Abstract
Kidneys are highly complex organs, playing a crucial role in human physiopathology, as they are implicated in vital processes, such as fluid filtration and vasomotor tone regulation. There is growing evidence that gap junctions are major determinants of renal physiopathology. It has been demonstrated that their expression or channel activity may vary depending on physiological and pathological situations within distinct renal compartments. While some studies have focused on the role of connexins in renal physiology, our knowledge regarding the functional relevance of pannexins is still very limited. In this paper, we provide an overview of the involvement of connexins, pannexins and their channels in various physiological processes related to different renal compartments.
Keywords: Connexin, Pannexin, Renal physiology
Introduction
Although kidneys only represent about 0.4 % of the total body weight, they receive 20 % of the cardiac output. This high flow is essential for refined regulation of body fluid volumes and solute concentrations, which in turn are dependent on tight control of glomerular filtration and excretory functions of the kidneys. Physiological and pathological processes involved in kidney function and dysfunction are as complex as its structure. Since renal function involves numerous interactions between same and different cells types within the same and/or among distinct renal compartments, it was inevitable to consider gap junctions (GJs) in this context. The presence of connexins (Cxs) in the kidney was first detected in the early 1960s in humans by electronic microscopy [1]. Since then, several studies demonstrated the expression of some members of the Cx family in all renal cell types in humans and rodents [2–8]. Although impairment of GJs and hemichannels (HCs) has been reported to exert substantial impact in renal diseases [9–15], our knowledge regarding the involvement of GJs in renal physiology is still limited. In addition, even though recent studies have associated high or decreased pannexin (Panx) expression with a wide range of human diseases, their role in kidney is poorly known [16]. Recently, two members of the Panx family have been identified in renal vasculature and the tubular compartment [17, 18]. However, the field of the Panx biology is quite young and the role of Panxs in renal function is not elucidated. In this review, we will first briefly discuss the expression of Cxs and Panxs in distinct renal compartments and then focus on their potential roles in renal physiology.
Connexin and pannexin distribution in the kidney
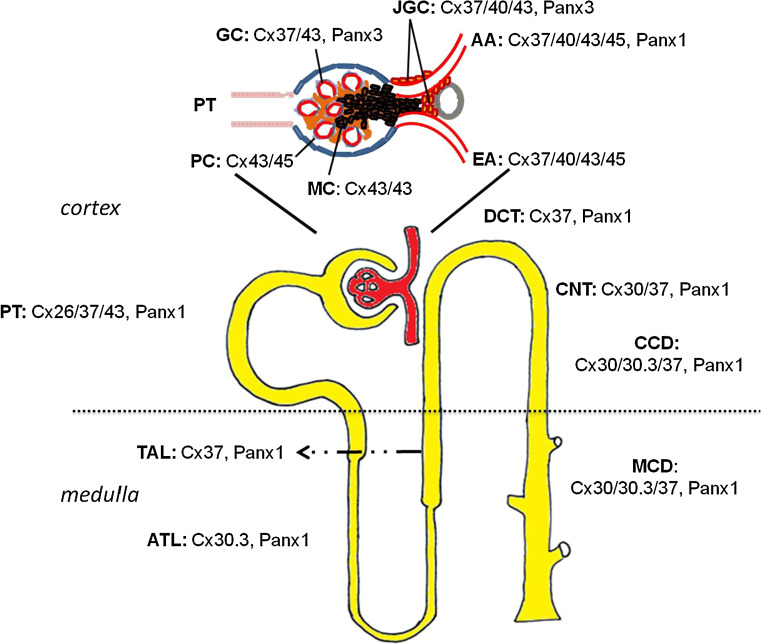
Previous studies reported that mRNA transcripts of about half of the Cx family are expressed in human and rodent kidney, including Cx26, Cx30.3, Cx31, Cx32, Cx37, Cx40, Cx43, Cx45 and Cx46 [8, 19]. However, Cx mRNA and protein expression do not always correlate, implying that mRNA data should be routinely verified at the protein level. Unfortunately, several currently used Cx antibodies display limitations and have generated conflicting data about Cx expression and localization depending on the experimental settings. Although most studies have focused on the contribution of vascular Cx proteins to renal hemodynamics, accumulating evidence suggests an essential physiological role for these proteins in tubular epithelial function. Regarding the Panx family, our knowledge is still limited, as today there are only two studies reporting their expression throughout different renal compartments [17, 18]. It should be noted though that Panx localization in some experiments was performed in paraffin-embedded tissues [17]. Flaws, such as lack of a strong signal in immunofluorescence and poor antigenicity compared to frozen sections, could mean that expression levels and localization sites of Panx proteins are underestimated. Localization of Cx and Panx isoforms within distinct renal compartments is illustrated in Fig. 1.
Fig. 1.
Schematic localization of connexin and pannexin isoforms in the kidney (AA, afferent arteriole, ATL ascending thin limb of the loop of Henle, CCD cortical collecting duct, CNT connecting tubule, DCT distal convoluted tubule, EA efferent arteriole, GC glomerular capillaries, JGC juxtaglomerular cells, MC mesangial cells, MCD medullary collecting duct, PC podocytes, PT proximal tubule, TAL thick ascending limb of the loop of Henle)
Renal vasculature
Cx37, Cx40, Cx43 and Cx45 are expressed in the renal vasculature, forming not only endothelial-to-endothelial or smooth muscle-to-smooth muscle junctions, but also myoendothelial coupling within afferent and efferent arterioles [7, 20, 21]. Panx1 was recently detected mainly in the endothelium of renal arteries and to a lesser extent in smooth muscle cells [17]. In contrast, no Panx isoforms were found at the myoendothelial junctions [17].
