Abstract
Many organs, such as lungs, nerves, blood and lymphatic vessels, consist of complex networks that carry flows of information, gases, and nutrients within the body. The morphogenetic patterning that generates these organs involves the coordinated action of developmental signaling cues that guide migration of specialized cells. Precision guidance of endothelial tip cells by vascular endothelial growth factors (VEGFs) is well established, and several families of neural guidance molecules have been identified to exert guidance function in both the nervous and the vascular systems. This review discusses recent advances in VEGF research, focusing on the emerging role of neural guidance molecules as key regulators of VEGF function during vascular development and on the novel role of VEGFs in neural cell migration and nerve wiring.
Keywords: Axon guidance, Angiogenesis, VEGF, Tip cell, Growth cone
Introduction
The nervous and the vascular systems are highly branched, ramified networks extending into nearly every part of the human body. The near-perfect alignment of some blood vessels with peripheral nerve fibers probably reflects mutual dependency between the two systems. Indeed the nervous system requires vascularization to ensure nutrient and oxygen supply, and nerve cells control vascular caliber and blood flow to tissue. The close structural resemblance between the nervous and vascular networks has raised the question of whether the developmental mechanisms that shape the non-random spatial patterns of nerves and vessels may be conserved. An important step in identifying these common mechanisms was the characterization of specialized endothelial cells situated at the tips of developing vascular sprouts [1, 2]. These so-called tip cells extend filopodia- and lamellipodia-like processes in the direction of vascular expansion and exert pulling forces on non-migrating trailing cells, the stalk cells, which form the capillary lumen and exhibit proliferative behavior. Endothelial cells with filopodia are also seen at the tip of vessels in the lymphatic system [3, 4]. Tip cells are for developing vessels, the counterparts of the neuronal growth cones, described almost a century earlier by neurologist Cajal, which are found at the leading edge of both growing axons and migrating neurons [5, 6]. Endothelial tip cells and neuronal growth cones function as sensory and motor structures that regulate substrate-adhesion and migration as well as directional sensing of external cues.
Precision guidance of endothelial tip cells is ensured by several growth factors, among which VEGFs and their receptors (VEGFRs) play a major role. Over the past years, it has become apparent that endothelial tip cells also respond to neural guidance signals that are employed to ensure precise wiring in the developing nervous system. These include the four canonical families of guidance cues: Netrins, which bind to UNC5 and DCC family receptors, Slits which bind Robo receptors, Semaphorins, whose receptors are Neuropilins and/or Plexins, and Ephrins and their Eph receptors. Each family of guidance receptors has at least one member expressed by developing blood vessels, and recent evidence indicates that neural guidance signaling cues directly regulate tip cell migratory behavior and patterning of blood vessels (reviewed in [7, 8]). Taking this parallel further, studies have investigated a reciprocal implication of factors involved in the development and physiology of the vascular system as potential regulators of nervous system wiring [9], and recent works have demonstrated the important roles of VEGFs and VEGFRs in neuron migration and axon guidance (see below).
Intense research in the vascular system has identified the responsible intracellular signal transduction networks that mediate VEGF effect on cell migration as well as the other regulatory functions of VEGF on endothelial cell proliferation, survival, and vascular permeability (reviewed in [10]). A major challenge now is to fully understand how VEGF signaling is regulated to evoke cell type-specific and context-dependent responses. For example, what are the cell intrinsic differences in the initiation of VEGF receptor signaling that favor a migratory response in tip cells while inducing proliferation in stalk cells? Do receptors for VEGF and neural guidance cues interact at the plasma membrane to specify or diversify incoming signals? Does VEGF use similar transduction networks to stimulate migration and guidance of tip cells and neuronal growth cones? This review focuses on recent advances addressing these questions and contains two sections. We will first discuss experimental advances that establish critical, mechanistic links between neural guidance factors and VEGF signaling during tip cell migration and vascular patterning. In the second part of this review, we will summarize the novel activities of VEGFs and VEGFRs in neuronal patterning and highlight the essential differences that emerge between modes of VEGF signaling in vascular and neural systems.
Functional links between axon guidance molecules and VEGF signaling in vascular patterning
Two distinct mechanisms, called vasculogenesis and angiogenesis, operate during vascular network formation in the embryo. Vasculogenesis is the de novo assembly of the primordial vessels by differentiation, migration, and aggregation of mesodermally derived endothelial cell progenitors. Later on, angiogenesis, which defines the formation of new blood vessels from these pre-existing vessels, expands and remodels the primary vascular network into more complex vascular structures. The cellular events accompanying the sprouting of new capillaries during angiogenesis are tightly regulated by pro-angiogenic VEGF signals. The VEGF family includes six homologous factors: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and the placenta growth factor (PlGF). The secreted signaling protein VEGF-A and its associated receptor VEGFR2 are the main effectors of vascular growth and pattern formation [2]. VEGF-A exists in different isoforms that are generated by alternative splicing of a single gene: VEGF-A120, VEGF-A164, VEGF-A188 in mice (human VEGF-A is one amino-acid longer: VEGF-A121, VEGF-A165, VEGF-A189). These isoforms differ in their ability to bind heparan sulfate proteoglycans (HSPG): VEGF-A120 lacks heparin-binding affinity and is freely diffusible, VEGF-A188 has the highest affinity to HSPG and is retained in the extracellular matrix after secretion, and VEGF-A164, the most common splicing isoform generated from the VEGF-A gene has intermediate properties [11]. Collectively, the expression of the various isoforms builds steep extracellular gradients of VEGF-A in developing tissues, which promote the polarization of tip cells and the directional extension of filopodia. In addition, VEGF-A/VEGFR2, together with Delta/Notch signaling, patterns endothelial cell population into tip and stalk cells (reviewed in [12]). Recent studies have suggested that dynamic regulatory interactions between the VEGF-C/VEGFR3 axis and the Delta/Notch pathway regulate this process as well [13–15].
Shortly after the development of blood vessels, the blind-end network of lymphatic vessels develops. The primary lymphatic sacs originate by lymphatic endothelial cells budding from veins. The lymphatic vessels then spread from these sacs into surrounding tissue and organs through a process known as lymphangiogenesis (reviewed in [16, 17]). Although less well understood, the cellular mechanisms of lymphangiogenic spouting are believed to be similar to those described for angiogenesis. However, one molecular difference is in the role of VEGF-C and its receptor VEGFR3 as the most potent lymphangiogenic factors controlling tip cell filopodia extension [3, 18, 19].
Similar to neuronal axons, vascular sprouts can also be guided by attractive or repulsive cues that belong to the four major families of neural guidance cues. Because the instructive roles of neural factors on the guidance of tip cells have been discussed in details elsewhere [7, 8, 20], we will not discuss them here. Instead, the following chapters will present evidence that supports additional roles for neural guidance molecules in fine-tuning VEGF ligand/receptor activities during physiological angiogenesis and lymphangiogenesis.
Neuropilins
Neuropilin-1 conveys VEGF-A signaling during angiogenesis
Neuropilin-1 is a type-I transmembrane receptor capable of mediating different types of protein–protein interactions. The function of Neuropilin-1 and their Plexin co-receptors has been studied extensively in the nervous system, where they play important roles in axonal patterning as a receptor for some class 3 (secreted) Semaphorins [21, 22]. In addition to its action in neurons, Neuropilin-1 is also expressed in endothelial cells of growing blood vessels [23] and can bind the heparin-binding VEGF-A164 isoform [24].
During development, Neuropilin-1 is dispensable for vasculogenesis, but is required for angiogenic remodeling of the primary vascular system. Evidence for the in vivo role of Neuropilin-1 comes from analysis of Neuropilin-1-null mice, which are embryonic lethal, and whose most notable defects are impaired yolk sac vasculature [25, 26], reduced vascular sprouting and branching in the embryo [26, 27] and defective great vessel remodeling. Defects in vascular plexus remodeling are also observed in the developing retina after inhibiting Neuropilin-1 with function blocking antibodies [28]. Moreover, mice with conditional deletion of Neuropilin-1 in endothelial cells exhibit similar vascular defects than that seen in the null allele [29], indicating that Neuropilin-1 is required within endothelial cells for vascular development. Further analyses have indicated that the primary role for Neuropilin-1 in angiogenesis is as a regulator of endothelial cell migration. It has been demonstrated in vivo that altered vascular morphology of yolk sacs in Neuropilin-1 mutants directly correlates with an abnormal pattern of endothelial cell migration while endothelial cell proliferation is unaffected [25]. Another detailed study on brain vasculature of Neuropilin-1 mutants has revealed that Neuropilin-1 is not required for endothelial sprout initiation and growth, but rather directs vessels by promoting selective filopodia extension of the tip cells [27].
A major question has been to determine whether the vascular defects in Neuropilin-1 mutants are due to deficiency of Semaphorin-Neuropilin-1 and/or VEGF-A-Neuropilin-1 signaling. Some angiogenesis defects have been reported in Sema3a null mice bred onto one specific genetic background [30]. However, other studies have reported that the vasculature of Sema3a mutants is indistinguishable from that of wild-type mice in this and other genetic backgrounds [31, 32] and that mice expressing a Neuropilin-1 variant defective for Semaphorin binding (Neuropilin-1sema−) also lack an observable vascular phenotype [29, 32]. In fact, two recent reports indicate that the function of Sema3A/Neuropilin-1 in vessel formation is restricted to the lymphatic system, where it is crucial for lymphatic valve development [31, 33]. On the other hand, mice producing only VEGF-A120, a non-Neuropilin-1 binding isoform [2, 34], show defective neural vascularization [32]. Moreover, inhibition of Neuropilin-1 function in the retina with an antibody specific for the VEGF binding domain reproduces the defects observed in Neuropilin-1-null mutants [25]. Thus, the opinion is now widely accepted that Semaphorin signaling through Neuropilin-1 is not essential to the formation of blood vessels networks, but that Neuropilin-1 preferentially transmits VEGF-A164 signaling in the vascular system.
The mechanisms by which Neuropilin-1 mediates VEGF-A164 activity are still unclear. Neuropilin-1 has a small cytoplasmic domain (consisting of 44 amino acids) lacking intrinsic catalytic function and so far there is sparse evidence that it may be able to directly transduce signals into cells [35]. In fact, no defect in developmental angiogenesis has been noted in mice expressing a Neuropilin-1 variant deleted from it cytoplasmic domain [36], indicating that the extracellular domain of Neuropilin-1 is sufficient to regulate vessel patterning. Thus, Neuropilin-1 is unlikely to transmit VEGF-A164 signal itself and rather acts as a co-activator of VEGFR2. Indeed, Neuropilin-1 has been shown to enhance the binding of VEGF-A164 to VEGFR2, and both Neuropilin-1 and VEGFR2 are required for enhanced chemotaxis of endothelial cells toward a gradient of VEGF-A165 [24]. Importantly, Neuropilin-1 is dispensable for the proliferative effect of VEGF-A on endothelial cells [28]. The mechanism by which Neuropilin-1 regulates cell migration response is poorly understood. It has been proposed that blocking Neuropilin-1 function in endothelial cells reduces VEGFR2 surface expression and tyrosine phosphorylation levels [28, 37]. However, these effects are modest and too general to fit with a role of Neuropilin-1 in determining outcome of VEGF-A signaling. It is therefore expected that Neuropilin-1 may also have more specific influence on downstream pathways of VEGFR2. For example, a recent report has established that VEGF-A-induced tyrosine phosphorylation of the scaffold protein p130Cas, which plays an essential role in chemotactic endothelial cell migration, is strictly dependent on the expression of Neuropilin-1 [38], thus identifying a potential mechanism for the selective effect of Neuropilin-1 on VEGF-A-mediated endothelial cell migration.
