Abstract
Alzheimer’s disease (AD) is pathologically characterized by the presence of misfolded proteins such as amyloid beta (Aβ) in senile plaques, and hyperphosphorylated tau and truncated tau in neurofibrillary tangles (NFT). The BRI2 protein inhibits Aβ aggregation via its BRICHOS domain and regulates critical proteins involved in initiating the amyloid cascade, which has been hypothesized to be central in AD pathogenesis. We recently detected the deposition of BRI2 ectodomain associated with Aβ plaques and concomitant changes in its processing enzymes in early stages of AD. Here, we aimed to investigate the effects of recombinant BRI2 ectodomain (rBRI276–266) on Aβ aggregation and on important molecular pathways involved in early stages of AD, including the unfolded protein response (UPR), phosphorylation and truncation of tau, as well as apoptosis. We found that rBRI276–266 delays Aβ fibril formation, although less efficiently than the BRI2 BRICHOS domain (BRI2 residues 113–231). In human neuroblastoma SH-SY5Y cells, rBRI276–266 slightly decreased cell viability and increased up to two-fold the Bax/Bcl-2 ratio and the subsequent activity of caspases 3 and 9, indicating activation of apoptosis. rBRI276–266 upregulated the chaperone BiP but did not modify the mRNA expression of other UPR markers (CHOP and Xbp-1). Strikingly, rBRI276–266 induced the activation of GSK3β but not the phosphorylation of tau. However, exposure to rBRI276–266 significantly induced the truncation of tau, indicating that BRI2 ectodomain can contribute to NFT formation. Since BRI2 can also regulate the metabolism of Aβ, the current data suggests that BRI2 ectodomain is a potential nexus between Aβ, tau pathology and neurodegeneration.
Keywords: Alzheimer’s disease, ITM2b, BRICHOS, Aggregation, Fibrillation, Apoptosis
Introduction
Alzheimer’s disease (AD), the most common form of dementia, is pathologically characterized by the accumulation and aggregation of misfolded proteins: the amyloid β peptide (Aβ) in senile plaques and hyperphosphorylated tau (p-tau) in neurofibrillary tangles (NFTs) [1, 2]. AD shares clinical and pathological similarities with familial British and Danish dementias (FBD and FDD). FBD and FDD originate from mutations in the BRI2 coding gene, causing amyloid angiopathy and NFTs similar to those observed in AD [3–6]. BRI2 is processed by different enzymes (furin [7], ADAM10 and SPPL2b [8] ) leading to the secretion of peptides of different molecular weights. Although the physiological function of BRI2 remains unknown, BRI2 ectodomain contains a BRICHOS domain (BRI2-BRICHOS), which is able to delay Aβ fibrillation [9–11]. In addition, several studies have revealed that BRI2 can regulate the proteostasis of critical proteins involved in AD pathogenesis, such as the amyloid precursor protein (APP) [12–18], insulin degrading enzyme (IDE) [19] and β-secretase 1 (BACE1) [20]. BRI2 could have a key role in memory performance, since loss of wild-type BRI2 function rather than amyloidogenesis of the mutated BRI2 fragments correlated with memory deficits and impaired synaptic plasticity in FBD and FDD mice models [21, 22]. Moreover, the recently characterized double-transgenic tg-FDD-tau model accumulated wild-type BRI2 and showed enhanced phosphorylation and truncation of tau before amyloid deposition [23], suggesting that BRI2 accumulation (or the subsequent loss of function) may facilitate NFT formation.
Taken together, previous studies suggest that the lack of BRI2 or reduced BRI2 functionality could play an important role in AD (reviewed in [24]). Interestingly, we recently found deposits of BRI2 ectodomain associated with Aβ plaques in early stages of AD [25]. In addition, the levels of BRI2-processing enzymes were also significantly changed in post-mortem hippocampus of AD patients compared to controls. Those results led us to hypothesize that in AD there is an aberrant BRI2 processing that promotes the secretion of the BRI2 ectodomain, leading to the formation of BRI2 aggregates, which may reduce BRI2 functionality as judged by the decrease of BRI2-APP complexes in AD hippocampus [25].
A common denominator in the pathogenesis of multiple neurodegenerative disorders is the abnormal folding, processing and/or clearance of different proteins leading to the formation of aberrant protein complexes. Those aberrant processes can influence protein activity promoting a pathological cascade via loss of physiological function, gain of toxic function or combination of both [26]. Thus, the presence of larger BRI2 forms in early stages of AD [25] besides influencing BRI2 physiological function may also have important direct effects on cell homeostasis, as shown for different protein aggregates (e.g. Aβ, α-synuclein, prion proteins) [27–32]. The presence of aggregated proteins and extracellular stimulus can induce stress in the endoplasmic reticulum (ER) and the subsequent activation the unfolded protein response (UPR) in order to restore cell homeostasis [33, 34]. When ER stress remains unmitigated, an apoptotic cell signalling cascade will be initiated, ultimately leading to cell death [33, 35].
Thus, we investigated whether the BRI2 ectodomain can affect Aβ fibrillation and if exposure to recombinant BRI2 ectodomain disrupts cell homeostasis in vitro. In addition, we investigated the possible involvement of aggregated recombinant BRI2 accumulation in tau phosphorylation and truncation.
Materials and methods
Cell culture
Human neuroblastoma SH-SY5Y and SK-N-SH cells were cultured in Dulbecco’s modified essential medium (DMEM)/Nutrient Mix F-12 (1:1; Gibco–BRL, Gaithersburg, MD, USA). Mouse neuroblastoma N2a cells were cultured in DMEM medium. Both mediums were supplemented with 10 U penicillin/mL, 100 µg/mL streptomycin (Gibco–BRL), 10 % fetal calf serum and 2 mM l-glutamine (Gibco–BRL). Cells were maintained at 37 °C in a humidified incubator with 5 % CO2/95 % air. Differentiation of SH-SY5Y and SK-N-SH cells was induced by incubation with 10 µM of retinoic acid (RA) for 5–6 days (Sigma, St Louis, MO, USA).
Expression and purification of recombinant BRI276–266 and BRI2 BRICHOS113–231
Recombinant human BRI2 ectodomain (76–266; rBRI2) was expressed and purified from E. coli as previously described [36]. A fragment corresponding to human BRI2 positions 113–231 was expressed as a soluble fusion protein together with His6 and thioredoxin in E. coli. Bacteria were cultured in LB medium with 100 μg/ml ampicillin at 30 °C for 16 h, and protein expression was induced by adding 0.5 mM isopropylthiogalactoside (IPTG). After 4 h at 25 °C, cells were harvested by centrifugation, resuspended in 20 mM sodium phosphate buffer, pH 7.5 and stored at −20 °C. The cells were lysed by lysozyme (1 mg/ml) for 30 min and incubated with DNase and 2 mM MgCl2 for 30 min on ice. The cell lysate was centrifuged at 6,000×g for 20 min, and the pellet was suspended in 2 M urea in 20 mM sodium phosphate buffer, pH 7.5, and sonicated for 5 min. After centrifugation at 24,000×g for 30 min at 4 °C, the supernatant was filtered through a 5 μm filter and loaded on a 2.5-ml nickel-agarose column (Qiagen, Ltd., West Sussex, UK). The column was washed with 50 ml of 2 M urea in 20 mM phosphate buffer, pH 7.5, then with 50 ml of 1 M urea in 20 mM sodium phosphate buffer, pH 7.5, and finally with 50 ml 20 mM sodium phosphate buffer, 20 mM imidazole, pH 7.5. The protein was then eluted with 200 mM imidazole in 20 mM sodium phosphate buffer, pH 7.5, dialyzed against 20 mM sodium phosphate buffer, pH 7.5, cleaved by thrombin for 16 h at 4 °C (enzyme/substrate weight ratio of 0.002) to remove the thioredoxin and His6 tag, and then reapplied to a Ni2+ column to remove the released tag. Concentration was determined from A280 using a molar extinction coefficient, ε = 9,065 × M−1 × cm−1.
