Abstract
Genomic instability is one of the representative causes in genetic disorder, where the proper cellular response to DNA damage is essential in maintaining genomic stability. ATM and the Mre11-Rad50-Nbs1 (MRN) complex play critical roles in the cellular response to DNA damage such as DNA double-strand break (DSB). In this study, we report that Smad7 is indispensible in DNA damage response as a novel component of MRN complex. Smad7 enhances cell survival against DNA damage by accelerating ATM dependent DNA repair signaling. In Smad7-deficient mouse embryonic fibroblast cells, the loss of Smad7 decreases ATM activation and inhibits recruitment of ATM to the sites of DSBs. Smad7 interacts with Nbs1, a member of MRN complex, and enhances the interaction between ATM and Nbs1 upon DNA damage response, leading to phosphorylation of downstream substrates. Ectopic expression of Smad7 in the skin of mice enhances the phosphorylation of ATM upon X-irradiation. We found that effect of Smad7 on enhancing DNA repair is independent of its inhibitory activity of TGF-β signaling. Taken together, our results highlight a critical function of Smad7 in DSB response and establish the novel mechanism in which Smad7 facilitates the recruitment of ATM to the MRN complex through direct interaction with Nbs1.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-014-1687-z) contains supplementary material, which is available to authorized users.
Keywords: Carcinogenesis, DNA damage response (DDR), DNA damage checkpoint, Genotoxic stress, Neocarzinostatin (NCS)
Introduction
The human body faces diverse genotoxic assaults from endogenous cellular processes, including reactive oxygen species (ROS) produced during aerobic metabolism, as well as exogenous agents like ultraviolet (UV) or ionizing ration (IR) and various chemicals [1, 2]. Facing the consequent cellular DNA lesion, cells either immediately repair the damage by activating DNA damage response (DDR) proteins or induce suicidal cell death. DNA double-strand break (DSB) is an example of such lesions, where incorrect repair of DSBs ultimately leads to genetic disorders, such as cancer [3, 4].
Ataxia telangiectasia mutated (ATM) is a serine/threonine protein kinase that plays a central role in DSB repair. Inactive mutations in ATM cause the genetic disorder ataxia-telangiectasia (A-T), which is characterized by progressive cerebellar ataxia, immune deficiencies, radiation sensitivity, chromosome instability, premature aging, gonadal atrophy, and an increased risk of various cancers [5]. The Mre11-Rad50-Nbs1 (MRN) complex, when DSB is detected, recruits ATM to the sites of DNA damage and initiates the signaling process [6, 7]. Nbs1 in MRN complex interacts with ATM and it modulates nuclear localization and activity of Mre11 and Rad50. The MRN complex facilitates activation of ATM and enhances the affinity of ATM for DNA damage response (DDR) proteins, including H2AX, p53, 53BP1, Mdm2, Chk2, MDC1 and Brca1 which are involved in DNA repair and cell cycle check point control [4, 8]. Inactive ATM dimers dissociate to become active monomers through the intermolecular autophosphorylation of Serine 1981 (S1981), which initiates cellular ATM kinase activity [9]. H2AX, a downstream effector of ATM, is phosphorylated by ATM at serine 139 and forms nuclear foci at the initial stage of the repair process [10]. ATM also phosphorylates MDC1 and recruits phosphorylated H2AX (γH2AX) to the damage site. This cascade leads to ubiquitination of γH2AX that stabilizes 53BP1 and Brca1 at the regions of DNA damage [11–13].
Smad7 is an inhibitory Smad protein responsible for regulation of TGF-β signaling in various cellular processes, including cell proliferation, differentiation, the immune response, cell adhesion and migration, and apoptosis through negative-feedback mechanisms. Smad7 inhibits TGF-β signaling by blocking TGF-β type I receptor-mediated R-Smad activation [14] or by binding to the Smad-response element and interferes with the formation of functional Smad-DNA complexes in the nucleus [15]. In addition to its antagonistic role in TGF-β signaling, Smad7 participates in several other cellular functions such as liver fibrosis, skin wound healing, radiation-induced oral mucositis and apoptosis [16–21]. A recent report also suggests a possibility that Smad7 is associated with DNA repair system by showing localization of Smad7 in nuclear foci upon DNA damage response [22]. However, it is still unclear through what mechanism of Smad7 is involved in DSB repair process and its functional role.
In this study, we identified a new role of Smad7 in DSB repair. We demonstrated that Smad7 accelerates ATM dependent DNA repair signaling in cells and skin of mice. Loss of Smad7 results in the failure of ATM phosphorylation, leading to the defect in DSB repair process shown in Smad7−/− mouse embryonic fibroblast (MEF) cells. We revealed a novel mechanism in which Smad7 serves as a regulator of ATM activation in the DNA damage repair process, interacting directly with the MRN complex through Nbs1 and enhancing the interaction between ATM and Nbs1.
Materials and methods
Cell culture and treatment
Cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured according to ATCC guidelines. Smad7+/− and Smad7−/− MEF cells were provided by Dr. YJ Lee (Gachon University). Cells stably expressing Smad7 were made by using pLPCX retroviral vector. Flag tagged Smad7 was inserted into pLPCX and viruses were produced according to the manufacturer’s protocol (Cell Biolabs, Inc., San Diego, CA, USA). After infection, 2 μg/ml puromycin was treated for selection of cells expressing Smad7. Cells containing empty pLPCX vector were used as a control. The radiomimetic compound neocarzinostatin (Sigma, St. Louis, MO, USA) was used to induce the DNA double-strand breaks.
Plasmid constructs and transfection
All ATM and Smad7 constructs were generated using RT-PCR. The amplified ATM fragments were cloned into the pCMV-Myc vector. Smad7 constructs were amplified and inserted into either pEBG-GST or pFlag-CMV-6 vectors. The full-length sequence was introduced into pCS4-3HA to generate the Nbs1 construct. The ATM-S1893A mutant and Smad7-GSD (G244A, S247A, and D248A) were generated using site-directed mutagenesis. The cells were transfected with FuGENE6 (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer’s protocol.
Antibodies
Antibodies against Myc (9E10), HA (Y11), Smad6/7 (N-19), GST and phospho-ATM (sc-47739) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The α-tubulin, β-actin, and Flag (M2) antibodies were purchased from Sigma–Aldrich (St. Louis, MO, USA). The γ-H2AX (JBW301) antibody was purchased from Upstate Biotechnology (Temecula, CA, USA). Phospho-ATM, ATM, NBS1, Mre11, Rad50, γ-H2AX, and GFP antibodies were obtained from Abcam, Cambridge, MA, USA. The Chk2 pAb (T68) was purchased from Cell Signaling, Inc. (Danvers, MA, USA), and the Smad7 antibody was purchased from the Imgenex Corporation (San Diego, CA, USA).
Cell survival assay
The cells were seeded onto 60-mm dishes and treated with NCS at concentrations ranging from 0 to 80 ng/ml. The cells were incubated for 10–14 days at 37 °C with 5 % CO2, and the surviving colonies were stained with 2 % methylene blue in 50 % ethanol. The colonies were counted using ImageJ software (NIH, Bethesda, MD).
Comet assay
The cells were treated with 0 or 200 ng/ml NCS and incubated for 0 or 4 h for recovery. Single-cell gel electrophoresis assays were performed using a Comet Assay kit (Trevigen, Gaithersburg, MD, 20877) according to the manufacturer’s instructions. The cells were analyzed using a Zeiss LSM META 510 confocal microscope (Carl Zeiss, Sachsen, Thüringen, Germany). The percent tail moment was determined using CometScore16 software.
G2/M checkpoint analysis
Cells were incubated in the presence or absence of 200 ng/ml NCS, and then harvested 3 h after treatment. The G2/M checkpoint analysis was performed as previously described, with modifications [23]. The fixed cells were incubated with an anti-phospho-histone H3 (S10)-Alexa Fluor 488-conjugated antibody (Cell Signaling Technology) and stained with propidium iodide. The cells were analyzed using fluorescence activated cell sorting (FACS). The percentage of phospho-H3-positive cells was analyzed using CELLQUEST (Becton–Dickinson).
