Abstract
Shiga toxin-producing Escherichia coli bacteria cause hemorrhagic colitis and hemolytic uremic syndrome in humans. Currently, only supportive treatment is available for diagnosed patients. We show here that 24-h pretreatment with an ether lipid precursor, the alkylglycerol sn-1-O-hexadecylglycerol (HG), protects HEp-2 cells against Shiga toxin and Shiga toxin 2. Also the endothelial cell lines HMEC-1 and HBMEC are protected against Shiga toxins after HG pretreatment. In contrast, the corresponding acylglycerol, dl-α-palmitin, has no effect on Shiga toxicity. Although HG treatment provides a strong protection (~30 times higher IC50) against Shiga toxin, only a moderate reduction in toxin binding was observed, suggesting that retrograde transport of the toxin from the plasma membrane to the cytosol is perturbed. Furthermore, endocytosis of Shiga toxin and retrograde sorting from endosomes to the Golgi apparatus remain intact, but transport from the Golgi to the endoplasmic reticulum is inhibited by HG treatment. As previously described, HG reduces the total level of all quantified glycosphingolipids to 50–70 % of control, including the Shiga toxin receptor globotriaosylceramide (Gb3), in HEp-2 cells. In accordance with this, we find that interfering with Gb3 biosynthesis by siRNA-mediated knockdown of Gb3 synthase for 24 h causes a similar cytotoxic protection and only a moderate reduction in toxin binding (to 70 % of control cells). Alkylglycerols, including HG, have been administered to humans for investigation of therapeutic roles in disorders where ether lipid biosynthesis is deficient, as well as in cancer therapy. Further studies may reveal if HG can also have a therapeutic potential in Shiga toxin-producing E. coli infections.
Keywords: Shiga toxin, Globotriaosylceramide, Endocytosis, Intracellular transport, Ether lipids, Alkylglycerol
Introduction
Shiga toxins are the major virulence factors released by the bacteria Shigella dysenteriae and Shiga toxin-producing Escherichia coli (STEC) [1]. Infections by STEC may cause hemorrhagic colitis and the more serious hemolytic uremic syndrome (HUS), which may result in death [2]. The prototypic Shiga family member, Shiga toxin, is produced by S. dysenteriae, while STEC express Shiga toxin 1, which is virtually identical to Shiga toxin, and/or one of several variants of the immunologically distinct Shiga toxin 2. All Shiga toxins have an AB5 structure with a catalytically active A subunit and a B subunit pentamer, which binds to the target cell surface. In humans, the only identified receptor for Shiga toxins is the neutral glycosphingolipid globotriaosylceramide (Gb3) [3]. Expression of Gb3 in humans is restricted to certain cell types [4], including kidney endothelial cells, which are the main target in HUS [2]. However, neurological symptoms can also be associated with such infections due to Gb3 expression on brain endothelial cells and neurons [5]. From the plasma membrane of the target cell, the toxin is internalized by clathrin-dependent and clathrin-independent endocytosis, and thereafter sorted from early endosomes to the trans-Golgi network (TGN) [6]. The toxin is further transported retrogradely to the endoplasmic reticulum (ER) [7], where the A1 fragment of the toxin is released and translocated across the ER membrane to the cytosol [8]. In the cytosol, the A1 fragment inhibits protein synthesis by catalytical inactivation of the 60S ribosomal subunit [9]. On a longer term, some cell types also undergo Shiga toxin-induced apoptosis [10].
Although a range of proteins required for efficient uptake and retrograde transport of Shiga toxins in target cells have been identified [1], far less is known about how the lipid composition of membranes affects the transport. The accessibility of the trisaccharide headgroup of the Gb3 receptor is thought to be dependent both on the Gb3 isoform, as well as on the surrounding membrane microenvironment [3], and can be modulated by phospholipid content [11] and by cholesterol levels [12–14]. Binding to Gb3 associated with lipid rafts/detergent-resistant membranes (DRMs) is suggested to be required for toxin-mediated signaling [15–17], efficient retrograde transport and cytotoxicity [18–20], and may also define Shiga toxin-pathology in kidneys [21].
The major lipid constituents of cellular membranes are phospholipids [22]. The most common phospholipids consist of a glycerol backbone with fatty acids attached through ester linkages at the sn-1 and sn-2. However, an important subgroup of phospholipids is defined by the presence of an ether-linked alkyl or alkenyl chain at the sn-1 position of the glycerol backbone. Several possible biological roles have been attributed to ether-linked phospholipids [23, 24]; (i) they serve as mediators of membrane structure and dynamics by decreasing fluidity, increasing order, and promoting fusion and fission events, (ii) they serve as endogenous antioxidants, and (iii) they may serve as storages of polyunsaturated fatty acid and lipid mediators, which may be released through plasmalogen-selective phospholipase A2 hydrolysis. The biological importance of these lipids is illustrated by the severe pathologies observed in patients with deficient ether lipid biosynthesis causing the malformation syndrome rhizomelic chondrodysplasia punctata [23], and by ether lipid-deficient mice, which suffer from several abnormalities in the central nervous system, arrest in spermatogenesis, and development of cataract [25]. Ether phospholipids are formed from dihydroxyacetone phosphate in a biosynthetic pathway initiated in peroxisomes, distinct from that of diacyl phospholipids [24]. The alkylglycerol backbone of naturally occurring ether lipids primarily contains 16:0, 18:0, or 18:1 hydrocarbon chains [26]. In a possible scavenging pathway, ether phospholipids can also be formed from alkylglycerols, which enter the pathway through phosphorylation by an alkylglycerol kinase [27], but the sequence of this enzyme is not yet characterized [28]. Dietary alkylglycerols have been shown to be incorporated into tissue plasmalogens of rodents and humans [29], and alkylglycerols are able to restore cellular levels of ether phospholipids both in vitro [30, 31] and in ether-phospholipid deficient mice [32]. In addition to the potential therapeutic role in treating plasmalogen deficiency, alkylglycerols have been studied as anti-cancer agents [26].
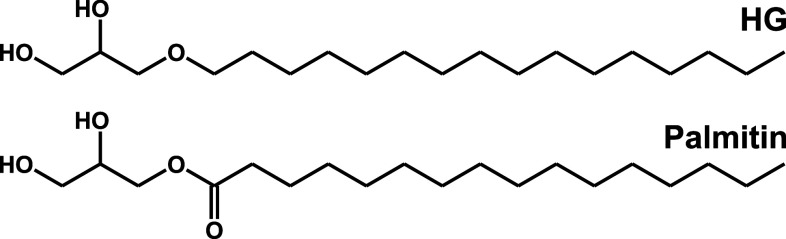
We and others have previously found that the intracellular sorting and toxicity of Shiga toxins are dependent on the glycosphingolipid contents of the cell [3, 33]. In another study [34] we have described how the addition of the ether lipid precursor, the alkylglycerol sn-1-O-hexadecylglycerol (HG; Fig. 1), but not the corresponding acylglycerol dl-α-palmitin, causes major changes in the lipidome of HEp-2 cells, including glycosphingolipids. In the present study, we report that the addition of HG strongly protects against Shiga toxins, and that this protection is accompanied not only by a reduced availability of Gb3 receptors for binding at the cell surface, but also an inefficient retrograde transport of the toxin from the Golgi to the ER.
Fig. 1.
The chemical structure of the ether lipid precursor sn-1-O-hexadecylglycerol (HG) and its ester-linked analog dl-α-palmitin
Materials and methods
Reagents
Shiga holotoxin was a kind gift from Dr. J. L. Kozlov (Academy of Sciences of Russia, Moscow, Russia) and Dr. J. E. Brown (USAMRIID, Fort Detrick, MD, USA). A non-toxic Shiga toxin 1-mutant (Stx1-mut) [35] was purified as previously described [36] from the pSW09 plasmid, which was a kind gift from Dr. A. D. O’Brien (Uniformed Services University of the Health Sciences, Bethesda, MD, USA). Shiga toxin 2 was purified from NVH 734 phage DNA1 [37] as described below. Ricin and cholera toxin were from Sigma-Aldrich (St. Louis, MO, USA). Diphtheria toxin was from Connaught Laboratories. Modeccin was prepared as previously described [38]. sn-1-O-hexadecylglycerol (HG) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and dl-α-palmitin was from Sigma-Aldrich. Bovine liver phosphatidylinositol (PI) and lysophosphatidylinositol (LPI) were from Avanti Polar Lipids (Alabaster, AL, USA). The following primary antibodies were used: mouse anti-Shiga toxin 3C10 (Toxin Technology, Sarasota, FL, USA), rabbit anti-giantin (Covance Inc., Princeton, NJ, USA), rabbit anti-PDI (Santa Cruz Biotechnology), sheep anti-TGN46 (AbD Serotec, Kidlington, UK), mouse anti-flotillin-1, mouse anti-calnexin, and mouse anti-SNX1 (all BD Biosciences, Franklin Lakes, NJ, USA). Fluorophore-labeled and HRP-labeled secondary antibodies were from Jackson ImmunoResearch. l-[3,4,5-3H(N)]leucine, Na125I, and d-[2-3H(N)]mannose was from PerkinElmer (Waltham, MA, USA). Other chemicals used were from Sigma-Aldrich unless otherwise stated.
