fiber biobleaching
Removing of natural pigments, other non-cellulosic matter and impurities from cellulosic material before fiber dyeing and finishing, a process termed bleaching, is a mandatory step in textile processing. Laccases have emerged as a fiber-preserving alternative to the most commonly used bleaching agent, hydrogen peroxide [5, 6]. When combined with hydrogen peroxide produced by glucose oxidase, laccase treatment of linen fabric resulted into effective whiteness increase and modification of amount and type of lignin surface functional groups [7]. A synergistic effect on oxidation/removal of the natural coloring matter of cotton has been observed coupling laccase with ultrasound treatment, the latter allowing an improved diffusion of the enzyme throughout the textile structure [8]. Enhanced bleaching efficiency by a combination of laccase and ultrasound treatment has been also verified on linen fabrics. In this study, the time/dye uptake isotherms were also enhanced after combined bleaching treatment [9]. More recently, a pilot reactor for laccase–hydrogen peroxide cotton bleaching assisted by ultrasound was scaled up and efficiently applied to fabrics treatment [10]. In addition, Tian and coworkers [11] developed what they called the “green” short-flow process to treat cotton fabric in an energy-saving way. The process, combining hydrogen peroxide with laccase-mediated system (LMS) pretreatment allowed a lower dosage of H2O2, reduced bleaching temperature and shortened bleaching time.
By oxidizing the indigo dye on denim garments into uncolored compounds, laccase treatment has allowed obtaining the desired wash light effect on blue denim jeans without using harsh oxidative agents such as sodium hypochlorite. The use of laccases for denim processing dates back to 1996, when the first commercial laccase preparation DeniliteI™ was launched on the market by Novozyme. Since then, customized formulation of laccases has been developed to target specific garment wet process conditions, as deeply reviewed by Rodriguez-Couto [12]. Solis-Olba applied an LMS based on ABTS to denim decoloration, verifying that denim fibers were not damaged during the treatment and also the environmental impact of the process was reduced since denim decoloration residual water can be easily biodegraded [13]. Montazer and Maryan [14] verified that the combination of laccases with cellulases in denim processing helped to improve the lightness and decrease staining on both back of garment and on white pocket. An example of effective application of laccase treatment for stone washing effects of denim fabric without using a mediator has also been reported [15].
Functional modification of textile fibers
Laccases have also been used to attach functional molecules on textile fibers. Modification of protein fibers, such as wool and cotton, allows improving their properties. Water repellence is often associated to self-cleaning properties, antioxidant and antimicrobial features, which are desired qualities in finishing design.
Grafting of wool with the bi-functional phenolic compound nordihydroguaiaretic acid (NDGA) through a laccase-catalyzed reaction in an aqueous ethanol mixture improves wool properties [16]. NDGA grafting on wool provides antioxidant activity and is efficient in protecting wool against the undesirable photoyellowing caused by UV radiation, probably due to the formation of inter/intramolecular bridges and cross-links among wool proteins. Shrink resistance, crease recovery angle and tensile strength of the modified samples also increase compared to the untreated ones.
Antibacterial properties toward Bacillus subtilis and Escherichia coli were imparted to flax fibers and fabrics through laccase-assisted modification with ferulic acid or hydroquinone [17]. Antibacterial properties were also imparted to wool fibers by a laccase (Trametes sp.)-mediated grafting of lauryl gallate [18]. Recently, Silva and coworkers [19] exploited the ascomycete Myceliophthora thermophila laccase to oxidize flavonoids naturally existing in flax fibers, covalently binding the o-quinones formed to the amino groups of chitosan or/and to catechin. Antibacterial activity of linen samples grafted with both chitosan and catechin was tested against Staphylococcus aureus and E. coli. The latter showed susceptibility only to chitosan-treated samples, whereas S. aureus was equally sensitive to chitosan and catechin actions.
Fiber wettability has also been modified by laccase-assisted grafting of ad hoc selected molecules. Kim and coworkers [20] have reported the synthesis of laccase-catalyzed protein–flavonoid conjugates and following anchoring of conjugates onto cationized fibers. By the anchoring of α-casein–catechin conjugates onto flax fibers, the surface became hydrophilic, with a lower contact angle (48°). Wool water-repellent property was modified through an enzymatic coating of wool with lauryl gallate [18].
The exploitation of LMS for the anti-shrinking treatment of wool has been patented [21] and also investigated by Lantto and coauthors [22]. Although molecular reasons causing this effect have not been examined in depth, it seems probable that laccase action would promote intermolecular cross-links of wool fibers, thereby decreasing elasticity and thus reducing the tendency to shrinkage. More recently, Zhang and coworkers [23] have exploited a high-redox potential laccase from Aspergillus to prepare conductive cotton by laccase-catalyzed in situ polymerization of conductive polyaniline (PANI). Electrical properties of cotton, such as antistatic property and electromagnetic shielding effect, were significantly enhanced, highlighting the potential for using common textile materials in flexible electronic devices.
Textile dyeing
The use of laccases to synthesize colorants in situ has come out as a “green” way for textile dyeing at mild process conditions. Laccase-mediated coupling and grafting reactions have also been used for coloring various textiles [17, 24–29]. In the first attempt of cellulose textiles dyeing by Hadzhiyska and coworkers [25], cotton cellulose was dyed in situ by a polymeric dye generated by oxidative coupling of colorless 2,5-diaminobenzenesulfonic acid (DABSA) and 1-hydroxyphenol (catechol) with laccase-treated fibers, obtaining up to 70 % of dye fixation. However, it has been reported that all fabrics showed high wash fastness but low light and friction resistance. Schröder and coworkers [17] used a response surface methodology (RSM) approach to determine the best conditions for laccase-induced coating of flax fibers and fabrics by a Trametes hirsuta laccase. Different phenols have been analyzed for their potential as monomers for enzyme-catalyzed polymerization: all the methoxyphenols showed different coloration with weak fastness properties. Kim and coworkers [26, 28] have reported the utilization of the natural flavonoids present in the cotton as anchors to attach other phenolic compounds to the fiber surface. A T. hirsuta laccase was used to catalyze the oxidation of flavonoids in solution producing quinines, further polymerized and grafted onto surface of the cotton, providing a yellow to brown coloration, depending on the external flavonoids used and on the reaction conditions. The natural flavonoids present in the cotton were found to play an important role on the grafting reaction, improving dyeing and color fastness. The washing and friction fastness test of flavonoids colorized cotton showed good results confirming the feasibility of this new and promising coloration technique. An important improvement in the wet rubbing fastness of the dyed sample has been observed by Blanco and coworkers [29]. In their work, there is a covalent fixation of the in situ generated polymeric pigment. Aromatic amine moieties of DABSA introduced onto tosylated cotton were coupled and copolymerized with a catechol into colored product covalently fixed on the fabric upon oxidation with laccase. The controlled amination of cellulose in a first step, and the subsequent coloration allowed for up to 95 % pigment fixation on the fabric. Tzanov and coworkers [24] have also proved the ability of laccases for wool dyeing. They used a dye bath prepared with a dye precursor (DABSA), dye modifiers (catechol and resorcinol) and laccase, in the absence of any dyeing auxiliaries, carrying out the reaction at pH and temperature values safe to the wool material. Furthermore, they have shown that by prolonging the contact time between wool, enzyme, precursor and modifier, deeper colors were obtained in contrast to the conventional process, where deeper colors are attained by increasing the amount of dye.
In situ laccase-assisted overdyeing using catechol and catechin as dye precursors has been reported by Guimares [30]. Laccase-generated polymers gave rise to new coloration states from dark brown to green-yellow and replaced dyes in the overdyeing process. Through this process, overdyeing of denim was successfully achieved with acceptable levels in terms of durability. Also leather was colored by laccase action using dihydroxynaphthalenes as substrates [27]. The colored products are lightfast and bound to the natural collagen by a covalent tanning manner.
New routes for dye synthesis
Textile dyes occupy an important fraction of chemical industry market. Although consumption of dyes by the textile industry already accounts for two-thirds of the total dyestuff market, recent world dyes and organic pigments industry have estimated a further 3.5 % annual growth for the period 2013–2018 [31]. Due to their toxicity and the harsh operative conditions required for their synthesis, this growth of demand has been accompanied by stringent legislation regarding removal of dyes from industrial effluents and has encouraged the development of eco-friendly processes for their disposal as well as for their synthesis. In this scenario, laccases have shown to play a pivotal and double role: being efficient biocatalysts for biodegradation of synthetic dyes and attractive enzymes for coupling reactions leading to the production of new colored products. Laccase-mediated remediation of textile wastewaters has not been reviewed in this paragraph.
Laccases are known to synthesize many natural compounds in nature, through oxidative coupling reactions. This ability can be translated in vitro into powerful synthetic routes towards new colored natural products. Small colorless aromatic compounds such as phenols, aminophenols, diamines are oxidized by laccase to aryloxy radicals, which may undergo further non-enzymatic reactions leading to formation of colored products [32]. Experimental evidence has shown that laccase is able to catalyze the formation of colored products (from yellow/brown to red and blue) by oxidation of benzene derivatives containing at least two substituents (comprising amino, hydroxyl, and methoxy groups) [32, 33]. Thus, derivatization of various phenolic and non-phenolic substrates may represent a strategy to expand the range of these potential precursors. Presence of mediators has been shown to promote color formation, such as in the case of trimerization of indole with formation of a yellow compound in the presence of 2,2,6,6-tetramethylpiperidin-1-yloxy (TEMPO) [34]. Also, inorganic polyoxometalates have been found to act as better mediators than the organic one, 1-hydroxybenzotriazole (HBT) in the laccase-catalyzed synthesis of polycatechols, assuring better flax coloration, color fixation and resistance [35]. A variety of secondary amines characterized by biologically activities and intense colors were obtained during C–N coupling of 3-methylcatechol with different primary linear, branched-chained and cyclic amines, mediated by both native and recombinant laccases [36]. Phenoxazinones are also another class of compounds with excellent coloring properties, non-toxic and obtainable through laccase-catalyzed oxidation of many aminophenolic compounds [37–39]. Azo-dyes, by far the most important class of commercial dyes, have been synthesized by oxidative coupling reaction between different substrates [40, 41]. In an interesting example, Enaud and coworkers [42, 43] synthesized a novel azo-dye containing two sulfonated anthraquinonic chromophores by a laccase immobilized on an inexpensive carrier. The acid dye obtained is neither cytotoxic nor mutagenic demonstrating good dying properties in polyamide fibers.
Food industry
Food industry benefits from laccase application in different sectors and for multiple purposes: from modification of food sensory parameters and texture to improvement of products shelf-life and determination of certain compounds in beverages. As a fact, many laccase substrates, mostly phenols, thiol-containing proteins and unsaturated fatty acids are fundamental components of various foods and beverages, thus their modification may lead to new functionalities, quality improvement and cost reduction [44].
Baking industry
Application of laccases in baking process has been first reported by Labat et al. [45] describing laccase ability to cross-link arabinoxylans (AX) matrix in dough through dimerization of the esterified ferulic acid, causing increased strength, stability, and reduced stickiness of dough, and thus resulting into its improved machinability. In addition, an increased volume and improved crumb structure and softness of the backed products have been observed. Enhancement of maximum resistance and decreased extensibility has been obtained by laccase treatment of both flour and gluten doughs [46]. The hardening effect was higher in flour dough, where laccase action is prevalent on AX fraction, with respect to gluten dough, where laccases react with gluten protein matrix with less efficiency. Similar results have been obtained on gluten-free oat flour, where laccase treatment was shown to not affect oat globulins [47]. Prolonged incubation with laccase caused softening phenomena, probably due to radical-catalyzed break-down of the cross-linked AX network [46]. Firmness of fresh oat bread and tightness of wheat dough were found to be increased after laccase treatment, as a consequence of the reduction of water-extractable arabinoxylan (WEAX) content following AX cross-linking [48]. When combined with xylanases, laccase treatment further improved textures of both oat and oat–wheat breads [48]. Coupled laccase and protease supplementation, combined with endo-β glucanase side activity of the laccase preparation, also improved bread-making performances. In this case, the effect of hydrolytic activities prevailed over laccase promoted protein cross-linking, by reducing the formation of globular aggregates and positively affecting flour pasting properties [49].
Food sensory parameters
Consumer’s perception of the quality of a food product is strictly influenced by its exterior aspect and odor. Laccases have been applied to the improvement of food sensory parameters by acting on processes affecting physico-chemical deterioration of food products, or enhancing their flavor and taste properties.
Tsuchiya et al. [50] have patented the use of a recombinant laccase and cholorogenic acid to control the malodor of cysteine in different products, including foodstuff. They have shown that enzymatically treated cysteines have a reduced H2S-related smell with respect to untreated ones.