Aβ42 preparations
Aβ42 peptide (American peptide, Sunnyvale, CA, USA) was reconstituted in MilliQ water to a final concentration of 225.5 µM. For aggregation kinetics, Met-Aβ1-42 (hereafter referred to as Aβ42) was expressed in E. coli BL21 from synthetic genes and purified in batch format using ion exchange and size exclusion steps as previously described [37], which results in highly pure monomeric peptide. Purified peptide was aliquoted in low-bind Eppendorf tubes (Axygene) and stored at −20 °C. The peptide concentration was determined using an extinction coefficient of 1,424 M−1 cm−1 for (A280–300).
Coomasie staining
rBRI276–266 (1 μg) was prepared in sample buffer [100 mM Tris, 0.2 % (w/v) bromophenol blue and 20 % (v/v) glycerol] with or without dithiothreitol (DTT, 10 mM) and/or 4 % (v/v) of SDS to analyze the effects of different reducing and denaturing conditions. Samples prepared in complete buffer (+DTT/+SDS) or buffer without SDS (+DTT/−SDS) were boiled at 95 °C for 5 min. Proteins were separated by electrophoresis on 10 % (w/v) SDS–polyacrylamide gel (SDS-PAGE) and gel was then incubated in fixing solution (50 % ethanol, 10 % acetic acid) during 30 min. For protein staining, gel was incubated during 2 h with GelCode® Blue Stain Reagent (Thermo Scientific, Waltham, MA, USA). After washing with MilliQ water, gel was incubated overnight with destain solution (50 % methanol, 10 % acetic acid) until protein bands could be clearly detected.
Size exclusion chromatography
Size exclusion chromatography was performed on a Superdex200HR column (GE Healthcare) using an ÄKTA basic FPLC system (GE Healthcare). The column was equilibrated and operated in degassed buffer (20 mM sodium phosphate, pH 7.5). 70 nmol recombinant BRI276–266 or BRI2 BRICHOS was injected from a 50 μl loop and chromatograms recorded by monitoring the absorbance at 280 nm.
Aggregation kinetics
Aggregation kinetics was studied by recording the thioflavin T (ThT) fluorescence intensity as a function of time in a plate reader (FLUOStar Galaxy from BMG Labtech, Offenberg, Germany). The fluorescence was recorded using bottom optics in half-area 96-well polyethylene glycol-coated black polystyrene plates with clear bottom (Corning Glass, 3881) using a 440 nm excitation filter and a 490 nm emission filter. Aβ42 monomer was isolated by size exclusion chromatography over a Superdex 75 column (GE Healthcare) in 20 mM sodium phosphate, 200 µM EDTA, 0.02 % NaN3 at pH 8, and kept on ice. Every sample was supplemented with 10 μM ThT from a 1 mM stock solution. To each well was added 80 μl of the ice-cold Aβ42 solution, and the plate was immediately placed in the fluorescence reader at 37 °C, and incubated under quiescent conditions with readings made every 17 min. Aβ42 (3 µM) was studied alone or coincubated with 0.6 µM of BRI2-BRICHOS or BRI276–266.
Cell viability test
The effect of rBRI276–266 on cell viability and on Aβ42 induced toxicity was assessed by measuring residual cellular redox activity with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma, St Louis, MO, USA) as previously described [38]. MTT assay is a sensitive and rapid indicator of amyloid toxicity [39].
Twenty-four hours after seeding (6 × 104 per well in a 48-well plate), medium was renewed by fresh medium containing rBRI276–266 at 0.2, 0.5 and 2 µM with or without 0.5 µM Aβ42. After 24 h incubation, the conditioned medium was removed and cells were then incubated with 0.5 mg/ml MTT in Na-buffer (132 mM NaCl, 4 mM KCl, 1.2 mM Na2HPO4, 1.4 mM MgCl2, 6 mM glucose, 10 mM HEPES, and 1 mM CaCl2, pH 7.4) for 2 h at 37 °C. The blue formazan crystals formed were dissolved in an equal volume of 0.04 M HCl in isopropanol and quantified spectrophotometrically by measuring the absorbance at 570 nm using a microplater reader (SpectraMax Plus 384, Molecular Devices, California, USA). Results were normalized and expressed as the percentage of the absorbance determined in untreated cells (control) within each experiment.
Western blotting
To obtain lysates, SH-SY5Y cells (9 × 104 per well in a 24-well plate) were washed twice with PBS and scraped in ice-cold lysis buffer (25 mM HEPES, 1 mM EDTA, 1 mM EGTA, 2 mM MgCl2, 100 µM PMSF, 2 mM DTT, 2 mM Na3VO4, 50 mM NaF pH 7.5) containing 1:1,000 of protein inhibitors cocktail (1 μg/ml leupeptin, pepstatin A, chymostatin and antipain). The cellular extracts immediately underwent three consecutive cycles of freezing/thawing with liquid nitrogen. Cell lysates were then centrifuged for 10 min at 20,800×g at 4 °C and the supernatant was collected. Protein concentration was determined using a Bio-Rad protein assay (BIO-RAD, Hercules, California, USA). Equal amounts of protein (20 µg) were prepared in sample buffer (100 mM Tris, 100 mM DTT, 4 % (v/v) SDS, 0.2 % (w/v) bromophenol blue and 20 % (v/v) glycerol), denatured at 95 °C for 5 min and proteins were separated by electrophoresis on 10 % (w/v) SDS–polyacrylamide gel (SDS-PAGE). Proteins were then transferred to PVDF membranes, which were further blocked for 30 min at RT with 5 % (w/v) BSA in Tris-buffered saline (150 mM NaCl, 50 mM Tris, pH 7.6) with 0.1 % (w/v) Tween 20 (TBS-T) or Odyssey blocking buffer (LI-COR bioscience, Lincoln, Nebraska USA) for detection of tau isoforms. The membranes were next incubated overnight at 4 °C with the following primary monoclonal antibodies in TBS-T: mouse anti-GSK3β and anti-GSK-3β(pY216) (1:1,000; BD Biosciences, San José, CA, USA), anti-cleaved-Tau (Asp421) clone C3 and total tau (tau-5) (1:500; Life Technologies, Carlsbad, USA) or rabbit antibody anti-Bcl-2 or anti-Bax (1:1,000; Cell Signaling, Danvers, Massachusetts, USA). Control of protein loading was performed using a primary mouse anti-α-tubulin (1:20,000, Cell signaling) or anti-actin (clone AC-40, 1:1,000; Sigma-Aldrich, Saint Louis, MO, USA) antibodies. After washing, membranes were incubated for 1 h at RT with an alkaline phosphatase (1:20,000; GE Healthcare, Amersham, UK) or biotinilyted (1:1,000 DAKO, Glostrup, Denmark) conjugated secondary anti-mouse or anti-rabbit antibody. Bands of immunoreactive proteins were visualized after incubation of the membrane with ECF™ substrate (GE Healthcare) during approximately 5 min on a Versa Doc 3000 Imaging System or after incubation with IRDye® Infrared Dye (1:15,000, LI-COR) for 45 min on the LI-COR Odyssey scanner. Densitometric analysis was performed using ImageLab Software (Bio-Rad, Hercules, CA, USA) or ImageJ 1.45 (NIH, Bethesda, USA). The ratios between Bax/Bcl-2, P-GSK-3β/GSK-3β and TauC3/t-tau were calculated and results were normalized to values in untreated cells.
Analysis of caspase-3 and -9 activities
In order to investigate the activation of caspases-3 and -9, cell lysates from SH-SY5Y cells treated with and without rBRI276–266 were prepared as described above, but in the absence of Na3VO4, and NaF. Cellular extracts containing 40 μg of protein were incubated with 0.1 mM Ac-DEVD-pNA (chromogenic substrate for caspase-3, Calbiochem, Darmstadt, Germany) or Ac-LEHD-pNA (chromogenic substrate for caspase-9, Calbiochem) in reaction buffer (25 mM HEPES-Na, 10 % (w/v) sucrose, 10 mM DTT, and 0.1 % (w/v) CHAPS, pH 7.4) for 2 h at 37 °C. Caspase activity was determined by measuring substrate cleavage at 405 nm in a microplate reader (SpectraMax Plus 384) and results were expressed relatively to the untreated cells.
