Abstract
The recent advances in surgery and radiation therapy have significantly improved the prognosis of patients with primary cancer, and the major challenge of cancer treatment now is metastatic disease development. The 5-year survival rate of cancer patients who have distant metastasis at diagnosis is extremely low, suggesting that prediction and early detection of metastasis would definitely improve their prognosis because suitable patient therapeutic management and treatment strategy can be provided. Cancer cells from a primary site give rise to a metastatic tumor via a number of steps which require the involvement and altered expression of many regulators. These regulators may serve as biomarkers for predicting metastasis. Over the past few years, numerous regulators have been found correlating with metastasis. In this review, we summarize the findings of a number of potential biomarkers that are involved in cadherin–catenin interaction, integrin signaling, PI3K/Akt/mTOR signaling and cancer stem cell identification in gastrointestinal cancers. We will also discuss how certain biomarkers are associated with the tumor microenvironment that favors cancer metastasis.
Keywords: Metastasis, Hepatocellular carcinoma, Colorectal cancer, Gastric cancer, Pancreatic cancer, Esophageal cancer
Introduction
Although surgery and radiation therapy effectively control many cancers at their primary sites, the development of metastatic disease signals poor prognosis [1]. Metastatic tumors, rather than the primary ones, are responsible for 90 % of all cancer deaths [2]. According to Cancer Facts and Figures 2010, if metastasis has developed at diagnosis, the 5-year relative survival rates in gastrointestinal cancers are very low (2 to 3 % for liver, colon, pancreas and esophagus cancer, and 11 % for stomach cancer). In other words, the prognosis of gastrointestinal cancer patients can be greatly improved by early detection or prediction of the metastatic potential of the primary tumor. This urges the need of biomarker development. In this review, we will discuss biomarkers identified in gastrointestinal cancers including hepatocellular carcinoma (HCC), colorectal cancer (CRC), gastric cancer (GC), pancreatic cancer (PC) and esophageal cancer (EC).
Biomarkers are indicators of specific biological state, which is crucial for patient therapeutic management and treatment strategy. Currently, there are some established biomarkers reported, including markers already used in clinic such as CEA, CA19-9 and AFP. Other established tumor/predictive markers such as ras oncogene mutations, mucin 4, LSA or CD31, yet their correlations with metastasis of gastrointestinal cancers are limiting and will not be discussed in this review. CEA has been extensively studied in GC, CRC and PC. According to the ASCO 2006 recommendations, although high preoperative CEA (>5 ng/mL) may correlate with poorer prognosis of CRC patients, data are insufficient to support the use of CEA to determine whether to treat a patient with adjuvant therapy [3]. In accordance, the preoperative CEA level did not show significant association with metastasis in most gastrointestinal cancer studies [4, 5]. Regular monitoring of postoperative CEA level has been suggested for detecting recurrent or metastatic disease in asymptomatic CRC patients [3]; however, a rising CEA level may occur in patients with non-malignant liver disease since clearance of CEA is affected by impaired liver function [6, 7]. Moreover, CEA level could be increased by the application of a new therapy, such as oxaliplatin, during the first 4–6 weeks of application [8, 9]. Therefore, it is necessary to include other markers to monitor the patient disease status more accurately. CA19-9 has been suggested, but present data are still insufficient for supporting their use in this purpose [3]. The application of CA19-9 in monitoring recurrence and metastasis is still controversial according to the results from different study groups [10–12]. Serum alpha-fetoprotein (AFP) is widely used as a surveillance and detection test for HCC among patients with cirrhosis, yet its performance is limited, particularly in early stage HCC [13–16]. Moreover, to the best of our knowledge, its correlation with metastasis of HCC has not been reported. AFP was also studied in AFP-producing GC, yet the correlation of elevated AFP with liver metastasis lacks consistency [17, 18].
Moreover, according to the clinical studies of these established biomarkers, it shows that elevation of their levels identifies a group of high risk patients to develop metastatic disease, whereas those with low levels still have a certain chance to develop metastasis. It is possibly due to heterozygous biology of tumors from different patients. These clinical results urge that additional biomarkers with higher sensitivity and specificity are necessary for predicting the presence of future metastasis. In some recent studies, the newly identified biomarkers even showed better prognostic values over the above conventional biomarkers.
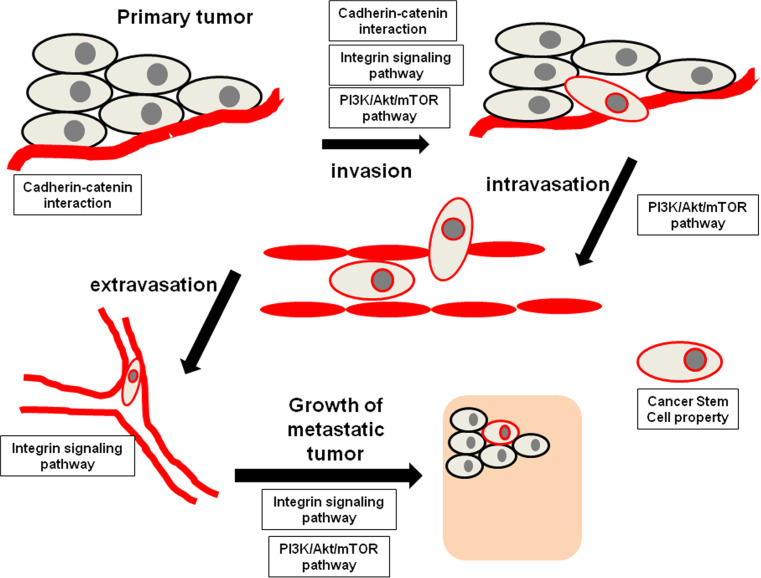
Understanding the molecular mechanism of metastasis provides a basis for the discovery of biomarkers that predict future development of metastasis. Basically, cancer cells from a primary site give rise to a metastatic tumor through the following processes: epithelial-mesenchymal transition (EMT), cancer cell invasion and migration, intravasation, survival within the circulation, extravasation, colonization at distant organs, and metastatic tumor angiogenesis [19]. Molecular pathways such as cadherin–catenin interaction, integrin signaling and PI3K/Akt/mTOR signaling have been demonstrated to regulate the cancer metastasis processes (Fig. 1), thus genes involved in these pathways might serve as biomarkers for predicting future metastasis. Moreover, the presence of a subpopulation of cancer stem cells (CSCs) within a bulk tumor is found to be responsible for the development of metastasis; hence, surface makers which identify CSCs are potential biomarker for predicting metastasis. The recent findings and updates of these potential biomarkers are summarized in Table 1.
Fig. 1.
Involvement of cadherin-catenin interaction, integrin signaling pathway and PI3K/Akt/mTOR pathway in the cancer metastasis process, whereas cancer cells processing cancer stem cell properties are more prominent in the metastasis process
Table 1.
Recent findings and updates of biomarkers for predicting future metastasis of gastrointestinal cancers
| Gene | Origin | Measurement | Correlation | Ref |
|---|---|---|---|---|
| Cadherin–catenin interaction | ||||
| E-cadherin | HCC |
IHC (< or = 90 % tumor cells: reduced) (>90 % tumor cells: normal) |
Reduced expression was correlated with invasion and metastasis | [21] |
| CRC |
IHC (<80 % tumor cells: reduced) (>80 % tumor cells: preserved) |
Reduced membranous expression: Lymph node metastasis Recurrence |
[22] | |
| GC |
IHC Staining intensity (tumor cells as strong as normal epithelial cells: preserved) (weaker in tumor cells: reduced) |
Reduced expression had greater extent of: Lymph node metastasis Lymphatic invasion Venous invasion |
[24] | |
| GC |
IHC (normal: strong and membranous) (abnormal: others) |
Abnormal expression: Lymph node metastasis Lymphatic invasion |
[23] | |
| GC |
qRT-PCR of RNA extracted from primary specimens Tumor vs. normal mucosa (< or = 2: down-regulation) (>2: up-regulation) |
Down-regulation was correlated with distant metastasis | [25] | |
| Soluble E-cadherin | GC |
Serum level by ELISA (cut-off: >10,000 ng/ml) |
Higher than cut-off value: Recurrence |
[28] |
| GC |
Serum level by ELISA (cut-off: >7,025 ng/ml) |
Higher than cut-off value: Tumor (T4) depth invasion |
[29] | |
| HCC |
Serum level by ELISA (cut-off: > or = 8,000 ng/ml) |
Higher than cut-off value: Early recurrence Extrahepatic metastasis |
[30] | |
| Snail | HCC | qRT-PCR of RNA extracted from PBMCs | Snail mRNA level was 18.8-fold higher in HCC with extrahepatic metastasis | [39] |
| HCC | qRT-PCR of RNA extracted from primary specimens | Snail mRNA level was significantly higher in HCC with capsular invasion | [46] | |
| HCC | qRT-PCR of RNA extracted from primary specimens | Snail mRNA level was significantly higher in HCC with portal vein invasion and intrahepatic metastasis | [47] | |
| EC |
IHC (>10 % tumor cells: positive) (<10 % tumor cells: negative) |
Tumors with positive Snail expression invaded deeper (p = 0.0385), had more distant lymph node metastasis | [49] | |
| Slug | CRC |
IHC (<10 % tumor cells: negative) (>10 % tumor cells: positive) |
Positive expression correlated with distant metastasis | [50] |
| GC |
qRT-PCR of RNA extracted from primary specimens Tumor vs. normal mucosa (>2: upregulation) (< or = 2: downregulation) |
Upregulation was correlated with distant metastasis | [25] | |
| GC |
IHC (>10 % tumor cells: positive) (<10 % tumor cells: negative) |
Positive expression had greater extent of: Depth of tumor invasion Lymph node metastasis Lymphatic invasion Venous invasion Peritoneal recurrence |
[24] | |
| ESCC |
IHC (>10 % tumor cells: positive) (<10 % tumor cells: negative) |
Positive expression correlated with lymphatic invasion and venous invasion | [51] | |
| Twist | HCC | Tissue microarray by immunostaining of primary and metastatic HCCs | Twist expression associated with HCC metastasis | [54] |
| HCC |
IHC Staining (Extent + percentage >2: positive) (Extent + percentage <2: negative) |
Positive expression: Intrahepatic and extrahepatic metastasis |
[55] | |
| HCC |
IHC (< or = 10 % tumor cells: negative) (>10 % tumor cells: positive) |
Positive expression: Invasion and metastasis |
[21] | |
| HCC | IHC |
Nuclear overexpression: Metastasis |
[53] | |
| CRC | qRT-PCR | mRNA expression in tumor tissues correlated with lymph node metastasis | [56] | |
| GC |
IHC Staining (Extent + percentage > or = 3: positive) (Extent + percentage < 3: negative) |
Higher Twist expression: Lymph node metastasis |
[58] | |
| GC | IHC |
Twist positive expression: Lymph node metastasis |
[59] | |
| GC |
IHC Staining intensity (0–1: low) (2–3: high) |
High expression: Depth of invasion Lymph node metastasis Distant metastasis |
[57] | |
| EC | IHC |
Twist positive expression: Lymphatic metastasis |
[60] | |
| EC |
qRT-PCR normalized with GAPDH (>twofold: overexpression) (<twofold: underexpression) |
Overexpression of Twist: Depth of tumor invasion Lymph node metastasis |
[61] | |
| EC | IHC | Twist expression correlated with distant metastasis after esophagectopmy | [62] | |
| EC |
IHC Twist expression: (negative or weak: low) (moderate or strong: high) |
High level of twist expression associated with a higher risk for the patient to develop distant metastasis | [63] | |
| Vimentin | HCC | IHC | Upregulation in metastatic HCC as compared to primary cases | [41] |
| GC | qRT-PCR of primary specimens |
mRNA expression: Recurrence Distant metastasis |
[40] | |
| GC |
qRT-PCR of bone marrow RNA normalized with GAPDH (>threshold: positive) (<threshold: negative) |
Positive expression: Tumor invasion Lymph node metastasis Lymphatic invasion |
[44] | |
| ZEB1 | HCC |
Western blotting ZEB1 vs. GAPDH intensity (>0.3: high) (<0.3: low) |
High expression: Intrahepatic metastasis Vascular invasion Early recurrence |
[68] |
| Nanog | CRC | IHC | Lymph node metastasis | [72] |
| p28GANK | HCC |
IHC Staining density Lower than the median: low Higher than the median: high |
High expression: Vascular invasion Intrahepatic metastasis Distant metastasis |
[73] |
| EIF5A2 | HCC | qRT-PCR | High expression was correlated with tumor venous infiltration | [71] |
| HCC | qRT-PCR | Overexpression was correlated with metastasis | [70] | |
| CRC |
IHC ROC curve to determine the cut-off score for the overexpression |
Overexpression: Lymph node metastasis Distant metastasis |
[69] | |
| PRL-3 | CRC | ISH | mRNA expression was elevated in nearly all metastatic lesions comparing to normal colon and non-metastatic CRCs | [76] |
| CRC |
ISH (>10 % tumor cells showed elevated level comparing to internal control: high) (>90 % tumor cells showed no elevated level comparing to internal control: low) |
High expression detected more frequently in CRCs with liver and lung metastasis, and higher in CRCs with venous invasion | [77] | |
| CRC | IHC | Positive expression was a predictor for liver metastasis | [79] | |
| CRC |
qRT-PCR Normalized by expression in normal mucoses |
High expression: Liver or lung metastasis Vascular or lymphatic invasion |
[78] | |
| CRC |
IHC Expression (< or = 5 %: low) (6–30 %: median) (>30 % tumor cells: strong) |
Increase percentage of strong expression in tumor bud of patient: Lymph and blood vessel invasion |
[80] | |
| CRC | qRT-PCR | Mean expression was significantly higher in CRC patients with lymphatic invasion, vascular invasion or liver metastasis | [75] | |
| GC |
IHC Number of stained cells and staining intensity Almost no positive cells: negative 5–50 % tumor cells showed weak to moderate staining: low >50 % tumor cells showed strong staining: high |
Expression associated with: Lymphatic invasion Venous invasion Extent of lymph node metastasis Nodal metastasis |
[81] | |
| GC |
ISH Staining compared to internal control: (>25 % tumor cells showed higher intensity: high expression) (Negative or no increase in staining: low/none expression) |
Incidence of high PRL-3 expression: Lymphatic invasion Venous invasion Extent of lymph node metastasis Nodal metastasis |
[84] | |
| GC |
IHC Staining <10 % tumor cells with weak staining: low 10–50 % positive: moderate >50 % tumor cells with strong inetnsity: strong |
High expression: Depth of cancer invasion Lymph node metastasis |
[83] | |
| GC |
IHC Staining score no stained cells: 0 faint intensity or <10 % staining cells: 1 moderate intensity: 2 strong intensity: 3 |
Overexpression (score 2 or 3): Lymphatic permeation Vascular permeation Lymph node metastasis |
[82] | |
| GC |
IHC Staining <5 % tumor cells: negative >5 % tumor cells: positive |
Positive expression Extent of lymph node metastasis Lymphatic invasion |
[85] | |
| HCC |
qRT-PCR Expression level expressed as PRL-3/β-actin |
High expression correlated with vascular invasion | [86] | |
| HCC |
qRT-PCR Expression level expressed as PRL-3/18S rRNA IHC Percentage of stained cells <20 % tumor cells: negative > or = 20 % tumor cells: positive |
High mRNA expression: Portal vein invasion Vascular invasion Positive expression: Hepatic vein invasion Vascular invasion Tumor stage |
[87] | |
| EC |
IHC Immunoreactive score: intensity × extent score (range from 0 to 12) (> or =4: positive) (<4: negative) |
PRL-3 overexpression: Lymph node metastasis Vascular invasion |
[89] | |
| EC |
Semiquantitative RT-PCR Expression level expressed as PRL-3/β-actin |
Frequency and level of PRL-3 expression was higher in ESCC with lymph node metastasis | [88] | |
| Stathmin | HCC |
qRT-PCR Expression (tumor:adjacent liver > threefold: overexpression) |
Overexpression: Local invasion Early HCC recurrence HCC recurrence |
[91] |
| CRC |
IHC Overall protein expression score was calculated by score of staining intensity × score of staining area (< or = 8: low) (>8: high) |
High expression: Tumor invasion Lymph node status |
[90] | |
| β-Catenin | HCC | IHC |
Positive staining: Microvascular invasion High tumor node metastasis stage Concomitant expressed with HIF-1α: Intrahepatic metastasis Microvascular invasion |
[99] |
| GC | IHC |
Positive staining: Reduced in mucinous gastric cancer which was more aggressive and metastatic |
[102] | |
| GC | IHC | Loss of membranous or appearance of nuclear expression correlated with lymph node metastasis | [101] | |
| CRC |
IHC Expression on cell–cell boundaries of tumor cells compared with normal epithelial cells: (Equal to normal: preserved) (Weaker or variable: Reduced) |
Reduced expression: Lymph node metastasis Metastasis |
[103] | |
| CRC |
IHC Membranous expression (>80 % tumor cells: preserved) (<80 % tumor cells: reduced) |
Reduced expression: Lymph node metastasis |
[22] | |
| CRC |
IHC Nuclear expression |
Positive nuclear accumulation was associated with lymph node metastasis | [104] | |
| CRC |
IHC Cytoplasmic expression (< or = 10 %: negative) (>10 %: positive) |
Positive cytoplasmic expression: Lymph node metastasis Distant metastasis |
[105] | |
| CRC |
IHC Expression (Similar to normal colonic or rectal crypt: membranous) (Nuclear accumulation throughout the tumor: NA) (Nuclear accumulation at tumor invasive front only: NAinv) |
NAinv pattern correlated with distant metastasis | [106] | |
| Gastroentero-pancreatic |
IHC Expression (Subcellular localisation of immunostaining: membranous, nuclear or cytoplasmic) |
Nuclear expression correlated with metastasis | [107] | |
| EC |
IHC Membranous staining >70 % cells: preserved expression <70 % cells: reduced expression |