Immunoprecipitation and western blot analysis
The cells were lysed in RIPA buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 % Nonidet P-40, 0.5 % sodium deoxycholate, 0.1 % sodium dodecyl sulfate and a protease inhibitor cocktail (Roche Diagnostic Corp.). For immunoprecipitation, the cell lysate (500 μg–1 mg) was incubated with the appropriate primary antibodies overnight at 4 °C. Dynabeads Protein G (Invitrogen, Carlsbad, CA, USA) was added to the antibody-bound protein samples and incubated at 4 °C according to the manufacturer’s instructions. After three or four washes, the bead-bound proteins were eluted in SDS sample buffer, separated in an SDS-PAGE gel, and electro-transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The protein expression was analyzed by western blotting using appropriate antibodies.
Immunofluorescence
Immunofluorescence analysis was performed as previously described [24]. The cells were fixed at the indicated time points after NCS treatment. The nuclear foci were examined with a Zeiss LSM META 510 confocal microscope (Carl Zeiss, Sachsen, Thüringen, Germany) and counted using ImageJ software (available for free from NIH, Bethesda, MD).
X-irradiation-induced DNA damage
Ten each 6-week-old B6 (control) and Smad7TG mice were used in this study [25]. The mice were exposed to 5 Gy X-irradiation and sacrificed at the indicated time points. The mouse skin tissues were collected for further study.
Immunohistochemistry
Paraffin-embedded mouse skin sections were immunostained as previously described [26].
Statistical analysis
All quantitative data are presented as the mean ± SD. The statistical analysis was performed using an unpaired t test. p values <0.05 were considered statistically significant.
Results
Smad7 accelerates DSB repair
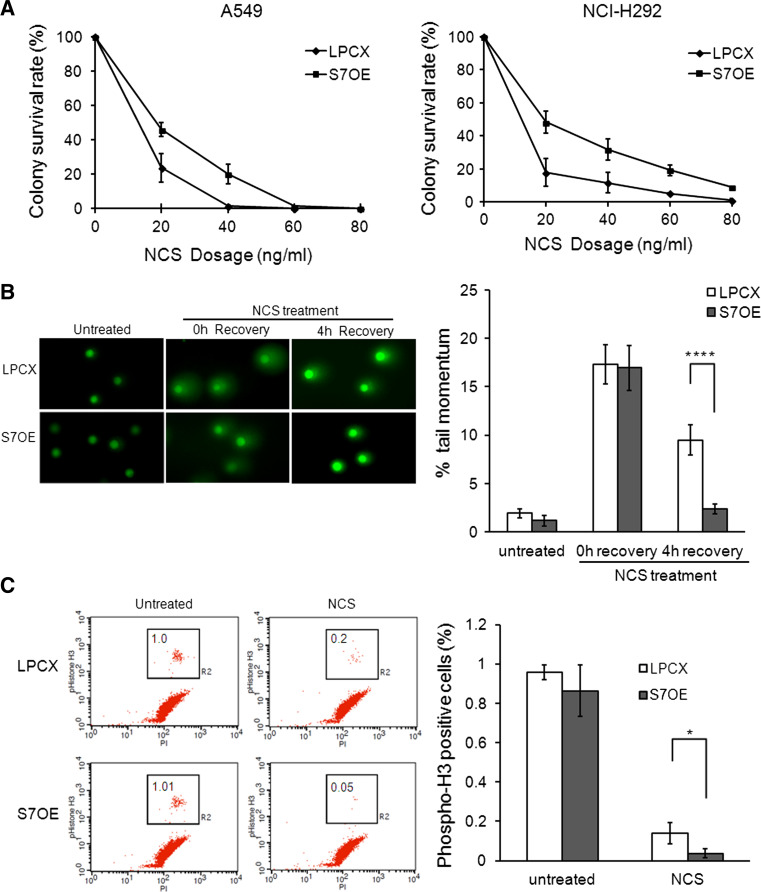
To investigate whether Smad7 has an effect on the DSB response, we examined cell survival using a colony formation assay after neocarzinostatin (NCS, a radiomimetic drug)-induced DNA damage in control (LPCX) and Smad7-overexpressing (S7OE) A549 and NCI-H292 lung cancer cells. We observed increased colony survival rates in Smad7-overexpressing cells compared to control cells at several dosages of NCS (Fig. 1a). To determine whether the overexpression of Smad7 affects DNA repair ability, we performed a comet assay. We treated cells with NCS and allowed them to recover for 0 or 4 h. As shown in Fig. 1b, after 4 h of recovery, the tail moment was significantly lower (approximately fourfold, p < 0.0001) in the Smad7-overexpressing cells than in the control cells. This result indicates that Smad7 increases cell survival by enhancing DNA repair.
Fig. 1.
Smad7 enhances DSB response. a The cells were treated with NCS (from 20 to 80 ng/ml) and incubated for 10–14 days in control or Smad7-overexpressing (S7OE) A549 and NCI-H292 cells. The surviving colonies were stained with 2 % methylene blue. The number of surviving colonies was counted. At least three independent experiments were performed for each analysis. b Single-cell electrophoresis was performed in control or S7OE A549 cells treated with or without 200 ng/ml NCS. The NCS-treated cells were allowed to recover for 0 or 4 h. To evaluate DNA damage repair, the % tail moment was calculated using the Cometscore16 program. Student’s t test showed that the % tail moment was significantly different in LPCX and S7OE cells at p < 0.0001 (****). c The activation of the G2/M checkpoint was examined in control and S7OE A549 cells at 3 h after treatment with 200 ng/ml NCS. The cells were fixed in 1 % formaldehyde and stained with Alexa Fluor 488-conjugated anti-phospho-histone H3 (S10) antibody and propidium iodide. The percentage of cells in M phase was calculated through FACS analysis. The percentage of phospho-H3-positive cells was significantly different between LPCX and S7OE cells at p < 0.05 (*), according to Student’s t test
When DNA is damaged, cells activate the G2/M checkpoint to prevent cells from entering the mitosis for repair and to stop the proliferation of damaged cells. We investigated G2/M checkpoint activation through detection of phospho-histone H3 (S10) positive cells after NCS-induced DNA damage using fluorescence activated cell sorting (FACS) assay. No significant difference in the percentage of cells in M phase was observed between control and Smad7-overexpressing cells in the absence of NCS treatment. In contrast, 3 h after NCS treatment, we found that a significantly smaller percentage of the cell population (approximately fourfold) had entered mitosis in Smad7-overexpressing cells compared to control cells (Fig. 1c). Similar result was observed 1 h after NCS treatment (Online Resource 1A). The decrease in mitotic cell numbers in Smad7-overexpressing cells was not due to faster M/G1 transition or due to mitotic cell death after DNA damage (Online Resource 1B). These results indicate that Smad7 promotes G2 checkpoint arrest after DNA damage for repair. We also examined the activation of Chk2, a known checkpoint kinase that controls cell cycle, in response to DSB. The activation of Chk2 was increased in Smad7-overexpressing A549 and NCI-H292 cells compared with that in the control cells at 1 h after NCS treatment (Online Resource 2). Taken together, these results suggest that Smad7 plays a critical role in the DNA damage checkpoint.