Laccase-catalyzed deoxygenation of vegetable oils has been shown to prevent oxidation of linoleic and linolenic acids responsible for generation of undesirable volatile compounds [51]. The authors have also demonstrated that addition of flavoring agents, such as anthocyanin, a spice, and other phenolic compounds available as food ingredients has a very positive influence on the deoxygenation rate by acting as laccase substrates too.
A method for enhancing color in tea-based foodstuff by the means of Pleurotus laccases has also been patented [52]. The oxidation step converted the colorless catechins in the leaves to a complex mixture of yellow and orange to dark brown substances, such as theaflavins and thearubigins. Also, cacao of superior taste and flavor has been obtained by treatment of cacao nib by Coriolus versicolor laccase [53]. Reduction of tannin content in cocoa pod husk after laccase treatment has been found to improve its nutritive value when used as ingredient for animal feeds [54].
Wine and beer stabilization
Wine stabilization is one of the main processes demanding laccases in food industry. Polyphenols in wine play a major role in determining aroma, color and taste of wine, which are strictly related to the particular phenolic composition. However, the same compounds are also the main actors involved in madeirization process causing turbidity, color intensification and aroma and flavor alteration. Laccase treatment aimed at reducing polyphenol content has been reported as an effective method to preserve wine quality, since the use of enzymes would selectively remove target-specific polyphenols without undesirably altering wine’s organoleptic characteristics. Laccases fit well with process requirement, due to their stability in acidic medium and reversible inhibition with sulfite [55]. Although the efficiency in polyphenol removal has been demonstrated, many authors underlined the need to add supplementary steps to wine production process, such as sulfur treatment, clarification and filtration to remove the oxidized products produced by laccase [56]. Other authors have also confirmed the potential of laccases in pre-fermentative treatment when coupled with conventional clarifiers such as proteins and polyvinylpyrrolidone (PVPP) [56]. Differential selectivity in phenol removal has been highlighted by Minussi et al. [57] in the treatment of red and white wines. Red wine treatment reduced phenolic compounds but mainly affected those responsible for the must antioxidant properties. On the other hand, treatment of white wines has shown higher reduction in total phenols than in the total antioxidant potential, resulting into reducing processing costs and increased storability of white wines.
Another negative aspect connected to wine storage is the characteristic cork off-flavor imparted to wine in contact with cork stoppers, which is related to phenols naturally present in cork and/or produced by microbial degradation. The use of laccase for the treatment of cork stoppers for wine bottles has been patented as an effective method to reduce the cork taint by phenols oxidation [58].
Similarly, the addition of laccase to fermented beer has been shown to improve its storage stability by scavenging the oxygen, which is responsible for the development of catty taint in bottled beer through reaction with fatty acids, amino acids and proteins [59]. Laccase treatment reduces the excess of naturally occurring proanthocyanidines and polyphenols that cause protein precipitation and therefore haze formation in beer [59].
Fruit juice clarification
Analogously to what happens during wine storage, phenols naturally occurring in fruit juices undergo interaction with proteins resulting into formation of haze or sediments. In addition, juice browning also imputable to both enzymatic and chemical oxidation phenomena involving phenols turns out into a final product which is less appealing to the consumers. Several authors have proposed the use of laccases as stabilizing agents, due to their ability to oxidize most of phenols in juices. Contradictory results have been published. Sammartino et al. [60] demonstrated that enzymatically treated apple juice was less stable than the one stabilized by conventional methods. Also, laccase treatment increased susceptibility to browning during storage [61, 62]. On the other hand, effective phenol removal and improved juice stabilization have been reported [56]. When laccase treatment was followed by a filtration step, juice quality could be highly improved [63, 64]. Neifar et al. [65] coupled a laccase treatment of pomegranate juice optimized by RSM with ultrafiltration, obtaining a reduction of polyphenol content and antioxidant capacity, but a concomitant increase of clarity of about 30 % versus untreated juice. More recently, Gassara-Chatti [66] has utilized a formulation of ligninolytic enzymes from Phanerochaete chrysosporium encapsulated in polyacrylamide hydrogel for clarification of mixed juice of berry and pomegranate, resulting in a significant polyphenolic reduction and clarity amelioration with respect to treatment with free enzymes.
Value-added food products
Laccases have been applied to enzymatic gelation of sugar beet pectin (SBP), a food ingredient capable of generating gels after oxidative cross-linking of ferulic acid [67]. Such kinds of gels are of particular interest in food industry due to their thermo-irreversibility, allowing a foodstuff to be heated without losing its gel structure. When added to three different food products, black currant juice, milk, and chopped heat-treated meat emulsion, gelation occurred in all the cases, although in the first two some unwanted side effects occurred [67]. Laccase-induced gelation of SBPs has been found to be highly related to molecular weight, acetylation and methylation degree of different pectin preparations [68]. Moreover, gelling rate and mechanical properties of laccase-induced gels have been proved to be greatly dependent on the amount of laccase activity used: higher enzymatic activity promoted the formation of softer gel due to the short reaction time available for the right arrangement of molecules. On the contrary, lower activity levels assured the formation of correct and permanent cross-links and led to a more rigid gel [68].
Another interesting application demanding laccases concerns the improvement of the stability of globular protein-coated lipid droplets to environmental stresses. As a fact, such kinds of emulsifiers are widely utilized in food industries, although their application is restricted to a limited range of matrixes because of their sensitivity to changes in pH, ionic strength and temperature. Laccases have been found to increase emulsion stability of oil droplets coated by multilayered biopolymer interfaces by promoting cross-linking among β-lactoglobulins in the primary interfacial layer as well as among beet pectin molecules constituting the secondary adsorbed layer. Also protein–polysaccharide cross-links between layers could be responsible for the observed increased emulsion stability to salt in a wider pH range [69]. Laccases also catalyzed the cross-linking of interfacial layers in multilayered oil-in water emulsions obtained by electrostatic deposition of pectin onto a fish gelatin interfacial membrane [70]. Cross-linking occurred exclusively in the layers and not between droplets, since no aggregates were formed. Resulting emulsions were characterized by enhanced stability in a broad pH range (3.5–10). In another example, oil-in water emulsions coated by laccase-mediated cross-linked sugar beet pectin were characterized by smaller and well-dispersed droplets improving their long-term stability [71].
Ma et al. [72] used laccase for the cross-linking of whey protein isolates (WPI) to improve their emulsification properties. Chemically modified and enzymatically treated WPI has been shown to enhance storage stability of WPI-stabilized emulsions. More recently, a mixed solution of WPI, SBP and laccase was microemulsified as nanodroplets in an organic phase [73]. Caffeine was encapsulated into the nanodroplets as a model compound to test their applicability as vehicles for drugs and/or nutraceuticals. Although stable conjugated particles of spherical shape and nanoscalar size were obtained, the authors recommended this method for encapsulation of non-oxidative nutraceuticals and biomaterials since antioxidants such as caffeine may counteract with the oxidizing action of laccase during cross-linking of biopolymers.
Laccase-mediated cross-linking of food proteins has also been used to develop added-value products in the food industry [71, 74], as well as to influence protein digestibility and reduce their allergenicity [75, 76]. β-Lactoglobulin (BLG), accounting for approximately 10–15 % of total milk proteins, is an important nutrient of dairy products and, at the same time, one of the major milk allergens causing serious health risk in cow’s milk-allergic patients [77]. A Trametes versicolor laccase has been used to cross-link BLG phenolic groups in the presence of a sour cherry extract as a natural source of phenolic mediators [76]. Enzymatic processing by cross-linking may be used to tailor BLG properties, by improving its safety and facilitating its digestibility, while conserving beneficial health effects, such as the radical-scavenging activity of peptides released during enzymatic digestion.
Compound detection in beverages
Different biosensors based on laccases have been developed to measure polyphenols in food products, such as wine, beer and tea. Effective potential of a biosensor based on immobilized laccase has been first verified by Ghindilis et al. [78] for detection of tannin in tea of different brands. Since then, a lot of reports concerning the development of laccase-based biosensors have been published in the scientific literature. Laccases from different Trametes species were immobilized on carbon nanotube screen-printed electrodes for determination of polyphenols index in wines [79]. Reported data have shown that biosensor performance is dependent on the laccase source used. Ibarra-Escutia et al. [80] optimized an amperometric biosensor based on a T. versicolor laccase for monitoring phenol compounds in tea infusion. This biosensor displays an excellent stability in the operative conditions and exhibits good performances in terms of response time and sensibility, allowing accurate sample analysis without any pretreatment step. Also, a disposable biosensor was developed for phenol detection in tea infusions [81]. Finally, a bioelectronic tongue based on lipid nanostructured layers containing tyrosinases and laccases has been recently developed by Medina-Plaza and coauthors [82]. The enzymes have been incorporated into a biomimetic environment provided by a Langmuir–Blodgett film of arachidic acid. Lutetium bisphthalocyanine (LuPc2) has also been introduced in the films to act as electron mediator. This multisensory system discriminates phenols according to the number of phenolic groups attached to the structure as well as grapes of different varieties according to their phenolic content. A combination of factors contributes to the high performances of this biosensor: the high functionality of the enzyme obtained using a biomimetic immobilization, the signal enhancement caused by the LuPc2 mediator, the improvement in the selectivity induced by the enzymes and the complementarity of the enzymatic activities.
Laccase-mediated polymer synthesis
In the frame of industrial application, laccase ability to synthesize polymers is of outstanding importance. Indeed, polymeric materials are essential for everyday life, being present in many and different fields, such as food, textile, electronics, communications, transportations, pharmacy, and medicine [83]. Even if current polymer production schemes are highly optimized, the huge quantity of annually produced polymers demands a greener technology, particularly in the perspective of toxic formaldehyde reduction. Hence, it is not surprising that also enzymes are being engaged for polymer synthesis [83], being laccases and peroxidases dominating this field [84]. In a polymerization reaction, laccase achieves its natural work with the initial formation of radicals from a typical substrate (aromatic with hydroxyl group or groups). Then, radicals can undergo polymerization reactions towards homopolymeric materials [85]. It is also possible to create new heteromolecular hybrid molecules coupling a laccase substrate and a non-laccase substrate (variable reaction partner) [85] (Fig. 1). Radical polymerization lacks any control on the structure and morphology of the final product, thus if a defined molecule is pursued, it is necessary to find opportune reaction conditions, as reviewed by Hollmann and Arends [84]. Short reaction time, defined molar concentrations [86], presence of templates [87], substrate engineering [88, 89], and use of co-solvent [90, 91] are some of the more frequently applied strategies to obtain defined polymers.
Despite the proved capability, short-term implementation of enzyme-mediated polymer synthesis on industrial scale is still hampered by economic issues. However, the high efforts put in this field let foresee a fast revolution in polymer chemistry in the next decade.
Polymer synthesis by laccases has already been reviewed by different authors [83–85, 92, 93]; the aim of this paragraph is to underline those applications we believe more promising or more ready for a real industrial exploitation.
Flavonoids
Flavonoids are benzo-γ-pyrone derivatives consisting of phenolic and pyrane rings [94] endowed with biological and pharmacological effects, including antioxidant, anti-mutagenic, anti-carcinogenic, anti-viral and anti-inflammatory properties [95]. High molecular weight polyphenols have been reported to exhibit enhanced biological properties with longer circulation time in vivo [96]. As a fact, in the last decade it has been demonstrated that oxidative coupling of flavonoids generates polymers with increased antioxidant activity. Kurisawa and coworkers [97] have employed a Myceliophthora laccase to catalyze the oxidative polymerization of catechin—a flavonoid found in green tea and wine—in a mixture of a polar organic solvent and buffer. Polymer yield and composition were improved testing different solvent composition. When compared with monomeric catechin, the synthesized polymer exhibited significantly improved superoxide scavenging activity and xanthine oxidase (XO) inhibitory activity. Jadahav and Singhal [98] used a laccase conjugated to Arabic gum to perform catechin polymerization. Free laccase produced water-insoluble cross-linked oligomer, whereas conjugated laccase produced water-soluble linear oligomer endowed with high antioxidant activity and reducing power. An improved antioxidant activity if compared with the corresponding monomer has also been observed in the polymerization of quercetin and kaempferol with a laccase from Ustilago maydis [99]. More recently, the oxidative grafting of flavonoids to modify/impart polymer properties is gaining increased attention, as described in other sections.
Artificial urushi
“Urushi” is a Japanese traditional coating material showing excellent toughness, brilliance and solvent resistance for a long period in comparison with synthetic coatings. It is a natural resinous sap obtained by the Rhus vernicifera tree through the cross-linking of urushiol monomers [100]. The urushiol structure is a catechol derivative, with unsaturated hydrocarbon chain consisting of monoenes, dienes, and trienes at the 3-, or 4-position of catechol. In nature, the film forming of urushiols proceeds via laccase catalysis under air. It can be regarded as the only example of a practical natural paint that utilizes in vitro enzymatic catalysis for hardening [100]. New urushiol analogs were developed to overcome the difficulty to chemically synthesize natural urushiols [101, 102]. “Artificial urushi” may be defined as the cross-linked film prepared by in vitro laccase-catalyzed oxidation of new urushiol analogs, in which the unsaturated group is connected with the phenolic group through an ester linkage. The artificial urushi prepared by laccase shows hardness and brilliance comparable to those of natural urushi [100–102].
