Abstract
The head is innervated by 12 cranial nerves (I–XII) that regulate its sensory and motor functions. Cranial nerves are composed of sensory, motor, or mixed neuronal populations. Sensory neurons perceive generally somatic sensations such as pressure, pain, and temperature. These neurons are also involved in smell, vision, taste, and hearing. Motor neurons ensure the motility of all muscles and glands. Innervation plays an essential role in the development of the various orofacial structures during embryogenesis. Hypoplastic cranial nerves often lead to abnormal development of their target organs and tissues. For example, Möbius syndrome is a congenital disease characterized by defective innervation (i.e., abducens (VI) and facial (VII) nerves), deafness, tooth anomalies, and cleft palate. Hence, it is obvious that the peripheral nervous system is needed for both development and function of orofacial structures. Nerves have a limited capacity to regenerate. However, neural stem cells, which could be used as sources for neural tissue maintenance and repair, have been found in adult neuronal tissues. Similarly, various adult stem cell populations have been isolated from almost all organs of the human body. Stem cells are tightly regulated by their microenvironment, the stem cell niche. Deregulation of adult stem cell behavior results in the development of pathologies such as tumor formation or early tissue senescence. It is thus essential to understand the factors that regulate the functions and maintenance of stem cells. Yet, the potential importance of innervation in the regulation of stem cells and/or their niches in most organs and tissues is largely unexplored. This review focuses on the potential role of innervation in the development and homeostasis of orofacial structures and discusses its possible association with stem cell populations during tissue repair.
Keywords: Innervation, Taste bud, Cornea, Salivary gland, Tooth, Stem cells, Regenerative medicine
Introduction
The head is innervated by 12 cranial nerves (I–XII) that regulate its sensory and motor functions [1]. Cranial nerves are composed of sensory, motor, or mixed neuronal populations. Sensory neurons perceive generally somatic sensations such as pressure, pain, and temperature. These neurons are also involved in smell, vision, taste, and hearing. Motor neurons ensure the motility of all muscles and glands.
Innervation plays an essential role in the development of the various orofacial structures during embryogenesis. Hypoplastic cranial nerves often lead to abnormal development of their target organs and tissues. For example, Möbius syndrome is a congenital disease characterized by defective innervation (i.e., abducens (VI) and facial (VII) nerves), deafness, tooth anomalies, and cleft palate [2]. Hence, it is obvious that the peripheral nervous system is needed for both development and function of orofacial structures.
Nerves have a limited capacity to regenerate. However, neural stem cells, which could be used as sources for neural tissue maintenance and repair [3, 4], have been found in adult neuronal tissues. Similarly, various adult stem cell populations have been isolated from almost all organs of the human body. Stem cells are tightly regulated by their microenvironment, the stem cell niche [5]. Deregulation of adult stem cell behavior results in the development of pathologies such as tumor formation or early tissue senescence. It is thus essential to understand the factors that regulate the functions and maintenance of stem cells [6]. Yet, the potential importance of innervation in the regulation of stem cells and/or their niches in most organs and tissues is largely unexplored.
This review focuses on the potential role of innervation in the development and homeostasis of orofacial structures and discusses its possible association with stem cell populations during tissue repair.
Signaling molecules implicated in axon guidance
All organs are innervated by specific neuronal cells, which are responsible for their motility, sensitivity, and homeostasis. Axonal growth towards its target tissues is guided by the growth cone, which senses molecular cues that are present in the environment and on the surface of contacting cells. The growth cone leads axonal growth through long distances, a process that necessitates the cooperation of diverse cellular and molecular mechanisms [1] (Table 1) (Fig. 1a).
Table 1.
Key signaling molecules and receptors involved in axon guidance
| Molecules | Receptors | Effects on axonal guidance and growth |
|---|---|---|
|
Neurotrophins (NGF, BNDF, NT-3, NT-4) |
p75NTR, TrkA, TrkB, TrkC (with different affinities) [10] | Promoters of neuronal and axonal survival; chemoattractant on short distances [10] |
| Netrins | DCC receptor family, UNC5 receptor family [8] | Chemoattractant on DCC, chemorepellent on UNC5/UNC5-DCC receptors [8] |
| Semaphorins | Plexins, neuropilins, integrins [1, 67] | Generally repulsive [1, 67] |
| Ephrins | Eph kinases [9] | Contact repulsion [9] |
| Extracellular matrix (ECM) components (e.g., laminin, fibronectin) | Mainly integrins [67] | Contact adhesion or repulsion [67] |
|
Cell adhesion molecules (CAMs) (IgSF-CAMs, cadherins) |
Homophilic or heterophilic interactions within class [67] | Contact adhesion or repulsion [67] |
|
Prototypic myelin inhibitors (Nogo-A, MAG, OMgp) |
NgR1, NgR2, Lingo1, p75NTR, TNFR, PirB, integrins [11, 12] | Inhibitors of axonal growth and regeneration; restriction of axonal plasticity [11, 12] |
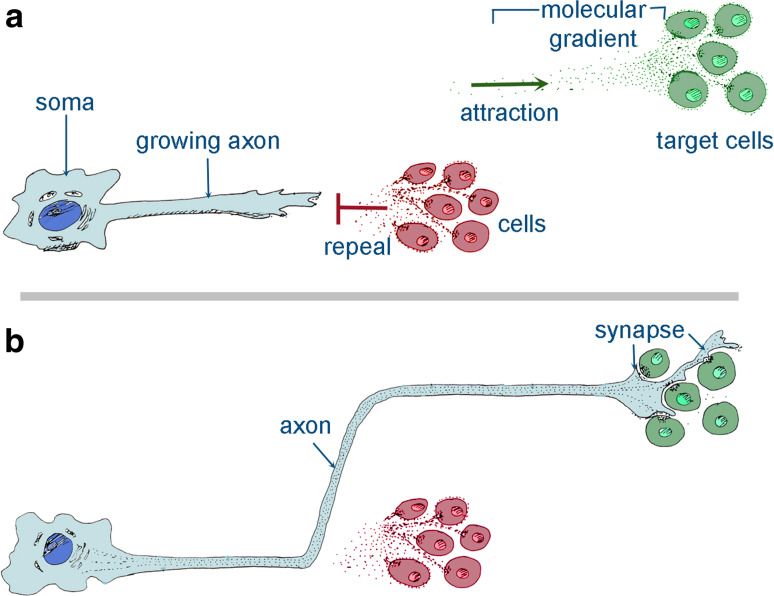
Fig. 1.
