Abstract
In highly polarized and elongated cells such as neurons, Tau protein must enter and move down the axon to fulfill its biological task of stabilizing axonal microtubules. Therefore, cellular systems for distributing Tau molecules are needed. This review discusses different mechanisms that have been proposed to contribute to the dispersion of Tau molecules in neurons. They include (1) directed transport along microtubules as cargo of tubulin complexes and/or motor proteins, (2) diffusion, either through the cytosolic space or along microtubules, and (3) mRNA-based mechanisms such as transport of Tau mRNA into axons and local translation. Diffusion along the microtubule lattice or through the cytosol appear to be the major mechanisms for axonal distribution of Tau protein in the short-to-intermediate range over distances of up to a millimetre. The high diffusion coefficients ensure that Tau can distribute evenly throughout the axonal volume as well as along microtubules. Motor protein-dependent transport of Tau dominates over longer distances and time scales. At low near-physiological levels, Tau is co-transported along with short microtubules from cell bodies into axons by cytoplasmic dynein and kinesin family members at rates of slow axonal transport.
Keywords: Alzheimer disease, Tau protein, Diffusion, Axonal transport, Kinesin, Dynein, Motor protein, Microtubules
Properties of Tau
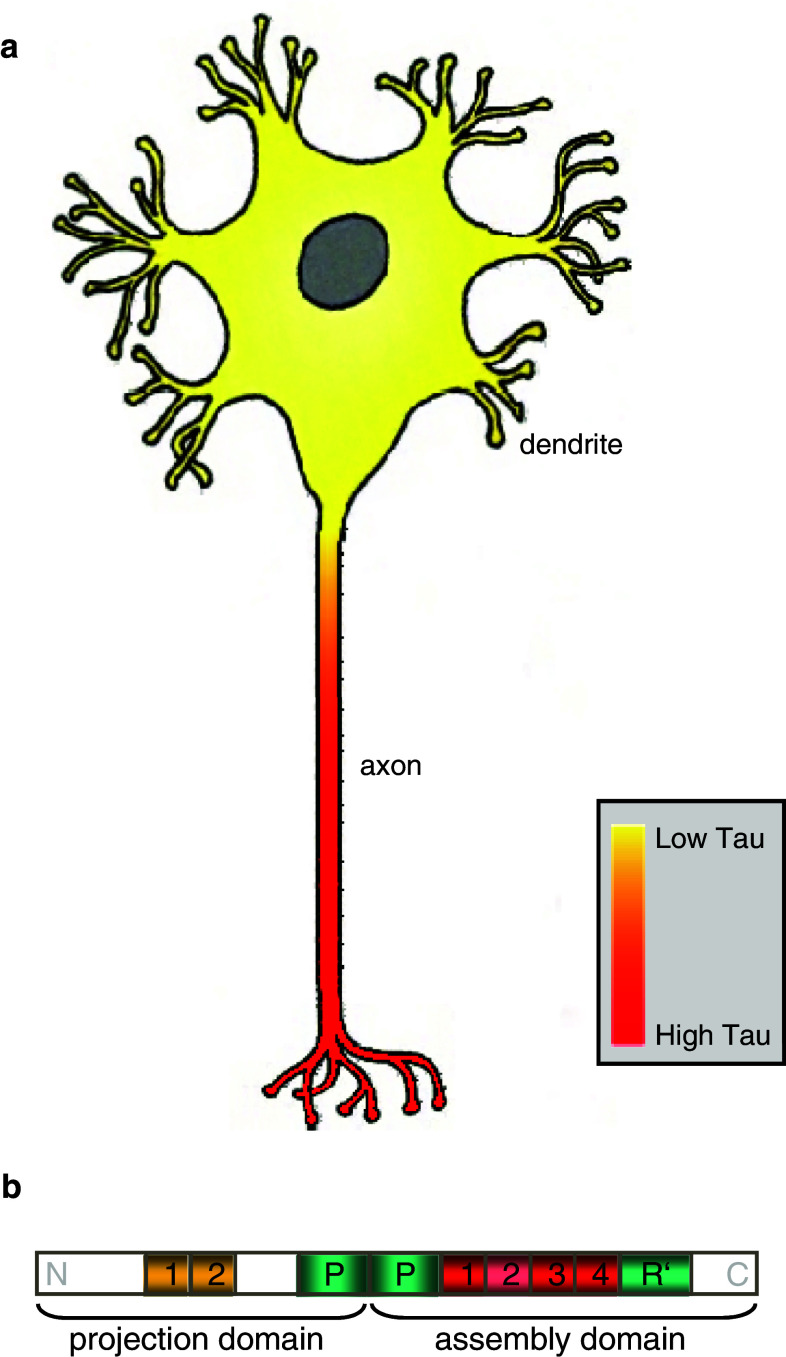
Tau is a structural microtubule-associated protein (MAP) which is located predominantly in the axons of neurons of the central nervous system (CNS) [14, 25, 56, 71, 75], with a proximal–distal gradient [15, 54, 71] (Fig. 1a). A decade after its discovery [117], Tau protein began to attract special interest because of its association with neurodegenerative diseases such as Alzheimer disease (reviews [31, 70]) or fronto-temporal dementia and Parkinsonism linked to chromosome 17 (FTDP17; review [32]). In the case of Alzheimer disease, Tau together with Amyloid-β are believed to be the two key factors contributing to neurodegeneration [121] (reviews [46, 59, 78]). Here, Tau accumulates in neurons, mislocalizes, and forms pathological filamentous aggregates called paired helical filaments (PHF) [114], which coalesce into neurofibrillary tangles [17, 31, 36, 57, 62, 119]. Amyloid-β-triggered Tau missorting into dendrites leads to spastin-mediated microtubule breakdown and spine loss [120], indicating the severe cellular consequences of Tau misregulation.
Fig. 1.
a Illustration of differential Tau expression levels in soma and axons of mature neurons showing a predominant axonal location of Tau protein. b Overview of the longest Tau isoform hTau40 (also called 2N4R or 4RL) with amino-(N)- and carboxy-terminal (C) regions as indicated. The repeats N1, N2 of the projection domain are highlighted in yellow while the repeats R1–R4 of the microtubule assembly domain are depicted in red. Repeat R2 is shown in light red as its presence is Tau isoform-dependent. Proline-rich domains (P and R′) are shown in light green
In neurons, the physiological function of Tau protein as the main axonal MAP is to support assembly and stabilization of axonal microtubules [21, 25, 35], which enables microtubules to fulfill their role as tracks for the motor-dependent axonal transport of vesicles, neurofilaments, and organelles such as mitochondria [93]. Hence, Tau molecules are analogous to ties or clips of microtubule tracks. Tau affects microtubule dynamic instability [83] and posttranslational modifications of microtubules [85], can interact with the neuronal plasma membrane [16], and anchors enzymes to microtubules [61, 64, 100]. Moreover, Tau can alter the mechanical properties of microtubules in vitro by enhancing their stiffness [20, 84, 91]. Tau protein was also suggested to function as a spacer between adjacent microtubules [19], although this role may be fulfilled better by larger MAPs such as MAP2.
In the human CNS, six main developmentally regulated Tau isoforms are found, which are derived by alternative mRNA splicing [4, 5, 33, 34] from the Tau gene (MAPT) encoded on chromosome 17q21 [82]. These Tau isoforms differ in their domain composition and overall length ranging from 352 to 441 aa [4]. A general overview of the longest Tau isoform in the CNS, termed htau40, Tau 4RL or Tau 2N4R, is illustrated in Fig. 1b. Tau molecules can be separated by chymotryptic cleavage into a mainly acidic amino-terminal “projection domain” (residues 1–197) and a “microtubule assembly domain” with the carboxy-terminal tail domain (residues 198–441) [44]. These two major domains can be further subdivided into several domains [37]: the “projection domain” can contain no, one or two insertions (N) of 29 residues each, while the core of the basic and proline-rich “microtubule assembly domain” (residues 244–368) comprises three or four semi-conserved pseudo-repeats (R) of ~31 amino acids. Different isoforms contain either repeats R1–R4 or R1 plus R3–R4, with three-repeat isoforms occurring preferentially in the fetal stage. These repeats promote microtubule assembly but bind on their own only with low affinity to microtubules. On either side of the microtubule assembly repeats, there are the so-called repeat-flanking “jaws” of Tau–microtubule interaction [87]: ~40 residues spanning proline-rich domains (P and R′, residues 151–198–240 and 369–400, respectively) which bind strongly to microtubules, thus strongly enhancing Tau binding and positioning on the microtubule lattice. Hence, efficient microtubule assembly can be achieved by the catalytic repeat domains [37, 87]. The amino-terminal “projection domain” protrudes away from the microtubule surface, and several distinct roles have been proposed for the projection domain including interaction with other cytoskeletal proteins [29, 44], membranes, and kinases [12, 16, 61, 64, 77, 100].
