Abstract
p75NTR, the common receptor for both neurotrophins and proneurotrophins, has been widely studied because of its role in many tissues, including the nervous system. More recently, a close relationship between p75NTR expression and pluripotency has been described. p75NTR was shown to be expressed in various types of stem cells and has been used to prospectively isolate stem cells with different degrees of potency. Here, we give an overview of the current knowledge on p75NTR in stem cells, ranging from embryonic to adult stem cells, and cancer stem cells. In an attempt to address its potential role in the control of stem cell biology, the molecular mechanisms underlying p75NTR signaling in different models are also highlighted. p75NTR-mediated functions include survival, apoptosis, migration, and differentiation, and depend on cell type, (pro)neurotrophin binding, interacting transmembrane co-receptors expression, intracellular adaptor molecule availability, and post-translational modifications, such as regulated proteolytic processing. It is therefore conceivable that p75NTR can modulate cell-fate decisions through its highly ramified signaling pathways. Thus, elucidating the potential implications of p75NTR activity as well as the underlying molecular mechanisms of p75NTR will shed new light on the biology of both normal and cancer stem cells.
Keywords: p75NTR, Neurotrophins, Signaling pathways, Stem cells, Cancer stem cells
Introduction
Since the discovery of pluripotent embryonic stem cells (ESCs), first isolated in 1981 [1], the ability of these cells to differentiate into three germ layers (ectoderm, mesoderm, and endoderm) and then into fully specialized cells [2] has opened many enticing perspectives in tissue regeneration and cell therapy. The comprehension of stem cell biology has become even more important with the discovery of cancer stem cells (CSCs) and their fundamental role in tumor development [3]. ESCs, adult stem cells, and CSCs share common features. They are all long-lived cells with the ability to renew through mitotic cell divisions and to differentiate into more specialized cell types. However, the stem cells are quite different in terms of potency, ranging from pluripotency in ESCs to multipotency, bipotency, and unipotency, with increasing degrees of commitment of transit amplifying/progenitor cells. Nevertheless, with the exception of hematopoietic stem cells, specific markers have not yet been identified. Studies of specific cell-surface markers are essential for distinguishing ESCs, adult stem cells, and CSCs from their destined-to-differentiate transit amplifying daughters to better understand mechanisms governing stem cell renewal and differentiation.
Among increasing prospective stem cell markers, the p75 neurotrophin receptor (p75NTR, also known as NGFR or CD271) enriches the stem/progenitor subset in several models, presenting varying degrees of potency. p75NTR has been widely described for its signaling role as the common receptor for neurotrophins and proneurotrophins and, as such, p75NTR can exert a plethora of functions according to cell context [4, 5]. The aim of this review is to overview what is known about p75NTR in stem cell biology. Based upon the well-known and diverse signaling functions of p75NTR, we also attempt to address its potential role in the control of stem cell proliferation and differentiation.
Diverse functions of p75NTR, the common receptor for neurotrophins and proneurotrophins
Since p75NTR was identified as a bona fide neural crest stem cell (NCSC) marker [6], it has been widely used to isolate putative stem cells from neural crest-derived tissues and its involvement in mesenchymal stem cell (MSC) differentiation along the osteogenic, adipogenic, chondrogenic, and myogenic lineages has been exploited. Nonetheless, p75NTR expression and function in vivo, as well as its underlying mechanisms in stem cell biology, have not yet been sufficiently addressed. In this chapter, we will briefly overview what is known about p75NTR-mediated signaling, which may give us a clue about the role of p75NTR in stem cells.
p75NTR is the common receptor for all neurotrophins, which includes nerve growth factor (NGF), brain-derived neutrophic factor (BDNF), neurotrophin 3 (NT-3), and neurotrophin 4/5 (NT-4/5). Neurotrophins are generated from the enzymatic processing of their precursors (proneurotrophins). More recently, p75NTR has been reported to mediate the biological effects of proneurotrophins in several types of cells [7–9]. Nevertheless, proneurotrophins’ in vivo activities still have to be demonstrated.
p75NTR is a 427-amino-acid transmembrane receptor containing an extracellular stalk domain, a single transmembrane domain, and a cytoplasmic domain. The presence of four cysteine-rich domains in the extracellular part of p75NTR affiliates it with the TNF receptor superfamily and is responsible for receptor conformation and ligand binding [10]. An N-glycosylation site and several O-glycosylation sites in the extracellular domain are implicated in the membrane targeting of the protein as well as in ligand binding [11]. The transmembrane domain consists of a unique helix, where the highly conserved cysteine C257 plays an important role in receptor dimerization, in conformational changes induced by ligand binding and in signal transduction [12]. The intracellular domain, which is highly conserved between species, does not have intrinsic catalytic activity, and it owes its signaling ability to its association with cytoplasmic partners through different regions. Three regions within the intracellular domain are important for p75NTR activity: (i) the chopper and (ii) death domain, the activation of which induces apoptosis [13], and (iii) the conserved SPV (tripeptide serine-proline-valine), which is a consensus sequence for Post-synaptic Disc-large Zona protein binding domains. The associated partners allow for assembly of protein complexes by acting as signaling platforms. The p75NTR intracellular domain is also subject to several post-translational modifications, such as palmitoylation [14] and phosphorylation, at several amino acid residues. For more specific details about p75NTR, extensive descriptions of its structure and processing are reviewed in [15, 16].
p75NTR signal transduction pathways are extremely variable because they are strongly dependent on cell type, cell differentiation status, neurotrophin binding, availability of intracellular adaptor molecule availability, and interacting transmembrane co-receptors and post-translational modification expression [17]. This leads to divergent cellular responses, including cell survival [18], apoptosis [13, 19], neurite outgrowth and retraction [20], myelination [21], cell cycle regulation [22], cell migration and invasion [23, 24], and progenitor differentiation [25] (Fig. 1).
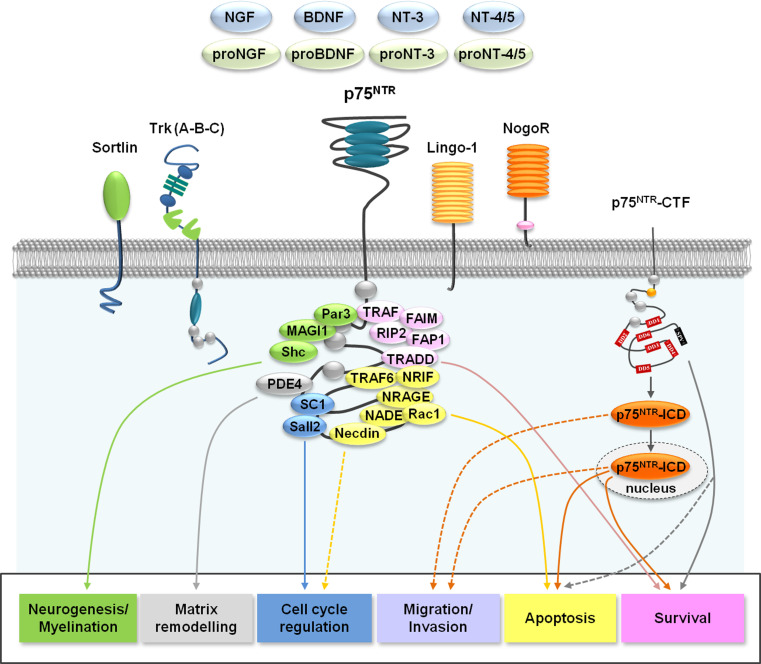
Fig. 1.
Schematic overview of p75NTR interactions. p75NTR can bind all neurotrophins and proneurotrophins, can dimerize, and can interact directly with Trk receptors, sortilin, Nogo-receptor, and Lingo1. The recruitment of intracellular proteins by p75NTR activates downstream signaling cascades, leading to different biological responses. p75NTR cleavage may also induce signaling mediated by the C-terminal fragment (CTF) or the intracellular fragment (ICD) of the receptor, generally leading to cell survival or apoptosis. ICD may be translocated to the nucleus to mediate its cellular responses (dotted arrows indicate that the exact molecular mechanisms leading to migration/invasion are unknown). In yellow are intracellular partners leading to pro-apoptotic signaling: NRAGE neurotrophin receptor-interacting MAGE homolog, NADE p75NTR-associated cell death executor, NRIF neurotrophin receptor interacting factor, Rac1 Ras-related C3 botulinum toxin substrate 1, TRAF TNF receptor-associated factor. In pink are partners leading to pro-survival signaling: RIP2 receptor-interacting protein 2, FAP1 Fas-associated protein 1, FAIM Fas apoptosis inhibitor molecule, TRADD TNF receptor-associated death domain protein. In blue are partners leading to cell cycle arrest: SC1 Schwann cell factor-1, Sall2 Sal-like 2; Necdin. In green are partners related to neurogenesis and myelination: Par3 protease activated receptor 3, MAGI-1 membrane-associated guanylate kinase with inverted organization; Shc. In gray, PDE4 phosphodiesterase type 4, leading to cAMP degradation and matrix remodeling in Schwann cells
Influence of co-receptors on p75NTR signaling
Formation of p75NTR dimers has a strong regulatory effect on the activation of receptor signaling [12, 26]. Nevertheless, different biological effects of p75NTR can be explained by the ability of p75NTR to cooperate with other receptors to form multimeric/heteromeric complexes. Indeed, apart from its interaction with specific tyrosine kinase receptors of neurotrophins (TrkA for NGF, TrkB for BDNF and NT-4/5, TrkC for NT-3), p75NTR participates in several signaling platforms by interacting with an increasing list of co-receptors, including sortilin (SORT1), Nogo receptor (NogoR), and LINGO-1 [27, 28] (Fig. 1). Interactions with co-receptors seem to be dependent on p75NTR cellular localization, the state of cellular differentiation, and its post-translational modifications [17].
p75NTR and Trk receptors can interact both in synergistic or antagonistic manners, and their association or mutual control has been extensively investigated [29]. The formation of a p75NTR/Trk complex was shown to facilitate the affinity and selectivity of each neurotrophin for its Trk receptor (k d = 10−11 M), most likely by the induction of conformational changes in its intracellular and extracellular domains and exposing a high affinity site for association with neurotrophins [30]. Recently, a direct interaction between p75NTR and TrkA has been demonstrated, even in the absence of NGF [31], and the possibility cannot be excluded that other proteins may be associated with this complex.