Neuropilin-2 conveys VEGF-C signaling during lymphangiogenesis
Neuropilin-2 is a transmembrane receptor closely related to Neuropilin-1 that is also expressed on neurons and endothelial cells. Neuropilin-2 has been initially characterized as a receptor for selected class 3 Semaphorins, and plays a key role as a partner in receptor signaling complexes, for example with Plexins, in nervous system patterning [39, 40]. However, like Neuropilin-1, it also acts as an isoform-specific VEGF-A receptor, which binds to VEGF-A164 as well as VEGF-A144, a rare VEGF-A variant expressed in the placenta and in epithelial ovarian carcinoma cells [41–43]. In addition, Neuropilin-2 also binds to the lymphangiogenic factor VEGF-C [44]. During development, Neuropilin-2 is initially co-expressed with Neuropilin-1 on primary vessels, but later on its expression become restricted to veins and lymphatic vessels [45]. Unlike Neuropilin-1-deficient mice that die early during development, Neuropilin-2 knockout mice are viable and show a grossly normal blood vascular system [45]. However, Neuropilin-2 deficiency results in impaired lymphatic vascular system, with normal formation of the main lymphatic vessels but a reduction of the small lymphatic vessels and capillaries [19, 45]. Time course analysis of lymphatic network formation after in vivo modulation of Neuropilin-2 with a blocking antibody indicated that these defects result from a specific reduction of lymphatic sprouting [19]. At the time of sprout formation, Neuropilin-2 expression is particularly strong in lymphatic tip cells and their filopodial extensions [19], suggesting a specific role of Neuropilin-2 on regulation of tip cell behavior. Indeed, loss of Neuropilin-2 function in vivo was associated with tip cells rarefaction and misshaping [19]. In vitro time-lapse analysis of lymphatic endothelial cell sprouting in the presence of blocking antibodies to Neuropilin-2 revealed that Neuropilin-2 is not essential for initiation of lymphangiogenic sprouting but is selectively required for sprout elongation, with its main function being to prevent stalling and retraction of tip cells [19].
It has now been clearly established that Neuropilin-2 mediates lymphangiogenic effects by acting as a co-receptor for VEGF-C signaling, a function reminiscent of that played by Neuropilin-1 in blood vessel development. In vivo evidence comes from the use of an anti-Neuropilin-2 antibody that selectively blocks the binding of VEGF-C to Neuropilin-2 while preserving the binding of other ligands, including the growth factors FGFs, HGF, and Semaphorins [46]. Neonatal mice treated with this antibody phenocopy the lymphatic defects reported in Neuropilin-2-null mice [19], indicating that Neuropilin-2 conveys VEGF-C signal in lymphatic endothelial cells. VEGF-C has the ability to bind to VEGFR2 and VEGFR3 receptors, both of which are expressed in developing lymphatic vessels and can interact with Neuropilin-2 [47]. Analysis of compound Neuropilin-2 +/−, vegfr3 +/− mice showed sprouting defects that were similar to the homozygous Neuropilin-2 −/− mice, providing direct evidence for a genetic interaction between Neuropilin-2 and VEGFR3 [19]. On the other hand, double heterozygous Neuropilin-2 +/−, vegfr2 +/− mice showed normal lymphatic development [19]. Thus, Neuropilin-2 acts as a co-receptor with VEGFR3 to modulate VEGF-C function in lymphatic development.
Neuropilin-2 regulates lymphatic endothelial tip cell migration induced by VEGF-C but does not appear to participate in other functions of VEGF-C during lymphatic network formation, such as lymphatic endothelial cell proliferation. From a mechanistic point of view, very little is known about VEGF-C/VEGFR3 signal modulation by Neuropilin-2. It has been proposed that Neuropilin-2 increases VEGF-C signaling through VEGFR3, but this is unlikely to be the sole explanation [46, 47]. Interestingly, in VEGF-C-treated lymphatic endothelial cells, Neuropilin-2 co-localizes with VEGFR3 in endocytic vesicles [48]. While receptor internalization is required for proper VEGF-C signaling, the presence of Neuropilin-2 is not an absolute requirement for VEGFR3 endocytosis [48]. However, the PDZ (postsynaptic density 95, PSD-85; discs large, Dlg; zonula occludens-1, ZO-1) adaptor protein Synectin, reported to be implicated in intracellular trafficking of endocytosed membrane receptors [49–52], has been shown to genetically interact with VEGFR3 and Neuropilin-2 to regulate lymphangiogenic sprouting in zebrafish [53]. Since Synectin can directly bind to Neuropilin-2 [54], it is tempting to speculate that Neuropilin-2 is required for intracellular trafficking of endocytosed VEGFR3 and its co-localization with specific downstream signaling molecules that produce its functional outcome.
In conclusion, Neuropilins are important key regulators of vessel remodeling and sprouting. Like in the nervous system, where both receptors are primarily involved in guidance of motile neuronal growth cones, Neuropilin-1 and -2 are required in the vascular system to confer specificity to the ability of VEGFs to stimulate and guide the movement of motile tip cells. While Neuropilin/Semaphorin signaling in neurons is essentially involved in repulsive activities, Neuropilin/VEGF signaling is instead permissive to vessel sprouting. These negative or positive effects of Neuropilin ligands depend on the different signaling co-receptors involved (Plexins in repulsion versus VEGFRs in attraction). Although Neuropilins may have been considered as simple molecular adapters, bringing together ligands and signaling co-receptors, it is undeniable that they play additional pivotal roles in modulating the signaling capacity of their partner receptors, whether they are VEGFRs or Plexins [55]. A future challenge will be to fully understand the mechanisms by which Neuropilins modulate their incoming signals and whether Neuropilin/VEGF signaling in tip cells can operate independently of VEGFRs, as it has been reported in neural cells (see below).
Eph and ephrins
Eph receptors are receptor tyrosine kinases that function together with their membrane-bound ephrin ligands in a number of different developmental processes including neuronal migration (reviewed in [56]) and axon guidance (reviewed in [4, 57, 58]). Eph/ephrin complexes present the interesting feature to signal bidirectionally: forward signals that depend on Eph kinase activity propagate in the receptor-expressing cell, and reverse signals are also transmitted in the ephrin-expressing cell (reviewed in [57]). In the developing vascular system, the transmembrane ligand ephrin-B2 is exclusively expressed on arteries, whereas its cognate EphB4 receptor is expressed on veins [59, 60]. Studies of EphB4 and ephrin-B2 null mice have suggested a requirement for reciprocal signaling between Eph receptors and ephrins in vascular morphogenesis [59–64]. However, the respective function and mechanism of action of EphB4 forward and ephrin-B2 reverse signaling in vivo remain unclear. Recent data in zebrafish have indicated that EphB4/ephrin-B2 interactions regulate sorting and segregation of arterial and venous angioblasts during the formation of the first embryonic artery (dorsal aorta) and vein (cardinal vein), which arise from a common precursor vessel [65]. Interestingly, in this system, VEGF-A signaling has been shown to regulate the expression of ephrin-B2 in arterial angioblasts, therefore limiting their ventral migration and excluding them from the EphB4-expressing venous vessel [65].
More recently, a novel molecular relationship between ephrin-B2 and VEGF signaling was revealed to be essential for vessel sprouting. Analysis of mice expressing an ephrin-B2 variant mutated in the PDZ binding motif—which provides docking sites for intracellular signaling molecules [66]—showed that ephrin-B2 signaling is required for sprouting activity during both blood vessel and lymphatic vessel development [67, 68]. These observations, combined with the fact that ephrin-B2 localizes to the filopodia of tip cells [68], suggest a role of ephrin-B2 in regulating endothelial cell motility. Indeed, microinjection of ephrin-B2 encoding plasmid in endothelial cells induces protrusion of filopodia and cell migration, and these effects are dependent on ephrin-B2 PDZ signaling ([18, 68].
Defective angiogenesis and lymphangiogenesis in ephrin-B2 mouse mutants appear to be the result of altered cellular responses to VEGF ligands. Indeed, ephrin-B2-deficient endothelial cells show reduced chemotaxis to VEGF-A164 in vitro. The level of VEGFR2 gene expression is unaffected in these cells [18, 61], however, receptor trafficking is severely compromised. Indeed, ephrin-B2 was found to physically interact with VEGFR2 to induce its internalization [68]. Internalization of VEGFR2 has been previously shown to allow its signaling from intracellular compartments, which is required for efficient VEGFR2 receptor activity [69]. Consistently, phosphorylation of VEGFR2 on intracellular tyrosine residues and activation of the downstream pro-angiogenic signal Akt is compromised in ephrin-B2 PDZ signaling mutants [68]. In a similar way, VEGF-C induced internalization of VEGFR3 was inhibited in endothelial cells from ephrin-B2 knockout mice, leading to reduction of VEGFR3 tyrosine phosphorylation and activation of Akt and Rac1, a regulator of cell motility and protrusion formation [18].
It remains to be understood how ephrin-B2 becomes activated in sprouting vessels to regulate VEGFR function. Interestingly, the EphB4 receptor is also expressed on sprouting lymphatic and blood vascular capillaries [59, 60, 67, 70]. Thus, a model is emerging where EphB4/ephrin-B2 interactions, occurring during direct cell–cell contacts between endothelial cells or within the same cell, activates ephrin-B2 PDZ signaling to control VEGF-induced tip cell filopodial dynamics.
Semaphorins and Plexins
As mentioned in previous chapters, Semaphorin signaling through Neuropilins is dispensable in most, if not all, aspects of vascular development. Despite this, some Semaphorin ligands have been proven essential for vascular development through mechanisms that involve the endothelial cell-enriched PlexinD1 receptor [71–73]. The best example is Sema3E, an atypical class 3 (secreted) Semaphorin, which binds directly and with high affinity to PlexinD1, independently of Neuropilins [72]. Sema3E functions as a guidance cue to coordinate circuit formation in the central nervous system [55, 74]. In addition, Sema3E guides migration of endothelial cells during both vasculogenesis [75] and sprouting angiogenesis [72] through repulsive interactions with PlexinD1 receptor. Another Semaphorin, the transmembrane Sema4A, also binds to PlexinD1, although with 10-fold less affinity than Sema3E [76]. However, since Sema4A-null mice show grossly normal vascular development and patterning, it is unclear what role, if any, Sema4A plays in embryonic vessel formation [76].
Accumulating evidence indicates the existence of functional relationships between Semaphorin/PlexinD1 and VEGF-A signaling. First evidence was initially reported in angiogenesis model in vitro, where Sema3E and Sema4A ligands were found to inhibit VEGF-A164 signal [76, 77]. In two recent articles, the repressive effect of PlexinD1 signaling on VEGF-A activity has been found essential for tip cell formation and vascular patterning in vivo, but the mechanisms involved appear to differ.
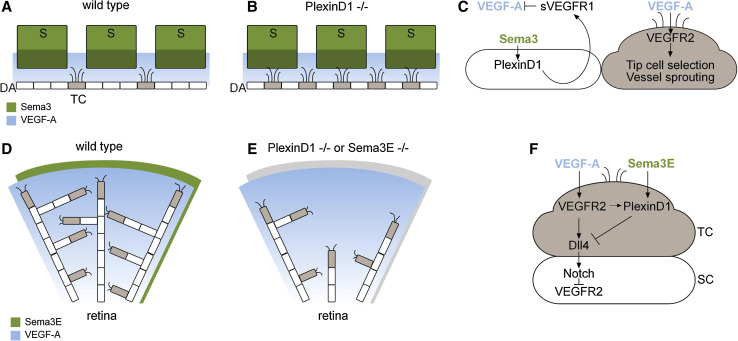
The first study investigated a role of Semaphorin/PlexinD1 signaling in spatially confining the sprouting of new blood vessels in the embryo [78]. During development, trunk intersomitic vessels (ISVs) emerge from the dorsal aorta by sprouting into the spaces that separate individual somites. VEGF-A signaling mediates the selection of tip cells within the parent vessel that will initiate the sprouts (reviewed in [12]). However, tip cell selection in the dorsal aorta occurs in a uniform VEGF-A environment (Fig. 1a; [78]), raising the question of the mechanisms limiting sprouting of ISVs to the intersomitic spaces. PlexinD1 deficiency in obd (out of bounds) zebrafish embryos was found to result in excessive sprouting from the dorsal aorta, with ectopic sprouts that were not confined to intersomitic spaces (Fig. 1b; [78]). These defects are causally related to enhanced VEGF-A signaling in obd mutants [78]. From a mechanistic point of view, Semaphorin/PlexinD1 signaling in endothelial cells has been shown to induce the production of a soluble form of VEGFR1, designated sVEGFR1, both in vitro and in vivo [78]. Generated by differential splicing of the VEGFR1 mRNA, sVEGFR1 carries only the extracellular domain of VEGFR1 as well as a 31-amino-acid stretch derived from an intron, and acts as an inhibitor of VEGF-A bioactivity by binding and sequestering VEGF-A. Endothelial cells that express sVEGFR1, and therefore have reduced VEGF-A signaling activity, are expected to be less likely to adopt a tip cell phenotype and sprouting behavior (Fig. 1c). Indeed, endothelial cell-specific overexpression of sVEGFR1 inhibited ISV sprouting in zebrafish embryos. On the contrary, partial reduction of both PlexinD1 and sVEGFR1 resulted in aberrant sprouting of endothelial cells from the dorsal aorta, confirming a genetic interaction between both molecules [78]. Thus, a model to set up local discontinuities in angiogenic potential along the dorsal aorta and ensure proper patterning of the trunk vasculature has emerged where somite-derived Semaphorin(s) act in a paracrine manner on PlexinD1-expressing endothelial cells to antagonize VEGF-A signal (Fig. 1c). As a consequence, only endothelial cells that occupy the intersomitic spaces and do not receive Semaphorin signals have the potential to be selected as tip cells and extend sprouts.