Tau is a highly soluble, natively unfolded, and intrinsically disordered protein [80, 95], with only a low content of transient secondary structure. Because of their disordered character, Tau molecules are very voluminous in solution, yet can adopt an overall “paperclip”-like conformation where the amino- and carboxy-terminal regions can fold back onto the “assembly domain” [48].
Tau protein promotes self-assembly of α/β-heterodimeric tubulin to microtubules and stabilizes microtubules by binding to their surface. Because of its disordered and very variable structure, the exact binding site of Tau on microtubules as well as its microtubule-bound conformation proved difficult to resolve. Different structural and biochemical approaches suggested that Tau might have more than one conformation or binding site on microtubules [1, 3, 18, 35, 45, 52, 68, 69, 92, 94]. Binding of Tau to microtubules was shown to be (at least partly) of electrostatic nature and involves the negatively charged E-hook of tubulin [38, 42, 65, 72, 88, 97]. In neurons, about 1 μM Tau compared to 20–40 μM tubulin was found [21], whereas higher Tau:tubulin ratios (~0.5) can be achieved for Tau binding to microtubules in vitro [37, 68].
During neuronal maturation, there is a shift in Tau isoforms from short Tau isoforms in the fetal brain (0N3R) to longer ones (up to 2N4R), accompanied by a decrease of phosphorylation [25, 58]. At the same time, Tau protein becomes redistributed from a ubiquitous distribution in fetal neurons to a pronounced polar distribution in the axonal compartment of mature neurons. How Tau is sorted into axons and/or depleted from the somatodendritic compartment is still not well understood, although several pathways have been discovered which may operate in parallel [8, 43, 51, 63, 110]. Nevertheless, in highly polarized and elongated cells such as neurons, Tau generated in the cell body needs to move into and down the axon to fulfill its task of stabilizing axonal microtubules. Therefore, mechanisms of transportation for Tau molecules in neurons are needed.
In this short review article, we focus on cellular mechanisms for Tau redistribution and on the impact of Tau on the function of molecular motors involved in axonal transport.
Motor protein-driven transport of Tau
The most intuitive mechanism for Tau transportation in neurons is directed active transport by motor proteins such as kinesin family members and cytoplasmic dynein. This could be achieved in different ways: (1) with Tau as a cargo of motor proteins or (2) with Tau dragged along with other mobile motor-driven structures such as small microtubules.
Tau has indeed been found to be subject to active transport. In neurons, the protein is normally transported along axons with overall rates of 0.2–0.4 mm/day (about 0.002 μm/s on average) [74, 110, 122], consistent with rates of slow axonal transport (slow component a) but significantly faster than transport rates of tubulin of 0.1–0.2 mm/day (about 0.001 μm/s) [74]. This is ~500-fold slower than typical rates of microtubule motors (~1 μm/s) which appears to disqualify them as transporters. However, the discrepancy could be removed by considering that slow motion can be generated by fast motors if they act in a discontinuous stop-and-go fashion [55, 111]. Alternatively, the slow axonal transport rate can be explained by diffusion of Tau through the cytosol which can be surprisingly fast and efficient over short distances (Fig. 2) [55]. Some hints at direct motor protein participation in Tau dispersion have been reported in recent years. As an indication of kinesin-mediated Tau transportation, it has been described that Tau can interact directly with kinesin-1 molecules via kinesin light chains 1 and 2 [111] (Fig. 3), and a disturbed kinesin light chain–Tau interaction results in Tau accumulation and Tau-dependent neurodegeneration [26, 27]. Additionally, PTL-1, the C. elegans homolog of Tau, has been shown to physically interact with UNC-104, a class-3 kinesin motor, with which it co-migrates in vivo. Hence, it was hypothesized that Tau/PTL-1 is transported alternatingly by kinesin-3 and kinesin-1 motors in cells [108]. Furthermore, it has been suggested that the rate of Tau transportation by motor proteins could be modulated by Tau phosphorylation. In cell experiments, inhibition of the axonal kinase GSK3β reduced Tau phosphorylation and led to decreased overall rates of axonal transport of Tau [23]. Based on previous observations of kinesin-1 light chain–Tau interactions [111], it was hypothesized that Tau binding to kinesin-1 via kinesin light chains could be tuned in a Tau phosphorylation-dependent manner [23]. Results from respective binding studies using phosphomimicking Tau constructs, however, could not be confirmed in later studies [89]. At elevated concentrations, Tau forms globular accumulations which occur first at the distal ends of axons and can move along microtubules bi-directionally and in a stop-and-go manner, with velocities typical for motor-driven transport [110, 111], although there is a debate whether this is due to a physiological transport mechanism or a sign of degenerating axons [55]. Such local Tau protein accumulations have been described recently to result from local mechanical microtubule disruption during traumatic brain injury [105], arguing against the idea that movement of such Tau accumulations might be part of the regular axonal transport of Tau protein in neurons.
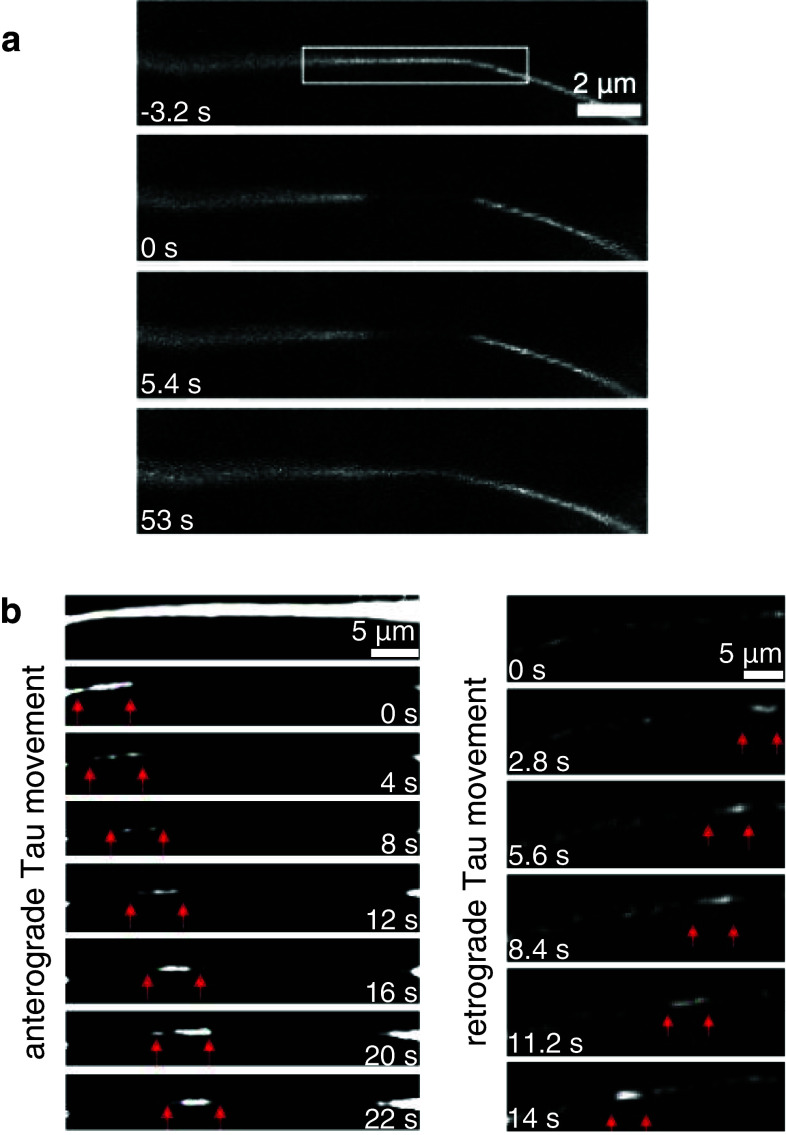
Fig. 2.