RNA isolation and cDNA synthesis
For RNA isolation, SH-SY5Y cells (9 × 104 per well in a 24-well plate) were harvested and total RNA was isolated using TriPure isolation reagent (Roche Applied Science, Basel, Switzerland) following the manufacturer’s specifications. RNA concentration and purity were assessed by OD measurements at 260 and 280 nm on a NanoDrop spectrophotometer (Thermo Scientific). cDNA synthesis was performed using 0.5 μg of RNA and 0.5 μg Oligo(dT)12–18 primer (Life technologies, Carlsbad, California, USA) as previously described [40].
Real-time qPCR
Real-time qPCR was performed using the Light Cycler 480 system (Roche Applied Science). Oligonucleotide primers (Sigma, Saint Louis, MO, USA) used for qPCR are listed in Table 1. Eukaryotic Elongation factor (EEF), Binding immunoglobulin protein (BiP) and CCAAT-enhancer-binding protein homologous protein (CHOP) cDNA were measured using Fluorescent reporter probe method. Reaction was performed using 1 μl contained cDNA, 0.05 μl Universal Library probe (Roche Applied Science), 0.1 μl forward primer, 0.1 μl reverse primer and 2.5 μl 2 × LightCycler 480 Probes Master (Roche Applied Science). Unspliced and spliced X-box binding protein 1 (Xbp-1) cDNA were measured using SYBRGreen dsDNA dye. Reaction was performed using 1 μl cDNA, 0.25 μl forward primer, 0.25 μl reverse primer and 5 μl 2 × SYBRGreen Master (Roche Applied Science). qPCR was performed as previously described [40]. Tunicamycin (0.1 µg/ml; Sigma) was used as a positive control for UPR activation. Data was analyzed using LightCycler Software (Roche).
Table 1.
Primers and probes used for qPCR 480 light cycler
| Gene | Primers (5′-3′) | Probe no. |
|---|---|---|
| BiP | FW: GCTGGCCTAAATGTTATGAGGA | 7 |
| RV: CCACCCAGGTCAAAC | ||
| CHOP | FW: AAGGCACTGAGCGTATCATGT | 21 |
| RV: TGAAGATACACTTCCTTCTTGAACA | ||
| hEEF | FW: CAATGGCAAAATCTCACTGC | 63 |
| RV: AACCTCATCTCTATTAAAAACACCAAA | ||
| Xbp-1 (unspliced) | FW: GACAGAGAGCCAAGCTAATGTGG | Sybr.Green |
| RV: ATCCAGTAGGCAGGAAGAT | ||
| Xbp-1 (spliced) | FW: AAGACAGCGCTTGGGGATGG | Sybr.Green |
| RV: CTGACCTGCTGCGGAC |
Probe numbers refer to numbers in the Roche universal probe library
Total tau and hyperphosphorylated tau
The concentration of total tau (t-tau) and phosphorylated tau (p-tau181) in the cell lysates (2 and 20 µg respectively) was measured using INNOTEST®hTAU Ag or INNOTEST®PHOSPHO-TAU(181P) (Innogenetics, Gent, Belgium) ELISA Kits. Phosphorylation of tau231 (p-tau231) was analyzed by Multi Array® PhosphoTau (Thr 231)/t-tau immunosorbent assay (mesoscale discovery, MSD, Rockville, MD, USA). Assays were performed following manufacturer´s instructions. The ratio p-tau/t-tau was calculated and results were normalized to control values. Intra-assay coefficients of variation (CV) for t-tau, p-tau181 and p-tau231 were 1.8, 2.51 and 1.30 % respectively.
Statistical analysis
Data were expressed as mean ± SEM of measurements from at least three independent experiments performed in duplicates or triplicates. Mean differences were analysed using non-parametric Mann–Whitney U test in GraphPad Prism Software (San Diego, CA, USA). Differences were considered significant for p values <0.05.
Results
Characterization of recombinant BRI2 ectodomain
Coomasie staining of rBRI2 ectodomain showed that rBRI2 ectodomain forms aggregates of different molecular weights as previously described [25] (Fig. 1a). Under reducing and denaturing conditions, rBRI276–266 was observed not only at its expected molecular weight of 25 kDa but also at 50 kDa. Larger complexes were observed under non-reducing conditions. Size exclusion chromatography showed that BRI2 BRICHOS elutes in several different fractions corresponding to complexes of different molecular weight (33, 80 336 and >600 kDa). However, rBRI2 ectodomain elutes only in one fraction at 6–8 ml, corresponding to larger complexes with a molecular mass higher than 600 kDa (Fig. 1b).
Fig. 1.
Recombinant BRI276–266 form oligomeric aggregates of different molecular weights. a Coomasie staining of rBRI276–266 in different reducing conditions showed several bands at different molecular weights. b Gel filtration of rBRI276–266 and BRI2 BRICHOS shows peaks that elutes in the void volume indicating the formation of different complexes. Numbers indicate the molecular mass of the corresponding complex in kDa
Recombinant BRI2 ectodomain delays Aβ42 fibril formation less efficiently than BRI2 BRICHOS domain
Thioflavin T (ThT) was used as a reporter for fibril formation in kinetic experiments of Aβ42 alone or with different concentrations of the rBRI2 ectodomain and the BRI2 BRICHOS proteins. The midpoint of the aggregation process, t 1/2 were obtained by fitting a sigmoidal function to each kinetic trace and was plotted versus molar ratio of corresponding proteins/Aβ42. The data showed that both rBRI2 ectodomain and BRI2-BRICHOS delayed the aggregation of Aβ42, and only substoichiometric amounts of the proteins are required (Fig. 2a). The half-times in the presence of BRICHOS were compared with the uninhibited case (Aβ42 alone) (Fig. 2b). These experiments showed that while BRI2-BRICHOS domain efficiently prevented Aβ42 conversion into ThT positive aggregates for more than 24 h, ThT positive aggregates of Aβ42 started to form already after 10 h when the rBRI2 ectodomain was used (Fig. 2a, b).
Fig. 2.
Recombinant BRI2 ectodomain delays Aβ42 less efficiently than BRI2 BRICHOS domain. a Aggregation kinetics as monitored by ThT fluorescence of 3 µM Aβ42 in the absence (black) or presence of 0.2 (0.6 µM) equivalents of rBRI276–266 (red) or BRI2-BRICHOS (green). Every kinetic trace is the average of 4 measurements with the standard deviations plotted as error bars on each time point. b Relative half-time of fibrillation versus molar ratio protein/Aβ42 for rBRI276–266 (black dots) and Bri2 BRICHOS (open squares). Numbers are taken from the average of 4 kinetic traces, and the data are plotted relative to the half-time obtained in the absence of BRI2 protein
Recombinant BRI2 ectodomain induced apoptotic cell death
The neurotoxicity of the rBRI2 ectodomain was investigated in SH-SY5Y human neuroblastoma cells. Incubation with rBRI2 ectodomain at 0.2, 0.5 and 2 µM for 24 h decreased mildly, but significantly, MTT reduction by 10 % in SH-SY5Y cells (Fig. 3a) and N2a cells (data not shown). Differentiated SH-SY5Y and SK-N-SH cells have been widely used to analyze the neurotoxic effects of Aβ [41, 42]. The exposure of rBRI2 ectodomain to RA-differentiated SH-SY5Y (Fig. 3b) or SK-N-SH (data not shown) cells led also to a 10 % decrease of MTT reduction indicating no differences between differentiated and non-differentiated cells. Therefore, subsequent experiments were performed only with undifferentiated SH-SY5Y cells. Since deposits of BRI2 were associated with Aβ plaques in the AD hippocampus [25], we investigated whether the co-incubation of rBRI2 ectodomain could modify the toxicity induced by Aβ42 peptides. The decrease of MTT reduction induced by the Aβ42 was not modified by the co-incubation with rBRI2 ectodomain (Fig. 3c).