Fig. 1.
Functional relationships between Semaphorin/PlexinD1 and VEGF-A signaling in angiogenic sprouting. a Schematic of endothelial sprouting during the development of trunk intersomitic vessels (ISVs) in the zebrafish embryo. Class 3 Semaphorins (Sema3) secreted from the somites act on endothelial cells of the dorsal aorta to antagonize VEGF-A activity and restricts sprout formation to the intersomitic spaces. b Loss of Sema3-PlexinD1 activity in endothelial cells (PlexinD1−/−) alters the abundance and distribution of sprouts along the dorsal aorta. c Sema3-PlexinD1 signaling acts as a repressor of tip cell formation and endothelial sprouting by inducing the secretion of sVEGFR1, which antagonizes VEGF-A pro-angiogenic activity. d Schematic of angiogenic sprouting in the early postnatal mouse retina. The centrifugal expansion of retinal vessels depends on a gradient of VEGF-A laid down by astrocytes. Sema3E is secreted from the neural retina and is evenly distributed throughout the retina. e Genetic ablation of Sema3E (Sema3E−/−) or endothelial PlexinD1 (PlexinD1−/−) disrupts the pattern of retinal vascular sprouting and decreases tip cell numbers. f PlexinD1 expression is induced by VEGF-A at the forefront of sprouting vessels. In turn, Sema3E-PlexinD1 signaling negatively regulates VEGF-A-induced Delta-Notch signaling, which controls the balance between tip and stalk cells. DA dorsal aorta; S somite; SC stalk cell; sVEGFR1 soluble VEGFR1; TC tip cell
The second study further identified a reciprocal regulation of VEGF-A and Semaphorin/PlexinD1 signaling pathways that regulates the formation of the proper number of tip cells required for correct sprouting and branching patterns [79]. The expression of PlexinD1 in vessels is highly dynamic: it is elevated in tip cells and actively growing vessels but almost completely absent from quiescent vessels [79]. Evidence shows that PlexinD1 expression is directly regulated by VEGF-A/VEGFR2 signaling [79, 80]. In mouse neonates, inhibiting VEGFR2 function using blocking antibodies abolished PlexinD1 expression in the retinal vasculature, whereas treatment with VEGF-A leads to expansion of PlexinD1 expression on retinal vessels, especially in the central retina where low levels of VEGF-A are normally detected [79]. Thus, VEGF-A acts on endothelial cells to induce PlexinD1 expression, rendering them sensitive to the paracrine activity of Sema3E, which is expressed by the neural retina. In turn, Sema3E/PlexinD1 negatively regulates the Delta-Notch signaling pathway that functions downstream of VEGF-A signaling to control tip- and stalk cell fate. VEGF-A signaling through VEGFR2 is known to promote the production of the membrane-bound Notch ligand Delta-like 4 (Dll4) in tip cells, which activates Notch receptors in adjacent cells to laterally suppress tip cell fate [81–83]. In the neonatal mouse retina, gain of function of Sema3E was shown to decrease Dll4 production in the vasculature, whereas loss of Sema3E function led to an opposite increase in Dll4 expression and a subsequent increase of Notch1 receptor activation in adjacent cells [79]. Enhanced Dll4-Notch signaling in Sema3E or PlexinD1-deficient mice was accompanied by reduced tip cell formation, disrupted endothelial cell sprouting and caused uneven vessel growth in the retina [79]. These vascular patterning defects could be rescued by lowering Notch activity in the mutants using a gamma secretase inhibitor, which blocks Notch endoproteolysis [79]. Hence, this study defines a negative feedback loop by which VEGF-A stimulates the expression of PlexinD1, a key upstream regulator of the VEGF-A-induced Delta/Notch signaling pathway that acts to shape the vascular network finely (Fig. 1d–f).
The above studies reveal essential roles of Semaphorin/PlexinD1 in establishing the adequate ratio between tip and stalk cells required for correct sprouting and branching patterns. However, tip cell/stalk cell assignment is transient and continued competition and positional shuffling between tip and stalk cells is observed along extending vessel sprouts both in vitro and in vivo [84, 85]. It is currently unknown how a tip cell switches to a stalk cell but it is interesting to postulate that the dynamic regulation of the Delta/Notch lateral inhibition and/or sVEGFR1 expression by Semaphorin/PlexinD1 signaling might cause the tip cell to lose its competitive position over the followers and to become a stalk cell. In support of this idea, elegant mosaic experiments in the zebrafish embryo indicated that endothelial cells deficient for PlexinD1 expression have a higher probability of acquiring the leading position when competing with wild-type neighboring cells [78].
Robo4
Robo4 is another neural guidance family member, which has been shown to regulate VEGF-A signaling in endothelial cells. Robo4 belongs to the roundabout proteins family, which encodes transmembrane receptors for secreted repellent Slit proteins. Robo1 to 3 have an important function in axon guidance in the nervous system. In contrast, Robo4 is mostly expressed in the vascular system [86], while its expression and function in the developing nervous system remains poorly described [87].
Robo4 has first been suggested to mediate the repellent function of Slit proteins, mainly Slit2, in endothelial cells [88]. More recently, an alternative model of Robo4 action has been proposed, in which Slit/Robo4 signal antagonizes VEGF-A signaling. This effect is mediated through inhibition of signaling pathways downstream of VEGFR2 activation, including the Src-Rac1 and GIT1 (ARF GTPase-activating protein)-Arf6 signaling axis [89, 90]. However, controversy remains as to whether Robo4 is able to directly bind Slit ligand [91] and it is presumed that reception of Slit requires a co-receptor, such as Robo1 or the heparin sulfate proteoglycan Syndecan [92–94]. Interestingly, a search for Robo4-binding partners has revealed that Robo4 can act as a ligand for another vascular-specific “axon guidance” receptor, UNC5B [95]. Importantly, Robo4/UNC5B signaling also counteracts VEGF-A/VEGFR2 signaling in endothelial cells by decreasing Src activation and association of phosphorylated Src to VEGFR2 [95].
Despite evidence of cross-talks between Robo4 and VEGF-A signaling, mice treated with anti-Robo4 antibodies or Robo4 null mice show normal development of intersomitic, retinal, and cephalic vasculature [89, 95], indicating that Robo4 function is dispensable for vascular patterning. This may be related to the fact that Robo4 is preferentially expressed by endothelial stalk cells and not tip cells. In fact, it has been shown in several experimental models of pathological angiogenesis that activation of Robo4 on stalk cells helps in maintaining blood vessel integrity at least in part by counteracting VEGF-A activity [89, 96]. Nevertheless, Robo4 could in some contexts regulate VEGF-A-mediated sprouting angiogenesis, as suggested by the finding that Robo4 null mice show enhanced blood vessel development in the mammary gland during pregnancy [90].
Conclusion
Recent studies on the roles of neural guidance molecules in vascular patterning have revealed that in addition to their conventional function as guidance cues for sprouting vessels, they also regulate VEGF signaling, either positively or negatively, during tip cell selection and migration in developmental angiogenesis. Some neural guidance receptors interact with and regulate VEGFR activity directly, while others intersect with VEGFR signaling pathways. Together, these findings have led to the hypothesis that neural guidance molecules may be used to regulate VEGF/VEGFR signaling in pathological contexts where vascular growth is not desired or, conversely, where VEGF treatment is ineffective. In these contexts, strategies to target regulators of VEGF, such as Neuropilins, PlexinD1, or Robo4 proteins, have already shown success [28, 46, 77, 80, 89].
VEGF in nervous system wiring and patterning
Over the past few years, a growing body of evidence has established that the VEGF family of ligands and VEGFRs are expressed in neural cells of the developing brain and nervous system [97–101]. In vitro studies have established that VEGF-A signaling controls multiple aspects of neural development, including proliferation, survival, cell migration, and neurite outgrowth [98, 102–109]. However, the intricate relationship between nervous and vascular cells has made it difficult to study the roles of VEGF-A in the developing nervous system in vivo, as VEGF-A may act directly on neural cells or indirectly by influencing the development of the blood vessels providing oxygen and nutrients to brain tissue. Clear evidence for a direct function of VEGF-A on neural progenitor cell proliferation and differentiation has been provided by studies of neurogenesis in the embryonic chick retina, which is entirely devoid of blood vessels [110]. In contrast, in the mouse brain, the proliferative and neurotrophic effects of VEGF-A on developing neurons is largely believed to be a secondary consequence of its activity on brain vascularization [111], although evidence for a direct survival activity of VEGF-A has been recently reported for gonadotropin releasing hormon (GnRH) neurons ([112]; Fig. 4a). In this part of the review, we will focus on recent in vivo studies that demonstrate a direct function of VEGF-A as a chemotropic cue for migrating neurons and axonal growth cones, hence allowing VEGF-A to be added to the growing list of brain-wiring molecules. A central concept that is emerging from these studies is that Neuropilin-1 and VEGFR2 receptors, which cooperate in mediating the activity of VEGF-A in the vascular system, instead act independently from each other to relay the neural guidance functions of VEGF-A.
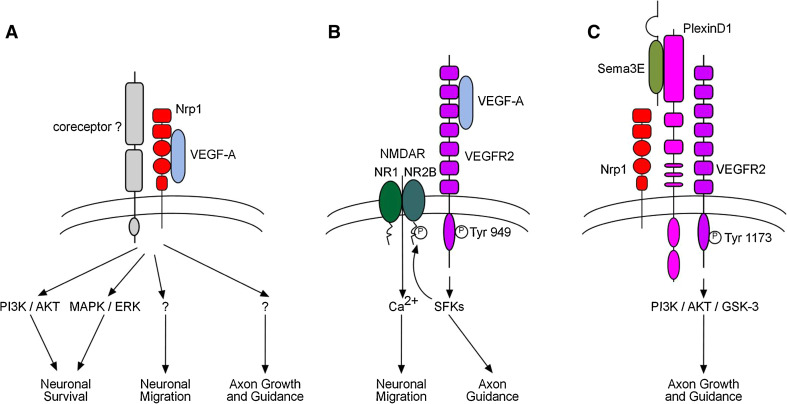
Fig. 4.
