Abstract
The great interest that scientists have for adiponectin is primarily due to its central metabolic role. Indeed, the major function of this adipokine is the control of glucose homeostasis that it exerts regulating liver and muscle metabolism. Adiponectin has insulin-sensitizing action and leads to down-regulation of hepatic gluconeogenesis and an increase of fatty acid oxidation. In addition, adiponectin is reported to play an important role in the inhibition of inflammation. The hormone is secreted in full-length form, which can either assemble into complexes or be converted into globular form by proteolytic cleavage. Over the past few years, emerging publications reveal a more varied and pleiotropic action of this hormone. Many studies emphasize a key role of adiponectin during tissue regeneration and show that adiponectin deficiency greatly inhibits the mechanisms underlying tissue renewal. This review deals with the role of adiponectin in tissue regeneration, mainly referring to skeletal muscle regeneration, a process in which adiponectin is deeply involved. In this tissue, globular adiponectin increases proliferation, migration and myogenic properties of both resident stem cells (namely satellite cells) and non-resident muscle precursors (namely mesoangioblasts). Furthermore, skeletal muscle could be a site for the local production of the globular form that occurs in an inflamed environment. Overall, these recent findings contribute to highlight an intriguing function of adiponectin in addition to its well-recognized metabolic action.
Keywords: Adiponectin, Tissue regeneration, Differentiation
Introduction
The metabolic action of adiponectin
Adiponectin is one of the most concentrated hormones in the blood (between 0.5 and 30 μg/ml), thus accounting for 0.01 % of total plasma proteins [1]. Significantly decreased plasma levels of adiponectin have been observed in obese/diabetic mice and humans [1–3] as well as in patients with cardiovascular diseases [4], hypertension [5], and metabolic syndrome [6].
Initially considered as a hormone produced exclusively by adipose tissue [7], it is currently known that adiponectin is locally secreted by various cell types. Primary human osteoblasts [8] and murine osteoblastic and osteoclastic cells express adiponectin and adiponectin receptors [9], thus suggesting that adiponectin can affect bone homeostasis through both endocrine and autocrine/paracrine mechanisms. Adiponectin is also produced by both human and rat placentas [10]. In this tissue, the genomic expression and secretion of adiponectin is modulated by cytokines such as tumor necrosis factor-α, interferon-γ, interleukin-6, and leptin [11]. Moreover, isolated murine and human cardiomyocytes produce adiponectin [12]. In murine heart, as well as in microvascular endothelium and white adipose tissue, adiponectin expression occurs through a particular regulation. In fact, adiponectin expression is regulated by the hypoxia inducible factor-1 (HIF-1) [13]. HIF-1-induced adiponectin is associated with improved myocardial viability in obese/diabetic mice and with increased preservation of left ventricular function, thus suggesting that local production of adiponectin by cardiomyocytes and microvascular endothelial cells may control cardiac function [13]. Expression of adiponectin is also observed in the pituitary gland where this adipokine regulates its own production. [14]. Another important site of adiponectin production is skeletal muscle, which is discussed in paragraph “Role of adiponectin in skeletal muscle regeneration”.
Adiponectin is secreted in “full-length” (fAd) form, which can be cleaved into smaller “globular” (gAd) form by the elastase secreted by activated monocytes and/or neutrophils [15, 16]. Monomers of fAd can assemble forming three different multimers: low molecular weight (LMW) trimers, middle molecular weight (MMW) hexamers, and high molecular weight (HMW) 12–18 multimers [17, 18]. The different forms of adiponectin exert their biological action through the binding with specific receptors. Two G-protein-independent, seven–transmembrane spanning receptors, called AdipoR1 and AdipoR2 were isolated [19]. AdipoR1 is ubiquitously expressed, whereas AdipoR2 is predominantly expressed in the liver. Furthermore, T-cadherin has been identified as a potential receptor for HMW adiponectin [20].
fAd (which has higher affinity for AdipoR2) and gAd (which has higher affinity for AdipoR1) have different target tissues in which they activate different signaling pathways [21–24]. In skeletal muscle, both gAd and fAd activate AMP kinase (AMPK), thereby stimulating phosphorylation of acetyl-coenzymeA-carboxylase (ACC), fatty acid oxidation, and glucose uptake. In the liver, only fAd activates AMPK thereby reducing molecules involved in gluconeogenesis and increasing phosphorylation of ACC and fatty acid oxidation. Both in liver and in skeletal muscle, activation of peroxisome proliferator-activated receptor α (PPARα) is important to decrease triglyceride content [23, 25, 26]. These metabolic actions of adiponectin are associated with the increase of insulin sensitivity in vivo [27–29].
