Abstract
Epithelial homeostasis within the epidermis is maintained by means of multiple cell–cell adhesion complexes such as adherens junctions, tight junctions, gap junctions, and desmosomes. These complexes co-operate in the formation and the regulation of the epidermal barrier. Disruption of the epidermal barrier through the deregulation of the above complexes is the cause behind a number of skin disorders such as psoriasis, dermatitis, keratosis, and others. During epithelial-to-mesenchymal transition (EMT), epithelial cells lose their adhesive capacities and gain mesenchymal properties. ZEB transcription factors are key inducers of EMT. In order to gain a better understanding of the functional role of ZEB2 in epidermal homeostasis, we generated a mouse model with conditional overexpression of Zeb2 in the epidermis. Our analysis revealed that Zeb2 expression in the epidermis leads to hyperproliferation due to the combined downregulation of different tight junction proteins compromising the epidermal barrier. Using two epidermis-specific in vivo models and in vitro promoter assays, we identified occludin as a new Zeb2 target gene. Immunohistological analysis performed on human skin biopsies covering various pathogeneses revealed ZEB2 expression in the epidermis of pemphigus vulgaris. Collectively, our data support the notion for a potential role of ZEB2 in intracellular signaling of this disease.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-014-1589-0) contains supplementary material, which is available to authorized users.
Keywords: EMT, ZEB2, Tight junctions, Skin
Introduction
The two major cell types forming the metazoan body, epithelial and mesenchymal cells, differ both morphologically and functionally. Epithelial cells are characterized by apical-basal polarization and by the close contact they maintain with neighboring cells through adherens junctions, tight junctions, desmosomes and gap junctions, creating a rigid three-dimensional structure that can support organ function [1]. In contrast, mesenchymal cells show anterior-posterior polarization and no durable adhesive properties, forming irregular structures without a uniform composition or density. The main distinctive feature of mesenchymal cells is their ability to actively migrate as individual cells [2].
Cells can switch between the epithelial and mesenchymal types through a process called epithelial-to-mesenchymal transition (EMT), which involves the dynamic remodeling of junctions, polarity, motility, and adhesive properties. EMT is reversible, and the reverse process is known as mesenchymal-to-epithelial transition (MET) [3, 4]. The same transcription factors that regulate EMT during development [5] can drive EMT during cancer progression [6]. These factors belong to three major families: the Snail, the ZEB, and the bHLH families. They directly repress various epithelial-specific genes, including E-cadherin, and maintain as such the mesenchymal phenotype.
Skin is the largest organ of the body and consists of an epidermis overlying the dermis and the deeper adipose tissue. The epidermis has a pivotal role in forming an essential barrier between the body and the outer environment, protecting against mechanical and chemical insult, as well as excessive water loss and dehydration [7]. The stratum corneum and cell–cell junctions in the suprabasal layers participate in barrier formation and function. Adherens junctions, tight junctions, and desmosomes between cells of the epidermal layers form a “fence” and actively control paracellular movement of water and other molecules through the epidermis [8–11]. Disruption of the epidermal barrier leads to various disorders as has been shown by different in vivo models. Deletion of E-cadherin and claudin-1 in vivo leads to neonatal lethality due to extensive water loss highlighting the role of these proteins in the maintenance of the water barrier function of the epidermis [8, 10]. The stratum corneum also plays a very important role as loss-of-function mutations on the Filaggrin gene in humans are considered the cause of the skin disorder ichthyosis vulgaris [12]. However, regulation of the epidermal barrier might be more complex, as recent studies have highlighted the importance of the collaboration between different proteins in its maintenance [13]. In cases of injury, EMT is activated in epithelial cells as part of the physiological responses necessary for the healing of the wound. In the skin, wound healing is a multistep process, which includes three distinct and overlapping phases: inflammation, tissue remodeling, and re-epithelialization. Initially, mechanical and inflammatory stimuli activate basal and suprabasal layers of keratinocytes at the edges of the wound, which in turn recapitulate part of the EMT process [14]. Snail2 (Slug) is important for the regulation of the process as it is expressed in the basal keratinocytes [15]. Recent publications have also highlighted the role of Snail in promoting cutaneous inflammation and hyperplasia [16].
Despite the information about the role of the Snail-family of transcription factors in maintaining skin homeostasis, nothing is known about the ZEB family, which are also potent inducers of EMT. With this in mind, we performed immunohistological analysis for ZEB2 in human skin samples with various pathologies, which revealed ZEB2 expression in the epidermis of pemphigus vulgaris patients. Following our findings, we generated and characterized a ROSA26 locus-based conditional expression mouse model for the transcription factor Zeb2. Using this model, we directed Zeb2 expression in the epidermis using two discrete, yet very similar, Cre lines, and we were able to demonstrate how the combined downregulation of different tight junction components jeopardizes the integrity of the epidermal barrier possibly contributing to the disorder.
Materials and methods
Animal experimentation and handling
All mice were housed in the SPF facility of the Inflammation Research Center (IRC-VIB, Ghent University). All experiments were performed in the same facility according to the regulations and guidelines of the Ethics Committee for care and use of laboratory animals of Ghent University.
Immunohistochemical analysis
Tissues were fixed overnight in 4 % paraformaldehyde and then embedded in paraffin. Sections of 5 μm were stained with hematoxylin-eosin for morphological analysis or processed for immunohistochemical analysis. The different proteins were detected by standard procedures. Details of the procedures can be found in Suppl. Materials and methods and details of the antibodies and their dilutions are provided in Suppl. Table S3.
Barrier functionality assays
The in situ skin permeability assay using Toluidine Blue was performed as previously described [17]. The embryos were immediately photographed at the end of the treatment. The Lucifer Yellow penetration assay was performed as previously described [18] in P4.5 neonates. The penetration of the dye was assessed by immunofluorescence microscopy on frozen skin sections. Trans-epidermal water loss (TEWL) and stratum corneum hydration levels were measured using a TEWAmeter (Courage and Khazaka, TM210) and a Corneometer (Courage and Khazaka, CM825), respectively. Tight junction permeability assay was performed on wild-type and transgenic P4.5 neonates as previously described. For the tracing of biotin, 5-μm frozen sections were incubated with Streptavidin-Alexa594 for 1 h at room temperature in the dark. Biotin tracing was performed together with the incubation of the secondary antibody during the immunofluorescent analysis of the skin.