VEGF signaling in the developing nervous system. Schematic outline of the interactions of VEGF-A with its receptors Neuropilin-1 (a) and VEGFR2 (b) in neural cells. The network of intracellular signal transduction pathways results in biological responses such as neural survival, migration, axon growth, and guidance, which are required for nervous system patterning and wiring. c Schematic diagram of Sema3E receptor complex. VEGFR2 heterodimerizes with its coreceptors Neuropilin-1 and PlexinD1 and transduces growth-promoting and attractive Sema3E signal. See the main text for details. ERK extracellular signal-regulated kinases; GSK-3 glycogen synthase kinase-3; MAPK mitogen-activated protein kinase; NMDAR N-methyl-d-aspartic acid receptor; Nrp1 Neuropilin-1; PI3K phosphoinositide 3-kinase; SFKs Src family kinases; Tyr tyrosine residue
Control of neuronal migration
VEGF-A/Neuropilin-1 controls neuronal migration
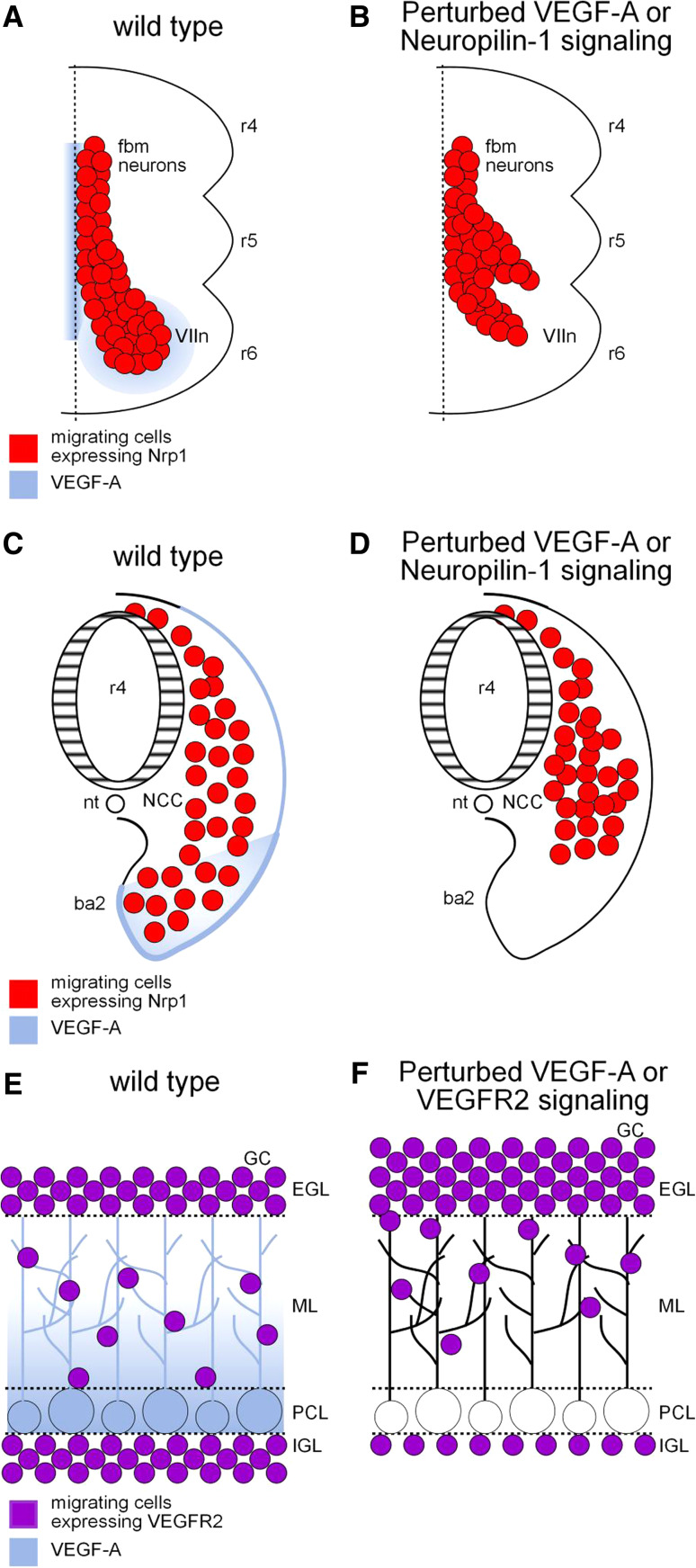
VEGF-A has first been reported to control neural cell migration in a study on the development of the facial motor nucleus, which contains branchiomotor neurons that control the movement of the muscles of facial expression (Fig. 2a, b; [113]). In the mouse, facial branchiomotor (fbm) neurons are generated in the hindbrain segment, or rhombomere, 4 and migrate caudally into rhombomere 6 where they form the facial motor nucleus. Concomitantly, fbm axons exit the neural tube and extend into the second branchial arch (ba2) to make synapses on their appropriate target muscles. The migration behavior of fbm neurons is severely perturbed in Neuropilin-1-null embryos, leading to mispositioned and misshaped facial motor nuclei [113]. In contrast, normal fbm migration was found in endothelial cell-specific Neuropilin-1 mutants, indicating that somata migration defects in full Neuropilin-1 knockout were not secondary to vascular defects [113]. In fact, proper somata migration was shown to rely on a chemotropic activity of VEGF-A164, which is expressed along the migratory path of fbm neurons and in a domain corresponding to the facial nucleus assembly site. Indeed, blocking VEGF-A164 function using blocking antibodies or using mouse embryos expressing only the VEGF-A120 isoform caused somata migration defects similar to that observed in Neuropilin-1-deficient mice (Fig. 2a, b). Interestingly, in addition to its effect on somata migration, Neuropilin-1 also regulates axonal projections of fbm neurons into ba2, which appear defasciculated in Neuropilin-1-null embryos. This effect, however, was found independent of VEGF-A164 function and was rather due to a loss of response to the axon guidance molecule Sema3A, another ligand of Neuropilin-1 [113]. Thus, the different Neuropilin-1 ligands appear to cooperate to pattern different compartments of the facial nerve, with VEGF-A controlling somata migration within the hindbrain and Sema3A guiding axons in the periphery.
Fig. 2.
VEGF-A regulates neural cell migration. a Schematic of facial branchiomotor neuron migration in the segmented mouse hindbrain. Facial branchiomotor neurons express Neuropilin-1 and migrate along a VEGF-A164-rich pathway from rhombomere (r) 4 into r6 where they form the facial motor nucleus (VIIn). b Disruption of VEGF-A164-Neuropilin-1 signaling leads to mispositioned and mishaped facial motor nucleus. c Schematic of neural crest cell migration from r4 to the second branchial arch in the chick embryo. VEGF-A is expressed in the ectoderm overlying the NCC migratory stream and in the second branchial arch tissue. d Neural crest cells fail to properly invade the second branchial arch when VEGF-A or Neuropilin-1 signaling is disrupted. e Schematic of cerebellar granule cell migration during early postnatal mouse development. Granule cell precursors in the external granule cell layer proliferate, then differentiate and migrate through the molecular layer past the Purkinje cells to their destination, the internal granule cell layer. A radial concentration gradient of VEGF-A isoforms provide one of the directional signals responsible for the inward migration of VEGFR2-expressing granule cells. f Inhibition of VEGF-A-VEGFR2 signaling delays granule cell migration. ba2 second branchial arch; EGL external granular cell layer; fbm neurons facial branchiomotor neurons; GC granule cells; IGL internal granular cell layer; ML molecular layer; NCC neural crest cells; nt notochord; PCL Purkinje cell layer; r, rhombomere; VIIn facial motor nucleus
A prominent role of Sema3A, and a general lack of effect of VEGF-A, in the patterning of peripheral axon projections has been confirmed in the developing mouse limb [32], although, as we will discuss below, VEGF-A has the potential to guide axons in the central nervous system. The expression of VEGF-A in peripheral tissues is primarily involved in patterning of the vascular network instead [32]. Nevertheless, a recent study reported that peripheral expression of VEGF-A also contributes to the migration of neural crest cells (NCCs), a stem cell population that delaminates from the developing neural tube and migrates to different regions of the embryo where they differentiate into various cell types (Fig. 2c, d). In the cranial region, NCC derivatives include neurons and glia of the cranial ganglia as well as bone and cartilage of the face and neck [114–117]. An RNAi–based approach in the chick embryo has identified a cell-autonomous function of Neuropilin-1 in regulating the entry of cranial NCCs derived from rhombomere 4 into ba2 [118]. VEGF-A is expressed in the surface ectoderm of the growing ba2 that overlays the rhombomere 4 NCC migratory stream, suggesting a potential influence of VEGF-A on NCC migration [119]. This was indeed demonstrated in vivo where ectopic sources of VEGF-A165 chemoattracted cranial NCCs through a mechanism requiring Neuropilin-1 in NCCs [119]. Moreover, NCCs failed to migrate properly into ba2 when VEGF-A signal was disrupted using the competitive antagonist sVEGFR1 (Fig. 2c, d; [119]).
Together, these studies indicate that VEGF-A/Neuropilin-1 signaling controls the migration of developing neurons and NCCs, some of which contribute to neurons and glia of the peripheral nervous system. It remains to be seen whether Neuropilin-1 functions on its own or with a co-receptor in VEGF-A signaling (Fig. 4a). Although VEGFR2 is expressed in chick cranial NCCs [119], its ability to act a signal transducing component in VEGF-A-induced migration has not been directly evaluated in this model. Neuropilin-1 can also partner with several other receptors, such as Plexins [22], IgCAM (L1, CHL1) [120, 121], Roundabout (Robo1) [122], and c-Met [123–125], all of which are expressed in neural cells.
VEGF-A/VEGFR2 signaling in control of neuronal migration
Although the expression of VEGFR2 in a variety of neuronal and glial cell types has been recognized for about a decade, its function in the developing nervous system has remained unknown until very recently. This probably stems from the fact that nervous system-specific deletion of Vegfr2 in mice did not cause any gross cerebral abnormalities, in contrast to the severe effects that lowering VEGF-A levels had on brain development [111]. Therefore it has often been argued that neural VEGFR2 only plays a minor role, if any, in mediating VEGF-A activity in development. In fact, the first function of neural VEGFR2 discovered was independent of VEGF-A family ligands, but involved VEGFR2 acting as a co-receptor for Neuropilin-1 and PlexinD1 in semaphorin signaling (see below, [126]). Shortly after, a role for VEGF-A/VEGFR2 was identified during neuronal migration in the developing mouse cerebellar cortex (Fig. 2e, f; [127, 128]).
During early postnatal development in the mouse, the granule cells (GCs), the most abundant interneurons in the cerebellum, proliferate in the external granular cell layer (EGL) and migrate radially across the molecular layers (ML) past the Purkinje cell layer (PCL) to form the internal granular cell layer (IGL). The Bergmann glia, a specialized type of astrocyte with radial processes, provides a scaffold for GC migration [129]. In addition, molecular cues provide motogenic and chemotactic signals that orchestrate the migration of GCs toward their proper target positions [130–132]. VEGF-A has recently been identified as a novel extrinsic factor that controls GCs directional migration. In the postnatal mouse cerebellum, migrating GCs express VEGFR2 and VEGF-A is expressed by Purkinje cells and to a lesser degree by Bergmann glia [128]. The use of transgenic mice expressing only VEGF-A120, an isoform lacking affinity for HSPGs, revealed diffuse VEGF-A staining distant from the cell bodies of PCL, whereas mice predominantly expressing the heparin-binding VEGF-A188 isoform localize VEGF-A distribution around the cell bodies and dendrites of Purkinje cells [128]. Thus VEGF-A distributes along a radial concentration gradient, with high levels in the PCL and progressively lower levels in the upper layers (Fig. 2e; [128]). Loss- and gain-of-function experiments performed in vivo or in cerebellar slices have shown that VEGF-A attracts migration of GCs from the EGL toward the IGL (Fig. 2f). This effect, however, was transient, and GCs eventually resumed migration in adult mice. Elegant experiments additionally provided evidence that the matrix binding activity of VEGF-A is required for this activity. Indeed in knock-in mice expressing only the heparin-binding, short range isoform VEGF-A188, GC migration was enhanced. Conversely, in knock-in mice expressing only the diffusible VEGF-A120 isoform, fewer GC reached their final destination in the IGL [128].