Hepatic insulin sensitivity in humans is associated with circulating adiponectin concentration [30–32]. The amount of adiponectin in the plasma negatively correlates with endogenous glucose production in healthy [2], severely obese [3, 33] and type 2 diabetic individuals [1, 30, 32]. Furthermore, the increase in plasma adiponectin after weight loss are associated with improvements in hepatic insulin sensitivity in severely obese females [34]. The enhancement of hepatic insulin sensitivity by fAd leads to the down-regulation of the expression of key gluconeogenic genes, including glucose 6-phosphatase and phosphoenolpyruvate carboxykinase [35]. AMPK plays an essential role in the decrease of glucose production since AMPK activation by adiponectin in both isolated hepatocytes and in murine liver in vivo was associated with a reduction of circulating glucose levels [24]. In addition, adiponectin-mediated regulation of hepatic glucose production is abolished in a liver-specific AMPK knockout mouse model [36]. Although the true mechanism underlying the insulin-sensitization of adiponectin in liver has not been yet defined, it is reported that fAd enhances hepatic insulin sensitivity through the up-regulation of insulin receptor substrate-2 (IRS-2) via the macrophage-secreted interleukin-6 [37]. In addition, a redox-based molecular mechanism for the insulin-sensitizing effect of gAd in hepatic cells has been suggested. Indeed, gAd provokes a ligand-independent trans-phosphorylation of insulin receptor that occurs through the production of reactive oxygen species. The oxidants generated following gAd stimulation are important second messengers for glucose oxidation and glycogen production in hepatic cells [38]. Recent data report the involvement of a cross-talk between adiponectin and fibroblast growth factor 21 (FGF21) in the insulin-sensitizing effect of adiponectin. FGF21, a metabolic hormone that regulates glucose and lipid homeostasis and insulin sensitivity [39], enhances adiponectin secretion by adipocytes and increases circulating adiponectin in mice [40, 41]. Interestingly, FGF21 decreases accumulation of ceramides in obese animals in an adiponectin-dependent fashion. Indeed, adiponectin-knockout (KO) mice are refractory to changes in energy expenditure and ceramide-lowering effects due to FGF21 administration [40], thus suggesting that FGF21-adiponectin cross-talk can have a key role in reducing the aberrant accumulation of lipids associated with insulin resistance.
Insulin sensitivity in skeletal muscle is greatly improved by the administration of gAd to lipoatrophic mice [23] and by gAd overexpression in ob/ob mice [26]. The final effect is the improvement of peripheral insulin sensitivity partly due to an increase in fatty acid oxidation. Fatty acid oxidation in skeletal muscle occurs through the inhibitory phosphorylation of acetyl-CoA carboxylase leading to a decrease of malonyl CoA and thus promoting fatty acid entry into the mitochondria [19, 24]. In addition, Zhao et al. [42] have recently reported that gAd increases muscle insulin uptake by recruiting muscle microvasculature thus contributing to its insulin-sensitizing action.
Adiponectin as a tissue-regenerating hormone
Role of adiponectin in skeletal muscle regeneration
Alongside the metabolic and insulin-sensitizing role in skeletal muscle, several observations suggest that gAd is involved in another important muscle process, i.e., muscle regeneration. For the first time, it has been reported that gAd drives myoblasts into myogenic program. Indeed, gAd blocks myoblast cell cycle entry and then induces the expression of specific skeletal muscle markers such as myosin heavy chain and caveolin-3, as well as provoking the fusion of cells into multinucleated syncytia [43].
Satellite cells, a population of stem cells resident beneath the basal lamina, are involved in the regeneration of adult skeletal muscles. When muscle damage occurs, satellite cells are immediately activated; they begin to proliferate and induce the expression of myogenic regulatory factors [44]. Activation of satellite cells requires the phosphorylation of p38 mitogen-activated protein kinase (MAPK) [45]. Conversely, p38 MAPK inhibition prevents MyoD induction, thus blocking satellite cell activation and proliferation [45]. gAd is involved in satellite cell activation, since it induces a strong activation of p38 MAPK, both in intact fibers and in isolated satellite cells [15]. Whether gAd induces satellite cell proliferation is unproven at present and this topic needs to be studied more deeply. Another key step during regeneration of adult skeletal muscle is the migration of satellite cells towards the injured site [46]. gAd elicits a specific motile program in satellite cells through both the activation of the small GTPase Rac1 and the expression of Snail and Twist transcription factors [15]. gAd-induced motile program enhances metalloproteinase-2 secretion, thus permitting the degradation of extracellular matrix and facilitating satellite cell arrival to the site of damage. In addition, gAd also takes part to attract both satellite cells and macrophages towards the myotubes and participates in muscle fiber formation thus inducing myogenesis in satellite cells [15].