Fig. 3.

Recombinant BRI276–266 induced the activation of the apoptosis pathway in human neuroblastoma cells. a–c Cell viability MTT assay after 24 h incubation of (a) non-differentiated SH-SY5Y cells with 0.2, 0.5 or 2 µM rBRI276–266; b differentiated SH-SY5Y cells with 0.5 µM rBRI276–266; c non-differentiated SH-SY5Y cells with Aβ42 peptide (0.5 µM) in the presence or absence of rBRI276–266 (0.5 µM). d Representative western blot showing Bax and Bcl-2 expression in cell lysates from SH-SY5Y cells treated with rBRI276–266 (0.5 μM) during 24 h. 20 μg of protein was loaded, and reactivity against α-tubulin was used as a loading control. Reactivity against Bax (e) and Bcl-2 (f) was quantified and corrected for α-tubulin levels. g Bax/Bcl-2 ratio in control and treated cells. h, i Quantification of caspase-3 (h) and caspase-9 (i) like activities in cell lysates using the colorimetric substrates Ac-LEHD-pNA and Ac-DEVD-pNA respectively. Results were normalized to untreated cells (control) and represent the mean ± SEM of at least four independent experiments. *p < 0.05, **p < 0.01
Since a slight but significant reduction of cell viability was observed after rBRI276–266 exposure, we hypothesized that the rBRI2 ectodomain may promote the activation of apoptosis. Incubation of SH-SY5Y cells with 0.5 µM of rBRI276–266 led to a significant increase in the levels of the pro-apoptotic protein Bax (Fig. 3d, e), together with a decrease in the levels of the anti-apoptotic Bcl-2 protein (Fig. 3d, f) and a subsequent two-fold increase in the Bax/Bcl-2 ratio (Fig. 3g). To further investigate the role of BRI2 in apoptosis, we also analyzed the activity of caspases 3 and 9, which are downstream effectors of the apoptotic pathway [43]. The incubation with rBRI2 ectodomain led to a significant two-fold increase in the activity of both caspases 3 and 9 (Fig. 3h, i). These data indicate rBRI2 ectodomain induced an apoptotic response in SH-SY5Y cells.
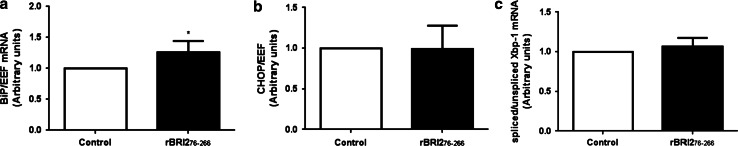
Recombinant BRI2 ectodomain increased the mRNA of BiP but did not modify the mRNA of CHOP or Xbp-1
Since deposits of BRI2 ectodomain have been observed in early stages of AD, we investigated whether rBRI2 ectodomain activates the classical mediators involved in the UPR (BiP, CHOP and Xbp-1), which may explain the apoptotic response. Incubation of SH-SY5Y cells with rBRI2 ectodomain slightly increased the mRNA levels of BiP (Fig. 4a), but not the mRNA levels of CHOP and Xbp-1 (Fig. 4b, c). Taken together, these data indicate that the UPR is not involved in the apoptotic cell death of SH-SY5Y observed upon rBRI2 exposure.
Fig. 4.
Recombinant BRI276–266 did not induce the activation of the UPR in human neuroblastoma cells. mRNA expression levels of BiP (a), CHOP (b) and spliced/unspliced Xbp-1 mRNA ratio (c). SH-SY5Y cells were treated with rBRI276–266 (0.5 µM) during 24 h and mRNA was isolated as described in “Materials and methods”. Relative mRNA of BiP, CHOP, spliced and unspliced Xbp-1 corrected EEF was determined using q-PCR. Results were normalized to untreated cells (control) within each experiment and represent the mean ± SEM of at least four independent experiments. *p < 0.05
Recombinant BRI2 ectodomain induced the activation of GSK3β and increased the levels of truncated tau at D421
Neurofibrillary tangles are mainly formed by aggregates of abnormal post translationally modified forms of tau such as hyper-phosphorylated tau and truncated tau [1, 44]. Glycogen synthase kinase 3 beta (GSK3β) is the major kinase involved in tau phosphorylation and it is active after phosphorylation of its tyrosine at position 216 (p-GSK3β-Y216) [45, 46]. We investigated whether incubation with rBRI2 ectodomain promoted activation of GSK3β and the subsequent phosphorylation of tau at threonine 181 and 231. We observed that incubation of SH-SY5Y cells with rBRI276–266 led to increased levels of p-GSK3β-Y216 and subsequent p-GSK3β-Y216/t-GSK3β ratio, indicating an activation of GSK3β (Fig. 5a–c). However, no differences in the levels of p-tau181 or p-tau231 were observed after incubation with rBRI2ectodomain (Fig. 5d, e). Strikingly, the levels of t-tau measured by the ELISA kit were mildly but significantly decreased in cells incubated with rBRI2 ectodomain (Fig. 5f). Interestingly, incubation with rBRI2 ectodomain led to increased levels of the truncated tau C3/t-tau ratio (Fig. 5g, h), indicating that rBRI276–266 induced the truncation of tau at D421. In summary, the results showed that rBRI2 ectodomain induced the activation of GSK3β, and increased the truncation of tau at D421.
Fig. 5.

Recombinant BRI276–266 induced the phosphorylation of GSK3β (Y216) and increased the levels of truncated tau at D421 in human neuroblastoma cells. a Western blot showing anti-GSK3β total and anti-p-GSK3β (Y216) expression in cell lysates from SH-SY5Y cells treated with rBRI276–266 (0.5 μM) during 24 h. 20 μg protein were loaded, and reactivity against α-tubulin was used as a loading control. b Reactivity against anti-GSK3β total and anti-p-GSK3β (Y216) was quantified and corrected for α-tubulin or actin levels. c p-GSK3β/total-GSK3β ratio was calculated in control and treated cells. d, e The corresponding p-tau/t-tau ratio for the levels of p-tau181 (d) or p-tau231 (e) were calculated in cell lysates from control and treated cells using highly specific ELISA and MSD kits respectively. f Levels of t-tau in cell lysates using specific ELISA kit. g Western blot showing anti-tau total and anti-truncated tau at D421 (tau C3) expression in cell lysates from SH-SY5Y cells treated with rBRI276–266 (0.5 μM) during 24 h. 20 μg protein was loaded, and reactivity against actin was used as a loading control. h Reactivity against anti-total tau and anti-truncated tau C3 was quantified and corrected for actin levels truncated/t-tau ratio was calculated in control and treated cells. Results were normalized to untreated cells (control) and represent the mean ± SEM of at least four independent experiments. **p < 0.01
Discussion
Protein conformation-dependent neurotoxicity is a common key molecular pathway involved in different neurodegenerative diseases including AD [47, 48]. Several studies have shown that protein misfolding and aggregation (e.g. of Aβ, α-synuclein, prion protein) can lead to synaptic dysfunction and neuronal apoptosis [27–32]. Interestingly, we previously showed an accumulation of BRI2 ectodomain associated with Aβ plaques in early stages of AD [25]. We hypothesize that the observed decrease in the levels of the enzymes involved in the shedding of BRI2 ectodomain (furin and ADAM10 [7, 8, 25, 49, 50]) leads to reduced cleavage of the BRI2 ectodomain. Together with the increased levels of SPPL2b [25], the enzyme cleaving in the BRI2 transmembrane domain [8], this may promote the release of an un-processed BRI2 ectodomain (BRI2 residues 76–266) instead of the shorter BRI2 peptides. The larger BRI2 fragment may then aggregate leading to the accumulation and loss of function of BRI2 [25]. The analysis of rBRI276–266 under reducing and denaturing conditions showed not only the expected band at 25 kDa but also a band at 50 kDa, which suggest the formation of strong bonds similar to those observed in human hippocampus using an antibody raised against rBRI276–266 [25]. Similar strong aggregates have been detected for other molecules such as the Aβ peptide [51]. The formation of BRI2 homodimers via covalent (i.e. disulfide bonds) and non-covalent interactions in the ectodomain have been previously reported [52]. Thus, the same interactions likely explain the larger BRI2 complexes that were also observed under non-reducing conditions. Further characterization of rBRI276–266 by size exclusion chromatography revealed that indeed rBRI276–266 forms large complexes >600 kDa. These data further confirm the aggregation state of recombinant BRI2 ectodomain [25], as also seen for the recombinant form of BRI2 residues 90–236 [9].