The chemotactic activity of VEGF-A for GCs is mediated by direct activation of VEGFR2 in GCs, independently of its angiogenic activity, since selective inactivation of Vegfr2 in GCs using an inducible Cre/Lox-mediated transgenic approach was found to inhibit GC migration (Fig. 2e, f; [128]). Importantly, this activity appears independent of Neuropilins, whose expression is not detectable in migrating GCs [128]. In fact, analysis of the signaling mechanism downstream of VEGF-A has implicated N-methyl d-aspartate (NMDA)-type glutamate receptors, which have been previously shown to regulate the radial migration of GCs [133–136]. In migrating GCs, NMDA receptors are composed of NR1 and NR2B subunits, with the latter being particularly important in regulating the calcium influx that helps the cells migrate to their final position [135]. Stimulation of VEGFR2 by VEGF-A has been shown to induce clustering of VEGFR2 and NR2B, to increase tyrosine phosphorylation of NR2B via Src Family Kinase and to amplify NMDA-mediated calcium influx and currents in GCs (Fig. 4b; [127]). These findings thus identify VEGF-A/VEGFR2 signaling as a critical pathway involved in the proper migration of neurons and revealed an unexpected cross-talk between VEGF-A and a receptor of a classical neurotransmitter.
Role of VEGF family ligands in the migration of glial cells
VEGF family ligands and receptors are expressed in glial cells of the developing brain [100, 137, 138]. In vitro, VEGF-A, VEGF-C, and VEGF-D have been reported to exert a chemokinetic effect on glial cell precursors, including oligodendrocyte precursor cells or OPCs [98, 100, 137]. The migratory effect of VEGF-A on OPCs from postnatal cerebral cortex is mediated by VEGFR2 and involves the production of reactive oxygen species and activation of two focal adhesion–associated proteins, focal adhesion kinase (FAK), and paxillin [98]. VEGF-C, on the other hand, has been reported to increase the non-directional migration of OPCs from the embryonic optic nerve. VEGF-C likely acts through VEGFR3, since a recombinant mutant VEGF-C, which is unable to bind to VEGFR2 and therefore selectively activates VEGFR3 [139], efficiently stimulates OPC migration [100]. Yet, the physiological and developmental significance of these in vitro responses is unclear, and although Vegfc-deficient mouse embryos showed a reduction in the number of OPCs colonizing the optic nerve [100], it remains to be determined whether this results from a direct effect of VEGF-C on OPC migration or from another biological function of VEGF-C.
Control of axon growth and guidance
Concomitant with the migration of neuronal cell bodies to their final destination in the developing nervous system, neurons extend axons toward long-distance targets. The directed growth of neuronal axons is regulated by attractive or repulsive extracellular signals that exert their actions either locally or after diffusing away from their source of origin. The list of instructive cues for growing axons has grown considerably since the discovery, in the late 1990s, of four families of canonical guidance cues (Semaphorins, Ephrins, Netrins and Slits). It now includes a number of signals primarily involved in other aspects of embryo development that also serve a role in axon guidance, one of the best examples being the morphogen family proteins (reviewed in [140]). The following chapters will review recent studies, which provide novel in vivo evidence that VEGF-A and VEGFR2 can function together or independently from each other to control axonal growth cone behavior and pattern projections in the nervous system.
VEGFR2 conveys Semaphorin signal during axon development
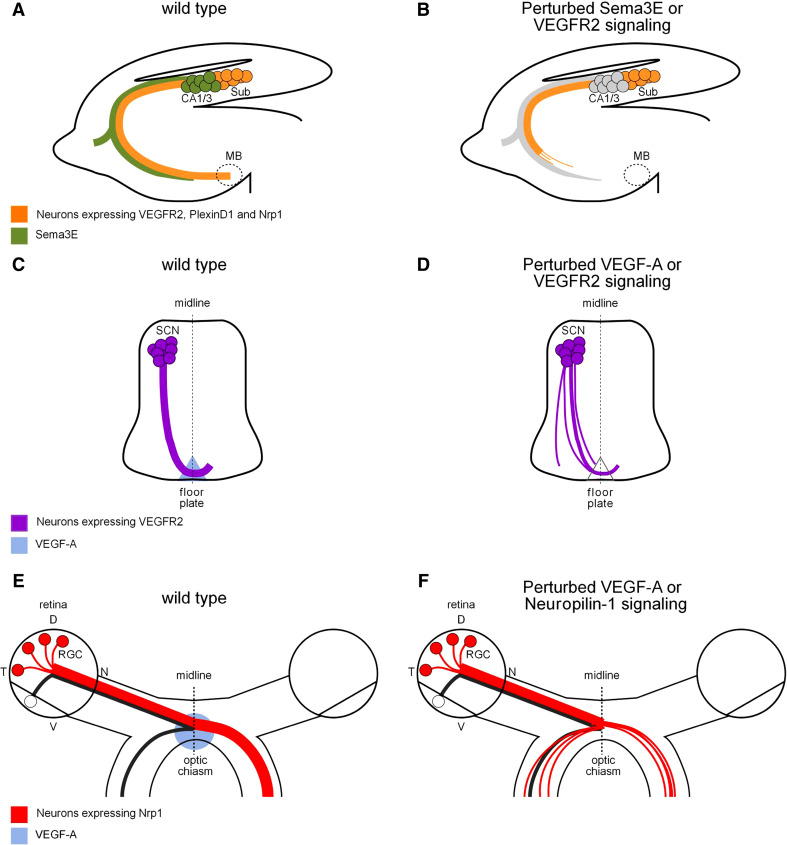
The first evidence that VEGFR2 can direct axon growth in vivo comes from the study of the development of the hippocampal formation, a key structure for the coding of anxiety, learning, and memory in the brain. The subiculum is a pivotal subregion of the hippocampal formation, whose projections form the major hippocampal output path to the mammillary bodies [141–143]. In the prenatal rodent brain, VEGFR2 is expressed in neurons of the subiculum around the time that they elaborate their axonal projections [99, 126]. It is also detected in glial fibrillary acidic protein (GFAP)-positive astroglial cells in the fimbria of the hippocampus, through which the fibers of the subiculum pass [126]. Analysis of mice with a conditional inactivation of Vegfr2 in neurons and glial cells (Nestin-Cre) revealed an abnormal development of the subiculo-mammillary tract, with axons failing to fasciculate properly and to project all the way down to their target (Fig. 3a, b; [126]). In contrast, GFAP-Cre-mediated deletion of Vegfr2 did not affect the development of subiculo-mammillary projections (F. Mann and J. Haigh, unpubl. obs.). Thus, VEGFR2 appeared to be required cell-autonomously for the proper development of subicular projections.
Fig. 3.
Novel role for VEGF-A in axonal wiring. a Schematic representation of the pathways taken by subiculo-mammillary projections in the developing mouse brain. VEGFR2 expression by subicular axons is involved in the recognition mechanism of the attractive/growth-promoting factor Sema3E, supplied locally by efferent CA1/3 axons. b Genetic ablation of Sema3E or neural VEGFR2 results in a hypoplastic subiculo-mammillary tract with few axons reaching their appropriate target, even at adult stages. c Schematic of spinal commissural axon projection toward and across the floor plate in the mouse embryo. Commissural axons express VEGFR2 and are attracted to the ventral midline by VEGF-A secreted from the floor plate. d Deleting VEGFR2 in spinal commissural neurons or lowering VEGF-A levels in the floor plate cause commissural axon pathway defects, including defasciculation and axonal misprojections to the lateral edge of the spinal cord. e Schematic representation of the routing of retinal ganglion cell axons at the optic chiasm to the appropriate hemisphere of the mouse brain. Ganglion cells giving rise to uncrossed axons are located in the ventrotemporal retina, whereas ganglion cells in the other retinal quadrants cross over the optic chiasm. Crossing axons express Neuropilin-1 and are guided across the optic chiasm by VEGF-A164. f Disrupted VEGF-A164-Neuropilin-1 signaling induces axon defasciculation and ipsilateral misprojections. CA1/3 Cornu Ammonis 1 and 3; D dorsal retina; MB mammillary bodies; N nasal retina; RGC retinal ganglion cells; SCN spinal commissural neurons; Sub subiculum; T temporal retina; V ventral retina
The impairment in subiculo-mammillary tract development in the absence of neural VEGFR2 suggested that the receptor is involved in the detection of environmental cues promoting and/or directing axon growth. Surprisingly, none of the VEGF family ligands known to interact with VEGFR2 (including VEGF-A164, VEGF-A120, VEGF-C and VEGF-D) were able to stimulate elongation of subicular axons in vitro [126]. Instead, neural cell-specific Vegfr2 mutants showed a subiculo-mammillary phenotype similar to the previously reported Sema3E null mice (Fig. 3a, b; [55]), pointing to a possible link between Semaphorin signaling and VEGFR2 function. While Semaphorins most frequently act as repulsive guidance cues, instead, Sema3E attracts subicular axons and enhances their growth along the subiculo-mammillary pathway [55]. Subicular neurons harvested from Vegfr2 mutants, however, failed to respond to Sema3E in vitro [126]. On a mechanistic point of view, it has been shown that VEGFR2 is part of a tripartite receptor complex for Sema3E, which comprises the Sema3E binding subunit PlexinD1 as well as the “gating” co-receptor Neuropilin-1 required to switch responses to Sema3E from repulsion to attraction (Fig. 4c; [55, 126]). In attempts to reconstitute a functional receptor complex in heterologous neuronal cells, co-expression of VEGFR2 with truncated PlexinD1 and Neuropilin-1 both lacking intracellular domains was sufficient to enable axon growth response to Sema3E, indicating that VEGFR2 serves as the signal transducing subunit [126]. Binding of Sema3E to the trimeric PlexinD1/Neuropilin-1/VEGFR2 receptor complex induces tyrosine phosphorylation of VEGFR2 on Tyr 1175 and 1214, with Tyr 1175 being sufficient to activate the PI3K/Akt/GSK-3 signaling pathway, which is essential for subicular axon growth response to Sema3E (Fig. 4c). Here, the downstream cascade is not followed further but it is intriguing to speculate an involvement of CRMP2 or other microtubule associated proteins, which have been shown to regulate axon growth though GSK-3 [144–146].
Together, these results revealed a novel interplay between vascular and neural guidance molecules and an unexpected VEGF-independent function of VEGFR2, which can regulate growth and guidance of neuronal axons in response to Semaphorin cues.
VEGF-A as an axon guidance cue
Two recent reports have provided physiological evidence that VEGF-A exerts a direct effect on growing axons to pattern neuronal connections in the mouse central nervous system ([147, 148]; Fig. 3c–f). These studies investigated how axon tracts, known as commissures, cross the midline that separates the two halves of the nervous system. One of the most studied models of crossing regulation is the development of commissural interneurons of the spinal cord. Commissural interneurons located in the dorsal aspect of the spinal cord grow to the ventral midline in response to long-range chemoattractants emanating from this region. Two major guidance cues have been implicated in commissural interneurons guidance, including Netrin-1 and the morphogen Sonic Hedgehog (Shh) (reviewed in [149]). A search for additional guidance factors has revealed that the growth factor VEGF-A is expressed and secreted by the midline floor plate at the time when commissural axons project ventrally [148]. Lowering VEGF-A expression in the floor plate with a Hoxa1-cre based recombination approach, without affecting the other floor plate chemoattractants, caused commissural axons to grow in a disorganized and defasciculated manner. While the majority of them eventually reached the midline, a subset of fibers was found projecting ectopically to the lateral edge of the spinal cord (Fig. 3c–d; [148]). Thus, VEGF-A appears to function as an attractive guidance cue to direct the growth of commissural interneuron axons towards the midline.
The other study reports the expression of VEGF-A at the optic chiasm midline, a structure where retinal axons from either eye enter the brain and diverge to project ipsilaterally or contralaterally, a first step toward the generation of binocular vision (Fig. 3e, f; [147]). The mechanisms driving ipsilaterally projecting axons have begun to be understood and involve ephrin-B ligands in repulsive sorting of ipsilaterally projecting axons in the chiasm [150, 151]. In contrast, the mechanisms that allow contralaterally projecting axons to extend across the chiasm midline have remained unknown. An important function of VEGF-A164 in this process has been demonstrated through the analysis of retinal projections in mice expressing VEGF-A120 only. Despite normal vascular development and appropriate expression of other midline cues, the mice displayed an increased proportion of axons that did not grow across the optic chiasm and projected into the ipsilateral optic tract, which appeared abnormally defasciculated (Fig. 3e, f; [147]). This study thus supplied additional evidence that VEGF-A164 is an essential cue that provides growth-promoting and chemoattractive signal for midline crossing axons.
