Abstract
Heat shock (HS) is one of the best-studied exogenous cellular stresses. The cellular response to HS utilizes ancient molecular networks that are based primarily on the action of stress-induced heat shock proteins and HS factors. However, in one way or another, all cellular compartments and metabolic processes are involved in such a response. In this review, we aimed to summarize the experimental data concerning all aspects of the HS response in mammalian cells, such as HS-induced structural and functional alterations of cell membranes, the cytoskeleton and cellular organelles; the associated pathways that result in different modes of cell death and cell cycle arrest; and the effects of HS on transcription, splicing, translation, DNA repair, and replication.
Keywords: Heat shock, Heat shock proteins, DNA replication, DNA damage, Transcription
Introduction
Heat shock (HS, also known as heat stress and hyperthermia) is one of the primary organismal and cellular stressors. It represents the subjection of cells or whole organisms to an abnormally high environmental temperature. The research history of HS dates back to studies of heat-induced mutations in Drosophila performed from the late 1910s to the mid-1930s [1–5]. Studies of HS response mechanisms started with the paper of Ferruccio Ritossa [6], published in 1962, which described the induction of a new chromosomal puffing pattern (i.e., transcriptional activation) in Drosophila salivary glands that were exposed to heat. This work led to the discovery of heat-induced proteins named thereafter heat shock proteins (HSPs) [7, 8]. Although the initially discovered and most-studied function of HSPs (particularly HSP70 and HSP90) was facilitation of protein folding and assembly [9, 10], it is now clear that not only molecular chaperones’ expression is induced by heat. The transcription of more than 100 genes, such as encoding factors participating in protein folding, degradation, transport, RNA repair, metabolic pathways, and so forth, is upregulated under HS conditions [11–13]. The amazing aspect of the HS response is that it represents one of the most ancient molecular pathways—the HS response is not only conserved across species but also very similar under different stress conditions (cold shock, oxidative stress, etc.) [14, 15]. During the decades of studies on the HS response, the primary interest has been analyzing the functions of stress-induced proteins (primarily HSPs). Accordingly, virtually all of the recently published reviews on the HS response have focused primarily on HSPs [13, 16, 17]. Nevertheless, a considerable amount of experimental data have been obtained by studying the effects of HS on nucleic acids and nucleic acid-associated processes (replication, repair, transcription, etc.) as well as the consequences of HS on a cellular level, such as cell-cycle alterations and the induction of programmed cell death pathways. In this review, we attempt to summarize all aspects of the HS response in higher eukaryotes (primarily mammals). The paper is divided into two major sections that review the cellular and molecular aspects of the HS response, with a special focus on DNA replication and repair processes.
Cellular level of response
Cell membranes
It seems logical to start this review with a description of how HS affects cell organization and cellular components. Biomembranes, including both plasma and organelle membranes, are delicate sensors of the temperature of the surrounding environment [18–20]. One of the main effects that hyperthermia exerts on cellular membranes is fluidization, i.e., an increase in membrane liquidity accompanied by the formation of nonpolar hexagonal structures in the lipid bilayer [21]. It has been demonstrated that changes in cytoplasmic membrane fluidity caused by an increase in the saturation level of lipid fatty acids induce the expression of genes encoding HSPs [22]. Subsequent accumulation of HSPs at the plasma membrane rigidifies it, thereby restoring normal fluidity of the membrane [21, 23]. It might be hypothesized that changes in membrane fluidity compose a specific component of the cellular response to temperature stress. However, fluidization also leads to the destabilization of membrane components, including protein and lipid domains. This destabilization results in changes in cell surface morphology [24], disruptions of intercellular interactions [25], changes in the surface charge [26], and changes in the membrane potential of the cell due to Na+/H+-ion exchange channels and K+-ATPase dysfunction [27–29]. Under HS conditions, the entrance of extracellular calcium ions leads to the activation of calmodulin-dependent protein kinases [30], the induction of inositol triphosphate synthesis and the activation of other Ca2+-dependent signaling pathways [29], including the expression of HSP72 and HSP90 [31]. Furthermore, HS-dependent fluidization may enhance TRAIL-induced apoptosis in certain lymphoid cells [32, 33].
Nevertheless, in mammalian cells, membranes per se do not play the role of primary temperature sensors. This role is performed by the TRP (transient receptor potential) channel family, which consists of 30 transmembrane channels, six of which are temperature sensitive [34]. Among these channels, vanilloid TRP receptors, in particular TRPV1 and TRPV2, with different activation thresholds, are able to sense temperatures above 42 °C [35].
Cytoskeleton
The cytoskeleton is primarily responsible for intracellular organization and for different cellular activities, including motion and division of cells, positioning of intracellular membrane organelles, transport of macromolecules, and regulation of protein biosynthesis [36]. The effect of HS on the cytoskeleton depends primarily on the force and duration of the exposure to increased temperature as well as on the cell type [37]. One of the characteristic reactions to temperature stress is a change in cell form, which manifests as a rounding and shrinking caused by modifications of the cytoskeleton [38], including the reorganization of microfilaments [39]. HS results in the collapse of actin stress fibers and F-actin accumulation in the central cellular compartments [38, 39]. Tubulin microtubules also react to stress. It has been demonstrated that under HS conditions in the H1299 cell line, microtubules are retracted from the cell periphery, their network is disorganized and tubulin is accumulated in condensed aggregates in the perinuclear space [39]. HS induces the amplification of microtubule organization centers (centrosomes), which can lead to multipolar mitosis and the subsequent formation of giant flattened or multinuclear cells, a hallmark of mitotic catastrophe [40–42]. Hyperthermia also promotes the reorganization of vimentin fibers [43], which compose one of the types of intermediate filaments. During thermal stress, vimentin is totally aggregated in perinuclear complexes, but its organization is restored after prolonged incubation of cells at normal physiological conditions [39]. Similarly, tubulin and actin components are able to restore their structure after exposure to hyperthermia. Moreover, such restoration is a prognostic criterion of cell survival [44].
Cytoplasm and cellular organelles
Heat stress provokes a number of remarkable morphological changes inside the cell. These changes involve cytoplasmic organelles such as mitochondria, the endoplasmic reticulum, the Golgi apparatus, and, of course, the cell nucleus. HS results in mitochondrial swelling, an increase in their visible size, and an expansion of the space inside the cristae [45–47]. The endoplasmic reticulum and Golgi apparatus are fragmented under stress conditions [45]. The structure of the nucleus is also transformed. First, under HS conditions, the nucleolus tends to undergo disintegration [48]. This phenomenon can be explained by the release of multiple proteins from the nucleolus during heat stress. Nucleolin, for example, dissociates from the nucleolar compartment under HS conditions and inhibits DNA synthesis through binding to the RPA protein [49]. Another nucleolar protein, nucleophosmin (B23), also relocates into the nuclear space under conditions of hyperthermia [50]. Therefore, the nucleolus is considered to be a kind of “storage room” for stress proteins [51].
One of the remarkable effects of HS on the nucleus is the formation of separate electron dense granules, so-called “stress bodies” [52]. These stress bodies are relatively large structures ranging in size from 0.3 to 3 μm. In humans, they are associated with pericentromeric heterochromatin, particularly the region 9q12, which contains satellite III [52]. Stress granules are composed of ribonucleoprotein complexes, which include heat shock factor 1 (HSF1), satellite III transcripts with associated RNA processing factors SF1/ASF, and also CBP protein and RNA polymerase II [53, 54]. The role of stress granules during HS is still unclear. The noncoding RNA of satellite III, which plays a central role in forming and supporting stress granules, may control pre-mRNA processing via specific binding to certain splicing factors [54–56].
Another type of stress granules is formed in the cytoplasm. These granules are detected in higher eukaryotes at increased temperatures or under conditions of glucose deprivation, UV irradiation, or viral infections [57]. The formation of cytoplasmic stress granules (CSG) is believed to be caused by changes in the functions of translation machinery [58]. A large number of housekeeping gene mRNAs dissociate from polysomes and accumulate in the CSG, where they are degraded or become translationally repressed. Translationally silent mRNA in CSG is preserved in the form of an initiatory complex lacking the triple complex (eIF2/GTP/Met-tRNA). At the same time, CSG contains the small ribosome subunit, initiation factors (phosphorylated eIF2α, eIF3, eIF4G, eIF4E, PABP1), and some other RNA-binding proteins [59], including the ARE-binding proteins HUR and TTP and two RNA-binding proteins, TIA-1 (T cell intracellular antigen) and TIAR (TIA-1-related) [59]. The TIA-1 and TIAR proteins contain prion-like domains that cause the degradation of untranslated RNA under stress conditions. According to some observations, ARE-dependent translational inactivation and mRNA degradation also take place in CSG [58].
Cell death
It is not surprising that massive destruction and reorganization of cellular components lead to a general decrease in cell viability and to cell death. Induction of cell death depends on the strength and duration of HS [39, 60]. Under conditions of acute hyperthermia over 45.5 °C, cells die exclusively through necrosis [61, 62]. Two modes of cellular death have been identified in cell populations subjected to heat stress below this temperature: rapid mode and slow mode [63]. The latter is the result of delayed consequences of HS, such as centrosome damage, cell division aberrations, and mitotic catastrophe [39, 41]. “Rapid” cell death occurs either at the moment of HS or within several hours after HS and is caused mainly by cellular devastations and the inhibition of macromolecular synthesis, as described above [63, 64]. The induction of apoptosis at hyperthermia also contributes to the “rapid” mode of cellular death [64, 65]. The probability of inducing apoptosis depends not only on the intensity of the HS but also on the cell type [66]. Thus, hyperthermia turns on the apoptotic program in HL60 and U937 cell lines but not in K562 cells [66]. It might depend on differences in the expression levels of antiapoptotic members of Bcl-2 family proteins [67]. Nevertheless, the apoptosis induced by HS is not altogether classical. During hyperthermia, caspase-3 is activated by some undefined caspase-like proteases, whereas the classical initiatory caspases (such as caspase-2, -8, or -9) and their complexes are not involved [68]. It is worth mentioning that the molecular mechanism of heat-induced apoptosis still constitutes a matter of discussions (reviewed in Ref. [69]).