The p75NTR/sortilin complex is known to induce cell death following proneurotrophin binding [32, 33]. Signaling pathways connected to the ternary complex proNT/p75NTR/sortilin are poorly described. The cytoplasmic tail of sortilin has the potential to recruit specific protein partners to induce its own signaling and/or to facilitate p75NTR-mediated signals.
Finally, the trimeric complex with NogoR and LINGO-1 receptors is known to bind to Nogo-66, myelin-associated glycoprotein (MAG), or oligodendrocyte myelin glycoprotein to inhibit neurite growth by activating RhoA [34–36].
p75NTR signaling by the recruitment of intracellular partners
p75NTR, like other members of the TNFR superfamily, does not have intrinsic enzymatic activity, and it owes its signaling to the recruitment of intracellular binding proteins, leading to the activation of different signaling pathways. These signaling pathways have been predominately established in neuronal models and in rat PC12 (pheochromocytoma) cells where, depending on the cellular context, they mediate survival, apoptosis, cell cycle arrest, myelination, or neurogenesis. There is a wide array of proteins that have been demonstrated to interact with the intracellular domain of p75NTR (Fig. 1). These include:
Neurotrophin receptor-interacting MAGE homolog (NRAGE) [37], NADE (p75NTR-associated cell death executor) [38], neurotrophin receptor interacting factor (NRIF) [39], Ras-related C3 botulinum toxin substrate 1 (Rac1) and TNF receptor-associated factor 6 [40], leading to pro-apoptotic signaling mainly through activation of the JNK pathway;
TRAFs [41], receptor interacting protein 2 (RIP2) [42], Fas-associated protein 1 (FAP1) [43], Fas apoptosis inhibitor molecule (FAIM) [44] and TNF receptor-associated death domain protein (TRADD) [45], leading to pro-survival signaling through the activation of the nuclear factor-κB (NF-κB) transcription factor;
Schwann cell factor-1 (SC1), Sal-like 2 (Sall2), and Necdin, leading to cell cycle arrest [22];
Protease-activated receptor 3 (Par3), which is implicated in Schwann cell myelination [46];
Phosphodiesterase type 4 (PDE4), leading to cAMP degradation and matrix remodeling in Schwann cells [47];
Membrane-associated guanylate kinase with inverted organization (MAGI-1) [48] and Shc [49], both of which are involved in neurite extension.
p75NTR proteolytic processing
The regulated proteolysis of p75NTR has largely been described in neurons. p75NTR undergoes extracellular cleavage by the metalloproteases ADAM17/TACE, releasing the ectodomain of the receptor to form a 28-kDa membrane-bound C-terminal fragment (p75-CTF) (Fig. 1). The p75-CTF is subsequently cleaved within the transmembrane domain by γ-secretase and gives rise to the soluble p75 intracellular domain (p75-ICD). The p75-ICD has been reported to be involved in cell death [50] or the survival [51] of neurons and in glioma cell invasion [52]. Whether these cleavages are regulated by neurotrophin binding or by co-receptors as well as their connection to p75NTR signaling are still a source of debate. It has been reported that in PC12 and HEK293 cell lines, TrkA activation increases p75NTR cleavage by ADAM17 [53, 54]. Several studies documented nuclear translocation of the p75NTR-ICD fragment, suggesting a direct or indirect transcriptional activity of the receptor [55]. Although in the majority of cases p75NTR-CTF was described as a transient form without signaling functions, this fragment has been more recently shown to be involved in the survival of breast cancer cells [56] and in promoting cell death in neurons when over-expressed in a form that cannot be cleaved to generate the ICD [50].
Despite the diversity of p75NTR signaling in adult cells, no specific signaling has been documented in a stem cell context. However, we can assume that in the case of stem cells, p75NTR is likely to function in a cell-context-dependent manner. Moreover, p75NTR activation has been shown to crosstalk with canonic signaling pathways involved in stem cell phenotypes, such as Notch and Wnt pathways. Indeed, NGF/p75NTR activation was described to be able to modulate the expression of hes1/5, which are target genes of the Notch signaling pathway, through NF-κB activation [57]. During axon regeneration, NGF regulates the activities of GSK-3β and ILK, in addition to PI3K and Akt [58]. By inactivating GSK-3β, NGF participates in the activation of the Wnt pathway, allowing for the stabilization of APC and β-catenin [59]. Moreover, Trk receptors directly phosphorylate β-catenin at the Y142 upon neurotrophin binding, driving β-catenin translocation to the nucleus [60]. Through β-catenin translocation, Wnt factors and NTs may regulate stem cell fate decisions by activating T cell-factor/lymphoid-enhancing-factor-driven gene transcription [61]. BDNF, for instance, seems to contribute to proliferation and neuronal and oligodendrocytic differentiation of NSCs in vitro by triggering the Wnt/β-catenin signaling pathway [62].
Expression and potential roles of p75NTR in stem cells
Increasing amounts of data describe the expression of p75NTR in both stem cells and well-differentiated cells. In many cases, p75NTR has been used solely or in combination to identify stem/progenitor subsets with varying degrees of commitment, progressing from embryonic to adult tissues. In this chapter, we attempt to perform an up-to-date overview of both the expression and potential roles of p75NTR in stem cells according to their potency and germ layer origin (Table 1).
Table 1.
p75NTR expression and functions in stem cells
| Cell type | Model | Plasticity | Origin | Ligand | Co-receptor | Functional outcome | Reference |
|---|---|---|---|---|---|---|---|
| Embryonic SC | Mouse | Totipotent | Blastocyst | NGF | TrkA | Proliferation | [65] |
| Human | Totipotent | Blastocyst | TrkC | Survival | [63, 64] | ||
| Neural crest | Human | Pluripotent | Neural crest | [67] | |||
| Esophageal keratinocytes SC | Human | Multipotent | Ectoderm | [136] | |||
| Laryngeal squamous SC | Human | Multipotent | Ectoderm | [81] | |||
| Epidermal keratinocytes | Human | Multipotent | Ectoderm | [82] | |||
| Hair follicle keratinocyte SC | Mouse | Multipotent | Ectoderm | Apoptosis | [85] | ||
| Corneal stromal and epithelial SC | Mouse | Multipotent | Neural crest | [83] | |||
| Oral mucosa SC | Human | Multipotent | Ectoderm | [84] | |||
| Dorsal root ganglion SC | Rat | Multipotent | Neural crest | NogoR | [76] | ||
| Gut SC | Human | Multipotent | Neural crest | [77] | |||
| Sciatic nerve SC | Rat | Multipotent | Neural crest | [72] | |||
| Enteric neural SC | Human, rodents | Multipotent | Neural crest | NT-3 | TrkC | Survival/multipotency | [78, 79] |
| Dental pulp SC | Human | Multipotent | Ectoderm | NGF | multipotency/differentiation | [90] | |
| CNS neural SC | Human, RAT | Multipotent | Ectoderm | NGF, NT-3, BDNF, proNGF | TrkC | Proliferation/multipotency/differentiation | [97–99] |
| Mesenchymal SC | Human, mouse | Pluripotent/multipotent | Mesoderm | NogoR, sortilin | [65] | ||
| Bone-marrow SC | Human | Multipotent | Meso ectoderm | NGF | [107] | ||
| Adipose SC | Human, mouse | Multipotent | Mesoderm | [109] | |||
| Testis SC | Human, mouse, rat | Multipotent | Mesoderm | NGF, NT-3 | TrkB | Differentiation | [154] |
| Skeletal muscle SC | Human | Multipotent | Mesoderm | BDNF | Differentiation | [115] | |
| Trachea epithelium SC | Mouse | Multipotent | Endoderm | NT-3 | [117] | ||
| Hepatic stellate SC | Human, rat | Multipotent | Endoderm | Differentiation | [122] | ||
| Melanoma CSC | Human | Multipotent | Ectoderm | NGF | Selfrenewal/multipotency | [128] | |
| Oral squamous CSC | Human | Multipotent | Ectoderm | [133] | |||
| Esophageal squamous CSC | Human | Multipotent | Ectoderm | [136] | |||
| Breast CSC | Human | Multipotent | Ectoderm | [146] | |||
| Hypopharyngeal CSC | Human | Multipotent | Ectoderm | [148] | |||
| Neuroblastoma CSC | Human | Multipotent | Ectoderm | [149] |
p75NTR expression has been found in numerous types of stem cells. In some cases, neurotrophins have been reported to be functionally implicated as indicated in the table. However, the precise functions of p75NTR in stem cells clearly remain to be studied
p75NTR in embryonic stem cells
Embryonic stem cells are pluripotent stem cells originating from the inner mass of the blastocyst that have the dual ability to self-renew and to differentiate into all cell types within the embryo and the adult (Fig. 2). Some evidence concerning the role of p75NTR in human ESCs remains controversial. Schuldiner et al. [63] found that p75NTR is expressed in a human ESC line derived from human blastocysts, and its mRNA is down-regulated upon differentiation in monolayer culture. In the same cells, Pyle found only a transient or low expression of this receptor and supported the idea that BDNF, NT-3, and NT-4/5 neurotrophins sustain hESC survival through their binding to TrkB and TrkC receptors [64]. Nevertheless, whether the low amount of p75NTR detected in these cells might be implicated in the activation of TRK signaling pathways or in the neurotrophin response was not assessed, leaving the role of p75NTR unresolved.