While both studies support a role of VEGF-A in regulating axon guidance and fasciculation, they also highlight important differences in the modes of action of VEGF-A between the two systems. The first difference lies in the cell surface receptor mediating VEGF-A signal. Indeed, the two VEGF-A binding receptors VEGFR2 and Neuropilin-1 present mutually exclusive expression profiles in midline crossing axons of the spinal cord and retina (Fig. 3c–f). Spinal commissural neurons express VEGFR2, but not Neuropilin-1, and conditional deletion of Vegfr2 in the dorsal spinal cord (Wnt1-cre) of mouse embryos caused defects in commissural axon pathfinding, which appeared qualitatively similar, albeit more severe, to those caused by lowering VEGF-A levels at the midline [148]. The possibility that axonal VEGFR2 acts downstream of a midline-derived Sema3E signal was ruled out, since Sema3E does not affect the growth of commissural axon either in vitro or in vivo [148]. In contrast, neurons of the retina that send their axons to the contralateral half of the brain express Neuropilin-1, but not VEGFR2 [147]. In vivo loss of Neuropilin-1 function induced ectopic ipsilateral projections, similar to what observed in mice expressing only the non-Neuropilin-binding VEGF-A120 isoform [147]. Moreover, development of visual projections proceeded normally in mouse embryos expressing the Neuropilin-1sema− variant, confirming that Neuropilin-1 mediates VEGF-A, but not Semaphorin signals, in this system [147]. Thus, unlike the situation in the vascular system where VEGF-A directs endothelial cell migration by signaling through a Neuropilin-1-VEGFR2 complex, VEGF-A selectively engages one or the other molecule to mediate its effects on developing axons.
Comparisons between the two studies reveal further differences in the range at which the guidance effects of VEGF-A are manifested. In the spinal cord, VEGF-A drives pre-crossing commissural axon growth toward the midline, indicating that it functions far away from its source of production, as a long-range guidance cue. In the chiasm model, however, axon extension toward the midline is independent of VEGF-A, which only regulates axon behavior at the midline choice point, indicating a local, short range action. VEGF-A isoform-specific signaling through Neuropilin-1 and VEGFR2 receptors could explain these differences. Indeed, in vitro and in vivo Neuropilin-1 mediates the guidance function of VEGF-A164, but not that of the freely diffusible VEGF-A120 [147], suggesting that retinal axons sense and respond to cell and matrix-bound VEGF-A isoforms locally expressed at the optic chiasm. Whether different VEGF-A isoforms have differential effects on VEGFR2-expressing commissural axons in the spinal cord has not been addressed. However, since VEGFR2 can bind all VEGF-A isoforms, one can expect that VEGFR2-expressing commissural axons respond to both VEGF-A120 and VEGF-A164, which may diffuse from the spinal cord midline and together form a long-range concentration gradient [2]. Differences between short and long-range actions of VEGF-A could also be due to proteolytic processing by the serine protease plasmin [152, 153] or matrix metalloproteinases (MMPs) [154], two mechanisms that contribute to regulate the bioavailability of VEGF-A by generating soluble VEGF-A species. In this context, it is particularly interesting to note that tissue-type plasminogen activator (tPA), an enzyme which converts plasminogen to plasmin, is expressed specifically in the floor plate of the developing spinal cord, but no in the optic chiasm [155]. Locally high concentration of plasmin at the spinal cord midline may thus serves to cleave bound forms of VEGF-A, releasing a soluble factor that exerts its effect at some distance from the midline.
Experiments that have examined in details the effect of VEGF-A on axon behavior in vitro revealed an additional level of complexity. VEGF-A exerts a chemoattractive effect on both spinal and retinal commissural axons, however VEGF-A can stimulate the outgrowth of retinal axons, but not spinal cord commissural axons [147, 148]. It can be argued that axon growth and guidance are the result of the same pathways: directional turning of axons being viewed as an asymmetric outgrowth response in a non-uniform concentration of guidance cues. Consistently, several guidance factors have the ability to regulate both axon growth and guidance. There are, however, exceptions. Some factors, including Sema7A and Neuregulin-1, display axon outgrowth activity, while having no guidance activity on the same neuronal population [156, 157]. On the other hand, the midline factor Shh, similar to VEGF-A, attracts spinal commissural axons, but does not promote their growth [158]. The reasons underlying these differential activities remain unknown. The lack of outgrowth response in VEGF-A-mediated signaling through VEGFR2 is intriguing given the fact that signaling by VEGFR2 receptor controls axon outgrowth in response to Sema3E in another neuronal cell type [126]. One possibility is that ligand-dependent activation of different downstream signaling pathways produces different cellular responses. For example, VEGFR2 mediates the outgrowth effect of Sema3E through activation of the PI3K/Akt/GSK3 signaling pathway [126], whereas the guidance effect of VEGF-A relies on VEGFR2-induced activation of Src kinase ([148], Fig. 4b). By making a parallel with the vascular system, where internalization of VEGFR2 is required for Akt activation (see above), it can be hypothesized that VEGFR2 may undergo different intracellular trafficking pathways when signaling axon outgrowth or axon guidance.
Responsiveness of navigating axons to extracellular guidance cues is under constant and tight regulation. Silencing of responses to attractive midline-derived cues is particularly important to enable crossing commissural axons to grow past this intermediate target and progress along their pathway (reviewed in [159]). How spinal commissural axons modulate their sensitivity to VEGF-A at the midline is not known. Studies in the vascular system have provided several mechanisms by which VEGF-A signal can be silenced, including expression of the “decoy receptor” sVEGFR1 or up-regulation of the novel family of VEGFxxxb splice variants that act as competitive antagonists of VEGFR2 [160]. It will be interesting to determine whether similar mechanisms operate in the developing nervous system. Furthermore, almost all conventional axon guidance cues have bifunctional activities, acting as attractants or repellents, and several mechanisms have been proposed to regulate the switch between these activities. While VEGF-A has been clearly shown to exert attractive effect on commissural axons, it now remains to be determined if it can repellent or inhibit these or other axon types. So far, it has been reported that axonal outgrowth of cultured subicular neurons is reduced in the presence of exogenous VEGF-A120 [126]. Whether this in vitro observation has in vivo significance awaits further study.
Conclusions
VEGF induces endothelial cells to sprout and migrate to form new blood vessels during vascular development. The directed growth of nascent vessels responds to gradients of VEGF as well as other dynamic cues expressed in the extracellular environment, such as members of the “classical” neural guidance families that cooperate to provide instructions for proper patterning of vascular networks. In addition, neural guidance molecules have functions other than guidance in endothelial cells and exert control over VEGF-mediated tip cell selection and migration. VEGF also influences neuronal growth cone migration and directional guidance in the developing nervous system. In the future, it will be interesting to examine how vascular and neural guidance cues are integrated in growth cones in order to establish stereotypical neuronal pathways and how molecular interplays between VEGFRs and classical guidance receptors participate in the patterning process.
Acknowledgments
We are grateful to J.L. Thomas, A. Eichmann, and C. Ruhrberg for critical reading of the manuscript. This work was supported in part by the Centre National de la Recherche Scientifique, the Aix-Marseille University, the Institut Universitaire de France, the Agence Nationale de la Recherche (ANR-10-BLAN-1412, Angioneurins), the Fédération pour la Recherche sur le Cerveau and the Institut National du Cancer (2011-139).
References
- 1.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16(20):2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benest AV, Harper SJ, Herttuala SY, Alitalo K, Bates DO. VEGF-C induced angiogenesis preferentially occurs at a distance from lymphangiogenesis. Cardiovasc Res. 2008;78(2):315–323. doi: 10.1093/cvr/cvm094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu NJ, Henkemeyer M. Ephrin reverse signaling in axon guidance and synaptogenesis. Semin Cell Dev Biol. 2012;23(1):58–64. doi: 10.1016/j.semcdb.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cajal SRy (1890) A quelle epoque apparaissent les expansions des cellules nerveuses de la moelle épinière du poulet? Anat Anz 5:609–613
- 6.Ono K, Shokunbi T, Nagata I, Tokunaga A, Yasui Y, Nakatsuji N. Filopodia and growth cones in the vertically migrating granule cells of the postnatal mouse cerebellum. Exp Brain Res. 1997;117(1):17–29. doi: 10.1007/PL00005787. [DOI] [PubMed] [Google Scholar]
- 7.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2(5):a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436(7048):193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 9.Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452(7188):759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2(7):a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4(12):1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4(4):241–246. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484(7392):110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 14.Nicoli S, Knyphausen CP, Zhu LJ, Lakshmanan A, Lawson ND. miR-221 is required for endothelial tip cell behaviors during vascular development. Dev Cell. 2012;22(2):418–429. doi: 10.1016/j.devcel.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomaki A, Aranda E, Miura N, Yla-Herttuala S, Fruttiger M, Makinen T, Eichmann A, Pollard JW, Gerhardt H, Alitalo K. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13(10):1202–1213. doi: 10.1038/ncb2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliver G. Lymphatic vasculature development. Nat Rev Immunol. 2004;4(1):35–45. doi: 10.1038/nri1258. [DOI] [PubMed] [Google Scholar]
- 17.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465(7297):483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivee B, Del Toro R, Suchting S, Medvinsky A, Silva J, Yang J, Thomas JL, Koch AW, Alitalo K, Eichmann A, Bagri A. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol. 2010;188(1):115–130. doi: 10.1083/jcb.200903137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelfand MV, Hong S, Gu C. Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning. Trends Cell Biol. 2009;19(3):99–110. doi: 10.1016/j.tcb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99(1):59–69. doi: 10.1016/S0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 22.Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99(1):71–80. doi: 10.1016/S0092-8674(00)80063-X. [DOI] [PubMed] [Google Scholar]
- 23.Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121(12):4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- 24.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. doi: 10.1016/S0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 25.Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 2008;135(14):2479–2488. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 26.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126(21):4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 27.Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231(3):503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 28.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le Couter J, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11(1):53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5(1):45–57. doi: 10.1016/S1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Puschel AW, Bussolino F. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424(6947):391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 31.Bouvree K, Brunet I, Del Toro R, Gordon E, Prahst C, Cristofaro B, Mathivet T, Xu Y, Soueid J, Fortuna V, Miura N, Aigrot MS, Maden CH, Ruhrberg C, Thomas JL, Eichmann A. Semaphorin3A, neuropilin-1, and PLEXINA1 are required for lymphatic valve formation. Circ Res. 2012;111(4):437–445. doi: 10.1161/CIRCRESAHA.112.269316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vieira JM, Schwarz Q, Ruhrberg C. Role of the neuropilin ligands VEGF164 and SEMA3A in neuronal and vascular patterning in the mouse. Novartis Found Symp. 2007;283:230–235. doi: 10.1002/9780470319413.ch18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jurisic G, Maby-El Hajjami H, Karaman S, Ochsenbein AM, Alitalo A, Siddiqui SS, Ochoa Pereira C, Petrova TV, Detmar M. An unexpected role of semaphorin3A-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ Res. 2012;111(4):426–436. doi: 10.1161/CIRCRESAHA.112.269399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmeliet P, Collen D. Role of vascular endothelial growth factor and vascular endothelial growth factor receptors in vascular development. Curr Top Microbiol Immunol. 1999;237:133–158. doi: 10.1007/978-3-642-59953-8_7. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Mukhopadhyay D, Xu X. C terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. FASEB J. 2006;20(9):1513–1515. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- 36.Fantin A, Schwarz Q, Davidson K, Normando EM, Denti L, Ruhrberg C. The cytoplasmic domain of neuropilin 1 is dispensable for angiogenesis, but promotes the spatial separation of retinal arteries and veins. Development. 2011;138(19):4185–4191. doi: 10.1242/dev.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitaker GB, Limberg BJ, Rosenbaum JS. Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121) J Biol Chem. 2001;276(27):25520–25531. doi: 10.1074/jbc.M102315200. [DOI] [PubMed] [Google Scholar]