Muscle regeneration involves both resident (i.e., satellite cells) and non-resident cells with myogenic properties that are recruited into the muscle following damage. Among non-resident precursors, a variety of different cells with intrinsic myogenic properties have been isolated. These include adipose-tissue derived stem cells [47], mesoangioblasts [48], pericytes [49], muscle-derived stem cells [50], side-population cells [51–53], Ac133 + cells [54], stem and/or precursor cells from muscle endothelium [55], and synovium [56]. Mesoangioblasts, multipotent progenitors of mesodermal tissues, particularly attracted scientific attention for their possible use in stem cell therapy since they ameliorated some myopathies in animal models. For example, intra-arterial delivery of mesoangioblasts corrects morphology and function of muscles in sarcoglycan-null mice (Sgca-null) and dystrophic dogs, where it induces extensive recovery of normal dystrophin expression [48, 57–59]. In addition to the successful role on satellite cells, recent results highlight that gAd positively affects mesoangioblast features. In particular, gAd counteracts some of the main disadvantages met using non-resident stem cells for gene therapy such as their limited survival upon systemic injection and their effective differentiation into myofibers [60–62]. gAd increases survival of mesoangioblasts and protects them by both growth factor- and anchorage-withdrawal by repressing the apoptotic and anoikis pathways. Furthermore, gAd increases myogenic properties of mesoangioblasts both in vitro and in vivo. Indeed, the positive effect of gAd on mesoangioblasts is evident in Sgca-null dystrophic muscles. Ex vivo treatment of mesoangioblasts with gAd and their subsequent injection in dystrophic muscles ameliorates in vivo mesoangioblast survival and greatly improves their engraftment in diseased muscles [63].
It is important to underline that skeletal muscle is an important site of adiponectin production [64–66]. Myotubes produce and secrete functional fAd through a PPARγ-dependent mechanism [67] and the amount of myotube-produced fAd increases in the pro-oxidant/inflammatory microenvironment related to damage [43, 64, 65]. A much-debated point is how and in what conditions gAd is produced, since the modalities of its production are still unclear. Waki et al. [16] reported that fAd can be cleaved by leukocyte elastase secreted from activated monocytes and/or neutrophils. This mechanism could be a possible source of the generation of gAd in the plasma. The inflamed microenvironment generated following damage could be involved in gAd production in skeletal muscle. The injured muscle favors the recruitment of macrophages, which exert a beneficial role in skeletal muscle regeneration. Macrophages participate in the transplantation of myogenic cells [68], in the increase of in vitro myoblast proliferation [69] and in the inhibition of apoptosis in satellite cells [70], thus indicating that the recruitment of macrophages into the site of muscle damage is a key step during regeneration. In this regard, gAd is more able to attract macrophages than fAd [15]. Furthermore, gAd proves to be more efficient than fAd in activating a motile program and in inducing the degradation of the extracellular matrix in satellite cells [15], thus demonstrating the key role of gAd during this process. fAd, secreted both by myotubes and satellite cells, can be cleaved in gAd by activated macrophages [15, 43, 64] thus likely creating an area with a high amount of gAd useful for muscle regeneration. Hence, an inflamed microenvironment could be a crucial step for gAd generation, thus suggesting that the skeletal muscle could be an autocrine source of gAd production. To this day, only one paper managed to demonstrate the presence of gAd in the bloodstream [71]. This is probably due to the very low amount of gAd circulating. So, it is likely that most gAd is locally produced. Hence, gAd production would be restricted to specific sites and to particular physiological conditions that allow the proteolytic cleavage of fAd into gAd. This can happen in skeletal muscle in response to an injury when an inflamed microenvironment is established. Thus, skeletal muscle may represent an autocrine system for gAd production that leads to increased hormone concentration in neighboring muscle fibers.
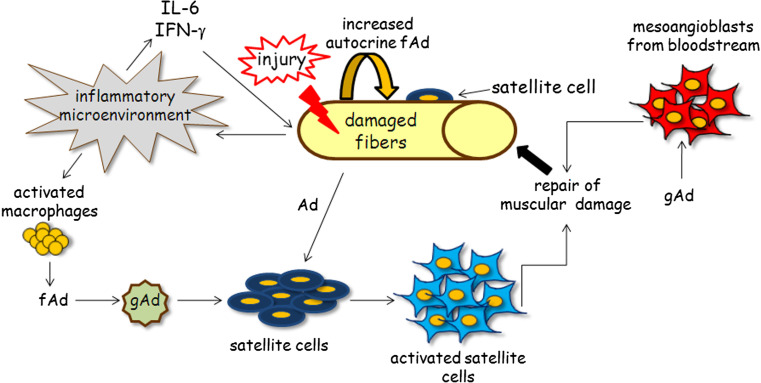
This evidence shows a scenario in which the effect of gAd on skeletal muscle is deeply pleiotropic and variegated. Beyond its involvement in the metabolic routes of skeletal muscle cells, gAd also plays an important function in muscle regeneration. Primarily, gAd acts on different cell muscle populations and muscle precursors. Indeed, gAd regulates proliferation, survival, and myogenic properties of satellite cells and acts as a chemo-attractant factor for non-resident stem cells that are recruited to the site of damage. Furthermore, gAd improves and maximizes muscle regeneration by activating myogenesis both in satellite cells and in recruited non-resident muscle progenitors (Fig. 1). In agreement with these findings, an impaired muscle regeneration is observed in obese and diabetic mice, pathologies correlated to adiponectinemia [72].