Smad7 increases the activation of ATM and H2AX in response to DNA damage
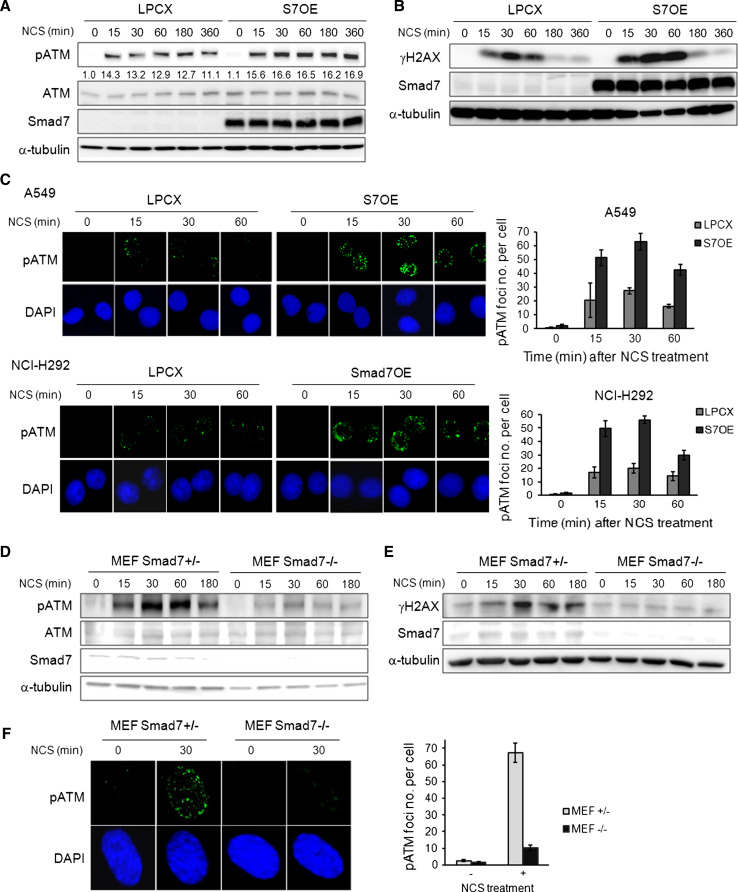
As ATM is a critical modulator that initiates DNA repair process in DSB response, we investigated whether Smad7 affects ATM activation in the DSB response pathway. We examined the phosphorylation level of ATM by using phospho-ATM (S1981) antibody in A549, which showed a strong activation of ATM and p53 [27], and NCI-H292 lung cancer cells after NCS treatment [23]. The activation of ATM was observed at a relatively early time point, approximately 15 min after NCS treatment in control and Smad7-overexpressing A549 and NCI-H292 lung cancer cells. The overexpression of Smad7 increased the phosphorylation of ATM upon NCS treatment (Fig. 2a; Online Resource 3a). We also observed that overexpression of Smad7 increased the phosphorylation of H2AX at a relatively early time points, 15 and 30 min, in response to DSB stress (Fig. 2b; Online Resource 3b). We investigated the effect of Smad7 on DNA repair efficiency by observing the nuclear foci formed by the DDR machinery after DSB induction. Smad7 overexpression enhanced the formation of nuclear foci at the DSBs, as shown by immunofluorescence with phospho-ATM- or γH2AX-specific antibodies (Fig. 2c; Online Resource 4). These data imply that Smad7 enhances DNA repair by increasing the activation of ATM and H2AX and suggest that Smad7 might be an upstream regulator of ATM.
Fig. 2.
Smad7 enhances the activity of ATM. a The expression of phospho-ATM was examined in control and S7OE A549 cells at the indicated time points after treatment with 200 ng/ml NCS. The anti-Flag antibody was used to detect Flag-tagged Smad7. Equal protein loading was confirmed by α-tubulin detection. Ratio pATM/ATM was calculated by densitometric analysis. b Control or S7OE A549 cells were treated with 200 ng/ml NCS, and the expression of γH2AX was examined at the indicated time points. Smad7 antibody was used to detect Smad7 overexpression. α-tubulin was used as a loading control. c Phospho-ATM nuclear foci formation was assessed in control and S7OE A549 and NCI-H292 cells at the indicated time points after treatment with 100 ng/ml NCS. DAPI was used for nuclear staining. d The ATM phosphorylation in Smad7+/− and Smad7−/− MEF cells was examined at the indicated time points after treatment with 200 ng/ml NCS. Endogenous Smad7 levels were assessed using an anti-Smad7 antibody. Equal protein loading was confirmed by α-tubulin detection. e The activation of H2AX was examined in Smad7+/− and Smad7−/− MEF cells at the indicated time points upon 200 ng/ml NCS treatment. Endogenous Smad7 levels were detected using an anti-Smad7 antibody. Equal protein loading was confirmed by α-tubulin detection. f Smad7+/− and Smad7−/− MEF cells were treated with 100 ng/ml NCS for 30 min. The cells were then fixed and incubated with a phospho-ATM-specific antibody to stain the nuclear foci and DAPI to stain the nuclear DNA
The loss of Smad7 leads to failures in ATM phosphorylation and nuclear foci formation at the site of DNA damage after NCS treatment
Next, we examined whether the loss of Smad7 affects the activation of ATM by investigating ATM phosphorylation in Smad7+/− and Smad7−/− MEF cells after NCS treatment. The loss of Smad7 dramatically reduced the activation of ATM at 15–180 min after NCS treatment without changing the total ATM expression (Fig. 2d). We also observed that activation of γH2AX is suppressed in both Smad7−/− MEF cells and Smad7 knock-down cells in response to NCS treatment (Fig. 2e; Online Resource 5). When we examined phospho-ATM nuclear foci formation in MEF cells 1 h after NCS treatment, the number of nuclear foci was greatly increased, by 27-fold, in Smad7+/− MEF cells, but only, by 6.8-fold, in Smad7−/− MEF cells (Fig. 2f). We also observed that depletion of Smad7 sensitizes cells to NCS-inducing DNA damage (Online Resource 6). In summary, loss of Smad7 leads to failure of nuclear foci formation and impaired recruitment of activated ATM to the site of DSB.
Smad7 co-localizes with DNA damage sensors at the sites of DNA damage and interacts with the ATM/MRN complex upon NCS treatment
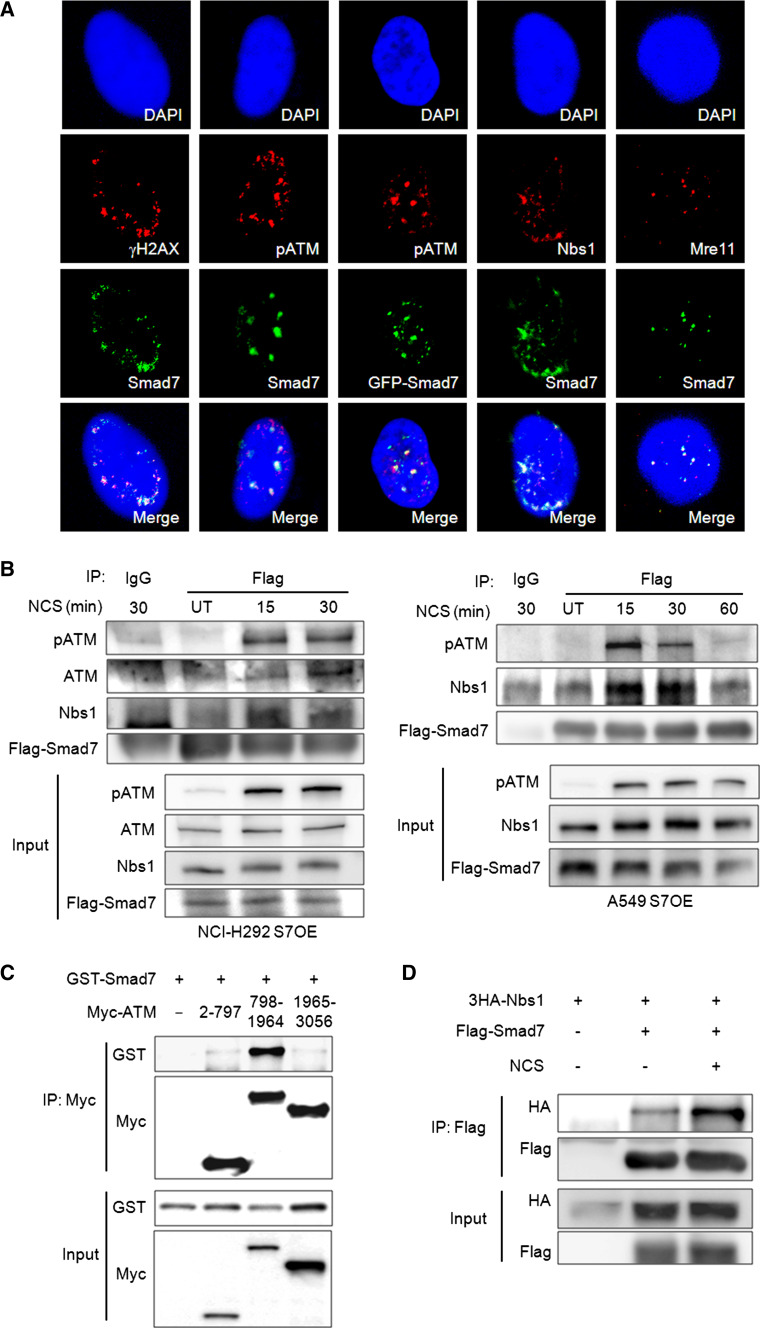
The recruitment of DDR elements to DSBs is essential cellular response to activate repair machinery. The recruited proteins appear as discrete nuclear foci, and these foci serve as an indicator of the DNA repair response. Therefore, we tested whether Smad7 is also recruited to nuclear foci when cells are exposed to a DSB-inducing agent. We observed that Smad7 was recruited to nuclear foci and approximately 72 or 74 % of Smad7 were co-localized with γH2AX or phospho-ATM in response to DSB induction (Fig. 3a). We also confirmed the co-localization of Smad7 with other DNA damage sensors, Nbs1 and Mre11 at NCS-induced DSBs. These results imply that Smad7 may play an important role in DSB response as a DDR element.