Previous studies showed that the BRI2-BRICHOS domain, which is part of the BRI2 protein fragment released after ADAM10 processing [8], inhibits Aβ aggregation and fibrillation very efficiently [9, 11]. We previously suggested that the deposits/aggregates of BRI2 ectodomain observed in early stages of AD hippocampus could lead to a loss of BRI2 function [25], including its inhibitory function on Aβ aggregation and fibrillation. The results of this study further support this hypothesis since we observed that the anti-Aβ aggregation activity of rBRI2 ectodomain is significantly lower than that of the recombinant BRI2 BRICHOS domain (residues 113–231). However, despite both BRI2 containing BRICHOS domain (90–236) [9] and BRI2 BRICHOS domain (residues 113–231) form different larger complexes, they still act in delaying Aβ fibril formation. Thus, further studies are needed to reveal the relation between BRI2 protein oligomeric states and activity. It should also be noted that rBRI276–266 includes the Bri23 peptide part, which is not the case for the fragments covering residues 90–236 or 113–231, and that it is still unknown to what extent this affects function.
Similarly to aggregated proteins such as Aβ or α-synuclein, the accumulation of BRI2 ectodomain in AD may lead to a gain of toxic function and have a negative effect on cell homeostasis [27–29, 53]. To address this question, we first analyzed the toxicity of rBRI2 ectodomain in neuronal cells using the MTT assay. In the current experimental setup, the addition of rBRI2 ectodomain led to a 10 % decrease in cell viability indicating that BRI2 ectodomain can be slightly neurotoxic. More specific and sensitive analysis showed that BRI276–266 clearly induced an apoptotic response by increasing the levels of the pro-apoptotic protein Bax and decreasing the levels of the anti-apoptotic protein Bcl-2. The increased activity of caspases 3 and 9, downstream effectors in the apoptosis cascade [43], further confirm the pro-apoptotic effects of exposure to rBRI2 ectodomain. Strikingly, the cell viability changes observed after incubation with rBRI276–266 do not resemble the large changes observed in the survival and pro-apoptotic proteins. It is possible that the changes observed in the apoptotic pathway still in an early stage, which cannot be fully recognized by the MTT assay. In addition, the activation of compensatory mechanisms that ultimately may prevent cell death can neither be excluded.
Overexpression of a shorter BRI2 form (ITM2Bs, 1–210) also activated apoptosis in a murine T cell line, which was caused by the pro-apoptotic conserved BH3 domain in the cytosolic part of BRI2 [54–56]. However, since rBRI276–266 does not contain the BH3 domain, we propose that the observed apoptosis is mediated by other causes, possibly conformation dependent mechanisms, as observed for Aβ [57, 58]. Co-incubation of rBRI276–266 with Aβ42 did not increase the toxicity induced by Aβ42. On the contrary, co-incubation led to a slight but not significant decrease in the Aβ42-induced toxicity. Since rBRI276–266 was able to delay Aβ42 fibrillation in our experiments, their co-incubation may delay the aggregation and fibrillation of Aβ42, possibly mediating the decrease in the Aβ42-induced toxicity. Whether recombinant BRI2 BRICHOS is similarly able to decrease Aβ42-induced toxicity by delaying Aβ42 aggregation and fibrillation remains to be investigated. Recently it was shown in a Drosophila model of Aβ aggregation and toxicity, that the BRICHOS domain of the lung surfactant protein C (proSP-C) prevents the toxic effect of expressed Aβ42 in the fly brain [59]. Recombinant proSP-C BRICHOS domain has been shown to have a similar effect in vitro on Aβ fibril formation as BRI2 BRICHOS [11], which supports the hypothesis that the BRI2 BRICHOS domain is also capable of decreasing the Aβ42-induced toxicity, and not only affect the fibrillation.
Misfolded proteins and extracellular stimuli can lead to ER stress and Ca2+ release, which can activate apoptosis [33, 34]. ER stress induces the UPR, a coordinated adaptive cell response focused on restoring cell homeostasis [33, 35]. Noteworthy, increased expression of UPR markers has been observed in early stages of AD [60–62]. Compelling evidence suggests that Aβ can induce an ER stress-mediated apoptosis [63–66]. In a recent study, inhibition of the UPR prevented neurodegeneration independently of the primary pathogen in a mouse model of prion disease [67]. Thus, the UPR is an important response and a causative contributor to the pathogenesis of misfolded protein disorders [68]. In the UPR, misfolded proteins activate three independent pathways that ultimately lead to an increased transcription of several genes including the ER chaperone BiP, the pro-apoptotic protein CHOP and the cellular stress transcription factor Xbp-1 [35]. Here, we showed that exogenous rBRI276–266 induced a slight up-regulation of BiP mRNA but it did not modify the mRNA levels of either CHOP or sXbp-1. Thus, the UPR presumably is not a major player during the apoptosis induced by exogenous rBRI2 ectodomain. However, the increase on BiP mRNA levels suggests that rBRI2 aggregates may initiate a mild stress of the ER and the subsequent release of Ca2+ similarly to Aβ [34, 64]. It has been shown that apoptotic stimuli and ER-Ca2+ release in neuronal cells can also lead to the activation of GSK3β, providing an alternative pathway leading to neuronal death [69, 70]. Interestingly, rBRI276–266 increased the phosphorylation and activation of GSK3β, suggesting that the apoptotic response induced by rBRI276–266 might be mediated via p-GSK3β-Y216. Alternatively, apoptosis could be also mediated via cell surface death receptors or by other cytotoxic stress pathways (i.e. heat shock response) [71, 72], which remains to be investigated.
We have previously shown that NFT formation correlated with the formation of BRI2 deposits in post-mortem human AD tissue [25], suggesting a close relationship between BRI2 accumulation and NFT development. NFTs are one of the classical neuropathological hallmarks in AD and consist of intracellular aggregates of abnormal hyperphosphorylated and truncated tau [1, 44, 73]. According to the amyloid cascade hypothesis, the observed association between Aβ deposits and NFTs suggests that amyloid-induced toxicity may trigger neurofibrillary pathology [74, 75]. However, the exact mechanism by which amyloidosis may facilitate NFTs formation remains to be elucidated. In the last years, several studies suggested that truncation of tau by caspases (preferentially caspase-3) is an early and crucial event in tau pathogenesis [44, 76, 77]. However, it remains controversial whether tau truncation precedes tau hyperphosphorylation or if it occurs once NFT are formed [78]. Our data showed that while rBRI276–266 led to increased levels of p-GSK3β-Y216, it did not modify the levels of p-tau181 or p-tau231 in SH-SY5Y cells. Besides tau phosphorylation, GSK3β can regulate other important proteins involved in AD pathogenesis such as presenilin 1 (PS1) [79, 80] or BACE1 [81], both involved in the processing of APP and production of Aβ [82, 83]. Intriguingly, previous studies have shown contradictory results when studying the effects of GSK3β in APP processing and Aβ production [79, 84–86]. In addition GSK3β participates also in inflammatory mechanisms by promoting the secretion of different cytokines including IL-12 [87], an inflammation pathway that regulated not only Aβ pathology but also spatial memory in a transgenic mouse model [88, 89]. Thus, future studies are needed to unravel whether rBRI2 ectodomain can modulate APP processing enzymes or inflammatory markers via activation of GSK3β.