Fig. 1.
Role of adiponectin in skeletal muscle regeneration. Beyond its metabolic role, gAd exerts a significant function as a regenerating hormone in skeletal muscle. Skeletal muscle represents an autocrine circuit of fAd production, the amount of which is further increased when an injury occurs. Indeed, damage develops an inflammatory environment that leads to adiponectin production via two different mechanisms: (1) secretion of IL-6 and IFN-γ, which induces the up-regulation of adiponectin expression by skeletal muscle; (2) recruitment of activated macrophages secreting fAd that, in turn, is cleaved into gAd. gAd plays a major role in skeletal muscle regeneration, acting on different cell populations involved in tissue regeneration. gAd acts on satellite cells by inducing their activation and migration towards the damaged muscle site. gAd also acts as a chemo-attractant factor for mesoangioblasts, non-resident muscle progenitor cells, recruited to the injured region. Here, gAd promotes myogenesis of both satellite cells and mesoangioblasts, thus concurring to rebuild the damaged fibers
Role of adiponectin in the regeneration of non-muscle tissues
Regeneration of damaged tissue begins with the activation of stem cells in the stem niche. Many factors in the microenvironment surrounding the stem niche induce stem cell activation [73]. In addition to the effect on satellite cells, as described in the previous paragraph, some evidence reports gAd as a hormone inducing the proliferation of both hemopoietic and adult hippocampal neural stem/progenitor cells [74, 75]. Hemopoietic stem niche produces fAd and hemopoietic stem cells express adiponectin receptors AdipoR1 and AdipoR2. In these cells, gAd increases proliferation both in vitro and in vivo [74]. In the latter, the hormone is more efficient in reconstituting lethally irradiated hosts in long-term transplantation assays [74]. In agreement, Zhang et al. [75] report a novel function of gAd in the regulation of adult hippocampal neural stem/progenitor cells. gAd enhances the proliferation of progenitor cells in a dose- and time-dependent manner, without affecting apoptosis and differentiation towards neuronal or glial lineage. The increased proliferation of adult stem cells by gAd occurs through the activation of AMPK and p38 MAPK signaling pathways. In turn, p38 MAPK leads to the inhibitory phosphorylation on Ser-389 of glycogen synthase kinase 3β. The final effect is the nuclear accumulation of β catenin, a well-reported glycogen synthase kinase 3β substrate [75, 76].
The use of mice genetically modified for adiponectin has allowed to demonstrate the involvement of the hormone in the regeneration of different tissues. For example, Ezaki et al. [77] demonstrate that adiponectin KO mice show a delayed liver regeneration after partial hepatectomy compared to wild-type mice. During liver regeneration in adiponectin KO mice, hepatic cells exhibit delayed DNA replication and increased lipid accumulation, suggesting a possible involvement of altered fat metabolism during liver regeneration [77]. In agreement, Shu et al. [78] show that adiponectin KO mice display decreased liver mass growth, hindered hepatocyte proliferation, and increased hepatic lipid accumulation. The deletion or the overexpression of fAd in mouse model reveals a specific role of adiponectin in promoting functional renal recovery after podocyte ablation [79]. The specific activation of apoptosis in mouse podocytes provokes kidney damage that resembles human kidney disease. The renal damage is completely recovered in healthy mice whereas mice lacking or overexpressing fAd show opposite results. In particular, mice lacking adiponectin develop irreversible albuminuria and renal failure, while those overexpressing fAd show a more rapid recovery and a decreased formation of fibrosis compared to control mice [79]. Furthermore, adiponectin KO mice show a significantly delayed wound closure compared with wild-type mice [80]. In particular, fAd promotes the proliferation and the migration of healthy human keratinocytes through AdipoR1/AdipoR2 and the MAPK signaling pathway. Systemic and topical administration of the hormone in adiponectin-deficient and diabetic db/db mice ameliorates wound repair, thus indicating fAd as a potent mediator of cutaneous wound healing [80].