Fig. 3.
Smad7 interacts with ATM and Nbs1 upon DSB response and co-localizes with them at nuclear foci. a To determine whether Smad7 co-localized with Nbs1, Mre11, γH2AX or phospho-ATM at the site of DNA damage, S7OE A549 cells were treated with 50 ng/ml NCS for 1 h. b Whole cell lysates were prepared at the indicated time points after treatment with 200 ng/ml NCS in S7OE A549 and NCI-H292 cells. Flag-tagged Smad7 was pulled down using an anti-Flag antibody, and western blotting was performed using the indicated antibodies. c GST-tagged Smad7 was co-expressed with a Myc-tagged ATM fragment in 293T cells. ATM fragments described in supplemental material Fig. S4 were immunoprecipitated with the Myc antibody, and the interacting Smad7 was detected using an anti-GST antibody. d To confirm the Nbs1-Smad7 interaction, Smad7 was immunoprecipitated with the Flag-specific antibody and the HA-specific antibody was used to detect the interacting Nbs1. Cells were treated with 200 ng/ml NCS to induce DSB stress
To determine whether Smad7-induced activation and nuclear foci formation of ATM are mediated by the physical interaction of Smad7 with the ATM/MRN complex, we performed an immunoprecipitation assay. We used a Flag-specific antibody rather than the Smad7-specific antibody to immunoprecipitate Flag-tagged Smad7 due to this antibody’s limited capacity. ATM and Smad7 binding was maximized at 30 min after NCS treatment in NCI-H292 cells (Fig. 3b). A strong interaction of Smad7 with phospho-ATM was detected at 15 min after NCS treatment and persisted until 30 min (Fig. 3b). We also observed that Smad7 interacted with Nbs1, a member of the MRN complex, with an association pattern similar to that observed for phospho-ATM (Fig. 3b). We also confirmed Smad7-ATM and Smad7-Nbs1 interaction through transient overexpression system. To verify the important binding region of ATM to Smad7, we used DNA constructs expressing ATM fragments. We observed that Smad7 bound primarily to the middle region (798–1964 aa) of ATM, containing the HEAT7 repeats which is known to interact with Nbs1 (Fig. 3c; Online Resource 7) [28–30]. We also verified enhanced Smad7-Nbs1 interaction upon NCS treatment (Fig. 3d). These data indicate that the interaction of Smad7 with ATM and Nbs1 is induced by DSBs.
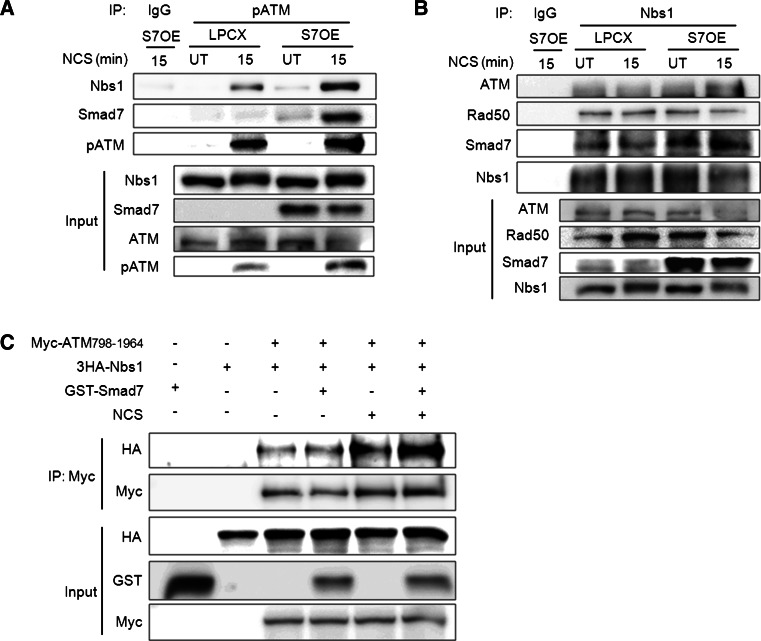
Smad7 enhances the interaction between ATM and Nbs1 after DNA damage
The association of Smad7 with ATM and Nbs1 led us to examine whether Smad7 facilitates the recruitment of ATM to the MRN complex upon DSBs response. The interaction between phospho-ATM and Nbs1 was greatly increased in Smad7-overexpressing cells compared with control cells at 15 min after NCS treatment. We also confirmed that the interaction of phospho-ATM with Smad7 in Smad7-overexpressing A549 cells in response to NCS treatment (Fig. 4a). Next, we performed an immunoprecipitation assay using Nbs1-specific antibody. The binding of Nbs1 and ATM was gradually increased in Smad7-overexpressing cells compared with control cells at 15 min after NCS treatment. However, overexpression of Smad7 had no effect on Nbs1-Rad50 interaction. The interaction between Nbs1 and Smad7 also confirmed (Fig. 4b). Next, we investigated whether the interaction of ATM with Nbs1 is enhanced in the presence of Smad7 in response to NCS treatment using a transient overexpression system in vitro. The binding between ATM and Nbs1 was dramatically increased after NCS treatment, and this interaction was significantly enhanced by addition of Smad7 (Fig. 4c). These data imply that Smad7 facilitates the recruitment of ATM to the MRN complex through Nbs1 at DSBs.
Fig. 4.
