Abstract
Asparagine (N)-linked protein glycosylation, which takes place in the eukaryotic endoplasmic reticulum (ER), is important for protein folding, quality control and the intracellular trafficking of secretory and membrane proteins. It is known that, during N-glycosylation, considerable amounts of lipid-linked oligosaccharides (LLOs), the glycan donor substrates for N-glycosylation, are hydrolyzed to form free N-glycans (FNGs) by unidentified mechanisms. FNGs are also generated in the cytosol by the enzymatic deglycosylation of misfolded glycoproteins during ER-associated degradation. FNGs derived from LLOs and misfolded glycoproteins are eventually merged into one pool in the cytosol and the various glycan structures are processed to a near homogenous glycoform. This article summarizes the current state of our knowledge concerning the formation and catabolism of FNGs.
Keywords: Free N-glycan, Oligosaccharyltransferase, Pyrophosphatase, Peptide:N-glycanase, Yeast, Mammals
Introduction
In the ER lumen, nascent polypeptides emerging from the protein-conducting channel are frequently modified with glycans at selected asparagine residues within the consensus sequences (N-X-S/T, X ≠ P) (Fig. 1) [1, 2]. This process, which is referred to as N-glycosylation, is one of the most frequent co- and post-translational modifications in eukaryotes [3]. In the past three decades, extensive genetic and biochemical studies on mutant cells derived from Saccharomyces cerevisiae harboring N-glycosylation defects have led to the identification of most of the genes that are involved in the biosynthesis of the core glycan. In sharp contrast, our knowledge of aspects of degradation of N-glycans is very incomplete. It is generally believed that lysosomes are the predominant organelle for the degradation of various glycoconjugates [4]. However, an accumulating body of evidence suggests that the non-lysosomal degradation of N-glycans is ubiquitous [5, 6]. Above all, one of the outstanding questions that remain to be clarified is the molecular mechanisms underlying the generation and degradation of “free”, unconjugated forms of glycans, which are structurally related to N-glycans (free N-glycans; FNGs). Since our last comprehensive review on this subject [7], significant progress has been made and the findings reveal the existence of more diverse, nevertheless sophisticated, pathways than had ever been thought. In this review, we update our knowledge regarding the generation and degradation of FNGs with a focus on mammalian cells and S. cerevisiae.
Fig. 1.

Biosynthesis of N-glycans in the ER of mammalian cells. Reactions utilizing UDP-Glc, UDP-GlcNAc and GDP-Man are boxed with cyan, magenta and green, respectively (dashed lines). Reactions utilizing Dol-P-Man and Dol-P-Glc are indicated green and cyan arrows, respectively. Red arrow indicates an unidentified pathway
Biosynthesis of N-glycans in the ER
The biosynthesis of N-glycans in the ER consists of two steps in all eukaryotic cells: (1) the preassembly of LLOs as glycan donor substrates and (2) their transfer to polypeptide chains (Fig. 1). LLO biosynthesis is a biphasic process initiated by the synthesis of Man5GlcNAc2-PP-Dol at the cytosolic side of the ER membrane by the sequential action of DPAGT1 (Alg7 in S. cerevisiae) [8, 9], the Alg13–Alg14 heterodimer complex [10–14], Alg1 [15–17], Alg2 [18, 19] and Alg11 [20, 21]. The donor substrates for all of these glycosyltransferases are two nucleotide sugars (UDP-GlcNAc and GDP-Man) (Fig. 1, red and green dashed boxes). In this connection, it is particularly noteworthy that the ALG13, ALG14, ALG1, and ALG2 genes from S. cerevisiae have been successfully expressed in Escherichia coli, and as a result, Man3-4GlcNAc2-PP-undecaprenol can be produced in the engineered E. coli [22]. However, the issue of specifically how the fourth mannose residue is added remains to be clarified.
Man5GlcNAc2-PP-Dol, the final product in the cytosolic process of LLO biosynthesis, is flipped into the luminal side of the ER membrane. Genetic studies using S. cerevisiae revealed that Rft1 is required for the transbilayer movement of LLOs [23], while biochemical reconstitution studies suggest the occurrence of an RFT1-independent flipping process [24–26]. Irrespective of the mechanisms involved, Man5GlcNAc2-PP-Dol oriented toward the ER lumen undergoes four additional mannose transfer reactions catalyzed by enzymes utilizing Dol-P-Man, Alg3 [19, 27, 28], Alg9 [29, 30] and Alg12 [31–34] (Fig. 1, green arrows). The synthesized LLO intermediate, Man9GlcNAc2-PP-Dol, is further glucosylated by enzymes utilizing Dol-P-Glc Alg6 [19, 35, 36], Alg8 [37, 38] and Alg10 [39] (Fig. 1, cyan arrows), generating the fully assembled Glc3Man9GlcNAc2-PP-Dol. The core glycan is transferred en bloc onto growing polypeptide chains in the ER lumen by the oligosaccharyltransferase (OST), leaving PP-Dol as a byproduct. The PP-Dol is then dephosphorylated to P-Dol by DOLPP1 (Cwh8 in S. cerevisiae) [40–42] and recycled as the acceptor substrate in LLO biosynthesis [43]. This cycle is generally referred to as the “dolichol cycle”.
In mammals and S. cerevisiae, OST associates with the translocon complex [44–49], and often catalyzes co-translational N-glycosylation [47, 50]. OST also catalyzes the post-translational N-glycosylation of the acceptor sites that are located in a flexible region of a protein [47, 51]. In co-translational glycosylation, the acceptor sites should be located > 15 amino acid residues away from the luminal surface of the ER membrane [52]. The cryo-electron microscopic structure of S. cerevisiae OST indicates that the active site may be located ~3 nm (corresponding to ~15 amino acids) above the membrane [53], while the crystal structures of bacterial OST suggest that the active site is formed at the juxtamembrane region [54]. The minimum acceptor sequence for fulfilling the requirement for N-glycosylation is a tripeptide (N-X-S/T, X ≠ P) [55], and OST can modify many proteins carrying the N-glycosylation consensus sequences. By contrast, OST shows a strict specificity for the donor substrate and preferentially utilizes the fully assembled LLO in vitro [56, 57]. Although OST also can utilize truncated LLOs as short as GlcNAc2-PP-Dol with a reduced efficiency in vitro [58, 59], an ordered assembly of LLOs (Fig. 1) ensures the preferential transfer of the fully assembled oligosaccharide onto a protein by OST under normal conditions [34, 60]. However, a subset of glycoproteins carrying N,N′-diacetylchitobiose has been detected in several types of glucose-starved cancer cells [61–63], implying that such conditions somehow allow OST to utilize GlcNAc2-PP-Dol, which is synthesized at the cytosolic side of the ER membrane, for N-glycosylation reactions in the ER lumen. The N-glycosylation reaction in vitro is usually activated by the addition of manganese ion [55], while the endogenous co-factor(s) for OST has not yet been determined.
In animals and S. cerevisiae, OST is the multi-membrane protein complex consisted of the conserved catalytic subunit Stt3 and several accessary subunits (Table 1) [1]. In contrast, some protists, such as Trypanosoma brucei and Leishmania major, possess only the Stt3 subunit as the OST enzyme, but instead have multiple numbers of STT3 genes (Table 1) [64–68]. The exogenous expression of T. brucei Stt3 and L. major Stt3 can suppress lethality as well as N-glycosylation defects in stt3∆ yeast mutant cells [66–68]. The multiplication of the STT3 gene is also found in animals and plants, where they have two STT3 genes (STT3A and STT3B) [57]. However, S. cerevisiae and other fungi have a single copy of the STT3 gene, which is closely related to metazoan STT3B. In mammalian cells, Stt3A and Stt3B exclusively form two distinct OST complexes. The Stt3A complex is believed to exclusively catalyze co-translational N-glycosylation by associating with the translocon [47], while the Stt3B complex can mediate the post-translational N-glycosylation of the sites that are skipped by the Stt3A complex [47, 69–72]. In S. cerevisiae, Stt3 forms two OST complexes containing either Ost3 or its paralog, Ost6 [45, 73], both of which have thioredoxin activity and enhances the efficiency of the N-glycosylation of sites near cysteine residues, presumably by hydrophobic interactions and the formation of mixed-disulfide bonds with substrate polypeptides [74, 75]. The mammalian Stt3B complex also associates with a thioredoxin-like protein MagT1 [57, 76] that forms mixed-disulfide bonds with substrate proteins [72]. The issue of why eukaryotic cells use multiple OST complexes is not fully understood, but it has been proposed that they complement substrate specificity and maximize N-glycosylation efficiency [77].
Table 1.
Genes encoding OST subunits in S. cerevisiae, Homo sapiens, L. major, T. brucei and C. jejuni
| Genes encoding OST subunits | ||||
|---|---|---|---|---|
| S. cerevisiae | H. sapiens | L. major | T. brucei | C. jejuni |
| STT3 |
STT3A STT3B |
LMJF_35_1130 (STT3A) LMJF_35_1140 (STT3B) LMJF_35_1150 (STT3C) LMJF_35_1160 (STT3D) |
Tb927.5.890 (STT3A) Tb927.5.900 (STT3B) Tb927.5.910 (STT3C) |
pglB |
| WBP1 | DDOST/OST48 | – | – | – |
| SWP1 | RPN2 | – | – | – |
| OST1 | RPN1 | – | – | – |
| OST2 | DAD1 | – | – | – |
| OST3/OST6 | TUSC3/MAGT1 | – | – | – |
| OST4 | OST4 | – | – | – |
| OST5 | N.D. | – | – | – |
N.D. not determined
The pathogenic bacterium Campylobacter jejuni expresses N-glycosylation machinery that is encoded by the pgl (protein glycosylation) gene cluster [78]. In C. jejuni, OST is a single subunit-type enzyme encoded by the pglB gene (Table 1), which is homologous to the eukaryotic STT3 [79]. LLOs synthesized at the inner membrane of C. jejuni are quite distinct from those found in eukaryotes in terms of sugar compositions (GlcNAc, Man and Glc in eukaryotes and bacillosamine, GalNAc and Glc in C. jejuni) [80], as well as lipid structure (dolichol in eukaryotes and undecaprenol in C. jejuni) [81]. Unlike STT3 in mammals and S. cerevisiae, pglB is not required for the cell growth of C. jejuni under normal culture conditions, while the deletion of pglB causes severe growth defects under conditions of osmotic stress [82].
Glycoprotein quality control in the ER
In the ER lumen of mammalian cells and S. cerevisiae, the folding processes of nascent polypeptides are strictly monitored by a system called ER quality control (ERQC) [83, 84]. In this system, concerted actions of ER-localized chaperones, modifying enzymes and lectins play critical roles in distinguishing native glycoproteins from misfolded or unfolded ones and in selectively transporting the former to their final destinations. On the other hand, the terminally misfolded glycoproteins that are formed in the ER lumen are eliminated by ER-associated degradation (ERAD), which involves the retrotranslocation of misfolded glycoproteins back to the cytosol followed by their proteasomal degradation. The detailed molecular mechanisms of ERQC and ERAD have been summarized in excellent review articles [85–87].
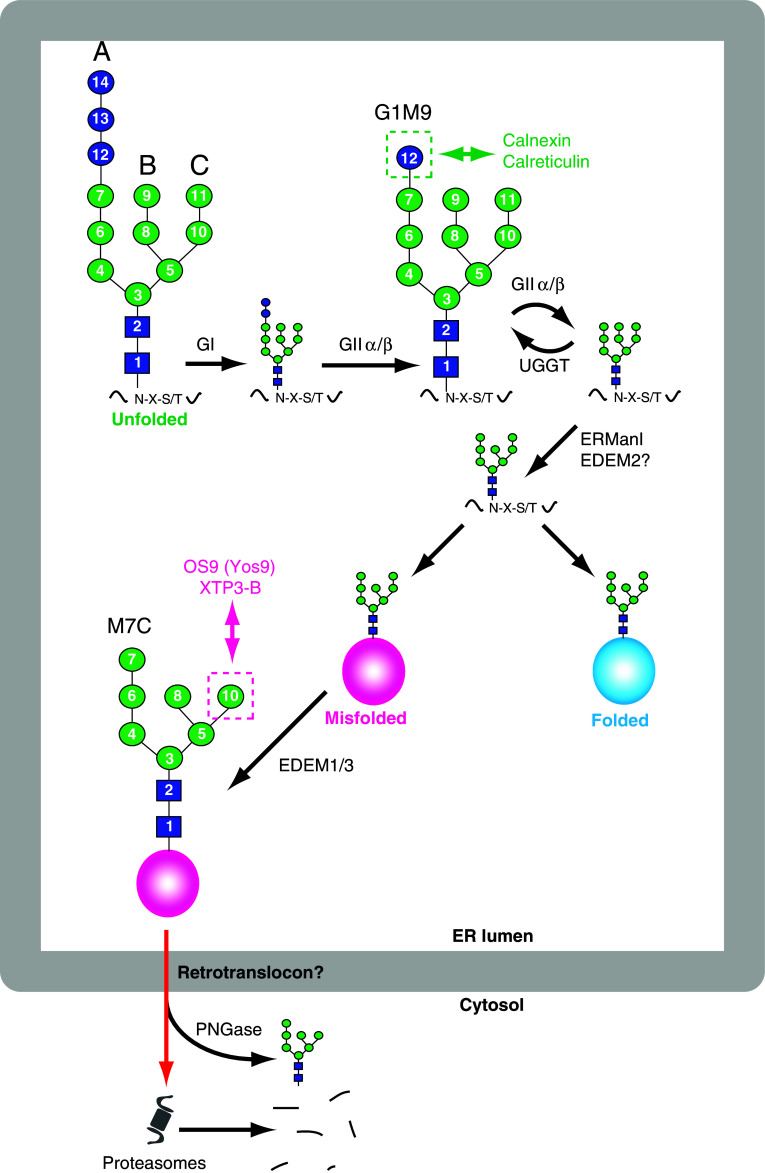
In both ERQC and ERAD, there are N-glycan structures that dictate the folding status of the carrier polypeptides (Fig. 2) [88, 89]. After N-glycosylation in the ER lumen, two glucose residues of the Glc3Man9GlcNAc2 (Fig. 2, residues 13 and 14) are rapidly removed by ER glucosidases (GI and GIIα/β in mammals; Gls1 and Gls2/Gtb1 in S. cerevisiae). Calnexin (CNX) and calreticulin (CRT) recognize the glucose residue of the G1M9 isomer (Fig. 2, residue 12) and may bind to the nascent glycoproteins, which facilitates productive folding. In contrast, Cne1, the yeast ortholog of CNX, is dispensable for the folding of nascent glycoproteins, although this lectin binds to the G1M9 isomer [90, 91]. The removal of the last glucose residue, also catalyzed by ER glucosidase II (Fig. 2, residue 12), occurs relatively slowly compared to the first reaction [92]. If the nascent glycoproteins bearing non-glucosylated N-glycans are recognized as being in unfolded states by the UDP-glucose:glycoprotein glucosyltransferase (UGGT), they are reglucosylated by this enzyme, thus allowing CNX/CRT to interact again with unfolded glycoproteins. It appears that the genome of S. cerevisiae does not have a gene ortholog of UGGT. During the CNX/CRT cycle [93, 94], the external α1,2-linked mannose residue at the B-arm (Fig. 2, residue 9) may be trimmed, presumably by ERManI (Mns1 in S. cerevisiae) [95] or the ER degradation-enhancing mannosidase-like protein 2 (EDEM2) [96]. When glycoproteins fold properly, they exit the ER and are transported to their final destinations. If glycoproteins fail to acquire correct folding or subunit assembly states, the external α1,2-linked mannose residue at the C-arm (Fig. 2, residue 11) is removed by EDEM1/3 (Htm1 in S. cerevisiae), which results in the α1,6-linked mannose residue being exposed (Fig. 2, residue 10) [89, 97, 98]. The degradation lectins (OS9 and/or XTP3-B/Erlectin in mammals; Yos9 in S. cerevisiae) recognize the exposed α1,6-linked mannose residue at the C-arm (Fig. 2, residue 10) and direct misfolded glycoproteins to ERAD by association with SEL1L (Hrd3 in S. cerevisiae) [99, 100], an ER membrane protein that forms a complex with the “retrotranslocon” [84, 101, 102]. Especially for misfolded (glyco)proteins generated in the ER lumen, they need to cross the ER membrane before their proteasomal degradation. The molecular mechanism underlying the retrotranslocation process is not currently understood. However, the central player of the retrotranslocation channel is the subject of debate, and so far HRD1 (Hrd1 in S. cerevisiae), Derlin-1 (Der1 in S. cerevisiae) and the Sec61 complex (Sec61α–Sec61β–Sec61γ in mammals and Sec61–Sbh1–Sss1 in S. cerevisiae) have been proposed to be involved in this process [103]. HRD1 is an E3 ubiquitin ligase that adds and elongates ubiquitin chains on misfolded (glyco)proteins during their retrotranslocation, and the polyubiquitination recruits the AAA-ATPase p97/VCP (Cdc48 in yeast) and 26S proteasomes to the ERAD machinery, promoting the extraction and degradation of misfolded (glyco)proteins [104]. During substrate extraction, N-glycans on misfolded glycoproteins are removed by the cytosolic peptide:N-glycanase (PNGase) [105–107], releasing FNGs in the cytosol. In mammalian cells, PNGase forms a complex with p97, HR23, and 26S proteasomes at the periphery of the ER membrane [108–110]. It has been postulated that the removal of bulky side chains, such as N-glycans, may facilitate the degradation of proteins by cylinder-shaped 26S proteasomes, as the proteins must enter the inside the cylinder, where the proteolytic activities reside. Yeast Png1 also forms a complex with Rad23, an ortholog of HR23, which may couple deglycosylation and proteasomal degradation [111, 112]. It should be noted that the ERAD process is implicated, not only in the degradation of misfolded proteins, but also in the generation of FNGs in the cytosol [6, 113, 114].
Fig. 2.
ERQC and ERAD for glycoproteins in mammalian cells. In the ER, glycan trimming is a temporally regulated process that generates glycan epitopes, which play crucial roles in protein folding, sorting and ERAD. Nascent glycoproteins are subjected to a productive folding process aided by lectin chaperones (CNX and CRT), GII and UGGT. When glycoproteins are terminally misfolded, they are segregated from the correctly folded molecules by the actions of EDEMs and degradation lectins (OS9 and XTPB-3), which initiate the retrotranslocation of misfolded glycoproteins. During the retrotranslocation of misfolded glycoproteins, the bulky N-glycans are removed by PNGase, releasing neutral FNGs in the cytosol. The deglycosylated polypeptides are degraded by proteasomes. The number for each sugar indicates the order of their assembly in LLO biosynthesis. A, B and C: A-, B- and C-arms. Red arrow indicates an unidentified pathway
Generation of neutral FNGs in the ER lumen of mammalian cells
In addition to the N-glycosylation function of OST, it has been proposed that OST may have hydrolytic activity for LLOs to generate neutral FNGs in the ER lumen (Fig. 3). This assumption is based on experimental findings showing that the LLO-degrading enzyme preferentially hydrolyzes Glc3Man9GlcNAc2-PP-Dol over biosynthetic intermediates, requires divalent cations, and is inhibited by N-glycosylatable peptides [115], all of which are in good agreement with the enzymatic properties of OST. Major questions that remain to be clarified concern (1) the hydrolytic activity of OST, (2) the STT3 isoform that is responsible for the hydrolysis, and (3) the biological function of the LLO hydrolysis.
Fig. 3.