Renal endothelium
Some studies reported endothelial expression of Cx40, Cx37 and Cx43 in the entire renal vasculature of rodents. Preglomerular vasculature strongly expresses Cx40 and Cx37, while production of Cx43 is weaker and irregular [19]. In postglomerular vessels, some discrepancies in endothelial Cx expression between mice and rats have been described. For instance, endothelial cells of murine efferent arterioles express only Cx43 [7], whereas the same cells harbor Cx37 in rats [21]. Moreover, vasa recta expresses Cx37 and Cx40, but not Cx43 in mice, whereas all three Cx species are present in its rat counterpart [19]. Panx3 was found to be expressed in the endothelium of renal arterioles [17].
Vascular smooth muscle cells
In contrast to renal endothelium, Cx expression in vascular smooth muscle cells (VSMCs) is less clear. Nevertheless, Cx45 was suggested to be the major Cx isoform expressed in these cells. Mice in which the Cx45 gene-coding region was replaced by lacZ showed a strong staining in the media of interlobular, efferent and afferent arterioles [22]. Some studies showed Cx37 and Cx43 presence in renal VSMCs [7, 12, 23], but others failed to reproduce these findings [19].
Glomerulus
Glomerular endothelium
Cx40 has been detected in glomerular endothelial cells [21, 24]. In addition, Cx37 expression was noticed in intraglomerular capillaries, while expression of Cx43 was only minor [12, 14, 25]. Furthermore, Panx3 presence has been seen in these cells [17].
Mesangial cells
The entire intraglomerular mesangium expresses Cx40. Cx37 was found only in mesangial cells at the vascular pole of the glomerulus, while Cx43 was detected in mesangial cells of rat glomeruli [7, 13, 24].
Podocytes
Cx43 is abundantly produced by human and rat podocytes [10, 13], but is only present in small quantities in mouse podocytes (unpublished observations). In addition, a single study reported Cx45 staining in peripheral glomerular cells, presumably podocytes [26].
Juxtaglomerular apparatus
With the exception of macula densa cells, the juxtaglomerular apparatus (JGA) has been shown to be extensively coupled by GJs in humans and rodents [27, 28]. Interestingly, GJs are more numerous between the renin-containing granular cells than between other parts of afferent arterioles [5]. Cx40 is the predominant Cx species in the JGA, expressed by both granular and extraglomerular mesangial cells. The same cells also display Cx37 expression, albeit only minimally [21]. Cx45 was also found in renin-producing JGA cells in mice [20]. The expression of Cx43 is a matter of debate, as unlike previous studies [19, 24, 29, 30], only Kurtz and collaborators reported that Cx43 is expressed in the JGA of adult mice [27]. Recent studies also show the expression of Panx3 as very distinct punctuate stains throughout the JGA, whereas Panx1 and Panx2 are undetectable [17].
Tubules
In situ and ex vivo reverse transcriptase-polymerase chain reaction analysis revealed expression of many Cx isoforms in tubular cells, including Cx30, Cx36, Cx37, Cx40, Cx43, Cx45, Cx46 and Cx50 [19]. However, in many cases, immunohistochemistry experiments either could not confirm these results or provided conflicting data. It is of interest that the expression pattern of these Cx species is cell type-specific. For instance, in cortical collecting ducts, Cx37 is expressed by principal cells basolaterally, whereas Cx30 in the same segment is restricted to intercalated cells at the luminal surface in the form of HCs [25, 31]. A recent study showed Panx1 expression in several tubular segments, including proximal tubules, thin descending limbs and collecting ducts, along their apical cell membranes [19].
Role of connexins and pannexins in renal physiology
Gap junctions and renal microcirculation
Cx proteins contribute to renal microcirculation control, most likely due to their ability to regulate renal vascular conductance, endothelium-derived vasodilatation and autoregulatory mechanisms (Table 1).
Table 1.
The role of major connexin and pannexin isoforms in renal functions
| VCR | EDV | Renal autoregulation | Tubular function | Blood pressure | |
|---|---|---|---|---|---|
| Cx37 | ++ | + | +++ | + | + |
| Cx40 | +++ | +++ | +++ | – | +++ |
| Cx43 | – | ++ | ++ | – | + |
| Cx30 | – | – | – | ++ | – |
| Panx1 | – | – | ? | ++ | ? |
| Panx3 | – | – | ± | – | ? |
EDV endothelium-derived vasodilatation, VC vascular-conducted responses
Vascular-conducted responses
Vascular-conducted responses are characterized by a distant propagated vasoconstriction or vasodilatation initiated by a local electrical or metabolic stimulation of an arteriole. These responses are most likely spread through GJs in the vascular beds and play a major role in the regulation of blood flow in microcirculation and maintenance of vascular resistance. Propagated vasoconstriction in kidney takes place in renal microcirculation mainly in afferent and interlobular arterioles [32, 33]. It has been shown that propagated vasodilatation after acetylcholine application, but not vasoconstriction, is partially disturbed in Cx40 knock-out (KO) mice [34]. In addition, potassium chloride-propagated vasoconstriction is blunted in Cx37 KO mice, indicating a high level of specialization and selectivity of Cx subtypes for different metabolic signals [35]. Electrical signals can also produce the same response by eliciting calcium waves passing through GJs. Electrical stimulation of preglomerular arterioles from Cx40 KO mice fail to induce this response [8]. Moreover, in the same study, calcium response in isolated preglomerular vessels from rats was blocked by GJ inhibitors. Although both smooth muscle cells and endothelial cells are responsible for this response, it appears that in renal microcirculation, endothelial cell function is more GJ-dependent, since these cells are highly coupled via Cx proteins [36]. In a recent study using an ex vivo rat kidney perfusion technique, the effect of Cx-blocking peptides infusion on phenylephrine-induced vasoconstriction was examined. The authors showed that Cx43 plays a pivotal role in regulating renal vascular resistance, as administration of 43Gap26 significantly elevated perfusion pressure. In addition, infusion of 40Gap27 considerably suppressed the increase in perfusion pressure induced by phenylephrine, indicating that Cx40 attenuates phenylephrine-induced vasoconstriction [37]. Finally, blocking peptides directed against Cx37, Cx40, Cx43 or Cx45 had no effect on conducted calcium responses in isolated rat interlobular arteries [38].