Experiments performed using mesenchymal progenitor cells established the involvement of fAd in the differentiation towards osteoblasts [81]. fAd stimulates osteoblast differentiation through cyclooxygenase-2-dependent mechanism and mediates osteogenesis through AdipoR1 leading to p38 MAPK activation. Activated p38 MAPK promotes c-Jun phosphorylation, an essential step for osteoblast differentiation via cyclooxygenase-2. Furthermore, fAd activates the osteogenic transcription factor Runx2, leading to osteogenic markers and cyclooxygenase-2 expression thus enhancing osteogenesis [81]. fAd is also involved in the recruitment of progenitor endothelial cells (EPC) [82]. EPC recruitment is significantly reduced in type 2 diabetes mellitus (T2 DM), which is characterized by severe vascular disease mainly caused by the imbalance between endothelial injury and hampered endothelial repair [83]. Several studies report that T2 DM could affect the function of circulating EPC, by impairing migration [84, 85], differentiation towards a mature endothelium, adhesive properties [86], proliferative rate [87, 88], and capacity to be incorporated into vascular structures [89]. Recent findings suggest that T2 DM and subsequent oxidative damage impede the interaction between the vascular wall and normal EPC through a mechanism that could be reversed by heme-oxygenase-1, fAd, and phospho-AMPK [82].
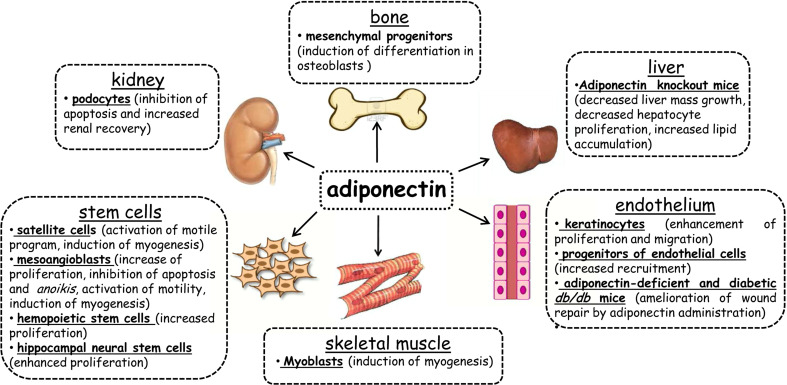
Finally, the relative failure of pro-angiogenic cell therapy through transplantation with bone marrow mononuclear (BM-MN) cells has been correlated with adiponectinemia [90]. fAd seems to play a critical role in stimulating BM-MN cell survival, proliferation, and pro-angiogenic function. Hence, if the recipient of cell therapy is adiponectin deficient, the transplanted BM-MN cells entirely lose their pro-angiogenic efficacy. Whereas, if the recipient has normal adiponectin levels, transplanted BM-MN cells remain fully functional even when the adiponectin receptor is silenced. These findings suggest that hypo-adiponectinemia per se does not significantly impair BM-MN cell functionality. Conversely, adiponectinemia in the recipient animal influences the therapeutic activity of transplanted BM-MN cells [90]. Figure 2 shows the tissues in which adiponectin has a reported role during regeneration.
Fig. 2.
Adiponectin participates in tissue regeneration. The figure shows the cell lines and/or the animal models used to demonstrate the involvement of adiponectin in tissue regeneration. The obtained results for each tissue are reported (detailed text in paragraph “Role of adiponectin in the regeneration of non-muscle tissues”). Both fAd and gAd play a role in tissue regeneration. gAd acts on satellite cells in skeletal muscle by inducing cell motility and myogenesis [15]; on mesoangioblasts by activating proliferation, cell motility, myogenesis, and inhibiting both apoptosis and anoikis [60]; on hemopoietic stem cells [71] and hippocampal neural stem cells by inducing cell proliferation [72]. In skeletal muscle, gAd promotes the differentiation of myoblasts into myotubes [40]. In the kidney, fAd acts by inhibiting apoptosis in podocytes and by supporting renal recovery [76]. Depletion of fAd in murine liver leads to a decrease in mass growth and hepatocyte proliferation [74] and an increase in lipid accumulation [75]. In the bone, fAd promotes the differentiation of mesenchymal progenitors into osteoblasts [77]. In the endothelium, fAd promotes the enhancement of proliferation and the migration of keratinocytes [88], ameliorates wound repair in adiponectin-deficient and diabetic db/db mice [88] and increases the recruitment of endothelial cell precursors [78]
Conclusions
Overall, these data shed a new light on adiponectin. The physiological functions of adiponectin appear more pleiotropic and varied and, above all, not restricted to its metabolic role. Interestingly, both fAd and gAd appear to be involved in tissue regeneration, although in different tissues. These observations give rise to some questions about, for example, the physiological relevance of the oligomeric forms of adiponectin in tissue regeneration. In addition, it would be interesting to know if the anti-diabetic effect of adiponectin may also be involved in tissue preservation. Some data suggest that adiponectin regulates pancreatic cell growth [91]. In regards to this, we could wonder if adiponectin is involved in the preservation of integrity and functionality of the pancreas. Indeed, these data highlight an important role of adiponectin on tissue regeneration, thus providing a possible tool for new healing therapies.