Schematic representation showing the mechanisms of axonal growth. Axonal growth is guided by gradients of repealing (in red) and attracting (in green) signals (a). In response to those signals, axons are guided to the target organs where they form synapses (b)
During early embryogenesis, neuronal guidance is ensured by pioneer axons that sprout following highly stereotyped paths towards their target organs. At this stage, distances are much shorter than in late embryogenesis, allowing the pioneer axons to reach their targets easily. Pioneer axons indicate the routes that the trailing axons must follow in order to successfully innervate the target tissue [7]. Secreted signaling molecules that are expressed in various locations of the developing head guide growing axons. Furthermore, axonal behavior is influenced by the composition of the extracellular matrix (ECM) and the cells that the axons encounter during their growth. ECM and neighboring cells can exert stimulatory or inhibitory effects on axonal growth, depending on the specific receptors expressed in the various neuronal populations [7] (Fig. 1a). Adhesive proteins that either allow or block adhesion to the ECM or cells ensure a type of axonal guidance. ECM molecules and cell adhesion molecules (CAMs) are expressed on either neuronal or non-neuronal cells. For example, the ECM protein laminin promotes axonal adhesion and growth. Laminin binds to integrin receptors that are located on the growth cone and the growing axon. Axons have preferences for specific laminin isoforms or other distinctive ECM proteins depending on the class of the integrin receptors that they express. Neurons and glia cells express a variety of CAMs combinations, which allow or inhibit their adhesion. CAMs can be either calcium-dependent (e.g., cadherins) or independent (e.g., Ig superfamily). Homophilic interactions between these molecules are the basis of a strong interaction between pioneer axons and the trailing axons that will later innervate the target tissue. These molecules also mediate the repulsion between pioneer axons and axons not belonging to the same nerve, thus avoiding inappropriate innervation of a given tissue/organ [7].
Tropic guidance molecules can be secreted and/or associated to membranes and are involved in axonal guidance as they provide directional instructions to the growth cones. The most notable among these molecules are the semaphorins, netrins, and ephrins. Semaphorins are membrane-associated proteins that generally inhibit axonal growth [7]. Netrins are laminin-related secreted chemotactic molecules that can either attract or repel axonal growth, depending on the receptor (i.e., DCC or UNC15) that is expressed in the growth cone [8]. Ephrins are membrane-bound proteins that exert inhibitory effects on axonal growth upon binding to their Eph kinases receptors [9]. Secreted tropic molecules (e.g., netrins) can act over a distance, creating gradients that allow nerve fibers to follow a specific path. Alternatively, guidance molecules can be bound to cell surfaces (most of the semaphorins and all ephrins) [7], allowing the growing cones to interact only with cells that are expressing them (Fig. 1b). Membrane-associated molecules can also generate gradients via differential localization and expression on the cell surface of the various cell populations [7].
Trophic molecules promote neuronal survival, growth cone motility, and axonal outgrowth. Neurotrophins (NTs) are the most studied trophic molecules. Four NTs are expressed in mammals: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4). NTs stimulate axonal growth and act as short-range chemoattractants. NTs interact with specific receptors: p75 neurotrophin receptor (p75NTR), TrkA, TrkB, and TrkC. Each NT binds to these receptors with different affinities [10]. Depending on the set of receptors expressed, axons can be more responsive to some NTs than to others.
Myelin-associated inhibitors such as Nogo-A also regulate axonal growth and maturation. Identified in adult myelin, these molecules exert a strong inhibitory effect on axonal regrowth following injury [11]. Nogo-A regulates axonal plasticity and has a key role in inhibiting axon regrowth in the damaged CNS [12]. Nogo-A is strongly expressed in cranial nerves and in different organs of the orofacial complex. The role of Nogo-A outside the CNS is largely unknown, but its localization in orofacial tissues suggests that this molecule is important for the establishment of correct craniofacial innervation.
Axon-to-target signaling
Axons secrete diverse combinations of neurotransmitters in the proximity of their target tissues/organs, thus exerting a wide variety of effects on postsynaptic structures. Conversely, several organs signal back to the neurons via these same neurotransmitters. Once secreted, neurotransmitters bind to specific receptors that activate or inhibit defined intracellular signaling cascades [1] (Table 2).
Table 2.
Main neurotransmitters and receptors involved in the innervation of orofacial organs
| Neurotransmitter | Main receptors | Action site |
|---|---|---|
| Acetylcholine | nAchR, mAchR | CNS; sympathetic, parasympathetic NS; motoneurons at neuromuscular junction |
| Biogenic amines | ||
| Norepinephrine | α, β adrenergic receptors | CNS; sympathetic NS |
| Epinephrine | α, β adrenergic receptors | CNS; sympathetic NS |
| Dopamine | D1-like, D2-like receptors | CNS; PNS |
| Serotonin (5-HT) | 5-HT receptors | CNS; PNS |
| Amino acids | ||
| GABA | GABAA, GABAB | CNS; PNS |
| Glycine | GlyR, NMDAR | CNS; PNS |
| Glutamate | NMDAR, kainate receptors, AMPAR, mGluR | CNS; PNS |
| Neuropeptides | ||
| Substance P | NK1 receptor | CNS; PNS |
| NPY | Y1–Y5 receptors | CNS; PNS |
| CGRP | CALCLR, RAMP1 | CNS; PNS |
Cranial nerves are composed of sympathetic, parasympathetic, motor, and sensory neurons. Sympathetic and parasympathetic nerves, together with the enteric system, compose the autonomic nervous system that regulates organ function at an unconscious level. The autonomic nervous system maintains homeostasis in visceral organs and determines responses to stress conditions. Sympathetic nerves signal to target organs and tissues by secreting mainly epinephrine and norepinephrine. Their targets generally express the G-protein-coupled adrenergic receptors [1]. The parasympathetic system is complementary to the sympathetic system. Parasympathetic nerves signal to their target organs by releasing acetylcholine. Their target organs express the M1–M5 muscarinic receptors that induce specific intracellular responses to acetylcholine [1]. Motor neurons instruct muscles to contract via neuromuscular junctions. Motor neurons secrete acetylcholine, which signals to nicotinic or muscarinic receptors that are expressed in the muscles [1]. Sensory neurons are dedicated to convert external stimuli into electric stimuli that are translated in the central nervous system. Sensory neurons receive signals from all types of sensory receptors, which convert a variety of stimuli such as mechanical, thermic, and luminous into electric signals that are finally conveyed to the central nervous system [1]. Sensory fibers can also secrete various neuropeptides such as substance P, neurokinines, and CGRP onto the innervated organs.