Smad7 enhances the interaction between ATM and Nbs1 in response to NCS treatment. a, b Untreated A549 cells (UT) or A549 cells treated with NCS for 15 min were harvested. Whole cell lysates were immunoprecipitated using an a anti-phospho-ATM or b anti-Nbs1 antibody and western blotting was performed using the indicated antibodies. Normal IgG was used as a negative control for the immunoprecipitation. The overexpression of Smad7 was confirmed using a Smad7-specific antibody. c To investigate whether Smad7 facilitates the interaction between ATM and Nbs1, Myc-ATM-798-1964, 3HA-Nbs1 and GST-Smad7 were co-transfected into 293 T cells. The cells were treated with 200 ng/ml NCS to determine the effect of NCS-induced DSB on the ATM-Nbs1 interaction. Whole cell lysates were immunoprecipitated using a Myc antibody to pull-down ATM, and western blotting was performed to detect the protein interaction
Mapping of interacting domains in ATM/Smad7 and Nbs1/Smad7
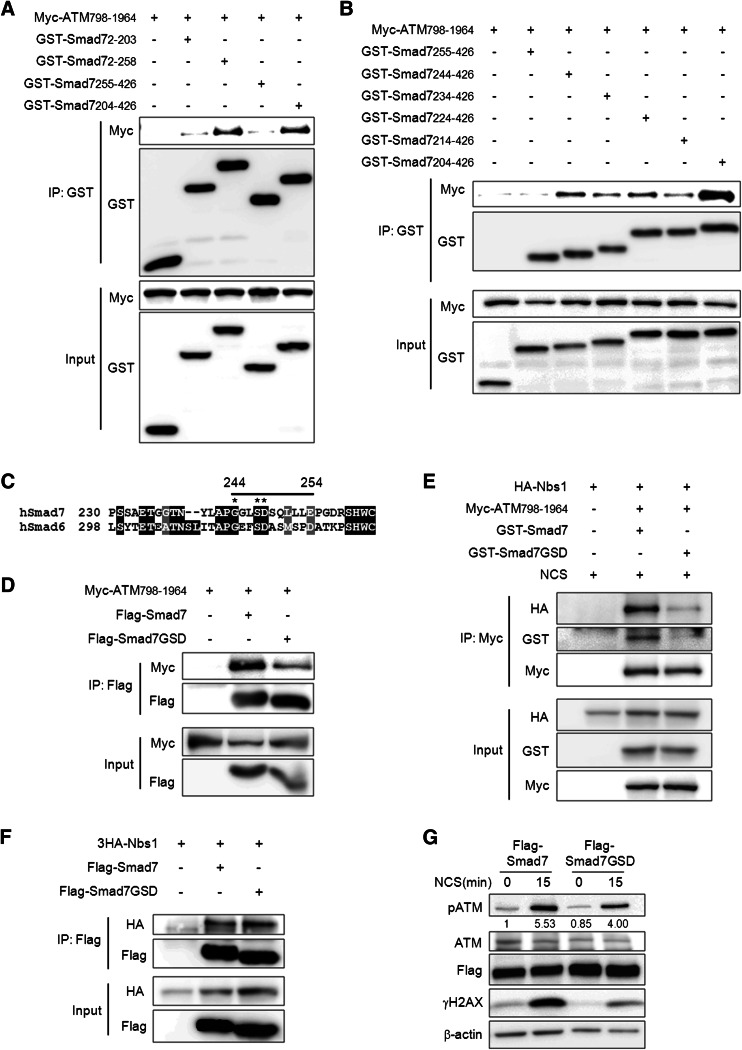
To map the ATM-binding domain in Smad7, we used Smad7 deletion constructs (Online Resource 8). Amino acids 204–255 of Smad7 are responsible for its interaction with ATM (Fig. 5a). To better define the residues of the Smad7 protein that are responsible for its binding to ATM, we generated a series of deletion mutants of the Smad7 204–426 aa fragment (Online Resource 8A). A Smad7 deletion mutant lacking residues 244–254 failed to interact with ATM (Fig. 5b), suggesting that residues 244–254 in Smad7 are critical for its interaction with ATM. Because Smad6, another inhibitory Smad that has a similar structure and function with Smad7, also interacted with ATM, we compared residues 244–254 of Smad7 with the corresponding domain of Smad6 (Fig. 5c; Online Resource 8b). To further define the ATM-binding sequences in residues 244–254 of Smad7, we generated three amino acid substitution mutants of Smad7. Substitutions of Ala at Gly 244, Ser 247, and Asp 248 in Smad7, which are also conserved in Smad6, significantly reduced the interaction of Smad7 with ATM 798–1964 aa (Fig. 5d). Interestingly, GSD mutations in Smad7 not only reduced the interaction of Smad7 with ATM but also markedly decreased the interaction between ATM and Nbs1 in response to NCS treatment compared to wild type, indicating that GSD sites in Smad7 are crucially involved in interaction between Smad7 and ATM and the complex formation of ATM/Nbs1 (Fig. 5e). However, GSD mutations in Smad7 had no effect on the interaction of Nbs1 with Smad7 (Fig. 5f). Next, to verify whether interference of the Smad7/Nbs1/ATM complex formation by GSD mutations affects ATM activity, we examined the expression of phospho-ATM and γH2AX. GSD mutations in Smad7 significantly reduced the activation of ATM and H2AX compared with wild type Smad7 at 15 min after NCS treatment (Fig. 5g). These data imply that the interaction of Smad7 with ATM is critical for the Nbs1-ATM binding and the MRN complex-mediated activation of ATM by DSB stress.
Fig. 5.
Identification of the binding sites that mediate the interaction of ATM and Smad7. a, b To identify the region of Smad7 responsible for its interaction with ATM 798-1964, Smad7 fragments described in supplemental material Fig. S5 were pulled-down using Glutathione Sepharose 4b beads, and a Myc-specific antibody was used to detect the interacting ATM. c Alignment of hSmad7 and hSmad6. The straight black line indicates amino acids 244–254 of Smad7. *indicate sites that were mutated. d To identify the sites that are important for the interaction of Smad7 and ATM-798-1964, we mutated three sites (G244A, S247A, and D248A) on Smad7 and examined the interactions of the mutated proteins with ATM 798-196. Whole cell lysates from the wild type or mutant (Smad7-GSD) Smad7 were immunoprecipitated using an anti-Flag antibody, and western blotting was performed using an anti-Myc antibody to detect the interacting ATM. e Cells were transfected with Myc-ATM-798-1964, 3HA-Nbs1 and GST-Smad7 or GST-Smad7-GSD mutant. The cells were treated with 200 ng/ml NCS to induce DSB. Whole cell lysates were immunoprecipitated using anti-Myc antibody and western blotting was performed using indicated antibody. f To identify whether Smad7-GSD mutant also affect the interactions with Nbs1, whole cell lysates from the wild type or Smad7-GSD mutant was immunoprecipitated using an anti-Flag antibody, and western blotting was performed using anti-HA antibody to detect the interacting Nbs1. g Wild or mutant (Smad7-GSD) Smad7 construct was transiently transfected into A549 cells. Cells were treated with 200 ng/ml NCS for 15 min and the expression of phospho-ATM or γH2AX was examined. Equal protein loading was confirmed by β-actin detection. Ratio pATM/ATM was calculated by densitometric analysis
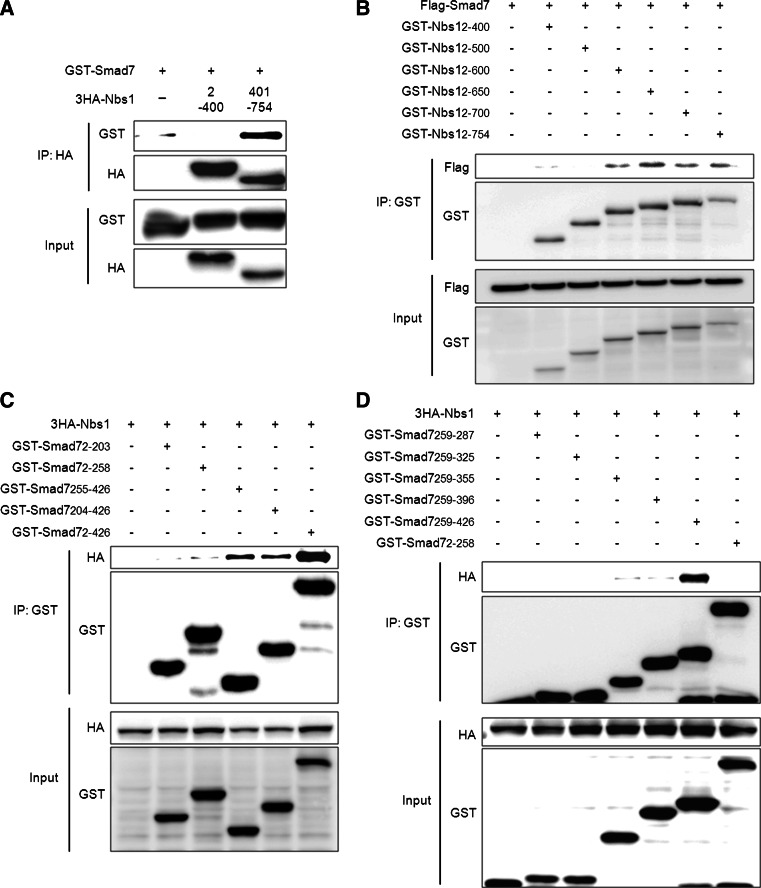
To investigate the relationship between Smad7 and Nbs1 via mapping Smad7-Nbs1 binding domains, we examined the interaction between Smad7 and Nbs1 fragments. We identified that the C-terminal region of Nbs1 (401–754 aa) interacted with Smad7 (Fig. 6a). To map the specific binding region of Nbs1 with Smad7, we used Nbs1 deletion mutants. The Smad7 interacted with the Nbs1 fragments containing 500–600 aa (Fig. 6b). We also identified the domains of Smad7 that interact with Nbs1 using deletion mutants of Smad7. Nbs1 only interacted with Smad7 deletion mutants containing MH2 domain (255–426 aa) (Fig. 6c). To investigate specific binding region of the Nbs1-binding regions in MH2 domain of Smad7, we used Smad7 deletion mutants. Our results showed that the residues 397–426 of Smad7 are critical for its interaction with Nbs1 (Fig. 6d; Online Resource 9).