Abbreviations
- AMPK
AMP kinase
- fAd
Full-length adiponectin
- gAd
Globular adiponectin
- HMW
High molecular weight
- LMW
Low molecular weight
- MAPK
Mitogen-activated protein kinase
- MMW
Middle molecular weight
References
- 1.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.ATV.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 2.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 3.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 4.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.ATV.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 5.Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, Kishida K, Maeda N, Nagasawa A, Kobayashi H, Hiraoka H, Komai N, Kaibe M, Rakugi H, Ogihara T, Matsuzawa Y. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 6.Trujillo ME, Scherer PE. Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 7.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 8.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–849. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, Terauchi Y, Kadowaki T, Takeuchi Y, Fukumoto S, Ikeda T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006;99:196–208. doi: 10.1002/jcb.20890. [DOI] [PubMed] [Google Scholar]
- 10.Caminos JE, Nogueiras R, Gallego R, Bravo S, Tovar S, Garcia-Caballero T, Casanueva FF, Dieguez C. Expression and regulation of adiponectin and receptor in human and rat placenta. J Clin Endocrinol Metab. 2005;90:4276–4286. doi: 10.1210/jc.2004-0930. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Tan B, Karteris E, Zervou S, Digby J, Hillhouse EW, Vatish M, Randeva HS. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 2006;49:1292–1302. doi: 10.1007/s00125-006-0194-7. [DOI] [PubMed] [Google Scholar]
- 12.Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Dieguez C, Gualillo O, Gonzalez-Juanatey JR, Lago F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163–5169. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 13.Natarajan R, Salloum FN, Fisher BJ, Kukreja RC, Fowler AA., III Hypoxia inducible factor-1 upregulates adiponectin in diabetic mouse hearts and attenuates post-ischemic injury. J Cardiovasc Pharmacol. 2008;51:178–187. doi: 10.1097/FJC.0b013e31815f248d. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, Castano JP, Malagon MM. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007;148:401–410. doi: 10.1210/en.2006-1019. [DOI] [PubMed] [Google Scholar]
- 15.Fiaschi T, Giannoni E, Taddei ML, Chiarugi P. Globular adiponectin activates motility and regenerative traits of muscle satellite cells. PLoS One. 2012;7:e34782. doi: 10.1371/journal.pone.0034782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, Uchida S, Tsuchida A, Takekawa S, Kadowaki T. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–796. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- 17.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 18.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 20.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Addabbo F, Nacci C, De BL, Leo V, Tarquinio M, Quon MJ, Montagnani M. Globular adiponectin counteracts VCAM-1-mediated monocyte adhesion via AdipoR1/NF-kappaB/COX-2 signaling in human aortic endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:E1143–E1154. doi: 10.1152/ajpendo.00208.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286:13460–13469. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 25.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 27.Tishinsky JM, Robinson LE, Dyck DJ. Insulin-sensitizing properties of adiponectin. Biochimie. 2012;94:2131–2136. doi: 10.1016/j.biochi.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi T, Hara K, Kubota N, Terauchi Y, Tobe K, Froguel P, Nagai R, Kadowaki T. Dual roles of adiponectin/Acrp30 in vivo as an anti-diabetic and anti-atherogenic adipokine. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:243–254. doi: 10.2174/1568008033340090. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj M, Suraamornkul S, Piper P, Hardies LJ, Glass L, Cersosimo E, Pratipanawatr T, Miyazaki Y, DeFronzo RA. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:200–206. doi: 10.1210/jc.2003-031315. [DOI] [PubMed] [Google Scholar]
- 31.Magkos F, Fabbrini E, Patterson BW, Eagon JC, Klein S. Portal vein and systemic adiponectin concentrations are closely linked with hepatic glucose and lipoprotein kinetics in extremely obese subjects. Metabolism. 2011;60:1641–1648. doi: 10.1016/j.metabol.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefan N, Stumvoll M, Vozarova B, Weyer C, Funahashi T, Matsuzawa Y, Bogardus C, Tataranni PA. Plasma adiponectin and endogenous glucose production in humans. Diabetes Care. 2003;26:3315–3319. doi: 10.2337/diacare.26.12.3315. [DOI] [PubMed] [Google Scholar]
