Abstract
Recognized as a “disease modifier”, physical activity (PA) is increasingly viewed as a more holistic, cost-saving method for prevention, treatment and management of human disease conditions. The traditional view that PA engages the monoaminergic and endorphinergic systems has been challenged by the discovery of the endocannabinoid system (ECS), composed of endogenous lipids, their target receptors, and metabolic enzymes. Indeed, direct and indirect evidence suggests that the ECS might mediate some of the PA-triggered effects throughout the body. Moreover, it is now emerging that PA itself is able to modulate ECS in different ways. Against this background, in the present review we shall discuss evidence of the cross-talk between PA and the ECS, ranging from brain to peripheral districts and highlighting how ECS must be tightly regulated during PA, in order to maintain its beneficial effects on cognition, mood, and nociception, while avoiding impaired energy metabolism, oxidative stress, and inflammatory processes.
Keywords: Adaptive responses, Endocannabinoids, Exercise, Health benefit, Physical activity
Physical activity (PA) at a glance
Physical activity (PA) is widely defined as any movement produced by skeletal muscles that results in energy expenditure, while physical exercise is a subset of PA that is structured, planned, and repetitive [1]. In humans, PA can be done in multiple ways that differ for type, intensity, frequency, and duration. For instance, aerobic exercise involves dynamic activity of large groups of muscles, while resistance exercise specifically increases muscular strength, power, and endurance by using smaller groups of muscles [1]. Also, the intensity of the exercise protocol is important: fatty acids represent the main source of energy at low and moderate intensities, while at high intensities, glycogen and phosphocreatine become the main fuels [1, 2].
As discussed in more detail in the next sections, a correctly performed PA improves cognitive performance [3], and affects the brain reward system [4] by inducing a sense of satisfaction. By modulating several signaling mediators, exercise influences mood [3] and nociception [5], modulates immune system functions [6], as well as whole-body energy metabolism [7]. These features contribute to the concept that PA is not only important for a healthy everyday life, but may also help to prevent and even treat chronic diseases that afflict modern society because of a sedentary lifestyle [8]. In particular, the beneficial effect of physical exercise has been clearly documented under different pathophysiological conditions affecting the central nervous system (CNS) [8], as well as peripheral tissues [9–11].
It seems noteworthy that exercise can become harmful in the case of unaccustomed, vigorous, or eccentric muscle actions that overstretch and hence, disrupt sarcomeres, and induce oxidative stress and inflammatory responses [12]. Although the body has been shown to adapt to harmful exercises [12], damaging activities can clearly promote or aggravate pathological conditions [12, 13].
The endocannabinoid system (ECS)
Endocannabinoids (eCBs)
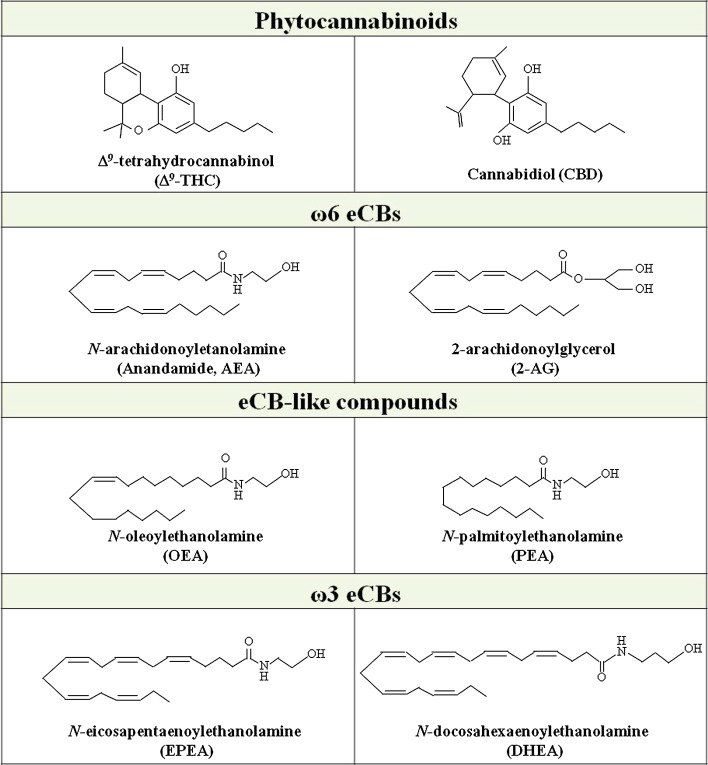
In the early 80s, several authors investigated the biological background of exercise-wide effects. Early hypotheses relied on the well-documented antidepressant and anxiolytic effect of PA [14], and pointed to a possible role of monoamines [15] and endorphines [16] in exercise neurochemistry. In the last decade, the evidence that PA induces some of the psychotropic effects elicited by the Cannabis sativa active ingredient Δ9-tetrahydrocannabinol (Δ9-THC, Fig. 1), like bliss, euphoria, and peacefulness, strengthened the hypothesis that endocannabinoids (eCBs) might mediate, at least in part, the central and peripheral effects of exercise [14].
Fig. 1.
Chemical structure of the main plant-derived and endogenous cannabinoids
The eCBs are lipid mediators including esters, amides, and ethers of arachidonic acid [the most active ω-6 polyunsaturated fatty acid (PUFA) in our body], which mimic the effects of Δ9-THC primarily by binding to and activating type-1 (CB1) and type-2 (CB2) cannabinoid receptors [17, 18]. The most studied eCBs are N-arachidonoylethanolamine (anandamide, AEA), an N-acylethanolamine (NAE) [19], and 2-arachidonoylglycerol (2-AG), a monoacylglycerol [20, 21] (Fig. 1). The NAE family also includes the appetite-suppressor N-oleoylethanolamine (OEA) and the anti-inflammatory and anti-proliferative N-palmitoylethanolamine (PEA) (Fig. 1) [22]. Both OEA and PEA are considered eCB-like compounds rather than authentic eCBs, because they usually do not bind to CB1 or CB2, but have some cannabimimetic or non-cannabimimetic actions through the activation of other molecular targets (see below) [22].
The eCB family also includes other bioactive members, like 2-arachidonoylglycerol ether (noladin ether) [23], O-arachidonoylethanolamine (virodhamine) [24] and N-arachidonoyldopamine (NADA) [25]. More recently, both N-docosahexaenoylethanolamine (DHEA) and N-eicosapentaenoylethanolamine (EPEA) (derived from the dietary ω-3 PUFA docosahexaenoic acid and eicosapentaenoic acid, respectively) (Fig. 1) have been shown to activate both CB1 and CB2 [26]. Although a clear understanding of the biological and pathophysiological relevance in vivo of these “ω-3 eCBs” is still missing, they are receiving growing attention as potential anti-proliferative agents [27].
eCB-binding proteins
AEA and 2-AG activate different signaling pathways depending on the specific receptor engaged [17, 18]. CB1 and CB2 are G-protein coupled receptors (GPCRs) that represent the main targets of eCBs [17, 18]. CB1 is highly expressed in brain areas that control emotionality, cognition, memory, motor, and nociception, and that include cortex, limbic system, hippocampus, cerebellum, and several nuclei of the basal ganglia. CB1 is also expressed in peripheral cells and tissues, including adipose tissue, liver, and skeletal muscle [17, 18]. CB2 is mainly present in the immune system [28], but is also expressed within the CNS [29], where it might play a relevant role in coping with insults [30].
Both CB1 and CB2 are coupled to Gi/o proteins, thus inhibiting adenylyl cyclase and stimulating different members of the mitogen-activated protein kinase (MAPK) family [17, 18]. CB1 can also activate A-type and inwardly rectifying K+ currents and inhibit N- and P/Q-type calcium currents [17, 18]. In addition, CB1 has been recently shown to stimulate Gs proteins [18], and to be modulated by specific accessory proteins [17].
Accumulated experimental evidence indicates that endogenous, plant-derived (like Δ9-THC and the non-psychoactive substance cannabidiol, CBD) (Fig. 1) and synthetic cannabinoids (synthocannabinoids) can activate the G protein-coupled receptor 55 (GPR55) [31, 32]. This orphan receptor shares low sequence homology with CB1 and CB2, is coupled to Gαq and Gα12/13, and has been proposed as a possible “CB3”. It regulates several biological processes both in the CNS and peripherally, by activating small GTPases (like Rho) and distinct MAPK kinases [31, 32].
In addition to CB receptors, AEA but not 2-AG binds to transient receptor potential vanilloid type 1 (TRPV1) Ca2+ channels, highly expressed in a subset of primary sensory neurons, both centrally and peripherally, and in various non-neuronal cells [33]. By triggering signaling cascades associated with Ca2+ homeostasis, the AEA-TRPV1 interaction (that occurs at the inner side of the plasma membrane) leads to the control of CNS functions including nociception [34], and of basic biological processes like the induction of apoptosis [35, 36].
Finally, both AEA and 2-AG bind (directly or indirectly) to nuclear peroxisome proliferator-activated receptors (PPARs) α and γ, thus regulating lipid and glucose metabolism [37, 38], as well as inflammatory responses [39].
Concerning eCB-like compounds, both OEA and PEA seem to act through the activation of PPAR-α, thus either regulating feeding and body weight (OEA) or reducing inflammatory responses (PEA) [22, 40]. However, some effects of PEA might also depend on the activation of CB receptors, as well as of TRPV1 [22, 40], thus suggesting that eCB-like compounds deserve further investigation.
eCB metabolic enzymes
eCBs are produced in response to numerous stimuli (i.e., neuronal activity, glucocorticoids, insulin, and cytokines) [41–45] by a variety of cells throughout the body, from brain [41] to peripheral tissues like adipose tissue [38], muscles [45], heart, kidney [41], and immune cells [46, 47], just to name a few.
Indeed the biological activity of eCBs is tightly regulated by their metabolism [48]. The main biosynthetic enzyme of AEA is a Ca2+-dependent N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD) [40], which is specific for NAEs and releases AEA from N-arachidonoylphosphatidylethanolamine (NArPE). Additional routes allow AEA biosynthesis [41]. For instance, a tyrosine phosphatase can release AEA from phospho-AEA, which in turn derives from NArPE through the action of a phospholipase C [49]. Moreover, the finding that mice lacking the NAPE-PLD gene have unaltered NAE levels in their brain allowed researchers to demonstrate that NAE biosynthesis also occurs through the sequential action of the α–β hydrolase enzyme ABH4, and of glycerophosphodiesterase 1 [50]. AEA is inactivated by a two-step process: cellular uptake and intracellular degradation. As yet, several hypotheses have been made to explain AEA transport across plasma membrane, including passive diffusion, carrier-mediated bidirectional transport through a specific eCB membrane transporter (EMT), and caveolae-related endocytosis [51, 52]. Moreover, it has recently been shown that AEA, once transported inside the cell, is shuttled to its final destinations by intracellular transporters, like fatty acid-binding proteins 5 and 7, heat-shock protein 70, albumin, and fatty acid amide hydrolase (FAAH)-like transporter [48, and references therein]. In this context, a relevant role is played by adiposomes that are intracellular lipid droplets representing a dynamic reservoir for AEA sequestration and a platform for accumulation, trafficking, metabolism, and signaling of this eCB [48, 53]. The most relevant degradative enzyme of AEA is the serine hydrolase FAAH (now called FAAH-1) that hydrolyzes it into arachidonic acid and ethanolamine [54, 55]. Additionally, AEA hydrolysis can be catalyzed by a newly discovered FAAH-2 [56], or by a lysosomal N-acylethanolamine-hydrolyzing acid amidase [41]. In this context, it should be recalled that both OEA and PEA share, at least in part, the catabolic routes of AEA [22, 40]. Therefore, they might potentiate the activity of true eCBs by inhibiting their degradation, through the so-called “entourage effect” [22].