Luciferase promoter assays
The human occludin promoter construct used in our experiments was a kind offer from Prof. Dr. J. Ikenouchi (Kyoto University, Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering) [19]. The wild-type and mutant E-cadherin promoter plasmids, as well as the ZEB2-expressing plasmid, have been previously characterized [20, 21]. Mutagenesis of the E-box located in the hOccludin promoter was performed by using the QuickChange Multi Site-directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) following the manufacturer’s instructions. The hOCC-luciferase reporter was transiently transfected together with the ZEB2-expression vector in MCF7/AZ cells by using FuGene6 (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s instructions. For normalization purposes, the pUT651 plasmid (Eurogentec, Cologne, Germany), encoding β-galactosidase, was co-transfected in each setting. Luciferase and β-galactosidase activities were measured using the Galacto-Star one-step kit (Applied Biosystems, Carlsbad, CA, USA). Both luciferase and β-galactosidase activities were measured with the GloMax 96 Microplate Luminometer (Promega, Madison, WI, USA).
Protein isolation and Western-blot analysis
For protein isolation, the skin of K5Cre;ROSA26-Zeb2 tg/+ neonates at P4.5 was incubated overnight at 4 °C on top of Dispase II (2.5 U/ml; Roche) in KSF medium (Lonza, Basel, Switzerland). The next day, the epidermis was mechanically separated from the dermis and ground into small pieces, which were then lysed in 1× Laemmli buffer (63 mM Tris–HCl, pH 6.8, 10 % glycerol, 2 % SDS). Lysates containing 30 μg of protein were used for Western-blot analysis.
Results
Immunohistological characterization of ZEB2 in various human skin disorders
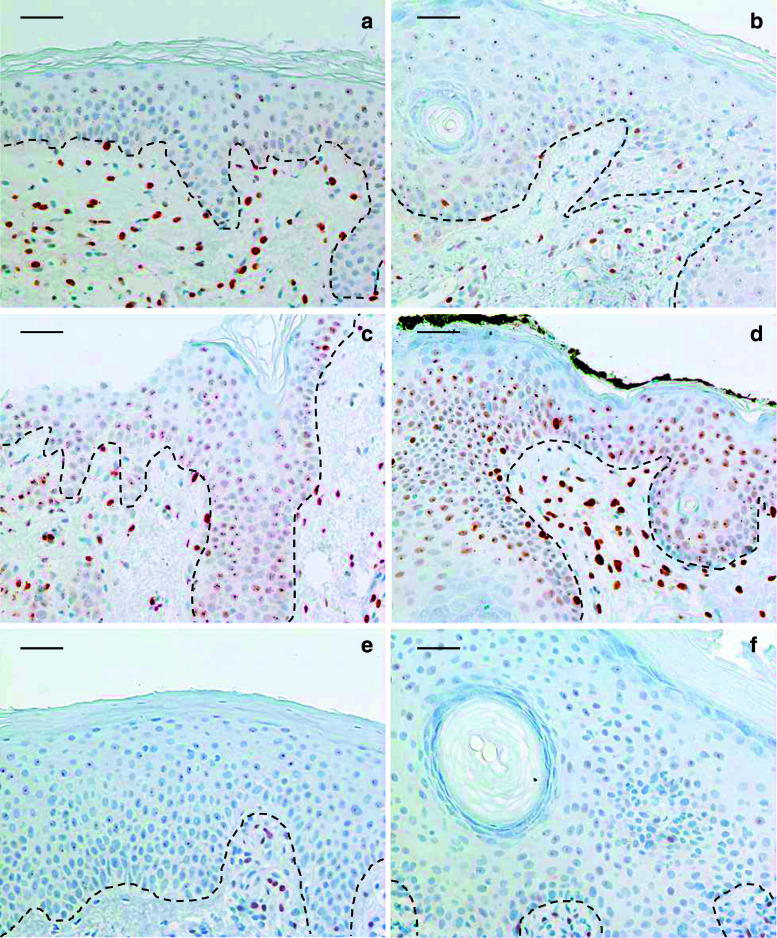
We performed an immunohistochemical analysis for ZEB2 in human skin samples covering various skin pathogeneses (Suppl. Table S1). ZEB2 expression is always prominently present in at least part of the fibroblasts in normal skin and affected skin. A clear positive staining in keratinocytes was only evident in pemphigus vulgaris (Fig. 1). Pemphigus vulgaris is an autoimmune disease, which affects intercellular adhesion through the deregulation of intercellular proteins [22]. Acantholysis or disruption of cell–cell adhesion associated with this disease is accompanied by intracellular signaling which is only poorly understood. Since the normal epidermis is negative for ZEB2, we decided to study the functional consequences of ZEB2 expression in the epidermis in vivo.
Fig. 1.
Immunohistochemical analysis of ZEB2 in human samples. Immunohistochemical analysis using an antibody recognizing human ZEB2 was performed on a series of human biopsies covering various diseases (summarized in Table S1). This analysis showed that ZEB2 protein is present in epithelial cells of pemphigus vulgaris (c, d), but not in epithelial cells of non-affected skin (a), lupus (b), eczema (e), and lichen planus (f). Bars 40 μm
Generation of ROSA26-Zeb2tg/tg transgenic mice
To generate conditional Zeb2 transgenic mice, we used a Gateway-compatible vector for targeting the ROSA26 locus (Suppl. Fig. S1a) [23]. Homozygote ROSA26-Zeb2 tg/tg mice appeared completely normal and no breeding or morphological abnormalities were observed. We verified the presence of the ROSA26-Zeb2 tg/+ and Zeb2 tg/tg transgene in germline-transmitting mice by Southern blot (Suppl. Fig. S1b) and confirmed the genotype by PCR on tail genomic DNA using transgene-specific primers to detect the presence of the transgene (Suppl. Fig. S1c).