The regulatory function of neurotransmitters on adult organs is intensively studied. However, little information exists concerning the role of neurotransmitters in development and repair of target tissues. Recent results suggest a role for innervation in organogenesis, but the molecular and cellular mechanisms linking innervation with organ formation and repair remain largely unknown. Neurotransmitters may affect development and regeneration of organs through the activation of similar pathways that trigger when they are signaling to mature organs. Innervation could also affect tissue development and repair via exosomes, vesicular bodies that are released by all cells. Exosomes mediate the secretion of proteins and transmitters as well as mRNAs and microRNAs [13]. These released substances can affect neighboring cells, either through well-known signaling pathways or, in the case of mRNA and miRNA secretion, by having a direct effect on gene expression [13]. In the nervous system, exosomes have been proposed to participate in myelin formation, neurite outgrowth, and neuronal survival [14]. In light of these observations, exosomes could be excellent mediators of neuronal-derived signals affecting the development of target organs.
Innervation and taste buds
Taste buds are formed by a group of receptor cells, which transduce and convert chemical stimuli into neural signals to convey the sense of taste. Taste buds reside in three types of taste papillae of the tongue and palate [15, 16] (Fig. 2a): fungiform papillae are concentrated in the anterior part of the tongue, while foliate papillae and circumvallate papillae reside in the posterior region. A fourth type of papillae, the foliate papillae, does not contain taste buds.
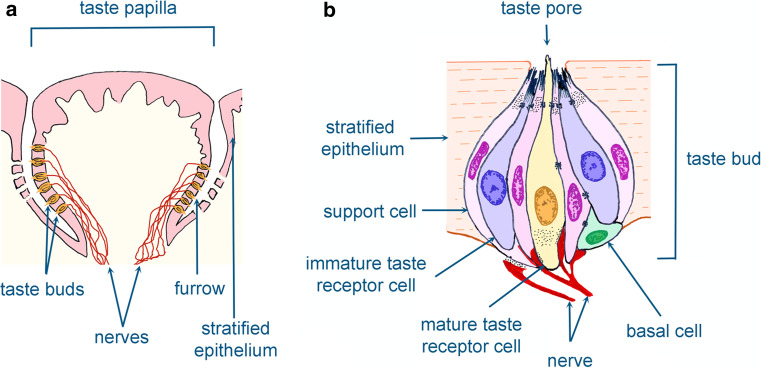
Fig. 2.
Schematic illustration showing the taste buds and taste papillae. Taste buds are located in the taste papillae of the tongue and palate (a). Cellular composition of the taste buds that are innervated by sensory neurons are shown in (b)
Taste buds are composed of a heterogeneous population of four cell types [17] (Fig. 2b). Type I or support cells are involved in synaptic clearance from neurotransmitters and ion level regulation in the extracellular space. Type II (premature) and type III (mature) cells are the effective receptors, binding sweet, bitter, umami, and sour compounds [17]. Type IV consists of basal cells that are poorly characterized. It is believed that these cells represent a reservoir of taste progenitor cells [17].
Developmentally, taste bud cells differ from other sensory cells since they originate from local epithelium rather than from neurogenic ectoderm. Nevertheless, taste bud receptor cells express voltage-gated channels and release neurotransmitters onto postsynaptic neurons [15, 17]. Unlike neurons and other receptor cells, bud cells are continuously regenerating [15, 18]. The placodes of the taste papillae first appear on the antero-dorsal surface of the tongue and after interaction with the underlying mesenchyme give rise to the fungiform papillae. Thereafter, taste buds start to differentiate with slightly different timing in the various areas of the tongue. Taste buds located in the posterior soft palate develop first, during embryogenesis, while the vallate and foliate papillae develop postnatally [18].
Taste buds are innervated by gustatory neurons of the geniculate ganglion via the chorda tympani nerve, while the remaining tongue epithelium is innervated by somatosensory neurons of the trigeminal ganglion via the lingual nerve [19]. The lingual nerve contains sympathetic and parasympathetic nerve fibers. Well-defined numbers of gustatory neurons innervate the taste buds in a region-specific manner [19]. Nerve fibers reach the fungiform papillae placodes shortly after exploring the nearby epithelium where the taste papillae will develop. Later, fibers from the chorda tympani start to branch and innervate the developing fungiform papillae [19]. It is believed that the pattern of the taste papillae is established before innervation. However, scattered sensory axons have been detected in the tongue mesenchyme at earlier stages in its development [20], thus preventing the complete exclusion of a role for innervation in taste bud initiation [20]. In contrast, it has been shown that innervation is essential for both later development and regeneration of taste buds [20].
In rodents, bilateral resection of the glossopharyngeal nerves impairs taste bud growth [21, 22], while re-establishment of innervation allows continuation of their development [21]. Additionally, innervation is required for the maintenance of taste buds: resection of the glossopharyngeal nerves in adult rodents leads to taste bud degeneration within a few weeks [21, 22]. This effect is reversible since the regeneration of nerve fibers permits the formation of functional new taste buds [21, 22]. These results support the notion that innervation is important for both homeostasis and regeneration of taste buds.