Conducting polymers
Conducting polymers, such as polyaniline (PANI), polythiophene (PEDOT), and polypyrrole (PP), have attracted a great attention in the last years.
PANI is regarded as one of the most important conducting polymers. Due to its good electrical and optical properties, as well as its high environmental stability, it can be used as active component of organic lightweight batteries, microelectronics, optical display, for anticorrosive protection, in bioanalysis, etc. [83]. PANI is commonly synthesized by chemical or electrochemical oxidation of aniline monomer under very harsh conditions and yielding a product with a poor solubility in common solvents [83]. Among all the forms of PANI only the emeraldine salt is conductive, and it is usually obtained from the emeraldine base via protonation of its imine sites in acidic conditions (doping) [103]. Problems concerning reaction conditions and PANI solubility can be overcome thanks to biotechnology, e.g., PANI can be enzymatically synthesized by oxidoreductases (glucose oxidase, peroxidase, laccase) in the presence of templates. Many studies deal with the use of peroxidases, but more recently also laccases are increasingly being used, thanks to their ability to work using only air (dioxygen) as cofactor [83]. When PANI polymerization is conducted in the absence of templates, extensive branching is observed and this is not desirable for conducting materials. Besides sulfonated polystyrene (SPS), the first used template in laccase-mediated PANI synthesis [104], many other templates have been used, such as other negatively charged polymers [105, 106], negatively charged micelles [23, 107–110], and negatively charged vesicles [111]. Shumakovich and coworkers [106] have performed a comparison of chemical and enzymatic methods of aniline polymerization. The high-redox potential T. hirsuta laccase was used in the synthesis of polyaniline in the presence of poly(2-acrylamido-2-methyl-1-propanesulfonic acid) (PAMPS) as template. PANI nanoparticles obtained by the two methods are morphologically distinctive. In addition, the mechanism of enzymatic aniline polymerization differs from that of the chemical synthesis. The latter proceeds with a significant induction period, which is not observed in the laccase-catalyzed reaction. With the aim to repeatedly use the enzyme, Vasileva and colleagues [105] used an immobilized laccase on CM cellulose to perform PANI synthesis in the presence of PAMS as template. The conductance of the interpolymer complex synthesized using the immobilized laccase is close to the value of PANI synthesized using the free enzyme, and comparable with the value of the chemically obtained PANI. During recent years, great interest has been given to the synthesis of chiral conducting polymers because they are very promising for practical application in different fields, e.g., for creating surface-modified electrodes, electrochemical asymmetric synthesis, chiral chromatography, and chiral membrane separating technology [112]. A fungal laccase from T. hirsuta was used for the synthesis of chiral polyaniline in the presence of optically active compounds, either in a homogeneous way (in the bulk solution) and heterogeneous one (on the surface of glass slides, in situ synthesis). The optical activity of the enzymatically synthesized polymer in the doped and de-doped forms was confirmed using Circular Dichroism spectroscopy. When the low redox potential laccase from R. vernicifera was used for PANI polymerization, the presence of mediator was required to increase the rate of the reaction [110]. Conducting polyaniline synthesized by the LMS method is produced with a higher yield and displays five times higher conductivity than that obtained in the absence of mediator.
3,4-ethylenedioxythiophene (EDOT) has attracted significant attention in recent years since EDOT polymers (PEDOT) have demonstrated potential applications in organic field effect transistors, organic light emitting diodes and photovoltaic cells [113]. Due to high redox potential of the monomer, EDOT cannot be oxidized in the presence of only laccases, thus it is necessary to use a redox mediator. A T. hirsuta laccase was used in the presence of potassium octocyanomolybdate(4+) as high potential redox mediator to catalyze the reaction of EDOT oxidation and to perform synthesis of PEDOT using PAMPS as template. UV–vis and FTIR spectra confirmed the formation of water dispersible conducting polymer [113].
Polypyrrole (PP) is a biocompatible conducting polymer endowed with a high conductivity, stability in the conducting state, and interesting redox and electromechanical features [114]. An LMS based on a T. versicolor laccase and ABTS was used for the synthesis of PP [115]. The presence of ABTS increased the rate of the reaction and allowed to produce oligomers with higher molecular weight with respect to those obtained without mediator. The conductivity of these polymers, both with and without ABTS, is similar to that of PP prepared electrochemically or chemically.
New materials
The recent synthesis of a novel photoluminescent material [116] is herein cited as a symptomatic case of viable future chances given by laccase-mediated polymer synthesis. A T. versicolor laccase was used for the oxidative polymerization of 4-fluoroguaiacol in the presence of 30 vol % organic co-solvent [108]. The novel synthesized material displays fluorescence with emissions in blue, green and red, thus it may be used as a component in optoelectronic devices. Obtained results have also led to demonstrate that the coupling of the repeated units proceeds by defluorination to attain oxy-phenol propagation with coupling by the C4 phenyl carbon.
Polymer grafting
Polymers with new and/or improved properties can be obtained not only by new synthetic routes, but also modifying existing polymers with selected molecules, using the so-called grafting technology [31, 93, 117]. Coupling of low-molecular weight molecules to polymers can be achieved through functional groups located at the polymer terminal, in the main chain or in the side chain [83]. This enzymatic modification of existing materials, yielding products with modified properties, has an immense potential in different industrial sectors, such as food, textile, pulp and paper, wood, and also cosmetic to a lesser extent. In this paragraph, selected cases of polymer modification of lignocellulosic materials will be reviewed, since examples of grafting in other fields are described in the corresponding sections (“Functional modification of textile fibers”, “Textile dyeing”, “Baking industry”, “Value-added food products”, “Pharmaceutical and cosmetic industries”).
Lignocellulosic materials
Laccase-mediated polymer modification covers different aspects in the pulp and paper industry. The potential of laccase-assisted biografting of phenolic acids for improving strength properties of kraft paper made from high kappa pulps is discussed in several papers [112–124]. The main focus of Chandra studies has been the grafting of phenolic acids to high-lignin content softwood kraft fibers mediated by a Trametes villosa laccase [118–122]. Results indicated that improvements of hydrogen bonding between fibers and creation of phenoxy radical cross-links within the sheets increased paper strength [122]. Recently, Witayakran and Ragauskas [123] have investigated the possibility of grafting various amino acids onto high-lignin content pulps obtaining an increasing of carboxyl group content and paper properties. The ability to use laccases to selectively graft amino acids to lignin-rich pulp fibers provides a new and unique fiber modification technology which will have many future opportunities. In most cases, treatment of flax and sisal pulp with laccases (from Pycnoporus cinnabarinus and T. villosa), with or without a mediator, in the presence of different phenolic compounds, improved strength-related properties (particularly wet tensile strength) in the resulting paper [125–128].
Recently, there has been an increase in innovative research aimed at developing functional packaging material with antimicrobial properties [129, 130]. Several bioactive phenolic compounds (acids, essential oil components and dopamine) were grafted onto the surface of unbleached kraft liner fibers using a laccase from T. pubescens [129]. Obtained results have shown that the handsheet papers obtained by laccase antibacterial surface process (LASP) display a greater efficacy against gram-positive and gram-negative bacteria, if compared with paper treated only with monomeric phenol derivatives. Antimicrobial activity was proved to be function of grafted structure, time of the treatment and concentration of phenol derivatives. Among the tested essential oil compounds, isoeugenol is the most effective: isoeugenol/LASP, besides killing S. aureus, displays a bacteriostatic effect on the more resistant spore forming gram-positive Bacillus subtilis. A P. cinnabarinus laccase was used to initiate the grafting of three different antimicrobial phenol structures (syringaldehyde, acetosyringone and p-coumaric acid) onto unbleached flax fibers with a high cellulose content [130]. Grafted pulps presented high antimicrobial activity against three bacteria analyzed (S. aureus, Pseudomonas aeruginosa and Klebsiella pneumoniae).
Wood (Picea abies) veneers and pulp were treated with tannins, as natural antibacterial phenols, in the presence or absence of commercial M. thermophila laccase with the aim to impart antibacterial properties to the substrates [131]. The treatment in the presence of laccase significantly improved the antibacterial resistance of veneers and paper made from tannin-treated pulp against S. aureus, while a more modest protective effect was observed against E. coli. Authors have also provided mechanistic evidence of the binding of catechin and gallic acid onto putative lignin monomers. Catechin was bonded to the phenolic molecules mainly through 5–5 linkages, while bonding to monomers with sinapyl units was mainly through 4–O–5 linkages. Bridging molecules such as 4-hydroxy-3-methoxybenzylurea, compatible with melamine resin, were attached to fiber lignin by means of laccase with the aim of boosting the disinfecting properties of phenol resin utilized for the impregnated kraft paper of certain environments where high hygienic requirements exist, such as hospitals and kitchens [132].
In an effort to use green chemistry technology to increase wood surface hydrophobicity, laccase-mediated grafting of pulps has been successfully tested. Fluorophenols and alkylamines were coupled, mediated by a T. hirsuta laccase, to complex lignin models to increase hydrophobicity of wood veneers [133–135]. The proposed mechanism has shown that the fluorophenols were bonded to sinapyl units via 4–O–5 linkages, while coupling to guaiacyl units occurred through 5–5 linkages. The alkyl amines were bonded onto simple phenolics and lignin models through the –NH2 thereby establishing a –C–O–N and a –C–N bond. The advantage of laccase-mediated covalent binding of molecules onto wood surface is that the grafted molecules are not readily displaced and released into the environment.
Several approaches of in situ laccase-created radicals in wood chips have been investigated as a way of producing binderless (artificial adhesive-free) wood such as Medium Density Fiberboards (MDFs) and Particle Boards (PBs), commonly used for interior building and furnishing [136].
Many attempts have been made at increasing bonding between the fibers or other constituent particles by their chemical modification. The activation of lignin for bonding can be carried out by oxidative enzymes [137–139]. Particularly, the auto-adhesion of beechwood (Fagus sylvatica) fibers was enhanced by a pretreatment of the fibers with a T. villosa laccase (Novozymes A/S) [139]. The mechanism of enzymatic catalyzed bonding is linked to the generation of stable radicals in lignin by oxidation. Fiberboards made from laccase-treated fibers have a high wet strength compared to boards made from untreated fibers. Furthermore, the surfaces of laccase-treated fibers have shown a markedly increased hydrophobicity, as well as a change in the chemical composition, indicating that lignin extractives precipitated on the fiber surfaces. Another approach to substitute synthetic resins in wood composite production is a two-component system in which laccase-treated technical lignin, such as kraft lignin or lignosulfonate, functions as the adhesive. This method has been applied to the production of PB [140–142], even if the use of water-soluble lignosulfonates generates products displaying poor dimensional stability [141]. A variant process has also been described where a laccase-oxidized lignin-based adhesive, with 1 % methylene diphenyl diisocyanate, resulted in PBs with doubled tensile strength and reduced swelling in water [143].
Other examples
Membrane fouling is a serious problem in membrane filtration due to adsorption of components from the feed [144]. Among other factors, also the (surface) material of the membrane affects adsorption. Poly(ether sulfone) (PES) is a popular hydrophobic material for membranes. A (more) hydrophilic membrane surface may reduce (or even prevent) adsorption [145]. A laccase-mediated grafting of PES with 4-hydroxybenzoic acid and gallic acid in controlled reaction conditions has proved to have clear potential for reduction of fouling by proteins, polysaccharides, and polyphenols, probably related to the more hydrophilic surface [146].
Pharmaceutical and cosmetic industries
Laccases can catalyze the synthesis of various therapeutics, such as antibiotics, antimicrobials and anti-cancer drugs. The usefulness of laccases for the amination of new antibiotics has been demonstrated. Many potential aromatic substituents of penicillins and cephalosporins are natural laccase substrates. Mikolasch and coworkers synthesized different novel β-lactam antibiotics (e.g., cephalosporins, penicillins and carbacephems) through laccase-catalyzed amination of amino β-lactams and 2,5-dihydroxyphenylacetic acid, 2,5-dihydroxybenzoic acid or catechols [147–150]. Novel cyclosporin analogs can be obtained through a laccase-mediated oxidation [151].
Recently, the laccase-catalyzed synthesis of anhydrovinblastine, mitomycin and structurally similar anti-cancer compounds has been reported. Anhydrovinblastine, useful in the leukemia treatment, was synthesized in a coupling process of catharine and vindoline [152, 153]. It has been demonstrated that laccases from M. thermophila and P. cinnabarinus synthesize 5-alkylamino- and 2,5-bis (alkylamino)-(1-4)-benzoquinones, structurally similar to mitomycin, a chemotherapeutic agent used to treat different cancers [154]. Synthesis of other anti-cancer molecules, such as aminonaphthoquinones and p-benzoquinone, has been, respectively, obtained in a monoamination and diamination laccase-catalyzed reaction [155, 156]. Moreover, laccases from different organisms exhibit cytotoxic or antiproliferative effects against the tumor cells, mainly MCF7 and Hep G2 [157–161].