The analysis of Zeb2-transgene expression in vivo was performed by intercrossing these mice with various tissue-specific Cre strains. Non-Cre ROSA26-Zeb2 tg/tg mice appeared normal and were indistinguishable from the non-ROSA26 wild-type mice and were used as control for our subsequent analyses and will be referred to as the wild type. To confirm the functionality of the ROSA26-Zeb2 transgene, in vivo ROSA26-Zeb2tg/+ or ROSA26-Zeb2tg/tg mice were genetically intercrossed with the ubiquitously expressing Sox2-Cre line prior to our epidermal-specific Cre lines. This intercrossing led to severe developmental defects and embryonic lethality prior to embryonic day (E) 9.5, thereby demonstrating activity of the transgene (Suppl. Fig. S2).
Expression of Zeb2 in the mouse epidermis
To specifically analyze the effects of Zeb2 expression in the skin epidermis, ROSA26-Zeb2 tg/tg mice were crossed with two different epithelial-specific Cre strains: K14-Cre (cytokeratin-14) and K5-Cre (cytokeratin-5) [24, 25]. Both of these strains are known to drive Cre expression in the basal layer of the skin epidermis, but their timing is slightly different (Suppl. Fig. S3a). Removal of the floxed stop cassette places the expression of Zeb2 under the control of the ROSA26 promoter in both cases (Suppl. Fig. S1a).
A mild skin abnormality was observed in heterozygous K14Cre;ROSA26-Zeb2 tg/+ mice. Starting at the age of 3.5 weeks, these mice showed scruffy hair and occasionally moderate hair loss. Breeding to homozygosity of the ROSA26-Zeb2 locus resulted in more pronounced hair loss and sporadic scaly crusts on the snouts and ears (Suppl. Fig. S3b-c). Transgenic neonatal mice were born in the expected Mendelian ratios, and the severity of the phenotypic aberrations was related to the Zeb2 levels. Both heterozygous K14Cre;ROSA26-Zeb2 tg/+ and homozygous K14Cre;ROSA26-Zeb2 tg/tg mice lived up to 2 years without any apparent problems. Morphological analysis of the skin revealed intense hyperproliferation of the epidermis of the K14Cre;ROSA26-Zeb2 tg/tg mice compared to wild-type mice (Fig. 2a).
Fig. 2.
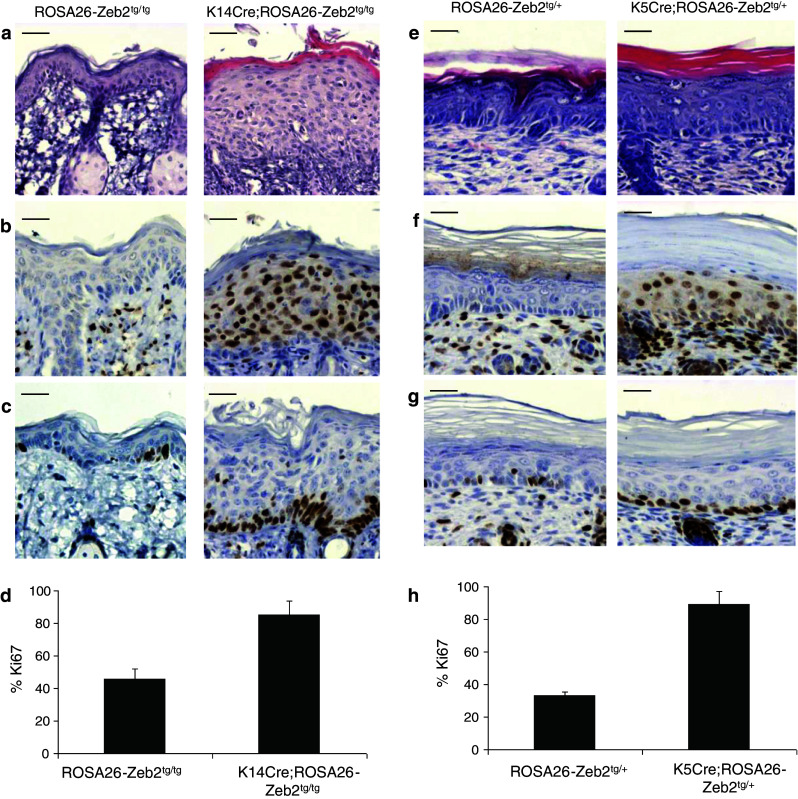
Expression of Zeb2 in the epidermis of K14Cre;ROSA26-Zeb2 tg/tg and K5Cre;ROSA26-Zeb2 tg/+mice. Hematoxylin-eosin staining on wild-type and transgenic skin revealed that Zeb2 expression in the epidermis results in hyperproliferation in adult K14Cre;ROSA26-Zeb2 tg/tg mice (a) and in K5Cre;ROSA26-Zeb2 tg/+ P4.5 neonates (e). Zeb2 is present in all epidermal layers in both strains (b and f) as seen by immunohistochemistry using a mouse Zeb2-specific antibody. Analysis for the proliferation marker Ki67 revealed that the basal layer is actively proliferating in both strains (c and g). Quantification of the number of Ki67-positive cells confirmed this observation (d and h). The graphs depict the average percentage ± SD of Ki67-positive cells in the basal layer (100 %) counted in four different low magnification photographs (p < 0.001 in both d and h). Bars 40 μm
Heterozygous K5Cre;ROSA26-Zeb2 tg/+ mice did not live beyond 6.5 days post-partum (P6.5). Newborn K5Cre;ROSA26-Zeb2 tg/+ pups were morphologically indistinguishable from their wild-type counterparts, however starting 2 days after birth they developed skin defects that aggravated until the sixth day (Suppl. Fig. S3d). At P4.5, the K5Cre;ROSA26-Zeb2 tg/+ pups were significantly smaller and showed shiny, inflexible skin, experiencing difficulties in movement. The epidermis of the K5Cre;ROSA26-Zeb2 tg/+ pups showed also extensive hyperproliferation (Fig. 2e), but there were no apparent differences in the dermis (Suppl. Fig. S4).