Fibers innervating the taste buds require different combinations of trophic factors in order to develop and survive. BDNF, NT-3, and NT-4 are produced by a variety of cell subpopulations in taste buds, but also in the surrounding non-gustatory epithelium (NT-3, NT-4) [19, 23, 24]. The taste bud cells express BDNF that acts as a chemoattractant molecule. NT-4 is mainly expressed by the non-gustatory tongue epithelium and, although known to signal mainly via the same receptor as BDNF, it repels growing nerve fibers from the tongue epithelium [19]. Due to the different responsiveness of these tissues to neurotrophins, their genetic ablation in taste buds may lead to a selective loss of specific neurons. Indeed, BDNF deletion results in a significant decrease of the gustatory innervation, while trigeminal somatosensory innervation is less affected. Defective gustatory innervation leads to an important reduction of taste buds in foliate and circumvallate papillae, with a subsequent impairment of taste [23]. Conversely, specifically driven overexpression of BDNF in taste buds of adult animals increases gustatory innervation, which results in wider taste buds with supernumerary cells when compared to those of wild-type animals [25]. A less severe loss of gustatory innervation and taste buds has been observed in NT-4 knockout mice [24]. BDNF and NT-4 signal through the same cell surface receptors, TrkB and p75NTR. The simultaneous ablation of BDNF and NT-4 results in the most severe loss of neurons and taste buds, a phenotype comparable to that observed in TrkB knockout mice. These mice lose more than 90 % of the gustatory innervation and most of their taste buds [26]. NT-3 signals via TrkB and TrkC and is required for the somatosensory innervation of the taste buds [27]. Although NT-3 deletion leads to a massive reduction of somatosensory neurons, taste bud development and taste perception were not affected [27]. Nevertheless, the loss of taste buds in BDNF/NT-3 knockout mice was larger when compared to that of BDNF mutant mice [27]. In vitro studies have also shown that taste buds cannot develop without innervation [20]. Although the different types of papillae can develop in the tongue of the rat when cultured in vitro in the absence of innervation, the development of functional taste buds is compromised [20, 28]. Taken together, these in vivo and in vitro data suggest that innervation is essential for the development of taste buds.
The relationship between stemness-related genes and innervation in taste buds has been explored recently. A link between Sox2 expression and innervation has been reported for taste buds [29]. Sox2 is expressed in taste buds and the surrounding epithelial cells [18]. Molecules of the BMP and WNT signaling pathways regulate Sox2 expression in these tissues [30]. Taste bud epithelial cells differentiate into keratinocytes instead of sensory cells after Sox2 deletion [18]. Interestingly, this phenotype closely resembles that obtained after surgical denervation [31]. Up-regulation of BMP-7 induces Sox2 expression, which leads to the formation of ectopic taste buds and papillae in the tongue. Under these conditions, Sox2 expression is induced ectopically through a process that is independent of innervation [30]. However, Sox2 expression is lost after denervation of taste buds [29]. All these observations indicate crosstalk between innervation and taste bud progenitor cells through a BMP/Sox2/WNT regulatory loop.
Innervation and salivary glands
Salivary glands are responsible for the synthesis and secretion of serous, mucous, or both types of proteins that facilitate food chewing, swallowing, and further digestion. Two types of salivary glands exist in mammals: the major glands, which produce 90 % of the saliva, and the minor glands [32, 33]. All major salivary glands originate from the embryonic ectoderm and include the parotid, sublingual, and submandibular glands. However, these three glands differ in their structure: parotid glands have elongated ducts, while sublingual and submandibular glands are compacted. Similar to other organs of ectodermal origin, salivary glands develop through epithelial–mesenchymal interactions [6]. Their development starts as epithelial thickenings that invaginate into the mesenchyme and give rise to epithelial buds that progressively branch [33]. At this stage (the pseudoglandular stage), the lumen is formed in the primary duct. During the next stage (called canalicular stage) the lumen appears in most of the ducts. However, the lumen reaches the developing epithelial buds later, during the terminal bud stage. At more advanced developmental stages, terminal tubular cells and proacinar cells, which undergo further differentiation, contribute to the salivary gland epithelium. The mature salivary gland is composed of ductal, acinar, and myoepithelial cells. Based on the duct type in which they reside, the ductal cells can be distinguished into three different lineages: intercalated, striated, and granular cells. Similarly, depending on the composition of their secretion, acinar cells are divided into serous or mucous cells [33] (Fig. 3a).
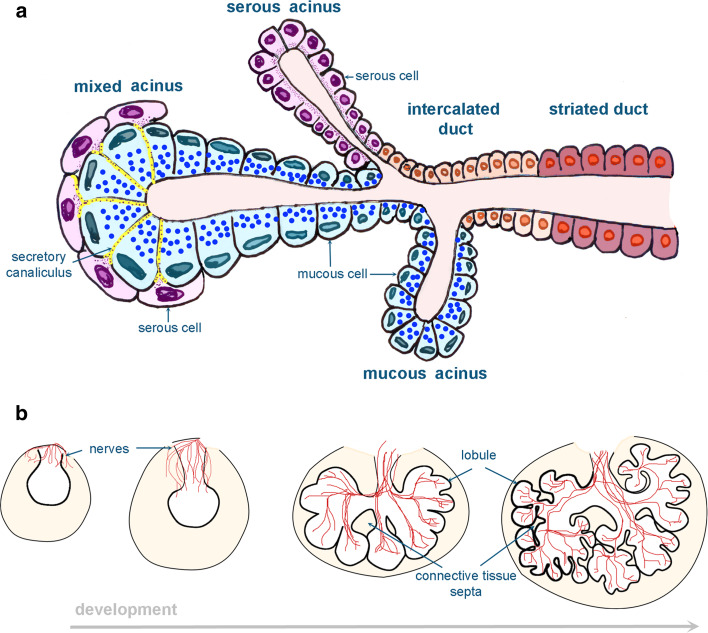
Fig. 3.
Schematic representation of the salivary glands. Structure and cellular composition of mature salivary glands (a) and parasympathetic innervation during salivary gland development (b)
Salivary secretion is a reflex mediated by the autonomic innervation. Innervation regulates not only the secretory function of the gland but also its morphogenesis and differentiation. Sympathetic denervation in rodents causes hypotrophic alterations of the acinar cells and decreases their granule content [34]. In mice, parasympathetic axons surrounding the salivary epithelial bud are first detected at E12 [35]. These parasympathetic axons follow the branching pattern of the developing salivary gland epithelium (Fig. 3b). This concomitant axonal outgrowth from the submandibular ganglion regulates epithelial salivary gland branching, and thus gland morphogenesis. Indeed, isolated E11 mouse submandibular epithelia cultured in vitro in absence of parasympathetic ganglia develop glands with a reduced number of branches [36]. The development of the appropriate number of terminal epithelial buds is dependent on acetylcholine and its muscarinic receptor M1 that is located on the surface of epithelial cells. This has been evidenced after addition of inhibitors of acetylcholine/M1 signaling in the medium of an in vitro culture system of salivary glands [36]. The reduced number of progenitor epithelial gland cells, assessed by quantification of cells expressing progenitor/stem cell markers, is mainly responsible for the diminished number of branches [36]. In the absence of parasympathetic ganglia, the expression of salivary gland progenitor/stem cell markers such as keratin5, keratin15, and aquaporin3 decreases significantly. Thus, the parasympathetic innervation affects salivary epithelial morphogenesis by regulating the pool of gland progenitor/stem cells. A potential therapeutic role of parasympathetic innervation in salivary gland regeneration could be suggested, since increased expression of the stemness markers keratin5 and keratin15 was observed in denervated adult salivary glands treated with carbachol, which is an equivalent of acetylcholine [36]. Salivary glands produce high quantities of NGF and express all neurotrophin receptors [37–39]. Genetic ablation of either NGF [40, 41] or TrkA receptor [42] leads to defective sympathetic innervation of the salivary gland parenchyma. Taken together, these data suggest that the molecular crosstalk between salivary glands and neurons is important for the correct development of both structures.