Cell lines
HEp-2, HEK-293, and PC-3 cells were obtained from ATCC/LGC (Manassas, VA, USA). HEp-2 and HEK-293 were grown at 5 % CO2 in Dulbecco’s modified Eagle’s medium GlutaMAX (Invitrogen, Carlsbad, CA, USA) with 10 % (v/v) fetal calf serum (FCS, PAA Laboratories, Linz, Austria) supplemented with 100 U/ml penicillin and 100 U/ml streptomycin (Invitrogen). PC-3 cells were grown at 5 % CO2 in DMEM:F12 1:1 GlutaMAX (Invitrogen) with 7 % (v/v) FCS and 100 U/ml penicillin and 100 U/ml streptomycin. HMEC-1 (Human dermal microvascular endothelial cells) cells were a kind gift from Prof. P. Jennings (Innsbruck Medical University, Innsbruck, Austria), and grown at 5 % CO2 in MCDB 131 medium (Invitrogen) with 10 % (v/v) FCS, GlutaMAX (Invitrogen), 10 ng/ml EGF (Sigma-Aldrich) and 0.2 µg/ml hydrocortisone (Sigma-Aldrich). HBMEC (Human brain microvascular endothelial cells) were a kind gift from Prof. J. Müthing (Institute of Hygiene, Münster, Germany), and were grown in EGM-2 (Endothelial Growth Medium-2, Lonza, Basel, Switzerland). Cells were seeded 1 day prior to experiments. Experiments with addition of lipids were performed by adding HG (dissolved in ethanol), palmitin (dissolved in ethanol), PI (dissolved in chloroform), LPI (dissolved in ethanol:water, 1:1 v/v) or 0.1 % (v/v) ethanol (control) to the growth medium.
Transfection
Cells were seeded in six-well plates 1 day prior to transfection with siRNA. Non-targeting control siRNA was from Dharmacon (Lafayette, CO, USA). Gb3 synthase siRNA was from Qiagen (Venlo, Netherlands), and in this paper Hs_A4GALT_2_FlexiTube is designated ‘Gb3 synthase siRNA 1’ and Hs_A4GALT_3_FlexiTube is ‘Gb3 synthase siRNA 2’. Cells were transfected using Lipofectamine RNAiMax from Invitrogen, according to the manufacturer’s protocol. Cells were incubated for 4 h with siRNA and transfection reagent in DMEM GlutaMAX without serum, before replacing the medium with DMEM GlutaMAX supplemented with 10 % (v/v) FCS, 100 U/ml penicillin, and 100 U/ml streptomycin.
Quantitative real-time PCR
When performing experiments with siRNA-treated cells, the efficiency of the knockdown at the mRNA level was quantified in parallel by performing reverse transcription followed by quantitative polymerase chain reaction analysis (qRT-PCR). RNA isolation, reverse transcription and the qRT-PCR analysis was performed as previously described [39], using RNeasy Plus Mini Kit (Qiagen), iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) and LightCycler 480 SYBR green 1 master mix (Roche, Basel, Switzerland). TBP (TATA box binding protein) was used as an internal reference gene, with primers from MWG Biotech (Ebersberg, Germany). The Quantitect Primer Assay for Gb3 synthase (Hs_A4GALT_1_SG) was from Qiagen. The analysis was performed using a LightCycler 480 Real-Time PCR system (Roche).
Purification of Shiga toxin 2
An expression plasmid was constructed by amplifying stx 2 AB from E. coli NVH 734 phage DNA1 [37] using primers stx 2-F2 (5′-ATGAAGTGTATATTATTTAAA-3′) and stx 2-down (5′-GAATTCTCAGTCATTATTAAACTG-3′), and cloning the product covering position 24437–25678 in GenBank accession JQ011318.1 into a pEXP5-CT/TOPO vector (Invitrogen). Shiga toxin 2 was expressed from the resulting pEXP5-CT/TOPO-stx 2 AB in E. coli BL21pLysS cells. Toxin production was induced with 1 mM isopropylthio-β-galactoside for 3.5 h and the cell pellet was lysed by repeated freeze/thawing before treatment with DNase. The cell lysate was precipitated with (NH4)2SO4, dialyzed against 25 mM Tris–HCl, pH 7.5, applied on a DEAE-Sephacel column (GE Healthcare, Little Chalfont, UK) and fractionated with a 0–0.5 M NaCl gradient. Each fraction was analyzed for toxic activity by a [3H]leucine incorporation assay. Toxin-containing fractions were pooled and applied on a hydroxyapatite column using a sodium phosphate gradient of 0–0.25 M at pH 6.8 for elution. Toxin-containing fractions from hydroxyapatite chromatography were pooled and diluted 1:1 in 200 mM sodium acetate, pH 4.5. Aliquots of 10–20 ml were applied on a 1 ml Mono S 5/50 column (GE Healthcare) using an ÄKTA explorer 10 chromatography system (GE Healthcare). Proteins in the sample were separated and eluted with a 0–1,000 mM NaCl gradient in 200 mM sodium acetate, pH 4.5. Stx2 eluted at approximately 100 mM NaCl. Fractions from cation-exchange chromatography were pooled and concentrated ten times by ultrafiltration with a Vivaspin 20 device (MWCO 10,000 Da; Sartorius, Göttingen, Germany). Aliquots of 0.5 ml were applied on a Superdex 200 10/300 column (GE Healthcare) and run isocratically with 20 mM Tris pH 7.5, 100 mM NaCl. Stx2 eluted in one symmetrical peak at retention volume 14.5 ml. Fractions from gel filtration were pooled and concentrated seven times by ultrafiltration with a Vivaspin 20 device. Protein concentration was measured with a Bradford assay (Bio-Rad Protein Assay; Bio-Rad).
Toxicity assay
Cells were seeded in 24-well plates 1 day prior to pretreatment with lipid precursors or transfection with siRNA. Cells were washed with leucine-free HEPES-buffered medium before Shiga toxin 1, Shiga toxin 2, ricin, modeccin, or diphtheria toxin was added in increasing concentrations. In experiments using lipid precursors, these were also present during incubation with toxins. After incubation for 3 h, the medium was replaced by leucine-free medium containing 1 µCi/ml [3H]leucine for 20 min at 37 °C to radioactively label newly synthesized proteins. The proteins were precipitated with 5 % (w/v) trichloroacetic acid (TCA), washed once in TCA and dissolved in 0.1 M KOH. The protein synthesis was quantified by measuring the amount of incorporated [3H]leucine measured by β-counting on a Tri-Carb 2100TR Liquid Scintillation Analyzer (Packard BioScience/PerkinElmer).
Cholera toxin-induced cAMP production
HG- and control-treated HEK-293 or PC-3 cells were incubated with HEPES-buffered medium supplemented with 0.2 mM IBMX (Sigma-Aldrich) for 15 min before addition of cholera toxin (Sigma-Aldrich). Due to different cholera toxin sensitivities of the cell lines, HEK-293 cells were incubated with 10 ng/ml cholera toxin for 0, 30, or 60 min, whereas PC-3 cells were incubated with 2 µg/ml cholera toxin for 0, 1, or 2 h. The cells were subsequently washed with PBS and lysed in 100–200 µl AlphaLisa immunoassay buffer (PerkinElmer) supplemented with 0.2 mM IBMX and a mixture of Complete protease inhibitors (Roche). cAMP levels were determined by the AlphaScreen cAMP Assay Kit (PerkinElmer). The cAMP standard curve was generated according to the manufacturer’s description. Triplicates (5 µl) of the standard curve and of each sample were incubated with cAMP acceptor beads and a detection mix containing biotinylated cAMP and streptavidin-coated donor beads following the manufacturer’s protocol. The AlphaScreen signal was detected using a Synergy 2 Multi-Mode Microplate Reader (Bio-Tek Instruments, Inc, Winooski, VT, USA) equipped with a bandpass 680/30 excitation filter, a bandpass 570/100 emission filter, and a 635-nm dichroic mirror, and data was obtained using the Gen5software (Bio-Tek Instruments, Inc).
Binding and endocytosis of biotinylated Shiga toxin
Total cell-associated and endocytosed biotinylated Shiga toxin was quantified as previously described [40]. Briefly, after preincubation with lipid precursors or siRNA transfection, cells were incubated with 40 ng/ml biotinylated Shiga toxin, containing a reducible EZ-link Sulfo-NHS-SS-Biotin (Pierce Biotechnology, Rockford, IL, USA), for 20 min in HEPES-buffered medium at 37 °C. This incubation time is too short for the toxin to reach the ER and its reducing environment. The cells were washed with cold buffer (0.14 M NaCl, 2 mM CaCl2, 20 mM HEPES, pH 8.6) before treating half the cells with sodium 2-mercaptoethanesulfonate (MESNa, Sigma-Aldrich) for 30 min to reduce the SS-biotin from the cell surface-bound toxin and thereby determine the amount of internalized toxin. The other half of the cells were mock-treated to determine the amount of total-associated toxin (internalized + cell surface-bound toxin). All cells were then washed and lysed in a lysis buffer [0.1 M NaCl, 5 mM MgCl2, 1 % (v/v) Triton X-100, 20 mM HEPES, 60 mM n-octyl-β-pyranoside, pH 7.4]. The lysates were mixed with 0.1 mg/ml streptavidin-coated Dynabeads (Invitrogen) and 0.5 µg/ml BV-TAG-labeled monoclonal anti-Shiga toxin antibody containing a Tris(bipyridine)-chelated ruthenium (II) atom (BioVeris Corporation/Roche) in assay diluent [0.2 % (w/v) BSA, 0.5 % (v/v) Tween-20 in PBS] for 1.5 h with gentle shaking. The amount of streptavidin-captured BV-TAG-labeled Shiga toxin was quantified using the electrochemiluminescent detection system M1R Analyzer (BioVeris Corporation/Roche).
Binding of 125I-labeled Shiga toxin 1-mutant
Stx1-mut was labeled with 125I using the IODO-GEN Iodination Reagent (Pierce Biotechnology) according to the manufacturer’s protocol. After pretreatment with or without HG, cells were incubated up to 3 h at 37 °C in HEPES-buffered medium in the presence of 10 ng/ml (~40 cpm/pg) 125I-labeled Stx1-mut. After removing the medium, washing three times with PBS, and dissolving the cells in 0.1 M KOH, the total amount of cell-associated toxin was quantified using an LKB Wallac 1261 Multigamma γ-counter (LKB Instruments, Mount Waverley, Australia).