- 33.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 34.Lin E, Phillips LS, Ziegler TR, Schmotzer B, Wu K, Gu LH, Khaitan L, Lynch SA, Torres WE, Smith CD, Gletsu-Miller N. Increases in adiponectin predict improved liver, but not peripheral, insulin sensitivity in severely obese women during weight loss. Diabetes. 2007;56:735–742. doi: 10.2337/db06-1161. [DOI] [PubMed] [Google Scholar]
- 35.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B. Liver adenosine monophosphate-activated kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology. 2006;147:2432–2441. doi: 10.1210/en.2005-0898. [DOI] [PubMed] [Google Scholar]
- 37.Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, Kobayashi M, Iwane A, Sasako T, Okazaki Y, Ohsugi M, Takamoto I, Yamashita S, Asahara H, Akira S, Kasuga M, Kadowaki T. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 2011;13:401–412. doi: 10.1016/j.cmet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Fiaschi T, Buricchi F, Cozzi G, Matthias S, Parri M, Raugei G, Ramponi G, Chiarugi P. Redox-dependent and ligand-independent trans-activation of insulin receptor by globular adiponectin. Hepatology. 2007;46:130–139. doi: 10.1002/hep.21643. [DOI] [PubMed] [Google Scholar]
- 39.Woo YC, Xu A, Wang Y, Lam KS. Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clin Endocrinol (Oxf) 2013;78:489–496. doi: 10.1111/cen.12095. [DOI] [PubMed] [Google Scholar]
- 40.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A, Scherer PE. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, Li X. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Zhao L, Chai W, Fu Z, Dong Z, Aylor KW, Barrett EJ, Cao W, Liu Z. Globular adiponectin enhances muscle insulin action via microvascular recruitment and increased insulin delivery. Circ Res. 2013;112:1263–1271. doi: 10.1161/CIRCRESAHA.111.300388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiaschi T, Cirelli D, Comito G, Gelmini S, Ramponi G, Serio M, Chiarugi P. Globular adiponectin induces differentiation and fusion of skeletal muscle cells. Cell Res. 2009;19:584–597. doi: 10.1038/cr.2009.39. [DOI] [PubMed] [Google Scholar]
- 44.Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Jones NC, Tyner KJ, Nibarger L, Stanley HM, Cornelison DD, Fedorov YV, Olwin BB. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J Cell Biol. 2005;169:105–116. doi: 10.1083/jcb.200408066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carmeli E, Moas M, Reznick AZ, Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve. 2004;29:191–197. doi: 10.1002/mus.10529. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B, Villageois A, Bagnis C, Breittmayer JP, Groux H, Ailhaud G, Dani C. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minasi MG, Riminucci M, De AL, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De MR, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 49.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 50.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bachrach E, Li S, Perez AL, Schienda J, Liadaki K, Volinski J, Flint A, Chamberlain J, Kunkel LM. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci USA. 2004;101:3581–3586. doi: 10.1073/pnas.0400373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/S0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 54.Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D’Antona G, Tonlorenzi R, Porretti L, Gavina M, Mamchaoui K, Pellegrino MA, Furling D, Mouly V, Butler-Browne GS, Bottinelli R, Cossu G, Bresolin N. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J. Clin. Invest. 2004;114:182–195. doi: 10.1172/JCI20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De BC, Dell’Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160:909–918. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, Constantin G, Torrente Y, Cossu G. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol. 2006;174:231–243. doi: 10.1083/jcb.200512085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, Bottinelli R, Cossu G. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 59.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 60.Cossu G, Bianco P. Mesoangioblasts–vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Oreffo RO, Cooper C, Mason C, Clements M. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1:169–178. doi: 10.1385/SCR:1:2:169. [DOI] [PubMed] [Google Scholar]
- 62.Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 63.Fiaschi T, Tedesco FS, Giannoni E, Diaz-Manera J, Parri M, Cossu G, Chiarugi P. Globular adiponectin as a complete mesoangioblast regulator: role in proliferation, survival, motility, and skeletal muscle differentiation. Mol Biol Cell. 2010;21:848–859. doi: 10.1091/mbc.E09-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology. 2004;145:5589–5597. doi: 10.1210/en.2004-0503. [DOI] [PubMed] [Google Scholar]
- 65.Delaigle AM, Senou M, Guiot Y, Many MC, Brichard SM. Induction of adiponectin in skeletal muscle of type 2 diabetic mice: in vivo and in vitro studies. Diabetologia. 2006;49:1311–1323. doi: 10.1007/s00125-006-0210-y. [DOI] [PubMed] [Google Scholar]