Reduced expression correlated with invasion depth and lymph node metastasis | [108] | |
| CDK8 | GC | IHC | Expression was associated with lymph node metastasis | [111] |
| VLDLR II | GC |
IHC Average intensity = intensity × percentage area stained |
High expression: Lymph node metastasis Distant metastasis Advanced TNM stage |
[113] |
| L1 | CRC |
IHC (>5 % tumor cells: positive) (<5 % tumor cells: negative) |
Positive expression Distant metastasis Recurrence |
[22] |
| PC |
IHC (>10 % tumor cells: positive) (<10 % tumor cells: negative) |
L1CAM positive expression correlated with lymph node involvement and distant metastasis | [110] | |
| PC |
IHC Staining score = intensity × percentage of cells (<30: negative) (> or = 30: positive) |
L1CAM positive expression correlated with node involvement and vascular invasion | [109] | |
| Soluble L1 | Gastrointestinal stromal tumor |
Serum level by ELISA (>2 ng/ml: high) (<2 ng/ml: low) |
High level associated with recurrence | [97] |
| TC1 | GC | IHC |
Overexpression: TNM stage depth of invasion lymph node metastasis lymphatic infiltration |
[114] |
| EPLIN | CRC |
Microarray IHC |
Expression of transcripts was lower in metastatic colon cancer Expression was significantly decreased in lymph node metastatic tumors |
[116] |
| NM23-H1 | HCC | IHC | Expression was negatively correlated with intrahepatic metastasis | [117] |
| GC | Northern blot analysis | Downregulation correlated with serosal invasion and nodal metastasis | [118] | |
| CRC | IHC | Expression was negatively correlated with lymph node metastasis | [121] | |
| CRC |
IHC Score based on intensity and percentage of stained area |
Underexpression was correlated with distant metastasis | [105] | |
| EC |
IHC (<10 % of cells: negative) (10–50 %: positive) (>50 %: strongly positive) |
Decreased expression of NM23H1 correlated with lymph node metastasis | [119] | |
| EC |
IHC (<10 % of cells: negative) (10–50 %: positive) (>50 %: strongly positive) |
Decreased expression of NM23H1 correlated with tumor invasion and lymph node metastasis | [120] | |
| c-myc | CRC |
IHC Score based on intensity and percentage of stained area |
Expression was correlated with distant metastasis | [105] |
| Integrin signaling | ||||
| Serum β1integrin | GC |
Serum level by ELISA Compared with cut-off value (5.2 μg/ml) |
Higher serum level: Lymph node metastasis Remote metastasis |
[124] |
| Blood β1integrin | GC |
qRT-PCR of blood RNA Expression level of targets mRNA was expressed as 2–ΔCt, ΔCt = Ct(targets mRNA) − Ct(GAPDH) |
Higher mRNA expression: Higher TNM stage Lymphatic metastasis Distant metastasis |
[125] |
| PBMC β1integrin | PC PBMC |
Triplex qRT-PCR Assay The copy number was quantified with regard to the standard curve |
The expression correlated with liver metastasis and clinical stage | [128] |
| PC PBMC |
Triplex qRT-PCR Assay The copy number was quantified with regard to the standard curve |
The expression correlated with clinical stage, lymph node and liver metastasis | [129] | |
| β1integrin | HCC | Western blotting to detect phospho-β1integrin (T788/789) | Positive expression was correlated with vascular invasion | [136] |
| GC |
IHC Staining index = intensity × proportion of positive cells (< or = 3: low) (>3: high) |
High expression: Depth of invasion Vessel invasion Lymph node metastasis Distant metastasis TNM stage |
[137] | |
| α2β1 and α3β1 integrins | GC | IHC |
α2β1 expression: Lymph node metastasis Liver metastasis α3β1 expression: Liver metastasis Peritoneal metastasis |
[138] |
| α3 integrin | HCC |
IHC Mean number of positive stained cells in 10 randomly chosen microscopic fields |
Mean number of positive stained cells was higher in HCC with metastasis than that without metastasis | [139] |
| αvβ3 integrin | PC |
IHC Intensity no, faint or equivocal: negative unequivocal or strong: positive |
Positive expression correlated with advance stage and node involvement | [140] |
| Plasma OPN | GC | Blood sample by ELISA |
Higher median plasma level: Serosal invasion Lymph node metastasis Lymphatic invasion Venous invasion Liver metastasis |
[131] |
| HCC | Blood sample by ELISA |
Higher plasma level: TNM stages Recurrence |
[133] | |
| HCC |
Blood sample by ELISA (cutoff value based on ROC curve:100 ng/ml) (<100 ng/ml: low) (> or = 100 ng/ml: high) |
Higher plasma level group: Recurrence |
[132] | |
| ESCC | Blood sample by enzyme immunoassay | High OPN level was associated with lymph node metastasis | [134] | |
| OPN | CRC |
qRT-PCR normalized by mRNA level of β-actin Cutoff value based on log-rank plot analysis: 0.276 (mRNA level < 0.276: low) (mRNA level > or = 0.276: high |
High expression: Lymph node metastasis Lymphatic invasion Venous invasion TNM stage |
[141] |
| HCC |
RT-PCR and signal measured by gel electrophoresis mRNA level determined by ratio of signal intensity to that of S26 (< or = 0.6: no overexpression) (>0.6: overexpression) |
mRNA overexpression: Portal vein invasion Early recurrence |
[142] | |
| HCC | cDNA microarray |
Overexpression: Good diagnostic value for metastatic HCC patients |
[143] | |
| GC |
IHC Staining: (negative: −) (weak or focal expression: 1+) (moderate expression with focal strong expression: 2+) (strong expression: 3+) |
High protein expression: Tumor depth Hematogenous metastasis Positive expression (1 + to 3 +): Tumor depth Regional lymph node metastasis Hematogenous metastasis |
[144] | |
| OPN fragment | HCC | qRT-PCR | Overexpression was detected in metastatic HCC as compared with non-metastatic samples | [145] |
| Cyr61 | GC |
qRT-PCR of blood RNA Expression level of targets mRNA was expressed as 2–ΔCt, ΔCt = Ct(targets mRNA) − Ct(GAPDH). |
Higher mRNA expression: Higher TNM stage Distant metastasis |
[125] |
| GC |
IHC Intensity (<50 % tumor cells: low) (>50 % tumor cells: high) |
High intensity correlated with status of lymph node metastasis | [126] | |
| ESCC |
qRT-PCR Expression level of targets mRNA was expressed as 2–ΔCt, ΔCt = Ct(targets mRNA) − Ct(β-actin). |
High expression correlated with regional lymph node metastasis | [147] | |
| Cyr61 and CTGF | HCC |
qRT-PCR Relative yield of PCR product to that of β-actin |
High expression correlated with portal vein invasion | [148] |
| SPARC | GC |
IHC Staining index (< or = 3: low) (> or = 4: high) |
High expression: Depth of invasion Vessel invasion Lymph node metastasis Distant metastasis TNM stage |
[137] |
| GC |
IHC Staining (no or trace staining: negative) (moderate or strong staining : positive) |
Positive staining: Depth of invasion Lymph node metastasis Lymphatic invasion Perineural invasion |
[150] | |
| ESCC |
IHC Staining (No detectable or only trace staining: negative) (Low, moderate or high levels: positive) |
High SPARC expression associated with lymph node metastasis | [151] | |
| Serum ANGPTL4 | HCC |
Serum level by ELISA Cutoff value determined by ROC analysis: 93.5 ng/ml (<93.5: negative) (> or = 93.5: positive) |
High serum level: Macrovascular invasion Intrahepatic metastasis |
[127] |
| ANGPTL4 | CRC | IHC |
Expression: Depth of invasion Venous invasion |
[152] |
| GC |
IHC Percentage of cells stained: (0–10 % tumor cells: negative) (>10 % tumor cells: positive) |
Positive: Depth of invasion Lymph node metastasis Lymph duct invasion Venous invasion TNM stage |
[234] | |
| ILK | CRC |
IHC Intensity: (negative: 0) (weak staining: 1) (moderate staining: 2) (strong staining: 3) |
Expression: Depth of invasion Lymph node metastasis |
[155] |
| GC |
RT-PCR IHC Staining: (<50 % tumor cells: weak) (>50 % tumor cells: strong) |
Positive expression was correlated with lymph node metastasis Strong expression: Depth of invasion Lymph node metastasis |
[156] | |
| Tβ4 | CRC |
IHC Score = intensity × area (<mean score: low) (>mean score: high) |
High expression was associated with distant metastasis | [160] |
| Cten | CRC |
IHC Assessed using H-score (<150: low) (>150: high) |
High expression: Lymph node metastasis Extra-mural vascular invasion Distant metastasis |
[161] |
| FAK and phospho FAK (Tyr397) | HCC |
IHC Staining: (< or = 20 % tumor cells: negative) (>20 % tumor cells: positive) FAK mRNA by qRT-PCR |
Positive expression: Capsular invasion Vascular invasion Intrahepatic metastasis Overexpression: Advance TNM stage Metastasis |
[163] |
| FAK | GC |
IHC Staining: (<10 % tumor cells: no staining) (> or = 10 %: Positive) Gene amplification by FISH FAK/CEB8 ratio: (> or = 2 in more than 10 % tumor cells: high) |
Positive expression: Depth of invasion Nodal metastasis Distant metastasis Lymphatic invasion Venous invasion Perineural invasion High level amplification: Nodal metastasis Distant metastasis Lymphatic invasion Venous invasion Perineural invasion |
[164] |
| GC or CRC l |
IHC Staining: (negative to mild: low) (moderate to strong: high) |
High expression: Extent of invasion Metastasis |
[165] | |
| EC |
IHC More than 40 % cells stained more intensely than the normal epithelium: overexpression |
Overexpression: Depth of tumour invasion Presence of regional lymph node metastasis Number of lymph node metastases |
[166] | |
| PC |
IHC More than 5 % tumor cells were positively stained was considered positive |
Positive staining correlated with distant metastasis | [167] | |
| MAP4K4 | HCC |
IHC Cutoff determined by median percentage of stained tumor cells: 10 % (> or = 10 %: high) (<10 %: low) |
High expression: Intrahepatic metastasis TNM stage |
[169] |
| CRC |
IHC Staining score = intensity × area (<4: low) (> or = 4: high) |
High expression: Lymph node metastasis Tumor invasion |
[170] | |
| PC |
IHC Cytoplasmic staining (> or = 10 % tumor cells: positive) (<10 % tumor cells: negative) |
Positive staining: Recurrence/Distant metastasis Average number of positive lymph nodes |
[171] | |
| Grb7 | HCC |
IHC Staining: (<20 % tumor cells: negative) (>20 % tumor cells: positive) |
Positive expression: Portal venous invasion Hepatic venous invasion Intrahepatic metastasis |
[172] |
| PC |
IHC Staining: (>20 % tumor cells: positive) (<20 % tumor cells: negative) |
Positive expression correlated with lymph node metastasis | [173] | |
| LOXL2 | GC |
IHC Staining = intensity × percentage of stained cells (no staining: 0) (faint, moderate to strong in 0 to 25 % cells: 1) (moderate or strong in 25 to 50 % cells: 2) (Strong in > or = 50 % cells: 3) |
Higher expression Lymph node metastasis Tumor invasion |
[174] |
| Egfl7 | HCC |
IHC Staining: (< or = 10 %: negative) (>10 %: positive) |
Positive expression: Tumor nodule numbers Capsular formation Venous invasion |
[175] |
| Egfl8 | CRC | qRT-PCR |
Downregulation Distant metastasis TNM stage |
[176] |
| SCARA5 | HCC |
IHC Intensity (0: negative) (1: low) (2: Moderate) (3: High) (4: Strong) |
Protein level was significantly lower in HCC with portal vein tumor thrombosis (PVTT), which was associated with cellular invasion, venous permeation, and perhaps even metastasis | [179] |
| PI3K/Akt/mTOR signaling | ||||
| Phospho Akt | GC |
IHC >10 % tumor cells with moderate to strong staining: positive Others: negative |
Overexpression: Depth of invasion Lymph node metastasis |
[179] |
| ESCC |
IHC Staining >50 % tumor cells stained positive: High expression < or = 50 % tumor cells stained positive: Low expression |
High expression correlated with depth of tumor invasion | [186] | |
| Phospho mTOR | GC |
IHC Staining of tumor cells (no staining or staining weaker than normal: underexpression) (staining similar to normal: normal) (staining stronger than normal: overexpression) |
Overexpression was correlated with lymph node metastasis | [187] |
| mTOR | GC |
IHC Scoring = intensity × percentage of stained cells No expression (Score 0): negative Any expression (Score 1-12): positive |
Higher expression was correlated with distant metastasis in foregut. | [188] |
| Phospho mTOR | GC |
IHC Scoring = extent × percentage of stained cells (< or = 2: negative) (>2: positive) |
Expression was correlated with extent of lymph node metastasis | [189] |
| mTOR | CRC |
IHC Scoring = intensity × percentage of stained cells (<20: negative) (>20: positive) |
Expression was correlated with depth of tumor infiltration | [190] |
| HOXB7 | CRC |
IHC Intensity (moderate or strong staining with at least 50 % stained cells: high) (no or weak staining with less than 50 % stained cells: low) |
High level was correlated with distant metastasis | [191] |
| PDA |
IHC Histoscore = staining intensity × percent tumor cell staining |
High expression correlated with lymph node metastasis | [192] | |
| YKL-40 | GC |
IHC Staining index = intensity × percentage of stained cells (<4: no overexpression) (> or = 4: overexpression) |
Overexpression: Tumor invasiveness Lymphatic metastasis TNM stage |
[183] |
| BMI1 | GC |
IHC >10 % tumor cells with moderate to strong staining: positive Others: negative |
Higher expression correlated with lymph node metastasis | [185] |
| PC |
IHC Cases with >10 % of cells stained nuclear were regarded as positive |
Positive staining was associated with lymph node metastasis | [193] | |
| ESCC |
IHC Score: intensity × percentage of stained cells (<4: low overexpression) (> or = 4: high expression) |
High expression was associated with lymph node metastasis | [194] | |
| Cancer stem cell identification | ||||
| CD26 | CRC | Percentage of CD26+ cells determined by flow cytometry |
Higher percentage: Microscopic vascular invasion Distant metastasis |
[197] |
| CD133 | CRC |
qRT-PCR of blood RNA Expression expressed as ratio to GAPDH (>cut-off (3.4 × 10−4): Positive (<cut-off (3.4 × 10−4): Negative |
Positive expression: Hematogenous metastasis |
[198] |
| CRC |
qRT-PCR of PBMC Amount of target was normalized to GAPDH and relative to calibrator Cutoff value: 4.79 |
mRNA level > or = 4.79 was correlated with recurrence | [199] | |
| GC |
IHC RT-PCR Expression as brightness scale value (BSV) ratio of CD133 strip and control strip |
Positive protein expression: Lymph node metastasis TNM stage Lymphatic vessel infiltration Depth of tumor invasion mRNA expression was correlated with lymph node metastasis |
[201] | |
| PC | IHC | Expression correlated with venous invasion | [203] | |
| PC |
IHC Tumor cells distinctly stained: positive |
Positive staining: lymphatic invasion lymph node metastasis |
[204] | |
| CD24 | GC | IHC |
Positive expression: Lymphatic invasion Vascular invasion |
[206] |
| HCC |
IHC Staining score = intensity × percentage of stained cells (0 to 3: negative) (4 to 9: positive) |
High expression: Diffused intrahepatic recurrence Distant metastasis |
[205] | |
| HCC |
qRT-PCR Expression cutoff value of tumor/non-tumor: 3 (<3: low) (>3: high) |
High expression: Recurrence in the 1st year after surgery Venous infiltration |
[207] | |
| PC |
IHC Staining score = intensity × percentage of stained cells (<2: negative) (between 2 and 6: weakly positive) (>6: strongly positive) |
Positive expression: Nodal metastasis Microscopic lymphatic invasion Venous invasion Neural invasion |
[208] | |
| CD133 and CD44 | HCC |
IHC Score: (none: 0) (0.01 to 5 % tumor cells: 1) (> or = 5 % tumor cells: 2) Percentage of CD133/CD44 cells determined by flow cytometry |
CD133 score was stronger in metastatic group and associated with vascular invasion CD44 score was higher in metastatic group, and higher in tumor edge as compared with the tumor bulk More CD133/CD44 cells were associated with portal vein metastasis |
[202] |
| Epithelial and variant CD44 | CRC | RT-PCR of RNA extracted from PBMC | Expression rate in PBMC was correlated with TNM stage and could predict distant metastasis | [211] |
| CD44 | PC |
IHC quantity of immunopositive cells (≤1 % positive cells: negative) (2–20 % positive cells: weakly positive) (21–50 % positive cells: moderately positive) (51–100 % positive cells: strongly positive) |
Expression was significantly higher in carcinomas with lymph node metastasis or distant metastasis | [212] |
| ALDH1 | ESCC | IHC | Increased nuclear accumulation correlated with lymph node metastasis | [213] |
| ESCC |
IHC Percentage of positive tumor cells (> or = 10 %: positive) (<10 %: negative) |
Positive expression correlated with recurrence | [214] | |
| GC |
IHC Percentage of positive tumor cells (> or = 10 %: positive) (<10 %: negative) |
Positive expression correlated with tumor stage and TNM stage | [215] | |
In the following, we will list some recently identified proteins which showed variation in clinical metastatic and non-metastatic tumors, and discuss how these potential biomarkers, detected in peripheral blood and/or primary tumors, are associated with metastasis of gastrointestinal cancers. Focus will be placed on biomarkers present in peripheral blood due to the ease of detection and the possibility of post-operative monitoring of the patient. Finally, we will briefly discuss how certain proteins are involved in tumor microenvironment modification to facilitate cancer cell metastasis.