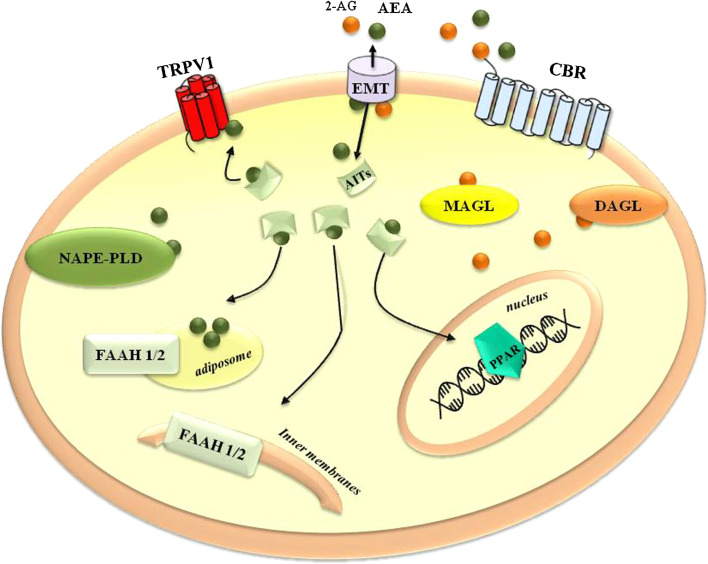
Also the biosynthetic pathway of 2-AG begins with the hydrolysis of its phospolipid precursors, by the means of phospholipase C or phosphatidic acid phosphohydrolase that generate diacylglycerol [41]. The latter is then converted to 2-AG by sn-1-diacylglycerol lipases (DAGLs) α and β, which are abundant in adult and developing nervous tissues, respectively [57]. After its cellular uptake, most likely through the same EMT that transports AEA, 2-AG is hydrolyzed by monoacylglycerol lipase (MAGL) [58], although FAAH, and more interestingly, other lipases like αβ-hydrolases 6 and 12 [59, 60], can contribute to its degradation. A schematic representation of the ECS is reported in Fig. 2.
Fig. 2.
Schematic representation of the ECS. 2-AG 2-arachidonoylglycerol, AEA anandamide, AITs anandamide intracellular transporters, CBR cannabinoid receptors, DAGL diacylglycerol lipase, EMT endocannabinoid membrane transporter, FAAH-1/2 fatty acid amide hydrolase 1 and 2, MAGL monoacylglycerol lipase, NAPE-PLD N-acylphosphatidylethanolamine-hydrolyzing phospholipase D, PPAR nuclear peroxisome proliferator-activated receptor, TRPV1 transient receptor potential vanilloid type 1. See text for further details
Although the primary fate of AEA and 2-AG seems to be their inactivation via hydrolysis, they might also be oxidized by cyclooxygenase-2 and lipoxygenase isozymes, thus producing oxidized eCBs that are involved in regulating brain synaptic transmission and other biological processes [61, 62].
Changes in the ECS during PA
To our knowledge, the first experimental study aimed at investigating the influence of PA on ECS in humans was carried out in 2003 by Sparling and coworkers [63], who showed increased plasma AEA content after 45 min of moderate intensity exercise on a treadmill or cycle ergometer. Since then, other human studies have shown increased blood concentrations of AEA, but interestingly not of 2-AG, after 30–45 min and up to 5 h of aerobic exercise [64–67]. Of note, the eCB-like compounds PEA and OEA have been shown to parallel the changes of AEA during 60 min of moderate aerobic exercise, immediately followed by 30 min of intense exercise, reaching a peak during the subsequent 15 min of recovery [65].
A dependence of the increase of AEA concentration on exercise intensity has also been documented. Plasma levels of AEA significantly increased upon 30 min of moderate exercise (heart rate of 72 and 83 %), but not at lower and significantly higher exercise intensities, where the age-adjusted maximal heart rate was 44 and 92 %, respectively [67]. Finally, AEA levels were enhanced by combining exercise with mild hypobaric and hypoxic conditions due to high altitude [64].
Moreover, our group has recently shown that an active lifestyle upregulates lymphocyte FAAH activity through an interleukin (IL)-6-dependent stimulation of the FAAH gene promoter [42]. Finally, two additional studies have investigated the effects of human chronic exercise combined with dietary restriction on ECS. In the first study, a 1-year life intervention was able to lower AEA and 2-AG plasma levels in viscerally obese men, where an overstimulated ECS is known to promote weight gain and metabolic impairment [68]. The second study demonstrated that the expression of the FAAH-encoding gene was lowered in abdominal adipose tissue of overweight or obese women, who followed concomitant aerobic and diet programs [69].
Animal studies support these investigations, confirming that eCBs are also modulated in the CNS [70, 71]. For instance, 8 days of voluntary exercise is able to increase AEA levels, CB1 density, and activity in rat hippocampus [70]. A very recent study has reported that in rats, acute aerobic exercise [a single bout on a rodent treadmill, until fatigue (≈50 min), after 3 days of training] triggers eCB-dependent analgesic effects at central and peripheral levels [71]. The latter is the first study to report increased 2-AG levels after a moderate acute aerobic exercise protocol, and is among the few articles indicating a positive peripheral effect of eCBs. Indeed, that eCBs increase during exercise nicely supports the belief in positive effects of PA on the CNS, but not on peripheral tissues (see below).
It seems of particular interest that short exercise protocols are accompanied by the upregulation of eCB signaling in murine striatum [72], whereas longer training is paralleled by reduced CB1 expression in the striatum and hippocampus of adolescent rats [73], and adipose tissue of rats on a high-fat diet (HFD) [74]. Similarly, acute aerobic exercise affects plasma levels of AEA, but usually not of 2-AG [63–67], which is instead affected by chronic exercise only when combined with caloric restriction [68]. Altogether, these results seem to suggest that ECS not only activates different pathways depending on exercise length, but can even adapt to a physically active lifestyle [42]. This hypothesis is further supported by a transversal study conducted on humans and dogs (two cursorial species), and ferrets (a non-cursorial species). AEA, but not 2-AG, levels were found to increase in humans who ran for 30 min at moderate intensity, and in dogs that walked or ran for the same time and at the same intensity; in contrast, no changes were reported in ferrets under the same experimental conditions [66]. Humans and dogs have evolved a behavior suitable for running with a neurobiological motivational reward, and hence, a reduction in anxiety and pain, in order to improve their fitness condition; instead, ferrets are not adapted to long-distance running at high speeds, but rather use high accelerations over short distances [66]. In the light of these evolutionary differences, the available data appear supportive of a role for eCBs in PA of those mammals that are “made for” endurance exercise [66].
Implications of the ECS in motor activity: from brain to muscle
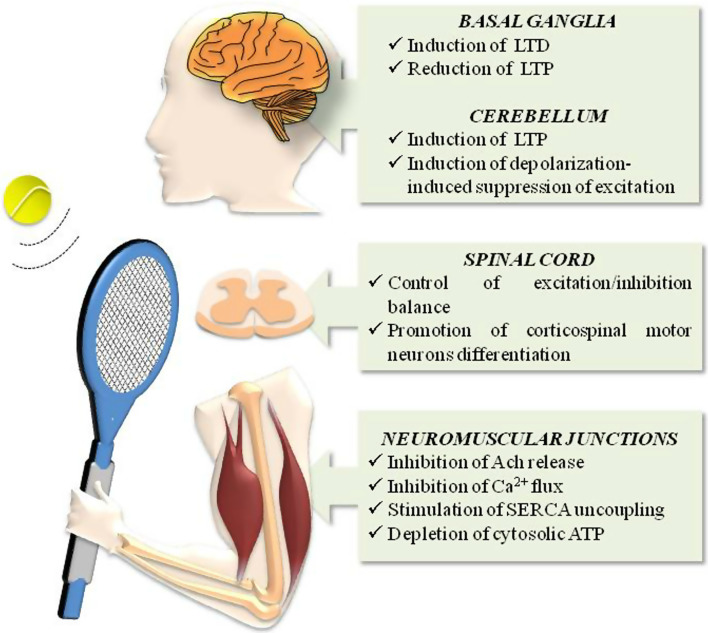
Every movement, whether simple or not, is based on a complex network of connections extending from different brain areas to muscles [75] and that, as schematically depicted in Fig. 3, have been shown to be influenced by the ECS. Solid experimental data, for instance, indicate that the ECS controls motor activity by interacting with the dopaminergic system in the basal ganglia network. Here, (1) CB1 co-localizes with both type-1 and type-2 dopamine (D1 and D2) receptors [18]; (2) AEA production is enhanced by D2 receptor activation [43], thus inducing a process known as long-term depression (LTD) in high-frequency-stimulated striatal slices; and (3) a CB1 blockade leads to long-term potentiation (LTP) expression [76].
Fig. 3.
Schematic representation of the main effects triggered by ECS in body areas involved in locomotor activity. ACh acetylcholine, LTD long-term depression, LTP long-term potentiation, SERCA sarcoplasmic reticulum Ca2+-ATPase. See text for further details
In the cerebellum, synaptic activation of Purkinje cells triggers the release of 2-AG that retrogradely mediates LTP and depolarization-induced suppression of excitation, thus contributing to the coordination, precision, and accurate timing of movement [77].
The ECS, and particularly CB1, is also implicated in the regulation of contraction frequency. In the isolated lamprey spinal cord, eCBs released upon type-1 metabotropic glutamate receptor (mGluR1) activation induce LTD during rhythmic locomotion, in a CB1-dependent manner [78]. More recently, it has been reported that 2-AG is mobilized from neurons of the spinal network during locomotor activity, and acts via CB1 in synergy with nitric oxide (NO), to shift the balance between excitation and inhibition, and to potentiate locomotor frequency [79]. Interestingly, CB1 has been shown to positively regulate transcription factors that control corticospinal motor neuron differentiation [80]. Accordingly, CB−/−1 mice show alterations in corticospinal motor neuron generation and subcerebral connectivity that cause defective skilled motor function [80].