Innervation and cornea
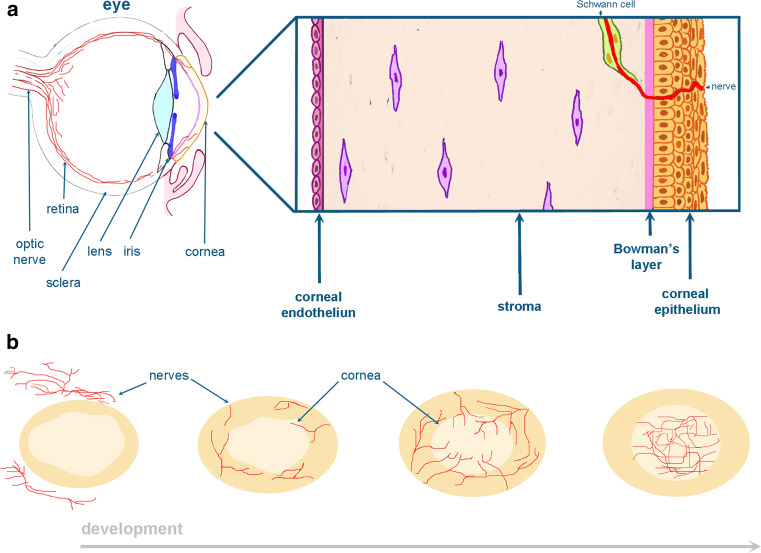
Cornea is an avascular and transparent tissue located in the most anterior part of the eye (Fig. 4a). Cornea development starts shortly after the detachment of the lens vesicle from the surface ectoderm. At this stage, ectomesenchymal cells migrate into the space between the anterior epithelium of the lens vesicle and the surface ectoderm [43]. This mesenchyme progressively condenses to form several layers that are separated by a loose extracellular matrix. The posteriorly located mesenchymal cells flatten and form the corneal endothelium. The surface ectoderm forms the corneal epithelium that is separated from the mesenchyme by a thin layer of collagen fibers known as the Bowman’s layer [43]. Mesenchymal cells between the corneal endothelium and epithelium differentiate into the highly specialized corneal stromal fibroblasts. The corneal epithelium thickens and forms a multilayer after the opening of the eye-lids (Fig. 4a). Corneal opacity or scarring is a major cause for blindness worldwide [44] and it occurs as a consequence of a disrupted wound-healing process. Molecules that are expressed in the corneal epithelium such as Notch1 play a key role during corneal wound healing [45]. However, it is also possible that neighboring tissues may be involved in the regulation of the corneal epithelium healing capacity and thus in the maintenance of cornea physiology. For instance, innervation of the cornea is indispensable for the maintenance of its transparency, which allows for the penetration of the external light onto the retina [46]. The cornea is densely innervated (300–400 times greater than skin) by sensory fibers from the ophthalmic nerve of the trigeminal ganglion and to a lesser degree by sympathetic neurons [47]. Nerve bundles are initially projected into the corneal periphery and radially innervate the anterior third of the mesenchymal stroma and the corneal epithelium only later [48]. Subsequently, stromal nerves ramify into radial patterns at the points of entry that directly innervate the developing epithelial layer. Finally, the nerve bundles branch and project towards the center of the cornea and the epithelium (Fig. 4b).
Fig. 4.
Schematic representation of the cornea. The stratified multilayer corneal epithelium is separated from the stroma by the Bowman’s layer (a). During corneal development, nerve bundles are projected from the corneal periphery towards the presumptive cornea, thereafter bifurcate and innervate the corneal stroma and finally their branches penetrate into the corneal epithelium (b)
Trigeminal specific denervation of the cornea can be caused after chemical and/or physical injury of the ocular surface (e.g., surgeries for cataract or trigeminal neuralgia treatment), systemic or central nervous system disorders and viral infections (e.g., Herpes simplex or Zoster) [46, 47]. Corneal denervation triggers a defective differentiation of epithelial corneal cells as well as an unsatisfactory healing process after corneal injury. This pathology of the corneal epithelium, known as neurotrophic keratopathy, severely reduces vision. Several treatments have been proposed for the reparation of these corneal epithelial defects, including the application of molecules such as substance P, insulin-like growth factor and NGF, which can facilitate the closure of the wound [47]. It has been shown that the levels of NGF are increased in the cornea after injury. This NGF increase is impaired in denervated cornea, which shows a delayed healing [49]. NGF treatment of denervated cornea improved corneal healing and increased the expression levels of the corneal stem cell marker p63 [50]. It has been proposed that the number of corneal stem cells decrease after corneal denervation [50]. Taken together, these data suggest that innervation may be essential to activate or maintain corneal epithelial stem cells after injury by up-regulating NGF signaling. On the other hand, TrkA deletion severely affects corneal innervation [51]. Thus, a crosstalk between corneal epithelium and neurons might be necessary for cornea development and homeostasis.
Innervation and teeth
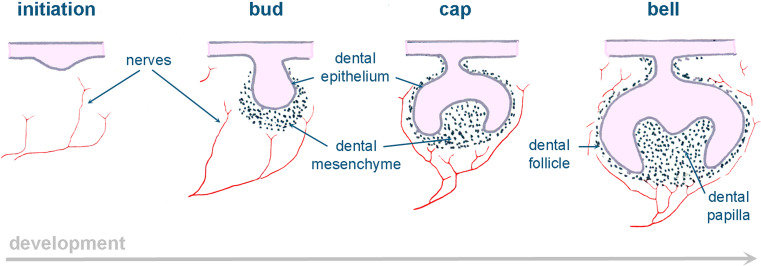
Teeth are organs that develop as a result of sequential and reciprocal interactions between the oral ectoderm and cranial neural crest-derived mesenchyme. Epithelial-derived ameloblasts synthesize the organic components of the enamel and mesenchyme-derived odontoblasts secrete the matrix of dentin [52]. Tooth development starts with a thickening in the oral epithelium that invaginates into the underlying mesenchyme and progressively forms a bud, around which the mesenchyme condenses. During the cap and bell stages, the epithelium folds around the mesenchyme that forms the dental follicle and dental pulp (Fig. 5). Pulp cells adjacent to the dental epithelium differentiate into odontoblasts and secrete dentin, while epithelial cells adjacent to the pulp differentiate into ameloblasts and secrete enamel [52].