Shiga toxin 1-mutant A1 release
HG- and control-pretreated HEp-2 cells were incubated with 10 ng/ml (~40 cpm/pg) 125I-labeled Stx1-mut for 5 h in HEPES-buffered medium at 37 °C. Cells were then incubated with 1 mM N-ethylmaleimide for 5 min at 37 °C. After removing the medium, non-reducing sample buffer was added to the cells, before boiling and separating the proteins by SDS-PAGE. The content of the gel was transferred to a polyvinylidene difluoride membrane (Bio-Rad), and investigated by autoradiography.
Mannosylation of Shiga B-sulfglyc
Shiga B-sulfglyc containing two sulfation sites and three partly overlapping N-glycosylation sites was purified as previously described [41]. After 24-h pretreatment with 20 µM HG or 0.1 % (v/v) ethanol (control), cells were washed twice with glucose-free DMEM (Invitrogen) supplemented with 1.8 µl/ml d-(+)glucose solution [10 % (w/v)] and incubated with 0.1 mCi/ml [3H]mannose for 3 h at 37 °C in the same medium, including 20 µM HG or 0.1 % (v/v) ethanol. Then, 1 µg/ml Shiga B-sulfglyc was added, and the cells were incubated for 3 h to allow the toxin to be transported to the ER. The Golgi mannosidase II inhibitor swainsonine (1 µg/ml, Sigma-Aldrich) was added together with the toxin to inhibit deglycosylation during the incubation period. The cells were washed in ice-cold PBS and lysed in lysis buffer [0.1 M NaCl, 5 mM MgCl2, 1 % (v/v) Triton X-100, 20 mM HEPES, Complete protease inhibitors, 60 mM n-octyl-β-pyranoside]. Shiga B-sulfglyc was immunoprecipitated from cleared lysates overnight at 4 °C using Protein A Sepharose beads (GE Healthcare) with anti-Shiga toxin antibody (3C10) adsorbed. After immunoprecipitation the beads were washed twice in 0.35 % (v/v) Triton X-100 in PBS, resuspended in sample buffer and boiled. The immunoprecipitate was loaded on a gel and separated using SDS-PAGE, before transfer to polyvinylidene difluoride membrane and investigation by autoradiography.
Immunofluorescence confocal microscopy
Cells were seeded on glass coverslips and pretreated with lipid precursors, LPI or siRNA. Cells were incubated with 250 ng/ml Shiga toxin or Stx1-mut in HEPES-buffered medium at 37 °C. To allow the toxin to be transported to the Golgi apparatus, cells were treated continuously for 45 min, and to allow the toxin to be transported to the ER, cells were incubated for 1 h before chasing for 3 h. In experiments using lipid precursors, these were present throughout the incubation period. Cells were washed with PBS and fixed in 10 % (v/v) formalin (Sigma-Aldrich) for 20 min. The cells were then washed again with PBS, permeabilized with 0.1 % (v/v) Triton X-100 for 5 min, and washed again with PBS; 5 % (v/v) FCS in PBS was used as blocking solution for 30 min, before incubating samples with primary antibodies in 5 % (v/v) FCS in PBS overnight at 4 °C. Cells were then washed again with blocking solution and incubated with appropriate fluorophore-labeled secondary antibodies for 1 h. After a final washing step with PBS, the samples were mounted in ProLong Gold with DAPI (4′6-diamidino-2-phenylindole; Invitrogen) overnight at 37 °C. Images of the samples were acquired using a Zeiss LSM 780 laser scanning confocal microscope and analyzed using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
DRM assay
DRMs were prepared mainly as described previously [42]. Briefly, control and HG-treated HEp-2 cells were incubated with 125I-labeled Stx1-mut in HEPES-buffered medium at 37 °C for 1 h. The cells were then washed three times with ice-cold PBS before they were harvested in ice-cold PBS by scraping with a cell scraper. The cells were pelleted by centrifugation and resuspended in ice-cold lysis buffer [150 mm NaCl, 1 mm EDTA, 50 mm Tris–HCl, pH 7.4, 1 % (v/v) Triton X-100, Complete protease inhibitor mixture] by gentle mixing at 4 °C for 30 min. The lysates were centrifuged at 800 × g for 10 min at 4 °C, and the postnuclear supernatants were then transferred to new tubes and centrifuged at 100,000 × g for 1 h at 4 °C. The amount of toxin in the resulting supernatants (non-DRM) and pellets (DRM) was quantified using an LKB Wallac 1261 Multigamma γ-counter. Equal amounts of proteins from the DRM fraction and the soluble fraction were separated by SDS-PAGE, and the distributions of the lipid raft marker flotillin-1 and the non-raft marker calnexin [43] in DRM and non-DRM fractions were determined by immunoblotting.
Mass spectrometry lipidomics
Cells were transfected with siRNA 1 day prior to harvesting for lipid analysis. Cells were then washed in HEPES-buffered medium before addition of HEPES-buffered medium containing trypsin/EDTA and incubation at 37 °C with 5 % CO2 until cells detached. Cells were then given HEPES-buffered medium and collected in microfuge tubes, centrifuged, and washed with PBS. The cells were then centrifuged again, and after removing the supernatant, the cell pellets were frozen at −80 °C. To determine protein content cells were lysed in 0.1 M NaCl, 10 mM Na2HPO4 (pH 7.4), 1 mM EDTA, 1 % (v/v) Triton X-100, supplemented with a mixture of Complete protease inhibitors, and lysates were analyzed using Pierce BCA protein assay kit as described by the manufacturer.
Lipids were extracted from approximately 1.5 million cells containing 215–230 µg protein using a modified Folch lipid extraction procedure [44]. Deuterium-labeled or heptadecanoyl-based synthetic internal standards for ceramide, glucosylceramide (GlcCer), lactosylceramide (LacCer) and Gb3 were added to the samples and used for quantification of endogenous lipids as previously described [45, 46]. After lipid extraction, samples were reconstituted in chloroform:methanol (1:2, v/v) and stored at −20 °C before performing MS analysis.
Reverse-phase ultra high pressure liquid chromatography (UHPLC) was used to analyze sphingolipids, as previously described [47], using an Acquity BEH C18, 2.1 × 50 mm column with a particle size of 1.7 µm (Waters, Milford, MA, USA) coupled to a hybrid triple quadrupole/linear ion trap mass spectrometer (QTRAP 5500, AB SCIEX, Foster City, CA, USA). A 25-min gradient using 10 mM ammonium acetate in water with 0.1 % (v/v) formic acid (mobile phase A) and 10 mM ammonium acetate in acetonitrile:2-propanol (4:3, v/v) containing 0.1 % (v/v) formic acid (mobile phase B) was used. Multiple reaction monitoring (MRM) was used for quantification of sphingolipids.
The MS lipidomic analyses were performed in a laboratory (Zora Biosciences) used to work according to GLP (Good Laboratory Practice) with published validation data showing <15 % variation for most lipid species [46].
The MS data files were processed using Lipid Profiler and MultiQuant software for producing a list of lipid names and peak areas as previously described [45]. To separate background noise from lipid peaks, a stringent cut-off was applied. Masses and counts of detected peaks were converted into a list of corresponding lipid names, and lipids were normalized to their respective internal standard [45]. The concentrations of molecular lipids are presented as pmol/µg protein. Quality control samples were used to remove technical outliers and lipid species that were detected below the lower limit of quantification, and thereby monitor the general quality of the lipid extraction and mass spectrometry analyses [46].
Results
HG protects HEp-2, HBMEC, and HMEC-1 cell lines against Shiga toxins
We have previously found that interfering with the glycosphingolipid synthesis in HEp-2 cells protects against Shiga toxins [33]. After observing that the addition of the ether lipid precursor HG to HEp-2 cells alters the cellular glycosphingolipid content [34], we tested if HG treatment of HEp-2 cells also offers a similar protection against Shiga toxin. Also, the increase in plasmalogens might in itself affect intracellular sorting. Indeed, we found that pretreatment with 20 µM HG for 24 h gave a strong protection against Shiga toxin in these cells (Fig. 2a). On average 30 times more toxin was needed to inhibit 50 % of the protein synthesis in HG-treated cells compared to control cells. Treatment with the ester analog of HG, dl-α-palmitin, does not alter the Gb3 content of HEp-2 cells [34], and did not affect the sensitivity towards Shiga toxin in these cells (Fig. 2a).
Fig. 2.
HG protects HEp-2 cells, HBMEC, and HMEC-1 cells against Shiga toxins. Inhibition of protein synthesis by Shiga toxins was determined by quantifying the incorporation of [3H]leucine in HEp-2 cells preincubated for 24 h with the indicated concentrations of HG, palmitin or 0.1 % (v/v) ethanol (control), before incubating cells with increasing concentrations of Shiga toxin (a) or Shiga toxin 2 (b). For Shiga toxin the average ± standard error of the mean of four individual experiments is shown, and for Shiga toxin 2 the average ± deviation of two individual experiments is shown. The endothelial cell lines HBMEC (c) and HMEC-1 (d) were incubated with 10 or 20 µM HG or 0.1 % (v/v) ethanol (control) for 24 h, before addition of increasing concentrations of Shiga toxin 2 for 3 h, and quantification of incorporated [3H]leucine. For HBMEC and HMEC-1 one representative experiment is shown
Although slightly less potent against cultured cells than Shiga toxin/Shiga toxin 1 [20], Shiga toxin 2 is more frequently associated with human pathologies [48], and we therefore tested if the HG treatment also protected HEp-2 cells against this subtype of the Shiga toxin family. We found that the sensitivity of HEp-2 cells to Shiga toxin 2 was strongly reduced after treatment with HG, making the cells virtually resistant to Shiga toxin 2 cytotoxicity (Fig. 2b). To further strengthen the relevance towards human pathology, we tested whether a similar protection could be obtained in endothelial cells, which are the physiological targets of Shiga toxins. We performed the same toxicity assay on human brain microvascular endothelial cells (HBMEC) and human dermal microvascular endothelial cells (HMEC-1), and found that HG also conferred a strong protection against Shiga toxin (data not shown) and Shiga toxin 2 in these cell types (Fig. 2c, d). Thus, we found that 24-h pretreatment with HG protected both HEp-2 cells and the endothelial cell lines HBMEC and HMEC-1 against Shiga toxin and Shiga toxin 2. The protective effect seems to be specific for Shiga toxins, as HG failed to protect HEp-2 cells against other protein toxins such as ricin, modeccin and diphtheria toxin (data not shown). Furthermore, we found that HG did not protect against cholera toxin (data not shown). As HEp-2 cells were insensitive to cholera toxin, these experiments were performed in HEK-293 and PC-3 cell lines.