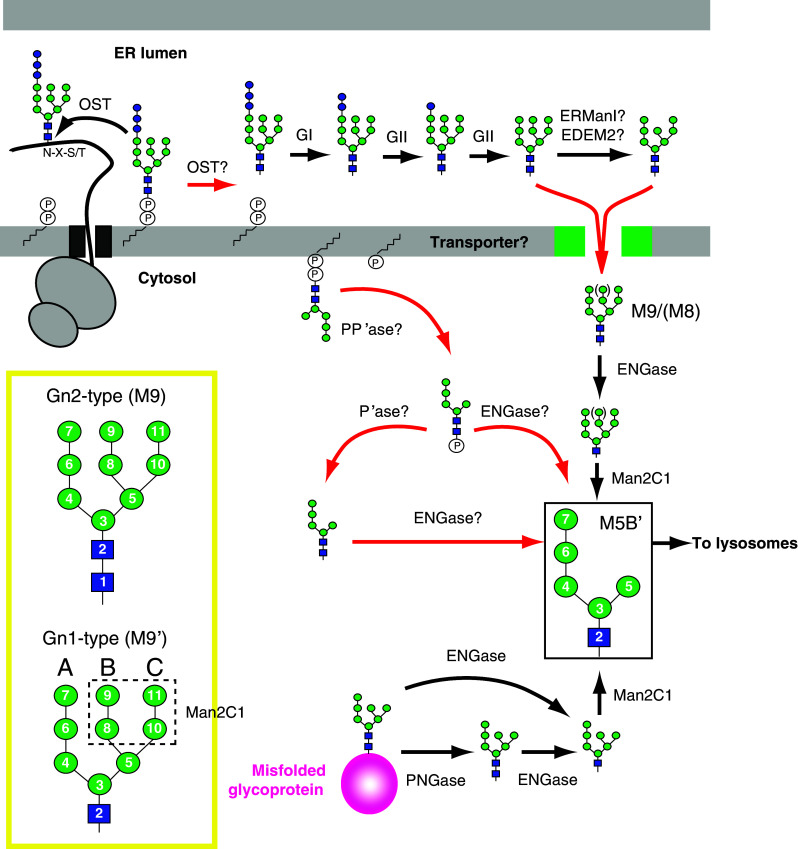
Formation and processing of neutral and phosphorylated FNGs derived from LLOs and misfolded glycoproteins in mammalian cells. In the ER lumen, a putative enzyme, proposed to be OST, hydrolyzes the fully assembled LLO and releases Glc3Man9GlcNAc2. The luminal FNG is rapidly trimmed by GI/II and may be ERManI or EDEM2. The deglucosylated FNGs are transported to the cytosol through a putative transporter. In the cytosol, neutral FNGs are generated from misfolded glycoproteins by PNGase. Neutral FNGs derived from LLOs and misfolded glycoproteins are processed by ENGase and Man2C1, generating the M5B′ isomer (in solid box) in the cytosol. LLOs are also degraded by a putative LLO-PP’ase. The topology of the LLO-PP’ase reaction is tentatively depicted on the cytosolic side of the ER membrane. The fate of phosphorylated FNGs is not known, but ENGase and a putative phosphatase are potential candidate enzymes for the catabolism of phosphorylated FNGs. An example for Gn1 and Gn2 types of the M9 isoform (M9′ and M9, respectively) is shown in the yellow box. Mannose residues removed by Man2C1 are boxed with dashed line. The number for each sugar indicates the order of their assembly in LLO biosynthesis. A, B and C: A-, B- and C-arms. Red arrows indicate unidentified pathways
The only way to unambiguously demonstrate the hydrolytic activity of OST would be to purify the enzymatically active OST and test whether the OST produces FNGs from LLOs. In the case of S. cerevisiae OST, FLAG tagging of the C-terminus of OST4 permits the immuno-purification of both of the OST3 and OST6 complexes to be achieved in a single step [116]. Although no such facile method for purifying mammalian OST has been developed, mammalian STT3A and STT3B complexes have been separately purified from canine pancreatic microsomes by conventional chromatography [57]. It is also extremely important to identify the STT3 isoform that catalyzes LLO hydrolysis in vivo, which would definitely provide deeper mechanistic insights into glycoprotein biosynthesis. We envision that simple knockdown/knockout studies for STT3s would not provide conclusive evidence, because the cytosolic deglycosylating enzymes, i.e., PNGase [117] and ENGase [118], are also involved in the generation of FNGs in living cells (see below). Therefore, both of the deglycosylating enzymes would need to be ablated prior to gene manipulation for STT3s and the evaluation of the effects on the production of FNGs.
In mammalian cells, the efficiency of LLO hydrolysis is estimated to be ~35 % of that of N-glycosylation [119], indicating that a substantial fraction of the mature LLO is hydrolyzed and not used for N-glycosylation. It appears that this energy-waste does not interfere with N-glycosylation reactions, since excess amounts of the mature LLO are normally synthesized [120, 121]. The question then arises as to what is the biological function of LLO hydrolysis? Previous studies have proposed that, in the absence of a sufficient supply of acceptor substrates for OST, the hydrolysis of Glc3Man9GlcNAc2-PP-Dol occurs, thus balancing the amounts of donor and acceptor substrates [119, 122]. However, this hypothesis is most unlikely, because incubation with translation inhibitors completely inhibits the generation of FNGs [123]. Moreover, the translation attenuation evoked by interferon-inducible RNA-dependent protein kinase (PKR)-like ER kinase (PERK) signaling does not reduce the extent of LLO hydrolysis [124]. Apart from these observations, Gao et al. [121, 124, 125] proposed that the frequency of LLO hydrolysis is regulated by the intracellular levels of Man 6-P. Their initial observations showed that, in streptolysin O-permeabilized cells, the addition of Man 6-P (10–100 μM) specifically induced the reduction of mature LLO and an increase in the corresponding FNG [121]. A remarkable elevation of Man 6-P has been detected in herpes simplex virus 1 (HSV-1)-infected mouse embryonic fibroblasts (MEFs) [124] and mannose 6-phosphate isomerase (MPI)-deficient mouse embryonic fibroblasts (MEFs) that had been cultured in medium supplemented with extremely high concentrations (500 μM) of mannose [126]. The MPI-deficient MEFs produce abnormally low levels of mature LLO by unknown mechanisms, while the loss of LLO has no effect on global N-glycosylation [126]. On the other hand, an HSV-1-infection apparently induces the hypoglycosylation of a viral glycoprotein in wild-type MEFs [124]. Although the N-glycosylation status of proteins produced in host cells needs to be carefully analyzed, it is tempting to speculate that, in virus-infected cells, Man 6-P-mediated LLO degradation may prevent the production of viral glycoproteins [124, 125].
Transport of neutral FNGs from the ER lumen to the cytosol in mammalian cells
When free Glc3Man9GlcNAc2 is generated in the ER lumen, the FNG is rapidly processed by ER mannosidases and/or glucosidases and transported out to the cytosol (Fig. 3). Since FNGs are bulky hydrophilic molecules, they should be able to cross the ER membrane through a transporter. This translocation process is thought to be mediated by a putative ATP- and Ca2+-dependent FNG transporter [127]. In addition to ATP, unidentified cytosolic factors are also required in FNG transport assay using reconstituted microsomes [128]. Although the biological function, as well as the molecular mechanism, of FNG transport remains to be clarified, one can imagine that the persistent retention of neutral FNGs in the ER may impair the glycoprotein quality control machinery regulated by glycan-recognizing proteins.
In vitro experiments indicate that the FNG transporter recognizes mannose, but not GlcNAc residues of FNGs [129], and non-glucosylated FNGs were found to be the preferred substrates over glucosylated ones [127, 128]. What then would be the fate of glucosylated FNGs that are formed in the ER lumen upon the inhibition of ER glucosidases? It has been reported that, under such conditions, glucosylated FNGs somehow reach the Golgi apparatus and the Glc1-3Man unit at the A-arm (Fig. 2, residues 7 and 12–14) of the FNGs may be cleaved off by a Golgi endo-α-mannosidase [130, 131]. The cleavage allows the structural remodeling of high-mannose-type FNGs to sialylated, complex-type FNGs (sialyl FNGs) by Golgi glycosidases and glycosyltransferases and the sialyl FNGs are then secreted to and detected in the culture medium [132]. While the fate of the extracellular sialyl FNG is currently unknown, it is tempting to speculate that sialyl FNGs in sera (see the section “Occurrence of sialyl FNGs inside and outside of mammalian cells” below) may be formed by a similar mechanism.
Generation of neutral FNGs in the cytosol of mammalian cells
It has been postulated that the deglycosylation reaction catalyzed by PNGase facilitates the degradation of the polypeptide backbone by proteasomes [105, 133, 134]. PNGase-mediated deglycosylation has been observed for a few model glycoproteins [118, 135–138]. Of these, a plant-derived, mutant form of the ricin toxin A chain (RTA) is the only model misfolded glycoprotein determined so far that is degraded in a PNGase-dependent manner [118]. Interestingly, in the absence of PNGase, ENGase deglycosylates the mutant form of RTA, producing the aggregation-prone, N-GlcNAc-modified protein [118]. This finding clearly demonstrates that ENGase can also act directly on glycoproteins in the cytosol of mammalian cells to generate FNGs (Fig. 3). However, the physiological substrates for ENGase, if any, need to be identified.
The knockdown of NGLY1 (the gene encoding human PNGase) in human cells results in a partial reduction of very specific neutral FNGs (Man9GlcNAc2 and Glc1Man9GlcNAc2), whereas a large majority of other FNGs remain unaffected by the NGLY1 knockdown [117]. This observation implies that misfolded glycoproteins that had been trapped and released during the calnexin/calreticulin cycle (Fig. 2) may be targeted to ERAD and undergo PNGase-mediated deglycosylation. Although complete knockout studies on NGLY1 will be required to draw any conclusions, the experimental findings indicate that, in mammalian cells, bulk FNGs are produced in a PNGase-independent manner, and indicate that PNGase-mediated deglycosylation may occur in a glycan structure-specific fashion. In any event, the biological importance of PNGase in mammalian systems is now well appreciated because patients harboring NGLY1 mutations have now been identified [107, 139–143]. The patients showed multiple symptoms including global developmental delay, multifocal epilepsy, involuntary movement, abnormal liver function, and the absence of tears [139, 141, 143]. However, the pathological mechanism responsible for the NGLY1 disorder is unclear. The establishment of model organisms with NGLY1 mutations and the identification of endogenous substrates for NGLY1 would be expected to provide mechanistic insights into the pathology of the NGLY1 disorder.
Processing of neutral FNGs in the cytosol of mammalian cells
It has long been believed that lysosomes are the predominant organelle for the degradation of biological macromolecules, including glycoconjugates. In this context, the occurrence of cytosolic glycosidases had attracted less attention in glycan catabolism, especially after the demonstration that lysosomes function as degradation factories [5, 144].
In the cytosol, FNGs derived from LLOs and misfolded glycoproteins are eventually merged into one pool and are then sequentially processed to a near homogenous glycoform (Fig. 3, the M5B′ isoform; Man5GlcNAc1) by the cytosolic endo-β-N-acetylglucosaminidase (ENGase) and Man2C1 (Fig. 3). Extensive structural studies of neutral FNGs have shown that there are two types of FNGs [115, 119, 127, 145–148]: One contains an N,N′-diacetylchitobiose core at the reducing end of FNGs (Gn2 type) and does the other a single GlcNAc (Gn1 type) predominated in the cytosol (Fig. 3, structural models shown in yellow box). The Gn2-type structure can be generated by either the hydrolysis of LLOs or the deglycosylation of misfolded glycoproteins by PNGase, but such FNGs rarely accumulate in the cytosol. On the other hand, high levels of the Gn1-type structure are found in the cytosol and are believed to be primarily the product of ENGase [149, 150], which converts Gn2-type FNGs to the Gn1 type by removing the innermost GlcNAc residue (Fig. 3, ENGase). The cytosolic ENGase has been biochemically purified and characterized [151], and the gene encoding this enzyme (Engase) has been identified [149, 150]. Engase knockdown in mammalian cells results in a dramatic accumulation of the Gn2 type of cytosolic neutral FNGs, which is accompanied by an increased number of mannose residues (Fig. 3, M9/(M8); Man8–9GlcNAc2) [117].
The “cytosolic” neutral α-mannosidase, referred to as Man2C1, occurs in various animal sources [152–165]. It has been shown that the knockout of Man2C1 in mice results in a dramatic accumulation of Gn1 type of FNGs with Man8–9 (Man8–9GlcNAc1) in the cytosol of all tissues tested [166], strongly indicating that the action of Man2C1 is responsible for generating M5B′ in the cytosol (Fig. 3). Biochemical studies of Man2C1 indicate that this enzyme preferentially trims Gn1-type FNGs over the Gn2 type [157, 158, 161, 167]. This substrate preference may explain the reason why Engase knockdown resulted in accumulation of M8B and M9 (Fig. 3) in the cytosol [117]. Man2C1 preferentially trims mannose residues on the B- and C-arms of FNGs with the reversed order of the mannose assembly in LLO biosynthesis (Fig. 3, Gn1 type in yellow box) [157].
In addition to the accumulation of larger Gn1-type FNGs, Man2C1 knockout mice exhibit multiple tissue damage, such as the degeneration of neurons, the glia, liver and kidney [166]. Although the molecular mechanism underlying these organ defects in Man2C1-null mice is currently unclear, it has been reported that the suppression of Man2C1 expression in cultured cells results in cell growth retardation and apoptosis [168–171]. In contrast to the cases for gene suppression, the overexpression of Man2C1 has been implicated in tumorigenesis [171–173]. In prostate cancer, Man2C1 is prone to be overexpressed, directly binds to PTEN, a tumor suppressor catalyzing the dephosphorylation of phosphatidylinositol 3,4,5-triphosphate, and suppresses its phosphatase activity, thereby promoting tumor growth [173]. In this study, it was suggested that multiple domains of Man2C1 including its catalytic site are involved in this PTEN interaction, whereas the issue of whether the catalytic activity of Man2C1 is required for tumor progression is not known with certainty. Interestingly, Man2C1 has an enzyme-independent function in suppressing mitochondria-dependent apoptosis [169], which may aid in Man2C1-overexpressing cancer cells to escape from programmed cell death. Recent studies have identified inhibitors for Man2C1 [174, 175]. These compounds show an inhibitory effect in intact cells, serving as new tools for evaluating the biological functions of Man2C1.
It has been repeatedly reported that Man2C1 overexpression alters glycosylation patterns: A reduction in concanavalin A staining in splenocytes from human Man2C1-transgenic mice [172] and the underglycosylation of a null Hong Kong variant of α1-antitrypsin in Man2C1-overexpressing HeLa cells [176]. Although the precise mechanism of how the overexpression of Man2C1 can affect the N-glycosylation process occurring in the luminal side of the membrane remains to be clarified, it has been implied that excess amounts of Man2C1 may inhibit the glucosylation steps of LLO biosynthesis [176]. It should be noted that Man2C1 is present, not only as a soluble form in the cytosol, but also as a membrane-associated form [168, 177]. Although it has been proposed that cytosolic Man2C1 is the likely precursor of an ER mannosidase [177, 178], no definitive evidence for the luminal occurrence of Man2C1 has been reported.
Transport of neutral FNGs from the cytosol to the lysosomes in mammalian cells
It has been shown that the final product of ENGase and Man2C1 in the cytosol, which is mainly composed of M5B′ (Fig. 3), is eventually degraded via a mechanism involving a cytosol–lysosome transport of FNGs mediated by an ATP-driven lysosomal transporter [179, 180]. However, the molecular nature of this transporter remains unknown. Biochemical studies showed that the transport process accepts both Gn2 and Gn1 types of FNGs, shows a strong preference for FNGs with shorter mannose residues, does not tolerate the phosphorylation of the reducing end of the innermost GlcNAc residue, and is inhibited by GlcNAc, but not mannose [180]. The lysosomal degradation of the transported FNGs results in the production of monosaccharides. Although their precise fates are currently unknown, it is reasonable to assume that the monosaccharides are recycled to synthesize nucleotide sugars for glycosylation reactions.
Occurrence of sialyl FNGs inside and outside of mammalian cells
The occurrence of sialyl FNGs, which contain galactose and sialic acid residues, has been reported in the urine from patients of lysosomal storage diseases, such as sialidosis and galactosialidosis [181–185]. It is generally thought that these sialyl FNGs leak from lysosomes upon the lysis of sick cells [186]. On the other hand, the intracellular accumulation of sialyl FNGs was also observed in mouse liver [147], various cultured cells [187] autophagy-defective cells [188], and various cancer tissues, but not in normal epithelial tissues [189, 190]. It is tempting to speculate that the sialyl FNGs found in cancer tissues may serve as useful, specific tumor markers. It has also been shown that the knockdown of Slc17a5, the gene encoding a lysosomal sialic acid transporter (known as sialin), causes a significant delay in the accumulation of sialyl FNGs upon induction of autophagy defect [188]. More recently, it was shown that the protein level of sialin is increased by shutting down of Atg5 expression, which causes a loss of autophagosome formation [191]. Sialin upregulation is not caused by general defects of lysosomes, as the protein level of LAMP1, another lysosomal membrane protein, remains unchanged under the same conditions [191]. How sialin levels are regulated by the autophagy process, as well as how it is correlated with the cytosolic accumulation of sialyl FNGs, is an intriguing question. All of these findings clearly show the occurrence of FNGs composed of, not only high-mannose-type glycans, but also complex-type glycans with galactose and sialic acid residues in the cytosol of mammalian cells. However, it is not known whether the catabolic pathway for the sialyl FNGs resides in the cytosol, except that the overexpression of cytosolic sialidase, Neu2, can reduce the levels of the FNGs that have accumulated in the cytosol of human stomach cancer-derived cultured cells MKN45 [187]. Further studies will clearly be needed to develop a better understanding of the molecular mechanism underlying the catabolic pathway of complex-type FNGs.
More recently, “extracellular” complex-type FNGs, which are structurally related to complex-type N-glycans, were identified in human sera [192]. While the most intracellular, complex-type FNGs are of the Gn1 type [147, 181–185, 187–190], the extracellular complex-type FNGs were exclusively the Gn2 type [192]. This finding implies that the intra- and extracellular complex-type FNGs may have different origins. How these complex-type FNGs are formed, as well as the nature of their biological roles, will be interesting issues that remain to be explored.
FNGs generated in the cytosol of S. cerevisiae
In S. cerevisiae, a large majority (~95 %) of the cytosolic FNGs, which are exclusively of the Gn2 type due to the lack of ENGase, are produced in a PNGase-dependent manner (Fig. 4a) [193–195]. The importance of PNGase-mediated deglycosylation in the glycoprotein ERAD was first demonstrated in S. cerevisiae: The deletion of PNG1 (the yeast ortholog of PNGase) appears to decelerate ERAD of a plant-derived ricin toxin A chain [112]. Although a few Png1-dependent ERAD substrates are now available [112, 196–199], no physiological substrates for Png1 have been identified. Therefore, the current model of glycoprotein ERAD in S. cerevisiae is thoroughly dependent on studies in which a limited number of non-physiological substrates have been used. It has been proposed that the sequential mannose trimming by Mns1 and/or Htm1 plays crucial roles in targeting misfolded glycoproteins to ERAD by recruiting a degradation lectin Yos9 [88, 198, 200]. On the other hand, when we analyzed the detailed structures of FNGs as a read-out of a global glycoprotein ERAD, it was found that the most abundant FNG was Man8GlcNAc2 lacking one external α1,2-linked mannose at the B-arm (M8B, Fig. 4b), but not Man7GlcNAc2 (M7C, Fig. 4b), the product of Mns1 and Htm1 [195]. This experimental finding clearly indicates that not all N-glycans that are present on misfolded glycoproteins are necessarily trimmed by Htm1. Interestingly, ~10 % of FNGs were found to contain an internal α1,6-linked mannose residue on their A-arm, which is added by the Golgi-resident mannosyltransferase Och1 (Fig. 4b) [195, 201]. This observation indicates that some misfolded glycoproteins travel to the Golgi prior to retrotranslocation. Although it is unclear what factors control mannose trimming and addition, Och1-modified M8B (Fig. 4b) is increased upon the induction of the unfolded protein response (UPR), while the amounts of Och1-modified M7C (Fig. 4b) were negligibly affected by UPR [195].