- 38.Evans IM, Yamaji M, Britton G, Pellet-Many C, Lockie C, Zachary IC, Frankel P. Neuropilin-1 signaling through p130Cas tyrosine phosphorylation is essential for growth factor-dependent migration of glioma and endothelial cells. Mol Cell Biol. 2011;31(6):1174–1185. doi: 10.1128/MCB.00903-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Puschel AW, Sanes JR, Castellani V. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron. 2005;48(1):63–75. doi: 10.1016/j.neuron.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 40.Kolk SM, Gunput RA, Tran TS, van den Heuvel DM, Prasad AA, Hellemons AJ, Adolfs Y, Ginty DD, Kolodkin AL, Burbach JP, Smidt MP, Pasterkamp RJ. Semaphorin 3F is a bifunctional guidance cue for dopaminergic axons and controls their fasciculation, channeling, rostral growth, and intracortical targeting. J Neurosci. 2009;29(40):12542–12557. doi: 10.1523/JNEUROSCI.2521-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung CY, Singh M, Ebaugh MJ, Brace RA. Vascular endothelial growth factor gene expression in ovine placenta and fetal membranes. Am J Obstet Gynecol. 1995;173(3 Pt 1):753–759. doi: 10.1016/0002-9378(95)90335-6. [DOI] [PubMed] [Google Scholar]
- 42.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J Biol Chem. 2000;275(38):29922. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 43.Poltorak Z, Cohen T, Sivan R, Kandelis Y, Spira G, Vlodavsky I, Keshet E, Neufeld G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem. 1997;272(11):7151–7158. doi: 10.1074/jbc.272.11.7151. [DOI] [PubMed] [Google Scholar]
- 44.Karkkainen MJ, Jussila L, Ferrell RE, Finegold DN, Alitalo K. Molecular regulation of lymphangiogenesis and targets for tissue oedema. Trends Mol Med. 2001;7(1):18–22. doi: 10.1016/S1471-4914(00)01864-5. [DOI] [PubMed] [Google Scholar]
- 45.Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129(20):4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 46.Caunt M, Mak J, Liang WC, Stawicki S, Pan Q, Tong RK, Kowalski J, Ho C, Reslan HB, Ross J, Berry L, Kasman I, Zlot C, Cheng Z, Le Couter J, Filvaroff EH, Plowman G, Peale F, French D, Carano R, Koch AW, Wu Y, Watts RJ, Tessier-Lavigne M, Bagri A. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13(4):331–342. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 47.Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, Mandron M, Herault JP, Neufeld G, Savi P, Herbert JM, Bono F. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108(4):1243–1250. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- 48.Karpanen T, Wirzenius M, Makinen T, Veikkola T, Haisma HJ, Achen MG, Stacker SA, Pytowski B, Yla-Herttuala S, Alitalo K. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am J Pathol. 2006;169(2):708–718. doi: 10.2353/ajpath.2006.051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ballmer-Hofer K, Andersson AE, Ratcliffe LE, Berger P. Neuropilin-1 promotes VEGFR-2 trafficking through Rab11 vesicles thereby specifying signal output. Blood. 2011;118(3):816–826. doi: 10.1182/blood-2011-01-328773. [DOI] [PubMed] [Google Scholar]
- 50.Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, Giordano FJ, Carmeliet P, Simons M. VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev Cell. 2010;18(5):713–724. doi: 10.1016/j.devcel.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naccache SN, Hasson T, Horowitz A. Binding of internalized receptors to the PDZ domain of GIPC/synectin recruits myosin VI to endocytic vesicles. Proc Natl Acad Sci USA. 2006;103(34):12735–12740. doi: 10.1073/pnas.0605317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salikhova A, Wang L, Lanahan AA, Liu M, Simons M, Leenders WP, Mukhopadhyay D, Horowitz A. Vascular endothelial growth factor and semaphorin induce neuropilin-1 endocytosis via separate pathways. Circ Res. 2008;103(6):e71–e79. doi: 10.1161/CIRCRESAHA.108.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hermans K, Claes F, Vandevelde W, Zheng W, Geudens I, Orsenigo F, De Smet F, Gjini E, Anthonis K, Ren B, Kerjaschki D, Autiero M, Ny A, Simons M, Dewerchin M, Schulte-Merker S, Dejana E, Alitalo K, Carmeliet P. Role of synectin in lymphatic development in zebrafish and frogs. Blood. 2010;116(17):3356–3366. doi: 10.1182/blood-2009-11-254557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai H, Reed RR. Cloning and characterization of neuropilin-1-interacting protein: a PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci. 1999;19(15):6519–6527. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, Segu L, Buhot MC, Jessell TM, Henderson CE, Mann F. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56(5):807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodger J, Salvatore L, Migani P. Should i stay or should i go? Ephs and ephrins in neuronal migration. Neurosignals. 2012;20(3):190–201. doi: 10.1159/000333784. [DOI] [PubMed] [Google Scholar]
- 57.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17(5):230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Triplett JW, Feldheim DA. Eph and ephrin signaling in the formation of topographic maps. Semin Cell Dev Biol. 2012;23(1):7–15. doi: 10.1016/j.semcdb.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13(3):295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–753. doi: 10.1016/S0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 61.Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104(1):57–69. doi: 10.1016/S0092-8674(01)00191-X. [DOI] [PubMed] [Google Scholar]
- 62.Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev Biol. 2004;271(2):263–271. doi: 10.1016/j.ydbio.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 63.Gerety E, Siegel A. Thoracic lymph node visualization after a peritoneal leak study. Clin Nucl Med. 2001;26(2):157–158. doi: 10.1097/00003072-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 64.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4(3):403–414. doi: 10.1016/S1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 65.Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326(5950):294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R. EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol Cell. 2002;9(4):725–737. doi: 10.1016/S1097-2765(02)00488-4. [DOI] [PubMed] [Google Scholar]
- 67.Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19(3):397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465(7297):487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 69.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174(4):593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, Folkman J, Gimbrone MA, Jr, Anderson DJ. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol. 2001;230(2):139–150. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- 71.Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7(1):107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307(5707):265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 73.Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7(1):117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 74.Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459(7248):842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meadows SM, Fletcher PJ, Moran C, Xu K, Neufeld G, Chauvet S, Mann F, Krieg PA, Cleaver O. Integration of repulsive guidance cues generates avascular zones that shape mammalian blood vessels. Circ Res. 2012;110(1):34–46. doi: 10.1161/CIRCRESAHA.111.249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, Hori M. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J. 2007;26(5):1373–1384. doi: 10.1038/sj.emboj.7601589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moriya J, Minamino T, Tateno K, Okada S, Uemura A, Shimizu I, Yokoyama M, Nojima A, Okada M, Koga H, Komuro I. Inhibition of semaphorin as a novel strategy for therapeutic angiogenesis. Circ Res. 2010;106(2):391–398. doi: 10.1161/CIRCRESAHA.109.210815. [DOI] [PubMed] [Google Scholar]
- 78.Zygmunt T, Gay CM, Blondelle J, Singh MK, Flaherty KM, Means PC, Herwig L, Krudewig A, Belting HG, Affolter M, Epstein JA, Torres-Vazquez J. Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Dev Cell. 2011;21(2):301–314. doi: 10.1016/j.devcel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 2011;25(13):1399–1411. doi: 10.1101/gad.2042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011;121(5):1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 82.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21(20):2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 83.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA. 2007;104(9):3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arima S, Nishiyama K, Ko T, Arima Y, Hakozaki Y, Sugihara K, Koseki H, Uchijima Y, Kurihara Y, Kurihara H. Angiogenic morphogenesis driven by dynamic and heterogeneous collective endothelial cell movement. Development. 2011;138(21):4763–4776. doi: 10.1242/dev.068023. [DOI] [PubMed] [Google Scholar]
- 85.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12(10):943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 86.Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 2002;79(4):547–552. doi: 10.1006/geno.2002.6745. [DOI] [PubMed] [Google Scholar]
- 87.Zheng W, Geng AQ, Li PF, Wang Y, Yuan XB. Robo4 regulates the radial migration of newborn neurons in developing neocortex. Cereb Cortex. 2011;22(11):2587–601. doi: 10.1093/cercor/bhr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261(1):251–267. doi: 10.1016/S0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 89.Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS, Lindblom P, Seth P, Frias A, Nishiya N, Ginsberg MH, Gerhardt H, Zhang K, Li DY. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14(4):448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marlow R, Binnewies M, Sorensen LK, Monica SD, Strickland P, Forsberg EC, Li DY, Hinck L. Vascular Robo4 restricts proangiogenic VEGF signaling in breast. Proc Natl Acad Sci USA. 2010;107(23):10520–10525. doi: 10.1073/pnas.1001896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suchting S, Heal P, Tahtis K, Stewart LM, Bicknell R. Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. FASEB J. 2005;19(1):121–123. doi: 10.1096/fj.04-1991fje. [DOI] [PubMed] [Google Scholar]
- 92.Johnson KG, Ghose A, Epstein E, Lincecum J, O’Connor MB, Van Vactor D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol. 2004;14(6):499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 93.Sheldon H, Andre M, Legg JA, Heal P, Herbert JM, Sainson R, Sharma AS, Kitajewski JK, Heath VL, Bicknell R. Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. FASEB J. 2009;23(2):513–522. doi: 10.1096/fj.07-098269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steigemann P, Molitor A, Fellert S, Jackle H, Vorbruggen G. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr Biol. 2004;14(3):225–230. doi: 10.1016/j.cub.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 95.Koch AW, Mathivet T, Larrivee B, Tong RK, Kowalski J, Pibouin-Fragner L, Bouvree K, Stawicki S, Nicholes K, Rathore N, Scales SJ, Luis E, del Toro R, Freitas C, Breant C, Michaud A, Corvol P, Thomas JL, Wu Y, Peale F, Watts RJ, Tessier-Lavigne M, Bagri A, Eichmann A. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev Cell. 2011;20(1):33–46. doi: 10.1016/j.devcel.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 96.London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, Day CW, Barnard DL, Zimmerman GA, Krasnow MA, Li DY. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2:23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hashimoto T, Zhang XM, Yang XJ. Expression of the Flk1 receptor and its ligand VEGF in the developing chick central nervous system. Gene Expr Patterns. 2003;3(1):109–113. doi: 10.1016/S1567-133X(02)00065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hayakawa K, Pham LD, Som AT, Lee BJ, Guo S, Lo EH, Arai K. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci. 2011;31(29):10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim HY, Choi JS, Cha JH, Choi JY, Lee MY. Expression of vascular endothelial growth factor receptors Flt-1 and Flk-1 in embryonic rat forebrain. Neurosci Lett. 2007;425(2):131–135. doi: 10.1016/j.neulet.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 100.Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, Haiko P, Karkkainen MJ, Yuan L, Muriel MP, Chatzopoulou E, Breant C, Zalc B, Carmeliet P, Alitalo K, Eichmann A, Thomas JL. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci. 2006;9(3):340–348. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- 101.Yang SZ, Zhang LM, Huang YL, Sun FY. Distribution of Flk-1 and Flt-1 receptors in neonatal and adult rat brains. Anat Rec A Discov Mol Cell Evol Biol. 2003;274(1):851–856. doi: 10.1002/ar.a.10103. [DOI] [PubMed] [Google Scholar]