HG reduces the binding of Shiga toxin to HEp-2 cells
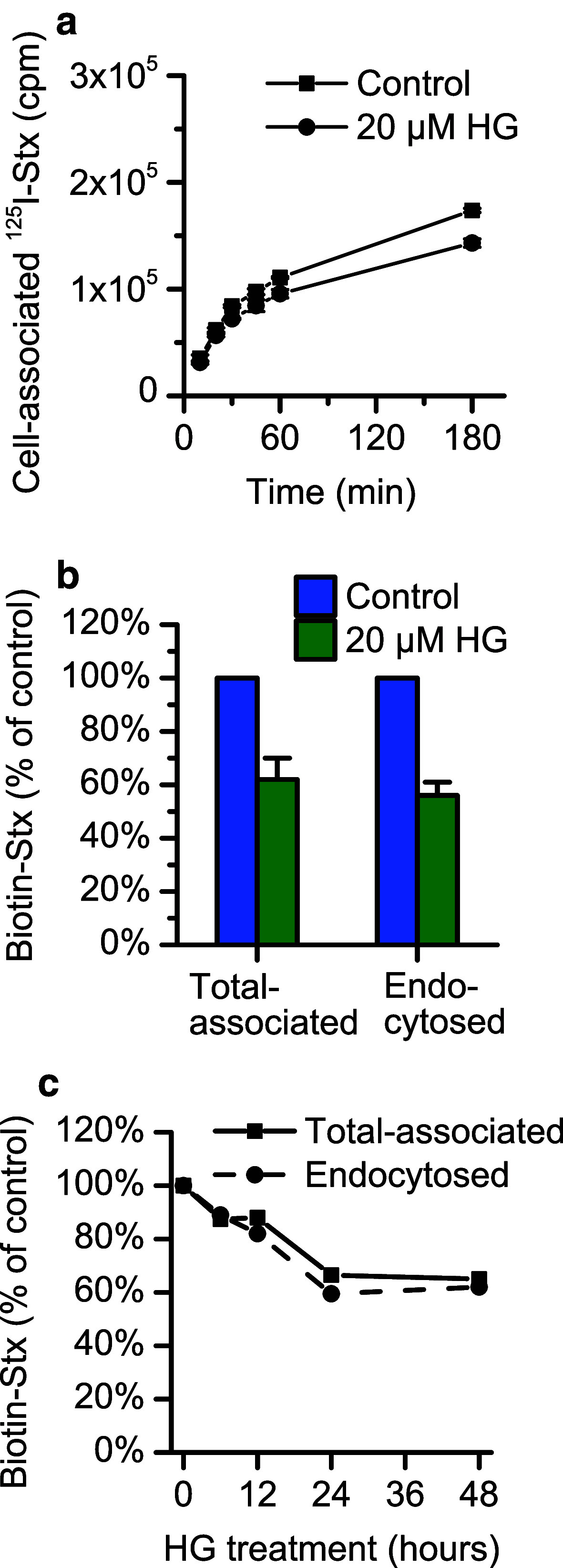
As previously shown [34], HG treatment reduces the total cellular level of the Shiga toxin receptor, Gb3, in HEp-2 cells. After 24 h the levels of all Gb3 species are reduced to about 70 % in HG-treated cells compared to control cells. We therefore wanted to investigate the binding of Shiga toxin to these cells, and found that the binding of an 125I-labeled Stx1-mut [36] was somewhat reduced (to 85–95 % of control) in HG-treated cells (Fig. 3a). Also the binding of biotinylated Shiga toxin was found to be reduced (to 60 % of control) after 24 h HG-treatment (Fig. 3b). By using the membrane-impermeable reducing agent MESNa to cleave the biotin from the non-internalized Shiga toxin, we also determined that the HG treatment did not have any effect on the endocytic rate of the toxin, i.e., the reduction in uptake was the same as for the bound toxin (Fig. 3b). We also found that the binding of biotinylated toxin was progressively reduced for incubation times with HG up to 24 h, but no additional reduction was observed by prolonging the incubation time from 24 to 48 h (Fig. 3c).
Fig. 3.
The binding of Shiga toxin is reduced after pretreatment with HG. a Shiga toxin binding in HEp-2 cells was determined by quantification of total cell-associated 125I-labeled Stx1-mut after 24-h pretreatment with 20 µM HG or 0.1 % (v/v) ethanol. The cells were incubated with 10 ng/ml toxin for the indicated times before removing the medium and measuring toxin associated with cells. The figure shows a representative experiment with each point representing mean ± deviation between two replicates. b Quantification of binding and endocytosis of Shiga toxin in HEp-2 cells after 24-h pretreatment with 20 µM HG or 0.1 % (v/v) ethanol (control) was performed using biotinylated Shiga toxin. After 20-min incubation with 40 ng/ml toxin, cells were lysed, and the lysates were incubated with streptavidin-coated beads and Ru(II)-labeled anti-Shiga toxin antibody. The total cell-associated Shiga toxin was determined by measuring the electrochemiluminescence produced by the Ru(II)-tag. To determine the amount of endocytosed toxin, half of the cells were treated with MESNa prior to lysis to remove biotin from non-internalized toxin at the cell surface. The figure shows the mean of three individual experiments ± standard error of the mean. c HEp-2 cells were incubated up to 48 h with 20 µM HG or 0.1 % (v/v) ethanol (control), and the total cell association and endocytosis of biotin-Shiga toxin were measured as previously described at each time point. The figure shows data from one representative experiment
Taken together, the binding of Shiga toxin is reduced to the same extent as Gb3, but it is unlikely that the strong protection against Shiga toxicity observed after HG treatment is caused only by this moderate reduction in binding.
The retrograde transport of Shiga toxin to the ER is inhibited by HG
As only a moderate reduction in Shiga toxin binding was found after HG treatment, we set out to investigate any possible effects on retrograde transport of the toxin, from the plasma membrane to the ER. After internalization from the plasma membrane, the toxin is sorted from early endosomes to the Golgi apparatus. To examine if this transport was impaired, we quantified the colocalization of Shiga toxin with the TGN marker TGN46 and the Golgi-resident protein giantin in HG-treated HEp-2 cells. Cells were pretreated with HG for 24 h before incubation with Shiga toxin for 45 min at 37 °C, and visualization by immunofluorescence microscopy of fixed and stained cells. These experiments showed that there was no effect of HG on the transport to the Golgi apparatus (Fig. 4a). Association with lipid rafts/detergent-resistant membranes (DRMs) has been shown to be required for the transport of the toxin from the plasma membrane to the Golgi [18], and in accordance with an intact Golgi transport we found no decrease in the association of Shiga toxin with DRMs after HG treatment (Fig. 4b). As expected from the reduction in binding observed in the binding assays, there was slightly less toxin associated with the HG-treated cells compared to control cells (data not shown).
Fig. 4.
Intracellular transport of Shiga toxin after HG treatment. a After 24-h pretreatment with 20 µM HG or 0.1 % (v/v) ethanol (control), cells were incubated with 250 ng/ml Shiga toxin for 45 min at 37 °C. The samples were fixed in 10 % (v/v) formalin, permeabilized with 0.1 % (v/v) Triton X-100 in PBS, and stained with anti-Shiga toxin and anti-giantin primary antibodies and appropriate secondary antibodies. To visualize the cell nuclei samples were mounted in the presence of DAPI. b Following 24-h treatment with HG, HEp-2 cells were incubated with 125I-labeled Stx1-mut for 1 h in HEPES-buffered medium at 37 °C. Cells were washed and harvested in PBS, centrifuged, and incubated with a lysis buffer containing 1 % (v/v) Triton X-100 for 30 min at 4 °C. After removal of nuclei and cell debris by centrifugation, the postnuclear supernatant was centrifuged at 100,000 × g for 1 h. The distribution of the toxin was determined by quantifying the 125I-Stx1-mut signal in the resulting pellet (DRM fraction) and supernatant (non-DRM). The graph shows the mean ± standard error of the mean for n = 4 experiments. p = 0.28, paired Student’s t test. The distribution of the lipid raft marker flotillin-1 and the non-raft marker calnexin in the two fractions was determined by immunoblotting, where the SDS-PAGE gel was loaded with equal amounts of total protein. c HEp-2 cells were pretreated with 20 µM HG or 0.1 % (v/v) ethanol (control). Cells were then incubated with 250 ng/ml Stx1-mut for 1 h, washed, and then incubated further for 3 h to allow toxin transport to the ER. The samples were prepared as in a using anti-Shiga toxin, anti-PDI, and anti-TGN46 primary antibodies. Scale bar 10 µm. d The ratio of ER/Golgi localization of Stx1-mut was determined by quantifying the intensity of the signal colocalizing with PDI or TGN46 in each cell. The figure shows a representative experiment where individually quantified cells have been plotted with increasing ER/Golgi ratio. n = 36 cells for control and n = 41 for HG. e HEp-2 cells pretreated with 20 µM HG or 0.1 % (v/v) ethanol (control) were incubated at 37 °C with 10 ng/ml 125I-labeled Stx1-mut for 5 h. The cells were then incubated with N-ethylmaleimide for 5 min, and analyzed by non-reducing SDS-PAGE. The proteins were transferred from gel to polyvinylidene difluoride membrane and visualized by autoradiography. f HEp-2 cells were pretreated with 20 µM HG or 0.1 % (v/v) ethanol (control), and incubated in glucose-free medium supplemented with 1.8 µl/ml glucose solution (10 %) and 0.1 mCi/ml [3H]mannose for 3 h at 37 °C, with HG or ethanol present. The cells were then incubated with 1 µg/ml StxB-sulfglyc and swainsonine for another 3 h at 37 °C. After incubation with toxin the cells were lysed, and StxB-sulfglyc was immunoprecipitated and analyzed using SDS-PAGE and autoradiography
To assess the next step in the retrograde transport, we incubated with Stx1-mut for 4 h at 37 °C to allow the toxin to be transported to the ER (Fig. 4c). By staining the samples with antibodies against the ER-resident protein PDI (protein disulfide isomerase) and the TGN marker TGN46, we were able to quantify the amount of Shiga toxin localized to either compartment. We expressed this as an ER-to-Golgi ratio and found this ratio to be higher in control cells than in HG-treated cells, indicating that the transport to the ER is inhibited (Fig. 4d).