Fig. 6.
Identification of the binding regions that are critical for Nbs1-Smad7 interaction. a GST-tagged Smad7 was transfected along with a 3HA-tagged Nbs1 fragment. Whole cell lysates were pulled-down with the HA-specific antibody and the interacting Smad7 was detected using an anti-GST antibody. b To identify the specific binding region of Nbs1 that is important for its interaction with Smad7, Nbs1 fragments were immunoprecipitated with Glutathione Sepharose 4b beads, and the interacting Smad7 was detected using the Flag-specific antibody. c, d To identify the region of Smad7 that is important for its interaction with Nbs1, all Smad7 fragments described in Online Resource 8 were immunoprecipitated using Glutathione Sepharose 4b beads, and the interacting Nbs1 was detected using the HA-specific antibody
Smad7 increases the activation of ATM and γH2AX in response to DNA damage in vivo in a mouse model
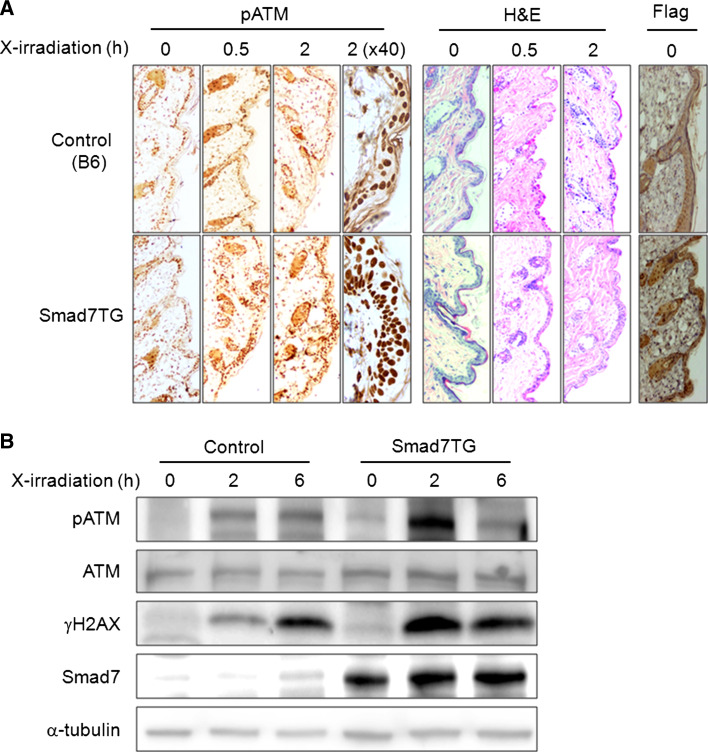
To elucidate the function of Smad7 overexpression in the DNA damage response in vivo, we examined the expression of phospho-ATM and γH2AX in the skin of control mice (B6) and in transgenic mice (Smad7TG) bearing Smad7 under the control of a keratin-K5 promoter (K5.Smad7) after DNA damage [25]. We treated the mice with a relatively low dose of X-irradiation (5 Gy) and assessed the protein expression in the mouse skin using immunohistochemistry or immunoblotting at the indicated times after treatment. The phosphorylation of ATM was dramatically increased in Smad7TG mouse skin compared with the control mouse skin at 6 h after radiation (Fig. 7a). Images of the epidermis that showed significant differences in phosphorylation were examined at higher magnification (40×) to allow a better comparison of wild type and Smad7TG (Fig. 7a). We also observed that the phosphorylation of ATM and H2AX was much higher in the Smad7TG mouse than in the control at 2 h after X-irradiation (Fig. 7b). These data indicates that Smad7 accelerates activation of DNA repair signaling for maintaining homeostasis in vivo as well as in vitro.
Fig. 7.
Ectopic expression of Smad7 increases the activation of ATM and γH2AX in mouse skin in response to X-irradiation. Control (B6) and Smad7 transgenic (Smad7TG) mice were exposed to X-irradiation (5 Gy) and sacrificed 0, 0.5 or 2 h after X-irradiation. a The mouse skin sections were paraffin embedded and subsequently prepared and analyzed with immunohistochemistry using phospho-ATM antibody. The mouse skin sections were also analyzed under high power magnification (40×) 2 h after X-irradiation. The anti-Flag antibody was used to confirm the overexpression of Smad7. H and E staining was performed to stain the nuclei. b Whole cell lysates were prepared from mouse skin samples, and the protein expression levels were examined by western blotting with the appropriate antibodies. A Smad7-specific antibody was used to detect Smad7 overexpression, and α-tubulin was detected to confirm equal protein loading
Discussion
Smad7 is an inhibitory Smad that negatively regulates the TGF-β signaling pathway [14]. In addition, Smad7 participates in several cellular processes in many systems [15, 17, 20, 21, 31]. Smad7 sensitizes tumor cells to tumor necrosis factor-α and other death signals by modulating the NF-κB survival pathway [32, 33]. Smad7 regulates gene transcription through interactions with histone deacetylases such as HDAC1 and SIRT1 and the acetyltransferase p300 [34, 35]. A recent report showed that Smad7 was recruited into DSB sites induced by IR [22]. However, it is still unknown how Smad7 regulates DSB repair process. Here we have identified Smad7 as a critical regulator of DNA damage repair process by directly interacting with MRN complex. The overexpression of Smad7 enhanced the activation of DSB response elements and promoted DNA damage repair, thus increasing cell survival, and the loss of Smad7 reduced the activation of DNA repair-related proteins in response to genotoxic stress (Figs. 1, 2, 7). Furthermore, we revealed that Smad7 regulates the ATM-mediated DSB response pathways through direct interactions with ATM and Nbs1 (Fig. 3).