Fig. 4.
Formation and processing of neutral FNGs derived from LLOs and misfolded glycoproteins in S. cerevisiae. a OST hydrolyzes Glc3Man8–9GlcNAc2-PP-Dol. The released Glc3Man8–9GlcNAc2 is trimmed by ER glycosidases (Gls1, Gls2 and Mns1). The processed FNGs are transported to the cytosol through an uncharacterized transporter and catabolized to Man1GlcNAc2 by Ams1. Png1 also generates neutral FNGs by deglycosylating misfolded glycoproteins in the cytosol, which are also processed by Ams1. b Glycan isoforms for Man7–8GlcNAc2 and their Och1-modified forms produced in S. cerevisiae. Mannose residues added by Och1 were indicated by down arrowheads. The number for each sugar indicates the order of their assembly in LLO biosynthesis. A, B and C: A-, B- and C-arms. Red arrow indicates an unidentified pathway
As discussed above, FNGs serve as a powerful tool for the dissection of glycoprotein ERAD in S. cerevisiae. However, it is clear that this concept is not applicable to mammalian cells, as bulk FNGs appear to be produced independent of the action of PNGase [117]. However, confusingly, there are several literature reports claiming that the FNGs found in mammalian cells represent the flow of glycoproteins to ERAD [123, 202–204]. In mammalian cells, very specific FNGs (G0–1Man9GlcNAc2) are produced in a PNGase-dependent manner [117]. Therefore, extensive structural and quantitative analyses on these FNG structures, together with the knockout of NGLY1 (PNGase ortholog in mammals), should be empirically carried out to assess the contribution of glycoprotein ERAD to the generation of FNGs in mammalian cells.
An overall picture of FNG catabolism, as well as the degradation of glycoproteins, is not fully understood in S. cerevisiae, since none of the glycosidases involved in these processes have been identified, except for the cytosol/vacuolar α-mannosidase (Ams1) [205]. Ams1 removes all α-linked mannose residues from FNGs and generates Man1GlcNAc2 (Fig. 4a) [193–195, 206]. Ams1 is first synthesized in the cytosol and is then transported to vacuoles via a cytosol-to-vacuole targeting (CVT) pathway mediated by Atg19 [207–211]. When ATG19 is deleted to block CVT pathway, Man1–3GlcNAc2 largely accumulates in the mutant cells [195]. This circumstantial evidence suggests that FNG catabolism can occur in the cytosol. However, it should be noted that S. cerevisiae produces Atg34 [212, 213], a paralog of Atg19, which functions as a cargo of Ams1 only under starvation conditions [213]. Further studies will be needed to clarify the involvement of Atg34 in the catabolism of FNG. The conversion of FNGs to Man1GlcNAc2 has been observed in post-diauxic and stationary phases [206] when the expression of Ams1 is upregulated [195]. Although the biological function of Ams1 is currently unknown, it is tempting to speculate that the Ams1-mediated catabolism of FNGs may generate mannose as a carbon source to compensate for the depletion of glucose in the stationary phase.
FNGs generated in the ER of S. cerevisiae
In S. cerevisiae, ~5 % of the FNGs are produced in an OST-dependent manner (Fig. 5) [194]. The S. cerevisiae OST complex is composed of 8 subunits including 5 essential gene (STT3, WBP1, SWP1, OST1 and OST2) and 3 non-essential gene (OST4, OST5 and either OST3 or its paralog OST6) products (Table 1) [1, 2, 77]. It was found that the maximal N-glycosylation activity of OST is required for the efficient hydrolysis of LLOs [194]. In yeast, Ost3 and Ost6 are exclusively incorporated into two distinct OST complexes, resulting in the formation of Ost3- and Ost6-containing OST complexes [45, 73, 214]. Although the deletion of either OST3 or OST6 causes the hypoglycosylation of a subset of glycoproteins [74, 215], only the deletion of OST3 drastically reduces FNG levels [194]. The underlying mechanism responsible for this is unknown, but it has been reported that the Ost3-containing OST complex is much more abundant (~80 % of the total OST) than the Ost6-containing complex [73]. In addition to OST subunits, LLO structures also modulate the hydrolytic activity of OST. It has been shown that the deletion of ALG6, which results in the production of Man9GlcNAc2-PP-Dol, causes hypoglycosylation [36] and a severe reduction in FNG levels [194]. However, the overproduction of the fully assembled LLO does not result in a proportional increase in FNGs and N-glycans [194], indicating that neither the hydrolysis nor the N-glycosylation is regulated by LLO levels.
Fig. 5.
Model for the metabolic regulation of the degradation of LLOs by LLO-PP’ase. Under normal-glucose conditions (left), GDP-Man is efficiently synthesized from glucose (Glc) and utilized by ALG2 in LLO biosynthesis. When glucose availability becomes low under glucose starvation (right), GDP-Man biosynthesis is downregulated, causing the arrest of the second ALG2 reaction by unknown mechanisms
The regulation of the hydrolytic activity of yeast OST appears to be quite complex. However, protozoan OST may serve as a powerful tool for dissecting the regulatory mechanism, because these organisms have a much simpler OST composition than yeast OST [1, 64–68, 77] and the OST has a relaxed specificity with respect to glycan donor substrates [66–68, 216, 217]. As discussed above, the genome of L. major encodes four STT3 genes (STT3A-D) as the sole OST subunit [65, 67, 68]. It has been reported that L. major Stt3D can be functionally expressed in S. cerevisiae [67, 68] and that non-glucosylated LLOs are utilized as donor substrates for this N-glycosylation [67]. Interestingly, it was found that L. major STT3D produces FNGs from Man9GlcNAc2-PP-Dol in alg6Δ cells where the endogenous OST barely generates FNGs [194]. The mutagenesis of L. major STT3D revealed that an Ala substitution at Glu102, which consists of a putative Asp–Xaa–Asp-like motif (102Glu-Phe-Asp104), causes the nearly complete loss of N-glycosylation in vivo, whereas the hydrolytic activity was reduced by only ~50 % [194]. To ensure normal N-glycosylation, the OST-mediated hydrolysis of LLOs should be tightly regulated. Further studies will be needed to clarify precisely how such regulation is achieved.
Processing and catabolism of FNGs produced in the ER lumen of S. cerevisiae
In S. cerevisiae, the Glc3Man9GlcNAc2 released from the fully assembled LLO by OST is rapidly trimmed by ER glycosidases (Fig. 4a) [194]. Such glycan trimming can be considered to be a part of the catabolic pathway of FNGs, because no apparent catabolic α-glucosidases are produced by S. cerevisiae. Based on the glycan structures of FNGs identified in png1∆ ams1∆ cells (M7C, M8B and their Och1-modified forms, see Fig. 4b for their glycan structures), it appears that ER glucosidases (Gls1 and Gls2) and ER mannosidase (Mns1) are involved in the glycan trimming [194]. For the formation of the M7C isoform, the involvement of Htm1 is unlikely, because this mannosidase has a very low specificity for FNGs [200]. Although it is unclear how M7C is generated in an Htm1-independent manner, an LLO analysis indicated that the lipid-linked triglucosylated M8C (Fig. 4a) may be the source of this isoform. It should be noted that the luminal FNGs acquire the Och1 modification [194]. The fact that no Och1-modified LLOs were detected [194] suggests that Och1 directly modifies FNGs. This assumption is consistent with the observation that fluorescently labeled oligosaccharides can serve as acceptor substrates for Och1 in vitro [218].
What is the fate of luminal FNGs? It has been reported that luminal FNGs are intracellularly catabolized to Man1GlcNAc2 by the cytosolic form of Ams1 [194]. In this context, the ER-to-cytosol transport of FNGs should be required to allow the cytosolic Ams1 to gain direct access to substrates. Although a similar transport system has been identified in mammalian cells [127, 128], it is unclear whether mammals and yeast share a common molecular mechanism, as the gene encoding a FNG transporter has not yet been identified in either organism. In addition to the ER-to-cytosol transport, FNGs are subject to Och1 modification [194], suggesting that they may be transported to the Golgi via a secretory pathway. However, it is currently unknown whether Och1-modified FNGs are retrieved back from the Golgi to the ER or whether they are further transported out of the cells.
Generation of phosphorylated FNGs
The occurrence of phosphorylated FNGs was first reported by Hsu et al. [219]. It was found that, during the incubation of [14C]-mannose with microsomes from a mouse myeloma, a considerable amount of [14C]-mannose is converted into a soluble oligosaccharide, estimated to be Man5GlcNAc2-P. It was suggested that a phosphate group is attached to the reducing end of the innermost GlcNAc, since glucosaminitol was generated after sodium borohydride treatment only when the phosphate group was removed by treatment with alkaline phosphatase. Cacan et al. [220] subsequently showed that similar phosphorylated FNGs are produced in intact rat-spleen lymphocytes. It was also shown that an endo-H treatment of the phosphorylated FNGs generates GlcNAc-P, supporting the earlier idea that the phosphate group is attached to the reducing end of the GlcNAc residue. However, no direct experimental evidence to show the number of phosphate groups and the attachment site has yet appeared in the literature. Based on the structure of phosphorylated FNGs, it was proposed that LLOs are degraded by an unidentified LLO-pyrophosphatase (LLO-PP’ase) [220]. Indeed, such enzymatic activity has been detected in microsomes from yeast [221] and the human liver [222].
Although the occurrence of LLO-PP’ase has been known for more than three decades, the LLO-PP’ase gene has not yet been identified. This is largely due to the fact that the biological relevance of the degradation reaction is still unclear. However, recent studies have shown that the generation of phosphorylated FNGs occurs when LLO biosynthesis is impaired. Peric et al. [223] and Vleugels et al. [222] reported that phosphorylated FNGs are actively produced in cells derived from congenital disorders of glycosylation type I (CDG-I), but not in healthy donor cells. CDG-I is caused by mutations in genes that are involved in the biosynthesis of LLOs and their transfer to proteins [140]. It has been reported that the glycan structures of phosphorylated FNGs (Man1–7GlcNAc2-P) are highly correlated with those of LLO intermediates that are abnormally produced in CDG-I cells [222, 223]. Interestingly, it was found that phosphorylated FNGs are almost exclusively detected in the cytosol [222, 223], although the substrate LLOs are assumed to be distributed on the both sides of the ER membrane. Identification of the LLO-PP’ase gene will be required to answer this question.
It has long been known that, in mammalian cells, glucose starvation causes the arrest of the maturation of LLOs, thereby resulting in hypoglycosylation [224–229]. Quite recently, we reported that, in mouse embryonic fibroblasts (MEFs), glucose starvation results in a dramatic loss of LLOs and a substantial accumulation of Man2GlcNAc2-P in the cytosol [120]. Because only low levels of the phosphorylated form of FNG are generated under normal-glucose conditions, this indicates that the degradation of LLOs is tightly regulated by the availability of glucose. It was also found that glucose starvation results in a reduction of intracellular GDP-Man levels, and that the selective shutdown of the GDP-Man biosynthetic pathway is sufficient to induce the degradation of Man2GlcNAc2-PP-Dol, even when glucose levels are normal [120]. This observation clearly indicates that glucose starvation downregulates GDP-Man biosynthesis, which causes the arrest of the maturation of LLOs with the synthesis of Man2GlcNAc2-PP-Dol and facilitates its degradation by LLO-PP’ase (Fig. 5). Man2GlcNAc2-PP-Dol was found to be a biosynthetic intermediate synthesized by ALG2 (Fig. 1). This finding implies that whether or not the second ALG2 reaction proceeds may be a fate determinant for Man2GlcNAc2-PP-Dol to be degraded or subjected to biosynthesis. Although the mechanism underlying the fate determination is unknown, one plausible explanation is that the availability of intracellular GDP-Man is involved in this process (Fig. 5). Kinetic studies of each ALG2 reaction will be required to validate this attractive hypothesis.
In eukaryotes, reports on the occurrence of phosphorylated FNGs have been limited to mammalian cells thus far [120, 219, 220, 222, 223]. While LLO-PP’ase activity has been detected in microsomes prepared from S. cerevisiae [221], no experimental evidence has been provided to show that S. cerevisiae produces phosphorylated FNGs. The issue of how widespread is the distribution of LLO-PP’ase in eukaryotes remains unclear at present.
FNGs in other organisms
It should be noted here that there are also abundant reports of the occurrence of FNGs in organisms other than mammals and budding yeast.
Non-mammalian vertebrates
The detailed structures of cytosolic FNGs in the hen oviduct have been determined [148]. Major structures observed were high-mannose-type, Gn1-type FNGs, while Gn2-type high-mannose-type FNGs were also detected [148]. The major structure of FNGs was M5B′ (Fig. 6), and the structures of other FNGs were also found to be very similar to those found in mammalian cells [148, 174, 230]. The cytoplasmic PNGase [231], ENGase [149, 151, 232] and Man2C1 [164] in the hen oviduct have also been biochemically characterized.
Fig. 6.
Two glycan isoforms of Man5GlcNAc1 found in D. melanogaster, hen oviduct and mammalian cells. In D. melanogaster and C. elegans, M5A′ (left) is abundantly found in the soluble oligosaccharide fraction. In contrast, M5B′ (right) is dominant in the cytosol of hen oviduct and various mammalian cells. The number for each sugar indicates the order of their assembly in LLO biosynthesis
Sialylated, complex-type FNGs are found in hen’s egg yolk [233]. The structures have been determined to be α2-6-linked disialyl-biantennary glycans with either Gn1 or Gn2 types. Based on structural similarity, these sialylated complex-type FNGs are most likely derived from sialylglycopeptide (SGP), an abundant glycopeptides found in egg yolk. However, the responsible enzymes involved in the formation of sialyl FNGs have not been identified [233].
In fish, Gn2-type sialylated complex-type FNGs have also been identified in unfertilized eggs or embryos of various fish species [234–239]. PNGase activity is potentially involved in the formation of Gn2-type complex-type FNGs, which were identified in early embryos of the medaka fish [240]. The PNGase from medaka embryos requires an acidic pH for optimal activity, and is most likely a lysosomal enzyme and, therefore, distinct from the cytosolic PNGase [241]. In some cases, Gn1-type sialylated complex-type FNGs are also observed [234, 239]. In this case, ENGase activity, which is potentially responsible for the formation of the Gn1-type FNGs, was observed in Zebrafish embryos [239]. However, the nature of this enzyme remains unclarified.
Plants
It has long been proposed that FNGs have biological activities with respect to growth, differentiation, fruit ripening and senescence in plants [242–249], and FNGs have been identified in seedlings, stems, developing fruits and culture cells of various plants [250–264]. FNGs in plants are classified into two subclasses according to their structures: Gn2-type, plant complex-type FNGs bearing a core α1,3-linked fucose or a β1,2-linked xylose and Gn1-type, high-mannose-type FNGs. The former is believed to be generated by the action of a plant-specific acidic PNGase [246, 247, 252, 254, 265–277]. Interestingly, multiple genes encoding acidic PNGases can be found in plants—2 genes Arabidopsis thaliana and 6 genes in Lycopersicum esculentum—and it has been proposed that there may be two types of acidic PNGases present in different subcellular locations in plants [263]. In contrast, Gn1-type, high-mannose-type FNGs are mainly observed in the cytosol [261], and the cytosolic ENGase is believed to be responsible for their formation. ENGase activity has been widely reported in plants [246, 247, 254, 261, 264, 270, 272, 276, 278–287]. Arabidopsis thaliana possesses two ENGase genes, both of which are found to be active [264, 286]. Arabidopsis thaliana bearing mutations in both ENGase genes was generated [264], revealing that the conversion of Gn2-type to Gn1-type FNGs is suppressed in this mutant plant. It was also found that there is no significant reduction in the levels of FNGs, implying that the action of ENGase to glycoproteins or possibly LLOs may not be a dominant source of FNGs in this plant.
More recently, the third type of FNGs has been identified; Gn1-type, complex-type FNGs were found in a rice cell culture medium [263]. In their paper, the authors proposed two possible mechanisms for the occurrence of this unusual glycan structure in the extracellular space. One possibility concerns the existence of a putative plant-specific chitobiase, which catalyzes the hydrolysis of the chitobiosyl linkage of GlcNAcβ1,4(Fucα1,3)GlcNAc of plant-type, complex-type FNGs [263]. Another possibility is that the extracellular Gn1-type, complex-type FNGs may be originated from the cytosol: The cytosolic Gn1-type, high-mannose-type FNGs produced by ENGase are translocated to the ER lumen by unknown mechanisms, processed by Golgi-resident glycosidases and glycosyltransferases, and are then secreted to the extracellular space [263]. It will be extremely interesting to know how these glycans are formed and distributed in plants, especially because the cytosolic ENGase that has been characterized thus far cannot act on plant complex-type glycans [249].
Plants also have the gene encoding the cytosolic PNGase. Arabidopsis thaliana PNGase exhibits a PNGase activity when expressed in yeast [288, 289]. How much this enzyme contributes to the generation of FNGs in plants remains to be determined.
Insects
It has been shown that high-mannose-type FNGs with both Gn1 and Gn2 types are present in the soluble fraction of larva of Drosophila melanogaster [290]. The most abundant structure observed was Man5GlcNAc1 (M5A′, Fig. 6), which is structurally different from the one abundantly found in the cytosol of mammalian cells (M5B′, Fig. 6). When a mutant of pngl, a gene encoding an ortholog of the cytosolic PNGase in D. melanogaster, is analyzed, a similar amount of FNGs are observed to wild-type flies, strongly indicating that most of the FNGs in flies are formed in a PNGase-independent manner.
Worms
A detailed structural analysis of FNGs has been carried out in Caenorhabditis elegans [291, 292]. In the wild-type worms, all of the FNGs detected were found to be the high-mannose-type, 94 % of which were the Gn1 type [291]. The most abundant structure observed, as was the case with D. melanogaster, was M5A′ (Fig. 6) [291]. When a mutant of the gene encoding the cytosolic ENGase [150] was generated, major FNGs became the Gn2 type, together with some reduction (<50 %) in the total amount of FNGs, clearly indicating that the cytosolic ENGase is involved in their catabolism [291]. It is interesting to note that ~30 % of FNGs detected still remained as the Gn1 type. Enzyme(s) responsible for the formation of these Gn1-type FNGs remains unclarified. In C. elegans, the cytosolic PNGase has been shown to be dual functional as PNGase and thioredoxin [293, 294]. While mutants for the gene encoding the PNGase has been isolated as the ones exhibiting the abnormal axon branching of the specific neuron [295], FNGs in this mutant have not yet been analyzed.
Bacteria
N-Glycosylation reactions are not restricted to eukaryotes, but occur over all domains of life including bacteria and archaea [77]. The key catalytic enzymes (STT3 in eukaryotes; PglB in bacteria; AglB in archaea) are orthologous to each other. As discussed above, the first bacterial N-glycosylation system encoded by the pgl gene cluster was described in C. jejuni [78]. In C. jejuni, FNGs comprise as much as 2.5 % of the dry cell weight [296], and the ratio of C. jejuni FNGs to N-glycans was determined to be 10:1 under standard laboratory growth conditions [297], indicating that the hydrolytic activity of PglB proteins is relatively efficient, compared to yeast OST [194]. It was also shown that Campylobacter species contain an N-glycosylation system and also release structurally identical FNGs, indicating that the formation of FNGs is a general phenomenon for these bacteria [296, 298]. Since the FNGs produced by C. jejuni are not detected in the culture medium [82], it is possible that they are not secreted into the extracellular space. FNGs cannot be detected in other pgl mutants producing smaller N-glycan structures, while the N-glycosylation of proteins has been observed [299].