Immunohistochemical analysis of the skin of both strains using an anti-Zeb2 antibody showed nuclear localization of Zeb2 in all layers of the epidermis of the transgenic mice, whereas the epidermis of the wild-type mice was negative (Fig. 2b, f). Additionally, immunostaining for the proliferation marker Ki67 showed that the entire basal layer of transgenic epidermis was strongly positive (Fig. 2c–d, g–h). We characterized the K5Cre;ROSA26-Zeb2 tg/+ mice further in more detail given the severity of the observed phenotype in these neonatal mice.
Epidermal barrier impairment in K5Cre;ROSA26-Zeb2tg/+ mice
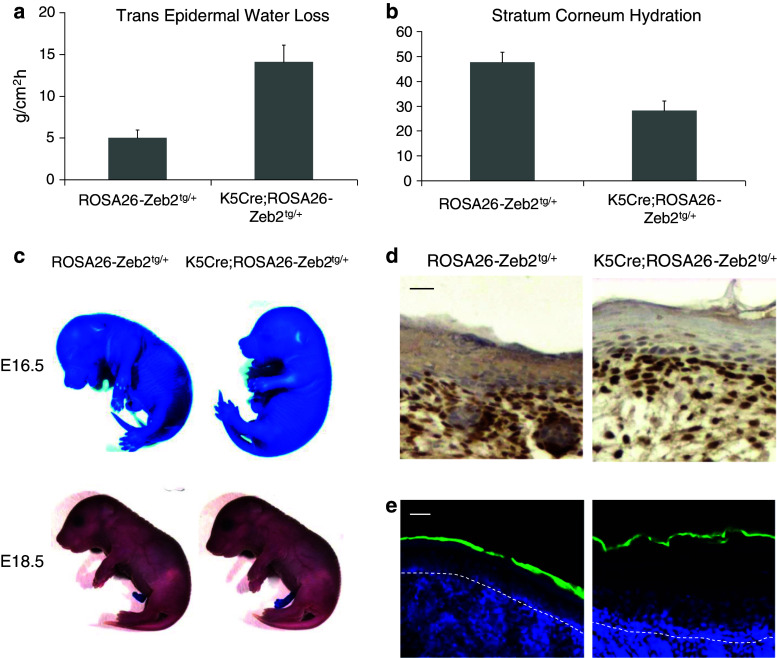
The macroscopic appearance of the K5Cre;ROSA26-Zeb2 tg/+ pups was suggestive of deregulation of the epidermal barrier. Indeed, P4.5 K5Cre;ROSA26-Zeb2 tg/+ neonates exhibited significantly increased trans epidermal water loss (TEWL) and reduced stratum corneum hydration levels compared to their wild-type counterparts (Fig. 3a–b). During embryonic development, a functional epidermal barrier is formed between embryonic days E16.5 and E17.5. To evaluate the functionality of the outside-to-inside barrier, a Toluidine Blue penetration assay was performed on K5Cre;ROSA26-Zeb2 tg/+ embryos before and after barrier formation. No difference between wild-type and transgenic embryos was observed, despite the presence of ectopic Zeb2 in the transgenic epidermis (Fig. 3c, d). Similarly, the functionality of the stratum corneum was verified in both transgenic and wild-type K5Cre;ROSA26-Zeb2 tg/+ neonates by a Lucifer Yellow penetration assay (Fig. 3e) showing no difference between wild-type and transgenic mice.
Fig. 3.
Expression of Zeb2 in the epidermis of K5Cre;ROSA26-Zeb2 tg/+mice affects the functionality of the epidermal barrier. Trans-epidermal water loss (TEWL) (a) and stratum corneum hydration level (b) of P4.5 K5Cre;ROSA26-Zeb2 tg/+ and wild-type neonates was measured using specialized equipment described in the Materials and methods section. The graphs show the average ± SD of five mice per group. The K5Cre;ROSA26-Zeb2 tg/+ mice exhibited significantly higher water loss through their skin than their wild-type littermates (a and b) (p < 0.001). The evaluation of outside-to-inside barrier was done using two different methods both in utero and also after birth. Toluidine Blue penetration assay was performed on E16.5 and E18.5 embryos (c). No difference in the establishment of the epidermal barrier between K5Cre;ROSA26-Zeb2 tg/+ and wild-type embryos is seen, although Zeb2 is already present in the epidermis of the embryos at E16.5 as shown by immunohistochemistry (d). No difference in Lucifer Yellow penetration between transgenic and wild-type P4.5 neonates (e). Bars 20 μm (d), 10 μm (e)
To examine the integrity of the inside-to-outside epidermal barrier, we injected P4.5 pups intradermally with biotin and assessed its diffusion through the epidermal layers. In the wild-type epidermis, biotin moved through the stratum granulosum, where it was held between the granular cells due to the presence of tight junctions. The tight junctions at the border between the stratum granulosum and the stratum corneum are marked by “free end” structures (Fig. 4a, arrows). In the K5Cre;ROSA26-Zeb2 tg/+ epidermis, biotin could penetrate through the stratum granulosum and reach the stratum corneum, indicating that the tight junctions are absent or not functional (Fig. 4a).
Fig. 4.