Endothelium-derived vasodilatation
It is well known that endothelium can change vascular wall contractility either by releasing vasoactive agents, such as prostaglandins and nitric oxide (NO), or by radial spreading of the initial endothelial hyperpolarization to the vascular media, resulting in muscle relaxation. The latter type of vasodilation has been attributed to endothelium-derived hyperpolarizing factor (EDHF) and was described to be dependent on myoendothelial junctions (MEJs) [39, 40]. Blocking Cx40 and Cx43 channel function with 40Gap27 and 43Gap27, respectively, inhibited EDHF in isolated rat renal arteries [41]. Of note, under these experimental conditions, the inhibitory effect of 43Gap27 was greater than that of 40Gap27. By contrast, in human mesenteric arteries, myoendothelial GJ activity was consistent with Cx37 expression and distribution [42]. Endothelium-derived vasodilatation is also related to NO activity. NO is produced from l-arginine by the action of endothelial nitric oxide synthase (eNOS) and diffuses to smooth muscle cells, thereby mediating vasodilatation [43]. A direct link between Cx40 and eNOS expression and function has been well established, since Cx40 KO mice showed reduced expression of eNOS, which possibly increases the vascular resistance via T-type calcium channels [44]. A recent study provided evidence that both Cx40 and Cx37 participate in eNOS regulation in vivo. In mice subjected to the 1-kidney 1-clip procedure, a model of volume-dependent hypertension, the interaction of Cx40 and Cx37 with eNOS was enhanced, resulting in increased NO release. Mice lacking Cx40 featured decreased levels of eNOS [45]. Moreover, NO itself had opposite effects on different Cxs expressed within the vascular wall, as it decreased the functional coupling of Cx37-consisting GJs, whereas it increased de novo formation of Cx40-containing GJs [46]. Regarding Cx43 and NO interaction, it has been hypothesized that endothelial Cx43 plays a key role in the production and/or action of NO. Indeed, endothelial cell-specific Cx43 KO mice are hypotensive and bradycardic compared to heterozygous or floxed counterparts. This hypotension is associated with elevated plasma levels of NO as well as angiotensin I and II [47]. By contrast, Theis and colleagues showed that mice lacking endothelial Cx43 do not exhibit any blood pressure abnormalities and respond normally to NG-nitro-l-arginine, a potent NO synthase inhibitor [48]. These discrepancies may be due to differences in the genetic background of the mouse strains used.
Autoregulation of renal blood flow
Autoregulation of renal blood flow describes the capacity of the vascular bed to maintain its perfusion constant despite variations in levels of arterial pressure. This function is particularly pronounced in the kidney and is based on two major mechanisms, namely tubuloglomerular feedback (TGF) and myogenic response (MR). TGF is a highly regulated process leading to vasoconstriction of afferent arterioles in response to increased luminal concentration of sodium chloride at the macula densa in the early distal tubule. The concentration of sodium chloride reaching the macula densa is dependent on the rate of tubular flow, with larger flow resulting in a higher distal tubular concentration. Increased arterial pressure will enhance tubular flow due to enhanced glomerular filtration and reduced proximal tubular reabsorption. This will raise the sodium chloride concentration at the macula densa and cause afferent arteriolar vasoconstriction, providing restoration of filtration and autoregulation of renal blood flow [49]. Molecular mechanisms involved in TGF regulation have been extensively studied. Several studies support the concept that initial absorption of sodium chloride through sodium–potassium-chloride cotransporters results in adenosine triphosphate (ATP) release from macula densa cells, most likely mediated through changes in intracellular concentrations of calcium, chloride and sodium, depolarization or cell swelling [50]. There are two hypotheses regarding the role of ATP release. The first concept says that ATP directly activates specific ATP purinoceptors, such as P2X1, located on afferent arterioles [51]. The second concept supports that ATP is converted to adenosine by ectonucleotidases in the interstitial space of the JGA, which then acts on A1 adenosine receptors of the P1 group of purinoceptors. Activation of these receptors results in increased levels of intracellular calcium in macula densa cells. Calcium ions can then spread rapidly to all JGA components, provoking vasoconstriction of afferent arterioles and inhibition of renin release [51, 53]. The contribution of Cxs to TGF has been studied using Cx-blocking peptides, mainly in rats. Indeed, autoregulation of renal blood flow and glomerular filtration rate in the whole kidney required GJ coupling, involving Cx37 and Cx40, but not Cx43. This contribution requires ATP release, rather than direct intercellular diffusion of calcium waves [54, 55]. These observations are in line with immunohistochemical studies, showing expression of Cx37 and Cx40 in renin-secreting cells of the JGA, Cx40 in extraglomerular mesangial cells, but absence of Cx43 from both these sites [21]. Expression of Panx3 has also been recently reported in the JGA of mice as well as in endothelial cells of renal cortical arteries [17]. Given that Panx3 has been shown to release ATP, a role for TGF regulation via purinergic signaling cascades cannot be excluded [16]. In contrast to previous studies [21, 55, 56], Piao and colleagues showed a pivotal role of Cx43 in perfusion pressure [37]. These discrepancies in the involvement of different Cx species in renal autoregulatory mechanisms could be related to different experimental settings and the stability of different blocking peptides. Cx40 KO mice were found to have an impaired steady-state autoregulatory response to a steep increase in renal perfusion pressure [57]. Interestingly, mice in which Cx40 is replaced by Cx45 have weaker steady-state autoregulation and TGF than wild-type mice, but stronger than Cx40 KO mice, suggesting that Cx45 can partially mimic Cx40 functions [26].