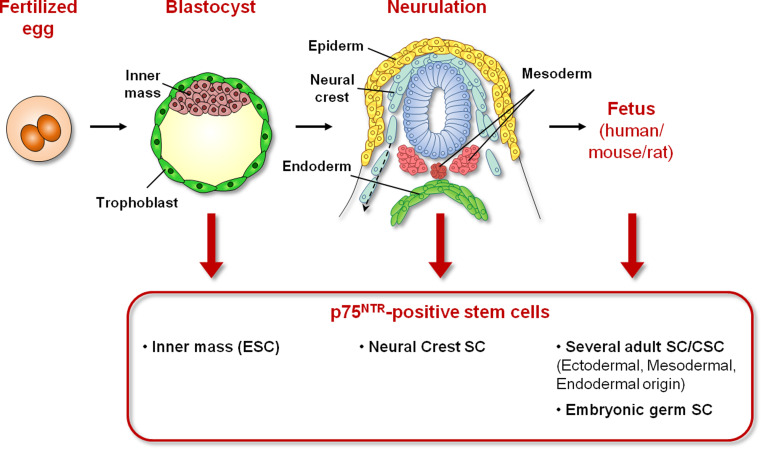
Fig. 2.
p75NTR-positive stem cells are present in many cellular models with different degrees of commitment. The fusion of gametes and formation of a diploid zygote determines the establishment of a multicellular embryo. Cells from the inner cell mass of the blastocyst (ESCs) present p75NTR transcripts. p75NTR-positive cells are present in multipotent migrating NCSCs (represented here during the neurulation stage) as well as in many fetal and post-natal tissues. It is worth noting that cell types other than neural crest-derived tissues present a subset of p75NTR-positive stem cells, demonstrating a more primitive origin of these cells
p75NTR was clearly found in the mouse embryo at the early blastocyst stage (3.5 days post-coitum, dpc) in Oct4-positive cells as well as in mouse ES cell lines [65]. Its expression persists in the inner cell mass of the blastocyst (4.5 dpc), while p75NTR transcripts are not present in trophoblast cells [66]. In particular, p75NTR is associated with mouse ESC proliferation upon NGF treatment through its interaction with the TrkA receptor [65]. Interestingly, primordial germ cells (unipotent stem cells) isolated from 11.5 dpc mouse gonads are found to be p75NTR-negative but become p75NTR-positive when dedifferentiated into pluripotent embryonic germ stem cells in vitro [65], suggesting that p75NTR is expressed by more primitive stem cells located high in the stem cell hierarchy.
Nevertheless, the close relationship between p75NTR expression and pluripotency thus far described indicates that neurotrophin signaling may be a key regulator of proliferation and survival in ESCs. Further studies are clearly needed to understand the precise dynamics of neurotrophin/p75NTR actions and the underlying mechanisms.
p75NTR is a robust marker of neural crest SCs
It has been clearly established that p75NTR is a robust marker of NCSCs, as p75NTR has been successfully used to isolate NCSCs from fetal and adult tissues [67]. The neural crest is a transient population of multipotent stem cells arising at the lateral edge of the dorsal neural tube in vertebrates [68] (Fig. 2). These cells migrate extensively into the embryo before aggregating to form a vast array of cell types, including the neurons and glia of the peripheral nervous system, endocrine cells in the adrenal and thyroid glands, melanocytes, craniofacial cartilage and skeletal cells, among others [69–71].
Jiang et al. were able to generate functional NCSCs in vitro by FACS-sorting p75NTR-positive cells from human ESCs whose differentiation was induced by stromal fibroblasts. These p75NTR-positive cells readily form neurospheres in suspension culture, self-renew to form secondary spheres, and give rise to multiple neural crest lineages, including peripheral nerves, glial cells, and myofibroblastic cells. Importantly, these cells migrate and differentiate into neural crest derivatives when transplanted into developing chick embryos in vivo, demonstrating functional NCSC properties [6].
The persistence of p75NTR staining in stem cells originating from NCSCs in vivo was evaluated in E14.5 rat sciatic nerve tissue. Starting from post-migratory neural crest cells of the fetal peripheral nerve (which is thought to contain only glial precursors), a p75NTR +/P0- (a peripheral myelin protein) fraction was identified as highly enriched in cells functionally indistinguishable from NCSCs in vitro. This subpopulation self-renewed in clonal assays and generated neurons, Schwann cells, and smooth muscle-like myofibroblasts [68, 72].
NCSC migration and fate are driven by environmental signals. For instance, the maintenance of an undifferentiated state as well as the persistent expression of p75NTR in NCSC are both supported by the combinatorial Wnt/bone morphogenic protein pathways [73, 74]. Neurotrophins act in combination with different factors to mediate different fates. Indeed, NGF, BDNF, or NT-3 act in concert with stem cell factor (SCF) to mediate cell death and melanocytic lineage through the p75NTR receptor, while the combination of FGF-2 and NT3 promotes expression of sympathetic neuroblast markers, and SCF and BDNF are involved in directing neural crest cells into a sensory neuron lineage [75]. These data emphasize the importance of the concerted action of neurotrophins and p75NTR in NCSC proliferation, survival, and differentiation. Based on the differentiation process, it cannot be excluded that neurotrophins may participate in NCSC migration and homing of p75NTR-positive cells as well as in their specific differentiation once stem cells have reached their destination.
p75NTR in adult stem cells
Adult stem cells are organ- or tissue-specific stem cells. These stem cells may be multipotent, bipotent, or unipotent, and maintain continuous cellular turnover to provide regenerative capacity in continually renewing tissues and reparative capacity in post-mitotic tissues. In the adult, the presence of p75NTR characterizes various stem/progenitor cell types, such as bone marrow stem cells; muscle stem cells (satellite cells); liver stem cells (stellate cells); keratinocytes of the basal layer of the epidermis, of the corneal limbal epithelium and of squamous epithelia; and stem cells of the oral and esophageal mucosa. In general, p75NTR is considered to be the most specific marker of MSCs, which are endowed with adipogenic, osteogenic, and chondrogenic potential [65], and of stem cells of all neural crest-derived tissues [71] (Fig. 2).
Ectodermal origin
Several tissues originating from migratory NCSCs through a series of progressive restrictions in developmental fate have been shown to maintain a number of multipotent/bipotent undifferentiated cells bearing p75NTR expression. For instance, stem cells from dorsal root ganglion [76], adult gut [77], and sciatic nerve [72] have a subpopulation of p75NTR-positive cells, displaying multipotency and sphere forming potential. Enteric neural stem cells isolated from the myoenteric plexus of both rodents and humans were shown to contain a rather homogeneous population of neural crest-derived cells that exhibit high proliferation, low apoptosis, and high expression of p75NTR, as well as expression of neuronal precursor markers including Nestin, Ret, and Sox10 [78]. Differentiation of these cells in the enteric nervous system is driven by a combination of NT-3 and other neutrophic factors, through the up-regulation of TrkC and the concomitant down-regulation of p75NTR [79]. We can therefore infer that in these cells p75NTR may maintain the undifferentiated phenotype and survival of stem cells.
A p75NTR-positive cell fraction has been found to be a useful stem/progenitor cell marker in many regenerative epithelia, such as esophageal keratinocytes [80], laryngeal squamous epithelial cells [81], and oral [25], epidermal [82], and corneal keratinocytes [83]. p75NTR expression is mainly restricted to the basal cell layer where keratinocyte stem cells are thought to reside [25]. In epithelial cells, p75NTR distinguished a relatively immature keratinocyte subset, slow-cycling in vivo and presenting a strong regenerative potential in vitro [80]. In particular, in human oral mucosal epithelium, p75NTR-positive cells were shown to be Ki67-negative in vivo and to present a higher clonal growth potential in vitro, demonstrating the importance of this receptor for the maintenance of a stem cell pool through the induction of a quiescent state [25]. Importantly, the influence of neurotrophins on the activation of these quiescent p75NTR-positive cells has not been tested. In human adult oral mucosa lamina propria, p75NTR-positive stem cells are self-renewing cells that co-express Oct4 and partially express Sox2 and Nanog transcription factors [84]. p75NTR is also found to be involved in controlling the fate of murine keratinocyte SCs through cell–cell interactions, where p75NTR-related signaling contributes to the control of hair follicle regression, most likely by driving apoptosis [85, 86]. A recent study has identified pluripotent p75NTR +/P0+ stem cells in the skin bulge that were shown to differentiate into all cell types in the adult [87]. Finally, NGF is able to drive healing in a model of corneal denervation by stimulating stem cell proliferation through the induction of p75NTR, TrkA, and p63 (an epithelial stem cell marker) expression [88]. These results highlight the fact that NGF and p75NTR represent pleiotropic factors affecting stem cell self-renewal in regenerating epithelia.
p75NTR has been proposed to be a marker of neural crest-derived dental pulp stem cells. Deciduous dental pulp stem cells (DDPSC) have been characterized as a multipotent stem cell population with the ability to differentiate into mesodermal and neural cell lineages [89]. p75NTR-positive DDPSCs have a high self-renewal capacity and the ability to migrate from the deciduous dental pulp tissues [90]. The adipogenic, osteogenic, chondrogenic, and neurogenic differentiation potential of p75NTR-positive stem cells has been evaluated by their ability to form lipid droplets, mineralized nodules, and cartilage extracellular matrix [91, 92]. A study performed in 1996 by Luukko et al. found a direct correlation between the development of tooth innervation and the expression of neurotrophin receptors, postulating that p75NTR alone may mediate neurotrophin effects during the determination and differentiation of odontoblast and ameloblast cell lineages. p75NTR transcripts were first observed in the tooth germ during its transition from the bud to the cap stage in El6. One possible ligand for p75NTR could be NGF, which has been found in developing and adult rat teeth [93]. Another study detected NGF, proNGF, and p75NTR in rat incisors [94]. This indicates that p75NTR and its ligands are important for tooth development and are maintained in adult dental pulp stem cells.
p75NTR is also widely expressed in several cell types of the central nervous system (mainly derived from the neural tube), where neurotrophins and growth factors play an important role in the regulation of several biological processes at various stages of development [95] and after neural injury (glial cell damage, axonal degeneration, and traumatic injury) [96]. p75NTR is a specific marker defining a population of highly proliferative subventricular-zone stem or precursor cells responsible for neuron production [97]. In neuronal stem cells, p75NTR was shown to be essential for the induction of oligodendrocyte differentiation. BDNF mediates its effects on neurite differentiation by activating TrkB and p75NTR, while NGF and NT3 were found to induce differentiation through an Erk1/2 signaling pathway [97, 98]. In the same cells, proNGF/p75NTR signals reduced oligodendrocyte differentiation by the induction of cell cycle arrest [8], while truncated TrkC/p75NTR signaling is involved in neural differentiation [99]. p75NTR is rapidly downregulated in neurons during progenitors differentiation [100] while ectopical overexpression of p75NTR has been reported to induce cell death of GABA-ergic neurons in the dorsal telencephalon of chick embryos [101].