Studies on vertebrate striated neuromuscular junctions have shown that activation of CB1 in nerve terminals (where the receptor is highly expressed) inhibits acetylcholine release [81]; similarly, in isolated fast and slow muscles of frog, CB1 has been reported to reduce tension evoked by caffeine, which is known to act on sarcoplasmic calcium release [82]. In this context, it should be stressed that calcium handling and energy supply are important events for movement. Indeed, ATP is necessary for muscle contraction, and also for key calcium-handling proteins like sarcoplasmic reticulum Ca2+-ATPase (SERCA), whose uncoupling causes muscle fatigue [83, 84]. NADA stimulates SERCA uncoupling with subsequent cytoplasmic ATP depletion [85], while both AEA and AM404 (an EMT inhibitor) directly inhibit Ca2+ flux in transverse tubule membrane vesicles derived from rabbit skeletal muscle cells [86, 87].
Concerning the general effects of systemic administration of (e)CBs, it is known that the locomotor activity of mice and rats is enhanced by AEA and other CB1 agonists in some studies [88, 89], but is decreased in others [90–92]. Moreover, the finding that both the activation and the blockade of CB1 decreased running activity in mice selectively bred for high voluntary wheel running, as well as in their wild-type littermates [93, 94], further confirms that the effects of ECS on movement not only depend on variables in the experimental paradigm (e.g., strain, sex, age, and locomotor measurements), but also on the intrinsic complexity of the system per se.
Interplay between PA and ECS in the CNS
Several experimental data support the hypothesis that ECS might, at least in part, explain PA effects on brain functions, because: (1) CB1 is the most abundant GPCR in the brain participating in neuronal plasticity [18]; (2) eCBs are involved in several brain responses that greatly overlap with the positive effects of exercise; (3) eCBs are able to cross the blood–brain barrier [95]; and (4) exercise increases eCB plasma levels [64–67].
Cognition
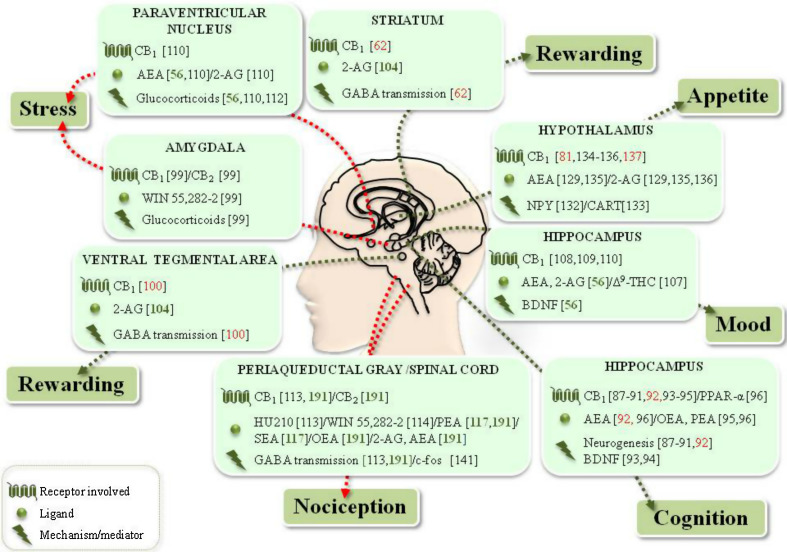
Physical activity improves brain plasticity and cognition by increasing hippocampal neurogenesis and cell proliferation, as well as dendritic length and complexity [3, 96]. Moreover, exercise critically influences the pleiotropic substance and mood controller brain-derived neurotrophic factor (BDNF), responsible for the development, regeneration, survival, and maintenance of neurons [3, 97]. In addition, ECS, and particularly CB1, is involved in the modulation of neurogenesis (Fig. 4). Indeed, neural progenitor cells express high levels of CB1, able to promote in vitro neuroprogenitor differentiation and maturation, via inhibition of the extracellular-signal-regulated kinase 1/2 (ERK1/2) signaling pathway [98]. In vivo, CB1 contributes to embryonic and adult hippocampal neurogenesis [99], which coherently decreases in CB−/−1 mice [100], along with impaired progenitor cell proliferation and astrogliogenesis [101]. It seems noteworthy that increased hippocampal neurogenesis correlates with both high AEA levels and increased CB1 activity in the same brain area [70]. Finally, eCBs positively regulate BDNF activity in the brain: reduced levels of this neurotrophin are detected in the brain of CB−/−1 mice [102], whereas increased BDNF content is found upon CB1 activation [103] (Fig. 4).
Fig. 4.
Schematic representation of the interplay between PA and ECS in the CNS. Red arrows indicate negative effects, green arrows indicate positive effects. Specific references are included for each item. References in red PA/ECS interplay confirmed in the specific brain area, references in green suggested PA/ECS interplay for the indicated function, but not necessarily related to the particular area, references in black ECS effects not yet proven to be related to exercise. See text for further details
An interplay between ECS and PA in neurogenesis has been recently suggested [70]. Daily administration of the CB1 agonist AM251 to rats with free access to a running wheel for 8 days abrogates a voluntary exercise-induced increase in cell proliferation [70] (Fig. 4). Yet, these data seem to be at variance with another study that failed to show any difference in hippocampal neurogenesis between CB−/−1 mice allowed free access to a running wheel for 6 weeks and their wild-type littermates [91]. Nonetheless, these discrepancies might depend on differences in species (rats vs mice), experimental approach (pharmacological vs genetic), and/or duration of running (8 days vs 6 weeks).
Pharmacological elevations of AEA (via inhibition of FAAH activity) enhance learning and memory in rats, in a CB1- and PPAR-α-dependent manner [104, 105] (Fig. 4). In this context, it should be recalled that OEA and PEA are also substrates of FAAH, and that these eCBs-like compounds, though inactive at CB1, bind to and activate PPAR-α [105]. Therefore, it is tempting to speculate that both OEA and PEA might contribute to AEA-induced improvement of hippocampal functions via PPAR-α signaling. On the other hand, other studies failed to confirm such a positive effect of eCBs. Indeed, FAAH and EMT inhibitors (URB597 and AM404, respectively), as well as CB1 agonists (Δ9-THC, HU-210 or R-methanandamide) were found to negatively influence working memory and/or short-term memory of rats [106]. These apparently conflicting data might be explained by recalling that pharmacological approaches might increase the eCBs content above physiological levels, thus affecting other brain processes that, in turn, might reduce performance in cognition tests [107, 108].
Mood
Due to the increased demand for energy, wheel running activates the hypothalamic–pituitary–adrenal (HPA) axis, thus raising circulating levels of glucocorticoids, endocrine signals that change in adaptation to stress [109, 110]. Moreover, acute and chronic exercise influences the activity of BDNF [3, 65], and reduction of the latter substance in the hippocampus (observed, for example, following stress-dependent activation of the HPA axis) critically contributes to stress vulnerability and depression [3]. Accordingly, voluntary running has been found to positively correlate with hippocampal BDNF levels in a rodent model of depression and anxiety [3].
(e)CBs and BDNF might interact in mood control (Fig. 4). For example, Δ9-THC treatment of intact and olfactory bulbectomized rats (an animal model of depression) increased BDNF expression in hippocampus and frontal cortex, thus exerting anti-depressant properties [111] (Fig. 4). Pharmacological inhibition of FAAH elicited anxiolytic-like and antidepressant-like effects in chronically and mildly stressed rats (a behavioral model with high isomorphism to human depression) [112]. Instead, a blockade of CB1 precipitated depression-like symptoms in clinical trial subjects [113].
eCBs have been also shown to regulate the HPA axis (Fig. 4). Glucocorticoids, indeed, quickly increase brain levels of eCBs, which suppress excitatory inputs in the paraventricular nucleus, rapidly shutting down the HPA axis in a CB1-dependent manner [44]. Involvement of the ECS in stress response is also supported by a study performed on a rat model of post-traumatic stress disorder, showing that WIN55,212-2 (injected systemically or in basolateral amygdale) prevents stress-induced effects [108]. Accordingly, CB−/−1 mice exhibit an increased susceptibility and neuroendocrine response to chronic stress [114, 115].
Finally, a recent study enrolling healthy, trained male cyclists showed that intense aerobic PA behaves as a physiological stressor, able to increase plasma AEA levels, that in turn might mediate the neuroplastic and anti-depressant effects of exercise through a BDNF-dependent mechanism [65] (Table 1; Fig. 4).
Table 1.
Effect of PA on the ECS in humans
| Subjects | Exercise protocol | Main finding | Reference |
|---|---|---|---|
| Normal weight male runners (n = 8), cyclists (n = 8), controls (n = 8) | Acute exercise. Running on a treadmill/cycling on a stationary bike for 45 min (HRmax = 70–80 %) | AEA content increases in plasma | [63] |
| Asymptomatic obese men (n = 49) | Chronic exercise. 1-year life intervention (diet plus exercise program, but with no indication on intensity) | Decrease of plasma AEA and 2-AG levels correlates with reduced visceral adipose tissue | [68] |
| Trained healthy volunteers (n = 12) | Acute exercise. A: strenuous hiking below an altitude of 2,100 m; B: strenuous hiking and descent the next day; C: passive ascent with helicopter (no indication on intensity parameter in any protocols) |
Plasma AEA content increases with altitude Plasma 2-AG content does not change |
[64] |
| Overweight/obese women: caloric restriction alone (n = 9) or plus moderate (n = 13) or high intensity (n = 8) exercise | Chronic exercise. 20-week intervention: caloric restriction alone (VO2max or in combination with 3 day/week of moderate (HRmax = 45–50 % for 15–55 min) or vigorous (HRmax = 70–75 % for 15–30 min) intensity exercise | FAAH gene expression decreases in abdominal adipose tissue, if exercise is combined to caloric restriction | [69] |
| Sedentary or minimally active (<60 min/week) females (n = 8) and males (n = 4) | Chronic exercise. 10 interventions during 2 weeks with a 30 min treadmill exercise (HR = 60 %) | Chronic exercise decreases craving for marijuana | [119] |
| Humans (n = 10), mixed-breed dogs (n = 8) and ferrets (n = 8) | Acute exercise. 30 min treadmill running (HRmax = 72 %) or walking (HRmax = 45 %) | Plasma AEA content increases only in cursorial animals | [66] |
| Females (n = 4), males (n = 6) | Acute exercise. 30 min treadmill running at low (HRmax = 44 %), moderate (HRmax = 72 and 83 %), or high (HRmax = 92 %) intensities | Plasma AEA content increases only at moderate intensities | [67] |
| Healthy, trained male cyclists (n = 11) | Acute exercise. Ergometric bicycle: 60 min moderate exercise (55 % of maximal trial power output), followed by 30 min intense exercise (75 % of maximal trial power output) and 15 min recovery | Increased plasma AEA, PEA, and OEA levels correlate with those of BDNF and cortisol | [65] |
| 16 healthy males: sedentary (n = 8) and active, regularly practicing running, swimming, cycling for 8.1 ± 1.2 h/week (n = 8) | Chronic exercise. Regular aerobic activity. Samples collected in resting conditions (i.e., no exercise 12 h prior to testing) | FAAH activity is higher in lymphocytes from active subjects | [42] |
HR max maximum heart rate, VO 2max maximal oxygen uptake
Rewarding
Cognitive processes are also influenced by reward circuitry and by dopamine signaling [107], which are crucial in processing environmental rewarding stimuli, in drug addiction, and in voluntary exercise [4, 72, 116]. Expanding on the reward system, it should be recalled that dopamine and eCBs are intimately connected and cooperate in a fine-tuned manner, thus influencing reward due to food, drugs of abuse, and drugs of electrical brain stimulation [117]. Moreover, much like addictive drugs, exercise activates dopamine transmission [4]; consequently, it might reduce drug abuse-dependence by counteracting positive-reinforcing effects produced by cocaine and marijuana [118, 119] (Table 1).