The second mechanism in renal autoregulation is MR. Smooth muscle cells contract in response to stretching force [58]. In the case of VSMCs, a rise in intraluminal pressure leads to vasoconstriction, which not only overcomes the passive distension of the elastic vascular wall, but, at least in small resistance vessels, also reduces the diameter below the one at lower pressure. This enhances vascular resistance at higher pressure and allows for autoregulation of flow [58]. The relationship between GJs and MR in the kidney has not been fully determined. However, in isolated rat mesenteric arteries, inhibition of GJ activity by Cx37 and Cx43 blocking peptides attenuates MR. This effect is related to GJs between VSMCs, which may contribute to this response by controlling early signaling events, such as coordinating smooth muscle cell depolarization or mechanosensitivity of VSMCs, but not synchronized calcium signaling [59]. Moreover, isolated cerebral arteries treated with nonselective GJ uncouplers showed inhibited myogenic tone [60]. However, in several vascular beds, endothelium removal resulted in the loss of synchronized calcium oscillations in VSMCs [61]. This may suggest a possible role of MEJs, connecting the two cellular layers, in controlling MR by coordinating synchronized calcium signaling.
Gap junctions and tubular function
The main role of the renal tubular compartment is the reabsorption of the glomerular filtrate. Reabsorption relies on a highly regulated set of physiological processes, involving specific primary and secondary active transport mechanisms that accomplish the return of a wide variety of nutritionally important ions or molecules from the plasma filtrate as it passes along the tubular system of the nephron. These processes are mediated by several transporters located in the tubular surface and are energy-consuming. The function of epithelial Cxs could be related to purinergic P2 receptors activation and/or propagating the effect of this activation. Purinergic P2 receptors have been suggested to contribute to tubular function, as they are expressed nearly by all nephron segments [62]. Mechanical stimulation is known to promote release of nucleotides, such as ATP, and trigger autocrine and paracrine activation of purinergic P2 receptors in renal epithelia regulating salt and water reabsorption. Activation of purinergic P2 receptors was proposed to be responsible, at least partially, for the flow-induced intracellular calcium response in the renal tubule [63]. Increased intracellular calcium levels affect different tubular cells in different ways. Thus, principal cells of cortical collecting ducts are responding to luminal and to a lesser extent to basolateral ATP by inhibiting sodium reabsorption via reducing sodium channel nonneuronal 1 activity [64]. In inner medulla, purinergic P2 receptors activation was proposed to balance the effect of vasopressin in urine concentration [65]. At present, there is some evidence to support a relation between Cx30 and epithelial function in distal nephron segments. Sipos and colleagues found that the luminal HCs formed by Cx30 have an integral role in pressure natriuresis by releasing ATP into the tubular fluid, which inhibits salt and water reabsorption [66]. In addition, Cx30 KO mice display hyperactive sodium channel nonneuronal 1 activity due to diminished ATP-mediated inhibition [67]. The role of other epithelial Cxs in tubular function is still unknown. However, rats treated with low-salt diet showed a significant increase in Cx37 levels in renal cortex, which may indicate a functional role for this Cx species in renal tubules [25]. In addition, a recent study from Hanner and collaborators demonstrated a major role for Panx1-based channels in ATP release. Indeed, urinary ATP levels were reduced by 30 % in Panx1 KO mice compared to wild-type mice. Since Panx1 was located at the apical membrane of various tubular segments, the authors suggested that Panx1-based channels may regulate ATP release and further participate in the control of renal epithelial fluid, electrolyte transport and vascular functions via purinergic signaling [18].
Gap junctions and blood pressure
The kidney regulates blood pressure through two distinct mechanisms, namely by means of the control of salt and water excretion, and via the control of renin secretion. Since these processes require highly coordinated interactions between vascular and tubular cells, several studies have considered a role for GJs in this context. The role of Cx43 in the regulation of blood pressure is still a matter of debate. Mice with endothelial deletion of Cx43 were reported to be either hypotensive or normotensive [47, 48]. However, in hypotensive mice, decreased blood pressure was not associated with renin secretion. Along the same line, intrarenal infusion of Cx43 blocking peptides exerted no influence on renin secretion or on blood pressure [21]. In contrast to these reports, it has been demonstrated that replacement of Cx43 by Cx32 in mice leads to lower concentrations of circulating renin associated with slightly decreased blood pressure. Interestingly, in the kidneys of these mice, the number of renin-expressing cells was reduced [68]. Even though Cx37 is expressed in the preglomerular endothelium and by renin-secreting cells, Cx37 KO mice showed normal blood pressure and renin secretion [69]. However, intrarenal infusion of Cx37 blocking peptides in rats showed an acute increase in both renin secretion and blood pressure [69]. In addition, it has been reported that renin expression, plasma renin activity and blood pressure were all increased in genetically engineered mice with reduced JGA Cx45 expression [20].