Interestingly, in addition to neutral FNGs, mono- and di-phosphorylated FNGs are also detected by MS analysis in Campylobacter lari [296, 298], while the exact site for the phosphorylation has not yet been fully determined. Whether the similar phosphorylated FNG-releasing system from lipid-linked oligosaccharides found in mammals exists in bacteria remains to be seen.
Perspectives
Extensive efforts in the last decade have resulted in a better understanding of the mechanisms responsible for the formation and processing of FNGs in eukaryotic and prokaryotic cells. In particular, the hypothetical hydrolytic activity of OST has now been experimentally demonstrated both in C. jejuni and S. cerevisiae. Major questions in this context include whether the OST-mediated hydrolysis of LLOs is a universal phenomenon, and how OST balances the hydrolysis and N-glycosylation. To address these issues, an empirical validation of the hydrolytic activity of OST in each organism and the identification of key amino acid residues or co-factors defining the balance, as well as a crystallographic analysis of the OST-LLO-peptide complex, will be needed. Another important issue concerns the unidentified components involved in the formation and transport of FNGs, such as LLO-PP’ase and ER/lysosome transporters for FNGs. The action of LLO-PP’ase appears to be limited under normal conditions, presumably to avoid the undesired degradation of LLOs, and may have important roles under patho/physiological conditions, such as CDG-I and glucose starvation. FNG transporters are closely involved in the catabolism of FNGs. The identification of their genes will open a new avenue for researches to investigate the biological importance of FNG production and catabolism.
The issue of whether FNGs or their catabolic pathways have any biological functions remains an unanswered question. We believe that a careful phenotypic analysis of organisms that have impairments in FNG catabolism should be carried out to elucidate the function of FNGs and their catabolism. For example, the mutation of ENGase in C. elegans dramatically changes the FNG pool from Gn1 to Gn2 structures and this structural change slightly shortened the life span [291]. Interestingly, the supplementation of GlcNAc has been shown to prolong the life of C. elegans by improving ER protein quality control, increasing ERAD activity, and inducing autophagy [300]. Therefore, it is tempting to speculate that the impairment of the Gn2-to-Gn1 conversion of FNGs in mutant worms may reduce the GlcNAc pool, thereby somehow affecting their life span. This attractive hypothesis, however, requires further experimental validations. In the case of C. jejuni, the deletion of pglB, which causes the loss of LLO-hydrolytic and N-glycosylation activity, renders the cells susceptible to osmotic stress [82]. It, therefore, would be extremely interesting to determine which enzymatic activity (i.e., LLO hydrolysis vs N-glycosylation) is important for osmotic regulation. Regarding non-lysosomal glycan degradation, the knockout of Man2C1 in mice apparently causes damage to multiple tissues, which are accompanied by the accumulation of considerable levels of Gn1-type of FNGs with untrimmed mannose residues [166]. As Man2C1 has both enzyme-dependent [168] and -independent [169] functions, knock-in studies will be necessary to test whether an enzyme-dead form of Man2C1 can complement the phenotypes in Man2C1-null mice.
As discussed above, PNGase (NGLY1) biology has attracted widespread interest of researchers in diverse fields since patients harboring mutations in this gene have been identified. The development of clinical approaches for the treatment of these patients is an urgent issue, while the knowledge of physiological functions of PNGase has been quite limited in mammals. Further basic researches directed to the functions of PNGase are required to accelerate clinical research. In any sense, it is imperative to note here that before the discovery of NGLY1 genetic deficiency patients, there were very few who believed that the cytoplasmic PNGase is a biologically important molecule, especially because png1 mutant yeast cells did not exhibit any significant phenotypes [197]. The unexpected findings of the importance of NGLY1 serve as good testimony that we will never be able to predict the biological significance of molecules involved in novel biological pathways, and we, therefore, should be prepared to make tenacious efforts to identify new genes that are involved in the generation and degradation of FNGs. We feel fortunate that there are so many interesting questions remaining to be explored regarding this subject.
It should also be noted that, among the distinct FNG generation pathways, a dominant one can be completely different between mammalian cells and S. cerevisiae, i.e., the PNGase-independent pathway appears to be the predominant path for the former, while most FNGs are generated by PNGase in S. cerevisiae. It is, therefore, important to clarify the mechanism underlying the generation and degradation of FNGs in different organisms, which promises to provide deeper insights into the evolutional conservation, as well as the biological importance, of these processes.
Acknowledgments
We are grateful to the members of Glycometabolome Team for sharing their passion for science as well as fruitful discussions. TS would like to thank Drs. Christine Szymanski and Harald Nothaft (University of Alberta, Canada) for their invaluable input on FNG generation in bacteria. Research regarding the generation and degradation of FNG by the Glycometabolome Team have been supported by the following research grants: Mizutani Foundation for Glycoscience, CREST (Japan Science and Technology Agency), Kakiuchi Memorial Research Grant (The Japanese Biochemical Society) (to T. S.); Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (24770134 and 26650040 to Y. H.; 22570148, 25291030 and 26110725 to T. S.); Incentive Research Projects (RIKEN) to Y. H. and H. H.
References
- 1.Kelleher DJ, Gilmore R. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology. 2006;16(4):47R–62R. doi: 10.1093/glycob/cwj066. [DOI] [PubMed] [Google Scholar]
- 2.Aebi M. N-linked protein glycosylation in the ER. Biochim Biophys Acta. 2013;1833(11):2430–2437. doi: 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473(1):4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 4.Winchester B. Lysosomal metabolism of glycoproteins. Glycobiology. 2005;15(6):1R–15R. doi: 10.1093/glycob/cwi041. [DOI] [PubMed] [Google Scholar]
- 5.Funakoshi Y, Suzuki T. Glycobiology in the cytosol: the bitter side of a sweet world. Biochim Biophys Acta. 2009;1790(2):81–94. doi: 10.1016/j.bbagen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T, Harada Y. Non-lysosomal degradation pathway for N-linked glycans and dolichol-linked oligosaccharides. Biochem Biophys Res Commun. 2014;453(2):213–219. doi: 10.1016/j.bbrc.2014.05.075. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Funakoshi Y. Free N-linked oligosaccharide chains: formation and degradation. Glycoconj J. 2006;23(5–6):291–302. doi: 10.1007/s10719-006-6975-x. [DOI] [PubMed] [Google Scholar]
- 8.Barnes G, Hansen WJ, Holcomb CL, Rine J. Asparagine-linked glycosylation in Saccharomyces cerevisiae: genetic analysis of an early step. Mol Cell Biol. 1984;4(11):2381–2388. doi: 10.1128/mcb.4.11.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X, Rush JS, Karaoglu D, Krasnewich D, Lubinsky MS, Waechter CJ, Gilmore R, Freeze HH. Deficiency of UDP-GlcNAc: dolichol phosphate N-Acetylglucosamine-1 phosphate transferase (DPAGT1) causes a novel congenital disorder of glycosylation Type Ij. Hum Mutat. 2003;22(2):144–150. doi: 10.1002/humu.10239. [DOI] [PubMed] [Google Scholar]
- 10.Gao XD, Moriyama S, Miura N, Dean N, Nishimura S. Interaction between the C termini of Alg13 and Alg14 mediates formation of the active UDP-N-acetylglucosamine transferase complex. J Biol Chem. 2008;283(47):32534–32541. doi: 10.1074/jbc.M804060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao XD, Tachikawa H, Sato T, Jigami Y, Dean N. Alg14 recruits Alg13 to the cytoplasmic face of the endoplasmic reticulum to form a novel bipartite UDP-N-acetylglucosamine transferase required for the second step of N-linked glycosylation. J Biol Chem. 2005;280(43):36254–36262. doi: 10.1074/jbc.M507569200. [DOI] [PubMed] [Google Scholar]
- 12.Bickel T, Lehle L, Schwarz M, Aebi M, Jakob CA. Biosynthesis of lipid-linked oligosaccharides in Saccharomyces cerevisiae: Alg13p and Alg14p form a complex required for the formation of GlcNAc(2)-PP-dolichol. J Biol Chem. 2005;280(41):34500–34506. doi: 10.1074/jbc.M506358200. [DOI] [PubMed] [Google Scholar]
- 13.Bissar-Tadmouri N, Donahue WL, Al-Gazali L, Nelson SF, Bayrak-Toydemir P, Kantarci S. X chromosome exome sequencing reveals a novel ALG13 mutation in a nonsyndromic intellectual disability family with multiple affected male siblings. Am J Med Genet A. 2014;164A(1):164–169. doi: 10.1002/ajmg.a.36233. [DOI] [PubMed] [Google Scholar]
- 14.Cossins J, Belaya K, Hicks D, Salih MA, Finlayson S, Carboni N, Liu WW, Maxwell S, Zoltowska K, Farsani GT, Laval S, Seidhamed MZ, Donnelly P, Bentley D, McGowan SJ, Muller J, Palace J, Lochmuller H, Beeson D. Congenital myasthenic syndromes due to mutations in ALG2 and ALG14. Brain. 2013;136(Pt 3):944–956. doi: 10.1093/brain/awt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz M, Thiel C, Lubbehusen J, Dorland B, de Koning T, von Figura K, Lehle L, Korner C. Deficiency of GDP-Man:GlcNAc2-PP-dolichol mannosyltransferase causes congenital disorder of glycosylation type Ik. Am J Hum Genet. 2004;74(3):472–481. doi: 10.1086/382492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huffaker TC, Robbins PW. Temperature-sensitive yeast mutants deficient in asparagine-linked glycosylation. J Biol Chem. 1982;257(6):3203–3210. [PubMed] [Google Scholar]
- 17.Couto JR, Huffaker TC, Robbins PW. Cloning and expression in Escherichia coli of a yeast mannosyltransferase from the asparagine-linked glycosylation pathway. J Biol Chem. 1984;259(1):378–382. [PubMed] [Google Scholar]
- 18.Thiel C, Schwarz M, Peng J, Grzmil M, Hasilik M, Braulke T, Kohlschutter A, von Figura K, Lehle L, Korner C. A new type of congenital disorders of glycosylation (CDG-Ii) provides new insights into the early steps of dolichol-linked oligosaccharide biosynthesis. J Biol Chem. 2003;278(25):22498–22505. doi: 10.1074/jbc.M302850200. [DOI] [PubMed] [Google Scholar]
- 19.Huffaker TC, Robbins PW. Yeast mutants deficient in protein glycosylation. Proc Natl Acad Sci USA. 1983;80(24):7466–7470. doi: 10.1073/pnas.80.24.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rind N, Schmeiser V, Thiel C, Absmanner B, Lubbehusen J, Hocks J, Apeshiotis N, Wilichowski E, Lehle L, Korner C. A severe human metabolic disease caused by deficiency of the endoplasmatic mannosyltransferase hALG11 leads to congenital disorder of glycosylation-Ip. Hum Mol Genet. 2010;19(8):1413–1424. doi: 10.1093/hmg/ddq016. [DOI] [PubMed] [Google Scholar]
- 21.Cipollo JF, Trimble RB, Chi JH, Yan Q, Dean N. The yeast ALG11 gene specifies addition of the terminal alpha 1,2-Man to the Man5GlcNAc2-PP-dolichol N-glycosylation intermediate formed on the cytosolic side of the endoplasmic reticulum. J Biol Chem. 2001;276(24):21828–21840. doi: 10.1074/jbc.M010896200. [DOI] [PubMed] [Google Scholar]
- 22.Valderrama-Rincon JD, Fisher AC, Merritt JH, Fan YY, Reading CA, Chhiba K, Heiss C, Azadi P, Aebi M, DeLisa MP. An engineered eukaryotic protein glycosylation pathway in Escherichia coli . Nat Chem Biol. 2012;8(5):434–436. doi: 10.1038/nchembio.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helenius J, Ng DT, Marolda CL, Walter P, Valvano MA, Aebi M. Translocation of lipid-linked oligosaccharides across the ER membrane requires Rft1 protein. Nature. 2002;415(6870):447–450. doi: 10.1038/415447a. [DOI] [PubMed] [Google Scholar]
- 24.Frank CG, Sanyal S, Rush JS, Waechter CJ, Menon AK. Does Rft1 flip an N-glycan lipid precursor? Nature. 2008;454(7204):E3–E4. doi: 10.1038/nature07165. [DOI] [PubMed] [Google Scholar]
- 25.Rush JS, Gao N, Lehrman MA, Matveev S, Waechter CJ. Suppression of Rft1 expression does not impair the transbilayer movement of Man5GlcNAc2-P-P-dolichol in sealed microsomes from yeast. J Biol Chem. 2009;284(30):19835–19842. doi: 10.1074/jbc.M109.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanyal S, Menon AK. Specific transbilayer translocation of dolichol-linked oligosaccharides by an endoplasmic reticulum flippase. Proc Natl Acad Sci USA. 2009;106(3):767–772. doi: 10.1073/pnas.0810225106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schollen E, Grunewald S, Keldermans L, Albrecht B, Korner C, Matthijs G. CDG-Id caused by homozygosity for an ALG3 mutation due to segmental maternal isodisomy UPD3(q21.3-qter) Eur J Med Genet. 2005;48(2):153–158. doi: 10.1016/j.ejmg.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Aebi M, Gassenhuber J, Domdey H, te Heesen S. Cloning and characterization of the ALG3 gene of Saccharomyces cerevisiae . Glycobiology. 1996;6(4):439–444. doi: 10.1093/glycob/6.4.439. [DOI] [PubMed] [Google Scholar]
- 29.Frank CG, Grubenmann CE, Eyaid W, Berger EG, Aebi M, Hennet T. Identification and functional analysis of a defect in the human ALG9 gene: definition of congenital disorder of glycosylation type IL. Am J Hum Genet. 2004;75(1):146–150. doi: 10.1086/422367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burda P, te Heesen S, Brachat A, Wach A, Dusterhoft A, Aebi M. Stepwise assembly of the lipid-linked oligosaccharide in the endoplasmic reticulum of Saccharomyces cerevisiae: identification of the ALG9 gene encoding a putative mannosyl transferase. Proc Natl Acad Sci USA. 1996;93(14):7160–7165. doi: 10.1073/pnas.93.14.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cipollo JF, Trimble RB. The Saccharomyces cerevisiae alg12∆ mutant reveals a role for the middle-arm α1,2Man- and upper-arm α1,2Manα1,6Man- residues of Glc3Man9GlcNAc2-PP-Dol in regulating glycoprotein glycan processing in the endoplasmic reticulum and Golgi apparatus. Glycobiology. 2002;12(11):749–762. doi: 10.1093/glycob/cwf082. [DOI] [PubMed] [Google Scholar]
- 32.Grubenmann CE, Frank CG, Kjaergaard S, Berger EG, Aebi M, Hennet T. ALG12 mannosyltransferase defect in congenital disorder of glycosylation type lg. Hum Mol Genet. 2002;11(19):2331–2339. doi: 10.1093/hmg/11.19.2331. [DOI] [PubMed] [Google Scholar]
- 33.Chantret I, Dupre T, Delenda C, Bucher S, Dancourt J, Barnier A, Charollais A, Heron D, Bader-Meunier B, Danos O, Seta N, Durand G, Oriol R, Codogno P, Moore SE. Congenital disorders of glycosylation type Ig is defined by a deficiency in dolichyl-P-mannose:Man7GlcNAc2-PP-dolichyl mannosyltransferase. J Biol Chem. 2002;277(28):25815–25822. doi: 10.1074/jbc.M203285200. [DOI] [PubMed] [Google Scholar]
- 34.Burda P, Jakob CA, Beinhauer J, Hegemann JH, Aebi M. Ordered assembly of the asymmetrically branched lipid-linked oligosaccharide in the endoplasmic reticulum is ensured by the substrate specificity of the individual glycosyltransferases. Glycobiology. 1999;9(6):617–625. doi: 10.1093/glycob/9.6.617. [DOI] [PubMed] [Google Scholar]
- 35.Imbach T, Burda P, Kuhnert P, Wevers RA, Aebi M, Berger EG, Hennet T. A mutation in the human ortholog of the Saccharomyces cerevisiae ALG6 gene causes carbohydrate-deficient glycoprotein syndrome type-Ic. Proc Natl Acad Sci USA. 1999;96(12):6982–6987. doi: 10.1073/pnas.96.12.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiss G, te Heesen S, Zimmerman J, Robbins PW, Aebi M. Isolation of the ALG6 locus of Saccharomyces cerevisiae required for glucosylation in the N-linked glycosylation pathway. Glycobiology. 1996;6(5):493–498. doi: 10.1093/glycob/6.5.493. [DOI] [PubMed] [Google Scholar]
- 37.Chantret I, Dancourt J, Dupre T, Delenda C, Bucher S, Vuillaumier-Barrot S, Ogier de Baulny H, Peletan C, Danos O, Seta N, Durand G, Oriol R, Codogno P, Moore SE. A deficiency in dolichyl-P-glucose:Glc1Man9GlcNAc2-PP-dolichyl α3-glucosyltransferase defines a new subtype of congenital disorders of glycosylation. J Biol Chem. 2003;278(11):9962–9971. doi: 10.1074/jbc.M211950200. [DOI] [PubMed] [Google Scholar]
- 38.Stagljar I, te Heesen S, Aebi M. New phenotype of mutations deficient in glucosylation of the lipid-linked oligosaccharide: cloning of the ALG8 locus. Proc Natl Acad Sci USA. 1994;91(13):5977–5981. doi: 10.1073/pnas.91.13.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burda P, Aebi M. The ALG10 locus of Saccharomyces cerevisiae encodes the alpha-1,2 glucosyltransferase of the endoplasmic reticulum: the terminal glucose of the lipid-linked oligosaccharide is required for efficient N-linked glycosylation. Glycobiology. 1998;8(5):455–462. doi: 10.1093/glycob/8.5.455. [DOI] [PubMed] [Google Scholar]
- 40.Rush JS, Cho SK, Jiang S, Hofmann SL, Waechter CJ. Identification and characterization of a cDNA encoding a dolichyl pyrophosphate phosphatase located in the endoplasmic reticulum of mammalian cells. J Biol Chem. 2002;277(47):45226–45234. doi: 10.1074/jbc.M207076200. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez F, Rush JS, Toke DA, Han GS, Quinn JE, Carman GM, Choi JY, Voelker DR, Aebi M, Waechter CJ. The CWH8 gene encodes a dolichyl pyrophosphate phosphatase with a luminally oriented active site in the endoplasmic reticulum of Saccharomyces cerevisiae . J Biol Chem. 2001;276(44):41455–41464. doi: 10.1074/jbc.M105544200. [DOI] [PubMed] [Google Scholar]