Fig. 5.
Schematic illustration showing innervation during embryonic tooth development
Both unmyelinated and myelinated sensory nerves, as well as unmyelinated sympathetic neurons, innervate the mature tooth. Sensory nerves originate from the trigeminal ganglion, while sympathetic adrenergic nerves project from the superior cervical ganglion [53]. Myelinated and unmyelinated axons are mixed and enter the tooth pulp from the apex. Although most of these fibers innervate the pulp, some of them project into the dentin through the dentinal tubules. Sensory fibers convey sensory information to the central nervous system, while sympathetic nerves regulate blood flow into the pulp and are involved in the process of dentinogenesis [54]. Initially, fibers from the trigeminal nerve are located below the dental placode or tooth primordium [55] (Fig. 5). At the bud and cap stages, nerve fibers are absent in dental epithelium and papilla, in contrast to the developing dental follicle that is highly innervated [55]. The first axons penetrate the dental pulp when dentin deposition starts. A massive neuronal ingrowth will contribute to the rich pulp innervation that is completed soon after tooth eruption [55].
Different neurotrophins and axon guidance molecules regulate tooth innervation. Neurotrophic factors such as NGF, NT-3, NT-4, and BDNF and their receptors are already expressed in both dental epithelium and mesenchyme before the bud stage [56, 57]. These molecules generally attract the growth cones. Nerve ingrowth towards the dental epithelium and the dental papilla is actively inhibited and regulated by semaphorins, and by semaphorin3a in particular [58]. Semaphorin3a is expressed in the dental follicle and directs the growth of trigeminal axons along precise paths [58]. Tooth innervation is therefore actively inhibited throughout the early stages of development.
The role of innervation in tooth initiation and development is controversial. Nerve fibers are absent in the proximity of the developing tooth epithelium from the earliest stages of its development [53, 59]. In this context, the mouse toothless region (called diastema) has been used to study the role of innervation in tooth formation. The diastemal area contains three rudimentary tooth structures that develop until the bud stage. This region is not innervated at the initiation and bud stages [60], thus excluding innervation from the process of tooth initiation. Nevertheless, it cannot be excluded that individual nerve fibers may contact the presumptive tooth epithelium before tooth initiation. In fact, several studies have shown that nerve fibers exist in close proximity to the oral epithelium before dental placode formation [61, 62]. However, organotypic in vitro and ex vivo cultures have suggested that tooth initiation and development can proceed without any neuronal contribution [63]. Indeed, entire teeth have been developed in ex vivo cultures regardless of the presence or absence of trigeminal ganglia [63].
In contrast, innervation is clearly necessary for tooth development in fishes [64]. Denervation of the lower jaw in teleost fishes leads to the complete arrest of tooth formation and substitution [64]. Tooth germs were not detectable in denervated jaws, indicating that innervation is essential for tooth initiation [64]. Although significant, all information regarding the role of innervation in tooth development is based on descriptions of nerve fiber localization and ex vivo and in vitro experiments. This information cannot be considered fully representative of the actual developmental processes in vivo, and need confirmation through in vivo manipulation of tooth development. Fishes and other non-mammalian vertebrates regrow teeth throughout their life. In these models, it is possible to observe the effects of denervation on the initiation and development of the successive generations of teeth. In contrast, most mammals have only one or two generations of teeth. The initiation and development of teeth in these animals occur during embryogenesis, making surgical denervation before or during tooth initiation and development impossible. The ideal approach would consist of the ablation of nerve fibers innervating the tooth using genetic tools such as knocking out different neurotrophins.
Conclusions: perspectives
Regeneration of human tissues and organs that would restore their physiological function is the ultimate goal of regenerative medicine. This could be achieved by transplantation of stem cells to the injured sites. Stem/progenitor cells are the driving force of morphogenesis and organ homeostasis due to their ability to self-renew and give rise to a variety of cell types. These cells are strongly influenced by the surrounding environment, which is commonly referred to as niche. A key challenge in regenerative medicine consists of recreating the ideal cellular and molecular environment where stem/progenitor cells will be able to fulfill their function. Suitable scaffolds in combination with specific signaling molecules could mimic the appropriate niche. For this purpose, it is fundamental to understand the mechanisms that influence the physiology of the various stem cells niches as well as organ development, renewal, and repair. Several studies have demonstrated the active role of innervation in the development and maintenance of orofacial organs/tissues. Denervation of these structures affects their development, maintenance, and regenerative potential. In many tissues of the body, innervation regulates the proliferation and differentiation of stem/progenitor cells, providing molecular signals that are necessary for their functions [65, 66]. These mechanisms have clinical relevance. Injured adult organs, including salivary glands, do not regenerate after damage by therapeutic irradiation, despite the presence of stem and progenitor cells. In these patients, parasympathetic innervation of salivary glands is strongly compromised; its restoration through the addition of specific neurotrophic factors resulted in salivary gland regeneration in vitro. [67]. Proper innervation is also needed to obtain fully functional organs. Bioengineered salivary glands, for example, require specific innervation from parasympathetic, sympathetic, and afferent fibers in order to respond correctly to stimuli and produce saliva [68]. However, the vast majority of research on regenerative medicine has neglected the important role of innervation in the regulation of the stem cell fate and behavior after transplantation. In this context, better knowledge of the molecules involved in the crosstalk between nerves and tissue-specific stem cells might provide crucial information that would significantly improve stem cell-based regenerative therapies.
Acknowledgments
The authors wish to thank the European Science Foundation (ESF) COST Action 1005 NAMABIO for the financial support of the short-term mission No. 020913-033584 (P.P.). This work was supported by the Swiss National Foundation (SNSF) grant 31003A-135633 (T.A.M.), and funds from the University of Zurich (L.J.-R., P.P., T.M.).