a Diffusion of Tau in the cytosol. Photobleaching and diffusive Tau recovery of CFP-Tau8R from both sides into a 3-μm area of an axon (modified from [55]). b Bidirectional axonal transport of Tau in small filamentous structures (indicated by red arrows) in retinal ganglion cell axons. In the left panel, anterograde movement of CFP-Tau containing structures (with an average speed of ~0.6 μm/s and instantaneous velocities of ~0.2–1.3 μm/s) is visible after subtraction of background fluorescence signal caused by diffusive recovery. The right panel depicts retrograde movement of CFP-Tau containing structures with an average speed of ~0.4 μm/s and instantaneous velocities of ~0.2–0.9 μm/s (modified from [55])
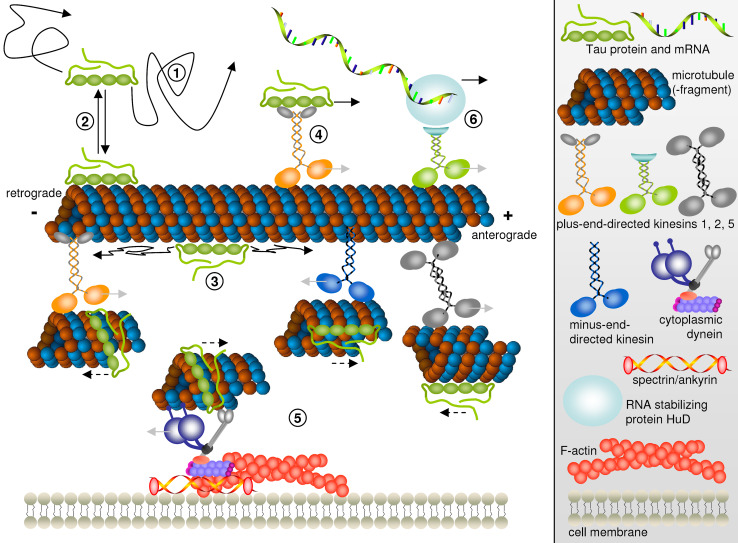
Fig. 3.
Proposed mechanisms of Tau dispersion in cells. Free diffusing Tau molecules in the cytosol (1) in rapid equilibrium with Tau bound to microtubules (2). On microtubules, Tau is free to diffuse along the microtubule lattice (3). Motor-dependent Tau transport by kinesin molecules (4) or piggybacking on short microtubule fragments translocated by kinesin family members or cytoplasmic dynein (5). Transport of HuD-bound Tau mRNA by kinesin-2 followed by local translation in the axon (6). Light gray arrows indicate the directions of motor protein movement while solid black arrows denote the directions of Tau protein or mRNA motion by diffusion or as cargo of kinesin motor proteins. Note that in (5), analogous to an in vitro microtubule gliding assay, motor proteins (different kinesins or dynein) being hooked up to structures such as immobile microtubules or the actin network push small microtubule fragments and bound Tau into the opposite direction of their own walking direction. This Tau movement along with microtubule fragments is indicated by dashed black arrows
Another possible mechanism for the motor-driven axonal transport of Tau is the co-transport along with microtubules. Axonal microtubules are mostly long and stationary [90], yet movement of short microtubules within axons has been observed by tubulin fluorescence and photobleaching experiments [2, 41, 116]. Such short microtubules have therefore been postulated to be the transport units of tubulin in neurons. Motion of small microtubules occurs in both axonal directions. About half of the anterograde movement of small microtubule fragments is dependent on the actin network while retrograde movement is independent of actin [40, 79, 93]. The anterograde traffic of short microtubule fragments is maintained by cytoplasmic dynein hooked up to the stationary actin cytoskeleton or to spectrin-β3 [9, 41, 53, 86, 116] and by minus-end-directed kinesin family members hooked up to stationary microtubules [28, 30, 99]. Analogous to in vitro microtubule gliding assays on surfaces of immobilized motor proteins, in cells, tethered motor proteins push these microtubule fragments to the opposite side instead of walking to their preferred microtubule end. Thus, retrograde microtubule transport can be achieved by plus-end-directed kinesin family members like kinesin-1 and kinesin-5 [39, 66]. Such “piggybacking” of Tau on small microtubule fragments (Fig. 3), which are transported along the axon by motor proteins with rapid motion bursts and saltatory characteristics and whose structure is not known in detail, appears to be the main transport mechanism for Tau under near-physiological conditions.
Local Tau protein synthesis in the axon from transported Tau mRNA
One other possible mechanism for Tau dispersion in neurons is the transport not of the protein but of the respective Tau mRNA followed by local translation in the axon. Recently, it has been suggested by a few reports from one group that proteins such as Tau can be synthesized locally from mRNA specifically targeted to the axonal compartment (review [101]). This can be achieved by the formation of RNA- and ribosome-containing ribonucleoprotein (RNP) complexes which are transported to periaxoplasmic ribosomal plaques (PARPs), possible centers of local Tau translation. Specific targeting to the axon requires an axonal localization signal within the 3′ untranslated region (3′UTR) of Tau mRNA [7]. This axonal “zipcode” can be recognized by HuD [6], a mRNA-binding protein that can regulate mRNA stability. A fraction of Tau RNP complexes additionally contain KIF3A, a subunit of kinesin-2, and associate with microtubules [8]. This suggests that active anterograde transport of Tau RNP complexes utilizes kinesin-2 motor proteins (Fig. 3), while kinesin-1 motors seem not to be involved [8]. Like axonal transport of Tau protein synthesized in the cell body, trafficking of Tau mRNA and local axonal Tau translation relies on uncompromised intracellular active transport by motor proteins.
Impact of Tau on microtubule-dependent motor molecules
Elevated expression of Tau in neurons slows down intracellular transport and dramatically alters the distribution of transported organelles. Net inhibition of anterograde transport may cause a predominance of retrograde transport and can lead to the accumulation of cell organelles such as mitochondria in the cell body, resulting in the starvation and decay of cell processes [102, 109].
How could Tau interfere with axonal transport? Several mechanisms of the pathological function of Tau protein during neurodegeneration have been suggested: (1) destabilization of microtubules by loss-of-function of hyper-phosphorylated Tau (which is therefore detached from microtubules), (2) toxic gain-of-function by Tau filament formation with inhibition of fast anterograde kinesin-driven axonal transport through activation of axonal phosphotransferases, (3) mechanic clogging of the axon through excess microtubules over-stabilized by elevated Tau [107], and (4) direct interference of kinesin- and dynein-dependent axonal transport by microtubule-bound Tau.
Regulation of motor protein function and therefore intracellular transport could be achieved by different strategies, such as switching the motor on or off, changing the direction of movement, changing the velocity of the motor protein, increase or decrease of the distance over which the motor protein can process, premature release of the transported cargo, and regulation of motor protein attachment to its cytoskeletal track. The molecular motors involved in the axonal transport of Tau protein themselves rely on their cyclic interaction with their cytoskeletal tracks. In neurons, the microtubule tracks are decorated with MAPs like Tau. Although the exact Tau binding site on the microtubule lattice is not known, it appears to partially overlap with binding sites for other proteins, e.g., molecular motors such as kinesin and dynein [38, 72, 88]. Thus, Tau could influence processes like axonal transport at the level of motor protein function. Interference with axonal transport and microtubule-dependent motor molecule functions has been observed both in vitro and in cell experiments [38, 96, 106, 109]. For example, overexpression of Tau in neurons changes the distribution of organelles transported via microtubule-dependent motor proteins and slows down intracellular transport by preferential impairment of plus-end-directed transport mediated by kinesin motor proteins, an effect that could also be observed in vitro [24, 113]. In the case of kinesin-1, elevated concentrations of Tau lead to a reduction of kinesin (re-)attachment rate to microtubules [96], and decrease the run length of kinesin molecules [24, 73, 109] or the number of engaged kinesin molecules per cargo [112], although with overall undiminished kinesin velocities. Likewise, kinesin-3 seems to be negatively affected by the presence of Tau protein. In an opposite approach to Tau overexpression, from Tau/PTL-1 knock-out experiments, it was concluded that, although the travelling velocity of kinesin-3 remains unchanged in the presence of Tau/PTL-1, motor run length is decreased and detachment is enhanced [108]. While kinesin motors tend to detach from microtubules more readily in the presence of Tau on microtubules, dynein can even reverse its direction temporarily to circumvent the Tau obstacle [24].