Cell cycle
Cellular sensitivity to hyperthermia depends on the stage of the cell cycle. The results of various studies show that cells in S phase or mitosis are more sensitive to high temperatures than those in G1 or G2 phases [70–72]. In S-phase cells, hyperthermia first affects DNA replication, which is drastically suppressed under stress conditions. This topic is discussed in more detail in the section “DNA replication”.
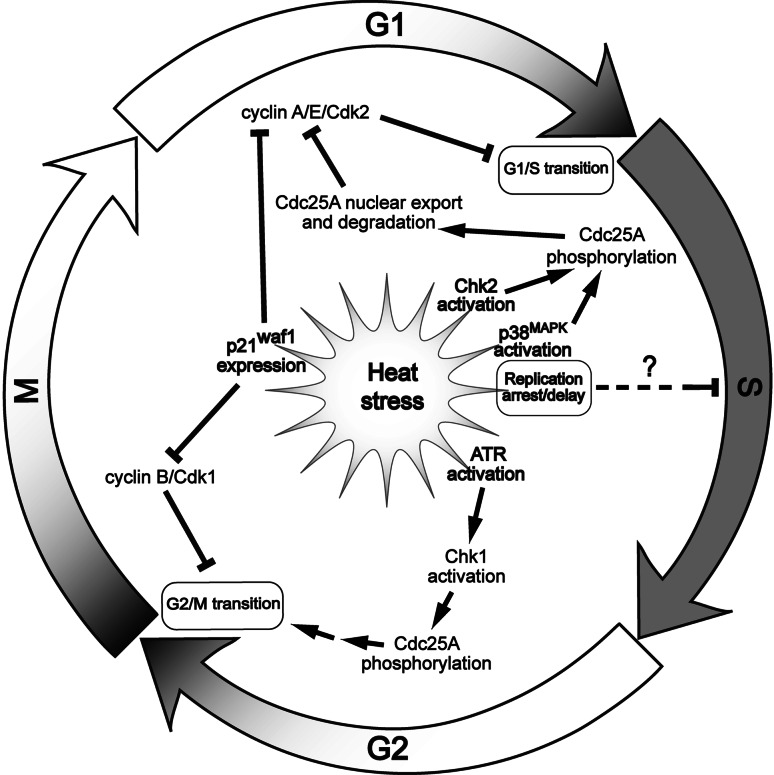
Exposure of an unsynchronized cell population to heat stress usually causes an arrest in the cell cycle (Fig. 1). However, the duration and phase specificity of the arrest depend on the type of cell culture. For example, in MCF7 BUS, MCF7 GS и T47D cell lines, HS induces G0/G1 arrest, while it does not at all affect MCF7 BB, MDA-MB-231 cells [73]. This difference does not depend on the p53 status of the cells. Apart from G0/G1 arrest, HS, like ionizing irradiation, induces arrest in G2/M. However, during hyperthermia, the arrest at G2/M phase occurs earlier and persists for less time than a radiation-induced block [73]. Studies in Jurkat and HeLa cells showed that the G2/M arrest under HS conditions is controlled by the ATR/Chk1-signaling pathway and is indispensable for the prevention of caspase-3-dependent apoptosis (Fig. 1) [74]. In some cell lines, moderate heat stress induces arrest of the cell cycle in G1 phase, which seems to be caused by the proteolytic degradation of the Cdc25A phosphatase [75]. Proteolysis of Cdc25A starts with the activation of the p38MAPK and Chk2 kinases, which phosphorylate Cdc25A. Such phosphorylation enables the dissociation of Cdc25A from the complex with HSP90 and subsequent translocation from the nucleus to the cytoplasm. In the cytoplasm, the phosphorylated form of Cdc25A forms a complex with the ubiquitin ligase SCFβ−TrCP and the protein 14-3-3, which leads to the degradation of Cdc25A (Fig. 1) [75].
Fig. 1.
Simplified general model of the heat stress-induced inhibition of the cell cycle progression (see the text for further discussion). ↑ activating or positive effect; ⊥ inhibitory or negative effect
Studies on normal skin fibroblasts have demonstrated that HS causes G1/S arrest mediated by p53, which under hyperthermic conditions is translocated to the cell nucleus, where it activates p21waf1 expression. In turn, p21 inhibits the cyclin-CDK protein kinase complex [76]. Analogous results were obtained in A172 glioma cells and U937 leukemic cells [77] but not in HeLa cells with a defect in the p53 gene [76]. Nevertheless, heat-induced overexpression of p21waf1 is not always p53 dependent. For example, hyperthermia induces p21waf1-mediated G1 arrest in the p53-deficient cells T98G and MDAH041 [77], which means that cell arrest in the G1/S phase of the cell cycle as a result of HS can be both p53 dependent and independent. Some authors have reported that HS leads to G1 arrest in yeast cells by inhibiting the transcription of the elements of the STAT system, including cyclins CLN1 and CLN2 [78, 79]. In summary, one may conclude that HS conditions can block cell cycle progression at practically any phase (Fig. 1).
Molecular level of response
Heat shock proteins (HSPs) and heat shock factors (HSFs)
The first studies of the HS response were performed by the Italian researcher Ferruccio Ritossa. Fifty years ago, he discovered the formation of temperature-activated chromosome puffs in Drosophila [6]. At that time, Ritossa demonstrated that active mRNA synthesis occurs in those puffs. This phenomenon remained a mystery and did not raise sufficient interest in the academic community [80]; however, more than 10 years later, the formation of these temperature-activated puffs was linked to the expression of a particular group of proteins that were therefore called HSPs [7, 8, 81]. Despite the fact that a new nomenclature of HSPs exists [82], we use their old custumal names throughout the review. The HSP family is usually divided into two classes according to their molecular weight. The first class contains small HSPs, with molecular weights of less than 40 kDa: HSP32, HSP27, HSP20, and others [83]. The second class includes HSPs with high molecular weights (60–100 kDa): HSP60, HSP70, HSP90, and HSP110 [64]. Synthesis of these proteins under stress conditions is a highly conserved mechanism of the cell response and is common among all living organisms [81, 84]. Moreover, HSPs are synthesized in response to not only HS but also other types of cellular stress, such as hypoxia [85], ischemia [86], hypothermia [87], virus infections [88], as well as the effects of heavy metals and ethanol [89]. Moreover, HSPs are expressed at a low level under normal conditions. This observation can be explained by the fact that HSPs are molecular chaperones for protein folding that play a central role in protein homeostasis [90, 91]. Many HSPs play the role of peculiar “assistants” for supporting protein conformational stability and the proper folding, assembly, and disassembly of protein complexes. HSPs prevent random protein aggregation, particularly during temperature denaturation [91, 92]. Molecular chaperones such as HSP70 and HSP90 preferentially bind to hydrophobic regions of unfolded polypeptide chains [93] and recruit auxiliary factors to perform ATP-dependent restoration of the protein molecule [84, 93]. However, the functions of HSP70 and HSP90 family members are not restricted to the maintenance of protein homeostasis. For example, they may be released (secreted) from cells [94–96] and are likely to participate in paracrine or autocrine interactions with adjacent cells [94, 96]. Some data suggest that secreted HSPs promote cell motility [97]. Finally, HSP90 was reported to participate in various DNA damage response pathways [98, 99]. Small HSPs such as HSP27 also participate in protein folding but perform it in an ATP-independent manner [84]. Refolding processes are carried out not only in the cytoplasm but also in the cell nucleus by some HSPs, such as HSP27, HSP72, or HSP90 [100]. For example, under conditions of hyperthermia, the HSP72 protein is translocated to the cell nucleus and accumulates in the nucleolus, where it presumably stabilizes the process of ribosome assembly [101]. Therefore, HSPs stand at the frontier of cellular defense against HS and other types of stress. Overexpression of HSPs provides “thermotolerance” for cells for a certain period of time, i.e., by providing temporary resistance to subsequent stresses [102]. This cytoprotective role of HSPs is not limited to their chaperone function. HSPs are capable of preventing cell death, in particular by blocking the induction of apoptosis [103]. In this way, HSP27 interacts with cytochrome c, whereas HSP70 and HSP90 bind to Apaf-1, thus inhibiting apoptosome formation and blocking the postmitochondrial apoptotic cascade [104]. The interaction of HSP70 with flavoprotein AIF blocks the induction of caspase-independent apoptosis [103, 104]. Under conditions of hyperthermia, the expression level of HSPs is increased to a certain temperature limit. Upon reaching this limit, the expression of HSPs begins to drop, which is likely caused by the destruction of thermolabile elements of the transcription system [91]. This threshold temperature is different for different cell types, but on approaching this threshold, the number of cells that die through apoptosis is substantially increased [64].
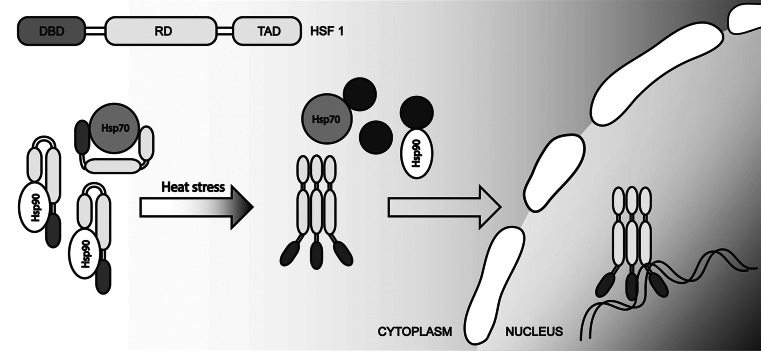
Two partially independent systems are responsible for activating the expression of genes encoding HSPs. The first system consists of heat shock transcription factors (HSFs) that bind to HS regulatory elements (heat shock elements, HSE), which usually localize to the promoter regions of a number of genes [105]. The second system consists of transcription factors that are responsible for nonspecific stress (Msn2 and Msn4) [106] that interact with stress response elements (STRE). This system is typical found in yeast. The mammalian HSF family includes four factors, HSF1-4 [107]. The most-studied factor among them is the transcription factor HSF1. It contains a helix-turn-helix DNA-binding domain and an oligomerization domain that enables trimerization [108]. Moreover, HSF1 contains two activation domains [109]. The diversity of HSE elements provides for shrewd expression regulation of HSPs. Thus, in higher eukaryotes, the activation of HSFs occurs through the trimerization of HSF1 under stress conditions, while yeast cells contain such trimers even before exposure to stress [110]. Under normal conditions, the majority of HSFs are retained in complexes with HSP70 and HSP90 chaperones that interact with the activation domain of HSF1 and thereby block its activation potential [111]. Under heat stress, HSP70 and HSP90 switch to interactions with denatured proteins, releasing HSF1 and stimulating self-expression. This increase in expression continues to the point where the number of molecular chaperones is sufficient to block the activation domain of HSF1 (Fig. 2) [13, 112].