The MRN complex recognizes DSBs and recruits ATM to DSBs to initiate repair [7]. The interaction of Nbs1 with ATM promotes the recruitment of ATM to DSBs and the MRN-dependent activation of ATM [6, 28, 36–38]. Recent papers have revealed new components of the MRN complex that regulates the ATM-MRN interaction and the recruitment of ATM to the DSBs. The tandem BRCT repeats of 53BP1 interact directly with the MRN complex through Rad50, which promotes the ATM-dependent phosphorylation of substrates, amplifying the accumulation of MRN and ATM at DSBs [39]. Furthermore, Skp2 binds to Nbs1 and triggers Nbs1 ubiquitination, which facilitates the interaction between Nbs1 and ATM, promoting ATM recruitment to the DNA nuclear foci for its activation in response to DSBs [40]. However, it still needs to be established how the ATM-MRN interaction and recruitment of ATM to the DSBs are regulated by new components of the MRN complex. In this study, we have described that Smad7 binds directly to Nbs1 and promotes the recruitment of ATM to DSBs by enhancing the affinity of Nbs1 for ATM in response to DNA damage (Fig. 4). GSD mutation in Smad7 which reduced the interaction of Smad7 with ATM also decreased the ATM-Nbs1 interaction (Fig. 5e). Our finding suggests that Smad7 is a novel component of the MRN complex that regulates ATM activity in response to DSBs.
The relationship between TGF-β signaling and ATM activation has been described in several previous studies [27, 41–43]. The loss of TGF-β signaling attenuates the phosphorylation of ATM and the activity of downstream DNA damage response substrates in response to genotoxic stress [41]. In another study, TGF-β1 antisense was shown to reduce the phosphorylation of ATM, p53, and Chk2 after ionizing irradiation in A549 cells, thus impacting DNA repair [27]. Dubrovskaet et al. [42] showed that Smad3 interacts with BRCA1 upon TGF-β1 treatment and suppresses BRCA-1-dependent DSB repair in human epithelial cells. It is also reported that TGF-β1 inhibited DNA repair and reduced the rate of cell survival by down-regulating the protein expression of Rad51, which is known to be involved in DNA DSB repair in Mu1Lu cells. The TGF-β receptor-dependent activation of Smad3 and Smad4 is crucial for this event [43]. A recent publication reported the possibility that Smad7 could associate with nuclear foci formation in response to DSB, and suggested that this association may depend on TβRI in human fibroblasts 82-6 cells [22]. However, a different study suggested that TβRI and Smad2 are not involved in the rapid activation of ATM and p53 in A549 cells [27]. Smad7 overexpression in SNU638 cells that lacks a functional TGF-β type II receptor [44] enhanced the phosphorylation of ATM and Chk2 at 15 min after NCS treatment (Online Resource 10). An in vivo study using a Smad7 transgenic mouse, which expresses barely detectable levels of the type I and II for TGF-β receptors [45], also showed an increase in the phosphorylation of ATM in its epidermis in response to X-irradiation (Fig. 7). These findings indicate that various signaling intermediates of TGF-β signaling pathway may target different DNA damage response substrates during DSB response, and this may occur dependent on cell-type. Smad7 expression is induced by TGF-β family members or other stimuli such as halofuginone (a widely used alkaloid coccidiostat) and IFN-γ [46, 47], suggesting that Smad7 expression induced by various stimuli may participate in the DSB damage repair pathway. Interestingly, we found that Smad6, another inhibitory Smad protein, also interact with ATM (Online Resource 11), indicating that inhibitory Smad proteins may regulate DNA repair responses to TGF-β. This is distinct from the traditional function defined for the inhibitory Smad protein as an inhibitor of TGF-β signal. Since there are several papers showed that Smad2 and Smad3 also have a role in DSB repair [22, 42], we tested whether Smad2, 3 and 4 interact with ATM. We could detect the interaction of Smad3 and ATM (unpublished data). We are currently investigating the biological significance of Smad3 interaction with ATM.
In summary, the results of the present study demonstrate a new function for Smad7 in the nucleus as a modulator of the DNA repair pathway.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank Wang, XJ for the K7.Smad7 transgenic mice and Yoon, K, Choi, CY, and Kim, TK for technical support. This work was supported by Basic Science Research Program (NRF 2011-0014281) and the Bio-Synergy Research Project (NRF-2012M3A9C4048735) of the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF).
Conflict of interest
All authors declare that they have no competing interests.
References
- 1.Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26(56):7773–7779. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- 2.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability—an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11(3):220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 3.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23(5):687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 4.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432(7015):316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 5.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9(10):759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 6.Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22(20):5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paull TT, Lee JH. The Mre11/Rad50/Nbs1 complex and its role as a DNA double-strand break sensor for ATM. Cell Cycle. 2005;4(6):737–740. doi: 10.4161/cc.4.6.1715. [DOI] [PubMed] [Google Scholar]
- 8.Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60(21):5934–5936. [PubMed] [Google Scholar]
- 9.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421(6922):499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 10.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276(45):42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 11.Derheimer FA, Kastan MB. Multiple roles of ATM in monitoring and maintaining DNA integrity. FEBS Lett. 2010;584(17):3675–3681. doi: 10.1016/j.febslet.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozlov SV, Graham ME, Peng C, Chen P, Robinson PJ, Lavin MF. Involvement of novel autophosphorylation sites in ATM activation. EMBO J. 2006;25(15):3504–3514. doi: 10.1038/sj.emboj.7601231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, Sweeney FD, Panier S, Mendez M, Wildenhain J, Thomson TM, Pelletier L, Jackson SP, Durocher D. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318(5856):1637–1640. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh S, Landstrom M, Hermansson A, Itoh F, Heldin CH, Heldin NE, ten Dijke P. Transforming growth factor beta1 induces nuclear export of inhibitory Smad7. J Biol Chem. 1998;273(44):29195–29201. doi: 10.1074/jbc.273.44.29195. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng XH, Meng A, Chen YG. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol. 2007;27(12):4488–4499. doi: 10.1128/MCB.01636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Ekman M, Thakur N, Bu S, Davoodpour P, Grimsby S, Tagami S, Heldin CH, Landstrom M. TGFbeta1-induced activation of ATM and p53 mediates apoptosis in a Smad7-dependent manner. Cell Cycle. 2006;5(23):2787–2795. doi: 10.4161/cc.5.23.3523. [DOI] [PubMed] [Google Scholar]
- 17.Okado T, Terada Y, Tanaka H, Inoshita S, Nakao A, Sasaki S. Smad7 mediates transforming growth factor-beta-induced apoptosis in mesangial cells. Kidney Int. 2002;62(4):1178–1186. doi: 10.1111/j.1523-1755.2002.kid583.x. [DOI] [PubMed] [Google Scholar]
- 18.Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, Ten Dijke P, Gressner AM. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003;125(1):178–191. doi: 10.1016/S0016-5085(03)00666-8. [DOI] [PubMed] [Google Scholar]