Inhibitory effects on microtubule-based motor proteins have been suggested both for the projection domain and for the assembly domain of Tau. In agreement with that, inhibition of kinesin and dynein functions by Tau was found to be Tau isoform-dependent, yet the results are contradictory. On one hand, the projection domain of Tau has been suggested to inhibit binding of kinesin or cytoplasmic dynein to microtubules by steric or electrostatic hindrance [38]. On the other hand, the projection domain has also been attributed to assist binding of motor proteins such as the dynein/dynactin complex to microtubules [67]. Moreover, Tau fragments comprising just the microtubule assembly domain were sufficient to inhibit kinesin and dynein in single molecule studies, and the absence of the projection domain even increased inhibition [24], whereas other studies indicate that motor inhibition can be achieved by a small amino-terminal sequence of Tau without the need for the microtubule assembly domain [50, 60]. Others found no effect whatsoever of non-aggregated Tau on fast axonal vesicle transport [76] and concluded that aggregation of Tau is needed to disturb kinesin-driven transport. This inhibitory effect of aggregated Tau was suggested to be driven by the activation of a signaling cascade comprising protein phosphatase 1 (PP1) which dephosphorylates the axonal kinase GSK3β. Activated GSK3β then phosphorylates kinesin-1 light chains thus leading to dissociation of kinesin from its cargo and inhibition of anterograde fast axonal transport [50, 60]. Activation of PP1, however, could be prevented by phosphorylation of a small amino-terminal sequence of Tau by Fyn [49]. Additionally, cargo-selective impairment of kinesin-driven anterograde fast axonal transport by hyper-phosphorylated Tau has also been reported to be the result of a pathological phospho-Tau/c-Jun N-terminal kinase-interacting protein 1 (JIP1) interaction, which interferes with the physiological JIP1/kinesin light chain interaction [47].
Overall, the majority of studies suggest a concentration-dependent inhibition of kinesin and dynein function by Tau constructs. Direct inhibition is achieved mainly through interference with the attachment of the motors to their microtubule tracks [24, 73, 96, 112, 113], with usually decreasing motor run lengths but no major changes in motion velocity. The inhibitory impact on molecular motors does not correlate with Tau binding affinity to microtubules or microtubule assembly properties [96]. Accordingly, a shorter Tau isoform lacking the amino-terminal inserts and repeat R2 of the microtubule assembly domain (isoform 0N3R), and therefore reduced microtubule affinity, was found to be a more potent inhibitor of kinesin and dynein, again with a stronger impact on kinesin [24, 73, 96, 112, 113]. Yet again, one other study found a larger effect on mitochondrial transport by a four-microtubule assembly repeat Tau construct compared to one with only three repeats [103].
Tau dispersion by diffusion in the cytosol
As described above, pathologically elevated levels of Tau protein can inhibit microtubule-dependent motor proteins such as kinesin family members and cytoplasmic dynein, thus impeding proper vesicle and organelle distribution as well as Tau’s own motor-dependent transport along axons. However, even under these inhibitory conditions, Tau itself can move significantly into axons over ranges of millimetres and is able to enter cell processes and to distribute along axons [102]. This prompted the question of how Tau could manage to travel into and down the axon despite its general negative effect on microtubule-based traffic.
A partial solution to this contradiction is that Tau can diffuse rather rapidly in cells, in spite of its preferred association with microtubules. The faster axonal transport rates of Tau compared to tubulin already indicate that Tau molecules interact dynamically with their microtubule tracks [74]. Live cell fluorescence microscopy, fluorescence recovery after photobleaching (FRAP), and fluorescence speckle microscopy experiments on neurons revealed that Tau is highly dynamic and diffuses rapidly in the cytosol, with diffusion coefficients of ~3 μm2/s [55] and microtubule dwell times of ~3–4 s which become even shorter upon phosphorylation [55, 91]. At physiological Tau levels, re-entry of fluorescent Tau into the photobleached zone occurred within minutes from both ends of a several-micrometer-long bleached axon stretch, emphasizing rapid diffusion (Fig. 2). Experiments using photoconvertible Tau constructs revealed some directional bias with somewhat enhanced Tau spreading to the distal direction, which was explained by Tau diffusion superimposed on slow anterograde Tau transport [63]. Consistent with Tau diffusion in the cytosol, entry of Tau into the axon is concentration-dependent, and diffusion of Tau can promote the entry of Tau into axons over distances of millimetres and periods of days [55].
If cytosolic diffusion substantially contributes to Tau movement over short-to-intermediate ranges in neurons, how can the equilibrium between microtubule-bound and freely diffusing Tau be controlled? One tool to regulate the occupancy of Tau along axonal microtubules is phosphorylation/de-phosphorylation [10, 13, 55]. In Alzheimer disease and other tauopathies, neurofibrillary changes of abnormally hyper-phosphorylated Tau are key lesions [98] pointing towards the physiological and pathological relevance of Tau phosphorylation. There are 80 putative serine or threonine phosphorylation sites within the longest CNS Tau isoform, htau40. Phosphorylation of Tau, especially of KXGS motifs in the repeats of the microtubule assembly domain, tends to detach Tau from the microtubule resulting in destabilization of microtubules [13, 104]. Accordingly, FRAP was faster in axons transfected with pseudo-phosphorylated 4KXGE-mutant Tau protein than in axons with wild-type and non-phosphorylatable 4KXGA Tau protein. Pseudo-phosphorylated Tau that was mainly detached from microtubules diffused rapidly with diffusion coefficients of ~11 μm2/s and also showed a weaker axonal localization. It was concluded that this is a result from phosphorylation-induced detachment of Tau followed by almost free Tau diffusion within the cytosol of axons [55]. Tau constructs with enhanced microtubule affinity resulted in a reduced apparent diffusion coefficient, lower detachment rates, and stronger axonal localization [55]. Hence, the apparent diffusion coefficient of a given Tau construct is influenced by the ratio of free to microtubule-bound Tau and therefore dependent on its affinity to microtubules. Microtubule affinity can be modulated by Tau phosphorylation with higher degrees of phosphorylation leading to faster apparent diffusion and cellular dispersion. This concept also agrees well with recent observations which have been interpreted dissentingly: in cell experiments, increased levels of Tau phosphorylation led to increased overall rates of axonal transport of Tau, and decreased phosphorylation levels to decreased rates. From that, it was concluded that Tau phosphorylation modulates axonal transport rates of Tau by regulating Tau binding to kinesin-1 light chains [23, 89]. However, experiments to prove phosphorylation-modulated kinesin-1 light chain binding of Tau using (de-)phosphomimicking Tau constructs were not consistent [23, 89], leaving this issue unclear. Alternatively, such observations of increased overall rates of axonal transport of phosphorylated or phosphomimicking Tau constructs can be explained by phosphorylation-induced decreased affinity to microtubules leading to more pronounced Tau diffusion through the cytosolic space.
Phosphorylation-induced low microtubule affinity of Tau, allowing the protein to diffuse freely in the cytosol, also has consequences for the cellular sorting of Tau in neurons. Recently, a microtubule-dependent retrograde barrier in the axon initial segment was discovered as a rectifying mechanism involved in cellular Tau sorting [63]. This diffusion barrier allows Tau to enter the axon but prevents retrograde flow back towards soma and dendrites. The retrograde diffusion barrier enables neurons to trap Tau in the axon but breaks down when Tau is detached from microtubules due to phosphorylation in its repeat domain, resulting in an increased appearance of Tau in the cell body and dendrites as observed in neurodegenerative diseases [63]. This demonstrates that Tau movement by diffusion through the cytosol is relevant not only during physiological distribution of Tau in neurons but also during development of pathological missorting of Tau protein.