Fig. 2.
Heat stress-induced activation of HSF1 (see the text for further discussion)
Posttranslational modifications of HSFs, such as phosphorylation, acetylation and SUMOylation, also regulate their binding to HSE. For example, HSF1 contains no less than 12 serine residues that are subjected to phosphorylation. Serine residues in positions 230 and 326 are phosphorylated under stress conditions that activate HSF1 [107]. SUMOylation of HSF1 has an opposite effect, as it weakens the transactivation function of this factor. Phosphorylation and SUMOylation occur immediately after HS, whereas the acetylation of HSF1 is a postponed process. Stress-induced acetylation is regulated by the balance between p300-CBP acetyltransferase and SIRT1 (NAD+-dependent sirtuin) deacetylase. An increase in the expression and activity of SIRT1 promotes the prolonged binding of HSF1 to the promoter of the HSP70 gene, whereas a decrease in SIRT1 expression leads to the acetylation of HSF1 and a weakening of DNA-binding activity without a direct influence on the structure of the HSF1 homotrimer [113].
Discussions concerning the functional activity of HSFs usually focus on their ability to activate the transcription of specific genes that mostly encode HSPs. However, more than a hundred different genes are activated during thermal stress, and an even greater number of them are repressed [12, 13, 114]. The decrease in the general level of transcription indicates that during hyperthermia, transcriptional repression prevails over activation [115]. This is due not only to temperature damaging the transcriptional machinery but also to the precise regulation of transcriptional activity during HS.
Transcription, splicing, and translation
As was previously described, HSFs are indispensable for stress-dependent activation of the expression of certain genes. The transcription level of many genes, including HSP40, HSP70, HSP90, HSP105, HSP47 (SERPINH2), and BAG3 (modulator of chaperon activity of HSP70), is specifically increased during HS [12, 114]. The expression of chaperone regulators (HSP70 binding protein ST13, HSP90 co-chaperon TEBP, FK506-binding protein) as well as proteins composing the posttranslational modification system (transglutaminase, proline-4-hydroxylase α) is also increased during HS [114]. Furthermore, a specific increase in transcription levels under heat stress conditions is typical for genes encoding proteasome degradation system components (proteasome subunit PSMD10), signal transduction (G-protein GNAS, transcription factor GABPB2, and p21), membrane transport (phospholamban, cation transporter SLC22A3) and general metabolism (cytochrome-b5-reductase, arylamine N-methyltransferase) [114]. Such kinds of increases in transcription are characteristic of a vast majority of cell lines. Nevertheless, certain genes are differently expressed in response to HS, depending on the cell line. For example, a sharp increase in the expression of the transcription factors c-jun and c-fos in response to HS was observed in HeLa and K562 cells but not in primary human fibroblasts [114].
For a long time, the mechanisms of gene repression during heat stress remained unknown. It is now understood that noncoding RNA can play the role of transcriptional repressor during heat stress [116]. RNA encoded by Alu-repeats (one of the types of human SINE) binds to RNA polymerase II during HS, blocking the formation of the preinitiation complex on the promoter and thereby inhibiting transcription [117–119]. An analogous mechanism supported by SINE B2 RNA exists in mice [116]. Meanwhile, RNA polymerase II, which transcribes active genes such as HSP70 during HS, does not bind to this RNA [117]. It is assumed that certain factors induced under hyperthermia conditions, such as another noncoding RNA, enable SINE-RNA-mediated gene repression to be overcome. A noncoding RNA called HSR1 (heat shock RNA 1) is known to participate in transcriptional regulation of heat-inducible genes, stimulating the trimerization of HSF1 [120]. Therefore, during HS, noncoding RNAs can be either transcriptional activators or repressors.
The regulation of gene expression during hyperthermia occurs at the post-transcriptional level as well. According to observations in the H1299 cell line, the increase in p21 expression during heat stress is associated with its mRNA stabilization rather than an increase in transcription [121]. The reverse mechanism also exists. The transcription factor TFDP1 and cyclin D1 expression inhibition during hyperthermia is determined foremost by the destabilization and enhanced degradation of transcripts [121]. MicroRNAs can also contribute to posttranscriptional gene expression regulation during hyperthermia. Studies with skin fibroblasts demonstrated that during heat stress, different changes occur in the expression level of 123 microRNAs—83 are suppressed and 40 are activated. Moreover, of all the expected targets for these microRNAs, the majority, such as DNAJB2, HSPA4L (HSP70L), HSPA6, HOXB8, and HOXC8, are components of the cellular stress response machinery [122].
RNA processing is also a subject for temperature inactivation [123–125]. Incubation of cells at sublethal temperatures leads to reversal inhibition of mRNA splicing. In HeLa cells exposed to HS, inactivation of splicing is linked to the destruction of small nuclear ribonucleoprotein complexes (snRNP) [124, 126]. During moderate hyperthermia (43 °C), the triple complex U4/U5/U6 of snRNP is degraded [127], whereas during acute HS (46 °C), U1 and U2 are inactivated as well [126]. Alterations in snRNP particles were observed during heat stress, which points to the high sensitivity level of ribonucleoprotein complexes to hyperthermic conditions [128]. At the same time, the inhibitory effect of HS on RNA processing is not limited to destruction. It turns out that the inhibition of splicing during hyperthermia can be regulated. Splicing repressor protein SRp38 is dephosphorylated during heat stress and binds to U1 snRNP, blocking U1-mediated recognition of 5′-splice-sites of unprocessed RNAs [129, 130]. Another possible regulation of splicing during HS is stress granule assembly in the nucleus (see Sect. “Cytoplasm and cellular organelles”). The noncoding transcript of the satellite III, which is a structural basis of nuclear stress granules, can bind some splicing factors, thus regulating their common availability [54, 131].
DNA damage response
Different DNA repair systems are targets of HS. The system of base excision repair (BER) is impaired by hyperthermia through inactivation of DNA-polymerase β (pol β), one of the key enzymes of BER [132, 133]}. HSP70 associated with DNA-polymerase β [134, 135], together with HSP27, stimulates and restores its activity after heat stress, which apparently results in the eventual development of thermotolerance [136]. UV-light irradiation and several types of alkylating agents lead to the cross-linking of DNA strands and pyrimidine-dimer formation. In eukaryotic cells, such DNA lesions are eliminated through nucleotide excision repair (NER) [137–139]. Hyperthermia has a great impact on this system. For example, the expression of one of the central components of NER, protein XPA, is suppressed under heat stress conditions [140]. In addition, NER of UV-light-induced pyrimidine dimers was shown to be extensively repressed during HS [141].
All organisms possess systems to repair DNA double-stranded breaks (DSBs). Two competing pathways are used by higher eukaryotes for DSB repair: homologous recombination (HR) and non-homologous end joining (NHEJ) [142, 143]. Cells exposed to hyperthermia become more susceptible to agents that induce DSBs, particularly to ionizing radiation [144]. This phenomenon is called thermal radiosensitivity. The possible cause of this effect is the ability of heat stress to inhibit both systems for DNA repair, HR, and NHEJ [144–146]. The effect of hyperthermia on NHEJ results in the aggregation of Ku protein, which is a subunit of DNA-dependent protein kinase. This aggregation leads to an inactivation of the DNA-binding activity of Ku protein [147] but is not the main reason for heat-induced radiosensitivity. Hyperthermia has a more pronounced and versatile impact on the HR repair system. The primary sensors of DNA damage in this system are the phosphorylated form of histone H2AX (γH2AX) and the proteins MDC1 and 53BP1 [148], which produce foci at the sites of DSBs. Exposure of cells to heat stress before and after the induction of DSBs interferes with the formation of 53BP1 foci [149]. At the site of damage, 53BP1 becomes a platform for the recruitment and assembly of other proteins, in particular the SMC1 protein, phosphorylated on serine residue 957 [150]. Therefore, hyperthermia-caused inhibition of 53BP1 foci assembly at sites of damage restricts the formation of p957S-SMC1 repair clusters. Interestingly, such effects are typically not observed in thermotolerant cells with high HSP70 expression levels [149].
The central element of the HR repair system is the MRN complex, which contains the Mre11/Rad50 dimer and Nbs1 [151]. At the first stage of repair through the HR pathway, Mre11/Rad50/Nbs1, CtIP and BRCA1 become associated at the site of the double-stranded break [152]. This complex has nuclease activity, which enables the generation of single-stranded stretches of DNA. These stretches of DNA are indispensable for recruiting RPA protein [153]. Moderate hyperthermia (41 °C) does not change the ability of Mre11 to associate with DSBs [154]. Under conditions of acute hyperthermia (42–45 °C), Mre11, Rad50, and Nbs1 are translocated from the nucleus to the cytoplasm via a CRM1-mediated nuclear export system [155]. This leads to the disruption of regulatory mechanisms. At the same time, this effect is not characteristic for all cell types. For example, such translocations of the MRN-complex components were totally absent at 44 °C in the culture of human fibroblasts [156]. Some studies have reported that moderate HS (42 °C and more) targeting protein BRCA1 leads to degradation through the ubiquitin–proteasome pathway. However, whether moderate HS disrupts MRN-complex formation has not been demonstrated [157].