- 42.van Berkel MA, Rieger M, te Heesen S, Ram AF, van den Ende H, Aebi M, Klis FM. The Saccharomyces cerevisiae CWH8 gene is required for full levels of dolichol-linked oligosaccharides in the endoplasmic reticulum and for efficient N-glycosylation. Glycobiology. 1999;9(3):243–253. doi: 10.1093/glycob/9.3.243. [DOI] [PubMed] [Google Scholar]
- 43.Rush JS, Gao N, Lehrman MA, Waechter CJ. Recycling of dolichyl monophosphate to the cytoplasmic leaflet of the endoplasmic reticulum after the cleavage of dolichyl pyrophosphate on the lumenal monolayer. J Biol Chem. 2008;283(7):4087–4093. doi: 10.1074/jbc.M707067200. [DOI] [PubMed] [Google Scholar]
- 44.Chavan M, Yan A, Lennarz WJ. Subunits of the translocon interact with components of the oligosaccharyl transferase complex. J Biol Chem. 2005;280(24):22917–22924. doi: 10.1074/jbc.M502858200. [DOI] [PubMed] [Google Scholar]
- 45.Yan A, Lennarz WJ. Two oligosaccharyl transferase complexes exist in yeast and associate with two different translocons. Glycobiology. 2005;15(12):1407–1415. doi: 10.1093/glycob/cwj026. [DOI] [PubMed] [Google Scholar]
- 46.Harada Y, Li H, Lennarz WJ. Oligosaccharyltransferase directly binds to ribosome at a location near the translocon-binding site. Proc Natl Acad Sci USA. 2009;106(17):6945–6949. doi: 10.1073/pnas.0812489106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz-Canada C, Kelleher DJ, Gilmore R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell. 2009;136(2):272–283. doi: 10.1016/j.cell.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shibatani T, David LL, McCormack AL, Frueh K, Skach WR. Proteomic analysis of mammalian oligosaccharyltransferase reveals multiple subcomplexes that contain Sec61, TRAP, and two potential new subunits. Biochemistry. 2005;44(16):5982–5992. doi: 10.1021/bi047328f. [DOI] [PubMed] [Google Scholar]
- 49.Pfeffer S, Dudek J, Gogala M, Schorr S, Linxweiler J, Lang S, Becker T, Beckmann R, Zimmermann R, Forster F. Structure of the mammalian oligosaccharyl-transferase complex in the native ER protein translocon. Nat Commun. 2014;5:3072. doi: 10.1038/ncomms4072. [DOI] [PubMed] [Google Scholar]
- 50.Lau JT, Welply JK, Shenbagamurthi P, Naider F, Lennarz WJ. Substrate recognition by oligosaccharyl transferase. Inhibition of co-translational glycosylation by acceptor peptides. J Biol Chem. 1983;258(24):15255–15260. [PubMed] [Google Scholar]
- 51.Duvet S, Op De Beeck A, Cocquerel L, Wychowski C, Cacan R, Dubuisson J. Glycosylation of the hepatitis C virus envelope protein E1 occurs posttranslationally in a mannosylphosphoryldolichol-deficient CHO mutant cell line. Glycobiology. 2002;12(2):95–101. doi: 10.1093/glycob/12.2.95. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson IM, von Heijne G. Determination of the distance between the oligosaccharyltransferase active site and the endoplasmic reticulum membrane. J Biol Chem. 1993;268(8):5798–5801. [PubMed] [Google Scholar]
- 53.Li H, Chavan M, Schindelin H, Lennarz WJ. Structure of the oligosaccharyl transferase complex at 12 A resolution. Structure. 2008;16(3):432–440. doi: 10.1016/j.str.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 54.Lizak C, Gerber S, Numao S, Aebi M, Locher KP. X-ray structure of a bacterial oligosaccharyltransferase. Nature. 2011;474(7351):350–355. doi: 10.1038/nature10151. [DOI] [PubMed] [Google Scholar]
- 55.Welply JK, Shenbagamurthi P, Lennarz WJ, Naider F. Substrate recognition by oligosaccharyltransferase. Studies on glycosylation of modified Asn-X-Thr/Ser tripeptides. J Biol Chem. 1983;258(19):11856–11863. [PubMed] [Google Scholar]
- 56.Karaoglu D, Kelleher DJ, Gilmore R. Allosteric regulation provides a molecular mechanism for preferential utilization of the fully assembled dolichol-linked oligosaccharide by the yeast oligosaccharyltransferase. Biochemistry. 2001;40(40):12193–12206. doi: 10.1021/bi0111911. [DOI] [PubMed] [Google Scholar]
- 57.Kelleher DJ, Karaoglu D, Mandon EC, Gilmore R. Oligosaccharyltransferase isoforms that contain different catalytic STT3 subunits have distinct enzymatic properties. Mol Cell. 2003;12(1):101–111. doi: 10.1016/s1097-2765(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 58.Sharma CB, Lehle L, Tanner W. N-Glycosylation of yeast proteins. Characterization of the solubilized oligosaccharyl transferase. Eur J Biochem. 1981;116(1):101–108. doi: 10.1111/j.1432-1033.1981.tb05306.x. [DOI] [PubMed] [Google Scholar]
- 59.Breuer W, Bause E. Oligosaccharyl transferase is a constitutive component of an oligomeric protein complex from pig liver endoplasmic reticulum. Eur J Biochem. 1995;228(3):689–696. [PubMed] [Google Scholar]
- 60.Frank CG, Aebi M. ALG9 mannosyltransferase is involved in two different steps of lipid-linked oligosaccharide biosynthesis. Glycobiology. 2005;15(11):1156–1163. doi: 10.1093/glycob/cwj002. [DOI] [PubMed] [Google Scholar]
- 61.Isono T. O-GlcNAc-specific antibody CTD110.6 cross-reacts with N-GlcNAc2-modified proteins induced under glucose deprivation. PLoS ONE. 2011;6(4):e18959. doi: 10.1371/journal.pone.0018959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Isono T, Chano T, Okabe H, Suzaki M. Study of global transcriptional changes of N-GlcNAc2 proteins-producing T24 bladder carcinoma cells under glucose deprivation. PLoS ONE. 2013;8(4):e60397. doi: 10.1371/journal.pone.0060397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Isono T, Chano T, Kitamura A, Yuasa T. Glucose deprivation induces G2/M transition-arrest and cell death in N-GlcNAc2-modified protein-producing renal carcinoma cells. PLoS ONE. 2014;9(5):e96168. doi: 10.1371/journal.pone.0096168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, Lennard NJ, Caler E, Hamlin NE, Haas B, Bohme U, Hannick L, Aslett MA, Shallom J, Marcello L, Hou L, Wickstead B, Alsmark UC, Arrowsmith C, Atkin RJ, Barron AJ, Bringaud F, Brooks K, Carrington M, Cherevach I, Chillingworth TJ, Churcher C, Clark LN, Corton CH, Cronin A, Davies RM, Doggett J, Djikeng A, Feldblyum T, Field MC, Fraser A, Goodhead I, Hance Z, Harper D, Harris BR, Hauser H, Hostetler J, Ivens A, Jagels K, Johnson D, Johnson J, Jones K, Kerhornou AX, Koo H, Larke N, Landfear S, Larkin C, Leech V, Line A, Lord A, Macleod A, Mooney PJ, Moule S, Martin DM, Morgan GW, Mungall K, Norbertczak H, Ormond D, Pai G, Peacock CS, Peterson J, Quail MA, Rabbinowitsch E, Rajandream MA, Reitter C, Salzberg SL, Sanders M, Schobel S, Sharp S, Simmonds M, Simpson AJ, Tallon L, Turner CM, Tait A, Tivey AR, Van Aken S, Walker D, Wanless D, Wang S, White B, White O, Whitehead S, Woodward J, Wortman J, Adams MD, Embley TM, Gull K, Ullu E, Barry JD, Fairlamb AH, Opperdoes F, Barrell BG, Donelson JE, Hall N, Fraser CM, Melville SE, El-Sayed NM. The genome of the African trypanosome Trypanosoma brucei . Science. 2005;309(5733):416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 65.Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, Sisk E, Rajandream MA, Adlem E, Aert R, Anupama A, Apostolou Z, Attipoe P, Bason N, Bauser C, Beck A, Beverley SM, Bianchettin G, Borzym K, Bothe G, Bruschi CV, Collins M, Cadag E, Ciarloni L, Clayton C, Coulson RM, Cronin A, Cruz AK, Davies RM, De Gaudenzi J, Dobson DE, Duesterhoeft A, Fazelina G, Fosker N, Frasch AC, Fraser A, Fuchs M, Gabel C, Goble A, Goffeau A, Harris D, Hertz-Fowler C, Hilbert H, Horn D, Huang Y, Klages S, Knights A, Kube M, Larke N, Litvin L, Lord A, Louie T, Marra M, Masuy D, Matthews K, Michaeli S, Mottram JC, Muller-Auer S, Munden H, Nelson S, Norbertczak H, Oliver K, O’Neil S, Pentony M, Pohl TM, Price C, Purnelle B, Quail MA, Rabbinowitsch E, Reinhardt R, Rieger M, Rinta J, Robben J, Robertson L, Ruiz JC, Rutter S, Saunders D, Schafer M, Schein J, Schwartz DC, Seeger K, Seyler A, Sharp S, Shin H, Sivam D, Squares R, Squares S, Tosato V, Vogt C, Volckaert G, Wambutt R, Warren T, Wedler H, Woodward J, Zhou S, Zimmermann W, Smith DF, Blackwell JM, Stuart KD, Barrell B, Myler PJ. The genome of the kinetoplastid parasite, Leishmania major . Science. 2005;309(5733):436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Izquierdo L, Schulz BL, Rodrigues JA, Guther ML, Procter JB, Barton GJ, Aebi M, Ferguson MA. Distinct donor and acceptor specificities of Trypanosoma brucei oligosaccharyltransferases. EMBO J. 2009;28(17):2650–2661. doi: 10.1038/emboj.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nasab FP, Schulz BL, Gamarro F, Parodi AJ, Aebi M. All in one: Leishmania major STT3 proteins substitute for the whole oligosaccharyltransferase complex in Saccharomyces cerevisiae . Mol Biol Cell. 2008;19(9):3758–3768. doi: 10.1091/mbc.E08-05-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hese K, Otto C, Routier FH, Lehle L. The yeast oligosaccharyltransferase complex can be replaced by STT3 from Leishmania major . Glycobiology. 2009;19(2):160–171. doi: 10.1093/glycob/cwn118. [DOI] [PubMed] [Google Scholar]
- 69.Bas T, Gao GY, Lvov A, Chandrasekhar KD, Gilmore R, Kobertz WR. Post-translational N-glycosylation of type I transmembrane KCNE1 peptides: implications for membrane protein biogenesis and disease. J Biol Chem. 2011;286(32):28150–28159. doi: 10.1074/jbc.M111.235168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shrimal S, Gilmore R. Glycosylation of closely spaced acceptor sites in human glycoproteins. J Cell Sci. 2013;126(Pt 23):5513–5523. doi: 10.1242/jcs.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shrimal S, Trueman SF, Gilmore R. Extreme C-terminal sites are posttranslocationally glycosylated by the STT3B isoform of the OST. J Cell Biol. 2013;201(1):81–95. doi: 10.1083/jcb.201301031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cherepanova NA, Shrimal S, Gilmore R. Oxidoreductase activity is necessary for N-glycosylation of cysteine-proximal acceptor sites in glycoproteins. J Cell Biol. 2014;206(4):525–539. doi: 10.1083/jcb.201404083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spirig U, Bodmer D, Wacker M, Burda P, Aebi M. The 3.4-kDa Ost4 protein is required for the assembly of two distinct oligosaccharyltransferase complexes in yeast. Glycobiology. 2005;15(12):1396–1406. doi: 10.1093/glycob/cwj025. [DOI] [PubMed] [Google Scholar]
- 74.Schulz BL, Stirnimann CU, Grimshaw JP, Brozzo MS, Fritsch F, Mohorko E, Capitani G, Glockshuber R, Grutter MG, Aebi M. Oxidoreductase activity of oligosaccharyltransferase subunits Ost3p and Ost6p defines site-specific glycosylation efficiency. Proc Natl Acad Sci USA. 2009;106(27):11061–11066. doi: 10.1073/pnas.0812515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jamaluddin MF, Bailey UM, Tan NY, Stark AP, Schulz BL. Polypeptide binding specificities of Saccharomyces cerevisiae oligosaccharyltransferase accessory proteins Ost3p and Ost6p. Protein Sci. 2011;20(5):849–855. doi: 10.1002/pro.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohorko E, Owen RL, Malojcic G, Brozzo MS, Aebi M, Glockshuber R. Structural basis of substrate specificity of human oligosaccharyl transferase subunit N33/Tusc3 and its role in regulating protein N-glycosylation. Structure. 2014;22(4):590–601. doi: 10.1016/j.str.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 77.Schwarz F, Aebi M. Mechanisms and principles of N-linked protein glycosylation. Curr Opin Struct Biol. 2011;21(5):576–582. doi: 10.1016/j.sbi.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 78.Szymanski CM, Yao R, Ewing CP, Trust TJ, Guerry P. Evidence for a system of general protein glycosylation in Campylobacter jejuni . Mol Microbiol. 1999;32(5):1022–1030. doi: 10.1046/j.1365-2958.1999.01415.x. [DOI] [PubMed] [Google Scholar]
- 79.Wacker M, Linton D, Hitchen PG, Nita-Lazar M, Haslam SM, North SJ, Panico M, Morris HR, Dell A, Wren BW, Aebi M. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli . Science. 2002;298(5599):1790–1793. doi: 10.1126/science.298.5599.1790. [DOI] [PubMed] [Google Scholar]
- 80.Young NM, Brisson JR, Kelly J, Watson DC, Tessier L, Lanthier PH, Jarrell HC, Cadotte N, St Michael F, Aberg E, Szymanski CM. Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium, Campylobacter jejuni . J Biol Chem. 2002;277(45):42530–42539. doi: 10.1074/jbc.M206114200. [DOI] [PubMed] [Google Scholar]
- 81.Glover KJ, Weerapana E, Imperiali B. In vitro assembly of the undecaprenylpyrophosphate-linked heptasaccharide for prokaryotic N-linked glycosylation. Proc Natl Acad Sci USA. 2005;102(40):14255–14259. doi: 10.1073/pnas.0507311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nothaft H, Liu X, McNally DJ, Li J, Szymanski CM. Study of free oligosaccharides derived from the bacterial N-glycosylation pathway. Proc Natl Acad Sci USA. 2009;106(35):15019–15024. doi: 10.1073/pnas.0903078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gething MJ, McCammon K, Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- 84.Hampton RY, Sommer T. Finding the will and the way of ERAD substrate retrotranslocation. Curr Opin Cell Biol. 2012;24(4):460–466. doi: 10.1016/j.ceb.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 85.Burda P, Aebi M. The dolichol pathway of N-linked glycosylation. Biochim Biophys Acta. 1999;1426(2):239–257. doi: 10.1016/s0304-4165(98)00127-5. [DOI] [PubMed] [Google Scholar]
- 86.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 87.Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426(6968):891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 88.Aebi M, Bernasconi R, Clerc S, Molinari M. N-glycan structures: recognition and processing in the ER. Trends Biochem Sci. 2010;35(2):74–82. doi: 10.1016/j.tibs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Maattanen P, Gehring K, Bergeron JJ, Thomas DY. Protein quality control in the ER: the recognition of misfolded proteins. Semin Cell Dev Biol. 2010;21(5):500–511. doi: 10.1016/j.semcdb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 90.Kostova Z, Wolf DH. For whom the bell tolls: protein quality control of the endoplasmic reticulum and the ubiquitin-proteasome connection. EMBO J. 2003;22(10):2309–2317. doi: 10.1093/emboj/cdg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu X, Azakami H, Kato A. P-domain and lectin site are involved in the chaperone function of Saccharomyces cerevisiae calnexin homologue. FEBS Lett. 2004;570(1–3):155–160. doi: 10.1016/j.febslet.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 92.Totani K, Ihara Y, Matsuo I, Ito Y. Substrate specificity analysis of endoplasmic reticulum glucosidase II using synthetic high mannose-type glycans. J Biol Chem. 2006;281(42):31502–31508. doi: 10.1074/jbc.M605457200. [DOI] [PubMed] [Google Scholar]
- 93.Caramelo JJ, Parodi AJ. Getting in and out from calnexin/calreticulin cycles. J Biol Chem. 2008;283(16):10221–10225. doi: 10.1074/jbc.R700048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Alessio C, Caramelo JJ, Parodi AJ. UDP-GlC:glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control. Semin Cell Dev Biol. 2010;21(5):491–499. doi: 10.1016/j.semcdb.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13(7):448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ninagawa S, Okada T, Sumitomo Y, Kamiya Y, Kato K, Horimoto S, Ishikawa T, Takeda S, Sakuma T, Yamamoto T, Mori K. EDEM2 initiates mammalian glycoprotein ERAD by catalyzing the first mannose trimming step. J Cell Biol. 2014;206(3):347–356. doi: 10.1083/jcb.201404075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clerc S, Hirsch C, Oggier DM, Deprez P, Jakob C, Sommer T, Aebi M. Htm1 protein generates the N-glycan signal for glycoprotein degradation in the endoplasmic reticulum. J Cell Biol. 2009;184(1):159–172. doi: 10.1083/jcb.200809198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Quan EM, Kamiya Y, Kamiya D, Denic V, Weibezahn J, Kato K, Weissman JS. Defining the glycan destruction signal for endoplasmic reticulum-associated degradation. Mol Cell. 2008;32(6):870–877. doi: 10.1016/j.molcel.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hosokawa N, Kamiya Y, Kamiya D, Kato K, Nagata K. Human OS-9, a lectin required for glycoprotein endoplasmic reticulum-associated degradation, recognizes mannose-trimmed N-glycans. J Biol Chem. 2009;284(25):17061–17068. doi: 10.1074/jbc.M809725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hosokawa N, Wada I, Nagasawa K, Moriyama T, Okawa K, Nagata K. Human XTP3-B forms an endoplasmic reticulum quality control scaffold with the HRD1-SEL1L ubiquitin ligase complex and BiP. J Biol Chem. 2008;283(30):20914–20924. doi: 10.1074/jbc.M709336200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carvalho P, Goder V, Rapoport TA. Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell. 2006;126(2):361–373. doi: 10.1016/j.cell.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 102.Denic V, Quan EM, Weissman JS. A luminal surveillance complex that selects misfolded glycoproteins for ER-associated degradation. Cell. 2006;126(2):349–359. doi: 10.1016/j.cell.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 103.Hebert DN, Bernasconi R, Molinari M. ERAD substrates: which way out? Semin Cell Dev Biol. 2010;21(5):526–532. doi: 10.1016/j.semcdb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 104.Zhang T, Ye Y. The final moments of misfolded proteins en route to the proteasome. DNA Cell Biol. 2014;33(8):477–483. doi: 10.1089/dna.2014.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suzuki T, Park H, Lennarz WJ. Cytoplasmic peptide:N-glycanase (PNGase) in eukaryotic cells: occurrence, primary structure, and potential functions. FASEB J. 2002;16(7):635–641. doi: 10.1096/fj.01-0889rev. [DOI] [PubMed] [Google Scholar]