During neurodevelopment, p75NTR-interacting proteins NADE, NRIF and SC-1 are co-localized with p75NTR. The spatial and temporal ratio of NADE and NRIF seems to make individual neuroblasts or glioblasts more or less susceptible to p75NTR-mediated apoptosis; SC-1 expression increases over time and seems to mediate p75NTR-induced growth arrest during glial development [102]. Interestingly, after traumatic brain injury, NGF stimulates the expression of biomarkers associated with neural stem cell characteristics, such as Nestin [103], and induces stem cell migration and differentiation to developing and/or degenerating CNS regions. Altogether, these results present p75NTR as the core of complex signaling pathways driven by neurotrophins and Trk receptors. The neural stem cell system could therefore represent an ideal model to examine in depth how p75NTR can switch from one biological response to another.
Mesodermal origin
Mesenchymal stem cells (MSCs) are multipotent stromal cells present in adult marrow that have the potential to differentiate into lineages of mesenchymal tissues, including bone, cartilage, fat, tendon, muscle, and marrow stroma [104]. The staining of mesenchymal cells by anti-p75NTR antibodies was first reported by Thomson et al. [105]. In these cells, p75NTR acts as a key regulator of the maintenance of the undifferentiated status with a pivotal role in the regulation of MSC differentiation into osteogenic, adipogenic, chondrogenic, and myogenic lineages. To assess the effect of p75NTR on universal mesenchymal differentiation, Mikami et al. [90] constitutively expressed the human p75NTR protein in murine multipotent MSCs (C3H10T1/2 cells). p75NTR was shown to directly inhibit the differentiation of MSCs into multiple cell types, most likely through inhibition of transcription factors, including Runx2 and OSX, which are essential for osteoblast differentiation and for expression of the chondrogenesis marker Sox9 and the myogenic marker Myf5. In hMSC, activation of the NogoR-p75NTR complex leads to p75NTR-mediated cell differentiation in a Trk- and neurotrophin-independent manner [90]. Moreover, increased expression of sortilin has been detected during early osteogenic differentiation where sortilin overexpression enhances mineralization by hMSC-derived cells after osteogenic differentiation, but it is not implicated in adipocyte commitment [106]. Thus, the differentiation promoting or inhibiting effects of p75NTR can be influenced by the absence or the presence of co-receptors. p75NTR is highly expressed in freshly isolated bone marrow mesenchymal cells when maintained in non-stimulated in vitro cultures and is rapidly down-regulated upon differentiation [107]. NGF was shown to drive bone marrow stem cell differentiation through the regulation of the Akt and MAPK signaling pathways [108]. Moreover, p75NTR distinguished a subset of multipotent adipose tissue-derived stem cells, which can be driven to differentiate into mature adipocytes, osteoblasts, chondrocytes, smooth muscle cells, and neuronal cells [109]. Further study showed that adipose tissue-derived stem cells, isolated with an anti p75NTR antibody, exhibited a high osteogenic differentiation potential [110].
Neurotrophins and their receptors are expressed in a differentiation-regulated and tissue compartment-regulated fashion during testicular and epididymal development in mice, rats, and humans [111, 112]. p75NTR is expressed at a very early stage of gonadal formation in the mouse (12.5 dpc), while no Trk receptor or neurotrophin immunoreactivity was detected [111]. Later in development, p75NTR/NT-3/truncated-TrkB co-expression leads to epididymal smooth muscle cell differentiation in the mouse, but TrkA expression is connected to mesonephric tubule formation. In particular, an NGF gradient directly regulates proliferation and differentiation of Leydig stem cells and the peritubular myoid cell lineage [113], demonstrating that neurotrophins and their receptors play a pivotal role in the regulation of cell differentiation in the developing testis and epididymis.
p75NTR is displayed on human and rodent adult muscle stem cells in vivo (satellite cells) and is a key regulator of myogenesis, through BDNF binding [114, 115]. It should be emphasized that in all cases, p75NTR expression begins well before such embryonal structures become innervated, and the receptor is down-regulated when embryo myoblasts differentiate into myotubes [116].
Endodermal origin
The role of p75NTR and neurotrophin signaling in tissues of endodermal origin has been less thoroughly examined. The pseudostratified epithelium of the mouse trachea and human airways was shown to contain a population of basal cells functioning as stem cells and involved in tissue repair. A transcriptional profile performed by Rock et al. [117] noted that basal cells are enriched in Ngfr as well as in Ntf3 (encoding for the NT-3 neurotrophin) transcripts, suggesting the possibility of an autocrine loop within this population.
Precursors of hepatic stellate cells, whose endodermal origin is still elusive [118, 119], express neurotrophins and their receptors, including p75NTR [120]. p75NTR has been found to regulate transdifferentiation of hepatic stellate cells into myofibroblasts [120, 121], apparently without neurotrophin ligands or co-receptor interaction and through the activation of Rho signaling [122]. In particular, the intracellular domain of p75NTR is critical to quiescent hepatic stellate cells activation and hepatocyte proliferation, whereas p75NTR−/− hepatic stellate cells exhibit significantly reduced differentiation [122].
p75NTR in cancer stem cells
Tumors usually are heterogeneous and comprise cells with different capacities to proliferate and differentiate. This cellular heterogeneity depends on the presence of so-called CSCs, which are defined as cells that can induce de novo tumor formation, self-renewal in vivo, and re-establish the cellular composition of the parental tumor.
Most of these cells have been isolated from whole tumor cell populations based on the expression of markers that characterize the stem cell compartment in the normal tissue of origin. In the context of cancer, several studies describe a tight connection between CSCs and p75NTR expression, especially in melanoma, squamous cell carcinoma, and breast cancer.
Melanoma
Over the last decade, many authors have raised questions about the existence of a tumorigenic CSC population in melanoma. In 2005, Fang et al. [123] reported on a subpopulation of melanoma cells with characteristics of primitive progenitors for melanocytes that could give rise to a broad range of cell types. Since then, these cells have been studied extensively, even though the existence of human melanoma stem cells is still debated [124]. Indeed, the tumorigenicity of melanoma cells has been posited to be variable depending on the degree of immunodeficiency in recipient mice as well as on the extracellular environment into which melanoma cells are transplanted, leading to an overestimation of the tumorigenic potential of melanoma cells. Nevertheless, transplanted cells that are able to recapitulate melanoma heterogeneity are unlikely to be originating from non-tumorigenic or differentiated cells.
Melanocytes have a neural crest origin, and all the neurotrophins and their receptors are expressed when reverting to embryonic phenotype melanoma cells, although their expression is weak in normal melanocytes. p75NTR/NGF signaling is known to be implicated in melanoma cell proliferation and migration [125] and has been associated with increased resistance of brain metastases [126, 127]. Starting from the fact that malignant melanoma, like normal melanocytes, derives from the neural crest lineage, Boiko et al. in 2010 found that melanoma p75NTR-positive cells were able to initiate and maintain tumor growth in vivo in fully immunocompromised mouse models [T-, B-, and NK-deficient Rag2−/−γc−/− mice (RG) mice]. These cells were more metastatic and able to re-establish the original p75NTR expression heterogeneity of the primary tumor, confirming the multilineage potency of these cells. However, the discovery that a small p75NTR-negative fraction was also able to generate tumors in these mouse models may imply that the tumor-initiating ability is not exclusively a feature of p75NTR-positive cells [128] or that both p75NTR-positive and p75NTR-negative cells characterize melanoma stem cells at different stages. A phenotype switch appears to be a dominant phenomenon of melanoma stem cells and may be a major obstacle in their eradication [129–131]. Other groups confirmed that p75NTR-positive melanoma cells have the capacity for self-renewal and sustain long-term tumor growth in vivo and that the incidence of p75NTR-positive cells in patient biopsies is associated with poor prognosis for melanoma [132], indicating that p75NTR may represent a marker of a stem/progenitor cell subset within melanoma cells.
Squamous cell carcinoma
In squamous cell carcinomas, p75NTR is known to play a pro-tumoral role. It is, for instance, associated with poor prognoses and a risk of local recurrence of oral cancers [133], where it is expressed in undifferentiated cell populations in oral leukoplakia and in oral squamous cell carcinoma (OSCC). This finding indicates that p75NTR could be a useful prognostic marker of OSCC [134]. p75NTR is correlated with perineural invasion in skin cancers [135] and is found in 50 % of esophageal squamous cell carcinomas (ESCC), where it is diffusely distributed in poorly differentiated tumors, suggesting that p75NTR is expressed in the actively proliferating, undifferentiated cell component of each tumor [136]. In ESCC specimens, p63, a keratinocyte stem cell marker, was confined mainly to p75NTR-positive cells, which furthermore expressed lower levels of differentiation markers such as involucrin, cytokeratin 13, β1-integrin and β4-integrin [137]. p75NTR-positive cells have the ability to self-renew and are resistant to chemotherapy, suggesting that it may be necessary for survival and maintenance of ESCC.
Breast cancer
The involvement of neurotrophins in breast cancer is well documented. Indeed, breast cancer epithelial cells produce and secrete NGF, proNGF, BDNF, and NT4/5, which act on the same cells through an autocrine loop [138–140]. Normal and cancerous breast cells express both p75NTR and TrkA, but only cancer cells respond to NGF treatment with enhanced proliferation, survival, and invasion [140–142]. p75NTR in breast cancer has been described as a marker of myoepithelial cells [143], and it has been used to identify a subset of cells with basal-like activity. Recent studies have shown that its overexpression increases the survival of breast cancer cells [144], or upon BDNF and NT-4/5 stimulation, recruiting the adapter TRADD to stimulate the NF-κB pathway and, consequently, cell survival [145]. A study by Kim et al. [146] showed that cultures derived from p75NTR-positive clones of MCF-7, BT474, and BT549 cell lines as well as of primary tumor-derived cells, were able to recapitulate a pattern of heterogeneity similar to that of the global population, generating both basal-like and luminal-like compartments. In addition, p75NTR-positive cells presented enhanced expression of miRNA 205, 221 and 222, which positively correlated with the maintenance of mammary epithelial progenitor cells in mice [146, 147].
Other cancers
Recently, p75NTR has been shown to define a stem cell-like population in hypopharyngeal cancer, exhibiting tumor initiation, self-renewal, and chemoresistance [148]. P75NTR is also expressed by neuroblastoma stem cells (SH-SY5Y neuroblastoma clone), as cells expressing p75NTR in association with c-kit and CD133 were found to display a highly clonogenic potency and a substantial plasticity [149].
Concluding remarks
Despite the great interest engendered by stem cells, the manipulation of these cells has remained challenging because of the lack of specific markers to unequivocally define stem cells with different potencies and to distinguish them from transit amplifying cells in each cellular model. With the exception of the hematopoietic system, molecular mechanisms underlying stem cell differentiation are still poorly understood. Additionally, the potential functions of numerous prospective stem cell markers remain to be determined.