The second stage of HR involves recruiting recombinase Rad51 and protein BRCA2 to the breakage site [153]. HS completely blocks this step. This is explained by fast proteasome-dependent degradation of protein BRCA2 that has already occurred at 42 °C [154, 158]. Therefore, hyperthermia interferes with HR at almost all of the essential steps.
The influence of HS on DNA repair systems underlies the phenomenon significant for cancer treatment, thermal (or heat) radiosensitization [159]. It represents the increased sensitivity of heat-treated cells to ionizing radiation (IR). The exact mechanism of radiosensitization is still being discussed. The most popular hypothesis considers thermal radiosensitization to be a result of inhibition of IR-induced DNA damage repair [159–163].
Apart from the inhibition of the repair systems, HS itself is a DNA-damaging factor. Until recently, hyperthermia was thought to provoke the formation of DNA single-stranded breaks by inhibiting replication [145, 164, 165]. This issue is discussed in more detail in the next section. In the remainder of this section, we focus on a problem that remains to be discussed; are DNA DSBs formed under HS conditions? A number of research groups have demonstrated that HS induces the phosphorylation of histone H2AX [149, 166–169], which is one of the first steps in DSB recognition and repair [170]. Several authors directly showed the formation of DSBs after HS using pulse-field gel electrophoresis, DNA comet assays [60, 146], and alkaline elution of DNA [167]. However, other authors that used these or other methods (the DNA halo method, detection of G1/G2 chromosomal aberrations or pulse-field gel electrophoresis) failed to detect DSBs in cells after moderate HS (43 °C, 1 h) [166]. Nevertheless, induction of cell type-independent phosphorylation of H2AX was observed in mammalian cells during hyperthermia with a broad range of exposure temperatures (42–45.5 °C) [169]. Our recently published study reports that HS does induce the formation of DSBs in human cell cultures. This conclusion is strongly supported by the results of neutral SCGE analysis and by a TdT incorporation assay [171]. Interestingly, the formation of DSBs under HS conditions is restricted to non-S-phase (G1 and G2) cells. In G1 and G2 cells, HS-induced DSBs are marked by H2AX phosphorylation, which is ATM dependent [171]. The mechanism of HS-induced DSB formation remains unclear. There are numerous possible triggers for such lesions: the production of reactive oxygen species [172], an increase in retroelement activity [173, 174], and the inhibition of the DNA repair system as well as the consequent slowing down or blocking of the repair of endogenous, spontaneously forming DSBs [154, 158, 175].
DNA replication
HS inhibits multiple processes associated with DNA replication, including the initiation of new replicons [176, 177], the elongation of replication forks [178], and the maturation of chromatin [179]. In many research studies, the impact of HS on replication was studied using an in vitro replication system. The results of these experiments show that extracts from cells exposed to HS are less replicatively active than control cell extracts. Nevertheless, even after prolonged HS at 44 °C, cell extracts retain relatively high level of replication activity [180]. This observation suggests that the inhibition of replication during heat stress is a regulated process that is implemented not merely by the irreversible denaturation of thermolabile replication factors. RPA protein was presumed to be one of the targets of such regulatory mechanisms [49]. Although the hypothesis that phosphorylation of RPA regulates replication during heat stress has not been proved [181], it has been demonstrated that during HS, the nucleolar protein nucleolin is translocated to nucleoplasm and bound to RPA, thereby blocking DNA replication [182]. In vitro analysis also confirmed that such an interaction strongly inhibits replication [49]. Interestingly, this regulatory mechanism is in itself thermosensitive and is inactivated during prolonged heat stress [49, 180]. The regulated nature of HS-induced inhibition of DNA replication is also supported by its gradualism: different temperature conditions lead to a progressive decrease in the apparent speed of replication fork progression (42 and 44 °C) and finally to a total arrest of replication at 45.5 °C [171]. It is notable that after HS-induced arrest, replication forks are mostly restarted upon cell cultivation under normal conditions. Furthermore, it appears that the phosphorylation of H2AX is necessary to prevent arrested replication forks from collapse [171]. Interestingly, under HS conditions, H2AX is phosphorylated at replication sites by DNA-dependent protein kinase (DNA-PK), while the phosphorylation of H2AX at DSBs is mediated by ATM [171]. The role of DNA-PK in replication-associated H2AX phosphorylation may be due to its association with RPA at replication forks [183].
Dewey and coauthors [184] for the first time demonstrated that S-phase cells are hypersensitive to thermoinducible cell death. In S-phase cells, hyperthermia results in persisting single-stranded DNA regions, which may be the source of chromosomal aberrations [185]. Indeed, after HS in S-phase cells, their number greatly exceeds that observed in G1 and G2 [186]. Furthermore, the level of thermoinducible cell death correlates with the number of such aberrations [178]. In case of aphidicolin-induced replicative block, the level of cell death under heat stress conditions is reduced compared to cases in which the cells with active replication are exposed to heat stress [187]. This effect was explained by the fact that in S-phase cells, thermoinducible lesions are not repaired in time and therefore are later converted to lethal devastations. Thus, replication arrest facilitates more efficient performance of repair systems [188]. This conclusion agrees with experiments that have demonstrated that the inhibition of replication for 2 h averts S-phase hypersensitivity after a 15-min HS at 45 °C.
Conclusions and perspectives
When we started working with HS a couple of years ago, we could not find any articles that summarized all aspects of the cellular response to heat stress. Most of the reviews published in recent years have been devoted to the regulation, functions and clinical applications of HSPs [13, 16, 17, 189]. Other important aspects of the cellular response to heat stress were overlooked. The present review is aimed to correct this gap in the literature. In this “textbook”-like article, we tried to summarize data concerning virtually all aspects of the HS response in mammals.
Although the HS response has been extensively studied for decades, we found that very little was known about the effects of HS on nucleic acids and nucleic acid-associated processes, such as DNA replication and repair, transcriptional repression, and epigenetic changes. Accordingly, we focused our own studies on the impacts of heat stress on genome organization and integrity and DNA replication. The results obtained [171, 190] demonstrate that HS is still a very powerful model system for studying alterations in all major intracellular processes. Emergence of new research tools always stimulates rapid progress in science. In modern molecular biology, so-called full-genome studies provide substantial novel data. Nevertheless, such powerful approaches as transcriptome analysis were not yet used to study cellular response to HS. It is obvious now that we need high-quality heat stress transcriptome data (obtained using next-generation sequencing techniques) to elucidate the cell stress response. It is commonly believed that under HS conditions, the expression of the majority of cellular genes is suppressed. However, the exact list of these genes remains obscure. Even more obscure are the molecular mechanisms involved in mediating transcriptional repression during HS conditions. As discussed above, there are also several important unanswered questions from studies of HS influence on DNA replication and repair processes. The data concerning the impact of heat stress on genome integrity remain controversial. This important area certainly deserves more attention from the scientific community. Finally, it is worth mentioning that the HS response is powerful model system for studying epigenetic changes associated with the cell stress response. Here, again, utilization of modern genomic approaches (including chromatin immunoprecipitation followed by deep sequencing) could provide important new findings. The final aim is, of course, to obtain a comprehensive picture of the cellular responses to different stresses, which can be achieved only by integrating the data regarding the impact of stresses on a variety of cellular processes and which is a task of modern system biology.
Acknowledgments
This work was supported in part by grants from the Ministry of Science and Education of the Russian Federation (8126, 8052), grants from the Russian Foundation for Basic Research (12-04-33040, 13-04-00604), and a grant from the Presidium of the Russian Academy of Sciences (MCB grant).