- 106.Hirayama H, Hosomi A, Suzuki T. Physiological and molecular functions of the cytosolic peptide:N-glycanase. Semin Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki T. The cytoplasmic peptide:N-glycanase (Ngly1): basic science encounters a human genetic disorder. J Biochem. 2015;157(1):23–34. doi: 10.1093/jb/mvu068. [DOI] [PubMed] [Google Scholar]
- 108.Li G, Zhou X, Zhao G, Schindelin H, Lennarz WJ. Multiple modes of interaction of the deglycosylation enzyme, mouse peptide N-glycanase, with the proteasome. Proc Natl Acad Sci USA. 2005;102(44):15809–15814. doi: 10.1073/pnas.0507155102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li G, Zhao G, Zhou X, Schindelin H, Lennarz WJ. The AAA ATPase p97 links peptide N-glycanase to the endoplasmic reticulum-associated E3 ligase autocrine motility factor receptor. Proc Natl Acad Sci USA. 2006;103(22):8348–8353. doi: 10.1073/pnas.0602747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park H, Suzuki T, Lennarz WJ. Identification of proteins that interact with mammalian peptide:N-glycanase and implicate this hydrolase in the proteasome-dependent pathway for protein degradation. Proc Natl Acad Sci USA. 2001;98(20):11163–11168. doi: 10.1073/pnas.201393498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suzuki T, Park H, Kwofie MA, Lennarz WJ. Rad23 provides a link between the Png1 deglycosylating enzyme and the 26 S proteasome in yeast. J Biol Chem. 2001;276(24):21601–21607. doi: 10.1074/jbc.M100826200. [DOI] [PubMed] [Google Scholar]
- 112.Kim I, Ahn J, Liu C, Tanabe K, Apodaca J, Suzuki T, Rao H. The Png1-Rad23 complex regulates glycoprotein turnover. J Cell Biol. 2006;172(2):211–219. doi: 10.1083/jcb.200507149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suzuki T. Cytoplasmic peptide:N-glycanase and catabolic pathway for free N-glycans in the cytosol. Semin Cell Dev Biol. 2007;18(6):762–769. doi: 10.1016/j.semcdb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 114.Chantret I, Moore SE. Free oligosaccharide regulation during mammalian protein N-glycosylation. Glycobiology. 2008;18(3):210–224. doi: 10.1093/glycob/cwn003. [DOI] [PubMed] [Google Scholar]
- 115.Anumula KR, Spiro RG. Release of glucose-containing polymannose oligosaccharides during glycoprotein biosynthesis. Studies with thyroid microsomal enzymes and slices. J Biol Chem. 1983;258(24):15274–15282. [PubMed] [Google Scholar]
- 116.Chavan M, Chen Z, Li G, Schindelin H, Lennarz WJ, Li H. Dimeric organization of the yeast oligosaccharyl transferase complex. Proc Natl Acad Sci USA. 2006;103(24):8947–8952. doi: 10.1073/pnas.0603262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chantret I, Fasseu M, Zaoui K, Le Bizec C, Yaye HS, Dupre T, Moore SE. Identification of roles for peptide: N-glycanase and endo-β-N-acetylglucosaminidase (Engase1p) during protein N-glycosylation in human HepG2 cells. PLoS ONE. 2010;5(7):e11734. doi: 10.1371/journal.pone.0011734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang C, Harada Y, Hosomi A, Masahara-Negishi Y, Seino J, Fujihira H, Funakoshi Y, Suzuki T, Dohmae N. Endo-β-N-acetylglucosaminidase forms N-GlcNAc protein aggregates during ER-associated degradation in Ngly1-defective cells. Proc Natl Acad Sci USA. 2015;112(5):1398–1403. doi: 10.1073/pnas.1414593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spiro MJ, Spiro RG. Potential regulation of N-glycosylation precursor through oligosaccharide-lipid hydrolase action and glucosyltransferase-glucosidase shuttle. J Biol Chem. 1991;266(8):5311–5317. [PubMed] [Google Scholar]
- 120.Harada Y, Nakajima K, Masahara-Negishi Y, Freeze HH, Angata T, Taniguchi N, Suzuki T. Metabolically programmed quality control system for dolichol-linked oligosaccharides. Proc Natl Acad Sci USA. 2013;110(48):19366–19371. doi: 10.1073/pnas.1312187110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gao N, Shang J, Lehrman MA. Analysis of glycosylation in CDG-Ia fibroblasts by fluorophore-assisted carbohydrate electrophoresis: implications for extracellular glucose and intracellular mannose 6-phosphate. J Biol Chem. 2005;280(18):17901–17909. doi: 10.1074/jbc.M500510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Villers C, Cacan R, Mir AM, Labiau O, Verbert A. Release of oligomannoside-type glycans as a marker of the degradation of newly synthesized glycoproteins. Biochem J. 1994;298(Pt 1):135–142. doi: 10.1042/bj2980135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Duvet S, Foulquier F, Mir AM, Chirat F, Cacan R. Discrimination between lumenal and cytosolic sites of deglycosylation in endoplasmic reticulum-associated degradation of glycoproteins by using benzyl mannose in CHO cell lines. Glycobiology. 2004;14(9):841–849. doi: 10.1093/glycob/cwh103. [DOI] [PubMed] [Google Scholar]
- 124.Gao N, Shang J, Huynh D, Manthati VL, Arias C, Harding HP, Kaufman RJ, Mohr I, Ron D, Falck JR, Lehrman MA. Mannose-6-phosphate regulates destruction of lipid-linked oligosaccharides. Mol Biol Cell. 2011;22(17):2994–3009. doi: 10.1091/mbc.E11-04-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao N, Lehrman MA. Mannose-6-phosphate: a regulator of LLO destruction. Methods Mol Biol. 2013;1022:277–282. doi: 10.1007/978-1-62703-465-4_20. [DOI] [PubMed] [Google Scholar]
- 126.Higashidani A, Bode L, Nishikawa A, Freeze HH. Exogenous mannose does not raise steady state mannose-6-phosphate pools of normal or N-glycosylation-deficient human fibroblasts. Mol Genet Metab. 2009;96(4):268–272. doi: 10.1016/j.ymgme.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moore SE, Bauvy C, Codogno P. Endoplasmic reticulum-to-cytosol transport of free polymannose oligosaccharides in permeabilized HepG2 cells. EMBO J. 1995;14(23):6034–6042. doi: 10.1002/j.1460-2075.1995.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haga Y, Totani K, Ito Y, Suzuki T. Establishment of a real-time analytical method for free oligosaccharide transport from the ER to the cytosol. Glycobiology. 2009;19(9):987–994. doi: 10.1093/glycob/cwp075. [DOI] [PubMed] [Google Scholar]
- 129.Moore SE. Transport of free polymannose-type oligosaccharides from the endoplasmic reticulum into the cytosol is inhibited by mannosides and requires a thapsigargin-sensitive calcium store. Glycobiology. 1998;8(4):373–381. doi: 10.1093/glycob/8.4.373. [DOI] [PubMed] [Google Scholar]
- 130.Moore SE, Spiro RG. Demonstration that Golgi endo-α-d-mannosidase provides a glucosidase-independent pathway for the formation of complex N-linked oligosaccharides of glycoproteins. J Biol Chem. 1990;265(22):13104–13112. [PubMed] [Google Scholar]
- 131.Spiro MJ, Bhoyroo VD, Spiro RG. Molecular cloning and expression of rat liver endo-α-mannosidase, an N-linked oligosaccharide processing enzyme. J Biol Chem. 1997;272(46):29356–29363. doi: 10.1074/jbc.272.46.29356. [DOI] [PubMed] [Google Scholar]
- 132.Durrant C, Moore SE. Perturbation of free oligosaccharide trafficking in endoplasmic reticulum glucosidase I-deficient and castanospermine-treated cells. Biochem J. 2002;365(Pt 1):239–247. doi: 10.1042/BJ20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hirsch C, Blom D, Ploegh HL. A role for N-glycanase in the cytosolic turnover of glycoproteins. EMBO J. 2003;22(5):1036–1046. doi: 10.1093/emboj/cdg107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Blom D, Hirsch C, Stern P, Tortorella D, Ploegh HL. A glycosylated type I membrane protein becomes cytosolic when peptide: N-glycanase is compromised. EMBO J. 2004;23(3):650–658. doi: 10.1038/sj.emboj.7600090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wiertz EJ, Jones TR, Sun L, Bogyo M, Geuze HJ, Ploegh HL. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84(5):769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 136.Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384(6608):432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 137.Huppa JB, Ploegh HL. The alpha chain of the T cell antigen receptor is degraded in the cytosol. Immunity. 1997;7(1):113–122. doi: 10.1016/s1074-7613(00)80514-2. [DOI] [PubMed] [Google Scholar]
- 138.Yu H, Kaung G, Kobayashi S, Kopito RR. Cytosolic degradation of T-cell receptor alpha chains by the proteasome. J Biol Chem. 1997;272(33):20800–20804. doi: 10.1074/jbc.272.33.20800. [DOI] [PubMed] [Google Scholar]
- 139.Need AC, Shashi V, Hitomi Y, Schoch K, Shianna KV, McDonald MT, Meisler MH, Goldstein DB. Clinical application of exome sequencing in undiagnosed genetic conditions. J Med Genet. 2012;49(6):353–361. doi: 10.1136/jmedgenet-2012-100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Freeze HH. Understanding human glycosylation disorders: biochemistry leads the charge. J Biol Chem. 2013;288(10):6936–6945. doi: 10.1074/jbc.R112.429274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Enns GM, Shashi V, Bainbridge M, Gambello MJ, Zahir FR, Bast T, Crimian R, Schoch K, Platt J, Cox R, Bernstein JA, Scavina M, Walter RS, Bibb A, Jones M, Hegde M, Graham BH, Need AC, Oviedo A, Schaaf CP, Boyle S, Butte AJ, Chen R, Clark MJ, Haraksingh R, Cowan TM, He P, Langlois S, Zoghbi HY, Snyder M, Gibbs RA, Freeze HH, Goldstein DB. Mutations in NGLY1 cause an inherited disorder of the endoplasmic reticulum-associated degradation pathway. Genet Med. 2014;16(10):751–758. doi: 10.1038/gim.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Might M, Wilsey M. The shifting model in clinical diagnostics: how next-generation sequencing and families are altering the way rare diseases are discovered, studied, and treated. Genet Med. 2014;16(10):736–737. doi: 10.1038/gim.2014.23. [DOI] [PubMed] [Google Scholar]
- 143.Caglayan AO, Comu S, Baranoski JF, Parman Y, Kaymakcalan H, Akgumus GT, Caglar C, Dolen D, Erson-Omay EZ, Harmanci AS, Mishra-Gorur K, Freeze HH, Yasuno K, Bilguvar K, Gunel M. NGLY1 mutation causes neuromotor impairment, intellectual disability, and neuropathy. Eur J Med Genet. 2015;58(1):39–43. doi: 10.1016/j.ejmg.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haeuw JF, Michalski JC, Strecker G, Spik G, Montreuil J. Cytosolic glycosidases: do they exist? Glycobiology. 1991;1(5):487–492. doi: 10.1093/glycob/1.5.487. [DOI] [PubMed] [Google Scholar]
- 145.Moore SE, Spiro RG. Intracellular compartmentalization and degradation of free polymannose oligosaccharides released during glycoprotein biosynthesis. J Biol Chem. 1994;269(17):12715–12721. [PubMed] [Google Scholar]
- 146.Kmiecik D, Herman V, Stroop CJ, Michalski JC, Mir AM, Labiau O, Verbert A, Cacan R. Catabolism of glycan moieties of lipid intermediates leads to a single Man5GlcNAc oligosaccharide isomer: a study with permeabilized CHO cells. Glycobiology. 1995;5(5):483–494. doi: 10.1093/glycob/5.5.483. [DOI] [PubMed] [Google Scholar]
- 147.Ohashi S, Iwai K, Mega T, Hase S. Quantitation and isomeric structure analysis of free oligosaccharides present in the cytosol fraction of mouse liver: detection of a free disialobiantennary oligosaccharide and glucosylated oligomannosides. J Biochem. 1999;126(5):852–858. doi: 10.1093/oxfordjournals.jbchem.a022526. [DOI] [PubMed] [Google Scholar]
- 148.Iwai K, Mega T, Hase S. Detection of Man5GlcNAc and related free oligomannosides in the cytosol fraction of hen oviduct. J Biochem. 1999;125(1):70–74. doi: 10.1093/oxfordjournals.jbchem.a022270. [DOI] [PubMed] [Google Scholar]
- 149.Suzuki T, Yano K, Sugimoto S, Kitajima K, Lennarz WJ, Inoue S, Inoue Y, Emori Y. Endo-β-N-acetylglucosaminidase, an enzyme involved in processing of free oligosaccharides in the cytosol. Proc Natl Acad Sci USA. 2002;99(15):9691–9696. doi: 10.1073/pnas.152333599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kato T, Fujita K, Takeuchi M, Kobayashi K, Natsuka S, Ikura K, Kumagai H, Yamamoto K. Identification of an endo-β-N-acetylglucosaminidase gene in Caenorhabditis elegans and its expression in Escherichia coli . Glycobiology. 2002;12(10):581–587. doi: 10.1093/glycob/cwf073. [DOI] [PubMed] [Google Scholar]
- 151.Kato T, Hatanaka K, Mega T, Hase S. Purification and characterization of endo-β-N-acetylglucosaminidase from hen oviduct. J Biochem. 1997;122(6):1167–1173. doi: 10.1093/oxfordjournals.jbchem.a021877. [DOI] [PubMed] [Google Scholar]
- 152.Shoup VA, Touster O. Purification and characterization of the α-d-mannosidase of rat liver cytosol. J Biol Chem. 1976;251(13):3845–3852. [PubMed] [Google Scholar]
- 153.Mathur R, Balasubramanian AS. Cobalt-ion chelate affinity chromatography for the purification of brain neutral α-d-mannosidase and its separation from acid α-d-mannosidase. Biochem J. 1984;222(1):261–264. doi: 10.1042/bj2220261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bischoff J, Kornfeld R. The soluble form of rat liver alpha-mannosidase is immunologically related to the endoplasmic reticulum membrane α-mannosidase. J Biol Chem. 1986;261(10):4758–4765. [PubMed] [Google Scholar]
- 155.Tulsiani DR, Touster O. Substrate specificities of rat kidney lysosomal and cytosolic α-d-mannosidases and effects of swainsonine suggest a role of the cytosolic enzyme in glycoprotein catabolism. J Biol Chem. 1987;262(14):6506–6514. [PubMed] [Google Scholar]
- 156.Mathur R, Panneerselvam K, Balasubramanian AS. Co2+-mediated time- and temperature-dependent activation of neutral α-d-mannosidase from monkey brain. Biochem J. 1988;253(3):677–685. doi: 10.1042/bj2530677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haeuw JF, Strecker G, Wieruszeski JM, Montreuil J, Michalski JC. Substrate specificity of rat liver cytosolic α-d-mannosidase. Novel degradative pathway for oligomannosidic type glycans. Eur J Biochem. 1991;202(3):1257–1268. doi: 10.1111/j.1432-1033.1991.tb16498.x. [DOI] [PubMed] [Google Scholar]
- 158.Oku H, Hase S, Ikenaka T. Purification and characterization of neutral alpha-mannosidase that is activated by Co2+ from Japanese quail oviduct. J Biochem. 1991;110(1):29–34. doi: 10.1093/oxfordjournals.jbchem.a123538. [DOI] [PubMed] [Google Scholar]
- 159.Al Daher S, De Gasperi R, Daniel P, Hirani S, Warren C, Winchester B. Substrate specificity of human liver neutral α-mannosidase. Biochem J. 1992;286(Pt 1):47–53. doi: 10.1042/bj2860047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.De Gasperi R, Al Daher S, Winchester BG, Warren CD. Substrate specificity of the bovine and feline neutral α-mannosidases. Biochem J. 1992;286(Pt 1):55–63. doi: 10.1042/bj2860055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Grard T, Saint-Pol A, Haeuw JF, Alonso C, Wieruszeski JM, Strecker G, Michalski JC. Soluble forms of α-d-mannosidases from rat liver. Separation and characterization of two enzymic forms with different substrate specificities. Eur J Biochem. 1994;223(1):99–106. doi: 10.1111/j.1432-1033.1994.tb18970.x. [DOI] [PubMed] [Google Scholar]
- 162.Grard T, Herman V, Saint-Pol A, Kmiecik D, Labiau O, Mir AM, Alonso C, Verbert A, Cacan R, Michalski JC. Oligomannosides or oligosaccharide-lipids as potential substrates for rat liver cytosolic α-d-mannosidase. Biochem J. 1996;316(Pt 3):787–792. doi: 10.1042/bj3160787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kumano M, Omichi K, Hase S. Substrate specificity of bovine liver cytosolic neutral α-mannosidase activated by Co2+ . J Biochem. 1996;119(5):991–997. doi: 10.1093/oxfordjournals.jbchem.a021340. [DOI] [PubMed] [Google Scholar]
- 164.Yamashiro K, Itoh H, Yamagishi M, Natsuka S, Mega T, Hase S. Purification and characterization of neutral α-mannosidase from hen oviduct: studies on the activation mechanism of Co2+ . J Biochem. 1997;122(6):1174–1181. doi: 10.1093/oxfordjournals.jbchem.a021878. [DOI] [PubMed] [Google Scholar]
- 165.Yamagishi M, Ishimizu T, Natsuka S, Hase S. Co(II)-regulated substrate specificity of cytosolic alpha-mannosidase. J Biochem. 2002;132(2):253–256. doi: 10.1093/oxfordjournals.jbchem.a003218. [DOI] [PubMed] [Google Scholar]
- 166.Paciotti S, Persichetti E, Klein K, Tasegian A, Duvet S, Hartmann D, Gieselmann V, Beccari T. Accumulation of free oligosaccharides and tissue damage in cytosolic α-mannosidase (Man2c1)-deficient mice. J Biol Chem. 2014;289(14):9611–9622. doi: 10.1074/jbc.M114.550509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Oku H, Hase S. Studies on the substrate specificity of neutral alpha-mannosidase purified from Japanese quail oviduct by using sugar chains from glycoproteins. J Biochem. 1991;110(6):982–989. doi: 10.1093/oxfordjournals.jbchem.a123700. [DOI] [PubMed] [Google Scholar]
- 168.Suzuki T, Hara I, Nakano M, Shigeta M, Nakagawa T, Kondo A, Funakoshi Y, Taniguchi N. Man2C1, an α-mannosidase, is involved in the trimming of free oligosaccharides in the cytosol. Biochem J. 2006;400(1):33–41. doi: 10.1042/BJ20060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang L, Suzuki T. Dual functions for cytosolic alpha-mannosidase (Man2C1): its down-regulation causes mitochondria-dependent apoptosis independently of its α-mannosidase activity. J Biol Chem. 2013;288(17):11887–11896. doi: 10.1074/jbc.M112.425702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tian Y, Ju JY, Zhou YQ, Liu Y, Zhu LP. Inhibition of alpha-mannosidase Man2c1 gene expression suppresses growth of esophageal carcinoma cells through mitotic arrest and apoptosis. Cancer Sci. 2008;99(12):2428–2434. doi: 10.1111/j.1349-7006.2008.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yue W, Jin YL, Shi GX, Liu Y, Gao Y, Zhao FT, Zhu LP. Suppression of 6A8 α-mannosidase gene expression reduced the potentiality of growth and metastasis of human nasopharyngeal carcinoma. Int J Cancer. 2004;108(2):189–195. doi: 10.1002/ijc.11536. [DOI] [PubMed] [Google Scholar]