It seems apparent that the reinforcing properties of exercise are mediated by the ECS in mouse striatum, where voluntary exercise alters sensitivity of CB1 in controlling γ-aminobutyric acid (GABA) transmission (Fig. 4) [72]. Indeed, the synaptic response to the selective CB1 agonist HU210 was normal after a single day of exposure to a running wheel or to sucrose consumption, but it was potentiated after 7 days of treatment. Remarkably, GABA functions slowly returned to normal after treatment discontinuation [72], thus confirming that the GABA receptor adapts to CB1 signaling in response to running activity. In keeping with these data, conditional deletion of CB1 selectively associated with GABA neurons leads to decreased wheel-running performance in mice [116]. Moreover, negative effects of intra-ventral tegmental area (VTA) administration of CB1 antagonists on voluntary exercise were lost in CB−/−1GABA mice, thus indicating that VTA dopaminergic activity is also controlled by eCBs during voluntary exercise [116] (Fig. 4).
Finally, in obese Zucker rats that were allowed to press a lever in order to reach a running wheel, 2-AG was seen to decrease the rewarding effect of exercise in obese rats in a dose-dependent manner, by modulating both lever press and revolution on the running wheel [120] (Fig. 4).
Pain
Pain reduction during exercise is crucial to improving performance and allowing individuals to continue exercise. The mechanism of analgesia associated with exercise is not yet fully understood [5]; nonetheless, several pieces of data suggest that ECS might be involved in exercise-mediated analgesic effects (Fig. 4). eCBs and their molecular targets are, indeed, present in pain-related regions [34], where they have been shown to exert analgesic properties by the retrograde activation of CB1 [121, 122]. Such an effect is potentiated by the pharmacological or genetic inactivation of FAAH [123, 124]. Coherently, levels of the two eCB congeners, PEA and N-stearoylethanolamine (both substrates of FAAH), have been found to increase after low-force exercise in women with chronic widespread or neck/shoulder pain, in association with reduced pain intensity [125].
Since eCBs are lipids that are able to readily cross the blood–brain barrier [95], it is conceivable that increased eCB concentrations in blood, observed during exercise, might lead to central reduction in pain perception, thus possibly contributing to an immediate feeling of well-being. Indeed, acute aerobic exercise has been demonstrated to increase plasma levels of AEA, 2-AG, PEA, and OEA in rats. Systemic and intrathecal injections of specific antagonists of CB1 and CB2, as well inhibitors of eCB transport and hydrolysis, showed that eCBs and their congeners contribute to exercise analgesic effects, either centrally or peripherally. Of note, the wide pharmacological approach used in this study was able to indicate a likely CB1 and CB2 involvement in the eCB anti-nociceptive effects during this exercise protocol [71], a fact that should be further explored in the future in order to deepen our knowledge on exercise and ECS.
Appetite
Exercise can raise energy expenditure several fold, so that compensatory mechanisms are activated at both central (i.e., in hypothalamus) and peripheral levels, with an impact on food intake [126].
High-intensity training increases the orexigenic neuropeptide Y (NPY) [127, 128]; similarly, acute exercise protocol increases agouti-related protein (AGRP) plasma levels [128, 129], while the anorexigenic pro-opiomelanocortin and cocaine- and amphetamine-regulated transcript (CART) are reduced [128].
Physical activity also influences release of peripheral orexigenic/anorexigenic mediators that enable communication among different energy-related tissues and the brain. In particular, exercise decreases the release of anorexigenic leptin [130, 131], while raising circulating levels of orexigenic adiponectin (both deriving from adipocytes) [131]. The orexigenic hormone ghrelin (primarily secreted from stomach) seems less responsive, although some studies reported an exercise-dependent decrease [130]. Nevertheless, most of these energy signals are strongly dependent on exercise type, duration, and intensity [132]. Leptin release, indeed, is affected by long-term exercise, while adiponectin levels are influenced by short-term exercise [132]. Likewise, acute exercise elevates post-prandial concentrations of peptide YY (a gut anorexigenic hormone), but not of ghrelin [133], while resistance exercise decreases ghrelin levels, but not peptide YY content [134].
eCBs influence feeding behavior by mainly acting on the hypothalamus, thus contributing to energy homeostasis modulation [for complete reviews, see Refs 45, 135]. 2-AG and AEA, indeed, increase feeding behavior in rodents [136] (Fig. 4). Their levels, reduced by leptin [137] and enhanced by ghrelin and glucocorticoids [44, 138], significantly increase in the hypothalamus during fasting, thus returning to basal levels after feeding [45]. Although levels of AEA are less sensitive to food restriction [45], this eCB increases NPY release in vitro [139]. Finally, FAAH−/− mice have reduced levels of CART in appetite-related hypothalamus areas [140]. To date, it is clear that (e)CB effects on feeding are CB1-dependent. Indeed, CB−/−1 (much like FAAH−/−) mice are lean, hypophagic, and resistant to diet-induced obesity [141, 142], while CART-deficient mice are resistant to inhibition of food intake dependent on rimonabant (a CB1 antagonist) [140]. Genetically and diet-induced obese mice have recently been reported to have more CB1-expressing inhibitory inputs and 2-AG biosynthetic enzyme DAGL in the lateral hypothalamus, compared with lean littermates, and such alterations are reverted to lean mice conditions upon leptin administration [143].
Interestingly, the influence of wheel running (over a 6-week period) and genotype (CB−/− vs CB+/+ mice) on food intake has been documented [91]; accordingly, voluntary exercise on a running wheel enhances the CB1 inverse agonist-dependent reduction of appetite in mice after 1 day of treatment [144].
Interplay between PA and the ECS in peripheral tissues
Peripheral energy homeostasis
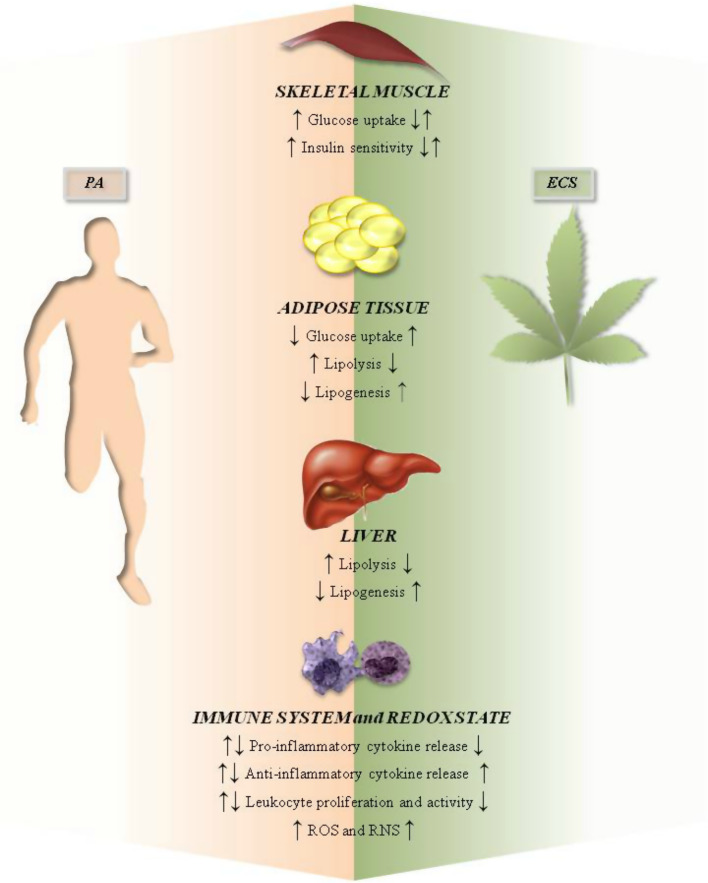
Due to increased energy demand during exercise, PA induces the metabolic remodeling of several tissues that contain lipid and carbohydrate stores, and thus it exerts a general positive effect on peripheral energy homeostasis. Indeed, PA improves insulin sensitivity and metabolic flexibility in muscles [145, 146], diminishes accumulation of lipids in liver [147, 148], and increases lipolysis in adipose tissue by: (1) activating the sympathetic nervous system; (2) increasing adipose tissue blood flow; and (3) stimulating fatty acid oxidation [149] (Fig. 5).
Fig. 5.
Schematic representation of effects of PA and the ECS at the peripheral level. ROS reactive oxygen species, RNS reactive nitrogen species. ↑: increase; ↓:decrease; ↑↓: increase or decrease depending on exercise intensity, type, and duration (when referred to PA), and on activated pathways (when referred to ECS). See text for further details
Conversely, eCB tone mainly exerts a negative influence on peripheral energy homeostasis [45, 135, 150]. For instance, AEA-treated soleus muscles from lean rats showed decreased basal and insulin-induced glucose uptake [151]. Accordingly, a CB1 blockade in L6 rat myotubes increased glucose uptake through phosphatidylinositol 3-kinase/protein kinase B (Akt) [152] and ERK1/2 kinases [153], without affecting the expression of GLUT1 or GLUT4 glucose transporters [152] (Fig. 5).
To date, no effect has been found on glucose uptake using CB2 or TRPV1 antagonists [152], though it has been reported that CB−/−2 mice, which did not develop diet-induced obesity and insulin resistance, increased insulin-mediated glucose uptake in skeletal muscles [154]. Treatment of primary skeletal muscle cells with AEA or with a pre-adipocyte-conditioned medium inhibited insulin-dependent glucose uptake, decreased Akt phosphorylation, and activated ERK1/2 and p38-MAPKs [155]. Moreover, AEA has been found to further disturb the insulin pathway, by increasing insulin receptor substrate 1 phosphorylation at Ser307, and thus triggering an inhibitory action [155].