- 66.Krause MP, Liu Y, Vu V, Chan L, Xu A, Riddell MC, Sweeney G, Hawke TJ. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. Am J Physiol Cell Physiol. 2008;295:C203–C212. doi: 10.1152/ajpcell.00030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amin RH, Mathews ST, Camp HS, Ding L, Leff T. Selective activation of PPARgamma in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2010;298:E28–E37. doi: 10.1152/ajpendo.00446.2009. [DOI] [PubMed] [Google Scholar]
- 68.Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord. 1999;9:72–80. doi: 10.1016/S0960-8966(98)00111-4. [DOI] [PubMed] [Google Scholar]
- 69.Cantini M, Giurisato E, Radu C, Tiozzo S, Pampinella F, Senigaglia D, Zaniolo G, Mazzoleni F, Vitiello L. Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurol Sci. 2002;23:189–194. doi: 10.1007/s100720200060. [DOI] [PubMed] [Google Scholar]
- 70.Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163:1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.98.4.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nguyen MH, Cheng M, Koh TJ. Impaired muscle regeneration in Ob/ob and Db/db mice. Sci World J. 2011;11:1525–1535. doi: 10.1100/tsw.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, Sankar U, Reya T. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol. 2007;178:3511–3520. doi: 10.4049/jimmunol.178.6.3511. [DOI] [PubMed] [Google Scholar]
- 75.Zhang D, Guo M, Zhang W, Lu XY. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3beta (GSK-3beta)/beta-catenin signaling cascade. J Biol Chem. 2011;286:44913–44920. doi: 10.1074/jbc.M111.310052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ezaki H, Yoshida Y, Saji Y, Takemura T, Fukushima J, Matsumoto H, Kamada Y, Wada A, Igura T, Kihara S, Funahashi T, Shimomura I, Tamura S, Kiso S, Hayashi N. Delayed liver regeneration after partial hepatectomy in adiponectin knockout mice. Biochem Biophys Res Commun. 2009;378:68–72. doi: 10.1016/j.bbrc.2008.10.176. [DOI] [PubMed] [Google Scholar]
- 78.Shu RZ, Zhang F, Wang F, Feng DC, Li XH, Ren WH, Wu XL, Yang X, Liao XD, Huang L, Wang ZG. Adiponectin deficiency impairs liver regeneration through attenuating STAT3 phosphorylation in mice. Lab Invest. 2009;89:1043–1052. doi: 10.1038/labinvest.2009.63. [DOI] [PubMed] [Google Scholar]
- 79.Rutkowski JM, Wang ZV, Park AS, Zhang J, Zhang D, Hu MC, Moe OW, Susztak K, Scherer PE. Adiponectin promotes functional recovery after podocyte ablation. J Am Soc Nephrol. 2013;24:268–282. doi: 10.1681/ASN.2012040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shibata S, Tada Y, Asano Y, Hau CS, Kato T, Saeki H, Yamauchi T, Kubota N, Kadowaki T, Sato S. Adiponectin regulates cutaneous wound healing by promoting keratinocyte proliferation and migration via the ERK signaling pathway. J Immunol. 2012;189:3231–3241. doi: 10.4049/jimmunol.1101739. [DOI] [PubMed] [Google Scholar]
- 81.Lee HW, Kim SY, Kim AY, Lee EJ, Choi JY, Kim JB. Adiponectin stimulates osteoblast differentiation through induction of COX2 in mesenchymal progenitor cells. Stem Cells. 2009;27:2254–2262. doi: 10.1002/stem.144. [DOI] [PubMed] [Google Scholar]
- 82.Sambuceti G, Morbelli S, Vanella L, Kusmic C, Marini C, Massollo M, Augeri C, Corselli M, Ghersi C, Chiavarina B, Rodella LF, L’Abbate A, Drummond G, Abraham NG, Frassoni F. Diabetes impairs the vascular recruitment of normal stem cells by oxidant damage, reversed by increases in pAMPK, heme oxygenase-1, and adiponectin. Stem Cells. 2009;27:399–407. doi: 10.1634/stemcells.2008-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bahia L, Aguiar LG, Villela N, Bottino D, Godoy-Matos AF, Geloneze B, Tambascia M, Bouskela E. Relationship between adipokines, inflammation, and vascular reactivity in lean controls and obese subjects with metabolic syndrome. Clinics (Sao Paulo) 2006;61:433–440. doi: 10.1590/S1807-59322006000500010. [DOI] [PubMed] [Google Scholar]
- 84.Esper RJ, Vilarino JO, Machado RA, Paragano A. Endothelial dysfunction in normal and abnormal glucose metabolism. Adv Cardiol. 2008;45:17–43. doi: 10.1159/000115120. [DOI] [PubMed] [Google Scholar]
- 85.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 86.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 87.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 88.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.CIR.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 89.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 90.Eren P, Camus S, Matrone G, Ebrahimian TG, Francois D, Tedgui A, Sebastien SJ, Blanc-Brude OP. Adiponectinemia controls pro-angiogenic cell therapy. Stem Cells. 2009;27:2712–2721. doi: 10.1002/stem.219. [DOI] [PubMed] [Google Scholar]
- 91.Rao JR, Keating DJ, Chen C, Parkington HC. Adiponectin increases insulin content and cell proliferation in MIN6 cells via PPARgamma-dependent and PPARgamma-independent mechanisms. Diabetes Obes Metab. 2012;14:983–989. doi: 10.1111/j.1463-1326.2012.01626.x. [DOI] [PubMed] [Google Scholar]