In contrast to the above-mentioned Cxs, different studies corroborate that Cx40 is highly important for the control of renin secretion and hence of blood pressure. Intrarenal infusion of Cx40 blocking peptides enhanced both renin secretion and blood pressure in rats, while Cx40 KO mice were found to be hypertensive [21, 70]. Of note, these mice showed impaired autoregulation of renal blood flow. Under normal conditions, elevated blood pressure should suppress renin secretion from the kidneys as a negative feedback, allowing preservation of normal blood pressure. This control was defective in the absence of Cx40. Interestingly, selective deletion of Cx40 in renin-producing cells, but not in endothelium, fully mimics the phenotype of global Cx40 deletion [71]. In addition, generation of mice carrying a loss-of-mutation in Cx40 with impaired pore function, which has been recently discovered in humans, showed a similar renin phenotype to that of Cx40 KO mice [72]. The molecular mechanisms via which GJs control renin secretion are poorly known. Some studies suggest that calcium may be a relevant signal passing through Cx40-based GJs in the control of renin secretion [52]. As Panx3 expression has been recently described within the JGA [17], its implication in the regulation of blood pressure cannot be ruled out. Generation of Panx3 KO mice would be useful in this respect.
Conclusions and perspectives
There is accumulating evidence that GJs play crucial role in renal physiology, as alteration of GJ activity contributes to structural and functional damage, leading to several kidney diseases [12–15, 73]. In this paper, the major relevant processes that are indispensable for the maintenance of renal homeostasis and function have been discussed. Despite the already existing tools, such as Cx KO mice and Cx-blocking peptides, our knowledge about Cx implication in renal physiology is still limited. Moreover, whether required communication for renal homeostasis occurs via GJs or HCs is currently poorly understood [19, 36, 74]. The recent development of specific HC blocking peptides will allow us to further study molecular mechanisms underlying Cx signaling in renal functions [75–77]. Furthermore, cell type-specific deletion or overexpression of different Cx isoforms in mice will be a valuable approach to study the role of these proteins in renal physiological processes. The role of the Panx family in renal physiology is still unclear, although some studies reported their involvement in several diseases in humans and rodents. The use of Panx-deficient mice will be of major interest to further increase our knowledge in Panx channel biology in the kidney.
Acknowledgments
The authors would like to thank Dr. Christos Chatziantoniou and Pr. Jean-Claude Dussaule for the helpful discussions. Support for this study was provided by the INSERM, the University Paris VI and the Foundation of Medical Research (FRM). A.A. is a doctoral fellow of a University Paris VI—Palestinian Universities cooperation program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Abbreviations
- ATP
Adenosine triphosphate
- Cx(s)
Connexin(s)
- EDHF
Endothelium-derived hyperpolarizing factor
- eNOS
Endothelial nitric oxide synthase
- GJ(s)
Gap junction(s)
- HC(s)
Hemichannel(s)
- JGA
Juxtaglomerular apparatus
- KO
Knock-out
- MEJ(s)
Myoendothelial junction(s)
- MR
Myogenic response
- NO
Nitric oxide
- Panx(s)
Pannexin(s)
- TGF
Tubuloglomerular feedback
- VSMC(s)
Vascular smooth muscle cell(s)
References
- 1.Biava C, West M. Fine structure of normal human juxtaglomerular cells. I. General structure and intercellular relationships. Am J Pathol. 1966;49(4):679–721. [PMC free article] [PubMed] [Google Scholar]
- 2.Boll HU, Forssmann WG, Taugner R. Studies on the juxtaglomerular apparatus. IV. Freeze-fracturing of membrane surfaces. Cell Tissue Res. 1975;161(4):459–469. doi: 10.1007/BF00224136. [DOI] [PubMed] [Google Scholar]
- 3.Pricam C, Humbert F, Perrelet A, Orci L. Gap junctions in mesangial and lacis cells. J Cell Biol. 1974;63(1):349–354. doi: 10.1083/jcb.63.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mink D, Schiller A, Kriz W, Taugner R. Interendothelial junctions in kidney vessels. Cell Tissue Res. 1984;236(3):567–576. doi: 10.1007/BF00217224. [DOI] [PubMed] [Google Scholar]
- 5.Taugner R, Kirchheim H, Forssmann WG. Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and Tupaia belangeri. Cell Tissue Res. 1984;235(2):319–325. doi: 10.1007/BF00217856. [DOI] [PubMed] [Google Scholar]
- 6.Butterweck A, Gergs U, Elfgang C, Willecke K, Traub O. Immunochemical characterization of the gap junction protein connexin45 in mouse kidney and transfected human HeLa cells. J Membr Biol. 1994;141(3):247–256. doi: 10.1007/BF00235134. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Hill CE. Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney Int. 2005;68(3):1171–1185. doi: 10.1111/j.1523-1755.2005.00509.x. [DOI] [PubMed] [Google Scholar]
- 8.Wagner C. Function of connexins in the renal circulation. Kidney Int. 2008;73(5):547–555. doi: 10.1038/sj.ki.5002720. [DOI] [PubMed] [Google Scholar]