- 172.Xiang ZG, Jiang DD, Liu Y, Zhang LF, Zhu LP. hMan2c1 transgene promotes tumor progress in mice. Transgenic Res. 2010;19(1):67–75. doi: 10.1007/s11248-009-9299-3. [DOI] [PubMed] [Google Scholar]
- 173.He L, Fan C, Kapoor A, Ingram AJ, Rybak AP, Austin RC, Dickhout J, Cutz JC, Scholey J, Tang D. α-Mannosidase 2C1 attenuates PTEN function in prostate cancer cells. Nat Commun. 2011;2:307. doi: 10.1038/ncomms1309. [DOI] [PubMed] [Google Scholar]
- 174.Kato A, Wang L, Ishii K, Seino J, Asano N, Suzuki T. Calystegine B3 as a specific inhibitor for cytoplasmic α-mannosidase, Man2C1. J Biochem. 2011;149(4):415–422. doi: 10.1093/jb/mvq153. [DOI] [PubMed] [Google Scholar]
- 175.Butters TD, Alonzi DS, Kukushkin NV, Ren Y, Bleriot Y. Novel mannosidase inhibitors probe glycoprotein degradation pathways in cells. Glycoconj J. 2009;26(9):1109–1116. doi: 10.1007/s10719-009-9231-3. [DOI] [PubMed] [Google Scholar]
- 176.Bernon C, Carre Y, Kuokkanen E, Slomianny MC, Mir AM, Krzewinski F, Cacan R, Heikinheimo P, Morelle W, Michalski JC, Foulquier F, Duvet S. Overexpression of Man2C1 leads to protein underglycosylation and upregulation of endoplasmic reticulum-associated degradation pathway. Glycobiology. 2011;21(3):363–375. doi: 10.1093/glycob/cwq169. [DOI] [PubMed] [Google Scholar]
- 177.Weng S, Spiro RG. Endoplasmic reticulum kifunensine-resistant α-mannosidase is enzymatically and immunologically related to the cytosolic α-mannosidase. Arch Biochem Biophys. 1996;325(1):113–123. doi: 10.1006/abbi.1996.0014. [DOI] [PubMed] [Google Scholar]
- 178.Weng S, Spiro RG. Demonstration that a kifunensine-resistant α-mannosidase with a unique processing action on N-linked oligosaccharides occurs in rat liver endoplasmic reticulum and various cultured cells. J Biol Chem. 1993;268(34):25656–25663. [PubMed] [Google Scholar]
- 179.Saint-Pol A, Bauvy C, Codogno P, Moore SE. Transfer of free polymannose-type oligosaccharides from the cytosol to lysosomes in cultured human hepatocellular carcinoma HepG2 cells. J Cell Biol. 1997;136(1):45–59. doi: 10.1083/jcb.136.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Saint-Pol A, Codogno P, Moore SE. Cytosol-to-lysosome transport of free polymannose-type oligosaccharides. Kinetic and specificity studies using rat liver lysosomes. J Biol Chem. 1999;274(19):13547–13555. doi: 10.1074/jbc.274.19.13547. [DOI] [PubMed] [Google Scholar]
- 181.Strecker G, Herlant-Peers MC, Fournet B, Montreul J. Structure of seven oligosaccharides excreted in the urine of a patient with Sandhoff’s disease (GM2 gangliosidosis-variant O) Eur J Biochem. 1977;81(1):165–171. doi: 10.1111/j.1432-1033.1977.tb11937.x. [DOI] [PubMed] [Google Scholar]
- 182.Strecker G, Peers MC, Michalski JC, Hondi-Assah T, Fournet B, Spik G, Montreuil J, Farriaux JP, Maroteaux P, Durand P. Structure of nine sialyl-oligosaccharides accumulated in urine of eleven patients with three different types of sialidosis. Mucolipidosis II and two new types of mucolipidosis. Eur J Biochem. 1977;75(2):391–403. doi: 10.1111/j.1432-1033.1977.tb11540.x. [DOI] [PubMed] [Google Scholar]
- 183.Kuriyama M, Ariga T, Ando S, Suzuki M, Yamada T, Miyatake T. Four positional isomers of sialyloligosaccharides isolated from the urine of a patient with sialidosis. J Biol Chem. 1981;256(23):12316–12321. [PubMed] [Google Scholar]
- 184.Kuriyama M, Ariga T, Ando S, Suzuki M, Yamada T, Miyatake T. Urinary sialyloligosaccharides in adult type sialidosis: occurrence of two positional isomers. Jpn J Exp Med. 1981;51(2):129–132. [PubMed] [Google Scholar]
- 185.van Pelt J, Hard K, Kamerling JP, Vliegenthart JF, Reuser AJ, Galjaard H. Isolation and structural characterization of twenty-one sialyloligosaccharides from galactosialidosis urine. An intact N,N′-diacetylchitobiose unit at the reducing end of a diantennary structure. Biol Chem Hoppe Seyler. 1989;370(3):191–203. doi: 10.1515/bchm3.1989.370.1.191. [DOI] [PubMed] [Google Scholar]
- 186.Michalski JC. Normal and pathological catabolism of glycoproteins. In: Montereuil J, Vliegenthart JFG, Schachter H, editors. Glycoproteins and disease, new comprehensive biochemistry. Amsterdam: Elsevier; 1996. pp. 55–97. [Google Scholar]
- 187.Ishizuka A, Hashimto Y, Naka R, Kinoshita M, Kakehi K, Seino J, Funakoshi Y, Suzuki T, Kameyama A, Narimatsu H. Accumulation of free complex-type N-glycans in MKN7 and MKN45 stomach cancer cells. Biochem J. 2008;413(2):227–237. doi: 10.1042/BJ20071562. [DOI] [PubMed] [Google Scholar]
- 188.Seino J, Wang L, Harada Y, Huang C, Ishii K, Mizushima N, Suzuki T. Basal autophagy is required for the efficient catabolism of sialyloligosaccharides. J Biol Chem. 2013;288(37):26898–26907. doi: 10.1074/jbc.M113.464503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yabu M, Korekane H, Hatano K, Kaneda Y, Nonomura N, Sato C, Kitajima K, Miyamoto Y. Occurrence of free deaminoneuraminic acid (KDN)-containing complex-type N-glycans in human prostate cancers. Glycobiology. 2013;23(6):634–642. doi: 10.1093/glycob/cws132. [DOI] [PubMed] [Google Scholar]
- 190.Yabu M, Korekane H, Takahashi H, Ohigashi H, Ishikawa O, Miyamoto Y. Accumulation of free Neu5Ac-containing complex-type N-glycans in human pancreatic cancers. Glycoconj J. 2013;30(3):247–256. doi: 10.1007/s10719-012-9435-9. [DOI] [PubMed] [Google Scholar]
- 191.Huang C, Seino J, Wang L, Haga Y, Suzuki T. Autophagy regulates the stability of sialin, a lysosomal sialic acid transporter. Biosci Biotechnol Biochem. 2014 doi: 10.1080/09168451.2014.991682. [DOI] [PubMed] [Google Scholar]
- 192.Iwatsuka K, Watanabe S, Kinoshita M, Kamisue K, Yamada KM, Hayakawa T, Suzuki T, Kakehi K. Free glycans derived from glycoproteins present in human sera. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;928:16–21. doi: 10.1016/j.jchromb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 193.Chantret I, Frenoy JP, Moore SE. Free-oligosaccharide control in the yeast Saccharomyces cerevisiae: roles for peptide:N-glycanase (Png1p) and vacuolar mannosidase (Ams1p) Biochem J. 2003;373(Pt 3):901–908. doi: 10.1042/BJ20030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Harada Y, Buser R, Ngwa EM, Hirayama H, Aebi M, Suzuki T. Eukaryotic oligosaccharyltransferase generates free oligosaccharides during N-glycosylation. J Biol Chem. 2013;288(45):32673–32684. doi: 10.1074/jbc.M113.486985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hirayama H, Seino J, Kitajima T, Jigami Y, Suzuki T. Free oligosaccharides to monitor glycoprotein endoplasmic reticulum-associated degradation in Saccharomyces cerevisiae . J Biol Chem. 2010;285(16):12390–12404. doi: 10.1074/jbc.M109.082081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Finger A, Knop M, Wolf DH. Analysis of two mutated vacuolar proteins reveals a degradation pathway in the endoplasmic reticulum or a related compartment of yeast. Eur J Biochem. 1993;218(2):565–574. doi: 10.1111/j.1432-1033.1993.tb18410.x. [DOI] [PubMed] [Google Scholar]
- 197.Suzuki T, Park H, Hollingsworth NM, Sternglanz R, Lennarz WJ. PNG1, a yeast gene encoding a highly conserved peptide:N-glycanase. J Cell Biol. 2000;149(5):1039–1052. doi: 10.1083/jcb.149.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hosomi A, Tanabe K, Hirayama H, Kim I, Rao H, Suzuki T. Identification of an Htm1 (EDEM)-dependent, Mns1-independent endoplasmic reticulum-associated degradation (ERAD) pathway in Saccharomyces cerevisiae: application of a novel assay for glycoprotein ERAD. J Biol Chem. 2010;285(32):24324–24334. doi: 10.1074/jbc.M109.095919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hosomi A, Suzuki T. Cytoplasmic peptide:N-glycanase cleaves N-glycans on a carboxypeptidase Y mutant during ERAD in Saccharomyces cerevisiae . Biochim Biophys Acta. 2015;4:612–619. doi: 10.1016/j.bbagen.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 200.Gauss R, Kanehara K, Carvalho P, Ng DT, Aebi M. A complex of Pdi1p and the mannosidase Htm1p initiates clearance of unfolded glycoproteins from the endoplasmic reticulum. Mol Cell. 2011;42(6):782–793. doi: 10.1016/j.molcel.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 201.Hirayama H, Suzuki T. Metabolism of free oligosaccharides is facilitated in the och1∆ mutant of Saccharomyces cerevisiae . Glycobiology. 2011;21(10):1341–1348. doi: 10.1093/glycob/cwr073. [DOI] [PubMed] [Google Scholar]
- 202.Kukushkin NV, Alonzi DS, Dwek RA, Butters TD. Demonstration that endoplasmic reticulum-associated degradation of glycoproteins can occur downstream of processing by endomannosidase. Biochem J. 2011;438(1):133–142. doi: 10.1042/BJ20110186. [DOI] [PubMed] [Google Scholar]
- 203.Alonzi DS, Kukushkin NV, Allman SA, Hakki Z, Williams SJ, Pierce L, Dwek RA, Butters TD. Glycoprotein misfolding in the endoplasmic reticulum: identification of released oligosaccharides reveals a second ER-associated degradation pathway for Golgi-retrieved proteins. Cell Mol Life Sci. 2013;70(15):2799–2814. doi: 10.1007/s00018-013-1304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Karaivanova VK, Spiro RG. Effect of proteasome inhibitors on the release into the cytosol of free polymannose oligosaccharides from glycoproteins. Glycobiology. 2000;10(7):727–735. doi: 10.1093/glycob/10.7.727. [DOI] [PubMed] [Google Scholar]
- 205.Yoshihisa T, Anraku Y. Nucleotide sequence of AMS1, the structure gene of vacuolar alpha-mannosidase of Saccharomyces cerevisiae . Biochem Biophys Res Commun. 1989;163(2):908–915. doi: 10.1016/0006-291x(89)92308-5. [DOI] [PubMed] [Google Scholar]
- 206.Chantret I, Kodali VP, Lahmouich C, Harvey DJ, Moore SE. Endoplasmic reticulum-associated degradation (ERAD) and free oligosaccharide generation in Saccharomyces cerevisiae . J Biol Chem. 2011;286(48):41786–41800. doi: 10.1074/jbc.M111.251371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yoshihisa T, Anraku Y. A novel pathway of import of alpha-mannosidase, a marker enzyme of vacuolar membrane, in Saccharomyces cerevisiae . J Biol Chem. 1990;265(36):22418–22425. [PubMed] [Google Scholar]
- 208.Hutchins MU, Klionsky DJ. Vacuolar localization of oligomeric α-mannosidase requires the cytoplasm to vacuole targeting and autophagy pathway components in Saccharomyces cerevisiae . J Biol Chem. 2001;276(23):20491–20498. doi: 10.1074/jbc.M101150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Scott SV, Guan J, Hutchins MU, Kim J, Klionsky DJ. Cvt19 is a receptor for the cytoplasm-to-vacuole targeting pathway. Mol Cell. 2001;7(6):1131–1141. doi: 10.1016/s1097-2765(01)00263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shintani T, Huang WP, Stromhaug PE, Klionsky DJ. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev Cell. 2002;3(6):825–837. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shintani T, Klionsky DJ. Cargo proteins facilitate the formation of transport vesicles in the cytoplasm to vacuole targeting pathway. J Biol Chem. 2004;279(29):29889–29894. doi: 10.1074/jbc.M404399200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Watanabe Y, Noda NN, Kumeta H, Suzuki K, Ohsumi Y, Inagaki F. Selective transport of alpha-mannosidase by autophagic pathways: structural basis for cargo recognition by Atg19 and Atg34. J Biol Chem. 2010;285(39):30026–30033. doi: 10.1074/jbc.M110.143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Suzuki K, Kondo C, Morimoto M, Ohsumi Y. Selective transport of α-mannosidase by autophagic pathways: identification of a novel receptor, Atg34p. J Biol Chem. 2010;285(39):30019–30025. doi: 10.1074/jbc.M110.143511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schwarz M, Knauer R, Lehle L. Yeast oligosaccharyltransferase consists of two functionally distinct sub-complexes, specified by either the Ost3p or Ost6p subunit. FEBS Lett. 2005;579(29):6564–6568. doi: 10.1016/j.febslet.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 215.Schulz BL, Aebi M. Analysis of glycosylation site occupancy reveals a role for Ost3p and Ost6p in site-specific N-glycosylation efficiency. Mol Cell Proteomics. 2009;8(2):357–364. doi: 10.1074/mcp.M800219-MCP200. [DOI] [PubMed] [Google Scholar]
- 216.Castro O, Movsichoff F, Parodi AJ. Preferential transfer of the complete glycan is determined by the oligosaccharyltransferase complex and not by the catalytic subunit. Proc Natl Acad Sci USA. 2006;103(40):14756–14760. doi: 10.1073/pnas.0607086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kelleher DJ, Banerjee S, Cura AJ, Samuelson J, Gilmore R. Dolichol-linked oligosaccharide selection by the oligosaccharyltransferase in protist and fungal organisms. J Cell Biol. 2007;177(1):29–37. doi: 10.1083/jcb.200611079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kitajima T, Chiba Y, Jigami Y. Saccharomyces cerevisiae alpha1,6-mannosyltransferase has a catalytic potential to transfer a second mannose molecule. FEBS J. 2006;273(22):5074–5085. doi: 10.1111/j.1742-4658.2006.05505.x. [DOI] [PubMed] [Google Scholar]
- 219.Hsu AF, Baynes JW, Heath EC. The role of a dolichol-oligosaccharide as an intermediate in glycoprotein biosynthesis. Proc Natl Acad Sci USA. 1974;71(6):2391–2395. doi: 10.1073/pnas.71.6.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cacan R, Hoflack B, Verbert A. Fate of oligosaccharide-lipid intermediates synthesized by resting rat-spleen lymphocytes. Eur J Biochem. 1980;106(2):473–479. doi: 10.1111/j.1432-1033.1980.tb04594.x. [DOI] [PubMed] [Google Scholar]
- 221.Belard M, Cacan R, Verbert A. Characterization of an oligosaccharide-pyrophosphodolichol pyrophosphatase activity in yeast. Biochem J. 1988;255(1):235–242. [PMC free article] [PubMed] [Google Scholar]
- 222.Vleugels W, Duvet S, Peanne R, Mir AM, Cacan R, Michalski JC, Matthijs G, Foulquier F. Identification of phosphorylated oligosaccharides in cells of patients with a congenital disorders of glycosylation (CDG-I) Biochimie. 2011;93(5):823–833. doi: 10.1016/j.biochi.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 223.Peric D, Durrant-Arico C, Delenda C, Dupre T, De Lonlay P, de Baulny HO, Pelatan C, Bader-Meunier B, Danos O, Chantret I, Moore SE. The compartmentalisation of phosphorylated free oligosaccharides in cells from a CDG Ig patient reveals a novel ER-to-cytosol translocation process. PLoS ONE. 2010;5(7):e11675. doi: 10.1371/journal.pone.0011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stark NJ, Heath EC. Glucose-dependent glycosylation of secretory glycoprotein in mouse myeloma cells. Arch Biochem Biophys. 1979;192(2):599–609. doi: 10.1016/0003-9861(79)90131-0. [DOI] [PubMed] [Google Scholar]
- 225.Gershman H, Robbins PW. Transitory effects of glucose starvation on the synthesis of dolichol-linked oligosaccharides in mammalian cells. J Biol Chem. 1981;256(15):7774–7780. [PubMed] [Google Scholar]
- 226.Rearick JI, Chapman A, Kornfeld S. Glucose starvation alters lipid-linked oligosaccharide biosynthesis in Chinese hamster ovary cells. J Biol Chem. 1981;256(12):6255–6261. [PubMed] [Google Scholar]
- 227.Turco SJ, Pickard JL. Altered G-protein glycosylation in vesicular stomatitis virus-infected glucose-deprived baby hamster kidney cells. J Biol Chem. 1982;257(15):8674–8679. [PubMed] [Google Scholar]
- 228.Baumann H, Jahreis GP. Glucose starvation leads in rat hepatoma cells to partially N-glycosylated glycoproteins including alpha 1-acid glycoproteins. Identification by endoglycolytic digestions in polyacrylamide gels. J Biol Chem. 1983;258(6):3942–3949. [PubMed] [Google Scholar]
- 229.Doerrler WT, Lehrman MA. Regulation of the dolichol pathway in human fibroblasts by the endoplasmic reticulum unfolded protein response. Proc Natl Acad Sci USA. 1999;96(23):13050–13055. doi: 10.1073/pnas.96.23.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yanagida K, Natsuka S, Hase S. Structural diversity of cytosolic free oligosaccharides in the human hepatoma cell line, HepG2. Glycobiology. 2006;16(4):294–304. doi: 10.1093/glycob/cwj074. [DOI] [PubMed] [Google Scholar]
- 231.Suzuki T, Kitajima K, Emori Y, Inoue Y, Inoue S. Site-specific de-N-glycosylation of diglycosylated ovalbumin in hen oviduct by endogenous peptide: N-glycanase as a quality control system for newly synthesized proteins. Proc Natl Acad Sci USA. 1997;94(12):6244–6249. doi: 10.1073/pnas.94.12.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tarentino AL, Maley F. Purification and properties of an endo-β-N-acetylglucosaminidase from hen oviduct. J Biol Chem. 1976;251(21):6537–6543. [PubMed] [Google Scholar]
- 233.Seko A, Koketsu M, Nishizono M, Enoki Y, Ibrahim HR, Juneja LR, Kim M, Yamamoto T. Occurence of a sialylglycopeptide and free sialylglycans in hen’s egg yolk. Biochim Biophys Acta. 1997;1335(1–2):23–32. doi: 10.1016/s0304-4165(96)00118-3. [DOI] [PubMed] [Google Scholar]
