Abstract
Borna disease virus (BDV) persistently infects neurons of the central nervous system of various hosts, including rats. Since type I IFN-mediated antiviral response efficiently blocks BDV replication in primary rat embryo fibroblasts, it has been speculated that BDV is not effectively sensed by the host innate immune system in the nervous system. To test this assumption, organotypical rat hippocampal slice cultures were infected with BDV for up to 4 weeks. This resulted in the secretion of IFN and the up-regulation of IFN-stimulated genes. Using the rat Mx protein as a specific marker for IFN-induced gene expression, astrocytes and microglial cells were found to be Mx positive, whereas neurons, the major cell type in which BDV is replicating, lacked detectable levels of Mx protein. In uninfected cultures, neurons also remained Mx negative even after treatment with high concentrations of IFN-α. This non-responsiveness correlated with a lack of detectable nuclear translocation of both pSTAT1 and pSTAT2 in these cells. Consistently, neuronal dissemination of BDV was not prevented by treatment with IFN-α. These data suggest that the poor innate immune response in rat neurons renders this cell type highly susceptible to BDV infection even in the presence of exogenous IFN-α. Intriguingly, in contrast to rat neurons, IFN-α treatment of mouse neurons resulted in the up-regulation of Mx proteins and block of BDV replication, indicating species-specific differences in the type I IFN response of neurons between mice and rats.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-013-1402-5) contains supplementary material, which is available to authorized users.
Keywords: Neurotropic viruses, Viral persistence, Innate immunity, Hippocampus
Introduction
Infection with Borna disease virus (BDV), a non-segmented negative-strand RNA virus, causes viral persistence in the central nervous system (CNS) of a broad range of mammalian species [1]. Although there is serological evidence for human BDV infections, several studies indicate the absence of a productive infection with detectable amounts of viral transcripts [2]. Remarkably, reverse-transcribed BDV transcripts entered the germ line of virtually all vertebrates approximately 40 million years ago, including human ancestors, indicating that infection had occurred in the past [3]. Experimental infection of warm-blooded animals, especially of the highly susceptible rats, is widely used to study BDV-induced pathogenesis and behavioral changes. In adult rats, BDV infection induces an immune-mediated disease with a high mortality rate, whereas infection of newborn rats provokes a persistent infection of the limbic system with a preference for the hippocampus [4]. Although BDV is non-cytolytic in primary neuronal cultures, infection of Lewis newborns results in the selective neuronal degeneration of the dentate gyrus (DG) in the hippocampus [4]. The BDV-induced neuronal cell death can also be observed in infected organotypic hippocampal slice cultures from newborn Lewis rats [4]. The susceptibility to BDV-mediated neuronal degeneration in the hippocampus is dependent on the host genetic background. In contrast to Lewis rats, selective neuronal loss is absent in BDV-infected hippocampal slice cultures of Sprague–Dawley (SD) rats [5], offering the possibility to study viral persistence in the absence of cell death also in complex brain tissues.
Protection from invading viruses by the innate immunity critically depends on the rapid production of type I (IFN-α/β) and type III Interferon (IFN-λ). A diverse family of intracellular pattern recognition receptors (PPRs), including Toll-like receptor (TLR) 3, retinoic acid-inducible gene-I (RIG-I) and melanoma differentiation-associated protein 5 (MDA5) sense the presence of viral RNA [6]. TLR3 recognizes double-stranded RNA in endosomes, whereas protein kinase R (PKR), RIG-I, and MDA5 have affinity to cytosolic viral RNA structures [6]. RIG-I also senses 5′ tri-phosphate viral genome ends [6]. The interaction of these cellular factors with viral RNA structures induces a signal cascade that results in the nuclear translocation of phosphorylated interferon regulatory factor (IRF) 3/IRF7 and the expression of cytokines, including IFN-α/β [6]. Upon receptor binding, IFN-α/β triggers a signaling pathway via STAT1/STAT2 phosphorylation and subsequent nuclear translocation of these transcription factors. This results in the induction of several hundreds of IFN-stimulated genes (ISGs) and the corresponding gene products, including the antivirally active Mx proteins [6]. Expression of Mx is strictly mediated by type I and III IFN and thus represents a reliable and widely used marker to identify cells in an IFN-induced antiviral state [7].
Amongst several known viral counter measurements to avoid the induction of IFN by intracellular pattern recognition receptors [6], BDV developed a unique replication strategy to prevent the formation of viral 5′ tri-phosphates at the viral genome ends [8]. This is achieved by cleavage of four nucleotides from the 5′ end to generate a 5′ mono-phosphorylated genome end [9], which is not recognized by RIG-I [8]. The tight association of the viral genome with the host chromatin might further prevent recognition by cellular innate immune sensors [10]. Besides escaping from the recognition by innate immune sensors, the viral phosphoprotein (BDV-P) was shown to interfere with the expression of type I IFN by blocking IKKε/TBK1-dependent phosphorylation of IRF3 in a reporter assay system [11]. However, there is evidence that treatment with type I IFN prevents BDV infection of various cell cultures, including rat embryo fibroblasts [12]. Consistently, BDV replication is also diminished in mice constitutively expressing IFN-α1 in the CNS [13].
However, little is known about the innate immune response against BDV in rat neuronal tissue and whether type I IFN abrogates BDV replication in these cells as observed in primary rat fibroblasts [12]. Here we show that infection of rat organotypic hippocampal slice cultures results in the up-regulation of IFN-induced genes. As judged by Mx staining, ISG induction is detected almost exclusively in astrocytes and microglial cells but not in neurons. We provide evidence that continuous treatment with type-I IFN fails to prevent the establishment of a persistent BDV infection in rat neurons. Moreover, this failure to build up a robust innate immune response in rat neurons appears to be species-specific feature and is not observed in neuronal tissue of mice.
Materials and methods
Hippocampal slice cultures and primary dissociated neuronal cultures
Hippocampal slice cultures and primary neuronal cultures were dissected from SD and LEW rats, BALB/c or Mx1-positive C57BL/6 [14] mouse neonates (P0–P2) as described [4]. Briefly, to obtain the primary neurons, hippocampi were first collected in cold modified Eagle’s medium (MEM) (Gibco, CA) supplemented with Glutamax (Invitrogen, CA), cut with scissors into smaller pieces, trypsinized (0.25 % trypsin) for 10 min at 37 °C and terminated by adding fetal calf serum (PAA, NJ). The samples were transferred to dissociation buffer (HBSS supplemented with 10 mM HEPES, BSA 3 mg/ml, 12 mM MgSO4, DNase I and trypsin inhibitor from soybean (Applichem, Germany) and triturated for 10–15 times using sterile glass Pasteur pipettes. The dissociated cell suspension was collected from the supernatant after centrifugation for 5 min at 100 g. Neurons were enriched by two centrifugation steps at 800 g for 5 min and finally seeded on the Poly-d-Lysine-coated cover slips in 48-well plate with Dulbecco’s modified Eagle’s medium (DMEM)(Gibco, CA) containing 10 % fetal calf serum at 37 °C with 8 % CO2. The medium was changed to Neurobasal-A (Gibco, CA) supplied with B27-supplement (Gibco, CA) and Glutamax after 1 day for optimal neuron growth. The organotypic hippocampal slice culture and dissociated neuronal cultures were infected with BDV with 1,000 focus forming units (FFU).