- 9.Hillis GS, Duthie LA, Brown PA, Simpson JG, MacLeod AM, Haites NE. Upregulation and co-localization of connexin43 and cellular adhesion molecules in inflammatory renal disease. J Pathol. 1997;182(4):373–379. doi: 10.1002/(SICI)1096-9896(199708)182:4<373::AID-PATH858>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 10.Sawai K, Mukoyama M, Mori K, Yokoi H, Koshikawa M, Yoshioka T, Takeda R, Sugawara A, Kuwahara T, Saleem MA, Ogawa O, Nakao K. Redistribution of connexin43 expression in glomerular podocytes predicts poor renal prognosis in patients with type 2 diabetes and overt nephropathy. Nephrol Dial Transplant. 2006;21(9):2472–2477. doi: 10.1093/ndt/gfl260. [DOI] [PubMed] [Google Scholar]
- 11.Alonso F, Krattinger N, Mazzolai L, Simon A, Waeber G, Meda P, Haefliger JA. An angiotensin II- and NF-kappaB-dependent mechanism increases connexin 43 in murine arteries targeted by renin-dependent hypertension. Cardiovasc Res. 2010;87(1):166–176. doi: 10.1093/cvr/cvq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toubas J, Beck S, Pageaud AL, Huby AC, Mael-Ainin M, Dussaule JC, Chatziantoniou C, Chadjichristos CE. Alteration of connexin expression is an early signal for chronic kidney disease. Am J Physiol Renal Physiol. 2011;301(1):F24–F32. doi: 10.1152/ajprenal.00255.2010. [DOI] [PubMed] [Google Scholar]
- 13.Morioka T, Okada S, Nameta M, Kamal F, Yanakieva-Georgieva NT, Yao J, Sato A, Piao H, Oite T. Glomerular expression of connexin 40 and connexin 43 in rat experimental glomerulonephritis. Clin Exp Nephrol. 2013;17(2):191–204. doi: 10.1007/s10157-012-0687-2. [DOI] [PubMed] [Google Scholar]
- 14.Abed A, Toubas J, Kavvadas P, Authier F, Alfieri C, Boffa JJ, Dussaule JC, Chatziantoniou C, Chadjichristos CE. Targeting Cx43 protects against the progression of experimental chronic kidney disease in mice. Kidney Int. 2014;86(4):768–779. doi: 10.1038/ki.2014.108. [DOI] [PubMed] [Google Scholar]
- 15.Hills CE, Price GW, Squires PE. Mind the gap: connexins and cell–cell communication in the diabetic kidney. Diabetologia. 2015;58(2):233–241. doi: 10.1007/s00125-014-3427-1. [DOI] [PubMed] [Google Scholar]
- 16.Penuela S, Harland L, Simek J, Laird DW. Pannexin channels and their links to human disease. Biochem J. 2014;461(3):371–381. doi: 10.1042/BJ20140447. [DOI] [PubMed] [Google Scholar]
- 17.Lohman AW, Billaud M, Straub AC, Johnstone SR, Best AK, Lee M, Barr K, Penuela S, Laird DW, Isakson BE. Expression of pannexin isoforms in the systemic murine arterial network. J Vasc Res. 2012;49(5):405–416. doi: 10.1159/000338758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Renal Physiol. 2012;303(10):F1454–F1459. doi: 10.1152/ajprenal.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol. 2010;298(5):R1143–R1155. doi: 10.1152/ajpregu.00808.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanner F, von Maltzahn J, Maxeiner S, Toma I, Sipos A, Krüger O, Willecke K, Peti-Peterdi J. Connexin45 is expressed in the juxtaglomerular apparatus and is involved in the regulation of renin secretion and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R371–R380. doi: 10.1152/ajpregu.00468.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takenaka T, Inoue T, Kanno Y, Okada H, Meaney KR, Hill CE, Suzuki H. Expression and role of connexins in the rat renal vasculature. Kidney Int. 2008;73(4):415–422. doi: 10.1038/sj.ki.5002673. [DOI] [PubMed] [Google Scholar]
- 22.Krüger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000;127(19):4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- 23.Barajas L, Liu L, Tucker M. Localization of connexin43 in rat kidney. Kidney Int. 1994;46(3):621–626. doi: 10.1038/ki.1994.314. [DOI] [PubMed] [Google Scholar]
- 24.Hwan Seul K, Beyer EC. Heterogeneous localization of connexin40 in the renal vasculature. Microvasc Res. 2000;59(1):140–148. doi: 10.1006/mvre.1999.2216. [DOI] [PubMed] [Google Scholar]
- 25.Stoessel A, Himmerkus N, Bleich M, Bachmann S, Theilig F. Connexin 37 is localized in renal epithelia and responds to changes in dietary salt intake. Am J Physiol Renal Physiol. 2010;298(1):F216–F223. doi: 10.1152/ajprenal.00295.2009. [DOI] [PubMed] [Google Scholar]
- 26.Just A, Kurtz L, de Wit C, Wagner C, Kurtz A, Arendshorst WJ. Connexin 40 mediates the tubuloglomerular feedback contribution to renal blood flow autoregulation. J Am Soc Nephrol. 2009;20(7):1577–1585. doi: 10.1681/ASN.2008090943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurtz L, Janssen-Bienhold U, Kurtz A, Wagner C. Connexin expression in renin-producing cells. J Am Soc Nephrol. 2009;20(3):506–512. doi: 10.1681/ASN.2008030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtz L, Madsen K, Kurt B, Jensen BL, Walter S, Banas B, Wagner C, Kurtz A. High-level connexin expression in the human juxtaglomerular apparatus. Nephron Physiol. 2010;116(1):1–8. doi: 10.1159/000315658. [DOI] [PubMed] [Google Scholar]
- 29.Haefliger JA, Demotz S, Braissant O, Suter E, Waeber B, Nicod P, Meda P. Connexins 40 and 43 are differentially regulated within the kidneys of rats with renovascular hypertension. Kidney Int. 2001;60(1):190–201. doi: 10.1046/j.1523-1755.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 30.Arensbak B, Mikkelsen HB, Gustafsson F, Christensen T, Holstein-Rathlou NH. Expression of connexin 37, 40, and 43 mRNA and protein in renal preglomerular arterioles. Histochem Cell Biol. 2001;115(6):479–487. doi: 10.1007/s004180100275. [DOI] [PubMed] [Google Scholar]