Cadherin–catenin interaction
At the cellular level, the early stages of cancer metastasis are characterized by cancer cell dissemination, which is the loss of contact with neighboring cells and an increase in invasive capacity. Such process is known as EMT, in which epithelial cells acquire a fibroblast-like morphology, enhanced motility, and gene expression patterns characteristic of mesenchymal cells. Loss of E-cadherin expression is a hallmark of EMT. A low level of E-cadherin advantages tumor cells in breaking the adhesion junctions and detaching from adjacent cells, so that these cells can invade and metastasize to distant organs [20]. In accordance, loss of E-cadherin expression in primary tumors associated with more invasive and metastatic tumors in HCC [21], CRC [22], GC [23–25], PC [26] and EC patients [27].
E-cadherin is lost from the cell surface upon proteolytic cleavage. Such process results in an 80-kDa degradation fragment known as soluble E-cadherin (sE-cadherin) which can be detected in the peripheral blood of cancer patients and acts as a non-invasive prognostic biomarker. In a GC study, there was no significant difference in preopertative sE-cadherin level among patients with and without recurrence (6,290 vs. 5,747 ng/ml, p = 0.8), whereas a significant increase in postoperative sE-cadherin level at months 3, 6, 9, 12, 18, and 21 was observed in the group with recurrence but not in that without recurrence [28]. Moreover, the authors indicated that a sE-cadherin level cut-off value of 10,000 ng/ml at 6 months postsurgery provided optimum sensitivity (59 %) for predicting recurrence, which was significantly better than the sensitivity of CEA (6 %) using the conventional cut-off value (5 ng/ml), and level of sE-cadherin but not CEA higher than the cut-off value predicted recurrence independently [28]. In another GC study, patients with preoperative sE-cadherin level over 7,025 ng/ml were also more likely to have tumor (T4) depth invasion [29]. High level of serum sE-cadherin (>8,000 ng/ml) also correlated with early recurrence and extrahepatic metastasis in HCC patients [30]. In CRC patients, preoperative serum sE-cadherin concentration was significantly higher in stage IV (9,471 ng/ml) than in stage I to III patients (~6,500 ng/ml), and significantly higher in patients with hepatic metastasis (9,637 vs. 6,648 ng/ml) and metachronous hepatic metastasis (10,540 vs. 6,018 ng/ml) [31]. Recently, Chung et al. [32] demonstrated the detection of serum sE-cadherin in esophageal squamous cell carcinoma (ESCC) patients and suggested that sE-cadherin is a potential prognostic marker. These studies demonstrated the high potential of sE-cadherin as metastatic biomarker in gastrointestinal cancers, though further investigations are warranted to determine the cut-off value, sensitivity and specificity of sE-cadherin in predicting metastasis of CRC, PC and EC patients.
It should be taken into account that the serum sE-cadherin level could be affected by other factors in addition to tumor progression and development of metastasis. For example, Weiss et al. [33] reported an elevation of serum sE-cadherin concentration in patients with familial adenomatous polyposis. Serum sE-cadherin level also increased with age in both GC patients and control subjects [34]. These factors should be taken into account when interpreting the patient’s sE-cadherin level. Moreover, there was discrepancy in the level of serum sE-cadherin in patients of the same category measured among different studies. For example, comparing the results of Okugawa et al. [31] and Weiss et al. [33], the mean serum sE-cadherin level in early stage (I to II) CRC patients was over 6,000 and 4,900 ng/ml, respectively, and in late stage patients (III to IV) was ~7,800 and 6,100 ng/ml, respectively. Since similar level of serum sE-cadherin was detected in healthy control in their studies (~4,500 and 4,800 ng/ml, respectively), their measurement technique was probably not the reason for the discrepancy. Such discrepancy could be due to difference in sample size; hence, a more accurate level shall be obtained by recruiting more patients in each category. We believed that serum sE-cadherin level would be a useful biomarker for predicting development of metastasis in the future.
The E-cadherin expression and EMT process are regulated by certain transcriptional factors, common examples include Snail [35, 36], Slug [37] and Twist [38]. It is not surprising that alteration of their levels correlated with cancer metastasis. In a HCC study, positive Snail mRNA expression in peripheral blood mononuclear cells (PBMCs) isolated from peripheral blood of patients was used to identify presence of circulating tumor cells (CTCs), which was associated with metastatic potential [39]. The authors showed that Snail mRNA expression was 26.6 folds higher in HCC patients with extrahepatic metastasis when compared with those without extrahepatic metastasis. When the cut-off level of Snail mRNA expression ratio (GAPDH normalized) was set at 1 log10, the positive and negative predictive values for extrahepatic metastasis were 88.2 and 81.3 %, respectively. Furthermore, the authors demonstrated that Snail mRNA expression was a better prognostic biomarker than serum AFP level. Though serum AFP level was high in all HCC cases with extrahepatic metastasis, it showed variation in those without metastasis. In contrast, Snail mRNA levels were low in most HCC patients without metastasis and high in those with metastasis. More importantly, the authors showed a decline of Snail mRNA expression in all of the six HCC patients after complete remission by repeated transarterial chemobilization. Though the complete set of post-surgery follow-up data for Snail mRNA has not been completed, their study demonstrated the potential of Snail mRNA expression as a surrogate marker for disease monitoring in HCC.
Expression of another EMT marker vimentin in primary tumor-associated with invasion and/or metastatic disease of HCC and GC patients [40, 41]. In GC patients vimentin mRNA was detectable in bone-marrow, which is an important reservoir of tumor cells and from which they recirculate into distant organs such as liver or lungs [42, 43]. Patients with positive expression of vimentin (cut-off value: 95 % confidence interval of normal) showed significantly higher frequency of tumor invasion (71.5 %), lymph node metastasis (78.6 %) and lymphatic invasion (66.8 %) [44]. However, in the group with no bone-marrow vimentin mRNA expression, more than half of the patients still showed these unfavorable prognostic features, indicating that the false negative rate was also high. In another study, vimentin methylation in pre-operative serum of CRC patients was studied [45]. They showed that comparing with patients showing no vimentin methylation, those showing vimentin methylation had a significantly higher risk of liver metastasis (10 vs. 50 %), peritoneal dissemination (5 vs. 75 %), and distant metastasis (0 vs. 50 %). However, the sample sizes of patient with vimentin methylation, liver metastasis, peritoneal dissemination, and distant metastasis were small. It is necessary to increase the number of patients in those groups to validate the prognostic value of vimentin methylation in CRC patients.
The expression of these EMT regulators in primary specimens of certain types of gastrointestinal cancers also correlated with metastasis. High snail expression in HCC, PC and ESCC specimens correlated with aggressiveness, invasion and/or metastasis [46–49]. Slug expression correlated with invasion and metastasis of GC, CRC and ESCC [24, 25, 50, 51], yet its mRNA and protein expressions showed no correlation with metastatic features of HCC [46, 52]. Twist expression associated with metastasis of HCC [21, 53–55], lymph node metastasis of CRC [56], distant and lymph node metastases of GC [57–59], as well as depth of tumor invasion, lymphatic metastasis and distant metastasis of ESCC [60–63]. Twist has been implicated in tumor progression of PC, yet solid association with metastasis is lacking [64]. Nonetheless, these studies suggested that Twist expression in primary tumor is a potential biomarker for predicting development of metastatic disease in gastrointestinal carcinomas. Besides inducing EMT in tumor cells, Twist was implicated in a process named vasculogenic mimicry, which is a unique property of aggressive tumor cells [65]. Extended investigation showed that up-regulated twist expression forms a complex with Bcl-2 and facilitates its translocation to the nucleus, leading to MMP-9 overexpression and affecting HCC angiogenesis, which is a critical step in distant metastasis [53, 66, 67], further supporting its crucial roles in HCC metastasis.
Furthermore, the correlations of other EMT regulators or their associated proteins with metastatic clinicopathological parameters have been reported, strengthening the importance of EMT regulators in the development of metastasis and their potentials as biomarkers for predicting metastasis. For example, recently, high protein expression of Zeb1 in tumor tissue correlated with intrahepatic metastasis and vascular invasion in HCC [68]. Putative oncoprotein EIF5A2 which was capable of inducing EMT in vitro, its upregulation significantly correlated with metastasis in CRC and HCC [69–71]. Overexpression of Nanog induced expressions of snail and slug in vitro and correlated with lymph node metastasis in CRC [72]. Oncoprotein p28Gank induced E-cadherin down-regulation and EMT through alteration of Twist, showed significantly higher expression in HCCs with vascular invasion and intrahepatic and distant metastases [73].
Phosphatase of regenerating liver-3 (PRL-3), which is transcriptionally activated by Snail [74], showed increased expression in metastatic CRC [75–80], GC [81–85], HCC [86, 87] and ESCC [88, 89]. Recent studies identified stathmin and polyC-RNA-binding protein 1 (PCBP1) as PRL-3 associated proteins. Stathmin is a key oncoprotein which interacts with PRL-3 and promotes tumor cell invasion and metastasis in vitro [90, 91]. Overexpression of stathmin strongly correlated with tumor invasion in HCC and CRC [90, 91]. PCBP1 was capable of downregulating PRL-3 translation in vitro and negatively correlated with PRL-3 protein levels in GC and CRC specimens [92]. Despite the lack of clinical data currently, PCBP-1 has been suggested as a potential metastatic suppressor in HCC [93]. These observations demonstrated the significance of PRL-3 and its associated genes in tumor metastasis and their potential as biomarkers in metastatic cancers.
β-catenin is an epithelial marker which interacts with E-cadherin in coordinating cell–cell adhesion, as well as the central mediator of the Wnt/β-catenin pathway which contributes to tumorigenesis. Plasma β-catenin mRNA level positively correlated with tumor stage of CRC patients, and the level was significantly decreased in 16 of 19 CRC patients after tumor removal [94], suggesting that monitoring of plasma β-catenin mRNA level might be a useful biomarker for monitoring disease progression and detecting early recurrence. Serum β-catenin level has also been detected and correlated with the development of HCV-associated HCC [95]. Further investigations are warranted to examine the prognostic value of blood β-catenin level in HCC and other gastrointestinal cancers.
A recent report identified L1, a neural cell adhesion molecule, as a new target gene of Wnt/β-catenin-T cell factor (TCF) signaling [96]. Recently, Zander et al. [97] demonstrated that the median serum L1 level at the time of diagnosis in gastrointestinal stromal tumor (GIST) patients with tumor recurrence was significantly higher (1.7 vs. 1.1 ng/ml). Moreover, the 5-year recurrence-free survival rate in GIST patients with low L1 level (<2 ng/ml) was 52 %, while the rate in the high L1 level group was only 19 % [97]. We think monitoring of post-operative L1 level in GC patients could be detective of early recurrence.