Virus stocks preparation and IFN treatment
The virus stocks He/80 [4] and the recombinant mouse adapted strain BDVLRD [15], designated rBDVLRD, and VSV encoding the green fluorescence protein (GFP) (VSV-GFP) [16] were obtained as described. Recombinant human IFN-α B/D hybrid [17] was used. Since IFN-α B/D hybrid exhibits species-specific differences in antiviral activity, the IFN concentration applied on rat and mouse cells and hippocampal slice cultures was adjusted to concentrations that result in comparable antiviral effects observed in embryonic fibroblast cultures of each species [17]. We used throughout the experiments 105 U/ml for rat and 103 U/ml IFN-α for mouse cells. If indicated, 103 U/ml rat IFN-α/β was used [12].
Antibodies
BDV-N-specific and the BDV-P-specific polyclonal rabbit antisera [18, 19], mouse anti-β-tubulin antibody (Sigma, MO), mouse anti-Mx1 antibody (kindly provided by G. Kochs), rabbit anti-Iba-1 polyclonal antibody (Wako, VA), rabbit anti-GFAP monoclonal antibodies (Dako, Denmark), rabbit-anti-phospho-STAT1 (Tyr701) antibody (Cell Signaling, MA), rabbit-anti-phospho-STAT2 (Tyr690) antibody (Abcam, United Kingdom), rabbit-anti-IFN-α receptor 1 antibody (LifeSpan Biosciences, WA), mouse anti-FluA-NP monoclonal antibody (AbD Serotec, United Kingdom), chicken anti-Map-2 polyclonal antibody (Abcam, United Kingdom), mouse anti-NeuN monoclonal (cloneA60) antibody (Millipore, MA), goat anti-mouse or rabbit antibodies conjugated with horseradish peroxidase (Jackson ImmunoResearch, PA), goat anti-mouse antibody conjugated with Cy3 (Jackson ImmunoResearch, PA) or Alexa 647 (Invitrogen, CA), goat anti-rabbit antibody conjugated with Alexa 488 (Invitrogen, CA), goat anti-rabbit antibody conjugated with biotin (Jackson ImmunoResearch, PA), goat-anti-chicken antibody conjugated with Cy3 (Abcam, United Kingdom) and mouse anti-NeuN monoclonal antibody conjugated with Alexa 488 (Millipore, MA).
Interferon titration
Determination of IFN levels in cell culture medium were carried out as described previously [20]. In brief, rat C6 cells [21] were first seeded in 96-well plate and then incubated with known concentrations of rat IFN (Lee Biomolecular Research Inc., CA) or culture supernatant of the hippocampal slice culture for 24 h. Cells were then infected with VSV-GFP in DMEM containing 0.3 % bovine serum albumin (BSA) for 15 h. The GFP signal intensities were determined by a fluorescence reader (TECAN, Switzerland). A linear standard curve was obtained from GFP signal observed with increasing concentration of IFN, which was used to determine IFN concentration in the slice culture supernatant.
Western blotting
Slice cultures were lysed in Laemmli buffer [22], homogenized by ultrasonication and analyzed by Western blot using 13 % of SDS–polyacrylamide electrophoresis gel. Protein extracts were then transferred on the polyvinylidene difluoride membrane (Millipore, MA) and blocked with 5 % skimmed milk in PBS containing 0.1 % Tween-20 (Sigma, MO). Membrane was hybridized with first antibody 4 °C overnight followed by the secondary antibodies for 1 h at room temperature. ECL Plus Western Blot Detection Reagents (Pierce, IL) were added and proteins were analyzed via Odyssey Infrared Imaging System (LI-COR, NE).
Immunofluorescence analysis
Hippocampal slice cultures were fixed with 4 % PFA for 2 h and re-sliced into 50 μm sections as described [4]. The free-floating sections were first permeabilized with 0.1 M phosphate buffer (PB) containing 0.1 % Triton X-100 (Sigma, MO) and 5 % normal goat serum (NGS) (Vector Labs, CA) for 1 h. Staining with primary antibodies was performed overnight at 4 °C in PB containing 1 % NGS. The sections were washed three times for 5 min in PB and incubated for 2 h at room temperature with secondary antibodies in PB with 1 % BSA (Sigma, MO). For double immunofluorescence analysis with mouse anti-Mx1 and mouse anti-NeuN antibodies, staining for Mx1 was completed first. Subsequent to blocking with 7 % normal mouse serum (Jackson ImmunoResearch, PA), slices were incubated with mouse anti-NeuN antibody conjugated with Alexa 488. TO-PRO-3 (Molecular Probes, OR), further designated ToPro3, was used for nuclear staining. Finally, the slices were mounted for confocal microscope TCS SP2 (Leica, Germany) analysis. Primary neurons were fixed with 4 % PFA for 15 min, washed with phosphate buffer saline (PBS) and permeabilized with 0.1 % Triton X-100 for 10 min and subsequently blocked by PBS containing 5 % NGS and 1 % BSA. Cells with primary antibodies were incubated at 4 °C overnight and washed three times for 5 min. Incubation with secondary antibodies were performed at room temperature for 1 h. Ultraviolet (UV) channel was used with biotin-conjugated secondary antibody labeled with Streptavidin-AMCA (Jackson ImmunoResearch, PA). Cell nuclei were stained with DAPI (Molecular Probes, OR) before mounting the slides. Samples were analyzed using Zeiss, Germany ApoTome. For staining of the IFN-α receptor (IFNAR), neurons were fixed with 4 % PFA for 15 min, subsequently washed with phosphate buffer saline (PBS), incubated with anti-IFNAR1 antibody and stained with Cy3-labeled secondary antibody. Cells were subsequently permeabilized by 0.1 % Triton X-100 for 10 min and incubated for Map-2 followed by the secondary antibody conjugated with Alexa® 488. The fluorescence signals were analyzed by confocal microscope TCS SP2.
Phospho-STAT quantification
Immunofluorescence images from neurons and astrocytes stained against pSTAT1 or pSTAT2 were obtained by Zeiss, Germany ApoTome and processed under the same software (AxioVision, Zeiss, Germany) settings. The fluorescence intensities of pSTAT1/pSTAT2 along the dashed line across cell nuclei, identified by DAPI staining, were determined by ImageJ and exported as histograms. pSTAT1/pSTAT2 mean fluorescence intensities were calculated with ImageJ and represent the fluorescence signal of the whole nuclei divided by the area of the nucleus.