A close relationship between the expression of p75NTR and pluripotency has been noted by increasing amounts of data. p75NTR is noteworthy for its wide expression by several cellular models bearing different degrees of plasticity. Although it has been shown that p75NTR is expressed by ESCs, by migrating NCSCs, by adult stem cells originating from different germ layers and by an increasing number of CSCs (Table 1; Fig. 2), its real function in most of these cells remains enigmatic. The signaling pathways that are connected to p75NTR and the absence of catalytic activity in this receptor make it difficult to precisely define how this receptor acts. As stated above, p75NTR signaling can be modulated by its level of expression, dimerization, ligand-binding, interaction with co-receptors, intracellular partner recruitment and post-translational modifications. Thus, when considering the role of p75NTR in biological process, it is impossible to generalize a unique mode of action. Nevertheless, as was discussed above, p75NTR is implicated in the regulation of stem cell differentiation in several cell models, including mesenchymal, dental pulp, testis and bone marrow stem cells. This action could be achieved through the induction of a quiescent state, as documented in neuronal and hematopoietic stem cells or in breast cancer cells. We hypothesize that p75NTR could be a “fate decision” protein that enables stem cells to maintain their potency and to engage in differentiation according to the molecular and cellular context. Indeed, p75NTR is important for potency maintenance, and its expression is often down-regulated as a cell engages in a differentiation program. An example of how p75NTR may serve as a bi-directional switch in response to different stimuli is given by neuronal stem cells, where differentiation is strictly dependent on p75NTR ligand expression. In these cells, oligodendrocyte differentiation is induced by NGF and NT-3 and inhibited by proNGF in a p75NTR-dependent manner [8, 98]. A mechanism facilitating p75NTR signal transduction could be its translocation in lipid rafts as a consequence of its phosphorylation at Ser304 by cAMP-PKA, as has been observed in cerebellar neurons [150]. Lipid rafts are cholesterol- and sphingolipid-rich microdomains in cell membranes that are believed to function in cellular signaling by concentrating or separating specific molecules in a unique lipid environment. Lipid rafts were shown to participate in the maintenance of ESC self-renewal [151] as well as in hematopoietic stem cell activation from quiescence [152].
The interest in p75NTR in stem cell biology also comes from the observation that developmental cells (especially neural stem cells) produce neutrophic factors that act on themselves and on surrounding tissues via autocrine and paracrine mechanisms [153].
Therefore, despite the physiological complexity and technological challenges, elucidating the potential influence of p75NTR as well as the underlying molecular mechanisms in a context-defined manner will shed new light on the biology of both normal and CSCs.
Acknowledgments
This research was supported by the Institut National de la Santé et de la Recherche Médicale (Inserm), The Université de Lille 1.
References
- 1.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Zeng J, Cen L, Chen Y, Wang X, Yao G, Wang W, Qi W, Kong K. Multiple roles of the p75 neurotrophin receptor in the nervous system. J Int Med Res. 2009;37:281–288. doi: 10.1177/147323000903700201. [DOI] [PubMed] [Google Scholar]
- 5.Schor NF. The p75 neurotrophin receptor in human development and disease. Prog Neurobiol. 2005;77:201–214. doi: 10.1016/j.pneurobio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Jiang X, Gwye Y, McKeown SJ, Bronner-Fraser M, Lutzko C, Lawlor ER. Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2009;18:1059–1070. doi: 10.1089/scd.2008.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong J, Zhou L, Yang M, Lim Y, Zhu Y, Fu D, Li Z, Zhong J, Xiao Z, Zhou X-F. ProBDNF and its receptors are upregulated in glioma and inhibit the growth of glioma cells in vitro. Neuro Oncol. 2013;15:990–1007. doi: 10.1093/neuonc/not039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo J, Wang J, Liang C, Yan J, Wang Y, Liu G, Jiang Z, Zhang L, Wang X, Wang Y, et al. proNGF inhibits proliferation and oligodendrogenesis of postnatal hippocampal neural stem/progenitor cells through p75NTR in vitro. Stem Cell Res. 2013;11:874–887. doi: 10.1016/j.scr.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Bartkowska K, Turlejski K, Djavadian RL. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol Exp (Wars) 2010;70:454–467. doi: 10.55782/ane-2010-1816. [DOI] [PubMed] [Google Scholar]
- 10.He X-L, Garcia KC. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science. 2004;304:870–875. doi: 10.1126/science.1095190. [DOI] [PubMed] [Google Scholar]
- 11.Large TH, Weskamp G, Helder JC, Radeke MJ, Misko TP, Shooter EM, Reichardt LF. Structure and developmental expression of the nerve growth factor receptor in the chicken central nervous system. Neuron. 1989;2:1123–1134. doi: 10.1016/0896-6273(89)90179-7. [DOI] [PubMed] [Google Scholar]
- 12.Vilar M, Charalampopoulos I, Kenchappa RS, Simi A, Karaca E, Reversi A, Choi S, Bothwell M, Mingarro I, Friedman WJ, et al. Activation of the p75 neurotrophin receptor through conformational rearrangement of disulphide-linked receptor dimers. Neuron. 2009;62:72–83. doi: 10.1016/j.neuron.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulson EJ, Reid K, Shipham KM, Morley S, Kilpatrick TJ, Bartlett PF. The role of neurotransmission and the Chopper domain in p75 neurotrophin receptor death signaling. Prog Brain Res. 2004;146:41–62. doi: 10.1016/S0079-6123(03)46003-2. [DOI] [PubMed] [Google Scholar]
- 14.Barker PA, Barbee G, Misko TP, Shooter EM. The low affinity neurotrophin receptor, p75LNTR, is palmitoylated by thioester formation through cysteine 279. J Biol Chem. 1994;269:30645–30650. [PubMed] [Google Scholar]
- 15.Skeldal S, Matusica D, Nykjaer A, Coulson EJ. Proteolytic processing of the p75 neurotrophin receptor: a prerequisite for signalling?: neuronal life, growth and death signalling are crucially regulated by intra-membrane proteolysis and trafficking of p75(NTR) BioEssays. 2011;33:614–625. doi: 10.1002/bies.201100036. [DOI] [PubMed] [Google Scholar]
- 16.Underwood CK, Coulson EJ. The p75 neurotrophin receptor. Int J Biochem Cell Biol. 2008;40:1664–1668. doi: 10.1016/j.biocel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 18.Gentry JJ, Casaccia-Bonnefil P, Carter BD. Nerve growth factor activation of nuclear factor kappaB through its p75 receptor is an anti-apoptotic signal in RN22 schwannoma cells. J Biol Chem. 2000;275:7558–7565. doi: 10.1074/jbc.275.11.7558. [DOI] [PubMed] [Google Scholar]
- 19.Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 21.Notterpek L. Neurotrophins in myelination: a new role for a puzzling receptor. Trends Neurosci. 2003;26:232–234. doi: 10.1016/S0166-2236(03)00099-7. [DOI] [PubMed] [Google Scholar]
- 22.Chittka A, Arevalo JC, Rodriguez-Guzman M, Pérez P, Chao MV, Sendtner M. The p75NTR-interacting protein SC1 inhibits cell cycle progression by transcriptional repression of cyclin E. J Cell Biol. 2004;164:985–996. doi: 10.1083/jcb.200301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann JL, Menter DG, Hamada J, Marchetti D, Nakajima M, Nicolson GL. Mediation of NGF-stimulated extracellular matrix invasion by the human melanoma low-affinity p75 neurotrophin receptor: melanoma p75 functions independently of trkA. Mol Biol Cell. 1993;4:1205–1216. doi: 10.1091/mbc.4.11.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston ALM, Lun X, Rahn JJ, Liacini A, Wang L, Hamilton MG, Parney IF, Hempstead BL, Robbins SM, Forsyth PA, et al. The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol. 2007;5:e212. doi: 10.1371/journal.pbio.0050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura T, Endo K, Kinoshita S. Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells. 2007;25:628–638. doi: 10.1634/stemcells.2006-0494. [DOI] [PubMed] [Google Scholar]
- 26.Vilar M, Charalampopoulos I, Kenchappa RS, Reversi A, Klos-Applequist JM, Karaca E, Simi A, Spuch C, Choi S, Friedman WJ, et al. Ligand-independent signaling by disulfide-crosslinked dimers of the p75 neurotrophin receptor. J Cell Sci. 2009;122:3351–3357. doi: 10.1242/jcs.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Bronfman FC, Fainzilber M. Multi-tasking by the p75 neurotrophin receptor: sortilin things out? EMBO Rep. 2004;5:867–871. doi: 10.1038/sj.embor.7400219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teng KK, Hempstead BL. Neurotrophins and their receptors: signaling trios in complex biological systems. Cell Mol Life Sci. 2004;61:35–48. doi: 10.1007/s00018-003-3099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esposito D, Patel P, Stephens RM, Perez P, Chao MV, Kaplan DR, Hempstead BL. The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J Biol Chem. 2001;276:32687–32695. doi: 10.1074/jbc.M011674200. [DOI] [PubMed] [Google Scholar]