- 31.McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol. 2005;289(6):F1304–F1312. doi: 10.1152/ajprenal.00203.2005. [DOI] [PubMed] [Google Scholar]
- 32.Steinhausen M, Endlich K, Nobiling R, Parekh N, Schütt F. Electrically induced vasomotor responses and their propagation in rat renal vessels in vivo. J Physiol. 1997;505(Pt2):493–501. doi: 10.1111/j.1469-7793.1997.493bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner AJ, Holstein-Rathlou NH, Marsh DJ. Internephron coupling by conducted vasomotor responses in normotensive and spontaneously hypertensive rats. Am J Physiol. 1997;272(3):F372–F379. doi: 10.1152/ajprenal.1997.272.3.F372. [DOI] [PubMed] [Google Scholar]
- 34.de Wit C, Roos F, Bolz SS, Pohl U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics. 2003;13(2):169–177. doi: 10.1152/physiolgenomics.00169.2002. [DOI] [PubMed] [Google Scholar]
- 35.McKinnon RL, Lidington D, Bolon M, Ouellette Y, Kidder GM, Tyml K. Reduced arteriolar conducted vasoconstriction in septic mouse cremaster muscle is mediated by nNOS-derived NO. Cardiovasc Res. 2006;69(1):236–244. doi: 10.1016/j.cardiores.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Sorensen CM, Holstein-Rathlou NH. Cell–cell communication in the kidney microcirculation. Microcirculation. 2012;19(5):451–460. doi: 10.1111/j.1549-8719.2011.00149.x. [DOI] [PubMed] [Google Scholar]
- 37.Piao H, Sato A, Nozawa Y, Sun W, Morioka T, Oite T. Effects of connexin-mimetic peptides on perfusion pressure in response to phenylephrine in isolated, perfused rat kidneys. Clin Exp Nephrol. 2011;15(2):203–211. doi: 10.1007/s10157-010-0382-0. [DOI] [PubMed] [Google Scholar]
- 38.Sorensen CM, Salomonsson M, Braunstein TH, Nielsen MS, Holstein-Rathlou NH. Connexin mimetic peptides fail to inhibit vascular conducted calcium responses in renal arterioles. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R840–R847. doi: 10.1152/ajpregu.00491.2007. [DOI] [PubMed] [Google Scholar]
- 39.Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11(2):251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffith TM, Chaytor AT, Edwards DH. The obligatory link: role of gap junctional communication in endothelium-dependent smooth muscle hyperpolarization. Pharmacol Res. 2004;49(6):551–564. doi: 10.1016/j.phrs.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Karagiannis J, Rand M, Li CG. Role of gap junctions in endothelium-derived hyperpolarizing factor-mediated vasodilatation in rat renal artery. Acta Pharmacol Sin. 2004;25(8):1031–1037. [PubMed] [Google Scholar]
- 42.Chadha PS, Liu L, Rikard-Bell M, Senadheera S, Howitt SL, Bertrand RL, Grayson TH, Murphy TV, Sandow SL. Endothelium-dependent vasodilation in human mesenteric artery is primarily mediated by myoendothelial gap junctions intermediate conductance calcium-activated K+ channel and nitric oxide. J Pharmacol Exp Ther. 2011;336(3):701–708. doi: 10.1124/jpet.110.165795. [DOI] [PubMed] [Google Scholar]
- 43.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 44.Sorensen CM, Giese I, Braunstein TH, Brasen JC, Salomonsson M, Holstein-Rathlou NH. Role of connexin40 in the autoregulatory response of the afferent arteriole. Am J Physiol Renal Physiol. 2012;303(6):F855–F863. doi: 10.1152/ajprenal.00026.2012. [DOI] [PubMed] [Google Scholar]
- 45.Le Gal L, Alonso F, Mazzolai L, Meda P, Haefliger JA. Interplay between connexin40 and nitric oxide signaling during hypertension. Hypertension. 2015;65(4):910–950. doi: 10.1161/HYPERTENSIONAHA.114.04775. [DOI] [PubMed] [Google Scholar]
- 46.Kameritsch P, Hoffmann A, Pohl U. Opposing effects of nitric oxide on different connexins expressed in the vascular system. Cell Commun Adhes. 2003;10(4–6):305–309. doi: 10.1080/714040444. [DOI] [PubMed] [Google Scholar]
- 47.Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci USA. 2001;9(17):9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theis M, de Wit C, Schlaeger TM, Eckardt D, Kruger O, Doring B, Risau W, Deutsch U, Pohl U, Willecke K. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis. 2001;29(1):1–13. doi: 10.1002/1526-968X(200101)29:1<1::AID-GENE1000>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 49.Just A. Mechanisms of renal blood flow autoregulation: dynamics and contributions. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R1–R17. doi: 10.1152/ajpregu.00332.2006. [DOI] [PubMed] [Google Scholar]
- 50.Bell PD, Lapointe JY, Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol. 2003;65:481–500. doi: 10.1146/annurev.physiol.65.050102.085730. [DOI] [PubMed] [Google Scholar]
- 51.Nishiyama A, Navar LG. ATP mediates tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R273–R275. doi: 10.1152/ajpregu.00071.2002. [DOI] [PubMed] [Google Scholar]
- 52.Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol. 2006;291(2):F473–F480. doi: 10.1152/ajprenal.00425.2005. [DOI] [PubMed] [Google Scholar]
- 53.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501–529. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 54.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA. 2002;99(15):9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takenaka T, Inoue T, Kanno Y, Okada H, Hill CE, Suzuki H. Connexins 37 and 40 transduce purinergic signals mediating renal autoregulation. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):R1–R11. doi: 10.1152/ajpregu.00269.2007. [DOI] [PubMed] [Google Scholar]