Laccases from Tricholoma giganteum, Lepiota ventrisospora, Lentinus edodes, Pleurotus cornucopiae, Coprinus comatus, Lentinus tigrinus, Hericium coralloides, Agaricus placomyces, and Abortiporus biennis have been reported as inhibitors against HIV-1 reverse transcriptase activity [162–169]. Another laccase has been reported to possess the capability of fighting aceruloplasminemia, restoring the iron homeostasis [170].
Additionally, laccase application has been reported in the generation of iodine [171–174], a well-known antifungal compound, generally used as disinfectant. Laccases are also able to catalyze the formation of other antimicrobial compounds, such as the cinnabarinic acid [175], or to have antibacterial and antifungal activity themselves. Recently, Grover and coworkers have developed a laccase-nanotube-based paint, showing a bactericidal and sporicidal activity [176].
Furthermore, an increasing interest has been focused on laccase application in the cosmetics as a biocatalyst in the hair dye synthesis and in the formulation of personal hygiene products, including deodorant, toothpaste, mouthwash, detergent and soap. Laccase-based hair dying is an emerging field of research. Traditional chemical-based hair coloring products are often irritant, difficult to handle and not safe. Hydrogen peroxide (H2O2) and phenylenediamines are the most used chemicals in hair dying. They are allergenic and carcinogenic [177, 178] and can, respectively, cause severe hair damage [179, 180]. Laccase can act as an oxidizing agent, substituting H2O2. For this reason, laccase-based hair dyes are less irritant [181, 182]. Recent works have shown that laccase-catalyzed polymerization of natural phenols is applicable to the development of new cosmetic pigments [181]. A laccase from T. versicolor and natural plant-derived phenolic compounds have been used to produce a colorful array of eco-friendly dyes [183]. Jeon and coworkers [184] have studied laccase-catalyzed in situ polymerization of natural phenols to form products for hair dyeing. Three kinds of two-monomer combinations for gray hair dyeing were developed, namely, gallic acid/syringic acid, catechin/catechol and ferulic acid/syringic acid, generating brown, black and red colorations, respectively. Laccase polymerization of natural phenols can provide stable colored polymers, since color changes in all dyed hairs tested were negligible, even after repeated shampooing with detergent excess. The alkaline laccase secreted by Flammulina velutipes has been tested for hair coloring [185]. Lately, Chen and coworkers have proposed the laccase from the actinomycete Thermobifida fusca as an excellent thermo-alkali-stable laccase for hair coloring [186]. Fang et al. have demonstrated that the bacterial laccase Lac15D displays a constant activity toward dye precursors and their combinations under alkaline conditions, suggesting its application in hair coloring [187].
In the cosmetic field, laccases are also used for skin lightening [188, 189]. Laccases may not only find utility in the oxidative reaction, production of pigments and melanins, but also in the reduction of melanin-derived blemishes [190]. In addition, laccases can oxidize various thiols and other sulfur-containing compounds, responsible of malodor or even kill the microbes that generate offensive molecules [191]. For this ability, these enzymes have been introduced in deodorant for personal hygiene [192], oral care products [193] and soaps [194].
The importance of these oxidoreductases as efficient catalysts in cosmetic field is underlined by the increasing number of patents registered in the last years [195–209].
Conclusion
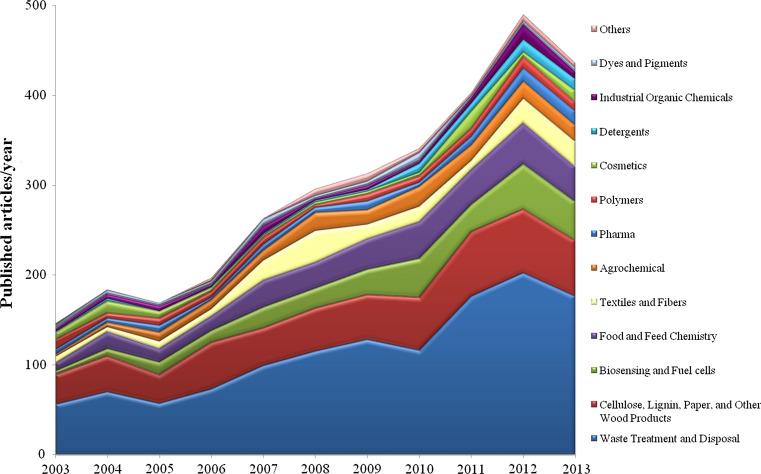
Figure 2 summarizes and classifies the scientific literature published in the last 10 years about implementation of laccases in several industrial fields. The trend is positive for most of the analyzed sectors, with applications in waste water treatment occupying the widest slice of published articles. Articles concerning modification of natural polymers such as lignin and cellulose with related applications in wood and paper industries also represent an important portion of the applicative research on laccases. Other emerging sectors are represented by laccase exploitation in biosensing and fuel cells industry, cosmetics, biopolymer synthesis and in food and textile fields. Also the use of laccases as green catalysts for the synthesis of high-added value organics is emerging as a new, although still poorly exploited, sector of applicative research.
Fig. 2.
Data are from Chemical Abstracts Service/SciFinder Scholar based on the combined CAS and Medline databases with duplicated removed. Source articles include journal articles and are referred to the decade 2003–2013. Categories used for classification of journal articles are based on the following CAS section title: Waste treatment and disposal; water, cellulose, lignin, paper, and other wood products; electrochemical, radiational, and thermal energy technology; electrochemistry; food and feed chemistry; textile and fibers; fertilizers, soils, and plant nutrition; agrochemical bioregulators; pharmaceuticals; pharmacology; essential oils and cosmetics; toxicology; surface active agents and detergents; heterocyclic compounds (more than one hetero atom); heterocyclic compounds (one hetero atom); industrial organic chemicals, leather, fats, and waxes; coatings, inks, and related products; dyes, organic pigments, fluorescent brighteners, and photographic sensitizers; industrial carbohydrates; surface chemistry and colloids; terpenes and terpenoids; plastics fabrication and uses; plastics manufacture and processing; chemistry of synthetic high polymers; biomolecules and their synthetic analogs; alkaloids; amino acids, peptides, and proteins
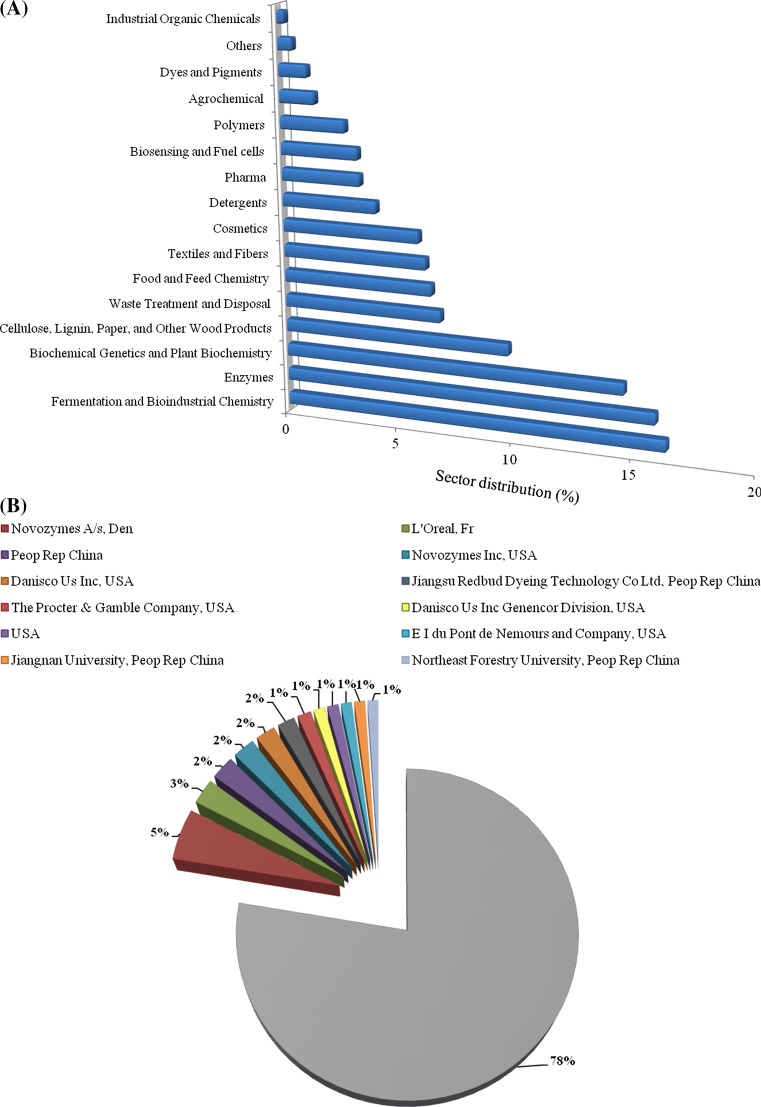
The active research on potential laccase industrial applications has stimulated the publication of a great number of patents in several sectors. Figure 3 reports the relative percentage of patents distribution in different sectors, referred to the decade 2003–2013. The widest slice of patents concerns methods of fermentation and optimization of laccase production, followed by inventions about enzyme modification aimed at its conjugation and/or immobilization for different uses (enzymes category in Fig. 3a). Analyzing the distribution of patent owners (Fig. 3b), Novozyme (Novo Nordisk, Denmark) owns the most of the totality of patents (5 %), followed by L’Oreal with 3 %. The biggest slice of the pie graph (78 %) is occupied by companies or Research centers possessing less than 1 % of total number of published patents.
Fig. 3.
a Distribution of patents deposited in the decade 2003–2013, according to classification into CA section titles. Data are from Chemical Abstracts Service/SciFinder Scholar based on the combined CAS and Medline databases with duplicated removed. b Distribution of patents deposited in the decade 2003–2013 among the owners (companies and research institutions). The gray slice corresponds to owners possessing less than 1 % of total published patents
Despite this plethora of applications, laccase potential was not fully exploited, due to several issues mainly connected with the economy and the efficiency of the enzyme in the operating conditions. Recent efforts to abundantly produce these enzymes, to improve enzyme activity and/or stability through immobilization and protein engineering, are boosting laccase exploitation at industrial level. Since the first commercial product based on laccase enzyme, launched in 1996 by Novozyme (Novo Nordisk, Denmark), widespread companies have been engaged in producing this enzyme in several formulation and for different purposes, mainly for the textile and food industries [2]. Less enzymatic formulations are available for pulp and paper industries, probably for a kind of reserve towards the integration of enzymes in an “ancient” and consolidated process flow, such as paper production. Most of the new companies are located in the Asian continent, where, despite global crises, industry is expanding, probably also due to the less bureaucratic constraints for industrial production and lower salaries of employees in these regions [2]. On the other hand, the increasing demand of specific and eco-friendly alternative biocatalysts has nowadays induced, also in Europe, the creation of new small companies offering customized formulations of laccases to target specific process conditions.
Acknowledgments
This work was supported by grants from the European project “Optimized oxidoreductases for medium and large scale industrial biotransformations, INDOX” (KBBE-2013-7-613549) and from the Italian MIUR (Ministero dell’Università e della Ricerca Scientifica Progetti di Rilevante Interesse Nazionale) PRIN 2009STNWX3. Cinzia Pezzella is recipient of a fellowship funded by POR Campania FSE 2007/2013 “Campania Research in Experimental Medicine, Project CREME” (CUP B25B09000050007).