- 31.Iacaruso MF, Galli S, Martí M, Villalta JI, Estrin DA, Jares-Erijman EA, Pietrasanta LI. Structural model for p75(NTR)-TrkA intracellular domain interaction: a combined FRET and bioinformatics study. J Mol Biol. 2011;414:681–698. doi: 10.1016/j.jmb.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 33.Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen Z-Y, Lee FS, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- 35.Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 36.Niederöst B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertrand MJM, Kenchappa RS, Andrieu D, Leclercq-Smekens M, Nguyen HNT, Carter BD, Muscatelli F, Barker PA, De Backer O. NRAGE, a p75NTR adaptor protein, is required for developmental apoptosis in vivo. Cell Death Differ. 2008;15:1921–1929. doi: 10.1038/cdd.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukai J, Hachiya T, Shoji-Hoshino S, Kimura MT, Nadano D, Suvanto P, Hanaoka T, Li Y, Irie S, Greene LA, et al. NADE, a p75NTR-associated cell death executor, is involved in signal transduction mediated by the common neurotrophin receptor p75NTR . J Biol Chem. 2000;275:17566–17570. doi: 10.1074/jbc.C000140200. [DOI] [PubMed] [Google Scholar]
- 39.Linggi MS, Burke TL, Williams BB, Harrington A, Kraemer R, Hempstead BL, Yoon SO, Carter BD. Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor. J Biol Chem. 2005;280:13801–13808. doi: 10.1074/jbc.M410435200. [DOI] [PubMed] [Google Scholar]
- 40.Geetha T, Zheng C, McGregor WC, Douglas White B, Diaz-Meco MT, Moscat J, Babu JR. TRAF6 and p62 inhibit amyloid β-induced neuronal death through p75 neurotrophin receptor. Neurochem Int. 2012;61:1289–1293. doi: 10.1016/j.neuint.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye X, Mehlen P, Rabizadeh S, VanArsdale T, Zhang H, Shin H, Wang JJ, Leo E, Zapata J, Hauser CA, et al. TRAF family proteins interact with the common neurotrophin receptor and modulate apoptosis induction. J Biol Chem. 1999;274:30202–30208. doi: 10.1074/jbc.274.42.30202. [DOI] [PubMed] [Google Scholar]
- 42.Khursigara G, Bertin J, Yano H, Moffett H, DiStefano PS, Chao MV. A prosurvival function for the p75 receptor death domain mediated via the caspase recruitment domain receptor-interacting protein 2. J Neurosci. 2001;21:5854–5863. doi: 10.1523/JNEUROSCI.21-16-05854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irie S, Hachiya T, Rabizadeh S, Maruyama W, Mukai J, Li Y, Reed JC, Bredesen DE, Sato TA. Functional interaction of Fas-associated phosphatase-1 (FAP-1) with p75(NTR) and their effect on NF-kappaB activation. FEBS Lett. 1999;460:191–198. doi: 10.1016/s0014-5793(99)01324-1. [DOI] [PubMed] [Google Scholar]
- 44.Sole C, Dolcet X, Segura MF, Gutierrez H, Diaz-Meco M-T, Gozzelino R, Sanchis D, Bayascas JR, Gallego C, Moscat J, et al. The death receptor antagonist FAIM promotes neurite outgrowth by a mechanism that depends on ERK and NF-kappa B signaling. J Cell Biol. 2004;167:479–492. doi: 10.1083/jcb.200403093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Yazidi-Belkoura I, Adriaenssens E, Dollé L, Descamps S, Hondermarck H. Tumor necrosis factor receptor-associated death domain protein is involved in the neurotrophin receptor-mediated antiapoptotic activity of nerve growth factor in breast cancer cells. J Biol Chem. 2003;278:16952–16956. doi: 10.1074/jbc.M300631200. [DOI] [PubMed] [Google Scholar]
- 46.Chan JR, Jolicoeur C, Yamauchi J, Elliott J, Fawcett JP, Ng BK, Cayouette M. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–836. doi: 10.1126/science.1134069. [DOI] [PubMed] [Google Scholar]
- 47.Sachs BD, Baillie GS, McCall JR, Passino MA, Schachtrup C, Wallace DA, Dunlop AJ, MacKenzie KF, Klussmann E, Lynch MJ, et al. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J Cell Biol. 2007;177:1119–1132. doi: 10.1083/jcb.200701040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito H, Morishita R, Iwamoto I, Mizuno M, Nagata K-I. MAGI-1 acts as a scaffolding molecule for NGF receptor-mediated signaling pathway. Biochim Biophys Acta. 2013;1833:2302–2310. doi: 10.1016/j.bbamcr.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Epa WR, Markovska K, Barrett GL. The p75 neurotrophin receptor enhances TrkA signalling by binding to Shc and augmenting its phosphorylation. J Neurochem. 2004;89:344–353. doi: 10.1111/j.1471-4159.2004.02344.x. [DOI] [PubMed] [Google Scholar]
- 50.Underwood CK, Reid K, May LM, Bartlett PF, Coulson EJ. Palmitoylation of the C-terminal fragment of p75(NTR) regulates death signaling and is required for subsequent cleavage by gamma-secretase. Mol Cell Neurosci. 2008;37:346–358. doi: 10.1016/j.mcn.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Kommaddi RP, Thomas R, Ceni C, Daigneault K, Barker PA. Trk-dependent ADAM17 activation facilitates neurotrophin survival signaling. FASEB J. 2011;25:2061–2070. doi: 10.1096/fj.10-173740. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Rahn JJ, Lun X, Sun B, Kelly JJP, Weiss S, Robbins SM, Forsyth PA, Senger DL. Gamma-secretase represents a therapeutic target for the treatment of invasive glioma mediated by the p75 neurotrophin receptor. PLoS Biol. 2008;6:e289. doi: 10.1371/journal.pbio.0060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urra S, Escudero CA, Ramos P, Lisbona F, Allende E, Covarrubias P, Parraguez JI, Zampieri N, Chao MV, Annaert W, et al. TrkA receptor activation by nerve growth factor induces shedding of the p75 neurotrophin receptor followed by endosomal gamma-secretase-mediated release of the p75 intracellular domain. J Biol Chem. 2007;282:7606–7615. doi: 10.1074/jbc.M610458200. [DOI] [PubMed] [Google Scholar]
- 54.Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci. 2003;23:5425–5436. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parkhurst CN, Zampieri N, Chao MV. Nuclear localization of the p75 neurotrophin receptor intracellular domain. J Biol Chem. 2010;285:5361–5368. doi: 10.1074/jbc.M109.045054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verbeke S, Tomellini E, Dhamani F, Meignan S, Adriaenssens E, Bourhis XL. Extracellular cleavage of the p75 neurotrophin receptor is implicated in its pro-survival effect in breast cancer cells. FEBS Lett. 2013;587(16):2591–2596. doi: 10.1016/j.febslet.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 57.Salama-Cohen P, Arévalo M-A, Meier J, Grantyn R, Rodríguez-Tébar A. NGF controls dendrite development in hippocampal neurons by binding to p75NTR and modulating the cellular targets of Notch. Mol Biol Cell. 2005;16:339–347. doi: 10.1091/mbc.E04-05-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou F-Q, Zhou J, Dedhar S, Wu Y-H, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 59.Arévalo JC, Chao MV. Axonal growth: where neurotrophins meet Wnts. Curr Opin Cell Biol. 2005;17:112–115. doi: 10.1016/j.ceb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 60.David MD, Yeramian A, Duñach M, Llovera M, Cantí C, de Herreros AG, Comella JX, Herreros J. Signalling by neurotrophins and hepatocyte growth factor regulates axon morphogenesis by differential beta-catenin phosphorylation. J Cell Sci. 2008;121:2718–2730. doi: 10.1242/jcs.029660. [DOI] [PubMed] [Google Scholar]
- 61.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen B-Y, Wang X, Wang Z-Y, Wang Y-Z, Chen L-W, Luo Z-J. Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. J Neurosci Res. 2013;91:30–41. doi: 10.1002/jnr.23138. [DOI] [PubMed] [Google Scholar]
- 63.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24:344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 65.Moscatelli I, Pierantozzi E, Camaioni A, Siracusa G, Campagnolo L. p75 neurotrophin receptor is involved in proliferation of undifferentiated mouse embryonic stem cells. Exp Cell Res. 2009;315:3220–3232. doi: 10.1016/j.yexcr.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 66.Kawamura K, Kawamura N, Sato W, Fukuda J, Kumagai J, Tanaka T. Brain-derived neurotrophic factor promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival. Endocrinology. 2009;150:3774–3782. doi: 10.1210/en.2009-0213. [DOI] [PubMed] [Google Scholar]
- 67.Betters E, Liu Y, Kjaeldgaard A, Sundström E, García-Castro MI. Analysis of early human neural crest development. Dev Biol. 2010;344:578–592. doi: 10.1016/j.ydbio.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crane JF, Trainor PA. Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol. 2006;22:267–286. doi: 10.1146/annurev.cellbio.22.010305.103814. [DOI] [PubMed] [Google Scholar]
- 69.Le Douarin NM, Calloni GW, Dupin E. The stem cells of the neural crest. Cell Cycle. 2008;7:1013–1019. doi: 10.4161/cc.7.8.5641. [DOI] [PubMed] [Google Scholar]
- 70.Delfino-Machín M, Chipperfield TR, Rodrigues FSLM, Kelsh RN. The proliferating field of neural crest stem cells. Dev Dyn. 2007;236:3242–3254. doi: 10.1002/dvdy.21314. [DOI] [PubMed] [Google Scholar]
- 71.Dupin E, Sommer L. Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol. 2012;366:83–95. doi: 10.1016/j.ydbio.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 72.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 73.Kléber M, Lee H-Y, Wurdak H, Buchstaller J, Riccomagno MM, Ittner LM, Suter U, Epstein DJ, Sommer L. Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J Cell Biol. 2005;169:309–320. doi: 10.1083/jcb.200411095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 75.Sieber-Blum M. Growth factor synergism and antagonism in early neural crest development. Biochem Cell Biol. 1998;76:1039–1050. [PubMed] [Google Scholar]
- 76.Li H-Y, Say EHM, Zhou X-F. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 2007;25:2053–2065. doi: 10.1634/stemcells.2007-0080. [DOI] [PubMed] [Google Scholar]