Interestingly, in this experimental setup, incubation with rBRI276–266 led not only to an increased activity of caspase 3 but also to increased levels of truncated tau at D421. Importantly, truncation of tau at D421 has been observed in AD [90, 91] and this truncated form has been suggested to have an important involvement in the formation of NFT [44, 73, 76, 92]. Strikingly, decreased levels of t-tau were found after incubation with rBRI2 ectodomain. We suggest that incubation with rBRI2 ectodomain and the subsequent increased activity of caspase 3 may promote the truncation and assembly of tau [93], masking some of the epitopes recognized by the antibodies of the t-tau immunoassay and thus leading to the observed decrease levels in t-tau. Thus, our results suggest that the presence of BRI2 aggregates may induce the truncation of tau at D421 and promote the formation of NFT in AD.
Taken together, our results reveal that the rBRI2 ectodomain was remarkably less efficient on preventing Aβ fibrillation than the BRI2 BRICHOS domain, supporting the hypothesis that un-processed BRI2 ectodomain and subsequent aggregation disrupts BRI2 functionality (i.e. easing amyloid fibrillation). Moreover, the rBRI2 ectodomain besides inducing an apoptotic response and activation of GSK3β, it also increased the levels of truncated tau at D421, suggesting that BRI2 ectodomain oligomers can be involved in NFT formation. Since BRI2 is able to regulate APP processing and Aβ load, we propose that modifications in BRI2 can play an important role of several aspects of AD pathogenesis. On one hand, the accumulation of BRI2 ectodomain in early stages of AD likely leads to a loss of BRI2 function [25], influencing APP processing and Aβ metabolism [11–13, 19, 20, 94]. On top of that, our current results suggest that the presence BRI2 aggregates may participate in the neurodegenerative process through truncation of tau and an activation of the apoptosis pathway (Fig. 6). Nevertheless, it is important to define the specific conformational structure and sequencing of the BRI2 form that is modified in AD and thus, specific antibodies covering a wide range of the BRI2 protein need to be developed. In addition, other aspects need to be further investigated including a thorough analysis of the effects of aggregated BRI2 on AD related mechanisms (e.g. APP processing, ER-Ca2+ release and inflammation). The further understanding of BRI2 in AD may open new insight in the development of disease-modifying therapies trying to restore the normal function of BRI2.
Fig. 6.
Hypothetical model illustrating the possible consequences of aggregated BRI2 ectodomain. Aggregated BRI2 ectodomain may have important consequences on the development of AD hallmarks as proposed in the “Discussion”. However, it is important to note that exact characteristics of the larger BRI2 forms and deposits observed in human AD tissue remain to be clarified
Acknowledgments
We acknowledge Kees van Uffelen, Dorine Wouters and Irina van Geffen from the Neurochemistry Laboratory of the Clinical Chemistry department at the VUmc for their technical assistance. This work was supported by the Erasmus Mundus Joint Doctorate Program (EMJD 2009-2013, Action 1B, Grant 159302-1-2009-1-NL-ERA, European Neuroscience Campus Network) and the Swedish Research Council. We also acknowledge the grant from the German scientific network for dementia research BMBF KNDD rpAD (BMBF 01GI1010A) to C.K, and a grant from the Forschungskommission of the Medical Faculty Heinrich Heine University Düsseldorf to A.M.S.
Conflict of interest
None of the authors have any competing interest.
References
- 1.Grundke-Iqbal I, Iqbal K, Tung YC, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selkoe DJ. The molecular of Alzheimer’s pathology disease review. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 3.Vidal R, Frangione B, Rostagno a, et al. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399:776–781. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- 4.Vidal R, Revesz T, Rostagno a, et al. A decamer duplication in the 3′ region of the BRI gene originates an amyloid peptide that is associated with dementia in a Danish kindred. Proc Natl Acad Sci USA. 2000;97:4920–4925. doi: 10.1073/pnas.080076097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rostagno A, Tomidokoro Y, Lashley T, et al. Chromosome 13 dementias. Cell Mol Life Sci. 2005;62:1814–1825. doi: 10.1007/s00018-005-5092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garringer HJ, Murrell J, D’Adamio L, et al. Modeling familial British and Danish dementia. Brain Struct Funct. 2010;214:235–244. doi: 10.1007/s00429-009-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SH, Wang R, Gordon DJ, et al. Furin mediates enhanced production of fibrillogenic ABri peptides in familial British dementia. Nat Neurosci. 1999;2:984–988. doi: 10.1038/14783. [DOI] [PubMed] [Google Scholar]
- 8.Martin L, Fluhrer R, Reiss K, et al. Regulated intramembrane proteolysis of Bri2 (Itm2b) by ADAM10 and SPPL2a/SPPL2b. J Biol Chem. 2008;283:1644–1652. doi: 10.1074/jbc.M706661200. [DOI] [PubMed] [Google Scholar]
- 9.Peng S, Fitzen M, Jörnvall H, Johansson J. The extracellular domain of Bri2 (ITM2B) binds the ABri peptide (1–23) and amyloid beta-peptide (Abeta1-40): implications for Bri2 effects on processing of amyloid precursor protein and Abeta aggregation. Biochem Biophys Res Commun. 2010;393:356–361. doi: 10.1016/j.bbrc.2009.12.122. [DOI] [PubMed] [Google Scholar]
- 10.Willander H, Hermansson E, Johansson J, Presto J. BRICHOS domain associated with lung fibrosis, dementia and cancer–a chaperone that prevents amyloid fibril formation? FEBS J. 2011;278:3893–3904. doi: 10.1111/j.1742-4658.2011.08209.x. [DOI] [PubMed] [Google Scholar]
- 11.Willander H, Presto J, Askarieh G, et al. BRICHOS domains efficiently delay fibrillation of amyloid β-peptide. J Biol Chem. 2012;287:31608–31617. doi: 10.1074/jbc.M112.393157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fotinopoulou A, Tsachaki M, Vlavaki M, et al. BRI2 interacts with amyloid precursor protein (APP) and regulates amyloid beta (Abeta) production. J Biol Chem. 2005;280:30768–30772. doi: 10.1074/jbc.C500231200. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda S, Giliberto L, Matsuda Y, et al. The familial dementia BRI2 gene binds the Alzheimer gene amyloid-beta precursor protein and inhibits amyloid-beta production. J Biol Chem. 2005;280:28912–28916. doi: 10.1074/jbc.C500217200. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda S, Giliberto L, Matsuda Y, et al. BRI2 inhibits amyloid beta-peptide precursor protein processing by interfering with the docking of secretases to the substrate. J Neurosci. 2008;28:8668–8676. doi: 10.1523/JNEUROSCI.2094-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda S, Matsuda Y, Snapp EL, D’Adamio L. Maturation of BRI2 generates a specific inhibitor that reduces APP processing at the plasma membrane and in endocytic vesicles. Neurobiol Aging. 2009;32:1400–1408. doi: 10.1016/j.neurobiolaging.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda S, Tamayev R, D’Adamio L. Increased AβPP processing in familial Danish dementia patients. J Alzheimers Dis. 2011;27:385–391. doi: 10.3233/JAD-2011-110785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamayev R, Matsuda S, Giliberto L, et al. APP heterozygosity averts memory deficit in knockin mice expressing the Danish dementia BRI2 mutant. EMBO J. 2011;30:2501–2509. doi: 10.1038/emboj.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamayev R, D’Adamio L. Memory deficits of British dementia knock-in mice are prevented by APP haploinsufficiency. J Neurosci. 