To exert its cytotoxic action, the A1 fragment of Shiga toxin is translocated across the ER membrane to the cytosol. The A1 release occurs in the ER lumen by reduction of the disulfide bond connecting the A1 and the A2 fragments. As the A1 fragment has a size of approximately 27.5 kDa, it can be distinguished from the 32-kDa A subunit (A1 + A2). We found that the released A1 fragment from 125I-labeled Stx1-mut could be detected after 5-h incubation in control cells, but not in HG-treated cells, supporting the finding that transport to the ER is inhibited (Fig. 4e).
We also found that the mannosylation of a Shiga toxin B molecule containing glycosylation sites, StxB-sulfglyc, was strongly reduced in HG-treated HEp-2 cells (Fig. 4f). Since the mannosylation process occurs exclusively in the ER, this result is in agreement with the finding that transport of Shiga toxin to the ER is less efficient after HG treatment.
In conclusion, we found that Shiga toxin is transported to the Golgi apparatus after HG treatment, but that further retrograde transport to the ER is impaired in these cells.
Reducing Gb3 levels by knocking down Gb3 synthase mimics the protection observed after HG-treatment
As treatment with HG reduces the levels of glycosphingolipids, including the Shiga toxin receptor Gb3 [34], we tested whether specifically reducing Gb3 levels by knocking down Gb3 synthase would produce a similar phenotype. We found that already 24 h after transfection with siRNA against Gb3 synthase, HEp-2 cells were strongly protected against Shiga toxin (Fig. 5a). More than 60 times more Shiga toxin was needed to inhibit the protein synthesis by 50 % in cells treated with Gb3 synthase siRNA than in cells treated with non-targeting siRNA. The binding and endocytosis of biotinylated Shiga toxin were reduced to the same extent as when treating cells with HG (Fig. 5b). The two different siRNA oligos used to knock down Gb3 synthase, produced a knock-down efficiency of 80–90 % when comparing the amount of mRNA with control cells (Fig. 5c). As for HG, the retrograde transport of Shiga toxin to the Golgi is unaffected when analyzing siRNA-treated cells by immunofluorescence, suggesting that the protection obtained with siRNA-treated cells is also due to inhibition of the subsequent retrograde pathway (Fig. 5d).
Fig. 5.
Knockdown of Gb3 synthase for 24 h protects against Shiga toxin. a After 24-h siRNA-mediated knockdown of Gb3 synthase by two different oligos, the protein synthesis was quantified by measuring the incorporation of [3H]leucine after 3 h incubation with increasing concentrations of Shiga toxin. b Quantification of binding and endocytosis of Shiga toxin in HEp-2 cells after knockdown of Gb3 synthase was performed using biotinylated Shiga toxin. After 20-min incubation with 40 ng/ml toxin, cells were lysed, and the lysates were incubated with streptavidin-coated beads and Ru(II)-labeled anti-Shiga toxin antibody. The total cell-associated Shiga toxin was determined by measuring the electrochemiluminescence produced by the Ru(II)-tag. To determine the amount of endocytosed toxin, half of the cells were treated with MESNa prior to lysis to remove biotin from non-internalized toxin at the cell surface. The figure shows the mean ± standard error of the mean of six individual experiments for siRNA 1 and two experiments for siRNA 2. c After knocking down Gb3 synthase by siRNA for 24 h, RNA was isolated and the level of Gb3 synthase mRNA was determined by RT-qPCR. The relative amount of Gb3 synthase transcript was related to the reference gene TBP, and the levels are shown as % of the level in control cells treated with non-targeting siRNA. d HEp-2 cells were transfected with Gb3 synthase (oligo 1) or non-targeting siRNA. Cells were then incubated with 250 ng/ml Shiga toxin for 45 min to allow the toxin to be transported to the Golgi. The samples were fixed in 10 % (v/v) formalin, permeabilized with 0.1 % (v/v) Triton X-100 in PBS, and stained with anti-Shiga toxin and anti-giantin primary antibodies, and appropriate secondary antibodies. To visualize the cell nuclei samples were mounted in the presence of DAPI. Scale bar 10 µm
When it comes to glycosphingolipids, treatment with HG reduced both GlcCer and LacCer, as well as Gb3 [34]. By analyzing the lipidome of cells treated with siRNA against Gb3 synthase we found no or only very minor changes in the levels of GlcCer (Fig. 6a), but there was an accumulation of LacCer (Fig. 6b) and reduction of Gb3 to 70–80 % (Fig. 6c). Importantly, there was virtually no change in the species distribution. In conclusion, Gb3 synthase knockdown and HG treatment had different effects on GlcCer and LacCer, while the effect on the levels of Gb3 was similar (Fig. 6d).
Fig. 6.
Quantitative MS analysis of glycosphingolipids after Gb3 synthase knockdown. HEp-2 cells were treated with non-targeting or Gb3 synthase siRNA, harvested and analyzed by mass spectrometry. The figure shows quantification of the major species of GlcCer (a), LacCer (b), and Gb3 (c) from one of two experiments. d The relative change in the total lipids of each class of glycosphingolipids after 24-h siRNA-mediated knockdown of Gb3 synthase. Previously obtained data of HG [34] are included for comparison
Exogenously supplied LPI protects against Shiga toxin
When analyzing the lipidome of HG-treated cells, the largest observed effect was a dramatic increase of LPI species, which in total increased about 50 times [34]. We therefore performed an experiment where we added LPI exogenously to HEp-2 cells to see if this would affect the toxicity of Shiga toxin. Indeed, we found that LPI provided a strong protection (15 ± 4.6 times higher IC50, average of 4 experiments) against Shiga toxin after 30-min pretreatment (Fig. 7a). Furthermore, we investigated the binding of 125I-labeled Stx1-mut in these cells. In contrast to in HG-treated cells we found the binding to be nearly abolished after 30 min with LPI (Fig. 7b). This was also confirmed by immunofluorescence (Fig. 7c). Also, PI species increased somewhat after HG treatment [34], but the addition of this lipid to the cells had no effect on Shiga toxicity (data not shown). Although we cannot exclude that the HG-induced increase in LPI also contributes to the reduced binding of Shiga toxin in HG-treated cells, the effect caused by exogenously added LPI seems to be different, as the impact on toxin binding is much larger.
Fig. 7.
LPI protects against Shiga toxin by reducing toxin binding to cells. a HEp-2 cells were treated with 10 µM LPI or 0.05 % (v/v) ethanol (control) for 30 min before increasing concentrations of Shiga toxin were added. Protein synthesis was measured 3 h later by quantifying the incorporation of [3H]leucine. One representative experiment out of four is shown. b Shiga toxin binding to HEp-2 cells at 37 °C was determined by quantification of total cell-associated 125I-labeled Stx1-mut. The cells were pretreated with 10 µM LPI or 0.05 % (v/v) ethanol for 30 min at 37 °C, and then incubated with 10 ng/ml toxin in the presence of LPI or ethanol for 20 min before removal of the medium, three times wash in PBS, and measurement of toxin associated with the cells. The figure shows the mean and deviation of two experiments. c HEp-2 cells were pretreated with LPI or ethanol (control) as described above, before continuous incubation with Stx1-mut in the presence of LPI or ethanol at 37 °C for 45 min. Cells were fixed, stained with an anti-Shiga toxin antibody (red) and appropriate secondary antibodies, mounted in the presence of DAPI (blue), and analyzed by immunofluorescence microscopy. d Total cell-associated Shiga toxin was determined by quantifying the immunofluorescence signal in control cells and LPI-treated cells, and is expressed as % of control. The figure shows the quantification for approximately 1,500 cells of each condition
Discussion
We show here that an ether lipid precursor, the alkylglycerol HG, is able to protect both HEp-2 cells and the endothelial cell lines HBMEC and HMEC-1 against Shiga toxin and Shiga toxin 2. The protective effect is specific to Shiga toxins, as HG did not protect against other toxins, including ricin, cholera toxin, diphtheria toxin and modeccin. Since several of these toxins are transported all the way from the cell surface to endosomes, Golgi and the ER before entering the cytosol, this demonstrates that retrograde transport is not blocked in general. The strong protection against Shiga toxin is accompanied by only a moderate reduction in toxin binding and endocytosis, a reduction which presumably can be explained by our previous observations that HG treatment reduces Gb3 levels [34]. We also found that the retrograde Shiga toxin transport to the Golgi was not changed after HG treatment, but that the toxin was less efficiently transported to the ER.