- 77.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Becker L, Kulkarni S, Tiwari G, Micci M-A, Pasricha PJ. Divergent fate and origin of neurosphere-like bodies from different layers of the gut. Am J Physiol Gastrointest Liver Physiol. 2012;302:G958–G965. doi: 10.1152/ajpgi.00511.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chalazonitis A. Neurotrophin-3 in the development of the enteric nervous system. Prog Brain Res. 2004;146:243–263. doi: 10.1016/S0079-6123(03)46016-0. [DOI] [PubMed] [Google Scholar]
- 80.Okumura T, Shimada Y, Imamura M, Yasumoto S. Neurotrophin receptor p75(NTR) characterizes human esophageal keratinocyte stem cells in vitro. Oncogene. 2003;22:4017–4026. doi: 10.1038/sj.onc.1206525. [DOI] [PubMed] [Google Scholar]
- 81.Li X, Shen Y, Di B, Li J, Geng J, Lu X, He Z. Biological and clinical significance of p75NTR expression in laryngeal squamous epithelia and laryngocarcinoma. Acta Otolaryngol. 2012;132:314–324. doi: 10.3109/00016489.2011.639086. [DOI] [PubMed] [Google Scholar]
- 82.Truzzi F, Marconi A, Atzei P, Panza MC, Lotti R, Dallaglio K, Tiberio R, Palazzo E, Vaschieri C, Pincelli C. p75 neurotrophin receptor mediates apoptosis in transit-amplifying cells and its overexpression restores cell death in psoriatic keratinocytes. Cell Death Differ. 2011;18:948–958. doi: 10.1038/cdd.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Girolamo N, Sarris M, Chui J, Cheema H, Coroneo MT, Wakefield D. Localization of the low-affinity nerve growth factor receptor p75 in human limbal epithelial cells. J Cell Mol Med. 2008;12:2799–2811. doi: 10.1111/j.1582-4934.2008.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marynka-Kalmani K, Treves S, Yafee M, Rachima H, Gafni Y, Cohen MA, Pitaru S. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells. 2010;28:984–995. doi: 10.1002/stem.425. [DOI] [PubMed] [Google Scholar]
- 85.Botchkarev VA, Botchkareva NV, Albers KM, Chen LH, Welker P, Paus R. A role for p75 neurotrophin receptor in the control of apoptosis-driven hair follicle regression. FASEB J. 2000;14:1931–1942. doi: 10.1096/fj.99-0930com. [DOI] [PubMed] [Google Scholar]
- 86.Botchkarev VA, Yaar M, Gilchrest BA, Paus R. p75 Neurotrophin receptor antagonist retards apoptosis-driven hair follicle involution (catagen) J Invest Dermatol. 2003;120:168–169. doi: 10.1046/j.1523-1747.2003.12003.x. [DOI] [PubMed] [Google Scholar]
- 87.Wong CE, Paratore C, Dours-Zimmermann MT, Rochat A, Pietri T, Suter U, Zimmermann DR, Dufour S, Thiery JP, Meijer D, et al. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175:1005–1015. doi: 10.1083/jcb.200606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lambiase A, Aloe L, Mantelli F, Sacchetti M, Perrella E, Bianchi P, Rocco ML, Bonini S. Capsaicin-induced corneal sensory denervation and healing impairment are reversed by NGF treatment. Invest Ophthalmol Vis Sci. 2012;53:8280–8287. doi: 10.1167/iovs.12-10593. [DOI] [PubMed] [Google Scholar]
- 89.Martens W, Wolfs E, Struys T, Politis C, Bronckaers A, Lambrichts I. Expression pattern of basal markers in human dental pulp stem cells and tissue. Cells Tissues Organs. 2012;196(6):490–500. doi: 10.1159/000338654. [DOI] [PubMed] [Google Scholar]
- 90.Mikami Y, Ishii Y, Watanabe N, Shirakawa T, Suzuki S, Irie S, Isokawa K, Honda MJ. CD271/p75(NTR) inhibits the differentiation of mesenchymal stem cells into osteogenic, adipogenic, chondrogenic, and myogenic lineages. Stem Cells Dev. 2011;20:901–913. doi: 10.1089/scd.2010.0299. [DOI] [PubMed] [Google Scholar]
- 91.Wen X, Liu L, Deng M, Zhang L, Liu R, Xing Y, Zhou X, Nie X. Characterization of p75(+) ectomesenchymal stem cells from rat embryonic facial process tissue. Biochem Biophys Res Commun. 2012;427:5–10. doi: 10.1016/j.bbrc.2012.08.109. [DOI] [PubMed] [Google Scholar]
- 92.Stevens A, Zuliani T, Olejnik C, LeRoy H, Obriot H, Kerr-Conte J, Formstecher P, Bailliez Y, Polakowska RR. Human dental pulp stem cells differentiate into neural crest-derived melanocytes and have label-retaining and sphere-forming abilities. Stem Cells Dev. 2008;17:1175–1184. doi: 10.1089/scd.2008.0012. [DOI] [PubMed] [Google Scholar]
- 93.Luukko K, Moshnyakov M, Sainio K, Saarma M, Sariola H, Thesleff I. Expression of neurotrophin receptors during rat tooth development is developmentally regulated, independent of innervation, and suggests functions in the regulation of morphogenesis and innervation. Dev Dyn. 1996;206:87–99. doi: 10.1002/(SICI)1097-0177(199605)206:1<87::AID-AJA8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 94.Mitsiadis TA, Couble P, Dicou E, Rudkin BB, Magloire H. Patterns of nerve growth factor (NGF), proNGF, and p75 NGF receptor expression in the rat incisor: comparison with expression in the molar. Differentiation. 1993;54:161–175. doi: 10.1111/j.1432-0436.1993.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 95.Cragnolini AB, Huang Y, Gokina P, Friedman WJ. Nerve growth factor attenuates proliferation of astrocytes via the p75 neurotrophin receptor. Glia. 2009;57:1386–1392. doi: 10.1002/glia.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ibáñez CF, Simi A. p75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci. 2012;35:431–440. doi: 10.1016/j.tins.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 97.Young KM, Merson TD, Sotthibundhu A, Coulson EJ, Bartlett PF. p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J Neurosci. 2007;27:5146–5155. doi: 10.1523/JNEUROSCI.0654-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Du Y, Fischer TZ, Clinton-Luke P, Lercher LD, Dreyfus CF. Distinct effects of p75 in mediating actions of neurotrophins on basal forebrain oligodendrocytes. Mol Cell Neurosci. 2006;31:366–375. doi: 10.1016/j.mcn.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Hapner SJ, Boeshore KL, Large TH, Lefcort F. Neural differentiation promoted by truncated trkC receptors in collaboration with p75(NTR) Dev Biol. 1998;201:90–100. doi: 10.1006/dbio.1998.8970. [DOI] [PubMed] [Google Scholar]
- 100.Bibel M, Richter J, Schrenk K, Tucker KL, Staiger V, Korte M, Goetz M, Barde Y-A. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat Neurosci. 2004;7:1003–1009. doi: 10.1038/nn1301. [DOI] [PubMed] [Google Scholar]
- 101.Nikoletopoulou V, Plachta N, Allen ND, Pinto L, Götz M, Barde Y-A. Neurotrophin receptor-mediated death of misspecified neurons generated from embryonic stem cells lacking Pax6. Cell Stem Cell. 2007;1:529–540. doi: 10.1016/j.stem.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 102.Kendall SE, Ryczko MC, Mehan M, Verdi JM. Characterization of NADE, NRIF and SC-1 gene expression during mouse neurogenesis. Brain Res Dev Brain Res. 2003;144:151–158. doi: 10.1016/s0165-3806(03)00166-4. [DOI] [PubMed] [Google Scholar]
- 103.Lu J, Frerich JM, Turtzo LC, Li S, Chiang J, Yang C, Wang X, Zhang C, Wu C, Sun Z, et al. Histone deacetylase inhibitors are neuroprotective and preserve NGF-mediated cell survival following traumatic brain injury. Proc Natl Acad Sci USA. 2013;110:10747–10752. doi: 10.1073/pnas.1308950110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 105.Thomson TM, Rettig WJ, Chesa PG, Green SH, Mena AC, Old LJ. Expression of human nerve growth factor receptor on cells derived from all three germ layers. Exp Cell Res. 1988;174:533–539. doi: 10.1016/0014-4827(88)90323-0. [DOI] [PubMed] [Google Scholar]
- 106.Maeda S, Nobukuni T, Shimo-Onoda K, Hayashi K, Yone K, Komiya S, Inoue I. Sortilin is upregulated during osteoblastic differentiation of mesenchymal stem cells and promotes extracellular matrix mineralization. J Cell Physiol. 2002;193:73–79. doi: 10.1002/jcp.10151. [DOI] [PubMed] [Google Scholar]
- 107.Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 108.Yuan J, Huang G, Xiao Z, Lin L, Han T. Overexpression of β-NGF promotes differentiation of bone marrow mesenchymal stem cells into neurons through regulation of AKT and MAPK pathway. Mol Cell Biochem. 2013;383(1–2):201–211. doi: 10.1007/s11010-013-1768-6. [DOI] [PubMed] [Google Scholar]
- 109.Yamamoto N, Akamatsu H, Hasegawa S, Yamada T, Nakata S, Ohkuma M, Miyachi E-I, Marunouchi T, Matsunaga K. Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci. 2007;48:43–52. doi: 10.1016/j.jdermsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 110.Rada T, Reis RL, Gomes ME. Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev. 2011;7:64–76. doi: 10.1007/s12015-010-9147-0. [DOI] [PubMed] [Google Scholar]
- 111.Russo MA, Giustizieri ML, Favale A, Fantini MC, Campagnolo L, Konda D, Germano F, Farini D, Manna C, Siracusa G. Spatiotemporal patterns of expression of neurotrophins and neurotrophin receptors in mice suggest functional roles in testicular and epididymal morphogenesis. Biol Reprod. 1999;61:1123–1132. doi: 10.1095/biolreprod61.4.1123. [DOI] [PubMed] [Google Scholar]
- 112.Russo MA, Giustizieri ML, Farini D, Campagnolo L, De Felici M, Siracusa G. Expression of the p75 neurotrophin receptor in the developing and adult testis of the rat. Int J Dev Biol Suppl. 1996;1:227S–228S. [PubMed] [Google Scholar]
- 113.Zhang L, Wang H, Yang Y, Liu H, Zhang Q, Xiang Q, Ge R, Su Z, Huang Y. NGF induces adult stem Leydig cells to proliferate and differentiate during Leydig cell regeneration. Biochem Biophys Res Commun. 2013;436:300–305. doi: 10.1016/j.bbrc.2013.05.098. [DOI] [PubMed] [Google Scholar]
- 114.Colombo E, Romaggi S, Medico E, Menon R, Mora M, Falcone C, Lochmüller H, Confalonieri P, Mantegazza R, Morandi L, et al. Human neurotrophin receptor p75NTR defines differentiation-oriented skeletal muscle precursor cells: implications for muscle regeneration. J Neuropathol Exp Neurol. 2011;70:133–142. doi: 10.1097/NEN.0b013e3182084391. [DOI] [PubMed] [Google Scholar]