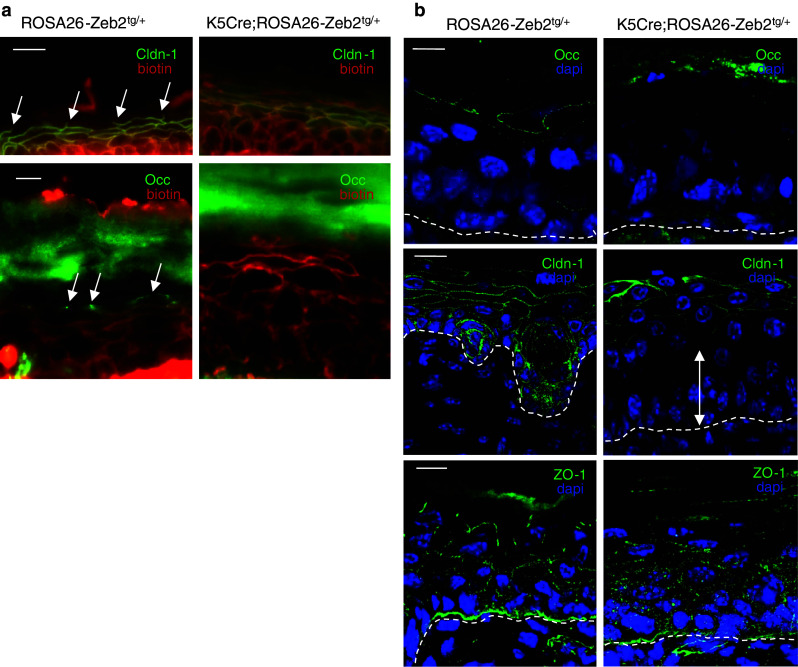
Occludin is absent from the epidermis of K5Cre;ROSA26-Zeb2 tg/+neonates, leading to loss of function of the inside-outside epidermal barrier. a Intradermal injection of biotin and subsequent tracing with fluorescently labeled streptavidin shows that biotin diffusion in wild-type epidermis stops at the tight junctions of the granular layer, whereas in the K5Cre;ROSA26-Zeb2 tg/+ epidermis biotin passes through and reaches the outer surface of the epidermis. The “free ends” (arrows) marking the tight junctions formed between the stratum granulosum and the cornified envelope are absent in the Zeb2 transgenic epidermis. b Occludin is missing in the epidermis of P4.5 K5Cre;ROSA26-Zeb2 tg/+ pups. Claudin-1 is disturbed in the lower epidermal layers (white arrow) but not in the stratum granulosum, while ZO-1 is only mildly disturbed. Bars 20 μm (a) and 10 μm (b)
In the wild-type epidermis, claudin-1 is present in all epidermal layers. However, immunohistochemical analysis of the epidermis of K5Cre;ROSA26-Zeb2 tg/+ pups showed absence of claudin-1 in the lower epidermal layers and its presence suprabasally. The loss of claudin-1 from the basal layer and its maintenance in the granular layer suggests that another protein is involved in the specific phenotype of these mice (Fig. 4b). Tight junctions formed properly by claudin-1 and occludin stop the diffusion of biotin to the stratum corneum in the wild-type epidermis, but this was not the case in the Zeb2-expressing transgenic epidermis, where no occludin is present (Fig. 4a and b). Claudin-4, a known target of Zeb2 [26], was also absent from the transgenic epidermis (Suppl. Fig. S6a). Examination of other tight junction proteins, such as Claudin-3, did not reveal major alterations (Suppl. Fig. S5c), but a moderate disturbance of the pattern of ZO-1 was observed (Fig. 4b).
Detailed examination of adherens junctions and desmosomes in wild-type and Zeb2-expressing epidermis did not reveal major differences at the protein level. Both E-cadherin and β-catenin remain at the membranes of the cells in all epidermal layers and various desmosomal proteins are also properly localized (Suppl. Fig. S5a-d). Additionally, examination of differentiation markers including filaggrin, keratin-1, and keratin-14 indicated physiological differentiation of the epidermal cells in the transgenic epidermis despite its increased thickness (Suppl. Fig. S5e-f and Suppl. Fig. S6b). Additionally, lipid differentiation also occurs normally as examined by Nile Red staining, which did not reveal any difference between Zeb2-expressing and wild-type epidermis (Suppl. Fig. S6c).
ZEB2 directly regulates occludin in vitro
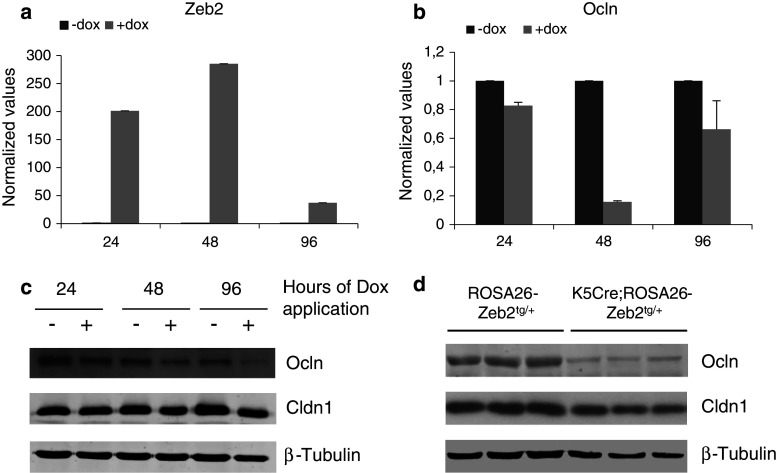
To verify our in vivo observations further, we used the human epidermoid carcinoma cell line A431 with Tet-On (doxycycline)-inducible ZEB2 expression (Fig. 5a) [26, 27]. Indeed, induction of ZEB2 expression for 24, 48, or 96 h led to a decrease in occludin mRNA and protein in these cells (Fig. 5b–c). In vivo, total occludin levels disappeared from the epidermis of P4.5 K5Cre;ROSA26-Zeb2 tg/+ neonates while total claudin-1 was only slightly decreased compared to their wild-type littermates (Fig. 5d). To examine whether the downregulation of claudin-1 in the basal layer was due to transgenic Zeb2 expression, primary keratinocytes from the epidermis of newborn wild-type and K5Cre;ROSA26-Zeb2 tg/+ mice were isolated and analyzed. Notably, Zeb2-expressing keratinocytes developed an EMT-morphology whereas their wild-type counterparts did not (Suppl. Fig. S7a). The presence of Zeb2 was confirmed at both the mRNA and protein levels (Suppl. Fig. S7b-c). Moreover, an inverse correlation between Zeb2 and claudin-1 levels was observed, while E-cadherin remained unaltered in both cell types (Suppl. Fig. S7b-c).
Fig. 5.