β-catenin expression in primary tumors and its correlation with metastasis have been demonstrated by numerous studies. While membranous β-catenin determines an epithelial phenotype which is less aggressive, nuclear β-catenin represents a transcriptional regulator and main effector of the Wnt signaling pathway [98]. A recent study showed that β-catenin-positive staining correlated with increased microvascular invasion and high tumor-node-metastasis (TNM) stage in HCC [99]. In the same study, the authors further demonstrated a strong positive correlation between β-catenin and HIF-1α expression levels in HCC tissues, and patients with concomitant HIF-1α- and β-catenin-positive expressions had a higher incidence of intrahepatic metastasis and microvascular invasion compared with patients with expression of either HIF-1α or β-catenin alone [99]. An indirect correlation between β-catenin level and development of metastasis in HCC has also been demonstrated in another recent study, which showed that loss of membranous β-catenin reactivity may be associated with the occurrence of peritoneal seeding that is significantly associated with lymph node metastasis [100]. In GC, reduced membranous expression or nuclear translocalization of β-catenin correlated with lymph node metastasis [101]. In addition, β-catenin expression was reduced in mucinous tumors which showed a more aggressive and metastatic phenotype [102]. In CRC, reduced expression of membranous β-catenin was significantly associated with liver and lymph node metastases [22, 103], whereas β-catenin nuclear accumulation or cytoplasmic expression correlated with lymph node metastasis and/or distant metastasis [104, 105]. The presence of nuclear accumulation of β-catenin at the tumor invasive front also significantly correlated with distant metastasis [106]. In gastroenteropancreatic endocrine tumors, nuclear expression of β-catenin also correlated with development of metastasis [107]. On the other hand, reduced expression of membranous β-catenin detected immunohistochemically in the resected cancer tissues of ESCC correlated with invasive depth and lymph node metastasis [108].
Certain factors regulating β-catenin expression and membranous/nuclear distribution in clinical specimens correlated with the metastatic potential of gastrointestinal cancers. The expression of L1 in primary tumors correlated with distant metastasis of CRC [22], as well as node involvement, vascular invasion and distant metastasis of PC [109, 110]. In GC patients, CDK8 expression was associated with β-catenin activation, which together showed a significant positive correlation with lymph node metastasis [111]. VLDLR II overexpression enhanced the activation of β-catenin/TCF signaling in vitro [112] and its expression correlated with β-catenin level and lymph node and distant metastases in GC [113]. Expression of TC1, a novel regulator of the Wnt/β-catenin signaling pathway, correlated with TNM stage, depth of invasion, lymph node metastasis and lymphatic infiltration of GC [114]. TC1 level also correlated with β-catenin target genes involved in cancer invasiveness, including cyclin D1, MMP-7 (matrilysin), MMP-14, CD44, c-Met, and LAMC2 [114]. Recently, EPLIN and NM23-H1 were demonstrated as metastatic suppressor genes and their down-regulation promoted the nuclear translocation of β-catenin in vitro [115, 116]. EPLIN expression was significantly decreased in lymph node metastatic tumors as compared with primary tumors in CRC [116], whereas NM23-H1 downregulation correlated with intrahepatic metastasis of HCC [117], serosal invasion and nodal metastasis of GC [118], as well as tumor invasion and lymph node metastasis of ESCC [119, 120]. In recent years, the significance of NM23-H1 in CRC has also been documented by two studies, in which underexpression of NM23-H1 correlated with lymph node metastasis and/or distant metastasis [105, 121]. In one study, Chen et al. [105] further showed that overexpression of c-Myc (another Wnt signaling gene) was associated with lymph node metastasis and distant metastasis.
Integrin signaling pathway
Integrins are transmembranous glycoproteins that mediate cell-to-cell and cell-to-matrix interactions and contribute to cancer progression, invasiveness, and metastasis [122]. Integrins directly bind components of the extracellular matrix (ECM) and provide the traction necessary for cell motility and invasion. To date, 24 distinct integrin heterodimers have been identified, formed by the combination of 18 α-subunits and eight β-subunits and preferentially binding to distinct ECM proteins [123].
The prognostic significance of blood β1 integrin level of GC patients was examined in two studies [124, 125]. In one study, quantitative PCR was performed to determine the expression of β1 integrin in peripheral blood karyocyte and the expression level was expressed as 2 − ΔCt (ΔCt = Ct(β1-integrin) − Ct(GAPDH)). β1 integrin level was significantly higher in patients with higher TNM stage (0.012 vs. 0.007) and distant metastasis (0.020 vs. 0.008) [125]. Moreover, their study showed that peripheral blood karyocyte CYR61 level, which positively correlated with the status of lymph node metastasis of GC patients through elevating functional COX-2 by an integrin-αvβ3/NF-κB-dependent pathway [126], was significantly increased in patients with higher TNM stage (0.025 vs. 0.014) and distant metastasis (0.08 vs. 0.016) [125]. ANGPTL4 acted as an important regulator in metastasis of HCC in β1-integrin-dependent signaling in vitro, and its serum protein level in HCC patients correlated with intrahepatic metastasis and macrovascular invasion [127]. In another GC study, ELISA was applied to determine the preoperative serum level of β1 integrin. Patients with lymph node metastasis and distant metastasis had higher β1 integrin level [124]. However, though the author mentioned the serum β1 integrin level of GC patient (4.8 μg/ml) was significantly higher than that of normal (2.1 μg/ml), there was no information on the level in groups of TNM staging, lymph node metastasis and distant metastasis. These values are crucial for developing the cut-off value to distinguish patients of different risk to develop metastasis. In two PC studies, β1 integrin level in PBMCs detected by qRT-PCR correlated with stage, lymph node and liver metastasis [128, 129]. These studies demonstrated the probability of using integrin and integrin-associated genes as metastatic biomarkers. However, different detection methods have been applied to determine the blood β1 integrin level, it would be better to standardize a method to quantify the blood β1 integrin level in order to set the cut-off value for identifying cancer patients with higher risk to metastasis.
Osteopontin (OPN) is a secreted phosphoprotein that interacts with integrins αvβ3, αvβ1 and αvβ5 and may contribute to tumor invasion and metastasis via integrin-mediated signaling [130]. Plasma OPN level was significantly higher in GC patients with serosal invasion, lymph node metastasis, lymphatic invasion, venous invasion and liver metastasis [131]. Using a cut-off of 111.2 ng/ml, the sensitivity and specificity of plasma OPN in the prognosis of liver metastasis in GC patients were 75 and 83.1 %, respectively [131]. However, the proportion of patients with liver metastasis in that study was small (eight of 132 patients), it is necessary to include more GC patients with metastatic disease for validating the efficacy of using plasma OPN as prognostic biomarker. Plasma OPN level in HCC patients also correlated with TNM stage and/or recurrence [132, 133]. In Sun et al’s study, high pre-operative plasma OPN level (over 100 ng/ml) as determined by ELISA assay correlated with recurrence (52.2 vs. 24.4 %). A cut-off value at 100 ng/ml gave a sensitivity of 47.8 % and specificity of 75.6 %. Additionally, their study showed that using AFP cut-off value at 20 ng/ml, the sensitivity and specificity for predicting recurrence are 87.0 and 46.6 %, whereas the OPN level was similar in AFP high and low groups (82.5 vs. 83.0, p = 0.788). These results suggested that OPN level could be applied in addition to AFP for the purpose of increasing the sensitivity for identifying high risk patients who show no elevation of AFP level. In Zhang et al’s study, the authors also demonstrated that plasma OPN level was significantly higher in patients with recurrence (213.6 vs. 153.7 ng/ml), and patients with higher OPN (over 200 ng/ml) had higher recurrence rates than those with lower OPN level (81.1 vs. 39.3 %). Additionally, the OPN level determined in Zhang et al’s study measured was generally higher than that in Sun’s study, probably due to using a different ELISA kit and protocol. Shimada’s study demonstrated that high plasma OPN level was associated with lymph node metastasis in ESCC patients, which was, in addition, a more accurate metastatic marker than CEA in their study [134]. The role of OPN as diagnostic marker in CRC patients has been investigated, yet the serum OPN level was not significantly different from normal [135]. However, we believe that it is still necessary to examine its correlation with metastasis as OPN could be involved in cancer invasion and metastasis rather than in tumor development. In another study, the sensitivity of pre-operative serum OPN (cut-off at 95 % specificity) was low in lower stage CRC patients (stage 0–3: 23.2 %), whereas the sensitivity increased to 54.4 % in stage IV patients [135]. These results suggested that OPN could serve as a non-invasive prognostic marker for tumor progression, invasion and metastasis in gastrointestinal cancers.
In addition, expressions of integrin and associated proteins in primary tumors have been found to correlate with invasion/metastasis in gastrointestinal cancers. A recent study detected phospho-β1 integrin in HCC specimens from all patients with vascular invasion and in paired peritumoral specimens with metastatic nodules, whereas none of the HCC and peritumoral specimens without vascular invasion showed phospho-β1 integrin expression [136]. In GC, β1 integrin expression correlated with depth of invasion, vessel invasion, and lymph node and distant metastases [137]. Moreover, the expression of α2β1 integrin was associated with liver metastasis while that of α3β1 integrin-associated with lymph node and peritoneal metastasis [138]. α3 integrin was involved in HCC invasiveness and metastasis formation, and its overexpression might be caused by TGF-β1 [139]. In PC patients, expression of αvβ3 integrin significantly correlated with higher stage and lymph node metastasis [140].
Investigations have discovered that the expression of OPN in primary tumors significantly correlated with lymph node metastasis, lymphatic invasion and venous invasion in CRC [141] and metastasis in HCC [142, 143]. In GC, OPN protein expression was also significantly associated with hematogenous metastasis [144]. Moreover, Takafuji et al. [145] reported that increased expression of an OPN splice variant which is generated by proteolytic cleavage of OPN by MMP-9 was associated with clinical metastatic HCC. The association of OPN with metastasis in clinical specimens has not been demonstrated in PC so far. Interestingly, OPN expression was associated with improved survival though it was found to be overexpressed in PC patients when compared with controls subjects [146].
Several studies showed that CYR61, SPARC and ANGPTL4 were implicated in the development of metastasis in an integrin-related manner. Secreted protein Cyr61 belongs to the CYR61-CTGF-NOV (CCN) gene family. Kuo et al. [126] also reported that high expression level of Cyr61 was frequently observed in invasive gastric adenocarcinoma and Cyr61 level positively correlated with the status of lymph node metastasis of GC patients and ESCC patients [147], through elevating functional COX-2 by an integrin-αvβ3/NF-κB-dependent pathway [126]. Moreover, mRNA expressions of CYR61 and another CCN family member CTGF were higher in HCC with venous invasion [148]. SPARC protected cells from stress-induced apoptosis in vitro by interaction with β1 integrin [149]. Recently, SPARC expression was found to correlate with β1 integrin level, invasion and metastasis in GC [137], which is in accordance with the previous finding with GC that higher expression of SPARC was significantly associated with lymph node metastasis, lymphatic invasion and perineural invasion [150]. High protein expression of SPARC also correlated with lymph node metastasis in ESCC [151]. The expression of ANGPTL4 in tumor lesions correlated with depth of tumor invasion and venous invasion in CRC and GC, and all CRC patients with distant metastasis showed immunopositivity for ANGPTL4 [152].
Integrins transmit signals by interacting with cytoskeletal signaling and adaptor proteins to form structures known as focal adhesions. Although intrinsic enzymatic activity is absent in the cytoplasmic domain of integrin receptors, by clustering integrins recruit and activate kinases such as focal adhesion kinase (FAK) and integrin-linked kinase (ILK) [123, 153]. In the following, the correlation between metastatic potential and expressions of ILK and FAK is discussed, and so do their associated proteins.
ILK is a multifunctional non-receptor serine/threonine protein kinase that binds to the cytoplasmic tail of integrin β1 and β3 subunits and transduces many signals initiated by the interactions between cells and ECM [154]. In CRC, the level of ILK expression strongly correlated with tumor invasion and was significantly higher in metastatic tumors [155]. ILK may have an important role in the progression of human CRC, possibly through in vivo regulation of the β-catenin, E-cadherin and Akt pathways [155]. Significant association was also detected between ILK mRNA expression and nodal metastasis in GC [156]. In HCC, ILK expression level showed a significant stepwise increase along with tumor progression, whereas down-regulation of ILK significantly suppressed cell growth, motility and invasion in HCC in vitro [157]. However, clinical evidence demonstrating the correlation between ILK expression and development of metastasis in HCC is still lacking.
ILK was also associated with Tβ4 and Cten which were found to be correlated with metastasis recently. In a previous study, overexpression of Tβ4 (a G-actin-sequestering peptide) was observed in liver metastasis in patients of CRC, indicating that tumor cells expressing high level of Tβ4 was more metastatic [158]. A strong positive correlation between Tβ4 and ILK expression in CRC was later established and it is postulated that ILK is the most critical mediator of Tβ4-induced effects [159]. Recently, Tang et al. [160] showed that CRC patients with higher Tβ4 expression levels displayed a marked increase in the incidence of subsequent distant metastasis, and that the expressions of Tβ4 and ILK positively correlated in metastatic CRC, which may act together in developing distant metastasis. Cten is a member of the Tensin gene family which is localized to the cytoplasmic tails of integrins at focal adhesions. Albasri et al. [161] reported that high Cten expression was associated with lymph node metastasis, extramural vascular invasion and distant metastasis in CRC. They also showed that ILK was regulated by Cten and mediated Cten-induced cell motility in vitro.
FAK is a cytoplasmic non-receptor protein tyrosine kinase and a crucial mediator of integrin and growth factor signaling [162]. The significance of FAK in metastasis has been highlighted in certain studies. For example, increased levels of FAK and phospho-FAK (tyr397) correlated with vascular invasion and intrahepatic metastasis of HCC [163]. In GC, FAK gene amplification or protein expression was associated with nodal metastasis, distant metastasis, lymphatic invasion, venous invasion and/or perineural invasion [164]. In a previous study, enhanced expression of FAK correlated with depth of invasion and lymph node metastasis in GC and CRC [165]. FAK overexpression also correlated with depth of tumor invasion, presence of regional lymph node metastasis, number of lymph node metastases in ESCC [166], as well as distant metastasis in pancreatic ductal adenocarcinoma [167], though such correlation could not be demonstrated in another PC study [168].
Moreover, FAK is associated with many metastasis-related proteins. Overexpression of mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4) in HCC significantly associated with intrahepatic metastasis, and down-regulation of MAP4K4 resulted in enhanced apoptosis and repression of the JNK pathway downstream of FAK in vitro [169]. MAP4K4 protein expression also associated with tumor invasion and lymph node metastasis of CRC [170]. Moreover, MAP4K4 correlated with higher frequency of recurrence/metastasis and increased number of positive lymph nodes in pancreatic ductal adenocarcinoma [171]. FAK protein expression significantly correlated with growth factor receptor-bound protein 7 (Grb7), whereas positive staining of Grb7 protein correlated with portal venous invasion, hepatic venous invasion and intrahepatic metastasis in HCC [172] and lymph node metastasis in PC [173]. Elevated level of secreted LOXL2 in GC was significantly associated with depth of tumor invasion and lymph node metastasis via the Src/FAK pathway [174]. The expression level of a secreted protein epidermal growth factor-like domain 7 (Egfl7) which facilitates FAK phosphorylation was found to be significantly higher in HCC with venous invasion [175]. Interestingly, downregulation of Egfl8 expression significantly correlated with distant metastasis in CRC [176]. Egfl8 is the only known paralog of Egfl7 and the proteins they encode share the same overall domain structure [177, 178]. Whether Egfl8 acts as a metastasis inhibitor by competing with Egfl7 in tumors needs further investigation. Downregulation of a candidate tumor suppressor gene SCARA5 was associated with vascular invasion in HCC, and SCARA5 was physically associated with FAK and inhibited the FAK signaling pathway [179]. In the same study, the authors demonstrated that SCARA5 was also downregulated in GC specimens as compared with normal gastric epithelium, suggesting that SCARA5 might be a predictive biomarker of metastasis in multiple cancer types [179].
PI3K/Akt/mTOR pathway
The phosphoinositide-3 kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway is an important cellular pathway involved in cell invasion, angiogenesis and drug response [180–182]. Overexpression of YKL-40 was associated with Akt activation [183] and high serum YKL-40 level predicted poor prognosis in HCC patients [184]. A significantly higher proportion of patients in TNM stage III to IV (68.3 %) showed elevated serum YKL-40 (>113 ng/ml) when compared with those in stage I to II (49.0 %). Moreover, patients with elevated YKL level showed significantly shorter relapse-free survival than patients with normal level [184].