- 115.Colombo E, Bedogni F, Lorenzetti I, Landsberger N, Previtali SC, Farina C. Autocrine and immune cell-derived BDNF in human skeletal muscle: implications for myogenesis and tissue regeneration. J Pathol. 2013;231:190–198. doi: 10.1002/path.4228. [DOI] [PubMed] [Google Scholar]
- 116.Mousavi K, Jasmin BJ. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J Neurosci. 2006;26:5739–5749. doi: 10.1523/JNEUROSCI.5398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Geerts A. On the origin of stellate cells: mesodermal, endodermal or neuro-ectodermal? J Hepatol. 2004;40:331–334. doi: 10.1016/j.jhep.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 119.Zaret KS. Hepatocyte differentiation: from the endoderm and beyond. Curr Opin Genet Dev. 2001;11:568–574. doi: 10.1016/s0959-437x(00)00234-3. [DOI] [PubMed] [Google Scholar]
- 120.Cassiman D, Denef C, Desmet VJ, Roskams T. Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology. 2001;33:148–158. doi: 10.1053/jhep.2001.20793. [DOI] [PubMed] [Google Scholar]
- 121.Kendall TJ, Hennedige S, Aucott RL, Hartland SN, Vernon MA, Benyon RC, Iredale JP. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology. 2009;49:901–910. doi: 10.1002/hep.22701. [DOI] [PubMed] [Google Scholar]
- 122.Passino MA, Adams RA, Sikorski SL, Akassoglou K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR . Science. 2007;315:1853–1856. doi: 10.1126/science.1137603. [DOI] [PubMed] [Google Scholar]
- 123.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 124.Dirks P. Cancer stem cells: invitation to a second round. Nature. 2010;466:40–41. doi: 10.1038/466040a. [DOI] [PubMed] [Google Scholar]
- 125.Truzzi F, Marconi A, Lotti R, Dallaglio K, French LE, Hempstead BL, Pincelli C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J Invest Dermatol. 2008;128:2031–2040. doi: 10.1038/jid.2008.21. [DOI] [PubMed] [Google Scholar]
- 126.Denkins Y, Reiland J, Roy M, Sinnappah-Kang ND, Galjour J, Murry BP, Blust J, Aucoin R, Marchetti D. Brain metastases in melanoma: roles of neurotrophins. Neuro Oncol. 2004;6:154–165. doi: 10.1215/S115285170300067X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marchetti D, McQuillan DJ, Spohn WC, Carson DD, Nicolson GL. Neurotrophin stimulation of human melanoma cell invasion: selected enhancement of heparanase activity and heparanase degradation of specific heparan sulfate subpopulations. Cancer Res. 1996;56:2856–2863. [PubMed] [Google Scholar]
- 128.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eichhoff OM, Weeraratna A, Zipser MC, Denat L, Widmer DS, Xu M, Kriegl L, Kirchner T, Larue L, Dummer R, et al. Differential LEF1 and TCF4 expression is involved in melanoma cell phenotype switching. Pigment Cell Melanoma Res. 2011;24:631–642. doi: 10.1111/j.1755-148X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 130.Touil Y, Zuliani T, Wolowczuk I, Kuranda K, Prochazkova J, Andrieux J, Le Roy H, Mortier L, Vandomme J, Jouy N, et al. The PI3K/AKT signaling pathway controls the quiescence of the low-Rhodamine123-retention cell compartment enriched for melanoma stem cell activity. Stem Cells. 2012;31:641–651. doi: 10.1002/stem.1333. [DOI] [PubMed] [Google Scholar]
- 131.Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T, Herlyn M. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, Seifert B, Moch H, Dummer R, van den Broek M, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098–3109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 133.Søland TM, Brusevold IJ, Koppang HS, Schenck K, Bryne M. Nerve growth factor receptor (p75 NTR) and pattern of invasion predict poor prognosis in oral squamous cell carcinoma. Histopathology. 2008;53:62–72. doi: 10.1111/j.1365-2559.2008.03063.x. [DOI] [PubMed] [Google Scholar]
- 134.Kiyosue T, Kawano S, Matsubara R, Goto Y, Hirano M, Jinno T, Toyoshima T, Kitamura R, Oobu K, Nakamura S. Immunohistochemical location of the p75 neurotrophin receptor (p75NTR) in oral leukoplakia and oral squamous cell carcinoma. Int J Clin Oncol. 2011;18(1):154–163. doi: 10.1007/s10147-011-0358-4. [DOI] [PubMed] [Google Scholar]
- 135.Lewis Kelso R, Colome-Grimmer MI, Uchida T, Wang HQ, Wagner RF., Jr p75(NGFR) immunostaining for the detection of perineural invasion by cutaneous squamous cell carcinoma. Dermatol Surg. 2006;32:177–183. doi: 10.1111/j.1524-4725.2006.32032.x. [DOI] [PubMed] [Google Scholar]
- 136.Okumura T, Tsunoda S, Mori Y, Ito T, Kikuchi K, Wang TC, Yasumoto S, Shimada Y. The biological role of the low-affinity p75 neurotrophin receptor in esophageal squamous cell carcinoma. Clin Cancer Res. 2006;12:5096–5103. doi: 10.1158/1078-0432.CCR-05-2852. [DOI] [PubMed] [Google Scholar]
- 137.Huang S-D, Yuan Y, Liu X-H, Gong D-J, Bai C-G, Wang F, Luo J-H, Xu Z-Y. Self-renewal and chemotherapy resistance of p75NTR-positive cells in esophageal squamous cell carcinomas. BMC Cancer. 2009;9:9. doi: 10.1186/1471-2407-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Demont Y, Corbet C, Page A, Ataman-Önal Y, Choquet-Kastylevsky G, Fliniaux I, Le Bourhis X, Toillon R-A, Bradshaw RA, Hondermarck H. Pro-nerve growth factor induces autocrine stimulation of breast cancer cell invasion through tropomyosin-related kinase A (TrkA) and sortilin protein. J Biol Chem. 2012;287:1923–1931. doi: 10.1074/jbc.M110.211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vanhecke E, Adriaenssens E, Verbeke S, Meignan S, Germain E, Berteaux N, Nurcombe V, Le Bourhis X, Hondermarck H. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin Cancer Res. 2011;17:1741–1752. doi: 10.1158/1078-0432.CCR-10-1890. [DOI] [PubMed] [Google Scholar]
- 140.Descamps S, Pawlowski V, Révillion F, Hornez L, Hebbar M, Boilly B, Hondermarck H, Peyrat JP. Expression of nerve growth factor receptors and their prognostic value in human breast cancer. Cancer Res. 2001;61:4337–4340. [PubMed] [Google Scholar]
- 141.Descamps S, Lebourhis X, Delehedde M, Boilly B, Hondermarck H. Nerve growth factor is mitogenic for cancerous but not normal human breast epithelial cells. J Biol Chem. 1998;273:16659–16662. doi: 10.1074/jbc.273.27.16659. [DOI] [PubMed] [Google Scholar]
- 142.Dollé L, Oliveira M-J, Bruyneel E, Hondermarck H, Bracke M. Nerve growth factor mediates its pro-invasive effect in parallel with the release of a soluble E-cadherin fragment from breast cancer MCF-7/AZ cells. J Dairy Res. 2005;72:20–26. doi: 10.1017/s0022029905001160. [DOI] [PubMed] [Google Scholar]
- 143.Popnikolov NK, Cavone SM, Schultz PM, Garcia FU. Diagnostic utility of p75 neurotrophin receptor (p75NTR) as a marker of breast myoepithelial cells. Mod Pathol. 2005;18:1535–1541. doi: 10.1038/modpathol.3800487. [DOI] [PubMed] [Google Scholar]
- 144.Verbeke S, Meignan S, Lagadec C, Germain E, Hondermarck H, Adriaenssens E, Le Bourhis X. Overexpression of p75(NTR) increases survival of breast cancer cells through p21(waf1) Cell Signal. 2010;22:1864–1873. doi: 10.1016/j.cellsig.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 145.Descamps S, Toillon RA, Adriaenssens E, Pawlowski V, Cool SM, Nurcombe V, Le Bourhis X, Boilly B, Peyrat JP, Hondermarck H. Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem. 2001;276:17864–17870. doi: 10.1074/jbc.M010499200. [DOI] [PubMed] [Google Scholar]
- 146.Kim J, Villadsen R, Sørlie T, Fogh L, Grønlund SZ, Fridriksdottir AJ, Kuhn I, Rank F, Wielenga VT, Solvang H, et al. Tumor initiating but differentiated luminal-like breast cancer cells are highly invasive in the absence of basal-like activity. Proc Natl Acad Sci USA. 2012;109:6124–6129. doi: 10.1073/pnas.1203203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ibarra I, Erlich Y, Muthuswamy SK, Sachidanandam R, Hannon GJ. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 2007;21:3238–3243. doi: 10.1101/gad.1616307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Imai T, Tamai K, Oizumi S, Oyama K, Yamaguchi K, Sato I, Satoh K, Matsuura K, Saijo S, Sugamura K, et al. CD271 defines a stem cell-like population in hypopharyngeal cancer. PLoS ONE. 2013;8:e62002. doi: 10.1371/journal.pone.0062002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Biagiotti T, D’Amico M, Marzi I, Di Gennaro P, Arcangeli A, Wanke E, Olivotto M. Cell renewing in neuroblastoma: electrophysiological and immunocytochemical characterization of stem cells and derivatives. Stem Cells. 2006;24:443–453. doi: 10.1634/stemcells.2004-0264. [DOI] [PubMed] [Google Scholar]
- 150.Higuchi H, Yamashita T, Yoshikawa H, Tohyama M. PKA phosphorylates the p75 receptor and regulates its localization to lipid rafts. EMBO J. 2003;22:1790–1800. doi: 10.1093/emboj/cdg177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee MY, Ryu JM, Lee SH, Park JH, Han HJ. Lipid rafts play an important role for maintenance of embryonic stem cell self-renewal. J Lipid Res. 2010;51:2082–2089. doi: 10.1194/jlr.M001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yamazaki S, Iwama A, Morita Y, Eto K, Ema H, Nakauchi H. Cytokine signaling, lipid raft clustering, and HSC hibernation. Ann N Y Acad Sci. 2007;1106:54–63. doi: 10.1196/annals.1392.017. [DOI] [PubMed] [Google Scholar]
- 153.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 154.Campagnolo L, Russo MA, Puglianiello A, Favale A, Siracusa G. Mesenchymal cell precursors of peritubular smooth muscle cells of the mouse testis can be identified by the presence of the p75 neurotrophin receptor. Biol Reprod. 2001;64:464–472. doi: 10.1095/biolreprod64.2.464. [DOI] [PubMed] [Google Scholar]