Analysis of the RNA and protein levels of occludin and claudin-1 in the inducible cell line A431-ZEB2 and in Zeb2-expressing epidermis. a, b Quantitative RT-PCR analysis of ZEB2 and occludin levels in the inducible cell line A431-ZEB2 upon doxycycline administration for different time points (24, 48, and 96 h). The quantitative data are normalized against the average of two reference genes and the bars represent relative levels compared to the non-induced cells. This analysis clearly shows that the upregulation of ZEB2 expression leads to a downregulation of occludin mRNA levels. c, d Western-blot analysis of the protein expression levels of occludin and claudin-1 in the A431-ZEB2 cell line (c) and in P4.5 K5Cre;ROSA26-Zeb2 tg/+ epidermis (d). This protein analysis confirms the qPCR data and suggests that the occludin downregulation is an effect of ZEB2 expression
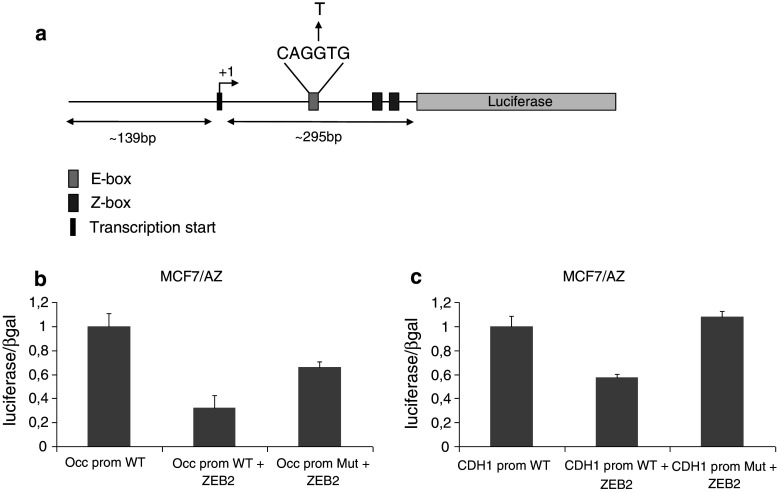
In an effort to examine whether the repression of occludin in the transgenic epidermis is a direct effect of ZEB2 activity on the occludin promoter, we performed transient transfection assays on human epithelial cells in vitro. The human occludin promoter contains two possible transcription initiation sites and one E-box (CAGGTG), which lies downstream of them (Fig. 6a) [28]. To confirm the activity of ZEB2 on the occludin promoter, we mutated the E-box and transfected both wild-type and mutant promoters in MCF7/AZ cells with a ZEB2-expressing vector. ZEB2 caused a nearly 70 % decrease in the activity of the occludin promoter, and use of the mutant promoter diminished this effect (Fig. 6b–c).
Fig. 6.
Analysis of the hOccludin promoter in MCF7/AZ cells. a Graphic overview of the human occludin promoter. The promoter fragment is approximately 440 bp and contains one of the two transcription initiation sites. The mutant construct carries the mutation of the E-box from CAGGTG to CAGTTG. b The ZEB2 expression plasmid was co-transfected in MCF7/AZ cells together with wild-type or mutant occludin promoter constructs. The graph depicts luciferase activity measured 48 h after transfection and the luciferase values are normalized against β-galactosidase expressed from a co-transfected plasmid. The values are scaled relative to the wild-type promoter activity. c The E-cadherin promoter was used as an experimental control. The bars represent the average of two independent transfection experiments in both b and c
Discussion
EMT is a transient dynamic process associated with both physiological situations such as embryonic development and wound healing, and pathological conditions, such as fibrosis and cancer progression [29]. Despite the extensive studies performed on ZEB family members, the functional consequences of their expression in adult epithelial tissues are not fully understood. Immunohistochemical analysis of ZEB2 in a series of human skin biopsies covering various skin pathologies revealed elevated ZEB2 expression levels only in the epidermis of pemphigus vulgaris samples. Pemphigus vulgaris is an autoimmune disease caused by antibodies directed primarily against the desmosomal proteins desmoglein-1 and -3 [22]. However, recent evidence showed that pemphigus sera contains also antibodies directed against other desmosomal and non-desmosomal proteins including E-cadherin, desmocollins, plakoglobin, desmoplakin, tight junctions, and collagen XII [22]. As such, pemphigus vulgaris is thought to result from synergistic effects of autoantibodies targeting different kinds of keratinocyte cell membrane antigens, however, downstream signaling contributing to the loss of intercellular connections is still poorly understood. The prominent presence of ZEB2 in the epidermis of pemphigus patients prompted us to study the functional consequences of Zeb2 expression in a transgenic mouse model, using two similar Cre-lines. Rosa26-Zeb2 expression in the epidermis following K5-Cre-based recombination leads to death a few days after birth due to excessive water loss through the skin. Examination of the epidermal barrier revealed that the neonates died because the inside-outside barrier was no longer functional, whereas the outside-inside barrier remained unaffected. The bidirectionality of the epidermal barrier depends on the proper formation of different parts of the epidermis. The maturation of the cornified layer is responsible for the functionality of the outside-inside barrier, whereas adherent and tight junctions regulate inside-outside skin permeability [8]. E-cadherin and other genes of cell–cell junctions are direct target genes of the ZEB transcription factors during EMT [20, 26]. Interestingly, in one in vivo model, epidermal deletion of E-cadherin leads to early neonatal death due to dehydration, a phenotype very similar to our model [10]. However, neither E-cadherin nor desmosomes were affected in the K5Cre;ROSA26-Zeb2 tg/+ epidermis compared to wild-type mice. Additional analysis for various differentiation markers (K14, K1, Filaggrin) and lipid differentiation (Nile Red) did not reveal major differences between wild-type and Zeb2-expressing epidermis. Similarly, E-cadherin downregulation in a K14-Snail in vivo model was not observed until the malignant conversion of the epithelial cells had taken place [30], suggesting that in some cases, an additional oncogenic event is necessary to observe the full spectrum of EMT.