Tau dispersion by diffusion along microtubules
Until recently, models for the dispersion of Tau protein only considered co-transport of bound Tau with short microtubule fragments, kinesin-driven Tau transport, and rapid Tau diffusion in the cytoplasm. Consequently, microtubule-bound Tau was believed to be stationary on a given microtubule or transported microtubule fragment. However, single molecule TIRF microscopy experiments have recently revealed that Tau molecules can also diffuse along microtubules guided by the microtubule lattice [42] (Figs. 3, 4). This provides experimental evidence for an additional mechanism of Tau transport in neurons, which has been hypothesized before [118], partly based on observations that microtubules can diffuse along their axis on methylcellulose-coated glass surfaces [81]. Individual Tau molecules diffuse in vitro for several seconds on microtubules, consistent with microtubule dwell times found previously in cell experiments [55, 91]. During these transient interactions, Tau molecules can slide along microtubules with diffusion coefficients of up to ~0.5 μm2/s, thus making contact with ~3 μm of microtubule length in a single encounter [42]. Diffusive interactions have been suggested previously for DNA binding proteins searching for their specific target sequence along their DNA substrate [11, 115].
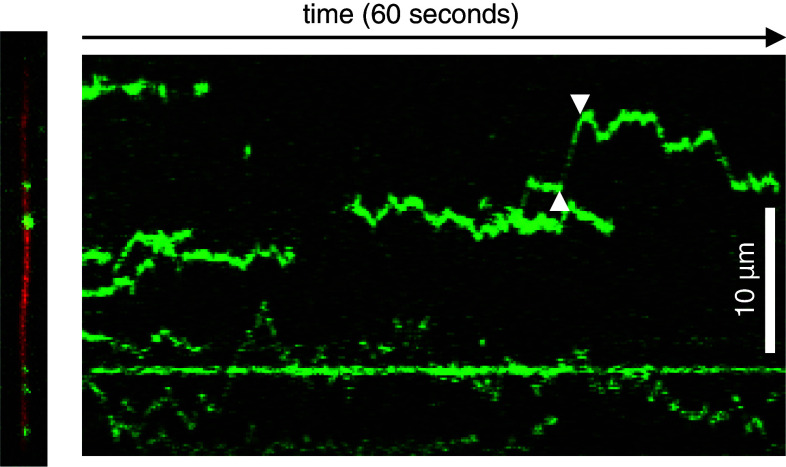
Fig. 4.
Diffusion of Tau molecules along microtubules. Left TIRF microscopy snapshot of Tau molecules (green) diffusing along an immobilized microtubule (27.5 μm, red) in vitro. Right The respective kymograph (plot of Tau fluorescence along the microtubule axis versus time) clearly shows diffusive movement of individual Tau molecules along the microtubule of instantaneous velocities of up to 2.7 μm/s (between white arrowheads)
Tau diffusion along microtubules does not cease or decelerate even at elevated Tau concentrations, but is sensitive to changes of ionic strength and pH as it requires the negatively charged carboxy-terminus of tubulin as a binding partner [42]. Microtubule lattice diffusion is also not restricted to one protofilament, as diffusing Tau molecules are able to change easily from one to another protofilament even of intersecting microtubules [42]. General advantages of Tau diffusion in the cytosol or guided by the microtubule lattice would be that (1) both ends of the microtubule can be targeted without a need for external chemical energy and (2) Brownian motion is faster than active transport over short distances of up to 1 μm. Moreover, (3) such a mechanism could avoid local accumulations of Tau on microtubules due to frequent transitions between protofilaments, thus leading to a more homogeneous distribution of Tau. Diffusing Tau molecules could also give way to passing kinesin or dynein motors (as postulated in [22]) as individual Tau molecules are still mobile on microtubules even at Tau levels where kinesin inhibition is observed [42].
Conclusion
Different mechanisms contribute to the dispersion of Tau molecules in highly polarized and elongated cells such as neurons (Fig. 3): (1) freely diffusing Tau molecules in the cytosol are in rapid equilibrium (2) with Tau bound to microtubules; (3) when interacting with microtubules, Tau molecules are able to diffuse along the microtubule lattice, rather than being restricted to one binding site; (4) motor-dependent Tau transport as cargo by kinesin molecules or (5) piggybacking on short microtubule fragments translocated by kinesin molecules or cytoplasmic dynein; (6) additionally, Tau mRNA can be transported by kinesin motors and locally synthesized in the axon.
Rapid diffusion in the cytosol and diffusion along the microtubule lattice appear to be the major mechanisms for axonal distribution of Tau protein in the short-to-intermediate range over distances of up to a millimetre. The sufficiently high diffusion coefficients ensure that Tau can distribute evenly throughout the axonal volume as well as along microtubules. Microtubule-dependent transport of Tau driven by motor proteins such as cytoplasmic dynein and kinesin family members dominates over longer distances and times. At low near-physiological levels, Tau is co-transported with microtubule fragments from cell bodies into axons, moving at instantaneous velocities of ~1 μm/s. At high concentrations, Tau forms local accumulations moving bi-directionally along microtubules with speeds of ~0.3 μm/s. Since these globular clusters at first appear at distal endings of axons, they might indicate an early stage of neurite degeneration or represent clusters of Tau locally synthesized from Tau mRNA which was actively targeted to the axonal compartment.
Acknowledgments
Work performed in the Scholz or Mandelkow groups was partially supported by Deutsche Forschungsgemeinschaft (DFG) Research Unit FOR629 grants to T.S and E.M. We thank Dietmar J. Manstein for organizing Research Unit FOR629 as well as Walter Steffen and Bernhard Brenner for helpful discussions.