- 56.De Vriese AS, Van de Voorde J, Lameire NH. Effects of connexin-mimetic peptides on nitric oxide synthase- and cyclooxygenase-independent renal vasodilation. Kidney Int. 2002;61(1):177–185. doi: 10.1046/j.1523-1755.2002.00122.x. [DOI] [PubMed] [Google Scholar]
- 57.Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol. 2007;18(4):1103–1111. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- 58.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Earley S, Resta TC, Walker BR. Disruption of smooth muscle gap junctions attenuates myogenic vasoconstriction of mesenteric resistance arteries. Am J Physiol Heart Circ Physiol. 2004;287(6):H2677–H2686. doi: 10.1152/ajpheart.00016.2004. [DOI] [PubMed] [Google Scholar]
- 60.Lagaud G, Karicheti V, Knot HJ, Christ GJ, Laher I. Inhibitors of gap junctions attenuate myogenic tone in cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;283(6):H2177–H2186. doi: 10.1152/ajpheart.00605.2001. [DOI] [PubMed] [Google Scholar]
- 61.Peng H, Matchkov V, Ivarsen A, Aalkjaer C, Nilsson H. Hypothesis for the initiation of vasomotion. Circ Res. 2001;88(8):810–850. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- 62.Turner CM, Elliott JI, Tam FW. P2 receptors in renal pathophysiology. Purinergic Signal. 2009;5(4):13–20. doi: 10.1007/s11302-009-9153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol. 2007;18(7):2062–2070. doi: 10.1681/ASN.2006070700. [DOI] [PubMed] [Google Scholar]
- 64.Wildman SS, Marks J, Churchill LJ, Peppiatt CM, Chraibi A, Shirley DG, Horisberger JD, King BF, Unwin RJ. Regulatory interdependence of cloned epithelial Na+ channels and P2X receptors. J Am Soc Nephrol. 2005;16(9):2586–2597. doi: 10.1681/ASN.2005020130. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK. Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am J Physiol Renal Physiol. 2008;295(6):F1715–F1724. doi: 10.1152/ajprenal.90311.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol. 2009;20(8):1724–1732. doi: 10.1681/ASN.2008101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD. Diminished paracrine regulation of the epithelial Na + channel by purinergic signaling in mice lacking connexin 30. J Biol Chem. 2011;286(2):1054–1060. doi: 10.1074/jbc.M110.176552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haefliger JA, Krattinger N, Martin D, Pedrazzini T, Capponi A, Doring B, Plum A, Charollais A, Willecke K, Meda P. Connexin43-dependent mechanism modulates renin secretion and hypertension. J Clin Invest. 2006;116(2):405–413. doi: 10.1172/JCI23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wagner C, Kurtz L, Schweda F, Simon AM, Kurtz A. Connexin 37 is dispensable for the control of the renin system and for positioning of renin-producing cells in the kidney. Pflugers Arch. 2009;459(1):151–158. doi: 10.1007/s00424-009-0707-6. [DOI] [PubMed] [Google Scholar]
- 70.Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100(4):556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 71.Wagner C, Jobs A, Schweda F, Kurtz L, Kurt B, Lopez ML, Gomez RA, van Veen TA, de Wit C, Kurtz A. Selective deletion of Connexin 40 in renin-producing cells impairs renal baroreceptor function and is associated with arterial hypertension. Kidney Int. 2010;78(8):762–768. doi: 10.1038/ki.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lubkemeier I, Machura K, Kurtz L, Neubauer B, Dobrowolski R, Schweda F, Wagner C, Willecke K, Kurtz A. The connexin 40 A96S mutation causes renin-dependent hypertension. J Am Soc Nephrol. 2011;22(6):1031–1040. doi: 10.1681/ASN.2010101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abed A, Dussaule JC, Boffa JJ, Chatziantoniou C, Chadjichristos CE. Connexins in renal endothelial function and dysfunction. Cardiovasc Hematol Disord: Drug Targets. 2014;14(1):15–21. doi: 10.2174/1871529X14666140401105827. [DOI] [PubMed] [Google Scholar]
- 74.Kurtz A. Renal connexins and blood pressure. Biochim Biophys Acta. 2012;1818(8):1903–1908. doi: 10.1016/j.bbamem.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 75.Wang N, De Vuyst E, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, Lai CP, De Bock M, Decrock E, Bol M, Vinken M, Rogiers V, Tavernier J, Evans WH, Naus CC, Bukauskas FF, Sipido KR, Heusch G, Schulz R, Bultynck G, Leybaert L. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2013;108(1):309. doi: 10.1007/s00395-012-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iyyathurai J, D’hondt C, Wang N, De Bock M, Himpens B, Retamal MA, Stehberg J, Leybaert L, Bultynck G. Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: gap junctions versus hemichannels. Neuropharmacology. 2013;75:491–505. doi: 10.1016/j.neuropharm.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 77.Abudara V, Bechberger J, Freitas-Andrade M, De Bock M, Wang N, Bultynck G, Naus CC, Leybaert L, Giaume C. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front Cell Neurosci. 2014;21(8):306. doi: 10.3389/fncel.2014.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]