References
- 1.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. 4. New York: McGraw-Hill; 2000. [Google Scholar]
- 2.Rizos M, Negron RJ, Serman N. Möbius syndrome with dental involvement: a case report and literature review. The Cleft Palate Craniofacial J. 1998;35(3):262–268. doi: 10.1597/1545-1569(1998)035<0262:MBSWDI>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1):25–34. doi: 10.1016/S0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 4.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 5.Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. Stem cell niches in mammals. Exp Cell Res. 2007;313(16):3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Rojo L, Granchi Z, Graf D, Mitsiadis TA. Stem cell fate determination during development and regeneration of ectodermal organs. Front Physiol. 2012;3:107. doi: 10.3389/fphys.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raper J, Mason C. Cellular strategies of axonal pathfinding. Cold Spring Harb Perspect Biol. 2010;2(9):a001933. doi: 10.1101/cshperspect.a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138(11):2153–2169. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- 9.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17(5):230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7(8):617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 2010;11(12):799–811. doi: 10.1038/nrn2936. [DOI] [PubMed] [Google Scholar]
- 13.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 14.Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Kramer-Albers EM. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7):e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thirumangalathu S, Harlow DE, Driskell AL, Krimm RF, Barlow LA. Fate mapping of mammalian embryonic taste bud progenitors. Development. 2009;136(9):1519–1528. doi: 10.1242/dev.029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310(5753):1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190(3):285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okubo T, Pevny LH, Hogan BL. Sox2 is required for development of taste bud sensory cells. Genes Dev. 2006;20(19):2654–2659. doi: 10.1101/gad.1457106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez GF, Krimm RF. Epithelial overexpression of BDNF and NT4 produces distinct gustatory axon morphologies that disrupt initial targeting. Dev Biol. 2006;292(2):457–468. doi: 10.1016/j.ydbio.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oakley B, Witt M. Building sensory receptors on the tongue. J Neurocytol. 2004;33(6):631–646. doi: 10.1007/s11068-005-3332-0. [DOI] [PubMed] [Google Scholar]
- 21.Hosley MA, Hughes SE, Morton LL, Oakley B. A sensitive period for the neural induction of taste buds. J Neurosci. 1987;7(7):2075–2080. doi: 10.1523/JNEUROSCI.07-07-02075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yee C, Bartel DL, Finger TE. Effects of glossopharyngeal nerve section on the expression of neurotrophins and their receptors in lingual taste buds of adult mice. J Comp Neurol. 2005;490(4):371–390. doi: 10.1002/cne.20670. [DOI] [PubMed] [Google Scholar]
- 23.Nosrat CA, Blomlof J, ElShamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997;124(7):1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- 24.Liebl DJ, Mbiene JP, Parada LF. NT4/5 mutant mice have deficiency in gustatory papillae and taste bud formation. Dev Biol. 1999;213(2):378–389. doi: 10.1006/dbio.1999.9385. [DOI] [PubMed] [Google Scholar]
- 25.Krimm RF, Miller KK, Kitzman PH, Davis BM, Albers KM. Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Dev Biol. 2001;232(2):508–521. doi: 10.1006/dbio.2001.0190. [DOI] [PubMed] [Google Scholar]
- 26.Patel AV, Huang T, Krimm RF. Lingual and palatal gustatory afferents each depend on both BDNF and NT-4, but the dependence is greater for lingual than palatal afferents. J Comp Neurol. 2010;518(16):3290–3301. doi: 10.1002/cne.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nosrat IV, Agerman K, Marinescu A, Ernfors P, Nosrat CA. Lingual deficits in neurotrophin double knockout mice. J Neurocytol. 2004;33(6):607–615. doi: 10.1007/s11068-005-3330-2. [DOI] [PubMed] [Google Scholar]
- 28.Mbiene JP, Maccallum DK, Mistretta CM. Organ cultures of embryonic rat tongue support tongue and gustatory papilla morphogenesis in vitro without intact sensory ganglia. J Comp Neurol. 1997;377(3):324–340. doi: 10.1002/(SICI)1096-9861(19970120)377:3<324::AID-CNE2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki Y. Expression of Sox2 in mouse taste buds and its relation to innervation. Cell Tissue Res. 2008;332(3):393–401. doi: 10.1007/s00441-008-0600-1. [DOI] [PubMed] [Google Scholar]
- 30.Beites CL, Hollenbeck PL, Kim J, Lovell-Badge R, Lander AD, Calof AL. Follistatin modulates a BMP autoregulatory loop to control the size and patterning of sensory domains in the developing tongue. Development. 2009;136(13):2187–2197. doi: 10.1242/dev.030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosley MA, Hughes SE, Oakley B. Neural induction of taste buds. J Comp Neurol. 1987;260(2):224–232. doi: 10.1002/cne.902600206. [DOI] [PubMed] [Google Scholar]
- 32.Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133(1):3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Tucker AS. Salivary gland development. Semin Cell Dev Biol. 2007;18(2):237–244. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Henriksson R, Carlsoo B, Danielsson A, Sundstrom S, Jonsson G. Developmental influences of the sympathetic nervous system on rat parotid gland. J Neurol Sci. 1985;71(2–3):183–191. doi: 10.1016/0022-510X(85)90058-9. [DOI] [PubMed] [Google Scholar]
- 35.Coughlin MD. Early development of parasympathetic nerves in the mouse submandibular gland. Dev Biol. 1975;43(1):123–139. doi: 10.1016/0012-1606(75)90136-0. [DOI] [PubMed] [Google Scholar]
- 36.Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 2010;329(5999):1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy RA, Saide JD, Blanchard MH, Young M. Nerve growth factor in mouse serum and saliva: role of the submandibular gland. Proc Natl Acad Sci USA. 1977;74(6):2330–2333. doi: 10.1073/pnas.74.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Vicente JC, Garcia-Suarez O, Esteban I, Santamaria J, Vega JA. Immunohistochemical localization of neurotrophins and neurotrophin receptors in human and mouse salivary glands. Ann Anat. 1998;180(2):157–163. doi: 10.1016/S0940-9602(98)80016-2. [DOI] [PubMed] [Google Scholar]