Microarray analysis
Hippocampal slice cultures were prepared from newborn Lewis and SD rats (P0-P2). Half of the samples remained uninfected, whereas the other half was infected with 1,000 FFU of BDV strain He/80 for 23 days. Total RNA from a pool of 12 slices was prepared and subjected to Agilent array analysis covering the whole rat genome. To calculate the significance of the regulated genes, a twofold change in expression in different groups was taken into account and a p value cutoff 0.05 was considered. Multiple testing corrections were performed based on Benjamin-Hochberg method. The results have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series (accession number GSE41540) (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE41540).
Results
BDV infection of neuronal tissues results in the induction of IFN stimulated gene products
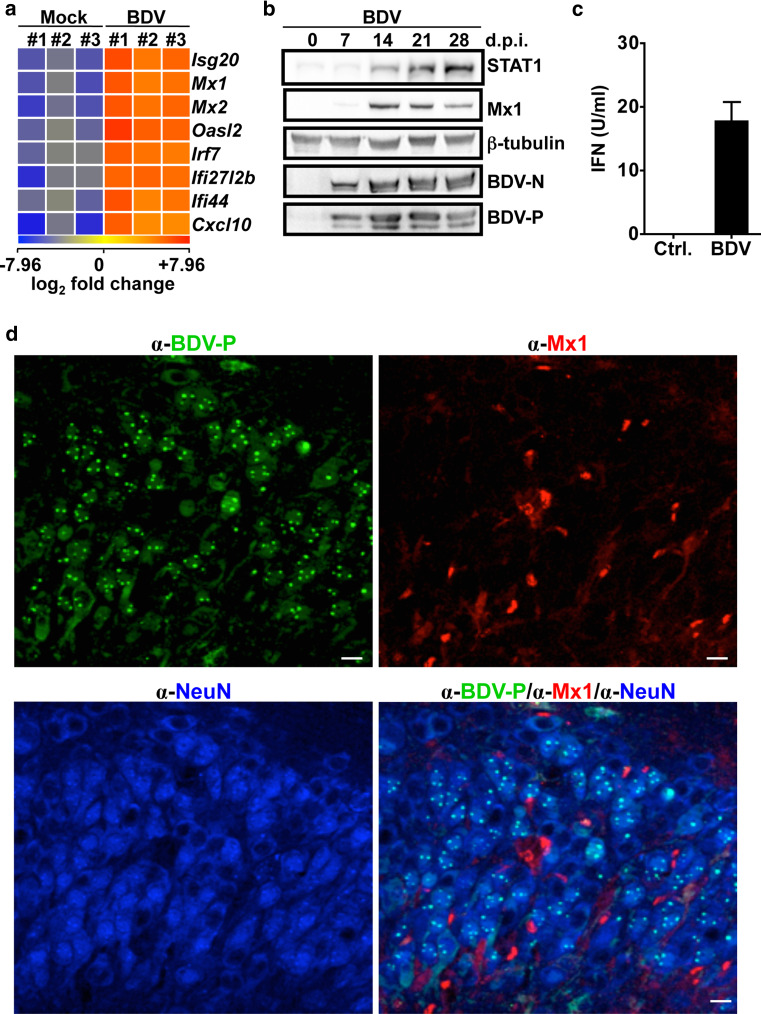
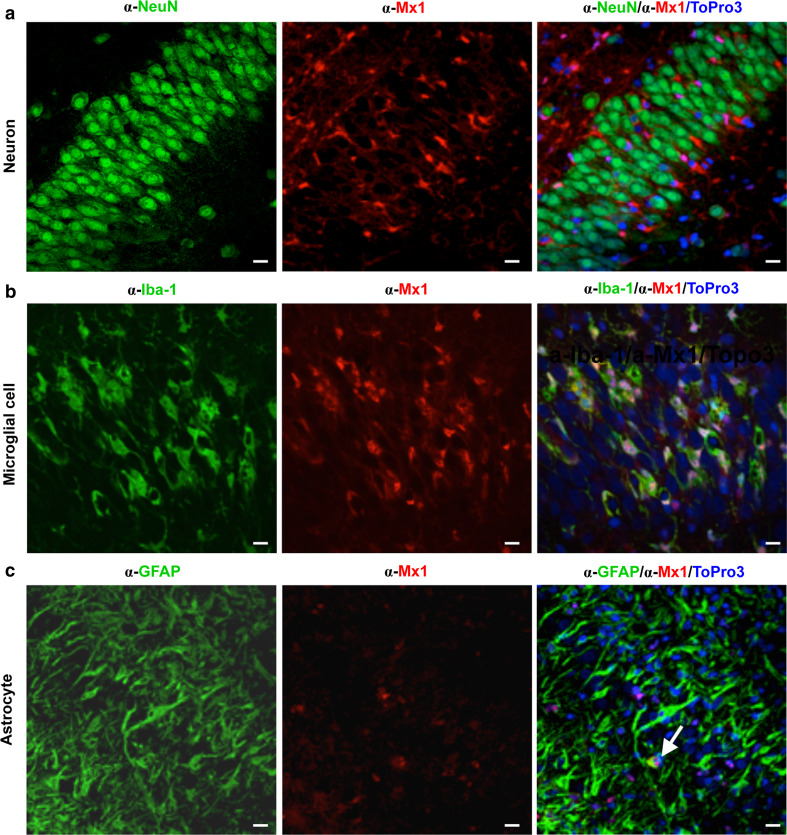
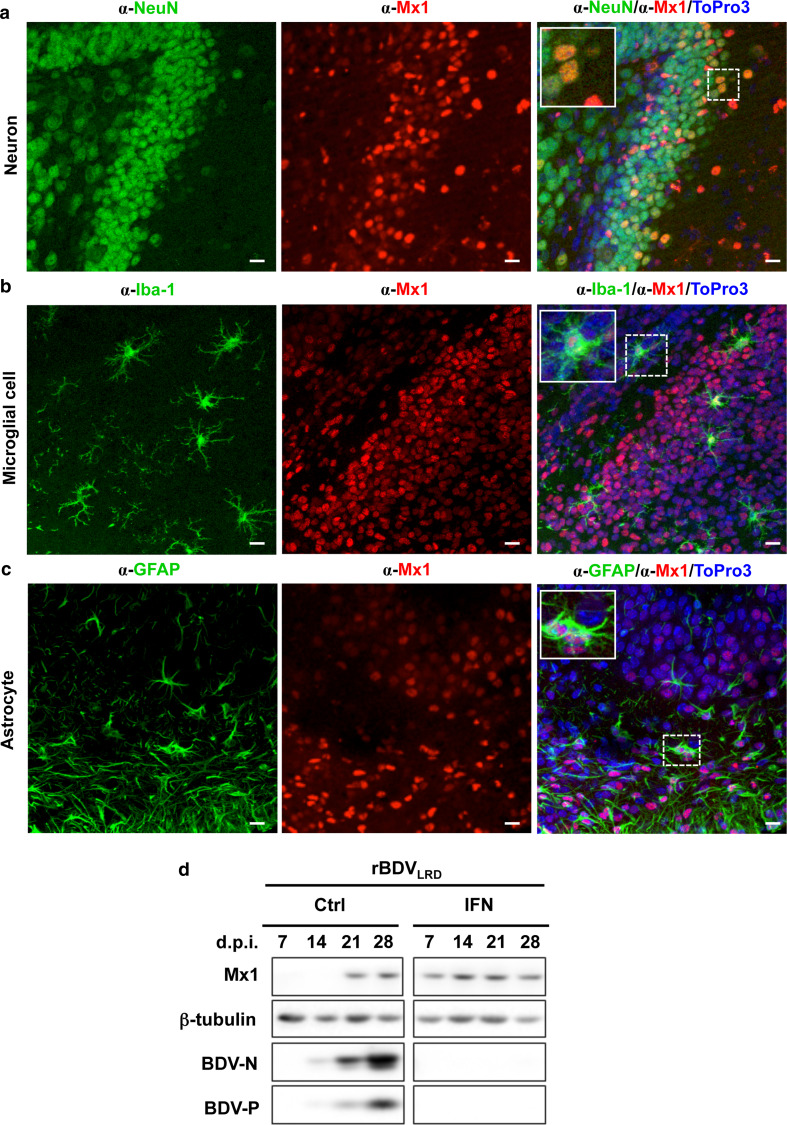
To determine whether BDV is sensed by the innate immune system in complex neuronal tissues, we performed microarray analyses using polyA+ mRNA from mock-infected or BDV-infected organotypic hippocampal slice cultures of Sprague–Dawley (SD) neonates 23 days post-infection (p.i.). This revealed changes in mRNA transcript levels of more than 500 genes (Table S1), including known IFN-induced genes coding for Mx1 or Mx2 (Fig. 1a) or STAT1 (Table S1). Similar up-regulation of IFN-induced genes was also observed in BDV-infected hippocampal slice cultures of Lewis rats (Table S2). Western blot analysis confirmed the presence of enhanced levels of either Mx1 or STAT1 protein upon BDV infection (Fig. 1b). Consistently, IFN was detected in the supernatant of BDV-infected cultures, albeit at low concentrations (ca. 15 U/ml, Fig. 1c). Immunofluorescence analysis of re-sliced 50 μm sections of the hippocampal cultures revealed that only a subset of Mx1-positive cells were found in infected cultures (Fig. 1d), while no Mx1-positive cells were observed in uninfected cultures (data not shown). Intriguingly, co-staining with neuron- and BDV-specific antibodies revealed that Mx1 was only present in non-neuronal cells, whereas the viral antigen (BDV-P) was found almost exclusively in neurons (Fig. 1d), suggesting that interferon response occurs mainly in non-neuronal cells. To identify the Mx1-expressing cell types in the absence of infection, we treated uninfected hippocampal slice cultures with 105 U/ml of human IFN-α B/D hybrid [17], further designated IFN-α, for 3 weeks and stained sections of these cultures with antibodies specific for Mx1 and either neuronal cells (α-Neu-N), microglial cells (α-Iba-1) or astrocytes (α-GFAP). Similar to BDV-infected cultures (Fig. 1d), Mx1 protein could not be detected in neurons after IFN treatment (Fig. 2a). However, the vast majority of Mx1-positive cells were found to be microglial cells (Fig. 2b), whereas only few astrocytes did stain for Mx1 (Fig. 2c). Similarly, in BDV-infected hippocampus cultures of SD rats, Mx1 expression was not detectable in neurons, whereas microglial cells were found to be Mx1-positive (Fig. S1a). The same cell type-specific Mx1 expression pattern was observed in hippocampus cultures treated with 103 U/ml of rat IFN-α/β [12] for 3 weeks (Fig. S1b). Together, these results suggest that BDV infection induces a detectable up-regulation of IFN-induced gene transcripts in non-neuronal cells, namely microglial cells and to a minor extent in astrocytes.