2012;32:5481–5485. doi: 10.1523/JNEUROSCI.5193-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilger E, Buehler A, Woelfing H, et al. BRI2 regulates β-amyloid degradation by increasing levels of secreted insulin degrading enzyme (IDE) J Biol Chem. 2011;286:37446–37457. doi: 10.1074/jbc.M111.288373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsachaki M, Fotinopoulou A, Slavi N, et al. BRI2 interacts with BACE1 and regulates its cellular levels by promoting its degradation and reducing its mRNA levels. Curr Alzheimer Res. 2013;10:532–541. doi: 10.2174/1567205011310050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamayev R, Giliberto L, Li W, et al. Memory deficits due to familial British dementia Bri2 mutation are caused by loss of BRI2 function rather than amyloidosis. J Neurosci. 2010;30:14915–14924. doi: 10.1523/JNEUROSCI.3917-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamayev R, Matsuda S, Fà M, et al. Danish dementia mice suggest that loss of function and not the amyloid cascade causes synaptic plasticity and memory deficits. PNAS. 2010;107:20822–20827. doi: 10.1073/pnas.1011689107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garringer HJ, Murrell J, Sammeta N, et al. Increased tau phosphorylation and tau truncation, and decreased synaptophysin levels in mutant BRI2/tau transgenic mice. PLoS ONE. 2013;8:e56426. doi: 10.1371/journal.pone.0056426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Campo M, Teunissen CE. Role of BRI2 in dementia. J Alzheimers Dis. 2014;40:481–494. doi: 10.3233/JAD-131364. [DOI] [PubMed] [Google Scholar]
- 25.Del Campo M, Hoozemans JJM, Dekkers L-L, et al. BRI2-BRICHOS is increased in human amyloid plaques in early stages of Alzheimer’s disease. Neurobiol Aging. 2014;35:1596–1604. doi: 10.1016/j.neurobiolaging.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J. 2008;27:336–349. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein WL. Synaptotoxic amyloid-β oligomers: a molecular basis for the cause, diagnosis, and treatment of Alzheimer’s disease? J Alzheimers Dis. 2013;33(Suppl 1):S49–S65. doi: 10.3233/JAD-2012-129039. [DOI] [PubMed] [Google Scholar]
- 28.Brown DR. Oligomeric alpha-synuclein and its role in neuronal death. IUBMB Life. 2010;62:334–339. doi: 10.1002/iub.316. [DOI] [PubMed] [Google Scholar]
- 29.Bossy-Wetzel E, Schwarzenbacher R, Lipton SA (2004) Molecular pathways to neurodegeneration. Nat Med Suppl:S2–S9. doi:10.1038/nm1067 [DOI] [PubMed]
- 30.Forloni G, Angeretti N, Chiesa R, et al. Neurotoxicity of a prion protein fragment. Nature. 1993;362:543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- 31.El-Agnaf OM, Jakes R, Curran MD, et al. Aggregates from mutant and wild-type alpha-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of beta-sheet and amyloid-like filaments. FEBS Lett. 1998;440:71–75. doi: 10.1016/S0014-5793(98)01418-5. [DOI] [PubMed] [Google Scholar]
- 32.El-Agnaf OM, Mahil DS, Patel BP, Austen BM. Oligomerization and toxicity of beta-amyloid-42 implicated in Alzheimer’s disease. Biochem Biophys Res Commun. 2000;273:1003–1007. doi: 10.1006/bbrc.2000.3051. [DOI] [PubMed] [Google Scholar]
- 33.Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol. 2010;189:783–794. doi: 10.1083/jcb.201003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreiro E, Oliveira CR, Pereira C. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-beta peptide. J Neurosci Res. 2004;76:872–880. doi: 10.1002/jnr.20135. [DOI] [PubMed] [Google Scholar]
- 35.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 36.Korth C, Stierli B, Streit P, et al. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature. 1997;390:74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- 37.Walsh DM, Thulin E, Minogue AM, et al. A facile method for expression and purification of the Alzheimer’s disease-associated amyloid beta-peptide. FEBS J. 2009;276:1266–1281. doi: 10.1111/j.1742-4658.2008.06862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonseca ACRG, Ferreiro E, Oliveira CR, et al. Activation of the endoplasmic reticulum stress response by the amyloid-beta 1–40 peptide in brain endothelial cells. Biochim Biophys Acta. 2013;1832:2191–2203. doi: 10.1016/j.bbadis.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Schubert D. Cytotoxic amyloid peptides inhibit cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction by enhancing MTT formazan exocytosis. J Neurochem. 1997;69:2285–2293. doi: 10.1046/j.1471-4159.1997.69062285.x. [DOI] [PubMed] [Google Scholar]
- 40.Nijholt DAT, de Graaf TR, van Haastert ES, et al. Endoplasmic reticulum stress activates autophagy but not the proteasome in neuronal cells: implications for Alzheimer’s disease. Cell Death Differ. 2011;18:1071–1081. doi: 10.1038/cdd.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Datki Z, Juhász A, Gálfi M, et al. Method for measuring neurotoxicity of aggregating polypeptides with the MTT assay on differentiated neuroblastoma cells. Brain Res Bull. 2003;62:223–229. doi: 10.1016/j.brainresbull.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Chafekar SM, Baas F, Scheper W. Oligomer-specific Abeta toxicity in cell models is mediated by selective uptake. Biochim Biophys Acta. 2008;1782:523–531. doi: 10.1016/j.bbadis.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Marsden VS, Strasser A. Control of apoptosis in the immune system: bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 44.Rissman RA, Poon WW, Blurton-Jones M, et al. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–130. doi: 10.1172/JCI200420640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovestone S, Reynolds CH, Latimer D, et al. Alzheimer’s disease-like phosphorylation of the microtubule-associated protein tau by glycogen synthase kinase-3 in transfected mammalian cells. Curr Biol. 1994;4:1077–1086. doi: 10.1016/S0960-9822(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 46.Hughes K, Nikolakaki E, Plyte SE, et al. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soto C, Estrada L. Protein misfolding and neurodegeneration. Arch Neurol. 2008;65:184–189. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- 48.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 49.Endres K, Fahrenholz F. Regulation of alpha-secretase ADAM10 expression and activity. Exp Brain Res. 2012;217:343–352. doi: 10.1007/s00221-011-2885-7. [DOI] [PubMed] [Google Scholar]
- 50.Tyler SJ, Dawbarn D, Wilcock GK, Allen SJ. α- and β-secretase: profound changes in Alzheimer’s disease. Boichem Res Commun. 2002;299:373–376. doi: 10.1016/S0006-291X(02)02635-9. [DOI] [PubMed] [Google Scholar]
- 51.Bouter Y, Dietrich K, Wittnam JL, et al. N-truncated amyloid β (Aβ) 4-42 forms stable aggregates and induces acute and long-lasting behavioral deficits. Acta Neuropathol. 2013;126:189–205. doi: 10.1007/s00401-013-1129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsachaki M, Ghiso J, Rostagno A, Efthimiopoulos S. BRI2 homodimerizes with the involvement of intermolecular disulfide bonds. Neurobiol Aging. 2010;31:88–98. doi: 10.1016/j.neurobiolaging.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bemporad F, Chiti F. Protein misfolded oligomers: experimental approaches, mechanism of formation, and structure-toxicity relationships. Chem Biol. 2012;19:315–327. doi: 10.1016/j.chembiol.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Fleischer A, Ayllón V, Dumoutier L, et al. Proapoptotic activity of ITM2B(s), a BH3-only protein induced upon IL-2-deprivation which interacts with Bcl-2. Oncogene. 2002;21:3181–3189. doi: 10.1038/sj.onc.1205464. [DOI] [PubMed] [Google Scholar]