We have previously shown that HG has major effects on the lipidome of HEp-2 cells [34]. As expected, HG increased the levels of ether-linked phospholipids with C16 in the sn-1 position, probably by entering the biosynthetic pathway through an alkylglycerol kinase activity. More surprisingly, HG increased the levels of phosphatidylinositol and caused a huge accumulation of LPI. In addition to these effects, HG treatment unexpectedly reduced all major species of GlcCer, LacCer and Gb3 (Fig. 6d), although how addition of an alkylglycerol can affect the levels of glycosphingolipids is currently not understood. Additionally, an intermediate in the biosynthesis of ether-linked phospholipids, alkylacylglycerol, can be used as a substrate for ceramide galactosyl transferase to form galactosylalkylacylglycerol (GalEAG), which may be used as a substrate by cerebroside sulfotransferase to generate sulfated GalEAG, known as seminolipid [49]. Thus, the addition of HG could theoretically result in increased amounts of GalEAG or seminolipid, which may have an effect on the retrograde transport of Shiga toxin. However, since these galactoglycerolipids have been published to be present mainly in semen, they were not included in our lipidomic study [34], and whether they are present in HEp-2 cells and affected by the HG treatment is currently not known. In contrast to HG, the corresponding acylglycerol, palmitin, did not protect against Shiga toxin, and caused an increase in GlcCer without having any effect on LacCer and Gb3 [34].
Whether the inhibited intracellular transport of Shiga toxin is due to changes in ether-linked phospholipids, phosphatidylinositols or glycosphingolipids is not clear. Structurally, the ether bond of ether-linked phospholipids brings the aliphatic chains closer together, resulting in these chains protruding deeper into the membrane bilayer. This decreases membrane fluidity, increases order, and may promote fusion and fission events [23]. The increased order may decrease the binding of proteins to the Golgi membranes and could thereby affect transport [50]. Certain plasmalogens are found to be enriched in DRMs [51, 52], and those with an ethanolamine headgroup are required for the transport of cholesterol from the cell surface or endocytic compartments to the ER in Chinese hamster ovary cells, illustrating that specific classes of ether-linked lipids may play essential roles in intracellular transport [53]. Interestingly, an intact ether lipid biosynthesis is also required for the generation of alkyl-containing glycosylphosphatidylinositol-anchored proteins, which are typical components of membrane microdomains [54]. Phosphorylated phosphatidylinositol (phosphoinositides) are known to be important for cell signaling and membrane trafficking [55], but we currently do not know if HG affects phosphoinositides and whether this may contribute to Shiga protection.
In the current study, we find that although there is a sufficient amount of Gb3 molecules available for binding of toxin to the cell surface, as shown by the modest reduction in toxin binding after HG treatment, the Gb3 molecules are unable to efficiently bring the toxin to the ER, which is needed for A1 translocation and toxicity. Also when specifically targeting the last step in the biosynthesis of Gb3 by treating cells with siRNA against Gb3 synthase for as short as 24 h, we observe a similar phenotype as for the HG treatment, i.e., a strong protection against the toxin with only a minor reduction in toxin binding and total levels of Gb3. One possibility is that there is a pool of rapidly depleted Gb3 which is required for efficient retrograde toxin transport, while the remaining pool of Gb3 receptors maintains toxin binding and endocytosis without being able to transport the toxin efficiently to the ER. This would be somewhat similar to the findings of Falguières et al. [56], who identified functionally different pools of Gb3 receptors in HeLa cells. They reported that Shiga toxin recruits Gb3 to DRMs, and that Gb3 receptors that were internalized with the toxin were replenished with Gb3 from internal stores. Although the recovered Gb3 receptors at the plasma membrane were able to internalize Shiga toxin, they did not associate with DRMs, and were unable to transport the toxin retrogradely to the Golgi and the ER. An important difference from the results shown here is that Shiga toxin is transported to the Golgi in HG-treated cells, but not to the ER. Lack of association with DRM is also proposed as an explanation of why some Gb3-expressing cells are resistant to Shiga toxicity, as the toxin fails to reach the Golgi and the ER and is instead sorted to lysosomes in these cells [18, 19]. In this study, we found no significant change in the association of Shiga toxin with DRMs after the addition of HG, which is in accordance with the intact transport of toxin to the TGN/Golgi in HG-treated cells. In addition, previous results indicate that Gb3 with relatively short N-amidated chains favor retrograde sorting of Shiga toxin [57, 58], but after HG or Gb3 synthase siRNA treatment we observe no preferential depletion of Gb3 species. It should however be noted that local changes may occur. Also, we cannot rule out that lipid changes induced by HG alter the accessibility to some of the B pentamer binding sites by modulating the membrane microenvironment of the Gb3 receptors. Several studies have reported that the receptor conformation and ligand accessibility can be modulated by surrounding lipids such as cholesterol [12–14]. Interestingly, studies of a Shiga toxin 2-neutralizing peptide have identified that receptor interaction with a particular binding site in the B pentamer (site 3) is required for proper sorting from the Golgi to the ER, and that toxin binding to this site may involve hydrophobic interplay with the ceramide part of Gb3, as well as interaction with the trisaccharide [59]. In a recent study, we have also found that HEp-2 cells growing in low density are more sensitive to Shiga toxin than high-density cells, despite the fact that the high-density cells had slightly higher total levels of Gb3, showing that Shiga toxicity is not directly related only to Gb3 levels but may also depend on changes in the composition of other lipid classes [60]. Interestingly, the phosphatase LPP3 was recently demonstrated to be required both for COPI-dependent and COPI-independent retrograde transport, and the lack of diacylglycerol seemed to be responsible for the inhibition of transport obtained with reduced LPP3 activity, as addition of the diacylglycerol analog, dioctanoylglycerol, could overcome the block in retrograde transport [61]. However, a similar treatment did not change the HG-induced protection against Shiga toxin (our unpublished data).
Reduced levels of the glycosphingolipid GlcCer has earlier been associated with protection against Shiga toxicity in cultured cells [33, 62]. In a previous study, we have shown that newly synthesized glycosphingolipids are required for efficient Shiga toxin transport to the Golgi [33], demonstrated by inhibiting glycosphingolipid synthesis using the inhibitors Fumonisin B1 and PDMP (dl-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol). This also led to a redistribution of the retromer component SNX1 (Sorting nexin 1), which is known to play a role in the retrograde sorting of Shiga toxin and other cargo from endosomes to the TGN [63, 64]. By using PDMP to inhibit glycosphingolipid synthesis there was a shift in the composition of species, and especially C16:0 species disappeared rapidly. A shift in lipid species composition might affect membrane curvature and endosomal recruitment of SNXs. On the other hand, an inhibition of toxin transport to the Golgi caused by other factors might also be involved in the shift of SNX1/SNX2 localization. Although HG also reduced the levels of several glycosphingolipids, there was no effect on the lipid species distribution. Importantly, the retrograde transport of Shiga toxin from endosomes to the Golgi was not affected in HG-treated cells, and there was no change in the localization or total level of SNX1 or other retromer subunits (our unpublished data). Interestingly, the species composition did not change after siRNA treatment against Gb3 synthase, and as the toxin is transported to the Golgi, the inhibition occurs also in this case later in the retrograde transport pathway. That PDMP treatment may have different effects on sorting and turnover of lipids than attenuation of Gb3 synthase is not surprising. PDMP has been reported to change intra-Golgi pH [65] and may induce structural changes in the Golgi compartment, as observed when interfering with glucosylceramide synthase [66]. GlcCer was reported to interact with the lumenal part of proteins and affect sorting [67]. In addition, a change in GlcCer and LacCer may interfere with the ability of Gb3 to interact with cholesterol [13]. So why is there a change in Golgi-to-ER transport of Shiga toxin? We know that HG does not cause a general block in retrograde transport, since the toxicity of ricin (which binds both glycoproteins and glycolipids) and cholera toxin (which binds the glycosphingolipid GM1) is not affected. Although both Shiga toxins and cholera toxin bind glycolipid receptors, different requirements for the retrograde transport from the Golgi to the ER have been reported [68, 69], and it is therefore not surprising that the effect of HG may not be the same for these toxins. Currently, we cannot answer why HG changes the Golgi-to-ER transport of Shiga toxin, but future studies of intra-Golgi transport of Shiga toxin are likely not only to reveal more details about how this toxin reaches its cytosolic target, but they will help us to clarify the complex machinery involved in Golgi structure and function.
We have previously published that HG treatment increases levels of PI and LPI [34], and we therefore performed experiments where these lipids were supplied exogenously. The addition of PI had no effect on Shiga toxicity, while the addition of LPI had a strong protective effect against Shiga toxin. As LPI seems to rapidly decrease the binding of Shiga toxin to the cells (Fig. 7), the effect of exogenously added LPI is different from the effect involved in the HG-mediated protection. Furthermore, we do not now whether the exogenously supplied LPI distributes to the same subcellular compartments as the LPI produced after the addition of HG. However, we cannot exclude that the HG-induced increase in LPI also contributes to the reduced toxin binding and toxin protection observed in HG-treated cells.
A natural source of alkylglycerols, including HG, is shark liver oil (SLO), which has been used in folk medicine for treating various conditions, and which is now available as over-the-counter health supplements. Several studies have investigated possible beneficial effects of SLO and alkylglycerols on the immune system and in cancer treatment in humans and various animals, and few unwanted effects have been reported in these studies [26]. For instance, the effects of SLO supplementation has been studied by administering high doses of SLO constituents, including alkylglycerols (3.6 g per day), to volunteers for 4 weeks [70]. The authors found the high-dose SLO supplementation to have a beneficial effect on the immune response against pathogen infections. The supplementation also markedly affected lipid metabolism and cholesterol balance, although in all the individuals the metabolism normalized spontaneously after the experiment.