Fig. 1.
BDV infection results in the induction of IFN-induced genes in neuronal tissues. a Transcriptome analysis of mock- or BDV-infected rat hippocampal slices cultures 21 days post-infection (d.p.i.). The significant changes (unpaired Student’s t test, p < 0.05) in RNA expression from three independent pools (#1–#3) of slice cultures are shown. Expression signals were log2-transformed and color coded such that lower signals are displayed as blue, and higher as red. b Determination of viral (BDV-N, BDV-P) or cellular (Mx1, Stat1, ß-tubulin) protein levels in pools of infected SD-derived hippocampal slice cultures at the indicated days p.i. c IFN concentration determined in the supernatant of BDV-infected or uninfected hippocampal slice cultures 21 days p.i. Error bars indicate the standard deviation of three independent experiments. d CA3 pyramidal cells of BDV-infected SD hippocampus slice cultures on 21 days p.i. were analyzed for the expression of BDV-P (green), Mx1 (red) and NeuN (blue), a specific marker for neurons. Scale bars 10 μm
Fig. 2.
IFN-α treatment results in the expression of Mx1 in microglial cells and astrocytes. SD hippocampal slice cultures were incubated in the presence of IFN-α (105 U/ml). Medium was changed three times per week over 3 weeks. The tissue cultures were fixed on day 21 and stained for the presence of Mx1 (α-Mx1) in neurons (α-NeuN) of the CA3 pyramidal cell layer (a), microglial cells (α-Iba-1) (CA3 pyramidal cell layer) (b), or astrocytes (α-GFAP) in the CA1 pyramidal cell layer (c), by using the indicated cell type-specific antibodies. Cell nuclei were stained with ToPro3. Scale bars 10 μm
Nuclear accumulation of both pSTAT1 and pSTAT2 is impaired in rat neurons after stimulation with IFN
The observation that neurons fail to express detectable levels of Mx1 after IFN-α treatment, prompted us to speculate that the IFN signaling pathway, such as the activation and nuclear translocation of phosphorylated STAT1 (pSTAT1) as well as pSTAT2 might be impaired in these cells. To visualize the translocation of these proteins, we prepared dissociated primary neuronal cultures from hippocampi of newborn SD rats, which mainly contain neurons and astrocytes, and treated them with 105 U/ml of IFN-α. As shown in Fig. 3a, b, treatment with IFN-α for 30 min resulted in a peak in nuclear accumulation of pSTAT1 and pSTAT2 in astrocytes. After 60 min, the signal intensities of pSTAT1 and pSTAT2 in the nucleus again decreased almost to the levels observed in non-treated cells. In sharp contrast, neurons did not show increased nuclear levels of pSTAT1 and pSTAT2 at either early (15 min) or late time points (60 min) of IFN treatment at comparable exposure times (Fig. 3c–e). However, especially in untreated neurons, we observed elevated basal levels of pSTAT2 (Fig. 3d, f). These results may suggest that neurons mount only a poor immune response after treatment with IFN-α due to inefficient translocation of newly activated STAT1 and STAT2. However, consistent with findings of others [23], IFN-α receptor 1 is expressed on the surface of neurons (Fig. 3g), suggesting that this lack of immune response is not due to the absence of IFNA-α/β receptors.