References
- 1.Plough HH. The effect of temperature on linkage in the second chromosome of Drosophila . Proc Natl Acad Sci USA. 1917;3:553–555. doi: 10.1073/pnas.3.9.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts E. Fluctuations in a recessive Mendelian character and selection. J Exp Zool. 1918;27:157–192. [Google Scholar]
- 3.Muller HJ. Variation due to change in the individual gene. Am Nat. 1922;56:32–50. [Google Scholar]
- 4.Plough HH, Ives PT. Heat induced mutations in Drosophila . Proc Natl Acad Sci USA. 1934;20:268–273. doi: 10.1073/pnas.20.5.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plough HH, Ives PT. Induction of mutations by high temperature in Drosophila . Genetics. 1935;20:42–69. doi: 10.1093/genetics/20.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritossa FM. A new puffing pattern induced by temperature shock and DNP in Drosophila . Experientia. 1962;18:571–573. [Google Scholar]
- 7.Ashburner M, Bonner JJ. The induction of gene activity in Drosophila by heat shock. Cell. 1979;17:241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- 8.Peterson NS, Moller G, Mitchell HK. Genetic mapping of the coding regions for three heat-shock proteins in Drosophila melanogaster . Genetics. 1979;92:891–902. doi: 10.1093/genetics/92.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 10.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 11.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabuchi Y, Takasaki I, Wada S, Zhao QL, Hori T, Nomura T, Ohtsuka K, Kondo T. Genes and genetic networks responsive to mild hyperthermia in human lymphoma U937 cells. Int J Hyperth. 2008;24:613–622. doi: 10.1080/02656730802140777. [DOI] [PubMed] [Google Scholar]
- 13.Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- 16.Morimoto RI. The heat shock response: systems biology of proteotoxic stress in aging and disease. Cold Spring Harb Symp Quant Biol. 2011;76:91–99. doi: 10.1101/sqb.2012.76.010637. [DOI] [PubMed] [Google Scholar]
- 17.Shamovsky I, Nudler E. New insights into the mechanism of heat shock response activation. Cell Mol Life Sci. 2008;65:855–861. doi: 10.1007/s00018-008-7458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigh L, Horvath I, Maresca B, Harwood JL. Can the stress protein response be controlled by ‘membrane-lipid therapy’? Trends Biochem Sci. 2007;32:357–363. doi: 10.1016/j.tibs.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Vigh L, Nakamoto H, Landry J, Gomez-Munoz A, Harwood JL, Horvath I. Membrane regulation of the stress response from prokaryotic models to mammalian cells. Ann N Y Acad Sci. 2007;1113:40–51. doi: 10.1196/annals.1391.027. [DOI] [PubMed] [Google Scholar]
- 20.Balogh G, Horvath I, Nagy E, Hoyk Z, Benko S, Bensaude O, Vigh L. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005;272:6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- 21.Vigh L, Maresca B, Harwood JL. Does the membrane’s physical state control the expression of heat shock and other genes? Trends Biochem Sci. 1998;23:369–374. doi: 10.1016/s0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- 22.Carratu L, Franceschelli S, Pardini CL, Kobayashi GS, Horvath I, Vigh L, Maresca B. Membrane lipid perturbation modifies the set point of the temperature of heat shock response in yeast. Proc Natl Acad Sci USA. 1996;93:3870–3875. doi: 10.1073/pnas.93.9.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torok Z, Horvath I, Goloubinoff P, Kovacs E, Glatz A, Balogh G, Vigh L. Evidence for a lipochaperonin: association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc Natl Acad Sci USA. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin PS, Lui PS, Tsai S. Heat induced ultrastructural injuries in lymphoid cells. Exp Mol Pathol. 1978;29:281–290. doi: 10.1016/0014-4800(78)90071-0. [DOI] [PubMed] [Google Scholar]
- 25.Hamada N, Kodama S, Suzuki K, Watanabe M. Gap junctional intercellular communication and cellular response to heat stress. Carcinogenesis. 2003;24:1723–1728. doi: 10.1093/carcin/bgg135. [DOI] [PubMed] [Google Scholar]
- 26.Sato C, Nakayama T, Kojima K, Nishimoto Y, Nakamura W. Effects of hyperthermia on cell surface charge and cell survival in mastocytoma cells. Cancer Res. 1981;41:4107–4110. [PubMed] [Google Scholar]
- 27.Mikkelsen RB, Koch B. Thermosensitivity of the membrane potential of normal and simian virus 40-transformed hamster lymphocytes. Cancer Res. 1981;41:209–215. [PubMed] [Google Scholar]
- 28.Nishida T, Akagi K, Tanaka Y. Correlation between cell killing effect and cell membrane potential after heat treatment: analysis using fluorescent dye and flow cytometry. Int J Hyperth. 1997;13:227–234. doi: 10.3109/02656739709012385. [DOI] [PubMed] [Google Scholar]
- 29.Park HG, Han SI, Oh SY, Kang HS. Cellular responses to mild heat stress. Cell Mol Life Sci. 2005;62:10–23. doi: 10.1007/s00018-004-4208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevenson MA, Calderwood SK, Hahn GM. Effect of hyperthermia (45 degrees C) on calcium flux in Chinese hamster ovary HA-1 fibroblasts and its potential role in cytotoxicity and heat resistance. Cancer Res. 1987;47:3712–3717. [PubMed] [Google Scholar]
- 31.Kiang JG, Gist ID, Tsokos GC. Regulation of heat shock protein 72 kDa and 90 kDa in human breast cancer MDA-MB-231 cells. Mol Cell Biochem. 2000;204:169–178. doi: 10.1023/a:1007016822939. [DOI] [PubMed] [Google Scholar]
- 32.Moulin M, Arrigo AP. Long lasting heat shock stimulation of TRAIL-induced apoptosis in transformed T lymphocytes. Exp Cell Res. 2006;312:1765–1784. doi: 10.1016/j.yexcr.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Moulin M, Carpentier S, Levade T, Arrigo AP. Potential roles of membrane fluidity and ceramide in hyperthermia and alcohol stimulation of TRAIL apoptosis. Apoptosis. 2007;12:1703–1720. doi: 10.1007/s10495-007-0096-2. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Zhang X, McNaughton PA. Modulation of temperature-sensitive TRP channels. Semin Cell Dev Biol. 2006;17:638–645. doi: 10.1016/j.semcdb.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- 36.Pollard TD. The cytoskeleton, cellular motility and the reductionist agenda. Nature. 2003;422:741–745. doi: 10.1038/nature01598. [DOI] [PubMed] [Google Scholar]
- 37.Armour EP, McEachern D, Wang Z, Corry PM, Martinez A. Sensitivity of human cells to mild hyperthermia. Cancer Res. 1993;53:2740–2744. [PubMed] [Google Scholar]
- 38.Luchetti F, Mannello F, Canonico B, Battistelli M, Burattini S, Falcieri E, Papa S. Integrin and cytoskeleton behaviour in human neuroblastoma cells during hyperthermia-related apoptosis. Apoptosis. 2004;9:635–648. doi: 10.1023/B:APPT.0000038043.03799.6f. [DOI] [PubMed] [Google Scholar]
- 39.Pawlik A, Nowak JM, Grzanka D, Gackowska L, Michalkiewicz J, Grzanka A. Hyperthermia induces cytoskeletal alterations and mitotic catastrophe in p53-deficient H1299 lung cancer cells. Acta Histochem. 2012;115(1):8–15. doi: 10.1016/j.acthis.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Vidair CA, Doxsey SJ, Dewey WC. Heat shock alters centrosome organization leading to mitotic dysfunction and cell death. J Cell Physiol. 1993;154:443–455. doi: 10.1002/jcp.1041540302. [DOI] [PubMed] [Google Scholar]
- 41.Nakahata K, Miyakoda M, Suzuki K, Kodama S, Watanabe M. Heat shock induces centrosomal dysfunction, and causes non-apoptotic mitotic catastrophe in human tumour cells. Int J Hyperth. 2002;18:332–343. doi: 10.1080/02656730210129736. [DOI] [PubMed] [Google Scholar]
- 42.Gupta RK, Srinivas UK. Heat shock induces chromosomal instability in near-tetraploid embryonal carcinoma cells. Cancer Biol Ther. 2008;7:1471–1480. doi: 10.4161/cbt.7.9.6428. [DOI] [PubMed] [Google Scholar]
- 43.Wang TT, Chiang AS, Chu JJ, Cheng TJ, Chen TM, Lai YK. Concomitant alterations in distribution of 70 kDa heat shock proteins, cytoskeleton and organelles in heat shocked 9L cells. Int J Biochem Cell Biol. 1998;30:745–759. doi: 10.1016/s1357-2725(97)00133-7. [DOI] [PubMed] [Google Scholar]
- 44.Coss RA, Alden ME, Wachsberger PR, Smith NN. Response of the microtubular cytoskeleton following hyperthermia as a prognostic indicator of survival of Chinese hamster ovary cells. Int J Radiat Oncol Biol Phys. 1996;34:403–410. doi: 10.1016/0360-3016(95)02039-x. [DOI] [PubMed] [Google Scholar]
- 45.Welch WJ, Suhan JP. Morphological study of the mammalian stress response: characterization of changes in cytoplasmic organelles, cytoskeleton, and nucleoli, and appearance of intranuclear actin filaments in rat fibroblasts after heat-shock treatment. J Cell Biol. 1985;101:1198–1211. doi: 10.1083/jcb.101.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole A, Armour EP. Ultrastructural study of mitochondrial damage in CHO cells exposed to hyperthermia. Radiat Res. 1988;115:421–435. [PubMed] [Google Scholar]
- 47.Rivera RM, Kelley KL, Erdos GW, Hansen PJ. Alterations in ultrastructural morphology of two-cell bovine embryos produced in vitro and in vivo following a physiologically relevant heat shock. Biol Reprod. 2003;69:2068–2077. doi: 10.1095/biolreprod.103.020347. [DOI] [PubMed] [Google Scholar]
- 48.Iliakis GE, Pantelias GE. Effects of hyperthermia on chromatin condensation and nucleoli disintegration as visualized by induction of premature chromosome condensation in interphase mammalian cells. Cancer Res. 1989;49:1254–1260. [PubMed] [Google Scholar]
- 49.Wang Y, Guan J, Wang H, Leeper D, Iliakis G. Regulation of DNA replication after heat shock by replication protein a-nucleolin interactions. J Biol Chem. 2001;276:20579–20588. doi: 10.1074/jbc.M100874200. [DOI] [PubMed] [Google Scholar]