References
- 1.Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G. Laccases: a never-ending story. Cell Mol Life Sci. 2010;67(3):369–385. doi: 10.1007/s00018-009-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piscitelli A, Pezzella C, Lettera V, Giardina P, Faraco V, Sannia G (2013) Fungal laccases: structure, function and application. In: Maria de Lourdes TMP and Rai M (eds) Fungal enzymes: progress and prospects. pp 113–151
- 3.Jeon JR, Baldrian P, Murugesan K, Chang YS. Laccase-catalysed oxidations of naturally occurring phenols: from in vivo biosynthetic pathways to green synthetic applications. Microb Biotechnol. 2012;5(3):318–332. doi: 10.1111/j.1751-7915.2011.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeon JR, Chang YS. Laccase-mediated oxidation of small organics: bifunctional roles for versatile applications. Trends Biotechnol. 2013;31(6):335–341. doi: 10.1016/j.tibtech.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Pereira L, Bastos C, Tzanov T, Cavaco-Paulo A, Guebitz GM. Environmentally friendly bleaching of cotton using laccases. Environ Chem Lett. 2005;3:66–69. [Google Scholar]
- 6.Tzanov T, Basto C, Gübitz GM, Cavaco-Paulo A. Laccases to improve the whiteness in a conventional bleaching of Cotton. Macromol Mater Eng. 2003;288:807–810. [Google Scholar]
- 7.Ren X, Buschle-Diller G. Oxidoreductases for modification of linen fibers. Colloid Surface A. 2007;299:15–21. [Google Scholar]
- 8.Basto C, Tzanov T, Cavaco-Paulo A. Combined ultrasound-laccase assisted bleaching of cotton. Ultrason Sonochem. 2007;14:350–354. doi: 10.1016/j.ultsonch.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Okeil A, El-Shafie A, El Zawahry MM. Ecofriendly laccase–hydrogen peroxide/ultrasound-assisted bleaching of linen fabrics and its influence on dyeing efficiency. Ultrason Sonochem. 2010;17:383–390. doi: 10.1016/j.ultsonch.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Goncalves I, Herrero-Yniesta V, Perales Arce I, Escrigas Castaneda M, Cavaco-Paulo A, Silva C. Ultrasonic pilot-scale reactor for enzymatic bleaching of cotton fabrics. Ultrason Sonochem. 2014;21:1535–1543. doi: 10.1016/j.ultsonch.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Tian L, Branford-White C, Wang W, Nie H, Zhu L. Laccase-mediated system pretreatment to enhance the effect of hydrogen peroxide bleaching of cotton fabric. Int J Biol Macromol. 2012;50:782–787. doi: 10.1016/j.ijbiomac.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Couto S. Laccases for denim bleaching: an eco-friendly alternative. Open Textil J. 2012;5:1–7. [Google Scholar]
- 13.Solís-Oba M, Almendáriz J, Viniegra-González G. Biotechnological treatment for colorless denim and textil wastewater treatment with laccase and ABTS. Rev Int Contam ambient. 2008;24:5–11. [Google Scholar]
- 14.Montazer M, Sadeghian Maryan A. Influences of different enzymatic treatment on denim garment. Appl Biochem Biotech. 2010;160:2114–2128. doi: 10.1007/s12010-009-8727-4. [DOI] [PubMed] [Google Scholar]
- 15.Pazarlıoğlu NK, Sariişik M, Telefoncu A. Laccase: production by Trametes versicolor and application to denim washing. Process Biochem. 2005;40:1673–1678. [Google Scholar]
- 16.Hossain KhMG, González MD, Juan AR, Tzanov T. Enzyme-mediated coupling of a bi-functional phenolic compound onto wool to enhance its physical, mechanical and functional properties. Enzyme Microb Tech. 2010;46:326–330. [Google Scholar]
- 17.Schröder M, Aichernig N, Gübitz GM, Kokol V. Enzymatic coating of lignocellulosic surfaces with polyphenols. Biotechnol J. 2007;2:334–341. doi: 10.1002/biot.200600209. [DOI] [PubMed] [Google Scholar]
- 18.Hossain KhMG, González MD, Lozano GR, Tzanov T. Multifunctional modification of wool using an enzymatic process in aqueous-organic media. J Biotechnol. 2009;141:58–63. doi: 10.1016/j.jbiotec.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Silva C, Matamá T, Kim SY, Padrão J, Nugroho Prasetyo E, Kudanga T, Nyanhongo GS, Guebitz GM, Casal M, Cavaco-Paulo A. Antimicrobial and antioxidant linen via laccase-assisted grafting. React Funct Polym. 2011;71:713–720. [Google Scholar]
- 20.Kim SY, Cavaco-Paulo A. Laccase-catalysed protein–flavonoid conjugates for flax fibre modification. Appl Microbiol Biotechnol. 2012;93:585–600. doi: 10.1007/s00253-011-3524-8. [DOI] [PubMed] [Google Scholar]
- 21.Yoon MY (1998) Process for improved shrink resistance in wool. WO Patent 98/27264
- 22.Lantto R, Schänberg C, Buchert J. Effects of laccase-mediator combinations on wool. Textile Res J. 2004;74:713–717. [Google Scholar]
- 23.Zhang Y, Dong A, Wang Q, Fan X, Cavaco-Paulo A, Zhang Y. Conductive cotton prepared by polyaniline in situ polymerization using laccase. Appl Biochem Biotech. 2014;174:820–831. doi: 10.1007/s12010-014-1094-9. [DOI] [PubMed] [Google Scholar]
- 24.Tzanov T, Silva C, Zille A, Oliveira J, Cavaco-Paulo A. Effect of some process parameters in enzymatic dyeing of wool. Appl Biochem Biotech. 2003;111:1–14. doi: 10.1385/abab:111:1:1. [DOI] [PubMed] [Google Scholar]
- 25.Hadzhiyska H, Calafell M, Gibert JM, Dagà JM, Tzanov T. Laccase-assisted dyeing of cotton. Biotechnol Lett. 2006;28:755–759. doi: 10.1007/s10529-006-9043-5. [DOI] [PubMed] [Google Scholar]
- 26.Kim SY, Moldes D, Cavaco-Paulo A. Laccase for enzymatic coloration of unbleached cotton. Enzyme Microb Tech. 2007;40:1788–1793. [Google Scholar]
- 27.Suparno O, Covington AD, Evans CS. Application of diphenols for dyeing. J Soc Leath Tech Ch. 2007;91:139–141. [Google Scholar]
- 28.Kim SY, Lopez C, Guebitz GM, Cavaco-Paulo A. Biological coloration of flax fabrics with flavonoids using laccase from Trametes hirsuta . Eng Life Sci. 2008;8:324–330. [Google Scholar]
- 29.Blanco DC, González DM, Monmany DM, Tzanov T. Dyeing properties, synthesis, isolation and characterization of an in situ generated phenolic pigment, covalently bound to cotton. Enzyme Microb Technol. 2009;44:380–385. [Google Scholar]
- 30.Guimarães C, Kim S, Silva C, Cavaco-Paulo A. In situ laccase-assisted overdyeing of denim using flavonoids. Biotechnol J. 2011;6:1272–1279. doi: 10.1002/biot.201100201. [DOI] [PubMed] [Google Scholar]
- 31.Kudanga T, Nyanhongo GS, Guebitz GM, Burton S. Potential applications of laccase-mediated coupling and grafting reactions: a review. Enzyme Microb Technol. 2011;48:195–208. doi: 10.1016/j.enzmictec.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Polak J, Jarosz-Wilkołazka A. Structure/redox potential relationship of simple organic compounds as potential precursors of dyes for laccase-mediated transformation. Biotechnol Prog. 2012;23:93–102. doi: 10.1002/btpr.713. [DOI] [PubMed] [Google Scholar]
- 33.Polak J, Jarosz-Wilkolazka A. Whole-cell fungal transformation of precursors into dyes. Microb Cell Fact. 2010;9:51. doi: 10.1186/1475-2859-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganachaud Ch, Garfagnoli V, Tron T, Iacazio G. Trimerisation of indole through laccase catalysis. Tetrahedron Lett. 2008;49:2476–2478. [Google Scholar]
- 35.Kim S, Silva C, Evtuguin DV, Gamelas JAF, Cavaco-Paulo A. Polyoxometalate/laccase-mediated oxidative polymerization of catechol for textile dyeing. Appl Microbiol Biotechnol. 2011;89:981–987. doi: 10.1007/s00253-010-2932-5. [DOI] [PubMed] [Google Scholar]
- 36.Herter S, Mikolasch A, Michalik D, Hammer E, Schauer F, et al. C–N coupling of 3-methylcatechol with primary amines using native and recombinant laccases from Trametes versicolor and Pycnoporus cinnabarinus . Tetrahedron. 2011;67:9311–9321. [Google Scholar]
- 37.Forte S, Polak J, Valensin D, Taddei M, Basosi R, Vanhulle S, et al. Synthesis and structural characterization of a novel phenoxazinone dye by use of a fungal laccase. J Mol Catal B Enzym. 2010;63:116–120. [Google Scholar]
- 38.Bruyneel F, Enaud E, Billottet L, Vanhulle S, Marchand-Brynaert J. Regioselective synthesis of 3-hydroxyorthanilic acid and its biotransformation into a novel phenoxazinone dye by use of laccase. Eur J Org Chem. 2008;2008:72–79. [Google Scholar]
- 39.Bruyneel F, Payen O, Rescigno A, Tinant B, Marchand-Brynaert J. Laccase mediated synthesis of novel substitutes phenoxazine chromophores featuring tuneable water solubility. Chem Eur J. 2009;15:8283–8295. doi: 10.1002/chem.200900681. [DOI] [PubMed] [Google Scholar]
- 40.Setti L, Giuliani S, Spinozzi G, Pifferi PG. Laccase catalyzed-oxidative coupling of 3-methyl 2-benzothiazoline hydrazone and methoxyphenols. Enzyme Microb Tech. 1999;25:285–289. [Google Scholar]
- 41.Martorana A, Bernini C, Valensin D, Sinicropi A, Pogni R, Basosi R, et al. Insights into the homocoupling reaction of 4-methylamino benzoic acid mediated by Trametes versicolor laccase. Mol BioSyst. 2011;7:2967–2969. doi: 10.1039/c1mb05301a. [DOI] [PubMed] [Google Scholar]
- 42.Enaud E, Trovaslet M, Bruyneel F, Billottet L, Karaaslan R, Sener ME, et al. A novel azoanthraquinone dye made through innovative enzymatic process. Dye Pigments. 2010;85:99–108. [Google Scholar]
- 43.Enaud E, Bols ChM, Casas Infantes A, Groslambert S, Hercher Ch, Iacazio G et al (2010) New azo dyes. WO Patent 2010/003969, 14 Jan 2010
- 44.Osma JF, Toca-Herrera JL, Rodriguez-Couto S. Uses of laccases in the food industry. Enzyme Res. 2010;2010:918761. doi: 10.4061/2010/918761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Labat E, Morel MH, Rouau X. Wheat gluten phenolic acids: occurrence and fate upon mixing. J Agric Food Chem. 2000;48:6280–6283. doi: 10.1021/jf0006271. [DOI] [PubMed] [Google Scholar]
- 46.Selinheimo E, Kruus K, Buchert J, Hopia A, Autio K. Effects of laccase, xylanase and their combination on the rheological properties of wheat doughs. J Cereal Science. 2006;43:152–159. [Google Scholar]
- 47.Flander L, Holopainen U, Kruus K, Buchert J. Effects of tyrosinase and laccase on oat proteins and quality parameters of gluten-free oat breads. J Agr Food Chem. 2011;59:8385–8390. doi: 10.1021/jf200872r. [DOI] [PubMed] [Google Scholar]
- 48.Flander L, Rouau X, Morel MH, Autio K, Seppanen-Laakso T, Kruus K, Buchert J. Effects of laccase and xylanase on the chemical and rheological properties of oat and wheat doughs. J Agr Food Chem. 2008;56:5732–5742. doi: 10.1021/jf800264a. [DOI] [PubMed] [Google Scholar]
- 49.Renzetti S, Courtin CM, Delcour JA, Arendt EK. Oxidative and proteolytic enzyme preparations as promising improvers for oat bread formulations: rheological, biochemical and microstructural background. Food Chem. 2010;119:1465–1473. [Google Scholar]
- 50.Tsuchiya R, Petersen BR, Christensen S (2000) Oxidoreductases for reduction of malodor. US Patent 6074631 A
- 51.Petersen BR, Mathiasen TE, Peelen B, Andersen H (1996) Use of laccase for deoxygenation of oil-containing product such as salad dressing. PCT international application WO 9635768 A1
- 52.Bouwens EM, Trivedi K, Van Vliet C, Winkel C (1999) Method of enhancing color in a tea based foodstuff. US Patent 5879730 A
- 53.Takemori T, Ito Y, Ito M, Yoshama M (1992) Flavor and taste improvement of cacao nib by enzymatic treatment. Japan Kokai Tokkyo Koho JP 04126037 A2
- 54.Mensah CA, Adamafio NA, Amaning-Kwarteng K, Rodrigues FK. Reduced tannin content of laccase-treated cocoa (Theobroma cacao) pod husk. Int J Biol Chem. 2012;6:31–36. [Google Scholar]