Analysis of the tight junction proteins in the K5Cre;ROSA26-Zeb2 tg/+ epidermis revealed a disturbance in the patterns of claudin-1, claudin-4, and ZO-1. Mice deficient for claudin-1 die soon after birth due to massive transepidermal water loss (TEWL) [8]. Mice deficient for claudin-4 show urogenital defects, however their epidermal barrier function remains intact [31]. Occludin, which is also important for formation of the tight junctions in the epidermis, was completely absent in K5Cre;ROSA26-Zeb2 tg/+ epidermis. Mice lacking only occludin do not show any skin defects [32]; these mice are born in expected Mendelian ratios but exhibit several other defects, including chronic inflammation, hyperplasia of the gastric epithelium, calcification of the brain, and abnormalities in testis, salivary glands and bones. Nonetheless, the barrier function of the intestinal epithelium is normal and the tight junctions are present despite the complete absence of occludin [32]. Taken together these data support the hypothesis that the observed phenotype of our K5Cre;ROSA26-Zeb2 tg/+ mice is the result of the targeting of multiple members of the tight junction proteins. Indeed, when we overexpressed occludin in the epidermoid cell line A431 with inducible ZEB2 expression we could not rescue the loss of transepithelial resistance (TER) associated with ZEB2 expression (data not shown).
Further in vitro analysis showed that the occludin promoter activity is repressed by the binding of ZEB2 to the E-box located downstream of the transcription initiation site. This repression is alleviated when the E-box is mutated, supporting the notion that the relationship between ZEB2 and occludin expression is a direct one. Nevertheless, the occludin promoter also contains two Z-boxes (CACCT) downstream of the E-box, which might also serve as binding sites for the ZEB factors [33] and could be responsible for the incomplete restoration of the promoter activity levels to the wild-type situation. A second transcript of occludin that results from alternative splicing has also been reported, but its role in skin and other tissues has not yet been investigated in detail [34]. Other well-studied EMT-inducing transcription factors, such as Snail and Slug, have also been shown to downregulate occludin during EMT [19, 35]. Our data show that the combined down-regulation of claudin-1, claudin-4, and occludin contribute to the phenotype of the K5Cre;ROSA26-Zeb2 tg/+ mice. This result indicates that the epidermal barrier requires both the integrity and the interaction of different proteins in complex functional networks. Likewise, a recent report about the combined loss of E- and P-cadherins in the epidermis revealed different phenotypes and new roles in tissue physiology resulting from the participation of these proteins in highly complex networks, which could not be seen in the single-molecule studies [13]. Disturbance in the expression levels or the distribution of tight junction proteins has been associated with various skin disorders. Reduced levels of claudin-1 have been associated with atopic dermatitis and early stage but not late psoriasis [36, 37]. Also, premature stop in protein synthesis of claudin-1 leads to a syndromic form of ichthyosis in humans [38], while reduced levels of claudin-1 have also been correlated with breast cancer recurrence [39]. With respect to occludin, although reduced levels have been associated with various diseases including cancer [40], there is to our knowledge no direct link with a skin disorder to this day. In our analysis we could clearly show a reduction of claudin-1 in the basal layer of the epidermis from all pemphigus vulgaris patients we examined irrespective of ZEB2 presence (Suppl. Fig. S8).
Here we hypothesize that although the widespread loss of the epidermal barrier that occurs in skin diseases like pemphigus vulgaris and pemphigus foliaceus might be triggered by antibodies against several adhesion proteins of the epidermis, it may be further propagated by intracellular signaling mediated by ZEB2. Interestingly, the specific loss of an adhesion molecule like E-cadherin can result in the upregulation of EMT-inducing transcription factors like Twist and ZEB1, which in turn drive epithelial dedifferentiation [41]. This suggests that in pemphigus vulgaris antibodies against conditionally pathogenic antigens can initiate the disease, which is then further facilitated by the upregulation of a transcription factor like ZEB2. Further research is underway to link ZEB2 upregulation in the epidermis as an alternative mechanism to explain the apparent absence of autoantibodies in a subclass of pemphigus patients. Our conditional mouse model is a valuable tool for studying the contribution of ZEB2 under the scope of other diseases such as epithelial cancer progression, as well as other tissues with a known role for ZEB2 such as embryonic myelination in the nervous system [42] and embryonic hematopoiesis [43].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We acknowledge Dr. Amin Bredan for critical reading of the manuscript and the members of our research group for valuable discussions. We would also like to thank Prof. Dr. J. Ikenouchi (Kyoto University, Dept. of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering) for kindly providing us with the human occludin promoter construct. This research was funded by grants from V.I.B.-International PhD Program in Life Sciences, the FWO, the geconcerteerde onderzoeksacties of Ghent University, the Stichting tegen Kanker and the EU-FP7 framework program TuMIC 2008-201662.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shook D, Keller R. Mechanisms, mechanics and function of epithelial–mesenchymal transitions in early development. Mech Dev. 2003;120(11):1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Nieto MA. The ins and outs of the epithelial-to-mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95(1):333–339. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 7.Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17(12):1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 8.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156(6):1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinkle CL, Lechler T, Pasolli HA, Fuchs E. Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc Natl Acad Sci USA. 2004;101(2):552–557. doi: 10.1073/pnas.0307437100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. Embo J. 2005;24(6):1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol. 2001;3(12):1076–1085. doi: 10.1038/ncb1201-1076. [DOI] [PubMed] [Google Scholar]