Importantly, AEA was also able to induce basal glucose uptake on its own at high concentrations (10 μM), perhaps due to 5′ AMP-activated protein kinase (AMPK) activation [156] (Fig. 5). Indeed, two distinct pools of GLUT4 exist in muscle cells [157] and insulin regulates GLUT4 trafficking principally via the PI3K/Akt pathway; instead, other stimuli like exercise appear to do so (at least in part) via activation of the stress kinase AMPK2 [158]. In line with this, AEA treatment of human primary skeletal muscle myotubes increased AMPK-α mRNA expression [61]. In the same study, AEA (5 μM) was also able to increase the expression of PPAR-γ co-activator-1α, a transcription factor engaged to regulate mitochondrial biogenesis, glucose uptake, and fat oxidation [61]. The latter data might suggest a possible eCB positive effect on muscles, through alternative pathways that could also be targeted by an eCB-like compound, like OEA and PEA. Should this be the case, further studies on eCB-triggered signaling might provide further support to the beneficial effect of an exercise-dependent rise of plasma eCBs.
eCB molecular targets, which are present in several adipose depots, such as visceral and subcutaneous adipose tissues, are linked to energy homeostasis in different ways (Fig. 5). While CB−/−2 mice fed HFD maintain insulin sensitivity and do not show signs of obesity-induced inflammation [154], GPR55 expression is elevated in obese subjects and correlates with visceral adipose tissue; moreover, its activation increases Ca2+ levels and expression of lipogenic enzymes in differentiated adipocytes [159]. CB1 activation stimulates glucose uptake in adipocytes [38], and enhances their endocrine function, by increasing visfatin expression and decreasing adiponectin expression [160]. Once again, these effects appear in contrast with those generally elicited by PA [131, 132]. Finally, rimonabant treatment in HFD rats produces profound metabolic remodeling of adipocytes, and hence it counteracts fat accumulation by: (1) affecting glycolysis, via induction of glyceraldehyde-3-phosphate dehydrogenase expression [161]; (2) influencing the tricarboxylic acid cycle and β-oxidation pathway, via increase of carnitine acetyltransferase and carnitine palmitoyltransferase II expression [162]; and (3) impairing lipogenesis, via inhibition of stearoyl-Coenzyme A desaturase 1 expression [163] and pre-adipocyte proliferation [161]. It has also been postulated that ECS might modulate adipose tissue biology by interacting with PPARδ, shown to play a role in adipose remodeling during endurance exercise [164]. In agreement with this hypothesis, HFD rats showed all signs of metabolic syndrome, like adipocyte hypertrophy, elevated CB1 expression, and reduced PPARδ, which disappeared after a chronic exercise consisting of swimming for 1 h per day thrice a week, for 6 months [74].
Moreover, a study enrolling viscerally obese subjects, who followed a lifestyle program combining a healthy diet and physical activity for 1 year, showed an improved profile of conventional metabolic risk factors, body weight loss, waist circumference reduction, and a 7 % decrease in plasma AEA and a 62 % decrease in plasma 2-AG levels [68] (Table 1). Coherently, lean and agouti mice, given AM251 (a CB1 antagonist) combined with voluntary exercise, lost more body weight than mice supplemented with the CB1 antagonist alone [144]. Since obesity and physical inactivity affect insulin and leptin, which both negatively regulate eCB levels [137, 150, 165], it is conceivable that in obese and sedentary subjects, increased eCB tone might depend on the lack of control exerted by the two hormones. Moreover, given that the upregulation of eCB tone affects peripheral energy homeostasis in favor of fat accumulation, a compensatory mechanism aimed at limiting the excessive production of eCBs upon exercise might exist in order to prevent anabolic processes and to favor catabolism and energy supply. In this context, it should be stressed that the in vivo biological actions of eCBs are tightly regulated by a metabolic control, where FAAH has been recognized as a major player [166]. Incidentally, we found that physically active subjects (practicing regular aerobic exercise for about 8 h per week) have increased lymphocyte FAAH activity when compared to sedentary individuals [42].
The liver is a key organ for the storage and disposal of carbohydrates, proteins, and fats, and it is known to be influenced by both PA [146] and ECS [45, 135] (Fig. 5). HFD mice, indeed, showed increased CB1 expression and AEA levels in hepatic cells [167, 168]. Consistently, a CB1 blockade decreased liver steatosis and dyslipidemia [167, 168]. On the other hand, at least in healthy subjects it seems that previous chronic exercise (8-week training) does not affect liver ECS response to fasting-refeeding cycles; indeed, in rats fed with fructose (an animal model of liver steatosis), diet-dependent increases of CB1, CB2 and FAAH expression were not affected by 8 weeks of treadmill exercise, and instead lowered plasma levels of free fatty acids and triacylglycerols [169].
Collectively, only a few reports confirm [71], suggest, or hypothesize [61, 156] a positive peripheral eCB effect during exercise. Thus, it would be very important to further investigate eCB-dependent mechanisms and/or their tissue specificity, in order to better understand the relationship between PA and ECS. At any rate, it has been convincingly demonstrated that the major role of eCBs at the periphery is opposite to that of PA and, if dysregulated, might also lead to pathological conditions [45, 150]. Therefore, it is conceivable that eCB metabolic routes are modulated to control eCB tone, a hypothesis that has been already demonstrated for FAAH in lymphocytes of subjects practicing regular physical activity [42]. Proving this concept also for other ECS elements might be an asset in the future.
Immune system and redox state
A growing body of evidence points to the ability of exercise to induce, in a type-, duration-, and intensity-dependent manner, physiological changes in the innate and adaptive immune system (Fig. 5). Acute exercise (1) increases the number of circulating monocytes, neutrophils, and lymphocytes [170]; (2) reduces the CD4+/CD8+ T cell ratio [171]; (3) increases mobilization of memory (but not naïve) lymphocytes [171]; (4) enhances the number and activity of natural killer cells [172]; and (5) markedly increases pro-inflammatory cytokines, such as IL-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α [173]. Nonetheless, all parameters returned to basal levels within 24 h from the end of exercise [13]. Instead, prolonged exercise or frequent bouts of vigorous exercise temporarily depress immune system, thus resulting in higher susceptibility to infections, particularly among athletes [13]. Against this background, it is well-recognized that regular PA exerts general positive effects in preventing and reducing systemic inflammation, by: (1) downregulating the release of pro-inflammatory cytokines from monocytes and macrophages; (2) decreasing the number of circulating activated immune cells [174]; and (3) increasing the amount of blood T regulatory (Treg) cells [175]. By reducing the accumulation of visceral adipose tissue (a condition often occurring in the case of a sedentary lifestyle), exercise also decreases macrophage infiltration in this organ, thus contributing to lower levels of pro-inflammatory cytokines in the blood [176]. In this context, a key role is played by skeletal muscle that, during contraction, releases myokines like IL-6, IL-8, and IL-15 [177]. Among them, IL-6 plays a metabolic role by increasing insulin sensitivity, hepatic glucose production, lipolysis, and fat oxidation [177, 178], and promotes an anti-inflammatory environment that suppresses the long-term effects of elevated pro-inflammatory cytokines [179].
Regular PA also promotes the generation of reactive oxygen and nitrogen species (ROS and RNS, respectively), which positively regulate redox-sensitive transcription factors (NF-κB and AP-1) [180], force production by contracting muscles [181], exert a beneficial impact on vascular functions [182] and induce satellite cell proliferation and differentiation [183]. The downside is that, when exercise is inappropriate, ROS and RSN levels dramatically increase, thus triggering oxidative stress that contributes to muscle fatigue and damage, as well as to pro-inflammatory responses [181, 184–186] (Fig. 5).
All ECS components are highly expressed in the immune system, where they are differentially modulated depending on leukocyte activation state: CB2 (undetectable in naïve T cells) and FAAH increase and decrease, respectively, in activated lymphocytes [46, 187–189], while AEA content augments (due to upregulated NAPE-PLD activity) in LPS-stimulated macrophages [46, 47].
The crucial role of ECS in immune responses is well-recognized [28] (Fig. 5): for instance, AEA/CB2 interaction strongly suppresses CD4+/CD8+ T lymphocyte proliferation, impairs the pro-inflammatory responses of both T helper (Th) 17 and Th1 cells [189], and stimulates anti-inflammatory activity of Treg and Th2 cells [190] (Fig. 5).
The ECS (particularly CB1) also modulates the redox state, by regulating inducible and endothelial nitric oxide synthase expression, mitochondrial biogenesis, respiration rate, and ROS/RNS generation [79, 191–194]. Furthermore, CBD has been shown to increase the production of ROS that, in turn, trigger CD4+ and CD8+ T cell apoptosis [195] (Fig. 5).
Overall, based on its ability to modulate immune responses, ECS might play a role in exercise-related changes of immune functions and cellular redox state. The interplay among eCB tone, exercise, and the immune system is, indeed, evident in subjects habitually practicing moderate exercise, who show increased FAAH activity in their T lymphocytes (but not in other blood cells), when compared to sedentary individuals. Such an upregulation, which is mediated by factors released during exercise, including IL-6 [42], IL-10 (whose release is enhanced by IL-6), and IL-4 [6, 179], represents a compensatory mechanism occurring in active individuals and aimed at avoiding an excessive increment of eCBs upon exercise. Indeed, high eCB levels exert the same effects as prolonged exercise, i.e., depression of the immune system; therefore, both PA and eCB tone should be maintained in an optimal range, in order to favor an anti-inflammatory environment able to counteract side effects like muscle damage and immune suppression [6, 179].
Concluding remarks and future directions
A growing body of evidences strongly indicates an interplay between PA and eCB tone, both centrally and peripherally. Indeed, eCBs are crucial in controlling locomotor activity, and much like exercise, they positively affect cognitive functions, nociception, and other brain processes. Thus, it is conceivable that some exercise-dependent effects in the CNS might depend (at least in part) on eCB signaling. This hypothesis is strongly supported by several human studies showing that exercise is able to increase the circulating levels of eCBs in a type-, intensity- and time-dependent manner. Yet, in the majority of reports, unlike exercise, eCB tone negatively affects peripheral energy homeostasis towards fat accumulation, which better describes an obesity-directed condition or a sedentary lifestyle. It seems necessary, therefore, that eCBs are tightly regulated during PA, in order to maintain their beneficial effects while avoiding impaired energy metabolism, oxidative stress, and inflammatory processes.
Otherwise, future research should probably aim at clarifying these aspects, for example by exploring the influence of different exercise protocols on distinct proteins of the ECS, as well as on different eCB-dependent signaling cascades. As yet, data on the effect of combining both exercise and diet on human eCB levels are still very few, and are all based on chronic exercise protocols. Nonetheless, it is well-known that dietary fat composition influences tissue levels of eCBs [196]: a standard Western diet rich in ω-6 PUFA (i.e., linoleic and arachidonic acids) enhances AEA and 2-AG levels, while reducing the levels of ω-3 PUFAs and their derivatives (e.g., DHEA and EPEA) [197].
Collectively, exercise is increasingly seen as a “disease-modifier”, i.e., a powerful tool to help prevent and treat several diseases, from metabolic disorders to neurodegenerative pathologies. A better understanding of the role of eCB in exercise could further increase the therapeutic efficacy of exercise protocols. This could also broaden exercise-based treatments to diseases with motor deficit, cognitive impairment, as well as oxidative or inflammatory disturbances. Indeed, eCBs might also be an alternative target for drug design, keeping in mind that conventional medicines imply tolerance, drug resistance, or unwanted side-effects.
In conclusion, the study of the combined effects of exercise and eCBs can lead to a more holistic, cost-saving clinical approach in related (and rather common) behaviors, like nicotine and alcohol dependence.