Fig. 3.
Absence of pSTAT1 and pSTAT2 nuclear translocation in IFN-α treated rat neurons. a–d Primary dissociated neuronal cultures of rat hippocampi were treated with IFN-α (105 U/ml) for the indicated time points and for the presence of phosphorylated STAT1 (α-pSTAT1) or STAT2 (α-pSTAT1) in astrocytes (α-GFAP) or neurons (α-Map-2) Scale bars 10 μm. Lower panels indicate the fluorescence intensity along the dashed line shown in the middle panels. e, f Statistical analysis of the mean fluorescence intensity (MFI) of pSTAT1 and pSTAT2 in astrocytes (n = 30) and neurons (n = 30) treated with IFN-α (105 U/ml) for the indicated time points. g IFN-α receptor 1 (IFNAR1) surface staining (α-IFNAR1) of neurons (α-Map-2). IFNAR1 staining was carried out with non-permeabilized neurons. For subsequent staining with Map-2, cell were extensively washed, treated with detergent and subsequently stained with anti-Map-2 antibodies. Ctrl w/o 1st AB, staining of non-permeabilized neurons with secondary antibodies only. Scale bars 10 μm
IFN treatment does not abrogate but delays the viral dissemination of BDV
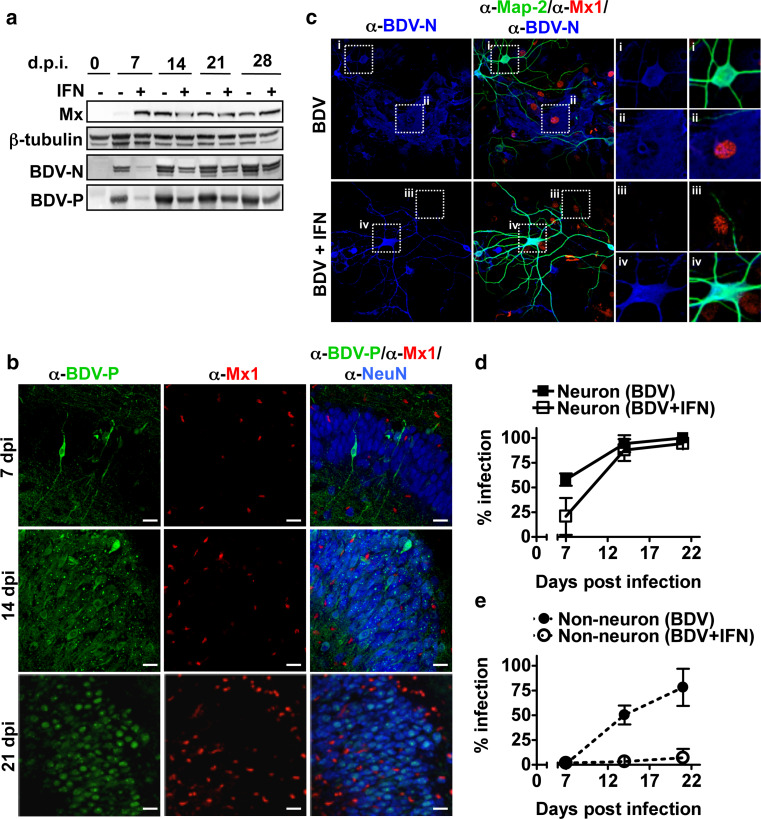
The lack of both Mx1 staining and nuclear translocation of pSTAT1 in neurons after IFN-α treatment suggests that the IFN-induced antiviral activity is circumvented by BDV through spreading within the neuronal network. To test this, BDV-infected hippocampal slice cultures were maintained in the presence or absence of IFN-α-containing media (105 U/ml). At 7 days post-infection, the IFN-α treatment resulted in reduced levels of both BDV-P and the viral nucleoprotein (BDV-N) (Fig. 4a). However, over the course of infection this difference leveled out and at 28 days p.i. only a minor difference was observed (Fig. 4a). As expected, IFN treatment during the course of infection did not result in detectable expression of Mx1 in neurons (Fig. 4b). Similar results were obtained with BDV-infected hippocampal slice cultures maintained in the presence or absence of rat IFN-α/β-containing media (103 U/ml) (Fig. S2a). Treatment with rat IFN-α/β (103 U/ml) prior infection also did not abrogate BDV infection (Fig. 2a). Comparable results were also observed in BDV-infected dissociated neuronal cultures (Fig. 4c). In these cultures both neurons and non-neuronal cells are susceptible to BDV infection (Fig. 4c–e). In neurons, BDV spread was partially inhibited in the presence of IFN-α at 7 days p.i., whereas at later time points all cells became infected (Fig. 4d). In contrast, IFN-α treatment prevented the productive infection of non-neurons (Fig. 4e). These results suggest that neurons can mount an innate immune response that is, however, insufficient to prevent neuronal spread of BDV.
Fig. 4.
BDV replication in neurons is not abrogated by treatment with type I IFN. a BDV-infected hippocampal slice cultures from SD rats were either treated or untreated with IFN-α (105 U/ml) after 3 days of virus infection. The medium was changed three times per week over 3 weeks. At the indicated time point post-infection, the cell extract was prepared from a pool of at least four cultures and analyzed by Western blotting for the presence of the indicated proteins. b Staining of hippocampal sections of BDV-infected rat slice cultures at the indicated time point p.i. using antiserum against BDV-P together with antibodies against Mx1 (α-Mx1) and NeuN (α-NeuN), a neuron-specific marker. Scale bars 20 μm. Upper panel CA1 pyramidal layer; Middle and lower panel CA3 pyramidal layer. c Dissociated neuronal cultures from the hippocampus of SD rats were infected with BDV (1,000 FFU) in the presence or absence of IFN-α for 21 days and stained for the presence of BDV-N (α-BDV-N), Mx1 (α-Mx1) and a neuronal marker Map-2 (α-Map-2). Scale bars 30 μm. Right four panels (i–iv) represent magnifications of the indicated areas. The infection rate of neurons (d) or non-neuronal cells (e) was determined by counting BDV-positive cells in Map-2-positive and -negative cells, respectively
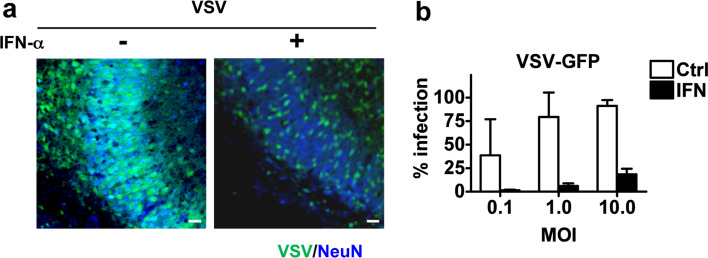
To further evaluate the antiviral state of neurons, rat hippocampal slice cultures from SD newborns were pre-treated with IFN-α (105 U/ml) for 24 h and subsequently infected with vesicular stomatitis virus (VSV) [16], which is known to be highly sensitive to IFN-α treatment [24]. As shown in Fig. 5a, pretreatment of hippocampal slice cultures with IFN-α significantly reduced the number of VSV-infected cells. Similar results were observed in dissociated neuronal cultures (Fig. 5b). Compared to untreated cultures, the number of VSV-infected cells was significantly reduced in IFN-treated cultures at low (0.1) or high (10) multiplicity of infection (MOI). Thus although rat neurons lack detectable Mx expression, these cells are able to mount a weak IFN-induced antiviral state.