Abbreviations
- 3′UTR
3′ Untranslated region
- aa
Amino acids
- CNS
Central nervous system
- FRAP
Fluorescence recovery after photobleaching
- FTDP17
Fronto-temporal dementia and Parkinsonism linked to chromosome 17
- JIP1
c-Jun N-terminal kinase-interacting protein 1
- MAP
Microtubule-associated protein
- PARPs
Periaxoplasmic ribosomal plaques
- PHF
Paired helical filaments
- PP1
Protein phosphatase 1
- RNP
Ribonucleoprotein
- TIRF
Total internal reflection fluorescence
References
- 1.Ackmann M, Wiech H, Mandelkow E. Nonsaturable binding indicates clustering of tau on the microtubule surface in a paired helical filament-like conformation. J Biol Chem. 2000;275(39):30335–30343. doi: 10.1074/jbc.M002590200. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosome are transported into the axon. J Cell Sci. 1995;108(Pt 8):2761–2769. doi: 10.1242/jcs.108.8.2761. [DOI] [PubMed] [Google Scholar]
- 3.Al-Bassam J, Ozer RS, Safer D, Halpain S, Milligan RA. MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J Cell Biol. 2002;157(7):1187–1196. doi: 10.1083/jcb.200201048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreadis A. Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Biophys Acta. 2005;1739(2–3):91–103. doi: 10.1016/j.bbadis.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31(43):10626–10633. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- 6.Aranda-Abreu GE, Behar L, Chung S, Furneaux H, Ginzburg I. Embryonic lethal abnormal vision—like RNA—binding proteins regulate neurite outgrowth and tau expression in PC12 cells. J Neurosci. 1999;19(16):6907–6917. doi: 10.1523/JNEUROSCI.19-16-06907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronov S, Aranda G, Behar L, Ginzburg I. Axonal tau mRNA localization coincides with tau protein in living neuronal cells and depends on axonal targeting signal. J Neurosci. 2001;21(17):6577–6587. doi: 10.1523/JNEUROSCI.21-17-06577.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronov S, Aranda G, Behar L, Ginzburg I. Visualization of translated tau protein in the axons of neuronal P19 cells and characterization of tau RNP granules. J Cell Sci. 2002;115(Pt 19):3817–3827. doi: 10.1242/jcs.00058. [DOI] [PubMed] [Google Scholar]
- 9.Baas PW, Vidya Nadar C, Myers KA. Axonal transport of microtubules: the long and short of it. Traffic. 2006;7(5):490–498. doi: 10.1111/j.1600-0854.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 10.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8(9):663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 11.Berg OG, Winter RB, von Hippel PH. Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry. 1981;20(24):6929–6948. doi: 10.1021/bi00527a028. [DOI] [PubMed] [Google Scholar]
- 12.Bhaskar K, Yen SH, Lee G. Disease-related modifications in tau affect the interaction between Fyn and Tau. J Biol Chem. 2005;280(42):35119–35125. doi: 10.1074/jbc.M505895200. [DOI] [PubMed] [Google Scholar]
- 13.Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11(1):153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- 14.Binder LI, Frankfurter A, Rebhun LI. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black MM, Slaughter T, Moshiach S, Obrocka M, Fischer I. Tau is enriched on dynamic microtubules in the distal region of growing axons. J Neurosci. 1996;16(11):3601–3619. doi: 10.1523/JNEUROSCI.16-11-03601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandt R, Leger J, Lee G. Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J Cell Biol. 1995;131(5):1327–1340. doi: 10.1083/jcb.131.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brion JP, Couck AM, Passareiro E, Flament-Durand J. Neurofibrillary tangles of Alzheimer’s disease: an immunohistochemical study. J Submicrosc Cytol. 1985;17(1):89–96. [PubMed] [Google Scholar]
- 18.Butner KA, Kirschner MW. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991;115(3):717–730. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360(6405):674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- 20.Choi MC, Raviv U, Miller HP, Gaylord MR, Kiris E, Ventimiglia D, Needleman DJ, Kim MW, Wilson L, Feinstein SC, Safinya CR. Human microtubule-associated-protein tau regulates the number of protofilaments in microtubules: a synchrotron X-ray scattering study. Biophys J. 2009;97(2):519–527. doi: 10.1016/j.bpj.2009.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleveland DW, Hwo SY, Kirschner MW. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977;116(2):227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- 22.Cooper JR, Wordeman L. The diffusive interaction of microtubule binding proteins. Curr Opin Cell Biol. 2009;21(1):68–73. doi: 10.1016/j.ceb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuchillo-Ibanez I, Seereeram A, Byers HL, Leung KY, Ward MA, Anderton BH, Hanger DP. Phosphorylation of tau regulates its axonal transport by controlling its binding to kinesin. FASEB J. 2008;22(9):3186–3195. doi: 10.1096/fj.08-109181. [DOI] [PubMed] [Google Scholar]
- 24.Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319(5866):1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol. 1986;103(6 Pt 2):2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falzone TL, Gunawardena S, McCleary D, Reis GF, Goldstein LS. Kinesin-1 transport reductions enhance human tau hyperphosphorylation, aggregation and neurodegeneration in animal models of tauopathies. Hum Mol Genet. 2010;19(22):4399–4408. doi: 10.1093/hmg/ddq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falzone TL, Stokin GB, Lillo C, Rodrigues EM, Westerman EL, Williams DS, Goldstein LS. Axonal stress kinase activation and tau misbehavior induced by kinesin-1 transport defects. J Neurosci. 2009;29(18):5758–5767. doi: 10.1523/JNEUROSCI.0780-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fink G, Hajdo L, Skowronek KJ, Reuther C, Kasprzak AA, Diez S. The mitotic kinesin-14 Ncd drives directional microtubule-microtubule sliding. Nat Cell Biol. 2009;11(6):717–723. doi: 10.1038/ncb1877. [DOI] [PubMed] [Google Scholar]
- 29.Fulga TA, Elson-Schwab I, Khurana V, Steinhilb ML, Spires TL, Hyman BT, Feany MB. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol. 2007;9(2):139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- 30.Furuta K, Toyoshima YY. Minus-end-directed motor Ncd exhibits processive movement that is enhanced by microtubule bundling in vitro. Curr Biol. 2008;18(2):152–157. doi: 10.1016/j.cub.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 31.Garcia ML, Cleveland DW. Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr Opin Cell Biol. 2001;13(1):41–48. doi: 10.1016/s0955-0674(00)00172-1. [DOI] [PubMed] [Google Scholar]
- 32.Goedert M, Ghetti B, Spillantini MG. Frontotemporal dementia: implications for understanding Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(2):a006254. doi: 10.1101/cshperspect.a006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta. 2005;1739(2–3):240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 1989;8(2):393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goode BL, Denis PE, Panda D, Radeke MJ, Miller HP, Wilson L, Feinstein SC. Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol Biol Cell. 1997;8(2):353–365. doi: 10.1091/mbc.8.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994;33(32):9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- 38.Hagiwara H, Yorifuji H, Sato-Yoshitake R, Hirokawa N. Competition between motor molecules (kinesin and cytoplasmic dynein) and fibrous microtubule-associated proteins in binding to microtubules. J Biol Chem. 1994;269(5):3581–3589. [PubMed] [Google Scholar]
- 39.Haque SA, Hasaka TP, Brooks AD, Lobanov PV, Baas PW. Monastrol, a prototype anti-cancer drug that inhibits a mitotic kinesin, induces rapid bursts of axonal outgrowth from cultured postmitotic neurons. Cell Motil Cytoskeleton. 2004;58(1):10–16. doi: 10.1002/cm.10176. [DOI] [PubMed] [Google Scholar]
- 40.Hasaka TP, Myers KA, Baas PW. Role of actin filaments in the axonal transport of microtubules. J Neurosci. 2004;24(50):11291–11301. doi: 10.1523/JNEUROSCI.3443-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Y, Francis F, Myers KA, Yu W, Black MM, Baas PW. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J Cell Biol. 2005;168(5):697–703. doi: 10.1083/jcb.200407191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinrichs MH, Jalal A, Brenner B, Mandelkow E, Kumar S, Scholz T. Tau protein diffuses along the microtubule lattice. J Biol Chem. 2012;287(46):38559–38568. doi: 10.1074/jbc.M112.369785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirokawa N, Funakoshi T, Sato-Harada R, Kanai Y. Selective stabilization of tau in axons and microtubule-associated protein 2C in cell bodies and dendrites contributes to polarized localization of cytoskeletal proteins in mature neurons. J Cell Biol. 1996;132(4):667–679. doi: 10.1083/jcb.132.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirokawa N, Shiomura Y, Okabe S. Tau proteins: the molecular structure and mode of binding on microtubules. J Cell Biol. 1988;107(4):1449–1459. doi: 10.1083/jcb.107.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoenger A, Gross H. Structural investigations into microtubule-MAP complexes. Methods Cell Biol. 2008;84:425–444. doi: 10.1016/S0091-679X(07)84014-3. [DOI] [PubMed] [Google Scholar]