- 50.Vanderwaal RP, Maggi LB, Jr, Weber JD, Hunt CR, Roti Roti JL. Nucleophosmin redistribution following heat shock: a role in heat-induced radiosensitization. Cancer Res. 2009;69:6454–6462. doi: 10.1158/0008-5472.CAN-08-4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boulon S, Westman BJ, Hutten S, Boisvert FM, Lamond AI. The nucleolus under stress. Mol Cell. 2010;40:216–227. doi: 10.1016/j.molcel.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jolly C, Konecny L, Grady DL, Kutskova YA, Cotto JJ, Morimoto RI, Vourc’h C. In vivo binding of active heat shock transcription factor 1 to human chromosome 9 heterochromatin during stress. J Cell Biol. 2002;156:775–781. doi: 10.1083/jcb.200109018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denegri M, Moralli D, Rocchi M, Biggiogera M, Raimondi E, Cobianchi F, De Carli L, Riva S, Biamonti G. Human chromosomes 9, 12, and 15 contain the nucleation sites of stress-induced nuclear bodies. Mol Biol Cell. 2002;13:2069–2079. doi: 10.1091/mbc.01-12-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biamonti G. Nuclear stress bodies: a heterochromatin affair? Nat Rev Mol Cell Biol. 2004;5:493–498. doi: 10.1038/nrm1405. [DOI] [PubMed] [Google Scholar]
- 55.Rizzi N, Denegri M, Chiodi I, Corioni M, Valgardsdottir R, Cobianchi F, Riva S, Biamonti G. Transcriptional activation of a constitutive heterochromatic domain of the human genome in response to heat shock. Mol Biol Cell. 2004;15:543–551. doi: 10.1091/mbc.E03-07-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valgardsdottir R, Chiodi I, Giordano M, Rossi A, Bazzini S, Ghigna C, Riva S, Biamonti G. Transcription of satellite III non-coding RNAs is a general stress response in human cells. Nucleic Acids Res. 2008;36:423–434. doi: 10.1093/nar/gkm1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson P, Kedersha N. Stressful initiations. J Cell Sci. 2002;115:3227–3234. doi: 10.1242/jcs.115.16.3227. [DOI] [PubMed] [Google Scholar]
- 58.Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi A, Matsumoto H, Nagayama K, Kitano M, Hirose S, Tanaka H, Mori E, Yamakawa N, Yasumoto J, Yuki K, Ohnishi K, Ohnishi T. Evidence for the involvement of double-strand breaks in heat-induced cell killing. Cancer Res. 2004;64:8839–8845. doi: 10.1158/0008-5472.CAN-04-1876. [DOI] [PubMed] [Google Scholar]
- 61.Harmon BV, Corder AM, Collins RJ, Gobe GC, Allen J, Allan DJ, Kerr JF. Cell death induced in a murine mastocytoma by 42–47 degrees C heating in vitro: evidence that the form of death changes from apoptosis to necrosis above a critical heat load. Int J Radiat Biol. 1990;58:845–858. doi: 10.1080/09553009014552221. [DOI] [PubMed] [Google Scholar]
- 62.VanderWaal R, Malyapa RS, Higashikubo R, Roti Roti JL. A comparison of the modes and kinetics of heat-induced cell killing in HeLa and L5178Y cells. Radiat Res. 1997;148:455–462. [PubMed] [Google Scholar]
- 63.Vidair CA, Dewey WC. Two distinct modes of hyperthermic cell death. Radiat Res. 1988;116:157–171. [PubMed] [Google Scholar]
- 64.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 65.O’Neill KL, Fairbairn DW, Smith MJ, Poe BS. Critical parameters influencing hyperthermia-induced apoptosis in human lymphoid cell lines. Apoptosis. 1998;3:369–375. doi: 10.1023/a:1009689407261. [DOI] [PubMed] [Google Scholar]
- 66.Falcieri E, Luchetti F, Burattini S, Canonico B, Santi S, Papa S. Lineage-related sensitivity to apoptosis in human tumor cells undergoing hyperthermia. Histochem Cell Biol. 2000;113:135–144. doi: 10.1007/s004180050016. [DOI] [PubMed] [Google Scholar]
- 67.Amarante-Mendes GP, McGahon AJ, Nishioka WK, Afar DE, Witte ON, Green DR. Bcl-2-independent Bcr-Abl-mediated resistance to apoptosis: protection is correlated with up regulation of Bcl-xL. Oncogene. 1998;16:1383–1390. doi: 10.1038/sj.onc.1201664. [DOI] [PubMed] [Google Scholar]
- 68.Milleron RS, Bratton SB. Heat shock induces apoptosis independently of any known initiator caspase-activating complex. J Biol Chem. 2006;281:16991–17000. doi: 10.1074/jbc.M512754200. [DOI] [PubMed] [Google Scholar]
- 69.Milleron RS, Bratton SB. ‘Heated’ debates in apoptosis. Cell Mol Life Sci. 2007;64:2329–2333. doi: 10.1007/s00018-007-7135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palzer RJ, Heidelberger C. Studies on the quantitative biology of hyperthermic killing of HeLa cells. Cancer Res. 1973;33:415–421. [PubMed] [Google Scholar]
- 71.Westra A, Dewey WC. Variation in sensitivity to heat shock during the cell-cycle of Chinese hamster cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19:467–477. doi: 10.1080/09553007114550601. [DOI] [PubMed] [Google Scholar]
- 72.Bhuyan BK, Day KJ, Edgerton CE, Ogunbase O. Sensitivity of different cell lines and of different phases in the cell cycle to hyperthermia. Cancer Res. 1977;37:3780–3784. [PubMed] [Google Scholar]
- 73.Valenzuela MT, Nunez MI, Villalobos M, Siles E, McMillan TJ, Pedraza V, Ruiz de Almodovar JM. A comparison of p53 and p16 expression in human tumor cells treated with hyperthermia or ionizing radiation. Int J Cancer. 1997;72:307–312. doi: 10.1002/(sici)1097-0215(19970717)72:2<307::aid-ijc18>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 74.Furusawa Y, Iizumi T, Fujiwara Y, Zhao QL, Tabuchi Y, Nomura T, Kondo T. Inhibition of checkpoint kinase 1 abrogates G2/M checkpoint activation and promotes apoptosis under heat stress. Apoptosis. 2012;17:102–112. doi: 10.1007/s10495-011-0660-7. [DOI] [PubMed] [Google Scholar]
- 75.Madlener S, Rosner M, Krieger S, Giessrigl B, Gridling M, Vo TP, Leisser C, Lackner A, Raab I, Grusch M, Hengstschlager M, Dolznig H, Krupitza G. Short 42 degrees C heat shock induces phosphorylation and degradation of Cdc25A which depends on p38MAPK, Chk2 and 14.3.3. Hum Mol Genet. 2009;18:1990–2000. doi: 10.1093/hmg/ddp123. [DOI] [PubMed] [Google Scholar]
- 76.Nitta M, Okamura H, Aizawa S, Yamaizumi M. Heat shock induces transient p53-dependent cell cycle arrest at G1/S. Oncogene. 1997;15:561–568. doi: 10.1038/sj.onc.1201210. [DOI] [PubMed] [Google Scholar]
- 77.Fuse T, Yamada K, Asai K, Kato T, Nakanishi M. Heat shock-mediated cell cycle arrest is accompanied by induction of p21 CKI. Biochem Biophys Res Commun. 1996;225:759–763. doi: 10.1006/bbrc.1996.1247. [DOI] [PubMed] [Google Scholar]
- 78.Nunes E, Siede W. Hyperthermia and paraquat-induced G1 arrest in the yeast Saccharomyces cerevisiae is independent of the RAD9 gene. Radiat Environ Biophys. 1996;35:55–57. doi: 10.1007/BF01211243. [DOI] [PubMed] [Google Scholar]
- 79.Rowley A, Johnston GC, Butler B, Werner-Washburne M, Singer RA. Heat shock-mediated cell cycle blockage and G1 cyclin expression in the yeast Saccharomyces cerevisiae . Mol Cell Biol. 1993;13:1034–1041. doi: 10.1128/mcb.13.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Maio A, Santoro MG, Tanguay RM, Hightower LE. Ferruccio Ritossa’s scientific legacy 50 years after his discovery of the heat shock response: a new view of biology, a new society, and a new journal. Cell Stress Chaperones. 2012;17:139–143. doi: 10.1007/s12192-012-0320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 82.Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- 84.Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging—a mini-review. Gerontology. 2009;55:550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel B, Khaliq A, Jarvis-Evans J, Boulton M, Arrol S, Mackness M, McLeod D. Hypoxia induces HSP 70 gene expression in human hepatoma (HEP G2) cells. Biochem Mol Biol Int. 1995;36:907–912. [PubMed] [Google Scholar]
- 86.Richard V, Kaeffer N, Thuillez C. Delayed protection of the ischemic heart—from pathophysiology to therapeutic applications. Fundam Clin Pharmacol. 1996;10:409–415. doi: 10.1111/j.1472-8206.1996.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 87.Yang XM, Baxter GF, Heads RJ, Yellon DM, Downey JM, Cohen MV. Infarct limitation of the second window of protection in a conscious rabbit model. Cardiovasc Res. 1996;31:777–783. doi: 10.1016/0008-6363(96)00026-0. [DOI] [PubMed] [Google Scholar]
- 88.Collins PL, Hightower LE. Newcastle disease virus stimulates the cellular accumulation of stress (heat shock) mRNAs and proteins. J Virol. 1982;44:703–707. doi: 10.1128/jvi.44.2.703-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plesset J, Palm C, McLaughlin CS. Induction of heat shock proteins and thermotolerance by ethanol in Saccharomyces cerevisiae . Biochem Biophys Res Commun. 1982;108:1340–1345. doi: 10.1016/0006-291x(82)92147-7. [DOI] [PubMed] [Google Scholar]
- 90.Freeman BC, Michels A, Song J, Kampinga HH, Morimoto RI. Analysis of molecular chaperone activities using in vitro and in vivo approaches. Methods Mol Biol. 2000;99:393–419. doi: 10.1385/1-59259-054-3:393. [DOI] [PubMed] [Google Scholar]
- 91.Diller KR. Stress protein expression kinetics. Annu Rev Biomed Eng. 2006;8:403–424. doi: 10.1146/annurev.bioeng.7.060804.100449. [DOI] [PubMed] [Google Scholar]
- 92.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 93.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Evdonin AL, Guzhova IV, Margulis BA, Medvedeva ND. Extracellular heat shock protein 70 mediates heat stress-induced epidermal growth factor receptor transactivation in A431 carcinoma cells. FEBS Lett. 2006;580:6674–6678. doi: 10.1016/j.febslet.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 95.Evdonin AL, Martynova MG, Bystrova OA, Guzhova IV, Margulis BA, Medvedeva ND. The release of Hsp70 from A431 carcinoma cells is mediated by secretory-like granules. Eur J Cell Biol. 2006;85:443–455. doi: 10.1016/j.ejcb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 96.Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods. 2007;43:168–175. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li W, Sahu D, Tsen F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim Biophys Acta. 2012;1823:730–741. doi: 10.1016/j.bbamcr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oda T, Hayano T, Miyaso H, Takahashi N, Yamashita T. Hsp90 regulates the Fanconi anemia DNA damage response pathway. Blood. 2007;109:5016–5026. doi: 10.1182/blood-2006-08-038638. [DOI] [PubMed] [Google Scholar]