There is currently no available treatment for STEC-infected patients apart from supportive care. The possibility of neutralizing the toxin by receptor-mimics or monoclonal antibodies have been investigated in clinical trials [71, 72], and some compounds have been shown to have a potential in treatment by inhibiting the intracellular transport of Shiga toxin [73, 74]. Currently available data suggest that HG is well tolerated by humans and animals, even at high doses [26]. Combined with the strong protection against both Shiga toxin and Shiga toxin 2 observed in cultured cells, including endothelial cells, this makes HG an interesting candidate for further studies in the continued search for safe treatment of STEC infections.
Acknowledgments
The work performed by the Oslo group has been supported by South-Eastern Norway Regional Health Authority, The Norwegian Cancer Society, and The Research Council of Norway. We thank Anne-Grethe Myrann for technical assistance with cell experiments, and Sirpa Sutela-Tuominen for assistance with lipidomic experiments.
References
- 1.Bergan J, Dyve Lingelem AB, Simm R, Skotland T, Sandvig K. Shiga toxins. Toxicon. 2012;60:1085–1107. doi: 10.1016/j.toxicon.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Melton-Celsa A, Mohawk K, Teel L, O’Brien A. Pathogenesis of Shiga-toxin producing Escherichia coli . Curr Top Microbiol Immunol. 2012;357:67–103. doi: 10.1007/82_2011_176. [DOI] [PubMed] [Google Scholar]
- 3.Lingwood CA, Binnington B, Manis A, Branch DR. Globotriaosyl ceramide receptor function—where membrane structure and pathology intersect. FEBS Lett. 2010;584:1879–1886. doi: 10.1016/j.febslet.2009.11.089. [DOI] [PubMed] [Google Scholar]
- 4.Engedal N, Skotland T, Torgersen ML, Sandvig K. Shiga toxin and its use in targeted cancer therapy and imaging. Microb Biotechnol. 2011;4:32–46. doi: 10.1111/j.1751-7915.2010.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathanson S, Kwon T, Elmaleh M, Charbit M, Launay EA, et al. Acute neurological involvement in diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2010;5:1218–1228. doi: 10.2215/CJN.08921209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandvig K, Bergan J, Dyve A-B, Skotland T, Torgersen ML. Endocytosis and retrograde transport of Shiga toxin. Toxicon. 2010;56:1181–1185. doi: 10.1016/j.toxicon.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Sandvig K, Garred O, Prydz K, Kozlov JV, Hansen SH, et al. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature. 1992;358:510–512. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- 8.Spooner RA, Lord JM. How ricin and Shiga toxin reach the cytosol of target cells: retrotranslocation from the endoplasmic reticulum. Curr Top Microbiol Immunol. 2012;357:19–40. doi: 10.1007/82_2011_154. [DOI] [PubMed] [Google Scholar]
- 9.Tumer NE, Li X-P. Interaction of ricin and Shiga toxins with ribosomes. Curr Top Microbiol Immunol. 2012;357:1–18. doi: 10.1007/82_2011_174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tesh VL. Induction of apoptosis by Shiga toxins. Future Microbiol. 2010;5:431–453. doi: 10.2217/fmb.10.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arab S, Lingwood CA. Influence of phospholipid chain length on verotoxin/globotriaosyl ceramide binding in model membranes: comparison of a supported bilayer film and liposomes. Glycoconj J. 1996;13:159–166. doi: 10.1007/BF00731490. [DOI] [PubMed] [Google Scholar]
- 12.Lingwood D, Binnington B, Róg T, Vattulainen I, Grzybek M, et al. Cholesterol modulates glycolipid conformation and receptor activity. Nat Chem Biol. 2011;7:260–262. doi: 10.1038/nchembio.551. [DOI] [PubMed] [Google Scholar]
- 13.Mahfoud R, Manis A, Binnington B, Ackerley C, Lingwood CA. A major fraction of glycosphingolipids in model and cellular cholesterol-containing membranes is undetectable by their binding proteins. J Biol Chem. 2010;285:36049–36059. doi: 10.1074/jbc.M110.110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yahi N, Aulas A, Fantini J. How cholesterol constrains glycolipid conformation for optimal recognition of Alzheimer’s beta amyloid peptide (Abeta1-40) Plos One. 2010;5:e9079. doi: 10.1371/journal.pone.0009079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katagiri YU, Mori T, Nakajima H, Katagiri C, Taguchi T, et al. Activation of Src family kinase yes induced by Shiga toxin binding to globotriaosyl ceramide (Gb3/CD77) in low density, detergent-insoluble microdomains. J Biol Chem. 1999;274:35278–35282. doi: 10.1074/jbc.274.49.35278. [DOI] [PubMed] [Google Scholar]
- 16.Mori T, Kiyokawa N, Katagiri YU, Taguchi T, Suzuki T, et al. Globotriaosyl ceramide (CD77/Gb3) in the glycolipid-enriched membrane domain participates in B-cell receptor-mediated apoptosis by regulating lyn kinase activity in human B cells. Exp Hematol. 2000;28:1260–1268. doi: 10.1016/S0301-472X(00)00538-5. [DOI] [PubMed] [Google Scholar]
- 17.Takenouchi H, Kiyokawa N, Taguchi T, Matsui J, Katagiri YU, et al. Shiga toxin binding to globotriaosyl ceramide induces intracellular signals that mediate cytoskeleton remodeling in human renal carcinoma-derived cells. J Cell Sci. 2004;117:3911–3922. doi: 10.1242/jcs.01246. [DOI] [PubMed] [Google Scholar]
- 18.Falguières T, Mallard F, Baron C, Hanau D, Lingwood C, et al. Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol Biol Cell. 2001;12:2453–2468. doi: 10.1091/mbc.12.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoey DEE, Sharp L, Currie C, Lingwood CA, Gally DL, et al. Verotoxin 1 binding to intestinal crypt epithelial cells results in localization to lysosomes and abrogation of toxicity. Cell Microbiol. 2003;5:85–97. doi: 10.1046/j.1462-5822.2003.00254.x. [DOI] [PubMed] [Google Scholar]
- 20.Tam P, Mahfoud R, Nutikka A, Khine AA, Binnington B, et al. Differential intracellular transport and binding of verotoxin 1 and verotoxin 2 to globotriaosylceramide-containing lipid assemblies. J Cell Physiol. 2008;216:750–763. doi: 10.1002/jcp.21456. [DOI] [PubMed] [Google Scholar]
- 21.Khan F, Proulx F, Lingwood CA. Detergent-resistant globotriaosyl ceramide may define verotoxin/glomeruli-restricted hemolytic uremic syndrome pathology. Kidney Int. 2009;75:1209–1216. doi: 10.1038/ki.2009.7. [DOI] [PubMed] [Google Scholar]
- 22.Van Meer G, de Kroon AIPM. Lipid map of the mammalian cell. J Cell Sci. 2011;124:5–8. doi: 10.1242/jcs.071233. [DOI] [PubMed] [Google Scholar]
- 23.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog Lipid Res. 2001;40:199–229. doi: 10.1016/S0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 25.Gorgas K, Teigler A, Komljenovic D, Just WW. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta. 2006;1763:1511–1526. doi: 10.1016/j.bbamcr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 26.Iannitti T, Palmieri B. An update on the therapeutic role of alkylglycerols. Mar Drugs. 2010;8:2267–2300. doi: 10.3390/md8082267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chae K, Piantadosi C, Snyder F. An alternate enzymic route for the synthesis of the alkyl analog of phosphatidic acid involving alkylglycerol. Biochem Biophys Res Commun. 1973;51:119–124. doi: 10.1016/0006-291X(73)90516-0. [DOI] [PubMed] [Google Scholar]
- 28.Watschinger K, Werner ER. Orphan enzymes in ether lipid metabolism. Biochimie. 2013;95:59–65. doi: 10.1016/j.biochi.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das AK, Holmes RD, Wilson GN, Hajra AK. Dietary ether lipid incorporation into tissue plasmalogens of humans and rodents. Lipids. 1992;27:401–405. doi: 10.1007/BF02536379. [DOI] [PubMed] [Google Scholar]
- 30.Schrakamp G, Schalkwijk CG, Schutgens RB, Wanders RJ, Tager JM, et al. Plasmalogen biosynthesis in peroxisomal disorders: fatty alcohol versus alkylglycerol precursors. J Lipid Res. 1988;29:325–334. [PubMed] [Google Scholar]
- 31.Styger R, Wiesmann UN, Honegger UE. Plasmalogen content and beta-adrenoceptor signalling in fibroblasts from patients with Zellweger syndrome. Effects of hexadecylglycerol. Biochim Biophys Acta. 2002;1585:39–43. doi: 10.1016/S1388-1981(02)00320-7. [DOI] [PubMed] [Google Scholar]
- 32.Brites P, Ferreira AS, da Silva TF, Sousa VF, Malheiro AR, et al. Alkyl-glycerol rescues plasmalogen levels and pathology of ether-phospholipid deficient mice. Plos One. 2011;6:e28539. doi: 10.1371/journal.pone.0028539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raa H, Grimmer S, Schwudke D, Bergan J, Wälchli S, et al. Glycosphingolipid requirements for endosome-to-Golgi transport of Shiga toxin. Traffic. 2009;10:868–882. doi: 10.1111/j.1600-0854.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 34.Bergan J, Skotland T, Sylvänne T, Simolin H, Ekroos K, et al. The ether lipid precursor hexadecylglycerol causes major changes in the lipidome of HEp-2 cells. Plos One. 2013;8:e75904. doi: 10.1371/journal.pone.0075904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen SX, Teel LD, Judge NA, O’Brien AD. Genetic toxoids of Shiga toxin types 1 and 2 protect mice against homologous but not heterologous toxin challenge. Vaccine. 2006;24:1142–1148. doi: 10.1016/j.vaccine.2005.08.094. [DOI] [PubMed] [Google Scholar]