Acknowledgments
We apologize in advance to all investigators whose research could not be appropriately quoted due to space limitations. We wish to thank all colleagues who have contributed over the past 15 years to our studies of the endocannabinoid system and its impact on human health and disease. Financial support from Fondazione TERCAS (Grant n° 2009–2012), Ministero dell’Istruzione, dell’Università e della Ricerca (grant n° PRIN 2010–2011), Fondazione Italiana Sclerosi Multipla (FISM grant 2010), to M.M., and from Regione Lazio (grant n° 00011377/2010–2013) to M.T. is also gratefully acknowledged.
Footnotes
V. Gasperi and M. Maccarrone are equally senior authors.
Contributor Information
Valeria Gasperi, Phone: +39-06-72596465, FAX: +39-06-72596463, Email: gasperi@med.uniroma2.it.
Mauro Maccarrone, Phone: +39-06-225419169, FAX: +39-06-22541456, Email: m.maccarrone@unicampus.it.
References
- 1.Howley ET. Type of activity: resistance, aerobic and leisure versus occupational PA. Med Sci Sports Exerc. 2001;33:S364–S369. doi: 10.1097/00005768-200106001-00005. [DOI] [PubMed] [Google Scholar]
- 2.Romijn JA, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 3.Yau SY, et al. Effects of voluntary running on plasma levels of neurotrophins, hippocampal cell proliferation and learning and memory in stressed rats. Neuroscience. 2012;222:289–301. doi: 10.1016/j.neuroscience.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Knab AM, Lightfoot JT. Does the difference between physically active and couch potato lie in the dopamine system? Int J Biol Sci. 2010;6:133–150. doi: 10.7150/ijbs.6.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nijs J, et al. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician. 2012;151:ES205–213. [PubMed] [Google Scholar]
- 6.Gleeson M, Walsh NP. British Association of Sport and Exercise Sciences. The BASES expert statement on exercise, immunity, and infection. J Sports Sci. 2012;30:321–324. doi: 10.1080/02640414.2011.627371. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen L, Hojman P. Muscle-to-organ cross talk mediated by myokines. Adipocyte. 2012;1:164–167. doi: 10.4161/adip.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voss MW, et al. Exercise, brain, and cognition across the life span. J Appl Physiol. 2011;111:1505–1513. doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mora S, et al. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexanderson H, Lundberg IE. Exercise as a therapeutic modality in patients with idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2012;24:201–207. doi: 10.1097/BOR.0b013e32834f19f5. [DOI] [PubMed] [Google Scholar]
- 11.Church T. Exercise in obesity, metabolic syndrome, and diabetes. Prog Cardiovasc Dis. 2011;53:412–418. doi: 10.1016/j.pcad.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537:333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaldivar F, et al. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J Appl Physiol. 2006;100:1124–1133. doi: 10.1152/japplphysiol.00562.2005. [DOI] [PubMed] [Google Scholar]
- 14.Carek PJ, et al. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med. 2011;41:15–28. doi: 10.2190/PM.41.1.c. [DOI] [PubMed] [Google Scholar]
- 15.Waters RP, et al. Selection for increased voluntary wheel-running affects behavior and brain monoamines in mice. Brain Res. 2013;1508:9–22. doi: 10.1016/j.brainres.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milman S, et al. Opioid receptor blockade prevents exercise-associated autonomic failure in humans. Diabetes. 2012;61:1609–1615. doi: 10.2337/db11-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howlett AC, et al. CB(1) cannabinoid receptors and their associated proteins. Curr Med Chem. 2010;17:1382–1393. doi: 10.2174/092986710790980023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pertwee RG, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2 . Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 20.Sugiura T, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 21.Mechoulam R, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 22.De Petrocellis L, Di Marzo V. An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract Res Clin Endocrinol Metab. 2009;23:1–15. doi: 10.1016/j.beem.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Hanus, et al. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter AC, et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- 25.Huang SM, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown I, et al. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis. 2010;31:1584–1591. doi: 10.1093/carcin/bgq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovito D, et al. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARγ activation in MCF-7 breast cancer cells. J Cell Physiol. 2013;228:1314–1322. doi: 10.1002/jcp.24288. [DOI] [PubMed] [Google Scholar]
- 28.Klein TW, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 29.Patel KD, et al. Cannabinoid CB(2) receptors in health and disease. Curr Med Chem. 2010;17:1393–1410. doi: 10.2174/092986710790980041. [DOI] [PubMed] [Google Scholar]
- 30.Viscomi MT, et al. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci. 2009;29:4564–4570. doi: 10.1523/JNEUROSCI.0786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross RA. The enigmatic pharmacology of GPR55. Trends Pharmacol Sci. 2009;30:156–163. doi: 10.1016/j.tips.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Gasperi V, et al. GPR55 and its interaction with membrane lipids: comparison with other endocannabinoid-binding receptors. Curr Med Chem. 2013;20:64–78. [PubMed] [Google Scholar]
- 33.Di Marzo V, De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem. 2010;17:1430–1449. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- 34.Greco R, et al. The endocannabinoid system and migraine. Exp Neurol. 2010;224:85–91. doi: 10.1016/j.expneurol.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 35.Maccarrone M, et al. Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J Biol Chem. 2000;275:31938–31945. doi: 10.1074/jbc.M005722200. [DOI] [PubMed] [Google Scholar]
- 36.Stock K, et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat Med. 2012;18:1232–1238. doi: 10.1038/nm.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouaboula M, et al. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 38.Gasperi V, et al. Endocannabinoids in adipocytes during differentiation and their role in glucose uptake. Cell Mol Life Sci. 2007;64:219–229. doi: 10.1007/s00018-006-6445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rockwell CE, et al. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70:101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto Y, et al. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- 41.Ueda N, et al. Metabolism of endocannabinoids and related N-acylethanolamines: canonical and alternative pathways. FEBS J. 2013;280:1874–1894. doi: 10.1111/febs.12152. [DOI] [PubMed] [Google Scholar]
- 42.Gasperi V, et al. The fatty acid amide hydrolase in lymphocytes from sedentary and active subjects. Med Sci Sports Exerc. 2014;46:24–32. doi: 10.1249/MSS.0b013e3182a10ce6. [DOI] [PubMed] [Google Scholar]
- 43.Giuffrida A, et al. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 44.Malcher-Lopes R, et al. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Maccarrone M, et al. Lipopolysaccharide downregulates fatty acid amide hydrolase expression and increases anandamide levels in human peripheral lymphocytes. Arch Biochem Biophys. 2001;393:321–328. doi: 10.1006/abbi.2001.2500. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, et al. Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J Biol Chem. 2003;278:45034–45039. doi: 10.1074/jbc.M306062200. [DOI] [PubMed] [Google Scholar]
- 48.Maccarrone M, et al. Intracellular trafficking of anandamide: new concepts for signaling. Trends Biochem Sci. 2010;35:601–608. doi: 10.1016/j.tibs.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon GM, Cravatt BF. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol BioSyst. 2010;6(8):1411–1418. doi: 10.1039/c000237b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chicca A, et al. Evidence for bidirectional endocannabinoid transport across cell membranes. J Biol Chem. 2012;287:34660–34682. doi: 10.1074/jbc.M112.373241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fowler CJ. Anandamide uptake explained? Trends Pharmacol Sci. 2012;33:181–185. doi: 10.1016/j.tips.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Oddi S, et al. Evidence for the intracellular accumulation of anandamide in adiposomes. Cell Mol Life Sci. 2008;65:840–850. doi: 10.1007/s00018-008-7494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 55.Fezza F, et al. Fatty acid amide hydrolase: a gate-keeper of the endocannabinoid system. Subcell Biochem. 2008;49:101–132. doi: 10.1007/978-1-4020-8831-5_4. [DOI] [PubMed] [Google Scholar]
- 56.Wei BQ, et al. A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem. 2006;281:36569–36578. doi: 10.1074/jbc.M606646200. [DOI] [PubMed] [Google Scholar]
- 57.Bisogno T, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dinh TP, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blankman JL, et al. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marrs WR, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valdeolivas S, et al. The inhibition of 2-arachidonoyl-glycerol (2-AG) biosynthesis, rather than enhancing striatal damage, protects striatal neurons from malonate-induced death: a potential role of cyclooxygenase-2-dependent metabolism of 2-AG. Cell Death Dis. 2013;4:e862. doi: 10.1038/cddis.2013.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nomura DK. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334(6057):809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sparling PB, Dietrich A, et al. Exercise activates the endocannabinoid system. Neuroreport. 2003;14:2209–2211. doi: 10.1097/00001756-200312020-00015. [DOI] [PubMed] [Google Scholar]
- 64.Feuerecker M, et al. Effects of exercise stress on the endocannabinoid system in humans under field conditions. Eur J Appl Physiol. 2012;112:2777–2781. doi: 10.1007/s00421-011-2237-0. [DOI] [PubMed] [Google Scholar]
- 65.Heyman E, et al. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Raichlen DA, et al. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high’. J Exp Biol. 2012;215:1331–1336. doi: 10.1242/jeb.063677. [DOI] [PubMed] [Google Scholar]
- 67.Raichlen DA, et al. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur J Appl Physiol. 2012;113:869–875. doi: 10.1007/s00421-012-2495-5. [DOI] [PubMed] [Google Scholar]
- 68.Di Marzo V, et al. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 2009;52:213–217. doi: 10.1007/s00125-008-1178-6. [DOI] [PubMed] [Google Scholar]
- 69.You T, et al. Adipose tissue endocannabinoid system gene expression: depot differences and effects of diet and exercise. Lipids Health Dis. 2011;10:194. doi: 10.1186/1476-511X-10-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill MN, et al. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–523. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galdino G, et al. The endocannabinoid system mediates aerobic exercise-induced antinociception in rats. Neuropharmacology. 2013;77C:313–324. doi: 10.1016/j.neuropharm.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 72.De Chiara V, et al. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology. 2010;35:374–387. doi: 10.1038/npp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gomes da Silva S, et al. Physical exercise in adolescence changes CB1 cannabinoid receptor expression in the rat brain. Neurochem Int. 2010;57:492–496. doi: 10.1016/j.neuint.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Yan ZC, et al. Exercise reduces adipose tissue via cannabinoid receptor type 1 which is regulated by peroxisome proliferator-activated receptor-delta. Biochem Biophys Res Commun. 2007;354:427–433. doi: 10.1016/j.bbrc.2006.12.213. [DOI] [PubMed] [Google Scholar]