- 55.Tanriöven D, Ekşi A. Phenolic compounds in pear juice from different cultivars. Food Chem. 2005;93:89–93. [Google Scholar]
- 56.Minussi RC, Pastore GM, Durán N. Potential applications of laccase in the food industry. Trends Food Sci Tech. 2002;13:205–216. [Google Scholar]
- 57.Minussi RC, Rossi M, Bologna L, Rotilio D, Pastore GM, Durán N. Phenols removal in musts: strategy for wine stabilization by laccase. J Mol Catal B-Enzym. 2007;45:102–107. [Google Scholar]
- 58.Conrad LS, Sponholz WR, Berker O (2000) Treatment of cork with a phenol oxidizing enzyme. US Patent 6152966
- 59.Mathiasen TE (1995) Laccase and beer storage. PCT international application, WO 9521240 A2
- 60.Sammartino M, Piacquadio P, De Stefano G, Sciancalepore V. Apple juice stabilization by conventional and innovative methods. Ind. Bevande. 1998;27:367–369. [Google Scholar]
- 61.Giovanelli G, Ravasini G. Apple juice stabilization by combined enzyme-membrane filtration process. Lebensm Wiss Technol. 1993;26:1–7. [Google Scholar]
- 62.Gökmen V, Borneman Z, Nijhuis HH. Improved ultrafiltration for color reduction and stabilization of apple juice. J Food Sci. 1998;63:504–507. [Google Scholar]
- 63.Ritter G, Maier G, Schoepplein E, Dietrich H. The application of polyphenoloxidase in the processing of apple juice. Bulletin de Liaison-Groupe Polyphenols. 1992;16:209–212. [Google Scholar]
- 64.Cantarelli C, Giovanelli G. Stabilization of pome and grape juice against phenolic deterioration by enzymic treatments. Inst Fruchtsaft Union Wiss Tech Komm. 1990;21:35–57. [Google Scholar]
- 65.Neifar M, Ellouze-Ghorbel R, Kamoun A, Baklouti S, Mokni A, Jaouani A, Ellouze-Chaabouni S. Effective clarification of pomegranate juice using laccase treatment optimized by response surface methodology followed by ultrafiltration. J Food Process Eng. 2011;34:1199–1219. [Google Scholar]
- 66.Gassara-Chatti F, Brar SK, Ajila CM, Verma M, Tyagi RD, Valero JR. Encapsulation of ligninolytic enzymes and its application in clarification of juice. Food Chem. 2013;137:18–24. doi: 10.1016/j.foodchem.2012.09.083. [DOI] [PubMed] [Google Scholar]
- 67.Norsker M, Jensen M, Adler-Nissen J. Enzymatic gelation of sugar beet pectin in food products. Food Hydrocolloid. 2000;14:237–243. [Google Scholar]
- 68.Kuuva T, Lantto R, Reinikainen T, Buchert J, Autio K. Rheological properties of laccase-induced sugar beet pectin gels. Food Hydrocolloid. 2003;17:679–684. [Google Scholar]
- 69.Littoz F, McClements DJ. Bio-mimetic approach to improving emulsion stability: cross-linking adsorbed beet pectin layers using laccase. Food Hydrocolloid. 2008;22:1203–1211. [Google Scholar]
- 70.Zeeb B, Gibis M, Fischer L, Weiss J. Crosslinking of interfacial layers in multilayered oil-in-water emulsions using laccase: characterization and pH-stability. Food Hydrocolloid. 2012;27:126–136. [Google Scholar]
- 71.Jung J, Wicker L. Laccase mediated conjugation of heat treated beta-lactoglobulin and sugar beet pectin. Carbohyd Polym. 2012;89:1244–1249. doi: 10.1016/j.carbpol.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 72.Ma H, Forssell P, Partanen R, Buchert J, Boer H. Improving laccase catalyzed cross-linking of whey protein isolate and their application as emulsifiers. J Agric Food Chem. 2011;59:1406–1414. doi: 10.1021/jf103591p. [DOI] [PubMed] [Google Scholar]
- 73.Gazme B, Madadlou A. Fabrication of whey protein–pectin conjugate particles through laccase-induced gelation of microemulsified nanodroplets. Food Hydrocolloid. 2014;40:189–195. [Google Scholar]
- 74.Mattinen ML, Kruus K, Buchert J, Nielsen JH, Andersen HJ, Steffensen CL. Laccase-catalysed polymerization of tyrosine-containing peptides. FEBS J. 2005;272:3640–3650. doi: 10.1111/j.1742-4658.2005.04786.x. [DOI] [PubMed] [Google Scholar]
- 75.Stanic D, Monogioudi E, Dilek E, Radosavljevic J, Atanaskovic-Markovic M, Vuckovic O, Raija L, Mattinen M, Buchert J, Cirkovic Velickovic T. Digestibility and allergenicity assessment of enzymatically crosslinked beta-casein. Mol Nutr Food Res. 2010;54:1273–1284. doi: 10.1002/mnfr.200900184. [DOI] [PubMed] [Google Scholar]
- 76.Tantoush Z, Stanic D, Stojadinovic M, Ognjenovic J, Mihajlovic L, Atanaskovic-Markovic M, Cirkovic T. Digestibility and allergenicity of b-lactoglobulin following laccase-mediated cross-linking in the presence of sour cherry phenolics. Food Chem. 2011;125:84–91. [Google Scholar]
- 77.Kontopidis G, Holt C, Sawyer L. Beta-lactoglobulin: binding properties, structure, and function. J Dairy Sci. 2004;87:785–796. doi: 10.3168/jds.S0022-0302(04)73222-1. [DOI] [PubMed] [Google Scholar]
- 78.Ghindilis AL, Gavrilova VP, Yaropolov AI. Laccase-based biosensor for determination of polyphenols: determination of catechols in tea. Biosens Bioelectron. 1992;7:127–131. doi: 10.1016/0956-5663(92)90017-h. [DOI] [PubMed] [Google Scholar]
- 79.Di Fusco M, Tortolini C, Deriu D, Mazzei F. Laccase-based biosensor for the determination of polyphenol index in wine. Talanta. 2010;81:235–240. doi: 10.1016/j.talanta.2009.11.063. [DOI] [PubMed] [Google Scholar]
- 80.Ibarra-Escutia P, Gomez JJ, Calas-Blanchard C, Marty JL, Ramirez-Silva MT. Amperometric biosensor based on a high resolution photopolymer deposited onto a screen-printed electrode for phenolic compounds monitoring in tea infusions. Talanta. 2010;81:1636–1642. doi: 10.1016/j.talanta.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 81.Eremia SA, Vasilescu I, Radoi A, Litescu SC, Radu GL. Disposable biosensor based on platinum nanoparticles-reduced graphene oxide-laccase biocomposite for the determination of total polyphenolic content. Talanta. 2013;110:164–170. doi: 10.1016/j.talanta.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 82.Medina-Plaza C, de Saja JA, Rodriguez-Mendez ML. Bioelectronic tongue based on lipidic nanostructured layers containing phenol oxidases and lutetium bisphthalocyanine for the analysis of grapes. Biosens Bioelectron. 2014;57:276–283. doi: 10.1016/j.bios.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 83.Kobayashi S, Makino A. Enzymatic polymer synthesis: an opportunity for green polymer chemistry. Chem Rev. 2009;109:5288–5353. doi: 10.1021/cr900165z. [DOI] [PubMed] [Google Scholar]
- 84.Hollmann F, Arends IWCE. Enzyme initiated radical polymerizations. Polymers. 2012;4:759–793. [Google Scholar]
- 85.Mikolasch A, Schauer F. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol. 2009;82:605–624. doi: 10.1007/s00253-009-1869-z. [DOI] [PubMed] [Google Scholar]
- 86.Hollmann F, Gumulya Y, Toelle C, Liese A, Thum O. Evaluation of the laccase from Myceliophthora thermophila as industrial biocatalyst for polymerization reactions. Macromolecules. 2008;41:8520–8524. [Google Scholar]
- 87.Walde P, Guo ZW. Enzyme-catalyzed chemical structure-controlling template polymerization. Soft Matter. 2011;7:316–331. [Google Scholar]
- 88.Mita N, Tawaki S, Uyama H, Kobayashi S. Structural control in enzymatic oxidative polymerization of phenols with varying the solvent and substituent nature. Chem Lett. 2002;31:402–403. [Google Scholar]
- 89.Ikeda R, Sugihara J, Uyama H, Kobayashi S. Enzymatic oxidative polymerization of 4-hydroxybenzoic acid derivatives to poly(phenylene oxide)s. Polym Int. 1998;47:295–301. [Google Scholar]
- 90.Mita N, Tawaki S, Uyama H, Kobayashi S. Precise structure control of enzymatically synthesized polyphenols. Bull Chem Soc Jpn. 2004;77:1523–1527. [Google Scholar]
- 91.Mita N, Tawaki SI, Uyama H, Kobayashi S. Laccase-catalyzed oxidative polymerization of phenols. Macromol Biosci. 2003;3:253–257. [Google Scholar]
- 92.Witayakran S, Ragauskas AJ. Synthetic applications of laccase in green chemistry. Adv Synth Catal. 2009;35:1187–1209. [Google Scholar]
- 93.Piscitelli A, Amore A, Faraco V. Last advances in synthesis of added value compounds by laccase-mediated biocatalysis. Curr Org Chem. 2012;16:2508–2524. [Google Scholar]
- 94.Uyama H. Artificial polymeric flavonoids: synthesis and applications. Macromol Biosci. 2007;7:410–422. doi: 10.1002/mabi.200700005. [DOI] [PubMed] [Google Scholar]
- 95.Es-Safi NE, Ghidouche S, Ducrot PH. Flavonoids: hemisynthesis, reactivity, characterization and free radical scavenging activity. Molecules. 2007;12:2228–2258. doi: 10.3390/12092228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agr Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 97.Kurisawa M, Chung JE, Uyama H, Kobayashi S. Laccase-catalyzed synthesis and antioxidant property of poly(catechin) Macromol Biosci. 2003;3:758–764. [Google Scholar]
- 98.Jadhav SB, Singhal RS. Laccase–gum Arabic conjugate for preparation of water-soluble oligomer of catechin with enhanced antioxidant activity. Food Chem. 2014;150:9–16. doi: 10.1016/j.foodchem.2013.10.127. [DOI] [PubMed] [Google Scholar]
- 99.Desentis-Mendoza RM, Hernandez-Sanchez H, Moreno A, Emilio RDC, Chel-Guerrero L, Tamariz J, Jaramillo-Flores ME. Enzymatic polymerization of phenolic compounds using laccase and tyrosinase from Ustilago maydis . Biomacromol. 2006;7:1845–1854. doi: 10.1021/bm060159p. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi S, Uyama H, Ikeda R. Artificial urushi. Chem Eur J. 2001;7:4755–4760. doi: 10.1002/1521-3765(20011119)7:22<4754::aid-chem4754>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 101.Kobayashi S, Ikeda R, Oyabu H, Tanaka H. Artificial Urushi: design, synthesis, and enzymatic curing of new urushiol analogues. Chem Lett. 2000;29:1214–1215. [Google Scholar]
- 102.Ikeda R, Tanaka H, Oyabu H, Uyama H, Kobayashi S. Preparation of artificial urushi via an environmentally benign process. Bull Chem Soc Japan. 2001;74:1067–1073. [Google Scholar]
- 103.Otrokhov GV, Morozova OV, Vasil’eva IS, Shumakovich GP, Zaitseva EA, Khlupova ME, Yaropolov AI. Biocatalytic synthesis of conducting polymers and prospects for its application. Biochemistry (Moscow) 2013;78:1539–1553. doi: 10.1134/S0006297913130117. [DOI] [PubMed] [Google Scholar]
- 104.Karamyshev AV, Shleev SV, Koroleva OV, Yaropolov AI, Sakharov IY. Laccase-catalyzed synthesis of conducting polyaniline. Enzyme Microb Technol. 2003;33:556–564. [Google Scholar]
- 105.Vasil’eva IS, Morozova OV, Shumakovich GP, Yaropolov AI. Synthesis of electroconductive polyaniline using immobilized laccase. Appl Biochem Microbiol. 2009;45:27–30. [PubMed] [Google Scholar]
- 106.Shumakovich GP, Vasil’eva IS, Morozova OV, Khomenkov VG, Staroverova IN, Budashov IA, Kurochkin IN, Boyeva JA, Sergeyev VG, Yaropolov AI. A comparative study of water dispersible polyaniline nanocomposites prepared by laccase-catalyzed and chemical methods. J Appl Polym Sci. 2010;117:1544–1550. [Google Scholar]
- 107.Streltsov AV, Shumakovich GP, Morozova OV, Gorbacheva MA, Yaropolov AI. Micellar laccase-catalyzed synthesis of electroconductive polyaniline. Appl Biochem Microb. 2008;44:264–270. [PubMed] [Google Scholar]
- 108.Streltsov AV, Morozova OV, Arkharova NA, Klechkovskaya VV, Staroverova IN, Shumakovich GP, Yaropolov AI. Synthesis and characterization of conducting polyaniline prepared by laccase-catalyzed method in sodium dodecylbenzenesulfonate micellar solutions. J Appl Pol Sci. 2009;114:928–934. [Google Scholar]