- 99.Stecklein SR, Kumaraswamy E, Behbod F, Wang W, Chaguturu V, Harlan-Williams LM, Jensen RA. BRCA1 and HSP90 cooperate in homologous and non-homologous DNA double-strand-break repair and G2/M checkpoint activation. Proc Natl Acad Sci USA. 2012;109:13650–13655. doi: 10.1073/pnas.1203326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehlen P, Arrigo AP. The serum-induced phosphorylation of mammalian hsp27 correlates with changes in its intracellular localization and levels of oligomerization. Eur J Biochem. 1994;221:327–334. doi: 10.1111/j.1432-1033.1994.tb18744.x. [DOI] [PubMed] [Google Scholar]
- 101.Welch WJ, Feramisco JR. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984;259:4501–4513. [PubMed] [Google Scholar]
- 102.Kampinga HH. Thermotolerance in mammalian cells. Protein denaturation and aggregation, and stress proteins. J Cell Sci. 1993;104(Pt 1):11–17. doi: 10.1242/jcs.104.1.11. [DOI] [PubMed] [Google Scholar]
- 103.Garrido C, Solary E. A role of HSPs in apoptosis through “protein triage”? Cell Death Differ. 2003;10:619–620. doi: 10.1038/sj.cdd.4401229. [DOI] [PubMed] [Google Scholar]
- 104.Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- 105.Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 106.Marchler G, Schuller C, Adam G, Ruis H. A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J. 1993;12:1997–2003. doi: 10.1002/j.1460-2075.1993.tb05849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harrison CJ, Bohm AA, Nelson HC. Crystal structure of the DNA binding domain of the heat shock transcription factor. Science. 1994;263:224–227. doi: 10.1126/science.8284672. [DOI] [PubMed] [Google Scholar]
- 109.Sorger PK. Heat shock factor and the heat shock response. Cell. 1991;65:363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- 110.Giardina C, Lis JT. Dynamic protein-DNA architecture of a yeast heat shock promoter. Mol Cell Biol. 1995;15:2737–2744. doi: 10.1128/mcb.15.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 113.Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murray JI, Whitfield ML, Trinklein ND, Myers RM, Brown PO, Botstein D. Diverse and specific gene expression responses to stresses in cultured human cells. Mol Biol Cell. 2004;15:2361–2374. doi: 10.1091/mbc.E03-11-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Streffer C. Aspects of biochemical effects by hyperthermia. Natl Cancer Inst Monogr. 1982;61:11–17. [PubMed] [Google Scholar]
- 116.Allen TA, Von Kaenel S, Goodrich JA, Kugel JF. The SINE-encoded mouse B2 RNA represses mRNA transcription in response to heat shock. Nat Struct Mol Biol. 2004;11:816–821. doi: 10.1038/nsmb813. [DOI] [PubMed] [Google Scholar]
- 117.Yakovchuk P, Goodrich JA, Kugel JF. B2 RNA and Alu RNA repress transcription by disrupting contacts between RNA polymerase II and promoter DNA within assembled complexes. Proc Natl Acad Sci USA. 2009;106:5569–5574. doi: 10.1073/pnas.0810738106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mariner PD, Walters RD, Espinoza CA, Drullinger LF, Wagner SD, Kugel JF, Goodrich JA. Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol Cell. 2008;29:499–509. doi: 10.1016/j.molcel.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 119.Espinoza CA, Goodrich JA, Kugel JF. Characterization of the structure, function, and mechanism of B2 RNA, an ncRNA repressor of RNA polymerase II transcription. RNA. 2007;13:583–596. doi: 10.1261/rna.310307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440:556–560. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- 121.Fan J, Yang X, Wang W, Wood WH, 3rd, Becker KG, Gorospe M. Global analysis of stress-regulated mRNA turnover by using cDNA arrays. Proc Natl Acad Sci USA. 2002;99:10611–10616. doi: 10.1073/pnas.162212399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wilmink GJ, Roth CL, Ibey BL, Ketchum N, Bernhard J, Cerna CZ, Roach WP. Identification of microRNAs associated with hyperthermia-induced cellular stress response. Cell Stress Chaperones. 2010;15:1027–1038. doi: 10.1007/s12192-010-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vogel JL, Parsell DA, Lindquist S. Heat-shock proteins Hsp104 and Hsp70 reactivate mRNA splicing after heat inactivation. Curr Biol. 1995;5:306–317. doi: 10.1016/s0960-9822(95)00061-3. [DOI] [PubMed] [Google Scholar]
- 124.Bond U. Heat shock but not other stress inducers leads to the disruption of a sub-set of snRNPs and inhibition of in vitro splicing in HeLa cells. EMBO J. 1988;7:3509–3518. doi: 10.1002/j.1460-2075.1988.tb03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yost HJ, Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986;45:185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]
- 126.Shukla RR, Dominski Z, Zwierzynski T, Kole R. Inactivation of splicing factors in HeLa cells subjected to heat shock. J Biol Chem. 1990;265:20377–20383. [PubMed] [Google Scholar]
- 127.Utans U, Behrens SE, Luhrmann R, Kole R, Kramer A. A splicing factor that is inactivated during in vivo heat shock is functionally equivalent to the [U4/U6.U5] triple snRNP-specific proteins. Genes Dev. 1992;6:631–641. doi: 10.1101/gad.6.4.631. [DOI] [PubMed] [Google Scholar]
- 128.Gattoni R, Mahe D, Mahl P, Fischer N, Mattei MG, Stevenin J, Fuchs JP. The human hnRNP-M proteins: structure and relation with early heat shock-induced splicing arrest and chromosome mapping. Nucleic Acids Res. 1996;24:2535–2542. doi: 10.1093/nar/24.13.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shin C, Feng Y, Manley JL. Dephosphorylated SRp38 acts as a splicing repressor in response to heat shock. Nature. 2004;427:553–558. doi: 10.1038/nature02288. [DOI] [PubMed] [Google Scholar]
- 130.Shin C, Kleiman FE, Manley JL. Multiple properties of the splicing repressor SRp38 distinguish it from typical SR proteins. Mol Cell Biol. 2005;25:8334–8343. doi: 10.1128/MCB.25.18.8334-8343.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Metz A, Soret J, Vourc’h C, Tazi J, Jolly C. A key role for stress-induced satellite III transcripts in the relocalization of splicing factors into nuclear stress granules. J Cell Sci. 2004;117:4551–4558. doi: 10.1242/jcs.01329. [DOI] [PubMed] [Google Scholar]
- 132.Spiro IJ, Denman DL, Dewey WC. Effect of hyperthermia on isolated DNA polymerase-beta. Radiat Res. 1983;95:68–77. [PubMed] [Google Scholar]
- 133.Dikomey E, Becker W, Wielckens K. Reduction of DNA-polymerase beta activity of CHO cells by single and combined heat treatments. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;52:775–785. doi: 10.1080/09553008714552291. [DOI] [PubMed] [Google Scholar]
- 134.Mendez F, Sandigursky M, Franklin WA, Kenny MK, Kureekattil R, Bases R. Heat-shock proteins associated with base excision repair enzymes in HeLa cells. Radiat Res. 2000;153:186–195. doi: 10.1667/0033-7587(2000)153[0186:hspawb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 135.Mendez F, Kozin E, Bases R. Heat shock protein 70 stimulation of the deoxyribonucleic acid base excision repair enzyme polymerase beta. Cell Stress Chaperones. 2003;8:153–161. doi: 10.1379/1466-1268(2003)008<0153:hspsot>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takahashi A, Yamakawa N, Mori E, Ohnishi K, Yokota S, Sugo N, Aratani Y, Koyama H, Ohnishi T. Development of thermotolerance requires interaction between polymerase-beta and heat shock proteins. Cancer Sci. 2008;99:973–978. doi: 10.1111/j.1349-7006.2008.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Drablos F, Feyzi E, Aas PA, Vaagbo CB, Kavli B, Bratlie MS, Pena-Diaz J, Otterlei M, Slupphaug G, Krokan HE. Alkylation damage in DNA and RNA—repair mechanisms and medical significance. DNA Repair (Amst) 2004;3:1389–1407. doi: 10.1016/j.dnarep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 138.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–1295. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 139.Kamileri I, Karakasilioti I, Garinis GA. Nucleotide excision repair: new tricks with old bricks. Trends Genet. 2012;28:566–573. doi: 10.1016/j.tig.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 140.Muenyi CS, States VA, Masters JH, Fan TW, Helm CW, States JC. Sodium arsenite and hyperthermia modulate cisplatin-DNA damage responses and enhance platinum accumulation in murine metastatic ovarian cancer xenograft after hyperthermic intraperitoneal chemotherapy (HIPEC) J Ovarian Res. 2011;4:9. doi: 10.1186/1757-2215-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schmidt-Rose T, Pollet D, Will K, Bergemann J, Wittern KP. Analysis of UV-B-induced DNA damage and its repair in heat-shocked skin cells. J Photochem Photobiol B. 1999;53:144–152. doi: 10.1016/s1011-1344(99)00141-4. [DOI] [PubMed] [Google Scholar]
- 142.Couedel C, Mills KD, Barchi M, Shen L, Olshen A, Johnson RD, Nussenzweig A, Essers J, Kanaar R, Li GC, Alt FW, Jasin M. Collaboration of homologous recombination and nonhomologous end-joining factors for the survival and integrity of mice and cells. Genes Dev. 2004;18:1293–1304. doi: 10.1101/gad.1209204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 144.Dewey WC, Sapareto SA, Betten DA. Hyperthermic radiosensitization of synchronous Chinese hamster cells: relationship between lethality and chromosomal aberrations. Radiat Res. 1978;76:48–59. [PubMed] [Google Scholar]
- 145.Corry PM, Robinson S, Getz S. Hyperthermic effects on DNA repair mechanisms. Radiology. 1977;123:475–482. doi: 10.1148/123.2.475. [DOI] [PubMed] [Google Scholar]