The significance of metastatic biomarkers involved in PI3K/Akt/mTOR pathway is also demonstrated in primary tumors. Overexpression of phospho-Akt positively correlated with depth of invasion and lymph node metastasis in GC [185], and depth of tumor invasion in ESCC patients without receiving preoperative chemotherapy [186]. Upregulation of the activated form of phospho-mTOR significantly correlated with lymph node metastasis in Chinese GC patients [187]. Furthermore, overexpression of mTOR and phospho-mTOR correlated with VEGF overexpression and tumor microvessel density, providing a strong clinical evidence of the critical role of the mTOR pathway in GC angiogenesis, which is an important step of metastasis [187]. In gastroenteropancreatic neuroendocrine tumors (GEP-NET), mTOR expression in foregut tumors was higher when distant metastasis was present [188]. In pT2b GC, phospho-mTOR expression was associated with the extent of lymph node metastasis [189]. Significant correlation was also found between mTOR signaling activity and the depth of CRC infiltration, which indicates that an increase in mTOR signaling activation might facilitate invasive growth of tumor cells [190]. HOXB7 protein expression significantly correlated with distant metastasis in CRC, and its expression was associated with phosphorylated level of Akt in vitro [191]. Expression of HOXB7 also correlated with lymph node metastasis of pancreatic ductal adenocarcinoma patients [192] Overexpression of YKL-40 associated with Akt activation, tumor invasiveness and lymphatic metastasis in GC [183].
Expression of oncogene BMI1 correlated with lymph node metastasis and phospho-Akt level in GC, and BMI1 regulated Akt activity in vitro [185]. It was also associated with lymph node metastasis in PC patients [193] and ESCC patients [194]. In HCC, however, BMI1 did not correlate significantly with any metastatic feature [195, 196]. The potential of BMI1 to act as a predictive biomarker for metastasis requires further investigation.
Cancer stem cell (CSCs) identification
There is growing evidence that the presence of a subpopulation of CSCs within a bulk tumor is responsible for the development of metastasis, hence determination of CSCs in patient provides useful information for predicting the risk of metastasis development. Our group recently identified CD26 as a CSC marker in CRC and showed that CD26 expression correlated with metastasis. The presence of CD26+ cells was significantly associated with microscopic vascular invasion and distant metastasis [197]. Moreover, CD26+ cells were capable of forming liver metastasis when injected into murine ceca, and the tumorigenic capacity was further enhanced by co-expression of CD133 and CD44; on the other hand, CD26− cells were incapable of forming liver metastasis. Furthermore, we demonstrated that CD26+ cells were resistant to chemotherapy which might enhance the development of further metastasis by enriching the CD26+ CSC subpopulation [197]. We also isolated CD26+ CSCs from the peripheral blood of CRC patients which closely correlated with the expression of CD26 in the tumors (our unpublished data), indicating the potential of peripheral blood CD26 level as a biomarker for predicting metastasis.
The mRNA expression (cut-off value: CD133/GAPDH = 3.4 × 10−4) of another putative CSC marker, CD133, in the presurgery peripheral blood of CRC patients who had undergone curative surgery showed the highest specificity for hematogenous metastasis when compared with general markers including CEA and CK [198]. Elevated CD133 mRNA in PBMCs also predicted CRC recurrence [199]. The median CD133 mRNA level was significantly higher in patients with recurrent disease (2 delta Ct method: 4.2 vs. 0.0017). The positive predictive value for recurrence (cut-off at 4.79) was 85 %. Moreover, in a study investigating the CD133 mRNA level in PBMCs collected from patients with CRC, renal cell carcinoma, prostate cancer and head and neck cancer, CD133 expression was significantly higher in patients with bone metastasis as compared with those without bone metastasis (163 vs. 0 copies) [200].
Recently, CD133 expression in primary lesions of GC was reported to be closely related to lymphatic metastasis [201]. In another recent study, Hou et al. reported that CD133+CD44+/high defined a subgroup of tumor cells responsible for hematogenous metastasis of liver cancer and the number of CD133+CD44+ tumor cells was associated with portal vein metastasis. They further showed that the presence of CD133 and high expression of CD44 in xenografts were necessary for producing intrahepatic or lung metastasis in nude mice [202]. Moreover, CD133 expression correlated with venous invasion of pancreatic ductal adenocarcinoma patients [203], and lymphatic invasion and lymph node metastasis in another PC study [204].
Previous studies reported that high CD24 expression was associated with invasion and metastasis in HCC and GC [205, 206]. Recently, Lee et al. identified CD24 as a CSC marker in HCC and found that the expression of CD24 was dramatically higher in their chemoresistant xenograft model and HCC cell lines. They showed that CD24+ HCC cells were more tumorigenic in vitro and in vivo and had a general overexpression of stemness-associated genes. Patients whose tumors had high CD24 expression had a significantly higher risk of tumor recurrence in the first year after surgery and more frequently had venous infiltration [207]. In accordance with this finding, PC with nodal metastasis, microscopic lymphatic invasion, venous invasion and neural invasion were more frequent in the CD24-positive group than in CD24-negative group [208].
Though the clinical relevance of CD44/CD54 and EpCAM/CD44 to metastasis is lacking so far, they have been associated with identification of CSCs in GC. Chen et al. recently identified CD44+ and CD54+ as CSC markers in GC. In their study, they showed that CD44+C54+ tumor cell subpopulation in the peripheral blood from GC patients possessed CSC properties [209]. In another study by Han et al., EpCAM+/CD44+ cells were isolated as CSCs from GC tissues [210]. Moreover, variant CD44 mRNA in the peripheral blood collect during surgery was associated with distant metastasis in patients with CRC [211]. CD44 expression was also significantly higher in PC with lymph node metastasis or distant metastasis [212].
Aldehyde dehydrongenase 1 (ALDH1) has been proposed as one of the possible candidates for a CSC marker. A recent ESCC study which recruited patients who underwent esophagectomy showed demonstrated that ALDH1 was mainly expressed in ESCC cell nucleus, and its expression correlated with lymph node metastasis [213]. The significance of ALDH1 was confirmed in another ESCC study that ALDH1 expression in primary tumor was associated with postoperative recurrence and prognosis [214], suggesting that patients with ALDH1 high tumors required better postoperative monitoring and maybe a more aggressive postoperative treatment is required. Similarly, ALDH1-positive GC cases also displayed more advanced T stage and TNM stage than ALDH1-negative cases [215]. In contrast, low expression of ALDH1 was associated with poor prognosis of HCC, CRC and PC patients [216–218].
Besides, another recent study which screened the expressions of a series of biomarkers in patients with recurrent HCC suggested that employment of a set of biomarkers will reinforce both the sensitivity and specificity of the predictive model. The study constructed a simplified predictive model with the biomarkers tested including CD133, CD44, nestin and MVD (determined by CD34 immunostaining) that overcame the limitation of narrow subpopulation of a single biomarker [219].
Tumor markers related to tumor microenvironment
Recently, increasing evidence has suggested that the tumor microenvironment plays crucial roles to seeding and metastatic potential of primary tumors. Such tumor stroma is made up of other cell types including fibroblasts, myofibroblasts, granulocytes, macrophages, mesenchymal stem cells and lymphocytes, as well as extracellular matrix, vasculature and lymphatic systems and other non-cell factors (Reviewed in [220]). Some of these cell types additionally change phenotype and become accessories to tumorigenesis and metastasis, and are, therefore, referred to as cancer-associated fibroblasts (CAF) and tumor-associated macrophages (TAM).
Recently, SPARC and OPN, which are involved in integrin signaling pathway, have been implicated in modification of the tumor microenvironment. In CRC patients, the expression of SPARC in mesenchymal and stromal cells showed a statistically significant difference in patients with lymph node metastasis and negatively correlated with VEGF level in CRC tissue [221], indicating that SPARC might inhibit the invasion and metastasis of tumor. This observation was supported by another study that SPARC expression in the microenvironment stromal cells was inversely correlated with lymphovascular invasion of CRC [222]. In pancreatic ductal adenocarcinoma, SPARC is overexpressed by fibroblast in the tumor microenvironment [223]. In another study by Imano and co-authors, they showed that OPN induced by macrophages contributed to metachronous liver metastases in colorectal cancer [224].
CSCs play an important role in regulation of the tumor microenvironment which favours the development of metatasis. For example, the presence of a hospitable environment is crucial for survival and outgrowth of cancer cells arriving a distant organ. A recent publication by Camussi and coworkers connects the formation of pre-metastatic niche with CSCs [225]. In their study, they demonstrated that microvesicles released from CD105+ CSCs in renal carcinomas were able to trigger angiogenesis and significantly enhanced the metastatic ability of cancer cells. CD105 expression in EC and in adjacent non-tumorous esophagus also played a prognostic role of recurrence. CD105 stained microvessels in and around the tumor, but showed weak or almost no staining in normal esophageal-tissue blood vessels, indicating its higher specificity for tumor vasculature than pan-endothelial markers [225]. Bellone et al. [226] find that a diffuse pattern of CD105 staining in the adjacent non-tumours esophagus can predict early recurrence. Angiogenesis, as demonstrated by CD105 staining in the nontumourous esophagus may enhance the growth of intraesophageal metastasis or development of multicentric tumors, and thus contribute to postoperative recurrence. These results suggested the importance of CD105 in the formation of premetastatic niche. Microvessel density (MVD) as determined by CD105 correlated with blood vessel invasion, distant metastasis, and formation of ascites in GC patients [227]. Moreover, CD34 was universally expressed in blood vessels within benign and malignant tissues, whereas CD105 expression was minimal in benign tissues but stronger in gastric carcinoma, suggesting that CD105 is a more specific prognostic marker in the evaluation of prognosis in gastric carcinoma. CD105-MVD was also significantly correlated with microscopic venous invasion in HCC patients [228]. Active angiogenesis as highlighted by diffuse CD105 staining of the microvessels in the adjacent non-tumorous liver tissues is predictive of early recurrence of HCC patients [229]. In colorectal cancer patients, CD105-vessels count in the carcinoma specimens can identify patients at high risk of metastatic disease [230, 231]. CD105 was also a useful predictor for the recurrence of resected GC and may have a specific association with the development of locoregional and hematogenous recurrence [232].
Many cancers will develop as a result of a chronic inflammatory state due to infections, for example, HCC by Hepatitis B and C; CRC by ulcerative colitis; GC by Helicobacter pylori and EC by chronic reflux esophagitis. These chronic inflammatory conditions help to establish a tumor microenvironment for tumor growth and progression. Putative inflammatory biomarkers of the “tumor microenvironment” have gained recognition for elucidating the biology of cancer, and may provide additional diagnostic, monitoring and therapeutic opportunities. In liver cancer, Budhu et al. [233] identified a unique set of immune response signature biomarker signature of early stage HCC and the tumor microenvironment. The pro-inflammatory cytokines including TNF, IFNG and IL1 are significantly down-regulated whereas anti-inflammatory cytokines such as IL4, IL5, IL8 and IL10 are highly elevated in livers with metastatic HCC [233]. These studies implicate a real potential for increasing the accuracy of liver cancer diagnosis and prognosis, as well as in monitoring recurrence.
Conclusion
Numerous regulators involved in the molecular mechanism of metastasis have been identified, which provides us a more thorough understanding of the development of metastatic tumors. These regulators are potential biomarkers for predicting metastasis. In this review, we’ve summarized and discussed some recent findings of potential biomarkers. A number of potential biomarkers have been demonstrated in gastrointestinal cancer patients, which provides additional choices of biomarkers to increase the sensitivity and specificity. Moreover, non-invasive biomarkers provide the possibility to monitor patient disease status after operation, yet their post-operation levels have not been monitored in most studies. It is possible that the post-operation level could predict the presence of metastasis with higher sensitivity and specificity when compared with the pre-operation level.
Biomarkers for predicting metastasis may also serve as therapeutic targets in the inhibition of metastasis development. Though at present there seems no “perfect biomarker” which precisely identifies all patients with metastatic tumor, the current findings are helpful in screening for high risk patients who require more careful monitoring or more aggressive treatment. It is worthwhile to carry on further investigation into the molecular mechanism of metastasis, identification of new regulators, and examination of clinicopathological parameters with combination of biomarkers.