- 109.Shumakovich G, Streltsov A, Gorshina E, Rusinova T, Kurova V, Vasil’eva I, Otrokhov G, Morozova O, Yaropolov A. Laccase-catalyzed oxidative polymerization of aniline dimer (N-phenyl-1,4-phenylenediamine) in aqueous micellar solution of sodium dodecylbenzenesulfonate. J Mol Catal B Enzym. 2011;69:83–88. [Google Scholar]
- 110.Shumakovich GP, Kurova V, Vasil’eva IS, Pankratov D, Otrokhov G, Morozova O, Yaropolov A. Laccase-mediated synthesis of conducting polyaniline. J Mol Catal B Enzym. 2012;77:105–110. [Google Scholar]
- 111.Junker K, Kissner R, Rakvin B, Guo Z, Willeke M, Busato S, Weber T, Walde P. The use of Trametes versicolor laccase for the polymerization of aniline in the presence of vesicles as templates. Enzyme Microb Technol. 2014;55:72–84. doi: 10.1016/j.enzmictec.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 112.Vasil’eva IS, Morozova OV, Shumakovich GP, Shleev SV, Sakharov IY, Yaropolov AI. Laccase-catalyzed synthesis of optically active polyaniline. Synth Met. 2007;157:684–689. [Google Scholar]
- 113.Shumakovich GP, Otrokhov G, Vasil’eva IS, Pankratov D, Morozova O, Yaropolov A. Laccase-mediated polymerization of 3,4-ethylenedioxythiophene (EDOT) J Mol Catal B Enzym. 2012;81:66–68. [Google Scholar]
- 114.George PM, Lyckman AW, LaVan DA, Hegde A, Leung Y, Avasare R, Testa C, Alexander PM, Langer R, Sur M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials. 2005;26:3511–3519. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 115.Song YK, Palmore GTR. Conductive Polypyrrole via enzyme catalysis. J Phys Chem B. 2005;109:19278–19287. doi: 10.1021/jp0514978. [DOI] [PubMed] [Google Scholar]
- 116.López J, Alonso-Omlin EM, Hernández-Alcántara JM, Bárzana E, Gimeno M. Novel photoluminescent material by laccase-mediated polymerization of 4-fluoroguaiacol throughout defluorination. J Mol Catal B Enzymatic. 2014;109:70–75. [Google Scholar]
- 117.Nyanhongo GS, Kudanga T, Nugroho Prasetyo E, Gübitz GM. Enzymatic polymer functionalisation: advances in laccase and peroxidase derived lignocellulose functional polymers. Adv Biochem Eng Biotechnol. 2011;125:47–68. doi: 10.1007/10_2010_86. [DOI] [PubMed] [Google Scholar]
- 118.Chandra RP, Ragauskas AJ (2001) Laccase: the renegade of fiber modification. In: Tappi pulping conference. pp 1041–1051
- 119.Chandra RP, Ragauskas AJ. Elucidating the effects of laccase on the physical properties of high-kappa kraft pulps. Prog Biotechnol. 2002;21:165–172. [Google Scholar]
- 120.Chandra RP, Wolfaardt F, Ragauskas AJ. Biografting of celestine blue onto a high kappa kraft pulp. ACS Sym Ser. 2003;855:66–80. [Google Scholar]
- 121.Chandra RP, Lehtonen LK, Ragauskas AJ. Modification of high lignin content kraft pulps with laccase to improve paper strength properties. Laccase treatment in the presence of gallic acid. Biotechnol Prog. 2004;20:255–261. doi: 10.1021/bp0300366. [DOI] [PubMed] [Google Scholar]
- 122.Chandra RP, Felby C, Ragauskas AJ. Improving laccase-facilitated grafting of 4-hydroxybenzoic acid to high-kappa kraft pulps. J Wood Chem Technol. 2004;24:69–81. [Google Scholar]
- 123.Witayakran S, Ragauskas AJ. Modification of high-lignin softwood kraft pulp with laccase and amino acids. Enzyme Microb Technol. 2009;44:176–181. [Google Scholar]
- 124.Fillat A, Colom JF, Vidal T. A new approach to the biobleaching of flax pulp with laccase using natural mediators. Bioresour Technol. 2010;101:4104–4110. doi: 10.1016/j.biortech.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 125.Aracri E, Fillat A, Colom JF, Gutiérrez A, Del Río JC, Martínez AT, Vidal T. Enzymatic grafting of simple phenols on flax and sisal pulp fibres using laccases. Bioresour Technol. 2010;101:8211–8216. doi: 10.1016/j.biortech.2010.05.080. [DOI] [PubMed] [Google Scholar]
- 126.Aracri E, Roncero MB, Vidal T. Studying the effects of laccase-catalysed grafting of ferulic acid on sisal pulp fibers. Bioresour Technol. 2011;102:7555–7560. doi: 10.1016/j.biortech.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 127.Aracri E, Vidal T, Ragauskas AJ. Wet strength development in sisal cellulose fibers by effect of a laccase–TEMPO treatment. Carbohyd Polym. 2011;84:1384–1390. [Google Scholar]
- 128.Aracri E, Valls C, Vidal T. Paper strength improvement by oxidative modification of sisal cellulose fibers with laccase–TEMPO system: influence of the process variables. Carbohyd Polym. 2012;88:830–837. [Google Scholar]
- 129.Elegir G, Kindl A, Sadocco P, Orlandi M. Development of antimicrobial cellulose packaging through laccase-mediated grafting of phenolic compounds. Enzyme Microb Technol. 2008;43:84–92. [Google Scholar]
- 130.Fillat A, Gallardo O, Vidal T, Pastor FIJ, Díaz P, Roncero MB. Enzymatic grafting of natural phenols to flax fibres: development of antimicrobial properties. Carbohyd Polym. 2012;87:146–152. doi: 10.1016/j.carbpol.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 131.Widsten P, Heathcote C, Kandelbauer A, Guebitz G, Nyanhongo GS, Nugroho Prasetyo E, Kudanga T. Enzymatic surface functionalisation of lignocellulosic materials with tannins for enhancing antibacterial properties. Proc Biochem. 2010;45:1072–1081. [Google Scholar]
- 132.Fackler K, Kuncinger T, Ters T, Srebotnik E. Laccase-catalyzed functionalization with 4-hydroxy-3-methoxybenzylurea significantly improves internal bond of particle boards. Holzforschung. 2008;62:223–229. [Google Scholar]
- 133.Kudanga T, Nugroho Prasetyo E, Sipilä J, Nyanhongo GS, Guebitz G. Enzymatic grafting of functional molecules to the lignin model dibenzodioxocin and lignocellulose material. Enzyme Microb Technol. 2010;46:272–280. [Google Scholar]
- 134.Kudanga T, Nugroho Prasetyo E, Widsten P, Kandelbauer A, Jury S, Heathcote C, Sipilä J, Weber H, Nyanhongo GS, Guebitz GM. Laccase-catalyzed covalent coupling of fluorophenols increases lignocellulose surface hydrophobicity. Bioresour Technol. 2010;101:2793–2799. doi: 10.1016/j.biortech.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 135.Kudanga T, Nugroho Prasetyo E, Sipilä J, Guebitz GM, Nyanhongo GS. Reactivity of long chain alkylamines to lignin moieties: implications on hydrophobicity of lignocellulose materials. J Biotechnol. 2010;149:81–87. doi: 10.1016/j.jbiotec.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 136.Widsten P, Kandelbauer A. Laccase applications in the forest products industry: a review. Enzyme Microb Technol. 2008;42:293–307. [Google Scholar]
- 137.Felby C, Pedersen LS, Nielsen BR. Enhanced auto-adhesion of wood fibers using phenol oxidases. Holzforschung. 1997;51:281–286. [Google Scholar]
- 138.Widsten P, Laine JE, Tuominen S, Qvintus-Leino P. Effect of high defibration temperature on the properties of medium-density fiberboard (MDF) made from laccase-treated hardwood fibers. J Adhes Sci Technol. 2003;17:67–78. [Google Scholar]
- 139.Felby C, Thygesen LG, Sanadi A, Barsberg S. Native lignin for bonding of fiber boards-evaluation of bonding mechanisms in boards made from laccase-treated fibers of beech (Fagus sylvatica) Ind Crop Prod. 2004;20:181–189. [Google Scholar]
- 140.Kharazipour A, Hüttermann A, Kühne G, Rong M (1993) Process for glueing wood chips and articles produced by this process. Eur Pat Appl EP0565109
- 141.Kharazipour A, Mai C, Hüttermann A. Polyphenols for compounded materials. Polym Degrad Stabil. 1998;59:237–243. [Google Scholar]
- 142.Qvintus-Leino P, Widsten P, Tuominen S, Laine J, Kunnas J (2003) Method of producing compressed layered structures such as fiberboard or similar wood-based product. Int Pat Appl WO03047826
- 143.Hüttermann A, Mai C, Kharazipour A. Modification of lignin for the production of new compounded materials. Appl Microbiol Biotechnol. 2001;55:387–394. doi: 10.1007/s002530000590. [DOI] [PubMed] [Google Scholar]
- 144.Tsapikouni TS, Missirlis YF. Protein–material interactions: from micro-to-nano scale. Mater Sci Eng B. 2008;152:2–7. [Google Scholar]
- 145.Nady N, Schroën K, Franssen MCR, van Lagen B, Murali S, Boom RM, Mohy Eldin MS, Zuilhof H. Mild and highly flexible enzyme-catalyzed modification of poly(ethersulfone) membranes. ACS Appl Mater Interfaces. 2011;3:801–810. doi: 10.1021/am101155e. [DOI] [PubMed] [Google Scholar]
- 146.Nady N, Schroën K, Franssen MCR, Fokkink R, Mohy Eldin MS, Zuilhof H, Boom RM. Enzyme-catalyzed modification of PES surfaces: reduction in adsorption of BSA, dextrin and tannin. J Colloid Interface Sci. 2012;378:191–200. doi: 10.1016/j.jcis.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 147.Mikolasch A, Niedermeyer THJ, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Gesell M, Hessel s Julich WD, Lindequist U. Novel penicillins synthesized by biotransformation using laccase from Trametes spec. Chem Pharm Bull. 2006;54(5):632–638. doi: 10.1248/cpb.54.632. [DOI] [PubMed] [Google Scholar]
- 148.Mikolasch A, Niedermeyer THJ, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Salazar MG, Hessel S, Julich WD, Lindequist U. Novel cephalosporins synthesized by amination of 2,5-dihydroxybenzoic acid derivatives using fungal laccases II. Chem Pharm Bull. 2007;55(3):412–416. doi: 10.1248/cpb.55.412. [DOI] [PubMed] [Google Scholar]
- 149.Mikolasch A, Wurster M, Lalk M, Witt S, Seefeldt S, Hammer E, Shauer F, Julich WD, Lindequist U. Novel b-lactam antibiotics synthesized by amination of catechols using fungal laccase. Chem Pharm Bull. 2008;56(7):902–907. doi: 10.1248/cpb.56.902. [DOI] [PubMed] [Google Scholar]
- 150.Mikolasch A, Manda K, Schlüter R, Lalk M, Witt S, Seefeldt S, Hammer E, Schauer F, Hammer E, Jülich WD, Lindequist U. Comparative analyses of laccase-catalyzed amination reactions for production of novel β-lactam antibiotics . Biotechnol Appl Bioc. 2012;59:295–306. doi: 10.1002/bab.1026. [DOI] [PubMed] [Google Scholar]
- 151.Yang Z, Pattamana K, Molino BF, Haydar SN, Cao Y, Bois F, Maeng JH, Hemenway MS, Rich JO, Khmelnitsky YL, Friedrich TD, Peace D, Michels PC. Novel Oxidation of cyclosporin A: preparation of cyclosporin methyl vinyl ketone (Cs-MVK) Synlett. 2009;18:2935–2938. [Google Scholar]
- 152.Fontana G, Baldelli E, Riva S, Danieli B (2009) Process for the preparation of bisindole alkaloid derivatives WO 2009153025 A1
- 153.Sagui F, Chirivì C, Fontana G, Nicotra S, Passarella D, Riva S, Danieli B. Laccase-catalyzed coupling of catharanthine and vindoline: an efficient approach to the bis indole alkaloid anhydrovinblastine. Tetrahedron. 2009;65:312–317. [Google Scholar]
- 154.Herter S, Michalik D, Mikolasch A, Schmidt M, Wohlgemuth R, Bornscheuer, Schauer F. Laccase-mediated synthesis of 2-methoxy-3-methyl-5-(alkylamino)-and 3-methyl-2,5-bis(alkylamino)-[1,4]-benzoquinones. J Mol Catal B Enzym. 2013;90:91–97. [Google Scholar]
- 155.Wellington KW, Steenkamp P, Brady D. Diamination by N-coupling using a commercial laccase. Bioorgan Med Chem. 2010;18(4):1406–1414. doi: 10.1016/j.bmc.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 156.Wellington KW, Kolesnikova NI. A laccase-catalysed one-pot synthesis of aminonaphthoquinones and their anticancer activity. Bioorgan Med Chem. 2012;20(14):4472–4481. doi: 10.1016/j.bmc.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 157.Ravikumar G, Gimathi DG, Kalaiselvi M, Devaki K, Uma C. Antioxidant property and anti-proliferative activity of recombinant laccase from Hypsizygus ulmarius against breast cancer cell line (MCF7) Int J Res Pharm Biomed Sci. 2013;4:1148–1152. [Google Scholar]