- 36.Kvalvaag AS, Pust S, Sundet KI, Engedal N, Simm R, et al. The ERM proteins ezrin and moesin regulate retrograde Shiga toxin transport. Traffic. 2013;14:839–852. doi: 10.1111/tra.12077. [DOI] [PubMed] [Google Scholar]
- 37.L’Abée-Lund TM, Jørgensen HJ, O’Sullivan K, Bohlin J, Ligård G, et al. The highly virulent 2006 Norwegian EHEC O103:H25 outbreak strain is related to the 2011 German O104:H4 outbreak strain. Plos One. 2012;7:e31413. doi: 10.1371/journal.pone.0031413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsnes S, Haylett T, Sandvig K. The toxic lectin modeccin. Methods Enzymol. 1982;83:357–362. doi: 10.1016/0076-6879(82)83030-9. [DOI] [PubMed] [Google Scholar]
- 39.Dyve AB, Bergan J, Utskarpen A, Sandvig K. Sorting nexin 8 regulates endosome-to-Golgi transport. Biochem Biophys Res Commun. 2009;390:109–114. doi: 10.1016/j.bbrc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 40.Torgersen ML, Wälchli S, Grimmer S, Skånland SS, Sandvig K. Protein kinase Cdelta is activated by Shiga toxin and regulates its transport. J Biol Chem. 2007;282:16317–16328. doi: 10.1074/jbc.M610886200. [DOI] [PubMed] [Google Scholar]
- 41.Dyve Lingelem AB, Bergan J, Sandvig K. Inhibitors of intravesicular acidification protect against Shiga toxin in a pH-independent manner. Traffic. 2012;13:443–454. doi: 10.1111/j.1600-0854.2011.01319.x. [DOI] [PubMed] [Google Scholar]
- 42.Grimmer S, Spilsberg B, Hanada K, Sandvig K. Depletion of sphingolipids facilitates endosome to Golgi transport of ricin. Traffic. 2006;7:1243–1253. doi: 10.1111/j.1600-0854.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- 43.Lingwood D, Simons K. Detergent resistance as a tool in membrane research. Nat Protoc. 2007;2:2159–2165. doi: 10.1038/nprot.2007.294. [DOI] [PubMed] [Google Scholar]
- 44.Ekroos K, Chernushevich IV, Simons K, Shevchenko A. Quantitative profiling of phospholipids by multiple precursor ion scanning on a hybrid quadrupole time-of-flight mass spectrometer. Anal Chem. 2002;74:941–949. doi: 10.1021/ac015655c. [DOI] [PubMed] [Google Scholar]
- 45.Ejsing CS, Duchoslav E, Sampaio J, Simons K, Bonner R, et al. Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal Chem. 2006;78:6202–6214. doi: 10.1021/ac060545x. [DOI] [PubMed] [Google Scholar]
- 46.Jung HR, Sylvänne T, Koistinen KM, Tarasov K, Kauhanen D, et al. High throughput quantitative molecular lipidomics. Biochim Biophys Acta. 2011;1811:925–934. doi: 10.1016/j.bbalip.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 2005;36:207–224. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, et al. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol. 1999;37:497–503. doi: 10.1128/jcm.37.3.497-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honke K. Biosynthesis and biological function of sulfoglycolipids. Proc Jpn Acad Ser B Phys Biol Sci. 2013;89:129–138. doi: 10.2183/pjab.89.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bigay J, Antonny B. Curvature, lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity. Dev Cell. 2012;23:886–895. doi: 10.1016/j.devcel.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Honsho M, Yagita Y, Kinoshita N, Fujiki Y. Isolation and characterization of mutant animal cell line defective in alkyl-dihydroxyacetonephosphate synthase: localization and transport of plasmalogens to post-Golgi compartments. Biochim Biophys Acta. 2008;1783:1857–1865. doi: 10.1016/j.bbamcr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 52.Pike LJ, Han X, Chung K-N, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 53.Munn NJ, Arnio E, Liu D, Zoeller RA, Liscum L. Deficiency in ethanolamine plasmalogen leads to altered cholesterol transport. J Lipid Res. 2003;44:182–192. doi: 10.1194/jlr.M200363-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Kanzawa N, Maeda Y, Ogiso H, Murakami Y, Taguchi R, et al. Peroxisome dependency of alkyl-containing GPI-anchor biosynthesis in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2009;106:17711–17716. doi: 10.1073/pnas.0904762106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayinger P. Phosphoinositides and vesicular membrane traffic. Biochim Biophys Acta. 2012;1821:1104–1113. doi: 10.1016/j.bbalip.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falguières T, Römer W, Amessou M, Afonso C, Wolf C, et al. Functionally different pools of Shiga toxin receptor, globotriaosyl ceramide, in HeLa cells. FEBS J. 2006;273:5205–5218. doi: 10.1111/j.1742-4658.2006.05516.x. [DOI] [PubMed] [Google Scholar]
- 57.Arab S, Lingwood CA. Intracellular targeting of the endoplasmic reticulum/nuclear envelope by retrograde transport may determine cell hypersensitivity to verotoxin via globotriaosyl ceramide fatty acid isoform traffic. J Cell Physiol. 1998;177:646–660. doi: 10.1002/(SICI)1097-4652(199812)177:4<646::AID-JCP15>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 58.Sandvig K, Ryd M, Garred O, Schweda E, Holm PK, et al. Retrograde transport from the Golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J Cell Biol. 1994;126:53–64. doi: 10.1083/jcb.126.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishikawa K, Watanabe M, Kita E, Igai K, Omata K, et al. A multivalent peptide library approach identifies a novel Shiga toxin inhibitor that induces aberrant cellular transport of the toxin. FASEB J. 2006;20:2597–2599. doi: 10.1096/fj.06-6572fje. [DOI] [PubMed] [Google Scholar]
- 60.Kavaliauskiene S, Nymark C-M, Bergan J, Simm R, Sylvänne T, et al. Cell density-induced changes in lipid composition and intracellular trafficking. Cell Mol Life Sci. 2014;71:1097–1116. doi: 10.1007/s00018-013-1441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gutiérrez-Martínez E, Fernández-Ulibarri I, Lázaro-Diéguez F, Johannes L, Pyne S, et al. Lipid phosphate phosphatase 3 participates in transport carrier formation and protein trafficking in the early secretory pathway. J Cell Sci. 2013;126:2641–2655. doi: 10.1242/jcs.117705. [DOI] [PubMed] [Google Scholar]
- 62.Smith DC, Sillence DJ, Falguières T, Jarvis RM, Johannes L, et al. The association of Shiga-like toxin with detergent-resistant membranes is modulated by glucosylceramide and is an essential requirement in the endoplasmic reticulum for a cytotoxic effect. Mol Biol Cell. 2006;17:1375–1387. doi: 10.1091/mbc.E05-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Utskarpen A, Slagsvold HH, Dyve AB, Skånland SS, Sandvig K. SNX1 and SNX2 mediate retrograde transport of Shiga toxin. Biochem Biophys Res Commun. 2007;358:566–570. doi: 10.1016/j.bbrc.2007.04.159. [DOI] [PubMed] [Google Scholar]
- 64.Bujny MV, Popoff V, Johannes L, Cullen PJ. The retromer component sorting nexin-1 is required for efficient retrograde transport of Shiga toxin from early endosome to the trans Golgi network. J Cell Sci. 2007;120:2010–2021. doi: 10.1242/jcs.003111. [DOI] [PubMed] [Google Scholar]
- 65.Van der Poel S, Wolthoorn J, van den Heuvel D, Egmond M, Groux-Degroote S, et al. Hyperacidification of trans-Golgi network and endo/lysosomes in melanocytes by glucosylceramide-dependent V-ATPase activity. Traffic. 2011;12:1634–1647. doi: 10.1111/j.1600-0854.2011.01263.x. [DOI] [PubMed] [Google Scholar]
- 66.D’Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- 67.Sprong H, Degroote S, Claessens T, van Drunen J, Oorschot V, et al. Glycosphingolipids are required for sorting melanosomal proteins in the Golgi complex. J Cell Biol. 2001;155:369–380. doi: 10.1083/jcb.200106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sandvig K, Skotland T, van Deurs B, Klokk TI. Retrograde transport of protein toxins through the Golgi apparatus. Histochem Cell Biol. 2013;140:317–326. doi: 10.1007/s00418-013-1111-z. [DOI] [PubMed] [Google Scholar]
- 69.Sandvig K, Bergan J, Kavaliauskiene S, Skotland T. Lipid requirements for entry of protein toxins into cells. Prog Lipid Res. 2014;54C:1–13. doi: 10.1016/j.plipres.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Lewkowicz P, Banasik M, Głowacka E, Lewkowicz N, Tchórzewski H. Effect of high doses of shark liver oil supplementation on T cell polarization and peripheral blood polymorphonuclear cell function. Pol Merkur Lekarski. 2005;18:686–692. [PubMed] [Google Scholar]
- 71.Bitzan M. Treatment options for HUS secondary to Escherichia coli O157:H7. Kidney Int. 2009;75:S62–S66. doi: 10.1038/ki.2008.624. [DOI] [PubMed] [Google Scholar]
- 72.Nishikawa K. Recent progress of Shiga toxin neutralizer for treatment of infections by Shiga toxin-producing Escherichia coli . Arch Immunol Ther Exp (Warsz) 2011;59:239–247. doi: 10.1007/s00005-011-0130-5. [DOI] [PubMed] [Google Scholar]
- 73.Stechmann B, Bai S-K, Gobbo E, Lopez R, Merer G, et al. Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell. 2010;141:231–242. doi: 10.1016/j.cell.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 74.Mukhopadhyay S, Linstedt AD. Manganese blocks intracellular trafficking of Shiga toxin and protects against Shiga toxicosis. Science. 2012;335:332–335. doi: 10.1126/science.1215930. [DOI] [PMC free article] [PubMed] [Google Scholar]