- 55.Fleischer A, Ayllon V, Rebollo A. ITM2BS regulates apoptosis by inducing loss of mitochondrial membrane potential. Eur J Immunol. 2002;32:3498–3505. doi: 10.1002/1521-4141(200212)32:12<3498::AID-IMMU3498>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 56.Fleischer A, Rebollo A. Induction of p53-independent apoptosis by the BH3-only protein ITM2Bs. FEBS Lett. 2004;557:283–287. doi: 10.1016/S0014-5793(03)01519-9. [DOI] [PubMed] [Google Scholar]
- 57.Lesné SE, Sherman M, Grant M, et al. Brain amyloid-β oligomers in ageing and Alzheimer’s disease. Brain. 2013;136:1383–1398. doi: 10.1093/brain/awt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zahs KR, Ashe KH. β-Amyloid oligomers in aging and Alzheimer’s disease. Front Aging Neurosci. 2013;5:28. doi: 10.3389/fnagi.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermansson E, Schultz S, Crowther D, et al. The chaperone domain BRICHOS prevents CNS toxicity of amyloid-β peptide in Drosophila melanogaster . Dis Model Mech. 2014;7:659–665. doi: 10.1242/dmm.014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unterberger U, Höftberger R, Gelpi E, et al. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J Neuropathol Exp Neurol. 2006;65:348–357. doi: 10.1097/01.jnen.0000218445.30535.6f. [DOI] [PubMed] [Google Scholar]
- 61.Hoozemans JJM, van Haastert ES, Nijholt DAT, et al. Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neurodegener Dis. 2012;10:212–215. doi: 10.1159/000334536. [DOI] [PubMed] [Google Scholar]
- 62.Hoozemans JJM, Veerhuis R, Van Haastert ES, et al. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol. 2005;110:165–172. doi: 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- 63.Costa RO, Ferreiro E, Martins I, et al. Amyloid β-induced ER stress is enhanced under mitochondrial dysfunction conditions. Neurobiol Aging. 2012;33(824):e5–e16. doi: 10.1016/j.neurobiolaging.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Resende R, Ferreiro E, Pereira C, Resende de Oliveira C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1–42: involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience. 2008;155:725–737. doi: 10.1016/j.neuroscience.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 65.Chafekar SM, Hoozemans JJM, Zwart R, et al. Abeta 1–42 induces mild endoplasmic reticulum stress in an aggregation state-dependent manner. Antioxid Redox Signal. 2007;9:2245–2254. doi: 10.1089/ars.2007.1797. [DOI] [PubMed] [Google Scholar]
- 66.Ferreiro E, Resende R, Costa R, et al. An endoplasmic-reticulum-specific apoptotic pathway is involved in prion and amyloid-beta peptides neurotoxicity. Neurobiol Dis. 2006;23:669–678. doi: 10.1016/j.nbd.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 67.Moreno JA, Halliday M, Molloy C, et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med. 2013;5:206ra138. doi: 10.1126/scitranslmed.3006767. [DOI] [PubMed] [Google Scholar]
- 68.Scheper W, Hoozemans JJM. Endoplasmic reticulum protein quality control in neurodegenerative disease: the good, the bad and the therapy. Curr Med Chem. 2009;16:615–626. doi: 10.2174/092986709787458506. [DOI] [PubMed] [Google Scholar]
- 69.Song L, De Sarno P, Jope RS. Central role of glycogen synthase kinase-3beta in endoplasmic reticulum stress-induced caspase-3 activation. J Biol Chem. 2002;277:44701–44708. doi: 10.1074/jbc.M206047200. [DOI] [PubMed] [Google Scholar]
- 70.Jacobs KM, Bhave SR, Ferraro DJ, et al. GSK-3β: a bifunctional role in cell death pathways. Int J Cell Biol. 2012;2012:930710. doi: 10.1155/2012/930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilhelmus MMM, Otte-Höller I, Wesseling P, et al. Specific association of small heat shock proteins with the pathological hallmarks of Alzheimer’s disease brains. Neuropathol Appl Neurobiol. 2006;32:119–130. doi: 10.1111/j.1365-2990.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- 72.Milleron RS, Bratton SB. Heat shock induces apoptosis independently of any known initiator caspase-activating complex. J Biol Chem. 2006;281:16991–17000. doi: 10.1074/jbc.M512754200. [DOI] [PubMed] [Google Scholar]
- 73.Gamblin TC, Chen F, Zambrano A, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reitz C. Alzheimer’s disease and the amyloid cascade hypothesis: a critical review. Int J Alzheimers Dis. 2012;2012:369808. doi: 10.1155/2012/369808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 76.De Calignon A, Fox LM, Pitstick R, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao H, Zhao W, Lok K, et al. A synergic role of caspase-6 and caspase-3 in tau truncation at D421 induced by H2O2 . Cell Mol Neurobiol. 2014;34:369–378. doi: 10.1007/s10571-013-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gendron TF, Petrucelli L. The role of tau in neurodegeneration. Mol Neurodegener. 2009;4:13. doi: 10.1186/1750-1326-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uemura K, Kuzuya A, Shimozono Y, et al. GSK3beta activity modifies the localization and function of presenilin 1. J Biol Chem. 2007;282:15823–15832. doi: 10.1074/jbc.M610708200. [DOI] [PubMed] [Google Scholar]
- 80.Twomey C, McCarthy JV. Presenilin-1 is an unprimed glycogen synthase kinase-3beta substrate. FEBS Lett. 2006;580:4015–4020. doi: 10.1016/j.febslet.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 81.Ly PTT, Wu Y, Zou H et al (2013) Inhibition of GSK3 β -mediated BACE1 expression reduces Alzheimer-associated phenotypes. J Clin Invest 123(1):224–235. doi: 10.1172/JCI64516.224 [DOI] [PMC free article] [PubMed]
- 82.Cai H, Wang Y, McCarthy D, et al. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 83.De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 84.Jaworski T, Dewachter I, Lechat B, et al. GSK-3α/β kinases and amyloid production in vivo. Nature. 2011;480:E4–E5. doi: 10.1038/nature10615. [DOI] [PubMed] [Google Scholar]
- 85.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature. 2003;17:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 86.Maesako M, Uemura K, Kubota M, et al. Effect of glycogen synthase kinase 3 β-mediated presenilin 1 phosphorylation on amyloid β production is negatively regulated by insulin receptor cleavage. Neuroscience. 2011;177:298–307. doi: 10.1016/j.neuroscience.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 87.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vom Berg J, Prokop S, Miller KR, et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat Med. 2012;18:1812–1819. doi: 10.1038/nm.2965. [DOI] [PubMed] [Google Scholar]
- 89.Tan M-S, Yu J-T, Jiang T, et al. IL12/23 p40 inhibition ameliorates Alzheimer’s disease-associated neuropathology and spatial memory in SAMP8 mice. J Alzheimers Dis. 2014;38:633–646. doi: 10.3233/JAD-131148. [DOI] [PubMed] [Google Scholar]
- 90.Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, et al. Tau truncation during neurofibrillary tangle evolution in Alzheimer’s disease. Neurobiol Aging. 2005;26:1015–1022. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 91.Basurto-Islas G, Luna-Muñoz J, Guillozet-Bongaarts AL, et al. Accumulation of aspartic acid421- and glutamic acid391-cleaved tau in neurofibrillary tangles correlates with progression in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:470–483. doi: 10.1097/NEN.0b013e31817275c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Q, Zhang X, Sun A. Truncated tau at D421 is associated with neurodegeneration and tangle formation in the brain of Alzheimer transgenic models. Acta Neuropathol. 2009;117:687–697. doi: 10.1007/s00401-009-0491-6. [DOI] [PubMed] [Google Scholar]
- 93.Lee S, Shea TB. Caspase-mediated truncation of tau potentiates aggregation. Int J Alzheimers Dis. 2012;2012:731063. doi: 10.1155/2012/731063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim J, Miller VM, Levites Y, et al. BRI2 (ITM2b) inhibits Aβ deposition in vivo. J Neurosci. 2008;28:6030–6036. doi: 10.1523/JNEUROSCI.0891-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]