- 158.Zhang GQ, Tian T, Liu YP, Wang HX, Chen QJ. A laccase with anti-proliferative activity against tumor cells from a white root fungus Abortiporus biennis . Process Biochem. 2011;46:2336–2340. [Google Scholar]
- 159.Hu DD, Zhang RY, Zhang GQ, Wang HX, Ng TB. A laccase with antiproliferative activity against tumor cells from an edible mushroom, white common Agrocybe cylindracea . Phytomedicine. 2011;18:374–379. doi: 10.1016/j.phymed.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 160.Othman AM, Elshafei AM, Hassan MM, Haroun AM, Elsayed MA, Farrag AA. Purification, biochemical characterization and applications of Pleurotus ostreatus ARC 280 laccase. Br Microbiol Res J. 2014;4(12):1418–1439. [Google Scholar]
- 161.Wong JH, Ng TB, Jiang Y, Liu F, Sze SC, Zhang KY. Purification and characterization of a laccase with inhibitory activity toward HIV-1 reverse transcriptase and tumor cells from an edible mushroom (Pleurotus cornucopiae) Protein Peptide Lett. 2010;17(8):1040–1047. doi: 10.2174/092986610791498966. [DOI] [PubMed] [Google Scholar]
- 162.Wang HX, Ng TB. Purification of a novel low-molecular-mass laccase with HIV-1 reverse transcriptase inhibitory activity from the mushroom Trichoderma gigantum . Biochem Bioph Res Co. 2004;315:450–454. doi: 10.1016/j.bbrc.2004.01.064. [DOI] [PubMed] [Google Scholar]
- 163.Zhang GQ, Chen QJ, Wang HX, Ng TB. A laccase with inhibitory activity against HIV-1 reverse transcriptase from mycorrhizal fungus Lepiota ventrisospora . J Mol Catal B Enzym. 2013;85–86:31–36. [Google Scholar]
- 164.Sun J, Wang H, Ng TB. Isolation of a laccase with HIV-1 reverse transcriptase inhibitory activity from fresh fruiting bodies of the Lentinus edodes (Shiitake mushroom) Indian J Biochem Bio. 2011;48:88–94. [PubMed] [Google Scholar]
- 165.Sun J, Chen QJ, Cao QQ, Wu YY, Xu LJ, Zhu MJ, Ng TB, Wang HX, Zhang GQ. A laccase with antiproliferative and HIV-I reverse transcriptase inhibitory activities from the mycorrhizal fungus Agaricus placomyces . J Biomed Biotechnol. 2012 doi: 10.1155/2012/736472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu X, Huang C, Chen Q, Wang H, Zhang J. A novel laccase with inhibitory activity towards HIV-I reverse transcriptase and antiproliferative effects on tumor cells from the fermentation broth of mushroom Pleurotus cornucopiae . Biomed Chromatogr. 2014;28(4):548–553. doi: 10.1002/bmc.3068. [DOI] [PubMed] [Google Scholar]
- 167.Zhao S, Rong CB, Kong C, Liu Y, Xu F, Miao DJ, Wang SX, Wang HX, Zhang GQ. A novel laccase with potent antiproliferative and HIV-1 reverse transcriptase inhibitory activities from mycelia of mushroom Coprinus comatus . J Biomed Biotechnol. 2014 doi: 10.1155/2014/417461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu L, Wang HX, Ng T. A laccase with HIV-1 reverse transcriptase inhibitor activity from the broth of mycelial culture of the mushroom Lentinus tigrinus . J Biomed Biotechnol. 2012 doi: 10.1155/2012/536725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zou YJ, Wang HX, Ng TB, Huang CY, Zhang JX. Purification and characterization of a novel laccase from the edible mushroom Hericium coralloides . J Microbiol. 2012;50(1):72–78. doi: 10.1007/s12275-012-1372-6. [DOI] [PubMed] [Google Scholar]
- 170.Harris ZL, Davis-Kaplan SR, Gitlin JD, Kaplan J. A fungal multicopper oxidase restores iron homeostasis in aceruloplasminemia. Blood. 2004;103:4672–4673. doi: 10.1182/blood-2003-11-4060. [DOI] [PubMed] [Google Scholar]
- 171.Kulys J, Bratkovskaja I, Vidziunaite R. Laccase-catalysed iodide oxidation in presence of methyl syringate. Biotechnol Bioeng. 2005;92(1):124–128. doi: 10.1002/bit.20610. [DOI] [PubMed] [Google Scholar]
- 172.Xu F (1998) Method of producing iodine by use of a copper containing oxidase enzyme. US 5766896 A
- 173.Ihssen J, Schubert M, Thony-Meyer L, Richter M. Laccase Catalyzed Synthesis of Iodonated Phenolic Compounds with Antifungal Activity. PLoS One. 2013;9(3):e89924. doi: 10.1371/journal.pone.0089924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Christensen BE, Danielsen S, Oestergaard LH (2007) Methods and compositions for killing spores. WO 2006094975 A3
- 175.Eggert C. Laccase-catalyzed formation of cinnabarinic acid is responsible for antibacterial activity of Pycnoporus cinnabarinus . Microbiol Res. 1997;152:315–318. doi: 10.1016/S0944-5013(97)80046-8. [DOI] [PubMed] [Google Scholar]
- 176.Grover N, Borkar IV, Dinu CZ, Kane RS, Dordick JS. Laccase- and chloroperoxidase-nanotube paint composites with bactericidal and sporicidal activity. Enzyme Microb Tech. 2012;50:271–279. doi: 10.1016/j.enzmictec.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 177.Chen SC, Chen CH, Chern CL, Hsu LS, Huang YC, Chung KT, Chye SM. p-Phenylenediamine induces p53-mediated apoptosis in Mardin-Darby canine kidney cells. Toxicol In Vitro. 2006;20:801–807. doi: 10.1016/j.tiv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 178.Huang YC, Hung WC, Kang WY, Chen WT, Chai CY. p-Phenylenediamine induced DNA damage in SV-40 immortalized human uroepithelial cells and expression of mutant p53 and COX-2 proteins. Toxicol Lett. 2007;170:116–123. doi: 10.1016/j.toxlet.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 179.Araujo R, Fernandes M, Cavaco-Paulo A, Gomes A. Biology of human hair: know your hair to control it. Adv Biochem Eng Biot. 2011;13:121–143. doi: 10.1007/10_2010_88. [DOI] [PubMed] [Google Scholar]
- 180.Takada K, Nakamura A, Matsuo N, Inoue A, Someya K, Shimogaki H. Influence of oxidative and/or reductive treatment on human hair (I): analysis of hair-damage after oxidative and/or reductive treatment. J Oleo Sci. 2003;52:541–548. [Google Scholar]
- 181.Fu J, Nyanhongo GS, Gubitz GM, Cavaco Paulo A, Kim S. Enzymatic colouration with laccase and peroxidases: recent progress. Biocatal Biotransfor. 2012;30(1):125–140. [Google Scholar]
- 182.Lavanya C, Dhankar R, Chhikara S, Sheoran S. Degradation of toxic dyes: a review. Int J Curr Microbiol App Sci. 2014;3(6):189–199. [Google Scholar]
- 183.Salame TM, Yarden O, Hadar Y. Pleurotus ostreatus manganese-dependent peroxidase silencing impairs decolourization of Orange II. Microb Biotechnol. 2010;3:93–106. doi: 10.1111/j.1751-7915.2009.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jeon JR, Kim EJ, Murugesan K, Park HK, Kim YM, Kwon JH, Kim WG, Lee JY, Chang YS. Laccase-catalysed polymeric dye synthesis from plant-derived phenols for potential application in hair dyeing: enzymatic colourations driven by homo- or hetero-polymer synthesis. Microb Biotechnol. 2010;3:324–335. doi: 10.1111/j.1751-7915.2009.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Otsuka Saito K, Ikeda R, Endo K, Tsujino Y, Takagi M, Tamiya E. Isolation of a novel alkaline-induced laccase from Flammulina velutipes and its application for hair coloring. J Biosci Bioeng. 2012;113(5):575–579. doi: 10.1016/j.jbiosc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 186.Chen CY, Huang YC, Wei CM, Meng M, Liu WH, Yang CH. Properties of the newly isolated extracellular thermo-alkali-stable laccase from thermophilic actinomycetes, Thermobifida fusca and its application in dye intermediates oxidation. AMB Express. 2013;3:49. doi: 10.1186/2191-0855-3-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fang Z, Zhou P, Chang F, Yin Q, Fang W, Yuan J, Zhang X, Xiao Y. Structure-based rational design to enhance the solubility and thermostability of a bacterial laccase Lac15. PLoS One. 2014;9(7):e102423. doi: 10.1371/journal.pone.0102423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Golz-Berner K, Walzel B, Zastrow L, Doucet O (2004) Cosmetic or dermatological preparation with skin-lightening proteins. WO2004017931
- 189.Kunamneni A, Plou FJ, Ballesteros A, Alcalde M. Laccases and their applications: a patent review. Recent Pat Biotechnol. 2008;2(1):10–24. doi: 10.2174/187220808783330965. [DOI] [PubMed] [Google Scholar]
- 190.Nagai M, Kawata M, Watanabe H, Ogawa M, Saito K, Takesawa T, Kanda K, Sato T. Important role of fungal intracellular laccase for melanin synthesis:purification and characterization of an intracellular laccase from Lentinula edodes fruit body. Microbiology. 2003;149:2455–2462. doi: 10.1099/mic.0.26414-0. [DOI] [PubMed] [Google Scholar]
- 191.Madhavi V, Lele SS. Laccase: properties and applications. BioResources. 2009;4:4. [Google Scholar]
- 192.Takase T, Narise A, Sakurai K (2011) Deodorant composition. WO2011105042 A1
- 193.Brinch DS, Pederson PB. Toxicological studies on laccase from Myceliophthora thermophila expressed in Aspergillus oryzae . Regul Toxicol Pharm. 2002;35(3):296–307. doi: 10.1006/rtph.2002.1538. [DOI] [PubMed] [Google Scholar]
- 194.Mano N, Durand F (2013) Laccase of Podospora anserina and uses of same. WO2013175399 A1
- 195.Bhogal RK, Casey J, Ganguli S, Hunter KJ, Koek JH, Redfern SP (2013) Hair colouring composition. WO2013189966 A2
- 196.Doucet O, Golz-Berner K, Walzel B, Zastrow L. Cosmetic or dermatological preparation with skin-lightening proteins. WO. 2004;2004017931:A1. [Google Scholar]
- 197.Koike K (2002). Multiple agent type hair dye. JP2002255764
- 198.Lang G, Cotteret J (2002) Ready to use hair dye containing oxidation coloring agent, cationic polymers and enzyme; hydrogen peroxide free; homogeneous, intense; nondegrading or decolouring of keratinous fibers. US 6471730 B1
- 199.Lang G, Cotteret J (2004) Composition for the oxidation dyeing of keratinous fibres containing a laccase and dyeing method using this composition. US2004255401 A1
- 200.Nakajima M, Fujita H, Kikuchi Y, Kobayashi I (2002) Cosmetic mixture for the oxidation tinting of keratin fibres, containing in a support material suitable for tinting keratin fibres (a) at least one laccase-type enzyme; (b) at least one polymer thickener selected from polymers. US 20020043731 A1
- 201.Onuki T, Nogucji M, Mitamura J (2000) Oxidative hair dye com-position containing laccase. WO0037030
- 202.Pereira R, Burgaud H (2005) Producing tetraazapentamethine compounds comprises reacting an azine compound with an oxidizing agent, useful for dyeing keratinic fibers, e.g. hair. FR2863487 A1
- 203.PLoS G (2005) Oxidation dyeing method using N-acetyclysteine as a reducing agent and laccase as an oxidising agent. US 6840964 B1
- 204.PLoS G (2001) Composition for oxidative dyeing of keratinous fibres and dyeing process using same. EP1138318 A2
- 205.Pruche F, Saint LP, Bernards B (2000) Hydroxystilbene compounds of formula (I) used as components in oxidation dye systems especially for dyeing hair. EP1013260
- 206.Shichiri S, Morita K, Koike K (2003) Hair cosmetic. JP2003055175 A2
- 207.Riva S. Laccases blue enzymes for green chemistry. Trends Biotechnol. 2006;24:219–226. doi: 10.1016/j.tibtech.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 208.Sorensen NH (2001) Method for dyeing dry hair. WO2001068042 A1
- 209.Tsuji K, Yoshino T, Asai Y (2002) Composition for bleaching melamine. JP2002012535 A2