- 12.Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000;11(4):383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- 13.Tinkle CL, Pasolli HA, Stokes N, Fuchs E. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc Natl Acad Sci USA. 2008;105(40):15405–15410. doi: 10.1073/pnas.0807374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savagner P, Kusewitt DF, Carver EA, Magnino F, Choi C, Gridley T, Hudson LG. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol. 2005;202(3):858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- 15.Arnoux V, Nassour M, L’Helgoualc’h A, Hipskind RA, Savagner P. Erk5 controls Slug expression and keratinocyte activation during wound healing. Mol Biol Cell. 2008;19(11):4738–4749. doi: 10.1091/mbc.E07-10-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du F, Nakamura Y, Tan TL, Lee P, Lee R, Yu B, Jamora C. Expression of snail in epidermal keratinocytes promotes cutaneous inflammation and hyperplasia conducive to tumor formation. Cancer Res. 2010;70(24):10080–10089. doi: 10.1158/0008-5472.CAN-10-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardman MJ, Sisi P, Banbury DN, Byrne C. Patterned acquisition of skin barrier function during development. Development. 1998;125(8):1541–1552. doi: 10.1242/dev.125.8.1541. [DOI] [PubMed] [Google Scholar]
- 18.Thomason HA, Scothern A, McHarg S, Garrod DR. Desmosomes: adhesive strength and signalling in health and disease. Biochem J. 2010;429(3):419–433. doi: 10.1042/BJ20100567. [DOI] [PubMed] [Google Scholar]
- 19.Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116(Pt 10):1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- 20.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7(6):1267–1278. doi: 10.1016/S1097-2765(01)00260-X. [DOI] [PubMed] [Google Scholar]
- 21.Verschueren K, Remacle JE, Collart C, Kraft H, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT, Bodmer R, Smith JC, Huylebroeck D. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5′-CACCT sequences in candidate target genes. J Biol Chem. 1999;274(29):20489–20498. doi: 10.1074/jbc.274.29.20489. [DOI] [PubMed] [Google Scholar]
- 22.Cirillo N, Cozzani E, Carrozzo M, Grando SA. Urban legends: pemphigus vulgaris. Oral Dis. 2012;18(5):442–458. doi: 10.1111/j.1601-0825.2011.01899.x. [DOI] [PubMed] [Google Scholar]
- 23.Nyabi O, Naessens M, Haigh K, Gembarska A, Goossens S, Maetens M, De Clercq S, Drogat B, Haenebalcke L, Bartunkova S, De Vos I, De Craene B, Karimi M, Berx G, Nagy A, Hilson P, Marine JC, Haigh JJ. Efficient mouse transgenesis using Gateway-compatible ROSA26 locus targeting vectors and F1 hybrid ES cells. Nucleic Acids Res. 2009;37(7):e55. doi: 10.1093/nar/gkp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, Peterse JL, Cardiff RD, Berns A, Jonkers J. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10(5):437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez A, Page A, Gandarillas A, Zanet J, Pibre S, Vidal M, Tusell L, Genesca A, Whitaker DA, Melton DW, Jorcano JL. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39(1):52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- 26.Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van Roy F, Berx G. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell–cell junctions. Nucleic Acids Res. 2005;33(20):6566–6578. doi: 10.1093/nar/gki965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mejlvang J, Kriajevska M, Vandewalle C, Chernova T, Sayan AE, Berx G, Mellon JK, Tulchinsky E. Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell. 2007;18(11):4615–4624. doi: 10.1091/mbc.E07-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 2000;113(Pt 11):2085–2090. doi: 10.1242/jcs.113.11.2085. [DOI] [PubMed] [Google Scholar]
- 29.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Craene B, Denecker G, Vermassen P, Taminau J, Mauch C, Derore A, Jonkers J, Fuchs E, Berx G. Epidermal Snail expression drives skin cancer initiation and progression through enhanced cytoprotection, epidermal stem/progenitor cell expansion and enhanced metastatic potential. Cell Death Differ. 2014;21(2):310–320. doi: 10.1038/cdd.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita H, Hamazaki Y, Noda Y, Oshima M, Minato N. Claudin-4 deficiency results in urothelial hyperplasia and lethal hydronephrosis. PLoS One. 2012;7(12):e52272. doi: 10.1371/journal.pone.0052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11(12):4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spaderna S, Schmalhofer O, Hlubek F, Berx G, Eger A, Merkel S, Jung A, Kirchner T, Brabletz T. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology. 2006;131(3):830–840. doi: 10.1053/j.gastro.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Muresan Z, Paul DL, Goodenough DA. Occludin 1B, a variant of the tight junction protein occludin. Mol Biol Cell. 2000;11(2):627–634. doi: 10.1091/mbc.11.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Zheng M, Liu G, Xia W, McKeown-Longo PJ, Hung MC, Zhao J. Kruppel-like factor 8 induces epithelial-to-mesenchymal transition and epithelial cell invasion. Cancer Res. 2007;67(15):7184–7193. doi: 10.1158/0008-5472.CAN-06-4729. [DOI] [PubMed] [Google Scholar]
- 36.De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, Berger AE, Zhang K, Vidyasagar S, Yoshida T, Boguniewicz M, Hata T, Schneider LC, Hanifin JM, Gallo RL, Novak N, Weidinger S, Beaty TH, Leung DY, Barnes KC, Beck LA. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127(3):773–786. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirschner N, Poetzl C, Von den Driesch P, Wladykowski E, Moll I, Behne MJ, Brandner JM. Alteration of tight junction proteins is an early event in psoriasis: putative involvement of proinflammatory cytokines. Am J Pathol. 2009;175(3):1095–1106. doi: 10.2353/ajpath.2009.080973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grosse B, Cassio D, Yousef N, Bernardo C, Jacquemin E, Gonzales E. Claudin-1 involved in neonatal ichthyosis sclerosing cholangitis syndrome regulates hepatic paracellular permeability. Hepatology. 2012;55(4):1249–1259. doi: 10.1002/hep.24761. [DOI] [PubMed] [Google Scholar]
- 39.Morohashi S, Kusumi T, Sato F, Odagiri H, Chiba H, Yoshihara S, Hakamada K, Sasaki M, Kijima H. Decreased expression of claudin-1 correlates with recurrence status in breast cancer. Int J Mol Med. 2007;20(2):139–143. [PubMed] [Google Scholar]
- 40.Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57(6):883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 42.Weng Q, Chen Y, Wang H, Xu X, Yang B, He Q, Shou W, Higashi Y, van den Berghe V, Seuntjens E, Kernie SG, Bukshpun P, Sherr EH, Huylebroeck D, Lu QR. Dual-mode modulation of Smad signaling by Smad-interacting protein Sip1 is required for myelination in the central nervous system. Neuron. 2012;73(4):713–728. doi: 10.1016/j.neuron.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goossens S, Janzen V, Bartunkova S, Yokomizo T, Drogat B, Crisan M, Haigh K, Seuntjens E, Umans L, Riedt T, Bogaert P, Haenebalcke L, Berx G, Dzierzak E, Huylebroeck D, Haigh JJ. The EMT regulator Zeb2/Sip1 is essential for murine embryonic hematopoietic stem/progenitor cell differentiation and mobilization. Blood. 2011;117(21):5620–5630. doi: 10.1182/blood-2010-08-300236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.