Fig. 5.
IFN treatment of rat neurons prevents VSV replication. a Rat hippocampal slice cultures were treated or untreated with IFN-α (105 U/ml) for 24 h and then infected with VSV-GFP coding for the green fluorescence protein (GFP). VSV-infected cells were visualized in the CA3 pyramidal cell layer by GFP autofluorescence 15 h p.i. Scale bars 20 μm. b Dissociated rat neuronal cultures were treated with IFN-α (105 U/ml) for 24 h and followed by an infection with the indicated MOI of VSV-GFP. The infection rate was monitored 24 h p.i. by GFP autofluorescence
Species difference in IFN responsiveness between mouse and rat neurons
Transgenic mice constitutively expressing mouse IFN-α in astrocytes of the CNS are known to induce Mx1 expression in neurons and efficiently block BDV replication [13], suggesting that mouse neurons differ in their response to IFN compared to rat neurons. Indeed, IFN treatment of hippocampal slice cultures from Mx1-positive BALB/c mice resulted predominantly in neuronal expression of mouse Mx1 (mMx1) (Fig. 6a). In addition, mMx1-specific signals were also observed in microglial cells and astrocytes (Fig. 6b, c). The staining of mMx1 in neurons might suggest that these cells can mount a stronger IFN response than rat neurons. To address this in more detail, we infected the Mx1-positive C57/B6 mouse slice culture with the mouse-adapted BDV strain (rBDVLRD) [25]. Continuous treatment with IFN-α (103 U/ml) was sufficient to block virus replication, while the non-treated cultures were persistently infected (Fig. 6d). These results suggest that there is a species-specific difference in the IFN responsiveness of neurons between mice and rats.
Fig. 6.
Mouse neurons mount a robust innate immune response against BDV. a–c Hippocampal slice culture from Mx1-positive BALB/c mice were prepared and kept in medium containing IFN-α for 4 weeks. Sections of the slice cultures were subjected to immunofluorescence analysis using cell type-specific antibodies recognizing (a) neurons (α-NeuN) in the dentate gyrus (inset shows granule cells at higher magnification), b microglial cells (α-Iba-1) in the hippocampus subfield, including hilus, granule cell layer and subiculum (enlarged cell locate at the hilus) or c astrocytes (α-GFAP) in the CA3 region (enlarged cell is located at the stratum oriens) and Mx1-specific antibodies. Cell nuclei were stained with ToPro3. Scale bars 10 μm. d Hippocampal slice cultures from Mx1-positive C57/B6 mice were infected with rBDVLRD in the presence or absence of IFN. The nutrient medium in the hippocampal cultures containing IFN was changed three times per week over 4 weeks of the incubation period. Western blot analysis was carried out for the determination of viral (BDV-N, BDV-P) or cellular (Mx1, ß-tubulin) protein levels at the indicated days post-infection
Discussion
BDV infection in rat embryo fibroblasts and other non-neuronal cells is efficiently prevented by type I IFN treatment [12] and BDV developed a replication strategy that avoids recognition of the innate immune system [8, 9]. Here we show that unknown innate immune sensors detect BDV infection in organotypical hippocampal slice cultures, resulting in the up-regulation of IFN-induced genes. We provide further evidence that BDV is nevertheless able to persistently infect hippocampal neurons even in the presence of high levels of IFN-α, by exploiting their poor innate immune response. In sharp contrast, neurons of mice can mount a robust innate immune response after IFN-α treatment and block BDV infection, indicating species-specific differences in the type I IFN response in neurons between mice and rats.
The presence of IFN in the medium of BDV-infected hippocampal tissue cultures of SD rats indicates that BDV infection can be recognized by PPRs. We therefore assume that the unusual replication strategy of BDV (generation of 5′-monophosphate genome ends) to escape detection by RIG-I does not entirely prevent the detection by other PPRs [11]. The nature of the recognition receptors remains to be shown, but could include the toll like receptor 7 (TLR-7), since this receptor is expressed on both astrocytes and microglial cells and initiates the induction of IFN in both cell types [26]. However, other host defense signaling pathways might be considered too, including the IFN induction signaling pathway induced by viral fusion [27, 28] and the possibility that 5′-monophosphate at the genome end might not completely block recognition by RIG-I. The cell type responsible for the induction of IFN also remains to be shown. In principle, neurons, astrocytes and microglial cells have the capacity to produce IFN [26, 29, 30]. Astrocytes, however, seem to be prime candidates, since they are readily infected in cultures [31], are known to produce IFN [26] and are activated by BDV [32]. This activation might be accompanied with abortive infections, resulting in a low frequency of BDV-antigen-positive astrocytes in infected hippocampal slice cultures or in newborn rats [33].
Intriguingly, although BDV-infection of dissociated neuronal cultures results in the expression of Mx1 in non-neuronal cells, viral replication seems to be impaired in these cells only after additional treatment with IFN-α. This is consistent with the observation that rat Mx proteins show no antiviral activity against BDV (Schwemmle et al., unpublished data). Since Mx1 is highly sensitive marker for IFN-induced genes and since even low concentrations of IFN is sufficient to induce abundant expression of this protein, we therefore speculate that the levels of those ISGs that abrogate BDV replication might not be reached without additional IFN treatment. However, which ISGs are involved in the inhibition of BDV replication remains to be shown.