References
- 1.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 2.Yang SY, et al. Growth factors and their receptors in cancer metastases. Front Biosci. 2011;16:531–538. doi: 10.2741/3703. [DOI] [PubMed] [Google Scholar]
- 3.Locker GY, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24(33):5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 4.Ishihara S, et al. Prognostic significance of response to preoperative radiotherapy, lymph node metastasis, and CEA level in patients undergoing total mesorectal excision of rectal cancer. Int J Colorectal Dis. 2010;25(12):1417–1425. doi: 10.1007/s00384-010-1051-1. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher RH. Carcinoembryonic antigen. Ann Intern Med. 1986;104(1):66–73. doi: 10.7326/0003-4819-104-1-66. [DOI] [PubMed] [Google Scholar]
- 6.Begent RH. The value of carcinoembryonic antigen measurement in clinical practice. Ann Clin Biochem. 1984;21(Pt 4):231–238. doi: 10.1177/000456328402100401. [DOI] [PubMed] [Google Scholar]
- 7.Thomas P, et al. The structure, metabolism and function of the carcinoembryonic antigen gene family. Biochim Biophys Acta. 1990;1032(2–3):177–189. doi: 10.1016/0304-419x(90)90003-j. [DOI] [PubMed] [Google Scholar]
- 8.Sorbye H, Dahl O. Carcinoembryonic antigen surge in metastatic colorectal cancer patients responding to oxaliplatin combination chemotherapy: implications for tumor marker monitoring and guidelines. J Clin Oncol. 2003;21(23):4466–4467. doi: 10.1200/JCO.2003.99.200. [DOI] [PubMed] [Google Scholar]
- 9.Sorbye H, Dahl O. Transient CEA increase at start of oxaliplatin combination therapy for metastatic colorectal cancer. Acta Oncol. 2004;43(5):495–498. doi: 10.1080/02841860410032380. [DOI] [PubMed] [Google Scholar]
- 10.Sato H, et al. Significance of serum concentrations of E-selectin and CA19-9 in the prognosis of colorectal cancer. Jpn J Clin Oncol. 2010;40(11):1073–1080. doi: 10.1093/jjco/hyq095. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, et al. The relationships between perioperative CEA, CA 19–9, and CA 72–4 and recurrence in gastric cancer patients after curative radical gastrectomy. J Surg Oncol. 2011;104(6):585–591. doi: 10.1002/jso.21919. [DOI] [PubMed] [Google Scholar]
- 12.Yakabe T, et al. Clinical significance of CEA and CA19-9 in postoperative follow-up of colorectal cancer. Ann Surg Oncol. 2010;17(9):2349–2356. doi: 10.1245/s10434-010-1004-5. [DOI] [PubMed] [Google Scholar]
- 13.Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(Suppl 4):14–22. doi: 10.1634/theoncologist.2010-S4-14. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 15.Di Bisceglie AM. Issues in screening and surveillance for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S104–S107. doi: 10.1053/j.gastro.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Daniele B, et al. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S108–S112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Ucar E, et al. Prognostic value of preoperative CEA, CA 19–9, CA 72–4, and AFP levels in gastric cancer. Adv Ther. 2008;25(10):1075–1084. doi: 10.1007/s12325-008-0100-4. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima K, et al. Impact of preoperative serum carcinoembryonic antigen, CA 19–9 and alpha fetoprotein levels in gastric cancer patients. Tumour Biol. 1998;19(6):464–469. doi: 10.1159/000030038. [DOI] [PubMed] [Google Scholar]
- 19.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3(1):55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 20.Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198(1):11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhang CH, et al. Activation of STAT3 signal pathway correlates with twist and E-cadherin expression in hepatocellular carcinoma and their clinical significance. J Surg Res. 2010;174(1):120–129. doi: 10.1016/j.jss.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Boo YJ, et al. L1 expression as a marker for poor prognosis, tumor progression, and short survival in patients with colorectal cancer. Ann Surg Oncol. 2007;14(5):1703–1711. doi: 10.1245/s10434-006-9281-8. [DOI] [PubMed] [Google Scholar]
- 23.Saad AA, et al. Prognostic significance of E-cadherin expression and peripheral blood micrometastasis in gastric carcinoma patients. Ann Surg Oncol. 2010;17(11):3059–3067. doi: 10.1245/s10434-010-1151-8. [DOI] [PubMed] [Google Scholar]
- 24.Uchikado Y, et al. Increased slug and decreased E-cadherin expression is related to poor prognosis in patients with gastric cancer. Gastric Cancer. 2011;14(1):41–49. doi: 10.1007/s10120-011-0004-x. [DOI] [PubMed] [Google Scholar]
- 25.Castro Alves C, et al. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol. 2007;211(5):507–515. doi: 10.1002/path.2138. [DOI] [PubMed] [Google Scholar]
- 26.Joo YE, et al. Expression of E-cadherin, alpha- and beta-catenins in patients with pancreatic adenocarcinoma. Pancreatology. 2002;2(2):129–137. doi: 10.1159/000055903. [DOI] [PubMed] [Google Scholar]
- 27.Zhao XJ, et al. Expression of e-cadherin and beta-catenin in human esophageal squamous cell carcinoma: relationships with prognosis. World J Gastroenterol. 2003;9(2):225–232. doi: 10.3748/wjg.v9.i2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan AO, et al. Early prediction of tumor recurrence after curative resection of gastric carcinoma by measuring soluble E-cadherin. Cancer. 2005;104(4):740–746. doi: 10.1002/cncr.21260. [DOI] [PubMed] [Google Scholar]
- 29.Chan AO, et al. Soluble E-cadherin is a valid prognostic marker in gastric carcinoma. Gut. 2001;48(6):808–811. doi: 10.1136/gut.48.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soyama A, et al. Significance of the serum level of soluble E-cadherin in patients with HCC. Hepatogastroenterology. 2008;55(85):1390–1393. [PubMed] [Google Scholar]
- 31.Okugawa Y, et al. Clinical significance of serum soluble E-cadherin in colorectal carcinoma. J Surg Res. 2011;175(2):e67–e73. doi: 10.1016/j.jss.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Chung Y, et al. Serum soluble E-cadherin is a potential prognostic marker in esophageal squamous cell carcinoma. Dis Esophagus. 2011;24(1):49–55. doi: 10.1111/j.1442-2050.2010.01093.x. [DOI] [PubMed] [Google Scholar]
- 33.Weiss JV, et al. Soluble E-cadherin as a serum biomarker candidate: elevated levels in patients with late-stage colorectal carcinoma and FAP. Int J Cancer. 2011;128(6):1384–1392. doi: 10.1002/ijc.25438. [DOI] [PubMed] [Google Scholar]
- 34.Pedrazzani C, et al. Influence of age on soluble E-cadherin serum levels prevents its utility as a disease marker in gastric cancer patients. Scand J Gastroenterol. 2008;43(6):765–766. doi: 10.1080/00365520802037370. [DOI] [PubMed] [Google Scholar]
- 35.Batlle E, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2(2):84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 36.Cano A, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 37.Zhang K, et al. Slug enhances invasion ability of pancreatic cancer cells through upregulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling. Lab Invest. 2011;91(3):426–438. doi: 10.1038/labinvest.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 39.Min AL, et al. High expression of snail mRNA in blood from hepatocellular carcinoma patients with extra-hepatic metastasis. Clin Exp Metastasis. 2009;26(7):759–767. doi: 10.1007/s10585-009-9275-6. [DOI] [PubMed] [Google Scholar]
- 40.Otsuki S, et al. Vimentin expression is associated with decreased survival in gastric cancer. Oncol Rep. 2011;25(5):1235–1242. doi: 10.3892/or.2011.1185. [DOI] [PubMed] [Google Scholar]
- 41.Hu L, et al. Association of vimentin overexpression and hepatocellular carcinoma metastasis. Oncogene. 2004;23(1):298–302. doi: 10.1038/sj.onc.1206483. [DOI] [PubMed] [Google Scholar]
- 42.Gal A, et al. Sustained TGF beta exposure suppresses smad and non-smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene. 2008;27(9):1218–1230. doi: 10.1038/sj.onc.1210741. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwatsuki M, et al. The clinical significance of vimentin-expressing gastric cancer cells in bone marrow. Ann Surg Oncol. 2010;17(9):2526–2533. doi: 10.1245/s10434-010-1041-0. [DOI] [PubMed] [Google Scholar]
- 45.Shirahata A, et al. Detection of vimentin (VIM) methylation in the serum of colorectal cancer patients. Anticancer Res. 2010;30(12):5015–5018. [PubMed] [Google Scholar]
- 46.Sugimachi K, et al. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin Cancer Res. 2003;9(7):2657–2664. [PubMed] [Google Scholar]
- 47.Miyoshi A, et al. Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br J Cancer. 2005;92(2):252–258. doi: 10.1038/sj.bjc.6602266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin T, et al. Expression of snail in pancreatic cancer promotes metastasis and chemoresistance. J Surg Res. 2007;141(2):196–203. doi: 10.1016/j.jss.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 49.Natsugoe S, et al. Snail plays a key role in E-cadherin-preserved esophageal squamous cell carcinoma. Oncol Rep. 2007;17(3):517–523. [PubMed] [Google Scholar]
- 50.Shioiri M, et al. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br J Cancer. 2006;94(12):1816–1822. doi: 10.1038/sj.bjc.6603193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uchikado Y, et al. Slug expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11(3):1174–1180. [PubMed] [Google Scholar]
- 52.Yang MH, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50(5):1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- 53.Zhao XL, et al. Promotion of hepatocellular carcinoma metastasis through matrix metalloproteinase activation by epithelial-mesenchymal transition regulator Twist1. J Cell Mol Med. 2011;15(3):691–700. doi: 10.1111/j.1582-4934.2010.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee TK, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12(18):5369–5376. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- 55.Niu RF, et al. Up-regulation of twist induces angiogenesis and correlates with metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26(3):385–394. [PubMed] [Google Scholar]
- 56.Gomez I, et al. TWIST1 is expressed in colorectal carcinomas and predicts patient survival. PLoS ONE. 2011;6(3):e18023. doi: 10.1371/journal.pone.0018023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ru GQ, et al. Upregulation of twist in gastric carcinoma associated with tumor invasion and poor prognosis. Pathol Oncol Res. 2011;17(2):341–347. doi: 10.1007/s12253-010-9332-0. [DOI] [PubMed] [Google Scholar]
- 58.Yan-Qi Z, et al. Expression and significance of TWIST basic helix-loop-helix protein over-expression in gastric cancer. Pathology. 2007;39(5):470–475. doi: 10.1080/00313020701570053. [DOI] [PubMed] [Google Scholar]
- 59.Ohk Sung C, et al. Twist1 is up-regulated in gastric cancer-associated fibroblasts with poor clinical outcomes. Am J Pathol. 2011;179(4):1827–1838. doi: 10.1016/j.ajpath.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gong T, et al. Nuclear expression of twist promotes lymphatic metastasis in esophageal squamous cell carcinoma. Cancer Biol Ther. 2012;13(8):606–613. doi: 10.4161/cbt.19851. [DOI] [PubMed] [Google Scholar]
- 61.Forghanifard MM, et al. Expression analysis elucidates the roles of MAML1 and Twist1 in esophageal squamous cell carcinoma aggressiveness and metastasis. Ann Surg Oncol. 2012;19(3):743–749. doi: 10.1245/s10434-011-2074-8. [DOI] [PubMed] [Google Scholar]
- 62.Xie F, Li K, Ouyang X. Twist, an independent prognostic marker for predicting distant metastasis and survival rates of esophageal squamous cell carcinoma patients. Clin Exp Metastasis. 2009;26(8):1025–1032. doi: 10.1007/s10585-009-9292-5. [DOI] [PubMed] [Google Scholar]
- 63.Yuen HF, et al. Upregulation of twist in oesophageal squamous cell carcinoma is associated with neoplastic transformation and distant metastasis. J Clin Pathol. 2007;60(5):510–514. doi: 10.1136/jcp.2006.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohuchida K, et al. Twist, a novel oncogene, is upregulated in pancreatic cancer: clinical implication of twist expression in pancreatic juice. Int J Cancer. 2007;120(8):1634–1640. doi: 10.1002/ijc.22295. [DOI] [PubMed] [Google Scholar]
- 65.Sun T, et al. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology. 2010;51(2):545–556. doi: 10.1002/hep.23311. [DOI] [PubMed] [Google Scholar]
- 66.Che N, et al. The role of Twist1 in hepatocellular carcinoma angiogenesis: a clinical study. Hum Pathol. 2011;42(6):840–847. doi: 10.1016/j.humpath.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 67.Sun T, et al. Promotion of tumor cell metastasis and vasculogenic mimicry by way of transcription coactivation by Bcl-2 and Twist1: a study of hepatocellular carcinoma. Hepatology. 2011;54(5):1690–1706. doi: 10.1002/hep.24543. [DOI] [PubMed] [Google Scholar]
- 68.Zhou YM, et al. Clinicopathological significance of ZEB1 protein in patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;19(5):1700–1706. doi: 10.1245/s10434-011-1772-6. [DOI] [PubMed] [Google Scholar]
- 69.Zhu W, et al. Overexpression of EIF5A2 promotes colorectal carcinoma cell aggressiveness by upregulating MTA1 through C-myc to induce epithelial-mesenchymaltransition. Gut. 2011;61(4):562–575. doi: 10.1136/gutjnl-2011-300207. [DOI] [PubMed] [Google Scholar]
- 70.Tang DJ, et al. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology. 2010;51(4):1255–1263. doi: 10.1002/hep.23451. [DOI] [PubMed] [Google Scholar]
- 71.Lee NP, et al. Prognostic significance and therapeutic potential of eukaryotic translation initiation factor 5A (eIF5A) in hepatocellular carcinoma. Int J Cancer. 2010;127(4):968–976. doi: 10.1002/ijc.25100. [DOI] [PubMed] [Google Scholar]
- 72.Meng HM, et al. Overexpression of nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol Ther. 2010;9(4):295–302. doi: 10.4161/cbt.9.4.10666. [DOI] [PubMed] [Google Scholar]
- 73.Fu J, et al. p28GANK overexpression accelerates hepatocellular carcinoma invasiveness and metastasis via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1alpha pathways. Hepatology. 2011;53(1):181–192. doi: 10.1002/hep.24015. [DOI] [PubMed] [Google Scholar]
- 74.Zheng P, et al. Snail as a key regulator of PRL-3 gene in colorectal cancer. Cancer Biol Ther. 2011;12(8):742–749. doi: 10.4161/cbt.12.8.15981. [DOI] [PubMed] [Google Scholar]
- 75.Kim NW, et al. Correlation between liver metastases and the level of PRL-3 mRNA expression in patients with primary colorectal cancer. J Korean Soc Coloproctol. 2011;27(5):231–236. doi: 10.3393/jksc.2011.27.5.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bardelli A, et al. PRL-3 expression in metastatic cancers. Clin Cancer Res. 2003;9(15):5607–5615. [PubMed] [Google Scholar]
- 77.Kato H, et al. High expression of PRL-3 promotes cancer cell motility and liver metastasis in human colorectal cancer: a predictive molecular marker of metachronous liver and lung metastases. Clin Cancer Res. 2004;10(21):7318–7328. doi: 10.1158/1078-0432.CCR-04-0485. [DOI] [PubMed] [Google Scholar]
- 78.Mollevi DG, et al. PRL-3 is essentially overexpressed in primary colorectal tumours and associates with tumour aggressiveness. Br J Cancer. 2008;99(10):1718–1725. doi: 10.1038/sj.bjc.6604747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng L, et al. The association of the expression level of protein tyrosine phosphatase PRL-3 protein with liver metastasis and prognosis of patients with colorectal cancer. J Cancer Res Clin Oncol. 2004;130(9):521–526. doi: 10.1007/s00432-004-0563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guzinska-Ustymowicz K, et al. Immunohistochemical assessment of PRL-3 (PTP4A3) expression in tumor buds, invasion front, central region of tumor and metastases of colorectal cancer. Adv Med Sci. 2011;56(1):39–43. doi: 10.2478/v10039-011-0015-1. [DOI] [PubMed] [Google Scholar]
- 81.Miskad UA, et al. Expression of PRL-3 phosphatase in human gastric carcinomas: close correlation with invasion and metastasis. Pathobiology. 2004;71(4):176–184. doi: 10.1159/000078671. [DOI] [PubMed] [Google Scholar]
- 82.Ooki A, et al. Phosphatase of regenerating liver-3 as a prognostic biomarker in histologically node-negative gastric cancer. Oncol Rep. 2009;21(6):1467–1475. doi: 10.3892/or_00000376. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z, et al. Expression and prognostic impact of PRL-3 in lymph node metastasis of gastric cancer: its molecular mechanism was investigated using artificial microRNA interference. Int J Cancer. 2008;123(6):1439–1447. doi: 10.1002/ijc.23643. [DOI] [PubMed] [Google Scholar]