- 234.Inoue S, Iwasaki M, Ishii K, Kitajima K, Inoue Y. Isolation and structures of glycoprotein-derived free sialooligosaccharides from the unfertilized eggs of Tribolodon hakonensis, a dace. Intracellular accumulation of a novel class of biantennary disialooligosaccharides. J Biol Chem. 1989;264(31):18520–18526. [PubMed] [Google Scholar]
- 235.Ishii K, Iwasaki M, Inoue S, Kenny PT, Komura H, Inoue Y. Free sialooligosaccharides found in the unfertilized eggs of a freshwater trout, Plecoglossus altivelis. A large storage pool of complex-type bi-, tri-, and tetraantennary sialooligosaccharides. J Biol Chem. 1989;264(3):1623–1630. [PubMed] [Google Scholar]
- 236.Seko A, Kitajima K, Inoue S, Inoue Y. Identification of free glycan chain liberated by de-N-glycosylation of the cortical alveolar glycopolyprotein (hyosophorin) during early embryogenesis of the Medaka fish, Oryzias latipes . Biochem Biophys Res Commun. 1991;180(3):1165–1171. doi: 10.1016/s0006-291x(05)81318-x. [DOI] [PubMed] [Google Scholar]
- 237.Iwasaki M, Seko A, Kitajima K, Inoue Y, Inoue S. Fish egg glycophosphoproteins have species-specific N-linked glycan units previously found in a storage pool of free glycan chains. J Biol Chem. 1992;267(34):24287–24296. [PubMed] [Google Scholar]
- 238.Plancke Y, Delplace F, Wieruszeski JM, Maes E, Strecker G. Isolation and structures of glycoprotein-derived free oligosaccharides from the unfertilized eggs of Scyliorhinus caniculus. Characterization of the sequences galactose(α 1–4)galactose(β 1-3)-N-acetylglucosamine and N-acetylneuraminic acid(α 2-6)galactose(β 1-3)-N-acetylglucosamine. Eur J Biochem. 1996;235(1–2):199–206. doi: 10.1111/j.1432-1033.1996.00199.x. [DOI] [PubMed] [Google Scholar]
- 239.Moriguchi K, Takemoto T, Aoki T, Nakakita S, Natsuka S, Hase S. Free oligosaccharides with Lewis × structure expressed in the segmentation period of zebrafish embryo. J Biochem. 2007;142(2):213–227. doi: 10.1093/jb/mvm128. [DOI] [PubMed] [Google Scholar]
- 240.Seko A, Kitajima K, Inoue Y, Inoue S. Peptide:N-glycosidase activity found in the early embryos of Oryzias latipes (Medaka fish). The first demonstration of the occurrence of peptide: N-glycosidase in animal cells and its implication for the presence of a de-N-glycosylation system in living organisms. J Biol Chem. 1991;266(33):22110–22114. [PubMed] [Google Scholar]
- 241.Seko A, Kitajima K, Iwamatsu T, Inoue Y, Inoue S. Identification of two discrete peptide: N-glycanases in Oryzias latipes during embryogenesis. Glycobiology. 1999;9(9):887–895. doi: 10.1093/glycob/9.9.887. [DOI] [PubMed] [Google Scholar]
- 242.Priem B, Gross KC. Mannosyl-containing and xylosyl-containing glycans promote tomato (Lycopersicon-Esculentum Mill) fruit ripening. Plant Physiol. 1992;98(1):399–401. doi: 10.1104/pp.98.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yunovitz H, Gross KC. Effect of tunicamycin on metabolism of unconjugated N-glycans in relation to regulation of tomato fruit ripening. Phytochemistry. 1994;37(3):663–668. doi: 10.1016/s0031-9422(00)90334-0. [DOI] [PubMed] [Google Scholar]
- 244.Yunovitz H, Gross KC. Delay of tomato fruit ripening by an oligosaccharide N-glycan: interactions with Iaa, galactose and lectins. Physiol Plant. 1994;90(1):152–156. [Google Scholar]
- 245.Priem B, Morvan H, Gross KC. Unconjugated N-glycans as a new class of plant oligosaccharins. Biochem Soc Trans. 1994;22(2):398–402. doi: 10.1042/bst0220398. [DOI] [PubMed] [Google Scholar]
- 246.Berger S, Menudier A, Julien R, Karamanos Y. Endo-N-acetyl-beta-d-glucosaminidase and peptide-N4-(N-acetyl-glucosaminyl) asparagine amidase activities during germination of Raphanus sativus . Phytochemistry. 1995;39(3):481–487. doi: 10.1016/0031-9422(95)00001-n. [DOI] [PubMed] [Google Scholar]
- 247.Berger S, Menudier A, Julien R, Karamanos Y. Do de-N-glycosylation enzymes have an important role in plant cells? Biochimie. 1995;77(9):751–760. doi: 10.1016/0300-9084(96)88193-4. [DOI] [PubMed] [Google Scholar]
- 248.Morvan H, Lhernould S. Unconjugated N-glycans probing or signalling in plant tissues. Plant Physiol Biochem. 1996;34(3):335–341. [Google Scholar]
- 249.Maeda M, Kimura Y. Structural features of free N-glycans occurring in plants and functional features of de-N-glycosylation enzymes, ENGase, and PNGase: the presence of unusual plant complex type N-glycans. Front Plant Sci. 2014;5:429. doi: 10.3389/fpls.2014.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Priem B, Morvan H, Hafez AMA, Morvan C. Influence of plant glycan of the oligomannoside type on the growth of flax plantlets. Comptes Rendus De L Academie Des Sciences Serie Iii-Sciences De La Vie-Life Sciences. 1990;311(11):411–416. [Google Scholar]
- 251.Priem B, Solokwan J, Wieruszeski JM, Strecker G, Nazih H, Morvan H. Isolation and characterization of free glycans of the oligomannoside type from the extracellular medium of a plant-cell suspension. Glycoconj J. 1990;7(2):121–132. [Google Scholar]
- 252.Lhernould S, Karamanos Y, Bourgerie S, Strecker G, Julien R, Morvan H. Peptide-N(4)-(N-acetylglucosaminyl)asparagine amidase (Pngase) activity could explain the occurrence of extracellular xylomannosides in a plant-cell suspension. Glycoconj J. 1992;9(4):191–197. doi: 10.1007/BF00731164. [DOI] [PubMed] [Google Scholar]
- 253.Priem B, Gitti R, Bush CA, Gross KC. Structure of 10 free N-glycans in ripening tomato fruit: arabinose is a constituent of a plant N-glycan. Plant Physiol. 1993;102(2):445–458. doi: 10.1104/pp.102.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lhernould S, Karamanos Y, Priem B, Morvan H. Carbon starvation increases endoglycosidase activities and production of unconjugated N-glycans in silene alba cell-suspension cultures. Plant Physiol. 1994;106(2):779–784. doi: 10.1104/pp.106.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yunovitz H, Livsey JN, Gross KC. Unconjugated Man(5)GlcNac occurs in vegetative tissues of tomato. Phytochemistry. 1996;42(3):607–610. doi: 10.1016/0031-9422(95)00956-6. [DOI] [PubMed] [Google Scholar]
- 256.Faugeron C, Lhernould S, Lemoine J, Costa G, Morvan H. Identification of unconjugated N-glycans in strawberry plants. Plant Physiol Biochem. 1997;35(11):891–895. [Google Scholar]
- 257.Faugeron C, Lhernould S, Maes E, Lerouge P, Strecker G, Morvan H. Tomato plant leaves also contain unconjugated N-glycans. Plant Physiol Biochem. 1997;35(1):73–79. [Google Scholar]
- 258.Kimura Y, Takagi S, Shiraishi T. Occurrence of free N-glycans in pea (Pisum sativum L.) seedlings. Biosci Biotechnol Biochem. 1997;61(5):924–926. doi: 10.1271/bbb.61.924. [DOI] [PubMed] [Google Scholar]
- 259.Kimura Y, Kitahara E. Structural analysis of free N-glycans occurring in soybean seedlings and dry seeds. Biosci Biotechnol Biochem. 2000;64(9):1847–1855. doi: 10.1271/bbb.64.1847. [DOI] [PubMed] [Google Scholar]
- 260.Kimura Y, Matsuo S. Free N-glycans already occur at an early stage of seed development. J Biochem. 2000;127(6):1013–1019. doi: 10.1093/oxfordjournals.jbchem.a022692. [DOI] [PubMed] [Google Scholar]
- 261.Kimura Y, Matsuo S, Tsurusaki S, Kimura M, Hara-Nishimura I, Nishimura M. Subcellular localization of endo-β-N-acetylglucosaminidase and high-mannose type free N-glycans in plant cell. Biochim Biophys Acta. 2002;1570(1):38–46. doi: 10.1016/s0304-4165(02)00149-6. [DOI] [PubMed] [Google Scholar]
- 262.Nakamura K, Inoue M, Yoshiie T, Hosoi K, Kimura Y. Changes in structural features of free N-glycan and endoglycosidase activity during tomato fruit ripening. Biosci Biotechnol Biochem. 2008;72(11):2936–2945. doi: 10.1271/bbb.80414. [DOI] [PubMed] [Google Scholar]
- 263.Maeda M, Kimura M, Kimura Y. Intracellular and extracellular free N-glycans produced by plant cells: occurrence of unusual plant complex-type free N-glycans in extracellular spaces. J Biochem. 2010;148(6):681–692. doi: 10.1093/jb/mvq102. [DOI] [PubMed] [Google Scholar]
- 264.Kimura Y, Takeoka Y, Inoue M, Maeda M, Fujiyama K. Double-knockout of putative endo-β-N-acetylglucosaminidase (ENGase) genes in Arabidopsis thaliana: loss of ENGase activity induced accumulation of high-mannose type free N-glycans bearing N, N′-acetylchitobiosyl unit. Biosci Biotechnol Biochem. 2011;75(5):1019–1021. doi: 10.1271/bbb.110148. [DOI] [PubMed] [Google Scholar]
- 265.Takahashi N. Demonstration of a new amidase acting on glycopeptides. Biochem Biophys Res Commun. 1977;76(4):1194–1201. doi: 10.1016/0006-291x(77)90982-2. [DOI] [PubMed] [Google Scholar]
- 266.Takahashi N, Nishibe H. Some characteristics of a new glycopeptidase acting on aspartylglycosylamine linkages. J Biochem. 1978;84(6):1467–1473. doi: 10.1093/oxfordjournals.jbchem.a132270. [DOI] [PubMed] [Google Scholar]
- 267.Sugiyama K, Ishihara H, Tejima S, Takahashi N. Demonstration of a new glycopeptidase, from Jack-Bean meal, acting on aspartylglucosylamine linkages. Biochem Biophys Res Commun. 1983;112(1):155–160. doi: 10.1016/0006-291x(83)91810-7. [DOI] [PubMed] [Google Scholar]
- 268.Taga EM, Waheed A, Van Etten RL. Structural and chemical characterization of a homogeneous peptide N-glycosidase from almond. Biochemistry. 1984;23(5):815–822. doi: 10.1021/bi00300a006. [DOI] [PubMed] [Google Scholar]
- 269.Plummer TH, Jr, Phelan AW, Tarentino AL. Detection and quantification of peptide-N4-(N-acetyl-β-glucosaminyl)asparagine amidases. Eur J Biochem. 1987;163(1):167–173. doi: 10.1111/j.1432-1033.1987.tb10751.x. [DOI] [PubMed] [Google Scholar]
- 270.Yet MG, Wold F. Purification and characterization of two glycopeptide hydrolases from jack beans. J Biol Chem. 1988;263(1):118–122. [PubMed] [Google Scholar]
- 271.Lhernould S, Karamanos Y, Lerouge P, Morvan H. Characterization of the peptide-N-4-(N-Acetylglucosaminyl) asparagine amidase (Pngase Se) from silene alba cells. Glycoconj J. 1995;12(1):94–98. doi: 10.1007/BF00731874. [DOI] [PubMed] [Google Scholar]
- 272.Berger S, Menudier A, Julien R, Karamanos Y. Regulation of de-N-glycosylation enzymes in germinating radish seeds. Plant Physiol. 1996;112(1):259–264. doi: 10.1104/pp.112.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Altmann F, Paschinger K, Dalik T, Vorauer K. Characterisation of peptide-N4-(N-acetyl-β-glucosaminyl)asparagine amidase A and its N-glycans. Eur J Biochem. 1998;252(1):118–123. doi: 10.1046/j.1432-1327.1998.2520118.x. [DOI] [PubMed] [Google Scholar]
- 274.Kimura Y, Ohno A. A new peptide-N-4-(acetyl-β-glucosaminyl)asparagine amidase from soybean (Glycine max) seeds: purification and substrate specificity. Biosci Biotechnol Biochem. 1998;62(2):412–418. doi: 10.1271/bbb.62.412. [DOI] [PubMed] [Google Scholar]
- 275.Chang T, Kuo MC, Khoo KH, Inoue S, Inoue Y. Developmentally regulated expression of a peptide:N-glycanase during germination of rice seeds (Oryza sativa) and its purification and characterization. J Biol Chem. 2000;275(1):129–134. doi: 10.1074/jbc.275.1.129. [DOI] [PubMed] [Google Scholar]
- 276.Vuylsteker C, Cuvellier G, Berger S, Faugeron C, Karamanos Y. Evidence of two enzymes performing the de-N-glycosylation of proteins in barley: expression during germination, localization within the grain and set-up during grain formation. J Exp Bot. 2000;51(346):839–845. [PubMed] [Google Scholar]
- 277.Hossain MA, Nakano R, Nakamura K, Kimura Y. Molecular identification and characterization of an acidic peptide:N-glycanase from tomato (Lycopersicum esculentum) fruits. J Biochem. 2010;147(2):157–165. doi: 10.1093/jb/mvp157. [DOI] [PubMed] [Google Scholar]
- 278.Chien S, Weinburg R, Li S, Li Y. Endo-β-N-acetylglucosaminidase from fig latex. Biochem Biophys Res Commun. 1976;76(2):317–323. doi: 10.1016/0006-291x(77)90727-6. [DOI] [PubMed] [Google Scholar]
- 279.Ogata-Arakawa M, Muramatsu T, Kobata A. Partial purification and characterization of an endo-β-N-acetylglucosaminidase from fig extract. J Biochem. 1977;82(2):611–614. [PubMed] [Google Scholar]
- 280.Li SC, Asakawa M, Hirabayashi Y, Li Y. Isolation of two endo-β-N-acetylglucosaminidases from fig latex. Biochim Biophys Acta. 1981;660(2):278–283. doi: 10.1016/0005-2744(81)90171-6. [DOI] [PubMed] [Google Scholar]
- 281.Nishiyama A, Nishimoto T, Yamaguchi H. A novel endo-β-N-acetyl-glucosaminidase from shoots of phyllostachys-heterocycla var pubescens. Agric Biol Chem. 1991;55(4):1155–1158. [Google Scholar]
- 282.Kimura Y, Iwata K, Sumi Y, Takagi S. Purification and substrate specificity of an endo-β-N-acetylglucosaminidase from pea (Pisum sativum) seeds. Biosci Biotechnol Biochem. 1996;60(2):228–232. doi: 10.1271/bbb.60.228. [DOI] [PubMed] [Google Scholar]
- 283.Kimura Y, Matsuo S, Takagi S. Enzymatic properties of a Ginkgo biloba endo-β-N-acetylglucosaminidase and N-glycan structures of storage glycoproteins in the seeds. Biosci Biotechnol Biochem. 1998;62(2):253–261. doi: 10.1271/bbb.62.253. [DOI] [PubMed] [Google Scholar]
- 284.Kimura Y, Tokuda T, Ohno A, Tanaka H, Ishiguro Y. Enzymatic properties of endo-β-N-acetylglucosaminidases from developing tomato fruits and soybean seeds: substrate specificity of plant origin endoglycosidase. Biochim Biophys Acta. 1998;1381(1):27–36. doi: 10.1016/s0304-4165(97)00155-4. [DOI] [PubMed] [Google Scholar]
- 285.Nakamura K, Inoue M, Maeda M, Nakano R, Hosoi K, Fujiyama K, Kimura Y. Molecular cloning and gene expression analysis of tomato endo-β-N-acetylglucosaminidase, an endoglycosidase involved in the production of high-mannose type free N-glycans during tomato fruit ripening. Biosci Biotechnol Biochem. 2009;73(2):461–464. doi: 10.1271/bbb.80889. [DOI] [PubMed] [Google Scholar]
- 286.Fischl RM, Stadlmann J, Grass J, Altmann F, Leonard R. The two endo-β-N-acetylglucosaminidase genes from Arabidopsis thaliana encode cytoplasmic enzymes controlling free N-glycan levels. Plant Mol Biol. 2011;77(3):275–284. doi: 10.1007/s11103-011-9808-7. [DOI] [PubMed] [Google Scholar]
- 287.Kim YC, Jahren N, Stone MD, Udeshi ND, Markowski TW, Witthuhn BA, Shabanowitz J, Hunt DF, Olszewski NE. Identification and origin of N-linked beta-d-N-acetylglucosamine monosaccharide modifications on Arabidopsis proteins. Plant Physiol. 2013;161(1):455–464. doi: 10.1104/pp.112.208900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Diepold A, Li G, Lennarz WJ, Nurnberger T, Brunner F. The Arabidopsis AtPNG1 gene encodes a peptide: N-glycanase. Plant J. 2007;52(1):94–104. doi: 10.1111/j.1365-313X.2007.03215.x. [DOI] [PubMed] [Google Scholar]
- 289.Masahara-Negishi Y, Hosomi A, Della Mea M, Serafini-Fracassini D, Suzuki T. A plant peptide: N-glycanase orthologue facilitates glycoprotein ER-associated degradation in yeast. Biochim Biophys Acta. 2012;1820(10):1457–1462. doi: 10.1016/j.bbagen.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 290.Funakoshi Y, Negishi Y, Gergen JP, Seino J, Ishii K, Lennarz WJ, Matsuo I, Ito Y, Taniguchi N, Suzuki T. Evidence for an essential deglycosylation-independent activity of PNGase in Drosophila melanogaster . PLoS ONE. 2010;5(5):e10545. doi: 10.1371/journal.pone.0010545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kato T, Kitamura K, Maeda M, Kimura Y, Katayama T, Ashida H, Yamamoto K. Free oligosaccharides in the cytosol of Caenorhabditis elegans are generated through endoplasmic reticulum-golgi trafficking. J Biol Chem. 2007;282(30):22080–22088. doi: 10.1074/jbc.M700805200. [DOI] [PubMed] [Google Scholar]
- 292.Katoh T, Ashida H, Yamamoto K. Generation and metabolism of cytosolic free oligosaccharides in Caenorhabditis elegans . Trends Glycosci Glycotechnol. 2009;21(119):163–177. [Google Scholar]
- 293.Kato T, Kawahara A, Ashida H, Yamamoto K. Unique peptide:N-glycanase of Caenorhabditis elegans has activity of protein disulphide reductase as well as of deglycosylation. J Biochem. 2007;142(2):175–181. doi: 10.1093/jb/mvm117. [DOI] [PubMed] [Google Scholar]
- 294.Suzuki T, Tanabe K, Hara I, Taniguchi N, Colavita A. Dual enzymatic properties of the cytoplasmic peptide: N-glycanase in C. elegans . Biochem Biophys Res Commun. 2007;358(3):837–841. doi: 10.1016/j.bbrc.2007.04.199. [DOI] [PubMed] [Google Scholar]
- 295.Habibi-Babadi N, Su A, de Carvalho CE, Colavita A. The N-glycanase png-1 acts to limit axon branching during organ formation in Caenorhabditis elegans . J Neurosci. 2010;30(5):1766–1776. doi: 10.1523/JNEUROSCI.4962-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Dwivedi R, Nothaft H, Reiz B, Whittal RM, Szymanski CM. Generation of free oligosaccharides from bacterial protein N-linked glycosylation systems. Biopolymers. 2013;99(10):772–783. doi: 10.1002/bip.22296. [DOI] [PubMed] [Google Scholar]
- 297.Liu X, McNally DJ, Nothaft H, Szymanski CM, Brisson JR, Li J. Mass spectrometry-based glycomics strategy for exploring N-linked glycosylation in eukaryotes and bacteria. Anal Chem. 2006;78(17):6081–6087. doi: 10.1021/ac060516m. [DOI] [PubMed] [Google Scholar]
- 298.Nothaft H, Scott NE, Vinogradov E, Liu X, Hu R, Beadle B, Fodor C, Miller WG, Li J, Cordwell SJ, Szymanski CM. Diversity in the protein N-glycosylation pathways within the Campylobacter genus. Mol Cell Proteomics. 2012;11(11):1203–1219. doi: 10.1074/mcp.M112.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nothaft H, Liu X, Li J, Szymanski CM. Campylobacter jejuni free oligosaccharides: function and fate. Virulence. 2010;1(6):546–550. doi: 10.4161/viru.1.6.13801. [DOI] [PubMed] [Google Scholar]
- 300.Denzel MS, Storm NJ, Gutschmidt A, Baddi R, Hinze Y, Jarosch E, Sommer T, Hoppe T, Antebi A. Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell. 2014;156(6):1167–1178. doi: 10.1016/j.cell.2014.01.061. [DOI] [PubMed] [Google Scholar]