Although no dynamic change of both pSTAT1 and pSTAT2 nuclear translocation was observed in rat neurons after IFN-α treatment, these cells were protected against VSV infection. This suggests that IFN-α induced signal transduction events in rat neurons still occur. This is consistent with the observation that tyrosine kinase 2 (Tyk2), an IFNAR-associated protein required for subsequent phosphorylation of STAT1 and STAT2, is activated in rat neurons after IFN treatment [23]. This might suggest that residual pSTAT1 and/or pSTAT2 translocation occurs in rat neurons. However, the levels of imported pSTAT1 and pSTAT2 might be too low to be detected by immunofluorescence analysis. In addition, the elevated basal levels of pSTAT1 and pSTAT2, which do not contribute to the antiviral state of neurons, further complicate this type of analysis. In sharp contrast, IFN-α treatment of mouse neurons resulted in a robust antiviral state, preventing BDV replication (Fig. 6d). This is consistent with the observation that both STAT1 and STAT2 are readily phosphorylated after IFN treatment in mouse neurons [34]. Thus, the extent of phosphorylation and activation of these signal transduction molecules might vary between mice and rat neurons and account for the observed differences in mounting a robust antiviral response against virus infection. However, we cannot exclude the possibility that the species-specific differences are also caused by reduced IFN-α/β receptor densities between rat and mouse neurons although rat neurons do express the IFN-α receptor 1 (Fig. 3g) [23].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 Rat IFN-α/β treatment results in the expression of Mx1 in microglial cells. SD hippocampal slice cultures were either BDV-infected (a) or treated with rat IFN-α/β (103 U/ml) (b). Medium was changed three times per week over 3 weeks. The tissue cultures were fixed on day 21 and stained for the presence of Mx1 (α-Mx1), microglial cells (α-Iba-1) or neurons (α-NeuN). Cell nuclei were visualized with ToPro3. Scale bars, 10 µm (TIFF 4059 kb)
Fig. S2 BDV replication in neurons is not abrogated by treatment with rat type I IFN. (a) BDV-infected hippocampal slice cultures from SD rats were either treated or untreated with rat IFN-α/β (103 U/ml) after 3 days of virus infection. The medium was changed three times per week over 4 weeks. At the indicated time point post-infection, the cell extract was prepared from a pool of at least four cultures and analyzed by Western blotting for the presence of the indicated proteins. (b) Rat hippocampal slice cultures from SD rats were either pretreated with 103 U/ml of rat IFN-α/β for 6 h (IFN pretreated) or left untreated (untreated) and subsequently infected with BDV (1,000 FFU). Medium containing 1,000 U of rat IFN-α/β was changed three times per week in pretreated cultures, whereas no additional IFN was added to the untreated cultures. 21 days post-infection (dpi) viral dissemination was determined by immunofluorescence using BDV-P-specific antibodies. Cell nucleus was visualized by TpPro-3. Scale bars, 10 µm (TIFF 6830 kb)
Acknowledgments
We thank Thomas Michiels, Friedemann Weber, Peter Staeheli, and Georg Kochs for critical reading of the manuscript and Simone Zenker for excellent technical assistance. The study was supported by the Deutsche Forschungsgemeinschaft (DFG) to MS (SCHW 632), BH (He 1520), and in part by the Excellence Initiative of the German Federal and State Governments (GSC-4, Spemann Graduate School).
Contributor Information
Bernd Heimrich, Phone: +49-761-2038418, FAX: +49-761-2038417, Email: bernd.heimrich@zfn.uni-freiburg.de.
Martin Schwemmle, Phone: +49-761-2036526, FAX: +49-761-2036639, Email: martin.schwemmle@uniklinik-freiburg.de.
References
- 1.Hornig M, Briese T, Lipkin WI. Borna disease virus. J Neurovirol. 2003;9(2):259–273. doi: 10.1080/13550280390194064. [DOI] [PubMed] [Google Scholar]
- 2.Hornig M, Briese T, Licinio J, Khabbaz RF, Altshuler LL, Potkin SG, Schwemmle M, Siemetzki U, Mintz J, Honkavuori K, Kraemer HC, Egan MF, Whybrow PC, Bunney WE, Lipkin WI. Absence of evidence for bornavirus infection in schizophrenia, bipolar disorder and major depressive disorder. Mol Psychiatry. 2012;17(5):486–493. doi: 10.1038/mp.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horie M, Honda T, Suzuki Y, Kobayashi Y, Daito T, Oshida T, Ikuta K, Jern P, Gojobori T, Coffin JM, Tomonaga K. Endogenous non-retroviral RNA virus elements in mammalian genomes. Nature. 2010;463(7277):84–87. doi: 10.1038/nature08695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer D, Fischer H, Schneider U, Heimrich B, Schwemmle M. Borna disease virus replication in organotypic hippocampal slice cultures from rats results in selective damage of dentate granule cells. J Virol. 2005;79(18):11716–11723. doi: 10.1128/JVI.79.18.11716-11723.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu YJ, Schulz H, Lin CC, Saar K, Patone G, Fischer H, Hubner N, Heimrich B, Schwemmle M. Borna disease virus-induced neuronal degeneration dependent on host genetic background and prevented by soluble factors. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1214939110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8(12):911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haller O, Kochs G, Weber F. Interferon, Mx, and viral countermeasures. Cytokine Growth Factor Rev. 2007;18(5–6):425–433. doi: 10.1016/j.cytogfr.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habjan M, Andersson I, Klingstrom J, Schumann M, Martin A, Zimmermann P, Wagner V, Pichlmair A, Schneider U, Muhlberger E, Mirazimi A, Weber F. Processing of genome 5′ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS ONE. 2008;3(4):e2032. doi: 10.1371/journal.pone.0002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin A, Hoefs N, Tadewaldt J, Staeheli P, Schneider U. Genomic RNAs of Borna disease virus are elongated on internal template motifs after realignment of the 3′ termini. Proc Natl Acad Sci USA. 2011;108(17):7206–7211. doi: 10.1073/pnas.1016759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto Y, Hayashi Y, Omori H, Honda T, Daito T, Horie M, Ikuta K, Fujino K, Nakamura S, Schneider U, Chase G, Yoshimori T, Schwemmle M, Tomonaga K. Bornavirus closely associates and segregates with host chromosomes to ensure persistent intranuclear infection. Cell Host Microbe. 2011;11(5):492–503. doi: 10.1016/j.chom.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Unterstab G, Ludwig S, Anton A, Planz O, Dauber B, Krappmann D, Heins G, Ehrhardt C, Wolff T. Viral targeting of the interferon-{beta}-inducing Traf family member-associated NF-{kappa}B activator (TANK)-binding kinase-1. Proc Natl Acad Sci USA. 2005;102(38):13640–13645. doi: 10.1073/pnas.0502883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallensleben W, Staeheli P. Inhibition of Borna disease virus multiplication by interferon: cell line differences in susceptibility. Arch Virol. 1999;144(6):1209–1216. doi: 10.1007/s007050050580. [DOI] [PubMed] [Google Scholar]