- 75.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 76.Ade KK, Lovinger DM. Anandamide regulates postnatal development of long-term synaptic plasticity in the rat dorsolateral striatum. J Neurosci. 2007;27:2403–2439. doi: 10.1523/JNEUROSCI.2916-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su LD, et al. Retrograde cPLA(2)α/arachidonic acid/2-AG signaling is essential for cerebellar depolarization-induced suppression of excitation and long-term potentiation. Cerebellum. 2013;12:297–299. doi: 10.1007/s12311-012-0444-9. [DOI] [PubMed] [Google Scholar]
- 78.Kyriakatos A, El Manira A. Long-term plasticity of the spinal locomotor circuitry mediated by endocannabinoid and nitric oxide signaling. J Neurosci. 2007;27:12664–12674. doi: 10.1523/JNEUROSCI.3174-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song J, et al. Gating the polarity of endocannabinoid-mediated synaptic plasticity by nitric oxide in the spinal locomotor network. J Neurosci. 2012;32:5097–5105. doi: 10.1523/JNEUROSCI.5850-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Díaz-Alonso J, et al. The CB(1) cannabinoid receptor drives corticospinal motor neuron differentiation through the Ctip2/Satb2 transcriptional regulation axis. J Neurosci. 2012;32:16651–16665. doi: 10.1523/JNEUROSCI.0681-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Newman Z, et al. Endocannabinoids mediate muscarine-induced synaptic depression at the vertebrate neuromuscular junction. Eur J Neurosci. 2007;25:1619–1630. doi: 10.1111/j.1460-9568.2007.05422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huerta M, et al. Effects of cannabinoids on caffeine contractures in slow and fast skeletal muscle fibers of the frog. J Membr Biol. 2009;229:91–99. doi: 10.1007/s00232-009-9174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.James RS, et al. Variation in expression of calcium-handling proteins is associated with inter-individual differences in mechanical performance of rat (Rattus norvegicus) skeletal muscle. J Exp Biol. 2011;214:3542–3548. doi: 10.1242/jeb.058305. [DOI] [PubMed] [Google Scholar]
- 84.Seebacher F, et al. How well do muscle biomechanics predict whole-animal locomotor performance? The role of Ca2+ handling. J Exp Biol. 2012;215:1847–1853. doi: 10.1242/jeb.067918. [DOI] [PubMed] [Google Scholar]
- 85.Mahmmoud YA, Gaster M. Uncoupling of sarcoplasmic reticulum Ca2+-ATPase by N-arachidonoyl dopamine. Members of the endocannabinoid family as thermogenic drugs. Br J Pharmacol. 2012;166:2060–2069. doi: 10.1111/j.1476-5381.2012.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oz M, et al. Endogenous cannabinoid anandamide directly inhibits voltage-dependent Ca(2+) fluxes in rabbit T-tubule membranes. Eur J Pharmacol. 2000;404:13–20. doi: 10.1016/s0014-2999(00)00396-4. [DOI] [PubMed] [Google Scholar]
- 87.Alptekin A, et al. The effects of anandamide transport inhibitor AM404 on voltage-dependent calcium channels. Eur J Pharmacol. 2010;634:10–15. doi: 10.1016/j.ejphar.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 88.Wiley JL. Sex-dependent effects of delta 9-tetrahydrocannabinol on locomotor activity in mice. Neurosci Lett. 2003;352:77–80. doi: 10.1016/j.neulet.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 89.Pandolfo P, et al. Increased sensitivity of adolescent spontaneously hypertensive rats, an animal model of attention deficit hyperactivity disorder, to the locomotor stimulation induced by the cannabinoid receptor agonist WIN 55,212-2. Eur J Pharmacol. 2007;563:141–148. doi: 10.1016/j.ejphar.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 90.Smirnov MS, Kiyatkin EA. Behavioral and temperature effects of delta 9-tetrahydrocannabinol in human-relevant doses in rats. Brain Res. 2008;1228:145–160. doi: 10.1016/j.brainres.2008.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dubreucq S, et al. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Exp Neurol. 2010;224:106–113. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 92.Tallett AJ, et al. Grooming, scratching and feeding: role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacology. 2007;195:27–39. doi: 10.1007/s00213-007-0880-2. [DOI] [PubMed] [Google Scholar]
- 93.Keeney BK, et al. Differential response to a selective cannabinoid receptor antagonist (SR141716: rimonabant) in female mice from lines selectively bred for high voluntary wheel-running behaviour. Behav Pharmacol. 2008;19:812–820. doi: 10.1097/FBP.0b013e32831c3b6b. [DOI] [PubMed] [Google Scholar]
- 94.Keeney BK, et al. Sex differences in cannabinoid receptor-1 (CB1) pharmacology in mice selectively bred for high voluntary wheel-running behavior. Pharmacol Biochem Behav. 2012;101:528–537. doi: 10.1016/j.pbb.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 95.Maccarrone M, et al. Regulation by cannabinoid receptors of anandamide transport across the blood-brain barrier and through other endothelial cells. Thromb Haemost. 2006;95:117–127. [PubMed] [Google Scholar]
- 96.Stranahan AM, et al. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular Med. 2008;10:118–127. doi: 10.1007/s12017-008-8027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duric V, Duman RS. Depression and treatment response: dynamic interplay of signaling pathways and altered neural processes. Cell Mol Life Sci. 2013;70:39–53. doi: 10.1007/s00018-012-1020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Compagnucci C, et al. Type-1 (CB1) cannabinoid receptor promotes neuronal differentiation and maturation of neural stem cells. PLoS One. 2013;8(1):e54271. doi: 10.1371/journal.pone.0054271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang W, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jin K, et al. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol Pharmacol. 2004;66:204–208. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- 101.Aguado T, et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aso E, et al. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. J Neurochem. 2008;105:565–572. doi: 10.1111/j.1471-4159.2007.05149.x. [DOI] [PubMed] [Google Scholar]
- 103.Butovsky E, et al. In vivo up-regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to Delta-tetrahydrocannabinol. J Neurochem. 2005;93:802–811. doi: 10.1111/j.1471-4159.2005.03074.x. [DOI] [PubMed] [Google Scholar]
- 104.Varvel SA, et al. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32:1032–1041. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- 105.Mazzola C, et al. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-alpha nuclear receptors. Learn Mem. 2009;16:332–337. doi: 10.1101/lm.1145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goonawardena AV, et al. Pharmacological elevation of anandamide impairs short-term memory by altering the neurophysiology in the hippocampus. Neuropharmacology. 2011;61:1016–1025. doi: 10.1016/j.neuropharm.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiew KS, Braver TS. Positive affect versus reward: emotional and motivational influences on cognitive control. Front Psychol. 2011;2:279. doi: 10.3389/fpsyg.2011.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ganon-Elazar E, Akirav I. Cannabinoids prevent the development of behavioral and endocrine alterations in a rat model of intense stress. Neuropsychopharmacology. 2012;37(2):456–466. doi: 10.1038/npp.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Campeau S, et al. Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. J Neuroendocrinol. 2010;22:872–888. doi: 10.1111/j.1365-2826.2010.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bisicchia E, et al. Activation of type-2 cannabinoid receptor inhibits neuroprotective and antiinflammatory actions of glucocorticoid receptor α: when one is better than two. Cell Mol Life Sci. 2013;70:2191–2204. doi: 10.1007/s00018-012-1253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elbatsh MM, et al. Antidepressant-like effects of Δ9-tetrahydrocannabinol and rimonabant in the olfactory bulbectomised rat model of depression. Pharmacol Biochem Behav. 2012;102:357–365. doi: 10.1016/j.pbb.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 112.Bortolato M, et al. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 113.Le Foll B, et al. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology. 2009;205:171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martin M, et al. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- 115.Steiner MA, et al. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology. 2008;33:54–67. doi: 10.1016/j.psyneuen.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dubreucq S, et al. Ventral tegmental area cannabinoid type-1 receptors control voluntary exercise performance. Biol Psychiatry. 2012;73:895–903. doi: 10.1016/j.biopsych.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 117.Solinas M, et al. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith MA, et al. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Buchowski MS, et al. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS One. 2011;6:e17465. doi: 10.1371/journal.pone.0017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith SL, Rasmussen EB. Effects of 2-AG on the reinforcing properties of wheel activity in obese and lean Zucker rats. Behav Pharmacol. 2010;21:292–300. doi: 10.1097/FBP.0b013e32833aec4d. [DOI] [PubMed] [Google Scholar]
- 121.Drew LJ, et al. Activation of spinal cannabinoid 1 receptors inhibits C-fibre driven hyperexcitable neuronal responses and increases [35S]GTPgammaS binding in the dorsal horn of the spinal cord of noninflamed and inflamed rats. Eur J Neurosci. 2002;12:2079–2086. doi: 10.1046/j.1460-9568.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- 122.Tsou K, et al. Suppression of noxious stimulus-evoked expression of Fos protein-like immunoreactivity in rat spinal cord by a selective cannabinoid agonist. Neuroscience. 1996;70:791–798. doi: 10.1016/s0306-4522(96)83015-6. [DOI] [PubMed] [Google Scholar]
- 123.Lichtman AH, et al. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 124.Caprioli A, et al. The novel reversible fatty acid amide hydrolase inhibitor ST4070 increases endocannabinoid brain levels and counteracts neuropathic pain in different animal models. J Pharmacol Exp Ther. 2012;342:188–195. doi: 10.1124/jpet.111.191403. [DOI] [PubMed] [Google Scholar]
- 125.Ghafouri N, et al. Palmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivity. Pain. 2013;154:1649–1658. doi: 10.1016/j.pain.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 126.Blundell JE, et al. Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Dis Model Mech. 2012;5:608–613. doi: 10.1242/dmm.009837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rämson R, et al. The effect of 4-week training period on plasma neuropeptide Y, leptin and ghrelin responses in male rowers. Eur J Appl Physiol. 2012;112:1873–1880. doi: 10.1007/s00421-011-2166-y. [DOI] [PubMed] [Google Scholar]
- 128.de Rijke CE, et al. Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel-running rats. J Mol Endocrinol. 2005;35:381–390. doi: 10.1677/jme.1.01808. [DOI] [PubMed] [Google Scholar]
- 129.Ghanbari-Niaki A, et al. Plasma agouti-related protein (AGRP), growth hormone, insulin responses to a single circuit-resistance exercise in male college students. Peptides. 2007;28:1035–1039. doi: 10.1016/j.peptides.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 130.Pil-Byung C, et al. Effects of exercise program on appetite-regulating hormones, inflammatory mediators, lipid profiles, and body composition in healthy men. J Sports Med Phys Fitness. 2011;51:654–663. [PubMed] [Google Scholar]
- 131.Shadid S, et al. Diet/Exercise versus pioglitazone: effects of insulin sensitization with decreasing or increasing fat mass on adipokines and inflammatory markers. J Clin Endocrinol Metab. 2006;91:3418–3425. doi: 10.1210/jc.2006-0015. [DOI] [PubMed] [Google Scholar]
- 132.Bouassida A, et al. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br J Sports Med. 2010;44:620–630. doi: 10.1136/bjsm.2008.046151. [DOI] [PubMed] [Google Scholar]
- 133.Martins C, et al. Effects of exercise on gut peptides, energy intake and appetite. J Endocrinol. 2007;193:251–258. doi: 10.1677/JOE-06-0030. [DOI] [PubMed] [Google Scholar]