- 46.Ittner LM, Gotz J. Amyloid-beta and tau–a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12(2):65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 47.Ittner LM, Ke YD, Gotz J. Phosphorylated Tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J Biol Chem. 2009;284(31):20909–20916. doi: 10.1074/jbc.M109.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeganathan S, von Bergen M, Brutlach H, Steinhoff HJ, Mandelkow E. Global hairpin folding of tau in solution. Biochemistry. 2006;45(7):2283–2293. doi: 10.1021/bi0521543. [DOI] [PubMed] [Google Scholar]
- 49.Kanaan NM, Morfini G, Pigino G, LaPointe NE, Andreadis A, Song Y, Leitman E, Binder LI, Brady ST. Phosphorylation in the amino terminus of tau prevents inhibition of anterograde axonal transport. Neurobiol Aging. 2012;33(4):826.e815–826.e830. doi: 10.1016/j.neurobiolaging.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanaan NM, Morfini GA, LaPointe NE, Pigino GF, Patterson KR, Song Y, Andreadis A, Fu Y, Brady ST, Binder LI. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J Neurosci. 2011;31(27):9858–9868. doi: 10.1523/JNEUROSCI.0560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanai Y, Hirokawa N. Sorting mechanisms of tau and MAP2 in neurons: suppressed axonal transit of MAP2 and locally regulated microtubule binding. Neuron. 1995;14(2):421–432. doi: 10.1016/0896-6273(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 52.Kar S, Fan J, Smith MJ, Goedert M, Amos LA. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 2003;22(1):70–77. doi: 10.1093/emboj/cdg001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10(12):854–865. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kempf M, Clement A, Faissner A, Lee G, Brandt R. Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament-dependent manner. J Neurosci. 1996;16(18):5583–5592. doi: 10.1523/JNEUROSCI.16-18-05583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konzack S, Thies E, Marx A, Mandelkow EM, Mandelkow E. Swimming against the tide: mobility of the microtubule-associated protein tau in neurons. J Neurosci. 2007;27(37):9916–9927. doi: 10.1523/JNEUROSCI.0927-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kosik KS, Finch EA. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. J Neurosci. 1987;7(10):3142–3153. doi: 10.1523/JNEUROSCI.07-10-03142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA. 1986;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kosik KS, Orecchio LD, Bakalis S, Neve RL. Developmentally regulated expression of specific tau sequences. Neuron. 1989;2(4):1389–1397. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- 59.Kreplak L, Aebi U. From the polymorphism of amyloid fibrils to their assembly mechanism and cytotoxicity. Adv Protein Chem. 2006;73:217–233. doi: 10.1016/S0065-3233(06)73007-8. [DOI] [PubMed] [Google Scholar]
- 60.LaPointe NE, Morfini G, Pigino G, Gaisina IN, Kozikowski AP, Binder LI, Brady ST. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. J Neurosci Res. 2009;87(2):440–451. doi: 10.1002/jnr.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee G, Newman ST, Gard DL, Band H, Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J Cell Sci. 1998;111(Pt 21):3167–3177. doi: 10.1242/jcs.111.21.3167. [DOI] [PubMed] [Google Scholar]
- 62.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 63.Li X, Kumar Y, Zempel H, Mandelkow EM, Biernat J, Mandelkow E. Novel diffusion barrier for axonal retention of Tau in neurons and its failure in neurodegeneration. EMBO J. 2011;30(23):4825–4837. doi: 10.1038/emboj.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao H, Li Y, Brautigan DL, Gundersen GG. Protein phosphatase 1 is targeted to microtubules by the microtubule-associated protein Tau. J Biol Chem. 1998;273(34):21901–21908. doi: 10.1074/jbc.273.34.21901. [DOI] [PubMed] [Google Scholar]
- 65.Littauer UZ, Giveon D, Thierauf M, Ginzburg I, Ponstingl H. Common and distinct tubulin binding sites for microtubule-associated proteins. Proc Natl Acad Sci USA. 1986;83(19):7162–7166. doi: 10.1073/pnas.83.19.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu H, Ali MY, Bookwalter CS, Warshaw DM, Trybus KM. Diffusive movement of processive kinesin-1 on microtubules. Traffic. 2009;10(10):1429–1438. doi: 10.1111/j.1600-0854.2009.00964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magnani E, Fan J, Gasparini L, Golding M, Williams M, Schiavo G, Goedert M, Amos LA, Spillantini MG. Interaction of tau protein with the dynactin complex. EMBO J. 2007;26(21):4546–4554. doi: 10.1038/sj.emboj.7601878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Makrides V, Massie MR, Feinstein SC, Lew J. Evidence for two distinct binding sites for tau on microtubules. Proc Natl Acad Sci USA. 2004;101(17):6746–6751. doi: 10.1073/pnas.0400992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Makrides V, Shen TE, Bhatia R, Smith BL, Thimm J, Lal R, Feinstein SC. Microtubule-dependent oligomerization of tau. Implications for physiological tau function and tauopathies. J Biol Chem. 2003;278(35):33298–33304. doi: 10.1074/jbc.M305207200. [DOI] [PubMed] [Google Scholar]
- 70.Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012;2(7):a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mandell JW, Banker GA. A spatial gradient of tau protein phosphorylation in nascent axons. J Neurosci. 1996;16(18):5727–5740. doi: 10.1523/JNEUROSCI.16-18-05727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marya PK, Syed Z, Fraylich PE, Eagles PA. Kinesin and tau bind to distinct sites on microtubules. J Cell Sci. 1994;107(Pt 1):339–344. doi: 10.1242/jcs.107.1.339. [DOI] [PubMed] [Google Scholar]
- 73.McVicker DP, Chrin LR, Berger CL. The nucleotide-binding state of microtubules modulates kinesin processivity and the ability of Tau to inhibit kinesin-mediated transport. J Biol Chem. 2011;286(50):42873–42880. doi: 10.1074/jbc.M111.292987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mercken M, Fischer I, Kosik KS, Nixon RA. Three distinct axonal transport rates for tau, tubulin, and other microtubule-associated proteins: evidence for dynamic interactions of tau with microtubules in vivo. J Neurosci. 1995;15(12):8259–8267. doi: 10.1523/JNEUROSCI.15-12-08259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Migheli A, Butler M, Brown K, Shelanski ML. Light and electron microscope localization of the microtubule-associated tau protein in rat brain. J Neurosci. 1988;8(6):1846–1851. doi: 10.1523/JNEUROSCI.08-06-01846.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morfini G, Pigino G, Mizuno N, Kikkawa M, Brady ST. Tau binding to microtubules does not directly affect microtubule-based vesicle motility. J Neurosci Res. 2007;85(12):2620–2630. doi: 10.1002/jnr.21154. [DOI] [PubMed] [Google Scholar]
- 77.Morishima-Kawashima M, Kosik KS. The pool of map kinase associated with microtubules is small but constitutively active. Mol Biol Cell. 1996;7(6):893–905. doi: 10.1091/mbc.7.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70(3):410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morris RL, Hollenbeck PJ. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 1993;104(Pt 3):917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- 80.Mukrasch MD, Bibow S, Korukottu J, Jeganathan S, Biernat J, Griesinger C, Mandelkow E, Zweckstetter M. Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol. 2009;7(2):e34. doi: 10.1371/journal.pbio.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakata T, Sato-Yoshitake R, Okada Y, Noda Y, Hirokawa N. Thermal drift is enough to drive a single microtubule along its axis even in the absence of motor proteins. Biophys J. 1993;65(6):2504–2510. doi: 10.1016/S0006-3495(93)81304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neve RL, Harris P, Kosik KS, Kurnit DM, Donlon TA. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986;387(3):271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- 83.Panda D, Goode BL, Feinstein SC, Wilson L. Kinetic stabilization of microtubule dynamics at steady state by tau and microtubule-binding domains of tau. Biochemistry. 1995;34(35):11117–11127. doi: 10.1021/bi00035a017. [DOI] [PubMed] [Google Scholar]
- 84.Peck A, Sargin ME, LaPointe NE, Rose K, Manjunath BS, Feinstein SC, Wilson L. Tau isoform-specific modulation of kinesin-driven microtubule gliding rates and trajectories as determined with tau-stabilized microtubules. Cytoskeleton. 2011;68(1):44–55. doi: 10.1002/cm.20494. [DOI] [PubMed] [Google Scholar]