- 146.Wong RS, Dynlacht JR, Cedervall B, Dewey WC. Analysis by pulsed-field gel electrophoresis of DNA double-strand breaks induced by heat and/or X-irradiation in bulk and replicating DNA of CHO cells. Int J Radiat Biol. 1995;68:141–152. doi: 10.1080/09553009514551041. [DOI] [PubMed] [Google Scholar]
- 147.Burgman P, Ouyang H, Peterson S, Chen DJ, Li GC. Heat inactivation of Ku autoantigen: possible role in hyperthermic radiosensitization. Cancer Res. 1997;57:2847–2850. [PubMed] [Google Scholar]
- 148.Stucki M, Jackson SP. gammaH2AX and MDC1: anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair (Amst) 2006;5:534–543. doi: 10.1016/j.dnarep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 149.Laszlo A, Fleischer I. Heat-induced perturbations of DNA damage signaling pathways are modulated by molecular chaperones. Cancer Res. 2009;69:2042–2049. doi: 10.1158/0008-5472.CAN-08-1639. [DOI] [PubMed] [Google Scholar]
- 150.Adams MM, Carpenter PB. Tying the loose ends together in DNA double strand break repair with 53BP1. Cell Div. 2006;1:19. doi: 10.1186/1747-1028-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Carney JP, Maser RS, Olivares H, Davis EM, Le Beau M, Yates JR, 3rd, Hays L, Morgan WF, Petrini JH. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 152.D’Amours D, Jackson SP. The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat Rev Mol Cell Biol. 2002;3:317–327. doi: 10.1038/nrm805. [DOI] [PubMed] [Google Scholar]
- 153.San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 154.Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, Zelensky A, van Bree C, Stalpers LJ, Buist MR, Soullie T, Rens J, Verhagen HJ, O’Connor MJ, Franken NA, Ten Hagen TL, Kanaar R, Aten JA. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA. 2011;108:9851–9856. doi: 10.1073/pnas.1101053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Seno JD, Dynlacht JR. Intracellular redistribution and modification of proteins of the Mre11/Rad50/Nbs1 DNA repair complex following irradiation and heat-shock. J Cell Physiol. 2004;199:157–170. doi: 10.1002/jcp.10475. [DOI] [PubMed] [Google Scholar]
- 156.Ohnishi K, Scuric Z, Yau D, Schiestl RH, Okamoto N, Takahashi A, Ohnishi T. Heat-induced phosphorylation of NBS1 in human skin fibroblast cells. J Cell Biochem. 2006;99:1642–1650. doi: 10.1002/jcb.20995. [DOI] [PubMed] [Google Scholar]
- 157.Xian Ma Y, Fan S, Xiong J, Yuan RQ, Meng Q, Gao M, Goldberg ID, Fuqua SA, Pestell RG, Rosen EM. Role of BRCA1 in heat shock response. Oncogene. 2003;22:10–27. doi: 10.1038/sj.onc.1206061. [DOI] [PubMed] [Google Scholar]
- 158.Eppink B, Krawczyk PM, Stap J, Kanaar R. Hyperthermia-induced DNA repair deficiency suggests novel therapeutic anti-cancer strategies. Int J Hyperth. 2012;28:509–517. doi: 10.3109/02656736.2012.695427. [DOI] [PubMed] [Google Scholar]
- 159.Kampinga HH, Dynlacht JR, Dikomey E. Mechanism of radiosensitization by hyperthermia (> or = 43 degrees C) as derived from studies with DNA repair defective mutant cell lines. Int J Hyperth. 2004;20:131–139. doi: 10.1080/02656730310001627713. [DOI] [PubMed] [Google Scholar]
- 160.Batuello CN, Kelley MR, Dynlacht JR. Role of Ape1 and base excision repair in the radioresponse and heat-radiosensitization of HeLa Cells. Anticancer Res. 2009;29:1319–1325. [PMC free article] [PubMed] [Google Scholar]
- 161.Dynlacht JR, Batuello CN, Lopez JT, Kim KK, Turchi JJ. Identification of Mre11 as a target for heat radiosensitization. Radiat Res. 2011;176:323–332. doi: 10.1667/rr2594.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pandita TK, Pandita S, Bhaumik SR. Molecular parameters of hyperthermia for radiosensitization. Crit Rev Eukaryot Gene Expr. 2009;19:235–251. doi: 10.1615/critreveukargeneexpr.v19.i3.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Iliakis G, Wu W, Wang M. DNA double-strand break repair inhibition as a cause of heat radiosensitization: re-evaluation considering backup pathways of NHEJ. Int J Hyperth. 2008;24:17–29. doi: 10.1080/02656730701784782. [DOI] [PubMed] [Google Scholar]
- 164.Jorritsma JB, Konings AW. The occurrence of DNA strand breaks after hyperthermic treatments of mammalian cells with and without radiation. Radiat Res. 1984;98:198–208. [PubMed] [Google Scholar]
- 165.Warters RL, Brizgys LM, Axtell-Bartlett J. DNA damage production in CHO cells at elevated temperatures. J Cell Physiol. 1985;124:481–486. doi: 10.1002/jcp.1041240318. [DOI] [PubMed] [Google Scholar]
- 166.Hunt CR, Pandita RK, Laszlo A, Higashikubo R, Agarwal M, Kitamura T, Gupta A, Rief N, Horikoshi N, Baskaran R, Lee JH, Lobrich M, Paull TT, Roti Roti JL, Pandita TK. Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res. 2007;67:3010–3017. doi: 10.1158/0008-5472.CAN-06-4328. [DOI] [PubMed] [Google Scholar]
- 167.Kaneko H, Igarashi K, Kataoka K, Miura M. Heat shock induces phosphorylation of histone H2AX in mammalian cells. Biochem Biophys Res Commun. 2005;328:1101–1106. doi: 10.1016/j.bbrc.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 168.Laszlo A, Fleischer I. The heat-induced gamma-H2AX response does not play a role in hyperthermic cell killing. Int J Hyperth. 2009;25:199–209. doi: 10.1080/02656730802631775. [DOI] [PubMed] [Google Scholar]
- 169.Takahashi A, Mori E, Somakos GI, Ohnishi K, Ohnishi T. Heat induces gammaH2AX foci formation in mammalian cells. Mutat Res. 2008;656:88–92. doi: 10.1016/j.mrgentox.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 170.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Velichko AK, Petrova NV, Kantidze OL, Razin SV. Dual effect of heat shock on DNA replication and genome integrity. Mol Biol Cell. 2012;23:3450–3460. doi: 10.1091/mbc.E11-12-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bruskov VI, Malakhova LV, Masalimov ZK, Chernikov AV. Heat-induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA. Nucleic Acids Res. 2002;30:1354–1363. doi: 10.1093/nar/30.6.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gasior SL, Wakeman TP, Xu B, Deininger PL. The human LINE-1 retrotransposon creates DNA double-strand breaks. J Mol Biol. 2006;357:1383–1393. doi: 10.1016/j.jmb.2006.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morales JF, Snow ET, Murnane JP. Environmental factors affecting transcription of the human L1 retrotransposon. II. Stressors. Mutagenesis. 2003;18:151–158. doi: 10.1093/mutage/18.2.151. [DOI] [PubMed] [Google Scholar]
- 175.Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci USA. 2003;100:12871–12876. doi: 10.1073/pnas.2135498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Warters RL, Stone OL. The effects of hyperthermia on DNA replication in HeLa cells. Radiat Res. 1983;93:71–84. [PubMed] [Google Scholar]
- 177.Warters RL, Stone OL. Histone protein and DNA synthesis in HeLa cells after thermal shock. J Cell Physiol. 1984;118:153–160. doi: 10.1002/jcp.1041180207. [DOI] [PubMed] [Google Scholar]
- 178.Wong RS, Kapp LN, Dewey WC. DNA fork displacement rate measurements in heated Chinese hamster ovary cells. Biochim Biophys Acta. 1989;1007:224–227. doi: 10.1016/0167-4781(89)90043-2. [DOI] [PubMed] [Google Scholar]
- 179.Warters RL, Lyons BW. Inhibition of replicon cluster ligation into chromosomal DNA at elevated temperatures. J Cell Physiol. 1990;142:365–371. doi: 10.1002/jcp.1041420220. [DOI] [PubMed] [Google Scholar]
- 180.Iliakis G, Krieg T, Guan J, Wang Y, Leeper D. Evidence for an S-phase checkpoint regulating DNA replication after heat shock: a review. Int J Hyperth. 2004;20:240–249. doi: 10.1080/02656730310001656379. [DOI] [PubMed] [Google Scholar]
- 181.Wang Y, Perrault AR, Iliakis G. Replication protein A as a potential regulator of DNA replication in cells exposed to hyperthermia. Radiat Res. 1998;149:284–293. [PubMed] [Google Scholar]
- 182.Daniely Y, Borowiec JA. Formation of a complex between nucleolin and replication protein A after cell stress prevents initiation of DNA replication. J Cell Biol. 2000;149:799–810. doi: 10.1083/jcb.149.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shao RG, Cao CX, Zhang H, Kohn KW, Wold MS, Pommier Y. Replication-mediated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA:DNA-PK complexes. EMBO J. 1999;18:1397–1406. doi: 10.1093/emboj/18.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dewey WC, Westra A, Miller HH, Nagasawa H. Heat-induced lethality and chromosomal damage in synchronized Chinese hamster cells treated with 5-bromodeoxyuridine. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;20:505–520. doi: 10.1080/09553007114551421. [DOI] [PubMed] [Google Scholar]
- 185.Wong RS, Thompson LL, Dewey WC. Recovery from effects of heat on DNA synthesis in Chinese hamster ovary cells. Radiat Res. 1988;114:125–137. [PubMed] [Google Scholar]
- 186.Bhuyan BK. Kinetics of cell kill by hyperthermia. Cancer Res. 1979;39:2277–2284. [PubMed] [Google Scholar]
- 187.VanderWaal RP, Griffith CL, Wright WD, Borrelli MJ, Roti JL. Delaying S-phase progression rescues cells from heat-induced S-phase hypertoxicity. J Cell Physiol. 2001;187:236–243. doi: 10.1002/jcp.1073. [DOI] [PubMed] [Google Scholar]
- 188.Roti Roti JL. Cellular responses to hyperthermia (40–46 degrees C): cell killing and molecular events. Int J Hyperth. 2008;24:3–15. doi: 10.1080/02656730701769841. [DOI] [PubMed] [Google Scholar]
- 189.Goloudina AR, Demidov ON, Garrido C. Inhibition of HSP70: a challenging anti-cancer strategy. Cancer Lett. 2012;325:117–124. doi: 10.1016/j.canlet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 190.Velichko AK, Kantidze OL, Razin SV. HP1alpha is not necessary for the structural maintenance of centromeric heterochromatin. Epigenetics. 2011;6:380–387. doi: 10.4161/epi.6.3.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]