- 134.Broom DR, et al. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol. 2009;296:R29–R35. doi: 10.1152/ajpregu.90706.2008. [DOI] [PubMed] [Google Scholar]
- 135.Maccarrone M, et al. The endocannabinoid system and its relevance for nutrition. Annu Rev Nutr. 2010;30:423–440. doi: 10.1146/annurev.nutr.012809.104701. [DOI] [PubMed] [Google Scholar]
- 136.Kirkham TC, et al. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Di Marzo V, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 138.Kola B, et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- 139.Gamber KM, et al. Cannabinoids augment the release of neuropeptide Y in the rat hypothalamus. Neuropharmacology. 2005;49:646–652. doi: 10.1016/j.neuropharm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 140.Osei-Hyiaman D, et al. Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology. 2005;81:273–282. doi: 10.1159/000087925. [DOI] [PubMed] [Google Scholar]
- 141.Wiley JL, et al. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Soria-Gómez E, et al. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol. 2007;151:1109–1116. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cristino L, et al. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc Natl Acad Sci USA. 2013;110:E2229–E2238. doi: 10.1073/pnas.1219485110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou D, Shearman LP. Voluntary exercise augments acute effects of CB1-receptor inverse agonist on body weight loss in obese and lean mice. Pharmacol Biochem Behav. 2004;77:117–125. doi: 10.1016/j.pbb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 145.Chinsomboon J, et al. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci USA. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goodpaster BH, et al. Enhanced fat oxidation through PA is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–2197. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 147.Lavoie JM, Gauthier MS. Regulation of fat metabolism in the liver: link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci. 2006;63:1393–1409. doi: 10.1007/s00018-006-6600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Perseghin G, et al. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30:683–688. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- 149.Jeppesen J, Kiens B. Regulation and limitations to fatty acid oxidation during exercise. J Physiol. 2012;590:1059–1068. doi: 10.1113/jphysiol.2011.225011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Di Marzo V, et al. Role of insulin as a negative regulator of plasma endocannabinoid levels in obese and nonobese subjects. Eur J Endocrinol. 2009;161:715–722. doi: 10.1530/EJE-09-0643. [DOI] [PubMed] [Google Scholar]
- 151.Lindborg KA, et al. Effects of in vitro antagonism of endocannabinoid-1 receptors on the glucose transport system in normal and insulin-resistant rat skeletal muscle. Diabetes Obes Metab. 2010;12:722–730. doi: 10.1111/j.1463-1326.2010.01227.x. [DOI] [PubMed] [Google Scholar]
- 152.Esposito I, et al. The cannabinoid CB1 receptor antagonist rimonabant stimulates 2-deoxyglucose uptake in skeletal muscle cells by regulating the expression of phosphatidylinositol-3-kinase. Mol Pharmacol. 2008;74:1678–1686. doi: 10.1124/mol.108.049205. [DOI] [PubMed] [Google Scholar]
- 153.Lipina C, et al. Regulation of MAP kinase-directed mitogenic and protein kinase B-mediated signaling by cannabinoid receptor type 1 in skeletal muscle cells. Diabetes. 2010;59:375–385. doi: 10.2337/db09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 154.Agudo J, et al. Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia. 2010;53:2629–2640. doi: 10.1007/s00125-010-1894-6. [DOI] [PubMed] [Google Scholar]
- 155.Aguirre V, et al. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 156.Eckardt K, et al. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia. 2009;52(4):664–674. doi: 10.1007/s00125-008-1240-4. [DOI] [PubMed] [Google Scholar]
- 157.Fazakerley DJ, et al. Kinetic evidence for unique regulation of GLUT4 trafficking by insulin and AMP-activated protein kinase activators in L6 myotubes. J Biol Chem. 2010;285(3):1653–1660. doi: 10.1074/jbc.M109.051185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang J, Holman GD. Long-term metformin treatment stimulates cardiomyocyte glucose transport through an AMP-activated protein kinase-dependent reduction in GLUT4 endocytosis. Endocrinology. 2006;147(6):2728–2736. doi: 10.1210/en.2005-1433. [DOI] [PubMed] [Google Scholar]
- 159.Moreno-Navarrete JM, et al. The L-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes. 2012;61:281–291. doi: 10.2337/db11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Perwitz N, et al. Cannabinoid receptor signaling directly inhibits thermogenesis and alters expression of adiponectin and visfatin. Horm Metab Res. 2006;38:356–368. doi: 10.1055/s-2006-925401. [DOI] [PubMed] [Google Scholar]
- 161.Gary-Bobo M, et al. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits cell proliferation and increases markers of adipocyte maturation in cultured mouse 3T3 F442A preadipocytes. Mol Pharmacol. 2006;69:471–478. doi: 10.1124/mol.105.015040. [DOI] [PubMed] [Google Scholar]
- 162.Jbilo O, et al. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 2005;19:1567–1569. doi: 10.1096/fj.04-3177fje. [DOI] [PubMed] [Google Scholar]
- 163.Nogueiras R, et al. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57:2977–2991. doi: 10.2337/db08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fredenrich A, Grimaldi PA. PPAR delta: an uncompletely known nuclear receptor. Diabetes Metab. 2005;31:23–27. doi: 10.1016/s1262-3636(07)70162-3. [DOI] [PubMed] [Google Scholar]
- 165.Maccarrone M, et al. Differential regulation of fatty acid amide hydrolase promoter in human immune cells and neuronal cells by leptin and progesterone. Eur J Biochem. 2004;271:4666–4676. doi: 10.1111/j.1432-1033.2004.04427.x. [DOI] [PubMed] [Google Scholar]
- 166.Cravatt BF, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Osei-Hyiaman D, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jourdan T, et al. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes. 2010;59:926–934. doi: 10.2337/db09-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yasari S, et al. Effects of exercise training on molecular markers of lipogenesis and lipid partitioning in fructose-induced liver fat accumulation. J Nutr Metab. 2012;2012:181687. doi: 10.1155/2012/181687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Simpson RJ. Aging, persistent viral infections, and immunosenescence: can exercise “make space”? Exerc Sport Sci Rev. 2011;39:23–33. doi: 10.1097/JES.0b013e318201f39d. [DOI] [PubMed] [Google Scholar]
- 171.Gannon GA, et al. Differential cell adhesion molecule expression and lymphocyte mobilisation during prolonged aerobic exercise. Eur J Appl Physiol. 2001;84:272–282. doi: 10.1007/s004210000374. [DOI] [PubMed] [Google Scholar]
- 172.Pedersen BK, et al. Modulation of natural killer cell activity in peripheral blood by physical exercise. Scand J Immunol. 1988;27:673–678. doi: 10.1111/j.1365-3083.1988.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 173.Scott JP, et al. Effect of exercise intensity on the cytokine response to an acute bout of running. Med Sci Sports Exerc. 2011;43:2297–2306. doi: 10.1249/MSS.0b013e31822113a9. [DOI] [PubMed] [Google Scholar]
- 174.Timmerman KL, et al. Exercise training-induced lowering of inflammatory (CD14+ CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84:1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 175.Wang J, et al. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand J Med Sci Sports. 2011;22:643–652. doi: 10.1111/j.1600-0838.2010.01288.x. [DOI] [PubMed] [Google Scholar]
- 176.Kawanishi N, et al. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- 177.Pedersen BK. The diseasome of physical inactivity—and the role of myokines in muscle—fat cross talk. J Physiol. 2009;587:5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pournot H, et al. Time-course of changes in inflammatory response after whole-body cryotherapy multi exposures following severe exercise. PLoS One. 2011;6:e22748. doi: 10.1371/journal.pone.0022748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pedersen BK. Muscular interleukin-6 and its role as an energy sensor. Med Sci Sports Exerc. 2012;44:392–396. doi: 10.1249/MSS.0b013e31822f94ac. [DOI] [PubMed] [Google Scholar]
- 180.Brooks SV, et al. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor kappaB activation. J Physiol. 2008;586:3979–3990. doi: 10.1113/jphysiol.2008.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cobley JN, et al. N-Acetylcysteine’s attenuation of fatigue after repeated bouts of intermittent exercise: practical implications for tournament situations. Int J Sport Nutr Exerc Metab. 2011;21:451–461. doi: 10.1123/ijsnem.21.6.451. [DOI] [PubMed] [Google Scholar]
- 182.Sun MW, et al. Effects of different levels of exercise volume on endothelium-dependent vasodilation: roles of nitric oxide synthase and heme oxygenase. Hypertens Res. 2008;31:805–816. doi: 10.1291/hypres.31.805. [DOI] [PubMed] [Google Scholar]
- 183.Radak Z, et al. Nitric oxide: is it the cause of muscle soreness? Nitric Oxide. 2012;26:89–94. doi: 10.1016/j.niox.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 184.Powers SK, et al. Reactive oxygen species: impact on skeletal muscle. Compr Physiol. 2011;1:941–969. doi: 10.1002/cphy.c100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Villalta SA, et al. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18:482–496. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chiurchiù V, Maccarrone M. Chronic inflammatory disorders and their redox control: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:2605–2641. doi: 10.1089/ars.2010.3547. [DOI] [PubMed] [Google Scholar]
- 187.Coopman K, et al. Temporal variation in CB2R levels following T lymphocyte activation: evidence that cannabinoids modulate CXCL12-induced chemotaxis. Int Immunopharmacol. 2007;7:360–371. doi: 10.1016/j.intimp.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 188.Smith SR, et al. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J Pharmacol Exp Ther. 2000;293:136–150. [PubMed] [Google Scholar]
- 189.Cencioni MT, et al. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS One. 2010;5:e8688. doi: 10.1371/journal.pone.0008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pandey R, et al. Endocannabinoids and immune regulation. Pharmacol Res. 2009;60:85–92. doi: 10.1016/j.phrs.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Maccarrone M, et al. Anandamide uptake by human endothelial cells and its regulation by nitric oxide. J Biol Chem. 2000;275:13484–13492. doi: 10.1074/jbc.275.18.13484. [DOI] [PubMed] [Google Scholar]
- 192.Tedesco L, et al. Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: the role of eNOS, p38 MAPK, and AMPK pathways. Diabetes. 2010;59:2826–2836. doi: 10.2337/db09-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mukhopadhyay P, et al. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic Biol Med. 2011;50:179–195. doi: 10.1016/j.freeradbiomed.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Romero TR, et al. Involvement of the l-arginine/nitric oxide/cyclic guanosine monophosphate pathway in peripheral antinociception induced by N-palmitoyl-ethanolamine in rats. J Neurosci Res. 2012;90:1474–1479. doi: 10.1002/jnr.22797. [DOI] [PubMed] [Google Scholar]
- 195.Lee CY, et al. A comparative study on cannabidiol-induced apoptosis in murine thymocytes and EL-4 thymoma cells. Int Immunopharmacol. 2008;8:732–740. doi: 10.1016/j.intimp.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 196.Kim J, et al. Fat to treat fat: emerging relationship between dietary PUFA, endocannabinoids, and obesity. Prostaglandins Other Lipid Mediat. 2013;104–105:32–41. doi: 10.1016/j.prostaglandins.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 197.Ailhaud G, et al. Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res. 2006;45:203–236. doi: 10.1016/j.plipres.2006.01.003. [DOI] [PubMed] [Google Scholar]