- 85.Perez M, Santa-Maria I, Gomez de Barreda E, Zhu X, Cuadros R, Cabrero JR, Sanchez-Madrid F, Dawson HN, Vitek MP, Perry G, Smith MA, Avila J. Tau–an inhibitor of deacetylase HDAC6 function. J Neurochem. 2009;109(6):1756–1766. doi: 10.1111/j.1471-4159.2009.06102.x. [DOI] [PubMed] [Google Scholar]
- 86.Pfister KK. Cytoplasmic dynein and microtubule transport in the axon: the action connection. Mol Neurobiol. 1999;20(2–3):81–91. doi: 10.1007/BF02742435. [DOI] [PubMed] [Google Scholar]
- 87.Preuss U, Biernat J, Mandelkow EM, Mandelkow E. The ‘jaws’ model of tau-microtubule interaction examined in CHO cells. J Cell Sci. 1997;110(Pt 6):789–800. doi: 10.1242/jcs.110.6.789. [DOI] [PubMed] [Google Scholar]
- 88.Rodionov VI, Gyoeva FK, Kashina AS, Kuznetsov SA, Gelfand VI. Microtubule-associated proteins and microtubule-based translocators have different binding sites on tubulin molecule. J Biol Chem. 1990;265(10):5702–5707. [PubMed] [Google Scholar]
- 89.Rodriguez-Martin T, Cuchillo-Ibanez I, Noble W, Nyenya F, Anderton BH, Hanger DP. Tau phosphorylation affects its axonal transport and degradation. Neurobiol Aging. 2013;34(9):2146–2157. doi: 10.1016/j.neurobiolaging.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sabry J, O’Connor TP, Kirschner MW. Axonal transport of tubulin in Ti1 pioneer neurons in situ. Neuron. 1995;14(6):1247–1256. doi: 10.1016/0896-6273(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 91.Samsonov A, Yu JZ, Rasenick M, Popov SV. Tau interaction with microtubules in vivo. J Cell Sci. 2004;117(Pt 25):6129–6141. doi: 10.1242/jcs.01531. [DOI] [PubMed] [Google Scholar]
- 92.Santarella RA, Skiniotis G, Goldie KN, Tittmann P, Gross H, Mandelkow EM, Mandelkow E, Hoenger A. Surface-decoration of microtubules by human tau. J Mol Biol. 2004;339(3):539–553. doi: 10.1016/j.jmb.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 93.Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J Cell Sci. 2012;125(Pt 9):2095–2104. doi: 10.1242/jcs.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schaap IA, Hoffmann B, Carrasco C, Merkel R, Schmidt CF. Tau protein binding forms a 1 nm thick layer along protofilaments without affecting the radial elasticity of microtubules. J Struct Biol. 2007;158(3):282–292. doi: 10.1016/j.jsb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 95.Schweers O, Schonbrunn-Hanebeck E, Marx A, Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J Biol Chem. 1994;269(39):24290–24297. [PubMed] [Google Scholar]
- 96.Seitz A, Kojima H, Oiwa K, Mandelkow EM, Song YH, Mandelkow E. Single-molecule investigation of the interference between kinesin, tau and MAP2c. EMBO J. 2002;21(18):4896–4905. doi: 10.1093/emboj/cdf503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Serrano L, Montejo de Garcini E, Hernandez MA, Avila J. Localization of the tubulin binding site for tau protein. Eur J Biochem. 1985;153(3):595–600. doi: 10.1111/j.1432-1033.1985.tb09342.x. [DOI] [PubMed] [Google Scholar]
- 98.Shahani N, Brandt R. Functions and malfunctions of the tau proteins. Cell Mol Life Sci. 2002;59(10):1668–1680. doi: 10.1007/PL00012495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharp DJ, Kuriyama R, Essner R, Baas PW. Expression of a minus-end-directed motor protein induces Sf9 cells to form axon-like processes with uniform microtubule polarity orientation. J Cell Sci. 1997;110(Pt 19):2373–2380. doi: 10.1242/jcs.110.19.2373. [DOI] [PubMed] [Google Scholar]
- 100.Sontag E, Nunbhakdi-Craig V, Lee G, Brandt R, Kamibayashi C, Kuret J, White CL, 3rd, Mumby MC, Bloom GS. Molecular interactions among protein phosphatase 2A, tau, and microtubules. Implications for the regulation of tau phosphorylation and the development of tauopathies. J Biol Chem. 1999;274(36):25490–25498. doi: 10.1074/jbc.274.36.25490. [DOI] [PubMed] [Google Scholar]
- 101.Sotelo-Silveira JR, Calliari A, Kun A, Koenig E, Sotelo JR. RNA trafficking in axons. Traffic. 2006;7(5):508–515. doi: 10.1111/j.1600-0854.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 102.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol. 2002;156(6):1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stoothoff W, Jones PB, Spires-Jones TL, Joyner D, Chhabra E, Bercury K, Fan Z, Xie H, Bacskai B, Edd J, Irimia D, Hyman BT. Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J Neurochem. 2009;111(2):417–427. doi: 10.1111/j.1471-4159.2009.06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stoothoff WH, Johnson GV. Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta. 2005;1739(2–3):280–297. doi: 10.1016/j.bbadis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 105.Tang-Schomer MD, Johnson VE, Baas PW, Stewart W, Smith DH. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol. 2012;233(1):364–372. doi: 10.1016/j.expneurol.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Terwel D, Dewachter I, Van Leuven F. Axonal transport, tau protein, and neurodegeneration in Alzheimer’s disease. Neuromolecular Med. 2002;2(2):151–165. doi: 10.1385/NMM:2:2:151. [DOI] [PubMed] [Google Scholar]
- 107.Thies E, Mandelkow EM. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J Neurosci. 2007;27(11):2896–2907. doi: 10.1523/JNEUROSCI.4674-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tien NW, Wu GH, Hsu CC, Chang CY, Wagner OI. Tau/PTL-1 associates with kinesin-3 KIF1A/UNC-104 and affects the motor’s motility characteristics in C. elegans neurons. Neurobiol Dis. 2011;43(2):495–506. doi: 10.1016/j.nbd.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 109.Trinczek B, Ebneth A, Mandelkow EM, Mandelkow E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J Cell Sci. 1999;112(Pt 14):2355–2367. doi: 10.1242/jcs.112.14.2355. [DOI] [PubMed] [Google Scholar]
- 110.Utton MA, Connell J, Asuni AA, van Slegtenhorst M, Hutton M, de Silva R, Lees AJ, Miller CC, Anderton BH. The slow axonal transport of the microtubule-associated protein tau and the transport rates of different isoforms and mutants in cultured neurons. J Neurosci. 2002;22(15):6394–6400. doi: 10.1523/JNEUROSCI.22-15-06394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Utton MA, Noble WJ, Hill JE, Anderton BH, Hanger DP. Molecular motors implicated in the axonal transport of tau and alpha-synuclein. J Cell Sci. 2005;118(Pt 20):4645–4654. doi: 10.1242/jcs.02558. [DOI] [PubMed] [Google Scholar]
- 112.Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP. Multiple-motor based transport and its regulation by Tau. Proc Natl Acad Sci USA. 2007;104(1):87–92. doi: 10.1073/pnas.0607919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vershinin M, Xu J, Razafsky DS, King SJ, Gross SP. Tuning microtubule-based transport through filamentous MAPs: the problem of dynein. Traffic. 2008;9(6):882–892. doi: 10.1111/j.1600-0854.2008.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.von Bergen M, Barghorn S, Muller SA, Pickhardt M, Biernat J, Mandelkow EM, Davies P, Aebi U, Mandelkow E. The core of tau-paired helical filaments studied by scanning transmission electron microscopy and limited proteolysis. Biochemistry. 2006;45(20):6446–6457. doi: 10.1021/bi052530j. [DOI] [PubMed] [Google Scholar]
- 115.von Hippel PH, Berg OG. Facilitated target location in biological systems. J Biol Chem. 1989;264(2):675–678. [PubMed] [Google Scholar]
- 116.Wang L, Brown A. Rapid movement of microtubules in axons. Curr Biol. 2002;12(17):1496–1501. doi: 10.1016/s0960-9822(02)01078-3. [DOI] [PubMed] [Google Scholar]
- 117.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA. 1975;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weissmann C, Reyher HJ, Gauthier A, Steinhoff HJ, Junge W, Brandt R. Microtubule binding and trapping at the tip of neurites regulate tau motion in living neurons. Traffic. 2009;10(11):1655–1668. doi: 10.1111/j.1600-0854.2009.00977.x. [DOI] [PubMed] [Google Scholar]
- 119.Wood JG, Mirra SS, Pollock NJ, Binder LI. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci USA. 1986;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zempel H, Luedtke J, Kumar Y, Biernat J, Dawson H, Mandelkow E, Mandelkow EM. Amyloid-beta oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 2013;32(22):2920–2937. doi: 10.1038/emboj.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2 +) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30(36):11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang B, Higuchi M, Yoshiyama Y, Ishihara T, Forman MS, Martinez D, Joyce S, Trojanowski JQ, Lee VM. Retarded axonal transport of R406 W mutant tau in transgenic mice with a neurodegenerative tauopathy. J Neurosci. 2004;24(19):4657–4667. doi: 10.1523/JNEUROSCI.0797-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]