- 84.Miskad UA, et al. High PRL-3 expression in human gastric cancer is a marker of metastasis and grades of malignancies: an in situ hybridization study. Virchows Arch. 2007;450(3):303–310. doi: 10.1007/s00428-006-0361-8. [DOI] [PubMed] [Google Scholar]
- 85.Pryczynicz A, et al. PTP4A3 (PRL-3) expression correlate with lymphatic metastases in gastric cancer. Folia Histochem Cytobiol. 2010;48(4):632–636. doi: 10.2478/v10042-010-0070-7. [DOI] [PubMed] [Google Scholar]
- 86.Zhao WB, et al. Evaluation of PRL-3 expression, and its correlation with angiogenesis and invasion in hepatocellular carcinoma. Int J Mol Med. 2008;22(2):187–192. [PubMed] [Google Scholar]
- 87.Mayinuer A, et al. Upregulation of protein tyrosine phosphatase type IVA member 3 (PTP4A3/PRL-3) is associated with tumor differentiation and a poor prognosis in human hepatocellular carcinoma. Ann Surg Oncol. 2012;20(1):305–317. doi: 10.1245/s10434-012-2395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu YQ, et al. Expression of phosphatase of regenerating liver 1 and 3 mRNA in esophageal squamous cell carcinoma. Arch Pathol Lab Med. 2008;132(8):1307–1312. doi: 10.5858/2008-132-1307-EOPORL. [DOI] [PubMed] [Google Scholar]
- 89.Ooki A, et al. Phosphatase of regenerating liver-3 as a convergent therapeutic target for lymph node metastasis in esophageal squamous cell carcinoma. Int J Cancer. 2010;127(3):543–554. doi: 10.1002/ijc.25082. [DOI] [PubMed] [Google Scholar]
- 90.Zheng P, et al. Stathmin, a new target of PRL-3 identified by proteomic methods, plays a key role in progression and metastasis of colorectal cancer. J Proteome Res. 2010;9(10):4897–4905. doi: 10.1021/pr100712t. [DOI] [PubMed] [Google Scholar]
- 91.Hsieh SY, et al. Stathmin1 overexpression associated with polyploidy, tumor-cell invasion, early recurrence, and poor prognosis in human hepatoma. Mol Carcinog. 2010;49(5):476–487. doi: 10.1002/mc.20627. [DOI] [PubMed] [Google Scholar]
- 92.Wang H, et al. PCBP1 suppresses the translation of metastasis-associated PRL-3 phosphatase. Cancer Cell. 2010;18(1):52–62. doi: 10.1016/j.ccr.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 93.Zhang T, et al. PCBP-1 regulates alternative splicing of the CD44 gene and inhibits invasion in human hepatoma cell line HepG2 cells. Mol Cancer. 2010;9:72. doi: 10.1186/1476-4598-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wong SC, et al. Quantification of plasma beta-catenin mRNA in colorectal cancer and adenoma patients. Clin Cancer Res. 2004;10(5):1613–1617. doi: 10.1158/1078-0432.CCR-1168-3. [DOI] [PubMed] [Google Scholar]
- 95.Zekri AR, et al. Serum levels of beta-catenin as a potential marker for genotype 4/hepatitis C-associated hepatocellular carcinoma. Oncol Rep. 2011;26(4):825–831. doi: 10.3892/or.2011.1355. [DOI] [PubMed] [Google Scholar]
- 96.Gavert N, et al. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol. 2005;168(4):633–642. doi: 10.1083/jcb.200408051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zander H, et al. Circulating levels of cell adhesion molecule L1 as a prognostic marker in gastrointestinal stromal tumor patients. BMC Cancer. 2011;11(189):1–7. doi: 10.1186/1471-2407-11-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brabletz T, Jung A, Kirchner T. Beta-catenin and the morphogenesis of colorectal cancer. Virchows Arch. 2002;441(1):1–11. doi: 10.1007/s00428-002-0642-9. [DOI] [PubMed] [Google Scholar]
- 99.Liu L, et al. Activation of beta-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin Cancer Res. 2010;16(10):2740–2750. doi: 10.1158/1078-0432.CCR-09-2610. [DOI] [PubMed] [Google Scholar]
- 100.Matsukuma S, Sato K. Peritoneal seeding of hepatocellular carcinoma: clinicopathological characteristics of 17 autopsy cases. Pathol Int. 2011;61(6):356–362. doi: 10.1111/j.1440-1827.2011.02669.x. [DOI] [PubMed] [Google Scholar]
- 101.Cheng XX, et al. Correlation of Wnt-2 expression and beta-catenin intracellular accumulation in Chinese gastric cancers: relevance with tumour dissemination. Cancer Lett. 2005;223(2):339–347. doi: 10.1016/j.canlet.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 102.Choi MG, et al. Mucinous gastric cancer presents with more advanced tumor stage and weaker beta-catenin expression than nonmucinous cancer. Ann Surg Oncol. 2010;17(11):3053–3058. doi: 10.1245/s10434-010-1184-z. [DOI] [PubMed] [Google Scholar]
- 103.Takayama T, et al. Aberrant expression and phosphorylation of beta-catenin in human colorectal cancer. Br J Cancer. 1998;77(4):605–613. doi: 10.1038/bjc.1998.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miyamoto S, et al. Nuclear beta-catenin accumulation as a prognostic factor in Dukes’ D human colorectal cancers. Oncol Rep. 2004;12(2):245–251. [PubMed] [Google Scholar]
- 105.Chen WC, et al. Survey of molecular profiling during human colon cancer development and progression by immunohistochemical staining on tissue microarray. World J Gastroenterol. 2007;13(5):699–708. doi: 10.3748/wjg.v13.i5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang B, et al. Beta-catenin and ras oncogenes detect most human colorectal cancer. Clin Cancer Res. 2003;9(8):3073–3079. [PubMed] [Google Scholar]
- 107.Hervieu V, et al. Expression of beta-catenin in gastroenteropancreatic endocrine tumours: a study of 229 cases. J Clin Pathol. 2006;59(12):1300–1304. doi: 10.1136/jcp.2005.035097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang W, Xue L, Wang P. Prognostic value of beta-catenin, c-myc, and cyclin D1 expressions in patients with esophageal squamous cell carcinoma. Med Oncol. 2011;28(1):163–169. doi: 10.1007/s12032-010-9436-0. [DOI] [PubMed] [Google Scholar]
- 109.Ben QW, et al. Positive expression of L1-CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2010;17(8):2213–2221. doi: 10.1245/s10434-010-0955-x. [DOI] [PubMed] [Google Scholar]
- 110.Tsutsumi S, et al. L1 Cell adhesion molecule (L1CAM) expression at the cancer invasive front is a novel prognostic marker of pancreatic ductal adenocarcinoma. J Surg Oncol. 2011;103(7):669–673. doi: 10.1002/jso.21880. [DOI] [PubMed] [Google Scholar]
- 111.Kim MY, Han SI, Lim SC. Roles of cyclin-dependent kinase 8 and beta-catenin in the oncogenesis and progression of gastric adenocarcinoma. Int J Oncol. 2011;38(5):1375–1383. doi: 10.3892/ijo.2011.948. [DOI] [PubMed] [Google Scholar]
- 112.Yang P, et al. Enhanced activity of very low density lipoprotein receptor II promotes SGC7901 cell proliferation and migration. Life Sci. 2009;84(13–14):402–408. doi: 10.1016/j.lfs.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 113.He L, et al. Up-regulated expression of type II very low density lipoprotein receptor correlates with cancer metastasis and has a potential link to beta-catenin in different cancers. BMC Cancer. 2010;10:601. doi: 10.1186/1471-2407-10-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim B, et al. TC1(C8orf4) correlates with Wnt/beta-catenin target genes and aggressive biological behavior in gastric cancer. Clin Cancer Res. 2006;12(11 Pt 1):3541–3548. doi: 10.1158/1078-0432.CCR-05-2440. [DOI] [PubMed] [Google Scholar]
- 115.Boissan M, et al. Implication of metastasis suppressor NM23-H1 in maintaining adherens junctions and limiting the invasive potential of human cancer cells. Cancer Res. 2010;70(19):7710–7722. doi: 10.1158/0008-5472.CAN-10-1887. [DOI] [PubMed] [Google Scholar]
- 116.Zhang S, et al. EPLIN downregulation promotes epithelial-mesenchymal transition in prostate cancer cells and correlates with clinical lymph node metastasis. Oncogene. 2011;30(50):4941–4952. doi: 10.1038/onc.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yamaguchi A, et al. Expression of human nm23-H1 and nm23-H2 proteins in hepatocellular carcinoma. Cancer. 1994;73(9):2280–2284. doi: 10.1002/1097-0142(19940501)73:9<2280::AID-CNCR2820730908>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 118.Kodera Y, et al. Expression of nm23 H-1 RNA levels in human gastric cancer tissues. A negative correlation with nodal metastasis. Cancer. 1994;73(2):259–265. doi: 10.1002/1097-0142(19940115)73:2<259::AID-CNCR2820730205>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 119.Liu WK, et al. The relationship between cyclooxygenase-2, CD44v6, and nm23H1 in esophageal squamous cell carcinoma. Onkologie. 2009;32(10):574–578. doi: 10.1159/000232346. [DOI] [PubMed] [Google Scholar]
- 120.Liu WK, et al. The relationship between HPV16 and expression of CD44v6, nm23H1 in esophageal squamous cell carcinoma. Arch Virol. 2005;150(5):991–1001. doi: 10.1007/s00705-004-0454-0. [DOI] [PubMed] [Google Scholar]
- 121.Elagoz S, et al. The intratumoral microvessel density and expression of bFGF and nm23-H1 in colorectal cancer. Pathol Oncol Res. 2006;12(1):21–27. doi: 10.1007/BF02893427. [DOI] [PubMed] [Google Scholar]
- 122.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20(3):203–213. doi: 10.1023/A:1022983000355. [DOI] [PubMed] [Google Scholar]
- 123.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ke JJ, Shao QS, Ling ZQ. Expression of E-selectin, integrin beta1 and immunoglobulin superfamily member in human gastric carcinoma cells and its clinicopathologic significance. World J Gastroenterol. 2006;12(22):3609–3611. doi: 10.3748/wjg.v12.i22.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao ZS, et al. Expression and prognostic significance of CEACAM6, ITGB1, and CYR61 in peripheral blood of patients with gastric cancer. J Surg Oncol. 2011;104(5):525–529. doi: 10.1002/jso.21984. [DOI] [PubMed] [Google Scholar]
- 126.Kuo ML, et al. Cyr61 induces gastric cancer cell motility/invasion via activation of the integrin/nuclear factor-kappa B/cyclooxygenase-2 signaling pathway. Clin Cancer Res. 2005;11(16):5809–5820. doi: 10.1158/1078-0432.CCR-04-2639. [DOI] [PubMed] [Google Scholar]
- 127.Li H, et al. HIF-1alpha-activated ANGPTL4 contributes to tumor metastasis via VCAM-1/integrin beta1 signaling in human hepatocellular carcinoma. Hepatology. 2011;54(3):910–919. doi: 10.1002/hep.24479. [DOI] [PubMed] [Google Scholar]
- 128.Zhou G, et al. Detection and clinical significance of CD44v6 and integrin-beta1 in pancreatic cancer patients using a triplex real-time RT-PCR assay. Appl Biochem Biotechnol. 2012;167(8):2257–2268. doi: 10.1007/s12010-012-9752-2. [DOI] [PubMed] [Google Scholar]
- 129.Zhou G, et al. The efficacy evaluation of cryosurgery in pancreatic cancer patients with the expression of CD44v6, integrin-beta1, CA199, and CEA. Mol Biotechnol. 2012;52(1):59–67. doi: 10.1007/s12033-011-9474-7. [DOI] [PubMed] [Google Scholar]
- 130.Lu JG, et al. Overexpression of osteopontin and integrin alphav in laryngeal and hypopharyngeal carcinomas associated with differentiation and metastasis. J Cancer Res Clin Oncol. 2011;137(11):1613–1618. doi: 10.1007/s00432-011-1024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu CY, et al. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56(6):782–789. doi: 10.1136/gut.2006.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun J, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with TNM stage-I of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136(1):1–7. doi: 10.1007/s00432-009-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang H, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132(11):709–717. doi: 10.1007/s00432-006-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shimada Y, et al. Clinical significance of osteopontin in esophageal squamous cell carcinoma: comparison with common tumor markers. Oncology. 2005;68(2–3):285–292. doi: 10.1159/000086961. [DOI] [PubMed] [Google Scholar]
- 135.Wild N, et al. A combination of serum markers for the early detection of colorectal cancer. Clin Cancer Res. 2010;16(24):6111–6121. doi: 10.1158/1078-0432.CCR-10-0119. [DOI] [PubMed] [Google Scholar]
- 136.Fransvea E, et al. Targeting transforming growth factor (TGF)-betaRI inhibits activation of beta1 integrin and blocks vascular invasion in hepatocellular carcinoma. Hepatology. 2009;49(3):839–850. doi: 10.1002/hep.22731. [DOI] [PubMed] [Google Scholar]
- 137.Zhao ZS, et al. SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2010;16(1):260–268. doi: 10.1158/1078-0432.CCR-09-1247. [DOI] [PubMed] [Google Scholar]
- 138.Ura H, et al. Separate functions of alpha2beta1 and alpha3beta1 integrins in the metastatic process of human gastric carcinoma. Surg Today. 1998;28(10):1001–1006. doi: 10.1007/BF02483952. [DOI] [PubMed] [Google Scholar]
- 139.Giannelli G, et al. Transforming growth factor-beta1 triggers hepatocellular carcinoma invasiveness via alpha3beta1 integrin. Am J Pathol. 2002;161(1):183–193. doi: 10.1016/S0002-9440(10)64170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hosotani R, et al. Expression of integrin alphaVbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25(2):e30–e35. doi: 10.1097/00006676-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 141.Likui W, Hong W, Shuwen Z. Clinical significance of the upregulated osteopontin mRNA expression in human colorectal cancer. J Gastrointest Surg. 2010;14(1):74–81. doi: 10.1007/s11605-009-1035-z. [DOI] [PubMed] [Google Scholar]
- 142.Pan HW, et al. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003;98(1):119–127. doi: 10.1002/cncr.11487. [DOI] [PubMed] [Google Scholar]
- 143.Ye QH, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9(4):416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 144.Higashiyama M, et al. Prognostic significance of osteopontin expression in human gastric carcinoma. Ann Surg Oncol. 2007;14(12):3419–3427. doi: 10.1245/s10434-007-9564-8. [DOI] [PubMed] [Google Scholar]
- 145.Takafuji V, et al. An osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene. 2007;26(44):6361–6371. doi: 10.1038/sj.onc.1210463. [DOI] [PubMed] [Google Scholar]
- 146.Collins AL, et al. Osteopontin expression is associated with improved survival in patients with pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19(8):2673–2678. doi: 10.1245/s10434-012-2337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang X, et al. Prognostic significance of altered expression of SDC2 and CYR61 in esophageal squamous cell carcinoma. Oncol Rep. 2009;21(4):1123–1129. doi: 10.3892/or_00000332. [DOI] [PubMed] [Google Scholar]
- 148.Zeng ZJ, et al. Expressions of cysteine-rich61, connective tissue growth factor and Nov genes in hepatocellular carcinoma and their clinical significance. World J Gastroenterol. 2004;10(23):3414–3418. doi: 10.3748/wjg.v10.i23.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Weaver MS, Workman G, Sage EH. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase. J Biol Chem. 2008;283(33):22826–22837. doi: 10.1074/jbc.M706563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang CS, et al. Overexpression of SPARC gene in human gastric carcinoma and its clinic-pathologic significance. Br J Cancer. 2004;91(11):1924–1930. doi: 10.1038/sj.bjc.6602213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Che Y, et al. The differential expression of SPARC in esophageal squamous cell carcinoma. Int J Mol Med. 2006;17(6):1027–1033. [PubMed] [Google Scholar]
- 152.Nakayama T, et al. Expression of angiopoietin-like 4 (ANGPTL4) in human colorectal cancer: ANGPTL4 promotes venous invasion and distant metastasis. Oncol Rep. 2011;25(4):929–935. doi: 10.3892/or.2011.1176. [DOI] [PubMed] [Google Scholar]
- 153.Makrilia N, et al. Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest. 2009;27(10):1023–1037. doi: 10.3109/07357900902769749. [DOI] [PubMed] [Google Scholar]
- 154.Hannigan GE, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379(6560):91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 155.Bravou V, et al. ILK over-expression in human colon cancer progression correlates with activation of beta-catenin, down-regulation of E-cadherin and activation of the Akt-FKHR pathway. J Pathol. 2006;208(1):91–99. doi: 10.1002/path.1860. [DOI] [PubMed] [Google Scholar]
- 156.Ito R, et al. Expression of integrin-linked kinase is closely correlated with invasion and metastasis of gastric carcinoma. Virchows Arch. 2003;442(2):118–123. doi: 10.1007/s00428-002-0718-6. [DOI] [PubMed] [Google Scholar]