- 102.Foehring D, Brand-Saberi B, Theiss C. VEGF-induced growth cone enhancement is diminished by inhibiting tyrosine-residue 1214 of VEGFR-2. Cells Tissues Organs. 2012;196(3):195–205. doi: 10.1159/000334600. [DOI] [PubMed] [Google Scholar]
- 103.Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66(3):236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- 104.Long JB, Jay SM, Segal SS, Madri JA. VEGF-A and Semaphorin3A: modulators of vascular sympathetic innervation. Dev Biol. 2009;334(1):119–132. doi: 10.1016/j.ydbio.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mani N, Khaibullina A, Krum JM, Rosenstein JM. Vascular endothelial growth factor enhances migration of astroglial cells in subventricular zone neurosphere cultures. J Neurosci Res. 2010;88(2):248–257. doi: 10.1002/jnr.22197. [DOI] [PubMed] [Google Scholar]
- 106.Rosenstein JM, Mani N, Khaibullina A, Krum JM. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23(35):11036–11044. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res. 1999;846(2):219–228. doi: 10.1016/S0006-8993(99)02056-9. [DOI] [PubMed] [Google Scholar]
- 108.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19(14):5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12(12):4243–4254. doi: 10.1046/j.0953-816X.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 110.Hashimoto T, Zhang XM, Chen BY, Yang XJ. VEGF activates divergent intracellular signaling components to regulate retinal progenitor cell proliferation and neuronal differentiation. Development. 2006;133(11):2201–2210. doi: 10.1242/dev.02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, Wagner EF, Betsholtz C, Nagy A. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262(2):225–241. doi: 10.1016/S0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 112.Cariboni A, Davidson K, Dozio E, Memi F, Schwarz Q, Stossi F, Parnavelas JG, Ruhrberg C. VEGF signalling controls GnRH neuron survival via NRP1 independently of KDR and blood vessels. Development. 2011;138(17):3723–3733. doi: 10.1242/dev.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwarz Q, Gu C, Fujisawa H, Sabelko K, Gertsenstein M, Nagy A, Taniguchi M, Kolodkin AL, Ginty DD, Shima DT, Ruhrberg C. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 2004;18(22):2822–2834. doi: 10.1101/gad.322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baker CV, Bronner-Fraser M. The origins of the neural crest. Part II: an evolutionary perspective. Mech Dev. 1997;69(1–2):13–29. doi: 10.1016/S0925-4773(97)00129-9. [DOI] [PubMed] [Google Scholar]
- 115.Baker CV, Bronner-Fraser M. The origins of the neural crest. Part I: embryonic induction. Mech Dev. 1997;69(1–2):3–11. doi: 10.1016/S0925-4773(97)00132-9. [DOI] [PubMed] [Google Scholar]
- 116.Kalcheim C, Le Douarin NM. Requirement of a neural tube signal for the differentiation of neural crest cells into dorsal root ganglia. Dev Biol. 1986;116(2):451–466. doi: 10.1016/0012-1606(86)90146-6. [DOI] [PubMed] [Google Scholar]
- 117.Le Douarin NM. The avian embryo as a model to study the development of the neural crest: a long and still ongoing story. Mech Dev. 2004;121(9):1089–1102. doi: 10.1016/j.mod.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 118.McLennan R, Kulesa PM. In vivo analysis reveals a critical role for neuropilin-1 in cranial neural crest cell migration in chick. Dev Biol. 2007;301(1):227–239. doi: 10.1016/j.ydbio.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 119.McLennan R, Teddy JM, Kasemeier-Kulesa JC, Romine MH, Kulesa PM. Vascular endothelial growth factor (VEGF) regulates cranial neural crest migration in vivo. Dev Biol. 2010;339(1):114–125. doi: 10.1016/j.ydbio.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27(2):237–249. doi: 10.1016/S0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- 121.Wright AG, Demyanenko GP, Powell A, Schachner M, Enriquez-Barreto L, Tran TS, Polleux F, Maness PF. Close homolog of L1 and neuropilin 1 mediate guidance of thalamocortical axons at the ventral telencephalon. J Neurosci. 2007;27(50):13667–13679. doi: 10.1523/JNEUROSCI.2888-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hernandez-Miranda LR, Cariboni A, Faux C, Ruhrberg C, Cho JH, Cloutier JF, Eickholt BJ, Parnavelas JG, Andrews WD. Robo1 regulates semaphorin signaling to guide the migration of cortical interneurons through the ventral forebrain. J Neurosci. 2011;31(16):6174–6187. doi: 10.1523/JNEUROSCI.5464-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu B, Guo P, Bar-Joseph I, Imanishi Y, Jarzynka MJ, Bogler O, Mikkelsen T, Hirose T, Nishikawa R, Cheng SY. Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene. 2007;26(38):5577–5586. doi: 10.1038/sj.onc.1210348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matsushita A, Gotze T, Korc M. Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res. 2007;67(21):10309–10316. doi: 10.1158/0008-5472.CAN-07-3256. [DOI] [PubMed] [Google Scholar]
- 125.Zhang S, Zhau HE, Osunkoya AO, Iqbal S, Yang X, Fan S, Chen Z, Wang R, Marshall FF, Chung LW, Wu D. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol Cancer. 2010;9:9. doi: 10.1186/1476-4598-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bellon A, Luchino J, Haigh K, Rougon G, Haigh J, Chauvet S, Mann F. VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron. 2010;66(2):205–219. doi: 10.1016/j.neuron.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 127.Meissirel C, Ruiz de Almodovar C, Knevels E, Coulon C, Chounlamountri N, Segura I, de Rossi P, Vinckier S, Anthonis K, Deleglise B, de Mol M, Ali C, Dassonville K, Loyens E, Honnorat J, Michotte Y, Rogemond V, Smolders I, Voets T, Vivien D, Vanden Berghe P, Van den Bosch L, Robberecht W, Chedotal A, Oliviero S, Dewerchin M, Schmucker D, Thomasset N, Salin P, Carmeliet P. VEGF modulates NMDA receptors activity in cerebellar granule cells through Src-family kinases before synapse formation. Proc Natl Acad Sci USA. 2011;108(33):13782–13787. doi: 10.1073/pnas.1100341108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ruiz de Almodovar C, Coulon C, Salin PA, Knevels E, Chounlamountri N, Poesen K, Hermans K, Lambrechts D, Van Geyte K, Dhondt J, Dresselaers T, Renaud J, Aragones J, Zacchigna S, Geudens I, Gall D, Stroobants S, Mutin M, Dassonville K, Storkebaum E, Jordan BF, Eriksson U, Moons L, D’Hooge R, Haigh JJ, Belin MF, Schiffmann S, Van Hecke P, Gallez B, Vinckier S, Chedotal A, Honnorat J, Thomasset N, Carmeliet P, Meissirel C. Matrix-binding vascular endothelial growth factor (VEGF) isoforms guide granule cell migration in the cerebellum via VEGF receptor Flk1. J Neurosci. 2010;30(45):15052–15066. doi: 10.1523/JNEUROSCI.0477-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971;141(3):283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- 130.Borghesani PR, Peyrin JM, Klein R, Rubin J, Carter AR, Schwartz PM, Luster A, Corfas G, Segal RA. BDNF stimulates migration of cerebellar granule cells. Development. 2002;129(6):1435–1442. doi: 10.1242/dev.129.6.1435. [DOI] [PubMed] [Google Scholar]
- 131.Kerjan G, Dolan J, Haumaitre C, Schneider-Maunoury S, Fujisawa H, Mitchell KJ, Chedotal A. The transmembrane semaphorin Sema6A controls cerebellar granule cell migration. Nat Neurosci. 2005;8(11):1516–1524. doi: 10.1038/nn1555. [DOI] [PubMed] [Google Scholar]
- 132.Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19(1):39–50. doi: 10.1016/S0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 133.Mancini JD, Atchison WD. The NR2B subunit in NMDA receptors is functionally important during cerebellar granule cell migration. Neurosci Lett. 2007;429(2–3):87–90. doi: 10.1016/j.neulet.2007.09.079. [DOI] [PubMed] [Google Scholar]
- 134.Rossi DJ, Slater NT. The developmental onset of NMDA receptor-channel activity during neuronal migration. Neuropharmacology. 1993;32(11):1239–1248. doi: 10.1016/0028-3908(93)90018-X. [DOI] [PubMed] [Google Scholar]
- 135.Tarnok K, Czondor K, Jelitai M, Czirok A, Schlett K. NMDA receptor NR2B subunit over-expression increases cerebellar granule cell migratory activity. J Neurochem. 2008;104(3):818–829. doi: 10.1111/j.1471-4159.2007.05051.x. [DOI] [PubMed] [Google Scholar]
- 136.Watanabe M, Mishina M, Inoue Y. Distinct distributions of five NMDA receptor channel subunit mRNAs in the brainstem. J Comp Neurol. 1994;343(4):520–531. doi: 10.1002/cne.903430403. [DOI] [PubMed] [Google Scholar]
- 137.Kranich S, Hattermann K, Specht A, Lucius R, Mentlein R. VEGFR-3/Flt-4 mediates proliferation and chemotaxis in glial precursor cells. Neurochem Int. 2009;55(8):747–753. doi: 10.1016/j.neuint.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 138.Wuestefeld R, Chen J, Meller K, Brand-Saberi B, Theiss C. Impact of VEGF on astrocytes: analysis of gap junctional intercellular communication, proliferation, and motility. Glia. 2012;60(6):936–947. doi: 10.1002/glia.22325. [DOI] [PubMed] [Google Scholar]
- 139.Joukov V, Kumar V, Sorsa T, Arighi E, Weich H, Saksela O, Alitalo K. A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation, and vascular permeability activities. J Biol Chem. 1998;273(12):6599–6602. doi: 10.1074/jbc.273.12.6599. [DOI] [PubMed] [Google Scholar]
- 140.Kolodkin AL, Tessier-Lavigne M (2011) Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol 3 (6) [DOI] [PMC free article] [PubMed]
- 141.Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J Comp Neurol. 2000;419(2):205–222. doi: 10.1002/(SICI)1096-9861(20000403)419:2<205::AID-CNE5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 142.Krout KE, Belzer RE, Loewy AD. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2002;448(1):53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- 143.Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172(1):49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- 144.Cole AR, Knebel A, Morrice NA, Robertson LA, Irving AJ, Connolly CN, Sutherland C. GSK-3 phosphorylation of the Alzheimer epitope within collapsin response mediator proteins regulates axon elongation in primary neurons. J Biol Chem. 2004;279(48):50176–50180. doi: 10.1074/jbc.C400412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, Kaibuchi K. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002;4(8):583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- 146.Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4(8):781–782. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- 147.Erskine L, Reijntjes S, Pratt T, Denti L, Schwarz Q, Vieira JM, Alakakone B, Shewan D, Ruhrberg C. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70(5):951–965. doi: 10.1016/j.neuron.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ruiz de Almodovar C, Fabre PJ, Knevels E, Coulon C, Segura I, Haddick PC, Aerts L, Delattin N, Strasser G, Oh WJ, Lange C, Vinckier S, Haigh J, Fouquet C, Gu C, Alitalo K, Castellani V, Tessier-Lavigne M, Chedotal A, Charron F, Carmeliet P. VEGF mediates commissural axon chemoattraction through its receptor Flk1. Neuron. 2011;70(5):966–978. doi: 10.1016/j.neuron.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dickson BJ, Zou Y. Navigating intermediate targets: the nervous system midline. Cold Spring Harb Perspect Biol. 2010;2(8):a002055. doi: 10.1101/cshperspect.a002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nakagawa S, Brennan C, Johnson KG, Shewan D, Harris WA, Holt CE. Ephrin-B regulates the Ipsilateral routing of retinal axons at the optic chiasm. Neuron. 2000;25(3):599–610. doi: 10.1016/S0896-6273(00)81063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39(6):919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 152.Claffey KP, Senger DR, Spiegelman BM. Structural requirements for dimerization, glycosylation, secretion, and biological function of VPF/VEGF. Biochim Biophys Acta. 1995;1246(1):1–9. doi: 10.1016/0167-4838(94)00144-6. [DOI] [PubMed] [Google Scholar]
- 153.Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, Ferrara N. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996;271(10):5638–5646. doi: 10.1074/jbc.271.10.5638. [DOI] [PubMed] [Google Scholar]
- 154.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169(4):681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sumi Y, Dent MA, Owen DE, Seeley PJ, Morris RJ. The expression of tissue and urokinase-type plasminogen activators in neural development suggests different modes of proteolytic involvement in neuronal growth. Development. 1992;116(3):625–637. doi: 10.1242/dev.116.3.625. [DOI] [PubMed] [Google Scholar]
- 156.Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marin O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125(1):127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424(6947):398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 158.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113(1):11–23. doi: 10.1016/S0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 159.Chedotal A. Further tales of the midline. Curr Opin Neurobiol. 2011;21(1):68–75. doi: 10.1016/j.conb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 160.Harris S, Craze M, Newton J, Fisher M, Shima DT, Tozer GM, Kanthou C. Do anti-angiogenic VEGF (VEGFxxxb) isoforms exist? A cautionary tale. PLoS One. 2012;7(5):e35231. doi: 10.1371/journal.pone.0035231. [DOI] [PMC free article] [PubMed] [Google Scholar]