- 13.Staeheli P, Sentandreu M, Pagenstecher A, Hausmann J. Alpha/beta interferon promotes transcription and inhibits replication of Borna disease virus in persistently infected cells. J Virol. 2001;75(17):8216–8223. doi: 10.1128/JVI.75.17.8216-8223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm D, Staeheli P, Hufbauer M, Koerner I, Martinez-Sobrido L, Solorzano A, Garcia-Sastre A, Haller O, Kochs G. Replication fitness determines high virulence of influenza A virus in mice carrying functional Mx1 resistance gene. Proc Natl Acad Sci USA. 2007;104(16):6806–6811. doi: 10.1073/pnas.0701849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmid S, Mayer D, Schneider U, Schwemmle M. Functional characterization of the major and minor phosphorylation sites of the P protein of Borna disease virus. J Virol. 2007;81(11):5497–5507. doi: 10.1128/JVI.02233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann M, Wu YJ, Gerber M, Berger-Rentsch M, Heimrich B, Schwemmle M, Zimmer G. Fusion-active glycoprotein G mediates the cytotoxicity of vesicular stomatitis virus M mutants lacking host shut-off activity. J Gen Virol. 2011;91(Pt 11):2782–2793. doi: 10.1099/vir.0.023978-0. [DOI] [PubMed] [Google Scholar]
- 17.Horisberger MA, de Staritzky K. A recombinant human interferon-alpha B/D hybrid with a broad host-range. J Gen Virol. 1987;68(Pt 3):945–948. doi: 10.1099/0022-1317-68-3-945. [DOI] [PubMed] [Google Scholar]
- 18.Schneider U, Schwemmle M, Staeheli P. Genome trimming: a unique strategy for replication control employed by Borna disease virus. Proc Natl Acad Sci USA. 2005;102(9):3441–3446. doi: 10.1073/pnas.0405965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider U, Naegele M, Staeheli P, Schwemmle M. Active Borna disease virus polymerase complex requires a distinct nucleoprotein-to-phosphoprotein ratio but no viral X protein. J Virol. 2003;77(21):11781–11789. doi: 10.1128/JVI.77.21.11781-11789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuri T, Habjan M, Penski N, Weber F. Species-independent bioassay for sensitive quantification of antiviral type I interferons. Virol J. 2010;7:50. doi: 10.1186/1743-422X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbone KM, Rubin SA, Sierra-Honigmann AM, Lederman HM. Characterization of a glial cell line persistently infected with Borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J Virol. 1993;67(3):1453–1460. doi: 10.1128/jvi.67.3.1453-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli UK, Teaff N, D’Ambrosia J. Maturation of the head of bacteriophage T4. III. DNA packaging into preformed heads. J Mol Biol. 1974;88(4):749–765. doi: 10.1016/0022-2836(74)90397-0. [DOI] [PubMed] [Google Scholar]
- 23.Stadler K, Bierwirth C, Stoenica L, Battefeld A, Reetz O, Mix E, Schuchmann S, Velmans T, Rosenberger K, Brauer AU, Lehnardt S, Nitsch R, Budt M, Wolff T, Kole MH, Strauss U. Elevation in type I interferons inhibits HCN1 and slows cortical neuronal oscillations. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs305. [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein S, Familletti PC, Pestka S. Convenient assay for interferons. J Virol. 1981;37(2):755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackermann A, Kugel D, Schneider U, Staeheli P. Enhanced polymerase activity confers replication competence of Borna disease virus in mice. J Gen Virol. 2007;88(Pt 11):3130–3132. doi: 10.1099/vir.0.83170-0. [DOI] [PubMed] [Google Scholar]
- 26.Butchi NB, Du M, Peterson KE. Interactions between TLR7 and TLR9 agonists and receptors regulate innate immune responses by astrocytes and microglia. Glia. 2010;58(6):650–664. doi: 10.1002/glia.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paladino P, Cummings DT, Noyce RS, Mossman KL. The IFN-independent response to virus particle entry provides a first line of antiviral defense that is independent of TLRs and retinoic acid-inducible gene I. J Immunol. 2006;177(11):8008–8016. doi: 10.4049/jimmunol.177.11.8008. [DOI] [PubMed] [Google Scholar]
- 28.Holm CK, Jensen SB, Jakobsen MR, Cheshenko N, Horan KA, Moeller HB, Gonzalez-Dosal R, Rasmussen SB, Christensen MH, Yarovinsky TO, Rixon FJ, Herold BC, Fitzgerald KA, Paludan SR. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat Immunol. 2012;13(8):737–743. doi: 10.1038/ni.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T. Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci USA. 2006;103(20):7835–7840. doi: 10.1073/pnas.0602460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kallfass C, Ackerman A, Lienenklaus S, Weiss S, Heimrich B, Staeheli P. Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice. J Virol. 2012;86(20):11223–11230. doi: 10.1128/JVI.01093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richt JA, Stitz L. Borna disease virus-infected astrocytes function in vitro as antigen-presenting and target cells for virus-specific CD4-bearing lymphocytes. Arch Virol. 1992;124(1–2):95–109. doi: 10.1007/BF01314628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ovanesov MV, Ayhan Y, Wolbert C, Moldovan K, Sauder C, Pletnikov MV. Astrocytes play a key role in activation of microglia by persistent Borna disease virus infection. J Neuroinflammation. 2008;5:50. doi: 10.1186/1742-2094-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Dunia D, Volmer R, Mayer D, Schwemmle M. Borna disease virus interference with neuronal plasticity. Virus Res. 2005;111(2):224–234. doi: 10.1016/j.virusres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Yin J, Gardner CL, Burke CW, Ryman KD, Klimstra WB. Similarities and differences in antagonism of neuron alpha/beta interferon responses by Venezuelan equine encephalitis and Sindbis alphaviruses. J Virol. 2009;83(19):10036–10047. doi: 10.1128/JVI.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Rat IFN-α/β treatment results in the expression of Mx1 in microglial cells. SD hippocampal slice cultures were either BDV-infected (a) or treated with rat IFN-α/β (103 U/ml) (b). Medium was changed three times per week over 3 weeks. The tissue cultures were fixed on day 21 and stained for the presence of Mx1 (α-Mx1), microglial cells (α-Iba-1) or neurons (α-NeuN). Cell nuclei were visualized with ToPro3. Scale bars, 10 µm (TIFF 4059 kb)
Fig. S2 BDV replication in neurons is not abrogated by treatment with rat type I IFN. (a) BDV-infected hippocampal slice cultures from SD rats were either treated or untreated with rat IFN-α/β (103 U/ml) after 3 days of virus infection. The medium was changed three times per week over 4 weeks. At the indicated time point post-infection, the cell extract was prepared from a pool of at least four cultures and analyzed by Western blotting for the presence of the indicated proteins. (b) Rat hippocampal slice cultures from SD rats were either pretreated with 103 U/ml of rat IFN-α/β for 6 h (IFN pretreated) or left untreated (untreated) and subsequently infected with BDV (1,000 FFU). Medium containing 1,000 U of rat IFN-α/β was changed three times per week in pretreated cultures, whereas no additional IFN was added to the untreated cultures. 21 days post-infection (dpi) viral dissemination was determined by immunofluorescence using BDV-P-specific antibodies. Cell nucleus was visualized by TpPro-3. Scale bars, 10 µm (TIFF 6830 kb)