- 19.Lan HY. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol. 2003;14(6):1535–1548. doi: 10.1097/01.ASN.0000067632.04658.B8. [DOI] [PubMed] [Google Scholar]
- 20.Saika S, Ikeda K, Yamanaka O, Miyamoto T, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Nakajima Y, Kao WW, Flanders KC, Roberts AB. Expression of Smad7 in mouse eyes accelerates healing of corneal tissue after exposure to alkali. Am J Pathol. 2005;166(5):1405–1418. doi: 10.1016/S0002-9440(10)62358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han G, Li F, Ten Dijke P, Wang XJ. Temporal smad7 transgene induction in mouse epidermis accelerates skin wound healing. Am J Pathol. 2011;179(4):1768–1779. doi: 10.1016/j.ajpath.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Saha J, Hada M, Anderson JA, Pluth JM, O’Neill P, Cucinotta FA. Novel Smad proteins localize to IR-induced double-strand breaks: interplay between TGFbeta and ATM pathways. Nucleic Acids Res. 2013;41(2):933–942. doi: 10.1093/nar/gks1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Kang Y, Khare V, Jin ZY, Kang MY, Yoon Y, Hyun JW, Chung MH, Cho SI, Jun JY, Chang IY, You HJ. The p53-inducible gene 3 (PIG3) contributes to early cellular response to DNA damage. Oncogene. 2010;29(10):1431–1450. doi: 10.1038/onc.2009.438. [DOI] [PubMed] [Google Scholar]
- 24.Noon AT, Shibata A, Rief N, Lobrich M, Stewart GS, Jeggo PA, Goodarzi AA. 53BP1-dependent robust localized KAP-1 phosphorylation is essential for heterochromatic DNA double-strand break repair. Nat Cell Biol. 2010;12(2):177–184. doi: 10.1038/ncb2017. [DOI] [PubMed] [Google Scholar]
- 25.He W, Li AG, Wang D, Han S, Zheng B, Goumans MJ, Ten Dijke P, Wang XJ. Overexpression of Smad7 results in severe pathological alterations in multiple epithelial tissues. EMBO J. 2002;21(11):2580–2590. doi: 10.1093/emboj/21.11.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falanga V, Schrayer D, Cha J, Butmarc J, Carson P, Roberts AB, Kim SJ. Full-thickness wounding of the mouse tail as a model for delayed wound healing: accelerated wound closure in Smad3 knock-out mice. Wound Repair Regen. 2004;12(3):320–326. doi: 10.1111/j.1067-1927.2004.012316.x. [DOI] [PubMed] [Google Scholar]
- 27.Wiegman EM, Blaese MA, Loeffler H, Coppes RP, Rodemann HP. TGFbeta-1 dependent fast stimulation of ATM and p53 phosphorylation following exposure to ionizing radiation does not involve TGFbeta-receptor I signalling. Radiother Oncol. 2007;83(3):289–295. doi: 10.1016/j.radonc.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Cariveau MJ, Tang X, Cui XL, Xu B. Characterization of an NBS1 C-terminal peptide that can inhibit ataxia telangiectasia mutated (ATM)-mediated DNA damage responses and enhance radiosensitivity. Mol Pharmacol. 2007;72(2):320–326. doi: 10.1124/mol.107.036681. [DOI] [PubMed] [Google Scholar]
- 29.You Z, Chahwan C, Bailis J, Hunter T, Russell P. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol Cell Biol. 2005;25(13):5363–5379. doi: 10.1128/MCB.25.13.5363-5379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry J, Kleckner N. The ATRs, ATMs, and TORs are giant HEAT repeat proteins. Cell. 2003;112(2):151–155. doi: 10.1016/S0092-8674(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 31.Han G, Bian L, Li F, Cotrim A, Wang D, Lu J, Deng Y, Bird G, Sowers A, Mitchell JB, Gutkind JS, Zhao R, Raben D, ten Dijke P, Refaeli Y, Zhang Q, Wang XJ. Preventive and therapeutic effects of Smad7 on radiation-induced oral mucositis. Nat Med. 2013;19(4):421–428. doi: 10.1038/nm.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong S, Lee C, Kim SJ. Smad7 sensitizes tumor necrosis factor induced apoptosis through the inhibition of antiapoptotic gene expression by suppressing activation of the nuclear factor-kappaB pathway. Cancer Res. 2007;67(19):9577–9583. doi: 10.1158/0008-5472.CAN-07-1179. [DOI] [PubMed] [Google Scholar]
- 33.Hong S, Lim S, Li AG, Lee C, Lee YS, Lee EK, Park SH, Wang XJ, Kim SJ. Smad7 binds to the adaptors TAB 2 and TAB 3 to block recruitment of the kinase TAK1 to the adaptor TRAF2. Nat Immunol. 2007;8(5):504–513. doi: 10.1038/ni1451. [DOI] [PubMed] [Google Scholar]
- 34.Simonsson M, Heldin CH, Ericsson J, Gronroos E. The balance between acetylation and deacetylation controls Smad7 stability. J Biol Chem. 2005;280(23):21797–21803. doi: 10.1074/jbc.M503134200. [DOI] [PubMed] [Google Scholar]
- 35.Gronroos E, Hellman U, Heldin CH, Ericsson J. Control of Smad7 stability by competition between acetylation and ubiquitination. Mol Cell. 2002;10(3):483–493. doi: 10.1016/S1097-2765(02)00639-1. [DOI] [PubMed] [Google Scholar]
- 36.Costanzo V, Paull T, Gottesman M, Gautier J. Mre11 assembles linear DNA fragments into DNA damage signaling complexes. PLoS Biol. 2004;2(5):E110. doi: 10.1371/journal.pbio.0020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308(5721):551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd J, Chapman JR, Clapperton JA, Haire LF, Hartsuiker E, Li J, Carr AM, Jackson SP, Smerdon SJ. A supramodular FHA/BRCT-repeat architecture mediates Nbs1 adaptor function in response to DNA damage. Cell. 2009;139(1):100–111. doi: 10.1016/j.cell.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Goodarzi AA, Jeggo PA, Paull TT. 53BP1 promotes ATM activity through direct interactions with the MRN complex. EMBO J. 2010;29(3):574–585. doi: 10.1038/emboj.2009.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J, Zhang X, Zhang L, Wu CY, Rezaeian AH, Chan CH, Li JM, Wang J, Gao Y, Han F, Jeong YS, Yuan X, Khanna KK, Jin J, Zeng YX, Lin HK. Skp2 E3 ligase integrates ATM activation and homologous recombination repair by ubiquitinating NBS1. Mol Cell. 2012;46(3):351–361. doi: 10.1016/j.molcel.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirshner J, Jobling MF, Pajares MJ, Ravani SA, Glick AB, Lavin MJ, Koslov S, Shiloh Y, Barcellos-Hoff MH. Inhibition of transforming growth factor-beta1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Res. 2006;66(22):10861–10869. doi: 10.1158/0008-5472.CAN-06-2565. [DOI] [PubMed] [Google Scholar]
- 42.Dubrovska A, Kanamoto T, Lomnytska M, Heldin CH, Volodko N, Souchelnytskyi S. TGFbeta1/Smad3 counteracts BRCA1-dependent repair of DNA damage. Oncogene. 2005;24(14):2289–2297. doi: 10.1038/sj.onc.1208443. [DOI] [PubMed] [Google Scholar]
- 43.Kanamoto T, Hellman U, Heldin CH, Souchelnytskyi S. Functional proteomics of transforming growth factor-beta1-stimulated Mv1Lu epithelial cells: Rad51 as a target of TGFbeta1-dependent regulation of DNA repair. EMBO J. 2002;21(5):1219–1230. doi: 10.1093/emboj/21.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang J, Park K, Bang YJ, Kim WS, Kim D, Kim SJ. Expression of transforming growth factor beta type II receptor reduces tumorigenicity in human gastric cancer cells. Cancer Res. 1997;57(14):2856–2859. [PubMed] [Google Scholar]
- 45.Han G, Li AG, Liang YY, Owens P, He W, Lu S, Yoshimatsu Y, Wang D, Ten Dijke P, Lin X, Wang XJ. Smad7-induced beta-catenin degradation alters epidermal appendage development. Dev Cell. 2006;11(3):301–312. doi: 10.1016/j.devcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 46.Xavier S, Piek E, Fujii M, Javelaud D, Mauviel A, Flanders KC, Samuni AM, Felici A, Reiss M, Yarkoni S, Sowers A, Mitchell JB, Roberts AB, Russo A. Amelioration of radiation-induced fibrosis: inhibition of transforming growth factor-beta signaling by halofuginone. J Biol Chem. 2004;279(15):15167–15176. doi: 10.1074/jbc.M309798200. [DOI] [PubMed] [Google Scholar]
- 47.Monteleone G, Del Vecchio Blanco G, Palmieri G, Vavassori P, Monteleone I, Colantoni A, Battista S, Spagnoli LG, Romano M, Borrelli M, MacDonald TT, Pallone F. Induction and regulation of Smad7 in the gastric mucosa of patients with Helicobacter pylori infection. Gastroenterology. 2004;126(3):674–682. doi: 10.1053/j.gastro.2003.11.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.