- 157.Chan J, et al. Integrin-linked kinase overexpression and its oncogenic role in promoting tumorigenicity of hepatocellular carcinoma. PLoS ONE. 2011;6(2):e16984. doi: 10.1371/journal.pone.0016984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang WS, et al. Overexpression of the thymosin beta-4 gene is associated with increased invasion of SW480 colon carcinoma cells and the distant metastasis of human colorectal carcinoma. Oncogene. 2004;23(39):6666–6671. doi: 10.1038/sj.onc.1207888. [DOI] [PubMed] [Google Scholar]
- 159.Huang HC, et al. Thymosin beta4 triggers an epithelial-mesenchymal transition in colorectal carcinoma by upregulating integrin-linked kinase. Oncogene. 2007;26(19):2781–2790. doi: 10.1038/sj.onc.1210078. [DOI] [PubMed] [Google Scholar]
- 160.Tang MC, et al. Thymosin beta 4 induces colon cancer cell migration and clinical metastasis via enhancing ILK/IQGAP1/Rac1 signal transduction pathway. Cancer Lett. 2011;308(2):162–171. doi: 10.1016/j.canlet.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 161.Albasri A, et al. Cten signals through integrin-linked kinase (ILK) and may promote metastasis in colorectal cancer. Oncogene. 2011;30(26):2997–3002. doi: 10.1038/onc.2011.26. [DOI] [PubMed] [Google Scholar]
- 162.Schwock J, Dhani N, Hedley DW. Targeting focal adhesion kinase signaling in tumor growth and metastasis. Expert Opin Ther Targets. 2010;14(1):77–94. doi: 10.1517/14728220903460340. [DOI] [PubMed] [Google Scholar]
- 163.Chen JS, et al. FAK is involved in invasion and metastasis of hepatocellular carcinoma. Clin Exp Metastasis. 2010;27(2):71–82. doi: 10.1007/s10585-010-9306-3. [DOI] [PubMed] [Google Scholar]
- 164.Park JH, et al. Focal adhesion kinase (FAK) gene amplification and its clinical implications in gastric cancer. Hum Pathol. 2010;41(12):1664–1673. doi: 10.1016/j.humpath.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 165.Su JM, et al. Expression of focal adhesion kinase and alpha5 and beta1 integrins in carcinomas and its clinical significance. World J Gastroenterol. 2002;8(4):613–618. doi: 10.3748/wjg.v8.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Miyazaki T, et al. FAK overexpression is correlated with tumour invasiveness and lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer. 2003;89(1):140–145. doi: 10.1038/sj.bjc.6601050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chatzizacharias NA, et al. Evaluation of the clinical significance of focal adhesion kinase and SRC expression in human pancreatic ductal adenocarcinoma. Pancreas. 2010;39(6):930–936. doi: 10.1097/MPA.0b013e3181d7abcc. [DOI] [PubMed] [Google Scholar]
- 168.Furuyama K, et al. Clinical significance of focal adhesion kinase in resectable pancreatic cancer. World J Surg. 2006;30(2):219–226. doi: 10.1007/s00268-005-0165-z. [DOI] [PubMed] [Google Scholar]
- 169.Liu AW, et al. ShRNA-targeted MAP4K4 inhibits hepatocellular carcinoma growth. Clin Cancer Res. 2011;17(4):710–720. doi: 10.1158/1078-0432.CCR-10-0331. [DOI] [PubMed] [Google Scholar]
- 170.Hao JM, et al. A five-gene signature as a potential predictor of metastasis and survival in colorectal cancer. J Pathol. 2010;220(4):475–489. doi: 10.1002/path.2668. [DOI] [PubMed] [Google Scholar]
- 171.Liang JJ, et al. Expression of MAP4K4 is associated with worse prognosis in patients with stage II pancreatic ductal adenocarcinoma. Clin Cancer Res. 2008;14(21):7043–7049. doi: 10.1158/1078-0432.CCR-08-0381. [DOI] [PubMed] [Google Scholar]
- 172.Itoh S, et al. Role of growth factor receptor bound protein 7 in hepatocellular carcinoma. Mol Cancer Res. 2007;5(7):667–673. doi: 10.1158/1541-7786.MCR-06-0282. [DOI] [PubMed] [Google Scholar]
- 173.Tanaka S, et al. Specific peptide ligand for Grb7 signal transduction protein and pancreatic cancer metastasis. J Natl Cancer Inst. 2006;98(7):491–498. doi: 10.1093/jnci/djj105. [DOI] [PubMed] [Google Scholar]
- 174.Peng L, et al. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30(10):1660–1669. doi: 10.1093/carcin/bgp178. [DOI] [PubMed] [Google Scholar]
- 175.Wu F, et al. Novel role for epidermal growth factor-like domain 7 in metastasis of human hepatocellular carcinoma. Hepatology. 2009;50(6):1839–1850. doi: 10.1002/hep.23197. [DOI] [PubMed] [Google Scholar]
- 176.Wu F, et al. Down-regulation of EGFL8: a novel prognostic biomarker for patients with colorectal cancer. Anticancer Res. 2011;31(6):2249–2254. [PubMed] [Google Scholar]
- 177.Parker LH, et al. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428(6984):754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- 178.Fitch MJ, et al. Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn. 2004;230(2):316–324. doi: 10.1002/dvdy.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huang J, et al. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120(1):223–241. doi: 10.1172/JCI38012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen M, et al. Genetic variations of the PI3K-AKT-mTOR pathway and clinical outcome in muscle invasive and metastatic bladder cancer patients. Carcinogenesis. 2010;31(8):1387–1391. doi: 10.1093/carcin/bgq110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784(1):150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 182.Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bi J, et al. Overexpression of YKL-40 is an independent prognostic marker in gastric cancer. Hum Pathol. 2009;40(12):1790–1797. doi: 10.1016/j.humpath.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 184.Zhu CB, et al. Elevated serum YKL-40 level predicts poor prognosis in hepatocellular carcinoma after surgery. Ann Surg Oncol. 2012;19(3):817–825. doi: 10.1245/s10434-011-2026-3. [DOI] [PubMed] [Google Scholar]
- 185.Zhang XW, et al. BMI1 and Mel-18 oppositely regulate carcinogenesis and progression of gastric cancer. Mol Cancer. 2010;9:40. doi: 10.1186/1476-4598-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yoshioka A, et al. The activation of Akt during preoperative chemotherapy for esophageal cancer correlates with poor prognosis. Oncol Rep. 2008;19(5):1099–1107. [PubMed] [Google Scholar]
- 187.Yu G, et al. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of Chinese patients with gastric cancer. Clin Cancer Res. 2009;15(5):1821–1829. doi: 10.1158/1078-0432.CCR-08-2138. [DOI] [PubMed] [Google Scholar]
- 188.Kasajima A, et al. mTOR expression and activity patterns in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2011;18(1):181–192. doi: 10.1677/ERC-10-0126. [DOI] [PubMed] [Google Scholar]
- 189.An JY, et al. Prognostic role of p-mTOR expression in cancer tissues and metastatic lymph nodes in pT2b gastric cancer. Int J Cancer. 2010;126(12):2904–2913. doi: 10.1002/ijc.24872. [DOI] [PubMed] [Google Scholar]
- 190.Zhang YJ, et al. mTOR signaling pathway is a target for the treatment of colorectal cancer. Ann Surg Oncol. 2009;16(9):2617–2628. doi: 10.1245/s10434-009-0555-9. [DOI] [PubMed] [Google Scholar]
- 191.Liao WT, et al. HOXB7 as a prognostic factor and mediator of colorectal cancer progression. Clin Cancer Res. 2011;17(11):3569–3578. doi: 10.1158/1078-0432.CCR-10-2533. [DOI] [PubMed] [Google Scholar]
- 192.Nguyen Kovochich A, et al. HOXB7 promotes invasion and predicts survival in pancreatic adenocarcinoma. Cancer. 2012;119(3):529–539. doi: 10.1002/cncr.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Song W, et al. Bmi-1 is related to proliferation, survival and poor prognosis in pancreatic cancer. Cancer Sci. 2010;101(7):1754–1760. doi: 10.1111/j.1349-7006.2010.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu WL, et al. Prognostic relevance of Bmi-1 expression and autoantibodies in esophageal squamous cell carcinoma. BMC Cancer. 2010;10:467. doi: 10.1186/1471-2407-10-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang H, et al. Increased polycomb-group oncogene Bmi-1 expression correlates with poor prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134(5):535–541. doi: 10.1007/s00432-007-0316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sasaki M, et al. The overexpression of polycomb group proteins Bmi1 and EZH2 is associated with the progression and aggressive biological behavior of hepatocellular carcinoma. Lab Invest. 2008;88(8):873–882. doi: 10.1038/labinvest.2008.52. [DOI] [PubMed] [Google Scholar]
- 197.Pang R, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6(6):603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 198.Iinuma H, et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol. 2011;29(12):1547–1555. doi: 10.1200/JCO.2010.30.5151. [DOI] [PubMed] [Google Scholar]
- 199.Lin EH, et al. Elevated circulating endothelial progenitor marker CD133 messenger RNA levels predict colon cancer recurrence. Cancer. 2007;110(3):534–542. doi: 10.1002/cncr.22774. [DOI] [PubMed] [Google Scholar]
- 200.Mehra N, et al. Progenitor marker CD133 mRNA is elevated in peripheral blood of cancer patients with bone metastases. Clin Cancer Res. 2006;12(16):4859–4866. doi: 10.1158/1078-0432.CCR-06-0422. [DOI] [PubMed] [Google Scholar]
- 201.Yu JW, et al. Expressions and clinical significances of CD133 protein and CD133 mRNA in primary lesion of gastric adenocacinoma. J Exp Clin Cancer Res. 2010;29:141. doi: 10.1186/1756-9966-29-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hou Y, et al. The critical role of CD133(+)CD44(+/high) tumor cells in hematogenous metastasis of liver cancers. Cell Res. 2011;22(1):259–272. doi: 10.1038/cr.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kure S, et al. Expression of cancer stem cell markers in pancreatic intraepithelial neoplasias and pancreatic ductal adenocarcinomas. Int J Oncol. 2012;41(4):1314–1324. doi: 10.3892/ijo.2012.1565. [DOI] [PubMed] [Google Scholar]
- 204.Maeda S, et al. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer. 2008;98(8):1389–1397. doi: 10.1038/sj.bjc.6604307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang XR, et al. CD24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clin Cancer Res. 2009;15(17):5518–5527. doi: 10.1158/1078-0432.CCR-09-0151. [DOI] [PubMed] [Google Scholar]
- 206.Darwish NS, et al. Prognostic significance of CD24 expression in gastric carcinoma. Cancer Res Treat. 2004;36(5):298–302. doi: 10.4143/crt.2004.36.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lee TK, et al. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9(1):50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 208.Ikenaga N, et al. Characterization of CD24 expression in intraductal papillary mucinous neoplasms and ductal carcinoma of the pancreas. Hum Pathol. 2010;41(10):1466–1474. doi: 10.1016/j.humpath.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 209.Chen T, et al. Identification and expansion of cancer stem cells in tumor tissues and peripheral blood derived from gastric adenocarcinoma patients. Cell Res. 2011;22(1):248–258. doi: 10.1038/cr.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Han ME, et al. Cancer spheres from gastric cancer patients provide an ideal model system for cancer stem cell research. Cell Mol Life Sci. 2011;68(21):3589–3605. doi: 10.1007/s00018-011-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yokoyama S, Yamaue H. Prediction of distant metastasis by using reverse transcriptase-polymerase chain reaction for epithelial and variant CD44 mRNA in the peripheral blood of patients with colorectal cancer. Arch Surg. 2002;137(9):1069–1073. doi: 10.1001/archsurg.137.9.1069. [DOI] [PubMed] [Google Scholar]
- 212.Bunger S, et al. Pancreatic carcinoma cell lines reflect frequency and variability of cancer stem cell markers in clinical tissue. Eur Surg Res. 2012;49(2):88–98. doi: 10.1159/000341669. [DOI] [PubMed] [Google Scholar]
- 213.Wang Y, et al. Cancer stem cell marker ALDH1 expression is associated with lymph node metastasis and poor survival in esophageal squamous cell carcinoma: a study from high incidence area of northern China. Dis Esophagus. 2012;25(6):560–565. doi: 10.1111/j.1442-2050.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- 214.Minato T, et al. Aldehyde dehydrogenase 1 expression is associated with poor prognosis in patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2012;20(1):209–217. doi: 10.1245/s10434-012-2535-8. [DOI] [PubMed] [Google Scholar]
- 215.Wakamatsu Y, et al. Expression of cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of gastric cancer. Pathol Int. 2012;62(2):112–119. doi: 10.1111/j.1440-1827.2011.02760.x. [DOI] [PubMed] [Google Scholar]
- 216.Chen XQ, He JR, Wang HY. Decreased expression of ALDH1L1 is associated with a poor prognosis in hepatocellular carcinoma. Med Oncol. 2012;29(3):1843–1849. doi: 10.1007/s12032-011-0075-x. [DOI] [PubMed] [Google Scholar]
- 217.Hessman CJ, et al. Loss of expression of the cancer stem cell marker aldehyde dehydrogenase 1 correlates with advanced-stage colorectal cancer. Am J Surg. 2012;203(5):649–653. doi: 10.1016/j.amjsurg.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kahlert C, et al. Low expression of aldehyde dehydrogenase 1A1 (ALDH1A1) is a prognostic marker for poor survival in pancreatic cancer. BMC Cancer. 2011;11:275. doi: 10.1186/1471-2407-11-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yang XR, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59(7):953–962. doi: 10.1136/gut.2008.176271. [DOI] [PubMed] [Google Scholar]
- 220.Lorusso G, Ruegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol. 2008;130(6):1091–1103. doi: 10.1007/s00418-008-0530-8. [DOI] [PubMed] [Google Scholar]
- 221.Liang JF, et al. Relationship and prognostic significance of SPARC and VEGF protein expression in colon cancer. J Exp Clin Cancer Res. 2010;29:71. doi: 10.1186/1756-9966-29-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yoshimura T, et al. Lymphovascular invasion of colorectal cancer is correlated to SPARC expression in the tumor stromal microenvironment. Epigenetics. 2011;6(8):1001–1011. doi: 10.4161/epi.6.8.16063. [DOI] [PubMed] [Google Scholar]
- 223.Infante JR, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25(3):319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 224.Imano M, et al. Osteopontin induced by macrophages contribute to metachronous liver metastases in colorectal cancer. Am Surg. 2011;77(11):1515–1520. [PubMed] [Google Scholar]
- 225.Wang JM, et al. A monoclonal antibody detects heterogeneity in vascular endothelium of tumours and normal tissues. Int J Cancer. 1993;54(3):363–370. doi: 10.1002/ijc.2910540303. [DOI] [PubMed] [Google Scholar]
- 226.Bellone G, et al. Transforming growth factor-beta binding receptor endoglin (CD105) expression in esophageal cancer and in adjacent nontumorous esophagus as prognostic predictor of recurrence. Ann Surg Oncol. 2007;14(11):3232–3242. doi: 10.1245/s10434-007-9528-z. [DOI] [PubMed] [Google Scholar]
- 227.Ding S, et al. Comparative evaluation of microvessel density determined by CD34 or CD105 in benign and malignant gastric lesions. Hum Pathol. 2006;37(7):861–866. doi: 10.1016/j.humpath.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 228.Yang LY, et al. Correlation between CD105 expression and postoperative recurrence and metastasis of hepatocellular carcinoma. BMC Cancer. 2006;6:110. doi: 10.1186/1471-2407-6-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ho JW, et al. Clinicopathological and prognostic implications of endoglin (CD105) expression in hepatocellular carcinoma and its adjacent non-tumorous liver. World J Gastroenterol. 2005;11(2):176–181. doi: 10.3748/wjg.v11.i2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Romani AA, et al. The risk of developing metastatic disease in colorectal cancer is related to CD105-positive vessel count. J Surg Oncol. 2006;93(6):446–455. doi: 10.1002/jso.20456. [DOI] [PubMed] [Google Scholar]
- 231.Saad RS, et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in colorectal cancer. Mod Pathol. 2004;17(2):197–203. doi: 10.1038/modpathol.3800034. [DOI] [PubMed] [Google Scholar]
- 232.Koyama Y, et al. Overexpression of endoglin (CD105) is associated with recurrence in radically resected gastric cancer. Exp Ther Med. 2010;1(4):627–633. doi: 10.3892/etm_00000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Budhu A, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10(2):99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]