- 39.Naesse EP, Schreurs O, Messelt E, Hayashi K, Schenck K. Distribution of nerve growth factor, pro-nerve growth factor, and their receptors in human salivary glands. Eur J Oral Sci. 2013;121(1):13–20. doi: 10.1111/eos.12008. [DOI] [PubMed] [Google Scholar]
- 40.Ghasemlou N, Krol KM, Macdonald DR, Kawaja MD. Comparison of target innervation by sympathetic axons in adult wild-type and heterozygous mice for nerve growth factor or its receptor trkA. J Pineal Res. 2004;37(4):230–240. doi: 10.1111/j.1600-079X.2004.00160.x. [DOI] [PubMed] [Google Scholar]
- 41.Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci. 2004;24(3):743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fagan AM, Zhang H, Landis S, Smeyne RJ, Silos-Santiago I, Barbacid M. TrkA, but not TrkC, receptors are essential for survival of sympathetic neurons in vivo. J Neurosci. 1996;16(19):6208–6218. doi: 10.1523/JNEUROSCI.16-19-06208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. BioEssays. 2004;26(4):374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- 45.Vauclair S, Majo F, Durham AD, Ghyselinck NB, Barrandon Y, Radtke F. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev Cell. 2007;13(2):242–253. doi: 10.1016/j.devcel.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Nishida T, Yanai R. Advances in treatment for neurotrophic keratopathy. Curr Opin Ophthalmol. 2009;20(4):276–281. doi: 10.1097/ICU.0b013e32832b758f. [DOI] [PubMed] [Google Scholar]
- 47.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–542. doi: 10.1016/S0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 48.McKenna CC, Lwigale PY. Innervation of the mouse cornea during development. Invest Ophthalmol Vis Sci. 2011;52(1):30–35. doi: 10.1167/iovs.10-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambiase A, Aloe L, Mantelli F, Sacchetti M, Perrella E, Bianchi P, Rocco ML, Bonini S. Capsaicin-induced corneal sensory denervation and healing impairment are reversed by NGF treatment. Invest Ophthalmol Vis Sci. 2012;53(13):8280–8287. doi: 10.1167/iovs.12-10593. [DOI] [PubMed] [Google Scholar]
- 50.Ueno H, Ferrari G, Hattori T, Saban DR, Katikireddy KR, Chauhan SK, Dana R. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest Ophthalmol Vis Sci. 2012;53(2):867–872. doi: 10.1167/iovs.11-8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Castro F, Silos-Santiago I, Lopez de Armentia M, Barbacid M, Belmonte C. Corneal innervation and sensitivity to noxious stimuli in trkA knockout mice. Eur J Neurosci. 1998;10(1):146–152. doi: 10.1046/j.1460-9568.1998.00037.x. [DOI] [PubMed] [Google Scholar]
- 52.Mitsiadis TA, Graf D. Cell fate determination during tooth development and regeneration. Birth Defects Res Part C Embryo today. 2009;87(3):199–211. doi: 10.1002/bdrc.20160. [DOI] [PubMed] [Google Scholar]
- 53.Luukko K. Immunohistochemical localization of nerve fibres during development of embryonic rat molar using peripherin and protein gene product 9.5 antibodies. Arch Oral Biol. 1997;42(3):189–195. doi: 10.1016/S0003-9969(97)00004-6. [DOI] [PubMed] [Google Scholar]
- 54.Johnsen DC. Innervation of teeth: qualitative, quantitative, and developmental assessment. J Dental Res. 1985;64:555–563. doi: 10.1177/002203458506400410. [DOI] [PubMed] [Google Scholar]
- 55.Mohamed SS, Atkinson ME. A histological study of the innervation of developing mouse teeth. J Anat. 1983;136(Pt 4):735–749. [PMC free article] [PubMed] [Google Scholar]
- 56.Mitsiadis TA, Luukko K. Neurotrophins in odontogenesis. Int J Dev Biol. 1995;39(1):195–202. [PubMed] [Google Scholar]
- 57.Luukko K, Arumae U, Karavanov A, Moshnyakov M, Sainio K, Sariola H, Saarma M, Thesleff I. Neurotrophin mRNA expression in the developing tooth suggests multiple roles in innervation and organogenesis. Dev Dyn. 1997;210(2):117–129. doi: 10.1002/(SICI)1097-0177(199710)210:2<117::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 58.Kettunen P, Loes S, Furmanek T, Fjeld K, Kvinnsland IH, Behar O, Yagi T, Fujisawa H, Vainio S, Taniguchi M, Luukko K. Coordination of trigeminal axon navigation and patterning with tooth organ formation: epithelial-mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development. 2005;132(2):323–334. doi: 10.1242/dev.01541. [DOI] [PubMed] [Google Scholar]
- 59.Pearson AA. The early innervation of the developing deciduous teeth. J Anat. 1977;123(Pt 3):563–577. [PMC free article] [PubMed] [Google Scholar]
- 60.Loes S, Kettunen P, Kvinnsland H, Luukko K. Mouse rudimentary diastema tooth primordia are devoid of peripheral nerve fibers. Anat Embryol. 2002;205(3):187–191. doi: 10.1007/s00429-002-0247-8. [DOI] [PubMed] [Google Scholar]
- 61.Kollar EJ, Lumsden AG. Tooth morphogenesis: the role of the innervation during induction and pattern formation. Journal de biologie buccale. 1979;7(1):49–60. [PubMed] [Google Scholar]
- 62.Stainier DY, Gilbert W. Pioneer neurons in the mouse trigeminal sensory system. Proc Natl Acad Sci USA. 1990;87(3):923–927. doi: 10.1073/pnas.87.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lumsden AG, Buchanan JA. An experimental study of timing and topography of early tooth development in the mouse embryo with an analysis of the role of innervation. Arch Oral Biol. 1986;31(5):301–311. doi: 10.1016/0003-9969(86)90044-0. [DOI] [PubMed] [Google Scholar]
- 64.Tuisku F, Hildebrand C. Evidence for a neural influence on tooth germ generation in a polyphyodont species. Dev Biol. 1994;165(1):1–9. doi: 10.1006/dbio.1994.1228. [DOI] [PubMed] [Google Scholar]
- 65.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 66.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8(5):552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, Hoffman MP. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun. 2013;4:1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ogawa M, Oshima M, Imamura A, Sekine Y, Ishida K, Yamashita K, Nakajima K, Hirayama M, Tachikawa T, Tsuji T. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2498. doi: 10.1038/ncomms3498. [DOI] [PMC free article] [PubMed] [Google Scholar]